CHAPTER 225 Clinical Features and Management of Cerebral Palsy
Cerebral palsy (CP) is “a group of disorders affecting the development of movement and posture, causing activity limitation, that are attributed to non progressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of cerebral palsy are often accompanied by disturbances of sensation, cognition, communication, perception and/or behavioral and/or by a seizure disorder.”1
The estimated prevalence of CP is 1.5 to 3.0 per 1000 live births worldwide, with differences among countries, racial/ethnic groups, and socioeconomic classes.2,3 In most cases the cause of CP is not well defined, and it may vary with the subtype; prematurity and low birth weight are major risk factors for CP, especially bilateral spastic CP associated with periventricular white matter injury. Although birth asphyxia is uncommon, it is associated with the dyskinetic CP subtypes.3 Multiple genetic and metabolic factors may predispose infants to an increased risk for brain injury and CP.4
Diagnosis and Classification
Children with CP have a delay in motor development and abnormal motor findings on examination. Evaluation begins with a detailed history and physical and neurological examination, including careful documentation of prenatal, perinatal, and neonatal events and risk factors, developmental milestones, family history, general medical history, and a detailed survey to look for associated impairments (e.g., seizures, language disturbance, vision and hearing loss, nutritional difficulties). All CP patients should undergo magnetic resonance imaging, which may help reveal the underlying brain injury,5 although neither imaging nor laboratory tests can prove the diagnosis. An atypical history, clinical features, or imaging findings should trigger further evaluation, especially if there is any history of developmental regression or loss of skills.3,6 Disorders that masquerade as CP include familial spastic paraparesis and dopa-responsive dystonia. Some patients, including those with typical risk factors for CP (twin gestation, prematurity, periventricular leukomalacia) have “CP plus” and are found to have a coexisting metabolic disorder (e.g., mitochondrial encephalomyopathy).
There is currently no universally accepted classification system for CP, but since the mid-1900s, CP has most commonly been classified into “spastic” or “dyskinetic” subtypes based on the predominant type of tone abnormalities or movement disorders, or both, present. Spastic CP is the most common and is further divided into spastic diplegia (legs more than the arms), spastic quadriplegia (arms equal to or more than the legs), and spastic hemiplegia (involvement of one side of the body). Dyskinetic CP is divided into dystonic and choreoathetoid forms. A small subset of patients may be classified as ataxic. However, there is great disparity among practitioners regarding the use of these terms, such that a given patient may be classified differently by different clinicians.7 This is a major weakness in the field, and correcting it is a priority for furthering research and treatment.
In 2000, the Surveillance for Cerebral Palsy in Europe group adopted a standardized system for database and registry purposes that is used throughout Europe and Australia. Patients are classified as “spastic bilateral” or “spastic unilateral.” Individuals with variable tone are classified as “dyskinetic” and then further classified as dystonic or choreoathetoid/hyperkinetic. Those with hypotonia and ataxia are classified as “ataxic.” Some patients are nonclassifiable. Additionally, clinically useful functional scales for both gross motor function (Gross Motor Function Classification Scale [GMFCS],8 Functional Mobility Scale9) and fine motor/hand function (Manual Ability Classification Scale,10 Bimanual Function Measure11) are now available to allow improved description of motor function in patients with CP. A Communication Function Classification System is currently being validated (M. J. Cooley Hidecker, personal communication).
Associated Impairments
Neurological Impairments
Sensation
Visual disorders are common in children with CP but are often missed or inaccurately diagnosed.12 Over half of children with spastic CP and no obvious visual deficit will nonetheless have visual perception disorders.13 The severity of the visual deficit tends to correlate with the severity of CP involvement and is increased with a history of prematurity, severity of periventricular leukomalacia, and the presence of dyskinetic CP.12,14
Children with multiple visual impairments are often misdiagnosed as having untreatable “cortical visual impairment,” which leads to devastating consequences on communication, learning, social interactions, and quality of life. True cortical visual impairment affects approximately 16% of patients.12 Detailed assessment may be required to untangle the various contributors to the visual impairment but may lead to dramatic improvement in vision through surgery (e.g., strabismus correction, lens replacement, laser treatment) and corrective lenses.
Even children with the mildest spastic CP are likely to have deficits in tactile and proprioceptive sensation,15 and their severity may correlate with the severity of the motor dysfunction.16
Communication
Communication difficulties are present in nearly 60% of all patients with CP, including up to 90% of children with quadriplegic or dyskinetic CP, or both.17
Epilepsy
Approximately a third of all children with CP have epilepsy. The prevalence varies with the subtype of CP, from 16% in children with diplegic CP to 50% in those with quadriplegic CP.17 The prognosis depends on the subtype of CP, brain imaging abnormality, and cognitive ability. Children with CP and epilepsy are much less likely to achieve freedom from seizures with or without antiepileptic drugs, and they are more likely to require polytherapy.18
Cognition/Behavior
Median intelligence quotient (IQ) scores are lower in children with CP, with 40% to 65% having an estimated IQ of less than 70. Detailed neuropsychological testing should be performed before a child starts school. Accurate assessment is difficult if not impossible in many children because of their multiple other impairments. Thus, low estimates of intellect should be interpreted cautiously. Children with severe CP and limited or absent communication skills often have spared nonverbal reasoning abilities. Conversely, nonverbal learning disorders may be missed in a child with superior verbal skills.19
Health Impairments
Impaired motor function leads to significant health problems. Children with severe mobility impairments tend to be less healthy and spend more time in bed and more days in the hospital, are more likely to be malnourished, and are often taking more medications than their nondisabled peers.20,21 Additionally, risk for fracture, hip dislocation, and scoliosis is elevated in nonambulatory patients with CP.22–24
Gastrointestinal
Moderate gastrointestinal symptoms occur in up to 90% of children with CP, including one or more of the following: drooling, swallowing problems (30% to 60%), regular vomiting (30%), gastroesophageal reflux disease, and delayed gastric emptying (70%).25–28 Even mild feeding dysfunction is significant and associated with lower body weight and reduced body fat stores.
Renal
Approximately two thirds of patients with CP have symptoms of voiding dysfunction, including difficulty voiding and daytime urinary incontinence. The prevalence of significant pathology (increased detrusor muscle contraction, reduced detrusor muscle compliance, reduced bladder capacity, inability to relax the external urethral sphincter, and high postvoid residuals) may be as high as 97%29–33 and can increase the risk for progressive decline in upper urinary tract function and renal disease.34,35 Urinary tract infections are also common in children with CP29,36 and can lead to scarring and chronic renal insufficiency.37
Improving Mobility and Motor Function (“I Want to Walk”): How Do We Help Them?
Improving a child’s ability to move is a major goal for most parents (and children). Children enjoy moving! The secondary consequences of immobility are high. Most children with CP have inadequate physical fitness, which further contributes to their poor health, pain, and secondary impairments.38
Physical and Occupational Therapy
Physical and occupational therapy occurs in many different settings, including traditional (home, school, private clinic) and nontraditional (e.g., therapeutic horseback riding39,40 and adapted sports). The therapist has a pivotal role as a teacher to help patients master new motor skills and teach parents (and older patients) exercises (stretching, strengthening, balance) and adaptive techniques (assistive technology for activities of daily living, access to computers or communication devices) that they can integrate into their daily life.
A variety of new tools are available to the physical therapist, such as electrical stimulation, which may have a positive effect on range of motion,41–43 and treadmill training with partial body weight support, which may be beneficial for a moderately severely impaired child (GMFCS III or IV).44 Ankle-foot orthoses are commonly prescribed for children who have a dynamic equinus abnormality, may be more effective in younger children,45 and can improve their ability to perform sit-to-stand transitions.46 The most effective type and “dose” or frequency of physical therapy remains controversial. Recent studies suggest that intermittent bursts of intense therapy for a few weeks with several weeks off between sessions may be as effective as continuous therapy 1 to 2 times per week.47
In the upper extremity, constraint-induced therapy and forced use have re-emerged as a useful tool. In clinical trials, restraint of the child’s better functioning hand/upper limb in combination with occupational therapy improves function in the affected limb (new motor skills and increased dexterity) for at least 6 months.48–52 Repeat courses of treatment lead to additional improvement.53 Botulinum neurotoxin (BoNT) combined with occupational therapy is another effective strategy.54 Recent, exciting advances include the use of robotics to improve upper extremity function.55
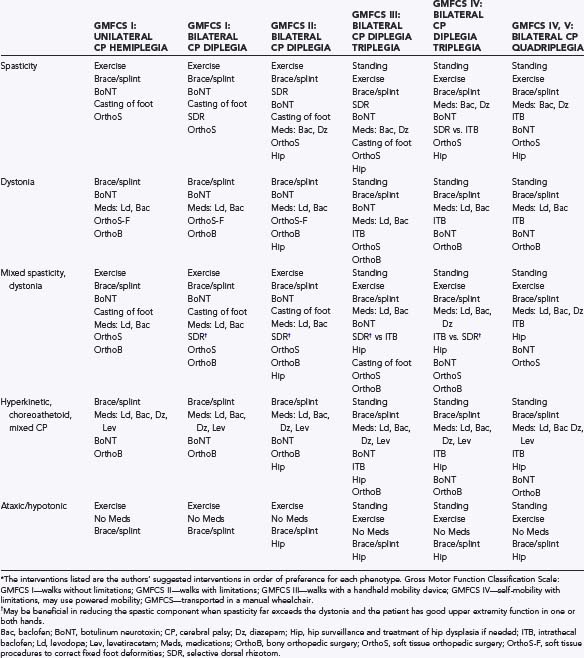
Therapy programs along with overall management of a child’s hypertonia and mobility impairments must be goal-driven and tailored specifically to each child’s needs (Table 225-1). For example, children with spasticity need regular stretching of spastic muscles to optimize range of motion and prevent contractures. Strengthening exercises to reverse disuse atrophy may improve gait and do not worsen the spasticity.56–59 Conversely, in children with generalized dystonia and hyperkinetic CP who are constantly moving or contracting their muscles, both stretching and strengthening may prove impractical if not impossible.
Management of Hypertonia and Spasticity in Children with Cerebral Palsy
Hypertonia is defined as abnormally increased resistance to externally imposed movement about a joint. The Task Force on Childhood Motor Disorders recently developed a consensus definition of spasticity as “hypertonia in which one or both of the following signs are present: (1) resistance to externally imposed movement increases with increasing speed of stretch and varies with the direction of joint movement, and/or (2) resistance to externally imposed movement rises rapidly above a threshold speed or joint angle.”60
Oral Medications
Baclofen
Baclofen is a γ-aminobutyric acid type B receptor (GABAB) agonist that is well recognized to be efficacious for spasticity of spinal cord origin in adults. There are limited studies in childhood addressing the use of oral baclofen and even fewer double-blind, placebo-controlled studies. Milla and Jackson showed in a double-blind crossover trial that baclofen reduces spasticity and allows more passive and active movements than placebo does.61 However, in a separate trial, there was no effect measured by the Pediatric Evaluation of Disability Inventory or the Modified Tardieu Scale. On the Goal Attainment Scale there was some functional improvement.62
Reports of side effects with baclofen use are varied. Confusion and sedation are a concern, although this tends to improve with use. The initial dose is traditionally 2.5 mg/day and is gradually increased up to a maximum of 20 to 60 mg/day.63 As with most medications, baclofen must be weaned slowly to avoid a withdrawal syndrome, which includes increased spasticity, mental confusion, and possibly seizures.64
Tizanidine
Tizanidine is a centrally acting α2-adrenergic agonist that reduces tonic stretch reflexes by presynaptic inhibition. Only one English-language trial of tizanidine in children has been published, and it showed that tizanidine was superior to oral baclofen as an adjunct to BoNT-A in patients with gastrocnemius spasticity, as measured by the Gross Motor Function Measure and caregiver questionnaire.65 Sedation and frequent dosing have been reported as limiting factors in adults, although in patients who have difficulty with sleep initiation, the sedative quality can actually be an advantage.
Diazepam
Diazepam is a benzodiazepine that acts by facilitating the postsynaptic action of GABA. Studies from the 1960s indicate its capacity to reduce spasticity. More recently, Mathew and colleagues compared diazepam with placebo in 180 children and demonstrated its ability to reduce muscle overactivity.66 A comparison of diazepam and dantrolene showed that both agents were equally effective and that a combination of the two was superior to either alone.67 The usual dosage of diazepam is 0.12 to 0.8 mg/kg up to the adult maximum. This dosage is divided into three to four doses across the day. Sedation is a known side effect of the benzodiazepines. Thus, use as a sleep aid may also be an added advantage.
Dantrolene Sodium
Dantrolene inhibits release of calcium from the sarcoplasmic reticulum. In two double-blind, crossover studies, spasticity was reported to be reduced67,68; however, in another placebo-controlled study, the reduction in spasticity could not be verified.69 The side effect profile is also a limiting factor. Diffuse weakness and sedation despite the peripheral mode of action have been noted. Moreover, hepatotoxicity is a concern for adults and thus considered a risk factor for children as well.
Focal Spasticity
Botulinum Toxin
Well in excess of 100 studies involving BoNT-A in children with CP have been published, the majority of which were positive, with spasticity significantly reduced even at 3 months after injection.70 Functional improvements after BoNT injection have been demonstrated in the lower71–73 and upper53 extremities. BoNT has also been used to reduce pain.74
Dosing recommendations for BoNT-A as Botox have been developed over time by consensus.75 Dosing must be individualized and is based on multiple parameters, including the child’s weight, muscle bulk, degree of spasticity, and previous response to therapy, if any. The lowest effective dose should be used, and an injection interval of at least 3 months is recommended to minimize the risk for antibody formation. Most children tolerate the injections because of the very short time frame required to complete them. Some children may need a topical anesthetic or light sedation.
Adverse effects of BoNT injections are usually mild and transient. There is pain at the time of injection, and a mild flu-like syndrome has been reported. Excessive weakness has also been reported, as has migration of medication, as mentioned earlier.76,77
Serial Casting
Serial casting of the ankle is an effective treatment of ankle equinus and, when combined with BoNT injections, produces results superior to those of BoNT alone.78 It has been recommended that casting should follow injections by 2 to 4 weeks to make use of the peak effect of the toxin.79
Multisegmental and Generalized Spasticity
Selective Dorsal Rhizotomy
Selective dorsal rhizotomy (SDR) has been used to treat moderate to severe lower extremity spasticity since the 1980s (see Chapter 227).80–83 A patient who is selected for SDR is typically ambulatory or nearly ambulatory (GMFCS II to III, sometimes GMFCS I) and between 4 and 7 years of age, has spared cognitive skills and is motivated to walk, has some degree of preserved selective motor control in the lower extremities, has adequate strength, and exhibits minimal if any dystonia. Additionally, it is critical that the family commit to a rigorous daily physical therapy program for 6 to 12 months.
Intrathecal Baclofen
Intrathecal baclofen is a technique in which a very low dose of baclofen is delivered into the intrathecal space by way of a catheter attached to a programmable, implantable pump (see Chapter 226).84,85 The dose is less than 1% of that delivered orally because of the direct delivery system to the central nervous system; such delivery reduces the principal side effect of sedation. Intrathecal baclofen is generally a consideration in children who have multisegmental or generalized spasticity/hypertonia, particularly those in whom lower extremity spasticity is a major component. Upper extremity spasticity can also be treated, particularly when the catheter tip is placed at the high thoracic or even cervical level.
Orthopedic Surgery
Despite aggressive therapies and management of spasticity/hypertonia, many children with spastic CP eventually require orthopedic surgery to treat secondary deformities (muscle contractures, hip dysplasia, bony deformities).86 Correction of hip subluxation typically involves adductor tenotomies, femoral osteotomy, or acetabular repair (or any combination of these procedures). An equinovarus foot is the most common deformity in CP and can become fixed and refractory to bracing, casting, or BoNT. Surgical treatments include tendon- or muscle-lengthening procedures, or both, to restore heel cord range. In children with severe varus posturing of the foot (as can be seen in focal spasticity or mixed spastic-dystonic disorders), split-tendon transfers may be of some benefit. Progressive hamstring contractures can occur after growth spurts, and hamstring tenotomies are commonly performed in adolescent patients with CP. Osteotomies to derotate the femur or tibia may be needed to correct rotational deformities when internal or external foot progression impedes gait or these deformities place undue strain on the knee.
Dystonia and Mixed Movement Disorders in Cerebral Palsy
Dystonia is defined as a movement disorder in which involuntary sustained or intermittent muscle contractions cause twisting and repetitive movements, abnormal postures, or both.60 Dystonia is often due to basal ganglia injury.
Dystonia may not be evident early in a patient’s life (before the age of 3 or 4 years) and may then evolve and progress over time.87–89 Patients may manifest significant dystonia at 12 or 13 years of age. This has obvious implications when considering that earlier interventions such as SDR might have been performed earlier in life. The development of unrecognized dystonia may lead to the erroneous conclusion that SDR had failed.
Dystonia can severely interfere with a patient’s daily function, and treatment choices are limited. Oral medications are often used but have been of controversial efficacy. Diazepam in particular has been tried in children with this movement disorder, but its clinical efficacy in ameliorating athetosis indicates that generalized relaxation is a major component of its action.90 A recent open-label, multicenter, clinical trial of trihexyphenidyl in children with CP showed some evidence of efficacy in the upper extremity 15 weeks after starting treatment, but not at 9 weeks. Children with hyperkinetic-dystonic movement disorders appeared to worsen during trihexyphenidyl therapy but returned to baseline after tapering from the medication.91 An additional pilot study of 14 children with generalized dystonia treated with trihexyphenidyl revealed improvement in both the Goal Attainment Scale and Canadian Occupational Performance Measure but not in the primary outcome measure, the Barry-Albright Dystonia Score.92 Side effects were frequent in both studies.
Levodopa/carbidopa has been used clinically because of its known action on other movement disorders, but clinical trials in children with CP are lacking. Levetiracetam has recently been reported to be helpful in managing paroxysmal kinesigenic choreoathetosis93 and may improve balance and fine motor skills in children with choreoathetosis.94–96
Tetrabenazine, a monoamine-depleting medication, has been used for multiple hyperkinetic movement disorders for more than 30 years. It has recently been approved in the United States for use in Huntington’s disease. Its use in patients with CP and associated hyperkinetic movement disorders has been anecdotal.97,98
Oral baclofen has been used for hyperkinetic movement disorders with minimal success by clinical report. Several studies have reported improvement of generalized secondary dystonia with intrathecal baclofen: improved comfort and ease of care in 85% and functional improvement in approximately 33%.99 Interesting clinical data have come from an open-label study of 10 patients with either severe secondary generalized or heredodegenerative dystonia and the use of intraventricular baclofen for dystonia.100 Eight of the 10 patients responded to the infusion with significant improvement as measured by the Barry-Albright Dystonia Score. The medication is hypothesized to act at the cortical level.
Bilateral pallidal deep brain stimulation has also gained interest in the care of children and adults with CP and associated dystonia after its successful use for many cases of idiopathic and secondary dystonia of other causes.101,102 In a prospective pilot multicenter study of 13 adult patients with dystonic-choreoathetoid CP, improvements were seen in the Burke-Fahn-Marsden dystonia rating scale, as well as measures of functional disability, pain, and mental health–related quality of life at 1 year. The posterolateroventral region of the globus pallidus pars interna was deemed the optimal target.102
Conclusion
The highest standard of care for children with CP requires a team of experts working together who understand the importance of a “leave-no-stone-unturned” approach to systematically identify and treat the motor disorders, other neurological disorders, and myriad associated impairments affecting these children. There is a great dichotomy in the treatment and prognosis of children with different functional levels and CP subtypes. Ambulatory children with mild spastic CP have more options from which to choose and are likely to do well.103 Treatment options for children with severe motor impairment and complicated mixed movement disorders are much fewer and less effective, and these children are at higher risk for medical complications and poor health. The neurosurgeon has the capacity to make a powerful impact on the lives of these children. Procedures such as SDR and intrathecal baclofen in the right clinical setting can change the life of a child for the better. Yet there is much more work to be done to develop neurosurgical techniques that will reach even the most severely disabled children who are currently “locked in” their uncooperative bodies waiting for their chance at full participation and optimal quality of life.
Albright AL, Cervi A, Singletary J. Intrathecal baclofen for spasticity in cerebral palsy. JAMA. 1991;265:1418-1422.
Albright AL, Ferson SS. Intraventricular baclofen for dystonia: techniques and outcomes. J Neurosurg Pediatr. 2009;3:11-14.
Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA. 2006;296:1602-1608.
Beckung E, Hagberg G. Neuroimpairments, activity limitations, and participation restrictions in children with cerebral palsy. Dev Med Child Neurol. 2002;44:309-316.
Bross S, Honeck P, Kwon ST, et al. Correlation between motor function and lower urinary tract dysfunction in patients with infantile cerebral palsy. Neurourol Urodyn. 2007;26:222-227.
Buckon CE, Thomas SS, Piatt JHJr, et al. Selective dorsal rhizotomy versus orthopedic surgery: a multidimensional assessment of outcome efficacy. Arch Phys Med Rehabil. 2004;85:457-465.
Carlsson M, Hagberg G, Olsson I. Clinical and aetiological aspects of epilepsy in children with cerebral palsy. Dev Med Child Neurol. 2003;45:371-376.
Charles JR, Wolf SL, Schneider JA, et al. Efficacy of a child-friendly form of constraint-induced movement therapy in hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2006;48:635-642.
Christiansen AS, Lange C. Intermittent versus continuous physiotherapy in children with cerebral palsy. Dev Med Child Neurol. 2008;50:290-293.
Del Giudice E, Staiano A, Capano G, et al. Gastrointestinal manifestations in children with cerebral palsy. Brain Dev. 1999;21:307-311.
Engsberg JR, Ross SA, Park TS. Changes in ankle spasticity and strength following selective dorsal rhizotomy and physical therapy for spastic cerebral palsy. J Neurosurg. 1999;91:727-732.
Fehlings D, Rang M, Glazier J, et al. An evaluation of botulinum-A toxin injections to improve upper extremity function in children with hemiplegic cerebral palsy. J Pediatr. 2000;137:331-337.
Ghasia F, Brunstrom J, Gordon M, et al. Frequency and severity of visual sensory and motor deficits in children with cerebral palsy: gross motor function classification scale. Invest Ophthalmol Vis Sci. 2008;49:572-580.
Glanzman AM, Kim H, Swaminathan K, et al. Efficacy of botulinum toxin A, serial casting, and combined treatment for spastic equinus: a retrospective analysis. Dev Med Child Neurol. 2004;46:807-811.
Gracies JM, Nance P, Elovic E, et al. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl. 1997;6:S92-S120.
Hägglund G, Lauge-Pedersen H, Wagner P, et al. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskelet Disord. 2007;8:101.
Liptak GS, O’Donnell M, Conaway M, et al. Health status of children with moderate to severe cerebral palsy. Dev Med Child Neurol. 2001;43:364-370.
Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214-223.
Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8-14.
Russman BS, Gormley MEJr, Tilton A. Cerebral palsy: a rational approach to a treatment protocol, and the role of botulinum toxin in treatment. In: Brashear A, Mayer NH, editors. Spasticity and Other Forms of Muscle Overactivity in the Upper Motor Neuron Syndrome: Etiology, Evaluation, Management, and the Role of Botulinum Toxin. New York: WE MOVE; 2008:179-192.
Sanger TD, Delgado MR, Gaebler-Spira D, et al. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:e89-e97.
Schaefer GB. Genetics considerations in cerebral palsy. Semin Pediatr Neurol. 2008;15:21-26.
Sigurdardóttir S, Thórkelsson T, Halldórsdóttir M, et al. Trends in prevalence and characteristics of cerebral palsy among Icelandic children born 1990 to 2003. Dev Med Child Neurol. 2009;51:356-363.
Steenbeek D, Meester-Delver A, Becher JG, et al. The effect of botulinum toxin type A treatment of the lower extremity on the level of functional abilities in children with cerebral palsy: evaluation with goal attainment scaling. Clin Rehabil. 2005;19:274-282.
Vidailhet M, Yelnik J, Lagrange C, et al. French SPIDY-2 Study Group. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study. Lancet Neurol. 2009;8:709-717.
Vles GF, Hendriksen JG, Visschers A, et al. Levetiracetam therapy for treatment of choreoathetosis in dyskinetic cerebral palsy. Dev Med Child Neurol. 2009;51:487-490.
Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547-554.
1 Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8-14.
2 Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547-554.
3 Nelson KB, Chang T. Is cerebral palsy preventable?”. Curr Opin Neurol. 2008;21:129-135.
4 O’Callaghan ME, MacLennan AH, Haan EA, et al. South Australian Cerebral Palsy Research Group. The genomic basis of cerebral palsy: a HuGE systematic literature review. Hum Genet. 2009;126:149-172.
5 Ashwal S, Russman BS, Blasco PA, et al. Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology.. 2004;23(62):851-863.
6 Schaefer GB. Genetics considerations in cerebral palsy. Semin Pediatr Neurol. 2008;15:21-26.
7 Blair E, Stanley F. Interobserver agreement in the classification of cerebral palsy. Dev Med Child Neurol. 1985;27:615-622.
8 Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214-223.
9 Graham HK, Harvey A, Rodda J, et al. The Functional Mobility Scale (FMS). J Pediatr Orthop. 2004;24:514-520.
10 Eliasson AC, Krumlinde-Sundholm L, Rösblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549-554.
11 Beckung E, Hagberg G. Neuroimpairments, activity limitations, and participation restrictions in children with cerebral palsy. Dev Med Child Neurol. 2002;44:309-316.
12 Ghasia F, Brunstrom J, Gordon M, et al. Frequency and severity of visual sensory and motor deficits in children with cerebral palsy: gross motor function classification scale. Invest Ophthalmol Vis Sci. 2008;49:572-580.
13 Kozeis N, Anogeianaki A, Mitova DT, et al. Visual function and execution of microsaccades related to reading skills, in cerebral palsied children. Int J Neurosci. 2006;116:1347-1358.
14 Pagliano E, Fedrizzi E, Erbetta A, et al. Cognitive profiles and visuoperceptual abilities in preterm and term spastic diplegic children with periventricular leukomalacia. J Child Neurol. 2007;22:282-288.
15 Wingert JR, Burton H, Sinclair RJ, et al. Tactile sensory abilities in cerebral palsy: deficits in roughness and object discrimination. Dev Med Child Neurol. 2008;50:832-838.
16 Hoon AH Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51:697-704.
17 Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA. 2006;296:1602-1608.
18 Carlsson M, Hagberg G, Olsson I. Clinical and aetiological aspects of epilepsy in children with cerebral palsy. Dev Med Child Neurol. 2003;45:371-376.
19 Sigurdardóttir S, Thórkelsson T, Halldórsdóttir M, et al. Trends in prevalence and characteristics of cerebral palsy among Icelandic children born 1990 to 2003. Dev Med Child Neurol. 2009;51:356-363.
20 Liptak GS, O’Donnell M, Conaway M, et al. Health status of children with moderate to severe cerebral palsy. Dev Med Child Neurol. 2001;43:364-370.
21 Stevenson RD, Conaway M, Chumlea WC, et al. Growth and health in children with moderate-to-severe cerebral palsy. Pediatrics. 2006;118:1010-1018.
22 Henderson RC, Lark RK, Gurka MJ, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5.
23 Hägglund G, Lauge-Pedersen H, Wagner P, et al. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskelet Disord. 2007;8:101.
24 Driscoll SW, Skinner J. Musculoskeletal complications of neuromuscular disease in children. Phys Med Rehabil Clin N Am. 2008;19:163-194. viii
25 Reilly S, Skuse D. Characteristics and management of feeding problems of young children with cerebral palsy. Dev Med Child Neurol. 1992;34:379-388.
26 Del Giudice E, Staiano A, Capano G, et al. Gastrointestinal manifestations in children with cerebral palsy. Brain Dev. 1999;21:307-311.
27 Fung EB, Samson-Fang L, Stallings VA, et al. Feeding dysfunction is associated with poor growth and health status in children with cerebral palsy. J Am Diet Assoc. 2002;102:361-373.
28 Campanozzi A, Capano G, Miele E, et al. Impact of malnutrition on gastrointestinal disorders and gross motor abilities in children with cerebral palsy. Brain Dev. 2007;29:25-29.
29 Bross S, Honeck P, Kwon ST, et al. Correlation between motor function and lower urinary tract dysfunction in patients with infantile cerebral palsy. Neurourol Urodyn. 2007;26:222-227.
30 Karaman MI, Kaya C, Caskurlu T, et al. Urodynamic findings in children with cerebral palsy. Int J Urol. 2005;12:717-720.
31 Mayo ME. Lower urinary tract dysfunction in cerebral palsy. J Urol. 1992;147:419-420.
32 Decter RM, Bauer SB, Khoshbin S, et al. Urodynamic assessment of children with cerebral palsy. J Urol. 1987;138:1110-1112.
33 Houle AM, Vernet O, Jednak R, et al. Bladder function before and after selective dorsal rhizotomy in children with cerebral palsy. J Urol. 1998;160:1088-1091.
34 McGuire EJ, Cespedes RD, O’Connell HE. Leak-point pressures. Urol Clin North Am. 1996;23:253-262.
35 Kari JA. Neuropathic bladder as a cause of chronic renal failure in children in developing countries. Pediatr Nephrol. 2006;21:517-520.
36 Ozturk M, Oktem F, Kisioglu N, et al. Bladder and bowel control in children with cerebral palsy: case-control study. Croat Med J. 2006;47:264-270.
37 Jahnukainen T, Chen M, Celsi G. Mechanisms of renal damage owing to infection. Pediatr Nephrol. 2005;20:1043-1053.
38 Fowler EG, Kolobe T HA, Damiano D, et al. Promotion of physical fitness and prevention of secondary conditions for children with cerebral palsy: Section on Pediatric Research Summit Proceedings. Phys Ther. 2007;87:1495-1510.
39 McGibbon NH, Benda W, Duncan BR, et al. Immediate and long-term effects of hippotherapy on symmetry of adductor muscle activity and functional ability in children with spastic cerebral palsy. Arch Phys Med Rehabil. 2009;90:966-974.
40 Davis E, Davies B, Wolfe R, et al. A randomized controlled trial of the impact of therapeutic horse riding on the quality of life, health, and function of children with cerebral palsy. Dev Med Child Neurol. 2009;51:111-119.
41 Khalili MA, Hajihassanie A. Electrical simulation in addition to passive stretch has a small effect on spasticity and contracture in children with cerebral palsy: a randomised within-participant controlled trial. Aust J Physiother. 2008;54:185-189.
42 van der Linden ML, Hazlewood ME, Hillman SJ, et al. Functional electrical stimulation to the dorsiflexors and quadriceps in children with cerebral palsy. Pediatr Phys Ther. 2008;20:23-29.
43 Al-Abdulwahab SS, Al-Khatrawi WM. Neuromuscular electrical stimulation of the gluteus medius improves the gait of children with cerebral palsy. NeuroRehabilitation. 2009;24:209-217.
44 Willoughby KL, Dodd KJ, Shields N. A systematic review of the effectiveness of treadmill training for children with cerebral palsy. Disabil Rehabil. 2009;19:1-9.
45 Bjornson KF, Schmale GA, Mczyk-Foster A, et al. The effect of dynamic ankle foot orthoses on function in children with cerebral palsy. J Pediatr Orthop. 2006;26:773-776.
46 Park ES, Park CI, Chang HJ, et al. The effect of hinged ankle-foot orthoses on sit-to-stand transfer in children with spastic cerebral palsy. Arch Phys Med Rehabil. 2004;85:2053-2057.
47 Christiansen AS, Lange C. Intermittent versus continuous physiotherapy in children with cerebral palsy. Dev Med Child Neurol. 2008;50:290-293.
48 Taub E, Ramey SL, DeLuca S, et al. Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetrical motor impairment. Pediatrics. 2004;113:305-312.
49 Charles JR, Wolf SL, Schneider JA, et al. Efficacy of a child-friendly form of constraint-induced movement therapy in hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2006;48:635-642.
50 Deluca SC, Echols K, Law CR, et al. Intensive pediatric constraint-induced therapy for children with cerebral palsy: randomized, controlled, crossover trial. J Child Neurol. 2006;21:931-938.
51 Gordon AM, Chinnan A, Gill S, et al. Both constraint-induced movement therapy and bimanual training lead to improved performance of upper extremity function in children with hemiplegia. Dev Med Child Neurol. 2008;50:957-958.
52 Sung IY, Ryu JS, Pyun SB, et al. Efficacy of forced-use therapy in hemiplegic cerebral palsy. Arch Phys Med Rehabil. 2005;86:2195-2198.
53 Charles JR, Gordon AM. A repeated course of constraint-induced movement therapy results in further improvement. Dev Med Child Neurol. 2007;49:770-773.
54 Fehlings D, Rang M, Glazier J, et al. An evaluation of botulinum-A toxin injections to improve upper extremity function in children with hemiplegic cerebral palsy. J Pediatr. 2000;137:331-337.
55 Frascarelli F, Masia L, DiRosa G, et al. The impact of robotic rehabilitation in children with acquired or congenital movement disorders. Eur J Phys Rehabil Med. 2009;45:135-141.
56 Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young people with cerebral palsy. Dev Med Child Neurol. 2003;45:652-657.
57 Patikas D, Wolf SI, Mund K, et al. Effects of a postoperative strength-training program on the walking ability of children with cerebral palsy: a randomized controlled trial. Arch Phys Med Rehabil. 2006;87:619-626.
58 Unger M, Faure M, Frieg A. Strength training in adolescent learners with cerebral palsy: a randomized controlled trial. Clin Rehabil. 2006;20:469-477.
59 Scholtes VA, Dallmeijer AJ, Rameckers EA, et al. Lower limb strength training in children with cerebral palsy—a randomized controlled trial protocol for functional strength training based on progressive resistance exercise principles. BMC Pediatr. 2008;8:41.
60 Sanger TD, Delgado MR, Gaebler-Spira D, et al. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:e89-e97.
61 Milla PJ, Jackson AD. A controlled trial of baclofen in children with cerebral palsy. J Int Med Res. 1977;5:398-404.
62 Scheinberg A, Hall K, Lam LT, et al. Oral baclofen in children with cerebral palsy: a double-blind cross-over pilot study. J Paediatr Child Health. 2006;42:715-720.
63 Gracies JM, Nance P, Elovic E, et al. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl. 1997;6:S92-S120.
64 Verrotti A, Greco R, Spalice A, et al. Pharmacotherapy of spasticity in children with cerebral palsy. Pediatr Neurol. 2006;34:1-6.
65 Dai AI, Wasay M, Awan S. Botulinum toxin type A with oral baclofen versus oral tizanidine: a nonrandomized pilot comparison in patients with cerebral palsy and spastic equinus foot deformity. J Child Neurol. 2008;23:1464-1466.
66 Mathew A, Mathew MC, Thomas M, et al. The efficacy of diazepam in enhancing motor function in children with spastic cerebral palsy. J Trop Pediatr. 2005;51:109-113.
67 Nogen AG. Medical treatment for spasticity in children with cerebral palsy. Childs Brain. 1976;2:304-308.
68 Haslam RH, Walcher JR, Lietman PS, et al. Dantrolene sodium in children with spasticity. Arch Phys Med Rehab. 1974;55:384-388.
69 Joynt RL, Leonard JAJr. Dantrolene sodium suspension in treatment of spastic cerebral palsy. Dev Med Child Neurol. 1980;22:755-767.
70 Corry IS, Cosgrove AP, Walsh EG, et al. Botulinum toxin A in the hemiplegic upper limb: a double-blind trial. Dev Med Child Neurol. 1997;39:185-193.
71 Love SC, Valentine JP, Blair EM, et al. The effect of botulinum toxin type A on the functional ability of the child with spastic hemiplegia a randomized controlled trial. Eur J Neurol. 2001;8(suppl 5)):50-58.
72 Ubhi T, Bhakta BB, Ives HL, et al. Randomised double blind placebo controlled trial of the effect of botulinum toxin on walking in cerebral palsy. Arch Dis Child. 2000;83:481-487.
73 Steenbeek D, Meester-Delver A, Becher JG, et al. The effect of botulinum toxin type A treatment of the lower extremity on the level of functional abilities in children with cerebral palsy: evaluation with goal attainment scaling. Clin Rehabil. 2005;19:274-282.
74 Barwood S, Baillieu C, Boyd R, et al. Analgesic effects of botulinum toxin A: a randomized, placebo-controlled clinical trial. Dev Med Child Neurol. 2000;42:116-121.
75 Russman BS, Gormley MEJr, Tilton A. Cerebral palsy: a rational approach to a treatment protocol, and the role of botulinum toxin in treatment. In: Brashear A, Mayer NH, editors. Spasticity and Other Forms of Muscle Overactivity in the Upper Motor Neuron Syndrome: Etiology, Evaluation, Management, and the Role of Botulinum Toxin. New York: WE MOVE; 2008:179-192.
76 Boyd RN, Graham JEA, Nattrass GR, et al. Medium-term response characterisation and risk factor analysis of botulinum toxin type A in the management of spasticity in children with cerebral palsy. Eur J Neurol. 1999;6(suppl):S37-S45.
77 Delgado MR. The use of botulinum toxin type A in children with cerebral palsy: a retrospective study. Eur J Neurol. 1999;6(suppl 4):S11-S18.
78 Glanzman AM, Kim H, Swaminathan K, et al. Efficacy of botulinum toxin A, serial casting, and combined treatment for spastic equinus: a retrospective analysis. Dev Med Child Neurol. 2004;46:807-811.
79 Newman CJ, Kennedy A, Walsh M, et al. A pilot study of delayed versus immediate serial casting after botulinum toxin injection for partially reducible spastic equinus. J Pediatr Orthop. 2007;27:882-885.
80 Smyth MD, Peacock WJ. The surgical treatment of spasticity. Muscle Nerve. 2000;23:153-163.
81 Hays RM, McLaughlin JF, Bjornson KF, et al. Electrophysiological monitoring during selective dorsal rhizotomy, and spasticity and GMFM performance. Dev Med Child Neurol. 1998;40:233-238.
82 Engsberg JR, Ross SA, Park TS. Changes in ankle spasticity and strength following selective dorsal rhizotomy and physical therapy for spastic cerebral palsy. J Neurosurg. 1999;91:727-732.
83 Engsberg JR, Ross SA, Wagner JM, et al. Changes in hip spasticity and strength following selective dorsal rhizotomy and physical therapy for spastic cerebral palsy. Dev Med Child Neurol. 2002;44:220-226.
84 Albright AL, Cervi A, Singletary J. Intrathecal baclofen for spasticity in cerebral palsy. JAMA. 1991;265:1418-1422.
85 Gilmartin R, Bruce D, Storrs BB, et al. Intrathecal baclofen for management of spastic cerebral palsy: multicenter trial. J Child Neurol. 2000;15:71-77.
86 Miller F. Surgical techniques. In: Miller F, editor. Cerebral Palsy. New York: Springer; 2005:865-1024.
87 Saint Hilaire MH, Burke RE, Bressman SB, et al. Delayed-onset dystonia due to perinatal or early childhood asphyxia. Neurology. 1991;41:216-222.
88 Cerovac N, Petrovic I, Klein C, et al. Delayed-onset dystonia due to perinatal asphyxia: a prospective study. Mov Disord. 2007;22:2426-2429.
89 Kostic VS, Covickovic-Sternic N, Svetel-Stojanovic M, et al. [Delayed-onset dystonia due to asphyxia in the perinatal period.]. Srp Arh Celok Lek. 1997;125(3-4):84-88.
90 Marsh HO. Diazepam in incapacitating cerebral palsied children. JAMA. 1965;191:797-800.
91 Sanger TD, Bastian A, Brunstrom J, et al. Child Motor Study Group. Prospective open-label clinical trial of trihexyphenidyl in children with secondary dystonia due to cerebral palsy. J Child Neurol. 2007;22:530-537.
92 Rice J, Waugh MC. Pilot study on trihexyphenidyl in the treatment of dystonia in children with cerebral palsy. J Child Neurol. 2009;24:176-182.
93 Chatterjee A, Louis ED, Frucht S. Levetiracetam in the treatment of paroxysmal kinesigenic choreoathetosis. Mov Disord. 2002;17:614-615.
94 Recio MV, Hauser RA, Louis ED, et al. Chorea in a patient with cerebral palsy: treatment with levetiracetam. Mov Disord. 2005;20:762-764.
95 Mink JW. Is levetiracetam a treatment option for dyskinetic cerebral palsy? Dev Med Child Neurol. 2009;51:418-419.
96 Vles GF, Hendriksen JG, Visschers A, et al. Levetiracetam therapy for treatment of choreoathetosis in dyskinetic cerebral palsy. Dev Med Child Neurol. 2009;51:487-490.
97 Heggarty H, Wright T. Tetrabenazine in athetoid cerebral palsy. Dev Med Child Neurol. 1974;16:137-142.
98 Kenney C, Jankovic J. Tetrabenazine in the treatment of hyperkinetic movement disorders. Exp Rev Neurother. 2006;6:7-17.
99 Albright AL, Barry MJ, Shafton DH, et al. Intrathecal baclofen for generalized dystonia. Dev Med Child Neurol. 2001;43:652-657.
100 Albright AL, Ferson SS. Intraventricular baclofen for dystonia: techniques and outcomes. J Neurosurg Pediatr. 2009;3:11-14.
101 Katsakiori PF, Kefalopoulou Z, Markaki E, et al. Deep brain stimulation for secondary dystonia: results in 8 patients. Acta Neurochir (Wien). 2009;151:473-478.
102 Vidailhet M, Yelnik J, Lagrange C, et al. French SPIDY-2 Study Group. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study. Lancet Neurol. 2009;8:709-717. 1
103 Buckon CE, Thomas SS, Piatt JHJr, et al. Selective dorsal rhizotomy versus orthopedic surgery: a multidimensional assessment of outcome efficacy. Arch Phys Med Rehabil. 2004;85:457-465.