Chapter 10 Clinical Breast Problems and Unusual Breast Conditions
The Male Breast: Gynecomastia and Male Breast Cancer
Broad categories of conditions causing gynecomastia include high serum estrogen levels from endogenous or exogenous sources, low serum testosterone levels, endocrine disorders (hyperthyroidism or hypothyroidism), systemic disorders (cirrhosis, chronic renal failure with maintenance by dialysis, chronic obstructive pulmonary disease), drug-induced (cimetidine, spironolactone, ergotamine, marijuana, anabolic steroids, estrogen for prostate cancer), tumors (adrenal carcinoma, testicular tumors, pituitary adenoma), or idiopathic (Box 10-1). Gynecomastia can occur at any age, but it may be seen in particular in neonates as a result of maternal estrogens circulating to the fetus through the placenta, in healthy adolescent boys 1 year after the onset of puberty because of high estradiol levels, or in older men as a result of decreasing serum testosterone levels.
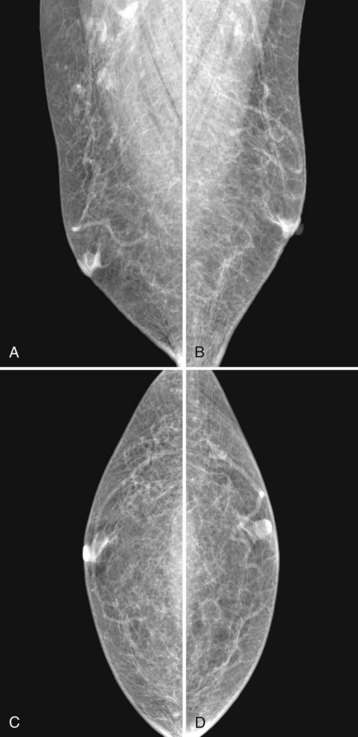
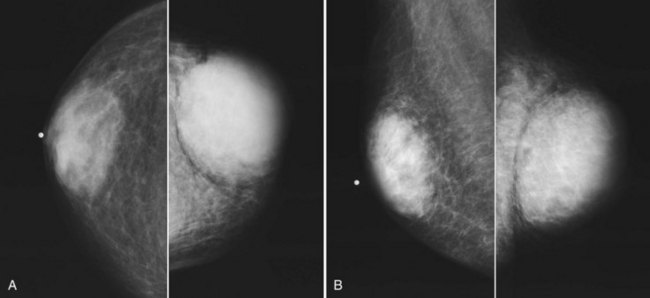
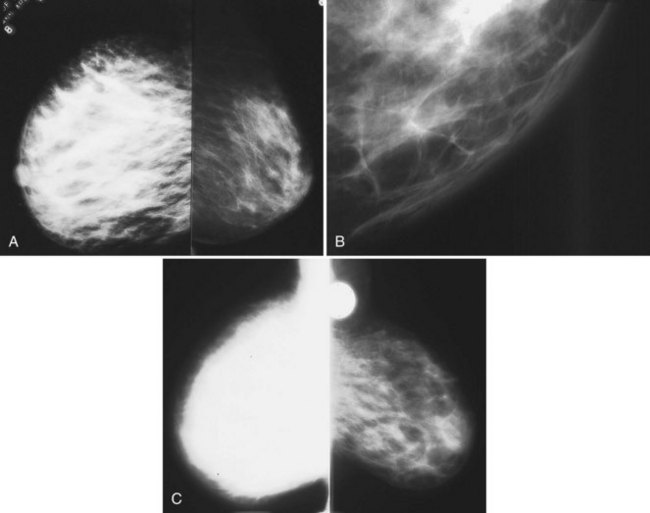
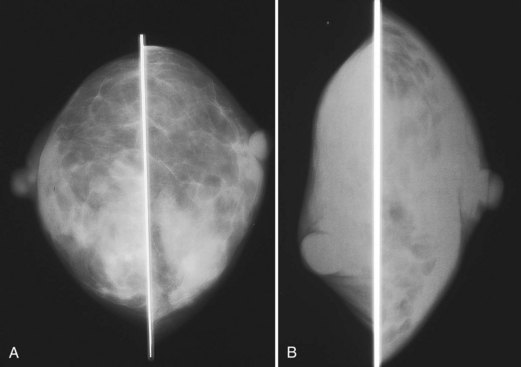
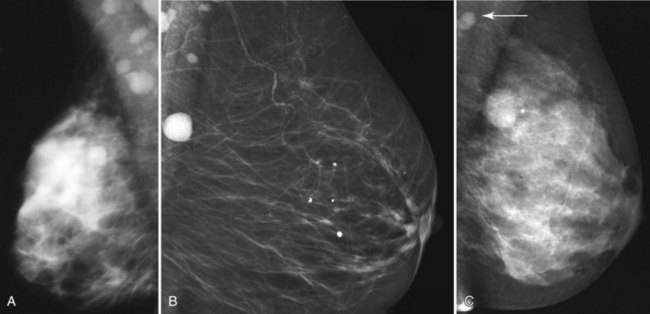
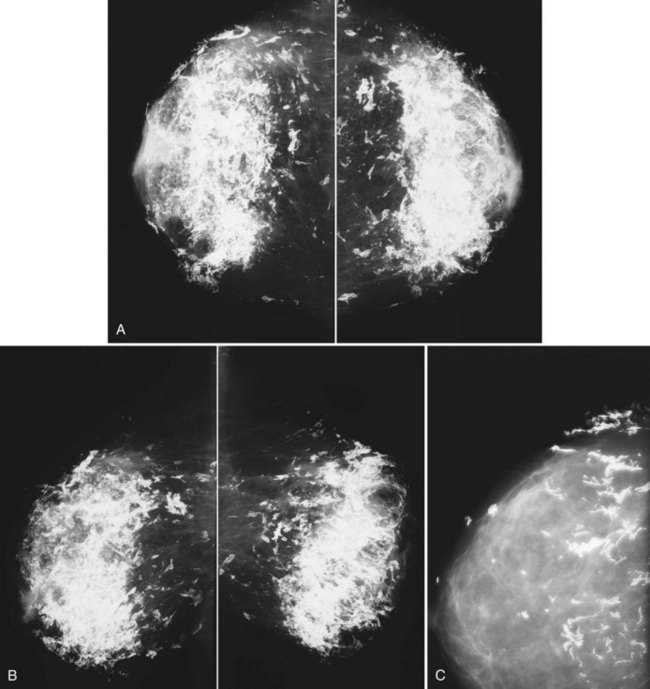
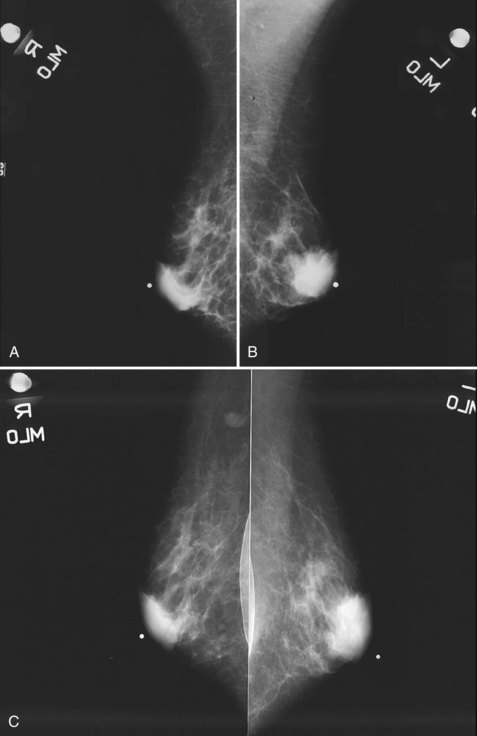
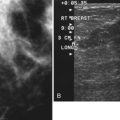
Mammography is performed in men in the same fashion as in women. On the mammogram, the normal male breast consists of fat without obvious fibroglandular tissue, and the pectoralis muscles are usually larger than in women (Fig. 10-1A to D). In both pseudogynecomastia and in women with Turner syndrome, mammograms consist mostly of fat, similar to the normal male breast (see Fig. 10-1E and F).
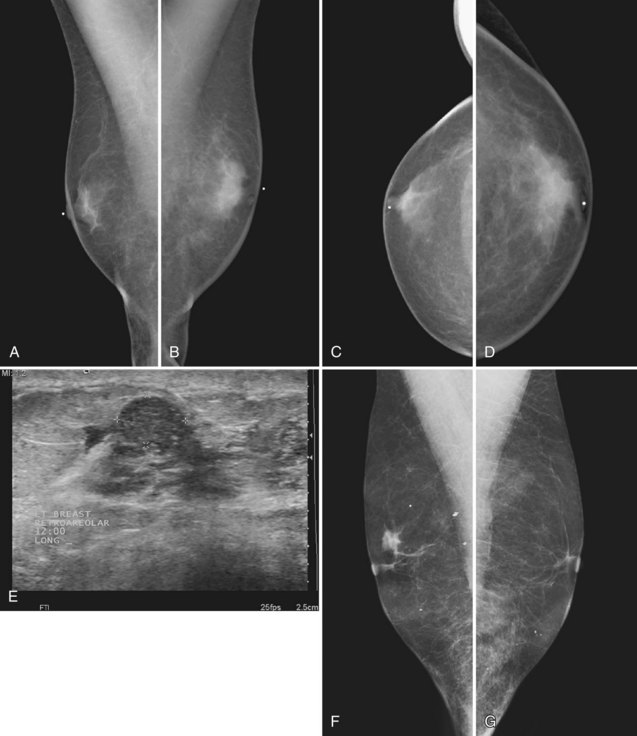
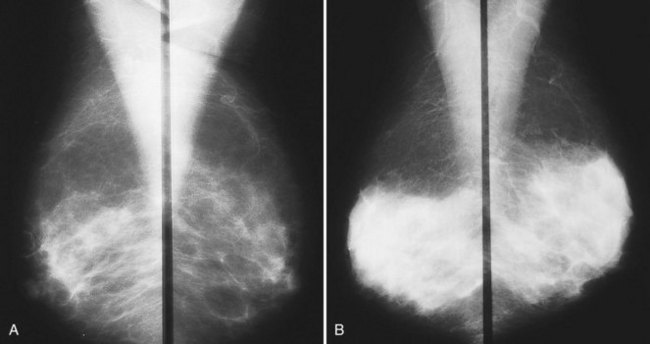
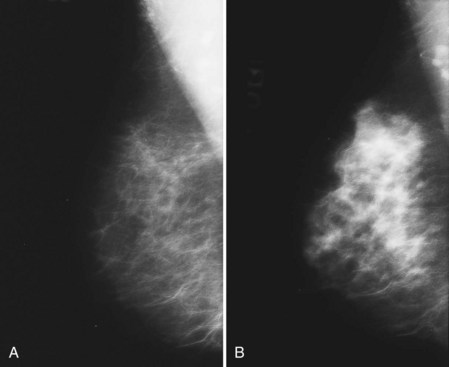
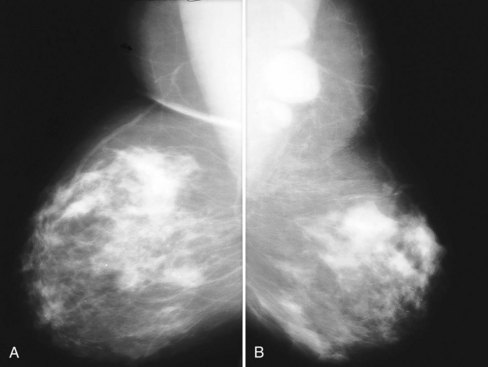
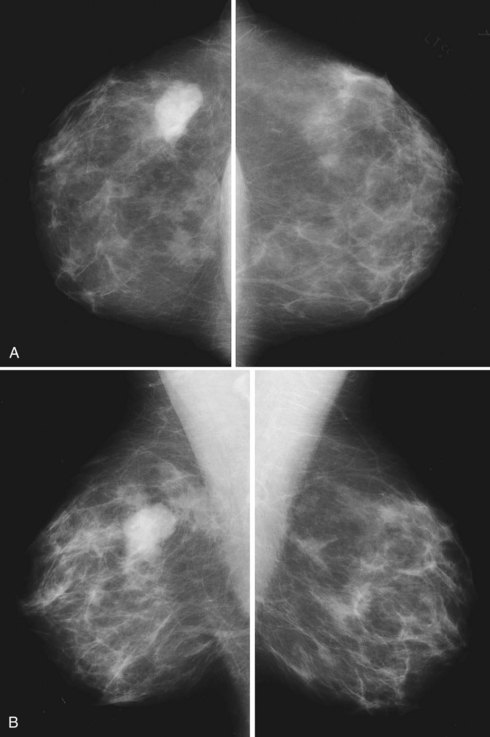
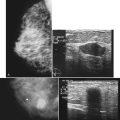
On the mammogram, gynecomastia is shown as glandular tissue in the subareolar region that is symmetric or asymmetric, unilateral or bilateral. In a large series by Gunhan-Bilgen and colleagues, gynecomastia was unilateral in 45% and bilateral in 55% of 206 cases on mammograms. In the early phases of gynecomastia, the glandular tissue takes on a flamelike dendritic appearance consisting of thin strands of glandular tissue extending from the nipple, similar to fingers extending posteriorly toward the chest wall (Table 10-1). With continued proliferation of breast ducts, the glandular tissue takes on a triangular nodular shape behind the nipple in the subareolar region that can be symmetric or asymmetric (Fig. 10-2). If the etiology of the gynecomastia is not eliminated, the proliferation may progress to the appearance of diffuse dense tissue in the later stromal fibrotic phase that is irreversible (Fig. 10-3). On ultrasound, gynecomastia shows hypoechoic flamelike, fingerlike, or triangular structures extending posteriorly toward the chest wall from the nipple (Fig. 10-4). Pseudogynecomastia shows only fatty tissue on the mammogram and is distinguished from gynecomastia by the absence of glandular tissue.
| Type | Mammography | Gynecomastia |
|---|---|---|
| Normal | Fatty breast | N/A |
| Pseudogynecomastia | Fatty breast | N/A |
| Dendritic | Prominent radiating extensions | Epithelial hyperplasia |
| Nodular | Fan-shaped triangular density | Later phase |
| Diffuse | Diffuse density | Dense fibrotic phase |
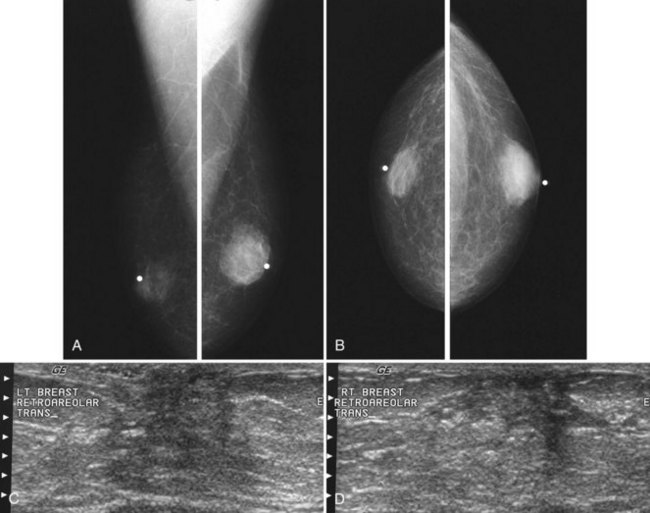
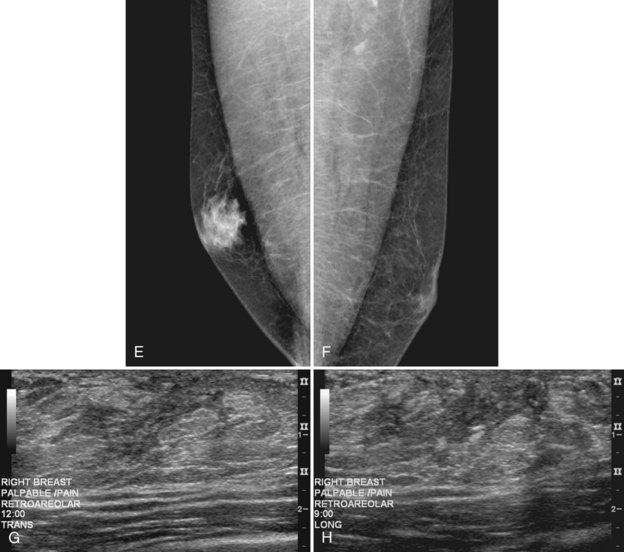
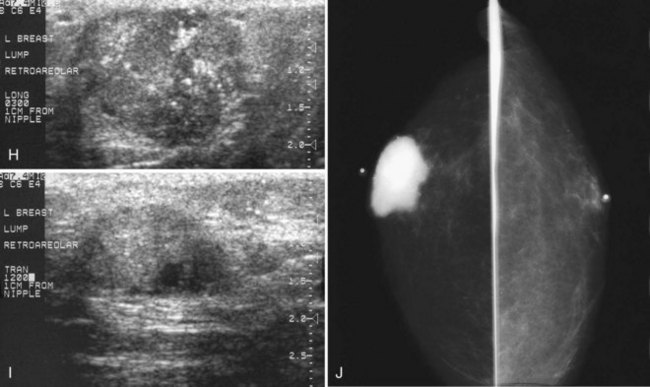
Male breast cancer accounts for less than 1% of all cancers found in men and is usually diagnosed at or around age 60, older than the mean age for the diagnosis of breast cancer in women (Box 10-2). Male breast cancer has the same prognosis as breast cancer in women, but it is often detected at a higher stage than in women because of delay in diagnosis; up to 50% of men have axillary adenopathy at initial evaluation. Risk factors include Klinefelter syndrome, high estrogen levels such as from prostate cancer treatment, and the development of mumps orchitis at an older age. Male breast cancer is generally manifested as a hard, painless, subareolar mass eccentric to the nipple. When not subareolar, cancers in men are usually found in the upper outer quadrant. Clinical symptoms of nipple discharge or ulceration are not rare in association with male breast cancer.
On mammography, male breast cancers are generally dense noncalcified masses with variable margin patterns located in the subareolar region (Figs. 10-5 and 10-6). Calcifications are less common in male than female breast cancer, although calcifications may be present. On ultrasound, male breast cancers are described as masses with well-circumscribed or irregular margins. Concomitant findings of skin thickening, adenopathy, and skin ulceration are associated with a poor prognosis. Breast cancers in men have the histologic appearance of invasive ductal cancer in 85% of cases, with most of the remaining tumors being medullary, papillary, and intracystic papillary tumors. An associated component of ductal carcinoma in situ (DCIS) may be present. Invasive lobular carcinoma is rare. Treatment of breast cancer is the same for men as for women and consists of surgery, axillary node dissection, chemotherapy, radiation therapy for invasive tumors, or any combination of these treatments; the prognosis is identical as that for women.
Pregnant Patients and Pregnancy-Associated Breast Cancer
Pregnancy produces a proliferation of glandular breast tissue that results in breast enlargement and nodularity; rarely, the condition progresses to gigantomastia or enlargement of multiple fibroadenomas. Breast masses are difficult to manage in a pregnant patient because of the surrounding breast nodularity and size increase over time. Most masses occurring in pregnancy are benign and include benign lactational adenomas, fibroadenomas, galactoceles, and abscesses (Box 10-3), but the diagnosis of exclusion is pregnancy-associated breast cancer.
Pregnancy-associated breast cancer is defined as breast cancer discovered during pregnancy or within 1 year of delivery (Box 10-4). The incidence of breast cancer in pregnant women is 0.2% to 3.8% of all breast cancers, or 1 in every 3000 to 10,000 pregnancies. Most pregnancy-associated breast cancers are invasive ductal cancer. These cancers are generally manifested as a hard mass, but they may be associated with bloody nipple discharge or findings of breast edema. The usual initial imaging test in a pregnant patient is breast ultrasound. Many patients are reluctant to undergo mammography because of concern about the effect of radiation on the fetus. However, if cancer is a clinical concern, it is important to perform mammography as part of the evaluation and in particular to detect the presence of suspicious calcifications that are often nonpalpable. The amount of scattered radiation delivered to the fetus is minimal and can be further reduced with lead shielding. Swinford and colleagues showed that breast density on mammography ranges from scattered fibroglandular density in pregnant patients to heterogeneously dense or dense breasts in a lactating patient. In their series, mammography was as useful as it is in nonpregnant women with clinical signs and symptoms of breast disease. In lactating patients, breast density can be reduced on the mammogram by pumping milk from the breasts before the study.
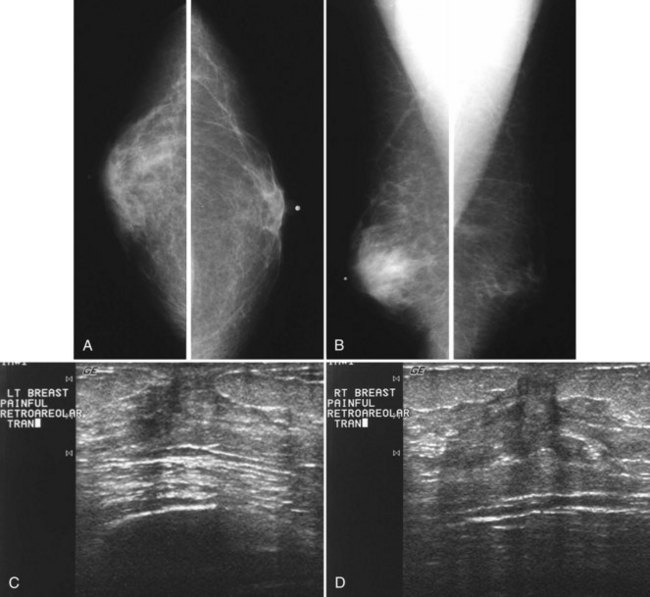
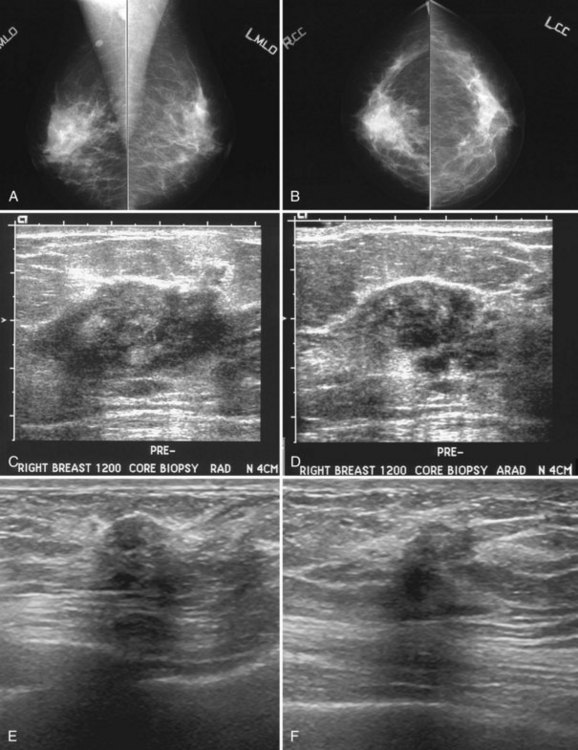
Benign conditions are the most frequent cause of breast masses in pregnant or lactating patients, and cancer is much less common. Lactational mastitis is a common complication of breast-feeding in which the breast becomes painful, indurated, and tender, usually as a result of Staphylococcus aureus infection. A cracked nipple may be the port of entry for the infecting bacteria, but it can be prevented by good nipple hygiene and care, along with frequent nursing to avoid breast engorgement. Treatment is administration of antibiotics and continuation of breast-feeding. On occasion, antibiotic therapy is not sufficient to treat mastitis. If a hot, swollen, painful breast does not respond to antibiotics, ultrasound may identify an abscess and guide percutaneous drainage. On mammography, an abscess is a developing asymmetry or mass in a background of breast edema; it does not usually contain gas and is frequently located in the subareolar region (Fig. 10-7A and B). On ultrasound, abscesses are fluid-filled structures with irregular margins in the early phase, but circumscribed margins develop in the later phase as the walls of the abscess form. The abscess may contain debris or multiple septations, which may be drained under ultrasound guidance, but some residua may remain because of thick debris. Ultrasound-guided percutaneous drainage may be curative in small abscesses or palliative until surgical drainage can be performed in large abscesses. Some investigators report using ultrasound-guided aspiration, with abscess irrigation and instillation of antibiotics directly into the abscess cavity, to aid in resolution of the abscess.
Both fibroadenomas and lactating adenomas are solid benign tumors diagnosed during pregnancy. Growth of preexisting fibroadenomas may be stimulated by the elevated hormone levels of pregnancy, and the fibroadenoma may become clinically apparent. Infarction of fibroadenomas has been reported in the literature during pregnancy as well. Presenting as a firm, painless palpable lump that occurs late in prgenancy or during lactation, the lactating adenoma is a circumscribed, lobulated mass containing distended tubules with an epithelial lining. The mass can enlarge rapidly and regress after cessation of lactation. Ultrasound typically shows an oval, well-defined hypoechoic mass that may contain echogenic bands representing the fibrotic bands seen on pathology (see Fig. 10-7C and D). Whether lactating adenoma represents change stimulated by hormonal alterations in a fibroadenoma or tubular adenoma or whether the tumors arise de novo has not been resolved.
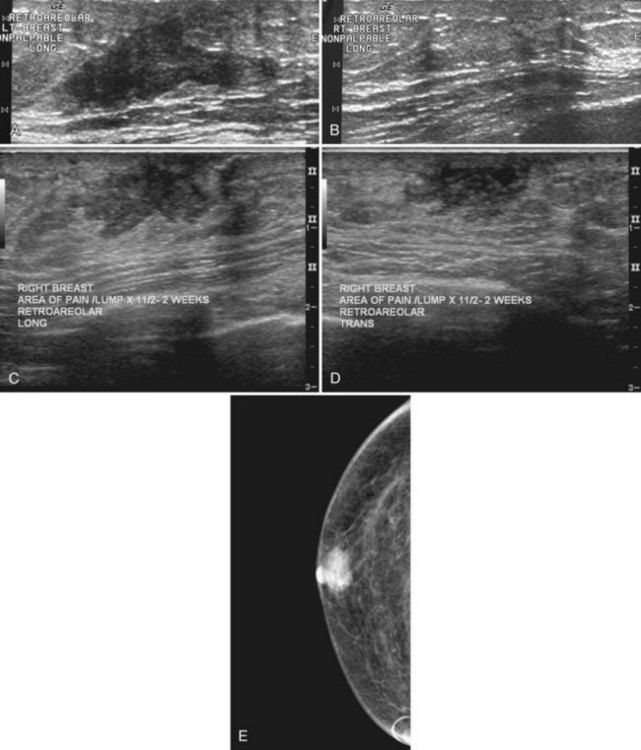
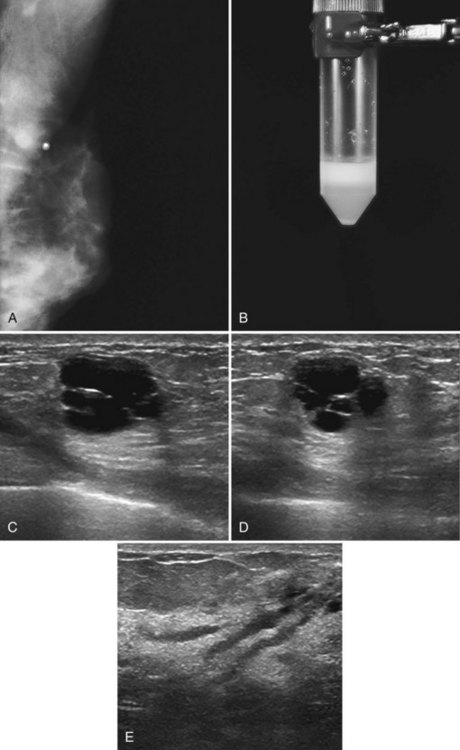
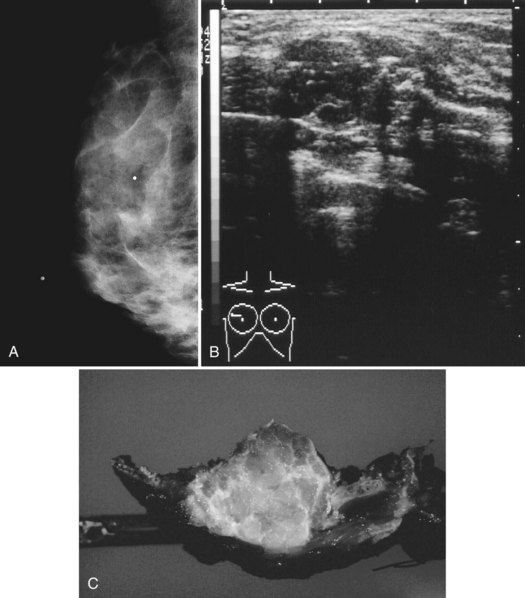
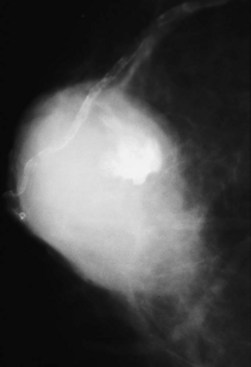
A galactocele produces a fluid-filled breast mass that can mimic a benign or malignant solid breast mass. On mammography, a galactocele is a round or oval, circumscribed mass of equal- or low-density (Fig. 10-8A and B). Because a galactocele is filled with milk, the creamy portions of the milk may rise to the nondependent part of the galactocele and produce a rare, but pathognomonic fluid-fluid or fat-fluid appearance on the horizontal beam image (lateral-medial view) at mammography. Ultrasound shows a fluid-filled mass that can have a wide range of sonographic appearances, depending on the relative amount of fluid and solid milk components within it. Galactoceles that are mostly fluid-filled have well-defined margins with thin echogenic walls (see Fig. 10-8C to E). Galactoceles containing more solid components of milk show variable findings, ranging from homogeneous medium-level echoes to heterogeneous contents with fluid clefts. Both distal acoustic enhancement and acoustic shadowing may be seen. The diagnosis is made by an appropriate history of childbirth and lactation, with aspiration yielding milky fluid and leading to resolution of the mass. Aspiration is usually therapeutic.
Probably Benign Findings (Bi-Rads® Category 3)
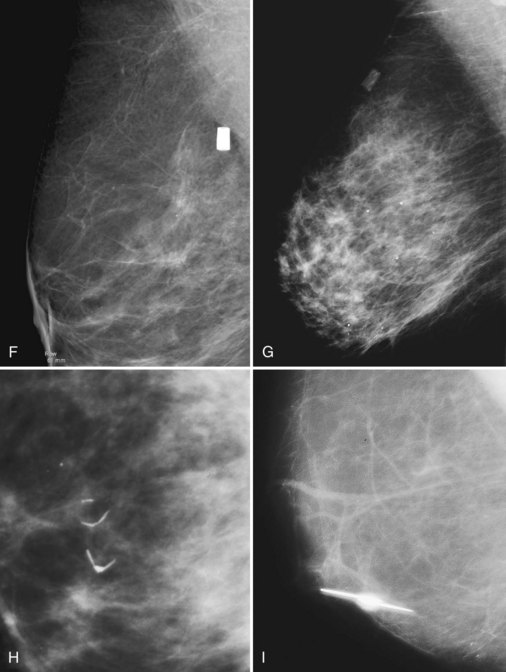
Mammography detects small cancers, but it can also uncover nonpalpable benign-appearing lesions indeterminate for malignancy. Fine-detail diagnostic mammographic views and ultrasound in appropriate cases show that some indeterminate findings are typically benign and the patient can therefore resume screening. Other findings have a low probability (<2%) of malignancy after an appropriate workup that serves as a baseline for follow-up studies (Box 10-5). Sickles, Varas and colleagues, and Yasmeen and colleagues have independently provided data that Breast Imaging Reporting and Database System (BI-RADS®) category 3, or probably benign, findings carry a less than 2% chance of malignancy. Probably benign BI-RADS® category 3 lesions were found in 5%, 3%, and 5% of all screening studies after recall in their series, respectively. Probably benign findings included single or multiple clusters of small, round or oval calcifications; nonpalpable and noncalcified, round or lobulated, circumscribed solid masses; and nonpalpable focal asymmetry containing interspersed fat and concave scalloped margins that resemble fibroglandular tissue at diagnostic evaluation (Fig. 10-9).
Box 10-5
Probably Benign Findings (BI-RADS® Category 3)
Found in 5% of all screening cases after recall and diagnostic workup
Clustered small round or oval calcifications at magnification mammography
Noncalcified oval or lobulated, primarily well-circumscribed solid masses
Asymmetric densities resembling fibroglandular tissue at diagnostic evaluation
From Rosen EL, Baker JA, Soo MS: Malignant lesions initially subjected to short-term mammographic follow-up, Radiology 223:221–228, 2002.
Nipple Discharge and Galactography
The most frequent causes of both nonbloody and bloody nipple discharge are benign conditions. The most common mass producing a bloody nipple discharge is a benign intraductal papilloma, with only approximately 5% of women found to have malignancy at biopsy. The bloody nipple discharge associated with papillomas is due to twisting of the papilloma on its fibrovascular stalk and subsequent infarction and bleeding. Other causes of bloody discharge are cancer, benign findings such as duct hyperplasia/ectasia, and pregnancy as a result of rapidly proliferating breast tissue. Causes of nonbloody nipple discharge are fibrocystic change, medications acting as dopamine receptor blockers or dopamine-depleting drugs, rapid breast growth during adolescence, chronic nipple squeezing, or tumors producing prolactin or prolactin-like substances (Table 10-2).
| Color | Cause |
|---|---|
| Clear or creamy | Duct ectasia |
| Green, white, blue, black | Cysts, duct ectasia |
| Milky |
The mammogram is frequently negative in the setting of nipple discharge (Table 10-3). Mammographic findings described in association with nipple discharge include a negative mammogram, a single dilated duct in isolation, or a small mass containing calcifications in either papilloma or malignancy (Fig. 10-10A to D). Ultrasound is frequently negative in women with nipple discharge, or fluid-filled dilated ducts without an intraductal mass in the retroareolar region may be seen. Solid masses in a fluid-filled duct may represent debris, a papilloma, or cancer.
Table 10-3 Imaging of Nipple Discharge
| Modality | Finding |
|---|---|
| Mammography | |
| Ultrasound | |
| Galactogram | |
| Magnetic resonance imaging |
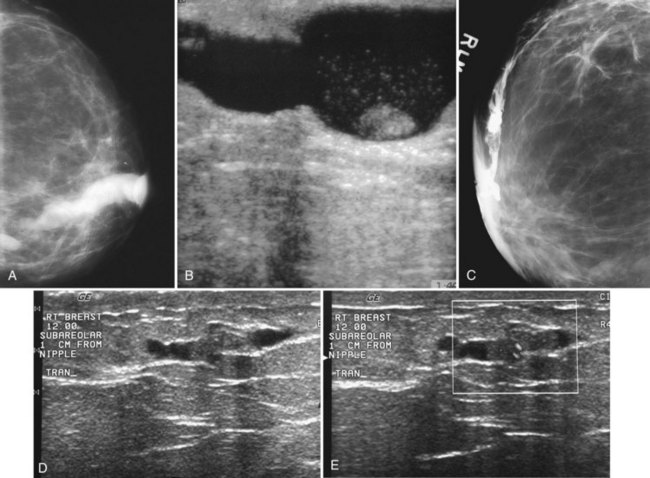
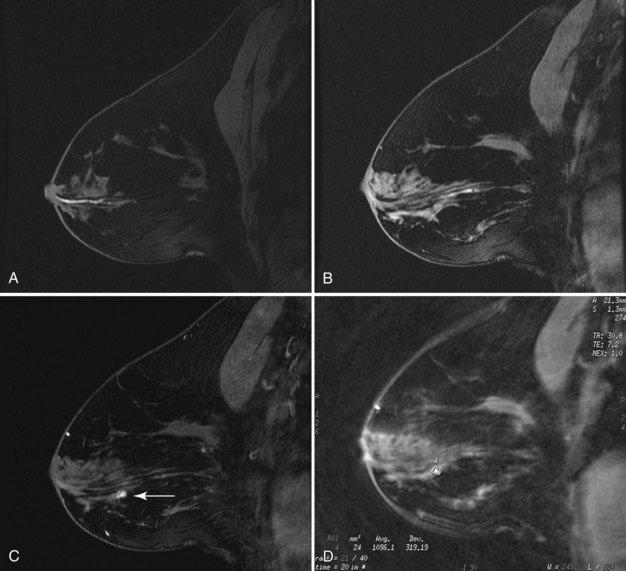
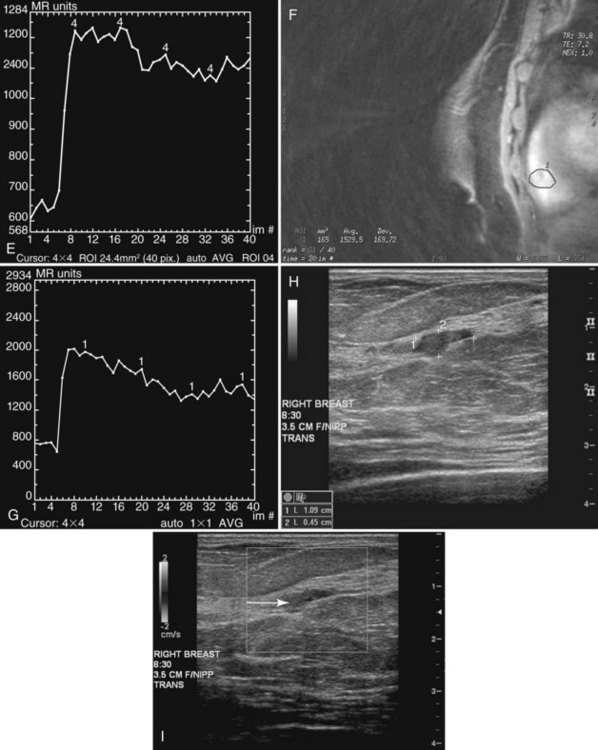
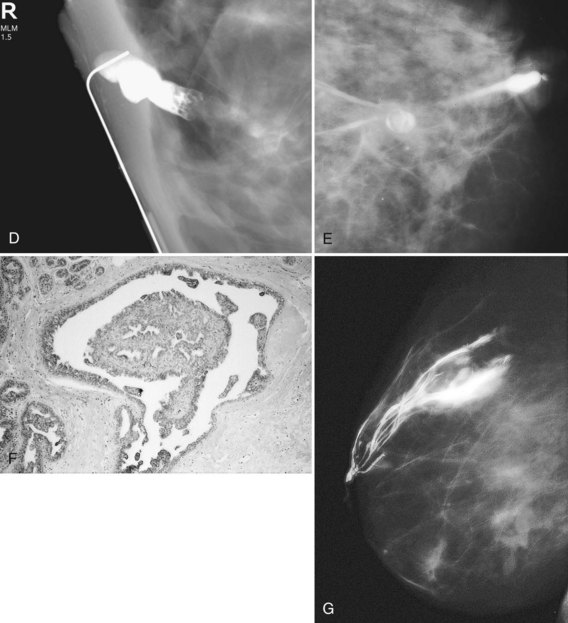
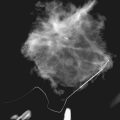
Papillomas on MRI deserve special mention because they mimic cancer by producing a round enhancing mass that frequently has rapid initial early enhancement and a late plateau or washout on kinetic curve analysis, indistinguishable from invasive cancer (Fig. 10-11). For this reason, papillomas are a common cause of false-positive MRI-guided breast biopsies. On MRI, intraductal papillomas can have three patterns. The first pattern is a small circumscribed enhancing mass at the terminus of a dilated breast duct, corresponding to the filling defect seen on galactography. The second pattern is an irregular, rapidly enhancing mass with occasional spiculation or rim enhancement in women without nipple discharge; this is the pattern that cannot be distinguished from invasive breast cancer. Finally, despite the presence of a papilloma, MRI may be negative, with the papilloma undetected on both contrast-enhanced and fat-suppressed T1-weighted studies.
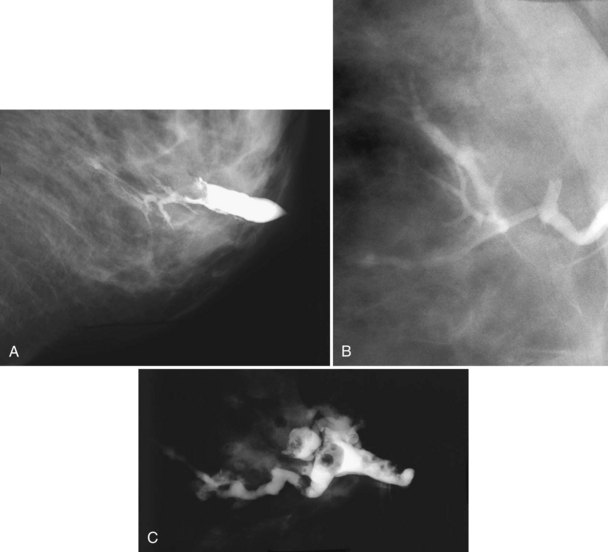
A normal duct arborizes from a single entry point on the nipple into smaller ducts extending over almost an entire quadrant of the breast. Normal ducts are thin and smooth-walled and have no filling defects or wall irregularities (Fig. 10-12A). Ductal ectasia is not uncommon; occasionally, normal cysts or lobules fill from the dilated ducts (see Fig. 10-12B). Ectatic ducts without a filling defect are usually normal. However, despite a normal galactogram, surgical excision of the discharging duct may reveal papillomas or cancer (i.e., false-negative study; see Fig. 10-12C).
Ducts containing malignancy or papillomas are typically dilated between the tumor and the nipple. Positive galactograms show a filling defect, an abrupt duct cutoff, or luminal irregularity and distortion. Tumors causing the abnormal findings may be located inside a fluid-filled dilated duct or may compress the duct from outside the duct walls (Fig. 10-13A to D). On occasion, masses, either papilloma or intracystic cancer, may become encysted (see Fig. 10-13D and E). Air bubbles produce filling defects that mimic papilloma or cancer, but they are usually sharply defined and round and change position inside the duct on repeat injection, unlike fixed intraductal tumors. On the galactogram, extravasation is seen as contrast extending outside the duct lumen into the breast tissue and obscuring the underlying breast tissue and ducts (see Fig. 10-13F). In the rare instance of lymphatic or venous uptake of extravasated contrast, a draining tubular structure leading away from the extravasation site can be seen.
Breast Edema
On clinical examination, breast edema may be evident as peau d’orange (a term signifying thickening and elevation of the skin around tethered hair follicles, similar in appearance to an orange peel), and the edematous breast may be larger than the contralateral side. The differential diagnosis for breast edema depends on whether the edema is unilateral or bilateral (Box 10-6). Unilateral breast edema is due to mastitis, inflammatory cancer, local obstruction of lymph nodes, trauma, radiation therapy, or coumarin necrosis (Fig. 10-14). Bilateral breast edema is due to systemic etiologies, such as congestive heart failure, liver disease, anasarca, renal failure, or other conditions that can cause edema elsewhere in the body. Alternatively, bilateral lymphadenopathy or superior vena cava obstruction for any reason may cause bilateral breast edema (Fig. 10-15).
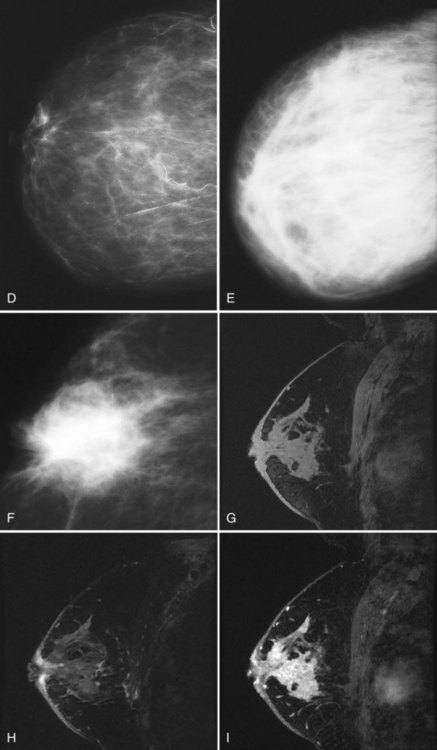
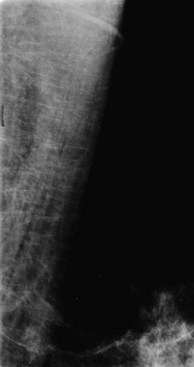
The differential diagnosis for increased bilateral breast density on mammography includes breast edema, exogenous hormone therapy, and weight loss. Increased breast density from edema is due to fluid overload. Increased breast density from exogenous hormone therapy is due to hormonally driven proliferation of stromal and epithelial breast tissue (Fig. 10-16). Increased breast density due to weight loss is not due to any increase in breast tissue, but is due to loss of fat (Fig. 10-17). A history of recent weight loss should lead to the correct diagnosis.
To disinguish between breast edema and exogenous hormone therapy or weight loss, the radiologist looks for skin thickening, which is found only with breast edema (Box 10-7). The increased breast density from breast edema can occur anywhere; skin thickening in dependent portions of the breast is often seen. The increased breast density from exogenous hormone therapy is usually bilateral, occurs in regions where breast tissue was previously present, and shows no skin thickening.
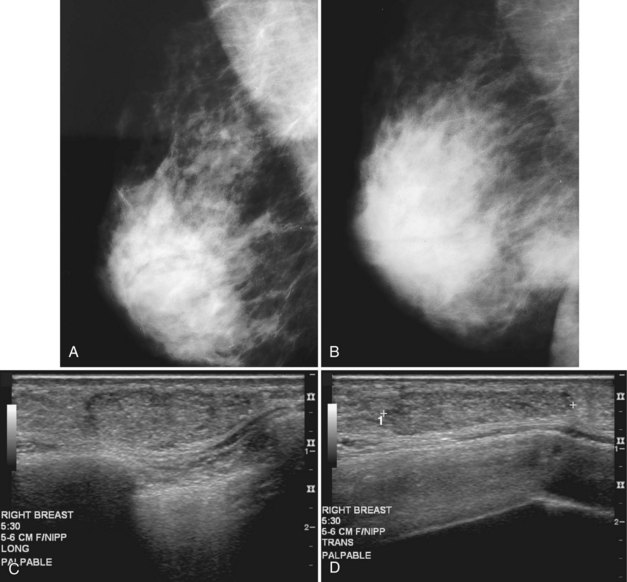
Inflammatory cancer, a rare (1% of all cancers) aggressive breast cancer with a poor prognosis, is the most important differential diagnosis for unilateral breast edema. The definition of inflammatory cancer varies, but it usually has the clinical signs of an enlarging erythematous breast with peau d’orange, and it should be distinguished from locally advanced breast cancer, producing a focal, red, raised skin metastasis. Inflammatory cancer is often mistaken for mastitis because of its clinical features, but it does not respond to antibiotics. Mammography of inflammatory cancer reveals findings of breast edema (skin thickening, diffuse increased breast density, trabecular thickening), but it may also demonstrate findings of cancer, including a breast mass, asymmetric focal density, microcalcifications, nipple retraction, or axillary adenopathy. Ultrasound may show findings of breast edema in 96% of cases, masses in 80%, and dilated lymphatic channels in 68%. On MRI, one report of inflammatory cancer described a “patch enhancement” pattern with some “areas of focal enhancement” and washout on the late phase of the dynamic curve. The enhancement rate on MRI for inflammatory cancer is reported to be quite rapid in the initial postcontrast phase and slightly less rapid in mastitis. The MRI shows breast edema, skin thickening, and skin enhancement (see Fig. 10-14G to I). On biopsy, breast cancer is present in the dermal lymphatics in 80% of cases. The usual management is biopsy to make the diagnosis of inflammatory cancer, neoadjuvant chemotherapy with or without subsequent surgery, and radiation therapy, depending on tumor response, or any combination of these modalities.
An extremely rare cause of unilateral breast edema is necrosis of the breast from coumarin (warfarin [Coumadin]) therapy. Necrosis occurs more commonly in the abdomen, buttocks, and thighs, rather than in breast, and it occurs in 0.01% to 1% of coumarin-treated patients (Box 10-8). Although it has been associated with protein C or protein S deficiency, the exact mechanism of coumarin-induced necrosis is unknown. Painful lesions, swelling, and petechiae from thrombosis of small vessels and inflammation occur after the initiation of coumarin treatment; large hemorrhagic bullae result and develop to full-thickness fat and skin necrosis. Discontinuing the use of coumarin is recommended. Heparin or other anticoagulants may be necessary in patients who require sustained anticoagulation in the short term. Heparin-induced skin necrosis has also been reported in association with type II heparin-induced thrombocytopenia, but heparin is often used in the setting of coumarin necrosis. In some cases, the skin lesions heal spontaneously after shallow tissue sloughing. In other cases, skin grafts are required; in extreme cases, mastectomy is required.
Hormone Changes
Exogenous hormone replacement therapy, pregnancy, and lactation reverse the trend toward fatty breast tissue by causing a proliferation of the glandular elements and periductal stroma of the breast, thereby resulting in a denser mammogram. Unlike breast edema, only the breast tissue becomes denser, and the skin does not become thickened more than 2 to 3 mm as it does with breast edema (Fig. 10-18).
Axillary Lymphadenopathy
Axillary lymphadenopathy is visualized on mammography as replacement of the fatty hilum of lymph nodes by dense tissue, a rounded shape of the lymph nodes, and an overall generalized increased density with or without lymph node enlargement (Fig. 10-19). Abnormal lymph nodes may also contain calcifications, gold deposits mimicking calcifications from treatment of rheumatoid arthritis, or silicone from a previously ruptured breast implant. The differential diagnosis for axillary adenopathy without a definite breast mass varies for unilateral versus bilateral findings (Box 10-9). Causes of unilateral axillary adenopathy include metastatic breast cancer and mastitis. Bilateral axillary adenopathy is usually due to systemic etiologies, such as infection, collagen vascular diseases such as rheumatoid arthritis, lymphoma, leukemia, or metastatic tumor.
“Calcific” particles in abnormal axillary lymph nodes may represent calcified metastasis from breast cancer or calcifying infections such as tuberculosis (Box 10-10). In the case of tuberculous mastitis, patients have axillary swelling and breast enlargement without a breast mass, as well as enlarged dense or matted axillary lymph nodes or breast edema with or without findings of pulmonary tuberculosis. The finding of macrocalcifications rather than pleomorphic microcalcifications in the lymph nodes may suggest tuberculous mastitis, but biopsy is necessary to exclude metastatic breast cancer. Migration of silicone into axillary lymph nodes from ruptured silicone breast implants or migration of gold particles from therapy for rheumatoid arthritis may mimic calcifications in lymph nodes, but the clinical history should provide clues to the correct diagnosis.
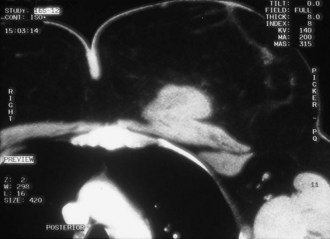
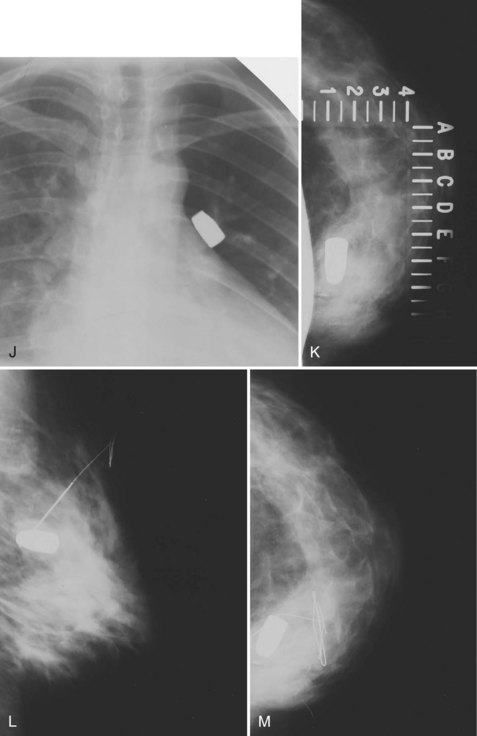
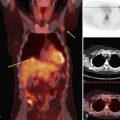
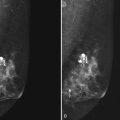
Primary breast cancer presenting as isolated lymph node metastasis in the setting of normal mammographic and physical examination findings is an uncommon clinical problem that accounts for less than 1% of all breast cancers (Fig. 10-20). Both breast ultrasound and contrast-enhanced breast MRI have been used to detect the primary breast cancer in this scenario, with improved results in comparison to mammography, and breast conservation rather than mastectomy is a potential option once the primary tumor is found (Fig. 10-21). Diagnosis of a primary cancer within the breast is clinically contributory, because an occult primary malignancy in the breast would be considered locoregional rather than metastatic disease in patients who present with axillary lymphadenopathy of unknown primary. In some cases the primary breast cancer is never identified. In a pathology series by Haupt and colleagues, in which 43 women with this clinical dilemma were reviewed, the primary tumor was found in 31 (72%) specimens but never identified in the remaining 12. Survival rates between the two groups were similar, and the 12 women in whom a tumor was never discovered did have another primary malignancy detected in the follow-up period.
Paget Disease of the Nipple
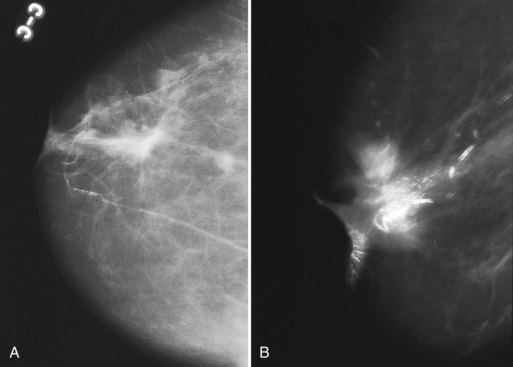
The nipple and mammogram are normal in almost 50% of cases despite clinical signs of Paget disease and the presence of an underlying breast cancer (Box 10-11). On abnormal mammograms, the underlying malignancy has the appearance of suspicious microcalcifications, a spiculated mass, or both. The cancer is often located in the subareolar region or deep in the breast and does not necessarily lie directly adjacent to the nipple or areola (Fig. 10-22A). In women with Paget disease, skin or areolar thickening, nipple retraction, subareolar masses, or calcifications leading to the nipple should be viewed with suspicion on mammography (see Fig. 10-22B). Spot compression magnification mammography of the nipple and retroareolar region is often helpful in identifying subtle abnormalities. Conversely, nipple–areolar abnormalities or thickening detected at mammography should be correlated with the physical examination to exclude clinical findings of Paget disease.
Sarcomas
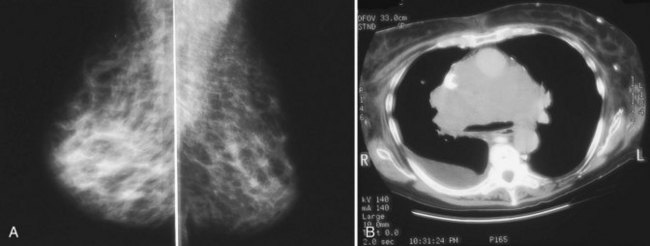
Sarcomas are rare tumors of the breast or the underlying chest wall, and their classification depends on the cell type involved (Fig. 10-23). Ultrasound shows a hypoechoic mass and may be helpful in determining whether the origin of the sarcoma is from the breast or chest wall (Fig. 10-24A and B). Mammography shows high-density masses without calcifications or spiculation, unless the tumor has osseous elements (Fig. 10-25). MRI is useful for demonstrating pectoralis muscle or chest wall involvement, because sarcomas tend to be large and locally invasive.
Mondor Disease
Mondor disease is acute thrombophlebitis of the superficial veins of the breast (Box 10-12). It is rare, is often associated with trauma or recent surgery, and has been reported to occur after sonography-guided or stereotactic core biopsy, but may also be idiopathic in origin. Patients report acute pain, discomfort, and tenderness along the lateral aspect of the breast, the chest wall, or the region of the thrombosed vein; they may also report a cordlike, painful elongated mass just below the skin. Extension of the arm may produce a long narrow furrow in the skin as a result of retraction from the thrombosed vein, similar to skin dimpling from breast cancer.
Case reports describe negative mammographic findings in women with Mondor disease or, rarely, a long linear or tubular density on the mammogram corresponding to the thrombosed vein (Fig. 10-26). Case reports of ultrasound in Mondor disease show a noncompressible hypoechoic tubular cord in the subcutaneous tissue, with or without flow on color Doppler imaging, depending on the degree of recanalization.
Granulomatous Mastitis
Women undergoing mammography are found to have asymmetric density, focal asymmetric or ill-defined breast masses, or negative results. Calcifications are not a feature. On ultrasound, findings include irregular masses, focal regions of inhomogeneous patterns associated with hypoechoic tubular/nodular structures, or decreased parenchymal echogenicity with acoustic shadowing, all suggestive of malignancy (Fig. 10-27A to D).
Diabetic Mastopathy
Diabetic mastopathy produces hard, irregular, sometimes painful mobile breast masses that may be recurrent or bilateral in patients with a history of long-term insulin-dependent diabetes, in younger premenopausal diabetic women, or in rare patients with thyroid disease (Box 10-13). Diabetic mastopathy is due to an autoimmune reaction to the accumulation of abnormal matrix proteins caused by hyperglycemia. It leads to atrophy and obliteration of glandular breast tissue and the production of fibrosis, which forms a hard mass simulating breast cancer. Because of the hardness of the mass, needle biopsy is often performed, but it may be insufficient for diagnosis and therefore may necessitate histologic sampling. Pathologic examination reveals fibrosis with a dense lymphocytic infiltration around breast lobules and ducts.
Mammography shows a regional asymmetric density with ill-defined margins but no microcalcifications or dense glandular tissue. Ultrasound demonstrates a hypoechoic mass or region displaying marked acoustic shadowing in most cases, findings suggestive of scirrhous breast cancer (see Fig. 10-27E).
Desmoid Tumor
On mammography, desmoid tumors are spiculated masses. Ultrasound shows a hypoechoic shadowing mass. Because these masses simulate spiculated breast cancer, biopsy is required (Fig. 10-28).
Treatment of desmoid tumors is complete local surgical excision. Recurrence of desmoid tumor is less likely with wide excision and clear histologic margins. Tumor recurrence usually occurs within 3 years of excision, and for this reason breast reconstruction is generally delayed for 3 years. Because surgical trauma has been associated with recurrence, informed consent is necessary before breast reconstruction. Recurrences are treated by radical excision, just as the primary tumor is. Radiation therapy is used as an alternative to surgery for tumors in which complete excision would result in a poor functional outcome or for some tumors with positive margins (Box 10-14).
Trichinosis
Trichinosis is caused by the ingestion of raw or undercooked meat containing encysted larvae of the Trichinella genus. Diarrhea is produced during the intestinal phase of adult development, and then myositis, fever, and periorbital edema develop during larval migration (Box 10-15). After gastric digestion releases the encysted larvae, the larvae migrate into the intestinal mucosa, mature, and mate. The adult female releases new larvae into mucosal blood vessels, and the larvae are distributed throughout the body over a period of 4 to 6 weeks. The larvae enter skeletal muscles, most commonly the diaphragm, tongue, periorbital muscles, deltoid, pectoralis, gastrocnemius, and intercostal muscles, where the larvae encyst and calcify in 6 to 18 months, with a further life span of 5 to 10 years in the encysted form. During migration, larvae may also produce myocarditis, pneumonitis, or central nervous system symptoms from vasculitis of small arteries or capillaries, but encystment does not usually occur in these locations. Ingestion of the encysted larvae by a new host perpetuates the life cycle of the organism. In the United States, most Trichinella infections are asymptomatic and are acquired by ingesting undercooked pork, feral meat, wild boar, bear, or walrus.
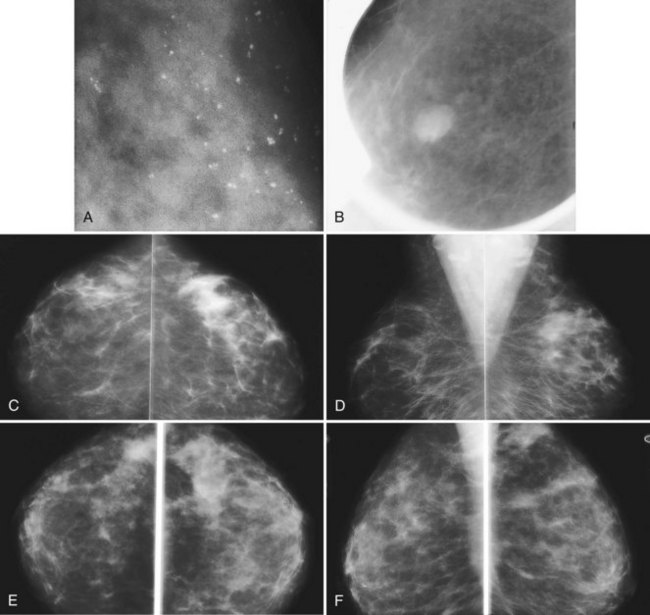
On mammography, the calcified encysted larvae are seen as tiny linear calcifications smaller than 1 mm that are aligned along the long axis of the pectoralis muscle, parallel to the muscular fibers (Fig. 10-29). Because the calcifications are within the muscle, they should not be mistaken for breast cancer. At this point patients are asymptomatic.
Dermatomyositis
Dermatomyositis and some collagen vascular diseases can rarely produce calcifications within the soft tissues of the arms and legs. In the breast, bizarre sheetlike calcifications form in a configuration similar to that of fat necrosis; the calcifications align along the breast tissues and generally point at the nipple (Fig. 10-30). Dermatomyositis is not a specific indicator of breast cancer. However, mammography is often performed because of an association of dermatomyositis with malignancies.
Foreign Bodies
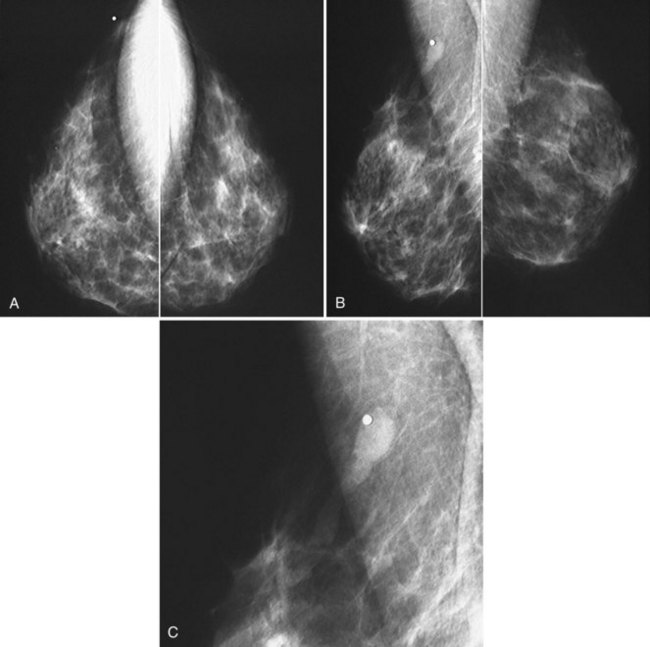
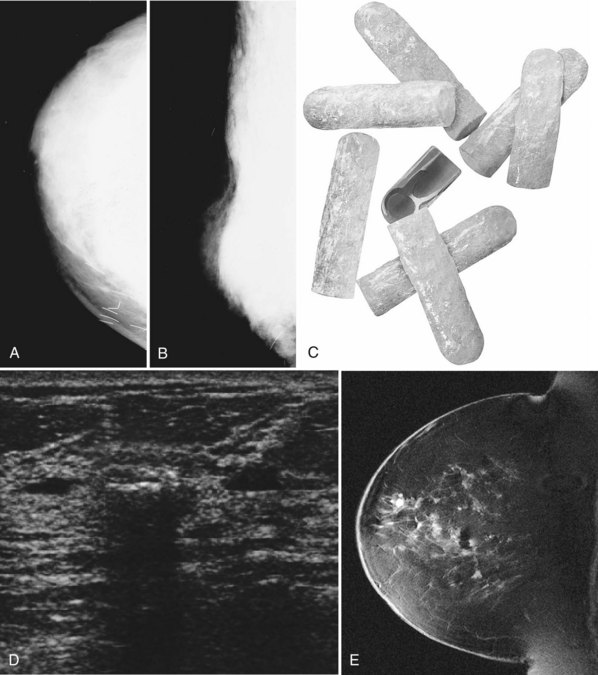
Foreign bodies can be seen within the breast on mammography. Some acupuncture practitioners break acupuncture needle tips off in the breast tissue after placement, and the tiny sheared-off metallic needle tip fragments can be seen inside the breast tissue (Fig. 10-31A and B).
The most common foreign bodies seen in the breast on mammography are metallic markers placed percutaneously after core needle biopsy guided by stereotaxis, ultrasound, or MRI (see Fig. 10-31C to E). The markers have different shapes, depending on the manufacturer, and may contain a pellet or pellets that are visible by ultrasound. For patients who desire removal of the markers, case reports describe the use of an 11-gauge stereotactic vacuum-assisted probe technique that can remove the markers percutaneously. Percutaneous breast biopsy devices may produce tiny residual metallic shavings or fragments from the biopsy probe or needle itself and are usually not seen on the mammogram. These fragments are ferromagnetic and can occasionally cause signal voids on MRI.
Round Dacron Hickman catheter cuffs may be left inside the breast after removal of a Hickman catheter. The rounded, short tubelike structure made of Dacron has a characteristic appearance in the upper part of the breast on mammography (see Fig. 10-31F and G).
Sutures used to close breast cancer biopsy sites may calcify after lumpectomy and radiation therapy, thereby delaying absorption of the suture material and promoting calcification. The result is linear or curvilinear calcification of the suture; the diagnosis can be made if the suture still contains a knot (see Fig. 10-31H).
Other materials may lodge in the breast, and the clinical history may help in the diagnosis (see Fig. 10-30I to M).
Neurofibromatosis
The skin lesions of neurofibroma can mimic a deep breast tissue mass and limit evaluation of the breast. However, like any skin lesion, neurofibromas may be outlined with air, and correlation with the breast history form is advisable. Furthermore, the description of cutaneous findings on the technologist’s sheet should enable the radiologist to distinguish neurofibromas on the skin from masses of ductal origin inside the breast (Fig. 10-32).
Poland Syndrome
Poland syndrome is an absence of the pectoralis muscle on x-ray. It is usually unilateral. The mammogram shows absence of the pectoralis muscle on one side (Fig. 10-33).
Breast Cancer Missed by Mammography
Cancer may not be detected on previous mammograms for several reasons (Box 10-16). First, the tumor may have a morphology that is undetectable on the mammographic background on which it is displayed and is therefore mammographically occult.
Box 10-17 shows factors that may contribute to cancer being missed on previous mammograms. Birdwell and colleagues reviewed possible reasons why tumors were not identified on previous mammograms. They postulated that findings were hidden among many other findings (“busy breasts”) or that distracting findings other than the cancer were present on the film. Other contributing factors included dense breast tissue, small calcifications or masses that may have been overlooked, cancers hiding in the axilla and simulating lymph nodes, linear microcalcifications simulating vascular calcifications, findings seen on only one mammographic view, and findings at the edge of the film or at the edge of the glandular tissue, producing either a tent sign or concavity that was missed at the time of screening. Of note, most of these cancers were located in the upper outer quadrant, where 50% of all cancers occur. Also of note, not all the cancers that were missed were small, inasmuch as at least half the tumors were 1 cm or larger at the time that they were missed.
Box 10-17 Factors Contributing to Missed Cancers on Mammography
Asymmetries
Asymmetries differ from true masses in that they may be seen primarily in one of two standard mammographic views, display concave-outward (rather than convex-outward) margins, and have lucent fat interspersed rather than being densest in the center (Box 10-18). Asymmetries are often normal findings in the breast, but they may occasionally represent a subtle malignancy, particularly lobular carcinoma. Hence, appropriate imaging evaluation is crucial in diagnosing these often subtle cancers while avoiding an unacceptably high false-positive rate.
Box 10-18 Breast Asymmetries
Asymmetry is seen in one standard mammographic view and likely represents summation
Global asymmetry occupies at least one quadrant and likely represents normal asymmetry between breasts
Focal asymmetry is seen in two mammographic views but does not fulfill criteria of mass
Developing asymmetry is focal asymmetry that is new or increasing and generally requires biopsy
Key Elements
The normal male breast shows only fat on mammography.
Gynecomastia is unilateral or bilateral, symmetric or asymmetric, and is shown as glandular tissue in a retroareolar flamelike dendritic, triangular nodular, or diffuse appearance on mammography.
Gynecomastia causes breast lumps and pain and has physiologic, drug-related, and medical-related etiologies.
Breast cancer in men is rare, is manifested as a mass eccentric to the nipple or in the upper outer quadrant, and has the same prognosis as breast cancer in women.
Breast cancer in men develops at 1% of the rate in women, occurs in older men, and on mammography is usually a noncalcified spiculated or circumscribed retroareolar or periareolar mass.
Pregnancy-related conditions include mastitis, lactational adenoma, enlarging fibroadenoma, galactocele, and pregnancy-associated breast cancer.
Pregnancy-associated breast cancer is defined as cancer diagnosed during pregnancy or within 1 year of delivery.
Stage for stage, the prognosis for pregnancy-associated breast cancer is the same as for nonpregnant women.
On mammography, pregnancy-associated breast cancer is detected as masses or pleomorphic calcifications.
Probably benign findings (BI-RADS® category 3) include single or multiple clusters of small, round or oval calcifications, circumscribed masses, and nonpalpable focal asymmetries that resemble fibroglandular tissue at diagnostic evaluation.
To identify subtle signs of malignancy and definitively benign entities, screen-detected findings should be recalled for full diagnostic imaging evaluation (including fine-detail diagnostic mammographic views and often ultrasound) before rendering a BI-RADS® category 3 assessment and assigning to short-term imaging follow-up.
Nipple discharge characteristics that should be investigated are new, bloody, or spontaneously occurring copious serous discharge.
Mammograms and ultrasound are frequently negative in the setting of nipple discharge.
A positive galactogram shows a filling defect, an abrupt duct cutoff, or luminal irregularity.
The differential diagnosis of intraductal masses on galactography includes papilloma, cancer, debris, and an air bubble.
Unilateral breast edema may be caused by mastitis, inflammatory cancer, local obstruction of lymph nodes, trauma, radiation therapy, or coumarin necrosis.
Bilateral breast edema is due to systemic etiologies, such as congestive heart failure, liver disease, anasarca, renal failure, bilateral lymphadenopathy, or superior vena cava syndrome.
Although breast edema, exogenous hormone therapy, and weight loss all result in increased breast density, distinction between these causes is made by the presence of skin thickening, which is seen only with breast edema.
Inflammatory cancer is the most important differential diagnosis for unilateral breast edema and is a rare (1% of all cancers) aggressive breast cancer with a poor prognosis.
The most common cause of mastitis is S. aureus; rare causes include tuberculosis, syphilis, hydatid disease, and molluscum contagiosum.
An extremely rare cause of unilateral breast edema is coumarin (warfarin [Coumadin]) therapy, producing necrosis of the breast.
Axillary lymphadenopathy on mammography is shown as replacement of the fatty hilum of lymph nodes by dense tissue, a rounded lymph node shape, and overall generalized increased density with or without lymph node enlargement.
The differential for abnormal lymph nodes containing “calcifications” includes calcifying metastatic disease, granulomatous disease, gold deposits from therapy for rheumatoid arthritis mimicking calcifications, or silicone from a previously ruptured breast implant.
The differential for unilateral axillary adenopathy includes metastatic breast cancer or mastitis.
The differential for bilateral axillary adenopathy is systemic conditions such as infection, collagen vascular diseases such as rheumatoid arthritis, lymphoma, leukemia, and metastatic tumor.
Primary breast cancer manifested as isolated lymph node metastasis in women with normal mammographic findings and normal physical examinations is uncommon and accounts for less than 1% of all breast cancers.
Paget disease of the nipple heralds an underlying breast cancer with a high rate of overexpression of the c-erb-B2 oncogene.
Women with Paget disease of the nipple have a bright red nipple, eczematous nipple changes that may extend to the areola, and subsequent ulceration or nipple destruction.
Sarcomas are rare malignant tumors of the breast or underlying chest wall. Their classification depends on the cell type; mammography shows high-density masses without calcifications or spiculation.
Mondor disease is a rare benign and self-limited acute thrombophlebitis of the superficial veins of the breast. It is often associated with trauma or recent surgery and produces a tender palpable cord extending toward the outer portion of the breast.
Mammography is usually negative in women with Mondor disease or rarely shows a long linear or tubular density corresponding to the thrombosed vein.
Granulomatous mastitis is a rare benign cause of a breast mass in young premenopausal women after their last childbirth; it has been correlated with breast-feeding and oral contraceptive use, and a possible autoimmune component has been implicated in its etiology.
Patients with granulomatous mastitis may have galactorrhea, inflammation, a breast mass, induration, and skin ulcerations. Treatment is surgery, but the recurrence rate is high.
Diabetic mastopathy is a benign cause of hard, irregular, sometimes painful mobile breast masses in long-term insulin-dependent diabetes, younger premenopausal diabetic women, or rare patients with thyroid disease.
Diabetic mastopathy is due to an autoimmune reaction to the accumulation of abnormal matrix proteins caused by hyperglycemia. It produces a hard fibrotic mass with a lymphocytic reaction; treatment is surgery, but the recurrence rate is high.
Desmoid tumor is also known as an extra-abdominal desmoid or fibromatosis.
Desmoid tumor is an infiltrative, locally aggressive fibroblastic/myofibroblastic process that is treated by surgery, may recur locally, may be multicentric, is associated with previous trauma or surgery, and has been reported in women with breast implants.
Trichinosis is caused by ingesting raw or undercooked meat containing encysted larvae of the Trichinella genus. The larvae give rise to tiny linear calcifications in the pectoralis muscles and not in the breast.
Dermatomyositis and some collagen vascular diseases can rarely produce bizarre sheetlike calcifications that are found to align along the breast tissues on mammography.
Foreign bodies in the breast seen on mammography include percutaneous metallic markers, acupuncture needle tips, hookwire fragments, calcifying sutures, vascular clips to mark breast cancer cavities for planning radiation therapy, Dacron Hickman catheter cuffs, and other foreign objects.
Hidradenitis suppurativa is a benign condition that produces breast lumps representing hidradenitis of the apocrine sweat glands in the axilla, between the breasts, and in the inframammary folds.
Neurofibromatosis is an autosomal dominant disease also known as von Recklinghausen disease (type I); affected patients may have café au lait skin lesions and neurofibromas of the neural plexus or peripheral nerve sheaths. The skin lesions can cause apparent breast masses on mammography.
Missed breast cancers may be due to cancers that are occult on the mammogram, nonspecific findings, atypical findings, or misinterpretation of the classic features of cancer.
Factors influencing why cancers were missed on previous mammograms include findings hidden among many other findings, distracting findings, dense breast tissue, overlooked small calcifications or masses, location simulating lymph nodes, simulation of vascular calcifications, visualization on only one mammographic view, and findings at the edge of the film or at the edge of glandular tissue.
There are four categories of asymmetries in the BI-RADS® lexicon: asymmetry, global asymmetry, focal asymmetry, developing asymmetry. Developing asymmetry is the most suspicious and is associated with a likelihood of malignancy of 13% when identified at screening and 27% when identified at diagnostic mammography.
The absence of a sonographic correlate does not obviate biopsy in cases of developing asymmetry. The role of MRI in the evaluation of asymmetries remains to be defined.
Ad-El DD, Meirovitz A, Weinberg A, et al. Warfarin skin necrosis: local and systemic factors. Br J Plast Surg. 2000;53:624-626.
Ahn BY, Kim HH, Moon WK, et al. Pregnancy- and lactation-associated breast cancer: mammographic and sonographic findings. J Ultrasound Med. 2003;22:491-499.
American College of Radiology. ACR Breast Imaging Reporting and Data System. In: Breast Imaging Atlas. Reston, VA: American College of Radiology; 2003.
Bayer U, Horn LC, Schulz HG. Bilateral, tumorlike diabetic mastopathy—progression and regression of the disease during 5-year follow up. Eur J Radiol. 1998;26:248-253.
Bejanga BI. Mondor’s disease: analysis of 30 cases. J R Coll Surg Edinb. 1992;37:322-324.
Bergkvist L, Frodis E, Hedborg-Mellander C, Hansen J. Management of accidentally found pathological lymph nodes on routine screening mammography. Eur J Surg Oncol. 1996;22:250-253.
Berkowitz JE, Gatewood OM, Goldblum LE, Gayler BW. Hormonal replacement therapy: mammographic manifestations. Radiology. 1990;174:199-201.
Birdwell RL, Ikeda DM, O’Shaughnessy KF, Sickles EA. Mammographic characteristics of 115 missed cancers later detected with screening mammography and the potential utility of computer-aided detection. Radiology. 2001;219:192-202.
Brenner RJ. Follow-up as an alternative to biopsy for probably benign mammographically detected abnormalities. Curr Opin Radiol. 1991;3:588-592.
Brenner RJ. Percutaneous removal of postbiopsy marking clip in the breast using stereotactic technique. AJR Am J Roentgenol. 2001;176:417-419.
Bruwer A, Nelson GW, Spark RP. Punctate intranodal gold deposits simulating microcalcifications on mammograms. Radiology. 1987;163:87-88.
Camuto PM, Zetrenne E, Ponn T. Diabetic mastopathy: a report of 5 cases and a review of the literature. Arch Surg. 2000;135:1190-1193.
Chow JS, Smith DN, Kaelin CM, Meyer JE. Case report: galactography-guided wire localization of an intraductal papilloma. Clin Radiol. 2001;56:72-73.
Crowe DJ, Helvie MA, Wilson TE. Breast infection. Mammographic and sonographic findings with clinical correlation. Invest Radiol. 1995;30:582-587.
Cyrlak D, Wong CH. Mammographic changes in postmenopausal women undergoing hormonal replacement therapy. AJR Am J Roetgenol. 1993;161:1177-1183.
Dale PS, Wardlaw JC, Wootton DG, et al. Desmoid tumor occurring after reconstruction mammaplasty for breast carcinoma. Ann Plast Surg. 1995;35:515-518.
Daniel BL, Gardner RW, Birdwell RL, et al. Magnetic resonance imaging of intraductal papilloma of the breast. Magn Reson Imaging. 2003;21:887-892.
Dershaw DD, Moore MP, Liberman L, Deutch BM. Inflammatory breast carcinoma: mammographic findings. Radiology. 1994;190:831-834.
Diesing D, Axt-Fliedner R, Hornung D, et al. Granulomatous mastitis. Arch Gynecol Obstet. 2004;269(4):233-236.
Doberl A, Tobiassen T, Rasmussen T. Treatment of recurrent cyclical mastodynia in patients with fibrocystic breast disease. A double-blind placebo-controlled study—the Hjorring project. Acta Obstet Gynecol Scand. 1984;Suppl 123:177-184.
Dooley WC. Ductal lavage, nipple aspiration, and ductoscopy for breast cancer diagnosis. Curr Oncol Rep. 2003;5:63-65.
Duijm LE, Guit GL, Hendriks JH, et al. Value of breast imaging in women with painful breasts: observational follow up study. BMJ. 1998;317:1492-1495.
Eby PR, DeMartini WB, Peacock S, et al. Cancer yield of probably benign breast MR examinations. J Magn Reson Imaging. 2007;26:950-955.
Elson BC, Ikeda DM, Andersson I, Wattsgard C. Fibrosarcoma of the breast: mammographic findings in five cases. AJR Am J Roentgenol. 1992;158:993-995.
Evans GF, Anthony T, Turnage RH, et al. The diagnostic accuracy of mammography in the evaluation of male breast disease. Am J Surg. 2001;181:96-100.
Finder CA, Kisielewski RW, Kedas AM. Residual metal shavings and fragments associated with large-core biopsy needles: a follow-up. Radiology. 1998;208:833-834.
Garstin WI, Kaufman Z, Michell MJ, Baum M. Fibrous mastopathy in insulin dependent diabetics. Clin Radiol. 1991;44:89-91.
Godwin Y, McCulloch TA, Sully L. Extra-abdominal desmoid tumour of the breast: review of the primary management and the implications for breast reconstruction. Br J Plast Surg. 2001;54:268-271.
Graf O, Helbich TH, Hopf G, et al. Probably benign breast masses at US: is follow-up an acceptable alternative to biopsy? Radiology. 2007;244:87-93.
Gunhan-Bilgen I, Bozkaya H, Ustun EE, Memis A. Male breast disease: clinical, mammographic, and ultrasonographic features. Eur J Radiol. 2002;43:246-255.
Gunhan-Bilgen I, Ustun EE, Memis A. Inflammatory breast carcinoma: mammographic, ultrasonographic, clinical, and pathologic findings in 142 cases. Radiology. 2002;223:829-838.
Harenberg J, Hoffmann U, Huhle G, et al. Cutaneous reactions to anticoagulants. Recognition and management. Am J Clin Dermatol. 2001;2(2):69-75.
Harvey JA, Nicholson BT, Cohen MA. Finding early invasive breast cancers: a practical approach. Radiology. 2008;248:61-76.
Haupt HM, Rosen PP, Kinne DW. Breast carcinoma presenting with axillary lymph node metastases. An analysis of specific histopathologic features. Am J Surg Pathol. 1985;9:165-175.
Homer MJ, Smith TJ. Asymmetric breast tissue. Radiology. 1989;173:577-578.
Hook GW, Ikeda DM. Treatment of breast abscesses with US-guided percutaneous needle drainage without indwelling catheter placement. Radiology. 1999;213:579-582.
Ikeda DM, Birdwell RL, O’Shaughnessy KF, et al. Analysis of 172 subtle findings on prior normal mammograms in women with breast cancer detected at follow-up screening. Radiology. 2003;226:494-503.
Ikeda DM, Helvie MA, Frank TS, et al. Paget disease of the nipple: radiologic–pathologic correlation. Radiology. 1993;189:89-94.
Ikeda DM, Sickles EA. Mammographic demonstration of pectoral muscle microcalcifications. AJR Am J Roentgenol. 1988;151:475-476.
Kedas AM, Byrd LJ, Kisielewski RW. Residual metal shavings and fragments associated with large-core breast biopsy. Radiology. 1996;200:585.
Kerlikowske K, Smith-Bindman R, Abraham LA, et al. Breast cancer yield for screening mammographic examinations with recommendation for short-interval follow-up. Radiology. 2005;234:684-692.
Kopans DB, Swann CA, White G, et al. Asymmetric breast tissue. Radiology. 1989;171:639-643.
Kushwaha AC, Whitman GJ, Stelling CB, et al. Primary inflammatory carcinoma of the breast: retrospective review of mammographic findings. AJR Am J Roentgenol. 2000;174:535-538.
Leibman AJ, Kossoff MB. Mammography in women with axillary lymphadenopathy and normal breasts on physical examination: value in detecting occult breast carcinoma. AJR Am J Roentgenol. 1992;159:493-495.
Leung JWT, Kornguth PJ, Gotway MB. Utility of targeted sonography in the evaluation of focal breast pain. J Ultrasound Med. 2002;21:521-526.
Leung JWT, Sickles EA. Multiple bilateral masses detected on screening mammography: assessment of need for recall imaging. AJR Am J Roentgenol. 2000;175:23-29.
Leung JWT, Sickles EA. The probably benign assessment. Radiol Clin North Am. 2007;23:773-789.
Leung JWT, Sickles EA. Developing asymmetry identified on mammography: correlation with imaging outcome and pathologic findings. AJR Am J Roentgenol. 2007;188:667-675.
Liberman L, Giess CS, Dershaw DD, et al. Imaging of pregnancy-associated breast cancer. Radiology. 1994;191:245-248.
Liberman L, Morris EA, Benton CL, et al. Probably benign lesions at breast magnetic resonance imaging: preliminary experience in high-risk women. Cancer. 2003;98:377-388.
Liu GJ, Chen WG, Duan G, et al. Mammographic findings of gynecomastia. Di Yi Jun Yi Da Xue Xue Bao. 2002;22:839-840.
Matsuoka K, Ohsumi S, Takashima S, et al. Occult breast carcinoma presenting with axillary lymph node metastases: follow-up of 11 patients. Breast Cancer. 2003;10:330-334.
Memis A, Bilgen I, Ustun EE, et al. Granulomatous mastitis: imaging findings with histopathologic correlation. Clin Radiol. 2002;57:1001-1006.
Montrey JS, Levy JA, Brenner RJ. Wire fragments after needle localization. AJR Am J Roentgenol. 1996;167:1267-1269.
Murakami R, Kumita S, Yamaguchi K, Ueda T. Diabetic mastopathy mimicking breast cancer. Clin Imaging. 2009;33:234-236.
Murray ME, Given-Wilson RM. The clinical importance of axillary lymphadenopathy detected on screening mammography. Clin Radiol. 1997;52:458-461.
Orel SG, Dougherty CS, Reynolds C, et al. MR imaging in patients with nipple discharge: initial experience. Radiology. 2000;216:248-254.
Park YM, Kim EK, Lee JH, et al. Palpable breast masses with probably benign morphology at sonography: can biopsy be deferred? Acta Radiol. 2008;49:1104-1111.
Parsi K, Younger I, Gallo J. Warfarin-induced skin necrosis associated with acquired protein C deficiency. Australas J Dermatol. 2003;44:57-61.
Raza S, Chikarmane SA, Neilsen SS, et al. BI-RADS 3, 4, and 5 lesions: value of US in management—follow-up and outcome. Radiology. 2008;248:773-781.
Rieber A, Tomczak RJ, Mergo PJ, et al. MRI of the breast in the differential diagnosis of mastitis versus inflammatory carcinoma and follow-up. J Comput Assist Tomogr. 1997;21:128-132.
Rosen EL, Baker JA, Soo MS. Malignant lesions initially subjected to short-term mammographic follow-up. Radiology. 2002;223:221-228.
Sachs DD, Gordon AT. Hidradenitis suppurativa of glands of Moll. Arch Ophthalmol. 1967;77:635-636.
Sadowski EA, Kelcz F. Frequency of malignancy in lesions classified as probably benign after dynamic contrast-enhanced breast MRI examination. J Magn Reson Imaging. 2005;21:556-564.
Sawhney S, Petkovska L, Ramadan S, et al. Sonographic appearances of galactoceles. J Clin Ultrasound. 2002;30:18-22.
Schnarkowski P, Kessler M, Arnholdt H, Helmberger T. Angiosarcoma of the breast: mammographic, sonographic, and pathological findings. Eur J Radiol. 1997;24:54-56.
Serels S, Melman A. Tamoxifen as treatment for gynecomastia and mastodynia resulting from hormonal deprivation. J Urol. 1998;159:1309.
Shin JH, Han BK, Ko EY, et al. Probably benign breast masses diagnosed by sonography: is there a difference in the cancer rate according to palpability? AJR Am J Roentgenol. 2009;192:187-191.
Sickles EA. Mammographic features of 300 consecutive nonpalpable breast cancers. AJR Am J Roentgenol. 1986;146:661-663.
Sickles EA. Periodic mammographic follow-up of probably benign lesions: results in 3,184 consecutive cases. Radiology. 1991;179:463-468.
Sickles EA. Nonpalpable, circumscribed, noncalcified solid breast masses: likelihood of malignancy based on lesion size and age of patient. Radiology. 1994;192:439-442.
Sickles EA. Probably benign breast lesions: when should follow-up be recommended and what is the optimal follow-up protocol? Radiology. 1999;213:11-14.
Sickles EA. The spectrum of breast asymmetries: imaging features, work-up, management. Radiol Clin North Am. 2007;45:765-771.
Stomper PC, Waddell BE, Edge SB, Klippenstein DL. Breast MRI in the evaluation of patients with occult primary breast carcinoma. Breast J. 1999;5:230-234.
Swinford AE, Adler DD, Garver KA. Mammographic appearance of the breasts during pregnancy and lactation: false assumptions. Acad Radiol. 1998;5:467-472.
Talele AC, Slanetz PJ, Edmister WB, et al. The lactating breast: MRI findings and literature review. Breast J. 2003;9:237-240.
Tilanus-Linthorst MM, Obdeijn AI, Bontenbal M, Oudkerk M. MRI in patients with axillary metastases of occult breast carcinoma. Breast Cancer Res Treat. 1997;44:179-182.
Varas X, Leborgne JH, Leborgne F, et al. Revisiting the mammographic follow-up of BI-RADS category 3 lesions. AJR Am J Roentgenol. 2002;179:691-695.
Woo JC, Yu T, Hurd TC. Breast cancer in pregnancy: a literature review. Arch Surg. 2003;138:91-99.
Yasmeen S, Romano PS, Pettinger M, et al. Frequency and predictive value of a mammographic recommendation for short-interval follow-up. J Natl Cancer Inst. 2003;95:429-436.
Youk JH, Kim EK, Ko KH, Kim MJ. Asymmetric mammographic findings based on the fourth edition of BI-RADS: types, evaluation, and management. Radiographics. 2009;29:e33.