Cleft–Orthognathic Surgery
The Unilateral Cleft Lip and Palate Deformity
• Facial Growth Implications of Cleft Palate Repair in the Infant with Unilateral Cleft Lip and Palate
• Timing of Orthognathic Surgery
• Residual Deformities in the Adolescent with Unilateral Cleft Lip and Palate
• Orthodontic Considerations in the Patient with Unilateral Cleft Lip and Palate with a Jaw Deformity
• Immediate Presurgical Re-Assessment
• Orthognathic Approach for Unilateral Cleft Lip and Palate Deformities
• Clinical Management after Initial Surgical Healing
• Orthognathic Surgery for Unilateral Cleft Lip and Palate: Review of Study
• Skeletal Stability after Modified Le Fort I for Unilateral Cleft Lip and Palate: Review of Study
In 1880, the pioneer orthodontist Normal W. Kingsley stated the following in his remarks about surgical operations as a means of correcting cleft palate: “Although the practice [of cleft palate repair] has been tested in thousands of cases by the most eminent surgeons of their time, it has resulted in such uniformity of failure [i.e., severe jaw deformities], that it should have been utterly abandoned years ago.”85
In 1920, another preeminent orthodontist of his day, Calvin Case, voiced a similar sentiment: “[L]et us hope that the proportion of [cleft palate] surgical failures will be greatly lessened in the future and that well-informed, honest surgeons … accept only the most favorable cases with a determination to follow them through with proper interest.”16
In 1965, Vilray Kazanjian, who was a leading maxillofacial surgeon of the first half of the twentieth century, reminded us that “the relative value of surgery to repair a cleft palate has been intelligently questioned for over 150 years.”83
Facial Growth Implications of Cleft Palate Repair in the Infant with Unilateral Cleft Lip and Palate
Effects of Cleft Palate Repair in Infancy
The management of individuals with unilateral cleft lip and palate (UCLP) presents specific clinical challenges for the maxillofacial surgeon, the orthodontist, and the restorative dental team. Orthognathic surgery is a procedure that should be considered as part of the treatment algorithm for patients with UCLP. Ross completed a multicenter, long-term facial growth study to assess the need for orthognathic surgery among individuals born with complete UCLP who had undergone primary lip and palate repair during childhood. He concluded that, even by the most conservative standards and in conjunction with maximum compensating orthodontic camouflage maneuvers, at least 25% of adolescents with UCLP required orthognathic surgery to achieve even the limited objective of a neutralized occlusion.148 His research indicated that only 25% of adolescents with UCLP had near-normal maxillary growth and that another 50% were in a borderline category with some degree of maxillary hypoplasia. Ross stated that individuals who were born with a cleft lip and palate have an intrinsic deficiency in the midfacial skeleton that is made worse by operations. More recently, Mulliken and colleagues reviewed the prevalence of Le Fort I osteotomies among patients with cleft lip and palate who were treated at Boston Children’s Hospital.55 They found that 48% of UCLP patients who underwent repair in infancy later required orthognathic surgery. The study also showed that the need for orthognathic surgery is dependent on the severity of the cleft type as well as the number and extent of previous operative procedures. Similarly, The Hospital for Sick Children in Toronto, Ontario, Canada, found that 48.3% of their patients (i.e. treated since infancy) with complete UCLP required orthognathic surgery. When they looked at all patients with UCLP who were referred to their center, they found that 59.4% needed jaw surgery.27 A retrospective cohort study of five prominent cleft palate centers in North America compared the maxillomandibular relationships of individuals with non-syndromal complete UCLP (n = 169).63 The one center that incorporated primary alveolar bone grafting showed especially poor maxillary growth, with 66% of its patients requiring orthognathic surgery. Interestingly, at the one center in which a single surgeon performed all of the surgeries with the use of a more delayed approach to cleft palate repair and whose patients underwent no revisions until they were 14 years old showed the lowest need for orthognathic surgery at less than 25%.
Saperstein and colleagues described the facial growth of children with complete clefting of the primary palate (i.e., the lip through the incisal foramen) but with an intact secondary palate (i.e., the incisal foramen through the uvula).151 This was a retrospective, cross-sectional analysis of non-syndromal patients with unilateral complete clefting of the primary palate as compared with those with unilateral complete clefting of both the primary and secondary palates. Angular and linear measurements of the midfacial region were made on lateral cephalograms. The study groups included those with unilateral complete clefting of the primary palate (n = 25) and those with unilateral complete clefting of the primary and secondary palate (n = 18). The study documented that individuals with a cleft of only the primary palate who underwent lip repair during infancy typically had a normal or even slightly forward maxillary position as compared with age-matched controls. This was in contrast with children with a cleft of both the primary and secondary palates who underwent lip repair followed by palate repair before they were 1 year old; this group showed a high incidence of maxillary deficiency. The study clarified that the cleft palate repair carried out before 1 year of age—and not the cleft lip repair itself—was responsible for the high incidence of midface hypoplasia.
Effects of Achieving Bone Fusion Across the Cleft Alveolus during Infancy
Reconstruction of the alveolar process in patients with UCLP is an essential part of cleft care. Accomplishing this goal provides support for the alar base of the nose; the teeth in the cleft region; and the periodontium that surrounds those teeth (Figs. 32-1 and 32-2). There are three basic methods that have been described for the closure of the cleft alveolus: 1) primary bone grafting; 2) secondary bone grafting; and 3) gingival periosteoplasty (GPP).56 The method of primary bone grafting is now generally recognized to result in severe midface growth disturbance and has therefore been universally abandoned throughout the world.63,148 The use of secondary (mixed dentition) bone grafting is generally recognized as an effective method to avoid the problem of additional midface growth disturbance and to successfully achieve support for the alar base; to provide bone for the eruption of the canine through the grafted cleft site; and to establish effective periodontal support (Figs. 32-3 through 32-6).1
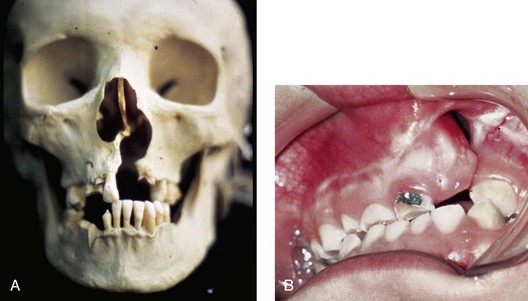
Figure 32-1 A, The maxillofacial skeleton of an adult with unrepaired unilateral cleft lip and palate (UCLP) is shown. Typical UCLP deformities of the hard palate, the alveolus, the nasal septum, the floor of the nose, the nasal spine, and the pyriform rims are demonstrated. B, An occlusal view of a child born with UCLP who presented during the mixed dentition with a residual alveolar/palatal skeletal defect and a labial and palatal oronasal fistula is also shown.
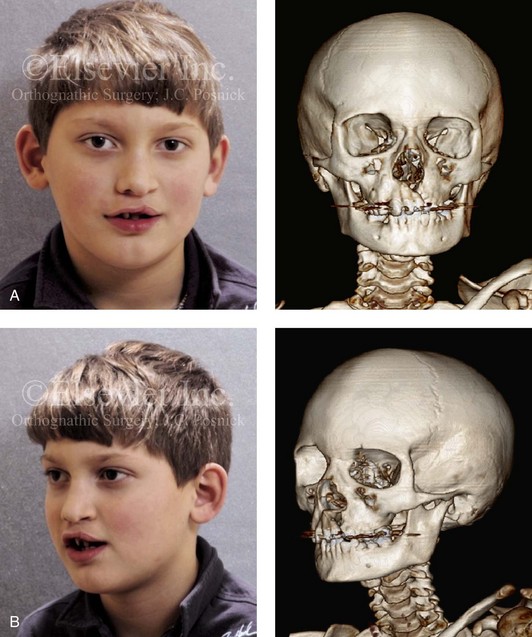
Figure 32-2 A 6-year-old boy who was born with a UCLP is shown after soft tissue lip and palate repair in infancy. A, Frontal and B, oblique facial and computed tomography scan views during the mixed dentition demonstrate residual cleft skeletal defects and deformities that were present before grafting and fistula closure.
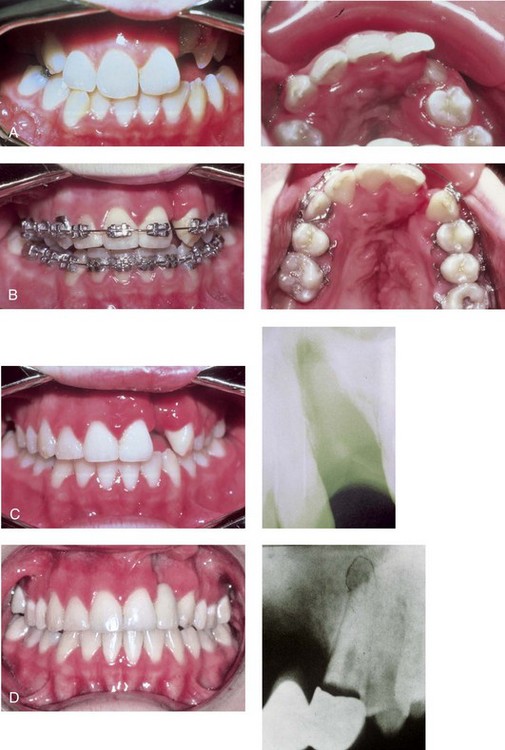
Figure 32-3 An example of the suboptimal management of a unilateral alveolar/palatal cleft. A child who was born with UCLP underwent soft tissue lip and palate repair during infancy. She underwent orthodontic treatment and achieved a satisfactory overjet and overbite. The alveolar/palatal cleft skeletal defects and the residual labial and palatal oronasal fistula were ignored. The maxillary lateral incisor tooth was inadequate and required extraction. The canine erupted adjacent to the cleft, and the dental gap was orthodontically retained. Dental rehabilitation was accomplished with a three-unit bridge to manage the cleft–dental gap. Limited periodontal (bone) support of the cleft-adjacent teeth (the canine and the central incisor) resulted in a periodontic–endodontic problem with a loss of the canine and poor support of the central incisor. These problems would not have occurred had effective bone grafting and fistula closure been carried out during the mixed dentition. A, Occlusal and palatal views during the mixed dentition before the full eruption of the canine through the non-grafted alveolar cleft. B, Occlusal and palatal views are also shown during orthodontic alignment (extraction of right bicuspid was carried out). C, Occlusal view at the completion of orthodontic alignment. A periapical radiograph indicates limited bony support of the cleft-adjacent teeth. D, An occlusal view is next shown after the placement of a three-unit bridge across the cleft–dental gap. A periapical radiograph confirms that, after several years, a periodontal–endodontic problem resulted in irreversible injury to the cleft-adjacent canine.
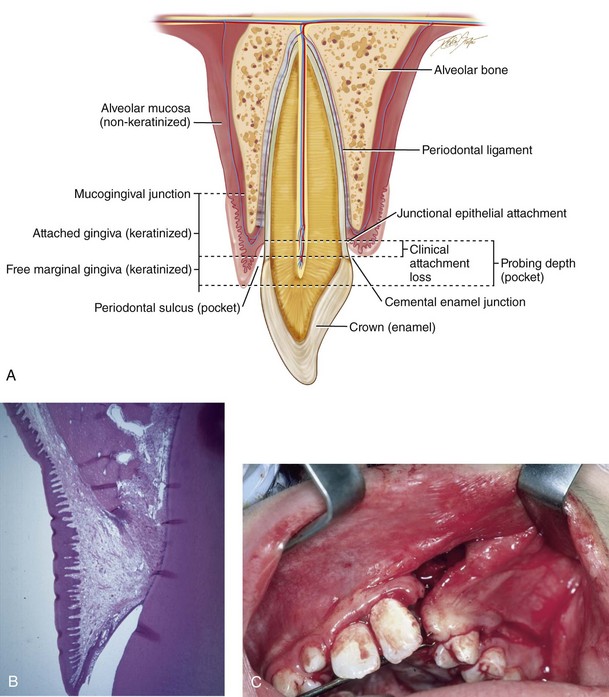
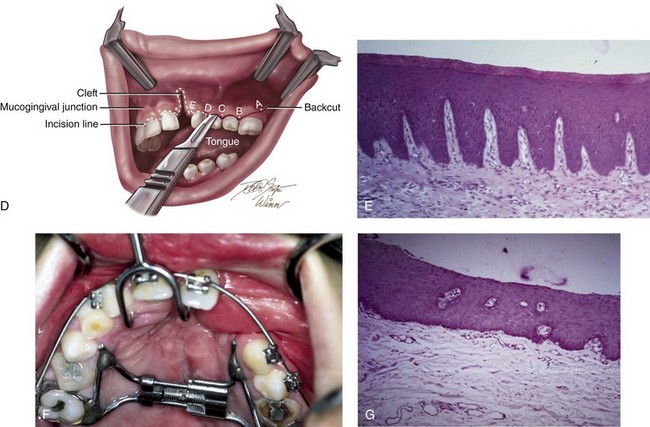
Figure 32-4 The importance of maintaining keratinized tissue adjacent to the cervical margin of each tooth for long-term periodontal health cannot be overemphasized (see Chapter 6). A, Illustration of a cross-section of dentoalveolar anatomy that indicates the location of the gingiva (keratinized mucosa) adjacent to the tooth surface. B, Low-power histomicrograph cross-section of the dentoalveolar anatomy that indicates the microscopic character of the keratinized mucosa adjacent to the tooth surface. Just deep to the keratinized epithelium rete pegs, organized fibrous tissue can be seen. C, Intraoperative view of an elevated mucogingival flap advanced anteriorly for the closure of the labial aspect of the cleft oronasal fistula. The flap brings the gingiva into the region where the canine will erupt. D, Illustration of incision placement for the elevation of the mucogingival flap. The flap splits the attached gingiva posteriorly adjacent to the first molar so that the advancing flap brings keratinized tissue into the cleft site without denuding the attached gingiva from the posterior teeth. E, Low-power histomicrograph that demonstrates keratinized epithelium, organized fibrous tissue, and rete pegs. F, Palatal view of a patient with UCLP during the mixed dentition. This patient underwent the closure of an oronasal fistula using a buccal “finger” flap by another surgeon. This is not the preferred way to rearrange the intraoral tissue for fistula closure; the labial vestibule is destroyed, and non-keratinized buccal mucosa is brought into the region where the canine will erupt. G, Low-power histomicrograph of non-keratinized buccal mucosa. Note the thin layer of non-keratinized epithelium with the underlying loose areolar connective tissue. Part D modified from an original illustration by Bill Winn.
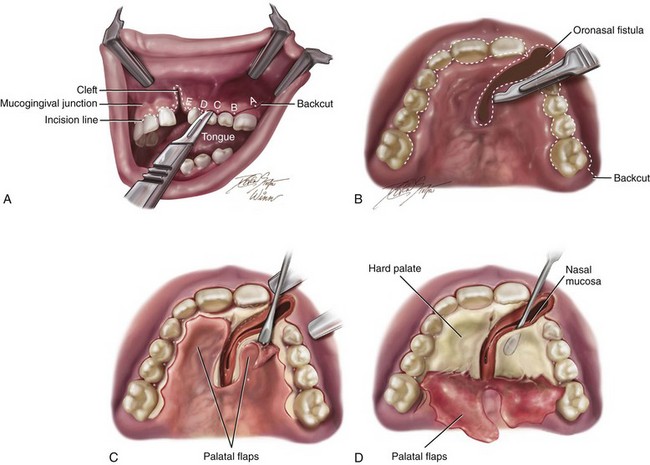
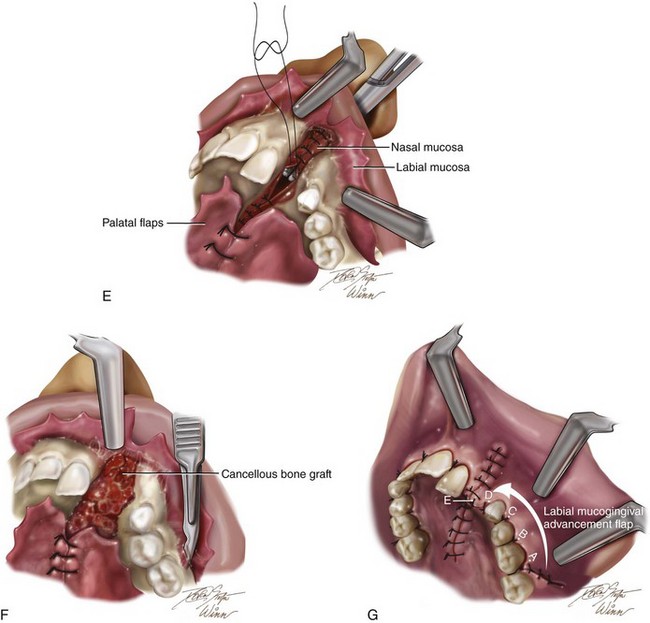
Figure 32-5 Illustrations of basic technique for bone grafting and the management of residual oronasal fistula during the mixed dentition of a child with a UCLP. If required, orthodontic expansion to correct the arch width precedes mixed-dentition bone grafting. A, A view of the left labial mucogingival incision in progress. B, Attention is turned to the sharp palate for the separation of the oral and nasal mucosa at the site of the fistula and then the elevation of the flaps. C, Subperiosteal elevation of the palatal flaps. D, With the palatal flaps elevated, the nasal mucosa is separated from the floor of the nose on each side. Left and right labial flaps have already been elevated. The nasal mucosa is further separated from the bony surface along the distal aspect of the central incision for later closure. Part A modified from an original illustration by Bill Winn. E, Suturing of the nasal flaps for watertight closure is in progress. A hemostat is placed through the left nostril to demonstrate the partially sutured nasal floor. The palatal flaps have been sutured together for fistula closure. F, After the nasal flaps are sutured, iliac cancellous bone graft is packed into the palatal, alveolar, and floor-of-the-nose skeletal defects. G, Left and right labial and palatal flaps are advanced for watertight closure. By advancing the left labial mucogingival flap, the keratinized tissue has been placed over the alveolar ridge, where the canine will eventually erupt. This is accomplished by splitting the attached gingiva at the last molar.
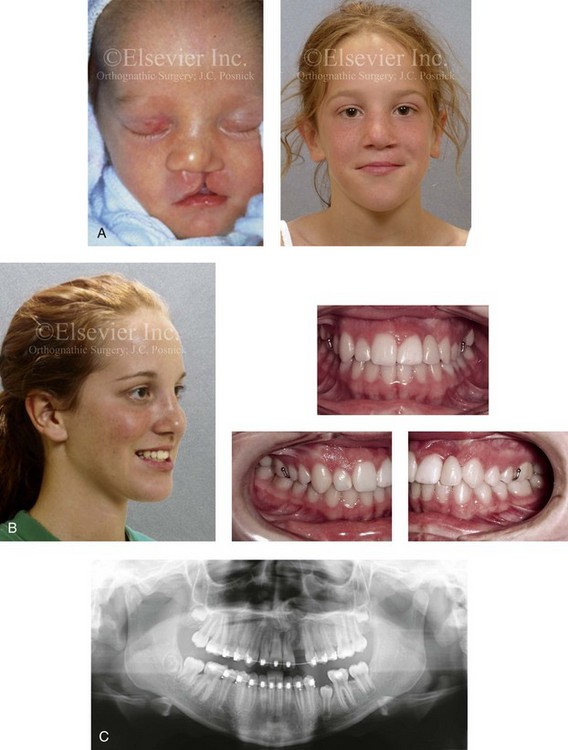
Figure 32-6 A 16-year-old girl was born with van der Woude syndrome, including UCLP and lower lip pits. She has been managed by this surgeon since her birth. She underwent lip and palate repair during her early childhood, which was followed by successful bone grafting and fistula closure during the mixed dentition. She had normal maxillary growth and underwent standard orthodontic treatment with the maintenance of the cleft–dental gap. She underwent an open rhinoplasty that included the use of a septal cartilage (caudal strut) graft when she was 15 years old. There is sufficient bone volume and attached gingiva for a dental implant, which is planned for when she is 18 years old. A, Facial views at the time of birth and during the mixed dentition, just before alveolar/palatal bone grafting. B, Facial and occlusal views taken when the patient was a teenager after rhinoplasty and before dental implant placement. A removable retainer that includes the missing lateral incisor is in place. C, Panorex image taken when the patient was a teenager during orthodontic treatment indicates successful bone grafting of the alveolar cleft.
The technique of GPP was first described by Skoog in 1965 as a method to achieve fusion across the cleft alveolus at the time of lip repair. The goal was to “remove” the cleft during infancy with the hope that no harm would result.156 Millard included the use of the Latham device to position the alveolar ridges close to one another before going forward with the GPP procedure at the time of lip repair.105,106 Grayson and Cutting later proposed the use of a nasoalveolar molding device before the GPP and primary lip repair to accomplish the same alveolar ridge fusion. Several studies confirm that the GPP procedure has a high osteogenic potential that results in the deposition of bone to achieve successful fusion of the alveolar ridge. Unfortunately, clinical studies do not confirm sufficient bone fill to consistently allow for the eruption of the canine through the ridge and to provide ideal periodontal support. Since the late 1970s, clinical studies have been carried out to assess the long-term effects of GPP on midfacial growth in cleft patients.
In 1999, Millard and colleagues used serial dental casts to evaluate the effects of GPP on maxillomandibular relationships.106 They found a greater frequency of anterior crossbite in the GPP group than in the non-GPP group. They also confirmed that the GPP group had a shorter anteroposterior length of the maxilla as compared with the non-GPP group at 6 years of age. Similar findings of poor midface growth have been documented by Berkowitz and colleagues,9 Matic and Powers,99 and others.70,169 In 2005, Renkielska and colleagues evaluated the impact of GPP on occlusal relationships with the use of the Goslon Yardstick occlusal grading system.143 They found that patients treated with GPP had a poor occlusal relationship and a Goslon Yardstick score of 4 and 5, thus indicating need for orthognathic surgery. They reaffirmed that the inclusion of the alveolar process in the primary lip repair increased severe occlusal maldevelopment.
More recently, Hsieh and colleagues completed a retrospective clinical study to evaluate the effects of GPP on facial growth in patients with UCLP.74 Sixty-two consecutive patients with non-syndromal complete UCLP with records from when they were 5 years old were included in the study. All of the patients had received nasoalveolar molding treatment before primary lip repair. Those individuals who underwent GPP at the time of lip repair (n = 26) were placed in one group. Those that did not undergo GPP at the time of lip repair (n = 36) were placed in another group. Cephalometry was used to evaluate facial growth at 5 years of age in the two treatment groups. GPP was found to have significant negative effects on the maxillary position (i.e., diminished horizontal projection), the intermaxillary position (i.e., negative overjet), the maxillary length, and the maxillary alveolar length at the age of 5 years. The authors concluded that, in patients with UCLP, the sagittal growth of the maxilla was significantly adversely affected by the GPP procedure. As a result of their study, the Chang Gung Cleft Center no longer uses the GPP technique.
Coordinated Team Approach
Care of the patient with UCLP is best delivered by an integrated group of specialists who evaluate and provide comprehensive definitive care. It is no longer acceptable for individual practitioners (e.g., surgeons, orthodontists, restorative dentists, speech pathologists, otolaryngologists) to carry out extended treatment without considering all aspects of the patient’s care and without discussing options with members of the team.82,90
A frequent road block to successful reconstruction and dental rehabilitation of the midface-deficient adolescent with UCLP—especially in those who present with other residual clefting problems (see the section about residual deformities later in this chapter)—is disagreement between clinicians about the indications, the most effective techniques, and the timing of intervention.8,104,123 The surgeon, the orthodontist, the dental and medical team, and the patient and his or her family must first agree about the dental, occlusal, speech, upper airway, and aesthetic objectives; only then can effective treatment go forward.
The advantage of coordinated care was confirmed by the Eurocleft Study, which found a lack of association between high-intensity disjointed treatment and favorable results. In other words, the greater the number of operations and the greater the number of years of orthodontic appliances worn (i.e., heavy burden of care), the worse the outcome.25,108
Treatment Protocol
The orthodontist provides interceptive treatment during the mixed dentition in association with bone grafting and carries out definitive orthodontic treatment in conjunction with orthognathic surgery, when indicated. From the mixed dentition phase, the cleft orthodontist should recognize the patient with UCLP who may require orthognathic surgery.* The institution of extensive camouflage (dental compensatory) treatment is likely to jeopardize periodontal health and lead to late dental relapse. Proceeding with a compromised (camouflage) orthodontic approach should only be entered into with full disclosure to the family and other treating clinicians.
Before orthognathic surgery, a speech pathologist evaluates the patient to characterize VP function and to identify articulation errors that result from the cleft palate jaw deformity and the dental malocclusion (see Chapter 8). A baseline evaluation is important, because VP function may deteriorate after maxillary advancement. A nasoendoscopic guided speech assessment is useful to provide maximum objective data.180 VP closure that is adequate before surgery may become borderline afterward, and VP closure that is borderline may become inadequate. Studies document that only a small percentage of patients require a primary pharyngeal flap or flap revision after maxillary advancement.19,20,57,60,79,86,91,95,102,125,170,180 Articulatory distortions that result from malocclusion are also identified and cause-and-effect relationships are determined. The successful orthodontic and surgical correction of crossbites, open bite, cleft–dental gaps, negative overjet, and residual oronasal fistulas represents the most effective way to correct the identified articulation distortions (see Chapter 8). Unfortunately, the use of “oral–motor therapy” is still often applied to manage both jaw-deformity–related articulation errors and VP insufficiency; however, this type of treatment has not been proven beneficial for dynamic speech.
A thorough evaluation of the upper airway is conducted to assess for areas of obstruction (see Chapter 10). Studies suggest an increased prevalence of sleep-disordered breathing and obstructive sleep apnea in patients with cleft palate, especially in the presence of Robin sequence. A formal sleep study (i.e., an attended polysomnogram) is performed if there is a suggestion of obstructive sleep apnea (see Chapter 26). If indicated, simultaneous intranasal procedures (e.g., septoplasty; reduction of the inferior turbinates; recontouring of the nasal apertures, the floor of the nose, and the anterior nasal spine) should be carried out at the time of orthognathic surgery (see Chapters 10 and 15).150
Timing of Orthognathic Surgery
Definitive correction of the jaw deformity is best carried out when the skeleton is mature and before the patient finishes high school.82,90 Maxillofacial growth is generally complete between the ages of 14 and 16 years in girls and 16 and 18 years in boys. However, skeletal growth is variable and may be further gauged by an analysis of sequential lateral cephalometric radiographs taken at 6-month intervals (see Chapter 17). The patient’s and the family’s preferences for the timing of the operation on the basis of psychosocial and functional needs are also taken into account.
As early as 1986, investigators showed that, if maxillary advancement is performed during the mixed dentition in a patient with a cleft palate, then another orthognathic procedure will be necessary for definitive correction after skeletal maturity is reached.181–184 All research to date indicates that a Le Fort I osteotomy carried out during the mixed dentition in a patient with clefting—whether with standard or distraction osteogenesis (DO) techniques—results in no further horizontal maxillary growth (see the controversies section later in this chapter).21,41,40,75
Residual Deformities in the Adolescent with Unilateral Cleft Lip and Palate
Correction of the residual skeletal, soft-tissue, and dental deformities in the adolescent patient with UCLP challenges the ingenuity and skill of the orthognathic surgeon and the cleft team. The central deformity is maxillary hypoplasia (Figs. 32-7, 32-8, and 32-9), and it is frequently combined with residual oronasal fistula, bone defects, intranasal obstruction, soft-tissue scarring, and, occasionally, VP dysfunction (Figs. 32-10 through 32-21).84,167 In addition, the maxillary lateral incisor at the cleft site is usually congenitally absent or deficient, thereby resulting in a cleft–dental gap. Secondary deformities of the nose, the mandible, and the chin region are also common.
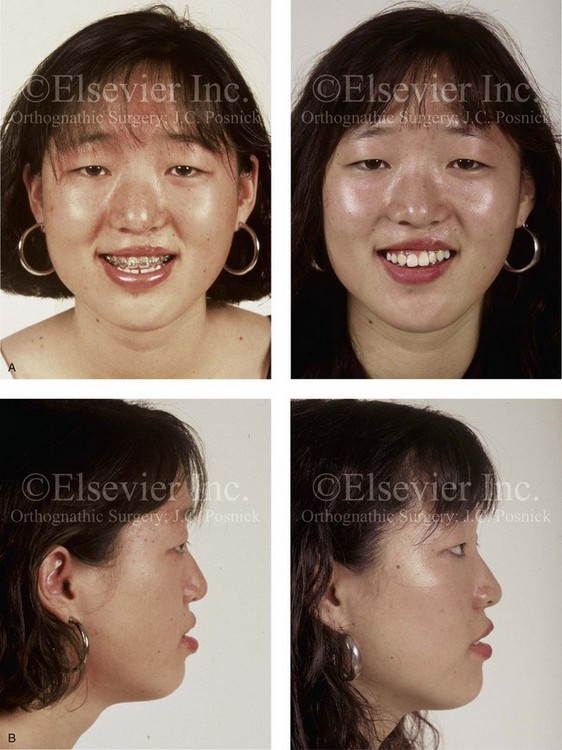
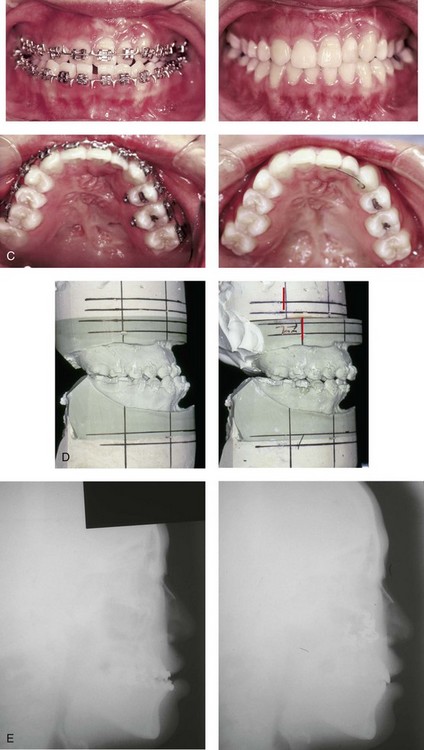
Figure 32-7 A 19-year-old woman was born with UCLP on the left side and underwent lip and palate repair followed by effective bone grafting and fistula closure during the mixed dentition. She has a useful lateral incisor at the cleft site but is missing the lateral incisor on the non-clefted side. A bicuspid has also been removed on the cleft side. As a teenager, she was referred to this surgeon and underwent a combined orthodontic and orthognathic surgical approach. The procedure included a standard Le Fort I osteotomy (horizontal advancement, vertical lengthening) and interpositional grafting. A, Frontal views with smile before and after reconstruction. B, Profile views before and after reconstruction. C, Occlusal and palatal views before and after reconstruction. D, Articulated dental casts that indicate analytic model planning. E, Lateral cephalometric radiographs before and after reconstruction. Note that, by limiting surgery to the maxilla, the cant (which also involved the mandible) cannot be corrected.
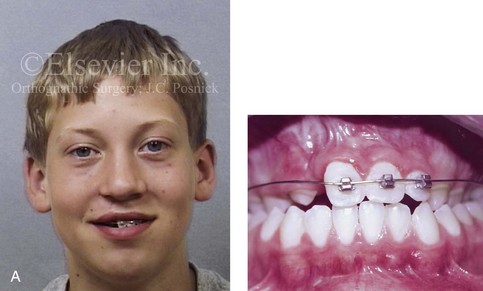
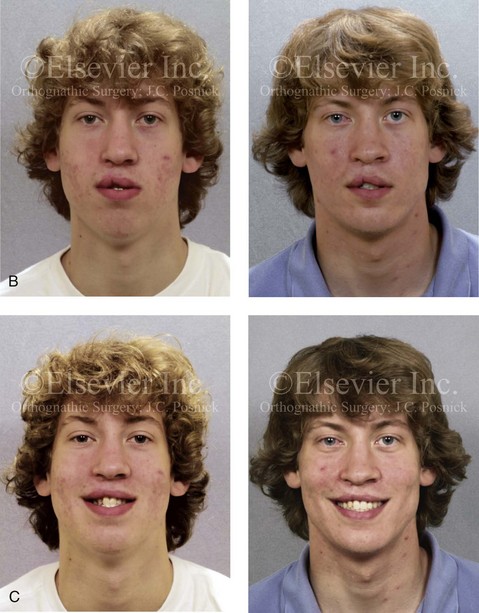
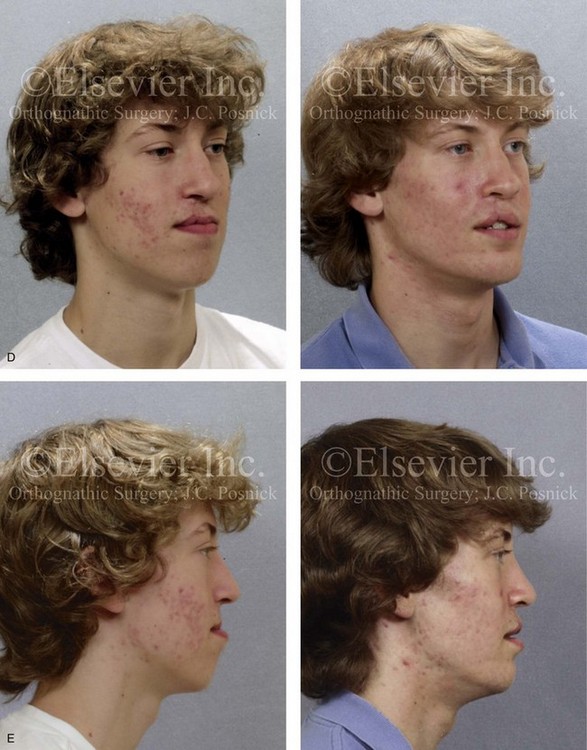
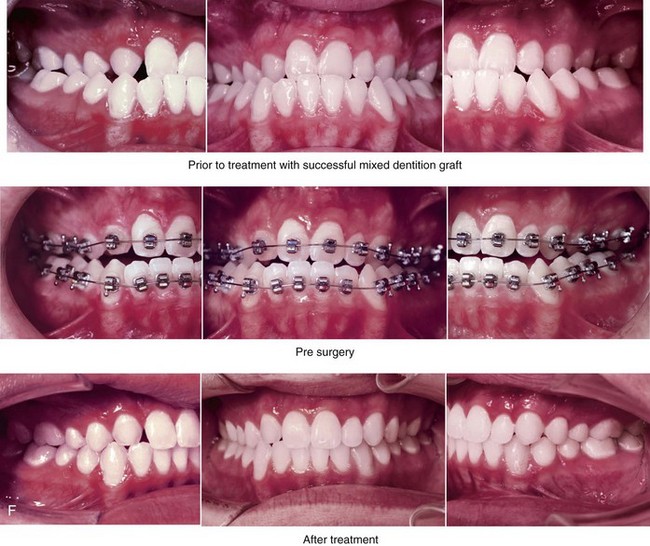
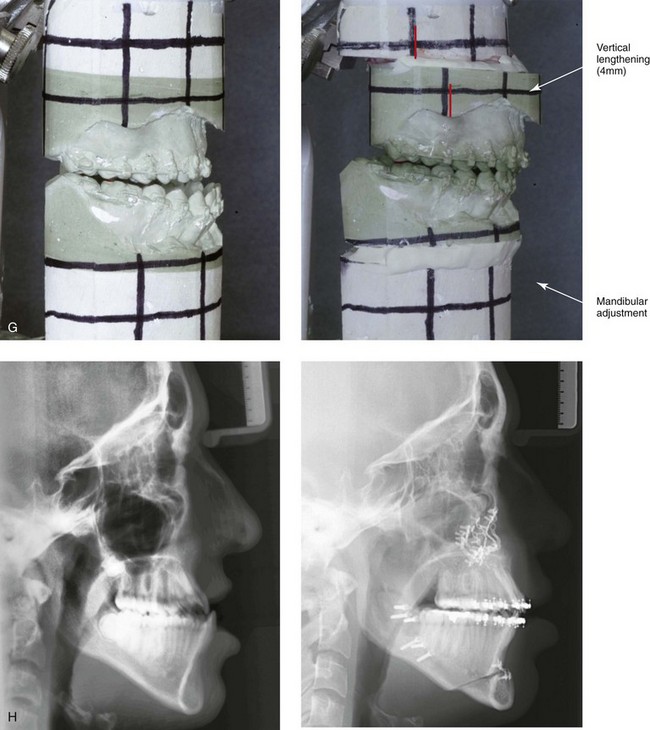
Figure 32-8 A 20-year-old man was born with UCLP on the right side. Primary lip and palate repair were carried out at another institution. He was referred to this surgeon and underwent successful bone grafting and fistula closure during the mixed dentition. He underwent orthodontic closure of the cleft–dental gap and limited alignment by the time he was 14 years old. Final orthodontic decompensation was later carried out in combination with orthognathic surgery when he was 19 years old. The patient’s procedures included maxillary Le Fort I osteotomy (horizontal advancement, vertical shortening, midline correction, cant correction, and clockwise rotation) with interpositional grafting; sagittal split ramus osteotomies (counterclockwise rotation and asymmetry correction); osseous genioplasty (horizontal advancement); and septoplasty, inferior turbinate reduction, and recontouring of the floor of the nose. A, The patient is shown during the mixed dentition after successful bone grafting and fistula closure. B, Frontal views in repose before and after reconstruction. C, Frontal views with smile before and after reconstruction. D, Oblique facial views before and after reconstruction. E, Profile views before and after reconstruction. F, Occlusal views before definitive orthodontics, with decompensation in progress before surgery, and after reconstruction. G, Articulated dental casts that indicate analytic model planning. H, Lateral cephalometric radiographs before and after reconstruction.
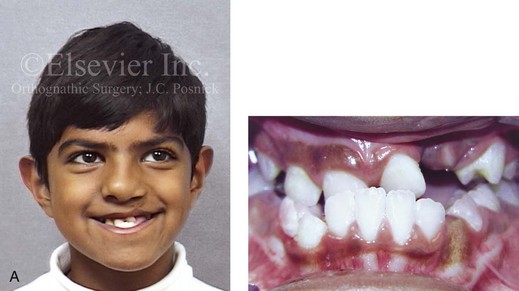

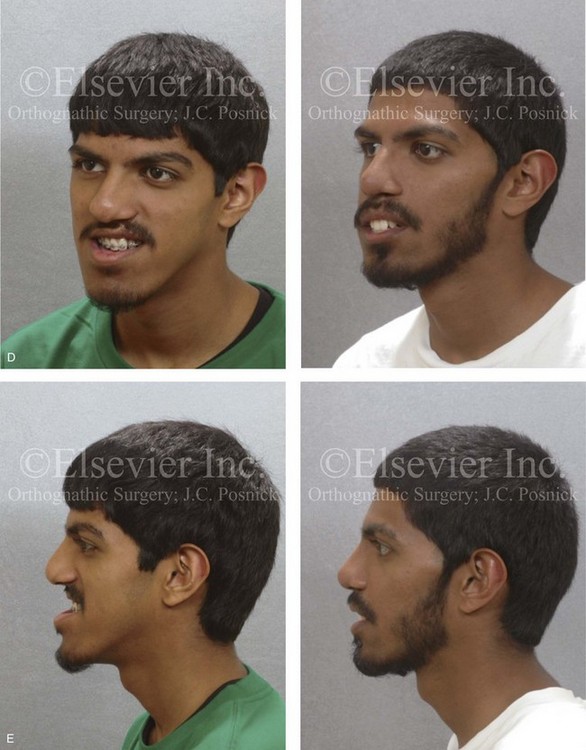
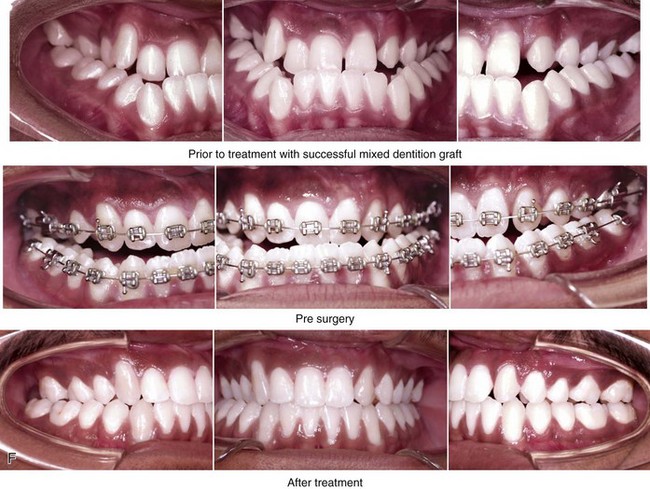
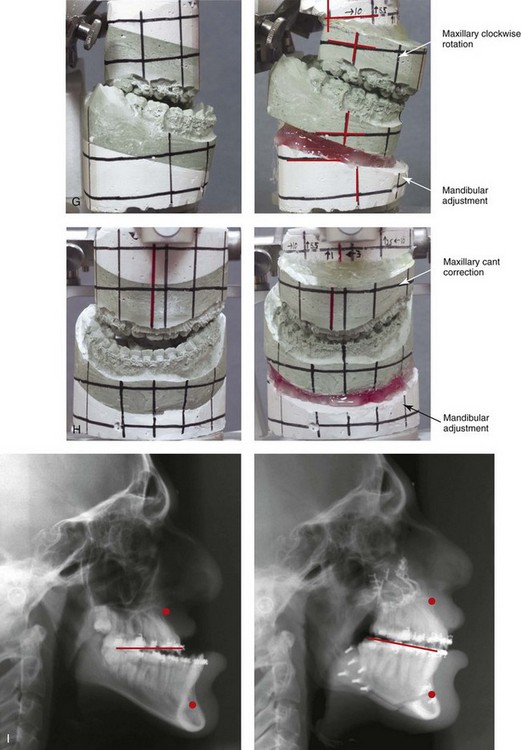
Figure 32-9 A high school senior was born with a complete UCLP on the left side. He underwent lip and palate repair at another institution. He was then referred to this surgeon and underwent successful bone grafting and fistula closure during the mixed dentition. He developed a jaw deformity that was characterized by maxillary deficiency and secondary deformities of the mandible and the intranasal cavity. He underwent a combined orthodontic and surgical approach. Orthodontic decompensation included cleft–dental gap closure (absent lateral incisor). He then underwent jaw reconstruction. The patient’s procedures included maxillary Le Fort I osteotomy (horizontal advancement, vertical shortening, midline correction, cant correction, and clockwise rotation) with interpositional grafting; sagittal split ramus osteotomies (clockwise rotation and asymmetry correction); osseous genioplasty (vertical shortening); and septoplasty, inferior turbinate reduction, and recontouring of the floor of the nose. Six months after successful orthognathic surgery, the patient underwent cleft rhinoplasty including rib cartilage (caudal strut) grafting. A, The patient is shown during the mixed dentition before bone grafting and fistula closure. B, Frontal views in repose before and after reconstruction. C, Frontal views with smile before and after reconstruction. D, Oblique facial views before and after reconstruction. E, Profile views before and after reconstruction. F, Occlusal views before definitive orthodontics, with orthodontic dental decompensation in progress, and after reconstruction. G & H, Articulated dental casts that indicate analytic model planning. Note that, with the clockwise rotation of the mandible, the pogonion moves posterior while the incisors do not. I, Lateral cephalometric radiographs before and after reconstruction.
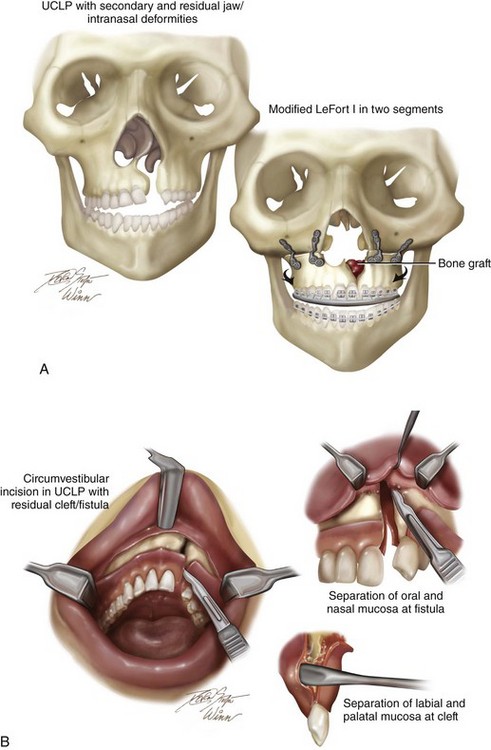
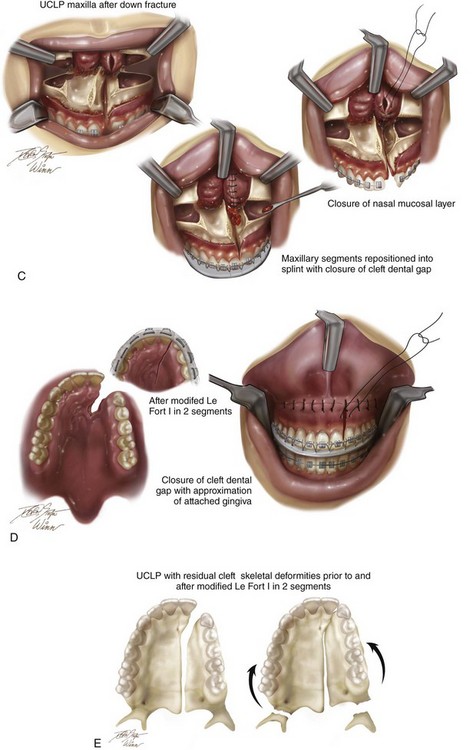
Figure 32-10 Illustrations of modified Le Fort I osteotomy in two segments as carried out in a patient with UCLP who did not undergo successful grafting during the mixed dentition and who presents as an adult with a jaw deformity. A, Frontal view of maxillofacial skeleton before and after Le Fort I osteotomy in two segments. The inferior turbinates have been reduced, and a submucous resection of the deviated septum has been performed. The nasal floor and the nasal spine have been recontoured with a rotary drill. Cancellous iliac bone graft has also been placed along the cleft nasal floor. Corticocancellous iliac graft will also be placed in gaps along the anterior maxilla on each side. B, Circumvestibular and perifistular incisions for exposure to complete osteotomies for down-fracture and later fistula closure. C, Down-fractured Le Fort I osteotomy in two segments after the submucosal resection of the septum, the reduction of the inferior turbinates through the nasal mucosa opening, and then watertight nasal-side closure. D, Wound closure of both the labial and palatal aspects after differential segmental repositioning. E, Palatal view of bony segments before and after repositioning. Parts A-E modified from an original illustration by Bill Winn.
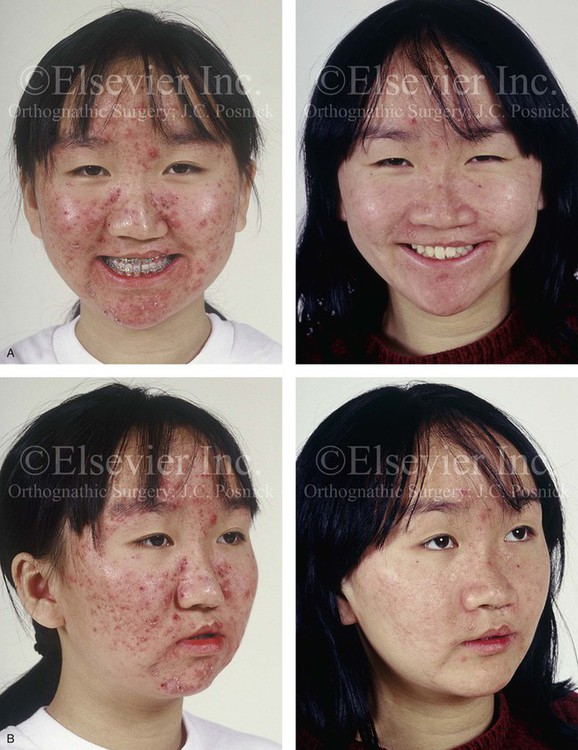
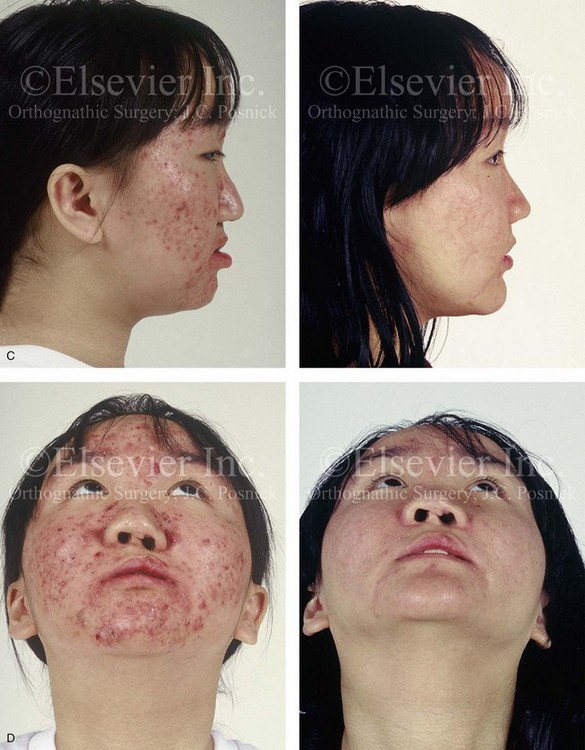
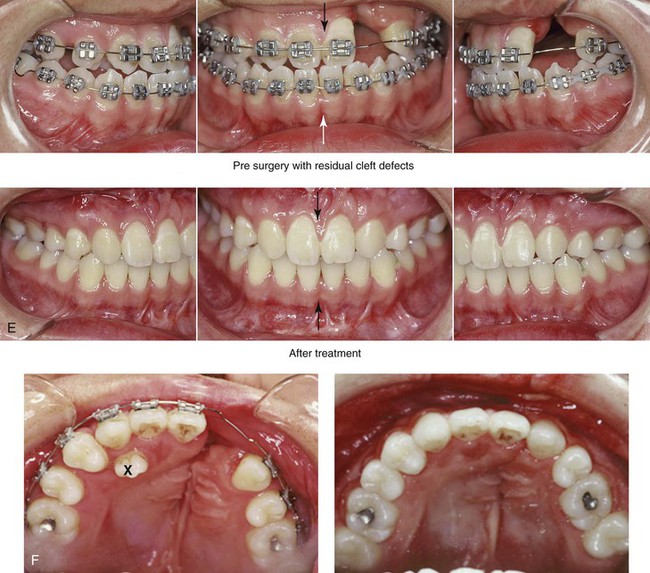
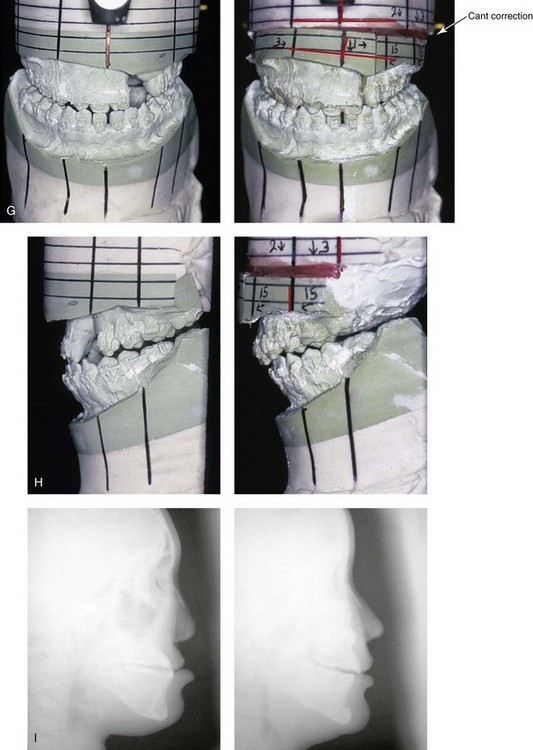
Figure 32-11 A 17-year-old girl who was born with UCLP. She underwent lip and palate repair during childhood, but she did not undergo effective bone grafting during the mixed dentition. A useful lateral incisor is not present at the cleft site. She was referred to this surgeon and underwent a comprehensive orthodontic and orthognathic surgical approach. The patient’s procedures included a modified Le Fort I osteotomy in two segments (differential repositioning of the segments) with interpositional grafting as well as closure of the oronasal fistula, the cleft–dental gap, and the alveolar defect; an osseous genioplasty (vertical reduction and horizontal advancement); and septoplasty, inferior turbinate reduction, nasal floor recontouring. A, Frontal views with smile before and after reconstruction. B, Oblique views before and after reconstruction. C, Profile views before and after reconstruction. D, Worm’s-eye views before and after reconstruction. E, Occlusal views with orthodontics in progress and after reconstruction. F, Palatal views with orthodontics in progress and after reconstruction. Note: The maxillary dentition is reconstructed with only eleven teeth. This provides a low maintenance dentition for the patient going forward. G and H, Views of articulated dental casts that indicate analytic model planning. I, Lateral cephalometric radiographs before and after reconstruction. A, E (top Center), E (bottom center), F, From Posnick JC, Tompson B: Modification of the maxillary Le Fort I osteotomy in cleft–orthognathic surgery: the unilateral cleft lip and palate deformity, J Oral Maxillofac Surg 50:666-675, 1992.
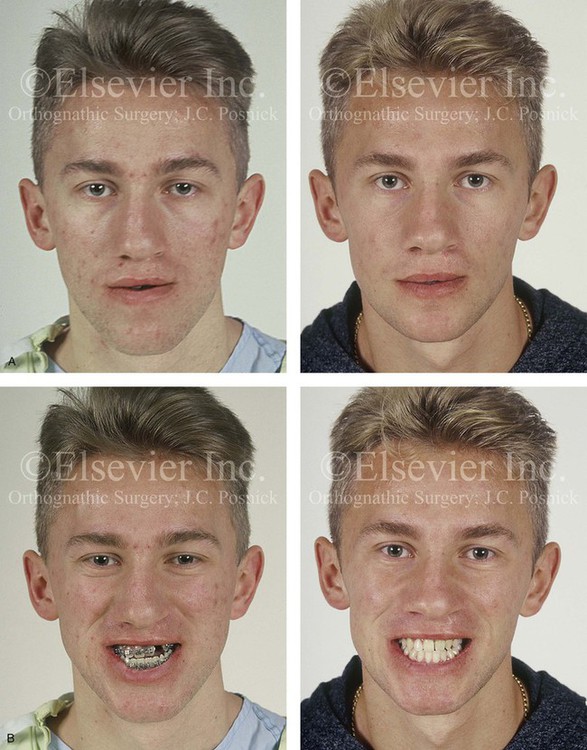
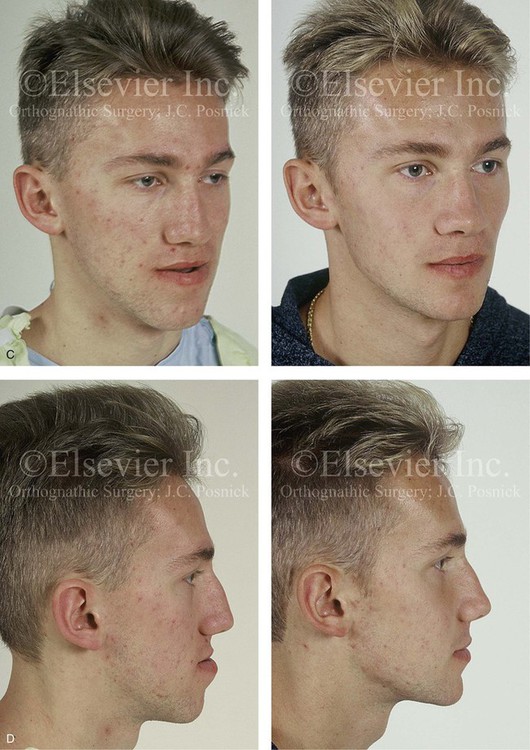
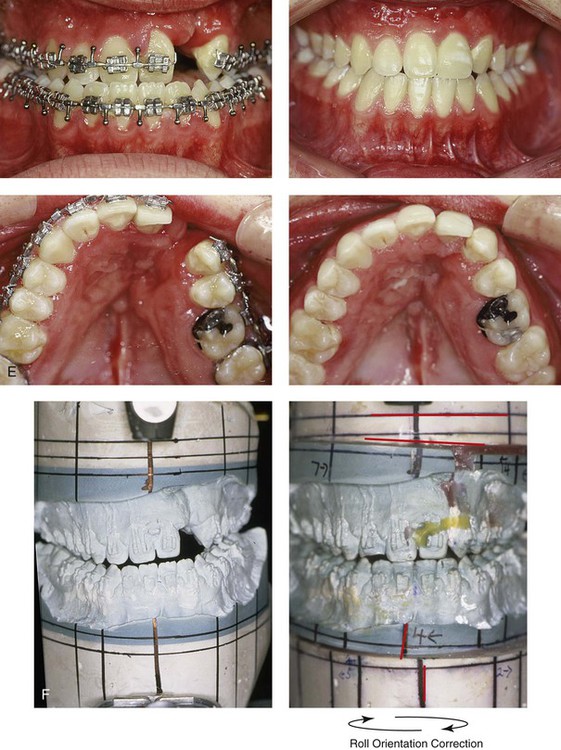
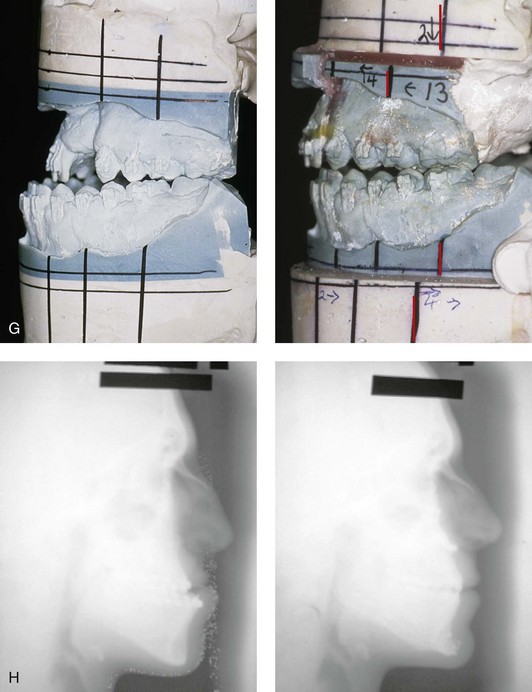
Figure 32-12 A 19-year-old man who was born with UCLP. He underwent lip and palate repair during childhood, but he did not undergo effective bone grafting during the mixed dentition. A useful lateral incisor is not present at the cleft site. He was referred to this surgeon and underwent a combined orthodontic and orthognathic surgical approach. The patient’s procedures included a modified Le Fort I osteotomy in two segments (differential repositioning of the segments) with interpositional grafting, the correction of occlusal canting, and the closure of the oronasal fistula, the alveolar defect, and the cleft–dental gap; bilateral sagittal ramus osteotomies (correction of asymmetry); osseous genioplasty (vertical reduction and horizontal advancement); and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A, Frontal views in repose before and after reconstruction. B, Frontal views with smile before and after reconstruction. C, Oblique views before and after reconstruction. D, Profile views before and after reconstruction. E, Occlusal and palatal views with orthodontics in progress and after reconstruction. F and G, Articulated dental casts that indicate analytic model planning. H, Lateral cephalometric radiographs before and after reconstruction. B, D, E, From Posnick JC, Tompson B: Modification of the maxillary Le Fort I osteotomy in cleft–orthognathic surgery: the unilateral cleft lip and palate deformity, J Oral Maxillofac Surg 50:666-675, 1992.

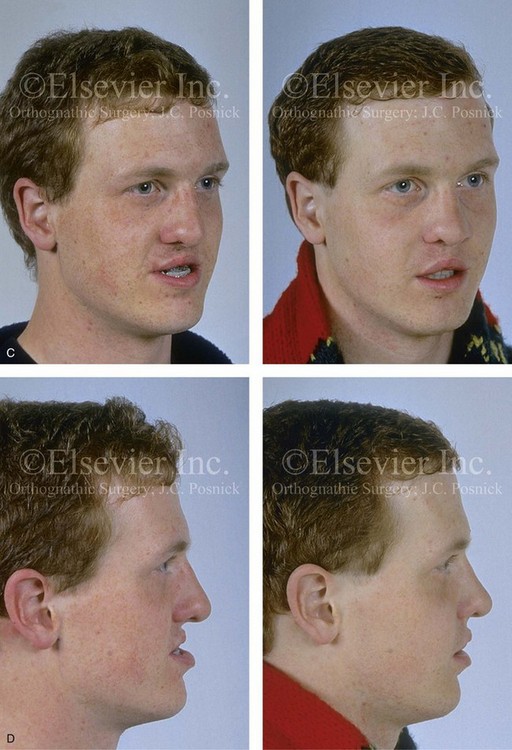
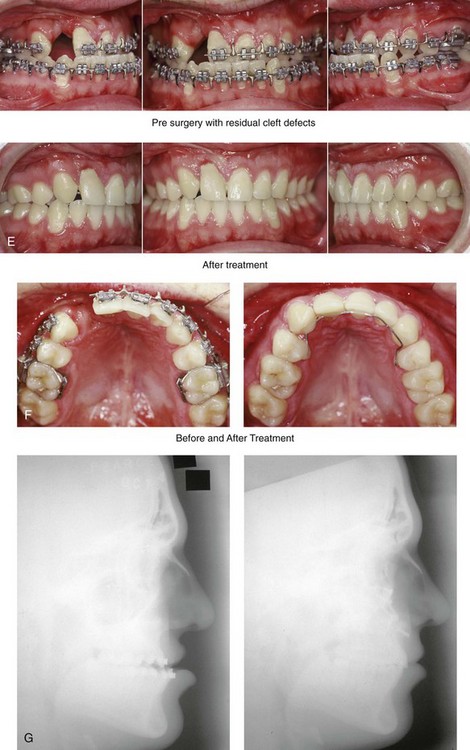
Figure 32-13 A 17-year-old boy who was born with UCLP. He underwent lip and palate repair during childhood, but he did not undergo effective bone grafting during the mixed dentition. The lateral incisor is not present at the cleft site. He was referred to this surgeon as a teenager and underwent a combined orthodontic and orthognathic surgical approach. The patient’s procedures included a modified Le Fort I osteotomy in two segments (differential repositioning of the segments) with interpositional grafting and closure of the oronasal fistula, the alveolar defect, and the cleft–dental gap; and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A, Frontal views in repose before and after reconstruction. B, Frontal views with smile before and after reconstruction. C, Oblique views before and after reconstruction. D, Profile views before and after reconstruction. E, Occlusal views with orthodontics in progress and after reconstruction. F, Palatal views with orthodontics in progress and after reconstruction. G, Lateral cephalometric radiographs before and after reconstruction. B, D, E, F, From Posnick JC, Dagys AP: Skeletal stability and relapse patterns after Le Fort I maxillary osteotomy fixed with miniplates: the unilateral cleft lip and palate deformity, Plast Reconstr Surg 94:924-932, 1994.
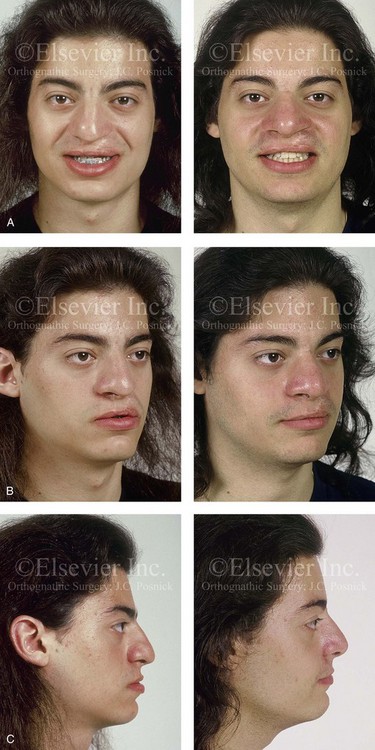
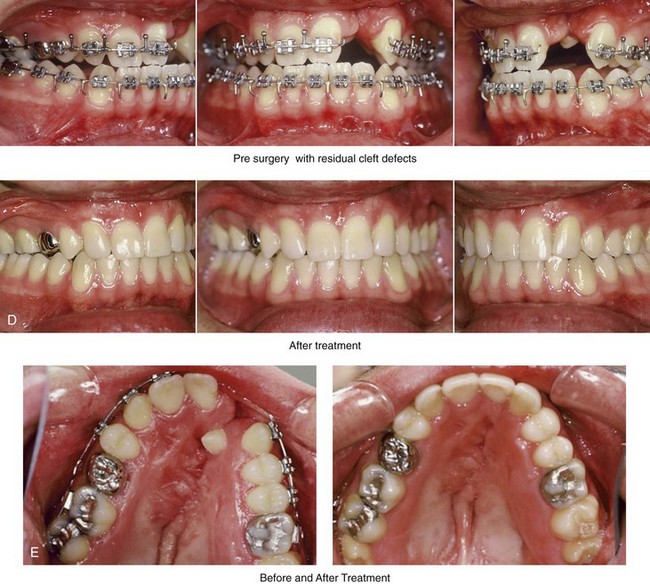
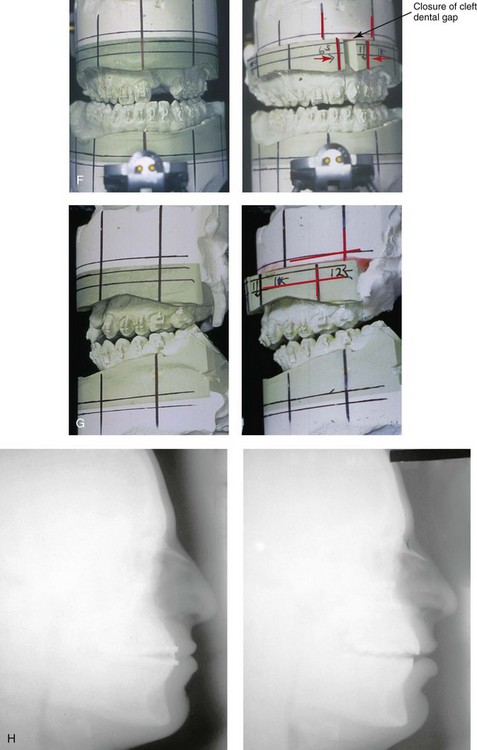
Figure 32-14 A 20-year-old man who was born with UCLP on the left side. He underwent lip and palate repair during childhood, but he did not undergo effective bone grafting during the mixed dentition. A useful lateral incisor is not present at the cleft site. He has a retained primary molar and an absent lateral incisor on the non-cleft side. He was referred to this surgeon as a young adult and underwent a combined orthodontic and orthognathic surgical approach. The patient’s procedures included a modified Le Fort I osteotomy in two segments (differential repositioning of the segments) with interpositional grafting and closure of the oronasal fistula, the alveolar defect, and the cleft–dental gap; and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A, Frontal views with smile before and after reconstruction. B, Oblique views before and after reconstruction. C, Profile views before and after reconstruction. D, Occlusal views with orthodontics in progress and after reconstruction. E, Palatal views with orthodontics in progress and after reconstruction. F and G, Articulated dental casts that indicate analytic model planning. H, Lateral cephalometric radiographs before and after reconstruction. A, C, D, F, From Posnick JC, Dagys AP: Skeletal stability and relapse patterns after Le Fort I maxillary osteotomy fixed with miniplates: the unilateral cleft lip and palate deformity, Plast Reconstr Surg 94:924-932, 1994.
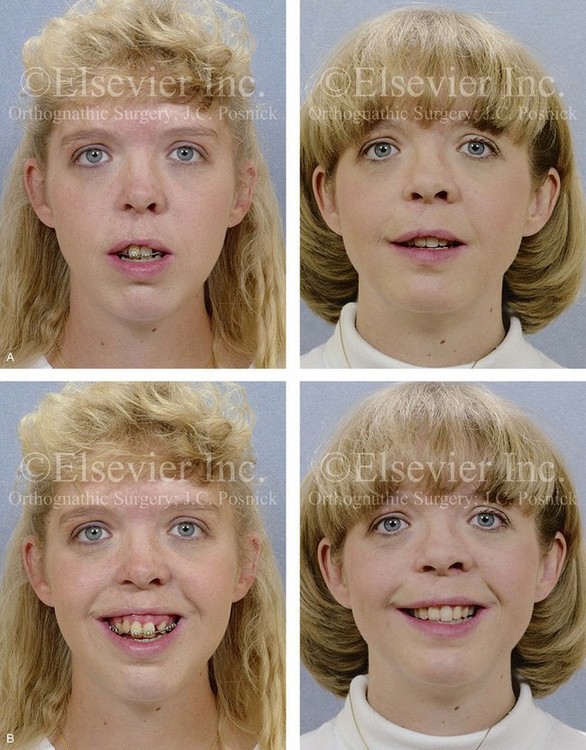
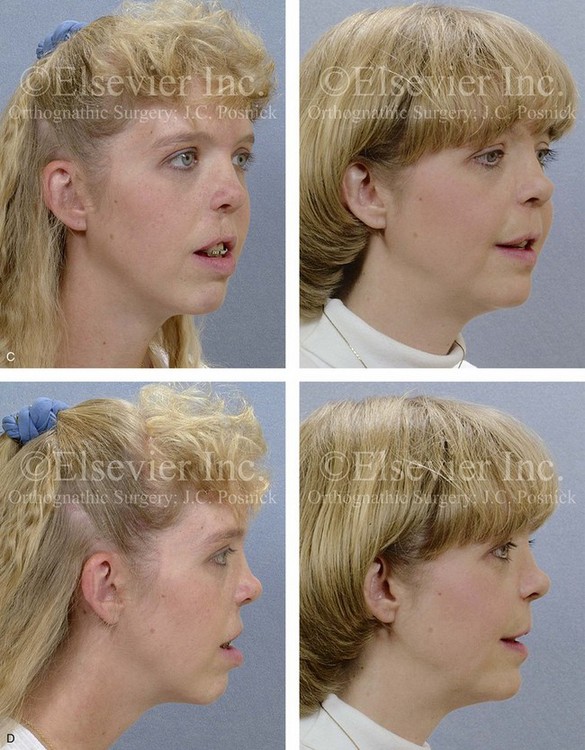
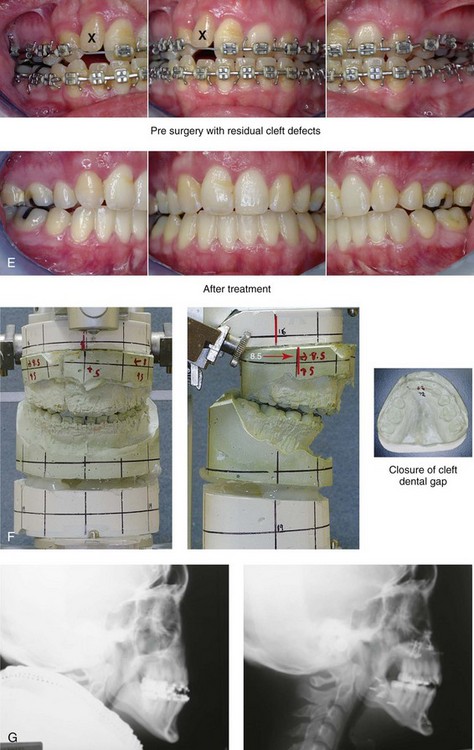
Figure 32-15 A 24-year-old schoolteacher who was born with UCLP. She underwent 4 bicuspid extractions as part of earlier orthodontic treatment. The retained lateral incisor at the cleft does not have an adequate root and required extraction. She was referred to this surgeon as an adult and underwent a combined orthodontic and orthognathic surgical approach. The patient’s procedures included a modified Le Fort I osteotomy in two segments (differential repositioning of the segments) and closure of the oronasal fistula, the alveolar cleft, and the cleft–dental gap; bilateral sagittal split ramus osteotomies; osseous genioplasty; and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A, Frontal views in repose before and after reconstruction. B, Frontal views with smile before and after reconstruction. C, Oblique views before and after reconstruction. D, Profile views before and after reconstruction. E, Occlusal views with orthodontics in progress and after reconstruction and dental rehabilitation. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and after reconstruction.
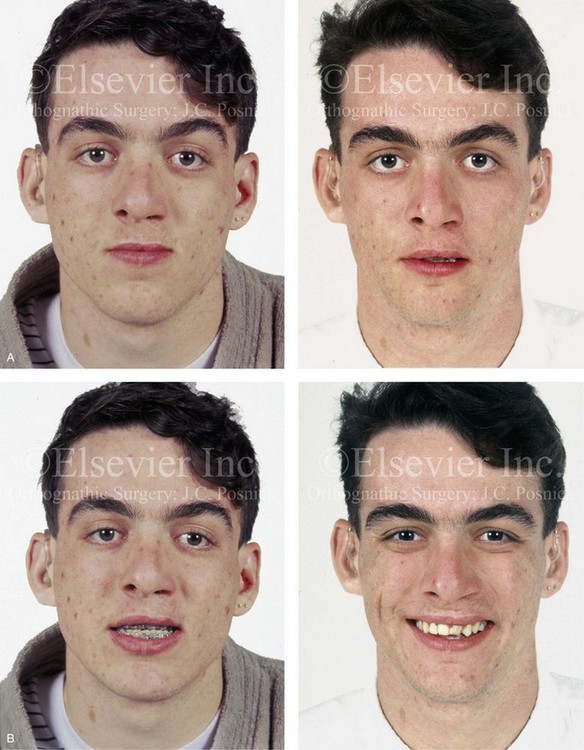
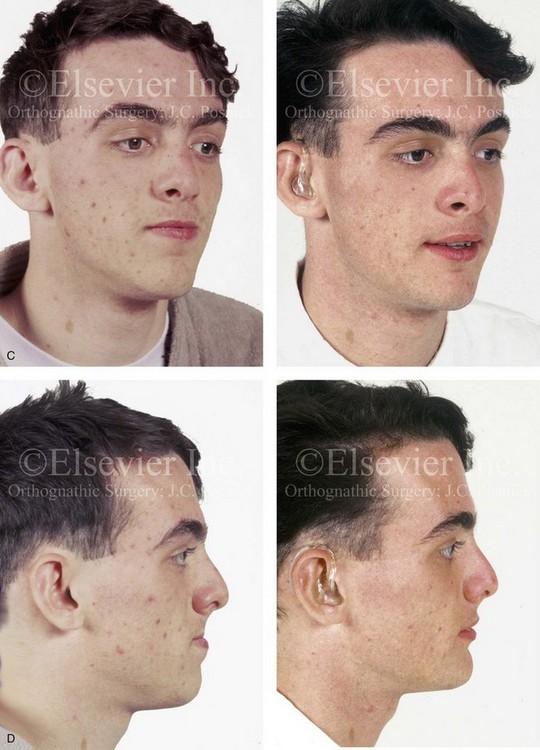
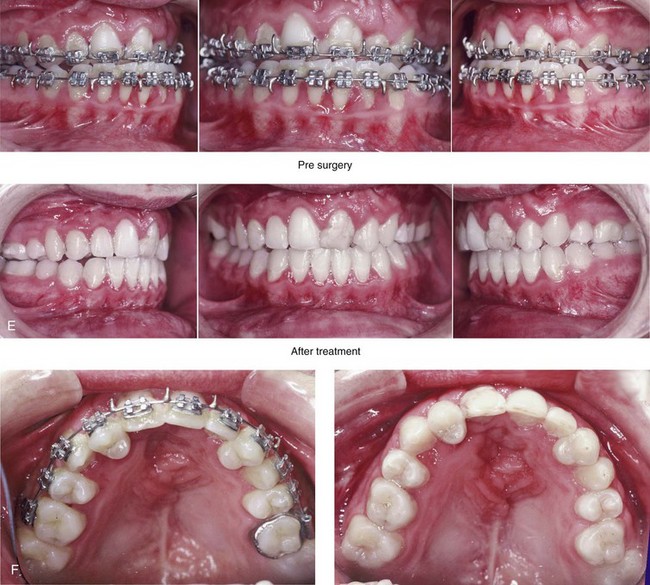
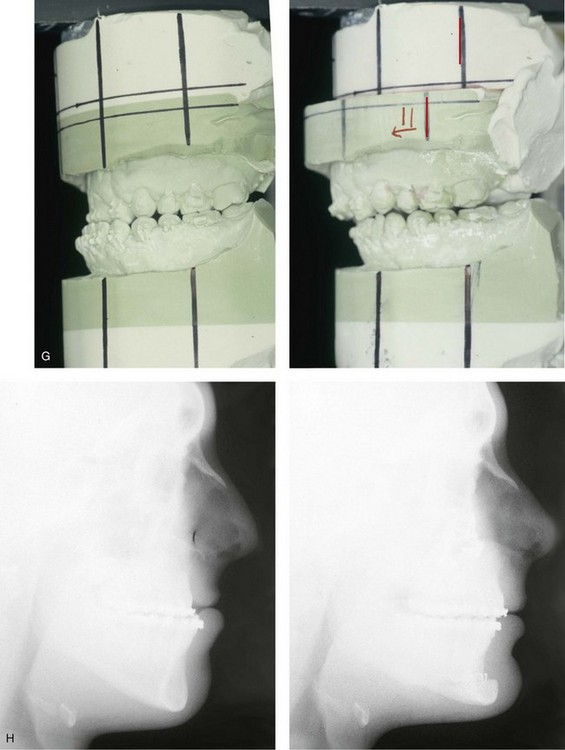
Figure 32-16 A 17-year-old boy who was born with UCLP. He was referred to this surgeon during the late mixed dentition and underwent effective bone grafting. He was congenitally missing his lateral incisor at the cleft site. There is transposition of the canine and the first bicuspids on each side of the upper jaw. He underwent a combined orthodontic and orthognathic surgical approach. The patient’s procedures included a standard Le Fort I osteotomy (horizontal advancement) with interpositional grafting; osseous genioplasty (vertical reduction and horizontal advancement); and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A, Frontal views in repose before and after reconstruction. B, Frontal views with smile before and after reconstruction. C, Oblique views before and after reconstruction. D, Profile views before and after reconstruction. E, Occlusal views with orthodontics in progress and after reconstruction. F, Palatal views with orthodontics in progress and after reconstruction. G, Articulated dental casts that indicate analytic model planning. H, Lateral cephalometric radiographs before and after reconstruction. Note that cosmetic recontouring and modification of the maxillary dental crowns would be beneficial.
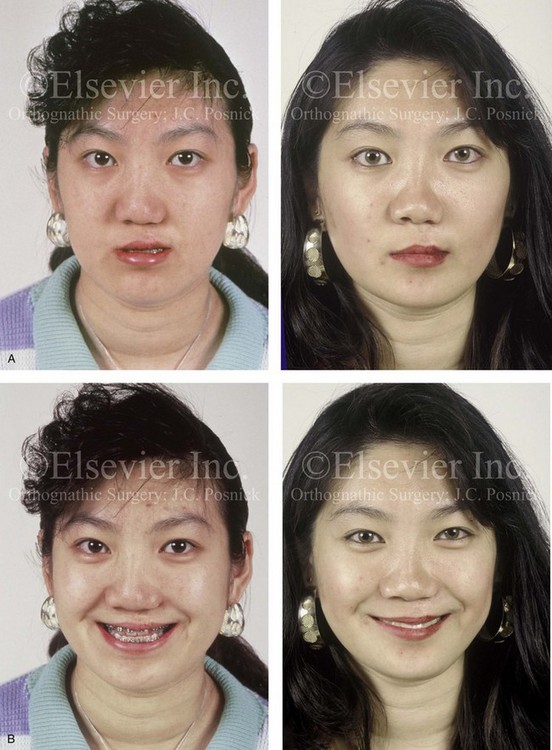
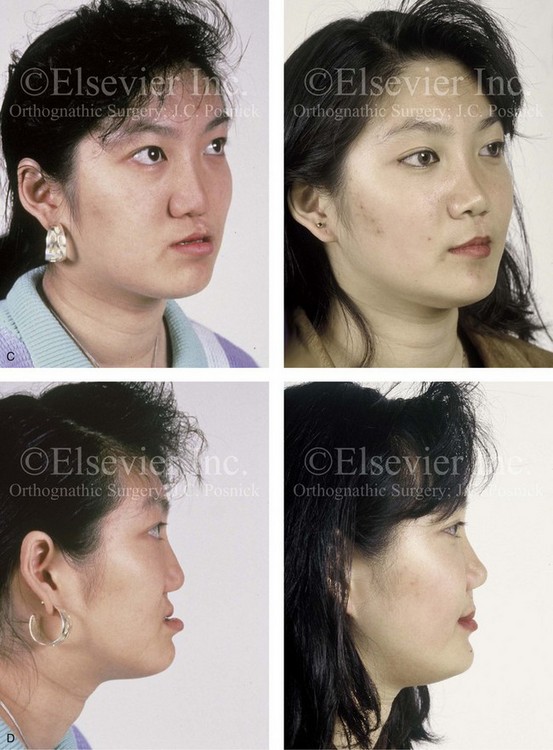
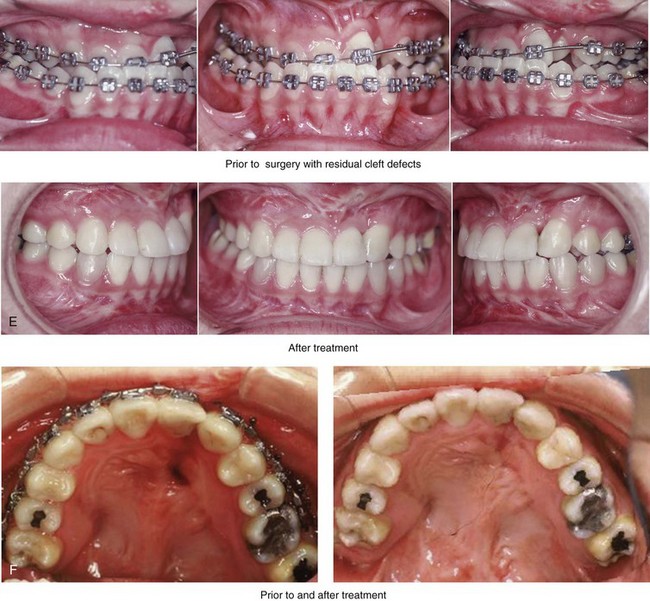
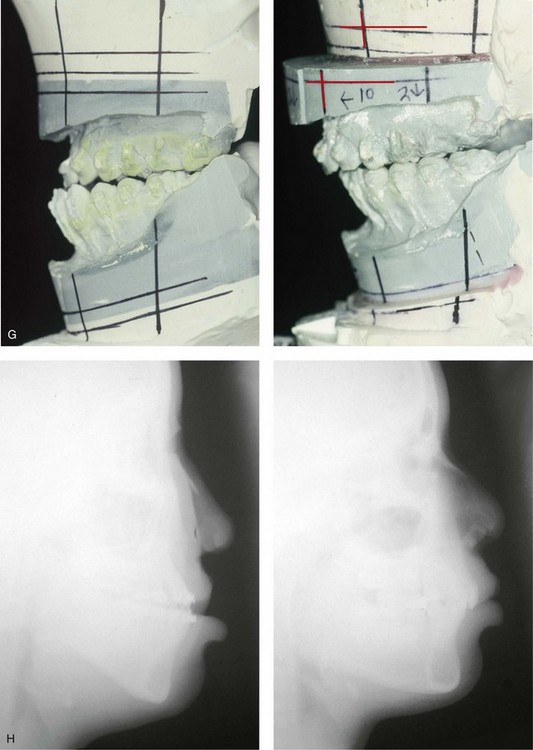
Figure 32-17 A 16-year-old girl who was born with UCLP. She was referred to this surgeon and underwent a combined orthodontic and orthognathic surgical approach. The patient’s procedures included a modified Le Fort I osteotomy in two segments (differential repositioning of the segments) and the closure of the oronasal fistula, the alveolar defect, and the cleft–dental gap; and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A and B, Frontal views in repose before and after reconstruction. B, Frontal views with smile before and after reconstruction. C and D, Oblique facial views before and after reconstruction. D, Profile views before and after reconstruction. E, Occlusal views with orthodontics in progress and after reconstruction. F, Palatal views with orthodontics in progress and after reconstruction. G, Articulated dental casts that indicate analytic model planning. H, Lateral cephalometric radiographs before and after reconstruction. From Posnick JC: Orthognathic surgery in the cleft patient. In Russell RC, ed: Instructional courses, Plastic Surgery Educational Foundation, vol 4, St. Louis, Mo, 1991, Mosby–Year Book, p 129.
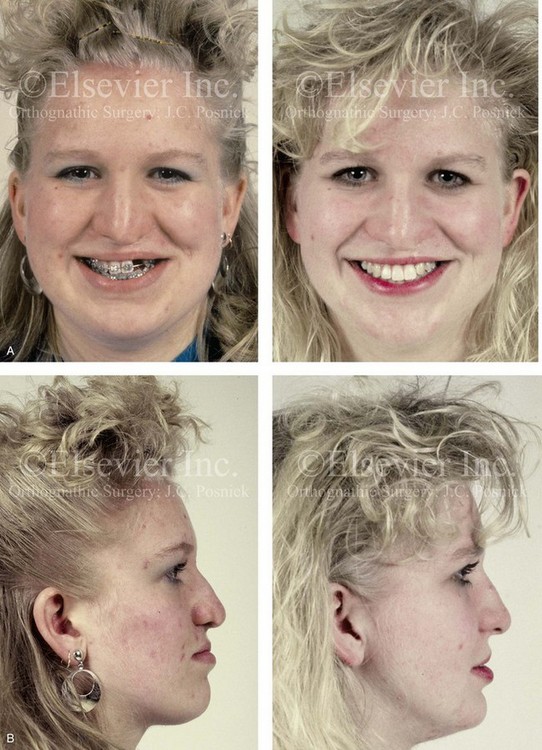
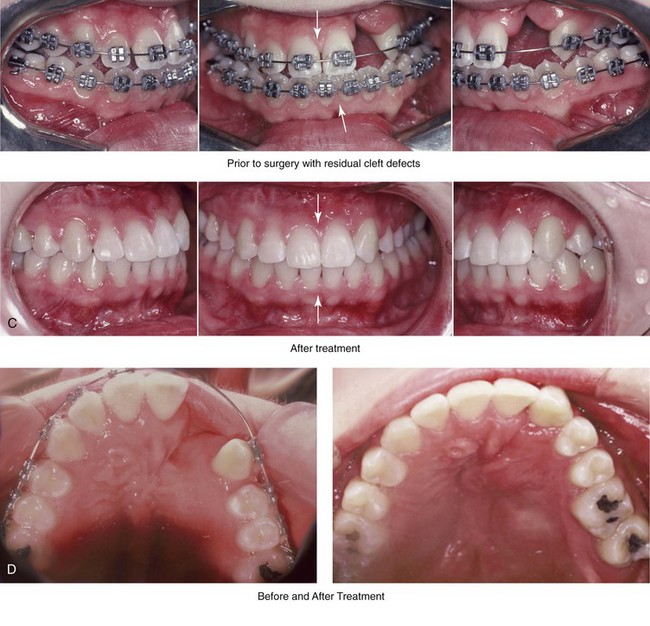
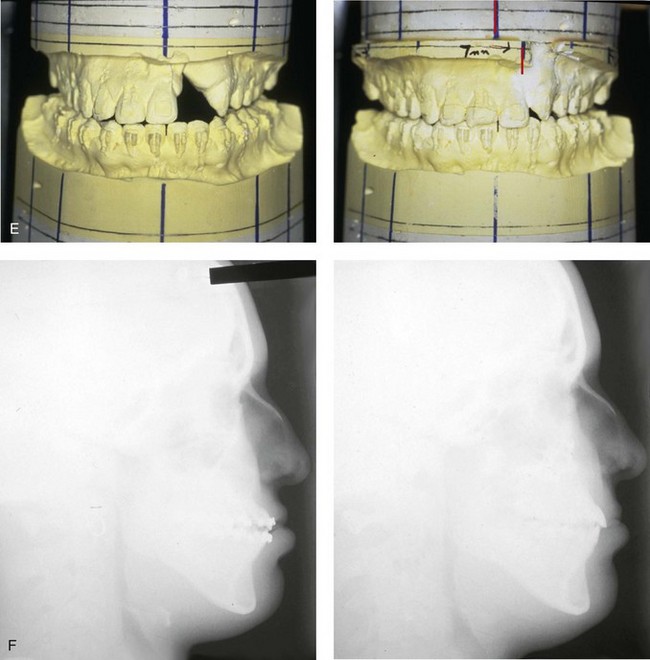
Figure 32-18 A 24-year-old woman who was born with UCLP. She did not undergo bone grafts in the mixed dentition. She is missing the lateral incisor on the cleft side. She was referred to this surgeon as an adult and underwent a combined orthodontic and orthognathic surgical approach. The patient’s procedures included a modified Le Fort I osteotomy in two segments (differential repositioning of the segments) and closure of oronasal fistula, the alveolar cleft, and the cleft–dental gap; bilateral sagittal split ramus osteotomies; osseous genioplasty; and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A, Frontal views with smile before and after reconstruction. B, Profile views before and after reconstruction. C, Occlusal views with orthodontics in progress and after reconstruction and dental rehabilitation. D, Palatal views before and after reconstruction. E, Articulated dental casts that indicate analytic model planning. F, Lateral cephalometric radiographs before and after reconstruction. Note that the palatal fistula was successfully closed. Cosmetic modification of the maxillary anterior teeth was recommended.
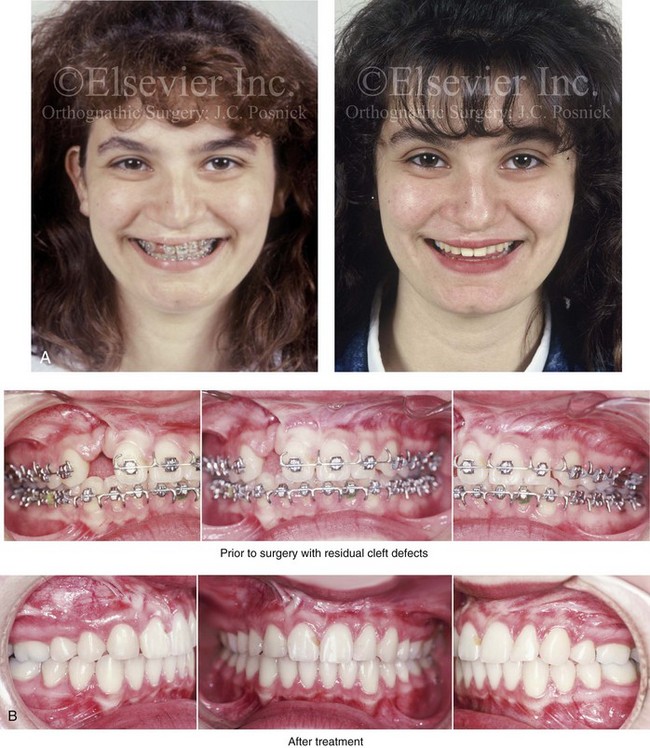
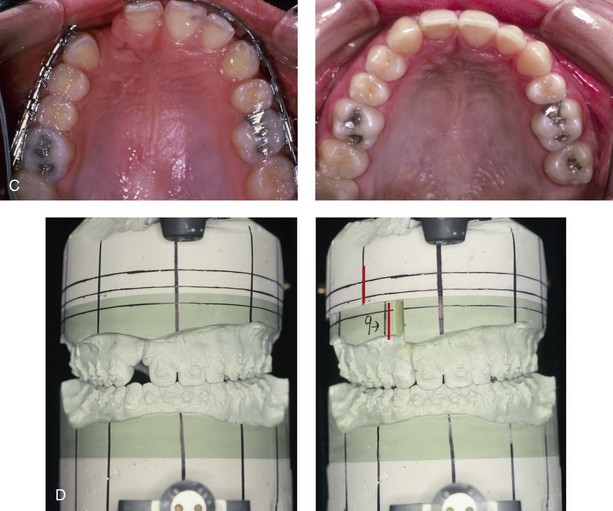
Figure 32-19 A 17-year-old girl who was born with UCLP. She did not undergo successful bone grafting in the mixed dentition. She is missing the lateral incisor on the cleft side. The first bicuspid was extracted on the non-cleft side. She was referred to this surgeon as a teenager and underwent a combined orthodontic and orthognathic surgical approach. The maxilla had good horizontal projection and vertical height. Of the options available to manage the residual upper dentoalveolar needs, a right posterior segmental osteotomy was completed to close the oronasal fistula, the alveolar defect, and the cleft–dental gap. A, Frontal views with smile before and after reconstruction. B, Occlusal views with orthodontics in progress and after reconstruction. C, Palatal views with orthodontics in progress and after reconstruction. D, Articulated dental casts that indicate analytic model planning.
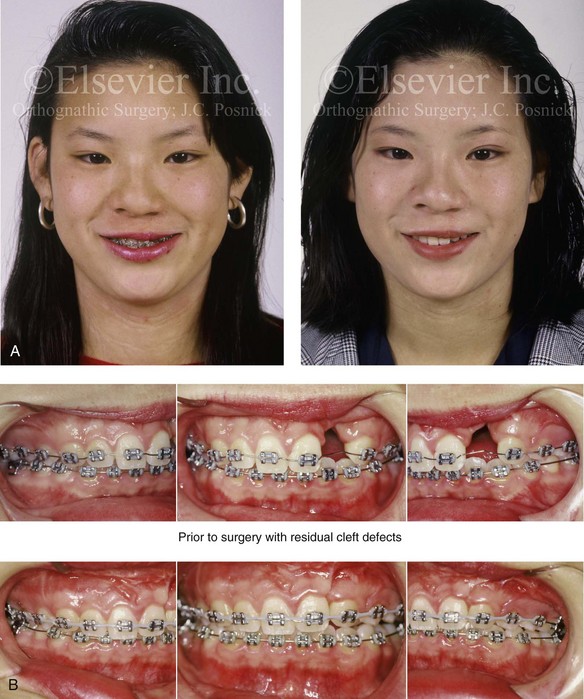
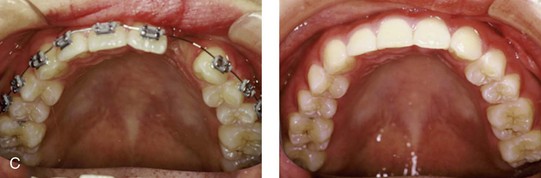
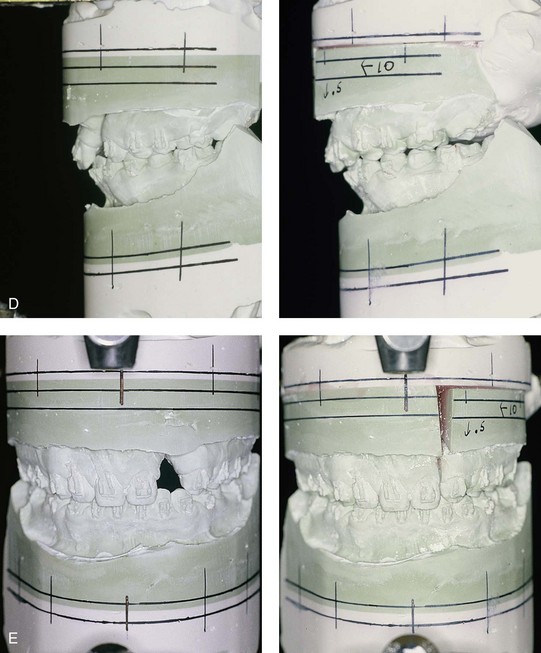
Figure 32-20 A 17-year-old girl who was born with UCLP. She underwent lip and palate repair during childhood, but she did not undergo effective bone grafting during the mixed dentition. The lateral incisor is not present at the cleft site. She was referred to this surgeon and underwent a combined orthodontic and orthognathic surgical approach. The maxilla had good horizontal projection and vertical height. Of the options available to manage the residual upper dentoalveolar needs, a right posterior segmental osteotomy was completed to close the oronasal fistula, the alveolar defect, and the cleft–dental gap. A, Frontal views with smile before and after reconstruction. B, Occlusal views with orthodontics in progress and after reconstruction. C, Palatal views with orthodontics in progress and after reconstruction. D and E, Articulated dental casts that indicate analytic model planning.
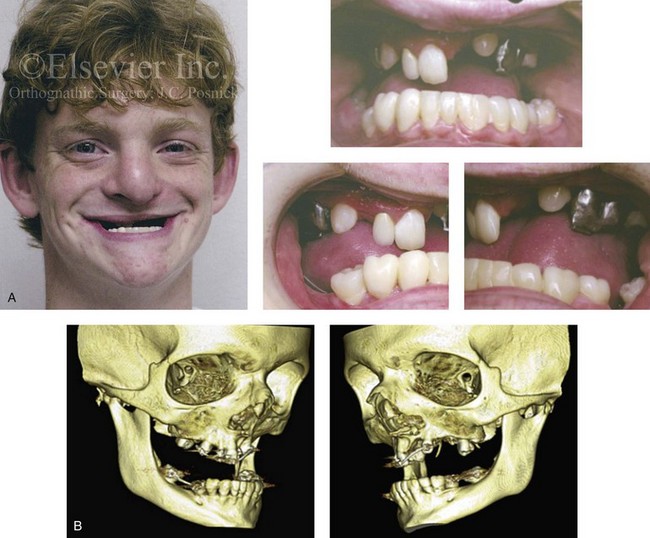
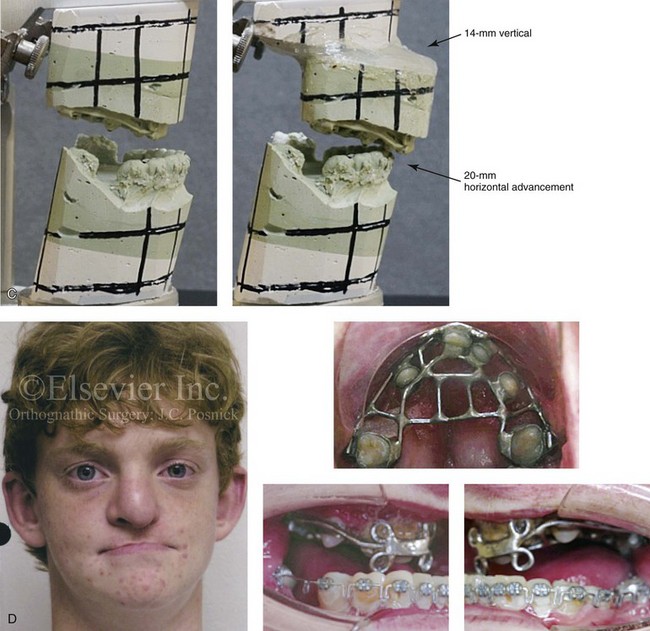
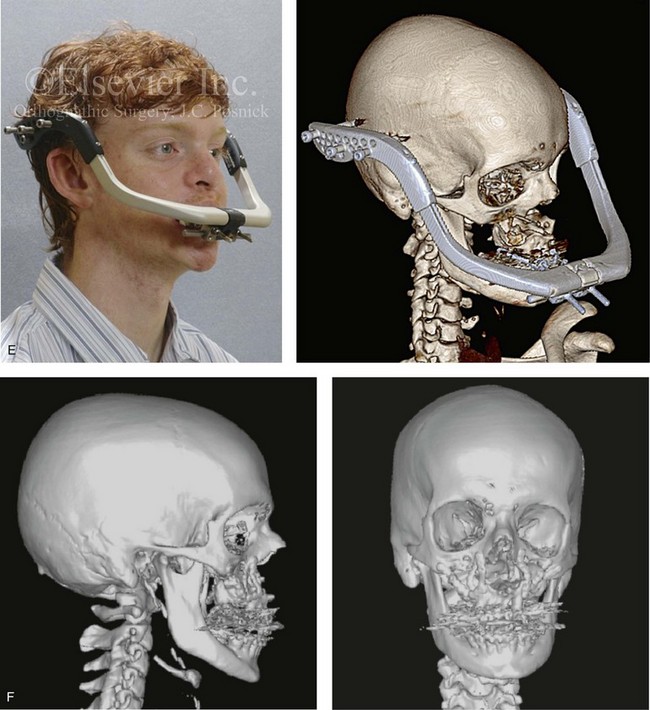
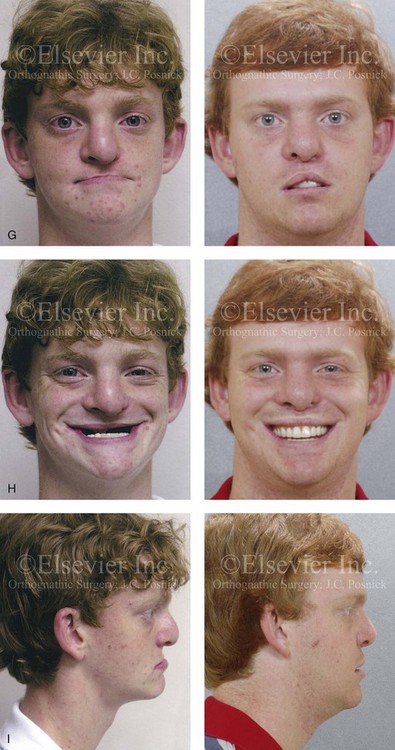
Figure 32-21 A teenage boy who was born with ectodermal dysplasia and complete UCLP on the left side. He underwent lip and palate closure during infancy. There are multiple congenital missing teeth in each arch. He was referred to this surgeon for evaluation when he was 14 years old. He retains only six long-term useful teeth in the maxilla (first and second molars) and displaced left and right canines. The maxilla is vertically and horizontally deficient. The mandible has satisfactory symmetry and horizontal projection. The patient underwent evaluations by specialists, including an orthodontist, a prosthodontist, a periodontist, a surgeon, a speech pathologist, an otolaryngologist, and a geneticist. Reconstruction and dental rehabilitation were felt to require the surgical repositioning of the maxilla followed by an overdenture. In the mandible, crown and bridge rehabilitation would be carried out. The prosthodontist requested 18 mm of horizontal advancement and 14 mm of vertical lengthening of the maxilla. A two-stage approach to maxillary reconstruction was undertaken.
Stage I surgery included the following: 1) nasotracheal intubation; 2) Le Fort I osteotomy with down fracture and disimpaction; 3) septoplasty and inferior turbinate reduction; 4) application of a MED I external distraction device; and 5) securing of the MED I device to a prefabricated in-place chrome cobalt appliance fixed to the maxillary dentition. Successful outpatient distraction of the maxilla to the preferred position was accomplished over a 10-day time frame.
Stage II surgery involved the patient’s return to the operating room for the following: 1) awake fiber-optic nasotracheal intubation; 2) removal of the MED I device; 3) harvesting of the anterior iliac corticocancellous graft; 4) reopening of the circumvestibular incision; 5) securing a prefabricated splint to the maxilla and then applying intermaxillary fixation (18 mm of advancement); 6) rotation of the maxillomandibular complex to achieve the desired vertical dimension (14 mm of lengthening); 7) application of plate and screw fixation to the maxilla; 8) crafting and inset of corticocancellous grafts to the left and right anterior maxilla; and 9) plate and screw fixation of each graft to the native maxilla. After 6 weeks, the maxilla achieved initial bone healing. The patient returned to a more regular diet and sports activities at that time. Six months postoperatively, the patient underwent a pharyngeal flap procedure to achieve velopharyngeal competence and an open rhinoplasty procedure that included a rib cartilage (caudal strut) graft. Dental rehabilitation included fixed bridgework in the mandibular arch and overdenture construction for the maxilla. A, Frontal and occlusal views when the patient was 14 years old. B, Computed tomography scan views that indicate the extent of maxillary hypoplasia. C, Articulated dental casts that indicate analytic model planning. D, Facial and occlusal views before surgery with a prefabricated chrome–cobalt appliance fixed to the maxillary dentition. E, The patient is shown after Le Fort I osteotomy followed by 10 days of MED I distraction to achieve the preferred maxillary position. A computed tomography scan is also shown 10 days after the Le Fort I procedure to demonstrate the advanced maxilla just before the removal of the MED I device. F, Computed tomography scan views are shown after the stage II procedures with the maxilla in its new location and now secured with bone graft and plate and screw fixation. G, Frontal facial views in repose before and after reconstruction and rehabilitation. H, Frontal views with smile before and after reconstruction and rehabilitation. I, Profile views before and after reconstruction and rehabilitation.
The prevalence of these residual clefting deformities in mature patients with UCLP varies widely depending on the primary cleft surgeon’s philosophy, available expertise, the individual’s intrinsic biologic growth potential, and the patient’s and family’s interests. Published clinical surveys of individuals who were born with complete UCLP and treated at established cleft centers provide insight into alveolar cleft management. The studies indicate that, despite a cleft team’s best efforts, a number of children will not make themselves available for mixed-dentition grafting (i.e., before the eruption of the cleft side canine). In addition, a percentage of those who do undergo such procedures will have problems with grafting and require other means of reconstruction and dental rehabilitation. Williams and colleagues documented that, in the United Kingdom, only 84% of children with complete UCLP arrived for mixed-dentition grafting.177 McIntyre and colleagues documented that only 75.4% of Scottish children with complete UCLP were grafted during the mixed dentition.103 Daskalogiannakis and colleagues documented that, at The Hospital for Sick Children in Toronto, children with complete UCLP underwent mixed-dentition grafting only 56.1% of the time.27 On a more encouraging note, Felstead and colleagues documented a 94% rate of radiographically successful mixed-dentition bone grafting in a consecutive series of children with UCLP or bilateral cleft lip and palate (BCLP) who were treated by a single surgeon (n = 53 alveolar cleft sites).39 Despite a clinician’s preferred approach to cleft management during infancy, childhood, and early adolescence and despite that clinician’s best efforts, a subgroup of patients with UCLP patients will present during or after adolescence with multiple cleft-related problems that may include the following:
1. Maxillary hypoplasia. The maxilla is often vertically short and canted (upward on the cleft side), and the maxillary dental midline is usually shifted off of the facial midline toward the clefted side. Arch-width deficiency that results in a dental crossbite is usually present. The hypoplastic maxilla is retruded in the horizontal plane, thereby resulting in a concave midface profile. There will be an Angle Class III malocclusion and negative overjet. In general, the greater and lesser maxillary segments vary with regard to the degree of dysplasia, thereby making it difficult to achieve a satisfactory occlusion and facial appearance by repositioning the maxilla in one unit. Separation in two segments (through the cleft) with differential repositioning is often required for the management of the arch shape, even if successful mixed-dentition grafting was accomplished.
2. Residual oronasal fistula. Despite a preference for oronasal fistula closure during the mixed dentition, the UCLP candidate for orthognathic surgery will often have residual labial and palatal fistulas. Previous attempts at closure may have failed. Furthermore, buccal (non-keratinized) mucosa may have been placed over the cleft site, thereby resulting in a lack of attached gingiva (keratinized mucosa) in the tooth-bearing region and a loss of vestibular depth.
3. Residual bony defects. In the patient with UCLP who has not been successfully or adequately grafted during the mixed dentition, a bony defect not just at the alveolus but throughout the hard palate and the floor of the nose may exist. This results in an inferiorly displaced or deficient floor of the nose and nasal sill with rotation of the anterior nasal spine and the septum toward the cleft. There may also be inadequate alveolar bony, gingival, and periodontal support for the teeth adjacent to the cleft.
4. Cleft–dental gap. Studies confirm that, approximately 93% of the time in patients with UCLP, the lateral incisor is either congenitally absent or inadequate at the cleft site.163,164 A hypoplastic lateral incisor or a supernumerary tooth may be present adjacent to the cleft, but it will have inadequate root development. Orthodontic closure of this dental gap by bodily moving the canine tooth into the bone-grafted lateral incisor location is generally considered the preferred approach. For a variety of reasons, this may not have occurred at the time of referral for orthognathic surgery. At times, there is mesial angulation of the canine (i.e., crown tipped) into a marginally grafted or non-grafted cleft site, while the apex of the root remains distal. The result may be a full or partial dental crown gap at the cleft site between the central incisor and the canine but with an even greater separation of the roots.
5. Chin dysplasia. The patient with UCLP will frequently suffer with a lifelong history of obstructed nasal breathing and an open-mouth posture. This is often the result of anatomic deformities, including septal deviations, inferior turbinate hypertrophy, an irregular nasal floor, and nasal vestibular stenosis. The presence of a pharyngeal flap since childhood may further increase an open-mouth breathing tendency. The resulting chin deformities that occur during growth are often characterized by excess vertical length and horizontal retrusion.
6. Mandibular dysplasia. True mandibular prognathism or retrognathism is uncommon in the patient with UCLP. However, the need for mandibular osteotomies is frequent. This should be limited to the correction of secondary deformities that result in facial asymmetries and skeletal distortions and the occasional true anteroposterior discrepancy.88,89,171
7. Nasal obstruction and sinus blockage. Obstructed breathing through the nose and frequent bouts of sinusitis are typical among patients with UCLP. This results from a combination of septal deviation; enlarged inferior turbinates; deformities of the nasal aperture, the floor of the nose, and the anterior nasal spine region; and stenosis of the nasal vestibule.
8. VP dysfunction. It has been shown that approximately 20% of patients with a repaired cleft palate will have VP insufficiency by the time they are 5 years old. The adolescent with UCLP who arrives for the evaluation of a cleft jaw deformity may have already undergone a pharyngoplasty with a pharyngeal flap. The planned Le Fort I osteotomy with advancement will alter the upper airway and may also negatively affect VP function, as described later in this chapter.
Orthodontic Considerations in the Patient with Unilateral Cleft Lip and Palate with a Jaw Deformity
The adolescent or adult patient with UCLP who presents with maxillary hypoplasia and ineffective bone grafting performed earlier during his or her childhood will have two maxillary segments separated by a cleft (see Figs. 32-11 through 32-21). Each segment will have a degree of skeletal dysplasia in all three planes of space. From an orthodontic perspective, each segment should be evaluated and treated individually in anticipation of Le Fort I segmental repositioning.
There is variability with regard to the number of permanent incisors and the amount of alveolar bone in the anterior aspect of the UCLP maxilla. A lateral incisor-like tooth is frequently found along the edge of the cleft in the lateral segment. When a poorly formed lateral incisor is present, it should be extracted in the interest of long-term function and dental rehabilitation. Cassolato and colleagues have documented that, in patients with complete UCLP, the lateral incisor on the cleft side is normal and maintained in only 7% of cases.17 Unerupted supernumerary teeth are also extracted either at the time of bone grafting during the mixed dentition or in conjunction with orthognathic surgery. The central incisor on the cleft side also has a high probability of being malformed.
A potential disadvantage of the modified Le Fort I approach in a patient with UCLP is that the differential advancement of the lesser dentoalveolar segment shifts the preoperative anterior dental gap (i.e., the lateral incisor region) into the posterior region (i.e., the second molar region) (see Fig. 32-5 and discussion later in this chapter). This is no different than the occlusal changes that will occur when the mixed-dentition bone-grafted cleft–dental gap (i.e., with absent lateral incisor) is orthodontically closed by canine substitution. Unfortunately, the mandibular second molar on the cleft side may no longer have an opposing maxillary molar. For this reason, the maxillary second molar should be orthodontically included in the arch form. Occasionally there will be a non-impacted maxillary wisdom tooth that can oppose the mandibular second molar. Articulated dental models with the maxillary segments in their proposed postoperative positions are analyzed to confirm that the posterior maxillomandibular occlusion will be acceptable.
Immediate Presurgical Assessment
Approximately 4 to 6 weeks before the operation, the orthodontist will confirm that the preoperative orthodontic objectives have been met and then place the passive surgical wires. The surgeon takes the final records, which include alginate impressions of the maxillary and mandibular arches, a centric relation bite registration, a face-bow registration, and direct facial measurements. The patient’s medical and dental records (e.g., radiographs, consultation reports, facial and occlusal photographs, dental models, special studies) are reviewed. Decisions are finalized with regard to the preferred vector changes (repositioning) of the jaws and the precise linear (millimeter) distances and angles to be accomplished in each jaw for the desired result (see Chapter 12).2,38,44,45,68,76,94,100,101 Analytic model planning is carried out on the articulated dental casts, and splints are fabricated. The splints assist with the achievement of the precise occlusion and the preferred facial aesthetics that have been decided on preoperatively (see Chapter 13).
Orthognathic Approach for Unilateral Cleft Lip and Palate Deformities
Evolution of Surgical Technique
Historically, the literature warned of possible complications with maxillary osteotomy among patients with UCLP but provided only limited and often confusing descriptions of techniques to guide the orthognathic surgeon in the performance of safe, reliable osteotomies to solve these complex problems.158
As with other aspects of orthognathic surgery, Hugo Obwegeser’s milestone contributions to cleft skeletal reconstruction are important (see Chapter 2). By the late 1960s, Obwegeser succeeded in advancing a cleft maxilla to the preferred location without the need for a compromised mandibular setback approach. He eventually felt comfortable with cleft maxillary advancements of up to 20 mm. He also realized that the adequate mobilization of the Le Fort I down-fracture was the key step in advancing the maxilla, whether the patient had clefting or not. With experience, the value of simultaneously closing the cleft–dental gap by moving the lesser segment further forward to position the canine in the lateral incisor location was appreciated. This differential segmental repositioning would essentially replace an anterior dental gap for an edentulous space in the posterior maxilla.
Early on, other surgeons without Obwegeser’s level of expertise reported complications after advancing the maxilla in patients with UCLP.113–117,119,120 In 1974, Willmar described the problems that occurred in 17 patients with UCLP who underwent Le Fort I osteotomy. One patient had aseptic necrosis and a partial loss of the lesser segment of the maxilla.178 In 1974, Georgiade suggested that a camouflage approach with mandibular osteotomies and set-back was preferred to maxillary advancement to avoid complications.51 Kiehn and others and Des Prez and Kiehn warned of blood-supply problems that might occur with maxillary osteotomies in patients with cleft lip and palate.30,83 In 1975, Henderson and Jackson reported combining lip-scar revision, anterior fistula closure, and maxillary osteotomy in a one-stage procedure.69 Their concept was innovative, but they did not specify details of their technique. In 1978, Jackson described the technique of Le Fort I osteotomy in patients with cleft lip and palate and noted that, if a large fistula was present, extensive flap mobilization for closure was required, and the blood supply might be compromised.77
Historically, surgeons were leery of completing a Le Fort I down-fracture and full mobilization in patients with UCLP because they feared flap necrosis with a subsequent loss of bone and teeth.6,7,31–33,52,53,87 In 1980, Tideman and colleagues proposed the segmental palatal osteotomy for patients with cleft lips and palates.168 This procedure required significant subperiosteal degloving with the potential for compromised flap circulation but without providing the needed direct exposure for full disimpaction. In 1980, Sinn also reported on the simultaneous Le Fort I advancement, oronasal fistula repair, and bone grafting of the alveolar cleft.155 He stressed the importance of preserving a vertical soft-tissue pedicle, which was similar to that described by Tideman and colleagues. He completed osteotomies through tunnels rather than under direct vision and used a cheek rotation flap for oral-side closure of the labial and palatal oronasal fistulas. With this technique, non-keratinized buccal mucosa (rather than attached gingiva) was brought into the cleft tooth-bearing region. In 1984, Ward-Booth and colleagues described the results of Le Fort II osteotomy in 13 patients with clefts for the management of midface hypoplasia in an attempt to improve blood supply to the alveolar segments.175 The residual fistulas were not simultaneously closed, and fixation occurred with direct wires, intermaxillary fixation, and external appliances. In 1985, James and Brook described another variation for the correction of maxillary hypoplasia in patients with cleft lips and palates via the transection of the hard palate.78 They raised an extensive palatal flap and used three vertical stab incisions for exposure on the labial aspects of the maxilla. Access was then achieved through subperiosteal tunneling and without direct exposure. Fixation was with intermaxillary fixation and a halo head frame, which was maintained for 10 weeks. A second procedure was required for bone grafting and palatal fistula closure. The authors expressed concern that a more direct down-fracture of the maxilla would result in vascular compromise to the segments. In 1985, Poole and others proposed an additional modification of the Le Fort I osteotomy to be used in patients with cleft palates.128 With their technique, a partial-thickness palatal flap was elevated, which left the greater palatine vessels in situ and allowed the maxilla to be repositioned anteriorly, without displacement of the soft palate. Poole and colleagues believed that this approach would limit interference with VP function; they also used small vertical incisions on the labial aspect that required tunneling and lacked direct exposure for osteotomies, disimpaction, fistula closure, bone-graft placement, and plate and screw fixation. Fixation in this series was with intermaxillary fixation, direct wires, and a halo craniomaxillofacial head frame.128
During the 1980s and the 1990s, Posnick used and refined Obwegeser’s original techniques involving Le Fort I osteotomy for the treatment of UCLP deformities.129–142 A key aspect was the circumvestibular incision, which allowed for direct exposure for dissection, osteotomies, disimpaction, fistula closure, septoplasty, inferior turbinate reduction, pyriform aperture recontouring, bone grafting, and the application of plate and screw fixation (see Fig. 32-5). This was found to be a reliable approach that did not involve the risk of circulation injury to the greater and lesser dento–osseous–musculo–mucosal segments. The visibility provided by the circumvestibular incision made possible the incorporation of routine closure of the cleft–dental gap through differential maxillary segmental repositioning without necrosis of the bone or any loss of teeth. This method also closes the cleft dead space and brings together the labial and palatal flaps without the need for subperiosteal undermining, which allows for the closure of recalcitrant oronasal fistulas without tension and the establishment of the periodontal health of the cleft adjacent teeth. The down-fracture provides ideal exposure for septoplasty; for the reduction of the hypertrophic inferior turbinates; and for the recontouring of the pyriform rims, the floor of the nose, and the anterior nasal spine. The success of this approach, as initially carried out by Obwegeser,114–117,119,120 was confirmed by Bell’s demonstration of the blood supply to these maxillary segments in animal studies.6–7 The clinical advantages were then further documented by Posnick and colleagues in a consecutive series of UCLP patients.129–142
Since the mid 1990s, the literature concerning cleft orthognathic surgery has mostly focused on use of DO techniques to avoid the need for complete intraoperative mobilization of the maxilla in the hopes of improved long-term stability.166 Although the issue of skeletal stability and dental relapse for precise long-term occlusion is important, it should not overshadow the value of achieving the other planned improvements of the airway and enhanced facial aesthetics.129–142 Unfortunately, the use of DO techniques has not altered the challenges of cleft jaw surgery, which include the following: 1) a lack of postoperative growth when maxillary advancement is carried out in children; 2) a worsening of VP function in some patients; 3) injury to the teeth and bones if circulation to the segments is not maintained; 4) facial and intraoral sensory loss; 5) suboptimal facial aesthetics when basic principles are not followed; and 6) concern for postoperative skeletal or dental relapse (i.e., residual malocclusion). In addition, DO techniques require a prolonged and labor-intensive postoperative convalescence for the patient and family as compared with the standard approach. Despite these disadvantages, DO does offer hope to the occasional patient with UCLP who also has severe maxillary hypoplasia and anodontia (see Fig. 32-21 and the section about controversies later in this chapter).
Standard Le Fort I Osteotomy
The adolescent or adult CLP patient with a jaw deformity but without a residual fistula and with an intact alveolar ridge of adequate height and volume in the area of the cleft may have been born without alveolar clefting or had a successful graft.34 For those with adequate alveolar ridge height and volume, a closed palate, and sufficient periodontal support, a standard Le Fort I osteotomy can be performed (see Figs. 32-8, 32-9, and 32-10). A segmental maxillary osteotomy to adjust the arch width, to correct the vertical dimension, or to close the cleft dental gap to avoid the need for a prosthetic lateral incisor may also be necessary in some of these patients (see Chapter 15). Unfortunately, even in the 21st century, a number of adults and adolescents with UCLP with maxillary hypoplasia also present with alveolar defects and oronasal fistulae. For these patients, a modified (two-segment) Le Fort I osteotomy should be considered, as discussed in the next section of this chapter (see Figs. 32-11 through 32-21).129–142
Modified Le Fort I Osteotomy (Two Segments)
In unilateral cleft cases, the dental gap of the [absent] lateral incisor can be eliminated by advancing the lateral alveolar process so that the canine is positioned next to the central incisor. The canine then subsequently is contoured to match the appearance of the lateral incisor.118,119
Subperichondrial and subperiosteal dissection of the deviated vomer, the perpendicular plate of the ethmoid, and the quadrangular cartilage is accomplished after the maxilla has been down-fractured. Resection of the deviated and buckled aspects of the septum (i.e., the bone and cartilage) is done with a rongeurs while preserving the structural components of the cartilaginous septum (i.e., the dorsal strut and the caudal strut) that are necessary to prevent a late saddle-nose deformity (see Chapter 15). If the inferior turbinates are enlarged, they are also reduced to improve the airway. The nasal mucosa flaps are sutured for a watertight nasal-side closure. If indicated, the impacted maxillary third molars are removed from above through the maxillary sinus (see Chapter 15).
The down-fractured maxilla may already be in two segments if the bony cleft was unrepaired. If the maxilla is intact but needs correction of the arch form, then it is separated using a reciprocating saw with short straight blade. The approximation of the segments to close the cleft–dental gap can occur after bony spurs are shaved from the alveolus along the distal aspect of the central incisor and the mesial aspect of the canine using a rotary drill with a watermelon bur. Care is taken to avoid penetrating the lamina dura, which would expose the dental root and may result in external root resorption. The maxillary segments are then ligated into a prefabricated acrylic occlusal splint. The segmental repositioning closes the cleft–dental gap, brings the alveolar ridges together, and approximates the labial and palatal mucosal soft tissues for oral-side fistula closure. The differential segmental repositioning approximates the mucosal edges along the palate, so they do not need to be sutured. In fact, to limit invaginations of the oral mucosa, the excision of redundant palatal mucosa is generally required just before the segments are placed in the splint. The placement of the splinted maxilla onto the mandibular dentition should be passive. The extent of maxillary advancement is based on the preferred occlusion and the facial aesthetics determined preoperatively. The ideal vertical dimension is achieved intraoperatively on the basis of the preoperative plan (see Chapter 12). The maxillary osteotomy sites are fixed in place with titanium miniplates and screws at each zygomatic buttress and pyriform rim in accordance with the principles originally described by Luhr.92 An additional microplate is frequently applied horizontally across the cleft site to stabilize the closure of the cleft–dental gap. The intermaxillary fixation is released, and the occlusion is checked.
The lateral nasal rims, the floor of the nose, and the anterior nasal spine region are recontoured using a rotary drill with a watermelon bur to improve the nasal airway and to enhance nasal aesthetics (see Chapter 15). The dead space associated with the alveolar cleft has already been closed by the differential segmental repositioning. Autogenous iliac cancellous bone graft may be packed along the floor of the nose to raise the ipsilateral sill and across the cleft palate to stabilize the segments. Generally, a crafted iliac corticocancellous bloc graft is placed in between the zygomatic and pyriform fixation plates on each side. Each graft is tightly wedged into the space created by the horizontal advancement and the vertical lengthening (see Chapters 15 and 18). An additional microplate is contoured and secured across the osteotomy, and it also incorporates the graft. With the cleft–dental gap surgically closed, the redundant labial mucosa is excised. Attached gingiva and mucosal edges are approximated without tension and then directly sutured without the need for rotation flaps.
Mandibular and chin osteotomies to correct secondary deformities and facial asymmetries are frequently planned. If so, the osteotomies are completed and secured with plate and screw fixation in standard sequence (see Chapter 15). After the completion of all of the osteotomies and the placement of fixation and grafts, the wounds are closed. The upper and lower teeth are approximated with orthodontic elastics. When maxillary segmental osteotomies are completed, the splint remains wired to the upper teeth postoperatively (see the orthodontic considerations section earlier in this chapter).
Avoiding Pitfalls
Horizontal Advancement and Vertical Lengthening of the Cleft Maxilla
In general, to achieve full skeletal correction, all or most of the negative overjet should be managed in the maxilla. The sagitally deficient maxilla in a patient with UCLP will also typically need vertical lengthening. When the maxilla is fully mobilized and repositioned as described previously, stabilization with titanium plates and screws (i.e., a plate at each zygomatic buttress and each pyriform aperture) is performed. The placement of a bloc corticocancellous graft wedged into the dead space between the zygomatic buttress and the pyriform aperture plate on each side is generally necessary (see Chapter 15). Further stabilization across each graft with a titanium plate and screws is also required (see Chapter 15).
Managing the Nasal Cavity and the Nose
In the patient with UCLP, the inferior turbinates will often be enlarged, asymmetric, and partially blocking the nasal cavity. The reduction of enlarged inferior turbinates will be beneficial for breathing and sinus drainage. The procedure is accomplished through the Le Fort down-fracture (see Chapter 15 and Fig. 32-10).
In the patient with UCLP, the septal bone and cartilage generally show significant deviation and thickening, thus further obstructing the airway. The completion of a submucous resection of the deviated portions of the septum will be helpful to open the airway. The preservation of caudal and dorsal cartilaginous struts will prevent a saddle deformity; this is accomplished through the Le Fort down-fracture (see Chapter 15 and Fig. 32-10).
In the patient with UCLP, the pyriform rims are generally constricted and asymmetric. The nasal floor is uneven, and the anterior nasal spine is deviated. After Le Fort down-fracture, the recontouring of the pyriform rims and the nasal floor and spine using a rotary drill with a watermelon bur is helpful to open the airway and improve nasal aesthetics (see Chapter 15 and Fig. 32-10).
Attempts to “control” the nasal soft-tissue envelope with suturing techniques (e.g., the alar cinch stitch) after Le Fort I osteotomy are often suggested. In my experience, manipulating the nasal soft tissues with suturing techniques after Le Fort I may be counterproductive to both the airway and the facial aesthetics. We anticipate that the nasal soft tissues will be redraped over the repositioned maxilla and the recontoured pyriform rims, nasal floor, and nasal spine for an overall improvement in aesthetics. A definitive rhinoplasty to fully correct the UCL nasal deformities is often beneficial; this is best postponed until 6 to 12 months after jaw reconstruction (see Chapter 38).
Immediately after surgery, the nasal cavity is not packed, and the septum is not stented. Coagulated blood will partially block nasal breathing. The clots and scab are expected to dislodge and be self-eliminating after the underlying mucosa is initially healed (i.e., 5 to 7 days after surgery). The use of saline nasal sprays and the institution of sinus precautions are helpful during this time (see Chapter 11).
Management of the Mandibular Deformity
In the individual with UCLP, the mandible is often secondarily deformed. In a previously published study, 70% of the patients with UCLP had secondarily deformed mandibles to the extent that sagittal split ramus osteotomies were beneficial to improve facial symmetry and proportions.141 The mandibular repositioning is not carried out in an attempt to avoid maxillary advancement but rather to improve overall facial morphology (see Chapter 15).
Management of the Chin Deformity
In the patient with UCLP with maxillary hypoplasia, the chin is often secondarily deformed, with increased vertical length and a flat pogonion. An intraoral oblique inferior border osteotomy is carried out with repositioning and reshaping of the distal chin to accomplish the preferred morphology (see Chapters 15 and 37).
Clinical Management after Initial Surgical Healing
Managing the details of the in-hospital and at-home convalescence during the initial healing of the orthognathic patient are essential for a successful outcome (see Chapter 11). Cephalometric and dental radiographs and facial and occlusal photographs are obtained at standard postoperative intervals to document patient healing. The orthodontist sees the patient within 24 hours of splint removal (approximately 5 weeks after surgery) and replaces the maxillary sectional arch wires with a rigid continuous arch wire. The maxillary teeth are ligated together to maintain the surgical dental-gap closure, the horizontal advancement, and the transverse expansion. Active orthodontic maintenance and finishing are started. The use of a transpalatal appliance (i.e., a wire or a palatal plate) may also be used to stabilize the new arch form. Close monitoring by an orthodontist for skeletal and dental shifts during the first 6 months after surgery is essential.
Orthognathic Surgery for Unilateral Cleft Lip and Palate: Review of Study
Patients and Methods
Posnick and Tompson prospectively assessed the cleft deformity and clinical results of 66 consecutive adolescents and young adults (age range, 15 to 25 years; mean, 18 years) with UCLP who underwent orthognathic surgery by one surgeon (Posnick) with the use of a single surgical protocol during a 6-year time period.141 All patients underwent perioperative orthodontic treatment and were judged to be skeletally mature at the time of jaw surgery. The clinical follow-up period after maxillary advancement ranged from 1 to 7 years (mean, 40 months). The countries of origin of the patients varied (e.g., Canada, United States, Eastern Europe, Southeast Asia), as did their races (e.g., Caucasian, Asian, African). These patients had their cleft lips and palates repaired by many different surgeons who were using a variety of protocols. The number and extent of previous revisions of the lip, nose, and palate varied greatly (range, 1 to 9 procedures). Many patients had undergone multiple attempts at closure of the residual oronasal fistula and at the reconstruction of the alveolar clefts with bone graft. Seven of the 66 patients with UCLP had previously undergone orthognathic surgery by another surgeon; all seven had residual maxillary hypoplasia, a recalcitrant oronasal fistula, and a cleft–dental gap.
Skeletal Stability after Modified Le Fort I for Unilateral Cleft Lip and Palate Deformity: Review of Study
Patients and Methods
Posnick and colleagues reviewed medical records and cephalometric radiographs and completed current surgical and orthodontic clinical examination of all patients (n = 45) with UCLP who had undergone Le Fort I osteotomy by a single surgeon (Posnick) during a 3-year period.139 The following information was noted: all previous maxillofacial procedures, details of the orthognathic procedures, osteotomy stabilization techniques used, the presence of a pharyngoplasty, the extent of bone grafting, the segmentalization of the Le Fort I osteotomy, perioperative orthodontics, the age at surgery, the age at final follow up, the amount of overjet and overbite at the 1 year or more postoperative visit, and perioperative morbidity.
The serial radiographs for each patient were analyzed with a modified version of the method described by Bachmayer and colleagues.5,6 On each preoperative tracing, the horizontal and vertical coordinates that represented the patient’s natural head position were constructed so that they passed through the sella. These reference lines were transferred and became the x-y grid template onto which all of the postoperative maxillary positional changes could be traced and measured. The end result was a Cartesian coordinate system that illustrated the horizontal and vertical directional changes of the maxilla at intervals after surgery. The measurements of the differences in the position of the maxilla at different postoperative points were taken from the grids and were calculated to within 0.5 mm. In addition, the incisor overjet and overbite measurements from the 1-year (or more) postoperative cephalogram were documented and compared with the measurements taken at the final (1 year or more postoperative) clinical assessment. Differences between groups (e.g., pharyngeal flap in place) were assessed via Student’s t-test. Any association between the magnitude of surgical change and the degree of relapse was examined with the Pearson correlation coefficient (r) and the least-squares linear regression analysis. Correlations were considered significant when the P value was less than .05.
Controversies and Unresolved Issues
Staging of Maxillary Reconstruction
The described modified Le Fort I segmental osteotomies as a method of managing end-stage oronasal fistulas, alveolar defects, and cleft–dental gaps in patients with UCLP who have maxillary hypoplasia is not intended to replace standard techniques and the accepted sequencing of treatment. We always prefer secondary bone grafting during the mixed dentition typically with orthodontic closure of the cleft dental gap. However, the method described does offer an alternative approach when the opportunity for grafting during the mixed dentition (i.e., before the eruption of the permanent canine) is lost and a jaw deformity also exists. Published studies from major cleft centers in the United Kingdom, Scotland, and Canada confirm that, despite best efforts, a number of patients in the mixed dentition will not arrive for treatment, and a percentage of those who do will undergo unsuccessful grafting.39,103,108,177 A two-stage approach to the adolescent or adult with maxillary hypoplasia, residual alveolar clefts, oronasal fistulas, a cleft–dental gap, and nasal obstruction is not cost- or time-effective and, in my experience, the potential overall morbidity is increased. It is in these patients with UCLP that the modified Le Fort I osteotomy offers a reasonable opportunity for the resolution of residual end-stage problems (e.g., maxillary hypoplasia, alveolar defect, residual fistulas, cleft–dental gap, nasal obstruction) in a safe and effective way.
In the patient with UCLP, there are several options for the management of a cleft–dental gap.* From both facial aesthetic and dental health perspectives, the long-term use of a removable partial denture is always a second choice. Fixed bridgework is a viable alternative, but this requires the partial destruction of adjacent normal teeth, it may look artificial, it requires replacement at intervals throughout the patient’s life, and it demands ongoing meticulous oral hygiene (i.e., high-maintenance dentition). Placement of a single-tooth osseous integrated implant is an attractive alternative, but the implant’s aesthetic success is dependent on adequate bone height, width, and volume and sufficient attached gingiva.62,93,122,124,146,165,166,172,174,185 This is not routinely established at the cleft site, even after bone and gingival grafting. In addition, it requires staged surgical procedures, and it also produces a high-maintenance dentition. Coordinated comprehensive periodontal and prosthetic rehabilitation is required, and even then failure to provide a natural-looking smile may occur. In a published study conducted at The Hospital for Sick Children in Toronto, only 10% of complete UCLP lateral incisor dental gaps were managed with a dental implant and crown.17 Other dental refinements for a patient’s dysmorphic and often hypoplastic and decayed anterior maxillary teeth include porcelain-veneer buildups, composite bonding, sculpting of the teeth, and bleaching techniques. Each of these refinements may improve the patient’s function, smile aesthetics, and self-esteem.
A major advantage of the described modified Le Fort I (segmental) osteotomies in the patient with UCLP is its ability to simultaneously close the cleft dead space, the residual oronasal fistulas, and the alveolar defect and to stabilize the dentoalveolar segments. The study by Posnick and colleagues documents the improved long-term periodontal health achieved in the cleft dentoalveolar regions when this technique is used.141 The long-term benefits to the patient of the resulting low-maintenance dentition cannot be over stated. Another advantage of this approach occurs when the lesser segment is advanced to attain this goal. By doing so, a relative arch-width expansion is gained at the mandibular molar and the premolar region without the actual need for a lateral shift of the whole segment. This limits any arch-width relapse tendencies.
Velopharyngeal Function after Le Fort I Advancement
Uncertainties about VP function and the management of an in-place pharyngeal flap should no longer be limiting factors when orthognathic surgery is necessary in a patient with a cleft. A nasoendoscopic guided examination by a speech pathologist and a surgeon who are familiar with cleft anatomy can reasonably predict current and expected VP function in a patient who is scheduled for a Le Fort I osteotomy (see Chapter 8). When postoperative VP deterioration is anticipated, the patient and family are counseled about the sequencing of treatment. Clinical studies have now documented that VP function will deteriorate in a similar fashion when either DO or standard Le Fort I osteotomy techniques are used.19,20,57,60,79,86 Despite the frequent need for significant maxillary advancement to normalize the skeleton and the facial aesthetics in patients with UCLP, I have not had to transect an in-place pharyngeal flap to achieve maxillary mobilization. Our research and that of others confirms that a pharyngeal flap in place at the time of Le Fort I osteotomy does not increase complications nor does it result in a higher incidence of relapse.141 The definitive reassessment of VP function after cleft Le Fort I advancement can be carried out 3 months after surgery. A primary or revision pharyngeal flap can be safely carried out within 6 months after surgery in conjunction with cleft rhinoplasty or labial revision, if indicated.
Mixed Dentition Le Fort I Osteotomy
By the mid 1980s, research clarified that, if jaw surgery is undertaken in the growing patient with a cleft palate, another procedure to advance the maxilla will likely be required when skeletal maturity is reached.181–184 More recently, several investigators again tested this theory by proceeding with mixed-dentition Le Fort I procedures using DO techniques. All research to date indicates that the Le Fort I advancement carried out during the mixed dentition in the patient with clefting—whether with standard or DO techniques—results in no significant further horizontal growth.21,26,40,41,59,61,75,126 As the mandible continues to grow, an Angle Class III malocclusion will occur with the need for either additional Le Fort I advancement or mandibular set-back.
Skeletal Relapse after Le Fort I Osteotomy in Patients with Clefting
There are more than 100 published articles reviewing skeletal stability and relapse in patients with clefting who have undergone Le Fort I advancement with the use of either standard osteotomy or DO techniques.* Proponents of DO techniques frequently state that, when more than 10 mm of horizontal maxillary advancement are required, the use of standard osteotomies with plate and screw fixation and bone grafting may lead to a greater degree of relapse. However, these studies do not report convincing data that demonstrate significant differences in relapse patterns between the two techniques. To give this situation some perspective, it should be noted that only 5% of patients with clefting who are undergoing Le Fort I advancement will require more than 10 mm of horizontal advancement at the incisors. All clinicians agree that this subgroup of patients is the most challenging to treat, but the reasons for this go beyond the degree of horizontal maxillary deficiency. These patients are also likely to present with multiple residual end-stage deformities (see the section about residual skeletal deformities earlier in this chapter), multiple missing teeth, and previously failed surgical procedures.
Aksu and colleagues documented a horizontal maxillary (skeletal) relapse of 22% in patients with cleft lips and palates after Le Fort I osteotomy with the use of DO techniques.4 He and colleagues reported their results for adolescent patients with repaired cleft lips and palates and maxillary hypoplasia who then underwent Le Fort I advancement with the use of an external DO device.65 The patients (n = 17) were treated at one center between 2000 and 2006, and they had at least 1 year of follow up and a full set of records. DO treatment was started on day 5 (1 mm per day) and continued until a Class II occlusion (i.e., overcorrection) was achieved. Consolidation time ranged from 4 to 12 weeks, and this was followed by several more months of face mask therapy. The first four patients (two UCLP and two BCLP) who were treated developed fibrous non-union, and all four required reoperation and rigid (i.e., titanium plate and screw) fixation to achieve union. The authors then extended the consolidation period to a minimum of 12 weeks for the remaining patients (n = 13); all of these patients achieved bony union. In the group that achieved satisfactory bony union (13 out of 17 patients; 76%), the mean horizontal relapse was 11.9%, with 5 of 13 patients (38%) developing no better than end-to-end occlusion. Another orthognathic procedure was necessary in 5 of the 13 patients (38%) who initially achieved bony union to obtain a satisfactory occlusion. Overall, 9 out of 17 patients (53%) required two orthognathic procedures. In 2011, Chen and colleagues reported a 30.7% incidence of horizontal skeletal relapse 1 year after Le Fort I osteotomy in patients with clefts who were treated with DO techniques (i.e., the RED device). This was in a consecutive series of patients who presented with Class III malocclusion as a result of cleft maxillary hypoplasia and who agreed to a combined orthodontic and surgical approach.21 Posnick and colleagues documented the degree of horizontal relapse and overall occlusal success when using the modified Le Fort I technique as described in this chapter for patients with clefts. They measured horizontal change, stability, and information concerning the maintenance of overjet and overbite from 1-year or more postoperative cephalometric radiographs and clinical examinations.141 The results were reported in accordance with cleft type: patients with UCLP had 6.9 mm mean advancement with 5.3 mm maintained; 94% of patients with BCLP maintained positive overjet for the long term; and patients with isolate cleft palate had 6.1 mm mean advancement with 5.1 mm maintained. Interestingly, the degree of relapse that was documented was less than that generally reported by clinicians who use DO approaches (see earlier).
Standard Approach versus Distraction Osteogenesis Approach to Le Fort I Advancement
As Obwegeser stated in the 1960s and as was confirmed by Precious and colleagues, “relapse in cleft patients [after Le Fort I advancement] may well be more related to failure to adequately mobilize the maxilla and free it of abnormal soft tissue attachments than anything inherent in the osteotomy or the specific [cleft] diagnosis.”113–117,119,120
I agree with Obwegeser when he stated the following: “Today, many surgeons will resort to the use of distraction devices to gradually advance the cleft maxilla. Although in some circumstances this may be appropriate, it should be remembered that most cleft patients can be treated efficiently, even when requiring significant advancement with the classic Le Fort I type procedure.”119
Observations About the Distraction Osteogenesis Approach
• DO techniques are always the patient’s second choice when compared with the standard approach of Le Fort I osteotomy with rigid (plate and screw) internal fixation. The length of convalescence after surgery involving DO is longer; it will include at least 3 months of limited diet and physical activities, and it is generally followed by several additional months of face mask therapy. The DO device is also awkward and socially embarrassing, as it typically blocks the patient’s visual fields (e.g., the RED, BLUE, or GREEN external DO devices). Furthermore, there is no overall reduction in the potential for perioperative complications with DO techniques.
• If the cleft maxilla is adequately down-fractured, mobilized, and stabilized with interpositional bone grafts and rigid plate and screw fixation, it has a high probability of healing as planned with a predictable and less extensive convalescence than would be necessary with DO.
• An experienced orthognathic surgeon will be more confident with his or her ability to down-fracture and fully mobilize the cleft maxilla than a less experienced surgeon. Therefore, the less experienced surgeon will more likely select DO techniques to avoid the personal stress of the intraoperative maxillary mobilization process.
• Even for the experienced cleft orthognathic surgeon, there will be the occasional patient in whom the extent of maxillary hypoplasia and associated deformities (e.g., missing teeth, lack of alveolar bone) will lead him or her to choose a DO technique. Despite its protracted healing requirements and its limited ability to correct all of the cleft deformities, the DO device’s gradual stretching ability can mobilize even the most recalcitrant maxilla (see Fig. 32-21).
References
1. Abyholm, F, Bergland, O, Semb, G. Secondary bone grafting of alveolar clefts. Scand J Plast Reconstr Surg. 1981; 15:127.
2. Al-Waheidi, EMH, Harradine, NWT, Orth, M. Soft tissue profile changes in patients with cleft lip and palate following maxillary osteotomies. Cleft Palate Craniofac J. 1998; 35:535–543.
3. Araujo, A, Schendel, SA, Wolfort, LM, Epker, BN. Total maxillary advancement with and without bone grafting. J Oral Surg. 1978; 36:849–858.
4. Aksu, M, Saglam-Aydinatay, B, Akcan, CA, et al. Skeletal and dental stability after maxillary distraction with a rigid external device in adult cleft lip and palate patients. J Oral Maxillofac Surg. 2010; 68:254–259.
5. Bachmayer, DI, Ross, RB, Munro, IR. Maxillary growth following Le Fort III advancement surgery in Crouzon, Apert, and Pfeiffer syndromes. Am J Orthod Dentofacial Orthop. 1986; 90:420.
6. Bell, WH, Levy, BM. Revascularization and bone healing after posterior maxillary osteotomy. J Oral Surg. 1971; 29:313.
7. Bell, WH, You, ZH, Finn, RA, et al. Wound healing after multisegmental Le Fort I osteotomy and transection of the descending palatine vessels. J Oral Maxillofac Surg. 1995; 53:1425.
8. Berkowitz, S. State of the art in cleft palate orofacial growth and dentistry: a historical perspective. Am J Orthod. 1978; 74:564–576.
9. Berkowitz, S, Mejia, M, Bystrik, A. A comparison of the effects of the Latham-Millard procedure with those of a conservative treatment approach for dental occlusion and facial aesthetics in unilateral and bilateral complete cleft lip and palate: part 1. Dental occlusion. Plast Reconstr Surg. 2004; 113:1–18.
10. Boyne, PJ, Sands, NR. Secondary bone grafting of residual alveolar and palatal clefts. J Oral Surg. 1972; 30:87.
11. Braun, TW. Discussion of) Modification of the maxillary Le Fort I osteotomy in cleft-orthognathic surgery: The unilateral cleft lip and palate deformity. J Oral Maxillofac Surg. 1992; 50:675.
12. Braun, TW, Sotereanos, GC. Orthognathic and secondary cleft reconstruction of adolescent patients with cleft palate. J Oral Surg. 1980; 38:425.
13. Braun, TW, Sotereanos, GC. Orthognathic surgical reconstruction of cleft palate deformities in adolescents. J Oral Surg. 1981; 39:255.
14. Canady, JW, Thompson, SA, Colburn, A. Craniofacial growth after iatrogenic cleft palate repair in a fetal bovine model. Cleft Palate Craniofac J. 1997; 34:69.
15. Capelozza Filho, L, Normando, AD, da Silva Filho, OG. Isolated influences of lip and palate surgery on facial growth: Comparison of operated and unoperated male adults with UCLP. Cleft Palate Craniofac J. 1996; 33:51–56.
16. Case, CS. A practical treatise on the techniques and principles of dental orthopedia and prosthetic correction of cleft palate, ed 2. Chicago: The C. S. Case Co; 1922.
17. Cassolato, SF, Ross, B, Daskalogiannakis, J, et al. Treatment of dental anomalies in children with complete unilateral cleft lip and palate at SickKids Hospital, Toronto. Cleft Palate Craniofac J. 2009; 46(2):166–172.
18. Champy, M. Surgical treatment of midface deformities. Head Neck Surg. 1980; 2:451.
19. Chanchareonsook, N, Samman, N, Whitehill, TL. The effect of cranio-maxillofacial osteotomies and distraction osteogenesis on speech and velopharyngeal status: A critical review. Cleft Palate Craniofac J. 2006; 43:477–487.
20. Chanchareonsook, N, Whitehill, TL, Samman, N. Speech outcome and velopharyngeal function in cleft palate: Comparison of Le Fort I maxillary osteotomy and distraction osteogenesis—early results. Cleft Palate Craniofac J. 2007; 44:23–32.
21. Chen, PK, Por, YC, Liou, EJ, Chang, FC. Maxillary distraction osteogenesis in the adolescent cleft patient: Three-dimensional computed tomography analysis of linear and volumetric changes over five years. Cleft Palate Craniofac J. 2011; 40:445–454.
22. Cheung, LK, Chua, HD. A meta-analysis of cleft maxillary osteotomy and distraction osteogenesis. Int J Oral Maxillofac Surg. 2006; 35:14.
23. Cho, BC, Kyung, HM. Distraction osteogenesis of the hypoplastic midface using a rigid external distraction system: The results of a one- to six-year follow-up. Plast Reconstr Surg. 2006; 118:1201.
24. Cohen, SR, Burnstein, FD, Stewart, MB, Rathburn, MA. Maxillary-midface distraction in children with cleft lip and palate: A preliminary report. Plast Reconstr Surg. 1997; 99:1421–1428.
25. Cohen, SR, Corrigan, M, Wilmot, J, Trotman, CA. Cumulative operative procedures in patients aged 14 years and older with unilateral or bilateral cleft lip and palate. Plast Reconstr Surg. 1995; 96:267–271.
26. Correa Normando, AD, da Silva Filho, OG, Capelozza Filho, L. Influence of surgery on maxillary growth in cleft lip and/or palate patients. J Craniomaxillofac Surg. 1992; 20:111.
27. Daskalogiannakis, J, Mehta, M. The need for orthognathic surgery in patients with repaired complete unilateral and complete bilateral cleft lip and palate. Cleft Palate Craniofac J. 2009; 46:498–502.
28. Daskalogiannakis, J, Ross, RB. Effect of alveolar bone grafting in the mixed dentition on maxillary growth in complete unilateral cleft lip and palate patients. Cleft Palate Craniofac J. 1997; 34:455.
29. DeLuke, DM, Marchand, A, Robles, EC, Fox, P. Facial growth and the need for orthognathic surgery after cleft palate repair: Literature review and report of 28 cases. J Oral Maxillofac Surg. 1997; 55:694–698.
30. Des Prez, JD, Kiehn, CL. Surgical positioning of the maxilla: Symposium on management of cleft lip and palate and associated deformities. Ann Plast Reconstr Surg. 1974; 8:222.
31. Dodson, TB, Neuenschwander, MC, Bays, RA. Intraoperative assessment of maxillary perfusion during Le Fort I osteotomy. J Oral Maxillofac Surg. 1994; 52:827.
32. Dodson, TB, Neuenschwander, MC. Maxillary perfusion during Le Fort I osteotomy after ligation of the descending palatine artery. J Oral Maxillofac Surg. 1997; 55:51.
33. Drommer, R. Selecting angiographic studies prior to Le Fort I osteotomy in patients with cleft lip and palate. J Maxillofac Surg. 1979; 7:264.
34. Drommer, R. The history of the “Le Fort I osteotomy. ”. J Maxillofac Surg. 1986; 14:119–122.
35. Drommer, R, Luhr, HG. The stabilization of osteotomized maxillary segments with Luhr miniplates in secondary cleft surgery. J Maxillofac Surg. 1981; 9:166–169.
36. Erbe, M, Stoelinga, PJW, Leenen, RJ. Long-term results of segmental repositioning of the maxilla in cleft palate patients without previously grafted alveolo-palatal clefts. J Craniomaxillofac Surg. 1996; 24:109–117.
37. Escenazi, LB, Schendel, SA. An analysis of Le Fort I maxillary advancement in cleft lip and palate patients. Plast Reconstr Surg. 1992; 90:779–786.
38. Ewing, M, Ross, RB. Soft tissue response to orthognathic surgery in persons with unilateral cleft lip and palate. Cleft Palate Craniofac J. 1993; 30:320–327.
39. Felstead, AM, Deacon, S, Revington, P. The outcome for secondary alveolar bone grafting in the southwest UK region post-CSAG. Cleft Palate Craniofacial. 2010; 47:359–362.
40. Figueroa, AA, Polley, JW, Friede, H, et al. Long-term skeletal stability after maxillary advancement with distraction osteogenesis using a rigid external distraction device in cleft maxillary deformities. Plast Reconstr Surg. 2004; 114:1382.
41. Figueroa, AA, Polley, JW, Ko, EW. Maxillary distraction for the management of cleft maxillary hypoplasia with a rigid external distraction system. Semin Orthod. 1999; 5:46.
42. Filho, LC. Isolated influences of lip and palate surgery on facial growth: Comparison of operated and unoperated male adults. Cleft Palate Craniofac J. 1996; 33:51.
43. Fitzpatrick, B. Midface osteotomy in the adolescent cleft patient. Aust Dent J. 1977; 22:338.
44. Freihofer, HPM, Jr. The lip profile after correction of retro-maxillism in cleft and non-cleft patients. J Maxillofac Surg. 1976; 4:136–141.
45. Freihofer, HPM, Jr. Changes in nasal profile after maxillary advancement in cleft and non-cleft patients. J Maxillofac Surg. 1977; 5:20–27.
46. Freihofer, HPM, Jr. Results of osteotomies of the facial skeleton in adolescence. J Maxillofac Surg. 1977; 5:267.
47. Friede, H, Lilja, J. Dentofacial morphology in adolescent or early adult patients with cleft lip and palate after a treatment regime that included vomer flap surgery and pushback palate repair. Scand J Plast Reconstr Hand Surg. 1994; 28:113–121.
48. Fudalej, P, Hortis-Dzierzbicka, M, Dudkiewicz, Z, Semb, G. Dental arch relationship in children with complete unilateral cleft lip and palate following Warsaw (one-stage repair) and Oslo protocols. Cleft Palate Craniofac J. 2009; 46:648–653.
49. Fudalej, P, Hortis-Dzierzbicka, M, Obloj, B, et al. Treatment outcome after one-stage repair in children with complete unilateral cleft lip and palate assessed with the Goslon Yardstick. Cleft Palate Craniofac J. 2009; 46:374–380.
50. Garrison, BT, Lapp, TH, Bussard, DA. The stability of the Le Fort I maxillary osteotomies in patients with simultaneous alveolar cleft bone grafts. J Oral Maxillofac Surg. 1987; 45:761.
51. Georgiade, NG. Mandibular osteotomy for the correction of facial disproportion in the cleft lip and palate patient. Symposium on management of cleft lip and palate and associated deformities. Plast Reconstr Surg. 1974; 8:238.
52. Gillies, HD, Millard, DR, Jr. The principles and art of plastic surgery. Boston: Little, Brown; 1957.
53. Gillies, HD, Rowe, NL. L’ostéotomie du maxillaire supérieur enoisagée essentiellement dans le cas de bec-de-lièvre total. Rev Stomatol. 1954; 55:545–552.
54. Gnoinski, W. Early identification of candidates for corrective maxillary osteotomy in cleft lip and palate group. Scand J Plast Reconstr Surg. 1987; 21:39.
55. Good, PM, Mulliken, JB, Padwa, BL. Frequency of Le Fort I osteotomy after repaired cleft lip and palate or cleft palate. Cleft Palate Craniofac J. 2007; 44:396–401.
56. Grayson, BH, Cutting, CB. Presurgical nasoalveolar orthopedic molding in primary correction of the nose, lip, and alveolus of infants bone with unilateral and bilateral clefts. Cleft Palate Craniofac J. 2011; 38:193–198.
57. Guyette, TW, Polley, JW, Figueroa, A, Smith, BE. Changes in speech following maxillary distraction osteogenesis. Cleft Palate Craniofac J. 2001; 38:199–205.
58. Hall, HD, Posnick, JC. Early results of secondary bone grafts in 106 alveolar clefts. J Oral Maxillofac Surg. 1984; 41:289.
59. Harada, K, Baba, Y, Ohyama, K, et al. Maxillary distraction osteogenesis for cleft lip and palate children using an external, adjustable, rigid distraction device: A report of 2 cases. J Oral Maxillofac Surg. 2001; 59:1492.
60. Harada, K, Ishii, Y, Ishii, M, et al. Effect of maxillary distraction osteogenesis on velopharyngeal function: A pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 93:538.
61. Harada, K, Sato, M, Omura, K. Long-term maxillomandibular skeletal and dental changes in children with cleft lip and palate after maxillary distraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:292.
62. Harrison, JW. Dental implants to rehabilitate a patient with an unrepaired complete cleft. Cleft Palate Craniofac J. 1992; 29:485.
63. Hathaway, R, Daskalogiannakis, J, Mercado, A, et al. The Americleft study: An inter-center study of treatment outcomes for patients with unilateral cleft lip and palate part 2. Dental arch relationship. Cleft Palate Craniofac J. 2011; 48:244–251.
64. Hathaway, RR, Eppley, BLE, Hennon, DK, et al. Primary alveolar cleft bone grafting in UCLP: Arch dimensions at age 8. J Craniofac Surg. 1999; 10:58–67.
65. He, Dongmei, Genecov, DG, Barcelo, R. Nonunion of the external maxillary distraction in cleft lip and palate: Analysis of possible reasons. J Oral Maxillofac Surg. 2010; 68:2402–2411.
66. Hedemark, A, Freihofer, HP, Jr. The behavior of the maxilla in vertical movements after Le Fort I osteotomy. J Maxillofac Surg. 1978; 6:244.
67. Heliövaara, A, Ranta, R, Hukki, J, Rintala, A. Skeletal stability of Le Fort I osteotomy in patients with unilateral cleft lip and palate. Scand J Plast Reconstr Surg Hand Surg. 2001; 35:43–49.
68. Heliövaara, A, Hukki, J, Ranta, R, et al. Soft tissue profile changes after Le Fort I osteotomy in UCLP patients. J Craniomaxillofac Surg. 2000; 28:25–30.
69. Henderson, D, Jackson, IT. Combined cleft lip revision, anterior fistula closure and maxillary osteotomy: A one-stage procedure. Br J Oral Surg. 1975; 13:33.
70. Henkel, KO, Gundlach, KKH. Analysis of primary gingivoperiosteoplasty in alveolar cleft repair. Part I: facial growth. J Craniomaxillofac Surg. 1997; 25:266–269.
71. Hirano, A, Suzuki, H. Factors related to relapse after Le Fort I maxillary advancement osteotomy in patients with cleft lip and palate. Cleft Palate Craniofac J. 2001; 38:1–10.
72. Hochban, W, Ganss, C, Austermann, KH. Long-term results after maxillary advancement in patients with clefts. Cleft Palate Craniofac J. 1993; 30:237–243.
73. Houston, WJB, James, DR, Jones, E, Kavvadia, S. Le Fort I maxillary osteotomies in cleft palate cases. Surgical changes and stability. J Craniomaxillofac Surg. 1989; 17:9–15.
74. Hsieh, CH, Ko, EW, Chen, PK, Huang, CS. The effects of gingivoperiosteoplasty on facial growth in patients with complete unilateral cleft lip and palate. Cleft Palate Craniofac J. 2010; 47:439–446.
75. Huang, CS, Harikrishnan, P, Liao, YF, et al. Long-term follow-up after maxillary distraction osteogenesis in growing children with cleft lip and palate. Cleft Palate Craniofac J. 2007; 44:274.
76. Hui, E, Hägg, EU, Tideman, H. Soft tissue changes following maxillary osteotomies in cleft lip and palate and non-cleft patients. J Craniomaxillofac Surg. 1994; 22:182–186.
77. Jackson, IT. Cleft and jaw deformities. In: Symposium on Reconstruction of Jaw Deformities. C. V. Mosby Co. ; 1978:113.
78. James, D, Brook, K. Maxillary hypoplasia in patients with cleft lip and palate deformity—the alternative surgical approach. Eur J Orthop. 1985; 7:231.
79. Janulewicz, J, Costello, BJ, Buckley, MJ, et al. The effects of Le Fort I osteotomies on velopharyngeal and speech functions in cleft patients. J Oral Maxillofac Surg. 2004; 62:308–314.
80. Jorgenson, RJ, Shapiro, SD, Odiner, KL. Studies on facial growth and arch size in cleft lip and plate. J Craniofac Genet Dev Biol. 1984; 4:33.
81. Kanno, T, Mitsugi, M, Hosoe, M, et al. Long-term skeletal stability after maxillary advancement with distraction osteogenesis in nongrowing patients. J Oral Maxillofac Surg. 2008; 66:1833–1846.
82. Kapp-Simon, KA. Psychological interventions for the adolescent with cleft lip and palate. Cleft Palate Craniofac J. 1995; 32:104.
83. Kazanjian, VH. Remembrance of things past. Plast Reconstr Surg. 1965; 35(1):5–13.
84. Kiehn, CL, DesPrez, JD, Brown, F. Maxillary osteotomy for late correction of occlusion and appearance in cleft lip and palate patients. Plast Reconstr Surg. 1968; 42:203.
85. Kingsley, NW. A treatise on oral deformities (as a branch of mechanical surgery). New York: Appleton & Co; 1880.
86. Ko, EW, Figueroa, AA, Guyette, TW, et al. Velopharyngeal changes after maxillary advancement in cleft patients with distraction osteogenesis using a rigid external distraction device: 1-year cephalometric follow-up. J Craniofac Surg. 1999; 10:312–320.
87. Lanigan, DT. Wound healing after multisegmental Le Fort I osteotomy and transection of the descending palatine vessels [discussion]. J Oral Maxillofac Surg. 1995; 53:1433.
88. Laspos, CP, Kyrkanides, S, Moss, ME, et al. Mandibular and maxillary asymmetry in individuals with unilateral cleft lip and palate. Cleft Palate Craniofac J. 1997; 34:232.
89. Laspos, CP, Kyrkanides, S, Tallents, RH, et al. Mandibular asymmetry in noncleft and unilateral cleft lip and palate individuals. Cleft Palate Craniofac J. 1997; 34:410.
90. Leonard, BJ, Brust, JD, Abrahams, G, et al. Self-concept of children and adolescents with cleft lip and/or palate. Cleft Palate Craniofac J. 1991; 28:347.
91. Linton, JL. Comparative study of diagnostic measures in borderline surgical cases of unilateral cleft lip and palate and noncleft class III malocclusions. Am J Orthod Dentofacial Orthop. 1998; 113:526–537.
92. Luhr, HG. Zur stabilen osteosynthese bei unterkiefer-frakturen. Dtsch Zahnarztl Z. 1968; 23:754.
93. Lund, TW, Wade, M. Use of osseointegrated implants to support a maxillary denture for a patient with repaired cleft lip and palate. Cleft Palate Craniofac J. 1993; 30:418.
94. Mansour, S, Burstone, C, Legan, H. An evaluation of soft-tissue changes resulting from Le Fort I maxillary surgery. Am J Orthod. 1983; 84:37–47.
95. Marrinan, EM, LaBrie, RA, Mulliken, JB. Velopharyngeal function in nonsyndromic cleft palate: Relevance of surgical technique, age at repair, and cleft type. Cleft Palate Craniofac J. 1998; 35:95–100.
96. Mars, M, Asher-McDade, C, Brattström, V, et al. A six-center international study of treatment outcomes in patients with clefts of the lip and palate. Part 3: Dental arch relationships. Cleft Palate Craniofac J. 1992; 29:405–408.
97. Mars, M, Houston, WJB. A preliminary study of facial growth and morphology in unoperated male unilateral cleft lip and palate subjects over 13 years of age. Cleft Palate J. 1990; 27:7–10.
98. Mars, M, Plint, DA, Houston, WJB, et al. The Goslon Yardstick: A new system of assessing dental arch relationships in children with unilateral clefts of the lip and palate. Cleft Palate J. 1987; 24:314–322.
99. Matic, DB, Power, SM. The effects of gingivoperiosteoplasty following alveolar molding with a pun-retained Latham appliance versus secondary bone grafting on midfacial growth in patients with unilateral clefts. Plast Reconstr Surg. 2008; 122:863–870.
100. McCance, AM, Moss, JP, Fright, WR, et al. Three-dimensional analysis techniques. Part 1: Three-dimensional soft-tissue analysis of 24 adult cleft palate patients following Le Fort I maxillary advancement: A preliminary report. Cleft Palate Craniofac J. 1997; 34:36.
101. McCance, AM, Orth, M, Moss, JP, et al. Three-dimensional analysis techniques. Part 4: Three-dimensional analysis of bone, and soft tissue to bone ratio of movements in 24 cleft patients following Le Fort I osteotomy: A preliminary report. Cleft Palate Craniofac J. 1997; 43:58–62.
102. McComb, R, Marrinan, E, Nuss, RC, et al. Predictors of velopharyngeal insufficiency after Le Fort I maxillary advancement in patients with cleft palate. J Oral Maxillofac Surg. 2011; 69:2226–2232.
103. McIntyre, GT, Devlin, MF. Secondary alveolar bone grafting (CLEFTSiS) 2000-2004. Cleft Palate Craniofac J. 2010; 47:66–72.
104. McKinstry, RE. Cleft palate dental care: A historical perspective. Arlington, Va: ABI Publications; 2000.
105. Millard, DR, Jr., Latham, RA. Improved primary surgical and dental treatment of clefts. Plast Reconstr Surg. 1990; 86:856–871.
106. Millard, DR, Latham, RA, Huifen, X, et al. Cleft lip and palate treated by presurgical orthopedics, gingivoperiosteoplasty, and lip adhesion (POPLA) compared with previous lip adhesion method: a preliminary study of serial dental casts. Plast Reconstr Surg. 1999; 103:1630–1644.
107. Molina, F, Ortiz Monasterio, F, de la Paz Aguilar, M, Barrera, J. Maxillary distraction: Aesthetic and functional benefits in cleft lip-palate and prognathic patients during mixed dentition. Plast Reconstr Surg. 1998; 101:951.
108. Mølsted, K, Brattström, V, Prahl-Andersen, B, et al. The Eurocleft study: Intercenter study of treatment outcomes in patients with complete cleft lip and palate. Part 3: Dental arch relationships. Cleft Palate Craniofac J. 2005; 42:78–82.
109. Motohashi, N, Kuroda, T, Filho, LC, et al. P-A cephalometric analysis of nonoperated adult cleft lip and palate. Cleft Palate Craniofac J. 1994; 31:193.
110. Nanda, SK. Patterns of vertical growth in the face. Am J Orthod Dentofacial Orthop. 1988; 93:103–116.
111. Nollet, PJPM, Katsaros, C, van’t Hof, MA, et al. Treatment outcome after two-stage palatal closure in unilateral cleft lip and palate: A comparison with Eurocleft. Cleft Palate Craniofac J. 2005; 42:512–516.
112. Nordquist, GG, McNeill, RW. Orthodontic vs restorative treatment of the congenitally absent lateral incisor—long-term periodontal and occlusal evaluation. J Periodontol. 1975; 46:139–143.
113. Obwegeser, HL, Correction of the facial appearance of harelip and cleft palate patients by surgery on the jaws. 1966.
114. Obwegeser, HL. Surgery as an adjunct to orthodontics in normal and cleft palate patients. Trans Eur Orthod Soc. 1967; 42:343–353.
115. Obwegeser, HL, Surgical correction of deformities of the jaws in adult cleft cases. 1969.
116. Obwegeser, HL. Surgical correction of small or retrodisplaced maxillae: The “dish-face” deformity. Plast Reconstr Surg. 1969; 43:351.
117. Obwegeser, HL. Surgical correction of maxillary deformities. In: Grabb WC, Rosenstein SW, Bzoch KR, eds. Cleft lip and palate. Boston: Little and Crown; 1971:515–556.
118. Obwegeser, HL. Chirurgische behandlungsmoglichkeiten von sekundardeformierungen bei spaltpatienten. Fortschr. Kieferorthop. 1988; 49:272–296.
119. Obwegeser, HL. Orthognathic surgery and a tale of how three procedures came to be: A letter to the next generations of surgeons. Clin Plast Surg. 2007; 34:331–355.
120. Obwegeser, HL, Lello, GE, Farmand, M, Correction of secondary cleft deformities. Surgical correction of dentofacial deformities. New Concepts. Bell, WH, eds. Surgical correction of dentofacial deformities. New Concepts; Vol III, Vol 13. Saunders, Philadelphia, 1985:592–638.
121. Palmer, CR, Hamlen, M, Ross, RB, Lindsay, WK. Cleft palate repair: Comparison of the results of two surgical techniques. Can J Surg. 1969; 12:32–67.
122. Parel, SM, Branemark, PI, Jansson, T. Osseointegration in maxillofacial prosthetics: Part I: Intraoral applications. J Prosthet Dent. 1986; 55:490.
123. Perko, M. The history of treatment of cleft lip and palate. Prog Pediatr Surg. 1986; 20:239–248.
124. Perrott, D, Sharma, AB, Vargevik, K. Endosseous implants for pediatric patients: Unknown factors, indications, contraindications, and special considerations. Oral Maxillofac Surg Clin North Am. 1994; 6:79.
125. Phillips, JH, Klaiman, P, Delorey, R, MacDonald, DB. Predictors of velopharyngeal insufficiency in cleft palate orthognathic surgery. Plast Reconstr Surg. 2005; 115:681–686.
126. Polley, JW, Figueroa, AA. Management of severe maxillary deficiency in childhood and adolescence through distraction osteogenesis with an external, adjustable, rigid distraction device. J Craniofac Surg. 1997; 8:181.
127. Polley, JW, Figueroa, AA. Rigid external distraction: Its application in cleft maxillary deformities. Plast Reconstr Surg. 1998; 102:1360.
128. Poole, MD, Robinson, PP, Nunn, ME. Maxillary advancement in cleft lip and palate patients: A modification of the Le Fort I osteotomy and preliminary results. J Maxillofac Surg. 1986; 14:123.
129. Posnick, JC. (Discussion of) Orthognathic surgery in cleft patients treated by early bone grafting. Plast Reconstr Surg. 1991; 87:840.
130. Posnick, JC, Orthognathic surgery in the cleft patient. Instructional courses, plastic surgery education foundation. Russel, RC, eds. Instructional courses, plastic surgery education foundation; Vol 4. CV Mosby Co, St Louis, Mo, 1991:129–157.
131. Posnick, JC. Orthognathic surgery for the cleft lip and palate patient. Semin Orthod. 1996; 2(3):205–214.
132. Posnick, JC. The treatment of secondary and residual dentofacial deformities in the cleft patient. Surgical and orthodontic therapy. Clin Plast Surg. 1997; 24(3):583–597.
133. Posnick, JC, Cleft lip and palate: Bone grafting and management of residual oro-nasal fistula. Craniofacial and maxillofacial surgery in children and young adults. Posnick, JC, eds. Craniofacial and maxillofacial surgery in children and young adults; Vol 33. WB Saunders Co, Philadelphia, 2000:827–859.
134. Posnick, JC, Cleft-orthognathic surgery: The unilateral cleft lip and palate deformity. Craniofacial and maxillofacial surgery in children and young adults. Posnick, JC, eds. Craniofacial and maxillofacial surgery in children and young adults; Vol 34. WB Saunders Co, Philadelphia, 2000:860–907.
135. Posnick, JC, The staging of cleft lip and palate reconstruction: Infancy through adolescence. Craniofacial and maxillofacial surgery in children and young adults. Posnick, JC, eds. Craniofacial and maxillofacial surgery in children and young adults; Vol 32. WB Saunders Co, Philadelphia, 2000:785–826.
136. Posnick, JC, Agnihotri, N. Managing chronic nasal airway obstruction at the time of orthognathic surgery: A twofer. J Oral Maxillofac Surg. 2011; 69:695–701.
137. Posnick, JC, Al-Qattan, MM, Pron, G. Facial sensibility in cleft and non-cleft adolescents one year after undergoing Le Fort I osteotomy. Plast Reconstr Surg. 1994; 194(3):431–435.
138. Posnick, JC, Dagys, AP. Skeletal stability and relapse patterns after Le Fort I maxillary osteotomy fixed with miniplates: The unilateral cleft lip and palate deformity. Plast Reconstr Surg. 1994; 94(7):924–932.
139. Posnick, JC, Ewing, MP. Skeletal stability after Le Fort I maxillary advancement in patients with unilateral cleft lip and palate. Plast Reconstr Surg. 1990; 85(5):706–710.
140. Posnick, JC, Tompson, B. Modification of the maxillary Le Fort I osteotomy in cleft-orthognathic surgery: The unilateral cleft lip and palate deformity. J Oral Maxillofac Surg. 1992; 50(7):666–675.
141. Posnick, JC, Tompson, B. Cleft-orthognathic surgery: Complications and long-term results. Plast Reconstr Surg. 1995; 96(2):255–266.
142. Posnick, JC, Ricalde, P. Cleft-orthognathic surgery. Clin Plast Surg. 2004; 31(2):315–330.
143. Renkielska, A, Wojtaszek-Slominska, A, Dobke, M. Early cleft lip repair in children with unilateral complete cleft lip and palate: a case against primary alveolar repair. Ann Plast Surg. 2005; 54:595–597.
144. Roberts, HG, Semb, G, Hathorn, I, Killingback, N. Facial growth in patients with unilateral clefts of the lip and palate: A two-center study. Cleft Palate Craniofac J. 1996; 31:372–375.
145. Robertsson, S, Mohlin, B. The congenitally missing upper lateral incisor: A retrospective study of orthodontic space closure versus restorative treatment. Eur J Orthod. 2000; 22:697–710.
146. Ronchi, P, Chiapasco, M, Frattini, D. Endosseous implants for prosthetic rehabilitation in bone grafted alveolar clefts. J Craniomaxillofac Surg. 1995; 23:382.
147. Rosenstein, SW. Facial growth and the need for orthognathic surgery after cleft palate repair: Literature review and report of 28 cases [discussion]. J Oral Maxillofac Surg. 1997; 55:698.
148. Ross, BR. Treatment variables affecting facial growth in complete unilateral cleft lip and palate. Part 7: An overview of treatment and facial growth. Cleft Palate J. 1987; 24:71–77.
149. Samman, N, Cheung, LK, Tideman, H. A comparison of alveolar bone grafting with and without simultaneous maxillary osteotomies in cleft palate patients. Int J Oral Maxillofac Surg. 1994; 23:65–70.
150. Sandham, A, Murray, JAM. Nasal septal deformity in unilateral cleft lip and palate. Cleft Palate Craniofac J. 1993; 30:222.
151. Saperstein, EL, Kennedy, DL, Mulliken, JB, Padwa, BL. Facial growth in children with complete cleft of the primary palate and intact secondary palate. J Oral Maxillofac Surg. 2012; 70:e66–e71.
152. Schnitt, DE, Agir, H, David, DJ. From birth to maturity: A group of patients who have completed their protocol management. Part I. Unilateral cleft lip and palate. Plast Reconstr Surg. 2004; 113:805–817.
153. Shaw, WC, Asher-McDade, C, Brattstrom, V, et al. A six-center international study of treatment outcomes in patients with clefts of the lip and palate. Cleft Palate Craniofac J. 1992; 29:393.
154. Sinko, K, Caacbay, E, Eagsch, R, et al. The Goslon Yardstick in patients with unilateral cleft lip and palate: Review of a Vienna sample. Cleft Palate Craniofac J. 2008; 45:87–91.
155. Sinn, DP. Simultaneous maxillary expansion and advancement, repair of oronasal fistula, and bone grafting of the alveolar cleft. In: Bell WH, Proffit WR, White RP, eds. Surgical correction of dentofacial deformities. Philadelphia: WB Saunders, 1980.
156. Skoog, T. The use of periosteal flaps in the repair of cleft of the primary palate. Cleft Palate J. 1965; 2:332–339.
157. Smabel, Z. Treatment effects on facial development in patients with unilateral cleft lip and palate. Cleft Palate Craniofac J. 1994; 31:437.
158. Steinkamm, W, Die pseudo-progenie und ihre behandlung. 1938.
159. Stoelinga, PJ, Haers, PE, Lennen, RJ, et al. Late management of secondarily grafted clefts. Int J Oral Maxillofac Surg. 1990; 19:91.
160. Stoelinga, PJ, vd Vijver, HR, Leenen, RJ, et al. The prevention of relapse after maxillary osteotomies in cleft palate patients. J Craniomaxillofac Surg. 1987; 15:325.
161. Susami, T, Ogihara, Y, Matsuzaki, M, et al. Assessment of dental arch relationships in Japanese patients with unilateral cleft lip and palate. Cleft Palate Craniofac J. 2006; 43:96–102.
162. Suzuki, EY, Motohashi, N, Ohyama, K. Longitudinal dento-skeletal changes in UCLP patients following maxillary distraction osteogenesis using RED system. J Med Dent Sci. 2004; 51:27–33.
163. Suzuki, A, Takahama, Y. Maxillary lateral incisor of subjects with cleft lip and/or palate: Part 1. Cleft Palate Craniofac J. 1992; 29:376.
164. Suzuki, A, Takahama, Y. Maxillary lateral incisor of subjects with cleft lip and/or palate: Part 2. Cleft Palate Craniofac J. 1992; 29:380.
165. Takahashi, T, Fukuda, M, Yamaguchi, T, et al. Use of an osseointegrated implant for dental rehabilitation after cleft repair by periosteoplasty: A case report. Cleft Palate Craniofac J. 1997; 35:268.
166. Takahashi, T, Fukuda, M, Yamaguchi, T, et al. Use of endosseous implants for dental reconstruction of patients with grafted alveolar clefts. J Oral Maxillofac Surg. 1997; 55:576.
167. Tessier, P, Tulasne, JF. Secondary repair of cleft lip deformity. Clin Plast Surg. 1984; 11:747.
168. Tideman, H, Stoelinga, P, Gallia, L. Le Fort I advancement with segmental palatal osteotomies in patients with cleft palates. J Oral Surg. 1980; 38:196.
169. Tomanova, M, Mullerova, Z. Growth of the dental arch in patients with complete unilateral cleft lip and palate after primary periosteoplasty. Acta Chir Plast. 1994; 36:119–123.
170. Trindale, IE, Yamashita, RP, Suguimoto, RM, et al. Effects of orthognathic surgery on speech and breathing of subjects with cleft lip and palate: Acoustic and aerodynamic assessment. Cleft Palate Craniofac J. 2003; 40:54–64.
171. Tulloch, JFC. A six-center international study of treatment outcome in patients with clefts of the lip and palate: Evaluation of maxillary asymmetry (commentary). Cleft Palate Craniofac J. 1993; 30:22.
172. Turvey, TA. Use of the Branemark implant in the cleft palate patient. Cleft Palate Craniofac J. 1991; 28:304.
173. Turvey, TA, Vig, KWL, Fonseca, RJ. Maxillary advancement and contouring in the presence of cleft lip and palate. In: Turvey TA, Vig KWL, Fonseca RJ, eds. Facial clefts and craniosynostosis: Principles and management. Philadelphia: Saunders; 1996:441–503.
174. Verdi, FJ, Jr., Shanzi, GL, Cohen, SR, et al. Use of the Branemark implant in the cleft palate patient. Cleft Palate Craniofac J. 1991; 28:301.
175. Ward-Booth, RP, Bhatia, SN, Moos, KF. A cephalometric analysis of the Le Fort II osteotomy in the adult cleft patient. J Maxillofac Surg. 1984; 12:208.
176. Westbrook, MT, Jr., West, RA, McNeil, RW. Simultaneous maxillary advancement and closure of bilateral alveolar clefts and oronasal fistulas. J Oral Maxillofac Surg. 1983; 41:257.
177. Williams, AC, Bearn, D, Mildinhall, S, et al. Cleft lip and palate care in the United Kingdom—the Clinical Standards Advisory Group (CSAG) study. Part 2: Dentofacial outcomes and patient satisfaction. Cleft Palate Craniofac J. 2001; 38:24–29.
178. Willmar, K. On Le Fort I osteotomy: A follow-up study of 106 operated patients with maxillo-facial deformity. Scand J Plast Reconstr Surg. 1974; 12(Suppl 1):1–68.
179. Wiltfang, J, Hirschfelder, U, Neukam, FW, et al. Long-term results of distraction osteogenesis of the maxilla and midface. Br J Oral Maxillofac Surg. 2002; 40:473.
180. Witzel, MA, Munro, IR. Velopharyngeal insufficiency after maxillary advancement. Cleft Palate J. 1977; 14:176.
181. Wolford, LM. Effects of orthognathic surgery on nasal form and function in the cleft patient. Cleft Palate Craniofac J. 1992; 29:546–555.
182. Wolford, LM, Cassano, DS, Cottrell, DA, et al. Orthognathic surgery in the young cleft patient: Preliminary study on subsequent facial growth. J Oral Maxillofac Surg. 2008; 66:2524–2536.
183. Wolford, LM, Karras, SC, Mehra, P. Considerations for orthognathic surgery during growth: Part 1. Mandibular deformities. Am J Orthod Dentofacial Orthop. 2001; 119:95.
184. Wolford, LM, Karras, SC, Mehra, P. Considerations for orthognathic surgery during growth: Part 2. Maxillary deformities. Am J Orthod Dentofacial Orthop. 2001; 119:102.
185. Zachrisson, BU, Stenvik, A. Single implants—optimal therapy for missing lateral incisors? Am J Orthod Dentofacial Orthop. 2004; 126:13A–15A.
*References 14, 15, 28, 29, 42, 47–49, 54, 80, 98, 96, 97, 109–111, 121, 144, 147, 152–154, 157, 161
*References 1, 10, 17, 58, 62, 64, 74, 93, 112, 122, 124, 145, 146, 159, 161, 163, 165, 166, 172, 174, 176, 185
*References 3, 11–13, 18, 21–24, 27, 35–37, 40, 41, 43, 46, 50, 55, 59, 61, 65–67, 71–73, 81, 107, 127, 129–142, 149, 160, 162, 168, 173, 179