12 Clinical Trials of Cardiac Resynchronization Therapy
Pacemakers and Defibrillators
Treatment of the heart failure disease substrate with device-based therapy such as CRT has expanded the potential of a heart failure device that not only treats heart failure, but also diagnoses and prevents heart failure exacerbations and arrhythmic events, aiding overall patient management and tracking. Patients with advanced heart failure can receive contemporary medical therapy combined appropriately with electrical resynchronization. Also, their outcome may be predicted by powerful point-of-source data sets that allow for daily diagnostic measures and patient participation in disease management.1–10
Resynchronization devices now represent about 40% of all implantable cardioverter-defibrillators (ICDs) used in the United States.11 Review of clinical trial data shows that at least one third of patients for whom ICDs are indicated also qualify for a CRT with defibrillator (CRT-D) device.12,13 This proportion is likely to increase with expansion of CRT therapy to patients with mild to moderate heart failure.14 Although the implant procedure for a CRT-D is technically more challenging than that for an ICD, CRT has the added advantages of making patients feel better, causing reverse ventricular remodeling, and reducing heart failure hospitalizations—three important goals when offering device therapy to a population with heart failure.2,9,15,16 Despite the evidence base and guidelines, CRT is underutilized in eligible patients, with significant variation in age, gender, QRS duration, care provider, insurance status, and geographic location of practices.17
This chapter also reviews advances in lead technology, and device features for delivering CRT itself or expanding CRT, such as remote device follow-up and enhanced CRT–heart failure diagnostic devices.18–23
 Heart Failure and QRS Delay: Scope of the Problem
Heart Failure and QRS Delay: Scope of the Problem
The greatest expense for the U.S. Medicare Trust Fund is the treatment of heart failure.24–28 The majority of this expense is in the acute management of heart failure hospitalizations, which often require intensive care unit management.26 There are not only about 1 million heart failure hospitalizations yearly, but also 300,000 heart failure deaths. These deaths are primarily caused by progressive pump dysfunction and sudden cardiac death, both of which can be addressed with a CRT and pacemaker (CRT-P) or CRT-D device.8,9 As heart failure severity increases, characterized by both mechanical and electrical remodeling, the primary cause of cardiovascular death typically is pump failure. Conversely, as heart failure functional class improves, electrical instability plays a more prominent role, such that the absolute number of sudden deaths in the patient with advanced heart failure and QRS prolongation is significant, accounting for about one third of all deaths.29,30
When it accompanies heart failure from systolic dysfunction, QRS delay (≥120 msec) itself adds significant morbidity and mortality.31–36 In fact, mortality rates progressively increase as intraventricular conduction delay increases. The latter may also predispose heart failure patients to an increased risk of ventricular arrhythmias by acting as a substrate for reentrant ventricular tachycardia.37
Affecting 30% to 50% of patients with New York Heart Association (NYHA) Class III or IV heart failure, QRS delay, predominantly left bundle branch block (LBBB), impairs cardiac function by introducing intraventricular dyssynchrony. Severe mechanical left ventricular (LV) dyssynchrony is observed in 60% to 70% of patients with QRS duration of 120 msec or greater. It was previously thought that this group of patients with advanced heart failure would be most likely to benefit from resynchronization therapy; this chapter reviews data disproving this assumption. Conduction delay also worsens atrioventricular (AV) and interventricular (VV) dyssynchrony, whereas intraventricular dyssynchrony results in worsening LV function, as measured by the rise of left ventricular pressure (dP/dt) and filling times.38–41 Interestingly, even in patients with right bundle branch block (RBBB) or intraventricular conduction delay, significant electrical delay to the left ventricle is observed on detailed activation mapping, suggesting that RBBB in this setting often represents “concealed” LBBB.42,43
 Studies of CRT in the Acute Setting: How Does It Work?
Studies of CRT in the Acute Setting: How Does It Work?
Cardiac resynchronization therapy is defined as the stimulation of the left ventricle or simultaneous stimulation of both the right and the left ventricle after atrial sensed or paced events or in atrial fibrillation (AF). CRT works by multiple mechanisms, ranging from structural changes (e.g., favorable ventricular remodeling, improved peak oxygen consumption, reduced mitral regurgitation) to cellular and molecular changes (e.g., improved adrenergic-stimulated myocyte function, decreased neurohormonal activation, altered ionic currents and calcium homeostasis). In an elegant study, Tomaselli’s group recently demonstrated that CRT abbreviates the dyssynchronous heart failure–induced prolongation of action potential in cells isolated from the LV lateral wall. Aiba et al.44 conclude that CRT partially reverses the cellular triggers and substrate for arrhythmias in this pacing-induced model of heart failure. CRT works by partially or totally correcting AV, VV, and most importantly left intraventricular dyssynchronies, leading to reverse remodeling and restoring adverse electrophysiologic, neurohormonal, and anatomic changes that result from heart failure.40,44–50 The predominant beneficial effects are on measures of systolic function, as summarized in Table 12-1 and discussed in detail in Chapter 9.
TABLE 12-1 Mechanisms of Acute Improvement in Cardiac Function with Cardiac Resynchronization Therapy (CRT)
| Type of CRT | Mechanisms |
|---|---|
| Atrioventricular (AV) resynchronization | Diminished mitral regurgitation |
| Lengthened diastolic filling time | |
| Optimization of filling pattern | |
| Inter/intraventricular resynchronization | Increases in left ventricular efficiency/systolic blood pressure, dP/dt, pulse pressure, stroke volume, and stroke work |
| Decrease in left ventricular end-systolic volume |
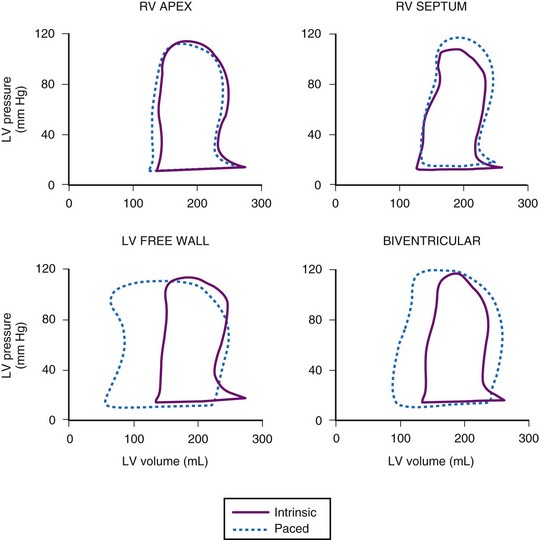
In the first closed-chest study of CRT, in 27 subjects with heart failure and QRS delay, Blanc et al.45 demonstrated improvements in systolic blood pressure, pulmonary capillary wedge pressure, and V-wave amplitude with LV or biventricular (BiV) stimulation compared with baseline or right ventricular (RV) pacing. Kass et al.39 and Aurrichio et al.46 subsequently demonstrated that the effects of CRT could be further optimized by AV delay timing to achieve immediate increases in dP/dt and pulse pressure of 12% to 25% with LV or BiV stimulation, in a total of 45 patients. Figure 12-1 demonstrates the effects of pacing site on pressure-volume loops obtained in a patient with heart failure and LBBB. Neither RV pacing site alters the abnormal loop. However, both left ventricular free wall (LVFW) and BiV stimulation result in reduced LV end-systolic volume and increased stroke volume and stroke work (increased loop width and area). These changes correlated with improved pulse pressure. Interestingly, in the patient with AF and heart block, greater immediate improvement in LV function is achieved with BiV or LV-RV offset stimulation than with single-site LV stimulation, presumably because VV dyssynchrony induced by LV-only stimulation is avoided in the setting of heart block.49
Acute predictors of a beneficial hemodynamic response to CRT were identified to be baseline extent of QRS delay (but not subsequent shortening with pacing) and mechanical dyssynchrony.41,50 LVFW rather than true anterior LV stimulation sites appear to elicit a more robust acute hemodynamic response.48 However, rigorous endocardial mapping has recently shown a high degree of individual variability for the best pacing site.51 The authors concluded that in a homogeneous group of patients with nonischemic dilated cardiomyopathy, the optimal pacing site cannot be predicted, and pacing from the coronary sinus or midlateral wall is rarely optimal.
Speckle tracking analysis of torsion suggests that an acute improvement in LV twist after CRT predicts LV reverse remodeling.52 Similar findings in the PROMISE-CRT study support the hypothesis that acute changes in radial mechanical dyssynchrony are associated with LV reverse remodeling.53
 Controlled Trials of CRT Devices
Controlled Trials of CRT Devices
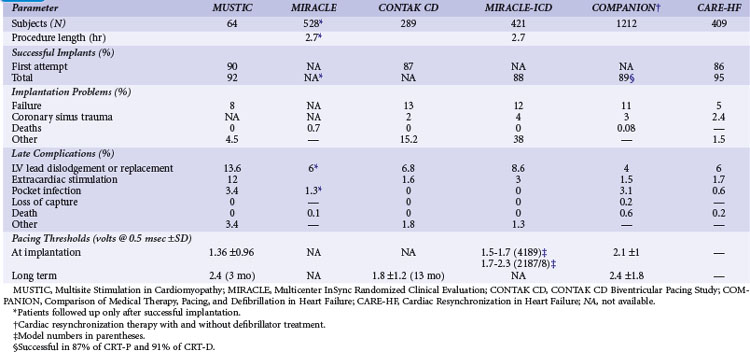
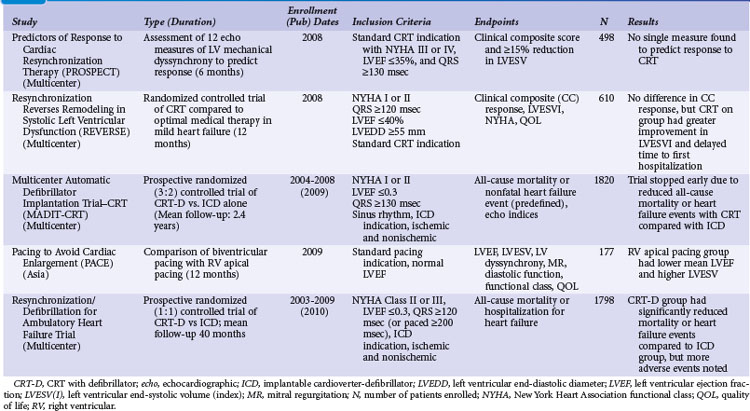
Table 12-2 summarizes the design, inclusion criteria, and results of the early controlled clinical trials of CRT-P and CRT-D devices. In general, inclusion criteria were similar: symptomatic, mostly NYHA Class III and IV heart failure (except REVERSE and MADIT-CRT, later trials that enrolled NYHA I and II patients), left ventricular ejection fraction (LVEF) of less than 0.35, prolonged QRS duration (>120, >130, or >150 msec), and stability of proved medical therapies for heart failure before enrollment.3,5,7–10,54–57
TABLE 12-2 Early Controlled Trials of CRT Alone or with Implantable Cardioverter-Defibrillator (ICD)
Only two early trials used epicardial LV leads, placed through limited thoracotomy for LV stimulation.4,54 The Multisite Stimulation in Cardiomyopathy–Sinus Rhythm (MUSTIC-SR) trial used a stylet-driven coronary sinus lead; the other trials used over-the-wire leads to achieve LV stimulation through a coronary sinus branch vein.3,7–10,54–57 The earliest U.S. CRT study using an epicardial LV lead, the VIGOR-CHF trial, was not completed because of insufficient patient enrollment for the primary functional endpoint of peak oxygen uptake (peak Vo2). In addition, with the emergence of the coronary sinus branch vein lead, patients and physicians became reluctant to continue using a more invasive procedure for LV stimulation. The echocardiographic substudy, however, as with the first European Pacing Therapies in Congestive Heart Failure (PATH-I) study, demonstrated improvement in several measures of response to CRT, comparable to levels seen in studies using transvenous LV stimulation for CRT.7,40,54 The VIGOR-CHF trial demonstrated a decrease in LV and left atrial volumes as well as improvements in LV outflow tract and aortic velocity time integrals and myocardial performance indices with just 12 weeks of CRT. The severity or grade of mitral regurgitation as well as mitral deceleration (a measure of improvement of diastolic LV function) also improved.
In the two early U.S. trials that were the basis for attaining the initial approvals from the U.S. Food and Drug Administration (FDA) for CRT-defibrillator (CONTAK CD and MIRACLE-ICD; see later), patients with NYHA Class II were included, but FDA labeling was not requested for this patient subset and was granted only for patients with NYHA III and IV.8,57,58 Exclusion criteria included the presence of an implanted device and requirement for bradycardia pacing support or permanent AF. The early U.S. trials used parallel design; devices were implanted in all patients, who were then randomly assigned to “CRT on” or “CRT off” status for 6 months. Two principal investigators at each enrolling center were designated in most trials, so the physician managing the medical therapies (heart failure) was blinded as to the treatment assignment, and the implanting physician (electrophysiologist) followed the device performance.
The study endpoints in CRT trials have evolved over time. Although all trials have included safety and efficacy endpoints, the initial trials assessed only measures of heart failure functional status, LV systolic function (LVEF), and LV remodeling (LV end-systolic and diastolic dimensions). The later, larger studies targeted mortality and hospitalization endpoints (Table 12-3). The use of these multiple endpoint measures is standard for heart failure trials evaluating medical therapies and has highlighted the issue of defining “benefit” from CRT.1,6 One can define “response” as consisting only of symptom improvement, or one can require that all three measures of heart failure show benefit, as outlined in Table 12-3. To complicate the issue further, no 1 : 1 correlation seems to exist between these measures of response. Again, the CONTAK CD and MIRACLE-ICD studies enrolled some patients with NYHA Class II in addition to those with NYHA III and IV functional status. In the NYHA II group, significant improvements in measures of functional status were not uniformly observed, although some patients experienced a positive reverse-remodeling response.57,58 This has been confirmed in the much larger REVERSE study, where CRT in patients with NYHA Class I/II heart failure resulted in major structural and functional reverse remodeling at 1 year, with the greatest changes in patients with nonischemic cardiomyopathy.15 Clearly, CRT has a positive effect on decreasing LV size, and the subsequent MADIT-CRT study demonstrated that CRT favorably alters the natural history of heart failure by reducing hospitalization.14,59
| Measure | Endpoints |
|---|---|
| Functional status |
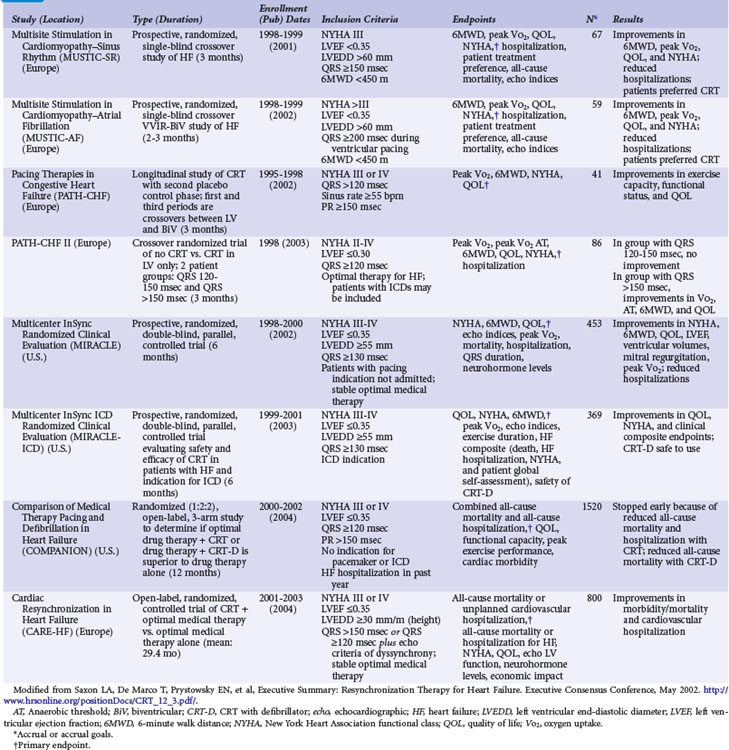
Multisite Stimulation in Cardiomyopathy Studies
The 2001-2002 European Multisite Stimulation in Cardiomyopathy (MUSTIC) studies provided the first long-term controlled trial data on the efficacy of CRT, delivered as BiV stimulation, for 3-month intervals, compared with normal sinus rhythm or continuous RV-based pacing in AF patients.3,56 Figure 12-2 illustrates the crossover study design of the MUSTIC and MUSTIC–Atrial Fibrillation studies. Although only 48 patients completed the two 3-month crossover study periods, all leads placed in the trial were transvenous, with no significant safety issues. Eligibility for patient enrollment included NYHA Class III with QRS longer than 150 msec. In patients with normal sinus rhythm, quality of life (QOL) score improved by 32%, 6-minute walk distance (6MWD) improved by 23%, and peak Vo2 improved by 8%. Although the study was not statistically powered to determine a reduction in rate of hospitalization, hospitalizations after CRT initiation decreased by two thirds.3 At the end of the crossover phase, patients (who were blinded to treatment) were asked to choose which 3-month period they preferred; 85% chose the pacing period during which they had been assigned to VDD, 10% had no preference, and 4% chose ODO (no pacing). Four patients had severe episodes of congestive heart failure exacerbation during the ODO pacing period.
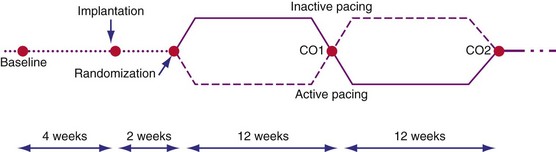
Figure 12-2 Crossover study design of MUSTIC studies.
(From Cazeau S, Leclercq C, Lavergne T, et al: Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 344:873-880, 2001.)
In the patients with permanent AF and continuous RV pacing, 37 of 59 who underwent CRT implantation completed both 3-month crossover phases and had documentation of 97% to 100% CRT delivery. Because of the significant number of dropouts (42%), the intention-to-treat analysis did not show a significant improvement with CRT. In the 37 patients with a complete data set and documentation of CRT, QOL measure did not improve, but 6MWD and peak Vo2 increased significantly, by 9% (P = .05) and 13% (P = .04), respectively.56 When the entire 6-month crossover phase is considered, 10 of 44 patients were hospitalized for heart failure decompensation during RV pacing, whereas only three were hospitalized for heart failure during the CRT period; 85% of patients preferred the CRT period. There was a trend toward a better QOL among patients with CRT (11% improvement; P = .09). Subsequent uncontrolled trials in patients with permanent AF and continuous RV pacing have shown a more robust improvement in these measures, as well as a reverse-remodeling response with CRT compared with RV pacing alone.60,61 The PAVE trial also showed improvement with CRT but did not require heart failure caused by systolic dysfunction for enrollment.62
Pacing Therapies in Congestive Heart Failure
The two Pacing Therapies in Congestive Heart Failure (PATH-I and PATH-II) European studies were groundbreaking in that chronic device programming was based on acute hemodynamic measures of cardiac performance. In addition, these same measures were used to optimize AV-delay programming.54,55 Begun in 1995 and completed in 1998, these trials enrolled patients with NYHA Class III or IV congestive heart failure, sinus rate higher than 55 beats per minute (bpm), and QRS duration longer than 120 msec.
In PATH-I, patients were crossed-over between LV and BiV stimulation with a 1-month interval of no stimulation. A second study phase lasted 9 months and used the CRT mode that achieved what the follow-up physician determined was the most optimal mode. There were no differences in the acute or chronic response of patients whether programmed to LV or BiV CRT. Statistically significant improvements in peak Vo2 anaerobic threshold (24% improvement; P < = .001), 6MWD (25%; P < .001) and QOL (59%; P < .001) were observed at 3 months and 12 months of follow-up. Of 29 patients followed to 12 months, 21 improved from NYHA Class III or IV to Class I or II. Heart failure hospitalizations decreased from 76% in the year before implantation to 31% during the year after implantation. Importantly, LBBB was the type of conduction delay in more than 87% of patients; most CRT trials enroll up to 30% of patients with either intraventricular condition delay (IVCD) or RBBB.4–69 This difference may explain why LV stimulation in PATH-I resulted in only an equivalent response to BiV stimulation to achieve CRT, although the study was not statistically powered to demonstrate a difference between the two modalities, and the long-term data were pooled from both modes. The best that one can conclude is that in small numbers of patients who undergo hemodynamic optimization programming during implantation that shows equivalence between LV and BiV stimulation to achieve CRT, long-term symptom responses appear to be equivalent.
Extending the observations from PATH-I, PATH-II evaluated LV-only CRT compared with no CRT in a 3-month crossover design.55 In all patients with LBBB (88% of subjects), LV pacing was identified as the optimal single-chamber pacing mode (compared with RV only) on the basis of immediate hemodynamic response, and AV delay timing was optimized in all patients. Patients were further divided by QRS duration according to whether the QRS was more than 120 msec but less than 150 msec (“short QRS”) or more than 150 msec (“long QRS”). Unfortunately, only 35 patients, slightly less than one half of all patients enrolled, completed both 3-month crossover intervals. Nonetheless, the study did demonstrate improvements in peak Vo2, anaerobic threshold, 6MWD, and QOL in the patients with long QRS. For example, 71% of the long-QRS group and 38% of the short-QRS group had an increase in the peak Vo2 of more than 1 mL/kg/min with active pacing. This was the first study to demonstrate that QRS duration predicts the magnitude of symptom response to CRT delivered as LV-only stimulation. Subgroup analysis of all but one of the larger U.S. long-term studies of BiV CRT also suggests that the magnitude of benefit may be greater in patients with longer QRS duration at baseline.5,8–10,57 The EARTH trial will compare chronic LV to BiV stimulation and RV stimulation and assess differences in symptoms and in echocardiographic and metabolic exercise test performance, according to stimulation mode in CRT candidates.63
Multicenter InSync Randomized Clinical Evaluation
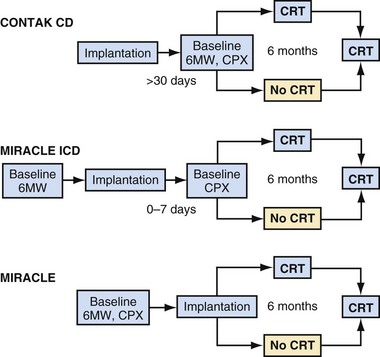
The Multicenter InSync Randomized Clinical Evaluation (MIRACLE) study was the only U.S. trial of CRT for heart failure that used a CRT-pacemaker device only.5 The number of patients randomly allocated in U.S. clinical trials was much greater than those in the European trials until the CARE-HF trial. All 453 patients enrolled in the MIRACLE study underwent implantation of the CRT device and then random assignment to “CRT on” or “CRT off” status for 6 months. Figure 12-3 illustrates the study design of the MIRACLE, MIRACLE-ICD, and CONTAK CD U.S. trials. Unlike the COMPANION trial, in which patients were randomly assigned after consent was obtained and before device implantation, these earlier U.S. trials randomly assigned patients only after a successful CRT implant. The U.S. trials also employed strict protocol-mandated criteria on appropriate and stable heart failure medical regimen requirements before consent and device implantation.
The primary endpoints, including 6MWD, QOL score, and NYHA functional class, were all favorably influenced by CRT, and the effects of CRT were apparent as early as 1 month after therapy initiation. Patients who underwent CRT showed 13% improvement in 6MWD, 13% improvement in QOL, about 1-mL/kg/min improvement in exercise capacity, and an increase in total exercise time of approximately 60 seconds. Unlike the European and acute hemodynamic studies, neither baseline QRS duration nor type of bundle branch block influenced response to CRT in the MIRACLE study. The secondary endpoints, Vo2 and LVEF, also improved with CRT, as did episodes of heart failure worsening, including heart failure hospitalizations. At 6 months, CRT was associated with decreased LV end-diastolic volume (LVEDV) and LV end-systolic volume, reduced LV mass, increased LVEF (+3.6%), decreased mitral regurgitation jet area (−2.5 cm2), and improvement in the clinical composite heart failure score. Improvements in LVEDV and LVEF were twofold greater in patients with nonischemic cardiomyopathy. CRT resulted in significant improvements in NYHA class and LVEF, regardless of age.64
CONTAK CD and Multicenter InSync ICD Randomized Clinical Evaluation
Concurrent with the MIRACLE trial enrollment, two large-scale trials of CRT-defibrillator for patients with heart failure and primary or secondary indications for an ICD were also enrolling subjects, the Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) and the CONTAK CD Biventricular Pacing Study. Unlike the MIRACLE study, the 950 patients randomly assigned to different therapies in the CRT-D studies had primarily ischemic cardiomyopathy (61%-75%,) and about one half of the patients had a secondary indication for the ICD.8,57 In patients with NYHA Class III or IV status, both studies demonstrated improvements in functional measures of heart failure status. An ongoing debate concerns the impact of CRT and reverse remodeling on ventricular arrhythmias. Neither study showed a difference in the incidence of treated episodes of ventricular tachycardia or ventricular fibrillation (VT/VF) with CRT on or off, indicating a neutral effect of CRT on the arrhythmia substrate early after device implantation. A subsequent analysis of the MIRACLE-ICD data indicated that patients with secondary ICD indications experienced more ICD therapies for VT, whereas those with primary ICD indications had more therapy for VF.65 The incidence of ICD therapy, as expected, was higher in those with secondary prevention indications. In CONTAK CD, the incidence of ICD therapy over the 6-month follow-up was 16% for both VT and VF. In contrast, in the InSync ICD Italian Registry, a significant reduction in ventricular arrhythmias and shock therapies was reported and correlated with the degree of ventricular remodeling at 12 months.66 Similar findings were seen in the InSync-III Marquis study, in which anatomic responders to CRT demonstrated fewer premature ventricular contractions (PVCs), runs of PVCs, and therapies for VT/VF.67
Improvements in LVEF and ventricular size and dimension and degree of mitral regurgitation were noted in the VIGOR-CHF, MIRACLE, MIRACLE-ICD, and CONTAK CD studies, all of which had core echocardiographic laboratories performing analysis, with excellent intraobserver and interobserver variability.7,40 As mentioned, even the patients with NYHA Class II benefited from CRT in terms of an echocardiographic response of reverse remodeling.16,57 These CRT-related effects were independent of the use of β-blocker therapy.7 This finding suggests that CRT can exert beneficial effects on the remodeling process across a spectrum of heart failure severity, similar to that observed with angiotensin-converting enzyme (ACE) inhibitor therapy.1 A subsequent study of the effects of CRT on ventricular function, volume, and dimension has shown that the beneficial effects occur as early as 4 weeks and are sustained for a time even after CRT is suspended, indicating that CRT affects cardiac structure.68
Another measure of heart failure progression, level of plasma neurohormones, did not improve or worsen with CRT in the MIRACLE-ICD or MIRACLE study. This neutral effect may be a result of optimization of medical therapy with neurohormonal antagonists before device implantation or inadequate duration of follow-up.7
Comparison of Medical Therapy, Pacing, and Defibrillation on Heart Failure
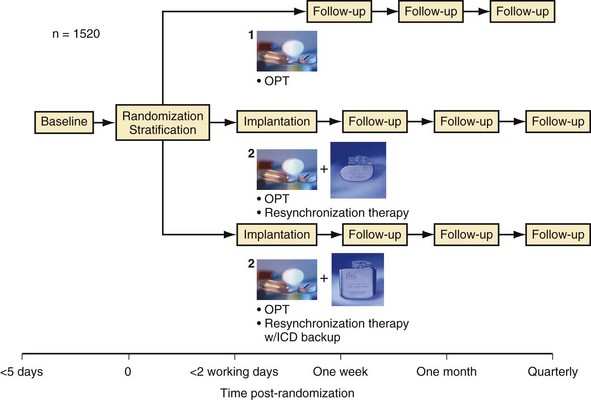
The Comparison of Medical Therapy, Pacing, and Defibrillation on Heart Failure (COMPANION) study was the first and only U.S. trial statistically powered to assess the impact of CRT on hospitalization and mortality endpoints.9,69 During COMPANION’s design, it was unclear whether ICD therapy in addition to CRT would reduce mortality in advanced heart failure compared with medical therapy. Therefore, the study randomly assigned patients to optimal medical (pharmacologic) therapy for heart failure (OPT), a CRT with pacemaker (CRT-P) alone, or a CRT with an ICD (CRT-D). The patients were assigned in a 1 : 2 : 2 ratio, respectively, to maximize the number of patients receiving devices. Statistical power was insufficient to compare CRT with CRT-D directly (both were compared with OPT), but the highest-order secondary endpoint was mortality. To enrich the anticipated event rate in the trial, patients also needed to have heart failure hospitalization in the previous year, but not in the month preceding enrollment, and to be receiving stable medical therapy at enrollment. Unlike the prior trials of CRT, patients were assigned for therapy and data were analyzed after they had provided informed consent, not after they had undergone a successful implantation.
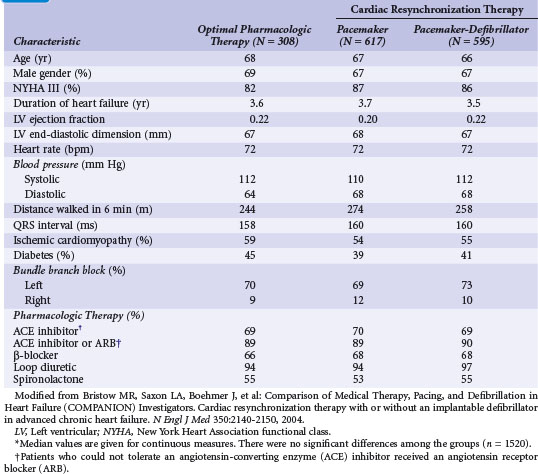
Figure 12-4 provides the design of the COMPANION study, and Table 12-4 lists the clinical characteristics of COMPANION patients. As with MIRACLE patients, and unlike those in the CRT-D studies, an equal proportion of patients in COMPANION had both ischemic and nonischemic etiologies for LV dysfunction. This was the first trial to enroll patients with advanced heart failure who were medically treated with “triple therapy,” consisting of ACE inhibitors, β-receptor blockers, and aldosterone antagonists.
The primary study endpoint was a composite of all-cause hospitalization and mortality, and the secondary endpoint was mortality (1520 patients enrolled). Figure 12-5 shows the event-free survival curves for the primary and secondary endpoints and demonstrates the 20% 12-month reduction in death or hospitalization from any cause observed with both CRT and CRT-D devices compared with OPT. The risk of one of these events was 68% in OPT patients, attesting to the severity of heart failure in this population. Although CRT-P reduced mortality by 24%, this was not statistically significant (P = .06). CRT-D alone reduced mortality by 36% (P = .003) compared with OPT. Close inspection of the mortality curves shows that CRT survival parallels OPT survival until 6 months, when CRT shows benefit. In contrast, the CRT-D and OPT curves separate immediately.
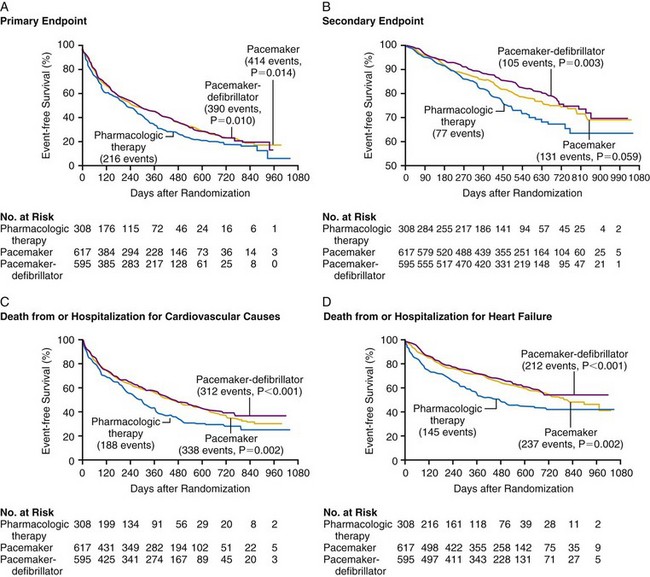
Figure 12-5 Kaplan-Meier estimates in COMPANION study.
(From Bristow MR, Saxon LA, Boehmer J, et al: Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 21;350:2140-2150, 2004.)
These observations suggest that the ICD portion of the CRT-D has an immediate effect to prevent sudden arrhythmic death, whereas the reductions in sudden death with CRT alone may be mediated through stabilization of heart failure status, which may be time dependent. Subsequent analysis showed that the reduced mortality with CRT-D resulted from a decrease in sudden cardiac death, and that the true reduction in hospitalizations resulted from a decrease in heart failure hospitalization, as adjudicated by an events committee.69,70 Subgroup analyses were remarkably consistent in demonstrating CRT benefit in all patient subgroups. There appeared to be equal benefit in women and men, in either ischemic or nonischemic etiologies of heart failure, regardless of LVEF greater or less than 20% and LV size greater or less than 67 mm. Those patients with longer QRS duration did appear to experience greater benefit with CRT, as did those with LBBB rather than RBBB or IVCD. Figure 12-6 provides the subgroup analysis from the COMPANION study, comparing CRT and CRT-D with OPT therapy for the primary and secondary endpoints.
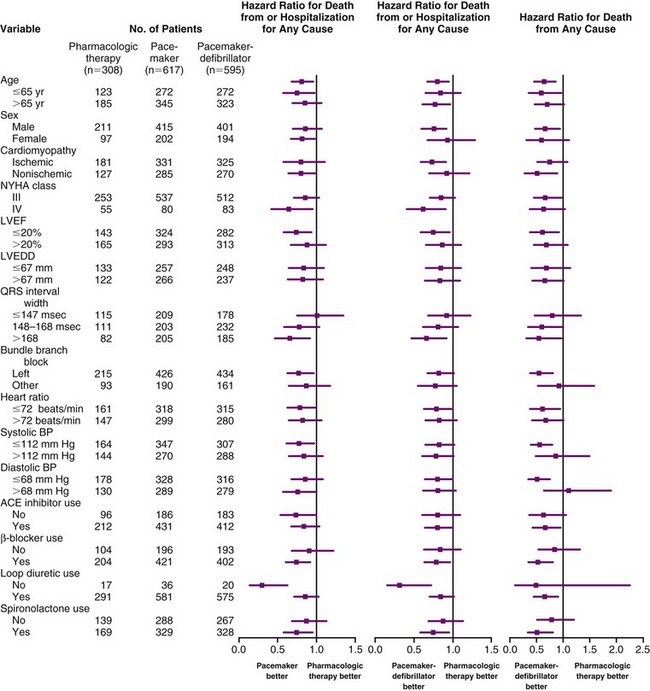
Figure 12-6 Patient characteristics and hazard ratios in COMPANION study.
(From Bristow MR, Saxon LA, Boehmer J, et al: Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators: Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350:2140-2150, 2004.)
Any concern that the implantation of a CRT device may destabilize the usually very ill NYHA Class IV patient was addressed in a post hoc analysis of COMPANION.71 Although these devices did not impact significantly on heart failure deaths, both CRT and CRT-D significantly improved time to all-cause mortality and hospitalizations in the NYHA Class IV subset of patients. Time to sudden death was significantly reduced in the CRT-D group.
The COMPANION data expand the role of CRT to achieve the three primary therapeutic goals in treating patients with heart failure: to improve symptoms, retard disease progression, and reduce rates of hospitalization and mortality. There are two primary reasons for selecting a CRT-D over CRT-P alone. The COMPANION data support early sudden death protection with CRT-D, and the majority of patients with CRT indications also have ICD indications in single-chamber, primary prevention ICD trials.13,14 The 12-month and 24-month appropriate ICD therapy rate was 12% and 19%, respectively, higher than observed in the primary prevention ICD trials. Appropriate ICD therapy was predictive of risk of subsequent hospitalization and death, suggesting that sustained ventricular arrhythmias are a harbinger of worsening heart failure in CRT-D recipients.72
Cardiac Resynchronization-Heart Failure Study
The Cardiac Resynchronization-Heart Failure (CARE-HF) study, enrolling 813 patients at 82 European centers, compared CRT only with optimal medical therapy.10 Over a mean follow-up of 29 months, CRT resulted in significant reductions in the primary composite endpoint of death or cardiovascular hospitalization. Reductions were also achieved in the secondary endpoint of mortality. Figure 12-7 illustrates the Kaplan-Meier curves for these endpoints.
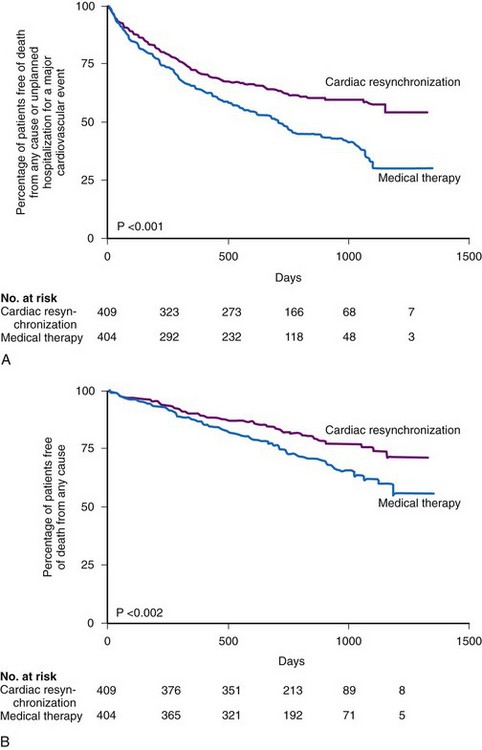
Figure 12-7 Kaplan-Meier estimates in CARE-HF study.
(From Cleland JG, Daubert JC, Erdmann E, et al: Cardiac Resynchronization-Heart Failure [CARE-HF] Study Investigators: The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352:1539-1549, 2005.)
Comparing CARE-HF with COMPANION shows COMPANION patients to be a sicker group, perhaps because heart failure hospitalization was required in the year before enrollment.9 The 12-month mortality rate in the medical therapy group in CARE-HF was 12.6%, versus 19% in COMPANION. Only 38% of the patients in CARE-HF had coronary artery disease, compared with 56% in COMPANION; the mean LVEF was 25% in CARE-HF, compared with 21% in COMPANION; and a higher percentage of patients in COMPANION had NYHA Class IV status (16% vs. 6.5% in CARE-HF). Another issue is the positive effect of CRT on mortality in CARE-HF. The relative risk reduction with CRT in CARE-HF patients was equivalent to that of the COMPANION CRT-D patients (36%). The COMPANION CRT-P group’s 24% reduction in mortality was not statistically significant. These differences may be a result of the shorter duration of follow-up in COMPANION; with longer follow-up, the value may have become significant. Another issue was the risk of sudden death in the CARE-HF patients given CRT; although only 8% of these patients were adjudicated as dying “suddenly,” these accounted for 37% of all deaths. COMPANION data indicate such deaths may have been prevented with a CRT-D device.69
The CRT-P patients in CARE-HF had a reduced risk of sudden death at extended follow-up of 37 months, supporting the concept that chronic stabilization of heart failure status reduces arrhythmias73,74 (Fig. 12-8).
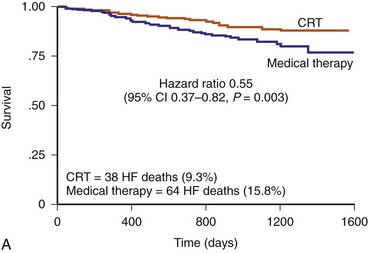
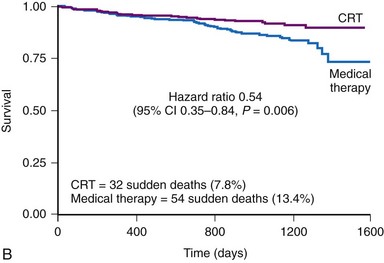
Figure 12-8 Kaplan–Meier estimates in CARE-HF extension phase.
(From Cleland JGF, Daubert JC, Erdmann E, et al: Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase] Eur Heart J 27:1928-1932, 2006.)
The CARE-HF trial answered several other important issues. CRT induces sustained LV reverse remodeling, with the most marked effects in the first 3 to 9 months and continuing up to 29 months.75 The benefits of CRT in patients with and without an ischemic etiology were similar in relative terms.76 Before CARE-HF, follow-up beyond 12 months was available to far too few patients undergoing CRT. This is the first large trial to demonstrate benefit with respect to biomarker measurements as a surrogate of congestive heart failure severity with CRT. A nonsignificant decrease in N-terminal pro–brain natriuretic peptide (NT-BNP) was noted at 3 months, but by 18 months, there was a dramatic decrease of more than 1100 pg/mL (P = .0016). CARE-HF was the first large clinical trial to select patients on the basis of a wide QRS (>149 msec) or >120 msec and the presence of at least two of three markers of dyssynchrony: (1) aortic preejection delay greater than 140 msec, (2) interventricular mechanical delay more than 40 msec, and (3) delayed activation of the posterolateral LV wall, as judged by M-mode and pulsed-wave Doppler.
The incremental benefit of a BiV defibrillator (CRT-D) over a BiV pacemaker (CRT-P) in patients with LV systolic dysfunction remains uncertain. The CRT-D device is more expensive than a CRT-P device and has higher long-term costs because of a shorter battery life. Decision-analysis models suggest that the cost of both is within acceptable standards for QOL years, but that the CRT-P is associated with less costs than the CRT-D device.77–79 Appropriate ICD therapy in CRT patients predicts a much worse outcome, and the number needed to treat (NNT) analysis in COMPANION patients favors the placement of the ICD with CRT therapy.72 Longer-term comparative clinical trials are needed to determine cost-effectiveness because the CRT-D costs are incurred at implant. Although sudden death appears to be reduced with long-term follow-up of CRT-P devices,73 sudden deaths certainly increase in CRT-P versus CRT-D recipients.74 None of the trials reported differences in the efficacy of CRT or CRT-D across patient subgroups, which are unlikely to be detected in underpowered post hoc analyses.
Although the rate of sudden death is lower in patients receiving a defibrillator, one study found no difference in long-term survival in patients receiving CRT-P versus CRT-D.80 In the prospective multicenter MONA LISA cohort study of 198 patients with a CRT-P device for standard indications, prevalence of sustained VT was ascertained from stored intracardiac electrograms (EGMs); capture of EGMs was triggered by a minimal number of programmed ventricular cycles at a minimal programmed cycle length.81 In all patients, devices were also programmed to facilitate the detection of sustained VT by bipolar and optimized ventricular sensing (spontaneous ventricular rate >150 bpm and >64 cycles). Within 1 year after implantation, mortality rate was 11.7%. Although the incidence of sustained VT was only 4.3%, these patients had a significantly higher risk of all-cause mortality and sudden cardiac death than those without sustained VT. This study highlights the value of remote monitoring to detect patients for urgent upgrade, although this is also associated with increased costs, and not all patients will have VT detected before a sudden fatal arrhythmia. Also, sustained episodes of VT could worsen heart failure symptom status and result in syncope.
Prevention of Heart Failure Events
Two pivotal trials studied the effect of CRT in patients with mild heart failure, REVERSE and MADIT.
Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction
The Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study was the first randomized controlled trial (RCT) of CRT in NYHA Class II patients or in NYHA Class I patients with previous heart failure symptoms.59 Patients were randomized to CRT on or off, with a clinical composite endpoint scoring patients as improved, unchanged, or worsened; intergroup efficacy of CRT was limited to percent patients who worsened at 12 months, because asymptomatic Class I patients cannot improve their symptom status. The prospectively powered secondary endpoint was LV end-systolic volume index (LVESVI). The mean LVEF was 26.7%, mean QRS was 153 msec, and all patients were in sinus rhythm receiving OPT. A CRT-D was implanted in 83% of successfully implanted patients, with even distribution between ischemic and nonischemic arms. AV delay was optimized by echocardiography in all patients, regardless of randomization assignment, before the final programming. Although there was no significant difference in the primary endpoint between the two groups, the active CRT group benefited from reduced heart failure hospitalization and improved ventricular function, with a highly significant reduction in LVESVI59 (Fig. 12-9).
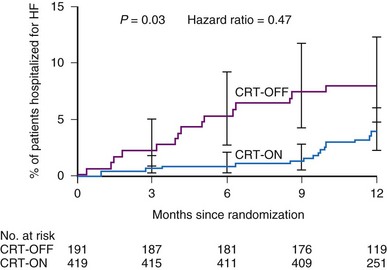
Figure 12-9 Hazard ratios in REVERSE study.
Time to first heart failure hospitalization in first 12 months in CRT-OFF and CRT-ON groups.
(From Linde C, Abraham WT, Gold MR, et al: Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol l52:1834-1843, 2008.)
The REVERSE European cohort of 287 patients had a longer follow-up over 24 months and were much younger with fewer comorbidities than the non-European sample. This cohort demonstrated a significant difference in primary endpoint between the two study groups, which became evident as early as 6 months and persisted for 24 months. A longer follow-up may have allowed the therapeutic effects of CRT to manifest in patients expected to have a slow progression of disease.15
Multi-Center Automatic Defibrillator Implantation Trial–CRT
The Multi-Center Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT) study was an RCT comparing CRT-D devices with RV-based ICDs in patients with QRS greater than 130 msec, ejection fraction (EF) of 30% or less, sinus rhythm, ischemic etiology (NYHA Class I or II) or nonischemic etiology (NYHA II only), and a guideline indication for an ICD.14 The primary endpoint was a composite of all-cause mortality and nonfatal heart failure events (1820 patients enrolled). Over an average follow-up of 2.4 years, the CRT-D group had a 34% risk reduction in the primary endpoint. The trial was stopped early once the interim analysis identified the superiority of CRT-D (Fig. 12-10).
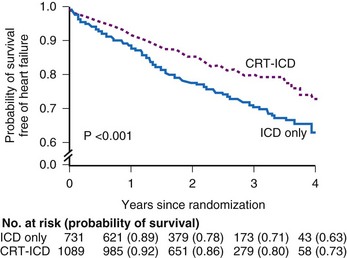
Figure 12-10 Kaplan-Meier estimates in MADIT-CRT.
Probability of survival that is free of heart failure.
(From Moss AJ, Hall WJ, Cannom DS, et al: MADIT-CRT Trial Investigators: Cardiac resynchronization therapy for the prevention of heart-failure events. N Engl J Med 361:1329-1338, 2009.)
The primary endpoint was driven almost entirely by a reduction in the risk of heart failure events, because the annual mortality rate was only 3% in each treatment group. The benefit was evident in patients with both ischemic and nonischemic cardiomyopathy. The outcome curves diverge within the first 2 months, suggesting an early benefit on heart failure hospitalization and other events requiring treatment. Interestingly, prespecified subgroup analysis showed that women derived a greater benefit from CRT-D independent of QRS duration. Patients with QRS of 150 msec or greater derived greater benefit, in keeping with previous trials. No benefit was observed among patients with right bundle branch block. Echocardiographic evaluation showed significant reductions in LV volumes and a mean improvement in EF of 11% at 1 year. Importantly, the results cannot be attributed to an increase in RV pacing–induced heart failure in the ICD group because the demand pacing rate was programmed to 40 ppm. The FDA advisory panel has voted for approval on expansion of current CRT indications.82
Further evidence of the beneficial effects of CRT in patients with mild-to-moderate heart failure has recently been provided by the results of the RAFT study.83 In this trial, 1798 patients with NYHA class II or III heart failure, a QRS duration of 120 msec or more, and a left ventricular ejection fraction of 30% or less were randomly assigned to receive either an ICD alone or an ICD plus CRT. Both groups were on OPT and were followed for an average of 3 years. The primary end point was death from any cause or hospitalization for heart failure, whichever came first. There was a 25% risk reduction in the primary end point in favor of the ICD-CRT group with a similar significant risk reduction in the individual components of the primary end point. The significant improvement in mortality and heart failure hospitalization is a novel observation, as the MADIT-CRT study showed benefit only in heart failure hospitalization. The mean follow-up was longer in the RAFT study and this or other differences in the study populations not apparent from the clinical characteristics data may explain the improvement in mortality observed in RAFT and not in MADIT-CRT. The data from RAFT certainly lend support to the use of CRT in patients with less manifest symptoms of heart failure who have depressed ventricular function and QRS delay. A final important finding of the RAFT study is that the rate of adverse events within 30 days of device implantation was significantly higher in patients in the ICD-CRT group compared to the ICD group. These data indicate that even though LV coronary sinus vein lead implantation can be successfully achieved with current tools by the majority of implanting electrophysiologists, the procedure still carries significant incremental risk compared to a standard left-sided ICD implant. This is particularly important as, in practical terms, the patient populations most apt to increase as a result of the RAFT and MADIT-CRT studies are those undergoing ICD device change-outs with minimally symptomatic heart failure who have continuous RV pacing or native conduction delay.
 Procedural Safety and Left Ventricular Lead Performance
Procedural Safety and Left Ventricular Lead Performance
In all the clinical trials subsequent to the PATH-I study and in patients enrolled early in the CONTAK CD study, LV lead placement was through a coronary sinus branch vein with an over-the-wire lead. In general, choice of lateral LV wall sites was based on trial results of short-term implants showing that the most robust hemodynamic response occurs at this site in patients with LBBB.48 Despite initial concerns about the additional risk of LV branch vein lead placement, particularly given the additional skills required and the extensive anatomic remodeling in advanced heart failure that makes access to the coronary sinus and its branch veins more challenging, the procedural safety and performance of LV leads have been very good (Table 12-5). A learning curve appears to be involved in CRT implantation, and CONTAK CD found that the threshold for attaining a higher than 95% rate of successful LV implantation was 15 cases.84
The most common complication of LV lead placement is coronary sinus trauma, which occurs at a rate of 2% to 4%, although the incidence of true perforation resulting in cardiac tamponade is less than 1%. LV lead dislodgement requiring repositioning occurs in up to 8% of implants. Diaphragmatic stimulation requiring reoperation for lead repositioning occurs in 1% to 4% of patients, but this risk has been lowered with the introduction of bipolar LV leads.85 The reoperation rate for LV lead–related complications was similar in REVERSE (8%), CARE-HF (7%), and RAFT (6.9%).87 In MADIT-CRT the left ventricular lead required repositioning in 4% of patients.
The long-term performance of several LV leads has been found to be safe and effective over time.88–90 Integrated bipolar RV defibrillator leads have a significantly lower incidence of RV anodal stimulation than dedicated bipolar RV defibrillator leads.91
The evolution of LV leads has driven the success of CRT. The high implant success rate (97%) seen in REVERSE undoubtedly represents an improvement in implantation techniques and lead technologies compared with earlier CRT studies. A new generation of smaller 4 French (4F) leads is currently in use, and further developments are anticipated with multipolar leads.92 The Quartet LV pacing lead features four pacing electrodes with up to 10 pacing configurations. Multiple vectors allow pacing around scar tissue and help overcome problems with high capture thresholds and diaphragmatic stimulation without the need for surgical repositioning of the lead.
 Trials of Long-Term CRT in Special Populations
Trials of Long-Term CRT in Special Populations
Although the incidence of atrial fibrillation in patients with advanced heart failure is 30% or higher, the effects of CRT in this patient population have been poorly studied. One study compared the results of 25 months of CRT in 162 patients with permanent AF, including 48 patients who had His bundle ablation, with results in 511 patients in sinus rhythm. Only those in the His ablation subgroup were shown to benefit from symptomatic improvement, suggesting that the LV requires to be paced consistently for CRT to be successful.93
Post AV Nodal Ablation Evaluation
The Post AV Nodal Ablation Evaluation (PAVE) study compared CRT with RV pacing in patients with permanent AF who were undergoing AV nodal ablation.62 Entry criteria for the study did not require patients to have symptomatic heart failure or systolic dysfunction. However, patients were required to have exercise limitation, defined as the inability to walk farther than 450 m during a 6MWD test, which was also the primary study endpoint. Peak Vo2 and QOL scores were assessed as secondary endpoints.
We can conclude from this trial that CRT does provide greater improvement than RV pacing in the functional status of patients undergoing AV nodal ablation if they have significant baseline limitation of exercise capacity. However, the need for His bundle ablation has been challenged in an observational study of 295 consecutive patients with NYHA Class III or IV heart failure, EF of 35% or less, and QRS of 120 msec or greater.94 Over follow-up of 6.8 years, no differences were found between patients in AF or sinus rhythm in any of the mortality or morbidity endpoints, even without AV junction ablation. The percentage of BiV pacing achieved in patients in AF was 87%. A very recent meta-analysis, which compared outcomes of CRT between heart failure patients with atrial fibrillation versus sinus rhythm shows that atrial fibrillation is associated with a lower likelihood of reverse remodeling and increased risk of clinical non-response and death compared to sinus rhythm.95 AV node ablation in selected patients with atrial fibrillation appeared to improve the rates of clinical response and survival. However, important differences exist between the device programming features for these two rhythms, and only a properly designed randomized trial can address the role of AV junction ablation in patients with permanent AF undergoing CRT.
Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS
Although CRT is clearly effective in patients with dyssynchrony from a wide QRS, more than one third of patients with a “narrow” QRS also display some degree of mechanical dyssynchrony. The Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS (RethinQ) trial has been the only trial to study the effect of CRT in patients with heart failure limited to a narrow QRS.96 Criteria for enrollment were ischemic or nonischemic cardiomyopathy with EF of 35% or less, NYHA Class III, QRS of 130 msec or less, and one of two echocardiographic measures of dyssynchrony: an opposing wall delay of 65 msec or longer on tissue Doppler imaging (TDI) or a septal-to-posterior wall delay of 130 msec or greater on M-mode imaging.
ESTEEM-CRT and Echo-CRT
A smaller study, ESTEEM-CRT, used the standard deviation of time to peak velocity of 12 echocardiographic segments to select patients with mechanical dyssynchrony, but with a QRS of 120 msec or less. It also found no improvement in exercise performance or reverse remodeling over 6 months despite invasive dP/dtmax testing for AV optimization at implant.97
We can conclude that current echocardiographic criteria are unable to identify patients with mechanical but no electrical dyssynchrony who would benefit from CRT. Trials in progress include Echocardiography Guided Cardiac Resynchronization Therapy (Echo-CRT), an international trial enrolling patients with QRS of 130 msec or less and evidence of dyssynchrony, as assessed either by speckle tracking, radial strain, septal–posterior wall delay of 130 msec or longer or by color TDI with an opposing wall delay of 80 msec or greater. The primary endpoint will evaluate the effect of CRT on all-cause mortality or first hospitalization for worsening heart failure.98
Pacing to Avoid Cardiac Enlargement
The optimal pacing mode and site(s) for patients with normal LV systolic function remains uncertain. RV apical pacing has been associated with deleterious LV function in observational studies.99,100 The role of biventricular pacing in preventing adverse LV remodeling in patients with bradycardia and normal LVEF was recently reported. The Pacing to Avoid Cardiac Enlargement (PACE) trial randomly assigned 177 patients with a BiV pacemaker to receive BiV pacing or RV apical pacing.101 At 12 months, mean LVEF assessed by three-dimensional echocardiography was significantly lower in the RV pacing group, with an absolute difference of 7.4%, and LV end-systolic volume was significantly higher. There were no differences in heart failure hospitalization, functional capacity, or QOL between the two groups.
 Trials Evaluating Device Features and Programming
Trials Evaluating Device Features and Programming
A number of ongoing or completed clinical trials have evaluated the use of CRT device features or programming options. Some features, such as atrial antitachycardia pacing and defibrillation, are available in an FDA-approved RV ICD and have been tested for safety and efficacy in a CRT device.20 In other cases, such as studies evaluating the usefulness of RV/LV offset programming for CRT, the feature is specific to a CRT device and intended to maximize response to therapy.102–104
RENEWAL Study
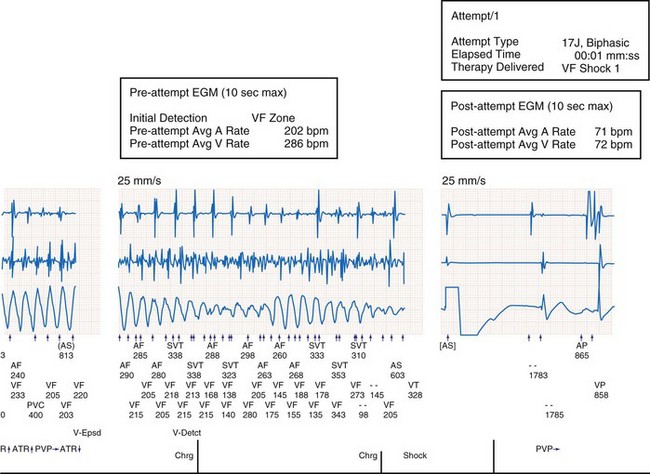
In the multicenter RENEWAL 3 AVT clinical study of CRT-D in patients with AF, the safety and efficacy of a defibrillator capable of advanced atrial therapies and diagnostics, including atrial defibrillation, was tested in a CRT device.20 In this single-arm study of 138 patients with paroxysmal or persistent AF in the year before enrollment, the delivery of atrial and ventricular antitachycardia therapies was not compromised by CRT delivery. Conversely, CRT did not impair the delivery of antitachycardia therapies. Figure 12-11 shows AF occurring in the presence of VF; both atrial and ventricular diagnostics and therapies were programmed “on.” The device correctly sensed both AF and VF and prioritized treatment of VF with a 17-J shock, which converted the rhythm to atrially paced CRT.
DECREASE-HF and Rhythm-II-ICD
Two trials have investigated if an optimal RV and LV pacing sequence yields the best global cardiac performance in CRT recipients. The Device Evaluation of CONTAK RENEWAL-2 and EASYTRAK-2: Assessment of Safety and Effectiveness in Heart Failure (DECREASE-HF) was the first prospective randomized trial comparing LV only or interventricular (VV) offset against simultaneous BiV stimulation to achieve CRT.102 306 patients (NYHA III or IV heart failure, LVEF ≤35%, QRS ≥150 msec) were randomized to three arms in a 1 : 1 : 1 ratio. To ensure ventricular pacing, programming of the AV delay was performed via the Expert Ease device feature, which uses QRS duration, lead location, and the patient’s native AV interval to determine the optimal programmed atrioventricular delay, as measured from intracardiac electrograms. In patients randomized to sequential BiV pacing, the VV timing was programmed with LV activation preceding RV activation by 20 to 80 msec. Echocardiographic assessments were performed at baseline, 3 months, and 6 months to determine the effects of the method of CRT delivery on cardiac function. All groups had a significant reduction in LV volume and an improvement of LVEF (median increase of 6.7%) with a trend toward greater improvement with simultaneous BiV pacing.
The multicenter, single blind, randomized Resynchronization for the Hemodynamic Treatment for Heart Failure Management II–ICD (RHYTHM-II-ICD) trial compared the effects of optimized VV delay programming to simultaneous BiV stimulation on clinical outcomes.103 A post hoc analysis studied ventricular remodeling based on paired echocardiographic recordings made over 6-month follow-up.104 Significant improvements in functional status and LV indices were seen in both study groups, but optimization of the VV delay conferred no incremental benefit compared with simultaneous BiV stimulation. These results suggest that individual optimization and programming of VV settings is necessary. Further data will be available in the Evaluation of Resynchronization Therapy in Heart Failure (EARTH) study, which is comparing LV pacing with BiV pacing with a primary endpoint of total exercise duration at a submaximal workload at 12 months.105 Currently, VV timing offset is offered in two FDA-approved CRT devices.106 The data in support of this method of CRT delivery come from a MIRACLE-ICD substudy.
Atrioventricular Optimization Studies
The issue of AV delay programming has not been systematically evaluated in large clinical trials of long-term CRT. Uncontrolled data on short-term and long-term therapies based largely on echocardiographic assessment of mitral inflow and forward output have shown that for most subjects, AV delay programming during atrial sensing is optimal at 100 to 130 msec, although exceptions exist.46 One report demonstrated that altering programming in implanted CRT devices from atrially sensed VDD stimulation to DDD stimulation actually worsened intraventricular LV synchrony and curtailed LV filling and worsened the myocardial performance index, a measure of systolic and diastolic function.107–108 These effects are most likely caused by delay in intra-atrial conduction times. Compared to atrial sensing, pacing from a long-term implanted right atrial lead results in delayed left atrial activation which, in turn, reduces LV filling time. This important observation suggests that echocardiographic evaluation and repeated AV delay optimization may be indicated in patients with CRT devices who require atrial rate support, to ensure that CRT efficacy is not compromised (Fig. 12-12).
This hypothesis was tested in a prospective multicenter double-blind study using the QuickOpt method in FREEDOM (A Frequent Optimization Study using the QuickOpt Method), which compared frequent (every 3 months) AV/PV and VV delay optimization with a single empirical or optimized setting.109 The primary outcome was a heart failure clinical composite score at 12 months. 1647 patients were randomized in a 1 : 1 fashion and stratified by etiology of cardiomyopathy. The majority of patients were in NYHA Class III and received a primary prevention CRT-D device. There was no significant treatment difference in the primary or secondary endpoint between the 2 groups or in the prespecified subgroup analysis based on etiology. Although to date no randomized study has demonstrated the need for regular adjustment, the Clinical Evaluation of Advanced Resynchronization (CLEAR) study was proof of principle that optimization of pacemaker settings by means of a sensor that measures peak endocardial acceleration can reduce the rate of CRT nonresponders.110
Several small studies have previously demonstrated improved clinical outcomes with echocardiographically guided optimization of the AV delay compared with an empirically set AV delay.111,112 The SMART-AV trial, which was recently reported, randomized 980 patients in a 1 : 1: 1 ratio to a fixed empiric AV delay (120 msec), echocardiographically optimized AV delay or AV delay optimized using SmartDelay, an electrogram based algorithm113. The primary endpoint was left ventricular end-systolic volume. Secondary end points included NYHA class, quality of life score, 6-minute walk distance, left ventricular end-diastolic volume and LVEF. The inclusion criteria for CRT-D were as in previous trials and, following randomization, patients underwent echocardiographic imaging at 3 and 6 months. In contrast to the FREEDOM trial, AV delay optimization was performed after 3 months of follow up in the SD arm only. At 6 months, the change in LVESV for the SD arm was no different than either the echo or fixed arms. No significant differences were noted in any of the secondary structural or functional end points by optimization group. We can conclude from these trials that the routine use of AV optimization does not offer any advantage over nominal settings but still has a role to play in managing patients who are non-responders.
The magnitude of BiV pacing that confers the optimal clinical benefit was reported in a post hoc analysis of mortality and heart failure hospitalization in 1812 patients undergoing CRT in two trials: CRT RENEWAL and REFLEx. Subjects were grouped according to percent BiV pacing quartiles. The greatest magnitude of benefit occurred with greater than 92% pacing.114
Atrial fibrillation and atrial flutter are common cardiac arrhythmias associated with an increased risk of stroke particularly in patients with congestive heart failure caused by LV systolic dysfunction. Because these arrhythmias may occur intermittently, initiation of prophylactic anticoagulation is frequently delayed until patients are symptomatic or ECG evidence is obtained. The IMPACT study is a multicenter randomized trial designed to test the hypothesis that oral anticoagulant therapy guided by episodes of atrial high rate detected and stored by a CRT device improves clinical outcomes, by reducing the combined rate of stroke, systemic embolism, and major bleeding, compared with conventional management in CRT recipients.115
 Screening Echocardiography and LV Lead Position for CRT Response
Screening Echocardiography and LV Lead Position for CRT Response
Depending on how “response” is defined, the major clinical trials of CRT indicate that the magnitude of response to CRT is clinically significant for a variety of endpoints, but that a symptomatic improvement in functional endpoints in not seen in all CRT recipients. Table 12-6 summarizes the data from RCTs. Together, the data indicate that depending on the endpoint measure, up to 30% of patients may not receive at least one of the potential benefits of CRT. A leading contributor to CRT response is believed to be intraventricular dyssynchrony, and investigators have focused on measures of mechanical dyssynchrony to improve the rate of response to therapy.116–119 However, no study has demonstrated that screening for mechanical dyssynchrony either predicts outcome or is superior in patient selection to QRS or electrical delay.
TABLE 12-6 Magnitude of Response to CRT in Randomized Controlled Trials (RCTs)*
| Outline Measure | Magnitude Summary |
|---|---|
| Functional | |
| Functional class improvement (% improving one NYHA class) | 62 to 85 |
| Quality-of-life score improvement | −18 to −24 |
| 6-minute walk distance improvement (m) | 25 to 46 |
| Peak Vo2 improvement (mL/kg/min) | 0.8 to 2 |
| Heart Failure Progression | |
| LV ejection fraction | 0.03 to 0.11 |
| LV end-systolic volume index (mL/m2) | −3 to −7 |
| LV end-diastolic dimension index (mm/m2) | −3 to −6 |
| Heart Failure Outcome (%) | |
| Relative risk reduction in heart failure hospitalization/mortality | 34 to 52 |
| Relative risk reduction in cardiovascular hospitalization/mortality | 36 |
NYHA, New York Heart Association functional class (I-IV); Vo2, oxygen uptake.
* Data from references 2 to; 3-month to 3-year follow-up.
Echocardiography, because it is noninvasive, is the most widely used method for short-term and long-term assessments of both baseline mechanical dyssynchrony and response to therapy. Also, the advent of tissue Doppler ultrasound methods has led to a host of measures that assess regional and particularly intraventricular dyssynchrony.116–117 Up to 50% of patients without QRS delay who have depressed LVEF and symptomatic heart failure also have mechanical dyssynchrony.120–121 Further, uncontrolled trials indicate that these patients demonstrate response to CRT that is similar in magnitude to that in patients with wide QRS delay.121,122 Despite being in use for 10 years, however, there are no published acquisition standards for assessing mechanical dyssynchrony by TDI or strain rate imaging. It is also not clear if patients with QRS delay but without echocardiographic evidence of mechanical dyssynchrony show response to CRT. Nonetheless, these various echocardiographic measures provide an elegant method for assessing mechanical dyssynchrony and resynchronization and offer important ancillary information. Based on current data, however, measures of mechanical dyssynchrony cannot be used to identify CRT candidates, and there are no multicenter trial data to indicate that findings of mechanical dyssynchrony on echocardiography can predict either clinical or reverse-remodeling response to CRT. Echocardiographic measures of dyssynchrony can be valuable in identifying ventricular regions that are scarred and may be used to customize programming, particularly in patients who are not feeling better or not doing more after CRT implant.116–122
Predictors of Response to CRT
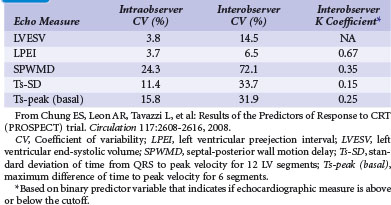
The Predictors of Response to CRT (PROSPECT) trial was the first prospective multicenter study that evaluated 12 common echocardiographic parameters of mechanical dyssynchrony using both conventional and TDI-based methods.123 The study sought to determine if measures of dyssynchrony were correlated with a positive CRT response, using a validated clinical composite score and a measure of reverse remodeling consisting of 15% or more reduction in LV end-systolic volume (LVESV) at 6 months. Fifty-three centers in Europe, Hong Kong, and the United States enrolled 498 patients with standard CRT indications. Three core echocardiography laboratories were used, and all centers received detailed training on echocardiographic acquisition. There was no correlation between clinical or reverse-remodeling response and the 12 dyssynchrony measures. Despite training at all core laboratories, poor intraobserver and interobserver reproducibility and quality of echocardiograms precluded evaluation of many studies (Table 12-7). Thus, at present, QRS duration remains the only prospectively validated parameter for selecting patients who would benefit from CRT.
There is no uniform definition of response to CRT. Most trials have used either a clinical response based on distance walked in 6 minutes or improvement in NYHA functional class over time or an echocardiographic response based on changes in EF or LVESV. Agreement among these response criteria has failed the test of scrutiny. Recently, agreement among 15 response criteria selected from the 26 most-cited publications on predicting response to CRT was assessed in 426 patients from PROSPECT.124 Seven criteria were based on echocardiography, seven on clinical measures, and one was based on a combination of both. The level of agreement not caused by chance among these 15 criteria was poor in 75% and strong in only 4% of cases. The level of agreement between echocardiographic and clinical response criteria was particularly poor and almost equal to the level of agreement predicted by chance.
Despite early acute studies suggesting that LV coronary sinus branch vein position is essential to achieving optimal CRT response,46 the influence of LV lead position on chronic outcomes such as mortality and hospitalization seems minimal. In an analysis of lead position in patients enrolled in COMPANION, LV lead position did not influence the major event-driven outcomes of mortality and hospitalization or impact clinical response.125 A similar conclusion was drawn by a Danish retrospective review of 567 consecutive patients treated with CRT over 10 years.126 A presumed “optimal” LV lead position between 2 and 5 o’clock in the short axis circumference and basal or middle ventricle in the long axis did not correlate with a lower mortality or better clinical response. These findings argue against rejecting a coronary sinus branch vein that achieves stable LV pacing only because it is not in a lateral or posterolateral position. Similarly, there seems little justification for proceeding to a thoracotomy implant if a nonlateral branch vein can be identified that results in stable LV pacing.
Table 12-8 summarizes recent controlled CRT trials.
 Future Populations for Cardiac Resynchronization Therapy
Future Populations for Cardiac Resynchronization Therapy
In general, the new randomized clinical trials of CRT are attempting to build on observations made in previous studies to identify additional patients with heart failure who may benefit from CRT. These studies are targeting those scheduled to undergo implantation or who already have an implanted device because of heart failure and who have RV pacing–induced LBBB.99 Because of the potential for worsening of heart failure from RV pacing–induced dyssynchrony, as well as the findings of uncontrolled studies that “upgrading” RV devices to CRT devices in patients with heart failure improves symptoms and ventricular function, upgrading of RV devices is a common practice in experienced CRT implantation centers and meets Class IIa guidelines but falls outside FDA labeling.60,61 There is currently no randomized trial evaluating CRT in this setting.
Conventional versus Multisite Pacing for Bradyarrhythmia Therapy
A CRT device may also have a role in ameliorating pacing-induced dyssynchrony in that group of patients who have depressed ventricular function and a guideline indication for pacing. The Conventional versus Multisite Pacing for Bradyarrhythmia Therapy (COMBAT) study compared biventricular pacing (BiVP) with right ventricular apical pacing (RVP) in patients with LV dysfunction and AV block.127 This prospective, multicenter, randomized double-blind crossover study enrolled 60 patients with LVEF of 40% or less, receiving optimal medical therapy, in NYHA Class II to IV, and with AV block resulting in a Class I indication for pacing. All patients had a BiV pacemaker implanted and were then randomized to receive either RVP-BiVP-RVP or BiVP-RVP-BiVP. At the end of each 3-month crossover period, patients were evaluated for the primary endpoints of QOL and NYHA functional class. Secondary endpoints included 6MWT, Vo2max, mortality, and echocardiographic indices. Over a mean follow-up of 17.5 months, BiVP was associated with significant improvements in QOL, NYHA class, LVEF, and LVESV compared with RVP. However, the effects of pacing mode on 6MWT and Vo2max were not significantly different in COMBAT, which included a relatively large proportion of patients with Chagas cardiomyopathy.
BLOCK-HF and BioPace Studies
The Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK-HF) study is evaluating the role of CRT in patients with depressed LVEF (≤0.45) and AV block in whom a pacemaker is indicated. The study is assessing whether CRT limits the clinical progression of heart failure compared with DDD RV-based pacing. The primary composite endpoint consists of mortality, morbidity, and cardiac function.128
The Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BioPace) study is randomizing 1200 patients with conventional bradycardia indication to RV or BiVP with or without AV synchrony, depending on atrial rhythm and regardless of underlying EF. The primary endpoint is total mortality.129
 Managing Heart Failure with CRT Devices: Additional Device Features
Managing Heart Failure with CRT Devices: Additional Device Features
A number of device features offer more comprehensive and frequent assessment in the management of patients with advanced heart failure who have a CRT device. Assessment of clinical status before an acute exacerbation of heart failure requiring urgent care is a therapeutic goal and adds both clinical benefit and economic value to CRT-Ds.130 Features predictive of worsening heart failure, such as heart rate variability, are already present in some CRT devices.131 Also, CRT reduced minimum heart rate and increased standard deviation (SD) of the R-R interval. A deterioration of these measures may predict worsening heart failure before it is clinically apparent. Further, these measures can be obtained remotely from the device at frequent intervals. In one study, continuous heart rate variability, as measured by SD of 5-minute median atrial-atrial intervals sensed by the CRT device, was associated with mortality and hospitalization risk.132
Another available feature is thoracic impedance monitoring, measured between the RV lead and the pulse generator. Acute decreases in intrathoracic impedance may reflect increased fluid retention indicative of worsening heart failure. The role of thoracic impedance changes in CRT recipients was systematically analyzed in a study of 532 heart failure patients. An audible alert decreased the rate of combined death and heart failure hospitalization, with a low rate of undetected events.133 The FAST trial demonstrated that impedance measures were superior to weight changes in CRT-D recipients for predicting heart failure exacerbations.134 However, false-positive readings can be observed in up to 75% of patients with impedance changes. These measures, however, whether used alone or combined with other measures (e.g., symptoms or arrhythmia burden), particularly if collected remotely, hold promise for the long-term management of CRT recipients, who face a high risk of worsening heart failure despite the device.70
The relationship between atrial tachyarrhythmias and fluid overload has been retrospectively analyzed in 59 ambulatory patients with LV systolic dysfunction and OptiVol index–capable devices.135 Tachyarrhythmias were not only more prevalent in patients with worsening pulmonary congestion episodes but also preceded 43% of these episodes.
Widely available since 2006 for CRT-Ds, remote monitoring allows daily surveillance of device function and patient status.21,22 Such systems, using telephone or radiofrequency (RF) communication, allow the physician to monitor device function with the patient at home and provide secure access to the Internet for frequent review of device data. The opportunity for better patient management with “virtual” patient encounters is currently being studied, with reports indicating earlier detection of device failure and better management of tachyarrhythmias and heart failure.136,137 This is likely to translate into greater patient safety and enhanced patient and clinician satisfaction. In addition, other aspects of the patient’s condition, such as weight and blood pressure, can be added to the remote transmission to provide a broader view of the patient’s clinical status for all physicians involved in treating the CRT patient.
The impact of the magnitude of RV pacing on mortality was analyzed in the ALTITUDE study using data from more than 100,000 patients in the Boston Scientific LATITUDE remote-monitoring system.138 The mean implant duration was 38 ±20 months. Compared to the RV pacing group, with more than 5% pacing, the group with less than 5% pacing was associated with 43% lower mortality. These results confirm those of the DAVID trial.99 Another prospectively defined question addressed in ALTITUDE was the impact of continuous versus more intermittent biventricular pacing on mortality. The percentage of BiVP meaningfully influenced mortality. Median and mean BiVP was 98% and 93%, respectively, in this real-world setting. Mortality was inversely related to the percentage of BiVP, with an optimal cutoff of 97%; lower BiVP was associated with worse mortality outcomes. This suggests that efforts to optimize the percentage of BiVP, such as AV nodal ablation, are worthwhile. Similar analysis of data, which can reveal programming strategies that reduce inappropriate ICD shocks, points to the power of remote-monitoring systems. Long-term survival in CRT-D patients followed remotely appears superior to those followed in clinic, according to a recent report of over 40,000 CRT recipients.139 This is most likely a result of increased patient engagement and early diagnosis of arrhythmias (e.g., AF) or worsening heart failure.
Currently, a stand-alone device that may be incorporated into a CRT system, the implantable hemodynamic monitor, measures pulmonary artery pressures and provides a reliable surrogate for pulmonary artery diastolic pressure both at rest and with activity.140 The Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) trial enrolled 274 patients from 40 U.S. centers with NYHA Class III/IV status and at least one heart failure hospitalization within 6 months while undergoing optimal medical therapy. The primary endpoint was the rate of combined heart failure hospitalizations, emergency department visits, and urgent visits. The trial showed that access to these measurements may aid in the long-term management of patients with heart failure.19 The concept of adding CRT or defibrillator capability to a cardiac restraint or support device is also being investigated.141
The Hemodynamically Guided Home Self-Therapy in Severe Heart Failure Patients (HOMEOSTASIS) Study Group recently reported on the outcomes in 40 patients in NYHA Class III or IV heart failure who, irrespective of LVEF, had been implanted with an investigational left atrial (LA) pressure monitor.23 After an initial blinded phase of 3 months, patients received individualized therapy instructions guided by LA pressures on a handheld module. Over median follow-up of 25 months, there was a significant reduction in mean daily LA pressure, which translated to a better event-free survival than in the observation period. However, this small, nonrandomized feasibility study limits the conclusions about the safety and clinical effectiveness of this strategy. The LA pressure monitor is being evaluated in the multicenter Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy (LAPTOP-HF) trial and will be implanted alone or with a CRT or ICD device.142
Other techniques delivering electrical impulses to the heart to treat heart failure are in development. Cardiac contractility modulation (CCM) signals deliver high-current impulses from a lead to the heart during the absolute refractory period and do not elicit a new action potential. In a subgroup of the patients with wide QRS, CCM signals were also applied simultaneously with BiV pacing; the effects of CRT and CCM on acute contractile performance, as quantified by dP/dtmax, were additive in most patients.3
The initial experiences with long-term CCM signal applications were obtained in patients with NYHA Class III symptoms and QRS duration of 120 msec or less.143,144 To date, however, completed studies of CCM are nonrandomized, are nonblinded (therefore subject to placebo effect), and have small sample sizes. In a recent European, randomized double-blind study (FIX-CHF-4) of CCM in patients with symptomatic heart failure, patients with EF of 35% or less who were in NYHA Class II or III received a CCM pulse generator and (based on design of MUSTIC) completed two 3-month crossover study periods.145 Although a strong placebo effect was seen in the first 12-week period, there was a significant difference in peak Vo2 and QOL scores between groups at 24 weeks. A further study (FIX-HF-5) now completed in the United States is testing the safety and efficacy of CCM as a treatment for heart failure.142 If these studies show CCM treatment in patients with normal QRS duration to be as safe and effective as CRT in patients with prolonged QRS, a new, easily deployable treatment would be available to patients with otherwise untreatable symptoms. Future studies could also evaluate whether CCM is effective in patients with wide QRS unresponsive to CRT, and if combining CRT with CCM is more effective than CRT alone. Testing these hypotheses would be facilitated by development of a single device that incorporates pacing, antitachycardia therapies, and CCM.
Incorporation of microelectromechanical systems (MEMS) technology into LV leads themselves is another area of interest. Development of short-term and long-term leads with nanotechnology would allow assessment of acute hemodynamic and regional cardiac performance.146
1 Jessup M, Abraham WT, Casey DE, et al. 2009 Focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977-2016.
2 Hunt SA, Abraham WT, Chin M, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure), developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation, endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154-e235.
3 Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. N Engl J Med, 344;24. 2001:873-880.
4 Saxon LA, Boehmer JP, Hummel J, et al. The VIGOR CHF and VENTAK CHF Investigators. Biventricular pacing in patients with congestive heart failure: two prospective randomized trials. Am J Cardiol. 1999;83(5B):120D-123D.
5 Abraham WT, Fisher WG, Smith AL, et al. MIRACLE Study Group. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845-1853.
6 Saxon LA, Ellenbogen KA. Resynchronization therapy for the treatment of heart failure. Circulation. 2003;108(9):1044-1048.
7 St John Sutton MG, Plappert T, Abraham WT, et al. Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group: Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107(15):1985-1990.
8 Young JB, Abraham WT, Smith AL, et al. Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure. The MIRACLE ICD Trial. JAMA. 2003;289(20):2685-2694.
9 Bristow MR, Saxon LA, Boehmer J, et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140-2150.
10 Cleland JG, Daubert JC, Erdmann E, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539-1549.
11 Hammill SC, Kremers MS, Kadish AH, et al. National ICD Registry Annual Report. 2008 Review of the ICD Registry’s third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6(9):1397-1401.
12 Moss AJ, Zareba W, Hall WJ, et al. The Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877-883.
13 Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. N Engl J Med, 352;3. 2005:225-237.
14 Moss AJ, Hall WJ, Cannom DS, et al. Cardiac resynchronization therapy for the prevention of heart failure events. MADIT-CRT Trial Investigators. N Engl J Med, 361;14. 2009:1329-1338.
15 Daubert C, Gold MR, Abraham WT, et al. Reverse Study Group: Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54(20):1837-1846.
16 St John Sutton M, Ghio S, Plappert T, et al. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association Class I/II heart failure. Circulation. 2009;120(19):1858-1865.
17 Curtis AB, Yancy CW, Albert NM, et al. Cardiac resynchronization therapy utilization for heart failure: findings from IMPROVE HF. Am Heart J. 2009;158(6):956-964.
18 Mann DL, Acker MA, Jessup M, et al. Clinical evaluation of the CorCap Cardiac Support Device in patients with dilated cardiomyopathy. Acorn Trial Principal Investigators and Study Coordinators. Ann Thorac Surg, 84;4. 2007:1226-1235.
19 Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. COMPASS-HF Study Group. J Am Coll Cardiol, 51;11. 2008:1073-1079.
20 Saxon LA, Greenfield RA, Crandall BG, et al. Results of the multicenter RENEWAL 3 AVT clinical study of cardiac resynchronization defibrillator therapy in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(5):520-525.
21 Medtronic CareLink Network. http://www.medtronic.com/carelink/.
22 Guidant Corporation. LATITUDE Patient Management. https://www.latitude.guidant.com/.
23 Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Hemodynamically Guided Home Self-Therapy in Severe Heart Failure Patients (HOMEOSTASIS) Study Group. Circulation, 121;9. 2010:1086-1095.
24 Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117(19):2544-2565.
25 Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure–related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52(6):428-434.
26 Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480-486.
27 Liao L, Allen LA, Whellan DJ. Economic burden of heart failure in the elderly. Pharmacoeconomics. 2008;26(6):447-462.
28 http://www.cdc.gov/dhdsp/library/pdfs/fs_heart_failure.pdf.
29 Cohn JN, Goldstein SO, Greenberg BH, et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N Engl J Med. 1998;339(25):1810-1816.
30 Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709-717.
31 Silvet H, Amin J, Padmanabhan S, et al. Prognostic implications of increased QRS duration in patients with moderate and severe left ventricular systolic dysfunction. Am J Cardiol. 2001;88(2):182-185. A6
32 Baldasseroni S, Opasich C, Gorini M, et al. Left bundle branch block is associated with increased 1-year sudden death and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian Network on Congestive Heart Failure. Am Heart J. 2002;143(3):398-405.
33 Stephenson K, Skali H, McMurray JJ, et al. Long-term outcomes of left bundle branch block in high-risk survivors of acute myocardial infarction: the VALIANT experience. Heart Rhythm. 2007;4(3):308-313.
34 Hawkins NM, Wang D, McMurray JJ, et al. Prevalence and prognostic impact of bundle branch block in patients with heart failure: evidence from the CHARM programme. CHARM Investigators and Committees. Eur J Heart Fail, 9;5. 2007:510-517.
35 Tabrizi F, Englund A, Rosenqvist M, et al. Influence of left bundle branch block on long-term mortality in a population with heart failure. Eur Heart J. 2007;28(20):2449-2455.
36 Clark AL, Goode K, Cleland JG. The prevalence and incidence of left bundle branch block in ambulant patients with chronic heart failure. Eur J Heart Fail. 2008;10(7):696-702.
37 Horwich T, Lee SJ, Saxon L. Usefulness of QRS prolongation in predicting risk of inducible monomorphic ventricular tachycardia in patients referred for electrophysiologic studies. Am J Cardiol. 2003;92(7):804-809.
38 Steendijk P, Tulner SA, Schreuder JJ, et al. Quantification of left ventricular mechanical dyssynchrony by conductance catheter in heart failure patients. Am J Physiol Heart Circ Physiol. 2004;286(2):H723-H730.
39 Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99(12):1567-1573.
40 Saxon LA, De Marco T, Schafer J, et al. Effects of chronic biventricular stimulation for resynchronization on echocardiographic measures of remodeling. VIGOR-CHF Investigators. Circulation, 105;11. 2002:1304-1310.
41 Leclercq C, Faris O, Tunin R, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106(14):1760-1763.
42 Fantoni C, Kawabata M, Massaro R, et al. Right and left ventricular activation sequence in patients with heart failure and right bundle branch block: a detailed analysis using three-dimensional non-fluoroscopic electroanatomic mapping system. J Cardiovasc Electrophysiol. 2005;16(2):112-119.
43 Saxon LA. If only rights were really lefts. J Cardiovasc Electrophysiol. 2005;16(7):120-121.
44 Aiba T, Hesketh GG, Barth AS, et al. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009;119(9):1220-1230.
45 Blanc JJ, Etienne Y, Gilard M, et al. Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation. 1997;96(10):3273-3277.
46 Auricchio A, Stellbrink C, Block M, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99(23):2993-3001.
47 Nelson GS, Berger RD, Fetics BJ, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102(25):3053-3059.
48 Butter C, Auricchio A, Stellbrink C, et al. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Pacing Therapy for Chronic Heart Failure II Study Group. Circulation, 104;25. 2001:3026-3029.
49 Hay I, Melenovsky V, Fetics BJ, et al. Short-term effects of right-left heart sequential cardiac resynchronization in patients with heart failure, chronic atrial fibrillation, and atrioventricular nodal block. Circulation. 2004;110(22):3404-3410.
50 Nelson GS, Curry CW, Wyman BT, et al. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation. 2000;101(23):2703-2709.
51 Derval N, Steendijk P, Gula LJ, et al. Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites. J Am Coll Cardiol. 2010;55(6):566-575.
52 Bertini M, Marsan NA, Delgado V, et al. Effects of cardiac resynchronization therapy on left ventricular twist. J Am Coll Cardiol. 2009;54(14):1317-1325.
53 Bank AJ, Kaufman CL, Kelly AS, et al. Results of the Prospective Minnesota Study of ECHO/TDI in Cardiac Resynchronization Therapy (PROMISE-CRT) study. J Card Fail. 2009;15(5):401-409.
54 Auricchio A, Stellbrink C, Sack S, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. J Am Coll Cardiol, 39;12. 2002:2026-2033.
55 Auricchio A, Stellbrink C, Butter C, et al. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. Pacing Therapies in Congestive Heart Failure II Study Group, Guidant Heart Failure Research Group. J Am Coll Cardiol, 42;12. 2003:2125-2157.
56 Linde C, Braunschweig F, Gadler F, et al. Long-term improvements in quality of life by biventricular pacing in patients with chronic heart failure: results from the Multisite Stimulation in Cardiomyopathy study (MUSTIC). Am J Cardiol. 2003;91(9):1090-1095.
57 Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42(8):1454-1459.
58 Abraham WT, Young JB, Leon AR, et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic heart failure. Circulation. 2004;110(18):2864-2868.
59 Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;l52(23):1834-1843.
60 Baker CM, Christopher TJ, Smith PF, et al. Addition of a left ventricular lead to conventional pacing systems in patients with congestive heart failure: feasibility, safety, and early results in 60 consecutive patients. Pacing Clin Electrophysiol. 2002;25(8):1166-1671.
61 Horwich T, Foster E, De Marco T, et al. Effects of resynchronization therapy on cardiac function in pacemaker patients “upgraded” to biventricular devices. J Cardiovasc Electrophysiol. 2004;15(11):1284-1289.
62 Doshi RN, Daoud EG, Fellows C, et al. Left ventricular–based cardiac stimulation Post AV Nodal Ablation Evaluation (the PAVE study). J Cardiovasc Electrophysiol. 2005;16(11):1160-1165.
63 http://clinicaltrials.gov/ct2/show/NCT00900549.
64 Kron J, Aranda JMJr, Miles WM, et al. Benefit of cardiac resynchronization in elderly patients: results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) trials. J Interv Card Electrophysiol. 2009;25(2):91-96.
65 Wilkoff BL, Hess M, Young J, Abraham WT. Differences in tachyarrhythmia detection and implantable cardioverter defibrillator therapy by primary or secondary prevention indication in cardiac resynchronization therapy patients. J Cardiovasc Electrophysiol. 2004;15(9):1002-1009.
66 Di Biase L, Gasparini M, Lunati M, et al. Antiarrhythmic effect of reverse ventricular remodeling induced by cardiac resynchronization therapy: the InSync ICD (Implantable Cardioverter-Defibrillator) Italian Registry. J Am Coll Cardiol. 2008;52(18):1442-1449.
67 Markowitz SM, Lewen JM, Wiggenhorn CJ, et al. Relationship of reverse anatomical remodeling and ventricular arrhythmias after cardiac resynchronization. J Cardiovasc Electrophysiol. 2009;20(3):293-298.
68 Yu CM, Chau E, Sanderson JE, et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002;105(4):438-445.
69 Carson P, Anand I, O’Connor C, et al. Mode of death in advanced heart failure. The Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46(12):2329-2334.
70 Anand IS, Carson P, Galle E, et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation. 2009;119(7):969-977. 24
71 Lindenfeld J, Feldman AM, Saxon L, et al. Effects of cardiac resynchronization therapy with or without a defibrillator on survival and hospitalizations in patients with New York Heart Association Class IV heart failure. Circulation. 2007;115(2):204-212.
72 Saxon LA, Bristow MR, Boehmer J, et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial. Circulation. 2006;114(45):2766-2772.
73 Cleland JGF, Daubert JC, Erdmann E, et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J. 2006;27(16):1928-1932.
74 Saxon LA. More is better with cardiac resynchronization therapy—but is it enough? Eur Heart J. 2006;27(16):1891-1892.
75 Ghio S, Freemantle N, Scelsi L, et al. Long-term left ventricular reverse remodeling with cardiac resynchronization therapy: results from the CARE-HF trial. Eur J Heart Fail. 2009;11(5):480-488.
76 Wikstrom G, Blomström-Lundqvist C, Andren B, et al. The effects of aetiology on outcome in patients treated with cardiac resynchronization therapy in the CARE-HF trial. CARE-HF Study Investigators. Eur Heart J, 30;7. 2009:782-788.
77 Yao G, Freemantle N, Calvert MJ, et al. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J. 2007;28(1):42-51.
78 Feldman AM, de Lissovoy G, Bristow MR, et al. Cost-effectiveness of cardiac resynchronization therapy in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46(12):2311-2321.
79 Fox M, Anderson R, Dean J, et al. The clinical effectiveness and cost-effectiveness of cardiac resynchronization (biventricular pacing) for heart failure: systematic review and economic model. In Health Technology Assessment. 2007. http://www.ncchta.org/fullmono/mon1147.pdf.
80 Stabile G, Solimene F, Bertaglia E, et al. Long-term outcomes of CRT-PM versus CRT-D recipients. Pacing Clin Electrophysiol. 2009;32(Suppl 1):141-145.
81 Boveda S, Marijon E, Jacob S, et al. Incidence and prognostic significance of sustained ventricular tachycardias in heart failure patients implanted with biventricular pacemakers without a back-up defibrillator: results from the prospective, multicentre, MONA LISA cohort study. Eur Heart J. 2009;30(10):1237-1244.
82 http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/ucm230212.htm.
83 Tang AS, Wells GA, Talajic M, et al. Cardiac resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363(25):2385-2395.
84 Higgins S, Giudici M, Hummel J, et al. Procedure time and success rates for the placement of a coronary venous lead designed for left ventricular pacing [abstract]. J Am Coll Cardiol. 2002;39:77A.
85 Saxon LA, Bristow MR, DeMarco T, Krueger SK. Procedural outcomes and device performance in the COMPANION trial of resynchronization therapy for heart failure. Circulation. 2004;110(17):SIII-443.
86 Tang AS, Wells GA, Talajic M, et al. Cardiac resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363(25):2385-2395.
87 Gras D, Böcker D, Lunati M, et al. Implantation of cardiac resynchronization therapy systems in the CARE-HF trial: procedural success rate and safety. Europace. 2007;9(7):516-522.
88 http://www.accessdata.fda.gov/cdrh_docs/pdf/P010012b.pdf.
89 Johnson WB, Abraham WT, Young JB, et al. Long-term performance of the attain Model 4193 left ventricular lead. InSync Registry Investigators. Pacing Clin Electrophysiol, 32;9. 2009:1111-1116.
90 Epstein AE, Baker JH2nd, Beau SL, et al. Performance of the St. Jude Medical Riata leads. Heart Rhythm. 2009;6(2):204-209.
91 Freedman RA, Petrakian A, Boyce K, et al. Performance of dedicated versus integrated bipolar defibrillator leads with CRT-defibrillators: results from a Prospective Multicenter Study. Pacing Clin Electrophysiol. 2009;2(2):157-165.
92 http://clinicaltrials.gov/ct2/show/NCT00964938.
93 Gasparini M, Auricchio A, Regoli F, et al. Four-year efficacy of cardiac resynchronization therapy on exercise tolerance and disease progression: the importance of performing atrioventricular junction ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2006;48(4):734-743.
94 Khadjooi K, Foley PW, Chalil S, et al. Long-term effects of cardiac resynchronization therapy in patients with atrial fibrillation. Heart. 2008;94:879-883.
95 Wilton SB, Leung AA, Ghali WA, et al. Outcomes of cardiac resynchronization therapy in patients with versus without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2011. doi:10.1016/j.hrthm.2011.02.014
96 Beshai JF, Grimm RA, Nagueh SF, et al. RethinQ Study Investigators: Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357(24):2461-2471.
97 Leon AR, Niazi I, Herrman K, et al. Chronic evaluation of CRT in narrow QRS patients with mechanical dyssynchrony from a multicenter study: ESTEEM-CRT. Heart Rhythm. 2008;5(5S):23-24.
98 http://clinicaltrials.gov/ct2/show/NCT00683696.
99 Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial. DAVID Trial Investigators. JAMA, 288;24. 2002:3115-3123.
100 Thambo JB, Bordachar P, Garrigue S, et al. Detrimental ventricular remodelling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. 2004;110(25):3766-3772.
101 Yu CM, Chan JY, Zhang Q, et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361(22):2123-2134.
102 Rao RK, Kumar UN, Schafer J, et al. Reduced ventricular volumes and improved systolic function with cardiac resynchronization therapy: a randomized trial comparing simultaneous biventricular pacing, sequential biventricular pacing, and left ventricular pacing. Circulation. 2007;115(16):2136-2144.
103 Boriani G, Müller CP, Seidl KH, et al. Randomized comparison of simultaneous biventricular stimulation versus optimized interventricular delay in cardiac resynchronization therapy. The Resynchronization for the HemodYnamic Treatment for Heart Failure Management II implantable cardioverter defibrillator (RHYTHM-II-ICD) study. Am Heart J. 2006;151(5):1050-1058.
104 Boriani G, Biffi M, Müller CP, et al. A prospective randomized evaluation of VV delay optimization in CRT-D recipients: echocardiographic observations from the RHYTHM-II-ICD study. Pacing Clin Electrophysiol. 2009;32(10 Suppl 1):120-125.
105 http://clinicaltrials.gov/ct2/show/NCT00901212.
106 US Food and Drug Administration, Center for Devices and Radiological Health. Premarket Approval (PMA) Database. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm.
107 Bernheim A, Ammann P, Sticherling C, et al. Right atrial pacing impairs cardiac function during resynchronization therapy: acute effects of DDD pacing compared to VDD pacing. J Am Coll Cardiol. 2005;45(9):1482-1487.
108 Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function—a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26(6):357-366.
109 Abraham WT, Gras DS: Randomized Clinical Trial to Assess the Safety and Efficacy of Frequent Optimization of Cardiac Resynchronization Therapy using the QuickOpt Method (FREEDOM) Trial Results. Presented at Late breaking Clinical Trials, Heart Rhythm Society Annual Scientific Sessions May 2010.
110 Ritter P, Nägele H, Lunati M, et al. Clinical benefit of cardiac resynchronization therapy in patients optimized by Sonr or standard methods: final results from the CLEAR study. Europace. 2010;12(Suppl 1):50.
111 Sawhney NS, Waggoner AD, Garhwal S, et al. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm. 2004;1(5):562-567.
112 Morales MA, Startari U, Panchetti L, et al. Atrioventricular delay optimization by Doppler-derived left ventricular dP/dt improves 6-month outcome of resynchronized patients. Pacing Clin Electrophysiol. 2006;29(6):564-568.
113 Ellenbogen KA, Gold MR, Meyer TE, et al: Primary results from the SmartDelay determined AV optimization: A comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: A randomized trial comparing empirical, echocardiography-guided and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. DOI: 10.1161/CIRCULATIONAHA.110992552 Accessed on 11/21/2010.
114 Koplan BA, Kaplan AJ, Weiner S, et al. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure: is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol. 2009;53(4):355-360.
115 Ip J, Waldo AL, Lip GY, et al. Multicenter randomized study of anticoagulation guided by remote rhythm monitoring in patients with implantable cardioverter-defibrillator and CRT-D devices: rationale, design, and clinical characteristics of the initially enrolled cohort The IMPACT study. Am Heart J. 2009;158(3):364-370.
116 Bax JJ, Ansalone G, Breithardt OA, et al. Echocardiographic evaluation of cardiac resynchronization therapy: ready for routine clinical use? A critical appraisal. J Am Coll Cardiol. 2004;44(1):1-9.
117 Ansalone G, Giannantoni P, Ricci R, et al. Doppler myocardial imaging in patients with heart failure receiving biventricular pacing treatment. Am Heart J. 2001;142(5):881-886.
118 Sogaard P, Egeblad H, Pedersen AK, et al. Sequential versus simultaneous biventricular resynchronization for severe heart failure: evaluation by tissue Doppler imaging. Circulation. 2002;106(16):2078-2084.
119 Sogaard P, Egeblad H, Kim WY, et al. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long-term cardiac resynchronization therapy. J Am Coll Cardiol. 2002;40(4):723-730.
120 Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89(1):54-60.
121 Achilli A, Sassara M, Ficili S, et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol. 2003;42(12):2117-2124.
122 Gasparini M, Mantica M, Galimberti P, et al. Beneficial effects of biventricular pacing in patients with a “narrow” QRS. Pacing Clin Electrophysiol. 2003;26(1 Pt 2):169-174.
123 Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608-2616.
124 Fornwalt BK, Sprague WW, BeDell P, et al. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121(18):1985-1991.
125 Saxon LA, Olshansky B, Volosin K, et al. Influence of left ventricular lead location on outcomes in the COMPANION study. J Cardiovasc Electrophysiol. 2009;20(7):769-772.
126 Kronborg MB, Albertsen AE, Nielsen JC, Mortensen PT. Long-term clinical outcome and left ventricular lead position in cardiac resynchronization therapy. Europace. 2009;11(9):1177-1182.
127 Filho MM, De Siqueira SF, Costa R, et al. Conventional versus biventricular pacing in heart failure and bradyarrhythmia: the COMBAT study. J Cardiac Fail. 2010;16(4):293-300.
128 Curtis AB, Adamson PB, Chung E, et al. Biventricular versus right ventricular pacing in patients with AV block (BLOCK HF): clinical study design and rationale. J Cardiovasc Electrophysiol. 2007;18(9):965-971.
129 Funck RC, Blanc JJ, Mueller HH, et al. Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BioPace) study. Europace. 2006;8(8):629-635.
130 Saxon LA. Improvement in heart rate turbulence as a measure of response to beta-blocker therapy for heart failure. J Cardiovasc Electrophysiol. 2004;15(7):757-758.
131 Medtronic. InSync Sentry CRT-D Device http://www.medtronic.com/for-healthcare-professionals/products-therapies/cardiac-rhythm/cardiac-resynchronization-therapy-devices/historical-crt-devices/index.htm
132 Adamson PB, Smith AL, Abraham WT, et al. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110(16):2389-2394.
133 Catanzariti D, Lunati M, Landolina M, et al. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Italian Clinical Service OptiVol-CRT Group. Pacing Clin Electrophysiol, 32;3. 2009:363-370.
134 O’Connor CM, Koch WJ, Mann DL. Highlights of the 2009 Scientific Sessions of the Heart Failure Society of America. J Card Fail. 2010;16(1):2-8.
135 Jhanjee R, Templeton GA, Sattiraju S, et al. Relationship of paroxysmal atrial tachyarrhythmias to volume overload: assessment by implanted transpulmonary impedance monitoring. Circ Arrhythm Electrophysiol. 2009;2(5):488-494.
136 Santini M, Ricci RP, Lunati M, et al. Remote monitoring of patients with biventricular defibrillators through the CareLink system improves clinical management of arrhythmias and heart failure episodes. J Interv Card Electrophysiol. 2009;24(1):53-61.
137 Hauck M, Bauer A, Voss F, et al. “Home monitoring” for early detection of implantable cardioverter-defibrillator failure: a single-center prospective observational study. Clin Res Cardiol. 2009;98(1):19-24.
138 Hayes DL, Boehmer JP, Day J, et al. Right ventricular pacing impacts mortality in a large cohort of ICD recipients. Heart Rhythm. 2009;6(5 Suppl 1):75.
139 Saxon LA, Hayes DL, Gilliam FR, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 2010;122(23):2359-2367.
140 Braunschweig F, Linde C, Adamson PB, et al. Continuous central hemodynamic measurements during the six-minute walk test and daily life in patients with chronic heart failure. Eur J Heart Fail. 2009;11(6):594-601.
141 Starling RC, Jessup M, Oh JK, et al. Sustained benefits of the CorCap Cardiac Support Device on left ventricular remodeling: three year follow-up results from the Acorn clinical trial. Ann Thorac Surg. 2007;84(4):1236-1242.
142 http:// www.clinicaltrials.gov.
143 Pappone C, Rosanio S, Burkhoff D, et al. Cardiac contractility modulation by electric currents applied during the refractory period in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;90(12):1307-1313.
144 Pappone C, Augello G, Rosanio S, et al. First human chronic experience with cardiac contractility modulation by nonexcitatory electrical currents for treating systolic heart failure: Mid-term safety and efficacy results from a multicenter study. J Cardiovasc Electrophysiol. 2004;15(4):418-427.
145 Borggrefe MM, Lawo T, Butter C, et al. Randomized, double-blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J. 2008;29(8):1019-1028.
146 MEMS and Nanotechnology Clearinghouse. What is MEMS technology? http://www.memsnet.org/mems/what-is.html.