CHAPTER 18 Chondrocyte Transplantation Techniques
Developing concepts and methods that can restore hyaline articular cartilage has been the goal of researchers and clinicians for centuries.1 In 1994, Brittberg and coworkers2 reported on the first successful use of autologous chondrocytes in human subjects with full-thickness articular cartilage lesions of the knee using a two-step implantation technique. Since its first description, chondrocyte transplantation has been performed in more than 15,000 patients worldwide and many research studies have clinically validated this surgical technique as a reliable method for biologic restoration of hyaline-like articular cartilage.3–13 Based on the evolution and better understanding of the treatment of articular cartilage defects and cell-based implantation techniques in recent years, this demanding surgical technique has undergone several technical modifications. These recent developments can help reduce patient morbidity and technique-specific complications. Chondrocyte implantation techniques continue to evolve using modern tissue engineering technologies.
PATIENT EVALUATION
Diagnostic Imaging
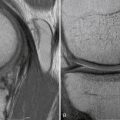
Plain radiographs, including weight-bearing anteroposterior (AP) and lateral views, Rosenberg and tunnel views, long-leg films, and Merchant views, can help identify osteochondral lesions, joint space narrowing, patellar maltracking, or lower extremity malalignment. Cartilage-sensitive magnetic resonance imaging (MRI) presents a sensitive, specific, and accurate tool for noninvasive diagnosis of articular cartilage injury.14,15 Images should be obtained in three planes; using fast spin-echo imaging with a repetition time (TR) of 3500 to 5000 msec and moderate echo time (TE) provides high contrast resolution among articular cartilage, subchondral bone, and joint fluid. Cartilage-sensitive MRI provides useful information about meniscal and ligamentous status, subchondral bone, lesion size, and depth Because of the pathologic changes in the surrounding cartilage, the final size of the defect intraoperatively is usually larger than the defect size measured on preoperative MRI. In addition to its use for preoperative diagnosis, cartilage-sensitive MRI can be helpful for postoperative evaluation of cartilage repair. Routine postoperative MRI is recommended to evaluate the cartilage repair tissue and detect potential complications, such as graft hypertrophy. If available, newer technologies, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), and T1 rho relaxation mapping, can be used to obtain additional details about cartilage morphology pre- and postoperatively.15
TREATMENT
Indications and Contraindications
Chondrocyte implantation is indicated for symptomatic, high-grade chondral and osteochondral defects of the knee in active patients who are physiologically too young for arthroplasty. This technique can be successfully used as a first-line treatment of femoral condylar, trochlear, and patellar lesions larger than 2 cm2, or used as a revision technique for failed prior cartilage repair procedures.8,11–13 The technique can be successfully used for isolated, multiple, and kissing cartilage defects. Prerequisites for successful autologous chondrocyte transplantation include adequate range of motion, appropriate axial alignment or patellar tracking, ligamentous stability, and the ability to comply with the postoperative rehabilitation protocol. Adjuvant procedures may be indicated to correct coexisting knee pathology; these can be performed simultaneously with chondrocyte transplantation without negative effects of the complex procedures on the postoperative functional outcome and activity level.
Arthroscopic Technique
Autologous chondrocyte transplantation of the knee is carried out as a two-stage procedure.
Stage 1: Chondrocyte Harvest
Step 1. Arthroscopic Evaluation.
The first stage involves a thorough diagnostic arthroscopy. In addition to identification of the known articular cartilage defect, the meniscal and ligamentous status is evaluated and the entire articular cartilage is inspected to rule out any additional cartilage defects. Partial meniscectomy or meniscal repair can be performed at the time of this arthroscopy because it minimally affects the course after the initial arthroscopy and can decrease operative time during the second-stage procedure. However, surgical treatment of meniscal deficiency or ligamentous pathology should be performed simultaneously with autologous chondrocyte implantation to avoid repetitive operative joint trauma and prolonged rehabilitation. During the first-stage arthroscopy, the cartilage defect is identified and existing cartilage flaps are débrided back to a stable and healthy peripheral margin. Following thorough débridement, the size of the articular cartilage lesion is measured and its containment documented. The knee is taken through a full range of motion and the arc of motion during which the lesion articulates with the opposing joint surface is carefully recorded (Fig. 18-1). The opposing joint surface should always be carefully inspected for the presence of a kissing lesion or signs of low grade cartilage injury.
Stage 2: Chondrocyte Implantation
Step 1. Addressing Concomitant Pathology.
Malalignment, ligamentous instability, and/or meniscal injury and deficiency are known to contribute to the development of articular cartilage lesions and surgically addressing these concomitant pathologies is critical for an effective and durable articular cartilage repair.11–13 Concomitant ligament reconstruction, meniscal allograft, or osteotomy should be performed before autologous chondrocyte implantation. Isolated or combined adjuvant procedures, including anterior cruciate ligament (ACL) reconstruction, high tibial osteotomy, or meniscal allograft and repair do not negatively affect postoperative activity levels after autologous chondrocyte transplantation.9 Simultaneous rather than staged adjuvant procedures avoid prolonged rehabilitation, promote postoperative activity, and provide a significant cost benefit.
Step 2. Chondrocyte Implantation.
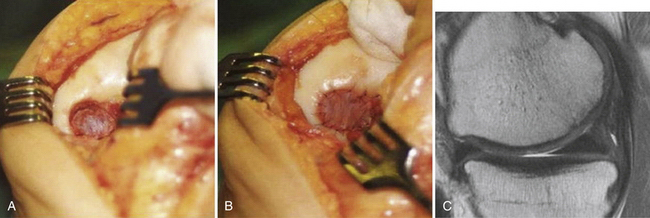
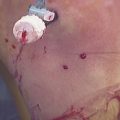
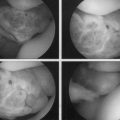
Chondrocyte implantation is performed using a parapatellar arthrotomy in a tourniquet-controlled bloodless field. The cartilage defect is again débrided back to a healthy cartilage margin and the calcified cartilage is carefully débrided without violating the subchondral bone using a ring curette (Fig. 18-2A). If bleeding is encountered, hemostasis of the defect bed can be achieved by application of thrombin or fibrin glue. Intralesional osteophytes can be débrided using a 4-mm burr without compromising the subchondral bone. A template of the defect is created with sterile aluminum foil or paper and then used to harvest an appropriately sized periosteal flap from the proximal medial border of the tibia. When using periosteum, the tissue patch should be slightly larger than the defect because of a tendency of the periosteum to contract. Any adherent fatty or connective tissue needs to be carefully removed to minimize the potential for graft hypertrophy. The periosteal flap is then sutured flush to the articular cartilage defect using interrupted 6-0 Vicryl sutures (Ethicon; Somerville, NJ), with the cambium layer facing into the defect (see Fig. 18-2B). As an alternative to the periosteum, a type I or III collagen membrane can be used to cover the defect (Fig. 18-3). This allows for smaller incisions, reduces patient morbidity, and minimizes the potential for graft hypertrophy. The rim of the periosteal flap or collagen membrane is sealed watertight with fibrin glue (Tisseel; Baxter; Deerfield, Ill) except for one corner, where the implanted chondrocytes are injected into the defect. Following cell injection, the remaining corner of the periosteal flap is secured with sutures and sealed with fibrin glue. If a tourniquet was used, it should be released and meticulous hemostasis should be obtained. Drains are not normally used so as to avoid injury to the periosteal cover or fibrin seal of the defect from direct abrasion by the drain during postoperative joint mobilization. A compression dressing is placed and cryotherapy used routinely. Bracing is not necessary for isolated chondrocyte transplantations but may be used if simultaneous adjuvant procedures require postoperative protection.
Deep Chondral or Osteochondral Defects
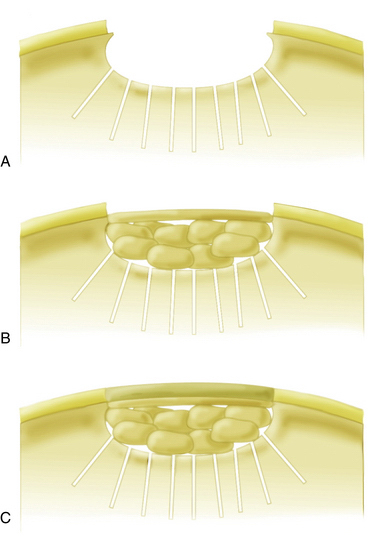
In patients with lesions deeper than 1 cm, autologous bone grafting should be performed in combination with autologous chondrocyte implantation (ACI). Implantation of chondrocytes in these cases is performed using the sandwich technique. The osseous defect is filled with cancellous bone graft from the iliac crest or proximal tibia to the level of the subchondral plate. One periosteal flap or collagen membrane sized for the defect is then anchored with the facing toward the joint using fibrin glue applied between the patch and bone graft. A second sized periosteal flap or membrane is then placed facing into the defect and secured to the surrounding cartilage with interrupted 6-0 Vicryl sutures. The rim is again sealed with fibrin glue and the cultured chondrocytes are implanted between the two periosteal flaps or collagen membranes (sandwich technique; Fig. 18-4).
PEARLS& PITFALLS
PEARLS
Postoperative Rehabilitation Protocol
Postoperative management varies, depending on the lesion location, size, and concomitant procedures. Rehabilitation is based on the biology of the cartilage repair tissue. During the initial proliferation phase (0 to 4 weeks), the developing repair cartilage tissue requires protection and weight bearing is avoided in tibiofemoral defects. Patients with patellofemoral cartilage lesions immediately start weight bearing in a knee brace locked in full extension. Continuous passive motion (CPM) used for 6 hours/day for 4 weeks should be implemented. Closed kinetic chain exercises such as stationary biking are allowed at 2 weeks; limited arc-strengthening exercises are initiated with range of motion modifications based on the location of the lesion and patellar contact pattern observed at the time of surgery. In the second matrix production phase (4 to 8 weeks), weight bearing is advanced by 25% per week depending on lesion size, location, and the patient’s symptoms. Pain and swelling indicate too rapid progression of rehabilitative exercises and should be reduced. The third maturation phase is characterized by low-impact activities and advanced based on the patient’s symptoms. Running is usually allowed at about 6 to 8 months after chondrocyte transplantation while cutting and pivoting exercises are avoided until 8 to 10 months postoperatively. Early weight bearing has been shown to promote postoperative function.18 Magnetic resonance imaging (MRI) is recommended before return to high-impact pivoting activities (see Fig. 18-2C).
COMPLICATIONS
Complications after chondrocyte transplantation include stiffness and adhesions in up to 10% of patients. Graft hypertrophy is seen in 25% to 63% of postoperative MRI images but has been described as clinically symptomatic in only 13% to 15%.2–15 Symptomatic hypertrophy can be effectively treated by arthroscopic chondroplasty. Hypertrophy may lead to partial detachment or graft delamination. Substituting the periosteum with collagen membranes or second-generation MACI effectively reduces the risk for periosteal hypertrophy.19,20 Graft failure has been described in 6% to 7% of patients. Grafts usually fail between 12 and 24 months after surgery and frequently show central degeneration. Treatment with revision chondrocyte implantation has been shown to be effective in many cases.13 All patients with graft failure should be carefully evaluated for the presence of subtle instability, axial malalignment, or patellar maltracking, which have been shown to lead to lower success rates after autologous chondrocyte transplantation.
OUTCOMES
Autologous chondrocyte transplantation has been successfully used for hyaline-like restoration of full-thickness articular cartilage lesions in the knee with long-term durability of functional improvement of up to 11 years.11,12 Good to excellent results were found in 92% of isolated femoral condyle lesions, 89% of osteochondritis dissecans, 75% of femoral condyle lesions with concomitant anterior cruciate ligament reconstruction, 67% of patients with multiple lesions, and 65% of patellar lesions.2–13 Return to sport is possible in 33% to 96% of patients, with the best return rates in competitive and adolescent athletes. Patients with single lesions, younger age, and short preoperative intervals have the best results.9,10 Accelerated weight bearing can improve long-term functional results.18 Correcting axial malalignment, patellar maltracking, and ligamentous laxity is critical for the functional improvement.
CONCLUSIONS
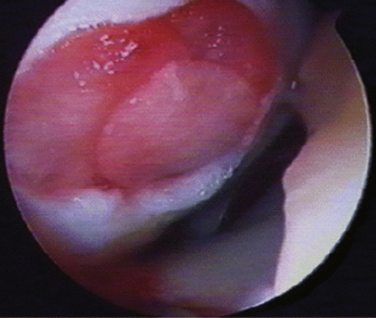
Scaffold-associated second-generation autologous cartilage transplantation techniques use three-dimensional biodegradable scaffolds to support the chondrocytes temporarily until they are replaced by matrix components synthesized from the implanted cells. MACI has been used with promising results in Europe and Australia but is not yet routinely available in the United States.19,20 The use of the biomatrix seeded with chondrocytes reduces surgical invasiveness and has the theoretic advantages of less chondrocyte leakage, more homogeneous chondrocyte distribution, and less graft hypertrophy. Arthroscopic MACI has been described, with improvement of knee function in up to 90% (Fig. 18-5).
Future developments are aimed at improving cellular matrix production by using more productive characterized chondrocytes with more sophisticated bioactive scaffolds that include growth factors and stimulate a more natural spatial distribution of chondrocytes within the repair cartilage.21–23 Other promising future approaches include identification and selective expansion of specific chondrocytes subpopulations capable of producing more hyaline-like repair cartilage tissue and implantation of neocartilage tissue produced in specifically designed bioreactors.24
1. Mandelbaum BT, Browne JE, Fu F, et al. Articular cartilage lesions of the knee. Am J Sports Med. 2000;26:853-861.
2. Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895.
3. Bentley G, Biant LC, Carrington RW, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-230.
4. Fu F, Zurakowski D, Browne, et al. Autologous chondrocyte implantation versus debridement for treatment of full-thickness chondral defects of the knee: an observational cohort study with 3-year follow-up. Am J Sports Med. 2005;33:1658-1666.
5. Horas U, Pelinkovic D, Aigner T. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: A prospective comparative trial. J Bone Joint Surg Am. 2003;85:185-192.
6. Knutsen G, Drogset JO, Engebertson L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105-2112.
7. Knutsen, G, Engebretsen L, Ludvigsen TC, et al. Autologous chrondrocyte implantation compared with microfracture in the knee. J Bone Joint Surg Am. 2004;86:455-464.
8. Mandelbaum B, Browne JE, Fu F, et al. Treatment outcomes of autologous chondrocyte transplantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35:915-921.
9. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33:1639-1646.
10. Mithöfer K, Minas T, Peterson L, et al. Functional outcome of articular cartilage repair in adolescent athletes. Am J Sports Med. 2005;33:1147-1153.
11. Peterson L, Minas T, Brittberg M, et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
12. Peterson L, Brittberg M, Kiviranta I, et al. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12.
13. Zaslav K, Cole BJ, Brewster R, et al. A Prospective study of autologous chondrocyte transplantation in patients with failed prior treatment for articular cartilage defect of the knee. Results of the study of the treatment of articular repair (STAR) clinical trial. Am J Sports Med. 2009;37:42-55.
14. Brown WE, Potter HG, Marx RG, et al. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res. 2004;(422):214-223.
15. Potter HG, Chong LR. Magnetic resonance imaging assessment of chondral lesions and repair. J Bone Joint Surg Am. 2009;91(suppl 1):126-131.
16. Mithoefer K, Williams RJ, Potter HG, et al. The microfracture technique for treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-1920.
17. Hambly K, Bobic V, Wondrasch B, et al. Autologous chondrocyte implantation postoperative care and rehabilitation: science and practice. Am J Sports Med. 2006;34:1020-1038.
18. Kreuz PC, Steinwachs M, Erggelet C, et al. Importance of sports in cartilage regeneration after autologous chondrocyte implantation. Am J Sports Med. 2007;35:1261-1268.
19. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomized study. J Bone Joint Surg Br. 2005;87:640-645.
20. Kon E, Gobbi A, Filardo G, et al. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee. Prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33-41.
21. Saris DBF, Vanlauwe J, Victor J, et al. Comparison of characterized chondrocyte implantation versus microfracture in the treatment of symptomatic cartilage defects of the knee: results after three years. Paper presented at: Proceedings of the 8th World Congress of the International Cartilage Repair Society; May 23-26, 2009; Miami.
22. Saris DBF, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235-246.
23. Mithoefer K, McAdams T, Scopp J, Mandelbaum B. Emerging options for treatment of articular cartilage injury in the athlete. Clin Sports Med. 2009;28:25-40.
24. Crawford D, Heveran C, Cannon D, et al. An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. Am J Sports Med. 2009;37:1334-1343.