Clinical Applications of Doppler Ultrasound in Obstetrics
The Uteroplacental Circulation
The uterine artery is a branch of the anterior division of the internal iliac artery, and divides further into four arcuate arteries, each of which divides into more than 25 spiral arteries. There are therefore between 100 and 200 spiral arteries which enter the intervillus space. During early pregnancy, trophoblast cells invade this space and disrupt the wall of the spiral arteries as part of the process of placental formation. There are two separate waves of invasion. Between implantation and 10 weeks, the trophoblastic invasion is limited to the decidual layer. From about 14 weeks until 22 weeks, the invasion extends as far as the spiral arteries. This invasion of the spiral arteries affects the resistance to blood flow within the spiral arteries and thereby in the arcuate and main uterine arteries (Fig. 15-1).1
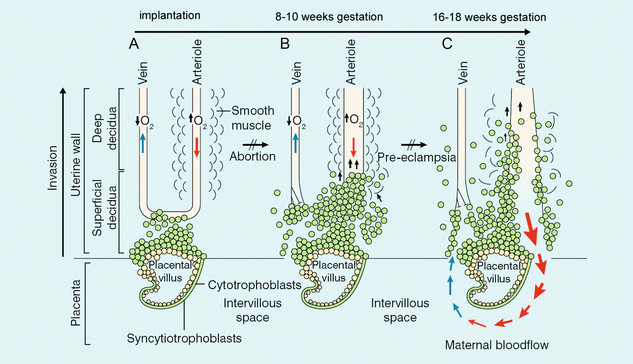
FIGURE 15-1 Endovascular trophoblast invasion into the endometrium occurs in two waves: (A) shows the situation shortly after implantation; (B) at 8–10 weeks the decidual segments of the spiral arteries are invaded; (C) at 16–18 weeks the myometrial segments are invaded. This results in low resistance to blood flow on the maternal side of the placental bed in a normal pregnancy.
METHOD OF EXAMINATION
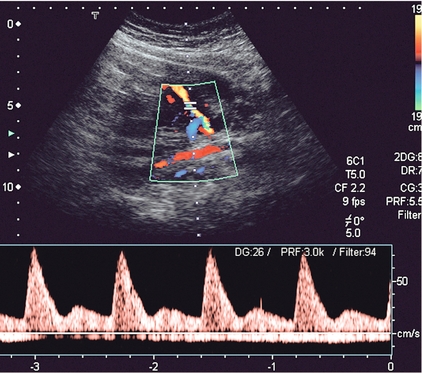
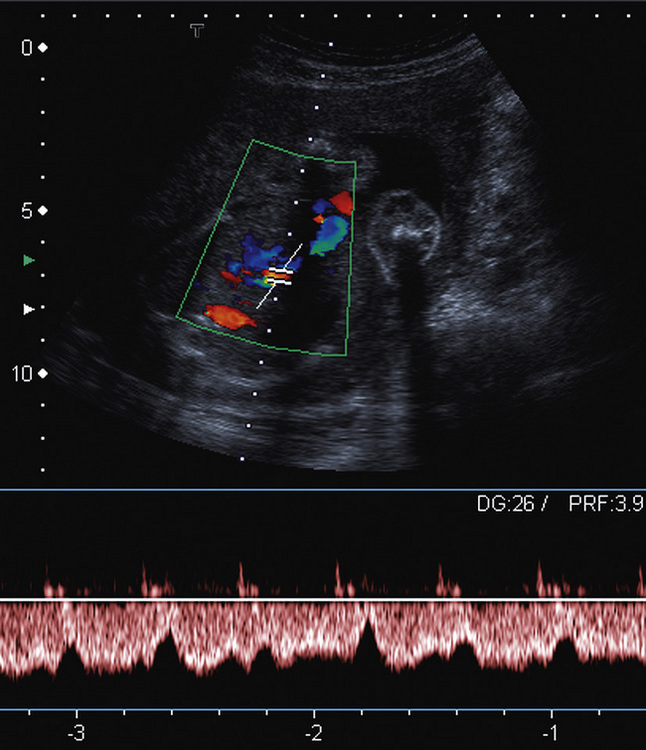
The complexity of the uteroplacental circulation makes accurate identification of the vessel under study difficult with either continuous wave or duplex Doppler ultrasound. Originally flow velocity waveforms were obtained using these modalities by angling the transducer towards the cervix on either side of the lower quadrant of the uterus.2,3 However the uterine arteries are more accurately identified using colour Doppler, which is now the recommended technique to study this artery. The region lateral to the lower uterus is examined and the external iliac artery and the adjacent vein are identified. The uterine artery crosses the external iliac artery on its course from the internal iliac artery to the body of the uterus. It is important to angle the transducer to improve the angle of insonation whilst maintaining vessel identification on colour Doppler. Spectral waveforms are obtained by placing the pulsed Doppler range gate within the vessel at this point (Fig. 15-2A).4 The spectral waveform from the normal uteroplacental system is unidirectional, of low pulsatility, and demonstrates frequencies throughout the cardiac cycle (Fig. 15-2B). This is a result of the trophoblastic invasion of the spiral arteries; end-diastolic frequencies increase to a maximum at 24–26 weeks of gestation (see Fig. 15-7).5
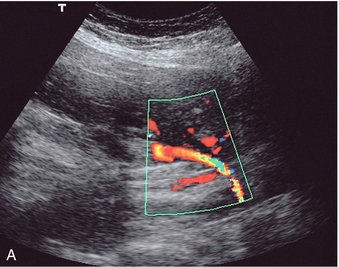
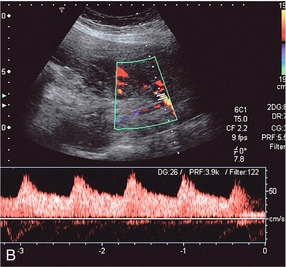
FIGURE 15-2 (A) Course of the left uterine artery as it crosses over the iliac vessels. (B) Normal uterine artery flow in the early third trimester demonstrating high diastolic flow.
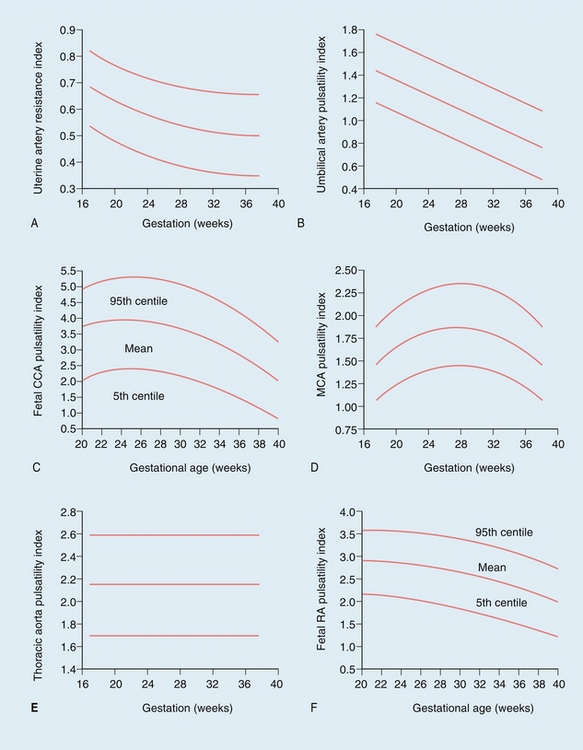
FIGURE 15-7 Normal values during pregnancy for (A) uterine artery resistance index (RI), and (B) umbilical artery pulsatility index (PI). Fetal common carotid artery (CCA) PI (C) falls steeply after 32 weeks gestation, mirrored by the middle cerebral artery (MCA) PI (D). Normal values are also shown for the fetal descending thoracic aorta PI (E) and renal artery (RA) PI (F). (Reproduced with permission from Pearce, References ranges and sources of variation for indices of pulsed Doppler flow velocity waveforms from the uteroplacental and fetal circulation, British J Obstet Gynaecol, 1988; 95(3): 248–256.)
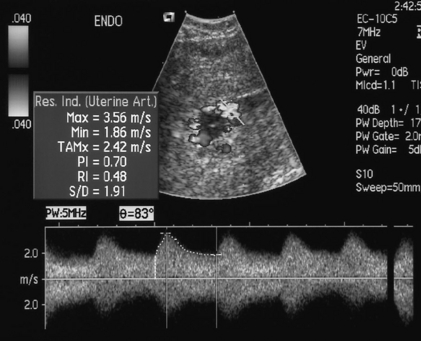
It is possible to calculate several indices to quantify blood flow; however, the most commonly used in the uterine artery is the resistance index (RI) (see Fig. 15-7A). After 26 weeks of gestation the normal range of the resistance index is between 0.45 and 0.58. Decreased end-diastolic flow with a consequently raised resistance index above 0.58 is considered abnormal, as is a notch in early diastole in either uterine artery, suggesting failure of trophoblastic invasion of the spiral arteries (Fig.15-3).6,7
PROBLEMS AND PITFALLS
A comparative histological study of third-trimester placental biopsy at caesarean section8 has demonstrated that the uterine artery flow impedance reflects impaired trophoblast migration. Nonetheless, there are a number of reasons for caution. Impedance to flow varies throughout the uteroplacental circulation, with the lowest value seen in the arcuate vessels on the placental side of the uterus and the highest value seen in the uterine arteries on the non-placental side of the uterus.9 The physiological variations and anatomical complexities of the uteroplacental vascular tree make it difficult to obtain accurate and reproducible measurements using continuous wave Doppler, with interobserver variations ranging from 3.9 to 17%.10,11 In later pregnancy, between 37 and 40 weeks, maternal position may also alter flow patterns, with the umbilical artery resistance being higher in the supine position than in the decubitus.12 Furthermore, variations in uterine activity (Fig. 15-4), maternal heart rate and exercise also significantly alter the waveform.13,14 The examination should therefore be performed with the mother resting and only during a period of uterine inactivity. The effect of exercise is more marked in complicated pregnancies, but there are no data evaluating the effect of exercise to improve sensitivity or specificity of Doppler in predicting fetal outcome. The time of day, or recent eating by the mother do not appear to have an effect on the uterine artery flow.14,15 Most of the antihypertensive drugs appear to have no effect on fetomaternal blood flow.16 However, nifedipine appears to produce a reduction in umbilical artery resistance, and this has therefore been suggested as a better drug to use during pregnancy.17 The effect of smoking on blood flow has been somewhat controversial with researchers reporting either no effect or a significant effect from smoking a cigarette.18,19 However, because of the dispute and the potential for cigarette smoking to alter flow patterns in chronic smokers, this author advocates that patients should not smoke for at least 1 hour prior to a Doppler study.
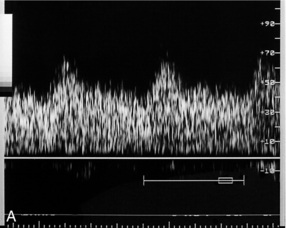
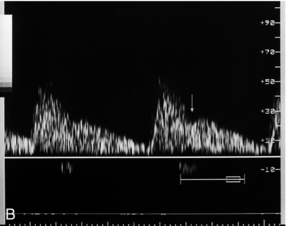
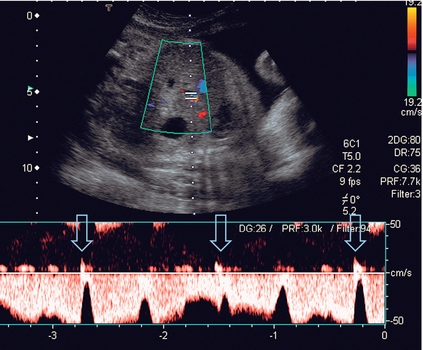
FIGURE 15-4 (A) Uterine artery in labour. Blood flow characteristics in the uterine artery between contractions are normal. (B) During a contraction the overall flow velocity in the uterine artery is reduced. However, the diastolic velocity is reduced further than the peak systolic velocity and there is an early diastolic notch (arrow).
PATHOLOGY
Although superficially attractive, the use of uterine artery Doppler, even in primiparous mothers where the risk of hypertensive complications is greater, is neither sensitive nor specific in the detection of pre-eclampsia, or small-for-gestational-age infants.20 This is typical for results seen in low- and moderate-risk pregnancies. The routine use of uterine artery Doppler studies to screen low-risk populations for subsequent development of pre-eclampsia and intrauterine growth retardation (IUGR) has a poor positive predictive value. Furthermore, screening can only be useful if there is an intervention available which will improve the outcome, and treatment options for both conditions are limited with, currently, no proven interventions to prevent either pre-eclampsia or IUGR. Therefore, in low-risk populations, using uterine artery Doppler studies as a screening tool is not recommended by national authorities, such as the National Institute for Health and Clinical Excellence (NICE) in England and Wales.21
COEXISTENT MATERNAL DISEASE
Uterine artery Doppler appears to be of significantly greater utility when there is pre-existing maternal disease. In chronic renal disease, for example, an abnormal artery waveform predicts pre-eclampsia and IUGR with a high degree of accuracy. Only 8% of patients with negative Doppler findings in one study developed complications of pregnancy.22 By contrast, although the resistance index is increased in the uterine arteries of diabetics with a morphological vasculopathy, there is no relationship with short- or long-term diabetic control and it is not a good predictor of diabetes-related fetal morbidity; presumably because these changes are reflecting the risk of acidosis as a result of hypoxia rather than metabolic acidosis.23,24 In patients with pre-existing, essential hypertension, uterine artery Doppler appears to be useful in defining groups of patients who are at risk of developing complications. If the systolic blood pressure is greater than 140 mmHg, then resistance indices in both uterine arteries is increased. If the systolic blood pressure is less than 140 mmHg, three separate groups may be identified: those with (a) bilateral or (b) unilateral abnormalities of the waveform within the uterine arteries; and (c) those with an entirely normal uterine artery flow. The prognosis appears to be related to the degree of abnormality of uterine artery flow. In systemic lupus erythematosus, one study suggests that an abnormal uterine artery Doppler will identify all those pregnancies with an adverse outcome.25
Doppler Examination of the Fetoplacental Circulation
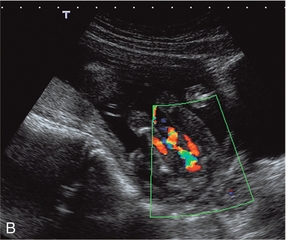
The placenta rather than the lungs is the organ of gaseous exchange in the fetus. Two umbilical arteries convey deoxygenated blood from the fetus to the placenta, and one umbilical vein returns oxygenated blood from the placenta to the fetal inferior vena cava. The umbilical arteries take origin from the fetal internal iliac arteries coursing alongside the lateral walls of the bladder in the urachus to the umbilical insertion. The umbilical vein courses posteriorly and cephalad in the falciform ligament to join the left branch of the portal vein. The oxygenated blood is then shunted through the liver to the inferior vena cava by the ductus venosus. Colour Doppler confers some advantages in the evaluation of the umbilical artery over duplex Doppler alone. Identification of a free loop of umbilical cord and the umbilical artery on colour Doppler allows unambiguous sampling of the correct vessel at the most appropriate angle to get the best waveform. Spectral waveforms are obtained by placing the pulsed Doppler range gate within the vessel. Research studies correlating abnormal umbilical artery Doppler findings to prenatal outcome relate to sampling of a free loop of umbilical cord. In clinical use, Doppler waveforms must therefore be recorded from a free loop of the cord. However in situations where there is oligohydramnios, and particularly in twin pregnancies with a ‘stuck twin’, sampling flow from the umbilical arteries within the fetal abdomen as they course alongside the bladder may be the only option to achieve satisfactory Doppler signals (Fig 15-5).
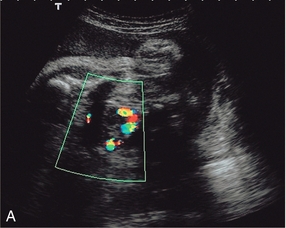
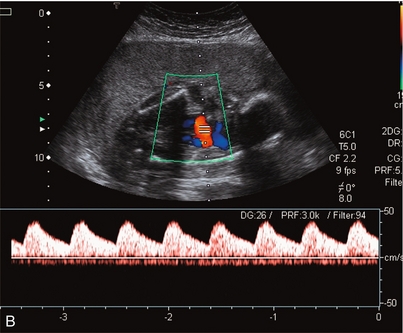
FIGURE 15-5 (A) Relative oligohydramnios. Colour flow Doppler demonstrating umbilical arteries in the pelvis on either side of an empty bladder. (B) Relative oligohydramnios sampling from the umbilical artery within the pelvis demonstrates normal umbilical artery flow patterns.
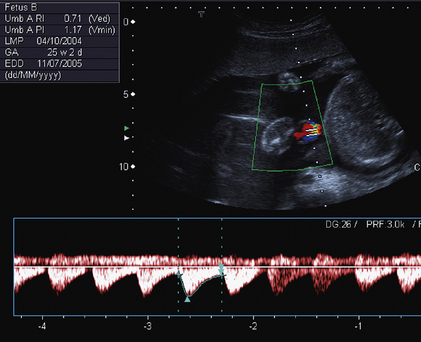
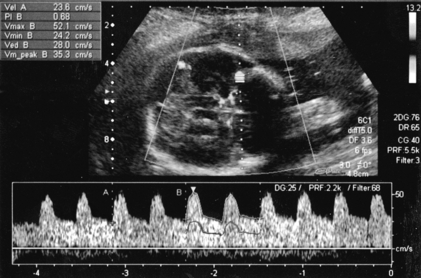
Doppler waveforms within the umbilical artery change with gestational age in a similar fashion to those of the uterine artery (Fig. 15-6). Resistance and pulsatility indices demonstrate a gradual reduction with increase in gestational age. Normal values of the pulsatility index are shown in Figure 15.7.
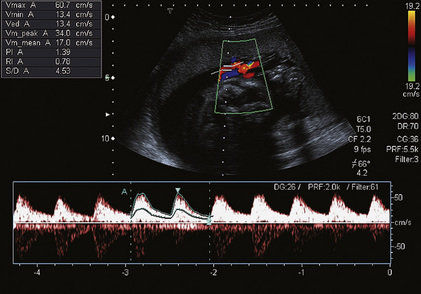
FIGURE 15-6 Umbilical artery waveform at 26 weeks gestation. Note the PI of 1.39 which is just above the mean value for this gestation.
PROBLEMS AND PITFALLS
Variations in fetal heart rate26 and the presence of fetal breathing movement27 may significantly alter the arterial waveforms, although within the physiological range of 120–160 beats per minute it is not necessary to correct indices for fetal heart rate.28 Nonetheless, it is important to examine umbilical artery waveforms during a period of fetal inactivity and in the absence of fetal breathing; duplex and colour Doppler ultrasound are of value in enabling the waveform to be sampled rapidly and accurately. Measurement of three consecutive cardiac cycles reduces the coefficient of variation of measurements to less than 5%.29
PATHOLOGY AND APPLICATIONS
TABLE 15-1
Indications for Doppler Examination in Pregnancy
| Uterine artery | Umbilical artery | Middle cerebral artery |
| High risk screening | Intrauterine growth retardation | Intrauterine growth retardation |
| Pre-existing disease, e.g. renal disease, systemic lupus erythematosus | Post dates | Fetal anaemia |
| Previous IUGR | Fetal abnormality | |
| Trophoblastic disease |
TABLE 15-2
| Uterine artery | Umbilical artery | Middle cerebral artery |
| Raised indices | Raised indices | Decreased indices |
| Notch | Absent end-diastolic flow | Raised maximum velocities |
| Reversed end-diastolic flow |
TABLE 15-3
Factors Contributing to Abnormal Flow
| Uterine artery | Umbilical artery | Middle cerebral artery |
| Pre-existing disease | Fetal activity | Direct cerebral compression |
| Exercise | Breathing | |
| Uterine activity | Cord compression | |
| Antihypertensives ?Cigarettes |
?Cigarettes |
Intrauterine Growth Retardation
Normal waveforms from the umbilical artery are unidirectional and demonstrate forward flow throughout the cardiac cycle. Decreased end-diastolic flow (Fig. 15-8) and consequently raised Doppler indices are considered abnormal and are thought to reflect increased placental resistance caused by damage to placental tertiary villi.30 In more extreme cases, end-diastolic flow may be absent (Fig. 15-9) or even reversed (Fig.15-10).
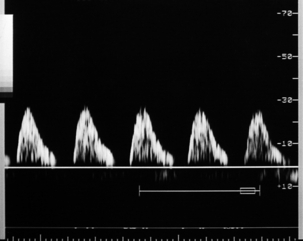
FIGURE 15-9 Severe intrauterine growth retardation with absent end-diastolic flow in the umbilical artery.
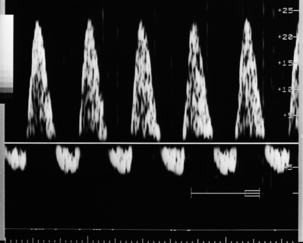
FIGURE 15-10 Reversed diastolic flow in the umbilical artery. This implies a fetus at risk of significant morbidity/mortality.
Reduction in end-diastolic flow velocities within the umbilical artery waveform is thought to occur as a result of reduced tertiary villi formation and therefore could be an indicator of placental dysfunction, IUGR and fetal distress. In spite of the attraction of the hypothesis that Doppler would provide an early indicator of failure of the tertiary villus, it has not proved to be of value as a primary screening tool in low-risk pregnancies for IUGR. However, in high-risk pregnancies, particularly those in which there is pregnancy-induced hypertension, or which have other clinical or sonographic evidence of fetal growth retardation, the umbilical artery waveform is a good indicator of fetal compromise. Progressive reduction in the diastolic component of umbilical artery flow mirrors the risk and severity of potential fetal compromise.31,32 Furthermore, not only may fetuses at increased risk be identified and managed more intensively but high-risk pregnancies, such as those with established IUGR or pregnancy-induced hypertension but with normal umbilical artery waveforms appear to carry no greater risk of fetal morbidity or fetal loss than a normal pregnancy. The corrected perinatal mortality rate is reduced when the results of umbilical artery Doppler are made available to clinicians, who are then able to act with more appropriate and timely intervention.33 The finding of absent or reversed end-diastolic flow in a pregnancy with established IUGR is an indication for the serious consideration of early delivery.34 However in late third trimester, as the umbilical artery pulsatility index normally decreases (Fig. 15-7B), using absent or reversed end diastolic flow in the umbilical artery is not sensitive for predicting poor outcome. This is because the flow in the umbilical vessels nearer term becomes so large it is very difficult for placental pathology to result in absent or reversed flows and the sonographer can be falsely reassured by positive flows although there may be progressive placental pathology. Fetuses with evidence of cerebral redistribution and adverse neurological sequelae can have umbilical artery PI values still within the normal range.35
Post Dates Pregnancy
The management of a post dates pregnancy is extremely difficult. No single parameter has been established that will confidently predict outcome and, although there has been some enthusiasm for a role for Doppler in the assessment of post dates pregnancy, there is not universal agreement about its value.36 In this author’s view, therefore, umbilical artery Doppler should be looked upon as one of the factors that may contribute to the monitoring of post dates pregnancies. Absence or reversal of diastolic flow is an indication for immediate delivery and in this situation, an operative delivery may need to be considered.
Twin and Higher-Order Pregnancy
In uncomplicated multiple pregnancy, there is the same progressive decrease in placental resistance as is seen in singleton pregnancies, with a consequent fall in indices with increasing gestational age. Doppler appears to be of no value in predicting adverse outcomes in unselected pregnancies.37 However, in pregnancies with discordant growth patterns, Doppler analysis of the umbilical arteries appears to be a useful adjunct to serial growth measurements, allowing recognition of high-risk twin pregnancies which require more intense surveillance38 (Fig. 15-11).
Diabetes
Both pre-existing vascular disease and hypertensive disorders of pregnancy are common in mothers with diabetes. In these cases, the value of an abnormal umbilical artery Doppler signal has the same significance in identification of uteroplacental insufficiency as in the non-diabetic population. However, diabetic pregnancies are also at risk of metabolic complications and Doppler flow patterns will not detect these complications, it is therefore vital that a normal umbilical artery flow does not give either the clinician or the mother false reassurance.22
Fetal Anaemia
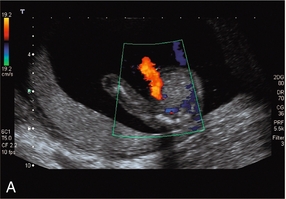
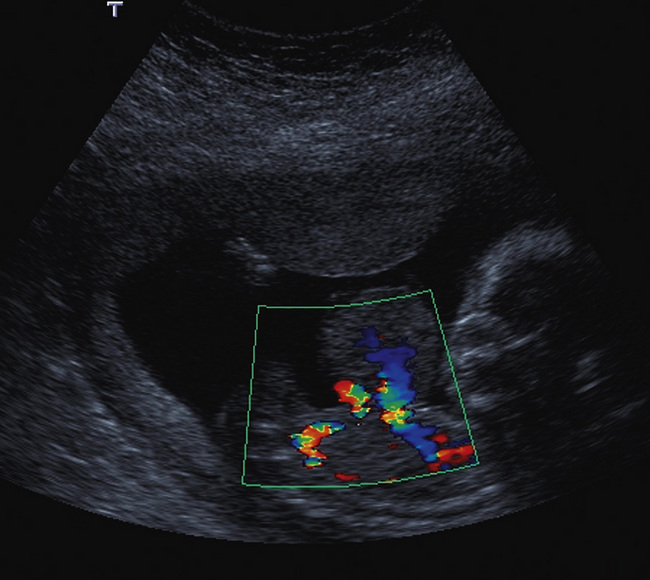
Performing middle cerebral artery Doppler studies (Fig. 15-12) in cases of suspected fetal anaemia has revolutionised the management of this condition. Chronic fetal anaemia is usually secondary to a maternal infection such as parvovirus, or alloimmunisation. The physiological response to chronic anaemia is the development of a hyperdynamic fetal circulation. Raised peak velocities in the middle cerebral artery are linked to fetal anaemia39,40 (Fig. 15-12C). Use of this technique accurately predicts anaemic fetuses, allowing the timing of any invasive testing to be at the point when fetal transfusions are clinically required. Middle cerebral artery Doppler peak velocities increase with gestational age.40 In cases of acute fetal anaemia secondary to fetomaternal haemorrhage, Doppler studies are not helpful, and urgent delivery is indicated to allow neonatal resuscitation.
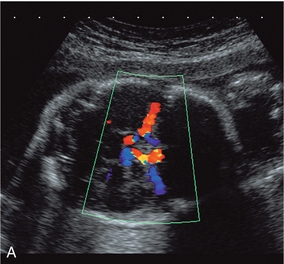
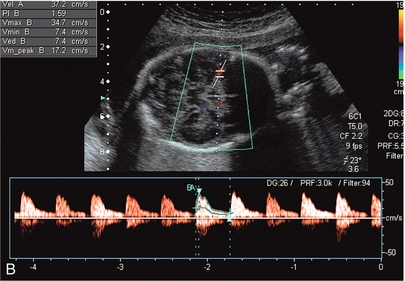
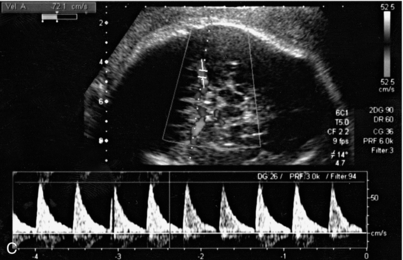
FIGURE 15-12 (A) Colour flow Doppler of the circle of Willis in the fetus. The middle cerebral arteries are demonstrated. (B) Spectral flow within the normal middle cerebral artery. Pulsatility index of 1.6 and a peak velocity of 37 cm/s. (C) Fetal anaemia increase in peak systolic flow velocity to 72 cm/s is demonstrated.
FETAL ABNORMALITY
Fetuses with autosomal trisomy may also have abnormal placentation with reduced tertiary villi formation.41 An abnormal umbilical artery waveform should therefore prompt a detailed study of fetal structural anatomy and an assessment of amniotic fluid volume; karyotyping should also be considered.34 This is particularly important in the presence of early-onset growth retardation (both symmetrical and asymmetrical), which has up to 20% association with abnormal karyotype.
Colour flow Doppler is of value in demonstrating abnormalities of the cord including true knots and a single umbilical artery (Fig. 15-13). Similarly, the use of colour Doppler will aid in the diagnosis of abdominal wall abnormalities such as omphaloceles and ectopia vesicae (Fig. 15-14).
The Fetal Circulation
THE FETAL CEREBRAL CIRCULATION
Whilst the fetal carotid artery can be examined, the middle cerebral artery is the most commonly interrogated of the intracerebral vessels. The skull is scanned as if to perform a biparietal diameter measurement. A colour Doppler examination is then performed in a plane slightly closer to the base of the skull, where the middle cerebral artery will be identified as a vessel coursing towards the probe from the circle of Willis in the Sylvian fissure (Fig. 15-12A).
The middle cerebral artery pulsatility index rises until 32 weeks of gestation and then falls steeply (Fig. 15-7).42
Fetal haemodynamics suggest a brain-sparing effect in IUGR and evidence of cerebral redistribution by a decrease in the pulsatility index in the middle cerebral artery in the presence of established growth retardation is commonly considered an indicator for delivery43,44 (Fig. 15-15). The presence of abnormal Doppler studies can also aid in the postnatal management of growth-restricted neonates who are at greater risk of ischaemic complications such as necrotising enterocolitis as a result of their intrauterine adaptations to a suboptimal environment.45,46 Clinicians vary as to the stage in the progress of growth retardation at any given gestation when they will decide that the fetus should be delivered; there is no evidence that early intervention improves perinatal outcome.47
THE RENAL ARTERIES
Oligohydramnios consequent upon poor renal perfusion is a cardinal sign of IUGR. Although Doppler evaluation of the renal arteries is not used in clinical practice, colour Doppler of the renal vessels (Fig. 15-16) is useful to assess congenital renal abnormalities, such as renal agenesis, where the artery is also absent, although care should be taken as hypoplastic arteries may be difficult to demonstrate on ultrasound, and enlarged adrenal glands supplied by large adrenal vessels may be misidentified as kidney.
THE DUCTUS VENOSUS
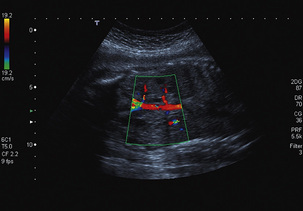
The ductus venosus is identified within the liver by following the umbilical vein in a sagittal plane into the fetal liver using colour Doppler. The ductus venosus arises at the junction of the umbilical vein and the left branch of the portal vein angling posteriorly and cephalad towards the inferior vena cava with which it makes an angle of 45 to 60°. Colour flow will demonstrate a high-velocity pattern with aliasing at low pulse repetition frequency (Fig. 15-17).
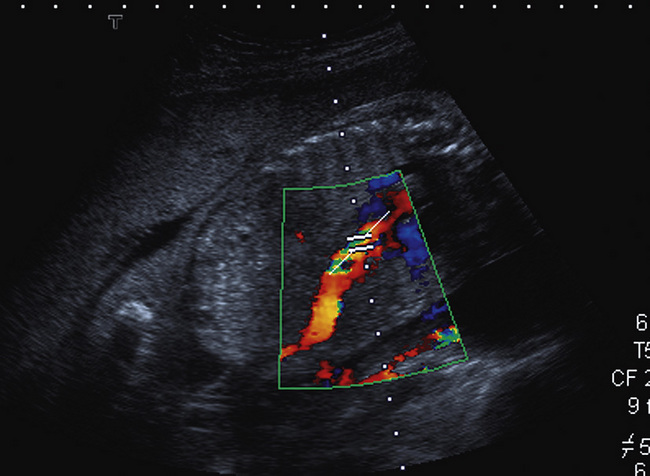
FIGURE 15-17 Oblique scan through the fetal abdomen with colour flow Doppler demonstrating the aliased flow within the ductus venosus. The Doppler sample volume is placed within the area of maximum aliasing.
Normal Doppler blood flow studies of the ductus venosus show a triphasic forward-flowing cycle reflecting ventricular systole, ventricular diastole and atrial systole (Fig. 15-18). Spectral analysis can therefore be used to reflect fetal ventricular function and cardiac afterload (Fig. 15-19). In the presence of end-stage hypoxaemia with hypoxic cardiomyopathy, there is a fall in cardiac function and hence rise in central venous pressure. This can be identified by absent end-diastolic, or reverse flow in the ductus venosus. The presence of reverse flow is associated with decreased fetal-maternal perfusion and fetal death.48,49 In severely growth-restricted fetuses where the gestational age is close to viability, use of this technique can help guide the clinician in deciding the exact moment at which delivery is required.
PLACENTAL ABNORMALITIES
The diagnosis of placenta praevia is largely performed by ultrasound imaging. Reporting of placental site at the routine second trimester transabdominal ultrasound has described incidences of low-lying placenta in excess of 40%,50 so routine screening for placenta praevia in the second trimester is therefore not now widely used. By contrast, when there is antepartum haemorrhage, ultrasound is used to establish the relationship of the placenta to the internal os. This is more commonly performed using transvaginal ultrasound. The addition of colour Doppler allows the demonstration of the rare but potentially serious vasa praevia. In this situation a vessel, or vessels, can be seen coursing from the margin of the placenta in close proximity to, or covering, the internal os. Similarly, other placental abnormalities can be documented: velamentous insertion of the cord can be identified with colour Doppler and chorioangioma can be differentiated from a placental haematoma by the demonstration of a rich vascular supply.51
Postpartum
There are changes in the blood flow characteristics of the uterine artery when there are retained products of conception: patients with residual trophoblast exhibit a resistance index in the myometrial vessels of 0.35 ± 0.1; whereas those without residual trophoblast have resistance indices in the myometrial vessels of 0.54 ± 0.15. However, the combination of low-impedance flow and intrauterine material is common after abortion and does not necessarily imply retained products of conception. It may simply reflect physiological involution; the temporal course of return to non-pregnant flow characteristics in the uterine vessels has not been documented52 (Fig. 15-20).
Trophoblastic Disease
Transvaginal ultrasound will demonstrate uterine abnormalities in persistent gestational trophoblastic tumour. However, the sensitivity of ultrasound imaging is only 70%, although abnormal uterine artery waveforms are seen in 90% of cases with persistent gestational trophoblastic tumour and raised uterine artery impedance may predict resistance to chemotherapy.53,54 However, magnetic resonance imaging (MRI) appears to be more sensitive in the detection of trophoblastic tumour, as it is more accurate at identifying myometrial invasion and therefore establishing a diagnosis of choriocarcinoma.
Maternal Haemodynamics
DOPPLER ULTRASOUND OF THE MATERNAL KIDNEY IN PREGNANCY
Doppler blood flow characteristics in the maternal kidney appear to alter little during pregnancy. Although certain authors report a slight reduction in the mean resistance index in renal artery examinations, this is not statistically significant. There is no doubt that volume flow is increased in pregnancy but this appears to be mediated largely by vasodilatation of the large supply vessels, although the absolute changes in flow velocity have not been investigated. There is no change in pulsatility or resistance indices in patients with essential hypertension.55 There has been no documented change in these indices in pregnancy-induced hypertension. However, there is significant increase in acceleration time within the intrarenal vessels. This is suggestive of proximal vasospasm as being at least partly responsible for the pathogenesis of pregnancy-induced hypertension.56,57
There is no change in Doppler indices in patients presenting with progressive physiological dilatation of the collecting system of the kidneys during pregnancy. In the past this has been attributed to a combination of hormonal effects, together with a degree of mechanical obstruction of the ureter by the enlarging uterus. There is no correlation between the degree of dilatation of the collecting system and any change in the resistance index in uncomplicated cases, but it may be clinically useful in cases of suspected obstruction where Doppler ultrasound may show a significant difference between the affected and unaffected kidney. The obstructed kidney will usually show a resistance index differing by 0.04 or more than that in the unobstructed kidney. Resistance indices can be used as a discriminator in deciding which patients with loin pain in association with pregnancy should proceed to an intravenous urogram.58
Conclusions
The role of Doppler in obstetrics is an essential technology. The advent of colour Doppler has enabled more precise examination of the uteroplacental and fetoplacental circulations, together with more intricate examination of the fetal vasculature. However, fetomaternal haemodynamics are complex, showing variation in their response to many maternal and fetal physiological and pathological states. Currently Doppler methods appear capable only of detecting gross changes in placentation and gross changes of fetal well-being. For example, umbilical artery waveforms may be normal in a morphologically normal but small placenta. However, in experimentally induced acute hypoxia there is no alteration in fetomaternal flow as detected by Doppler techniques.59
In the same way, the role of uterine artery Doppler in the management of pregnancy is not clear cut. While apparently of value in patients with pre-existing maternal disease, its findings are not sufficiently sensitive or specific, nor does the use of screening Doppler affect perinatal morbidity and outcome.60 Doppler ultrasound cannot be recommended as a screening procedure for the identification of pregnancy complications in low-risk pregnancies. Doppler examination of the uterine and umbilical arteries between 18 and 26 weeks of gestation in high-risk pregnancies may be predictive of significant pregnancy complications, but at present there is insufficient evidence to suggest that the level of surveillance in the Doppler-negative group can be relaxed.
Doppler has most potential in the management of high-risk pregnancies such as those with pre-existing maternal disease, previously identified placental disease and those at high risk of fetal IUGR, or where IUGR is already established. Umbilical artery studies help identify fetuses at risk of hypoxia and acidaemia; when the results are applied clinically they contribute to reduced perinatal mortality rates. Doppler ultrasound of the fetal circulation may identify chronic hypoxia and there is evidence that documentation of redistribution of flow is important in the identification of fetal compromise (Tables 15-2 and 15-3). Doppler ultrasound has significantly impacted on the care of severe fetal anaemia.
REFERENCES
1. Pijnenborg, R., Dixon, G., Robertson, W. B., et al. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980; 1:3–19.
2. Bewley, S., Campbell, S., Cooper, D. Uteroplacental Doppler flow velocity waveforms in the second-trimester. A complex circulation. Br J Obstet Gynaecol. 1989; 96:1040–1046.
3. Schulman, H., Winter, D., Farmakides, G., et al. Doppler examinations of the umbilical and uterine arteries during pregnancy. Clin Obstet Gynecol. 1989; 32:738–745.
4. Bower, S., Vyas, S., Campbell, S., et al. Colour Doppler imaging of the uterine artery in pregnancy: normal ranges of impedance to blood flow, mean velocity and volume flow. Ultrasound Obstet Gynecol. 1992; 2:261–265.
5. Schulman, H., Fleischer, A., Farmakides, G., et al. Development of uterine artery compliance in pregnancy as detected by Doppler ultrasound. Am J Obstet Gynecol. 1986; 155:1031–1036.
6. Fleischer, A., Schulman, H., Farmakides, G., et al. Uterine artery Doppler velocimetry in pregnant women with hypertension. Am J Obstet Gynecol. 1986; 154:806–813.
7. Bower, S., Schuchter, K., Campbell, S. Doppler ultrasound screening as part of routine antenatal screening: prediction of pre-eclampsia and growth retardation. Br J Obstet Gynaecol. 1993; 100:989–994.
8. Voigt, H. J., Becker, V. Doppler flow measurements and histomorphology of the placental bed in uteroplacental insufficiency. J Perinat Med. 1992; 20:139–147.
9. Kofinas, A. D., Espeland, M., Swain, M., et al. Correcting umbilical artery flow velocity waveforms for fetal heart rate is unnecessary. Am J Obstet Gynecol. 1989; 160:704–707.
10. Rightmire, D. A., Campbell, S. Fetal and maternal Doppler blood flow parameters in postterm pregnancies. Obstet Gynecol. 1987; 69:891–894.
11. Bewley, S., Cooper, D., Campbell, S. Doppler investigation of uteroplacental blood flow resistance in the second trimester: a screening study for pre-eclampsia and intrauterine growth retardation. Br J Obstet Gynaecol. 1991; 98:871–879.
12. Qu, L. R., Kan, A., Masahiro, N. Fetal circulation in relation to various maternal body positions. Chung Hua Fu Chan Ko Tsa Chih. 1994; 29:589–591.
13. Mulders, L. G., Jongsma, H. W., Wijn, P. F., et al. The uterine artery blood flow velocity waveform: reproducibility and results in normal pregnancy. Early Hum Dev. 1988; 17:55–70.
14. Morrow, R., Ritchie, K. Doppler ultrasound fetal velocimetry and its role in obstetrics. Clin Perinatol. 1989; 16:771–778.
15. Hastie, S. J., Howie, C. A., Whittle, M. J., et al. Daily variability of umbilical and lateral uterine wall artery blood velocity waveform measurements. Br J Obstet Gynaecol. 1988; 95:571–574.
16. Duggan, P. M., McCowan, L. M., Stewart, A. W. Antihypertensive drug effects on placental flow velocity wave forms in pregnant women with severe hypertension. Aust N Z J Obstet Gynaecol. 1992; 32:335–338.
17. Hirose, S., Yamada, A., Kasugai, T., et al. The effect of nifedipine and dipyridamole on the Doppler blood flow waveforms of umbilical and uterine arteries in hypertensive pregnant women. Asia Oceania J Obstet Gynaecol. 1992; 18:187–193.
18. Castro, L. C., Allen, R., Ogunyemi, D., et al. Cigarette smoking during pregnancy: acute effects on uterine flow velocity waveform. Obstet Gynecol. 1993; 81:551–555.
19. Morrow, R. J., Ritchie, J. W., Bull, S. B. Maternal cigarette smoking: the effects on umbilical and uterine blood flow velocity. Am J Obstet Gynecol. 1998; 159:1069–1071.
20. North, R. A., Ferrier, C., Long, D., et al. Uterine artery flow velocity waveforms in the second trimester for the prediction of pre-eclampsia and fetal growth retardation. Obstet Gynecol. 1994; 83:378–386.
21. NICE Clinical Guideline 62, Antenatal Care. www.nice.org.uk/CG062fullguideline; March 2008.
22. Ferrier, C., North, R. A., Becker, G., et al. Uterine artery waveform as a predictor of pregnancy outcome in women with underlying renal disease. Clin Nephrol. 1994; 42:362–368.
23. Johnstone, F. D., Steel, J. M., Haddad, N. G., et al. Doppler umbilical artery flow velocity waveforms in diabetic pregnancy. Br J Obstet Gynaecol. 1992; 99:135–140.
24. Ben-Ami, M., Battino, S., Geslevich, Y., et al. A random single Doppler study of the umbilical artery in the evaluation of pregnancies complicated by diabetes. Am J Perinatol. 1995; 12:437–438.
25. Kofinas, A. D., Penry, M., Simon, N. V., et al. Interrelationship and clinical significance of increased resistance in the uterine arteries in patients with hypertension or pre-eclampsia or both. Am J Obstet Gynecol. 1992; 166:601–606.
26. Giles, W. B., Trudinger, B. J., Baird, P. J. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol. 1985; 92:31–38.
27. Mires, G., Dempster, J., Patel, N. B., et al. The effect of fetal heart rate on umbilical artery flow velocity waveforms. Br J Obstet Gynaecol. 1987; 94:665–669.
28. Gill, R. W., Trudinger, B. J., Garrett, W. J., et al. Fetal umbilical venous flow measured in utero by pulsed Doppler and B-mode ultrasound. Am J Obstet Gynecol. 1980; 139:720–725.
29. Kofinas, A. D., Penry, M., Swain, M., et al. The effect of placental laterality on uterine artery resistance and development of pre-eclampsia and intrauterine growth retardation. Am J Obstet Gynecol. 1989; 161:1536–1539.
30. Erskine, R. L. A., Ritchie, J. W. K. Umbilical artery blood flow characteristics in normal and growth-retarded fetuses. Br J Obstet Gynaecol. 1985; 92:605–610.
31. McParland, P. Modern approach to the poorly grown fetus. Ir Med J. 1992; 85:88–89.
32. Trudinger, B. J., Cook, C. M., Giles, W. B., et al. Fetal umbilical artery velocity waveforms and subsequent neonatal outcome. Br J Obstet Gynaecol. 1991; 98:378–384.
33. Neilson, J. P., Doppler ultrasound in high-risk pregnanciesEnkin M. W., Keirse M. J. N. C., Renfrew M. J., et al, eds. Pregnancy and childbirth module. Cochrane Database of Systematic Reviews, No. 03889. BMJ Publishing, London, 1993.
34. Poulain, P., Palaric, J. C., Paris-Liado, J., et al. Fetal umbilical Doppler in a population of 541 high-risk pregnancies: prediction of perinatal mortality and morbidity. Doppler Study Group. Eur J Obstet Gynecol Reprod Biol. 1994; 54:191–196.
35. Cruz-Martinez, R., Figueras, F., Oros, D., et al. Cerebral blood perfusion and neurobehavioral performance in full term small-for-gestational-age. Am J Obstet Gynaecol. 2009; 201:474e1–474e7.
36. Zimmermann, P., Alback, T., Koskinen, J., et al. Doppler flow velocimetry of the umbilical artery, uteroplacental arteries and fetal middle cerebral artery in prolonged pregnancy. Ultrasound Obstet Gynecol. 1995; 5:189–197.
37. Faber, R., Viehweg, B., Burkhardt, U. Predictive value of Doppler ultrasound findings in twin pregnancies. Zentralbl Gynäkol. 1995; 117:353–357.
38. Giles, W. B., Trudinger, B. J., Cook, C. M., et al. Umbilical artery flow velocity waveforms and twin pregnancy outcome. Obstet Gynecol. 1988; 72:894–897.
39. Vyas, S., Nicolaides, K. H., Campbell, S. Doppler examination of the middle cerebral artery in anaemic fetuses. Am J Obstet Gynecol. 1990; 162:1066–1068.
40. Mari, G. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. N Engl J Med. 2000; 342:9–14.
41. Rochelson, B., Kaplan, C., Guzman, E., et al. A quantitative analysis of placental vasculature in the third-trimester fetus with autosomal trisomy. Obstet Gynecol. 1990; 75:59–63.
42. Vyas, S., Nicolaides, K. H., Bower, S., et al. Middle cerebral artery flow velocity waveforms in fetal hypoxaemia. Br J Obstet Gynaecol. 1990; 97:797–803.
43. Rowlands, D. J., Vyas, S. K., Longitudinal study of fetal middle cerebral artery flow velocity waveforms preceding fetal death [Comment in Br J Obstet Gynaecol 103(8):852]. Br J Obstet Gynaecol 1995; 102:888–890.
44. Society for Maternal-Fetal Medicine Publications Committee, Berkley, E., Chauhan, S. P., Abuhamad, A. Doppler assessment of the fetus with intrauterine growth restriction. Am J Obstet Gynecol. 2012; 206:300–308.
45. Hackett, G. A., Campbell, S., Gamsu, H., et al. Doppler studies in the growth-retarded fetus and prediction of neonatal necrotising enterocolitis, haemorrhage, and neonatal morbidity. Br Med J. 1987; 294:13–16.
46. Laurin, J., Marsal, K., Persson, P. H., et al. Ultrasound measurements of fetal blood flow in predicting fetal outcome. Br J Obstet Gynaecol. 1987; 94:940–948.
47. The GRIT Trial Study Group. Infant wellbeing at 2 years of age in Growth Restriction Intervention Trial (GRIT): multicentred randomized controlled trail. Lancet. 2004; 364:513–520.
48. Gudmunsson, S., Tulzer, G., Huhta, J. C., et al. Venous Doppler velocimetry in fetuses with absent end-diastolic blood velocity in the umbilical artery. J Matern Fetal Invest. 1993; 3:196.
49. Baschat, A. A., Gembruch, U. Triphasic umbilical venous blood flow with prolonged intrauterine survival in intrauterine growth retardation. Ultrasound Obstet Gynecol. 1996; 8:201–205.
50. Ott, W. Placenta praevia. Ultrasound Obstet Gynecol. 1993; 139:1493–1494.
51. Heinonen, S., Ryynanen, M., Kirkinen, P., et al. Perinatal diagnostic evaluation of velamentous umbilical cord insertion: clinical, Doppler, and ultrasonic findings. Obstet Gynecol. 1996; 87:112–117.
52. Dillon, E. H., Case, C. Q., Ramos, I. M., et al. Endovaginal ultrasound and Doppler findings after first-trimester abortion. Radiology. 1993; 186:87–91.
53. Dobkin, G. R., Berkowitz, R. S., Goldstein, D. P., et al. Duplex ultrasonography for persistent gestational trophoblastic tumor. J Reprod Med. 1991; 36:14–16.
54. Long, M. G., Boultbee, J. E., Langley, R., et al. Doppler assessment of the uterine circulation and the clinical behaviour of gestational trophoblastic tumours requiring chemotherapy. Br J Cancer. 1992; 66:883–887.
55. Zeeman, G. G., McIntire, D. D., Twickler, D. M. Maternal and fetal artery Doppler findings in women with chronic hypertension who subsequently developed superimposed pre-eclampsia. J Matern Fetal Neonatal Med. 2003; 14(5):318–323.
56. Myake, H., Nakai, A., Kshino, T., et al. Doppler velocimetry of maternal renal circulation in pregnancy induced hypertension. J Clin Ultrasound. 2001; 29(8):449–455.
57. Nakai, A., Asakura, H., Oya, A., et al. Pulsed Doppler US findings of renal interlobar arteries in pregnancy induced hypertension. Radiology. 1999; 213(2):423–428.
58. Weston, M. J., Dubbins, P. A. The diagnosis of obstruction: colour Doppler ultrasonography of renal blood flow and ureteric jets. Curr Opin Urol. 1994; 4:69–74.
59. Trudinger, B. J., Giles, W. B., Cook, C. M. Flow velocity waveforms in the maternal uteroplacental and fetal umbilical placental circulations. Am J Obstet Gynecol. 1985; 152:155–163.
60. Newnham, J. P., O’Dea, M. R., Reid, K. P., et al. Doppler flow velocity waveform analysis in high-risk pregnancies: a randomised control trial. Br J Obstet Gynaecol. 1991; 98:956–963.