10 Clinical Trials of Atrial and Ventricular Pacing Modes
 History and Rationale for Different Pacing Modes
History and Rationale for Different Pacing Modes
Cardiac pacing has been developed to treat syncope caused by bradycardia and asystole, initially in patients with permanent third-degree atrioventricular (AV) block. For this purpose, a single lead implanted in the ventricle and pacing at a prespecified rate was sufficient. This simple method of pacing (later coded “VOO 70”) was able to demonstrate a mortality reduction in patients with AV block despite that pacemaker implantation required open-chest surgery at that time.1 Cardiac pacing in permanent third-degree AV block was found not only to prevent syncope, but also to improve symptoms, exercise capacity, and prognosis. It was therefore also used for other patients with bradycardia, such as from intermittent high-degree AV block and sinus node disease (SND).
With technical advancement, the limitations of fixed-rate ventricular pacing were overcome by the development of sensors to provide rate-adaptive (R) ventricular pacing. Rate-adaptive pacing was used to improve the hemodynamic benefit of ventricular single-chamber pacing by providing ventricular rates that take into account the metabolic needs of the patient. In patients with SND, however, ventricular single-chamber pacing in VVI and VVI(R) modes did not reduce mortality2 and could even cause symptoms that were later termed pacemaker syndrome.3 To treat SND, atrial single-chamber pacing has been used, particularly in the Scandinavian countries,4 although many physicians remained skeptical about atrial pacing because it could not prevent asystole if AV block occurred.5 However, a number of advantages of atrial versus ventricular single-chamber pacing were observed, including the absence of pacemaker syndrome, better cardiac output and exercise capacity, resolution of mitral regurgitation that could be induced by ventricular pacing, and a lower incidence of atrial fibrillation.
Comparison Studies
Ventricular Pacing with Versus Without Rate Response (VVI vs. VVIR)
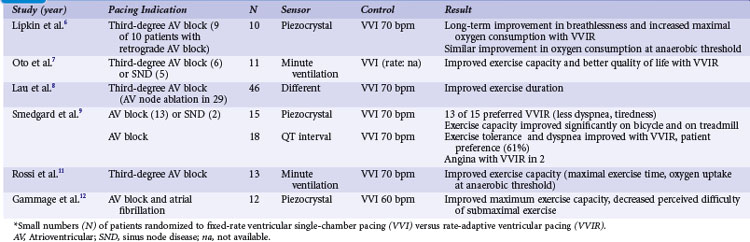
Crossover studies in the 1980s and early 1990s randomized small numbers of patients to fixed-rate ventricular single-chamber pacing (VVI) versus rate-adaptive ventricular pacing (VVIR) with the use of different sensors6–12 (Table 10-1). These studies unequivocally favor rate-adaptive pacing and showed an improvement in submaximal and maximal exercise in terms of objective data (e.g., exercise duration, maximal exercise capacity in watts or duration, VO2 at anaerobic threshold) or subjective data (e.g., pacing mode preference, symptom scores/questionnaires) compared with fixed-rate VVI pacing. This advantage was still present in studies that included patients with SND or retrograde AV conduction. The most striking advantage of VVIR over VVI pacing was observed in patients with AV node ablation for atrial fibrillation.
Ventricular Pacing with Rate Response Versus Dual-Chamber Pacing (VVIR vs. VDD/DDD/DDDR)
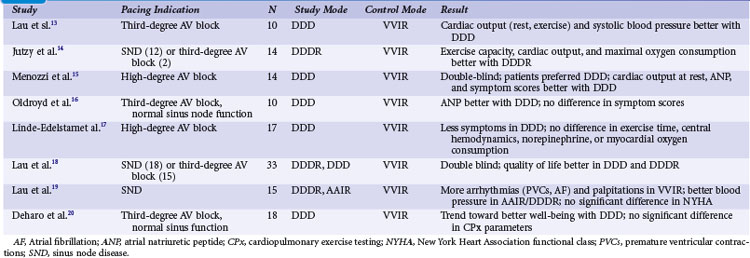
With the advent of dual-chamber pacing, a number of trials tested if rate-adaptive ventricular pacing (VVIR) with an artificial sensor driving the heart rate was comparable to dual-chamber pacing, in which the sinus node determines the heart rate. Investigators observed that sensors frequently reacted inappropriately and were therefore inferior to a chronotropically competent sinus node to increase the heart rate linearly according to metabolic needs during exercise.13 Most studies showed a superiority of dual-chamber pacing in terms of exercise capacity, symptoms, and hemodynamic parameters13–20 (Table 10-2).
Ventricular Single-Chamber Pacing Versus Atrial Synchronized Ventricular Pacing (VVI vs. VDD)
The question if dual-chamber pacing with ventricular pacing synchronized to atrial activity was superior to single-chamber ventricular pacing was evaluated early. In 16 patients with high-degree AV block, Kruse and Rydén21 found a significant increase in maximal exercise capacity in VDD compared to VVI pacing. Interestingly, the extent of improvement was the same for patients older and younger than 65 years. At comparable exercise levels, the atrial rate was significantly lower in VDD than VVI pacing. In a crossover design, 13 patients underwent invasive hemodynamic measurements after 3 months of VDD and VVI pacing.22 During VDD pacing, cardiac output was significantly higher (>30%), particularly during exercise, because of the heart rate increase in VDD and despite a stroke volume increase in VVI pacing. Arteriovenous oxygen difference was significantly higher during VVI pacing. Arterial blood lactate was significantly higher in VVI than VDD pacing during exercise. Heart size was significantly smaller with VDD pacing. In a questionnaire on symptoms and pacemaker choice, patients preferred the VDD mode.
Single-Lead VDD Pacing Versus Dual-Chamber DDD Pacing in Atrioventricular Block
The VDD pacing mode offers the advantage of using only one lead, typically tripolar or quadripolar, with two floating rings that sense atrial activity.23 This pacing mode may be similar to DDD pacing with two leads in patients with AV block and normal sinus node function. However, no RCTs have been performed comparing VDD and DDD pacing. A retrospective study analyzed 1214 consecutive patients with pacemaker implantation for AV block (36.5% VVI; 32.9% DDD; 30.6% VDD).24 At implantation, operation and fluoroscopic times were longer in DDD than in VDD and VVI systems. During follow-up of 5 years, complications requiring surgical interventions occurred 2.3 times more frequently for DDD systems than VDD systems, mainly because of a higher incidence of early atrial lead dysfunction.
Dual-Chamber Pacing in Chronotropic Incompetence or Paroxysmal Atrial Fibrillation: DDD(R) with Mode Switching
Sulke et al.25 compared DDDR, DDD, DDIR, and VVIR pacing modes in 22 patients with high-degree AV block and chronotropic incompetence. In a randomized double-blind crossover design, patients were paced in each of these modes for 4 weeks. Patients were asked their preference, received three questionnaires for subjective assessment, and underwent treadmill testing, mental stress testing, testing of everyday activities (e.g., suitcase lifting, staircase climbing), and echocardiographic evaluation. Scores for subjective grading of well-being were highest for DDDR pacing, similar for DDD and DDIR modes, and lowest for VVIR pacing. DDDR pacing was the preferred mode in 59% of patients, VVIR mode was the least acceptable mode for 73% of patients. Treadmill testing was best in DDDR mode; nontracking modes underresponded to mental stress; and all rate-adaptive modes overreacted to staircase descent. Cardiac output was best in DDDR mode and non-tracking modes showed varying values in patients with AV block. This study suggested that rate-responsive dual-chamber tracking mode is superior to nontracking dual-chamber pacing, dual-chamber pacing without rate-response, and single-chamber ventricular pacing in patients with AV block and chronotropic incompetence, based on both objective and subjective measurements.
Another study compared different pacing modes in 48 patients with AV block and paroxysmal atrial tachyarrhythmias.26 Patients received dual-chamber systems programmed to DDIR mode for 30 days. In a randomized double-blind crossover manner, devices were then programmed for 4 weeks each to DDDR with mode switching, DDDR with limitation of the upper tracking rate, and VVIR. Patients received transtelephonic self-activated devices to transmit electrocardiograms whenever they perceived symptoms. In addition, at the end of each 4-week study period, devices were interrogated; patients underwent 24-hour Holter recordings, received three symptom questionnaires, and at the end of the study were asked for their preferred pacing period. Patients with intolerable symptoms were allowed to cross over early to the next pacing mode. Different devices with fast- and slow-reacting mode-switching algorithms were used. In this study, one third of patients requested early crossover from VVIR to another mode because of intolerable symptoms, most likely pacemaker syndrome. In DDDR pacing without mode switching but limited upper tracking rate, 19% of patients requested early crossover (29% of patients with documented atrial tachyarrhythmias during this period). Only one patient with DDDR and mode switching requested early crossover because of inappropriate tracking of atrial tachyarrhythmia. Patients with fast-reacting mode-switching algorithms in particular preferred DDDR over VVIR mode. Symptom questionnaires favored DDDR with mode switching over DDDR with limited upper racking rates, DDIR, and VVIR pacing. The authors conclude that dual-chamber pacing with tracking and mode switching to a nontracking mode, whenever this automatic function is fast and reliable, is the preferred pacing mode for patients with AV block and paroxysmal atrial tachyarrhythmias (Fig. 10-1).
Dual-Chamber Pacing with Versus Without Rate Response (DDD vs. DDDR)
The need for a sensor in patients with chronotropic incompetence has received little attention after dual-chamber pacing became available and resolved this problem for patients with AV block and normal sinus function. Most studies subsequently compared different sensors.27,28 Additionally, the definition of “chronotropic incompetence” varied widely.
An early study evaluated cardiopulmonary exercise testing with the DDDR versus DDD pacing modes in eight patients.29 Unfortunately, this was a mix of patients, five with AV block and three with sinus node disease, and five with and three without chronotropic incompetence. Chronotropic incompetence was defined as a maximum heart rate at exercise before pacemaker implantation of less than 110 beats per minute (bpm), but not whether it was caused by AV block (resolved with DDD pacing alone) or sinus node dysfunction (requires rate-adaptive pacing). In a crossover study, patients were randomized to DDD or DDDR, each for 3 weeks, and performed an exercise test and completed a symptom evaluation form at the end of each study period. Rate-adaptive pacing was associated with a higher oxygen uptake at the anaerobic threshold and maximum exercise. However, exercise time and subjective symptoms were not different in the whole patient group, only in patients with chronotropic incompetence, and symptoms of exercise intolerance were better with DDDR pacing.
In a study in 63 patients with a DDD pacemaker, a symptom-limited exercise test was performed 1 month after implantation.30 With chronotropic (sinus node) incompetence defined as a maximum heart rate of less than 60% of the age-predicted maximum or less than 100 bpm, 25 patients were then randomized to perform exercise testing in DDD or DDDR mode on two consecutive days. To minimize bias, patients and physicians were blinded with respect to the pacing mode. Paired analysis revealed a statistically significant improvement in the DDDR mode, with higher heart rates at maximum exercise (113 vs. 84 bpm), longer duration of total exercise and time to anaerobic threshold, and higher oxygen uptake at maximum exercise and anaerobic threshold.
Only one large RCT performed according to the principles of evidence-based medicine (EBM) specifically assessed the benefit of rate-adaptive pacing in the dual-chamber mode in patients with sinus rhythm.31 Unfortunately, the single-blind Advanced Elements of Pacing Randomized Controlled Trial (ADEPT) included a mix of patients with SND (~66%) and AV block. After an exercise test 1 month after implantation, 872 patients were enrolled. To be eligible, patients needed to complete at least stage 2 on the Chronotropic Assessment Exercise Protocol and to have a heart rate at peak exercise of 80% or greater of the age-predicted maximum. The mean age was 71 years, however, so patients with a maximum heart rate not exceeding 120 bpm at maximum exercise could be included. Patients were randomized to DDD versus DDDR pacing (dual-sensor system; maximum adaptive rate, 220 bpm minus age) with rate-adaptive AV delay shortening in a parallel study design. After 12 months, general QOL (measured by Short-Form 36 General Health Survey [SF-36], Ferrans and Powers Quality of Life Index) and cardiovascular functional status (Specific Activity Scale and Duke Activity Status Index) were assessed by questionnaires, together with the maximum exercise time at treadmill testing 6 months after randomization. ADEPT found no specific or general differences in scores of the activity and QOL questionnaires. The total exercise time was not different between DDD and DDDR pacing. Independent of discussions about the ideal sensor(s), severe limitations included the following:
 Atrial Versus Ventricular Single-Chamber Pacing in Sinus Node Disease
Atrial Versus Ventricular Single-Chamber Pacing in Sinus Node Disease
In 1994, Andersen et al.34 published the first RCT on pacing mode. A total of 225 patients with SND were randomized to receive a single-chamber pacemaker in the right atrium or ventricle. Of note, the study excluded patients with AV block of any degree (including first-degree AV block with PQ interval >220/260 msec), Wenckebach block at less than 100 bpm, atrial fibrillation (AF) more than 50% of the time (or with pauses >3 seconds or ventricular rates <40 bpm), or bifascicular bundle branch block. In total, 827 of 1052 screened patients were excluded from study participation, predominantly because of AV block (n = 552). Study outcome parameters were mortality, AF, thromboembolism (TE), and heart failure (HF). From retrospective studies, the authors expected AF in 30%, TE in 15%, and mortality in 15% of patients in the ventricular pacing group. The study intent was to demonstrate a 50% reduction in AF and HF and a 66% reduction in TE and mortality with atrial single-chamber pacing, which would have required 100 patients in each group. This rather optimistic prediction of the capabilities of physiologic pacing reflects the widespread belief in its superiority over ventricular pacing at the time of conception of this study.
Andersen et al.35 extended the follow-up until the last patient completed 5 years in the assigned pacing mode. By that time, the first patients enrolled into the trial had completed an 8-year follow-up (mean, 5.5 years). This prolonged follow-up demonstrated a significantly lower mortality in atrial versus ventricular pacing (relative risk reduction [RRR], 34%), mainly from a reduction in cardiovascular mortality (Table 10-3). Similarly, atrial fibrillation was less likely in the atrial pacing group (RRR, 46% vs. ventricular pacing) as were TE events (RRR, 53%). Only 4 of 110 patients developed AV block during the study period (annual rate, 0.6%), two of whom had a right bundle branch block at baseline. The authors detected retrograde conduction in 63% of patients randomized to VVI pacing.
TABLE 10-3 Clinical Events in Atrial versus Ventricular Pacing for Sick Sinus Syndrome (Danish Study)*
| Clinical Event | Ventricular (n = 115) | Atrial (n = 110) |
|---|---|---|
| All-cause mortality | 50% | 34%† |
| Cardiovascular mortality | 34% | 17%† |
| Stroke (cerebrovascular accident) | 23% | 12%† |
| NYHA functional class | Mean 1.3 | Mean 1.5† |
| Permanent atrial fibrillation | 8% | 19%† |
* Results from long-term follow-up.
† P < .05 for lower incidence respectively; better value with AAIR.
From Andersen HR, Nielsen JC, Thomsen PE, et al: Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet 350:1210-1216, 1997.
Critical Appraisal of Danish Study
The Danish study34,35 was the first large-scale RCT on pacing mode selection, at a time when the discussion was whether it was ethically possible to implant VVI(R) pacemaker systems in SND patients. Also, it is as yet the most positive RCT in favor of atrial-based pacing. Compared with RCTs on dual- versus single-chamber ventricular pacing, however, the number of patients in the Danish trial was rather small; the expectations of a 50% AF and HF reduction and even 66% TE and mortality reduction by atrial versus ventricular single-chamber pacing were too optimistic to show a significant difference in such a small patient group. Prolongation of follow-up to cope with this error in sample size calculation was not prespecified, thus arguably not allowed in a prospective trial. Therefore, results resemble a “post hoc” analysis and are only hypothesis generating. However, all results at 3.3 and 5.5 years point to atrial pacing being associated with more favorable clinical results than ventricular pacing. In no study outcome parameter was ventricular better (significantly or nonsignificantly) than atrial pacing.
The Danish trial proposed effective criteria to select patients for AAIR pacing with low risk of AV block. The need to measure the Wenckebach block point at study entry in addition to the PQ interval, however, is not clear. During the study, no development of AV block was predictable by a progressive reduction in the heart rate at the Wenckebach block point.36 However, only a few patients who had documented second-degree or third-degree AV block received an upgrade from AAI to DDD. An observational study found an annual incidence of upgrade from AAI to DDD of 1.7% as a result of documented AV block or AF with slow ventricular rate in 399 patients followed for 4.6 years.37 Also of note was a 0.8% additional annual reoperation rate for the addition of a ventricular lead, mainly because of atrial lead problems. Therefore, 2.5% of AAI systems were surgically changed to VVIR or DDDR per year.
There is renewed interest in the Andersen et al.34,35 data because of the deleterious effects of unnecessary right ventricular pacing observed in the DDDR mode38 (see later). In this context, observations from the Danish trial on development of heart failure with VVI pacing in sinus node disease are important.39 The Danish trial observed an increase in New York Heart Association (NYHA) functional class during follow-up in 31% of patients in the ventricular versus 9% in the atrial group (P < .0005). The incidence of crural edema and dyspnea, together with the need for diuretics during the 5.5-year study period, was significantly higher in patients with ventricular pacing, and the left ventricular fractional shortening decreased significantly only in the ventricular group, not in the atrial pacing group. At the same time, the left atrial diameter increased significantly more during ventricular pacing, from 34 to 41 mm. Interestingly, despite the left atrial dilatation in ventricular pacing in SND, a higher incidence of AF was evident only after 5.5 years, not at 3.3 years of follow-up, despite that TE events were observed more frequently in ventricular pacing already at 3.3 years. Of note, 12 of the 39 patients who developed TE during follow-up never had documentation of AF, neither before randomization nor during follow-up.40 Documentation may have missed AF in a significant number of patients because only 12-lead ECG was used at follow-up. Pacemaker memory functions likely would have documented a much higher incidence of AF.41
 Ventricular Versus Dual-Chamber Pacing
Ventricular Versus Dual-Chamber Pacing
Nonrandomized Early Studies
Starting from impressive observations of improved hemodynamics with VAT and VDD compared to VVI pacing in patients with AV block,22 many publications documented the benefits of “physiologic” versus ventricular pacing from the earlier 1980s to the late 1990s. Various parameters were used to define the presence or absence of this benefit (Table 10-4). Outcomes of dual-chamber or atrial versus ventricular single-chamber pacing particularly note hemodynamic improvement with dual-chamber pacing and range from case reports to patient series.42 Of these multiple outcome parameters, four were frequently chosen as endpoints in prospective, large-scale RCTs: mortality, stroke, atrial fibrillation, and heart failure. Whereas studies of the early 1980s usually focused on hemodynamic parameters, the concept of clinical studies on pacing shifted toward investigating the impact of different pacing modes on clinical events such as mortality, particularly after the CAST study, in which antiarrhythmic drugs successfully suppressed ventricular ectopy but were associated with an increased mortality.
TABLE 10-4 Evaluation Parameters for Impact of Pacing Modes on Cardiac Performance
| Area | Parameters |
|---|---|
| Clinical |
LV, Left ventricular; NYHA, New York Heart Association.
Mortality
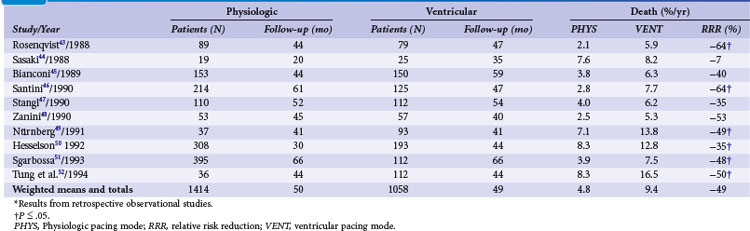
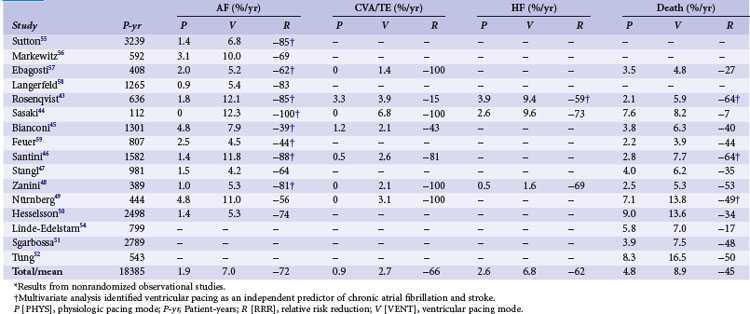
Retrospective early trials showed a significant reduction of mortality for atrial and dual-chamber pacing compared to ventricular single-chamber pacing, predominantly in patients with sinus node disease43–52 (Tables 10-5 and 10-6). However, mortality rates varied widely for VVI (15%-48%) compared to atrial or dual-chamber pacing (7%-27%).50
TABLE 10-6 Survival in Patients with Atrioventricular Block and Other Indications in Relation to Pacing Mode*
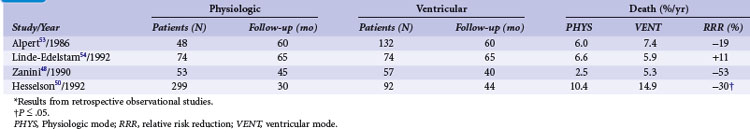
In high-degree AV block, Alpert et al.53 found no difference in all-cause mortality between 132 patients with ventricular compared to 48 patients with dual-chamber systems. Similarly, in 148 patients with AV block (74 with VVI and 74 with VDD pacemaker systems), Linde-Edelstam et al.54 found no difference in mortality in the entire patient group. However, both studies found that in patients with preexisting heart failure, VVI pacing was associated with a significantly higher mortality than VDD pacing. A study of 1245 patients (77% with AV block; ventricular pacing in 97%, dual-chamber pacing in only 3%), suggested that survival was related to the mode of pacing.48 Analyzing 391 patients with AV block or carotid sinus hypersensitivity, Hesselson et al.50 demonstrated a significant mortality benefit during a 7 year follow-up with DDD (54%) versus VVI pacing (33%).
Atrial Fibrillation and Thromboembolism
Starting in the 1980s, almost all retrospective studies showed a lower incidence of AF in patients with atrial/dual-chamber pacing compared to ventricular single-chamber pacing43–52,54–59 (Table 10-7). This was particularly evident for SND.
Heart Failure
Several studies identified ventricular versus “physiologic” pacing mode as an independent predictor of mortality in patients with congestive heart failure (CHF),54 whereas others did not confirm this observation.51 Overall, retrospective studies suggest a large improvement in clinical outcomes with the use of atrial and dual-chamber pacing versus ventricular pacing systems, with a 45% RRR in mortality, 62% reduction in HF, 66% reduction in stroke, and a 72% reduction in AF (see Table 10-7). These data were used to calculate sample size in the Danish trial of AAI versus VVI pacing.34
Medicare Trial
As with other investigators in the late 1980s, Lamas60 noted the problem of “retrograde,” or ventriculoatrial (VA) conduction during VVI pacing at Brigham and Women’s Hospital (Boston). This was a common phenomenon in pacemaker patients and a significant cause of adverse symptoms after VVI pacemaker implantation. Also, only a properly timed atrial systole contributed positively to systolic ventricular performance by the Frank-Starling mechanism which states that the extent of systolic myocardial fiber shortening depends on the degree of diastolic fiber stretch, or preload. Therefore, with the important atrial contribution to cardiac performance, dual-chamber pacing with restoration of normal cardiac physiology (AV synchrony and appropriate rate response) may be advantageous in patients with AV block or SND.
Lamas et al.61 sought to confirm the theoretical advantages of dual-chamber pacing in a large, retrospective analysis of a 20% random sample of all Medicare beneficiaries 65 or older who received a first pacemaker implantation (1988-1990) and whose vital status information was available for at least 1 year (n = 36,312). Dual-chamber pacemaker selection was an independent predictor of survival at 1 and 2 years, with a relative mortality risk reduction of 18%, even after controlling for potentially confounding factors. However, choice of dual- versus single-chamber pacing mode was biased by patient age, gender, indication for pacing, presence of CHF, and AF. Additionally, implantation in the western United States, rural provider, and hospitalization in a facility with 500 beds or more, a private hospital, or a hospital with a catheterization laboratory were also associated with dual- instead of single-chamber pacemaker selection. For this potential bias, the authors performed a prospective trial.
Randomized Studies
In the early 1980s, double-blind crossover studies prospectively compared VVI to dual-chamber pacing. Perrins et al.62 randomized 13 patients with AV block and a VDD pacemaker to dual-chamber or VVI mode. They found a better exercise capacity and reduction of symptoms during the period with dual-chamber pacing, as documented by patients’ diaries and monthly symptom scores. Similarly, Kristensson et al.63 randomized 44 patients to VVI or VDD for 3 weeks for each mode. They observed a statistically significant improvement of maximal exercise tolerance of 14% with AV synchronous pacing. Arterial lactate, respiratory rates, and perceived exertion ratings during submaximal exercise were higher on ventricular inhibited pacing. Symptom scores during the 3-week periods with VVI pacing were worse than with dual-chamber pacing. Most patients had a better functional class during AV synchronous pacing and preferred this pacing mode.
In one of the first prospective randomized, intention-to-treat trials on pacing mode selection, Mattioli et al.64 compared ventricular (VVI or VVIR) with physiologic pacing (AAI, DDD, DDDR, VDD) in 210 patients (SND in 110, AV block in 100) with a median age of 77 years. Only six patients received an AAI pacemaker. Patients with previously known AF were excluded from this study. A total of 56 patients (27%) developed AF during follow-up. The incidence of AF was 10% at 1 year, 23% at 3 years, and 31% at 5 years. The investigators observed an increased incidence of chronic AF in patients receiving ventricular pacing (P < .05). No difference was observed in the incidence of chronic AF between patients paced for AV block and patients paced for SND. In the subgroup of SND patients, the incidence of chronic AF was significantly less in patients receiving a physiologic than in those receiving a ventricular pacemaker (see Table 10-7).
Pacemaker Selection in the Elderly Study (PASE)
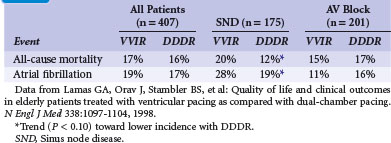
After their observations in the Medicare trial, Lamas et al.65 randomized 407 patients 65 or older with sinus rhythm and an indication for permanent pacing (SND or AV block) to single- or dual-chamber pacemaker therapy in the Pacemaker Selection in the Elderly Study. All patients received a dual-chamber device programmed to VVIR or DDDR according to the randomization result. The primary endpoint was health-related QOL (measured by SF-36); average age was 76. During the 30-month follow-up, QOL improved significantly after pacemaker implantation, but without a significant difference between VVIR and DDDR pacing modes, neither in QOL nor in clinical outcomes (cardiovascular events or death; Table 10-8). However, this refers to the intention-to-treat analysis while “on-treatment”, 26% percent of patients assigned to ventricular pacing were crossed over and paced in the dual-chamber mode because of symptoms of pacemaker syndrome.
The PASE subgroup of patients with SND had a significantly better QOL in some subscales of the SF-36 and a better cardiovascular functional status with dual-chamber pacing than with ventricular pacing. A trend toward lower all-cause mortality, lower incidence of AF, and reduced incidence of the combined endpoint of stroke, hospitalization for heart failure, or all-cause mortality favoring dual-chamber pacing was seen in patients with SND, but not in those with AV block (see Table 10-8). The authors concluded that pacemaker therapy improves QOL, but that the benefits associated with dual-chamber pacing versus ventricular pacing were moderate and observed only in the subgroup of SND patients.
Critical Appraisal
PASE was the first large multicenter RCT on dual-chamber versus ventricular single-chamber pacing performed according to EBM guidelines. The results were primarily interpreted as disappointing, showing only a marginal or even no advantage of dual-chamber pacing. However, the most relevant clinical finding may be the crossover rate of 26% from VVIR to DDDR pacing because of symptoms potentially caused or worsened by single-chamber pacing. In PASE, all patients had received dual-chamber devices, and randomization to single- or dual-chamber pacing was performed by device programming (“software randomization”). Patients randomized to VVIR pacing thus were fortunate because crossover did not require an additional surgical intervention, only reprogramming. Therefore, it was argued that crossover may have been performed because of bias on the side of physicians who attributed symptoms during VVIR pacing inappropriately to “pacemaker syndrome,” despite that per protocol, investigators were required to discuss each case individually with the principal investigator. An analysis of causes and results of crossover from VVIR to DDDR pacing, however, showed that the most common reason for crossover was “severe pacemaker syndrome” (77%), defined as a constellation of fatigue, dyspnea, effort intolerance, paroxysmal nocturnal dyspnea, neck fullness, presyncope, cannon A waves, blood pressure (BP) reduction with ventricular pacing, and CHF initiation or exacerbation.66 The most common individual symptoms were fatigue (62%), effort intolerance (64%), dyspnea (55%), and presyncope (34%). The diagnosis “pacemaker syndrome” or “intolerance to VVIR” in these patients with crossover is supported by the observation that, in contrast to other patients, their QOL deteriorated significantly after pacemaker implantation before crossover and improved significantly after reprogramming to dual-chamber pacing. The use of β-blockers, the presence of nonischemic cardiomyopathy, and systolic BP less than 110 mm Hg during ventricular pacing (relative risk of crossing over = 2.6) predicted intolerance to VVIR. However, the sensitivity of a decrease in paced systolic BP to less than 110 mm Hg at implantation for predicting intolerance to VVIR pacing was only 36%, the positive predictive power only 48%. The presence of VA conduction at implant did not predict the development of intolerance to VVIR pacing, although VA conduction or cannon A waves were present in almost 50% of crossover patients.
Mode Selection Trial in Sinus Node Dysfunction (MOST)
The group of investigators of PASE followed their observation that differences in outcome parameters between VVIR and DDDR were (if present at all) more pronounced in sinus node disease, performing a pacing mode comparison in SND patients.67 A total of 2010 patients were randomized to dual-chamber (1014) or ventricular pacing (996) and followed for 33 months. The incidence of the primary endpoint (all-cause mortality or nonfatal stroke) did not differ significantly (21.5% in DDDR, 23.0% in VVIR). Adjusted analyses revealed that in patients assigned to DDDR pacing, the risk of AF was 23% lower (21% vs. 27%) and HF scores were better. Heart failure hospitalization was reduced by 27%, the composite endpoint death, stroke, or HF hospitalization was reduced by 15% (Table 10-9). Dual-chamber pacing resulted in significantly better QOL for six of the eight SF-36 subscales during the 4 years of follow-up. The authors concluded that in SND patients, dual-chamber pacing does not increase stroke-free survival compared to ventricular single-chamber pacing, but DDDR significantly reduces AF risk and HF symptoms and improves QOL.
TABLE 10-9 Clinical Events in Mode Selection Trial in Sinus Node Dysfunction (MOST)
| Clinical Event | VVIR (n = 996) | DDDR (n = 1014) |
|---|---|---|
| Death | 23.0% | 21.5% |
| Stroke | 4.9% | 4.0% |
| Heart failure hospitalization | 12.3% | 10.2%* |
| Atrial fibrillation | 27.1% | 21.4%* |
* P < .05 for lower incidence with DDDR.
Data from Lamas GA, Lee KL, Sweeney MO, et al: Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 346:1854-1862, 2002.
Critical Appraisal
The MOST trial had the ambitious aim to assess clinical events (mortality, stroke), AF incidence, and symptoms (HF, pacemaker syndrome, QOL) in VVIR versus DDDR pacing for SND. While the latter two endpoints favored dual-chamber pacing, the first and most important endpoint (mortality) did not demonstrate a significant difference. Therefore, the MOST trial was frequently regarded as a negative trial, showing no significant benefit of dual-chamber compared to ventricular single-chamber pacing. In-depth analyses of mortality68 and stroke risk69 showed no advantage of dual-chamber pacing, and QOL was better in dual-chamber mode in several items, particularly related to physical function.70 However, there were two important lessons from MOST: (1) pacemaker syndrome is frequent in SND and VVIR pacing,71 and (2) unnecessary right ventricular pacing in SND is detrimental.38
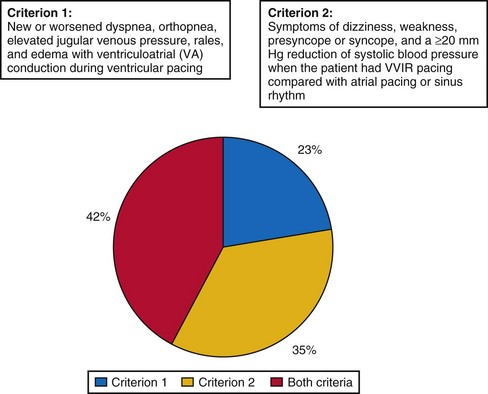
A strong argument in favor of dual-chamber pacing is the observation of severe pacemaker syndrome in approximately 20% of SND patients treated by VVIR pacing.71 The investigators in MOST attempted to prevent the major weakness of PASE, where the crossover rate from single- to dual-chamber pacing of 26% was so high that it may have prevented any meaningful intention-to-treat analysis of the difference between the two pacing modes. In MOST, therefore, the diagnosis of pacemaker syndrome strictly required documentation of new symptoms (e.g., dyspnea, syncope) together with VA conduction or systolic BP reduction of 20 mm Hg or more during VVI pacing compared to intrinsic rhythm. Even in the presence of pacemaker syndrome, crossover of pacing mode had to be confirmed by the clinical coordinating center; other attempts to resolve pacemaker syndrome (e.g., reprogram pacing rate, deactivate sensor) were required; and only if these failed, the centers could ask the principal investigator to review the indication for crossover to dual-chamber mode.
Despite these rigid requirements, 20% of patients programmed to VVIR met criteria for pacemaker syndrome and required crossover from single- to dual-chamber. Interestingly, pacemaker syndrome could basically develop at any time after implantation: At 6 months, it was present and required reprogramming in 14% of patients with VVIR pacing, in 16% at 1 year, in 18% at 2 years, and in 20% at 4 years. Slow sinus rates but not the presence or absence of VA conduction before pacemaker implantation predicted the development of pacemaker syndrome. QOL in patients with pacemaker syndrome was significantly worse than in patients without this complication of VVIR pacing, and it improved significantly after crossover to dual-chamber mode. Therefore, pacemaker syndrome with 20% incidence can be regarded as the most frequent complication of ventricular single-chamber pacing in SND, with significant impact on patients’ symptoms and QOL (Fig. 10-2).
A second post hoc analysis of MOST detected one of the basic problems of studies on mode selection and changed the practice of cardiac pacing. Sweeney et al.38 found that the percentage of right ventricular (RV) pacing was strongly associated with the risk of developing HF and AF. This refers to patients with SND and narrow QRS at baseline. Interestingly, this association was more striking for dual-chamber than single-chamber pacing. From these results, hypothetically, a “badly timed” (i.e., too early) ventricular stimulus may be more detrimental in dual-chamber pacing, where the pacing mode forces adverse AV timing to be present in every cycle, versus single-chamber ventricular pacing, where (besides pacemaker syndrome with VA conduction) only some cycles have by chance a detrimental AV relation, whereas in most cardiac cycles, atrium and ventricle are dissociated.
Canadian Trial of Physiologic Pacing (CTOPP)
After PASE in 1998, the Canadian Trial of Physiologic Pacing was the second large RCT on pacing mode selection.72 It enrolled patients scheduled for pacemaker implantation without permanent atrial fibrillation at 32 Canadian centers. Patients were randomized to receive either a ventricular pacemaker or a “physiologic pacemaker” (i.e., atrial single or dual chamber) and followed for an average of 3 years. The primary outcome was stroke or death from a cardiovascular cause; secondary outcomes were all-cause mortality, AF development, and hospitalization for heart failure. CTOPP randomized 2568 patients, 1094 received a physiologic system (dual-chamber or atrial single-chamber pacemaker), and 1474 a ventricular single-chamber device. The annual rate of stroke and death from cardiovascular causes were similar in both groups (5.5% with ventricular pacing, 4.9% with physiologic pacing; P = 0.33) (Table 10-10). The incidence of AF was lower with physiologic versus ventricular pacing (annual rate, 5.3% vs. 6.6%; P = .05) with the difference emerging only 2 years after implantation. All-cause mortality and HF hospitalization were not significantly different, whereas postoperative complications occurred more frequently with physiologic pacing (9.0%), which consisted of atrial single-chamber pacing in only 5% and ventricular single-chamber pacing in another 5% of patients because of problems to implant the atrial lead. The authors of CTOPP concluded that physiologic pacing provides little or no benefit over ventricular pacing with regard to stroke prevention or cardiovascular death.
TABLE 10-10 Clinical Events in Canadian Trial of Physiologic Pacing (CTOPP)
| Clinical Event (annual) | VVIR (n = 1474) | AAIR/DDDR (n = 1094) |
|---|---|---|
| Stroke | 1.1% | 1.0% |
| Heart failure hospitalization | 3.5% | 3.1% |
| Atrial fibrillation | 6.6% | 5.3%* |
* P ≤ .05 for lower incidence with DDDR.
Data from Connolly SJ, Kerr CR, Gent M, et al: Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. N Engl J Med 342:1385-1391, 2000.
Critical Appraisal
A post hoc analysis supported the criticism that the lack of benefit of physiologic pacing may derive from CTOPP including many patients with only intermittent and rare bradycardia. Tang et al.73 compared patients with an unpaced heart rate before pacemaker implantation of ≤60 bpm to patients with an unpaced heart rate above 60 bpm. Risk of cardiovascular death or stroke was significantly higher in patients with ventricular single-chamber pacing than with physiologic pacing, if the unpaced heart rate before pacemaker implantation was ≤60 bpm, whereas patients with an unpaced heart rate greater than 60 bpm derived no benefit in this study endpoint from physiologic pacing. Similarly, only patients with an unpaced heart rate ≤60 bpm showed a better exercise capacity, as measured by 6-minute hall walk test with dual-chamber pacing, whereas pacing mode did not affect exercise capacity in patients with an unpaced heart rate greater than 60 bpm.74
One of the major criticisms of CTOPP refers to the lack of experience with atrial leads and dual-chamber pacing by many centers that enrolled a large number of patients.75 The results that centers were unable to implant an atrial lead in 5% of patients, that incidence of postoperative complications for dual-chamber pacemaker implantation was 9%, and that more than 17% of dual-chamber devices were reprogrammed to VVI at 5-year follow-up reflect the lack of experience in implanting and following dual-chamber devices in some of the centers that participated in CTOPP.
As an argument in favor of dual-chamber pacing, a prespecified subanalysis found “chronic atrial fibrillation” (defined as AF lasting >1 week) occurred significantly more often in ventricular single-chamber pacing.76 This trend was even more pronounced in 1474 patients from CTOPP in whom follow-up was extended to a mean of 6.4 years.77 However, even in this extended study period, no difference could be observed between dual-chamber and ventricular single-chamber pacing with regard to cardiovascular mortality and stroke. Additionally, the effect of pacing mode on AF incidence was regarded as less important after publication of the AFFIRM trial.78
In conclusion, CTOPP showed for the first time that even in a study with a large patient group and a long follow-up period, it is difficult to demonstrate an advantage in “hard endpoints” of dual- over single-chamber ventricular pacing. However, CTOPP was frequently regarded as a study with the “apparent intent of the trial to pattern cost control instead of optimize patient care.”75 Even in Canada, it never had an impact on pacing mode selection, which was characterized by a constant increase of the percentage of dual-chamber systems.
United Kingdom Pacing and Cardiovascular Events (UKPACE) Trial
Complementary to MOST, which randomized patients with SND to ventricular single- or dual-chamber pacing, the UKPACE trial randomized patients with AV block; 2021 patients age 70 or older with high-degree AV block randomly received a ventricular single-chamber or a dual-chamber pacemaker.79 In the single-chamber group, patients were further randomized to receive either fixed-rate or rate-adaptive pacing. The primary outcome was all-cause mortality; secondary outcomes were AF, heart failure, and stroke/transient ischemic attack (TIA)/systemic embolism. During the follow-up of 4.6 years, annual mortality did not differ (7.2% vs. 7.4% in single- vs. dual-chamber group) (Table 10-11). Surprisingly, there were also no significant differences between both groups in the incidence of AF, HF, or stroke/TIA/embolism. The authors concluded that in elderly patients with high-degree AV block, pacing mode does not influence all-cause mortality or the incidence of cardiovascular events.
TABLE 10-11 Clinical Events in United Kingdom Pacing and Cardiovascular Events (UKPACE) Trial
| Clinical Event (annual) | VVI/VVIR (n = 1009) | DDDR (n = 1012) |
|---|---|---|
| All-cause mortality | 7.2% | 7.4% |
| Cardiovascular mortality | 3.9% | 4.5%* |
| Thromboembolism | 2.1% | 1.7% |
| Heart failure hospitalization | 3.2% | 3.3% |
| Atrial fibrillation | 3.0% | 2.8% |
* P < .05 for higher incidence with DDDR.
Critical Appraisal
It had been expected that results of the UKPACE trial might favor dual-chamber pacing, because patients with high-degree AV block are frequently pacemaker dependent and require ventricular pacing continuously. Patients with SND in MOST were usually not pacemaker dependent and may therefore have had periods when they did not require pacing. This could dilute the advantageous effects of “physiologic” compared to ventricular single-chamber pacing. Elderly patients with AV block enrolled in UKPACE may have been particularly sensitive to suboptimal hemodynamic conditions produced by asynchronous pacing in the VVI and VVIR modes. In their trial design paper, the authors of UKPACE therefore hypothesized that “it may be that such patients have much to gain from the superior hemodynamics of optimal pacing in terms of functional capacity and quality of life, in addition to the possibility of reduced cardiovascular morbidity and improved survival.”80
The main finding of UKPACE, that dual-chamber pacing did not convey any advantages in terms of mortality, AF, stroke, or heart failure in elderly patients with AV block compared with single-chamber ventricular pacing, was therefore particularly disappointing. UKPACE was the only large RCT that found no difference in AF between single- and dual-chamber pacing. In fact, AF was detected more frequently in dual-chamber pacing during the first 18 months of the study, perhaps from a systematic bias. In dual-chamber devices, AF can easily be diagnosed by the atrial lead and electrogram (EGM). In VVI and VVIR devices, the detection of AF is limited to the surface electrocardiogram (ECG) and can be difficult in patients with complete AV block and VVIR pacing.81
Some studies on hemodynamics in pacing, particularly in the context of cardiac resynchronization therapy, have emphasized the importance of timing the AV delay to achieve acceptable results in dual-chamber pacing.82,83 Hemodynamic optimization of the AV delay had not been performed in UKPACE. On the contrary, programming instructions in UKPACE suggested the use of short AV delays (150 msec, with rate-adaptive shortening to 75 msec)80 to facilitate and ensure continuous ventricular pacing. In view of the deleterious effects of RV pacing, this may have negatively affected patients with dual-chamber devices (see Role of Prevention of Unnecessary Right Ventricular Pacing). It was not reported how many patients had only intermittent versus permanent second-degree or third-degree AV block. Continuous ventricular pacing may have been unnecessary and therefore disadvantageous in these patients. Programming of such short AV delays caused a significantly (P < .001) higher percentage of ventricular pacing in patients with dual-chamber pacing: Counters (available in 65% of patients) demonstrated a median of 99% RV pacing in dual-chamber mode versus 94% in VVI and 93% in VVIR at 1 month.79 Inappropriately short AV delays can lead to premature mitral valve closure, causing pulmonary venous regurgitation by nonphysiologic flow reversal during atrial systole.84 Because of device programming, this may have occurred more frequently in UKPACE than in other RCTs of pacing mode, leading to disadvantageous side effects that counteracted and precluded any potential benefit of dual-chamber pacing.
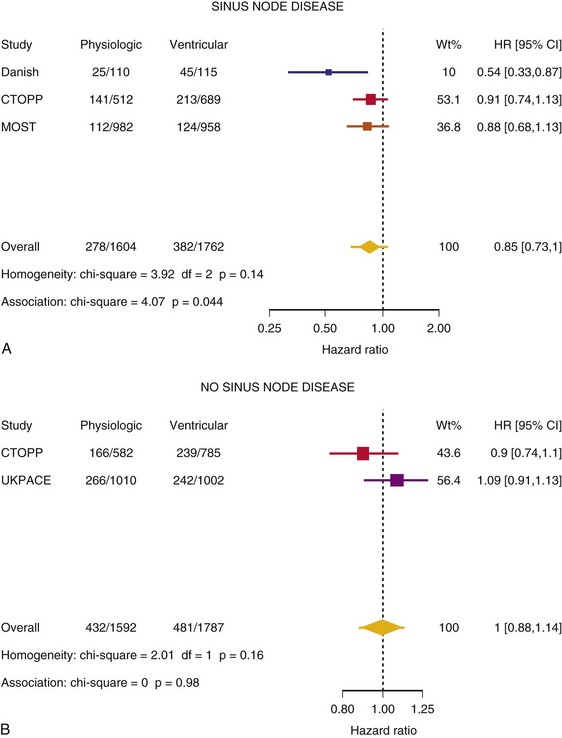
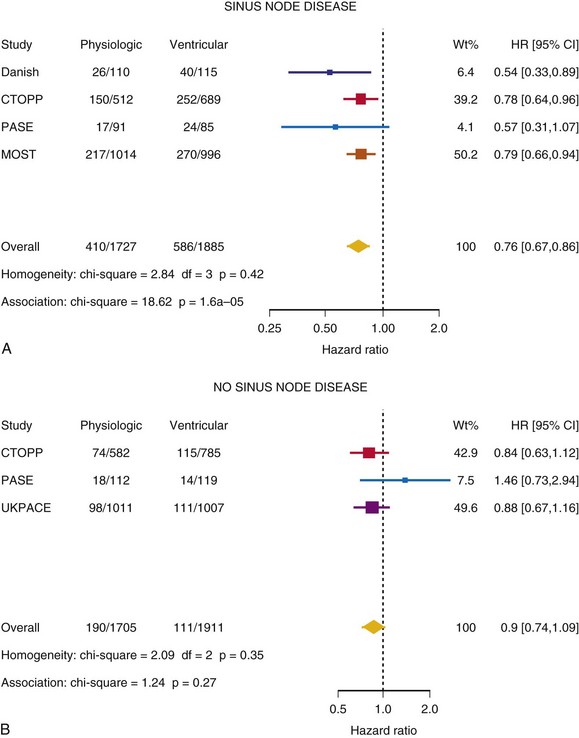
Meta-Analysis of Ventricular Versus Atrial-Based Pacing
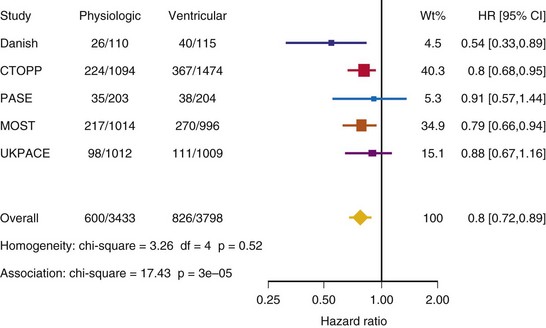
After UKPACE, Healey et al.85 performed a meta-analysis of the large trials on pacing mode selection (Danish study,34,35 PASE,65 CTOPP,72 MOST,67 UKPACE79). As expected, no advantage of dual-chamber pacing was found for mortality, for the combination of mortality and stroke, or for heart failure hospitalization (Figs. 10-3 to 10-8). The incidence of atrial fibrillation was lower in dual-chamber pacing. However, predominantly because of UKPACE results, a subanalysis showed a lower incidence of AF only for dual-chamber pacing in sinus node disease, but not in AV block. Even though this meta-analysis included the impressive number of 7231 patients, results cannot overcome the general limitations of the individual trials, such as selection bias and biased detection of AF when an atrial lead is present.
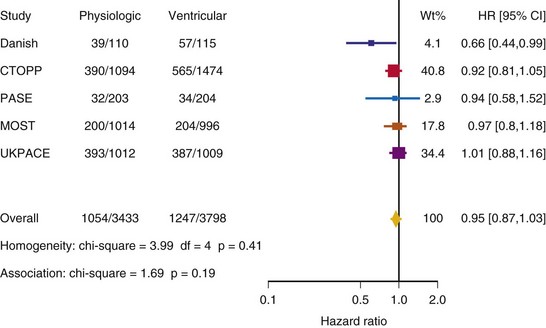
Figure 10-3 Pooled estimate of mortality in randomized trials of pacing mode.
Danish, Atrial versus ventricular pacing in sick sinus syndrome (Andersen et al.34,35); CI, Confidence interval; CTOPP, canadian Trial of Physiologic Pacing; HR, hazard ratio; MOST, Mode of Selection Trial in Sinus Node Dysfunction; PASE, Pacemaker Selection in the Elderly; UKPACE, United Kingdom Pacing and Cardiovascular Events trial.
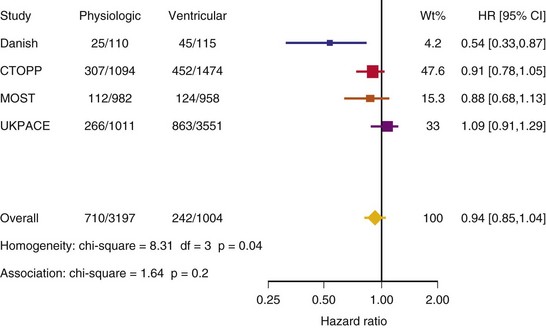
Figure 10-4 Pooled estimate of cardiovascular mortality or stroke in randomized trials of pacing mode.
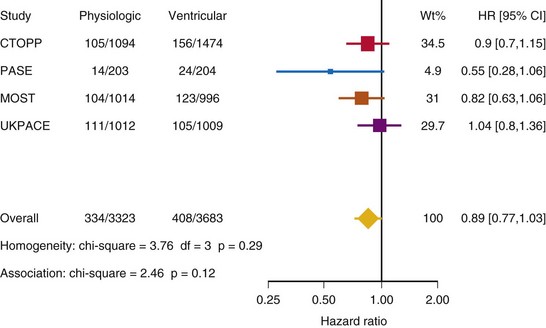
Figure 10-6 Pooled estimate of hospitalization for heart failure in randomized trials of pacing mode.
 Atrial Single-Chamber Versus Dual-Chamber Pacing: Clinical Trials
Atrial Single-Chamber Versus Dual-Chamber Pacing: Clinical Trials
In 2004, Masumoto et al.86 performed a retrospective analysis of 196 patients with sinus node disease who were implanted with dual-chamber devices programmed to DDD (n = 101) or AAI (n = 95) mode. Consecutive patients implanted between 1979 and 2000 were included if they had no second- or third-degree AV block, a maximum PR interval of 220 msec, and no left bundle branch block or bifascicular block. During a mean follow-up of 8 years, eight AAI devices had to be switched to DDD because high-degree AV block developed (incidence, 1.1%/yr). At 10 years, there was no need to add a ventricular lead in 89% of patients with AAI pacing. Excluding generator exchanges, 18 patients with DDD versus 17 with AAI system underwent reoperation, predominantly for lead failure. All-cause mortality was similar in both groups (10-year survival: 88% in AAI, 93% in DDD), as was freedom from permanent AF (94% in AAI, 91% in DDD) (Table 10-12). The authors concluded that AAI pacing can achieve similar clinical benefits as DDD pacing in sinus node disease with relevant AV or infrahisian conduction disturbances.
Nielsen et al.87 compared myocardial blood flow and left ventricular ejection fraction (LVEF) in 30 patients with SND randomized to AAI versus DDD pacing. After 22 months of pacing in the assigned mode, myocardial blood flow was assessed with positron emission tomography (PET) and LVEF measured with echocardiography. Patients with dual-chamber pacemakers were temporarily programmed to AAI to obtain an intraindividual comparison. Myocardial blood flow was similar in the groups with AAI and DDD pacing. However, blood flow significantly improved if the patient’s DDD pacing was temporarily programmed to AAI mode. LVEF was similar to baseline results after 22 months in AAI but had become significantly lower in patients with DDD pacing, from 61% to 56% (P = .01). However, if DDD pacing was temporarily changed to AAI, measurements of LVEF returned to baseline results. The authors concluded that dual-chamber compared with single-chamber AAI pacing has detrimental effects on myocardial blood flow and LVEF, which are still reversible after 22 months.
In a single-center pilot study, consecutive patients with sinus node disease were screened and enrolled if they had no bundle branch block (BBB), and the spontaneous PQ interval did not exceed 220 msec (patients ≤70 years) or 260 msec (patients >70 years).88 Of 952 patients with a first pacemaker implantation between 1994 and 1999, 755 were excluded from the study, mainly for AV block (48%), permanent AF (10%), or BBB (5%), and only 2% of patients did not give consent. The 177 enrolled patients were randomized to receive an AAIR pacing system (n = 54) or a DDDR system programmed to a short AV delay (150 msec, DDDR-s, n = 60) or a long AV delay (300 msec, DDDR-l, n = 63). Echocardiographic (left atrial diameter, left ventricular function and size) and clinical (AF, stroke or arterial embolism, mortality, heart failure) outcomes were assessed in these three modes of pacing in SND. During the study, patients in the DDDR-s group received pacing for a mean of 90%. Patients in the DDDR-l group still received 17% of (a priori unnecessary) ventricular pacing. After a mean follow-up of 3 years, patients randomized to AAIR did not show a significant change in left atrial diameter, left ventricular diameter or LVEF shortening compared to baseline. In contrast, left atrial diameter was increased and left ventricular shortening fraction decreased significantly in the DDDR-s patients. In the DDDR-l group, the left atrial diameter and the left ventricular end-systolic and end-diastolic diameters increased significantly during the 3-year follow-up. Atrial fibrillation in the 12-lead ECG during at least one follow-up visit was significantly less common in AAIR (7.4%) than in DDDR-s (23.3%) or DDDR-l (17.5%).89 During the study, 16 strokes occurred in 14 patients, but no peripheral embolism was observed. Clinical events, including all-cause mortality, cardiovascular mortality, development of CHF, and increased use of diuretics, did not differ among the three groups. However, progression of HF symptoms, although not significantly different among the groups, was seen, with an increase of at least one NYHA class in 31% of patients paced in AAIR mode versus 30% in DDDR-s and 46% in DDDR-l.
The study by Nielsen et al.87 would have required 450 patients for an 80% power to detect clinical or echocardiographic differences between AAIR and DDDR (no differences between DDDR-s and DDDR-l were expected). Inclusion was stopped when a multicenter trial (DANPACE) randomizing SND patients to AAIR versus DDDR pacing was initiated. However, the results of this study from Aarhus suggest that in patients with SND, narrow QRS and normal AV conduction dual-chamber pacing compared to AAIR pacing may result in deleterious effects in terms of left atrial enlargement, left ventricular volumes and ejection fraction, and AF incidence. Surprisingly, even in these select patients, programming the AV delay to very long values was unable to prevent a significant proportion of unnecessary right ventricular pacing. The observed proportion of ventricular pacing of 17% had detrimental effects in terms of left atrial dilatation and incidence of AF similar to DDDR pacing with short AV delay.
In a study following these observations, Albertsen et al.90 randomized 50 patients with sinus node disease to AAIR or DDDR (AV delay 220 msec paced and 200 msec sensed). Primary endpoint was dyssynchrony (evaluated by tissue-Doppler imaging with at least 1 segment with delayed longitudinal contraction) and LVEF (evaluated by 3D echocardiography) after 12 months compared to baseline. Physicians performing echocardiography were blinded with regard to pacing mode. Secondary endpoints referred to 6-minute walking test and NT-proBNP. During follow-up, patients with DDDR pacing received ventricular pacing for 66% of the time. Dyssynchrony slightly increased with DDDR pacing (mean of 1.3 delayed segments at baseline vs. 2.1 after 12 months) but not in AAIR pacing. Although LVEF decreased statistically significant with DDDR pacing (63% at baseline vs. 59% after 12 months; no change observed in AAIR pacing), there was no difference between AAIR and DDDR pacing after 12 months in NYHA class or NT-proBNP. These results confirm other observations of deteriorated left ventricular function with unnecessary right ventricular pacing, and suggest the development of left ventricular dyssynchrony as one cause. At the same time, echocardiographic changes from baseline did not translate into significant clinical changes in this small study.
The Danish multicenter randomized trial on single-lead atrial versus dual-chamber pacing in patients with sick sinus syndrome (DANPACE) was started in 1999.91 It compared AAIR and DDDR pacing in patients with SND but with no BBB or AV block (except for PR interval ≤220 msec for patients <70 years and ≤260 msec for those ≥70 years). This trial used hardware randomization. Primary endpoint was all-cause mortality; secondary endpoints included detection of atrial fibrillation during scheduled follow-up, progression to permanent AF, incidence of stroke, heart failure, and the need for reoperation. It planned to include 1900 patients followed for a mean of 5.5 years to detect an absolute 6% difference in all-cause mortality with a power of 80%. The trial was terminated after 1415 patients, implanted in 20 centers in Denmark, United Kingdom, and Canada, were followed for a mean of 5.5 years. No patient was lost to follow-up. Patients in the DDDR group had been programmed to an AV delay of ≤220 msec paced and ≤200 msec sensed with rate-adaptive shortening. This caused ventricular pacing for a mean of 65% of the time in DANPACE. All-cause mortality (30% in AAIR, 27% in DDDR) was not significantly different (P = 0.53) nor was there a difference in stroke, NYHA functional class, use of diuretics, or hospitalization for heart failure.
The incidence of AF related to pacing mode is different in DANPACE compared to results from a 2003 study by Nielsen et al.88 In this pilot study in 177 patients, the incidence of AF was significantly lower with AAIR pacing (7.4%) compared to DDDR with long AV delay (17.5%) and DDDR with short AV delay (23.3%). In the SAVE PACe trial, patients had significantly less persistent AF if randomized to a dual-chamber mode allowing single nonconducted P waves not to be tracked, to minimize ventricular pacing in the dual-chamber mode, than patients with conventional DDDR pacing92 (see Role of Prevention of Unnecessary Right Ventricular Pacing). However, patients with SND and conventional dual-chamber pacing in DANPACE received RV pacing for only 65% of the time versus 99% in SAVE PACe.
 Dual-Chamber Versus Ventricular Pacing in Defibrillator Patients
Dual-Chamber Versus Ventricular Pacing in Defibrillator Patients
The possibility of physiologic pacing in patients with an indication for an ICD and dual-chamber pacing was highly desired before it was available.93 However, the first studies on dual- versus single-chamber ICDs were disappointing, failing to show improved discrimination between ventricular and supraventricular tachycardia.94–96 Some newer studies question if single-chamber ICDs are comparable to dual-chamber ICDs in prevention of inappropriate therapy.97–99
However, one of the aims of dual-chamber pacing in ICD patients is the reduction in clinical events, particularly heart failure and HF progression caused by bradycardia, which frequently develops during treatment with β-blockers and amiodarone. Even an increased risk of ventricular tachyarrhythmia (e.g., from bradycardia-dependent arrhythmias) has been observed in ICDs that were programmed to a VVI 40-bpm backup pacing mode.100 The Dual Chamber and VVI Implantable Defibrillator (DAVID) trial therefore evaluated if dual-chamber pacing reduced the incidence of HF hospitalization and all-cause mortality compared to backup ventricular pacing in patients with a conventional indication for ICD therapy and heart failure (LVEF ≤40%) but no standard indication for antibradycardia pacing.101 In 37 U.S. centers, 506 patients were enrolled and randomized to DDDR pacing at 70 bpm versus VVI backup pacing at 40 bpm (software randomization, DDDR systems implanted in all patients). Programming of other parameters, particularly the AV delay, was left to the individual investigator. The mean sinus rate at baseline was 72 bpm, the mean PR interval of patients in DAVID was 184 msec, and 27% of patients had a BBB. At 6 months, 86% of patients received β-blockers and 27%, amiodarone. The trial was prematurely stopped at a mean follow-up of 8 months because, unexpectedly, patients with dual-chamber ICDs had a significantly higher incidence of HF hospitalization (43 [23%] at 1-year follow-up) and death (23 [10%] at 1 year) than patients with single-chamber ICDs (30 and 15, equivalent to 1-year incidence of 13% and 6.5%). This difference reached statistical significance in favor of single-chamber ICDs (Table 10-13).
TABLE 10-13 Clinical Endpoints in Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial at 1 Year

One major difference in patients with single-chamber ICDs was the significantly lower proportion of ventricular pacing. At the 6-month control, device counters documented that VVI 40-bpm back-up pacing was associated with a mean proportion of ventricular pacing of only 0.6% in these patients without bradycardia compared to 59.6% ventricular pacing in the DDDR mode. A post hoc analysis corroborated the hypothesis that right ventricular pacing was the cause of worse outcome in patients with dual-chamber ICDs.102 In 380 patients who had a 3-month follow-up and had not yet reached a study endpoint, a multivariate analysis demonstrated that the percent of cumulative RV pacing was correlated with death or HF hospitalization during further study. If patients with dual-chamber ICDs had RV pacing more than 40% of the time, they had a more than fivefold higher risk of HF hospitalization or death than patients with a dual-chamber ICD and RV pacing for 40% or less of the time (P = .02). Patients with DDDR pacing at 70 bpm and ≤40% RV pacing trended toward a better outcome than those with VVI 40-bpm backup pacing.
Another analysis assessed data in patients with “soft” indications for pacing, such as bradycardia, defined as sinus bradycardia of less than 60 bpm, and without a conventional indication for pacing.103 Results similar to the overall analysis were observed in these patients. However, the two soft indications are fundamentally different; patients with first-degree AV block may have experienced specifically deleterious effects of dual-chamber pacing because they likely were continuously paced (and unpaced in VVI 40-bpm group), whereas patients with sinus bradycardia might have specifically benefited from dual-chamber pacing as long as a sufficiently long programmed AV delay prohibited unnecessary RV pacing. However, this was not reported in this post hoc analysis.
These data were partly replicated in the second Multicenter Automatic Defibrillator Implantation Trial (MADIT-II), although differences between pacing modes in a primary prevention ICD trial did not reach statistical significance.104 In 404 patients who received a single-chamber ICD and 313 patients with a dual-chamber ICD for primary prevention of sudden cardiac death after myocardial infarction (LVEF ≤30%), the trend was toward an increase in hospitalization for heart failure in patients with dual-chamber devices. Because of the nonrandomized assignment to dual-chamber ICDs, however, patients who had received dual-chamber devices were older and had higher NYHA functional class, more frequent prior coronary artery bypass surgery, wider QRS complex, more frequent left BBB, atrial tachyarrhythmias, and higher nitrate urea levels.
The DAVID investigators performed a second study with VVI backup pacing at 40 bpm in patients with heart failure, an indication for ICD therapy, and no conventional indication for bradycardia pacing (DAVID-II).105 This time the dual-chamber group was programmed to AAI 70 bpm and tested for “non-inferiority” compared to backup VVI pacing at 40 bpm. In 29 U.S. centers, 543 patients were enrolled and implanted with a dual-chamber ICD programmable to the VVI and AAI pacing modes. Of note, 57 patients from the first DAVID trial who did not reach a study endpoint were also included. During a mean follow-up of 2.7 years, no significant difference was seen in the primary combined endpoint of time to HF hospitalization or death (incidence 11%, 17%, and 25% at 1, 2, and 3 years, respectively). In a subanalysis, asymptomatic sinus bradycardia at baseline was associated with a lower incidence of adverse outcome (12% vs. 26%) in patients randomized to VVI 40-bpm back-up or AAIR 70-bpm pacing.106
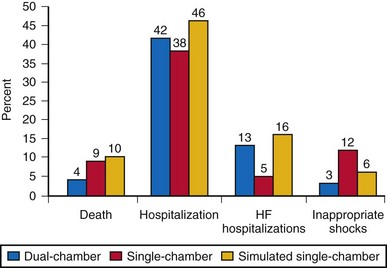
The question if dual-chamber ICDs have any potential to reduce adverse clinical events compared to single-chamber ICDs was assessed in the Dual Chamber and Atrial Tachyarrhythmias and Adverse Events Study (DATAS).107 Patients with Class I indication for an ICD (according to ACC/AHA 1998 guidelines) were included if permanent atrial tachyarrhythmias and an indication for dual-chamber pacing were absent. A total of 334 patients were then randomized to single-chamber ICD, dual-chamber ICD, or “simulated single-chamber ICD” (i.e., dual-chamber ICD implanted but programmed to single-chamber mode for pacing and discrimination between supraventricular and ventricular tachyarrhythmia). With crossover of the latter two groups after 8 months, all patients were followed for 16 months. The primary outcome was a composite of all-cause-mortality, need for cardiovascular hospitalization or intervention, two or more inappropriate shocks, and sustained symptomatic atrial tachyarrhythmia for 48 hours or longer. This composite was expressed as a prespecified score. Any one of these endpoints occurred in 65 patients with dual-chamber ICD (58%), 82 patients with single-chamber ICD (74%), and 84 patients with a dual-chamber ICD programmed to single-chamber parameters (76%). Mortality was 4% in dual-chamber, 9% in single-chamber, and 10% in simulated single-chamber ICD patients. The composite endpoint score was 33% lower for patients with dual-chamber ICDs (Fig. 10-9).
 Critical Appraisal of Pacing Mode Trials
Critical Appraisal of Pacing Mode Trials
The large randomized trials on pacing mode dramatically changed the perception of “physiologic pacing.” In studies of the late 1980s, the addition of an atrial lead to a ventricular lead was perceived as a great step forward in cardiac pacing. The dual-chamber pacing mode enabled ventricular pacing at a programmable time after atrial sensing (AV-synchronous pacing), which improved cardiac output significantly compared with “asynchronous” ventricular pacing in the VVI(R) mode, as well as reducing mitral regurgitation, right atrial pressure, and pulmonary capillary wedge pressure and resolving pacemaker syndrome in a significant number of patients. Retrospective studies confirmed these hemodynamic advantages of dual-chamber pacing by clinical data. However, these clinical data from retrospective and nonrandomized studies were questioned108,109 when selection bias became evident.61,110
CTOPP
The Canadian Trial of Physiologic Pacing is usually regarded as negative because three of four endpoints (stroke/cardiovascular events, all-cause mortality, heart failure) were not reduced with physiologic compared to ventricular pacing. Even the statistically significant difference in the development of AF emerged only after 2 years, and in light of the results of AFFIRM,78 was deemed not clinically relevant. At the same time, in patients who required pacing most of the time (“unpaced heart rate <60 bpm”), physiologic pacing significantly reduced stroke, cardiovascular mortality, and even all-cause mortality, rendering CTOPP the most positive trial on pacing mode selection in favor of physiologic pacing.
UKPACE
From the information published, the United Kingdom Pacing and Cardiovascular Events study is the most negative trial on pacing mode selection. It clearly showed that death can be prevented in AV block as effectively by single-chamber as by dual-chamber pacemakers. Any difference between these pacing modes with regard to exercise capacity, symptoms, and QOL remains unclear. The lack of difference between single- and dual-chamber pacing in AV block could be explained by patient selection bias in UKPACE (only 45% of eligible patients were enrolled) and by inappropriately short AV delays (per protocol 75-150 ms) with adverse hemodynamic consequences. The “forced” proportion of ventricular pacing of 99% in the dual-chamber mode which was significantly more than in VVI and VVIR pacing (93 and 94%) may theoretically have further deteriorated clinical results of dual-chamber pacing (see Role of Prevention of Unnecessary Right Ventricular Pacing).
Other Issues
First, randomized trials on pacing mode selection suffer from selection bias, particularly if hardware randomization is applied. From theoretical considerations and earlier studies, investigators have ethical concerns regarding whether pacemaker-dependent patients are at an increased risk for adverse events with ventricular single-chamber pacing. It is a strength of published trials (particularly CTOPP and UKPACE, which were hardware randomized) to keep track of why eligible patients were not enrolled into the trial: In CTOPP, only 16% of eligible but not enrolled patients declined consent, whereas in 56% the physician declined.72
Second, in a blinded trial, patient preference would be a powerful argument in favor of or against a pacing mode.25,26,62,63 Unfortunately, but logically, all large RCTs on pacing mode selection had a parallel trial design preventing intraindividual comparisons. Intraindividual comparisons could only be made in crossover patients (because of suspected pacemaker syndrome) from VVIR to DDDR pacing in PASE and MOST, showing a striking preference for DDDR pacing in these select patients.
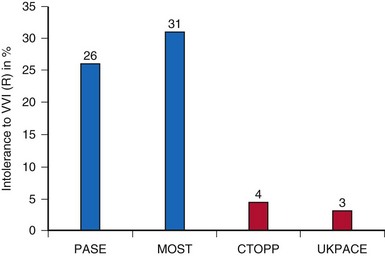
Third, the choice of software versus hardware randomization has both advantages and problems. If a patient “software randomized” to VVIR expresses symptoms, it is easy to reprogram the device to DDDR and see if symptoms are resolved. This may lead to an overestimation of the incidence of “VVIR intolerance” (26-31% in PASE and MOST). On the other hand, in hardware randomization, crossover to dual-chamber pacing requires surgical intervention with potential complications, medically and legally. It is therefore evident that the incidence of “VVIR intolerance” is underestimated in hardware-randomized trials (3.1% in UKPACE, 4.3% in CTOPP) (Fig. 10-10).
In summary, despite the RCT results on pacing mode selection, the perception that dual-chamber pacing is superior to single-chamber ventricular pacing in sinus rhythm still governs current guidelines111,112 and international physicians’ choices75,113 of pacing mode selection. However, trials have shown that (1) the beneficial effects of dual-chamber pacing may have been overestimated (see rationale and sample size calculation of the Danish trial and UKPACE), (2) dual-chamber pacing by itself may not convey hemodynamic benefits if not optimized to the patient (lead position, AV delay, pacing rates), (3) dual-chamber pacing is more complex and therefore associated with more pitfalls and potential for complications (atrial lead dislodgement, pacemaker mediated tachycardia), and (4) dual-chamber pacing is not as “innocent” as previously thought because it may cause more poorly timed right ventricular pacing than VVI(R), with potentially deleterious effects.
 Role of Prevention of Unnecessary Right Ventricular Pacing
Role of Prevention of Unnecessary Right Ventricular Pacing
Surprising data from the DAVID trial showed that compared with VVI 40-bpm backup pacing, DDDR pacing at 70 bpm produced more, not less, heart failure hospitalization and a slight, statistically not significant increase risk of mortality in patients with an ICD and no bradycardia101 (see earlier). In search of an explanation, it became obvious that right ventricular pacing was present in dual-chamber pacing much more frequently than in ventricular backup pacing (56% vs. 3% of patients with paced ventricular rhythm after 6 months). Similar results have been reported (independent of the presence of a single- or dual-chamber ICD) for patients in MADIT-II, where a cumulative proportion of ventricular pacing of more than 50% was associated with an almost twofold increased risk of new or worsened heart failure.114
A number of studies on histology,115 hemodynamics,116 and echocardiography117 have demonstrated deleterious acute and long-term consequences of RV apical pacing, their full clinical impact emerged only after DAVID.
The MOST trial enrolled only patients with sinus node disease; apart from 11% of patients with additional second- or third-degree AV block,67 patients who did not require ventricular pacing. Sweeney et al.38 investigated the effect of unnecessary RV pacing in patients with SND. They selected 1339 patients from MOST who had a narrow QRS (<120 msec) at baseline before pacing was instituted. Taken from pacemaker memory functions, 707 patients randomized to DDDR received RV pacing at an average 90% of the time. In contrast, in patients randomized to VVIR, RV pacing was present only 58% of the time. In a Cox proportional hazard model, RV pacing (again, unnecessary in SND patients) was a strong predictor of HF hospitalization in DDDR mode (Table 10-14). For percentages of RV pacing in the AV-synchronous mode up to 40%, every 10% increase in ventricular pacing linearly increased the relative risk increase for HF hospitalization by 54%. RV pacing in the DDDR mode more than 40% of the time versus less than 40% was associated with a 2.6-fold increased risk of HF hospitalization. This association was much more striking for DDDR than for VVIR pacing, in which RV pacing up to 80% of the time had only a minor impact on HF development, whereas pacing more than 80% of the time resulted in a 2.5-fold increased risk of HF hospitalization.
TABLE 10-14 Association of Percentage of Ventricular Pacing (VP) with Heart Failure Hospitalization (HF Hosp) and Atrial Fibrillation (AF) for Dual-Chamber and Single-Chamber Ventricular Pacing
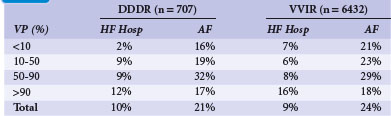
Similar to the increased risk of heart failure hospitalization, unnecessary right ventricular pacing also increased the risk of atrial fibrillation.38 Up to 80% to 85% of RV pacing in the DDDR or VVIR modes, the risk of AF linearly increased by approximately 1% for every percent increase in cumulative ventricular pacing. The authors concluded that unnecessary RV pacing in SND leads to ventricular desynchronization in asynchronous as well as in AV-synchronous RV pacing, and over a broad range of cumulative percentages of pacing, linearly increases the risk of development of HF and AF in patients without BBB.
The higher percentage of RV pacing in dual-chamber than in ventricular single-chamber pacing may be one explanation for the lack of superiority of this pacing mode in large trials. A post hoc analysis confirmed this for the DAVID trial.102 The authors dichotomized the group of patients with DDDR pacing at 70 bpm into those with 40% or less versus those with more than 40% RV pacing. In a Cox regression analysis, ventricular pacing 40% or less of the time was associated with slightly fewer clinical events than with VVI 40-bpm backup (P = .07). Patients with ventricular pacing more than 40% of the time in DDDR mode had a 5.3-fold increased risk of a study endpoint (death or HF hospitalization) than patients with 40% or less ventricular pacing.
Prevention of unnecessary RV pacing in patients with a dual-chamber pacemaker or ICD is difficult to achieve with prolonged settings for the AV delay alone. In a pilot study with 177 patients with SND and normal AV conduction, Nielsen et al.88 observed ventricular pacing still for 17% of the time in DDDR mode with a fixed AV delay as long as 300 msec. Beyond being ineffective, programming AV delays to such long values creates new problems, such as limitations of the upper tracking rate or AV desynchronization arrhythmias.118,119
A better way to prevent unnecessary RV pacing is therefore an automatic algorithm to provide AAIR pacing, with monitoring of AV conduction and an automatic switch to DDDR when AV block occurs beyond a single dropped beat. Two such algorithms developed for minimized ventricular pacing are MVP (Medtronic, Minneapolis) and SafeR (Sorin, Milano). For the SafeR algorithm, a reduction in RV pacing to less than 1% in patients without permanent AV conduction block (SND, paroxysmal AV block) was shown, together with safe switching to DDDR mode when necessary.120–122 For the MVP algorithm, its superiority in prevention of unnecessary RV pacing versus an extended AV search hysteresis has been demonstrated (0.9% in first-degree AV block vs. 80.6% ventricular pacing),123 maintaining 99% ventricular pacing in patients with permanent third-degree AV block. In SND patients, MVP consistently reduced the percentage of ventricular pacing to 1.4%,124,125 with a relative reduction of ventricular pacing of 99% in SND and 60% in paroxysmal AV block.125 Similar reductions in RV pacing have been achieved in ICD patients.126
The effect of consequent prevention of RV pacing on clinical outcomes was tested in 1065 SND patients in the SAVE-PACe trial.92 All patients had normal AV conduction and QRS duration less than 120 msec. After randomization to conventional dual-chamber pacing versus dual-chamber pacing with the addition of algorithms that specifically promote intrinsic AV conduction, patients were followed for 1.7 years, when the trial was stopped. RV pacing had been present for a median of 99% of the time in patients with conventional pacing; the addition of algorithms to promote spontaneous AV conduction and to preserve a narrow QRS limited unnecessary ventricular pacing to a median of 9%. The incidence of persistent AF was significantly lower (7.9%) in patients with dedicated algorithms to promote intrinsic AV conduction compared to patients with conventional dual-chamber pacing (12.7%; RRR, 40%). The need for any intervention for AF (cardioversion, catheter ablation) was also significantly reduced by 38%. Mortality was not significantly different (4.9% with minimized ventricular pacing, 5.4% with conventional dual-chamber pacing).
The MVP algorithm was also tested in DAVID-like patients with an indication for ICD therapy.127 In a non-inferiority design, the MVP trial enrolled 1030 patients with an ICD indication, sinus rhythm and no indication for pacing to dual-chamber/minimal pacing at 60 bpm versus VVI 40 bpm backup pacing. The primary endpoint was time to death, HF hospitalization, or HF-related urgent care. The trial was stopped after 2.4 years after an interim analysis suggested that it was highly unlikely that there would be a statistically significant difference with regard to the primary endpoint regardless of follow-up duration. In these patients with a mean LVEF of 35%, all-cause mortality was slightly but not significantly higher in the dual-chamber group (11% vs. 9%), mainly driven by a difference in noncardiac death (4.1% vs. 3.1%). Heart failure hospitalizations and related urgent care was necessary in 20.8% of patients with a dual-chamber device, versus 18.8% in patients randomized to VVI 40-bpm backup pacing. These differences were not statistically significantly different (P = 0.873). However, because of fewer events than expected, the confidence interval crossed the prespecified border for non-inferiority. Therefore, the MVP trial was unable to demonstrate non-inferiority of dual-chamber ICDs incorporating an algorithm for prevention of RV pacing compared to VVI backup pacing at 40 bpm. Of note (even though not prespecified), a striking difference in outcome was observed in a post hoc analysis for patients with a baseline PR interval ≥230 msec (mean, 260 msec). Although event rates in patients with PR <230 msec were virtually identical between single- and dual-chamber ICDs, patients with a PR ≥230 msec had a significantly higher event rate with dual-chamber pacing promoting intrinsic AV conduction.
Similarly, the Inhibition of Unnecessary RV Pacing with AVSH in ICDs (INTRINSIC) trial investigated if dual-chamber ICDs are “non-inferior” to single-chamber ICDs with VVI 40-bpm backup, if an AV hysteresis algorithm prevents unnecessary RV pacing. Patients with a standard indication for an ICD were enrolled. All patients whose AV search hysteresis algorithm achieved ventricular pacing less than 20% of the time at the 1-week control visit were randomized to receive VVI 40-bpm or DDDR 60-bpm pacing. Investigators had to amend the study protocol (prolong AV delay during activity of AV search hysteresis) because a larger-than-expected number of patients would otherwise have been excluded from randomization.128 Thus, of 1530 patients enrolled, a total of 988 with ventricular pacing less than 20% at the 1-week visit were randomized.129 During a mean follow-up of 10.4 months, the endpoints of death and HF hospitalization were more frequent in VVI backup 40 bpm (9.5%) than in DDDR pacing with AV search hysteresis (6.4%). Non-inferiority of DDDR with AV search hysteresis met a significance of P <.001, even with a trend for superiority of DDDR with AV search hysteresis (P = .07) with regard to primary endpoints. Also, all-cause mortality was nonsignificantly lower in the dual-chamber ICD arm (3.6% vs. 5.1%; P = 0.23). Of note, the event rate was higher in the observational arm (patients excluded from randomization because ventricular pacing was present >20% of the time despite use of AV search hysteresis algorithm), with a total of 12.5% events and an all-cause mortality of 5.5%.
 Trials Evaluating Right Ventricular Versus Biventricular Pacing
Trials Evaluating Right Ventricular Versus Biventricular Pacing
A growing body of evidence indicates that RV pacing leads to new-onset heart failure in a significant proportion (~25%) of patients with high-grade AV block and normal left ventricular function over the long term (>5 years), typically in patients who are older at implantation, those with a particularly wide QRS, and those with coronary artery disease.130 To date, however few prospective randomized controlled trials have compared biventricular (BiV) to conventional pacing for standard bradycardia indications.
In the left ventricular–based cardiac stimulation post–AV nodal ablation evaluation (PAVE) trial, Doshi et al.131 randomized 184 patients with permanent AF and AV node ablation to BiV versus RV pacing. After 6 months, LVEF was significantly better in patients with BiV pacing (46% vs. 41%; P = .03) as was the 6-minute walking distance (6MWD), but no significant difference in perceived QOL. Patients with LVEF less than 45% or NYHA Class II and III at baseline had greater improvement in 6MWD by BiV pacing compared to patients with normal LVEF or no HF symptoms. Although PAVE was an important step in the evaluation of cardiac resynchronization therapy (CRT) for standard pacing indications outside hemodynamic indications in HF patients, it was certainly too small with insufficient follow-up to investigate the full impact of CRT in patients without symptomatic HF.
Biventricular pacing was tested in patients with standard bradycardia indications and heart failure in the Homburg Biventricular Pacing Evaluation (HOBIPACE) study.132 In 30 patients with AV block and LVEF of 40% or less, BiV devices were implanted and randomized to RV versus BiV pacing for 3 months, then a 3-month crossover phase. In patients with and without AF, BiV pacing induced improvements in LVEF and left ventricular end-diastolic as well as end-systolic volumes, a reduction in NT-proBNP by 31%, an improved QOL score, improved exercise capacity, and improved maximum oxygen consumption and oxygen uptake at the anaerobic threshold during cardiopulmonary exercise testing. Although these were impressive results, 63% of the patients in HOBIPACE had a left BBB (mean QRS duration, 174 msec), meeting the conventional indication for CRT.
In the RD-CHF study, 44 permanently paced patients with continuous single-chamber RV pacing for a standard pacing indication (worsening CHF) who required a pacemaker generator change for battery depletion were upgraded to a BiV system and completed a randomized crossover study.133 All patients had NYHA Class III or IV with LVEF of 35% or less and echocardiographic signs of interventricular or intra-LV dyssynchrony. The mean paced QRS duration at baseline was 200 msec. During crossover with pacing in RV and BiV mode for 3 months each, QRS duration and LV preejection delay were significantly shortened by BiV pacing, and NYHA functional class, 6MWD, and QOL were significantly improved. Although these are important data in a population with a pacemaker indication, there is almost 100% overlap with current indications for CRT.
Albertsen et al.134 randomized 50 patients with AV block (permanent or paroxysmal) who were referred for a first pacemaker implantation to either RV dual-chamber or BiV pacing.134 Only four patients had a left BBB, and mean LVEF was 60% at baseline. All patients received a pacemaker capable of BiV pacing with three lead connectors, but only BiV-randomized patients received an LV lead (implantation success, 100%). During follow-up of 12 months, LVEF was unchanged in BiV pacing, with a minimal but statistically significant decrease to 57% with RV pacing. Tissue Doppler imaging revealed more LV dyssynchrony after 12 months with RV than BiV pacing (1.8 vs. 0.8 segments with delayed longitudinal contraction; P = .02). NT-proBNP levels, which were not high at baseline (122 pmol/L in DDDR: 91 pmol/L in BiV) remained unchanged with dual-chamber pacing but were significantly lower after 12 months with BiV pacing. In summary, Albertsen et al.134 were the first to investigate BiV pacing in a group of patients with AV block (unrelated to AV node ablation) requiring ventricular pacing, without an indication for CRT. Although only surrogate parameters for outcome were analyzed, the 12-month follow-up was sufficient to produce echocardiographic and serologic results that indicate a hemodynamic superiority of BiV pacing in patients with AV block and normal LV function at baseline.
To date, PACE represents the largest trial on biventricular pacing for standard pacing indications.135 In 177 patients with a pacing indication (AV block in 102, SND in 71, other indication in 4 patients) and normal systolic LV function (defined as LVEF >45%), a BiV pacemaker was implanted. Another 16 patients were excluded from randomization after an unsuccessful attempt of implantation of the LV lead. After stratification according to the presence or absence of diastolic dysfunction, patients were randomized to receive DDDR pacing (60-140 bpm) or BiV pacing. In patients with SND, the AV delay was programmed 20 msec shorter than intrinsic AV conduction. After 12 months, the primary endpoints (LVEF and LV end-systolic volume) were assessed. Left ventricular function was much better preserved in BiV pacing (LVEF = 62% vs. 55%, P <.001; end-systolic volume = 28 vs. 36 mL, P <.001) in patients with and without diastolic dysfunction at baseline. A significant deterioration in LVEF to less than 45% occurred in one patient (1%) during BiV versus eight (9%) during RV pacing (P = .02). Six patients with RV pacing and five with BiV pacing were hospitalized for heart failure; one patient with RV pacing died during the study. There was no significant difference in 6MWD or QOL using the SF-36 questionnaire. Echocardiographic data suggest that BiV pacing can prevent pacing-induced ventricular remodeling and heart failure in patients without significant systolic ventricular dysfunction. However, these are only surrogate parameters. PACE must be criticized for failed implant procedures that do not appear in the analysis, and for including SND patients with presumably normal PR interval and QRS duration, who underwent “forced” RV or BiV pacing. At least for RV pacing, it is known that heart failure can be caused by unnecessary pacing in these patients. The results of PACE therefore must be interpreted with caution.
Several studies on biventricular pacing for conventional pacing indications are ongoing. The PREVENT-HF trial has completed enrollment and follow-up of 108 randomized patients with a standard Class I or IIa indication for cardiac pacing and a projected need for ventricular pacing of at least 80% to RV or BiV pacing.136 After 12 months, LV end-diastolic volume is compared as the primary endpoint.
The Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BioPace) study enrolled 1800 patients in 2007 with a conventional indication for pacing, with a high probability that the ventricle will be predominantly paced.137 Patients with all NYHA functional classes were eligible, with no restriction in LVEF. Patients with an indication for primary prophylaxis of sudden cardiac death were allowed to receive an ICD instead of a pacemaker. There was a hardware randomization to RV versus BiV devices. The study is endpoint driven; follow-up will be terminated when 635 endpoints (all-cause mortality) have occurred.
In contrast, BLOCK-HF is a double-blind, software-randomized trial enrolling patients with a Class I or IIa pacing indication for second-degree or third-degree AV block or other pacing indications (e.g., first-degree AV block with pseudo–pacemaker syndrome, Wenckebach block rates <100 bpm, PR >300 msec).138 Patients must have LVEF of 50% or less (NYHA Class I-III) and are software-randomized to RV versus BiV pacing (ICD if an indication for primary prevention of sudden cardiac death is present). During a follow-up of at least 12 months, the primary endpoint in the projected 1636 enrolled patients will be a combination of all-cause mortality, HF-related urgent care, or a 15% or more increase in LV end-systolic volume index.
In summary, there is some evidence that biventricular pacing can prevent the deleterious effects of right ventricular pacing in a significant number of patients. However, the results of BioPace and BLOCK-HF should be evaluated before BiV pacing, with the additional expenditure of time, money, and effort at implantation and the additional hardware, as well as potential short-term and long-term complications, is applied in patients with a pacing indication involving an expected high percentage of ventricular pacing. In the meantime, routine placement of an LV lead for patients with AV block is not recommended by the pacing guidelines. Patients with an indication for CRT, according to REVERSE, MADIT-CRT, and RAFT (LVEF ≤40%; NYHA I-III if positive history of ≥Class II),139–141 may represent candidates that can benefit from biventricular devices based on the assumption that they would otherwise have a (paced) broad left bundle branch block (LBBB).
 Cost-Effectiveness of Dual-Chamber Pacing
Cost-Effectiveness of Dual-Chamber Pacing
In 1996, Sutton and Bourgeois142 published a cost-effectiveness analysis based on the (nonrandomized) studies available. They found a 10-year survival in sinus node disease with DDD pacing of 71%, versus only 57% with VVI pacing. The numbers for AV block were 61% with DDD and 51% with VVI pacing. For QOL, prevalence of heart failure was 60% lower with dual-chamber pacing; severe disability was present with VVI pacing in 36% of SND patients and 22% of AV block patients, but only for 8% and 3% of patients paced in dual-chamber mode. Assuming the implantation of a dual-chamber system costs 1.5 times more than a VVI system, this higher cost was offset by the higher cost of complications and side effects (stroke, disability, upgrade) during VVI pacing after 3 years. Assuming a battery life of 10 years, dual-chamber pacing was highly cost-effective in this analysis.
Cost-effectiveness was calculated for a subset of patients from the Canadian Trial of Physiologic Pacing.143 CTOPP authors calculated additional costs of approximately 3000 Canadian dollars ($) for implantation and follow-up of a dual-chamber versus single-chamber device. With a (nonsignificant) relative mortality reduction of 9.4% with dual-chamber pacing, the authors calculated a gain of 0.01 life-years with dual-chamber pacing, with costs of approximately $300,000 per life-year saved. Only in the subgroup of patients with an intrinsic heart rate less than 60 bpm would the cost per life-year be acceptable, at $16,000. A key consideration in this analysis is the need for reoperation because of pacemaker syndrome. In CTOPP, this was necessary in only 4.3% of patients randomized to VVI during 5 years,72 versus 25% to 30% in PASE and MOST, where “reoperation” could be replaced by reprogramming the devices.65,67
An analysis of the U.S. MOST trial found that dual-chamber pacing in patients with sinus node disease was cost-effective, with an average of $53,000 per life-year saved.144 However, depending on the statistical model and method, and exclusion or inclusion of QOL data, results varied between $3200 and $334,000.
A cost-effectiveness analysis by the British National Health Service in 2005 (before UKPACE results) took into account the lower incidence of AF and pacemaker syndrome in dual-chamber pacing, particularly in patients with SND.145 The higher incidence of complications in dual-chamber devices at implantation was also considered. The cost of the dual-chamber system, including all costs of complications and clinical events during a 5-year period, was assumed to be 7400  . The cost difference compared to single-chamber systems was small, about 700
. The cost difference compared to single-chamber systems was small, about 700  during 5 years. The cost for dual-chamber versus single-chamber ventricular pacing over 5 years was therefore estimated to be 8500
during 5 years. The cost for dual-chamber versus single-chamber ventricular pacing over 5 years was therefore estimated to be 8500  per QOL-year saved in patients with AV block and 9500
per QOL-year saved in patients with AV block and 9500  in patients with SND. This cost was reduced to approximately 5500
in patients with SND. This cost was reduced to approximately 5500  over 10 years. However, these calculations would dramatically change, to approximately 30,000
over 10 years. However, these calculations would dramatically change, to approximately 30,000  per QOL-year saved, if a lower incidence of pacemaker syndrome, similar to the CTOPP, would have been used.
per QOL-year saved, if a lower incidence of pacemaker syndrome, similar to the CTOPP, would have been used.
A “cost-efficacy analysis” has been performed retrospectively in patients with single-chamber versus dual-chamber ICDs using the Yale University Electrophysiology database.146 It included 453 patients who had received a single- or dual-chamber ICD between June 1997 (when the first dual-chamber ICD received FDA approval) and July 2001. Three strategies of pacing mode selection were assessed: (1) implantation of a single-chamber ICD with upgrade whenever needed, (2) implantation of a dual-chamber ICD in all patients, and (3) electrophysiologic (EP) study and implantation of a dual-chamber ICD only in patients with results indicating abnormal sinus or AV nodal function. Interestingly, the targeted, EP-guided device selection was the most expensive strategy (>$41,000); implantation of single-chamber ICDs with need for upgrade in an estimated 20% of patients cost $39,000. Surprisingly, implantation of dual-chamber ICDs in all patients would have been the least expensive strategy, only $36,000. Subsequent sensitivity analyses demonstrated that even with need to upgrade single-chamber ICDs in only 10% or even 5%, implantation of a dual-chamber ICD remained less expensive as long as it was no more than $1500 over a single-chamber ICD. An EP-guided approach would be least expensive in patients who underwent EP testing for other reasons (e.g., inducibility of ventricular tachyarrhythmias).
A recent analysis with assumptions representative for current systems calculated cost-effectiveness in a statistical model.147 The authors assumed that the implantation of dual-chamber devices instead of VVIR systems in 1000 patients would prevent AF in 36 and severe pacemaker syndrome in 168 patients, while requiring 13 additional hospitalizations from complications over 5 years. In this model, health benefits would need an incremental cost of 23  per patient and 0.09 QOL-years, equal to incremental costs of 260
per patient and 0.09 QOL-years, equal to incremental costs of 260  per QOL-year gained. Similar to other analyses, upgrade procedures for pacemaker syndrome would have the greatest impact on costs.
per QOL-year gained. Similar to other analyses, upgrade procedures for pacemaker syndrome would have the greatest impact on costs.
 Current Guidelines on Pacing Mode Selection
Current Guidelines on Pacing Mode Selection
The current American College of Cardiology/American Heart Association (ACC/AHA/HRS) guidelines on cardiac pacing reference the five pacing mode selection trials discussed (Danish, PASE, MOST, CTOPP, UKPACE).148 A major limitation of these trials was a rate of crossover and dropout of assigned pacing mode greater than 37%. Whenever a low percentage of pacing is expected (e.g., “very infrequent sinus pauses or infrequent AV block”), maintenance of AV synchrony during pacing likely does not seem important, and ventricular single-chamber pacing may be used as an alternative to dual-chamber pacing. However, progression of bradycardia is difficult to predict. Therefore, some patients with a seemingly rare need for pacing may develop bradycardia with permanent need of pacing (e.g., caused by β-blockers and antiarrhythmic drugs) and may then require a reoperation for an upgrade to dual-chamber pacing.
The current European Society of Cardiology (ESC) guidelines on pacing recommend only AAIR pacing and DDDR, with dedicated algorithms to prevent unnecessary right ventricular pacing in sinus node disease.149 Single-chamber ventricular pacing is discarded because of the higher risk of atrial fibrillation and the trend toward lower exercise capacity and more pacemaker syndrome in a Cochrane review.150 In patients with AV block, VVIR is recommended only in those without sinus rhythm. Otherwise, VDD, DDD, and DDDR devices are recommended, depending on chronotropic sinus node function. VVIR is mentioned only as a Class IIb indication (i.e., majority of experts would not chose this pacing mode in patients with AV block and sinus rhythm). Alternative RV pacing sites, such as septal, hisian, parahisian, and biventricular, are mentioned. However, no recommendation is provided for these pacing sites in AV block with narrow QRS.
1 Ohm OJ, Brejvik K. Patients with high-grade atrioventricular block treated and not treated with a pacemaker. Acta Med Scand. 1978;203:521-528.
2 Sasaki Y, Shimotori M, Akahane K, et al. Long-term follow-up of patients with sick sinus syndrome: a comparison of clinical aspects among unpaced, ventricular inhibited paced and physiologically paced groups. Pacing Clin Electrophysiol. 1988;11:1575-1583.
3 Ausubel K, Furman S. The pacemaker syndrome. Ann Intern Med. 1985;103:420-429.
4 Rosenqvist M, Brandt J, Schüller H. Long-term pacing in sinus node disease: effects of stimulation mode on cardiovascular morbidity and mortality. Am Heart J. 1988;116:16-22.
5 Barold SS. Permanent single chamber atrial pacing is obsolete. Pacing Clin Electrophysiol. 2001;24:271-275.
6 Lipkin DP, Buller N, Frenneaux M, et al. Randomised crossover trial of rate responsive Activitrax and conventional fixed rate ventricular pacing. Br Heart J. 1987;58:613-616.
7 Oto MA, Muderrisoglu H, Ozin MB, et al. Quality of life in patients with rate responsive pacemakers: a randomized, crossover study. Pacing Clin Electrophysiol. 1991;14:800-806.
8 Lau CP, Rushby J, Leigh-Jones M, et al. Symptomatology and quality of life in patients with rate-responsive pacemakers: a double-blind, randomized, crossover study. Clin Cardiol. 1989;12:505-512.
9 Smedgard P, Kristensson BE, Kruse I, et al. Rate-responsive pacing by means of activity sensing versus single rate ventricular pacing. Pacing Clin Electrophysiol. 1987;10:902-915.
10 Hedman A, Nordlander R. QT sensing rate responsive pacing compared to fixed rate ventricular inhibited pacing. Pacing Clin Electrophysiol. 1989;12:374-385.
11 Rossi P, Rognoni G, Occhetta E, et al. Respiration-dependent ventricular pacing compared with fixed ventricular and atrial-ventricular synchronous pacing: aerobic and hemodynamic variables. J Am Coll Cardiol. 1985;6:646-652.
12 Gammage M, Schofield S, Rankin I, et al. Benefit of single setting rate responsive ventricular pacing compared with fixed rate demand pacing in elderly patients. Pacing Clin Electrophysiol. 1991;14:174-180.
13 Lau CP, Wong CK, Leung WH, Liu WX. Superior cardiac hemodynamics of atrioventricular synchrony over rate responsive pacing at submaximal exercise: observations in activity sensing DDDR pacemakers. Pacing Clin Electrophysiol. 1990;13:1832-1837.
14 Jutzy RV, Florio J, Isaeff DM, et al. Comparative evaluation of rate modulated dual chamber and VVIR pacing. Pacing Clin Electrophysiol. 1990;13:1838-1846.
15 Menozzi C, Brignole M, Moracchini PV, et al. Intrapatient comparison between chronic VVIR and DDD pacing in patients affected by high degree AV block without heart failure. Pacing Clin Electrophysiol. 1990;13:1816-1822.
16 Oldroyd KG, Rae AP, Carter R, et al. Double blind crossover comparison of the effects of dual chamber pacing [DDD] and ventricular rate adaptive [VVIR] pacing on neuroendocrine variables, exercise performance, and symptoms in complete heart block. Br Heart J. 1991;65:188-193.
17 Linde-Edelstam C, Nordlander R, Unden AL, et al. Quality of life in patients treated with atrioventricular synchronous pacing compared to rate modulated ventricular pacing: a long-term, double-blind, crossover study. Pacing Clin Electrophysiol. 1992;17:1844-1848.
18 Lau CP, Tai YT, Lee PW, et al. Quality-of-life in DDDR pacing: atrioventricular synchrony or rate adaptation? Pacing Clin Electrophysiol. 1994;17:1838-1843.
19 Lau CP, Tai YT, Leung WH, et al. Rate adaptive pacing in sick sinus syndrome: effects of pacing modes and intrinsic conduction on physiological responses, arrhythmias, symptomatology and quality of life. Eur Heart J. 1994;15:1445-1455.
20 Deharo JC, Badier M, Thirion X, et al. A randomized, single-blind crossover comparison of the effects of chronic DDD and dual sensor VVIR pacing mode on quality-of-life and cardiopulmonary performance in complete heart block. Pacing Clin Electrophysiol. 1996;19:1320-1326.
21 Kruse I, Rydén L. Comparison of physical work capacity and systolic time intervals with ventricular inhibited and atrial synchronous ventricular inhibited pacing. Br Heart J. 1981;46:129-136.
22 Kruse I, Arnman K, Conradson TB, Rydén L. A comparison of the acute and long-term hemodynamic effects of ventricular inhibited and atrial synchronous ventricular inhibited pacing. Circulation. 1982;65:846-855.
23 Antonioli GE, Ansani L, Barbieri D, et al. Italian multicenter study on a single lead VDD pacing system using a narrow atrial dipole spacing. Pacing Clin Electrophysiol. 1992;15:1890-1893.
24 Wiegand UK, Bode F, Bonnemeier H, et al. Long-term complication rates in ventricular, single lead VDD, and dual chamber pacing. Pacing Clin Electrophysiol. 2003;26:1961-1969.
25 Sulke AN, Chambers J, Dristas A, et al. A randomized double-blind crossover comparison of four rate-responsive pacing modes. J Am Coll Cardiol. 1991;17:696-706.
26 Kamalvand K, Tan K, Kotsakis A, et al. Is mode switching beneficial? A randomized study in patients with paroxysmal atrial tachyarrhythmias. J Am Coll Cardiol. 1997;30:496-504.
27 Lau CP, Butrous GS, Ward DE, Camm AJ. Comparison of exercise performance of six rate-adaptive right ventricular cardiac pacemakers. Am J Cardiol. 1989;63:833-838.
28 Shukla HH, Flaker GC, Hellkamp AS, et al. Clinical and quality of life comparison of accelerometer, piezoelectric crystal, and blended sensors in DDDR-paced patients with sinus node dysfunction in the Mode Selection Trial (MOST). Pacing Clin Electrophysiol. 2005;28:762-770.
29 Capucci A, Boriani G, Specchia S, et al. Evaluation by cardiopulmonary exercise test of DDDR versus DDD pacing. Pacing Clin Electrophysiol. 1992;15:1908-1913.
30 Lazarus A, Mitchell K. A prospective multicenter study demonstrating clinical benefit with a new accelerometer-based DDDR pacemaker. Pacing Clin Electrophysiol. 1996;19:1694-1697.
31 Lamas GA, Knight JD, Sweeney MO, et al. Impact of rate-modulated pacing on quality of life and exercise capacity: evidence from the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT). Heart Rhythm. 2007;4:1125-1132.
32 Kindermann M, Schwaab B, Finkler N, et al. Defining the optimum upper heart rate limit during exercise: a study in pacemaker patients with heart failure. Eur Heart J. 2002;23:1301-1308.
33 Camm AJ, Fei L. Chronotropic incompetence. Part II. Clinical implications. Clin Cardiol. 1996;19:503-508.
34 Andersen HR, Thuesen L, Bagger JP, et al. Prospective randomised trial of atrial versus ventricular pacing in sick-sinus syndrome. Lancet. 1994;344:1523-1528.
35 Andersen HR, Nielsen JC, Thomsen PE, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210-1216.
36 Andersen HR, Nielsen JC, Thomsen PE, et al. Atrioventricular conduction during long-term follow-up of patients with sick sinus syndrome. Circulation. 1998;98:1315-1321.
37 Kristensen L, Nielsen JC, Pedersen AK, et al. AV block and changes in pacing mode during long-term follow-up of 399 consecutive patients with sick sinus syndrome treated with an AAI/AAIR pacemaker. Pacing Clin Electrophysiol. 2001;24:358-365.
38 Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932-2937.
39 Nielsen JC, Andersen HR, Thomsen PE, et al. Heart failure and echocardiographic changes during long-term follow-up of patients with sick sinus syndrome randomized to single-chamber atrial or ventricular pacing. Circulation. 1998;97:987-995.
40 Andersen HR, Nielsen JC, Thomsen PE, et al. Arterial thromboembolism in patients with sick sinus syndrome: prediction from pacing mode, atrial fibrillation, and echocardiographic findings. Heart. 1999;81:412-418.
41 Israel CW, Grönefeld G, Ehrlich JR, et al. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43:47-52.
42 Rediker DE, Eagle KA, Homma S, et al. Clinical and hemodynamic comparison of VVI versus DDD pacing in patients with DDD pacemakers. Am J Cardiol. 1988;61:323-329.
43 Rosenqvist M, Brandt J, Schüller H. Long-term pacing in sinus node disease: effects of stimulation mode on cardiovascular morbidity and mortality. Am Heart J. 1988;116:16-22.
44 Sasaki Y, Shimotori M, Akahane K, et al. Long-term follow-up of patients with sick sinus syndrome: a comparison of clinical aspects among unpaced, ventricular inhibited paced and physiologically paced groups. Pacing Clin Electrophysiol. 1988;11:1575-1583.
45 Bianconi L, Boccadamo R, Di Florio A, et al. Atrial versus ventricular stimulation in sick sinus syndrome: effect on morbidity and mortality [abstract]. Pacing Clin Electrophysiol. 1989;12:1236.
46 Santini M, Alexidou G, Ansalone G, et al. Relation of prognosis in sick sinus syndrome to age, conduction defect, and modes of permanent cardiac pacing. Am J Cardiol. 1990;65:729-735.
47 Stangl K, Seitz K, Wirtzfeld A, et al. Differences between atrial single chamber pacing (AAI) and ventricular single chamber pacing (VVI) with respect to prognosis and antiarrhythmic effect in patients with sick sinus syndrome. Pacing Clin Electrophysiol. 1990;13:2080-2085.
48 Zanini R, Facchinetti AI, Gallo G, et al. Morbidity and mortality of patients with sinus node disease: comparative effects of atrial and ventricular pacing. Pacing Clin Electrophysiol. 1990;13:2076-2079.
49 Nürnberg M, Frohner K, Podczeck A, et al. Is VVI pacing more dangerous than AV sequential pacing in patients with sick sinus syndrome? [abstract]. Pacing Clin Electrophysiol. 1991;14:674.
50 Hesselson AB, Parsonnet V, Bernstein AD, et al. Deleterious effect of long-term single-chamber ventricular pacing in patients with sick sinus syndrome: the hidden benefits of dual chamber pacing. J Am Coll Cardiol. 1992;19:1542-1549.
51 Sgarbossa EB, Pinski SL, Maloney JD, et al. Chronic atrial fibrillation and stroke in paced patients with sick sinus syndrome. Relevance of clinical characteristics and pacing modalities. Circulation. 1993;88:1045-1053.
52 Tung RT, Shen WK, Hayes DL, et al. Long-term survival after permanent pacemaker implantation for sick sinus syndrome. Am J Cardiol. 1994;74:1016-1020.
53 Alpert MA, Curtis JJ, Sanfelippo JF, et al. Comparative survival after permanent ventricular and dual chamber pacing for patients with chronic high degree atrioventricular block with and without preexistent congestive heart failure. J Am Coll Cardiol. 1986;7:925-932.
54 Linde-Edelstam C, Gullberg B, Norlander R, et al. Longevity in patients with high degree atrioventricular block paced in the atrial synchronous or the fixed rate ventricular inhibited mode. Pacing Clin Electrophysiol. 1992;15:304-313.
55 Sutton R, Kenny RA. The natural history of sick sinus syndrome. Pacing Clin Electrophysiol. 1986;9:1114-1116.
56 Markewitz A, Schad N, Hemmer W, et al. What is the most appropriate stimulation mode in patients with sinus node dysfunction? Pacing Clin Electrophysiol. 1986;9:1115-1120.
57 Ebagosti A, Guenoun M, Saadjian A, et al. Long-term follow-up of patients treated with VVI pacing and sequential pacing with reference to VA retrograde conduction. Pacing Clin Electrophysiol. 1988;11:1929-1934.
58 Langenfeld H, Schneider B, Grimm W, et al. The six-minute walk: an adequate exercise for pacemaker patients? Pacing Clin Electrophysiol. 1990;13:1761-1765.
59 Feuer JM, Shandling AH, Messenger JC, et al. Influence of cardiac pacing mode on the long-term development of atrial fibrillation. Am J Cardiol. 1989;64:1376-1379.
60 Lamas GA. Physiological consequences of normal atrioventricular conduction: applicability to modern cardiac pacing. J Card Surg. 1989;4:89-98.
61 Lamas GA, Pashos CL, Normand SL, McNeil B. Permanent pacemaker selection and subsequent survival in elderly Medicare pacemaker recipients. Circulation. 1995;91:1063-1069.
62 Perrins EJ, Morley CA, Chan SL, Sutton R. Randomized controlled trial of physiological and ventricular pacing. Br Heart J. 1983;50:112-117.
63 Kristensson BE, Amman K, Smedgard P, Ryden L. Physiological versus single-rate ventricular pacing: a double-blind cross-over study. Pacing Clin Electrophysiol. 1986;8:73-84.
64 Mattioli AV, Vivoli D, Mattioli G. Influence of pacing modalities on the incidence of atrial fibrillation in patients without prior atrial fibrillation. Eur Heart J. 1998;19:282-286.
65 Lamas GA, Orav J, Stambler BS, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. N Engl J Med. 1998;338:1097-1104.
66 Ellenbogen KA, Stambler BS, Orav EJ, et al. Clinical characteristics of patients intolerant to VVIR pacing. Am J Cardiol. 2000;86:59-63.
67 Lamas GA, Lee KL, Sweeney MO, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346:1854-1862.
68 Flaker G, Greenspon A, Tardiff B, et al. Death in patients with permanent pacemakers for sick sinus syndrome. Am Heart J. 2003;146:887-893.
69 Greenspon AJ, Hart RG, Dawson D, et al. Predictors of stroke in patients paced for sick sinus syndrome. J Am Coll Cardiol. 2004;43:1617-1622.
70 Fleischmann KE, Orav EJ, Lamas GA, et al. Atrial fibrillation and quality of life after pacemaker implantation for sick sinus syndrome: data from the Mode Selection Trial (MOST). Am Heart J. 2009;158:78-83.
71 Link MS, Hellkamp AS, Estes NA3rd, et al. High incidence of pacemaker syndrome in patients with sinus node dysfunction treated with ventricular-based pacing in the Mode Selection Trial (MOST). J Am Coll Cardiol. 2004;43:2066-2071.
72 Connolly SJ, Kerr CR, Gent M, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med. 2000;342:1385-1391.
73 Tang AS, Roberts RS, Kerr C, et al. Relationship between pacemaker dependency and the effect of pacing mode on cardiovascular outcomes. Circulation. 2001;103:3081-3085.
74 Baranchuk A, Healey JS, Thorpe KE, et al. The effect of atrial-based pacing on exercise capacity as measured by the 6-minute walk test: a substudy of the Canadian Trial of Physiologic Pacing (CTOPP). Heart Rhythm. 2007;4:1024-1028.
75 Tyers GF, Gao M, Hayden RI, et al. Use of physiologic pacing after the Canadian Trial of Physiologic Pacing. Pacing Clin Electrophysiol. 2005;28(suppl 1):68-69.
76 Skanes AC, Krahn AD, Yee R, et al. Progression to chronic atrial fibrillation after pacing: the Canadian Trial of Physiologic Pacing. CTOPP Investigators. J Am Coll Cardiol. 2001;38:167-172.
77 Kerr CR, Connolly SJ, Abdollah H, et al. Canadian Trial of Physiological Pacing: effects of physiological pacing during long-term follow-up. Circulation. 2004;109:357-362.
78 Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
79 Toff WD, Camm AJ, Skehan JD. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med. 2005;353:145-155.
80 Toff WD, Skehan JD, De Bono DP, Camm AJ. The United Kingdom Pacing and Cardiovascular Events (UKPACE) trial. Heart. 1997;78:221-223.
81 Patel AM, Westveer DC, Man KC, et al. Treatment of underlying atrial fibrillation: paced rhythm obscures recognition. J Am Coll Cardiol. 2000;36:784-787.
82 Auricchio A, Stellbrink C, Block M, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99:2993-3001.
83 Auricchio A, Ding J, Spinelli JC, et al. Cardiac resynchronization therapy restores optimal atrioventricular mechanical timing in heart failure patients with ventricular conduction delay. J Am Coll Cardiol. 2002;39:1163-1169.
84 Stierle U, Kruger D, Mitusch R, et al. Adverse pacemaker hemodynamics evaluated by pulmonary venous flow monitoring. Pacing Clin Electrophysiol. 1995;18:2028-2034.
85 Healey JS, Toff WD, Lamas GA, et al. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing: meta-analysis of randomized trials, using individual patient data. Circulation. 2006;114:11-17.
86 Masumoto H, Ueda Y, Kato R, et al. Long-term clinical performance of AAI pacing in patients with sick sinus syndrome: a comparison with dual-chamber pacing. Europace. 2004;6:444-450.
87 Nielsen JC, Bøttcher M, Nielsen TT, et al. Regional myocardial blood flow in patients with sick sinus syndrome randomized to long-term single chamber atrial or dual chamber pacing: effect of pacing mode and rate. J Am Coll Cardiol. 2000;35:1453-1461.
88 Nielsen JC, Kristensen L, Andersen HR, et al. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol. 2003;42:614-623.
89 Kristensen L, Nielsen JC, Mortensen PT, et al. Incidence of atrial fibrillation and thromboembolism in a randomised trial of atrial versus dual chamber pacing in 177 patients with sick sinus syndrome. Heart. 2004;90:661-666.
90 Albertsen AE, Nielsen JC, Poulsen SH, et al. DDD(R)-pacing, but not AAI(R)-pacing, induces left ventricular desynchronization in patients with sick sinus syndrome: tissue-Doppler and 3D echocardiographic evaluation in a randomized controlled comparison. Europace. 2008;10:127-133.
91 Nielsen JC, Thomsen PE, Højberg S, et al. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32:686-696.
92 Sweeney MO, Bank AJ, Nsah E, et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357:1000-1008.
93 Best PJ, Hayes DL, Stanton MS. The potential usage of dual chamber pacing in patients with implantable cardioverter-defibrillators. Pacing Clin Electrophysiol. 1999;22:79-85.
94 Kühlkamp V, Dörnberger V, Mewis C, et al. Clinical experience with the new detection algorithms for atrial fibrillation of a defibrillator with dual chamber sensing and pacing. J Cardiovasc Electrophysiol. 1999;10:905-915.
95 Deisenhofer I, Kolb C, Ndrepepa G, et al. Do current dual chamber cardioverter defibrillators have advantages over conventional single chamber cardioverter-defibrillators in reducing inappropriate therapies? A randomized, prospective study. J Cardiovasc Electrophysiol. 2001;12:134-142.
96 Theuns DA, Rivero-Ayerza M, Boersma E, Jordaens L. Prevention of inappropriate therapy in implantable defibrillators: a meta-analysis of clinical trials comparing single-chamber and dual-chamber arrhythmia discrimination algorithms. Int J Cardiol. 2008;125:352-357.
97 Friedman PA, McClelland RL, Bamlet WR, et al. Dual-chamber versus single-chamber detection enhancements for implantable defibrillator rhythm diagnosis: the detect supraventricular tachycardia study. Circulation. 2006;113:2871-2879.
98 Bänsch D, Steffgen F, Grönefeld G, et al. The 1+1 trial: a prospective trial of a dual- versus a single-chamber implantable defibrillator in patients with slow ventricular tachycardias. Circulation. 2004;110:1022-1029.
99 Dorian P, Philippon F, Thibault B, et al. Randomized controlled study of detection enhancements versus rate-only detection to prevent inappropriate therapy in a dual-chamber implantable cardioverter-defibrillator. Heart Rhythm. 2004;1:540-547.
100 Wietholt D, Kuehlkamp V, Meisel E, et al. Prevention of sustained ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators: the PREVENT study. J Interv Card Electrophysiol. 2003;9:383-389.
101 Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115-3123.
102 Sharma AD, Rizo-Patron C, Hallstrom AP, et al. Percent right ventricular pacing predicts outcomes in the DAVID Trial. Heart Rhythm. 2005;2(8):830-834.
103 Kutalek SP, Sharma AD, McWilliams MJ, et al. Effect of pacing for soft indications on mortality and heart failure in the dual chamber and VVI implantable defibrillator (DAVID) trial. Pacing Clin Electrophysiol. 2008;31:828-837.
104 Berenbom LD, Weiford BC, Vacek JL, et al. Differences in outcomes between patients treated with single- versus dual-chamber implantable cardioverter defibrillators: a substudy of the Multicenter Automatic Defibrillator Implantation Trial II. Ann Noninvasive Electrocardiol. 2005;10:429-435.
105 Wilkoff BL, Kudenchuk PJ, Buxton AE, et al. The DAVID (Dual Chamber and VVI Implantable Defibrillator) II trial. J Am Coll Cardiol. 2009;53:872-880.
106 Kudenchuk PJ, Hallstrom AP, Herre JM, Wilkoff BL. Heart rate, pacing, and outcome in the Dual Chamber and VVI Implantable Defibrillator (DAVID) trials. Heart Rhythm. 2009;6:1129-1135.
107 Almendral J, Arribas F, Wolpert C, et al. Dual-chamber defibrillators reduce clinically significant adverse events compared with single-chamber devices: results from the DATAS (Dual Chamber and Atrial Tachyarrhythmias Adverse Events Study) trial. Europace. 2008;10:528-535.
108 Connolly SJ, Kerr C, Gent M, Yusuf S. Dual-chamber versus ventricular pacing: critical appraisal of current data. Circulation. 1996;94:578-583.
109 Lamas GA, Estes NM3rd, Schneller S, Flaker GC. Does dual chamber or atrial pacing prevent atrial fibrillation? The need for a randomized controlled trial. Pacing Clin Electrophysiol. 1992;15:1109-1113.
110 Lamas GA, Prosser AP, Edery TP, et al. Age and sex bias in pacemaker selection [abstract]. Circulation. 1992;86(suppl I):449.
111 Vardas PE, Auricchio A, Blanc JJ, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy. The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Europace. 2007;9:959-998.
112 Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5:934-955.
113 Markewitz A. Annual report 2007 of the German Pacemaker Registry. Herzschrittmacherther Elektrophysiol. 2009;20:191-218.
114 Steinberg JS, Fischer A, Wang P, et al. The clinical implications of cumulative right ventricular pacing in the Multicenter Automatic Defibrillator Implantation Trial II. J Cardiovasc Electrophysiol. 2005;16:359-365.
115 Karpawich PP, Rabah R, Haas JE. Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol. 1999;22:1372-1377.
116 Lieberman R, Padeletti L, Schreuder J, et al. Ventricular pacing lead location alters systemic hemodynamics and left ventricular function in patients with and without reduced ejection fraction. J Am Coll Cardiol. 2006;48:1634-1641.
117 Thambo JB, Bordachar P, Garrigue S, et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. 2004;110:3766-3772.
118 Barold SS, Levine PA. Pacemaker repetitive nonreentrant ventriculoatrial synchronous rhythm. J Interv Card Electrophysiol. 2001;5:45-58.
119 Sweeney MO. Novel cause of spurious mode switching in dual-chamber pacemakers: atrioventricular desynchronization arrhythmia. J Cardiovasc Electrophysiol. 2002;13:616-619.
120 Fröhlig G, Gras D, Victor J, et al. Use of a new cardiac pacing mode designed to eliminate unnecessary ventricular pacing. Europace. 2006;8:96-101.
121 Savouré A, Fröhlig G, Galley D, et al. A new dual-chamber pacing mode to minimize ventricular pacing. Pacing Clin Electrophysiol. 2005;28(suppl 1):43-46.
122 Thibault B, Simpson C, Gagné CE, et al. Impact of AV conduction disorders on SafeR mode performance. Pacing Clin Electrophysiol. 2009;32(suppl 1):231-235.
123 Pürerfellner H, Brandt J, Israel C, et al. Comparison of two strategies to reduce ventricular pacing in pacemaker patients. Pacing Clin Electrophysiol. 2008;31:167-176.
124 Milasinovic G, Tscheliessnigg K, Boehmer A, et al. Percent ventricular pacing with managed ventricular pacing mode in standard pacemaker population. Europace. 2008;10:151-155.
125 Gillis AM, Pürerfellner H, Israel CW, et al. Reducing unnecessary right ventricular pacing with the managed ventricular pacing mode in patients with sinus node disease and AV block. Pacing Clin Electrophysiol. 2006;29:697-705.
126 Sweeney MO, Ellenbogen KA, Casavant D, et al. Multicenter, prospective, randomized safety and efficacy study of a new atrial-based managed ventricular pacing mode (MVP) in dual chamber ICDs. J Cardiovasc Electrophysiol. 2005;16:811-817.
127 Sweeney MO, Ellenbogen KA, Tang AS, et al. Atrial pacing or ventricular backup-only pacing in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:1552-1560.
128 Olshansky B, Day J, McGuire M, et al. Reduction of right ventricular pacing in patients with dual-chamber ICDs. Pacing Clin Electrophysiol. 2006;29:237-243.
129 Olshansky B, Day JD, Moore S, et al. Is dual-chamber programming inferior to single-chamber programming in an implantable cardioverter-defibrillator? Results of the INTRINSIC RV (Inhibition of Unnecessary RV Pacing with AVSH in ICDs) study. Circulation. 2007;115:9-16.
130 Zhang XH, Chen H, Siu CW, et al. New-onset heart failure after permanent right ventricular apical pacing in patients with acquired high-grade atrioventricular block and normal left ventricular function. J Cardiovasc Electrophysiol. 2008;19:136-141.
131 Doshi RN, Daoud EG, Fellows C, et al. Left ventricular–based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol. 2005;16:1160-1165.
132 Kindermann M, Hennen B, Jung J, et al. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: the Homburg Biventricular Pacing Evaluation (HOBIPACE). J Am Coll Cardiol. 2006;47:1927-1937.
133 Leclercq C, Cazeau S, Lellouche D, et al. Upgrading from single chamber right ventricular to biventricular pacing in permanently paced patients with worsening heart failure: the RD-CHF Study. Pacing Clin Electrophysiol. 2007;30(suppl 1):23-30.
134 Albertsen AE, Nielsen JC, Poulsen SH, et al. Biventricular pacing preserves left ventricular performance in patients with high-grade atrioventricular block: a randomized comparison with DDD(R) pacing in 50 consecutive patients. Europace. 2008;10:314-320.
135 Yu CM, Chan JY, Zhang Q, et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361:2123-2134.
136 De Teresa E, Gómez-Doblas JJ, Lamas G, et al. Preventing ventricular dysfunction in pacemaker patients without advanced heart failure: rationale and design of the PREVENT-HF study. Europace. 2007;9:442-446.
137 Funck RC, Blanc JJ, Mueller HH, et al. Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BioPace) study. Europace. 2006;8:629-635.
138 Curtis AB, Adamson PB, Chung E, et al. Biventricular versus right ventricular pacing in patients with AV block (BLOCK HF): clinical study design and rationale. J Cardiovasc Electrophysiol. 2007;18:965-971.
139 Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834-1843.
140 Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338.
141 Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385-2395.
142 Sutton R, Bourgeois I. Cost-benefit analysis of single- and dual-chamber pacing for sick sinus syndrome and atrioventricular block: an economic sensitivity analysis of the literature. Eur Heart J. 1996;17:574-582.
143 O’Brien BJ, Blackhouse G, Goeree R, et al. Cost-effectiveness of physiologic pacing: results of the Canadian Health Economic Assessment of Physiologic Pacing. Heart Rhythm. 2005;2:270-275.
144 Rinfret S, Cohen DJ, Lamas GA, et al. Cost-effectiveness of dual-chamber pacing compared with ventricular pacing for sinus node dysfunction. Circulation. 2005;111:165-172.
145 Castelnuovo E, Stein K, Pitt M, et al. The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation. Health Technol Assess. 2005;9(43):1-246. iii, xi-xiii
146 Goldberger Z, Elbel B, McPherson CA, et al. Cost advantage of dual-chamber versus single-chamber cardioverter-defibrillator implantation. J Am Coll Cardiol. 2005;46:850-857.
147 Deniz HB, Caro JJ, Ward A, et al. Economic and health consequences of managing bradycardia with dual-chamber compared to single-chamber ventricular pacemakers in Italy. J Cardiovasc Med. 2008;9:43-50.
148 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;117:e350e-408.
149 Vardas PE, Auricchio A, Blanc JJ, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy. The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Europace. 2007;9:959-998.
150 Dretzke J, Toff WD, Lip GY, et al: Dual chamber versus single chamber ventricular pacemakers for sick sinus syndrome and atrioventricular block. Cochrane Database Syst Rev 2:CD003710, 2004.