Chapter 34 Chronic Diseases and Wilderness Activities
For online-only figures, please go to www.expertconsult.com ![]()
Medical problems may occur during wilderness activities as a result of an acute injury, acquired infectious illness, or environmentally caused illness (heat, cold, or high-altitude illness). A preexisting medical condition may also cause complications during a trip or activity or may predispose the patient to environmentally caused illness. The few studies that report the epidemiology of medical problems in the wilderness show that most are due to traumatic injuries, and most deaths are due to falls or drowning.46,68,121 Cardiac disease is the most common medical illness that causes death in the wilderness. Cardiac disease, asthma, and diabetes are also reported as causes of medical illness during wilderness activities. Medical illness due to environmental exposure, such as heat, cold, or altitude illness, depends on the environment.46,96
Considerations for Wilderness Travel
Increased Physical Activity
Although some wilderness activities are of the sedentary variety (e.g., car-based safaris), many other activities involve physical exertion. The level of exertion may be comparable with that which the person already performs on a regular basis but may, in some cases, involve more activity than the person’s typical baseline. As a result, all wilderness travelers with underlying medical problems must consider the level of activity on their planned trip and whether they are capable of doing the work necessary for that activity. Many commercial outfitters label trips with a difficulty rating that takes into account the level of exertion, remoteness, and exposure to high altitude. Ratings generally include easy, moderate, and strenuous categories. This can be useful to the physician evaluating the patient before the trip, especially if the physician is not familiar with the environment or the activity that will be pursued, although the physician must be aware that these ratings are not based on hard criteria and what constitutes a “moderate” trip for one company may be labeled as a “strenuous” trip by another. Some companies that offer adventure trips require potential clients to complete a medical history questionnaire and may also have a consultant physician review the questionnaire and provide advice to select clients.77,78 Erb47 has developed a scale of trip difficulty and correlated that with objective parameters of exercise capacity required to complete that type of activity (see Table 101-2, Chapter 101). “Extreme-performance ventures” are the most physically demanding and require above-average exercise capacity for participation. This category would include activities such as mountaineering at high altitude and alpine climbing. “High-performance ventures,” such as high-altitude trekking or hunting, or jungle trekking are the next most demanding category of activities and require an average exercise capacity. The third level of wilderness trips, “recreational activities” requires just below-average exercise capacity and includes activities such as hiking on mild-moderate terrain in a variety of environments. The last category of wilderness trips is “therapeutic activities,” which may be appropriate for persons with chronic cardiovascular or pulmonary disease that limit activity. Even though Erb defines categories of wilderness trips and assigns objective exercise capacity parameters to them, formal cardiac stress testing or cardiopulmonary exercise testing is not required in most cases to determine if a patient has adequate exercise capacity to complete a planned wilderness trip. The most important predictor is successful completion by the patient of similar activities in the past. Still, formal cardiopulmonary exercise testing may be useful in patients with chronic medical conditions to define maximum exercise capacity objectively to select the appropriate level of a wilderness trip. This helps to ensure a more enjoyable and safe experience for patients and their trip partners.
Environmental Extremes and Chronic Medical Conditions
Cold
Heat
Susceptible individuals can improve heat tolerance through a program of heat acclimatization before or during their trip.5 Before a wilderness trip to a hot environment, exercising daily in the heat for limited periods of at least 1 hour’s duration for at least several days improves heat tolerance. If such pretrip training is not feasible, then the individual should restrict exertion to limited periods during cool parts of the day for the first week of the trip. Regardless of the acclimatization program, once a trip commences, individuals should maintain volume status through adequate intake of water or electrolyte drinks with copious clear urine output being a good indicator of adequate hydration status. Individuals engaged in prolonged bouts of exercise (lasting several hours or more) should supplement water intake with meals or salty foods to maintain electrolyte balance and prevent hyponatremia.
High Altitude
Important cardiovascular responses also occur that may not be tolerated well by all individuals. Increased sympathetic tone occurs acutely after ascent to high altitude and increases heart rate and blood pressure. Heart rate and blood pressure gradually decrease over several days at high altitude, but remain higher than sea level baseline values for the duration of stay at high altitude. Despite the increase in sympathetic tone, most persons with mild to moderate cardiovascular disease do well after ascent to moderate altitudes of approximately 2500 m (8202 feet),99,137 although individuals with unstable angina, severe cardiomyopathy, or poorly controlled hypertension might not tolerate such changes.
Chronic Medical Conditions and Wilderness Travel
Asthma
Asthma is a disorder of reversible airflow limitation marked by the presence of cough, wheezing, chest tightness, and shortness of breath. Affected individuals can have long symptom-free periods punctuated by exacerbations and worsening symptoms that are often triggered by stimuli such as respiratory infections, exercise, or allergen exposures. Given the high prevalence of the disorder in the general population, it is likely that many wilderness travelers suffer from this disorder. Despite this fact, little data are available as to how wilderness travel affects these patients. In the only prospective study of asthma patients engaged in wilderness travel, Golan and colleagues66 studied 203 patients with mild to moderate asthma presenting to a travel clinic before departure. Forty-three percent of these individuals reported an exacerbation during their trip, whereas 20% reported worsening asthma control and 16% reported the worst exacerbation of their life. The leading risk factors for exacerbations during the trips were frequent rescue inhaler use (>3 times per week) before the trip and participation in intensive physical activity during the trip itself. Pretrip exercise testing with pretest and post-test spirometry was not useful in predicting which patients would develop an exacerbation.
The first part of this evaluation is to review the state of the patient’s current symptoms and determine if the patient is on the appropriate pharmacologic regimen. The National Institutes of Health’s Guidelines for the Diagnosis and Management of Asthma118 provides definitions of categories of severity for patients with asthma and the appropriate treatment (Box 34-1 and Table 34-1). Pretrip evaluation for a patient with asthma should include a review of these guidelines in relation to the patient’s symptoms and consideration of escalating therapy before a wilderness trip. For example, a patient who usually uses inhaled bronchodilators only on an as-needed basis for mild persistent asthma may consider adding an inhaled corticosteroid for improved control, although this practice has never been formally studied for wilderness travel. Because a primary trigger for asthma on a wilderness trip may be exercise, consideration can be given to adding the leukotriene receptor blocker montelukast to the patient’s controller regimen, because this has been shown to be effective adjunctive therapy for exercise-induced asthma.97 Patients can also use short-acting β2 agonists before and during exercise.114 Individuals reporting worsening control or who are in the midst of a severe exacerbation should strongly consider postponing their trip until symptoms are under better control.
BOX 34-1
Classification of Asthma Severity in Patients 12 Years of Age and Older
Severe Persistent (Step 4 or 5 Treatment)
FEV1, Forced expiratory volume in 1 second; FVC, forced vital capacity.
Modified from National Asthma Education and Prevention Program: Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007, J Allergy Clin Immunol 120:S94, 2007.
TABLE 34-1 Stepwise Approach to Managing Asthma in Patients 12 Years of Age and Older
| Intermittent Asthma | |
| Step 1 | Short-acting β2-agonist as needed |
| Persistent Asthma | |
| Step 2 | Preferred: Low-dose inhaled corticosteroid |
| Alternative: Cromolyn, leukotriene receptor antagonist, nedocromil, or theophylline | |
| Step 3 | Preferred: Low-dose inhaled corticosteroid plus long-acting β2-agonist OR medium-dose inhaled corticosteroid |
| Alternative: Low-dose inhaled corticosteroid plus either leukotriene receptor antagonist or theophylline | |
| Step 4 | Preferred: Medium-dose inhaled corticosteroid plus long-acting β2-agonist Alterative: Medium-dose inhaled corticosteroid plus either leukotriene receptor antagonist or theophylline |
| Step 5 | Preferred: High-dose inhaled corticosteroid plus long-acting β2-agonist AND consider omalizumab for patients who have allergies |
| Step 6 | Preferred: High-dose inhaled corticosteroid plus long-acting β2-agonist plus oral corticosteroid AND consider omalizumab for patients who have allergies |
| Each step: Patient education, environmental control, and management of comorbidities. | |
| Steps 2-4: Consider subcutaneous allergen immunotherapy for patients who have allergic asthma. | |
| Quick relief of symptoms for all patients: Short acting β2-agonist as needed for symptoms. Intensity of treatment depends on severity of symptoms: up to three treatments at 20-min intervals as needed. Short course of oral systemic corticosteroids may be needed. | |
| Use of short-acting β2-agonist >2 days/wk for symptom relief (not prevention of exercise-induced bronchospasm) generally indicates inadequate control and the need to step up treatment. | |
| Step down if possible if asthma is well controlled at least 3 mo. | |
Modified from National Asthma Education and Prevention Program: Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007, J Allergy Clin Immunol 120:S94, 2007.
Another important part of the pretravel assessment is to devise a program for objectively monitoring disease status during the trip. Asthma patients commonly monitor asthma control using measurements of peak expiratory flow (PEF), an objective parameter measured after the patient inhales to total lung capacity and then forcefully exhales into a peak flow meter. Patients establish their baseline peak flows when their disease is under good control by performing the maneuver several times a day and recording the results in a diary. Typically, PEF is highest in the morning and lowest in the afternoon. The highest measured PEF becomes the baseline for the patient, and comparison of further measurements with that baseline can be used to identify disease exacerbation and therefore escalate therapy. The National Institutes of Health’s Guidelines for the Diagnosis and Management of Asthma118 recommends using a zone scheme for categorizing results of PEF: green is a PEF greater than 80% of personal best, yellow is a PEF 50% to 80% of personal best, and red is a PEF less than 50% of personal best. A PEF in the green zone indicates the patient should continue maintenance medications, whereas a PEF in the yellow or red zone requires adjustments in treatment according to a predetermined plan, as well as seeking evaluation by a physician.
An alternative to peak-flow monitoring is the PiKo-1, which measures both PEF and forced expiratory volume in 1 second (FEV1). This electronic device has a 2-year battery life and is small and easy to pack. Patients should be aware that the PiKo-1 and PEF meters, particularly variable orifice peak flow meters, may generate readings significantly lower than actual PEF under conditions of cold temperatures or high altitude.127,132 If concern exists about such problems on a trip, the individual should rely on an assessment of trends in the measured PEF rather than the absolute values.
With regard to specific types of wilderness activities, two types of activities that warrant further attention in the asthma patient are high-altitude travel and diving. The effect of high-altitude exposure on asthmatic patients has not been well studied, but available evidence suggests that patients with mild to moderate disease, well controlled at the time of their trip, can tolerate significant altitude exposure. Several studies have shown that exposure to elevations as high as 5000 m (16,404 feet) is associated with decreased bronchial hyperresponsiveness.2,32 A small study of patients with mild to moderate disease climbing Mt Kilimanjaro found a non–statistically significant improvement in PEF between 2700 and 4700 m (8858 and 15,420 feet), no difference in the incidence of acute mountain sickness or summit success compared with nonasthmatic patients, and no evidence of exacerbations during the excursion.156 Because of interindividual disease heterogeneity, however, it is difficult to draw broad conclusions that apply to all patients. In the end, how a patient fares at altitude may be a function of the particular triggers for their disease. Patients whose disease is triggered primarily by allergens may fare well at altitude,166 where, for example, the number of dust mites decreases with increasing elevation, whereas patients whose disease is triggered by breathing cold, dry air may have difficulty during mountaineering or ski excursions that include significant exposure to such conditions. Epidemiologic evidence suggests that asthma incidence is increased among cross-country skiers and ski mountaineers, athletes whose activities entail high levels of minute ventilation in cold, dry environments.42,94
The primary concern with scuba diving in patients with asthma is that active airflow obstruction could lead to air trapping and significantly increase the risk for pulmonary barotrauma with changes in barometric pressure on ascent back to the surface of the water. As discussed further in Chapter 77, asthma was previously considered an absolute contraindication to diving but is now permitted provided the patient is (1) an asymptomatic adult with a childhood history of asthma, (2) has well-controlled disease with known triggers, (3) has normal pulmonary function tests with a less than 20% change in peak expiratory flow after exercise, and (4) no evidence of cold- or exercise-induced bronchospasm.
Chronic Obstructive Pulmonary Disease
COPD is a syndrome of progressive airflow limitation caused by chronic inflammation of the airways and lung parenchyma with a prevalence of approximately 4 to 7 per 1000 persons in developed countries.6 The extent to which COPD limits an individual’s planned wilderness activities is a function of disease severity, assessment of which can be made using criteria specified by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).133 According to these guidelines, disease severity is graded based on the decrement in the patient’s postbronchodilator FEV1 of a forced vital capacity (FVC) maneuver (Table 34-2). Patients who fall in the mild disease category (FEV1 ≥80% predicted) will probably do well on a wilderness activity, provided the planned activity does not far exceed their usual exercise tolerance. Once patients meet the criteria for moderate disease (FEV1 50% to 80% predicted), careful evaluation is warranted to determine their suitability for the planned activity. Cardiopulmonary exercise testing4 should be considered to determine exercise capacity, which can then be compared with the expected level of exertion on the planned trip. Of note, patients whose disease may not appear too severe based on their pulmonary function test results may have significant air trapping during exercise that impairs pulmonary mechanics and leads to significant exercise limitation.124Patients in the severe (FEV1 30% to 50% predicted) or very severe (FEV1 ≤30% predicted) categories, or those with carbon dioxide retention or right heart failure, should be advised against any wilderness activity in which exertion above their baseline level of tolerance is expected, or if the planned trip is to a higher altitude than their current residence. Such patients may, however, tolerate car or horse-led activities such as safaris or fishing that do not require much in the way of physical exertion. In considering suitability for travel, it is important to remember that many patients with COPD have comorbid conditions, such as coronary artery disease, that may also affect their tolerance for a planned activity and that will require attention in the pretravel assessment.
TABLE 34-2 Global Initiative for Chronic Obstructive Lung Disease (GOLD) Classification of Severity of COPD and Therapy
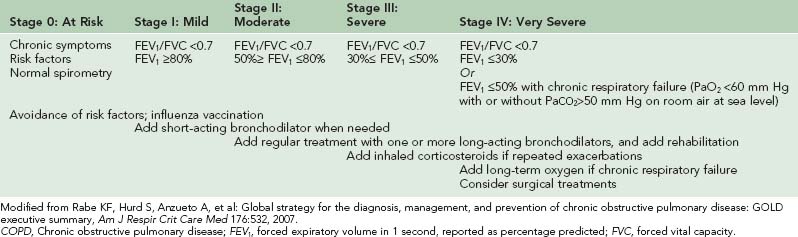
Once a decision is made that a patient can undertake a given activity, pretrip pulmonary evaluation is warranted to evaluate several important aspects of disease management around the time of the trip. The first element of this evaluation is to review the patient’s pharmacologic regimen. Inhaled bronchodilators (selective β2-agonists or anticholinergics) are the foundation of pharmacotherapy for COPD because of their capacity to alleviate symptoms, decrease frequency of disease exacerbations, and improve exercise tolerance by decreasing hyperinflation and airflow limitation.25,157 These medications have clear benefits, even in patients who may not meet official pulmonary function testing criteria for bronchodilator responsiveness (increase in FEV1 or FVC by 200 mL and 12% compared with prebronchodilator testing). As indicated in Table 34-2, patients with mild disease are typically on a regimen of short-acting bronchodilators used on an as-needed basis. The selective β2-agonist albuterol and the anticholinergic ipratropium bromide are equally effective for this purpose.157 In addition to as-needed short-acting bronchodilators, there is a need for patients with moderate disease severity to be started on scheduled long-acting bronchodilator therapy with either a long-acting β-agonist (salmeterol or formoterol) or the long-acting anticholinergic tiotropium. Patients are typically started on a single agent, but combination therapy with a β-agonist and anticholinergic can be used in those who fail to respond to monotherapy. Inhaled corticosteroids are considered for patients with poor symptom control on such therapy or frequent exacerbations, or whose disease falls in the severe or very severe categories.51
COPD patients with a baseline resting arterial partial pressure of oxygen (PaO2) less than 55 mm Hg or SpO2 less than 88% should be treated with continuous supplemental oxygen, because this has been shown to have a mortality benefit in this patient population. These patients should continue supplemental oxygen on any planned wilderness trip, whereas patients not regularly on oxygen, but whose exacerbations are associated with worsening hypoxemia, might consider supplemental oxygen for the purpose of their trip. The traditional supplemental medical oxygen delivery system is a continuous flow of 100% oxygen from a compressed gas cylinder delivered by nasal cannula. The disadvantage to this system is its inefficient use of oxygen, because only a small percentage of the oxygen delivered to the nose actually reaches the lungs. In a wilderness setting, the weight and space required to carry medical oxygen cylinders create a significant burden and limit the trip duration. A more efficient alternative is for the patient to use a pneumatic nonelectronic demand valve that delivers flow of oxygen only on inspiration.163 Portable liquid oxygen units with demand valves are an alternative to oxygen cylinders and offer the advantage of lighter weight for a comparable amount of oxygen but are more expensive. Any patient using a demand valve system for a wilderness trip should be evaluated during rest and during exercise to ensure that adequate oxygenation is maintained.162 Finally, portable oxygen concentrators are available that obviate the need for liquid or compressed gas cylinders and increase patient mobility. However, their usefulness is limited by short battery life.101 Depending on the length of the planned trip, access to power sources, or ability to carry spare batteries, they may not represent a suitable alternative.
Patients seeking to travel with supplemental oxygen need to be aware of important logistic issues that may affect their plans. For those traveling by car to their destination, there should be few problems bringing oxygen to their destination, but persons traveling by airplane may encounter significant problems. As a general rule, patients are not allowed to bring liquid or compressed gas oxygen cylinders on board aircraft as either carry-on or checked baggage. The Federal Aviation Administration permits the use of small portable oxygen concentrators on aircraft in the United States, but use may not be permitted on all airlines worldwide. Unfortunately, standard practices for supplemental oxygen vary across the airline industry, with the availability of service, feasibility of using personal systems, and the fees varying between countries, airlines, and domestic and international flights.104,172 Patients planning to obtain oxygen sources on arrival at their destination will need to confirm whether such sources are available, because access will vary based on whether the person is traveling in the developed or developing world. Even in the developed world, access to supplies might be limited in more remote settings.
Similar to asthma patients, two specific wilderness activities that deserve further attention in patients with COPD are high-altitude travel and diving. The biggest concern with regard to high-altitude travel is the potential for worsening hypoxemia. Few data are available about COPD patients in actual mountain environments, but data from the literature on COPD patients and commercial airline flights clearly indicate that patients with FEV1 values of 1 to 1.5 L experience significant hypoxemia when exposed to altitudes equivalent to 2440 m (8005 feet), with further drops in their PaO2 with minimal exertion, such as walking on flat ground or cycling at very modest work rates (20 to 30 W).101,103 Patients already using supplemental oxygen at home should increase their flow rates at high altitude and can consider portable pulse oximetry as a means to decide on the appropriate adjustment. Depending on their baseline disease severity, patients not already on supplemental oxygen should undergo pretravel assessment to determine the need for oxygen at high altitude. This can be done using either high altitude simulation testing40 or a variety of prediction rules that take into account various factors such as the PaO2 on room air at sea level, the FEV1, or the target altitude.31,67,116 Patients who develop symptomatic hypoxemia (PaO2 <50 to 55 mm Hg) during the high altitude simulation testing or who are predicted to have a PaO2 less than 50 to 55 mm Hg using one of the other prediction tools should be strongly encouraged to use supplemental oxygen at high altitude. Decisions to use oxygen should not be based on the PaO2 alone but should reflect whether or not the patient develops associated symptoms (dyspnea, light-headedness, dizziness, altered mental status, exercise intolerance). Patients who become hypoxemic (PaO2 <50 to 55 mm Hg) but remain asymptomatic with preserved exertion tolerance can travel without supplemental oxygen. They should monitor symptoms and oxygen saturation on arrival at high altitude using portable pulse oximetry or through periodic clinic visits and should carry a prescription for supplemental oxygen that can be filled at their destination if they develop problems following arrival. Patients whose PaO2 remains above these thresholds can travel without supplemental oxygen but may also consider monitoring symptoms and SpO2 during their sojourn.101
There are no data on the frequency of exacerbations at high altitude, whereas data on measures of airflow obstruction are limited and conflicting. As a result, any COPD patient traveling to high altitude must be prepared for the possibility of exacerbations as described above. There is no evidence to suggest that patients with severe bullous disease are at increased risk for pneumothorax at high altitude, despite the fall in barometric pressure.103
Sleep Apnea
Sleep-disordered breathing refers to respiratory disturbances that occur during sleep and includes entities such as obstructive sleep apnea (OSA), central sleep apnea (CSA), and sleep-related hypoventilation. OSA is the most common form of sleep-disordered breathing and is marked by the presence of repeated reductions (hypopneas) or cessation (apnea) of airflow that occur as a result of either partial or complete occlusion of the upper airway during sleep. Present in up to 28% of the general population, the disease is more common in men and among older individuals and may occur in people who lack other underlying medical problems.178 CSA is also marked by recurrent apneas or hypopneas, but, unlike in OSA, alterations in airflow occur because of changes in respiratory signaling and effort rather than upper airway occlusion. Although idiopathic forms of the disease occur, it is most frequently seen among individuals with severe cardiomyopathy. As indicated in Chapter 1, CSA is also common among otherwise healthy people at high altitude. Sleep-related hypoventilation refers to abnormally high arterial carbon dioxide tension levels during sleep and is usually seen in the context of severe obesity, obstructive lung diseases, and various neuromuscular disorders, such as muscular dystrophy or amyotrophic lateral sclerosis. The various forms of sleep-disordered breathing are significant not only because of their ability to disrupt sleep quality but also due to their adverse effects on daytime function, including, for example, excessive daytime somnolence and impaired concentration. In addition to treatments directed at the underlying disease (e.g., heart failure), the standard treatment approach for each of these disorders is nocturnal use of intermittent noninvasive positive pressure ventilation (NIPPV) or continuous positive airway pressure (CPAP). Individuals with these disorders who seek to travel into wilderness environments must consider several key issues before their trip, including (1) what will happen to the underlying disorder in that environment, (2) whether it is necessary to continue treatment while engaged in the wilderness activity, and (3) how to facilitate continued treatment if such treatment is deemed necessary. With regard to the first question, little is known about what happens to the various patterns of sleep-disordered breathing in the wilderness. There is little theoretic basis to expect changes in the incidence and severity of these problems when sleep is conducted at or near the same elevation at which the patient normally resides. Although changes in the severity of preexisting CSA following ascent to high altitude have not been studied, limited data suggest that the severity of OSA decreases considerably with ascent to high altitude. In one study of normal individuals, the OSA index fell from 5.5 + 6.9 events/hr to 0.1 + 0.3 events/hr at 5050 m (16,568 feet),22 whereas in a study of adults with moderate OSA at baseline, the obstructive respiratory disturbance index fell from 25.5 + 14.4 events/hr to 0.5 + 0.5 events/hr at 2750 m (9022 feet).21 Of note, however, was the fact that in both studies, the decrease in obstructive events was offset by marked increases in the frequency of central apneas. The reason for the observed changes was not elucidated in these studies, but they may be due to alterations in air density, increased respiratory drives, and increased upper airway tone.21 Because the various forms of sleep-disordered breathing are generally associated with intermittent nocturnal hypoxemia, it can be expected that high-altitude travel will lead to greater degrees of nocturnal desaturation, with the magnitude of hypoxemia likely being a function of the altitude attained, as well as the duration of apneas and hypopneas.
For individuals who plan to continue NIPPV or CPAP, the most important issue will be ensuring reliable access to power supplies. When such access is available (e.g., hotel, generators), travel should be relatively straightforward, because most machines are small and light enough to facilitate travel and can be brought on airplanes as carry-on luggage. Significant challenges arise, however, when power access is not available. Some manufacturers now make devices with rechargeable lithium batteries, but battery life is short, and use for more than 1 to 2 days would require access to recharging facilities or ample numbers of back-up batteries. An alternative is to obtain a special power cord from the device manufacturer that allows one to draw power off a 12-volt battery (deep cycle marine batteries offer the best battery life), but this option is limited by the size and weight of these batteries, expense, and logistic issues associated with obtaining a battery at the destination or traveling with one in hand.146 Individuals should contact the device manufacturer for information about the power consumption of their device to guide anticipated battery needs and should be aware that use of heated humidity systems will increase power needs. Finally, although most commercially available machines can run off the voltage levels used with outlets in the United States (110 to 120 V) and Europe (220 to 240 V), individuals should confirm the range for their machine and ensure that they are carrying the appropriate plug adapters.
Diabetes
Approximately 17.9 million people with a diagnosis of diabetes live in the United States (6% of the U.S. population), so it is likely that diabetes will be encountered in persons pursuing wilderness activities.119 Diabetes encompasses the disorders of type 1 diabetes, previously known as insulin-dependent or juvenile-onset diabetes, and type 2 diabetes, previously known as non–insulin-dependent or adult-onset diabetes. Approximately 5% to 10% of diabetic patients have type 1 disease, and 80% have type 2, with the remainder of cases due to other causes. The pathophysiology of type 1 diabetes results from inadequate insulin production, whereas the pathophysiology of type 2 diabetes is due to peripheral resistance to insulin action. Patients with type 1 diabetes must be treated with insulin, whereas type 2 diabetes may be treated with diet and exercise, oral and injectable hypoglycemic agents, or insulin.
A number of issues need consideration for diabetics pursuing wilderness activities. Diabetics may be remote from medical help and need to ensure that adequate medication is available. Carrying two or three times as much medication and devices (syringes, glucometer, glucose and ketone test strips) as anticipated and splitting up medication and medical device supplies among group members will mitigate against theft, loss, or unanticipated delays on longer trips (Table 34-3). Anticipate erratic meals, time changes, and increased levels of physical activity, and factor how they may change medication regimens. Engaging in nonroutine activities also creates certain safety issues for the patient with diabetes, so advising the patient on how to manage diabetes appropriately during exercise in an outdoor environment is essential. High-risk wilderness activities, such as mountaineering or rock climbing, where loss of focus and concentration may result in death, would not be appropriate for a diabetic patient who is susceptible to hypoglycemia, unless the person is constantly monitored by a climbing partner, does not climb in the lead, and is always secured by a rope. Similarly, solo wilderness activities may not be appropriate for diabetic patients who may become hypoglycemic.
| Insulin Supplies | |
| Insulin | Three times the amount anticipated for each type of insulin, stored at nonextreme temperatures |
| Insulin pens and needles (if applicable) | One extra pen and three times the anticipated number of needles |
| Pump supplies (if applicable) | Three to five times the amount anticipated |
| Syringes | Enough to cover the entire trip if on the pen or pump; two to three times the anticipated requirement if using syringes alone |
| Glucose meter | Two different meters with extra batteries for each |
| Glucose strips and lance/lancets | Three times anticipated number of strips for each meter, two lances, and three times the anticipated number of lancets; a supply of visually read strips should also be taken as a backup in the event of meter failure |
| Ketone strips | Two packages |
| Carbohydrates | |
| Dextrose tablets (rapid-acting carbohydrate) | One package (50 g) per day |
| Dried fruit and cookies (slower-acting carbohydrates) | Several individually wrapped packages per day |
| Glucagon kit (this must be protected from breakage and from freezing of the vehicle) | Two kits |
| Intravenous setup | One complete kit |
| Single-use sterile needles and syringes | Several 18-g and 10-mL syringes, respectively, in the event that medical treatment is required in a hospital or clinic with limited resources |
| Insulated packs | Enough to carry all supplies |
| Letter from physician | Listing supplies and their necessity, for international border crossings |
Note: Supplies should be packed and carried in a minimum of two independent sites (carried personally at all times by two people or by one person with the second set in a separate travel bag and/or at a nearby hotel).
Modified from Brubaker PL: Adventure travel and type 1 diabetes: The complicating effects of high altitude, Diabetes Care 28:2563, 2005.
The effect of increased exercise is important for both type 1 and type 2 diabetic patients because it may precipitate hypoglycemia or hyperglycemia, depending on the timing of the last dose of insulin and blood glucose level at the onset of exercise.70,173 The normal response to exercise in nondiabetic persons is a decrease in insulin secretion as serum glucose levels fall because of uptake of glucose by exercising muscle, and an increase in hepatic glucose release in response to catecholamines, glucagon, and growth hormone to maintain blood glucose levels. Patients with type 1 diabetes who are hypoinsulinemic when they exercise (as may occur if they excessively decrease their insulin in an effort to accommodate the increased physical activity) may become hyperglycemic because of increased hepatic release of glucose into the blood in addition to insufficient insulin to allow glucose to enter the cells. Thus hyperglycemia results in decreased exercise capacity, because exercising muscle is depleted of glucose as a result of insufficient insulin to enable glucose to enter the cells. Under these conditions, the substrate for fuel becomes free fatty acids released from adipocytes, with generation of ketone bodies by the liver. Hypovolemia may occur due to glycosuria; if the process persists, diabetic ketoacidosis may ensue.
Another factor contributing to hypoglycemia in the exercising patient with type 1 diabetes is increased exogenous insulin mobilization from subcutaneous tissue because of increased blood flow.60,85 Inadvertent intramuscular injection would exaggerate this phenomenon. It is important for patients with type 1 diabetes to administer their dose of subcutaneous insulin before exercise in a location away from exercising muscle. They should avoid injections into the arms and legs, instead using the abdomen or back of the neck. Insulin absorption is fastest and most consistent when it is injected into the abdomen. Absorption is generally slower from the arms and slowest from the thighs or buttocks, but the rate may be more inconsistent depending on the type of exercise. If the planned exercise uses primarily arm muscles, such as sport climbing, then it may be beneficial to give the insulin injection in the abdomen to avoid increased absorption from the exercising arm muscles. Likewise, if the planned activity is hiking or skiing, then it would be beneficial to use the arm or abdomen to avoid increased absorption from the exercising leg muscles. Absorption of subcutaneous insulin during exercise may also depend on the type of insulin used. A study of the long-acting insulin analog glargine, injected subcutaneously into the thigh on the evening before an intense 30-minute exercise session in patients with type 1 diabetes, did not show an increased rate of absorption, but plasma glucose fell during exercise.130
Another measure to prevent exercise-associated hypoglycemia is to reduce the dose of insulin that will be in effect during exercise.109 The best strategy for a patient with type 1 diabetes is to monitor blood glucose before, during, and after exercise to predict changes, and adjust insulin doses accordingly. This means that before a wilderness trip, the type 1 diabetic patient should exercise daily at a level of physical activity similar to that anticipated on the wilderness trip, so that adjustments in insulin dosing can be made. Differences in nutritional intake, in addition to increased exercise, during the wilderness trip should also be anticipated when planning insulin regimens. Patients with type 1 diabetes may engage in strenuous physical activity without experiencing problems, but it is important for the patient to focus on the timing of exercise in relation to meals and insulin dosing.86,159 It is also important to anticipate the nature of the exercise that will be undertaken. Long endurance activities have different implications for type 1 diabetes management (more risk for hypoglycemia) than do short bursts of high-intensity exercise (more risk for hyperglycemia).70
Another consideration is whether regular insulin or a rapidly acting insulin analog (lispro or aspart) is used for prandial dosing. Rapidly acting insulin alters the timing of exercise-related hypoglycemia. Patients who exercise early in the postprandial period (1 to 3 hours after a meal) require a decrease in the dose of rapidly acting insulin, whereas those who exercise later (3 to 5 hours) require a smaller or no change.79 A predictable exercise schedule on a wilderness trip would be useful for the patient with type 1 diabetes, although this may be difficult depending on the type of trip and the environment.
Other members of the group need to be aware of signs and symptoms of hypoglycemia in the diabetic patient and how to render appropriate treatment. This is especially important for patients with type 1 diabetes, but also is significant for patients with type 2 diabetes who are on oral hypoglycemic drugs or insulin, because this group of diabetic patients also experiences a decrease in serum glucose during exercise.131,173 Preventive measures include ingesting extra food in the form of 15 to 30 g (0.5 to 1 oz) of quickly absorbed carbohydrate (e.g., glucose tablets, whole milk, hard candies, or juice), which should be taken 15 to 30 minutes before exercise and approximately every 30 minutes during exercise.69,70 Patients are also at risk for late hypoglycemia (i.e., 4 to 8 hours after the termination of exercise) because of replenishment of depleted glycogen stores. This can usually be avoided by ingesting slowly absorbed carbohydrates (dried fruit, granola bars, or trail mix) immediately after exercise.152 Fluid intake should also be increased to ensure adequate hydration, because dehydration prolongs insulin action by decreasing insulin clearance from the blood.
Activity limitations for diabetic patients also depend on associated complications. Patients with proliferative retinopathy should avoid activities that can rapidly or explosively elevate intraocular pressure because vitreous hemorrhage may occur. Bleeding from damaged retinal capillaries may also occur during a Valsalva maneuver, while lifting heavy objects, or during a collision. An expert should do an examination for early signs of retinopathy at least once a year.41,169
Ensuring that insulin does not freeze and glucose testing equipment works properly are also important for the insulin-dependent diabetic patient in the wilderness. Strategies to ensure that insulin does not freeze include carrying the medication inside a jacket next to the body or storing the insulin in an insulated container and putting the container in the sleeping bag at night. Glucose testing equipment should be reliable, rapid, and used frequently to help identify impending hypoglycemia and increase carbohydrate ingestion before incapacitation occurs. The accuracy of glucose testing equipment can be affected by high altitude and cold. Both overestimation and underestimation of glycemia and of standard glucose control solutions have been reported for all types of glucose meters.19 Glucose meters using the oxygen-insensitive enzyme glucose dehydrogenase may perform better at high altitude than those using the enzyme glucose oxidase, but both types perform poorly at low temperatures.123 High glucose levels seem to be misreported to a greater extent at altitude than are low to normal glucose levels.19 The use of multiple meters with control glucose solutions can lend some confidence. Carrying glucose monitoring equipment next to the skin may prevent the problems associated with battery malfunction at cold temperatures.
Provided that the insulin-treated patient with diabetes has reviewed all the factors associated with a wilderness trip that may be complicated by diabetes and has chosen to undertake the preparation required and accept the risks, a treatment plan needs to be generated. The most challenging wilderness trips for maintaining glucose control in diabetic patients are those where the level of exercise is consistently increased above usual. Intensive diabetes management can be achieved with multiple daily injections of insulin or an insulin pump and numerous checks of blood glucose throughout the day (Tables 34-4 and 34-5). A standard strategy to offset the increased efficiency of insulin due to exercise is to ingest 15 to 30 g (0.5 to 1 oz) of carbohydrate for every half hour of moderate aerobic exercise. Monitoring blood glucose during periods of exercise of several hours’ duration is important.70,173 Adjusting the dose of insulin downward may be required if carbohydrate supplementation does not prevent hypoglycemia. It may be necessary to decrease the dose of insulin by 30% or more.41,109 Blood glucose should be checked more often early during a wilderness trip to assess how to balance insulin, carbohydrate intake, and exercise. It is also critical to prevent delayed hypoglycemia associated with exercise that often occurs after muscle and liver glycogen stores have been depleted and not replenished. The use of intermediate-acting insulin may need to be shifted from the afternoon or early evening to bedtime, or a long-acting insulin preparation that does not peak may be used. Adequate hydration is essential to ensure that the duration of action of insulin is not increased.
TABLE 34-4 Matching Insulin Treatment Schedules With Exercise Schedules
| Treatment Type | Advantages | Disadvantages |
|---|---|---|
| Standard: two injections, mixed intermediate- and short-acting insulins | Easy to perform | Poor match with exercise, rigid time restraints, least likely to give good metabolic control and health |
| Intensive: three or more injections a day | Better control, more flexible timing, less hypoglycemia in the evening | More frequent testing, harder to learn |
| Extended: glargine for basal plus lispro/aspart for meals | Least amount of time rigidity, most protection against hypoglycemia, excellent metabolic control | Much more effort to master and do well |
| Continuous infusion (pump) | Most flexible (no injections most days), low hypoglycemia risk overnight, best metabolic control Basal insulin infusion rate can be adjusted to accommodate increased insulin sensitivity due to increased activity |
Needs expensive device, harder to master, must remove pump for some activities; need to carry syringes in case of mechanical failure Risk for infusion site infections |
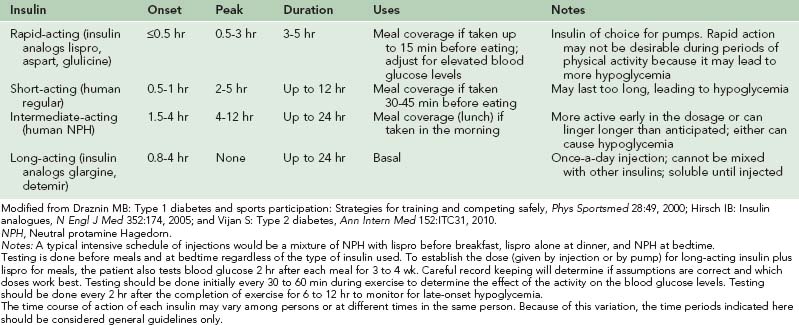
Modified from Draznin MB: Type 1 diabetes and sports participation: Strategies for training and competing safely, Phys Sportsmed 28:49, 2000.
Diabetic patients may pursue the same wilderness activities as do other individuals, provided they have the experience necessary to participate safely. This includes activities such as high-altitude mountaineering.95 On an expedition to Cho Oyu, an 8201-m (26,906-foot) peak in the Nepal Himalayas, six type 1 diabetic patients participated in the expedition and one reached the summit.125,126 These climbers were highly motivated and adhered to careful glucose monitoring, and all completed the expedition safely and free from long-term complications. Their cardiovascular parameters were comparable with those of nondiabetic control subjects on the same expedition, although the patients with diabetes did have a worsening in metabolic control and required higher insulin doses than at sea level.
Peripheral Arterial Disease
Peripheral arterial disease (PAD) is caused by atherosclerotic occlusion of the arteries of the legs. The most common symptomatic presentation is intermittent claudication, which is leg muscle discomfort on exertion that is relieved with rest. Many patients present with atypical symptoms, including leg fatigue, difficulty walking, and leg pain not typical of cluadication.175 Among men over the age of 60 years, 2% to 3% have symptomatic PAD, as do 1% to 2% of women. The prevalence of asymptomatic PAD, proved by a reduced ankle–brachial index less than or equal to 0.9 (measurement described below), is three to four times as great as that of symptomatic disease.151 Approximately 25% of patients with peripheral arterial disease have symptoms of claudication, and approximately 10% have critical ischemia.155 Because PAD is common, it may be present in persons intending to pursue wilderness activities, especially elders and those with other manifestations of systemic atherosclerosis. Severity of PAD is closely associated with the risk for myocardial infarction, ischemic stroke, and death from vascular causes. The major risk factors for PAD are older age (>40 years), cigarette smoking, and diabetes mellitus. Hyperlipidemia, hypertension, and hyperhomocysteinemia are also important risk factors.76
When evaluating a patient with PAD for a wilderness activity, it is helpful to characterize the severity of the disease and how it affects functional capacity. The most useful method for evaluating severity is the ankle-to-arm ratio of systolic blood pressure (ankle–brachial index), which is easily obtained with a standard blood pressure cuff and a Doppler device.76,175 Systolic blood pressure is measured by Doppler ultrasonography in each arm and in the dorsalis pedis and posterior tibial arteries in each ankle. The higher of the two arm pressures is selected, as is the higher of the two pressures in each ankle to calculate a left and right ankle–brachial index. An ankle-to-arm pressure ratio of 0.91 to 1.3 is normal. A ratio of 0.41 to 0.9 indicates mild to moderate peripheral arterial disease, and less than 0.4 is severe. A ratio of greater than 1.3 indicates a calcified noncompressible vessel. Patients with claudication, defined as walking-induced pain in one or both legs (primarily in the calves) that does not go away with continued walking and is relieved by rest, typically have ankle–brachial indexes in the mild to moderate range. Patients with critical ischemia have ankle–brachial indexes in the severe range.
Increased exercise is associated with wilderness activities. This may be a limiting factor for patients with peripheral vascular disease who suffer claudication. Hiking or trekking, however, are reasonable activities for patients with claudication if they engage in a regular exercise training program. The benefit of exercise in improving functional capacity in patients with claudication is well proved,73,155 and greater physical activity is associated with lower mortality.63 One meta-analysis concluded that exercise training improved pain-free walking time in patients with claudication by an average of 180% and improved maximal walking time by an average of 120%.62 Exercise at least 2 times per week, even in the presence of pain from intermittent claudication, increases walking time. Walking through the claudication pain is not harmful and increases walking distance.73 Even self-directed walking exercise programs of 3 times weekly prevent functional decline in patients with symptomatic and asymptomatic PAD.113
Exercise-induced increases in functional capacity and lessening of claudication symptoms are primarily due to improvements in endothelial vasodilator function, skeletal muscle metabolism, blood viscosity, and biomechanics of walking. Increases in leg blood flow and oxygen delivery may also occur but do not appear to account for the large improvements in exercise capacity that are observed. A supervised exercise program several times per week is recommended, but a regular self-directed exercise program can also have benefit.113 The key elements of each session include a warm-up period followed by walking on a track or treadmill at a workload that causes claudication in about 5 minutes. One continues at that workload until claudication of moderate severity occurs, then rests standing or sitting for a brief period to allow symptoms to subside, and repeats the exercise–rest pattern for the duration of the session, lasting initially approximately 30 minutes but working up to about an hour. This exercise strategy might also be used while hiking, repeating periods of exercise and rest as gauged by claudication symptoms, and could potentially lead to improvements in exercise capacity over time. A walking or hiking exercise program needs to be done regularly and sustained over months, or the benefits diminish.
Patients with PAD who wish to pursue wilderness activities or an exercise program should undergo evaluation for heart disease, hypertension, hyperlipidemia, and diabetes. Appropriate treatment and risk factor modification (including smoking cessation) should be initiated.73 In addition, an exercise test with 12-lead electrocardiographic monitoring should be performed to evaluate for cardiac ischemia. Patients with claudication may be limited by leg pain during a maximal exercise test, thus limiting stress on the heart, but information gained regarding heart rate and blood pressure response, work level at which claudication occurs, and exercise capacity is useful for estimating the type of wilderness activity that may be pursued and in formulating a prescription for exercise training.
Treatment of claudication should include a formal walking-based exercise program. In addition, several options exist for drug therapy. The Eighth ACCP Consensus Conference on Antithrombotic and Thrombolytic Therapy recommends lifelong antiplatelet therapy in patients with PAD: aspirin or clopidogrel for persons with coronary artery or cerebrovascular disease, and aspirin rather than clopidogrel for all others.151 Clopidogrel is a thienopyridine drug that inhibits platelet activation and is an alternative to aspirin, and it has fewer hematologic side effects than the related drug ticlopidine. Aspirin is significantly less expensive than clopidogrel and ticlopidine.73 Clopidogrel has U.S. Food and Drug Administration (FDA) approval for prevention of ischemic events in patients with PAD.76,151
Cilostazol and pentoxifylline are the two drugs approved in the United States for treating claudication. Cilostazol is a phosphodiesterase inhibitor that suppresses platelet aggregation and is a direct arterial vasodilator. Cilostazol improves both pain-free and maximal treadmill walking distances in patients with stable moderate to severe claudication161 and is recommended for patients with more severe disabling claudication.151 Because other oral phosphodiesterase inhibitors used for inotropic therapy have caused increased mortality in patients with advanced heart failure, cilostazol is contraindicated in heart failure of any severity. Pentoxifylline is a methylxanthine derivative that improves deformability of red cells, lowers plasma fibrinogen concentrations, and has antiplatelet effects. It is not as effective in improving walking ability as is cilostazol.36 The Eighth ACCP Consensus Conference did not recommend pentoxifylline for treatment of PAD.151
Raynaud’s Phenomenon
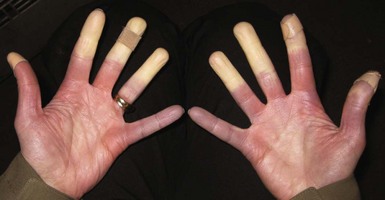
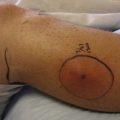
Raynaud’s phenomenon is a disorder of abnormal vasomotor control marked by the onset of pallor or cyanosis in the distal extremities due to vasospasm and decreased arterial blood flow.177 It can occur as an isolated problem (primary Raynaud’s phenomenon) or as part of collagen vascular diseases such as systemic sclerosis or systemic lupus erythematosus (secondary Raynaud’s phenomenon). Primary Raynaud’s phenomenon is more common in women than men and occurs in approximately 5% of the U.S. population.177 Raynaud’s phenomenon is an episodic phenomenon with attacks usually occurring in response to triggers such as cold (exposure of the extremity to cold or a when the patient’s body becomes cold), moisture, vibration, and emotional stress. Attacks begin with an ischemic phase marked by well-demarcated pallor that progresses to cyanosis of the digits (Figure 34-1). Changes typically start in one or several digits and spread symmetrically to all digits in the hands and/or feet, with the hands being a more common site of involvement than the feet in most individuals. These symptoms are often accompanied by pain, numbness, and burning sensations and are usually, although not always, followed by a hyperemic phase as circulation is restored to the affected areas. The classic three-phase color change occurs in approximately two-thirds of affected patients, whereas the rest have only pallor and cyanosis.14 In most cases the attack is self-limited and resolves after rewarming the hands or feet. In severe secondary Raynaud’s phenomenon, superficial ulceration or deep tissue necrosis with gangrene and amputation can occur.
Concern has also been raised about the risk for frostbite in Raynaud’s patients, because they have been shown to have lower finger temperatures with body cooling,136 slower rate of skin temperature rise with rewarming,170 and diminished cold-induced vasodilation (“hunting response”)83 when compared with healthy controls. Few studies, however, have determined whether the incidence of frostbite is increased relative to normal controls. Ervasti and colleagues49 compared Finnish military recruits with and without the disorder and found that the odds ratio for all degrees of frostbite among people with Raynaud’s phenomenon was 2.05 (95% CI, 1.72 to 2.44). The risk for “deep frostbite” (blisters, ulcers, and gangrene), however, was not different between the two groups. In another study of high-altitude travelers with primary Raynaud’s phenomenon, Luks and colleagues102 reported an incidence rate of 15%, but this study lacked a control group and the data were based on self-report rather than formal confirmation.
In addition to the issues of cold exposure in the wilderness, there is also theoretic concern that high-altitude travel could adversely affect Raynaud’s phenomenon patients. Any impairment in oxygen delivery stemming from arterial vasoconstriction might be worsened by low ambient oxygen conditions at high altitude, thereby increasing the severity or frequency of attacks and the risk for frostbite and/or gangrene. In the only study to address this question, Luks and colleagues102 surveyed 142 people with Raynaud’s phenomenon, 98% of whom had primary disease, and did not find evidence of worse problems at high altitude. Survey respondents spent 5 to 7 days per month at elevations greater than 2400 m (7874 feet) and engaged in a wide variety of activities, including winter sports (89% of respondents). Only 22% reported changing their pattern of activities in the mountains because of their disease. Of note, there was significant heterogeneity in the respondents’ perceptions of the frequency, duration, and severity of their disease at altitude, compared with their home elevation. Although the survey approach made it difficult to separate out the effects of hypobaria and cold at high altitude, this study does at least suggest that motivated individuals with primary Raynaud’s phenomenon can safely engage in many different activities, including winter sports at high elevations.
To prevent problems in cold or high-altitude environments, Raynaud’s patients should take steps to minimize the risk for serious attacks. For example, they might choose to travel in warmer climates or in the summer rather than the winter months. If winter activities are pursued, a location with a less severe winter environment can be chosen. For example, high-altitude mountaineering in South America is more equatorial and takes place in a warmer climate than does mountaineering in North America, Asia, or Europe. Appropriate cold-weather gear and clothing are essential to keep the entire body warm and help mitigate attacks. High-quality plastic mountaineering boots or ski boots and expedition-type mittens or gloves with space for disposable chemical hand warmers help in winter environments. Once on a trip, patients might avail themselves of a variety of strategies to prevent attacks. In the study by Luks and colleagues,102 for example, 89% of the respondents used multiple strategies on any given trip, with the most common tactics being wearing gloves or mittens at all times, chemical hand warmers, the use of liner gloves, and limiting exposure to moisture. Climbers with Raynaud’s phenomenon should also exercise good judgment and retreat early, before becoming overextended and exhausted. Nicotine and other drugs with peripheral vasoconstrictive effects, such as over-the-counter decongestants, should be avoided.
Patients might also consider the use of pharmacologic prophylaxis. Calcium channel blockers160 and phosphodiesterase inhibitors,24 for example, have been shown to decrease the frequency and severity of episodes of Raynaud’s phenomenon and may be used on an as-needed basis during the trip.24 Other pharmacologic agents that can be used for prevention include the α1-antagonist prazosin, the serotonin uptake inhibitor fluoxetine, the angiotensin receptor inhibitor losartan, and the direct vasodilator isoxsuprine.75,174 Calcium channel blockers are the pharmacologic agents of choice, but for those intolerant of calcium channel blockers, other agents may be tried. Benefits may also be idiosyncratic, so lack of response to calcium channel blockers is also an indication to try an alternative agent. Once an attack sets in, efforts should be made to warm the affected extremities and, in some cases, the entire body. By way of example, individuals can move to a warmer environment, don dry gloves and/or change into dry clothing, use chemical hand warmers, or place hands in warm water or on a warm surface.
Osteoarthritis
Osteoarthritis is a major cause of disability in adults and most commonly affects the joints of the hands, hips, knees, and cervical and lumbar spine. Uncommonly affected joints include the shoulder, elbow, and wrist. Factors in the evolution of osteoarthritis include initiation in either previously injured or susceptible joints; development, which is biochemically mediated and biomechanically driven; and clinical expression, which may be modified by factors such as weight and sex.34 The primary symptom of osteoarthritis is pain that is typically exacerbated by activity and relieved by rest. With more advanced disease, pain may occur with progressively less activity. Osteoarthritis can be inflammatory or noninflammatory. Patients with noninflammatory osteoarthritis complain primarily of joint pain and disability. Physical findings in affected joints include tenderness, bony prominence, and crepitus. Patients with inflammatory osteoarthritis complain of articular swelling, morning stiffness, and night pain. Signs of inflammation include joint effusion on examination or radiography, warmth on palpation of the joint, and synovitis on arthroscopic examination.
The degree of disability caused by hip or knee osteoarthritis is an important consideration for wilderness activities. Patients should be guided toward activities that are within their functional capability. Wilderness activities that cause increased weight bearing on lower extremity joints should be avoided. For example, hiking or trekking with a light pack or bicycling are recommended over activities, such as a multiday backpacking trip or mountaineering, where carrying heavy loads might be required. Risk for development of hip or knee osteoarthritis is also relevant to wilderness activities. There is increased risk for lower limb osteoarthritis associated with repetitive, high-impact sports, and that risk is increased with joint injury.34 When assessing risk for osteoarthritis from wilderness activities, the nature and intensity of the activity, presence of previous injury, and body mass index should all be considered. Recreational running does not appear to increase the risk for knee osteoarthritis,34,92 but how this applies to trail running is not known. The evidence that obesity is strongly associated with development of knee, and probably hip, osteoarthritis and that weight loss improves joint pain and function82,120 are relevant to wilderness activities where carrying heavy loads is required. Wilderness activities that repeatedly require carrying a heavy pack, or squatting and kneeling maneuvers, may cause progression of knee or hip osteoarthritis, especially if there has been previous injury to a joint or associated joint muscles, ligaments, or tendons.150
Very little information is available on the development of hand arthritis with wilderness activities such as climbing. One study suggests that rock climbing at a high standard for over 10 years may increase risk for osteoarthritis in certain joints of the hands.139
Measures that can be taken to improve functional capacity for wilderness activities include nonpharmacologic and pharmacologic treatments. The goal of management of osteoarthritis is to control pain and improve function and health-related quality of life with avoidance of therapeutic toxicity. Potential treatments include weight loss, exercise, biomechanical techniques, pharmacologic therapy, and surgery. Table 34-6 outlines a stepwise approach to management of osteoarthritis depending on severity.82
| Symptom Severity | |
|---|---|
| Nonpharmacologic Management | Mild ↓ Severe |
| Education, exercise, weight loss, appropriate footwear | |
| ↓ | |
| Further Nonpharmacologic Management | |
| Physiotherapy, braces | |
| Simple analgesics | |
| Acetaminophen | |
| ↓ | |
| Pharmacologic Management | |
| NSAIDs, opioids | |
| If effusion is present, aspirate and inject intra-articular corticosteroids | |
| ↓ | |
| Surgery | |
| Osteotomy, joint replacement |
NSAIDs, Nonsteroidal antiinflammatory drugs.
Exercise is the primary nonpharmacologic intervention for lower limb osteoarthritis that is directly related to wilderness activities. There is good evidence that strengthening and aerobic exercise can reduce pain and improve function and health status in patients with knee osteoarthritis.57 The evidence for hip osteoarthritis is not as compelling,58 but exercise is still recommended.50,138 Well-conditioned muscles and muscular balance are needed to attenuate impact loads and provide joint stability. Muscular conditioning may prevent exercise-related osteoarthritis.150 Muscular conditioning is achieved through well-designed exercise programs performed with supervision or as home exercise routines that include range-of-motion and flexibility exercise, muscle conditioning, and aerobic cardiovascular exercise.52,53,167
Biomechanical treatments for knee osteoarthritis are relevant to wilderness activities such as hiking and trekking and are helpful at reducing symptoms. For appropriate application, consultation with a physiatrist or sports medicine physician may be required. Shock-absorbing footwear reduces impact loading, heel wedges reduce loading of the medial knee joint surface, neoprene support sleeves increase proprioception and reduce feelings of instability, dynamic bracing controls lateral instability, and taping allows repositioning of the patella.52,53 Hiking poles are an additional method to help unload lower extremity joints while hiking or trekking and may be helpful for patients with osteoarthritis of the hip and knee. During downhill walking when not carrying an external load, the use of poles reduces forces on the knees,59,143 and with an external load, reduces forces on the ankles, knees, and hips.13
The major pharmacologic treatments for osteoarthritis include analgesics, nonsteroidal antiinflammatory drugs (NSAIDs), and intra-articular corticosteroids. The major goal of treatment with these agents is relief of pain, which usually is achieved with nonopioid analgesics. The nonprescription analgesic acetaminophen at doses up to 4 g/day is the recommended primary treatment.15,82,179 Patients with osteoarthritis who have mild to moderate pain will obtain a similar degree of pain relief with acetaminophen as with NSAIDs.52,53 Although it is one of the safest analgesics, acetaminophen can prolong the half-life of warfarin and can cause hepatic toxicity at therapeutic doses in patients with chronic alcohol abuse.
For patients who do not obtain adequate symptom relief with nonopioid analgesics, self-limited use of nonselective NSAIDs is an alternative after considering the risk for upper gastrointestinal and renal toxicity. Cyclooxygenase-2–selective NSAIDs are an alternative with less potential risk for gastrointestinal toxicity, but with an increased risk for serious cardiovascular events. The risk for adverse cardiovascular events may also apply to the nonselective NSAIDs, and they are recommended only for short-term use in the relief of pain12 (updated information is available at the FDA website: Rheumatology Information: Therapeutics for Osteoarthritis, http://www.fda.gov/Drugs/ResourcesForYou/HealthProfessionals/ucm106969.htm).
An alternative to NSAIDs for osteoarthritis pain not relieved by acetaminophen is the centrally acting oral analgesic tramadol. This is a synthetic opioid agonist that inhibits reuptake of norepinephrine and serotonin and has been approved by the FDA for treatment of moderate to severe pain. The efficacy of tramadol has been found to be comparable with that of ibuprofen in patients with hip and knee osteoarthritis.52,53,171
Another alternative therapy for osteoarthritis is the combination of glucosamine and chondroitin. These are compounds extracted from animal products that are absorbed by the gastrointestinal tract and appear to be capable of increasing proteoglycan synthesis in articular cartilage. One meta-analysis concluded that, despite methodologic flaws in many studies, these compounds are probably efficacious for treatment of osteoarthritis and have no significant side effects.35,82,110 Relevant to wilderness activities, glucosamine and chondroitin were effective in relieving symptoms of knee osteoarthritis in active members of the U.S. Navy diving and special warfare community.98
In persons with osteoarthritis of the hand or knee who have mild to moderate pain, use of topical analgesics, such as capsaicin cream, is appropriate as adjunctive treatment or monotherapy.35,82
Evidence supports short-term (up to 2 weeks) improvement in symptoms of osteoarthritis of the knee after intra-articular corticosteroid injection. Significant improvement may also occur for up to 16 to 24 weeks with a dose equivalent to 50 mg of prednisone.3,35,82 There is concern that multiple injections of intra-articular corticosteroids may promote disease progression; further study is required. Corticosteroid injection for knee osteoarthritis to provide benefit for the duration of a wilderness trip for up to 2 weeks seems reasonable105 and may provide enough pain relief and increase in function to make wilderness activity safer and more enjoyable.
After total joint arthroplasty, patients should be encouraged to remain physically active for general health and also for the quality of their bones. There is evidence that increased bone quality improves prosthesis fixation and decreases the incidence of early loosening. To recommend a certain activity after total knee or hip replacement, factors such as wear, joint load, intensity, and the type of prosthesis must be taken into account for each patient and sport. Because load exponentially influences the amount of wear, only activities with low joint loading, such as swimming, cycling, or walking, should be performed regularly for exercise. If an activity is performed intermittently for recreation, then activities with higher joint loads, such as skiing or hiking, may be acceptable. It is unwise to start technically demanding wilderness activities after total joint replacement because the joint loads and the risk for injuries are generally higher for these activities in unskilled individuals. Activity recommendations differ after total knee and total hip replacement. During activities such as hiking or jogging, high joint loads occur between 40 and 60 degrees of knee flexion, where many knee designs are not conforming and high stress will occur. It is prudent to be more conservative after total knee arthroplasty than after total hip arthroplasty for activities that exhibit high joint loads in knee flexion. After total knee replacement, patients should alternate activities such as walking and cycling. For mountain hiking, patients are advised to avoid descents or at least use hiking poles. Jogging or sports involving running should be discouraged after total knee replacement,89,90 although some higher-impact activity, such as tennis, may be well tolerated.147
Hematology
Anemia
The most common hematologic condition encountered is anemia. Although specific anemias have certain concerns discussed below, the universal effect of mild to moderate anemia is reduction in exercise capacity. As a rule, for every 1% fall in hematocrit maximum oxygen consumption (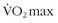 ) decreases 1% and endurance decreases by 2%.23,45,65,164 Thus anemic patients should be counseled that their ability to perform strenuous exercise will be less than that of traveling companions; they may not be able to keep up the same pace or hike as far.
) decreases 1% and endurance decreases by 2%.23,45,65,164 Thus anemic patients should be counseled that their ability to perform strenuous exercise will be less than that of traveling companions; they may not be able to keep up the same pace or hike as far.
Iron Deficiency
Iron deficiency is very common, affecting up to 40% of women and 1% to 5% of men. In athletes, especially runners, the incidence is increased to 50% to 80% of women and 10% to 17% of men.117,140 This increase in iron deficiency may reflect iron loss through gastrointestinal bleeding,26,29,111 sweat losses, urinary iron loss via hemolysis,26,72 and blockage of iron absorption by inflammation.128 Iron deficiency has multiple impacts on exercise ability. As noted above, lower hematocrit decreases exercise ability. Iron lack has a negative effect on exercise beyond the decrement in hematocrit. Studies have shown that repletion of iron improves 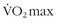 , exercise endurance, and strength, suggesting that lack of tissue stores of iron is detrimental.17,18,20,61,140 This may be due to iron deficiency first leading to loss of muscle iron stores because of the bone marrow iron demand.87 Iron deficiency has been shown to impair cold tolerance, perhaps because of alteration in thyroid hormone metabolism.7,16
, exercise endurance, and strength, suggesting that lack of tissue stores of iron is detrimental.17,18,20,61,140 This may be due to iron deficiency first leading to loss of muscle iron stores because of the bone marrow iron demand.87 Iron deficiency has been shown to impair cold tolerance, perhaps because of alteration in thyroid hormone metabolism.7,16
Going to altitude puts additional stresses on body iron stores. Although the initial increase in hematocrit at altitude is due to contraction of plasma volume, red cell production increases several days later. Studies have shown that after as little as 1 week at altitude, serum ferritin falls as iron is consumed to make more red cells.9,74,134 For example, Berglund and associates9 showed that after 10 days of breathing 14% oxygen, a 10% increase in hemoglobin was associated with a 46% decrease in ferritin.
Although serum ferritin is the best test for iron deficiency, the range of “normal” listed on the laboratory report may not be the appropriate level for athletes. Improvement can be seen in exercise ability and fatigue with the ferritin above 50 ng/mL.168 Climbers with low ferritin levels may not be able to mount an adequate hematocrit response to altitude and may have impaired exercise ability. In one study, climbers with ferritin levels above 50 to 100 ng/mL were the ones who performed the best.134 In theory, 250 mg of storage iron is required for every 1 g/dL increase in hemoglobin; that amount of iron is equivalent to 25 to 32 ng/mL of serum ferritin. It has been suggested that “ideal” hemoglobin for altitude should be 2.5 gm/dL higher than the usual range; increasing the hemoglobin by that amount would require a serum ferritin level of 62 to 80 ng/mL.9
Iron replacement therapy should be prescribed for any person with a serum ferritin under 50 ng/mL who is planning a high- or extreme-performance expedition or if serum ferritin is under 100 mg/mL in a person planning a prolonged trip to altitude. Given that 100 mg of oral iron decreases iron deficiency and improves vigor in women undergoing basic training for 9 weeks, women undergoing major treks or expeditions can consider this option.112 The best method of iron replacement is still controversial. One approach is to start with a single pill that contains at least 60 mg of elemental iron once a day; the gut cannot absorb more iron, and additional doses can lead to GI intolerance. Taking the pills with vitamin C can aid absorption. People taking iron should avoid certain foods, such as fiber and tea, within several hours of iron ingestion. Those who need iron replacement but who cannot tolerate or absorb oral iron can accomplish intravenous iron therapy.54
Glucose-6-Phosphate Dehydrogenase Deficiency
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an important cause of hereditary hemolytic syndromes with wilderness implications.10 G6PD deficiency is gender linked and most commonly affects males. The defect is in the hexose monophosphate shunt and renders the red cell unable to withstand oxidative stress. Most people with this disease have hemolysis only with such stressors as infections and intake of oxidative drugs. The two main subtypes of G6PD deficiency are African and Mediterranean. In the African type, the enzyme is unstable and older cells have diminished activity. Therefore, when these patients suffer hemolysis, it is self-limited because as the reticulocyte count increases, G6PD activity returns to normal. The Mediterranean type is caused by a defective enzyme and so tends to be more severe because with oxidative stress, G6PD activity does not keep pace with the reticulocyte count increase, and fatal hemolysis may result. G6PD deficiency is of concern because many medicines used in travel medicine can lead to sudden and severe hemolysis (Box 34-2). Many patients are unaware that they are G6PD deficient; their first symptom may be fulminant hemolysis with antimalarial drugs. In the field, acute hemolysis manifests as back pain and dark urine. Management is stopping the suspect drug and hydrating the patient. Ingestion of fava beans (an oxidative stress) in patients with the Mediterranean type of G6PD deficiency may cause severe hemolysis, which may prove fatal.
Sickle Cell Anemia and Trait
Eight percent of black patients and 0.08% to 0.5% of whites have sickle cell trait.56,107,149 Mostly clinically silent, there is a potential for problems in the wilderness. Sickling can occur in trait patients under moderately hypoxic conditions. Multiple case reports describe splenic crisis occurring at altitude or during airplane travel.56,107 Patients present with acute onset of severe left upper quadrant pain that may have a pleuritic component, nausea, and vomiting. Therapy is descent, oxygen, and pain control. Rarely in cases of large splenic infarction, splenectomy may be required.
Patients with sickle trait are more prone to dehydration due to a renal concentration defect. Maintenance of adequate fluid intake is very important. One study showed that just 45 minutes of exercise without fluids led to sickling in trait patients walking briskly in warm (33° C [91.4° F]) weather.8 Because patients cannot maximally concentrate their urine, the color of the urine cannot be used as a guide to hydration. If patients with sickle trait develop diarrhea or vomiting, very close attention should be paid to hydration status.
Concern about the risk for “sudden death” in people with sickle cell trait with strenuous exercise is valid.43,142 Data collected in the 1960s from army recruits in boot camp showed a 28-fold increased risk for death (absolute risk 1 : 3200).148 Review of these cases revealed that most patients had heatstroke, with resulting severe rhabdomyolysis causing their deaths. Practical advice for patients with sickle cell trait is to be cautious about dehydration and to avoid strenuous exercise in the heat, especially if they are deconditioned. Cramping of the legs can be an early warning sign of sickling and a sign to immediately stop exercising and begin to rehydrate.142
Hemophilia
Deficiency of factor VIII or IX occurs in 1 of 5000 males.153 Patients with severe hemophilia are at risk for severe bleeding, even with minor trauma. Bleeding most often occurs in muscles and joints, but intracranial hemorrhage is the leading cause of fatal hemorrhage. Patient can have severe arthritis because of repeated bleeding. A rough rule is that patients with less than 1% of normal levels of factor VIII or IX can have spontaneous bleeding, those with 1% to 15% of normal levels may have bleeding with minor trauma, and those with greater than 15% of normal factor levels may have bleeding with major trauma.
Factor concentrates to correct the coagulation defects are available. A suggested dosing scheme is given in Table 34-7. The World Federation of Hemophilia website is an invaluable guide to resources available for the traveler in many different countries (http://www.wfh.org/).
Hemophilia does not preclude travel or expedition travel. However, patients with hemophilia who are planning travel should take factor replacement with them, especially if they will be away from health care facilities. Only certain factors listed in Box 34-3 can be stored without refrigeration. The patient should also have the supplies necessary, such as alcohol wipes and needles, to inject the factors. At least one member of the traveling party should also be trained to infuse factor in case the patient is incapacitated.
Anticoagulation
Indications for chronic anticoagulation are increasing, especially because of recommendations for lifelong anticoagulation for idiopathic and recurrent deep vein thrombosis (DVT). Patients on warfarin and other oral anticoagulants pose special challenges.135 A varied diet, increased exertion, and potential travel-related illness can dramatically alter the level of anticoagulation. Unless the patient has access to a point-of-care international normalized ratio (INR) monitor, there may be no way to monitor the level of anticoagulation.
Well-controlled patients with stable INR can safely go several weeks without an INR check. This assumes that their diet is the same as their regular diet and there are no concurrent illnesses. For patients who plan to travel longer or for those who will be away from health care, the use of point-of-care INR monitors is advised. The patient should be very familiar with how to operate the machine and be able (or have a nomogram) to adjust their warfarin dose. A suggested nomogram is given in Table 34-8. Vitamin K tablets should be carried to allow treatment of very high INR. Another option for long-term therapy is to consider the use of low-molecular-weight (LMW) heparin injections. The dosing is weight based and does not need monitoring. The trade-off is the extra space required to pack the syringes and inconvenience of proper needle disposal. For the patient who has an absolute requirement for stable anticoagulation, use of LMW heparin is the safest alternative. In the future, availability of new oral anticoagulants that neither require monitoring nor have food or drug interactions will make management of travelers who need chronic anticoagulation much simpler.
| International Normalized Ratio (INR) | Dose Change |
|---|---|
| 1.1-1.4 | Day 1: Add 10%-20% total weekly dose (TWD)* |
| Weekly: Increase TWD by 10%-20% | |
| Return: 1 wk | |
| 1.5-1.9 | Day 1: Add 5%-10% of TWD |
| Weekly: Increase TWD by 5%-10% | |
| Return: 2 wk | |
| 2.0-3.0 | No Change |
| Return: 4 wk | |
| 3.1-3.9 | Day 1: Subtract 5%-10% TWD |
| Weekly: Reduce TWD by 10%-20% | |
| Return: 2 wk | |
| 4.0-5.0 | Day 1: No warfarin |
| Weekly: Reduce TWD by 10%-20% | |
| Return: 1 wk | |
| >5.0 | Stop warfarin until INR <3.0 |
| Decrease TWD by 20%-50% | |
| Return daily |
From DeLoughery TG: Oral anticoagulants. In Goodnight SH, Hathaway WE, editors: Disorders of hemostasis and thrombosis: A clinical guide, New York, 2001, McGraw-Hill Professional, pp 533-566. With permission.
Travel Thrombosis
Airplane travel has been considered a risk factor for DVT for over 40 years, but it is only recently that flight has been rigorously studied as a DVT risk factor. Case-controlled studies suggest a relative risk for thrombosis of threefold to fourfold with prolonged (>4 to 6 hours) travel, with a higher risk for travel times over 8 hours.1,28,84,158 The absolute risk for thrombosis is uncertain. For example, the overall risk for symptomatic pulmonary embolism is estimated to be 0.4 per million passengers, rising to 4 per million in the highest risk group. One study showed a DVT risk of 1 in 4600 following flight.88,93,129 In contrast, small prospective trials showed a calf vein thrombosis rate of 2% to 10%.144,145 The presence of risk factors, such as history of DVT or underlying hypercoagulable state, is important. Up to 70% to 90% of those with thrombosis had other risk factors for thrombosis.80,108
Pathogenesis of “traveler’s thrombosis” is controversial. Venous stasis appears to be the primary risk factor. Prolonged sitting is a risk factor for thrombosis, perhaps by increasing venous stasis. Although mild hypoxia due to the low air cabin pressure is often blamed in the popular press, there are currently no data to show activation of coagulation with mild hypoxic exposure.37 Preexisting risk factors for thrombosis are important. Most studies indicate that people who develop travel-related thrombosis have other risk factors such as history of thrombosis or estrogen use. The presence of long travel and these thrombotic risk factors raise the risk for thrombosis by 16-fold.108
The best method of prophylaxis is still to be determined. Ideally people should try to be up and exercising their legs at least once an hour. However, given crowded cabins and the security climate, this is unrealistic. Aspirin remains a popular recommendation but has been demonstrated in a randomized trial not to be effective for preventing traveler’s thrombosis and so should not be relied on for this purpose.27,64 Elastic stockings provide protection in trials but have the side effect of discomfort and superficial thrombosis.145 A single prophylactic dose of LMW heparin before the flight is effective, but this is inconvenient for most people.
A reasonable approach is to first assess a patient’s risk for thrombosis when traveling by plane for over 6 hours (Box 34-4). For most low-risk people, one could encourage foot movement and avoiding dehydration. For medium- and high-risk patients, stockings (knee-high 20 to 30 mm Hg compression pressure) should be recommended. In addition, the feasibility of adding LMW heparin on a case-by-case basis for high-risk patients should be considered.
The Asplenic Patient
Asplenic patients pose unique travel risks. Asplenic patients may have no other health problems but are at risk for overwhelming infections from a diverse group of infectious agents. The risk for overwhelming sepsis varies by indications for splenectomy but appears to be about 1%.11,91 Use of pneumococcal vaccine and recognition of this syndrome have helped lessen the risk, but travel can expose asplenic persons to novel (for them) infectious organisms.
The classic bacterial pathogen is the pneumococcus, but in older patients gram-negative bacteria are a common cause of infections.44 Unusual organisms causing overwhelming infections, such as Capnocytophaga from dog bite or babesiosis from tick bite, have been reported.38,176
Asplenic patients need to be counseled about the risk for overwhelming infections and should be vaccinated against pneumococcus, meningococcus, and Haemophilus influenzae.71,115 Patients previously vaccinated for pneumococcus should be revaccinated every 3 to 5 years.91 In addition, due to concerns about greater severity of malaria, they should be scrupulous with antimalarial prophylaxis.106,122 The role of prophylactic antibiotics is controversial for adults, but patients under the age of 18 should be on penicillin VK 250 mg orally twice a day. Patients should be advised to start antibiotics and seek medical care if they get a fever, shaking chills or lower-respiratory tract infections. A reasonable oral antibiotic for self-medicating is amoxicillin/clavulanic acid 875 mg twice a day.33 Patients who are bitten by dogs should also take antibiotics. Asplenic patients should wear an identification bracelet to alert health care providers.48
Oncology
Chemotherapy
Chemotherapy can cause a variety of long-term side effects (Box 34-5). Anthracyclines such as Adriamycin can lead to cardiac damage that may be well compensated until another stressor, such as altitude, is added. The risk for chronic heart failure is highest with doses over 300 mg/m2 but can be seen with any dose. Agents such as vincristine can lead to neuropathy that causes loss of dexterity.
Of particular concern is bleomycin, an antineoplastic agent that is part of a curative regimen for both germ cell tumors and Hodgkin’s disease. Bleomycin can lead to subtle lung damage that results in loss of diffusing capacity. For very competitive athletes with germ cell tumors, effective non–bleomycin-containing regimens can be used. Bleomycin can also interact with high concentrations of oxygen to cause fulminant lung toxicity. Fatal cases have been reported up to many months after cessation of therapy. Controversy remains as to whether patients who have had bleomycin can safely go scuba diving because of the high pressures of oxygen to which they will be exposed. For example, during a dive to 20 m (65.6 feet), the PIO2 is 0.63 atm. It will be 0.84 atm at 30 meters, potentially leading to lung toxicity.81 This notion has recently been challenged, such that diving can be considered for patients 6 months after receiving bleomycin if they did not suffer pulmonary toxicity.39 Patients who have received bleomycin should wear an identification bracelet in case emergency surgery is needed, so that the lowest possible oxygen concentration is used.
Certain agents used for hematologic malignancies, especially chronic lymphocytic leukemia, can lead to profound and prolonged T-cell immunosuppression that persists for months to years and puts patients at risk for unusual infections. These agents are fludarabine, cladribine, and alemtuzumab. The degrees of immunosuppression can be monitored by measuring CD4 counts. Patients with counts under 200/µL are severely immunocompromised, and travel is not recommended.30,141 Patients with higher CD4 counts still may have residual immunosuppression and may be prone to zoster outbreaks for years after chemotherapy.
Stem Cell (“Bone Marrow”) Transplantation
Timing of travel immunizations for transplant patients is an uncertain area.33,100 If given too soon, the immature immune system will not mount a response. Live vaccines are also a concern if immune function is not normal. An approach is given in Table 34-9.
Solid Organ Transplant Recipients
Improvements in immunosuppressive agents have decreased the incidence of rejection of transplanted organs in solid organ transplant recipients but have increased the risks for infection and malignancy.55 Newer immunosuppressive approaches, including use of sirolimus, mycophenolate mofetil, and T-cell and B-cell depletion, have largely replaced high-dose corticosteroids and azathioprine. Sources of infections include donor-derived infections, recipient-derived infections, nosocomial infections, and community-acquired infections. A predictable timeline for risk for infection is dictated by the time from transplant and the immunosuppressive regimen (Figure 34-2). In the first month after transplant donor-derived infection, recipient-derived infection, and nosocomial infection are the categories for infection risk. In the first 6 months after transplantation infectious risk is determined by whether Pneumocystis (trimethoprim-sulfamethoxazole) and antiviral prophylaxis medications (valacyclovir, high-dose acyclovir, ganciclovir, or valganciclovir) are used. After 6 months, the primary risk is community-acquired infections, some of which are “unusual” pathogens (e.g., aspergillus, atypical molds, Mucor species, Nocardia, Rhodococcus, and rare viruses).
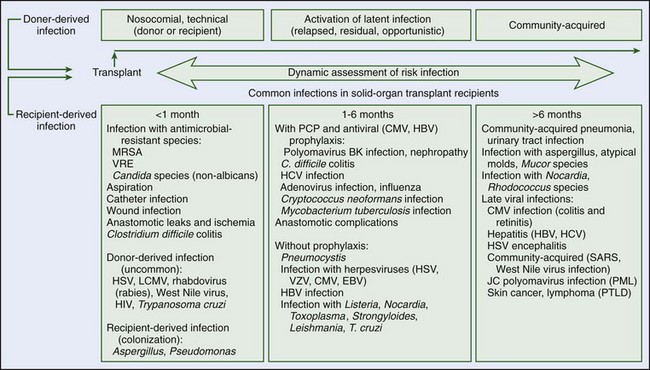
(Data from Fishman JA: Infection in solid-organ transplant recipients, N Engl J Med 357:2601, 2007.)
Wilderness activities and travel in rural areas or Third World countries can increase risk for exposure to potential infectious pathogens. Transplant recipients should wash their hands after food preparation and after contact with soil or feces. They should avoid well water and lake water (which may contain Cryptosporidium or Giardia species), undercooked meats, unwashed fruits and vegetables, and unpasteurized dairy products (which may contain Escherichia coli or Listeria monocytogenes).55 Despite these concerns, solid organ transplant recipients can travel safely outside the United States or Canada. In one study of 303 solid organ transplant recipients, 94% of whom were further than 1 year from transplant, only 24 (8%) became ill during travel.165 The most common illness was upper respiratory infection. Illness during travel was more common among those who visited high–infection risk areas (18.4% became ill during travel to Asia, Central or South America, Africa, or the Middle East), compared with low–infection risk areas (6.1% became ill). No travelers were diagnosed with “tropical” infections. Three of 24 ill travelers returned home early. Two travelers to high–infection risk areas developed transplant-related illness, one with post-transplant lymphoproliferative disease and the other with acute allograft rejection. Only 18% of travelers in this series were in rural areas, and only one traveler was camping, so risk for infection may be greater during some wilderness activities.
Vaccination is an important element of prevention of infection for transplant recipients. Immunization status should be carefully reviewed before transplant to ensure that all immunizations are up to date and any needed vaccinations administered while the patient is immunocompetent. Vaccination is generally less effective during immunosuppression. Pneumococcal vaccine is recommended every 3 to 5 years, and influenza vaccine is recommended annually. The immunologic protection from vaccines may be limited.55 Other vaccines are appropriate for patients who travel to regions where certain illnesses are endemic, and patients should seek advice from transplant and travel medicine specialists. Live vaccines are generally contraindicated after transplantation, because they may cause disseminated infection in immunocompromised hosts.55
Wilderness activities commonly require increased exercise; solid organ transplant recipients in general have lower-than-predicted exercise capacity.154 This includes recipients of heart, lung, liver, and kidney transplants. Although maximal oxygen consumption is decreased, cardiopulmonary function is appropriate for the level of work performed. Cardiac output is normal in response to exercise, oxygen desaturation does not occur, and ventilation is not a limiting factor. Anemia may play a role in decreasing oxygen-carrying capacity, although the degree of anemia is generally mild and does not explain the decrease in exercise capacity. Peripheral muscle work capacity or oxygen utilization by the tissues are proposed as causes of decreased exercise capacity. Indeed, many solid organ transplant recipients have endured long illnesses before transplant and have deconditioned muscle. Supervised exercise programs improve maximum oxygen consumption, but it still may be lower than predicted.154 Immunosuppressive drugs have also been suggested as a cause of peripheral myopathy. Despite results from studies showing decreased maximal exercise capacity in solid organ transplant recipients, clearly some are capable of high-performance athletic endeavors, including mountaineering, competitive recreational sports, and even competition at the Olympic level. Most transplant recipients return to a level of function at least as good as before transplant.
1 Adi Y, Bayliss S, Rouse A, et al. The association between air travel and deep vein thrombosis: Systematic review and meta-analysis. BMC Cardiovasc Disord. 2004;4:7.
2 Allegra L, Cogo A, Legnani D, et al. High altitude exposure reduces bronchial responsiveness to hypo-osmolar aerosol in lowland asthmatics. Eur Respir J. 1995;8:1842.
3 Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: Meta-analysis. BMJ. 2004;328:869.
4 ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211.
5 Backer H. Medical limitations to wilderness travel. Emerg Med Clin North Am. 1997;15:17.
6 Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269.
7 Beard JL, Borel MJ, Derr J. Impaired thermoregulation and thyroid function in iron-deficiency anemia. Am J Clin Nutr. 1990;52:813.
8 Bergeron MF, Cannon JG, Hall EL, et al. Erythrocyte sickling during exercise and thermal stress. Clin J Sport Med. 2004;14:354.
9 Berglund B, Gennser M, Ornhagen H, et al. Erythropoietin concentrations during 10 days of normobaric hypoxia under controlled environmental circumstances. Acta Physiol Scand. 2002;174:225.
10 Beutler E. G6PD deficiency. Blood. 1994;84:3613.
11 Bisharat N, Omari H, Lavi I, et al. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43:182.
12 Bjordal JM, Ljunggren AE, Klovning A, et al. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: Meta-analysis of randomised placebo controlled trials. BMJ. 2004;329:1317.
13 Bohne M, Abendroth-Smith J. Effects of hiking downhill using trekking poles while carrying external loads. Med Sci Sports Exerc. 2007;39:177.
14 Bowling JC, Dowd PM. Raynaud’s disease. Lancet. 2003;361:2078.
15 Bradley JD, Brandt KD, Katz BP, et al. Comparison of an antiinflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee. N Engl J Med. 1991;325:87.
16 Brigham D, Beard J. Iron and thermoregulation: A review. Crit Rev Food Sci Nutr. 1996;36:747.
17 Brownlie TIV, Utermohlen V, Hinton PS, et al. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr. 2002;75:734.
18 Brownlie TIV, Utermohlen V, Hinton PS, et al. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437.
19 Brubaker PL. Adventure travel and type 1 diabetes: The complicating effects of high altitude. Diabetes Care. 2005;28:2563.
20 Brutsaert TD, Hernandez-Cordero S, Rivera J, et al. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am J Clin Nutr. 2003;77:441.
21 Burgess KR, Cooper J, Rice A, et al. Effect of simulated altitude during sleep on moderate-severity OSA. Respirology. 2006;11:62.
22 Burgess KR, Johnson PL, Edwards N. Central and obstructive sleep apnoea during ascent to high altitude. Respirology. 2004;9:222.
23 Burtscher M. Endurance performance of the elderly mountaineer: Requirements, limitations, testing, and training. Wien Klin Wochenschr. 2004;116:703.
24 Caglayan E, Huntgeburth M, Karasch T, et al. Phosphodiesterase type 5 inhibition is a novel therapeutic option in Raynaud disease. Arch Intern Med. 2006;166:231.
25 Calverley PM, Burge PS, Spencer S, et al. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659.
26 Carlson DL, Mawdsley RH. Sports anemia: A review of the literature. Am J Sports Med. 1986;14:109.
27 Cesarone MR, Belcaro G, Nicolaides AN, et al. Venous thrombosis from air travel: The LONFLIT3 study—prevention with aspirin vs low-molecular-weight heparin (LMWH) in high-risk subjects: A randomized trial. Angiology. 2002;53:1.
28 Chandra D, Parisini E, Mozaffarian D. Meta-analysis: Travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180.
29 Chatard JC, Mujika I, Guy C, et al. Anaemia and iron deficiency in athletes: Practical recommendations for treatment. Sports Med. 1999;27:229.
30 Cheson BD. Infectious and immunosuppressive complications of purine analog therapy. J Clin Oncol. 1995;13:2431.
31 Christensen CC, Ryg MS, Refvem OK, et al. Effect of hypobaric hypoxia on blood gases in patients with restrictive lung disease. Eur Respir J. 2002;20:300.
32 Cogo A, Basnyat B, Legnani D, et al. Bronchial asthma and airway hyperresponsiveness at high altitude. Respiration. 1997;64:444.
33 Committee to Advise on Tropical Medicine and Travel (CATMAT): The immunocompromised traveller. An Advisory Committee Statement (ACS). Can Commun Dis Rep. 2007;33:1.
34 Conaghan PG. Update on osteoarthritis: I. Current concepts and the relation to exercise. Br J Sports Med. 2002;36:330.
35 Crosby J. Osteoarthritis: Managing without surgery. J Fam Pract. 2009;58:354.
36 Dawson DL, Cutler BS, Hiatt WR, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109:523.
37 DeLoughery TG, Robertson DG, Smith CA, et al. Moderate hypoxia suppresses exercise-induced procoagulant changes. Br J Haematol. 2004;125:369.
38 Deshmukh PM, Camp CJ, Rose FB, et al. Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee. Am J Med Sci. 2004;327:369.
39 de Wit R, Sleijfer S, Kaye SB, et al. Bleomycin and scuba diving: Where is the harm? Lancet Oncol. 2007;8:954.
40 Dine CJ, Kreider ME. Hypoxia altitude simulation test. Chest. 2008;133:1002.
41 Draznin MB. Type 1 diabetes and sports participation: Strategies for training and competing safely. Phys Sportsmed. 2000;28:49.
42 Durand F, Kippelen P, Ceugniet F, et al. Undiagnosed exercise-induced bronchoconstriction in ski-mountaineers. Int J Sports Med. 2005;26:233.
43 Eichner ER. Sports medicine pearls and pitfalls—sickle cell trait and athletes: Three clinical concerns. Curr Sports Med Rep. 2007;6:134.
44 Ejstrud P, Kristensen B, Hansen JB, et al. Risk and patterns of bacteraemia after splenectomy: A population-based study. Scand J Infect Dis. 2000;32:521.
45 Ekblom B, Berglund B. Effect of erythropoietin administration on maximal aerobic power. Scand J Med Sci Sports. 1991;1:88.
46 Ela GK. Epidemiology of wilderness search and rescue in New Hampshire, 1999-2001. Wilderness Environ Med. 2004;15:11.
47 Erb BD. Elders in the wilderness. In: Auerbach PS, editor. Wilderness medicine. ed 5. Philadelphia: Mosby; 2007:2072-2090.
48 Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents. 2003;21:181.
49 Ervasti O, Juopperi K, Kettunen P, et al. The occurrence of frostbite and its risk factors in young men. Int J Circumpolar Health. 2004;63:71.
50 Ettinger WHJr, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis: The Fitness Arthritis and Seniors Trial (FAST). JAMA. 1997;277:25.
51 Fabbri LM, Hurd SS. Global strategy for the diagnosis, management and prevention of COPD: 2003 Update. Eur Respir J. 2003;22:1.
52 Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: New insights: I. The disease and its risk factors. Ann Intern Med. 2000;133:635.
53 Felson DT, Lawrence RC, Hochberg MC, et al. Osteoarthritis: New insights: II. Treatment approaches. Ann Intern Med. 2000;133:726.
54 Fishbane S, Kowalski EA. The comparative safety of intravenous iron dextran, iron saccharate, and sodium ferric gluconate. Semin Dial. 2000;13:381.
55 Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601.
56 Franklin QJ, Compeggie M. Splenic syndrome in sickle cell trait: Four case presentations and a review of the literature. Mil Med. 1999;164:230.
57 Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: A metaanalysis of randomized controlled trials. J Rheumatol. 2009;36:1109.
58 Fransen M, McConnell S, Hernandez-Molina G, et al: Exercise for osteoarthritis of the hip, Cochrane Database Syst Rev: CD007912, 2009.
59 Fregly BJ, D’Lima DD, Colwell CWJr. Effective gait patterns for offloading the medial compartment of the knee. J Orthop Res. 2009;27:1016.
60 Frid A, Ostman J, Linde B. Hypoglycemia risk during exercise after intramuscular injection of insulin in thigh in IDDM. Diabetes Care. 1990;13:473.
61 Friedmann B, Weller E, Mairbaurl H, et al. Effects of iron repletion on blood volume and performance capacity in young athletes. Med Sci Sports Exerc. 2001;33:741.
62 Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975.
63 Garg PK, Tian L, Criqui MH, et al. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242.
64 Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133:381S.
65 Gledhill N, Warburton D, Jamnik V. Haemoglobin, blood volume, cardiac function, and aerobic power. Can J Appl Physiol. 1999;24:54.
66 Golan Y, Onn A, Villa Y, et al. Asthma in adventure travelers: A prospective study evaluating the occurrence and risk factors for acute exacerbations. Arch Intern Med. 2002;162:2421.
67 Gong HJr, Tashkin DP, Lee EY, et al. Hypoxia-altitude simulation test: Evaluation of patients with chronic airway obstruction. Am Rev Respir Dis. 1984;130:980.
68 Goodman T, Iserson KV, Strich H. Wilderness mortalities: A 13-year experience. Ann Emerg Med. 2001;37:279.
69 Grimm JJ, Ybarra J, Berne C, et al. A new table for prevention of hypoglycaemia during physical activity in type 1 diabetic patients. Diabetes Metab. 2004;30:465.
70 Guelfi KJ, Jones TW, Fournier PA. New insights into managing the risk of hypoglycaemia associated with intermittent high-intensity exercise in individuals with type 1 diabetes mellitus: Implications for existing guidelines. Sports Med. 2007;37:937.
71 Guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen: Working Party of the British Committee for Standards in Haematology Clinical Haematology Task Force. BMJ. 1996;312:430.
72 Hallberg L, Magnusson B. The etiology of “sports anemia:” A physiological adaptation of the oxygen-dissociation curve of hemoglobin to an unphysiological exercise load. Acta Med Scand. 1984;216:147.
73 Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. JAMA. 2006;295:547.
74 Heinicke K, Prommer N, Cajigal J, et al. Long-term exposure to intermittent hypoxia results in increased hemoglobin mass, reduced plasma volume, and elevated erythropoietin plasma levels in man. Eur J Appl Physiol. 2003;88:535.
75 Henness S, Wigley FM. Current drug therapy for scleroderma and secondary Raynaud’s phenomenon: Evidence-based review. Curr Opin Rheumatol. 2007;19:611.
76 Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608.
77 Hillebrandt D. A year’s experience as advisory doctor to a commercial mountaineering expedition company. High Alt Med Biol. 2002;3:409.
78 Hillebrandt D. Six selected cases from a year’s experience as advisory doctor to a commercial mountaineering expedition company. High Alt Med Biol. 2003;4:93.
79 Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174.
80 Hughes RJ, Hopkins RJ, Hill S, et al. Frequency of venous thromboembolism in low to moderate risk long distance air travellers: The New Zealand Air Traveller’s Thrombosis (NZATT) study. Lancet. 2003;362:2039.
81 Huls G, ten Bokkel Huinink D. Bleomycin and scuba diving: To dive or not to dive? Neth J Med. 2003;61:50.
82 Hunter DJ, Lo GH. The management of osteoarthritis: An overview and call to appropriate conservative treatment. Med Clin North Am. 2009;93:127.
83 Jobe JB, Goldman RF, Beetham WPJr. Comparison of the hunting reaction in normals and individuals with Raynaud’s disease. Aviat Space Environ Med. 1985;56:568.
84 Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: Record linkage study. BMJ. 2003;327:1072.
85 Koivisto VA, Felig P. Effects of leg exercise on insulin absorption in diabetic patients. N Engl J Med. 1978;298:79.
86 Koivisto VA, Sane T, Fyhrquist F, et al. Fuel and fluid homeostasis during long-term exercise in healthy subjects and type I diabetic patients. Diabetes Care. 1992;15:1736.
87 Kuchulakanti PK, Chu WW, Torguson R, et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation. 2006;113:1108.
88 Kuipers S, Cannegieter SC, Middeldorp S, et al. The absolute risk of venous thrombosis after air travel: A cohort study of 8,755 employees of international organisations. PLoS Med. 2007;4:e290.
89 Kuster MS. Exercise recommendations after total joint replacement: A review of the current literature and proposal of scientifically based guidelines. Sports Med. 2002;32:433.
90 Kuster MS, Spalinger E, Blanksby BA, et al. Endurance sports after total knee replacement: A biomechanical investigation. Med Sci Sports Exerc. 2000;32:721.
91 Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med. 2004;255:664.
92 Lane NE, Oehlert JW, Bloch DA, et al. The relationship of running to osteoarthritis of the knee and hip and bone mineral density of the lumbar spine: A 9 year longitudinal study. J Rheumatol. 1998;25:334.
93 Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med. 2001;345:779.
94 Larsson K, Ohlsen P, Larsson L, et al. High prevalence of asthma in cross country skiers. BMJ. 1993;307:1326.
95 Leal C. Going high with type 1 diabetes. High Alt Med Biol. 2005;6:14.
96 Leemon D, Schimelpfenig T. Wilderness injury, illness, and evacuation: National Outdoor Leadership School’s incident profiles, 1999-2002. Wilderness Environ Med. 2003;14:174.
97 Leff JA, Busse WW, Pearlman D, et al. Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339:147.
98 Leffler CT, Philippi AF, Leffler SG, et al. Glucosamine, chondroitin, and manganese ascorbate for degenerative joint disease of the knee or low back: A randomized, double-blind, placebo-controlled pilot study. Mil Med. 1999;164:85.
99 Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: The Tenth Mountain Division study. Circulation. 1997;96:1224.
100 Ljungman P, Cordonnier C, Einsele H, et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44:521.
101 Luks AM. Do lung disease patients need supplemental oxygen at high altitude? High Alt Med Biol. 2009;10:321.
102 Luks AM, Grissom CK, Jean D, et al. Can people with Raynaud’s phenomenon travel to high altitude? Wilderness Environ Med. 2009;20:129.
103 Luks AM, Swenson ER. Travel to high altitude with pre-existing lung disease. Eur Respir J. 2007;29:770.
104 Lyznicki JM, Williams MA, Deitchman SD, et al. Medical oxygen and air travel. Aviat Space Environ Med. 2000;71:827.
105 MacAuley D. Managing osteoarthritis of the knee. BMJ. 2004;329:1300.
106 Magill AJ. The prevention of malaria. Prim Care. 2002;29:815.
107 Mahony BS, Githens JH. Sickling crises and altitude: Occurrence in the Colorado patient population. Clin Pediatr (Phila). 1979;18:431.
108 Martinelli I, Taioli E, Battaglioli T, et al. Risk of venous thromboembolism after air travel: Interaction with thrombophilia and oral contraceptives. Arch Intern Med. 2003;163:2771.
109 Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Glucose response to intense aerobic exercise in type 1 diabetes: Maintenance of near euglycemia despite a drastic decrease in insulin dose. Diabetes Care. 2003;26:1316.
110 McAlindon TE, LaValley MP, Gulin JP, et al. Glucosamine and chondroitin for treatment of osteoarthritis: A systematic quality assessment and meta-analysis. JAMA. 2000;283:1469.
111 McCabe MEIII, Peura DA, Kadakia SC, et al. Gastrointestinal blood loss associated with running a marathon. Dig Dis Sci. 1986;31:1229.
112 McClung JP, Karl JP, Cable SJ, et al. Longitudinal decrements in iron status during military training in female soldiers. Br J Nutr. 2009;102:605.
113 McDermott MM, Liu K, Ferrucci L, et al. Physical performance in peripheral arterial disease: A slower rate of decline in patients who walk more. Ann Intern Med. 2006;144:10.
114 McFadden ERJr, Gilbert IA. Exercise-induced asthma. N Engl J Med. 1994;330:1362.
115 Mileno MD, Bia FJ. The compromised traveler. Infect Dis Clin North Am. 1998;12:369.
116 Muhm JM. Predicted arterial oxygenation at commercial aircraft cabin altitudes. Aviat Space Environ Med. 2004;75:905.
117 Nachtigall D, Nielsen P, Fischer R, et al. Iron deficiency in distance runners: A reinvestigation using Fe-labelling and non-invasive liver iron quantification. Int J Sports Med. 1996;17:473.
118 National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94.
119 National Diabetes Statistics, http://diabetes.niddk.nih.gov/dm/pubs/statistics/#allages, 2011.
120 Nevitt MC. Obesity outcomes in disease management: Clinical outcomes for osteoarthritis. Obes Res. 2002;10:33S.
121 Newman LM, Diekema DS, Shubkin CD, et al. Pediatric wilderness recreational deaths in western Washington State. Ann Emerg Med. 1998;32:687.
122 Newman RD, Parise ME, Barber AM, et al. Malaria-related deaths among U.S. travelers, 1963-2001. Ann Intern Med. 2004;141:547.
123 Oberg D, Ostenson CG. Performance of glucose dehydrogenase-and glucose oxidase-based blood glucose meters at high altitude and low temperature. Diabetes Care. 2005;28:1261.
124 O’Donnell DE, D’Arsigny C, Fitzpatrick M, et al. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: The role of lung hyperinflation. Am J Respir Crit Care Med. 2002;166:663.
125 Pavan P, Sarto P, Merlo L, et al. Extreme altitude mountaineering and type 1 diabetes: The Cho Oyu alpinisti in Alta Quota expedition. Diabetes Care. 2003;26:3196.
126 Pavan P, Sarto P, Merlo L, et al. Metabolic and cardiovascular parameters in type 1 diabetes at extreme altitude. Med Sci Sports Exerc. 2004;36:1283.
127 Pedersen OF, Miller MR, Sigsgaard T, et al. Portable peak flow meters: Physical characteristics, influence of temperature, altitude, and humidity. Eur Respir J. 1994;7:991.
128 Peeling P, Dawson B, Goodman C, et al. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol. 2008;103:381.
129 Perez-Rodriguez E, Jimenez D, Diaz G, et al. Incidence of air travel-related pulmonary embolism at the Madrid-Barajas airport. Arch Intern Med. 2003;163:2766.
130 Peter R, Luzio SD, Dunseath G, et al. Effects of exercise on the absorption of insulin glargine in patients with type 1 diabetes. Diabetes Care. 2005;28:560.
131 Poirier P, Tremblay A, Catellier C, et al. Impact of time interval from the last meal on glucose response to exercise in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2000;85:2860.
132 Pollard AJ, Mason NP, Barry PW, et al. Effect of altitude on spirometric parameters and the performance of peak flow meters. Thorax. 1996;51:175.
133 Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532.
134 Richalet JP, Souberbielle JC, Antezana AM, et al. Control of erythropoiesis in humans during prolonged exposure to the altitude of 6,542 m. Am J Physiol. 1994;266:R756.
135 Ringwald J, Strobel J, Eckstein R. Travel and oral anticoagulation. J Travel Med. 2009;16:276.
136 Rissanen S, Hassi J, Juopperi K, et al. Effects of whole body cooling on sensory perception and manual performance in subjects with Raynaud’s phenomenon. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:749.
137 Roach RC, Houston CS, Honigman B, et al. How well do older persons tolerate moderate altitude? West J Med. 1995;162:32.
138 Roddy E, Zhang W, Doherty M, et al. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee: The MOVE consensus. Rheumatology (Oxford). 2005;44:67.
139 Rohrbough JT, Mudge MK, Schilling RC, et al. Radiographic osteoarthritis in the hands of rock climbers. Am J Orthop (Belle Mead NJ). 1998;27:734.
140 Rowland TW, Deisroth MB, Green GM, et al. The effect of iron therapy on the exercise capacity of nonanemic iron-deficient adolescent runners. Am J Dis Child. 1988;142:165.
141 Samonis G, Kontoyiannis DP. Infectious complications of purine analog therapy. Curr Opin Infect Dis. 2001;14:409.
142 Sanchez CE, Jordan KM. Exertional sickness. Am J Med. 2010;123:27.
143 Schwameder H, Roithner R, Muller E, et al. Knee joint forces during downhill walking with hiking poles. J Sports Sci. 1999;17:969.
144 Schwarz T, Siegert G, Oettler W, et al. Venous thrombosis after long-haul flights. Arch Intern Med. 2003;163:2759.
145 Scurr JH, Machin SJ, Bailey-King S, et al. Frequency and prevention of symptomless deep-vein thrombosis in long-haul flights: A randomised trial. Lancet. 2001;357:1485.
146 Senske A. Battery powered CPAP machines. http://www.cpap-supply.com/Articles.asp?ID=146, 2007.
147 Seyler TM, Mont MA, Ragland PS, et al. Sports activity after total hip and knee arthroplasty: Specific recommendations concerning tennis. Sports Med. 2006;36:571.
148 Shaskey DJ, Green GA. Sports haematology. Sports Med. 2000;29:27.
149 Shepherd JE, Grabenstein JD. Immunizations for high-risk populations. J Am Pharm Assoc (Wash). 2001;41:839.
150 Shrier I. Muscle dysfunction versus wear and tear as a cause of exercise related osteoarthritis: An epidemiological update. Br J Sports Med. 2004;38:526.
151 Sobel M, Verhaeghe R. Antithrombotic therapy for peripheral artery occlusive disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:815S.
152 Soo K, Furler SM, Samaras K, et al. Glycemic responses to exercise in IDDM after simple and complex carbohydrate supplementation. Diabetes Care. 1996;19:575.
153 Soucie JM, Nuss R, Evatt B, et al. Mortality among males with hemophilia: Relations with source of medical care: The Hemophilia Surveillance System Project Investigators. Blood. 2000;96:437.
154 Stephenson AL, Yoshida EM, Abboud RT, et al. Impaired exercise performance after successful liver transplantation. Transplantation. 2001;72:1161.
155 Stewart KJ, Hiatt WR, Regensteiner JG, et al. Exercise training for claudication. N Engl J Med. 2002;347:1941.
156 Stokes S, Kalson N, Earl M, et al. Bronchial asthma on Mount Kilimanjaro is not a disadvantage. Thorax. 2008;63:936.
157 Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2689.
158 Tasker A, Akinola O, Cohen AT. Review of venous thromboembolism associated with air travel. Travel Med Infect Dis. 2004;2:75.
159 Temple MY, Bar-Or O, Riddell MC. The reliability and repeatability of the blood glucose response to prolonged exercise in adolescent boys with IDDM. Diabetes Care. 1995;18:326.
160 Thompson AE, Pope JE. Calcium channel blockers for primary Raynaud’s phenomenon: A meta-analysis. Rheumatology (Oxford). 2005;44:145.
161 Thompson PD, Zimet R, Forbes WP, et al. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. Am J Cardiol. 2002;90:1314.
162 Tiep BL, Barnett J, Schiffman G, et al. Maintaining oxygenation via demand oxygen delivery during rest and exercise. Respir Care. 2002;47:887.
163 Tiep BL, Carter R. Oxygen conserving devices. Waltham, Mass; 2008.
164 Tufts DA, Haas JD, Beard JL, et al. Distribution of hemoglobin and functional consequences of anemia in adult males at high altitude. Am J Clin Nutr. 1985;42:1.
165 Uslan DZ, Patel R, Virk A. International travel and exposure risks in solid-organ transplant recipients. Transplantation. 2008;86:407.
166 Valletta EA, Comis A, Del Col G, et al. Peak expiratory flow variation and bronchial hyperresponsiveness in asthmatic children during periods of antigen avoidance and reexposure. Allergy. 1995;50:366.
167 van Gool CH, Penninx BW, Kempen GI, et al. Effects of exercise adherence on physical function among overweight older adults with knee osteoarthritis. Arthritis Rheum. 2005;53:24.
168 Verdon F, Burnand B, Stubi CL, et al. Iron supplementation for unexplained fatigue in non-anaemic women: Double blind randomised placebo controlled trial. BMJ. 2003;326:1124.
169 Vijan S. Type 2 diabetes. Ann Intern Med. 2010;152:ITC31.
170 Virokannas H, Rintamaki H. Finger blood pressure and rewarming rate for screening and diagnosis of Raynaud’s phenomenon in workers exposed to vibration. Br J Ind Med. 1991;48:480.
171 Vorsanger G, Xiang J, Jordan D, et al. Post hoc analysis of a randomized, double-blind, placebo-controlled efficacy and tolerability study of tramadol extended release for the treatment of osteoarthritis pain in geriatric patients. Clin Ther. 2007;29:2520.
172 Walker J, Kelly PT, Beckert L. Airline policy for passengers requiring supplemental in-flight oxygen. Respirology. 2009;14:589.
173 Weltman NY, Saliba SA, Barrett EJ, et al. The use of exercise in the management of type 1 and type 2 diabetes. Clin Sports Med. 2009;28:423.
174 Wesseling H, den Heeten A, Wouda AA. Sublingual and oral isoxsuprine in patients with Raynaud’s phenomenon. Eur J Clin Pharmacol. 1981;20:329.
175 White C. Clinical practice: Intermittent claudication. N Engl J Med. 2007;356:1241.
176 White DJ, Talarico J, Chang HG, et al. Human babesiosis in New York State: Review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158:2149.
177 Wigley FM. Clinical practice: Raynaud’s phenomenon. N Engl J Med. 2002;347:1001.
178 Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217.
179 Zhang W, Jones A, Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? A meta-analysis of randomised controlled trials. Ann Rheum Dis. 2004;63:901.