Chapter 49 Chronic Pancreatitis, Stones, and Strictures
![]() Video related to this chapter’s topics: Pancreatic Duct Stone Extraction
Video related to this chapter’s topics: Pancreatic Duct Stone Extraction
Introduction
Chronic pancreatitis is an inflammatory condition that results in permanent structural changes in the pancreas, which can lead to impairment of exocrine and endocrine function.1 This disorder contrasts with acute pancreatitis in that the latter is nonprogressive, and the gland returns to histologic and functional normalcy once the acute event subsides. Most diagnostic and therapeutic efforts in chronic pancreatitis are directed toward evaluation and management of symptoms, primarily abdominal pain and steatorrhea. Although interpretation of data on the role of endoscopic therapy for management of chronic pancreatitis remains difficult, this area is rapidly expanding and is of great interest and challenge to gastrointestinal endoscopists.
Epidemiology
The incidence of chronic pancreatitis is in the range of 3 to 10 per 100,000 population in many parts of the world.2 The crude incidence rate for chronic pancreatitis per 100,000 population in Germany is 6.4; in Czech Republic, 7.9; and in Japan, 27.9.3–5 The peak incidence for chronic pancreatitis in Germany is in the age group 45 to 54 years, which is 10 years older than the peak age group for acute pancreatitis suggesting that chronic pancreatitis develops during this time frame following first attacks of acute pancreatitis. In a prospective study that evaluated patients with alcoholic chronic pancreatitis, an annual incidence of 8.2 cases per year per 100,000 population and an overall prevalence of 27.4 cases per year per 100,000 population were noted.6 The incidence rates in retrospective European and North American studies range from 2 to 10 per 100,000 cases per year.7,8 Chronic pancreatitis is more common in male patients3,5,6; the male-to-female ratio in Japan is 3.5.5 Compared with whites, blacks are two to three times more likely to be hospitalized for chronic pancreatitis than for alcoholic cirrhosis.9 The explanation for this observation is unclear but could be related to racial differences in diet, type or quantity of alcohol consumption, smoking, or ability to detoxify substances harmful to the liver or pancreas. The absence of any screening programs and unresolved debate on the “gold standard” for diagnosis of chronic pancreatitis make epidemiologic studies in this area more difficult and explain the wide range of variations noted among studies.
Pathophysiology
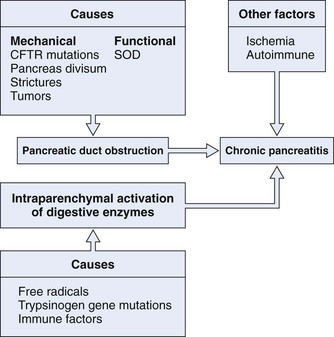
The pathogenesis of chronic pancreatitis seems to be multifactorial and is probably initiated by two distinct events (Fig. 49.1). The first event is a decrease in bicarbonate secretion that is due to either a mechanical or a functional ductal obstruction. Mechanical causes include strictures, sphincter of Oddi dysfunction, and tumors. Functional causes include mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene leading to impaired bicarbonate secretion. This impaired bicarbonate secretion has formed the basis for the secretin pancreatic function test. The second event involves intraparenchymal activation of digestive enzymes within the pancreatic gland. Ischemia, antioxidant stress, and sphincter of Oddi dysfunction are possible later events involved in perpetuating the disease process. This multifactorial model provides an explanation as to why no single therapy works in all patients with chronic pancreatitis.
Pancreatic Duct Obstruction
Proteinaceous plugs are one of the earliest findings noted in patients with chronic pancreatitis.10 It is theorized that increased glandular secretion of pancreatic proteins causes precipitation of proteinaceous plugs within the pancreatic ductal system. These plugs may act as a nidus for calcification that leads to stone formation. As ductal obstruction progresses, inflammatory changes and cell loss occur. The importance of these proteinaceous plugs in perpetuating changes within the pancreas is emphasized by studies that have shown relief of clinical symptoms after endoscopic removal of these plugs in patients with chronic pancreatitis.11,12 GP2 is a glycosyl phosphatidylinositol anchored protein that is cleaved from the zymogen granules and secreted into the pancreatic juice. This protein has been identified as a major component of intraductal plugs.13 Pancreatic acinar cells release another protein called lithostatine that prevents calcium carbonate precipitation and stone formation in pancreatic juice.14 Low levels of these proteins in patients with chronic pancreatitis may be another factor involved in stone formation.
Ischemia
Ischemia may be another important event in the pathogenesis of chronic pancreatitis. Animal models have shown that partial pancreatic duct ligation induces ductal hypertension and increased resistance to blood flow within the pancreas.15,16 Blood flow was found to be 40% of that observed in controls. Secretory stimulus reduced blood flow further as opposed to the normal increase. In patients with chronic pancreatitis, pancreatic interstitial pressure increases to a greater degree than in normal individuals owing to decreased glandular elasticity. The rapid relief of symptoms achieved by ductal decompression procedures suggests that ischemia plays a central role in the complex mechanisms involved in chronic pancreatitis.
Antioxidants
Nutritional depletion is frequently seen in patients with chronic pancreatitis. In particular, antioxidants such as selenium, vitamin C and E, and methionine are depleted.17,18 An imbalance between a decrease in antioxidants and an increased demand for them in pancreatic cells in chronic pancreatitis may lead to elevation in free radical formation, which is associated with lipid peroxidation and cellular impairment. Increased membrane lipid peroxidation, a marker of oxidative stress and free radical production, can also be seen in alcoholic chronic pancreatitis.19 It is postulated that alcohol causes a disproportionate increase in the secretion of trypsinogen leading to premature activation of digestive enzymes within the acinar or ductal cell systems.20
Autoimmunity
Chronic pancreatitis is also seen in association with autoimmune disorders such as Sjögren’s syndrome and primary biliary cirrhosis.21,22 Autoantibody to pancreatic antigens has been shown in patients with Sjögren’s syndrome and idiopathic chronic pancreatitis. Some cases of idiopathic chronic pancreatitis are associated with the expression of novel HLA-DR antigens on duct cells in combination with a localized T-cell inflammatory infiltrate, lending further credibility to an autoimmune pathogenesis.23
Interstitial Fibrosis
It is proposed that repeated episodes of acute pancreatitis initiate a sequence of perilobular fibrosis, duct distortion, and altered secretion and flow of pancreatic juice.24 Studies on the natural history of pancreatitis have shown that more frequent and more repeated attacks of acute pancreatitis lead to chronic changes as seen in the alcoholic type of chronic pancreatitis.25 The exact mechanisms involved in the pathophysiology of chronic pancreatitis are still elusive and unproven. Multiple exogenous factors may act in a genetically predisposed patient in an appropriate clinical setting, such as alcohol consumption, to trigger a cascade of events culminating in progressive destruction of pancreatic parenchyma and ensuing sequelae. Although the focus of therapies has been the inhibition of acinar cell secretion, further insights into the role of ductal bicarbonate secretion, ductal obstruction, and the relative contribution of ischemia and oxygen-derived free radicals in this process may provide new therapeutic avenues.
Etiology
Alcohol
Alcohol abuse accounts for 70% to 80% of cases of chronic pancreatitis (Table 49.1); the mechanism by which this occurs is unclear. The risk seems to be related to the duration and amount of alcohol consumed rather than the type of alcohol or the pattern of consumption.26 Intake of large amounts of alcohol (>50 g/day) has been shown to be associated with a shortened time to pancreatic calcification and survival.27 There is considerable variation in individual sensitivity to the toxicity of alcohol making it difficult to define a “safe” level of consumption. Only 5% to 10% of alcoholics develop chronic pancreatitis, suggesting that other unidentified factors may be important in the pathogenesis of the disease.28 Tobacco, although not important in the pathogenesis of alcoholic pancreatitis,29,30 has been implicated in the development of calcification in patients who already have chronic pancreatitis.31 A U.S.-based study showed an association between the presence of calcium-sensing receptor gene polymorphisms and chronic pancreatitis.32 The risk for developing chronic pancreatitis was significantly higher in patients with these gene polymorphisms who consumed moderate to heavy amounts of alcohol.
| Alcohol | 70% |
| Idiopathic | 10%–30% |
| Other | 10%–15% |
| Pancreatic duct obstruction (trauma, divisum, tumor, fibrosis) | |
| Hereditary (CFTR gene mutation, trypsinogen gene mutation) | |
| Hyperlipidemia | |
| Tropical |
CFTR, cystic fibrosis transmembrane conductance regulator.
Hereditary Pancreatitis
Hereditary pancreatitis is characterized by a young age at onset and prominent pancreatic calcifications. It is transmitted as an autosomal dominant trait, and nearly 80% of patients with the inherited defect develop chronic pancreatitis.33 Most affected individuals develop symptoms before age 20. In some kindreds, hereditary chronic pancreatitis has been mapped to the long arm of chromosome 7 (7q35), where a cluster of trypsinogen genes is located.34,35 Several mutations associated with chronic pancreatitis have been identified in this region. Although the exact consequences of these mutations on trypsin activity are unclear, they are known to interfere with trypsin inactivation or to enhance its activation, permitting autodigestion of the pancreas.36,37 Although mutations in the trypsinogen gene are specific for hereditary pancreatitis, not all affected family members develop chronic pancreatitis. The relationship between mutations of other genes associated with chronic pancreatitis, such as the CFTR and the trypsinogen genes, needs further elucidation.
Mutations of the Cystic Fibrosis Gene
Cystic fibrosis is due to mutations in the CFTR gene. Most patients with cystic fibrosis develop progressive pancreatic damage as a result of defective ductular and acinar pancreatic secretion.38 In some series, mutations in the CFTR gene have been identified in 13% to 37% of patients with idiopathic chronic pancreatitis who have no clinical evidence of cystic fibrosis.39,40 This percentage range could be an underestimation because currently available genetic screening tests identify only 18 to 23 of the most severe CFTR mutations that cause classic, childhood cystic fibrosis.
Tropical Pancreatitis
Tropical pancreatitis is a condition of unknown etiology that is seen commonly in younger individuals in south India and other parts of the tropics, where it is the most common cause of chronic pancreatitis. The pathology is characterized by large intraductal calculi, marked dilation of the pancreatic ducts, atrophy, and fibrosis. Clinically, most patients experience abdominal pain, diabetes mellitus, and fat malabsorption. The etiology of tropical pancreatitis is unknown. The cassava fruit had been implicated as an etiologic factor in this disorder, although it is no longer thought to be related.41 Mutations in the serine protease inhibitor SPINK1 have been identified in some patients.42,43
Ductal Obstruction
Obstruction of the pancreatic duct from any cause can lead to chronic pancreatitis. The histologic abnormalities that are induced may persist after relief of the obstruction. Sphincter of Oddi dysfunction seems to be associated with chronic pancreatitis. In one study of patients with chronic pancreatitis undergoing sphincter of Oddi manometry, more than 60% had sphincter of Oddi dysfunction.44
Pancreas divisum may cause chronic pancreatitis by producing a relative obstruction to flow of pancreatic juice at the minor papilla. It is estimated that less than 5% of patients with pancreas divisum develop pancreatic symptoms. The low frequency of symptoms has created controversy as to whether pancreas divisum and its associated small minor papilla orifice are ever a cause of obstructive pancreatitis. The arguments against an association are based on two major observations. First, some studies have found that the incidence of pancreas divisum is the same among patients with and without pancreatitis.45 Second, symptoms occur infrequently in patients with this anomaly. We believe that there is a group of patients with pancreas divisum who are subject to recurrent bouts of seemingly idiopathic pancreatitis. In these patients, the minor papilla orifice is so small that excessively high intrapancreatic dorsal ductal pressure occurs during active secretion, which may result in inadequate drainage, ductal distention, pain, and, in some cases, pancreatitis. To support this view, greater than 60% of patients with pancreas divisum and otherwise unexplained abdominal pain had relief of pain after surgical sphincteroplasty suggesting that obstruction to flow of secretion was the proximate cause of symptoms in these patients.46
Idiopathic Chronic Pancreatitis
An etiology for pancreatitis cannot be determined in 10% to 30% of patients with chronic pancreatitis despite extensive investigations. Concealed alcohol ingestion, hypersensitivity to small amounts of alcohol, unreported pancreatic trauma, and mutations in the CFTR and the trypsinogen genes may be contributing factors in at least a small proportion of patients with idiopathic chronic pancreatitis.47,48 Although in the past patients with idiopathic chronic pancreatitis were considered as a single group, data from the Mayo Clinic have defined an early and late onset form of idiopathic chronic pancreatitis.49 Age distribution at onset of symptoms showed a bimodal distribution of patients with early and late onset idiopathic chronic pancreatitis with a median age of 19.2 years for early onset and 56.2 years for late onset. No gender differences were observed among patients in either group. Pain was the predominant symptom in 96% of patients with early onset idiopathic pancreatitis but was present in only 54% of late onset idiopathic pancreatitis. Regardless of whether patients had early or late onset idiopathic pancreatitis, pain was the presenting symptom, and endocrine and exocrine insufficiency with pancreatic calcification was seen in both forms of the disease.
Clinical Features
Abdominal Pain
The mechanism for abdominal pain is poorly understood. Causes are perhaps multifactorial and include inflammation, duct obstruction, high pancreatic tissue pressure, fibrotic encasement of sensory nerves, and neuropathy characterized by both increased numbers and sizes of intrapancreatic sensory nerves and by inflammatory injury to the nerve sheaths allowing exposure of the neural elements to toxic substances.50,51 Pain is not in the spectrum of clinical symptoms in nearly one-fourth of patients with chronic pancreatitis.52 The view that chronic pain subsides in a substantial number of patients as the disease progresses to the point of organ failure53 has been widely accepted, but that process may take an unpredictable number of years or may never occur. Some studies suggest that the likelihood of spontaneous pain relief is low.54 In a study that evaluated the natural history of pain in chronic pancreatitis, pain decreased or disappeared in 67%, 64%, and 77% of early onset idiopathic, late onset idiopathic, and alcoholic pancreatitis over a median time of 25 years, 13 years, and 14 years.49
Pancreatic Insufficiency
Patients with severe pancreatic exocrine dysfunction cannot properly digest complex foods or absorb digestive breakdown products. Nevertheless, clinically significant protein and fat deficiencies do not occur until more than 90% of pancreatic function is lost.55 In a large natural history study, the median time for development of pancreatic insufficiency was 13.1 years in patients with alcoholic chronic pancreatitis, 16.1 years in patients with late onset idiopathic chronic pancreatitis, and 26.3 years in patients with early onset idiopathic chronic pancreatitis.48 Steatorrhea usually occurs before protein deficiencies because lipolytic activity decreases more quickly than proteolysis.56,57 Glucose intolerance occurs frequently in chronic pancreatitis, but overt diabetes mellitus usually occurs late in the course of disease. Patients with chronic calcific disease, particularly patients who develop early calcifications, may develop diabetes more frequently than patients with chronic noncalcific disease.58,59 Nearly 40% to 70% of patients with chronic pancreatitis develop diabetes on prolonged follow-up. In one study, the median time to develop diabetes was 19.8 years, 11.9 years, and 26.3 years in patients with alcoholic, late onset idiopathic, and early onset idiopathic chronic pancreatitis.49 In chronic pancreatitis, both insulin-producing beta cells and glucagon-producing alpha cells are destroyed. When exogenously administered insulin leads to hypoglycemia, the deficiency in glandular glucagon storage fails to correct the serum glucose levels back to normal leading to prolonged and severe hypoglycemia. The nature of diabetes in this patient population is brittle, and management is more complicated than that of patients with type 1 diabetes.
Pathology
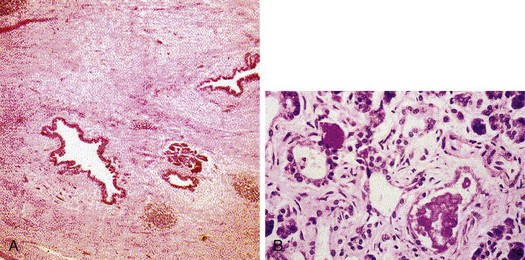
In early stages of chronic pancreatitis, glandular damage is patchy and uneven (Fig. 49.2). Areas of irregularly distributed fibrosis, reduced number and size of acini with relative sparing of the islets of Langerhans, and variable degrees of obstruction of pancreatic ducts of all sizes are seen.60 A chronic inflammatory infiltrate around lobules and ducts is usually present. The interlobular and intralobular ducts are dilated and contain protein plugs in their lumens. The ductal epithelium may be atrophied or hyperplastic or show squamous metaplasia, and ductal concretions may be evident. Remaining islets become embedded in sclerosed tissue or severely damaged lobules before they too disappear. Grossly, the gland is hard, sometimes with extremely dilated ducts and grossly visible calcified concretions. Pseudocyst formation is common.
Differential Diagnosis
The International Pancreatitis Study Group observed a standardized incidence ratio for pancreatic cancer of 26.3 among patients with chronic pancreatitis compared with an expected ratio of 2.13 that was calculated from country-specific incidence data and adjusted for age and sex.61 Similar to chronic pancreatitis, patients with pancreatic cancer can present with abdominal pain, weight loss, and jaundice. Findings suggestive of possible pancreatic cancer in a patient thought or known to have chronic pancreatitis include older age, absence of a history of alcohol use, weight loss, a protracted flare of symptoms, and the onset of significant constitutional symptoms. The physician should maintain a high index of suspicion for pancreatic cancer, particularly in any elderly patient presenting with a new-onset pancreatitis when common causes such as alcohol and gallstones have been excluded. Tumor markers such as CA 19.9 (for colorectal and pancreatic carcinomas) and carcinoembryonic antigen (CEA) are helpful if elevated, but normal values do not rule out pancreatic cancer. Computed tomography (CT) or endoscopic ultrasound (EUS)–guided biopsy may be required to establish the diagnosis in some patients.
Diagnosis
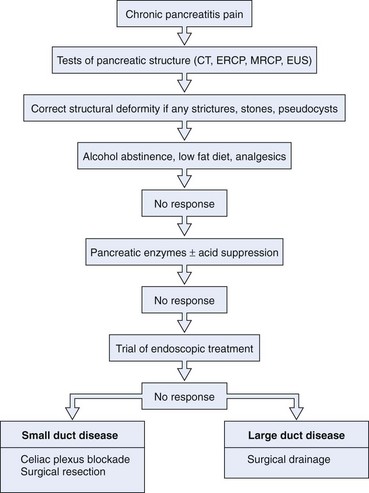
Tests for chronic pancreatitis can be classified into tests that evaluate the structure of the gland (parenchyma, ductal anatomy, or both) or its exocrine function (Table 49.2). The tests most widely used clinically are tests that assess structure. The clinical manifestations of pancreatic insufficiency are usually a late event in the course of chronic pancreatitis when more than 90% of the glandular tissue is not functioning either because of glandular dysfunction or because of fibrotic tissue replacement or proximal pancreatic duct obstruction. The most sensitive and accurate among pancreatic function tests is the secretin stimulation test. However, this test is invasive, its methodology is very time-consuming and demanding, and its diagnostic accuracy is not superior to endoscopic retrograde cholangiopancreatography (ERCP).62,63 Noninvasive pancreatic function tests yield sufficient diagnostic accuracy only in the advanced stages of the disease, and their sensitivity for detection of early or moderate chronic pancreatitis is low.64 Tests that evaluate pancreatic structure, although limited by sensitivity, are advantageous in that they are more widely available and are better standardized for clinical use.
| Structural Tests | Functional Tests | |
|---|---|---|
| Indirect | Direct | |
| X-ray | Serum enzymes (trypsinogen) | Secretin stimulation test |
| Ultrasound | Fecal tests (fat, elastase, chymoptrysin) | |
| CT | Urine tests (bentiromide, pancreolauryl) | |
| MRCP | ||
| ERCP | ||
| EUS | ||
CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; MRCP, magnetic resonance cholangiopancreatography.
Tests of Pancreatic Function
Invasive or Direct Pancreatic Function Tests (Secretin Stimulation Test)
Some studies have shown the secretin stimulation test to be slightly more sensitive than ERCP for the diagnosis of chronic pancreatitis, but the evaluation of all tests in the diagnosis of chronic pancreatitis is suspect because of the lack of a “gold standard.” Sensitivity ranges from 74% to 97%, and specificity ranges from 80% to 90%.63,65–69 The percentage of patients with an abnormal stimulation test and a normal pancreatogram ranges from 3% to 20%.66–69 When such patients were followed, two studies found that 90% of patients developed chronic pancreatitis.69,70 Conversely, these studies also identified a small group of patients (<10% on average) with a normal hormonal stimulation test but an abnormal pancreatogram. On long-term follow-up, chronic pancreatitis developed in 0% to 26% of patients.69,70 When the results of pancreatic function tests were compared with pancreatic histology, the overall sensitivity, specificity, and accuracy were 67%, 90%, and 81%.71 Limitations of this test are that it is not well accepted by patients, it is time-consuming and expensive, and it requires specialized equipment and methodology. The test has not been well standardized, and a consensus on the normal ranges for the test results is yet to be reached. The test is available in very few specialized pancreatic centers around the world.
Noninvasive or Indirect Pancreatic Function Tests
Serum Enzymes
Because chronic pancreatitis is a patchy, focal disease with significant parenchymal fibrosis, pancreatic serum enzyme levels (amylase and lipase) are not or only minimally elevated. Very low levels of serum trypsinogen (<20 ng/mL) are reasonably specific for chronic pancreatitis, but levels as low as this are seen only in very advanced stages of the disease where there is accompanying steatorrhea.55 In clinical practice, serum enzyme levels are helpful to identify an acute attack of chronic pancreatitis or for monitoring the disease evolution with abnormally low concentration once steatorrhea occurs.
Fecal Tests
Steatorrhea can be diagnosed qualitatively by Sudan staining of feces or quantitatively by determination of fecal fat excretion over 72 hours while the patient is consuming a 100 g/day fat diet for at least 3 days before the test. Excretion of more than 7 g of fat per day is diagnostic of malabsorption, although patients with steatorrhea often have values greater than 20 g/day. On qualitative analysis, more than six globules per high-power field is considered to be positive, but the patient must be ingesting adequate fat to allow measurable steatorrhea. In a landmark study on exocrine insufficiency, steatorrhea did not occur until more than 90% of the pancreas or more than 85% of pancreatic lipase had been destroyed.55 Stool fat analysis has limited sensitivity in chronic pancreatitis because patients with mild and moderate and often severe chronic pancreatitis in the absence of steatorrhea would not be detected by this technique. A novel method, near-infrared reflectance analysis (NIRA), may become the procedure of choice for evaluating fat malabsorption.72–74 NIRA is equally accurate but less time-consuming than a 72-hour fecal fat collection and allows for simultaneous measurement of fecal fat, nitrogen, and carbohydrates in a single sample. NIRA is being increasingly used in Europe and is available in some centers in the United States.
The low diagnostic value of fat malabsorption in chronic pancreatitis led to the discovery of individual pancreatic enzymes in stool specimen that have increased diagnostic sensitivity. Measurement of fecal chymotrypsin is abnormal in most patients with chronic pancreatitis and steatorrhea.65 Since inception of fecal chymotrypsin measurement into clinical use, its utility has been clearly established only in advanced chronic pancreatitis with exocrine insufficiency. This assay is unavailable in the United States at the present time. More recently, an assay to measure human pancreatic elastase in feces has been developed. The assay detects exclusively human elastase, and so no interference occurs with simultaneous therapeutic pancreatic enzyme supplementation. The diagnostic sensitivity and specificity of the fecal elastase test are superior to the fecal chymotrypsin test, although both tests are most accurate in advanced chronic pancreatitis.75 The fecal elastase test may be falsely abnormal in other diseases causing steatorrhea, such as short bowel syndrome or small bowel bacterial overgrowth syndrome. This test is available but not widely used in the United States.
Urine Tests
The principle of urine tests is based on the administration of a complex substrate, which is hydrolyzed by a specific pancreatic enzyme with the release of a pancreatic marker substance. This marker is absorbed from the gut and detected and quantitated either in urine or in serum. The bentiromide (N-benzoyl-l-tyrosyl-p-aminobenzoic acid [NBT-PABA]) test measures the presence of pancreatic chymotrypsin within the gut lumen, and the pancreolauryl test measures the presence of pancreatic arylesterases within the gut lumen. Both tests are accurate in advanced chronic pancreatitis, with sensitivities of 80% to 100%.65 Limitations of these tests lie in the fact that both tests require anatomic and functional integrity of the digestive systems. Although these tests have reasonable accuracy, they are both unavailable for clinical use in the United States at the present time.
Tests of Pancreatic Structure
Plain Abdominal Radiography
Calcifications within the pancreas are present on plain films in about one-third of patients with chronic pancreatitis (Fig. 49.3). Calcifications occur late in the natural history of chronic pancreatitis and may take 5 to 25 years to develop.49,53 Both anteroposterior and oblique views should be employed because small flecks of calcium can be lost in the spine if oblique views are not obtained. The finding of calcification is pathognomonic of chronic pancreatitis, but the sensitivity of this test is very low.
Abdominal Ultrasound
Ultrasound was the first technique that allowed complete imaging of the pancreas. Several morphologic characteristics of chronic pancreatitis are detectable by ultrasound, including irregular contours in the margin of the gland, dilation and irregularity of the main pancreatic duct, heterogeneity of the gland parenchyma, cysts within or adjacent to the pancreas, and the presence of calcifications.76 The sensitivity and specificity of ultrasound for the diagnosis of chronic pancreatitis are 60% to 70% and 80% to 90%.77 However, the detail to which the pancreas can be interrogated depends on the body habitus of the patient, the presence or absence of overlying bowel gas, and the experience and expertise of the sonographer.
Computed Tomography
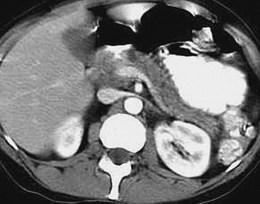
The sensitivity and specificity of CT for the diagnosis of chronic pancreatitis are 75% to 90% and 85%.65 The main advantage of CT is that it can be standardized and in virtually all cases can visualize the pancreas in its entirety. CT scan is the most sensitive test for detecting calcification, is accurate in detecting main pancreatic duct dilation, and can detect an irregular contour of the gland (Fig. 49.4).65,78,79 These features are characteristics of advanced chronic pancreatitis, and CT is quite good at detecting these changes. However, CT is poor at detecting subtle abnormalities in pancreatic parenchyma or changes in side branches of the pancreatic duct, which are commonly seen in milder forms of the disease. CT has good specificity but lacks sensitivity for diagnosis of chronic pancreatitis. The newer spiral CT scanners would be likely to produce better sensitivity.
Magnetic Resonance Cholangiopancreatography
Several small studies have reported on the utility of magnetic resonance cholangiopancreatography (MRCP) in assessing pancreatic duct morphology.80,81 MRCP agrees with ERCP in 70% to 80% of findings, with higher rates of agreement in studies using the most advanced image analysis techniques (Fig. 49.5). In studies that compared MRCP findings with ERCP, MRCP visualized the main pancreatic duct in the head, body, and tail in 79%, 64%, and 53% of cases.82 Correlation with ERCP with respect to main pancreatic duct dilation, narrowing, and filling defects was 83% to 92%, 70% to 92%, and 92% to 100%. The major disadvantage of MRCP compared with conventional cholangiography is a lower spatial resolution, such that MRCP continues to be partially limited in the assessment of fine detail, such as subtle side branch changes of chronic pancreatitis. Improvements in magnetic resonance (MR) imaging analysis are expected to continue to improve the image quality of MRCP, and image quality could approach ERCP in accuracy in the future.
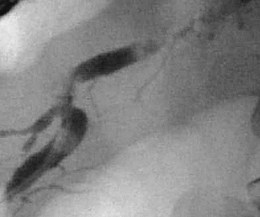
Endoscopic Retrograde Cholangiopancreatography
ERCP is the most widely used structural test for chronic pancreatitis (Fig. 49.6). In 1984, the Pancreatic Society of Great Britain and Ireland reached a consensus on ductographic definitions for chronic pancreatitis, known as “the Cambridge criteria,” which have become the most widely accepted criteria for interpreting pancreatography.83 The criteria are based on abnormalities seen in the main pancreatic duct and side branches (Table 49.3). In most studies, the sensitivity of ERCP is 70% to 90%, and specificity is 80% to 100%.63,65–69 ERCP is highly sensitive and specific in patients with advanced structural disease; less dramatic pancreatographic changes are less definitive.84,85 The ductographic abnormalities of chronic pancreatitis are not specific; age-related changes, morphologic changes such as in pancreatic cancer or recovery phase of acute pancreatitis, and injury induced by stent treatment can mimic chronic pancreatitis.
Table 49.3 Cambridge Grading of Chronic Pancreatitis by Endoscopic Retrograde Pancreatography
| Grade | Main Pancreatic Duct | Side Branches |
|---|---|---|
| Normal | Normal | Normal |
| Equivocal | Normal | <3 abnormal |
| Mild | Normal | >3 abnormal |
| Moderate | Abnormal | >3 abnormal |
| Severe | Abnormal, with at least one of the following | >3 abnormal |
| Large cavity (>10 mm) | ||
| Duct obstruction | ||
| Intraductal filling defects | ||
| Severe dilation or irregularity |
Adapted from Axon AT, Classen M, Cotton PB, et al: Pancreatography in chronic pancreatitis: International definitions. Gut 25:1107, 1984.
Chronic pancreatitis can involve the pancreatic parenchyma per se and completely spare the radiographically visible portions of the pancreatic ductal system leading to false-negative studies.86,87 Also, significant interobserver and intraobserver variability is noted in the interpretation of pancreatography.88 Much of this variability is related to interpretation of mild pancreatographic changes rather than to severe abnormalities. There are several limitations with ERCP as a diagnostic test for chronic pancreatitis. Reliable interpretation of ERCP depends on adequate filling of the pancreatic duct with dye such that the secondary branches are well visualized. However, inadequate opacification of ducts, especially the secondary ducts, occurs in at least 30% of cases.84 The procedure is invasive and is associated with a 3% to 7% chance of causing acute pancreatitis.89 This risk is low in patients with advanced disease and high in patients with mild disease, particularly patients with underlying sphincter of Oddi dysfunction.90
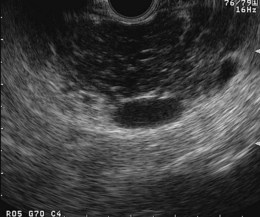
Endoscopic Ultrasound
Numerous EUS criteria for pancreatic disease have been described (Fig. 49.7). Lees and colleagues91,92 first described EUS findings in patients with clinical and radiologic evidence of chronic pancreatitis and characterized EUS criteria that distinguish normal from abnormal pancreas. Wiersema and associates93 refined their definitions and found that abnormal EUS changes occurred frequently in patients with abnormal endoscopic pancreatograms and were absent in healthy volunteers. Criteria for chronic pancreatitis that are specific to EUS can be divided into two groups (Table 49.4): parenchymal and ductal. Parenchymal criteria include inhomogeneity, hyperechoic foci, hyperechoic strands, cysts, and lobularity. Ductal criteria specific to EUS include obvious to more subtle ductal dilation (≥3 mm in the head, ≥2 mm in the body, ≥1 mm in the tail), hyperechoic main duct margins, irregular main duct margins, and visible side branches.
Table 49.4 Endoscopic Ultrasound Criteria for Chronic Pancreatitis
| Parenchymal Changes | Ductal Changes |
|---|---|
| Inhomogeneity | Ductal dilation |
| Hyperechoic foci | Hyperechoic main duct margins |
| Hyperechoic strands | Irregular main duct margins |
| Lobularity | Visible side branches |
| Pseudocysts |
Several studies show that the increasing EUS abnormalities correlate with the severity of pancreatographic changes94–97 and with reductions in secretin-stimulated duodenal bicarbonate.98 A quantitative analysis94 with nine possible criteria (hyperechoic foci, hyperechoic strands, lobularity, ductal dilation, ductal irregularity, hyperechoic duct margins, visible side branches, calcifications, and cysts) suggested that in a population at low to moderate risk of chronic pancreatitis EUS is most reliable only when it is either clearly normal (two or fewer criteria) or clearly abnormal (five or more criteria). When the threshold for normal is set at two or fewer criteria and the threshold for abnormal is set at five or more criteria, the predictive values are 85%.99 Some of the controversy regarding the accuracy of EUS may be due to studies that use three or four criteria (i.e., mild abnormalities) as threshold values to distinguish normal from abnormal EUS. When EUS is only mildly abnormal, endoscopic retrograde pancreatography and functional tests are often normal.
It is unclear whether minimal EUS changes reflect early chronic pancreatic disease. In a prospective study that compared EUS findings of the pancreas with surgical histopathology in 42 patients, there was a significant correlation between the number of EUS criteria and fibrosis score at histology (r = 0.85).100 Because EUS provides higher resolution imaging than previously used imaging methods and provides information on both the ducts and the parenchyma, it is logical to assume that it may detect abnormalities not described previously with tests used traditionally to study pancreatic morphology. Functional testing is said to become abnormal only after greater than 60% to 70% of pancreatic functional reserve is depleted.101 If this is the case, it may also be reasonable to expect that EUS could detect subtle structural changes that predate functional abnormalities. Finally, even severe chronic pancreatitis can be asymptomatic. EUS may show pancreatic abnormalities in asymptomatic individuals. It has been shown that alcohol consumption is often associated with asymptomatic abnormalities.102,103
Treatment
Medical Management
Pain Management
Abstinence from Alcohol
Early in the course of chronic pancreatitis, many patients experience recurrent acute attacks rather than chronic pain (Fig. 49.8). In these patients with recurrent attacks, a temporal relationship with alcohol binge can be commonly elucidated. In such patients, abstinence from alcohol may be of substantial benefit, in contrast to patients with end-stage disease and chronic pain. Mortality in chronic pancreatitis has been shown to be related to continued alcohol abuse.104 In a meta-analysis105 evaluating the effect of abstinence on pain, a substantial reduction in pain was associated with cessation of alcohol (continued pain in 26% of abstinent patients compared with 53% of patients who continued to consume alcohol). Also, in a large natural history study, continued drinking was associated with a higher risk of painful relapses.106 Cessation of alcohol seems to have a beneficial effect in preventing alcohol-induced complications and in prolonging life.
Pancreatic Enzyme Supplements
A trial of pancreatic enzyme supplements should be the initial strategy employed for patients with pain in chronic pancreatitis. The rationale for this therapy is based on suppression of feedback loops in the duodenum that regulate the release of cholecystokinin, the hormone that stimulates digestive enzyme secretion from the exocrine pancreas.107
Several randomized control trials have evaluated pancreatic enzymes as a method to provide pain relief. Two of the trials used non–enteric-coated enzymes and showed a reduction in pain compared with placebo.108,109 Four trials used enteric-coated enzymes (which may not be released until they reach the jejunum) and showed no benefit.110–113 In the two trials that showed pain reduction, female patients, patients with idiopathic pancreatitis, and patients with less advanced disease seemed to benefit most. Despite the lack of proof of clear-cut benefit, a more recent consensus review recommended a trial of pancreatic enzymes for pain relief.114 Such a trial should be considered particularly in patients with less advanced disease and in patients with idiopathic chronic pancreatitis.
Analgesics
Most patients with painful chronic pancreatitis require analgesics for symptom relief. Although there are no accurate estimates of the risk of narcotic addiction, most experts suggest it occurs in 10% to 20% of patients. This problem is observed to occur more commonly in patients with poor social support and in patients with a history of narcotic addiction. A useful strategy is to begin with nonnarcotic analgesics such as acetaminophen and nonsteroidal antiinflammatory drugs. If these agents fail to provide adequate symptom relief, narcotic agents may be administered. In many patients, coexistent depression lowers the visceral pain threshold, and the addition of an antidepressant is often useful. Also, antidepressants have a direct effect on pain and potentiate the effects of narcotic analgesia.115,116 Chronic narcotic analgesia may be required in patients with persistent significant pain. Long-acting narcotic agents generally are more effective than short-acting agents, which last only 3 to 4 hours.
Antioxidants
In a placebo-controlled double-blind trial of 127 patients randomly assigned to placebo or antioxidants,117 patients receiving antioxidants had significantly fewer painful days per month compared with the placebo group and required less analgesia for pain control. Also, more patients receiving antioxidants (32%) became pain-free compared with the placebo group (13%). This finding was confirmed by the lower levels of oxidative stress noted in patients receiving antioxidants.
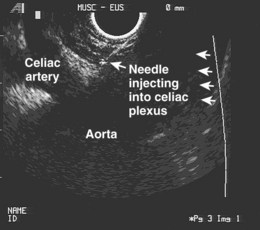
Celiac Plexus Nerve Block
Pancreatic pain is predominantly transmitted through the celiac plexus. Celiac plexus neurolysis, via a surgical or transcutaneous approach, has been used for many years to manage abdominal pain resulting from advanced malignancy.118,119 These approaches have had complications such as paralysis that might be overcome by better visualization of the region. The celiac artery is a landmark structure readily visualized on EUS (Fig. 49.9). Wiersema and Wiersema120 performed transgastric EUS-guided celiac plexus neurolysis and found that the success rate was similar to surgical or transcutaneous approaches. An injection of absolute ethanol that permanently destroys the plexus is referred to as a celiac plexus neurolysis, and an injection of corticosteroids that temporarily block the plexus is referred to as a celiac plexus block. EUS-guided celiac plexus block and neurolysis are safe and well-tolerated procedures that can be performed in an outpatient setting with conscious sedation. The procedures can be performed in 10 minutes or less. Mild complications include transient diarrhea (4% to 15%), transient orthostasis (1%), and transient increase in pain (9%). Major complications (2.5%) have included retroperitoneal bleeding and peripancreatic abscess.121
In a prospective study, Wiersema and associates122 evaluated patients with pancreatic malignancy and chronic pancreatitis treated by celiac plexus neurolysis and found that the initial pain scores were similar between the two patient groups. However, after 16 weeks of follow-up, the pain score improvement after celiac plexus neurolysis in patients with chronic pancreatitis was not found to be significant. The malignant disease group had a mean pain score of less than baseline. Celiac plexus neurolysis is not recommended for chronic pancreatitis. Gress and colleagues123 used injection of triamcinolone in 90 patients with chronic pancreatitis. At 8 weeks after the procedure, 55% of patients had a decreased score; this became 10% of patients by 24 weeks. Based on available data, the technique of performing celiac plexus block does not seem to affect treatment outcomes.124 In a prospective study of 51 patients with chronic pancreatitis and pain who underwent celiac plexus block at either one side or either side of the celiac trunk, there was no significant difference in short-term pain relief between both cohorts. Of patients, 57% who received a single injection had pain relief compared with 54% who received two injections.
Management of Pancreatic Insufficiency
Medium-Chain Triglycerides
Medium-chain triglycerides may provide extra calories in patients with weight loss and a poor response to diet and pancreatic enzyme therapy. In contrast to long-chain triglycerides, which require bile salts and pancreatic lipase, medium-chain triglycerides are readily degraded by gastric and pancreatic lipase and do not require the presence of bile. In addition, medium-chain triglycerides can be directly absorbed by the intestinal mucosa and are less of a stimulant to pancreatic secretion. In a pilot study of eight patients with chronic pancreatitis and postprandial pain, three to four cans of Peptamen (enriched in medium-chain triglycerides and hydrolyzed peptides) per day for 10 weeks resulted in improvement in pain, which in some patients was sustained after stopping this oral supplement.125 The mechanism of action may relate to the fact that administration of this internal formulation resulted in a minimal increase in plasma cholecystokinin levels or alternatively may be due to its antioxidant effects.
Management of Complications
Chronic pancreatitis may be associated with various complications. Splenic vein thrombosis, pseudoaneurysm formation, and common bile duct or duodenal strictures are occasionally encountered. Other complications such as pseudocyst formation and pancreatic ascites or pleural effusion are discussed in Chapter 47. Pancreatic ductal complications such as pancreatic stones and strictures are discussed subsequently.
Pseudoaneurysms
Pseudoaneurysm formation is a rare complication of chronic pancreatitis. The splenic artery is the most commonly involved vessel. Pseudoaneurysms form as a consequence of enzymatic digestion of muscular wall of the artery by a pseudocyst. The presence of unexplained anemia or any degree of gastrointestinal bleeding in a patient with chronic pancreatitis or a known pseudocyst should immediately raise the possibility of a pseudoaneurysm. When bleeding occurs, the mortality is 40% to 60%.126 Although contrast CT scanning and MR imaging with MR angiography may be diagnostic, mesenteric angiography permits confirmation and provides a means of therapy by embolization. Operative intervention for bleeding pseudoaneurysms is difficult and associated with a high morbidity and mortality.
Common Bile Duct Strictures
Intrapancreatic common bile duct strictures have been reported in 2.7% to 45.6% of patients with chronic pancreatitis.127,128 Common bile duct strictures can have serious sequelae of cholangitis, cholelithiasis, choledocholithiasis, intrahepatic stones, and secondary biliary cirrhosis. Deviere and colleagues128 evaluated the use of biliary stent placement in patients with biliary strictures secondary to chronic pancreatitis. They reported that endoscopic biliary drainage is an effective therapy for resolving cholangitis or jaundice in this patient subset. However, the long-term efficacy of this therapy was unsatisfactory because stricture resolution rarely occurred. Preliminary results using metal stents for this indication suggest they could be an effective alternative to operative biliary diversion. More than 90% of patients had no recurrence of strictures at 3 years, but longer follow-up and controlled trials are necessary to confirm these findings.129,130 In a nonrandomized retrospective study that compared surgical drainage procedures with stent insertion, Pitt and coworkers131 found a significantly higher success rate of 88% in the surgically treated group compared with 55% for patients managed by stent insertion.
Surgical Management
Pancreatic Duct Decompression
In patients with a dilated pancreatic duct, a Roux-en-Y side-to-side pancreaticojejunostomy is performed to drain the entire pancreatic duct from the tail to the duodenum. When necessary, a partial resection of the head of the gland is performed to ensure complete drainage. Operative mortality for this technique is less than 3%.132 Substantial improvement in symptoms is seen in 65% to 85% of patients.133,134 Although some authors reported that improvements in symptoms were durable over a follow-up of 7.9 years,128 others reported recurrence of symptoms in a substantial number of patients within 1 year.133–136 The explanation for this decline in effectiveness may be that the secondary ducts, which are not amendable to main duct drainage procedures, may subsequently become obstructed. Although improvement in pancreatic endocrine or exocrine function is not expected after pancreaticojejunostomy, steatorrhea and insulin-dependent diabetes arising as a consequence of surgery are not inevitable.132 However, some studies have noted progressive loss of endocrine and exocrine pancreatic function despite a lateral pancreaticojejunostomy.137–139
Pancreatic Resection
Pancreatic resection involves resection of a portion of the pancreas, usually the tail or head. This procedure is most appropriate when there is focal disease, particularly in the absence of pancreatic ductal dilation. In one series of patients who had no benefit from pancreaticojejunostomy, reoperation to resect the head of the pancreas led to a significant improvement in pain in a select group of patients.140 The reason for the improvement was unclear because there was no evidence of focal pancreatitis. The choice of distal or proximal pancreatectomy and the magnitude of resection are determined by the location and extent of disease. Distal resection is limited to patients with disease involving the tail, most commonly as a sequela of traumatic pancreatitis. Pancreaticoduodenectomy (Whipple’s procedure) has been advocated because the fibrosis of chronic pancreatitis is often more prominent in the head and uncinate process. This procedure often preserves enough islets in the tail to prevent diabetes. Duodenum-preserving resection of the pancreatic head is another alternative that has alleviated pain with lesser rates of exocrine and endocrine dysfunction.141
Steatorrhea can develop in 30% to 40% of patients undergoing simple drainage procedures and in 66% of patients undergoing extensive pancreatic resections.142–144 Diabetes mellitus can occur after pancreatic resection either as a consequence of surgery or secondarily from ongoing disease process. In a randomized trial that compared extended drainage and resection for pain relief,144 although there was no difference in pain score between both cohorts (94% vs. 95%), the global quality of life at 2 years was superior for patients who underwent drainage (71%) compared with resection (43%) procedures. However, at a long-term follow-up of 7 years, there was no significant difference in pain relief, quality of life, and pancreatic exocrine or endocrine functions between both cohorts.145
Total Pancreatectomy with Islet Cell Autotransplantation
Because chronic pancreatitis is progressive and has an unpredictable clinical course, there is an increasing trend to recommend total pancreatectomy as opposed to partial resection. When partial resections are performed, 30% of patients may require subsequent completion or salvage pancreatectomy. The main goal of total pancreatectomy is to achieve a durable treatment for chronic pain. The main long-term morbidity of total pancreatectomy is related to brittle diabetes. The addition of islet cell autotransplantation has dramatically reduced morbidity related to hypoglycemia and has achieved insulin independence in select patients. In a report of 26 patients who underwent total pancreatectomy and islet cell autotransplantation, 80% of patients reported decreased or eliminated use of narcotics for pain control at 6-month follow-up.146 However, all patients required a moderate dosage of insulin for management of diabetes. In patients undergoing resection procedures, such as Whipple’s procedure, islet cell autotransplantation of the tail can be attempted to decrease the risk of diabetes.147 Total pancreatectomy with islet cell autotransplantation is currently the only surgical option available for patients with small duct disease variant of chronic pancreatitis who have unremitting pain and without a focal inflammatory mass.
Endoscopic Management
Sphincter of Oddi Dysfunction
The role of sphincter of Oddi dysfunction as a cause of chronic pancreatitis is not completely understood. In a study that evaluated 104 patients with unexplained abdominal pain, 29% of patients diagnosed with sphincter of Oddi dysfunction had structural evidence of chronic pancreatitis.90 Two other studies made similar observations and found that basal sphincter pressures were elevated particularly in the pancreatic segment of the sphincter of Oddi.148,149 It is unknown whether the sphincter becomes dysfunctional sometimes as part of the overall general scarring process or has a role in the pathogenesis of chronic pancreatitis. Studies have shown that sphincter ablation therapy benefits 30% to 60% of patients with chronic pancreatitis who have manometrically proven sphincter of Oddi dysfunction.150,151 Bagley and colleagues152 reported a series of 67 patients with mild to moderate chronic pancreatitis who underwent empiric sphincterotomy or sphincteroplasty and found that 44% of patients during a 5-year follow-up period had pain relief. The utility of sphincter ablation therapy in patients with chronic pancreatitis awaits further study, probably in randomized controlled trials.
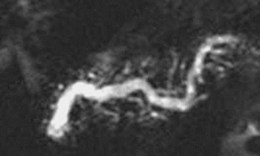
Pancreatic Duct Stones
Approximately one-third of patients with chronic pancreatitis have pancreatic stones. There is no close correlation between the presence of pancreatic duct stones and pain, and many patients with pancreatic duct stones report no pain. It is unclear if pancreatic calculi aggravate the clinical course of chronic pancreatitis or are the consequence of ongoing glandular destruction from persistent disease processes. It is postulated that pain in chronic pancreatitis is related to increased intrapancreatic pressure arising as a consequence of mechanical duct obstruction by pancreatic stones or strictures.153,154 This notion is supported by studies that show improvement in symptoms after ductal clearance of stones.155–159 Removal of pancreatic duct stones is recommended in patients with symptomatic chronic pancreatitis.
Treatment
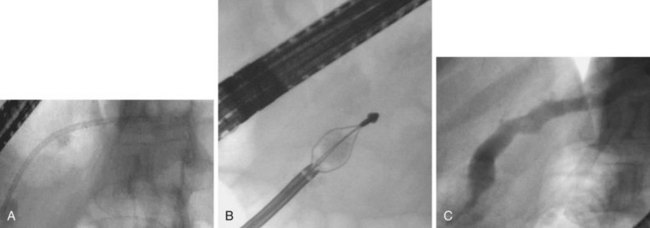
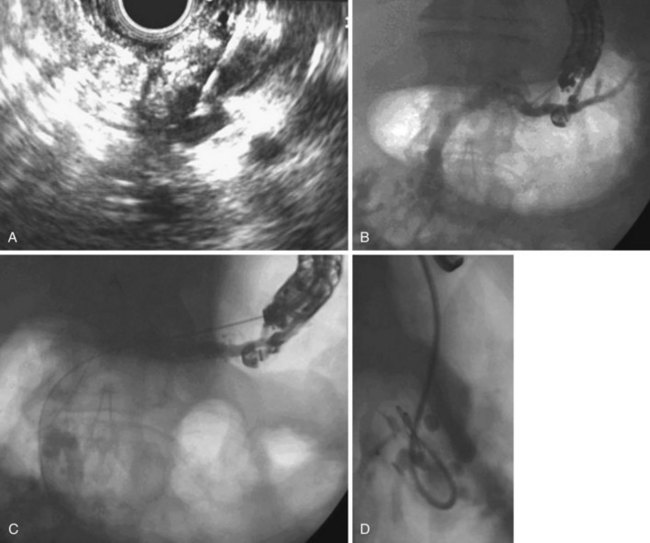
The most suitable features for successful endoscopic removal are small number of mobile stones in the duct without significant strictures. An impacted stone that impedes injection of contrast material into the pancreatic duct usually requires adjunctive therapy using extracorporeal shock wave lithotripsy (ESWL) or intraductal lithotripsy for clearance. Endoscopic management involves sphincterotomy, stricture dilation, and stone removal by baskets or balloons (Fig. 49.10).
Removal of pancreatic stones requires an adequate opening of the pancreatic orifice. There is often thickening and fibrosis or stenosis of the pancreatic orifice in chronic pancreatitis. Cholangiography and pancreatography are performed initially, and the termination of either duct is correctly assessed. Usually a biliary sphincterotomy is performed first to expose the pancreatic septum and to help assess the necessary extent of the pancreatic cut. Pancreatic sphincterotomy can be done using either a standard pull-type sphincterotome or a needle-knife to perform sphincterotomy over a previously placed pancreatic stent. In patients with pancreas divisum, a minor papilla sphincterotomy may be required and is usually performed by using a needle-knife over a previously placed dorsal duct pancreatic stent. The ability to remove a stone by endoscopic methods alone depends on stone size and number, location in the duct, presence of downstream stricture, and degree of impaction.157 Downstream strictures may require dilation either with catheters or with hydrostatic balloons.
Sherman and coworkers157 reported that endoscopic therapy was effective in 83% of patients presenting with chronic relapsing pancreatitis compared with 46% of patients presenting with continuous pain alone. Factors favoring successful endoscopic therapy included three or fewer stones, stones confined to head or body of the pancreas, stone size less than 10 mm, absence of impacted stones, and absence of downstream strictures.151,160 After successful stone removal, 25% of patients had regression of ductographic changes of chronic pancreatitis, and 42% had a decrease in the main pancreatic duct diameter. The only complication was pancreatitis encountered in 8% of patients. Studies have reported success rates with endoscopic therapy of 45% to 79% and improvement in symptoms of 60% to 90%.157,159,161 One study reported clinical improvement in steatorrhea in 73% of patients after endoscopic management.159
Electrohydraulic lithotripsy may be an effective adjunct to endoscopic treatment of pancreatic stones. In this technique, shocks are delivered in a fluid medium under direct visualization because inadvertent firing on tissue can cause perforation or bleeding. This technique requires the use of a baby pancreatoscope that is passed up the pancreatic duct to the stone. Although pancreatoscopy can be used to visualize directly probe contact with the stone and fragmentation, intraductal manipulation remains difficult and very limited.158,162 These smaller baby scopes have fragile control systems with limited one-way tip deflection, which may hinder accurate placement of the probe. The operating channel diameter in these scopes is 0.75 to 1.0 mm and accepts only specialized ultrathin accessories. The electrohydraulic lithotripsy probe is 1.9-Fr in diameter and can be used, but channels of 1.0 mm or less do not allow for much coaxial perfusion of saline necessary for lithotripsy. The saline is essential for transmission of the shock waves at the stone surface and for irrigation after the shock wave. The debris that is created after the shock waves obscures visibility and must be flushed away. Placement of a nasopancreatic tube (5-Fr) beyond the stone before pancreatoscopy is helpful for irrigation purposes. Advancing the pancreatoscope requires an ample pancreatic sphincterotomy for insertion and may require a guidewire to advance up a tortuous pancreatic duct. A 450-cm wire is placed into the duct beforehand, and the proximal stiffer end is backloaded into the baby scope. The stiff end of the wire should be slightly bent before backloading to help negotiate the elbow junction of the accessory port and the scope body.
The main advantage of endoscopic therapy in pancreatic stone management is that recurrence of symptoms secondary to migrated stone can be treated again by endoscopy with or without ESWL. Rate of repeat surgery for recurrent pain is 20% with a striking increase in morbidity and mortality after repeated surgery.163 Controlled trials comparing surgical and endoscopic therapies are awaited. ESWL has become almost indispensable to specialized centers treating many patients with advanced chronic pancreatitis.
ESWL is an effective adjunct to the nonsurgical endoscopic approach in chronic calcifying pancreatitis with complete or partial relief of symptoms in 80% of patients, which is comparable to the surgical literature.164,165 In one study, stones were successfully fragmented in 99% of patients resulting in a decrease in duct dilation in 90%.164 The main pancreatic duct was cleared of all stones in 59%. However, one of the challenges in pancreatic duct therapy is evaluating treatment efficacy. Disintegration of a stone can be considered successful when a decrease is seen in the radiographic density of the stone or the stone surface area. Also, the ability to show relief of ductal obstruction at deep cannulation of the pancreatic duct during ERCP is an indicator of treatment efficacy.164 Using the aforementioned criteria, the success rate of fragmentation has been approximately 76% to 100% in most series regardless of the shock wave system used.164–167 Most patients require endoscopic extraction of stone fragments after ESWL for complete clearance from the ductal system. Some authorities recommend that pancreatic sphincterotomy should be performed before ESWL to facilitate stone passage.161,168 With the exception of one report157 in which successful treatment was more frequent in patients with solitary stones (74% vs. 43% for multiple stones), successful fragmentation and stone clearance was not correlated with the initial size or the number of main pancreatic duct stones by others.166,169,170
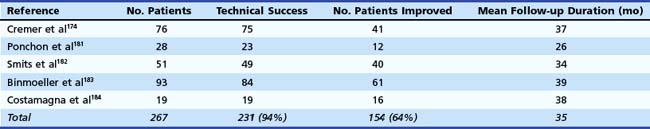
Repeat ESWL may be required if stones have incompletely disintegrated, which is often the case in patients with large or multiple stones. The reported mean number of treatment sessions required to complete lithotripsy has ranged from 1.3 to 4.1 per patient in most reports.161,167,168 The radiographic success of ESWL has been associated with clinical improvement (Table 49.5). Complete or partial pain relief was observed in 62% to 97% of the patients in the largest series during a mean follow-up ranging from 7 to 44 months.160,164,168,170–173 However, complete stone clearance was not required for symptom relief. Many patients gained weight because of a reduction in postprandial pain attacks, improvement in pancreatic function, or both. The number and location of stones, the presence of a stricture, or continued alcohol use did not seem to be associated with recurrent pain.164,168 As a result, ESWL does not have to be restricted to patients without these unfavorable clinical characteristics.
Table 49.5 Technical and Clinical Results of Extracorporeal Shock Wave Lithotripsy for Pancreatic Stones
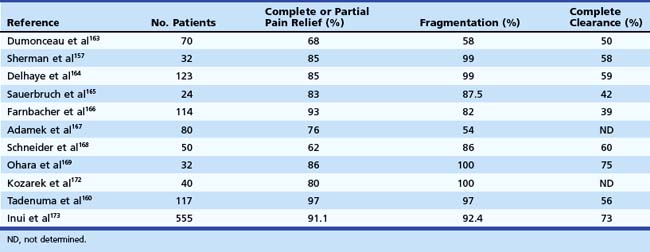
One study163 identified three independent predictors of pain relapse at long-term follow-up after ESWL therapy: (1) a high frequency of pain attacks before treatment (more than or at least two pain attacks during the 2 months before treatment), (2) a long duration of disease before treatment, and (3) the presence of a nonpapillary stenosis of the main pancreatic duct. This study suggests that ESWL in association with endoscopic therapy should be performed as early as possible in the course of chronic pancreatitis. In another study, pain relapse was noted to occur more frequently in patients who had incomplete removal of stones than in patients in whom ductal clearance was complete.160 Early ductal decompression of the main pancreatic duct may also help prevent further fibrosis, which can lead to pancreatic insufficiency. In addition, it may improve pancreatic function in patients who have already developed pancreatic insufficiency. Although some studies investigating this issue found that exocrine pancreatic function improved more often after treatment compared with endocrine pancreatic function, others have shown progressive deterioration in both exocrine and endocrine functions at long-term follow-up.162,164,170–173 In a multicenter study, the rate of recurrence of pancreatic duct stones after ESWL was reported to be 20% to 30%.173 Factors predictive of stone recurrence included ongoing alcohol use and the presence of pancreatic duct strictures. Complications in series using ESWL were primarily related to the endoscopic procedure.
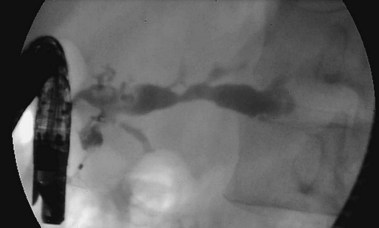
Pancreatic Strictures
Pancreatic duct strictures (Fig. 49.11) may be a complication of previously embedded stone or a consequence of acute inflammatory changes around the pancreatic duct.174 Pancreatic duct strictures may contribute to pain, recurrent acute pancreatitis, and exocrine insufficiency. Strictures may also be associated with stones, pseudocysts, and pancreatic malignancy.161,175–177 The mechanism of pain in patients with pancreatic strictures is poorly understood but may be attributable partly to pancreatic duct hypertension from obstruction caused by the stricture to the flow of pancreatic juice. Pancreatic duct strictures may be present in association with biliary strictures, and liver function test abnormalities, jaundice, and cholangitis may be presenting symptoms.
Diagnosis
No findings on pancreatography are absolutely specific for chronic pancreatitis. At ERCP, complete cutoff of the pancreatic duct implies an abrupt stop in the flow of contrast material at some point along the length of the pancreatic duct. Incomplete filling of the pancreatic duct can also portray a similar picture. Sufficient contrast material must be injected so that the secondary branches of the pancreatic duct can be seen downstream to the blockage; this confirms the presence of a functionally important stricture in the main pancreatic duct because it indicates that the contrast material has taken the path of least resistance into the secondary branches. During ERCP, it is important to attempt to cross the stricture with accessories to allow for complete imaging and tissue sampling by brush cytology, forceps, and needle aspirate. Adjunctive imaging modalities are also important in the differential diagnosis of pancreatic duct strictures. These include conventional studies such as CT and EUS. Both modalities can detect and differentiate chronic pancreatitis and pancreatic neoplasms in advanced stages and may assist in obtaining tissue diagnosis. The role of probe-based optical coherence tomography (OCT) was evaluated in 12 patients with pancreatic duct strictures. All patients underwent ERCP with brush cytology and OCT.178 Although a three-layer architecture was noted in all patients with nonmalignant strictures, the architecture was totally distorted in patients with malignancy. The accuracy of OCT for diagnosing malignancy was 100% compared with 66.7% for brush cytology. OCT remains an investigational modality at the present time that needs further evaluation.
Treatment
The appropriate duration of pancreatic stent placement is currently unknown. Most stents in diagnostic trials or for short-term therapy are left in place for 2 to 4 weeks. In contrast, stents for long-term therapy are left in place for several months. If the patient has improvement in symptoms, the stent can be removed and the patient can be followed clinically, stent therapy can be continued for a more prolonged period, or a surgical drainage procedure can be performed. The last option suggests that the results of endoscopic stent treatment would predict the surgical outcome. Two preliminary reports support this concept, but more studies are required.179,180 Quantitation of the degree of improvement in pancreatic disorders is often poorly defined. Generally, partial or complete symptom improvement indicates that intraductal hypertension was an etiologic factor. Continued improvement in symptoms after stent removal indicates adequate dilation of the narrowing. The results of stent insertion for dominant pancreatic duct strictures (Table 49.6) have been favorable, with technical success in 72% to 99%, relief of pain in 75% to 94%, and good long-term outcomes in 52% to 81%.174,181–183
Although long-term symptom resolution has been reported in more than 60% of patients, endoscopic resolution of strictures has been documented in only about one-third of patients managed by endoscopic stent placement.181,182 Although these data suggest that stricture resolution is not a prerequisite for symptom improvement, other concomitant therapies at the time of pancreatic stent placement such as pancreatic sphincterotomy or pancreatic stone removal may account for successful outcomes. It is also likely that pain in chronic pancreatitis tends to decrease over time as glandular destruction of the pancreas progresses in an uninhibited manner.53
In a study of 75 patients with pancreatic duct strictures and upstream dilation managed by placement of 10-Fr stents, Cremer and coworkers174 reported that 71 patients (94%) were improved over a follow-up of 3 years, with 40 patients (53%) symptom-free. Improvement in symptoms was associated with a decrease in the pancreatic duct diameter. Also, in a prospective study of 23 patients, Ponchon and associates181 reported that disappearance of stenosis at stent removal and a reduction in the pancreatic duct diameter by more than 2 mm were predictive of pain relief after pancreatic duct stent placement. Binmoeller and colleagues183 made a similar observation in their study of 93 patients with chronic pancreatitis and dominant pancreatic duct strictures managed by pancreatic duct stent placement. Although 74% of the patients experienced complete or partial symptom relief, most patients were found to have a regression of ductal dilation after successful stent treatment.
The placement of multiple stents results in successful obliteration of benign biliary strictures; the role of multiple stent placement in pancreatic duct strictures was evaluated in a more recent study of 19 patients.184 All patients with a single main pancreatic duct stricture underwent balloon dilation (6 to 10 mm) followed by placement of multiple stents (8.5-Fr to 11.5-Fr) across the stricture site. All stents were retrieved at a mean follow-up of 7 months. The median number of stents placed per patient was three, and the most common stent diameters used were 10-Fr and 11.5-Fr. During a mean follow-up of 38 months, 84% of patients were asymptomatic, and 10.5% developed symptom recurrence. Although all the above-described studies used conventional plastic stents, Cremer and coworkers,185 in a pilot study, reported their experience with self-expandable metal stents in patients with chronic pancreatitis. Stent placement through the major duodenal papilla was performed in 22 patients with relapsing dominant strictures of the main pancreatic duct. Successful placement, associated with an immediate decrease of pancreatic duct diameter and disappearance of pain, was noted in 100% of cases. Although no immediate complications were encountered, follow-up of these patients showed a high occlusion rate of these metal stents from mucosal hyperplasia.
Another pilot study evaluated the role of covered self-expandable metal stents (8-mm) in patients with refractory main pancreatic duct strictures.186 The stents were retrieved by endoscopy 3 months after placement. Although the pain scores improved significantly, three of six patients developed recurrent strictures warranting subsequent placement of large-caliber (10-mm) metal stents. Although this concept appears novel, more studies with a larger cohort of patients are needed to evaluate the role of covered metal stents in the management of benign pancreatic duct strictures. Direct comparative studies evaluating the efficacies of surgery and endoscopic therapy are required to identify a subset of patients who would benefit from either treatment modality.
Two prospective, randomized studies comparing surgical and endoscopic therapy in chronic pancreatitis have been reported in the literature.187,188 In the first study,187 140 patients with obstructive chronic pancreatitis were treated either by endoscopic therapy or by surgical resection or drainage procedures. Although immediate relief of symptoms was identical in both groups (51.6% in the endotherapy group vs. 42.1% in the surgical group), at 5 years of follow-up, complete absence of pain was more frequent after surgery (37% vs. 14%), with partial relief of pain being similar (49% vs. 51%). The increase in body weight was also greater by 20% to 25% in the surgical group, whereas new-onset diabetes mellitus developed with similar frequency in both groups (34% in the surgical group vs. 43% in the endotherapy group). In the second randomized trial of 39 patients with chronic pancreatitis and pain, patients managed surgically had lower pain scores and better quality of life at 2-year follow-up.188 Also, although only 32% of patients randomly assigned to endoscopy had better or partial pain relief, 75% of patients randomly assigned to surgery had better pain relief. There was no difference in the complications, length of hospital stay, and pancreatic function between both groups; however, patients undergoing endotherapy required more interventions.
Pancreatic stent therapy is not without consequences. Complications related directly to stent therapy include acute pancreatitis, pancreatic infection, pseudocyst formation, duct injury, stone formation, and migration.154,189 The rate of pancreatic stent occlusion appears similar to that of biliary stents.180 Most of these occlusions are without adverse clinical events, however, because pancreatic juice may siphon along the sides of the stent. Morphologic changes of the pancreatic duct directly related to stent placement occur in more than 50% of patients.190–193 It is uncertain what the long-term consequences of these stent-induced ductal changes are in most patients, although permanent new strictures are seen in a few patients. EUS identified parenchymal changes in 68% of patients who underwent short-term pancreatic stent treatment.85 Although such changes may have significant long-term consequences in patients with a normal pancreas, the outcomes in patients with advanced chronic pancreatitis seem less certain.
Future Trends
EUS has been advocated more recently as a means to establish pancreatic ductal drainage in patients after failed ERCP.194 This treatment can be accomplished either by rendezvous stent placement after passage of a guidewire into the main pancreatic duct and through the ampulla under EUS guidance or by transmural drainage of the main pancreatic duct via the stomach or duodenum (Fig. 49.12). However, in addition to technical difficulties, EUS-guided pancreatic duct drainage was associated with a complication rate of nearly 20% that included pancreatitis, perforation, bleeding, and death. Although clinicians have a keen interest in assessing the effects of medical intervention on outcomes related to morbidity and mortality, quality-of-life evaluation remains an area that has long been neglected. Quality of life may be defined as an individual’s overall satisfaction with life and one’s general sense of well-being.195 This definition may be focused further by limiting it to just health-related quality of life. Physicians have always attempted to integrate their patients’ well-being into therapeutic plans. However, health care providers have repeatedly been shown to be poor proxies for measuring quality of life.196 By using instruments that measure quality of life, clinicians can learn whether the patient truly benefits from therapeutic interventions, rather than relying solely on clinical indicators. There are currently numerous disease-specific instruments for conditions such as inflammatory bowel disease, arthritis, and cancer, but only one instrument is available for evaluating patients with chronic pancreatitis.197 Patient-centered outcomes and quality-of-life assessment are important areas in chronic pancreatitis that must be researched further to evaluate the impact of technical and technologic advances in this area.
1 Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332:1482-1490.
2 Lankisch PG, Banks PA. Pancreatitis. New York: Springer; 1998.
3 Lankisch PG, Assmus C, Maisonneuve P, et al. Epidemiology of pancreatic diseases in Luneburg County: A study in a defined German population. Pancreatology. 2002;2:469-477.
4 Dite P, Stary K, Novotny I, et al. Incidence of chronic pancreatitis in the Czech Republic. Eur J Gastroenterol Hepatol. 2001;13:749-750.
5 Lin Y, Tamakoshi A, Matsuno S, et al. Nationwide epidemiological screening of chronic pancreatitis in Japan. J Gastroenterol. 2000;35:136-141.
6 Copenhagen Pancreatic Study: An interim report from a prospective multicenter study. Scand J Gastroenterol. 1981;16:305.
7 Haemmerli UO, Hefti ML, Scmid M. Chronic pancreatitis in Zurich, 1958 through 1962. Biblio Gastroenterol. 1962;7:58.
8 O’Sullivan JN, Noberga FT, Morlock CG, et al. Acute and chronic pancreatitis in Rochester, Minnesota, 1940 to 1969. Gastroenterology. 1972;62:373-379.
9 Lowenfels AB, Maisonneuve P, Grover H, et al. Racial factors and the risk of chronic pancreatitis. Am J Gastroenterol. 1999;94:790-794.
10 Nakamura K, Sarles H, Payan H. Three dimensional reconstruction of the pancreatic ducts in chronic pancreatitis. Gastroenterology. 1972;62:942-949.
11 Harada H, Miyake H, Miki H, et al. Role of endoscopic elimination of protein plugs in the treatment of chronic pancreatitis. Gastroenterol Jpn. 1982;17:463-468.
12 Tsurumi T, Fujii Y, Takeda M, et al. A case of chronic pancreatitis successfully treated by endoscopic removal of protein plugs. Acta Med Okayama. 1984;38:169-174.
13 Freedman SD, Sakamoto K, Venu RP. GP2, the homologue to the renal cast protein uromodulin, is a major component of intraductal plugs in chronic pancreatitis. J Clin Invest. 1993;92:83-90.
14 Guy O, Robles-Diaz G, Adrich Z, et al. Protein content of precipitates present in pancreatic juice of alcoholic subjects and patients with chronic calcifying pancreatitis. Gastroenterology. 1983;84:102-107.
15 Karanjia ND, Widdison AL, Leung FW, et al. Blood flow alterations in chronic pancreatitis: Effects of secretory stimulation [abstract]. Gastroenterology. 1990;98:A221.
16 Karanjia ND, Singh SM, Widdison AL, et al. Pancreatic ductal and interstitial pressures in cats with chronic pancreatitis. Dig Dis Sci. 1992;37:268-273.
17 Rose P, Fraine E, Hunt LP, et al. Dietary antioxidants and chronic pancreatitis. Hum Nutr Clin Nutr. 1986;40:151-164.
18 Uden S, Acheson DW, Reeves J, et al. Antioxidants, enzyme induction, and chronic pancreatitis: A reappraisal following studies in patients on anticonvulsants. Eur J Clin Nutr. 1988;42:561-569.
19 Schoenberg MH, Buchler M, Pietrzyk C, et al. Lipid peroxidation and glutathione metabolism in chronic pancreatitis. Pancreas. 1995;10:36-43.
20 Sahel J, Sarles H. Modifications of pure human pancreatic juice induced by chronic alcohol consumption. Dig Dis Sci. 1979;24:897-905.
21 Epstein O, Chapman RW, Lake-Vakaar G, et al. The pancreas in primary biliary cirrhosis and primary sclerosing cholangitis. Gastroenterology. 1982;83:1172-1182.
22 Nishimori I, Yamamoto Y, Okazaki K, et al. Identification of autoantibodies to a pancreatic antigen in patients with idiopathic chronic pancreatitis and Sjögren’s syndrome. Pancreas. 1994;9:374-381.
23 Bovo P, Mirakian R, Merigo F, et al. HLA molecule expression on chronic pancreatitis specimens: Is there a role for autoimmunity? A preliminary study. Pancreas. 1987;2:350-356.
24 Ammann RW, Heitz PU, Kloppel G. Course of alcoholic chronic pancreatitis: A prospective clinicomorphological long-term study. Gastroenterology. 1996;111:224-231.
25 Ammann RW, Muellhaupt B. Progression of alcoholic acute to chronic pancreatitis. Gut. 1994;35:552.
26 Gastard J, Jobaud F, Farbos T, et al. Etiology and course of primary chronic pancreatitis in western France. Digestion. 1973;9:416-428.
27 Lankisch MR, Imoto M, Layyer P, et al. The effect of small amounts of alcohol on the clinical course of chronic pancreatitis. Mayo Clin Proc. 2001;76:242-251.
28 Bisceglie AM, Segal I. Cirrhosis and chronic pancreatitis in alcoholics. J Clin Gastroenterol. 1984;6:199-200.
29 Haber PS, Wilson JS, Pirola RC. Smoking and alcoholic pancreatitis. Pancreas. 1993;8:568-572.
30 Levy P, Mathurin P, Roqueplo A, et al. A multidimensional case control study of dietary, alcohol, and tobacco habits in alcoholic men with chronic pancreatitis. Pancreas. 1995;10:231-238.
31 Cavallini G, Talamini G, Vaona B, et al. Effect of alcohol and smoking on pancreatic lithogenesis in the course of chronic pancreatitis. Pancreas. 1994;9:42-46.
32 Muddana V, Lamb J, Greer JB, et al. Association between calcium sensing receptor gene polymorphisms and chronic pancreatitis in a US population: Role of serine protease inhibitor Kazal 1 type and alcohol. World J Gastroenterol. 2008;14:4486-4491.
33 Sossenheimer MJ, Aston CE, Preston RA, et al. Clinical characteristics of hereditary pancreatitis in a large family, based on high risk haplotype. Am J Gastroenterol. 1997;92:1113-1116.
34 Le Bodic LL, Bignon JD, Raguenes O, et al. The hereditary pancreatitis gene maps to long arm of chromosome 7. Hum Mol Genet. 1996;5:549-554.
35 Whitcomb DC, Preston RA, Aston CE, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996;110:1975-1980.
36 Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141-145.
37 Teich N, Ockenga J, Hoffmeister A, et al. Chronic pancreatitis associated with an activation peptide mutation that facilitates trypsin activation. Gastroenterology. 2000;119:461-465.
38 Kopelman H, Corey M, Gaskin K, et al. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in cystic fibrosis pancreas. Gastroenterology. 1988;95:349-355.
39 Cohn JA, Friedman KJ, Noone PG, et al. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653-658.
40 Sharer N, Schwarz M, Malone G, et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med. 1998;339:645-652.
41 Sarles H, Augustine P, Laugier R, et al. Pancreatic lesions and modifications of pancreatic juice in tropical chronic pancreatitis. Dig Dis Sci. 1994;39:1337-1344.
42 Bhatia E, Choudhuri G, Sikora SS, et al. Tropical calcific pancreatitis: Strong association with SPINK1 trypsin inhibitor mutations. Gastroenterology. 2002;123:1020-1025.
43 Schneider A, Suman A, Rossi L, et al. SPINK1/PSTI mutations are associated with tropical pancreatitis and type II diabetes mellitus in Bangladesh. Gastroenterology. 2002;123:1026-1030.
44 Vestergaard H, Kruse A, Rokkjaer M, et al. Endoscopic manometry of the sphincter of Oddi and the pancreatic and biliary ducts in patients with chronic pancreatitis. Scand J Gastroenterol. 1994;29:188-192.
45 Delhaye M, Engelholm L, Cremer M. Pancreas divisum: Congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology. 1985;89:951-958.
46 Lehman GA, Sherman S. Pancreas divisum: Diagnosis, clinical significance, and management alternatives. Gastrointest Endosc Clin N Am. 1995;5:145-170.
47 Witt H, Luck W, Becker M. A signal peptide cleavage site mutation in the cationic trypsinogen gene is strongly associated with chronic pancreatitis. Gastroenterology. 1999;117:7-10.
48 Creighton J, Lyall R, Wilson DI, et al. Mutations in the cationic trypsinogen gene in patients with chronic pancreatitis. Lancet. 1999;354:42-43.
49 Layer P, Yamamoto H, Kalthoff L, et al. The different courses of early- and late-onset idiopathic and alcoholic pancreatitis. Gastroenterology. 1994;107:1481-1487.
50 Bockman DE, Buchler MW, Malfertheiner P, et al. Analysis of nerves in chronic pancreatitis. Gastroenterology. 1988;94:1459-1469.
51 Buchler MW, Weihe E, Friess H, et al. Changes in peptidergic innervation in chronic pancreatitis. Pancreas. 1992;7:183-192.
52 Lankisch PG, Lohr-Happe A, Otto J, et al. Natural course in chronic pancreatitis. Digestion. 1993;54:148-155.
53 Ammann RW, Akovbiantz A, Largiader F, et al. Course and outcome of chronic pancreatitis: Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820-828.
54 Lankisch PG, Seidensticker F, Lohr-Happe A, et al. The course of pain is the same in alcohol- and nonalcohol-induced chronic pancreatitis. Pancreas. 1995;10:338-341.
55 DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813-815.
56 Mergener K, Baillie J. Chronic pancreatitis. Lancet. 1997;350:1379-1385.
57 Toskes PP, Hansell J, Cerda J, et al. Vitamin B12 malabsorption in chronic pancreatic insufficiency. N Engl J Med. 1971;284:627-632.
58 Del Prato S, Tiengo A. Pancreatic diabetes. Diabetes Rev. 1993;1:260-265.
59 Malka D, Hammel P, Sauvanet A, et al. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology. 2000;119:1324-1332.
60 Crawford JM, Cotran RS. The pancreas. In: Cotran RS, editor. Robbins pathologic basis of disease. ed 6. Philadelphia: Saunders; 1999:902-929.
61 Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437.
62 Malfertheiner P, Buchler M. Correlation of imaging and function in chronic pancreatitis. Radiol Clin North Am. 1989;27:51-64.
63 Bozkurt T, Braun U, Leferink S, et al. Comparison of pancreatic morphology and exocrine functional impairment in patients with chronic pancreatitis. Gut. 1994;35:1132-1136.
64 Lankisch PG. Function tests in the diagnosis of chronic pancreatitis. Int J Pancreatol. 1993;14:9-20.
65 Niederau C, Grendell JH. Diagnosis of chronic pancreatitis. Gastroenterology. 1985;88:1973-1995.
66 Braganza JM, Hunt LP, Warwick F. Relationship between pancreatic exocrine function and ductal morphology in chronic pancreatitis. Gastroenterology. 1982;82:1341-1347.
67 Girdwood AH, Hatfield ARW, Bornman PC, et al. Structure and function in noncalcific pancreatitis. Dig Dis Sci. 1984;29:721-726.
68 Malfertheiner P, Buchler M, Stanescu A, et al. Exocrine pancreatic function in correlation to ductal and parenchymal morphology in chronic pancreatitis. Hepatogastroenterology. 1986;33:110-114.
69 Lankisch PG, Seidensticker F, Otto J, et al. Secretin-pancreozymin test (SPT) and endoscopic retrograde cholangiopancreatography (ERCP): Both are necessary for diagnosing or excluding chronic pancreatitis. Pancreas. 1996;12:149-152.
70 Lambiase L, Forsmark CE, Toskes PP. Secretin test diagnoses chronic pancreatitis earlier than ERCP [abstract]. Gastroenterology. 1993;104:A315.
71 Hayakawa T, Kondo T, Shibata T, et al. Relationship between pancreatic exocrine function and histological changes in chronic pancreatitis. Am J Gastroenterol. 1992;87:1170-1174.
72 Stein J. New fecal tests in the diagnosis of exocrine pancreatic insufficiency. In: Malfertheiner P, Ditschuneit H, editors. Diagnostic procedures in pancreatic disease. Berlin: Springer Verlag; 1997:277-289.
73 Stein J, Purschian B, Zeuzem S, et al. Quantification of fecal carbohydrates by near-infrared reflectance analysis. Clin Chem. 1996;42:309-312.
74 Bekers O, Postma C, Fischer JC, et al. Fecal nitrogen determination by near-infrared spectroscopy. Eur J Clin Chem Clin Biochem. 1996;34:561-563.
75 Dominguez E, Hieronymus C, Sauerbruch T, et al. Fecal elastase test: Evaluation of a new noninvasive pancreatic function test. Am J Gastroenterol. 1995;90:1834-1837.
76 Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756-759.
77 Bolondi L, Li Bassi S, Gaiani S, et al. Sonography of chronic pancreatitis. Radiol Clin North Am. 1989;27:815-833.
78 Ferucci JTJr, Wittenberg J, Black B, et al. Computed body tomography in chronic pancreatitis. Radiology. 1979;13:172-182.
79 Hessel SJ, Siegelman SS, McNeil NJ, et al. A prospective evaluation of computer tomography in ultrasound of the pancreas. Radiology. 1982;143:129-133.
80 Robinson PJ, Sheridan MB. Pancreatitis: Computed tomography and magnetic resonance imaging. Eur Radiol. 2000;10:401-408.
81 Sica JT, Braver J, Cooney MJ, et al. Comparison of endoscopic retrograde cholangiopancreatography with MR cholangiography in patients with pancreatitis. Radiology. 1999;210:605-610.
82 Takehara Y, Ichijo K, Tooyama N, et al. Breath-hold MR cholangiopancreatography with a long-echo-train fast-spin echo sequence in a surface coil in chronic pancreatitis. Radiology. 1994;92:73-78.
83 Axon AT, Classen M, Cotton PB, et al. Pancreatography in chronic pancreatitis: International definitions. Gut. 1984;25:1107-1112.
84 Forsmark CE, Toskes PP. What does an abnormal pancreatogram mean? Gastrointest Endosc Clin North Am. 1995;5:105-123.
85 Sherman S, Hawes RH, Savides TJ, et al. Stent-induced pancreatic ductal and parenchymal changes: Correlation of endoscopic ultrasound with ERCP. Gastrointest Endosc. 1996;44:276-282.
86 Walsh TN, Rode J, Theis BA, et al. Minimal change chronic pancreatitis. Gut. 1992;33:1566-1571.
87 Hayakawa T, Kondo T, Shibata T, et al. Relationship between pancreatic exocrine function and histological changes in chronic pancreatitis. Am J Gastroenterol. 1992;87:1170-1174.
88 Schmitz-Moormann P, Himmelmann GW, Brandes JW, et al. Comparative radiological and morphological study of human pancreas: Pancreatitis-like changes in postmortem ductograms and their morphological pattern. Possible implication for ERCP. Gut. 1985;26:406-414.
89 Sherman S, Lehman GA. Endoscopic therapy of pancreatic disease. Gastroenterologist. 1993;1:5-17.
90 Tarnasky PR, Hoffman BJ, Aabakken L, et al. Sphincter of Oddi dysfunction is associated with chronic pancreatitis. Am J Gastroenterol. 1997;92:1125-1129.
91 Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol. 1986;123:123-129.
92 Lees WR, Vallon AG, Denyer MR, et al. Prospective study of ultrasonography in chronic pancreatic disease. BMJ. 1979;1:162-164.
93 Wiersema MJ, Hawes RH, Lehman GA, et al. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555-564.
94 Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18-25.
95 Buscail L, Escourrou J, Moreau J, et al. Endoscopic ultrasonography in chronic pancreatitis: A comparative prospective study with conventional ultrasonography, computed tomography, and ERCP. Pancreas. 1995;10:251-257.
96 Dancygier H. Endoscopic ultrasonography in chronic pancreatitis. Gastrointest Endosc Clin N Am. 1995;5:795-804.
97 Natterman C, Goldschmidt AJW, Dancygier H. Endosonography in chronic pancreatitis: A comparison between endoscopic retrograde pancreatography and endoscopic ultrasonography. Endoscopy. 1993;25:565-570.
98 Catalano MF, Lahoti S, Geenen JE, et al. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48:11-17.
99 Bhutani MS, Hoffman BJ, Hawes RH. Diagnosis of pancreas divisum by endoscopic ultrasonography. Endoscopy. 1999;31:167-169.
100 Varadarajulu S, Eltoum I, Tamhane A, et al. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: A prospective tissue characterization study. Gastrointest Endosc. 2007;65:501-509.
101 Toskes PP. Diagnosis of chronic pancreatitis and exocrine insufficiency. Hosp Pract. 1985;20:97-100.
102 Sahai AV, Mishra G, Penman I, et al. EUS to detect evidence of pancreatic disease in patients with persistent or nonspecific dyspepsia. Gastrointest Endosc. 2000;52:153-159.
103 Bhutani MS. Endoscopic ultrasonography: Changes of chronic pancreatitis in asymptomatic and symptomatic alcoholic patients. J Ultrasound Med. 1999;18:455-462.
104 Lowenfels AB, Maisonneuve P, Cavallini G, et al. Prognosis of chronic pancreatitis: An international multicenter study. Am J Gastroenterol. 1994;89:1467-1471.
105 Strum WB. Abstinence in alcoholic chronic pancreatitis: Effect on pain and outcome. J Clin Gastroenterol. 1995;20:37-41.
106 Talamini G, Bassi C, Falconi M, et al. Pain relapses in the first 10 years of chronic pancreatitis. Am J Surg. 1996;171:565-569.
107 Dobrilla G. Management of chronic pancreatitis: Focus on enzyme replacement therapy. Int J Pancreatol. 1989;5(Suppl):17-29.
108 Slaff J, Jacobson D, Tillman CR, et al. Protease-specific suppression of pancreatic exocrine secretion. Gastroenterology. 1984;87:44-52.
109 Isaksson G, Ihse I. Pain reduction by an oral pancreatic enzyme preparation in chronic pancreatitis. Dig Dis Sci. 1993;28:97-102.
110 Halgreen H, Pederson NT, Worning H. Symptomatic effect of pancreatic enzyme therapy in patients with chronic pancreatitis. Scand J Gastroenterol. 1986;21:104-108.
111 Mossner J, Secknus R, Meyer J, et al. Treatment of pain with pancreatic extracts in chronic pancreatitis: Results of a prospective placebo-controlled multicenter trial. Digestion. 1992;53:54-66.
112 Malesci A, Gaia E, Fioretta A, et al. No effect of long-term treatment with pancreatic extract on recurrent abdominal pain in patients with chronic pancreatitis. Scand J Gastroenterol. 1995;30:392-398.
113 Larvin M, McMahon MJ, Thomas WEG, et al. Creon (enteric coated pancreatin microspheres) for the treatment of pain in chronic pancreatitis: A double-blind randomized placebo-controlled crossover trial [abstract]. Gastroenterology. 1991;100:A283.
114 AGA Technical Review: Treatment of pain in chronic pancreatitis. Gastroenterology. 1998;115:765-776.
115 Max MB, Schafer SC, Culnane M, et al. Amitryptiline, but not lorazepam, relieves postherpetic neuralgia. Neurology. 1988;38:1427-1432.
116 Ventafridda V, Bianchi M, Ripamonti C, et al. Studies on the effects of antidepressant drugs on the antinociceptive action of morphine and on plasma morphine in rat and man. Pain. 1990;43:155-162.
117 Bhardwaj P, Garg PK, Maulik SK, et al. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology. 2009;136:149-159.
118 Lillemore KD, Cameron JL, Kaufman HS, et al. Chemical splanchniectomy in patients with unresectable pancreatic cancer: A prospective randomized trial. Ann Surg. 1993;217:447-455.
119 Mercadante S. Celiac plexus block versus analgesics in pancreatic cancer pain. Pain. 1993;52:187-192.
120 Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:639-662.
121 Davies DD. Incidence of major complications of neurolytic celiac plexus block. J R Soc Med. 1993;86:224-266.
122 Wiersema MJ, Harada N, Wiersema LM. Endosonography guided celiac plexus neurolysis efficacy in chronic pancreatitis and malignant disease. Acta Endosc. 1998;28:67-79.
123 Gress F, Schmitt C, Sherman S, et al. A prospective randomized comparison of endoscopic ultrasound and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900-905.
124 LeBlan JK, DeWitt J, Johnson C, et al. A prospective randomized trial of 1 versus 2 injections during EUS-guided celiac plexus block for chronic pancreatitis pain. Gastrointest Endosc. 2009;69:835-842.
125 Shea J, Bishop M, Parker E, et al. An internal therapy containing medium chain triglycerides and hydrolyzed peptides reduces postprandial pain associated with chronic pancreatitis. Pancreatology. 2003;3:36-40.
126 Forsmark CE, Wilcox CM, Grendell JH. Endoscopy-negative upper gastrointestinal bleeding in a patient with chronic pancreatitis. Gastroenterology. 1992;102:320-329.
127 Draganov P, Hoffman B, Marsh W, et al. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc. 2002;55:680-686.
128 Deviere J, Devaere S, Baize M, et al. Endoscopic biliary drainage in chronic pancreatitis. Gastrointest Endosc. 1990;36:96-100.
129 Deviere J, Cremer M, Love J, et al. Management of common bile duct strictures caused by chronic pancreatitis with metal self-expandable stents. Gut. 1994;35:122-126.
130 Kahl S, Zimmermann S, Glasbrenner B, et al. Treatment of benign biliary strictures in chronic pancreatitis by self-expandable metal stents. Dig Dis Sci. 2002;20:199-203.
131 Pitt HA, Kaufman SL, Coleman J, et al. Benign postoperative biliary strictures: Operate or dilate. Ann Surg. 1989;210:417-425.
132 Prinz RA. Surgical drainage procedures. In: Howard J, Idezuki Y, Ihse I, Prinz R, editors. Surgical diseases of the pancreas. Baltimore: Williams & Wilkins; 1998:359-366.
133 Frey CF. Why and when to drain the pancreatic ductal system. In: Beger HG, Buchler M, Ditschuneit H, et al, editors. Chronic pancreatitis: Research and clinical management. Berlin: Springer-Verlag; 1990:415.
134 Prinz RA, Greenlee HB. Pancreatic duct drainage in 100 patients with chronic pancreatitis. Ann Surg. 1981;194:313-320.
135 Adams DB, Ford MC, Anderson MC. Outcomes after lateral pancreaticojejunostomy for chronic pancreatitis. Ann Surg. 1994;219:481-487.
136 Nealon WH, Thompson JC. Progressive loss of pancreatic function in chronic pancreatitis is delayed by main pancreatic duct decompression: A longitudinal prospective analysis of the modified Puestow procedure. Ann Surg. 1991;217:458-466.
137 White TT, Slavotinek AH. Results of surgical treatment of chronic pancreatitis. Ann Surg. 1979;189:217-224.
138 Warshaw AL, Popp JWJr, Schapiro RH. Long-term patency, pancreatic function, and pain relief after lateral pancreaticojejunostomy for chronic pancreatitis. Gastroenterology. 1980;79:289-293.
139 Taylor RH, Bagley FH, Braasch JW, et al. Ductal draining or resection for chronic pancreatitis. Am J Surg. 1981;141:28-33.
140 Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis: Strategies for salvage. Arch Surg. 1994;129:374-379.
141 Buchler MW, Freiss H, Muller MW, et al. Randomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis. Am J Surg. 1995;169:65-69.
142 Jimenez RE, Fernandez-del Castillo C, Rattner DW, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg. 2000;231:293-300.
143 Sakorafas GH, Farnell MB, Farley DR, et al. Long-term results after surgery for chronic pancreatitis. Int J Pancreatol. 2000;27:131-142.
144 Izbicki JR, Bloechle C, Broering DC, et al. Extended drainage versus resection in surgery for chronic pancreatitis: A prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head resection with the pylorus-preserving pancreaticoduodenectomy. Ann Surg. 1998;228:771-779.
145 Strate T, Bachmann K, Busch P, et al. Resection vs drainage in treatment of chronic pancreatitis: Long-term results of a randomized trial. Gastroenterology. 2008;134:1406-1411.
146 Argo JL, Contreras JL, Wesley MM, et al. Pancreatic resection with islet cell autotransplant for the treatment of severe chronic pancreatitis. Am Surg. 2008;74:530-536.
147 Rossi RL, Soeldner JS, Braasch JW, et al. Long-term results of pancreatic resection and segmental pancreatic autotransplantation for chronic pancreatitis. Am J Surg. 1990;159:51-57.
148 Vestergaard H, Krause A, Rokkjaer M, et al. Endoscopic manometry of the sphincter of Oddi and the pancreatic and biliary ducts in patients with chronic pancreatitis. Scand J Gastroenterol. 1994;29:188-192.
149 Ugljesic M, Bulajic M, Milosavljevic T, et al. Endoscopic manometry of the sphincter of Oddi and pancreatic duct in patients with chronic pancreatitis. Int J Pancreatol. 1996;19:191-195.
150 Sherman S, Hawes RH, Madura JA, et al. Comparison of intraoperative and endoscopic manometry of the sphincter of Oddi. Surg Gynecol Obstet. 1992;175:410-418.
151 Williamson RCN. Pancreatic sphincteroplasty: Indications and outcome. Ann R Coll Surg. 1988;70:205-211.
152 Bagley FH, Fraasch JW, Taylor RH, et al. Sphincterotomy or sphincteroplasty in the treatment of pathologically mild chronic pancreatitis. Am J Surg. 1981;141:418-422.
153 Geenen JE, Rolny P. Endoscopic therapy of acute and chronic pancreatitis. Gastrointest Endosc. 1991;37:377-382.
154 Seigel J, Veerappan A. Endoscopic management of pancreatic disorders: Potential risks of pancreatic prosthesis. Endoscopy. 1991;23:177-180.
155 Huibregtse K, Smits ME. Endoscopic management of diseases of the pancreas. Am J Gastroenterol. 1994;89:S66-S77.
156 Neuhaus H. Fragmentation of pancreatic stones by ESWL. Endoscopy. 1991;23:161-165.
157 Sherman S, Lehman GA, Hawes RH, et al. Pancreatic ductal stones: Frequency of successful endoscopic removal and improvement in symptoms. Gastrointest Endosc. 1991;37:511-517.
158 Kozarek RA, Ball TJ, Patterson GJ. Endoscopic approach to pancreatic duct calculi and obstructive pancreatitis. Am J Gastroenterol. 1992;87:600-603.
159 Cremer M, Deviere J, Delhaye M, et al. Endoscopic management of chronic pancreatitis. Acta Gastroent Belg. 1993;56:192-200.
160 Tadenuma H, Ishihara T, Yamaguchi T, et al. Long-term results of extracorporeal shockwave lithotripsy and endoscopic therapy for pancreatic stones. Clin Gastroenterol Hepatol. 2005;3:1128-1135.
161 Smits ME, Rauws EA, Tytgat GNJ, et al. Endoscopic treatment of pancreatic stones in patients with chronic pancreatitis. Gastrointest Endosc. 1996;43:556-560.
162 Neuhaus H, Hoffman W, Classen M. Laser lithotripsy of pancreatic and biliary stones via 3.4mm and 3.7mm miniscopes: First clinical results. Endoscopy. 1992;24:208-214.
163 Dumonceau JM, Deviere J, Le Moine O, et al. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: Long-term results. Gastrointest Endosc. 1996;43:547-555.
164 Delhaye M, Vandermeeren A, Baize M, et al. Extracorporeal shock wave lithotripsy of pancreatic calculi. Gastroenterology. 1992;102:610-620.
165 Sauerbruch T, Holl J, Sackmann M, et al. Extracorporeal lithotripsy of pancreatic stones in patients with chronic pancreatitis and pain: A prospective follow-up study. Gut. 1992;33:969-972.
166 Farnbacher MJ, Schoen C, Rabenstein T, et al. Pancreatic duct stones in chronic pancreatitis: Criteria for treatment intensity and success. Gastrointest Endosc. 2002;56:501-506.
167 Adamek HE, Jakobs R, Buttmann A, et al. Long term follow up of patients with chronic pancreatitis and pancreatic stones treated with extracorporeal shock wave lithotripsy. Gut. 1999;45:402-405.
168 Schneider HT, May A, Benninger J, et al. Piezoelectric shock wave lithotripsy of pancreatic duct stones. Am J Gastroenterol. 1994;89:2042-2048.
169 Ohara H, Hoshino M, Hayakawa T, et al. Single application extracorporeal shock wave lithotripsy is the first choice for patients with pancreatic duct stones. Am J Gastroenterol. 1996;91:1388-1394.
170 Schreiber F, Gurakuqi GCH, Pristautz H, et al. Sonographically-guided extracorporeal shock wave lithotripsy for pancreatic stones in patients with chronic pancreatitis. J Gastroenterol Hepatol. 1996;11:247-251.
171 Brand B, Kahl M, Sidhu S, et al. Prospective evaluation of morphology, function, and quality of life after extracorporeal shockwave lithotripsy and endoscopic treatment of chronic calcific pancreatitis. Am J Gastroenterol. 2000;95:3428-3438.
172 Kozarek RA, Brandabur JJ, Ball TJ, et al. Clinical outcomes in patients who undergo extracorporeal shock wave lithotripsy for chronic calcific pancreatitis. Gastrointest Endosc. 2002;56:496-500.
173 Inui K, Tazuma S, Yamaguchi T, et al. Treatment of pancreatic stones with extracorporeal shock wave lithotripsy: Results of a multicenter study. Pancreas. 2005;30:26-30.
174 Cremer M, Deviere J, Delhaye M, et al. Stenting in severe chronic pancreatitis: Results of medium-term follow-up in 76 patients. Endoscopy. 1991;23:171-176.
175 Nealon WH, Townsend CJ, Thompson JC. Operative drainage of the pancreatic duct delays functional improvement in patients with chronic pancreatitis: A prospective analysis. Ann Surg. 1988;208:321-329.
176 Barthet M, Sahel J, Bodiou BC, et al. Endoscopic transpapillary drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:208-213.
177 Catalano MF, Geenen GE, Schmalz MJ, et al. Treatment of pancreatic pseudocysts with ductal communication by transpapillary duct endoprosthesis. Gastrointest Endosc. 1995;42:214-218.
178 Testoni PA, Mariani A, Mangiavillano B, et al. Intraductal optical coherence tomography for investigating main pancreatic duct strictures. Am J Gastroenterol. 2007;102:269-274.
179 McHenry L, Gore DC, DeMaria EJ, et al. Endoscopic treatment of dilated-duct chronic pancreatitis with pancreatic stents: Preliminary results of a sham-controlled, blinded crossover trial to predict surgical outcome. Am J Gastroenterol. 1993;88:1536A.
180 DuVall GA, Schneider DM, Kortan P, et al. Is the outcome of endoscopic therapy of chronic pancreatitis predictive of surgical success. Gastrointest Endosc. 1996;43:405A.
181 Ponchon T, Bory RM, Medeluis F, et al. Endoscopic stenting for pain relief in chronic pancreatitis: Results of a standardized protocol. Gastrointest Endosc. 1995;42:452-456.
182 Smits ME, Badiga SM, Rauws AJ, et al. Long-term results of pancreatic stents in chronic pancreatitis. Gastrointest Endosc. 1995;42:461-467.
183 Binmoeller KF, Jue P, Seifert H, et al. Endoscopic pancreatic stent drainage in chronic pancreatitis and a dominant stricture: Long-term results. Endoscopy. 1995;27:638-644.
184 Costamagna G, Bulajic M, Tringali A, et al. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: Long-term results. Endoscopy. 2006;38:254-259.
185 Cremer M, Suge B, Delhoye M, et al. Expandable pancreatic metal stents (Wallstent) for chronic pancreatitis: First world series [abstract]. Gastroenterology. 1990;98:215.
186 Sauer B, Talreja J, Ellen K, et al. Temporary placement of a fully covered self-expandable metals stent in the pancreatic duct for management of symptomatic refractory chronic pancreatitis: Preliminary data (with videos). Gastrointest Endosc. 2008;68:1173-1178.
187 Dite P, Ruzicka M, Zboril V, et al. A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis. Endoscopy. 2003;35:553-558.
188 Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676-684.
189 Smit MT, Sherman S, Ikenberry SO, et al. Alterations in pancreatic duct morphology following polyethylene pancreatic duct stenting. Gastrointest Endosc. 1996;44:268-275.
190 Kozarek RA. Pancreatic stents can induce ductal changes consistent with chronic pancreatitis. Gastrointest Endosc. 1990;36:93-95.
191 Derfus GA, Geenen JE, Hogan WJ. Effect of endoscopic pancreatic duct stent placement on pancreatic ductal morphology. Gastrointest Endosc. 1990;36:206A.
192 Lehman GA, Sherman S, Nisi R, et al. Pancreas divisum: Results of minor papilla sphincterotomy. Gastrointest Endosc. 1996;44:268-275.
193 Eisen G, Coleman S, Troughton A, et al. Morphological changes in the pancreatic duct after stent placement for benign pancreatic disease. Gastrointest Endosc. 1994;40:107A.
194 Gines A, Varadarajulu S, Napoleon B. EUS 2008 Working Group Document. Evaluation of EUS-guided pancreatic-duct drainage. Gastrointest Endosc. 2009;69(2 Suppl):S43-S48.
195 Shumaker SA, Anderson RT, Czajkowski SM. Psychological test and scales. In: Spilker B, editor. Quality of life assessment in clinical trials. New York: Raven Press, 1990.
196 Barofsky I, Sugarbaker PH. Cancer. In: Spilker B, editor. Quality of life assessment in clinical trials. New York: Raven Press, 1990.
197 Eisen GM, Sandler RS, Coleman SD. Development of a disease specific measure for health related quality of life for individuals with chronic pancreatitis. Gastroenterology. 1995;108:A12.