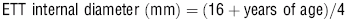Chapter 1 Childhood Resuscitation
3 What are the common causes of cardiopulmonary arrest in children?
Common causes of cardiopulmonary arrest in children are numerous, but most fit into the classifications of respiratory, infectious, cardiovascular, traumatic, or central nervous system (CNS) diseases (Table 1-1). Respiratory diseases and SIDS together consistently account for one-third to two-thirds of all pediatric cardiopulmonary arrests in published series.
Table 1-1 Common Causes of Cardiopulmonary Arrest in Children
| Respiratory | Central Nervous System |
| Pneumonia | Seizures, or complications thereof |
| Near drowning | Hydrocephalus, or shunt malfunction |
| Smoke inhalation | Tumor |
| Aspiration and obstruction | Meningitis |
| Apnea | Hemorrhage |
| Suffocation | Other |
| Bronchiolitis | Trauma |
| Cardiovascular | Sudden infant death syndrome |
| Congenital heart disease | Anaphylaxis |
| Congestive heart failure | Gastrointestinal hemorrhage |
| Pericarditis | Poisoning |
| Myocarditis | |
| Arrhythmia | |
| Septic shock |
8 After establishing a clear chain of command and assigning specific duties to all members of the resuscitation team, what should the order of priorities be?
9 What is the recommended way to establish a patent airway?
 The first attempt to establish airway patency should be through proper airway positioning. Often, this alone will be effective. Since most airway obstruction is due to the effect of gravity on the mandibular block of soft tissues, it can be relieved by either a head tilt–chin lift or jaw-thrust maneuver.
The first attempt to establish airway patency should be through proper airway positioning. Often, this alone will be effective. Since most airway obstruction is due to the effect of gravity on the mandibular block of soft tissues, it can be relieved by either a head tilt–chin lift or jaw-thrust maneuver.
 Vomitus or other foreign material can also obstruct airways. Inspect the airway for these materials, and suction early and frequently.
Vomitus or other foreign material can also obstruct airways. Inspect the airway for these materials, and suction early and frequently.
 In selected patients with altered levels of consciousness, nasopharyngeal or oropharyngeal airway stents are useful. Semiconscious children generally tolerate the softer nasopharyngeal airways better than the harder, less comfortable oropharyngeal airways. Children, such as those in postictal states, who have sustained spontaneous respiratory effort but have upper airway obstruction due to poor muscle tone often benefit from the use of these devices.
In selected patients with altered levels of consciousness, nasopharyngeal or oropharyngeal airway stents are useful. Semiconscious children generally tolerate the softer nasopharyngeal airways better than the harder, less comfortable oropharyngeal airways. Children, such as those in postictal states, who have sustained spontaneous respiratory effort but have upper airway obstruction due to poor muscle tone often benefit from the use of these devices.
 The laryngeal mask airway is a relatively new supraglottic advanced airway device that may be a very useful tool to the experienced user in certain situations. However, at this time, the American Heart Association states that there is insufficient evidence to recommend for or against the routine use of this device during arrests.
The laryngeal mask airway is a relatively new supraglottic advanced airway device that may be a very useful tool to the experienced user in certain situations. However, at this time, the American Heart Association states that there is insufficient evidence to recommend for or against the routine use of this device during arrests.
10 What is the recommended way to deliver supplemental oxygen to a child?
Supplemental oxygen can be delivered to a child by a variety of different means. For the sickest patient, oxygen should be delivered in the highest concentration and by the most direct method possible. Children who demonstrate spontaneous breathing might require less invasive means of administration of supplemental oxygen. Table 1-2 lists some different methods of oxygen delivery with their associated delivery capabilities.
Table 1-2 Methods of Oxygen Delivery and Their Delivery Capabilities
| Nasal cannula: 30–40% oxygen |
| Simple masks: 30–60% oxygen |
| Partial rebreather masks: 50–60% oxygen |
| Oxygen tents: 30–50% oxygen |
| Oxygen hoods: 80–90% oxygen |
| Nonrebreather masks: ~100% oxygen |
11 Which children require intubation?
 Inadequate central nervous system control of ventilation
Inadequate central nervous system control of ventilation
 Functional or anatomic airway obstruction
Functional or anatomic airway obstruction
 Strong potential for developing airway obstruction (e.g., inhalation airway burns, expanding airway hematoma)
Strong potential for developing airway obstruction (e.g., inhalation airway burns, expanding airway hematoma)
 Loss of protective airway reflexes
Loss of protective airway reflexes
 Excessive work of breathing, which might lead to fatigue and respiratory insufficiency
Excessive work of breathing, which might lead to fatigue and respiratory insufficiency
 Need for high airway pressures to maintain effective alveolar gas exchange
Need for high airway pressures to maintain effective alveolar gas exchange
 Need for mechanical ventilatory support
Need for mechanical ventilatory support
 Potential occurrence of any of the above during patient transport
Potential occurrence of any of the above during patient transport
12 When selecting an endotracheal tube, what sizing guidelines are suggested?
KEY POINTS: HOW TO DETERMINE THE PROPER PLACEMENT OF THE ETT
1 Check to see that the tube is inserted at a depth that is three times the internal diameter of the ETT (from the point of the patient’s central incisors).
2 Observe for symmetric chest expansion.
3 Auscultate for symmetric breath sounds.
4 Look for distention of the abdomen, indicating misplacement of the tube.
5 Measure end-tidal carbon dioxide using a colorimetric detector. In infants and children with a perfusing rhythm, a purple color on the device indicates a problem, whereas a yellow color implies that the tube is in the trachea.
13 How can I determine if ETT placement is appropriate?
Proper depth for ETT insertion from the point of the patient’s central incisors can be estimated to be three times the internal diameter of the ETT. Measurement of end-tidal carbon dioxide using a colorimetric detector, observation for symmetric chest expansion, and auscultation for symmetric breath sounds can help to ensure proper placement. Confirmation of placement is probably best determined with a chest radiograph. Prior to an x-ray, the colorimetric detector offers a rapid bedside determination to detect CO2 to confirm endotracheal tube placement (Fig. 1-1).
14 What are the best methods to assess a child’s circulatory status?
Assessment of a child’s circulatory status should always include appraisal of:
16 What are the golden rules of vascular access?
20 What role does drug therapy play in pediatric resuscitation?
 Epinephrine (to increase heart rate, myocardial contractility, and systemic vascular resistance)
Epinephrine (to increase heart rate, myocardial contractility, and systemic vascular resistance)
 Atropine (to increase heart rate in nonneonates)
Atropine (to increase heart rate in nonneonates)
 Dextrose (to increase glucose)
Dextrose (to increase glucose)
 Sodium bicarbonate (to increase pH)
Sodium bicarbonate (to increase pH)
 Amiodarone or procainamide (to reverse ventricular arrhythmias)
Amiodarone or procainamide (to reverse ventricular arrhythmias)
 Naloxone (to reverse the effects of narcotics)
Naloxone (to reverse the effects of narcotics)
 Adenosine (to reverse supraventricular tachycardia)
Adenosine (to reverse supraventricular tachycardia)
 Dopamine (to increase vasoconstriction and blood pressure)
Dopamine (to increase vasoconstriction and blood pressure)
21 What are the new recommendations regarding epinephrine administration during pediatric resuscitation?
PALS recommendations for pulseless arrest (PES, asystole)
 If the first asystole-countering dose is intravascularly (IV or IO route) administered: give as a standard dose (0.01 mg/kg). This can be delivered as 0.1 mL/kg of a 1:10,000 solution of epinephrine. Vasopressin is used in place of epinephrine for the first or second dose in adult resuscitations but is considered Class Indeterminate (not enough evidence to recommend for or against) in pediatric arrests.
If the first asystole-countering dose is intravascularly (IV or IO route) administered: give as a standard dose (0.01 mg/kg). This can be delivered as 0.1 mL/kg of a 1:10,000 solution of epinephrine. Vasopressin is used in place of epinephrine for the first or second dose in adult resuscitations but is considered Class Indeterminate (not enough evidence to recommend for or against) in pediatric arrests.
 If the first asystole-countering dose is endotracheally administered: give as a higher dose (0.1 mg/kg). This can be delivered as 0.1 mL/kg of a 1:1000 solution of epinephrine. An IV or IO route of administration is preferred, however, if at all possible.
If the first asystole-countering dose is endotracheally administered: give as a higher dose (0.1 mg/kg). This can be delivered as 0.1 mL/kg of a 1:1000 solution of epinephrine. An IV or IO route of administration is preferred, however, if at all possible.
 Higher-dose epinephrine (0.1 mg/kg; 0.1 mL/kg of a 1:1000 solution) is no longer routinely recommended for subsequent doses of epinephrine given through an IV or IO route.
Higher-dose epinephrine (0.1 mg/kg; 0.1 mL/kg of a 1:1000 solution) is no longer routinely recommended for subsequent doses of epinephrine given through an IV or IO route.
PALS recommendations for bradycardia
 Any intravascularly (IV or IO route) administered doses should be given as standard doses (0.01 mg/kg). This is generally delivered as 0.1 mL/kg of a 1:10,000 solution of epinephrine.
Any intravascularly (IV or IO route) administered doses should be given as standard doses (0.01 mg/kg). This is generally delivered as 0.1 mL/kg of a 1:10,000 solution of epinephrine.
 Any endotracheally administered doses should be given as higher doses (0.1 mg/kg). This can be delivered as 0.1 mL/kg of a 1:1000 solution of epinephrine.
Any endotracheally administered doses should be given as higher doses (0.1 mg/kg). This can be delivered as 0.1 mL/kg of a 1:1000 solution of epinephrine.
22 Which resuscitation drugs are effective when given via an endotracheal tube?
KEY POINTS: DRUGS THAT CAN BE GIVEN VIA THE ENDOTRACHEAL ROUTE
23 Are there minimum dosing requirements for any resuscitation drugs?
 Atropine (usual dose, 0.02 mg/kg) has a minimum dosing requirement for effective reversal of bradycardia. It appears that at doses lower than 0.1 mg, atropine exerts an effect that might actually worsen bradycardia. Thus, if its use is considered for reversal of bradycardia in a child who weighs less than 5 kg, a minimum of 0.1 mg should be administered.
Atropine (usual dose, 0.02 mg/kg) has a minimum dosing requirement for effective reversal of bradycardia. It appears that at doses lower than 0.1 mg, atropine exerts an effect that might actually worsen bradycardia. Thus, if its use is considered for reversal of bradycardia in a child who weighs less than 5 kg, a minimum of 0.1 mg should be administered.
 Dopamine also has different effects when administered at different doses. At lower doses (1–5 μg/kg/min), dopaminergic effects are seen. When administered at these lower doses, dopamine tends to augment renal blood flow and enhance urinary output. During resuscitation, dopamine typically is used to bolster blood pressure through increased vasoconstriction. For that α-adrenergic effect, higher doses (10–20 μg/kg/min) are required.
Dopamine also has different effects when administered at different doses. At lower doses (1–5 μg/kg/min), dopaminergic effects are seen. When administered at these lower doses, dopamine tends to augment renal blood flow and enhance urinary output. During resuscitation, dopamine typically is used to bolster blood pressure through increased vasoconstriction. For that α-adrenergic effect, higher doses (10–20 μg/kg/min) are required.
27 Is there an easy method to calculate mixtures of constant infusions of drugs?
Several methods are used. Here is one easy method:
 For constant infusion of drugs (epinephrine, isoproterenol) beginning at 0.1 μg/kg/min: 0.6 times the weight in kg equals the number of milligrams of drug to add to enough water to make a total of 100 mL of solution. The resultant solution is then infused at a rate of 1 mL per hour, delivering 0.1 μg/kg/min.
For constant infusion of drugs (epinephrine, isoproterenol) beginning at 0.1 μg/kg/min: 0.6 times the weight in kg equals the number of milligrams of drug to add to enough water to make a total of 100 mL of solution. The resultant solution is then infused at a rate of 1 mL per hour, delivering 0.1 μg/kg/min.
 For constant infusion of drugs (dopamine, dobutamine) beginning at 1 μg/kg/min: 6 times the weight in kg equals the number of milligrams of drug to add to enough water to make a total of 100 mL of solution. The resultant solution is then infused at a rate of 1 mL per hour, delivering 1 μg/kg/min.
For constant infusion of drugs (dopamine, dobutamine) beginning at 1 μg/kg/min: 6 times the weight in kg equals the number of milligrams of drug to add to enough water to make a total of 100 mL of solution. The resultant solution is then infused at a rate of 1 mL per hour, delivering 1 μg/kg/min.