Chapter 2 Clinical-Anatomical Syndromes of Ischemic Infarction
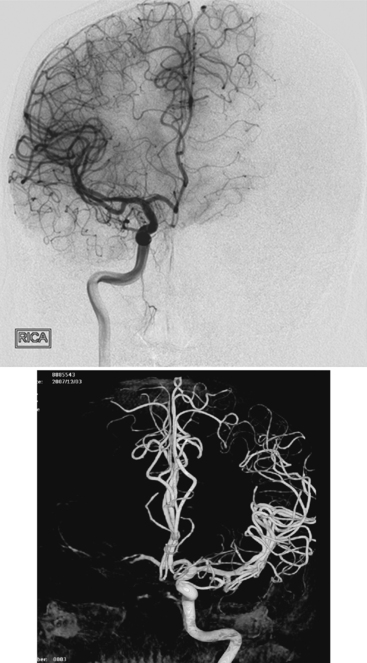
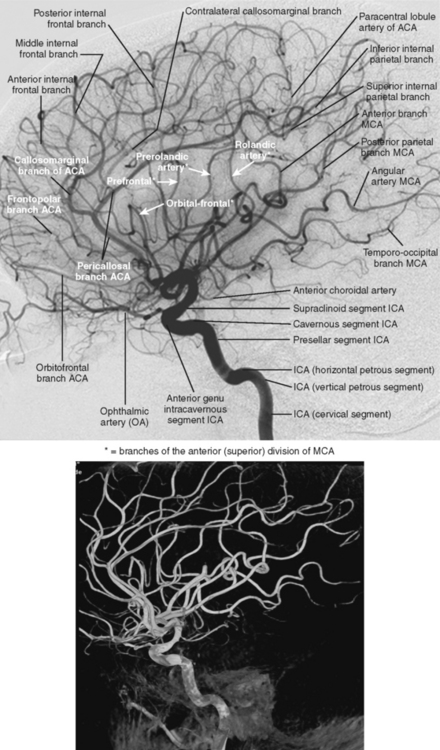
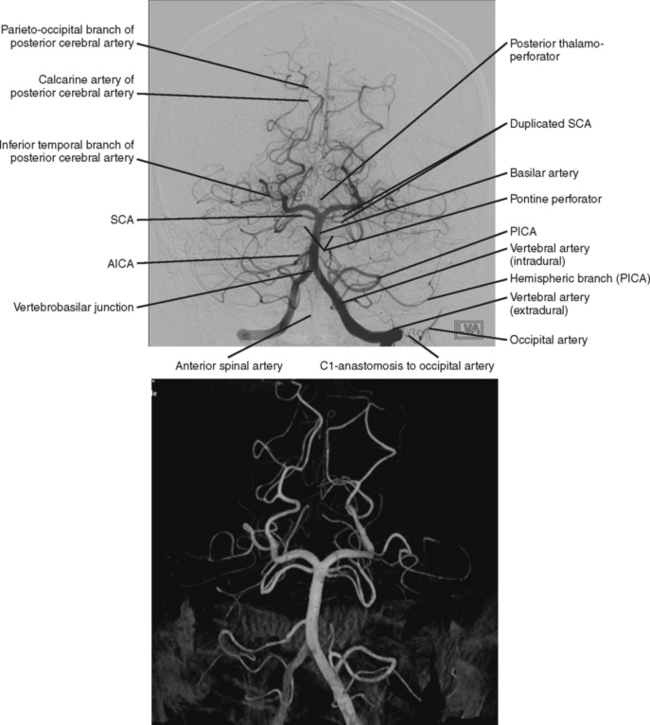
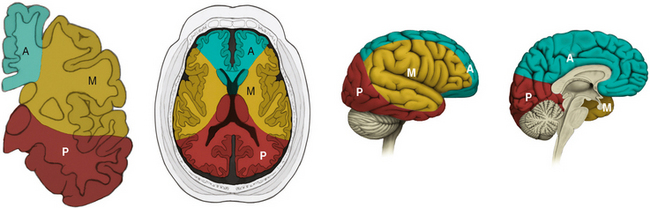
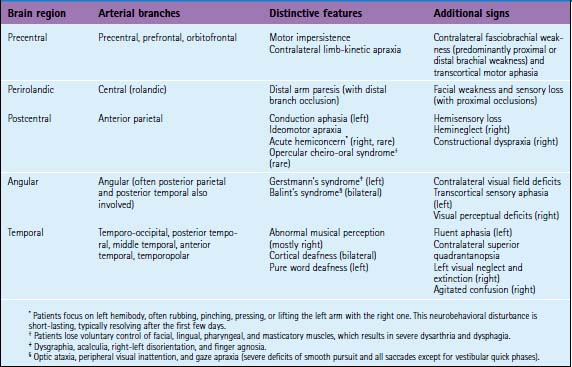
Ischemic stroke can be defined as a sudden focal neurological deficit corresponding to a vascular distribution. Brain imaging techniques allow us to visualize lesions with great anatomical precision. However, optimal interpretation of the information provided by neuroimaging requires having detailed knowledge of the arterial anatomy (Figures 2-1 through 2-4) and the vascular territories of the brain (Figure 2-5).
CAROTID BIFURCATION OCCLUSION
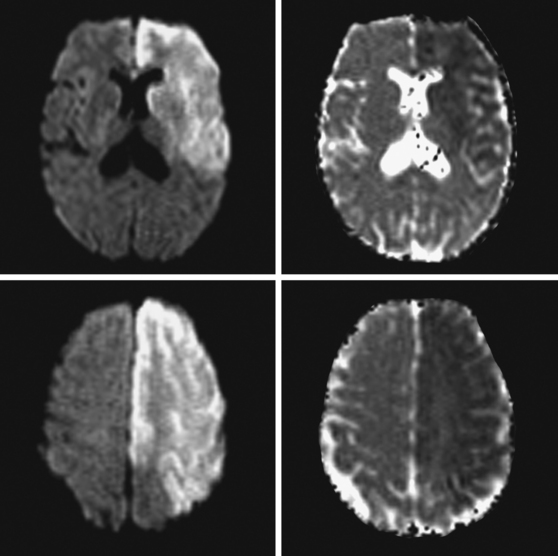
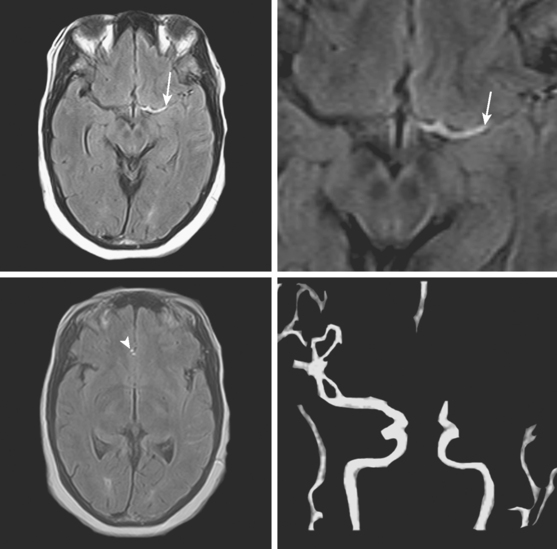
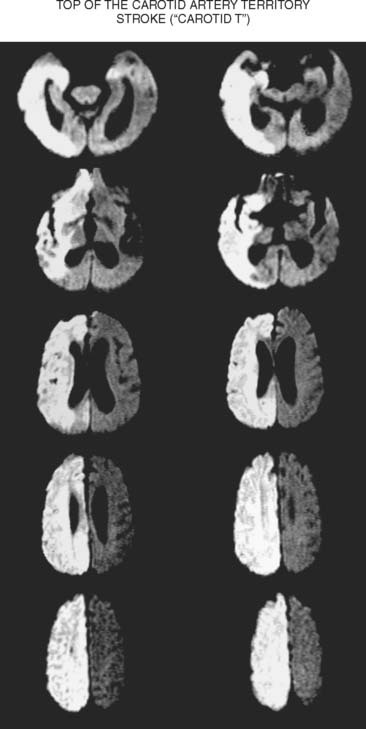
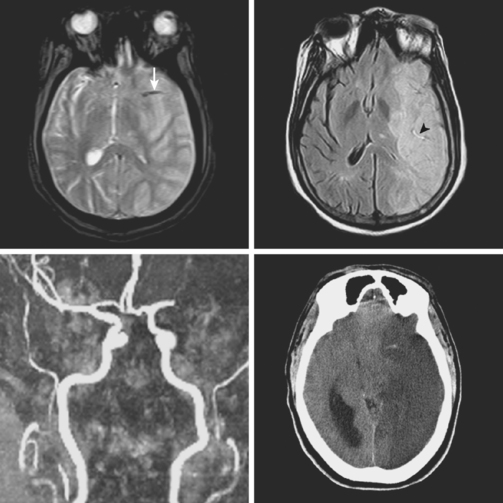
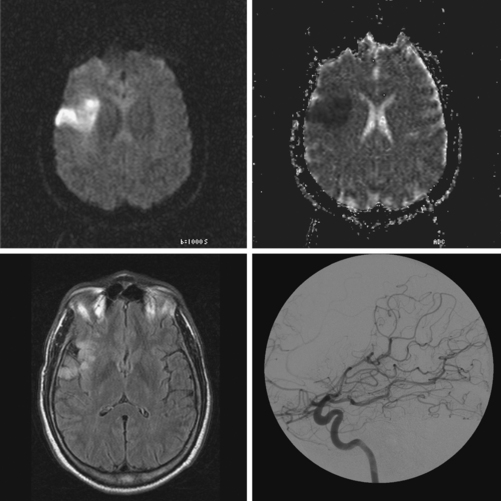
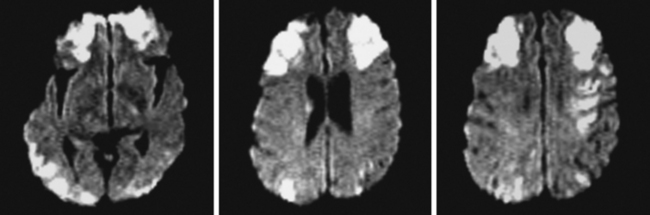
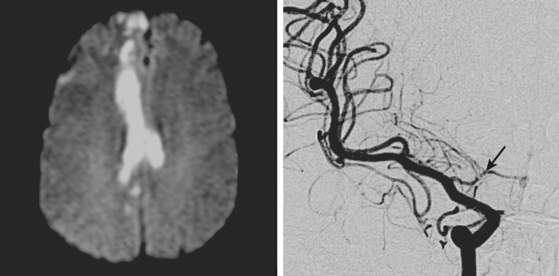
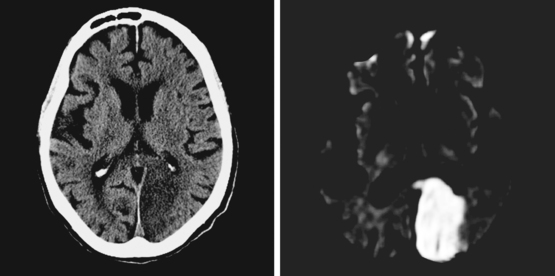
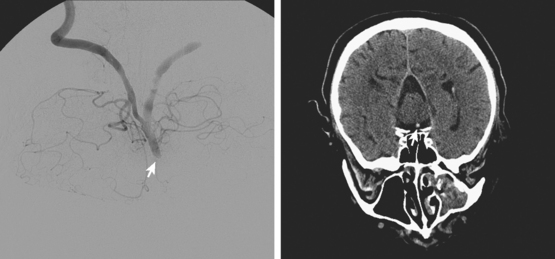
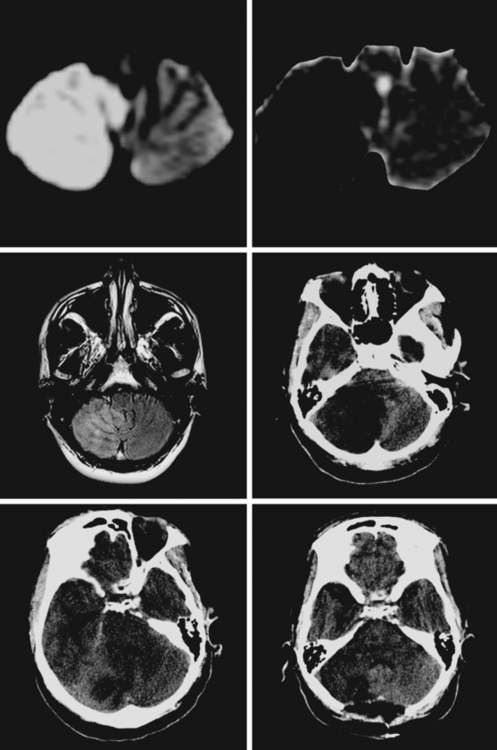
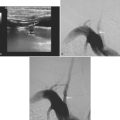
A 61-year-old man with history of coronary artery disease, previous myocardial infarction, and multiple vascular risk factors presented to the emergency department with global aphasia and right hemiplegia for more than 6 hours. On examination, he was drowsy and exhibited forced left gaze deviation, right hemianopia, right flaccid hemiplegia involving the arm and the leg to similar degree, and absent response to pain on the right side. Diffusion-weighted imagery (DWI) of the brain revealed a large area of ischemia in the left hemisphere, including the territories of the anterior and middle cerebral arteries (Figure 2-6). Fluid-attenuated inversion recovery (FLAIR) sequence showed no parenchymal hyperintensity but disclosed extensive hyperintense signal in the left middle cerebral artery consistent with fresh thrombus (Figure 2-7). Magnetic resonance angiography (MRA) of the intracranial circulation confirmed the presence of a left carotid terminus occlusion (Figure 2-7). The patient was subsequently diagnosed with acute myocardial infarction and a left ventricular mural thrombus. His neurological condition deteriorated over the following 48 hours, and he expired after care was restricted to palliative measures.
MIDDLE CEREBRAL ARTERY OCCLUSION
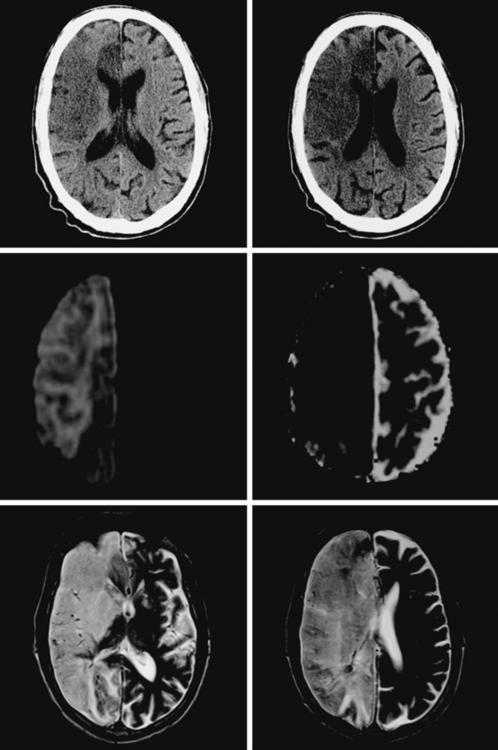
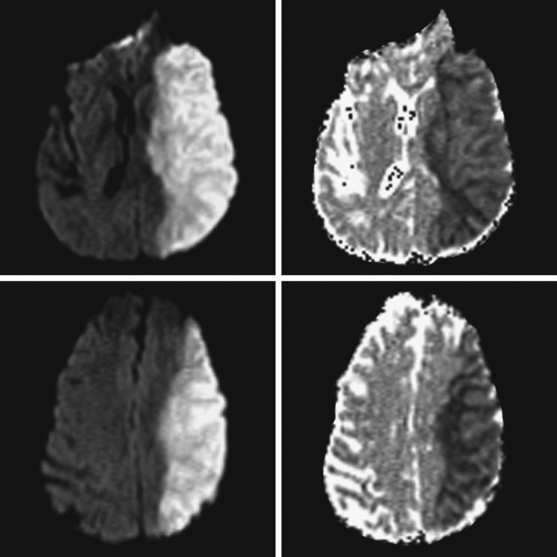
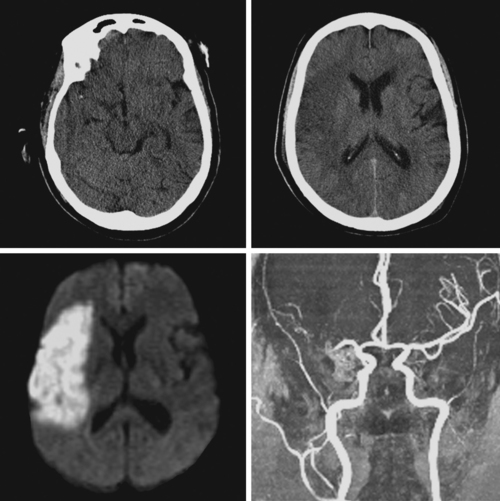
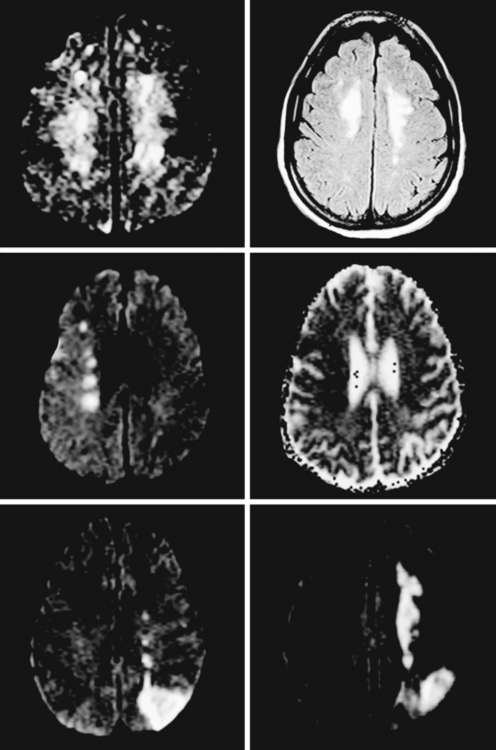
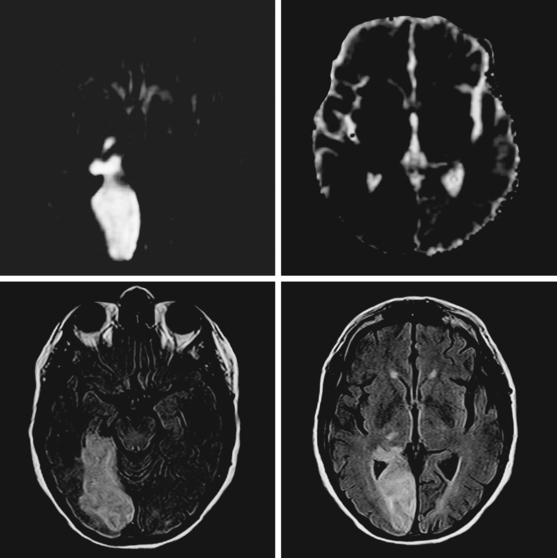
A 65-year-old man with history of hypertension presented with acute global aphasia and right flaccid hemiplegia involving the lower face, the arm, and, to a lesser degree, the leg. He also had a dense right visual field deficit and profound right sensory loss. Magnetic resonance imaging (MRI) with DWI revealed extensive ischemia in the territory of the left MCA (Figure 2-13). T2* sequence disclosed a hypointense signal in the left MCA indicative of acute vessel thrombosis and MRA confirmed the left M1 occlusion (Figure 2-14). Atrial fibrillation was noted on cardiac telemetry. Over the following 48 hours, the patient developed fatal brain swelling (see Figure 2-14).
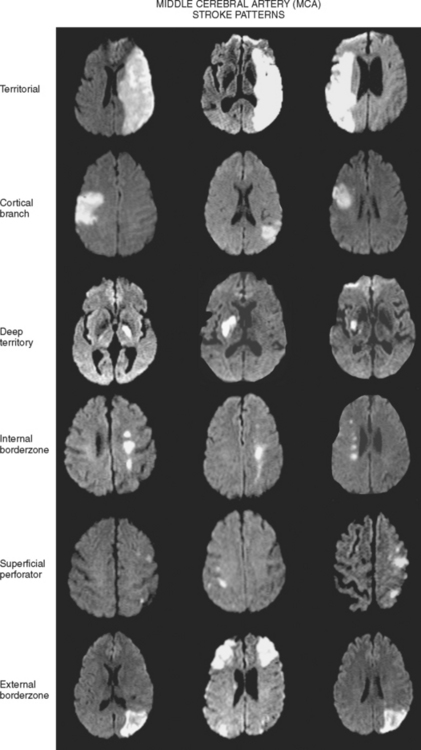
Territorial MCA Infarction
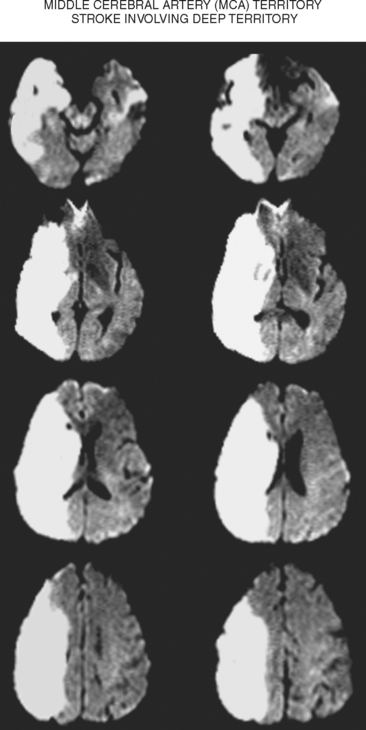
Deep Middle Cerebral Artery Infarction
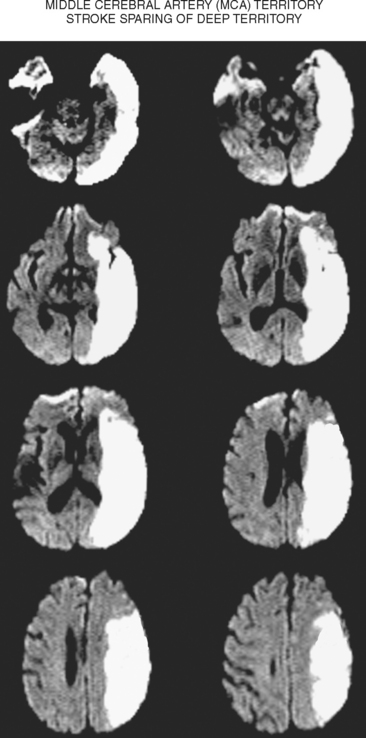
Superficial Divisional Middle Cerebral Artery Infarction
Superficial Cortical Infarctions
Hemispheric Border-Zone Infarctions
ANTERIOR CEREBRAL ARTERY OCCLUSION
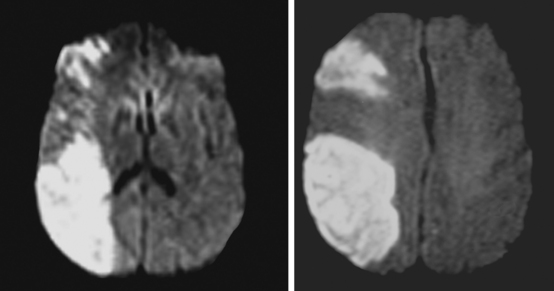
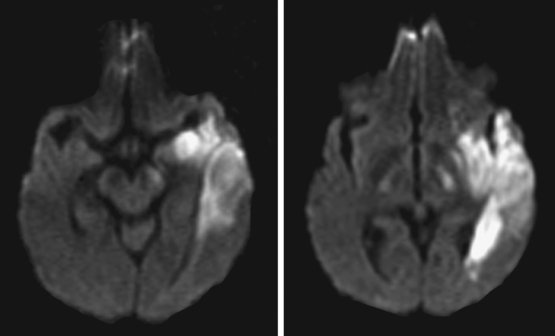
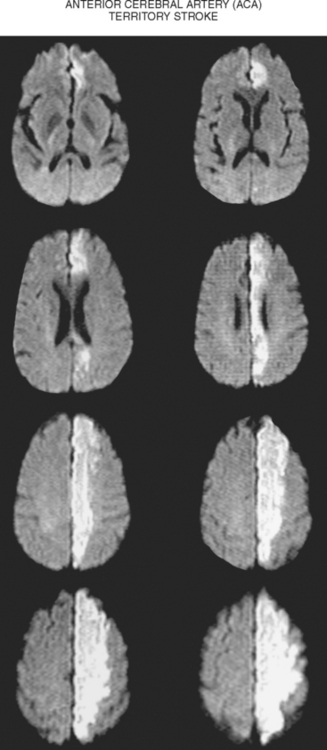
A 65-year-old man presented to the hospital with acute onset of left crural paresis. He had suffered a myocardial infarction 2 weeks before and was taking aspirin and clopidogrel for secondary prevention of coronary events. Examination revealed left leg weakness predominantly involving the proximal flexor muscles and associated with hyperreflexia. MRI demonstrated acute infarction in the right ACA distribution (Figure 2-24). Echocardiogram disclosed a mural thrombus overlying a hypokinetic segment of the left ventricle. The patient was anticoagulated and recovered well with physical therapy.
ANTERIOR CHOROIDAL ARTERY OCCLUSION
POSTERIOR CEREBRAL ARTERY OCCLUSION
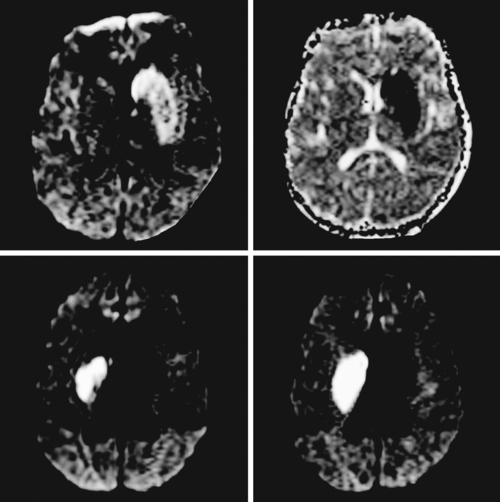
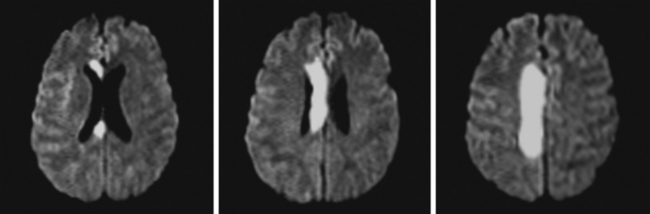
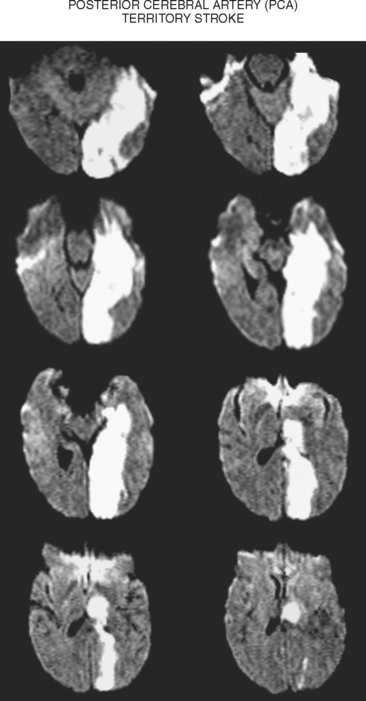
A 68-year-old woman with a history of atrial fibrillation on chronic anticoagulation presented to the hospital with acute behavioral changes, visual disturbances, and right hemiparesis. On examination, she was somnolent, had poor short-term recollection, dense right hemianopia, mild right hemiparesis, and profound right hypoesthesia. Her INR was subtherapeutic because the patient had recently stopped taking warfarin for a dental procedure, and she had opted not to replace it with subcutaneous low-molecular-weight heparin. MRI of her brain showed a large left PCA infarction and MRA disclosed proximal occlusion of this vessel (Figure 2-28). Despite some functional recovery over the following 6 months, her visual and cognitive disturbances remained disabling.
TABLE 2-2 Visual field disturbance caused by PCA infarction.
| Bilateral PCA infarction |
| Cortical blindness |
| Bilateral altitudinal hemianopia |
| Unilateral PCA infarction |
| Macular sparing hemianopia |
| Temporal crescent paring hemianopia |
| Quadrantanopia |
| Isolated macular hemianopia (rare) |
VERTEBROBASILAR DISEASE
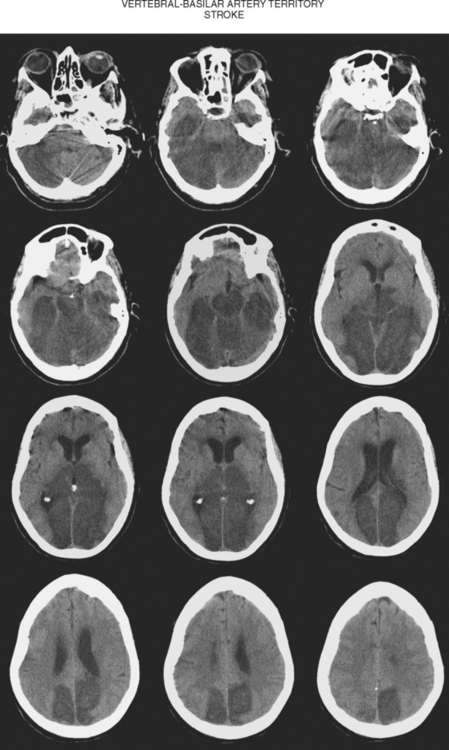
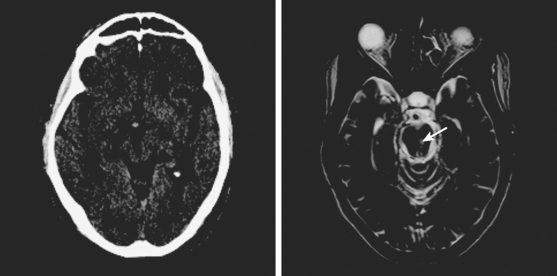
Figure 2-33 Computed tomography scan of the brain showing a massive infarction in the vertebrobasilar territory.
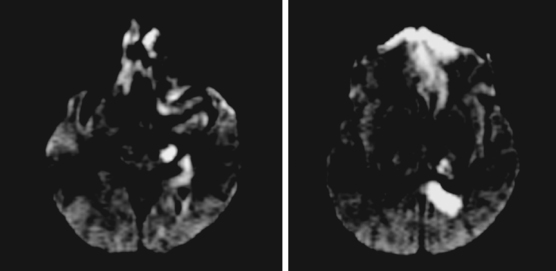
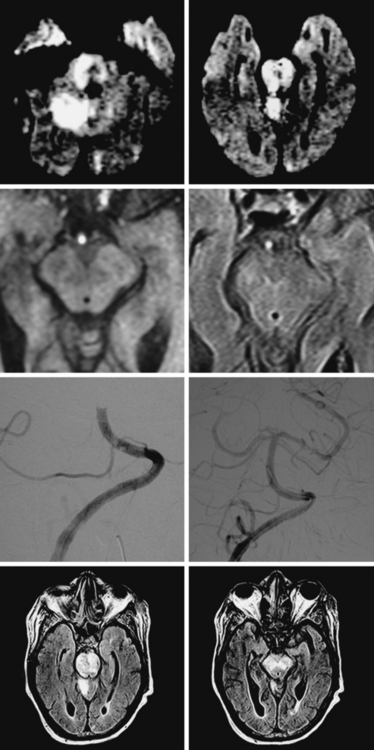
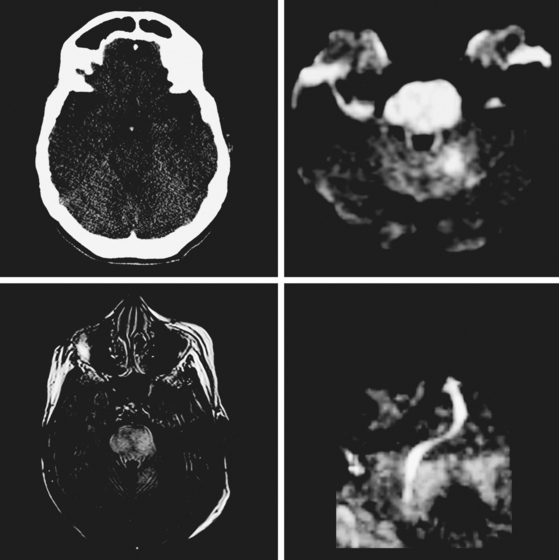
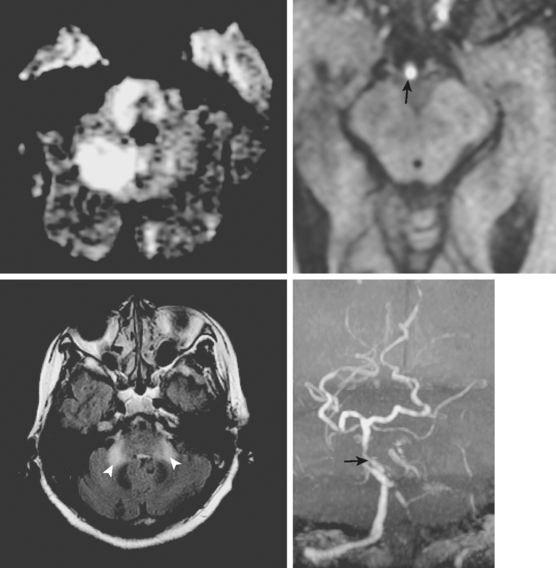
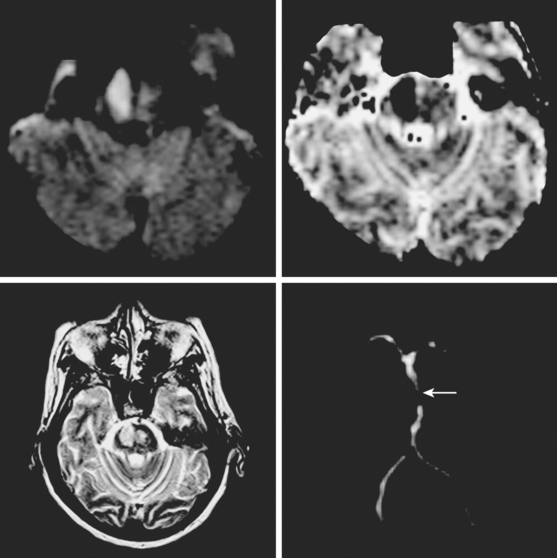
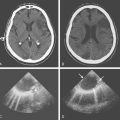
A 64-year-old woman was found unresponsive in her bathroom by her husband. She was intubated by the paramedics and transported to our emergency department. On arrival, she was comatose and breathing at a rate of 40 to 45 per minute. She was tachycardic and hypertensive. Her pupils were slightly anisocoric (3.5 mm on the left and 3 mm on the right), and responses to light were minimal on the left and absent on the right. Corneal and oculocephalic reflexes were preserved. Best motor responses to pain were in the form of withdrawal. DWI showed restricted diffusion in the midcerebellum and mesencephalon (Figure 2-34). A hyperintense signal in the basilar artery indicative of acute thrombosis was visualized on FLAIR. Conventional angiography confirmed occlusion of the basilar trunk. She underwent successful basilar recanalization by intra-arterial thrombolysis combined with mechanical disruption of the clot. Despite reperfusion, the patient failed to improve neurologically. Repeat MRI showed established infarction throughout the midbrain. Patient expired shortly after her family requested withdrawal of artificial life support.
| Combination of ophthalmoplegia with motor, sensory, or coordination deficits |
| Crossed motor or sensory findings |
| Acute ataxia with inability to walk |
| Sequential appearance of bilateral Babinski signs* |
| Sequential appearance of bilateral weakness |
| Acute reduction in the level of consciousness |
* It can also be seen in patients with incipient craniocaudal herniation from massive supratentorial infarctions.
CEREBELLAR INFARCTIONS
Posterior Inferior Cerebellar Artery Infarction
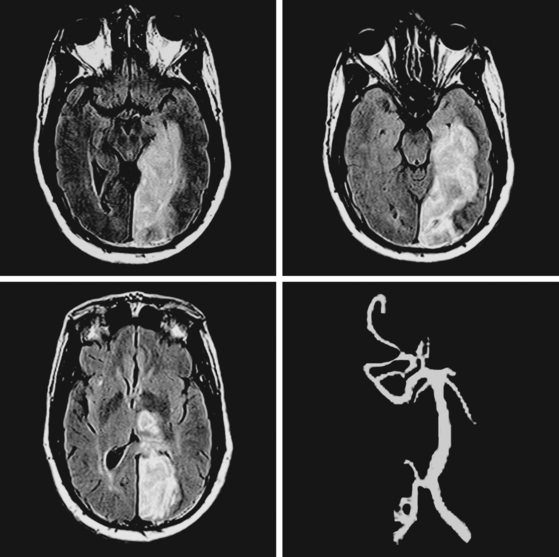
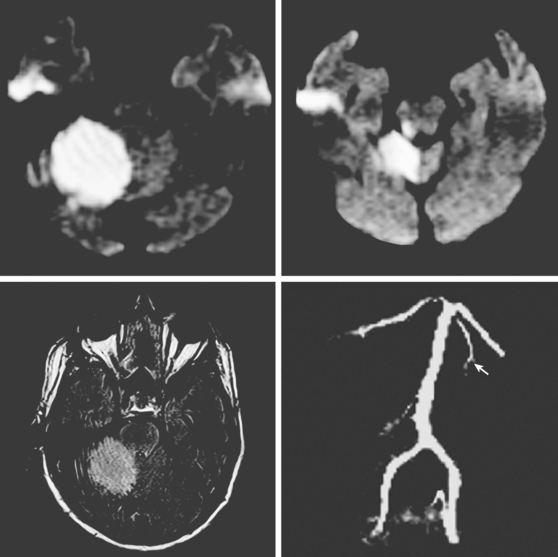
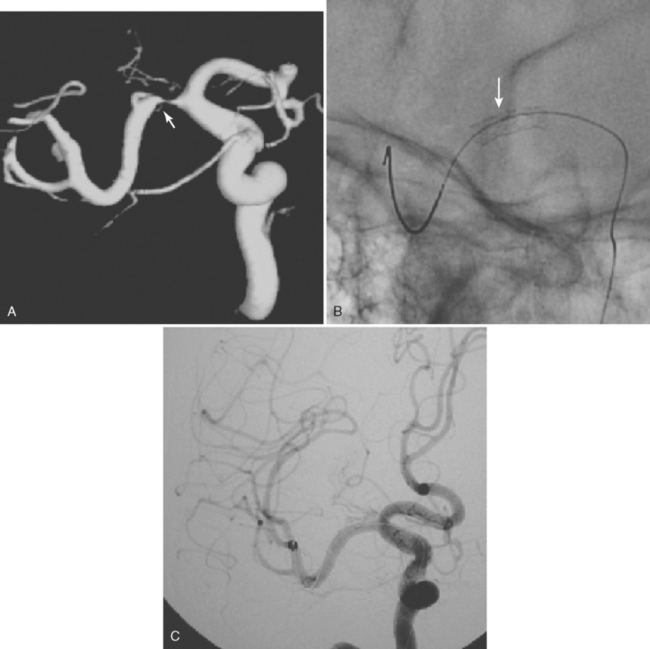
A 38-year-old woman presented with severe neck and posterior head pain, dizziness, gait imbalance, and right-hand clumsiness. Her examination predominantly demonstrated right appendicular ataxia. Brain imaging disclosed a large right PICA stroke with incipient mass effect (Figure 2-38). Vascular imaging revealed a right vertebral artery occlusion, most likely due to dissection. The patient was carefully monitored in the stroke unit, and as she developed mild confusion and restlessness, repeat imaging was performed showing worsening swelling with displacement of the fourth ventricle, effacement of the subarachnoid cisterns, distortion of the brainstem, and dilatation of the temporal horn of the right lateral ventricle. The patient immediately underwent suboccipital craniectomy. She improved substantially after surgery and achieved good functional recovery over the ensuing 6 months.
Anterior Inferior Cerebellar Artery Infarction
Superior Cerebellar Artery Infarction
Cerebellar Border-Zone Infarctions
BRAINSTEM INFARCTIONS
Medullary Infarctions
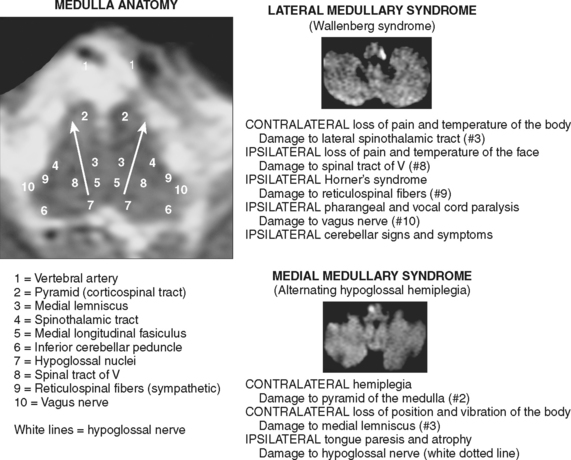
The main ischemic medullary syndromes are illustrated in Figure 2-42.
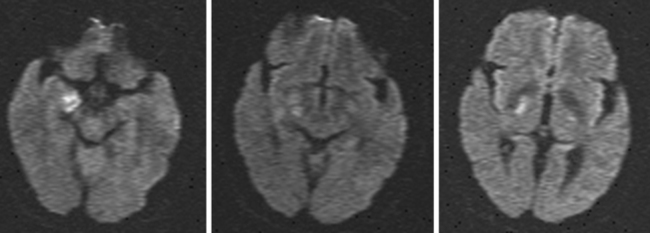
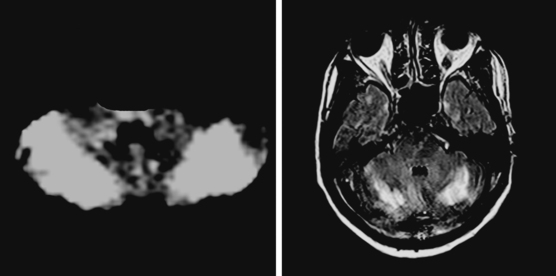
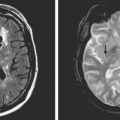
A 75-year-old man was admitted with sudden onset of dysphagia, dysarthria, and gait imbalance. He had long-standing history of hypertension and poorly controlled type 2 diabetes. On examination, he had left miosis and ptosis, dysarthria, difficulty swallowing his saliva, left ataxia, and decreased sensation to pain and temperature on the right side. He developed uncontrollable hiccups in the emergency department. CT scan was not informative, but DWI demonstrated a left lateral medullary infarction with associated ischemia of the left cerebellum (Figure 2-43). The PICA was not seen on noninvasive angiogram and considered to be occluded. Irregularities in other intracranial vessels indicated widespread intracranial atherosclerosis. The patient required temporary placement of a percutaneous gastrostomy tube for safe feeding but subsequently made a favorable recovery with moderate residual dysarthria and ataxia.
Wallenberg’s Syndrome
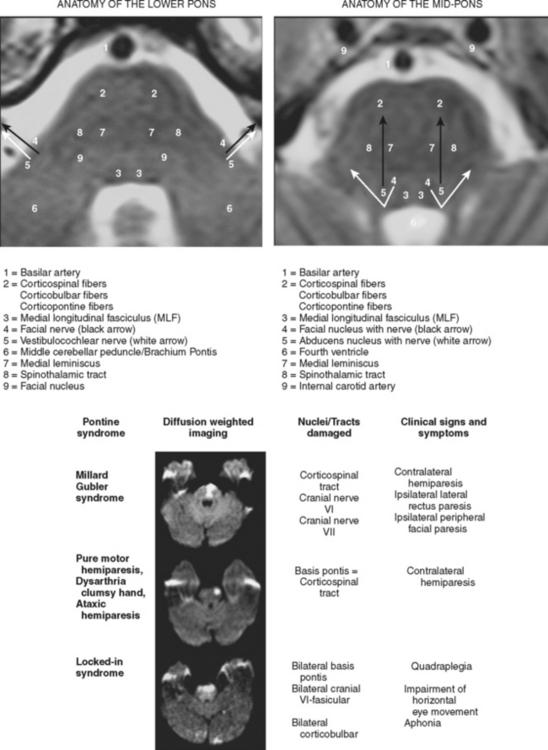
Pontine Infarctions
The main ischemic pontine syndromes are illistrated in Figure 2-44.
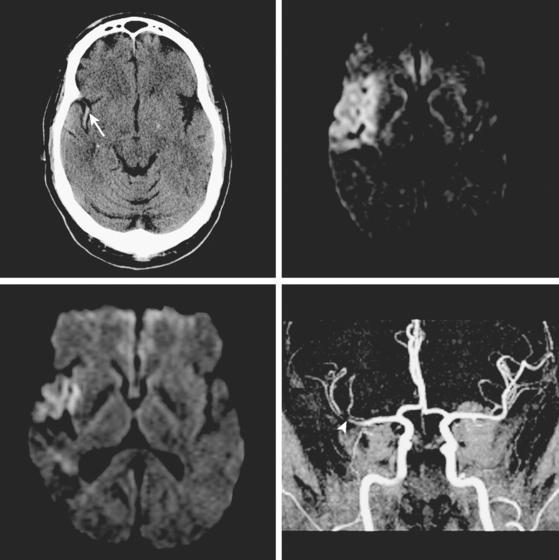
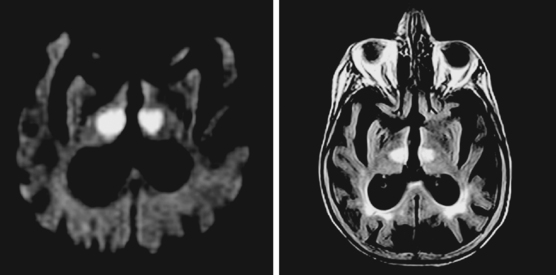
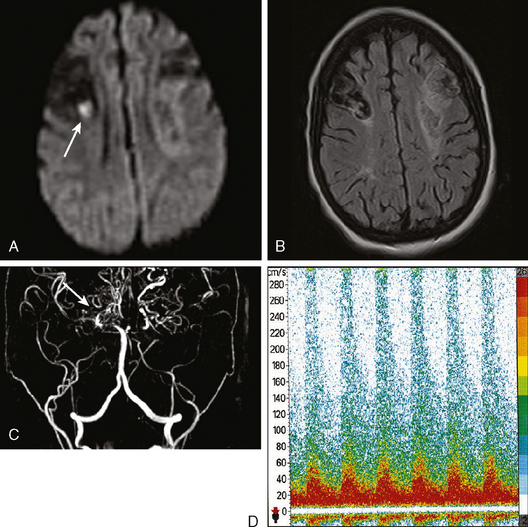
A 63-year-old man developed acute onset of slurred speech, gait imbalance, left-sided weakness, and horizontal diplopia. Initially he did not seek medical attention because “he did not like doctors who always found something wrong with him,” but after more than 12 hours of persistent deficits, his family convinced him to go to the hospital. On examination, he had mild dysarthria, right abducens palsy, right facial weakness, left arm and leg weakness, and mild axial and right appendicular ataxia. CT scan showed an area of possible hypoattenuation of the right pons, which was subsequently confirmed by MRI with DWI (Figure 2-45). MRA of the intracranial vessels disclosed atherosclerotic midbasilar stenosis (see Figure 2-45), which was likely the culprit for the pontine stroke by occluding a paramedian penetrating branch. During the hospitalization the patient was diagnosed with hypertension, diabetes mellitus, and hyperlipidemia. He was discharged on antiplatelet therapy, a statin, an angiotensin-converting enzyme (ACE) inhibitor, and an oral hypoglycemic agent. He achieved fair functional recovery with the help of intensive physical and occupational therapy. He remained compliant with the medications and has not had recurrent symptoms of posterior circulation ischemia.
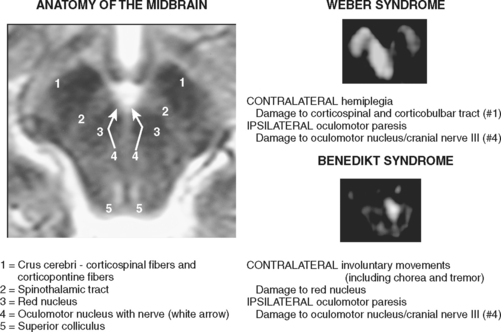
Midbrain Infarctions
The main ischemic midbrain syndromes are illustrated in Figure 2-46.
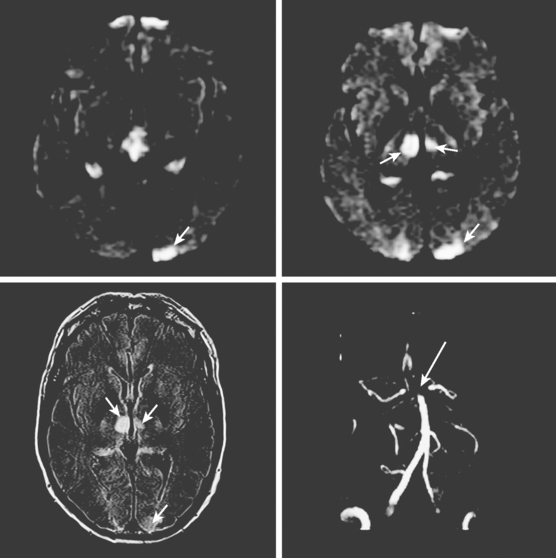
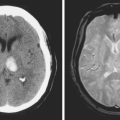
A 60-year-old man with history of hypertension and heavy smoking was acutely evaluated in the emergency department for sudden onset of diplopia and right hemiparesis. On examination, he was hypertensive and had a left oculomotor nerve palsy and right hemiparesis with upper motor neuron pattern. CT scan was suspicious for an area of hypoattenuation in the middle aspect of the midbrain, and MRI scan confirmed the diagnosis of acute mesencephalic infarction (Figure 2-47). No significant intracranial stenosis was noted on MRA. The patient was kept on antiplatelet therapy, and measures to control his vascular risk factors. The deficits remained stable and moderately disabling at 4 months. He has continued to smoke.
1 Kim EY, Lee SK, Kim DJ, Suh SH, Kim J, Heo JH, et al. Detection of thrombus in acute ischemic stroke: value of thin-section noncontrast-computed tomography. Stroke. 2005;36:2745-2747.
2 Assouline E, Benziane K, Reizine D, Guichard JP, Pico F, Merland JJ, et al. Intra-arterial thrombus visualized on T2* gradient echo imaging in acute ischemic stroke. Cerebrovasc Dis. 2005;20:6-11.
3 Sugg RM, Malkoff MD, Noser EA, Shaltoni HM, Weir R, Cacayorin ED, et al. Endovascular recanalization of internal carotid artery occlusion in acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:2591-2594.
4 Rabinstein AA, Wijdicks EF, Nichols DA. Complete recovery after early intraarterial recombinant tissue plasminogen activator thrombolysis of carotid T occlusion. AJNR Am J Neuroradiol. 2002;23:1596-1599.
5 Tomsick T, Brott T, Barsan W, Broderick J, Haley EC, Spilker J, et al. Prognostic value of the hyperdense middle cerebral artery sign and stroke scale score before ultraearly thrombolytic therapy. AJNR Am J Neuroradiol. 1996;17:79-85.
6 Qureshi AI, Ezzeddine MA, Nasar A, Suri MF, Kirmani JF, Janjua N, et al. Is IV tissue plasminogen activator beneficial in patients with hyperdense artery sign? Neurology. 2006;66:1171-1174.
7 Manno EM, Nichols DA, Fulgham JR, Wijdicks EF. Computed tomographic determinants of neurologic deterioration in patients with large middle cerebral artery infarctions. Mayo Clin Proc. 2003;78:156-160.
8 Agarwal P, Kumar S, Hariharan S, Eshkar N, Verro P, Cohen B, et al. Hyperdense middle cerebral artery sign: can it be used to select intra-arterial versus intravenous thrombolysis in acute ischemic stroke? Cerebrovasc Dis. 2004;17:182-190.
9 Marinkovic SV, Kovacevic MS, Marinkovic JM. Perforating branches of the middle cerebral artery. Microsurgical anatomy of their extracerebral segments. J Neurosurg. 1985;63:266-271.
10 Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol. 1979;36:65-73.
11 Nicolai A, Lazzarino LG, Biasutti E. Large striatocapsular infarcts: clinical features and risk factors. J Neurol. 1996;243:44-50.
12 Marinkovic SV, Milisavljevic MM, Kovacevic MS, Stevic ZD. Perforating branches of the middle cerebral artery. Microanatomy and clinical significance of their intracerebral segments. Stroke. 1985;16:1022-1029.
13 Fenelon G, Houeto JL. Unilateral parkinsonism following a large infarct in the territory of the lenticulostriate arteries. Mov Disord. 1997;12:1086-1090.
14 Caplan LR, Schmahmann JD, Kase CS, Feldmann E, Baquis G, Greenberg JP, et al. Caudate infarcts. Arch Neurol. 1990;47:133-143.
15 Bogousslavsky J, Van Melle G, Regli F. Middle cerebral artery pial territory infarcts: a study of the Lausanne Stroke Registry. Ann Neurol. 1989;25:555-560.
16 Caplan LR, Kelly M, Kase CS, Hier DB, White JL, Tatemichi T, et al. Infarcts of the inferior division of the right middle cerebral artery: mirror image of Wernicke’s aphasia. Neurology. 1986;36:1015-1020.
17 Fink JN, Selim MH, Kumar S, Voetsch B, Fong WC, Caplan LR. Insular cortex infarction in acute middle cerebral artery territory stroke: predictor of stroke severity and vascular lesion. Arch Neurol. 2005;62:1081-1085.
18 Abboud H, Berroir S, Labreuche J, Orjuela K, Amarenco P. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol. 2006;59:691-699.
19 Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, Arsava EM, et al. Neuroanatomic correlates of stroke-related myocardial injury. Neurology. 2006;66:1325-1329.
20 Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66:477-483.
21 Ay H, Arsava EM, Koroshetz WJ, Sorensen AG. Middle cerebral artery infarcts encompassing the insula are more prone to growth. Stroke. 2008;39:373-378.
22 Minematsu K, Yamaguchi T, Omae T. “Spectacular shrinking deficit”: rapid recovery from a major hemispheric syndrome by migration of an embolus. Neurology. 1992;42:157-162.
23 Baird AE, Donnan GA, Austin MC, McKay WJ. Early reperfusion in the “spectacular shrinking deficit” demonstrated by single-photon emission computed tomography. Neurology. 1995;45:1335-1339.
24 Sage JI, Van Uitert RL. Man-in-the-barrel syndrome. Neurology. 1986;36:1102-1103.
25 Hurley JP, Wood AE. Isolated man-in-the-barrel syndrome following cardiac surgery. Thorac Cardiovasc Surg. 1993;41:252-254.
26 Riggs HE, Rupp C. Variation in form of circle of Willis. The relation of the variations to collateral circulation: anatomic analysis. Arch Neurol. 1963;8:8-14.
27 Marinkovic S, Kovacevic M, Milisavljevic M. Hypoplasia of the proximal segment of the anterior cerebral artery. Anat Anz. 1989;168:145-154.
28 Dunker RO, Harris AB. Surgical anatomy of the proximal anterior cerebral artery. J Neurosurg. 1976;44:359-367.
29 Ogawa A, Suzuki M, Sakurai Y, Yoshimoto T. Vascular anomalies associated with aneurysms of the anterior communicating artery: microsurgical observations. J Neurosurg. 1990;72:706-709.
30 Caplan LR, Schmahmann JD, Kase CS, Feldmann E, Baquis G, Greenberg JP, et al. Caudate infarcts. Arch Neurol. 1990;47:133-143.
31 Nagaratnam N, Nagaratnam K, Ng K, Diu P. Akinetic mutism following stroke. J Clin Neurosci. 2004;11:25-30.
32 Gelmers HJ. Non-paralytic motor disturbances and speech disorders: the role of the supplementary motor area. J Neurol Neurosurg Psychiatry. 1983;46:1052-1054.
33 Hupperts RM, Lodder J, Heuts-van Raak EP, Kessels F. Infarcts in the anterior choroidal artery territory. Anatomical distribution, clinical syndromes, presumed pathogenesis and early outcome. Brain. 1994;117(Pt 4):825-834.
34 Takahashi S, Ishii K, Matsumoto K, Higano S, Ishibashi T, Suzuki M, et al. The anterior choroidal artery syndrome. II. CT and/or MR in angiographically verified cases. Neuroradiology. 1994;36:340-345.
35 Levy R, Duyckaerts C, Hauw JJ. Massive infarcts involving the territory of the anterior choroidal artery and cardioembolism. Stroke. 1995;26:609-613.
36 Friedman JA, Pichelmann MA, Piepgras DG, Atkinson JL, Maher CO, Meyer FB, et al. Ischemic complications of surgery for anterior choroidal artery aneurysms. J Neurosurg. 2001;94:565-572.
37 Bisaria KK. Anomalies of the posterior communicating artery and their potential clinical significance. J Neurosurg. 1984;60:572-576.
38 Pedroza A, Dujovny M, Artero JC, Umansky F, Berman SK, Diaz FG, et al. Microanatomy of the posterior communicating artery. Neurosurgery. 1987;20:228-235.
39 Waterston JA, Stark RJ, Gilligan BS. Paramedian thalamic and midbrain infarction: the “mesencephalothalamic syndrome.”. Clin Exp Neurol. 1987;24:45-53.
40 Auchus AP, Chen CP, Sodagar SN, Thong M, Sng EC. Single stroke dementia: insights from 12 cases in Singapore. J Neurol Sci. 2002;203–204:85-89.
41 Tay KY, King-Im JM, Trivedi RA, Higgins NJ, Cross JJ, Davies JR, et al. Imaging the vertebral artery. Eur Radiol. 2005;15:1329-1343.
42 Caplan LR, Wityk RJ, Glass TA, Tapia J, Pazdera L, Chang HM, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389-398.
43 Koch S, Amir M, Rabinstein AA, Reyes-Iglesias Y, Romano JG, Forteza A. Diffusion-weighted magnetic resonance imaging in symptomatic vertebrobasilar atherosclerosis and dissection. Arch Neurol. 2005;62:1228-1231.
44 Caplan LR. “Top of the basilar” syndrome. Neurology. 1980;30:72-79.
45 Kumral E, Kisabay A, Atac C. Lesion patterns and etiology of ischemia in the anterior inferior cerebellar artery territory involvement: a clinical–diffusion weighted–MRI study. Eur J Neurol. 2006;13:395-401.
46 Kumral E, Kisabay A, Atac C. Lesion patterns and etiology of ischemia in superior cerebellar artery territory infarcts. Cerebrovasc Dis. 2005;19:283-290.
47 Barth A, Bogousslavsky J, Regli F. The clinical and topographic spectrum of cerebellar infarcts: a clinical-magnetic resonance imaging correlation study. Ann Neurol. 1993;33:451-456.
48 Canaple S, Bogousslavsky J. Multiple large and small cerebellar infarcts. J Neurol Neurosurg Psychiatry. 1999;66:739-745.
49 Min WK, Kim YS, Kim JY, Park SP, Suh CK. Atherothrombotic cerebellar infarction: vascular lesion-MRI correlation of 31 cases. Stroke. 1999;30:2376-2381.
50 Koh MG, Phan TG, Atkinson JL, Wijdicks EF. Neuroimaging in deteriorating patients with cerebellar infarcts and mass effect. Stroke. 2000;31:2062-2067.
51 Jauss M, Muffelmann B, Krieger D, Zeumer H, Busse O. A computed tomography score for assessment of mass effect in space-occupying cerebellar infarction. J Neuroimaging. 2001;11:268-271.
52 Jauss M, Krieger D, Hornig C, Schramm J, Busse O. Surgical and medical management of patients with massive cerebellar infarctions: results of the German-Austrian Cerebellar Infarction Study. J Neurol. 1999;246:257-264.
53 Chaves CJ, Caplan LR, Chung CS, Tapia J, Amarenco P, Teal P, et al. Cerebellar infarcts in the New England Medical Center Posterior Circulation Stroke Registry. Neurology. 1994;44:1385-1390.
54 Amarenco P, Hauw JJ. Cerebellar infarction in the territory of the anterior and inferior cerebellar artery. A clinicopathological study of 20 cases. Brain. 1990;113:139-155.
55 Sohn SI, Lee H, Lee SR, Baloh RW. Cerebellar infarction in the territory of the medial branch of the superior cerebellar artery. Neurology. 2006;66:115-117.
56 Amarenco P, Hauw JJ. Cerebellar infarction in the territory of the superior cerebellar artery: a clinicopathologic study of 33 cases. Neurology. 1990;40:1383-1390.
57 Kim HA, Lee H, Sohn SI, Yi HA, Cho YW, Lee SR, et al. Bilateral infarcts in the territory of the superior cerebellar artery: clinical presentation, presumed cause, and outcome. J Neurol Sci. 2006;246:103-109.
58 Amarenco P, Kase CS, Rosengart A, Pessin MS, Bousser MG, Caplan LR. Very small (border zone) cerebellar infarcts. Distribution, causes, mechanisms and clinical features. Brain. 1993;116:161-186.
59 Sacco RL, Freddo L, Bello JA, Odel JG, Onesti ST, Mohr JP. Wallenberg’s lateral medullary syndrome. Clinical-magnetic resonance imaging correlations. Arch Neurol. 1993;50:609-614.
60 Sacco RL, Freddo L, Bello JA, Odel JG, Onesti ST, Mohr JP. Wallenberg’s lateral medullary syndrome. Clinical-magnetic resonance imaging correlations. Arch Neurol. 1993;50:609-614.
61 Milandre L, Lucchini P, Khalil R. [Lateral bulbar infarctions. Distribution, etiology and prognosis in 40 cases diagnosed by MRI]. Rev Neurol (Paris). 1995;151:714-721.
62 Frumkin LR, Baloh RW. Wallenberg’s syndrome following neck manipulation. Neurology. 1990;40:611-615.
63 Kitis O, Calli C, Yunten N, Kocaman A, Sirin H. Wallenberg’s lateral medullary syndrome: diffusion-weighted imaging findings. Acta Radiol. 2004;45:78-84.
64 Ross MA, Biller J, Adams HPJr, Dunn V. Magnetic resonance imaging in Wallenberg’s lateral medullary syndrome. Stroke. 1986;17:542-545.
65 Erro ME, Gallego J, Herrera M, Bermejo B. Isolated pontine infarcts: etiopathogenic mechanisms. Eur J Neurol. 2005;12:984-988.
66 Klein IF, Lavallee PC, Schouman-Claeys E, Amarenco P. High-resolution MRI identifies basilar artery plaques in paramedian pontine infarct. Neurology. 2005;64:551-552.
67 Kim JS, Kim J. Pure midbrain infarction: clinical, radiologic, and pathophysiologic findings. Neurology. 2005;64:1227-1232.
68 Kumral E, Bayulkem G, Akyol A, Yunten N, Sirin H, Sagduyu A. Mesencephalic and associated posterior circulation infarcts. Stroke. 2002;33:2224-2231.