CHAPTER 12 Clinical Methods for Sclerotherapy of Telangiectasias
Historical Review of Techniques
Sclerosing treatment for telangiectasias was ignored until the 1930s when Biegeleisen1 injected sclerosing agents intradermally or subcutaneously into the general area of capillary enlargement. However, this procedure caused severe necrosis and lack of effect on the telangiectasias. Biegeleisen then developed and popularized a method of ‘microinjection’ of telangiectasias with sclerosing agents through the use of an ‘extremely fine metal needle’ (later described as a handmade 32- or 33-gauge needle).2 Unfortunately, he used sodium morrhuate in the treatment of these fragile small vessels, which produced multiple complications, including pigmentation, cutaneous necrosis, and allergic reactions. Thereafter, sclerotherapy treatment of leg telangiectasias was thought of disparagingly by most practitioners3 until the 1970s when Alderman,4 Foley,5 Tretbar,6 and Shields and Jansen7 published reports of procedures that had achieved excellent results with few adverse sequelae. In these procedures, solutions less caustic to the telangiectasia were used – hypertonic saline (HS) with or without heparin and lidocaine (15% to 30%), and sodium tetradecyl sulfate (STS) 1% – as were techniques that ensured accurate placement of the solution into the blood vessels (use of 30-gauge needles).
Indication
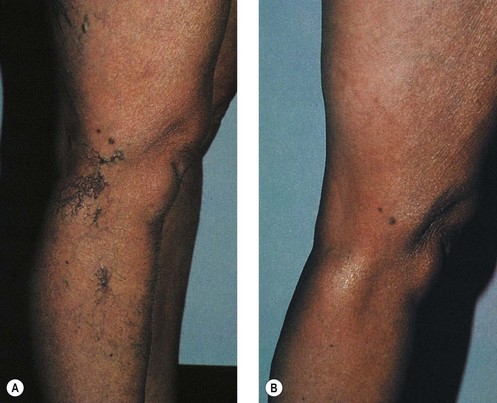
Microsclerotherapy is theoretically indicated for any small telangiectatic vessel or venule on the cutaneous surface. Best results are obtained on superficial linear or radiating vessels on the lower extremities. Telangiectasias on the face are less reliably responsive to microsclerotherapy because they probably have more of an arteriolar component and result from active vasodilation, but they can be treated successfully (see Chapter 4).8,9 In addition, bright-red telangiectasias on the leg that have a rapid refilling time after diascopy (applying pressure with a glass slide) with the patient recumbent (Fig. 12.1) are probably also supplied through arteriolar flow (see Chapter 3).10 These vessels are relatively recalcitrant to usual therapy and tend to recur after treatment. More importantly, these arteriolar leg veins are more likely to develop overlying cutaneous necrosis if sclerosing solutions reach the arteriolar feeding loop (see Chapter 8). They may be more effectively treated with the pulsed dye laser or intense pulsed light sources (see Chapter 13).
The description in Chapter 9 of the injection of varicose veins by first closing off the high-pressure reflux points with sclerosing solution, followed by sclerotherapy of remaining abnormal vessels, forms the basis for the rationale of compression sclerotherapy of varicose veins. The treatment of ‘spider’ leg veins should be just as rational.
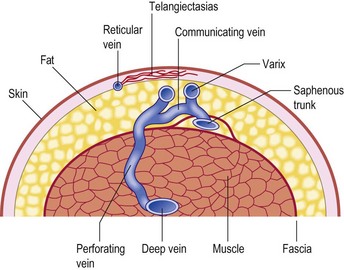
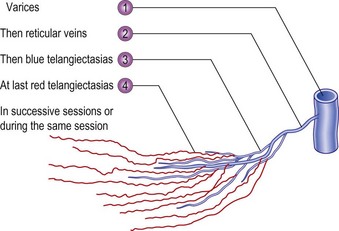
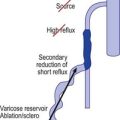
In the vast majority of cases, spider veins connect to underlying varicose veins either directly or through tributaries (Fig. 12.2) (see Chapter 3).11–13 This is typical on the lateral aspect of the thigh when the lateral network described by Albanese is visible (Fig. 12.3). Doppler examination shows that almost all visible blue reticular veins are connected to telangiectasia.14,15 Transillumination shows exactly the same pattern (Fig. 12.4). Therefore, as with varicose veins, treatment should be directed first at ‘plugging’ the leaking high-pressure outflow at its point of origin (Fig. 12.5). An appropriate analogy is to think of spider veins as the ‘fingers’ and the feeding varicose or reticular vein as the ‘arm’. Treatment should first be directed to the feeding arm and then, only if necessary, to the spider fingers. Mariani et al16 found that when telangiectasias were treated in this manner, 3-year follow-up of 109 patients showed enduring resolution of telangiectasias in 95%.
There are a number of advantages to this systematic approach to sclerotherapy. When sclerotherapy is performed in this manner, the spider veins often disappear without direct treatment, or decrease markedly in size, thus limiting the number of injections into the patient. The larger feeding vein is both easier to cannulate and less likely to rupture when injected with the sclerosing solution, thus minimizing the extent of extravasated red blood cells (RBCs) and solution. Theoretically, this method also should minimize the postsclerotherapy development of hyperpigmentation, cutaneous necrosis, telangiectatic matting (TM), and recurrence (see Chapter 8).
Injection Technique
Preinjection procedure
After a physical examination, including the use of noninvasive diagnostic techniques when appropriate (see Chapter 5), the patient is scheduled for a sclerotherapy session and given a questionnaire (Appendix C), consent form (Appendix D), and instructional material (Appendix G) to read and complete at home. Questions about the procedure are answered, and all reasonable and appropriate complications and adverse sequelae are addressed. An estimate of the approximate number of treatment sessions and the cost of treatment is given in writing to prevent any future misunderstandings. Insurance reimbursement policies are discussed and documented. Documentation of the relief of symptoms with compression stockings is helpful in gaining preauthorization of treatment from insurance companies, but in most cases insurance companies decide on the medical necessity for treatment based on the size (diameter) and type of vessel, not symptoms. If graduated compression stockings are planned to be applied after treatment, they are fitted at this time and given to the patient to wear before treatment. If the stockings produce a resolution of symptoms, the physician can assume that successful sclerotherapy will give the same result. In addition, wearing the stockings before treatment helps answer any questions about their fit to ensure they will be worn for the prescribed length of time after treatment.
With the patient standing on an elevated platform or stool, a complete set of photographs of the legs is taken from four different views, and the individual areas that will be treated are photographed up close (Fig. 12.6). Reticular veins and telangiectasias are photographed with the patient recumbent. Photographic documentation is important because patients frequently cannot remember exactly how their legs appeared before treatment. Any pretreatment pigmentation irregularities and scars may be blamed later on the sclerotherapy treatment, since patients usually look more closely at their legs once treatment has begun. In addition, when patients return in a few years with additional veins and telangiectasia, viewing pretreatment photographs will allay concerns regarding the possibility of unsuccessful previous treatment.
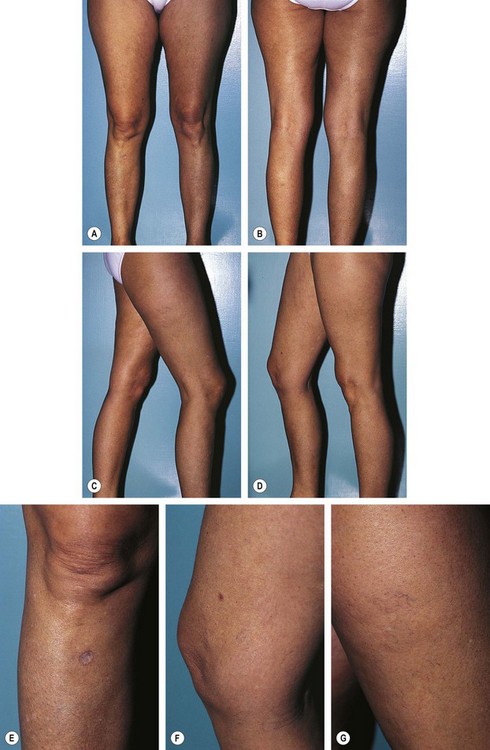
Figure 12.6 Standard photographs taken before treatment begins to allow accurate determinations of treatment outcome. Four standard views are taken at an F-stop of F8; close-up views are taken at an F-stop of F11, with macro-close-ups taken at an F-stop of F16. All photographs are taken with Kodachrome ASA 64 film as described in Chapter 15. A, Frontal view. B, Rear view. C, Right side (the right foot is always in front of the left foot). The right knee is slightly bent so the left inner thigh is better visualized. D, Left side (again with the right foot in front of the left). E, Documentation of scar from previous treatment for a verruca on the right anterior tibial area, which could later be thought of as a treatment scar. F, Documentation of dermatofibroma on the right medial knee area, which later could be thought of as punctate pigmentation from treatment. G, Close-up view of telangiectasia and venules (0.2–0.6 mm in diameter) on the right lateral thigh, along with pretreatment nonspecific light-brown pigmentation, which could later be thought of as postsclerotherapy hemosiderin pigmentation.
| Gauge | External diameter (mm) |
|---|---|
| 30 | 0.30 |
| 27 | 0.41 |
| 26 | 0.46 |
| 25 | 0.51 |
| 22 | 0.71 |
| 20 | 0.89 |
| 18 | 1.27 |
At the end of the treatment session, the treated areas are recorded on a diagrammatic chart to help check progress at follow-up examinations (Appendix E). Patients are given written postoperative instructions about activity and the disposition of their graduated compression stockings and/or bandages.
Preparation and visualization of the vessels
Microsclerotherapy of spider veins is performed with the patient in the supine position. Gravitational dilation of telangiectasias is unnecessary to minimize intravascular thrombosis. The skin is wiped with alcohol, making the telangiectasias more visible because of a change in the index of refraction of the skin. The glistening effect of alcohol renders the skin more transparent and helps clean the injection site. In addition, alcohol may cause some vasodilation of the telangiectasias. Alternatively, Sadick17 recommended that the skin be wiped with a solution of isopropyl alcohol 70% with acetic acid 0.5%. He found that this solution improves the angle of refraction better than alcohol alone.
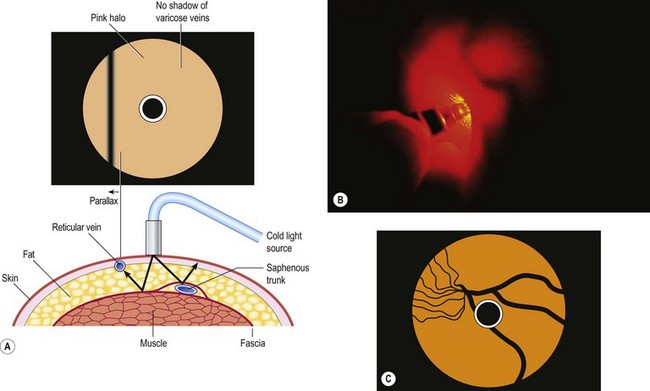
Scarborough and Bisaccia18 recommended rubbing a few drops of the sclerosing solution on the skin overlying the venules with a gloved finger. They used polidocanol (POL), which also contains alcohol in water as the diluent. We agree that visualization is enhanced with this technique once the initial effects of the isopropyl alcohol have worn off through evaporation. To further enhance visualization of the vessels, we recommend the use of magnifiers from 2.25× to 5× (see Chapter 15).
Equipment
Needle and syringe
Although visualization of the vessel is important in ensuring proper needle placement, the examiner actually enters the vessel ‘by feel’. This is particularly true in the injection of reticular varices. In this situation it is best to pierce the skin rapidly and advance the needle superficially over the vessel at a slight angle in a ‘double-piercing’ technique. Penetration of the vessel is ‘felt’, even when the examiner uses a 30-gauge needle. Some authors state that the ‘feel’ is enhanced with the use of a 26- or 27-gauge needle but we find this unnecessary.19 In this regard, the use of a glass syringe would best reflect an impedance to flow if a vessel were not properly cannulated. However, glass syringes are more cumbersome to use and regulations regarding sterile technique and other hazards have relegated glass to undesirable. With the availability of high-quality plastic syringes, a good feel can be obtained and the risk of transmitting blood-borne diseases obviated (see Chapter 15).
Ideally, the goal of microsclerotherapy is to cannulate the vessel, injecting sclerosing solution within and not outside of the vessel wall. Usually, a 30-gauge needle suffices for most vessels, although some physicians recommend the use of a 32- to 33-gauge needle to decrease the likelihood of inadvertent perivenular injection in the treatment of the smallest diameter vessels.20–23 The disadvantages of using a 32-gauge needle are that it dulls rather quickly and easily bends away from the targeted vein (see Chapter 15). Boxes of needles sometimes contain individually defectively sharpened and dull needles, which give a ‘scratchy’ sensation to the tip. The physician should never hesitate to change needles if a vein cannot be cannulated easily. It is usually not the ‘tough skin’ of the patient but a dull needle that makes injection difficult.
Injecting with a syringe of smaller diameter will increase the pressure of the liquid at the tip of the needle; this may cause more extravasation, more transparietal burn, and may increase the risk for ‘reverse flow’ injection and subsequent necrosis. It has been measured and calculated that for the same force applied to the piston, the pressure can almost double with a small syringe (Fig. 12.7).
One theoretical disadvantage to multiple injections with the same needle is that the needle will become dull.21 However, this was found not to occur on microscopic examination of the needle tips after eight injections into the skin (see Chapter 15).
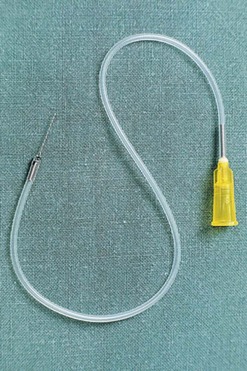
A small vein infusion set designed for sclerotherapy is available in various gauge and tubing lengths with 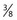 -inch long needles (Kawasumi Laboratories, STS Pharmaceuticals) (Fig. 12.8). These sets may provide enhanced control for cannulating small veins. In addition, the kink-resistant tubing allows for flow to ensure that the needle is in a vein and not an artery.
-inch long needles (Kawasumi Laboratories, STS Pharmaceuticals) (Fig. 12.8). These sets may provide enhanced control for cannulating small veins. In addition, the kink-resistant tubing allows for flow to ensure that the needle is in a vein and not an artery.
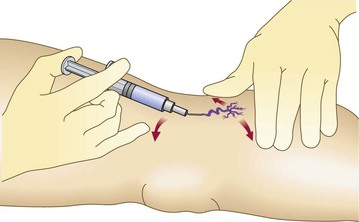
Skin tension
The skin must be taut to facilitate cannulation of the vessel. This can be accomplished with the help of an assistant who stretches the patient’s skin in at least two directions. Alternatively, with proper hand placement, the physician alone can produce three-point tension. Figure 12.9 illustrates the recommended technique for injection. The nondominant hand is used to stretch the skin adjacent to the treated vessel in two directions. Then the fifth finger of the dominant hand exerts countertraction in a third direction. With a little practice, even the most lax skin, such as that on the thighs, can be brought under tension with this technique. Skin laxity varies with patient age, adiposity, and location on the leg.
Depth of injection
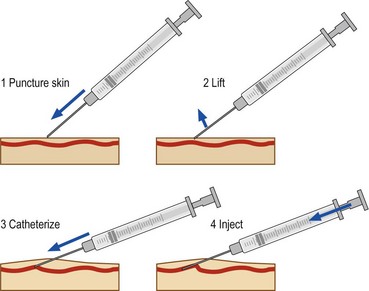
The location of most leg telangiectasias is in the upper dermis (see Chapter 1). The most common error in technique is to place the needle tip deep to the vessel. To enter the vessel at a less acute angle almost parallel to the skin surface, the physician should bend the needle to 145 degrees with the bevel up (Fig. 12.10).24 If the needle is not within the vessel, the solution will either leak out onto the skin or produce an immediate superficial wheal. At times, gentle upward traction can be applied as the needle is advanced to ensure superficial placement.
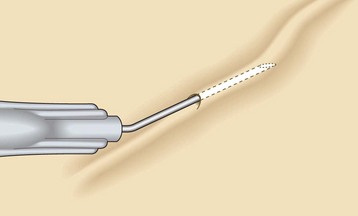
Figure 12.10 Needle is bent to 145 degrees with the bevel up to facilitate accurate insertion into the superficial telangiectasia.
(Reprinted from Goldman MP, Bennett RG: J Am Acad Dermatol 17:167, 1987, with permission from American Academy of Dermatology.)
Injection with the bevel of the needle up has the advantage of minimizing the chance of transecting the vessel. Inserting the needle bevel down may be easier, probably as the result of the vacuum produced by the bevel on the skin surface. An alternate technique is to puncture the skin very superficially, to hook it, to lift it a little in order to bring the vessel in the same axis as the needle and then to cannulate it (Fig. 12.11).
Air-bolus (block) or foam technique
The air-bolus technique – injecting a small amount of air to clear the vessel before instilling the sclerosing solution – is recommended by multiple physicians.4,5,19 It is thought to minimize the risk of inadvertent intradermal injection. This, however, may not occur, since once the vessel is cleared of blood, it is more difficult to see the progress of the sclerosing solution within its lumen. Therefore, others, including ourselves, have abandoned this technique.21,23 Another theoretical advantage of the air-bolus technique is the decreased risk of intravascular thrombosis. It is thought that if the vessel is cleared of blood by first injecting air, the risk of extravasation of RBCs may also be minimized. However, antegrade and retrograde filling of the treated vessel occurs after the injection. Thus, the air-bolus technique may not prevent or lessen the incidence of post-treatment pigmentation. It is necessary to stress the risk of visual disturbances when using the air-block technique.25,26
Foam injection
Another variant of the air-bolus technique that helps visualize clearing of the vessels is that of creating a foamy solution before injection. This can be achieved with the use of any ‘detergent’ class of sclerosing solution such as STS or POL (see Chapter 7). Green and Morgan27 added Haemacel to STS to accentuate bubble formation. It is also thought that the foam causes the sclerosing agent to interact more efficiently with the endothelium (see Chapters 7 and 9). We have found that foaming a detergent solution increases its potency at least twofold while decreasing its caustic toxicity fourfold when a solution-to-air ratio of 1 : 4 is used. Therefore, the use of foam in treating telangiectasia less than 1 mm in diameter is tricky. Until a method is devised to standardize the size and stability of foam, the physician cannot accurately predict the foam’s sclerosing strength. We reserve the use of foam for treating reticular and varicose veins.
To create foam, a small amount of a detergent solution can be drawn into a glass syringe. While the open end of the syringe is almost totally closed, the plunger is pulled back, allowing the introduction of air through the sides of the plunger and syringe. This technique produces foam of fair quality that degrades 50% over 1 to 2 minutes.28 Silicone is present in syringes in order to lubricate the barrel, making for easier compression on the plunger. We have found that the foam half-life varies over 50% for different syringes.29 Some phlebologists recommend using glass syringes to avoid this variability in foam half-life. The glass syringe method necessitates appropriate sterilization of the syringe between patients.
Other methods for producing long-lasting reproducible bubble diameter foam are under development and evaluation. Sclerosing foams can be made by adding air to the liquid solution or with a tensioactive agent and CO2, according to Cabrera et al.30,31
The main difference between sclerotherapy with a solution and with foam is the longer duration of foam within the vein and the concentration of the damaging nonpolar end of the detergent molecule on the endothelial surface. This promotes sclerosis of the treated vessel with a lower concentration of sclerosing solution. Several methods for preparation of simple sclerotherapy foam have been proposed by Monfreux,32 Benigni et al,33 Mingo-Garcia,34 Tessari,35 and Frullini.36 Tessari’s method makes foam with a three-way tap and two syringes.
Hennet37 used 3-mL glass syringes into which 0.3 to 0.4 mL of POL is made up as a 0.5% solution and then diluted with 0.1 to 0.2 mL of physiologic serum. The foam is stated to last for approximately 1 minute with this technique. The syringe is withdrawn until 0.6 to 1.3 mL of foam fills the syringe. The foam, as it is injected, prevents the secondary back-bleeding and is therefore in contact with the vessel wall. Hennet reported treating 10,262 patients with over 70,000 injections between November 1995 and September 1998. Less than 0.5 mL of foam was given in each injection, and compression was not used. He reported excellent results without significant adverse sequelae.
With Frullini’s method, the foam is simply produced in a disposable vial with the air contained in the vial or, preferably, with prior withdrawal of most of the sclerosing solution. A small connector is inserted into the vial, and simply pulling and pushing the liquid produces the foam (Figs 12.12 and 12.13). The key point is to generate a turbulence that produces the foam. Foam can be produced even without the connector by directly fitting the cone of the syringe into the rubber of the cap of the vial. However, the connector makes the maneuver easier and permits reuse of the system on the same patient in order to reproduce the foam for a second injection.

Figure 12.12 Syringe inserted into a vial containing approximately 0.2 mL of sodium tetradecyl sulfate 3%.
(From Frullini A: Dermatol Surg 26:705, 2000.)

Figure 12.13 Foam produced by rapid movement of the plunger in and out of the vial is stable for 5–10 min.
(From Frullini A: Dermatol Surg 26:705, 2000.)
Benigni and Sadoun38 compared the efficacy and adverse-effect profile of 0.25% POL foam with 0.25% POL liquid in treating telangiectasia. They reported a 20% improvement in efficacy with foam versus liquid, without an increase in adverse effects. This is contrary to Weiss,39 who found an increased incidence of pigmentation and TM when 0.1 or 0.2% STS foam was used as compared with liquid STS. Forty percent of patients treated with STS 0.1 or 0.2% foam into telangiectasia of less than 1 mm had 76% to 100% improvement and 82% had greater than 50% improvement. The incidence of pigmentation and TM increased to 20% each if a second treatment was required. Kern40 has shown that more pigmentation occurs with foam, and we have demonstrated41 that more side effects were observed with foam. Finally, and following the conclusion of the European consensus,42 we do not recommend the use of foam as primary treatment of telangiectatic veins.
Quantity of sclerosing solution per injection site
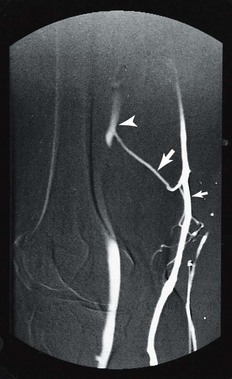
The maximum quantity of sclerosing solution that can be injected safely into a single site during treatment of varicose veins is detailed in Chapter 9. In short, the physician should not inject a volume that would travel undiluted easily into the deep venous system. This is especially important because contrast material has been noted to flow from telangiectasias directly into the deep venous system in 2 of 13 patients (13.3%) with digital subtraction phlebography (Fig. 12.14).43 It appears reasonable to inject solution only until the physician cannot see further progression of blood displacement. When this point occurs, the solution is most likely traveling deeper and possibly into perforating or deep veins.
The maximum amount of sclerosing solution that can be injected into leg telangiectasias is unclear. Although Duffy21 placed little emphasis on limiting the amount of solution injected at a single site, and Lary44 recommended the injection of up to 3 mL at a single site when injecting a reticular 3-mm diameter vein, multiple complications can and do occur from this technique. Excessive inflammation and inadvertent flow of the solution into a feeding varicose vein or arteriole can produce ulceration with injection and damage to the deep venous system. Thrombosis and emboli can occur when amounts greater than 1 mL are injected in a single site. For example, injection of 0.5 mL of solution into a 2-mm diameter vessel fills a length of 16 cm (see Chapter 8).
Ouvry and Davy45 recommend that the amount injected should be sufficient to produce blanching of vessels 1 to 2 cm around the point of injection. No more than 0.5 mL should be used to avoid the risk of initiating the formation of new telangiectasias around the edge of the treated area because of excessive inflammation. In this regard, the same area should not be retreated more often than every 4 to 6 weeks. In our practice it appears that TM occurs more frequently if reinjections are given to a previously treated area that is still undergoing resolution. This unresolved state can be appreciated clinically by slight inflammation and evidence of microthrombosis of vessels.
In addition to limiting the inflammatory reaction of sclerotherapy treatment, limiting the amount of solution may prevent pain and cramping when hypertonic solutions are used. Sadick17 has found that volumes greater than 0.3 mL of HS 23.4% caused the most pain and cramping. One way to minimize the pain from injection of HS is to dilute the HS with lidocaine to the appropriate concentration. One study noted a decrease in pain of 30% with use of this dilution technique.46
Some areas, especially the ankles, should not be injected with more than 1 mL of any sclerosing solution. In this area, the skin is thinnest, the distance between the deep and superficial venous system is the least, and swelling after treatment is common (see Chapter 8). From a purely legal point of view, French regulations (dictionnaire Vidal, Vidal Ed. Paris 2009) recommend not to exceed 10 mL of STS 3%, 2 ampules of 2 mL of POL (any dosage), and 10 mL of CG.
Concentration and strength of sclerosing solutions
Sclerosing solution concentration and strength are discussed in detail in Chapter 7, but a few points should be mentioned here. The most important concept in sclerotherapy is that of achieving optimal destruction of the blood vessel wall with the minimum concentration of sclerosing solution necessary; too much will lead to excessive complications and adverse sequelae, and too little will lead to ineffective sclerosis or recurrence from recanalization (see Chapter 8). The physician should always estimate conservatively when choosing the concentration and type of sclerosing solution.
Three randomized, double-blind, paired comparative human studies evaluated the results of different sclerosing solutions and concentrations in the treatment of leg telangiectasia. Carlin and Ratz47 tested POL 0.25%, STS 0.5%, and HS 20% with heparin, 100 U/mL (Heparsal), in the treatment of leg telangiectasia. They found that whereas HS and STS gave quicker clearing of telangiectasia with fewer injections, the overall level of improvement was identical for all agents. Polidocanol was the best-tolerated sclerosing solution, with the smallest number of adverse sequelae. In a follow-up study comparing POL in four concentrations – 0.25%, 0.5%, 0.75%, and 1.0% – Norris et al48 found that all concentrations were equally effective in treating leg telangiectasia. The POL 0.5% was ideal, with the least number of adverse effects and the most rapid clearing in their patients with leg telangiectasias 0.2 to 1.0 mm in diameter. Sadick49 compared HS in three concentrations – 23.4%, 11.7%, and 5.8% – and found that HS 11.7% was the minimal concentration of saline that produced the most effective vein sclerosis of vessels 1.0 mm in diameter while producing the least discomfort and morbidity.
A comparison of STS 0.25% and POL 0.5% in treating 61 patients with telangiectasias less than 1 mm in diameter demonstrated a similar degree of vessel disappearance of about 90% with one treatment.50 This study demonstrated that sclerosing solutions of equal potency act in a similar manner in treating telangiectasia.
However, some sclerosing agents have a better adverse sequelae pattern. Although the incidence of TM was similar, patients treated with STS had an incidence of pigmentation of 63% versus 32% with POL. Thibault51 found that STS is a stronger sclerosing agent than has been surmised and used a 0.12% concentration for treating telangiectasias greater than 1 mm in diameter, with a decreased incidence of pigmentation and TM.
Kern et al52 compared the efficacy of CG 100%, POL 0.25% solution, and POL 0.25% foam in the treatment of leg telangiectasias. A total of 150 patients were randomized to receive treatment. Foam was created by the Monfreux technique in a 1 : 4 solution-to-air ratio using glass syringes. The CG cleared vessels better than POL solution or foam and, although it was more painful (35/100 vs 20/100), CG did not cause any episodes of pigmentation or TM. More TM and pigmentation was produced with POL foam than with POL solution.
In a study by Leach and Goldman,53 glycerin 72% mixed 2 : 1 with lidocaine 1% with epinephrine was compared with STS 0.25% in 13 patients with leg telangiectasia of 0.2 to 0.4 mm in diameter. Patients were evaluated from 2 to 6 months postsclerotherapy for overall clinical improvement and incidence of adverse sequelae. Glycerin was comparable to STS in discomfort of injection but demonstrated a significant decrease in bruising, swelling, and postprocedural hyperpigmentation. Glycerin also demonstrated better, more rapid clearance of treated telangiectasias (Table 12.1).
Table 12.1 Summary of adverse events and efficacy by treatment group
| Adverse Event/Efficacy | Glycerin (n = 13) | STS (n =13) |
|---|---|---|
| Pain | (3) 23% | (2) 15% |
| Bruising | (1) 8% | (7) 54% |
| Swelling | (0) 0% | (3) 23% |
| Hyperpigmentation | (1) 8% | (12) 92% |
| Vessel clearance | (7) 54% | (1) 8% |
Reproduced from Leach B, Goldman MP: Dermatol Surg 29:612, 2003.
Munavalli et al54 confirmed the lack of adverse effects and efficacy of glycerin compared with STS 0.1% foam. Patients were treated with either glycerin 72% or STS 0.1% foam and analyzed at 6 weeks. Discomfort was the same with each agent. Pigmentation occurred in 18 of 20 patients treated with STS 0.1% foam and in only 1 of 20 patients treated with glycerin. Telangiectatic matting occurred in 4 of 20 STS patients versus 1 of 20 glycerin patients. Improvement was 90% for STS and 60% for glycerin. We recommend that leg telangiectasias less than 1 mm diameter be treated with POL 0.25%–0.5%, STS 0.1%–0.25%, CG, or HS 11.7%. Best efficacy and least adverse effects appear to occur when using glycerin 72% mixed 2 : 1 with lidocaine 1% with epinephrine.
Pressure of injection
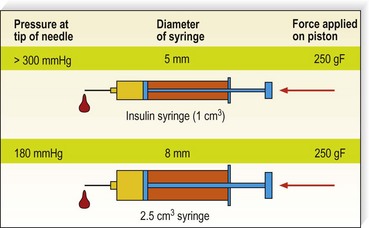
Another variable of technique is the pressure and rapidity of injection. If leg telangiectasias are injected under excessive force, they may rupture and result in extravasation of solution (see Chapter 8). Therefore, injections should be made with minimum pressure. This may be difficult on injection of sclerosing solutions of high viscosity, such as CG, since more pressure is required to push them through a 30-gauge needle. Duffy21 likens the proper injection pressure to that needed to fix a postage stamp to an envelope. In addition, the slower the injection, the longer the solution will be in contact with the vessel wall. Finally, injection pressure (with equal force applied to the piston) is inversely proportional to the square of the piston radius: P = F/S = F/πr2; P1/P2 = (r2/r1)2.* If the approximate piston radius of a 2-mL syringe is 8 mm and that for a 1-mL syringe is 5 mm, the applied force is 180 mmHg for a 2-mL syringe and more than 300 mm Hg for a 1-mL syringe.
In treatment of varicose veins, patient position and movement determine the length of time the solution will be in contact with the endothelium (see Chapter 9). With the injection of telangiectasias, however, the blood flow is not determined by muscle movement or body position but by many other factors, including environmental temperature, nervous stress, and the telangiectasias’ association with arterioles and underlying veins. Therefore, injections should be made slowly enough that it takes approximately 5 to 10 seconds to fill the vessel. Many times the vessel will remain filled with sclerosing solution if the plunger of the syringe is held with almost zero force while the needle remains motionless.
Post-Treatment Techniques
Immediately after injection of the sclerosing solution, the perivascular tissues may swell, producing a clinically visible occlusion of the vessel, or the vessels may go into spasm. This usually occurs if the injection is given slowly and if the sclerosing solution is of adequate strength. Indeed, several authors recommend that a given vessel be treated until such an effect occurs.19 This may require a second injection of a more concentrated solution (125%) into the same vessel during the same sclerotherapy session.
After hypertonic solutions are injected, the injected area should be massaged to minimize the stinging and help alleviate the associated muscle cramping by rapidly diluting the hyperosmotic solution outside of the treated vessel.21–2355 The slower the injection, the less the cramping. When the needle is withdrawn, a small amount of sclerosing solution may be deposited under the skin, causing a burning sensation. This usually occurs only with hypertonic solutions and CG. Massaging for 30 to 60 seconds alleviates the pain and prevents necrosis from the HS solution.22
All sclerosing solutions, even unadulterated HS (see Chapter 8), produce some degree of erythema or urtication or both with injection. Pruritus may be associated with this effect, especially when associated with urticarial lesions. This probably occurs as a result of histamine release caused by perivascular release of mast-cell mediators because of perivascular irritation or as a result of intravascular degranulation of basophils and other white blood cells destroyed by the direct toxic effects of the sclerosing solution. This reaction and its associated pruritus can be minimized by the application of a potent topical corticosteroid cream, thereby providing relief to the patient, especially if injected areas will be occluded with compression pads or stockings.
For telangiectasias that do not respond to standard compression sclerotherapy, added vasoconstriction induced by cold temperature may be helpful. Orbach56 found that vessels respond better to the injection of refrigerated sclerosing solution. Marteau and Marteau,57 using similar logic, advised applying cold compression pads after injection. Although these two techniques seem logical, we have not found them helpful as detergent sclerosants are theoretically more ‘active’ at higher temperatures.
Post-treatment bruising is common and bothersome to patients, even if its disappearance occurs over a few weeks without residual discoloration. It may be useful to recommend application of topical creams like Hirucrème (contains Hirudo medicinalis extract) or Hirudex, which has demonstrated efficacy on mild bruising.58 In case of local discomfort, anti-inflammatory nonsteroidal creams can be applied as well.
Post-treatment compression
For many phlebologists, compression of the sclerosed vessel with a 30- to 40-mmHg graduated compression stocking should be maintained for a minimum of 24 to 72 hours after treatment of leg telangiectasias.59 Postsclerosis compression serves a number of purposes. First, the pressure helps seal the irritated vascular lumen. Second, the pressure helps decrease the likelihood of recanalization of the sclerosed vessel, especially if compression is maintained for 1 to 2 weeks. Third, the possibility of clinical and symptomatic thrombosis is minimized, thus minimizing hyperpigmentation, TM, and recanalization after sclerosis. Although some physicians do not advocate postsclerosis compression,7,17,22,25,57,60 and consider that its only use is to protect against side effects of an inappropriate technique,61 the procedure is simple, safe, and its benefits likely, so its routine use may be recommended. In addition, most patients actually like the feel of the compression stocking while they are ambulatory. (A complete discussion on the use of compression in the treatment of varicose and telangiectatic leg veins is presented in Chapter 6.)
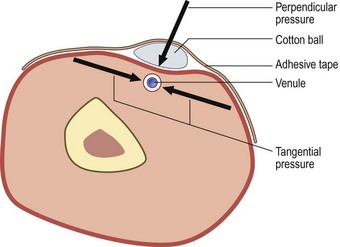
A second aspect of compression is represented by the local dressing applied on the injected vein. It usually consists of a cotton ball or pad and adhesive tape. It exerts double compression: perpendicular (by application of Laplace’s law) and tangential (by direct traction on the skin on both sides) (Fig. 12.15). This type of compression may be stronger than compression exerted by stockings. How long to keep it in place is unknown: advice ranges from 1 hour to more than 24 hours. This type of compression has at least one obvious advantage: it stops bleeding. Although bleeding is not usually a problem, especially if feeding reticular veins are treated first, some patients who have a decreased coagulability may benefit from local compression.
Microthrombectomy
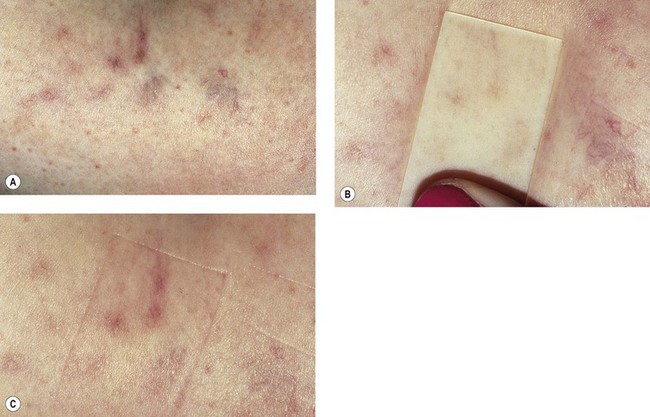
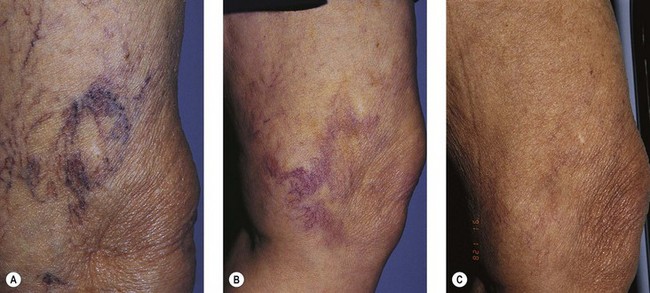
Spider veins (especially those bigger than 0.6 mm in diameter) and reticular veins are likely to form microthrombi after being injected. This phenomenon is less frequent with glycerin than with detergent solutions, especially foam. As discussed in Chapter 8, to prevent pigmentation, microthrombectomy is carried out as early as 1 to 2 weeks after injection. Microthrombectomy is easily executed with any needle, the important point being to puncture every millimeter of the ‘blue line’ of the microthrombi (Fig. 12.16A–C). In very small venules, manual pressure is unnecessary; a cotton ball is applied and kept in place for several hours with an adhesive tape.
Repeat Treatment Sessions
All patients are informed that successful treatment of a given telangiectasia may require more than one treatment (in general, and also on each treatment site). Patients easily understand that, like for wall painting, 2 or 3 thin layers give a better result than a thick one. As previously mentioned, the same vessel or the immediate area is not retreated for 4 to 6 weeks to allow resolution of the endosclerosis or controlled phlebitis to occur. Waiting also allows appreciation of the effectiveness of treatment with a given solution and concentration. If little change is apparent 6 weeks after injection, the second treatment can be performed with a stronger sclerosing agent or more concentrated solution. Different areas can be treated as often as every day, but the venous system of the leg is a complex interwoven network, so treatment of only one part may not prevent reflux pressure from another part promoting continued blood flow through the treated area (see Chapter 1). This leads to an increased incidence of complications from blood flow through a damaged endothelial system (see Chapter 8). Thus, in the treatment of superficial reticular and telangiectatic leg veins, the only real limiting factor is patient and physician motivation for treatment and adherence to using the maximum daily recommended amounts of sclerosing solution that can be injected (see Chapter 7). In addition, if compression is used, it may be best to wait until the pressure stocking has been removed for a few days before treatment is continued on the same leg.
Poor Results of Microsclerotherapy: How to Analyze the Reasons
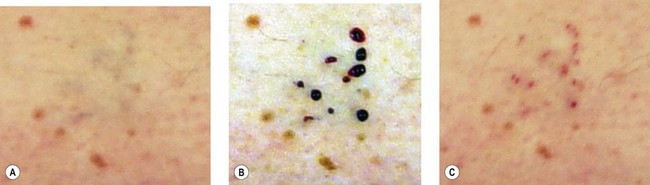
Objective poor results present in many forms (Fig. 12.17); they differ from subjective poor results, where patients are not satisfied despite a satisfactory outcome. Most poor results are related to an inappropriate treatment. Two types of error are possible:
Sclerotherapy Treatment of Facial Telangiectasia
Sclerotherapy treatment of facial telangiectasia has proved to be effective and safe (see Case study 12).8 However, there is a potential for sight-threatening complications from periocular vascular manipulation. This topic was reviewed and is abstracted here.62
Inadvertent intra-arterial injection of corticosteroid suspensions in the periocular region has been reported to lead to embolic occlusion in the ophthalmic artery distribution, causing blindness.63 Inadvertent intra-arterial injection is the likely factor permitting steroid particulate emboli to reach the retinal circulation. Severe visual loss has also been reported following intralesional steroid injection into a chalazion, again apparently causing retinal and choroidal embolic occlusion, presumably as a consequence of inadvertent intra-arterial injection.64 Since it is not a suspension, distal embolic phenomena would not be expected from an injection of STS. However, as an intravascular sclerosant agent it clearly presents a danger if it gains inadvertent access to normal vessels supplying the eye in therapeutic concentrations through the production of an embolus composed of denatured endothelial cells and blood cell elements (see Chapter 8). Presently accepted standards of injection technique, including careful placement of the needle, repeated aspiration, and careful stabilization of the syringe, do not guarantee that the physician can detect if the bevel is against or within the vessel wall if the vessel is constricted, or if there has been any intra-arterial placement of the needle.65
Although Green66 reports that despite the numerous and variable anastomoses between the superficial facial and deep orbital venous systems, injection into superficial eyelid veins is ‘highly unlikely’ to reach the orbit, his technique uses comparatively large volumes (1–3 mL) of STS. Since venous pressure is quite low, it is not inconceivable that intravenous eyelid injection could reach the orbit (where there are no venous valves) and hence the ocular adnexae, the central retinal vein, the choroidal vortex veins, or even the cavernous sinus through these anastomoses. Monocular blindness has been reported following STS injection into a venous malformation partially located in the orbit.67 Green’s technique uses compression only after delivery of the sclerosing solution. At that point, compression could conceivably force the solution into the orbital vessels through the variable anastomotic channels. Since the purpose of his compression is to delay the return of blood into the treated vein, the sclerosing solution forced upstream or downstream would not be significantly diluted necessarily and could be potentially dangerous.
Current treatments for cosmetically objectionable lower eyelid veins include direct cautery application through small cutaneous incisions and direct cautery plus surgical vein transection.68 These procedures pose no known risks to the remainder of the vascular system but could be complicated by cutaneous scars. Surgical removal of the vein through a 2-mm incision with a phlebectomy hook has also been shown to be effective.69 The vein can be tied off with an absorbable 6-0 suture with minimal bruising. However, this technique requires practice to avoid damage to perivascular tissue.
Our technique of sclerotherapy of lower eyelid veins has been reported previously as part of larger series.8 We recommend using very small quantities of Sclerodex (dextrose, sodium chloride, phenethyl alcohol) for treating facial telangiectasias and prominent periorbital veins. The technique is the use of 1 mL or less of sclerosant solution for 24 hours after proximal vein ligation with a 6-0 Prolene suture. The suture prevents backflow of the solution into the retro-orbital venous system. This allows vigorous massage of the area and complements immediate compression. Use of a hypertonic solution provides for effective sclerosis of the vein within the concentration gradient of the solution. A detergent sclerosing solution like STS may travel for many centimeters from the site of injection into areas that should not be sclerosed (see Chapters 7 and 8). This technique demonstrates a 90% to 100% improvement in 70% of patients after one treatment.70 No adverse effects have been reported.
Laser technologies, including the long-pulsed, dynamically cooled 1064-nm Nd:YAG laser (CoolTouch Varia, New Star Lasers, Rosemont, Calif.) has been reported to treat periorbital vessels 1 to 2 mm in diameter with a near 100% efficacy.71 Lasers of lower wavelength would not be expected to deliver sufficient energy at the correct depth to thermocoagulate a vessel of this size without producing excessive cutaneous thermal damage.72
Sclerotherapy Treatment of Essential Telangiectasia
Vessels of essential telangiectasia are extremely small, usually measuring less than 0.1 mm in diameter, and are often associated with a feeding arteriolar component (see Chapter 4). Lasers and intense pulsed light (IPL) are the easiest techniques to cause their involution (see Chapter 13). However, sclerotherapy may also be used to decrease the extent of the lesion. Sclerotherapy should be performed with a dilute solution. Lim and Kossard73 have successfully treated a 56-year-old patient with STS 0.08% with good efficacy and no adverse effects.
Conclusion
It is clear that sclerotherapy is the ‘gold standard’ for treating leg telangiectasia. In certain cases, lasers or IPL therapy is also effective and recommended (see Chapter 13). The lay press is filled with products that patients can apply to the legs to eliminate leg telangiectasia. A scientific evaluation of one of these products showed conclusively that a vitamin K-containing cream touted to eliminate vessels has no effect compared with placebo when used daily over 33 days.74 Although we as physicians think of medicine as a science, it is also an art. Therefore, the sclerotherapy technique just mentioned should not be perceived as dogma. Rather, it should serve as a logical outline for the physician in planning individualized treatment.
Case Study 1 Traumatic telangiectatic patch
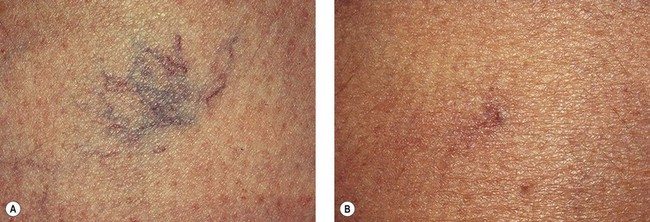
A 46-year-old woman was accidentally hit by a tennis ball while she was ‘playing the net’. A telangiectatic patch on the posterior medial thigh developed after resolution of the bruise 4 to 6 weeks after the initial injury and did not change in size or color over the following 4 years (Fig. 12.18A). The patch was treated on one occasion only, with injections of POL 0.5% in three locations to blanch the lesion completely. A total of 1 mL was used. A localized pressure dressing with an STD foam pad was placed and secured with adhesive tape for 3 days. Figure 12.18B, shows the same area 10 months after initial treatment.
Case Study 2 Unassociated telangiectasia
Figure 12.19A, shows the appearance of linear telangiectasia on the medial thigh of a 46-year-old woman, which was noted during her second pregnancy 22 years previously. The area was asymptomatic, and treatment was requested for cosmetic improvement. The appearance 6 months after a single treatment using approximately 3 mL of POL 0.5% is shown in Figure 12.19B. A 30- to 40-mmHg graduated compression stocking was worn for 3 days after the injection. Note some mild hyperpigmentation in one of the treated vessels.
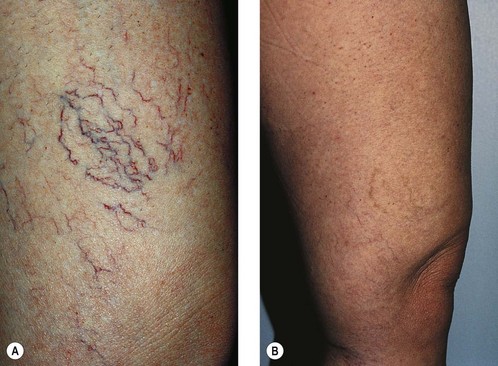
Case Study 3 Reticular vein unassociated with the saphenous system
A reticular vein 2 to 3 mm in diameter on the popliteal fossa in a 32-year-old woman is shown in Figure 12.20A. The same area 18 months after one treatment with 1 mL of POL 0.75% is shown in Figure 12.20B. The area was compressed for 72 hours after treatment with an STD foam pad under a 30- to 40-mmHg graduated compression stocking, after which the compression stocking alone was worn for 1 more week while the patient was ambulatory. In the intervening 18 months, the patient wore a 20-mmHg graduated compression stocking on a fairly consistent basis while she was ambulatory.
Case Study 4 Mixed reticular and telangiectatic veins
Reticular veins approximately 2 mm in diameter associated with multiple telangiectasias on the lateral distal thigh in a 32-year-old woman appeared during her second pregnancy 10 years previously (Fig. 12.21A). The patient requested treatment because of a dull aching that occurred in the area during menses and after prolonged standing. The treated veins are shown immediately after a total injection with 0.5 mL of POL 0.75% in Figure 12.21B, and Figure 12.21C shows the treated area 1 day after injection after removal of the STD pad and a 30- to 40-mmHg graduated compression stocking. Note the ecchymosis induced by the pressure dressing. Figure 12.21D, shows the area 1 week after injection. Intravascular thrombi were noted and drained at that time. Eight weeks after injection, the vessels were almost totally resolved. Some mild pigmentation was present in the distal aspect in which thrombosis was most extensive (Fig. 12.21E). Finally, Figure 12.21F, shows the area 22 months after initial treatment, demonstrating sustained resolution of the telangiectasia, reticular veins, and pigmentation.

Figure 12.21 Case Study 4. A, Reticular veins associated with multiple telangiectasias on lateral distal thigh. B, Immediately after injection. C, 1 day after injection and removal of pad and graduated pressure stocking. D, 1 week after injection. E, 8 weeks after injection. F, 22 months after treatment, with complete resolution.
Case Study 5 Extensive reticular and telangiectatic veins
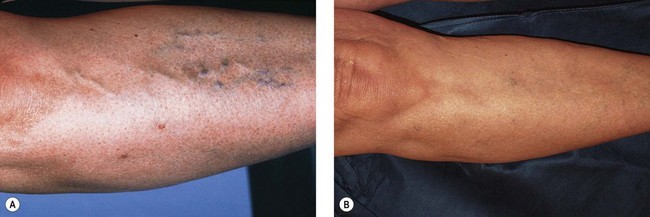
A 53-year-old woman had a 30-year history of asymptomatic reticular and telangiectatic leg veins that had been stable in appearance since her last of two pregnancies 20 years previously. She sought treatment for cosmetic reasons (Fig. 12.22A). A total of 10 mL of POL 0.75% was injected into all feeding reticular veins on the proximal and distal lateral thigh. Policocanol 0.5%, 2 mL, was injected into the portion of the telangiectatic mats on the lateral calf and knee, which did not blanch with the previous injection. STD foam pads were placed under a 30- to 40-mmHg graduated support stocking that was worn continually for 7 days after the procedure. When the stocking was removed, multiple small thrombi were drained. Figure 12.22B, shows the appearance of the treated area 25 months after the single treatment session.
Case Study 6 Treatment of cherry hemangiomas
A 48-year-old woman had multiple cherry hemangiomas on her abdomen and thighs and a 4-mm-diameter hemangioma located on her anterior thigh (Fig. 12.23A). Figure 12.23B, shows the appearance 5 months after injection with 0.1 mL of POL 0.75%. Note the slightly indented and hypopigmented scar, which was acceptable to the patient.
Case Study 7 Lateral subdermal plexis
A 59-year-old woman was seen initially with a leg ache from the lateral thigh veins that had been increasing in severity over the previous 2 years. Reticular and telangiectatic veins had been present since age 16. The lateral subdermal plexus, noted as having 2- to 3-mm diameter reticular veins, was incompetent to venous Doppler examination (Fig. 12.24A). Sclerotherapy began with injection of a total of 4 mL of POL 0.75% to all reticular veins on the left leg while the patient was supine. All telangiectasias that did not become inflamed after reticular vein injection were then sclerosed with a total of 6 mL of POL 0.5%. The leg was compressed for 72 hours with a 30- to 40-mmHg graduated compression stocking. A second treatment to the same leg was given 4 months later with 4 mL of POL 0.75% injected into reticular veins and 2 mL of POL 0.5% injected into remaining telangiectasias. The leg was compressed as previously described, and all veins and bruising resolved within 2 months. Figure 12.24B shows total resolution of all veins 1 year after the second treatment.
Case Study 8 Long-term follow-up of sclerotherapy treatment of telangiectasia
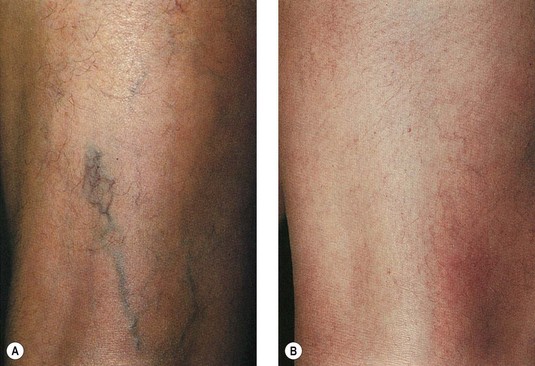
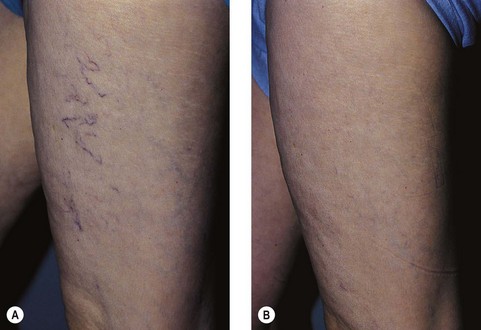
A 43-year-old woman presented with dilated pretibial reticular veins 2 to 4 mm in diameter (Fig. 12.25A). She gave a history of playing tennis and working on a hard floor. No evidence of truncal vein incompetence was found. She was treated with one session of sclerotherapy using 2 mL of 0.5% STS and then wore 30- to 40-mmHg graduated compression stockings 24 hours a day for 7 days. She reported complete resolution of her veins after 6 to 8 weeks and did not recall any post-treatment pigmentation. She presented 16 years later for treatment of new veins on her thighs (Fig. 12.25B).
Case Study 9 Long-term follow-up of sclerotherapy treatment of telangiectasia
A 52-year-old woman presented with scattered telangiectasia in patches without obvious feeding reticular veins on her anterior thigh (Fig. 12.26A). Veins measured 0.2 to 0.4 mm in diameter. She was treated with one session of sclerotherapy using 1.5 mL of 0.25% STS and then wore 30- to 40-mmHg graduated compression stockings 24 hours a day for 7 days. She reported complete resolution of her veins after 4 to 6 weeks and did not recall any post-treatment pigmentation. Figure 12.26B, shows the appearance of the treated area 6 years later when she presented for treatment of new veins on her calves.
Case Study 10 Treatment of telangiectasia and resulting telangiectatic matting
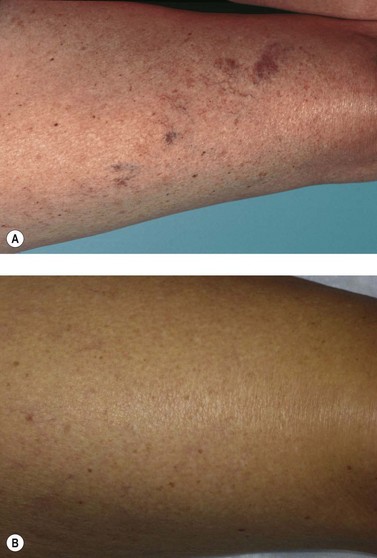
A 66-year-old woman was seen initially with extensive telangiectasias and venules 0.2 to 0.6 mm in diameter over the left lateral knee (Fig. 12.27A). These veins developed when she was near 40 years of age after she began estrogen replacement therapy. There was no previous family history of varicose veins. In addition to the cosmetic appearance that was disturbing when she wore shorts while playing golf, her legs ached continuously, especially over the telangiectasias. These vessels were treated with POL 0.5%, 2 mL, with the development of TM 4 weeks after treatment (Fig. 12.27B). Examination 3 months after initial treatment showed a ‘feeding’ reticular vein in the telangiectatic area. The feeding reticular vein, 2 mm in diameter, was treated with POL 0.75%, 1 mL, and the TM vessels were then treated with CG mixed 1 : 1 with lidocaine 1% with epinephrine, 1 mL, with resolution occurring in approximately 4 weeks. When the woman was examined 1 year later, the telangiectasia and leg pain had both resolved (Fig. 12.2C).
Case Study 11 Treatment of facial telangiectasia
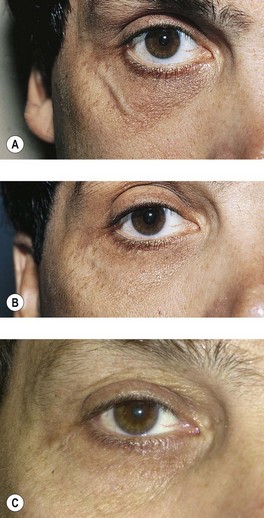
A 40-year-old man with a 20-year history of a prominent periorbital vein requested treatment (Fig. 12.28A). Physical examination showed a 2-mm venule in the lateral infraorbital region. A 6-0 Prolene suture was placed in the lateral canthal crease to close the vein circumferentially, and 0.2 mL of Sclerodex was injected slowly into the bulging vein. Immediate handheld pressure was applied with an ice pack and maintained by the patient for 5 minutes. The suture was removed the next day. Figure 12.28B, shows the appearance 8 weeks after treatment. A tiny coagulum is present in the resolving vein. Follow-up examination 12 years later showed continued elimination of the vein (Fig. 12.28C).
Case Study 12 Treatment of facial telangiectasia
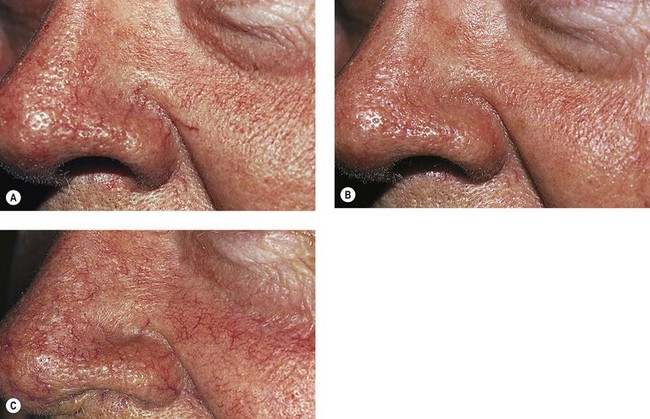
A 65-year-old man presented with prominent perinasal red telangiectasia (Fig. 12.29A). A slow infusion of POL 0.5% was given into the telangiectasia, and the solution was held in place until the vessel went into spasm. A total of 0.5 mL of solution was given in a single treatment session into nasal vessels. Three weeks after treatment, the vessels had resolved (Fig. 12.29B). When the patient was seen in follow-up 15 years later, the treated vessels were not present. New red telangiectasias were present but not as prominent as before treatment (Fig. 12.29C).
Case Study 13 Facial telangiectasias and venous malformation
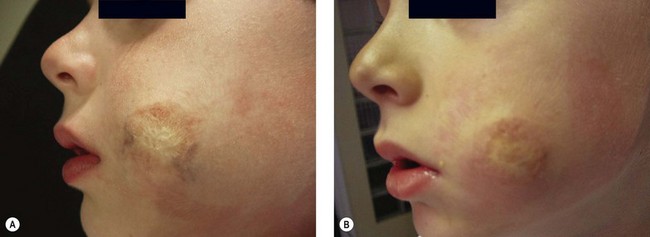
An 8-year-old boy had been treated 4 years before for an extensive venous malformation of the right cheek. The initial treatment had included embolization with Ethiblock and subsequent surgical removal of remaining material. A perilesional network of spider veins outlining the remaining scar was still present (Fig. 12.30A). Injection of 1 mL of 0.5% POL foam in three different points was challenging since the patient had also requested local anesthesia with EMLA cream, inducing a venous spasm. Two sessions gave a nice improvement, and patient satisfaction.
Case Study 14 Sclerotherapy of telangiectasias on venous malformation
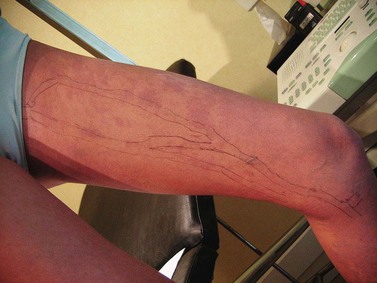
A 12-year-old girl presented with an extensive venous malformation of the left limb. Lesions were present at birth. One can observe the development of large competent superficial veins: great saphenous vein and accessory anteromedial extrafascial tributary (Fig. 12.31). A wide but light-pink port-wine stain extended the length of the limb, but both legs had the same length and circumferences. The girl was mainly concerned with the development of telangiectatic and reticular veins on the anterior, medial, and lateral aspects of the limb. Since reflux had not been detected, neither in the deep nor in the superficial venous networks, careful microsclerotherapy with POL 0.5% foam was carried out, improving the lesions with patient satisfaction after four sessions.
Case Study 15 Treatment of reticular chest veins
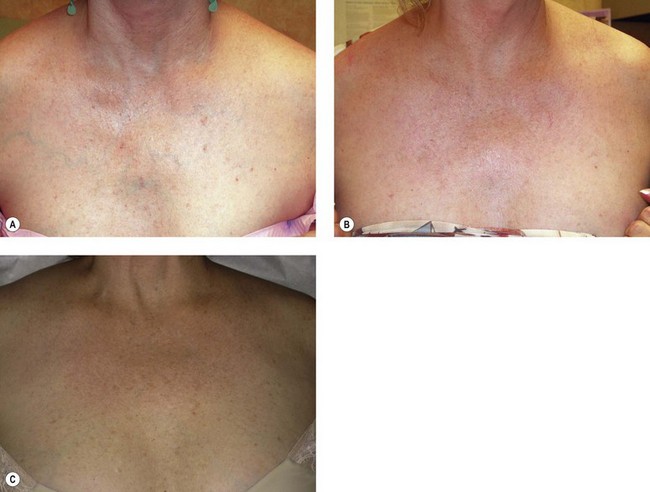
A 40-year-old woman developed dilated reticulated veins on her chest shortly after breast augmentation (Fig. 12.32A). Sclerotherapy was performed using STS 0.25% foam made by using 1 mL of STS and 4 mL of air. A total of 8 cm3 of foam (2 mL of solution) was used to infiltrate the entire anterior chest venous network. Figure 12.32B, shows the appearance 3 months after treatment.
1 Biegeleisen HI. Telangiectasia associated with varicose veins: treatment by a micro-injection technique. JAMA. 1934;102:2092.
2 Biegeleisen HI. Varicose veins, related diseases, and sclerotherapy: a guide for practitioners. Montreal: Eden Press; 1984.
3 Higgins TT, Kittel PB. Injection treatment with sodium morrhuate. Lancet. 1930;215:68.
4 Alderman DB. Therapy for essential cutaneous telangiectasias. Postgrad Med. 1977;61:91.
5 Foley WT. The eradication of venous blemishes. Cutis. 1975;15:665.
6 Tretbar LL. Spider angiomata: treatment with sclerosant injections. J Kans Med Soc. 1978;79:198.
7 Shields JL, Jansen GT. Therapy for superficial telangiectasias of the lower extremities. J Dermatol Surg Oncol. 1982;8:857.
8 Goldman MP, Weiss RA, Brody HJ, et al. Treatment of facial telangiectasia with sclerotherapy, laser surgery, and/or electrodesiccation: a review. J Dermatol Surg Oncol. 1993;19:899.
9 Prescott R. Treatment of facial telangiectasias by sclerotherapy. In: Raymond-Martimbeau P, Prescott R, Zummo M, editors. Phlébologie ’92. Paris: John Libbey Eurotext, 1992.
10 Merlen JF. Red telangiectasis, blue telangiectasis. Soc Fr Phlebol. 1970;22:167.
11 De Faria JL, Moraes IN. Histopathology of the telangiectasias associated with varicose veins. Dermatologia. 1963;127:321.
12 Raymond-Martimbeau P, Dupuis JL. Telangiectasias: incidence, classification, and relationship with the superficial and deep venous systems: a double-blind study. In: Negus D, Jantet G, Coleridge-Smith PD, editors. Phlebology ’95, Vol. 1. London: Springer; 1995:169-171.
13 Sommer A, Van Mierlo PL, Neumann HA, Kessels AG. Red and blue telangiectasias. Differences in oxygenation? Dermatol Surg. 1997;23:55.
14 Tretbar LL. The origin of reflux in incompetent blue reticular/telangiectasia veins. In: Davy A, Stemmer R, editors. Phlébologie ’89. Montrouge, France: John Libbey Eurotext, 1989.
15 Weiss RA, Weiss MA. Doppler ultrasound findings in reticular veins of the thigh subdermic lateral venous system and implications for sclerotherapy. J Dermatol Surg Oncol. 1993;19:947.
16 Mariani F, Bianchi V, Mancini S, Mancini S. Telangiectases in venous insufficiency: point of reflux and treatment strategy. Phlebology. 2000;15:38.
17 Sadick N. Treatment of varicose and telangiectatic leg veins with hypertonic saline: a comparative study of heparin and saline. J Dermatol Surg Oncol. 1990;16:24.
18 Scarborough DA, Bisaccia E. Sclerotherapy: translucidation of the skin prior to injection. J Dermatol Surg Oncol. 1989;15:498.
19 Marley W. Low dose sotradecol for small vessel sclerotherapy. Newsl North Am Soc Phlebol. 1989;3:3.
20 Eichenberger H. Results of phlebosclerosation with hydroxy-polyethoxydodecane. Zentralbl Phlebol. 1969;8:181.
21 Duffy DM. Small vessel sclerotherapy: an overview. Adv Dermatol. 1988;3:221.
22 Bodian E. Sclerotherapy. Dialogues Dermatol. 13(3), 1983.
23 Green D. Compression sclerotherapy techniques. Dermatol Clin. 1989;7:137.
24 Goldman MP, Bennett RG. Treatment of telangiectasia: a review. J Am Acad Dermatol. 1987;17:167.
25 Guex JJ. Indications for the sclerosing agent polidocanol. J Dermatol Surg Oncol. 1993;19:959.
26 Guex JJ, Allaert FA, Gillet JL, Chleir F. Immediate and midterm complications of sclerotherapy: report of a prospective multicenter registry of 12,173 sclerotherapy sessions. Dermatol Surg. 2005;31:123.
27 Green AR, Morgan BDG. Sclerotherapy for venous flare. Br J Plast Surg. 1985;38:241.
28 Rao J, Goldman MP. Stability of foam in sclerotherapy: difference between sodium tetradecyl sulfate and polidocanol and the type of connector used in the double syringe system (DSS) technique. Dermatol Surg. 2005;31:19.
29 Lai SW, Goldman MP. Does the relative silicone content of different syringes affect the stability of foam in sclerotherapy? J Drugs Dermatol. 2008;7:399.
30 Cabrera Garrido JR, Cabrera Garcia-Olmedo JR, Garcia-Olmedo Dominguez MA. Elargissement des limites de la sclérothérapie: noveaux produits sclérosants. Phlebologie. 1997;50:181.
31 Cabrera Garrido J. Los esclerosantes en microespuma contra la patología venosa. Noticias Medicas. 312, 1997.
32 Monfreux A. Traitement sclérosant des troncs saphènies et leurs collatérales de gros calibre par la méthode MUS. Phlebologie. 1997;50:351.
33 Benigni JP, Sadoun S, Thirion V, et al. Télangiectasies et varices réticulaires: traitement par la mousse d’Aetoxisclérol a 0.25%. Présentation d’une étude pilote. Phlebologie. 1999;3:283.
34 Mingo-Garcia J. Esclerosis venosa con espuma: foam medical system. Rev Esp Med Cir Cosmética. 1999;7:29.
35 Tessari L. Metodique extemporaine de la preparation de la ‘scleromousse’ en seringue de plastique monousage. Presented at the Society of French Phlebology, Paris, December 11, 1999.
36 Frullini A. New technique in producing sclerosing foam in a disposable syringe. Dermatol Surg. 2000;26:705.
37 Hennet JP. Three years’ experience with polidocanol foam in treatment of reticular veins and varicosities. Phlebologie. 1999;52:277.
38 Benigni J, Sadoun S. Telangiectasia: benefits of a foam sclerosing agent. J Phlebol. 2002;2:35.
39 Weiss RA. Comparison of liquid vs. foamed 0.1 and 0.2% STS on the treatment of leg telangiectasia. Presented at the 16th Annual Congress of the American College of Phlebology, Ft. Lauderdale, Fla., 2002.
40 Kern P, Ramelet AA, Wutschert R, et al. Single-blind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatol Surg. 2004;30:367.
41 Guex J-J, Allaert F-A, Gillet J-L, Chleir F. Immediate and mid-term complications of sclerotherapy, report of a prospective multi-center registry of 12,173 sclerotherapy sessions. J Dermatol Surg. 2005;31:123.
42 Breu F, Guggenbichler S, Wollmann JC. 2nd European consensus meeting on foam sclerotherapy. 28-30 April 2006. Tegernsee, Germany. Vasa. 2008;37(Suppl 71):1.
43 Bohler-Sommeregger K, Karnel F, Schuller-Petrovic S, Santler R. Do telangiectases communicate with the deep venous system? J Dermatol Surg Oncol. 1992;18:403.
44 Lary BG. Varicose veins and intracutaneous telangiectasia: combined treatment in 1500 cases. South Med J. 1987;80:1105.
45 Ouvry P, Davy A. Le traitement sclerosant des telangiectasies des membres inferieurs. Phlebologie. 1982;35:349.
46 Bukhari RH, Lohr JM, Paget DS, Hearn AT. Evaluation of lidocaine as an analgesic when added to hypertonic saline for sclerotherapy. J Vasc Surg. 1999;29:479.
47 Carlin MC, Ratz JL. Treatment of telangiectasia: comparison of sclerosing agents. J Dermatol Surg Oncol. 1987;13:1181.
48 Norris MJ, Carlin MC, Ratz JL. Treatment of essential telangiectasia: effects of increasing concentrations of polidocanol. J Am Acad Dermatol. 1989;20:643.
49 Sadick N. Sclerotherapy of varicose and telangiectatic leg veins: minimal sclerosant concentration of hypertonic saline and HS relationship to vessel diameter. J Dermatol Surg Oncol. 1991;17:65.
50 Goldman MP. Treatment of varicose and telangiectatic leg veins: double-blind prospective comparative trial between Aethoxysclerol and Sotradecol. Dermatol Surg. 2002;28:52.
51 Thibault PK. Sclerotherapy of varicose veins and telangiectasias: a 2-year experience with sodium tetradecyl sulfate. Aust N Z J Phlebol. 1999;3:25.
52 Kern P, Ramelet AA, Wutschert R, et al. Single-blind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatol Surg. 2004;30:367. discussion 372
53 Leach B, Goldman MP. Comparative trial between sodium tetradecyl sulfate and glycerin in the treatment of telangiectatic leg veins. Dermatol Surg. 2003;29:612.
54 Munavalli GS, Weiss MA, Beasley KL, Weiss RA. Prospective trial of 72% glycerin vs. foamed 0.1% sodium tetradecyl sulfate for thigh telangiectasia. Presented at the 18th Annual Congress of the American College of Phlebology. Ft. Myers, Fla., 2004.
55 Weiss R, Weiss M. Resolution of pain associated with varicose and telangiectatic leg veins after compression sclerotherapy. J Dermatol Surg Oncol. 1990;16:333.
56 Orbach J. A new look at sclerotherapy. Folia Angiologia. 1977;25:181.
57 Marteau J, Marteau J. Contribution de la cryotherapie en phlebologie. Phlebologie. 1978;31:191.
58 Stamenova PK, Marchetti T, Simeonov I. Efficacy and safety of topical hirudin (Hirudex®, Pharmafar, Torino, Italy): a double-blind, placebo-controlled study. Eur Rev Med Pharmacol Sci. 2001;5:37.
59 Kern P, Ramelet AA, Wütschert R, Hayoz D. Compression after sclerotherapy for telangiectasias and reticular leg veins: a randomized controlled study. J Vasc Surg. 2007;45(6):1212-1216.
60 Chrisman BB. Treatment of venous ectasias with hypertonic saline. Hawaii Med J. 1982;41:406.
61 Guex J-J. Inutilité de la compression après sclérothérapie des micro-varices et télangiectasies. Phlebologie. 1994;47:371.
62 Fante RG, Goldman MP. Editorial: removal of periocular veins by sclerotherapy. Ophthalmology. 2001;180:433.
63 Shafir R, Cohen M, Gur E. Blindness as a complication of subcutaneous nasal steroid injection. Plast Reconstr Surg. 1999;104:1180.
64 Thomas EL, Laborde RP. Retinal and choroidal vascular occlusion following intralesional corticosteroid injection of a chalazion. Ophthalmology. 1986;93:405.
65 McGrew RN, Wilson RS, Havener WH. Sudden blindness secondary to injections of common drugs in the head and neck. I. Clinical experiences. Otolaryngology. 1978;86:147.
66 Green D. Removal of periocular veins by sclerotherapy. Ophthalmology. 2001;108:442.
67 Siniluoto TM, Svendsen PA, Wikholm GM, et al. Percutaneous sclerotherapy of venous malformations of the head and neck using sodium tetradecyl sulphate (Sotradecol). Scand J Plast Reconstr Surg Hand Surg. 1997;31:145.
68 Kersten RC, Kulwin DR. Management of cosmetically objectionable veins in the lower eyelids. Arch Ophthalmol. 1989;107:278.
69 Weiss RA, Ramelet A-A. Removal of blue periocular lower eyelid veins by ambulatory phlebectomy. Dermatol Surg. 2002;28:43.
70 Bowes LE, Goldman MP. Sclerotherapy of reticular and telangiectatic veins of the face, hands and chest. Dermatol Surg. 2002;28:46.
71 Lai SW, Goldman MP. Treatment of facial reticular veins with dynamically cooled, variable spot-sized 1064 nm Nd:YAG laser. J Cosmet Dermatol. 2007;6:6.
72 Goldman MP, Fitzpatrick RE. Cutaneous laser surgery: the art and science of selective photothermolysis, 2nd ed. St Louis: Mosby; 1999.
73 Lim AC, Kossard S. Generalized essential telangiectasia: treatment with sodium tetradecyl sulfate sclerotherapy. Aust N Z J Phlebol. 2002;6:26.
74 McCoy S, Evans A, Tiller A, Malouf GM. A blinded prospective comparative trial of a topical vitamin K cream for the treatment of leg telangiectasias. Aust N Z J Phlebol. 2000;4:28.