Chapter 16 Chronic Gastrointestinal Bleeding
Introduction
Chronic gastrointestinal (GI) hemorrhage may be overt or occult. Overt bleeding is defined as chronic if it is persistent but not severe enough to cause circulatory compromise. It may be seen in the form of melena or red rectal bleeding. If bleeding is occult, the clinical presentation is anemia, and evidence of occult bleeding is found on testing of the stools. In some patients, chronic hemorrhage may be clinically interspersed with acute episodes.1 Acute GI bleeding is discussed in detail in other chapters.
Chronic GI bleeding includes common clinical scenarios, yet the meaning and diagnostic criteria for the different terms are not well delineated.2 Chronic bleeding from the gut is always significant; in particular, malignant tumors of the gut that are curable may be present. There is no universal agreement regarding the nomenclature of GI lesions that can cause chronic bleeding. Development of new technology, mainly wireless video capsule endoscopy and balloon-assisted enteroscopy has provided an opportunity to revisit the traditional classifications of the source of GI bleeding into upper or lower GI bleeding based on the location of the bleeding either proximal or distal to the ligament of Treitz.3
Some authors propose reclassifying GI bleeding into three categories: upper GI, mid-GI, and lower GI bleeding. Bleeding above the ampulla of Vater, within the reach of esophagogastroduodenoscopy (EGD), is defined as upper GI bleeding; small intestinal bleeding from the ampulla of Vater to the terminal ileum, best investigated by capsule endoscopy and balloon-assisted enteroscopy, is defined as mid-GI bleeding; and colonic bleeding, which can be evaluated by colonoscopy, is defined as lower GI bleeding. A simple classification is presented in Table 16.1. This chapter discusses some of the most frequent causes of chronic GI bleeding. Vascular lesions are an important cause of chronic GI bleeding. They may be solitary, multiple, or diffuse and may exist as isolated abnormalities or be part of a syndrome or a systemic disorder. The internationally accepted term for the endoscopic finding of a mucosally based vascular malformation is angiectasis. The categorization of vascular abnormalities in the GI tract has been inconsistent and a source of confusion.4,5 It can be based on histologic characteristics, gross appearance, or association with systemic diseases. These considerations permit categorization into three broad groups, as follows:
| Gastrointestinal Lesions | |
|---|---|
| Within Reach of Upper Endoscope | May Be beyond Reach of Upper Endoscope |
| Esophagitis | Celiac sprue |
| Cameron’s erosions | Crohn’s disease |
| Peptic ulcer disease | Intestinal lymphoma |
| Gastritis and erosions | Small bowel angiodysplasia |
| Duodenitis and erosions | Small bowel tumors |
| Angiodysplasia | Small bowel ulcers and erosions, including NSAID- and other drug-induced lesions |
| Portal hypertensive gastropathy | Small bowel diverticulosis |
| Gastroesophageal cancer | Small bowel varices |
| Gastric or duodenal polyps | Lymphangioma |
| Gastroduodenal lymphoma | Radiation enteritis |
| Partial gastrectomy | Blue rubber bleb nevus syndrome |
| GAVE | Osler-Weber-Rendu syndrome |
| Dieulafoy’s lesion | Small bowel polyposis syndromes |
| Gardner’s syndrome | |
| Amyloidosis | |
| Meckel’s diverticulum | |
| Hemosuccus pancreaticus, hemobilia | |
| Klippel-Trénaunay-Weber syndrome | |
| Colonic Lesions |
GAVE, Gastric antral vascular ectasia; IBD, inflammatory bowel disease; NSAID, nonsteroidal antiinflammatory drug.
Angiodysplasia of the Gastrointestinal Tract
Vascular ectasia of the GI tract, also referred to as angiodysplasia or less accurately as arteriovenous malformation, is a distinct clinical and pathologic entity.6–8 It is the most common vascular abnormality of the GI tract and probably the most frequent cause of lower intestinal bleeding in patients older than 60 years. Although the terms angiodysplasia and arteriovenous malformation have been used synonymously, the term angiodysplasia (Greek angeion, “vessel”; dys, “bad” or “difficult”; plasis, “a molding”), means a poorly formed vessel but with a lesser connotation of congenital origin than with the word malformation. Angiodysplasias are usually distinguished from telangiectasias, which, although anatomically similar, are usually referred to in the context of systemic or hereditary diseases. Because most vascular abnormalities are detected during endoscopy, a classification based on endoscopic appearance has been proposed.9 The classification system recognizes the location, size, and number of angiodysplasias.
Pathogenesis
Angiodysplasias are composed of ectatic, dilated, thin-walled vessels that are lined by endothelium alone or by only small amounts of smooth muscle. Their anatomy has been best shown by studies in which casts of the vessels were made by injecting a silicone material.10 These studies showed that dilated tortuous submucosal veins are the most prominent feature in angiodysplasias. Small arteriovenous communications are present because of incompetence of the precapillary sphincter. Enlarged arteries are also present in bigger angiodysplasias and may be associated with arteriovenous fistulas, which explains why bleeding can be a risk in some patients.
Histologic examination shows dilated vessels in the mucosa and submucosa, sometimes covered by a single layer of surface epithelium. These features are shared by angiodysplasias in the colon and stomach.11 Increased expression of angiogenic factors has been found in human colonic angiodysplasias.12 The pathogenesis of angiodysplasias is not well understood. Four theories have been proposed, as follows:
Epidemiology and Natural History
The prevalence of GI angiodysplasias in the overall population is not well known because asymptomatic individuals usually do not undergo endoscopic evaluation. Angiectases have been seen in 0.2% to 2.9% of “nonbleeding persons”15,16 and in 2.6% to 6.2% of patients evaluated specifically for occult blood in the stool, anemia, or hemorrhage.15–17 Angiodysplasias occur most often in the colon, where they are an important cause of lower GI bleeding, particularly in patients older than 60,18–20 although presentation in patients in their 30s has been described.21 There is no gender predilection.
Clinical Manifestations
Angiodysplasias can remain clinically silent or cause bleeding. The estimated incidence of active GI bleed in patients with angiodysplasia is less than 10%. These lesions may be located throughout the GI tract with a variable rate of bleeding associated with them and presentation ranging from hematemesis or hematochezia to occult anemia.3,22 Bleeding is usually chronic or recurrent and, in most cases, low grade and painless.
Patients with colonic angiodysplasia may present with hematochezia (0% to 60%), melena (0% to 26%), Hemoccult-positive stool (4% to 47%), or iron deficiency anemia (0% to 51%). Melena occurs in at least one-fourth of patients with colonic bleeding. The nature and degree of bleeding frequently vary in the same patient with different episodes, and patients may have bright red blood, maroon-colored stools, and melena on separate occasions. In 20% to 25% of episodes, only tarry stools are passed, and in 10% to 15% of patients, bleeding is evidenced only by iron deficiency anemia, with stools that are intermittently positive for occult blood.23 With effective diagnostic and therapeutic endoscopy, the percentage of patients who have had operations has decreased because most lesions are being identified and treated at the time of the first episode.24 Angiodysplasia can be confidently considered to be a source of GI bleeding in an anemic patient only if it is seen to be actively bleeding. The risk of bleeding in patients who are incidentally found to have nonbleeding colonic angiodysplasia is unknown. The number of lesions and the presence of coexisting coagulopathy or platelet dysfunction may increase the risk for bleeding. Patients who have bled from colonic angiodysplasias are at increased risk for subsequent bleeding.10
Stomach and Duodenum
Angiodysplasias of the stomach have been found to be the cause of blood loss in 4% to 7% of patients with GI bleeding.18,25 Angiodysplasias in the stomach or duodenum are found incidentally in approximately 50% of cases.26
The risk that an incidentally found gastric or duodenal angiodysplasia will subsequently bleed is uncertain. Patients who have bled from gastric or duodenal angiodysplasias do rebleed. This rebleeding was illustrated in a series of 30 patients with gastric or duodenal angiodysplasias; 77% had experienced at least one episode of overt bleeding before diagnosis.18
Small Intestine
Approximately 5% of patients presenting with GI hemorrhage have no source found by upper endoscopy and colonoscopy. In approximately 75% of these patients, responsible lesions can be detected in the small bowel. In patients presenting with obscure overt bleeding (defined as the presence of recurrent melena or hematochezia with normal evaluation by upper endoscopy and colonoscopy), small bowel angiectases are detected in 30% to 60% of examinations.3,27
Colon
The colon is the most common site of angiodysplasias in the GI tract; colonic lesions are most often found in the cecum and ascending colon. In some reported experiences, angiodysplasias of the colon account for approximately 20% to 30% of cases of acute lower GI bleeding, approaching the frequency of acute colonic diverticular bleeding.28 Foutch and colleagues29 noted the prevalence of angiodysplasia to be 0.93% from three prospective studies in which screening colonoscopies were performed in 964 asymptomatic individuals (mean age 61 years).
Conditions with Increased Prevalence
End-Stage Renal Disease
Angiodysplasia is the second most common cause of GI bleeding in patients with end-stage renal disease.30 These lesions account for about 20% of upper GI bleeds and 30% of lower GI bleeds30 and approximately 50% of recurrent upper GI bleeds.31 In a prospective study of upper GI hemorrhage over a 50-month period, vascular ectasia was the etiology of upper GI hemorrhage in 13% of patients with renal insufficiency and was the etiology of bleeding more often in patients with renal insufficiency than in patients with normal renal function.32 The prevalence of vascular ectasia as a cause of upper GI bleeding was related to the duration of renal failure and the requirement of hemodialysis. The lesions can occur anywhere along the GI tract and are usually multiple.31 The reason for the increased prevalence among patients with end-stage renal disease is unknown. One possible explanation is that the lesions are detected more frequently because of the increased risk of bleeding associated with uremia-induced platelet dysfunction.
von Willebrand’s Disease
The association of abnormal von Willebrand’s factor (vWF) is receiving increasing attention in the management of patients with bleeding GI angiectasias. von Willebrand’s disease is a bleeding disorder that results from a qualitative or quantitative defect in vWF. vWF is a complex multimeric glycoprotein present in platelets, plasma, and subendothelium. vWF is essential to platelet adhesion and aggregation at the site of vascular injury. In a study of patients with both bleeding and nonbleeding angiectasias of the GI tract and control patients with colonic diverticular hemorrhage, Veyradier and associates33 showed that most patients with bleeding angiectasias of the GI tract lack the largest multimers of vWF induced by a latent acquired form of von Willebrand’s disease. Because these specific multimers are the most effective in inducing platelet aggregation in high shear stress that is commonly present in the microcirculation of angiectasias, it was concluded their deficiency contributes to active bleeding.
An association between angiodysplasias and congenital or acquired von Willebrand’s disease had been reported previously.34,35 Patients with angiodysplasia found on endoscopy were prospectively tested for von Willebrand’s disease at the Mayo Clinic, but no associations were identified.
Aortic Stenosis
Approximately 50% of patients with bleeding vascular ectasias have evidence of cardiac disease, and 25% have been reported to have aortic stenosis. Bleeding from angiodysplasias in patients with aortic stenosis (Heyde’s syndrome) has been repeatedly reported but is highly controversial.36 In support of this relationship is the observation that bleeding may be improved after aortic valve replacement.37–39 Two possible explanations have been proposed to explain this observation. Patients with aortic stenosis may develop an acquired form of von Willebrand’s disease, which can be reversed after aortic valve replacement.33,40,41 The mechanism is thought to involve mechanical disruption of vWF multimers during turbulent passage through the narrowed valve.42 Patients with aortic stenosis may be more likely to bleed from existing angiodysplasias. The observation that angiodysplasias persist after aortic valve replacement despite the fact that bleeding stops supports this hypothesis.11,43 Another explanation is that existing angiodysplasias may bleed as a result of ischemic necrosis in patients who have a low cardiac output.13,43 However, this explanation is inconsistent with the observations that bleeding angiodysplasias have not been observed with other forms of heart disease associated with a low cardiac output and that a low cardiac output is a late complication of aortic stenosis.
Several retrospective, uncontrolled studies44,45 and a prospective, controlled investigation46 do not substantiate a causative role or association of aortic valve diseases with colonic angiectases. Replacement of the aortic valve for control of bleeding secondary to these vascular lesions is not universally accepted.24 A logical approach to patients with both lesions is to treat the colonic lesion first endoscopically, regardless of whether the patient’s cardiac status warrants surgery. If valve replacement is necessary and endoscopic therapy is unsuccessful, further endoscopic or surgical treatment of the colonic angiodysplasia should be delayed until after cardiac surgery. Further attempts at endoscopic treatment or surgical resection are indicated if bleeding recurs.47 The association between chronic GI bleeding in elderly patients and aortic stenosis becomes more relevant with the advent of transcatheter aortic valve implantation that can be offered even to elderly patients with comorbidities, which could make conventional surgery impossible.48
Progressive Systemic Sclerosis
Vascular lesions are a prominent feature of progressive systemic sclerosis, especially in the CREST variant.49 Angiodysplasias are usually distinguished from telangiectases, which, although anatomically similar, are usually referred to in the context of systemic or hereditary diseases. In patients with progressive systemic sclerosis, sites most frequently involved by telangiectases are the hands, lips, tongue, and face, but gastric, intestinal, and colorectal lesions have been reported. These lesions may be the source of occult or clinically significant bleeding and are best treated by endoscopic coagulation.50
Diagnosis
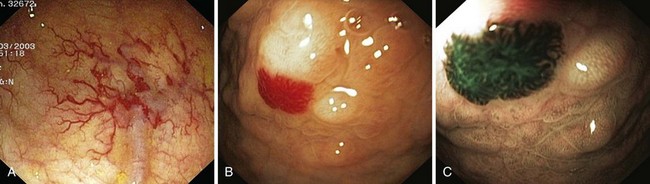
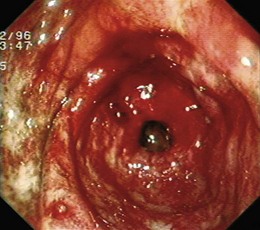
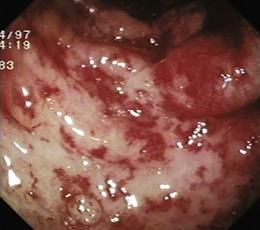
Angiectases have a characteristic appearance of a cherry-red, fernlike pattern of arborizing, ectatic blood vessels radiating from a central vessel (Fig. 16.1). This pattern should be specifically looked for because angiectases may be confused with other erythematous mucosal lesions or with normal vessels (Table 16.2).24,51 Because traumatic and endoscopic suction artifacts may resemble vascular lesions, all lesions must be evaluated immediately on insertion of the colonoscope, rather than during withdrawal. “Anemic halos” are often seen surrounding angiectases of the bowel. Although these “halos” do not differentiate the various types of vascular lesions, they distinguish true vascular lesions from artifacts.47 Newer alternative imaging options, such as narrow band imaging, allow precise discrimination of vascular structures from artifactual mucosal hemorrhage. Punch biopsy samples of vascular lesions obtained during endoscopy are usually nonspecific; the bleeding induced by performing biopsies of these abnormalities is not justified.
| Vascular | Nonvascular | Colitis |
|---|---|---|
| Arteriovenous malformations | Trauma | Ischemic |
| Angiomas | Polyps | Infectious |
| Phlebectasia | Adenomatous | Radiation (acute) |
| Varices | Hyperplastic | Inflammatory bowel disease |
| Venous stars | Lymphoid |
Angiectases may be difficult to visualize during colonoscopy in patients who do not have an optimal bowel cleaning. Because the appearance of angiectases is influenced by blood pressure, blood volume, and state of hydration, these lesions may not be evident in patients with severely reduced blood volumes or who are in shock until red blood cell and volume deficits are corrected. Cold water lavage of the colon, as is sometimes done to clean the luminal surface from debris during colonoscopy, reportedly may mask these lesions.52 Meperidine also has been implicated in masking lesions because of a transient decrease in mucosal blood flow. Minimizing use of meperidine and reversal with naloxone to increase the yield of detection have been advocated by some clinicians. Naloxone has been reported to enhance the appearance of normal vasculature in about 10% of patients and to cause angiectases to appear (2.7%) or increase in size (5.4%).53 Reversal of narcotic analgesia may affect the comfort of an examination, particularly if therapeutics are performed.
Some patients presenting with GI bleeding have no source found by upper endoscopy and colonoscopy.3 In these cases of obscure chronic GI bleeding, endoscopic examination of the small bowel has been limited by several factors. The length of the small intestine, in addition to its free intraperitoneal location, vigorous contractility, and overlying loops, confounds the usual diagnostic techniques, including barium studies, endoscopic intubation, and identification of specific sites by special imaging techniques of nuclear medicine scans and angiography. The bleeding rate may be slow or intermittent, not allowing identification by either angiography or radionuclide bleeding scan. Because of the inability to localize a bleeding site in the small bowel, patients with obscure GI bleeding from a small bowel source typically presented with prolonged occult blood loss or recurrent episodes of melena or maroon stool without a specific diagnosis. In this group of patients, previous noninvasive tests, such as small bowel follow-through, radioisotope-labeled red blood cell scan, and push enteroscopy, have had suboptimal diagnostic yields of 20% to 40%. Invasive methods, such as laparotomy or intraoperative enteroscopy, may improve the yield up to 70%.54
For patients with obscure chronic GI bleeding, an early diagnosis of the bleeding site was the exception rather than the norm until more recently with the development of capsule endoscopy and balloon-assisted enteroscopy. The last American Gastroenterological Association Institute medical position statement on obscure GI bleeding considered that for patients with obscure GI bleeding and associated anemia or overt bleeding, repeat endoscopic examinations can be worthwhile.55 Useful adjunctive diagnostic maneuvers include use of a cap-fitted endoscope to examine blind areas, such as the high lesser curve, under the incisura angularis, and the posterior wall of the duodenal bulb; use of a side-viewing endoscope to examine the ampulla in patients with suspected pancreaticobiliary pathology; and use of a push enteroscope to examine the C-loop of duodenum carefully after injection of glucagons. Although the yield is low (6%), repeat colonoscopy may be useful in the setting of prior poor bowel preparation. Use of naloxone may improve the detection of colonic angiectases that were not obvious at the index examination.3
Capsule Endoscopy
The first capsule endoscope, referred to as the M2A (“mouth to anus”), was approved for clinical use late in 2001. The capsule endoscope is a wireless miniature camera that can be swallowed to obtain images of the GI mucosa; the camera contains a light source, batteries, and a radio transmitter. Video images are transmitted via radiotelemetry to a skin surface patterned antenna that allows images to be captured and localizes the relative position of each image in the abdomen.56 In July 2003, the capsule endoscope was approved by the U.S. Food and Drug Administration (FDA) as a first-line tool for the detection of abnormalities of the small bowel, based on evidence provided by a meta-analysis (Fig. 16.2). M2A was renamed PillCam SB (SB means “small bowel”).57
Obscure GI bleeding is the main clinical indication for capsule endoscopy; approximately 70% to 80% of patients undergo capsule endoscopy for this indication. The American Society for Gastrointestinal Endoscopy (ASGE) Technology Assessment Committee concluded that capsule endoscopy provided superior yield compared with radiographic contrast studies and push enteroscopy.58 Two published meta-analyses support the utility of capsule endoscopy in obscure GI bleeding.59,60 The ASGE Technology Assessment Committee also concluded that capsule endoscopy is indicated not only for evaluating obscure GI bleeding, but also for evaluating unexplained iron deficiency anemia.58
The EndoCapsule EC type 1 is another small bowel capsule endoscope, developed by Olympus America (Center Valley, PA). This capsule uses a high-resolution CCD and an external real-time image viewer (External Viewer) monitor.61 A randomized study comparing these two types of capsule endoscope reported a statistically nonsignificant trend for the EndoCapsule to detect more bleeding sources in patients with suspected small bowel bleeding than the PillCam SB.62 Future capsule designs may emerge with expanded capabilities that include fluid sampling, mucosal biopsy, targeted labeling, and controlled movement. With future innovation and study, the indications for capsule endoscopy are likely to expand and become more focused.
Balloon-Assisted Enteroscopy
Push-and-pull enteroscopy with the double-balloon technique (double-balloon enteroscopy [DBE]) for inspection of the entire small bowel was first performed in the Western world in 2003.63 The earliest case report documenting the success of this method was published by Yamamoto and colleagues in 2001.63a
Double-Balloon Technique
The endoscope is introduced into the small bowel within a soft overtube. The balloon at the tip of the endoscope is inflated to hold it in place while the overtube, with deflated balloon, is passed distally until it reaches the tip of the endoscope. Both balloons are inflated, and the entire system is slowly pulled back; this results in an accordionlike sleeving of the small bowel onto the overtube. In the next step, the overtube balloon is kept inflated while the endoscope balloon is deflated and the endoscope is pushed 40 cm deeper into the small bowel. This method is repeated until the enteroscope has been passed as far as technically possible. In approximately 10% of cases, the entire small bowel can be visualized in a single session, typically via an antegrade approach. Carbon dioxide insufflation improves depth of insertion especially during antegrade approaches.64 In most cases, however, antegrade and retrograde examinations are required for the entire small bowel to be visualized. In this setting, the distalmost point in the small bowel that is reached after antegrade passage is tattooed to provide an endpoint during the retrograde examination.
Single-Balloon Enteroscopy
Antegrade DBE can examine three times the length of small bowel as push enteroscopy, with a corresponding increase in diagnostic yield.65 More recently, single-balloon enteroscopy (SBE) was introduced in the United States (Olympus America, Center Valley, PA).66 The single-balloon and double-balloon systems share many features, including scope length, diameter, accessory channel size, and overtube design. The most important design difference between the two systems is that the single-balloon enteroscope does not have a distal balloon on the scope; the only balloon is on the tip of the overtube. As a result, the sequence of steps to advance the scope through the small bowel is simplified in SBE.
Although there are some minor differences between the systems, use of either scope requires a technician to assist with handling of the overtube and balloon inflation and deflation. In addition, both systems require fluoroscopy to monitor scope position and sedation appropriate for prolonged procedures. Early clinical experience using SBE on average reported a depth of insertion (270 cm) and diagnostic yield (54%) similar to DBE with a shorter procedure time.67 Comparison studies between DBE and SBE are inherently difficult to design and carry out. For this reason, accurate comparisons are impossible. Choice of procedure has become a user preference.
Complications
Abdominal pain is common after DBE and can be lessened by the use of carbon dioxide insufflation. Diagnostic DBE has an overall complication rate of 1.7% (perforation 0.3%, bleeding 0.8%, pancreatitis 0.3%).68 The cause of pancreatitis is uncertain. Advancing the overtube and enteroscope into the jejunum before inflating balloons and avoiding excessive tension on the mesentery during push-pull cycles may limit this risk. Therapeutic DBE has a relatively high complication rate of 4.3% (polypectomy bleeding 3.3%, argon plasma coagulation [APC] perforation 1.2%, dilation perforation 2.9%). DBE evaluation of the entire small bowel is possible in 45% to 84% of patients in whom it is attempted, although it can rarely be achieved with antegrade DBE alone. In the evaluation of obscure GI bleeding, DBE has been reported to identify a bleeding source in 53% to 80% of cases.69,70
Three prospective studies compared capsule endoscopy with DBE. Combining the results of the three studies, capsule endoscopy and DBE agreed in 68% of all cases and in 63% of positive cases. A meta-analysis that included these three prospective studies and results published only in abstract form compared diagnostic outcomes between capsule endoscopy and DBE. There was no difference in diagnostic yield between capsule endoscopy and DBE.71 Another meta-analysis reached the same conclusion.72 A new enteroscopy technology consists of a 48-Fr rotating overtube with spiral threads (Discovery SB; Spirus Medical, Inc, Stoughton, MA). The overtube is backloaded on any enteroscope73 or on a pediatric colonoscope.74 After intubation of the stomach with the endoscope, the overtube is rotated clockwise through the upper GI tract until the spiral threads engage in the jejunum; once free in the abdominal cavity, clockwise spinning of the overtube results in rapid pleating of small bowel onto the overtube. In a preliminary report,75 average procedure time for spiral enteroscopy was shorter (32 minutes) than times reported for DBE and SBE. The diagnostic yield is lower (32%) compared with balloon-assisted enteroscopy. The depth of insertion into the small bowel and the overall safety of the large twisting overtube have not been shown.
Summary
Balloon-assisted enteroscopy seems to have superior diagnostic capability compared with push enteroscopy and equivalent yield compared with intraoperative enteroscopy without the associated morbidity of the latter procedure. Although balloon-assisted enteroscopy does not allow visualization of the entire small bowel in one examination, compared with capsule endoscopy, it has been shown to be associated with an equivalent detection rate, has the capability to detect lesions missed by capsule endoscopy, and offers the advantages of therapeutic treatment. In patients with a positive finding on capsule endoscopy, balloon-assisted enteroscopy provides a safe method to achieve a favorable clinical outcome, including significant reduction in recurrent bleeding and transfusion requirements.76 With the advent of balloon-assisted enteroscopy, intraoperative enteroscopy can be relegated to cases in which the success of balloon-assisted enteroscopy is limited by body habitus, the presence of adhesions, or other anatomic factors.3 Most existing trials have focused on the diagnostic value of capsule endoscopy, with few examining the impact on the long-term outcome of obscure GI bleeding. In a 1-year study based on telephone interviews, the negative predictive value of capsule endoscopy for clinical rebleeding was 87%.77 In another more recent report, the negative predictive value was 89%.78 Capsule endoscopy can guide the management of these small bowel lesions.
Similar to other authors,57,80 we79 recommend capsule endoscopy as a first-line investigation over balloon-assisted enteroscopy in view of its convenience, higher chance to visualize the entire small intestine, and similar diagnostic yield. A proposed algorithm for capsule endoscopy in cases of obscure GI bleeding is shown in Fig. 16.3. No test substitutes for good clinical judgment, however, and all small bowel diagnostic studies must be considered in difficult cases of obscure GI bleeding, particularly in a young patient.81
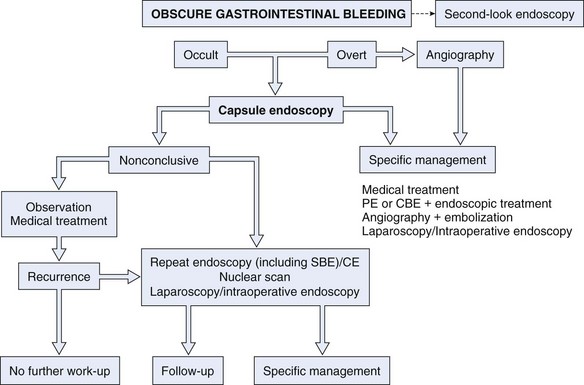
Fig. 16.3 Diagnostic algorithm for obscure gastrointestinal bleeding.
(Adapted from Nakamura T, Terano A: Capsule endoscopy: Past, present, and future. J Gastroenterol 43:93–99, 2008.)
In the setting of obscure overt bleeding, capsule endoscopy should be performed close to the bleeding episode. We recommend that a second endoscopist reread the capsule endoscopy study. We also recommend a second-look EGD with special attention to areas less optimally examined by capsule endoscopy, especially the duodenum, before concluding with a final diagnosis of obscure GI bleeding. Second-look capsule endoscopy has also been suggested for patients with a prior nondiagnostic capsule endoscopy. Bar-Meir and associates82 reported that 7 of 20 patients (35%) who underwent second-look capsule endoscopy had positive or suspicious findings. Another study showed that patients with a nondiagnostic capsule endoscopy test would benefit from a second-look capsule endoscopy if the bleeding presentation changed from occult to overt or if the hemoglobin value decreased 4 g/dL or more.83
Angiography is used to determine the site and nature of lesions during bleeding. It may permit therapy in patients who are bleeding and can identify some vascular lesions even when bleeding has ceased. The three reliable angiographic signs that help diagnose angiectases are a densely opacified, slowly emptying, dilated, tortuous vein; a vascular tuft, and an early-filling vein.84 A fourth sign, extravasation of contrast material, identifies the site of bleeding when bleeding volume is at least 0.5 mL/min but does not contribute to the diagnosis of an angiectasis. Nuclear scan is another diagnostic technique that can be used in selected patients.85,86 Provocative scintigraphy with heparin can enhance diagnosis with further localization of bleeding.87
Helical computed tomography (CT) angiography may provide another method to help diagnose angiectases. Accuracy may be high,88 although studies are needed to understand the role of this technique better in the management of patients with GI bleeding. In a report of 22 patients with obscure GI bleeding, CT enteroclysis was found to be inferior to capsule endoscopy in the detection of potential bleeding lesions such as angiectases in the small bowel.89 In another more recent study performed in 28 patients with obscure GI bleeding, capsule endoscopy detected more lesions (72%) than CT angiography (24%) or standard mesenteric angiography (56%).90 The aggressiveness of the approach should be individualized depending on the clinical circumstances. Evaluation of the small bowel may be deferred in patients with negative upper and lower endoscopy until or unless the patient is bleeding severely enough to require a transfusion.55
Management
Management of bleeding angiectases consists of three phases24: (1) diagnosis; (2) conversion of acute bleeding manifesting as an emergency to elective care by control of the acute hemorrhage; and (3) definitive treatment of the lesions by endoscopic ablation, angiography, or surgical removal. The natural history of colonic angiectases is benign in healthy, asymptomatic individuals, and the risk of bleeding is small.29 It is estimated that only about 50% of colonic lesions ever bleed. There is a risk of bleeding and perforation following attempts at endoscopic obliteration. For all these reasons, in incidentally found angiectases at all levels of the gut, endoscopic therapy is not warranted.91
Pharmacologic Treatment
Iron Formulations
Nonbleeding angiodysplasia detected during evaluation of occult bleeding or iron deficiency anemia should be considered to be causative. In patients with occult bleeding, bleeding from angiodysplasia may be more likely in patients who have multiple lesions and a bleeding diathesis (e.g., anticoagulation). As a result, a graduated approach with primary or adjunctive iron replacement therapy may be initiated, with pursuit of more aggressive therapeutic options guided by the clinical circumstances.91–93 The aims of iron replacement therapy should be to restore hemoglobin levels and mean corpuscular volume to normal and to replenish body stores.
Hormonal Therapy
Estrogen-progesterone combination hormonal therapy has been used to treat patients with angiectases of the GI tract.94,95 The effect, which is not immediate, seems to be estrogen dose–dependent. Hormonal therapy acts by enhancing microvascular circulation, coagulation, and vascular endothelial integrity. The most common combination schedule has been ethinyl estradiol 0.01 to 0.05 mg and norethisterone 1 to 3 mg. This therapy should be used in 6-month courses with pauses to reduce the incidence of adverse effects, mostly secondary to the estrogen component. The results of several prospective, controlled trials examining hormonal therapy have been divergent.96–99 In a long-term observational study, combination hormonal therapy was shown to stop bleeding in patients with occult GI bleeding of obscure origin, likely to have resulted from small bowel angiodysplasias.98 Although uncontrolled studies suggest that combination estrogen-progesterone therapy prevents bleeding episodes secondary to angiodysplasia, the evidence from the largest placebo-controlled trial to date suggests that this therapy is ineffective.100 These authors considered that efficacy of hormonal therapy in these patients remains to be proven by a large, randomized, placebo-controlled trial with long-term follow-up.
Octreotide
Reports of efficacy of octreotide in the treatment of angiodysplasia have been limited to case reports and small series in which a response has been reported in some patients.95,101–103 Octreotide produces vasoconstriction secondary to inhibitory effects on growth hormone and multiple GI vasodilator hormones and markedly reduces splanchnic blood flow. Its antiangiogenic properties have been shown in different tissues (eye, placenta, liver, and GI neuroendocrine tumors); however, applicability and utility in obscure GI bleeding remain unknown.
The dosage of octreotide can be tapered to the lowest quantity that prevents rebleeding. Response is immediate, and the drug can be administered intravenously (50 µg/hr) or subcutaneously (50 to 100 µg two or three times a day).95 Its subcutaneous administration and its longer half-life (90 to 100 minutes) make octreotide superior to somatostatin and allow use in the outpatient setting. A 6-month course of therapy has been used to treat most patients. The first comparative cohort study showing the benefit of long-term octreotide compared with placebo in preventing bleeding from angiodysplasia was reported more recently.104 Compared with placebo, octreotide markedly reduced the risk of rebleeding with a significant decrease in iron requirements, but there were no differences in hemoglobin levels or transfusion requirements between the two groups.
Sandostatin LAR Depot is a depot formulation of octreotide for long-term maintenance therapy currently approved for acromegaly and GI and pancreatic neuroendocrine tumors. Compared with conventional octreotide, Sandostatin LAR Depot is administered intramuscularly once a month with a similar efficacy and safety profile and does not require hospital admission, which makes it an attractive outpatient option for long-term therapy in patients with chronic GI bleeding. The effectiveness of Sandostatin LAR Depot at a dose of 20 mg intramuscularly once a month for obscure GI bleeding has been described in several small series.95,102,105–107 The main disadvantage of this drug formulation may be its cost compared with conventional octreotide. However, in very specific cases, it may prove to be cost-effective. The appropriate dose and schedule required for long-term therapy are unknown.
Thalidomide
Thalidomide is a drug with powerful immunomodulatory, antiinflammatory, and antiangiogenic effects that was withdrawn from the market in the 1960s because of its teratogenicity; it has been reintroduced more recently for the treatment of leprosy, multiple myeloma, and various tumors. Vascular endothelial growth factor (VEGF) has been identified as the key mediator for endothelial vessel formation in early phases of angiogenesis. High concentrations of VEGF result in aberrant angiogenesis with formation of angiectasias that lack a smooth muscle cell layer and are more susceptible to rupture and bleeding. VEGF-dependent angiogenesis is inhibited by thalidomide. Thalidomide is an innovative and promising therapeutic option for GI bleeding associated with angiodysplasia and can be used in refractory cases or when other drugs or therapies are contraindicated.95
Thalidomide is administered orally at a variable dose of 100 to 300 mg/day.95 No significant adverse effects have been reported except transient fatigue; however, thalidomide is contraindicated in patients with peripheral neuropathy and pregnant women and women with childbearing potential because of its teratogenic effects, and it should be used cautiously in patients with cardiovascular or neurologic disorders and hepatic or renal impairment. Owing to its immunosuppressant activity by blocking tumor necrosis factor, use of thalidomide may be also discouraged in patients at risk for infection or chronic infectious disease, especially patients with human immunodeficiency virus (HIV) infection. In all these clinical settings, Sandostatin LAR Depot may be safer than thalidomide.
There are few reports in the literature about the effectiveness of thalidomide for chronic GI bleeding. It was successfully used in a patient with von Willebrand’s disease and life-threatening bleeding caused by small bowel angiodysplasia refractory to other pharmacologic treatments and endoscopic cauterization with argon beam laser.108 It has also been proven effective in controlling bleeding from diffuse idiopathic angiodysplastic lesions in the small bowel.109,110 Bauditz and coworkers111 studied the effect of thalidomide at a dose of 100 mg daily for 3 months in three patients with chronic bleeding from small bowel angiodysplasia as evidenced by wireless capsule endoscopy. Bleeding was controlled in all cases for a median follow-up of 34 months despite drug discontinuation. Repeat capsule endoscopy after therapy revealed a substantial reduction in lesion numbers, size, and color intensity.
Miscellaneous Agents
Antifibrinolytics
Tranexamic acid is a synthetic lysine analogue that inhibits the conversion of plasmin to fibrinogen. It has been used successfully for chronic bleeding from angiodysplasia in patients with end-stage renal failure at doses of 10 to 20 mg/kg every 48 hours; it remains unclear whether long-term therapy or as-needed therapy is preferable during hemorrhagic crises.95,112 The main risk derived from the use of antifibrinolytics is thrombosis, so thrombophilia should be ruled out before prescribing them. Adverse events associated with tranexamic acid may be frequent, and use of these drugs is not supported by randomized controlled trials, which makes antifibrinolytics a last option for chronic GI bleeding. Because of their mechanism of action, antifibrinolytics may have a more important role in the treatment of patients with hematologic disorders.
Danazol
Danazol is an antigonadotropin drug with weak androgenic activity that blocks pituitary secretion of follicle-stimulating hormone and luteinizing hormone, leading to ectopic and normal endometrial tissue atrophy. It has been used for endometriosis and uterine bleeding disorders. Anecdotal reports suggest a partial improvement in patients with GI bleeding and hereditary hemorrhagic telangiectasia.44,45 Cosmetic stigmata (acne, hair loss, mild hirsutism) and uncommon but severe adverse effects (intracranial hypertension, peliosis hepatis, thrombosis, seizures) consign danazol to a secondary role in chronic GI bleeding, to be used when other therapies have failed.
Desmopressin
Desmopressin is a synthetic analogue of the antidiuretic hormone vasopressin that lacks vasopressor activity. It increases vWF and factor VIII levels and enhances hemostasis in patients with defective platelet function. It is indicated as a hemostatic agent for patients with hemophilia A and von Willebrand’s disease, and it can be administered intravenously, subcutaneously, or by intranasal spray. An isolated report showed a benefit of intravenous desmopressin for life-threatening GI bleeding in a patient with hereditary hemorrhagic telangiectasia and vWF deficiency, allowing elective colectomy and bleeding resolution.113
Recombinant Activated Factor VII
Recombinant activated human factor VII has been used mainly in upper GI hemorrhage related to cirrhosis or acute liver failure,114,115 although it has been used in other settings, including refractory bleeding after endoscopic sphincterotomy in patients with preexisting coagulopathy.116 Sporadic reports have shown the effectiveness of factor VIIa in patients with von Willebrand’s disease for chronic GI bleeding secondary to small bowel angiodysplasia or of unknown origin.117 Secondary myocardial and cerebrovascular infarctions have been described while using factor VIIa owing to its marked prothrombotic activity,118 so particular care should be taken in patients with a high-risk cardiovascular profile, and the indication should be assessed for each individual case.
Endoscopic Treatment
Bipolar Or Heater Probe Coagulation
Bipolar or heater probe coagulation is said to be effective for treatment of angiodysplasia,119,120 and these modalities have replaced monopolar coagulation.14,121
Sclerosant
Injection of a sclerosant, such as ethanolamine, has been used to obliterate lesions.122 Epinephrine injection works by volume tamponade of the bleeding vessel and transient vasoconstriction.123 Sclerosants should probably be avoided, however, because of the risk for bleeding from injection site ulceration and perforation.47
Band Ligation
Band ligation has been used to treat angiectasia of the stomach.124,125 The relatively small surface area incorporated into the ligation site (e.g., in GAVE or “watermelon stomach”) and, more so, the expense of the therapy outweigh any perceived benefits. The walls of the small and large intestine above the rectum (especially the right colon) are thinner than the stomach. For this reason, band ligation therapy cannot be advocated owing to the recognized risk for perforation.126,127
Lasers
Argon and neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers have been used in the past.128–130 These techniques require expensive equipment and specific training. More convenient and safer coagulation options have replaced laser therapy except for treatment of “watermelon stomach” (GAVE). APC has become the dominant therapy for GAVE. A few patients are refractory to this therapy. These patients most often respond to treatment with Nd:YAG laser.
Argon Plasma Coagulation
APC is a monopolar electrosurgical procedure in which electrical energy is transferred to the target tissue using ionized and conductive argon gas (argon plasma).131 The plasma beam follows the path of least electrical resistance. This phenomenon permits the argon plasma to be applied both en face and tangentially, allowing treatment of regions that are normally difficult to access. A second-generation APC (VIO APC; Erbe Elektromedizin, Tuebingen, Germany; and Beamer, Con Med, Utica, NY) offering a broader bandwith of APC modes compared with the earlier generators has been introduced more recently to GI endoscopy. The optional coagulation modes (e.g., “forced,” “pulsed,” and “precise”) of second-generation devices provide different coagulation tissue effects. These effects include a continuous energy output, pulsed energy (with high energy per pulse with longer pauses per pulse or a greater number of pulses with a lower energy per pulse with a constant voltage maintained for each pulse option).
APC has been used for various bleeding lesions, including angiectases.132,133 Its great advantage over laser or photodynamic therapy is the limited depth of tissue injury and lower cost. Shallow tissue injury is due to the fact that the argon stream always seeks electrically conductive areas of tissue, avoiding the coagulated zones, which have lost their electrical conductivity as a result of desiccation. Perforation of the cecum has been reported.132 APC seems to be most effective131 in the treatment of vascular lesions (GAVE, angiodysplasias, and Dieulafoy’s lesions).
The utility of obtaining a submucosal saline solution cushion before APC therapy to prevent deep tissue injury has been shown in a porcine model.134 Before APC, it is recommended to collapse the lumen partially while maintaining the treatment site in view. It is important to avoid thinning of the colonic wall with excessive air insufflation because this increases the risk for perforation during therapy, especially in the cecum.123
Cryotherapy
Safety and efficacy of cryotherapy have been reported for treatment of diffuse mucosal lesions of the GI tract.135 The effectiveness of endoscopic treatment of angiectases is difficult to assess owing to the absence of prospective controlled trials. Recurrent bleeding from cecal angiodysplasia seems to be reduced after laser photocoagulation or thermal ablation via heater probe or bipolar electrocoagulation, with long-term control of bleeding requiring more than one treatment.136 Aside from perforation, the main risks of thermal therapy for colonic angiectases are bleeding in 5% of patients and postcoagulation syndrome in 1.7% of cases.120 Endoscopic treatment of vascular lesions in patients known to have coagulation disorders carries an increased risk of procedure-induced hemorrhage.
When treating large lesions, some experienced endoscopists recommend47 to ablate first the periphery of the lesion to create a collar of edema that theoretically reduces the vascular supply to the lesion and diminishes the potential for immediate or delayed hemorrhage. Some clinicians have placed mechanical clips around the margins of large lesions also to reduce the blood supply and facilitate effective coagulation. Others typically target the central portion of the angiodysplasia because ensuing coagulation and edema obstruct the peripheral branches and limit the extent of coagulation needed. Also, for a very large angiodysplasia, epinephrine injection may contract the lesion and reduce the amount and area of coagulation needed for eradication.123 Recurrent bleeding can be expected in approximately 20% of patients with colonic angiectases and in a greater percentage of patients with associated coagulopathies, renal failure, portal hypertension, or additional upper GI vascular lesions.47 It is reasonable to attempt endoscopic therapy in patients with accessible lesions despite various coexisting morbidities that may allow only short-term success. Patients with multiple lesions or an underlying bleeding diathesis are less likely to benefit long-term from endoscopic therapy and are at increased risk of complications, especially delayed bleeding from treatment site thermally induced ulceration. Such patients benefit from any attempts to improve their underlying bleeding tendency.
In preparation for endoscopic ablation of vascular lesions, aspirin, nonsteroidal antiinflammatory drugs, anticoagulants, and antiplatelet agents should be withdrawn at least 1 week to 10 days before the procedure. Care should be taken not to distend the cecum fully because the wall would be thinned further, and the risk of perforation would be increased.24,123 After therapy, patients must be cautioned not to resume full doses of anticoagulants or antiplatelet agents for at least 1 week. Coagulated tissues are at their maximum of thermal injury by 5 to 10 days, and the onset of hemorrhage may be delayed.
Angiography
Localization of active bleeding permits embolization or infusion of vasopressin. Because angiography occasionally causes serious complications, such as arterial thrombosis, contrast reactions, and acute renal failure, its use before definitive surgical therapy has been questioned.137 Angiography should be reserved for patients with life-threatening bleeding, patients who are not surgical candidates, and patients in whom localization of lesions is desired before surgical resection. Intravenous or intraarterial vasopressin infusions through the angiographic catheter successfully arrest hemorrhage from vascular ectasias in more than 80% of patients in whom extravasation is demonstrated.16 The intravenous route seems to be as effective as the intraarterial route when the bleeding is in the left colon, but intraarterial administration is more successful when the bleeding is from the right colon or small bowel. Infarction of the sigmoid colon and severe arterial spasm and lower extremity ischemia have resulted from vasopressin infusions into the inferior mesenteric artery given at the same rate as used in the superior mesenteric artery. These complications may be avoided by infusing less than 0.4 U/min (the dose of superior mesenteric artery infusions), recognizing the lesser blood flow of the inferior mesenteric artery.
Surgery
In the latter two situations, right hemicolectomy is done as an elective procedure after active bleeding is controlled. The entire right half of the colon needs to be removed to ensure that no angiectases are left behind. Recurrent bleeding can be expected in 20% of patients. Subtotal colectomy should be performed only as a last resort in circumstances in which colonic bleeding is strongly suspected but the site and cause are unknown.24 Richter and colleagues16 studied the course of 101 patients with angiodysplasias to determine the natural history and compare the efficacy of medical therapy, endoscopic electrocoagulation, and surgery (right hemicolectomy). Similar rates of recurrent bleeding were observed for medically and endoscopically treated groups during a mean follow-up of 22 months. Surgically treated patients had a frequency of recurrent bleeding less than half that of the other groups.
Hereditary Hemorrhagic Telangiectasia
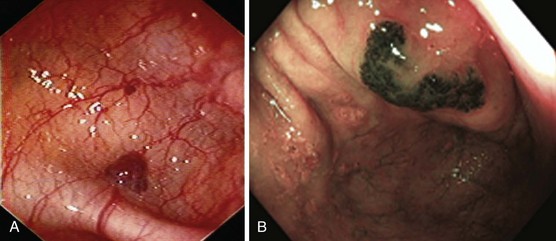
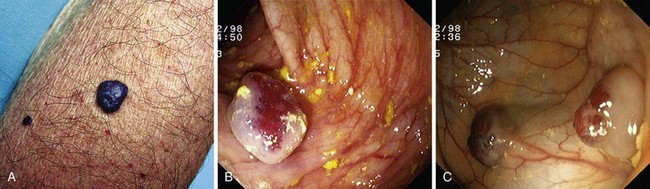
Hereditary hemorrhagic telangiectasia, or Rendu-Osler-Weber disease, is an uncommon, autosomal dominant disorder characterized by telangiectases and arteriovenous malformations that affect many organs, including the skin, periungual areas, lips, oral and nasopharyngeal membranes, tongue (Fig. 16.4A), lungs, GI tract (Fig. 16.4B), liver, and brain,138,139 and can result in bleeding. Lesions consist of irregular, ectatic, tortuous blood spaces lined by a single layer of endothelial cells and supported by a fine layer of fibrous connective tissue. In these vessels, no elastic lamina or muscular tissue is present, so they cannot contract; this property may explain why these lesions tend to bleed.24
Clinical Manifestations
Manifestations of hereditary hemorrhagic telangiectasia are not generally present at birth but develop with increasing age. Epistaxis is usually the earliest sign of disease, often occurring in childhood; pulmonary arteriovenous malformations become apparent from puberty; mucocutaneous and GI telangiectases develop progressively with age; and, finally, GI bleeding occurs well into adulthood.140 A family history has been reported in approximately 80% of patients with this disease141 but is less common in patients who manifest bleeding after age 50 years.47 Severe GI bleeding is unusual before the 5th or 6th decade142; occurs in 25% to 33% of patients with hereditary hemorrhagic telangiectasia; is challenging to treat; and can cause significant morbidity, resulting in severe anemia and high blood transfusion requirements.143,144
Endoscopy may reveal telangiectases in the stomach (see Fig. 16.4B), duodenum, small bowel, or colon that are punctiform, discrete, red spots or with a classic fernlike border similar to all other angiectases. Lesions are usually flat, although they may be slightly raised 1 to 3 mm, similar in size and appearance to angiectases of the nasal and oral mucosa.142 Telangiectases are more common in the stomach, duodenum, or jejunum than in the colon.140 They also may have a characteristic pale mucosal halo. Capsule endoscopy contributes significantly to defining the extent of small intestinal telangiectases and can be used to stage the disease to decide on the extent and form of endoscopic therapy.139 Selective mesenteric arteriography may localize the precise bleeding site, but in most cases this is unnecessary.145
Management
Therapies for GI bleeding range from various pharmacologic agents to endoscopic and surgical treatment. Drug therapies include ethinyl estradiol and norethisterone,96 danazol,146 and aminocaproic acid.147 Endoscopic therapy is the most effective form of treatment in stopping hemorrhage from actively bleeding lesions. Because of the multiplicity of lesions and the redevelopment of lesions, sometimes within weeks, bleeding recurs at varying intervals after therapy, depending on the extent and thoroughness of ablative therapies.141 There are several reports of endoscopic therapy, including sclerotherapy with sodium morrhuate,148 ethanol, and polidocanol149; monopolar and bipolar coagulation and heater probe150,151; APC149; YAG laser152; and clip application.153 Surgical therapy with resection of involved bowel has limited success because of recurrent disease140 but may be useful for emergency control of hemorrhage from discrete lesions identified as the source of the bleeding that do not respond to medical or endoscopic therapy.141 Surgical intervention has otherwise been more common owing to excessive bleeding from thermally induced ulcerations after endoscopic therapy and perforation.
Gastric Vascular Lesions in Patients with Cirrhosis
After variceal bleeding, hemorrhagic gastritis is the most frequent cause of upper GI bleeding in cirrhotic patients with portal hypertension.154 The term hemorrhagic gastritis has included bleeding from various nonvariceal mucosal lesions, such as multiple ulcerations, PHG, and GAVE.154–156 PHG and GAVE can cause acute and chronic upper GI blood loss.157 These conditions frequently, but not invariably, are diagnosed by upper endoscopy. Although they are fairly prevalent, only 15% to 20% of affected individuals experience symptomatic GI blood loss.
Portal Hypertensive Gastropathy
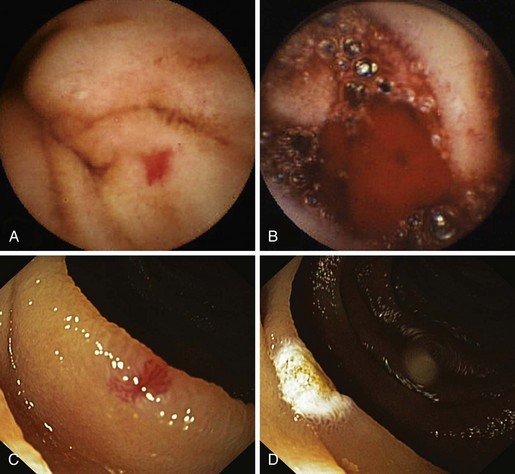
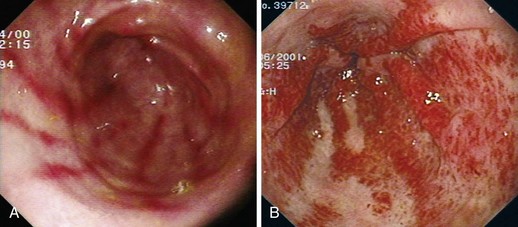
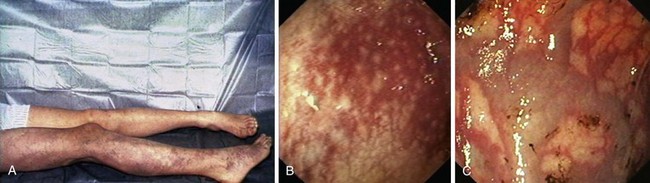
PHG describes the endoscopic appearance of gastric mucosa, with a characteristic mosaiclike pattern with or without red spots, seen in patients with cirrhotic or noncirrhotic portal hypertension. The mosaiclike pattern appears as a white reticular network separating areas of raised red or pink mucosa, resembling the skin of a snake (“snakeskin appearance”). PHG is seen mainly in the body and the fundus of the stomach but is also seen rarely in the gastric antrum (Fig. 16.5). These changes are not unique to gastric mucosa. Similar changes are rarely seen in the small intestine and colon, especially in the presence of extrahepatic portal hypertension. With the broad use of capsule endoscopy, different manifestations of portal hypertension have been reported, although their clinical implications are uncertain to date.158,159 When PHG is severe, it can include discrete cherry-red spots, fine pink speckling, or scarlatina-type rash, collectively called red marks.160 The characteristic histologic finding of PHG is dilated capillaries and venules in the mucosa and submucosa without erosion, inflammation, or fibrinous thrombi.161
Classification
There is no general consensus on the endoscopic classification of PHG. The most widely used classification is the one recommended by McCormack and colleagues,161 who classified PHG into mild and severe (Table 16.3). Mild PHG is defined by the presence of only a mosaiclike pattern, whereas severe PHG is diagnosed when red point lesions, cherry-red spots, or black-brown spots are present. The popularity of this classification relates in part to its simplicity and its ability to predict the risk of bleeding, with an increased risk of gastric hemorrhage in severe cases (38% to 62%) compared with mild cases (3.5% to 31%).155,156 Observed endoscopic findings often are of an intermediate severity, however, and are not well represented as being either mild or severe. Tanoue and colleagues162 and the New Italian Endoscopy Club162,163 have described a more detailed scoring system. Tanoue and colleagues162 classified PHG into four grades (grade 0 = none, grade 1 = mild, grade 2 = moderate, grade 3 = severe). This grading permits more informative description of the observed endoscopic findings. A simpler classification system such as recommended by McCormack and colleagues161 has a better intraobserver and interobserver agreement and reproducibility.164 Further work needs to be done to improve the currently available grading system.
Table 16.3 Classification of Portal Hypertensive Gastropathy
| McCormack et al161 | Tanoue et al162 | Primignani et al (NIEC)163 |
|---|---|---|
| Mild | Grade I | Mosaic pattern |
| Fine pink speckling (scarlatina-type rash) | Mild reddening | Mild—diffuse pink areola |
| Superficial reddening | Congestive mucosa | Moderate—flat red spot |
| Mosaic pattern | Severe—diffuse red areola | |
| Severe | Grade II | Red mark lesion |
| Discrete red spots | Severe redness and fine reticular pattern separating areas of raised edematous mucosa | Discrete |
| Diffuse hemorrhagic lesion | Confluent (diffuse) | |
| Grade III | Black brown spot | |
| Point bleeding + grade II |
NIEC, New Italian Endoscopic Club.
Prevalence
Prospective studies have reported that PHG is present in more than 50% of patients with cirrhosis.165,166 The reported prevalence varies widely because of patient selection, absence of uniform criteria and classification, and, more importantly, the differences in interobserver and intraobserver variation.167 Although PHG is usually seen in association with either esophageal or gastric varices, there is no direct linear correlation between portal pressure and the presence or severity of PHG.168 The cause of portal hypertension is unlikely to be a significant factor.169–171 The prevalence of PHG does not seem to have a linear relationship with the severity of liver disease, as reported by one study that showed the lowest prevalence of severe PHG in Child C patients.163 This finding may suggest that other, hitherto unidentified factors may play a role in the pathogenesis of PHG. There is general agreement, however, that the prevalence of PHG increases with variceal obliteration.170
Pathogenesis
Portal hypertension, and not liver disease, seems to be the key factor for the development of PHG because PHG is equally common in patients with portal hypertension with or without liver disease.172,173 The improvement or disappearance of PHG in many patients after transjugular intrahepatic portosystemic shunt (TIPS) and shunt surgery suggests that there is a significant association between PHG and portal hypertension. However, the severity and the presence of PHG do not have a linear correlation with the severity of portal hypertension.168 Chronic elevation in portal pressure and increased splenic circulation may increase gastric mucosal blood flow; this may be one explanation for the development of PHG, but actual measurement of gastric blood flow using Doppler has shown variable results, suggesting that there may be other explanations for the pathogenesis of PHG.174,175
The severity of PHG may increase with more advanced liver disease as shown in some studies, but the results are inconsistent.163,168,169 Many other explanations have been offered for the pathogenesis of PHG. Experimental evidence suggests that gastric mucosal defense mechanisms are impaired in the presence of portal hypertension.176–178 Aggravation of PHG after variceal banding or sclerotherapy might be caused by alterations in local hemodynamics after obliteration of the esophageal varices.179,180 Hepatofugal flow velocity is increased in the left gastric vein in direct relationship with the enlarging size of esophageal varices. Gastric mucosal blood flow is higher in cirrhotic patients whose extrahepatic collaterals are predominantly via esophageal varices. Obliteration of the esophageal collateral venous network may cause higher flow velocity in the gastric vascular bed. It has been speculated that the extravasation of sclerosant into the mediastinum during sclerotherapy may result in chemical vagotomy, and this may cause esophageal dysmotility and delayed gastric emptying, leading to the development of PHG.181 However, there is no direct evidence to suggest that delayed gastric emptying causes PHG.
Natural History and Complications
The presence and severity of PHG seem to change with time. Although some authors believe that PHG is a progressive lesion,166,182 others have observed that it may regress in a fair proportion of patients. In one study, during a follow-up of 2 years, the severity of PHG fluctuated with time in 25%, improved in 23%, remained stable in 29%, and deteriorated in 23%.163 The variability in results can be due to differences in the patient population, the time when the lesions appear, or the influence of endoscopic intervention for varices. Endoscopic sclerotherapy and banding seems to worsen the severity of PHG and increases the risk of bleeding.161,162,179,180 In one study, 44% of patients developed PHG after variceal eradication by sclerotherapy compared with only 9% before sclerotherapy.170 The results in this prospective study suggested that if gastropathy persists for more than 3 months, it is likely to persist for longer periods. In a few patients, PHG lesions may still regress within 6 months but are unlikely to regress beyond this period; when PHG was present before endoscopic therapy for varices, the lesions often persisted or progressed after obliteration of esophageal varices. The frequency of bleeding from PHG in these circumstances was high. For these reasons, the study investigators recommended that such patients be followed with serial endoscopies and that the benefits of β blocker therapy be evaluated as prophylaxis against PHG bleeding.170 However, the efficacy of serial endoscopies simply for monitoring of the progression of PHG remains to be proven.183
Management
Pharmacologic Treatment
Nonselective β Blockers
Nonselective β blockers, such as propranolol and nadolol, have been shown to reduce portal pressure and gastric mucosal blood flow. In small studies, propranolol has been shown to reduce bleeding related to PHG.184,185 In a randomized, controlled trial of 56 patients with PHG, multivariate analysis showed that absence of propranolol treatment was the only predictive variable for rebleeding.165 The use of propranolol in PHG leads to endoscopic improvement, a cessation of bleeding in acutely hemorrhaging patients, and a decreased incidence of rebleeding from severe PHG. Pharmacologic therapy is typically long-term because of evidence that discontinuation of therapy can lead to rebleeding from PHG. Other medications, such as prednisone, estrogen, and progesterone, are ineffective for bleeding-related PHG.
Vasoactive Agents
Given the proven beneficial effects of vasoactive agents such as somatostatin, octreotide, and terlipressin in variceal bleeding, their role in the management of PHG has also been evaluated. Somatostatin has been shown to reduce gastric mucosal blood flow by decreasing splanchnic blood flow.186 Similar effects are seen with vasopressin and terlipressin. In patients with acute bleeding, an uncontrolled study showed efficacy with somatostatin and octreotide.187 A more recent study showed that the decrease in portal pressure is only transient, however, and use of somatostatin or octreotide is unlikely to benefit patients with chronic PHG bleeding.188 Use of these agents should be limited to patients with acute bleeding. There are no clinical trials directly comparing the efficacy of propranolol versus octreotide for active hemorrhage related to PHG. Some authors use octreotide first in patients with acute bleeding because it is better tolerated than propranolol in this setting.189
Endoscopic Treatment
Endoscopic treatment does not have a significant role in the management of PHG bleeding because the bleeding is often mild and diffuse. If an active bleeding site is identified, it could be managed by injection of sclerosant or cauterization using the heater or bipolar probe. Although laser therapy has been shown to be safe and effective in reducing GAVE-associated bleeding, it is unknown whether laser therapy is effective or safe in patients with severe PHG when the predominant lesion is in the gastric antrum.190
Noninvasive And Invasive Surgery
Both TIPS and shunt surgery have been shown to be effective for PHG bleeding in anecdotal reports. In a study by Orloff and coworkers,191 portacaval shunting successfully controlled bleeding, and the gastric mucosa had reverted to normal on follow-up gastroscopies in all 12 patients with repeated bleeding unresponsive to propranolol. TIPS was shown to improve PHG in 9 of 10 patients.192 TIPS successfully stopped recurrent bleeding refractory to medical therapy in one of these patients. TIPS offers another therapeutic option for patients who have bleeding refractory to medical therapy, especially if they are poor candidates for surgical shunts.193 Despite these encouraging anecdotal reports, most authors believe189 that TIPS or shunt surgery should be used only as the last resort in these patients because the risks outweigh the benefits. Currently, the only treatment that could be recommended for prophylaxis of PHG bleeding is nonselective β blockers such as propranolol or nadolol. Pharmacologic therapy is typically long-term because of evidence that discontinuation of therapy can lead to rebleeding from PHG.193 Efficacy of therapy can be followed clinically and endoscopically; efficacy is evidenced by improved mucosal appearance and diminished portal hypertension.
Gastric Antral Vascular Ectasia
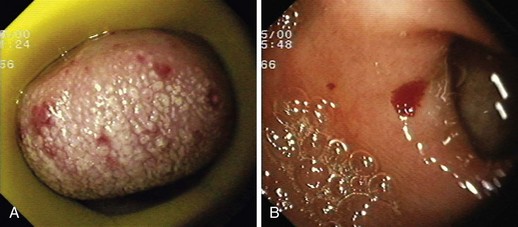
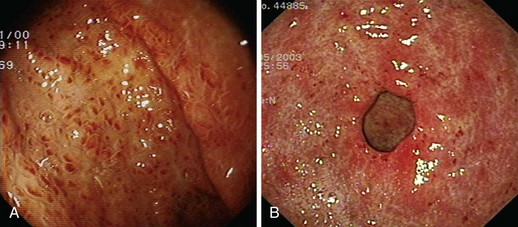
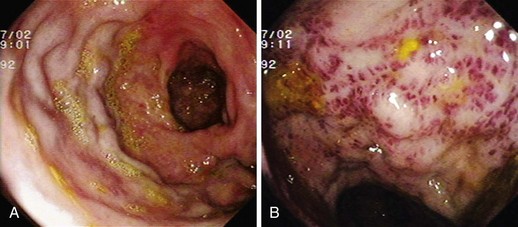
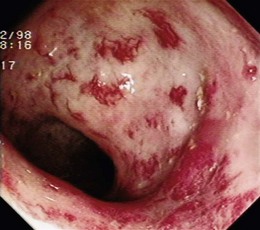
GAVE was first described in 1984 by Jabbari and colleagues.194 These authors coined “watermelon stomach” to describe GAVE because of its resemblance to the skin of a watermelon. GAVE describes vascular lesions of the antrum organized in a linear array on top of raised convoluted folds radiating outward from the pylorus, similar to spokes from a wheel, and resembling the dark stripes on the surface of a watermelon (Fig. 16.6A). The typical histologic appearance of GAVE includes marked dilation of capillaries and collecting venules in the gastric mucosa and submucosa with areas of intimal thickening characterized by fibromuscular hyperplasia, fibrohyalinosis, and thrombi.155,156,194 An increasing number of reports of GAVE have included some cases in which the endoscopic appearance showed spotty erythemas diffusely scattered in the antral area and coalesced (diffuse antral vascular ectasia or “honeycomb stomach”).4,25 Diffuse antral vascular ectasia is now recognized as the same entity as “watermelon stomach,” and both are regarded as GAVE (Fig. 16.6B).26
The diffuse form is the predominant pattern in patients with cirrhosis. Noncirrhotic patients with GAVE are typically middle-aged or older women; in these patients, GAVE is associated with achlorhydria, atrophic gastritis, CREST syndrome, and post–bone marrow transplantation.26,49,195
Pathogenesis
The pathogenesis of GAVE is poorly understood, and there is no single unifying hypothesis. Possible mechanisms include humoral factors and mechanical causes.155,156,196 Spahr and coworkers197 observed that GAVE was not improved by successful portal decompression after TIPS (one patient) or by endoscopic laser therapy (one patient), whereas the antral mucosal lesions disappeared completely after liver transplantation. GAVE could be related to increased secretion of yet unidentified vasoactive substances in the presence of liver disease; for example, glucagon and nitric oxide were found to be increased in cirrhotic patients. Local disturbances in vascular tone must be involved to explain that vascular ectasias develop specifically in the antrum. The proponents of a “mechanical” theory have suggested that chronic intermittent venous obstruction associated with powerful muscular contractions of the antrum and pylorus or repeated trauma associated with a loosely attached antral mucosa and prolapse of the antral mucosa through the pylorus may cause GAVE.194,198,199 The fibromuscular hyperplasia seen at histology in GAVE supports the hypothesis of repeated mechanical stress induced by gastric peristalsis.
Relationship between Portal Hypertensive Gastropathy and Gastric Antral Vascular Ectasia
Some researchers believe that GAVE and PHG are different manifestations of the same pathogenetic process, whereas others view them as separate entities.156 These lesions can be differentiated by endoscopic and histologic findings. PHG is always associated with cirrhosis and is observed mostly in the fundus and corpus of the stomach; mucosal red spots and the so-called mosaic pattern are present, and the histologic examination shows only microvascular ectasia in the mucosa without signs of inflammation. By contrast, GAVE can occur in cirrhotic and noncirrhotic patients. Lesions are found almost exclusively in the antrum, and the mucosal microvascular ectasias are either aggregated in linear stripes (as in “watermelon stomach”) or diffusely spread. Histologically, true ectasia of the mucosal microvasculature is present and associated with fibrin thrombi, fibrohyalinosis, and spindle cell proliferation. This constellation of findings can be identified within the body of the stomach in a patient with PHG but not a patient with GAVE.
In severe PHG, the mucosal vascular ectasias are linearly arrayed in the antrum and resemble GAVE. A GAVE-like appearance is commonly seen in patients with portal hypertension.164 The dilemma is whether this appearance is GAVE or severe PHG. As mentioned earlier, the histologic appearance of GAVE is confined to the antrum. Similar histologic examination of random biopsy specimens in the body of the stomach in patients with severe PHG can serve to discriminate between the two. In contrast to PHG, bleeding from GAVE is not known to respond to nonselective β blockers.156,200 However, a therapeutic challenge is not clinically useful to differentiate these two entities because the response to β blockers is variable in patients with PHG. Kamath and associates201 showed that compared with patients with PHG, patients with GAVE, in the absence of background mosaic appearance, did not respond to TIPS.
It has been suggested that the same gastric vascular alteration related to portal hypertension may appear grossly and histologically different depending on the anatomic location in the stomach.166 The hemodynamics of venous drainage of the antrum are different from the body or the fundus of the stomach. It can be argued that the “typical” histologic changes, fibrosis and thrombosis, seen in GAVE are also seen with more chronic and severe PHG confined to the antrum. The variable response to β blockers and TIPS may be a reflection of the advanced histologic changes.156 Some authors believe that the diagnosis of GAVE should be reserved for patients with the presence of typical, linear red lesions in the gastric antrum without the mosaiclike appearance of mucosal background in the rest of the stomach. When there is evidence of a mosaiclike pattern or evidence of more severe PHG in the body and fundus, some authors favor classifying it as a spectrum of PHG.164
Management
Pharmacologic Treatment
Combination Hormonal Therapy
Combination hormonal therapy has been shown to be effective in controlling bleeding from GAVE in some case reports and small trials.202–205 Hormonal therapy does not improve the endoscopic appearance of this lesion. As with other pharmacologic therapies, hormonal therapy provides an alternative to endoscopic therapy when extensive vascular abnormalities are present. It also eliminates the risk of circumferential scarring and stenosis, which can occur with the endoscopic modalities discussed.
Tranexamic Acid
One case report described the use of tranexamic acid for GAVE.206 Given the limited experience with antifibrinolytics for GI bleeding, the use of this agent should be reserved for refractory patients who have failed other measures.189
Octreotide
Octreotide was used in one trial with successful results.101 However, at least one case report failed to show a response of GAVE to octreotide.207
β Blockers
Treatment with β blockers has been reported to be successful in controlling bleeding from gastric mucosal lesions in cirrhotic patients. However, it is unclear in these reports whether patients bled from PHG or from GAVE.165,185 In the study from Spahr and coworkers,197 treatment with the nonselective β blocker nadolol did not control chronic bleeding from GAVE. In addition, the results of this study clearly showed that the decrease in portal hypertension after TIPS or shunt surgery was not effective in controlling chronic blood loss related to GAVE.197
Endoscopic Treatment
Neodymium:Yttrium-Aluminum-Garnet Laser Coagulation
In a trial by Bourke and associates,208 Nd:YAG laser coagulation was used in 11 consecutive patients with GAVE. Transfusion dependence was eliminated in two-thirds of the patients and decreased in the remaining patients. In this trial, an average of three sessions per patient was required; sucralfate was used to prevent iatrogenic ulceration. Endoscopic laser therapy is repeated every 8 to 12 weeks until most of the lesions disappear and the hemoglobin remains stable. Sufficient time must be allowed after laser photocoagulation therapy for healing of the thermally induced ulcers to occur and to maximize the assessment of residual vascular lesions. One study indicated that the mean number of treatments needed to obliterate the vascular lesions and eliminate the need for transfusions was less for Nd:YAG laser compared with APC (2.33 ± 0.27 vs. 5.75 ± 0.89).209
Heater Probe
The heater probe uses a thermal element that heats the device tip and results in tissue coagulation. Petrini and Johnston210 described the successful use of a heater probe for management of GAVE. An average of four sessions was required to eliminate the transfusion requirements and improve endoscopic appearance in 8 of 10 patients.
Argon Plasma Coagulation
As with the Nd:YAG laser, large surface areas of involved mucosa can be adequately treated with APC during a single endoscopic session. Several case reports have described the successful use of APC for the management of GAVE.211,212
Compared with Nd:YAG laser, APC is more convenient to use. The tangential approach to the vascular lesions, especially along the posterior wall of the antrum, makes APC an attractive tool for use compared with the laser device, which requires a more perpendicular approach. Among noncontact thermal endoscopic treatments for GAVE, APC has gained popularity in recent years and is now widely available in endoscopy units. The results from several small series and case reports so far have been similar to the results achieved with the Nd:YAG laser but with a superior safety profile.213 Long-term efficacy remains suboptimal, however, with a 22% incidence of recurrent bleeding and with up to 31% of patients requiring ongoing transfusions.156,214,215 Long-term sequelae of ablation include antral scarring and hyperplastic polyps.216 Most rebleeding episodes respond to repeated treatment, but these patients may require multiple sessions and commitment to ongoing endoscopic treatments.213
Higher power settings should be used for patients with GAVE to provide ablation of the mucosal and submucosal vascular abnormalities. Nd:YAG laser therapy provides a viable option for patients with refractory bleeding and should be offered to these patients. The major problem with GAVE is continued blood loss requiring repeated blood transfusions. Ikeda and colleagues217 presented a 10-year endoscopic follow-up study from discrete initial lesions to the full picture of GAVE. They reported that latent hemorrhage occurred at an early stage as the coalesced lesions formed and that the vascular lesions of GAVE should be eradicated at this stage. The underlying pathophysiology is unclear, and endoscopic ablative methods do not affect the origins of the problem, so there is a long-term potential for recurrent vascular lesions and bleeding.
Generally, Nd:YAG laser and APC are preferred over heater probe for the treatment of GAVE because of their ability to cover a greater surface area. However, the endoscopic therapeutic modality chosen also depends on the individual experience of the endoscopist and local availability.189
Other Endoscopic Techniques
Band Ligation
A retrospective study of thermal therapy (APC or multipolar electrocoagulation probe) compared with band ligation found that band ligation may offer a therapeutic option.218 The use of band ligation requires meticulous efforts to band ligate all of the involved mucosa, which is technically challenging and expensive. At the present time, this form of therapy cannot be advocated as superior to existing therapies. Newer mucosal ablation techniques,219 such as radiofrequency ablation and cryotherapy, have also been used to ablate GAVE in small pilot studies.
Cryotherapy
A study of 26 patients with various bleeding lesions (e.g., GAVE, arteriovenous malformations, radiation proctitis, radiation gastritis) were treated with cryotherapy with a mean of 3.6 sessions.135 These patients had previously undergone treatment with multipolar electrocoagulation and heater probe but continued to have bleeding. Cryotherapy with nitrous oxide was efficacious in causing hemostasis in 77% overall, with follow-up of 6 months. Another report included 12 patients with a diagnosis of GAVE and documented iron deficiency anemia. Eight patients had a history of overt GI bleeding. Eight patients (67%) had previously been treated with APC (median 6 sessions, range 1 to 10 sessions) and failed to respond or had a recurrence. Six patients (50%) had a complete response, and six patients (50%) had a partial response.220
Preliminary data suggest that cryotherapy may be a reasonable option for diffuse GAVE lesions. Although the etiology of GAVE is poorly understood, histopathology results show distinct abnormalities limited to mucosa and lamina propria, including superficial fibromuscular hyperplasia with capillary ectasia and microvascular thrombosis. Cryotherapy seems to work in GAVE by causing a deeper injury compared with APC with necrosis of mucosa and superficial submucosa, followed by reepithelialization. A major advantage of cryotherapy over APC is its ability to treat large areas of mucosa relatively quickly because ice formation occurs after 2 to 3 seconds of spraying the mucosa and is quickly followed by thawing.135
Effective and safe APC treatment requires an experienced endoscopist who is able to target the lesions accurately while maintaining a 1- to 3-mm distance from the mucosa for effective coagulation and avoidance of transmural injury especially because high-power settings are used. Cryotherapy treatment times for GAVE have not previously been reported. Cho and associates220 were able to treat greater than 90% of affected mucosa in 89% of cases, with a mean of 5 minutes. Because of its ability to spray a large area quickly, there was no significant difference in the duration with respect to the extent of GAVE. A reduction in the duration of treatment was observed with subsequent cryotherapy sessions, which may reflect the endoscopists’ learning curve with cryotherapy, the use of an overtube, and the reduction in the extent of the lesions to be treated. Limitations of cryotherapy include the need for specialized equipment and training. An overtube has been used to enable passive venting of carbon dioxide to reduce patient discomfort and the risk of perforation. The requirement for an overtube extends the duration of the procedure and adds technical difficulty and risk to the procedure.
Radiofrequency Ablation
A pilot study of six patients with GAVE treated with the HALO90 device (Barrx Medical, Sunnyvale, CA) showed improved hemoglobin concentrations in all patients after one to three treatments. Five of six patients were no longer dependent on transfusions.221 This device provided very superficial coagulation injury. Considerably more experience is needed to determine if this expensive form of therapy is justified.
Surgery
In the study from Spahr and coworkers,197 chronic bleeding was never controlled in one patient who underwent a surgical shunt despite a complete normalization of portal pressure. Similarly, it has been reported that portacaval shunt surgery proved ineffective in treating chronic GI blood loss related to GAVE in a patient with portal hypertension as a result of nodular regenerative hyperplasia.222 Antrectomy provides the most definitive therapy for GAVE as illustrated by low rates of rebleeding and anemia recurrence.223–225 Surgery carries a significant risk of mortality, however, in cirrhotic patients with portal hypertension. TIPS placement may make antrectomy easier and faster by avoiding operative blood losses related to the presence of collaterals secondary to portal hypertension. However, TIPS should be restricted to cirrhotic patients with good liver function, owing to the risk of TIPS-induced progressive liver failure.197 Given the comorbid illnesses typically associated with GAVE and the advanced age of most patients, surgery is considered salvage therapy for patients who have not responded to a reasonable trial of endoscopic or pharmacologic therapies.189
Portal Colopathy
Clinical Manifestations
Over the past 2 decades, awareness of the association between portal hypertension and changes in the intestinal circulation has increased. The fundamental pathologic change is a vasculopathy. Portal hypertensive vasculopathy most often involves the stomach (PHG) and can be a source of bleeding. The significance of small bowel involvement (enteropathy) is unknown. Colonic involvement (colopathy) has been associated with bleeding. Several studies have described the colonic findings associated with cirrhosis and portal hypertension.226–235 Colorectal lesions associated with portal hypertension and cirrhosis include hemorrhoids, varices (portosystemic collaterals that develop because of portal hypertension; Fig. 16.7A), focal and diffuse angiectases, and spiderlike vascular lesions. The term portal colopathy has been used to describe colonic lesions resembling the vascular lesions of portal gastropathy and the diffuse colitislike appearance seen in patients with portal hypertension (Fig. 16.7B).226,227 There is confusion regarding the diagnostic criteria and clinical significance of this condition. This confusion might be attributable to imprecise terminology, lack of uniform endoscopic descriptions, interobserver variability, and the absence of distinctive histopathologic features.236
In previously published studies, the prevalence of colonic abnormalities in patients with cirrhosis varied widely (Table 16.4), and the true prevalence of portal colopathy is unknown. Because all patients in some studies were referred for colonoscopy based on clinical indications,237 these reviews may have overestimated the true prevalence of portal colopathy. Conflicting data have been published regarding the relationship between the prevalence of portal colopathy and Child-Pugh classification in cirrhotic patients.226–228,233,235,237,238
In the study from Bini and associates,237 portal gastropathy, 2+ or larger esophageal varices, Child-Pugh class C cirrhosis, and the use of β blockers were independent predictors of portal colopathy; the use of β blockers was protective. Portal hypertensive colopathy and gastropathy might not be distinct entities but rather regional manifestations of portal hypertension. These entities might share similar mechanisms with regard to pathogenesis. The histology of the colitislike mucosal abnormalities from most patients with portal hypertension is nonspecific, mild inflammation. Several studies have shown that the endoscopic appearance of colonic mucosal edema and erythema is not associated with significant inflammatory infiltrates but rather mucosal vessel changes.227,237,239,240 Based on these findings, some authors suggest the use of the term colopathy when referring to the mucosal abnormalities of the colon in patients with cirrhosis.237 This term avoids the suggestion of the involvement of inflammatory cells in the pathogenesis of a disease that lacks a definitive etiologic mechanism.
Several endoscopic classification systems exist to grade the severity of mucosal changes in patients with portal gastropathy.161,163 To date, no classification systems exist for grading the severity of mucosal abnormalities in cirrhotic patients with portal colopathy, making comparisons between studies difficult. Based on their findings, Bini and associates237 proposed that portal colopathy could be classified into three grades: grade 1, erythema of the colonic mucosa; grade 2, erythema along with a mosaiclike appearance of the mucosa; and grade 3, vascular lesions of the colon, including cherry-red spots, telangiectases, or angiodysplasialike lesions. The same authors237 observed for the first time an increase in the prevalence of portal colopathy in cirrhotic patients who had undergone band ligation or sclerotherapy, or both, of esophageal varices. They suggested that, similar to PHG, it may be that portal colopathy is related to the levels of portal pressure and that obliteration of esophageal varices may cause a redistribution of blood flow in similar ways in the stomach and colon. Prospective studies are necessary to validate this observation.
Management
β-adrenergic blockers are usually the first-line therapy in patients with portal colopathy. The vascular lesions of portal colopathy are considered amenable to the same thermal therapies as GAVE and PHG, although no trials have been published at this time.24,226 TIPS may be useful as a second-line treatment in patients with recurrent portal hypertensive bleeding from colonic angiodysplasialike lesions who do not tolerate or are unresponsive to treatment with β-adrenergic blockers.241
Hemangiomas
Clinical Manifestations
Hemangiomas are the second most common vascular lesion of the colon. Considered by some investigators to be true neoplasms, they are generally thought to be hamartomas because most are present at birth. They may be solitary or multiple lesions limited to one segment of the GI tract, or they may be part of diffuse GI or multisystem angiomatoses. Individual hemangiomas may be classified as cavernous, capillary, or mixed types. Most are small (Fig. 16.8), ranging from a few millimeters to 2 cm, but larger lesions do occur, especially in the rectum (see section on cavernous hemangioma of the rectum). In the presence of GI bleeding, hemangiomas of the skin should suggest the possibility of associated bowel lesions.47 Hemangiomas causing upper GI hemorrhage are most commonly identified in the upper small intestine. These benign vascular tumors, almost all of which are cavernous hemangiomas, appear as single or multiple red, purple, or blue nodular lesions.
Management
Hemangiomas that are small, solitary, or few in number can be treated by colonoscopic coagulation using laser or other thermal mode. Colonoscopic excision of small lesions has been described,242 including removal of small lesions for the purpose of diagnosis in patients with multiple hemangiomas.243 Amano and colleagues244 reported electrosurgical snare polypectomy of a large, pedunculated, polypoid cavernous hemangioma that arose in the sigmoid colon in a 28-year-old man who presented with prolapse of the lesion through the anus. Although complications of colonoscopic excision of hemangiomas seem to be rare, the number of reports is small, and it is difficult to determine the safety of colonoscopic treatment. Safety may depend on size and gross morphology of the lesion (e.g., sessile vs. pedunculated) and the technical feasibility of endoscopic treatment. Large or multiple lesions usually require surgical resection of either the hemangioma alone or the involved segment of colon.
Cavernous Hemangioma of the Rectum
A distinct form of colonic hemangioma is cavernous hemangioma of the rectum.47 These lesions are usually not associated with other GI hemangiomas and are extensive, involving the entire rectum or portions of the rectosigmoid colon. The massive bleeding resulting from these rectal hemangiomas often necessitates excision of the rectum by either abdominal-perineal or low-anterior resection; ligation and embolization of major feeding vessels have been employed successfully. Attempts at local control have been valuable in some instances, but mostly these have been only temporarily effective.
Blue Rubber Bleb Nevus Syndrome
Clinical Manifestations
Blue rubber bleb nevus syndrome (BRBNS), also known as Bean’s syndrome, is a rare entity, characterized by cutaneous hemangiomas and vascular tumors of the GI tract, that can also involve other organs such as the brain, kidneys, lungs, eyes, oronasopharynx, parotids, liver, spleen, heart, pleura, peritoneum, pericardium, skeletal muscles, bladder, penis, and vulva.245–247 Although a familial history is infrequent, a few cases of autosomal dominant transmission have been reported,24 and one analysis identified a responsible locus on chromosome 9. The lesions were thought to be hemangiomas, but they are now considered to be venous malformations,24,248 which usually appear in early childhood and tend to increase in size and number with age. They are usually blue and raised, vary from 0.1 to 5 cm in diameter, have a wrinkled surface, and are easily compressible with light palpation. The contained blood can be emptied by direct pressure, leaving a wrinkled blue sac that slowly refills over several seconds or minutes.24 Skin lesions may be present throughout the body, particularly predominating on the upper limbs (Fig. 16.9A), trunk,249 and face.
GI lesions are usually multiple and may involve any portion of the GI tract but are most common in the small bowel and in the distal part of the colon.24 The characteristic GI lesion is a discrete mucosal nodule with an overlying central bluish red cap resembling a nipple, but a lesion may be a flat macular, dark bluish red spot or a frankly polypoid nodule with a central venous colored portion (Fig. 16.9B and C).246,250 Most patients are asymptomatic. Loss of blood from the GI tract is the major problem and commonly causes chronic anemia.250,251 The onset of GI bleeding may be at any time from early childhood to middle age.145 The lesions can also cause intestinal intussusception252 and numerous extraintestinal problems, such as orthopedic deformities associated with bone involvement,245 neurologic defects secondary to central nervous system involvement253 and spinal cord compression,254 pulmonary hypertension owing to vascular obliteration, hemothorax and hemopericardium,255 exophthalmos and loss of vision secondary to eye involvement,247 and disseminated intravascular coagulation and thrombocytopenia.256
Diagnosis
When blue rubber bleb nevus syndrome is suspected and depending on the clinical manifestations, diagnostic studies should include gastroscopy, colonoscopy, capsule endoscopy,257 enteroscopy,246 intraoperative enteroscopy,248 endoscopic ultrasound,258 enteroclysis, ultrasound, CT,251 magnetic resonance (MR) imaging,249,253,259 radionuclide tagged red blood cell scan,255 and angiography.260
Treatment
Most patients respond to supportive therapy, such as iron supplementation and blood transfusion when required.250 For recurrent bleeding, medical, endoscopic, and surgical therapies have been used. Medical therapy includes the use of corticosteroids,256,261 antifibrinolytic agents,262 γ-globulin,256 interferon,248,261 and octeotride.262 Endoscopic management seems to be safe and useful, although the lesions can be transmural, which implies a theoretical increase in risk for bowel perforation as a complication. There are several reports of endoscopic therapy, including sclerotherapy with absolute alcohol,263 sodium morrhuate,264 epinephrine and polidocanol,265 band ligation,251,265 polypectomy,249,251,266 bipolar coagulation,267 and laser therapy.267,268
Angiographic embolization can be useful for treating acute bleeding and preventing further bleeding.269 Successful surgical excision of the bleeding lesions or segmental resection of the involved bowel segment has been performed248,270 and can be aided by intraoperative endoscopy.248,271 Patients with an overwhelming number of lesions may require staged operative procedures.248
Klippel-Trénaunay-Weber Syndrome
Clinical Manifestations
Klippel-Trénaunay-Weber syndrome, also known as nevus vasculosus hypertrophicus, is a rare congenital vascular malformation, secondary to a generalized mesodermal development abnormality.272 Originally described by Klippel and Trénaunay in 1900, it is characterized by bony or soft tissue hypertrophy, usually affecting one extremity (Fig. 16.10A); hemangiomas or lymphangiomas or both; and varicosities or venous malformations, appearing at birth or in childhood.
Visceral hemangiomas have been described involving organs such as the GI tract (Fig. 16.10B), liver, spleen, bladder, kidney, lung, and heart.273,274 Involvement of the GI tract may be more common than previously believed, occurring in 20% of patients.274 GI hemorrhage is a potential serious complication secondary to diffuse vascular malformations involving the GI tract. GI bleeding usually begins in the 1st decade of life and may be recurrent and mild or severe.273 The severity may be enhanced by a consumption coagulopathy owing to intravascular clotting within the venous sinusoids of the hemangiomas.47 The most common reported cause of GI bleeding is attributed to diffuse cavernous hemangiomas of the distal colon and rectum,275 found in 1% to 12.5% of cases.273,276 Less frequent causes of GI bleeding are rectal or rectovaginal varices caused by obstruction of the internal iliac system, esophageal varices secondary to prehepatic portal hypertension owing to cavernous transformation or hypoplasia of the portal vein,277 and jejunal hemangiomas.278 Colonic obstruction and ascites secondary to massive hemangiomatous-lymphangiomatous retroperitoneal masses may occur.279
Diagnosis
Radiologic investigations play an important role in assessing colorectal involvement in Klippel-Trénaunay-Weber syndrome and occasionally finding the specific bleeding site. The presence of phleboliths on a plain abdominal radiograph in these very young patients suggests that they have angiomatosis.273 Barium studies can show luminal distensible narrowing, with a scalloped mucosal outline caused by the presence of varicosities or submucosal hemangiomas.47,280 CT and MR imaging of the abdomen and pelvis provide a simple, noninvasive means of assessing visceral hemangiomatous masses and identifying upward extension in the pelvis and abdomen.274,280 Abdominal angiography is required preoperatively for defining the anatomy and extent of the intestinal involvement to guide surgical intervention.278 At colonoscopy, the rectum and the lower part of the colon may show visible mucosal vessels or compressible nodules and extensive bluish angiomatous submucosal lesions. Biopsy of the lesions should be avoided because it may precipitate severe hemorrhage.273 Lesions in deeper layers of the wall can be assessed by endoscopic ultrasound.281
Treatment
Treatment varies from conservative to surgical measures, depending on the clinical symptoms and risk of complications. Transfusion dependency, life-threatening bleeding episodes, and poor quality of life require definitive surgical therapy.273,274 When the entire rectum is severely involved, surgery is sometimes necessary via an abdominoperineal resection of the involved rectum and colon, with a permanent colostomy276 or with a colon pouch anal anastomosis.282 Angiographic embolization may be useful if a specific active bleeding site is found273 or preoperatively.282 Endoscopic therapy has a limited role because of the commonly diffuse nature of the intestinal hemangiomas283 and is reserved for management of localized lesions or ablation of postoperative residual disease. The use of argon284 and Nd:YAG laser,283 sclerosis with formaldehyde and absolute alcohol,285 and placement of hemostatic clips in the rectum combined with arterial embolization286 have been reported with success. APC and photodynamic therapy273 may be very efficient, but their use in Klippel-Trénaunay-Weber syndrome has not yet been reported.
Radiation Injury of the Gastrointestinal Tract
Radiation Proctopathy
Clinical Manifestations
Radiotherapy is a common treatment modality used for several pelvic malignancies. Despite progress in radiation techniques, adjacent organs are still exposed to chronic radiation injury, which occurs in 2% to 25% of patients.287
The rectum, a fixed organ in the pelvis, has a glandular-type epithelium in which the cells undergo a rapid turnover. This organ is particularly vulnerable to ionizing radiation and to radiation-induced complications. The incidence of such complications is proportional to the dose and its degree of spreading and to the volume and method of irradiation (external or by brachytherapy).287 Intracavitary radiation delivery has been shown to increase the risk of developing chronic radiation proctopathy.288 Risk factors for radiation-induced damage include history of abdominal surgery, arteriosclerosis, obesity, diabetes, and concomitant chemotherapy.287,289,290
Radiation injury to the intestine has acute and chronic phases. Acute radiation injury occurs during or immediately after treatment and results from direct cellular damage. This injury inhibits division of the intestinal crypt stem cells, and the lamina propria becomes infiltrated with inflammatory cells resulting in loss of cellular function and mucosal inflammation. Clinical symptoms of acute radiation proctitis—diarrhea and tenesmus—are usually self-resolving, requiring only symptomatic treatment, and are not typically therapeutic challenges.291 Chronic radiation proctopathy is histologically characterized by submucosal collagen proliferation, endarteritis of arterioles, and thrombi in vessel lumens, leading to submucosal fibrosis, chronic mucosal ischemia, and progressive epithelial atrophy.292 Clinical symptoms of chronic radiation usually begin months to years after the initial radiation exposure, with a median of 8 to 16 months292,293 but with latencies as long as 30 years.294 Symptoms include rectal bleeding, diarrhea, rectal pain, fecal urgency, tenesmus, obstructed defecation (in patients who have developed stenosis), and less commonly fecal incontinence and fistulas. Many patients have multiple symptoms with a significantly negative impact on their daily activities.291 Bleeding may be severe enough to require frequent hospitalizations and blood transfusions; 55% of patients may require support with blood transfusions before undergoing definitive therapy.295
Diagnosis
Flexible Sigmoidoscopy And Colonoscopy
Flexible sigmoidoscopy and colonoscopy aid in diagnosis and determine the extent and severity of radiation injury. The findings include distinctive angiectases accumulated in the distal rectum, extending to and often including the dentate line along with friability and spontaneous bleeding (Fig. 16.11). Less frequently, there may be ulceration, stenosis, and fistulas. There is often a distinct margin between normal and abnormal tissue that relates to the edge of the radiation field.295 In some patients, especially women who have undergone radiation therapy after surgery for a gynecologic malignancy, there may be an associated segment of sigmoid colon with identical findings.
Treatment
Medical Therapies
The traditional approach to treatment of radiation proctopathy has been directed toward decreasing any inflammation with the use of antiinflammatory agents such as aminosalicylic acid derivatives and corticosteroids.291 The response to such agents is disappointing. Other approaches such as promoting healing in damaged tissue with short-chain fatty acid enemas,299 oral and rectal sucralfate,293,300 antioxidants and vitamins,291 or hormonal therapy with an estrogen-progesterone combination301 have been tried without significant success. The use of hyperbaric oxygen has been shown more recently to be helpful in patients who are not controlled with endoscopic techniques such as formalin, laser therapy, and APC. The response rate was 68%.302 The response rate for bleeding was 70% to 76%,302,303 48% showed complete resolution of bleeding, and 28% reported significantly fewer bleeding episodes.303 Although this therapy is expensive and inconvenient, requiring 20 to 40 treatment sessions,304,305 it should be offered to patients who fail conventional treatments.306
Endoscopic Therapies
Different endoscopic therapies have been described for rectal bleeding in this condition.
Topical Formalin
Topical formalin application for radiation injury was first reported in radiation-induced cystitis.307 Its mechanism of action is likely to be due to a local chemical cauterization of the inflamed or fragile telangiectatic rectal mucosa.295 The treatment is inexpensive and easy to perform. The technique involves the direct application of 4% formalin to the affected area in the rectum, either with formalin-soaked gauze308 or by direct instillation and timed retention (typically approximately 10 minutes).309 The application continues until mucosal blanching occurs, usually within 2 to 3 minutes.310 The perianal area should be protected with careful draping and the application of petroleum jelly around the anus and perianal skin to avoid formalin injury.296 The rectum is irrigated liberally after topical application to remove residual formalin. Formalin is contraindicated in the presence of fissures or ulceration. The short-term success of the technique with complete cessation of bleeding ranges from 59% to 100%.295,311 Recurrent symptomatic relapses have been treated successfully with repeated applications.
In patients with severe radiation proctitis, the administration of topical formalin may be more effective than APC.312 Complications have been described in 27% to 57% of patients311,313 and include painful fissures of the anal margins, perianal ulcerations,314 stool incontinence, and rectal ulcerations and strictures.309 Some authors think that this technique should be reserved for severe hemorrhagic proctitis refractory to medical or endoscopic treatment and that it should be thoroughly discussed in cases of anorectal radiation–induced stricture, prior anal incontinence, or treated anal cancer.311 Formalin offers an excellent option for patients who are on anticoagulation and have increased risk for bleeding from thermally induced ulceration after conventional coagulation therapies.
Bipolar Electrocoagulation and Heater Probe
Bipolar electrocoagulation and heater probe are more effective than medical therapy improving the quality of life.315
Laser
Argon,316 Nd:YAG,317 and KTP laser318 have been reported to be effective with a marked decrease in bleeding in 87% of treated patients.295 Repeated treatments were needed in the long-term because symptoms tended to recur.316 Laser therapy has the advantage of being a precise technique that does not involve tissue contact, is well tolerated, and improves activities of daily life. Disadvantages include its high cost; the need for protective precautions; and the risk of rectal ulcer,318 bowel perforation, and rectovaginal fistulas.295
Argon Plasma Coagulation
APC represents a safer, less expensive, and more widely available alternative to laser therapy.313 Many authors consider APC to be first-line therapy for radiation proctopathy. When the lesions are circumferential and numerous, a staged treatment may be useful allowing a sufficient period of time for healing of the previously treated area of at least 4 weeks.287 A median of 1.7 to 3.7 treatment sessions is necessary for control of bleeding.295,319,320 APC is relatively ineffective in patients with excessive bleeding, owing to the absorption of current by blood on the surface. It is important to minimize contact-induced bleeding during endoscopy to maximize access to the vascular lesions.
Care must be taken not to overdistend the bowel with argon gas insufflations.295 In the case of low rectal lesions, the use of a transparent cap at the tip of the colonoscope allows direct viewing of these lesions and of the upper part of the anal canal without the retroflexed position. Thanks to the cap, the endoscope remains close to the lesion but with sufficient distance to allow safe APC.321 One of the most common reasons for failed endoscopic therapy is the failure to coagulate the lesions at and immediately above the dentate line. There was significant improvement in rectal bleeding in many patients, with complete cessation of bleeding in 81% to 98%,319,322,323 providing long-lasting clinical remission.324
APC improves symptoms of diarrhea and tenesmus in 60% to 75% of cases325 with no obvious reason apparent. Patients with diarrhea and tenesmus should be advised that these symptoms may seem worsened after bleeding is controlled owing to the greater awareness of the symptoms in the absence of bleeding. Because of its limited depth of penetration, morbidity is usually minor and consists of gas bloating, tenesmus, transient abdominal or anal pain, and diarrhea in about 20% of cases.319 Major complications are exceptional but include rectovaginal fistula,326 chronic rectal ulceration, anal or rectal stricture,287 and perforation.327 Deliberate care should be taken to use the lowest power setting that provides a coagulum and to avoid overtreating, especially with persistent oozing after initial coagulation.
Endoscopic Cryotherapy
Endoscopic cryotherapy using nitrous oxide has been described more recently in a small group of patients with radiation proctitis with complete control of bleeding in all patients.135 It was also highly effective in patients with multiple angiectases (86%) and “watermelon stomach” (71%), but less effective in patients with radiation gastropathy and duodenopathy (40%). Cryotherapy was applied every 2 to 3 days until all lesions were ablated. The average number of therapeutic sessions needed to stop the bleeding was 3.6. Cryotherapy resulted in superficial tissue necrosis that was usually completely healed within 3 months after the final treatment sessions. Cryotherapy was remarkably safe with only benign self-limited adverse events.
Surgery
Surgical treatment is now considered a last resort and should be reserved for patients who have intractable symptoms that have failed to respond to medical therapy and patients with rectal strictures and fistulas because it is associated with frequent morbidity (9% to 79%)295,313 and mortality (3% to 13%).313,328,329 Proctectomy and Hartmann’s procedure, abdominoperianal resection, and coloanal anastomosis are the surgical options. Resection of the affected rectum necessitates dissection in an irradiated field with potential complications that range from bleeding to anastomotic leaks and sepsis.293
Radiation Gastropathy
Gastric complications secondary to radiotherapy are uncommon, but injury can occur when the stomach lies within the radiation field of an adjacent extragastric tumor.330 A high total dose, usually greater than 5000 rad, and a high daily fraction seem to be the main risk factors.331 There have been reports of chronic gastroduodenal ulcerations, gastritis, and duodenitis being diagnosed days to months after selective internal radiation therapy with resin-based microspheres loaded with yttrium-90. It is possible to find small spheres located inside the vessels in the biopsy specimens.332,333 Acute vasculopathy may progress to a prolonged and progressive endarteritis obliterans, vasculitis, and endothelial proliferation, leading to mucosal ischemia, ulceration, mucosal angiectases and fibrosis.334
In contrast to radiation proctopathy, GI bleeding from chronic radiation gastropathy is rare with very few cases reported.334–336 When it is present, there are multiple friable angiectases. These lead to melena and significant blood loss requiring multiple blood transfusions, prolonged hospitalizations, and repeated endoscopies. Endoscopically, the mucosa of the affected stomach (usually antrum) is friable and with multiple angiectases (Fig. 16.12). Other possible severe complications are perforation and gastric outlet obstruction.292
There is very little information in the literature on the management of radiation-induced complications in the stomach.292 Prolonged blood loss needs to be treated with iron and folic acid supplements and with transfusions. Aminocaproic acid334 and hyperbaric oxygen335 have been reported to be effective in control of bleeding in a few patients. Bleeding can be controlled by any endoscopic coagulation technique, with APC preferred336–338 and cryotherapy.135 Surgery may be necessary if other treatment fails,331,339 but is associated with high morbidity.334
Radiation Injury of the Small Bowel
The small intestine is particularly susceptible to radiation injury (Fig. 16.13).330 Radiation injury to the small intestine most often occurs in the clinical setting of radiotherapy for rectal, urologic, gynecologic, or retroperitoneal malignancies. The degree of injury seems to be proportional to radiation dosage delivered to the segment of small bowel that lies within the radiation field. The relative fixed position of the duodenum and distal ileum within the abdominal cavity make these portions particularly vulnerable to radiation-induced injury. Predisposing factors in the progression of radiation injury include excessive radiation, previous abdominal surgery that fixes loops of bowel in place, underlying cardiovascular disease, and an asthenic habitus.340 The mechanism of injury is vascular damage with progressive localized ischemia. The ischemia leads to angiectases and ulceration of the mucosa, fibrosis with stricturing, and, less frequently, fistula formation or perforation.
The clinical manifestations of chronic radiation typically occur 1 to 2 years after exposure and range from malabsorption, hemorrhage, bowel obstruction, fistulas, and abscess formation secondary to perforation.341 Bleeding can be managed with the same endoscopic techniques that are used in radiation proctitis. Balloon enteroscopy may be needed to access distal small bowel involvement and perform dilation of the diaphragmlike strictures, which resemble strictures seen with long-standing use of nonsteroidal antiinflammatory drugs. The treatment is difficult, and often surgery is necessary.340 Resection of the involved small bowel segment is preferable to bypass.137
Cameron Ulcers and Erosions
Patients with large hernias may develop Cameron ulcers or erosions. These mucosal lesions are usually located on the crest of the mucosal folds that make up the distal hiatal hernia rosette, on the lesser curve of the stomach, or at the level of the diaphragmatic hiatus.342 Cameron lesions can be found in 5% of all patients with hiatal hernias.343 The cause of Cameron lesions is still unclear, but it is thought that mechanical trauma secondary to diaphragmatic contraction from respiratory excursions and, perhaps, ischemia and acid mucosal injury play a primary role in their pathogenesis.344 Cameron lesions manifest clinically with chronic occult GI bleeding and associated iron deficiency anemia. These lesions can also manifest as acute upper GI bleeding in one-third of cases.343 Treatment includes antisecretory therapy and supplemental iron. In about one-third of patients, Cameron lesions may persist or recur despite antisecretory medication, in which case surgical repair of the hiatal hernia is required.343
1 Reuter SR, Redman HC. Gastrointestinal bleeding. In: Gastrointestinal Angiography. London: Saunders; 1997:218-268.
2 Zuckerman GR, Prakash C, Askin MP, et al. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:201-221.
3 Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. American Gastroenterological Association. Gastroenterology, 133. 1697-1717:2007.
4 Moore JD, Thompson NW, Appelman HD, et al. Arteriovenous malformations of the gastrointestinal tract. Arch Surg. 1976;111:381-389.
5 Duray PH, Marcal JMJr, LiVolsi VA, et al. Small intestinal angiodysplasia in the elderly. J Clin Gastroenterol. 1984;6:311-319.
6 Boley SJ, Sammartano R, Adams A, et al. On the nature and etiology of vascular ectasias of the colon: Degenerative lesions of aging. Gastroenterology. 1977;72:650-660.
7 Naveau S, Leger-Ravet MB, Houdayer C, et al. Nonhereditary colonic angiodysplasias: Histomorphometric approach to their pathogenesis. Dig Dis Sci. 1995;40:839-842.
8 Foutch PG. Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol. 1993;88:807-818.
9 Schmit A, Van Gossum A. Proposal for an endoscopic classification of digestive angiodysplasias for therapeutic trials. The European Club of Enteroscopy. Gastrointest Endosc. 1998;48:659.
10 Boley SJ, DiBiase A, Brandt LJ, et al. Lower intestinal bleeding in the elderly. Am J Surg. 1979;137:57-64.
11 Weaver GA, Alpern HD, Davis JS, et al. Gastrointestinal angiodysplasia associated with aortic valve disease: Part of a spectrum of angiodysplasia of the gut. Gastroenterology. 1979;77:1-11.
12 Junquera F, Saperas E, de Torres I, et al. Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol. 1999;94:1070-1076.
13 Baum S, Athanasoulis CA, Waltman AC, et al. Angiodysplasia of the right colon: A cause of gastrointestinal bleeding. AJR Am J Roentgenol. 1977;129:789-794.
14 Rogers BH. Endoscopic diagnosis and therapy of mucosal vascular abnormalities of the gastrointestinal tract occurring in elderly patients and associated with cardiac, vascular, and pulmonary disease. Gastrointest Endosc. 1980;26:134-138.
15 Danesh BJ, Spiliadis C, Williams CB, et al. Angiodysplasia—an uncommon cause of colonic bleeding: colonoscopic evaluation of 1,050 patients with rectal bleeding and anaemia. Int J Colorectal Dis. 1987;2:218-222.
16 Richter JM, Christensen MR, Colditz GA, et al. Angiodysplasia: natural history and efficacy of therapeutic interventions. Dig Dis Sci. 1989;34:1542-1546.
17 Rockey DC. Gastrointestinal tract evaluation in patients with iron deficiency anemia. Semin Gastrointest Dis. 1999;10:53-64.
18 Clouse RE, Costigan DJ, Mills BA, et al. Angiodysplasia as a cause of upper gastrointestinal bleeding. Arch Intern Med. 1985;145:458-461.
19 Richter JM, Hedberg SE, Athanasoulis CA, et al. Angiodysplasia: clinical presentation and colonoscopic diagnosis. Dig Dis Sci. 1984;29:481-485.
20 Boley SJ, Sammartano R, Brandt LJ, et al. Vascular ectasias of the colon. Surg Gynecol Obstet. 1979;149:353-359.
21 Jesudason SR, Devasia A, Mathen VI, et al. The pattern of angiodysplasia of the gastrointestinal tract in a tropical country. Surg Gynecol Obstet. 1985;161:525-531.
22 Regula J, Wronska E, Pachlewski J. Vascular lesions of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008;22:313-328.
23 Boley SJ, Brandt LJ. Vascular ectasias of the colon—1986. Dig Dis Sci. 1986;31(Suppl):26S-42S.
24 Brandt LJ. Vascular lesions of the gastrointestinal tract. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and liver disease: Pathophysiology, diagnosis, management. ed 8. Philadelphia: Saunders; 2006:757-777.
25 Moreto M, Figa M, Ojembarrena E, et al. Vascular malformations of the stomach and duodenum: An endoscopic classification. Endoscopy. 1986;18:227-229.
26 Marwick T, Kerlin P. Angiodysplasia of the upper gastrointestinal tract: Clinical spectrum in 41 cases. J Clin Gastroenterol. 1986;8:404-407.
27 Schmit A, Gay F, Adler M, et al. Diagnostic efficacy of push-enteroscopy and long-term follow-up of patients with small bowel angiodysplasias. Dig Dis Sci. 1996;41:2348-2352.
28 Browder W, Cerise EJ, Litwin MS. Impact of emergency angiography in massive lower gastrointestinal bleeding. Ann Surg. 1986;204:530-536.
29 Foutch PG, Rex DK, Lieberman DA. Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 1995;90:564-567.
30 Porush JG, Faubert PF. Chronic renal failure. In: Porush JG, Faubert PF, editors. Renal disease in the aged. Boston: Little, Brown; 1991:285.
31 Zuckerman GR, Cornette GL, Clouse RE, et al. Upper gastrointestinal bleeding in patients with chronic renal failure. Ann Intern Med. 1985;102:588-592.
32 Chalasani N, Cotsonis G, Wilcox CM. Upper gastrointestinal bleeding in patients with chronic renal failure: role of vascular ectasia. Am J Gastroenterol. 1996;91:2329-2332.
33 Veyradier A, Balian A, Wolf M, et al. Abnormal von Willebrand factor in bleeding angiodysplasias of the digestive tract. Gastroenterology. 2001;120:346-353.
34 Duray PH, Marcal JMJr, LiVolsi VA, et al. Gastrointestinal angiodysplasia: A possible component of von Willebrand’s disease. Hum Pathol. 1984;15:539-544.
35 Alhumood SA, Devine DV, Lawson L, et al. Idiopathic immune-mediated acquired von Willebrand’s disease in a patient with angiodysplasia: Demonstration of an unusual inhibitor causing a functional defect and rapid clearance of von Willebrand factor. Am J Hematol. 1999;60:151-157.
36 Imperiale TF, Ransohoff DF. Aortic stenosis, idiopathic gastrointestinal bleeding, and angiodysplasia: Is there an association? A methodologic critique of the literature. Gastroenterology. 1988;95:1670-1676.
37 King RM, Pluth JR, Giuliani ER. The association of unexplained gastrointestinal bleeding with calcific aortic stenosis. Ann Thorac Surg. 1987;44:514-516.
38 Cappell MS, Lebwohl O. Cessation of recurrent bleeding from gastrointestinal angiodysplasias after aortic valve replacement. Ann Intern Med. 1986;105:54-57.
39 Scheffer SM, Leatherman LL. Resolution of Heyde’s syndrome of aortic stenosis and gastrointestinal bleeding after aortic valve replacement. Ann Thorac Surg. 1986;42:477-480.
40 Warkentin TE, Moore JC, Morgan DG. Aortic stenosis and bleeding gastrointestinal angiodysplasia: Is acquired von Willebrand’s disease the link? Lancet. 1992;340:35-37.
41 Warkentin TE, Moore JC, Morgan DG. Gastrointestinal angiodysplasia and aortic stenosis. N Engl J Med. 2002;347:858-859.
42 O’Brien JR, Etherington MD, Brant J, et al. Decreased platelet function in aortic valve stenosis: High shear platelet activation then inactivation. Br Heart J. 1995;74:641-644.
43 Bourdette D, Greenberg B. Twelve-year history of gastrointestinal bleeding in a patient with calcific aortic stenosis and hemorrhagic telangiectasia. Dig Dis Sci. 1979;24:77-79.
44 Mehta PM, Heinsimer JA, Bryg RJ, et al. Reassessment of the association between gastrointestinal arteriovenous malformations and aortic stenosis. Am J Med. 1989;86:275-277.
45 Oneglia C, Sabatini T, Rusconi C, et al. Prevalence of aortic valve stenosis in patients affected by gastrointestinal angiodysplasia. Eur J Med. 1993;2:75-78.
46 Bhutani MS, Gupta SC, Markert RJ, et al. A prospective controlled evaluation of endoscopic detection of angiodysplasia and its association with aortic valve disease. Gastrointest Endosc. 1995;42:398-402.
47 Brandt LJ, Boley SJ. Vascular disorders of the colon. In: Sivak MVJ, editor. Gastroenterologic endoscopy. ed 2. Philadelphia: Saunders; 2000:1324-1350.
48 Mishra PK, Kovac J, de Caestecker J, et al. Intestinal angiodysplasia and aortic valve stenosis: Let’s not close the book on this association. Eur J Cardiothorac Surg. 2009;35:628-634.
49 Sjogren RW. Gastrointestinal features of scleroderma. Curr Opin Rheumatol. 1996;8:569-575.
50 Duchini A, Sessoms SL. Gastrointestinal hemorrhage in patients with systemic sclerosis and CREST syndrome. Am J Gastroenterol. 1998;93:1453-1456.
51 Stamm B, Heer M, Buhler H, et al. Mucosal biopsy of vascular ectasia (angiodysplasia) of the large bowel detected during routine colonoscopic examination. Histopathology. 1985;9:639-646.
52 Brandt LJ, Mukhopadhyay D. Masking of colon vascular ectasias by cold water lavage. Gastrointest Endosc. 1999;49:141-142.
53 Brandt LJ, Spinnell MK. Ability of naloxone to enhance the colonoscopic appearance of normal colon vasculature and colon vascular ectasias. Gastrointest Endosc. 1999;49:79-83.
54 Lai LH. Obscure GI bleeding: Is capsule endoscopy sufficient? Gastrointest Endosc. 2008;68:1128-1130.
55 Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA): Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1694-1696.
56 Muñoz-Navas M. Capsule endoscopy. World J Gastroenterol. 2009;15:1584-1586.
57 Nakamura T, Terano A. Capsule endoscopy: past, present, and future. J Gastroenterol. 2008;43:93-99.
58 Mishkin DS, Chuttani R, Croffie J, et al. ASGE Technology Status Evaluation Report: Wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539-545.
59 Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418.
60 Marmo R, Rotondano G, Piscopo R, et al. Meta-analysis: capsule enteroscopy vs. conventional modalities in diagnosis of small bowel diseases. Aliment Pharmacol Ther. 2005;22:595-604.
61 Gheorghe C, Iacob R, Bancila I. Olympus capsule endoscopy for small bowel examination. J Gastrointest Liver Dis. 2007;16:309-313.
62 Hartmann D, Eickhoff A, Damian U, et al. Diagnosis of small-bowel pathology using paired capsule endoscopy with two different devices: A randomized study. Endoscopy. 2007;39:1041-1045.
63 May A, Nachbar L, Wardak A, et al. Double-balloon enteroscopy: preliminary experience in patients with obscure gastrointestinal bleeding or chronic abdominal pain. Endoscopy. 2003;35:985-991.
63a Yamamoto H, Sekine Y, Sato Y, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220.
64 Domagk D, Bretthauer M, Lenz P, et al. Carbon dioxide insufflation improves intubation depth in double-balloon enteroscopy: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:1064-1067.
65 May A, Nachbar L, Schneider M, et al. Prospective comparison of push enteroscopy and push-and-pull enteroscopy in patients with suspected small-bowel bleeding. Am J Gastroenterol. 2006;101:2016-2024.
66 Kawamura T, Yasuda K, Tanaka K, et al. Clinical evaluation of a newly developed single-balloon enteroscope. Gastrointest Endosc. 2008;68:1112-1116.
67 Tsujikawa T, Saitoh Y, Andoh A, et al. Novel single-balloon enteroscopy for diagnosis and treatment of the small intestine: Preliminary experiences. Endoscopy. 2008;40:11-15.
68 Mensink PB, Haringsma J, Kucharzik T, et al. Complications of double balloon enteroscopy: A multicenter survey. Endoscopy. 2007;39:613-615.
69 Ross WA. Small-bowel imaging: Multiple paths to the last frontier. Gastrointest Endosc. 2008;68:1117-1121.
70 Ohmiya N, Yano T, Yamamoto H, et al. Diagnosis and treatment of obscure GI bleeding at double balloon endoscopy. Gastrointest Endosc. 2007;66(Suppl):S72-S77.
71 Pasha SF, Leighton JA, Das A, et al. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: A meta-analysis. Clin Gastroenterol Hepatol. 2008;6:671-676.
72 Chen X, Ran ZH, Tong JL. A meta-analysis of the yield of capsule endoscopy compared to double-balloon enteroscopy in patients with small bowel diseases. World J Gastroenterol. 2007;13:4372-4378.
73 Buscaglia JM, Dunbar KB, Okolo PI, et al. The spiral enteroscopy training initiative: results of a prospective study evaluating the Discovery SB overtube device during small enteroscopy (with video). Endoscopy. 2009;41:194-199.
74 Ackerman PA, Agrawal D, Chen W, et al. Spiral enteroscopy: A novel method of enteroscopy by using the Endo-Ease Discovery SB overtube and a pediatric colonoscope. Endoscopy. 2009;69:327-332.
75 Ackerman PA, Cantero D, Avila J, et al. The spiral enteroscopy experience in 101 consecutive patients: Safety and efficacy using the Discovery SB. Gastrointest Endosc. 2008;67:AB92-AB93.
76 Kaffes AJ, Siah C, Koo JH. Clinical outcomes after double-balloon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. 2007;66:304-309.
77 Saurin JC, Delvaux M, Vahedi K, et al. Clinical impact of capsule endoscopy compared to push enteroscopy: 1-year follow-up study. Endoscopy. 2005;37:318-323.
78 Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc. 2008;68:1122-1127.
79 Carretero C, Fernandez-Urien I, Betes M, Munoz-Navas M. Role of videocapsule endoscopy for gastrointestinal bleeding. World J Gastroenterol. 2008;14:5261-5264.
80 Sidhu R, Sanders DS, Morris AJ, et al. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125-136.
81 Semrad CE. Small bowel enteroscopy: territory conquered, future horizons. Curr Opin Gastroenterol. 2009;25:110-115.
82 Bar-Meir S, Eliakim R, Nadler M, et al. Second capsule endoscopy for patients with severe iron deficiency anemia. Gastrointest Endosc. 2004;60:711-713.
83 Viazis N, Papaxoinis K, Vlachogiannakos J, et al. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc. 2009;69:850-856.
84 Boley SJ, Sprayregen S, Sammartano RJ, et al. The pathophysiologic basis for the angiographic signs of vascular ectasias of the colon. Radiology. 1977;125:615-621.
85 Dusold R, Burke K, Carpentier W, et al. The accuracy of technetium-99m-labeled red cell scintigraphy in localizing gastrointestinal bleeding. Am J Gastroenterol. 1994;89:345-348.
86 Levy R, Barto W, Gani J. Retrospective study of the utility of nuclear scintigraphic-labelled red cell scanning for lower gastrointestinal bleeding. Aust N Z J Surg. 2003;73:205-209.
87 Brunnler T, Klebl F, Mundorff S, et al. Significance of scintigraphy for the localisation of obscure gastrointestinal bleedings. World J Gastroenterol. 2008;14:5015-5019.
88 Junquera F, Quiroga S, Saperas E, et al. Accuracy of helical computed tomographic angiography for the diagnosis of colonic angiodysplasia. Gastroenterology. 2000;119:293-299.
89 Voderholzer WA, Ortner M, Rogalla P, et al. Diagnostic yield of wireless capsule enteroscopy in comparison with computed tomography enteroclysis. Endoscopy. 2003;35:1009-1014.
90 Saperas E, Dot J, Videla S, et al. Capsule endoscopy versus computed tomographic or standard angiography for the diagnosis of obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:731-737.
91 Brandt LJ. A cecal angiodysplastic lesion is discovered during diagnostic colonoscopy performed for iron-deficiency anemia associated with stool positive for occult blood: What therapy would you recommend? Am J Gastroenterol. 1988;83:710-711.
92 Wilcox CM, Alexander LN, Clark WS. Prospective evaluation of the gastrointestinal tract in patients with iron deficiency and no systemic or gastrointestinal symptoms or signs. Am J Med. 1997;103:405-409.
93 Goddard AF, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. Gut. 2000;46(Suppl 3–4):IV1-IV5.
94 Granieri R, Mazzulla JP, Yarborough GW. Estrogen-progesterone therapy for recurrent gastrointestinal bleeding secondary to gastrointestinal angiodysplasia. Am J Gastroenterol. 1988;83:556-558.
95 Molina Infante J, Perez Gallardo B, Fernández Bermejo M. Update on medical therapy for obscure gastrointestinal hemorrhage [in Spanish]. Rev Esp Enferm Dig. 2007;99:457-462.
96 van Cutsem E, Rutgeerts P, Vantrappen G. Treatment of bleeding gastrointestinal vascular malformations with oestrogen-progesterone. Lancet. 1990;335:953-955.
97 Lewis BS, Salomon P, Rivera-MacMurray S, et al. Does hormonal therapy have any benefit for bleeding angiodysplasia? J Clin Gastroenterol. 1992;15:99-103.
98 Barkin JS, Ross BS. Medical therapy for chronic gastrointestinal bleeding of obscure origin. Am J Gastroenterol. 1998;93:1250-1254.
99 Marshall JK, Hunt RH. Hormonal therapy for bleeding gastrointestinal mucosal vascular abnormalities: A promising alternative. Eur J Gastroenterol Hepatol. 1997;9:521-525.
100 Junquera F, Feu F, Papo M, et al. A multicenter, randomized, clinical trial of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia. Gastroenterology. 2001;121:1073-1079.
101 Nardone G, Rocco A, Balzano T, et al. The efficacy of octreotide therapy in chronic bleeding due to vascular abnormalities of the gastrointestinal tract. Aliment Pharmacol Ther. 1999;13:1429-1436.
102 Orsi P, Guatti-Zuliani C, et al. Long-acting octreotide is effective in controlling rebleeding angiodysplasia of the gastrointestinal tract. Dig Liver Dis. 2001;33:330-334.
103 Bowers M, McNulty O, Mayne E. Octreotide in the treatment of gastrointestinal bleeding caused by angiodysplasia in two patients with von Willebrand’s disease. Br J Haematol. 2000;108:524-527.
104 Junquera F, Saperas E, Videla S, et al. Long-term efficacy of octreotide in the prevention of recurrent bleeding from gastrointestinal angiodysplasia. Am J Gastroenterol. 2007;102:254-260.
105 Krikis N, Tziomalos K, Perifanis V, et al. Treatment of recurrent gastrointestinal haemorrhage in a patient with von Willebrand’s disease with octreotide LAR and propranolol. Gut. 2005;54:171-172.
106 Tamagno G, Mioni R, De Carlo E, et al. Effects of a somatostatin analogue in occult gastrointestinal bleeding: a case report. Dig Liver Dis. 2004;36:843-846.
107 Molina-Infante J, Perez-Gallardo B, Gonzalez-Garcia G, et al. Octreotide LAR for severe obscure-overt gastrointestinal haemorrhage in high-risk patients on anticoagulation therapy. Gut. 2007;56:447.
108 Hirri HM, Green PJ, Lindsay J. Von Willebrand’s disease and angiodysplasia treated with thalidomide. Haemophilia. 2006;12:285-286.
109 Shurafa M, Kamboj G. Thalidomide for the treatment of bleeding angiodysplasias. Am J Gastroenterol. 2003;98:221-222.
110 Bauditz J, Schachschal G, Wedel S, et al. Thalidomide for treatment of severe intestinal bleeding. Gut. 2004;53:609-612.
111 Bauditz J, Lochs H, Voderholzer W. Macroscopic appearance of intestinal angiodysplasias under antiangiogenic treatment with thalidomide. Endoscopy. 2006;38:1036-1039.
112 Vujkovac B, Lavre J, Sabovic M. Successful treatment of bleeding from colonic angiodysplasias with tranexamic acid in a hemodialysis patient. Am J Kidney Dis. 1998;31:536-538.
113 Quitt M, Froom P, Veisler A, et al. The effect of desmopressin on massive gastrointestinal bleeding in hereditary telangiectasia unresponsive to treatment with cryoprecipitate. Arch Intern Med. 1990;150:1744-1746.
114 Romero-Castro R, Jimenez-Saenz M, Pellicer-Bautista F, et al. Recombinant-activated factor VII as hemostatic therapy in eight cases of severe hemorrhage from esophageal varices. Clin Gastroenterol Hepatol. 2004;2:78-84.
115 Bosch J, Thabut D, Bendtsen F, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123-1130.
116 Romero-Castro R, Jimenez-Saenz M, Pellicer-Bautista F, et al. Refractory bleeding after endoscopic sphincterotomy: A new indication for recombinant factor VII therapy? Am J Gastroenterol. 2004;99:2063-2065.
117 Meijer K, Peters FT, van der Meer J. Recurrent severe bleeding from gastrointestinal angiodysplasia in a patient with von Willebrand’s disease, controlled with recombinant factor VIIa. Blood Coagul Fibrinolysis. 2001;12:211-213.
118 Akyildiz M, Turan I, Ozutemiz O, et al. A cerebrovascular event after single-dose administration of recombinant factor VIIa in a patient with esophageal variceal bleeding. Dig Dis Sci. 2006;51:1647-1649.
119 Askin MP, Lewis BS. Push enteroscopic cauterization: Long-term follow-up of 83 patients with bleeding small intestinal angiodysplasia. Gastrointest Endosc. 1996;43:580-583.
120 Jensen DM, Machicado GA. Colonoscopy for diagnosis and treatment of severe lower gastrointestinal bleeding: Routine outcomes and cost analysis. Gastrointest Endosc Clin N Am. 1997;7:477-498.
121 Trudel JL, Fazio VW, Sivak MV. Colonoscopic diagnosis and treatment of arteriovenous malformations in chronic lower gastrointestinal bleeding: Clinical accuracy and efficacy. Dis Colon Rectum. 1988;31:107-110.
122 Bemvenuti GA, Julich MM. Ethanolamine injection for sclerotherapy of angiodysplasia of the colon. Endoscopy. 1998;30:564-569.
123 Wong Kee Song LM, Baron TH. Endoscopic management of acute lower gastrointestinal bleeding. Am J Gastroenterol. 2008;103:1881-1887.
124 Weilert F, Smith AC. Endoscopic band ligation of gastric angiodysplasia. N Z Med J. 1998;111:320.
125 Campo R, Brullet E. Endoscopic treatment of gastric angiodysplasia with elastic band ligation. Gastrointest Endosc. 1996;43:502-504.
126 Campo R, Brullet E, Montane JM, et al. Elastic band ligation in the bowel: Is it really safe? Gastrointest Endosc. 1998;47:105-106.
127 Goff JS. Endoscopic variceal ligation safety throughout the GI tract. Gastrointest Endosc. 2005;62:228-229.
128 Gostout CJ, Bowyer BA, Ahlquist DA, et al. Mucosal vascular malformations of the gastrointestinal tract: clinical observations and results of endoscopic neodymium: yttrium-aluminum-garnet laser therapy. Mayo Clin Proc. 1988;63:993-1003.
129 Naveau S, Aubert A, Poynard T, et al. Long-term results of treatment of vascular malformations of the gastrointestinal tract by neodymium YAG laser photocoagulation. Dig Dis Sci. 1990;35:821-826.
130 Cello JP, Grendell JH. Endoscopic laser treatment for gastrointestinal vascular ectasias. Ann Intern Med. 1986;104:352-354.
131 Manner H. Argon plasma coagulation therapy. Curr Opin Gastroenterol. 2008;24:612-616.
132 Wahab PJ, Mulder CJ, den Hartog G, et al. Argon plasma coagulation in flexible gastrointestinal endoscopy: Pilot experiences. Endoscopy. 1997;29:176-181.
133 Johanns W, Luis W, Janssen J, et al. Argon plasma coagulation (APC) in gastroenterology: Experimental and clinical experiences. Eur J Gastroenterol Hepatol. 1997;9:581-587.
134 Norton ID, Wang L, Levine SA, et al. Efficacy of colonic submucosal saline solution injection for the reduction of iatrogenic thermal injury. Gastrointest Endosc. 2002;56:95-99.
135 Kantsevoy SV, Cruz-Correa MR, Vaughn CA, et al. Endoscopic cryotherapy for the treatment of bleeding mucosal vascular lesions of the GI tract: A pilot study. Gastrointest Endosc. 2003;57:403-406.
136 Hutcheon DF, Kabelin J, Bulkley GB, et al. Effect of therapy on bleeding rates in gastrointestinal angiodysplasia. Am Surg. 1987;53:6-9.
137 Cohn SM, Moller BA, Zieg PM, et al. Angiography for preoperative evaluation in patients with lower gastrointestinal bleeding: Are the benefits worth the risks? Arch Surg. 1998;133:50-55.
138 Garcia-Tsao G, Korzenik JR, Young L, et al. Liver disease in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2000;343:931-936.
139 Longacre AV, Gross CP, Gallitelli M, et al. Diagnosis and management of gastrointestinal bleeding in patients with hereditary hemorrhagic telangiectasia. Am J Gastroenterol. 2003;98:59-65.
140 Begbie ME, Wallace GM, Shovlin CL. Hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): A view from the 21st century. Postgrad Med J. 2003;79:18-24.
141 Rockey DC. Gastrointestinal bleeding. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and liver disease: Pathophysiology, diagnosis, management. ed 8. Philadelphia: Saunders; 2006:255-299.
142 Guttmacher AE, Marchuk DA, White RIJr. Hereditary hemorrhagic telangiectasia. N Engl J Med. 1995;333:918-924.
143 Kjeldsen AD, Kjeldsen J. Gastrointestinal bleeding in patients with hereditary hemorrhagic telangiectasia. Am J Gastroenterol. 2000;95:415-418.
144 Sharma VK, Howden CW. Gastrointestinal and hepatic manifestations of hereditary hemorrhagic telangiectasia. Dig Dis. 1998;16:169-174.
145 Spencer J, Camillieri M. Chronic gastrointestinal haemorrhage. In: Bouchier IAD, Allan RN, Hodgson HJF, Keighley MRB, editors. Gastroenterology: Clinical science and practice. London: Saunders; 1993:988-1003.
146 Haq AU, Glass J, Netchvolodoff CV, et al. Hereditary hemorrhagic telangiectasia and danazol. Ann Intern Med. 1988;109:171.
147 Annichino-Bizzacchi JM, Facchini RM, Torresan MZ, et al. Hereditary hemorrhagic telangiectasia response to aminocaproic acid treatment. Thromb Res. 1999;96:73-76.
148 Young W, Gilbert V, Feinsat T, et al. The recurrent upper gastrointestinal bleeding in hereditary hemorrhagic telangiectasia (Osler’s disease) successfully treated by endoscopic sclerotherapy. Gastrointest Endosc. 1982;28:148-152.
149 Kitamura T, Tanabe S, Koizumi W, et al. Rendu-Osler-Weber disease successfully treated by argon plasma coagulation. Gastrointest Endosc. 2001;54:525-527.
150 Machicado GA, Jensen DM. Upper gastrointestinal angiomata: Diagnosis and treatment. Gastrointest Endosc Clin N Am. 1991;1:241-262.
151 Takazawa J, Motoya M, Okamura S, et al. A case of Rendu-Osler-Weber disease treated with electrocautery. Prog Dig Endosc. 1981;19:148-150.
152 Harada K, Mizushima K, Ono M, et al. A case of Osler’s disease treated with laser coagulation. Gastroenterol Endosc. 1980;22:400-407.
153 Iwanuma Y, Haba T, Maekawa T. A case of Rendu-Osler-Weber disease with bleeding from mucosal telangiectasia of the stomach treated by endoscopic hemostasis using clip. Prog Dig Endosc. 1994;45:180-181.
154 Rector WGJr, Reynolds TB. Risk factors for haemorrhage from oesophageal varices and acute gastric erosions. Clin Gastroenterol. 1985;14:139-153.
155 Quintero E, Pique JM, Bombi JA, et al. Gastric mucosal vascular ectasias causing bleeding in cirrhosis: A distinct entity associated with hypergastrinemia and low serum levels of pepsinogen I. Gastroenterology. 1987;93:1054-1061.
156 Payen JL, Cales P, Voigt JJ, et al. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138-144.
157 Ripoll C, Garcia-Tsao G. Treatment of gastropathy and gastric antral vascular ectasia in patients with portal hypertension. Curr Treat Options Gastroenterol. 2007;10:483-494.
158 Figueiredo P, Almeida N, Lerias C, et al. Effect of portal hypertension in the small bowel: an endoscopic approach. Dig Dis Sci. 2008;53:2144-2150.
159 Canlas KR, Dobozi BM, Lin S, et al. Using capsule endoscopy to identify GI tract lesions in cirrhotic patients with portal hypertension and chronic anemia. J Clin Gastroenterol. 2008;42:844-848.
160 Spina GP, Arcidiacono R, Bosch J, et al. Gastric endoscopic features in portal hypertension: Final report of a consensus conference, Milan, Italy, September 19, 1992. J Hepatol. 1994;21:461-467.
161 McCormack TT, Sims J, Eyre-Brook I, et al. Gastric lesions in portal hypertension: Inflammatory gastritis or congestive gastropathy? Gut. 1985;26:1226-1232.
162 Tanoue K, Hashizume M, Wada H, et al. Effects of endoscopic injection sclerotherapy on portal hypertensive gastropathy: A prospective study. Gastrointest Endosc. 1992;38:582-585.
163 Primignani M, Carpinelli L, Preatoni P, et al. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis: The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC). Gastroenterology. 2000;119:181-187.
164 Thuluvath PJ, Yoo HY. Portal hypertensive gastropathy. Am J Gastroenterol. 2002;97:2973-2978.
165 Perez-Ayuso RM, Pique JM, Bosch J, et al. Propranolol in prevention of recurrent bleeding from severe portal hypertensive gastropathy in cirrhosis. Lancet. 1991;337:1431-1434.
166 Pique JM. Portal hypertensive gastropathy. Baillieres Clin Gastroenterol. 1997;11:257-270.
167 Cales P, Zabotto B, Meskens C, et al. Gastroesophageal endoscopic features in cirrhosis. Observer variability, interassociations, and relationship to hepatic dysfunction. Gastroenterology. 1990;98:156-162.
168 Iwao T, Toyonaga A, Oho K, et al. Portal-hypertensive gastropathy develops less in patients with cirrhosis and fundal varices. J Hepatol. 1997;26:1235-1241.
169 Sarin SK, Sreenivas DV, Lahoti D, et al. Factors influencing development of portal hypertensive gastropathy in patients with portal hypertension. Gastroenterology. 1992;102:994-999.
170 Sarin SK, Shahi HM, Jain M, et al. The natural history of portal hypertensive gastropathy: Influence of variceal eradication. Am J Gastroenterol. 2000;95:2888-2893.
171 Gupta R, Saraswat VA, Kumar M, et al. Frequency and factors influencing portal hypertensive gastropathy and duodenopathy in cirrhotic portal hypertension. J Gastroenterol Hepatol. 1996;11:728-733.
172 Bayraktar Y, Balkanci F, Uzunalimoglu B, et al. Is portal hypertension due to liver cirrhosis a major factor in the development of portal hypertensive gastropathy? Am J Gastroenterol. 1996;91:554-558.
173 Masuko E, Homma H, Ohta H, et al. Rheologic analysis of gastric mucosal hemodynamics in patients with cirrhosis. Gastrointest Endosc. 1999;49:371-379.
174 Panes J, Bordas JM, Pique JM, et al. Increased gastric mucosal perfusion in cirrhotic patients with portal hypertensive gastropathy. Gastroenterology. 1992;103:1875-1882.
175 Ohta M, Hashizume M, Higashi H, et al. Portal and gastric mucosal hemodynamics in cirrhotic patients with portal-hypertensive gastropathy. Hepatology. 1994;20:1432-1436.
176 Nishizaki Y, Kaunitz JD, Oda M, et al. Impairment of gastric mucosal defenses measured in vivo in cirrhotic rats. Hepatology. 1994;20:445-452.
177 Ohta M, Yamaguchi S, Gotoh N, et al. Pathogenesis of portal hypertensive gastropathy: A clinical and experimental review. Surgery. 2002;131(Suppl):S165-S170.
178 Kawanaka H, Tomikawa M, Jones MK, et al. Defective mitogen-activated protein kinase (ERK2) signaling in gastric mucosa of portal hypertensive rats: Potential therapeutic implications. Hepatology. 2001;34:990-999.
179 Sarin SK, Misra SP, Singal A, et al. Evaluation of the incidence and significance of the “mosaic pattern” in patients with cirrhosis, noncirrhotic portal fibrosis, and extrahepatic obstruction. Am J Gastroenterol. 1988;83:1235-1239.
180 Lo GH, Lai KH, Cheng JS, et al. The effects of endoscopic variceal ligation and propranolol on portal hypertensive gastropathy: A prospective, controlled trial. Gastrointest Endosc. 2001;53:579-584.
181 Balan KK, Grime JS, Sutton R, et al. Do alterations in the rate of gastric emptying after injection sclerotherapy for oesophageal varices play any role in the development of portal hypertensive gastropathy? HPB Surg. 1999;11:141-148.
182 D’Amico G, Montalbano L, Traina M, et al. Natural history of congestive gastropathy in cirrhosis. The Liver Study Group of V. Cervello Hospital. Gastroenterology. 1990;99:1558-1564.
183 Gostout CJ. Portal hypertensive gastropathy: Much ado about nothing? Am J Gastroenterol. 2000;95:2682-2684.
184 Lebrec D, Poynard T, Hillon P, et al. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: A controlled study. N Engl J Med. 1981;305:1371-1374.
185 Hosking SW, Kennedy HJ, Seddon I, et al. The role of propranolol in congestive gastropathy of portal hypertension. Hepatology. 1987;7:437-441.
186 Li MK, Sung JJ, Woo KS, et al. Somatostatin reduces gastric mucosal blood flow in patients with portal hypertensive gastropathy: A randomized, double-blind crossover study. Dig Dis Sci. 1996;41:2440-2446.
187 Kouroumalis EA, Koutroubakis IE, Manousos ON. Somatostatin for acute severe bleeding from portal hypertensive gastropathy. Eur J Gastroenterol Hepatol. 1998;10:509-512.
188 Escorsell A, Bandi JC, Andreu V, et al. Desensitization to the effects of intravenous octreotide in cirrhotic patients with portal hypertension. Gastroenterology. 2001;120:161-169.
189 Garcia N, Sanyal AJ. Portal hypertensive gastropathy and gastric antral vascular ectasia. Curr Treat Options Gastroenterol. 2001;4:163-171.
190 Gostout CJ, Ahlquist DA, Radford CM, et al. Endoscopic laser therapy for watermelon stomach. Gastroenterology. 1989;96:1462-1465.
191 Orloff MJ, Orloff MS, Orloff SL, et al. Treatment of bleeding from portal hypertensive gastropathy by portacaval shunt. Hepatology. 1995;21:1011-1017.
192 Urata J, Yamashita Y, Tsuchigame T, et al. The effects of transjugular intrahepatic portosystemic shunt on portal hypertensive gastropathy. J Gastroenterol Hepatol. 1998;13:1061-1067.
193 Panes J, Pique JM. Therapeutic options for bleeding portal hypertensive gastropathy. J Gastroenterol Hepatol. 1998;13:977-979.
194 Jabbari M, Cherry R, Lough JO, et al. Gastric antral vascular ectasia: The watermelon stomach. Gastroenterology. 1984;87:1165-1170.
195 Toyota M, Hinoda Y, Nakagawa N, et al. Gastric antral vascular ectasia causing severe anemia. J Gastroenterol. 1996;31:710-713.
196 Perez-Ayuso RM, Pique JM, Saperas E, et al. Gastric vascular ectasias in cirrhosis: Association with hypoacidity not related to gastric atrophy. Scand J Gastroenterol. 1989;24:1073-1078.
197 Spahr L, Villeneuve JP, Dufresne MP, et al. Gastric antral vascular ectasia in cirrhotic patients: Absence of relation with portal hypertension. Gut. 1999;44:739-742.
198 Lee FI, Costello F, Flanagan N, et al. Diffuse antral vascular ectasia. Gastrointest Endosc. 1984;30:87-90.
199 Suit PF, Petras RE, Bauer TW, et al. Gastric antral vascular ectasia: A histologic and morphometric study of “the watermelon stomach.”. Am J Surg Pathol. 1987;11:750-757.
200 Gilliam JH3rd, Geisinger KR, Wu WC, et al. Endoscopic biopsy is diagnostic in gastric antral vascular ectasia: The “watermelon stomach.”. Dig Dis Sci. 1989;34:885-888.
201 Kamath PS, Lacerda M, Ahlquist DA, et al. Gastric mucosal responses to intrahepatic portosystemic shunting in patients with cirrhosis. Gastroenterology. 2000;118:905-911.
202 Tran A, Villeneuve JP, Bilodeau M, et al. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: An open pilot study. Am J Gastroenterol. 1999;94:2909-2911.
203 Moss SF, Ghosh P, Thomas DM, et al. Gastric antral vascular ectasia: Maintenance treatment with oestrogen-progesterone. Gut. 1992;33:715-717.
204 Schoonbroodt D, Horsmans Y, Hoang P, et al. Vascular gastric anomalies, CREST syndrome and primary biliary cirrhosis: Efficacy of ethinyl estradiol-norethisterone combination. Gastroenterol Clin Biol. 1994;18:649-651.
205 Manning RJ. Estrogen/progesterone treatment of diffuse antral vascular ectasia. Am J Gastroenterol. 1995;90:154-156.
206 McCormick PA, Ooi H, Crosbie O. Tranexamic acid for severe bleeding gastric antral vascular ectasia in cirrhosis. Gut. 1998;42:750-752.
207 Barbara G, De Giorgio R, Salvioli B, et al. Unsuccessful octreotide treatment of the watermelon stomach. J Clin Gastroenterol. 1998;26:345-346.
208 Bourke MJ, Hope RL, Boyd P, et al. Endoscopic laser therapy for watermelon stomach. J Gastroenterol Hepatol. 1996;11:832-834.
209 Bjorkman DJ, Buchi KN. Endoscopic laser therapy of the watermelon stomach. Lasers Surg Med. 1992;12:478-481.
210 Petrini JLJr, Johnston JH. Heat probe treatment for antral vascular ectasia. Gastrointest Endosc. 1989;35:324-328.
211 Eloubeidi MA, Branch MS. Clinical images: Watermelon stomach. Dig Dis. 1999;17:123.
212 Focke G, Seidl C, Grouls V. Treatment of watermelon stomach (GAVE syndrome) with endoscopic argon plasma coagulation (APC): A new therapy approach [in German]. Leber Magen Darm. 1996;26(254):257-259.
213 Sebastian S, O’Morain CA, Buckley MJ. Current therapeutic options for gastric antral vascular ectasia. Aliment Pharmacol Ther. 2003;18:157-165.
214 Herrera S, Bordas JM, Llach J, et al. The beneficial effects of argon plasma coagulation in the management of different types of gastric vascular ectasia lesions in patients admitted for GI hemorrhage. Gastrointest Endosc. 2008;68:440-446.
215 Kwan V, Bourke MJ, Williams SJ, et al. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: Experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol. 2006;101:58-63.
216 Dulai GS, Jensen DM. Treatment of watermelon stomach. Curr Treat Options Gastroenterol. 2006;9:175-180.
217 Ikeda M, Ishida H, Nakamura E, et al. An endoscopic follow-up study of the development of diffuse antral vascular ectasia. Endoscopy. 1996;28:390-393.
218 Wells CD, Harrison ME, Gurudu SR, et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008;68:231-236.
219 American Society for Gastrointestinal Endoscopy Technology Committee. Mucosal ablation devices. Gastrointest Endosc. 2008;68:1031-1042.
220 Cho S, Zanati S, Yong E, et al. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895-902.
221 Gross SA, Al-Haddad M, Gill KR, et al. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: A pilot study. Gastrointest Endosc. 2008;67:324-327.
222 Cales P, Voigt JJ, Payen JL, et al. Diffuse vascular ectasia of the antrum, duodenum, and jejunum in a patient with nodular regenerative hyperplasia: Lack of response to portosystemic shunt or gastrectomy. Gut. 1993;34:558-561.
223 Borsch G. Diffuse gastric antral vascular ectasia: The “watermelon stomach” revisited. Am J Gastroenterol. 1987;82:1333-1334.
224 Gostout CJ, Viggiano TR, Ahlquist DA, et al. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol. 1992;15:256-263.
225 Kruger R, Ryan ME, Dickson KB, et al. Diffuse vascular ectasia of the gastric antrum. Am J Gastroenterol. 1987;82:421-426.
226 Kozarek RA, Botoman VA, Bredfeldt JE, et al. Portal colopathy: Prospective study of colonoscopy in patients with portal hypertension. Gastroenterology. 1991;101:1192-1197.
227 Naveau S, Bedossa P, Poynard T, et al. Portal hypertensive colopathy: A new entity. Dig Dis Sci. 1991;36:1774-1781.
228 Chen LS, Lin HC, Lee FY, et al. Portal hypertensive colopathy in patients with cirrhosis. Scand J Gastroenterol. 1996;31:490-494.
229 Tam TN, Ng WW, Lee SD. Colonic mucosal changes in patients with liver cirrhosis. Gastrointest Endosc. 1995;42:408-412.
230 Rabinovitz M, Schade RR, Dindzans VJ, et al. Colonic disease in cirrhosis: An endoscopic evaluation in 412 patients. Gastroenterology. 1990;99:195-199.
231 Scandalis N, Archimandritis A, Kastanas K, et al. Colonic findings in cirrhotics with portal hypertension: A prospective colonoscopic and histological study. J Clin Gastroenterol. 1994;18:325-328.
232 Goenka MK, Kochhar R, Nagi B, et al. Rectosigmoid varices and other mucosal changes in patients with portal hypertension. Am J Gastroenterol. 1991;86:1185-1189.
233 Ganguly S, Sarin SK, Bhatia V, et al. The prevalence and spectrum of colonic lesions in patients with cirrhotic and noncirrhotic portal hypertension. Hepatology. 1995;21:1226-1231.
234 Misra SP, Dwivedi M, Misra V. Prevalence and factors influencing hemorrhoids, anorectal varices, and colopathy in patients with portal hypertension. Endoscopy. 1996;28:340-345.
235 Bresci G, Gambardella L, Parisi G, et al. Colonic disease in cirrhotic patients with portal hypertension: An endoscopic and clinical evaluation. J Clin Gastroenterol. 1998;26:222-227.
236 Viggiano TR, Gostout CJ. Portal hypertensive intestinal vasculopathy: A review of the clinical, endoscopic, and histopathologic features. Am J Gastroenterol. 1992;87:944-954.
237 Bini EJ, Lascarides CE, Micale PL, et al. Mucosal abnormalities of the colon in patients with portal hypertension: An endoscopic study. Gastrointest Endosc. 2000;52:511-516.
238 Ito K, Shiraki K, Sakai T, et al. Portal hypertensive colopathy in patients with liver cirrhosis. World J Gastroenterol. 2005;11:3127-3130.
239 Lamps LW, Hunt CM, Green A, et al. Alterations in colonic mucosal vessels in patients with cirrhosis and noncirrhotic portal hypertension. Hum Pathol. 1998;29:527-535.
240 Munakata A, Nakajima H, Sasaki Y, et al. Does portal hypertension modify colonic mucosal vasculature? Quantification of alteration by image processing and topology. Am J Gastroenterol. 1995;90:1997-2001.
241 Balzer C, Lotterer E, Kleber G, et al. Transjugular intrahepatic portosystemic shunt for bleeding angiodysplasia-like lesions in portal-hypertensive colopathy. Gastroenterology. 1998;115:167-172.
242 Fraiberg EN, Ahmed S. Colonoscopic excision of a polypoidal cavernous hemangioma of the cecum. Gastrointest Endosc. 1985;31:109.
243 Pontecorvo C, Lombardi S, Mottola L, et al. Hemangiomas of the large bowel: Report of a case. Dis Colon Rectum. 1983;26:818-820.
244 Amano K, Seko A, Nagura K, et al. A case of polypoid cavernous hemangioma of the sigmoid colon excised by colonoscopic polypectomy. Gastroenterol Jpn. 1993;28:712-718.
245 Beck PL, Aspinall AI, Kilvert VM, et al. Blue rubber bleb nevus syndrome. Gastrointest Endosc. 2002;56:598-600.
246 Oksuzoglu BC, Oksuzoglu G, Cakir U, et al. Blue rubber bleb nevus syndrome. Am J Gastroenterol. 1996;91:780-782.
247 Rodrigues D, Bourroul ML, Ferrer AP, et al. Blue rubber bleb nevus syndrome. Rev Hosp Clin Fac Med Sao Paulo. 2000;55:29-34.
248 Fishman SJ, Smitiers CJ, Folkman J, et al. Blue rubber bleb nevus syndrome: Surgical eradication of gastrointestinal bleeding. Ann Surg. 2005;241:523-528.
249 Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
250 Munoz-Navas M, Fernandez-Urien I, Espinet E, et al. Blue rubber bleb nevus syndrome: Three cases [in Spanish]. Rev Esp Enferm Dig. 2004;96:344-345.
251 Bak YT, Oh CH, Kim JH, et al. Blue rubber bleb nevus syndrome: Endoscopic removal of the gastrointestinal hemangiomas. Gastrointest Endosc. 1997;45:90-92.
252 Tyrrel RT, Baumgartner BR, Montemayor KA. Blue rubber bleb nevus syndrome: CT diagnosis of intussusception. AJR Am J Roentgenol. 1990;154:105-106.
253 Kim SJ. Blue rubber bleb nevus syndrome with central nervous system involvement. Pediatr Neurol. 2000;22:410-412.
254 Garen PD, Sahn EE. Spinal cord compression in blue rubber bleb nevus syndrome. Arch Dermatol. 1994;130:934-935.
255 Fernandes C, Silva A, Coelho A, et al. Blue rubber bleb naevus: Case report and literature review. Eur J Gastroenterol Hepatol. 1999;11:455-457.
256 Aihara M, Konuma Y, Okawa K, et al. Blue rubber bleb nevus syndrome with disseminated intravascular coagulation and thrombocytopenia: Successful treatment with high-dose intravenous gammaglobulin. Tohoku J Exp Med. 1991;163:111-117.
257 De Bona M, Bellumat A, De Boni M. Capsule endoscopy for the diagnosis and follow-up of blue rubber bleb nevus syndrome. Dig Liver Dis. 2005;37:451-453.
258 Romao Z, Pontes J, Lopes H, et al. Endosonography in the diagnosis of “blue rubber bleb nevus syndrome”: An uncommon cause of gastrointestinal tract bleeding. J Clin Gastroenterol. 1999;28:262-265.
259 Mechri M, Soyer P, Boudiaf M, et al. Small bowel involvement in blue rubber bleb nevus syndrome: MR imaging features. Abdom Imaging. 2009;34:448-451.
260 Jennings M, Ward P, Maddocks JL. Blue rubber bleb naevus disease: An uncommon cause of gastrointestinal tract bleeding. Gut. 1988;29:1408-1412.
261 Boente MD, Cordisco MR, Frontini MD, et al. Blue rubber bleb nevus (Bean syndrome): Evolution of four cases and clinical response to pharmacologic agents. Pediatr Dermatol. 1999;16:222-227.
262 Gonzalez D, Elizondo BJ, Haslag S, et al. Chronic subcutaneous octreotide decreases gastrointestinal blood loss in blue rubber-bleb nevus syndrome. J Pediatr Gastroenterol Nutr. 2001;33:183-188.
263 Dwivedi M, Misra SP. Blue rubber bleb nevus syndrome causing upper GI hemorrhage: A novel management approach and review. Gastrointest Endosc. 2002;55:943-946.
264 Arguedas MR, Wilcox CM. At the focal point: Blue rubber bleb nevus syndrome. Gastrointest Endosc. 1999;50:544.
265 Sala Felis T, Urquijo Ponce JJ, et al. Blue nevus syndrome: Endoscopic treatment by sclerosis and banding ligation. Gastroenterol Hepatol. 1999;22:136-138.
266 Shimada S, Namikawa K, Maeda K, et al. Endoscopic polypectomy under laparotomy throughout the alimentary tract for a patient with blue rubber bleb nevus syndrome. Gastrointest Endosc. 1997;45:423-427.
267 Maunoury V, Turck D, Brunetaud JM, et al. Blue rubber bleb nevus syndrome: 3 cases treated with a Nd:YAG laser and bipolar electrocoagulation. Gastroenterol Clin Biol. 1990;14:593-595.
268 Dieckmann K, Maurage C, Faure N, et al. Combined laser-steroid therapy in blue rubber bleb nevus syndrome: Case report and review of the literature. Eur J Pediatr Surg. 1994;4:372-374.
269 Yacoub M, Gnaoui A, Abroug S, et al. The “blue rubber bleb nevus” (Bean’s syndrome): Uncommon cause of gastrointestinal bleeding. Ann Pediatr (Paris). 1993;40:157-161.
270 Wong SH, Lau WY. Blue rubber-bleb nevus syndrome. Dis Colon Rectum. 1982;25:371-374.
271 Watanabe Y, Sato M, Tokui K, et al. Multiendoscope-assisted treatment for blue rubber bleb nevus syndrome. Surg Endosc. 2000;14:595.
272 Baskerville PA, Ackroyd JS, Browse NL. The etiology of the Klippel-Trenaunay syndrome. Ann Surg. 1985;202:624-627.
273 Wilson CL, Song LM, Chua H, et al. Bleeding from cavernous angiomatosis of the rectum in Klippel-Trenaunay syndrome: Report of three cases and literature review. Am J Gastroenterol. 2001;96:2783-2788.
274 Cha SH, Romeo MA, Neutze JA. Visceral manifestations of Klippel-Trénaunay syndrome. Radiographics. 2005;25:1694-1697.
275 Duque JM, Muñoz Navas M, Betes MT, et al. Colonic involvement in the Klippel-Trenaunay-Weber syndrome [in Spanish]. Rev Esp Enferm Dig. 2000;92:44-45.
276 Gandolfi L, Rossi A, Stasi G, et al. The Klippel-Trenaunay syndrome with colonic hemangioma. Gastrointest Endosc. 1987;33:442-445.
277 Bataller R, Sans M, Escorsell A, et al. Esophageal variceal bleeding caused by hypoplasia of the portal vein in a patient with the Klippel-Trenaunay syndrome. Am J Gastroenterol. 1998;93:275-276.
278 Brown R, Ohri SK, Ghosh P, et al. Case report: jejunal vascular malformation in Klippel-Trenaunay syndrome. Clin Radiol. 1991;44:134-136.
279 Telander RL, Kaufman BH, Gloviczki P, et al. Prognosis and management of lesions of the trunk in children with Klippel-Trenaunay syndrome. J Pediatr Surg. 1984;19:417-422.
280 Yeoman LJ, Shaw D. Computerized tomography appearances of pelvic haemangioma involving the large bowel in childhood. Pediatr Radiol. 1989;19:414-416.
281 Vazquez-Sequeiros E, Sorbi D, Kamath PS, et al. Klippel-Trenaunay-Weber syndrome: Role of EUS. Gastrointest Endosc. 2001;54:660-661.
282 Lehmann TG, Dux M, von Herbay A, et al. Klippel-Trenaunay syndrome with involvement of the rectum: Surgical therapy after interventional-radiologic preparation. Chirurg. 2000;71:228-233.
283 Myers BM. Treatment of colonic bleeding in Klippel-Trenaunay syndrome with combined partial colectomy and endoscopic laser. Dig Dis Sci. 1993;38:1351-1353.
284 Azizkhan RG. Life-threatening hematochezia from a rectosigmoid vascular malformation in Klippel-Trenaunay syndrome: Long-term palliation using an argon laser. J Pediatr Surg. 1991;26:1125-1128.
285 Garteiz Martinez D, Robledo Ogazon F, de la Fuente Lira M, et al. Rectorrhagia as complication of Klippel-Trenaunay syndrome [in Spanish]. Rev Gastroenterol Mex. 1999;64:181-185.
286 Natterer J, Joseph JM, Denys A, et al. Life-threatening rectal bleeding with Klippel-Trenaunay syndrome controlled by angiographic embolization and rectal clips. J Pediatr Gastroenterol Nutr. 2006;42:581-584.
287 Taieb S, Rolachon A, Cenni JC, et al. Effective use of argon plasma coagulation in the treatment of severe radiation proctitis. Dis Colon Rectum. 2001;44:1766-1771.
288 Deitel M, Vasic V. Major intestinal complications of radiotherapy. Am J Gastroenterol. 1979;72:65-70.
289 Cho KH, Lee CK, Levitt SH. Proctitis after conventional external radiation therapy for prostate cancer: Importance of minimizing posterior rectal dose. Radiology. 1995;195:699-703.
290 Cho LC, Antoine JE. Radiation injury to the gastrointestinal tract. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and liver disease: Pathophysiology, diagnosis, management. ed 8. Philadelphia: Saunders; 2006:813-826.
291 Kennedy M, Bruninga K, Mutlu EA, et al. Successful and sustained treatment of chronic radiation proctitis with antioxidant vitamins E and C. Am J Gastroenterol. 2001;96:1080-1084.
292 Coia LR, Myerson RJ, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31:1213-1236.
293 Gul YA, Prasannan S, Jabar FM, et al. Pharmacotherapy for chronic hemorrhagic radiation proctitis. World J Surg. 2002;26:1499-1502.
294 Lucarotti ME, Mountford RA, Bartolo DC. Surgical management of intestinal radiation injury. Dis Colon Rectum. 1991;34:865-869.
295 Tagkalidis PP, Tjandra JJ. Chronic radiation proctitis. Aust N Z J Surg. 2001;71:230-237.
296 Johnson RJ, Carrington BM. Pelvic radiation disease. Clin Radiol. 1992;45:4-12.
297 Varma JS, Smith AN, Busuttil A. Correlation of clinical and manometric abnormalities of rectal function following chronic radiation injury. Br J Surg. 1985;72:875-878.
298 Varma JS, Smith AN, Busuttil A. Function of the anal sphincters after chronic radiation injury. Gut. 1986;27:528-533.
299 Talley NA, Chen F, King D, et al. Short-chain fatty acids in the treatment of radiation proctitis: A randomized, double-blind, placebo-controlled, cross-over pilot trial. Dis Colon Rectum. 1997;40:1046-1050.
300 Sasai T, Hiraishi H, Suzuki Y, et al. Treatment of chronic post-radiation proctitis with oral administration of sucralfate. Am J Gastroenterol. 1998;93:1593-1595.
301 Wurzer H, Schafhalter-Zoppoth I, Brandstatter G, et al. Hormonal therapy in chronic radiation colitis. Am J Gastroenterol. 1998;93:2536-2538.
302 Marshall GT, Thiriby RC, Bredfeldt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
303 Dall’Era MA, Hampson NB, Hsi RA, et al. Hyperbaric oxygen therapy for radiation induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
304 Warren DC, Feehan P, Slade JB, et al. Chronic radiation proctitis treated with hyperbaric oxygen. Undersea Hyperb Med. 1997;24:181-184.
305 Woo TC, Joseph D, Oxer H. Hyperbaric oxygen treatment for radiation proctitis. Int J Radiat Oncol Biol Phys. 1997;38:619-622.
306 Jones K, Evans AW, Bristow RG, et al. Treatment of radiation proctitis with hyperbaric oxygen. Radiother Oncol. 2006;78:91-94.
307 Brown RB. A method of management of inoperable carcinoma of the bladder. Med J Aust. 1969;1:23-24.
308 Roche B, Chautems R, Marti MC. Application of formaldehyde for treatment of hemorrhagic radiation-induced proctitis. World J Surg. 1996;20:1092-1094.
309 Counter SF, Froese DP, Hart MJ. Prospective evaluation of formalin therapy for radiation proctitis. Am J Surg. 1999;177:396-398.
310 Seow-Choen F, Goh HS, Eu KW, et al. A simple and effective treatment for hemorrhagic radiation proctitis using formalin. Dis Colon Rectum. 1993;36:135-138.
311 De Parades V, Etienney I, Bauer P, et al. Formalin application in the treatment of chronic radiation-induced hemorrhagic proctitis: An effective but not risk-free procedure: A prospective study of 33 patients. Dis Colon Rectum. 2004;48:1535-1541.
312 Zinicola R, Rutter MD, Falasco G, et al. Haemorrhagic radiation proctitis: Endoscopic severity may be useful to guide therapy. Int J Colorectal Dis. 2003;18:439-444.
313 Phan J, Swanson DA, Levy LB, et al. Late rectal complications after prostate brachytherapy for localized prostate cancer. Cancer. 2009;115:1827-1839.
314 Saclarides TJ, King DG, Franklin JL, et al. Formalin instillation for refractory radiation-induced hemorrhagic proctitis: Report of 16 patients. Dis Colon Rectum. 1996;39:196-199.
315 Jensen DM, Machicado GA, Cheng S, et al. A randomized prospective study of endoscopic bipolar electrocoagulation and heater probe treatment of chronic rectal bleeding from radiation telangiectasia. Gastrointest Endosc. 1997;45:20-25.
316 Taylor JG, DiSario JA, Buchi KN. Argon laser therapy for hemorrhagic radiation proctitis: Long-term results. Gastrointest Endosc. 1993;39:641-644.
317 Barbatzas C, Spencer GM, Thorpe SM, et al. Nd:YAG laser treatment for bleeding from radiation proctitis. Endoscopy. 1996;28:497-500.
318 Taylor JG, Disario JA, Bjorkman DJ. KTP laser therapy for bleeding from chronic radiation proctopathy. Gastrointest Endosc. 2000;52:353-357.
319 Villavicencio RT, Rex D, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
320 Kaassis M, Oberti E, Burtin P, et al. Argon plasma coagulation for the treatment of hemorrhagic radiation proctitis. Endoscopy. 2000;32:673-676.
321 Coriat R, Wolfers C, Chaput U, et al. Treatment of radiation-induced rectal lesions with argon plasma coagulation: Use of a transparent cap. Endoscopy. 2008;40:E270.
322 Sebastian S, O’Connor H, O’Morain C, et al. Argon plasma coagulation as first-line treatment for chronic radiation proctopathy. J Gastroenterol Hepatol. 2004;19:1169-1173.
323 Dees J, Meijssen MA, Kuipers EJ. Argon plasma coagulation for radiation proctitis. Scand J Gastroenterol. 2006;243(Suppl):175-178.
324 Karamanolis G, Triantafyllou K, Tsiamoulos Z, et al. Argon plasma coagulation has a long-lasting therapeutic effect in patients with chronic radiation proctitis. Endoscopy. 2009;41:529-531.
325 Postgate A, Saunders B, Tjandra J, et al. Argon plasma coagulation in chronic radiation proctitis. Endoscopy. 2007;39:361-365.
326 Silva RA, Correia AJ, Dias LM, et al. Argon plasma coagulation therapy for hemorrhagic radiation proctosigmoiditis. Gastrointest Endosc. 1999;50:221-224.
327 Ben Soussan E, Mathieu N, Roque I, et al. Bowel explosion with colonic perforation during argon plasma coagulation for hemorrhagic radiation-induced proctitis. Gastrointest Endosc. 2003;57:412-413.
328 Swaroop VS, Gostout CJ. Endoscopic treatment of chronic radiation proctopathy. J Clin Gastroenterol. 1998;27:36-40.
329 Jao SW, Beart RW, Gunderson LL. Surgical treatment of radiation injuries of the colon and rectum. Am J Surg. 1986;151:272-277.
330 Cohn SM, Bickston S. Radiation injury in the gastrointestinal tract. In: Yamada T, editor. Textbook of gastroenterology. Philadelphia: Lippincott Williams & Wilkins; 2003:2760-2771.
331 Flobert C, Cellier C, Landi B, et al. Severe hemorrhagic gastritis of radiation origin. Gastroenterol Clin Biol. 1998;22:232-234.
332 Carretero C, Muñoz-Navas M, Betes M, et al. Gastroduodenal injury after radioembolization of hepatic tumors. Am J Gastroenterol. 2007;102:1216-1220.
333 Ogawa F, Mino-Kenudson M, Shimizu M, et al. Gastroduodenitis associated with yttrium 90-microsphere selective internal radiation. Arch Pathol Lab Med. 2008;132:1734-1738.
334 Grover N, Johnson A. Aminocaproic acid used to control upper gastrointestinal bleeding in radiation gastritis. Dig Dis Sci. 1997;42:982-984.
335 Kernstine KH, Greensmith JE, Johlin FC, et al. Hyperbaric oxygen treatment of hemorrhagic radiation-induced gastritis after esophagectomy. Ann Thorac Surg. 2005;80:1115-1117.
336 Morrow JB, Dumot JA, Vargo JJ. Radiation-induced hemorrhagic carditis treated with argon plasma coagulator. Gastrointest Endosc. 2000;51:498-499.
337 Shukuwa K, Kume K, Yamasaki M, et al. Argon plasma coagulation therapy for a hemorrhagic radiation-induced gastritis in patient with pancreatic cancer. Intern Med. 2007;46:975-977.
338 Wada S, Tamada K, Tomiyama T, et al. Endoscopic hemostasis for radiation-induced gastritis using argon plasma coagulation. J Gastroenterol Hepatol. 2003;18:1215-1218.
339 Yeung YP, Ho CM, Wong KH, et al. Surgical treatment of recalcitrant radiation-induced gastric erosions. Head Neck. 2000;22:303-306.
340 Sher ME, Bauer J. Radiation-induced enteropathy. Am J Gastroenterol. 1990;85:121-128.
341 Nguyen NP, Antoine JE, Dutta S, et al. Current concepts in radiation enteritis and implications for future clinical trials. Cancer. 2002;95:1151-1163.
342 Cameron AJ, Higgins JA. Linear gastric erosion: A lesion associated with large diaphragmatic hernia and chronic blood loss anemia. Gastroenterology. 1986;91:338-342.
343 Weston AP. Hiatal hernia with Cameron ulcers and erosions. Gastrointest Endosc Clin N Am. 1996;6:671-679.
344 Maganty K, Smith RL. Cameron lesions: Unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.