Chapter 42 Chronic Pain
There are among us those who haply please to think our business is to treat disease.
And all unknowingly lack this lesson still ’tis not the body, but the man is ill.
Historical Overview
Pain Defined
Pain is a subjective and entirely individually personal experience influenced by learning, context, and multiple psychosocial variables.201 Pain is not merely the end product of peripheral receptor stimulation and afferent signaling, but a complicated dynamic process of neural interplay with the noxious environment along ascending and descending peripheral, spinal cord, and brain networks. The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”203 Pain serves an adaptive function, a warning system designed to protect the organism from harm. With chronicity and neuraxial pathology, however, the nociceptive system can become maladaptive and reflect endogenous pathology instead of an exogenous state.265 Acute pain is usually a response to a “noxious” event (i.e., a mechanical, thermal, or chemical insult) causing depolarization of the nonspecialized transducers (the nociceptors). It is time-limited, and treatment should be aimed at removing the underlying pathologic process. Concurrent behaviors will be designed to avoid or remove the offending noxious stimulus. In contrast, chronic pain is designated 3 to 6 months after the initiating event and in many cases might not be associated with any obvious ongoing noxious event or pathologic process.219 Behavior can become pathologic as attempts to avoid the noxious element fail, fight or flight responses escalate to no purpose, etc. Chronic pain can differ from acute pain conditions in that underlying tissue pathology or injury begins to less directly correlate with levels of pain report. Whereas acute pain can be considered a physiologic response to tissue trauma or damage, chronic pain involves a more dynamic interplay of additional psychologic and behavioral mechanisms (Table 42-1).48 Chronic pain often is associated with disrupted sleep and declining function, and eventually can cease to serve any protective role. At this point pain can become a source of dysfunctional behaviors, suffering, and disability, often completely perplexing to the patient, as well as to the unprepared physician.
Environmental and affective factors can contribute to the persistence of pain and subsequent illness behaviors. The individual’s subjective response to chronic pain is shaped by the cognitive repertoire involved in attending to and anticipating noxious sensory signals, as well as in appraising events associated with those signals (Figure 42-1). Chronic painful conditions, when left untreated, can result in multiple problems, including unnecessary personal suffering for the patient, increased medical care use, overuse or misuse of psychoactive medications, iatrogenic complications secondary to inappropriate surgeries, excess disability, comorbid emotional problems (including increased risk of suicide), and increased economic and social costs. A multidisciplinary approach that addresses psychosocial and biologic factors and focuses on functional restoration in all areas of life is sine qua non.
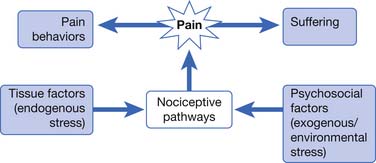
FIGURE 42-1 Processes of chronic pain.
(Modified from Kidd BL, Urban LA: Mechanisms of inflammatory pain, Br J Anaesth 87:3-11, 2001, with permission.)
The prepared physiatrist can offer a unique perspective and skill set to the assessment and management of chronic pain and the psychosocial sequelae. The rehabilitative interdisciplinary team approach, a model for the treatment of other chronic disability conditions (e.g., spinal cord injury, stroke-related disorders, and amputee-related conditions), is focused on maximizing independent physical function, improving psychosocial state, and returning patients to work and previous leisure pursuits, as well as maximizing patients’ reintegration into the community and subsequent improvement of general quality of life. To achieve these ambitious goals, as well as adding the goal of decreasing the pain to tolerable levels, the physiatrist must thoroughly understand and appreciate the biologic, psychologic, and socioeconomic implications of pain and pain-related disability. A list of pain terminology and definitions is included for review (Table 42-2).
| Term | Definition |
|---|---|
| Addiction | A chronic biopsychosocial disease characterized by impaired control over drug use, compulsive use, continued use despite harm, and craving |
| Allodynia | Pain caused by a stimulus that does not normally provoke pain |
| Analgesia | Absence of pain in response to stimulation that would normally be painful |
| Central pain | Pain initiated or caused by a primary lesion or dysfunction in the central nervous system |
| Dependence | A maladaptive pattern of drug use marked by tolerance and a drug class-specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood levels of drug, or administration of an antagonist |
| Dysesthesia | An unpleasant abnormal sensation, whether spontaneous or evoked |
| Hyperalgesia | An increased response to a stimulus that is normally painful |
| Hyperesthesia | Increased sensitivity to stimulation, excluding the special senses |
| Neurogenic pain | Pain initiated or caused by a primary lesion, dysfunction, or transitory perturbation in the peripheral or central nervous system |
| Neuropathic pain | Pain initiated or caused by a primary lesion or dysfunction in the nervous system∗ |
| Nociception | A receptor preferentially sensitive to a noxious stimulus that would become noxious if prolonged |
| Noxious stimulus | A noxious stimulus is one that is damaging to normal tissues |
| Pain | An unpleasant sensory and emotional experience associated with actual or potential tissue damage |
| Paresthesia | An abnormal sensation, whether spontaneous or evoked, that is not unpleasant |
| Peripheral neurogenic pain | Pain initiated or caused by a primary lesion, dysfunction, or transitory perturbation in the peripheral nervous system |
| Peripheral neuropathic pain | Pain initiated or caused by a primary lesion or dysfunction in the peripheral nervous system |
| Psychogenic pain | Pain not caused by an identifiable, somatic origin and that may reflect psychologic factors |
| Tolerance | A state of adaptation in which exposure to a drug induces changes that result in diminution of one or more of the drug’s effects over time |
∗ See also neurogenic pain and central pain. Peripheral neuropathic pain occurs when the lesion or dysfunction affects the peripheral nervous system. Central pain may be retained as the term when the lesion or dysfunction affects the central nervous system.
From Merskey H, Bogduk N: IASP Task Force on Taxonomy classification of chronic pain: description of chronic pain syndromes and definition of pain terms, Seattle, 1994, IASP Press, with permission.
Prevalence
Chronic pain and related suffering and disability represent an accelerating public health concern and a fiscal “black hole” in the U.S. economy. Prevalence rates of chronic pain vary widely, from 2% to 55% in general population studies,43,74,121,309 and probably realistically represent 30% to 40%.5,29 Prosaic diagnoses (i.e., chronic arthritic and musculoskeletal conditions and spine-related disorders) account for a large portion of reported pain and correlate with a high concurrent risk for disability.326 By 2030, physician-diagnosed arthritis and related disability is predicted to affect 71 million Americans. Chronic pain is an important cause of health-related loss of productivity in the workplace, in addition to medical, pharmacy costs, and absenteeism, surpassing combined costs related to cancer, hypertension, and depression.165 Projected expansion of the elderly population, as well as increased survival rates of the disabled population and individuals with life-shortening or previously terminal conditions, will lead to further increases in prevalence.
Reviews of chronic pain as a secondary problem in patients with a primary disability, such as spinal cord injury, amputation, cerebral palsy, and multiple sclerosis, have demonstrated even higher prevalence rates of intolerable pain (>70%), which can substantially add to disability. For example, a longitudinal study of chronic pain in adult cerebral palsy patients found that pain remained steady over a 2-year period, and that pain was unlikely to decrease spontaneously without treatment.132 Pain associated with rehabilitation diagnoses is often reported in multiple sites, not just the focal site of the primary injury,73,303 and can contribute to a more generalized loss of function and related disability.
The Cost of Chronic Pain
The progression from acute to chronic pain inevitably includes a greater impact in related psychologic and social functioning. Chronic pain-related impairment and disability have significant socioeconomic consequences as a result of high health care costs, lost wages and productivity, and the growing costs of disability benefits and other compensation.307 Conservative estimates of cost related to health care expenditure and lost productivity range from $70 to $120 billion annually.94,222 Chronic pain is responsible for 90 million physician visits, 14% of all prescriptions, and 50 million lost workdays per year.24,54 One third of the population suffers from chronic pain-related conditions, at a cost of $100 billion in related compensation, health care, and litigation costs.167 Stewart et al.287 found that 75% of pain-related productivity loss was on the job, not a result of absence from work.
History
The concept of pain has been part of the human experience closely woven into the cultural fabric of philosophy, politics, and religion since early times, including documented periods in ancient China, India, and Egypt. In a quest for analgesia and decreased suffering, individuals have tolerated rather barbaric and ineffective treatments, including purging, cupping, blistering, bleeding, leeching, heating, and freezing.271 In ancient Greece, Hippocrates (460 to 370 BC) hypothesized that four bodily fluids (“humors”) were responsible for the state of one’s personality, and with any physical and psychologic illness an imbalance of these humors could lead to pain. Early medical practitioners continued this quest for relief from pain and suffering with the use of various concoctions including the use of mandrake, one of the earliest known medicinal plants, as a means of inducing analgesia and anesthesia. Crude forms of opium have also been used. The “somniferant” sponge, a sponge saturated with a mixture of opium juices, mandrake root, and other plant extracts, was used for pain relief and in everyday medical treatments, and as a crude anesthetic for the inevitable and almost always painful surgical procedures.23
The history of our understanding of pain physiology and treatments evolved in parallel (to some extent) with general advancements in the field of medicine. During the European Dark Ages (400 to 1300 AD), a time of little progress in any field of science, Middle Eastern medicine and the treatment of pain flourished. For example, the writings of Turkish surgeon Sabuncuoğlu eloquently described procedures involving “cauterization” for the treatment of migraine headaches, dental pain, low back pain, and “sciatica.”102
The nineteenth and twentieth centuries included important advances in pharmacologic treatment for pain. Before this, crude forms of analgesia included the use of laudanum, a mixture of opium, alcohol, and other ingredients given with whiskey or other types of alcohol, for analgesia and also before surgical procedures. Serturner isolated morphine from opium in 1806, leading to advancement in isolation techniques and the subsequent production of morphine (in the 1820s), codeine (in 1832), and synthetic opioids such as methadone and fentanyl (in the 1940s). The chemist Felix Hoffman developed acetylsalicylic acid (aspirin) from extracts of willow tree bark as the first reliable analgesic for the relief of moderate pain.56 Alexander Wood’s development of the syringe made parenteral administration practical and convenient but also led to increased abuse and misuse of the opioids. Diacetylated morphine (heroin) was later introduced by the Bayer Company in 1898 as a cough suppressant.123 Abusers soon learned that the compound could be crushed into a powder and snorted, injected, or smoked with significant euphoric effects. The Harrison Narcotic Control Act was passed in 1914, limiting the use of morphine and other opiates to the care of a physician. Controversies related to the use of opioid medications for ongoing treatment of chronic nonmalignant and cancer-related pain have been a primary focus of federal regulatory and legislative scrutiny. Fear of iatrogenic addiction in the use of chronic opioid management, as well as variability between federal, state, and community-based laws, has led to ongoing physician fears for aggressive management of pain-related conditions. It remains an important evolving contemporary issue related to the treatment of chronic pain.
Many of the advancements in anesthesiology pain management developed from experiences in treating injured soldiers. Twentieth century surgical advancements included the works of René Leriche and his experience with treating wounded World War I soldiers. He eloquently described chronic pain states including phantom limb pain. His work also included descriptions of “sympathetic pain” arising from smooth muscle in peripheral blood vessels. Leriche championed surgical procedures directed at relieving sympathetic-mediated pain, with aggressive surgical procedures involving sympathetic ganglia and surgical “periarterial” sympathectomies. Another surgeon, William K. Beecher, helped to put forward the importance of psychologic factors in the experience of pain. As a young army physician in World War II, he observed significantly less pain reported and fewer requests for pain medication by soldiers severely wounded in battle compared with civilian patients in his practice with similar injuries.20 He went on to describe the “power of placebo,” underscoring the importance of meaning and distraction as important factors in the experience of pain.
The success of the gate control theory in the 1960s helped initiate pain management as a formal field of study. John Bonica formalized the idea of pain management as a multidisciplinary collaboration in the late 1940s. In 1974, Bonica organized a scientific meeting of leaders in the field of pain medicine, which established the IASP. The publication of the IASP’s journal, Pain, occurred 1 year later. The IASP created the first Taxonomy of Pain in 1979 and continues to publish clinical updates and guidelines regarding a number of pain-related conditions (i.e., headache, cancer pain, chronic nonmalignant pain, and complex regional pain syndrome). It also cultivates research related to further understanding of pain mechanisms and treatment developments. A number of agencies and professional societies remain active in the field of pain science and treatment, including the American Pain Society, American Academy of Pain Medicine, and multispecialty spine intervention-based societies such as the North American Spine Society and International Spinal Injection Society. In the past 2 decades, there has been an explosive growth of modern neuroscience that has led to advancement in understanding pain mechanisms and treatment. This is due in part to the financial impetus from the analgesic area, as well as the development of new tools such as functional imaging studies (positron emission tomography and functional magnetic resonance imaging), which have helped to demonstrate evidence of complex cortical networks related to pain perception.236,239
Recently the number of physiatrists becoming interested in pain medicine as a subspecialty has exponentially increased, with many pursuing formal fellowship training. Subspecialty certification in pain medicine is now offered by the American Board of Physical Medicine and Rehabilitation in cooperation with the American Board of Psychiatry and Neurology and the American Board of Anesthesiology. With the growing availability of pain fellowships and the increasing number of physiatrists specializing solely in pain medicine, advanced interventional pain management procedures (including fluoroscopically guided spinal interventions and placement of implantable pain devices) are increasingly being performed by physiatrists as part of a comprehensive treatment plan for chronic pain disorders (see Chapter 25).
Early History of Pain Theory: A Peripheral Perspective
The dualistic or mind–body controversy started with René Descartes’ (1596 to 1650) biomedical theories and can be seen as a precursor to specificity theory. He likened the pain system to a bell-ringing mechanism. The individual on the ground pulls the rope, ringing the bell in the tower. Similarly, placing the foot next to a burning flame would set particles in the foot in motion, traveling up the leg, back, and to the head, causing activation of pain. This theory, traditionally ascribed to Descartes, actually has earlier antecedents of Galen, based on the central position of the pineal gland, the center of the soul, and the sensory motor system.199
The specificity theory remained somewhat unchallenged until the nineteenth century, with the emergence of physiology as a more formal scientific field of study. Magendie (1783 to 1855) and his student, Claude Bernard (1813 to 1878), revolutionized the field of physiology by codifying the principles of observation, data recording, and analysis (considered heretic at the time!). Bernard was the first to publish observations about the relationship of the autonomic nervous system to pain, and one of his students, the American Civil War surgeon Silas Weir Mitchell, would go on to elucidate what he called causalgia (now complex regional pain syndrome type 2). The qualities of pain experience were thought to be associated with properties of sensory nerves. Johannes Müller was the first to elaborate on more specific neural pathways for pain in his theory of specific nerve energies (1842). Müller’s concept included the distinction of four major cutaneous modalities (i.e., touch, warmth, cold, and pain), each with its own projection system to the brain.199
Max von Frey expanded Müller’s idea of specific nerve energies to include a theory based on specific receptors. Von Frey proposed the presence of cutaneous sensitivity maps on the skin “mapped out” by anatomists of the day with a brand new experimental device, the microscope. First, a spotlike distribution of warmth and cold cutaneous sensitivity was mapped out with two devices: a pin on a string, to gauge pressure thresholds for pain, and snippets of horsetail hairs attached to a piece of wood, to map out distributions of “touch spots.” More formal standardized versions of these instruments continue to be used in contemporary sensory testing (i.e., von Frey filaments and von Frey hairs, respectively). Second, von Frey included his theory, later disproved, that there were specific pain receptors (free nerve endings) that varied in their distribution on the body to complement other specialized receptors identified around the same time (Meissner corpuscles, touch; Krause end bulbs, cold; and Ruffini end organs, warmth). Sherrington272 later postulated the existence of specialized cells or “nociceptors,” which could detect noxious sensations in terms of the lowest “lumen” or threshold.
An alternative to these specificity theories, the intensive (summation) theory, was formulated by Erb in 1874. Intensive theory proposed that each sensory transducer was capable of producing pain only if the stimulus reached a sufficient intensity.27 Goldscheider later (1894) refined the stimulus intensity and summation theories and proposed the pattern theory. In pattern theory, pain results after total output at the cellular level reaches a critical level, either by stimulation by nonnoxious stimuli or by pathologic conditions that enhance summation. The theory centered on the contention that all nerve fibers are alike, and that pain is produced by spatiotemporal patterns of neuronal impulses versus activity on “specific” nerve fibers.
Central Theories of Pain
Until the late 1800s, pain theory was based primarily on peripheral mechanisms and failed to explain persistent pain states. William Livingston’s work with injured soldiers in World War II, and later in chronic work-related injuries, suggested that some portion of chronic pain mechanisms might be related to more specific central nervous system dysfunction.164 Livingston’s summation theory stated that pathologic stimulation of sensory nerves after nerve injury could lead to reverberating circuits in neuron pools of the spinal cord, which could later be triggered by peripheral nonnoxious inputs. This volley of nerve impulses could lead to a vicious cycle between central and peripheral processes.158
Although debate continued among three basic pain theories, specificity ultimately prevailed and was universally accepted and practiced. An appreciation for cognitive and psychologic aspects of pain processing, although secondary, slowly emerged within the fourth theory of pain, proposed by Hardy, Wolff, and Goodell. Pain was separated into two components: the perception of pain (afferent) and the reaction to pain (efferent). Pain perception was thought as a more hardwired physiologic process, whereas reaction to pain was under the influence of complex psychologic and physiologic processes influenced by past experiences, the environment, and emotional state.119
Melzack and Wall’s gate control theory championed a more convergent view of pain processing. The spinal cord is not just a passive conduit for pain transmission but also an active modulator of pain signals. Activity in large myelinated afferent fibers theoretically activates dorsal horn encephalogenic interneurons that inhibit cephalad transmission in small unmyelinated primary afferent nociceptive fibers and the secondary transmission cells in the lateral spinothalamic tracts.202 Somatic afferents activate convergent wide dynamic range cells deep in the dorsal horn (lamina V), which project in the spinothalamic tract to higher somatosensory processing in the thalamus and cortex. In theory, inhibiting pain by rubbing the skin activates large-diameter afferents inhibiting small-diameter fiber activation of wide dynamic range cells, that is, “closing the gate.”
Melzack has extended his work with the gate control theory to include the more central neuromatrix theory based on concepts from cognitive neuroscience network theory.254 Dimensions of the pain experience are considered as output of the neuromatrix, which proposes a neurosignature of pain experience that is unique to each individual and is influenced by sensory, psychosocial, and genetic factors. This pattern is modulated by various sensory inputs from the environment and by cognitive events such as psychologic stress. In turn, these multiple parallel processing inputs contribute to the sensory, affective, and cognitive dimensions of the pain experience and subsequent behavior.
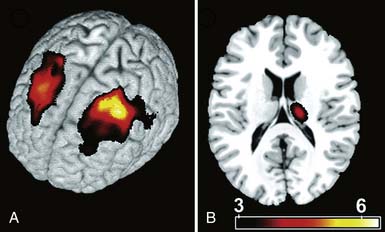
Recent advances in neuroimaging and the exploding field of neuroscience networking have offered greater insight into higher-level cerebral plasticity related to acute and chronic pain. Apkarian et al.6 studied brain morphologic changes with the use of high-resolution magnetic resonance imaging in a group of patients with chronic low back pain. Significant evidence of discrete central nervous system degeneration (gray matter atrophy) in the chronic pain patient group was demonstrated. Discrete thalamic and prefrontal cortex atrophy was reported at a rate approximately 5 to 10 times greater than that of normal age-related atrophy. This underscores the importance of appropriate and aggressive treatment of pain as a means of preventing possible long-term or permanent central nervous system changes. In addition, these findings add to the ongoing developments in neural plasticity of pain because these changes are not plastic but are perhaps permanent (Figure 42-2). The use of positron emission tomography and functional magnetic resonance imaging has offered accelerating insight into the main cerebral components of human nociceptive processing and networking at the brain and spinal cord levels.138
History of Contemporary Advancements in Psychologic Aspects of Pain
The twentieth century also provided significant growth in the fields of psychiatry and psychosomatic medicine. Sigmund Freud emphasized the potential link between psychologic and physical factors in a number of medical conditions. Later, disenchantment with Freud’s psychoanalytic principles led to the development of the field of psychosomatic medicine and the subsequent rapid development of the fields of health psychology and behavioral medicine in the 1970s.105 Physicians such as George Engel (1959) challenged the biomedical model of disease as inadequate, in that it failed to include the social, psychologic, and behavioral dimensions of illness. Engel’s classic article “Psychogenic” Pain and the Pain-Prone Patient discussed various contextual meanings of persistent pain and the importance of an individual’s interpretation of his or her pain. Sternbach argued that physiologic and affective perceptions of pain should be understood as learned responses under the control of environmental forces, and addressed psychophysiologic pain syndromes, including “stress-induced pain disorders.”286 Wilbert Fordyce later proposed an operant conditioning model of chronic pain based on an ends approach of identifying and treating pain behaviors. More contemporaneously, higher cognitive functioning in pain states (such as memory and emotive components) were embraced in the cognitive behavioral approach, led by health psychologists such as Dennis Turk and Frances Keefe, emphasizing the role of attributions, efficacy, personal control, and problem solving. Thoughts and beliefs could influence, and be influenced by, emotional and physiologic responses.302 This has contributed to the evolution of a more clinically pragmatic school of pain assessment and treatment: the biopsychosocial model. This model incorporates the physical, cognitive, affective, and behavioral components related to ongoing pain experience. In this context, biologic factors can initiate a physical disturbance, but psychosocial factors often influence pain perception, pain behavior, and the ongoing pain experience.86,301
Physiology and Pathophysiology of Pain
In a normal homeostatic state, cutaneous, visceral, and musculoskeletal pain serve as an alarm system to the body that indicates damage or potential damage in the environment. The purpose of nociception is to alert the organism to this potential damage so that avoidance behavior can be initiated. In contrast, chronic pain states might represent an alteration involving damage or injury to the central nervous system that serves no real protective role, reflecting a pathologic as opposed to physiologic state. The complex interaction between the initial stimulus of tissue injury and the subjective experience of nociception and acute and chronic pain can be described by four general processes known as transduction, transmission, modulation, and perception (Table 42-3).
| Stage | Description |
|---|---|
| Transduction (receptor activation) | One form of energy (thermal, mechanical, or chemical stimulus) is converted electrochemically into nerve impulses (action potentials) in primary afferents |
| Transmission | Coded information is transferred from primary afferent fibers to spinal cord dorsal horn and onto brain stem, thalamus, and higher cortical structures |
| Modulation | Involves activity- and signal-induced dorsal horn neural plasticity, which includes altered receptor and channel function (i.e., wind-up and central sensitization), gene expression,328 and changes in brain-mediated descending inhibition and facilitation |
| Perception | Begins with activation of sensory cortex. The cortex is in intimate communication with motor and prefrontal cortices, which initiate efferent responses, as well as more primitive structures involved in the emotive aspects of pain |
Transduction
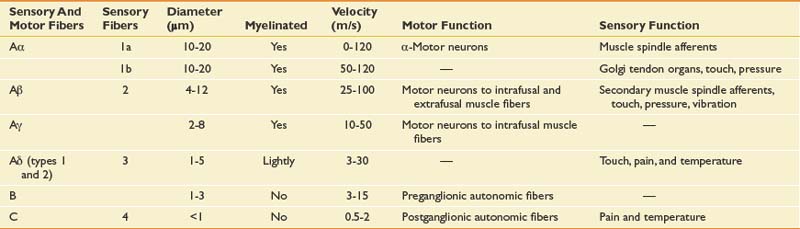
The principal receptors for pain are the branched endings of C and Aδ fibers (Table 42-4) in the skin, muscles, and joints. Damaging (or potentially damaging) energy in the cellular environment impacts the free nerve endings, and the complicated cellular processes of nociceptive transduction occur. Inflammatory cascades are concurrently activated (e.g., prostaglandin, leukotriene) and immediately become principal players in the transduction process. Recent histochemical studies have revealed two broad categories of C fibers: peptidergic and isolectin B4 binding. Peptidergic fibers contain a variety of peptide neurotransmitters, including substance P and calcitonin gene-related peptide (CGRP), and express tyrosine receptor kinase A receptors, which show high affinity for nerve growth factors. Peptidergic neurons appear to be key players in neurogenic inflammation (where the transduction cells themselves become active participants in the local inflammatory process) and other chronic inflammatory states.39,64,329 The other class, isolectin B4 binding, contains few neuropeptides but expresses a surface carbohydrate group selectivity binding to the plant lectin isolectin B4 and is supported by glial-derived neurotrophic factor.290 Isolectin B4 expresses P2X3 receptors, a subtype of ATP-gated ion channels.144 Differences in supporting trophic factors might be responsible for differing functional responses to painful stimuli between these distinct C-fiber types. Neurotrophins have emerged as potential factors for activity-dependent changes at the synapse and possibly subsequent central nervous system plasticity.169
Aδ nociceptors (also responders to noxious, thermal, and chemical stimuli) are most easily classified on functional grounds. Type 2 exhibit short response latencies to heat and are activated at relatively higher thresholds (43° C). Type 2 Aδ are responsible for the initial sensation of a burn stimulus. Type 1 Aδ exhibit longer response latencies and are activated at much higher temperatures (>50° C). Type 1 Aδ and nociceptive C fibers are more commonly associated with persistent painful sensations.44
Transmission
Cutaneous peripheral afferent neurons can be classified into three types based on diameter, structure, and conduction velocity of action potentials. In general, C fibers (thin, unmyelinated, slowly conducting; 0.5 to 2.0 m/s) and Aδ fibers (medium, thinly myelinated, rapidly conducting; 12 to 30 m/s) carry noxious stimuli, and Aβ fibers (large, myelinated, and fast; 30 to 100 m/s) carry innocuous stimuli (touch, vibration, and pressure), except in situations of peripheral or central sensitization (see Table 42-4). The percentage of distribution of nociceptors in the skin is roughly proportioned 70%, 10%, and 20%, respectively. With peripheral and central neuroplastic changes in Aβ fibers, innocuous stimuli might be perceived as painful, resulting in allodynia. Aδ nociceptors respond to intense mechanical and temperature stimuli, and with sensitization contribute to the process called hyperpathia, in which noxious stimuli become frankly more painful and the pain perception can last longer, even after the initial stimulus is removed. Most C fibers are polymodal transducers. Aβ fibers demonstrate encapsulated nerve endings involved in nonnociceptive function. Aδ fibers mediate the fast, prickling quality of pain, whereas C fibers mediate the slow, burning quality of pain. An additional class of nociceptors, the so-called silent or sleeping nociceptors, makes up approximately 10% to 20% of C fibers in the skin, joints, and viscera, and is normally unresponsive to acute noxious stimuli. With inflammation and tissue injury, these “silent” nociceptors are sensitized via activation of second-messenger systems and the release of a number of local chemical mediators (i.e., bradykinin, prostaglandins, serotonin, and histamine) and can contribute to temporal and spatial summation, increasing afferent input at the dorsal horn.46,106,265
Peripheral Sensitization
C fibers and Aδ receptors undergo changes in response to tissue injury such as inflammation, ischemia, and compression. These changes are marked at the peripheral terminals by the release of chemical mediators from damaged and inflammatory cells. The so-called inflammatory soup, rich in analgesic substances, causes a lowering of threshold for activation and subsequent evoked pain. Algogenic substances also activate second-messenger systems, which induce gene expression in the cell. Excitatory amino acids and neuropeptides (substance P, CGRP, and neurokinins) are released by peripheral and central nociceptive C fibers, inducing neurogenic inflammation. Neurogenic inflammation involves retrograde release of algogenic substances, which in turn excites other nearby nociceptors, creating local feed-forward loops of sensitization and activation.
Modulation
Primary afferents subserving distinct input from cutaneous, muscle, and visceral tissues converge at the dorsal horn. Several ascending pathways are involved in transferring and modulating this nociceptive input. At the cellular level, the influx of sodium is fundamental to electrical signaling and subsequent generation of action potentials and excitatory postsynaptic potentials. This is followed by calcium channel opening, contributing to more prolonged depolarization, as well as second-messenger molecular changes involved in more permanent neuroplastic central nervous system changes. At the synaptic terminal of the axon, action potentials lead to the release of neurotransmitters. Neurotransmitter release depends on specific ion channels, which are either ligand-gated, opening in response to binding of ligands to receptors, or voltage-gated, opening in response to changes in membrane potentials.258 Other targeted receptor and ion channels include vanilloid (capsaicin) receptor, heat-activated, ATP-gated purinergic receptor (P2X), proton-gated or acid-sensing ion channels, and voltage-gated sodium channels. The vanilloid receptor is a nonselective cation channel (vanilloid receptor 1) activated by elevated temperature (>43° C) and acidification.
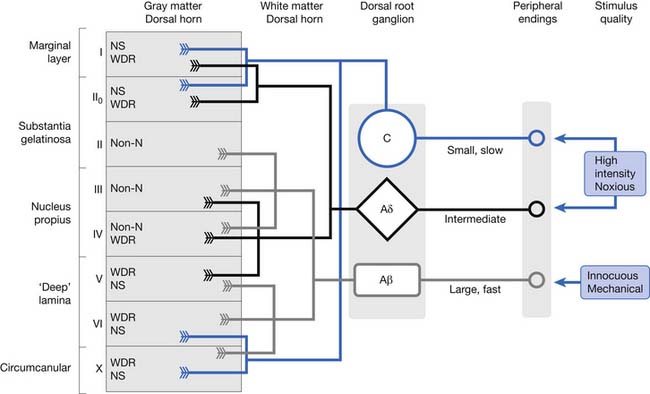
Aδ and C fibers convey nociceptive information primarily to superficial laminae (I and II) and deep laminae (V and VI) of the dorsal horn. Lamina I plays an important role in relaying information on the current state of tissues, including damaging mechanical stress, heat and cold, local metabolism (acid pH, hypoxia), cell breakdown (ATP, glutamate), mast cell activation (serotonin, bradykinin), and immune activity (cytokines).57 Aβ fibers transmit innocuous, mechanical stimuli to deeper laminae (III through VI). Lamina I cells are activated by nociceptive-specific neurons, whereas lamina V cells respond to wide dynamic range neurons of “wide” stimulus intensities. Wide dynamic range neurons receive input from mechanoreceptive Aβ fibers and nociceptive (Aδ and C) fibers (Figure 42-3). Normal synaptic transmission conduction of action potentials at the dorsal horn initiates neurotransmitter release. Low-intensity stimulations (i.e., brush, touch, or vibration) activate Aβ fibers only, releasing fast, glutamate-mediated postsynaptic currents. Fast excitatory transmission glutamate is coreleased presynaptically with neuropeptides such as substance P, CGRP, cholecystokinin, proteins (brain-derived neurotrophic factor), and glial-derived factors.179 Glutamate acts on a range of transmission cell receptors, such as N-methyl-D-aspartate (NMDA) (slow current), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (fast current), metabotropic glutamate receptors, and kainate ligand-gated ion channels. With normal transmission, sodium flows only through the AMPA receptor, whereas the NMDA receptor is blocked by magnesium. Prolonged depolarization of the postsynaptic cell causes voltage-dependent magnesium removal, opening the channel and allowing additional sodium and calcium to enter the cell. This amplified evoked response to subsequent input describes the process of wind-up.179
Central Sensitization
The term central sensitization describes a complex set of activation-dependent posttranslational changes occurring at the dorsal horn, brain stem, and higher cerebral sites. For example, at the dorsal horn, nociceptors release neurotransmitters (glutamate, substance P, and brain-derived neurotrophic factor) onto the transmission cells, which results in changes in activation of related receptors and channels (as described above). This results in an increase in calcium influx (and efflux from cytoplasmic organelles), contributing to potentiation of the cell by activation of calcium-dependent enzyme protein kinases (e.g., protein kinase C, cyclic AMP, and tyrosine receptor kinase).175 Posttranslational changes also include phosphorylation of NMDA and AMPA receptors, activation of second messengers such as nitric oxide, and central prostaglandin production.328
Ascending and Descending Modulation
Melzack and Casey’s classic descriptions of neuroanatomic pathways make a distinction between the lateral and medial pain systems corresponding to their relationship with the thalamus.201 The two systems are highly interdependent, the lateral (neospinothalamic) system generally representing sensory-discriminative dimensions, versus the medial (paleospinothalamic) system involving more motivational-affective and cognitive-evaluative dimensions of the pain experience. Additional ascending pathways, including the spinothalamic, spinomesencephalic, spinoreticular, spinolimbic, spinocervical, and dorsal column pathways, are described elsewhere.323
The lateral system projects to the ventral posterolateral and ventral posteromedial thalamic nuclei before projecting to the somatosensory and premotor cortices. The motor input is nearly as large as the sensory input, and this theoretically prepares the recipient of the painful input for the appropriate efferent (behavioral) response. The more medial pathway projects to the medial thalamic nuclei and limbic cortices, which include the anterior cingulated cortex, orbitofrontal cortex, and amygdala. The medial system involves important connections with periaqueductal gray, a key area involved in modulating nociceptive inhibition and behavioral responses to potentially threatening stimuli.219 Animal and human studies have identified the anterior cingulated cortex in regulating avoidance behaviors and the perception of pain unpleasantness.155 Only a small portion of these action potentials normally reach the thalamus and higher brain centers as a result of significant modulating or filtering effects at the spinal cord and brain stem. Of course, with prolonged pathology and inflammation these filters “break down,” contributing to central sensitization.
In addition to descending inhibition, the endogenous inhibitory system also includes local endogenous opioids (from periaqueductal gray), biogenic amines (serotonin and noradrenaline [norepinephrine]), and γ-aminobutyric acid (GABA), which generally act to inhibit pain signals. Important excitatory transmitters in this system include glutamate and substance P.151, 327 Besides descending inhibition from cortical areas, recent studies have suggested that descending facilitatory pathways might link brain stem and spinal cord areas via pronociceptive serotonergic281 and opioid mechanisms.79 These pronociceptive pathways could help explain the possible mechanism of persistent pain signs and symptoms, such as allodynia and hyperalgesia, that are common to chronic pain conditions.235
Pathways originating from the spinal cord dorsal horn activate brain structures involved in rudimentary aspects of the autonomic system response (i.e., escape, arousal, and fear), including the medulla and midbrain reticular formation, amygdala, hypothalamus, and thalamic nuclei.239 Activation of somatosensory cortices (S1–S2) provides information regarding the quality and intensity of pain.124 Affective aspects of the pain experience, such as pain unpleasantness, reflect more of the aversive qualities of the pain experience, such as the suffering component. Higher processing involves parietal and insular regions, contributing to an overall sense of intrusion and unpleasantness.243 Finally, convergence of these pathways with more frontal regions, such as the anterior cingulate cortex, is responsible for attention and emotional valence of the overall pain experience.
Although cutaneous and visceral pain share common cortical and subcortical networks, differences in response pattern, frequency, and processing might underlie differences in quality, affect, and resultant behavioral responses.289 Visceral pain has a more indistinct quality, poor localization, and in general is associated with autonomic markers such as bradycardia and hypotension. Cutaneous nociceptive reactions more classically involve protective reflexes such as tachycardia and hypertension.
Psychologic Issues Related to Chronic Pain
The physiatric approach to chronic pain conditions must include an understanding of the wide array of important psychologic (affective and cognitive) factors that impact the multidimensional experience of pain. Psychologic factors can serve to decrease or increase the subjective perception of pain and adjustment to ongoing pain-related disability. Affective factors usually include more negative emotions, such as depression, pain-related anxiety, and anger. Cognitive factors include catastrophizing, fear, helplessness, decreased self-efficacy, pain coping, readiness to change, and acceptance (Figure 42-4).
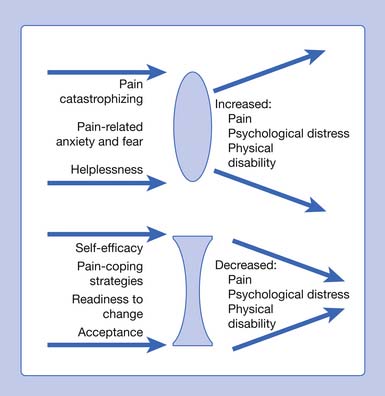
FIGURE 42-4 Factors associated with adjustment to pain.
(Modified from Keefe FJ, Rumble ME, Scipio CD, et al: Psychological aspects of persistent pain: current state of the science, J Pain 5:195–211, 2004, with permission.)
Affective Factors
Depression
A strong association between chronic pain and depression has been suggested.106,249,291 The prevalence estimates of major depression in patients with chronic pain conditions vary from 5% to 87%, and this variation could be due to a number of analytic factors, including the diagnostic criteria used, type of pain studied, and selection bias.9,83,99 Somatic symptoms of major depressive disorder can also be common in patients with chronic pain (i.e., change in appetite, change in weight, loss of energy, and sleep disturbance). The incidence of depression among chronic pain patients can be higher than with other chronic medical conditions.14 The presence of chronic pain might be related to longer durations of depressive symptoms.75,220 In general, most systematic reviews on the relationship between pain and depression suggest that chronic pain precedes depression.82 Predictors of depression in chronic pain include pain intensity, number of painful areas reported, frequency the severe pain is experienced, and a number of related psychosocial factors. Depressed patients can report higher levels of pain, be less active, report greater disability and life interference related to pain, and are more likely to display overt pain behaviors.148,156 Brown et al.32 examined the mediating factors of the relationship between chronic pain in patients with rheumatoid arthritis and decreased cognitive functioning, which included measures of inductive reasoning and working memory. Elevated depression mediated the relationship between higher levels of pain and reduced cognitive functioning,32 underscoring the importance of the complex relationship between depression, chronic pain, and functional impairment.
Anxiety
Anxiety related to pain is an important factor involved in maladaptive responses, behavioral interference, and affective distress. Heightened pain-related anxiety has been described as one of the most disabling aspects of ongoing chronic pain. It is closely related to avoidance activities (discussed below), which serve to promote ongoing pain, physical deconditioning, and social isolation.117 Anxiety as a psychologic construct in chronic pain has been developed by McCracken et al.188 as pain-related anxiety. Pain-related anxiety encompasses fear reactions across the cognitive, behavioral, and physiologic dimensions of pain. In chronic pain, it has been found to be a significant predictor of pain severity, disability, and pain behaviors.188
Anger
Ongoing failure to achieve pain relief and repeated unsuccessful attempts to escape pain have been shown to be associated with increased levels of anger and physiologic responses to pain, independent of pain intensity.3,131 In a study of patients presenting for chronic pain management, Okifuji et al.221 reported 70% of participants with angry feelings, most commonly with themselves (74%) and health care professionals (62%). In this study, anger toward oneself was associated with pain and depression, whereas “only anger” was related to perceived disability.
Conceptualizations of anger in chronic pain vary. A more classic definition of anger has been described as a “feeling involving a belief that a person one cares for has, intentionally or through neglect, been treated without respect, and a want to have that respect reestablished.”276 Anger as a construct has also been considered to be related to personality dispositions associated with unconscious conflicts,93 or as a reaction to the presence of ongoing unrelieved pain.78 Others have suggested that chronic pain might develop as a conversion-like symptom to suppress feelings of anger, and suppressed anger could be related negatively to adjustment to ongoing chronic pain.149 In contrast, “anger out” has also been linked to poor adjustment.35 These styles of anger management—suppression (anger in) and expression (anger out)—are distinguished from overt hostility. Hostility has been defined as “an attitude of cynical mistrustfulness, resentment, and interpersonal antagonism.”279 Burns35 has demonstrated how anger management style and hostility can affect maintenance and exacerbation of chronic low back pain via symptom-specific physiologic responses (i.e., increased muscle stress reactivity in lumbar paraspinals in patients with low back pain). This work was based on the studies of Flor et al.,85,87 who showed that patients with chronic low back pain exhibited greater stress-induced increases in electromyogram readings in lower paraspinal muscles compared with normal subjects. Anger and related physiologic responses are additional targets for pharmacologic and behavioral treatments, including relaxation training and other mind–body treatments.
Cognitive Factors
Many patients with chronic pain demonstrate a reduction in goal-directed activities and assume a more passive sedentary lifestyle. This further contributes to a downward spiral of inactivity, deconditioning, and increased somatic focus. Individual responses to pain are recognized as important variables of the pain experience and can be associated with a greater risk of maintaining pain-related disability. Patients who frequently have excessively negative thoughts about themselves, others, and the future are more likely to experience high levels of depression, low levels of activity, and increased tension.107,293 Pain beliefs (pain-related fear and self-efficacy), anger, and passive coping are important affective factors, which can significantly affect pain response, behavior, and function. Other neurocognitive factors, unique to each patient, including attention, expectation or anticipation, and appraisals, can contribute to maladaptive behaviors and can represent important targets for cognitive and behavioral interventions.29
Learning Factors
Operant Learning
Fordyce’s operant conditioning approach to pain serves as one of the earliest psychologic models for chronic pain.88 The model focuses primarily on observable behavioral manifestations of pain, which are subject to both reinforcement and avoidance learning. When an individual is exposed to a stimulus that causes tissue damage, an immediate response occurs that involves withdrawal or attempts to escape the stimulus. By successfully avoiding pain (i.e., “punishment”), the individual achieves a reduction in pain, thus rewarding the avoidance behavior. The acquisition of pain behaviors can be determined initially by the history of learned avoidance behaviors. In these cases, pain becomes a discriminating stimulus signaling behaviors that are pain reducing, such as rest and analgesic medication consumption. With time, pain-eliciting situations such as movement and activity cause anticipatory fear and are avoided. Over time, pain avoidance behaviors can generalize to other potentially painful stimuli, contributing to more inactivity and passivity.163,314 In a similar way, verbal expression of pain (e.g., complaining) and nonverbal pain behaviors (e.g., limping and grimacing) can be maintained by external reinforcement contingencies such as subtle rewards by significant others or family members who respond to these behaviors.
Waddell et al.315 identified a set of “nonorganic” signs that can be used as a simple clinical screening tool to help identify signs and symptoms of pain behavior (tenderness, simulation, distraction, and regional sensory and motor impairments). Although controversial, a study of nonorganic signs in a group of patients with low back pain found that demonstration of at least three of the five signs correlated with psychologic distress.315
Respondent Learning
The development of chronic pain can also be initiated and maintained by classical or respondent learning. In this case, an aversive stimulus is paired with a neutral stimulus and, with repeated exposures over time, the neutral stimulus will come to elicit an aversive response (i.e., fear). For example, a work-related lifting injury elicits an automatic response such as increased muscle tension and sympathetic arousal (fear and anxiety). Over time, fear and anxiety become associated with the once neutral stimuli of simple work-related activities, and lead to increased avoidance of the behavior.313 With chronic pain, the patient learns to anticipate negative consequences of activity, even in the absence of the noxious stimulus.
Fear of Movement
Kinesophobia, a term that describes an irrational and excessive fear of movement, physical activity, and reinjury, is exhibited by many patients with chronic pain.154 Fear of movement can be initially induced by classical conditioning but is reinforced through operant learning; by avoiding the conditioned anxiety and fear associated with movement, the patient never extinguishes the fear. It has been shown in studies to strongly correlate with other responses, such as catastrophic thinking and subsequent increased fear and avoidance behaviors in chronic low back pain patients. In this way, increased levels of fear and disability can occur independently of the experienced pain intensity.312 McCracken et al.190 found that increased fear and anxiety in low back pain subjects correlated with decreased range of motion and increased expectation of pain. Other studies in chronic low back pain have found pain-related fear and fear-avoidance beliefs as predictors of disability, decreased activities of daily living, and lost work time.193 In a Dutch study of patients presenting to a primary care clinic with chronic low back pain, approximately 70% of participants agreed to the statement “Simply being careful not to make unnecessary movements is the safest thing I can do to prevent back pain,” and approximately 50% endorsed the statement “Back pain always means that the body is injured.” In addition, patients who reported pain-related fear had increased risk for disability at 6 months (odds ratio = 4.6, 95% confidence interval).230
A cognitive behavioral model emphasizes two opposing behavioral responses: confrontation and avoidance. Waddell et al.’s conclusion that “fear of pain and what we do about pain can be more disabling than pain itself”316 underscores the importance of identifying and treating such maladaptive thinking and behavior in a physiatric approach to effectively managing chronic pain.
Behavioral Treatment Approaches
Operant Behavioral Techniques
Operant behavioral therapy refers to interventions focused on the observed behavior of the patient. As proposed by Fordyce,88 operant models of pain are based on both positive and negative reinforcement contingencies. Environment and social factors serve to maintain pain behaviors. For example, the verbal expression of pain and nonverbal pain behaviors (e.g., grimacing and guarding) can be maintained by both positive (attention from others, potential monetary gain) and negative reinforcement (nonoccurrence of aversive stimuli, avoidance of activity).146 Once identified, these behaviors serve as targets for treatment. Many times these behaviors need to be reinforced only intermittently. Operant behavioral therapy can be most useful and practical with patients demonstrating excessive pain behaviors despite limited tissue pathology, poor insight into the relationship of their own behavior and subjective experiences of pain, and operant-related issues (secondary gain).
Goals of operant behavioral therapy include encouraging the development and acquisition of more adaptive pain management strategies, which include establishing wellness behaviors and discouraging or reducing reinforcement of pain behaviors.55 The theory suggests that both wellness and pain behaviors can be shaped. Management techniques target unlearning these behaviors and serve as the basis of most functional restoration-based programs developed by Mayer and Gatchel.185 Operant behavioral therapy approaches can be delivered in individual sessions and in group settings. Operant behavioral therapy techniques that patients can master and apply include pacing and graded exercise, scheduling and/or limiting pain medications and passive treatments, and social reinforcement via spouse and family training.
Cognitive Behavioral Techniques
Cognitive therapy techniques are based on the notion that one’s cognitions can have an impact on mood, behavior, and physiologic function.17 Techniques used in pain management are designed to help patients notice and modify the negative thought patterns that contribute to ongoing pain and affective distress. These include cognitive restructuring, problem solving, distraction, and relapse prevention.318 Five primary assumptions underlie all cognitive behavioral therapy interventions (Box 42-1).28 Cognitive behavioral therapy is a flexible, viable, and empirically validated approach for effectively treating patients with persistent pain.55,211
BOX 42-1 Five Primary Assumptions That Underlie All Cognitive Behavioral Therapy Interventions
From Bradley LA, McKendree-Smith NL, Cianfrini LR: Cognitive-behavioral therapy interventions for pain associated with chronic illness, Semin Pain Med 1:44-54, 2003, with permission.
Sleep and Chronic Pain
Sleep is a dynamic, complex physiologic process that is required for survival. During sleep there is decreased sensitivity to the external environment and increased activity of the parasympathetic nervous system. Sympathetic nervous system activity is similar to that in wakefulness, except for during periods of rapid eye movement (REM). Breathing is irregular, and control of body temperature is altered. Sleep comprises alternating REM and non-REM (NREM) states that cycle at an ultradian rhythm of approximately 90 minutes.252
Sleep of 8 to 8.5 hours is considered restorative in adults. Sleep is entered through NREM, and the NREM–REM cycle occurs three to six times during a normal 8-hour sleep period. The stages of sleep are stage 1 (light sleep), stage 2, stage 3, stage 4 (deep or delta-wave sleep), and REM sleep. Stage 1 is a transitional stage between wakefulness and sleep. It comprises 2% to 5% of total sleep time and occurs when initially falling asleep and during brief arousal periods within sleep. Stage 2 is marked by sleep spindles and K complexes and occurs throughout the sleep period, accounting for 45% to 55% of total sleep time. NREM stages 3 and 4 occur primarily during the first third of sleep and are commonly referred to as delta-wave, slow-wave, or deep sleep. They account for approximately 20% of total sleep time. REM sleep is characterized by fast electroencephalograph activity, skeletal muscle atonia, REM, and bursts of autonomic activity. The last third of sleep is primarily spent in REM.252
The determinants of sleep are numerous and include homeostasis, the circadian rhythm, control via the ventrolateral preoptic nucleus, age, drugs, external temperature, medical and psychiatric disease, and other environmental factors.252,256 The ventrolateral preoptic nucleus has been shown to contain GABAergic and galaninergic neurons that are necessary for normal sleep.263 Lesions to this region have been shown to decrease both REM and NREM sleep by 55%, verifying their function in inhibiting the firing of cells involved in wakefulness.170 These inhibited neurons contain the neurotransmitters histamine, norepinephrine, serotonin, hypocretin, and glutamate. Age represents a strong determinant of sleep, as time spent in stages 3 and 4 decreases by 10% to 15%, latency to fall asleep increases, and the number and duration of overnight arousal periods increase in elderly persons compared with young adults.256
The interrelationship between disturbed sleep and chronic pain conditions is well documented for both adults and adolescents.208,209,226,231,278 Prevalence estimates of disturbed sleep range from approximately 50% to 90% depending on the clinical study population under evaluation.38,208,209 Although the nature of the relationship between pain and disturbed sleep is not well understood, a reciprocal association is suggested.2,278 Current research suggests a multifactorial relationship including depression, fear-avoidance behaviors, catastrophizing, and even treatments such as benzodiazepines and chronic opioid therapy.174 Patients with chronic pain can display frequent sleep fragmentation, longer sleep latency, and decreased overall quality of sleep.37 Sleep fragmentation is characterized by repetitive short interruptions in sleep and is a recognized factor in the cause of excessive daytime sleepiness.284 This inability to maintain sleep can be the most important factor in the treatment of disturbed sleep in individuals with chronic pain. The strength of this relationship between disturbed sleep and chronic pain cannot be underestimated. In fact, a study of 679 subjects with chronic widespread pain (CWP) found that although rapid onset of sleep, absence of early awakening, and restorative sleep were associated with the resolution of CWP, after adjusting for the effects of psychosocial factors, restorative sleep was independently associated with the resolution of chronic widespread pain.63
Assessment
The assessment of chronic pain involves a thorough physical examination and a comprehensive evaluation of pain intensity and psychosocial factors related to ongoing pain experience and interference with sleep, daily activities, family life, and employment. Subjective reports of pain intensity are an important part of the initial assessment and subsequent visits, and can include pain intensity numeric rating scales, visual and verbal analogue scales, and pain drawings. Self-monitored pain intensity ratings are both reliable and valid.133 Patient variability remains, however, when interpreting self-report measurement scales. Recent work has examined the level of change that best represents a clinically important improvement with the use of the numeric rating scale in monitoring pain response with drug treatment trials. Farrar et al.76 found that a reduction of approximately 30% represented a clinically important difference. A commonly used comprehensive measure of pain intensity, the McGill Pain Questionnaire Short Form, measures three dimensions of pain: sensory, affective, and evaluative. It uses 20 subclasses or groupings of pain adjectives, including sensory (e.g., “sharp,” “dull,” and “heavy”) and affective (e.g., “annoying,” “tiring,” and “exhausting”); it also includes pain drawings and the visual analogue scale.200
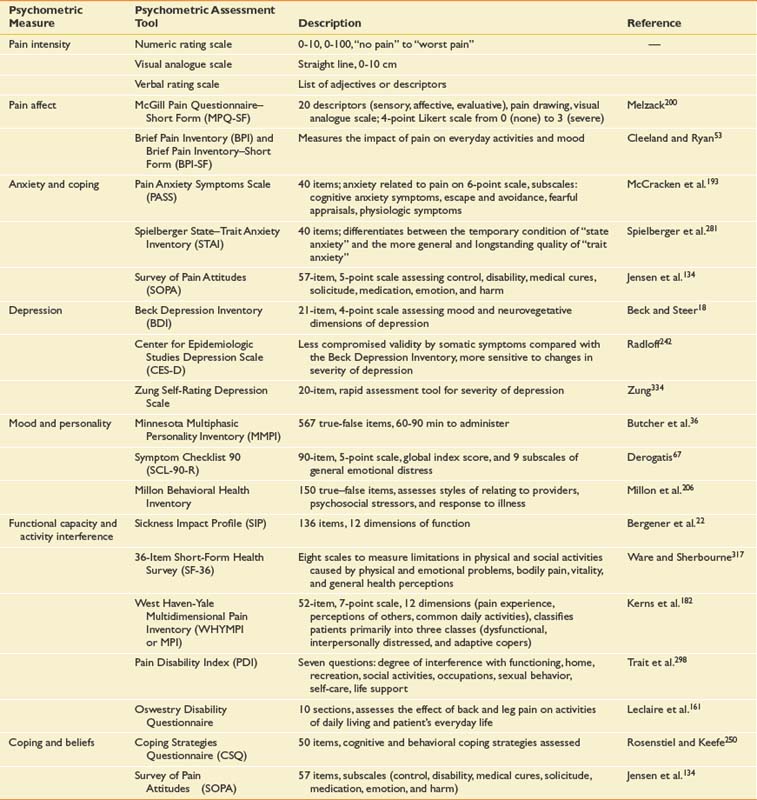
Additional psychometric measures can also be included in the initial assessment focusing on psychosocial factors such as mood (depression, anxiety, and anger), attitudes, beliefs, functional capacity, activity interference, and personality traits (Table 42-5). The use and combination of these different methods depend largely on the goal of the assessment. A semistructured interview by an experienced psychologist is the most comprehensive means of evaluating the psychologic state of the patient.28 A packet of self-reported questionnaires completed by the patient before the evaluation, measuring a wide spectrum of the multidimensional factors related to pain, can be used in isolation or as an adjunct to the psychologic and medical interview.
Pain disorder can be associated with a psychologic and/or a general medical condition.4
Treatment
The ultimate goal of a rehabilitation-based approach to chronic pain is the reduction of pain and the restoration of function. The physiatrist plays a critical role in the assessment and management of chronic pain conditions and leads the team of health care professionals in achieving this goal of maximal functional recovery. The treatment of chronic pain conditions has been practiced according to a number of different patient care models. Regardless of the setting, recent data suggest that chronic pain management is best addressed using a biopsychosocial assessment and approach to treatment.106,192,299 The traditional biomedical model fails because it focuses on the identification and treatment of a specific anatomic pain generator without accounting for the psychologic determinants involved in the pain experience. The treatment goals of chronic pain management encompass the acceptance and reduction of pain, maximal restoration of functional mobility, restoration of sleep, improvement in mood, return to leisure activity, and return to work (Box 42-2).
Pain Treatment Programs
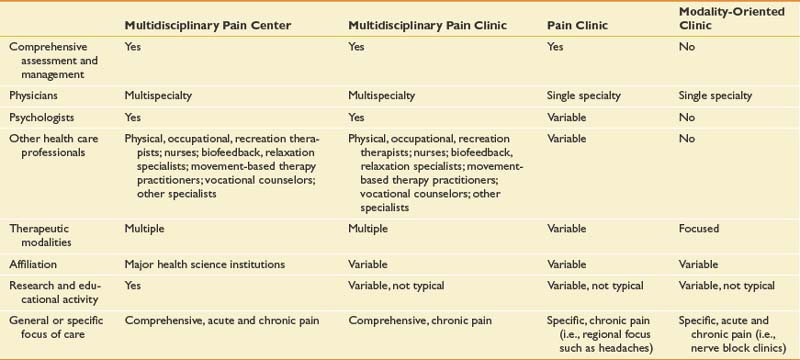
The IASP classifies four types of pain treatment programs (Table 42-6).166 In general, multidisciplinary treatment centers or clinics might or might not include a formal interdisciplinary collaboration model. Although the terms multidisciplinary and interdisciplinary are many times used interchangeably, multidisciplinary more formally refers to collaboration with members of different disciplines (including various medical specialists and therapists) managed by a leader who directs a range of ancillary services. Team members assess and treat patients independently and then share information. Interdisciplinary describes a deeper level of a consensus-based collaboration where the entire process (i.e., evaluation, goal setting, and treatment delivery) is orchestrated by the team, facilitated by regular face-to-face meetings, and primarily delivered within a single facility.186 Multidisciplinary and interdisciplinary program facilities can be accredited by the Commission on Accreditation of Rehabilitation Facilities, with established treatment standards and ongoing outcome measurement. The interdisciplinary team is commonly led by a physiatrist or other pain specialist and includes physical and occupational therapists, pain psychologists, relaxation training experts, vocational rehabilitation and therapeutic recreational specialists, social workers, and nurse educators (Box 42-3). A key process in multidisciplinary treatment is the comprehensive evaluation. This usually incorporates a thorough musculoskeletal evaluation, psychologic assessment, and, in patients with work-related injuries, a vocational rehabilitation interview. The evaluation enables the team to assess patient motivation and realistic goals for return to function and/or work. Those patients accepted for treatment are placed in a structured outpatient environment with one-on-one and group-based treatments.
Multidisciplinary and Interdisciplinary Approaches
Interdisciplinary Treatment
Interdisciplinary biopsychosocial rehabilitation-based programs have been increasingly and successfully used in the treatment of patients with chronic pain and related psychosocial dysfunction.8,103,116,141,247 Comprehensive reviews of the clinical and cost-effectiveness of interdisciplinary programs have demonstrated significant improvements in return to work, function, reduced health care use, and closure of disability claims.19,299 Positive functional results have been shown in patients classified as having both short-term and long-term disability at the onset of care.141 These comprehensive programs have also shown clear benefits over conventional management in regard to decreasing pain behavior and improving mood.
Scope and intensity varies, with most outpatient-based centers offering part-time (2 days/wk) or full-time (5 days/wk, 6 to 8 hr/day) programs lasting 4 to 6 weeks in total duration. The interdisciplinary model provides ongoing communication for all members of the treatment team, helping to facilitate patient progress while they progress the behavioral, cognitive, and active therapy treatments. Patients are discussed individually in a team conference format on a weekly basis, enabling ongoing communication of progress and adjustment of treatment goals. Physician follow-up visits two or three times per week are ideal for ongoing pharmacologic trials (targeted for improving mood, disturbed sleep, and analgesia) and encouraging progress across the multiple therapy domains.
At completion of treatment, patients are encouraged to continue with their own individually structured home exercise, aerobic, and stretching program. They should also be independent in their own use of various relaxation and pacing techniques. The identification of a chronic pain condition as a chronic disease imparts an important facet into the continued care of these patients. As with a chronic disease such as diabetes or hypertension, self-control and self-management of symptoms are critical for successful treatment. It logically follows that chronic pain should be treated as any other chronic disease or illness, in that regular follow-up evaluation and reassessment of psychosocial and physical function be performed.21,159,299
Multidisciplinary Team
Physical Therapy and Occupational Therapy
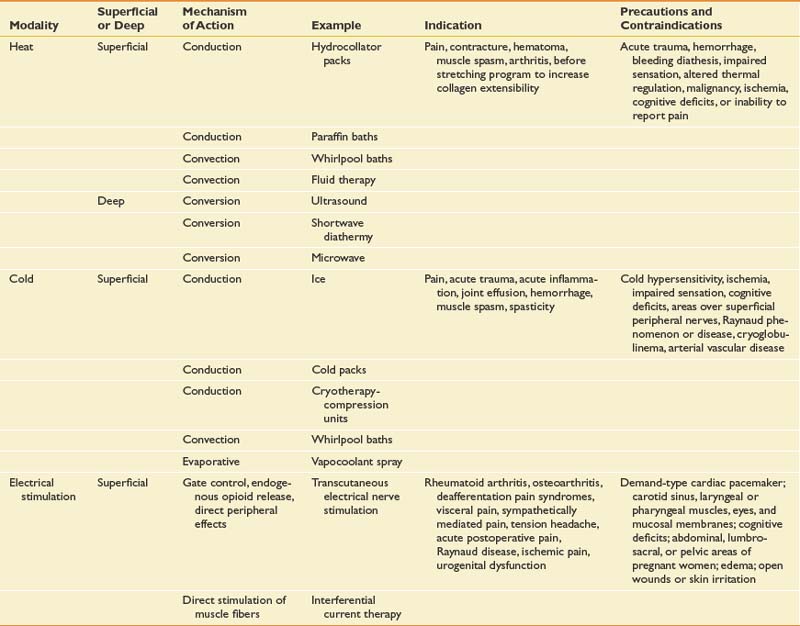
Physical and occupational therapists use active and passive therapeutic exercises, manual techniques, and passive physical modalities (Table 42-7) to address deficits in flexibility, strength, balance, neuromuscular control, posture, functional mobility, locomotion, and endurance. Both types of therapists help patients to overcome fear of movement. Although there is some crossover between the skill sets of physical and occupational therapists, they possess established core competencies that are fairly universal. Physical therapists specialize in gait training and locomotion, core stability, lower extremity biomechanics, and functional mobility, as well as activities of daily living such as bed mobility and transfers. They are also experts in the development of aerobic conditioning programs aimed at improving cardiopulmonary health and endurance. Occupational therapists typically concentrate on educating patients regarding proper posture and ergonomics related to upper limb functional activities such as lifting and computer usage. They address upper extremity-related activities of daily living including feeding, hygiene, grooming, bathing, and dressing. Physical and occupational therapists also play a primary role in the education of patients, family members, and other caregivers.
Psychology
Pain psychology assessment and therapeutic interventions focus on both cognitive and behavioral factors related to pain. As noted earlier, key psychologic factors involved in the development of and adaptation to chronic pain include anxiety and fear-avoidance behavior,147,189,313 pain catastrophizing,147,270,292,304 and helplessness.147 Factors identified with improvement in adjustment to chronic pain include self-efficacy, pain coping strategies, readiness to change, and acceptance.147 Psychologic intervention focuses on unlearning maladaptive responses and reactions to pain, while fostering self-efficacy, wellness, improved coping, perceived control, decreased catastrophizing, and acceptance (see Figure 42-4).147 Phases of individual and group-based treatment include education, skills training, application, and relapse prevention.
Vocational Rehabilitation
The Centers for Medicare and Medicaid Service define vocational rehabilitation as “the process of facilitating an individual in the choice of or return to a suitable vocation.”45 Title I of the Rehabilitation Act of 1973 describes the goods or services provided to render a individual with handicap employable. Vocational rehabilitation counselors should be involved with patient care early in the process of chronic pain management to ensure identification of employment as a long-term goal for the patient.
Vocational counselors participate in the analysis of current or previous job descriptions, provide suggestions for work accommodation or modification, and if necessary facilitate vocational testing and targeted retraining. At the end of the rehabilitation process, vocational rehabilitation can help coordinate functional capacity evaluation testing and finalize return to work issues (i.e., restrictions and level of work). Vocational rehabilitation counselors acquire information from the physical therapists, occupational therapists, and physicians to address instructions as to limitation of duty (full or limited) and the functional restrictions or modifications that might be required. These restrictions or modifications include sitting or standing tolerance, walking, pushing, pulling, stair usage, bending at the waist, sustained postures, hot or cold tolerance, data entry or other repetitive tasks, grip, tool usage, and vibration factors.
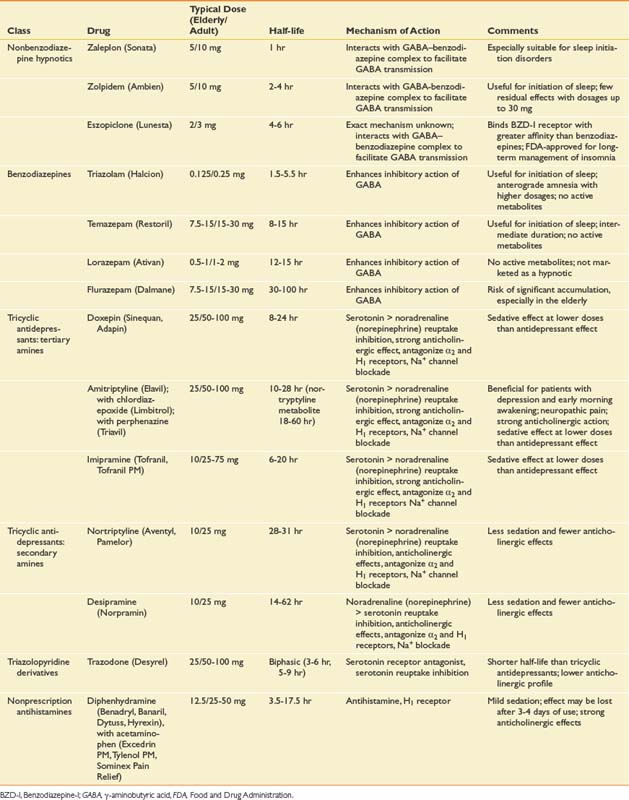
Medications
Nonsteroidal Antiinflammatory Drugs and Cyclooxygenase-2 Inhibitors
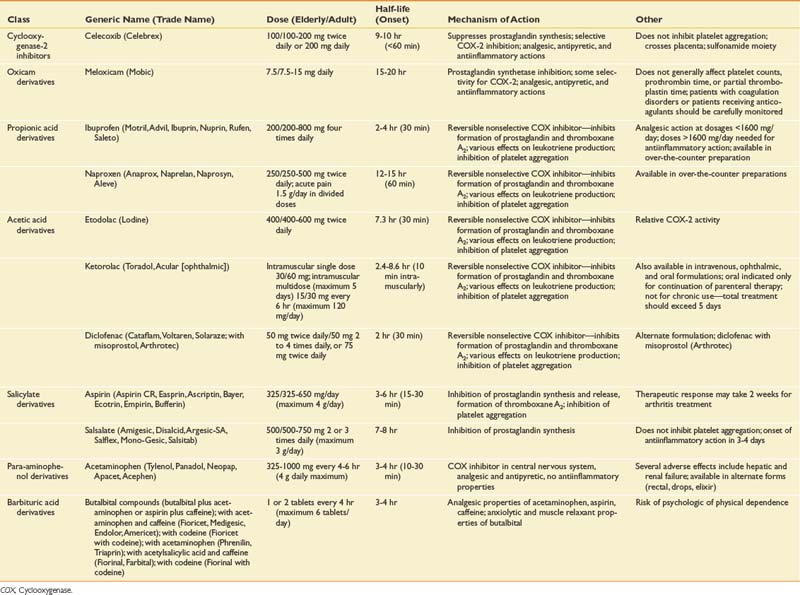
Conventional (i.e., nonspecific) nonsteroidal antiinflammatory drugs (NSAIDs) have been a first-line treatment for analgesia and the treatment of inflammatory conditions, including osteoarthritis, rheumatoid arthritis, and the various musculoskeletal-related conditions.288,308 COX-1 and COX-2 isoforms catalyze the conversion of arachidonic acid to prostaglandins. More recent classification of NSAIDs is as follows:
Conventional nonselective NSAIDs were found to offer effective analgesic responses but are limited by potential upper gastrointestinal bleeding and ulceration, renal toxicity, and platelet dysfunction.49
The isolation of the COX-2 protein in the early 1990s led to the development and release of a new class of NSAIDs, the COX-2 inhibitors. The oral COX-2 inhibitors available and approved by the Food and Drug Administration (FDA) at some time in the United States include celecoxib (Celebrex), rofecoxib (Vioxx), and valdecoxib (Bextra). Only celecoxib remains on the market in the United States. Meloxicam (Mobic) is an NSAID with preferential COX-2 selectivity that spares COX-1 at approved doses. It can be considered as a special agent in the COX-2 class (Table 42-8).225 COX-1 is constitutively expressed in most tissues, being responsible for homeostatic functions such as platelet aggregation and the maintenance of upper gastrointestinal mucosa integrity by producing protective prostaglandins. COX-2, a largely cytokine “inducible” constitutive isoenzyme, is primarily responsible for producing inflammation and pain. Animal neuropathic pain models have demonstrated up-regulation of COX-2 by early inflammatory changes in various types of peripheral nerve injury,173 as well as COX-2 induction in dorsal horn neurons and other regions of the central nervous system.261 This suggests COX-2 involvement with peripheral and central sensitization processes, as well as involvement in key mechanisms underlying neuroplastic changes in chronic pain states.
Major studies, including the Vioxx Gastrointestinal Outcomes Research (VIGOR) trial, the Celecoxib Long-term Arthritis Safety Study (CLASS), and the Safety and Efficacy Large-Scale Evaluation of COX Inhibition Therapies (SELECT) trial, demonstrated significant safety benefits of COX-2 inhibitors compared with nonselective NSAIDs with regard to reduced incidence of symptomatic gastric ulcers and renal toxicity.26,66,228,273 The VIGOR trial, however, reported a 2.38-fold increase in relative risk of cardiovascular events among study patients with rheumatoid arthritis randomly assigned to rofecoxib treatment.26 Reevaluation of the VIGOR study suggested that the higher rate of cardiovascular events in the treatment group (rofecoxib) compared with in the naproxen group could be due to additional cardioprotective effects of naproxen,59 although ongoing questions remain.
Despite billions of dollars in sales and the widespread use of COX-2 inhibitors, questions and concerns reemerged regarding the potential increased risk of cardiac events, including myocardial infarction and sudden cardiac death.152,213 Some have proposed that selective COX-2 inhibitors can decrease vascular prostacyclin (PGI2) production, interfering with the balance between prothrombotic and antithrombotic eicosanoids (thromboxane A2) and increasing the likelihood of a prothrombotic state manifested by possible cardiac events.118 Merck voluntarily withdrew rofecoxib from the market in 2004, after a trial involving the use of high-dose rofecoxib in adenomatous polyp disease found an increased risk for serious cardiovascular events in patients taking the drug compared with those patients on a traditional NSAID. Other studies have found conflicting results when looking at other agents and possible “class effects” with increased incidence of myocardial and renal events. Other studies subsequently demonstrated similar cardiac effects with naproxen, causing many practitioners to reassess the long-term use of nonselective NSAIDs and COX-2 inhibitors for management of chronic spine and osteoarthritic conditions.
Based on a 2004 review of available data from long-term placebo- and active-controlled clinical trials of NSAIDs, the U.S. FDA concluded that an increased risk of serious adverse cardiovascular events might be a class effect for NSAIDs (excluding aspirin) and requested that the package insert for all NSAIDs be revised to include a boxed warning to highlight the potential increased risk of cardiovascular events and the risk of serious and potentially life-threatening gastrointestinal bleeding.294 A number of topical diclofenac preparations have been approved in the United States for the management of sprains (diclofenac epolamine patch 1.3% [Flector Patch]) and pain related to osteoarthritis in joints amenable to topicals (diclofenac sodium gel 1% [Voltaren gel]). These topical preparations and over-the-counter products all carry the identical black box warning, although systemic effects of topical agents are much less than oral preparations (5% to 10%). Physicians need to assess relative risks versus potential benefits (analgesia, decreased stiffness, and improved function) on a case-by-case basis. Ongoing monitoring of blood pressure, cardiac, and renal status is recommended with acute and chronic use of both nonselective NSAIDs and selective COX-2 inhibitors.10,234
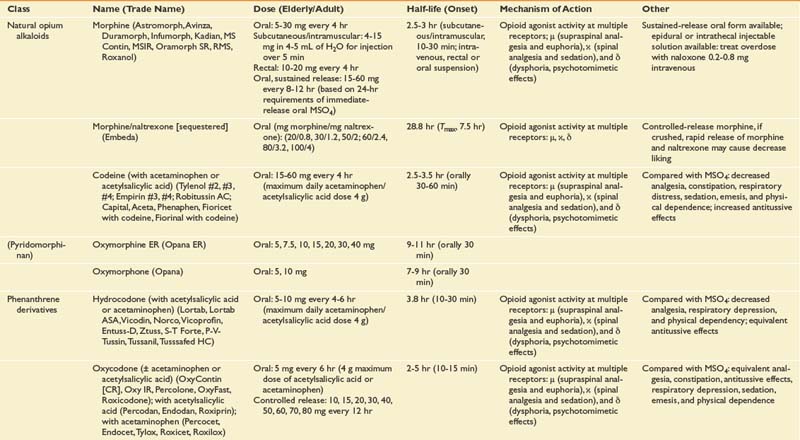
Opioid Analgesics
Opioid and opioid-like medications are potent analgesics (Table 42-9). Opioids work by binding to three receptor types (μ, δ, and κ) belonging to a G-protein receptor family. Presynaptic effects of opioids decrease calcium into the cell, inhibiting subsequent release of excitatory neurotransmitters (serotonin, norepinephrine, substance P, and glutamate). Postsynaptic effects include increasing potassium efflux, resulting in hyperpolarization of the neuron, decreasing synaptic transmission.279 At the brain stem level, opioids inhibit GABAergic transmission, leading to excitation of descending inhibition.126
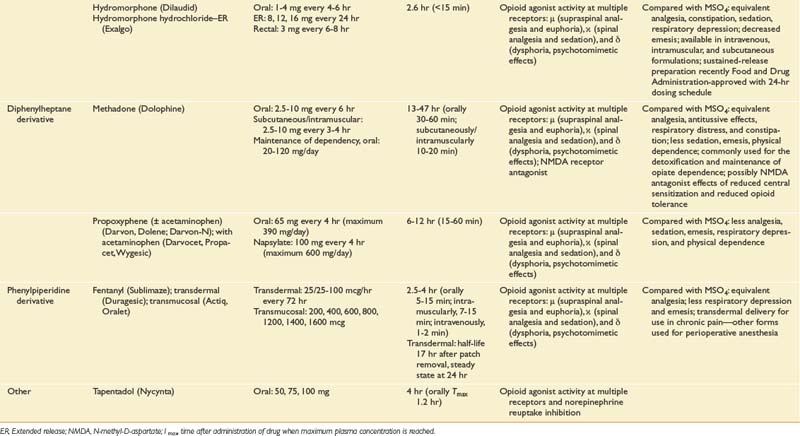
Longer-acting oral opioid medications are available and include extended release morphine (MS Contin, Avinza, and Kadian), oxycodone (OxyContin), oxymorphone (Opana) formulations, and transdermal fentanyl products (Duragesic). Transdermal fentanyl systems deliver the medication across the skin into a drug reservoir or depot beneath the dermis, enabling steady release directly into the bloodstream over a 72-hour period, again bypassing first-pass metabolism. Recent advancements in opioid management include rapid-onset fentanyl transmucosal and transbuccal (Actiq, Fentora, Onsolis) formulations approved by the FDA more specifically for breakthrough cancer-related pain. The highly lipophilic character of fentanyl allows for rapid absorption across the buccal mucosa, with transmucosal delivery providing peak serum levels less than approximately 25 minutes after initiating dosing depending on the formulation type. Abuse-resistant and -deterrent formulations are presently under development as a means of decreasing the abuse liability of an opioid. Morphine sulfate and naltrexone extended-release capsules (Embeda, King Pharmaceuticals, Bristol, Tenn.), extended-release morphine formulations combined with a sequestered low-dose naltrexone core, were the first of this potentially promising class of opioid to be approved by the FDA in 2009 for the management of pain. Crushing Embeda leads to significant release of the sequestered naltrexone, an opioid antagonist, decreasing the liking effect of the medicine, as studied in recreational drug users.283 The formulations’ ability to limit abuse and addiction has not been proven and will depend on subsequent postmarketing data surveillance.
Short-acting opioids include morphine, hydromorphone, and many combination opioid products that combine hydrocodone or oxycodone with acetaminophen or ibuprofen (Vicodin, Lortab, Percocet, and Vicoprofen). Tapentadol (Nucynta), approved by the FDA in 2009, is a short-acting opioid with a dual mechanism of action: traditional μ-agonism and norepinephrine reuptake inhibition. Noradrenergic effects can include increasing descending inhibition via blocking reuptake of norepinephrine centrally and/or norepinephrine effects more peripherally, limiting gastrointestinal adverse effects (i.e., nausea and vomiting).61,305 It is important to understand basic pharmacologic principles related to these agents, including absorption, bioavailability, plasma half-life, peak onset, and duration of analgesia, when selecting appropriate agents (see Table 42-9). For example, sustained-release oxycodone as sustained-release OxyContin provides a bimodal release system with bimodal peak serum release at 0.6 and 6.8 hours dosed on a twice-daily schedule. Variability of pharmacokinetics between patients can necessitate adjustment from standard dosing regimens. A recent survey of a large university-based chronic pain clinic found that a significant number of patients on chronic sustained-release oxycodone management required dosing more frequently than twice daily (every 8-hour dosing) (67%). Many patients in this clinic required greater than half the amount of rescue medication compared with the every 12-hour dosing group.181 Oxymorphone (Opana ER), a ketone-substituted morphine, is relatively more lipophilic than morphine and is relatively more potent with a longer duration of action.
Methadone
The use of methadone has experienced a rebirth in its use as a “novel” opioid analgesic. Long used in addiction medicine and opioid maintenance programs, this synthetic opioid has a number of potentially unique advantages compared with other opioids. Methadone hydrochloride is a relatively potent NMDA receptor antagonist. NMDA activation has been implicated as a key player in neural plasticity (i.e., central sensitization)194 and as a mediator of opioid tolerance.115 Methadone offers other potential advantages, including low price and no active metabolites. Clinicians prescribing methadone need to use caution,321 however, because methadone accumulates in tissues with repeated dosing, creating an extensive reservoir, and is highly protein-bound. Half-life varies from 7 hours to 5 days. Dosing changes must be done at least every 5 to 6 days. Morley and Makin210 describe standardized conversion regimens for converting oral morphine equivalents to methadone; for example, with 24-hour doses less than 300 mg, a fixed dose one tenth the actual morphine dose every 3 hours as required for 5 days. On day 6, the amount taken over the previous 2 days is averaged to a daily dose, which is then taken on a regular fixed twice-daily schedule.210
Outcomes of Opioid Management for Chronic Pain
Controversy continues regarding the use of long-term opioid therapy for chronic nonmalignant pain, and relates primarily to fears of possible development of iatrogenic addiction and abuse.140,300 Clinical practice guidelines support an underlying assumption that safe and effective therapy requires clinical skills and knowledge in both the principles of prescribing, ongoing assessment, and management of risks of opioid treatment (including abuse, addiction, and diversion).50 Important areas of management include patient risk stratification, informed consent and patient-centered goal setting, opioid trial and plan for discontinuation, abuse and diversion monitoring (urine toxicology screening), boundary setting and closer monitoring with high-risk patients, and understanding and effectively treating opioid-related adverse effects (i.e., constipation, nausea, somnolence, and endocrine dysfunction). Studies have demonstrated modest to moderate levels of analgesia in chronic pain but varied results when examining improvement in functional status.131,237,267 Moulin et al.212 examined the effects of sustained-release morphine with active placebo in a double-blind randomized controlled trial in soft tissue or musculoskeletal pain, and failed to demonstrate any functional or psychologic improvements despite significant pain reduction. In a study of patients on long-term opioid therapy, stable long-term pain control was achieved in those patients who finished a 12-month trial (56%) with sustained transdermal fentanyl at doses of approximately 90 mcg/hr.205 Others have demonstrated that chronic opioid therapy can lead to a so-called pain opioid downhill spiral characterized by loss of functional capacity and corresponding increase in depressed mood.267 Studies attempting to corroborate these findings found support for the downhill spiral but also that opioid use independently failed to explain a comparable amount of variance in illness behavior. On the contrary, associated benzodiazepine use was found to be associated with functional impairment, increased health care use, depression, and pain.51 Assessing patients’ individual psychosocial factors and analgesic response to COAT should incorporate ongoing monitoring of functional improvements and assessment for signs or symptoms of possible addictive or aberrant use (see Table 42-2 for definitions).319 The physician should be aware of local, state, and federal laws and regulations guiding controlled substance use. Careful individual patient assessment and monitoring, which can include urine and serum toxicology testing, are recommended.139
Legislative and Federal Scrutiny
The more liberal prescribing practices of the late 1990s have been slowed after the realization of increased incidence of addiction, abuse, recreational drug use among nonpain patients, and diversion. Increased focus on more comprehensive patient pain and psychologic assessment, as well as standardized office screening and monitoring practices (formal patient–physician treatment agreements), have become basic standards of practice for physicians choosing to prescribe opioids and other controlled substances. A “universal precautions” (Box 42-4) approach based on a similar approach to limiting the risk for infection in the infectious disease model has been developed as the basis for a more comprehensive assessment, monitoring, and treatment approach in prescribing opioids.112
Patient–physician treatment agreements have been published by a number of national pain organizations, including the American Pain Society and the American Academy of Pain Medicine. Potential complications of agreements include perpetuating stigmas on the patient as a result of the patient believing opioid prescribing to be a problem, the binding nature of contracts remaining unclear, and the existence of significant variability among the content of agreements. This variability includes many contracts with statements limiting driving, although such limitations are not clearly supported in the literature.84 The use of a trilateral opioid contract has been proposed, which includes the collaboration of the primary care physician, the pain physician, and the patient, as a means of effectively transferring care and responsibility for long-term opioid management back to the primary care physician once the individual opioid regimen is stabilized.84
Cognitive Functioning With Opioid Therapy
Controversy remains regarding the effects of COAT on psychomotor functioning.331 Haythornthwaite et al.122 studied the effects of chronic opioid therapy on cognition and mood in a group of chronic pain patients before and after achieving stable opioid doses. Besides reducing pain, long-acting opioid medication reduced anxiety and hostility without declines in cognition, while demonstrating improvements on measures of psychomotor speed and sustained attention.122 Fishbain et al.’s recent comprehensive review of studies81 found that there was moderate, generally consistent evidence for no impairment of psychomotor abilities, inconclusive evidence for no impairment on cognitive functioning, and strong consistent findings of no evidence of greater incidence of motor vehicle accidents or violations. Others have argued that transient cognitive and psychomotor impairment can be more evident during dose escalation and in opioid-naive patients.33
Endocrine Effects of Long-Term High-Dose Opioid Management
Animal models and some case study reviews in humans have suggested that chronic high-dose opioid therapy can cause abnormalities in hypothalamic–pituitary–adrenal axis and in hypothalamic–pituitary–gonadal secretion.60,248 Endocrine effects can include deceased testosterone, progesterone, and estradiol (resulting in decreased libido in men and women); amenorrhea; and reduced cortisol response to stress. These effects can be more common with administration of intraspinal opioids.1,224 The syndrome of opioid-induced androgen deficiency has been described in case series and can require additional screening and treatment (testosterone supplementation) by the prescribing physician.
Opioid Hyperalgesia and Pronociceptive Effects
Progress in understanding cellular and neuromodulatory mechanisms involved in addiction and pain treatment point to possible pronociceptive effects of chronic opioid therapy, independent of more widely accepted and understood neural mechanisms involved with the desensitization process of tolerance (a pharmacologic phenomenon characterized by the need to increase the dose over time to maintain the same opioid analgesic effect). Several mechanisms contribute to these cellular adaptive processes, including receptor desensitization most probably mediated by the NMDA receptor cascade.180 Repeated administration of opioids might not only contribute to tolerance but might also lead to a pronociceptive cascade of events (opioid-induced abnormal pain sensitivity) representing a sensitization process supported by evidence of increased spinal dynorphin, descending central facilitation, and activation of pronociceptive glutamate. Some clinicians have suggested maintaining opioid doses at the lowest level required to achieve analgesia as a means of limiting these cellular processes. Periodic opioid rotation and the use of NMDA receptor antagonists and other nonopioid medications within a rational polypharmacy approach have been suggested to limit escalating doses of opioids and possible pronociceptive and tolerance effects.13 Opioids with possible NMDA antagonist effects include methadone and propoxyphene. Nonopioid NMDA antagonists include dextromethorphan, ketamine, and memantine. Formal randomized placebo-controlled studies are lacking on the use of these medications.
Anticonvulsant Medications as a Treatment for Neuropathic Pain Conditions
Neuropathic pain has been defined as pain “initiated or caused by a primary lesion or dysfunction in the nervous system.”203 Neuropathic pain manifests as spontaneous pain (stimulus-independent, i.e., paresthesia and dysesthesia) or pain hypersensitivity caused by a stimulus after damage or changes in the sensory neurons (stimulus-evoked pain, i.e., allodynia and hyperalgesia).71 Peripheral mechanisms include sensitization of nociceptors by local chemical inflammatory changes (substance P, serotonin, bradykinin, histamine, and COX and lipoxygenase pathways); ectopic activity from damaged, demyelinated, or regenerating nerve sprouts; noradrenergic sensitivity; lowering of neuronal threshold for firing at ectopic areas by accumulation of sodium and calcium channels; and changes at the more proximal dorsal root ganglion (i.e., spontaneous activity).15 Peripheral changes can also lead to loss of central GABAergic inhibition, opioid receptor down-regulation, and interneuron cell death.322 Central nervous system changes are primarily due to phenotypic changes of Aβ and C fibers, sprouting of nerve fibers in deeper layers of dorsal horn laminae,330 and effects of central sensitization. Central sensitization is primarily mediated by the release of neurotransmitters (e.g., substance P, glutamate, CGRP, neurokinin A, and GABA), increased calcium flux, and activation of NMDA receptors.282
Understanding the basic physiologic neurotransmitter changes can help target the use of a single or a number of anticonvulsants in the management of chronic neuropathic pain states, including postherpetic neuralgia, diabetic peripheral neuropathy, spinal radiculopathy, trigeminal neuralgia, HIV-related neuropathic pain states, and small-fiber neuropathy. Recent treatment recommendations highlight the importance of a mechanistic approach to diagnosis and treatment, as well as to proposed first-line medications (i.e., gabapentin, 5% lidocaine [lignocaine] patch, opioid analgesics, tramadol, and TCAs) based on positive results from multiple randomized trials, and to second-line agents based on positive results from a single randomized controlled trial or inconsistent finding. Second-line agents in some cases have shown greater efficacy but are awaiting future randomized controlled trials.71
First-generation anticonvulsants include phenytoin and carbamazepine, which exert membrane-stabilizing effects by blocking sodium channels and reducing neuronal excitability, presumably in sensitized C nociceptors.68 The use of newer-generation anticonvulsants has made their incorporation into outpatient management more practical, given their more favorable metabolic and interaction profiles compared with traditional anticonvulsants. The older-generation antiepileptic drugs (i.e., phenytoin, phenobarbital, carbamazepine, and valproic acid) are effective agents for a number of seizure-related disorders, but they might be less practical options in outpatient chronic pain management because of much less favorable metabolic and interaction profiles compared with newer-generation agents, which can necessitate serum blood level and organ monitoring during titration.229 A brief update follows on newer-generation anticonvulsant and neuropathic agents, including key individual pharmacokinetic profiles and suggested therapeutic doses for chronic pain conditions. Most of the anticonvulsant agents have been used off-label, except those with FDA approval, including the 5% lidocaine patch (Lidoderm), gabapentin (Neurontin), and pregabalin (Lyrica) for postherpetic neuralgia and carbamazepine (Tegretol) for trigeminal neuralgia.
Gabapentin has also found wide off-label use for a number of chronic neuropathic pain conditions. Randomized, double-blind, placebo-controlled studies have demonstrated efficacy with analgesia in diabetic peripheral neuropathy (titrated from 900 to 3600 mg/day)11 and postherpetic neuralgia.253 These studies showed significant improvement in pain (average daily pain 4.2 vs. 6.0 with placebo), sleep, and physical function at similar maximum doses of 3600 mg/day and heterogeneous groups of neuropathic pain.269 Gabapentin, although structurally related to GABA, is an α2δ ligand. The α2δ receptor is a protein associated with neuronal voltage-gated calcium channels. Binding to this channel reduces presynaptic calcium influx into the cell at the dorsal horn, reducing the release of several neurotransmitters (glutamate, substance P, norepinephrine, and CGRP). A number of indirect GABAergic mechanisms have also been proposed. Multiple studies have demonstrated significantly reduced pain and improved sleep, mood, and quality of life at dosages between 1800 and 3600 mg/day. Side effects include somnolence and dizziness. Gabapentin’s unique pharmacokinetics lend to the necessity of using higher doses compared with other newer-generation anticonvulsants. With escalating dose titration, the intestinal active transport absorption system becomes saturated, decreasing the percentage of bioavailability, resulting in a nonlinear relationship between serum concentration and dosage. When this occurs, a significant increase in dosage is needed to see a relative increase in therapeutic response.
Pregabalin is also an α2δ ligand and is structurally related to gabapentin but with no intrinsic GABA activity. Studies have demonstrated efficacy in the management of postherpetic neuralgia,72,84,257 diabetic peripheral neuropathy,90,246,251 general peripheral neuropathy,92,128 and generalized anxiety disorder72,251 with doses between 150 and 600 mg/day. Pregabalin demonstrates linear pharmacokinetics and has a rapid onset of actions (within 1 hour), stable bioavailability independent of dose (approximately 90%), and an affinity for the α2δ subunit that is 6 times greater than that of gabapentin. Pregabalin appears to work by modulating voltage-gated calcium channels, decreasing calcium influx into the cell, and limiting release of excitatory neurotransmitters (substance P, glutamate, aspartate, and norepinephrine).80 Pregabalin (Lyrica) is approved by the FDA for postherpetic neuralgia, diabetic peripheral neuropathy, and the management of fibromyalgia in the United States. It is approved for neuropathic pain in Europe. Pregabalin’s relatively increased potency, linear pharmacokinetics, and stable bioavailability, compared with gabapentin, might diminish the need for prolonged dose titration.
Lamotrigine blocks voltage-dependent sodium channels and N-type calcium channels, and inhibits glutamate release.114,169 Dosages range from 50 to 400 mg/day. Efficacy has been demonstrated in studies of patients with trigeminal neuralgia332 and central poststroke pain310 resistant to other therapies.
Oxcarbazepine is an analogue of carbamazepine, without the epoxide metabolite. It is thought that the epoxide metabolite is a possible contributor to drug interactions and adverse events associated with carbamazepine use.195 Oxcarbazepine has shown efficacy in patients with postherpetic neuralgia, trigeminal neuralgia, and diabetic peripheral neuropathy at doses averaging between 600 and 1200 mg/day.42
Tiagabine is a novel selective GABA reuptake inhibitor, indicated for partial seizures, that has also been used off-label for chronic neuropathic pain, anxiety, and insomnia. Theoretically, increasing GABA levels at the synaptic cleft (dorsal horn and brain) might help to increase GABA’s inhibitory effects on neuronal excitability. Increased GABA levels have been associated with improved sleep, characterized by increasing time in NREM stage 3 and 4 sleep.182
Topiramate and zonisamide are broad-spectrum anticonvulsants with a number of proposed mechanisms, including inhibition of voltage-gated sodium channels, potentiation of GABAergic inhibition, and blocking excitatory glutamate activity and voltage-gated calcium channels. Inhibition of carbonic anhydrase and antiglutamate effects have been considered as mechanisms responsible for the clinically significant weight loss associated with these medications.30,95 The mechanism of levetiracetam, an agent with a chemical structure unrelated to that of other anticonvulsants, remains unclear but might include calcium channel effects.171 It is similar to gabapentin, having minimal drug–drug interactions, and is easily renally excreted.
Antidepressants
Antidepressants have demonstrated mixed efficacy in a number of chronic pain-related conditions (i.e., nociceptive, neuropathic, inflammatory, poststroke pain conditions, central pain states, and headache) and chronic pain-related disorders (i.e., depression, anxiety, and insomnia).41,259 Antidepressants can be divided into general classes: TCAs, selective serotonin reuptake inhibitors (SSRIs), selective serotonin–norepinephrine reuptake inhibitors (SNRIs), and triazolopyridines (i.e., trazodone and nefazodone). Analgesic effects of antidepressants have primarily been associated with peripheral and central norepinephrine and serotonin effects, but might also involve binding to opioid and NMDA receptor complexes, reducing intracellular Ca2+ accumulation, as well as binding to α-adrenoceptors and a number of ion channels (i.e., Na+, Ca2+, and K+).264 Emotional and painful symptoms of depression might be regulated by overlapping pathways for serotonin and norepinephrine at the brain and spinal cord, affecting mood, sleep, coping, and painful symptoms. A more divergent view of transmitter effects has also suggested that the noradrenergic system is involved with motivational activities, including energy, interest, and concentration, compared with serotonergic systems influencing behavioral activity (i.e., sexual function, appetite, and impulsiveness).65
Tricyclic Antidepressants and Selective Serotonin Reuptake Inhibitors
Tricyclic antidepressants have been found to be effective in controlled trials for a variety of chronic pain conditions.172 Their use as both potent antidepressants and sedating medications can fit into a number of therapeutic targets related to symptom management of chronic pain syndrome (e.g., pain, depression, and disturbed sleep) (Table 42-10). Dosing these medications initially at night can be of benefit for the relatively potent serotonergic, noradrenergic, and antihistaminergic effects. Noradrenergic side effects can be associated with autonomic (i.e., orthostatic hypotension, dizziness, and urinary retention), cardiac (i.e., tachycardia), and ocular (i.e., blurred vision) disturbances. Serotonergic effects can include increased gastric distress, agitation, and headaches.232 Antihistamine-mediated effects can include decreased gastric acid secretion and sedation. The so-called serotonin syndrome285 is a rare, reversible clinical syndrome and medical emergency associated with toxic serum and cerebrospinal fluid levels of serotonin. The syndrome is characterized by mental state dysfunction and autonomic and neurologic symptoms, and can occur with concomitant use of TCAs and other medications, including SSRIs207 and tramadol (a synthetic codeine analogue with noradrenergic and serotonergic effects).109,245
Analgesic effects of TCAs can be evident within 1 week of dose initiation, followed later by antidepressant effects with escalating dose titration.172,233 Although SSRI use has surpassed the use of traditional antidepressants because of a more tolerable side effect profile, analgesic effects of these compounds have been mixed in a number of controlled studies, including diabetic peripheral neuropathy183,184,274,275,277 and fibromyalgia.111,325 Studies involving the use of triazolopyridines, which are compounds with similar chemical properties to those of TCAs (including trazodone and nefazodone), have demonstrated little to no analgesic effects, although they can be beneficial in restoring sleep.62,91 A more rational polypharmacy approach can include the use of SSRIs dosed in the morning in conjunction with a more sedating TCA or TCA-like medication at night for pain-related insomnia.
Serotonin–Norepinephrine Reuptake Inhibitors
The newest class of antidepressants, dual monoamine reuptake inhibitors, was developed for the treatment of depression with a goal of providing shorter onset of antidepressant effects and fewer side effects as a result of their relative serotonin and norepinephrine selectivity. Mirtazapine is a potent antagonist of central α2-adrenergic receptors, an antagonist of 5-HT2 and 5-HT3 receptors, and an enhancer of norepinephrine and serotonin neurotransmission. Mirtazapine is indicated for the treatment of depression and can be used to enhance the efficacy of SSRIs. Its relatively sedating effects can have additional benefits for improving sleep in patients with chronic pain. Venlafaxine is a potent dual reuptake inhibitor of serotonin (at lower doses) and of norepinephrine and possibly dopamine (at higher doses), without binding to cholinergic, histaminic, or α1-adrenergic receptor sites. Analgesic effects have been found in animal models160 and in human studies, including a number of case reports and case series of heterogeneous chronic pain conditions,280,296 neuropathic pain states,295 and fibromyalgia.70 Duloxetine is a potent balanced reuptake inhibitor of both serotonin and norepinephrine and demonstrates a higher affinity for monoamine transporters compared with venlafaxine.37 In the United States, duloxetine is indicated for major depressive disorder, diabetic peripheral neuropathy, and management of fibromyalgia. A randomized, double-blind, placebo-controlled study of patients with primary fibromyalgia (60 mg twice daily) demonstrated improved fibromyalgia-related symptoms and pain severity independent of baseline depression in women greater than in men.7 Milnacipran (Savella), an SNRI, is approved for the management of fibromyalgia in the United States and for the treatment of depression in Europe and Asia. Studies in fibromyalgia patients (100 mg in divided doses) demonstrated clinically meaningful improvement in pain reduction, patient global impression of change, and physical function.52,196
Medication for Insomnia
Pain is an important factor related to sleep problems in community-based studies110 and can reflect a bidirectional relationship where pain might interrupt onset and quality of sleep, while pain intensity might be exacerbated by insufficient or nonrestorative sleep patterns.2 Pain clinic studies report more than 70% of patients report disturbed sleep.209,231 In one study of patients with chronic pain, McCracken and Iverson191 reported at least 90% of patients reporting at least one sleep disturbance. Severity of sleep disturbance has also been correlated with greater pain, depression, and disability.191,209,324 To complicate this relationship further, the effects of chronic opioid therapy on disordered sleep reveal conflicting results. Whereas multiple studies have noted that effective opioid analgesia results in improved sleep measures,31 in a study of patients on chronic methadone, long-term opioid therapy correlated with sleep apnea and apnea severity.320 Multiple classes of medications with a number of differing mechanisms are available for pain-related insomnia and can be incorporated into a rational polypharmacy approach (see Table 42-10).
From a historical perspective, treatment for pain-related insomnia has primarily relied on pharmacotherapy. Barbiturates, barbiturate-like compounds, and antihistamines were commonly used until the introduction of the benzodiazepines, which had fewer safety concerns and less potential for the development of tolerance. During the past decade, however, the use of benzodiazepines has decreased with the introduction of the nonbenzodiazepine hypnotics, also commonly referred to as the “Z drugs” (zolpidem and zaleplon).266 Like the benzodiazepines, the Z drugs facilitate GABAA transmission by preferential binding at the 1a receptor subunits (corresponding to benzodiazepine receptor subtype 1), and therefore are devoid of the significant muscle relaxant, anxiolytic, and anticonvulsant activity of traditional full-agonist benzodiazepines.262 A third nonbenzodiazepine hypnotic, eszopiclone, is approved by the FDA for the long-term management of insomnia and retains a greater half-life (5 to 5.8 hours) with evidence of greater sleep maintenance efficacy compared with the current relatively shorter half-life Z drugs.333 Some evidence suggests that eszopiclone (3 mg) might also have a positive effect on depression, anxiety, and quality of life. Whether these are primary effects of the medication or secondary effects of the improved sleep parameters is unclear.136 In addition to medications that affect the benzodiazepine receptor complex, there are a number of other drugs that have profound effects on sleep and wakefulness. Medications such as the TCAs and trazodone are especially useful in patients with disturbed sleep that is present with concurrent chronic conditions such as elevated anxiety levels, depression, and myofascial or neuropathic pain. These drugs increase total sleep time and NREM stage 2 sleep. They act by inhibition of norepinephrine and serotonin uptake and block histamine and acetylcholine. When choosing a medication to address disturbed sleep, the half-life of the medication should be considered to ensure that the medication is appropriate for the particular sleep disturbance. Patients with trouble initiating sleep might require shorter-acting medications, whereas those with fragmented sleep and frequent awakenings could more ideally benefit from medications with an intermediate to long half-life.
Little evidence supports the long-term use of benzodiazepines for the management of insomnia and anxiety in chronic pain.113 Some have suggested that chronic use might simply prevent rebound insomnia rather than promote restorative sleep.238,260 Chronic benzodiazepine use can lead to associated cognitive impairment, and it can increase risk for falls, produce rebound insomnia with prolonged use, disrupt normal sleep architecture, and promote misuse and abuse in patients with histories of substance-related disorders.113,142,157,255 Medications with other secondary sedating qualities might also be considered as part of this approach (i.e., muscle relaxers, TCAs and tricyclic-like antidepressants, and novel antidepressants). Older- and newer-generation anticonvulsants have been shown to decrease sleep latency and to increase total sleep time and slow-wave sleep.241 In general, opiates tend to produce sedation and have the effect of increasing sleep fragmentation and decreasing REM and stage 2 sleep. Antidepressants in the SSRI class are associated with both insomnia and somnolence, depending on the specific medication. They generally tend to decrease total sleep time and are less sedating than the TCAs. SSRIs can reduce REM sleep. A complete sleep history can help determine behavioral issues impacting insomnia, as well as the excessive use of nicotine and alcohol before bedtime. Nicotine use can lead to delayed sleep onset. Alcohol’s relatively sedating qualities might facilitate sleep by increasing slow-wave and reducing REM sleep, but it also might cause a rebound increase in sleep fragmentation later in the sleep cycle.
Novel mediation treatments for insomnia are currently in development. Orexin receptor antagonists are currently in Phase III clinical trials for the treatment of primary insomnia. This novel approach targets the orexin peptides produced by lateral hypothalamic neurons that are prominently involved in the maintenance of wakefulness. Pharmacologic blockade of orexin-1 and orexin-2 receptors has been shown to promote sleep in animals and humans.68,218
Topical Analgesics
The use of over-the-counter and prescription topical analgesics continues to grow. An increased understanding of nociceptor physiology, including a greater understanding of thermosensation, has been spurred by identification of proteins called vanilloid receptors, detectors of noxious heat, and subsequent identification of a new family of thermosensation receptors, the transient receptor protein vanilloid channel (TRPV) family.143 The vanilloid receptor (TRPV1) is a nonselective cation receptor activated by capsaicin, the pungent agent found in chili peppers. Another TRPV receptor, the cold- and menthol-sensitive receptor, has been identified and might contribute to a better understanding of cold thermosensation and the possible development of targeted cold-producing analgesics. Pharmacologic studies of menthol have suggested a possible κ-opioid receptor effect, providing additional analgesic properties to the substance.97 A number of prescription and over-the-counter topical therapies are available for the treatment of musculoskeletal and neuropathic pain states, including the lidocaine (lignocaine) patch, topical TCAs, capsaicin creams, and topical NSAIDs.
Prescription medications include lidocaine 5% patches that are indicated for postherpetic neuralgia. Lidocaine acts peripherally by blocking sodium channels. Randomized placebo-controlled studies have demonstrated analgesic efficacy in postherpetic neuralgia253 and focal peripheral neuropathic pain syndromes.198 Safety and decreased risk for systemic effects with multiple patches worn up to 24 hours at a time has more recently been reported.100
More widely used in Europe, topical TCAs such as doxepin and amitriptyline have demonstrated efficacy in a number of neuropathic pain states.108,187 Topical capsaicin, which depletes substance P and CGRP to produce a pharmacologic desensitization of nociceptors, has demonstrated efficacy in a number of trials, including diabetic peripheral neuropathy, HIV-associated neuropathic pain, and painful distal polyneuropathy.40,168,223 More widespread use in those with chronic pain is limited by poor patient tolerability of the necessary desensitization application process.
Over-the-counter topical analgesics include NSAIDs, capsaicin, and menthol-based products. Compounding pharmacies can serve a unique service in providing customized compounding of various creams and gels for topical use, including ketamine, gabapentin, cyclobenzaprine, and various NSAIDs.139
Mind–Body Medicine
Mind–body medicine describes a subset of medical care that attempts to tie together methods of ancient traditional Eastern healing techniques with the modern biopsychosocial model of health care. Therapies that fall under the guise of mind–body medicine include but are not limited to relaxation therapy, biofeedback, meditation, hypnosis, guided imagery, yoga, and t’ai chi. Many of these methods, such as meditation and relaxation therapy, display significant overlap in basic theory and mechanism, whereas other methods, such as t’ai chi and yoga, can be further subclassified as movement-based therapies. Two of the more commonly used mind–body medicine techniques, relaxation training and biofeedback, are described below.
Relaxation Training
Relaxation techniques are incorporated to some extent as an element of almost all mind–body medicine therapies, most notably in meditation, guided imagery, hypnosis, and biofeedback. The technique of progressive muscle relaxation as developed by Jacobson in 1938129 is perhaps the most popular form of relaxation training, although it has been modified and abbreviated by various practitioners. Progressive muscle relaxation teaches the patient to sequentially voluntarily contract and then relax various muscle groups. Through this voluntary cycle, the patient gains insight into the sensation of muscle tension, which can then facilitate subsequent muscular relaxation.
Progressive muscle relaxation has been studied in various chronic conditions, with strong support for its use in the treatment of anxiety, depression, headache, and insomnia, as well as chronic pain.12,16,25,125,215 Treatments are commonly combined with guided imagery or meditation, and a brief form of progressive muscle relaxation known as self-control relaxation can be taught to patients to practice during periods of acute flare-ups. Relaxation therapies are ideal behavioral techniques for patients with chronic pain conditions because they are easy to learn, use minimal health care resources, and are without side effects.
Biofeedback
The positive effects of biofeedback have been documented in numerous chronic pain conditions, including low back pain, headache, temporomandibular disorders, and fibromyalgia.16,34,58,311 It is an important form of therapeutic training for chronic pain conditions because it allows and encourages the patient to achieve an improved sense of self-control and self-efficacy.
Movement-Based Therapies
Aquatic Rehabilitation
The physical properties of water make it an ideal setting for the rehabilitation of selected chronic pain patients, such as those with significant fear and anxiety related to movement. The pool-based environment affords numerous advantages to land-based therapy because of the buoyancy and viscosity provided by water. The buoyant force reduces the effective weight of the patient in proportion to the depth of water. Weight-bearing loads are reduced to 40% of total body weight when standing in chest-deep water.120 With the patient in a floating state, the effects of gravity are eliminated. Aquatic-based therapy programs allow for graduated loads to tissue by progressively decreasing the depth at which therapy is performed. The viscosity of water provides resistance to movement equal to that of the force exerted by the patient. This resistance also varies with the speed of movement performed.240
Reduced self-reported levels of pain, anxiety, and depression have been shown in patients with fibromyalgia who participated in a pool-based physical therapy program.135 Other studies have reported improvements in pain, fatigue, social and physical function, and quality of life.177,178 Effects can last up to 24 months after participation in a pool-based exercise program.168 Other benefits include muscle relaxation, improved body awareness, cardiorespiratory fitness, balance, and coordination.240
Pilates
A scant body of literature supports its role in the treatment of chronic pain syndromes, and its use is experiential. Improvements in strength and flexibility, as well as static and dynamic posture in selected populations, have been reported.127
Yoga
Although there are a number of different yoga styles, the most popular forms of yoga focus on the performance of various postures (asanas), stretches, and controlled breathing (pranayamas). Yoga programs have been reported to improve balance and flexibility96,244; decrease disability and depression96; reduce blood pressure, heart rate, and anxiety244; and improve range of motion, muscular endurance, and lung capacity.244 The beneficial effects on pain might be derived from the control of stress and depression, and the relaxation, stretching, and strengthening of targeted muscles.96,103,104,216 Yoga can also be practiced in a group setting or with individual instruction.
Feldenkrais Method
The Feldenkrais method is a system of body retraining created by Moshe Feldenkrais, a Russian-born Israeli physicist. The method incorporates gentle stretching, reaching, and postural change sequences with a goal of improved kinesthetic awareness and psychologic well-being. The two complementary components of the Feldenkrais method—“awareness through movement,” which consists of verbal cues to movement, and “functional integration,” which involves touch to facilitate movement—can be practiced together or independently of each other. Movement sequences can replicate activities of daily living or can be abstract in nature.
Feldenkrais therapy has been reported to improve health-related quality of life and self-efficacy of pain in patients with persistent nonspecific musculoskeletal complaints.176 Other effects of the Feldenkrais method include decreased perceived stress, lowered anxiety,137 and improvement in mood.217 The slow, fluid movements can be performed by patients at any functional level. In addition, the technique can be taught in gravity-eliminated positions such as lying down.
T’ai Chi
In a sample of women diagnosed and treated for breast cancer, those involved in a program of t’ai chi demonstrated improvements in health-related quality of life and self-esteem compared with those who solely received psychosocial support.214 Other reported beneficial effects of t’ai chi include improved functional balance,153,162 quality of life, activity tolerance, cardiovascular function, pain management, flexibility, strength, and kinesthetic sense.146
1. Abs R., Verhelst J., Maeyaert J., et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215-2222.
2. Affleck G., Urrows S., Tennen H., et al. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363-368.
3. Aldrich S., Eccleston C., Crombez G. Worrying about chronic pain: vigilance to threat and misdirected problem solving. Behav Res Ther. 2000;38:457-470.
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, ed 4. Washington: The Association; 1994.
5. Andersson H.I. The epidemiology of chronic pain in a Swedish rural area. Qual Life Res. 1994;3:S19-S26.
6. Apkarian A.V., Sosa Y., Sonty S., et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410-10415.
7. Arnold L.M., Crofford L.Y., Wohlreich M. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974-2984.
8. Ashburn M.A., Staats P.S. Management of chronic pain. Lancet. 1999;353:1865-1869.
9. Atkinson J.H., Slater M.A., Grant I., et al. Depressed mood in chronic low back pain: relationship with stressful life event. Pain. 1988;35:47-55.
10. Aw T.J., Haas S.J., Liew D., et al. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med. 2005;165:1-7.
11. Backonja M., Beydoun A., Edwards K.R., et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831-1836.
12. Baird C.L., Sands L. A pilot study of the effectiveness of guided imagery with progressive muscle relaxation to reduce chronic pain and mobility difficulties of osteoarthritis. Pain Manage Nurs. 2004;5:97-104.
13. Ballantyne J.C., Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943-1953.
14. Banks S.M., Kerns R.D. Explaining high rates of depression in chronic pain: a diathesis–stress framework. Psychol Bull. 1996;119:95-110.
15. Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16(suppl 2):S12-S20.
16. Barrows K.A., Jacobs B.P. Mind–body medicine. An introduction and review of the literature. Med Clin North Am. 2002;86:11-31.
17. Beck A.T., Rush A.J., Shaw B.F., et al. Cognitive therapy of depression. New York: Guilford Press; 1979.
18. Beck A.T., Steer R.A. Beck Depression Inventory manual. New York: Psychological Corp; 1987.
19. Becker E., Horn S., Hussla B., et al. [Guidelines for the sociomedical assessment of performance in patients suffering from discopathy or associated diseases]. [in German] Gesundheitswesen. 2003;65(1):19-39.
20. Beecher H.K. Measurement of subjective responses: quantitative effects of drugs. New York: Oxford University Press; 1959.
21. Bendix A.F., Bendix T., Lund C., et al. A prospective, randomized, 5-year follow-up study of functional restoration in chronic low back pain patients. Eur Spine J. 1998;7:111-119.
22. Bergener M., Bobbit R.A., Carter W.B. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981;19:787-805.
23. Bergman N.A. The genesis of surgical anesthesia. Park Ridge: Wood Library–Museum of Anesthesiology; 1998.
24. Berry P.H., Chapman C.R., Covington E.C., et al. Pain: current understanding of assessment, management, and treatments. Oakbrook Terrace: Joint Commission on Accreditation of Healthcare Organizations; 2001.
25. Blanchard E.B., Nicholson N.L., Taylor A.E., et al. The role of regular home practice in the relaxation treatment of tension headache. J Consult Clin Psychol. 1991;59:467-470.
26. Bombardier C., Lain L., Reicin A., et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520-1528. VIGOR Study Group
27. Bonica J.J., Loeser J.D., et al. History of pain concepts and therapies. In: Loeser J.D., Butler S.H., Chapman C.R., editors. Bonica’s management of pain. Philadelphia: Lippincott Williams & Wilkins, 2001.
28. Bradley L.A., McKendree-Smith N.L., Cianfrini L.R. Cognitive-behavioral therapy interventions for pain associated with chronic illness. Semin Pain Med. 2003;1:44-54.
29. Brattberg G., Thorslund M., Wikman A. The prevalence of pain in a general population: the results of a postal survey in a county of Sweden. Pain. 1989;37:215-222.
30. Bray G.A., Hollander P., Klein S., et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11(6):722-733.
31. Brennan M.J., Lieberman J.A.3rd. Sleep disturbances in patients with chronic pain: effectively managing opioid analgesia to improve outcomes. Curr Med Res Opin. 2009 May;25(5):1045-1055.
32. Brown S.C., Glass J.M., Park D.C. The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain. 2002;96:279-284.
33. Bruera E., Macmillan K., Hanson K., et al. The cognitive effects of the administration of narcotic analgesics in patients with cancer pain. Pain. 1989;39:13-16.
34. Buckelew S.P., Conway R., Parker J., et al. Biofeedback/relaxation training and exercise interventions for fibromyalgia: a prospective trial. Arthritis Care Res. 1998;11:196-209.
35. Burns J.W. Anger management style and hostility: predicting symptom-specific physiological reactivity among chronic low back pain patients. J Behav Med. 1997;20:505-522.
36. Butcher J.N., Dahlstrom W.G., Graham J.R. MMPI-2: manual for administration and scoring. Minneapolis: University of Minnesota Press; 1989.
37. Bymaster F.P., Dreshfield-Ahmad L.J., Threlkeld P.G., et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes and other neuronal receptors. Neuropsychopharmacology. 2001;25:871-880.
38. Call-Schmidt T.A., Richardson S.J. Prevalence of sleep disturbance and its relationship to pain in adults with chronic pain. Pain Manage Nurs. 2003;4:124-133.
39. Cao Y.Q., Mantyh P.W., Carlson E.J., et al. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390-394.
40. Capsaicin Study Group. Treatment of painful diabetic peripheral neuropathy with topical capsaicin: a multicenter, double-blind, vehicle-controlled study. Arch Intern Med. 1991;151:2225-2229.
41. Cardenas D.D., Warms C.A., Turner J.A., et al. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96:365-373.
42. Carrazana E., Mikoshiba I. Rationale and evidence for the use of oxcarbazepine in neuropathic pain. J Pain Symptom Manage. 2003;25(5 suppl):S31-S35.
43. Catala E., Reig E., Artes M., et al. Prevalence of pain in the Spanish population: telephone survey in 5000 homes. Eur J Pain. 2002;6:133-140.
44. Caterina M.J., Julius D. Sense and specificity: a molecular identity for nociceptors. Curr Opin Neurobiol. 1999;9:525-530.
45. Centers for Medicare and Medicaid Services: Glossary. Online. Available: http://www.cms.hhs.gov/glossary. Accessed June 20, 2005.
46. Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95-138.
47. Chapman C.R., Okifuji A. Pain: basic mechanisms and conscious experience. In: Breitbart D., editor. Psychosocial aspects of pain: a handbook for healthcare providers, progress in pain research and management, vol 27. Seattle: IASP Press, 2004.
48. Chapman C.R., Stillman M. Pathological pain. In Kruger L., editor: Pain and thought, ed 2, New York: Academic Press, 1996.
49. Cheng J.F., Harris R.C. Cyclooxygenases, the kidney, and hypertension. Hypertension. 2004;43:525-530.
50. Chou R., Fanciullo G., Fine P., Adler J., et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:1123-1130.
51. Ciccone D.S., Just N., Bandilla E.B., et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20:180-192.
52. Clauw D., Mease P., Palmer R., et al. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30:1988-2004.
53. Cleeland C.S., Ryan K.M. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med. 1994;23:129-138.
54. Coda B.A., Bonica J.J. General considerations of acute pain. In Lowese J.D., Bonica J.J., editors: Bonica‘s management of pain, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2001.
55. Compas B.E., Keefe F.J., Haaga D.A., et al. Sampling of empirically supported psychological treatments from health psychology: smoking, chronic pain, cancer, and bulimia nervosa. J Consult Clin Psychol. 1998;66:89-112.
56. Costiglione A. The renaissance of medicine in Italy. Baltimore MD: Johns Hopkins Press; 1934.
57. Craig A.D. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1-30.
58. Crider A.B., Glaros A.G. A meta-analysis of EMG biofeedback treatment of temporomandibular disorders. J Orofac Pain. 1999;13:29-37.
59. Dalen J.E. Selective COX-2 inhibitors, NSAIDs, aspirin, and myocardial infarction. Arch Intern Med. 2002;162:1091-1092.
60. Daniell H.W. Hypogonadism in men consuming sustained-action oral opioids. J Pain. 2002;3(5):377-384.
61. Daniels E., Upmallis D., Okamoto A., Lange C., Haeussler J. A randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxydone IR, and placebo for postoperative (buniectomy) pain, Curr Med Res Opin. 2009;25:765-776.
62. Davidoff G., Guarracini M., Roth E., et al. Trazodone hydrochloride in the treatment of dysesthetic pain in traumatic myelopathy: a randomized, double-blind, placebo-controlled study. Pain. 1987;29:151-161.
63. Davies K.A., Macfarlance G.J., Nicholl B.I., et al. Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology (Oxford). 2008 Dec;47(12):1809-1813.
64. De Felipe C., Herrero J.F., O’Brien J.A., et al. Altered nociception, analgesia and aggression in mice lacing the receptor for substance P. Nature. 1998;392:394-397.
65. Delgado P.L. Common pathways of depression and pain. J Clin Psychiatry. 2004;65(suppl 12):16-19.
66. Dequeker J., Hawkey C., Kahan A., et al. Improvement in gastrointestinal tolerability of the selective cyclooxygenase COX-2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficacy Large Scale Evaluation of COX Inhibition Therapies (SELECT) trial in osteoarthritis. Br J Rheumatol. 1998;37:946-951.
67. Derogatis L.R. The SCL-90-R manual II: administration, scoring, and procedures. In Towson, MD. Clinical Psychometric Press; 1983.
68. Dray A. Kinins and their receptors in hyperalgesia. Can J Physiol Pharmacol. 1997;75:704-712.
69. Dugovic C., Shelton J.E., Aluisio L.E., et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330(1):142-151.
70. Dwight M.M., Arnold L.M., O‘Brien H., et al. An open clinical trial of venlafaxine treatment of fibromyalgia. Psychosomatics. 1998;39:14-17.
71. Dworkin R.H., Backonja M., Rowbotham M.C., et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524-1534.
72. Dworkin R.H., Corbin A.E., Young J.P., et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60:1274-1283.
73. Ehde D.M., Jensen M.P., Engel J.M., et al. Chronic pain secondary to disability: a review. Clin J Pain. 2003;19:3-17.
74. Elliott A.M., Smith B.H., Penny K.I., et al. The epidemiology of chronic pain in the community. Lancet. 1999;354(9186):1248-1252.
75. Estlander A.M., Takala E.P., Verkasalo M. Assessment of depression in chronic musculoskeletal pain patients. Clin J Pain. 1995;11:194-200.
76. Farrar J.T., Young J.P.Jr., LaMoreaux L., et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149-158.
77. Feltner D.E., Crockatt J.G., Dubovsky S.J., et al. A randomized, double-blind, placebo-controlled, fixed-dose, multicenter study of pregabalin in patients with generalized anxiety disorder. J Clin Psychopharm. 2003;23:240-249.
78. Fernandez E., Turk D.C. The scope and significance of anger in the experience of chronic pain. Pain. 1995;61:165-175.
79. Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5(7):565-575.
80. Fink K., Dooley D.J., Meder W.P., et al. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42(2):229-236.
81. Fishbain D.A., Cutler R.B., Rosomoff H.L., et al. Are opioid-dependent/tolerant patients impaired in driving-related skills? A structured evidence-based review. J Pain Symptom Manage. 2003;25(6):559-577.
82. Fishbain D.A., Cutler R.B., Rosomoff H.L., et al. Chronic pain associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116-137.
83. Fishbain D.A., Goldeberg M., Meagher B.R., et al. Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain. 1986;26:181-197.
84. Fishman S.M., Kreis P.G. The opioid contract. Clin J Pain. 2002;18:S70-S75.
85. Flor H., Birbaumer N., Schugens M.M., et al. Symptom-specific psychophysiological responses in chronic pain patients. Psychophysiology. 1992;29:452-460.
86. Flor H., Birbaumer N., Turk D.C. The psychobiology of chronic pain. Adv Behav Res Ther. 1990;12:47-84.
87. Flor H., Turk D.C., Burbaumer N. Assessment of stress-related psychophysiological reactions in chronic back pain patients. J Consult Clin Psychol. 1985;53:354-364.
88. Fordyce W.E. Behavioral methods of chronic pain and illness. St Louis: Mosby; 1976.
89. Frampton J.E., Foster R.H. Pregabalin: in the treatment of postherpetic neuralgia. Drugs. 2005;65:1111-1118.
90. Frampton J.E., Scott L.J. Pregabalin: in the treatment of painful diabetic peripheral neuropathy. Drugs. 2004;64:2813-2820.
91. Frank R.G., Beck N.C., Parker J.C., et al. Depression in rheumatoid arthritis. J Rheumatol. 1988;15:920-925.
92. Freynhagen R., Strojek K., Griesing T., et al. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomized, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115:254-263.
93. Fromm-Reichmann F. Principles of intensive psychotherapy. Chicago: University of Chicago Press; 1950.
94. Frymoyer J.W., Durett C.L. The economics of spinal disorders. In: Frymoyer J.W., editor. The adult spine. Philadelphia: Lippincott-Raven, 1997.
95. Gadde K.M., Franciscy D.M., Wagner H.R.II, et al. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289(14):1820-1825.
96. Galantino M.L., Bzdewka T.M., Eissler-Russo J., et al. The impact of modified Hatha yoga on chronic low back pain: a pilot study. Altern Ther Health Med. 2004;10:56-58.
97. Galeotti N., Mannelli L.D., Mazzanti G., et al. Menthol: a natural analgesic compound. Neurosci Lett. 2002;322:145-148.
98. Gallagher R.M. Treatment planning in pain medicine: integrating medical, physical, and behavioral therapies. Med Clin North Am. 1999;83:823-849.
99. Gallagher R.M., Moore P., Chernoff I. The reliability of depression diagnosis in chronic low back pain. Gen Hosp Psychiatry. 1995;17:399-413.
100. Gammaitoni A.R., Davis M.W. Pharmacokinetics and safety of continuously applied lidocaine patches 5%. Am J Health Syst Pharm. 2002;59:2215-2220.
101. Gamsa A. The role of psychological factors in chronic pain. II. A critical appraisal. Pain. 1994;57(1):17-29.
102. Ganidagli S., Cengiz M., Aksoy S., et al. Approach to painful disorders by Serefeddin Sabuncuoglu in the fifteenth century Ottoman period. Anesthesiology. 2004;100:165-169.
103. Garfinkel M.S., Schumacher H.R., Husain A., et al. Evaluation of a yoga based regimen for treatment of osteoarthritis of the hands. J Rheumatol. 1994;21:2341-2343.
104. Garfinkel M.S., Singhal A., Katz W.A., et al. Yoga-based intervention for carpal tunnel syndrome. JAMA. 1998;280:1601-1603.
105. Gatchel R.J. Perspectives on pain: a historical overview. In: Gatchel R.J., Turk D.C., editors. Psychosocial factors in pain. New York: Guilford Press, 1999.
106. Gebhart G.F. Visceral pain. Seattle: IASP Press; 1995.
107. Geisser M.E., Roth R.S., Theisen M.E., et al. Negative effect, self-report of depressive symptoms, and clinical depression: relation to experience of chronic pain. Clin J Pain. 2000;16:110-120.
108. Gerner P., Kao G., Srinivasa V., et al. Topical amitriptyline in healthy volunteers. Reg Anesth Pain Med. 2003;28:289-293.
109. Gilliam P.K. Serotonin syndrome: history and risk. Fundam Clin Pharmacol. 1998;12:482-491.
110. Giron M.S., Forsell Y., Bernsten C., et al. Sleep problems in a very old population: drug use and clinical correlates. J Gerontol A Biol Sci Med Sci. 2002;57:M236-M240.
111. Goldenberg D.L., Simms R.W., Geiger A., et al. High frequency of fibromyalgia in patients with chronic fatigue seen in a primary care practice. Arthritis Rheum. 1990;33:381-387.
112. Gourlay D.L., Heit H., Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
113. Griffiths R.R., Weerts E.M. Benzodiazepine self-administration in humans and laboratory animals: a implications for problems of long-term use and abuse. Psychopharmacology. 1997;134:1-37.
114. Grunze H., von Wegerer J., Greene R.W., et al. Modulation of calcium and potassium currents by lamotrigine. Neuropsychobiology. 1998;38(3):131-138.
115. Gudehithlu K.P., Reddy P.L., Bhargava H.N. Effect of morphine tolerance and abstinence on the binding of MK-801 to brain regions and spinal cord of the rat. Brain Res. 1994;639:269-274.
116. Guzman J., Esmail R., Karjalainen K., et al. Multidisciplinary rehabilitation for chronic low back pain: systematic review. BMJ. 2001;322:1511-1516.
117. Hadjistavropoulos H.D., LaChapelle D.L. Extent and nature of anxiety experienced during physical examination of chronic low back pain. Behav Res Ther. 2000;38:13-29.
118. Hankey G.J., Eikelboom J.W. Cyclooxygenase-2 inhibitors: are they really atherothrombotic, and if not, why not? Stroke. 2003;34:2736-2740.
119. Hardy J.D., Wolff H.G., Goodell H. Pain sensations and reactors. New York: Williams & Wilkins; 1952.
120. Harrison R.A., Hillman M., Bulstrode S. Loading of the lower limb when walking partially immersed: implications for clinical practice. Physiotherapy. 1992;78:164.
121. Hasselström J., Liu-Palmgren J., Rasjo-Wraak G. Prevalence of pain in general practice. Eur J Pain. 2002;6:375-385.
122. Haythornthwaite J.A., Menefee L.A., Quatrano-Piacentini A.L., et al. Outcome of chronic opioid therapy for non-cancer pain. J Pain Symptom Manage. 1998;15:185-194.
123. Higby G.J. Heroin and medical reasoning: the power of analogy. N Y State J Med. 1986;86:137-142.
124. Hofbauer R.K., Rainville P., Duncan G.H., et al. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86:402-411.
125. Holland J.C., Morrow G.R., Schmale A., et al. A randomized clinical trial of alprazolam versus progressive muscle relaxation in cancer patients with anxiety and depressive symptoms. J Clin Oncol. 1991;9:1004-1011.
126. Inturrisi C.E. Clinical pharmacology of opioids for pain. Clin J Pain. 2002;18:S3-S13.
127. Ives J.C., Sosnoff J. Beyond the mind–body exercise hype. Phys Sports Med. 2000;28:67-81.
128. Jaaskelainen S.K. Pregabalin for painful peripheral neuropathy. Lancet Neurol. 2005;4:207-208.
129. Jacobson E. Progress relaxation. Chicago: University of Chicago Press; 1938.
130. Jamison R.N., Raymond S.A., Slawsky E.A., et al. Opioid therapy for chronic noncancer back pain. Spine. 1996;23:2591-2600.
131. Janssen S.A., Philip S., Arntz A. The effect of failing to control pain: an experimental investigation. Pain. 2004;107:227-233.
132. Jensen M.P., Engel J.M., Hoffman A.J., et al. Natural history of chronic pain and pain treatment in adults with cerebral palsy. Am J Phys Med Rehabil. 2004;83:439-445.
133. Jensen M.P., Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk D.C., Melzack R., editors. Handbook of pain assessment. New York: Guilford Press, 2001.
134. Jensen M.P., Karoly P., Huger R. The development and preliminary validation of an instrument to assess patients’ attitudes toward pain. J Psychosom Res. 1987;31:393-400.
135. Jentoft E.S., Kvalvik A.G., Mengshoel A.M. Effects of pool-based and land-based aerobic exercise on women with fibromyalgia/chronic widespread muscle pain. Arthritis Care Res. 2001;45:42-47.
136. Joffe H., Petrillo L., Vigurea A., et al. Eszopiclone improves insomnia and depressive symptoms in perimenopausal and postmenopausal women with hot flashes: a randomized, double-blinded, placebo-controlled crossover trial. Am J Obstet Gynecol. 2009 Dec 23. [Epub ahead of print]
137. Johnson S.K., Frederick J., Kaufman M., et al. A controlled investigation of bodywork in multiple sclerosis. J Altern Complement Med. 1999;5:237-243.
138. Jones A.K., Kulkarni B., Derbyshire S.W. Pain mechanisms and their disorders. Br Med Bull. 2003;65:83-93.
139. Jones M. Chronic neuropathic pain: pharmacological interventions in the new millennium. Int J Pharm Compound. 2000;4(1):6-11.
140. Joranson D.E., Ryan K.M., Gilson A.M., et al. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283:1710-1714.
141. Jordan K.D., Mayer T.G., Gatchel R.J. Should extended disability be an exclusion criteria for tertiary rehabilitation? Socioeconomic outcomes of early versus late functional restoration in compensation spinal disorders. Spine. 1998;23:2110-2116.
142. Judd L.L., Ellinwood E., McAdams L.A. Cognitive performance and mood in patients with chronic insomnia during 14-day use of flurazepam and midazolam. J Clin Psychopharmacol. 1990;10:S56-S67.
143. Julius D. The molecular biology of thermosensation. In: Dostrovsky J.O., Carr D.B., Koltzenburg M., editors. Proceedings of the 10th World Congress on Pain: progress in pain research and management, vol 24. Seattle: IASP Press, 2003.
144. Julius D., Basbaum A.I. Molecular mechanisms of nociception. Nature. 2001;413:203-210.
145. Katz N., Fanciullo G. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18:S76-S82.
146. Keefe F.J., Lefebvre J.C. Behavior therapy. In: Melzack R., Wall P., editors. Textbook of pain. London: Churchill Livingstone, 1999.
147. Keefe F.J., Rumble M.E., Scipio C.D., et al. Psychological aspects of persistent pain: current state of the science. J Pain. 2004;5:195-211.
148. Keefe F.J., Wilkins R.H., Cook W.A., et al. Depression, pain and pain behavior. J Consult Clin Psychol. 1986;54:665-669.
149. Kerns R.D., Rosenberg R., Jacob M.C. Anger expression and chronic pain. J Behav Med. 1994;17:57-67.
150. Kerns R.D., Turk D.C., Rudy T.E. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain. 1985;23:345-356.
151. Kidd B.L., Urban L.A. Mechanisms of inflammatory pain. Br J Anaesth. 2001;87:3-11.
152. Kimmel S.E., Berlin J.A., Reily M., et al. Patients exposed to rofecoxib and Celebrex have different odds of nonfatal myocardial infarction. Ann Intern Med. 2005;142(3):157-164.
153. Klein P.J., Adams W.D. Comprehensive therapeutic benefits of Taiji: a critical review. Am J Phys Med Rehabil. 2004;83:735-745.
154. Kori S.H., Miller R.P., Todd D.D. Kinesophobia: a new view of chronic pain behavior. Pain Manage. 1990;3:35-43.
155. Koyana T., Kato K., Mikami A. During pain-avoidance neurons activated in the macaque anterior cingulated and caudate. Neurosci Lett. 2000;283:17-20.
156. Krause S.J., Weiner R.L., Tait R.C. Depression and pain behavior in patients with chronic pain. Clin J Pain. 1994;10:122-127.
157. Kripke D.F., Hauri P., Ancoli-Israel S., et al. Sleep evaluation in chronic insomniacs during 14-day use of flurazepam and midazolam. J Clin Psychopharmacol. 1990;10:32S-43S.
158. Kucharski A., Todd E. Pain: historical perspectives. In Warfield C.A., Bajwa Z., editors: Principles and practice of pain medicine, ed 2, New York: McGraw-Hill, 2004.
159. Lanes T.C., Gauron E.F., Spratt K.F., et al. Long-term follow-up of patients with chronic back pain treated in a multidisciplinary rehabilitation program. Spine. 1995;20:801-806.
160. Lang E., Hord A.H., Denson D. Venlafaxine hydrochoride relieves thermal hyperalgesia in rats with an experimental mononeuropathy. Pain. 1996;68:151-155.
161. Leclaire R., Blier F., Fortin L., et al. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22:68-71.
162. Li F., Harmer P., Fisher K.J., et al. Tai chi: improving functional balance and predicting subsequent falls in older persons. Med Sci Sports Exerc. 2004;36:2046-2052.
163. Linton S.J., Melin L. Behavioral analysis of chronic pain and its management. In: Hersen A.B.M., Iesler M., editors. Progress in behavior modification. New York: Academic Press, 1985.
164. Livingston W.K. Pain and suffering. Seattle: IASP Press; 1998.
165. Loeppke R., Taitel M., Richling D., et al. Health and productivity as a business strategy. J Occup Environ Med. 2007;49:712-721.
166. Loeser J.D. Desirable characteristics for pain treatment facilities. Seattle: IASP Press; 1992.
167. Loeser J.D., Butler S.H., Chapman C.R., et al, editors. Bonica’s management of pain. Philadelphia: Lippincott, 2001.
168. Low P.A., Opfer-Gehrking T., Dyck P.J., et al. Double-blind, placebo-controlled study of the application of capsaicin cream in chronic distal painful polyneuropathy. Pain. 1995;62:163-168.
169. Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86-98.
170. Lu J., Greco M.A., Shiromani P., et al. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830-3842.
171. Lukyanetz E.A., Shkryl V.M., Kostyuk P.G. Selective blockade of N-type calcium channels by levetiracetam. Epilepsia. 2002;43(1):9-18.
172. Lynch M.E. Antidepressants as analgesics: a review of randomized controlled studies. J Psychiatry Neurosci. 2001;261:30-36.
173. Ma W., Eisenach J.C. Cyclooxygenase-2 in infiltrating inflammatory cells in injured nerve is universally up-regulated following various types of peripheral nerve injury. Neuroscience. 2003;121:691-704.
174. MacDonald S., Linton S.J., Jansson-Frojmark M. Avoidant safety behaviours and catastrophizing: shared cognitive-behavioral processes abd consequences in co-morbid pain and sleep disorders. Int J Behav Med. 2008;15(3):201-210.
175. Malenka R.C., Nicoll R.A. Long-term potentiation: a decade of progress. Science. 1999;285(5435):1870-1874.
176. Malmgren-Olsson E.B., Branholm I.B. A comparison between three physiotherapy approaches with regard to health-related factors in patients with non-specific musculoskeletal disorders. Disabil Rehabil. 2002;24:308-317.
177. Mannerkorpi K., Ahlmén M., Ekdahl C. Six- and 24-month follow-up of pool exercise therapy and education for patients with fibromyalgia. Scand J Rheum. 2002;31:306-310.
178. Mannerkorpi K., Nyberg B., Ahlmen M., et al. Pool exercise combined with an education program for patients with fibromyalgia syndrome: a prospective, randomized study. J Rheumatol. 2000;27:2473-2481.
179. Mannion R.J., Woolf C.J. Pain mechanisms and management: a central perspective. Clin J Pain. 2000;16:S144-S156.
180. Mao J., Price D.D., Mayer D.J. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353-364.
181. Marcus D.A., Click R.M. Sustained-release oxycodone dosing survey of chronic pain patients. Clin J Pain. 2004;20:363-366.
182. Mathias S., Wetter T.C., Steiger A., et al. The GABA uptake inhibitor tiagabine promotes slow wave sleep in normal elderly subjects. Neurobiol Aging. 2001;22(2):247-253.
183. Max M.B., Culnane M., Schafer S.C., et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37:589-596.
184. Max M.B., Lynch S.A., Muir J., et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250-1256.
185. Mayer T.G., Gatchel R.J. Functional restoration for spinal disorders: the sports medicine approach. Philadelphia: Lea & Febiger; 1988.
186. McCallin A. Interdisciplinary practice: a matter of teamwork: an integrated literature review. J Clin Nurs. 2001;10:419-428.
187. McCleane G. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: a randomized, double-blind, placebo-controlled study. Br J Clin Pharmacol. 2000;49:574-579.
188. McCracken L.M., Gross R.T., Aikens J., et al. The assessment of anxiety and fear in persons with chronic pain: a comparison of instruments. Behav Res Ther. 1996;34:927-933.
189. McCracken L.M., Gross R.T., Eccleston C. Multimethod assessment of treatment process in chronic low back pain: comparison of reported pain-related anxiety with directly measured physical capacity. Behav Res Ther. 2002;40:585-594.
190. McCracken L.M., Gross R.T., Sorg P.J., et al. Prediction of pain in patients with chronic low back pain: effects of inaccurate prediction and pain-related anxiety. Behav Res Ther. 1993;31:647-652.
191. McCracken L.M., Iverson G.L. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manage. 2002;7(2):75-79.
192. McCracken L.M., Turk D.C. Behavioral and cognitive-behavioral treatment for chronic pain. Outcome, predictors of outcome, and treatment process. Spine. 2002;27:2564-2573.
193. McCracken L.M., Zayfert C., Gross R.T. The Pain Anxiety Symptom Scale: development and validation of a scale to measure fear of pain. Pain. 1992;50:67-73.
194. McDonald J.W., Johnson M.V. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Rev. 1990;15:41-70.
195. McLean M.J., Schmutz M., Wamil A.W., et al. Oxcarbazepine: mechanisms of action. Epilepsia. 1994;35(suppl 3):S5-S9.
196. McNamara J.O., Patel M., He X.P., et al. Glutamate receptor autoimmunity in Rasmussen’s encephalitis. Cold Spring Harb Symp Quant Biol. 1996;61:327-332.
197. Mease P., Clauw D., Gendreau M., et al. The efficacy and safety of milnacipran for treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:398-409.
198. Meier T., Wasner G., Faust M., et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003;106:151-158.
199. Melzack R. The puzzle of pain. New York: Basic Books; 1973.
200. Melzack R. The Short Form McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277-299.
201. Melzack R., Casey K.L. Sensory, motivational and central control determinants of pain: a new conceptual model. In: Kenshalo D., editor. The skin senses. Springfield: Thomas, 1966.
202. Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971-976.
203. Merskey H., Bogduk N. IASP Task Force on Taxonomy classification of chronic pain: description of chronic pain syndromes and definition of pain terms. Seattle: IASP Press; 1994.
204. Millan M.J. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1-164.
205. Milligan K., Lanteri-Minet M., Borchert K., et al. Evaluation of long-term efficacy and safety of transdermal fentanyl in the treatment of chronic noncancer pain. J Pain. 2001;2(4):197-204.
206. Millon T., Breen J.C., Meagher R.B. Professional Psychol. 1979;10:529-539.
207. Mittino D., Mula M., Monaco F. Serotonin syndrome associated with tramadol–sertraline coadministration. Clin Neuropharmacol. 2004;27:150-151.
208. Moldofsky H. Management of sleep disorders in fibromyalgia. Rheum Dis Clin North Am. 2002;28:353-365.
209. Morin C.M., Gibson D., Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14:311-314.
210. Morley J.S., Makin M.K. The use of methadone in cancer pain poorly responsive to other opioids. Pain Rev. 1998;5:51-58.
211. Morley S., Eccleston C., Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy and behavior therapy for chronic pain in adults, excluding headache. Pain. 1999;80:1-13.
212. Moulin D.E., Iezzi A., Amireh R., et al. Randomised trial of oral morphine for chronic non-cancer pain. Lancet. 1996;347(8995):143-147.
213. Mukherjee D., Nissen S.E., Topol E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286(8):954-959.
214. Mustian K.M., Katula J.A., Gill D.L., et al. Tai chi chuan, health-related quality of life and self-esteem: a randomized trial with breast cancer survivors. Support Care Cancer. 2004;12:871-876.
215. National Institutes of Health Technology Assessment Panel on Integration of Behavioral and Relaxation Approaches into the Treatment of Chronic Pain and Insomnia: integration of behavioral and relaxation approaches into the treatment of chronic pain and insomnia. JAMA. 1996;276(4):313-318.
216. Nayak N.N., Shankar K. Yoga: a therapeutic approach. Phys Med Rehabil Clin North Am. 2004;15:783-798.
217. Netz Y., Lidor R. Mood alterations in mindful versus aerobic exercise modes. J Psychol. 2003;137:405-419.
218. Neubauer D.N. Almorexant, a dual orexin receptor antagonist for the treatment of insomnia. Curr Opin Investig Drugs. 2010 Jan;11(1):101-110.
219. Nicholson K., Martelli M.F. The problem of pain. J Head Trauma Rehabil. 2004;19:2-9.
220. Ohayon M.M., Schatzberg A.F. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39-47.
221. Okifuji A., Turk D.C., Curran S.L. Anger in chronic pain: investigations of anger targets and intensity. J Psychosom Res. 1999;47:1-12.
222. Okifuji A., Turk D.C., Kalauokalani D. Clinical outcome and economic evaluation of multidisciplinary pain centers. In: Block A.R., Kremer E.F., Fernandez E., editors. Handbook of pain syndromes. Mahwah NJ: Erlbaum, 1999.
223. Paice J.A., Ferrans C.E., Lashley F.R., et al. Topical capsaicin in the management of HIV-associated peripheral neuropathy. J Pain Symptom Manage. 2000;19:45-52.
224. Paice J.A., Penn R.D., Ryan W.G. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage. 1994;9:126-131.
225. Pairet M., van Ryn J., Schierok H., et al. Differential inhibition of cyclooxygenase-1 and -2 by meloxicam and its 4′-isomer. Inflamm Res. 1998;47:270-276.
226. Palermo T.M., Fonareva I., Janosy N.R. Sleep quality and efficiency in adolescents with chronic pain: relationship with activity limitations and health-related quality of life. Behav Sleep Med. 2008;6(4):234-250.
227. Pande A.C., Crockatt J.G., Feltner D.E., et al. Pregabalin in generalized anxiety disorder: a placebo-controlled trial. Am J Psychiatry. 2003;160:533-540.
228. Pavelka K., Recker D.P., Verburg K.M. Valdecoxib is as effective as diclofenac in the management of rheumatoid arthritis with a lower incidence of gastroduodenal ulcers: results of a 26-week trial. Rheumatology (Oxford). 2003;42:1207-1215.
229. Perucca E. Established antiepileptic drugs. In: Brodie M.J., Treiman D.M., editors. Modern management of epilepsy. Baillière’s clinical neurology, Vol 5. London: Baillière-Tindall, 1996.
230. Picavet S.J., Vlaeyen J.W.S., Schouten J. Pain catastrophizing and kinesophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156:1028-1034.
231. Pillowsky I., Crettenden I., Townley M. Sleep disturbance in pain clinic patients. Pain. 1985;23:27-33.
232. Polatin P.B., Dersh J. Psychotropic medication in chronic spinal disorders. Spine J. 2004;4:436-450.
233. Polatin P.B., Mayer T.G. Occupational disorders and the management of chronic pain. Orthop Clin North Am. 1996;27:881-890.
234. Pope J.E., Anderson J.J., Felson D.T. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med. 1993;153:477-484.
235. Porreca F., Ossipov M.H., Gebhart G.F. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25(6):319-325.
236. Porro C.A., Baraldi P., Pagoni G., et al. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22(8):3206-3214.
237. Portenoy R.K. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manage. 1996;11:203-217.
238. Poyares D., Guilleminault C., Ohayon M.M., et al. Chronic benzodiazepine usage and withdrawal in insomnia patients. J Psychiatr Res. 2004;38:327-334.
239. Price D.D. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769-1772.
240. Prins J., Cutner D. Aquatic therapy in the rehabilitation of athletic injuries. Clin Sports Med. 1999;18:447-461.
241. Qureshi A., Lee-Chiong T. Medications and their effects on sleep. Med Clin North Am. 2004;88:751-766.
242. Radloff L. J Appl Psychol Measurement. 1977;1:385-401.
243. Rainville P., Duncan G.H., Price D.D., et al. Pain affect encoded in human anterior cingulated but not somatosensory cortex. Science. 1997;277:968-971.
244. Raub J.A. Psychophysiologic effects of Hatha yoga on musculoskeletal and cardiopulmonary function: a literature review. J Altern Complement Med. 2002;8:797-812.
245. Reus V.I., Rawitscher L. Possible interaction of tramadol and antidepressants. Am J Psychiatry. 2000;157:839.
246. Richter R.W., Portenoy R., Sharma U., et al. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized placebo-controlled trial. J Pain. 2005;6:253-260.
247. Robbins H., Gatchel R.J., Noe C., et al. A prospective one-year outcome study of interdisciplinary chronic pain management: compromising its efficacy by managed care policies. Anesth Analg. 2003;97:156-162.
248. Roberts L.J., Finch P.M., Pullan P.T., et al. Sex hormone suppression by intrathecal opioids: a prospective study. Clin J Pain. 2002;18:144-148.
249. Romano J.M., Turner J.A. Chronic pain and depression: does the evidence support a relationship? Psychol Bull. 1985;97:18-34.
250. Rosenstiel A.K., Keefe F.J. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33-44.
251. Rosenstock J., Tuchman M., LaMoreaux L., et al. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628-638.
252. Roth T. Characteristics and determinants of normal sleep. J Clin Psychiatry. 2004;65(suppl 16):8-11.
253. Rowbotham M.C., Davies P.S., Verkempinck C., et al. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996;65:39-44.
254. Rumelhart D.E., McClelland J.L., PDP Research Group. Parallel distributed processing: explorations in the microstructure of cognition. Cambridge: MIT Press; 1986.
255. Rush C.R., Griffiths R.R. Zolpidem, triazolam, and temazepam: behavioral and subject-rated effects in normal volunteers. J Clin Psychopharmacol. 1996;16:146-157.
256. Russo MB: Normal sleep, sleep physiology, and sleep deprivation: general principles. eMedicine 2004. Available: http://www.emedicine.com/neuro/topic444.htm. Accessed February 13, 2005.
257. Sabatowski R., Galvez R., Cherry D.A., et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomized placebo-controlled clinical trial. Pain. 2004;109:26-35.
258. Sadock B.J., Sadock V.A. The brain and behavior. In Kaplan & Sadock’s synopsis of psychiatry, ed 9. Philadelphia: Lippincott Williams & Wilkins, 2003.
259. Salerno S.M., Browning R., Jackson J.L. The effects of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med. 2002;162:19-24.
260. Salzman C., Watsky E. Rational prescribing of benzodiazepines. In: Hallstrom C., editor. Benzodiazepine dependence. Oxford: Oxford University Press, 1993.
261. Samad T.A., Moore K.A., Sapirstein A., et al. Interleukin-1β-mediated induction of COX-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471-475.
262. Sanger D.J., Depoortere H. The pharmacology and mechanism of action of zolpidem. CNS Drug Rev. 1998;4:323-340.
263. Saper C.B., Chou T.C., Scammell T.E. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726-731.
264. Sawynok J., Esser M.J., Reid A.R. Antidepressants as analgesics: an overview of central and peripheral mechanisms of action. J Psychiatry Neurosci. 2001;26:21-29.
265. Schaible H.G., Schmidt R.F. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988;60:2190-2195.
266. Scharf M.B., Roth T., Vogel G.W., et al. A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55:192-199.
267. Schofferman J. Long-term use of opioid analgesics for the treatment of chronic pain of non-malignant origin. J Pain Symptom Manage. 1993;8:279-288.
268. Scholz J., Woolf C.J. Can we conquer pain? Nat Neurosci. 2002;5(suppl):1062-1067.
269. Serpell M.G. Neuropathic Pain Study Group. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99:557-566.
270. Severijns R., van den Hout M., Vlaeyen J., et al. Pain catastrophising and general health status in a large Dutch community sample. Pain. 2002;99:367-376.
271. Shapiro A.K. Psychological aspects of medication. In: Lief H.I., Lief V.F., Lief R.N., editors. The psychological bases of medical practice. New York: Hoeber, 1963.
272. Sherrington C.S. Integrative action of the nervous system. New York: Scribner’s Sons; 1906.
273. Silverstein F.E., Faich G., Goldstein J.L., et al. Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA. 2000;284:1247-1255.
274. Sindrup S.H., Bjerre U., Dejgaard A., et al. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther. 1992;52:547-552.
275. Sindrup S.H., Gram L.F., Brosen K., et al. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain. 1990;42:135-144.
276. Smedslund J. How shall the concept of anger be defined? Theory Psychol. 1992;3:5-34.
277. Smith A.J. The analgesic effects of selective serotonin reuptake inhibitors. J Psychopharmacol. 1998;12:407-413.
278. Smith M.T., Haythornthwaite J.A. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119-132.
279. Smith T.W., Frohm K.D. What’s so unhealthy about hostility? Construct validity and psychosocial correlates of the Cook and Medley Ho scale. Health Psychol. 1985;4:503-520.
280. Songer D.A., Schulte H. Venlafaxine for the treatment of chronic pain. Am J Psychiatry. 1996;153:737.
281. Spielberger C.D., et al. Manual of the STAI. Palo Alto: Consulting Psychologists Press; 1970.
282. Stacey B.R. Management of peripheral neuropathic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S4-S16.
283. Stauffer J., Setnik B., Sokolowska M., et al. Subjective effects and safety of whole and tampered morphine sulfate and naltrexone hydrochloride extended-release capsules versus morphine solution and placebo in experienced non-dependent opioid users: a randomized, double-blind, placebo controlled, crossover study. Clin Drug Investig. 2009;29:777-790.
284. Stepanski E., Lamphere J., Badia P., et al. Sleep fragmentation and daytime sleepiness. Sleep. 1984;7:18-26.
285. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705-713.
286. Sternbach R.A. Principles of psychophysiology. New York: Academic Press; 1966.
287. Stewart W.F., Ricci J.A., Chee E., et al. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443-2454.
288. Stovitz S.D., Johnson R.J. NSAID and musculoskeletal treatment. Phys Sports Med. 31(1), 2003.
289. Strigo I.A., Duncan G.H., Boivin M., et al. Differentiation of visceral and cutaneous pain in the human brain. J Neurophysiol. 2003;89:3294-3303.
290. Stucky C.L., Gold M.S., Zhang X. Mechanisms of pain. Proc Natl Acad Sci U S A. 2001;98(21):11845-11846.
291. Sullivan M.J., Reesor K., Mikail S., et al. The treatment of depression in chronic low back pain: review and recommendations. Pain. 1992;50:5-13.
292. Sullivan M.J., Stanish W., Waite H., et al. Catastrophizing, pain, and disability in patients with soft tissue injuries. Pain. 1998;77:253-260.
293. Sullivan M.J., Thorn B., Haythornthwaite J.A., et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52-64.
294. Suzuki R., Rygh L.J., Dickenson A.H. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25(12):613-617.
295. Tasmuth T., Hartel B., Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. Eur J Pain. 2002;6:17-24.
296. Taylor K., Rowbotham M.C. Venlafaxine hydrochloride and chronic pain. West J Med. 1996;165:147-148.
297. Terman G., Bonica J.J. Spinal mechanisms and their modulation. In Loeser J.D., Butler S.D., Chapman C.R., et al, editors: Bonica’s management of pain, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2001.
298. Trait R.C., Pollard C.A., Margolis R.B., et al. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil. 1987;68:438-441.
299. Turk D.C. Clinical effectiveness and cost effectiveness of treatment for patients with chronic pain. Clin J Pain. 2002;18:355-365.
300. Turk D.C., Brody M.C., Okifuji E.A. Physicians’ attitudes and practices regarding the long-term prescribing of opioids for non-cancer pain. Pain. 1994;59:201-208.
301. Turk D.C., Flor H. Chronic pain: a biobehavioral perspective. In: Gatchel R.J., Turk D.C., editors. Psychosocial factors in pain: critical perspectives. New York: Guilford Press, 1999.
302. Turk D.C., Meichenbaum D., Genest M. Pain and behavioral medicine: a cognitive-behavioral perspective. New York: Guilford Press; 1983.
303. Turner J.A., Cardenas D.D., Warms C.A., et al. Chronic pain associated with spinal cord injuries: a community survey. Arch Phys Med Rehabil. 2001;82:501-509.
304. Turner J.A., Jensen M., Warms C., et al. Catastrophizing is associated with pain intensity, psychological distress, and pain-related disability among individuals with chronic pain after spinal cord injury. Pain. 2002;98:127-134.
305. Tzschentke T.M., Christoph T., Kogel B., Schiene K., et al. (–)-(1R,2R)-3-(3-Dimethylamino-1-ethyl-2-methyl-propylphenol Hydrochloride (Tapentadol HCl): a novel μ-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther. 2007;323:265-276.
306. US Food and Drug Administration: Alert for healthcare professionals: prescription non-steroidal anti-inflammatory drugs (NSAIDs). April 7, 2005. Available: http://www.fda.gov/cder/drug/InfoSheets/HCP/NS_NSAIDsHCP.pdf. Accessed June 16, 2005.
307. van Tulder M.W., Koes B.W., Bouter L.M. A cost-of-illness study of back pain in the Netherlands. Pain. 1995;62:233-240.
308. van Tulder M.W., Scholten R.J., Koes B.W., et al. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2000;25:2501-2513.
309. Verhaak P.F., Kerssens J.J., Dekker J., et al. Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain. 1998;77:231-239.
310. Vestergaard K., Andersen G., Gottrup H., et al. Lamotrigine for central poststroke pain: a randomized controlled trial. Neurology. 2001;56(2):184-190.
311. Vlaeyen J.W., Haazen I.W., Schuerman J.A., et al. Behavioral rehabilitation of chronic low back pain: comparison of an operant treatment, an operant-cognitive treatment and an operant-respondent treatment. Br J Clin Psychol. 1995;34:95-118.
312. Vlaeyen J.W., Kole-Snijders A.M., Boeren R.G., et al. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363-372.
313. Vlaeyen J.W., Linton S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317-332.
314. Vlaeyen J.W., Seelen H.A., Peters M., et al. Fear of movement/(re)injury and muscular reactivity in chronic low back pain patients: an experimental investigation. Pain. 1999;82:297-304.
315. Waddell G., McCulloch J.A., Kummel E., et al. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
316. Waddell G., Newton M., Henderson I., et al. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157-168.
317. Ware J.E., Sherbourne C.D. The MOS 36-Item Short Form Health Survey (SF-36). Med Care. 1992;30:473-483.
318. Waters S.J., Campbell L.C., Keefe F.J., et al. The essence of cognitive-behavioral pain management. In: Dworkin R.H., Breitbart W.S., editors. Psychosocial aspects of pain: a handbook for health care providers. progress in pain research and management, vol 27. Seattle: IASP Press, 2004.
319. Weaver M., Schnoll S. Abuse liability in opioid therapy for pain treatment in patients with an addiction history. Clin J Pain. 2002;18:S61-S69.
320. Webster L.R., Choi Y., Desai H., et al. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9(4):425-432.
321. Wheeler W.L., Dickerson E.D. Clinical application of methadone. Am J Hosp Palliat Care. 2000;17(3):196-203.
322. Willis W.D., Coggeshall R.E. Sensory mechanisms of the spinal cord. New York: Plenum Press; 1991.
323. Willis W.D., Westlund K.N. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2-31.
324. Wilson K.G., Eriksson M.Y., D’Eon J.L., et al. Major depression and insomnia in chronic pain. Clin J Pain. 2002;18:77-83.
325. Wolfe F., Cathey M.A., Hawley D.J. A double-blind placebo-controlled trial of fluoxetine in fibromyalgia. Scand J Rheumatol. 1994;23:255-259.
326. Woolf A.D., Pfeger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646-656.
327. Woolf C.J. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140:441-451.
328. Woolf C.J., Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999;96:7723-7730.
329. Woolf C.J., Mannion R.J., Neumann S. Null mutations lacking substance: elucidating pain mechanisms by genetic pharmacology. Neuron. 1998;20:1063-1066.
330. Woolf C.J., Shortland P., Coggeshall R.E. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75-78.
331. Zacny J.P. A review of the effects of opioids on psychomotor and cognitive functioning in humans. Exp Clin Psychopharmacol. 1995;3:432-466.
332. Zakrzewska J.M., Chaudhry Z., Nurmikko T.J., et al. Lamotrigine (Lamictal) in refractory trigeminal neuralgia: results from a double-blind placebo controlled crossover trial. Pain. 1997;73(2):223-230.
333. Zammit G.K., McNabb L.J., Caron J., et al. Efficacy and safety of eszopiclone across 6-weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20:1979-1991.
334. Zung W.W.K. A self-rating scale depression scale. Arch Gen Psychol. 1965;12:63-70.