|
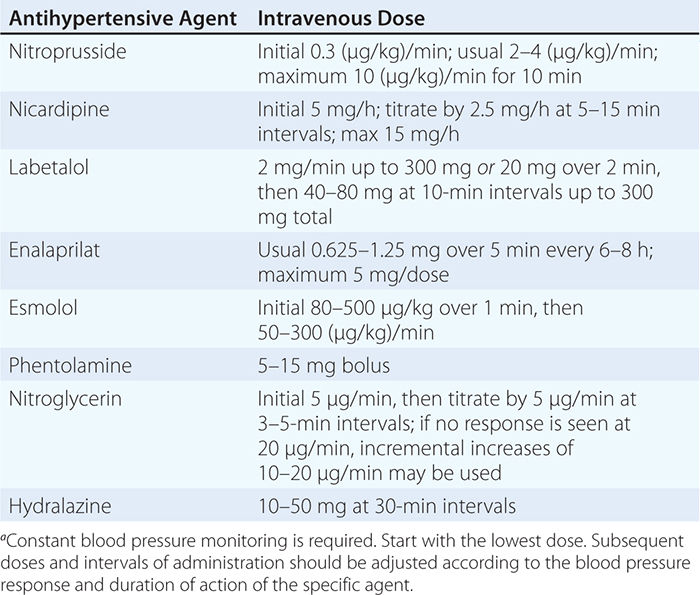
USUAL INTRAVENOUS DOSES OF ANTIHYPERTENSIVE AGENTS USED IN HYPERTENSIVE EMERGENCIESa |
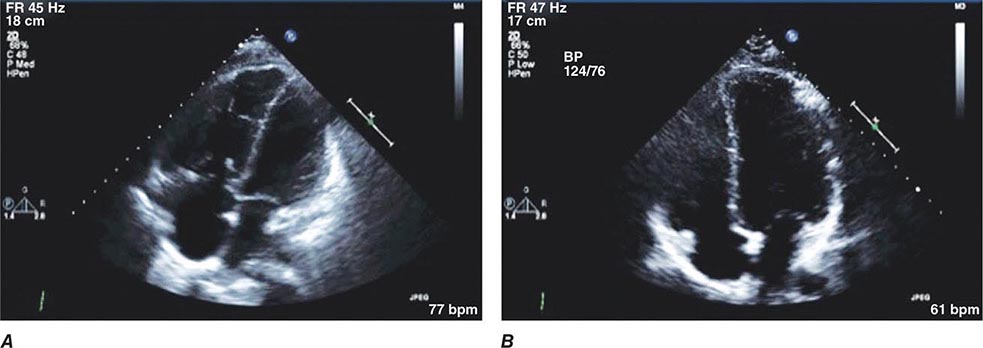
Malignant hypertension is a syndrome associated with an abrupt increase of blood pressure in a patient with underlying hypertension or related to the sudden onset of hypertension in a previously normotensive individual. The absolute level of blood pressure is not as important as its rate of rise. Pathologically, the syndrome is associated with diffuse necrotizing vasculitis, arteriolar thrombi, and fibrin deposition in arteriolar walls. Fibrinoid necrosis has been observed in arterioles of kidney, brain, retina, and other organs. Clinically, the syndrome is recognized by progressive retinopathy (arteriolar spasm, hemorrhages, exudates, and papilledema), deteriorating renal function with proteinuria, microangiopathic hemolytic anemia, and encephalopathy. Historic inquiry should include questions about the use of monoamine oxidase inhibitors and recreational drugs (e.g., cocaine, amphetamines).
Although blood pressure should be lowered rapidly in patients with hypertensive encephalopathy, there are inherent risks of overly aggressive therapy. In hypertensive individuals, the upper and lower limits of autoregulation of cerebral blood flow are shifted to higher levels of arterial pressure, and rapid lowering of blood pressure to below the lower limit of autoregulation may precipitate cerebral ischemia or infarction as a consequence of decreased cerebral blood flow. Renal and coronary blood flows also may decrease with overly aggressive acute therapy. The initial goal of therapy is to reduce mean arterial blood pressure by no more than 25% within minutes to 2 h or to a blood pressure in the range of 160/100–110 mmHg. This may be accomplished with IV nitroprusside, a short-acting vasodilator with a rapid onset of action that allows for minute-to-minute control of blood pressure. Parenteral labetalol and nicardipine are also effective agents for the treatment of hypertensive encephalopathy.
In patients with malignant hypertension without encephalopathy or another catastrophic event, it is preferable to reduce blood pressure over hours or longer rather than minutes. This goal may effectively be achieved initially with frequent dosing of short-acting oral agents such as captopril, clonidine, and labetalol.
Acute, transient blood pressure elevations that last days to weeks frequently occur after thrombotic and hemorrhagic strokes. Autoregulation of cerebral blood flow is impaired in ischemic cerebral tissue, and higher arterial pressures may be required to maintain cerebral blood flow. Although specific blood pressure targets have not been defined for patients with acute cerebrovascular events, aggressive reductions of blood pressure are to be avoided. With the increasing availability of improved methods for measuring cerebral blood flow (using CT technology), studies are in progress to evaluate the effects of different classes of antihypertensive agents on both blood pressure and cerebral blood flow after an acute stroke. Currently, in the absence of other indications for acute therapy, for patients with cerebral infarction who are not candidates for thrombolytic therapy, one recommended guideline is to institute antihypertensive therapy only for patients with a systolic blood pressure >220 mmHg or a diastolic blood pressure >130 mmHg. If thrombolytic therapy is to be used, the recommended goal blood pressure is <185 mmHg systolic pressure and <110 mmHg diastolic pressure. In patients with hemorrhagic stroke, suggested guidelines for initiating antihypertensive therapy are systolic >180 mmHg or diastolic pressure >130 mmHg. The management of hypertension after subarachnoid hemorrhage is controversial. Cautious reduction of blood pressure is indicated if mean arterial pressure is >130 mmHg.
In addition to pheochromocytoma, an adrenergic crisis due to catecholamine excess may be related to cocaine or amphetamine overdose, clonidine withdrawal, acute spinal cord injuries, and an interaction of tyramine-containing compounds with monoamine oxidase inhibitors. These patients may be treated with phentolamine or nitroprusside.
Treatment of hypertension in patients with acute aortic dissection is discussed in Chap. 301, and treatment of hypertension in pregnancy is discussed in Chap. 8.
299 |
Renovascular Disease |
The renal vasculature is unusually complex with rich arteriolar flow to the cortex in excess of metabolic requirements, consistent with its primary function as a filtering organ. After delivering blood to cortical glomeruli, the postglomerular circulation supplies deeper medullary segments that support energy-dependent solute transport at multiple levels of the renal tubule. These postglomerular vessels carry less blood, and high oxygen consumption leaves the deeper medullary regions at the margin of hypoxemia. Vascular disorders that commonly threaten the blood supply of the kidney include large-vessel atherosclerosis, fibromuscular diseases, and embolic disorders. Microvascular injury, including inflammatory and primary hematologic disorders, is described in Chap. 341.
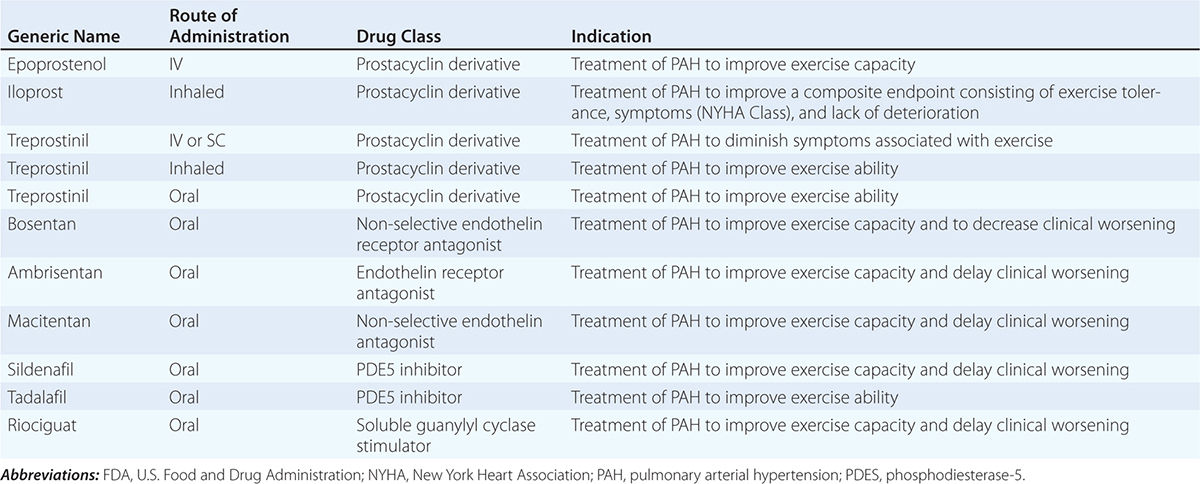
The glomerular capillary endothelium shares susceptibility to oxidative stress, pressure injury, and inflammation with other vascular territories. Rates of urinary albumin excretion (UAE) are predictive of systemic atherosclerotic disease events. Increased UAE may develop years before cardiovascular events. UAE and the risk of cardiovascular events are both reduced with pharmacologic therapy such as statins. Experimental studies demonstrate functional changes and rarefaction of renal microvessels under conditions of accelerated atherosclerosis and/or compromise of proximal perfusion pressures with large-vessel disease (Fig. 299-1).
FIGURE 299-1 Examples of micro-CT images from vessels defined by radiopaque casts injected into the renal vasculature. These illustrate the complex, dense cortical capillary network supplying the kidney cortex that can either proliferate or succumb to rarefaction under the influence of atherosclerosis and/or occlusive disease. Changes in blood supply are followed by tubulointerstitial fibrosis and loss of kidney function. MV, microvascular. (From LO Lerman, AR Chade: Curr Opin Nephrol Hyper 18:160, 2009, with permission.)
MACROVASCULAR DISEASE
Large-vessel renal artery occlusive disease can result from extrinsic compression of the vessel, fibromuscular dysplasia, or, most commonly, atherosclerotic disease. Any disorder that reduces perfusion pressure to the kidney can activate mechanisms that tend to restore renal pressures at the expense of developing systemic hypertension. Because restoration of perfusion pressures can reverse these pathways, renal artery stenosis is considered a specifically treatable “secondary” cause of hypertension.
Renal artery stenosis is common and often has only minor hemodynamic effects. Fibromuscular dysplasia (FMD) is reported in 3–5% of normal subjects presenting as potential kidney donors without hypertension. It may present clinically with hypertension in younger individuals (between age 15 and 50), most often women. FMD does not often threaten kidney function, but sometimes produces total occlusion and can be associated with renal artery aneurysms. Atherosclerotic renal artery stenosis (ARAS) is common in the general population (6.8% of a community-based sample above age 65), and the prevalence increases with age and for patients with other vascular conditions such as coronary artery disease (18–23%) and/or peripheral aortic or lower extremity disease (>30%). If untreated, ARAS progresses in nearly 50% of cases over a 5-year period, sometimes to total occlusion. Intensive treatment of arterial blood pressure and statin therapy appear to slow these rates and improve clinical outcomes.
Critical levels of stenosis lead to a reduction in perfusion pressure that activates the renin-angiotensin system, reduces sodium excretion, and activates sympathetic adrenergic pathways. These events lead to systemic hypertension characterized by angiotensin dependence in the early stages, widely varying pressures, loss of circadian blood pressure (BP) rhythms, and accelerated target organ injury, including left ventricular hypertrophy and renal fibrosis. Renovascular hypertension can be treated with agents that block the renin-angiotensin system and other drugs that modify these pressor pathways. It can also be treated with restoration of renal blood flow by either endovascular or surgical revascularization. Most patients require continued antihypertensive drug therapy because revascularization alone rarely lowers BP to normal.
ARAS and systemic hypertension tend to affect both the post-stenotic and contralateral kidneys, reducing overall glomerular filtration rate (GFR) in ARAS. When kidney function is threatened by large-vessel disease primarily, it has been labeled ischemic nephropathy. Moderately reduced blood flow that develops gradually is associated with reduced GFR and limited oxygen consumption with preserved tissue oxygenation. Hence, kidney function can remain stable during medical therapy, sometimes for years. With more advanced disease, reductions in cortical perfusion and frank tissue hypoxia develop. Unlike FMD, ARAS develops in patients with other risk factors for atherosclerosis and is commonly superimposed upon preexisting small-vessel disease in the kidney resulting from hypertension, aging, and diabetes. Nearly 85% of patients considered for renal revascularization have stage 3–5 chronic kidney disease (CKD) with GFR below 60 mL/min per 1.73 m2. The presence of ARAS is a strong predictor of morbidity- and mortality-related cardiovascular events, independent of whether renal revascularization is undertaken.
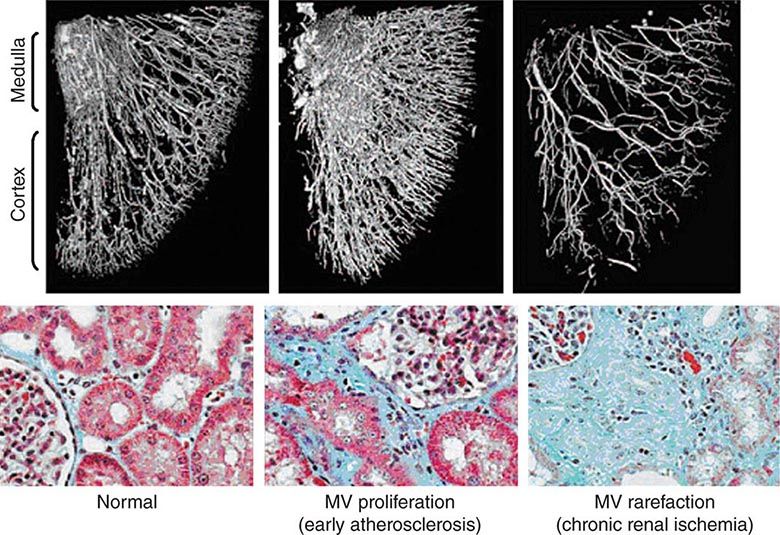
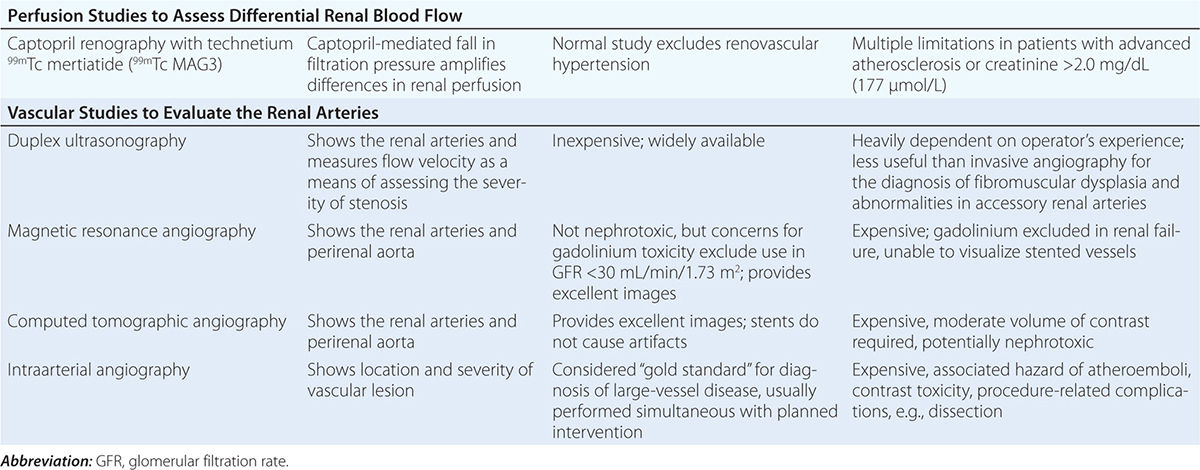
Diagnostic approaches to renal artery stenosis depend partly on the specific issues to be addressed. Noninvasive characterization of the renal vasculature may be achieved by several techniques, summarized in Table 299-1. Although activation of the renin-angiotensin system is a key step in developing renovascular hypertension, it is transient. Levels of renin activity are therefore subject to timing, the effects of drugs, and sodium intake, and do not reliably predict the response to vascular therapy. Renal artery velocities by Doppler ultrasound above 200 cm/s generally predict hemodynamically important lesions (above 60% vessel lumen occlusion), although treatment trials require velocity above 300 cm/s to avoid false positives. The renal resistive index has predictive value regarding the viability of the kidney. It remains operator- and institution-dependent, however. Captopril-enhanced renography has a strong negative predictive value when entirely normal. Magnetic resonance angiography (MRA) is now less often used, as gadolinium contrast has been associated with nephrogenic systemic fibrosis. Contrast-enhanced computed tomography (CT) with vascular reconstruction provides excellent vascular images and functional assessment, but carries a small risk of contrast toxicity.
|
SUMMARY OF IMAGING MODALITIES FOR EVALUATING THE KIDNEY VASCULATURE |
TREATMENT RENAL ARTERY STENOSIS
While restoring renal blood flow and perfusion seems intuitively beneficial for high-grade occlusive lesions, revascularization procedures also pose hazards and expense. Patients with FMD are commonly younger females with otherwise normal vessels and a long life expectancy. These patients often respond well to percutaneous renal artery angioplasty. If BP can be controlled to goal levels and kidney function remains stable in patients with ARAS, it may be argued that medical therapy with follow-up for disease progression is equally effective. Prospective trials up to now have failed to identify compelling benefits for interventional procedures regarding short-term results of BP and renal function, and long-term studies regarding cardiovascular outcomes, such as stroke, congestive heart failure, myocardial infarction, and end-stage renal failure, are not yet complete. Medical therapy should include blockade of the renin-angiotensin system, attainment of goal BPs, cessation of tobacco, statins, and aspirin. Renal revascularization is now often reserved for patients failing medical therapy or developing additional complications.
Techniques of renal revascularization are improving. With experienced operators, major complications occur in about 9% of cases, including renal artery dissection, capsular perforation, hemorrhage, and occasional atheroembolic disease. Although not common, atheroembolic disease can be catastrophic and accelerate both hypertension and kidney failure, precisely the events that revascularization is intended to prevent. Although renal blood flow usually can be restored by endovascular stenting, recovery of renal function is limited to about 25% of cases, with no change in 50% and some deterioration evident in others. Patients with rapid loss of kidney function, sometimes associated with antihypertensive drug therapy, or with vascular disease affecting the entire functioning kidney mass are more likely to recover function after restoring blood flow. When hypertension is refractory to effective therapy, revascularization offers real benefits. Table 299-2 summarizes currently accepted guidelines for considering renal revascularization.
|
CLINICAL FACTORS FAVORING MEDICAL THERAPY AND REVASCULARIZATION OR SURVEILLANCE FOR RENAL ARTERY STENOSIS |
Abbreviations: ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; GFR, glomerular filtration rate.
ATHEROEMBOLIC RENAL DISEASE
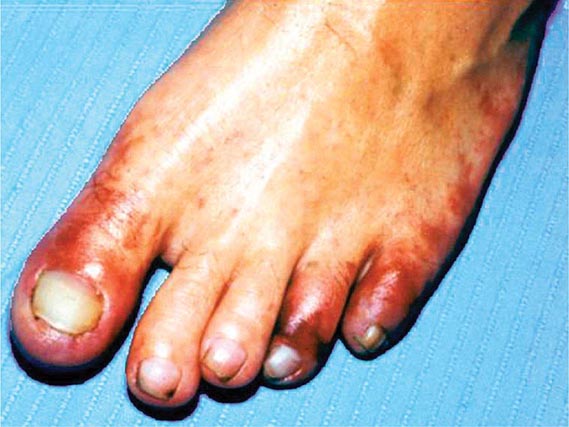
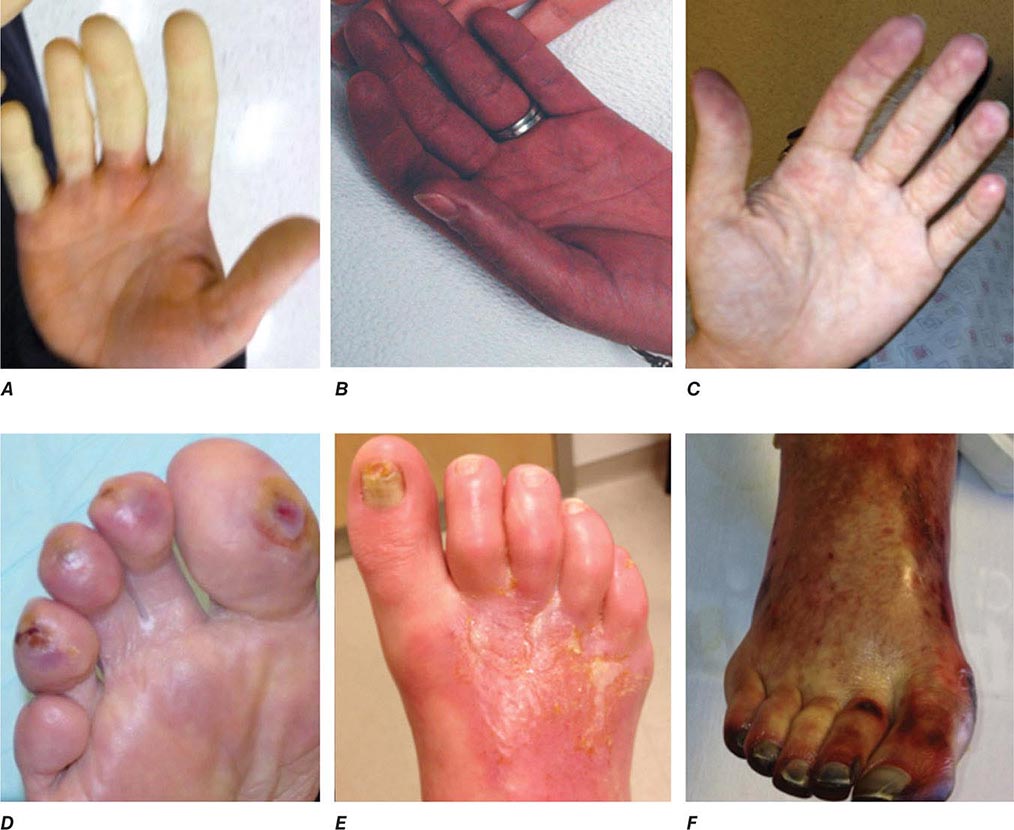
Emboli to the kidneys arise most frequently as a result of cholesterol crystals breaking free of atherosclerotic vascular plaque and lodging in downstream microvessels. Most clinical atheroembolic events follow angiographic procedures, often of the coronary vessels. It has been argued that nearly all vascular interventional procedures lead to plaque fracture and release of microemboli, but clinical manifestations develop only in a fraction of these. The incidence of clinical atheroemboli has been increasing with more vascular procedures and longer life spans. Atheroembolic renal disease is suspected in more than 3% of elderly subjects with end-stage renal disease (ESRD) and is likely underdiagnosed. It is more frequent in males with a history of diabetes, hypertension, and ischemic cardiac disease. Atheroemboli in the kidney are strongly associated with aortic aneurysmal disease and renal artery stenosis. Most clinical cases can be linked to precipitating events, such as angiography, vascular surgery, anticoagulation with heparin, thrombolytic therapy, or trauma. Clinical manifestations of this syndrome commonly develop between 1 and 14 days after an inciting event and may continue to develop for weeks thereafter. Systemic embolic disease manifestations, such as fever, abdominal pain, and weight loss, are present in less than half of patients, although cutaneous manifestations including livedo reticularis and localized toe gangrene may be more common. Worsening hypertension and deteriorating kidney function are common, sometimes reaching a malignant phase. Progressive renal failure can occur and require dialytic support. These cases often develop after a stuttering onset over many weeks and have an ominous prognosis. Mortality rate after 1 year reaches 38%, and although some may eventually recover sufficiently to no longer require dialysis, many do not.
Beyond the clinical manifestations above, laboratory findings include rising creatinine, transient eosinophilia (60–80%), elevated sedimentation rate, and hypocomplementemia (15%). Establishing this diagnosis can be difficult and is often by exclusion. Definitive diagnosis depends on kidney biopsy demonstrating microvessel occlusion with cholesterol crystals that leave a “cleft” in the vessel. Biopsies obtained from patients undergoing surgical revascularization of the kidney indicate that silent cholesterol emboli are frequently present before any further manipulation is performed.
No effective therapy is available for atheroembolic disease once it has developed. Withdrawal of anticoagulation is recommended. Late recovery of kidney function after supportive measures sometimes occurs, and statin therapy may improve outcome. The role of embolic protection devices in the renal circulation is unclear, but a few prospective trials have failed to demonstrate major benefits. These devices are limited to distal protection during the endovascular procedure and offer no protection from embolic debris after removal.
THROMBOEMBOLIC RENAL DISEASE
Thrombotic occlusion of renal vessels or branch arteries can lead to declining renal function and hypertension. It is difficult to diagnose and is often overlooked, especially in elderly patients. Thrombosis can develop as a result of local vessel abnormalities, such as local dissection, trauma, or inflammatory vasculitis. Local microdissections sometimes lead to patchy, transient areas of infarctions labeled “segmental arteriolar mediolysis.” Although hypercoagulability conditions sometimes present as renal artery thrombosis, this is rare. It can also derive from distant embolic events, e.g., the left atrium in patients with atrial fibrillation or from fat emboli originating from traumatized tissue, most commonly large bone fractures. Cardiac sources include vegetations from subacute bacterial endocarditis. Systemic emboli to the kidneys may also arise from the venous circulation if right-to-left shunting occurs, e.g., through a patent foramen ovale.
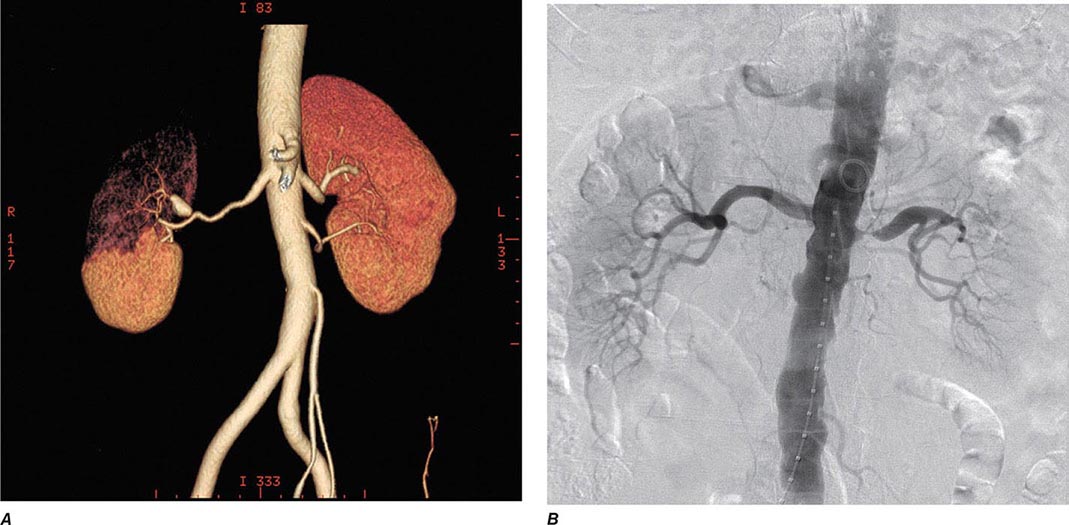
Clinical manifestations vary depending on the rapidity of onset and extent of occlusion. Acute arterial thrombosis may produce flank pain, fever, leukocytosis, nausea, and vomiting. If kidney infarction results, enzymes such as lactate dehydrogenase (LDH) rise to extreme levels. If both kidneys are affected, renal function will decline precipitously with a drop in urine output. If a single kidney is involved, renal functional changes may be minor. Hypertension related to sudden release of renin from ischemic tissue can develop rapidly, as long as some viable tissue in the “peri-infarct” border zone remains. If the infarct zone demarcates precisely, the rise in BP and renin activity may resolve. Diagnosis of renal infarction may be established by vascular imaging with MRI, CT angiography, or arteriography (Fig. 299-2).
FIGURE 299-2 A. CT angiogram illustrating loss of circulation to the upper pole of the right kidney in a patient with fibromuscular disease and a renal artery aneurysm. Activation of the renin-angiotensin system produced rapidly developing hypertension. B. Angiogram illustrating high-grade renal artery stenosis affecting the left kidney. This lesion is often part of widespread atherosclerosis and sometimes is an extension of aortic plaque. This lesion develops in older individuals with preexisting atherosclerotic risk factors.
MANAGEMENT OF ARTERIAL THROMBOSIS OF THE KIDNEY
Options for interventions of newly detected arterial occlusion include surgical reconstruction, anticoagulation, thrombolytic therapy, endovascular procedures, and supportive care, particularly antihypertensive drug therapy. Application of these methods depends on the patient’s overall condition, the precipitating factors (e.g., local trauma or systemic illness), the magnitude of renal tissue and function at risk, and the likelihood of recurrent events in the future. For unilateral disease, e.g., arterial dissection with thrombosis, supportive care with anticoagulation may suffice. Acute, bilateral occlusion is potentially catastrophic, producing anuric renal failure. Depending on the precipitating event, surgical or thrombolytic therapies can sometimes restore kidney viability.
MICROVASCULAR INJURY ASSOCIATED WITH HYPERTENSION
ARTERIOLONEPHROSCLEROSIS
“Malignant” Hypertension Although BP rises with age, it has long been recognized that some individuals develop rapidly progressive BP elevations with target organ injury including retinal hemorrhages, encephalopathy, and declining kidney function. Placebo arms during the controlled trials of hypertension therapy identified progression to severe levels in 20% of subjects over 5 years. If untreated, patients with target organ injury including papilledema and declining kidney function suffered mortality rates in excess of 50% over 6–12 months, hence the designation “malignant.” Postmortem studies of such patients identified vascular lesions, designated “fibrinoid necrosis,” with breakdown of the vessel wall, deposition of eosinophilic material including fibrin, and a perivascular cellular infiltrate. A separate lesion was identified in the larger interlobular arteries in many patients with hyperplastic proliferation of the vascular wall cellular elements, deposition of collagen, and separation of layers, designated the “onionskin” lesion. For many of these patients, fibrinoid necrosis led to obliteration of glomeruli and loss of tubular structures. Progressive kidney failure ensued and, without dialysis support, led to early mortality in untreated malignant-phase hypertension. These vascular changes could develop with pressure-related injury from a variety of hypertensive pathways, including but not limited to activation of the renin-angiotensin system and severe vasospasm associated with catecholamine release. Occasionally, endothelial injury is sufficient to induce microangiopathic hemolysis, as discussed below.
Antihypertensive therapy is the mainstay of therapy for malignant hypertension. With effective BP reduction, manifestations of vascular injury including microangiopathic hemolysis and renal dysfunction can improve over time. Whereas series reported before the era of drug therapy suggested that 1-year mortality rates exceeded 90%, current survival over 5 years exceeds 50%.
Malignant hypertension is less common in Western countries, although it persists in parts of the world where medical care and antihypertensive drug therapy are less available. It most commonly develops in patients with treated hypertension who neglect to take medications or who may use vasospastic drugs, such as cocaine. Renal abnormalities typically include rising serum creatinine and occasionally hematuria and proteinuria. Biochemical findings may include evidence of hemolysis (anemia, schistocytes, and reticulocytosis) and changes associated with kidney failure. African-American males are more likely to develop rapidly progressive hypertension and kidney failure than are whites in the United States. Genetic polymorphisms (first identified as MYH9, but now thought to be APOL1) that are common in the African-American population predispose to subtle focal sclerosing glomerular disease, with severe hypertension developing at younger ages secondary to renal disease in this instance.
“Hypertensive Nephrosclerosis” Based on experience with malignant hypertension and epidemiologic evidence linking BP with long-term risks of kidney failure, it has long been assumed that lesser degrees of hypertension induce less severe, but prevalent, changes in kidney vessels and loss of kidney function. As a result, a large portion of patients reaching ESRD without a specific etiologic diagnosis are assigned the designation “hypertensive nephrosclerosis.” Pathologic examination commonly identifies afferent arteriolar thickening with deposition of homogeneous eosinophilic material (hyaline arteriolosclerosis) associated with narrowing of vascular lumina. Clinical manifestations include retinal vessel changes associated with hypertension (arteriolar narrowing, crossing changes), left ventricular hypertrophy, and elevated BP. The role of these vascular changes in kidney function is unclear. Postmortem and biopsy samples from normotensive kidney donors demonstrate similar vessel changes associated with aging, dyslipidemia, and glucose intolerance. Although BP reduction does slow progression of proteinuric kidney diseases and is warranted to reduce the excessive cardiovascular risks associated with CKD, antihypertensive therapy does not alter the course of kidney dysfunction identified specifically as hypertensive nephrosclerosis.
300 |
Deep Venous Thrombosis and Pulmonary Thromboembolism |
EPIDEMIOLOGY
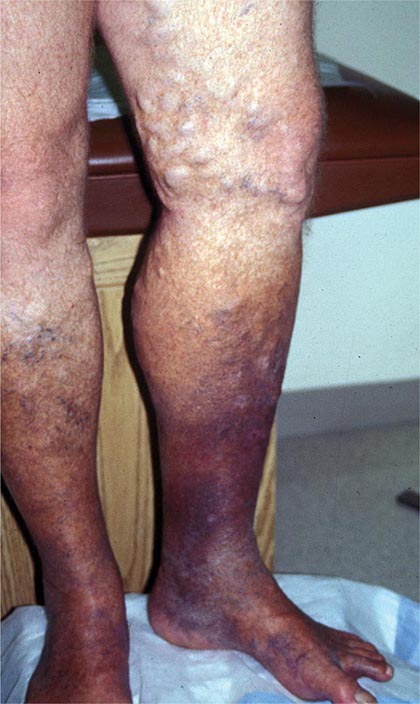
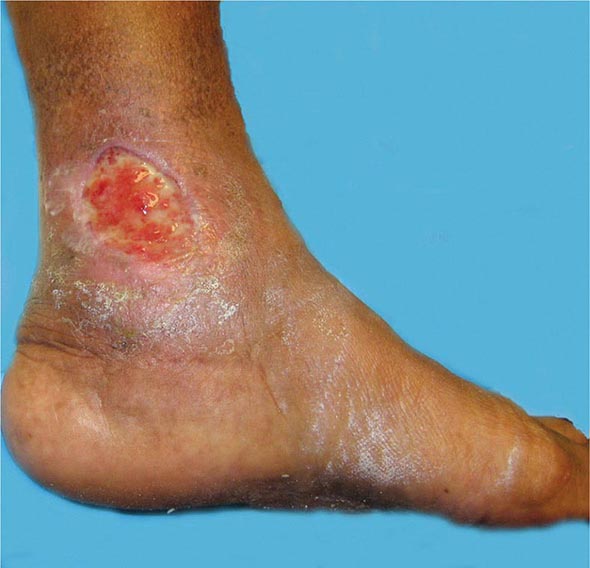
Venous thromboembolism (VTE) encompasses deep venous thrombosis (DVT) and pulmonary embolism (PE) and causes cardiovascular death and disability. In the United States, the Surgeon General estimates there are 100,000 to 180,000 deaths annually from PE and has declared that PE is the most common preventable cause of death among hospitalized patients. Survivors may succumb to the disabilities of chronic thromboembolic pulmonary hypertension or postthrombotic syndrome. Chronic thromboembolic pulmonary hypertension causes breathlessness, especially with exertion. Postthrombotic syndrome (also known as chronic venous insufficiency) damages the venous valves of the leg and causes ankle or calf swelling and leg aching, especially after prolonged standing. In its most severe form, postthrombotic syndrome causes skin ulceration (Fig. 300-1).
FIGURE 300-1 Skin ulceration in the lateral malleolus from postthrombotic syndrome of the leg.
PATHOPHYSIOLOGY
Inflammation and Platelet Activation Virchow’s triad of inflammation, hypercoagulability, and endothelial injury leads to recruitment of activated platelets, which release microparticles. These microparticles contain proinflammatory mediators that bind neutrophils, stimulating them to release their nuclear material and form web-like extracellular networks called neutrophil extracellular traps. These prothrombotic networks contain histones that stimulate platelet aggregation and promote platelet-dependent thrombin generation. Venous thrombi form and flourish in an environment of stasis, low oxygen tension, and upregulation of proinflammatory genes.
Prothrombotic States The two most common autosomal dominant genetic mutations are factor V Leiden, which causes resistance to the endogenous anticoagulant, activated protein C (which inactivates clotting factors V and VIII), and the prothrombin gene mutation, which increases the plasma prothrombin concentration (Chaps. 78 and 142). Antithrombin, protein C, and protein S are naturally occurring coagulation inhibitors. Deficiencies of these inhibitors are associated with VTE but are rare. Antiphospholipid antibody syndrome is the most common acquired cause of thrombophilia and is associated with venous or arterial thrombosis. Other common predisposing factors include cancer, obesity, cigarette smoking, systemic arterial hypertension, chronic obstructive pulmonary disease, chronic kidney disease, blood transfusion, long-haul air travel, air pollution, oral contraceptives, pregnancy, postmenopausal hormone replacement, surgery, and trauma.
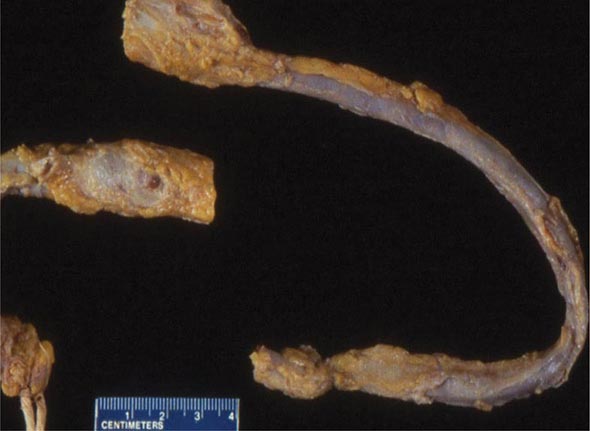
Embolization When deep venous thrombi (Fig. 300-2) detach from their site of formation, they embolize to the vena cava, right atrium, and right ventricle, and lodge in the pulmonary arterial circulation, thereby causing acute PE. Paradoxically, these thrombi occasionally embolize to the arterial circulation through a patent foramen ovale or atrial septal defect. Many patients with PE have no evidence of DVT because the clot has already embolized to the lungs.
FIGURE 300-2 Deep venous thrombosis at autopsy.
Physiology The most common gas exchange abnormalities are arterial hypoxemia and an increased alveolar-arterial O2 tension gradient, which represents the inefficiency of O2 transfer across the lungs. Anatomic dead space increases because breathed gas does not enter gas exchange units of the lung. Physiologic dead space increases because ventilation to gas exchange units exceeds venous blood flow through the pulmonary capillaries.
Other pathophysiologic abnormalities include:
1. Increased pulmonary vascular resistance due to vascular obstruction or platelet secretion of vasoconstricting neurohumoral agents such as serotonin. Release of vasoactive mediators can produce ventilation-perfusion mismatching at sites remote from the embolus, thereby accounting for discordance between a small PE and a large alveolar-arterial O2 gradient.
2. Impaired gas exchange due to increased alveolar dead space from vascular obstruction, hypoxemia from alveolar hypoventilation relative to perfusion in the nonobstructed lung, right-to-left shunting, or impaired carbon monoxide transfer due to loss of gas exchange surface.
3. Alveolar hyperventilation due to reflex stimulation of irritant receptors.
4. Increased airway resistance due to constriction of airways distal to the bronchi.
5. Decreased pulmonary compliance due to lung edema, lung hemorrhage, or loss of surfactant.
Pulmonary Hypertension, Right Ventricular (RV) Dysfunction, and RV Microinfarction Pulmonary artery obstruction causes a rise in pulmonary artery pressure and in pulmonary vascular resistance. When RV wall tension rises, RV dilation and dysfunction ensue, with release of the cardiac biomarker, brain natriuretic peptide. The interventricular septum bulges into and compresses an intrinsically normal left ventricle (LV). Diastolic LV dysfunction reduces LV distensibility and impairs LV filling. Increased RV wall tension also compresses the right coronary artery, limits myocardial oxygen supply, and precipitates right coronary artery ischemia and RV microinfarction, with release of cardiac biomarkers such as troponin. Underfilling of the LV may lead to a fall in LV cardiac output and systemic arterial pressure, with consequent circulatory collapse and death.
CLASSIFICATION OF PULMONARY EMBOLISM AND DEEP VENOUS THROMBOSIS
Pulmonary Embolism Massive PE accounts for 5–10% of cases, and is characterized by extensive thrombosis affecting at least half of the pulmonary vasculature. Dyspnea, syncope, hypotension, and cyanosis are hallmarks of massive PE. Patients with massive PE may present in cardiogenic shock and can die from multisystem organ failure. Submassive PE accounts for 20–25% of patients, and is characterized by RV dysfunction despite normal systemic arterial pressure. The combination of right heart failure and release of cardiac biomarkers indicates an increased likelihood of clinical deterioration. Low-risk PE constitutes about 70–75% of cases. These patients have an excellent prognosis.
Deep Venous Thrombosis Lower extremity DVT usually begins in the calf and propagates proximally to the popliteal vein, femoral vein, and iliac veins. Leg DVT is about 10 times more common than upper extremity DVT, which is often precipitated by placement of pacemakers, internal cardiac defibrillators, or indwelling central venous catheters. The likelihood of upper extremity DVT increases as the catheter diameter and number of lumens increase. Superficial venous thrombosis usually presents with erythema, tenderness, and a “palpable cord.” Patients are at risk for extension of the thrombosis to the deep venous system.
DIAGNOSIS
Clinical Evaluation PE is known as “the Great Masquerader.” Diagnosis is difficult because symptoms and signs are nonspecific. The most common symptom is unexplained breathlessness. When occult PE occurs concomitantly with overt congestive heart failure or pneumonia, clinical improvement often fails to occur despite standard medical treatment of the concomitant illness. This scenario presents a clinical clue to the possible coexistence of PE.
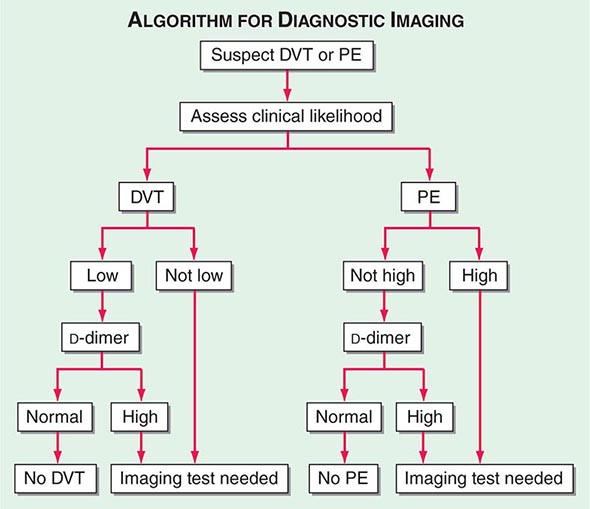
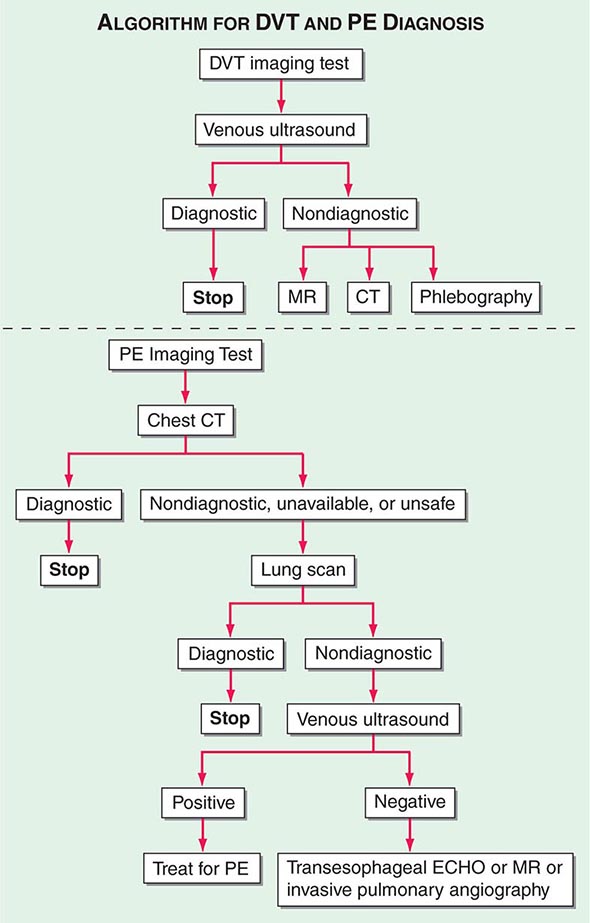
With DVT, the most common symptom is a cramp or “charley horse” in the lower calf that persists and intensifies over several days. Point score criteria help estimate the clinical likelihood of DVT and PE (Table 300-1). Patients with a low-to-moderate likelihood of DVT or PE should undergo initial diagnostic evaluation with D-dimer testing alone (see “Blood Tests”) without obligatory imaging tests (Fig. 300-3). However, patients with a high clinical likelihood of VTE should skip D-dimer testing and undergo imaging as the next step in the diagnostic algorithm.
|
CLINICAL DECISION RULES |
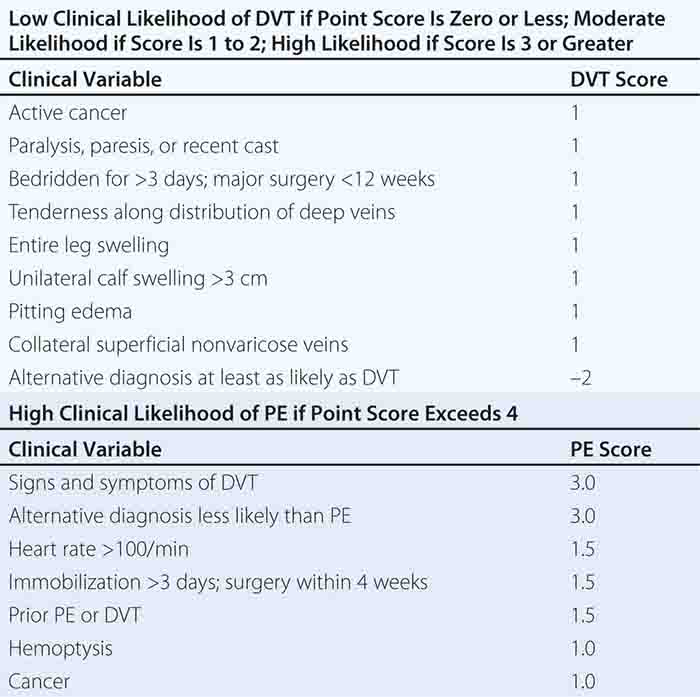
FIGURE 300-3 How to decide whether diagnostic imaging is needed. For assessment of clinical likelihood, see Table 300-1.
Clinical Pearls Not all leg pain is due to DVT, and not all dyspnea is due to PE (Table 300-2). Sudden, severe calf discomfort suggests a ruptured Baker’s cyst. Fever and chills usually herald cellulitis rather than DVT. Physical findings, if present, may consist only of mild palpation discomfort in the lower calf. However, massive DVT often presents with marked thigh swelling, tenderness, and erythema. If the leg is diffusely edematous, DVT is unlikely. More probable is an acute exacerbation of venous insufficiency due to postthrombotic syndrome. Upper extremity venous thrombosis may present with asymmetry in the supraclavicular fossa or in the circumference of the upper arms.
|
DIFFERENTIAL DIAGNOSIS |
Pulmonary infarction usually indicates a small PE. This condition is exquisitely painful because the thrombus lodges peripherally, near the innervation of pleural nerves. Nonthrombotic PE etiologies include fat embolism after pelvic or long bone fracture, tumor embolism, bone marrow, and air embolism. Cement embolism and bony fragment embolism can occur after total hip or knee replacement. Intravenous drug users may inject themselves with a wide array of substances that can embolize such as hair, talc, and cotton. Amniotic fluid embolism occurs when fetal membranes leak or tear at the placental margin.
Nonimaging Diagnostic Modalities • BLOOD TESTS The quantitative plasma D-dimer enzyme-linked immunosorbent assay (ELISA) rises in the presence of DVT or PE because of the breakdown of fibrin by plasmin. Elevation of D-dimer indicates endogenous although often clinically ineffective thrombolysis. The sensitivity of the D-dimer is >80% for DVT (including isolated calf DVT) and >95% for PE. The D-dimer is less sensitive for DVT than for PE because the DVT thrombus size is smaller. A normal D-dimer is a useful “rule out” test. However, the D-dimer assay is not specific. Levels increase in patients with myocardial infarction, pneumonia, sepsis, cancer, and the postoperative state and those in the second or third trimester of pregnancy. Therefore, D-dimer rarely has a useful role among hospitalized patients, because levels are frequently elevated due to systemic illness.
ELEVATED CARDIAC BIOMARKERS Serum troponin and plasma heart-type fatty acid–binding protein levels increase because of RV microinfarction. Myocardial stretch causes release of brain natriuretic peptide or NT-pro-brain natriuretic peptide.
ELECTROCARDIOGRAM The most frequently cited abnormality, in addition to sinus tachycardia, is the S1Q3T3 sign: an S wave in lead I, a Q wave in lead III, and an inverted T wave in lead III (Chap. 268). This finding is relatively specific but insensitive. RV strain and ischemia cause the most common abnormality, T-wave inversion in leads V1 to V4.
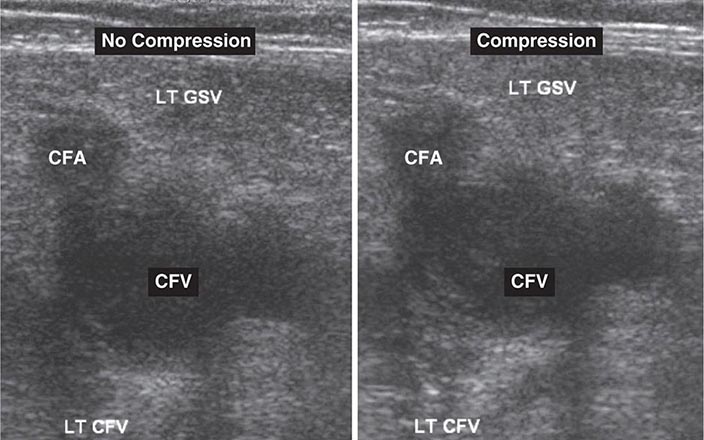
Noninvasive Imaging Modalities • VENOUS ULTRASONOGRAPHY Ultrasonography of the deep venous system relies on loss of vein compressibility as the primary criterion for DVT. When a normal vein is imaged in cross-section, it readily collapses with gentle manual pressure from the ultrasound transducer. This creates the illusion of a “wink.” With acute DVT, the vein loses its compressibility because of passive distention by acute thrombus. The diagnosis of acute DVT is even more secure when thrombus is directly visualized. It appears homogeneous and has low echogenicity (Fig. 300-4). The vein itself often appears mildly dilated, and collateral channels may be absent.
FIGURE 300-4 Venous ultrasound, with and without compression of the leg veins. CFA, common femoral artery; CFV, common femoral vein; GSV, great saphenous vein; LT, left.
Venous flow dynamics can be examined with Doppler imaging. Normally, manual calf compression causes augmentation of the Doppler flow pattern. Loss of normal respiratory variation is caused by an obstructing DVT or by any obstructive process within the pelvis. For patients with a technically poor or nondiagnostic venous ultrasound, one should consider alternative imaging modalities for DVT, such as computed tomography (CT) and magnetic resonance imaging.
CHEST ROENTGENOGRAPHY A normal or nearly normal chest x-ray often occurs in PE. Well-established abnormalities include focal oligemia (Westermark’s sign), a peripheral wedged-shaped density above the diaphragm (Hampton’s hump), and an enlarged right descending pulmonary artery (Palla’s sign).
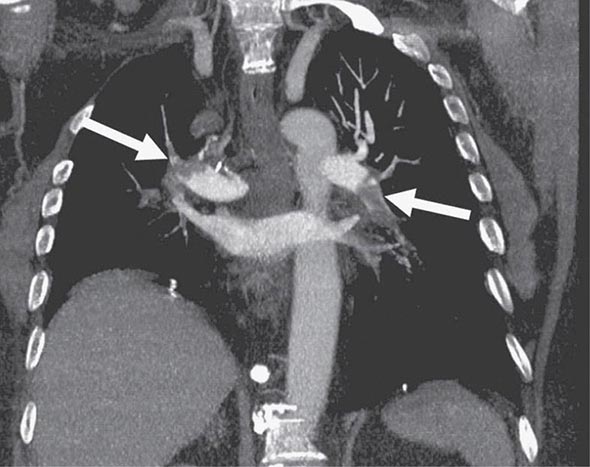
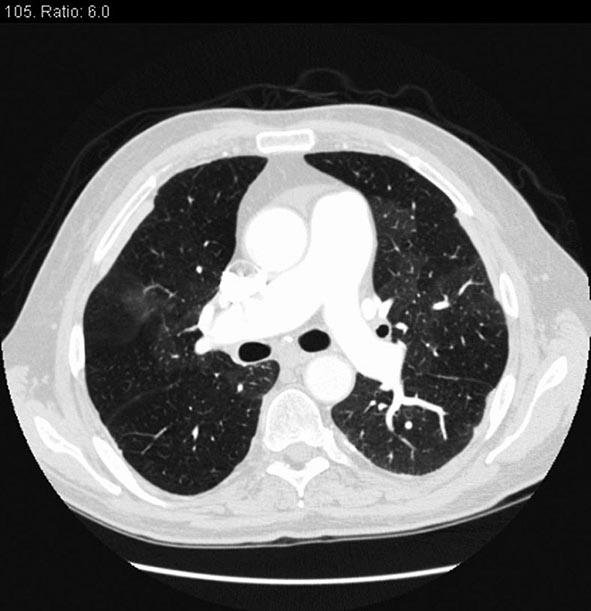
CHEST CT CT of the chest with intravenous contrast is the principal imaging test for the diagnosis of PE (Fig. 300-5). Multidetector-row spiral CT acquires all chest images with ≤1 mm of resolution during a short breath hold. Sixth-order branches can be visualized with resolution superior to that of conventional invasive contrast pulmonary angiography. The CT scan also provides an excellent four-chamber view of the heart. RV enlargement on chest CT indicates an increased likelihood of death within the next 30 days compared with PE patients who have normal RV size. When imaging is continued below the chest to the knee, pelvic and proximal leg DVT also can be diagnosed by CT scanning. In patients without PE, the lung parenchymal images may establish alternative diagnoses not apparent on chest x-ray that explain the presenting symptoms and signs such as pneumonia, emphysema, pulmonary fibrosis, pulmonary mass, and aortic pathology. Sometimes asymptomatic early-stage lung cancer is diagnosed incidentally.
FIGURE 300-5 Large bilateral proximal PE on a coronal chest CT image in a 54-year-old man with lung cancer and brain metastases. He had developed sudden onset of chest heaviness and shortness of breath while at home. There are filling defects in the main and segmental pulmonary arteries bilaterally (white arrows). Only the left upper lobe segmental artery is free of thrombus.
LUNG SCANNING Lung scanning has become a second-line diagnostic test for PE, used mostly for patients who cannot tolerate intravenous contrast. Small particulate aggregates of albumin labeled with a gamma-emitting radionuclide are injected intravenously and are trapped in the pulmonary capillary bed. The perfusion scan defect indicates absent or decreased blood flow, possibly due to PE. Ventilation scans, obtained with a radiolabeled inhaled gas such as xenon or krypton, improve the specificity of the perfusion scan. Abnormal ventilation scans indicate abnormal nonventilated lung, thereby providing possible explanations for perfusion defects other than acute PE, such as asthma and chronic obstructive pulmonary disease. A high-probability scan for PE is defined as two or more segmental perfusion defects in the presence of normal ventilation.
The diagnosis of PE is very unlikely in patients with normal and nearly normal scans and is about 90% certain in patients with high-probability scans. Unfortunately, most patients have nondiagnostic scans, and fewer than one-half of patients with angiographically confirmed PE have a high probability scan. As many as 40% of patients with high clinical suspicion for PE but “low-probability” scans do, in fact, have PE at angiography.
MAGNETIC RESONANCE (MR) (CONTRAST-ENHANCED) IMAGING When ultrasound is equivocal, MR venography with gadolinium contrast is an excellent imaging modality to diagnose DVT. MR pulmonary angiography may detect large proximal PE but is not reliable for smaller segmental and subsegmental PE.
ECHOCARDIOGRAPHY Echocardiography is not a reliable diagnostic imaging tool for acute PE because most patients with PE have normal echocardiograms. However, echocardiography is a very useful diagnostic tool for detecting conditions that may mimic PE, such as acute myocardial infarction, pericardial tamponade, and aortic dissection. Transthoracic echocardiography rarely images thrombus directly. The best-known indirect sign of PE on transthoracic echocardiography is McConnell’s sign: hypokinesis of the RV free wall with normal or hyperkinetic motion of the RV apex. One should consider transesophageal echocardiography when CT scanning facilities are not available or when a patient has renal failure or severe contrast allergy that precludes administration of contrast despite premedication with high-dose steroids. This imaging modality can identify saddle, right main, or left main PE.
Invasive Diagnostic Modalities • PULMONARY ANGIOGRAPHY Chest CT with contrast (see above) has virtually replaced invasive pulmonary angiography as a diagnostic test. Invasive catheter-based diagnostic testing is reserved for patients with technically unsatisfactory chest CTs and for those in whom an interventional procedure such as catheter-directed thrombolysis is planned. A definitive diagnosis of PE depends on visualization of an intraluminal filling defect in more than one projection. Secondary signs of PE include abrupt occlusion (“cut-off”) of vessels, segmental oligemia or avascularity, a prolonged arterial phase with slow filling, and tortuous, tapering peripheral vessels.
CONTRAST PHLEBOGRAPHY Venous ultrasonography has virtually replaced contrast phlebography as the diagnostic test for suspected DVT.
Integrated Diagnostic Approach An integrated diagnostic approach (Fig. 300-3) streamlines the workup of suspected DVT and PE (Fig. 300-6).
FIGURE 300-6 Imaging tests to diagnose DVT and PE. ECHO, echocardiography.
TREATMENT DEEP VENOUS THROMBOSIS
PRIMARY THERAPY
Primary therapy consists of clot dissolution with pharmacomechanical therapy that usually includes low-dose catheter-directed thrombolysis. This approach is reserved for patients with extensive femoral, iliofemoral, or upper extremity DVT. The open vein hypothesis postulates that patients who receive primary therapy will sustain less long-term damage to venous valves, with consequent lower rates of postthrombotic syndrome. A National Heart, Lung, and Blood Institute–sponsored randomized controlled trial called ATTRACT (NCT00790335) is testing this hypothesis.
SECONDARY PREVENTION
Anticoagulation or placement of an inferior vena caval filter constitutes secondary prevention of VTE. To lessen the severity of postthrombotic syndrome of the legs, below-knee graduated compression stockings may be prescribed, 30–40 mmHg, for 2 years after the DVT episode. They should be replaced every 3 months because they lose their elasticity.
TREATMENT PULMONARY EMBOLISM
RISK STRATIFICATION
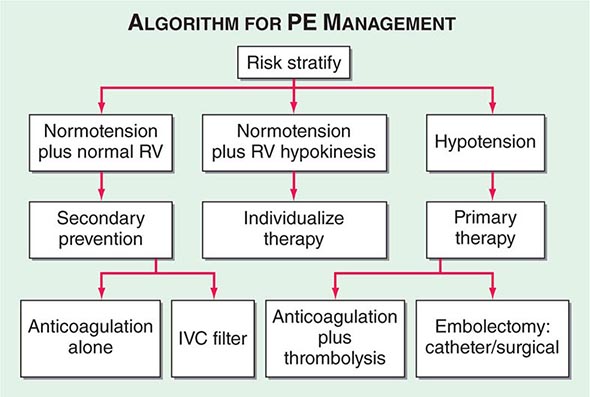
Hemodynamic instability, RV dysfunction on echocardiography, RV enlargement on chest CT, or elevation of the troponin level due to RV microinfarction portend a high risk of an adverse clinical outcome. When RV function remains normal in a hemodynamically stable patient, a good clinical outcome is highly likely with anticoagulation alone (Fig. 300-7).
FIGURE 300-7 Acute management of pulmonary thromboembolism. RV, right ventricular; IVC, inferior vena cava.
ANTICOAGULATION
Effective anticoagulation is the foundation for successful treatment of DVT and PE. There are three options: (1) the conventional strategy of parenteral therapy “bridged” to warfarin, (2) parenteral therapy “bridged” to a novel oral anticoagulant such as dabigatran (a direct thrombin inhibitor) or edoxaban (an anti-Xa agent), or (3) oral anticoagulation with rivaroxaban or apixaban (both are anti-Xa agents) with a loading dose followed by a maintenance dose as monotherapy without parenteral anticoagulation.
The three heparin-based parenteral anticoagulants are (1) unfractionated heparin (UFH), (2) low-molecular-weight heparin (LMWH), and (3) fondaparinux. For patients with suspected or proven heparin-induced thrombocytopenia, there are two parenteral direct thrombin inhibitors: argatroban and bivalirudin (Table 300-3).
|
ANTICOAGULATION OF VTE |
Unfractionated Heparin UFH anticoagulates by binding to and accelerating the activity of antithrombin, thus preventing additional thrombus formation. UFH is dosed to achieve a target activated partial thromboplastin time (aPTT) of 60–80 s. The most popular nomogram uses an initial bolus of 80 U/kg, followed by an initial infusion rate of 18 U/kg per h.
The major advantage of UFH is its short half-life, which is especially useful in patients in whom hour-to-hour control of the intensity of anticoagulation is desired.
Low-Molecular-Weight Heparins These fragments of UFH exhibit less binding to plasma proteins and endothelial cells and consequently have greater bioavailability, a more predictable dose response, and a longer half-life than does UFH. No monitoring or dose adjustment is needed unless the patient is markedly obese or has chronic kidney disease.
Fondaparinux Fondaparinux, an anti-Xa pentasaccharide, is administered as a weight-based once-daily subcutaneous injection in a prefilled syringe. No laboratory monitoring is required. Fondaparinux is synthesized in a laboratory and, unlike LMWH or UFH, is not derived from animal products. It does not cause heparin-induced thrombocytopenia. The dose must be adjusted downward for patients with renal dysfunction.
Warfarin This vitamin K antagonist prevents carboxylation activation of coagulation factors II, VII, IX, and X. The full effect of warfarin requires at least 5 days, even if the prothrombin time, used for monitoring, becomes elevated more rapidly. If warfarin is initiated as monotherapy during an acute thrombotic illness, a paradoxical exacerbation of hypercoagulability increases the likelihood of thrombosis. Overlapping UFH, LMWH, fondaparinux, or parenteral direct thrombin inhibitors with warfarin for at least 5 days will nullify the early procoagulant effect of warfarin.
WARFARIN DOSING In an average-size adult, warfarin is often initiated in a dose of 5 mg. The prothrombin time is standardized by calculating the international normalized ratio (INR), which assesses the anticoagulant effect of warfarin (Chap. 78). The target INR is usually 2.5, with a range of 2.0–3.0.
The warfarin dose is usually titrated empirically to achieve the target INR. Proper dosing is difficult because hundreds of drug-drug and drug-food interactions affect warfarin metabolism. Increasing age and systemic illness reduce the required warfarin dose. Pharmacogenomics may provide more precise initial dosing of warfarin. CYP2C9 variant alleles impair the hydroxylation of S-warfarin, thereby lowering the dose requirement. Variants in the gene encoding the vitamin K epoxide reductase complex 1 (VKORC1) can predict whether patients require low, moderate, or high warfarin doses.
Centralized anticoagulation clinics have improved the efficacy and safety of warfarin dosing. Patients can self-monitor their INR with a home point-of-care fingerstick machine and can occasionally be taught to self-dose their warfarin.
Novel Oral Anticoagulants Novel oral anticoagulants are administered in a fixed dose, establish effective anticoagulation within hours of ingestion, require no laboratory coagulation monitoring, and have few of the drug-drug or drug-food interactions that make warfarin so difficult to dose. Rivaroxaban, a factor Xa inhibitor, is approved for treatment of acute DVT and acute PE as monotherapy, without a parenteral “bridging” anticoagulant. Apixaban is likely to receive similar approval for oral monotherapy. Dabigatran, a direct thrombin inhibitor, and edoxaban, a factor Xa inhibitor, are likely to be approved for treatment of VTE after an initial course of parenteral anticoagulation.
Complications of Anticoagulants The most serious adverse effect of anticoagulation is hemorrhage. For life-threatening or intracranial hemorrhage due to heparin or LMWH, protamine sulfate can be administered. Heparin-induced thrombocytopenia is less common with LMWH than with UFH. There is no specific reversal agent for bleeding caused by fondaparinux, direct thrombin inhibitors, or factor Xa inhibitors.
Major bleeding from warfarin is best managed with prothrombin complex concentrate. With serious but non–life-threatening bleeding, fresh-frozen plasma or intravenous vitamin K can be used. Recombinant human coagulation factor VIIa (rFVIIa) is an off-label option to manage catastrophic bleeding from warfarin, but prothrombin complex concentrate is a better choice. Oral vitamin K is effective for managing minor bleeding or an excessively high INR in the absence of bleeding.
Duration of Anticoagulation For DVT isolated to an upper extremity or calf that has been provoked by surgery, trauma, estrogen, or an indwelling central venous catheter or pacemaker, 3 months of anticoagulation usually suffice. For an initial episode of provoked proximal leg DVT or PE, 3 to 6 months of anticoagulation are considered sufficient. For patients with cancer and VTE, prescribe LMWH as monotherapy without warfarin and continue anticoagulation indefinitely unless the patient is rendered cancer-free.
Among patients with idiopathic, unprovoked VTE, the recurrence rate is high after cessation of anticoagulation. VTE that occurs during long-haul air travel is considered unprovoked. Unprovoked VTE may be caused by an exacerbation of an underlying inflammatory state and can be conceptualized as a chronic illness, with latent periods between flares of recurrent episodes. American College of Chest Physicians (ACCP) guidelines recommend considering anticoagulation for an indefinite duration with a target INR between 2 and 3 for patients with idiopathic VTE. An alternative approach after the first 6 months of anticoagulation is to reduce the intensity of anticoagulation and to lower the target INR range to between 1.5 and 2.
Counterintuitively, the presence of genetic mutations such as heterozygous factor V Leiden and prothrombin gene mutation does not appear to increase the risk of recurrent VTE. However, patients with antiphospholipid antibody syndrome may warrant indefinite-duration anticoagulation, even if the initial VTE was provoked by trauma or surgery.
INFERIOR VENA CAVAL (IVC) FILTERS
The two principal indications for insertion of an IVC filter are (1) active bleeding that precludes anticoagulation and (2) recurrent venous thrombosis despite intensive anticoagulation. Prevention of recurrent PE in patients with right heart failure who are not candidates for fibrinolysis and prophylaxis of extremely high-risk patients are “softer” indications for filter placement. The filter itself may fail by permitting the passage of small-to medium-size clots. Large thrombi may embolize to the pulmonary arteries via collateral veins that develop. A more common complication is caval thrombosis with marked bilateral leg swelling.
Paradoxically, by providing a nidus for clot formation, filters increase the DVT rate, even though they usually prevent PE (over the short term). Retrievable filters can now be placed for patients with an anticipated temporary bleeding disorder or for patients at temporary high risk of PE, such as individuals undergoing bariatric surgery who have a prior history of perioperative PE. The filters can be retrieved up to several months after insertion unless thrombus forms and is trapped within the filter. The retrievable filter becomes permanent if it remains in place or if, for technical reasons such as rapid endothelialization, it cannot be removed.
MANAGEMENT OF MASSIVE PE
For patients with massive PE and hypotension, replete volume with 500 mL of normal saline. Additional fluid should be infused with extreme caution because excessive fluid administration exacerbates RV wall stress, causes more profound RV ischemia, and worsens LV compliance and filling by causing further interventricular septal shift toward the LV. Dopamine and dobutamine are first-line inotropic agents for treatment of PE-related shock. Maintain a low threshold for initiating these pressors. Often, a “trial-and-error” approach works best; other agents that may be effective include norepinephrine, vasopressin, or phenylephrine.
FIBRINOLYSIS
Successful fibrinolytic therapy rapidly reverses right heart failure and may result in a lower rate of death and recurrent PE by (1) dissolving much of the anatomically obstructing pulmonary arterial thrombus, (2) preventing the continued release of serotonin and other neurohumoral factors that exacerbate pulmonary hypertension, and (3) lysing much of the source of the thrombus in the pelvic or deep leg veins, thereby decreasing the likelihood of recurrent PE.
The preferred fibrinolytic regimen is 100 mg of recombinant tissue plasminogen activator (tPA) administered as a continuous peripheral intravenous infusion over 2 h. The sooner thrombolysis is administered, the more effective it is. However, this approach can be used for at least 14 days after the PE has occurred.
Contraindications to fibrinolysis include intracranial disease, recent surgery, and trauma. The overall major bleeding rate is about 10%, including a 1–3% risk of intracranial hemorrhage. Careful screening of patients for contraindications to fibrinolytic therapy (Chap. 295) is the best way to minimize bleeding risk.
The only Food and Drug Administration–approved indication for PE fibrinolysis is massive PE. For patients with submassive PE, who have preserved systolic blood pressure but moderate or severe RV dysfunction, use of fibrinolysis remains controversial. Results of a 1006-patient European multicentered randomized trial of submassive PE, using the thrombolytic agent tenecteplase, were published in 2014. Death or hemodynamic collapse within 7 days of randomization was reduced by 56% in the tenecteplase group. However, hemorrhagic stroke occurred in 2% of tenecteplase patients versus 0.2% in patients who only received heparin.
PHARMACOMECHANICAL CATHETER-DIRECTED THERAPY
Many patients have relative contraindications to full-dose thrombolysis. Pharmacomechanical catheter-directed therapy usually combines physical fragmentation or pulverization of thrombus with catheter-directed low-dose thrombolysis. Mechanical techniques include catheter maceration and intentional embolization of clot more distally, suction thrombectomy, rheolytic hydrolysis, and low-energy ultrasound-facilitated thrombolysis. The dose of alteplase can be markedly reduced, usually to a range of 20 to 25 mg instead of the peripheral intravenous systemic dose of 100 mg.
PULMONARY EMBOLECTOMY
The risk of major hemorrhage with systemically administered fibrinolysis has prompted a renaissance of interest in surgical embolectomy, an operation that had almost become extinct. More rapid referral before the onset of irreversible multisystem organ failure and improved surgical technique have resulted in a high survival rate.
PULMONARY THROMBOENDARTERECTOMY
Chronic thromboembolic pulmonary hypertension develops in 2–4% of acute PE patients. Therefore, PE patients who have initial pulmonary hypertension (usually diagnosed with Doppler echocardiography) should be followed up at about 6 weeks with a repeat echocardiogram to determine whether pulmonary arterial pressure has normalized. Patients impaired by dyspnea due to chronic thromboembolic pulmonary hypertension should be considered for pulmonary thromboendarterectomy, which, if successful, can markedly reduce, and sometimes even cure, pulmonary hypertension (Chap. 304). The operation requires median sternotomy, cardiopulmonary bypass, deep hypothermia, and periods of hypothermic circulatory arrest. The mortality rate at experienced centers is approximately 5%. Inoperable patients should be managed with pulmonary vasodilator therapy.
EMOTIONAL SUPPORT
Patients with VTE may feel overwhelmed when they learn that they are suffering from PE or DVT. Some have never previously encountered serious cardiovascular illness. They wonder whether they will be able to adapt to the new limitations imposed by anticoagulation. They worry about the health of their families and the genetic implications of their illness. Those who are advised to discontinue anticoagulation may feel especially vulnerable about the potential for suffering recurrent VTE. At Brigham and Woman’s Hospital, a physician-nurse–facilitated PE support group was initiated to address these concerns and has met monthly for more than 20 years.
PREVENTION OF VTE
Prevention of DVT and PE (Table 300-4) is of paramount importance because VTE is difficult to detect and poses a profound medical and economic burden. Low-dose UFH or LMWH is the most common form of in-hospital prophylaxis. Computerized reminder systems can increase the use of preventive measures and, at Brigham and Women’s Hospital, have reduced the symptomatic VTE rate by more than 40%. Audits of hospitals to ensure that prophylaxis protocols are being used will also increase utilization of preventive measures. Duration of prophylaxis is an important consideration. Extended-duration prophylaxis has not been shown to be both effective and safe in medically ill patients after hospital discharge in separate large trials that have tested enoxaparin, apixaban, and rivaroxaban. There is an ongoing trial of a novel oral anticoagulant, betrixaban, for extended-duration VTE prophylaxis in medically ill patients.
|
PREVENTION OF VENOUS THROMBOEMBOLISM AMONG HOSPITALIZED PATIENTS |
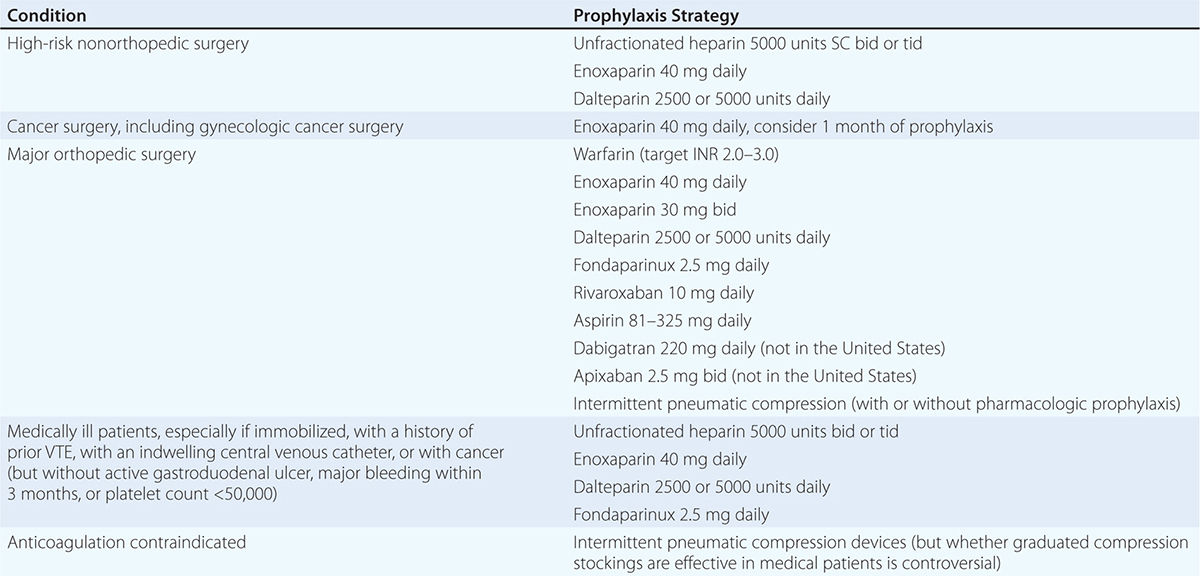
Patients who have undergone total hip or knee replacement or cancer surgery will benefit from extended pharmacologic VTE prophylaxis after hospital discharge. For hip replacement or extensive cancer surgery, the duration of prophylaxis is usually at least 1 month.
301 |
Diseases of the Aorta |
The aorta is the conduit through which blood ejected from the left ventricle is delivered to the systemic arterial bed. In adults, its diameter is approximately 3 cm at the origin and in the ascending portion, 2.5 cm in the descending portion in the thorax, and 1.8–2 cm in the abdomen. The aortic wall consists of a thin intima composed of endothelium, subendothelial connective tissue, and an internal elastic lamina; a thick tunica media composed of smooth muscle cells and extracellular matrix; and an adventitia composed primarily of connective tissue enclosing the vasa vasorum and nervi vascularis. In addition to the conduit function of the aorta, its viscoelastic and compliant properties serve a buffering function. The aorta is distended during systole to allow a portion of the stroke volume and elastic energy to be stored, and it recoils during diastole so that blood continues to flow to the periphery. Owing to its continuous exposure to high pulsatile pressure and shear stress, the aorta is particularly prone to injury and disease resulting from mechanical trauma. The aorta is also more prone to rupture than is any other vessel, especially with the development of aneurysmal dilation, since its wall tension, as governed by Laplace’s law (i.e., proportional to the product of pressure and radius), will be increased.
CONGENITAL ANOMALIES OF THE AORTA
Congenital anomalies of the aorta usually involve the aortic arch and its branches. Symptoms such as dysphagia, stridor, and cough may occur if an anomaly causes a ring around or otherwise compresses the esophagus or trachea. Anomalies associated with symptoms include double aortic arch, origin of the right subclavian artery distal to the left subclavian artery, and right-sided aortic arch with an aberrant left subclavian artery. A Kommerell’s diverticulum is an anatomic remnant of a right aortic arch. Most congenital anomalies of the aorta do not cause symptoms and are detected during catheter-based procedures. The diagnosis of suspected congenital anomalies of the aorta typically is confirmed by computed tomographic (CT) or magnetic resonance (MR) angiography. Surgery is used to treat symptomatic anomalies.
AORTIC ANEURYSM
An aneurysm is defined as a pathologic dilation of a segment of a blood vessel. A true aneurysm involves all three layers of the vessel wall and is distinguished from a pseudoaneurysm, in which the intimal and medial layers are disrupted and the dilated segment of the aorta is lined by adventitia only and, at times, by perivascular clot. Aneurysms also may be classified according to their gross appearance. A fusiform aneurysm affects the entire circumference of a segment of the vessel, resulting in a diffusely dilated artery. In contrast, a saccular aneurysm involves only a portion of the circumference, resulting in an outpouching of the vessel wall. Aortic aneurysms also are classified according to location, i.e., abdominal versus thoracic. Aneurysms of the descending thoracic aorta are usually contiguous with infradiaphragmatic aneurysms and are referred to as thoracoabdominal aortic aneurysms.
ETIOLOGY
Aortic aneurysms result from conditions that cause degradation or abnormal production of the structural components of the aortic wall: elastin and collagen. The causes of aortic aneurysms may be broadly categorized as degenerative disorders, genetic or developmental diseases, vasculitis, infections, and trauma (Table 301-1). Inflammation, oxidative stress, proteolysis, and biomechanical wall stress contribute to the degenerative processes that characterize most aneurysms of the abdominal and descending thoracic aorta. These are mediated by B cell and T cell lymphocytes, macrophages, inflammatory cytokines, and matrix metalloproteinases that degrade elastin and collagen and alter the tensile strength and ability of the aorta to accommodate pulsatile stretch. The associated histopathology demonstrates destruction of elastin and collagen, decreased vascular smooth muscle, in-growth of new blood vessels, and inflammation. Factors associated with degenerative aortic aneurysms include aging, cigarette smoking, hypercholesterolemia, hypertension, and male sex.
|
DISEASES OF THE AORTA: ETIOLOGY AND ASSOCIATED FACTORS |
The most common pathologic condition associated with degenerative aortic aneurysms is atherosclerosis. Many patients with aortic aneurysms have coexisting risk factors for atherosclerosis (Chap. 291e), as well as atherosclerosis in other blood vessels.
Medial degeneration, previously designated cystic medial necrosis, is the histopathologic term used to describe the degeneration of collagen and elastic fibers in the tunica media of the aorta as well as the loss of medial cells that are replaced by multiple clefts of mucoid material, such as proteoglycans. Medial degeneration characteristically affects the proximal aorta, results in circumferential weakness and dilation, and leads to the development of fusiform aneurysms involving the ascending aorta and the sinuses of Valsalva. This condition is particularly prevalent in patients with Marfan’s syndrome, Loeys-Dietz syndrome, Ehlers-Danlos syndrome type IV (Chap. 427), hypertension, congenital bicuspid aortic valves, and familial thoracic aortic aneurysm syndromes; sometimes it appears as an isolated condition in patients without any other apparent disease.
Familial clusterings of aortic aneurysms occur in 20% of patients, suggesting a hereditary basis for the disease. Mutations of the gene that encodes fibrillin-1 are present in patients with Marfan’s syndrome. Fibrillin-1 is an important component of extracellular microfibrils, which support the architecture of elastic fibers and other connective tissue. Deficiency of fibrillin-1 in the extracellular matrix leads to excessive signaling by transforming growth factor β (TGF-β). Loeys-Dietz syndrome is caused by mutations in the genes that encode TGF-β receptors 1 (TGFBR1) and 2 (TGFBR2). Increased signaling by TGF-β and mutations of TGFBR1 and TGFBR2 may cause thoracic aortic aneurysms. Mutations of type III procollagen have been implicated in Ehlers-Danlos type IV syndrome. Mutations of SMAD3, which encodes a downstream signaling protein involved with TGF binding to its receptors, have been described in a syndrome of thoracic aortic aneurysm; craniofacial, skeletal, and cutaneous anomalies; and osteoarthritis. Mutations of the genes encoding the smooth muscle–specific alpha-actin (ACTA2), smooth muscle cell–specific myosin heavy chain 11 (MHC11), and myosin light chain kinase (MYLK) and mutations of TGFBR2 and SMAD3 have been reported in some patients with nonsyndromic familial thoracic aortic aneurysms.
The infectious causes of aortic aneurysms include syphilis, tuberculosis, and other bacterial infections. Syphilis (Chap. 206) is a relatively uncommon cause of aortic aneurysm. Syphilitic periaortitis and mesoaortitis damage elastic fibers, resulting in thickening and weakening of the aortic wall. Approximately 90% of syphilitic aneurysms are located in the ascending aorta or aortic arch. Tuberculous aneurysms (Chap. 202) typically affect the thoracic aorta and result from direct extension of infection from hilar lymph nodes or contiguous abscesses as well as from bacterial seeding. Loss of aortic wall elasticity results from granulomatous destruction of the medial layer. A mycotic aneurysm is a rare condition that develops as a result of staphylococcal, streptococcal, Salmonella, or other bacterial or fungal infections of the aorta, usually at an atherosclerotic plaque. These aneurysms are usually saccular. Blood cultures are often positive and reveal the nature of the infective agent.
Vasculitides associated with aortic aneurysm include Takayasu’s arteritis and giant cell arteritis, which may cause aneurysms of the aortic arch and descending thoracic aorta. Spondyloarthropathies such as ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, relapsing polychondritis, and reactive arthritis (formerly known as Reiter’s syndrome) are associated with dilation of the ascending aorta. Aortic aneurysms occur in patients with Behçet’s syndrome (Chap. 387), Cogan’s syndrome, and IgG4-related systemic disease. Aortic aneurysms also result from idiopathic aortitis. Traumatic aneurysms may occur after penetrating or nonpenetrating chest trauma and most commonly affect the descending thoracic aorta just beyond the site of insertion of the ligamentum arteriosum. Chronic aortic dissections are associated with weakening of the aortic wall that may lead to the development of aneurysmal dilatation.
THORACIC AORTIC ANEURYSMS
The clinical manifestations and natural history of thoracic aortic aneurysms depend on their location. Medial degeneration is the most common pathology associated with ascending aortic aneurysms, whereas atherosclerosis is the condition most frequently associated with aneurysms of the descending thoracic aorta. The average growth rate of thoracic aneurysms is 0.1–0.2 cm per year. Thoracic aortic aneurysms associated with Marfan’s syndrome or aortic dissection may expand at a greater rate. The risk of rupture is related to the size of the aneurysm and the presence of symptoms, ranging approximately from 2–3% per year for thoracic aortic aneurysms <4.0 cm in diameter to 7% per year for those >6 cm in diameter. Most thoracic aortic aneurysms are asymptomatic; however, compression or erosion of adjacent tissue by aneurysms may cause symptoms such as chest pain, shortness of breath, cough, hoarseness, and dysphagia. Aneurysmal dilation of the ascending aorta may cause congestive heart failure as a consequence of aortic regurgitation, and compression of the superior vena cava may produce congestion of the head, neck, and upper extremities.
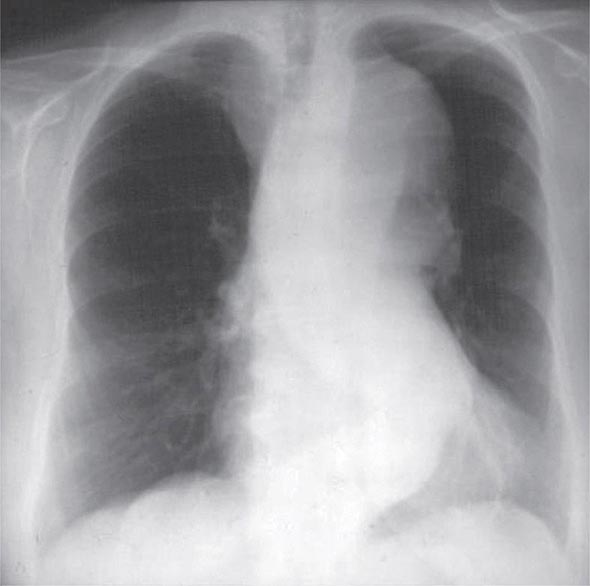
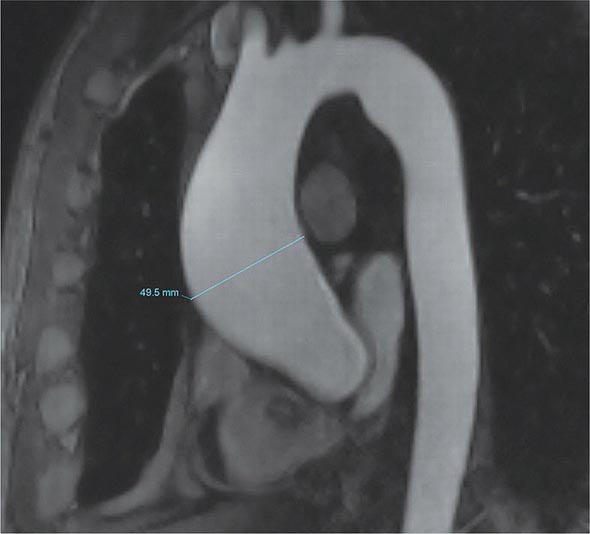
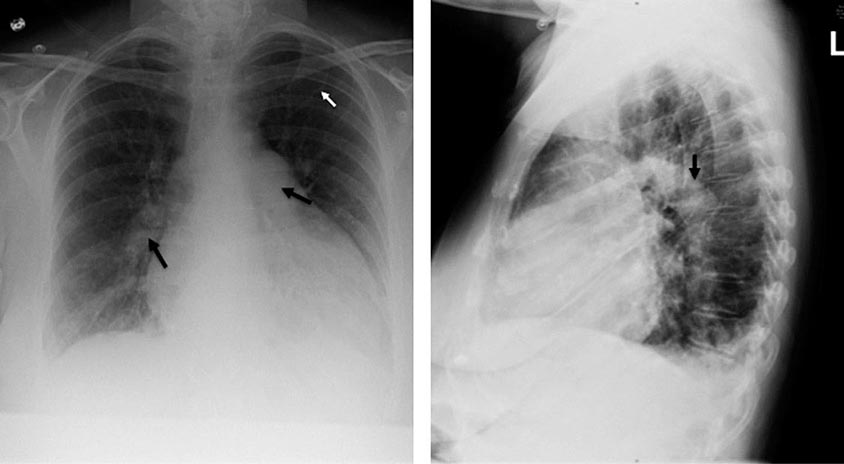
A chest x-ray may be the first test that suggests the diagnosis of a thoracic aortic aneurysm (Fig. 301-1). Findings include widening of the mediastinal shadow and displacement or compression of the trachea or left main stem bronchus. Echocardiography, particularly transesophageal echocardiography, can be used to assess the proximal ascending aorta and descending thoracic aorta. Contrast-enhanced CT, magnetic resonance imaging (MRI), and conventional invasive aortography are sensitive and specific tests for assessment of aneurysms of the thoracic aorta and involvement of branch vessels (Fig. 301-2). In asymptomatic patients whose aneurysms are too small to justify surgery, noninvasive testing with either contrast-enhanced CT or MRI should be performed at least every 6–12 months to monitor expansion.
FIGURE 301-1 A chest x-ray of a patient with a thoracic aortic aneurysm.
FIGURE 301-2 A magnetic resonance angiogram demonstrating a fusiform aneurysm of the ascending thoracic aorta. (Courtesy of Dr. Michael Steigner, Brigham and Women’s Hospital, Boston, MA, with permission.)
TREATMENT THORACIC AORTIC ANEURYSMS
β-Adrenergic blockers currently are recommended for patients with thoracic aortic aneurysms, particularly those with Marfan’s syndrome, who have evidence of aortic root dilatation to reduce the rate of further expansion. Additional medical therapy should be given as necessary to control hypertension. Recent studies indicate that angiotensin receptor antagonists and angiotensin-converting enzyme inhibitors reduce the rate of aortic dilation in patients with Marfan’s syndrome by blocking TGF-β signaling; clinical outcome trials of this treatment approach are in progress. Operative repair with placement of a prosthetic graft is indicated in patients with symptomatic ascending thoracic aortic aneurysms and for most asymptomatic aneurysms when the ascending aortic diameter is >5.5 cm. In patients with Marfan’s syndrome or bicuspid aortic valve, ascending thoracic aortic aneurysms of 4–5 cm should be considered for surgery. Operative repair is indicated for patients with descending thoracic aortic aneurysms when the diameter is >6 cm, and endovascular repair should be considered if feasible when the diameter is >5.5 cm. Repair is also recommended when the diameter of an aneurysm has increased >1 cm per year.
ABDOMINAL AORTIC ANEURYSMS
Abdominal aortic aneurysms occur more frequently in males than in females, and the incidence increases with age. Abdominal aortic aneurysms ≥4.0 cm may affect 1–2% of men older than 50 years. At least 90% of all abdominal aortic aneurysms >4.0 cm are related to atherosclerotic disease, and most of these aneurysms are below the level of the renal arteries. Prognosis is related to both the size of the aneurysm and the severity of coexisting coronary artery and cerebrovascular disease. The risk of rupture increases with the size of the aneurysm: the 5-year risk for aneurysms <5 cm is 1–2%, whereas it is 20–40% for aneurysms >5 cm in diameter. The formation of mural thrombi within aneurysms may predispose to peripheral embolization.
An abdominal aortic aneurysm commonly produces no symptoms. It usually is detected on routine examination as a palpable, pulsatile, expansile, and nontender mass, or it is an incidental finding observed on an abdominal imaging study performed for other reasons. As abdominal aortic aneurysms expand, however, they may become painful. Some patients complain of strong pulsations in the abdomen; others experience pain in the chest, lower back, or scrotum. Aneurysmal pain is usually a harbinger of rupture and represents a medical emergency. More often, acute rupture occurs without any prior warning, and this complication is always life-threatening. Rarely, there is leakage of the aneurysm with severe pain and tenderness. Acute pain and hypotension occur with rupture of the aneurysm, which requires an emergency operation.
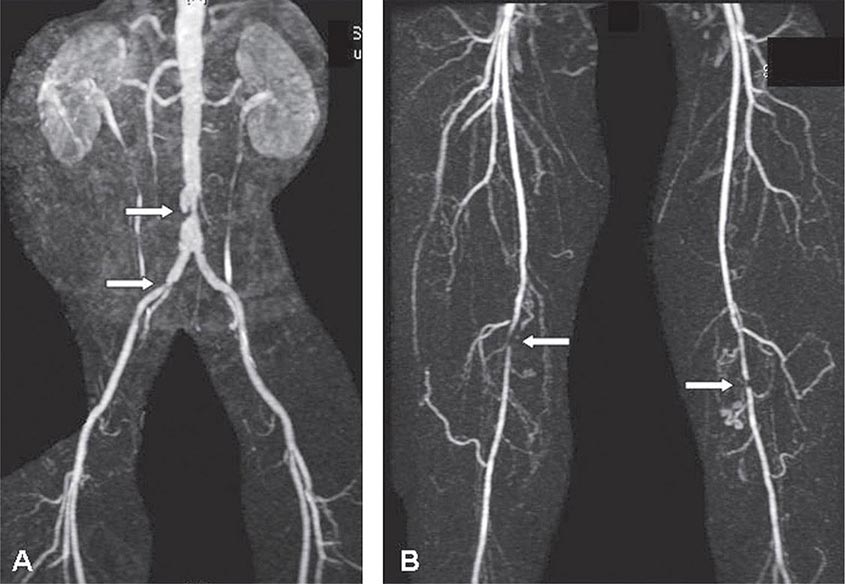
Abdominal radiography may demonstrate the calcified outline of the aneurysm; however, about 25% of aneurysms are not calcified and cannot be visualized by x-ray imaging. An abdominal ultrasound can delineate the transverse and longitudinal dimensions of an abdominal aortic aneurysm and may detect mural thrombus. Abdominal ultrasound is useful for serial documentation of aneurysm size and can be used to screen patients at risk for developing an aortic aneurysm. In one large study, ultrasound screening of men age 65–74 years was associated with a risk reduction in aneurysm-related death of 42%. For this reason, screening by ultrasonography is recommended for men age 65–75 years who have ever smoked. In addition, siblings or offspring of persons with abdominal aortic aneurysms, as well as individuals with thoracic aortic or peripheral arterial aneurysms, should be considered for screening for abdominal aortic aneurysms. CT with contrast and MRI are accurate noninvasive tests to determine the location and size of abdominal aortic aneurysms and to plan endovascular or open surgical repair (Fig. 301-3). Contrast aortography may be used for the evaluation of patients with aneurysms, but the procedure carries a small risk of complications such as bleeding, allergic reactions, and atheroembolism. Since the presence of mural thrombi may reduce the luminal size, aortography may underestimate the diameter of an aneurysm.
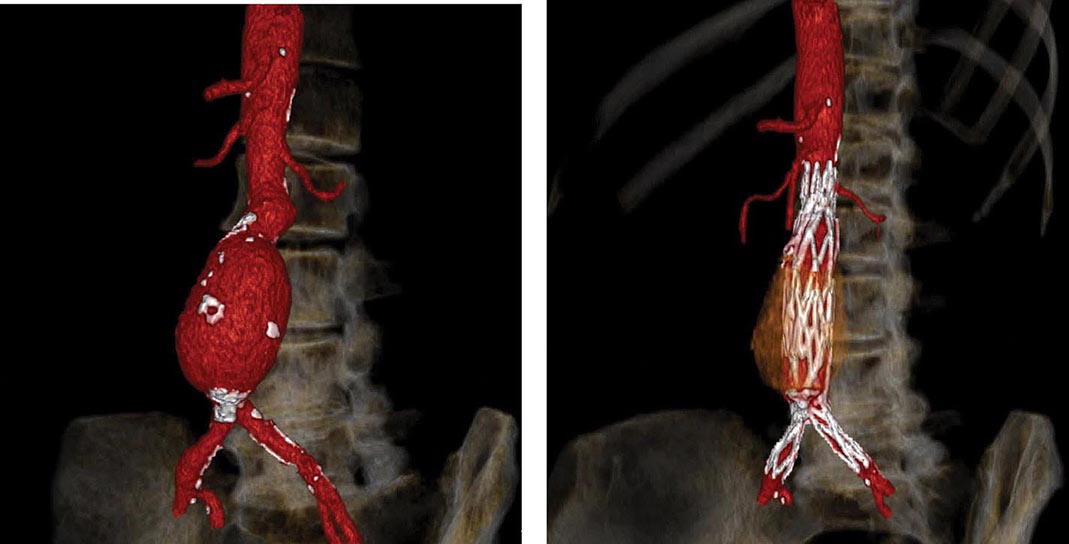
FIGURE 301-3 A computed tomographic angiogram depicting a fusiform abdominal aortic aneurysm before (left) and after (right) treatment with a bifurcated stent graft. (Courtesy of Drs. Elizabeth George and Frank Rybicki, Brigham and Women’s Hospital, Boston, MA, with permission.)
TREATMENT ABDOMINAL AORTIC ANEURYSMS
Operative repair of the aneurysm with insertion of a prosthetic graft or endovascular placement of an aortic stent graft (Fig. 301-3) is indicated for abdominal aortic aneurysms of any size that are expanding rapidly or are associated with symptoms. For asymptomatic aneurysms, abdominal aortic aneurysm repair is indicated if the diameter is >5.5 cm. In randomized trials of patients with abdominal aortic aneurysms <5.5 cm, there was no difference in the long-term (5- to 8-year) mortality rate between those followed with ultrasound surveillance and those undergoing elective surgical repair. Thus, serial noninvasive follow-up of smaller aneurysms (<5 cm) is an alternative to immediate repair. The decision to perform an open surgical operation or endovascular repair is based in part on the vascular anatomy and comorbid conditions. Endovascular repair of abdominal aortic aneurysms has a lower short-term morbidity rate but a comparable long-term mortality rate with open surgical reconstruction. Long-term surveillance with CT or MR aortography is indicated after endovascular repair to detect leaks and possible aneurysm expansion.
In surgical candidates, careful preoperative cardiac and general medical evaluations (followed by appropriate therapy for complicating conditions) are essential. Preexisting coronary artery disease, congestive heart failure, pulmonary disease, diabetes mellitus, and advanced age add to the risk of surgery. β-Adrenergic blockers decrease perioperative cardiovascular morbidity and mortality. With careful preoperative cardiac evaluation and postoperative care, the operative mortality rate approximates 1–2%. After acute rupture, the mortality rate of emergent operation is 45–50%. Endovascular repair with stent placement is an alternative approach to treat ruptured aneurysms and may be associated with a lower mortality rate.
ACUTE AORTIC SYNDROMES
The four major acute aortic syndromes are aortic rupture (discussed earlier), aortic dissection, intramural hematoma, and penetrating atherosclerotic ulcer. Aortic dissection is caused by a circumferential or, less frequently, transverse tear of the intima. It often occurs along the right lateral wall of the ascending aorta where the hydraulic shear stress is high. Another common site is the descending thoracic aorta just below the ligamentum arteriosum. The initiating event is either a primary intimal tear with secondary dissection into the media or a medial hemorrhage that dissects into and disrupts the intima. The pulsatile aortic flow then dissects along the elastic lamellar plates of the aorta and creates a false lumen. The dissection usually propagates distally down the descending aorta and into its major branches, but it may propagate proximally. Distal propagation may be limited by atherosclerotic plaque. In some cases, a secondary distal intimal disruption occurs, resulting in the reentry of blood from the false to the true lumen.
There are at least two important pathologic and radiologic variants of aortic dissection: intramural hematoma without an intimal flap and penetrating atherosclerotic ulcer. Acute intramural hematoma is thought to result from rupture of the vasa vasorum with hemorrhage into the wall of the aorta. Most of these hematomas occur in the descending thoracic aorta. Acute intramural hematomas may progress to dissection and rupture. Penetrating atherosclerotic ulcers are caused by erosion of a plaque into the aortic media, are usually localized, and are not associated with extensive propagation. They are found primarily in the middle and distal portions of the descending thoracic aorta and are associated with extensive atherosclerotic disease. The ulcer can erode beyond the internal elastic lamina, leading to medial hematoma, and may progress to false aneurysm formation or rupture.
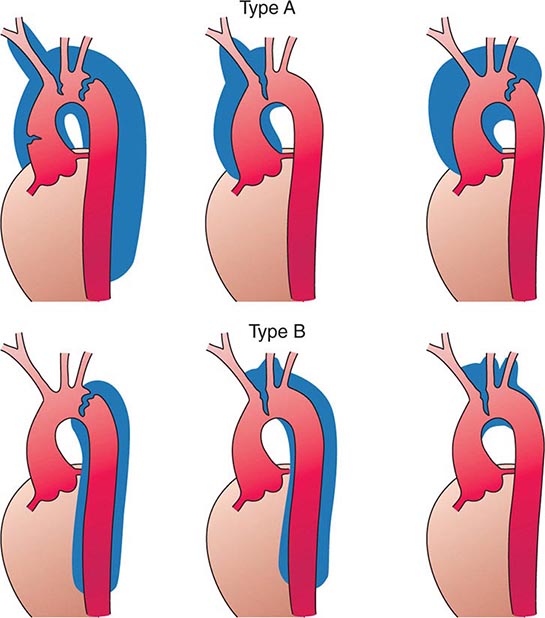
Several classification schemes have been developed for thoracic aortic dissections. DeBakey and colleagues initially classified aortic dissections as type I, in which an intimal tear occurs in the ascending aorta but involves the descending aorta as well; type II, in which the dissection is limited to the ascending aorta; and type III, in which the intimal tear is located in the descending aorta with distal propagation of the dissection (Fig. 301-4). Another classification (Stanford) is that of type A, in which the dissection involves the ascending aorta (proximal dissection), and type B, in which it is limited to the arch and/or descending aorta (distal dissection). From a management standpoint, classification of aortic dissections and intramural hematomas into type A or B is more practical and useful, since DeBakey types I and II are managed in a similar manner.
FIGURE 301-4 Classification of aortic dissections. Stanford classification: Type A dissections (top) involve the ascending aorta independent of site of tear and distal extension; type B dissections (bottom) involve transverse and/or descending aorta without involvement of the ascending aorta. DeBakey classification: Type I dissection involves ascending to descending aorta (top left); type II dissection is limited to ascending or transverse aorta, without descending aorta (top center + top right); type III dissection involves descending aorta only (bottom left). (From DC Miller, in RM Doroghazi, EE Slater [eds]: Aortic Dissection. New York, McGraw-Hill, 1983, with permission.)
The factors that predispose to aortic dissection include those associated with medial degeneration and others that increase aortic wall stress (Table 301-1). Systemic hypertension is a coexisting condition in 70% of patients. Aortic dissection is the major cause of morbidity and mortality in patients with Marfan’s syndrome (Chap. 427) or Loeys-Dietz syndrome, and similarly may affect patients with Ehlers-Danlos syndrome. The incidence also is increased in patients with inflammatory aortitis (i.e., Takayasu’s arteritis, giant cell arteritis), congenital aortic valve anomalies (e.g., bicuspid valve), coarctation of the aorta, and a history of aortic trauma. In addition, the risk of dissection is increased in otherwise normal women during the third trimester of pregnancy. Aortic dissection also may occur as a consequence of weight lifting, cocaine use, or deceleration injury.
CLINICAL MANIFESTATIONS
The peak incidence of aortic dissection is in the sixth and seventh decades. Men are more affected than women by a ratio of 2:1. The presentations of aortic dissection and its variants are the consequences of intimal tear, dissecting hematoma, occlusion of involved arteries, and compression of adjacent tissues. Acute aortic dissection presents with the sudden onset of pain (Chap. 19), which often is described as very severe and tearing and is associated with diaphoresis. The pain may be localized to the front or back of the chest, often the interscapular region, and typically migrates with propagation of the dissection. Other symptoms include syncope, dyspnea, and weakness. Physical findings may include hypertension or hypotension, loss of pulses, aortic regurgitation, pulmonary edema, and neurologic findings due to carotid artery obstruction (hemiplegia, hemianesthesia) or spinal cord ischemia (paraplegia). Bowel ischemia, hematuria, and myocardial ischemia have all been observed. These clinical manifestations reflect complications resulting from the dissection occluding the major arteries. Furthermore, clinical manifestations may result from the compression of adjacent structures (e.g., superior cervical ganglia, superior vena cava, bronchus, esophagus) by the expanding dissection, causing aneurysmal dilation, and include Horner’s syndrome, superior vena cava syndrome, hoarseness, dysphagia, and airway compromise. Hemopericardium and cardiac tamponade may complicate a type A lesion with retrograde dissection. Acute aortic regurgitation is an important and common (>50%) complication of proximal dissection. It is the outcome of either a circumferential tear that widens the aortic root or a disruption of the annulus by a dissecting hematoma that tears a leaflet(s) or displaces it, inferior to the line of closure. Signs of aortic regurgitation include bounding pulses, a wide pulse pressure, a diastolic murmur often radiating along the right sternal border, and evidence of congestive heart failure. The clinical manifestations depend on the severity of the regurgitation.
In dissections involving the ascending aorta, the chest x-ray often reveals a widened superior mediastinum. A pleural effusion (usually left-sided) also may be present. This effusion is typically serosanguineous and not indicative of rupture unless accompanied by hypotension and falling hematocrit. In dissections of the descending thoracic aorta, a widened mediastinum may be observed on chest x-ray. In addition, the descending aorta may appear to be wider than the ascending portion. An electrocardiogram that shows no evidence of myocardial ischemia is helpful in distinguishing aortic dissection from myocardial infarction. Rarely, the dissection involves the right or, less commonly, left coronary ostium and causes acute myocardial infarction.
The diagnosis of aortic dissection can be established by noninvasive techniques such as echocardiography, CT, and MRI. Aortography is used less commonly because of the accuracy of these noninvasive techniques. Transthoracic echocardiography can be performed simply and rapidly and has an overall sensitivity of 60–85% for aortic dissection. For diagnosing proximal ascending aortic dissections, its sensitivity exceeds 80%; it is less useful for detecting dissection of the arch and descending thoracic aorta. Transesophageal echocardiography requires greater skill and patient cooperation but is very accurate in identifying dissections of the ascending and descending thoracic aorta but not the arch, achieving 98% sensitivity and approximately 90% specificity. Echocardiography also provides important information regarding the presence and severity of aortic regurgitation and pericardial effusion. CT and MRI are both highly accurate in identifying the intimal flap and the extent of the dissection and involvement of major arteries; each has a sensitivity and specificity >90%. They are useful in recognizing intramural hemorrhage and penetrating ulcers. The relative utility of transesophageal echocardiography, CT, and MRI depends on the availability and expertise in individual institutions as well as on the hemodynamic stability of the patient, with CT and MRI obviously less suitable for unstable patients.
TREATMENT AORTIC DISSECTION
Medical therapy should be initiated as soon as the diagnosis is considered. The patient should be admitted to an intensive care unit for hemodynamic monitoring. Unless hypotension is present, therapy should be aimed at reducing cardiac contractility and systemic arterial pressure, and thus shear stress. For acute dissection, unless contraindicated, β-adrenergic blockers should be administered parenterally, using intravenous propranolol, metoprolol, or the short-acting esmolol to achieve a heart rate of approximately 60 beats/min. This should be accompanied by sodium nitroprusside infusion to lower systolic blood pressure to ≤120 mmHg. Labetalol (Chap. 298), a drug with both β- and α-adrenergic blocking properties, also may be used as a parenteral agent in acute therapy for dissection.
The calcium channel antagonists verapamil and diltiazem may be used intravenously if nitroprusside or β-adrenergic blockers cannot be employed. The addition of a parenteral angiotensin-converting enzyme (ACE) inhibitor such as enalaprilat to a β-adrenergic blocker also may be considered. Isolated use of a direct vasodilator such as hydralazine is contraindicated because these agents can increase hydraulic shear and may propagate the dissection.
Emergent or urgent surgical correction is the preferred treatment for acute ascending aortic dissections and intramural hematomas (type A) and for complicated type B dissections, including those characterized by propagation, compromise of major aortic branches, impending rupture, or continued pain. Surgery involves excision of the intimal flap, obliteration of the false lumen, and placement of an interposition graft. A composite valve-graft conduit is used if the aortic valve is disrupted. The overall in-hospital mortality rate after surgical treatment of patients with aortic dissection is reported to be 15–25%. The major causes of perioperative mortality and morbidity include myocardial infarction, paraplegia, renal failure, tamponade, hemorrhage, and sepsis. Endoluminal stent grafts may be considered in selected patients. Other transcatheter techniques, such as fenestration of the intimal flaps and stenting of narrowed branch vessels to increase flow to compromised organs, are used in selected patients. For uncomplicated and stable distal dissections and intramural hematomas (type B), medical therapy is the preferred treatment. The in-hospital mortality rate of medically treated patients with type B dissection is 10–20%. Long-term therapy for patients with aortic dissection and intramural hematomas (with or without surgery) consists of control of hypertension and reduction of cardiac contractility with the use of beta blockers plus other antihypertensive agents, such as ACE inhibitors or calcium antagonists. Patients with chronic type B dissection and intramural hematomas should be followed on an outpatient basis every 6–12 months with contrast-enhanced CT or MRI to detect propagation or expansion. Patients with Marfan’s syndrome are at high risk for postdissection complications. The long-term prognosis for patients with treated dissections is generally good with careful follow-up; the 10-year survival rate is approximately 60%.
CHRONIC ATHEROSCLEROTIC OCCLUSIVE DISEASE
Atherosclerosis may affect the thoracic and abdominal aorta. Occlusive aortic disease caused by atherosclerosis usually is confined to the distal abdominal aorta below the renal arteries. Frequently the disease extends to the iliac arteries (Chap. 302). Claudication characteristically involves the buttocks, thighs, and calves and may be associated with impotence in males (Leriche’s syndrome). The severity of the symptoms depends on the adequacy of collaterals. With sufficient collateral blood flow, a complete occlusion of the abdominal aorta may occur without the development of ischemic symptoms. The physical findings include the absence of femoral and other distal pulses bilaterally and the detection of an audible bruit over the abdomen (usually at or below the umbilicus) and the common femoral arteries. Atrophic skin, loss of hair, and coolness of the lower extremities usually are observed. In advanced ischemia, rubor on dependency and pallor on elevation can be seen.
The diagnosis usually is established by physical examination and noninvasive testing, including leg pressure measurements, Doppler velocity analysis, pulse volume recordings, and duplex ultrasonography. The anatomy may be defined by MRI, CT, or conventional aortography, typically performed when one is considering revascularization. Catheter-based endovascular or operative treatment is indicated in patients with lifestyle-limiting or debilitating symptoms of claudication and patients with critical limb ischemia.
ACUTE AORTIC OCCLUSION
Acute occlusion in the distal abdominal aorta constitutes a medical emergency because it threatens the viability of the lower extremities; it usually results from an occlusive (saddle) embolus that almost always originates from the heart. Rarely, acute occlusion may occur as the result of in situ thrombosis in a preexisting severely narrowed segment of the aorta.
The clinical picture is one of acute ischemia of the lower extremities. Severe rest pain, coolness, and pallor of the lower extremities and the absence of distal pulses bilaterally are the usual manifestations. Diagnosis should be established rapidly by MRI, CT, or aortography. Emergency thrombectomy or revascularization is indicated.
AORTITIS
Aortitis, a term referring to inflammatory disease of the aorta, may be caused by large vessel vasculitides such as Takayasu’s arteritis and giant cell arteritis, rheumatic and HLA-B27–associated spondyloarthropathies, Behçet’s syndrome, antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides, Cogan’s syndrome, IgG4-related systemic disease, and infections such as syphilis, tuberculosis, and Salmonella, or may be associated with retroperitoneal fibrosis. Aortitis may result in aneurysmal dilation and aortic regurgitation, occlusion of the aorta and its branch vessels, or acute aortic syndromes.
TAKAYASU’S ARTERITIS
This inflammatory disease often affects the ascending aorta and aortic arch, causing obstruction of the aorta and its major arteries. Takayasu’s arteritis is also termed pulseless disease because of the frequent occlusion of the large arteries originating from the aorta. It also may involve the descending thoracic and abdominal aorta and occlude large branches such as the renal arteries. Aortic aneurysms also may occur. The pathology is a panarteritis characterized by mononuclear cells and occasionally giant cells, with marked intimal hyperplasia, medial and adventitial thickening, and, in the chronic form, fibrotic occlusion. The disease is most prevalent in young females of Asian descent but does occur in women of other geographic and ethnic origins and also in young men. During the acute stage, fever, malaise, weight loss, and other systemic symptoms may be evident. Elevations of the erythrocyte sedimentation rate and C-reactive protein are common. The chronic stages of the disease, which is intermittently active, present with symptoms related to large artery occlusion, such as upper extremity claudication, cerebral ischemia, and syncope. The process is progressive, and there is no definitive therapy. Glucocorticoids and immunosuppressive agents are effective in some patients during the acute phase. Surgical bypass or endovascular intervention of a critically stenotic artery may be necessary.
GIANT CELL ARTERITIS
(See also Chap. 385) This vasculitis occurs in older individuals and affects women more often than men. Primarily large and medium-size arteries are affected. The pathology is that of focal granulomatous lesions involving the entire arterial wall; it may be associated with polymyalgia rheumatica. Obstruction of medium-size arteries (e.g., temporal and ophthalmic arteries) and major branches of the aorta and the development of aortitis and aortic regurgitation are important complications of the disease. High-dose glucocorticoid therapy may be effective when given early.
RHEUMATIC AORTITIS
Rheumatoid arthritis (Chap. 380), ankylosing spondylitis (Chap. 384), psoriatic arthritis (Chap. 384), reactive arthritis (formerly known as Reiter’s syndrome) (Chap. 384), relapsing polychondritis, and inflammatory bowel disorders may all be associated with aortitis involving the ascending aorta. The inflammatory lesions usually involve the ascending aorta and may extend to the sinuses of Valsalva, the mitral valve leaflets, and adjacent myocardium. The clinical manifestations are aneurysm, aortic regurgitation, and involvement of the cardiac conduction system.
IDIOPATHIC AORTITIS
Idiopathic abdominal aortitis is characterized by adventitial and periaortic inflammation with thickening of the aortic wall. It is associated with abdominal aortic aneurysms and idiopathic retroperitoneal fibrosis. Affected individuals may present with vague constitutional symptoms, fever, and abdominal pain. Retroperitoneal fibrosis can cause ureteral obstruction and hydronephrosis. Glucocorticoids and immunosuppressive agents may reduce the inflammation.
INFECTIVE AORTITIS
Infective aortitis may result from direct invasion of the aortic wall by bacterial pathogens such as Staphylococcus, Streptococcus, and Salmonella or by fungi. These bacteria cause aortitis by infecting the aorta at sites of atherosclerotic plaque. Bacterial proteases lead to degradation of collagen, and the ensuing destruction of the aortic wall leads to the formation of a saccular aneurysm referred to as a mycotic aneurysm. Mycotic aneurysms have a predilection for the suprarenal abdominal aorta. The pathologic characteristics of the aortic wall include acute and chronic inflammation, abscesses, hemorrhage, and necrosis. Mycotic aneurysms typically affect the elderly and occur in men three times more frequently than in women. Patients may present with fever, sepsis, and chest, back, or abdominal pain; there may have been a preceding diarrheal illness. Blood cultures are positive in the majority of patients. Both CT and MRI are useful to diagnose mycotic aneurysms. Treatment includes antibiotic therapy and surgical removal of the affected part of the aorta and revascularization of the lower extremities with grafts placed in uninfected tissue.
Syphilitic aortitis is a late manifestation of luetic infection (Chap. 206) that usually affects the proximal ascending aorta, particularly the aortic root, resulting in aortic dilation and aneurysm formation. Syphilitic aortitis occasionally may involve the aortic arch or the descending aorta. The aneurysms may be saccular or fusiform and are usually asymptomatic, but compression of and erosion into adjacent structures may result in symptoms; rupture also may occur.
The initial lesion is an obliterative endarteritis of the vasa vasorum, especially in the adventitia. This is an inflammatory response to the invasion of the adventitia by the spirochetes. Destruction of the aortic media occurs as the spirochetes spread into this layer, usually via the lymphatics accompanying the vasa vasorum. Destruction of collagen and elastic tissues leads to dilation of the aorta, scar formation, and calcification. These changes account for the characteristic radiographic appearance of linear calcification of the ascending aorta.
The disease typically presents as an incidental chest radiographic finding 15–30 years after initial infection. Symptoms may result from aortic regurgitation, narrowing of coronary ostia due to syphilitic aortitis, compression of adjacent structures (e.g., esophagus), or rupture. Diagnosis is established by a positive serologic test, i.e., rapid plasmin reagin (RPR) or fluorescent treponemal antibody. Treatment includes penicillin and surgical excision and repair.
302 |
Arterial Diseases of the Extremities |
PERIPHERAL ARTERY DISEASE
Peripheral artery disease (PAD) is defined as a clinical disorder in which there is a stenosis or occlusion in the aorta or the arteries of the limbs. Atherosclerosis is the leading cause of PAD in patients >40 years old. Other causes include thrombosis, embolism, vasculitis, fibromuscular dysplasia, entrapment, cystic adventitial disease, and trauma. The highest prevalence of atherosclerotic PAD occurs in the sixth and seventh decades of life. As in patients with atherosclerosis of the coronary and cerebral vasculature, there is an increased risk of developing PAD in cigarette smokers and in persons with diabetes mellitus, hypercholesterolemia, hypertension, or renal insufficiency.
Pathology (See also Chap. 291e) Segmental lesions that cause stenosis or occlusion are usually localized to large and medium-size vessels. The pathology of the lesions includes atherosclerotic plaques with calcium deposition, thinning of the media, patchy destruction of muscle and elastic fibers, fragmentation of the internal elastic lamina, and thrombi composed of platelets and fibrin. The primary sites of involvement are the abdominal aorta and iliac arteries (30% of symptomatic patients), the femoral and popliteal arteries (80–90% of patients), and the more distal vessels, including the tibial and peroneal arteries (40–50% of patients). Atherosclerotic lesions occur preferentially at arterial branch points, which are sites of increased turbulence, altered shear stress, and intimal injury. Involvement of the distal vasculature is most common in elderly individuals and patients with diabetes mellitus.
Clinical Evaluation Fewer than 50% of patients with PAD are symptomatic, although many have a slow or impaired gait. The most common symptom is intermittent claudication, which is defined as a pain, ache, cramp, numbness, or a sense of fatigue in the muscles; it occurs during exercise and is relieved by rest. The site of claudication is distal to the location of the occlusive lesion. For example, buttock, hip, thigh, and calf discomfort occurs in patients with aortoiliac disease, whereas calf claudication develops in patients with femoral-popliteal disease. Symptoms are far more common in the lower than in the upper extremities because of the higher incidence of obstructive lesions in the former region. In patients with severe arterial occlusive disease in whom resting blood flow cannot accommodate basal nutritional needs of the tissues, critical limb ischemia may develop. Patients complain of rest pain or a feeling of cold or numbness in the foot and toes. Frequently, these symptoms occur at night when the legs are horizontal and improve when the legs are in a dependent position. With severe ischemia, rest pain may be persistent.
Important physical findings of PAD include decreased or absent pulses distal to the obstruction, the presence of bruits over the narrowed artery, and muscle atrophy. With more severe disease, hair loss, thickened nails, smooth and shiny skin, reduced skin temperature, and pallor or cyanosis are common physical signs. In patients with critical limb ischemia, ulcers or gangrene may occur. Elevation of the legs and repeated flexing of the calf muscles produce pallor of the soles of the feet, whereas rubor, secondary to reactive hyperemia, may develop when the legs are dependent. The time required for rubor to develop or for the veins in the foot to fill when the patient’s legs are transferred from an elevated to a dependent position is related to the severity of the ischemia and the presence of collateral vessels. Patients with severe ischemia may develop peripheral edema because they keep their legs in a dependent position much of the time. Ischemic neuropathy can result in numbness and hyporeflexia.
Noninvasive Testing The history and physical examination are often sufficient to establish the diagnosis of PAD. An objective assessment of the presence and severity of disease is obtained by noninvasive techniques. Arterial pressure can be recorded noninvasively in the legs by placement of sphygmomanometric cuffs at the ankles and the use of a Doppler device to auscultate or record blood flow from the dorsalis pedis and posterior tibial arteries. Normally, systolic blood pressure in the legs and arms is similar. Indeed, ankle pressure may be slightly higher than arm pressure due to pulse-wave amplification. In the presence of hemodynamically significant stenoses, the systolic blood pressure in the leg is decreased. Thus, the ratio of the ankle and brachial artery pressures (termed the ankle:brachial index, or ABI) is 1.00–1.40 in normal individuals. ABI values of 0.91–0.99 are considered “borderline,” and those <0.90 are abnormal and diagnostic of PAD. ABIs >1.40 indicate noncompressible arteries secondary to vascular calcification.
Other noninvasive tests include segmental pressure measurements, segmental pulse volume recordings, duplex ultrasonography (which combines B-mode imaging and Doppler flow velocity waveform analysis examination), transcutaneous oximetry, and stress testing (usually using a treadmill). Placement of pneumatic cuffs enables assessment of systolic pressure along the legs. The presence of pressure gradients between sequential cuffs provides evidence of the presence and location of hemodynamically significant stenoses. In addition, the amplitude of the pulse volume contour becomes blunted in the presence of significant PAD. Duplex ultrasonography is used to image and detect stenotic lesions in native arteries and bypass grafts.
Treadmill testing allows the physician to assess functional limitations objectively. Decline of the ABI immediately after exercise provides further support for the diagnosis of PAD in patients with equivocal symptoms and findings on examination.
Magnetic resonance angiography (MRA), computed tomographic angiography (CTA), and conventional catheter-based angiography should not be used for routine diagnostic testing but are performed before potential revascularization (Fig. 302-1). Each test is useful in defining the anatomy to assist planning for endovascular and surgical revascularization procedures.
FIGURE 302-1 Magnetic resonance angiography of a patient with intermittent claudication, showing stenoses of the distal abdominal aorta and right iliac common iliac artery (A) and stenoses of the right and left superficial femoral arteries (B). (Courtesy of Dr. Edwin Gravereaux, with permission.)
Prognosis The natural history of patients with PAD is influenced primarily by the extent of coexisting coronary artery and cerebrovascular disease. Approximately one-third to one-half of patients with symptomatic PAD have evidence of coronary artery disease (CAD) based on clinical presentation and electrocardiogram, and over one-half have significant CAD by coronary angiography. Patients with PAD have a 15–30% 5-year mortality rate and a two- to sixfold increased risk of death from coronary heart disease. Mortality rates are highest in those with the most severe PAD. Measurement of ABI is useful for detecting PAD and identifying persons at risk for future atherothrombotic events. The likelihood of symptomatic progression of PAD is lower than the chance of succumbing to CAD. Approximately 75–80% of nondiabetic patients who present with mild to moderate claudication remain symptomatically stable. Deterioration is likely to occur in the remainder, with approximately 1–2% of the group ultimately developing critical limb ischemia each year. Approximately 25–30% of patients with critical limb ischemia undergo amputation within 1 year. The prognosis is worse in patients who continue to smoke cigarettes or have diabetes mellitus.
TREATMENT PERIPHERAL ARTERY DISEASE
Patients with PAD should receive therapies to reduce the risk of associated cardiovascular events, such as myocardial infarction and death, and to improve limb symptoms, prevent progression to critical limb ischemia, and preserve limb viability. Risk factor modification and antiplatelet therapy should be initiated to improve cardiovascular outcomes. The importance of discontinuing cigarette smoking cannot be overemphasized. The physician must assume a major role in this lifestyle modification. Counseling and adjunctive drug therapy with the nicotine patch, bupropion, or varenicline increase smoking cessation rates and reduce recidivism. It is important to control blood pressure in hypertensive patients. Angiotensin-converting enzyme inhibitors may reduce the risk of cardiovascular events in patients with symptomatic PAD. β-Adrenergic blockers do not worsen claudication and may be used to treat hypertension, especially in patients with coexistent CAD. Treatment of hypercholesterolemia with statins is advocated to reduce the risk of myocardial infarction, stroke, and death. The 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults recommends high intensity statin treatment in patients with atherosclerotic disorders, including peripheral artery disease. Platelet inhibitors, including aspirin and clopidogrel, reduce the risk of adverse cardiovascular events in patients with atherosclerosis and are recommended for patients with symptomatic PAD, including those with intermittent claudication or critical limb ischemia or prior lower extremity revascularization. Dual antiplatelet therapy with both aspirin and clopidogrel is not more effective than aspirin alone in reducing cardiovascular morbidity and mortality rates in patients with PAD. The anticoagulant warfarin is as effective as antiplatelet therapy in preventing adverse cardiovascular events but causes more major bleeding; therefore, it is not indicated to improve outcomes in patients with chronic PAD.
Therapies for intermittent claudication and critical limb ischemia include supportive measures, medications, nonoperative interventions, and surgery. Supportive measures include meticulous care of the feet, which should be kept clean and protected against excessive drying with moisturizing creams. Well-fitting and protective shoes are advised to reduce trauma. Elastic support hose should be avoided, as it reduces blood flow to the skin. In patients with critical limb ischemia, shock blocks under the head of the bed together with a canopy over the feet may improve perfusion pressure and ameliorate some of the rest pain.
Patients with claudication should be encouraged to exercise regularly and at progressively more strenuous levels. Supervised exercise training programs for 30- to 45-min sessions, three to five times per week for at least 12 weeks, prolong walking distance. Patients also should be advised to walk until nearly maximum claudication discomfort occurs and then rest until the symptoms resolve before resuming ambulation. The beneficial effect of supervised exercise training on walking performance in patients with claudication often is similar to or greater than that realized after a revascularization procedure. Pharmacologic treatment of PAD has not been as successful as the medical treatment of CAD (Chap. 293). In particular, vasodilators as a class have not proved to be beneficial. During exercise, peripheral vasodilation occurs distal to sites of significant arterial stenoses. As a result, perfusion pressure falls, often to levels lower than that generated in the interstitial tissue by the exercising muscle. Drugs such as α-adrenergic blocking agents, calcium channel antagonists, and other vasodilators have not been shown to be effective in patients with PAD.
Cilostazol, a phosphodiesterase inhibitor with vasodilator and antiplatelet properties, increases claudication distance by 40–60% and improves measures of quality of life. The mechanism of action accounting for its beneficial effects is not known. Pentoxifylline, a substituted xanthine derivative, increases blood flow to the microcirculation and enhances tissue oxygenation. Although several placebo-controlled studies have found that pentoxifylline increases the duration of exercise in patients with claudication, its efficacy has not been confirmed in all clinical trials. Statins and angiotensin-converting enzyme inhibitors appear promising for treatment of intermittent claudication in initial clinical trials, but more studies are needed to confirm the efficacy of each class of drugs. There is no definitive medical therapy for critical limb ischemia, although several studies have suggested that long-term parenteral administration of vasodilator prostaglandins decreases pain and facilitates healing of ulcers. Enthusiasm for therapy with angiogenic growth factors abated when clinical trials of intramuscular gene transfer of DNA encoding vascular endothelial growth factor, fibroblast growth factor, hepatocyte growth factor, or hypoxia-inducible factor 1α failed to demonstrate improvement in symptoms or outcomes in patients with intermittent claudication or critical limb ischemia. Clinical trials assessing the ability of bone marrow–derived vascular progenitor cells to promote angiogenesis and preserve limb viability in patients with critical limb ischemia are ongoing.
REVASCULARIZATION
Revascularization procedures, including catheter-based and surgical interventions, are usually indicated for patients with disabling, progressive, or severe symptoms of intermittent claudication despite medical therapy and for those with critical limb ischemia. MRA, CTA, or conventional angiography should be performed to assess vascular anatomy in patients who are being considered for revascularization. Nonoperative interventions include percutaneous transluminal angiography (PTA) and stent placement (Chap. 296e). PTA and stenting of the iliac artery are associated with higher success rates than are PTA and stenting of the femoral and popliteal arteries. Approximately 90–95% of iliac PTAs are initially successful, and the 3-year patency rate is >75%. Patency rates may be higher if a stent is placed in the iliac artery. The initial success rates for femoral-popliteal PTA and stenting are approximately 80%, with 60% 3-year patency rates. Patency rates are influenced by the severity of pretreatment stenoses; the prognosis of occlusive lesions is worse than that of nonocclusive stenotic lesions. The role of drug-eluting stents and drug-coated balloons in PAD is under investigation.
Several operative procedures are available for treating patients with aortoiliac and femoral-popliteal artery disease. The preferred operative procedure depends on the location and extent of the obstruction(s) and the general medical condition of the patient. Operative procedures for aortoiliac disease include aortobifemoral bypass, axillofemoral bypass, femoro-femoral bypass, and aortoiliac endarterectomy. The most frequently used procedure is the aortobifemoral bypass using knitted Dacron grafts. Immediate graft patency approaches 99%, and 5- and 10-year graft patency rates in survivors are >90% and 80%, respectively. Operative complications include myocardial infarction and stroke, infection of the graft, peripheral embolization, and sexual dysfunction from interruption of autonomic nerves in the pelvis. The operative mortality rate ranges from 1–3%, mostly due to ischemic heart disease.
Operative therapy for femoral-popliteal artery disease includes in situ and reverse autogenous saphenous vein bypass grafts, placement of polytetrafluoroethylene (PTFE) or other synthetic grafts, and thromboendarterectomy. The operative mortality rate ranges from 1–3%. The long-term patency rate depends on the type of graft used, the location of the distal anastomosis, and the patency of runoff vessels beyond the anastomosis. Patency rates of femoral-popliteal saphenous vein bypass grafts approach 90% at 1 year and 70–80% at 5 years. Five-year patency rates of infrapopliteal saphenous vein bypass grafts are 60–70%. In contrast, 5-year patency rates of infrapopliteal PTFE grafts are <30%.
Preoperative cardiac risk assessment may identify individuals who are especially likely to experience an adverse cardiac event during the perioperative period. Patients with angina, prior myocardial infarction, ventricular ectopy, heart failure, or diabetes are among those at increased risk. Stress testing with treadmill exercise (if feasible), radionuclide myocardial perfusion imaging, or echocardiography permits further stratification of risk in these patients (Chap. 296e). Patients with abnormal test results require close supervision and adjunctive management with anti-ischemic medications. β-Adrenergic blockers and statins reduce the risk of postoperative cardiovascular complications. Coronary angiography and coronary artery revascularization compared with optimal medical therapy do not improve outcomes in most patients undergoing peripheral vascular surgery, but cardiac catheterization should be considered in patients with unstable angina and angina refractory to medical therapy as well as those suspected of having left main or three-vessel CAD.
FIBROMUSCULAR DYSPLASIA
Fibromuscular dysplasia is a hyperplastic disorder that affects medium-size and small arteries. It occurs predominantly in females and usually involves the renal and carotid arteries but can affect extremity vessels such as the iliac and subclavian arteries. The histologic classification includes intimal fibroplasia (also classified as focal), medial dysplasia (multifocal), and adventitial hyperplasia. Medial dysplasia is subdivided into medial fibroplasia, perimedial fibroplasia, and medial hyperplasia. Medial fibroplasia is the most common type and is characterized by alternating areas of thinned media and fibromuscular ridges. The internal elastic lamina usually is preserved. The iliac arteries are the limb arteries most likely to be affected by fibromuscular dysplasia. It is identified angiographically by a “string of beads” appearance caused by thickened fibromuscular ridges contiguous with thin, less-involved portions of the arterial wall, which is typical of medial fibroplasia. When limb vessels are involved, clinical manifestations are similar to those for atherosclerosis, including claudication and rest pain. PTA and surgical reconstruction have been beneficial in patients with debilitating symptoms or threatened limbs.
THROMBOANGIITIS OBLITERANS
Thromboangiitis obliterans (Buerger’s disease) is an inflammatory occlusive vascular disorder involving small and medium-size arteries and veins in the distal upper and lower extremities. Cerebral, visceral, and coronary vessels may be affected rarely. This disorder develops most frequently in men <40 years of age. The prevalence is higher in Asians and individuals of Eastern European descent. Although the cause of thromboangiitis obliterans is not known, there is a definite relationship to cigarette smoking in patients with this disorder.
In the initial stages of thromboangiitis obliterans, polymorphonuclear leukocytes infiltrate the walls of the small and medium-size arteries and veins. The internal elastic lamina is preserved, and a cellular, inflammatory thrombus develops in the vascular lumen. As the disease progresses, mononuclear cells, fibroblasts, and giant cells replace the neutrophils. Later stages are characterized by perivascular fibrosis, organized thrombus, and recanalization.
The clinical features of thromboangiitis obliterans often include a triad of claudication of the affected extremity, Raynaud’s phenomenon, and migratory superficial vein thrombophlebitis. Claudication usually is confined to the calves and feet or the forearms and hands because this disorder primarily affects distal vessels. In the presence of severe digital ischemia, trophic nail changes, painful ulcerations, and gangrene may develop at the tips of the fingers or toes. The physical examination shows normal brachial and popliteal pulses but reduced or absent radial, ulnar, and/or tibial pulses. MRA, CTA, and conventional arteriography are helpful in making the diagnosis. Smooth, tapering segmental lesions in the distal vessels are characteristic, as are collateral vessels at sites of vascular occlusion. Proximal atherosclerotic disease is usually absent. The diagnosis can be confirmed by excisional biopsy and pathologic examination of an involved vessel.
There is no specific treatment except abstention from tobacco. The prognosis is worse in individuals who continue to smoke, but results are discouraging even in those who stop smoking. Arterial bypass of the larger vessels may be used in selected instances, as well as local debridement, depending on the symptoms and severity of ischemia. Antibiotics may be useful; anticoagulants and glucocorticoids are not helpful. If these measures fail, amputation may be required.
VASCULITIS
Other vasculitides may affect the arteries that supply the upper and lower extremities. Takayasu’s arteritis and giant cell (temporal) arteritis are discussed in Chap. 385.
ACUTE LIMB ISCHEMIA
Acute limb ischemia occurs when arterial occlusion results in the sudden cessation of blood flow to an extremity. The severity of ischemia and the viability of the extremity depend on the location and extent of the occlusion and the presence and subsequent development of collateral blood vessels. Principal causes of acute arterial occlusion include embolism, thrombus in situ, arterial dissection, and trauma.
The most common sources of arterial emboli are the heart, aorta, and large arteries. Cardiac disorders that cause thromboembolism include atrial fibrillation, both chronic and paroxysmal; acute myocardial infarction; ventricular aneurysm; cardiomyopathy; infectious and marantic endocarditis; thrombi associated with prosthetic heart valves; and atrial myxoma. Emboli to the distal vessels may also originate from proximal sites of atherosclerosis and aneurysms of the aorta and large vessels. Less frequently, an arterial occlusion results paradoxically from a venous thrombus that has entered the systemic circulation via a patent foramen ovale or another septal defect. Arterial emboli tend to lodge at vessel bifurcations because the vessel caliber decreases at those sites; in the lower extremities, emboli lodge most frequently in the femoral artery, followed by the iliac artery, aorta, and popliteal and tibioperoneal arteries.
Acute arterial thrombosis in situ occurs most frequently in atherosclerotic vessels at the site of an atherosclerotic plaque or aneurysm and in arterial bypass grafts. Trauma to an artery may disrupt continuity of blood flow and cause acute limb ischemia via formation of an acute arterial thrombus or by disruption of an artery’s integrity and extravasation of blood. Arterial occlusion may complicate arterial punctures and placement of catheters; it also may result from arterial dissection if the intimal flap obstructs the artery. Less common causes include thoracic outlet compression syndrome, which causes subclavian artery occlusion, and entrapment of the popliteal artery by abnormal placement of the medial head of the gastrocnemius muscle. Polycythemia and hypercoagulable disorders (Chaps. 131 and 141) are also associated with acute arterial thrombosis.
CLINICAL FEATURES
The symptoms of an acute arterial occlusion depend on the location, duration, and severity of the obstruction. Often, severe pain, paresthesia, numbness, and coldness develop in the involved extremity within 1 hour. Paralysis may occur with severe and persistent ischemia. Physical findings include loss of pulses distal to the occlusion, cyanosis or pallor, mottling, decreased skin temperature, muscle stiffening, loss of sensation, weakness, and/or absent deep tendon reflexes. If acute arterial occlusion occurs in the presence of an adequate collateral circulation, as is often the case in acute graft occlusion, the symptoms and findings may be less impressive. In this situation, the patient complains about an abrupt decrease in the distance walked before claudication occurs or of modest pain and paresthesia. Pallor and coolness are evident, but sensory and motor functions generally are preserved. The diagnosis of acute limb ischemia is usually apparent from the clinical presentation. In most circumstances, MRA, CTA, or catheter-based arteriography is used to confirm the diagnosis and demonstrate the location and extent of arterial occlusion.
TREATMENT ACUTE LIMB ISCHEMIA
Once the diagnosis is made, the patient should be anticoagulated with intravenous heparin to prevent propagation of the clot. In cases of severe ischemia of recent onset, particularly when limb viability is jeopardized, immediate intervention to ensure reperfusion is indicated. Catheter-directed thrombolysis/thrombectomy, surgical thromboembolectomy, and arterial bypass procedures are used to restore blood flow to the ischemic extremity promptly, particularly when a large proximal vessel is occluded.
Intraarterial thrombolytic therapy with recombinant tissue plasminogen activator, reteplase, or tenecteplase is most effective when acute arterial occlusion is recent (<2 weeks) and caused by a thrombus in an atherosclerotic vessel, arterial bypass graft, or occluded stent. Thrombolytic therapy is also indicated when the patient’s overall condition contraindicates surgical intervention or when smaller distal vessels are occluded, thus preventing surgical access. Meticulous observation for hemorrhagic complications is required during intraarterial thrombolytic therapy. Another endovascular approach to thrombus removal is percutaneous mechanical thrombectomy using devices that employ hydrodynamic forces or rotating baskets to fragment and remove the clot. These treatments may be used alone but usually are used in conjunction with pharmacologic thrombolysis. Surgical revascularization is preferred when restoration of blood flow must occur within 24 h to prevent limb loss or when symptoms of occlusion have been present for more than 2 weeks. Amputation is performed when the limb is not viable, as characterized by loss of sensation, paralysis, and the absence of Doppler-detected blood flow in both arteries and veins.
If the limb is not in jeopardy, a more conservative approach that includes observation and administration of anticoagulants may be taken. Anticoagulation prevents recurrent embolism and reduces the likelihood of thrombus propagation; it can be initiated with intravenous heparin and followed by oral warfarin. Recommended doses are the same as those used for deep vein thrombosis (Chap. 300). Emboli resulting from infective endocarditis, the presence of prosthetic heart valves, or atrial myxoma often require surgical intervention to remove the cause.
ATHEROEMBOLISM
Atheroembolism is another cause of limb ischemia. In this condition, multiple small deposits of fibrin, platelets, and cholesterol debris embolize from proximal atherosclerotic lesions or aneurysmal sites. Large protruding aortic atheromas are a source of emboli that may lead to limb ischemia, as well as stroke and renal insufficiency. Atheroembolism may occur after intraarterial procedures. Since atheroemboli to limbs tend to lodge in the small vessels of the muscle and skin and may not occlude the large vessels, distal pulses usually remain palpable. Patients complain of acute pain and tenderness at the site of embolization. Digital vascular occlusion may result in ischemia and the “blue toe” syndrome; digital necrosis and gangrene may develop (Fig. 302-2). Localized areas of tenderness, pallor, and livedo reticularis (see below) occur at sites of emboli. Skin or muscle biopsy may demonstrate cholesterol crystals.
FIGURE 302-2 Atheroembolism causing cyanotic discoloration and impending necrosis of the toes (“blue toe” syndrome).
Ischemia resulting from atheroemboli is notoriously difficult to treat. Usually neither surgical revascularization procedures nor thrombolytic therapy is helpful because of the multiplicity, composition, and distal location of the emboli. There is limited evidence that antithrombotic therapy with platelet inhibitors or anticoagulants prevents atheroembolism. Statins may stabilize plaque and potentially reduce the risk of atheroembolism. Surgical intervention to remove or bypass the atherosclerotic vessel or aneurysm that causes the recurrent atheroemboli may be necessary.
THORACIC OUTLET COMPRESSION SYNDROME
This is a symptom complex resulting from compression of the neurovascular bundle (artery, vein, or nerves) at the thoracic outlet as it courses through the neck and shoulder. Cervical ribs, abnormalities of the scalenus anticus muscle, proximity of the clavicle to the first rib, or abnormal insertion of the pectoralis minor muscle may compress the subclavian artery, subclavian vein, and brachial plexus as these structures pass from the thorax to the arm. Depending on the structures affected, thoracic outlet compression syndrome is divided into arterial, venous, and neurogenic forms. Patients with neurogenic thoracic outlet compression may develop shoulder and arm pain, weakness, and paresthesias. Patients with arterial compression may experience claudication, Raynaud’s phenomenon, and even ischemic tissue loss and gangrene. Venous compression may cause thrombosis of the subclavian and axillary veins; this is often associated with effort and is referred to as Paget-Schroetter syndrome.
POPLITEAL ARTERY ENTRAPMENT
Popliteal artery entrapment typically affects young athletic men and women when the gastrocnemius or popliteus muscle compresses the popliteal artery and causes intermittent claudication. Thrombosis, embolism, or popliteal artery aneurysm may occur. The pulse examination may be normal unless provocative maneuvers such as ankle dorsiflexion and plantar flexion are performed. The diagnosis is confirmed by duplex ultrasound, CTA, MRA, or conventional angiography. Treatment involves surgical release of the popliteal artery or vascular reconstruction.
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysms are the most common peripheral artery aneurysms. Approximately 50% are bilateral. Patients with popliteal artery aneurysms often have aneurysms of other arteries, especially the aorta. The most common clinical presentation is limb ischemia secondary to thrombosis or embolism. Rupture occurs less frequently. Other complications include compression of the adjacent popliteal vein or peroneal nerve. Popliteal artery aneurysm can be detected by palpation and confirmed by duplex ultrasonography. Repair is indicated for symptomatic aneurysms or when the diameter exceeds 2–3 cm, owing to the risk of thrombosis, embolism, or rupture.
ARTERIOVENOUS FISTULA
Abnormal communications between an artery and a vein, bypassing the capillary bed, may be congenital or acquired. Congenital arteriovenous fistulas are a result of persistent embryonic vessels that fail to differentiate into arteries and veins; they may be associated with birthmarks, can be located in almost any organ of the body, and frequently occur in the extremities. Acquired arteriovenous fistulas either are created to provide vascular access for hemodialysis or occur as a result of a penetrating injury such as a gunshot or knife wound or as complications of arterial catheterization or surgical dissection. An uncommon cause of arteriovenous fistula is rupture of an arterial aneurysm into a vein.
The clinical features depend on the location and size of the fistula. Frequently, a pulsatile mass is palpable, and a thrill and a bruit lasting throughout systole and diastole are present over the fistula. With long-standing fistulas, clinical manifestations of chronic venous insufficiency, including peripheral edema; large, tortuous varicose veins; and stasis pigmentation become apparent because of the high venous pressure. Evidence of ischemia may occur in the distal portion of the extremity. Skin temperature is higher over the arteriovenous fistula. Large arteriovenous fistulas may result in an increased cardiac output with consequent cardiomegaly and high-output heart failure (Chap. 279).
The diagnosis is often evident from the physical examination. Compression of a large arteriovenous fistula may cause reflex slowing of the heart rate (Nicoladoni-Branham sign). Duplex ultrasonography may detect an arteriovenous fistula, especially one that affects the femoral artery and vein at the site of catheter access. CTA and conventional angiography can confirm the diagnosis and are useful in demonstrating the site and size of the arteriovenous fistula.
Management of arteriovenous fistulas may involve surgery, radiotherapy, or embolization. Congenital arteriovenous fistulas are often difficult to treat because the communications may be numerous and extensive, and new communications frequently develop after ligation of the most obvious ones. Many of these lesions are best treated conservatively using elastic support hose to reduce the consequences of venous hypertension. Occasionally, embolization with autologous material, such as fat or muscle, or with hemostatic agents, such as gelatin sponges or silicon spheres, is used to obliterate the fistula. Acquired arteriovenous fistulas are usually amenable to surgical treatment that involves division or excision of the fistula. Occasionally, autogenous or synthetic grafting is necessary to reestablish continuity of the artery and vein.
RAYNAUD’S PHENOMENON
Raynaud’s phenomenon is characterized by episodic digital ischemia, manifested clinically by the sequential development of digital blanching, cyanosis, and rubor of the fingers or toes after cold exposure and subsequent rewarming. Emotional stress may also precipitate Raynaud’s phenomenon. The color changes are usually well demarcated and are confined to the fingers or toes. Typically, one or more digits will appear white when the patient is exposed to a cold environment or touches a cold object (Fig. 302-3A). The blanching, or pallor, represents the ischemic phase of the phenomenon and results from vasospasm of digital arteries. During the ischemic phase, capillaries and venules dilate, and cyanosis results from the deoxygenated blood that is present in these vessels. A sensation of cold or numbness or paresthesia of the digits often accompanies the phases of pallor and cyanosis.
FIGURE 302-3 Vascular diseases associated with temperature: (A) Raynaud’s phenomenon; (B) acrocyanosis; (C) livedo reticularis; (D) pernio; (E) erythromelalgia; and (F) frostbite.
With rewarming, the digital vasospasm resolves, and blood flow into the dilated arterioles and capillaries increases dramatically. This “reactive hyperemia” imparts a bright red color to the digits. In addition to rubor and warmth, patients often experience a throbbing, painful sensation during the hyperemic phase. Although the triphasic color response is typical of Raynaud’s phenomenon, some patients may develop only pallor and cyanosis; others may experience only cyanosis.
Raynaud’s phenomenon is broadly separated into two categories: idiopathic, termed primary Raynaud’s phenomenon, and secondary Raynaud’s phenomenon, which is associated with other disease states or known causes of vasospasm (Table 302-1).
|
CLASSIFICATION OF RAYNAUD’S PHENOMENON |
Primary Raynaud’s Phenomenon This appellation is applied when the secondary causes of Raynaud’s phenomenon have been excluded. Over 50% of patients with Raynaud’s phenomenon have the primary form. Women are affected about five times more often than men, and the age of presentation is usually between 20 and 40 years. The fingers are involved more frequently than the toes. Initial episodes may involve only one or two fingertips, but subsequent attacks may involve the entire finger and may include all the fingers. The toes are affected in 40% of patients. Although vasospasm of the toes usually occurs in patients with symptoms in the fingers, it may happen alone. Rarely, the earlobes, the tip of the nose, and the penis are involved. Raynaud’s phenomenon occurs frequently in patients who also have migraine headaches or variant angina. These associations suggest that there may be a common predisposing cause for the vasospasm.
Results of physical examination are often entirely normal; the radial, ulnar, and pedal pulses are normal. The fingers and toes may be cool between attacks and may perspire excessively. Thickening and tightening of the digital subcutaneous tissue (sclerodactyly) develop in 10% of patients. Angiography of the digits for diagnostic purposes is not indicated.
In general, patients with primary Raynaud’s disease have milder clinical manifestations. Fewer than 1% of these patients lose a part of a digit. After the diagnosis is made, the disease improves spontaneously in approximately 15% of patients and progresses in about 30%.
Secondary Causes of Raynaud’s Phenomenon Raynaud’s phenomenon occurs in 80–90% of patients with systemic sclerosis (scleroderma) and is the presenting symptom in 30% (Chap. 382). It may be the only symptom of scleroderma for many years. Abnormalities of the digital vessels may contribute to the development of Raynaud’s phenomenon in this disorder. Ischemic fingertip ulcers may develop and progress to gangrene and autoamputation. About 20% of patients with systemic lupus erythematosus (SLE) have Raynaud’s phenomenon (Chap. 378). Occasionally, persistent digital ischemia develops and may result in ulcers or gangrene. In most severe cases, the small vessels are occluded by a proliferative endarteritis. Raynaud’s phenomenon occurs in about 30% of patients with dermatomyositis or polymyositis (Chap. 388). It frequently develops in patients with rheumatoid arthritis and may be related to the intimal proliferation that occurs in the digital arteries.
Atherosclerosis of the extremities is a common cause of Raynaud’s phenomenon in men >50 years. Thromboangiitis obliterans is an uncommon cause of Raynaud’s phenomenon but should be considered in young men, particularly those who are cigarette smokers. The development of cold-induced pallor in these disorders may be confined to one or two digits of the involved extremity. Occasionally, Raynaud’s phenomenon may follow acute occlusion of large and medium-size arteries by a thrombus or embolus. Embolization of atheroembolic debris may cause digital ischemia. The latter situation often involves one or two digits and should not be confused with Raynaud’s phenomenon. In patients with thoracic outlet compression syndrome, Raynaud’s phenomenon may result from diminished intravascular pressure, stimulation of sympathetic fibers in the brachial plexus, or a combination of both. Raynaud’s phenomenon occurs in patients with primary pulmonary hypertension (Chap. 304); this is more than coincidental and may reflect a neurohumoral abnormality that affects both the pulmonary and digital circulations.
A variety of blood dyscrasias may be associated with Raynaud’s phenomenon. Cold-induced precipitation of plasma proteins, hyperviscosity, and aggregation of red cells and platelets may occur in patients with cold agglutinins, cryoglobulinemia, or cryofibrinogenemia. Hyperviscosity syndromes that accompany myeloproliferative disorders and lymphoplasmacytic lymphoma (Waldenström’s macroglobulinemia) should also be considered in the initial evaluation of patients with Raynaud’s phenomenon.
Raynaud’s phenomenon occurs often in patients whose vocations require the use of vibrating hand tools, such as chain saws or jackhammers. The frequency of Raynaud’s phenomenon also seems to be increased in pianists and keyboard operators. Electric shock injury to the hands or frostbite may lead to the later development of Raynaud’s phenomenon.
Several drugs have been causally implicated in Raynaud’s phenomenon. They include ergot preparations, methysergide, β-adrenergic receptor antagonists, and the chemotherapeutic agents bleomycin, vinblastine, cisplatin, and gemcitabine.
TREATMENT RAYNAUD’S PHENOMENON
Most patients with Raynaud’s phenomenon experience only mild and infrequent episodes. These patients need reassurance and should be instructed to dress warmly and avoid unnecessary cold exposure. In addition to gloves and mittens, patients should protect the trunk, head, and feet with warm clothing to prevent cold-induced reflex vasoconstriction. Tobacco use is contraindicated.
Drug treatment should be reserved for severe cases. Dihydropyridine calcium channel antagonists such as nifedipine, isradipine, felodipine, and amlodipine decrease the frequency and severity of Raynaud’s phenomenon. Diltiazem may be considered but is less effective. The postsynaptic α1-adrenergic antagonist prazosin has been used with favorable responses; doxazosin and terazosin may also be effective. Phosphodiesterase type 5 inhibitors such as sildenafil and tadalafil may improve symptoms in patients with secondary Raynaud’s phenomenon, as occurs with systemic sclerosis. Digital sympathectomy is helpful in some patients who are unresponsive to medical therapy.
ACROCYANOSIS
In this condition, there is arterial vasoconstriction and secondary dilation of the capillaries and venules with resulting persistent cyanosis of the hands and, less frequently, the feet. Cyanosis may be intensified by exposure to a cold environment. Acrocyanosis may be categorized as primary or secondary to an underlying condition. In primary acrocyanosis, women are affected much more frequently than men, and the age of onset is usually <30 years. Generally, patients are asymptomatic but seek medical attention because of the discoloration. The prognosis is favorable, and pain, ulcers, and gangrene do not occur. Examination reveals normal pulses, peripheral cyanosis, and moist palms (Fig. 302-3B). Trophic skin changes and ulcerations do not occur. The disorder can be distinguished from Raynaud’s phenomenon because it is persistent and not episodic, the discoloration extends proximally from the digits, and blanching does not occur. Ischemia secondary to arterial occlusive disease can usually be excluded by the presence of normal pulses. Central cyanosis and decreased arterial oxygen saturation are not present. Patients should be reassured and advised to dress warmly and avoid cold exposure. Pharmacologic intervention is not indicated.
Secondary acrocyanosis may result from hypoxemia, vasopressor medications, connective tissue diseases, atheroembolism, antiphospholipid antibodies, cold agglutinins, or cryoglobulins and is associated with anorexia nervosa and postural orthostatic tachycardia syndrome. Treatment should be directed at the underlying disorder.
LIVEDO RETICULARIS
In this condition, localized areas of the extremities develop a mottled or rete (netlike) appearance of reddish to blue discoloration (Fig. 302-3C). The mottled appearance may be more prominent after cold exposure. There are primary and secondary forms of livedo reticularis. The primary, or idiopathic, form of this disorder may be benign or associated with ulcerations. The benign form occurs more frequently in women than in men, and the most common age of onset is the third decade. Patients with the benign form are usually asymptomatic and seek attention for cosmetic reasons. These patients should be reassured and advised to avoid cold environments. No drug treatment is indicated. Primary livedo reticularis with ulceration is also called atrophie blanche en plaque. The ulcers are painful and may take months to heal. Secondary livedo reticularis can occur with atheroembolism (see above), SLE and other vasculitides, anticardiolipin antibodies, hyperviscosity, cryoglobulinemia, and Sneddon’s syndrome (ischemic stroke and livedo reticularis). Rarely, skin ulcerations develop.
PERNIO (CHILBLAINS)
Pernio is a vasculitic disorder associated with exposure to cold; acute forms have been described. Raised erythematous lesions develop on the lower part of the legs and feet in cold weather (Fig. 302-3D). They are associated with pruritus and a burning sensation, and they may blister and ulcerate. Pathologic examination demonstrates angiitis characterized by intimal proliferation and perivascular infiltration of mononuclear and polymorphonuclear leukocytes. Giant cells may be present in the subcutaneous tissue. Patients should avoid exposure to cold, and ulcers should be kept clean and protected with sterile dressings. Sympatholytic drugs and dihydropyridine calcium channel antagonists may be effective in some patients.
ERYTHROMELALGIA
This disorder is characterized by burning pain and erythema of the extremities (Fig. 302-3E). The feet are involved more frequently than the hands, and males are affected more frequently than females. Erythromelalgia may occur at any age but is most common in middle age. It may be primary (also termed erythermalgia) or secondary. Mutations in the SCN9A gene, which encodes the Nav1.7 voltage-gated sodium channel expressed in sensory and sympathetic nerves, has been described in inherited forms of erythromelalgia. The most common causes of secondary erythromelalgia are myeloproliferative disorders such as polycythemia vera and essential thrombocytosis. Less common causes include drugs, such as calcium channel blockers, bromocriptine, and pergolide; neuropathies; connective tissue diseases such as SLE; and paraneoplastic syndromes. Patients complain of burning in the extremities that is precipitated by exposure to a warm environment and aggravated by a dependent position. The symptoms are relieved by exposing the affected area to cool air or water or by elevation. Erythromelalgia can be distinguished from ischemia secondary to peripheral arterial disorders because the peripheral pulses are present. There is no specific treatment; aspirin may produce relief in patients with erythromelalgia secondary to myeloproliferative disease. Treatment of associated disorders in secondary erythromelalgia may be helpful.
FROSTBITE
In this condition, tissue damage results from severe environmental cold exposure or from direct contact with a very cold object. Tissue injury results from both freezing and vasoconstriction. Frostbite usually affects the distal aspects of the extremities or exposed parts of the face, such as the ears, nose, chin, and cheeks. Superficial frostbite involves the skin and subcutaneous tissue. Patients experience pain or paresthesia, and the skin appears white and waxy. After rewarming, there is cyanosis and erythema, wheal-and-flare formation, edema, and superficial blisters. Deep frostbite involves muscle, nerves, and deeper blood vessels. It may result in edema of the hand or foot, vesicles and bullae, tissue necrosis, and gangrene (Fig. 302-3F).
Initial treatment is rewarming, performed in an environment where reexposure to freezing conditions will not occur. Rewarming is accomplished by immersion of the affected part in a water bath at temperatures of 40°–44°C (104°–111°F). Massage, application of ice water, and extreme heat are contraindicated. The injured area should be cleansed with soap or antiseptic, and sterile dressings should be applied. Analgesics are often required during rewarming. Antibiotics are used if there is evidence of infection. The efficacy of sympathetic blocking drugs is not established. After recovery, the affected extremity may exhibit increased sensitivity to cold.
303 |
Chronic Venous Disease and Lymphedema |
CHRONIC VENOUS DISEASE
Chronic venous diseases range from telangiectasias and reticular veins, to varicose veins, to chronic venous insufficiency with edema, skin changes, and ulceration. This section of the chapter will focus on identification and treatment of varicose veins and chronic venous insufficiency, since these problems are encountered frequently by the internist. The estimated prevalence of varicose veins in the United States is approximately 15% in men and 30% in women. Chronic venous insufficiency with edema affects approximately 7.5% of men and 5% of women, and the prevalence increases with age ranging from 2% among those less than 50 years of age to 10% of those 70 years of age. Approximately 20% of patients with chronic venous insufficiency develop venous ulcers.
VENOUS ANATOMY
Veins in the extremities can be broadly classified as either superficial or deep. The superficial veins are located between the skin and deep fascia. In the legs, these include the great and small saphenous veins and their tributaries. The great saphenous vein is the longest vein in the body. It originates on the medial side of the foot and ascends anterior to the medial malleolus and then along the medial side of the calf and thigh, and drains into the common femoral vein. The small saphenous vein originates on the dorsolateral aspect of the foot, ascends posterior to the lateral malleolus and along the posterolateral aspect of the calf, and drains into the popliteal vein. The deep veins of the leg accompany the major arteries. There are usually paired peroneal, anterior tibial, and posterior tibial veins in the calf, which converge to form the popliteal vein. Soleal tributary veins drain into the posterior tibial or peroneal veins, and gastrocnemius tributary veins drain into the popliteal vein. The popliteal vein ascends in the thigh as the femoral vein. The confluence of the femoral vein and deep femoral vein form the common femoral vein, which ascends in the pelvis as the external iliac and then common iliac vein, which converges with the contralateral common iliac vein at the inferior vena cava. Perforating veins connect the superficial and deep systems in the legs at multiple locations, normally allowing blood to flow from the superficial to deep veins. In the arms, the superficial veins include the basilic, cephalic, and median cubital veins and their tributaries. The basilic and cephalic veins course along the medial and lateral aspects of the arm, respectively, and these are connected via the median cubital vein in the antecubital fossa. The deep veins of the arms accompany the major arteries and include the radial, ulnar, brachial, axillary, and subclavian veins. The subclavian vein converges with the internal jugular vein to form the brachiocephalic vein, which joins the contralateral brachiocephalic vein to form the superior vena cava. Bicuspid valves are present throughout the venous system to direct the flow of venous blood centrally.
Pathophysiology of Chronic Venous Disease Varicose veins are dilated, bulging, tortuous superficial veins, measuring at least 3 mm in diameter. The smaller and less tortuous reticular veins are dilated intradermal veins, which appear blue-green, measure 1 to 3 mm in diameter, and do not protrude from the skin surface. Telangiectasias, or spider veins, are small, dilated veins, less than 1 mm in diameter, located near the skin surface, and form blue, purple, or red linear, branching, or spider-web patterns.
Varicose veins can be categorized as primary or secondary. Primary varicose veins originate in the superficial system and result from defective structure and function of the valves of the saphenous veins, intrinsic weakness of the vein wall, and high intraluminal pressure. Approximately one-half of these patients have a family history of varicose veins. Other factors associated with primary varicose veins include aging, pregnancy, hormonal therapy, obesity, and prolonged standing. Secondary varicose veins result from venous hypertension, associated with deep venous insufficiency or deep venous obstruction, and incompetent perforating veins that cause enlargement of superficial veins. Arteriovenous fistulas also cause varicose veins in the affected limb.
Chronic venous insufficiency is a consequence of incompetent veins in which there is venous hypertension and extravasation of fluid and blood elements into the tissue of the limb. It may occur in patients with varicose veins but usually is caused by disease in the deep veins. It also is categorized as primary or secondary. Primary deep venous insufficiency is a consequence of an intrinsic structural or functional abnormality in the vein wall or venous valves leading to valvular reflux. Secondary deep venous insufficiency is caused by obstruction and/or valvular incompetence from previous deep vein thrombosis (Chap. 300). Deep venous insufficiency occurs following deep vein thrombosis, as the delicate valve leaflets become thickened and contracted and can no longer prevent retrograde flow of blood and the vein itself becomes rigid and thick walled. Although most veins recanalize after an episode of thrombosis, the large proximal veins may remain occluded. Secondary incompetence develops in distal valves because high pressures distend the vein and separate the leaflets. Other causes of secondary deep venous insufficiency include May-Thurner syndrome, where the left iliac vein is occluded or stenosed by extrinsic compression from the overlapping right common iliac artery; arteriovenous fistulas resulting in increased venous pressure; congenital deep vein agenesis or hypoplasia; and venous malformations as may occur in Klippel-Trénaunay-Weber and Parkes-Weber syndromes.
Clinical Presentation Patients with venous varicosities are often asymptomatic but still concerned about the cosmetic appearance of their legs. Superficial venous thrombosis may be a recurring problem, and, rarely, a varicosity ruptures and bleeds. Symptoms in patients with varicose veins or venous insufficiency, when they occur, include a dull ache, throbbing or heaviness, or pressure sensation in the legs typically after prolonged standing; these symptoms usually are relieved with leg elevation. Additional symptoms may include cramping, burning, pruritus, leg swelling, and skin ulceration.
The legs are examined in both the supine and standing positions. Visual inspection and palpation of the legs in the standing position confirm the presence of varicose veins. The location and extent of the varicose veins should be noted. Edema, stasis dermatitis, and skin ulceration near the ankle may be present if there is superficial venous insufficiency and venous hypertension. Findings of deep venous insufficiency include increased leg circumference, venous varicosities, edema, and skin changes. The edema, which is usually pitting, may be confined to the ankles, extend above the ankles to the knees, or involve the thighs in severe cases. Over time, the edema may become less pitting and more indurated. Dermatologic findings associated with venous stasis include hyperpigmentation, erythema, eczema, lipodermatosclerosis, atrophie blanche, and a phlebectasia corona. Lipodermatosclerosis is the combination of induration, hemosiderin deposition, and inflammation, and typically occurs in the lower part of the leg just above the ankle. Atrophie blanche is a white patch of scar tissue, often with focal telangiectasias and a hyperpigmented border; it usually develops near the medial malleolus. A phlebectasia corona is a fan-shaped pattern of intradermal veins near the ankle or on the foot. Skin ulceration may occur near the medial and lateral malleoli. A venous ulcer is often shallow and characterized by an irregular border, a base of granulation tissue, and the presence of exudate (Fig. 303-1).
FIGURE 303-1 Venous insufficiency with active venous ulcer near the medial malleolus. (Courtesy of Dr. Steven Dean, with permission.)
Bedside maneuvers can be used to distinguish primary varicose veins from secondary varicose veins caused by deep venous insufficiency. With the contemporary use of venous ultrasound (see below), however, these maneuvers are employed infrequently. The Brodie-Trendelenburg test is used to determine whether varicose veins are secondary to deep venous insufficiency. As the patient is lying supine, the leg is elevated and the veins allowed to empty. Then, a tourniquet is placed on the proximal part of the thigh and the patient is asked to stand. Filling of the varicose veins within 30 s indicates that the varicose veins are caused by deep venous insufficiency and incompetent perforating veins. Primary varicose veins with superficial venous insufficiency are the likely diagnosis if venous refilling occurs promptly after tourniquet removal. The Perthes test assesses the possibility of deep venous obstruction. A tourniquet is placed on the midthigh after the patient has stood, and the varicose veins are filled. The patient is then instructed to walk for 5 min. A patent deep venous system and competent perforating veins enable the superficial veins below the tourniquet to collapse. Deep venous obstruction is likely to be present if the superficial veins distend further with walking.
Differential Diagnosis The duration of leg edema helps to distinguish chronic venous insufficiency from acute deep vein thrombosis. Lymphedema, as discussed later in this chapter, is often confused with chronic venous insufficiency, and both may occur together. Other disorders that cause leg swelling should be considered and excluded when evaluating a patient with presumed venous insufficiency. Bilateral leg swelling occurs in patients with congestive heart failure, hypoalbuminemia secondary to nephrotic syndrome or severe hepatic disease, myxedema caused by hypothyroidism or pretibial myxedema associated with Graves’ disease, and with drugs such as dihydropyridine calcium channel blockers and thiazolidinediones. Unilateral causes of leg swelling also include ruptured leg muscles, hematomas secondary to trauma, and popliteal cysts. Cellulitis may cause erythema and swelling of the affected limb. Leg ulcers may be caused by severe peripheral artery disease and critical limb ischemia; neuropathies, particularly those associated with diabetes; and less commonly, skin cancer, vasculitis, or rarely as a complication of hydroxyurea. The location and characteristics of venous ulcers help to differentiate these from other causes.
Classification of Chronic Venous Disease The CEAP (clinical, etiologic, anatomic, pathophysiologic) classification schema incorporates the range of symptoms and signs of chronic venous disease to characterize its severity. It also broadly categorizes the etiology as congenital, primary, or secondary; identifies the affected veins as superficial, deep, or perforating; and characterizes the pathophysiology as reflux, obstruction, both, or neither (Table 303-1).
|
CEAP (CLINICAL, ETIOLOGIC, ANATOMIC, PATHOPHYSIOLOGIC) CLASSIFICATION |
Source: B Eklöf et al: J Vasc Surg 40:1248, 2004.
Diagnostic Testing The principal diagnostic test to evaluate patients with chronic venous disease is venous duplex ultrasonography. A venous duplex ultrasound examination uses a combination of B-mode imaging and spectral Doppler to detect the presence of venous obstruction and venous reflux in superficial and deep veins. Color-assisted Doppler ultrasound is useful to visualize venous flow patterns. Obstruction may be diagnosed by absence of flow, the presence of an echogenic thrombus within the vein, or failure of the vein to collapse when a compression maneuver is applied by the sonographer, the last implicating the presence of an intraluminal thrombus. Venous reflux is detected by prolonged reversal of venous flow direction during a Valsalva maneuver, particularly for the common femoral vein or saphenofemoral junction, or after compression and release of a cuff placed on the limb distal to the area being interrogated.
Some vascular laboratories use air or strange gauge plethysmography to assess the severity of venous reflux and complement findings from the venous ultrasound examination. Venous volume and venous refilling time are measured when the legs are placed in a dependent position and after calf exercise to quantify the severity of venous reflux and the efficiency of the calf muscle pump to affect venous return.
Magnetic resonance, computed tomographic, and conventional venography are rarely required to determine the cause and plan treatment for chronic venous insufficiency unless there is suspicion for pathology that might warrant intervention. These modalities are used to identify obstruction or stenosis of the inferior vena cava and iliofemoral veins, as may occur in patients with previous proximal deep vein thrombosis; occlusion of inferior vena cava filters; extrinsic compression from tumors; and May-Thurner syndrome.
TREATMENT CHRONIC VENOUS DISEASE
SUPPORTIVE MEASURES
Varicose veins usually are treated with conservative measures. Symptoms often decrease when the legs are elevated periodically, prolonged standing is avoided, and elastic support hose are worn. External compression with elastic stockings or stretch bandages provides a counterbalance to the hydrostatic pressure in the veins. Although compression garments may improve symptoms, they do not prevent progression of varicose veins. Graduated compression stockings with pressures of 20–30 mmHg are suitable for most patients with simple varicose veins, although pressures of 30–40 mmHg may be required for patients with manifestations of venous insufficiency such as edema and ulcers.
Patients with chronic venous insufficiency also should be advised to avoid prolonged standing or sitting; frequent leg elevation is helpful. Graded compression therapy consisting of stockings or multilayered compression bandages is the standard of care for advanced chronic venous insufficiency characterized by edema, skin changes, or venous ulcers defined as CEAP clinical class C3–C6. Graduated compression stockings of 30–40 mmHg are more effective than lesser grades for healing venous ulcers. The length of stocking depends on the distribution of edema. Calf-length stockings are tolerated better by most patients, particularly elderly patients; for patients with varicose veins or edema extending to the thigh, thigh-length stockings or panty hose should be considered. Overweight and obese patients should be advised to lose weight via caloric restriction and exercise.
In addition to a compression bandage or stocking, patients with venous ulcers also may be treated with low adherent absorbent dressings that take up exudates while maintaining a moist environment. Other types of dressings include hydrocolloid (an adhesive dressing comprised of polymers such as carboxymethylcellulose that absorbs exudates by forming a gel), hydrogel (a nonabsorbent dressing comprising over 80% water or glycerin that moisturizes wounds), foam (an absorbent dressing made with polymers such as polyurethane), and alginate (an absorbent, biodegradable dressing that is derived from seaweed), but there is little evidence that these are more effective than low adherent absorbent dressings. The choice of specific dressing depends on the amount of drainage, presence of infection, and integrity of the skin surrounding the ulcer. Antibiotics are not indicated unless the ulcer is infected. The multilayered compression bandage or graduated compression garment is then put over the dressing.
MEDICAL THERAPIES
There are no drugs approved by the U.S. Food and Drug Administration for the treatment of chronic venous insufficiency. Diuretics may reduce edema, but at the risk of volume depletion and compromise in renal function. Topical steroids may be used for a short period of time to treat inflammation associated with stasis dermatitis. Several herbal supplements, such as horse chestnut seed extract (aescin); flavonoids including diosmin, hesperidin, or the two combined as micronized purified flavonoid fraction; and French maritime pine bark extract, are touted to have venoconstrictive and anti-inflammatory properties. Although meta-analyses have suggested that aescin reduces edema, pruritus, and pain and that micronized purified flavonoid fraction in conjunction with compression therapy facilitates venous ulcer healing, there is insufficient evidence to recommend the general use of these substances in patients with chronic venous insufficiency.
INTERVENTIONAL AND SURGICAL THERAPIES
Ablative procedures, including endovenous thermal ablation, sclerotherapy, and surgery, are used to treat varicose veins in selected patients who have persistent symptoms, great saphenous vein incompetency, and complications of venous insufficiency including dermatitis, edema, and ulcers. Ablative therapy may also be indicated for cosmetic reasons.
Endovenous thermal ablation procedures of the saphenous veins include endovenous laser therapy and radiofrequency ablation. To ablate the great saphenous vein, a catheter is placed percutaneously and advanced from the level of the knee to just below the saphenofemoral junction via ultrasound guidance. Thermal energy is then delivered as the catheter is pulled back. The heat injures the endothelium and media and promotes thrombosis and fibrosis, resulting in venous occlusion. Average 1- and 5-year occlusion rates exceed 90% following endovenous laser therapy and are slightly less after radiofrequency ablation. Deep vein thrombosis of the common femoral vein adjacent to the saphenofemoral junction is an uncommon but potential complication of endovenous thermal ablation. Other adverse effects of thermal ablation procedures include pain, paresthesias, bruising, hematoma, and hyperpigmentation.
Sclerotherapy involves the injection of a chemical into a vein to cause fibrosis and obstruction. Sclerosing agents approved by the U.S. Food and Drug Administration include sodium tetradecyl sulfate, polidocanol, sodium morrhuate, and glycerin. The sclerosing agent is administered as a liquid or mixed with air or CO2/O2 to create a foam. It first is injected into the great saphenous vein or its affected tributaries, often with ultrasound guidance. Thereafter, smaller more distal veins and incompetent perforating veins are injected. Following completion of the procedure, elastic bandages are applied, or 30–40 mmHg compression stockings are worn for 1–2 weeks. Average 1- and 5-year occlusion rates are 81% and 74%, respectively, following sclerotherapy. Complications are uncommon and include deep vein thrombosis, hematomas, damage to adjacent saphenous or sural nerves, and infection. Anaphylaxis is a very rare but severe complication.
Surgical therapy usually involves ligation and stripping of the great and small saphenous veins. The procedure is performed under general anesthesia. Incisions are made at the groin and the upper calf. The great saphenous vein is ligated below the saphenofemoral junction, and a wire is inserted into the great saphenous vein and advanced distally. The proximal part of the great saphenous vein is secured to the wire and retrieved, i.e., stripped, via the calf incision. Stripping of the great saphenous vein below the knee and stripping of the small saphenous vein usually are not performed because of the respective risks of saphenous and sural nerve injury. Complications of great saphenous vein ligation and stripping include deep vein thrombosis, bleeding, hematoma, infection, and nerve injury. Recurrent varicose veins occur in up to 50% patients by 5 years, due to technical failures, deep venous insufficiency, and incompetent perforating veins.
Stab phlebectomy is another surgical treatment for of varicose veins. A small incision is made alongside the varicose vein, and it is avulsed by means of a forceps or hook. This procedure may be performed in conjunction with saphenous vein ligation and stripping or thermal ablation. Subfascial endoscopic perforator surgery (SEPS) uses endoscopy to identify and occlude incompetent perforating veins. It also may be performed along with other ablative procedures.
Endovascular interventions, surgical bypass, and reconstruction of the valves of the deep veins are performed when feasible to treat patients with advanced chronic venous insufficiency who have not responded to other therapies. Catheter-based interventions, usually involving placement of endovenous stents, may be considered to treat some patients with chronic occlusions of the iliac veins. Technical success rates exceed 85% in most series, and long-term patency is achieved in approximately 75% of these patients. Iliocaval bypass, femoroiliac venous bypass, and femorofemoral crossover venous bypass are procedures used occasionally to treat iliofemoral vein occlusion; saphenopopliteal vein bypass can be used to treat chronic femoropopliteal vein obstruction. Long-term patency rates for venous bypass procedures generally exceed 60% and are associated with improvement in symptoms. Surgical reconstruction of the valves of the deep veins and valve transfer procedures are used to treat valvular incompetence. Valvuloplasty involves tightening the valve by commissural apposition. With valve transfer procedures, a segment of vein with a competent valve, such as a brachial or axillary vein, or adjacent saphenous or deep femoral vein, is inserted as an interposition graft in the incompetent vein. Both valvuloplasty and vein transfer operations result in ulcer healing in the majority of patients, although success rates are somewhat better with valvuloplasty.
Lymphedema Lymphedema is a chronic condition caused by impaired transport of lymph and characterized by swelling of one or more limbs and occasionally the trunk and genitalia. Fluid accumulates in interstitial tissues when there is an imbalance between lymph production and lymph absorption, a process governed in large part by Starling forces. Deficiency, reflux, or obstruction of lymph vessels perturbs the ability of the lymphatic system to reabsorb proteins that had been filtered by blood vessels, and the tissue osmotic load promotes interstitial accumulation of water. Persistent lymphedema leads to inflammatory and immune responses characterized by infiltration of mononuclear cells, fibroblasts, and adipocytes, leading to adipose and collagen deposition in the skin and subcutaneous tissues.
Lymphatic Anatomy Lymphatic capillaries are blind-ended tubes formed by a single layer of endothelial cells. The absent or widely fenestrated basement membrane of lymphatic capillaries allows access to interstitial proteins and particles. Lymphatic capillaries merge to form microlymphatic precollector vessels, which contain few smooth muscle cells. The precollector vessels drain into collecting lymphatic vessels, which comprise endothelial cells, a basement membrane, smooth muscle, and bileaflet valves. The collecting lymphatic vessels in term merge to form larger lymphatic conduits. Analogous to venous anatomy, there are superficial and deep lymphatic vessels in the legs, which communicate at the popliteal and inguinal lymph nodes. Pelvic lymphatic vessels drain into the thoracic duct, which ascends from the abdomen to the thorax and connects with the left brachiocephalic vein. Lymph is propelled centrally by the phasic contractile activity of lymphatic smooth muscle and facilitated by the contractions of contiguous skeletal muscle. The presence of lymphatic valves ensures unidirectional flow.
Etiology Lymphedema may be categorized as primary or secondary (Table 303-2). The prevalence of primary lymphedema is approximately 1.15 per 100,000 persons less than 20 years of age. Females are affected more frequently than males. Primary lymphedema may be caused by agenesis, hypoplasia, hyperplasia, or obstruction of the lymphatic vessels. There are three clinical subtypes: congenital lymphedema, which appears shortly after birth; lymphedema praecox, which has its onset at the time of puberty; and lymphedema tarda, which usually begins after age 35. Familial forms of congenital lymphedema (Milroy’s disease) and lymphedema praecox (Meige’s disease) may be inherited in an autosomal dominant manner with variable penetrance; autosomal or sex-linked recessive forms are less common. Mutations in genes expressing vascular endothelial growth factor receptor 3 (VEGFR3), which is a determinant of lymphangiogenesis, have been described in patients with Milroy’s disease. A mutation on chromosome 15q is associated with the cholestasis-lymphedema syndrome. A mutation in the FOXC2 gene, which encodes a transcription factor that interacts with a signaling pathway involved in the development of lymphatic vessels, has been reported in patients with the lymphedema-distichiasis syndrome, in which lymphedema praecox occurs in patients who also have a double row of eyelashes. A mutation of SOX18, a transcription factor upstream of lymphatic endothelial cell differentiation, has been described in patients with lymphedema, alopecia, and telangiectasias (hypotrichosis, lymphedema, telangiectasia syndrome). Patients with a chromosomal aneuploidy, such as Turner’s syndrome, Klinefelter’s syndrome, or trisomy 18, 13, or 21, may develop lymphedema. Syndromic vascular anomalies associated with lymphedema include Klippel-Trénaunay syndrome, Parkes-Weber syndrome, and Hennekam’s syndrome. Other disorders associated with lymphedema include Noonan’s syndrome, yellow nail syndrome, intestinal lymphangiectasia syndrome, lymphangiomyomatosis, and neurofibromatosis type 1.
|
CAUSES OF LYMPHEDEMA |
Secondary lymphedema is an acquired condition that results from damage to or obstruction of previously normal lymphatic channels. Recurrent episodes of bacterial lymphangitis, usually caused by streptococci, are a very common cause of lymphedema. The most common cause of secondary lymphedema worldwide is lymphatic filariasis, affecting approximately 129 million children and adults worldwide and causing lymphedema and elephantiasis in 14 million of these affected individuals (Chap. 258). Recurrent bacterial lymphangitis by Streptococcus may result in chronic lymphedema. Other infectious causes include lymphogranuloma venereum and tuberculosis. In developed countries, the most common secondary cause of lymphedema is surgical excision or irradiation of axillary and inguinal lymph nodes for treatment of cancers, such as breast, cervical, endometrial, and prostate cancer, sarcomas, and malignant melanoma. Lymphedema of the arm occurs in 13% of breast cancer patients after axillary node dissection and in 22% after both surgery and radiotherapy. Lymphedema of the leg affects approximately 15% of patients with cancer after inguinal lymph node dissection. Tumors, such as prostate cancer and lymphoma, also can infiltrate and obstruct lymphatic vessels. Less common causes include contact dermatitis, rheumatoid arthritis, pregnancy, and self-induced or factitious lymphedema after application of tourniquets.
Clinical Presentation Lymphedema is generally a painless condition, but patients may experience a chronic dull, heavy sensation in the leg, and most often they are concerned about the appearance of the leg. Lymphedema of the lower extremity initially involves the foot and gradually progresses up the leg so that the entire limb becomes edematous (Fig. 303-2). In the early stages, the edema is soft and pits easily with pressure. Over time, subcutaneous adipose tissue accumulates, the limb enlarges further and loses its normal contour, and the toes appear square. Thickening of the skin is detected by Stemmer’s sign, which is the inability to tent the skin at the base of the toes. Peau d’orange is a term used to describe dimpling of the skin, resembling that of an orange peel, caused by lymphedema. In the chronic stages, the edema no longer pits and the limb acquires a woody texture as the tissues become indurated and fibrotic. The International Society of Lymphology describes four clinical stages of lymphedema (Table 303-3).
FIGURE 303-2 A. Lymphedema characterized by swelling of the leg, nonpitting edema, and squaring of the toes. (Courtesy of Dr. Marie Gerhard-Herman, with permission.) B. Advanced chronic stage of lymphedema illustrating the woody appearance of the leg with acanthosis and verrucous overgrowths. (Courtesy of Dr. Jeffrey Olin, with permission.)
|
STAGES OF LYMPHEDEMA |
Differential Diagnosis Lymphedema should be distinguished from other disorders that cause unilateral leg swelling, such as deep vein thrombosis and chronic venous insufficiency. In the latter condition, the edema is softer, and there is often evidence of a stasis dermatitis, hyperpigmentation, and superficial venous varicosities, as described earlier. Other causes of leg swelling that resemble lymphedema are myxedema and lipedema. Lipedema usually occurs in women and is caused by accumulation of adipose tissue in the leg from the thigh to the ankle with sparing of the feet.
Diagnostic Testing The evaluation of patients with lymphedema should include diagnostic studies to clarify the cause. Abdominal and pelvic ultrasound and computed tomography (CT) can be used to detect obstructing lesions such as neoplasms. Magnetic resonance imaging (MRI) of the affected limb may reveal a honeycomb pattern characteristic of lymphedema in the epifascial compartment and identify enlarged lymphatic channels and lymph nodes. MRI also is useful to distinguish lymphedema from lipedema. Lymphoscintigraphy and lymphangiography are rarely indicated, but either can be used to confirm the diagnosis or differentiate primary from secondary lymphedema. Lymphoscintigraphy involves the injection of radioactively labeled technetium-containing colloid into the distal subcutaneous tissue of the affected extremity, which is imaged with a scintigraphic camera to visualize lymphatic vessels and lymph nodes. Findings indicative of primary lymphedema include absent or delayed filling of the lymphatic vessels or dermal back flow caused by lymphatic reflux. Findings of secondary lymphedema include dilated lymphatic vessels distal to an area of obstruction. In lymphangiography, iodinated radiocontrast material is injected into a distal lymphatic vessel that has been isolated and cannulated. In primary lymphedema, lymphatic channels are absent, hypoplastic, or ectatic. In secondary lymphedema, lymphatic channels often appear dilated beneath the level of obstruction. The complexities of lymphatic cannulation and the risk of lymphangitis associated with the contrast agent limit the utility of lymphangiography. A novel technique of optical imaging with a near-infrared fluorescence dye may enable quantitative imaging of lymph flow.
TREATMENT LYMPHEDEMA
Patients with lymphedema of the lower extremities must be instructed to take meticulous care of their feet to prevent recurrent lymphangitis. Skin hygiene is important, and emollients can be used to prevent drying. Prophylactic antibiotics are often helpful, and fungal infection should be treated aggressively. Patients should be encouraged to participate in physical activity; frequent leg elevation can reduce the amount of edema. Psychosocial support is indicated to assist patients cope with anxiety or depression related to body image, self-esteem, functional disability, and fear of limb loss.
Physical therapy, including massage to facilitate lymphatic drainage, may be helpful. The type of massage used in decongestive physiotherapy for lymphedema involves mild compression of the skin of the affected extremity to dilate the lymphatic channels and enhance lymphatic motility. Multilayered, compressive bandages are applied after each massage session to reduce recurrent edema. After optimal reduction in limb volume by decongestive physiotherapy, patients can be fitted with graduated compression hose. Occasionally, intermittent pneumatic compression devices can be applied at home to facilitate reduction of the edema. Diuretics are contraindicated and may cause depletion of intravascular volume and metabolic abnormalities.
Liposuction in conjunction with decongestive physiotherapy may be considered to treat lymphedema, particularly postmastectomy lymphedema. Other surgical interventions are rarely used and often not successful in ameliorating lymphedema. Microsurgical lymphaticovenous anastomotic procedures have been performed to rechannel lymph flow from obstructed lymphatic vessels into the venous system. Limb reduction procedures to resect subcutaneous tissue and excessive skin are performed occasionally in severe cases of lymphedema to improve mobility.
Therapeutic lymphangiogenesis has been studied in rodent models of lymphedema, but not as yet in humans. Overexpression of vascular endothelial growth factor (VEGF) C generates new lymphatic vessels and improves lymphedema in a murine model of primary lymphedema, and administration of recombinant VEGF-C or VEGF-D stimulated lymphatic growth in preclinical models of postsurgical lymphedema. Clinical trials in patients with lymphedema are required to determine efficacy of gene transfer (cell-based) therapies for lymphedema.
304 |
Pulmonary Hypertension |
Pulmonary hypertension (PH) is a spectrum of diseases involving the pulmonary vasculature, and is defined as an elevation in pulmonary arterial pressures (mean pulmonary artery pressure >22 mmHg). Pulmonary arterial hypertension (PAH) is a relatively rare form of PH and is characterized by symptoms of dyspnea, chest pain, and syncope. If left untreated, the disease carries a high mortality rate, with the most common cause of death being decompensated right heart failure. There have been significant advances in this field in regard to understanding the pathogenesis, diagnosis, and classification of PAH. Despite these significant advances, there is still a substantial delay in diagnosis of up to 2 years. In many cases, patients whose primary complaint is dyspnea on exertion are frequently misdiagnosed with more common diseases such as asthma or chronic obstructive pulmonary disease. The availability of newer drugs has resulted in a radical change in the management of this disease with significant improvement in both quality of life and mortality. A delay in diagnosis results in an obvious delay in the initiation of appropriate treatment. Clinicians should be able to recognize the signs and symptoms of PH and to complete a systematic workup in patients suspected of having it. In this way, early diagnosis, prompt treatment, and improved outcomes for patients become achievable.
PATHOBIOLOGY
Vasoconstriction, vascular proliferation, thrombosis, and inflammation appear to underlie the development of PAH (Fig. 304-1). In long-standing PH, intimal proliferation and fibrosis, medial hypertrophy, and in situ thrombosis characterize the pathologic findings in the pulmonary vasculature. Vascular remodeling at earlier stages may be confined to the small pulmonary arteries. As the disease advances, intimal proliferation and pathologic remodeling progress, resulting in decreased compliance and increased elastance of the pulmonary vasculature. The outcome is a progressive increase in the right ventricular afterload or total pulmonary vascular resistance (PVR) and, thus, right ventricular work. In subjects with moderate to severe pulmonary vascular disease with significantly increased PVR, as the resting PVR increases, there will be a corresponding increase in mean pulmonary artery pressure (PAP) until the cardiac output (CO) is compromised and starts to fall. With a decline in CO, the PAP will fall. As CO declines as a result of increased afterload and decreased contractility, tachycardia is a compensatory response. Tachycardia decreases filling time and, thus, preload, and results in a reduced fraction of stroke volume available to distend the pulmonary vascular tree.
FIGURE 304-1 The left panels show examples of plexogenic pulmonary arteriopathy. These are obstructive and proliferative lesions of the small muscular pulmonary arteries, composed primarily of endothelial cells with intermixed inflammatory cells, myofibroblasts, and connective tissue components. The lower left panel demonstrates proliferating cells (red PCNA stained cells). Panels on the right demonstrate medial hypertrophy of muscular pulmonary arteries. (Photographs on the left are courtesy of Dr. Stephen Archer, Queen’s University School of Medicine, Kingston, Ontario, Canada.)
Abnormalities in multiple molecular pathways and genes that regulate the pulmonary vascular endothelial and smooth muscle cells have been identified (Table 304-1). These abnormalities include decreased expression of the voltage-regulated potassium channel, mutations in the bone morphogenetic protein receptor-2, increased tissue factor expression, overactivation of the serotonin transporter, hypoxia-induced activation of hypoxia-inducible factor-1α, and activation of nuclear factor of activated T cells. As a result, there is a decrease in apoptosis of the smooth muscle cells and the emergence of apoptosis-resistant endothelial cells that promote their accumulation and can obliterate the vascular lumen. In addition, thrombin deposition in the pulmonary vasculature from the prothrombotic state that develops as an independent abnormality or as a result of endothelial dysfunction may amplify vascular cell proliferation and the obliterative arteriopathy.
|
COMPONENTS OF THE PATHOGENESIS OF PULMONARY ARTERIAL HYPERTENSION |
Abbreviations: PDGF, platelet-derived growth factor; EGF, epidermal-derived growth factor; FGF, fetal-derived growth factor; VEGF, vascular endothelial-derived growth factor; MCP-1, monocyte chemoattractant protein-1; IL, interleukin.
DIAGNOSIS AND CLASSIFICATION
The diagnosis of PH can be missed without a reasonable index of suspicion. Dyspnea is the most common presenting symptom, but this complaint is far from specific for the diagnosis of PH. PH symptoms are insidious and overlap considerably with many common conditions, including asthma and other lung disease and cardiac disease. The symptoms of PH are often nonspecific and variable. Most patients will present with dyspnea and/or fatigue, whereas edema, chest pain, presyncope, and frank syncope are less common and associated with more advanced disease. On examination, there may be evidence of right ventricular failure with elevated jugular venous pressure, lower extremity edema, and ascites. Additionally, the cardiovascular examination may reveal an accentuated P2 component of the second heart sound, a right-sided S3 or S4, and a holosystolic tricuspid regurgitant murmur. It is also important to seek signs of the diseases that are often concurrent with PH: clubbing may be seen in some chronic lung diseases, sclerodactyly and telangiectasia may signify scleroderma, and crackles and systemic hypertension may be clues to left-sided systolic or diastolic heart failure.
Once clinical suspicion is raised, a systematic approach to diagnosis and assessment is essential. An echocardiogram with (if indicated) a bubble study is the most important screening test. Echocardiography is important for the diagnosis of PH and often essential for determining the cause. All forms of PH may demonstrate a hypertrophied and dilated right ventricle (Fig. 304-2) with elevated estimated pulmonary artery systolic pressure. Important additional information can be gleaned about specific etiologies of PH such as valvular disease, left ventricular systolic and diastolic function, intracardiac shunts, and other cardiac diseases.
FIGURE 304-2 A. Representative echocardiogram showing the apical four-chamber view from a patient with pulmonary hypertension demonstrating an enlarged right atrium and ventricle with some compression of the left side of the heart. B. Same echocardiographic view showing a normal echocardiogram.
Although the accuracy of Doppler echocardiography is often debated, a high-quality echocardiogram that is absolutely normal may obviate the need for further evaluation for PH. An echocardiogram is a screening test, whereas invasive hemodynamic monitoring is the gold standard for diagnosis and assessment of disease severity. With a normal echocardiogram, there may still be some concern for PH; this is particularly true if there is unexplained dyspnea or hypoxemia. In this setting, it is reasonable to proceed to right heart catheterization for definitive diagnosis. Alternatively, if the patient has a reasonable functional capacity, a cardiopulmonary exercise test may help to identify a true physiologic limitation as well as differentiate between cardiac and pulmonary causes of dyspnea. If this test is normal, there is no indication for a right heart catheterization. If a cardiovascular limitation to exercise is found, a right heart catheterization should be pursued.
If the echocardiogram or cardiopulmonary exercise test (CPET) suggests PH and the diagnosis is confirmed by catheterization, a reasonable effort must be made to establish the etiology because this will largely determine the therapeutic approach. A stepwise approach to evaluation is outlined below.
Chest imaging and lung function tests are essential because lung disease is an important cause of PH. A sign of PH that may be evident on chest x-ray include enlargement of the central pulmonary arteries associated with “vascular pruning,” a relative paucity of peripheral vessels (Fig. 304-3). Cardiomegaly, with specific evidence of right atrial and ventricular enlargement, can often be observed. The chest x-ray may also demonstrate significant interstitial lung disease or suggest hyperinflation from obstructive lung disease, which may be the underlying cause or contributor to the development of PH. High-resolution computed tomography (CT) may provide additional useful information. Classic findings of PH on CT include those found on chest x-ray: enlarged pulmonary arteries (Fig. 304-4), peripheral pruning of the small vessels, and enlarged right ventricle and atrium. However, high-resolution CT may also reveal signs of venous congestion including centrilobular ground-glass infiltrate and thickened septal lines. In the absence of left heart disease, these findings suggest pulmonary veno-occlusive disease, a rare cause of PAH that can be quite challenging to diagnose.
FIGURE 304-3 Posteroanterior (left) and lateral (right) chest radiograph showing enlarged pulmonary arteries (black arrows) and pruning of the distal pulmonary vasculature (white arrow) commonly seen with advanced pulmonary arterial hypertension.
FIGURE 304-4 Representative computed tomography scan of the chest demonstrating enlarged main pulmonary arteries. There is also a mosaic pattern evident in both lungs.
CT angiograms are commonly used to evaluate acute thromboembolic disease and have demonstrated excellent sensitivity and specificity for that purpose. Ventilation-perfusion (V·/Q·) scanning has traditionally been used for screening because of its high sensitivity and its role in qualifying patients for surgical intervention. The role of CT angiograms in the diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH) remains controversial, even with the advent of spiral CT. Although a negative V·/Q· virtually rules out CTEPH, some cases may be missed through the use of CT angiograms.
Pulmonary function tests are an important component of the evaluation. Although an isolated reduction in DLCO is the classic finding in PAH, results of pulmonary function tests may also suggest restrictive or obstructive lung diseases as the cause of dyspnea or PH. The 6-minute walk test is also important to evaluate the degree of exertional hypoxemia and limitation and to monitor progression and response to therapy.
Sleep-disordered breathing is another important cause of PH, but a sleep study is generally necessary only when indicated by the patient’s history. Nocturnal desaturation is a common finding in PH, even in the absence of sleep-disordered breathing. Thus, all patients should undergo nocturnal oximetry screening, regardless of whether classic symptoms of obstructive sleep apnea or obesity-hypoventilation syndrome are observed. Laboratory tests that are important for screening include an HIV test when clinically indicated. In addition, all patients should have antinuclear antibodies, rheumatoid factor, and scl-70 antibodies assessed to screen for the most common rheumatologic diseases associated with PH if clinically indicated. Liver function and hepatitis serology tests are important to screen for underlying liver disease. Finally, there is an increasing role for brain natriuretic peptide (BNP) testing in the diagnosis and management of PH. BNP and the N-terminus of its propeptide (NT-proBNP) correlate with right ventricular function, hemodynamic severity, and functional status in PAH.
Right heart catheterization with pulmonary vasodilator testing remains the gold standard both to establish the diagnosis of PH and to enable selection of appropriate medical therapy. The definition of precapillary PH or PAH requires (1) an increased mean pulmonary artery pressure (mPAP ≥25 mmHg); (2) a pulmonary capillary wedge pressure (PCWP), left atrial pressure, or left ventricular end-diastolic pressure ≤15 mmHg; and (3) PVR >3 Wood units. Postcapillary PH is differentiated from precapillary PH by a PCWP of ≥15 mmHg; this is further differentiated into passive, based on a transpulmonary gradient <12 mmHg, or reactive, based on a transpulmonary gradient >12 mmHg and an increased PVR. In either case, the CO may be normal or reduced.
Vasodilators with a short duration of action, such as inhaled nitric oxide, inhaled epoprostenol, or intravenous adenosine, are preferred for vasodilator testing. A decrease in mPAP by ≥10 mmHg to an absolute level of ≤40 mmHg without a decrease in CO is defined as a positive pulmonary vasodilator response, and responders are considered for long-term treatment with calcium channel blockers (CCBs). Less than 12% of patients are deemed vasoreactive during testing, and even fewer exhibit long-term responsiveness to CCBs. Acute vasodilator-induced reductions in PVR and mPAP predict better long-term survival even among patients not treated with CCBs. The need for invasive hemodynamic measurements to diagnose PH accurately poses an additional problem when evaluating older patients. Physicians are often reluctant to refer older patients for invasive procedures. However, the diagnosis of PH is increasing in the older population, at least in part because of increased awareness of this disease in the elderly and increased use of screening echocardiograms. Furthermore, the increased availability of oral and less complicated therapeutic options has encouraged the referral of older patients for evaluation and treatment.
PULMONARY HYPERTENSION AS A COMORBID DISEASE
PAH is just one of a number of disease classifications that affect the pulmonary vascular bed. PH was previously classified as primary or secondary, but as understanding of the various contributing diseases has increased, classification systems have attempted to group these diseases by clinical features to aid in diagnosis. The World Health Organization (WHO) formulated a clinical classification of the various manifestations of PH, of which PAH is a subgroup, according to similarities in pathophysiologic mechanisms and clinical presentation. PH is a diverse mix of pathologies in which the only unifying theme is elevated PAP relative to left atrial pressure. The categorization of PH was designed by convenience for the purpose of facilitating novel treatments to be tested across different presentations and is not based on a molecular understanding of the pathology and is not a guide for management decisions.
The current classification system, last revised in 2013 during the Fifth World Symposium on Pulmonary Hypertension, recognizes five categories of PH, including PAH, PH due to left heart disease, PH due to chronic lung disease, PH associated with chronic thromboemboli, and a group of miscellaneous diseases that only rarely cause PH.
Pulmonary Arterial Hypertension WHO Group I PH, pulmonary arterial hypertension (PAH), is a relatively rare cause of PH. PAH includes a group of diseases that result in pulmonary arterial precapillary remodeling marked by intimal fibrosis, increased medial thickness, pulmonary arteriolar occlusion, and classic plexiform lesions. PAH is defined as a sustained elevation in resting mPAP ≥25 mmHg, PVR>240 dyne·s/cm5, and PCWP or left ventricle end-diastolic pressure of ≤15 mmHg based on a right heart catheterization. With a normal PCWP and an elevated mPAP, these diseases demonstrate an increased transpulmonary gradient (mPAP – PCWP); in addition, the PVR is elevated.
Idiopathic pulmonary arterial hypertension (IPAH) is a progressive disease that leads to right heart failure and death. It is typically seen in young women. The National Institutes of Health registry, the first large registry of patients with PAH, reported that the average age at diagnosis was 36 years, with only 9% of patients with IPAH over the age of 60 at diagnosis. However, the more current clinical data suggest that the patient demographics are changing. The Pulmonary Hypertension Connection registry found that the average age of diagnosis for IPAH was 45 years, with 8.5% of patients older than 70 years at diagnosis. This finding is supported by data from the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), the largest cohort of PAH to date, which reported that the average age at diagnosis of IPAH was 44.9±0.6 years.
Other forms of PAH that deserve specific consideration in patients are those associated with HIV, connective tissue disease, and portal hypertension. Although HIV is a rare cause of PAH, this form of PAH is indistinguishable from IPAH and is an important cause of mortality in the HIV-infected population. Importantly, there is no correlation between the stage of HIV infection and the development of PAH.
Among connective tissue diseases, the prevalence of PAH has been established only for systemic sclerosis, especially in those with limited cutaneous scleroderma. Although the average age of scleroderma onset is 30 to 50 years old, patients who eventually develop scleroderma-associated PAH tend to be older at the time of scleroderma diagnosis. Outcomes of scleroderma are closely linked to the development of PAH and are associated with a poor prognosis, although modern therapies have improved outcomes.
Portopulmonary hypertension occurs in 2–10% of patients with established portal hypertension. Its occurrence appears to be independent of the cause of liver disease and is observed in patients with nonhepatic causes of portal hypertension. A hyperdynamic circulatory state is common, as in most patients with advanced liver disease; however, the same pulmonary vascular remodeling observed in other forms of PAH is seen in the pulmonary vascular bed in portopulmonary hypertension. It is important to distinguish this process from hepatopulmonary syndrome, which can also manifest with dyspnea and hypoxemia but is pathophysiologically distinct from portopulmonary hypertension in that abnormal vasodilation of the pulmonary vasculature leads to intrapulmonary shunting.
Pulmonary Hypertension Associated with Left Heart Disease WHO Group II PH includes patients with left heart systolic failure, aortic and mitral valve disease, and heart failure with preserved ejection fraction (HFpEF). PH can develop as a result of all of these conditions. The hallmark of Group II PH (i.e., PH due to left heart disease) is elevated left atrial pressure with resulting pulmonary venous hypertension. In general, the transpulmonary gradient and PVR remain normal. Although this phenomenon is well described in both left-sided valvular disease and left-sided systolic heart failure, studies suggest that HFpEF may carry a higher overall risk of PH.
Whatever the cause of elevated left atrial pressure (i.e., systolic or diastolic heart failure or valvular disease), the increased pulmonary venous pressure indirectly leads to a rise in pulmonary arterial pressure. The presence of PH portends a poor prognosis in all forms of heart failure. In particular, chronic pulmonary venous hypertension may lead to a reactive pulmonary arterial vasculopathy, seen as an elevated transpulmonary gradient (>12 mmHg) and elevated PVR (>3 Wood units). Pathologically, this process is marked by pulmonary arteriolar remodeling with intimal fibrosis and medial hyperplasia akin to that seen in PAH.
Pulmonary Hypertension Associated with Lung Disease Intrinsic lung disease is the second most common cause of PH, although its actual prevalence is difficult to ascertain. PH has been observed in both chronic obstructive lung disease and interstitial lung disease. It can also be seen in diseases with mixed obstructive/restrictive physiology: bronchiectasis, cystic fibrosis, mixed obstructive restrictive disease marked by fibrosis in the lower lung zones, and emphysema predominantly in the upper lung zones. As in patients with left heart disease, PH associated with chronic lung disease is usually modest; however, some of these patients appear to have PH “out of proportion” to their parenchymal lung disease, suggesting intrinsic pulmonary arterial disease. These patients typically have more severe PH, with results of pulmonary function tests demonstrating a very low DLCO.
Although PH is described in most forms of interstitial lung disease, it has been most extensively studied in idiopathic pulmonary fibrosis; however, the individual studies have been small. Early echocardiographic data suggested that the prevalence of PH in interstitial lung diseases was high, but invasive hemodynamic monitoring suggests that the incidence is considerably lower than originally believed. The diagnosis of PH portends poor outcome in pulmonary fibrosis.
Also included in Group III PH is sleep-disordered breathing. Sleep apnea has long been associated with PH. However, PH associated with sleep-disordered breathing is generally mild.
Pulmonary Hypertension Associated with Chronic Thromboembolic Disease The development of PH after chronic thromboembolic obstruction of the pulmonary arteries is well described, but its incidence is not known. The incidence of PH after a single pulmonary embolic event is thought to be quite low and likely increases following recurrent embolism. The risk factors for developing CTEPH are unclear. Many patients have no history of clinical venous thromboembolism. The pathogenesis of CTEPH is poorly understood. Obstruction of the proximal pulmonary vasculature is important and often the dominant factor; however, additional pulmonary vascular remodeling occurs. Approximately 10–15% of patients will develop a disease very similar clinically and pathologically to PAH after resection of the proximal thrombus.
OTHER DISORDERS AFFECTING THE PULMONARY VASCULATURE
Sarcoidosis Patients with sarcoidosis can develop PH as a result of lung involvement. Consequently, patients with sarcoidosis who present with progressive dyspnea and PH require a thorough evaluation. Although the majority of sarcoidosis patients with PH generally do not respond to therapy for PAH, a subset of patients with sarcoidosis and severe PH do have a beneficial response to therapy.
Sickle Cell Disease Cardiovascular system abnormalities are prominent in the clinical spectrum of sickle cell disease, including PH. The etiology is multifactorial, including hemolysis, hypoxemia, thromboembolism, chronic high CO, and chronic liver disease. The presence of PH in patients with sickle cell disease is rare.
Schistosomiasis Globally, schistosomiasis is one of the most common causes of PH. The development of PH occurs in the setting of hepatosplenic disease and portal hypertension. Studies suggest that inflammation from the infection triggers the pulmonary vascular changes that occur. The diagnosis is confirmed by finding the parasite ova in the urine or stool of patients with symptoms, which can be difficult. The efficacy of therapies directed toward PH in these patients is unknown.
PHARMACOLOGIC TREATMENT OF PAH
PH was a consistently fatal condition with no effective medical treatment options before 1996; however, since that time, there has been an upsurge in the development of novel therapeutic agents for PAH. There are several approved agents for PAH, including prostacyclin and prostacyclin analogues, phosphodiesterase-5 inhibitors, a soluble guanylyl cyclase stimulator, and endothelin receptor antagonists, that have improved the outlook dramatically. Although there is no cure for PAH, current pharmacologic therapies improve morbidity and, in some cases, mortality.
PROSTANOIDS
In PAH, endothelial dysfunction and platelet activation cause an imbalance of arachidonic acid metabolites with reduced prostacyclin levels and increased thromboxane A2 production. Prostacyclin (PGI2) activates cyclic adenosine monophosphate (cAMP)-dependent pathways that mediate vasodilation. PGI2 also has antiproliferative effects on vascular smooth muscle and inhibits platelet aggregation. Protein levels of prostacyclin synthase are decreased in pulmonary arteries of patients with PAH. This imbalance of mediators is addressed by the exogenous administration of prostanoids as therapy in advanced PAH.
Epoprostenol was the first prostanoid available for the management of PAH. Epoprostenol delivered as a continuous intravenous infusion improves functional capacity and survival in PAH. The efficacy of epoprostenol in WHO functional class 3 and 4 PAH patients was demonstrated in a clinical trial that showed improved quality of life, mPAP, PVR, 6-minute walk distance (6MWD), and mortality. Treprostinil has a longer half-life than epoprostenol (~4 h vs ~6 min), which allows for continuous subcutaneous and intravenous administration. Treprostinil has been shown to improve pulmonary hemodynamics, symptoms, exercise capacity, and survival in PAH.
Inhaled prostacyclins provide the beneficial effects of infused prostacyclin therapy without the inconvenience and side effects (risk of infection and infusion site reactions) of infusion catheters. Both inhaled iloprost and treprostinil have been approved for patients with WHO class 3 and 4 PAH. The main advantage of treprostinil is less frequent administration. Inhaled formulations can be efficacious in moderately symptomatic patients with PAH and may be appropriate when used in combination with an oral medication. Phosphodiesterase-5 (PDE5) inhibitors (e.g., sildenafil) increase cyclic guanosine monophosphate (cGMP) levels and activate cGMP-dependent signaling pathways that also mediate vasodilation and platelet inhibition. Thus, the addition of a PDE5 inhibitor augments the pulmonary hemodynamic and functional capacity benefits of prostanoids in PAH.
Endothelin Receptor Antagonists Endothelin receptor antagonists (ERAs) target endothelin-1 (ET-1), a potent endogenous vasoconstrictor and vascular smooth muscle mitogen that is elevated in PAH patients. Endothelin levels are increased coincident with increased PVR and mPAP and decreased CO and 6MWD.
ERAs block the binding of ET-1 to either endothelin receptor A (ET-A) and/or B (ET-B). ET-A receptors found on pulmonary artery smooth muscle cells mediate vasoconstriction. In the normal pulmonary vasculature, ET-B receptors are found on endothelial cells and mediate vasodilation via production of prostacyclin and nitric oxide as well as ET-1 clearance. Three ERAs approved for use in the United States are bosentan and macitentan both, nonselective receptor antagonists, and ambrisentan, a selective ET-A receptor antagonist.
Studies have shown that both bosentan and macitentan improve hemodynamics and exercise capacity and delay clinical worsening. The randomized, placebo-controlled, phase III Bosentan Randomized Trial of Endothelin Antagonist Therapy (BREATHE)-1 comparing bosentan with placebo demonstrated improved symptoms, 6MWD, and WHO functional class. The Endothelin Antagonist Trial in Mildly Symptomatic Pulmonary Arterial Hypertension Patients (EARLY) comparing bosentan with placebo demonstrated improved PVR and 6MWD.
Several studies, including the phase III, placebo-controlled Ambrisentan in Pulmonary Arterial Hypertension-1 (ARIES-1) trial, suggest that ambrisentan improves exercise tolerance, WHO functional class, hemodynamics, and quality of life in patients with PAH. There are no trial data to evaluate whether the selective ET-A receptor antagonism of ambrisentan has any advantage over the nonselective ET receptor antagonism of bosentan.
Phosphodiesterase Type-5 Inhibitors Nitric oxide derived from endothelial cells activates guanylyl cyclase, which, in turn, generates cGMP in vascular smooth muscle cells and platelets. cGMP is a second messenger that induces vasodilation through relaxation of the arterial smooth muscle cells and inhibits platelet activation. PDE5 enzymes metabolize cGMP. Therefore, cGMP PDE5 inhibitors prolong the vasodilatory effect of nitric oxide, especially within the pulmonary arterial bed where high concentrations of cGMP are found. There are currently two PDE5 inhibitors used for the treatment of PAH, sildenafil and tadalafil. Both agents have been shown to improve hemodynamics and 6MWD. Recently, the oral soluble guanylyl cyclase stimulator, riociguat, was approved for the treatment of both PAH and CTEPH.
Unmet and Future Research Needs in Pulmonary Hypertension Presently there are only three classes of therapy for patients with PAH, and even with therapy, the median survival for a person with PAH is only 5–6 years (Table 304-2). Although there are five subtypes of PH, current approved therapies only address one subtype. Not only do we need to expand the treatment options for patients with PAH, we also need to develop effective therapies for all patients with PH. Limited survival is, in part, a result of delay in diagnosis. Improved awareness among clinicians and patients could lead to more timely diagnosis that will affect the response to therapy and survival. PH needs to be diagnosed in a timely manner so that therapy can be initiated as soon as possible. Patients should also have the option of referral to a specialty center that focuses on treatment of patients with pulmonary vascular disease, which will ensure their access to state-of-the-art care and a multidisciplinary approach to care. Finally, there need to be continued efforts at developing new therapies that target the increasingly complex and overlapping pathways involved in the various forms of PH.
|
FDA-APPROVED THERAPIES FOR THE TREATMENT OF PAH |