61 Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a major cause of death and disability worldwide and is one of the most common reasons for intensive care unit (ICU) admission. Several monographs review this complex disorder in some detail.1,2 The intensivist’s view of COPD is predominantly physiologic, focusing on the impact of disrupted function on the individual’s normal homeostatic mechanisms. Although many important insights that have shaped our understanding of COPD have come from ICU studies, other aspects of this disorder must be considered if a rational approach to COPD management is to be developed.
Access to ICU care for sick COPD patients remains relatively inequitable among different healthcare systems. In North America and parts of Western Europe, most patients are offered ICU care, but in other relatively developed healthcare systems, such as in the United Kingdom, this is not the case. Even physicians in the same healthcare system differ significantly in their selection of patients for ICU referral.3 These choices may be influenced by local resource availability, but they are also conditioned by the generally pessimistic view of the outcome achievable with this treatment intervention. However, poor response to treatment in the ICU is not universal, and extended periods of mechanical ventilation are not invariably required to successfully manage patients with COPD.4 Nevertheless, intensivists often take a particularly bleak view of the prognosis of COPD patients compared with others entering their units. In one prospective study, intensivists estimated the survival of the sickest COPD patients to be 10% at 180 days post admission, when in fact it was 40%.5 In a survivor population after mechanical ventilation, 96% were happy to have received ventilator support, despite their continuing physical problems.6 Clearly, decisions about ventilator support should not be made in the emergency department without sufficient medical information or a proper discussion with the family. Supportive therapy should be offered until it is clear what the patient’s wishes are and what the likely outcome of treatment will be.
 Definition and Natural History
Definition and Natural History
“Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease with some extrapulmonary effects that may contribute to severity in individual patients. Its pulmonary component is characterized by airflow limitation that is not fully reversible. The airflow limitation is usually both progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gasses.”7
The emphasis here is on incompletely reversible airflow obstruction that is persistent and progressive. Symptoms and disability usually parallel these processes, although some individuals can apparently cope with a severe degree of airflow limitation without seeking medical help. Such patients finally present to the emergency room when they develop a severe exacerbation of COPD. In this situation, it is wisest to offer ventilatory support until the patient has at least had a chance to improve with conventional medical therapy. More common is a patient whose progressive illness is accompanied by repeated exacerbations, events that identify an accelerated decline in both lung function and health status.8,9 Such patients have often been hospitalized previously, and their response to treatment is usually clearly established.
The usual inhaled particles or gases that produce COPD are a complex mixture of hydrocarbons and particulates derived from tobacco smoke. These are the principal causes of COPD in the United States and western Europe,10 although other factors such as poor lung function during childhood, bronchial hyperresponsiveness, and low birth weight may also be important. The associated inflammatory changes, which persist when smoking stops,11,12 are thought to explain the airway and parenchymal destruction and fibrosis within the lung, although this has not been conclusively established as the only mechanism.
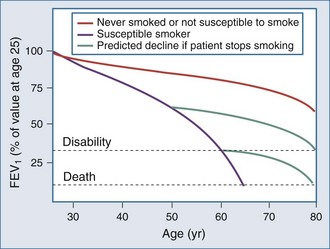
The natural history of COPD explains why the number of patients presenting for ICU care has not diminished in the last 3 decades as might be expected, given the overall reduction in tobacco consumption in Western countries. This is illustrated by the classic study of Fletcher and Peto, which has now been confirmed by longitudinal data from the Framingham study13,14 (Figure 61-1). Although the rate of decline of lung function is reduced in individuals who stop smoking, the lung function already lost is never regained, and even if the rate of decline of lung function returns to normal, these patients are still more likely to experience disability as they age. Thus, in an aging population that contains many former smokers, a significant number will still develop complications of COPD that require ICU care. The situation is complicated by the steadily rising number of women who smoke.15 Women are at least as susceptible as male smokers and more likely to be symptomatic. Thus an early fall in the number of COPD cases is being offset by the changing demographics of the current and ex-smoking population.
The important role of comorbidities in COPD has now been recognized.16 Most patients with significant symptoms due to COPD have at least one if not many comorbid diseases, especially cardiovascular problems.17 Whether the association is causal or an epiphenomenon is of little relevance in the ICU, where a high index of suspicion for undiagnosed comorbid disorders is a useful aid to effective management.
 Pathology
Pathology
The pathologic features of COPD depend on the stage of the illness and the part of the lung examined.18 Central airways show mucous gland hypertrophy and goblet cell metaplasia, whereas more peripheral airways show variable combinations of smooth muscle hypertrophy, peribronchial fibrosis, luminal occlusion by mucus, and enlarged lymphoid follicles. Alveoli are often but not invariably enlarged by the loss of alveolar walls, with an attendant loss of support for the small non-cartilaginous airways in this region of the lung. There is evidence of persistent inflammation, with neutrophils in the airway lumen and macrophages in the airway wall. CD8+ T lymphocytes are more prominent in this response than in bronchial inflammation of an asthmatic type, although intermediate states appear to exist.19 Inflammatory cells are also present adjacent to breaks in the alveolar wall.20 Overall, as the clinical and spirometric severity of the disease increases, so do the numbers of each cell population involved in the inflammatory process.21 In addition, extraluminal lymphoid follicles develop containing CD4+ lymphocytes, possibly reflecting a response to repeated infective exacerbations.21 Data obtained during exacerbations, though limited, support an increased role for neutrophils and, surprisingly, eosinophils.22
 Physiology
Physiology
The pathologic changes just described combine to produce the characteristic diagnostic finding of reduced forced expiratory flow (FEV1) at a given lung volume, which is usually assessed on a time base as an FEV1/forced vital capacity (FVC) ratio of less than 0.7. Technically, this should be 70% of the age-adjusted normal value for this ratio, because lung elastic recoil declines with age, even in healthy individuals. Use of the uncorrected ratio tends to overdiagnose COPD among the very elderly.23 In practice, however, this does not cause problems for COPD patients admitted for ICU care, because they are invariably more severely affected.
COPD affects all aspects of lung function, but its primary impact is a change in lung mechanics. This is traditionally analyzed in terms of the static (no flow) and dynamic (flow) properties of the respiratory system.24 Because chest wall mechanics are believed to be normal in COPD (although they are seldom measured directly), changes in the pressure-volume characteristics of the respiratory system are determined by alterations in lung compliance, often attributed to the loss of elastic recoil due to emphysema. How large a role this plays in changes in tissue compliance is not known. The resulting steeper slope, early-onset inspiratory plateau, and increase in end-expiratory lung volume are typical of the pressure-volume relationships in patients with COPD. Changes in end-expiratory lung volume and increases in residual volume change chest wall geometry favor a lower, flatter diaphragm and a more horizontal rib cage; these changes, in turn, impair the inspiratory muscles’ ability to develop pressure, and increase the overall work of breathing.25 Expiratory muscle activation is common in more severe COPD26,27 even at rest, and provides a useful clinical marker of respiratory distress. Flattening of the diaphragm redirects the axis of shortening of the skeletal muscle and often produces paradoxical in-drawing of the lower thoracic rib cage (so-called Hoover’s sign), which becomes more evident as pulmonary hyperinflation and respiratory drive to breathe rise. Patients with Hoover’s sign are more breathless and have more hyperinflation of their chest wall during exercise.28
The dynamics of the respiratory system are influenced by static properties but also differ significantly between inspiration and expiration. Maximum inspiratory flow is affected by inspiratory resistance as well as by the inspiratory muscles’ ability to develop pressure (and thus indirectly by chest wall geometry). Maximum expiratory flow is influenced by expiratory pressure generation and, more importantly, by the onset of volume-related airflow limitation, best described by the maximum expiratory flow-volume loop. As lung volume falls during expiration, airways close or become flow limited; hence, the flow at a specific lung volume is reduced. Although an assessment of flow (FEV1) relative to total volume change during expiration (FVC) is useful in defining COPD, an assessment of tidal flow limitation is more helpful in determining the degree of dyspnea experienced by the patient.29 More attention is now being paid to the determination of expiratory flow limitation under tidal conditions. In the past, detection was difficult, involving invasive measurements or reliance on body plethysmography, which tended to overestimate the incidence of tidal expiratory flow limitation. The development of the negative expiratory pressure test and, more recently, within-breath variation in respiratory system reactance has changed this.30 The within-breath method assesses more breaths, is less prone to observer error, and is likely to be automated in future for ICU application.31
In general, the lower the FEV1, the greater the likelihood that expiratory flow limitation is present. However, some COPD patients are not flow-limited on every breath and regulate their end-expiratory lung volume to try to minimize this. When respiratory drive rises (e.g., during exercise), during disease exacerbations, or when minute ventilation has to increase to maintain gas exchange during ventilator weaning, this resting variation in expiratory lung volume is likely to decrease. If expiratory flow and hence tidal volume are to increase, end-expiratory lung volume must rise; this further increases the work of breathing and the sensation of respiratory distress. This process, described as dynamic hyperinflation, has been clearly demonstrated during exercise and can be lessened by bronchodilator treatment which aids lung emptying.32
In the ICU, patients have a high respiratory drive during weaning and adopt a rapid, shallow breathing pattern. Total respiratory muscle work increases, in part because of the increased operating lung volumes, but also because of the presence of intrinsic positive end-expiratory pressure (PEEPi). This represents the pressure that must be developed to overcome residual expiratory driving pressure before inspiratory flow can begin.33 Calculating the size of this variable is fraught with technical difficulties beyond the problems of accurate placement of the balloon catheter system in intubated patients. Several methods have been proposed that correct for the effects of coexisting abdominal muscle activation, with recent work favoring a correction based on the total decay of gastric pressure.34 However, the need to compute this variable in clinical practice has been questioned.35
Gas Exchange
Arterial hypoxemia is common in COPD but becomes clinically significant only when the partial pressure of oxygen in arterial blood (PaO2) falls below 60 mm Hg, a problem largely confined to patients with an FEV1 below 35% of their predicted value. It arises predominantly due to ventilation-perfusion mismatching, often worsens during exercise, and is readily corrected by a small increase in the inspired oxygen concentration, unless the situation is made worse by secretion retention or severe pneumonia.36 Arterial hypercapnia is seen in some but not all hypoxemic patients who are clinically stable, but it is more frequent, at least temporarily, in hospitalized individuals.37 A combination of ventilation-perfusion mismatching due to an increase in physiologic dead space and a degree of effective alveolar hypoventilation explains this phenomenon. Acute rises in the partial pressure of arterial carbon dioxide (PaCO2) precipitate respiratory acidosis, a more reliable guide to prognosis and the need for ventilation than the PaCO2 itself.38,39
Control of Breathing
Despite years of study, there is no conclusive evidence that ventilatory control is abnormal in COPD patients. However, the response to sustained mechanical loading appears to be variable in healthy subjects40 and may explain why some individuals adopt the breathing patterns they do. Traditional techniques of studying respiratory control, which involve stimulation with exogenous CO2 or nitrogen, suggested that respiratory drive was reduced. However, studies using mouth occlusion pressure techniques or recording the electrical activation of inspiratory muscles suggest that respiratory drive is generally high, even in those COPD patients who tolerate relatively high levels of CO2.41–43 Studies of breathing pattern have been more instructive. In general, the lower the tidal volume, the higher the PaCO2.44 This is because the ratio of dead space (its fixed, predominantly anatomically determined volume) to tidal volume increases as the latter is reduced. Small tidal volumes are accompanied by an increased respiratory frequency to maintain the somewhat higher-than-normal level of minute ventilation. The resulting shortening of inspiratory time is also associated with hypercapnia.44 The system appears to be regulated to minimize peak inspiratory pressure generation, even at the cost of impaired gas exchange. There are theoretical reasons for believing that this is both energy efficient and likely to minimize the occurrence of inspiratory muscle fatigue.45 This also explains the usefulness of rapid, shallow breathing as an index of weaning failure when neuromechanical coupling in the respiratory system is under considerable stress.46
Pulmonary Circulation
In the past, considerable attention was paid to the determination of pulmonary artery pressure in COPD patients, but this is now thought to be less important. Undoubtedly, pulmonary artery pressure increases by day and at night47 in hypoxemic COPD patients, reflecting a combination of hypoxic vasoconstriction and pulmonary vascular remodeling. How important this is in the daily limitation of exercise reported by these patients is not clear, but it is known that treatment with domiciliary oxygen prevents disease progression48 and may even reduce pulmonary artery pressure. More specific attempts at therapy, including treatment with vasodilators, phosphodiesterase enzyme type V (PDEV) inhibitors, and nitric oxide—studied inside and outside the ICU—have been unsuccessful, usually resulting in unacceptable worsening of ventilation-perfusion mismatching.49 In general, assessment of pulmonary hypertension has fallen out of favor as part of a routine evaluation in COPD patients, but its occurrence is important to note when interpreting changes in central venous pressure in instrumented patients. Acute rises in pulmonary arterial pressure can follow a pulmonary embolism. Although this can lead to rather atypical COPD exacerbations with persistent hypoxemia,50 this is uncommon in routine practice.51
 Systemic Effects
Systemic Effects
There is good evidence that systemic (extrapulmonary) factors are important in COPD. Patients with a reduced body mass index die sooner than better-nourished individuals with a similar degree of pulmonary function impairment, although those who can gain weight fare better.52 There are data to show that peripheral muscle function is impaired,53 fiber type is altered,54 and exercise is associated with increased oxidative stress.55 The earlier concept of a specific COPD myopathy has now largely been abandoned, as the major burden falls on the lower limb muscles, with preserved function in the upper limb muscle groups. This likely reflects inactivity, which is worse in those with exacerbated COPD.56 Weakness of the quadriceps muscle is an independent guide to a poor prognosis.57 In contrast, the wealth of circulating biomarkers in COPD have contributed little to practical management so far.58
 Exacerbations
Exacerbations
An exacerbation of COPD is currently defined as sustained worsening of the patient’s condition from the stable state, beyond normal day-to-day variation, that is acute in onset and necessitates a change in regular medication.7 The key feature is the sustained change from usual daily symptoms. The operational requirement for a change in treatment is more arbitrary but is almost always present in patients referred for ICU care. Disease exacerbation is the principal cause of ICU admission with COPD, and patients commonly have or are at risk of developing significant respiratory failure, defined as a PaO2 below 60 mm Hg with or without an increase in PaCO2.59 The most common causes of exacerbation are listed in Table 61-1. Viral and bacterial infections are both relevant,54 with rhinoviruses commonly reported in most series; Haemophilus influenzae and Streptococcus pneumoniae are the principal microbial pathogens.60,61 Some patients, particularly those with a regular cough and green sputum production, develop persistent lower respiratory tract colonization, making the interpretation of qualitative microbiology difficult.62 Usually there is an increase in the absolute number of colony-forming units of microorganisms in these patients during exacerbations, reflecting an increased burden of infection, although more subtle changes have been reported involving the introduction of a different serotype of H. influenzae63 without substantial changes in the total bacterial load.64
TABLE 61-1 Causes of Chronic Obstructive Pulmonary Disease Exacerbation
* Clinical presentation is dominated by the primary illness, but respiratory failure can occur.
Not all exacerbations of COPD have an infectious precipitant, and changes in the degree of atmospheric pollution can precipitate events in some patients.65 How frequently individuals develop exacerbations after exposure to a specific precipitating event is not clear, although the likelihood of meeting the consensus definition rises as spirometric impairment worsens.66
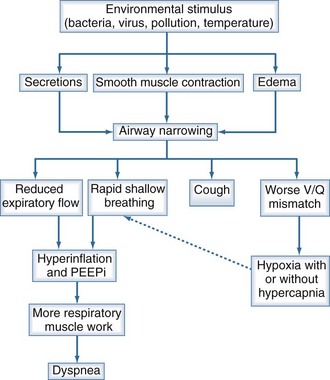
The physiologic consequences of increased airflow obstruction secondary to increased inflammation within the bronchial tree are summarized in Figure 61-2. Whatever the precipitant, the key event appears to be a change in lung mechanics. Previously, attention focused on alterations in respiratory system resistance, but more recent data emphasize that airway narrowing and closure may be more important, particularly by producing changes in operating lung volumes (see earlier discussion). Observations in patients recovering from hospitalized exacerbations have shown progressive improvements in respiratory system reactance (a measure of inspiratory resistance and flow limitation ) together with reductions in end-expiratory lung volume that are most evident in patients reporting less dyspnoea.67 These changes are larger than those in spirometry and help explain why the small changes in FEV1 associated with exacerbations can be associated with substantial deterioration in gas exchange and clinical well-being, leading to hospitalization.
Pneumonia is an important reason for hospitalization in COPD, is more frequently seen in these patients than in others, and is associated with worse outcomes.68 Pneumonia is diagnosed more frequently in patients taking the inhaled corticosteroid, fluticasone propionate,69 especially older patients with worse airflow obstruction.70 These pneumonias are not necessarily associated with poor outcome in terms of mortality or health status69 and are not seen with all types of inhaled corticosteroids.71,72 At present the benefit of inhaled corticosteroid treatment, especially combined with a long-acting inhaled bronchodilator, outweigh the apparent risk of increased pneumonia events.
 Clinical Features
Clinical Features
Key clinical features of the acute presentation of COPD are summarized in Table 61-2. In addition to obtaining an appropriate history and performing a physical examination, with particular attention to the respiratory rate, it is necessary to assess the degree of abnormal gas exchange and the presence of acidosis by measuring arterial blood gases. In the context of an exacerbation, more direct measurements of lung mechanics are usually impractical, and the severity of the mechanical problem is evaluated indirectly by its effect on gas exchange. An urgent chest radiograph is useful for identifying specific precipitating factors, particularly alveolar shadowing due to infection, the presence of a pneumothorax, or radiographic features of pulmonary edema. The last is especially important, because it is commonly associated with hypercapnic respiratory failure, with the combination of an increased ventilatory drive and poor perfusion of respiratory muscles, together with further impairment of ventilation-perfusion matching favoring CO2 retention. In this context, an electrocardiogram is invaluable to screen for both underlying ischemic heart disease and rhythm disturbances. If a major thromboembolic event is suspected on clinical grounds, quantitative D-dimer and urgent computed tomographic pulmonary angiography is the best way to establish this diagnosis. Simple laboratory tests, such as the hemoglobin and white cell count, can be valuable guides to the need for oxygenation and the likelihood of coexisting sepsis.
TABLE 61-2 Clinical Features of Chronic Obstructive Pulmonary Disease Exacerbation
|
Symptoms of upper respiratory tract infection (variable and should be accompanied by a major symptom)
|
 Intensive Care Unit Referral
Intensive Care Unit Referral
The need for ventilatory support is the primary reason for ICU referral among COPD patients. Although the various indications for mechanical ventilation (Table 61-3) vary in frequency from institution to institution, they represent the most common causes for ICU admission.
FIO2, inspired oxygen fraction; PaCO2, partial pressure of carbon dioxide in arterial blood; PaO2, partial pressure of oxygen in arterial blood.
 Principles of Treatment
Principles of Treatment
Treat Precipitating Factors
Bacterial infection is the most common reason for ICU admission in COPD patients. There is now good evidence that antibiotics shorten the symptomatic period, even when patients are treated with corticosteroids73; when given early, antibiotics are associated with lower mortality, fewer episodes of intubation, and shorter hospital stays.74 Intravenous therapy with antimicrobials is generally required in patients sick enough to merit ICU admission. Radiographic evidence of pneumonia likely requires a broadening of the antibiotic spectrum, but whether the infection is confined to the airways or involves the alveoli, antibiotic therapy should follow locally established guidelines designed to minimize the development of resistance within the ICU and to address known patterns of drug resistance in the community and the hospital. Broad-spectrum penicillins or, more commonly, cephalosporins are usually recommended, often with an intravenous macrolide. Some advocate the prophylactic use of a quinolone in COPD patients in the ICU,75 but this practice requires confirmation before being accepted as universally effective. Colonization with methicillin-resistant Staphylococcus aureus is a frequent problem and requires particular vigilance in the selection of antibiotics. Likewise, excessive use of broad-spectrum agents can produce superinfection, such as Clostridium difficile diarrhea. This can be particularly distressing in a patient with severe COPD and low body mass index and requires early identification and appropriate therapy.
The role of antiviral drugs, such as the neuraminidase inhibitors, in the management of acutely ill COPD patients remains to be determined. Surprisingly, H1N1 influenza infection has not been a major problem for COPD patients, possibly reflecting prior partial immunity.76 If this virus is diagnosed, the use of antivirals such as oseltamivir is prudent but not likely to have a major effect on the natural history of the episode. Similar considerations apply to other viral pneumonias.
Reduce Lung Volume and Increase Expiratory Flow
Agents that improve lung emptying, commonly by increasing airway caliber or preventing airway closure, interfere with the vicious circle of pulmonary hyperinflation described in Figure 61-2. This has been demonstrated in stable patients using exercise as a model of hyperinflation,32 but the data in spontaneously breathing COPD patients during exacerbations are much less satisfactory. Nonetheless, treatment with regular but high doses of short-acting nebulized β-agonists such as albuterol or ipratropium (2.5-5 mg or 250-500 µg, respectively), is usually recommended. There is no clear evidence that one drug is better than the other,77 and combination therapy is commonly used. The outcomes of the few studies conducted in this setting were based on FEV1 rather than symptoms or lung volume change. Intravenous theophylline, or one of its derivatives, is often added to these regimens but is no more effective than a placebo infusion.78,79
Reduce Pulmonary Inflammation
Several randomized, controlled trials have shown that oral corticosteroids shorten the duration of hospitalization and accelerate improvement of post-bronchodilator FEV1 during an exacerbation of COPD.80,81 Patients randomized to treatment with oral corticosteroids were less likely to relapse during the subsequent month and showed a number of other benefits, although these did not always reach statistical significance.82 There does not appear to be any additional benefit from using particularly high doses of corticosteroids or prolonging treatment beyond 10 days to 2 weeks. In the ICU, corticosteroid treatment is often given peremptorily to patients on mechanical ventilation; caution should be exercised, however, because these individuals are often at risk for relatively acute-onset corticosteroid myopathy.83 If corticosteroid therapy has been maintained for a longer-than-normal period, or if courses of oral corticosteroids to treat less serious exacerbations have been given frequently, a tapered dose-reduction plan should be introduced. Otherwise, treatment can be discontinued at the end of the normal 10 to 14 days. Patients given this therapy may benefit from subsequent treatment with inhaled corticosteroids, but they should be evaluated for significant side effects.69 Osteoporosis is particularly common in COPD patients, whether they receive corticosteroid treatment or not, and it is probably worth identifying in any individual who requires ICU care.84
Manage Gas Exchange
It is relatively easy to improve oxygenation in an uncomplicated exacerbation of COPD.85 Raising the inspired oxygen concentration to 28% to 35% is usually sufficient to achieve a PaO2 greater than 90 mm Hg. However, this can be accompanied by an undesirable increase in PaCO2, with its accompanying respiratory acidosis. Such an increase in PaCO2 impairs respiratory muscle function, at least during loaded breathing,86 and often precedes more serious clinical deterioration, including impairment of consciousness. The reasons for this effect have been debated for many years, with some advocating a reduction in respiratory drive from the carotid chemoreceptors, and others citing a worsening ventilation-perfusion match as the cause.85 Each view has evidence to support it, but the actual cause is likely a combination of both problems, with ventilation-perfusion mismatching being particularly important in severely ill patients, and hypoventilation playing a larger role in those not yet sick enough to require intubation.87
Although the phenomenon of oxygen-induced hypercapnia has been recognized for decades, it remains a real problem. In one large center in the United Kingdom, 34% of individuals showed evidence of oxygen-induced hypercapnia.39 The use of high-flow oxygen in the emergency room is widespread, as is the false sense of security provided by a high oxygen saturation. Many intensivists have legitimate concerns about the failure to adequately oxygenate COPD patients with compromised circulation, along with the attendant risk of unanticipated mortality. However, the solution is to carefully consider the risks of excessive or insufficient oxygen in a given individual, rather than to slavishly adhere to one view or the other. Patients whose problems are predominantly due to COPD and who have a normal hemoglobin and preserved cardiac output can maintain adequate tissue oxygen delivery with an oxygen saturation as low as 85%, and they will do quite well if an arterial oxygen saturation (SaO2) of 90% to 93% is maintained. The modest increase in inspired oxygen needed to achieve this (often 24%-28%) is accompanied by less hypercapnia and may avoid the need for ventilatory support. However, if cardiac output is impaired (reduced blood pressure, poor peripheral circulation) or tissue metabolic demands are increased (e.g., in sepsis secondary to pneumonia), a higher SaO2 will be required to ensure sufficient oxygen delivery; in this case, the consequences of any resultant hypercapnia, including the need for ventilatory support, must be accepted.
Oxygen can be delivered accurately by facemask, using the Venturi principle of entraining room air into the mask. This is a precise method of giving a known inspired oxygen concentration to COPD patients,88 but many patients dislodge facemasks and are unlikely to keep nasal prongs in place.89 Nasal prongs allow the patients to speak and drink, but the inspired oxygen fraction (FIO2) is more variable, and it may be necessary to monitor arterial blood gases more frequently. Institutions where nebulizers are used to deliver bronchodilator drugs should be cautious about nebulizing these drugs using wall oxygen, because this can produce severe hypercapnia. A better policy is to nebulize in air, with the patients keeping their nasal cannulas in place.
For many years, respiratory stimulants were used to waken semiconscious patients and permit physiotherapy and other forms of suction, but this approach was never tested scientifically and must be viewed with some skepticism. Although respiratory stimulants were recommended as a way of deferring the need for positive-pressure ventilation, the advent of nasal positive-pressure ventilation has changed this approach, and the only study that directly compared the effects of doxapram and this modality in COPD concluded that patients did better with noninvasive ventilation and were less likely to deteriorate.90 If chemical ventilatory stimulants are used, they should be considered a short-term means of sustaining the patient until a more appropriate method of ventilation can be instituted. Ultimately, some kind of mechanical ventilatory support is the best way to address the problems of hypercapnia.
 Noninvasive Ventilation
Noninvasive Ventilation
This topic is reviewed in detail in Chapter 51, but some key issues relevant to COPD are worth emphasizing. Many of the data supporting the use of noninvasive ventilation (NIV) were obtained in patients with hypercapnic respiratory failure due to COPD exacerbation, and several excellent reviews have analyzed these data.91,92
Noninvasive ventilation has a number of potentially beneficial effects in COPD. Intuitively, it seems reasonable to expect that it would increase tidal volume, improve CO2 elimination, and hence reduce respiratory drive. Studies of gas exchange using a multiple inert gas elimination methodology confirmed that CO2 elimination is increased, but overall ventilation-perfusion mismatch is not changed during NIV.93 A more important effect is the unloading of the respiratory muscles, which are often close to fatigue conditions in severe episodes of respiratory failure. By assuming some of the additional work required to overcome intrinsic PEEP, NIV directly reduces the drive to breathe, and the respiratory rate falls, a good prognostic feature.94 Data from randomized, controlled trials suggest that there is a mean fall of 3.1 breaths per minute (95% confidence interval 4.3 to 1.9) with the institution of NIV in COPD patients.91 This allows more effective emptying of the lungs and less dynamic hyperinflation. The resulting improvement in the intensity of breathlessness is usually a much earlier sign of successful NIV treatment in COPD than are changes in blood gas tensions, which often lag behind evidence of clinical improvement.
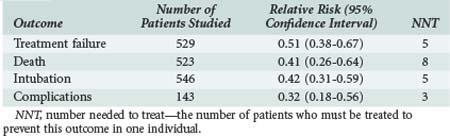
Evidence-based reviews provide a reasonable series of recommendations based on the relative effectiveness of NIV. Key points, including the number of patients needed to be treated to prevent one significant event or complication, are shown in Table 61-4. Noninvasive ventilation is associated with less treatment failure, lower mortality, fewer complications, and a lower intubation rate compared with conventional medical treatment. It reduces ICU or hospital stay by approximately 3 days and favorably influences gas exchange. With NIV, pH increases by a mean value of 0.03 (0.02-0.04), PaCO2 falls by 3 mm Hg (5.9-0.23 mm Hg), and PaO2 rises by 2 mm Hg (−2 to +6 mm Hg). The lower rate of nosocomial pneumonia associated with NIV is a particular advantage.
Data support the use of NIV as a first-line treatment in patients with exacerbations of COPD and moderate respiratory acidosis (pH < 7.35) despite medical treatment. In general, most patients with pH in the range of 7.3 to 7.35 survive without NIV, although the number patients needed to prevent one intubation is still only 10.95 As acidosis becomes more severe, the benefits of NIV become greater; this treatment should be encouraged in anyone with a pH less than 7.3. In patients with more severe acidosis (pH < 7.25), the benefit is less clear, and results in different trials suggest that such patients have a better outcome if they are managed in the ICU with mechanical ventilation and intubation; however, these trials are influenced by selection bias. In clinical practice, it is reasonable to offer a trial of NIV unless the patient has some of the established contraindications to this treatment (Table 61-5). Even then, there are occasions when NIV is appropriate first-line therapy—for example, if a patient does not wish to be intubated (as indicated in an advance directive) or has a “ceiling of treatment” determined by his or her prior health status.
Treatment failure, which occurs in approximately 30% of cases,96 reflects an inability to adapt to NIV or progression of the underlying disease. Recent data suggest that patients likely to subsequently fail with NIV can be prospectively identified by a high blood sugar on admission (irrespective of having diabetes), a raised respiratory rate, or a high APACHE 2 score. All these variables are relatively effective predictors of risk, but combining them increases their discriminant power.94 In COPD patients, failure to trigger the noninvasive ventilator or excess trigger sensitivity can lead to problems of coordination between patient and machine. Air leakage can be a problem when facemasks are used, the usual approach in patients with COPD. Reducing rather than increasing inspiratory positive airway pressure often lessens this complication and allows better patient-ventilator coordination. Some patients develop hypercapnia, occasionally due to rebreathing in the mask, but more often due to ineffective cough and retained secretions. Conversion to a nasal mask and chinstrap allows more effective cough without loss of ventilator support. Late failure (after 48 hours or more of NIV), suggested by worsening acidosis, is a poor prognostic sign; it usually reflects deterioration caused by the underlying lung disease. If this occurs, the institution of invasive mechanical ventilation needs to be considered.97 Patients treated in this way may have a better prognosis, although the interpretation of data is difficult, given the nonrandomized design of the relevant study. What is clear is that extending the period of NIV in a patient with physiologic evidence of deterioration is not likely to produce a successful result.
In addition to its role in the acute phase of respiratory failure, NIV can be valuable as a “bridge” in helping patients wean from intermittent positive-pressure ventilation. In an important multicenter prospective trial, Nava and colleagues randomized people who had failed a T-piece weaning trial to either NIV or further mechanical ventilation.98 Noninvasive ventilation was associated with fewer days of ventilatory support (10.2 versus 16.6, respectively), shorter ICU stay (15.1 versus 24 days), less nosocomial pneumonia, and better 60-day survival (92% versus 72%). These results were achieved in a unit with experience in NIV. The generic use of weaning by NIV has proven less successful, particularly if patients have significant cardiac disease or established acute respiratory distress syndrome (ARDS).99 However, further data from Spain have confirmed the value of this approach in hypercapnic patients limited primarily by COPD.100,101
 Mechanical Ventilation
Mechanical Ventilation
Mechanical ventilation should be considered when NIV is not appropriate (see Table 61-5) or has failed. Patients with a pH below 7.25 are more likely to require this therapy, although most physicians now offer a trial of NIV unless the patient is hemodynamically unstable or the treatment is contraindicated. Persistent significant hypoxemia despite treatment, hypotension, and impaired mental state are all predictors of imminent respiratory arrest and the need for intubation and institution of mechanical ventilation.
The major risk during intubation is hypotension. This reflects a combination of problems, including reduced venous return secondary to positive intrathoracic pressures, direct vasodilatation, and reduced sympathetic tone produced by the anesthetic agents. Reoxygenation of the patient with rapid-sequence induction of anesthesia is recommended, and this is normally accompanied by cricoid pressure during intubation to reduce the risk of aspiration, although the benefits of this technique remain unclear.102 Short-acting muscle relaxants are usually used. Because of concerns about the risk of hyperkalemia, nondepolarizing drugs are often preferred in this circumstance. Hypotension is normally combated with fluid replacement, and if it is persistent, it is sensible to disconnect the endotracheal tube from the ventilator and allow the patient to return to a true end-expiratory lung volume before resuming ventilation.
Ventilation Strategies
A wide range of ventilation strategies have been advocated for use in COPD, each with its own proponents; none has shown a clear advantage over its competitors, however. Familiarity with the equipment in the context of COPD patients is probably more important than the relatively minor differences between ventilator modes. The most commonly used approaches, together with their proposed advantages, are summarized in Table 61-6.
| Mode | Method | Comment |
|---|---|---|
| Assist-control | Preset tidal volume, patient triggered with backup rate | Patient still performs substantial work of breathing; dynamic hyperinflation worsens this |
| Spontaneous intermittent mandatory ventilation | Preset number of breaths of a preset volume—patient does the rest | Patient still makes an effort during part of machine breath—involves more patient work, especially at low respiratory rates |
| Pressure support ventilation | Pressure set to augment each inspiration—tidal volume depends on patient effort, pulmonary mechanics, and pressure applied | Basis of noninvasive ventilation therapy; pressure titrated to a respiratory rate below 27 breaths/min; asynchrony with machine breaths a problem at high pressures |
| Proportional assist ventilation | Flow and volume generated proportional to patient effort | Experimental technique; requires accurate measurement of elastance and resistance + an intact drive to breathe; proven effective in COPD patients |
Assisted Ventilation and Weaning
As acidosis resolves and oxygen requirements fall, it is possible to reduce the degree of sedation and allow the patient to make some contribution to ventilation before weaning. Several modes of ventilatory support are available in these circumstances, and again, there is no specific advantage of one over another.103,104 There is an impression, however, that reliance on spontaneous intermittent mandatory ventilation prolongs subsequent weaning. Although not universally accepted, there are good data supporting the use of spontaneous breathing trials in clinically stable COPD patients to determine when they are ready to wean.104–106 The ability to sustain ventilation in the absence of increasing CO2, worsening acidosis, or clinical distress (reflected by an increase in blood pressure, heart rate, or restlessness) is generally agreed to be a predictor of future weaning success. Although COPD patients are less likely to achieve these goals as early as other ICU patients, the reintubation rate in those who do meet these criteria is low.105,106 Unfortunately, breathing through the ventilator on a continuous positive airway pressure (CPAP) circuit may be associated with significant increases in inspiratory resistance,107 and it is sensible to use pressure support to offset some of this additional respiratory work. This reflects the necessity of identifying patients who can be weaned using the ventilator alone and those who need more prolonged support. In the latter circumstance, weaning supported by NIV is particularly helpful.
A variety of predictors of weaning success have been developed to try to identify when successful weaning will occur. Unfortunately, none has proved entirely reliable, and relatively few have been assessed prospectively. An empirical approach based on the criteria listed in Table 61-7 is widely used. An aggressive policy toward weaning is justified in COPD patients, because an inability to wean is invariably associated with a worse prognosis and prolonged ventilation.
 Nonventilatory Issues
Nonventilatory Issues
Therapy employed in spontaneously breathing patients is still required in those undergoing mechanical ventilation. High-dose nebulized bronchodilators are commonly used, singly and in combination,108,109 although it is important to pay attention to the details of drug delivery. Drug deposition within the ventilator circuit and endotracheal tube can lead to a significant loss of effective drug.93 When using a nebulized drug, the nebulizer should be placed in the inspiratory line at least 30 cm from the endotracheal tube; this allows the tubing to act as a spacer device and increases the respirable fraction.110 If a metered dose inhaler is used instead, it should always be given with some form of spacer device for the same reason. Parenteral corticosteroids are commonly administered. This is not without hazard, particularly because of the real risk of myopathy (see earlier). As noted previously, there does not seem to be any advantage in giving high doses of corticosteroids.
 Prognosis
Prognosis
The prognosis following an exacerbation of COPD is better than the gloomy outlook proposed by some physicians. Nonetheless, patients who experience exacerbations appear to have a more severe clinical course than those who do not, and they report a worse overall quality of life.111 Mortality after an ICU admission is significant, at least in North American series112; 10% to 15% of such subjects die as inpatients, and over the next 2 years, 30% to 60% die. Patients with a low FEV1, significant comorbidity, and a particularly poor performance status at home have the worst outlook.113 These factors should be considered when decisions about the requirement for ventilatory support are made. However, as noted already, the physician’s view of the very sick COPD patient can be unduly pessimistic. Exacerbations leave patients relatively immobile, and this has now been confirmed objectively.56 There are encouraging data suggesting that early rehabilitation can reduce subsequent hospital admissions, although the optimal place to organize such a program for patients post exacerbation has still to be determined.114
Changes in clinical practice continue to improve the outlook for COPD patients. The impact of NIV on their acute care has been enormous, as has closer adherence to evidence-based recommendations across the field of intensive care,115 something about which both practitioners and their patients can feel proud.
Key Points
Aaron SD, Vandemheen KL, Hebert P, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med. 2003;348(26):2618-2625.
Davies L, Angus RM, Calverley PMA. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354(9177):456-460.
Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1999;340(25):1941-1947.
Calverley PMA, MacNee W, Pride NB, Rennard SI, editors. Chronic Obstructive Pulmonary Disease, 2nd ed, London: Arnold, 2003.
Connors AFJ, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease: The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med. 1996;154(1):959-967. erratum, Am J Respir Crit Care Med 1997;155(4 Pt 1):386
Still the major study of outcomes in COPD patients managed in the ICU.
Lightowler JV, Wedzicha JA, Elliott MW, et al. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003;326(7382):185.
Soler N, Torres A, Ewig S, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1498-1505.
Younes M. Dynamic intrinsic PEEP (PEEP(i), dyn): Is it worth saving? Am J Respir Crit Care Med. 2000;162(5):1608-1609.
Thoughtful overview of a physiologically important but technically difficult measurement.
Ferrer M, Sellares J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet. 2009;374(9695):1082-1088.
1 Calverley PMA, MacNee W, Pride NB, Rennard SI, editors. Chronic Obstructive Pulmonary Disease, 2nd ed, London: Arnold, 2003.
2 Voelkel N, MacNee W, editors. Chronic Obstructive Pulmonary Disease. Hamilton: BC Dekker, 2002.
3 Wildman MJ, O’Dea J, Kostopoulou O, Tindall M, Walia S, Khan Z. Variation in intubation decisions for patients with chronic obstructive pulmonary disease in one critical care network. QJM. 2003 August;96(8):583-591.
4 Breen D, Churches T, Hawker F, Torzillo PJ. Acute respiratory failure secondary to chronic obstructive pulmonary disease treated in the intensive care unit: a long term follow up study. Thorax. 2002 January;57(1):29-33.
5 Wildman MJ, Sanderson C, Groves J, et al. Implications of prognostic pessimism in patients with chronic obstructive pulmonary disease (COPD) or asthma admitted to intensive care in the UK within the COPD and asthma outcome study (CAOS): multicentre observational cohort study. BMJ. 2007 December 1;335(7630):1132.
6 Wildman MJ, Sanderson CF, Groves J, et al. Survival and quality of life for patients with COPD or asthma admitted to intensive care in a UK multicentre cohort: the COPD and Asthma Outcome Study (CAOS). Thorax. 2009 February;64(2):128-132.
7 Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007 September 15;176(6):532-555.
8 Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002 October;57(10):847-852.
9 Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004 May;23(5):698-702.
10 Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007 September 1;370(9589):765-773.
11 Turato G, Di Stefano A, Maestrelli P, et al. Effect of smoking cessation on airway inflammation in chronic bronchitis. Am J Respir Crit Care Med. 1995 October;152(4 Pt 1):1262-1267.
12 Gamble E, Grootendorst DC, Hattotuwa K, et al. Airway mucosal inflammation in COPD is similar in smokers and ex-smokers: a pooled analysis. Eur Respir J. 2007 September;30(3):467-471.
13 Fletcher C, Peto R. The natural history of chronic airway obstruction. BMJ. 1977;1(6077):1645-1648.
14 Kohansal R, Martinez-Camblor P, Agusti A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009 July 1;180(1):3-10.
15 Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006 January;27(1):188-207.
16 Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009 May;33(5):1165-1185.
17 Crisafulli E, Costi S, Luppi F, et al. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax. 2008 June;63(6):487-492.
18 Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004 August 21;364(9435):709-721.
19 Saetta M, Di Stefano A, Turato G, et al. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998 March;157(3 Pt 1):822-826.
20 Saetta M, Ghezzo H, Kim WD, et al. Loss of alveolar attachments in smokers. A morphometric correlate of lung function impairment. Am Rev Respir Dis. 1985 October;132(4):894-900.
21 Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004 June 24;350(26):2645-2653.
22 Saetta M, Di Stefano A, Maestrelli P, et al. Airway eosinophilia and expression of interleukin-5 protein in asthma and in exacerbations of chronic bronchitis. Clinical & Experimental Allergy. 1996 July;26(7):766-774.
23 Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002 November;20(5):1117-1122.
24 Pride NB, Milic-Emili J. Lung mechanics. In: Calverley PMA, Pride NB, editors. Chronic Obstructive Pulmonary Disease. 1 ed. London: Edward Arnold; 1995:69-92.
25 Gibson GJ. Pulmonary hyperinflation a clinical overview. Eur Respir J. 1996 December;9(12):2640-2649.
26 Dodd DS, Brancatisano T, Engel LA. Chest wall mechanics during exercise in patients with severe chronic air-flow obstruction. Am Rev Respir Dis. 1984 January;129(1):33-38.
27 Gorini M, Misuri G, Duranti R, Iandelli I, Mancini M, Scano G. Abdominal muscle recruitment and PEEPi during bronchoconstriction in chronic obstructive pulmonary disease. Thorax. 1997 April;52(4):355-361.
28 Aliverti A, Quaranta M, Chakrabarti B, Albuquerque AL, Calverley PM. Paradoxical movement of the lower ribcage at rest and during exercise in COPD patients. Eur Respir J. 2009 January;33(1):49-60.
29 Eltayara L, Becklake MR, Volta CA, Milic-Emili J. Relationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996 December;154(6 Pt 1):1726-1734.
30 Calverley PM, Koulouris NG. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J. 2005 January;25(1):186-199.
31 Dellaca RL, Duffy N, Pompilio PP, et al. Expiratory flow limitation detected by forced oscillation and negative expiratory pressure. Eur Respir J. 2007 February;29(2):363-374.
32 O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006 April;3(2):180-184.
33 Pepe PE, Marini JJ. Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction: the auto-PEEP effect. Am Rev Respir Dis. 1982 July;126(1):166-170.
34 Zakynthinos SG, Vassilakopoulos T, Zakynthinos E, Roussos C. Accurate measurement of intrinsic positive end-expiratory pressure: How to detect and correct for expiratory muscle activity. Eur Respir J. 1997;10(3):522-529.
35 Younes M. Dynamic intrinsic PEEP (PEEP(i),dyn): is it worth saving? Am J Respir Crit Care Med. 2000 November;162(5):1608-1609.
36 Rodriguez-Roisin R, Barbera JA, Roca J. Pulmonary gas exchange. In: Calverley PMA, MacNee W, Pride NB, Rennard SI, editors. Chronic obstructive pulmonary disease. 2nd ed. London: Arnold; 2003:175-193.
37 Costello R, Deegan P, Fitzpatrick M, McNicholas WT. Reversible hypercapnia in chronic obstructive pulmonary disease: a distinct pattern of respiratory failure with a favorable prognosis. Am J Med. 1997 March;102(3):239-244.
38 Jeffrey AA, Warren PM, Flenley DC. Acute hypercapnic respiratory failure in patients with chronic obstructive lung disease: risk factors and use of guidelines for management. Thorax. 1992 January;47(1):34-40.
39 Plant PK, Owen JL, Elliott MW. One year period prevalence study of respiratory acidosis in acute exacerbations of COPD: implications for the provision of non-invasive ventilation and oxygen administration. Thorax. 2000 Jul;55(7):550-554.
40 Clague JE, Carter J, Pearson MG, Calverley PM. Effect of sustained inspiratory loading on respiratory sensation and CO2 responsiveness in normal humans. Clin Sci. 1996 October;91(4):513-518.
41 Parot S, Miara B, Milic-Emili J, Gautier H. Hypoxemia, hypercapnia, and breathing pattern in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1982 November;126(5):882-886.
42 Sorli J, Grassino A, Lorange G, Milic-Emili J. Control of breathing in patients with chronic obstructive lung disease. Clin Sci Mol Med. 1978 March;54(3):295-304.
43 De Troyer A, Leeper JB, McKenzie DK, Gandevia SC. Neural drive to the diaphragm in patients with severe COPD. Am J Respir Crit Care Med. 1997 April;155(4):1335-1340.
44 Gorini M, Misuri G, Corrado A, et al. Breathing pattern and carbon dioxide retention in severe chronic obstructive pulmonary disease. Thorax. 1996 July;51(7):677-683.
45 Poon CS, Lin SL, Knudson OB. Optimization character of inspiratory neural drive. J Appl Physiol. 1992 May;72(5):2005-2017.
46 Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991 May 23;324(21):1445-1450.
47 Douglas NJ, Calverley PM, Leggett RJ, et al. Transient hypoxaemia during sleep in chronic bronchitis and emphysema. Lancet. 1979 January 6;1(8106):1-4.
48 Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981 March 28;1(8222):681-686.
49 Barbera JA, Roger N, Roca J, Rovira I, Higenbottam TW, Rodriguez-Roisin R. Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet. 1996 February 17;347(8999):436-440.
50 Tillie-Leblond I, Marquette CH, Perez T, et al. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Ann Intern Med. 2006 March 21;144(6):390-396.
51 Rutschmann OT, Cornuz J, Poletti PA, et al. Should pulmonary embolism be suspected in exacerbation of chronic obstructive pulmonary disease? Thorax. 2007 February;62(2):121-125.
52 Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998 June;157(6 Pt 1):1791-1797.
53 Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153(3):976-980.
54 Maltais F, Sullivan MJ, LeBlanc P, et al. Altered expression of myosin heavy chain in the vastus lateralis muscle in patients with COPD. Eur Respir J. 1999 April;13(4):850-854.
55 Rabinovich RA, Ardite E, Troosters T, et al. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001 October 1;164(7):1114-1118. JID—9421642
56 Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006 March;129(3):536-544.
57 Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007 February;62(2):115-120.
58 Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004 July;59(7):574-580.
59 Calverley PMA. Chronic respiratory failure. In: Warrell DA, Cox TM, Firth JD, editors. Oxford Textbook of Medicine. 5th ed. Oxford: OUP; 2010:3467-3475.
60 Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001 November 1;164(9):1618-1623.
61 Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev. 2001 April;14(2):336-363.
62 Soler N, Agusti C, Angrill J, Puig DlB, Torres A. Bronchoscopic validation of the significance of sputum purulence in severe exacerbations of chronic obstructive pulmonary disease. Thorax. 2007 January;62(1):29-35.
63 Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002 August 15;347(7):465-471.
64 Sethi S, Sethi R, Eschberger K, et al. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007 August 15;176(4):356-361.
65 Delfino RJ, Murphy-Moulton AM, Burnett RT, Brook JR, Becklake MR. Effects of air pollution on emergency room visits for respiratory illnesses in Montreal, Quebec. Am J Respir Crit Care Med. 1997 February;155(2):568-576.
66 Jones PW, Willits LR, Burge PS, Calverley PM. Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):68-73.
67 Stevenson NJ, Walker PP, Costello RW, Calverley PM. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005 December 15;172(12):1510-1516.
68 Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006 August;28(2):346-351.
69 Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007 February 22;356(8):775-789.
70 Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009 September;34(3):641-647.
71 Calverley PM, Rennard S, Nelson HS, et al. One-year treatment with mometasone furoate in chronic obstructive pulmonary disease. Respir Res. 2008 November 13;9:73. 73
72 Sin DD, Tashkin D, Zhang X, et al. Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet. 2009 August 29;374(9691):712-719.
73 Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010 January 15;181(2):150-157.
74 Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010 May 26;303(20):2035-2042.
75 Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trial. Lancet. 2001 December 15;358(9298):2020-2025.
76 Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010 May 6;362(18):1708-1719.
77 Moayyedi P, Congleton J, Page RL, Pearson SB, Muers MF. Comparison of nebulised salbutamol and ipratropium bromide with salbutamol alone in the treatment of chronic obstructive pulmonary disease. Thorax. 1995 August;50(8):834-837.
78 Barr RG, Rowe BH, Camargo CAJr. Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta-analysis of randomised trials. BMJ. 2003 September 20;327(7416):643.
79 Duffy N, Walker P, Diamantea F, Calverley P, Davies L. Intravenous aminophylline in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Thorax. 2005;60(9):713-717.
80 Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1999;340(25):1941-1947.
81 Davies L, Angus RM, Calverley PMA. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354(9177):456-460.
82 Aaron SD, Vandemheen KL, Hebert P, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med. 2003 June 26;348(26):2618-2625.
83 Anzueto A. Muscle dysfunction in the intensive care unit. Clin Chest Med. 1999 June;20(2):435-452.
84 Ferguson GT, Calverley PM, Anderson JA, et al. Prevalence and progression of osteoporosis in Patients with COPD. Results from the Towards a Revolution in COPD Health study. Chest. 2009 July;136(6):1456-1465.
85 Barbera JA, Roca J, Ferrer A, Diaz FO, Roger N, Rodriguez-Roisin R. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 1997;10(6):1285-1291.
86 Juan G, Calverley P, Talamo C, Schnader J, Roussos C. Effect of carbon dioxide on diaphragmatic function in human beings. New Engl J Med. 1984 April 5;310(14):874-879.
87 Calverley PMA. Oxygen-induced hypercapnia revisited. Lancet. 2000;356(9241):1538-1539.
88 Bazuaye EA, Stone TN, Corris PA, Gibson GJ. Variability of inspired oxygen concentration with nasal cannulas. Thorax. 1992 August;47(8):609-611.
89 Costello RW, Liston R, McNicholas WT. Compliance at night with low flow oxygen therapy: a comparison of nasal cannulae and Venturi face masks. Thorax. 1995 April;50(4):405-406.
90 Angus RM, Ahmed AA, Fenwick LJ, Peacock AJ. Comparison of the acute effects on gas exchange of nasal ventilation and doxapram in exacerbations of chronic obstructive pulmonary disease [published erratum appears in Thorax 1997 Feb;52(2):204]. Thorax. 1996 October;51(10):1048-1050.
91 Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003 January 25;326(7382):185.
92 British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002 March;57(3):192-211.
93 Diaz O, Iglesia R, Ferrer M, et al. Effects of noninvasive ventilation on pulmonary gas exchange and hemodynamics during acute hypercapnic exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997 December;156(6):1840-1845.
94 Chakrabarti B, Angus RM, Agarwal SDr, Lane S, Calverley P. Hyperglycaemia as a predictor of outcome during non-invasive ventilation in decompensated COPD. Thorax. 2009;64(10):857-862.
95 Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000 June 3;355(9219):1931-1935.
96 Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. New Engl J Med. 1995 September 28;333(13):817-822.
97 Moretti M, Cilione C, Tampieri A, Fracchia C, Marchioni A, Nava S. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax. 2000 October;55(10):819-825.
98 Nava S, Ambrosino N, Clini E, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med. 1998 May 1;128(9):721-728.
99 Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004 June 10;350(24):2452-2460.
100 Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006 January 15;173(2):164-170.
101 Ferrer M, Sellares J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet. 2009;374(9695):1082-1088.
102 Davidson AC. The pulmonary physician in critical care: critical care management of respiratory failure resulting from COPD. Thorax. 2002 December;57(12):1079-1084.
103 Brochard L, Rauss A, Benito S, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994 October;150(4):896-903.
104 Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995 February 9;332(6):345-350.
105 Vallverdu I, Calaf N, Subirana M, Net A, Benito S, Mancebo J. Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med. 1998 December;158(6):1855-1862.
106 Alvisi R, Volta CA, Righini ER, et al. Predictors of weaning outcome in chronic obstructive pulmonary disease patients. Eur Respir J. 2000 April;15(4):656-662.
107 Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patient effort and dyspnea. Am J Respir Crit Care Med. 1997;155:1940-1948.
108 Dhand R, Duarte AG, Jubran A, et al. Dose-response to bronchodilator delivered by metered-dose inhaler in ventilator-supported patients. Am J Respir Crit Care Med. 1996 August;154(2 Pt 1):388-393.
109 Yang SC, Yang SP, Lee TS. Nebulized ipratropium bromide in ventilator-assisted patients with chronic bronchitis. Chest. 1994 May;105(5):1511-1515.
110 Fuller HD, Dolovich MB, Turpie FH, Newhouse MT. Efficiency of bronchodilator aerosol delivery to the lungs from the metered dose inhaler in mechanically ventilated patients. A study comparing four different actuator devices. Chest. 1994 January;105(1):214-218.
111 Connors AFJr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) [published erratum appears in Am J Respir Crit Care Med 1997 Jan;155(1):386]. Am J Respir Crit Care Med. 1996 October;154(4 Pt 1):959-967.
112 Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995 December 20;274(23):1852-1857.
113 Menzies R, Gibbons W, Goldberg P. Determinants of weaning and survival among patients with COPD who require mechanical ventilation for acute respiratory failure. Chest. 1989;95(2):398-405.
114 Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010 May;65(5):423-428.
115 Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med. 2008 January 15;177(2):170-177.