25 Clinical trials in cancer
Introduction
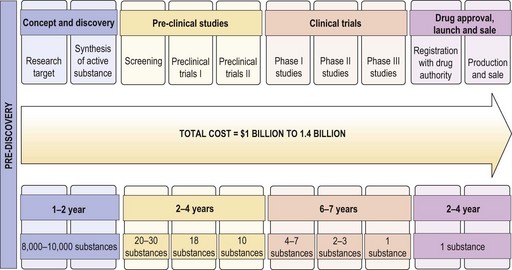
Traditionally the clinical efficacy of drugs is studied through several phases of clinical trial after initial pre clinical studies. Each of these trials is aimed at generating sufficient evidence on the safety, dosing, efficacy and feasibility of use. This process is expensive and time consuming (Figure 25.1), which often leads to very high costs of effective treatments preventing their general use and often prompting policy makers to adopt rationing strategies based on cost-effectiveness.
Clinical trials
Phase I
Phase I studies are the first human application of a new drug or drug combination. The aim of this phase is to establish the dose and schedule of the experimental agent for efficacy testing in a phase II study based on the maximum tolerated dose (MTD) of the new agent. Phase I studies are typically single arm, open label, sequential studies that include patients with good performance status (PS 0-2) and for whom there is no standard treatment option. The MTD is determined by progressively increasing the dose in small cohorts until the dose limiting toxicity (DLT) is achieved. DLT is defined as grade 4 non-haematological toxicity or a grade 3 or more haematological toxicity graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) (p. 38). MTD dose is defined as the dose below the level the DLT is met or the dose level at which the DLT is seen. There are a number of components to the design of a phase I study and there are various dose escalation methods to find out the maximum tolerated dose (Box 25.1). Phase I trial design is a specialized topic and is beyond the scope of this chapter (see Further reading).
Box 25.1
Components of phase I study design and methods of dose escalation
Dose escalation methods
Phase III
The aim of a phase III study (also called randomized controlled trials) is to establish the efficacy of a new treatment compared with an existing standard treatment or observation/placebo where there is no standard treatment. It also helps to establish whether the new treatment has less toxicity compared with the standard treatment. Randomization reduces bias by balancing the characteristics of study population. Design of a phase III study is complex (Box 25.2). It requires large patient numbers to ensure adequate statistical power which frequently necessitates international cooperation and is very costly. Modern trials are incorporating translational studies to try to target the new drug to the population most likely to benefit. This may affect the inclusion criteria e.g. presence of an EGFR mutation for entry into a trial for an antibody against the EGFR receptor.
Phase IV studies
Phase IV studies are post-marketing studies done to learn more about the side effects, safety, and long term risk-benefits of the drug in a wider context than in clinical trial. The various study designs are given in Box 25.3.
Box 25.3
Phase IV study designs
Recruiting patients into a clinical trial
A number of issues need to be considered when recruiting patients into clinical trial ranging from explaining the trial itself to giving a realistic picture of the potential risks and benefits. Consent is a complex process which does not simply refer to the signing of a form but with good clinical practice has to ensure that the patient has adequately understood what is involved and has given their consent freely. It should be made clear that consent can be withdrawn at any time without affecting their future care to avoid pressurizing already vulnerable individuals into believing they have to agree to please their medical carers. Good clinical practice also states that patients should be given adequate time to reflect on the information received and be able to discuss with family, friends or their general practitioner. Key areas to be covered when discussing a trial with a patient are listed in Box 25.4.
Box 25.4
Cancer clinical trials – what to tell the patient?
Advances in clinical trials
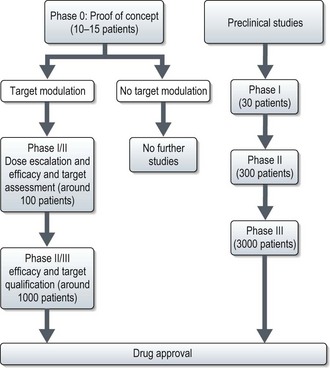
The current process of cancer clinical trial design is costly and lengthy. Studies show that the overall cost of introduction of a drug to clinical use is approximately 1 billion dollars and that for a target molecule is 1.4 billion dollars. This process also takes an average of 12–14 years. The phase 0 trial design has been proposed as a solution (Box 25.5). This may expedite the identification of new target molecules and bring potentially active molecules into routine clinical use faster.
Box 25.5
Phase 0 trial
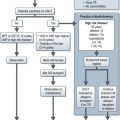
Phase 0 trial – are first-in-human trials with no therapeutic or diagnostic intent, in a limited number of patients (<15) using subtherapeutic doses over a limited period to establish a drug-target effect. (See Figure 25.2). These are established as means to address the pitfalls of phase I–III studies and to expedite the development of new investigational targeted agents. These methods may also be included within a classical phase I trial.
Under these studies, dose escalation is allowed provided it is to modulate the target, not to determine MTD. Phase 0 trials provide critical human PK and PD data which help to directly proceed to phase I/II study or a combination with an established agent, thus expediting the development of agents or combination of agents likely to have clinical activity. Using the new design, the National Cancer Institute evaluated the effect of a PARP inhibitor, ABT 888 in tumour tissue which led to a phase I study of ABT-888 with chemotherapy. The phase 0 study met its primary objective within 5 months, even before any of the planned phase I study started patient accrual (see Figure 25.2).
Oncology remains one of the most evidence-based specialties at the forefront of clinical trial development.
Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101(10):708-720.
Kummar S, Doroshow JH, Tomaszewski, et al. Phase 0 clinical trials: recommendations from the Task Force on Methodology for the Development of Innovative Cancer Therapies. Eur J Cancer. 2009;45(5):741-746.
Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS. Phase I clinical trial design in cancer drug development. J Clin Oncol. 2000;18:684-692.
Lee JJ, Feng L. Randomized phase II designs in cancer clinical trials: current status and future directions. J Clin Oncol. 2005;23(19):4450-4457.
Green S, Bendetti J, Crowley J. Clinical Trials in Oncology, Second edition. London: Chapman and Hall; 2003.
Eisenhauer EA, Twelves C, Buyse M. Phase I cancer clinical trials. Oxford: Oxford University Press; 2006.

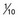 of the animal dose
of the animal dose