Chapter 11 Chronic venous insufficiency
AETIOLOGY AND EPIDEMIOLOGY
Chronic venous insufficiency (CVI) is a pathological disorder of the venous system, characterised by impaired venous blood flow in the lower limbs. The condition is manifested by pathological changes to the skin, subcutaneous tissue and vascular tissue.1 These manifestations, which can range from mild to severe, can be grouped into symptomatic complaints, such as leg heaviness, discomfort and pruritus, or advanced physical signs, including leg oedema, ochre pigmentation and lipodermatosclerosis. The condition, which is a precursor to varicose veins and venous leg ulceration, is not uncommon, affecting between 0.1% and 17% of men, and from 0.2% to 20% of women.1
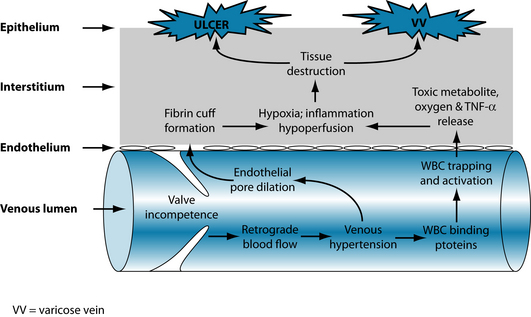
This chronic and sometimes disabling disorder is believed to originate from an episode of macrovascular injury, which may be attributed to lower limb surgery, trauma, deep vein thrombosis (DVT) or pregnancy. This insult to the venous system can lead to valvular incompetence, venous reflux (or retrograde blood flow), ambulatory venous hypertension, venous wall dilatation and a subsequent rise in capillary filtration. As well as contributing to the formation of interstitial oedema, increased capillary filtration may also lead to localised hypoxia, malnutrition and eventual tissue destruction (see Figure 11.1). The extravasation of fibrinogen and the consequent formation of pericapillary cuffs, and the intraluminal trapping of leucocytes and subsequent release of toxic metabolites, proteolytic enzymes and tissue necrosis factor alpha (TNF-α)2 are some of the mechanisms linking elevated capillary filtration pressure to changes in tissue perfusion and local architecture. The extravasation of fibrinogen and leucocyte products into pericapillary tissue may also mediate inflammation, suggesting that CVI may be a disease of chronic inflammation.2
RISK FACTORS
Many risk factors, both modifiable and non-modifiable, are claimed to be responsible for the pathogenesis of venous insufficiency. Occupations requiring periods of prolonged standing (such as nurses, flight attendants and factory workers) have been observed across a number of studies to have a higher prevalence and severity of CVI and varicose veins.1 This could be attributed in part to excessive lower limb venous congestion secondary to reduced calf-muscle pump activity. Evidence linking other modifiable risk factors to chronic venous insufficiency, such as obesity, smoking, constipation, hormone therapy and hypertension, has been inconsistent however.1
In terms of non-modifiable risk factors, both family history and increasing age appear to be associated with an increased risk of CVI.1 Advancing age is also correlated with an increased prevalence of varicose veins1 and venous leg ulceration.3 While female gender has been associated with an increased risk of varicose veins1 and venous leg ulceration,4 there is conflicting evidence regarding the link between sex and CVI risk.
CONVENTIONAL TREATMENT
There are a number of different approaches to the management of CVI. Two approaches often recommended in conventional practice are compression therapy and surgery. Compression therapy is advocated in conventional practice as it helps to reduce leg oedema, venous reflux, venous hypertension and lipodermatosclerosis, while improving deep vein blood flow velocity, capillary clearance, calf-muscle pump function, venous refilling time and venous ejection volume.5 Compression therapy targets a number of processes associated with the pathogenesis of venous insufficiency, with a meta-analysis of 11 randomised controlled trials (n = 1453) finding compression therapy (10–15 mmHg) to be significantly more effective than low grade compression, placebo stockings and no treatment at reducing the symptoms of CVI, including lower limb oedema and discomfort.6 These results need to be interpreted with caution, however, given the heterogeneous populations and diverse assessment techniques used in these studies. It is also possible that the reported effectiveness of compression therapy may not reflect that observed in clinical practice due to the poor level of compliance observed with this treatment. Some of the reasons for the poor adherence to compression therapy may relate to the long duration of therapy, the visible appearance of the stockings, associated discomfort, skin reactions, and the cost and maintenance of the therapy.7
The surgical restoration or removal of diseased vessels also may be advised in the overall management of chronic venous insufficiency. The array of surgical techniques that may be recommended include sclerotherapy, venous ligation and stripping, endovenous laser treatment, phlebectomy and valvuloplasty. Evidence from a meta-analysis of three randomised controlled trials (RCTs) suggests that venous ligation and valvuloplasty may be more effective than ligation alone in improving ambulatory venous pressure and quality of life.8 Another meta-analysis of three RCTs found subfascial endoscopic perforator vein surgery (SEPS) to be significantly more effective than conventional surgery at reducing venous ulcer recurrence, wound infection and length of hospital stay in patients with CVI.9 It is not clear from either of these reviews, however, whether the benefits of these techniques outweigh the risks and costs of surgery, and whether these approaches are relatively more effective (both economically and clinically) than the conservative management of CVI.
KEY TREATMENT PROTOCOLS
Venous integrity
Enzymatic degradation
The abnormal venous tone observed in chronic venous insufficiency may be linked to an increase in lysosomal enzyme activity, as evidenced by the elevated levels of these enzymes in patients with CVI,10 and in the exudate of venous ulcers.11 The lysosomal enzymes hyaluronidase and elastase are believed to be responsible for this extravascular and extracellular matrix degradation,12 and the subsequent increase in capillary permeability and oedema formation. It is therefore hypothesised that a reduction in lysosomal enzyme activity could decrease the symptoms of CVI by restoring venous elasticity and contractility through improvements in collagen biosynthesis10 and proteoglycan recovery.12
The saponins and sapogenins of Hedera helix and Aesculus hippocastanum have been shown to inhibit hyaluronidase activity in vitro,12 whereas Ruscus aculeatus saponins,12 rutin13 and grape seed procyanidins14 have been found to inhibit elastase activity in vitro. By attenuating overactive lysosomal enzyme activity, these compounds may shift the equilibrium between proteoglycan synthesis and degradation, towards net synthesis. This reduction in enzyme activity against capillary wall mucopolysaccharides may improve vessel wall integrity and subsequently reduce oedema formation.15–17 Although the saponins appear to be responsible for the anti-exudative and vascular-tightening effect of several of these plant extracts, the effect of other constituents (such as the flavonoids) cannot be dismissed.
Oxidative damage
Another process implicated in the pathogenesis of CVI is oxidative injury. This process involves the peroxidation of venous lipids, production of oxygen free radicals18–19 and consequent destruction of lipids, proteins, collagen, proteoglycan and hyaluronic acid.20 Agents that exhibit significant antioxidant activity may interrupt this cascade of events and, as a result, preserve venous tissue and improve venous integrity.
Many phlebotonic agents exhibit good antioxidant activity in experimental models, particularly free radical scavenging activity, including Aesalus hippocastanum horse chestnut seed extract, (HCSE),18 Centella asiatica flavonoids,21 Vaccinium myrtillus extract,22 grape seed extract, pine bark extract,23 quercetin and rutin.24 When the active-oxygen scavenging activity of 65 plant extracts were compared in vitro, HCSE and Hamamelis virginiana demonstrated the greatest antioxidant activity. Both extracts were found to be more potent than ascorbic acid and α-tocopherol in scavenging superoxide anions, but less effective than ascorbic acid in scavenging hydroxyl radicals and inhibiting singlet-oxygen generation.19 As an indicator of cell protection, Masaki et al.19 explored the effect that the plant extracts had on fibroblast survival. HCSE, witch hazel and English oak (Quercus robur) were the most protective, increasing fibroblast survival at least threefold. As fibroblasts are a key source of collagen, elastin, proteoglycans and matrix metalloproteinases,11 increasing fibroblast survival is likely to improve venous integrity.
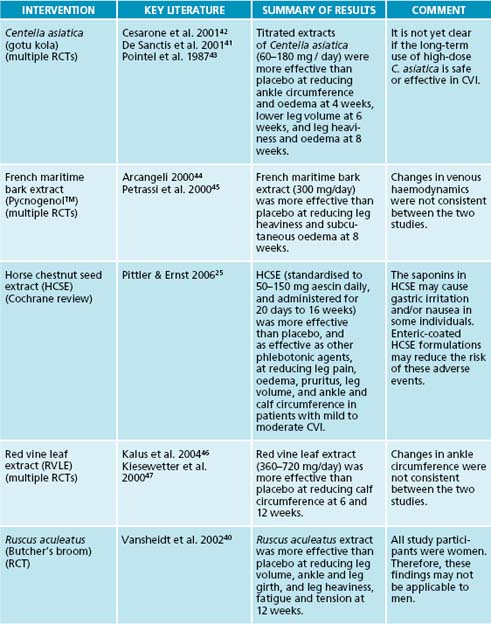
There is a large body of evidence to support the use of HCSE in the management of mild to moderate CVI. A Cochrane review of 17 RCTs found orally administered HCSE (standardised to 50–150 mg aescin daily, and administered for 20 days to 16 weeks (mean = 6 weeks)) to be more effective than placebo, and as effective as other phlebotonic agents, at reducing leg pain, oedema, pruritus, leg volume and ankle and calf circumference in patients with mild to moderate CVI.25 As for severe or advanced cases of CVI, it appears that HCSE may not be as effective.26
Inflammation
Chronic inflammation is another contributing factor in the development of CVI. It is hypothesised that the manifestation of venous hypertension leads to widened capillary pore diameter, causing intravascular components such as fibrinogen, erythrocytes and α2-macroglobulin to be leached into the interstitium.2 These potent chemoattractants up-regulate the expression of intracellular adhesion molecule 1 (ICAM-1) and, together with increased platelet reactivity,27 increase the expression of platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF). These growth factors trigger leucocyte migration. Once recruited, the white blood cells secrete or activate transforming growth factor-β1 (TGF-β1). Since TGF-β1 has been located in pericapillary cuffs, it is believed that this growth factor may be responsible for tissue remodelling and fibrosis, as well as capillary angiogenesis, increased capillary tortuosity and density.2 This factor may therefore contribute to some of the defining features of CVI, including varicose veins and lipodermatosclerosis. The increased expression of ICAM-1,28–30 TGF-β1,31 PDGF and VEGF32 in the dermis of patients with CVI lends some support to this sequelae of events.
Venotonic agents exhibiting antiinflammatory activity may attenuate the progression of CVI through a number of different pathways. Aescin (from HCSE)33 and Ruscus aculeatus extract,34 for instance, both inhibit histamine-induced vascular permeability in vivo, an important step in the pathogenesis of CVI. Aescin,33 grape seed extract35 and Vaccinium myrtillus anthocyanosides36 have also been shown to inhibit carrageen an-induced paw oedema, another measure of acute anti-inflammatory activity. French maritime pine bark extract (Pycnogenol™), on the other hand, exhibits anti-inflammatory activity by inhibiting nuclear factor kappa B (NFκB) and the proinflammatory cytokine interleukin-1 (IL-1);37 whereas rutin and quercetin reduce inflammation by inhibiting the secretion of TNF-α, IL-1, IL-6, IL-8 and immunoglobulin E-induced histamine release.38 Apart from grape seed extract,39 however, few of these agents have yet been found to inhibit the key chemical mediators of CVI pathogenesis, including PDGF, VEGF, ICAM-1 and TGF-β1.
The body of mechanistic data that supports many of these aforementioned treatments is supported by a growing body of clinical evidence. Many of these studies have, however, used complex formulations. This is problematic as it is almost impossible to extrapolate the effect of an individual agent from the effect of a complex formulation. Thus, when reviewing the best available evidence for these treatments, only those studies using monopreparations were included (see Table 11.1). In brief, evidence from RCTs indicate Ruscus aculeatus extract is statistically significantly superior to placebo in reducing leg volume, ankle and leg girth, and leg heaviness, fatigue and tension;40 titrated extracts of Centella asiatica are significantly more effective than placebo at reducing ankle circumference and oedema,41 lower leg volume42 and leg heaviness and oedema;43 Pycnogenol™ is significantly more effective than placebo at reducing leg heaviness and subcutaneous oedema;44–45 and red vine leaf extract is statistically significantly superior to placebo at reducing calf circumference.46–47
INTEGRATIVE MEDICAL CONSIDERATIONS
Reflexology
Reflexology is a tactile therapy that manipulates specific points in the hands, feet and/or ears to initiate a reflex or physiological response in distant organs and tissues. A single-blind RCT tested whether this treatment may be effective in CVI by randomly allocating 55 healthy pregnant women with foot oedema to one of three groups: relaxation foot reflexology, lymphatic foot reflexology and rest.48 Up to four 15-minute treatments were provided, though the mean number of visits in each group was not clear. The study found no statistically significant difference between groups in mean ankle and foot circumference measurements over time. While all groups demonstrated a significant reduction in pain, discomfort and tiredness, it is not clear if these symptoms related to the overall pregnancy or to CVI specifically. Thus, at this point in time, it is uncertain whether reflexology would be useful in alleviating the symptoms of venous insufficiency.
Example treatment
Herbal and nutritional prescription
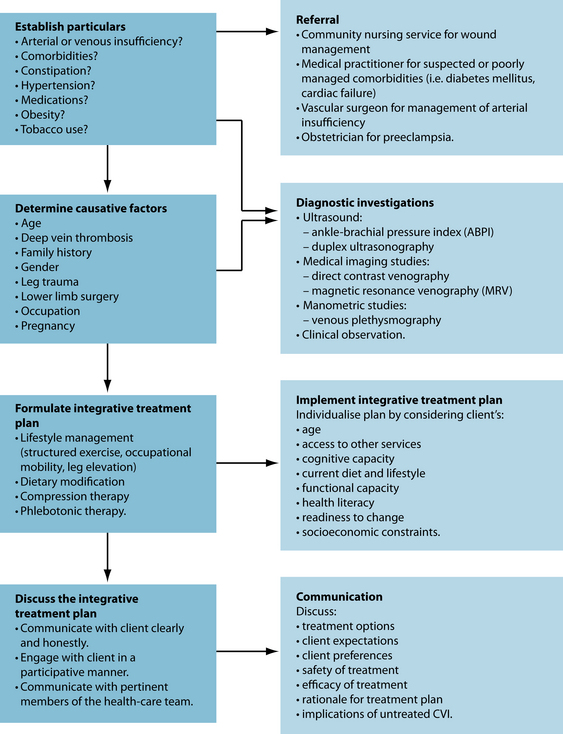
Once the underlying causes of the CVI have been identified, and measures introduced to address the causes (where possible), the naturopath should focus their attention on the pathological processes of the disease. Agents with venotonic activity, as well as
Herbal prescription
| Aesculus hippocastanum 1:2 | 25 mL |
| Centella asiatica 1:1 | 30 mL |
| Ruscus aculeatus 1:2 | 35 mL |
| Zingiber officinale 1:2 | 10 mL |
| 100 mL | |
| Dose: 7 mL b.d. (before meals) | |
antioxidant, anti-inflammatory, antienzymatic and antioedema effects, would be most desirable in this case. Interventions that exhibit more than one of these actions and, more importantly, have demonstrable clinical efficacy in patients with CVI should be afforded the highest priority in the naturopathic management of venous insufficiency. Examples of interventions that fulfil these criteria include horse chestnut seed extract, Ruscus aculeatus, Vaccinium myrtillus, Centella asiatica, French maritime pine bark extract, red vine leaf extract and grape seed extract. Many of these agents have been included in the herbal prescription outlined on the right. The inclusion of mixed bioflavonoids (as a nutritional prescription) is based on theoretical evidentiary support only–specifically, data from mechanistic studies.24,38
Dietary and lifestyle advice
Leg elevation is often recommended to people with CVI to help reduce lower limb venous pressure, leg discomfort and oedema. Although lower limb elevation (to 30 cm above heart level) has been shown to enhance microcirculatory flow velocity in liposclerotic skin of patients with chronic venous insufficiency,49 it is uncertain whether leg elevation offers any significant clinical benefit to patients with CVI. Given that many patients experience some relief of symptoms following lower limb elevation, there is no reason why this practice should not be recommended at this point in time.
A structured exercise program also may be recommended to individuals with CVI in order to facilitate calf-muscle pump function and reduce venous congestion. Findings
HAEMORRHOIDS
Overview
The underlying causes of haemorrhoidal disease are similar to that of varicose veins. As the portal venous system contains no valves, factors that increase venous congestion in the region–including intraabdominal pressure from causes such as strained defecation, pregnancy, cirrhosis of the liver or standing or sitting for prolonged periods of time–can hasten haemorrhoid formation.51–53 Haemorrhoids are common and tend to develop between the ages of 20 and 50 years–in the Western world they are extremely common and half of all people will experience them by the time they reach 50 years of age.53
Conventional treatment
Treatment for the removal of haemorrhoids revolves around a number of surgical options: rubber band ligation, cryotherapy and sphincterotomy.51–53 However, treatment in conventional medicine is increasingly focused towards prevention. The most common cause of haemorrhoids is constipation due to lack of fibre. Accordingly, conventional treatment centres around the promotion of a soft, bulky stool through dietary modification and/or supplementation.52 Topical treatment for pruritus ani or pain may also be recommended, most often in the form of steroid or anaesthetic medication.53
CAM interventions
Nutrition clearly needs to be considered. With overwhelming evidence to support increased fibre in the diet, appropriate dietary adjustments should be suggested. However, supplementation with bulking agents such as Ulmus fulva or Plantago ovata husks may also be beneficial. Supplementation with Plantago ovata husks is approved by Commission E for relieving constipation in haemorrhoids.15
A warm sitz bath may also be an effective and non-invasive treatment for haemorrhoids, most likely due to mechanisms involving relaxation of the internal anal sphincter.54
The internal use of herbs and nutrients for varicose veins and venous insufficiency suggested earlier in the chapter will be equally well indicated in restoring venous integrity in haemorrhoid treatment. Herbal medicines may be useful both internally and topically. However, few trials of herbal or clinical nutritional treatment in haemorrhoids exist. In one double-blind, placebo-controlled trial, internal use of Aesculum hippocastanum (equivalent to 40 mg aescin three times daily) for 6 days or more was found to relieve symptoms (82% compared to 32% with placebo) and reduce swelling (87% versus 38% with placebo) in 72 patients with acute symptamatic haemorrhoids.17
Topical therapy in most instances will provide only temporary relief of symptoms. Astringent therapy may be beneficial in restoring venous tone and has been traditionally used for this purpose.15,55 Astringent ointments containing Hamamelis virginiana, for instance, have been shown in clinical studies to be beneficial in alleviatins the symptoms of haemorrhoids, demonstrating similar efficacy to conventional topical applications.56,57 Other topical herbs that have been used include Matricaria recutita and Calendula officinalis.
from a small RCT (n = 31) showed that a supervised calf-muscle strength exercise program, together with compression hosiery, significantly improved mean venous ejection fraction at 6 months when compared to control. Between-group differences in venous reflux, venous severity scores and quality of life, however, were not statistically significant.50 Nevertheless, given that long periods of standing may contribute to the pathogenesis of CVI, it is probable that a structured exercise program may still be useful in preventing the onset and/or progression of the disease.
Expected outcomes and follow-up protocols
KEY POINTS
1. Beebe-Dimmer J.L., et al. The epidemiology of chronic venous insufficiency and varicose veins. Annals of Epidemiology. 2004;15:175-184.
2. Pappas P.J., et al. Causes of severe chronic venous insufficiency. Seminars in Vascular Surgery. 2005;18:30-35.
3. Baker S., Stacey M. Epidemiology of chronic leg ulcers in Australia. Australian & New Zealand Journal of Surgery. 1994;64(4):258-261.
4. Callam M., et al. Chronic ulcer of the leg: clinical history. British Medical Journal. 1987;294(6584):1389-1391.
5. Leach M.J. Making sense of the venous leg ulcer debate: a literature review. Journal of Wound Care. 2004;13(2):52-57.
6. Amsler F., Blattler W. Compression therapy for occupational leg symptoms and chronic venous disorders – a meta-analysis of randomised controlled trials. European Journal of Vascular and Endovascular Surgery,. 2008;35(3):366-372.
7. Raju S., et al. Use of compression stockings in chronic venous disease: patient compliance and efficacy. Annals of Vascular Surgery. 2008;21(6):790-795.
8. Hardy S.C., et al. Surgery for deep venous incompetence. Cochrane Database of Systematic Reviews. (3):2004.
9. Luebke T., Brunkwall J. Meta-analysis of subfascial endoscopic perforator vein surgery (SEPS) for chronic venous insufficiency. Phlebology. 2009;24(1):8-16.
10. Kreysel H., et al. A possible role of lysosomal enzymes in the pathogenesis of varicosis and the reduction in their serum activity by Venostasin. VASA. 1983;12(4):377-382.
11. Schultz G., Mast B. Molecular analysis of the environment of healing and chronic wounds: cytokines, proteases, and growth factors. Wounds. 1998;10(Supp F):1F-9F.
12. Facino R., et al. Anti-elastase and anti-hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: factors contributing to their efficacy in the treatment of venous insufficiency. Archiv der Pharmazie. 1995;328(10):720-724.
13. Selloum L., et al. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Experimental & Toxicologic Pathology. 2003;54(4):313-318.
14. Carini M., et al. Procyanidins from Vitis vinifera seeds inhibit the respiratory burst of activated human neutrophils and lysosomal enzyme release. Planta Medica. 2001;67(8):714-717.
15. Blumenthal M., editor. The complete German Commission E monographs: therapeutic guide to herbal medicines. Austin: American Botanical Council, 1998.
16. Gruenwald J., et al. PDR for herbal medicines. In Montvale. Medical Economics Company; 2000.
17. Sirtori C. Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacological Research. 2001;44(3):183-193.
18. Guillaume M., Padioleau F. Veinotonic effect, vascular protection, antiinflammatory and free radical scavenging properties of horse chestnut extract. Arzneimittel Forschung. 1994;44(1):25-35.
19. Masaki H., et al. Active-oxygen scavenging activity of plant extracts. Biological & Pharmaceutical Bulletin. 1995;18(1):162-166.
20. Yeoh S. The influence of iron and free radicals on chronic leg ulceration. Primary Intention. 2000;8(2):47-55.
21. Zheng C.J., Qin L.P. Chemical components of Centella asiatica and their bioactivities. Journal of Chinese Integrative Medicine. 2007;5(3):348-351.
22. Faria A., et al. Antioxidant properties of prepared blueberry (Vaccinium myrtillus) extracts. Journal of Agricultural & Food Chemistry. 2005;53(17):6896-6902.
23. Busserolles J., et al. In vivo antioxidant activity of procyanidin-rich extracts from grape seed and pine (Pinus maritima) bark in rats. International Journal for Vitamin & Nutrition Research. 2006;76(1):22-27.
24. Zhang J., et al. Free radical scavenging and cytoprotective activities of phenolic antioxidants. Molecular Nutrition & Food Research. 2006;50(11):996-1005.
25. Pittler M.H., Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database of Systematic Reviews. (1):2006. CD003230
26. Leach M.J., et al. Clinical efficacy of horsechestnut seed extract in the treatment of venous ulceration. Journal of Wound Care. 2006;15(4):159-167.
27. Lu X., Chen Y., Huang Y., Li W., Jiang M. Venous hypertension induces increased platelet reactivity and accumulation in patients with chronic venous insufficiency. Angiology. 2006;57(3):321-329.
28. Ciuffetti G., et al. Circulating leucocyte adhesion molecules in chronic venous insufficiency. VASA. 1999;28(3):156-159.
29. Peschen M., et al. Expression of the adhesion molecules ICAM-1, VCAM-1, LFA-1 and VLA-4 in the skin is modulated in progressing stages of chronic venous insufficiency. Acta Dermato-Venereologica. 1999;79(1):27-32.
30. Wilkinson L.S., et al. Leukocytes: their role in the etiopathogenesis of skin damage in venous disease. Journal of Vascular Surgery. 1993;17:669-675.
31. Pappas P.J., et al. Dermal tissue fibrosis in patients with chronic venous insufficiency is associated with increased transforming growth factor-β1 gene expression and protein production. Journal of Vascular Surgery. 1999;30(6):1129-1145.
32. Peschen M., et al. Increased expression of platelet-derived growth factor receptor alpha and beta and vascular endothelial growth factor in the skin of patients with chronic venous insufficiency. Archives of Dermatological Research. 1998;290(6):291-297.
33. Matsuda H., et al. Effects of escins Ia, Ib, IIa, and IIB from Horse chestnut, the seeds of Aesculus hippocastanum L., on acute inflammation in animals. Biological & Pharmaceutical Bulletin. 1997;20(10):1092-1095.
34. Bouskela E., et al. Possible mechanisms for the inhibitory effect of Ruscus extract on increased microvascular permeability induced by histamine in hamster cheek pouch. Journal of Cardiovascular Pharmacology. 1994;24(2):281-285.
35. Greenspan P., et al. Antiinflammatory properties of the muscadine grape (Vitis rotundifolia). Journal of Agricultural & Food Chemistry. 2005;53(22):8481-8488.
36. Lietti A., et al. Studies on Vaccinium myrtillus anthocyanosides. I. Vasoprotective and antiinflammatory activity. Arzneimittel Forschung. 1976;26(5):829-832.
37. Cho K.J., et al. Effect of bioflavonoids extracted from the bark of Pinus maritima on proinflammatory cytokine interleukin-1 production in lipopolysaccharide-stimulated RAW 264.7. Toxicology & Applied Pharmacology. 2000;168(1):64-71.
38. Park H.H., et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Archives of Pharmaceutical Research. 2008;31(10):1303-1311.
39. Wen W., et al. Grape seed extract inhibits angiogenesis via suppression of the vascular endothelial growth factor receptor signalling pathway. Cancer Prevention Research. 2008;1(7):554-561.
40. Vanscheidt W., et al. Efficacy and safety of a Butcher’s broom preparation (Ruscus aculeatus L. extract) compared to placebo in patients suffering from chronic venous insufficiency. Arzneimittel Forschung. 2002;52(4):243-250.
41. De Sanctis M.T., et al. Treatment of edema and increased capillary filtration in venous hypertension with total triterpenic fraction of Centella asiatica: a clinical, prospective, placebo-controlled, randomized, dose-ranging trial. Angiology. 2001;52(Suppl 2):S55-S59.
42. Cesarone M.R., et al. Microcirculatory effects of total triterpenic fraction of Centella asiatica in chronic venous hypertension: measurement by laser Doppler, TcPO2–CO2, and leg volumetry. Angiology. 2001;52(Suppl 2):S45-S48.
43. Pointel J.P., et al. Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology. 1987;38(1, Pt 1):46-50.
44. Arcangeli P. Pycnogenol in chronic venous insufficiency. Fitoterapia. 2000;71(3):236-244.
45. Petrassi C., et al. Pycnogenol in chronic venous insufficiency. Phytomedicine. 2000;7(5):383-388.
46. Kalus U., et al. Improvement of cutaneous microcirculation and oxygen supply in patients with chronic venous insufficiency by orally administered extract of red vine leaves AS 195: a randomised, double-blind, placebo-controlled, crossover study. Drugs in R&D. 2004;5(2):63-71.
47. Kiesewetter H., et al. Efficacy of orally administered extract of red vine leaf AS 195 (folia vitis viniferae) in chronic venous insufficiency (stages I-II). A randomized, double-blind, placebo-controlled trial. Arzneimittel Forschung. 2000;50(2):109-117.
48. Mollart L. Single-blind trial addressing the differential effects of two reflexology techniques versus rest, on ankle and foot oedema in late pregnancy. Complementary Therapies in Nursing and Midwifery. 2003;9(4):203-208.
49. Abu-Own A., et al. Effect of leg elevation on the skin microcirculation in chronic venous insufficiency. Journal of Vascular Surgery. 1994;20(5):705-710.
50. Padberg F.T., et al. Structured exercise improves calf muscle pump function in chronic venous insufficiency: a randomized trial. Journal of Vascular Surgery. 2004;39(1):79-87.
51. Chong P.S., Bartolo D.C. Hemorrhoids and fissure in ano. Gastroenterol Clin North Am. 2008;37(3):627-644.
52. Acheson A.G., Scholefield J.H. Management of haemorrhoids. BMJ. 2008;336(7640):380-383.
53. Murtagh J. General practice. Sydney: McGraw-Hill; 2006.
54. Shafik A. Role of warm-water bath in anorectal conditions. The ‘thermosphincteric reflex’. J Clin Gastroenterol. 1993;16(4):304-308.
55. Scientific Committee of the British Herbal Medical Association. British herbal pharmacopoeia. Bournemouth: British Herbal Medicine Association; 1983.
56. Drug therapy of hemorrhoids. Proven results of therapy with a hamamelis containing hemorrhoid ointment. Results of a meeting of experts. Fortschr Med. 1991;116(Supp):1-11.
57. Knoch H.G., et al. Ointment treatment of 1st degree hemorrhoids. Comparison of the effectiveness of a phytogenic preparation with two new ointments containing synthetic drugs. Fortschr Med. 1992;110(8):135-138.