Clinical Applications for Platelet Rich Plasma Therapy
Eric S. Honbo and Luga Podesta
Over the past several years, there has been significant interest in the use of biologic treatment of muscle, tendon, ligament, and bone injuries in orthopedic and sports medicine. The use of orthobiologic tissue grafts, such as platelet rich plasma (PRP) to stimulate and promote tissue healing and regeneration, has received increasing notoriety since first being reported in the February 2009 article “A Promising Treatment for Athletes, in Blood” in the New York Times. This article increased the public’s awareness of PRP to treat the NFL’s Pittsburgh Steelers football player Hines Ward before the 2009 Super Bowl.
The use of PRP to promote healing has been studied since the 1970s in both the veterinary and human literature. Ferrari and associates first reported using PRP in 1987 during cardiac surgery as an autologous transfusion component after open heart surgery to avoid homologous blood product transfusion.1 PRP has successfully been used in various specialties, such as maxillofacial surgery, cosmetic surgery, orthopedics, and podiatry, and for general wound healing.2–9 In humans, the higher concentrations of autologous growth factors and the secretory proteins found in PRP preparations are attributed to its ability to promote tissue healing and regeneration when applied to a variety of tissue.
Definition of PRP
Platelets are small, nonnucleated cell fragments in the peripheral blood known primarily for their role in homeostasis. The normal platelet count ranges from 150,000 µL to 400,000 µL. Platelets contain numerous proteins (growth factors), cytokines, and bioactive factors that initiate and regulate tissue healing.10 The fluid portion of blood—plasma—also contains clotting factors, proteins, and ions. PRP is the result of concentrating the platelet count to at least 1 million platelets per microliter in 5 mL of plasma.10,11
Platelet Function in Tissue Healing
Platelets contain two unique types of granules—alpha granules and dense granules. Alpha granules in platelets function as storage units containing a variety of hemostatic proteins, inactive growth factors, cytokines, and other proteins such as adhesion proteins. Dense granules store and release bioactive factors that promote platelet aggregation, tissue modulation, and regeneration including adenosine diphosphate (ADP), adenosine triphosphate (ATP), calcium, serotonin, histamine, and dopamine.12,13
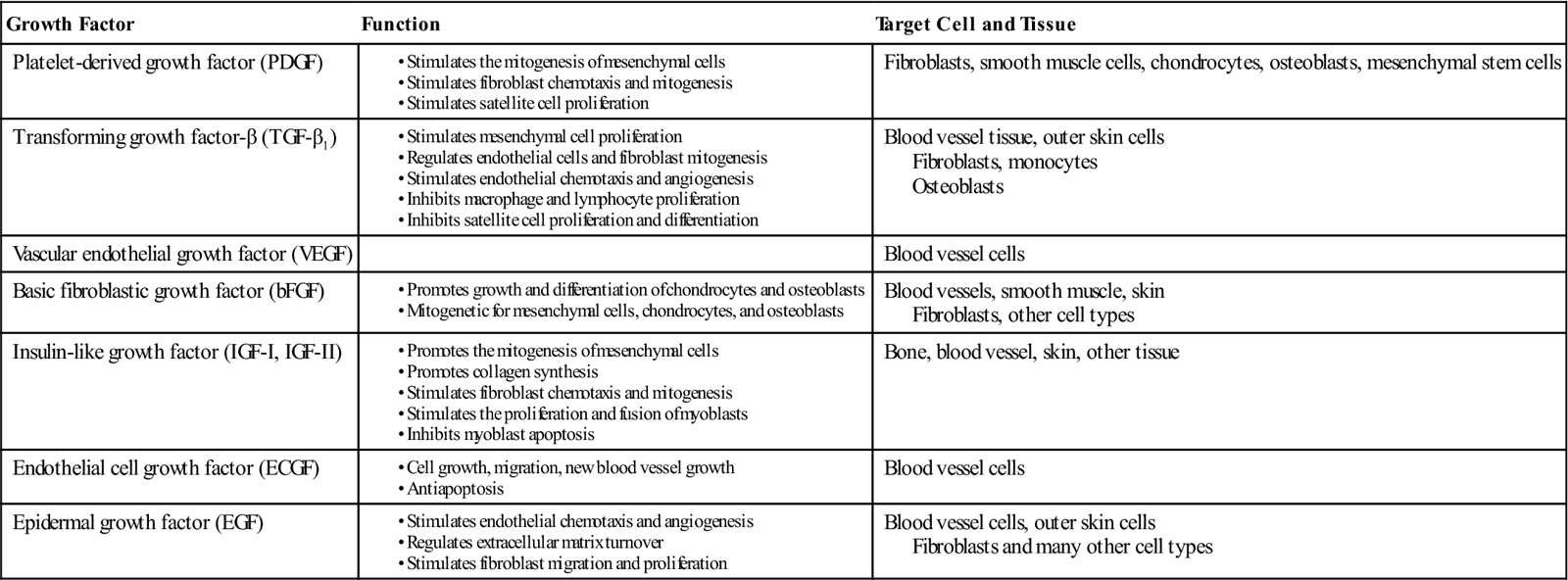
Growth factors found in these granules include platelet derived growth factor (PDGF), transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), basic fibroblastic growth factor (bFGF), insulin-like growth factor (IGF-I, IGF-II), endothelial cell growth factor (ECGF), and epidermal growth factor (EGF).4,6,10,14–16 Platelet activation is required for discharge of granule content (B5) (Table 10-1). Upon clotting, platelets are activated, resulting in degranulation and release of their growth factors from the alpha granules. Approximately 70% of the stored growth factors are released within the first 10 minutes. The majority of growth factor release occurs within the first hour after degranulation. Continued growth factor release has been shown to occur throughout the period of platelet viability, approximately 7 days.4,8,10
TABLE 10-1
Growth Factors in Platelet Rich Plasma
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
PRP is a mechanism to deliver a physiologically natural balance/ratio of growth factors, cytokines, and other bioactive proteins in supraphysiologic concentrations directly into an injured tissue to potentially optimize healing while maintaining the body’s homeostatic environment.4,17–19 Using PRP to treat a variety of soft tissue pathologies is appealing to the clinician because of its simplicity of acquisition and administration, relatively low cost when compared with surgical treatments, and absence of significant adverse effects. Since PRP is an autologous tissue graft, the risk of tissue rejection, immune response, or disease transmission is eliminated.
Tissue Healing
The healing process is defined as a complex and dynamic biologic progression that results in the restoration of anatomic structure and function. Tissue healing is a process characterized by a predictable cascade of biologic tissue response triggered by the injury itself. Physiologic healing progresses through three overlapping stages: stage 1, the acute inflammatory phase; stage 2, the proliferative or repair phase; and stage 3, the remodeling phase.
The inflammatory phase, stage 1 begins with a tissue injury. Platelets are stimulated to provide hemostasis by forming a clot. Platelets in the clot then degranulate and secrete several growth factors, hemostatic factors, and cytokines from alpha granules that are necessary in the early stages of the clotting cascade. Histamine and serotonin are released from the dense granules and function to increase capillary permeability, activate macrophages, and allow inflammatory cells greater access to the injury site.10,20,21 The inflammatory phase can last up to 72 hours and is characterized by localized pain, swelling, erythema, and increased local tissue temperature. The proliferative phase (stage 2) begins when polymorphonuclear leukocytes migrate to the inflamed tissue. During the ensuing 48 hours to 6 weeks, anatomic structures begin to be restored while tissue generation occurs. Fibroblasts begin to synthesize scar tissue and capillary neoformation begins to reestablish nutrients to the injured tissue. Stage 2 ends with the beginning of wound contracture. Stage 3 is characterized by collagen remodeling. The process of tissue remodeling can last from 3 weeks to 12 months.14
Formation of PRP
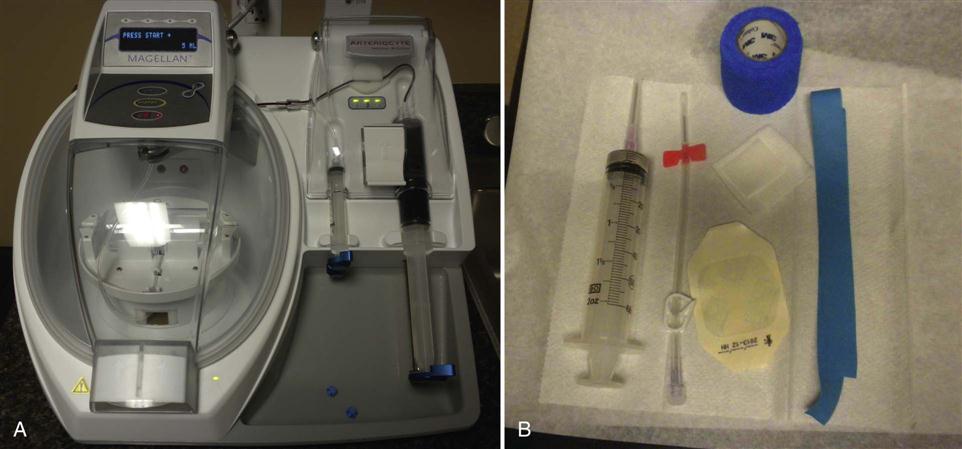
PRP can only be derived from anticoagulated whole blood. Since platelets form part of the clot in coagulated blood and are activated triggering degranulation—thereby releasing their bioactive proteins, growth factors, and cytokines—clotted blood is not an appropriate source of blood to obtain PRP. PRP preparation begins by adding citrate to whole blood. Citrate binds to ionized calcium inhibiting the clotting cascade. The anticoagulated blood then undergoes a centrifugation process to first separate red and white blood cells from plasma and platelets, and then a second centrifugation cycle further separating the platelet rich from the platelet poor plasma. There are a number of commercially available devices on the market that produce PRP (Fig. 10-1). Current systems available to generate PRP differ in the amount of whole blood needed for processing, the anticoagulant used, the speed of the centrifuge, and the time necessary to spin the blood. Systems also differ in the final volume of PRP produced and the total number of platelets present in the concentrated product. As defined by the American Red Cross, PRP has 5.5 × 1010 platelets or greater per 50 mL of concentrate equaling a 2 to 7 times increase compared with whole blood. Normal platelet counts can vary between individuals. Platelet concentrations can vary greatly ranging from 2.5 to 8.0 times the concentration found in whole blood depending on the commercial system used.4,22 Literature suggests that clinical benefits of platelet concentrations occur with the greatest predictability when a fourfold increase is achieved.4,23 Unfortunately, evidence is lacking with regard to the appropriate and most clinically beneficial concentration of platelets.
Leukocyte concentration in PRP has become a topic of debate. Leukocyte concentrations can vary depending on the PRP system used. There is concern that the release of acid hydrolases and proinflammatory proteases from leukocytes may act as cytotoxic agents causing secondary damage to cells.14,24
Procedural Technique for PRP Delivery
Treatment begins with the identification of the tissue (muscle, tendon, or ligament) and anatomic structures to be treated—after proper informed consent has been obtained and the procedure has been explained to the patient. Before treatment, pain medication is prescribed for the immediate postinjection period (3 to 5 days) and we instruct patients that nonsteroidal antiinflammatory medication cannot be used 2 to 3 weeks before and 6 to 8 weeks after the PRP treatment has been completed. Postinjection pain is common after PRP treatment. The duration and severity varies from patient to patient and with the specific tissue being treated. Before treatment, pre-PRP and post-PRP treatment instructions are discussed with patients and all questions are answered. The patient is advised that it is normal to experience an increase in pain at the injection site after the PRP treatment, which may last for several days (Box 10-1).
The treatment area is cleaned with an alcohol/Betadine prep solution or Hibeclens solution before injection. Blood is drawn using a large bore fenestrated needle. The amount of blood drawn is dependent on the amount of PRP required for treatment. On average, 60 mL whole blood (to obtain 5 mL PRP) is drawn from the patient. The whole blood is placed into the centrifuge and the separation process is begun requiring approximately 15 minutes. The treatment site is then anesthetized, first with an ethyl chloride spray followed by a local anesthetic injection of lidocaine.
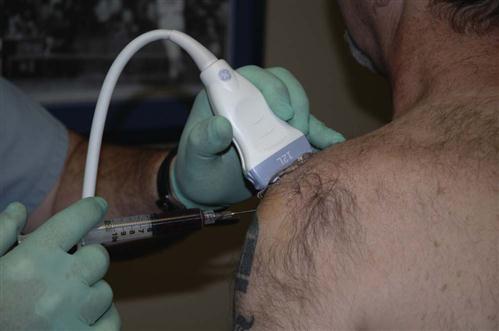
When the separation process has been completed, the PRP concentrate is then delivered into the injured tissue through a 22-gauge needle under direct musculoskeletal ultrasound (US) guidance (Fig. 10-2). The precise placement of the PRP preparation is extremely important for overall outcome and efficacy of the procedure. The exact technique of delivery is dependent on the location of the tissue treated myotendinous, teno-osseous, or ligamentous. A “peppering” technique is used when treating tendons and ligaments. It is also important to touch bone at the osseous interface. Layering the PRP graft throughout the entire injury site in muscle, tendon, and ligamentous injuries will also help ensure complete coverage with the PRP preparation.
When treatment is completed, a sterile Band-Aid is applied. Protective splinting or bracing may be recommended after treating large weight-bearing tendons, such as the Achilles tendon, or areas where an extensive percutaneous tenotomy “peppering” has been performed. Application of heat after the procedure for 15 minutes every 2 to 3 hours is often recommended for postinjection pain management.
Strenuous activity for the first 7 days posttreatment is discouraged. Establishing normal range of motion after the procedure and performing activities of daily living (ADL) are encouraged as soon as possible posttreatment. At 4 to 6 weeks, the patient is reassessed. If pain persists at the treatment site a second PRP treatment might be considered at that time.
Risks and Contraindication for PRP Treatment
PRP therapy is a safe and potentially very effective treatment modality for a variety of musculoskeletal soft tissue pathologies. Unfortunately, there is a paucity of randomized, placebo-controlled studies regarding treatment with PRP and its possible adverse effects. Although inherent risk is minimal, the same risks are present as with any percutaneous needle technique, including infection or puncturing a hollow organ. When treatment is conducted with standard sterile technique, the risk of transmitting an infection or developing an allergic reaction after treatment with these autologous tissue preparations is effectively eliminated. The most common complaint from patients after PRP treatments is localized pain from the PRP injection itself.
There are a number of conditions in which treatment with PRP is contraindicated. Absolute contraindications for the use of PRP include: platelet dysfunction syndromes, critical thrombocytopenia, hemodynamic instability, septicemia, and hypofibrinogenemia. PRP treatments are relatively contraindicated in those patients that consistently used antiinflammatory medications and systemic corticosteroid medications. It is also contraindicated in those who have received a corticosteroid injection at the treatment site within 14 days before treatment, have HGB levels less than 10 g/dL or platelet counts less than 105/µL, or have had recent fevers or illness, a rash at the donor or receptor site, bone cancer or hematopoietic cancer, or a history of, or an active infection with, Enterococcus, Pseudomonas, or Klebsiella.12,25
Clinical Application of PRP
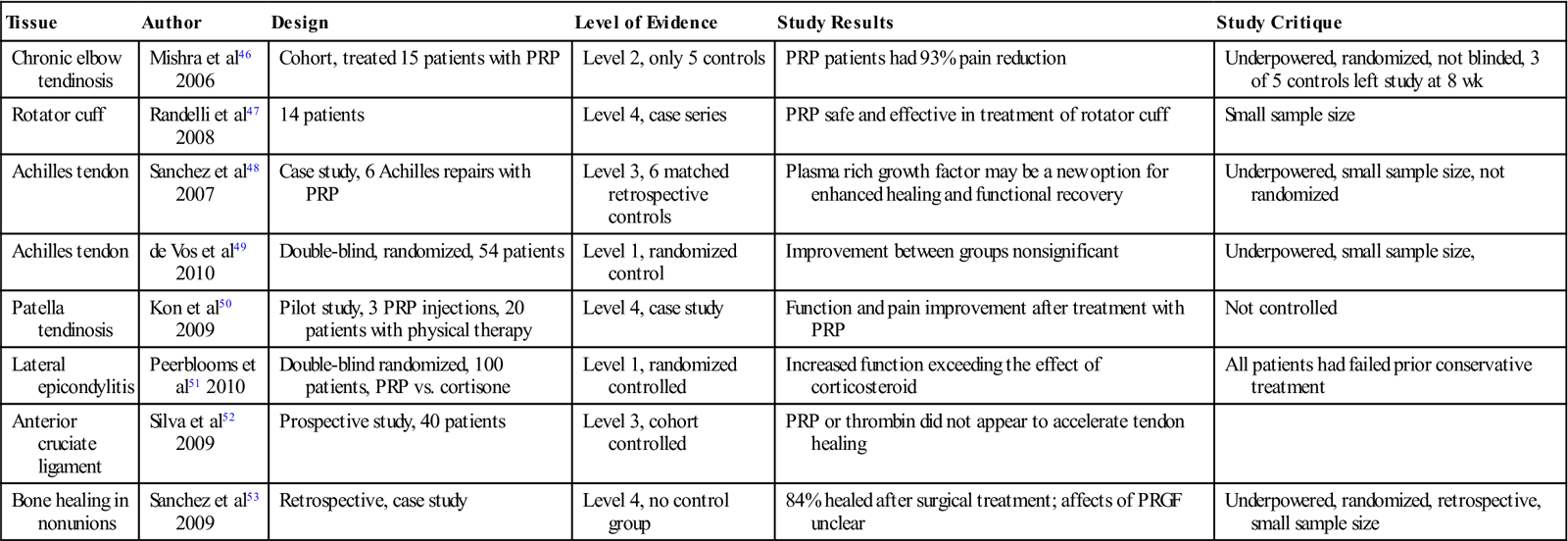
Despite the surge of interest in orthobiologic treatment modalities—and there recent widespread use for the treatment of a wide variety of soft tissue and boney injuries involving muscle, tendon, ligament, and articular cartilage—there remains a lack of animal and clinical studies demonstrating the efficacy of PRP. Although many studies report excellent outcomes, many of these studies unfortunately are limited case reports at best. Many of the current published studies are difficult to interpret because of the lack of standardization of PRP dosing, platelet acquisition and preparation, platelet concentration, growth factor quantity, number of treatments given, small patient sample sizes, and lack of control groups. Table 10-2 summarizes some of the recent published clinical studies regarding PRP.
TABLE 10-2
Human Clinical Treatment Trials Using Platelet Rich Plasma4,10
< ?comst?>
| Tissue | Author | Design | Level of Evidence | Study Results | Study Critique |
| Chronic elbow tendinosis | Mishra et al46 2006 | Cohort, treated 15 patients with PRP | Level 2, only 5 controls | PRP patients had 93% pain reduction | Underpowered, randomized, not blinded, 3 of 5 controls left study at 8 wk |
| Rotator cuff | Randelli et al47 2008 | 14 patients | Level 4, case series | PRP safe and effective in treatment of rotator cuff | Small sample size |
| Achilles tendon | Sanchez et al48 2007 | Case study, 6 Achilles repairs with PRP | Level 3, 6 matched retrospective controls | Plasma rich growth factor may be a new option for enhanced healing and functional recovery | Underpowered, small sample size, not randomized |
| Achilles tendon | de Vos et al49 2010 | Double-blind, randomized, 54 patients | Level 1, randomized control | Improvement between groups nonsignificant | Underpowered, small sample size, |
| Patella tendinosis | Kon et al50 2009 | Pilot study, 3 PRP injections, 20 patients with physical therapy | Level 4, case study | Function and pain improvement after treatment with PRP | Not controlled |
| Lateral epicondylitis | Peerblooms et al51 2010 | Double-blind randomized, 100 patients, PRP vs. cortisone | Level 1, randomized controlled | Increased function exceeding the effect of corticosteroid | All patients had failed prior conservative treatment |
| Anterior cruciate ligament | Silva et al52 2009 | Prospective study, 40 patients | Level 3, cohort controlled | PRP or thrombin did not appear to accelerate tendon healing | |
| Bone healing in nonunions | Sanchez et al53 2009 | Retrospective, case study | Level 4, no control group | 84% healed after surgical treatment; affects of PRGF unclear | Underpowered, randomized, retrospective, small sample size |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Regulation of PRP in Sports Medicine
The use of PRP in amateur and professional athletes remains controversial. In the United States, the use of PRP in professional sports, including the NFL, Major League Baseball, National Basketball Association, National Hockey League, Major League Soccer, National League Lacrosse, and Major League Lacrosse, is not regulated or prohibited. In addition, the National Collegiate Athletic Association currently does not regulate or prohibit the use of PRP in its participating institutions. Initially, the World Anti-Doping Agency (WADA) prohibited the use of platelet-derived preparations (PRP blood spinning) administered through an intramuscular route. Both the WADA and the U.S. Anti-doping Agency originally prohibited the injections of any growth factors affecting muscle, tendon, or ligament protein synthesis or degradation, vascularization, regenerative capacity, fiber type switching, or energy use.14 Athletes required a therapeutic use exemption if this mode of treatment is deemed necessary and recommended by a physician. Both organizations recently have changed their stance on PRP, since this is an autologous treatment of the patient’s own blood products that have not been treated with any nonautologous growth factors.
Therapy Guidelines for Rehabilitation
Rehabilitation progression following PRP injection is based on several individual factors: the combination of time since injection, the physiologic healing mechanism, patient’s health and age, severity of injury, tissue integrity, response to physical therapy treatment dosage, and adherence to appropriate home programs. The goal of rehabilitation following PRP injections is to progressively and therapeutically place appropriate amounts of physical stress to the injured tissue to help facilitate healing. General guidelines following physiology are listed in Box 10-2. Physical stress to the tissue (muscle tendon, ligament, and bone) may include tension, torsion, compression, and shear. The stress or loading is imparted via manual therapy techniques, dosed medical exercise therapy progressions, functional strengthening, and return to play phase exercises. There is limited evidence in the literature defining specific protocols following PRP injection and limited documentation regarding tissue healing time frames following PRP injection. There is no absolute progression or transition between phases and there can be variability between patients pending each individual case.
The goal following PRP injection is to promote adequate tissue healing such that the tissue is able to once again maximally withstand the physiologic stresses and forces placed upon it with daily functional demands or sporting activities. Collagen fibers run in parallel alignment, which affords the tissue to withstand tensile forces and unilateral stress placed upon it.26 The following information is based on clinical experience with patients who have undergone PRP injections to different tissues including muscle, tendon, bone, and ligament.
Phase I (Inflammatory Phase) (Table 10-3)
TIME: 0 to 7 days
TABLE 10-3
Platelet Rich Plasma Injection of the UCL (Inflammatory Phase)
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to this Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase I postinjection (0-7 days) |
< ?comen?>< ?comst1?>

< ?comst1?>
< ?comen1?>
GOALS: To allow the PRP to absorb at the injected tissue, to avoid cross-link disruption, and to facilitate integrity of cross link-formation
Phase I consists of early mobilization, gentle self-stretching, and weight-bearing functional activities to prevent the deleterious effects of immobilization and to promote tissue healing. Because of the elevated inflammatory response, the patient commonly feels an increase in pain for the next 1 to 3 days following the injection. Following PRP injection, the majority of the growth factors are released within the first hour of injection but continued release occurs up until about 7 days following injection.24 Thus, the home program for the first 7 days following PRP injection is aimed at avoiding disruption of this physiologic mechanism and includes: rest, gentle active elbow motion, submaximal isometric holds in all pain-free planes and ranges to help fiber alignment, and heat to control symptoms. Functional outcome tools, such as the Kerlan-Jobe orthopedic score, shoulder pain and disability index and patellofemoral index (SCOR), are commonly used to establish the patient’s baseline subjective functional status.
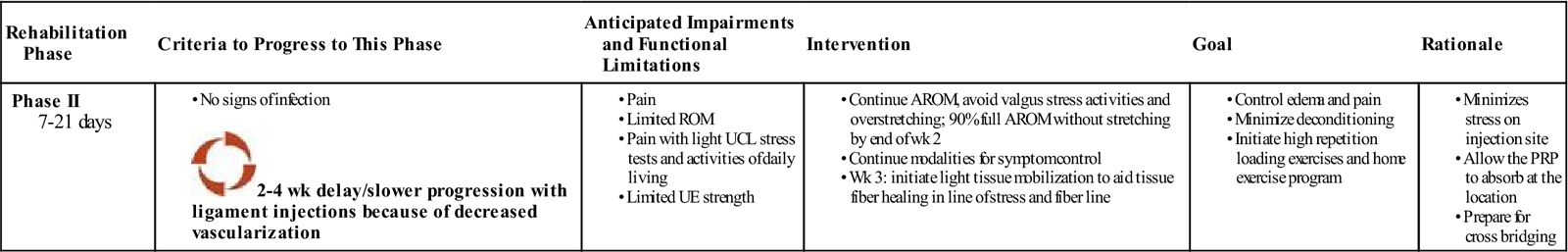
Phase II (Inflammatory Phase) (Table 10-4)
TIME: 7 to 21 days
TABLE 10-4
Platelet Rich Plasma Injection of the UCL (Inflammatory Phase)
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase II 7-21 days |
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
GOALS: To avoid disruption of collagen cross-link bridging and formation, and initiate early motion and high repetition loading exercises. Obtain 90% of full range of motion (ROM).
Phase II consists of continued gentle active elbow motion and increased activity at home. The patient should obtain greater than 90% of full ROM by the end of week 2. Light soft tissue mobilizations should commence to the ulnar collateral ligament (UCL) and common flexor origin and pronator teres at this time. ![]() However, to avoid disruption of collagen cross-link bridging and formation, deeper soft tissue techniques (transverse friction, etc.) are not implemented until the third week following injection. Gentle early motion of the elbow facilitates the physiologic tissue healing response following PRP injection, which proceeds through the inflammatory, reparative, and remodeling phases. Furthermore, early motion and self-stretching prevent joint adhesions, increases muscle contraction, muscle fiber size, and tension, and increases resting levels of glycogen and protein synthesis. Submaximal-maximal effort elbow isometrics performed three times per day are also initiated in an attempt to create light tension in the direction of the tendon fibers. Intermittent rest and care with resuming work and normal daily functional activities are also encouraged at home to help control postinjection symptoms and early inflammatory elevation.
However, to avoid disruption of collagen cross-link bridging and formation, deeper soft tissue techniques (transverse friction, etc.) are not implemented until the third week following injection. Gentle early motion of the elbow facilitates the physiologic tissue healing response following PRP injection, which proceeds through the inflammatory, reparative, and remodeling phases. Furthermore, early motion and self-stretching prevent joint adhesions, increases muscle contraction, muscle fiber size, and tension, and increases resting levels of glycogen and protein synthesis. Submaximal-maximal effort elbow isometrics performed three times per day are also initiated in an attempt to create light tension in the direction of the tendon fibers. Intermittent rest and care with resuming work and normal daily functional activities are also encouraged at home to help control postinjection symptoms and early inflammatory elevation.
Progressive full arc motion in the first two phases prevents ligament atrophy and increases ligament linear tissue stress and stiffness, particularly at the bone-ligament junction. Ligament-stressing exercises or functional activities, as well as excessive muscle or tendon tension, are avoided during this phase. There may be a 2- to 4-week delay with ligament healing because of decreased tissue vascularization. ![]() Exercises that exert tension on the UCL (valgus stress) are not begun until later (phase III).
Exercises that exert tension on the UCL (valgus stress) are not begun until later (phase III).
Early motion restoration aids connective tissue lubrication between collagen cross-links, increases collagen mass, decreases abnormal collagen cross-links, and prevents adhesion development. Following PRP injections, articular cartilage responds to early motion, intermittent compression, and decompression loading with improved metabolic activity and increased health of the cartilage matrix. Muscle, tendon, ligament, and bone tissue all respond favorably to motion. Restoring full elbow ROM is advocated during the first 10 to 14 days following PRP injection.
Modalities used in the first two phases of PRP rehabilitation can include US, laser, and electrical stimulation. The use of modalities during this phase is aimed at further stimulating tissue healing of the UCL and increasing local perfusion and oxygen delivery to the site. Nonthermal US is commonly used to facilitate tissue repair and regeneration in damaged tissue. There is research that supports the use of therapeutic US to increase bony and muscle tissue regeneration.27–29 However, most studies that support the use of nonthermal US and a laser to aid tissue healing are based on animal studies. It is still unclear if using a pulsed nonthermal US is more effective than a low-intensity continuous protocol in terms of proliferation and tissue healing. The use of laser treatment in patients with lateral epicondylosis was found to lower subjective overall pain levels, with reports of 90% to 100% relief in over 45% of the patients who were treated with a laser.30 Additionally, studies have found that the use of a low energy laser improves tensile strength and stiffness in repairing the medial collateral ligament in rats at 3 and 6 weeks after injury.31 When rehabilitating after tendon PRP, we have found positive results using Russian electrical stimulation to help increase endorphin release and minimize tissue response to loading and manual mobilizations using the following parameters: 2500 Hz frequency, 50 pps, 10/10 seconds duty cycle, and 2 seconds ramp time for 10 to 12 minutes.
Progressive loading with shoulder, elbow, and wrist exercises during phases II to IV is a critical component of the post-PRP injection treatment plan. The first two phases include use of a concentric low-load, higher repetition exercise regimen: 3 sets of 20 to 25 repetitions are recommended. Once the patient reaches 3 sets of 25 repetitions, the weight is increased by 1 lb and progresses from there. Proper postural alignment, proximal and distal joint positioning, and control throughout the range, etc., are emphasized. This submaximal intensity using higher repetition progression improves tissue vascularization, helps align collagen cross-links, promotes tissue healing, and enables the tissue to start adapting to controlled amounts of stress. Endurance training versus strengthening has been used in the early phases with success following PRP injections. Submaximal loading exercises reduce homeostasis, tissue breakdown, and symptom exacerbation during the first 2 to 4 weeks. Studies have demonstrated that resistance exercise is more effective in inducing acute muscle anabolism than high-load, low volume or work matched resistance exercise modes (isometrics).32
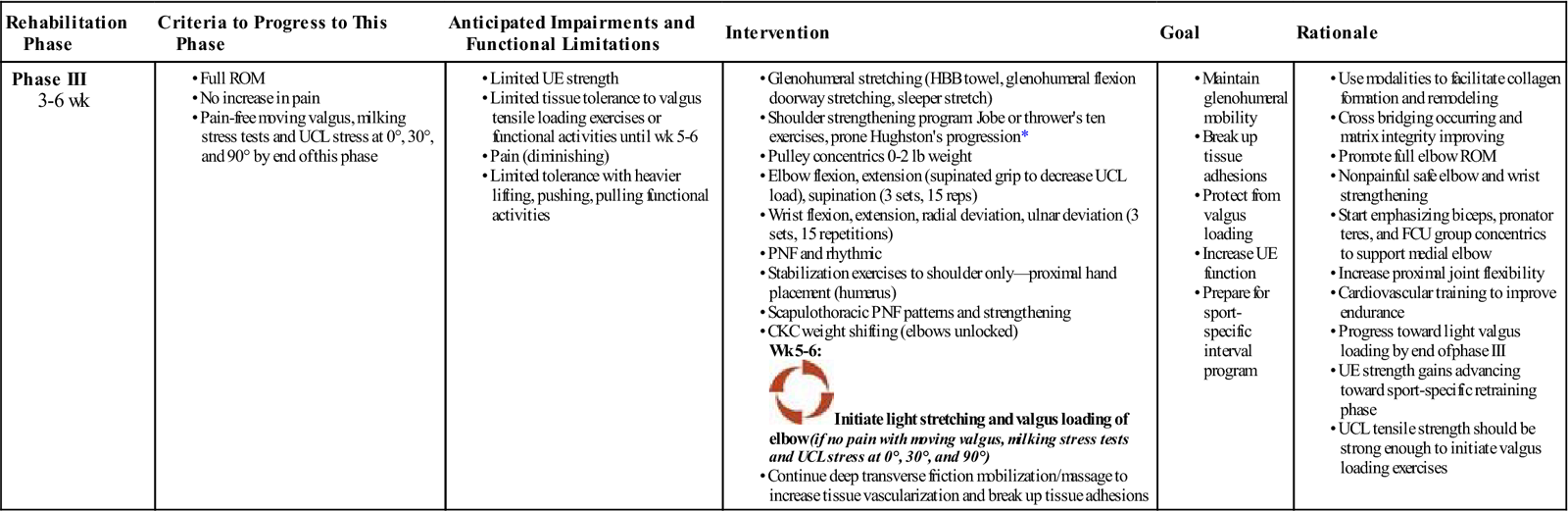
Phase III (Reparative Phase) (Table 10-5)
TIME: 3 to 6 weeks
TABLE 10-5
Platelet Rich Plasma Injection of the UCL (Reparative Phase)
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase III 3-6 wk |
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
< ?comst1?>< ?comen1?>*< ?comst1?>< ?comen1?>http://www.dynoswim.com/archives/ShoulderRotatorExer.pdf.
GOALS: Adjust exercise progression based on type of tissue and severity of injury; use of modalities to aid tissue proliferation (recommend pulsed US, laser, electrical stimulation); begin high repetition loading and concentric; begin functional activities
![]() NOTE: Avoid ligament stress for 4 weeks with ADLs and exercise; progress to eccentric weeks 4 to 6.
NOTE: Avoid ligament stress for 4 weeks with ADLs and exercise; progress to eccentric weeks 4 to 6.
Pain levels have typically lessened by the third week. Collagen synthesis is occurring and aligning in the longitudinal axis. At this point, the tissue is beginning to withstand tensile forces and loads. However, it is important to adjust exercise progression based on type of tissue and the severity of injury (ligament healing and proliferation take longer). Soft tissue mobilizations and progressive loading via resistance exercise are key components of the postinjection reparative and remodeling phases. The primary pathologic mechanism that leads to tendinopathy includes chronic microscopic tearing in hypovascular tendon tissue. These repetitive tears heal by scar formation as opposed to the normal tendon healing pathways of vascularization and inflammation mechanisms.33 Thus during the reparative and remodeling phase of muscle and tendon tissue, the use of soft tissue mobilization techniques (ASTYM, deep transverse friction mobilization, active release, Graston technique) in conjunction with appropriate exercise progressions is an important component of the healing process in order to help minimize scar formation and to promote anatomic tissue fiber healing in line of stress.
Deep transverse tissue massage (DTFM) and friction massage had been employed with positive results. As described by Cyriax,34 DTFM is an aggressive form of soft tissue mobilization in which localized pressure or distractive manipulation of tissues is directed tangentially across the longitudinally oriented collagen component of the injured tissue. To promote normal resolution of the collagen tissue, the tissue to be treated should be in a moderate stretch position (not painful).35 Deep transverse friction mobilizations and other soft tissue manipulation techniques have mechanical, physiologic, histologic, and neurologic effects on the tissue that facilitate the healing mechanism of PRP injections (Box 10-3). Reaction to DTFM may include rapid desensitization, latent posttreatment soreness, and moderate tissue bruising covering the area of tissue contact.34
Eccentric loading is initiated early in the reparative and remodeling phases at approximately weeks 4 to 6, depending on the individual patient’s status. Because of its positive effect on improving tissue integrity, strength, and function, eccentric loading is the other important component of the post-PRP injection rehabilitation. Eccentric contractions function to decelerate a limb, provide shock absorption, and generate forces 14% to 50% greater than a maximal concentric contraction does.36 This increased force generation improves musculotendinous integrity by inducing muscle hypertrophy and increased tensile strength, or by lengthening the musculotendinous unit.37
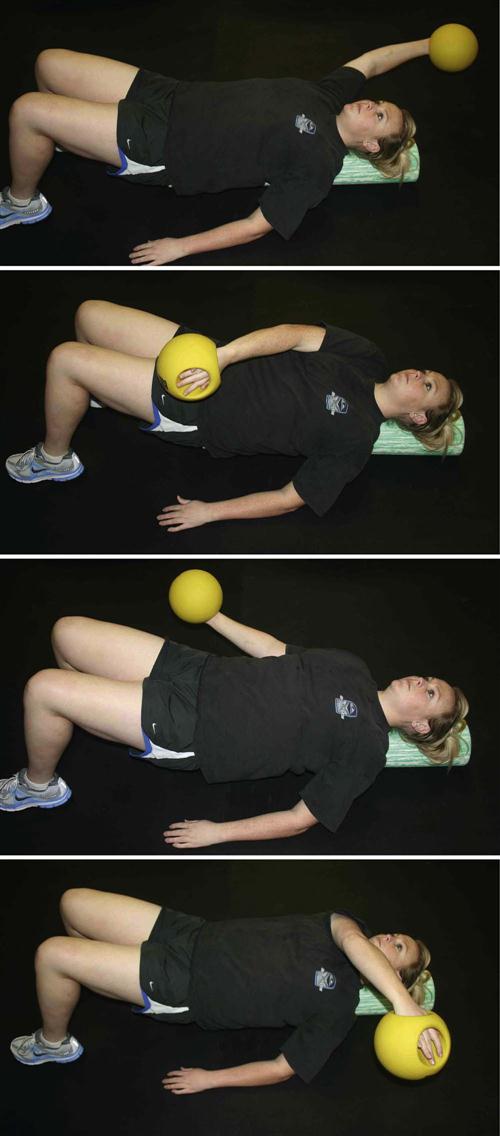
Unlike with concentric phase I to II exercises, eccentric loading has been shown to aid in stable angiogenesis in early tendon injury.38 Daily eccentric loading was found not to have any detrimental effect on tendon vascularity or microcirculation.38 A systematic review of tendinopathy found that eccentric exercises had the most clinical efficacy in regenerating function.39 Other studies have found that eccentric exercise progressions are an effective treatment for chronic tendinosis.40,41 Eccentric tendon loading exercise progressions are thus implemented into postinjection rehabilitation by week 4, depending upon the individual patient’s response through the first 2 to 4 weeks. By the end of week 6, more advanced exercise may begin (Figs. 10-3 through 10-8).
Whereas the prior table provides an overview of exercise progressions for UCL following PRP injection, examples of tendon treatment and exercise progressions are also provided (Boxes 10-4 and 10-5).
There is no clear consensus on the best nonoperative treatment for muscle injuries beyond immediate rest and antiinflammatory medications or modalities.28 In chronic tendinosis injuries, rest has been found to be a less effective treatment.39,42 In turn, eccentric exercise and loading has been shown in many studies to be beneficial in treating patients with tendinosis.37,38 However, the optimal dosage and frequency of eccentric loading for treating chronic tendinosis has not yet been established.43
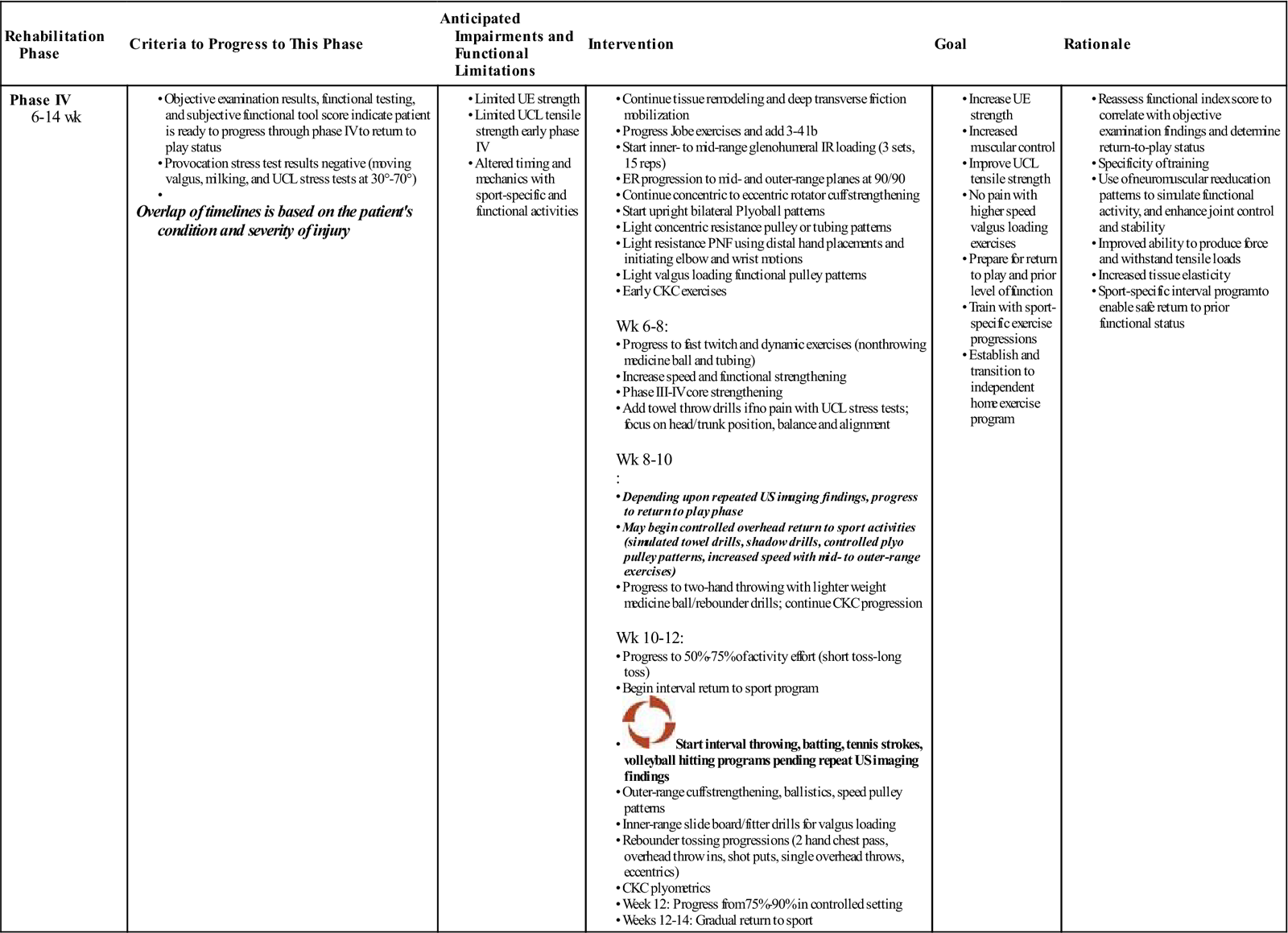
Phase IV (Remodeling Phase) (Table 10-6)
TIME: 6 to 12 weeks
TABLE 10-6
Platelet Rich Plasma Injection of the UCL (Reparative Phase)
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase IV 6-14 wk |
Overlap of timelines is based on the patient’s condition and severity of injury |
Wk 6-8: Wk 8-10 Wk 10-12: |
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
GOALS: Eccentric loading, plyometric training return to sport/activity; (depending upon individual sport and postinjection status [variable from patient to patient]); continue tissue remodeling facilitation with deep transverse friction and soft tissue mobilizations
Note: Diagnostic US (at approximately 8 weeks) may be repeated to determine the extent of healing; resume full functional or sporting activity in 10 to 12 weeks depending on progress with postinjection program.
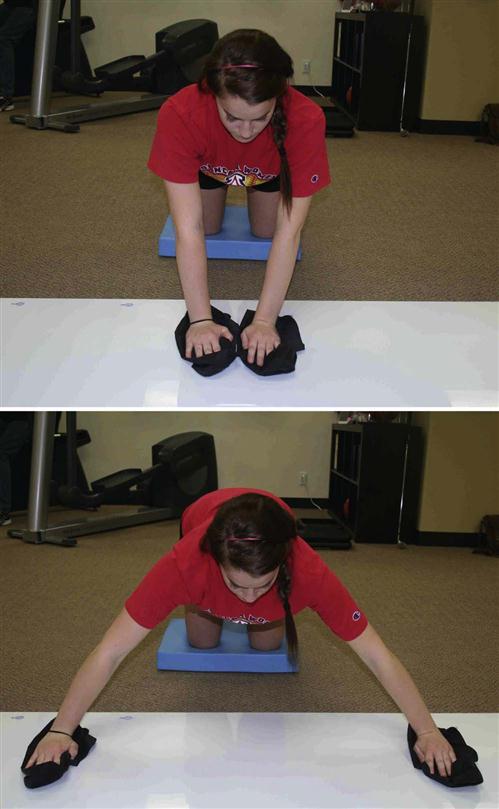
The injected tissue commonly demonstrates increased tensile strength by the remodeling phase. Tissue remodeling facilitation is continued in phase IV with the use of deep transverse friction and soft tissue mobilizations. Depending on the response to the eccentric strengthening progression, the patient progresses to speed and coordination drills, plyometrics, ballistics, and more explosive, sport-specific phase IV exercises (Figs. 10-9 through 10-18).
At this point connective tissue has improved tensile strength because its fiber orientation is better aligned and suited to withstand more demanding tensile stress.44 The functional strengthening, plyometrics, ballistics, neuromuscular power, and coordination exercises are performed at more intense levels to enable the patient to meet the demands of his or her sport or job activity. Typically, selective tissue tension tests (ligament stress tests, resistance muscle-tendon tests in lengthened position, weight-bearing and compression tests for bone) are nonprovocative. The use of follow-up functional tools is recommended to ascertain the patient’s readiness to resume higher level exercises, and return to sport or work. Studies have not yet been published regarding the efficacy of using scores on subjective functional tools or questionnaires to help determine when a patient is ready to safely resume a particular activity or sport given a certain subjective score.
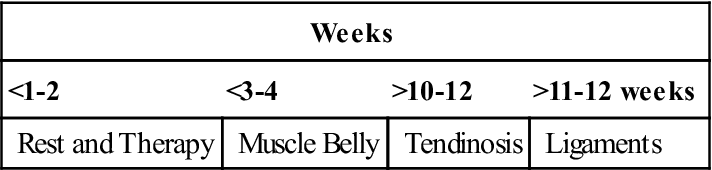
There is no clearly defined or objective means of determining when an athlete is able to safely return to play or a patient is able to return to a functional activity (job duty). A grading system has been used to describe tendinopathy.45 However, the use of a detailed clinical examination together with the repeat US imaging findings, as well as the patient’s subjective assertions and functional index score, are all used to assist the physician, therapist, and athletic trainer in determining when the patient is ready to resume the desired activity. Interval running programs, on field agility progressions, and interval throwing programs are initiated in phase IV. Although there are several notable documented cases in which an athlete has returned to play at an earlier time period following PRP injection, most patients have been able to resume full functional or sporting activity by 10 to 12 weeks (Table 10-7).
TABLE 10-7
Timeline for Return to Activity or Interval Return Sport Phase IV
< ?comst?>
| Weeks | |||
| <1-2 | <3-4 | >10-12 | >11-12 weeks |
| Rest and Therapy | Muscle Belly | Tendinosis | Ligaments |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Ligament healing may be delayed 2-4 weeks; avoid varus/valgus stress for 6 weeks.
Conclusions
The use of orthobiologic modalities such as PRP in orthopedics and sports medicine to deliver high concentrations of naturally occurring biologically active growth factors and proteins to the site of injury is very promising. However, there remain significant clinical and basic science questions that need to be answered regarding the use of PRP in clinical practice. Questions still remain regarding the optimal concentration of PRP, how many injections are optimal, and the timing of treatments in the acute and or chronic settings. Other questions that need to be addressed include what is the optimal physiologic environment for these injections to be performed and how can PRP be optimally used in specific tissue, including muscle, tendon, ligament, or bone. Questions regarding optimal post-PRP treatment rehabilitation also need to be defined. Although PRP is widely being used in clinical practice today, a significant amount of basic science and clinical research remains to be done to define the optimal use and overall safety of PRP therapy in clinical practice.
Clinical Case Review
1Paul is 2 weeks post-PRP injection. He is complaining of continued medial elbow pain. Is phonophoresis/iontophoresis an appropriate choice for treatment?
No. Nonsteroidal antiinflammatory medication cannot be used 2 to 3 weeks before and 6 to 8 weeks after the PRP treatment has been completed.
2Sam is 4 weeks post-PRP injection for a partial UCL tear. He continues to experience medial elbow pain that is limiting his progression through rehabilitation. What must be considered?
At this stage a call into the physician should be made and the potential of a second PRP injection should be considered.
3During the initial phases of rehabilitation, what exercises should be avoided?
Any exercise that places a valgus stress on the elbow (shoulder internal rotation and proprioceptive neuromuscular facilitation [PNF] patterns with distal hand placement should be avoided)
4Doug is 8 weeks postinjection and is making excellent progress. What must be considered before progressing to throwing activities?
Of prime concern is why the UCL was exposed and torn to begin with. Throwing mechanics should be a main concern. Doug should be performing exercises recommended in the Return to Throwing chapter, and videotaped initially and periodically thereafter to evaluate his throwing technique and progression through the throwing program.
5What is the effectiveness of PRP injections?
The success rate for PRP injections has been reported as high as 85% in some cases. The success of the treatment depends on a number of factors, the most important being choosing the appropriate patient. I have noted a high rate of success and publication of my data is pending. However, more studies need to be done to validate the effectiveness before PRP injection can be considered a routine procedure for soft tissue injuries.
6What is the best treatment course for an acute muscle injury?
There is no clear consensus on the best nonoperative treatment for muscle injuries beyond immediate rest and antiinflammatory medications or modalities.
7Is rest an effective tool in managing chronic soft tissue dysfunction (tendinosis)?
In the case of chronic tendinosis injuries, rest has been found to be a less effective treatment. In turn, eccentric exercise and loading has been shown in many studies to be beneficial in treating patients with tendinosis.