Chapter 11 Clinical Management of Heat-Related Illnesses
Exertional Heat Illness
Dehydration and heat exposure can impair exercise performance and contribute to various illnesses. Exertional heat illnesses are comprised of minor and serious disorders. Minor heat and dehydration-related illnesses include heat cramps, erythromelalgia, and heat syncope. Heat cramps are characterized by intense muscle spasms, typically in the legs, arms, and abdomen. Heat cramps result from fluid and electrolyte deficits and occur most often in persons who have not been fully acclimated to a combination of intense muscular activity and environmental heat. Individuals who are susceptible to heat cramps are often believed to be profuse sweaters who sustain large sweat sodium losses.13,106 Heat syncope (fainting) is characterized by dizziness and weakness during or after prolonged standing or after rapidly standing up from a lying or sitting position during heat exposure. Heat syncope results from blood pooling in the cutaneous and skeletal vasculature, and it occurs most commonly in dehydrated and inactive persons who are not acclimated.93 Erythromelalgia is characterized by pain and swelling in the feet and hands that is triggered by exposure to elevated temperatures.64
Serious illnesses include exertional heat injury (EHI) and EHS. These illnesses have many overlapping diagnostic features; it has been suggested that they exist along a continuum on the severity scale.17 Heat exhaustion is characterized by inability to sustain cardiac output in the presence of moderate (>38.5° C [101° F]) to high (>40° C [104° F]) body temperatures, and is frequently accompanied by hot skin and dehydration. EHI is a moderate to severe illness characterized by injury to an organ (e.g., liver, kidneys, gut, muscle) and that usually (but not always) involves a high Tco of more than 40° C (104° F). EHS is a severe illness that is characterized by central nervous system dysfunction (e.g., confusion, disorientation, impaired judgment) and that is usually accompanied by a Tco of more than 40.5° C (105° F). EHI and EHS can be complicated by cardiac arrhythmia, liver damage, rhabdomyolysis, coagulopathy, fluid and electrolyte imbalances, and kidney failure. Rhabdomyolysis is most often observed with novel and strenuous overexertion. Clinical evidence suggests that dehydration increases the likelihood or severity of acute renal failure associated with rhabdomyolysis.19,91 Among U.S. soldiers who have been hospitalized for serious heat illness, 25% had rhabdomyolysis, and 13% had acute renal failure.22
EHS is usually associated with prolonged exertion in a warm climate; however, in many instances, EHS occurs within the first 2 hours of exercise and not necessarily at high ambient temperatures.17,37 This is because exertion and environmental heat stress during the 72 hours that precede such an event strongly influence the individual’s susceptibility to heat illness.40 Using a Tco of 40.5° C (104.9° F) as a critical temperature to initially diagnose EHS is arbitrary. Mental status changes in an individual who is performing exertion in the heat should be the defining characteristic of heat stroke unless the individual has sustained head trauma. At the stage of collapse, profuse sweating is still likely to be present unless heatstroke develops in an already anhidrotic individual. Dry skin may be evident either in situations in which the climate is very dry and sweat evaporates easily or when heatstroke coincides with a severe degree of dehydration.36
On-Site Emergency Medical Treatment
The early diagnosis of heat illness can be critical to therapeutic success. Early warning signs include flushed face, hyperventilation, headache, dizziness, nausea, tingling arms, piloerection, chilliness, incoordination, and confusion.74 If the patient is alert and has no mental status changes, he or she can rest in the shade or indoors, and oral rehydration can be instituted with cold water or an electrolyte-replacement beverage. The concentration of carbohydrates in such a beverage should not exceed 6%; otherwise, gastric emptying and fluid absorption by the intestines may be delayed. Responders should target an intake of 1 to 2 L (0.9 to 1.8 qt) over 1 hour. If the patient does not improve or in fact worsens, he or she should be evaluated by a medical provider. All persons with suspected heat injuries should be observed to ensure that decompensation does not occur. The victim should continue to rest and drink over the next 24 hours. As a general rule, for every pound of weight lost by sweating, 0.5 qt (2 cups or 500 mL) of fluid should be consumed. It may require 36 hours to completely restore lost electrolytes and fluid volume to all body compartments via oral intake. After the acute episode, a medical provider should determine any possible host risk factors for heat illness and review with the victim the signs of heat illness and preventive measures to consider.
Any athlete who is performing exercise in warm weather and who develops mental status changes in the absence of trauma should be treated as an EHS victim until proved otherwise.36 EHS is a medical emergency. Rapid reduction of elevated Tco is the cornerstone of EHS management; the duration of hyperthermia may be the primary determinant of outcome.59,100 Cooling should not be delayed so that a temperature measurement can be obtained. Cooling measures should only be minimally delayed for vital resuscitation measures. Nevertheless, it is important to follow the ABCs (airway, breathing, and circulation) of stabilization while cooling efforts are initiated; see Box 11-1 for basic first aid information. Before 1950, the mortality rate with EHS was 40% to 75%.6,35 Long-term survival is directly related to rapid institution of resuscitative measures.49
In the field, the sick individual should be placed in the shade, and any restrictive clothing should be removed. There are multiple ways to cool victims in the field, with cold-water immersion (CWI) being the most effective modality.24,86 In a remote setting, this can be accomplished by using a small children’s pool filled with iced water. The victim should be submerged up to the shoulders and kept under immediate hands-on supervision at all times. Another expedient method in the field is to keep bed sheets soaked in a cooler full of iced water; the victim can then be wrapped in the cold sheets. Particular care should be given to covering the head and to resubmerging the sheets every few minutes to recool them.79 Ice packs can be applied to the groin, axillae, sides of the neck, and head to augment iced-sheet cooling. Cooling should continue until emergency medical services providers arrive. Nonmedical first responders should not attempt to evacuate heat stroke patients themselves, because this may distract from cooling efforts. If CWI or iced sheets are not available, the victim should be kept wet by applying large quantities (20 to 30 L [5.3 to 7.9 gal]) of tap water or water from any source, and the victim’s body should be constantly fanned. Cooling blankets are generally ineffective as a single modality for inducing the rapid lowering of body temperature required for treating heatstroke.
Emergency Medical Services Treatment
During evacuation, CWI is often not a viable method for treatment. Iced sheets and ice packs can be easily used en route during transport. Many EMS vehicles now carry refrigerated intravenous (IV) fluid to initiate induction of therapeutic hypothermia in cardiovascular emergencies. When used, chilled IV fluid (4° C [39° F]) should be peripherally administered.63 Vascular access should be established without delay by inserting a 12- or 14-gauge IV catheter. Administration of normal saline or lactated Ringer’s solution should be started. Recommendations vary regarding administration rate of fluids. Some clinicians advise a rate of 1200 mL (1.26 qt) over 4 hours,78 whereas others encourage a 2-L (2.11-qt) bolus over the first hour and an additional liter of fluid per hour for the next 3 hours.98 Patients should be placed on a cardiac monitor. Administration of supplemental oxygen may help to meet the victim’s increased metabolic demands, and it may also be used to treat hypoxia that is commonly associated with aspiration, pulmonary hemorrhage, pulmonary infarction, pneumonitis, or pulmonary edema.34,67 A blood glucose determination should be performed, and adults with blood sugar level less than 60 mg/dL should be treated with 1 ampule of 50% IV dextrose solution. Children should be treated with 2 to 4 cc/kg of 25% IV dextrose solution.
Hospital Emergency Medical Treatment
Patients with suspected heat stroke should be placed in a large treatment room to accommodate the needed number of staff. Patients are often combative and disoriented before reestablishing their baseline mental status. Aggressive cooling measures should continue until mental status returns to normal and Tco is 39° C (102° F).24 After discontinuation of cooling, Tco should be monitored every 5 minutes to ensure that it does not increase.
The Tco reported in the field for heatstroke victims may be significantly higher (e.g., 41.1° C [106.9° F]), than those documented in the hospital emergency department (e.g., 37.8° C [100° F]), because Tco may fall during transport to the hospital.99 Documenting only a mild elevation in Tco on arrival does not exclude the diagnosis of heatstroke. Central nervous system (CNS) disturbances (i.e., coma, convulsions, confusion, or agitation) that accompany hyperthermia may also result from CNS infections, sepsis, or other disease processes. Other diagnoses should be considered when the patient does not regain normal mental status when the Tco is normalized in less than 30 minutes. When Tco remains elevated for a longer time, there is a decreased likelihood that mental status will normalize with euthermia.17
The comparison of oral and rectal temperature values in heat-stressed underground miners showed a difference of approximately 1° C (2° F), with oral temperatures underestimating rectal values.108 Tympanic temperature responds more rapidly to cooling or heating than does rectal temperature, but it is influenced by changes in the skin temperature of the head and neck.44,68 The ear should be insulated from the environment to prevent cool ambient temperatures (<30° C [86 ° F]) from affecting this measurement. Esophageal temperature is the most accurate and responsive to changes in blood temperature, but its instrumentation is impractical in severely injured or unresponsive patients.
If airway control was not previously established and if the patient is still unconscious, a cuffed endotracheal tube should be inserted to protect against aspiration of oral secretions. Supplemental oxygen—and, when hypoxia (PaO2 <55 mm Hg) or hypotension is present, positive-pressure ventilation—should be provided. Overly vigorous fluid resuscitation may precipitate pulmonary edema, so careful monitoring is indicated. Ideally, 1 to 2 L (1.05 to 2.11 qt) of fluid should be administered during the first hour after collapse, and additional fluids should be administered until satisfactory urine output (0.5 cc/kg/hr in an adult and 1.0 cc/kg/hr in a child) is established.38 Most heatstroke victims arrive with a high cardiac index, low peripheral vascular resistance, and mild right-sided heart failure with elevated central venous pressure. Only moderate fluid replacement is indicated if effective cooling results in vasoconstriction and increased blood pressure. Providers should consider noninvasive intravascular volume monitoring or the minimally invasive monitoring of systolic volume variation and pulse pressure variation. If these methods are not adequate, a Swan-Ganz pulmonary artery catheter may be necessary to assess appropriate fluid supplementation. Some victims have a low cardiac index, hypotension, and elevated central venous pressure. These persons have been successfully treated with an isoproterenol drip (1 mg/min).78 Patients with a low cardiac index, low central venous pressure, hypotension, and low pulmonary capillary wedge pressure should receive fluid. Unless the patient has rhabdomyolysis, aggressive fluid hydration is seldom required after initial treatment.
As a result of drastic cooling, skin temperature may decrease enough to cause shivering. Administration of 12.5 mg of meperidine via slow IV push103 or of 5 mg of diazepam is effective to suppress shivering and to prevent an additional rise in Tco from metabolic heat production. If CWI is used, the increase in metabolic rate as a result of shivering will be more than offset by the high rate of heat transfer. Therefore the presence of shivering should not be a cause for concern when this method of cooling is used.24,83
Severe muscle cramping may be caused by electrolyte imbalances. Magnesium levels should be obtained. If magnesium levels are low, consideration may be given to the use of 50% IV magnesium sulfate (4 g in 250 mL of 5% dextrose injection at a rate that does not exceed 3 mL per minute).14,15
A Foley catheter should be placed to monitor urine output. Renal damage from myoglobinuria and hyperuricemia can be prevented by promoting renal blood flow by administering IV mannitol (0.25 mg/kg) or furosemide (1 mg/kg).98 If creatine phosphokinase (CPK) levels exceed 100,000 international units, alkalinize the urine of patients with exertional rhabdomyolysis; there is no advantage to alkalinization when levels are lower. Hemodialysis should be reconsidered if anuria, oliguria (<0.5 mL/kg of urine per hr for >6 hr), uremia, or hyperkalemia develops. Cooling and hydration usually correct acid–base abnormalities; however, serum electrolytes should be monitored and appropriate modifications of IV fluids made. Glucose should be monitored repeatedly, because either hypoglycemia or hyperglycemia may occur after EHS.95 Oral and gastric secretions are evacuated via a nasogastric tube that is connected to continuous low suction. Although antacids, proton pump inhibitors, and histamine-2 blockers have been used to prevent gastrointestinal bleeding, no studies to date demonstrate their efficacy for heatstroke victims.
Induced Hypothermia
Induced hypothermia is increasingly being used for many neurologic and cardiovascular emergencies, including acute stroke, neonatal hypoxic–ischemic encephalopathy, and after cardiac arrest.9,50,76,94 This therapeutic modality has not been evaluated for effectiveness in individuals with EHS, but may have a role in cooling severe refractory cases of EHS. There may be a role for a period of induced hypothermia after severe EHS.
Dantrolene
No drug has been found to have a significant effect for reducing Tco. Antipyretics are ineffective, because the thermoregulatory set point is not affected in heatstroke. Furthermore, antipyretics might be harmful, because they cannot be readily metabolized in the heat-affected liver. However, dantrolene has been used quite successfully for the treatment of several hypercatabolic syndromes, such as malignant hyperthermia, neuroleptic malignant syndrome, and other conditions that are characterized by muscular rigidity or spasticity.107,114 Dantrolene stabilizes the calcium (Ca2+)-release channel in muscle cells, thereby reducing the amount of Ca2+ released from cellular calcium stores. This lowers intracellular Ca2+ concentrations, muscle metabolic activity, muscle tone, and thus heat production.27,75 In some studies, dantrolene was claimed to be effective for the treatment of heatstroke, whereas in others it improved neither the rate of cooling nor survival.25,31,66,109 In six patients with rhabdomyolysis, intramuscular Ca2+ concentrations were 11 times higher than in controls, and dantrolene successfully lowered this elevated Ca2+.65 Collectively, the limited data available are at best inconsistent. Despite growing evidence for a possible benefit of dantrolene treatment in patients with heatstroke, justification for its routine use in such cases is not proved, although future clinical trials may change this assessment.
Moran and colleagues71 studied dantrolene in a hyperthermic rat model, and found it to be effective as a prophylactic agent in sedentary animals only. Dantrolene induced more rapid cooling by depressing Ca2+ entry into the sarcoplasm; this led to relaxation of peripheral blood vessels with attenuated production of metabolic heat. Dantrolene may also be effective in treating heatstroke by increasing the cooling rate. However, in other animal models, dantrolene was not superior to conventional cooling methods.117 As such, dantrolene is not recommended.
Clinical Manifestations
Clinical manifestations of heatstroke vary, depending on whether the victim suffers from classic heatstroke, which is a common disorder of older adults during heat waves and occurs in the form of epidemics, or EHS,1 which occurs when excess heat generated by muscular exercise exceeds the body’s ability to dissipate it (Table 11-1). Some overlap in presentation may occur; treatment with a medication (e.g., antihypertensive or antipsychotic) that places an older adult at risk for classic heatstroke also places an exercising individual at risk for EHS. The clinical picture of heatstroke usually follows a distinct pattern of events with three phases: (1) acute, (2) hematologic or enzymatic, and (3) late.38
| Characteristics | Classic | Exertional |
|---|---|---|
| Age group | Young children and older adults | Men between the ages of 15 and 45 yr |
| Health status | Chronically ill | Healthy |
| Concurrent activity | Sedentary | Strenuous exercise |
| Drug use | Diuretics, antidepressants, antihypertensives, anticholinergics, and antipsychotics | Usually none |
| Sweating | May be absent | Usually present |
| Lactic acidosis | Usually absent; poor prognosis if present | Common |
| Hyperkalemia | Usually absent | Often present |
| Hypocalcemia | Uncommon | Frequent |
| Hypoglycemia | Uncommon | Common |
| Creatine phosphokinase | Mildly elevated | Markedly elevated |
| Rhabdomyolysis | Unusual | Frequently severe |
| Hyperuricemia | Mild | Severe |
| Acute renal failure | <5% of patients | 25-30% of patients |
| Disseminated intravascular coagulation | Mild | Marked; poor prognosis |
| Mechanism | Poor dissipation of environmental heat | Excessive endogenous heat production and overwhelming heat-loss mechanisms |
Modified from Knochel JP, Reed G: Disorders of heat regulation. In Kleeman CR, Maxwell MH, Narin RG, editors: Clinical disorders of fluid and electrolyte metabolism, New York, 1987, McGraw-Hill.
Acute Phase
The acute phase of heatstroke is characterized by CNS manifestations. Because brain function is very sensitive to hyperthermia, this phase is present in all heatstroke patients. Early signs of CNS dysfunction are typically cerebellar and include ataxia, poor coordination, and dysarthria.116 Advanced signs of CNS depression include irritability, aggressiveness, stupor, delirium, and coma.2,21,102 Mental status changes usually resolve after return to a normal Tco. After a return to normothermia, persistence of coma is a poor prognostic sign.57,102 Other symptoms include fecal incontinence, flaccidity, and hemiplegia. Cerebellar symptoms may persist beyond the acute phase.69,102,116
Other common disturbances during the acute phase occur in the gastrointestinal and respiratory systems. Gastrointestinal dysfunction, including diarrhea and vomiting, often occurs. However, the latter may reflect translocation of toxic gram-negative bacterial lipopolysaccharide from the lumen of the intestines because of poor splanchnic perfusion as a result of hypotension caused by increased skin blood flow and from CNS impairment.18,41,102 Hyperventilation and elevation of Tco primarily lead to respiratory alkalosis, which in EHS may be masked by metabolic acidosis as a result of increased glycolysis and hyperlacticacidemia.26,74 Hypoxemia may be present in patients with respiratory complications.28,74,105 It should also be noted that oxygen consumption is elevated during hyperthermia, with a 10% to 13% increase for every ° C above euthermia.32
EHS shares many common findings with systemic inflammatory response syndrome (SIRS).17,97 Endotoxemia, hyperthermia, and other risk factors (e.g., preexisting infection) and stressors associated with EHS can trigger this exaggerated inflammatory response. Patients should be assessed for SIRS after admission with the use of the following criteria:16 (1) body temperature of less than 36° C (98.6° F) or of more than 38° C (100.4° F); a heart rate of more than 90 beats per minute, tachypnea, or an arterial partial pressure of carbon dioxide of less than 4.3 kPa (32 mm Hg); and a white blood cell count of less than 4000 cells/mm3 (4 × 109 cells/L) or of more than 12,000 cells/mm3 (12 × 109 cells/L) or the presence of more than 10% immature neutrophils. When two or more of these criteria are met, SIRS can be diagnosed. The presence of systemic inflammatory response markers during the acute phase predicts the severity of subsequent phases.
Hematologic and Enzymatic Phase
Hematologic and enzymatic disorders peak 24 to 48 hours after collapse. In the hematologic and enzymatic phase of EHS, hematologic, enzymatic, and other blood parameters are altered. In humans and experimental animals, hyperthermia results in leukocyte activation46 and in changes in lymphocyte subpopulations, both in absolute numbers and in percentages.1 Leukocytes may range from 20 to 30 × 103/mm3 or higher.10,51 In severe cases, leukocyte activation is associated with the systemic activation of coagulation cascades.55 In one study, all fatal cases of EHS involved disturbances in the blood coagulation system.10,101 Prothrombin time, partial thromboplastin time, and the level of fibrin split products increased with a fall in thrombocytes.38 Clotting dysfunction peaked 18 to 36 hours after the acute phase of heatstroke; 2 to 3 days after heatstroke, prothrombin levels fell to 17% to 45% of normal. Depending on the severity of heatstroke, thrombocyte values ranged between 110 × 103/mm3 and 0.100,101 This systemic inflammatory response state resembles gram-negative bacterial sepsis, and it appears that lipopolysaccharide (a cell-wall component of gram-negative bacteria) participates in the pathophysiology of EHS.43
Enzymes
One of the prominent and almost pathognomonic characteristics of EHS is the appearance of exceptionally high levels of certain cellular enzymes, which implies cell damage or death. Most patients with EHS show an elevation of serum CPK activity and myoglobinuria, which suggests damage to skeletal muscle.37 CPK values in the range of 103 to 104 international units were commonly found, with peak values occurring 24 to 48 hours after collapse.30,96 At a Tco of 41.8° to 42.2° C (107.24° to 107.96° F), aspartate aminotransferase and alanine aminotransferase levels rose by factors of 25 and 8, respectively, and bilirubin levels approximately doubled to 1.56 mg/dL.82
These rises in enzyme levels are related to tissue damage, which in turn depends on both Tco and its rates of rise and duration.73 CPK was the most sensitive to increases in Tco, and this was followed by lactate dehydrogenase, which is an index of generalized tissue damage. CPK levels in EHI and EHS typically peak at 24 to 48 hours. Rising values after this point should alert the clinician to the possibility of an occult compartment syndrome. It is not uncommon for a victim of EHI to complain of lower-extremity pain that declares itself as a compartment syndrome. During the 1994 Hajj, 26 heatstroke victims admitted to a heatstroke treatment unit had elevated levels of CPK, aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase; these levels remained high after 24 hours. Those who died had higher enzyme levels than did the survivors. Lactate dehydrogenase concentration was useful for distinguishing between those who died and those who had a rapid recovery. The enzymes were better prognostic indicators than were Tco, anion gap, and serum potassium level.
Late Phase
Acute renal failure is a common complication of severe cases, and occurs in approximately 25% to 30% of patients with EHS patients.60,67,100 Oliguria and anuria are characteristic features. During this phase, urine has been described as being like machine oil, with a high specific gravity.81,84,92,100 Usually present in the urine are red and white cells, hyaline and granular casts, and mild to moderate proteinuria.92 The etiology involves multiple causes,81 but a major one is reduced renal blood flow caused by heat-induced hypotension, hypohydration, and peripheral vasodilation. In addition, direct thermal injury may also lead to widespread renal tissue damage.92 Myoglobinuria and elevated blood viscosity that result from disseminated intravascular coagulation may further contribute to acute oliguric renal failure.84,92,104,111
EHS is usually manifested by increased serum levels of liver enzymes, but acute liver failure has also been reported.115,39 High bilirubin levels, which may last for several days, reflect hepatic dysfunction and hemolysis. In most cases of EHS, liver injury is usually asymptomatic and exhibits reversible elevation in plasma aminotransferase levels.48 Acute liver failure is documented in 5% of patients with EHS.61 Hypophosphatemia (<0.5 mmol/L) at the time of admission may predict occurrence of acute liver failure.39 Despite limited experience and the observation that EHS patients with extensive liver damage may recover spontaneously, orthotopic liver transplantation had been suggested as a potential treatment.12,47,89 Among 16 reported cases of EHS-induced liver failure,45 three patients underwent liver transplantation. In the conservatively managed group, eight patients recovered spontaneously, and five died. Concomitantly, all three patients who received transplantation died. Hadad and co-workers45 concluded that, because of the poor and limited results of liver transplantation after heatstroke, the interpretation of prognostic criteria is crucial before listing a patient for such surgery.
Sequelae And Aftercare
The combination of the rapid reduction of Tco, control of seizures, proper rehydration, and prompt evacuation to an emergency medical facility results in a 90% to 95% survival rate in heatstroke victims, with morbidity directly related to the duration of hyperthermia.17,97 A poor prognosis is associated with Tco of more than 41° C (105.8° F), prolonged duration of hyperthermia, hyperkalemia, acute renal failure, and elevated serum levels of liver enzymes. Therefore misdiagnosis, early inefficient treatment, and delay in evacuation are the major causes of clinical deterioration. Full recovery without evidence of neurologic impairment has been achieved even after coma of 24 hours’ duration and subsequent seizures.101 Persistence of coma after return to normothermia is a poor prognostic sign.102 Red blood cell apoptosis (i.e., cell blebbing, asymmetry, and shrinkage) on early peripheral blood smears is a very poor prognostic sign and may indicate extensive cellular necrosis. Neurologic deficits may persist, but usually only last for a limited period of 12 to 24 months and only rarely for longer.
CNS dysfunction becomes increasingly severe with prolonged duration of hyperthermia and associated circulatory failure. Nevertheless, coma that persists for up to 24 hours, even with subsequent seizures, is usually followed by complete recovery without evidence of mental or neurologic impairment.2,88 However, chronic disability may prevail for several weeks or months in the forms of cerebellar deficits, hemiparesis, aphasia, and mental deficiency.2,60,88 Only in exceptional cases, when coma persisted for more than 24 hours, did mental and neurologic impairment become chronic and prevail for years. However, in one study of classic heatstroke, 78% of patients had minimal to severe neurologic impairment, such as ataxia or dysathria.30 Long-term EHS victims may have increased mortality from heart, liver, and kidney disease.113
Prevention
Awareness of Host Risk Factors
Shapiro and Moran96 studied 82 cases of EHS in Israeli soldiers and concluded that at least one factor that predisposes an individual to heatstroke (e.g., diarrhea, lack of acclimatization, poor fitness) was associated with each case. Correcting those individual risk factors should lead to strategies that can prevent heatstroke. Any underlying condition that causes dehydration or increased heat production or that causes decreased dissipation of heat interferes with normal thermoregulatory mechanisms and predisposes an individual to heat injury. Older individuals are less tolerant to EHI than younger persons, and they are more susceptible to classic heatstroke because of decreased secretory ability of their sweat glands and decreased ability of their cardiovascular systems to increase blood flow to the skin. When healthy young adults exercise strenuously in the heat, EHS may occur despite the absence of host risk factors.
Elite and professional athletes, the general public, and the military have widely used ergogenic aids (e.g., the herb ephedra [ma huang]) that contain ephedrine to improve performance and for weight loss. Because ephedra increases metabolic rate, it has caused numerous cases of heat illness and deaths worldwide, and it has been banned. Because there are no clear ergogenic benefits to the use of ephedra alone, use of ephedra-containing substances should be discouraged.80
The ratio of basal metabolic rate to surface area is higher in children than in adults. As a result, a child’s skin temperature is higher for any given Tco. Although the secretory rates of sweat glands are lower in children, children have greater numbers of active sweat glands per area of skin than adults and overall greater sweat rates per unit area.54 Any reduction in the rate of sweating puts children especially at risk. The primary mechanism for heatstroke in young children is hot vehicle entrapment. Between 1998 and 2010, 462 children died from heatstroke as a result of vehicular hyperthermia.
The primary means of heat dissipation is production and evaporation of sweat. Any condition that reduces this process places the individual at risk for thermal injury. Poor physical conditioning, fatigue, sleep deprivation, cardiovascular disease, and lack of acclimation all limit the cardiovascular response to heat stress.40,56 Obesity places an individual at risk as a result of reduced cardiac output, the increased energy cost of moving extra mass, increased thermal insulation, and altered distribution of heat-activated sweat glands.72 Older adults and younger individuals show decreased efficiency of thermoregulatory functions and increased risk for heat injury.
Chronic conditions that may contribute to heat illness include diabetes mellitus, diabetes insipidus, spinal cord injury, eating disorders (especially bulimia), and mental retardation. Alcoholism and illicit drug use are among the 10 major risk factors for heatstroke in the general population.58 An important effect of alcohol consumption is inhibition of antidiuretic hormone secretion, which leads to relative dehydration. Autonomic dysfunction, which is present with many chronic diseases, impairs thermoregulation.52
Despite evidence that hypohydration limits physical performance, voluntary dehydration continues to be routine in certain athletic arenas.5,7,20,110 Wrestlers, jockeys, boxers, and bodybuilders commonly lose 3% to 5% of their body mass 1 to 2 days before competition. In addition to restricting fluid and food, they use other pathogenic weight-control measures, such as self-induced vomiting, laxatives, diuretics, and exposure to heat (e.g., saunas, hot tubs, “sauna suits”). Athletes who are undergoing rapid dehydration are at risk not only for heat injury but also for other serious medical conditions, such as pulmonary embolism.29
Box 11-2 highlights common medications that interfere with thermoregulation. Special attention should be paid to the role of antihistamines in reducing sweating. This class of medications is commonly obtained over the counter, so the general population should be warned about the dangers of exercising in the heat when they are taking antihistamines.
BOX 11-2
Drugs That Interfere With Thermoregulation
Although it has been widely believed that sustaining an episode of heatstroke predisposes the individual to future heat injury, this has been refuted in a recent study of heatstroke victims.8 Ten heatstroke patients were tested for their ability to acclimate to heat; by definition, the ability to acclimate to heat indicates heat tolerance. Nine of the patients demonstrated heat tolerance within 3 months after the heatstroke episode; the remaining patient acclimated to heat one year after his heat injury. In no case was heat intolerance permanent. Although individuals may show transient heat intolerance after thermal injury, evidence for permanent susceptibility to thermal injury is lacking.
Adaptation To Environmental Conditions
Clothing
Different regions of the body are not equivalent with regard to sweat production.53 The face and the scalp account for 50% of total sweat production, whereas the lower extremities contribute only 25%. When exercising under conditions of high heat load, maximal sweat evaporation is facilitated by maximal skin exposure. Clothing should be lightweight and absorbent. Although significant improvement has been made in the fabrication of athletic uniforms, the uniforms and protective gear required by certain branches of the military and public safety officers continue to add to the risk of heat injury. Developing protective clothing that permits for more effective heat dissipation is indicated. Uniforms should be modified to decrease the amount of extra protective equipment and head gear needed as much as is safely possible during the first week of training and during times of high heat stress conditions.
Activity
Behavioral actions can effectively minimize the occurrence of classic heatstroke. Lack of residential air conditioning places indigent persons at risk during heat waves. By sitting in a cool or tepid bath periodically throughout the day, the individual can decrease heat stress and thereby prevent heat injury. The more than 10,000 deaths during the 2003 heat wave in Europe could have been reduced by simple announcements by public health officials of this preventive measure. This type of “heat dumping” activity can also include taking cool showers instead of warm showers. In addition, the forearms and hands can be immersed into water that has been cooled to 10° to 20° C (50° to 68° F) for a period of 10 minutes. This action will achieve a reduction in Tco of 0.7° C (1.3° F) and provide the athlete with sustained capability to train in the heat.42
In addition to forearm and hand immersion in cool or cold water, the concept of cooling the palm by various devices, some of which add vacuum pressure to the palm in addition to cooling, has recently become popular. A comprehensive review23 of cooling rates of various cooling modalities has shown that this methodology is no more effective in lowering body core temperature than limb immersion in tap water (15° C [59° F]). Recent research on these devices has shown that the addition of negative pressure with cooling did not enhance any cooling effect.62 In addition, these devices have been shown to be ineffective in slowing the development of hyperthermia when used between exercise bouts4 and in the improvement of high-intensity, intermittent exercise.112 Cold or ice water has been reported to have a cooling power of 5 to 8 times greater than hand/palm cooling devices.23 Given the low cooling power of these devices, their use in a field or athletic setting, especially for treatment of heat injury or illness, should be questioned. This is also true when viewed in terms of cost vs. limb immersion modalities. Regarding use in a wilderness setting, these devices have additional drawbacks including the need for electrical power via batteries and the added weight of transport.
Modification of physical activity should not be based solely on any individual parameter of ambient temperature (Tamb), relative humidity, or solar radiation, because all of these contribute to heat load. The wet bulb globe temperature (WBGT) is an index of heat stress that incorporates all three factors. This value may be calculated (Table 11-2), or it may be obtained directly from portable digital heat-stress monitors that measure all three parameters simultaneously to compute the WBGT. When heat-stress monitors are used, care should be taken to ensure that they are calibrated yearly and that they are not left out in the heat for long periods of time without use. Care should also be taken to ensure that the device measures radiant heat, humidity, air movement, and shaded temperature to calculate WBGT. Devices that measure the heat stress index, relative humidity, and wind speed should not be used to estimate WGBT. Current recommendations from the American College of Sports Medicine for preventing exertional heat illness during workouts and competition are based on the WBGT.3 Most heat injuries occur during cooler WBGT periods as a result of cumulative heat exposure from preceding days. Some clinicians have proposed that heat-warning systems would base their warnings on cumulative heat stress rather than solely on the current WBGT.113 Other clinicians have suggested the use of syndromic surveillance to help alert the public about periods of high heat stress.11
TABLE 11-2 Determination of Wet Bulb Globe Temperature Heat Index
| Temperature (T, in ° F) | Factor | Example |
|---|---|---|
| Wet bulb T | ×0.7 | 78 × 0.7 = 54.6 |
| Dry bulb T | ×0.1 | 80 × 0.1 = 8.0 |
| Black globe T | ×0.2 | 100 × 0.2 = 20.0 |
| Heat index | 82.6 |
Wet bulb reflects humidity, dry bulb reflects ambient air temperature, and black globe reflects radiant heat load; the heat index is the sum of the three.
Alternative equation: Wet bulb globe temperature = (0.567 Tdb) + (0.393 Pa) + 3.94, where Tdb is dry bulb temperature and Pa is water vapor pressure.
Table 11-3 presents a suggested modification of sports activity that is also based on the WBGT. Although American College of Sports Medicine guidelines for the summer indicate that vigorous physical activity should be scheduled in the morning or evening, individuals should be cautioned that the highest humidity of the day is usually during early morning. In 1999, Montain and co-workers70 updated the fluid replacement guidelines for warm-weather training (see Chapter 70, Table 70-5). It is important to note that compliance with these recommendations does not remove all risk of heat injury. The development of another index of heat stress that provides a better basis for the prevention of EHS is indicated.
TABLE 11-3 Modification of Sports Activity on the Basis of Wet Bulb Globe Temperature
| Index | Limitation |
|---|---|
| <10° C (50° F) | There is a low risk for hyperthermia but a possible risk for hypothermia. |
| <18.3° C (65° F) | There is a low risk for heat illness. |
| 18.3°-22.8° C (65°-73° F) | There is a moderate risk toward the end of the workout. |
| 22.8°-27.8° C (73°-82° F) | Those at high risk for heat injury should not continue to train; all athletes should practice in shorts and T-shirts during the first week of training. |
| 27.8°-28.9° C (82°-84° F) | Care should be taken by all athletes to maintain adequate hydration. |
| 28.9°-31.1° C (85°-88° F) | Unacclimated persons should stop training; all outdoor drills in heavy uniforms should be canceled. |
| 31.1°-32.2° C (88°-90° F) | Acclimated athletes should exercise caution and continue workouts only at a reduced intensity; they should wear light clothing only. |
| ≥32.2° C (90° F) | Stop all training. |
Rav-Acha and colleagues85 assembled a case series of six fatal cases of EHS in the Israeli Defense Forces and examined the circumstances that led to the deaths. A significant association between the accumulation of predisposing factors and EHS totality was found. In almost all of the fatal cases, seven predisposing factors were noticed: (1) low physical fitness; (2) sleep deprivation; (3) high heat load; (4) high solar radiation; (5) physical exercise unmatched to physical fitness; (6) absence of proper medical triage; and (7) training during the hottest hours. These fatality factors primarily concerned organizational training regulations and not individual factors, which emphasizes the importance of proper guidelines and safety measures in a warm climate—rather than individual characteristics—for preventing EHS fatalities. It follows that a combination of predisposing factors that were already found to impair heat tolerance40 is a strong predictor of a grave prognosis. Dehydration was found in only two of the six cases reported.
Conditioning
The contribution of cardiovascular conditioning to thermoregulation is discussed in Chapter 4. Ideally, an individual should train under temperate or thermoneutral conditions before exercising in the heat. For the previously sedentary individual, an exercise regimen that incorporates 20 to 30 minutes of aerobic activity 3 to 4 days a week will improve cardiovascular function after 8 weeks.
Acclimatization
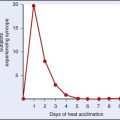
During initial exposure to a hot environment, workouts should be moderate in intensity and duration. A gradual increase in the time and intensity of physical exertion over 8 to 10 days should allow for optimal acclimatization.33 Early season high-school heat-stroke deaths are most likely to occur during the first 4 days of practice87; children require 10 to 14 days to achieve an appropriate acclimatization response. Acclimatization can be induced by simulating hot environmental conditions indoors. Aerobic activity should be conducted during exposure to the hot environment so that the individual can achieve optimal acclimatization.90 If symptoms of heat illness develop during the acclimation period, all physical activity should be stopped and appropriate interventions begun. Acclimatization is not facilitated by restricting fluid intake; in fact, conscious attention to fluid intake is required to prevent dehydration. As with physical conditioning, there are limits to the degree of protection that acclimatization provides from heat stress. Given a sufficiently hot and humid environment, no one is immune to heat injury.
1 Abderrezak B, Al-Hussein K, Adra C, et al. Distribution of peripheral blood leucocytes in acute heat stroke. J Appl Physiol. 1992;73:405.
2 Al-Khawashki MI, Mustafa MKY, Khogali M, et al. Clinical presentation of 172 heat stroke cases seen at Mina and Arafat. In: Khogali M, Hales JRS, editors. Heat stroke and temperature regulation. New York: Academic Press, 1983.
3 American College of Sports MedicineArmstrong LE, Casa DJ, et al. American College of Sports Medicine position stand: exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39:556.
4 Amorim FT, Yamada PM, Robergs RA, et al. Palm cooling does not reduce heat strain during exercise in a hot, dry environment. Appl Physiol Nutr Metab. 2010;35:480.
5 Amoroso P, Yore MM, Smith GS, et al. Analysis of military occupational specialties and hospitilization: Part 1. The 25 largest Army enlisted occupations, Technical Report T98-7. Natick Mass: U.S. Army Research Institute of Environmental Medicine; 1997.
6 Appenzeller O, Atkinson R. Sports medicine: Fitness training injuries. Baltimore: Urban and Schwarzenberg; 1981. pp 11-33
7 Armstrong LE, Costill DL, Fink WJ. Influence of diuretic-induced dehydration on competitive running performance. Med Sci Sports Exerc. 1985;17:456.
8 Armstrong LE, De Luca JP, Hubbard RW. Time course of recovery and heat acclimation ability of prior exertional heatstroke patients. Med Sci Sports Exerc. 1990;22:36.
9 Arrich J, Holzer M, Herkner H, et al: Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 7:CD004128, 2009.
10 Assia E, Epstein Y, Shapiro Y. Fatal heatstroke after a short march at night: A case report. Aviat Space Environ Med. 1985;56:441.
11 Bassil K. The relationship between temperature and 911 medical dispatch data for heat-related innless in Toronto, 2002-2005: An application of syndromic surveillance. Toronto: University of Toronto Research Repository; 2010.
12 Berger J, Hart J, Millis M, et al. Fulminant hepatic failure from heat stroke requiring liver transplantation. J Clin Gastroenterol. 2000;30:429.
13 Bergeron MF. Heat cramps: Fluid and electrolyte challenges during tennis in the heat. J Sci Med Sport. 2003;6:19.
14 Berkelhammer C, Bear RA. A clinical approach to common electrolyte problems: 4. Hypomagnesemia. Can Med Assoc J. 1985;132:360.
15 Bilbey DL, Prabhakaran VM. Muscle cramps and magnesium deficiency: Case reports. Can Fam Physician. 1996;42:1348.
16 Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest. 2009;136:e28.
17 Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978.
18 Brock-Utne JG, Gaffin SL, Wells MT, et al. Endotoxaemia in exhausted runners after a long-distance race. S Afr Med J. 1988;73:533.
19 Brown TP. Exertional rhabdomyolysis: Early recognition is key. Phys Sportsmed. 2004;32:15.
20 Caldwell JE. Diuretic therapy and exercise performance. Sports Med. 1987;4:290.
21 Carter BJ, Cammermeyer M. A phenomenology of heat injury: The predominance of confusion. Mil Med. 1988;153:118.
22 Carter RIII, Cheuvront SN, Williams JO, et al. Hospitalizations and death from heat illness in U.S. Army soldiers, 1980-2002. Med Sci Sports Exerc. 2005;37:1338.
23 Casa DJ, Armstrong LE, Ganio MS, et al. Exertional heat stroke in competitive athletes. Curr Sports Med Rep. 2005;4:309.
24 Casa DJ, McDermott BP, Lee EC, et al. Cold water immersion: The gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35:141.
25 Channa AB, Seraj MA, Saddique AA, et al. Is dantrolene effective in heat stroke patients? Crit Care Med. 1990;18:290.
26 Chao NHH. Clinical presentation of heat disorders. Yeo PPB. Lin NK. Headquarters Medical Services, Heat disorders, Singapore, 1985..
27 Chinet A, Giovannini P. Evidence by calorimetry for an activation of sodium-hydrogen exchange of young rat skeletal muscle in hypertonic media. J Physiol. 1989;415:409.
28 Clowes GHII, O’Donnell TFII. Heat stroke. N Engl J Med. 1974;291:564.
29 Croyle PH, Place RA, Hilgenberg AD. Massive pulmonary embolism in a high school wrestler. JAMA. 1979;241:827.
30 Dematte JE, O’Mara K, Buescher J, et al. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med. 1998;129:173.
31 Denborough MA. Heatstroke and malignant hyperpyrexia. Med J Aust. 1982;1:204.
32 Du Bois EF. The basal metabolism in fever. JAMA. 1921;77:352.
33 Eichna LW, Park CR, Nelson N, et al. Thermal regulation during acclimatization in a hot, dry (desert type) environment. Am J Physiol. 1950;163:585.
34 el-Kassimi FA, Al-Mashhadani S, Abdullah AK, et al. Adult respiratory distress syndrome and disseminated intravascular coagulation complicating heat stroke. Chest. 1986;90:571.
35 Ellis FP. Mortality from heat illness and heat-aggravated illness in the United States. Environ Res. 1972;5:1.
36 Epstein Y, Moran DS. Extremes of temperature and hydration. In: Keystone JS, Kozarsky PE, Freedman DO, editors. Travel medicine. London: Mosby, 2003.
37 Epstein Y, Moran DS, Shapiro Y, et al. Exertional heat stroke: A case series. Med Sci Sports Exerc. 1999;31:224.
38 Epstein Y, Sohar E, Shapiro Y. Exertional heatstroke: A preventable condition. Isr J Med Sci. 1995;31:454.
39 Garcin JM, Bronstein JA, Cremades S, et al. Acute liver failure is frequent during heat stroke. World J Gastroenterol. 2008;14:158.
40 Gardner JW, Kark JA, Karnei K, et al. Risk factors predicting exertional heat illness in male Marine Corps recuits. Med Sci Sports Exerc. 1996;28:939.
41 Gathiram P, Wells MT, Raidoo D, et al. Changes in lipopolysaccharide concentrations in hepatic portal and systemic arterial plasma during intestinal ischemia in monkeys. Circ Shock. 1989;27:103.
42 Giesbrecht GG, Jamieson C, Cahill F. Cooling hyperthermic firefighters by immersing forearms and hands in 10 degrees C and 20 degrees C water. Aviat Space Environ Med. 2007;78:561.
43 Graber CD, Reinhold RB, Breman JG, et al. Fatal heat stroke: Circulating endotoxin and gram-negative sepsis as complications. JAMA. 1971;216:1195.
44 Greenleaf JE, Castle BL. External auditory canal temperature as an estimate of core temperature. J Appl Physiol. 1972;32:194.
45 Hadad E, Ben-Ari Z, Heled Y, et al. Liver transplantation in exertional heat stroke: A medical dilemma. Intensive Care Med. 2004;30:1474.
46 Hammarstrom S, Hua XY, Dahle’n SE, et al. Microcirculatory effects of leukotrienes C4, D4, and E4 in the guinea pig. Prog Clin Biol Res. 1985;199:35.
47 Hassanein T, Perper JA, Tepperman L, et al. Liver failure occurring as a component of exertional heatstroke. Gastroenterology. 1991;100:1442.
48 Hassanein T, Razack A, Gavaler JS, et al. Heatstroke: Its clinical and pathological presentation, with particular attention to the liver. Am J Gastroenterol. 1992;87:1382.
49 Heled Y, Rav-Acha M, Shani Y, et al. The “golden hour” for heatstroke treatment. Mil Med. 2004;169:184.
50 Hemmen TM, Lyden PD. Induced hypothermia for acute stroke. Stroke. 2007;38:794.
51 Henderson A, Simon JW, Melia WM, et al. Heat illness: A report of 45 cases from Hong Kong. J R Army Med Corps. 1986;132:76.
52 Hensel HT. Neural processes in thermoregulation. Physiol Rev. 1973;53:948.
53 Herrmann F, Prose PH, Sulzberger MB. Studies on sweating. V. Studies of quantity and distribution of thermogenic sweat delivery to the skin. J Invest Dermatol. 1952;18:71.
54 Hori S. Adaptation to heat. Jpn J Physiol. 1995;45:921.
55 Huisse MG, Pease S, Hurtado-Nedelec M, et al. Leukocyte activation: the link between inflammation and coagulation during heatstroke: A study of patients during the 2003 heat wave in Paris. Crit Care Med. 2008;36:2288.
56 Kark JA, Burr PQ, Wenger CB, et al. Exertional heat illness in Marine Corps recruit training. Aviat Space Environ Med. 1996;67:354.
57 Khogali M. Heat stroke: An overview. In: Khogali M, Hales JRS, editors. Heat stroke and temperature regulation. New York: Academic Press, 1983.
58 Kilbourne EM, Choi K, Jones TS, et al. Risk factors for heatstroke: A case-control study. JAMA. 1982;247:3332.
59 Knochel JP. Environmental heat illness: An eclectic review. Arch Intern Med. 1974;33:841.
60 Knochel JP. Heat stroke and related heat stress disorders. Dis Mon. 1989;35:301.
61 Knochel JP, Reed G. Disorders of heat regulation. In: Maxwell MH, Kleema CR, Narins RG, editors. Clinical disorders of fluid and electrolyte metabolism. New York: McGraw-Hill; 1987:1197-1232.
62 Kuennen MR, Gillum TL, Amorim FT, et al. Palm cooling to reduce heat strain in subjects during simulated armoured vehicle transport. Eur J Appl Physiol. 2010;10:1217.
63 Leight AS, Sinclair WH, Patterson MJ. Influence of postexercise cooling techniques in heart rate variability in men. Exp Physiol. 2009;94:695.
64 Lipsker D. Images in clinical medicine: A white hand and a red hand-erythromelalgia. N Engl J Med. 2010;363:1463.
65 Lopez JR, Rojas B, Gonzalez MA, et al. Myoplasmic Ca2+ concentration during exertional rhabdomyolysis. Lancet. 1995;345:424.
66 Lydiatt JS, Hill GE. Treatment of heat stroke with dantrolene. JAMA. 1981;246:41.
67 Malamud N, Haymaker W, Custer RP. Heatstroke: A clinicopathological study of 125 fatal cases. Mil Surg. 1946;99:397.
68 McCaffrey TV, McCook RD, Wurster RD. Effect of head skin temperature on tympanic and oral temperature in man. J Appl Physiol. 1975;39:114.
69 Mehta AC, Baker RN. Persistent neurological deficits in heat stroke. Neurology. 1970;20:336.
70 Montain SJ, Latzka WA, Sawka MN. Fluid replacement recommendations for training in hot weather. Mil Med. 1999;164:502.
71 Moran D, Epstein Y, Wiener M, et al. Dantrolene and recovery from heat stroke. Aviat Space Environ Med. 1999;70:987.
72 Moran DS, Montain SJ, Pandolf KB. Evaluation of different levels of hydration using a new physiological strain index. Am J Physiol. 1998;275:R854.
73 Moran DS, Pandolf KB. Wet bulb globe temperature (WBGT): To what extent is GT essential? Aviat Space Environ Med. 1999;70:480.
74 Mustafa MK, Khogali M, Gumaa K. Respiratory pathophysiology in heat stroke. In: Khogali M, Hales JRS, editors. Heat stroke and temperature regulation. New York: Academic Press, 1983.
75 Nemeth ZH, Hasko G, Szabo C, et al. Calcium channel blockers and dantrolene differentially regulate the production of interleukin-12 and interferon-gamma in endotoxemic mice. Brain Res Bull. 1998;46:257.
76 Nolan JP, Morley PT, Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: An advisory statement by the Advancement Life support Task Force of the International Liaison committee on Resuscitation. Resuscitation. 2003;57:231.
77 Null J. Hypothermia deaths of children in vehicles. Department of Geosciences, San Francisco State University, 2010 http://ggweather.com/heat/
78 O’Donnell TF. Medical problems of recruit training: A research approach. US Navy Med. 1971;58:28.
79 O’Brien KK. Case studies of exertional heat injury/stroke in military populations. Med Sci Sports Exerc. 2003;35:5.
80 Oh RC, Henning JS. Exertional heatstroke in an infantry soldier taking ephedra-containing dietary supplements. Mil Med. 2003;168:429.
81 Pattison ME, Logan JL, Lee SM, et al. Exertional heat stroke and acute renal failure in a young woman. Am J Kidney Dis. 1988;11:184.
82 Pettigrew RT, Galt JM, Ludgate CM, et al. Circulatory and biochemical effects of whole body hyperthermia. Br J Surg. 1974;61:727.
83 Proulx CI, Ducharme MB, Kenny GP. Effect of water temperature on cooling efficiency during hyperthermia in humans. J Appl Physiol. 2003;94:1317.
84 Rajan SF, Robinson GH, Borwer JD. The pathogenesis of acute renal failure in heatstroke. South Med J. 1973;66:330.
85 Rav-Acha M, Hadad E, Epstein Y, et al. Fatal exertional heat stroke: A case series. Am J Med Sci. 2004;328:84.
86 Roberts WO. A 12-yr profile of medical injury and illness for the Twin Cities Marathon. Med Sci Sports Exerc. 2000;32:1549.
87 Roberts WO. Death in the heat: Can football heat stroke be prevented? Curr Sports Med Rep. 2004;3:1.
88 Royburt M, Epstein Y, Solomon Z, et al. Long-term psychological and physiological effects of heat stroke. Physiol Behav. 1993;54:265.
89 Saissy JM. Liver transplantation in a case of fulminant liver failure after exertion. Intensive Care Med. 1996;22:831.
90 Sawka MN, Young AJ. Physiological systems and their responses to conditions of heat and cold. In: Tipton CM, Sawka MN, Tate CA, et al, editors. ACSM’s advanced exercise physiology. Baltimore: Lippincott Williams & Wilkins, 2006.
91 Sayers SP, Clarkson PM. Exercise-induced rhabdomyolysis. Curr Sports Med Rep. 2002;1:59.
92 Schrier RW, Hano J, Keller HI, et al. Renal, metabolic, and circulatory responses to heat and exercise. Ann Intern Med. 1970;73:213.
93 Seto CK, Way D, O’Connor N. Environmental illness in athletes. Clin Sports Med. 2005;24:695.
94 Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574.
95 Shapiro Y, Cristal N. Hyperthermia and heat stroke: Effects on acid-base balance, blood electrolytes and hepato-renal function. In: Hales JRS, Richards D, editors. Heat stress: Physical exertion and environment. New York: Elsevier, 1987.
96 Shapiro Y, Moran DS. Heat stroke: A consequence of mal-adaptation to heat-exercise exposure. In: Pandolf KB, Takeda N, Singal PK, editors. Adaptation biology and medicine: Molecular bases. New Delhi: Narosa, 1999.
97 Shapiro Y, Rosenthal T, Sohar E. Experimental heatstroke: A model in dogs. Arch Intern Med. 1973;131:688.
98 Shapiro Y, Seidman DS. Field and clinical observations of exertional heat stroke patients. Med Sci Sports Exerc. 1990;22:6.
99 Shibolet S: The clinical picture of heat stroke: Proceedings of the Tel HaShomer Hospital. Tel Aviv, Israel, 1962, pp 80-83.
100 Shibolet S, Coll R, Gilat T, et al. Heatstroke: Its clinical picture and mechanism in 36 cases. Q J Med. 1967;36:525.
101 Shibolet S, Fisher S, Gilat T, et al. Fibrinolysis and hemorrhages in fatal heatstroke. N Engl J Med. 1962;266:169.
102 Shibolet S, Lancaster MC, Danon Y. Heat stroke: A review. Aviat Space Environ Med. 1976;47:280.
103 Silverstein JH, Apfelbaum JL, Barlow JC, et al. Practical guidelines for postanasthetic care. Anesthesiology. 2002;96:742.
104 Sohal RS, Sun SC, Colcolough HL, et al. Heat stroke: An electron microscopic study of endothelial cell damage and disseminated intravascular coagulation. Arch Intern Med. 1968;122:43.
105 Sprung CL, Portocarrero CJ, Fernaine AV, et al. The metabolic and respiratory alterations of heat stroke. Arch Intern Med. 1980;140:665.
106 Stofan J, Niksich D, Horswill CA, et al. Sweat and sodium losses in cramp-prone professional football players. Med Sci Sports Exerc. 2001;33:S256.
107 Strazis KP, Fox AW. Malignant hyperthermia: A review of published cases. Anesth Analg. 1993;77:297.
108 Strydom NB, Morrison J, Booyens J, et al. Comparison of oral and rectal temperatures during work in heat. J Appl Physiol. 1956;8:406.
109 Tayeb OS, Marzouki ZM. Effect of dantrolene pretreatment on heat stroke in sheep. Pharmacol Res. 1990;22:565.
110 Torranin C, Smith DP, Byrd RJ. The effect of acute thermal dehydration and rapid rehydration on isometric and istonic endurance. J Sports Med Phys Fitness. 1979;19:1.
111 Vertel RM, Knochel JP. Acute renal failure due to heat injury: An analysis of ten cases associated with a high incidence of myoglobinuria. Am J Med. 1967;43:435.
112 Walker TB, Zupan MF, McGregor JN, et al. Is performance of intermittent intense exercise enhanced by use of a commercial palm cooling device? J Strength Cond Res. 2009;23:2666.
113 Wallace RF, Kriebel D, Punnett L, et al. Prior heat illness hospitalization and risk of early death. Environ Res. 2007;104:290.
114 Ward A, Chaffman MO, Sorkin EM. Dantrolene. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in malignant hyperthermia, the neuroleptic malignant syndrome and an update of its use in muscle spasticity. Drugs. 1986;32:130.
115 Weigand K, Riediger C, Stremmel W, et al. Are heat stroke and physical exhaustion underestimated causes of acute hepatic failure? World J Gastroenterol. 2007;13:306.
116 Yaqub BA. Neurologic manifestations of heatstroke at the Mecca pilgrimage. Neurology. 1987;37:1004.
117 Zuckerman GB, Singer LP, Rubin DH, et al. Effects of dantrolene on cooling times and cardiovascular parameters in an immature porcine model of heatstroke. Crit Care Med. 1997;25:135.