Chapter 43 Choledocholithiasis
Introduction
Gallstone disease affects people of both genders from every society, race, and age group. It is estimated that 15% of Americans have gallstones. Approximately 700,000 cholecystectomies are performed each year in the United States, making it the most common reason for digestive disease admission to Western hospitals. More than 95% of biliary tract disorders are related to gallstones.1 Most bile duct stones are gallstones that have passed into the bile duct.
Cholelithiasis refers to gallbladder stones, and choledocholithiasis refers to stones in the bile ducts. Noncrumbling concretions larger than 2 mm in diameter are considered stones, and biliary microlithiasis refers to particles 2 mm or less in diameter, although there is no universally accepted definition. Choledocholithiasis can be classified as primary stones, which develop in the bile ducts, or secondary stones, which pass from the gallbladder. Choledocholithiasis can be subdivided further by the location of the stones, which may be intrahepatic or extrahepatic. Of patients with symptomatic gallstone disease, 5% to 15% have bile duct stones, yet greater than 90% of patients with choledocholithiasis also have cholelithiasis. Sludge is a suspension of cholesterol monohydrate crystals, calcium bilirubinate granules, and other calcium salts with or without microlithiasis in gallbladder mucus. Sludge is a form of gallstone disease and may predispose to macroscopic stones or cause pancreatitis and other morbidity directly.2
Cholelithiasis
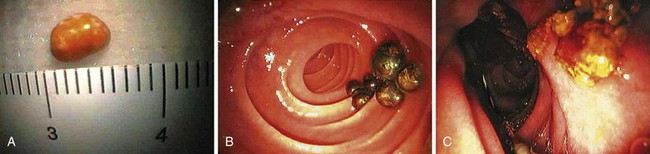
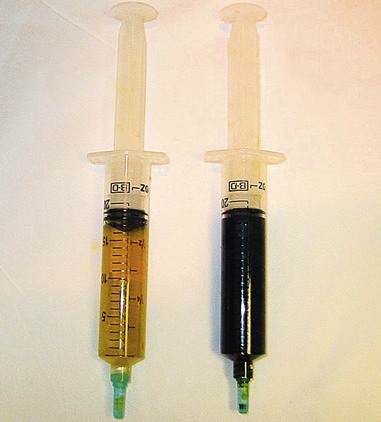
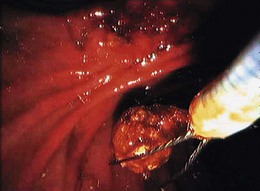
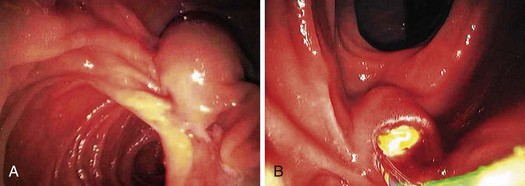
Most gallstones are composed primarily of cholesterol and are nodular and round with a golden color. A few gallstones contain mostly calcium bilirubinate and are round, hard, and black. Primary bile duct stones are composed of calcium salts of unconjugated bilirubin with variable amounts of cholesterol, protein, and bacteria. These stones are brown, are amorphous, and have an earthy texture. Fig. 43.1 shows examples of stones extracted from the bile duct.
Choledocholithiasis
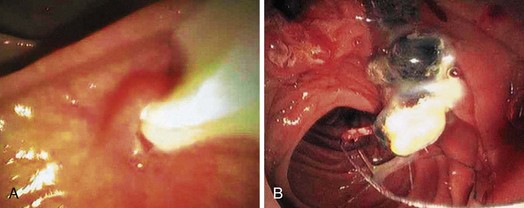
Epidemiologically, primary and secondary bile duct stones vary greatly. In Western societies, most bile duct stones are secondary, and the prevalence increases with age. Primary bile duct stones are more common in Asia. Primary stones are associated with bacterial contamination of the choledochus by biliary enteric anastomoses, sphincterotomy, stents, instrumentation, and portal bacteremia. Periampullary diverticula provide a site for bacterial proliferation with subsequent reflux into the bile duct (Fig. 43.2). Hemoglobinopathies may induce primary stones by providing a bilirubinate nidus for stone development. Foreign bodies including surgical clips and parasites may also introduce bacteria and serve as a nidus for stone formation, as shown in Fig. 43.3.3
Clinical Features and Diagnosis
The clinical presentation of biliary stones can be classified into three groups: (1) asymptomatic cholelithiasis or choledocholithiasis, (2) symptomatic gallstones (biliary colic), and (3) complications from gallstones (pancreatitis, cholecystitis, obstructive jaundice, cholangitis, gallbladder cancer, gallstone ileus). No symptoms occur in 60% to 80% of persons with gallstones, and the risk of progression is small. However, once symptoms develop, 35% to 50% of patients have recurrence within 1 year, and nearly 2% per year have complications.4
Biliary colic precedes complications in 90% of cases. The natural history of choledocholithiasis is unpredictable and not well described. Many common bile duct (CBD) stones are asymptomatic and pass into the duodenum without incident, whereas others lead to biliary colic, jaundice, cholangitis, or pancreatitis.5–7 Choledocholithiasis-associated biliary colic is similar to gallbladder-associated biliary colic. Untreated bile duct stone obstruction can cause secondary biliary cirrhosis, usually after about 5 years.8
Bile duct stones typically cause elevations in serum transaminases, alkaline phosphatase, and total bilirubin and ductal dilation on transabdominal ultrasound studies. However, CBD stones can be present with normal laboratory values. Total bilirubin levels range from normal to very high, and duration of obstruction does not correlate with serum bilirubin. Complete obstruction may cause a steady increase in serum bilirubin. Alkaline phosphatase is usually elevated up to fivefold in symptomatic patients. Acute biliary obstruction usually causes an initial disproportionate increase in transaminases.9
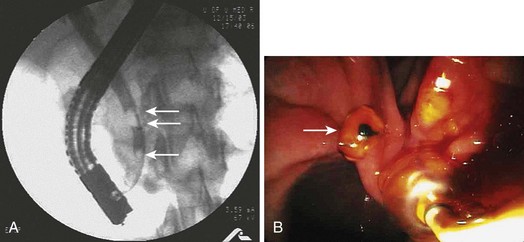
Imaging studies are the standard for the diagnosis of biliary stone disease. Transcutaneous abdominal ultrasound is the initial imaging study obtained when gallstone disease is suspected; sensitivity and specificity are greater than 95% for cholelithiasis and about 50% and 98% for bile duct stones.10 Spiral computed tomography (CT) is reported to be 82% sensitive and 97% specific for detecting bile duct stones, but many series have lower rates.11,12 Meta-analysis data show magnetic resonance cholangiopancreatography (MRCP) to be 92% sensitive and 97% specific for stones, but sensitivity declines for concretions 5 mm or less in diameter (Fig. 43.4).12,13
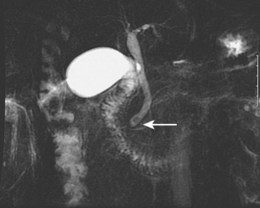
Fig. 43.4 Magnetic resonance cholangiopancreatography (MRCP) shows a 5-mm distal bile duct stone (arrow).
In a comparative study of 70 patients who underwent MRCP, transcutaneous ultrasound, or multislice CT, concordance was found between MRCP and ultrasound in 92% of cases for gallbladder lesions; however, statistically significant discordance occurred with ultrasound missing 16% of bile duct stones. Although there was concordance between MRCP and CT gallbladder imaging in 87% of patients, statistically significant discordance for bile duct lesions occurred in 35% of patients.14 Multiple studies show sensitivities and specificities for endoscopic retrograde cholangiopancreatography (ERCP) of 90% to 100% and 98% to 100%.12 Endoscopic ultrasound (EUS) can be performed with a dedicated echoendoscope with either 360-degree radial imaging or linear imaging or with an intraductal probe. EUS is performed similar to a standard endoscopy with ultrasound imaging of the extrahepatic bile duct done through the duodenal bulb or descending duodenum as shown in Fig. 43-5. Reported sensitivities and specificities for EUS are 84% to 100% and 96% to 100%.15
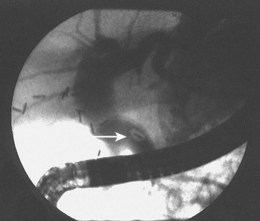
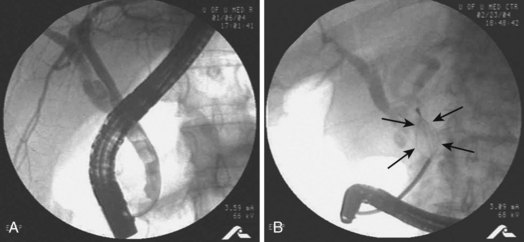
Preoperative diagnosis of CBD stones is not always necessary. Many authors advocate intraoperative cholangiography (IOC) as the diagnostic modality of choice in the setting of cholecystectomy with suspected CBD calculi. Laparoscopic IOC is technically successful in more than 90% of cases and is about 80% to 90% sensitive and 76% to 97% specific (Fig. 43.6).16,17 Fluoroscopic IOC has better diagnostic accuracy than static imaging. IOC is also used during cholecystectomy to verify biliary tree anatomy before dividing the cystic duct. IOC generally adds 5 to 25 minutes to the operation; however, it is usually faster, better tolerated, and less expensive than other invasive modalities.17 Laparoscopic intraoperative ultrasound is less widely employed than IOC. Yet, it has no radiation exposure, may be less time-consuming than IOC, and has sensitivity and specificity approaching 100%.17,18
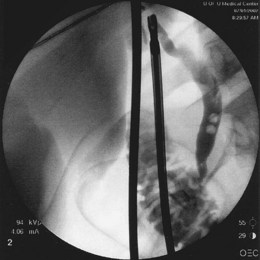
Fig. 43.6 Intraoperative cholangiogram with two radiolucent stones seen in the common bile duct (CBD).
(Courtesy of Robert Glasgow, MD.)
Although 5% to 15% of persons who have cholecystectomy have bile duct stones, it is not cost-effective to perform universal IOC or ERCP, or both, for detection and treatment. Clinical, laboratory, and imaging studies are used to stratify for risk of harboring CBD stones. Numerous studies have identified risk factors and developed scoring formulas for risk. The most commonly detected factors are elevations in serum transaminases, alkaline phosphatase, and bilirubin, and bile duct dilation to 8 mm or larger on transabdominal ultrasound. However, only about 50% to 75% of persons predicted to be at high risk have choledocholithiasis.1,19,20 MRCP or EUS may be useful to define the risk more precisely; however, these tests add cost, and EUS-guided stone extraction is experimental.21 When stones are suspected preoperatively, the options for diagnosis and therapy are preoperative ERCP, IOC, and laparoscopic common duct exploration (LCDE). For stones detected by IOC and LCDE, intraoperative or postoperative ERCP can be done and obtain similar results. ERCP entails increased resource use, however.22 Conversion to open common duct exploration is associated with significant morbidity and prolonged hospital and recovery times, and open common duct exploration should not be used routinely. Choledocholithiasis may be diagnosed after surgery with transabdominal ultrasound, CT, MRCP, EUS, or ERCP, depending on the index of suspicion, and subsequently treated with ERCP.17
Biliary Sludge, Microlithiasis, and Crystals
Biliary sludge, microlithiasis, and crystals are a form of gallstone disease and seem to have a natural history and clinical associations similar to macroscopic stone disease. Over the course of 3 years, 50% of persons with abdominal pain and sludge have spontaneous resolution of the sludge, 20% have sludge with no symptoms, 10% to 15% have symptoms develop or persist, and 5% to 15% acquire stones. Sludge, microlithiasis, and crystals account for about 75% of cases of acute recurrent pancreatitis in which the etiology is undiagnosed by history, physical examination, blood tests, and noninvasive imaging studies.23–25 Cholesterol monohydrate crystals or calcium bilirubinate granules or both can be found in the gallbladder or bile duct after cholecystectomy in 67% to 89% of these patients.25–27 These lesions are also associated with cholelithiasis not seen on noninvasive imaging studies, cholecystitis without macroscopic stones, and cholangitis.
The initial diagnosis of gallbladder sludge is often made by transabdominal ultrasound, which has been reported to be 86% sensitive at showing a dependent layer of nonshadowing, slowly mobile material.28 MRCP is more sensitive than transabdominal ultrasound for the diagnosis of sludge and microlithiasis.29 EUS is about 96% sensitive for detecting sludge and finds small gallbladder and bile duct stones missed on other studies.28,30 Intraductal ultrasound probes are highly sensitive but may yield false-positive results. For lesions larger than 1.4 mm, intraductal ultrasound is 71% sensitive and 75% specific for microlithiasis validated by microscopy.31 Direct microscopic examination of the bile is considered the diagnostic standard, is more sensitive than transcutaneous ultrasound or EUS, allows the determination of the type of particles in the sludge, and is required to diagnose microlithiasis in the absence of sludge. However, crystals may occur intermittently, and false-positive and false-negative results may occur.2
Duodenal aspiration after stimulation of gallbladder emptying is cumbersome and yields a contaminated specimen. ERCP with gallbladder cannulation and aspiration provides a relatively pure specimen and has the added advantage of allowing for imaging of the biliary tree and pancreas and sphincter of Oddi manometry when indicated.32
Gallbladder bile (B bile) is opaque dark green or black and is much more informative than lighter colored hepatic bile (A bile) for diagnosing microlithiasis and crystals (Fig. 43.7). Some authors have collected dark bile with cannulation of only the choledochus, but this may be time-consuming and unsuccessful.33 Radiographic contrast material in the bile may cause false-positive microscopic examinations. I collect a pure bile specimen by gallbladder cannulation under fluoroscopic guidance before contrast injection into the gallbladder.
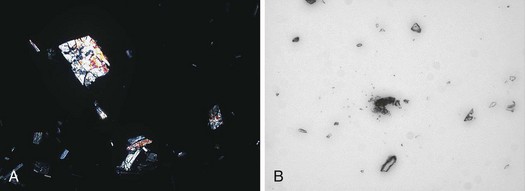
There is no consensus on the method of preparation, examination, and interpretation of bile specimens for microscopic crystal examination. Some authors have attempted to quantify the findings33; however, a qualitative examination is appropriate for clinical purposes.2 One suggested protocol is to centrifuge a 10- to 15-mL specimen at 3000g for 15 minutes and make a slide from the sediment. The slide is examined by light and polarizing microscopy at 100× (two crystals or granules per field or four per slide is considered a positive test). Cholesterol monohydrate crystals are rhomboid plaques with a notch that are multicolored on polarized examination, and bilirubinate granules are amorphous and red-brown colored as shown in Fig. 43.8. Leukocytes may be indicative of acute or chronic cholecystitis. It is difficult and unnecessary to maintain the specimen temperature strictly at 37° C, so the specimen should be centrifuged as quickly as possible. The intact specimen should not be stored because bacterial contamination can occur in specimens at room temperature and refrigerated specimens, and cholesterol crystal precipitation develops in frozen bile. However, the sediment may be frozen for later examination if necessary.2
Gallstone Pancreatitis
Gallstones cause about 35% of cases of acute pancreatitis in the United States, and about 25% of these cases are severe with mortality in 10%. Gallstones are recovered from the feces in 95% of patients with acute pancreatitis; however, only 7% of patients with gallstones develop pancreatitis.34 Soon after the onset of pancreatitis, 78% of affected persons have bile duct stones found at surgery or ERCP.35,36 However, delayed operations reveal CBD and impacted ampullary stones much less frequently.37
Small stones are thought to pass easily through the cystic duct and impact in the sphincter of Oddi; stones less than 5 mm in diameter are the most common.38 The causal relationship between gallstones and pancreatitis is confirmed by the finding that cholecystectomy or endoscopic sphincterotomy with removal of bile duct stones prevents recurrences.39 The pathophysiology may be due to obstruction of pancreatic juice outflow, reflux of offending substances into the pancreatic duct, or both.
The diagnosis of biliary pancreatitis is usually made by finding gallstones on transabdominal ultrasound in the absence of other known causes of pancreatitis, although EUS is more sensitive and specific and provides much better visualization of the bile duct.35 Abnormal liver enzymes and bilirubin and bile duct dilation on transabdominal ultrasound are frequently found but are nonspecific.40 It is important to stratify the severity of pancreatitis by various algorithms or failure of one or more organ systems because prognosis and therapy vary greatly between mild and severe cases. I use failure of one or more organ systems to categorize severe pancreatitis because algorithms are cumbersome and difficult to remember, include subjective criteria, and may require 48 hours of observation for a conclusion.41
Suppurative Cholangitis
Acute suppurative cholangitis develops in the setting of bacteria in the biliary system and bile duct obstruction. Bile duct stones are the cause of the obstruction in most cases. The bacteria enter the duct from the gut and proliferate resulting in increased intraductal pressure and forced translocation of bacteria and endotoxins into the hepatic sinusoids and bloodstream. In contrast to gallstone disease, women and men are affected equally with a median age of 50 to 60 years. Less frequently, benign and malignant strictures, obstructed stents, and side-to-side surgical anastomoses predispose to cholangitis. Mortality rates approach 100% in persons who fail conservative therapy and who do not have an adequate drainage procedure.42
The most common organisms are Escherichia coli, Enterococcus, Klebsiella, and Enterobacter. Pseudomonas, anaerobes, and skin and oral flora may be found after biliary instrumentation or surgery. Polymicrobial infection is more common in severe infections, and bile cultures are usually concordant.42 The classic clinical presentation is Charcot’s triad including fever, jaundice, and right upper quadrant abdominal pain; this occurs in 50% to 100% of patients. Reynold’s pentad adds altered mental status and hypotension to Charcot’s triad and occurs in less than 14% of patients. Typical laboratory abnormalities include leukocytosis, hyperbilirubinemia, elevated alkaline phosphatase, mildly increased transaminases, and occasionally elevated amylase.
Imaging findings on transabdominal ultrasound include stones or ductal dilation or both in 67% of patients. CT scans may show ductal dilation, the level of obstruction, and occasionally calcified CBD or gallstones or both. One study showed changes of papillitis to be 60% sensitive and 86% specific in homogeneous, hepatic contrast agent uptake to be 60% sensitive and 80% specific, and combined findings of papillitis and contrast agent uptake to be 97% specific for suppurative cholangitis.43 MRCP is reported to show distinctive changes in 92% of patients; however, because MRCP is without therapeutic capabilities, the utility is unclear. EUS may be beneficial in a clinically stable patient when ERCP is unsuccessful or undesirable because of increased procedure risks or pregnancy. Percutaneous transhepatic cholangiography (PTC) is sensitive and specific in 90% or more of affected patients and allows for therapy. However, it should not be considered as a first-line procedure because of high complication rates. ERCP is the procedure of choice because it affords the opportunity to provide diagnosis and definitive therapy with acceptable morbidity and mortality rates.
Treatment
Surgery
The management of CBD stones varies widely around the world. Therapeutic determinants include individual patient presentation; operative risk; and available expertise for LCDE, open CBD exploration, and ERCP. Laparoscopic cholecystectomy is the preferred initial approach to cholelithiasis with the benefit of decreased pain, shorter length of hospital stay, more rapid return to full activity, and less cost than open surgery. LCDE seems to have similar advantages over open CBD exploration and is cost-effective compared with ERCP.22 A transcystic duct approach is generally preferred to choledochotomy, resulting in shorter procedure duration and hospital stays and reduced need for T-tube placement. Some surgeons use a choledochotomy, however, depending on the stone. Stone extraction is generally achieved under fluoroscopic guidance with medical dilation of the sphincter of Oddi and flushing of small stones, by retrograde balloon or basket catheter techniques used to push stones through the native sphincter of Oddi, or after balloon dilation or antegrade sphincterotomy. Balloon dilation should be discouraged because of increased rates of pancreatitis. Choledochoscopic guidance may also be used with or without intracorporeal lithotripsy techniques.
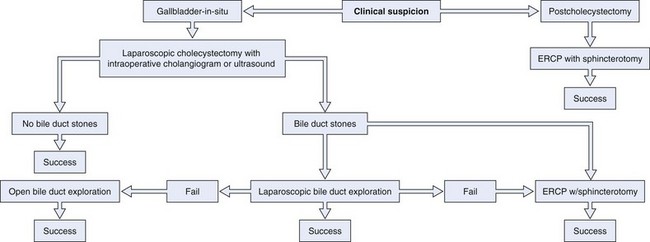
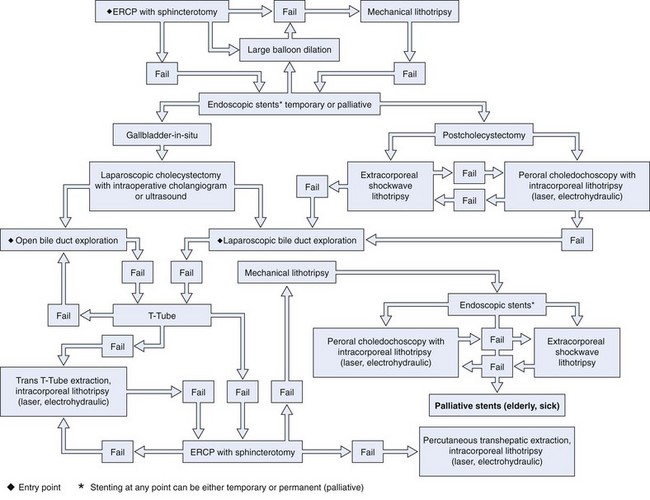
LCDE results in stone clearance in 75% to 90% of cases, and T-tube placement or antegrade biliary stent placement is required for incomplete drainage. LCDE adds about 1 hour to the operation. Transcystic duct LCDE requires hospital stays of about 1.5 days, and transductal procedures generally result in stays of up to 7.5 days. Morbidity and mortality rates are 10% and 1%.17 A treatment algorithm for preoperatively suspected bile duct stones managed by standard techniques is shown in Fig. 43.9. A treatment algorithm for bile duct stones requiring more specialized techniques is shown in Fig. 43.10. Stones detected after surgery are best managed by ERCP.
Endoscopic Therapy
ERCP with sphincterotomy is the most common method of treating bile duct stones in the United States, and more than 150,000 cases are performed each year. Overall, the procedure is ultimately successful in greater than 90% of patients with complications in about 10%.44 Successful clearance of the biliary tree of all stones depends on the size and number of stones and experience of the endoscopists. Reported rates of successful bile duct clearance range from about 60% to greater than 90% at the initial procedure45–48 to almost 100% with subsequent procedures at specialized centers.49–57 Failed cases are usually due to the inability to access the major papilla owing to surgically altered anatomy. Morbidity occurs in only 3% to 5% of patients from initial ERCP with sphincterotomy performed for stones, especially when performed within 30 days of laparoscopic cholecystectomy.48,58,59
Preoperative History and Considerations
Considerations in the medical history include prior intestinal surgery with altered anatomy such as Billroth or Roux-en-Y anastomoses, which could require a different approach and special instruments. Stones are common in pregnancy, and pregnancy could alter the timing and technique of ERCP. Preprocedure coagulation studies are not generally recommended but are appropriate in patients receiving anticoagulants or with biliary obstruction, who may develop a coagulopathy secondary to vitamin K malabsorption, which is significantly exacerbated in patients receiving warfarin. It is wise to have the international normalized ratio corrected to 1.5 or less if sphincterotomy is anticipated.60
Indications for general anesthesia include sepsis with hypotension; severe acute pancreatitis; significant cardiopulmonary disease; macroglossia; dysmorphic facies; obstructive sleep apnea; morbid obesity; and narcotic, benzodiazepine, or alcohol tolerance. Other considerations include a bowel purge in patients who have recently had a CT scan or other barium contrast study because residual barium in the colon can compromise cholangiography. Prophylactic antibiotics are not recommended for routine bile duct stone removal. However, antibiotics that cover enteric gram-negative organisms, enterococci, and perhaps Pseudomonas are recommended for cases of bile duct obstruction when there is a possibility of incomplete drainage.61
Therapeutic Techniques
Stones are extracted after cannulation has been achieved; the stone is identified on cholangiography, and the sphincterotomy is performed. A balloon or basket catheter is advanced upstream to the stone. The balloon is inflated and retracted, bringing the stone with it into the duodenum as shown in Fig. 43.11. A basket is positioned so that it completely opens and deploys such that the stone is totally engaged. It is often helpful to move or jiggle the basket gently up and down the affected segment of the duct to entrap the stone. The basket is left in the fully opened position and retracted into the duodenum, bringing the stone with it (Fig. 43.12). If the stone continually slips out of the basket, the basket may be closed around the stone to get a better grip. If this maneuver is done, it is important that the stone be small enough to pass through the distal duct and sphincterotomy orifice or that the basket be compatible with mechanical lithotripsy, to prevent basket and stone impaction in the duct. If multiple stones are present, the most downstream stone should be removed first also to prevent this type of impaction.
If the balloon or basket catheter is simply pulled out with the endoscope in the standard position, the stone is likely to be forced against the superior aspect of the duct deep to the duodenal wall. The catheter then slips out inferiorly to the stone. The balloon can rupture against the bridge on the endoscope, or the basket may slip off of the stone and out. Primary bile duct stones have an earthy consistency and crumble with extraction. It is often necessary to sweep the duct repeatedly with a balloon and perhaps irrigate the biliary tree with saline. Brown stone debris extracted into the duodenum is shown in Fig. 43.1C.
Unusual Situations
Pregnancy
About 8% of pregnant women develop cholelithiasis and frequently have symptoms. Cholecystectomy can often be postponed until after delivery; however, choledocholithiasis poses significant risk of cholangitis and pancreatitis and generally requires therapy. Potential risks to the fetus include sedatives and analgesics; radiation exposure; and sequelae of procedural complications such as hypoxia, pancreatitis, and sepsis. There are several small series in the literature of ERCP during pregnancy including during the first trimester. Biliary sphincterotomy, stone extraction, and stent placement were frequently done. Most patients delivered healthy full-term infants. Therapeutic outcomes were generally successful with acceptable morbidity.62
A reasonable approach is to perform transabdominal ultrasound for pregnant women who develop right upper quadrant or epigastric pain, abnormal serum hepatic enzymes, unexplained acute pancreatitis, or biliary sepsis. If gallstones are found and there is no pancreatitis, cholecystectomy may be postponed until after delivery if the symptoms and biochemical tests resolve.62 ERCP is indicated for choledocholithiasis or ductal dilation seen on ultrasound, persistent cholestasis, pancreatitis, or cholangitis. The patient should be considered for referral to a high-volume center with experience in these cases. Obstetric consultation should be obtained, and fetal monitoring should be considered during the procedure. The procedure may need to be performed in the supine or left lateral position. It may be wise to have an anesthesiologist administer the sedation or anesthesia and to consider endotracheal intubation if the patient is to be in the supine position.
Fetal radiation exposure should be minimized by lead shielding; using minimal fluoroscopy; and avoiding spot films, which give higher radiation exposure. Using these techniques, fetal radiation exposure can be contained to about 310 mrad, which is significantly below the accepted teratogenic dose.63 Radiation exposure to the fetus may be monitored with a radiation dosimetry badge placed on the mother’s abdomen over the uterine fundus.
Sphincterotomy and stent placement may be safely performed, and transpapillary gallbladder stent placement for cholecystitis has been suggested to postpone cholecystectomy until after delivery.62,64 Some authorities advocate performing the procedure with no fluoroscopy. One technique is to use wire-guided cannulation with visualization of bile flow around the wire, through a small-caliber stent, or with aspiration through the sphincterotome. Sphincterotomy may be performed in the standard fashion or with a needle-knife over a stent. Stones can be removed with balloons, baskets, or mechanical lithotripsy.65 Alternatively, a stent may be left in situ, and ERCP may be repeated after delivery.66 Cholangioscopy has been used to confirm ductal clearance.67 Ultrasound, rather than fluoroscopic, guidance has also been used.
Biliary Sludge, Microlithiasis, and Crystals
Traditional therapy for sludge, microlithiasis, and crystals is cholecystectomy, which generally cures relapsing pain and prevents recurrent pancreatitis. Ursodeoxycholic acid can dissolve cholesterol microlithiasis and crystals and prevents the recurrence of pancreatitis. However, the duration of effect is unknown.2 Endoscopic sphincterotomy prevents or reduces episodes of recurrent pancreatitis caused by sludge, microlithiasis, and crystals.33
Gallstone Pancreatitis
The usual therapy for mild to moderate disease is supportive until pancreatitis resolves and then laparoscopic cholecystectomy with IOC generally during the same hospital stay. Severe disease is best treated with intensive care monitoring, prophylactic antibiotics for extensive necrosis, enteral nutritional support, and urgent biliary drainage for signs of bile duct obstruction (jaundice, persistently abnormal liver tests, and dilated bile duct) or cholangitis. Early biliary surgery is associated with high rates of morbidity and mortality in severe pancreatitis.36 Cholecystectomy should be performed during the same hospital stay or as soon as possible afterward.
Urgent endoscopic retrograde cholangiography (ERC) with biliary sphincterotomy and stone extraction has been used to reduce morbidity and mortality.68 There are six published peer-reviewed, randomized controlled trials of ERC compared with conservative management in patients with acute pancreatitis, and there are six meta-analyses of these studies with conflicting results. The conflicting results are due to heterogeneity of patients, inclusion criteria, severity of disease, and inability to confirm the presence of stones. There are also different methodologies and endpoints.68–79 Extraction of obstructing stones is beneficial in relieving cholangitis and jaundice. There are several issues pertinent to interpreting this literature, as follows: (1) Does ERC prevent systemic or pancreatic morbidity? (2) Should ERC be done in all cases or only for cholangitis or biliary obstruction? (3) Is it beneficial to diagnose CBD stones with noninvasive imaging or EUS and then do ERC only if stones are seen? (4) Should sphincterotomy be done in all patients to provide drainage for unseen microlithiasis, sludge, or stones? (5) What is the optimal timing for ERCP?
Neoptolemos and colleagues69 randomly assigned 121 patients from a single center with suspected biliary pancreatitis to have ERC with sphincterotomy for bile duct stones within 72 hours of admission compared with conservative therapy. Subgroup analysis of patients with severe pancreatitis showed a statistically significant decrease in morbidity and a numerical benefit in mortality. Fan and coworkers70 randomly assigned 195 patients with pancreatitis of any etiology (including alcohol and hyperlipidemia) to receive ERC with sphincterotomy for choledocholithiasis within 24 hours of hospitalization or medical management. The overall outcomes were similar for the treatment and control groups; however, in patients with severe pancreatitis, morbidity was significantly less frequent at 13% versus 54% (P = .003). There was a trend toward improved mortality rates in the ERC group at 3% versus 18% (P = .097). Biliary sepsis occurred less commonly in patients with severe disease treated with ERC than conservative management at zero and 29% (P < .001). Issues with this study are inclusion of patients with pancreatitis of all causes and patients with cholangitis.
In a multicenter German study, 238 subjects were randomly assigned to ERC with sphincterotomy and stone extraction within 72 hours of symptom onset or conservative management. Patients with cholangitis or a total bilirubin of 5 mg/dL or greater were excluded. Of 112 patients randomly assigned to conservative management, 20 went on to ERC, and 13 had stones extracted. The overall morbidity rates were similar between the ERC and control groups, but the data were not stratified by severity of pancreatitis. There were more serious complications in the treatment group, mostly because of respiratory failure (P = .03), but fewer episodes of cholangitis. Death occurred in 14 treatment and 7 control patients; most deaths were due to respiratory failure. Pancreatic morbidity rates were similar between the groups at 23% and 22%. The authors concluded that early ERC with sphincterotomy is not beneficial in patients with acute biliary pancreatitis and no obstructive jaundice or cholangitis. Problems with this study were that there were significantly fewer patients with stones and severe disease than in the other studies and that 19 of the 22 centers enrolled fewer than two subjects per year on average. The rate of respiratory failure in the ERC group was higher than in other studies. Considering the low volume at some centers and the undue rates of respiratory failure, questions have been raised about the degree of endoscopic expertise and potential for procedure-related aspiration.71
Zhou and colleagues72 randomly assigned 45 patients with gallstone pancreatitis stratified by severity to have ERC within 24 hours or medical therapy. Sphincterotomy and extraction was done for small stones, and nasobiliary drainage was performed for large or no stones. Morbidity and length of hospitalization were significantly decreased in severe cases with ERC but not for mild disease. Issues with this study include the small sample size and that cholangitis was not explicitly excluded.
Another more recent randomized trial included 61 patients with gallstone pancreatitis and ampullary obstruction assigned to a control group of conservative therapy with selective ERC at 48 hours or a treatment group with ERC at 24 hours for persistent obstruction. In 9 of 31 control patients, obstruction did not spontaneously resolve, and 3 had ERC and sphincterotomy with no stones found. Among the treatment patients, obstruction resolved in 16, and 11 of 14 who had ERCP had stones removed. There was a statistically significant lower morbidity rate in the treatment group, and the authors concluded that these patients should have ERC within 48 hours. However, only 47% of the treatment patients had ERC.73
Oria and colleagues74 randomly assigned 103 patients with acute gallstone pancreatitis with elevated bilirubin and dilated bile ducts but no cholangitis to ERC with sphincterotomy for stones or conservative management, and outcomes were stratified by severity. Bile duct stones were detected in 72% of the ERC group and 42% of the conservative group at subsequent surgery. There were no statistically significant differences between the groups in morbidity, CT index, local complications, or mortality. Limitations of the study are the small sample size and inclusion of patients with mild to moderate disease.
Van Santvoort and coworkers80 performed a prospective multicenter, observational study of 153 patients with predicted severe biliary pancreatitis and no cholangitis. There were 81 patients who had ERC performed within 72 hours of admission for clinical indications. Morbidity occurred in fewer ERC patients with cholestasis than patients without cholestasis at 25% versus 54% (P = .02); this included fewer patients with greater than 30% of pancreatic necrosis at 8% and 31% (P = .01). ERC with or without papillotomy was also protective with statistical significance. For patients without cholestasis, morbidity rates were similar in the ERC and medically managed groups. Mortality rates were similar regardless of the treatment group. The authors opined that sphincterotomy may be beneficial in patients without choledocholithiasis seen at ERC by providing drainage for microlithiasis and sludge and stones that may have been missed.
There are six published meta-analyses of these randomized studies. ERC with or without sphincterotomy or conservative care was analyzed from four early trials including one published only in abstract. There were no exclusions for cholestasis or cholangitis. Morbidity and mortality rates were lower with ERC with statistical significance, and the number needed to treat to prevent complications and death was 7.6 and 25.5.68–71 A Cochrane systematic review was performed on three trials with 511 patients. Treatment included ERC with or without sphincterotomy, and the analysis was controlled for cholangitis. The odds of having complications were reduced only in treated patients with predicted severe gallstone pancreatitis.75 Petrov and colleagues76 analyzed three trials with 450 participants and controlled for cholangitis. Early ERC, with or without sphincterotomy, in predicted mild and severe biliary pancreatitis did not lead to reductions in morbidity or mortality.
Another systematic review assessed local pancreatic morbidity. This review included five studies with 717 patients with mild or severe biliary pancreatitis. Early ERC did not significantly reduce the risk of local pancreatic complications.77 Moretti and coworkers78 reported a meta-analysis of five studies with 702 patients with predicted severe gallstone pancreatitis and determined that early ERC with or without sphincterotomy in patients with severe pancreatitis reduced complications with a number needed to treat of 3. Mortality was not significantly affected. There was no significant decrease in morbidity for mild disease. A meta-analysis by Uy and colleagues79 involved two studies with 340 patients and was controlled for cholangitis; this showed no statistically significant benefit from early ERC.
Alternative approaches have been proposed. Liu and colleagues81 randomly assigned 140 patients with suspected biliary pancreatitis to have EUS with ERC and sphincterotomy only for stones or ERC within 24 hours of admission. All patients in the EUS group had bile duct stones detected, but 14% were missed in the ERC group (P = .001). Additionally, standard transcutaneous ultrasound plus ERC missed cholelithiasis in six patients. The morbidity and mortality rates and lengths of hospital stay were similar between the groups. The authors concluded that EUS could safely replace diagnostic ERCP to select patients for therapeutic ERCP. Percutaneous transhepatic cholecystotomy was shown in a randomized trial to have similar outcomes to ERC in severe gallstone pancreatitis. The authors suggested percutaneous transhepatic cholecystotomy as second-line therapy if ERC fails.82 The American Society for Gastrointestinal Endoscopy recommends the following for early ERC in acute biliary pancreatitis: (1) no early ERC in mild disease in the absence of clear evidence of a retained stone, (2) early ERC for concomitant cholangitis, (3) consideration of early ERC for evidence of obstruction, and (4) no recommendation for or against early ERC in patients with predicted severe acute biliary pancreatitis in the absence of overt biliary obstruction or cholangitis.83 The American Gastroenterological Association Institute and the American College of Gastroenterology have similar recommendations.84,85
From these data, ERC with sphincterotomy and stone extraction seems to improve outcomes in patients with severe biliary pancreatitis who have cholangitis or signs of biliary obstruction or both. The improved outcomes may be due to avoidance of and treatment for cholangitis or stone impaction or both; this may result in decreased systemic morbidity and progression to pancreatic necrosis. Some studies show a benefit to sphincterotomy even if no stones are present.80
One small study showed that about 90% of patients with gallstone pancreatitis do well for about 3 years after sphincterotomy and stone extraction without cholecystectomy.39 Close follow-up is prudent in this circumstance. ERCP is not generally indicated for mild to moderate biliary pancreatitis without signs of obstruction. Laparoscopic cholecystectomy with IOC soon after the pancreatitis has resolved is optimal. Employing MRCP or EUS, or both, to diagnose stones in a less invasive fashion and then perform ERC on patients with positive results seems reasonable.35,81,82
Acute Cholangitis
Initial therapy is medical with supportive care, blood cultures, and parenteral vitamin K supplementation and empiric antibiotics. Up to 90% of patients respond to medical therapy within 12 to 24 hours and may then undergo semielective biliary drainage by ERC. Patients who do not respond require urgent or emergent drainage, preferably by ERC.86–88 The choice of antibiotics should be based on local sensitivities and potential need for extended anaerobic coverage for elderly patients and patients with prior biliary instrumentation. Some appropriate regimens include monotherapy with fluoroquinolones (especially levofloxacin owing to expanded Enterococcus coverage), piperacillin/tazobactam, imipenem/cilastatin, or meropenem or therapy with extended-spectrum cephalosporins with or without metronidazole or clindamycin for enhanced anaerobic coverage. There are many other recommended regimens as well.42
Interventional radiographic drainage with PTC is successful in up to 90% of all patients with biliary obstruction, but morbidity, including bleeding, pseudoaneurysms, peritonitis, bile fistulas, infections, and strictures, occurs in 30% to 80%, and mortality occurs in 5% to 17% of patients with cholangitis.89 ERC is more effective, has lower morbidity and mortality rates, and is preferred over PTC.58,87 PTC may be indicated in unusual circumstances, however, such as intrahepatic stones, surgically altered duodenal anatomy, or failed ERC.
The traditional therapy for cholangitis is open surgery, but this is rarely performed as first-line therapy at the present time because of high rates of morbidity and mortality. In a randomized controlled trial in patients with cholangitis, Lai and colleagues87 showed that ERC with sphincterotomy compared with open choledochotomy was associated with morbidity in 34% and 66% and in-hospital mortality in 10% and 32%. Operative mortality is associated with the severity of illness at the time of surgery with reported rates of 40% for emergent, 16% for urgent, and 3% for elective procedures.86,90,91 Cholangitis may recur after open surgery with seemingly adequate drainage. In the setting of laparoscopic cholecystectomy, a systematic review showed that laparoscopic bile duct exploration is equivalent to ERC with sphincterotomy for bile duct stone clearance and morbidity and mortality but with shorter hospital stays.22,90 However, the outcomes of laparoscopic bile duct exploration in patients with cholangitis are unknown because only a few patients with mild cholangitis are reported, and none had severe cholangitis. One-stage laparoscopic cholecystectomy with bile duct exploration seems appropriate for cholangitis patients with gallstones who respond to medical therapy and are clinically stable and good surgical candidates. Such surgery should not be performed in patients who are unstable or who have had prior cholecystectomy.86,90,91
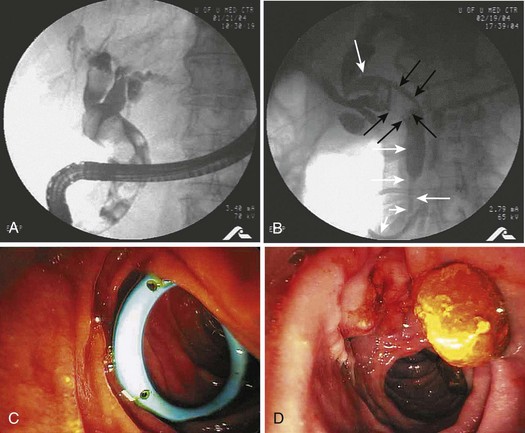
ERC with sphincterotomy and stone extraction is the preferred procedure for patients with acute cholangitis who are critically ill or have had prior cholecystectomy. It is also appropriate for stable patients with cholelithiasis when expertise in laparoscopic bile duct exploration is unavailable. ERC may be performed in the standard fashion in stable patients with normal coagulation studies. However, patients with cholangitis frequently have septic shock with associated hypotension, multiorgan failure, disseminated intravascular coagulopathy, and coagulopathy from vitamin K malabsorption secondary to biliary obstruction. In these circumstances, it is generally best to provide temporizing biliary drainage rapidly with stent placement or a nasobiliary drain without sphincterotomy or stone extraction. I do these procedures under general anesthesia with endotracheal intubation. It is wise to aspirate and decompress the biliary system before injecting contrast material to avoid increasing the biliary pressure further with potential to exacerbate hematogenous seeding with bacteria and endotoxins. A stent may be placed in the normal fashion by passing a guidewire upstream to the obstructing stone and then inserting the endoprosthesis. Pus under pressure may be seen to emanate around the cannulating catheter or through the stent (Fig. 43.13).
The outcomes of stent placement and nasobiliary drainage seem to be similar; I prefer stent placement because it is quicker, is easier, and has no external segment of the drain that could lead to accidental displacement.88 Recovery is often rapid after drainage, and ERCP can be repeated with definitive sphincterotomy and stone extraction when the patient is stable and the coagulopathy has resolved.
Endoscopic Bile Duct Stone Extraction as Definitive Therapy with the Gallbladder In Situ
Patients with choledocholithiasis who are fit for surgery should have bile duct stone clearance and cholecystectomy. Endoscopic sphincterotomy with bile duct stone extraction cures the initiating episode related to choledocholithiasis. The need for subsequent cholecystectomy in patients with an intact gallbladder with gallstones has long been debated. After biliary sphincterotomy, there is a marked decrease in lithogenicity of gallbladder and hepatic bile, which has been proposed to decrease clinical events.92 However, frequent symptoms and complications of leaving the gallbladder in situ include biliary colic, jaundice, cholecystitis, cholangitis, choledocholithiasis, pancreatitis bile leaks, and papillary stenosis. Predisposing factors include gallstones, complete opacification of the gallbladder at ERCP, bile duct dilation, and periampullary diverticula.93–96
McAlister and colleagues97 performed a Cochrane systematic review to evaluate clinical observation with cholecystectomy only if symptoms occur versus scheduled cholecystectomy shortly after sphincterotomy in patients who had prior endoscopic biliary sphincterotomy. This analysis comprised 5 randomized clinical trials involving 662 participants. Patients who were managed expectantly had higher rates of recurrent biliary pain (relative risk [RR] 14.56, 95% confidence interval [CI] 4.95 to 42.78, P < .0001), jaundice or cholangitis (RR 2.53, 95% CI 1.09 to 5.87, P = .03), and subsequent ERCP or other cholangiography (RR 2.36, 95% CI 1.29 to 4.32, P = .005). Cholecystectomy was ultimately performed in 35% of the observational group. There were 26 (7.9%) deaths in the cholecystectomy group and 47 (14.1%) in the observational group representing a 78% increased risk of mortality (RR 1.78, 95% CI 1.15 to 2.75, P = .01). The survival benefit from scheduled cholecystectomy was independent of trial design, inclusion of high-risk patients, or inclusion of any one of the five trials.
Endoscopic Balloon Dilation of the Sphincter of Oddi for Extraction of Bile Duct Stones
Endoscopic balloon dilation of the sphincter of Oddi can be used to open the orifice to allow extraction of stones. This procedure may be done in the intact sphincter with a relatively small-caliber balloon for standard extraction of small stones and mechanical lithotripsy for large stones. Balloon dilation may also be done after sphincterotomy with large-caliber balloons for removal of large stones; this is discussed subsequently in the section on Large Stones.
Balloon Dilation of the Intact Papilla
Endoscopic sphincterotomy is the standard of care for opening the sphincter of Oddi and allows for removal of bile duct stones. Sphincterotomy is associated with short-term morbidity in about 5% of patients.58 Medium-term (6 to 15 years) morbidity occurs in 6% to 24% of patients and can usually be managed with endoscopic techniques, but long-term outcomes remain largely unknown.98–100 Endoscopic balloon dilation of the sphincter of Oddi has been proposed to prevent short-term and late occurring morbidity from sphincterotomy. Persons who may benefit most from this procedure are the young, healthy patients with laparoscopic cholecystectomy who have sphincterotomy for choledocholithiasis.
The technique of endoscopic balloon dilation involves passing a wire-guided balloon dilation catheter to bridge the biliary sphincter segment. The balloon is inflated with diluted radiographic contrast material to maximum pressure. Some experts advocate slow inflation to maximum pressure, which is maintained for 15 seconds before deflation to minimize pancreatitis risk. Although the benefits are unproven, this seems unlikely to increase morbidity.101
Fluoroscopy is generally used to document complete inflation with obliteration of the waist in the middle of the balloon. The diameter of the balloon is generally equal to the smallest diameter of either the stone or the duct; 6-mm and 8-mm balloons are the most common. Stones are removed with standard balloon and basket techniques (Fig. 43.14) Wire-guided instruments facilitate the procedure. Some authorities place a prophylactic pancreatic duct stent to reduce risks of pancreatitis.102 For large stones, small-caliber dilation can be performed and followed with mechanical lithotripsy. Crushed fragments can be removed with extraction-balloon catheters.
Balloon dilation of the sphincter of Oddi is usually effective at enlarging the orifice for stone extraction. However, compared with sphincterotomy, it takes longer, has higher failure rates, requires increased use of mechanical lithotripsy, and is associated with higher morbidity rates owing to pancreatitis, including deaths.46,48,103 Balloon dilation may induce edema or spasm, or both, with obstruction of the pancreatic duct or papillary edema and impaction of stones not extracted. Pancreatitis or cholangitis may result.44,102,104 After sphincterotomy, most retained stones pass spontaneously. Some authors have proposed prophylactic placement of pancreatic duct stents to minimize balloon dilation–induced pancreatitis; but this has not been adequately studied.102
From four uncontrolled series of sphincter of Oddi balloon dilation involving 1296 patients, it appears that initial bile duct clearance was achieved in 72% of patients, with the remainder requiring two or more ERCPs and occasionally sphincterotomy, surgery, or other therapy. Mechanical lithotripsy was needed in 34%. Morbidity occurred in at least 7% to 19% and was mostly due to pancreatitis.105–108 Problems with interpreting these studies include that three series seem retrospective with potential underestimation of complications.105–107 All of these reports are from tertiary centers, the patients were relatively old, and standardized morbidity criteria were not consistently used.44 Gabexate was given to decrease morbidity from pancreatitis in one series108 and may have been used in others.105–107
Several randomized, controlled clinical trials from tertiary centers in Europe and Asia and a single multicenter study representing academic and community practices compared balloon dilation with sphincterotomy. The largest studies are summarized in Table 43.1.45–48109 The patients in the European and Asian studies were relatively old and sick, harbored large and numerous stones, often had periampullary diverticula, and did not always have cholecystectomy. Multiple procedures were frequently required, mechanical lithotripsy and precut sphincterotomy were often employed, procedures may have been terminated prematurely, and gabexate use was not always specified. The study methodologies were not always rigorous, lacking a predefined sample size, not verifying sequential enrollment or concealed allocation or both, and inconsistently using standardized criteria for overall and severe morbidity.45,47,109 At least one dilation patient who experienced morbidity was excluded from analysis.47 One study found no advantage to balloon dilation after 24 hours and after 12 months, and another study showed statistically significant increased rates of pancreatitis and overall morbidity with endoscopic balloon dilation.45,109
Table 43.1 Randomized Studies of Balloon Dilation of the Sphincter of Oddi Compared with Sphincterotomy for Stone Removal
DiSario and colleagues48 performed a randomized, controlled, multicenter, largely American study involving equal numbers of community and academic practices. The patients were representative of patients having laparoscopic cholecystectomy in routine clinical practice in that they were younger and healthier, had fewer and smaller stones, and did not have complicated anatomy, and most had cholecystectomy before or within 30 days of ERCP. The procedures were generally straightforward without mechanical lithotripsy or precut sphincterotomy. The study had a predefined primary endpoint, sample size determination, and an independent Oversight Board. The study was stopped at the first interim analysis because a statistically significant difference in the primary endpoint was found with more overall morbidity in the dilation group. There were also significantly more severe complications, two deaths from pancreatitis, and greater resource usage and patient days away for normal activities in the dilation group. Multivariate analysis showed that balloon dilation was the only factor associated with complications.
The American study48 and the study by Bergman and colleagues46 from a single referral center in the Netherlands (see Table 43.1) have analogous methodology, and the results can be compared.46,48 Overall and severe complications after balloon dilation occurred at similar rates of 18% and 4% in the Dutch study and 18% and 6.8% in the American study. However, the Dutch study showed overall and severe morbidity in 24% and 3% in the sphincterotomy group, whereas only 3.3% of American study participants had mild to moderate morbidity. These sphincterotomy-related morbidity rates reflect the older, sicker, and more complicated Dutch tertiary referral center patients and are much higher than the rates observed in younger and healthier patients treated in a broad spectrum of American practices.48
Two other large-scale multicenter studies showed less than 5% morbidity after sphincterotomy performed for stones.58,59 Papillary balloon dilation was found to be the most significant risk factor for pancreatitis in well-designed, multicenter series of 1966 ERCPs with an adjusted odds ratio of 4.5 (95% CI 1.51 to 113.46, P = .0027).110 A randomized, controlled clinical trial by Arnold and colleagues104 also showed excessive overall and severe complication rates with dilation compared with sphincterotomy that compelled the authors to terminate the study prematurely. A Cochrane systematic review showed that balloon dilation compared with sphincterotomy for stone extraction imparts increased risks of pancreatitis and is slightly less successful.103
Complication rates for sphincterotomy are increased to 12% to 23% in the larger, referral center, randomized controlled studies owing to the complicated patients and procedures. These morbidity rates are similar to the morbidity rates of balloon dilation of 15% to 17% in these series, which is appropriate for that patient population.45–47 However, these similarities reflect the selection bias of tertiary center patients and cannot be generalized to patients seen in routine practice. Morbidity rates for sphincterotomy from referral center patients are far greater than the 3% to 5% commonly seen in young and healthy patients undergoing laparoscopic cholecystectomy.48,58,59
The frequency and severity of morbidity of balloon dilation is unacceptable for routine clinical practice in the United States. Papillary balloon dilation has been proposed to avert the late-occurring complications of sphincterotomy, but it offers no clear benefit. There may be partial return of sphincter function 12 to 15 months after dilation. Chronic inflammation occurs 2 to 63 months after dilation with unknown clinical significance.111–114 Gallbladder motility seems normal 2 years after dilation but may be increased after sphincterotomy.115 Bile duct flow assessed by quantitative cholescintigraphy 1 to 3 years after ERC is unaffected by dilation but is significantly increased after sphincterotomy.116 Reflux of pancreatic enzymes into the bile duct as a measure of sphincter function was undetectable 1 year after either dilation or sphincterotomy.117
A multicenter Japanese study of 837 patients undergoing balloon dilation and followed for a mean of 4.4 years revealed biliary complications in 12.4% including recurrent stones in 8.8% and cholecystitis in 4.5%. Risk factors included lithotripsy at ERC and cholecystectomy either before or more than 30 days after ERC.105 Another retrospective series of 686 patients revealed a statistically significant difference in late recurrence of stones after dilation in 8% and sphincterotomy in 17%.114 A randomized study involving 104 patients followed for 16 months after ERC found similar rates of recurrent choledocholithiasis in 6.3% of dilation patients and 7.5% of sphincterotomy patients.118 Another small randomized trial revealed stone recurrence in 25% and 6.3% of dilation and sphincterotomy patients at about 1 year; with about 5 years of follow-up, this changed to 6.3% and 26.7% for dilation and sphincterotomy patients.119 Similar rates of morbidity of 29% for balloon dilation and 31% for sphincterotomy were reported at 10-year follow-up of the American multicenter, randomized study.98
Papillary balloon dilation may be appropriate for patients with severe coagulopathies and altered surgical anatomy that makes standard sphincterotomy more difficult and risky120–122; however, this approach may simply replace bleeding or perforation with pancreatitis. Pancreatic duct stent placement seems reasonable if papillary balloon dilation is used for these conditions. However, in routine clinical practice, balloon dilation of the sphincter of Oddi for extraction of bile duct stones is associated with high rates of morbidity and deaths from pancreatitis and should be avoided.
Difficult Stones
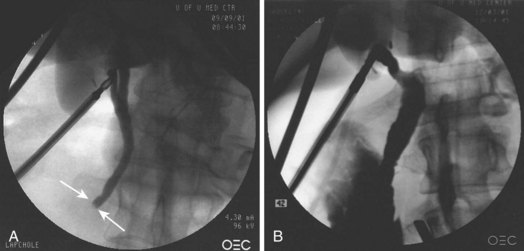
Impacted stones at the ampulla first should be approached by attempting to pass a guidewire or catheter around the stone and upstream into the duct or by dislodging the stone slightly upstream by pushing with a catheter (Fig. 43.15). If a wire or instrument can be passed above the stone, a sphincterotomy can be performed, and the stone can be extracted in the usual fashion. If instruments cannot pass the impacted stone, an access or precut papillotomy may be required. Using a needle-knife or a sphincterotome with the cutting wire extending over the distal tip, the attenuated papillary tissue encasing the impacted stone is incised in the direction of the bile duct at the 11 o’clock position. The incision is freehand with repeated smooth and shallow cuts in the same incision line until access is gained. This procedure requires an experienced endoscopist with excellent control of the instruments. Once the incision alleviates the impaction, the stone usually pops out spontaneously. However, it may be necessary to probe with the precut papillotome or an atraumatic guidewire until an instrument passes above the stone. Extension of the sphincterotomy can be performed with a standard sphincterotome.
Precut sphincterotomy is associated with increased rates of overall and severe complications compared with standard sphincterotomy. However, precutting is used to gain access to the duct in a complicated situation that requires therapy. The risks for precutting are less with a dilated bile duct and when the incision is made on an attenuated papilla, as is often the case with an impacted stone.58,123
Large Stones
Large stones are generally considered to be 10 mm or larger across the greatest span. They are often brown or mixed stones and are often impacted. Endoscopic extraction may be challenging because of the frequent presence of periampullary diverticula, the sheer volume of stone material, and the difficulty in entrapping very large stones in Dormia baskets. One series showed that only 12% of stones larger than 15 mm could be removed with standard sphincterotomy and techniques.124 A large opening is required to remove large stones effectively. The first endoscopic maneuver is to create a large sphincterotomy or to extend a prior sphincterotomy. If a guidewire or catheter can be passed upstream to the stone, it can often be removed with standard techniques, mechanical lithotripsy, or stent placement to provide drainage and break the stone over time. A relatively new technique is to perform large balloon dilation after sphincterotomy and remove the stones in the usual fashion or with mechanical lithotripsy.57 Alternatively, transendoscopic electrohydraulic lithotripsy (EHL), laser lithotripsy, or ESWL may be performed. One series of 108 patients with unsuccessful stone extraction by standard techniques showed success with mechanical lithotripsy in 33 patients, EHL in 65 patients, and ESWL in 7 of 10 patients with intrahepatic stones.56
Large-Caliber Balloon Dilation after Sphincterotomy
The biliary orifice can be safely opened to 12 to 20 mm by performing large-caliber balloon dilation after sphincterotomy. The sphincterotomy separates the biliary and pancreatic components of the sphincter and prevents the inflated balloon from compressing the pancreatic orifice. This approach does not seem to cause excessive rates of pancreatitis as with dilation of an intact papilla.48 The technique is to incise the ampullary mound partially or completely. A complete incision is favored by many authorities to minimize balloon-induced trauma to the sphincter. Patients with prior sphincterotomy can have large dilation without extension.
A wire-guided large-caliber balloon dilator of the type used for esophageal and gastrointestinal dilation is inserted over a guidewire to bridge the orifice. The balloon should be about the same diameter as the stone but no larger than the diameter of the duct. These balloons often do not have a radiopaque tip, and care must be taken to avoid inadvertent ductal perforation unseen on fluoroscopy. The balloon is positioned along the axis of the duct by advancing the duodenoscope and applying rightward torque. The balloon is inflated with dilute radiographic contrast material while maintaining position with endoscopic and fluoroscopic visualization. Once inflation is complete and obliteration of the waist on the balloon is verified, it may be deflated and removed. There is no standard time of maintained inflation. The stones may be extracted with balloons, baskets, or mechanical lithotripsy.57
From a compilation of 12 series involving 842 patients with large stones who had sphincterotomy followed by balloon dilation to about 15 mm (range 12 to 20 mm), 92% had total stone clearance at the first procedure, and 12% required mechanical lithotripsy. Morbidity occurred in 7%, mostly owing to mild to moderate bleeding, pancreatitis, perforation, and pain.125,126 Itoi and colleagues125 showed that ERC with sphincterotomy with standard techniques and mechanical lithotripsy had a mean procedure duration of 40 minutes compared with sphincterotomy with large-balloon dilation, which took 32 minutes (P < .05). These procedures also required 22 minutes and 13 minutes of fluoroscopy (P < .05). In a randomized study of 27 patients with large stones assigned to sphincterotomy plus large-balloon dilation and 28 patients assigned to sphincterotomy and standard techniques, no statistically significant differences were detected in success at stone removal at the first procedure, use of mechanical lithotripsy, procedure duration, or morbidity. However, the small sample size limits the validity of this study.127 Heo and colleagues128 randomly assigned 200 patients with large stones to have small sphincterotomy and large-balloon (12 to 20 mm) dilation or sphincterotomy with traditional techniques. Similar results were found for ductal clearance (97% vs. 98%); large stone extraction (94% vs. 97%); mechanical lithotripsy use (8% vs. 9%); and overall morbidity (5% vs. 7%) including pancreatitis (4% vs. 4%), cholecystitis (1% vs. 1%), and delayed bleeding (0 vs. 2%).
Intracorporeal Lithotripsy
In mechanical lithotripsy, when the stone is too large to be pulled out of the sphincterotomy, it is entrapped in a basket with securely welded wires. The basket is winched down against a metal sheath until the stone abuts the sheath, and continued pressure from retraction of the basket shatters the stone or breaks the basket (Fig. 43.16). Devices that pass through the working channel of the duodenoscope are manufactured as a single unit or a sheath that can be inserted over the long wire shaft of a basket that has become impacted at the bile duct orifice and then attached to a winch. Mechanical lithotripsy results in complete bile duct clearance in about 80% to 90% of patients, but 20% to 30% require multiple procedures. This technique is less likely to be successful with larger or impacted stones.129 Mechanical lithotripsy is a straightforward technology that should be present in all endoscopic units that perform stone extractions.
Intracorporeal lithotripsy can also be performed with EHL or laser probes under direct visualization via a cholangioscope passed through the duodenoscope. The probe can be aimed at the stone to prevent bile duct trauma. These techniques have traditionally required two endoscopists for the mother scope (duodenoscope) and the baby scope (4.5-mm or 3.0-mm cholangioscope). A light source and processing unit are required for each instrument as well as an irrigation system to maintain visualization and flush debris out of the bile duct. The endoscopist with the mother scope controls most of the motion of the baby scope, mainly with gentle torque on the duodenoscope insertion tube. The baby scope has tip deflection in only one axis. Single-user, partially disposable cholangioscopes are available that fasten to the duodenoscope. They have a reusable optical fiber, a working channel, and four-way tip deflection.129,130 Another technique in development uses a small-caliber gastroscope directed into the bile duct with a fixation balloon in place of the duodenoscope and cholangioscope system.131
EHL produces shock waves by generating high-voltage sparks that vaporize fluid and transmit this energy through a small-caliber (3-Fr, 1-mm) probe. The probe is positioned about 1 mm from the stone. Shock wave energy is activated by a foot switch and is repeated until the stone is fragmented. Centering balloons and basket catheters are also available to aim the probe under fluoroscopic guidance without choledochoscopy.132 However, aberrantly aimed shock waves can cause ductal trauma and perforation. EHL provides clearance of all stones and fragments in about 90% of patients with stones refractory to standard therapy. Morbidity is reported in about 9% of patients and is usually mild.130,133,134
Several laser lithotripsy systems have been used for bile duct stones. The most widely used are the holmium:yttrium aluminum garnet (YAG) and the frequency-doubled, double-pulse neodymium:YAG (FREDDY). High-power density laser light focused on a stone creates a plasma composed of a gaseous collection of ions and free electrons. An oscillating plasma bubble induces cavitation with tensile and compressive waves that shatter the stone surface.129
Holmium:YAG lasers are commercially available for treatment of bile duct stones. These lasers are commonly used for urinary tract stones. The laser delivers high-energy pulses of about 500 to 1000 mJ. Fibers come in several diameters ranging from 200 to 1000 µm and lengths of up to 4 m. Fibers are specifically designed for biliary use. The fibers must be used with a cholangioscope or centering balloon. Routine power settings are 0.6 to 1.0 J at 6 to 10 Hz for a total laser energy of 12 kJ. These are small, portable units that require no special plumbing. The FREDDY laser uses wavelengths of 532 to 1064 nm and generates up to 120 to 160 mJ (about 24 mJ at 532 nm). The pulse duration is 1.2 µsec at 160 mJ with single or dual pulse at adjustable rates of 1 Hz, 3 Hz, 5 Hz, or 10 Hz with standard 110 voltage AC. The recommended settings are 120 mJ single pulse and 3 to 5 Hz repetition rate, which can be increased to 160 mJ and 10 Hz. The fibers are 3.5 m long with a diameter of 420 µm and are reusable. The FREDDY laser causes minimal, if any, trauma to the duct and has been used with a cholangioscope and with centering balloons. These units are also mobile and do not require special plumbing.129
The holmium:YAG laser is reported to induce total clearance of intrahepatic and extrahepatic stones in 97% of patients from a compilation of several small studies.129 There are reports of complications from the holmium:YAG laser, but it is reasonable to expect similar rates with EHL and other lasers. From two series involving 69 patients with large bile duct stones treated with the FREDDY laser under cholangioscopic (10%) or fluoroscopic (90%) guidance, complete clearance was achieved in 91% with a mean of 1.5 sessions. Mild morbidity occurred in 20% (nine cases of hemobilia, four cases of pancreatitis, and two cases of cholangitis).129,134–136
Extracorporeal Shock Wave Lithotripsy
ESWL focuses high-pressure shock wave energy at a desired point while minimizing the pressure in the adjacent tissue. There are different methods to generate the shock waves, but all systems require the shock waves to travel through water to minimize energy loss. The contact with the patient is with a water-filled compressible bag with a gel. When shock waves traverse the stone, the surface becomes cavitated, and changes in acoustic impedance release compressive and tensile forces resulting in fragmentation. The properties of the stone that determine fragmentation are the size, microcrystalline structure, and architecture and not the chemical makeup.137
ESWL can achieve complete removal of refractory bile duct stones in about 84% of patients and partial clearance in 12% of patients. Complete clearance is more difficult with larger stones but not with multiple stones.138 About 13% of treated patients have stone recurrence within about 1 year; this may be due to retained fragments after ESWL. About 30% to 40% of treated patients have complications, including pain, hemobilia, cholangitis, sepsis, hematomas, pancreatitis, hematuria, ileus, and anesthesia effects. Mortality occurs in less than 1%; predisposing factors are advanced age, serious comorbidities, and concomitant cholangitis.129 Randomized controlled studies show that intracorporeal laser lithotripsy is more effective at achieving complete ductal clearance than ESWL (about 92% vs. 66%). However, complete clearance was achieved in similar proportions of patients randomly assigned to ESWL (79%) and EHL (75%).129 Crossing over to another lithotripsy modality improved the ductal clearance rates to 94% to 100%.139–142
PTC with antegrade instrumentation, choledochoscopy, or the modalities described previously is another option. PTC techniques achieve ductal clearance in 80% to 97% of cases but often require multiple procedures. Percutaneous tubes are usually left in place for several weeks. Minor complications are frequent and include pain, fever, and local infections. Serious morbidity from pancreatitis (10%), bleeding (2.5%), sepsis (2.5%), and pneumothoraces (0.5%) and deaths are reported. Other complications include subcapsular hepatic hematomas, hepatic artery aneurysms, cholangitis, peritonitis, bilomas, gallstone ileus, and premature tube displacement and may require surgery or other invasive procedures. Surgery for bile duct stones is generally reserved as a last resort.129
Stents
Biliary drainage with nasobiliary drains or stents is required to prevent cholangitis when large stones cannot be removed at ERCP (Fig. 43.17). Nasobiliary drains are cumbersome to place and are uncomfortable and unsightly for the patient. Stents may be used to temporize until a definitive procedure is performed or as long-term therapy in patients with advanced age or serious comorbidities and a severely limited life span. Midterm to long-term morbidity and mortality rates are high, however, and careful patient selection is crucial. The stents may act as a wick allowing bile drainage through or alongside the stent when obstruction occurs. Friction from the stent may wear down or break stones. Sphincterotomy is performed, and one or two straight or pigtail 7-Fr or 10-Fr stents can be placed in virtually all cases. Stents placed for several months induce stone shrinkage and disappearance and facilitate removal at subsequent ERCP.143 Ursodeoxycholic acid seems to facilitate this process.144
More recent cohort studies involving 196 elderly and sick patients treated with stent placement for refractory stones and followed for means of 2 to 39 months showed serious morbidity in 33%, usually resulting from cholangitis, and related mortality in 6%. Cholangitis developed at means ranging from 2 to 39 months.145–148 In comparative studies, there were significantly higher late morbidity rates with stent placement than routine stone extraction (36% vs. 14%), EHL (63% vs. 8%), or surgery (36% vs. 8%) and elevated overall mortality rates with stent placement over EHL (74% vs. 41%).49,146–148
Hepatolithiasis
Primary intrahepatic stones are associated with ductal strictures, and secondary stones are gallstones or bile duct stones that have refluxed into the intrahepatic system. Primary stones occur mainly in residents of eastern Asia who are rural dwellers and of lower socioeconomic status. These stones are associated with parasitic infestations including Clonorchis sinensis, biliary infections, congenital factors, ductal abnormalities, and intrahepatic cholangiocarcinoma. In white patients, primary intrahepatic stones are associated with prior hepatobiliary surgery, strictures, cystic fibrosis, and Caroli’s disease. The intrahepatic ducts are dilated and contain multiple stones, often of mixed composition. Patients have recurrent bouts of pain, fever, and jaundice that require repeated operations or other invasive therapies.149
Secondary intrahepatic stones may be treated with ERCP techniques with or without intracorporeal lithotripsy. However, primary stones are difficult to treat from a transpapillary approach because of duct angulation, multiple strictures, and peripheral stone impaction. Sphincterotomy should be avoided unless definitive therapy is ensured because of increased risks of recurrent cholangitis. However, clearance of intrahepatic stones was achieved in 64% of 36 patients treated with peroral cholangioscopic lithotripsy with morbidity in 1 (3%) and recurrences in 22% with almost 8 years of follow-up.150
PTC with intracorporeal lithotripsy results in complete stone clearance in 77% to 85% of patients with morbidity in 1.6% to 22%.151,152 With 1 to 22 years of follow-up, recurrence of stones or cholangitis developed in about 63% and was directly proportional to duration of follow-up at a median of 11 to 18 years. Symptomatic recurrence was more frequent in patients with ductal dilation and strictures and occurred sooner in patients with strictures. Cholangitis and cholangiocarcinoma developed more often in patients with residual stones than patients without residual stones (44% and 16% vs. 6.6% and 0.7%).151–152 ESWL has also been used successfully with lesser clearance rates.50,51,139,141,142,153 In combination with intracorporeal lithotripsy, however, more than 90% of intrahepatic stones can be cleared.44 Patients with stones associated with strictures do not respond as well.
Hepatic resection or hepaticojejunostomy, usually with intraoperative or postoperative choledochoscopy and lithotripsy, may be performed in selected patients with hepatolithiasis. Resection is generally indicated for segments with parenchymal atrophy, multiple abscesses, or intrahepatic cholangiocarcinomas. Patients with resection fare better than patients with biliary-enteric anastomoses, with operative morbidity in 20% and 16%, mortality in 2% and 6%, residual stones in 2% to 60% and 44% to 90%, recurrent stones in 16% and 33%, and cholangitis in 3% and 31%.154,155
Mirizzi’s Syndrome
Mirizzi’s syndrome is a rare disorder that occurs when a gallstone is entrapped in the gallbladder neck or cystic duct and causes obstruction or fistula of the bile duct. The appearance on direct cholangiography is an extrinsic obstruction with smooth tapering of the proximal and distal margins. Making the diagnosis requires an index of suspicion and careful interpretation of direct cholangiography and high-quality MRCP or intraductal ultrasound. Endoscopic therapy is challenging, and standard techniques usually fail. Bile duct stent placement and intracorporeal or extracorporeal lithotripsy can be successful, however.156 Surgery is often complicated, and open conversion is often required. However, laparoscopic techniques are possible, and it is important to have a preoperative diagnosis to minimize the complications and conversion rates.157
Sump Syndrome
The sump syndrome is an unusual condition in which there is a choledochoduodenal fistula with downstream impaction of stones or food material, or both, in the bile duct remnant and sphincter segment. This condition may cause pain, pancreatitis, and cholangitis. Therapy is with endoscopic sphincterotomy, and excellent immediate and long-term results are obtained.158
Small Stones
Stones that are 3 mm or less in diameter are generally considered to be small (Fig. 43.18). The clinical significance of these lesions is uncertain and may be negligible. After ESWL, all stone fragments 3 mm or less in diameter and almost all stones 3.5 to 5 mm in diameter have been shown to pass into the duodenum with rare symptoms and no complications.159 In a study of 539 cholecystectomy patients, 12% of the patients randomly assigned to have IOC had unsuspected bile duct stones detected with equivalent amounts expected in the control group. Similar symptomatic outcomes occurred in the IOC and control groups, and none of the control patients had clinically detected retained bile duct stones with 3 years of follow-up.5 In another study of 163 preoperative patients with gallstones and abnormal liver test results and normal ERC, 49% had sphincterotomy with small bile duct stones found in 26%. With more than 3 years of postoperative follow-up, none of the sphincterotomy patients had biliary complications.6 In another report, about 5% of 942 laparoscopic cholecystectomy patients who had routine IOC had filling defects indicative of stones, and a biliary catheter was left in place. A normal cholangiogram was seen at 48 hours in 26% and at 6 weeks in another 26%, but 48% had stones detected and removed by ERCP at 12 weeks.7
These data indicate that most small bile duct stones, particularly stones that are 3 mm or less in diameter, pass spontaneously without adverse sequela. However, if conservative management of these lesions is contemplated, clinical follow-up should be continued because small stones cause pancreatitis, and secondary biliary cirrhosis may develop in 1 to 3 years with intermittent ductal obstruction.8,38
Postoperative Care
Most patients undergoing ERCP are observed in a holding area or are hospitalized after the procedure. Postoperative management depends on the clinical situation and endoscopic maneuvers performed. Most patients who have had an uncomplicated ERCP with sphincterotomy for stones can be discharged on the same day with instructions to avoid anticoagulants, aspirin, nonsteroidal antiinflammatory drugs, and antiplatelet drugs to prevent hemorrhage. Patients at increased risk for pancreatitis because of younger age, female sex, small-caliber ducts, difficult cannulations, repeated pancreatic injections or instrumentation, or precut sphincterotomy should be vigorously hydrated, given only sips of water, and provided with analgesics and antiemetics for 12 to 24 hours after the procedure. Patients with cholangitis who are adequately drained should receive antibiotics for 3 to 5 days after ERCP.42
Outpatient Endoscopic Retrograde Cholangiopancreatography
ERCP is regularly performed on an outpatient basis. Patients considered for same-day discharge must be stratified based on risk of complications. Good candidates are patients with few comorbidities and adequate support systems who reside in proximity to the hospital. Additional risk factors include suspected sphincter of Oddi dysfunction, cirrhosis, difficult cannulation, precut sphincterotomy, and combined PTC and endoscopic access. About 44% of complications in patients planned for same-day discharge develop within 2 hours, and 79% develop within 6 hours.160 More recent studies show that up to 19% of planned outpatient procedures resulted in admission; most complications were detected in the endoscopy or recovery units, and few patients with complications returned from home.161,162
Recurrent Stones
Recurrent stones develop in about 10% to 20% of patients who have had sphincterotomy or papillary balloon dilation for stone extraction.163–165 Recurrence is usually with brown stones, and the clinical presentation is often cholangitis. The problem may be due to bile stasis and repeated infections. Risk factors for recurrence include older age, periampullary diverticula, a largely dilated bile duct, biliary strictures, mechanical lithotripsy, and a gallbladder in situ. Clinical follow-up with serum enzyme levels and transabdominal ultrasound with ERCP for abnormal findings may improve stone extraction rates and decrease occurrences of cholangitis.163,166,167 Surgery may be beneficial to remove stones that cannot be extracted by endoscopic means but has not been shown to decrease recurrence rates.167
Complications
ERCP with sphincterotomy for stones is successful in more than 90% of patients; morbidity is about 5%, and mortality is rare.58 High-risk patients may safely and successfully undergo ERCP with sedation and analgesia for appropriate indications. Patients with significant comorbidities and morbid obesity who require ERCP benefit from general anesthesia with endotracheal intubation to minimize the risks of cardiopulmonary complications.168 Patients with severe acute pancreatitis and suppurative cholangitis benefit from urgent ERCP and stone extraction or drainage but often require general anesthesia and maintenance of vital signs. Successful ERCP has been reported in five patients within 15 to 56 days after myocardial infarction for urgent indications without cardiovascular complications.169 Patients with severe coagulopathies and thrombocytopenia with urgent indications for stone extraction should have attempts made to correct the problem with vitamin K, plasma, or platelet transfusions.60 If the deficit cannot be corrected, stent placement or nasobiliary drainage may be performed without sphincterotomy, or papillary balloon dilation may be performed to minimize bleeding risks.88,120
1 Ko CW, Lee SP. Epidemiology and natural history of common bile duct stones and prediction of disease. Gastrointest Endosc. 2002;56(Suppl 6):S165-S169.
2 Ko CW, Sekijima JH, Lee SP. Biliary sludge. Ann Intern Med. 1999;130:301-311.
3 Lee JG, Leung JW. Choledocholithiasis, bacterial cholangitis, oriental intrahepatic stone disease, and parasitic disorders of the biliary tree. In: DiMarino AJ, Benjamin SB, editors. Gastrointestinal disease. An endoscopic approach. Malden, MA: Blackwell Science; 1997:852-870.
4 Gracie WA, Ransohoff DF. The natural history of silent gallstones: The innocent gallstone is not a myth. N Engl J Med. 1982;307:798-800.
5 Murison MS, Gartell PC, McGinn FP. Does selective preoperative cholangiography result in missed common bile duct stones? J R Coll Surg Edinb. 1993;38:220-224.
6 Siddique I, Mohan K, Khajah A, et al. Sphincterotomy in patients with gallstones, elevated LFTs and a normal CBD on ERCP. Hepatogastroenterology. 2003;50:1242-1245.
7 Collins C, Maguire D, Ireland A, et al. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: Natural history of choledocholithiasis revisited. Ann Surg. 2004;239:28-33.
8 Lakshmi MV, Sridharan GV, Butterworth D. Gallstone cirrhosis: Are we only seeing the tip of the iceberg? Br J Clin Pract. 1993;47:164-165.
9 George GO, Spiegelman GA, Barkin JS. Normal serum alkaline phosphatase: An unusual finding in early suppurative biliary obstruction. Am J Gastroenterol. 1993;88:771-778.
10 Gandolfi L, Torresan F, Solmi L, et al. The role of ultrasound in biliary and pancreatic disease. Eur J Ultrasound. 2003;16:141-159.
11 Mortele KJ, Ji H, Ros PR. CT and magnetic resonance imaging in pancreatic and biliary tract malignancies. Gastrointest Endosc. 2002;56:S206-S212.
12 Mark DH, Flamm CR, Aronson N. Evidence-based assessment of diagnostic modalities for common bile duct stones. Gastrointest Endosc. 2002;56:S190-S194.
13 Romagnuolo J, Bardou M, Rahme E, et al. Magnetic resonance cholangiopancreatography: A meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557.
14 Maurea S, Caleo O, Mollica C, et al. Comparative diagnostic evaluation with MR cholangiopancreatography, ultrasonography and CT in patients with pancreatobiliary disease. Radiology. 2009;114:390-402.
15 Tse F, Barkun AN, Armstrong D, et al. EUS: A meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc. 2008;67:235-244.
16 Montariol T, Msika S, Charlier A, et al. Diagnosis of asymptomatic common bile duct stones: Preoperative endoscopic ultrasonography versus intraoperative cholangiography—a multicenter, prospective controlled study. Surgery. 1998;124:6-13.
17 Petelin JB. Surgical management of common bile duct stones. Gastrointest Endosc. 2002;56:S183-S189.
18 Hublet A, Dili A, Lemaire J, et al. Laparoscopic ultrasonography as a good alternative to intraoperative cholangiography (IOC) during laparoscopic cholecystectomy: Results of prospective study. Acta Chir Belg. 2009;109:312-316.
19 Sun XD, Cai XY, Li JD, et al. Prospective study of scoring system in selective intraoperative cholangiography during laparoscopic cholecystectomy. World J Gastroenterol. 2003;9:865-867.
20 Topal B, Fiews S, Tomczyk K, et al. Clinical models are inaccurate in predicting bile duct stones in situ for patients with gallbladder. Surg Endosc. 2009;23:38-44.
21 Artifon EL, Kumar A, Eloubeidi MA, et al. Prospective randomized trial of EUS versus ERCP-guided common bile duct stone removal: An interim report (with video). Gastrointest Endosc. 2009;69:238-243.
22 Martin DJ, Vernon DR, Toouli J: Surgical versus endoscopic treatment for bile duct stones. Cochrane Database Syst Rev (2):CD003327, 2006.
23 Keizman D, Ish-Shalom M, Konikoff FM. The clinical significance of bile duct sludge: Is it different from bile duct stones? Surg Endosc. 2007;21:769-773.
24 Ros E, Navarro S, Bru C, et al. Occult microlithiasis in “idiopathic” acute pancreatitis: Prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology. 1991;101:1701-1709.
25 Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589-593.
26 Perez-Martin G, Gomez-Cerezo J, Codoceo C, et al. Bilirubinate granules: Main pathologic bile component in patients with idiopathic acute pancreatitis. Am J Gastroenterol. 1998;93:360-362.
27 Gloor B, Stahel PF, Muller CA, et al. Incidence and management of biliary pancreatitis in cholecystectomized patients. J Gastrointest Surg. 2003;7:372-377.
28 Ammori BJ, Boreham B, Lewis P, et al. The biochemical detection of biliary etiology of acute pancreatitis on admission: A revisit in the modern era of biliary imaging. Pancreas. 2003;26:e32-e35.
29 Calvo MM, Bujanda L, Heras I, et al. Magnetic resonance cholangiography versus ultrasound in the evaluation of the gallbladder. J Clin Gastroenterol. 2002;34:233-236.
30 Liu CL, Lo CM, Chan JK, et al. EUS for detection of occult cholelithiasis in patients with idiopathic pancreatitis. Gastrointest Endosc. 2000;51:28-32.
31 Kim BJ, Kang P, Lee JK, et al. Are the echogenicities on intraductal ultrasonography really biliary microlithiasis? Dig Dis Sci. 2010;55:836-841.
32 Choudhuri G, Agarwal DK, Saraswat VA, et al. Is duodenal bile representative of gallbladder bile? A comparative study. Scand J Gastroenterol. 1993;28:920-923.
33 Kohut M, Nowak A, Nowakowska-Dulawa E, et al. The frequency of bile duct crystals in patients with presumed biliary pancreatitis. Gastrointest Endosc. 2001;54:37-41.
34 Acosta JM, Ledesma CL. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974;290:484-487.
35 Chak A, Hawes RH, Cooper GS, et al. Prospective assessment of the utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc. 1999;49:599-604.
36 Kelly TR. Gallstone pancreatitis: The timing of surgery. Surgery. 1980;88:345-350.
37 Armstrong CP, Taylor TV, Jeacock J, et al. The biliary tract in patients with acute gallstone pancreatitis. Br J Surg. 1985;72:551-555.
38 Diehl AK, Holleman DRJr, Chapman JB, et al. Gallstone size and risk of pancreatitis. Arch Intern Med. 1997;157:1674-1678.
39 Kaw M, Al-Antably Y, Kaw P. Management of gallstone pancreatitis: Cholecystectomy or ERCP and endoscopic sphincterotomy. Gastrointest Endosc. 2002;56:61-65.
40 Tenner S, Dubner H, Steinberg W. Predicting gallstone pancreatitis with laboratory parameters: A meta-analysis. Am J Gastroenterol. 1994;89:1863-1866.
41 Bradley EL3rd. A clinically based classification system of acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg. 1993;128:586-590.
42 Tanaka A, Takada T, Kawarada Y, et al. Antimicrobial therapy for acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:59-67.
43 Lee NK, Kim S, Lee JW, et al. Discrimination of suppurative cholangitis from nonsuppurative cholangitis with computed tomography (CT). Eur J Radiol. 2009;69:528-535.
44 Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest Endosc. 1991;37:383-393.
45 Vlavianos P, Chopra K, Mandalia S, et al. Endoscopic balloon dilatation versus endoscopic sphincterotomy for the removal of bile duct stones: A prospective randomised trial. Gut. 2003;52:1165-1169.
46 Bergman JJ, Rauws EA, Fockens P, et al. Randomised trial of endoscopic balloon dilation versus endoscopic sphincterotomy for removal of bile duct stones. Lancet. 1997;349:1124-1129.
47 Fujita N, Maguchi H, Komatsu Y, et al. Endoscopic sphincterotomy and endoscopic papillary balloon dilatation for bile duct stones: A prospective randomized controlled multicenter study. Gastrointest Endosc. 2003;57:151-155.
48 DiSario JA, Freeman ML, Bjorkman DJ, et al. Endoscopic balloon dilation compared to spincterotomy for extraction of bile duct stones. Gastroenterology. 2004;127:1291-1299.
49 Hui CK, Lai KC, Ng M, et al. Retained common bile duct stones: A comparison between biliary stenting and complete clearance of stones by electrohydraulic lithotripsy. Aliment Pharmacol Ther. 2003;17:289-296.
50 Sackmann M, Holl J, Sauter GH, et al. Extracorporeal shock wave lithotripsy for clearance of bile duct stones resistant to endoscopic extraction. Gastrointest Endosc. 2001;53:27-32.
51 Ellis RD, Jenkins AP, Thompson RP, et al. Clearance of refractory bile duct stones with extracorporeal shockwave lithotripsy. Gut. 2000;47:728-731.
52 Hochberger J, Bayer J, May A, et al. Laser lithotripsy of difficult bile duct stones: Results in 60 patients using a rhodamine 6G dye laser with optical stone tissue detection system. Gut. 1998;43:823-829.
53 Cipolletta L, Costamagna G, Bianco MA, et al. Endoscopic mechanical lithotripsy of difficult common bile duct stones. Br J Surg. 1997;84:1407-1409.
54 Hintze RE, Adler A, Veltzke W. Outcome of mechanical lithotripsy of bile duct stones in an unselected series of 704 patients. Hepatogastroenterology. 1996;43:473-476.
55 Shaw MJ, Mackie RD, Moore JP, et al. Results of a multicenter trial using a mechanical lithotripter for the treatment of large bile duct stones. Am J Gastroenterol. 1993;88:730-733.
56 Binmoeller KF, Bruckner M, Thonke F, et al. Treatment of difficult bile duct stones using mechanical, electrohydraulic and extracorporeal shock wave lithotripsy. Endoscopy. 1993;25:201-206.
57 Attam R, Freeman ML. Endoscopic papillary large balloon dilation for large common bile duct stones. J Hepatobiliary Pancreat Surg. 2009;16:618-623.
58 Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918.
59 Cotton PB, Geenen JE, Sherman S, et al. Endoscopic sphincterotomy for stones by experts is safe, even in younger patients with normal ducts. Ann Surg. 1998;227:201-204.
60 Anderson MA, Ben-Menachem T, Gan SI, et al. ASGE Standards of Practice Committee. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060-1070.
61 Banerjee S, Shen B, Baron TH, et al. ASGE Standards of Practice Committee. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67:791-798.
62 Qureshi WA, Rajan E, Adler DG, et al. American Society for Gastrointestinal Endoscopy. ASGE Guideline: Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc. 2005;61:357-362.
63 Tham TC, Vandervoort J, Wong RC, et al. Safety of ERCP during pregnancy. Am J Gastroenterol. 2003;98:308-311.
64 Tang SJ, Mayo MJ, Rodriguez-Frias E, et al. Safety and utility of ERCP in pregnancy. Gastrointest Endosc. 2009;69:453-461.
65 Akcakaya A, Ozkan OV, Okan I, et al. Endoscopic retrograde cholangiopancreatography during pregnancy without radiation. World J Gastroenterol. 2009;15:3649-3652.
66 Sharma SS, Maharshi S. Two stage endoscopic approach for management of choledocholithiasis in pregnancy. J Gastrointest Liver Dis. 2008;17:83-85.
67 Shelton J, Linder JD, Rivera-Alsina ME, et al. Commitment, confirmation, and clearance: New techniques for nonradiation ERCP in pregnancy. Gastrointest Endosc. 2008;67:364-368.
68 Sharma VK, Howden CW. Metaanalysis of randomized controlled trials of endoscopic retrograde cholangiography and endoscopic sphincterotomy for the treatment of acute biliary pancreatitis. Am J Gastroenterol. 1999;94:3211-3214.
69 Neoptolemos JP, Carr-Locke DL, London NJ, et al. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983.
70 Fan ST, Lai EC, Mok FP, et al. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328:228-232.
71 Fölsch UR, Nitsche R, Ludtke R, et al. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. N Engl J Med. 1997;336:237-242.
72 Zhou MQ, Li NP, Lu RD. Duodenoscopy in treatment of acute gallstone pancreatitis. Hepatobiliary Pancreat Dis Int. 2002;1:608-610.
73 Acosta JM, Katkhouda N, Debian KA, et al. Early ductal decompression versus conservative management for gallstone pancreatitis with ampullary obstruction: A prospective randomized clinical trial. Ann Surg. 2006;243:33-40.
74 Oria A, Cimmino D, Ocampo C, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction: A randomized clinical trial. Ann Surg. 2007;245:10-17.
75 Ayub K, Imada R, Slavin J: Endoscopic retrograde cholangiopancreatography in gallstone-associated acute pancreatitis. Cochrane Database Syst Rev (18):CD003630, 2004.
76 Petrov MS, van Santvoort HC, Besselink MG, et al. Early endoscopic retrograde cholangiopancreatography versus conservative management in acute biliary pancreatitis without cholangitis: A meta-analysis of randomized trials. Ann Surg. 2008;247:250-257.
77 Petrov MS, Uchugina AF, Kukosh MV. Does endoscopic retrograde cholangiopancreatography reduce the risk of local pancreatic complications in acute pancreatitis? A systematic review and metaanalysis. Surg Endosc. 2008;22:2338-2343.
78 Moretti A, Papi C, Aratari A, et al. Is early endoscopic retrograde cholangiopancreatography useful in the management of acute biliary pancreatitis? A meta-analysis of randomized controlled trials. Dig Liver Dis. 2008:379-385.
79 Uy MC, Daez ML, Sy PP, et al. Early ERCP in acute gallstone pancreatitis without cholangitis: A meta-analysis. JOP. 2009;10:299-305.
80 van Santvoort HC, Besselink MG, de Vries AC, et al. Early endoscopic retrograde cholangiopancreatography in predicted severe acute biliary pancreatitis: A prospective multicenter study. Ann Surg. 2009;250:68-75.
81 Liu CL, Fan ST, Lo CM, et al. Comparison of early endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in the management of acute biliary pancreatitis: A prospective randomized study. Clin Gastroenterol Hepatol. 2005;3:1238-1244.
82 Yu W, Li W, Wang Z, et al. Early percutaneous transhepatic gallbladder drainage compared with endoscopic retrograde cholangiopancreatography and papillotomy treatment for severe gallstone associated acute pancreatitis. Postgrad Med J. 2007;83:187-191.
83 ASGE Standards of Practice CommitteeMaple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc. 2010;71:1-9.
84 Forsmark CE, Baillie J, AGA Institute Clinical Practice and Economics Committee; AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022-2044.
85 Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400.
86 Lai EC, Tam PC, Paterson IA, et al. Emergency surgery for severe acute cholangitis: The high-risk patients. Ann Surg. 1990;211:55-59.
87 Lai EC, Mok FP, Tan ES, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582-1586.
88 Lee DW, Chan AC, Lam YH, et al. Biliary decompression by nasobiliary catheter or biliary stent in acute suppurative cholangitis: A prospective randomized trial. Gastrointest Endosc. 2002;56:361-365.
89 Winick AB, Waybill PN, Venbrux AC. Complications of transhepatic biliary interventions. Tech Vasc Interv Radiol. 2001;4:200-206.
90 Cuschieri A. Management of patients with gallstones and ductal calculi. Lancet. 2002;360:739-740.
91 Poon RT, Liu CL, Lo CM, et al. Management of gallstone cholangitis in the era of laparoscopic cholecystectomy. Arch Surg. 2001;136:11-16.
92 Caroli-Bosch FX, Montet JC, Salmon L, et al. Effect of endoscopic sphincterotomy on bile lithogenicity in patients with gallbladder in situ. Endoscopy. 1999;31:437-441.
93 Hammarstrom LE, Holmin T, Stridbeck H. Endoscopic treatment of bile duct calculi in patients with gallbladder in situ: Long-term outcome and factors. Scand J Gastroenterol. 1996;31:294-301.
94 Schreurs WH, Vles WJ, Stuifbergen WH, et al. Endoscopic management of common bile duct stones leaving the gallbladder in situ: A cohort study with long-term follow-up. Dig Surg. 2004;21:60-64.
95 Targarona EM, Ayuso RM, Bordas JM, et al. Randomized trial of endoscopic sphincterotomy with gallbladder left in situ versus open surgery for common bile duct calculi in high-risk patients. Lancet. 1996;347:926-929.
96 Boerma D, Rauws EA, Keulemans YC, et al. Wait-and-see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile-duct stones: A randomised trial. Lancet. 2002;360:761-765.
97 McAlister VC, Davenport E, Renouf E: Cholecystectomy deferral in patients with endoscopic sphincterotomy. Cochrane Database Syst Rev (17):CD006233, 2007.
98 Folkers MT, DiSario JA, Adler DG. Long-term complications of endoscopic biliary sphincterotomy for choledocholithiasis: A North-American perspective. Am J Gastroenterol. 2009;104:2868-2869.
99 Sugiyama M, Atomi Y. Risk factors predictive of late complications after endoscopic sphincterotomy for bile duct stones: Long-term (more than 10 years) follow-up study. Am J Gastroenterol. 2002;97:2763-2767.
100 Bergman JJ, van der Mey S, Rauws EA, et al. Long-term follow-up after endoscopic sphincterotomy for bile duct stones in patients younger than 60 years of age. Gastrointest Endosc. 1996;44:643-649.
101 Tsujino T, Hiroyuki I, Komatsu Y, et al. Risk factors for pancreatitis in patients with common bile duct stones managed by endoscopic papillary balloon dilation. Am J Gastroenterol. 2005;100:38-42.
102 Aizawa T, Ueno N. Stent placement in the pancreatic duct prevents pancreatitis after endoscopic sphincter dilation for removal of bile duct stones. Gastrointest Endosc. 2001;54:209-213.
103 Weinberg BM, Shindy W, Lo S. Endoscopic balloon sphincter dilation (sphincteroplasty) versus sphincterotomy for common bile duct stones. Cochrane Database Syst Rev (4):CD004890, 2006.
104 Arnold JC, Benz C, Martin WR, et al. Endoscopic papillary balloon dilation vs. sphincterotomy for removal of common bile duct stones: A prospective randomized pilot study. Endoscopy. 2001;33:563-567.
105 Tsujino T, Kawabe T, Komatsu Y, et al. Endoscopic papillary balloon dilation for bile duct stone: Immediate and long-term outcomes in 1000 patients. Clin Gastroenterol Hepatol. 2007;5:130-137.
106 Mathuna PM, White P, Clarke E, et al. Endoscopic balloon sphincteroplasty (papillary dilation) for bile duct stones: Efficacy, safety, and follow-up in 100 patients. Gastrointest Endosc. 1995;42:468-474.
107 Komatsu Y, Kawabe T, Toda N, et al. Endoscopic papillary balloon dilation for the management of common bile duct stones: Experience of 226 cases. Endoscopy. 1998;30:12-17.
108 Ueno N, Ozawa Y. Pancreatitis induced by endoscopic balloon sphincter dilation and changes in serum amylase levels after the procedure. Gastrointest Endosc. 1999;49:472-476.
109 Watanabe H, Yoneda M, Tominaga K, et al. Comparison between endoscopic papillary balloon dilatation and endoscopic sphincterotomy for the treatment of common bile duct stones. J Gastroenterol. 2007;42:56-62.
110 Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: A prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434.
111 Yasuda I, Tomita E, Enya M, et al. Can endoscopic papillary balloon dilation really preserve sphincter of Oddi function? Gut. 2001;49:686-691.
112 Mac Mathuna P, Siegenberg D, Gibbons D, et al. The acute and long-term effect of balloon sphincteroplasty on papillary structure in pigs. Gastrointest Endosc. 1996;44:650-655.
113 Kawabe T, Komatsu Y, Isayama H, et al. Histological analysis of the papilla after endoscopic papillary balloon dilation. Hepatogastroenterology. 2003;50:919-923.
114 Kojima Y, Nakagawa H, Miyata A, et al. Long-term prognosis of bile duct stones: Endoscopic papillary balloon dilatation versus endoscopic sphincterotomy. Dig Endosc. 2010;22:21-24.
115 Sugiyama M, Atomi Y. Medium-term effects of endoscopic papillary balloon dilation on gallbladder motility. Gastrointest Endosc. 2001;54:459-463.
116 Isayama H, Komatsu Y, Inoue Y, et al. Preservation of the sphincter of Oddi after endoscopic papillary balloon dilation. Hepatogastroenterology. 2003;50:1787-1791.
117 Takezawa M, Kida Y, Kida M, et al. Influence of endoscopic papillary balloon dilation and sphincterotomy on sphincter of Oddi function: A randomized controlled trial. Endoscopy. 2003;36:631-637.
118 Lin CK, Lai KH, Chan HH, et al. Endoscopic balloon dilatation is a safe method in the management of common bile duct stones. Dig Liver Dis. 2004;36:68-72.
119 Tanaka S, Sawayama T, Yoshioka T. Endoscopic papillary balloon dilation and endoscopic sphincterotomy for bile duct stones: Long-term outcomes in a prospective randomized controlled trial. Gastrointest Endosc. 2004;59:614-618.
120 Kawabe T, Komatsu Y, Tada M, et al. Endoscopic papillary balloon dilation in cirrhotic patients: Removal of common bile duct stones without sphincterotomy. Endoscopy. 1996;28:694-698.
121 Bergman JJ, van Berkel AM, Bruno MJ, et al. A randomized trial of endoscopic balloon dilation and endoscopic sphincterotomy for removal of bile duct stones in patients with a prior Billroth II gastrectomy. Gastrointest Endosc. 2001;53:19-26.
122 Prat F, Fritsch J, Choury AD, et al. Endoscopic sphincteroclasy: A useful therapeutic tool for biliary endoscopy in Billroth II gastrectomy patients. Endoscopy. 1997;29:79-81.
123 Bruins Slot W, Schoeman MN, DiSario JA, et al. Needle-knife sphincterotomy as a precut procedure: A retrospective evaluation of efficacy and complications. Endoscopy. 1996;28:334-339.
124 Lauri A, Horton RC, Davidson BR, et al. Endoscopic extraction of bile duct stones: Management related to stone size. Gut. 1993;34:1718-1721.
125 Itoi T, Itokawa F, Sofuni A, et al. Endoscopic sphincterotomy combined with large balloon dilation can reduce the procedure time and fluoroscopy time for removal of large bile duct stones. Am J Gastroenterol. 104, 2009. 560–556
126 Attasaranya S, Cheon Y, Vittal H, et al. Large-diameter biliary orifice balloon dilation to aid in endoscopic bile duct stone retrieval: A multicenter series. Gastrointest Endosc. 2008;67:1046-1052.
127 Kim HG, Cheon YK, Cho YD, et al. Small sphincterotomy combined with endoscopic papillary large balloon dilation versus sphincterotomy. World J Gastroenterol. 2009;15:4298-4304.
128 Heo JH, Kang DH, Jung HJ, et al. Endoscopic sphincterotomy plus large-balloon dilation versus endoscopic sphincterotomy for removal of bile-duct stones. Gastrointest Endosc. 2007;66:720-726.
129 DiSario J, Chuttani R, Croffie J, et al. Biliary and pancreatic lithotripsy devices. Gastrointest Endosc. 2007;65:750-756.
130 Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: A clinical feasibility study (with video). Gastrointest Endosc. 2007;65:832-841.
131 Moon JH, Ko BM, Koo HC, et al. Direct peroral cholangioscopy using an ultra-slim upper endoscope for the treatment of retained bile duct stones. Am J Gastroenterol. 2009;104:2729-2733.
132 Lee JE, Moon JH, Choi HJ, et al. Endoscopic treatment of difficult bile duct stones by using a double-lumen basket for laser lithotripsy—a case series. Endoscopy. 2010;42:169-172.
133 Bland PJ, Lundmark M. Management of bile duct stones: Lithotripsy by laser, electrohydraulic, and ultrasonic techniques. Report of a series and a clinical review. Eur J Surg. 1998;164:403-409.
134 Piraka C, Shah RJ, Awadallah NS, et al. Transpapillary cholangioscopy-directed lithotripsy in patients with difficult bile duct stones. Clin Gastroenterol Hepatol. 2007;5:1333-1338.
135 Cho YD, Cheon YK, Moon JH, et al. Clinical role of frequency-doubled double-pulsed yttrium aluminum garnet laser technology for removing difficult bile duct stones (with videos). Gastrointest Endosc. 2009;70:684-689.
136 Kim TH, Oh HJ, Choi CS, et al. Clinical usefulness of transpapillary removal of common bile duct stones by frequency-doubled double pulse Nd:YAG laser. World J Gastroenterol. 2008;14:2863-2866.
137 Paumgartner G, Walter B. Cannon lecture. Shock-wave lithotripsy of gallstones. AJR Am J Roentgenol. 1989;153:235-242.
138 Tandan M, Reddy DN, Santosh D, et al. Extracorporeal shock wave lithotripsy of large difficult common bile duct stones: Efficacy and analysis of factors that favor stone fragmentation. J Gastroenterol Hepatol. 2009;24:1370-1374.
139 Adamek HE, Maier M, Jacobs R, et al. Management of retained bile duct stones: A prospective open trial comparing extracorporeal and intracorporeal lithotripsy. Gastrointest Endosc. 1996;44:40-47.
140 Jakobs R, Adamek HE, Maier M, et al. Fluoroscopically guided laser lithotripsy versus extracorporeal shock wave lithotripsy for retained bile duct stones: A prospective randomized study. Gut. 1997;40:678-682.
141 Neuhaus H, Zillinger C, Born P, et al. Randomized study of intracorporeal laser lithotripsy versus extracorporeal shock-wave lithotripsy for difficult bile duct stones. Gastrointest Endosc. 1998;47:327-334.
142 Bland KI, Jones RS, Maher JW, et al. Extracorporeal shock-wave lithotripsy of bile duct calculi. An interim report of the Dornier U.S. Bile Duct Lithotripsy Prospective Study. Ann Surg. 1989;209:743-753.
143 Li KW, Zhang XW, Ding J, et al. A prospective study of the efficacy of endoscopic biliary stenting on common bile duct stones. J Dig Dis. 2009;10:328-331.
144 Han J, Moon JH, Koo HC, et al. Effect of biliary stenting combined with ursodeoxycholic acid and terpene treatment on retained common bile duct stones in elderly patients: A multicenter study. Am J Gastroenterol. 2009;104:2418-2421.
145 Chan AC, Ng EK, Chung CS, et al. Common bile duct stones become smaller after endoscopic biliary stenting. Endoscopy. 1998;30:356-359.
146 De Palma GD, Catanzano C. Stenting or surgery for treatment of irretrievable common bile duct calculi in elderly patients? Am J Surg. 1999;178:390-393.
147 Bergman JJ, Rauws EA, Tijssen JG, et al. Biliary endoprostheses in elderly patients with endoscopically irretrievable common bile duct stones: Report on 117 patients. Gastrointest Endosc. 1995;42:195-201.
148 Chopra KB, Peters RA, O’Toole PA, et al. Randomized study of endoscopic biliary endoprosthesis versus duct clearance for bile duct stones in high-risk patients. Lancet. 1996;348:791-793.
149 Kim MH, Sekijima J, Lee SP. Primary intrahepatic stones. Am J Gastroenterol. 1995;90:540-548.
150 Okugawa T, Tsuyuguchi T, et al. Peroral cholangioscopic treatment of hepatolithiasis: Long-term results. Gastrointest Endosc. 2002;56:366-371.
151 Huang MH, Chen CH, Yang JC, et al. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655-2662.
152 Cheung MT, Wai SH, Kwok PC. Percutaneous transhepatic choledochoscopic removal of intrahepatic stones. Br J Surg. 2003;90:1409-1415.
153 Adamek HE, Schneider AR, Adamek MU, et al. Treatment of difficult intrahepatic stones by using extracorporeal and intracorporeal lithotripsy techniques: 10 years’ experience in 55 patients. Scand J Gastroenterol. 1999;34:1157-1161.
154 Uchiyama K, Onishi H, Tani M, et al. Indication and treatment of hepatolithiasis. Arch Surg. 2002;137:149-153.
155 Chen MF, Jan YY, Lee TY. Percutaneous transhepatic cholangioscopy. Br J Surg. 1987;74:728-730.
156 Tsuyuguchi T, Saisho H, Ishihara T, et al. Long-term follow-up after treatment of Mirizzi syndrome by peroral cholangioscopy. Gastrointest Endosc. 2000;52:639-644.
157 Antoniou SA, Antoniou GA, Makridis C. Laparoscopic treatment of Mirizzi syndrome: A systematic review. Surg Endosc. 2010;24:33-39.
158 Caroli-Bosc FX, Demarquay JF, Peten EP, et al. Endoscopic management of sump syndrome after choledochoduodenostomy: Retrospective analysis of 30 cases. Gastrointest Endosc. 2000;51:180-183.
159 Greiner L, Munks C, Heil W, et al. Gallbladder stone fragments in feces after biliary extracorporeal shock-wave lithotripsy. Gastroenterology. 1990;98:1620-1624.
160 Freeman ML, Nelson DB, Sherman S, et al. Same-day discharge after endoscopic biliary sphincterotomy: Observations from a prospective multicenter complication study. The Multicenter Endoscopic Sphincterotomy (MESH) Study Group. Gastrointest Endosc. 1999;49:580-586.
161 Fox CJ, Harry RA, Cairns SR. A prospective series of out-patient endoscopic retrograde cholangiopancreatography. Eur J Gastroenterol Hepatol. 2000;12:523-527.
162 Tham TC, Vandervoort J, Wong RC, et al. Therapeutic ERCP in outpatients. Gastrointest Endosc. 1997;45:225-230.
163 Lai KH, Lo GH, Lin CK, et al. Do patients with recurrent choledocholithiasis after endoscopic sphincterotomy benefit from regular follow-up? Gastrointest Endosc. 2002;55:523-526.
164 Ueno N, Ozawa Y, Aizawa T. Prognostic factors for recurrence of bile duct stones after endoscopic treatment by sphincter dilation. Gastrointest Endosc. 2003;58:336-340.
165 Ando T, Tsuyuguchi T, Okugawa T, et al. Risk factors for recurrent bile duct stones after endoscopic papillotomy. Gut. 2003;52:116-121.
166 Geenen DJ, Geenen JE, Jafri FM, et al. The role of surveillance endoscopic retrograde cholangiography in preventing episodic cholangitis in patients with recurrent common bile duct stones. Endoscopy. 1988;30:18-20.
167 Cetta F. Do surgical and endoscopic sphincterotomy prevent or facilitate recurrent common duct stone formation? Arch Surg. 1993;128:329-336.
168 Ramirez FC, McIntosh AS, Dennert B, et al. Emergency endoscopic retrograde cholangiopancreatography in critically ill patients. Gastrointest Endosc. 1998;47:368-371.
169 Capell MS. Endoscopic retrograde cholangiopancreatography with endoscopic sphincterotomy for symptomatic choledocholithiasis after recent myocardial infarction. Am J Gastroenterol. 1996;91:827-831.