Chapter 10 Cholangiocarcinoma
Epidemiology and Risk Factors
IHCC occurs most commonly in the sixth and seventh decades. There is male-to-female predominance of 3:2. The age-adjusted mortality increased from 0.15 to 0.66 per 100,000 persons from 1973 to 1997.1 The incidence of IHCC also increased from 0.13 to 0.67 per 100,000 from 1973 to 1997. The 5-year survival is less than 10%. The survival approaches 0% in the setting of intrahepatic metastasis. Surgery offers the best outcome.2
Anatomy
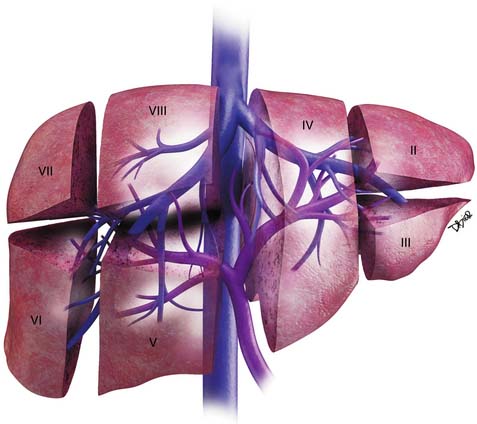
The liver is divided into the right and left lobes and subdivided into eight anatomic segments (Figure 10-1). The segments are defined by their portal vein supply and separated from each other by the hepatic veins. The caudate lobe is considered segment I. The portal supply to the caudate lobe arises commonly from the main portal vein but may be seen from the left or right portal veins. Segments II and III of the liver are in the left lobe and are supplied by the first and second lateral branches of the left portal vein, respectively. Segment IV is also in the left lobe of the liver and is separated from segments II and III by the left hepatic vein and the falciform ligament; it is supplied by the medial branches of the left portal vein. The right hepatic vein separates segments in the right lobe of the liver: segment VII from VIII and segment VI from V. Segments VII and VI are supplied by the posterior branch of the right portal vein, superior and inferior branches, respectively. Segments VIII and V are supplied by the anterior branch of the right portal vein, superior and inferior, respectively. The middle hepatic vein separates the right and left lobe of the liver (segment VIII from IV and segment V from IV). Segment V is also separated from segment IV by the GB fossa (see Figure 10-1).
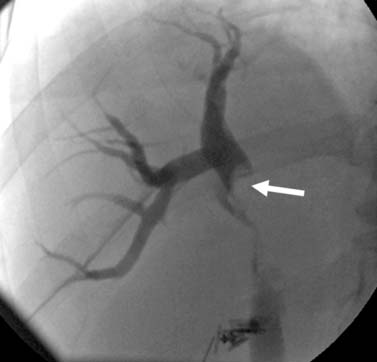
The liver has a variety of vascular variants. Michel’s classification describes 10 hepatic arterial variants.3 The most common, type I, has a common hepatic artery that arises from the celiac artery and bifurcates into the right and left hepatic artery, supplying the right and left lobes of the liver. Other common vascular variants include a replaced or accessory right hepatic artery from the superior mesenteric artery and a replaced or accessory left hepatic artery from the left gastric artery. There are also hepatic venous variants, for example, an accessory hepatic vein draining segment VI to the lower retrohepatic vena cava. Surgical intervention and transarterial chemoembolization (TACE) rely on the complete understanding of the liver arterial anatomy. Documentation of the vascular supply of the liver is essential for correct treatment planning.
Pathology
The gross pathology evaluation of IHCC reveals a firm, gray-white tumor. The tumors are usually large and solitary. Satellite nodules are not infrequent.4 The tumors with periductal infiltration tend to have a higher incidence of satellite nodules and also of nodal metastases. Although more commonly seen in HCC, large portal vein and hepatic vein tumor invasion can be detected on gross specimen evaluation. IHCCs occur in noncirrhotic livers.
Clinical Presentation
Patients with IHCC present with nonspecific symptoms such as weight loss, abdominal pain, nausea, and malaise.5 In addition and in contrast to other cholangiocarcinomas that may not present with signs of biliary obstruction, IHCC typically presents with signs and symptoms associated with a large mass in the liver. The symptoms include pain, discomfort, weight loss, and anorexia. An enlarged liver may be detected on physical examination. Some cases present with an incidental liver mass, and imaging studies or endoscopic evaluation are performed in search for the site of possible primary GI tumor. In these cases, the diagnosis of IHCC is made as the diagnosis of exclusion. In contrast to HCC, the patients do not present with ascites, cirrhosis, or portal hypertension.
Laboratory testing shows normal bilirubin, elevated CA19-9 (median 140 U/mL with > 60% with CA19.9 > 100 U/mL).6 The median carcinoembryonic antigen (CEA) is elevated near 2.1 ng/mL and is not significantly different between intrahepatic and extrahepatic tumors.6
Staging Classification
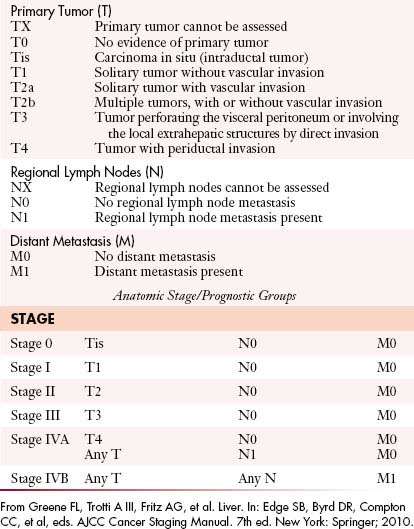
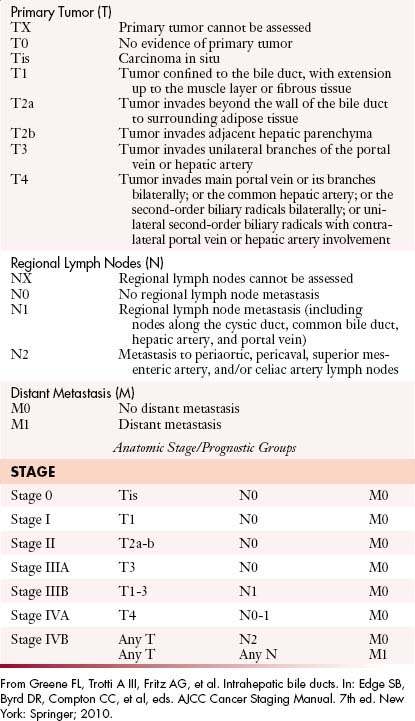
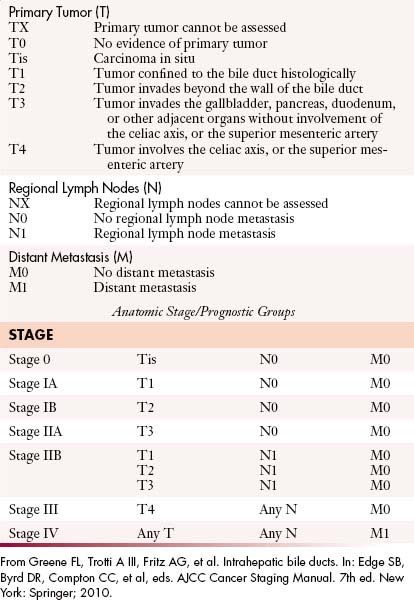
The most recent tumor-node-metastasis (TNM) staging criteria of the seventh edition of the American Joint Committee on Cancer (AJCC) is based on tumor number, size, and vascular invasion (T), local and distant metastatic adenopathy (N), and the presence of metastatic disease (M).6a The same staging is used for HCC (Table 10-1).
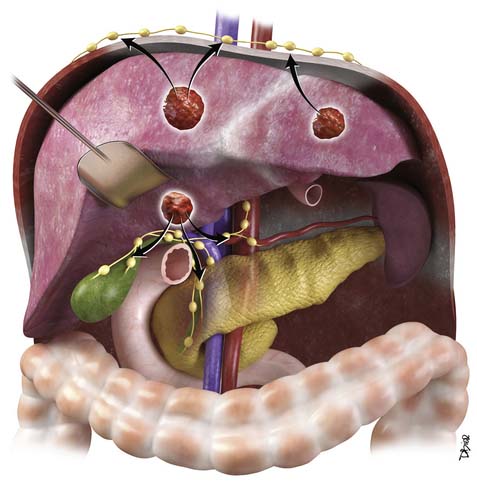
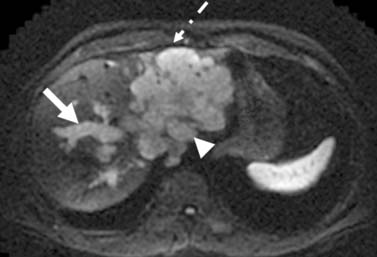
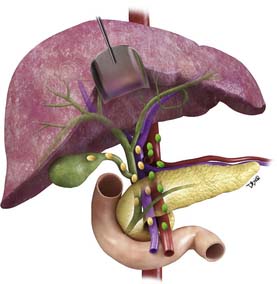
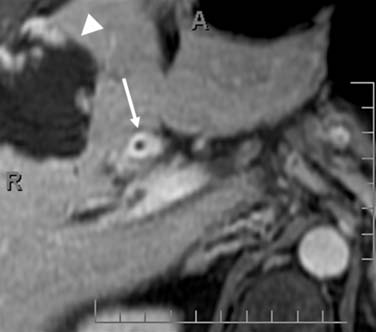
The nodal disease defines N1 and N2 nodes as regional or distant nodal spread of disease. In the central IHCC, the nodes along the hepatoduodenal ligament are considered N1 and nodes distant to this location are considered N2 (e.g., retroperitoneal nodes). Diaphragmatic nodes in the anterior, posterior, or middle diaphragmatic nodal stations may be considered as N1 nodes for IHCCs that are located near the dome of the liver (Figure 10-2). IHCC can present with distant metastases. Common sites of metastasis include the lungs, bone, adrenals, peritoneum, and brain. The lung metastases can be single or multiple nodules. The bone lesions are mostly lytic, and adrenal metastases are large, irregular masses. Thickening of omentum or discrete nodules are signs of peritoneal spread of tumor.
Patterns of Tumor Spread
IHCC may present with intrahepatic or extrahepatic tumor spread. The pattern of tumor spread is very similar to HCC.7 Similar to HCC, the most common type of tumor spread is intrahepatic via portal vein invasion. Biliary tract involvement is not common. The lymph nodes, skeleton, lung, and peritoneum are common sites of spread. Nodes along the hepatic hilum are a common site of tumor spread. The para-aortic, retropancreatic, and common hepatic nodes may also show tumor. For tumors in the left lobe of the liver, the left gastric, the paracardiac, and the nodes along the lesser curvature of the stomach may be involved (see Figure 10-2). Common distant lymph node involvement includes mediastinum and para-aortic nodes. For more peripheral tumors, the diaphragmatic nodes may be involved. Invasion through the liver capsule and extension to adjacent sites is more common in IHCC than in HCC. Extrahepatic spread has been reported as 50% to 67% based on autopsy series.8
Key Points Tumor spread of intrahepatic cholangiocarcinoma
Imaging
Primary Tumor
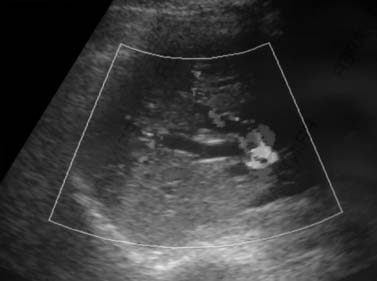
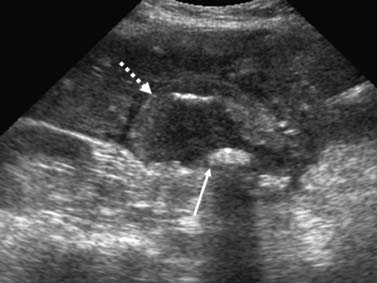
Similar to other GI malignancies, ultrasound (US) evaluation of the abdomen is commonly the first imaging modality to evaluate patients with IHCC. US provide a fast and relatively inexpensive evaluation. It is limited in that it is dependent on the experience of the operator and quality of the equipment. IHCC presents as a mass in the liver with variable echogenicity (Figure 10-3). Contrast-enhanced US will demonstrate relative hypoperfusion on the arterial phase of contrast administration and hypoenhancement on the portal venous phase.9 US may also be useful as guidance for fine-needle aspiration (FNA) biopsy and to evaluate vascular invasion with the aid of color Doppler techniques. US is very limited in the assessment of lymph node invasion and metastatic disease.
Computed tomography (CT) plays a major role in the clinical evaluation of patients with IHCC. The availability and short duration of the scan makes CT the major diagnostic tool in oncologic imaging, and IHCC is no exception. The CT imaging protocol of a patient with a liver mass is the multiphasic examination, same as HCC, obtained during the precontrast, late arterial, portal venous, and excretory phases of contrast administration. A bolus tracking technique is utilized to optimize the first phase of contrast administration. The images are obtained with at least a 16-slice multidetector row computed tomography (MDCT) scanner at 5 mm and reconstructed at 2.5 mm for each phase of contrast administration.
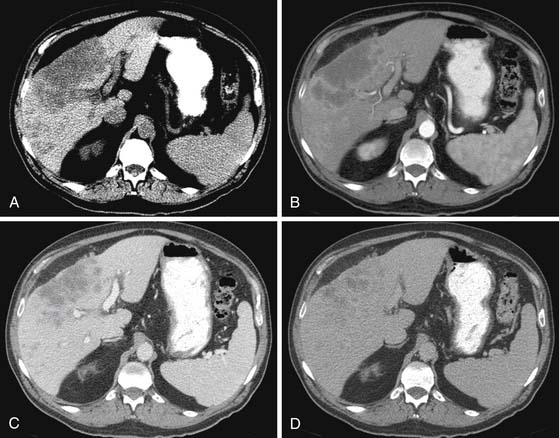
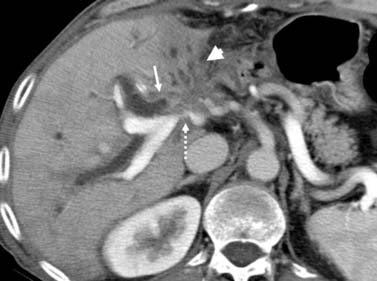
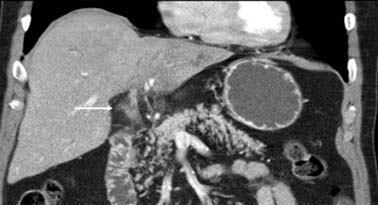
The IHCC will present as large, hypoattenuating masses in the precontrast images (Figure 10-4). During the late arterial phase of contrast administration, the enhancement, if present, is minimal and at the periphery (see Figure 10-4). On the portal venous phase, the enhancement will continue in a centripetal fashion and continue to progress on the delayed and excretory phases of contrast administration (see Figure 10-4). The CT findings of IHCC are very similar to those of hypovascular metastases, especially from the GI tract. Features that will suggest the diagnosis of IHCC include a large single mass, absence of a primary GI tumor, and absence of multiple nodules. Other CT findings seen on IHCC include calcifications and capsular retraction.
The multiphasic CT evaluation provides key information for surgical planning. This includes hepatic artery, portal vein, and hepatic vein anatomy. This is important in the setting of variant anatomy. Before surgery, calculations of future liver remnant (FLR) are made (FLR = future liver volume [FLV]/total expected liver volume [TELV]). In the setting of an FLR less than 20% (for a noncirrhotic liver) and less than 40% (for a cirrhotic liver), embolization of the portal vein is utilized with resultant compensatory hypertrophy and subsequent reduction in postoperative complications.10
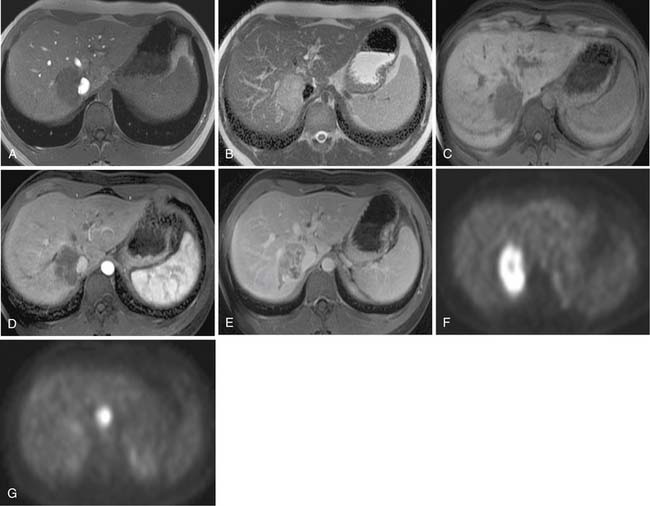
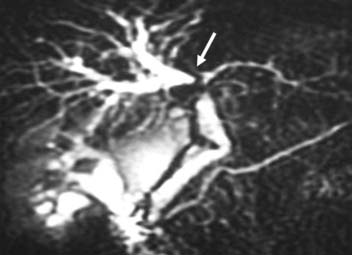
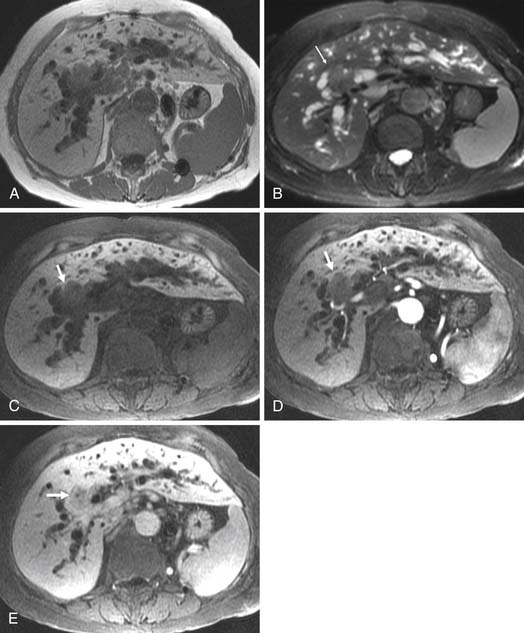
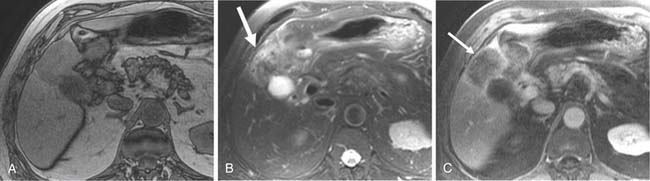
Two of the main reasons for the common use of CT for imaging patients with IHCC are its availability and short examination time. In contrast, magnetic resonance imaging (MRI) examinations are longer and may not be as readily available. However, MRI provides a higher contrast of lesion to liver on the various imaging sequences. The MRI protocol for the evaluation of patients with IHCC includes T1-weighted images (T1WI) and T2-weighted images (T2WI). The T1WI technique is a breathhold gradient echo sequence obtained at two echo times (2.1 [out-of-phase] and 4.2 [in-phase] msec), 1.5-T magnet. These two echoes can be obtained during a single repetition time (TR). The T2WIs consist of fast spin echo (FSE) and diffusion-weighted images (DWI). The FSE T2WI can be obtained with a respiratory-triggered technique or with breathhold technique. Our protocol utilizes a respiratory-triggered FSE sequence with time to excitation (TE) of 85 msec. On these noncontrast sequences, IHCC will demonstrate low signal relative to the liver on the T1WI, high signal on the T2WI, and restrictive diffusion on the DWI (Figure 10-5). On the T2WI, signal can be variable as a function of fibrosis (dark), mucous secretions (bright), or necrosis (bright).8,11 The darker central fibrosis may be a distinctive feature not commonly seen on metastatic disease to the liver.8 The DWI can be obtained at two different B values (0 and 500 sec/mm2). On this sequence, IHCC is usually hyperintense to liver at B of 0 and remains hyperintense to liver at B of 500 sec/mm2) (Figure 10-6). The larger B value allows the suppression of signal from vessels, bile ducts, and other fluids, resulting in an increased contrast between liver and lesion. The primary tumor as well as vascular invasion will have similar imaging features on the DWI. This is useful in the assessment of vascular invasion.
The postcontrast MRI evaluation consists of precontrast, late arterial, portal venous, delayed, and excretory phases of contrast administration. The timing of the injection is less critical than that for the evaluation of patients with HCC. The tumor will demonstrate peripheral enhancement with concentric fill-in (see Figure 10-5). Metastatic disease from colorectal cancer may show a pattern of enhancement similar to that seen on CT. The delayed complete enhancement may be seen only on the excretory phase or even later in the examination. In the setting of hepatocyte contrast administration, IHCC will not demonstrate uptake of contrast during the hepatocyte phase and will appear hypointense relative to the liver.3
Positron-emission tomography (PET) has become an important imaging modality in oncologic imaging. The most widely used radiopharmaceutical is fluoro-2-deoxy-D-glucose (FDG; see Figure 10-5). The benefits of PET/CT over CT include the increased accuracy in the detection of distant metastatic disease.12 Unfortunately, the sensitivity for detection of locoregional disease is less than 25%.13
Lymph Nodes
Lymph node metastases are a poor predictor of survival. In various series, the rate of positive lymph nodes found at surgery ranges from 31% to 59%.14–17 There is a reported negative effect on survival in the setting of lymph node metastases. In one series, the presence of three or more nodes had a 0% 3-year survival compared with 50% and 60% for one/two nodes and no nodes, respectively.16
Imaging features that may suggest tumor extension to the lymph nodes include size larger than 1 cm, low-density center, or delayed enhancement. CT has a high negative predictive value but a low positive predictive value for nodal involvement.18
Nodal stations that should be carefully examined in a patient with IHCC include the anterior, posterior, and middle diaphragmatic, celiac, hepatic artery, left gastric, and retroperitoneal nodal stations.19–21 Evaluation of retroperitoneal nodes is important in the search of N2 nodal stations. In our experience, DWI has shown to be very useful in the detection of lymph nodes (see Figure 10-6).
Metastatic Disease
The pattern of tumor spread for IHCC may be extrahepatic or intrahepatic. Intrahepatic spread may include extension of tumor into the portal vein. The portal vein tumor will demonstrate postcontrast enhancement features similar to those of IHCC.22
Preoperative imaging should detect satellite nodules because these increase the risk of dying by approximately 4 to 11 times that found when satellite nodules are not present.15,23 Thus, satellite nodules may be considered a relative contraindication to surgery.
Treatment
Surgery
The routine intraoperative lymph node dissection is not recommended because it has limited effect on survival.17 For the evaluation of disease in the lymph nodes, optimum imaging and attention to detail in preoperative imaging interpretation are essential in correctly identifying surgical patients.
The primary tumor usually presents as a large mass that invades adjacent structures requiring extended hepatectomy. An extended hepatectomy is defined as the resection of four or more Couinaud segments. In large series, it was required in approximately 50% of cases.24,25 The extended resection may include vascular and biliary resection.26,27 There is a significant difference in survival between an R0 and an R1 resection. The R0 survival group had a median overall survival (OS) of 46 months. The R1 group had a 5-month median OS and the only explored group was 7 months.26 The 5-year OS for resected patients is 23% to 40%.15,23,26 The OS for an R0 resection is 36% to 63%.28,29 The resection rate for patients explored for curative intent is 50% to 87%.2,25,30 Prognostic factors for recurrence include a positive margin, satellite lesions, lymph node metastases, and vascular invasion.2,15,23,31
The use of liver transplant for treatment of IHCCA has been reported.
Key Points Surgery for intrahepatic cholangiocarcinoma
Transarterial Chemoembolization
Various publications describe the application of TACE to the management of patients with IHCC. In multiple series, the median survival was 21 to 24 months after TACE.32–34 Chemotherapy agents used include mitomycin C, doxorubicin, and cisplatin.35 The median survival in the nonsurgical population is 6 to 12 months.
Radiofrequency Ablation
The principle of radiofrequency ablation (RFA) is to apply a localized thermal treatment resulting in tumor death. The high temperatures of 100°C reached during RFA will result in coagulative necrosis of the tumor and surrounding liver. There are limited reports of RFA of IHCC 3.36,37 The effectiveness of the treatment is a function of tumor size (best at < 3 cm) with complete necrosis of the treated tumors in some cases.37 The radiologic description of the tumor should indicate the proximity to major vessels, diaphragm, or other structures that may interfere with the technical success of this procedure. Tumor response to RFA is best evaluated with extent of necrosis rather than size criteria used in the Response Evaluation Criteria In Solid Tumors (RECIST) criteria.
Chemotherapy
Single agents and combination therapies have been evaluated in patients with IHCC. Single-class agents include 5-fluorouracil (5-FU), mitomycin C, and gemcitabine. These have demonstrated low response rates. Combination therapy including cisplatin/gemcitabine, 5-FU/cisplatin, carboplatin/5-FU, and epirubicin/5-FU have also demonstrated low response rates.
Radiotherapy
Radiation therapy (RT) in biliary cancers is administered in the adjuvant setting after surgical resection or for the palliation of advanced disease.38 Local recurrence is the most common type of failure after surgical resection, and therefore, adjuvant radiation has a strong rationale. Unfortunately, level one evidence regarding adjuvant RT after surgical resection is lacking because there are no randomized, controlled trials. At our institution, patients have been selectively referred for adjuvant chemoradiation when they have positive lymph nodes or positive margins. Typical doses of radiation are roughly 50 Gy administered over 5 to 6 weeks with concurrent fluoropyrimidine chemotherapy.
Surveillance
After RFA, on imaging, the ablated tissue will show changes owing to hemorrhage and necrosis (liquefactive and coagulative).39,40 The hemorrhage will result in high signal on T1WI, the coagulative necrosis will result in low signal on T2WI, and the liquefactive necrosis will result in high signal on the T2WI. The liquefactive necrosis will demonstrate nonrestrictive diffusion on DWI (B = 500) and increase in apparent diffusion coefficient (ADC).41
It is important to note that the WHO and RECIST criteria are suboptimal in the evaluation of post–RFA-treated IHCC. The size of the cavity following RFA treatment will appear larger than the size of the original tumor. In these first scans, the evaluation should be centered on the margin of the RFA cavity. The detection of a nodular enhancement, irregular wall, or the distortion of a smooth tumor margin is a sign of an unsuccessful treatment.40 Subtraction images in MRI using the precontrast as the mask for subtracted images can be very helpful in detecting intracavity enhancement.
On follow-up studies, successful RFA will demonstrate a subsequent decrease in size of the cavity.39 An increase in size of the cavity (on CT or MRI) indicates either interval hemorrhage or recurrent disease.42,43
Epidemiology
The risk factors are the same as for other cholangiocarcinomas and include a family history of congenital fibrosis or cysts (Caroli’s disease, choledochal cyst, polycystic liver, or congenital hepatic fibrosis). Also included as risk factors are PSC, ulcerative colitis (UC), drugs (oral contraceptives, methyldopa, and isoniazid), chemical exposure (thorotrast, radionucleides, asbestos, arsenic, and dioxin), and primary biliary cirrhosis.44 For patients with UC, the risk of EHCC is 0.5%. The risk also increases with biliary cirrhosis, cholelithiasis, alcoholic liver disease, diabetes, and chronic pancreatitis.45 Infectious pathogens associated with EHCC include liver flukes (Clonorchis sinensis).
Clinical Presentation
Serum markers CEA and CA19-9 are commonly elevated in EHCC.46,47 In a series of 57 patients with EHCC, the laboratory values for bilirubin, CA19-9, and CEA levels are elevated. The median bilirubin was 9.4 mg/mL, CA19-9 was 140 U/mL, and CEA was 2.1 ng/mL.6 In patients with PSC and a value of CA19-9 greater than 100 U/mL, there is a sensitivity and specificity of 89% and 86% for EHCC, respectively.47 The CA19-9 is not specific because a portion of the population cannot synthesize the protein or may be elevated secondary to inflammation rather than tumor.48 The laboratory values of CA19-9 are elevated to a higher degree in the setting of IHCC.6
Anatomy
The combination of hepatic artery, bile duct, and portal vein is considered the porta triad. These anatomic structures course together to the various segments of the liver along the portal vein supply. The intimate relationship between these structures can lead to an unfortunately poor prognosis even in the setting of early disease because a small bile duct tumor can extend to either the portal vein or the hepatic artery and make the patient potentially inoperable. To provide the best outcome and analysis of radiologic imaging, familiarity with the variant arterial supply to the liver is of great importance. Michel’s classification of the hepatic artery describes variants of arterial supply to the liver.3 The most common variants include a replaced/accessory right hepatic artery from the superior mesenteric artery and a replaced/accessory left hepatic artery from the left gastric artery.
Pathology
The EHCCs are mostly well-differentiated, mucin-producing adenocarcinomas.4 Histopathology will demonstrate an increased nucleocytoplasm ratio, nucleolar prominence, concentric layering of cellular stroma around neoplastic glands, and a combination of normal-appearing cells with cells having large nuclei with prominent nucleoli.4 The histology may also demonstrate stromal and perineural invasion. p53 gene expression is seen in 94%.49
Staging Classification
The staging for the bile duct tumors is based on the local extension of tumor (T), nodal disease (N), and distant metastases. The seventh edition of the AJCC TNM staging criteria for EHCC divides the tumors into proximal tumors and distal tumors (Tables 10-2 and 10-3).6a
Proximal Bile Duct Tumors
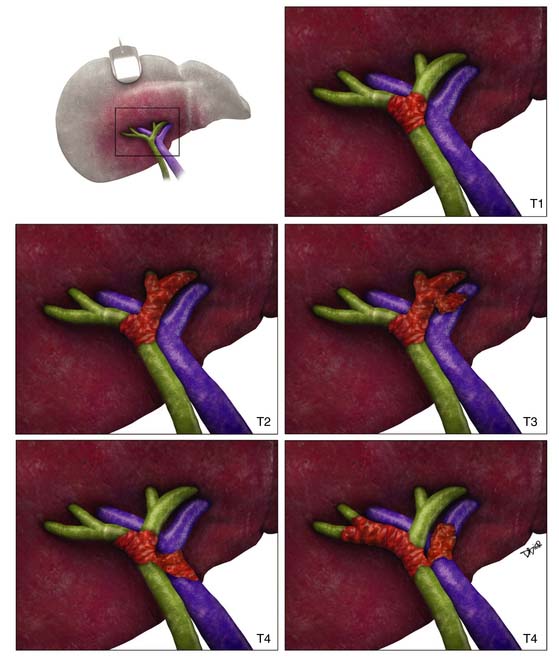
For the proximal bile duct tumors, the T staging is based on the thickness of the tumor. In the early stage, the tumor is confined to the bile duct (T1). In T2 staging, the tumor extends to the adipose tissue beyond the wall (T2a) or invades the liver (T2b). Unilateral extension into the portal vein or hepatic artery classifies the tumor as T3. T4 tumors are defined by the extension into the main portal vein and branches, common hepatic artery, contralateral vascular extension, and degree of second biliary radicle involvement (Figure 10-7).
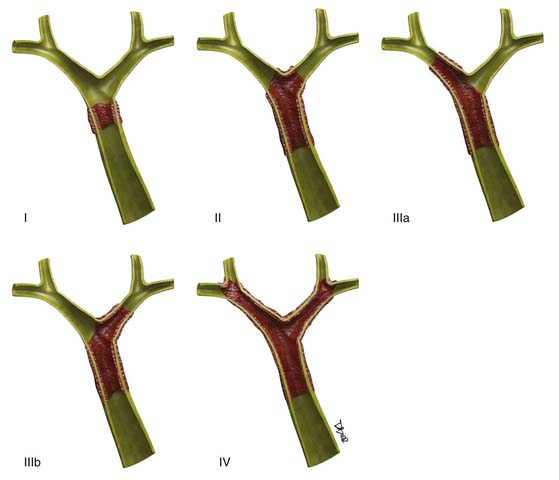
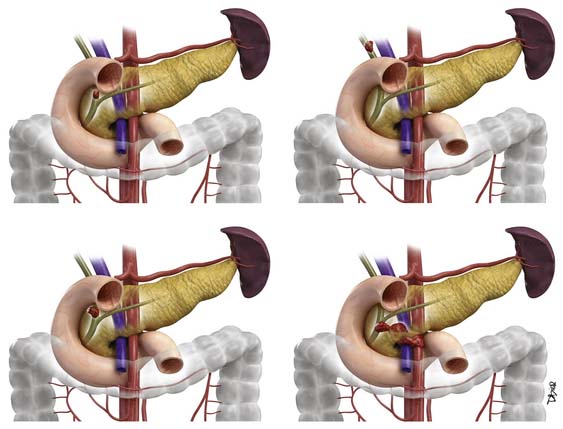
The hilar (also known as Klatzkin) tumors are also classified by the Bismuth-Corlette classification (Figure 10-8).49a This classification details the degree of biliary extension. There are five types of Klatzkin tumors: (I) common hepatic duct within 1 cm of the bifurcation, (II) tumors at the bifurcation without extension into the secondary biliary radicles, (IIIa) tumors extending to the right intrahepatic bile ducts, and (IIIb) tumors extending into the left intrahepatic bile ducts. Type IV tumors extend to the left and right intrahepatic bile ducts.
The N1 nodes for the perihilar tumors are nodes in the hilar, hepatoduodenal ligament, cystic, CBD, hepatic artery, and portal vein nodal stations. N2 nodes are periaortic, pericaval, superior mesenteric, and celiac nodal stations. The higher the T staging, the higher the prevalence of nodal spread of disease (Figure 10-9).
Distal Bile Duct Tumors
For the distal bile duct tumors, the T staging is based on tumors confined to the bile duct histologically (T1) or invade beyond the bile duct wall (T2). Tumors that invade adjacent organs without arterial vascular invasion are T3. T4 tumors have involvement of the celiac and superior mesentery arteries. This classification is similar to that for pancreatic tumors (Figure 10-10).
There are only two nodal classifications for the distal tumors, N1 and N0. N1 refers to the regional nodes that include hepatic artery, CBD, celiac, posterior and anterior pancreaticoduodenal, and superior mesenteric nodal stations. Similar to the management of cancer of the pancreas head, 12-nodal sampling is required as a minimum during pancreaticoduodenectomy.
Key Points Staging of extrahepatic biliary cancer
• T staging is based on thickness of the tumor and N2 nodes are only part of the proximal EHCC.
• Bismuth-Corlette classification of hilar tumors (Klatzkin) is based on the level of bile duct extension and has implications for therapeutic options.
• For distal tumors, nodal sampling is required similar to the management of ampullary and pancreatic head cancer.
Pattern of Tumor Spread
For the proximal bile duct tumors, the prevalence of lymphatic metastasis increases directly with T category and ranges from 30% to 53%.50 In proximal bile duct tumors, the nodes in the hepatoduodenal ligament, hilar, and periductal nodal stations are the most commonly involved. For the distal bile duct tumors, the regional nodes are the same as for tumors in the head of the pancreas. These include hepatic artery, CBD, and celiac nodal stations. The anterior and posterior pancreaticoduodenal nodes and superior mesenteric nodes are involved in the nodal spread of disease. For both types of EHCC, the evaluation of retroperitoneal nodes is essential because this may make the patient a nonsurgical candidate.
Imaging
Primary Tumor
Ultrasound
The initial clinical presentation of jaundice will require, in many instances, a US evaluation of the biliary tree. The detection of EHCC by US is dependent on the technology and the expertise of the ultrasonographer. The goals of the US examination in the setting of obstructive jaundice are to confirm biliary obstruction, evaluate the extent of obstruction, and identify the cause.1–3
The tumor may present as wall thickening or a polypoid intraluminal mass. The location of the tumor is inferred from the abrupt change in caliber of the bile ducts (Figure 10-11). Signs such as lobar atrophy and crowding of the vessels in addition to the presence of biliary dilatation raise the suspicion that the obstruction is malignant in etiology. Doppler evaluation of the portal vein in search of caliber change or changes in blood flow velocity may suggest vascular involvement.
US can detect the level of biliary obstruction in 71% to 100% of cases and the cause of obstruction in 57% to 96% of cases.51,52 In the setting of malignant disease, US shows a sensitivity of 83% to 93% for the detection of the primary tumor and 86% to 93% for portal vein involvement.51,53 Unfortunately, US has a high percentage of false-positive diagnoses of malignant biliary obstruction, higher than for CT or MRI.51
Endoscopic ultrasound (EUS) is a very useful modality for the diagnosis and staging of EHCC. EUS with FNA has a reported diagnostic accuracy of 89%.54 There is the risk of seeding into the peritoneum with EUS, but a reduced risk of biliary contamination (seen with brush cytology).54
Computed Tomography
CT is the most commonly used modality for the evaluation of patients with suspected biliary cancer. Multislice computed tomography (MSCT) provides high-speed and high-resolution imaging of the biliary tree. The images are acquired with a pancreas protocol. The technique is a biphasic scanning technique (parenchyma and delayed phase) after the intravenous administration of contrast by a power injector at 5 mL/sec.55 A bolus tracking technique is used for monitoring the contrast. The first phase (parenchyma phase) is acquired after a 20-second delay after the HU at the aorta at the level of the celiac artery reached 100 HU. The images are acquired during the pancreas-parenchyma phase and the delayed phase of contrast administration. The slice thickness is 2.5 mm and reconstructed at 0.63 mm. The thin-slice images are essential for optimum multiplanar reconstruction for the extent of tumor and vascular involvement.
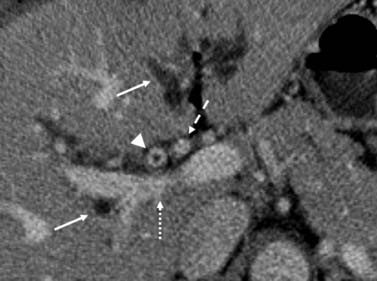
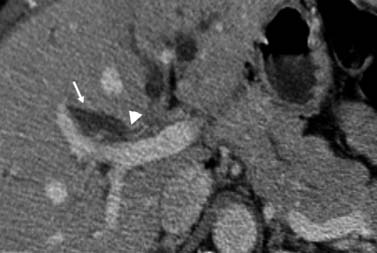
The features used to determine tumor extension and location are enhancement of the bile duct wall, abrupt caliber changes in the bile duct, an intraductal mass, and soft tissue attenuation in the bile duct (rather than bile/fluid attenuation) (Figures 10-12 and 10-13). The detection of bile duct wall enhancement should alert to the possibility of malignancy. The possibility of inflammation is also to be considered. The most distinctive feature is the presentation of an irregular circumferential thickening of the bile duct with proximal biliary dilatation and narrowing of the lumen (see Figures 10-12 and 10-13). The detection of soft tissue attenuation with obliteration of the fat surrounding the bile ducts and vessels in the hepatic hilum is to be considered as perineural and perivascular spread (Figure 10-14). The tumor invasion can also extend beyond the regional vessels to adjacent organs (liver, GB, and bowel).
MSCT with very thin images (0.63 mm) is used to create multiplanar reconstruction of the biliary tree (Figure 10-15). These 3D images are helpful in the conceptualization of the extent of tumor, regional extension, and relationship to regional vascular structures. This may be of great assistance in surgical planning.56,57
MSCT has a reported sensitivity and specificity of 94% and 79%, respectively, for tumor detection with a negative predictive value of 92% and an overall accuracy of 74.5%.58 This sensitivity has improved as a result of improved technique (thinner slices/multiphasic imaging) and improved expertise in image interpretation.
Key Points Computed tomography of extrahepatic biliary cancer
• Multiphasic pancreas protocol with 0.63-mm reconstruction is used for multiplanar imaging.
• Thickening of the bile duct in keeping with tumor may also be seen in inflammation (stent placement) or infection.
• Multiplanar reconstruction is useful for detecting extent of tumor and surgical planning.
Magnetic Resonance Imaging
MRI and magnetic resonance cholangiopancreatography (MRCP) are used in combination for the evaluation of patients with suspected biliary cancer. The protocol includes a routine multiphasic MRI of the abdomen. This protocol includes T1WI, T2WI, postgadolinium images, and DWI of the abdomen. The postgadolinium images are obtained during the arterial, portal venous, and delayed phases of contrast administration. The timing of image acquisition is optimized with a bolus tracking technique. The MRCP images are heavily T2WI (fluid sensitive) with TE greater than 200 msec. These images are acquired with thin slices (<3 mm) in the axial and coronal planes. Thick-slab (40-mm) images in the coronal, oblique coronal, and sagittal planes are obtained (Figure 10-16). These are radial images centered on the CBD. The thick-slab images correspond to images obtained during endoscopic retrograde cholangiopancreatography (ERCP). A single-shot fast spin echo (SSFSE) or Half fourier Acquisition Single shot Turbo spin Echo (HASTE) (Siemens) sequence is routinely used for the MRCP images. These sequences are fast and have low sensitivity to motion artifacts.
The imaging features of the primary tumor on MRCP are proximal bile duct dilatation with abrupt termination of the bile ducts (shoulder sign), thickening and enhancement of the bile duct wall, a mass in the lumen, lobar atrophy, and crowding of the vessels (Figure 10-17). Similar to CT, some of the imaging features are shared by non-neoplastic inflammatory processes (Figure 10-18). The tumors are usually hyperintense on T2WI and hypointense on T1WI and have a heterogeneous enhancement pattern (see Figure 10-17).59 In a significant number of patients, the cross-sectional images are obtained after the placement of a biliary stent. This results in interval resolution of biliary dilatation with the loss of the transition site of bile duct caliber attenuation. In addition, a newly placed stent can result in enhancement of the bile duct wall and infiltration of the periductal fat (see Figure 10-18). This may result in false-positive image interpretations. Careful review of prestent imaging is required before the diagnosis of vascular invasion by tumor.
The accuracy of MRI/MRCP for resectability ranges from 80% to 93.3%.60 MRI/MRCP was comparable with CT in a series evaluating tumor extent and respectability.61
The role of FDG-PET is limited in the evaluation of patients with bile duct tumors.19,62 The detection rates for FDG-PET were 74% for the infiltrating tumor and 96% for the nodular type of bile duct cancer. The specificity and sensitivity for nodal metastases were 97% and 33% for FDG-PET compared with 79% and 57% for MSCT.62 Evaluation of FDG-PET/CT for resectability has demonstrated improved accuracy over conventional imaging.63
Key Points Magnetic resonance imaging and magnetic resonance cholangiopancreatography of extrahepatic biliary cancer
• MRCP is a heavily T2WI that can be obtained with thin and thick slabs for visualization of the bile ducts.
• Thickening of the bile duct is in keeping with tumor and may also be seen in inflammation (stent placement) or infection.
• MRCP reveals abrupt termination of bile duct (shoulder sign) in keeping with tumor.
Percutaneous Cholangiography and Endoscopic Retrograde Cholangiopancreatography
Percutaneous transhepatic cholangiography (PTC) and ERCP are considered as gold standards for imaging the bile ducts. The biliary tree is visualized after the opacification with iodinated contrast. The imaging features of these techniques for malignancy are abrupt termination or irregular narrowing of the bile duct with proximal intrahepatic bile duct dilatation (Figure 10-19). The advantages of the intervention with ERCP include the ability to place a stent for bile duct decompression and to obtain a tissue sample for diagnosis. The major complications include sepsis, bile leak, hemorrhage, and even death.64 ERCP has limited value for the evaluation of extraluminal tumor spread.
Lymph Nodes
The radiologic evaluation of lymph nodes is based on size, morphology, and location. Nodes that are larger than 1 cm are more likely to be malignant. Nodes located near the primary tumor are also likely to be malignant, even if they measure less than 1 cm in minimum diameter. A round node is also likely to be malignant than an oval node. In the setting of prior biliary stent placement, reactive periportal nodes may be seen and may mimic metastatic disease.
MSCT has proved accurate for the prediction of nodal disease with an overall accuracy of 83.6% and a sensitivity and specificity of 53.3% and 95%, respectively. The accuracy for MRI is similar to CT in the characterization of para-aortic lymph node metastases.65 The accuracy of FDG-PET/CT is higher than CT alone (75.9% vs. 60.9%).63 DWI has shown to be very useful in the detection of lymph nodes. The high signal of the free fluid in the DWI will be suppressed with a B greater than 50 sec/mm2.
Metastatic Disease
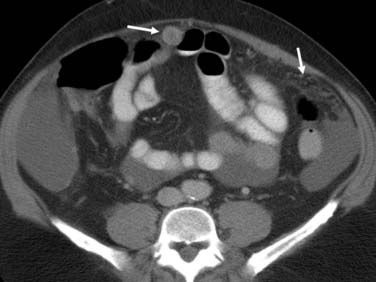
Metastatic disease from biliary tract tumors may be detected in the liver, peritoneum, and distant nodal stations. Liver metastases present as low-attenuation lesions on MSCT and are best seen during the portal venous phase of contrast administration. For MRI, liver metastases are hyperintense on T2WI and low signal on T1WI and demonstrate delayed enhancement. FDG-PET liver metastases may be hypermetabolic. In the setting of subcentimeter lesions in the liver, the distinction of benign and malignant disease is difficult with MSCT, MRI, or PET. In this setting, follow-up imaging at 3-month and 6-month intervals is suggested. Peritoneal disease usually presents as soft tissue nodules and is a common source of error in understaging patients with biliary cancer (Figure 10-20). These nodules demonstrate restrictive diffusion on DWI on MRI.
Treatment
Surgery
Surgery provides the only curative option for patients with EHCC. Unfortunately, 60% to 70% of patients are not suitable for curative resection. The need for imaging with exceptional quality is indispensable in the presurgical evaluation of potential surgical candidates. The contraindications to surgical resection include advanced disease, inadequate functional liver remnant, and patient factors. Findings of advanced disease that may make a patient unresectable include (1) metastatic disease (N2 nodes, peritoneal disease, or distant metastases), (2) bilateral segmental bile duct tumor extension, (3) contralateral portal vein or hepatic artery involvement, (4) contralateral lobar atrophy, (5) portal vein and bilateral portal vein extension, and (6) tumor extending into the bilateral hepatic artery branches.66–68 Patient factors such as significant medical comorbidities should be evaluated, and Eastern Cooperative Oncology Group (ECOG) scores of 0, 1, and 2 are candidates for surgery.69
Prior to surgery, in the setting of biliary obstruction, biliary drainage (percutaneous or endoluminal) has been suggested for presurgical management.70,71 This may reverse hepatic dysfunction due to biliary stasis. In the setting of stenting, preoperative antibiotics are recommended to reduce the risk of postoperative infection.
The surgical procedure may begin with a laparoscopic evaluation to assess resectability. For biliary cancer, laparoscopy has been recommended for staging. In one series, it prevented unnecessary resection in 42% of cases.72 The advent of improved imaging techniques has improved the accuracy of presurgical staging. For example, in a recent series with improved technique, MSCT demonstrated high accuracy in predicting resectability (94% correctly predicted resectability and 79% correctly predicted unresectability).58 The cases that were incorrectly predicted had small peritoneal disease and liver metastases.58
The surgical approach of hilar or proximal EHCC is different from that for distal bile duct tumors. For the hilar tumors, the resection may include cholecystectomy, resection of extrahepatic bile ducts, regional lymphadenectomy, hepatic lobar resection, caudate lobe resection, Roux-en-Y hepaticojejunostomy, and vascular reconstruction or resection. The regional nodes are hilar, cystic, pericoledochal, hepatic artery, portal, and posterior pancreaticoduodenal. The extent of hepatic resection depends on the Bismuth-Corlette classification. For type III tumors, the resection of the caudate lobe is required.68
For hilar tumors, the overall perioperative mortality is less than 5% owing to improved perioperative management.66,73,74 An R0 resection, lymph node involvement, and low-grade tumor are prognostic factors associated with long-term survival. However, in the setting of required portal vein resection (due to vascular invasion), the 5-year survival is diminished. In the setting of hepatic artery involvement, the resection of the hepatic artery does not show survival benefit.75 The application of hepatic resection has increased the number of negative surgical margins.68,76,77 Patients with major liver resection and R0 resection of the hilar cholangiocarcinoma had a 5-year survival of 30% to 65%.78 In patients without hepatic resection and only resection of the extrahepatic ducts, the 5-year survival was 0% to 25%.79 The 5-year survival for a R1 resection is less than 5%.
For distal tumors of the bile duct, the surgical approach is similar to the management of ampullary or pancreatic head cancer. The surgical intervention includes pancreaticoduodenectomy and sampling of at least 12 regional nodes. The median survival after surgery is 2 years with a 20% to 40% 5-year survival.50 The prognosis is improved in the papillary type tumors and in the setting of lymphatic, perineural, and vascular sparing by tumor.80–82 The 5-year survival rates are 20% to 50%.
Key Points Surgery for extrahepatic biliary cancer
Chemotherapy
Patients with unresectable EHCCs have a poor prognosis. There are number of studies of palliative chemotherapy associated with some improved OS in selected publications and some response rates.82a The use of single agents and combination therapies has been evaluated in patients with EHCC. Single-class agents include 5-fluorouracil (5-FU) or capecitabine, mitomycin C, and gemcitabine. These have demonstrated low response rates. The use of combination agents has improved response rates. Combination therapy included cisplatin/gemcitabine, 5-FU/cisplatin, carboplatin/5-FU, and epirubicin/5-FU. The studies cannot be compared because they include a combination of biliary cancers (IHCC, EHCC, and GB CA). For example, the response rate of capecitabine plus cisplatin is reported as 20% to 40%.83 Targeted therapy to epidermal growth factor receptor (EGFR) with erlotinib and with bevacizumab has been reported.84,85 Adjuvant chemotherapy after resection has been evaluated in some clinical trials.
The recent ABC trials (ABC-01 and -02) have changed the treatment paradigm in this disease.86,87 Based on the results of the randomized phase II study (ABC-01), the MRC initiated the ABC-02 study consisting of gemcitabine plus cisplatin versus gemcitabine as a single agent.86 The study enrolled 400 patients and demonstrated a significant survival advantage with the platinum-based combination (11 mo vs. 8 mo); the combination arm also had improved PFS. Similar survival figures have been reported with phase II studies of gemcitabine/capecitabine, GEMOX, and gemcitabine/irinotecan.
Radiotherapy
RT has been applied to the management of EHCC. The method of radiation delivery is varied including 3D conformal techniques, IMRT, and proton therapy. Some patients receive concurrent chemotherapy and others receive only radiation. In all instances, the proximity of the bowel mucosa often limits the dose of radiation that can be safely given. Although the role for adjuvant RT in the treatment of distal cholangiocarcinomas cannot be conclusively established, locoregional control with chemoradiation seems appropriate to consider in these patients.52,54 In the case of hilar cholangiocarcinomas, even with optimal margins after surgery, two in three patients have a recurrence within 2 years, with 60% being locoregional for hilar cholangiocarcinomas.55 This pattern of locoregional recurrence provides a rationale for adjuvant treatment with a locoregional modality such as RT with or without radiosensitizing chemotherapy. Unfortunately, most series evaluating postoperative adjuvant RT for hilar cholangiocarcinomas do not provide information on locoregional control. Nevertheless, multiple series postulate a survival advantage for postoperative adjuvant RT of hilar cholangiocarcinomas when compared with historical controls.56 At our institution, patients have been selectively referred for adjuvant chemoradiation when they have positive lymph nodes or positive margins. Adjuvant therapy has improved the outcome in these poor-prognosis patients such that their outcome is similar to that of node-negative/margin-negative patients, suggesting that treatment makes up for these poor prognostic features.57
For the majority of nonmetastatic patients who are unable to undergo complete surgical resection with negative margins, multiple small studies have concluded that RT improves OS when compared with no treatment (or stenting alone). However, these studies are constrained by the nonuniform criteria for determination of resectability, inclusion of patients with all EHCCs and GB CA, and/or the use of older radiographic imaging and RT techniques. In most series reporting chemoradiation outcomes for unresectable cholangiocarcinomas, the dose of external beam RT has been 50 Gy.88 Selected series have shown a benefit to dose escalation of cholangiocarcinoma.16,54,73,74 Although these studies are subject to selection bias and the possibility that smaller tumors received a higher dose of radiation, these results are promising. These results also validate the promising outcomes from the Mayo Clinic where unresectable hilar cholangiocarcinoma patients received 45 Gy of external beam RT and an intraluminal brachytherapy boost in the neoadjuvant setting prior to transplantation.75 Among 38 patients who eventually underwent transplants, explanted livers demonstrated no residual carcinoma in 16 patients, 9 of whom had unequivocal histologic or cytologic confirmation of tumor before administration of neoadjuvant therapy.
Photodynamic therapy (PDT) has been used in treating cholangiocarcinomas. The principle is to use a low-energy laser light via a percutaneous or an endoscopic approach to photosensitize a drug such as Photofrin. There is a reported improved median survival with PDT.89,90
Conclusion
Advances in imaging including MSCT with very thin slice images and MRI in combination with MRCP have resulted in a single study to answer all the questions in the evaluation of biliary obstruction. This includes answers to the presence of obstruction, the location of transition zone, and the cause of obstruction. In the particular setting of tumor, these techniques can provide the diagnosis and staging of the tumor including nodal disease and metastatic spread. Angiographic images of the arterial and vascular anatomy in multiple planes can be generated and detection of variant anatomy is readily available. The multiplanar reconstruction can create images of the biliary tree that mimic those of the traditionally used ERCP. This combination in improvement in image acquisition and processing has allowed a “one-stop” shop for CT and MRI. For EHCC, surgery is the only option for cure and correct interpretation will reduce the number of unnecessary surgeries. Laparoscopic evaluation also assists in the reduction of unnecessary surgeries. The role of chemotherapy, RT, and PDT continues to be evaluated and is promising. The management has to include a team approach with the radiologist, surgeon, oncologist, and radiation therapist working together for a successful outcome.
Epidemiology
GB stones, in particular cholesterol stones, are the most common risk factor. Gallstones are present in 74% to 92% of patients with GB CA.91 Other reported risk factors include congenital biliary cysts, environmental factors (tobacco), infectious factors (Salmonella typhi), PSC, and genetic factors.92 Porcelain GB, formerly believed to be associated with GB CA, has been shown to be totally unassociated with GB CA.93
The Surveillance, Epidemiology, and End Results (SEER) registry reports an incidence of 0.9 to 1.0 per 100,000. There is a geographic prevalence of GB CAs with the highest number of cancers located in Chile and Bolivia. Other locations with a higher incidence of GB CA are India, Japan, Israel, and Poland. There is a female preponderance with an average female-to-male ratio of 3:1. It tends to present in an older population with the greatest incidence in patients older than 65 years. The average age at presentation is 72 years with a median age of 73 years.94
Anatomy
The GB is part of the biliary system that is composed of the intrahepatic and extrahepatic bile ducts and the GB. The GB is divided into fundus, body, and neck (infundibulum or cystic duct). Most of the tumors are located in the fundus (60%). The body (30%) and neck (10%) account for the rest of the tumors.95
Important anatomic considerations in the management of GB CA are the location of the tumor in relation to the portal vein, bile ducts, and hepatic artery and its presence in the GB. For example, when the tumor extends to the CBD, it may be clinically and radiologically indistinct from bile duct tumors. These tumors may also invade the portal vein and hepatic artery. In contrast, the tumors in the fundus and body of the GB can readily invade segments IV and V of the liver owing to its anatomic proximity. The GB is attached to segment IV of the liver. In autopsy series, liver extension was reported in 65% of cases.96 Its propensity to invade the peritoneal cavity and seed wounds (e.g., laparoscopy ports) affects treatment planning.
Clinical Presentation
Gallstones are a common finding in GB CA with co-presentation in over 70% of patients. Although there is a high association, the recommendation for prophylactic cholecystectomy is reserved for high-risk populations, given the low incidence of the disease.97 The odds ratio for the development of cancer in patients with gallstones is dependent on the size of the stones, with an odds ratio close to 10 for stones larger than 3 cm compared with 2.4 for GB with stones smaller than 1 cm.98
Early diagnosis is rare except in the case of the incidental tumor discovered after a cholecystectomy for suspected cholecystitis. The early clinical presentation of GB CA overlaps with the signs and symptoms of gallstones. The presenting signs and symptoms may vary and depend on the stage of the disease. Distinctive features are persistent right upper quadrant pain, anorexia, weight loss, and palpable mass. More advanced disease may present with weight loss, hepatomegaly, and jaundice. In the setting of extension to the adjacent bowel, small or large bowel obstruction may be seen. The possibility of a fistulous communication to bowel may be seen in advanced cases.
There are various serum markers for patients with GB CA: CA242, CA19-9, CA15-3, CEA, and CA125.99 The best serum markers for GB CA are CA19-9 and CA125. The markers may be elevated in other malignancies and benign processes.100 An elevated marker has to be evaluated with a combination of history, physical examination, and diagnostic imaging results. These values should not be interpreted in isolation.
Pathology
Carcinomas, sarcomas, lymphoma, carcinoid, and other malignancies may be found in the GB. The most common malignancy is a primary GB CA with adenocarcinoma as the most common type of cancer with an incidence greater than 85%. Other less common histologies of GB CA are papillary adenocarcinoma, mucinous carcinoma, and squamous cell carcinoma.101
The most widely accepted sequence of development of GB CA is a stepwise progression from dysplasia to carcinoma rather than the adenoma-to-adenocarcinoma model. The sequential development appears to involve the stepwise progression from normal GB to metaplasia (due to chronic cholecystitis), to dysplasia, to carcinoma in situ, to invasive carcinoma.102,103 In this model, the initial insult results in chronic inflammation with resultant regeneration of the epithelium with resultant metaplasia. The metaplasia is similar to that seen in the intestine. In over 50% of the chronically inflamed GB, metaplasia has been found. The estimated time to progression from chronic cholecystitis to mucosal cancer is 10.9 and 9.2 years for females and males, respectively.104 The rate of progression from dysplasia to invasive carcinoma is estimated at 5 to 15 years.102
The gross pathology description of GB CA is either an intraluminal polypoid lesion or, more commonly, a diffusely infiltrating mass. The tumors are commonly described in three patterns: infiltrative (most common), nodular, and papillary (best prognosis) forms. This is the same classification used for other forms of cholangiocarcinomas. Other studies have used five subtypes for classification based on gross morphology: papillary, nodular, flat, filling, and massive.105
Histologic evaluation of GB CA (adenocarcinoma) reveals glands that are lined by cuboid or columnar cells that may contain mucin. There are several variants of adenocarcinomas: papillary, intestinal, mucinous, signet-ring cell, and clear cell.106 Some tumors may contain more than one of these variants. The best prognosis is seen in the papillary type. These tumors filled the GB before invading the wall. The intestinal type resembles intestinal epithelium. The mucinous and signet-ring adenocarcinomas consist of extracellular and intracytoplasmic mucin, respectively. The signet-ring type displaces the nuclei to the periphery. The clear cell types are composed of cords, sheets, nest, and trabeculae of clear cells. In contrast to the renal cell clear carcinoma, these tumors produce mucin. Most common tumor grades are moderately and poorly differentiated.
Gene expression has been evaluated in GB CA. p53 mutation is very common, found in 92% of invasive carcinomas.107 The K-ras mutation has been reported in 39% of GB CAs.107
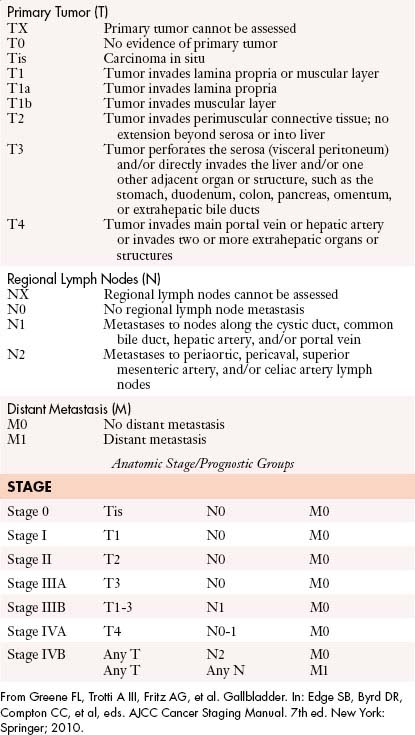
Staging
The AJCC staging system for GB CA is based on the TNM classification: tumor depth of invasion (T), nodal disease (N), and metastatic disease (M) (Table 10-4).
The nodal staging is based on regional nodes (N1) or no nodal involvement (N0). Similarly, M staging is based on the presence or absence of metastatic disease.50,58
Pattern of Tumor Spread
Direct extension to adjacent organs is the most common method of tumor spread. The lymphatic spread and subsequently vascular spread are less common. Other methods of dissemination of the disease include neural, intraperitoneal, and intraductal spread of tumor. The organs most frequently involved in order of frequency are liver, colon, duodenum, and pancreas.96
The nodal drainage of the GB begins with the cystic and pericholedochal nodes.108 From these nodes, there is extension to the periportal, hepatic artery, and posterior pancreatic nodal stations. The interaortocaval, celiac, and superior mesenteric nodes are also important routes in the spread of disease.109
Imaging
The sonographic imaging features of GB CA include wall thickening, gallstones, a mass in the GB, echogenic foci, discontinuous and echogenic mucosa, heterogeneous echogenicity, and a hypoechoic submucosal layer (Figure 10-21). The detection of echogenic foci may be related to detection of gallstones, calcifications in the wall, or calcifications in the tumor. The wall thickening and gallstones are signs more commonly related to acute cholecystitis. Wall thickening of more than 1 cm raises the concern for GB CA. The detection of heterogeneous echogenicity may be related to tumor necrosis.
The reported sensitivity of US in the diagnosis of GB ranges from 34% to 85%.110–112 Color Doppler has been suggested to assist in the distinction between benign and malignant processes.113 Although US may be useful in the detection of disease, for the detection of the extent of tumor spread, US is suboptimal.114 CT and MRI are more useful imaging modalities for staging.115
The normal GB on the contrast-enhanced CT demonstrates thin (<3 mm) homogeneous mucosal enhancement (Figure 10-22). The GB wall may appear thickened due to underdistention. But, similar to US, the detection of wall thickening (>1 cm) with mural irregularity on CT raises the suspicion for malignancy. Based on gross morphology, the carcinomas of the GB can be divided into papillary, nodular, flat, filling, and massive. The nodular, papillary, and filling types of tumors share the same imaging feature of a soft tissue–attenuation mass filling the low-attenuation fluid in the GB. Irregular thickening of the GB on the CT examination may be seen in flat tumors. A large mass that replaces the GB and adjacent liver parenchyma is an imaging feature of the massive type.
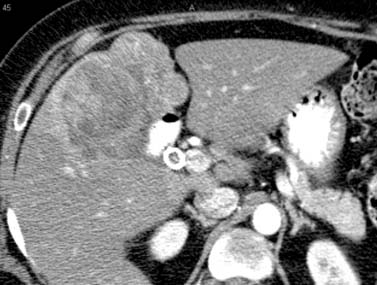
Figure 10-22 Delayed-phase postcontrast CT examination of the abdomen demonstrates a mass in the GB fossa with regional extension.
The CT examination may detect the presence of metastatic adenopathy, extension to the liver and other organs, or distant metastases that will assist in the staging of these tumors (see Figure 10-22).
The sensitivity of CT to detect tumor extension is better when there is advanced disease (T4), approaching 100%.115 When the stage is T3, the sensitivity decreases to 65% to 79%.115,116 The addition of multiplanar reconstruction will improve the accuracy of T staging of GB CA. The criterion for tumor extension to adjacent organs, bile ducts, or vessels is the disruption of fat planes between the tumor and the adjacent structures.
Using the CT criteria for nodes larger than 1 cm, for the presence of malignancy, the specificity is 99% for N1 and N2 nodal stations. Another imaging factor that suggests nodal spread of malignancy is the detection of necrotic nodes. The sensitivity of CT for the detection of N1 and N2 nodes are 36% and 47%, respectively.116,117 Hypodense nodes are more suspicious, and attention to the presence of suspicious interaortocaval and retropancreatic nodes is important (N2).
GB spread into the peritoneum is common. The imaging features of peritoneal deposits are discrete nodules and fat stranding of the low-attenuation peritoneal fat (see Figure 10-19). The detection of peritoneal disease is a challenge to the radiologist and may be very difficult. The sensitivity of CT for the detection of peritoneal metastases has been reported to be from 63% to 93%.116–118 The sensitivity of CT for the determination of resectability in the evaluation of vascular invasion, organ invasion, and metastases is 72.7% with an accuracy of 85%.116,119
On T1WI, the tumor is hypo- to isointense and the content of the GB may be hypo- or hyperintense depending on the fat-to-bile composition ratio of the GB fluid (Figure 10-23). The higher the fat content, the higher the signal on the T1WI. On the T2WI, the tumor is heterogeneous in signal intensity in the background of a fluid-filled (high-signal) GB (see Figure 10-23). The postgadolinium images demonstrate enhancement of the tumor. Similar to CT and US, a GB wall thickness greater than 1 cm with an irregular margin raises the concern of malignancy. MRI has been advocated to differentiate chronic cholecystitis from malignancy.120 During a multiphasic postgadolinium evaluation, tumor will enhance early, which can also be useful to assess for local tumor extension (see Figure 10-23). In the setting of prior surgical resection for acute cholecystitis, careful review of the wound and laparoscopic ports is essential. Any soft tissue attenuation mass is suspicious for metastatic implant.
MRI is useful to detect invasion to lymph nodes, portal vein, and hepatoduodenal nodes. The sensitivity and specificity of the combination of MRI and MRCP for the evaluation of GB CA for vascular invasion are 100% and 87%, respectively. For liver invasion, these are 67% and 89%, respectively. For lymph node involvement, these are 56% and 89%, respectively.121,122 In the setting of a tumor extension into the cystic duct and CBD, intrahepatic biliary dilatation has been evaluated with MRCP series. Biliary dilatation may result from enlarged metastatic nodes rather than tumor extension.
FDG-PET is evolving as an image modality in the setting of GB CA. It has been evaluated for identifying GB primary, nodal disease, and metastatic disease with sensitivities greater than 75%.123,124
Key Points Imaging of gallbladder cancer
• Multiphasic liver protocol is useful for presurgical planning.
• Mural irregularity and enhancement of the GB suggest malignancy.
• Hypodense/necrotic lymph nodes are more suspicious for malignancy.
• Wall thickening more than 1 cm raises concern for GB CA.
• MRCP is useful for biliary anatomy
• FDG-PET is used to evaluate metastatic and nodal spread of tumor.
Therapy
Surgery
GB CA is incidentally found in 2% of 70,000 routine cholecystectomies in the United States every year. The procedure carries a small risk of seeding cancer cells at the peritoneal surfaces and the laparoscopic port. If a patient is suspected of having a GB CA pre- or intraoperatively, he or she should undergo open laparotomy or percutaneous biopsy rather than laparoscopic resection.125,126
The initial step for resection of the GB is a laparoscopic survey of the primary tumor and metastatic disease. The recommended intraoperative nodal survey includes pancreaticoduodenal, interaortocaval, and superior mesenteric nodes. The hepatic artery and portal vein are skeletonized into the liver hilum. In cases in which the bile duct is resected, it would help in the evaluation of the hepatoduodenal ligament but may increase operative morbidity.127
The resection of the GB is dependent on the tumor stage and prior history of cholecystectomy. In the setting in which GB CA is detected before cholecystectomy for presumed cholecystitis, it would be best to postpone the cholecystectomy for a later time when a complete resection is possible. An incomplete cholecystectomy does not improve outcome on patients who later have a complete resection.105 For early-stage tumors (T1a), a simple cholecystectomy with negative margins is adequate. For T1b tumors, a more aggressive approach is to be considered. There are reported series with a 50% 1-year survival after only laparoscopic cholecystectomy in T1b tumors.128 The 5-year survival rate for T1a patients undergoing simple cholecystectomy ranges from 57% to 100%. The incidence of lymph node metastases in T1a patients after simple cholecystectomy is less than 10%. It is further reduced to 2.5% for patients who undergo cholecystectomy and regional lymphadenectomy. However, extended resection has a mortality rate of 2% to 5% and a major postoperative morbidity rate of over 13%.129,130
T2 lesions require en bloc resection of liver and regional nodes. The 5-year survival of patients with T2 lesions treated only with cholecystectomy is 20% to 40%. This increases to 80% after an en bloc hepatic resection.127,131 The degree of hepatic resection is dependent on the vascular extension of tumor. Careful review of preoperative images of the vascular anatomy will assist the surgeon in planning the surgical approach. In tumors in which the right portal vein is involved, a right hepatectomy is required. It is of note that the detection of residual disease at reexploration had a worse prognosis with a median survival of 14.6 months versus 72 months for patients with no residual disease.132
T3 lesions, similar to T2, require aggressive liver and regional node resection. In addition, all involved organs, for example, the stomach, duodenum, pancreas, or colon, are resected en bloc. The 5-year survival for the T3 tumors after a complete resection is 30% to 50%.127 In the setting of T4 disease, surgery has been considered if vascular reconstruction, biliary bypass, and complete en bloc resection of tumor are plausible. The benefit of this procedure is indeterminate.
In the setting of a T1, T2, or T3 lesion found incidentally in a laparoscopic specimen in an unsuspected patient, reexploration with excision of port sites has been recommended.133,134
Key Points Surgery for gallbladder cancer
Chemotherapy/Radiation Therapy
There is a high recurrence rate and locoregional disease in GB CA patients. Thus, adjuvant therapy has been explored. There have been reports of adjuvant RT with external beam radiation, brachytherapy, and intraoperative RT. The chemotherapeutic agent used was 5-FU. Some studies showed some improvements in survival.135,136 In prior studies, 5-FU showed an objective partial response rate of less than 12%. Gemcitabine has a response rate similar to that of 5-FU. Complete remission is very rare, and the median survival is 11 months or less.137 The duration of response is usually 3 to 6 months.
The recent ABC trials (ABC-01 and -02) have changed the treatment paradigm in this disease.86,87 Based on the results of the randomized phase II study (ABC-01),87 the MRC initiated the ABC-02 study, consisting of gemcitabine plus cisplatin versus gemcitabine as a single agent.86 The study enrolled 400 patients and demonstrated a significant survival advantage with the platinum-based combination (11 mo vs. 8 mo); the combination arm also had improved PFS. Similar survival figures have been reported with phase II studies of gemcitabine/capecitabine, GEMOX, and gemcitabine/irinotecan.
After reexploration for incidentally diagnosed GB CAs, most studies are small and nonrandomized and do not have sufficient power to address the survival benefit of chemoradiation. In contrast to cholangiocarcinomas, because the patterns of failure suggest a predominantly distant pattern of failure for GB CAs, it may be reasonable to establish that there is no unrecognized metastatic disease (over a course of systemic chemotherapy) before consolidating with chemoradiation therapy.
Based on population-based registries such as SEER, regionally advanced or hepatic-invasive GB CA is treated with adjuvant radiation in the United States and may result in survival improvement.138
Recent data indicate that EGFR and vascular endothelial growth factor receptor (VEGFR) are important targets in biliary cancer. Erlotinib is an active agent that has produced an 8% response rate and a median survival of 7 months for biliary cancer patients, some of whom had received prior therapy.139 Biweekly cetuximab is combined with gemcitabine and oxaliplatin in the ongoing BINGO (Biliary Cancers: EGFR Inhibitor, Gemcitabine and Oxaliplatin) trial. The interim safety analysis of this randomized study indicated no added toxicity with the addition of cetuximab; efficacy data are awaited. Investigators from Massachusetts General Hospital in Boston have combined gemcitabine and oxaliplatin with bevacizumab and demonstrated promising efficacy of this combination.140 In this study,85 FDG-PET scan was used to assess response; this could be particularly useful in biliary tract cancers, which are often nonmeasurable by RECIST. PET responses correlated with survival; further exploration of this imaging modality is warranted.
1. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357.
2. Nakagohri T., et al. Surgical outcome and prognostic factors in intrahepatic cholangiocarcinoma. World J Surg. 2008;32:2675-2680.
3. Michels N.A. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337-347.
4. Weinbren K., Mutum S.S. Pathological aspects of cholangiocarcinoma. J Pathol. 1983;139:217-238.
5. Fu X.H., et al. Clinicopathologic features, diagnosis and surgical treatment of intrahepatic cholangiocarcinoma in 104 patients. Hepatobiliary Pancreat Dis Int. 2004;3:279-283.
6. Singal A.G., et al. The clinical presentation and prognostic factors for intrahepatic and extrahepatic cholangiocarcinoma in a tertiary care centre. Aliment Pharmacol Ther. 2010;31:625-633.
6a. Greene F.L., Trotti A.III, Fritz A.G., et al. Intrahepatic bile ducts. Edge S.B., Byrd D.R., Compton C.C., et al. AJCC Cancer Staging Manual, 7th ed., New York: Springer, 2010.
7. Kaczynski J., Hansson G., Wallerstedt S. Incidence, etiologic aspects and clinicopathologic features in intrahepatic cholangiocellular carcinoma—a study of 51 cases from a low-endemicity area. Acta Oncol. 1998;37:77-83.
8. Choi B.I., Lee J.M., Han J.K. Imaging of intrahepatic and hilar cholangiocarcinoma. Abdom Imaging. 2004;29:548-557.
9. Chen L.D., et al. Enhancement patterns of intrahepatic cholangiocarcinoma: comparison between contrast-enhanced ultrasound and contrast-enhanced CT. Br J Radiol. 2008;81:881-889.
10. Madoff D.C., Abdalla E.K., Vauthey J.N. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779-790.
11. Manfredi R., et al. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155-164.
12. Breitenstein S., Apestegui C., Clavien P.A. Positron emission tomography (PET) for cholangiocarcinoma. HPB (Oxford). 2008;10:120-121.
13. Petrowsky H., et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43-50.
14. Yamamoto M., et al. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2001;8:154-157.
15. Ohtsuka M., et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525-1531.
16. Nakagawa T., et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005;29:728-733.
17. Shimada K., et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery. 2009;145:411-416.
18. Grobmyer S.R., et al. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg. 2006;244:260-264.
19. Li J., et al. Preoperative assessment of hilar cholangiocarcinoma by dual-modality PET/CT. J Surg Oncol. 2008;98:438-443.
20. Zhou X.D., et al. Intrahepatic cholangiocarcinoma: report of 272 patients compared with 5,829 patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1073-1080.
21. Saxena A., et al. Clinicopathologic and treatment-related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: a 19-year experience from an established Australian hepatobiliary unit. J Gastrointest Surg. 2010;14:1128-1138.
22. Uenishi T., et al. Portal thrombosis due to intrahepatic cholangiocarcinoma following successful treatment for hepatocellular carcinoma. Hepatogastroenterology. 2003;50:1140-1142.
23. Suzuki S., et al. Clinicopathological prognostic factors and impact of surgical treatment of mass-forming intrahepatic cholangiocarcinoma. World J Surg. 2002;26:687-693.
24. Valverde A., et al. Resection of intrahepatic cholangiocarcinoma: a Western experience. J Hepatobiliary Pancreat Surg. 1999;6:122-127.
25. Weber S.M., et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;93:384-391.
26. Weimann A., et al. Retrospective analysis of prognostic factors after liver resection and transplantation for cholangiocellular carcinoma. Br J Surg. 2000;87:1182-1187.
27. Madariaga J.R., et al. Liver resection combined with excision of vena cava. J Am Coll Surg. 2000;191:244-250.
28. Miwa S., et al. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol. 2006;41:893-900.
29. Morimoto Y., et al. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:432-440.
30. Hanazaki K., et al. Prognostic factors of intrahepatic cholangiocarcinoma after hepatic resection: univariate and multivariate analysis. Hepatogastroenterology. 2002;49:311-316.
31. Uenishi T., et al. Clinicopathological factors predicting outcome after resection of mass-forming intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:969-974.
32. Burger I., et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol. 2005;16:353-361.
33. Herber S., et al. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2007;30:1156-1165.
34. Kim J.H., et al. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer. 2008;113:1614-1622.
35. Liapi E., Geschwind J.F. Chemoembolization for primary and metastatic liver cancer. Cancer J. 2010;16:156-162.
36. Carrafiello G., et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33:835-839.
37. Chiou Y.Y., et al. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J Med Sci. 2005;21:304-309.
38. Beddar A.S., et al. 4D-CT imaging with synchronized intravenous contrast injection to improve delineation of liver tumors for treatment planning. Radiother Oncol. 2008;87:445-448.
39. Dromain C., et al. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology. 2002;223:255-262.
40. Kuszyk B.S., et al. Local tumor recurrence following hepatic cryoablation: radiologic-histopathologic correlation in a rabbit model. Radiology. 2000;217:477-486.
41. Okuma T., et al. Assessment of early treatment response after CT-guided radiofrequency ablation of unresectable lung tumours by diffusion-weighted MRI: a pilot study. Br J Radiol. 2009;82:989-994.
42. Limanond P., et al. Interpretation of CT and MRI after radiofrequency ablation of hepatic malignancies. AJR Am J Roentgenol. 2003;181:1635-1640.
43. Braga L., Semelka R.C. Magnetic resonance imaging features of focal liver lesions after intervention. Top Magn Reson Imaging. 2005;16:99-106.
44. Szendroi M., Nemeth L., Vajta G. Asbestos bodies in a bile duct cancer after occupational exposure. Environ Res. 1983;30:270-280.
45. Welzel T.M., et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221-1228.
46. Nichols J.C., et al. Diagnostic role of serum CA 19-9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clin Proc. 1993;68:874-879.
47. Patel A.H., et al. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204-207.
48. Chintanaboina J., et al. Transient marked elevation of serum CA 19-9 levels in a patient with acute cholangitis and biliary stent. South Med J. 2008;101:661.
49. Batheja N., et al. Expression of p53 and PCNA in cholangiocarcinoma and primary sclerosing cholangitis. Mod Pathol. 2000;13:1265-1268.
49a. Bismuth H., Corlette M.B. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170-178.
50. Greene F.L., et al. AJCC Cancer Staging Handbook from the AJCC Cancer Staging Manual. Available at Online version: http://online.statref.com/TOC.aspx?grpalias=JHU&FxId=73 Available to US Hopkins community
51. Gerhards M.F., et al. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg. 2001;88:48-51.
52. Neumaier C.E., et al. Staging of hilar cholangiocarcinoma with ultrasound. J Clin Ultrasound. 1995;23:173-178.
53. Hann L.E., et al. Cholangiocarcinoma at the hepatic hilus: sonographic findings. AJR Am J Roentgenol. 1997;168:985-989.
54. Fritscher-Ravens A., et al. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45-51.
55. Sahani D., et al. Using multidetector CT for preoperative vascular evaluation of liver neoplasms: technique and results. AJR Am J Roentgenol. 2002;179:53-59.
56. Uchida M., et al. Three-dimensional imaging of liver tumors using helical CT during intravenous injection of contrast medium. J Comput Assist Tomogr. 1999;23:435-540.
57. Nanashima A., et al. Three-dimensional cholangiography applying C-arm computed tomography in bile duct carcinoma: a new radiological technique. Hepatogastroenterology. 2009;56:615-618.
58. Aloia T.A., et al. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am J Surg. 2007;193:702-706.
59. Lee D.H., et al. MR imaging findings of early bile duct cancer. J Magn Reson Imaging. 2008;28:1466-1475.
60. Masselli G., et al. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur Radiol. 2008;18:2213-2221.
61. Park H.S., et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol. 2008;190:396-405.
62. Furukawa H., et al. Preoperative staging of biliary carcinoma using 18F-fluorodeoxyglucose PET: prospective comparison with PET+CT, MDCT and histopathology. Eur Radiol. 2008;18:2841-2847.
63. Kim J.Y., et al. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145-1151.
64. May G.R., Bender C.E., Williams H.J.Jr. Radiologic approaches to the treatment of benign and malignant biliary tract disease. Semin Liver Dis. 1987;7:334-342.
65. Kim Y.C., et al. Comparison of CT and MRI for presurgical characterization of para-aortic lymph nodes in patients with pancreatico-biliary carcinoma. World J Gastroenterol. 2008;14:2208-2212.
66. Gazzaniga G.M., et al. Surgery for hilar cholangiocarcinoma: an Italian experience. J Hepatobiliary Pancreat Surg. 2000;7:122-127.
67. Hemming A.W., et al. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693-699. discussion 699–702
68. Hidalgo E., et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur J Surg Oncol. 2008;34:787-794.
69. di Sebastiano P., et al. Surgical aspects in management of hepato-pancreatico-biliary tumours in the elderly. Best Pract Res Clin Gastroenterol. 2009;23:919-923.
70. Nagino M., et al. Preoperative biliary drainage for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:25-30.
71. Coss A., Byrne M.F. Preoperative biliary drainage in malignant obstruction: indications, techniques, and the debate over risk. Curr Gastroenterol Rep. 2009;11:145-149.
72. Connor S., et al. The utility of laparoscopic assessment in the preoperative staging of suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2005;9:476-480.
73. Hochwald S.N., et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261-266.
74. Nishio H., Nagino M., Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford). 2005;7:259-262.
75. Klempnauer J., et al. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 1997;15:947-954.
76. Kondo S., et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg. 2008;15:41-54.
77. Kosuge T., et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663-671.
78. Neuhaus P., et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808-818. discussion 819
79. Jarnagin W.R., et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-517. discussion 517–519
80. Murakami Y., et al. Pancreatoduodenectomy for distal cholangiocarcinoma: prognostic impact of lymph node metastasis. World J Surg. 2007;31:337-342. discussion 343–344
81. Murakami Y., et al. Prognostic significance of lymph node metastasis and surgical margin status for distal cholangiocarcinoma. J Surg Oncol. 2007;95:207-212.
82. Yoshida T., et al. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69-73.
82a. Ducreux M., Van Custem E., Van Laethem J.L., et al. A randomized phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41:398-403.
83. Lee D.H., et al. A phase I and pharmacologic study of belotecan in combination with cisplatin in patients with previously untreated extensive-stage disease small cell lung cancer. Clin Cancer Res. 2007;13:6182-6186.
84. Philip P.A., et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006;24:3069-3074.
85. Zhu A.X., et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48-54.
86. Valle J., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281.
87. Valle J.W., et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study—The UK ABC-01 Study. Br J Cancer. 2009;101:621-662.
88. Borghero Y., et al. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol. 2008;15:3147-3156.
89. Witzigmann H., et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006;244:230-239.
90. Ortner M.A. Photodynamic therapy in cholangiocarcinomas. Best Pract Res Clin Gastroenterol. 2004;18:147-154.
91. Nagorney D.M., McPherson G.A. Carcinoma of the gallbladder and extrahepatic bile ducts. Semin Oncol. 1988;15:106-115.
92. Chaurasia P., Thakur M.K., Shukla H.S. What causes cancer gallbladder? a review. HPB Surg. 1999;11:217-224.
93. Towfigh S., et al. Porcelain gallbladder is not associated with gallbladder carcinoma. Am Surg. 2001;67:7-10.
94. Henson D.E., Albores-Saavedra J., Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1493-1497.
95. Albores-Saavedra J., Henson D.E. Armed Forces Institute of Pathology (U.S.). Tumors of the gallbladder and extrahepatic bile ducts. Washington, DC: Armed Forces Institute of Pathology; 1986.
96. Sons H.U., Borchard F., Joel B.S. Carcinoma of the gallbladder: autopsy findings in 287 cases and review of the literature. J Surg Oncol. 1985;28:199-206.
97. Wood R., et al. Epidemiology of gallbladder cancer and trends in cholecystectomy rates in Scotland, 1968-1998. Eur J Cancer. 2003;39:2080-2086.
98. Diehl A.K. Gallstone size and the risk of gallbladder cancer. JAMA. 1983;250:2323-2326.
99. Shukla V.K., et al. Diagnostic value of serum CA242, CA 19-9, CA 15-3 and CA 125 in patients with carcinoma of the gallbladder. Trop Gastroenterol. 2006;27:160-165.
100. Murray M.D., Burton F.R., Di Bisceglie A.M. Markedly elevated serum CA 19-9 levels in association with a benign biliary stricture due to primary sclerosing cholangitis. J Clin Gastroenterol. 2007;41:115-117.
101. Carriaga M.T., Henson D.E. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(Suppl 1):171-190.
102. Goldin R.D., Roa J.C. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55:218-229.
103. Yamamoto M., Nakajo S., Tahara E. Dysplasia of the gallbladder. Its histogenesis and correlation to gallbladder adenocarcinoma. Pathol Res Pract. 1989;185:454-460.
104. Roa I., et al. Preneoplastic lesions and gallbladder cancer: an estimate of the period required for progression. Gastroenterology. 1996;111:232-236.
105. Fong Y., Jarnagin W., Blumgart L.H. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557-569.
106. Albores-Saavedra J., Henson D.E., Sobin L.H. The WHO histological classification of tumors of the gallbladder and extrahepatic bile ducts. A commentary on the second edition. Cancer. 1992;70:410-414.
107. Lazcano-Ponce E.C., et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349-364.
108. Wang J.D., et al. Role of regional lymphadenectomy in different stage of gallbladder carcinoma. Hepatogastroenterology. 2009;56:593-596.
109. Shirai Y., et al. Identification of the regional lymphatic system of the gallbladder by vital staining. Br J Surg. 1992;79:659-662.
110. Hawkins W.G., et al. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310-315.
111. Onoyama H., et al. Diagnostic imaging of early gallbladder cancer: retrospective study of 53 cases. World J Surg. 1999;23:708-712.
112. Tsuchiya Y. Early carcinoma of the gallbladder: macroscopic features and US findings. Radiology. 1991;179:171-175.
113. Komatsuda T., et al. Gallbladder carcinoma: color Doppler sonography. Abdom Imaging. 2000;25:194-197.
114. Bach A.M., et al. Gallbladder cancer: can ultrasonography evaluate extent of disease? J Ultrasound Med. 1998;17:303-309.
115. Kim S.J., et al. Accuracy of preoperative T-staging of gallbladder carcinoma using MDCT. AJR Am J Roentgenol. 2008;190:74-80.
116. Ohtani T., et al. Spread of gallbladder carcinoma: CT evaluation with pathologic correlation. Abdom Imaging. 1996;21:195-201.
117. Levin B. Gallbladder carcinoma. Ann Oncol. 1999;10(Suppl 4):129-130.
118. Kalra N., et al. MDCT in the staging of gallbladder carcinoma. AJR Am J Roentgenol. 2006;186:758-762.
119. Kumaran V., et al. The role of dual-phase helical CT in assessing resectability of carcinoma of the gallbladder. Eur Radiol. 2002;12:1993-1999.
120. Demachi H., et al. Dynamic MRI using a surface coil in chronic cholecystitis and gallbladder carcinoma: radiologic and histopathologic correlation. J Comput Assist Tomogr. 1997;21:643-651.
121. Choi J.Y., et al. Navigator-triggered isotropic three-dimensional magnetic resonance cholangiopancreatography in the diagnosis of malignant biliary obstructions: comparison with direct cholangiography. J Magn Reson Imaging. 2008;27:94-101.
122. Oikarinen H. Diagnostic imaging of carcinomas of the gallbladder and the bile ducts. Acta Radiol. 2006;47:345-358.
123. Koh T., et al. Differential diagnosis of gallbladder cancer using positron emission tomography with fluorine-18-labeled fluoro-deoxyglucose (FDG-PET). J Surg Oncol. 2003;84:74-81.
124. Anderson C.D., et al. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90-97.
125. Misra M.C., Guleria S. Management of cancer gallbladder found as a surprise on a resected gallbladder specimen. J Surg Oncol. 2006;93:690-698.
126. Kapoor V.K. Incidental gallbladder cancer. Am J Gastroenterol. 2001;96:627-629.
127. Bartlett D.L., et al. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639-646.
128. Principe A., et al. Radical surgery for gallbladder carcinoma: possibilities of survival. Hepatogastroenterology. 2006;53:660-664.
129. Abdalla E.K., Vauthey J.N. Extrahepatic bile duct resection: standard treatment for advanced gallbladder cancer? J Surg Oncol. 2006;94:269-270.
130. D’Amico D., et al. Current situation in the treatment of gallbladder cancer. Considerations on the utility of an extended resection. Hepatogastroenterology. 1991;38(Suppl 1):16-21.
131. Oertli D., Herzog U., Tondelli P. Primary carcinoma of the gallbladder: operative experience during a 16 year period. Eur J Surg. 1993;159:415-420.
132. Duffy A., et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Center (MSKCC). J Surg Oncol. 2008;98:485-489.
133. Fong Y., Heffernan N., Blumgart L.H. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive reresection is beneficial. Cancer. 1998;83:423-427.
134. Hueman M.T., Vollmer C.M.Jr., Pawlik T.M. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol. 2009;16:2101-2115.
135. Oswalt C.E., Cruz A.B.Jr. Effectiveness of chemotherapy in addition to surgery in treating carcinoma of the gallbladder. Rev Surg. 1977;34:436-438.
136. Todoroki T., et al. Resection combined with intraoperative radiation therapy (IORT) for stage IV (TNM) gallbladder carcinoma. World J Surg. 1991;15:357-366.
137. de Aretxabala X., et al. Chemoradiotherapy in gallbladder cancer. J Surg Oncol. 2006;93:699-704.
138. Wang S.J., et al. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26:2112-2117.
139. Thomas M.B. Targeted therapies for cancer of the gallbladder. Curr Opin Gastroenterol. 2008;24:372-376.
140. Malka D., Trarbach T., Fartoux L., et al. A multicener, randomized phase II trial of gemcitabine and oxaliplatin (GEMOX) alone or in combination with biweekly cetuximab in the first-line treatment of advanced biliary cancer: interim analysis of the BINGO trial. J Clin Oncol. 2009;27(Suppl):15s.