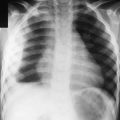
Chapter 218 Chlamydia trachomatis
218.1 Trachoma
Chidambaram JD, Alemayehu W, Melese M, et al. Effect of a single mass antibiotic distribution on the prevalence of infectious trachoma. JAMA. 2006;295:1142-1146.
Grassley NC, Ward ME, Ferris S, et al. The natural history of trachoma infection and disease in a Gambian cohort with frequent follow-up. PloS Negl Trop Dis. 2008;2:e341.
House JI, Ayele B, Porco TC, et al. Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster randomized trial. Lancet. 2009;373:1111-1118.
Nieuwenhuis RF, Ossewaarde JM, Götz HM, et al. Resurgence of lymphogranuloma venereum in Western Europe: an outbreak of Chlamydia trachomatis serovar L2 proctitis in the Netherlands among men who have had sex with men. Clin Infect Dis. 2004;39:996-1003.
Solomon AW, Peeling RW, Foster A, et al. Diagnosis and assessment of trachoma. Clin Microbiol Rev. 2004;17:982-1011.
Taylor HR. Elimination of blinding trachoma revolves around children. Lancet. 2009;373:1061-1063.
218.2 Genital Tract Infections
Diagnosis
The etiology of most cases of nonchlamydial nongonococcal urethritis is unknown, although Ureaplasma urealyticum and possibly Mycoplasma genitalium are implicated in up to one third of cases (Chapter 216). Proctocolitis may develop in individuals who have a rectal infection with an LGV strain (see Chapter 218.4, later).
Complications
Complications of genital chlamydial infections in women include perihepatitis (Fitz-Hugh-Curtis syndrome) and salpingitis. Of women with untreated chlamydial infection who develop pelvic inflammatory disease, up to 40% will have significant sequelae; approximately 17% will suffer from chronic pelvic pain, approximately 17% will become infertile, and approximately 9% will have an ectopic (tubal) pregnancy. Adolescent girls may be at higher risk for developing complications, especially salpingitis, than older women. Salpingitis in adolescent girls is also more likely to lead to tubal scarring, subsequent obstruction with secondary infertility, and increased risk for ectopic pregnancy. Approximately 50% of neonates born to pregnant women with untreated chlamydial infection will acquire C. trachomatis infection (see Chapter 218.3, next). Women with C. trachomatis infection have a 3-5-fold increased risk for acquiring HIV infection.
Bébéar C, de Barbeyrac B. Genital Chlamydia trachomatis infections. Clin Microbiol Infect. 2009;15:4-10.
Black CM, Driebe EM, Howard LA, et al. Multicenter study of nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in children being evaluated for sexual abuse. Pediatr Infect Dis J. 2009;28:608-613.
Centers for Disease Control and Prevention: Sexually transmitted diseases treatment guidelines 2010, MMWR (in press).
Gaydos CA, Theodore M, Dalesio N, et al. Comparison of three nucleic acid amplification tests for detection of Chlamydia trachomatis in urine specimens. J Clin Microbiol. 2004;42:3041-3045.
Girardet RG, Lahoti S, Howard LA, et al. The epidemiology of sexually transmitted infections in suspected child victims of sexual assault. Pediatrics. 2009;124:79-86.
Hammerschlag MR, Guillén CD. Medical and legal implications of testing for sexually transmitted infections in children. Clin Microbiol Rev. 2010;23:493-506.
Hwang L, Shafer MA. Chlamydia trachomatis infection in adolescents. Adv Pediatr. 2004;51:379-407.
Schachter J, McCormack WM, Chernesky MA, et al. Vaginal swabs are appropriate specimens for diagnosis of genital tract infection with Chlamydia trachomatis. J Clin Microbiol. 2003;41:3784-3789.
218.3 Conjunctivitis and Pneumonia in Newborns
Centers for Disease Control and Prevention: Sexually transmitted diseases treatment guidelines 2010, MMWR (in press.
Hammerschlag MR, Gelling M, Roblin PM, et al. Treatment of neonatal chlamydial conjunctivitis with azithromycin. Pediatr Infect Dis J. 1998;17:1049-1050.
Rours GIJG, Hammerschlag MR, De Faber JTHN, et al. Chlamydia trachomatis as a cause of neonatal conjunctivitis in Dutch infants. Pediatrics. 2008;121:e321-e326.