Chapter 1. Clinical reasoning in orthopaedic medicine
CHAPTER CONTENTS
SUMMARY
Orthopaedic medicine is based on the life’s work of the late Dr James Cyriax (1904–1985). He developed a method of assessing the soft tissues of the musculoskeletal system, employing a process of diagnosis by selective tension, which uses passive movements to test the inert structures and resisted movements to test the contractile structures.
This chapter is divided into two parts. The first discusses ‘referred symptoms’ and includes patterns and ‘rules’ of referral of pain and other symptoms from different structures. The second, ‘clinical examination’, describes the theory behind Cyriax’s logical method of subjective and objective examination, which, by reasoned elimination, leads to the incrimination of the tissue in which the lesion lies.
REFERRED SYMPTOMS
Patients usually complain of pain but there may be other symptoms that cause them to seek advice, such as stiffness, weakness, numbness and pins and needles. Since pain is the most usual complaint, it will form the basis of this discussion.
Whether right or wrong in their assessment, patients usually localize their pain as coming from a certain point and can describe the area of its spread, although sometimes only vaguely. Cyriax considered that all pain is referred and explored the pattern of referred pain to try to establish some rules that would help in its interpretation towards establishing its true source (Cyriax & Cyriax 1993).
The study of pain itself is a vast topic, most of which is outside the scope of this book, and the reader is referred for a detailed account to the many other sources that confine themselves to the in-depth study of this field. Nevertheless, pain and its behaviour are relevant to orthopaedic medicine, particularly in the assessment procedures, both towards the achievement of an accurate clinical diagnosis and as a guide to the effectiveness of the treatment techniques applied.
To be able to identify the source of the pain, a thorough knowledge of applied and functional anatomy is essential, coupled with an understanding of the behaviour of pain, particularly in relation to its ability to be referred to areas other than the causative site. It is acknowledged that other influences can affect the perception of pain and within this chapter referred pain will be discussed, with a brief consideration of psychosocial factors.
Discussion of the possible mechanisms and patterns of referred pain
It is commonly found in clinical practice that pain of visceral origin can mimic that of somatic origin (‘pain arising from noxious stimulation of one of the musculoskeletal components of the body’, Bogduk 2005) and vice versa. Pain arising from pathology in the heart, for example, may produce a spread of pain into the arm, imitating the pain of nerve root sleeve compression from a cervical lesion. Similarly, mid-thoracic back pain may arise from a stomach lesion. Visceral pain tends to be inflammatory in origin, since the viscera are relatively insensitive to mechanical pressure, whereas somatic pain can arise from either or both causes (Lundeburg & Ekholm 2002).
There have been several suggestions put forward for the mechanism of referred pain and the more significant ones are discussed here. McMahon et al (1995) cite Sinclair who suggested that primary sensory neurons have bifurcating axons which innervate both somatic and visceral structures. Some evidence was found for this theory, but some of the findings were challenged, particularly as such axons had failed to be demonstrated in appreciable numbers. While unable to find neurons with visceral and somatic fields, McMahon et al mention that Mense and colleagues did find a few single sensory neurons with receptive fields in two tissues, in both skin and muscle in the tail of a cat, but overall there was scant support for Sinclair’s suggestion.
More recently, Sameda et al (2003) have demonstrated peripheral axons dichotomizing (branching into two) into both the L5, L6 disc and groin skin in rats. This could provide a possible explanation for pain referred to the groin area from damage to the L5 and S1 nerve roots from L4–L5 and L5–S1 disc herniation in humans, in spite of the nerve supply of the groin area arising from the higher lumbar spinal nerves.
Evidence has been provided for the mechanism that visceral and somatic primary sensory neurons converge onto common spinal neurons, causing confusion in the ascending spinal pathways and leading to misinterpretation of the origin of the pain. The message from the primary lesion could be wrongly interpreted as coming from the area of pain referral (Vecchiet & Giamberardino 1997, Robinson 2003). This has been dubbed the convergence–projection theory.
A side-track from the ‘convergence–projection theory’ is the ‘convergence–facilitation theory’, attributed to McKenzie (cited in McMahon et al 1995), which claimed that the viscera are insensitive and that visceral afferent activity does not directly give rise to pain. It was suggested that an irritable focus was produced within the spinal cord, where somatic inputs would take over to produce abnormal referred pain in the appropriate segmental distribution. This theory was not generally accepted, however, since it denied that true visceral pain could exist. However, it did provide an explanation for heightened referred sensations, including that of secondary hyperalgesia (Vecchiet & Giamberardino 1997). Its basic concepts have been developed under the descriptor of ‘central sensitization’ that explains hyperalgesia and the prolongation of chronic pain as arising from the augmented response of signalling neurons in the central nervous system as a result of inflammation or compression of nerve structures (Niere 1991, Butler 1995, Mendelson 1995, Campbell & Meyer 2006).
As mentioned above, there are no separate ascending spinal pathways for the transmission of visceral pain, and sensations from the viscera are represented within the somatosensory pathways, that also transfer sensations from somatic structures (Galea 2002). This can lead to confusion in differential diagnosis between pain arising from visceral lesions and that arising from musculoskeletal lesions. Galea describes how the level of the spinal cord to which visceral afferent fibres project depends on their embryonic innervation and notes that many viscera migrate well away from their embryonic derivation during development, such that visceral referred pain may be perceived at remote sites.
Referred pain does not only present itself for misinterpretation between visceral and somatic structures but is also a phenomenon which may prevent accurate localization among the musculoskeletal tissues. Cyriax & Cyriax (1993) suggested that the misinterpretation of pain occurs at cortical level where stimuli arriving at certain cortical cells from the skin can be localized accurately to that area. When stimuli from other deeper tissues of the same segmental derivation reach those same cells, the sensory cortex makes assumptions on the basis of past experience and attributes the source of the pain to that same area of skin. This accounts for the dermatomal reference of pain but the theory can be extended to include the referral of other symptoms from structures within the same segment.
SEGMENTAL REFERENCE
Knowledge of the nerve supply of soft tissue structures, coupled with factors affecting segmental reference and the general rules of referred pain, is a useful aid to diagnosis. It will help to direct the clinician to the true source of the patient’s pain and so facilitate the application of effective treatment.
Several workers have tried to establish patterns of referred pain by examining the dermatomes. However, the dermatomes appear to vary according to the different methods for defining them. These mainly derive from embryonic development, observation of herpetic eruptions, areas of vasodilatation resulting from nerve root stimulation and the areas of tactile sensation remaining after rhizotomy (surgical severance) of spinal nerve roots, as described below. These dermatomes are referred to as embryonic, herpetic, vasodilatation and tactile, respectively.
Sir Henry Head (1900) laid the foundations for the mapping of dermatomes by analysing herpetic eruptions. Herpes zoster is an inflammatory lesion of the spinal ganglia which produces an eruption on the skin in the corresponding segmental cutaneous area. By defining the dermatomes in this way, little overlap was found and there was only slight variation between subjects studied.
Stricker & Bayliss, as described by Foerster (1933), used faradic stimulation of the distal part of a divided posterior root to produce vasodilatation. This produced a clearly defined area similar to the dermatomes defined in the isolation method, but slightly smaller in size.
The skin is supplied by both ventral and dorsal nerve roots. Foerster (1933) examined the areas of skin supplied by the dorsal roots which carry the afferent sensory fibres and efferent fibres producing vasodilatation. He described two methods of isolating the area of skin supplied by the dorsal nerve roots, using the terms ‘anatomical’ and ‘physiological’.
The anatomical method, attributed to Herringham & Bolk (Foerster 1933), involved the isolation of fibres arising from a single nerve root by dissection through the plexus and the peripheral nerves into the skin. It was impossible to follow the finest ramifications by this method but it largely demonstrated that there was little or no overlap of dermatomes classified in this way.
Within the physiological method of differentiation, Foerster (1933) describes how Sherrington divided the nerve roots above and below a single nerve root to map the area supplied by the intervening intact root in the monkey. There was such overlap of different dermatomes identified by this method that division of a single root produced no loss of sensibility. He used the same technique in humans and found the same pattern of overlap in the tactile dermatomes.
Lee et al (2008) set out to produce a novel dermatome map based on the available evidence drawn from experiments conducted over the past century (Fig. 1.1) They proposed that the overlapping of dermatomes and their variability deserved more emphasis and that to represent dermatomes as autonomous zones of cutaneous sensory innervation is unreliable.
 |
| Figure 1.1
The evidence-based dermatome map representing the most consistent tactile dermatomal areas for each spinal dorsal nerve root found in most individuals, based on the best available evidence. The dermatomal areas shown are NOT autonomous zones of cutaneous sensory innervation since, except in the midline where overlap is minimal, adjacent dermatomes overlap to a large and variable extent. Blank regions indicate areas of major variability and overlap. S3, S4 and S5 supply the perineum but are not shown for reasons of clarity.
From Lee M W L, McPhee R W, Stringer M D 2008 An evidence-based approach to human dermatomes. Clinical Anatomy 21:363–373. Reprinted with permission of John Wiley & Sons, Inc.
|
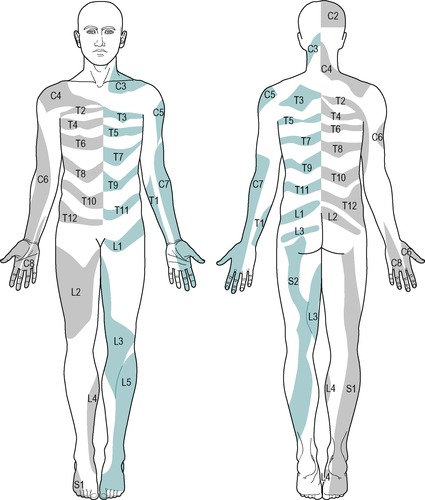
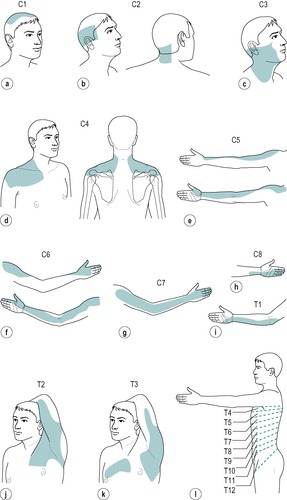
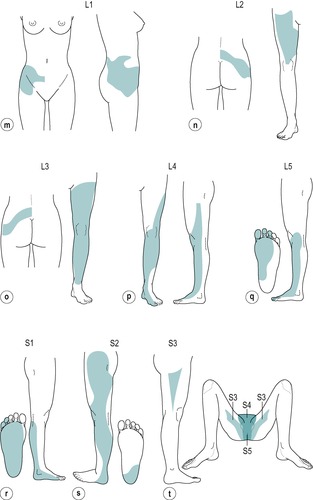
However, despite the experimental findings, the extent of individual dermatomes, especially in the limbs, is largely based on clinical evidence and Standring (2009) acknowledged that this leads to a wide variation between the opinions of different disciplines. The dermatomes given in this book are drawn mainly from the clinical experience of Cyriax and are different from those given in Gray’s Anatomy (Standring 2009), for example. In the authors’ experience, they provide a basic guide for clinical practice and are presented as shown in Figure 1.2.
 |
 |
| Figure 1.2
Dermatomes.
C1: top of head (Fig. 1.2a)
C2: side and back of the head, upper half of the ear, cheek and upper lip, nape of the neck (Fig. 1.2b)
C3: entire neck, lower mandible, chin, lower half of the ear (Fig. 1.2c)
C4: epaulette area of the shoulder (Fig. 1.2d)
C5: anterolateral aspect of the arm and forearm as far as the base of the thumb (Fig. 1.2e)
C6: anterolateral aspect of the arm and forearm, thenar eminence, thumb and index finger (Fig. 1.2f)
C7: posterior aspect of the arm and forearm, index, middle and ring fingers (Fig. 1.2g)
C8: medial aspect of the forearm, medial half of the hand, middle, ring and little fingers (Fig. 1.2h)
T1: medial aspect of the forearm, upper boundary uncertain (Fig. 1.2i)
T2: Y-shaped dermatome, medial condyle of humerus to axilla, branch to sternum and branch to scapula (Fig. 1.2j)
T3: area at front of chest, patch in axilla (Fig. 1.2k)
T4, 5, 6: circling trunk above, at and below the nipple area (Fig. 1.2l)
T7, 8: circling trunk at lower costal margin (Fig. 1.2l)
T9, 10, 11: circling the trunk, reaching the level of the umbilicus (Fig. 1.2l)
T12: margins uncertain, extends into groin, covers greater trochanter and the iliac crest (Fig. 1.2l)
L1: lower abdomen and groin, lumbar region between levels L2 and L4, upper, outer aspect of the buttock (Fig. 1.2m)
L2: two separate areas: lower lumbar region and upper buttock, whole of the front of the thigh (Fig. 1.2n)
L3: two separate areas: upper buttock, medial aspect and front of the thigh and leg as far as the medial malleolus (Fig. 1.2o)
L4: lateral aspect of the thigh, front of the leg crossing to the medial aspect of the foot, big toe only (Fig. 1.2p)
L5: lateral aspect of the leg, dorsum of the whole foot, first, second and third toes, inner half of the sole of the foot (Fig. 1.2q)
S1: sole of the foot, lateral two toes, lower half of the posterior aspect of the leg (Fig. 1.2r)
S2: posterior aspect of the whole thigh and leg, plantar aspect of the heel (Fig. 1.2s)
S3: circular area around the anus, medial aspect of the thigh (Fig. 1.2t)
S4: saddle area: anus, perineum, genitals, medial upper thigh (Fig. 1.2t)
S5: coccygeal area (Fig. 1.2t)
|
Nerve root dermatomes
In general the muscle groups lie under the dermatome which shares the same nerve supply. However, the dermatome and myotome may not overlie each other and there are some apparent exceptions to this rule. This can cause confusion in the consideration of referred pain, in that a lesion in the relevant muscle may appear to refer pain to an unrelated site and vice versa.
As mentioned above, the viscera may also refer pain to apparently unrelated sites and this should also be considered within differential diagnosis. Pappano & Bass (2006) present a case study describing referred shoulder pain preceding abdominal pain in a teenage girl with gastric perforation. The paper also highlights other conditions as referring to the shoulder including peritonitis, cholecystitis and subdiaphragmatic endometriosis. Post-laparoscopy shoulder pain is noted, where carbon dioxide used for inflation applies stretch to the diaphragm. Sloan (2008) provides further detail listing right-sided periscapular pain in gall bladder disease and interscapular pain in aortic dissection.
The most commonly encountered discrepancies are as follows:
• Scapular muscles are supplied by C4–C7, but underlie thoracic dermatomes
• Latissimus dorsi is supplied by C6–C8, but underlies thoracic and lumbar dermatomes
• Pectoralis major is supplied by C5–T1 but underlies thoracic dermatomes
• The heart, a thoracic structure, is supplied by C8–T4 and may refer pain into the arm, axilla and chest
• The diaphragm is supplied by C3–C5 and diaphragmatic irritation may lead to pain being felt in the epaulette region of the shoulder
• The gluteal muscles are supplied by L5, S1–S2 but underlie L1–L3 dermatomes
• The testicle is supplied by T11–T12, but underlies the S4 dermatome where pain may be felt locally or can be referred to the lower thoracic or upper lumbar regions.
Cyriax (1982) and Cyriax & Cyriax (1993) identified several factors that influence the referral of pain:
• Strength of the stimulus
• Position of the structure in the dermatome
• Depth of the structure
• Type or nature of the structure.
Strength of the stimulus
The more acutely inflamed or irritable the lesion (i.e. the greater the stimulus), the further into the dermatome will the symptoms be referred (Inman & Saunders 1944). For example, an acutely inflamed subacromial bursitis may refer its pain to the wrist, the distal extent of the C5 dermatome, and a lumbar lesion involving compression and inflammation of the L4 nerve root may refer pain and associated symptoms to the big toe, at the distal end of the L4 dermatome.
Position in the dermatome
Pain and tenderness tend to refer distally. Therefore a structure placed more proximally in the dermatome is capable of referring its symptoms over a greater distance to the end of the dermatome.
The length or distance of dermatomal referral is particularly obvious in the limbs, where the dermatomes tend to be long. However, if the dermatome is short, even with an acutely inflamed or irritable lesion, the reference will halt at the end of its dermatome, or the most distal dermatome in the event of more than one nerve supply.
Structures within the hand and forefoot are already at the distal end of their relevant dermatome. Referral of symptoms is therefore less and such lesions are easier to localize (Inman & Saunders 1944).
Depth of the structure
Skin is the only organ that provides precise localization of pain and is of course the most superficial structure (Gnatz 1991). Cyriax (1982) proposed that lesions in the more deeply placed structures tend to give greater reference of pain, which was also the finding of Kellgren (1939) and Inman & Saunders (1944). The deeper structures therefore give rise to greater misunderstanding in terms of clinical diagnosis.
Joint, ligament (e.g. medial collateral and anterior cruciate ligaments) and bursa lesions tend to conform to this assumption but a notable exception is provided by lesions within bone. Pain and tenderness arising from fractures or involvement of the cortical bone tend to be well localized, even though bone itself is the most deeply placed tissue in musculoskeletal terms. Lesions involving the cancellous part of bone may give the more typical pattern of referred pain.
Segmental pain arising from lesions in the deeply sited viscera can also be misleading.
Nature of the tissue
The factor of depth of the structure should perhaps be considered alongside that of the nature of the tissue, since studies to observe the effect of lesions in tissues of different nature and site on patterns of referred pain are hard to dissociate.
Kellgren (1938, 1939) observed patterns of referred pain induced by the injection of 6% hypertonic saline into deeply placed structures including muscle, tendon sheaths, fascia, periosteum and interspinous ligaments. On injection of muscle, diffuse referred pain was produced which appeared to follow a segmental pattern and was associated with deep tenderness rather than hyperaesthetic skin. Witting et al (2000) compared local and referred pain following intramuscular capsaicin injection into the brachioradialis muscle and intradermal injection in the skin above the muscle. Intradermal injection produced more intense but localized pain whereas referred pain was more marked after the intramuscular injection and was deeply located as well as referring to skin.
Kellgren also observed that pain arising from the limb muscles tended to refer to the region of the joints moved by these muscles, where it could easily be confused as arising from the joint itself. Farasyn (2007) adds support to the observation that muscles refer pain, describing that a local muscle lesion can give rise to a wider area of pain, separate from the tender local injury and often described as ‘burning’.
Tendon sheath and fascia gave sharply localized pain. Stimuli did not produce pain from articular cartilage or compact bone but when applied to cancellous bone a deep diffuse pain was produced. Stimulation of the interspinous ligaments gave rise to segmentally referred pain which, as in muscle, was associated with tenderness in the deeply placed structures.
Inman & Saunders (1944) noted a variability of sensitivity of the different structures beneath the skin, creating a ‘league table’ of those tissues with the highest sensitivity to those with the least, as follows: bone, ligaments, fibrous capsules of joints, tendons, fascia and muscle. These findings were supported in part by Kuslich et al (1991), who investigated tissues in the lumbar spine as potential sources of low back pain using progressive local anaesthetic during exploratory operation of the spine. Their emphasis too was that muscles, fascia and the periosteum and compact layer of bone (they did not test cancellous bone) were relatively insensitive. In contrast, in the work of Travell & Simons (1996) trigger points in muscle have been identified as a focus of hyperirritability which gives rise to local tenderness and referred pain.
It had been particularly noted by Inman & Saunders (1944) that capsules and ligaments were most sensitive close to their bony attachments, and therefore most likely to be pain-producing following trauma to these commonly injured sites.
Controversy has existed for many years as to whether the disc or the zygapophyseal joint is the primary source of back pain, especially when associated with pain in the limb. Aprill et al (1989) studied the reference of pain from the cervical zygapophyseal joints to establish whether each joint had a specific area of reference of pain in a segmental distribution. They found reasonably distinct and consistent segmental patterns of pain referral associated with joints between each of the levels of C2–C7, but there was no referral of pain into the arm from any of the tested levels. The paper acknowledged that the study had not set out to distinguish the pain arising from zygapophyseal joints from other potential sources.
In support of this pattern of referral, Cooper et al (2007) set out to determine the patterns of referred pain arising from cervical zygapophyseal joints by using diagnostic blocks to establish pain referral patterns in symptomatic subjects. There was considerable overlap of referral areas and patients described the symptoms in lines, spots or patches that were mostly confined to the neck and shoulder region. They reported some variation from the results of their previous studies conducted on non-symptomatic subjects and suggested that the outcome might be due to the length of time that patients had been experiencing symptoms.
Maintaining the emphasis on the nature of the tissue, two pain syndromes of somatic and radicular origin have been described associated with the spinal joints (Bogduk 2005). In somatic pain syndromes it is proposed that the source of the pain could be in any structure in the spine that receives a nerve supply, i.e. muscles, ligaments, zygapo-physeal joints, intervertebral discs, dura mater and dural nerve root sleeve. Somatic pain is not associated with neurological abnormalities and does not involve nerve root compression. The quality of somatic pain is described as dull, diffuse and difficult to localize.
For clarity, ‘ somatic pain’ is distinct from ‘ visceral pain’ where the noxious stimulation occurs within an organ. It is also distinct from ‘ neurogenic pain’ where the nociceptive information arises as a result of irritation or damage to the axons or cell bodies of a peripheral nerve, not to the nerve endings (Bogduk 2005).
A further distinction should be made between ‘ radiculopathy’ and ‘ radicular pain’.
Radiculopathy is a neurological condition where conduction is blocked in the axons of a spinal nerve or its roots. Conduction block in sensory axons results in numbness, and conduction block in motor axons results in weakness. Radiculopathy is a state of neurological loss and it does not result in pain, either in the back or limbs. However it may, or may not, be associated with radicular pain. The aetiology (cause) of both can be the same but the mechanisms are different.
In radicular pain syndromes, the radicular (root) pain arises as a result of irritation of a spinal nerve or its roots. Contrary to the traditional belief, it is not caused by compression or traction of nerve roots, unless the nerve root has become sensitized by previous damage (Kuslich et al 1991). Robinson (2003) confirmed this by stating that an inflammatory component or already damaged nerve root is necessary before a nerve root will produce pain.
Radicular pain occurs as the result of compression of dorsal root ganglia when activity occurs not only in nociceptive axons but also in A-beta fibres (Niere 1991, Bogduk 2005). Disc herniation is the most common cause of radicular pain. However, as well as compression of the dorsal root ganglion, from anatomical abnormalities, leakage of inflammatory mediators from the nucleus pulposus into the epidural space has also been proposed as a cause of radicular pain (Peng et al 2007). Both mechanisms may result in an inflammatory reaction causing hyperexcitability and spontaneous ectopic activity in the dorsal root ganglion, which is interpreted as pain. This can also add to central sensitization at the dorsal horn synapses (Niere 1991).
As discussed above, experimental compression of dorsal root ganglia or previously damaged nerve root has been shown to cause radicular pain. The clinical experiments of Smyth & Wright show this pain to be produced in a particular form, namely lancinating and shooting in quality, and referred in relatively narrow bands (Smyth Wright 1959, Bogduk 2005). Taking into account the overlapping nature of somatic and radicular pain referral patterns, Robinson (2003) suggests that the patient’s description of the pain itself may be more reliable than the location. Table 1.1 attempts to draw out the key differences between somatic and radicular pain, based on subjective and objective descriptors, to aid clinical reasoning and diagnosis.
| SOMATIC PAIN | RADICULAR PAIN | |
|---|---|---|
| Source of pain? | A deep musculoskeletal structure, e.g. dural nerve root sleeve, muscle, zygapophyseal joint, with a nerve supply from a specific segment. | Pain deriving from damage or irritation of the spinal nerve tissue, particularly the dorsal root ganglion. |
| Pain is referred where? | Segmentally referred dermatomal pain according to the nerve supply of the structure. | Segmental reference, more commonly to distal end of dermatome in limb (little back pain). |
| Pain is described as? | Deep ache, vague, poorly localized. | Lancinating, burning, severe, radiating, deep, strap-like, narrow, well localized, may be latent in nature. |
| Is there any neurology? | None associated. Nociceptive stimulation of somatic structures will not produce any neurological signs. | Yes. Usually associated with radiculopathy giving paraesthesia, numbness, muscle weakness and reduced reflexes in the appropriate segment. |
| Clinical tips | Somatic pain can precede, and often be associated with, radicular pain since the dural nerve sleeve is a somatic structure surrounding the spinal nerve root. | Once chronic, radicular pain becomes more difficult to differentiate from somatic pain due to central sensitization, i.e. increased excitability of pain-related central nervous system neurons and the widening out of the pain field. |
Bogduk (1994) proposed that somatic and radicular pain syndromes can coexist. For example, the annulus fibrosus of the disc may be a source of somatic low back pain but may also cause secondary compression by a posterolateral displacement causing compression of the nerve root leading to radicular pain. However, a lesion cannot selectively compress nociceptive axons; therefore, for compression to be the source of radicular pain, other neurological abnormalities associated with radiculopathy should be present, e.g. paraesthesia, numbness, muscle weakness and loss of reflexes (Bogduk 2005).
Kidd & Richardson (2002) further clarify that somatic referred pain is nociceptive, being initiated by stimulation of receptors on peripheral terminals of sensory fibres, by either damage or inflammation within somatic structures. In contrast, radicular pain is neuropathic and arises as a result of sensory fibres being abnormally stimulated along their course.
Grieve (1981) observed that ‘all root pain is referred pain, but not all referred pain is root pain’. Kellgren (1939), in charting the distribution of pain, demonstrated that injection of the interspinous ligaments of L2–S2 produced pain in the leg. However, as referred to above, Kuslich et al (1991) found that sciatica could only be produced by stretching or direct pressure on an already inflamed nerve root.
In Kuslich’s study, the zygapophyseal joint capsule was found to be tender in some instances but the pain was never referred to the leg. The zygapophyseal joint’s main significance in the production of low back pain was its ability to compress or irritate other local sensitive tissues, particularly with osteophyte formation, including the annulus fibrosus. The annulus fibrosus was demonstrated to be the tissue of origin in most cases of low back pain without pain or symptom referral into the limb. It should be noted that the sacroiliac joint was not included in either study to be able to establish its ability to produce leg pain.
O’Neill et al (2002) demonstrated that noxious stimulation of the intervertebral disc can give rise to low back and referred extremity pain, with the distal extent of pain produced depending on the intensity of stimulation. Nociceptors within the discal tissue may have become more sensitized in patients presenting with non-radicular leg pain allowing provocation of peripheral symptoms at lower thresholds of stimulation. They suggest that Kuslich et al (1991), discussed above, would have observed a similar pattern of pain referral on stimulation of the annulus, and other structures, if they had applied a stimulus of sufficient intensity.
O’Neill et al support Bogduk (2005) in describing referred leg pain from the somatic lumbar annulus fibrosus as being poorly localized, dull and aching, adding that it is usually less troublesome than the patient’s low back pain. They warn that it is important to be able to differentiate between referred pain arising from somatic structures and radicular pain associated with nerve root compression from disc herniation, because the two types of pain have different causal mechanisms and may therefore require different treatment. They add that the traditional description ‘pain radiating below the knee’ as representing radicular pain rather than referred somatic pain is unreliable.
As mentioned above Bogduk (2005) claims that for referred leg pain to be radicular in origin, arising from compression of a nerve root, it must be accompanied by other signs of compression – paraesthesia and muscle weakness. If these are absent, pain referred to the limb must be somatic in origin.
Schäfer et al (2009) have attempted to classify the causal mechanisms of leg pain into four groups that may help to guide treatment approaches, although they acknowledge that there is likely be some overlap. As well as radicular pain with motor loss and musculoskeletal (somatic) causes, they propose that leg pain can also arise from central and peripheral nerve sensitization.
Within this text, specific consideration of visceral referred pain is provided within the appropriate chapters as part of differential diagnosis. However, just as we have attempted to separate out the characteristics of somatic and radicular pain, Vecchiet & Giamberardino (1997) have described general aspects of visceral pain that can be borne in mind, particularly for those conditions requiring more urgent referral, for example myocardial infarction, pancreatitis, etc.
Initially, the pain is usually felt in the same site along the midline of the chest or abdomen, mainly in the lower sternal area and the epigastric region, regardless of the viscera affected. It is generally perceived as a deep, dull, vague sensation that is poorly defined and may not be able to be described. It can vary from a sense of discomfort to one of oppression or heaviness and may be associated with autonomic signs such as pallor, sweating, nausea, vomiting and changes in heart rate, blood pressure and temperature. Strong emotional reactions are also often present and include anxiety, anguish and sometimes even feelings of impending death.
Common features of segmentally referred pain were identified by Cyriax as ‘rules’ of referred pain (Cyriax 1982, Cyriax & Cyriax 1993). As with all pronounced ‘rules’, exceptions may be noted, but nonetheless they act as a general guideline in assessing pain behaviour and locating the causative lesion. He developed rules for referral of pain from unilateral structures as represented in the Box below.
• It does not cross the midline.
• It refers distally.
• It refers segmentally.
• It occupies part or all of its dermatome.
A notable exception to the rules of referred pain is provided by the dura mater which, as a centrally placed structure, produces so called ‘ multisegmental reference’ on compression or irritation.
Multisegmental reference of pain
Throughout his writing Cyriax referred to the phenomenon of ‘extrasegmental’ reference of pain (Cyriax 1982, Cyriax & Cyriax 1993). He used the term to explain the observation that symptoms arising from the dura mater do not obey the rules of referred pain, in terms of referring segmentally, but rather that a pattern of reference is produced which is ‘outside’ or ‘extra’ to that of the segment. With another interpretation, the terminology falls short of being ideal since it implies that the pain is not experienced within the segment in which the causative lesion is housed. This is not so and ‘multisegmental’ would perhaps be a more correct term.
The ventral aspect of the dura mater, the annulus fibrosus and other central spinal structures are innervated by the sinuvertebral nerve which sends branches to segments above and below its level of origin (Bogduk 2005). This could account for the multisegmental reference of pain arising from compression of central structures.
Multisegmentally referred pain is felt diffusely across several segments, usually as a dull background ache, and is often associated with tenderness or trigger spots (Fig. 1.3). It may vary and other unilateral symptoms might be superimposed upon it, as in lesions involving pressure on both the dura and the dural nerve root sleeve.
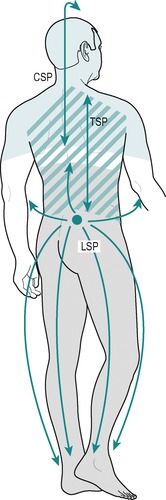 |
| Figure 1.3
Multisegmental pain. CSP = cervical spine pain; TSP = thoracic spine pain; LSP = lumbar spine pain.
|
Mention of the effect of compression of the dura mater leads to the general observation that lesions associated with compression of neural tissue produce varying patterns and nature of symptoms, according to the tissue involved. These will be listed as described in the Box facing, beginning with the dura mater itself.
Psychosocial factors
Whenever pain itself is the central focus for information towards clinical diagnosis, consideration needs to be given to both its sensory and affective aspects. Thus discussion within this section on the characteristics of referred pain has been based on the sensory component of pain, addressing the mechanism and factors of referred pain, particularly relating to its dermatomal distribution.
The concept of ‘central pain’ has been discussed, arising from changes within the central nervous system (Butler 1995, Mendelson 1995). However, notwithstanding the contribution of central pain to the chronic state, attention needs to be given to the affective aspects of pain as an emotional experience rather than as a pure sensation (Butler 1995). The so-called psychosocial factors (involving aspects of both social and psychological behaviour) can have varying effects on the patient’s perception of pain as well as distorting the account given to the clinician.
In addressing this issue in orthopaedic medicine, the term ‘psychogenic pain’ has traditionally been used. The term has survived the controversy on whether or not it exists and, according to the literature review of Mendelson (1995), the work of Engel, Walters and Merskey has led to a general acceptance that pain can be amplified or evoked by psychological factors. However, the term ‘psychosocial’ is currently more commonly used and several studies provide evidence that certain personality traits and experiences of individuals have predisposed them to the persisting cycle of chronic pain (Waddell 1998).
An initial organic basis should not be ignored however. Shaw et al (2007) looked at shared and independent associations of psychological factors among men with low back pain and suggested that early intervention will do much to avoid chronicity with its attendant psychosocial factors and persistent work disability.
Dura mater
• Multisegmental reference of pain and tenderness (Fig. 1.3).
Spinal cord
• No pain, multisegmental reference of paraesthesia, i.e. in both feet, or all extremities if associated with cervical lesions.
• May produce upper motor neuron lesion with spastic muscle weakness.
• May produce a spastic gait.
• May produce an extensor plantar response.
Dural nerve root sleeve
• Pain produced on compression of the tissue, i.e. the pressure or ‘compression phenomenon’ (Cyriax 1982).
• Segmental reference of pain in all or part of the dermatome.
• Difficult to ascribe an aspect to the pain, e.g. anterior or lateral.
• Difficult to define an edge to the pain.
Nerve root
• Neurological signs and symptoms are produced on compression through the dural sleeve:
– segmental reference of paraesthesia felt at the distal end of the dermatome
– lower motor neuron lesion with flaccid muscle weakness
– absent or reduced reflexes
– may become pain-sensitive.
Nerve trunk
• Symptoms produced on release of pressure, i.e. the ‘release phenomenon’ (Cyriax 1982), e.g. after sitting on a hard gate with pressure placed on the sciatic nerve trunk for a period of time, a shower of ‘painful pins and needles’ will be experienced in the leg until the nerve recovers.
• Sensation of deep painful paraesthesia in the cutaneous distribution of the nerve trunk.
• Some aspect; no edge to the symptoms.
• The longer the compression, the greater the length of time before the onset of pins and needles, e.g. the thoracic outlet syndrome produces diffuse pins and needles in all five digits of one or both hands after going to bed at night, several hours after the compression has been released from the brachial plexus.
Peripheral nerve
• Compression produces paraesthesia and numbness.
• Clear edge and aspect to the symptoms, e.g. carpal tunnel syndrome producing paraesthesia and/or numbness in the cutaneous distribution of the lateral three and a half digits on the palmar aspect of the hand, or meralgia paraesthetica involving compression of the lateral cutaneous nerve of the thigh, which is associated with a clearly demarcated area of paraesthesia and/or numbness in the anterolateral aspect of the thigh.
Identification of a significant psychological influence will allow for modification in the treatment approach adopted and certainly may provide a contraindication for some of the techniques used in orthopaedic medicine, most notably the manipulative treatments used for spinal pain.
Cyriax (1982) presented characteristics which, if recognized in patients, give an indication that affective influences are a predominant feature of the complaint and should be taken into account in arriving at a diagnosis, as well as in subsequent treatment selection or appropriate referral.
He suggested that any or all of the following features might be present:
• No recognizable pattern of symptoms or signs in the subjective or objective examination
• Poor cooperation in both sections of the examination
• Overenthusiastic assistance from the patient, often accompanied by an element of ‘triumph’ if the clinical diagnosis remains obscure
• Patients may seek the answer expected of them
• Mutually contradictory signs through an incomplete knowledge of the condition.
Juddering is a response occasionally noted on resisted testing but there is no evidence for any neurological condition which allows muscles to work in spasms of effort to produce such a juddering pattern. This is not the cogwheel resistance to movement encountered in parkinsonism. Severe pain usually produces an ‘all-or-nothing’ effort where resistance is lost completely as a result of pain, e.g. in resisted wrist extension as a test for tennis elbow where the wrist ‘breaks’ due to the sharp pain elicited.
Active movements are cited later in this chapter as a useful means of establishing the patient’s willingness to perform the movement. This can relate to the psychosocial influence as well as allowing consideration of any unwillingness caused by actual pain, or fear of producing it.
Waddell (1992, 1998), after extensive study in the field of problematic back pain, describes behavioural symptoms and signs that provide information and raise awareness of illness behaviour. In contrast, Adams et al (2002) suggest that the balance of back pain research has swung too far towards psychosocial issues to the neglect of the physical. The aim should certainly be to avoid acute back pain from becoming chronic to avoid the emergence of the dominant psychosocial component (Shaw et al 2007). However, to balance the focus on psychosocial issues, Waddell has traditionally emphasized the need for careful assessment, warning that a ‘galaxy of signs does not exclude a remediable condition’. On orthopaedic medicine courses, students are cautioned against the misinterpretation of unusual signs and symptoms by the statement: ‘beware of the bizarre but consistent patient’, whose behaviour could be indicative of underlying serious pathology.
Several effective approaches have been devised in the treatment of chronic pain (Chartered Society of Physiotherapy (2006a) and Chartered Society of Physiotherapy (2006b)). These are often based on the concept of increasing activity and fitness levels and promoting the maxim that ‘hurt does not equate to harm’. Skilled counselling may also be required. This text has set out to acknowledge the possible influence of psychosocial factors but does not intend to expand further on its management. The interested reader is referred to appropriate texts relating to this work.
CLINICAL EXAMINATION
Cyriax’s starting point in the development of orthopaedic medicine was the premise that all pain has a source. It was a simple extension of that logic that, to be effective, all treatment must reach the source and all treatment must benefit the lesion.
Cyriax was intrigued by the number of patients passing through orthopaedic clinics who presented with normal X-ray findings, and acknowledged the soft tissues as the source of the complaints. He devised a mechanism of clinical examination to establish the source of the pain which was logical and methodical, and was deliberately pared to the minimum procedure in order to discover in which tissue the lesion lay. His style of assessment conformed to the claim of Sir Robert Hutchinson in 1897, that ‘every good method of case taking should be both comprehensive and concise’ (Hunter & Bomford 1963).
An important emphasis of Cyriax’s examination procedure is that negative findings are as significant as the positive, eliminating from the enquiry those structures which are not at fault. The systematic approach he devised produces a set of findings that can be interpreted through logical reasoning, integrating the assessment with existing knowledge towards eventual clinical diagnosis. The term ‘diagnosis’ at this stage implies the hypothesis against which we all work, which becomes proven or disproven in light of the patient’s subsequent response.
In recent years, much work has been devoted to developing areas of specialist assessment. However, the orthopaedic medicine examination procedure gives clinicians a sound framework from which to start and any special examination techniques can be superimposed upon it. For example, once a basic spinal assessment has been carried out, extra neural tissue sensitizing tests can be included to search for aspects of neural tension, repeated or combined movements can be included, or localized joint palpation and mobility tests can be applied.
A further aim of the examination is to establish whether or not the lesion is suitable for the treatments offered in orthopaedic medicine or other allied treatment modalities, or whether the patient would more suitably be referred on for appropriate specialist opinion. More detailed tests such as blood tests, X-rays, computed tomography (CT) and magnetic resonance imaging (MRI) scans can be employed as necessary but do not form part of the basic examination procedure.
The orthopaedic medicine assessment procedure follows a set model for the collection of clinical data. It contains the following elements:
• Observation, noting face, gait and posture
• A detailed history
• Inspection for bony deformity, colour changes, muscle wasting and swelling
• Palpation for heat, swelling and synovial thickening
• State at rest
• Examination by selective tension, assessing active, passive and resisted movements
• Palpation for the site of the lesion, once the causative structure has been identified.
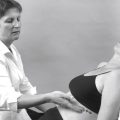
OBSERVATION
Note the patient’s face, posture and gait.
A general observation is made as the patient is met, or even before the meeting without the patient’s knowledge, for example when the patient is walking across the car park or approaching reception. Observe the patient’s face for any signs of sleeplessness and pain. Serious disease accompanied by unrelenting pain is usually indicated in the patient’s overall demeanour. It can be useful to note whether the patient’s appearance matches the history as important clues can be provided, particularly relating to the psychosocial component of the condition.
Certain postures indicate specific conditions. An antalgic posture (one assumed by the patient to avoid pain) is often adopted in neck pain as a wry neck, or as a lateral shift when associated with a lumbar lesion.
In the upper limb, note any apparent guarding, perhaps with an altered arm swing associated with a painful shoulder, elbow or even wrist.
The patient’s gait pattern may be altered, with a limp indicating pain or a leg length discrepancy. An altered stride may be due to limitation of movement at a joint, or protection from weight-bearing. The use of an aid such as a stick or crutches provides an obvious clue to the need for extra support to avoid or to assist with weight-bearing. As part of the overall observation, a check may also be made on whether such aids are being used correctly.
HISTORY
A complete history is taken from the patient to ascertain as much information as possible about the condition. At some joints the history is more relevant than at others with respect to diagnosis. The history of the spinal joints and knee joint, for instance, may give many clues to diagnosis whereas with many of the commonly encountered conditions of the shoulder or hip, such as capsulitis, the history gives almost no clues at all.
The term ‘history’ implies a chronological account of how the condition has progressed but this forms part of a broader compass that includes other aspects, such as aggravating and alleviating factors or past medical history. The history-taking is really the subjective part of the examination procedure and it is traditional in medical and physiotherapy practice to use a model for the collection of clinical data from the patient (Beckman & Frankel 1984).
Most models, including the model used in orthopaedic medicine, involve the categorizing of the subjective examination under different headings to ensure that all information is collected. However, if this pattern is adhered to too rigidly it can be restricting for the patient. The use of closed questions to steer patients’ responses results in interviews being physician-/physiotherapist-led and constant interruptions may restrict the fluency of patients’ accounts such that they are prevented from presenting the true nature of the problem (Beckman & Frankel 1984, Blau 1989).
Studies on the process of history-taking revealed that physicians interrupted and took control of the interview on average 18 s into the consultation, and that by asking specific closed questions they halted the spontaneous flow of information (Beckman & Frankel 1984). However, if allowed to continue uninterrupted and with no specific guidance, patients talked on average for less than 2 min, during which time most of the information required by the physician was disclosed (Blau 1989). This study was repeated by Wilkinson (1989) who found the time taken to be even shorter, with 89 of the 100 patients surveyed speaking for less than 1½ min and 41 of those for less than 30 s.
Bearing the previous points in mind, while consideration of the various categories adopted in orthopaedic medicine is necessary, in clinical practice it is better to begin the patient interview with an open question such as ‘What can I do for you?’ or ‘What brings you to me?’ and then to allow the history to be expressed in the patient’s own words. Anything not mentioned can be searched for after the patient has finished speaking.
Of course, not all patients will obligingly reveal their history and some guidance will be required to keep to the relevant. Nonetheless, a balance should still be sought between allowing the patient time to speak and controlling the interview to prevent irrelevant deviation (Blau 1989).
The model for history-taking in orthopaedic medicine will now be described. The clinician should note the relevant details from the history on the patient’s record card for subsequent interpretation while accepting that the process of clinical reasoning continues throughout the interview and that the ‘rambling thoughts of a clinician’ cannot – and should not – be impeded.
Age, occupation, sports, hobbies and lifestyle
The age of the patient may be relevant as certain conditions predominantly affect certain age groups. For example, children may suffer from Perthes’ disease or slipped epiphysis in the hip, pulled elbow, or loose bodies associated with osteochondritis dissecans in the knee. Adolescents may suffer from Osgood–Schlatter’s disease or maltracking problems in the knee. Mechanical lumbar lesions, ligament or tendon injuries are common in the middle-aged and degenerative osteoarthrosis is usually suffered by the elderly.
Occupation may be relevant in terms of the postures adopted while at work and the activities involved in the patient’s job. In this respect, it is important to explore the job’s requirements rather than merely to note the job title.
An overview of the patient’s general lifestyle may give clues to the cause of the problem and, relating to treatment, can provide an indication of the requirements for rehabilitation back to full activity. For example, tendon or muscle belly lesions frequently occur in sports requiring explosive activity, as in tennis and squash, and the specific requirements of the sport will need to be considered to be able to grade the rehabilitation programme appropriately. Those engaged in a more sedentary occupation or lifestyle might be prone to back pain and will need to be encouraged to increase their general activity as part of management and to prevent recurrence.
Site and spread
As referred to at the beginning of this chapter, patients usually complain of pain but there may be other symptoms that cause them to seek advice, such as stiffness, weakness, numbness and pins and needles, for example. Whatever the symptoms, much information can be gained towards diagnosis by establishing where the symptoms are and their overall spread. For instance, it is important to know not just where the symptoms are now but where they have been. A low back pain often travels into the leg but usually becomes less apparent in the back as it does so, a typical presentation of nerve root irritation of mechanical origin. A low back pain moving into the leg without remission in the back implies a spreading lesion and requires a different interpretation.
Several factors can influence the site and spread of symptoms and these were described in the first part of this chapter. The site and spread of the symptoms can be an indication of the overall irritability of the lesion as well as providing a clue to the structure at fault by considering the ‘rules’ (mentioned earlier) for the referral of symptoms that can arise from different somatic and neurological structures.
Onset and duration
The mode of onset of the lesion can provide indicators which lead to a clinical diagnosis and can also contribute to the treatment selection and overall prognosis of the condition. Was the onset sudden or gradual? Meniscal tears and acute muscle belly lesions come on suddenly whereas overuse tendon and bursa problems and nuclear disc lesions have a gradual onset. Fractures usually have a sudden onset associated with trauma but systemic conditions or serious pathology such as tumours have an insidious onset. None of these latter conditions is suitable for treatment within the specialism of orthopaedic medicine.
In the lumbar spine the mode of onset leads towards a particular hypothesis with regard to pathology, which then provides a guideline for treatment selection. For example, a gradual onset may respond more effectively to treatment with traction, while, in the absence of any contraindications, a sudden onset may require manipulative treatment.
The duration of the symptoms is important as an indicator of the acute or chronic stage in healing which in itself gives guidance for the treatment approach to be adopted, as well as indicating the likely effectiveness and progress of treatment applied. For example, the acutely sprained ligament will require a gentle approach to treatment aiming to reduce swelling to promote healing and to prevent adverse scar tissue formation. In contrast, the chronic ligamentous lesion will require a more aggressive approach to treatment aiming to restore function by breaking down any adverse adhesion formation with stretching and manipulative techniques as appropriate. A lumbar lesion of gradual onset is less likely to respond to treatment if it has been present for 3 months than if it has been present for 1 month.
Symptoms and behaviour
The way the pain behaves will assist diagnosis. Mechanical joint lesions are usually better for rest and worse on activity. Also, changing posture can alter the pain. Ligamentous lesions like movement and are usually worse after a period of resting. What is the daily pattern of the symptoms? Joint stiffness more marked in the morning indicates the presence of inflammation, and questions on how long it takes to ease will provide an indication of irritability or severity. If the lesion was associated with sudden trauma, could the patient continue with the activity to provide information on the severity of the lesion and/or instability?
Serious pathology may present with unrelenting pain; night pain is likely to be a feature. Generally speaking, benign musculoskeletal lesions do not present with constant, unremitting pain and there is usually some position of rest which eases pain, or some periods when the pain is easier or even absent. In distinguishing between serious pathology and an irritable musculoskeletal lesion it can be helpful to distinguish whether the pain prevents sleep or disturbs sleep, with more serious pathology possibly being implicated in the former. With musculoskeletal lesions, however, it may be the case that specific postures, such as sitting up for neck pain, have to be adopted to allow sleep.
There will be special questions at each joint which are relevant to the patient’s symptoms. For example, how do stairs affect knee pain? How does a cough or sneeze affect back pain? Does the patient with neck pain experience any dizziness? Pertinent questions asked at each joint region will aid diagnosis and, importantly, will also rule out any contraindications to treatment.
The clinician will also wish to investigate if there have been any previous episodes of similar complaints. Spinal pain may be a progression of the same condition and a lumbar disc lesion may characteristically present as increasing episodes of worsening pain.
Past medical history
Asking about the past and present existence of serious illness, unexplained weight loss, operations or accidents gives insight into the patient’s medical history. Past trauma or serious illness may be relevant to current diagnosis, e.g. old fracture of the tibia leading to degenerative changes in the ankle joint, or mastectomy for carcinoma followed by the formation of bony secondaries in the spine. As well as past medical history, establish general health and any ongoing conditions and treatment. Explore other previous or current musculoskeletal problems with previous episodes of the current complaint, any treatment given and the outcome of treatment.
Other joint involvement
This particularly gives clues to the patient’s pain being associated with systemic joint disease. It will alert the examiner to the presence of inflammatory arthritis, such as rheumatoid arthritis and ankylosing spondylitis, and also to degenerative osteoarthrosis, all of which have a distinctive pattern of onset and joints affected. Inflammatory joint disease, most notably rheumatoid arthritis, provides a contraindication to manipulative treatment, especially in the cervical spine.
Medications
These give a clue to past or underlying disease, e.g. the use of tamoxifen indicates a history of breast cancer, long-term steroids may indicate inflammatory joint disease. Anticoagulants are in themselves a contraindication for manipulation since manipulation may cause disruption of capillaries and the prolonged bleeding time induced by anticoagulant therapy may lead to haematoma formation. Steroids may not be so much a contraindication in themselves but consideration needs to be given to the underlying pathology which necessitates their prescription. Antidepressants can give an indication of the emotional state of patients which may provide a contraindication to some treatments. However, it should also be noted that low-dose antidepressants may be used as an adjunct to analgesics in the management of chronic pain.
The quantity and regularity of the dose of analgesic and non-steroidal anti-inflammatory drugs provide an indication of the level of pain being experienced by the patient and can be used as an objective marker to monitor the progress of treatment in terms of noting whether higher or lower doses are needed to control the pain.
INSPECTION
Having completed the history, the patient is inspected, suitably undressed and in a good light, paying particular attention to any bony deformity, colour changes, muscle wasting or swelling.
Bony deformity
This may include spinal asymmetry, leg length discrepancy, excessive valgus or varus deformity at joints, bony lumps or exostoses. Obvious distortion of bony or joint contours following trauma could indicate fracture or dislocation.
Colour changes
Redness may indicate the presence of acute inflammation as one of the four cardinal signs of inflammation: redness, heat, swelling and pain. It may be indicative of infection. There may be signs of bruising following trauma, that may be distal to the site of the lesion, or pallor, mottling or reddening associated with circulatory or sympathetic involvement. The presence of scars and rashes may also be indicative of relevant pathology.
Muscle wasting
Any obvious muscle wasting is noted. This may have its origin in a neurological condition, usually from nerve root compression at the relevant spinal level, as a result of reflex muscle inhibition associated with joint effusion (de Andrade et al 1965), or as a consequence of disuse. The cause of the wasting will usually become apparent as the examination proceeds.
Swelling
Swelling is indicative of the presence of inflammation resulting from trauma or overuse, as in tenosynovitis and bursitis or arthritis. Swelling which presents immediately indicates bleeding in the area. Other swellings may include haematomas, ganglia, lipomas and soft tissue nodules.
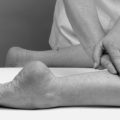
PALPATION
At this stage of the objective examination, peripheral joints are palpated for signs of inflammatory activity in the form of heat, swelling and synovial thickening. The area is not palpated for tenderness – this must wait until the end of the examination when the structure at fault has been identified through the rest of the examination procedure. It may also be necessary to palpate for peripheral pulses at this stage if circulatory disturbance is suspected.
Heat
The joint is palpated for heat using the back of the hand and comparing the same aspect on each side. The same hand is used throughout since the dominant hand may be a few degrees warmer than the non-dominant. Any bandaging or support that the patient has been wearing which could have warmed the area should be taken into account.
Swelling
Swelling is often apparent on inspection alone but may be palpated for, particularly to detect minor swelling, in the knee, for example.
Synovial thickening
Synovial thickening has a ‘boggy’ feel to it and indicates the presence and level of inflammation in a joint. It is particularly evident in rheumatoid arthritis at the wrist, ankle and knee.
STATE AT REST
The state at rest must be established before any examination requiring joint movement or muscle activity takes place, in order to provide a baseline against which to note the effect of any such movements. There may or may not be any symptoms at rest. Comparison can then be made with subsequent movements which may make the symptoms better or worse.
An open question such as ‘How do you feel as you are standing/sitting there?’ usually elicits the status quo and avoids the leading use of the word ‘pain’.
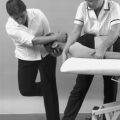
Based on the observation that normal tissue functions painlessly whereas abnormal tissue does not, Cyriax devised a method of applying appropriate stress to the structures surrounding each joint in order to test their function. Passive movements were employed to test the so-called inert structures such as joint capsules and ligaments, whereas resisted tests were used to test the contractile structures incorporating muscle, tendon and attachments to bone. This then was the method of applying selective tension to tissues (Cyriax 1982, Cyriax & Cyriax 1993) and the movements he propounded will now be described.
EXAMINATION BY SELECTIVE TENSION
Throughout this discussion of examination by selective tension and within the objective examination in the following regional chapters, comparison should always be made with the other side, where appropriate.
Active movements
Active movements give an idea of the range of movement available in the joint, the pain experienced by the patient and the power in the muscle groups. They are not carried out routinely at all joints, since they are non-selective and employ both inert and contractile tissues.
However, they are useful in establishing the willingness of the patient to perform the movements as an indicator of the level of pain being experienced within each range. In this role they can act as a guide to the range of movement available before applying passive stresses to the joint and can be used at any joint to gain that information if necessary.
Active movements can also be used to eliminate neighbouring joints as a potential source of pain. For example, as part of the shoulder examination, six active neck movements are performed. If the patient’s pain is not elicited then the cervical spine is eliminated from the enquiry at that initial stage.
They are also most important in illustrating how willing the patient is to move, with respect to psychosocial factors and particularly noting any unusual or bizarre responses. The appropriateness of the patient’s response can be significant in the subsequent selection of treatment techniques. This is helpful when assessing the so-called ‘emotional’ joints, i.e. the joints which are prone to an exaggeration of symptoms and which include all spinal areas, the shoulders and, to a lesser extent, the hips.
A particular sign known as the painful arc is demonstrated by active movement. By definition, a painful arc is an arc of pain with pain-free movement on either side. It implies that a structure is being pinched or compressed at that point of the movement and is a useful finding towards diagnosis. For example, a painful arc may be found on active abduction at the shoulder if a lesion lies in the subacromial bursa, or the supraspinatus, infraspinatus or subscapularis teno-osseous attachments, where it may be pinched as it passes under the coracoacromial arch before passing beyond and beneath it, out of harm’s way. The painful arc is not usually found in isolation and positive findings on other tests will further incriminate the causative structure.
Passive movements
Passive movements test the inert structures which include the joint capsule, joint menisci, ligaments, fascia, bursae, dura mater and the dural nerve root sleeve. Relaxed muscle and tendon can act as inert structures when they are stretched by their opposite passive movement. Passive movements principally give information on pain, range and end-feel.
The observation of pain and range of movement will be familiar to all clinicians working with soft tissue lesions. However, the notion of end-feel may be a new concept which is particularly valuable in the assessment of the soft tissues and is inherent in the orthopaedic medicine approach.
End-feel is the specific sensation imparted through the examiner’s hands at the extreme of passive movement (Cyriax 1982, Cyriax & Cyriax 1993).
Normal end-feel
Normal end-feel is divided into three categories: hard, soft and elastic. Normal bone-to-bone approximation, as in extension of the elbow, gives a characteristic hard end-feel to passive movement. A soft end-feel is characteristic of a stop to the movement brought about by approximation of tissue, as in passive knee or elbow flexion. An elastic end-feel is felt when the tissues are placed on a passive stretch and is the elastic resistance produced in the inert tissues at the end of range in normal joints. Examples are provided in stretching the end of range of lateral rotation at the shoulder or hip.
There may be a range of end-feels within the ‘elastic’ group, but all are indicative of normal tissue tension, such as the ‘leathery’ end-feel of passive pronation and supination of the forearm, or the even tighter ‘rubbery’ end-feel of ankle plantarflexion and wrist flexion where the resistance to the movement is in part provided by the tendons spanning the joint.
Pain is also a component at the extreme end of range of normal movements, acting as a protection in preventing continuation of movement to the point of producing tissue damage.
Abnormal end-feel
The sensation imparted in the abnormal end-feel falls into three categories: ‘hard’, springy, and empty.
The cause of the abnormal ‘hard’ end-feel is different from that of the normal hard bone-to-bone end-feel and it is a particular feature of the movements limited in arthritis.
In early arthritis, joint movement is initially limited by pain, and involuntary muscle spasm halts the movement (Cyriax 1982). This involuntary muscle spasm provides a brake to the movement and feels ‘hard’ to the examiner. Certainly the end-feel feels harder than expected, but in the early stages some elasticity is preserved. Early arthritis of the hip, for example, may produce a ‘hard’ end-feel on either passive flexion or medial rotation but it should be emphasized that this is not caused by bony degenerative change blocking the joint movement. This difference is important as it aids the clinician in the selection of treatment techniques.
In more advanced arthritis, the ‘hard’ end-feel is also due to capsular contracture (Cyriax 1982). The capsular resistance together with the involuntary muscle spasm may still allow some ‘give’ at the end of range, but it will not feel as elastic as the earlier stage. In late arthritis, bony changes may occur, leading to a genuine hard end-feel and the crepitus associated with advanced arthritis, as well as that arising from involuntary muscle spasm and capsular contracture.
An abnormal springy end-feel is associated with mechanical joint displacement, usually a loose body. The sensation imparted to the examiner is one of not quite getting to the end of range, with the joint springing or bouncing back. It is similar to the sensation of trying to close a door with a small piece of rubber or grit caught in the hinge which causes the door to spring back.
The abnormal end-feel associated with involuntary muscle spasm is different from that produced by voluntary muscle spasm. Voluntary muscle spasm comes in abruptly to halt the movement and indicates acute pain or serious pathology. If the pain is very severe it may also be associated with an empty end-feel.
An empty end-feel occurs where the examiner does not have the opportunity to appreciate the true end-feel because the patient calls a halt to the movement prematurely and urgently because of pain, often raising a hand to prevent further movement. The sensation imparted to the examiner is empty, as described, in that the complaint of serious pain comes on before any spasm or tissue resistance.
The empty end-feel is associated with serious pathology such as fracture, neoplasm or septic arthritis. A further cause may be a highly irritable lesion such as acute subacromial bursitis where the empty end-feel is instantly apparent on passive abduction of the shoulder.
The capsular pattern
The capsular pattern is a limitation of movement in a defined pattern which is specific to each joint and indicates the presence of an arthritis. The pattern varies from joint to joint and is characterized by limitation of movement in a fixed proportion. It is the same whatever the cause of the arthritis (Cyriax 1982, Cyriax & Cyriax 1993) and the history will suggest the form of arthritis, be it degenerative, inflammatory or traumatic. Table 1.2 provides a list of the capsular patterns for all the joints and spinal regions.
| The movements which become limited in the capsular pattern take on a characteristically ‘hard’ end-feel. | |
| JOINT | CAPSULAR PATTERN |
|---|---|
| Shoulder joint | Most limitation of lateral rotation Less limitation of abduction Least limitation of medial rotation |
| Elbow joint | More limitation of flexion than extension |
| Radioulnar joints | Pain at end of range of both rotations |
| Wrist joint | Equal limitation of flexion and extension Eventual fixation in the mid-position |
| Trapezio-first metacarpal joint | Most limitation of extension |
| Metacarpophalangeal joints | Limitation of radial deviation and extension Joints fix in flexion and drift into ulnar deviation |
| Interphalangeal joints | Slightly more limitation of flexion than extension |
| Cervical spine | Demonstrated by the cervical spine as a whole: Equal limitation of side flexions Equal limitation of rotations Some limitation of extension Usually full flexion |
| Thoracic spine | Demonstrated by the thoracic spine as a whole: Equal limitation of rotations Equal limitation of side flexions Some limitation of extension Usually full flexion |
| Hip joint | Most limitation of medial rotation Less limitation of flexion and abduction Least limitation of extension |
| Knee joint | More limitation of flexion than extension |
| Ankle joint | More limitation of plantarflexion than dorsiflexion |
| Subtalar joint | Increasing limitation of supination Eventual fixation in pronation |
| Mid-tarsal joint | Limitation of adduction and supination Forefoot fixes in abduction and pronation |
| First metatarsophalangeal joint | Marked limitation of extension Some limitation of flexion |
| Other metatarsophalangeal joints | May vary: Tend to fix in extension |
| Interphalangeal joints | Fix in flexion |
| Lumbar spine | Demonstrated by the lumbar spine as a whole: Limitation of extension Equal limitation of side flexions Usually full flexion |
Characteristically, the movements restricted in the capsular pattern take on the ‘hard’ end-feel of arthritis, different from the expected normal elastic capsular resistance.
The reason for the development of the capsular pattern could be the presence of joint effusion, causing the joint to assume the position of ease and/or protective muscle spasm (Eyring & Murray 1964). An effusion is often present in an arthritis and the acuteness of the condition determines the quantity of the effusion. Joints with symptomatic effusions are held in the position of ease mentioned above and movements out of this position produce pain.
Eyring & Murray (1964) noted a possible relationship between intra-articular pressure and pain. Experiments were conducted to determine the position of minimum pressure in various joints and it was observed that symptomatic joints with effusion spontaneously assume a position of minimum pressure, which coincides with that of minimum pain.
The involuntary muscle spasm, in protecting the painful, inflamed joint, prevents the use of the painful range of movement and if the range of movement is underused it will become limited. In arthritis, the individual joints resent some movements more than others, hence the capsule contracts disproportionately, making some movements more limited than others and giving rise to the characteristic pattern of limitation. For example, in the study conducted by Eyring & Murray the position of minimum pressure for the elbow was found to be between 30° and 70° of flexion. The pressure was not influenced by either pronation or supination. The capsular pattern of the elbow joint is proportionally more limitation of flexion than extension but without involvement of pronation or supination.
The capsular patterns described in Table 1.2 provide a useful guide to diagnosis of arthritis but research support is mixed. Pellecchia et al (1996) established that the Cyriax evaluation scheme was highly reliable in assessing patients with shoulder pain. Fritz et al (1998) found evidence to support the concept of the capsular pattern in the inflamed or osteoarthritic knee whereas, although they established some support for the capsular pattern in the knee, Bijl et al (1998) were unable to recommend it as a valid test. No support for the capsular pattern at the hip was provided by the studies of Bijl et al (1998) and Klãssbo et al (2003) and it was questioned by Sims (1999).
Non-capsular pattern
The non-capsular pattern of a joint is, quite simply, anything other than the capsular pattern. That is, it is a limitation of movement that does not conform to the capsular pattern and occurs as a result of a component or part of the joint being affected rather than the whole joint.
A mechanical lesion such as an intra-articular displacement produces the non-capsular pattern which may be evident at the spinal joints and in a loose body at the elbow joint or a meniscal tear at the knee. A mechanical lesion in a degenerative joint may produce a non-capsular pattern superimposed on the capsular pattern.
A ligamentous lesion will give pain and possible limitation of the movement which stretches the structure. However, sprains of ligaments which form an integral part of the capsule itself, such as the anterior talofibular ligament of the ankle and the medial collateral ligament of the knee, cause a secondary capsulitis and hence a non-capsular pattern is superimposed on the capsular pattern.
Since a contractile unit can be stretched by passive movement, the unit may respond as an inert structure when relaxed, producing pain when it is stretched by the passive movement in opposition to its functional movement. For example, the subscapularis muscle and tendon which produce medial rotation at the shoulder will be stretched by passive lateral rotation. This is also particularly evident in acute tenosynovitis when the involved tendon is pulled through its inflamed synovial sheath. In acute tenosynovitis at the wrist, for example, pain will be produced on the appropriate resisted test and the opposite passive movement, e.g. de Quervain’s tenosynovitis.
Resisted tests
Resisted isometric muscle tests assess the contractile unit – comprising the muscle, musculotendinous junction, tendon and the teno-osseous attachment to bone – for pain and power. The tendon is not strictly a contractile structure but is tested as part of the functional contractile unit. When assessing the resisted tests it is important to look for reproduction of the patient’s presenting pain and there are specific points to bear in mind which ensure that, as far as possible, only the contractile unit is being tested rather than the inert structures. It is accepted that, although joint movement may not be seen to occur, isometric muscle contraction will cause compression and joint shearing and, in the spinal joints, a rise in intradiscal pressure (Lamb 1994).
The joint should be placed in the mid-position with the inert structures relaxed so that no stress falls upon them. The muscle group is tested isometrically, as strongly as possible, so encouraging maximal voluntary contraction but without allowing movement to occur at the joint. This also ensures that minor lesions will be detected. Patient and examiner’s body positioning should be such that all muscles not being tested are eliminated from the testing procedure.
Resisted tests may produce any of several findings, each of which has a different implication. The test may be:
• Strong and painless – normal
• Strong and painful – contractile lesion
• Weak and painless – neurological weakness; complete rupture
• Weak and painful – partial rupture or serious pathology such as fracture or bone tumour
• Painful on repetition – claudication or provocation of overuse injury or less irritable lesion
• All resisted tests about the joint painful or juddering – serious pathology, or marked psychosocial component.
In addition to the active, passive and resisted tests, a brief neurological examination is included as part of the routine examination for a spinal joint, testing for root signs. (See Chs. 8, 9, 13 and 14).
Having completed the subjective and objective examination sequence the causative structure, if not its pathology, will, in almost all cases, have been identified (Cyriax 1982, Cyriax & Cyriax 1993).
PALPATION
At this stage palpation of the structure determined to be at fault may be made to identify the site of the lesion to which appropriate treatment can be applied.
If the diagnosis is still unclear further tests may be added either mechanically, for example using techniques of neural tension tests, combined, repeated or accessory movements, or with the use of blood tests, X-rays, electromyography (EMG) or scanning techniques, as mentioned above.
REFERENCES
Adams, M.; Bogduk, N.; Burton, K.; et al., The Biomechanics of Back Pain. ( 2002)Churchill Livingstone, Edinburgh.
Aprill, C.; Dwyer, A.; Bogduk, N., Cervical apophyseal joint pain patterns II: a clinical evaluation, Spine 15 (1989) 458–461.
Beckman, H.B.; Frankel, R.M., The effect of physician behaviour on the collection of data, Ann. Intern. Med. 101 (1984) 692–696.
Bijl, D.; van Baar, M.E.; Oostendorp, R.A.B.; et al., Validity of Cyriax’s concept capsular pattern for the diagnosis of osteoarthritis of hip and/or knee, Scand. J. Rheumatol. Suppl. 27 (1998) 347–351.
Blau, J.N., Time to let the patient speak, Br. Med. J. 298 (1989) 39.
Bogduk, N., Innervation, pain patterns and mechanisms of pain production, In: (Editor: Twomey, L.T.) Clinics in Physical Therapy, Physical Therapy of the Low Backsecond ed. ( 1994)Churchill Livingstone, Edinburgh, pp. 93–109.
Bogduk, N., Clinical Anatomy of the Lumbar Spine and Sacrum. fourth ed. ( 2005)Churchill Livingstone, Edinburgh.
Butler, D., Moving in on pain, In: (Editor: Shacklock, M.) Moving in on Pain ( 1995)Butterworth Heinemann, Oxford, pp. 8–12.
Campbell, J.N.; Meyer, R.A., Mechanisms of neuropathic pain, Neuron 52 (1) ( 2006) 77–92.
Chartered Society of Physiotherapy, 2006a. Clinical Guidelines for the Physiotherapy Management of Persistent Low Back Pain (LBP): part 1 Exercise. CSP, London.
Chartered Society of Physiotherapy, 2006b. Clinical Guidelines for the Physiotherapy Management of Persistent Low Back Pain (LBP): part 2 Manual Therapy. CSP, London.
Conesa, S.H.; Argote, M.L., A Visual Aid to the Examination of Nerve Roots. ( 1976)Baillière Tindall, London.
Cooper, G.; Bailey, B.; Bogduk, N., Cervical zygapophyseal joint pain maps, Am. Acad. Pain Med. 8 (2007) 344–352.
Cyriax, J., 8th edn.Textbook of Orthopaedic Medicine. vol. 1 ( 1982)Baillière Tindall, London.
Cyriax, J.; Cyriax, P., Cyriax’s Illustrated Manual of Orthopaedic Medicine. ( 1993)Butterworth Heinemann, Oxford.
de Andrade, J.R.; Grant, C.; Dixon, A.; St, J., Joint distension and reflex muscle inhibition in the knee, J. Bone Joint Surg. 47A (1965) 313–322.
Eyring, E.J.; Murray, W.R., The effect of joint position on the pressure of intraarticular effusion, J. Bone Joint Surg. 46A (1964) 1235–1241.
Farasyn, A., Referred muscle pain is primarily peripheral in origin: the barrier-dam theory, Med. Hypoth. 68 (2007) 144–150.
Foerster, O., The dermatomes in man, Brain 56 (1933) 1–39.
Fritz, J.M.; Delitto, A.; Erhard, R.E.; et al., An examination of the selective tissue tension scheme, with evidence for the concept of the capsular pattern at the knee, Phys. Ther. 10 (1998) 1046–1056.
Galea, M., Neuroanatomy of the nociceptive system, In: (Editors: Strong, J.; Unruh, A.M.; Wright, A.) Pain: A Textbook for Therapists ( 2002)Churchill Livingstone, Edinburgh.
Gnatz, S.M., Referred pain syndromes of the head and neck, Phys. Med. Rehab. 5 (3) ( 1991) 585–596.
Grieve, G., Common Vertebral Joint Problems. ( 1981)Churchill Livingstone, Edinburgh.
Head, H., The pathology of herpes zoster and its bearing on sensory localization, Brain 16 (1900) 353–523.
Hunter, D.; Bomford, R.R., Hutchison’s Clinical Methods. 14th edn. ( 1963)Baillière Tindall & Cassell, London.
Inman, V.T.; Saunders, J.B., Referred pain from skeletal structures, J. Nerv. Mental Dis. 99 (1944) 660–667.
Kellgren, J.H., Observations on referred pain from muscle, Clin. Sci. 3 (1938) 175–190.
Kellgren, J.H., On the distribution of pain arising from deep somatic structures with charts of segmental pain areas, Clin. Sci. 4 (1939) 35–46.
Kidd, B.L.; Richardson, P.M., How does neuropathophysiology affect the signs and symptoms of spinal disease?Best Pract. Res. Clin. Rheumatol. 16 (1) ( 2002) 31–42.
Klãssbo, M.; Harms-Ringdahl, K.; Larsson, G., Examination of passive ROM and capsular patterns of the hip, Phys. Res. Intern. 8 (1) ( 2003) 1–12.
Kuslich, S.D.; Ulstrom, C.L.; Michael, C.J., The tissue origin of low back pain and sciatica, Orthop. Clin. North Am. 22 (1991) 181–187.
Lamb, D.W., A review of manual therapy for spinal pain, In: (Editors: Boyling, J.D.; Palastanga, N.) Grieve’s Modern Manual Therapy2nd edn. ( 1994)Churchill Livingstone, Edinburgh.
Lee, M.W.L.; McPhee, R.W.; Stringer, M.D., An evidence-based approach to human dermatomes, Clin. Anat. 21 (2008) 363–373.
Lundeburg, T.; Ekholm, J., Pain – from periphery to brain, Dis. Rehab. 24 (8) ( 2002) 402–406.
McMahon, S.B.; Dmitrieva, N.; Koltzenburg, M., Visceral pain, Br. J. Anaesth. 75 (1995) 132–144.
Mendelson, G., Psychological and psychiatric aspects of pain, In: (Editor: Shacklock, M.) Moving in on Pain, ( 1995)Butterworth Heinemann, Oxford, pp. 66–89.
Niere, K., Understanding referred pain, Sports Train. Med. Rehab. 2 (1991) 247–256.
O’Neill, C.W.; Kurganky, M.E.; Derby, R.; et al., Disc stimulation and patterns of referred pain, Spine 27 (24) ( 2002) 2776–2781.
Pappano, D.A.; Bass, S., Referred shoulder pain preceding abdominal pain in a teenage girl with gastric perforation, Pediatr. Emerg. Care 22 (2006) 807–809.
Pellecchia, G.L.; Paolino, J.; Connell, J., Intertester reliability of the Cyriax evaluation in assessing patients with shoulder pain, J. Orthop. Sports Phys. Ther. 23 (1996) 34–38.
Peng, B.; Wenwen, W.; Zhenzhou, L.; et al., Chemical radiculitis, Pain 127 (2007) 11–16.
Robinson, J., Lower extremity pain of lumbar spine origin: differentiating somatic referred and radicular pain, J. Man. Manip. Ther. 11 (4) ( 2003) 223–234.
Sameda, H.; Takahashi, K.; Takahashi, T.; et al., Dorsal root ganglion neurons with dichotomising afferent fibres to both the lumbar disc and groin skin: a possible neuronal mechanism underlying referred groin pain in lower lumbar disc diseases, J. Bone Joint Surg. 85B (4) ( 2003) 600–603.
Schäfer, A.; Hall, T.; Briffa, K., Classification of low-back related leg pain – a proposed patho-mechanism-based approach, Man. Ther. 14 (2009) 222–230.
Sloan, J., Soft tissue injuries: introduction and basic principles, Emerg. Med. J. 25 (1) ( 2008) 33–37.
Shaw, W.S.; Means-Christensen, A.; Slater, M.A.; Patterson, T.L.; et al., Shared and independent associations of psychosocial factors on work status among men with subacute low back pain, Clin. J. Pain 23 (5) ( 2007) 409–416.
Sims, K., Assessment and treatment of hip osteoarthritis, Man. Ther. 4 (3) ( 1999) 136–144.
Smyth, M.J.; Wright, V., Sciatica and the intervertebral disc: an experimental study, J. Bone Joint Surg. 40A (1959) 1401–1418.
Standring, S., Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 40th edn ( 2009)Churchill Livingstone, Edinburgh.
Travell, J.G.; Simons, D.G., Trigger Point Flipcharts. ( 1996)Lippincott, Williams & Wilkins, Philadelphia.
Vecchiet, L.; Giamberardino, M.A., Referred pain: clinical significance, pathophysiology, and treatment, Phys. Med. Rehabil. Clin. N. Am. 8 (1) ( 1997) 119–136.
Waddell, G., Understanding the patient with back pain, In: (Editor: Jayson, M.) The Lumbar Spine and Back Pain4th edn ( 1992)Churchill Livingstone, Edinburgh, pp. 469–485.
Waddell, G., The Back Pain Revolution. ( 1998)Churchill Livingstone, Edinburgh.
Wilkinson, C., Time to let the patient speak [letter], Br. Med. J. 298 (1989) 389.
Witting, N.; Svensson, P.; Gottrup, H.; et al., Intramuscular and intradermal injection of capsaicin: a comparison of local and referred pain, Pain 84 (2000) 407–412.