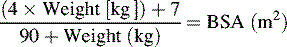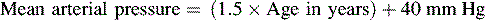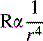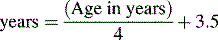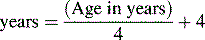1 Children Are Different
Pearls
• Children are not “little adults.” They are physically, physiologically, and emotionally immature. Critical care equipment, assessment techniques, and therapies must be appropriate for the child’s anatomy and physiology.
• The skilled critical care nurse is able to determine at a glance whether the child “looks good” or “looks bad.” This rapid evaluation includes visual and tactile assessment of the child’s color, perfusion, level of activity, and position. This assessment is similar to that described as the pediatric initial impression8a: level of consciousness, breathing, and color.
• Normal vital signs are not always appropriate vital signs in the seriously ill or injured patient. Tachycardia and tachypnea are usually appropriate when the child is in distress. Hypotension may be only a late sign of shock in children.
Psychosocial development
The child and parents or primary caretaker must be treated as a unit, and the parents should be allowed to remain with the child as much as possible. Visitation hours should be liberal, if not unlimited. Communication with the parents must be clear, frequent, and consistent. Joint nurse-physician rounds (involving all caregivers) that include the patient and family will help establish open and consistent communication within the healthcare team and with the family. Specific information about the child’s emotional and intellectual development and the response of the child and family to the child’s illness is included in Chapters 2 and 3.
General assessment
Initial Impression: “Looks Good” vs. “Looks Bad”
Every skilled critical care nurse and physician develops a systematic method for determining the severity of the patient’s condition, making both qualitative and quantitative assessments. Often, the initial, general impression of how the patient looks is more important than any single vital sign or clinical measurement.8a
The skilled critical care clinician can determine at a glance whether the patient “looks good” or “looks bad.” This determination requires a rapid visual evaluation of the child’s color, skin perfusion, level of consciousness (activity and responsiveness), breathing, and position of comfort (Box 1-1). Each portion of this assessment is reviewed in detail in this section.
Evaluation of Vital Signs
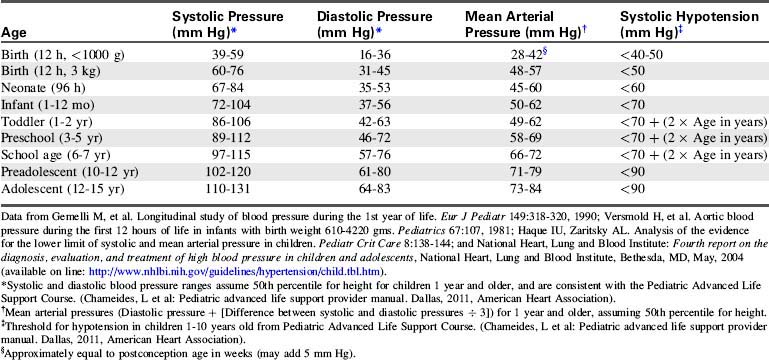
Normal vital signs ranges are provided in Tables 1-1 to 1-3. Evaluation of vital signs requires consideration of normal values for the child’s age,14a trends in the individual patient’s vital signs, and appropriate vital signs for the child’s condition. Remember that in children, shock can be present despite the observation of a normal blood pressure. Hypotension is often a late sign of shock in the pediatric patient.
| Age | Awake Heart Rate (beats/min) | Sleeping Heart Rate (beats/min) |
| Neonate | 100-205 | 90-160 |
| Infant | 100-180 | 90-160 |
| Toddler | 98-140 | 80-120 |
| Preschooler | 80-120 | 65-100 |
| School-aged child | 75-118 | 58-90 |
| Adolescent | 60-100 | 50-90 |
* Always consider the patient’s normal range and clinical condition. Heart rate will normally increase with fever or stress.
Table 1-2 Normal Respiratory Rates in Children*14a
| Age | Rate (breaths/min) |
| Infant | 30-53 |
| Toddler | 22-37 |
| Preschool | 20-28 |
| School age | 18-25 |
| Adolescent | 12-20 |
* Consider the patient’s normal range. The child’s respiratory rate is expected to increase in the presence of fever or stress.
Assessment Format
An additional alphabetical format may be useful for pediatric critical care nurses. This format uses the first seven letters of the alphabet to help the nurse recall the steps in a seven-point check (Box 1-2). The seven essential assessment points include the child’s airway (and aeration), brain (neurologic function), circulation, drips or drugs administered, electrolyte balance, fluids (including fluid balance and fluid administration rate), genitourinary and gastrointestinal function, and growth and development.17a When caring for the critically ill neonate, this format can be modified to create a nine-point check, with the addition of the letter H for heat, or thermoregulation, and the letter I for immunologic immaturity.
General characteristics
Thermoregulation
The nurse can reduce cold stress by maintaining a neutral thermal environment for the neonate. A neutral thermal environment is the environmental temperature at which the infant maintains a rectal temperature of 37° C with the lowest oxygen consumption. This neutral temperature should be maintained during all aspects of the infant’s care, especially during transport and diagnostic tests. Over-bed radiant warmers can help maintain the infant’s temperature without interfering with observation and care. The beds are equipped with servo-control devices to adjust heat output in response to changes in the infant’s skin temperature. Adjustable alarms indicate when excessive warming is required to maintain the infant’s temperature or when the infant’s temperature varies from the selected range. (For further information regarding warming devices, see Chapter 21)
Fluid Requirements and Fluid Therapy
The weight can be estimated from body length using a length-based tape (see section, Cardiac Arrest and Resuscitation) such as the Broselow resuscitation tape (Fig. 1-1; see also Evolve Fig. 1-1 in the Chapter 1 Supplement on the Evolve Website.)
The estimate of maintenance fluid requirements (Table 1-4) provides a baseline for tailoring the fluid administration rate for each patient. Actual fluid administration is tailored to the child’s clinical condition. Normal insensible water losses average 300 to 400 mL/m2 BSA per day. Fever increases insensible water losses by approximately 0.42 mL/kg per hour per degree Celsius elevation in temperature above 37° C.40 Radiant warmers, phototherapy, and the presence of diaphoresis or large burns also will increase a child’s insensible water loss. Fluid retention can diminish fluid requirements postoperatively and fluid retention typically develops in the presence of congestive heart failure, respiratory failure, or renal failure.
Table 1-4 Formulas for Estimating Daily Maintenance Fluid and Electrolyte Requirements for Children
| Daily Requirements | Hourly Requirements | |
| Fluid Requirements Estimated from Weight* | ||
| Newborn (up to 72 hr after birth) | 60-100 mL/kg (newborns are born with excess body water) | – |
| Up to 10 kg | 100 mL/kg (can increase up to 150 mL/kg to provide caloric requirements if renal and cardiac function are adequate) | 4 mL/kg |
| 11-20 kg | 1000 mL for the first 10 kg + 50 mL/kg for each kg over 10 kg | 40 mL for first 10 kg + 2 mL/kg for each kg over 10 kg |
| 21-30 kg | 1500 mL for the first 20 kg + 25 mL/kg for each kg over 20 kg | 60 mL for first 20 kg + 1 mL/kg for each kg over 20 kg |
| Fluid Requirements Estimated from Body Surface Area (BSA) | ||
| Maintenance | 1500 mL/m2 BSA | – |
| Insensible losses | 300-400 mL/m2 BSA | – |
| Electrolytes | ||
| Sodium (Na) | 2-4 mEq/kg | – |
| Potassium (K) | 1-2 mEq/kg | – |
| Chloride (Cl) | 2-3 mEq/kg | – |
| Calcium (Ca) | 0.5-3 mEq/kg | – |
| Phosphorous (Phos) | 0.5-2 mmol/kg | – |
| Magnesium (Mg) | 0.4-0.9 mEq/kg | – |
* The “maintenance” fluids calculated by these formulas must only be used as a starting point to determine the fluid requirements of an individual patient. If intravascular volume is adequate, children with cardiac, pulmonary, or renal failure or increased intracranial pressure should generally receive less than these calculated “maintenance” fluids. The formula utilizing body weight generally results in a generous “maintenance” fluid total.
Consistent with values from Barakat AY, Ichikawa I: Laboratory data. In Ichikawa I, editor: Pediatric textbook of fluids and electrolytes, Baltimore, 1990, Williams and Wilkins; and Tan JM: Nephrology. In Custer JW, Rau RE, editors: The Johns Hopkins Hospital Harriet Lane Handbook, ed 18, Philadelphia, 2009, Mosby-Elsevier.
Children have proportionally more body water than do adults. Total body water constitutes approximately 75% to 80% of the full-term infant’s weight and 60% to 70% of the body weight of the adult. During the first weeks of life, most body water is located in the extracellular compartment, and much of this water is exchanged daily. For this reason and because the infant kidney is less able to concentrate urine (see section, Renal Function), dehydration can develop rapidly if the infant’s fluid intake is compromised or fluid losses are excessive.
Intravenous fluids are provided to flush monitoring lines, dilute medications, replace volume loss, or provide nutrition. In the past, hypotonic crystalloids (e.g., 5% dextrose with 0.2% sodium chloride) were routinely used for pediatric maintenance and replacement fluids, with the assumption that critically ill patients are likely to retain sodium and water. However, children are much more likely to develop hyponatremia if hypotonic rather than isotonic solutions are used, and isotonic fluids do not increase risk of hypernatremia.2,9 At this time there is insufficient evidence to identify a single optimal intravenous fluid for pediatric maintenance therapy, so practitioners will need to individualize intravenous fluid selection for each patient.
Electrolyte, Glucose, and Calcium Balance
Sodium is the major intravascular ion, and acute changes in serum sodium concentration will affect serum osmolality and free water movement. Hyponatremia in the critically ill child can result from antidiuretic hormone excess (i.e., the syndrome of inappropriate antidiuretic hormone secretion) and liberal water administration in excess of sodium, including administration of hypotonic fluids.4 Hyponatremia can also result from excess sodium losses, such as those occurring with adrenocortical insufficiency (see Chapter 12).
Hypernatremia can result from excessive sodium administration or free water loss, such as that occurring with diabetes insipidus or vomiting. Hypernatremia in infants and young children is most frequently observed as a complication of dehydration. Cerebral hemorrhage and cerebral dysfunction have been reported after abrupt correction of hyponatremia in adults (i.e., rapid rise in serum sodium concentration),18 and similar complications are thought to occur in children. Rapid correction of hypernatremia can produce an acute fall in serum osmolality, with resultant intracellular free water shift and cerebral edema. In general, when correcting hyponatremia or hypernatremia the child’s serum sodium concentration should be changed at a maximum rate of 10 to 12 mEq/24 h (or an average of 0.5 mEq/h).
The serum potassium should be expected to fall as the child’s pH rises, and it will rise as the pH falls because hydrogen ion moves intracellularly in exchange for potassium. A low serum potassium concentration in a patient with acidosis is problematic, because it will drop even lower as the acidosis is corrected (see Chapter 12).
Several case series in adult38 and pediatric patients suggested that uncontrolled hyperglycemia, whether it is endogenous or exogenous in origin, can be harmful to critically ill patients and can increase complication rates and decrease survival. Although this is a significant concern, other studies have found contradictory evidence, and additional studies are underway to clarify these issues. Use of insulin infusion to prevent hyperglycemia was associated with reduced critical care unit mortality in adult studies38 and one multicenter pediatric study,39 but was also associated with increased hypoglycemic episodes.
The serum ionized calcium concentration (normal value is approximately 4.8 to 5.2 mg/dL or 1.2 to 1.38 mmol/L) is the “working” calcium, involved in nerve and muscle function.12 Therefore, the healthcare team monitors ionized and total calcium concentration during critical illness and provides supplementary calcium for documented hypocalcemia.
A fall in total or ionized calcium is observed frequently in critically ill infants and children. Ionized hypocalcemia has been reported after cardiac arrest in children with septic shock or renal failure.41 The phosphate in citrate phosphate dextran-preserved blood will precipitate with ionized calcium, so some transfusions may produce ionized hypocalcemia.
Abnormalities in magnesium balance are observed frequently in critically ill patients. Magnesium affects parathyroid function and contributes to control of the intracellular potassium concentration. As a result, hypomagnesemia can contribute to refractory hypocalcemia or hypokalemia. In addition, it can be associated with increased neuromuscular excitability, gastrointestinal dysfunction, and arrhythmias.18
Renal Function
Kidney weight doubles in the first 10 months of life, more as the result of proximal tubular growth than from an increase in glomerular size. The glomerular filtration rate (GFR) also increases significantly after birth; the GFR of the full-term neonate (per square meter of BSA) is approximately one third that of an adult. Renal blood flow and the GFR double during the first 2 weeks of life, and the GFR continues to increase during the first year. The GFR approaches adult values by approximately 3 years of age (Table 1-5).3,36 Until that time, the relatively low GFR and reduced tubular secretion can prolong the half-life of administered drugs (see Chapters 4 and 13).
| Age | Glomerular Filtration Rate (mL/min per 1.73 m2) |
| Premature infant | 6 |
| Full-term newborn | 8-60 |
| 1 month | 26-90 |
| 1 year | 63-150 |
| 3 years | 89-179 |
| 6 years | 79-170 |
| Adult male | 110-152 |
Regulation of acid-base balance by the newborn kidney is relatively efficient, although the infant kidney has less ability to secrete hydrogen ions or fixed acid than the adult kidney (this is exacerbated by limited dietary protein intake). As a result, renal compensation for metabolic acidosis may be limited in the neonate. Dehydration, hypotension, and hypoxemia all produce a marked fall in the infant’s GFR, so renal function can become compromised quickly during critical illness (for further information, see Chapter 13).
Pediatric Pharmacokinetics
Drug absorption, distribution, and elimination will be affected by age and clinical condition. Drug absorption is influenced by maturation of the gastrointestinal tract, liver, and kidney. Because the gastric pH is higher (less acidic) during the first 2 years of life, bioavailability of weak acids administered orally (e.g., phenytoin and phenobarbital) may be reduced in this age group, so higher doses (per kilogram body weight) may be required to achieve target serum concentrations of such drugs.1
Drug distribution is affected by cardiac output, organ blood flow, composition and relative size of body compartments, pH of body fluids, and extent of drug binding to plasma proteins and tissues. The higher proportion of water in the body during the first years of life increases the volume of distribution for hydrophilic drugs during these years.1 Even if a drug has a consistent volume of distribution (mg/kg body weight) for patients of all ages, the neonate has limited ability to eliminate some drugs, so those drugs will have longer half-lives and lower clearances when administered to neonates than to older children and adults. Even when loading doses for drugs are similar (per kilogram body weight) for neonates and adults, neonates will likely require lower maintenance doses of the drugs than will be required by adults.
Drug clearance is affected by metabolic, hepatic, and renal blood flow and function.1 Developmental changes associated with hepatic metabolism and renal secretion or filtration can slow down or speed up drug elimination. Several metabolic processes mature during the first months of life (see Fig. 4-7), and many drug elimination pathways continue to mature during the first years of life. Failure to recognize these developmental changes in children can lead to drug dosing errors and complications. By the end of the first year of life, liver metabolism and drug clearance are similar to those reported in older children and adults.
The nurse should be familiar with the pharmacokinetics and pharmacodynamics of all drugs administered to the patient (see also Chapter 4) and should evaluate drug dose in light of the patient’s organ perfusion and function and in light of clinical factors, such as drugs or conditions that may alter protein binding.
Nutrition and Gastrointestinal Function
The child has a higher metabolic rate than does the adult, and the child requires more calories per kilogram body weight (Table 1-6). Most of a child’s maintenance calories are needed for basal metabolism and growth, so the child typically requires a caloric intake that approaches the typical maintenance caloric intake even if the child is inactive.
Table 1-6 Estimated Normal Maintenance Caloric Requirements for Infants and Children
| Age | Kcal/kg per 24 hours | |
| 0-6 months | 90-110 | |
| 6-12 months | 80-100 | |
| 12-36 months | 75-90 | |
| 4-10 years | 65-75 | |
| >10 years, male | 40-55 | |
| >10 years, female | 38-50 | |
| Nutrient | Percent of total daily calories | |
| Carbohydrates |  |
|
| Fat | ||
| Protein | 7-15 | |
Critical illness, trauma, or burns will increase the child’s caloric requirements significantly, and fever will increase caloric requirements 12% per hour per degree Celsius elevation in temperature above 37° C.40 Unless intolerably large quantities of fluids are administered, maintenance calories cannot be provided through 5% or 10% dextrose intravenous fluids. Therefore, provision of parenteral nutrition or tube feedings must be planned early in the child’s hospitalization.
Cardiovascular function
Cardiac Output
Normal cardiac output is higher per kilogram body weight in the child than in the adult. The cardiac output at birth is 400 mL/kg per minute; it falls to approximately 200 mL/kg per minute within the first weeks of life and to 100 mL/kg per minute during adolescence.32
To allow direct comparison of cardiac outputs for patients of different ages and sizes, the cardiac index is usually calculated. The cardiac index is equal to the cardiac output per square meter of BSA (cardiac output ÷ BSA in m2); normal values are 3.5 to 4.5 L/min per m2 BSA in the child and 2.5 to 3.5 L/min per m2 BSA in the adult. A cardiac index of less than 2.1 to 2.5 L/min per m2 BSA is considered low cardiac output in a patient of any age.32
Heart Rate and Rhythm
Tachycardia is the most efficient method of increasing cardiac output in any patient, and it is the chief method of increasing cardiac output in the child. Tachycardia is normally observed when the child is frightened, febrile, or stressed. However, an increase in heart rate to extremely high levels may reduce cardiac output. If the ventricular rate exceeds 180 to 220 beats/min, ventricular diastolic filling time and coronary artery perfusion time are severely compromised, so stroke volume and cardiac output usually fall.30
Transient bradycardia may be normal in the infant or child, particularly during periods of sleep or times of vagal stimulation (such as that produced by suctioning, defecation, or feeding). Profound or persistent bradycardia, however, usually results in a fall in cardiac output and systemic perfusion. The most common cause of bradycardia in the child is hypoxia, so the initial treatment of bradycardia requires assessment and support of airway and ventilation. Symptomatic bradycardia (i.e., bradycardia associated with signs of poor perfusion) despite adequate oxygenation and ventilation is an ominous sign of deterioration and requires immediate resuscitation.8a,20,30
Many neonatal and pediatric arrhythmias are clinically benign, because they do not compromise systemic perfusion, and they are unlikely to convert to malignant arrhythmias. The significance of any arrhythmia is determined by its effects on the child’s systemic perfusion—the heart rhythm is either stable or unstable.8a,20,30
Unstable arrhythmias include those in which the ventricular rate is too slow to maintain effective perfusion, too fast to maintain systemic perfusion, or the rhythm results in ineffective perfusion (with loss of pulses). The most common clinically significant unstable arrhythmias observed in children are bradycardia and supraventricular tachycardia; children with cardiovascular disease and some with channelopathies or left ventricular outflow tract obstruction may demonstrate ventricular arrhythmias (see section, Cardiac Arrest and Resuscitation).
Factors Influencing Stroke Volume
Cardiac output can be affected by changes in the stroke volume. The stroke volume in the neonate is extremely small, averaging 1.5 mL/kg, or 5 mL, in the full-term newborn. This stroke volume increases with age and averages approximately 75 to 90 mL in the adolescent or adult.32
There is not a linear relationship between volume administered and preload (ventricular end-diastolic pressure [VEDP]) produced. The effect of volume administration on VEDP is influenced by ventricular compliance (i.e., the distensibility of the ventricle); compliance varies from patient to patient and can vary in the same patient. Neonatal myocardium is less compliant and has a smaller response to volume loading than the myocardium of older children and adults.27 When treating shock with bolus fluid administration, the critical care provider will titrate volume administration to patient response.
Cardiac contractility refers to efficiency of myocardial fiber shortening. Contractility can be impaired in postoperative patients or patients with ischemia, electrolyte or acid-base imbalance, coronary artery insufficiency, or infection. Neonatal myocardium is less compliant and contains less contractile mass than does adult myocardium, and the neonatal ventricle is thought to require higher VEDP to maximize stroke volume. Infant myocardium, however, actually has a higher ejection fraction than that of the older child or adult.32
Ventricular afterload is ventricular wall stress, commonly considered as impedance to ventricular ejection. Infants and children tolerate mild increases in ventricular afterload (such as may result from mild pulmonary or aortic stenosis), provided the afterload does not develop acutely. As in the adult, significant increases in ventricular afterload can produce heart failure and decreased cardiac output (see Chapters 6 and 8 for more information).
Response to Catecholamines
The developmental response to exogenous catecholamine administration is still under investigation. Published studies of catecholamine administration in children have used heterogeneous groups of patients (many ages, sizes, and clinical conditions), so it is difficult to generalize observations. The response of the myocardium and vascular tone to exogenous catecholamine administration will probably be affected more by the child’s clinical condition (e.g., whether downgrading or upgrading of receptors or in hepatic and renal dysfunction) than by the child’s age (see Chapters 6 and 8). Therefore, the correct dose of any vasoactive drug must be determined at the patient’s bedside with careful titration according to patient response. This drug titration requires ongoing assessment to maximize therapeutic effects and minimize side or toxic effects.
Signs of Shock
As noted previously, the infant with poor systemic perfusion can demonstrate temperature instability, and the child can develop a high core temperature in the face of profound reduction in skin blood flow. Subtle signs of poor systemic perfusion in the infant or young child include a change in level of consciousness or responsiveness and hypoglycemia (Box 1-3). Unlike in adults, however, hypotension is usually only a late sign of poor systemic perfusion in the child.
Box 1-3 Signs of Poor Systemic Perfusion
Cool skin, prolonged capillary refill
Oliguria (urine volume <l-2 mL/kg per hour)
The American Heart Association (AHA) Pediatric Advanced Life Support Guidelines20 define hypotension as a systolic blood pressure less than 60 mm Hg in term neonates (up to 28 days of age), and a systolic blood pressure less than 70 mm Hg in infants (1 to 12 months of age). In children 1 to 10 years old, systolic hypotension is present if the systolic pressure is less than 70 mm Hg plus twice the age in years.8a,20 This estimate corresponds to slightly higher than the fifth percentile systolic blood pressure for children of median height.17 For the same age group, the critically low mean arterial pressure (the fifth percentile mean arterial pressure for children of median height) can be estimated by the following formula17:
For children 10 years of age and older, a systolic blood pressure less than 90 mm Hg is considered hypotensive.8a,20,30
Treatment of congestive heart failure in any patient requires eliminating excess intravascular fluid and improving myocardial function. Diuretic therapy and limitation of fluid intake will eliminate excess intravascular fluid. Administration (and titration) of inotropic agents, inodilators or vasodilators can improve cardiovascular function. Digoxin derivatives are used less often in children than in adults, and potential benefits of use must be weighed against risk of toxicity. Because risk of toxicity is high in premature infants, digoxin is less likely to be used in this population. Digoxin can be used in infants with large ventricular septal defects and preoperative congestive heart failure and in older children who have structurally normal hearts and cardiomyopathy (see section, Congestive Heart Failure in Chapter 8).
Circulating Blood Volume
The child’s circulating blood volume is larger per kilogram body weight than that of the adult (Table 1-7). However, the child’s absolute blood volume is small, so quantitatively small blood loss can significantly reduce blood volume and systemic perfusion. A 25-mL blood loss in a 70-kg adult would represent loss of only 0.5% to 0.6% of blood volume. The same 25-mL blood loss in the 3-kg neonate constitutes a 10% hemorrhage.
| Age | Blood Volume (mL/kg) |
| Neonate | 80-85 |
| Infant | 75-80 |
| Child | 70-75 |
| Adolescent, adult | 65-70 |
As in the adult, systemic oxygen delivery is a product of arterial oxygen content and cardiac output. A threshold hemoglobin concentration for red blood cell (RBC) transfusion of approximately 7 g/dL (typically associated with a hematocrit of 20% to 21% or less) was found to be sufficient in stable critically ill children with adequate cardiovascular function.22 However, there are insufficient data to recommend a threshold hemoglobin concentration for premature infants or children with conditions such as severe hypoxemia, hemodynamic instability, or heart disease. The healthcare team must individualize transfusion approach, weighing the potential risks of transfusion and the need to optimize the hemoglobin concentration to support the child’s systemic oxygen delivery and cardiac output (see Chapter 15).
Cardiac Arrest and Resuscitation
Although there are differences in the epidemiology of out-of-hospital versus in-hospital pediatric cardiac arrest, most episodes of cardiac arrest in infants and children are associated with a terminal rhythm of bradycardia or pulseless electrical activity which, if untreated, progresses to asystole. Sudden arrhythmic arrest is much less common in infants and children than in adults.30
Recent analysis of in-hospital pediatric resuscitation data from the American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) in the United States,26 and data downloaded from automated external defibrillators in the prehospital setting33 support many of the widely held concepts regarding the epidemiology of pediatric arrest, reinforce the need to prevent arrest, and raise questions for additional studies.
In-hospital cardiac arrest often develops as a progression of respiratory failure and shock. Typically half or more of pediatric victims of in-hospital arrest have preexisting respiratory failure, and one third or more have shock, although these figures vary somewhat among reporting hospitals.25,26 When pediatric in-hospital respiratory failure or arrest with bradycardia is treated before the development of (pulseless) cardiac arrest, survival is generally high.14,25
Bradycardia, asystole, or pulseless electrical activity were recorded as initial rhythms in half or more of recent reports of in-hospital pediatric cardiac arrest, with survival to hospital discharge ranging from 22% to 40%.25,26 In the NRCPR analysis of first rhythm in cardiac arrest, children who received chest compressions for severe bradycardia with pulses had a significantly higher rate of survival to hospital discharge than those who had a pulseless arrest (60% versus 27%).14,26 In addition, children with bradycardia who received chest compressions had a higher survival rate than adults who arrested with a terminal bradycardic rhythm.26
An analysis of the initial rhythm of in-hospital cardiac arrest in the NRCPR confirmed age-related differences in initial in-hospital arrest rhythms and outcomes. Pediatric patients were more likely than adults to exhibit asystole and only about half as likely to exhibit ventricular fibrillation (VF). Pulseless electrical activity was also less common in children than in adults.26
Although VF and pulseless ventricular tachycardia (VT) are uncommon presenting rhythms for pediatric patients with in-hospital pulseless arrest, a “shockable” rhythm was present sometime during the course of one fourth of attempted in-hospital resuscitations in children.26 This report confirms the importance of training pediatric resuscitation team members in coordination of high-quality chest compressions with shock delivery.
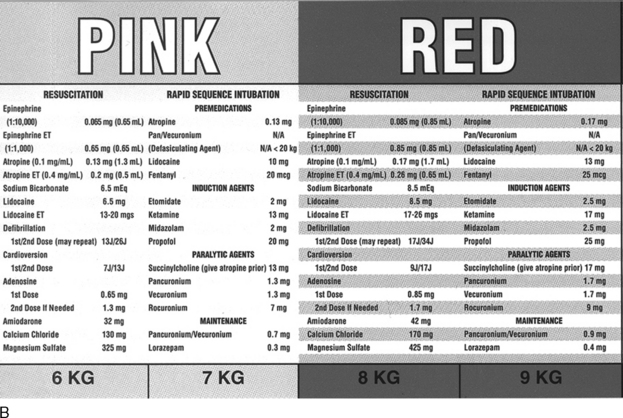
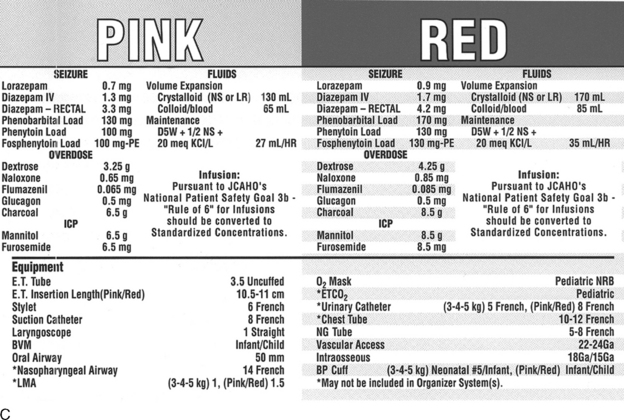
Pediatric advanced life support includes accurate and rapid preparation of appropriate drugs. Use of emergency drug and supply tables and tapes (see Fig. 1-1 earlier in chapter) will improve accuracy and eliminate the need for rapid calculations at a stressful time.23
Outcome of in-hospital pediatric resuscitation is undoubtedly influenced by the quality of CPR provided, including duration of “hands off” intervals, and specific periarrest interventions such as extracorporeal membrane oxygenation support, cardiorespiratory support and attempted defibrillation (including technique and dose). Each healthcare system that provides resuscitation is responsible for monitoring outcome and identifying areas for improvement (see Chapter 6).
There are limited data to characterize pediatric out-of-hospital cardiac arrest, although existing data (most recently obtained with automated external defibrillators) support the long-held belief that brady-asystolic rhythms are far more common than “shockable rhythms.”33 VF and VT are not common pediatric arrest rhythms in the out-of-hospital setting, especially in children 7 years of age and younger. Shockable rhythms are more likely to be present with sudden, witnessed collapse, particularly among adolescents.33
Although the frequency of sudden cardiac arrest in athletes is not known, extrapolation from a statewide survey in Minnesota suggests the annual incidence is approximately 1 per 200,000 athletes, with more than half of deaths attributed to hypertrophic cardiomyopathy (the leading cause), commotio cordis, or coronary artery anomalies.24 More recently, channelopathies causing long-QT syndrome have been identified as causes of sudden cardiac arrest.20 Sudden cardiac arrest in athletes is likely associated with VF or VT, and many episodes are witnessed. Immediate bystander CPR and early defibrillation with an automated external defibrillator can improve the chance of survival. Infants with congenital heart disease often develop ventricular arrhythmias. More data are needed regarding any modifications in resuscitation approach that these children might require.29
For adults with a return of spontaneous circulation after cardiac arrest, therapeutic hypothermia and protocols for hemodynamic support and respiratory care improved outcomes of patients admitted after cardiopulmonary arrest and return of spontaneous circulation.35 Although similar studies have not been reported in children, it is likely that improving post-resuscitation care can increase the rates of survival following cardiopulmonary arrest.
Respiratory function
The five major components of the respiratory system and their functions are listed in Table 1-8. Every component of the respiratory system is immature in the child, and this immaturity may contribute to the development of respiratory failure when respiratory dysfunction is present.
| Component | Function |
| Central nervous system | Control ventilation |
| Airways | Conduct gas to and from respiratory surface |
| Chest wall | Enclose lungs |
| Respiratory muscles | Contribute to expansion of chest wall and lung, stabilize chest wall, and maintain airway patency |
| Lung tissue | Surface for gas diffusion |
Airways
At birth, the full “adult” complement of conducting airways is present and the airway branching pattern is complete. These airways grow in size and length during childhood. Alveoli and respiratory bronchioles multiply after birth. The number of alveoli increases by more than 10-fold by adulthood, and the alveolar surface area increases by a factor of 20.6,37
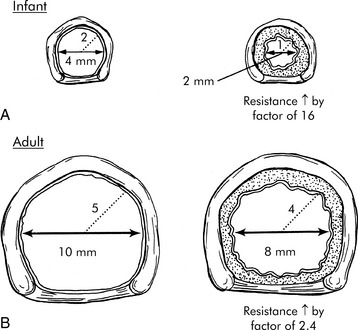
Small amounts of accumulated mucus, edema or airway constriction can have a minimal effect on the adult airway, but will often produce critical reduction in airway radius and critical increase in resistance to air flow and work of breathing in infants and young children (Fig. 1-2). Pediatric artificial airways are also small; they provide greater resistance to airflow than a normal natural airway and can quickly become obstructed by mucus.
The position and shape of the pediatric larynx is different from that of the adult. The pediatric larynx is more anterior and cephalad, and the articulation of the epiglottis with the larynx is more acute in children than in adults. These differences make the upper airway of infants more funnel-shaped than columnar. Pediatric intubation is often difficult for these reasons, and application of slight pressure on the cricoid cartilage may be necessary to displace the larynx posteriorly to facilitate intubation (although excessive pressure may make intubation more difficult).8a,20,30
Until a child is approximately 8 years old, the smallest diameter of the pediatric larynx is at the level of the cricoid cartilage, and maximum endotracheal tube size is limited by the size of this area. By comparison, the cricoid area of the adult larynx is relatively wide, so maximal adult endotracheal tube size usually is limited by the diameter of the adult larynx at the level of the vocal cords (Fig. 1-3). In the past, fear of tracheal injury prevented the use of cuffed endotracheal tubes in children. However, cuffed tubes are now used for children in the prehospital and hospital settings, and may be preferable to uncuffed tubes, particularly when there is a high risk of aspiration or when it is difficult to maintain sufficient airway pressures during assisted ventilation in a child with poor lung compliance or when a large glottic leak is present.20 The tube cuff pressure must be monitored and maintained per manufacturer’s recommendation (usually less than 20 to 25 cm H2O).30
There are several formulas to estimate pediatric endotracheal tube size, depth of insertion, and suction catheter size (Box 1-4). However, body length provides the most reliable parameter for selection of accurate endotracheal tube size.23
Box 1-4 Formulas for Estimating Endotracheal (ET) Tube Size, Depth of Insertion, and Suction Catheter Size8a,20,30
The pediatric trachea is much shorter than the adult trachea. The slightest downward or upward displacement of a pediatric endotracheal tube can move the tube into a mainstem bronchus or out of the trachea. Artificial airways must be securely taped in place, and nurses and therapists should monitor the tube insertion depth at the lip or nares, verifying position with auscultation and exhaled CO2 tension hourly and with any change in patient condition. Continuous monitoring of waveform capnography will enable immediate detection of inadvertent extubation (see Chapter 9) and may assist in evaluating the quality of resuscitation (see Chapter 6).
The tip of an orotracheal tube will move with changes in the child’s head position. Flexion of the neck will displace an orotracheal tube further into the trachea, and extension of the neck will move the tip of the orotracheal tube further out of the trachea (see Fig. 10-19).
Signs of Respiratory Distress
Signs of respiratory distress in children include: tachypnea, tachycardia, retractions, nasal flaring, grunting, mottled color, and change in responsiveness (Box 1-5). The infant may also demonstrate a weak cry. Hypercarbia, hypoxemia, or a fall in oxyhemoglobin saturation may also be documented. Apnea or gasping, decreased air movement, and alteration in perfusion can be late signs of respiratory distress, indicating impending arrest.
Neurologic function
Brain and Skull Growth
At birth, the brain is 25% of its mature adult weight. By 2½ years of age, the brain has achieved 75% of its mature adult weight.13 The growth in brain size is largely due to the development of fiber tracts and myelinization of neurons. This tremendous central nervous system growth during the first years of life adds uncertainty to the prediction of long-term consequences of early neurologic insults or injury. The child may recover with fewer sequelae than anticipated, because other areas of the brain begin to compensate for the injured areas; this is called plasticity. Subtle signs of unsuspected neurologic sequelae can manifest as learning disabilities when the child enters school.
Mortality is approximately the same following similar head injury in adults and children. However, children who survive head injury often demonstrate more complete recovery than do adult victims with similar injury. The Glasgow Coma Scale is less accurate in predicting the outcome of severe head injury in children than it is in adults, so modified pediatric coma scales have been published (see Chapter 11). A poor prognosis is indicated following head injury in children by the absence of spontaneous respiration, cardiovascular instability despite adequate volume resuscitation, flaccid paralysis, and fixed and dilated pupils. The presence of diabetes insipidus or disseminated intravascular coagulation can also indicate a poor prognosis.
Normal cerebral blood flow and cerebral perfusion pressure in the infant have not been definitively established. Adult cerebral blood flow averages approximately 50 mL/100 g brain tissue per minute, and the infant’s cerebral blood flow is thought to approximate 60% of that amount.19 The normal volume of cerebrospinal fluid production in children is unknown.
Criteria for brain death pronouncement in children require the use of accepted pediatric brain death criteria that are fundamentally the same as those used for adult. Clinical brain death criteria include absence of reversible cause and complete cessation of brain function (e.g., absence of cranial nerve function and absence of brainstem function, including absence of spontaneous respirations). For further information, see Chapter 11.
Neurologic Evaluation
Signs of increased intracranial pressure (Box 1-6) are the same in patients of all ages and include change in level of consciousness; decrease in spontaneous movement, movement in response to commands, and movement in response to painful stimulus; and pupil dilation with decreased constriction to light. In children, bradycardia, systolic hypertension, and altered breathing pattern are usually late signs of increased intracranial pressure and often indicate impending cerebral herniation.
Box 1-6 Signs of Increased Intracranial Pressure in Children
Decreased responsiveness (irritability, lethargy)
Decreased spontaneous movement and movement in response to commands
Decreased response to painful stimulus
Pupil dilation with decreased response to light
LATE: change in heart rate (tachycardia or bradycardia), hypertension, altered breathing pattern
Immune function and infection
Healthcare-Acquired (Nosocomial) Infections
Nosocomial infections can develop in as many as 12% of pediatric critical care patients.16 The sources of nosocomial infections in children differ from those reported in adults. Whereas the most common nosocomial infections observed in adult patients are urinary tract and wound infections, the most common nosocomial infections in pediatric patients are bloodstream infections (including catheter-related bloodstream infections [CRBSIs]) and ventilator-associated pneumonias (VAPs).8,31 The risk of pediatric nosocomial infection and sepsis increases with increased length of stay, each invasive device day, illness severity at admission, and depressed immune status.7
As in adults, nosocomial infections are reduced by strict hand washing before and after every patient contact, strict attention to aseptic technique, and specific bundled care to target common causes of infection such as CRBSIs and VAP (see section, Care of Vascular Monitoring Lines, and Chapters 16 and 22).8,15,28,31
Care of Vascular Monitoring Lines
CRBSIs are among the most common nosocomial infections in pediatric critical care. Risk factors include parenteral nutrition and antimicrobial therapy.34 Risk factors for patients in the pediatric cardiac critical care unit include unscheduled medical admission, noncardiac comorbidities, prolonged device use, and medical therapies such as extracorporeal membrane oxygenation.11
Although there is limited evidence to support specific strategies to prevent CRBSI in children, bundled therapies have been effective. Most multifaceted approaches are based on adult studies and include: (1) use of maximal sterile barrier precautions (e.g., cap, mask, sterile gown, sterile gloves, large sterile drape) during catheter placement; (2) use of 2% to 3% chlorhexidine gluconate/70% isopropyl alcohol or other appropriate antiseptic agents to prepare the skin before catheter placement and during routine care of the catheter insertion site; (3) prompt removal of catheters as soon as they are no longer required; and (4) strict adherence to appropriate hand hygiene practices, with annual handwashing campaigns.21,28,34 For additional information, see Chapters 16.
In studies performed in adult intensive care units, antiseptic impregnated catheters were associated with reduced rates of CRBSI (see Chapter 22). Studies performed in children have described delayed time to infection, but not reduced infection rate associated with the use of antibiotic-impregnated catheters.
Ventilator-Associated Pneumonia
Although the incidence of VAP is lower in children than adults, VAP remains the second most common cause of nosocomial infections children. The pathogenesis is poorly understood, but in children it is likely related to aspiration and immunodeficiency.15 VAP is a significant cause of increased critical care length of stay, increased mechanical ventilation days, and mortality in pediatric and adult critical care.
As with prevention of CRBSIs, the use of a multidisciplinary approach with bundled care protocols has been associated with a decreased prevalence of VAP. Strategies documented to reduce the risk of VAP in children include hand hygiene, elevation of the head of the bed, scheduled mouth care, and changing the ventilator circuit only when soiled.5 Use of heated ventilator circuits can reduce the pooling of water within the circuit.5 Additional factors associated with reduction of VAP in adults include: avoidance of nasotracheal intubation and the use of in-line suctioning to prevent the aspiration of pooled tracheal sections.10
1 Anderson G.D., Lynn A.M. Optimizing pediatric dosing: a developmental pharmacologic approach. Pharmacotherapy. 2009;29(6):680-690.
2 Armon K., et al. Hyponatremia and hypokalemia during intravenous fluid administration. Arch Dis Child. 2008;93(4):285-287. [Epub 2007, Jan 9]
3 Barakat A.Y., Ichikawa I. Laboratory data. In: Ichikawa I., editor. Pediatric textbook of fluids and electrolytes. Baltimore: Williams and Wilkins, 1990.
4 Beck C.E. Hypotonic versus isotonic maintenance intravenous fluid therapy in hospitalized children: a systematic review. Clin Pediatr. 2007;46(9):764-770.
5 Bigham M.T., et al. Ventilator-associated pneumonia in the pediatric intensive care unit: characterizing the problem and implementing a sustainable solution. J Pediatr. 2009;154(4):582-587.
6 Boyden E.A. Development and growth of the airways. In: Hodson W.A., editor. Development of the lung. New York: Marcel Dekker, 1977.
7 Carcillo J., et al. Rationale and design of the pediatric critical illness stress-induced immune suppression (CRISIS) Prevention Trial. J Parenter Enteral Nutr. 2009;33:368-374.
8 Centers for Disease Control and Prevention (CDC). 2003 National nosocomial infections surveillance (NNIS) system report: data summary from January 1992 through June 2003; Atlanta, Georgia: US Department of Health and Human Services, CDC: August
8a Chameides L.C., Samson R.A., Schexnayder S.M., Hazinski M.F., editors. Pediatric advanced life support provider manual. Dallas: American Heart Association, 2011.
9 Choong K., et al. Hypotonic versus isotonic saline in hospitalized children: a systematic review. Arch Dis Child. 2006;91:828-835.
10 Coffin S.E., et al. Strategies to Prevent Ventilator-Associated Pneumonia in Acute Care Hospitals. Infect Control Hosp Epidemiol. 2008;29:S31-S40.
11 Costello J.M., et al. Risk factors for central line-associated bloodstream infection in a pediatric cardiac intensive care unit. Pediatr Crit Care Med. 2009;10(4):453-459.
12 Custer J.W. Blood chemistries and body fluids. In Custer J.W., Rau R.E., editors: The Harriet Lane handbook, ed 18, Philadelphia: Mosby Elsevier, 2009.
13 Dobbing J., Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757.
14 Donoghue A., et al. Cardiopulmonary resuscitation for bradycardia with poor perfusion versus pulseless cardiac arrest. Pediatrics. 2009;124(6):1541-1548.
14a Felming S., et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377:1011-1018.
15 Foglia E., Meier M.D., Elward A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin Microbiol Rev. 2007;20(3):409-425.
16 Grohskopf L.A., et al. A national point-prevalence survey of pediatric intensive care unit-acquired infections in the United States. J Pediatr. 2002;140:432-438.
17 Haque I.U., Zaritsky A.L. Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr Crit Care. 2007;8:138-144.
17a Hazinski M.F. Nursing care of the critically ill child: the 7-point check. Pediatric Nursing.. 1985;11:453.
18 Huether S.E. The cellular environment; fluids and electrolytes, acids and bases. In: Mc Cance K.L., Huether S.E., editors. Pathophysiology: the biologic basis for disease in adults and children. Philadelphia: Elsevier, 2009.
19 Kirsch J.R., Traystman R.F., Rogers M.C. Cerebral blood flow measurement techniques in infants and children. Pediatrics. 1985;75:887.
20 Kleinman M.E., Chameides L., Schexnayder S.M., Samson R.A., et al. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S876-S908.
21 Kline A.M. Pediatric catheter-related bloodstream infections; latest strategies to decrease risk. AACN Clin Issues. 2005;16:185-198.
22 Lacroix J., et al. Transfusion strategies for patients in pediatric intensive care units. New Engl J Med. 2007;356:1609-1619.
23 Luten R., Zaritsky A. The sophistication of simplicity…optimizing emergency dosing. Acad Emerg Med. 2008;15(5):461-465.
24 Maron B.J. Sudden death in young athletes. N Engl J Med. 2003;349(11):1064-1075.
25 Meaney P.A., et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118(6):2424-2433.
26 Nadkarni V.M., et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50-57.
27 Notterman D.A. Pediatric pharmacotherapy. In Chernow B., editor: The Pharmacologic approach to the critically III patient, ed 3, Philadelphia: Williams and Wilkins, 1994.
28 O’Grady N.P., et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections. Clin Infect Dis. 2009;35:1281-1307.
29 Peddy S.B., et al. Cardiopulmonary resuscitation: special considerations for infants and children with cardiac disease. Cardiol Young. 2007;17(Suppl. 2):116-126.
30 Ralston M., et al. PALS provider manual. Dallas: American Heart Association; 2006.
31 Rowin M.E., Patel V.V., Christenson J.C. Pediatric intensive care unit nosocomial infections. Crit Care Clin. 2003;19:473-487.
32 Rudolph A.M. The changes in the circulation after birth. Circulation. 1970;41:343.
33 Smith B.T., Rea T.D., Eisenberg M.S. Ventricular fibrillation in pediatric cardiac arrest. Acad Emerg Med. 2006;13(5):525-529.
34 Smith M.J. Catheter-related bloodstream infections in children. Am J Infect Control. 2008;36(S173):e1-e3.
35 Sunde K., et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29-39.
36 Tan J.M. Nephrology. In Custer J.W., Rau R.E., editors: The Johns Hopkins Hospital. The Harriet Lane Handbook, ed 18, Philadelphia: Mosby Elsevier, 2009.
37 Tooley W.H. Lung growth in infancy and childhood. In Rudolph A.M., editor: Pediatrics, ed 18, Norwalk: Appleton-Century-Crofts, 1987.
38 Van den Berghe G., et al. Outcome benefit of intensive insulin therapy in the critically ill; insulin dose versus glycemic control. Crit Care Med. 2003;31:359-366.
39 Vlasselaers D., et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547-556.
40 Winters R.W. Maintenance fluid therapy. In: Winters R.W., editor. The body fluids in pediatrics. Boston: Little, Brown, & Co, 1973.
41 Zaritsky A., Nadkarni V., Getson P., Kuehl K. CPR in children. Ann Emerg Med. 1987;10(16):1107-1111.

 Be sure to check out the supplementary content available at
Be sure to check out the supplementary content available at