CHAPTER 1 CLINICAL ASSESSMENT OF MENTAL STATUS
Since the mid-1980s, the relationship between the clinical neurosciences of neurology, neuropsychology, and psychiatry has been the subject of several important textbooks that seek to cross these disciplinary boundaries.1–6 Lishman7 succinctly described the overlap between neurology and psychiatry as a “delicate balance … between our knowledge and understanding of the brain and our knowledge and understanding of people.” Neuropsychology, as a relative newcomer to this field, has added a third element to this balance, and the advent of new investigative imaging modalities and molecular biology technologies has further assisted in “closing the great divide.”8 At the heart of these converging relationships lies an increasing appreciation that a thorough assessment and formulation of individual patients is incomplete without reference to the knowledge and skills of other disciplines. The logical conclusion to this convergence is that disciplinary hybrids such as neuropsychiatry, behavioral neurology, and cognitive neuropsychology may be better replaced by the broader concept of a clinical neuroscience.
Although the core expertise of each discipline may differ, the clinical assessment always includes the documentation of a detailed history and the performance of an examination that is driven by a process of hypothesis testing. The major difference in assessment between disciplines is determined by their specific expertise, whether it be the neurological examination, the psychiatric mental state examination, or a detailed neuropsychological assessment. An element common to all is the assessment of cognition, which in neurological practice has been termed the mental status examination.9 In psychiatric practice, the term mental state/status examination refers to a broader assessment of the patient’s mental state and incorporates the cognitive assessment. Because most neurologists and psychiatrists are restricted to bedside assessments of patients and have limited access to neuropsychological expertise, all possible information must be incorporated into decisions regarding the patient’s cognitive abilities. The clinical skill of cognitive assessment extends far beyond the administration of a test and the generation of a score. A common error among junior clinicians is to base diagnosis on a test score and ignore the richness of cognitive information available from the remainder of their interaction with the patient.
NEUROCOGNITIVE HISTORY
Informant History
Although the key informant is usually a relative or caretaker, the clinician should seek information from as wide a range of sources as possible. Key informants may include a nursing home worker, neighbor, friend, or primary care physician. Such information may be crucial if the validity of the patient’s history is limited by impairments in insight regarding the nature of their deficits.
Integration of Neurobehavioral and Neurocognitive History
Neurobehavioral disturbances such as depression or psychosis are often the initial presenting feature in patients with neurocognitive impairment. Conversely, patients who initially present with the neurocognitive features of dementia may later exhibit psychotic symptoms; disinhibited, stereotypical, or inappropriate behavior; or mood and anxiety disturbance.10 Psychiatric disorders with associated behavioral disturbance may themselves manifest with neurocognitive impairments, such as the difficulties with sustained attention, memory, and processing speed seen in major depression11 or the working memory impairments seen in schizophrenia.12 Neurobehavioral impairment may herald future neurocognitive impairment, and vice versa. For example, frontotemporal dementias commonly manifest with neurobehavioral disturbance,13 and late-life depression may herald Alzheimer’s disease.14
Gross Functional Capacity
Patients and caretakers do not always have a specific complaint of “cognitive impairment.” Functional impairment as noticed by caretakers, relatives, or colleagues is commonly a precipitant for assessment. A thorough history of the pattern and nature of functional impairment provides valuable diagnostic information and may help treatment planning (Table 1-1).
Neurocognitive Baseline
An understanding of an individual’s premorbid neurocognitive function allows an estimate of the degree and rate of decline. In impaired individuals with superior premorbid intellect, cross-sectional assessment may be within accepted norms for age. Commonly used screening instruments such as the Mini-Mental State Examination (MMSE) have a significant ceiling effect (see Chapter 2 on neuropsychological assessment) and are likely to be insensitive to this decline.15 Conversely, individuals with long-standing premorbid intellectual impairment may be incorrectly classified as suffering a degenerative process such as dementia, when in fact their neurocognitive function is stable.
Baseline function can be estimated grossly from the patient’s maximal educational and occupational attainment and from mental state features such as vocabulary and capacity for abstract thinking. A number of assessment tools for estimating premorbid intellectual function have been devised and rely on the stability of semantic language functions independent of disease or age. The most well-known and widely used is the National Adult Reading Test.16 This probes reading ability for irregular words, which has been shown to be relatively preserved in many disorders.17 Other similar tools include the Wechsler Test of Adult Reading18 and the Cambridge Contextual Reading Test.19 This issue is also addressed in Chapter 2 on neuropsychological assessment.
FUNCTION IN NEUROCOGNITIVE DOMAINS
Attention and Orientation
Attention is not a unitary construct. Sustained, directed attention is the ability to attend to a specific stimulus without being distracted by extraneous internal or environmental stimuli. Other aspects of attention include the ability to share attention between simultaneous tasks (divided attention), the ability to switch attention between tasks, and the ability to attend to stimuli at different spatial locations. Disturbance of sustained, directed attention is most commonly reported in delirium (see Chapter 11) but may also be present with anxiety or mood disturbance, executive dysfunction (see Chapter 7), or dementias. Orientation is a more complex function involving the capacity to attend to stimuli and to process and retain information regarding locale and point in time.
The following questions are useful guides to areas for evaluation:
 Can the patient attend to, and persevere with, most tasks, or does the patient demonstrate difficulties completing a task or take much longer than would be expected (a measure of sustained, directed attention)?
Can the patient attend to, and persevere with, most tasks, or does the patient demonstrate difficulties completing a task or take much longer than would be expected (a measure of sustained, directed attention)?Language
Disorders of language are separated into aphasia (partial or total inability to articulate ideas or comprehend spoken or written language), alexia (inability to read), and agraphia (inability to write). An understanding of the large group of aphasias has been complicated by various nomenclatures, although the most recognized is that originally proposed by Benson and Geschwind,20 which divides the group into Broca’s, Wernicke’s, global, conduction, anomic, and transcortical aphasias. This is discussed in more detail in Chapter 3.
The following questions are useful guides to areas for evaluation:
 Has the patient had any difficulties with speech, such as in finding the right word or using the wrong word?
Has the patient had any difficulties with speech, such as in finding the right word or using the wrong word? Can the patient pronounce words correctly, or does he or she make errors in pronunciation? Are these errors consistent?
Can the patient pronounce words correctly, or does he or she make errors in pronunciation? Are these errors consistent?Visuospatial Functions
Visuospatial disturbance is common in dementias of all types, but it is most commonly seen with Alzheimer’s-type dementia. It is the presenting and dominant feature in the posterior variant, posterior cortical atrophy. Vascular or other acquired focal lesions of the parietal regions may also manifest with visuospatial difficulties. Aspects of visuoperceptual disturbance are discussed further in Chapter 5.
The following questions are useful guides to areas for evaluation:
 Is the patient able to dress himself or herself appropriately? Does he or she wear items of clothing the wrong way around, inside out, or fitted incorrectly? Of importance, however, is that often a dressing apraxia may result from visuoperceptual disturbances or spatial inattention.
Is the patient able to dress himself or herself appropriately? Does he or she wear items of clothing the wrong way around, inside out, or fitted incorrectly? Of importance, however, is that often a dressing apraxia may result from visuoperceptual disturbances or spatial inattention. Can the patient find his or her way around the house, to the shops, and in unfamiliar environments when directions are provided?
Can the patient find his or her way around the house, to the shops, and in unfamiliar environments when directions are provided? Can the patient still use everyday objects—eating utensils, grooming items (toothbrush, comb), or tools of his or her trade—appropriately?
Can the patient still use everyday objects—eating utensils, grooming items (toothbrush, comb), or tools of his or her trade—appropriately?Memory
Anterograde episodic memory is a general term for the registration, acquisition, storage, and subsequent retrieval of new information. Anterograde episodic memory impairment is perhaps the most common manifestation of neurocognitive disturbance. Anterograde episodic memory consists of several stages, beginning with the reception and registration of the information by a sensory modality, followed by the holding of the information temporarily in working memory (defined as the current content of consciousness); then storage in a more permanent form, which is enhanced by association with other already stored information and augmented by practice or rehearsal; and, finally, the process of retrieval. Each of these steps requires the integrity of the preceding steps, and any interruption in this hierarchy may disrupt anterograde episodic memory function. A detailed discussion of the various memory systems and their disorders is presented in Chapter 4.
The following questions are useful guides to areas for evaluation:
 Is the patient able to describe or relate recent world or local events that may have been heard on the radio, seen on television, or read in the newspaper?
Is the patient able to describe or relate recent world or local events that may have been heard on the radio, seen on television, or read in the newspaper?Executive Function
Executive functions are a group of complex functions that are based on the interaction with and executive control of basic processes such as attention, memory, and language. The prefrontal lobes and connected subcortical structures are crucial to the integrity of executive function, personality, and behavior. Cognitive functions that have been attributed to these networks include adaptive behavior, abstract conceptual ability, set-shifting/mental flexibility, problem-solving, planning, initiation, sequencing of behavior, and personality factors such as drive, motivation, and inhibition. Patients with the dysexecutive syndrome fail to anticipate changes, show poor planning ability, and do not learn from their errors. They are poor at self-guided learning and goal-setting, in that they perform normally on externally driven tasks but are poor at self-motivated tasks. Such patients are sensitive to interference from irrelevant stimuli, and may display both motor and cognitive perseveration. A more detailed overview of executive function and dysfunction can be found in Chapter 7.
The following questions are useful guides to areas for evaluation:
 Has there been any decline in appropriate social judgment, such as inappropriate familiarity with strangers or disinhibited sexual or aggressive behavior? Of note is that not all patients with executive dysfunction display these features.
Has there been any decline in appropriate social judgment, such as inappropriate familiarity with strangers or disinhibited sexual or aggressive behavior? Of note is that not all patients with executive dysfunction display these features.FUNCTION IN NEUROBEHAVIORAL DOMAINS
Mood
Mood is the sustained level of emotional tone, whereas affect refers to the patient’s emotional behavior. The emotional response of the examiner to the patient provides a further indicator of the patient’s emotional state. Patients with disorders of mood such as major depressive disorder often show impairments in attention and psychomotor speed.21,22 Bipolar disorder is associated with impairments in attention, verbal memory, and executive function. High rates of comorbid mood disturbance are associated with Parkinson’s disease,23 Huntington’s disease,24 and multiple sclerosis.25 Late-onset depression is being increasingly seen as a prodrome to the dementias.14
Inquiries about a patient’s mood state may include the following:
“How have you been feeling recently?”
“Do you have periods of time when you are always down?”
In patients with speech disorders, responding to such questioning may not be possible, and close informants can be asked directly, “Does he/she seem unhappier to you than usual?” Informants are able to comment on the reactivity of mood, inasmuch as depressed patients are often unable to “brighten up.” Patients who are depressed may describe themselves as feeling sad, unhappy, hopeless, useless, blue, or “flat.” This mood state is often accompanied by disturbances in sleep, appetite, concentration, and motivation. The depressed patient conveys sadness and misery or presents as anxious and irritable. Commonly used tools for rating the severity of depressive symptoms include the patient-rated Beck Depression Inventory26 and the clinician-rated Hamilton Depression Rating Scale.27 Patients with elevated mood states display euphoria, elation, and irritability in association with overactivity, accelerated thoughts, disinhibited behavior, reduced sleep, and grandiose ideas. Examiners who find themselves regularly suppressing a smile or giggle in interviews with patients should always ask themselves whether the patient’s mood is elevated. Reduced intensity and narrowing of affective responses is termed blunting of affect and is a key feature of schizophrenia.
Anxiety
Anxiety, a normal and adaptive component of human psychological function that allows for the identification of danger or threat, may become inappropriate and/or excessive. Anxiety disorders include panic disorder, characterized by panic attacks (discrete, relatively brief periods of intense anxiety accompanied by somatic symptoms of sympathetic drive), agoraphobia (recurrent fear of inability to escape from situations such as being in a crowd or an enclosed area), social anxiety (fear of social situations), specific phobia (of a discrete object, such as heights or water), and generalized anxiety, which leads to diffuse anxiety on most days.28 Pathological anxiety states have been associated with stroke,29 Huntington’s30 and Parkinson’s diseases,31 temporal lobe epilepsy,32 and thyrotoxicosis.33 A history of anxiety can be sought through inquiries about increasing or frequent worrying, its source, and the presence of concomitant physiological signs such as sweating, tachycardia, shortness of breath, and tremor and whether the degree of worry seems excessive to the patient or caretaker.
An anxiety disorder with a strong relationship to neurological disorders is obsessive-compulsive disorder, which is associated with intrusive recurrent thoughts (obsessions) and repetitive behaviors or mental acts (compulsions such as counting, checking, or cleaning). Obsessive-compulsive disorder has been strongly associated with Gilles de la Tourette syndrome34 and with the pediatric neuropsychiatric disorder pediatric autoimmune neuropsychiatric disorder associated with group A streptococci (PANDAS).35 Symptoms of obsessive-compulsive disorder can be ascertained by questions about compulsive behaviors, such as “Do you find yourself checking/cleaning things more than once or more often than you need to?”, and obsessions, such as “Do you have recurrent, intrusive thoughts/ideas/images/impulses?”
“Have you ever had the sense that the world around you is different or changed?” (derealization)
Thought Content and Form
In a number of neurological disorders, patients may develop abnormalities in their beliefs about themselves or the environment around them. Thought content can be seen as a spectrum from normal, reality-based thinking to delusions, which are fixed false ideas out of keeping with the patient’s cultural background or education.28 Delusions are categorized by the nature of their content, such as persecutory, grandiose, somatic, and erotomanic delusions or delusions of jealousy or reference. Delusions are classically associated with schizophrenia but are seen in patients with Huntington’s disease, temporal lobe epilepsy, leukodystrophies, systemic lupus erythematosus, and most forms of dementia.36 Persecutory delusions are commonly seen in delirium and substance intoxication. Organic delusions are particularly associated with diseases affecting the limbic system.37 In schizophrenia, delusions tend to be very well systematized (generally static and supported by an extensive belief system) and often have a bizarre nature, whereas in neurological disorders they are often poorly systematized and are predominantly persecutory.38
Perception
Perceptual experience occurs on a continuum, with “true” perception of stimuli at one end and frank hallucinations at the other.39 Hallucinations are defined as percept-like experiences in the absence of an external stimulus that are spontaneous and unwilled and cannot be readily controlled.40 Illusions, which are distortions or elaborations of a normal stimulus, also belong to this continuum. Hallucinations can be well formed and complex or poorly formed and simple,41 and they are categorized according to sensory modality. Complex and elaborate auditory hallucinations are the hallmark of schizophrenia but can occur in severe affective psychotic states. Poorly formed, fragmented auditory hallucinations may be present in organic states such as delirium. Special note should be made of complex visual hallucinations, which are often a feature of organic states. Examples include the Charles Bonnet syndrome and peduncular hallucinosis, where lesions to the visual pathways and to their terminations or ascending inhibitory afferent pathways, respectively, can result in strikingly vivid formed scenes, complex patterns, or groups of miniature figures or animals.42 Olfactory, gustatory, and tactile hallucinations are not commonly experienced in psychiatric illness and should alert the clinician to the possibility of neurological conditions such as complex partial seizures or neoplasia. For patients who are reluctant or unable to discuss their experiences, questioning caretakers about behavior that might represent a reaction to perceptual disturbance, such as responding to voices or gaze movements in response to visual phenomena, may prove useful.
Vegetative Function
Vegetative functions such as sleep, appetite, and sexual drive are often disturbed in patients with neurobehavioral disturbances, particularly disturbances of mood.43 Patients may complain of appetite disturbance with weight loss or gain; sleep disturbance with insomnia in initial, middle, or terminal sleep phases (the last known as early morning wakening, a characteristic of severe depression); altered libido; disturbances in energy and motivation; and impaired capacity to enjoy usual pursuits.
Insight and Judgment
A patient’s lack of insight into illness may reduce the validity of a history, such as the patient’s self-report of memory complaints44 or of adherence to prescribed treatments.45 Insight has been traditionally viewed in both psychological and cognitive frames. In the psychological frame, insight relates to acceptance of illness and the need for its treatment. Insight is impeded by denial of illness, which occurs to varying degrees and reflects psychological coping mechanisms that allow the patient to deal with the fear, hopelessness, or shame associated with sickness and its treatment.46 Cognitive models focus on the neurocognitive capacity of an individual to internalize, retain, and cognitively process the awareness of symptoms, attribute these to an illness, and appreciate the likely effects of accepting or refusing treatment.47 Cognitive models of insight were devised from the strong association of disorders in which disrupted frontal-executive function is the cognitive hallmark, such as frontotemporal dementia and schizophrenia, with poor insight.45,48 An apparent change in insight is likely to reflect an organic process, inasmuch as the psychological mechanisms that relate to insight tend to be personality based and thus relatively stable.49
The concept of insight overlaps with the neurological symptom of anosognosia, a lack of awareness of neurological deficit, first described by Babinski50 and most commonly seen in right hemisphere stroke. Although it most commonly characterizes a lack of awareness of hemiparesis, anosognosia can also occur for amnesia, apraxia, aphasia, cortical blindness, and prosopagnosia.51 The observation that most lesions associated with anosognosia involve the parietal lobes or related connections reinforces the role of the parietal lobe in awareness of illness and, in particular, the role of the right inferior parietal cortex in attention.52
OTHER ABNORMAL BEHAVIORS
A range of other neurobehavioral disturbances may alert the clinician to the possibility of associated cognitive or neurological impairment. Apathy, disinhibition, and stereotypies can prove diagnostically challenging because they may initially mimic other disorders. These behaviors are often seen in frontotemporal dementia, itself a mimicker of other neurobehavioral disorders, and are elements of the three behavioral subtypes of these disorders53 (see also Chapter 7). Apathy may manifest as apparent depression, disinhibition as mood elevation, and stereotypies as compulsive behaviors or dyskinesias. Utilization behavior and echo phenomena (echopraxia, echolalia) are uncommon but important neurobehavioral syndromes that may be easily missed or ignored. Finally, catatonia constitutes a medical emergency for which the underlying cause must be determined quickly to ensure appropriate treatment.
Apathy
Apathy is best defined as a state of reduced or absent motivation.54 In practice, apathy manifests as a reduction in the initiation of goal-directed behavior. Apathy is most common in disorders that disrupt the frontal-subcortical circuit, considered to be the substrate for motivated behavior. This circuit includes the anterior cingulate cortex, nucleus accumbens, globus pallidus, and thalamus.55 Disruption to these circuits with consequent apathy is seen in frontal-subcortical dementias such as Parkinson’s disease, Huntington’s disease,56,57 and frontotemporal dementia; traumatic brain injury58; schizophrenia59; and vascular insult.60 Apathy in many of these syndromes may respond to stimulant medication,61 and in schizophrenia, atypical antipsychotic agents may reduce apathy through increased dopamine release in the frontal cortex.62
On history, the differentiation of apathy from depression can be difficult, although patients with pure apathy often blandly deny feeling depressed and do not transmit a depressed affect. The other cardinal features of depression, such as neurovegetative disturbance, are often absent. Family and caretakers may become angry or resentful at a patient whose worsening apathy is perceived as a voluntary withdrawal. The distinction between apathy and depression is important, so that an apathetic patient with frontotemporal dementia does not receive an incorrect diagnosis of depression, which leads to delays in appropriate management, or, conversely, so that patient with a reversible clinical depression does not remain untreated.63
Disinhibition
Inhibition is the capacity to cognitively “cancel” a thought, response, or activity that is considered unintended, unwanted, or inappropriate. Social disinhibition is associated with insults to the nondominant inferior frontal cortex64 and linked subcortical structures. Lesions to these regions result in release of motor, affective, instinctive, or cognitive behaviors such as hyperactivity, elevated mood, hyperorality and hypersexuality, and accelerated or grandiose thinking.65 Disorders that produce disinhibition include focal vascular, neoplastic or traumatic lesions,66 and frontotemporal dementia.67 Cognitive disinhibition of automatic responses to stimuli is associated with lesions of the anterior cingulate cortex and impaired performance on tasks such as the Stroop test. (See also Chapter 7.)
Stereotypies
Stereotypies are repetitive, rhythmical, and invariant motor behaviors, without an apparent purpose or function, that can vary from simple motor behaviors such as rocking or hand waving to extraordinarily complex acts and rituals.68 They are one of the defining features of autism and are common in patients with mental retardation.69 Stereotypies are seen in adults with lesions or disorders affecting the frontostriatal circuit running between the dorsolateral frontal cortex and the head of the caudate nucleus.70 Frontotemporal dementias commonly manifest with stereotypic behaviors resulting from degeneration of the dorsolateral prefrontal cortex.71 Stimulant medications can produce complex stereotypies through a dopaminergic effect on the basal ganglia.72 Other repetitive motor behaviors such as compulsive behaviors and tics are seen in patients with Gilles de la Tourette syndrome and obsessive-compulsive disorder, both of which are considered to be associated with basal ganglia pathology.72,73 Of importance is that stereotypies, compulsions, complex tics, mannerisms (unusual or pathological styles of performing goal-directed activities, such as a bizarre gait and unusual ways of greeting people), and habits can often be difficult to distinguish purely on the basis of subjective observation. The context and history of the motor phenomena provide important diagnostic information.
Utilization Behavior
Utilization behavior refers to the phenomenon in which patients grasp and purposively use objects within their reach, even though this action may be inappropriate or out of context.74,75 It reflects an inability to inhibit an automatic action cued by an environmental stimulus. It has been described in patients with lesions affecting the prefrontal/subcortical circuits.75,76 Examples given by Lhermitte75 included “pouring” from an empty jug into a glass, using a knife and fork on a plate without food, and lighting multiple cigarettes for the examine while the first remains unsmoked. An awareness of utilization behavior is important because it may be misinterpreted as odd, eccentric, or even antisocial behavior by the inexperienced examiner.
Echolalia and Echopraxia
Echo phenomena are the unsolicited and stereotyped repetition of another person’s speech (echolalia) or actions (echopraxia). These phenomena are commonly observed in patients with schizophrenia, especially during acute illness or in catatonic states. They have been well described in patients with neurodevelopmental disorders, including Gilles de la Tourette syndrome and autism,77,78 and with various complex startle syndromes such as latah.79 Echolalia has been described in patients with dysphasia and left hemispheric lesions.80
RELEVANT HISTORY
Medical History
The effects of therapeutic drugs on the CNS must always be considered. Dopaminergic agents used in the treatment of Parkinson’s disease may cause psychosis,81 and anticholinergic agents impair cognition.82 Treatments used for systemic disorders may produce neurobehavioral states, as is seen in the depression associated with β-interferon used for viral hepatitis83 and the mania secondary to corticosteroid use for autoimmune disorders.84 Polypharmacy, particularly in the elderly, increases the likelihood of medication neurotoxicity.85 The possibility of drug withdrawal states warrants specific inquiry about the use of benzodiazepines, sedatives, hypnotics, and alcohol.
Psychiatric History
A psychiatric history allows the clinician to differentiate between a relapse of an established disorder and new-onset disease. The neurocognitive deficits of chronic schizophrenia may manifest as a dementing illness86; “hypofrontal” disinhibition and poor judgment may be the presenting symptoms of mania87; and patients with depression may present with a “pseudodementia.”88
Substance Use History
A thorough longitudinal alcohol history is essential for the verification of Korsakoff’s amnesia, alcoholic dementia, delirium tremens, and alcoholic hallucinosis, A smoking history should be obtained, including total exposure and past or current related illnesses. Stimulants such as amphetamines may produce neurobehavioral symptoms such as delusions and hallucinations,89 chronic marijuana usage may lead to psychosis and cognitive impairment,90 and inhalant solvent abuse has been associated with acute and chronic cognitive impairment, depression, and psychosis.91
NEUROBEHAVIORAL RATING SCALES
Neuropsychiatric Inventory
The NPI92 is a semistructured clinician interview of caretakers in which the severity and frequency of disturbance in 12 symptom domains is rated (Table 1-2). The NPI shows good interrater and test-retest reliability.92 It has been modified and validated for use in nursing homes (NPI-NH)93 and in various non-English versions.94–97 Scoring in subscales of the NPI has been shown to correlate strongly with those in other well-validated symptomatic scales, such as the Behavioral Pathologic Rating Scale for Alzheimer’s Disease (BEHAVE-AD) and Hamilton Rating Scale for Depression.98
TABLE 1-2 Symptom Domains Rated by the Neuropsychiatric Inventory
* Additional domains in the Neuropsychiatric Inventory for nursing homes.
The performance characteristics of the NPI have been established in a range of neurological conditions, including Alzheimer’s disease,99 Parkinson’s disease,100 frontotemporal dementia,101 progressive supranuclear palsy,102 corticobasal degeneration,103 mild cognitive impairment,104 Tourette disorder,105 subcortical vascular ischemia,106 multiple sclerosis,107 and Huntington’s disease.30 The NPI has a key role alongside cognitive scales in monitoring the noncognitive improvements with cholinesterase inhibitor treatment of various dementias, and it has been shown to reliably detect improvement in individuals treated with donepezil,108 galantamine,109 and rivastigmine.110
Neurobehavioral Rating Scale
The NRS is a 27-item, multidimensional instrument designed to measure neurobehavioral disturbance after traumatic brain injury.111 Based on the Brief Psychiatric Rating Scale,112 the NRS is a brief structured patient interview that takes 15 to 20 minutes to complete. It includes ratings of neurobehavioral symptoms, basic tests of cognition, and questions about the patient’s current level of function. The NRS has demonstrable utility in assessing a number of different organic neurobehavioral states, including traumatic brain injury,113–116 dementia,117,118 Parkinson’s disease,119 HIV-related dementia,120 and the post-endarterectomy state.121 As with the NPI, translations of the NRS into other languages have been made and are of proven validity.121
NEUROCOGNITIVE EXAMINATION
Many clinicians assess cognitive function by using standardized instruments such as the MMSE,122 which offer a brief, validated, and easily communicable approach. However, because most commonly used instruments have limitations, an understanding of the how to assess separate cognitive domains allows the clinician to tailor the examination to an individual presentation. The necessity of performing a formal neurocognitive examination warrants emphasis, because in the absence of a thorough and structured assessment, it is possible to miss a clear deficit. For example, patients who perform well verbally may mask significant impairments in other cognitive domains, such as memory or visuoperceptual function. Few neurologists would consider their assessment complete without a physical neurological examination of the patient or a careful history of the presenting symptoms. Although a complete neurocognitive assessment may be unnecessary for all patients, it is similarly a key component of the clinical assessment in a number of circumstances. This section describes an approach to a largely qualitative bedside cognitive assessment and serves as an introduction to the subject. More details regarding the examination of each domain and the disorders that such an examination may reveal can be found in subsequent chapters.
Principles of Cognitive Assessment
When to undertake neurocognitive examination
A neurocognitive examination should be undertaken whenever the reported complaint is a cognitive one, in clinical circumstances in which cognitive impairment occurs frequently (postsurgically, in cerebrovascular disease, in neoplasia, or in trauma), or when there is an unexplained history of personality, functional, or behavioral change. In many countries, standardized cognitive assessment is mandatory before the prescription of cholinesterase inhibitors for dementia.
Quantitative versus qualitative data
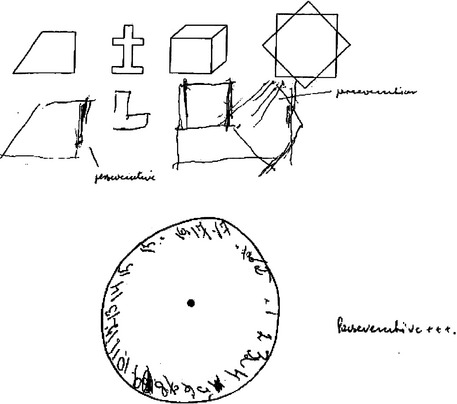
When quantitative screening or assessment instruments are used to gauge cognition in patients, it is important for the clinician to look beyond the quantitative result in making a diagnosis. For example, a score of 22 of 30 on the MMSE is below most accepted cutoffs for dementia, but it may not point definitively to organic cognitive impairment. Such a score may be seen, for example, in individuals of limited education, in patients sedated by medication, or in patients with psychiatric illness such as major depression or schizophrenia. The quality of a patient’s performance is as important as the numeric measure in many or most quantitative tests; examples include perseveration displayed on a serial subtraction task or in a copying or clock-drawing task (Fig. 1-1). Further details of the quantitative aspects of neuropsychological testing may be found in Chapter 2
Examination of Attention and Orientation
Attention has traditionally been tested by serial subtraction tasks and reverse spelling of words, such the serial sevens and “WORLD” backwards tasks in the MMSE. Such tasks are dependent on working memory, as well as on calculation and spelling, respectively, both of which are strongly related to educational background and both of which may be disrupted by focal lesions that do not otherwise impair attention.123 Reciting an overlearned sequence such as days of the week or months of the year in reverse order requires sustained attention and intact working memory; this test is very sensitive to disturbance of attentional processes and is generally understood across cultures and languages. Repeating a spoken sequence of digits, starting with two digits and increasing the length of the sequence with each correct attempt, is also a sensitive marker of attention, particularly when the patient needs to repeat the sequence in reverse, which is more difficult and places a greater load on working memory. Most subjects correctly complete 7 ± 2 digits forward and 5 ± 1 in reverse. Digit span testing depends on intact working memory, the frontal lobe–mediated brief store of visual or auditory information in current consciousness (e.g., remembering a telephone number before writing it down). Continuous performance tasks, which require the patient to respond when a particular stimulus is presented (e.g., the letter “A” in a list of random letters read by the examiner9) are minimally dependent on working memory but are good measures of sustained, directed attention. Finally, it is important for the clinician to be aware that attentional impairment may impair performance in other parts of the cognitive examination. If attention is markedly impaired, poor results on testing in other domains may not necessarily indicate that function in those domains is also impaired (Table 1-3).
Examination of Language
As with attentional disturbance, impaired language can affect many other aspects of the neurocognitive examination and hence should be tested early. A significant subjective understanding of the patient’s capacity for language can be gained during the clinical interview with regard to the degree of spontaneous speech, articulation, capacity for word-finding, and comprehension (Table 1-4).
Reading is the language task in which performance is most determined by educational status, and poor literacy is not uncommon in many patient populations. The capacities to read aloud and to understand written language are potentially dissociable and should be tested separately. Patients should be asked to read simple and then complex words, particularly comparing the pronunciation of orthographically regular (sounded as spelled) words such as “shed” to irregular words such as “rough,” as well as phrases such as “Close your eyes.” Writing should be tested to detect agraphia, which frequently accompanies aphasia. Agraphia is diagnosed when basic language errors, gross spelling errors, or paragraphias (word or syllable substitutions) are present. The patient should be asked to write sentences both spontaneously and in response to dictation, with the latter ideally containing a number of orthographically irregular words and/or homonyms (“The boy’s aunt made a large pie out of steak and dough”). Further details of language assessment can be found in Chapter 3.
Examination of Visuoperceptual/Visuoconstructional Function and Calculation
Visual and constructional impairments are often present with parieto-occipital lesions, particularly of the nondominant hemisphere, which, in combination with lesions of prefrontal regions, is important for spatially directed attention.124 When ventral occipitotemporal regions are affected, visual recognition is impaired, particularly for objects, written words (pure alexia), colors, or faces (prosopagnosia). Dorsal lesions result in impairment of visuospatial organization. A detailed exposition of disorders of visual perception may be found in Chapter 5. When a patient is unable to maintain attention to one side of his or her spatial field or body soma, visual and somatosensory neglect, respectively, are said to occur and are typically seen with nondominant parietal lesions. Lesions in the dominant parietal region may result in Gerstmann’s syndrome, which, in addition to agraphia, manifests with calculation dysfunction (acalculia), right/left disorientation, and naming inability for fingers (finger agnosia). A disorder of skilled movement, apraxia, occurs when lesions in a network that includes the inferior dominant parietal region result in the loss of the ability to perform the “formulas for movement” stored in this region of the brain (ideomotor apraxia) or the capacity to perform a series of actions such as putting a letter in an envelope, sealing it, and stamping it in the correct sequence (ideational apraxia).
Visuospatial organization is commonly tested by tasks of figure drawing or copying, such as figure copying or clock drawing, although the latter taps into many cognitive functions and can be considered as a cognitive screening test in its own right.125 A gradient from simple to complex figures should be used, and patients can also be asked to spontaneously draw figures such as a house or a tree. Many different ways to conduct and score clock drawing tasks have been published, although most require subjects to correctly place all numbers on the clock and appropriately place hands demonstrating a time in response to command. Most constructional tests are sensitive for visual neglect, which can also be tested by line bisection tasks. Orobuccal and limb praxis should be tested separately because they are controlled by different neural pathways. Dominant and nondominant sides should be tested separately. Patients should be asked to demonstrate a movement such as using a comb and to imitate the examiner’s movements. The latter can be done in a systematized manner by using the interlocking finger test.126
Calculation should be specifically tested by means of simple arithmetic in addition to functional real-life examples but is often tapped by the serial sevens test (Table 1-5).123
TABLE 1-5 Tests of Visuoconstructional Function, Prascis, and Calculation
Examination of Memory
“Memory disturbance” is most commonly used to refer to a disorder of episodic, declarative memory (memory for things that can be stated). This manifests as difficulty in recalling personally experienced material from the past and/or learning new information, and it is often found in dementing disorders such as Alzheimer’s disease and in amnestic disorders such as Korsakoff’s syndrome. Declarative memory has traditionally been divided into immediate, recent, and remote memory. Immediate memory is now more commonly described as working memory and refers to the very brief storage of the auditory and visual contents of current consciousness in the dominant perisylvian language areas of the cortex and the nondominant parietal cortex, respectively, and under frontal executive control. Recent memory is the term traditionally used by physicians to describe the capacity for new learning of verbal and visual material. The consolidation of recent memories is a function of the hippocampus, related structures in the medial temporal zones, and hippocampal outflow through the fornices to the mammillary bodies and thalamus. Remote memory is a time-based distinction, but the term may be used to imply that the relevant memories are consolidated and no longer hippocampally dependent. Remote memory is made up of autobiographical, episodic memory (memory for events) and semantic memory (memory for knowledge and words, dependent on anterior temporal neocortex). The nosology of the various memory systems and their disorders is covered in detail in Chapter 4.
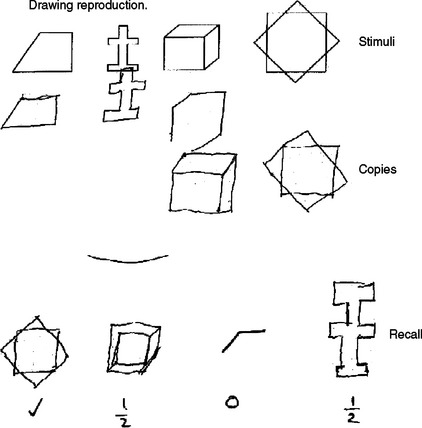
Working memory is usually tested by items such as digit span or by the immediate recall of a word list. Recent memory is generally tested with delayed recall of a word list, name and address, or a short story. Patients with a primary deficit in registration or encoding of memory (e.g., as in Alzheimer’s disease) demonstrate rapid forgetting and limited spontaneous recall and do not benefit from the provision of clues. Patients with lesions affecting dorsal prefrontal circuits have poor or inefficient retrieval of normally registered memory and show limited spontaneous recall but may benefit from cueing.127 Nonverbal memory should be tested through the reproduction of a visual figure after a delay. Ideally, these figures are constructions that do not lend themselves to a verbal description, as then patients can use intact verbal memory as a “workaround.” Testing for nonverbal recall is not included in the MMSE, but it is featured in other brief tools such as the Neuropsychiatry Unit Cognitive Screening Instrument (NUCOG)128 and is illustrated in Figure 1-2.
Remote memory is tested by the patient’s recall of personal history and his or her general fund of knowledge. The latter may be difficult to test in a standardized manner, inasmuch as commonly used questions about political leaders or historical events are strongly education- and culture-dependent (Table 1-6).
Examination of Executive Function
Control of executive functions is commonly ascribed to the prefrontal lobes and related subcortical structures, although this involves a deliberate simplification. Executive dysfunction may be seen in focal prefrontal lesions, frontotemporal dementias, or neurobehavioral syndromes such as schizophrenia and major depression, among other disorders. The basal ganglia and thalamus are connected to the frontal lobes through defined circuits, and lesions of these structures or circuits can manifest with executive impairment.129 A fuller account of executive functioning and the various features of the dysexecutive syndrome can be found in Chapter 7.
Set-shifting/sequencing is the ability of the patient to shift efficiently from one cognitive set to another and to inhibit no longer appropriate responses, and its impairment manifests as perseveration.130,131 The neural substrate for set-shifting involves the dorsolateral prefrontal cortex and its connections to basal ganglia and thalamus.132 The initiation of cognitive strategies is an integral but often overlooked element of the cognitive examination and is often the core deficit underlying apathy syndromes, particularly after occurrence of medial frontal lesions that affect the anterior cingulate region.65 Abstract thinking appears to be a function of the lateral frontal zones, and impairment of abstract thinking manifests as concreteness of thinking. The orbitomedial frontal zones are involved in the processing of emotion and in judgment and planning; lesions to this region, particularly on the nondominant side, manifest as disinhibition and poor judgment.65 The capacity to integrate abstractive and emotional functions, perhaps best illustrated in the appreciation of humor, relates to right frontal regions.133
Although impairments in set shifting can be observed behaviorally, they can be directly tested with tasks of motor or written sequencing. Motor sequencing is tested with the fist-edge-palm task, which is particularly impaired in patients with dominant frontal lesions but also at the extremes of age.134 Written sequences of alternating letters or shapes can also be used.135 Initiation can be assessed with tests of verbal fluency by initial letter (words beginning with a particular letter) in a limited time span. Most adults score 16 to 18 ± 4 words in 1 minute. Although abstract thinking is commonly tested with proverbs, these are notoriously culturally, educationally, and age sensitive and are best replaced by questions regarding similarities (e.g., “What is the similarity between a desk and a chair?” or “What is the difference between a painting and poetry?”). Capacity to inhibit, as well as manage, interference can be tested with a “go–no-go” task where the patient is asked to provide a response to one particular examiner’s instruction, while not responding to a second, but related, instruction, such as “Tap on the table when I tap it once, but don’t tap the table when I tap it twice” (Table 1-7).
Cortical Release Signs
Although they are technically not a part of the mental status examination, a brief discussion of cortical release signs or primitive reflexes is warranted. These signs include the grasp, palmomental/pollicomental, snout, and pout reflexes.74 The presence and nature of such signs in schizophrenia may be an index of severity of neurodevelopmental disturbance and may aid in assessing prognosis, expected outcome, development of medication side effects such as tardive dyskinesia, and treatment planning.136,137 In geriatric psychiatry, cortical release signs are valuable aids to illness staging138,139 and outcome prediction140 and may be diagnostic aids in particular subtypes such as frontotemporal dementia.141,142 Although the palmomental reflex is relatively nonspecific, the grasp reflex is virtually never present in healthy elderly subjects143–147 and should be considered an indicator of CNS disease in this population.
STANDARDIZED COGNITIVE ASSESSMENT INSTRUMENTS
Mini-Mental State Examination and the Modified Mini-Mental State Examination
The MMSE is the most widely used of a substantial number of available screening tests122 and is a popular clinical measure that has validated versions available in many languages. The MMSE consists of a variety of tasks, yields a summed score with a maximum of 30 points, and can generally be administered in less than 10 minutes. The tasks have been grouped into seven categories, each rationally representing a different function: orientation to time, orientation to place, registration, attention and calculation, spontaneous recall, language (naming, repetition, reading, and spontaneous writing), and visual construction. The traditional cutoff score for dementia in mixed samples is less than 24, but this requires adjustment depending on the desired sensitivity versus specificity (see Chapter 2).
The MMSE has significant content limitations. There is substantial variability in the recall of three words in healthy elderly patients, with low to moderate correlations between scores on this task and those on standard neuropsychological tests of memory.148 The MMSE does not assess long-delay recall, which can result in false-negative findings in the evaluation of relatively mild memory disorder and may fail to reveal amnesia.149 The “WORLD backwards” and serial sevens forms are not equivalent, and it has been recommended that they be replaced by the “months backwards” task.150 A significant concern with the structure of the MMSE is the lack of specific items that test executive function and spatial recall, and the American Neuropsychiatric Association has recommended that clinicians “supplement it with specific measures of spatial functions, delayed memory, and executive abilities.”151 Finally, a patient’s age and level of education significantly affect MMSE scores, and this needs to be taken into account when scores are interpreted.152,153 In response to these limitations, the more comprehensive Modified Mini-Mental State Examination has been developed and is being increasingly used in epidemiological and community-based surveys.154
Neurobehavioral Cognitive Status Examination
The Neurobehavioral Cognitive Status Examination (NCSE), now known as the Cognistat, was developed for use in neurosurgical patients and involves the “screen and metric” approach in 11 cognitive domains155: level of consciousness, orientation, attention, comprehension, repetition, naming, construction, memory, calculation, similarities, and judgment. The resultant profile provides a “pattern” of distribution of cognitive dysfunction across the relevant domains. The Cognistat has shown utility in a number of neurocognitive conditions, particularly dementias, but has proved less useful in populations with neurobehavioral or neuropsychiatric disorders. The Cognistat assesses some aspects of executive function but does not test sequencing, inhibition, or verbal fluency. It appears to be a reasonably sensitive tool for the detection of dementia, but at a significant cost to its specificity,156 and is subject to the same effects of age and education as is the MMSE.157 The screen and metric approach has been criticized for sacrificing sensitivity for expediency, resulting in false-negative results in some populations.158 The Cognistat takes significantly longer to complete than the MMSE.
Addenbrooke’s Cognitive Examination
Addenbrooke’s Cognitive Examination (ACE) was developed by Mathuranath and coworkers in Cambridge159 and was reported to be able to reliably differentiate frontotemporal from Alzheimer’s dementia, although this property has been questioned.160 It has shown utility in parkinsonian syndromes161 and in differentiating early dementia from affective disorders.162 Addenbrooke’s Cognitive Examination provides a total score out of 100 and includes all items from the MMSE, which allows the clinician to generate both scores. It provides unequal scores on scales of orientation, attention/concentration, verbal fluency, language, and visuospatial function. Calculating a ratio of scores in these areas may aid in the differentiation of dementia subtypes. A revised version of Addenbrooke’s Cognitive Examination is currently being developed by the Cambridge group.
Frontal Assessment Battery
In view of the generally low inclusion rates of executive function testing in most screening tools, a further option is to use a dedicated executive battery such as the Frontal Assessment Battery, which has shown good correlation with neuropsychological measures of cognitive function and discriminant validity for patients with frontal lobe disorders.163 The Frontal Assessment Battery may for be used to supplement the MMSE, because of its lack of executive function testing.
THE NEUROCOGNITIVE FORMULATION
Case Example
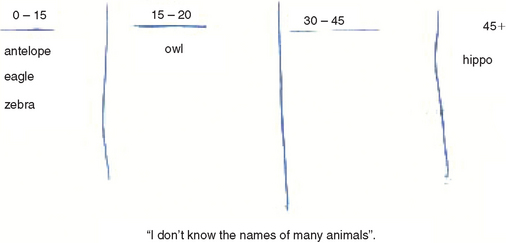
Mr. Jones, a 50-year-old executive with two teenaged children, presented for neurological assessment after a recent minor head injury in a car accident, on a background of 18 months of personality and functional change. Mr. Jones’ major complaint was of feeling rundown and lethargic, which had led his local doctor to prescribe antidepressants. His wife had noted that, for the last 18 months, he had been lacking motivation, had been forgetful, and had been allocated work duties well below his level of seniority. The family history revealed that Mr. Jones’ father had recently received a diagnosis of Parkinson’s disease after a 10-year history of gradual cognitive decline. Mr. Jones had been educated to tertiary level. There was no medical, psychiatric, or substance use history. On mental state examination, he manifested psychomotor retardation with poor grooming and a relative lack of concern regarding the recent events in his life. He did not describe depressed mood or communicate a depressed affect. He was oriented and performed well on attentional and memory testing. There were no abnormalities of visuoconstruction, praxis, left/right orientation, or calculation. He performed normally on language tasks. He had difficulty with a three-step motor sequencing task, could name only five animals in 1 minute (Fig. 1-3) and gave concrete responses on a task of similarities. He scored 29 out of 30 on the MMSE. Neurological examination findings were normal except for bilateral grasp reflexes.
CONCLUSION
The assessment of neurocognitive function extends far beyond the administration of cognitive tests and should include relevant aspects of history from a breadth of informants, as well as from the patient, and examination findings. A diagnosis is rarely made on the basis of a deficit in cognition alone. Even if the only positive finding is a single deficit in one cognitive domain, the clinician must always bear in mind Lishman’s “delicate balance”7 when discussing his or her formulation with the patients and caretakers and planning management and treatment appropriately.
Cummings JL, Mega MS. Neuropsychiatry and Behavioral Neuroscience. New York: Oxford University Press, 2003.
Mitchell AJ. Neuropsychiatry and Behavioral Neurology Explained. Philadelphia: Elsevier, 2003.
Price BH, Adams RD, Coyle JT. Neurology and psychiatry. Closing the great divide. Neurology. 2000;54:8.
Schiffer RB, Rao SM, Fogel BS. Neuropsychiatry, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2003.
Yudovsky SC, Hales RE. American Psychiatric Publishing Textbook of Neuropsychiatry and Clinical Neurosciences. Washington, DC: American Psychiatric Publishing, 2003.
1 Andrewes D. Neuropsychology: From Theory to Practice. East Sussex, UK: Psychology Press Ltd., 2001.
2 Mesulam M-M. Behavioral Neurology. Philadelphia: FA Davis, 1985.
3 Cummings J. Clinical Neuropsychiatry. Orlando, FL: Grune & Stratton, 1985.
4 Lishman WA. Organic Psychiatry: The Psychological Consequences of Cerebral Disorder. Oxford, UK: Blackwell Science, 1998.
5 Schiffer RB, Rao SM, Fogel BS. Neuropsychiatry. Philadelphia: Lippincott Williams & Wilkins, 2003.
6 Yudovsky SC, Hales RE. American Psychiatric Publishing Textbook of Neuropsychiatry and Clinical Neurosciences. Washington, DC: American Psychiatric Publishing, 2003.
7 Lishman AW. Neuropsychiatry: a delicate balance. Psychosomatics. 1992;33(1):4-9.
8 Price BH, Adams RD, Coyle JT. Neurology and psychiatry. Closing the great divide. Neurology. 2000;54:8.
9 Strub R, Black F. The Mental Status Examination in Neurology. Philadelphia: FA Davis, 2000.
10 Finkel S. Behavioral and psychological symptoms of dementia: a current focus for clinicians, researchers, and caregivers. J Clin Psychiatry. 2001;62(Suppl 21):3-6.
11 Marvel C, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatr Clin North Am. 2004;27:19-36.
12 Flashman L, Green M. Review of cognition and brain structure in schizophrenia: profiles, longitudinal course, and effects of treatment. Psychiatr Clin North Am. 2004;27:1-18.
13 Chow T. Frontotemporal dementias: clinical features and management. Semin Clin Neuropsychiatry. 2003;8:58-70.
14 Schweitzer I, Tuckwell V, O’Brien J, et al. Is late onset depression a prodrome to dementia? Int J Geriatr Psychiatry. 2002;17:995-1005.
15 Tombaugh T, McIntyre N. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922-935.
16 Nelson H, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the new adult reading test. Cortex. 1978;14:234-244.
17 Cummings J, Houlihan J, Hill M. The pattern of reading deterioration in dementia of the Alzheimer type: observations and implications. Brain Lang. 1986;29:315-323.
18 Wilkinson G. WRAT3 Administration Manual. Wilmington, DE: Wide Range, 1993.
19 Beardsall L, Huppert F. Improvement in NART word reading in demented and normal older persons using the Cambridge contextual reading test. J Clin Exp Neuropsychol. 1994;16:232-242.
20 Alexander M, Benson D. The aphasias and related disturbances. In: Joynt RJ, Griggs RC, editors. Clinical Neurology. Philadelphia: Lippincott; 1998:1-58.
21 Shenal B, Harrison D, Demaree H. The neuropsychology of depression: a literature review and preliminary model. Neuropsychol Rev. 2003;13:33-42.
22 Rogers M, Kasai K, Koji M, et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1-11.
23 Tandberg E, Larsen J, Aarsland D, et al. The occurrence of depression in Parkinson’s disease. A community-based study. Arch Neurol. 1996;53:175-179.
24 Folstein S, Abbott M, Chase G, et al. The association of affective disorder with Huntington’s disease in a case series and in families. Psychol Med. 1983;13:537-542.
25 Feinstein A. The Clinical Neuropsychiatry of Multiple Sclerosis. Cambridge, UK: Cambridge University Press, 1999.
26 Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571.
27 Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
28 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Press, 1994.
29 Castillo C, Schultz S, Robinson R. Clinical correlates of earlyonset and late-onset post-stroke generalized anxiety. Am J Psychiatry. 1995;152:1174-1179.
30 Paulsen J, Ready R, Hamilton J, et al. Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry. 2001;71:310-314.
31 Richard I, Schiffer R, Kurlan R. Anxiety and Parkinson’s disease. Mov Disord. 1996;8:501-506.
32 Young G, Chandarana P, Blume W, et al. Mesial temporal lobe seizures presenting as anxiety disorders. J Neuropsychiatry Clin Neurosci. 1995;7:352-357.
33 Simon N, Blacker D, Korbly N, et al. Hypothyroidism and hyperthyroidism in anxiety disorders revisited: new data and literature review. J Affect Disord. 2002;69:209-217.
34 George M, Trimble M, Ring H, et al. Obsessions in obsessive-compulsive disorder with and without Gilles de la Tourette’s syndrome. Am J Psychiatry. 1993;150:93-97.
35 Snider L, Swedo S. PANDAS: current status and directions for research. Mol Psychiatry. 2004;9:900-907.
36 Cummings J. Organic psychosis. Psychosomatics. 1988;29:16-26.
37 Cummings J. Organic delusions: phenomenology, anatomical correlates and review. Br J Psychiatry. 1985;146:184-187.
38 Cutting J. The phenomenology of acute organic psychosis. Comparison with acute schizophrenia. Br J Psychiatry. 1987;151:324-332.
39 van Os J. Is there a continuum of psychotic experiences in the general population? Epidemiol Psichiatr Soc. 2003;12:242-252.
40 Bentall R, Slade P. Reality testing and auditory hallucinations: a signal detection analysis. Br J Clin Psychol. 1985;24:159-169.
41 Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143:1088-1097.
42 Santhouse AM, Howard RJ, Ffytche DH. Visual hallucinatory syndromes and the anatomy of the visual brain. Brain. 2000;123(Pt 10):2055-2064.
43 Goldberg D, Bridges K, Duncan-Jones P, et al. Dimensions of neuroses seen in primary-care settings. Psychol Med. 1987;17:461-470.
44 Harwood D, Sultzer D, Wheatley M. Impaired insight in Alzheimer’s disease: association with cognitive deficits, psychiatric symptoms, and behavioural disturbances. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:83-88.
45 Lacro J, Dunn L, Dolder C, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892-909.
46 Johnson S, Orrell M. Insight, psychosis and ethnicity: a case-note study. Psychol Med. 1996;26:1081-1084.
47 David A. “To see ourselves as others see us.” Aubrey Lewis’ insight. Br J Psychiatry. 1999;175:210-216.
48 David A. Insight and psychosis. Br J Psychiatry. 1990;156:798-808.
49 McGlashan T. Recovery from mental illness and long-term outcome. J Nerv Ment Dis. 1987;175:681-685.
50 Babinski J. Contribution à l’étude des troubles mentaux dans l’hémiplégie organique cérébrale (anosognosie). Revue Neurologique. 1914;22:845-848.
51 Vuilleumier P. Anosognosia: the neurology of beliefs and uncertainties. Cortex. 2004;40:9-17.
52 Heilman K, Watson R. Mechanisms underlying the unilateral neglect syndrome. Adv Neurol. 1977;18:93-105.
53 Hodges J, Miller B. Frontotemporal dementia (Pick’s disease). In: Hodges J, editor. Early-Onset Dementia: A Multidisciplinary Approach. Oxford, UK: Oxford University Press; 2001:284-303.
54 Marin R. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147:22-30.
55 Mega M, Cummings J. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci. 1994;6:358-370.
56 Burns A, Folstein S, Brandt J, et al. Clinical assessment of irritability, aggression and apathy in Huntington’s and Alzheimer’s disease. J Nerv Ment Dis. 1990;178:20-26.
57 Starkstein S, Mayberg H, Preziosi T, et al. Reliability, validity and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4:134-139.
58 Andersson S, Krogstad J, Finset A. Apathy and depressed mood in acquired brain damage: relationship to lesion localization and psychophysiological reactivity. Psychol Med. 1999;29:447-456.
59 Roth R, Flashman L, Saykin A, et al. Apathy in schizophrenia: reduced frontal lobe volume and neuropsychological deficits. Am J Psychiatry. 2004;161:157-159.
60 Phillips S, Sangalang V, Sterns G. Basal forebrain infarction. A clinicopathologic correlation. Arch Neurol. 1987;44:1134-1138.
61 Marin R, Fogel B, Hawkins J, et al. Apathy: a treatable syndrome. J Neuropsychiatry Clin Neurosci. 1995;7:23-30.
62 Hertel P, Nomikos G, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berlin). 1996;124:74-86.
63 Levy M, Cummings J, Fairbanks L, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10:314-319.
64 Aron A, Robbins T, Poldrack R. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170-177.
65 Starkstein S, Robinson R. Mechanism of disinhibition after brain lesions. J Nerv Ment Dis. 1997;185:108-114.
66 Starkstein S, Boston J, Robinson R. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176:87-100.
67 Neary D, Snowden J, Northen B, et al. Dementia of frontal lobe type. J Neurol Neurosurg Psychiatry. 1988;51:353-361.
68 Cooper S, Dourish C. Neurobiology of Stereotyped Behaviour. London: Oxford University Press, 1990.
69 Lewis M, Gluck J, Bodfish J, et al. Neurobiological basis of stereotyped movement disorder in animals and humans. In: Sprague R, Newell K, editors. Stereotypy: Brain-Behavior Relationships. Washington, DC: American Psychological Association Press; 1996:37-67.
70 Stuss D, Benson D. Neuropsychological studies of the frontal lobes. Psychol Bull. 1984;95:3-24.
71 Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8:566-583.
72 Graybiel A, Canales J. The neurobiology of repetitive behaviors: clues to the neurobiology of Tourette syndrome. In: Cohen D, Goetz C, Jankovic J, editors. Tourette Syndrome. Philadelphia: Williams & Wilkins; 2001:123-131.
73 Cummings J. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann N Y Acad Sci. 1995;769:1-13.
74 Walterfang M, Velakoulis D. Cortical release signs in psychiatry. Aust N Z J Psychiatry. 2005;39:317-327.
75 Lhermitte F. “Utilization behaviour” and its relation to lesion of the frontal lobes. Brain. 1983;106:237-255.
76 Rudd R, Maruff P, MacCuspie-Moore C, et al. Stimulus relevance in eliciting utilisation behaviour. Case study in a patient with a caudate lesion. Cogn Neuropsychiatry. 1998;3:287-298.
77 Harris JC. Developmental Neuropsychiatry. Assessment, Diagnosis and Treatment of Developmental Disorders. New York: Oxford University Press, 1998.
78 Coffey CE, Brumback RA, editors. Pediatric Neuropsychiatry. Washington, DC: American Psychiatric Press, 1998.
79 Tanner CM, Chamberland J. Latah in Jakarta, Indonesia. Move Disord. 2001;16:526-529.
80 Schuler A. Echolalia. Issues and clinical applications. J Speech Hear Disord. 1979;44:411-434.
81 Wint D, Okun M, Fernandez H. Psychosis in Parkinson’s disease. J Geriatr Psychiatry Neurol. 2004;17:127-136.
82 Katzenschlager R, Sampaio C, Costa J, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. (2):2003. CD003735.
83 Loftis J, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82:175-190.
84 Brown E, Khan D, Nejtek V. The psychiatric side effects of corticosteroids. Ann Allergy Asthma Immunol. 1999;83:495-503.
85 Inouye S. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32:257-263.
86 Lawson W, Waldman I, Weinberger D. Schizophrenic dementia. Clinical and computed axial tomography correlates. J Nerv Ment Dis. 1988;176:207-212.
87 Shulman K. Disinhibition syndromes, secondary mania and bipolar disorder in old age. J Affect Disord. 1997;46:175-182.
88 Dobie D. Depression, dementia and pseudodementia. Semin Clin Neuropsychiatry. 2002;7:170-186.
89 Harris D, Batki S. Stimulant psychosis: symptom profile and acute clinical course. Am J Addict. 2000;9:28-37.
90 Kalant H. Adverse effects of cannabis on health: an update of the literature since 1996. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:849-863.
91 Westermeyer J. The psychiatrist and solvent-inhalant abuse: recognition, assessment and treatment. Am J Psychiatry. 1987;144:903-907.
92 Cummings J, Mega M, Gray K, et al. The Neuropsychiatric Inventory: a comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308-2314.
93 Wood S, Cummings J, Hsu M, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry. 2000;8:75-83.
94 Choi S, Na D, Kwon H. The Korean version of the Neuropsychiatric Inventory: a scoring tool for neuropsychiatric disturbance in dementia patients. J Korean Med Sci. 2000;15:609-615.
95 Leung V, Lam L, Chiu H. Validation study of the Chinese version of the Neuropsychiatric Inventory (CNPI). Int J Geriatr Psychiatry. 2001;16:789-793.
96 Vilalta-Franch J, Lozano-Gallego M, Hernandez-Ferrandiz M, et al. The Neuropsychiatric Inventory: psychometric properties of its adaptation into Spanish. Rev Neurol. 1999;29:15-19.
97 Politis A, Mayer L, Passa M, et al. Validity and reliability of the newly translated Hellenic Neuropsychiatric Inventory (H-NPI) applied to Greek outpatients with Alzheimer’s disease: a study of disturbing behaviors among referrals to a memory clinic. Int J Geriatr Psychiatry. 2004;19:203-208.
98 Reisberg B, Auer S, Monteiro I. Behavioral pathology in Alzheimer’s disease (BEHAVE-AD) rating scale. Int Psychogeriatr. 1996;8(Suppl 3):301-308.
99 Mega M, Cummings J, Fiorello T, et al. The spectrum of behavioural changes in Alzheimer’s disease. Neurology. 1996;46:130-135.
100 Aarsland D, Cummings J, Larsen J. Neuropsychiatric differences between Parkinson’s disease with dementia and Alzheimer’s disease. Int J Geriatr Psychiatry. 2001;16:184-191.
101 Mourik J, Rosso S, Niermeijer M, et al. Frontotemporal dementia: behavioral symptoms and caregiver distress. Dement Geriatr Cogn Disord. 2004;18:299-306.
102 Litvan I, Mega M, Cummings J, et al. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology. 1996;47:1184-1188.
103 Litvan I, Cummings J, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry. 1998;65:717-721.
104 Lyketsos C, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475-1483.
105 Kulisevsky J, Litvan I, Berthier M, et al. Neuropsychiatric assessment of Gilles de la Tourette patients: comparative study with other hyperkinetic and hypokinetic movement disorders. Mov Disord. 2001;16:1098-1104.
106 Aharon-Peretz J, Kliot D, Tomer D. Behavioral differences between white matter lacunar dementia and Alzheimer’s disease: a comparison on the neuropsychiatric inventory. Dement Geriatr Cogn Disord. 2000;11:294-298.
107 Diaz-Olavarrieta C, Cummings J, Velazquez J, et al. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci. 1999;11:51-57.
108 Holmes C, Wilkinson D, Dean C, et al. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology. 2004;63:214-219.
109 Monsch A, Giannakopoulos P. Effects of galantamine on behavioural and psychological disturbances and caregiver burden in patients with Alzheimer’s disease. Curr Med Res Opin. 2004;20:931-938.
110 Aupperle P, Koumaras B, Chen M, et al. Long-term effects of rivastigmine treatment on neuropsychiatric and behavioral disturbances in nursing home residents with moderate to severe Alzheimer’s disease: results of a 52-week open-label study. Curr Med Res Opin. 2004;20:1605-1612.
111 Levin H, High W, Goethe K, et al. The Neurobehavioural Rating Scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry. 1987;50:183-193.
112 Overall J, Gorham D. The brief psychiatric rating scale. Psychol Reports. 1962;10:799-812.
113 Vanier M, Mazaux J, Lambert J, et al. Assessment of neuropsychologic impairments after head injury: interrater reliability and factorial and criterion validity of the Neurobehavioral Rating Scale-Revised. Arch Phys Med Rehabil. 2000;81:796-806.
114 Merchant R, Bullock M, Carmack C, et al. A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann N Y Acad Sci. 1999;890:42-50.
115 Rapoport M, McCauley S, Levin H, et al. The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:123-132.
116 Vilkki J, Ahola K, Holst P, et al. Prediction of psychosocial recovery after head injury with cognitive tests and neurobehavioral ratings. J Clin Exp Neuropsychol. 1994;16:325-338.
117 Sultzer D, Berisford M, Gunay I. The Neurobehavioral Rating Scale: reliability in patients with dementia. J Psychiatr Res. 1995;29:185-191.
118 Pollock B, Mulsant B, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159:460-465.
119 Mathias J. Neurobehavioral functioning of persons with Parkinson’s disease. Appl Neuropsychol. 2003;10:57-68.
120 Hilton G, Sisson R, Freeman E. The Neurobehavioral Rating Scale: an interrater reliability study in the HIV seropositive population. J Neurosci Nurs. 1990;22:36-42.
121 Rabee H, Saadani M, Iqbal K, et al. Neurobehavioral effects of carotid endarterectomy. Saudi Med J. 2001;22:433-437.
122 Folstein M, Folstein S, McHugh P. “Mini Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
123 Manning R. The serial sevens test. Arch Intern Med. 1982;142:1192.
124 Critchley M. The Parietal Lobes. New York: Hafner, 1966.
125 Shulman K. Clock drawing: is it the ideal cognitive test? Int J Geriatr Psychiatry. 2000;15:548-561.
126 Moo L, Slotnick S, Tesoro M, et al. Interlocking finger test: a bedside screen for parietal lobe dysfunction. J Neurol Neurosurg Psychiatry. 2003;74:530-532.
127 Tulving E, Kapur S, Craik F, et al. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings [Review]. Proc Natl Acad Sci U S A. 1994;91:2016-2020.
128 Walterfang M, Velakoulis D, Gibbs A, et al. The NUCOG: construction and piloting of a cognitive screening instrument in a neuropsychiatric unit. Australas Psychiatry. 2003;11:325-329.
129 Marburg D. The effect of lesions in the centromedian nucleus of the thalamus on the monkey’s performance in delayed alternation and object reversal tasks. Int J Neurosci. 1973;5:207-214.
130 Ridley R. The psychology of perseverative and stereotyped behaviour. Prog Neurobiol. 1994;44:221-231.
131 Hotz G, Helm-Estabrooks N. Perseveration. Part I: a review. Brain Inj. 1995;9:151-159.
132 Nagahama Y, Okada T, Katsumi Y, et al. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex. 2001;11:85-92.
133 Shammi P, Stuss D. Humour appreciation: a role of the right frontal lobe. Brain. 1999;122:657-666.
134 Luria A. The Working Brain. New York: Basic Books, 1973.
135 Luria A. Frontal lobe syndromes. Vinken P, Bruyn G, editors. Handbook of Clinical Neurology. vol 2. New York: Elsevier; 1969:725-757.
136 Barnes T, Crichton P, Nelson H, et al. Primitive (developmental) reflexes, tardive dyskinesia and intellectual impairment in schizophrenia. Schizophr Res. 1995;16:47-52.
137 Youssef H, Waddington J. Primitive (developmental) reflexes and diffuse cerebral dysfunction in schizophrenia and bipolar affective disorder: over-representation in patients with tardive dyskinesia. Biol Psychiatry. 1988;23:791-796.
138 Franssen E, Reisberg B, Kluger A, et al. Cognition-independent neurologic symptoms in normal aging and probable Alzheimer’s disease. Arch Neurol. 1991;48:148-154.
139 Benesch C, McDaniel K, Cox C, et al. End-stage Alzheimer’s disease: Glasgow Coma Scale and the neurologic examination. Arch Neurol. 1993;50:1309-1315.
140 Burns A, Jacoby R, Levy R. Neurological signs in Alzheimer’s disease. Age Ageing. 1991;20:45-51.
141 Gregory C, Orrell M, Sahakian B, et al. Can frontotemporal dementia and Alzheimer’s disease be differentiated using a brief battery of tests? Int J Geriatr Psychiatry. 1997;12:375-383.
142 Sjögren M, Wallin A, Edman A. Symptomatological characteristics distinguish between frontotemporal dementia and vascular dementia with a dominant frontal lobe syndrome. Int J Geriatr Psychiatry. 1997;12:656-661.
143 Di Legge S, Di Piero V, Altieri M, et al. Usefulness of primitive reflexes in demented and non-demented cerebrovascular patients in daily clinical practice. Eur Neurol. 2001;45:104-110.
144 Hogan D, Ebly E. Primitive reflexes and dementia: results from the Canadian Study of Health and Aging. Age Ageing. 1995;24:375-381.
145 Jacobs L, Gossman M. Three primitive reflexes in normal adults. Neurology. 1980;30:184-188.
146 Jenkyn L, Reeves A, Warren T, et al. Neurologic signs in senescence. Arch Neurol. 1985;42:1154-1157.
147 Kobayashi S, Yamaguchi S, Okada K, et al. Primitive reflexes and MRI findings, cerebral blood flow in normal elderly. Gerontology. 1990;36:199-205.
148 Cullum M, Thompson L, Smernoff E. Three word recall as a measure of memory. J Clin Exp Neuropsychol. 1993;15:321-329.
149 Benedict R, Brandt J. Limitations of the Mini-Mental State Examination for the detection of amnesia. J Geriatr Psychiatry Neurol. 1993;5:233-237.
150 Galasko D, Klauber MR, Hofstetter CR, et al. The Mini-Mental State Examination in the early diagnosis of Alzheimer’s disease. Arch Neurol. 1990;47:49-52.
151 Malloy P, Cummings J, Coffey C, et al. Cognitive screening instruments in neuropsychiatry: a report of the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 1997;9:189-197.
152 O’Connor D, Pillott P. The influence of education, social class and sex on Mini-Mental State scores. Psychol Med. 1989;19:771-776.
153 Mungas D, Marshall S, Weldon M, et al. Age and education correction of Mini-Mental State Examination for English and Spanish speaking elderly. Neurology. 1996;46:700-706.
154 Teng EL, Chui H. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314-318.
155 Kiernan R, Mueller J, Langston J, et al. The Neurobehavioral Cognitive Status Examination: a brief but quantitative approach to cognitive assessment. Ann Intern Med. 1987;107:481-485.
156 Drane D, Osato S. Using the Neurobehavioral Cognitive Status Examination as a screening measure for older adults. Arch Clin Neuropsychol. 1997;12:139-143.
157 Drane DL, Yuspeh RL, Huthwaite JS, et al. Healthy older adult performance on a modified version of the Cognistat (NCSE): demographic issues and preliminary normative data. J Clin Exp Neuropsychol. 2003;25:133-144.
158 Oehlert M, Hass S, Freeman M, et al. The Neurobehavioral Cognitive Status Examination: accuracy of the “screen-metric” approach in a clinical sample. J Clin Psychol. 1997;53:733-737.
159 Mathuranath P, Nestor P, Berrios G, et al. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology. 2000;55:1613-1620.
160 Bier J, Ventura M, Donckels V, et al. Is the Addenbrooke’s Cognitive Examination effective to detect frontotemporal dementia? J Neurol. 2004;251:428-431.
161 Bak T, Rogers T, Crawford L, et al. Cognitive bedside assessment in atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2005;76:420-422.
162 Dudas R, Berrios G, Hodges J. The Addenbrooke’s Cognitive Examination (ACE) in the differential diagnosis of early dementias versus affective disorder. Am J Geriatr Psychiatry. 2005;13:218-226.
163 Dubois B, Slachevsky A, Litvan I, et al. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621-1626.