Chapter 4 Cleaning and Disinfecting Gastrointestinal Endoscopic Equipment
Introduction
Since the first report of fiberoptic GI endoscopy in 1961,1 the endoscope has undergone almost continuous evolution in design. Although most of these developments have been aimed at improving the diagnostic and therapeutic capability of GI endoscopy, the introduction of fully immersible endoscopes in 1983 greatly facilitated cleaning and disinfection of the internal channels of the endoscope.2,3 The development of video imaging technology, which provided a tremendous increase in the quality and resolution of the endoscopic image, had few implications for endoscope reprocessing. However, some changes have come at the cost of increasing complexity of design, presenting new challenges to cleaning and disinfection. The addition of an elevator lever to the duodenoscope allowed easier cannulation of the papilla during endoscopic retrograde cholangiopancreatography (ERCP), although the new exposed movable part at the distal tip of the instrument and the associated control-wire channel also added new reprocessing steps. A similar type of elevator is present on current endoscopic ultrasound (EUS) endoscopes, or echoendoscopes. Echoendoscopes also possess an additional channel to inflate a balloon at the tip (needed to create the acoustic interface) that must be cleaned and disinfected. The incorporation of a dedicated high-flow water irrigation channel (distinct from the standard air and water channels) in some models of endoscopes adds yet another channel that requires reprocessing (regardless of use) in addition to the external equipment that connect to this channel.
Current reprocessing guidelines are discussed in detail. These guidelines, although applicable to nearly all GI endoscopes, do not apply to sheathed endoscope systems. One endoscopic sheath system that is approved by the U.S. Food and Drug Administration (FDA) is commercially available.4–8 In contrast to the popular misconception of an “endoscope condom,” the sheath is actually a part of the endoscope insertion tube and contains several channels. Because this is a complete endoscope system, the sheaths are not compatible with other endoscopes. Although the sheath itself is disposable and does not need conventional cleaning and disinfection (i.e., a new sheath is used for each procedure), the control dials on the handpiece are not protected and do require reprocessing. These dials are removable and require conventional cleaning and disinfection or sterilization. There are two main disadvantages of the system: (1) The only currently marketed sheathed endoscope for use in the GI tract is a flexible sigmoidoscope; (2) the imaging technology of the instrument uses fiberoptic rather than video-chip technology.9 Readers should refer to the manufacturer’s instructions for reprocessing this type of endoscope.
Principles of Disinfection
Definitions
Cleaning is a term that is both simple to understand and difficult to define precisely in terms of a measurable endpoint. The official definition of cleaning used by the FDA is “the removal, usually with detergent and water, of adherent visible soil, blood, protein substances, and other debris from the surfaces, crevices, serrations, joints, and lumens of instruments, devices, and equipment by a manual or mechanical process that prepares the items for safe handling and/or further decontamination.”10 Although this definition seems straightforward, there is as yet no uniform consensus on how this process is operationally defined or what the endpoint of the process should be. How hot should the water be, and what concentration of detergent should be used? How many times should the cleaning brush be passed down the endoscope channels? What does “visibly clean” mean, and how can this be applied to the internal channels of an endoscope that cannot be examined? Many experimental methods can be used to determine the efficacy of cleaning by the detection of residual protein, carbohydrate, blood, or viral or bacterial RNA or DNA,11–16 although these are impractical for routine clinical use.
Despite the difficulty in precisely defining the process or the subsequent endpoint, there is ample evidence that endoscope cleaning (as currently performed) is an essential part of the disinfection process. Mechanical cleaning alone reduces microbial counts by approximately 103–106 (three to six logs), or a 99.9% to 99.9999% reduction.17–24 Cleaning is an integral part of any endoscope reprocessing regimen because failure to clean endoscopes or their accessories adequately can defeat disinfection or sterilization processes.25
For liquid chemical germicides (LCGs), high-level disinfection is operationally defined as the ability to kill 106 mycobacteria (a six-log reduction). The FDA defines a high-level disinfectant as a sterilant that is used for a shorter contact time.26 This difference in the way the same chemical is used to achieve different levels of disinfection and sterilization is important for endoscopy because the contact times for sterilization with any given LCG are generally much longer (hours) than for high-level disinfection (minutes) and may be detrimental to the endoscope. The relative resistance of various microorganisms to LCGs is shown in Box 4.1.
Box 4.1
Descending Order of Resistance of Microorganisms to Liquid Chemical Germicides
Modified from Bond WW, Ott BJ, Franke KA, et al: Effective use of liquid chemical germicides on medical devices: instrument design problems. In Block SS, editor: Disinfection, sterilization, and preservation, ed 4, Philadelphia, 1991, Lea & Febiger, pp 1097–1106.
Sterilization is the destruction or inactivation of all microorganisms, or the absence of all microbial life. As an endpoint, it is an absolute (sterile or not sterile). The process is operationally defined as a 12-log reduction of bacterial endospores.27 Not all sterilization processes are alike, however. Steam and dry heat are the most extensively characterized processes; both are thermal methods that do not require the same physical contact as LCGs to achieve sterilization, and the processes are routinely monitored by the use of biologic indicators (e.g., spore test strips) to show that sterilization has been achieved. Although theoretically sterilization could be achieved with LCGs, the FDA and other authorities have stated that these processes do not convey the same sterility assurance as other sterilization methods.26,28,29
The Spaulding classification system divides medical devices into categories based on the risk of infection involved with their use.30,31 With some modifications, this classification scheme is widely accepted nationally and internationally and has been used by the FDA, the Centers for Disease Control and Prevention (CDC), epidemiologists, microbiologists, and professional medical organizations to determine the degree of disinfection or sterilization needed for various medical instruments. Three categories of medical devices and their associated level of disinfection are recognized, as follows:
Disinfection and Gastrointestinal Endoscopy
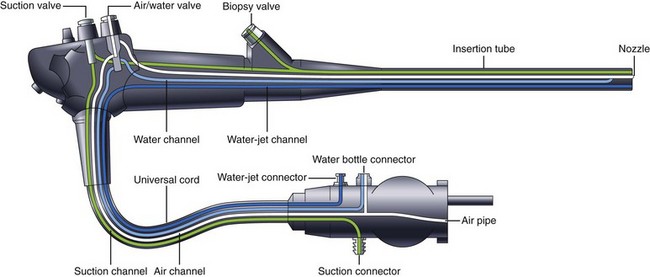
GI endoscopes are considered semicritical devices and should undergo at least high-level disinfection. This standard has been endorsed by the FDA32; the CDC33; and numerous professional medical organizations, including the American Society for Gastrointestinal Endoscopy (ASGE), the American College of Gastroenterology (ACG), the American Gastroenterology Association (AGA), the Society of Gastroenterology Nurses and Associates (SGNA), the Association of Perioperative Registered Nurses (AORN), the Association for Professionals in Infection Control and Epidemiology (APIC), and the American Society for Testing and Materials (ASTM).34–37 Because of design considerations, GI endoscopes can be a challenge to clean and disinfect. Endoscopes are heat-labile instruments and cannot be steam autoclaved. They possess several long, narrow internal channels with bends (Fig. 4.1) that require exposure to the LCG to achieve high-level disinfection. Generally, the air and water channels are too narrow to allow the passage of a cleaning brush (although the LCG is routinely circulated through this channel); however, one manufacturer has designed an endoscope with air and water channels that can be brushed.38 Despite the complex internal design, high-level disinfection is not difficult to achieve with rigorous adherence to currently accepted guidelines. Most accessory instruments used during endoscopy either contact the bloodstream (e.g., biopsy forceps, snares, and sphincterotomes) or enter sterile tissue spaces (e.g., biliary tract) and are classified as critical devices. As such, these devices require sterilization.
Most accessories used during GI endoscopy are labeled by the FDA for single use (i.e., disposable) and are intended to be discarded at the end of the procedure. Because these items are sterilized by the manufacturer, reprocessing is not an issue. However, some accessories are designed to be resterilized and reused and are designated as such by FDA. In this case, cleaning and sterilization is performed by the user according to the manufacturer’s instructions. The issue of sterilization of endoscopic accessories becomes considerably more complex when the reuse of single-use devices (SUDs) is considered. Although labeled for single use (disposable), many hospitals safely clean, resterilize, and reuse SUDs, resulting in decreased costs and reduced medical waste generation.39–42 Despite the absence of evidence suggesting that this practice resulted in patient injury, the FDA issued a guidance document on August 14, 2000, that altered the agency’s regulatory policy. The FDA considered the process of reprocessing (i.e., cleaning and sterilizing) a used SUD into a ready-for-patient-use device as “manufacturing,” and as a result hospitals or third-party reprocessing companies that reprocessed SUDs were required to follow the same regulations as the original equipment manufacturers: premarket notification and approval requirements, including 510(k) and premarket approval application (PMA); registration and listing; submission of adverse event reports; manufacturing and labeling requirements; tracking of devices; and correcting or removing from the market unsafe medical devices. Enforcement of these regulations was phased in over the subsequent 18 months (all aspects taking effect by February 14, 2002). The most onerous requirement was that a 510(k) or PMA was needed for each device that the institution intended to reprocess (both manufacturer and model-specific).43 The regulatory burden imposed by these requirements essentially eliminated the practice of reprocessing of SUDs by most hospitals.
Risks of Inadequate Disinfection
Before discussing the specifics of current guidelines for endoscope cleaning and disinfection, it is helpful to understand how guidelines evolved over time in response to episodes of infection to minimize or eliminate vulnerabilities in the reprocessing procedure. Initially, endoscopes were simply washed with tap water and detergent, followed by exposure to alcohol.44 In the 1970s, centers began using various “disinfectants” to reprocess endoscopes.45–52 The germicides used were generally antiseptic agents. Many of the agents that were considered to be effective at that time (e.g., alcohols, phenolics, iodophors, quaternary ammonium compounds, and chlorhexidine) have since been shown to be inadequate for high-level disinfection of GI endoscopes (Table 4.1).53
Table 4.1 Pathogens Reportedly Transmitted during Gastrointestinal Endoscopy
| Organism | Probable Cases | Failure in Reprocessing Guideline |
|---|---|---|
| Pseudomonas aeruginosa | 227 | Failure to clean/disinfect between patients |
| Inadequate cleaning | ||
| Inadequate disinfectant | ||
| Failure to disinfect all channels (particularly elevator channel) | ||
| Failure to disinfect/sterilize water bottle | ||
| Failure to dry with 70% alcohol | ||
| Faulty/contaminated AER (n = 143) | ||
| Salmonella spp. | 48 | Inadequate cleaning |
| Inadequate disinfectant | ||
| Failure to sterilize forceps | ||
| Helicobacter pylori | 10 | Forceps not cleaned or sterilized between patients |
| Inadequate cleaning | ||
| Inadequate disinfectant | ||
| Klebsiella pneumoniae | 5 | Failure to dry with 70% alcohol |
| Failure to disinfect elevator channel | ||
| Hepatitis C virus | 4 | Inadequate disinfectant |
| Inadequate exposure to LCG | ||
| Failure to disinfect all channels with LCG | ||
| Failure to sterilize forceps | ||
| Serratia marcescens | 2 | Inadequate disinfectant |
| Failure to dry with 70% alcohol | ||
| Failure to disinfect elevator channel | ||
| Enterobacter spp. | 2 | Inadequate cleaning |
| Inadequate disinfectant | ||
| Hepatitis B virus | 1 | Inadequate cleaning |
| Inadequate disinfectant | ||
| Failure to disinfect all channels with LCG | ||
| Trichosporon spp. | 1 | Failure to sterilize forceps |
AER, automatic endoscope reprocessor; LCG, liquid chemical germicide.
From Nelson DB: Infectious disease complications of GI endoscopy. Part II. Exogenous infections. Gastrointest Endosc 57:695–711, 2003, with permission from the American Society for Gastrointestinal Endoscopy.
To standardize the cleaning and disinfection process, the ASGE, the AGA, and the ACG published joint guidelines on endoscope reprocessing in 1988. Key components of these guidelines were the emphasis on thorough manual cleaning of the instrument and all channels, high-level disinfection with an approved LCG (with a 10-minute exposure for glutaraldehyde specified at that time), a water rinse to remove residual sterilant, and a final drying step with forced air. The handles of nonimmersible endoscopes were to be cleaned with alcohol.54 The British Society of Gastroenterology (BSG) published similar guidelines the same year, although notable differences included a recommended exposure time for glutaraldehyde of 4 minutes, the use of quaternary ammonium detergents as an acceptable second-line disinfectant, and only a brief mention of drying.55 One of the authors of the BSG guidelines interpreted the guidelines as applying only to the insertion tube (which had direct patient contact) rather than to the entire endoscope (particularly the control handpiece, which was not high-level disinfected) and recommended that if the handpiece was “extensively contaminated,” or if the next patient was known to be immunocompromised, only then was high-level disinfection of the entire instrument necessary. If the instrument was not submersible, cleaning with alcohol and chlorhexidine was “practical.”56
More recent guidelines in the United States from multiple organizations have been uniformly consistent (all endorsing a 20-minute exposure to glutaraldehyde at room temperature).34–3657 The importance of close adherence to reprocessing guidelines becomes apparent in the subsequent section. The major difference with formal guidelines originating outside the United States has been the endorsement of a shorter glutaraldehyde exposure time of 10 minutes.58–60 Actual facility practices in other countries can vary substantially, highlighting the difficulty in generalizing reports of infection to the experience in the United States.61–65
Specific Agents
The most commonly reported infectious agent transmitted during GI endoscopy is Pseudomonas aeruginosa, with 227 cases described in the medical literature (see Table 4.1).25 P. aeruginosa is an opportunistic pathogen that is widely found in the environment, and the organism thrives in a moist environment.66 Endoscopes and their ancillary equipment are a potential reservoir and may serve as a source of contamination. Early reports of Pseudomonas transmission during endoscopy (similar to reports of other organisms at that time) were generally related to inadequate cleaning or the use of inadequate disinfectants; however, later reports have centered around the following major areas: (1) flawed automatic endoscope reprocessor (AER) units (responsible for more than half of the reported cases), (2) failure to disinfect or sterilize the irrigation bottle of the endoscope regularly, (3) failure to recognize and disinfect the elevator channel of duodenoscopes, and (4) failure to dry the endoscope and all channels completely with a 70% alcohol solution followed by forced air.
There have been 48 cases of Salmonella species attributed to GI endoscopy.25 In these reports, failure to clean the internal instrument channels mechanically was a uniform occurrence, and this was usually compounded by the use of an ineffective disinfectant. Because these cases were relatively early in the evolution of endoscope reprocessing (and preceded the guidelines standardizing these protocols), it is not surprising that there have been no reported cases of Salmonella transmission since 1987.
The 10 reported cases of endoscopic Helicobacter pylori transmission are almost as interesting as the initial confirmatory study by Marshall with self-inoculation. In one case, the author underwent endoscopy immediately after the instrument had been used in a patient known to harbor H. pylori. The endoscope was reprocessed by wiping the insertion tube with a paper towel soaked with benzethonium chloride and sucking the “disinfectant” through the instrument channels without cleaning. Perhaps predictably, the author developed acute H. pylori infection.67 Another case was associated with endoscopic research dealing with H. pylori and was attributed to failure to clean and sterilize (or even disinfect) the endoscopic biopsy forceps between subjects (although reprocessing of the endoscope or other ancillary study equipment is not mentioned).68 The remaining cases were due to inadequate cleaning and the use of inadequate LCGs.
Much greater anxiety is associated with the possibility of transmission of viral infections. This anxiety is surprising because the viruses of greatest concern (i.e., hepatitis B virus [HBV], hepatitis C virus [HCV], and human immunodeficiency virus [HIV]) are among the easiest microorganisms to destroy with standard reprocessing. Before the advent of the reprocessing guidelines in 1988, there were three cases of HBV attributed to endoscopy. Two early reports suggested a temporal relationship between the use of an endoscope in an HBV-positive individual preceding the case and subsequent development of HBV infection, although in both cases no actual investigation was performed, and endoscope cleaning and disinfection were unacceptable by current standards.69,70 In the third case, subtyping of HBV was used to confirm that transmission was likely. In this instance, the air and water channels were not exposed to glutaraldehyde.71 Two more recent cases of HBV infection attributed to endoscopy are unlikely.25,72,73
There have been four cases of HCV transmission during GI endoscopy, all outside the United States. In three cases, a breach in currently accepted guidelines for endoscope reprocessing was reported.74,75 In the fourth case, the transmission was believed to be due to contamination of multidose vials used for sedation (and associated with the procedure but not the endoscope itself).76 This type of contamination was also the case in outbreaks of HCV at two endoscopy clinics in 2002 and 2007 in the United States. In the first case, the cause was initially attributed to deficient endoscope reprocessing practices by the lay press, but subsequent investigation by the New York State Department of Health determined that the cause was the improper reuse of needles and contamination of multidose vials.77 A similar cause was found for the transmission of HCV in at least six patients at a Nevada endoscopy clinic.78 These cases highlight the importance of general infection control practices, which are discussed later.
No cases of endoscopic transmission of HIV have been reported. Three studies have shown that glutaraldehyde disinfection of endoscopes contaminated with HIV completely eliminates the virus.79–81
There have been 317 putative episodes of transmission of infection reported in the medical literature. In the absence of defective equipment (notably the automated endoscope reprocessor), there has been a failure to follow currently accepted guidelines for cleaning and disinfection in each case.25 These deficient practices can be summarized as follows:
Liquid Chemical Germicides
The FDA defines a high-level disinfectant as a sterilant that is used under the same contact conditions except for a shorter contact time. LCGs were previously classified as sterilants by passing the Association of Official Analytical Chemists (AOAC) Sporicidal Test.82 Older LCGs (e.g., ≥2% glutaraldehyde) were approved by the FDA for sterilization and high-level disinfection (although the prolonged exposure time required made this impractical). However, more recently approved LCGs, such as 0.55% ortho-phthalaldehyde (Cidex OPA) and hypochlorite 400–450 ppm (Sterilox), that passed the AOAC Sporicidal Test have not been given an indication for device sterilization (i.e., high-level disinfection only). The FDA has approved many LCGs for use as high-level disinfectants or sterilants in the reprocessing of endoscopes and other reusable medical devices (Table 4.2). These include 2.4% to 3.5% glutaraldehyde, 3.4% glutaraldehyde/26% isopropanol, 1.12% glutaraldehyde/1.93% phenol/phenate, 0.55% to 0.60% ortho-phthaldehyde, 0.2% peracetic acid, 2.0% and 7.5% hydrogen peroxide, 8.3% hydrogen peroxide/7.0% peracetic acid, 7.35% hydrogen peroxide/0.23% peracetic acid, 1.0% hydrogen peroxide/0.08% peracetic acid, and hypochlorite/hypochlorous acid 400 ppm or greater active free chlorine.82
| FDA-Cleared Sterilants and High-Level Disinfectants for High-Level Disinfection of Endoscopes | Disinfectants Inadequate for High-Level Disinfection of Endoscopes (Examples) |
|---|---|
| 2.4%–3.5% glutaraldehyde | Phenolic solutions |
| Hexachlorophene | |
| 3.4% glutaraldehyde/26% isopropanol | Iodophor solutions |
| Povidone-iodine | |
| 1.12% glutaraldehyde/1.93% phenol/phenate | quaternary ammonium solutions |
| Benzalkonium chloride | |
| Benzethonium chloride | |
| Cetrimide | |
| 0.55% ortho-phthalaldehyde | Chlorhexidine |
| 0.60% ortho-phthalaldehyde | |
| 5.75% ortho-phthalaldehyde (diluted) | |
| 0.2% peracetic acid | Chlorhexidine/cetrimide |
| 2.0% hydrogen peroxide | Alkyldiaminoethylglycine hydrochloride |
| 7.5% hydrogen peroxide | |
| 8.3% hydrogen peroxide/7.0% peracetic acid | Ethyl or isopropyl alcohol* |
| 7.35% hydrogen peroxide/0.23% peracetic acid | |
| 1.0% hydrogen peroxide/0.08% peracetic acid | |
| Hypochlorite/hypochlorous acid 650–675 ppm (active free chlorine) | |
| Hypochlorite/hypochlorous acid 400–450 ppm (active free chlorine) |
FDA, U.S. Food and Drug Administration.
* When used for high-level disinfection; appropriate for terminal drying.
From Nelson DB: Infectious disease complications of GI endoscopy. Part II. Exogenous infections. Gastrointest Endosc 57:695–711, 2003, with permission from the American Society for Gastrointestinal Endoscopy.
Many LCGs are labeled for multiple reprocessing cycles for a specific time period. However, as these sterilants are reused, dilution occurs, which can reduce their effectiveness. Product-specific test strips should be used regularly to monitor these solutions to insure that they are above their minimum effective concentration (MEC). Solutions should be discarded whenever they fall below the MEC or when the use-life expires, whichever comes first. Users should consult with manufacturers of endoscopes and AERs (if used) for compatibility before selecting an LCG. Two agents (0.2% peracetic acid, 5.75% ortho-phthalaldehyde) are used for a single disinfection cycle and are not reusable (i.e., single use—each cycle requires new LCG); the hypochlorite/hypochlorous acid solution is generated from electrolysis of a saline solution for each cycle.83–85
Automatic Endoscope Reprocessors
Historically, cleaning and high-level disinfection of endoscopes has been performed manually. The high-level disinfection step involved placing mechanically cleaned endoscopes into a basin or container of LCG (usually glutaraldehyde) that was also circulated through the internal channels of the instrument. Exposure of endoscopy personnel to some LCGs has been reported to cause respiratory, nasal, and skin problems, however.86,87 AERs were designed to ensure that reprocessing is performed consistently and to replace some manual disinfection steps. In addition, AERs may minimize the exposure of endoscopy personnel to the LCG.88 It is crucial, however, that users understand that endoscopes must be mechanically cleaned before reprocessing in an AER. Although several devices are labeled by the FDA as “washer-disinfectors,” and one device has been approved to bypass the mechanical cleaning step, this has not been endorsed or sanctioned by any of the gastrointestinal societies that represent the end-users (mechanical cleaning is still recommended before the use of all AERs). It is also important to verify that the endoscope and the AER are compatible and use appropriate connectors.89
Cleaning and Disinfecting Endoscopes
A guideline for reprocessing GI endoscopes that has been endorsed by numerous gastroenterology, infection control, surgical, nursing, and hospital organizations contains detailed recommendations for this process.90 A similar guideline (although broader in scope) by the Healthcare Infection Control Practices Advisory Committee (HICPAC) of the CDC has been finalized.91 The pertinent steps to achieve high-level disinfection of endoscopes from these guidelines are summarized as follows:
Disinfection Procedure Compliance
Adherence to established guidelines for the cleaning and disinfection of endoscopes is imperative. When these guidelines are followed, the risk of transmission of infection is virtually eliminated; however, this is not a reason for complacency because compliance with existing reprocessing guidelines is not uniform. In 1991, Gorse and Messner92 surveyed 2030 SGNA members and found that compliance with existing guidelines was 67% in some areas. A collaborative study by the FDA and three state health departments published in 1992 investigated endoscope reprocessing at 26 health care facilities and found that 24% of patient-ready endoscopes were contaminated, and these were attributed to fundamental errors in the disinfection process.93,94
Although office endoscopy has been shown to be as safe as endoscopy practiced in more regulated settings (e.g., hospitals),95 the absence of formal infection control programs and personnel may leave the office setting more vulnerable to compliance issues with regard to endoscope reprocessing. In one study of 19 family practice and internal medicine offices performing flexible sigmoidoscopy, all were found to deviate from accepted reprocessing guidelines in at least one area.96 Although two more recent studies suggest that compliance with reprocessing guidelines has improved,97,98 there is room for further improvement. The challenge facing practitioners in the field of GI endoscopy is to ensure that compliance with these guidelines is universal, regardless of the practitioner or setting.
Reprocessing Personnel
Only trained personnel who understand the importance of strict adherence to established protocols should perform endoscope reprocessing (as a corollary, untrained personnel should not reprocess endoscopes). This training should include device-specific reprocessing instructions (for both the endoscope and the reprocessing equipment) and education regarding the biologic and chemical hazards associated with the cleaning and disinfection of endoscopes with LCGs. These individuals should meet annual competency standards for endoscope reprocessing. In addition, all health care personnel in the endoscopy suite should be trained in and adhere to standard infection control recommendations (e.g., standard precautions), including recommendations to protect both patients and health care workers.33 Personal protective equipment, such as gloves, gowns, eyewear, and respiratory protection devices, should be readily available. This equipment should be used, as appropriate, to protect reprocessing personnel from exposure to chemicals, blood, or other potentially infectious material.99–102
Novel Infectious Agents
Although Creutzfeldt-Jakob disease (CJD) and vCJD are rare, the impact of these diseases on endoscope reprocessing is addressed. CJD and vCJD are degenerative neurologic disorders transmitted by proteinaceous infectious agents called prions (although this is a simplification). Prions are unusually resistant to disinfection by conventional chemical high-level disinfectants or sterilants.103,104 The incidence of CJD in the United States is extremely low, with approximately 250 cases per year, or 0.97 cases per 1 million persons per year.105 Tissues and secretions that come into contact with the endoscope during procedures, such as saliva, gingival tissue, intestinal tissue, feces, and blood, are considered noninfectious by the World Health Organization.103 A draft statement on CJD and medical device reprocessing from the CDC concluded that current guidelines for cleaning and disinfection of these instruments need not be changed.35 Other infection control experts have concurred, citing the lack of exposure to high-risk tissue and the importance of mechanical cleaning in removing microbial contamination,31,104
The clinical relevance of the more recent finding of abnormal prion proteins in the olfactory (but not respiratory) epithelium of affected patients with regard to infection control or endoscope reprocessing is unclear.106 To date, there have been no reported cases in the world literature of transmission of CJD (or any other transmissible spongiform encephalopathy) by endoscopy. vCJD is a more recently recognized and even more rare syndrome that is believed to be due to consumption of beef products containing the bovine spongiform encephalopathy (BSE) agent, possibly requiring a susceptible genotype by the individual.107 The only case of the disease reported in the United States was found in a 22-year-old patient that had moved from the United Kingdom. Despite active surveillance since 1990, BSE has not been detected in the United States.108 In contrast to CJD, the prions associated with vCJD can be detected in the lymphoid tissue of affected individuals (e.g., tonsil, appendix, and possibly ileum and rectum).107,109–112 The prions in these tissues are present in lower concentrations and are approximately 50% less infective than central nervous system tissue when homogenated and injected intracerebrally in mice.113 The infectivity of intact tissue that might be encountered at endoscopy and the risk of subsequent transmission to another individual via gut inoculation are unknown but would undoubtedly be lower.
General Infection Control Practices
The importance of general infection control practices has been highlighted by the transmission of HCV to at least six individuals at a Nevada endoscopy clinic. The subsequent epidemiologic investigation revealed that the outbreak was due to unsafe injection practices, specifically the reuse of syringes and the use of single-use medical vials on multiple patients.78 A review of nonhospital health care–associated HBV and HCV transmission outbreaks in the United States over the last decade showed that in each case, failure to follow fundamental principles of infection control and aseptic technique was the cause.114 Although this problem is not unique to endoscopy, it is imperative that health care workers in endoscopy units understand and adhere to recommended infection control practices.
1 Hirschowitz BI. Endoscopic examination of the stomach and duodenal cap with the fiberscope. Lancet. 1961;1:1074-1078.
2 Ayliffe GAJ, Babb JR, Bradley CR. The immersible endoscope [letter]. Lancet. 1984;1:161.
3 Petersen BT. Gaining perspective on reprocessing of GI endoscopes. Gastrointest Endosc. 1999;50:287-291.
4 Rothstein RI, Littenberg B. Disposable, sheathed, flexible sigmoidoscopy: a prospective, multicenter, randomized trial. Gastrointest Endosc. 1995;41:566-572.
5 Schroy PC, Wilson S, Afdahl N. Feasibility of high-volume screening sigmoidoscopy using a flexible fiberoptic endoscope and a disposable sheath system. Am J Gastroenterol. 1996;91:1331-1337.
6 Sardinha TC, Wexner SD, Gilliland J, et al. Efficiency and productivity of a sheathed fiberoptic sigmoidoscope compared with a conventional sigmoidoscope. Dis Colon Rectum. 1997;40:1248-1253.
7 Mayinger B, Strenkert M, Hochberger J, et al. Disposable-sheath, flexible gastroscope system versus standard gastroscopes: a prospective, randomized trial. Gastrointest Endosc. 1999;50:461-467.
8 Bretthauer M, Hoff G, Thiis-Evensen E, et al. Use of a disposable sheath system for flexible sigmoidoscopy in decentralized colorectal cancer screening. Endoscopy. 2002;34:814-818.
9 ECRI. Endosheath endoscopic system. Health Devices. 2000;29:7-13.
10 Block SS. Definition of terms. In: Block SS, editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:19-28.
11 Knieler R. Manual cleaning and disinfection of flexible endoscopes—an approach to evaluating a combined procedure. J Hosp Infect. 2001;48(Suppl A):S84-S87.
12 Alfa MJ, Olson N, DeGagne P, et al. A survey of reprocessing methods, residual viable bioburden, and soil levels in patient-ready endoscopic retrograde cholangiopancreatography duodenoscopes used in Canadian centers. Infect Control Hosp Epidemiol. 2002;23:198-206.
13 Fantry GT, Zheng Q-X, James SP. Conventional cleaning and disinfection techniques eliminate the risk of endoscopic transmission of Helicobacter pylori. Am J Gastroenterol. 1995;90:227-232.
14 Deva AK, Vickery K, Zou J, et al. Detection of persistent vegetative bacteria and amplified viral nucleic acid from in-use testing of gastrointestinal endoscopes. J Hosp Infect. 1998;39:149-157.
15 Bécheur H, Harzic M, Colardelle P, et al. Contamination des endoscopes et des pinces à biopsies par le virus de l’hépatite C [French with English abstract]. Gastroenterol Clin Biol. 2000;24:906-910.
16 Deflandre J, Cajot O, Brixko C, et al. Risques de contamination par le virus de l’hépatite C des endoscopes utilisés dans un service hospitalier de gastroentérologie [French with English abstract]. Rev Med Liege. 2001;56:696-698.
17 Vesley D, Norlien KG, Nelson B, et al. Significant factors in the disinfection and sterilization of flexible endoscopes. Am J Infect Control. 1992;20:291-300.
18 Babb JR, Bradley CR. Endoscope reprocessing: where do we go from here? J Hosp Infect. 1995;30:543-551.
19 Urayama S, Kozarek RA, Sumida S, et al. Mycobacteria and glutaraldehyde: is high-level disinfection of endoscopes possible? Gastrointest Endosc. 1996;43:451-456.
20 Chu NS, McAlister D, Antonoplos PA. Natural bioburden levels detected on flexible gastrointestinal endoscopes after clinical use and manual cleaning. Gastrointest Endosc. 1998;48:137-142.
21 Cronmiller JR, Nelson DK, Salman G, et al. Antimicrobial efficacy of endoscopic disinfection procedures: a controlled, multifactorial investigation. Gastrointest Endosc. 1999;50:152-158.
22 Kovacs BJ, Chen YK, Kettering JD, et al. High-level disinfection of gastrointestinal endoscopes: are current guidelines adequate? Am J Gastroenterol. 1999;94:1546-1550.
23 Vesley D, Melson J, Patricia S. Microbial bioburden in endoscope reprocessing and an in-use evaluation of the high-level disinfection capabilities of Cidex PA. Gastroenterol Nurs. 1999;22:63-68.
24 Foliente RL, Kovacs BJ, Aprecio RM, et al. Efficacy of high-level disinfectants for reprocessing GI endoscopes in simulated in-use testing. Gastrointest Endosc. 2001;53:456-462.
25 Nelson DB. Infectious disease complications of GI endoscopy. Part II. Exogenous infections. Gastrointest Endosc. 2003;57:695-711.
26 U.S. Food and Drug Administration. Guidance for industry and FDA reviewers: Content and format of premarket notification [510(k) submissions for liquid chemical sterilants/high level disinfectants]. Rockville, MD: FDA; 2000.
27 Muscarella LF. What is disinfection, sterilization? [letter]. Gastrointest Endosc. 1999;50:301-303.
28 Muscarella LF. Are all sterilization processes alike? AORN J. 1998;67:966-976.
29 Rutala WA, Weber DJ. Low-temperature sterilization technologies: do we need to redefine sterilization? Infect Control Hosp Epidemiol. 1996;17:87-91.
30 Spaulding EH. Chemical disinfection and antisepsis in the hospital. J Hosp Res. 1972;9:5-31.
31 Favero MS, Bond WW. Disinfection of medical and surgical materials. In: Block SS, editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:881-917.
32 U.S. Food and Drug Administration. Draft guidance for the content of premarket notifications for endoscopes used in gastroenterology and urology. Rockville, MD: FDA; 1995.
33 Garner JS, Favero MS. CDC guideline for handwashing and hospital environmental control, 1985. Infect Control Hosp Epidemiol. 1986;7:231-243.
34 DiMarino AJJr, Leung J, Ravich W, et al. Reprocessing of flexible gastrointestinal endoscopes. Gastrointest Endosc. 1996;43:540-546.
35 Alvarado CJ, Reichelderfer M. APIC guidelines for infection prevention and control in flexible endoscopy. Am J Infect Control. 2000;28:138-155.
36 American Society for Testing and Materials. Standard practice for cleaning and disinfection of flexible fiberoptic and video endoscopes used in the examination of the hollow viscera. West Conshohocken, PA: ASTM; 2000.
37 Association of Perioperative Registered Nurses. Recommended practices for use and care of endoscopes. 2002 Standards, Recommended Practices, and Guidelines. Denver, CO: AORN; 2002. pp 229–232
38 Ishino Y, Ido K, Koiwai H, et al. Pitfalls in endoscope reprocessing: brushing of air and water channels is mandatory for high-level disinfection. Gastrointest Endosc. 2001;52:165-168.
39 Kozarek RA, Sumida SE, Raltz SL, et al. In vitro evaluation of wire integrity and ability to reprocess single-use sphincterotomes. Gastrointest Endosc. 1997;45:117-121.
40 Cohen J, Haber GB, Kortan P, et al. A prospective study of the repeated use of sterilized papillotomes and retrieval baskets for ERCP: quality and cost analysis. Gastrointest Endosc. 1997;45:122-127.
41 Kozarek RA, Raltz SL, Ball TJ, et al. Reuse of disposable sphincterotomes for diagnostic and therapeutic ERCP: a one-year prospective study. Gastrointest Endosc. 1999;49:39-42.
42 Roach SK, Kozarek RA, Raltz SL, et al. In vitro evaluation of integrity and sterilization of single-use argon beam plasma coagulation probes. Gastrointest Endosc. 1999;94:139-143.
43 U.S. Food and Drug Administration. Enforcement priorities for single-use devices reprocessed by third parties and hospitals. Rockville, MD: FDA; 2000.
44 Axon ATR, Phillips I, Cotton PB, et al. Disinfection of gastrointestinal fibre endoscopes. Lancet. 1974;1:656-658.
45 Whalen GE. Risks of hepatitis: what can be done? [letter]. Gastrointest Endosc. 1975;22:48-49.
46 Tolon M, Thofern E, Miederer SE. Disinfection procedures of fiberscopes in endoscopy departments. Endoscopy. 1976;8:24-29.
47 Dunkerley RC, Cromer MD, Edmiston CEJr, et al. Practical technique for adequate cleansing of endoscopes: a bacteriological study of pHisoHex and Betadine. Gastrointest Endosc. 1977;23:148-149.
48 Carr-Locke DL, Clayton P. Disinfection of upper gastrointestinal fibreoptic endoscopy equipment: an evaluation of a cetrimide chlorhexidine solution and glutaraldehyde. Gut. 1978;19:916-922.
49 Geenen JE, Pfeifer M, Simonsen L. Cleaning and disinfection of endoscopic equipment [letter]. Gastrointest Endosc. 1978;24:185-186.
50 Hedrick E. Cleaning and disinfection of flexible fiberoptic endoscopes used in gastrointestinal endoscopy. APIC J. 1978;6:8-9.
51 Lindstaedt H, Krizek L, Miederer SE, et al. Experience and problems in the disinfection of fibre endoscopes. Endoscopy. 1978;10:80-85.
52 Vennes JA, Geenen JE, Papp JP, et al. Endoscopically related infections and their prevention [letter]. Gastrointest Endosc. 1981;27:239-240.
53 Rutala WA. APIC guideline for selection and use of disinfectants. Am J Infect Control. 1996;24:313-342.
54 Infection control during gastrointestinal endoscopy: guidelines for clinical application. Gastrointest Endosc. 1988;34:37S-40S.
55 Weller IVD, Williams CB, Jeffries DJ, et al. Cleaning and disinfection of equipment for gastrointestinal flexible endoscopy: interim recommendations of a Working Party of the British Society of Gastroenterology. Gut. 1988;29:1134-1151.
56 Sobala GM, Lincoln C, Axon ATR. Does the endoscope control head need to be disinfected between examinations. Endoscopy. 1989;21:19-21.
57 Society of Gastroenterology Nurses and Associates, Inc. Standards of infection control in reprocessing of flexible gastrointestinal endoscopes. Gastroenterol Nurs. 2000;23:172-187.
58 British Society of Gastroenterology. Cleaning and disinfection of equipment for gastrointestinal endoscopy. Report of a Working Party of the British Society of Gastroenterology Endoscopy Committee. Gut. 1998;42:585-593.
59 European Society of Gastrointestinal Endoscopy. Guidelines on cleaning and disinfection in GI endoscopy. Endoscopy. 2000;32:77-83.
60 Leung JW. Working party report: care of endoscopes. Reprocessing of flexible endoscopes. J Gastroenterol Hepatol. 2000;15:G73-G77.
61 Arora A, Seth S, Tandon RK. Gastrointestinal endoscope disinfection practices in India: results of a national survey. Indian J Gastroenterol. 1992;11:62-64.
62 Akamatsu T, Tabata K, Hironga M, et al. Transmission of Helicobacter pylori infection via flexible fiberoptic endoscopy. Am J Infect Control. 1996;24:396-401.
63 Orsi GB, Filocamo A, Di Stefano L, et al. Italian national survey of digestive endoscopy disinfection procedures. Endoscopy. 1997;29:732-740.
64 Alvarez SZ, Kothari K, Novis B, et al. Disinfection of endoscopic equipment. Gastrointest Endosc. 1999;49:668-670.
65 Brullet E, Ramirez-Armengol JA, Campo R, Board of the Spanish Association for Digestive Endoscopy. Cleaning and disinfection practices in digestive endoscopy in Spain: results of a national survey. Endoscopy. 2001;33:864-868.
66 Pollack M. Pseudomonas aeruginosa. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practices of infectious diseases. ed 5. Philadelphia: Churchill Livingstone; 2000:2310-2335.
67 Miyaji H, Kohli Y, Azuma T, et al. Endoscopic cross-infection with Helicobacter pylori [letter]. Lancet. 1995;345:464.
68 Graham DY, Alpert LC, Smith JL, et al. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am J Gastroenterol. 1988;83:974-980.
69 Morris IM, Cattle DS, Smits BJ. Endoscopy and transmission of hepatitis B [letter]. Lancet. 1975;2:1152.
70 Seefeld U, Bansky G, Jaeger M, et al. Prevention of hepatitis B virus transmission by gastrointestinal fibrescope: successful disinfection with an aldehyde liquid. Endoscopy. 1981;13:238-239.
71 Birnie GG, Quigley EM, Clements GB, et al. Endoscopic transmission of hepatitis B virus. Gut. 1983;24:171-174.
72 Davis AR, Pink JM, Kowalik AM, et al. Multiple endoscopies in a Sydney blood donor found positive for hepatitis B and C antibodies. Med J Aust. 1996;164:571.
73 Federman DG, Kirsner RS. Leukocytoclastic vasculitis, hepatitis B, and the risk of endoscopy. Cutis. 1999;63:86-87.
74 Tennenbaum R, Colardelle P, Chochon M, et al. Hépatite C après cholangiographie rétrograde [French]. Gastroenterol Clin Biol. 1993;17:763-775.
75 Bronowicki J-P, Venard V, Botté C, et al. Patient-to-patient transmission of hepatitis C virus during colonoscopy. N Engl J Med. 1997;337:237-240.
76 Le Pogam S, Gondeau A, Bacq Y. Nosocomial transmission of hepatitis C virus [letter]. Ann Intern Med. 1999;131:794.
77 Thompson ND, Perz JF, Moorman AC, et al. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med. 2009;150:33-39.
78 Centers for Disease Control and Prevention (CDC). Acute hepatitis C virus infections attributed to unsafe injection practices at an endoscopy clinic—Nevada, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:513-517.
79 Classen M, Dancygier HII, Gürtler L, et al. Risk of transmitting HIV by endoscopes [letter]. Endoscopy. 1988;20:128.
80 Hanson PJV, Gor D, Clarke JR, et al. Contamination of endoscopes used in AIDS patients. Lancet. 1989;2:86-88.
81 Hanson PJV, Gor D, Jeffries DJ, et al. Elimination of high titre HIV from fiberoptic endoscopes. Gut. 1990;31:657-659.
82 U.S. Food and Drug Administration. FDA-cleared sterilants and high level disinfectants with general claims for processing reusable medical and dental devices—March 2009. Available at http://www.fda.gov/cdrh/ode/germlab.html Accessed July 1, 2009
83 Selkon JB, Babb JR, Morris R. Evaluation of the antimicrobial activity of a new super-oxidized water, Sterilox, for the disinfection of endoscopes. J Hosp Infect. 1999;41:59-70.
84 Tsuji S, Kawano S, Oshita M, et al. Endoscope disinfection using acidic electrolytic water. Endoscopy. 1999;31:528-535.
85 Nelson D. Newer technologies for endoscope disinfection: electrolyzed acid water and disposable component endoscope systems. Gastrointest Endosc Clin N Am. 2000;10:319-328.
86 Norbäck D. Skin and respiratory symptoms from exposure to alkaline glutaraldehyde in medical services. Scand J Work Environ Health. 1988;14:366-371.
87 Gannon PFG, Bright P, Campbell M, et al. Occupational asthma due to glutaraldehyde and formaldehyde in endoscopy and x ray departments. Thorax. 1995;50:156-159.
88 Muscarella LF. Advantages and limitations of automatic flexible endoscope reprocessors. Am J Infect Control. 1996;24:304-309.
89 Sorin M, Segal-Maurer S, Urban C, et al. Nosocomial transmission of imipenem-resistant Pseudomonas aeruginosa following bronchoscopy associated with improper connection to the STERIS System 1 processor. Infect Control Hosp Epidemiol. 2001;20:514-516.
90 Nelson DB, Jarvis WR, Rutala WA, et al. Multi-society guidelines for reprocessing flexible gastrointestinal endoscopes. Gastrointest Endosc. 2003;58:1-8.
91 Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee. Guideline for disinfection and sterilization in healthcare facilities. November 2008. Available at http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/Disinfection_Nov_2008.pdf Accessed July 1, 2009
92 Gorse GJ, Messner RL. Infection control practices in gastrointestinal endoscopy in the United States: a national survey. Infect Control Hosp Epidemiol. 1991;12:289-296.
93 Kaczmarek RG, Moore RMJr, John M, et al. Multi-state investigation of the actual disinfection/sterilization of endoscopes in health care facilities. Am J Med. 1992;92:257-261.
94 Reynolds CD, Rhinehart E, Dreyer P, et al. Variability in reprocessing policies and procedures for flexible fiberoptic endoscopes in Massachusetts hospitals. Am J Infect Control. 1992;20:283-290.
95 United States General Accounting Office. Medicare physician payments: medical settings and safety of endoscopic procedures. Washington, D.C.: U.S. General Accounting Office; 2002.
96 Jackson FW, Ball MD. Correction of deficiencies in flexible fiberoptic sigmoidoscope cleaning and disinfection technique in family practice and internal medicine offices. Arch Fam Med. 1997;6:578-582.
97 Cheung RJ, Ortiz D, DiMarino AJJr. GI endoscopic reprocessing practices in the United States. Gastrointest Endosc. 1999;50:362-368.
98 Muscarella LF. Current instrument reprocessing practices: results of a national survey. Gastroenterol Nurs. 2001;24:253-260.
99 Occupational Safety and Health Administration. Hazard Communication Standard: 29 CFR 1910.1200, Washington, D.C., OSHA. http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&tpl=/ecfrbrowse/Title29/29cfr1910_main_02.tpl. Accessed April 22, 2011
100 Occupational Safety and Health Administration. Occupational exposure to bloodborne pathogens: final rule. Fed Reg. 1991;56:64003-64182.
101 Carr-Locke DL, Conn MI, Faigel DO, et al. Personal protective equipment. Gastrointest Endosc. 1999;49:854-857.
102 Siegel JD, Rhinehart E, Jackson M, et alHealthcare Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. June 2007. Available at http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf Accessed July 1, 2009
103 World Health Organization. WHO infection control guidelines for transmissible spongiform encephalopathies. Geneva: World Health Organization; 1999.
104 Rutala WA, Weber DJ. Creutzfeldt-Jakob disease: recommendations for disinfection and sterilization. Clin Infect Dis. 2001;32:1348-1356.
105 Gibbons RV, Holman RC, Belay ED, et al. Creutzfeldt-Jakob disease in the United States:1979–1998 [letter]. JAMA. 2000;284:2322-2323.
106 Zanusso G, Ferrari S, Cardone F, et al. Detection of pathological prion protein in the olfactory epithelium in sporadic Creutzfeldt-Jakob disease. N Engl J Med. 2003;348:711-719.
107 Wadsworth JDF, Joiner S, Hill AF, et al. Tissue distribution of protease resistant prion in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171-180.
108 Centers for Disease Control and Prevention: BSE and CJD information and resources. Available at http://www.cdc.gov/ncidod/diseases/cjd/cjd.htm Accessed December 11, 2002
109 Hill AF, Zeidler M, Ironside JW, et al. Diagnosis of new variant Creuzfeldt-Jakob disease by tonsil biopsy [letter]. Lancet. 1997;349:99-100.
110 Hilton DA, Fathers E, Edwards P, et al. Prion immunoreactivity in appendix before clinical onset of variant Creuzfeldt-Jakob disease. Lancet. 1998;352:703-704.
111 Hill AF, Butterworth RJ, Joiner S, et al. Investigation of variant Creuzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet. 1999;353:183-189.
112 Ironside JW, Head MW, Bell JE, et al. Laboratory diagnosis of variant Creutzfeldt-Jakob disease. Histopathology. 2000;37:1-9.
113 Bruce ME, McConnell I, Will RG, et al. Detection of variant Creuzfeldt-Jakob disease infectivity in extraneural tissues [letter]. Lancet. 2001;358:208-209.
114 Thompson ND, Perz JF, Moorman AC, et al. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med. 2009;150:33-39.