Chapter 12 Clinical depression
CLASSIFICATION, EPIDEMIOLOGY AND AETIOLOGY
Depression is associated with normal emotions of sadness and loss, and can be seen as part of the natural adaptive response to life’s stressors. True ‘clinical depression’, however, is a disproportionate ongoing state of sadness, or absence of pleasure, that persists after the exogenous stressors have abated. Clinical depression is commonly characterised by either a low mood, or a loss of pleasure, in combination with changes in, for example, appetite, sleep and energy, and is often accompanied by feelings of guilt or worthlessness or suicidal thoughts.1 The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) classifies ‘Major Depressive Disorder’ (MDD) as a clinical depressive episode that lasts longer than 2 weeks, and is uncomplicated by recent grief, substance abuse or a medical condition.2 Depression presents a significant socioeconomic burden, with the condition being projected by the year 2020 to effect the second greatest increase in morbidity after cardiovascular disease.3 The lifetime prevalence of depressive disorders varies depending on the country, age, sex and socioeconomic group, and approximates about one in six people.4,5 The 12-month prevalence of MDD is approximately 5–8%, with women approximately twice as likely as men to experience an episode.4,5
The pathophysiology of MDD is complex, and to date no unified theory explaining the biological cause exists.1 The main premise concerning the biopathophysiology of MDD centres on monoamine impairment, involving:6–10
From a holistic perspective, the biological causes of depression are unique to the individual, and can be viewed biochemically as varying impairment of monoamine
THE FOUR HUMORS
A traditional view of depression terms the condition ‘melancholia’. This is based on the humoral model, which depicts four ‘humors’ (choleric, sanguine, phlegmatic and melancholic).17
Depression falls under the auspices of the melancholic humor, being embodied as ‘black bile’.
The liver from an energetic perspective in traditional Western folkloric medicine and from traditional Chinese medicine is considered to be the organ primarily involved with depression, and is seen to regulate emotions.17,18
activity, homocysteine, cortisol and BDNF, and inflammatory interactions. Psychologically, cognitive and behavioural causes (or manifestations) of MDD are also commonly present in variations of negative or erroneous thought patterns, or schemas, impaired self-mastery, challenged social roles, and depressogenic behaviours or lifestyle choices.11–13
Several biological and psychological models theorising the causes of depression have been proposed (reviewed below). The predominant biological model of depression in the last 60 years is the monoamine hypothesis.14 Other key biological theories involve the homocysteine hypothesis,15 and the inflammatory cytokine depression theory.8 A prominent psychological model is the stress-diathesis model, which promulgates the theory that a combination of vulnerabilities (genetic, parenting, health status and cognitions) are exploited by a life stressor, for example relationship break-up, job loss and family death.13,16 These stressful events may trigger a depressive disorder. Some scholars have advanced the theory of a biopsychosocial model, which aims to understand depression in terms of a dynamic interrelation between the biological, psychological and social causes (discussed later).12
RISK FACTORS
Various factors that increase the risk of MDD exist, and such an episode may in turn cause certain health disorders/issues. Genetic vulnerability may play an important part in the development of MDD. Genetic studies have revealed that polymorphisms relevant to monoaminergic neurotransmission exist in some people who experience MDD.19 Recent hypotheses suggest that genes related to neuro-protective/toxic/trophic processes, and to the overactivation of the hypothalamic–pituitary axis may be involved in the pathogenesis of MDD.19 Early life events or proximal stressful events increase the risk of an episode.20 Twin studies provide evidence of the effect of environmental stressors on depression and many studies have revealed that a range of stressful events are involved, affecting remission and relapse of the disorder. Recurrence of depressive episodes and early age at onset present with the greatest familial risk.21 Current evidence suggests that the primary risk factors involved in MDD are a complex interplay of genetics and exposure to depressogenic life events.
A consistent theme revealed by epidemiological data is that females have higher rates of MDD than men, approximating two times higher in some community samples.4 This is associated with a higher risk of first onset, and not due to differential persistence or recurrence. It appears that hormonal factors are not responsible (for example, oestrogen levels, pregnancy or the use of oral contraceptives). Biological vulnerabilities and environmental psychosocial factors appear to be responsible for the increased incidence of depression among women. Initial psychosocial triggers may occur in early teen years upon the onset of puberty, whereby gender difference markedly presents. As Kessler states,23 it is conceivable that MDD presents more commonly in females due to social and psychological influences, such as sex-role differences and an intrinsic propensity to ruminate. Another methodological possibility is that men’s depression may present with irritability rather than anhedonia, and as depression scales place less weight on irritability this may skew the results.
Practitioners should be aware of the existence of conditions that commonly co-occur with MDD. People who are clinically depressed have a far greater risk of having co-occurring generalised anxiety, sleep disorders and substance abuse or dependency.23 It should be noted that these conditions may cause MDD and may also result from MDD. Depression is also often misdiagnosed as ‘unipolar’ when in fact it is the presentation of the depressive phase of ‘bipolar’ depression.24 Appropriate screening needs to occur in patients presenting with depression. Initial questioning should assess the length and frequency of previous and current episodes, the severity, what triggers an episode, and whether they think about death regularly or have felt so low lately that they have considered suicide. Assessment should also include a drug and alcohol screen in addition to reviewing their sleep pattern and level of anxiety and stress. To assess any bipolarity of the depression, it is important to determine whether they have ever experienced several days or more of feeling very happy or ‘high’ in addition to behavioural changes such as a decreased need for sleep, rapidity of cognition or ideas, and any increases in planning, spending money or sexual drive (the bipolar spectrum discussed further below).24 Appropriate referral in the case of suspected alcohol/substance abuse or dependency or bipolar disorder is recommended, as complementary or alternative medicine (CAM) currently lacks evidence as a primary intervention in these areas (although CAM may be adjuvantly beneficial).
SUICIDE
CONVENTIONAL TREATMENT
Current medical treatment strategies for MDD primarily involves synthetic antidepressants (for example, tricyclics, monoamine oxidase inhibitors or selective serotonin reuptake inhibitors), and psychological interventions (for example, cognitive behavioural therapy (CBT), interpersonal therapy (IPT) and behavioural therapy (BT)).1,25 Medical treatment guidelines usually involve options such as providing counselling, CBT or IPT for mild depression, antidepressants and/or CBT for moderate depression, and antidepressants and ECT (and possibly hospitalisation) for severe depression.26,27 As only 30–40% of people achieve a satisfactory response to first-line antidepressant prescription, and approximately 40% do not achieve remission after several antidepressant prescriptions, further pharmacotherapeutic developments are currently being pursued.14,28 Future novel antidepressant mechanisms of action may involve modulating cytokines, secondary messengers, and glucocorticoid, opioid, dopaminergic or melatoninergic pathways.9
KEY TREATMENT PROTOCOLS
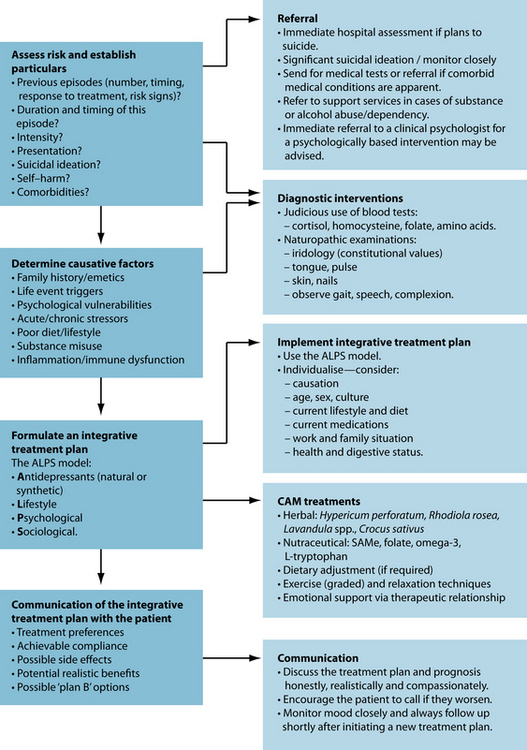
From a clinical perspective, the goal of treating MDD is to ameliorate the depression as safely and quickly as possible. Suicide is a great concern, and is a devastating potential consequence of MDD. If suicidal ideation is significant, or if self-harm is a distinct possibility at any stage, referral to a medical practitioner or to an emergency ward of hospital for immediate psychiatric assessment is crucial. The socioeconomic cost of untreated MDD is massive, and treated depression reduces the burden on health-care systems.29 Evidence advocates early intervention to effectively treat MDD, to enhance remission, and thereby subsequently decrease human suffering and socioeconomic burden.29
Although medical research has not currently advanced to the state of tailoring pharmacotherapy prescriptions to individual neurochemical or genetic profiles, ‘whole-system’ naturopathic diagnosis and treatment has an advantage in being able to prescribe in an individualised manner. First, in order to treat depression effectively, it helps to understand the psychological and biological factors that are involved. Causes of depression are multifaceted, and individual presentations vary markedly. Because of this, tailoring the prescription for the individual may assist in compliance and recovery. Causative factors can be classified into pre-existing ‘vulnerabilities’ to depression, which may be ‘triggered’ by a stressor (commonly a series of stressors or one key event), then ‘maintaining’ factors may exacerbate or prolong the episode.
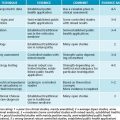
Several herbal medicines are particularly adept at affecting neuroreceptor binding and activity to achieve an antidepressant effect. Herbal medicines used to treat mental health disorders usually have central nervous system or endocrine-modulating activity.6 Common actions can involve monoamine activity modulation, stimulation or sedation of central nervous system activity, and regulation or support of healthy hypothalamic pituitary adrenal axis function (see Table 12.1).30
| TRADITIONAL ACTION | PROPOSED MECHANISMS | APPLICATIONS |
|---|---|---|
| Nervines (tonics, stimulants) | HPA-modulation, beta-adrenergic activity | Depression, fatigue, convalescence |
| Adaptogens, thymoleptics, antidepressants, tonics |
Biopsychosocial model of depression
The most suitable model consistent with the holistic paradigm is a biopsychosocial model.12 The essence of the model is that the cause of depression is multifactorial, with many interrelated influences involved in its growth. Genetics and biochemistry (biological), cognitions and personality traits (psychological), environmental factors (environmental) and social interactions (sociological) all affect the level of a person’s ‘vulnerability’ to a depressive disorder, which is commonly triggered by chronic or acute stressors. Protective factors are considered to be good genetics, balanced positive cognitions, healthy interpersonal relations and social support, and spirituality.11,31
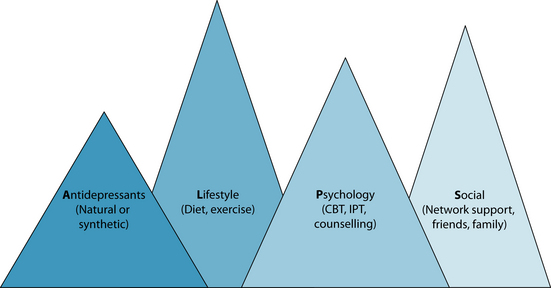
A balanced and integrative naturopathic treatment plan needs to address all aspects concerning the biopsychosocial model. Herbal, nutraceutical and dietary prescription can modulate the biological component of depression; psychological therapies and counselling support is advised to reconfigure negative cognitions, resolve underlying issues, and build resilience; and social concerns (for example, healthy work, lifestyle, exercise, rest balance, and sufficient family/friend/community interaction) should also be addressed. Depression may provide a context for developing meaning from the experience, thereby promoting spiritual growth. Displayed below is a model developed by the author for treating depression: the ALPS model (see Figure 12.1). This treatment model is based on the biopsychosocial model, outlining specific strategies for treating depression holistically. The model advocates a combined approach of antidepressant agents (natural or synthetic); lifestyle adjustments such as dietary improvement, and reduction of alcohol and caffeine, and increased relaxation and exercise; psychological interventions; and improved social functioning and integration.
Monoamine hypothesis
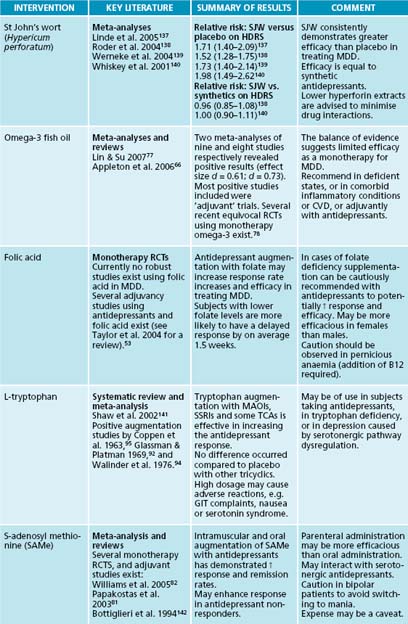
The monoamine hypothesis concerns the theory that depression is primarily caused by dysregulation of serotonin, dopamine and noradrenaline pathways (receptor activity and density, neurotransmitter production and neurochemical transport and transmission).9 Herbal and nutritional/dietary modulation may be helpful in modulating monoaminergic transmission. To date, the phytotherapy with the most evidence of monoamine modulation is Hypericum perforatum. Enough human clinical trials have been conducted for several meta-analyses to be conducted (see Table 12.2). All meta-analyses have revealed that H. perforatum provides a significant antidepressant effect compared to placebo, and an equivalent efficacy compared to synthetic antidepressants. H. perforatum has demonstrated several beneficial effects on modulating monoamine transmission. Although initial in vitro experiments suggested monoamine oxidase-inhibition by H. perforatum, further conducted experiments have not confirmed this activity.32 In vivo and in vitro studies have, however, revealed non-selective inhibition of the neuronal reuptake of serotonin, dopamine and norepinephrine.33 This activity is likely to occur in part via modulation of neurotransmitter transport systems (for example, via Na+ gradient membranes). Increased dopaminergic activity in the prefrontal cortex has been documented.34 A decreased degradation of neurochemicals and a sensitisation of and increased binding to various receptors (for example, GABA, glutamate and adenosine) have also been observed.35–37 It should be noted that some of the pharmacodynamic studies used intraponeal rather than oral administration; caution in extrapolating to humans is advised.
Aside from SJW, Rhodiola rosea and Crocus sativus currently possess the most evidence as monoamine and neuroendocrine modulators, and have provided preliminary human clinical evidence of efficacy in treating MDD.38,39 R. rosea is a stimulating adaptogen, which possesses antidepressant, anti-fatigue and tonic activity.39,40 A 6-week, phase III, three-arm randomised controlled trial (RCT) involving 91 subjects comparing R. rosea SHR-5 standardised extract (680 mg and 340 mg/day) with placebo demonstrated significant dose-dependent improvement on depression.41 It should be noted that the effect size was small, with a low response in comparison to a very low placebo response (usually there is a 20–50% reduction of depression in a placebo group); further studies need to be conducted to confirm efficacy. The phytochemicals salidroside, rosvarin, rosarin, rosin and tyrosol are considered to be the active constituents.42 In animal models, R. rosea has been documented to increase noradrenaline, dopamine and serotonin in the brainstem and hypothalamus, and to increase the blood–brain permeability to neurotransmitter precursors.43 Crocus sativus is developing clinical evidence as an effective antidepressant (reviewed later). Crocin and safranal are currently regarded as the constituents responsible for C. sativus’s antidepressant action.38 The mechanisms responsible for the antidepressant actions are purported to be mediated via reuptake inhibition of dopamine, norepinephrine and serotonin, and NMDA receptor antagonism.38 Safranal is posited to exert selective GABA-α agonism, and possible opioid receptor modulation, as demonstrated via intracerebroventricular administration in an animal model.44
Other herbal medicines that have been documented to exert monoamine modulation include Bacopa monnieri, Ginkgo biloba, Panax ginseng and Convolvulus pluricaulis; however, to date insufficient clinical trials have confirmed antidepressant effects in humans.45,46
HPA-axis modulation
In the last two decades, cortisol has achieved increased attention in the study of the pathogenesis of depression. Substantial evidence exists for the role of cortisol and the HPA axis in depression.47 Postmortem studies and cerebral spinal fluid sampling have found that corticotrophin-releasing hormone (CRF) can be elevated in samples from depressed patients.48 A combination of vulnerability factors (genetic, age and early life events) and precipitating factors (psychological, physiological stressors, substance misuse and comorbid disease) may provoke an increase in CRF. This stimulates the secretion of adrenocorticotropin hormone (ACTH), and subsequent cortisol release from the adrenal glands (see Section 5 on the endocrine system). In vitro and animal models have demonstrated that HPA-axis dysfunction and increased cortisol attenuate the production of BDNF in the brain.9 BDNF is an important growth factor that nourishes nerve cells, and lower BDNF is correlated with depressive states.1,19 Synthetic antidepressants and electroconvulsive therapy appear to regulate the HPA axis and increase the production of BDNF.47 In animal models, hypericin and the flavonoid derivatives have demonstrated to down-regulate plasma ACTH and corticosterone levels.31 In particular, an animal model demonstrated that 8 weeks of H. perforatum or hypericin administration decreased the expression of genes involved in the regulation of the HPA axis, and significantly decreased levels of CRH mRNA by 16–22% in the hypothalamic paraventricular nucleus (PVN) and serotonin 5-HT(1A) receptor mRNA by 11–17% in the hippocampus. Human studies have, however, found that H. perforatum increases salivary and serum cortisol levels.49,50 Importantly, while in vivo studies have shown that synthetic antidepressants can increase BDNF, H. perforatum does not prevent a decrease in stress-reduced BDNF.51 It should be noted that while evidence does suggest that HPA modulation does occur with H. perforatum administration, the complex pharmacodynamics of the effect has not been fully elucidated to date, with variables such as differing human or animal models, stress study methodology and types of H. perforatum extracts obfuscating the conclusion.
Herbal adaptogens and tonics may play a beneficial role in modulating ACTH (refer further to Section 5 on the endocrine system). Stimulating adaptogens such as Eleutherococcus senticosus, Schisandra chinensis and Rhodiola rosea have demonstrated significant adaptogenic effects, posited as occurring from HPTA modulation.42 Although E. senticosus, S. chinensis and other adaptogens such as Panax ginseng and Withania somnifera have not demonstrated specific antidepressant activity, they may provide a supportive role in depressive presentations with HPA-axis dysregulation.
Homocysteine hypothesis
The homocysteine hypothesis centres on the theory that genetic and environmental factors elevate levels of homocysteine, which in turn provokes changes in neuronal architecture and neurotransmission, resulting in depression.15,52 The sulfur compound homocysteine (formed from methionine) has been demonstrated to be directly toxic to neurons, and can induce DNA strand breakage. Higher serum levels of homocysteine have been noted in depressive populations compared to healthy controls.52 Metabolism of homocysteine to S-adenosyl methionine (SAMe) or back to methionine requires folate, B6 and B12. Folate is involved with the methylation pathways in the ‘one-carbon’ cycle, and is responsible for the metabolism and synthesis of various monoamines.52 Folate is also most notably involved with the synthesis of SAMe, an endogenous antidepressant formed from homocysteine. Folate deficiency is implicated in causing increased homocysteine levels, and has been consistently demonstrated in depressive populations and in poor responders to antidepressants.53,54 Folate deficiency has been reported in approximately one-third of people suffering from depressive disorders.54 Finally, a correlation has been discovered between methylenetetrahydrofolate reductase (a folate-metabolising enzyme) polymorphisms and depression, indicating a genetic link.55
Several studies exist assessing the antidepressant effect of folic acid in humans with concomitant antidepressant use.1,56,57 All of these studies yielded positive results with regard to enhancing antidepressant response rates or increasing the onset of response. An example of folic acid’s antidepressant activity is reflected in a controlled study using 500 μg of folic acid or placebo adjuvantly with 20 mg fluoxetine in 127 subjects with a Hamilton Depression Rating Scale (HDRS) of ≥ 20.57,58 The study demonstrated a statistically significant reduction after 10 weeks on the HDRS for women. This effect was not, however, replicated in the male sample. Along with a good dietary intake of folate-rich leafy vegetables or folic acid supplementation, a multivitamin high in B vitamins (especially B6 and B12) may assist in reducing homocysteine, and maintaining adequate levels of SAMe. This will also assist in maintenance of energy production, adrenal function and the creation of neurotransmitters.
Inflammatory factors causing depression
A cytokine-mediated pro-inflammatory event has been considered as a factor involved with the pathophysiology of MDD.8 Studies have demonstrated that otherwise healthy patients with depression have presented with activated inflammatory pathways.59 It has been posited that pro-inflammatory cytokines produced from inflammation may influence neuroendocrine function via entry through the ‘leaky regions’ of the brain (for example, the circumventricular organs), and subsequent modulation of cytokine specific transport molecules, or cytokine stimulation of vagal afferent fibres.8 Modulation of both CRT and neurotransmitters is known to be effected by cytokines. The main pro-inflammatory cytokines implicated in depressogenesis centres on IFN-α producing IL-1β, IL-6 and TNF-α cytokines (see Chapter 28 on autoimmunity). In laboratory studies, animals exposed to a variety of stressors have demonstrated an increase in these pro-inflammatory cytokines. Synthetic antidepressants have been shown to inhibit the production of various inflammatory cytokines, and to stimulate the production of anti-inflammatory cytokines.8 Although in its infancy, nascent research is evolving towards developing synthetic medicines that modulate cytokines with a regard to ameliorating depression.9
Attenuation of pro-inflammatory cytokines may be of benefit in individuals who present with either a preceding or comorbid inflammatory condition, or a chronic latent infection. Appropriate screening to determine any infections, or inflammatory process, with reference to the chronology of the onset of depression is advised. If an association is plausible, herbal medicines and nutrients that dampen the inflammatory cascade and attenuate the production of pro-inflammatory cytokines may be advised (see Section 2 on the respiratory system and and Section 1 on the gastrointestinal system). In brief, herbal and nutritional medicines that may potentially benefit the treatment of pro-inflammatory evoked MDD include Albizzia spp., Echinacea spp., vitamin C and bioflavonoids, and zincAlbizzia spp. (in particular A. lebbeck) have been documented to exert anti-inflammatory and antiallergic activity.60 In addition to this activity, anxiolytic and antidepressant effects have been demonstrated in animal models, and in the case of Albizzia julibrissan, the plant curiously is known as ‘happy bark’ in traditional Chinese medicine.61–63
Aside from the previously mentioned herbal and nutritional medicines, omega-3 fatty acids also have a role in reducing inflammation-based MDD.59 Epidemiological studies have demonstrated that a rise in depressive symptoms may be correlated with lower dietary omega-3 fish oil (eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)).64–67 Studies have also demonstrated that people with depression have a tendency towards a higher ratio of serum arachidonic acid to essential fatty acids, and an overall lower serum level of omega-3 compared to healthy controls.59,68–70 Urbanised Western cultures tend to have a far higher ratio of dietary omega-6 oils compared to omega-3 oils, and this has been regarded as a possible factor in the rise of depression over the last several decades.64,67 The pathophysiology occurring from a pro-omega-6 diet may involve an increased promotion of inflammatory eicosanoids, a lessening of BDNF and a decrease in neuronal cell membrane fluidity and communication.67,71 Evidence currently suggests that omega-3 fatty acids exert antidepressant activity via beneficial effects on neurotransmission.72 This may occur via modulation of neurotransmitter (norepinephrine, dopamine and serotonin) reuptake, degradation, synthesis and receptor binding.73,74 Animal models have demonstrated that omega-3 fatty acids increase serotonin and dopamine concentrations in the frontal cortex, and that a diet deficient in the nutrient decreases catecholamine synthesis.73,75,76 A recent human clinical trial demonstrated a significant increase in plasma concentrations of norepinephrine in healthy humans.74
Several human clinical trials have been conducted assessing the efficacy of EPA, DHA or a combination of both of these essential fatty acids.77 Clinical evidence regarding the use of essential fatty acids as a monotherapy is equivocal, with a mixture of positive and negative trials (see Table 12.2 at the end of the chapter for a review of the evidence).
ADDITIONAL THERAPEUTIC OPTIONS
S-adenosyl methionine (SAMe)
L-tryptophan
Crocus sativus (saffron)
This may in part be due to many studies using olive oil as an ‘inert’ control, and some studies using higher DHA to EPA ratios or DHA alone.78 Clinical trials using essential fatty acids adjuvantly with antidepressants have provided positive evidence of additional increased reduction of depression level.79 Current evidence supports the use of essential fatty acids adjuvantly with antidepressants, in cases of deficiency or if comorbid cardiovascular or inflammatory disorders are present.
The mood spectrum versus categorical diagnosis
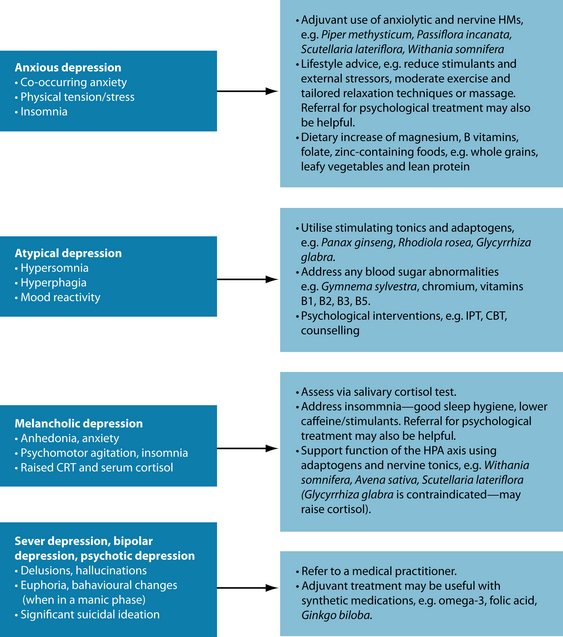
Naturopathic diagnosis of mood disorders reflects the holistic psychiatric medicine model, whereby individuals present with unique presentation of MDD, often oscillating between varying levels of depression and anxiety, and sometimes present with peaks of hypomania (for example, increased mental activity, socialisation, work and planning, and decreased sleep). An advantage of naturopathic practice is that prescriptions can be altered to flexibly accommodate the natural rhythm of mood disorders. While it is more applicable to treat the patient holistically (not just ‘the depression’), if the condition is viewed in terms of a discrete medical diagnosis, then specific treatment protocols and prescriptions can be instigated (see Figure 12.3).
INTEGRATIVE MEDICAL CONSIDERATIONS
Acupuncture and massage
The use of acupuncture to treat depressive disorders has been documented in traditional Chinese medicine (TCM) texts.110 In TCM the two main organs (energetically) involved in depression are the liver and the heart.18 Two primary patterns of depression are diagnosed in TCM: ‘Stagnation of Liver Qi’ (excess pattern) and ‘Deficiency of Qi, Blood, or Kidney Jing’ (deficient pattern).110 In principle, physical activity and exercise are regarded to ‘Move Qi and Blood’, thereby alleviating ‘Stagnation’, and to ‘Tonify Qi’(lung and spleen), thereby improving energy and vigour. A review of eight small-randomised controlled trials confirmed that acupuncture could significantly reduce the severity of depression on the HDRS or Beck Depression Scale.111 However, no significant effect of active acupuncture was found on the response rate or remission rate. Another review112 found a total of four RCTs meeting a minimum standard of methodological rigour (for example, a randomised sample and control groups used). Results of these studies revealed significant effects on reducing depression versus non-specific or sham acupuncture, and equivocal efficacy to tricyclic antidepressants. In one study, although acupuncture was equally effective to massage and sham acupuncture, only the true acupuncture provided sustained antidepressant effects. Acupuncture has been documented to interact with opioid pathways, and substances which modulate these pathways have been shown to have antidepressant activity.9,113,114 Other possible antidepressant mechanisms of action include the increased release of serotonin and norepinephrine, and CRT and cortisol modulation.116
Massage may also be of benefit in improving mood and reducing depression. Studies of varying methodological rigour have shown that massage increases relaxation, decreases stress and elevates the mood.115 A rigorous review of massage techniques in treating clinical depression commented that, while positive studies exist, a lack of evidence from RCTs does not support this intervention.116 While evidence currently does not support massage as a primary monotherapy in treating MDD, use of massage adjuvantly can be advised, especially in cases of co-occurring muscular tension.
Psychological intervention
As outlined under the ALPS model, psychological intervention is an important component in treating MDD. Guidelines support the use of psychological interventions such as cognitive behavioural therapy (CBT) and interpersonal therapy (IPT) in mild depression rather than synthetic medication.27 CBT and IPT are accepted psychological interventions, both having equal evidence of efficacy in treating MDD.25 CBT involves learning cognitive skills to ‘reprogram’ erroneous or negative thought patterns with positive balanced cognitions, and to institute positive behavioural modifications.117 The theory is based on the concept that a person’s negative, critical, erroneous thought patterns provoke deleterious emotional and physiological responses. By intervening before this cascade occurs, and establishing a positive balanced inner dialogue, this spiral can be avoided. IPT focuses on identifying problematic social situations that are depressogenic, and developing interpersonal techniques (such as social skills) to manage interpersonal relationships.117 By increasing confidence and competency in managing social interactions, a robust self-esteem may develop.
Adjuvant CAM treatments with antidepressants
If the patient is taking antidepressant medication, adjuvancy options are recommended (see Sarris et al.118 for a review). Adjuvant strategies with antidepressants involve combining an additional thymoleptic intervention to directly increase the antidepressant effect, or use a supplementary therapy to enhance activity, or reduce side effects by a synergistic interaction. Such prescription should be discussed between the physician and naturopath, and be closely monitored. The evidence regarding combining synthetic antidepressants and herbal medicines is currently unknown. Potential exists in combining antidepressant herbal medicines to increase the therapeutic effect in absent or partial responders to synthetic antidepressants. Consideration of serotonin syndrome or switching to bipolar (hypo)mania should, however, be given. Co-administration of herbal medicines may also have a potential role in addressing individual presentations or comorbid features of depression (see Figure 12.3), or to reduce side effects of antidepressants. Note the following:
Example treatment
Herbal and nutritional prescription
In the above case, the primary prescriptive protocol is to provide an antidepressant action to treat the depression. The co-occurring manifestations of fatigue, amotivation and hypersomnia can be addressed via stimulating tonics and adaptogens. In the above case, a dysregulation of serotonin may be responsible for the low mood; norepinephrine dysregulation may affect amotivation, hypersomnia and fatigue; while dopamine dysregulation may be responsible for anhedonia. Hypericum perforatum, Rhodiola rosea and Lavandula angustifolia should aid in the elevation of her mood. Panax ginseng, Rhodiola rosea and Glycyrrhiza glabra will assist in enhancing adrenal activity and invigorating her energy and motivation.101 Omega-3 may be of benefit in treating her depression (especially if she is deficient in it), and a multivitamin high in folate will provide the nutrients involved in the manufacture and transmission of neuroreceptors, while assisting the methylation pathway.
Dietary and lifestyle advice
Dietary programs designed to treat depression have to date not been rigorously evaluated. Although evidence supporting specific dietary advice is currently absent, a basic balanced diet (see Section 1 on the gastrointestinal system) including foods rich in a spectrum of nutrients can be recommended. Foods rich in folate, omega-3, tryptophan, B and C vitamins, zinc and magnesium are necessary for the production of neurotransmitters and neuronal communication.79 These include whole grains, lean meat, deep-sea fish, green leafy vegetables, coloured berries and nuts (walnuts, almonds).65,130
General lifestyle advice should focus on encouraging a balance between meaningful work, adequate rest and sleep, judicious exercise, positive social interaction and pleasurable hobbies. Behavioural therapy techniques have shown positive effects on reducing depression by training the person to reduce or better manage stressful situations, and to increase pleasurable activities that enhance self-esteem and self-mastery. If substance or alcohol dependence or misuse is apparent, supportive advice on curtailing this, or appropriate referral, should be communicated (see the case in Chapter 13 on chronic generalised anxiety for more detail).
Exercise or physical activity
Increasing physical activity is advised in cases of underactivity. Associations between greater physical activity and improved mood and wellbeing have been documented,131 and several RCTs support exercise as effective in managing MDD. A meta-analysis of 11 treatment-outcome studies of exercise on the treatment of depression showed a significant effect in favour of physical exercise compared with control conditions (routine care, wait list, meditation/relaxation or low-intensity exercise).132 A very large average effect size was obtained with all but two studies obtaining superior results from exercise than from control. However, many of these studies had methodological failings (for example, not using blind assessment or intention-to-treat analyses). Research strongly suggests that anabolic exercise of high intensity is more effective than low intensity.133 The biological antidepressant effects of exercise include a beneficial modulation of the HPA axis, increased expression of 5-HT, and increased levels of circulating testosterone (which may have a protective effect against depression).134 Evidence also exists for the use of yoga to reduce depression and improve mood. A review documented five RCTs using various types of yoga to treat MDD.135 While the studies reviewed all concluded positive results, the methodologies were poorly reported and thereby no firm conclusion can be reached. It is worthwhile highlighting that certain types of yoga may actually have greater antidepressant effect. ‘Mindfulness’ in exercise techniques such as yoga may potentially have greater efficacy than low-intensity, low-focus yoga, although evidence does not currently confirm this theory.136
Evidence for the type and amount of exercise for the management of MDD, currently favours anabolic over aerobic activity to gain the greatest benefits, and the intensity needs to be moderate to high and performed two or three times per week.133 Caveats exist regarding exercise prescription for MDD. Depression may be worsened if the person is unable to meet expectations, potentially promoting a sense of failure and guilt. This may be more likely to occur in severe MDD, especially where psychomotor retardation, hypersomnia, somnolescence, marked fatigue or anhedonia are present. Exercise plans should be instituted after a medical assessment, and initially commenced at a low intensity to allow for physical and psychological adaptation to occur to the new stimulus.
Expected outcomes and follow-up protocols
In the above case, reduction of depression and a return towards euthymia is expected within a month of commencing treatment. A depressive episode will commonly remit within 6 months (even without treatment due to the natural rhythmicity of MDD), although maintaining factors and the number of previous episodes may affect complete remission. Many people will have their depression alleviated simply by taking the step to seek treatment, making lifestyle adjustments, and from the interpersonal therapeutic relationship with the practitioner. If the depressive episode persisted and suicidality was still absent, a change of prescription would be warranted. Additional interventions such as SAMe or L-tryptophan augmentation may be helpful. If the condition worsened then medical referral would be advised. Depressive episodes are often diagnostically unstable, and thereby the patient should be monitored carefully to modulate the prescription according to any changes in symptoms. Changes that may occur include bipolar elements, anxiety, insomnia or changes in appetite, energy and cognition. After the depressive symptoms remit, treatment should be continued for 3–6 months to enhance the chance of remission.
Belmaker R.H., Agam G. Major depressive disorder. N Engl J Med. 2008;358(1):55-68.
Sarris J. Herbal medicines in the treatment of psychiatric disorders: a systematic review. Phytother Res. 2007;21(8):703-716.
Sarris J., et al. Major depressive disorder and nutritional medicine: A review of monotherapies and adjuvant treatments. Nutrition Reviews. 2009;67(3):125-131.
Werneke U., et al. Complementary medicines in psychiatry: Review of effectiveness and safety. Br J Psychiatry. 2006;188:109-121.
1. Belmaker R.H., Agam G. Major depressive disorder. N Engl J Med. 2008;358(1):55-68.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edn. Arlington: American Psychiatric Association; 2000.
3. WHO. Mental and neurological disorders. Depression. 2006. Online. Available: http://www.who.int/mental_health/management/depression/definition/en/.
4. Kessler R.C., et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105.
5. Alonso J., et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;420(420):21-27.
6. Antonijevic I.A. Depressive disorders – is it time to endorse different pathophysiologies? Psychoneuroendocrinology. 2006;31(1):1-15.
7. Ressler K.J., Nemeroff C.B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2-19.
8. Raison C.L., et al. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24-31.
9. Hindmarch I. Expanding the horizons of depression: beyond the monoamine hypothesis. Hum Psychopharmacol. 2001;16(3):203-218.
10. Plotsky P.M., et al. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am. 1998;21(2):293-307.
11. Southwick S.M., et al. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255-291.
12. Molina J. Understanding the biopsychosocial model. Int’l J Psychiatry in Medicine. 1983;13(1):29-36.
13. Haeffel G.J., Grigorenko E.L. Cognitive vulnerability to depression: exploring risk and resilience. Child Adolesc Psychiatr Clin N Am. 2007;16(2):435-448. x
14. Berton O., Nestler E.J. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7(2):137-151.
15. Folstein M., et al. The homocysteine hypothesis of depression. Am J Psychiatry. 2007;164(6):861-867.
16. Kessler R.C. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191-214.
17. Culpeper N. Culpeper’s complete herbal. Hertfordshire: Wordsworth Editions Ltd; 1652.
18. Maciocia G. The foundations of Chinese medicine. Singapore: Churchill Livingstone; 1989.
19. Levinson D.F. The genetics of depression: a review. Biol Psychiatry. 2006;60(2):84-92.
20. Tennant C. Life events, stress and depression: a review of recent findings. Aust N Z J Psychiatry. 2002;36(2):173-182.
21. Paykel E. Life events and affective disorders. Acta Psychiatr Scand Suppl.. 2003;418:61-66.
22. Australian Bureau of Statistics. Mental health in Australia: a snapshot. Australian Bureau of Statistics,. 2004.
23. Kessler R.C., et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
24. Miklowitz D.J., Johnson S.L. The psychopathology and treatment of bipolar disorder. Annu Rev Clin Psychol. 2006;2:199-235.
25. Parker G., Fletcher K. Treating depression with the evidence-based psychotherapies: a critique of the evidence. Acta Psychiatr Scand. 2007;115(5):352-359.
26. Ellen S., et al. Depression and anxiety: pharmacological treatment in general practice. Aust Fam Physician. 2007;36(4):222-228.
27. Ellis P. Australian and New Zealand clinical practice guidelines for the treatment of depression. Aust N Z J Psychiatry. 2004;38(6):389-407.
28. Warden D., et al. The STAR∗D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
29. Donohue J., Pincus H. Reducing the societal burden of depression: a review of economic costs, quality of care and effects of treatment. Pharmacoeconomics. 2007;25(1):7-24.
30. Spinella M. The psychopharmacology of herbal medicine: plant drugs that alter mind, brain and behavior. Cambridge: MIT Press; 2001.
31. Schotte C.K., et al. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety. 2006;23(5):312-324.
32. Butterweck V., Schimdt M. St John’s wort: role of active compounds for its mechanism of action and efficacy. Wien Med Wochenschr. 2007;157(13–14):356-361.
33. Muller W.E. Current St John’s wort research from mode of action to clinical efficacy. Pharmacol Res. 2003;47:101-109.
34. Yoshitake T., et al. Hypericum perforatum L (St John’s wort) preferentially increases extracellular dopamine levels in rat prefrontal cortex. Br J Pharmacol. 2004;142(3):414-418.
35. Butterweck V. Mechanism of action of St John’s wort in depression: what is known? CNS Drugs. 2003;17(8):539-562.
36. Zanoli P. Role of hyperforin in the pharmacological activities of St John’s Wort. CNS Drug Rev. 2004;10(3):203-218.
37. Mennini T., Gobbi M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004;75(9):1021-1027.
38. Schmidt M., et al. Saffron in phytotherapy: pharmacology and clinical uses. Wien Med Wochenschr. 2007;157(13–14):315-319.
39. Kelly G.S. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6(3):293-302.
40. Kucinskaite A., et al. [Experimental analysis of therapeutic properties of Rhodiola rosea L. and its possible application in medicine]. Medicina (Kaunas). 2004;40(7):614-619.
41. Darbinyan V., et al. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord J Psychiatry. 2007;61(5):343-348.
42. Panossian A. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005;19:819-838.
43. Kucinskaite A., et al. Evaluation of biologically active compounds in roots and rhizomes of Rhodiola rosea L. cultivated in Lithuania. Medicina (Kaunas). 2007;43(6):487-494.
44. Hosseinzadeh H., Sadeghnia H.R. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: involvement of GABAergic and opioids systems. Phytomedicine. 2007;14(4):256-262.
45. Sarris J. Herbal medicines in the treatment of psychiatric disorders: a systematic review. Phytother Res. 2007;21(8):703-716.
46. Kumar V. Potential medicinal plants for CNS disorders: an overview. Phytother Res. 2006;20(12):1023-1035.
47. Pariante C., et al. Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology. 2003;29:423-447.
48. Mitchell A.J. The role of corticotropin releasing factor in depressive illness: a critical review. Neurosci Biobehav Rev. 1998;22(5):635-651.
49. Schule C., et al. Neuroendocrine effects of Hypericum extract WS 5570 in 12 healthy male volunteers. Pharmacopsychiatry. 2001;34(Suppl 1):S127-S133.
50. Franklin M., et al. Effect of sub-chronic treatment with Jarsin (extract of St John’s wort, Hypericum perforatum) at two dose levels on evening salivary melatonin and cortisol concentrations in healthy male volunteers. Pharmacopsychiatry. 2006;39(1):13-15.
51. Butterweck V., et al. St John’s wort, hypericin, and imipramine: a comparative analysis of mRNA levels in brain areas involved in HPA axis control following short-term and long-term administration in normal and stressed rats. Mol Psychiatry. 2001;6(5):547-564.
52. Bottiglieri T., et al. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69(2):228-232.
53. Taylor M.J., et al. Folate for depressive disorders: systematic review and meta-analysis of randomized controlled trials. J Psychopharmacol. 2004;18(2):251-256.
54. Morris D.W., et al. Folate and unipolar depression. J Altern Complement Med. 2008;14(3):277-285.
55. Bjelland I., et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C→T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
56. Godfrey P.S., et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
57. Coppen A., Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000;60(2):121-130.
58. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
59. Dinan T., et al. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. J Psychiatr Res. 2009;43(4):471-476.
60. Johri R.K., et al. Effect of quercetin and Albizzia saponins on rat mast cell. Indian J Physiol Pharmacol. 1985;29(1):43-46.
61. Kim J.H., et al. Antidepressant-like effects of Albizzia julibrissin in mice: involvement of the 5-HT1A receptor system. Pharmacol Biochem Behav. 2007;87(1):41-47.
62. Chintawar S.D., et al. Nootropic activity of Albizzia lebbeck in mice. J Ethnopharmacol. 2002;81(3):299-305.
63. Une H.D., et al. Nootropic and anxiolytic activity of saponins of Albizzia lebbeck leaves. Pharmacol Biochem Behav. 2001;69(3–4):439-444.
64. Sanchez-Villegas A., et al. Mediterranean diet and depression. Public Health Nutr. 2006;9(8A):1104-1109.
65. Appleton K.M., et al. Depressed mood and n-3 polyunsaturated fatty acid intake from fish: non-linear or confounded association? Soc Psychiatry Psychiatr Epidemiol. 2007;42(2):100-104.
66. Appleton K.M., et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr. 2006;84(6):1308-1316.
67. Parker G., et al. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969-978.
68. Maes M., et al. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38(1):35-46.
69. Adams P.B., et al. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31(Suppl):S157-S161.
70. Edwards R., et al. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48(2–3):149-155.
71. Tassoni D., et al. The role of eicosanoids in the brain. Asia Pac J Clin Nutr. 2008;17(Suppl 1):220-228.
72. Williams A.L., et al. Do essential fatty acids have a role in the treatment of depression? J Affect Disord. 2006;93(1–3):117-123.
73. Chalon S., et al. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J Nutr. 1998;128(12):2512-2519.
74. Hamazaki K., et al. Effect of omega-3 fatty acid-containing phospholipids on blood catecholamine concentrations in healthy volunteers: a randomized, placebo-controlled, double-blind trial. Nutrition. 2005;21(6):705-710.
75. Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4–5):259-269.
76. Delion S., et al. Alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem. 1996;66(4):1582-1591.
77. Lin P.Y., Su K.P. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
78. Sarris J., et al. Major depressive disorder and nutritional medicine: a review of monotherapies and adjuvant treatments. Nutr Reviews. 2009;67(3):125-131.
79. Werneke U., et al. Complementary medicines in psychiatry: review of effectiveness and safety. Br J Psychiatry. 2006;188:109-121.
80. Papakostas G.I., et al. The relationship between serum folate, vitamin B12, and homocysteine levels in major depressive disorder and the timing of improvement with fluoxetine. Int J Neuropsychopharmacol. 2005;8(4):523-528.
81. Papakostas G.I., et al. S-adenosyl-methionine in depression: a comprehensive review of the literature. Curr Psychiatry Rep. 2003;5(6):460-466.
82. Williams A.L., et al. S-adenosylmethionine (SAMe) as treatment for depression: a systematic review. Clin Invest Med. 2005;28(3):132-139.
83. Alpert J.E., et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
84. Pancheri P., et al. A double-blind, randomized parallel-group, efficacy and safety study of intramuscular S-adenosyl-L-methionine 1,4-butanedisulphonate (SAMe) versus imipramine in patients with major depressive disorder. Int J Neuropsychopharmacol. 2002;5(4):287-294.
85. Salmaggi P., et al. Double-blind, placebo-controlled study of S-adenosyl-L-methionine in depressed postmenopausal women. Psychother Psychosom. 1993;59(1):34-40.
86. Agnoli A., et al. Effect of s-adenosyl-l-methionine (SAMe) upon depressive symptoms. J Psychiatr Res. 1976;13(1):43-54.
87. Berlanga C., et al. Efficacy of S-adenosyl-L-methionine in speeding the onset of action of imipramine. Psychiatry Res. 1992;44(3):257-262.
88. Rosenbaum J.F., et al. The antidepressant potential of oral S-adenosyl-l-methionine. Acta Psychiatr Scand. 1990;81(5):432-436.
89. Hood S.D., et al. Acute tryptophan depletion. Part I: rationale and methodology. Aust N Z J Psychiatry. 2005;39(7):558-564.
90. Roiser J., et al. The subjective and cognitive effects of acute phenylalanine and tyrosine depletion in patients recovered from depression. Neuropsychopharmacology. 2005;30:775-785.
91. Byerley W.F., et al. 5-Hydroxytryptophan: a review of its antidepressant efficacy and adverse effects. J Clin Psychopharmacol. 1987;7(3):127-137.
92. Glassman A.H., Platman S.R. Potentiation of a monoamine oxidase inhibitor by tryptophan. J Psychiatr Res. 1969;7(2):83-88.
93. Nardini M., et al. Treatment of depression with L-5-hydroxytryptophan combined with chlorimipramine, a double-blind study. Int J Clin Pharmacol Res. 1983;3(4):239-250.
94. Walinder J., et al. Potentiation of the antidepressant action of clomipramine by tryptophan. Arch Gen Psychiatry. 1976;33(11):1384-1389.
95. Coppen A., et al. Potentiation of the antidepressive effect of a monoamine-oxidase inhibitor by tryptophan. Lancet. 1963;1(7272):79-81.
96. Levitan R.D., et al. Preliminary randomized double-blind placebo-controlled trial of tryptophan combined with fluoxetine to treat major depressive disorder: antidepressant and hypnotic effects. J Psychiatry Neurosci. 2000;25(4):337-346.
97. Shaw D.M., et al. Tricyclic antidepressants and tryptophan in unipolar depression. Psychol Med. 1975;5(3):276-278.
98. Thomson J., et al. The treatment of depression in general practice: a comparison of L-tryptophan, amitriptyline, and a combination of L-tryptophan and amitriptyline with placebo. Psychol Med. 1982;12(4):741-751.
99. Ayuso Gutierrez J.L., et al. [Tryptophan and amitriptyline in the treatment of depression (double blind study)]. Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1973;1(3):471-476.
100. Mills S., Bone K. Principles and practice of phytotherapy. London: Churchill Livingstone; 2000.
101. Akhondzadeh S., et al. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement Altern Med. 2004;4:12.
102. Noorbala A.A., et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
103. Akhondzadeh B., et al. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: a pilot double-blind randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;30(2):439-442.
104. Akhondzadeh S., et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
105. Moshiri E., et al. Hesameddin Abbasi S, Akhondzadeh S. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13(9–10):607-611.
106. Benazzi F. Is there a continuity between bipolar and depressive disorders? Psychother Psychosom. 2007;76(2):70-76.
107. Cassano G.B., et al. Conceptual underpinnings and empirical support for the mood spectrum. Psychiatr Clin North Am. 2002;25(4):699-712. v
108. Magnusson A., Partonen T. The diagnosis, symptomatology, and epidemiology of seasonal affective disorder. CNS Spectr. 2005;10(8):625-634. quiz 621–614
109. Lambert G.W., et al. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;360(9348):1840-1842.
110. Maciocia G. The practice of Chinese medicine: the treatment of diseases with acupuncture and Chinese herbs. London: Churchill Livingstone; 1994.
111. Wang H., et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord.. 2008;111(2–3):125-134.
112. Leo R.J., Ligot J.S.Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1–3):13-22.
113. Cabyoglu M.T., et al. The mechanism of acupuncture and clinical applications. Int J Neurosci. 2006;116(2):115-125.
114. Wang S.M., et al. Acupuncture analgesia: I. The scientific basis. Anesth Analg. 2008;106(2):602-610.
115. Garner B., et al. Pilot study evaluating the effect of massage therapy on stress, anxiety and aggression in a young adult psychiatric inpatient unit. Aust N Z J Psychiatry. 2008;42(5):414-422.
116. Coelho H.F., et al. Massage therapy for the treatment of depression: a systematic review. Int J Clin Pract. 2008;62(2):325-333.
117. Markowitz J.C. Evidence-based psychotherapies for depression. J Occup Environ Med. 2008;50(4):437-440.
118. Sarris J., et al. Adjuvant use of nutritional and herbal medicines with antidepressants, mood stabilizers and benzodiazepines. J Psychiatr Res. 2009. In press
119. Chrubasik S., et al. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12(9):684-701.
120. Holtmann G., et al. Efficacy of artichoke leaf extract in the treatment of patients with functional dyspepsia: a six-week placebo-controlled, double-blind, multicentre trial. Aliment Pharmacol Ther. 2003;18(11–12):1099-1105.
121. Narimanian M., et al. Randomized trial of a fixed combination (KanJang) of herbal extracts containing Adhatoda vasica, Echinacea purpurea and Eleutherococcus senticosus in patients with upper respiratory tract infections. Phytomedicine. 2005;12(8):539-547.
122. Miyasaka L.S., et al. Passiflora for anxiety disorder. Cochrane Database Syst Rev. (1):2007;. CD004518
123. Pittler M.H., Ernst E. Kava extract for treating anxiety. Cochrane Database Syst Rev. (1):2006;. CD003383
124. Mahady G.B. Ginkgo biloba for the prevention and treatment of cardiovascular disease: a review of the literature. Journal of Cardiovascular Nursing. 2002;16(4):21.
125. Zhou W., et al. Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovasc Drug Rev.. Winter 2004;22(4):309-319.
126. Cohen A.J., Bartlik B. Ginkgo biloba for antidepressant-induced sexual dysfunction. J Sex Marital Ther. 1998;24(2):139-143.
127. Wheatley D. Triple-blind, placebo-controlled trial of Ginkgo biloba in sexual dysfunction due to antidepressant drugs. Hum Psychopharmacol. 2004;19(8):545-548.
128. Kang B.J., et al. A placebo-controlled, double-blind trial of Ginkgo biloba for antidepressant-induced sexual dysfunction. Hum Psychopharmacol. 2002;17(6):279-284.
129. Saller R., et al. An updated systematic review of the pharmacology of silymarin. Forsch Komplement Med. 2007;14(2):70-80.
130. Osiecki H. The physician’s handbook of clinical nutrition, 5th edn. Brisbane: Bioconcepts Publishing; 1998.
131. Brosse A.L., et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760.
132. Lawlor D.A., et al. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ (Clinical Research Ed.). 2001;322(7289):763-767.
133. Dunn A.L., et al. Exercise treatment for depression: efficacy and dose response. American Journal Of Preventive Medicine. 2005;28(1):1-8.
134. McIntyre R.S., et al. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology. 2006;31:1029-1035.
135. Pilkington K., et al. Yoga for depression: the research evidence. Journal Of Affective Disorders. 2005;89(1–3):13-24.
136. Tsang H.W., et al. Effects of mindful and non-mindful exercises on people with depression: a systematic review. Br J Clin Psychol. 2008;47(Pt 3):303-322.
137. Linde K., Knuppel L. Large-scale observational studies of hypericum extracts in patients with depressive disorders – a systematic review. Phytomedicine. 2005;12(1–2):148-157.
138. Roder C., et al. [Meta-analysis of effectiveness and tolerability of treatment of mild to moderate depression with St. John’s Wort]. Fortschr Neurol Psychiatr. 2004;72(6):330-343.
139. Werneke U., et al. How effective is St John’s wort? The evidence revisited. J Clin Psychiatry. 2004;65(5):611-617.
140. Whiskey E., et al. A systematic review and meta-analysis of Hypericum perforatum in depression: a comprehensive clinical review. Int J Clin Psychopharm. 2001;16:239-252.
141. Shaw K., et al. Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst Rev. (1):2002;. CD003198
142. Bottiglieri T., Hyland K. S-adenosylmethionine levels in psychiatric and neurological disorders: a review. Acta Neurol Scand Suppl. 1994;154:19-26.
143. Sarris J., et al. Depression and exercise. Journal of Complementary Medicine. 2008;3:48-50. 61
144. Doyne E.J., et al. Running versus weight lifting in the treatment of depression. Journal of Consulting and Clinical Psychology. 1987;55(5):748-754.