27 Chest Pain
 Initial Approach
Initial Approach
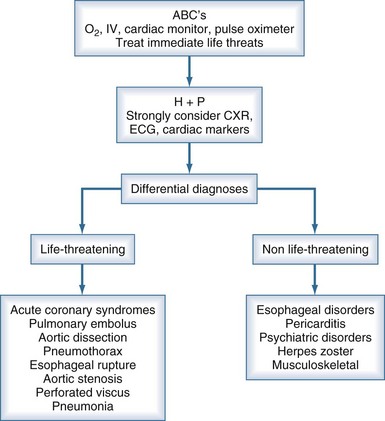
Several life-threatening conditions can cause chest pain in the critically ill, and the initial approach should focus on prompt evaluation and resuscitation of the airway, breathing, and circulation. Assess the patient’s level of consciousness, palpate the pulse, and listen to the breath sounds and heart. Obtain vital signs, including oxygen saturation by pulse oximetry, and ensure that the patient is on a cardiac monitor and has adequate intravenous (IV) access. Adhering to this algorithmic approach (Figure 27-1) in patients with chest pain will ensure that critical conditions such as hypoxemia, hypotension, tension pneumothorax, and unstable ventricular arrhythmias are quickly identified and treated. These conditions, as well as the life-threatening causes of chest pain discussed below, are covered in greater detail in other chapters in this textbook.
 History
History
After the initial evaluation and stabilization, obtain a more detailed history. If the patient can communicate, start with an open-ended question like “What’s going on, Mr. Jones?” Physicians typically interrupt patients within 23 seconds, but one should resist this temptation and allow the patient to describe their symptoms.1 Physicians often neglect to ask even basic questions about the quality of chest pain in patients with aortic dissection, and this omission is associated with a delay in diagnosis.2 The mnemonic OLDCAAR can help avoid this mistake (Table 27-1). Ask the bedside nurse about recent changes in the patient’s condition (e.g., changes in mental status, respiratory pattern, or recent medications). Lastly, a quick “chart dissection” should be performed, focusing on the findings noted on initial presentation, reason for ICU admission, past history, and recent progress notes.
| Domain | Suggested Questions |
|---|---|
| Onset | Sudden or gradual? Maximal pain at onset? |
| Location | Generalized or localized? Can you point with one finger to where it hurts? |
| Duration | When did it start? Just now, or did the pain occur earlier, but you didn’t want to bother anyone? Is it constant or intermittent? If intermittent, is there a trigger, or is it random? |
| Character | Sharp? Dull? Ache? Indigestion? Pressure? Tearing? Ripping? |
| Associated symptoms | “Dizzy”—vertiginous or presyncopal? Diaphoresis? Palpitations? Dyspnea? Nausea or vomiting? |
| Alleviating/aggravating | Position? Belching? Exertion? Deep breathing? Coughing? |
| Radiation | To the back? Jaw? Throat? Arm? Neck? Abdomen? |
 Differential Diagnoses
Differential Diagnoses
Potentially Life-Threatening Causes of Chest Pain
Acute Coronary Syndromes
ACS include unstable angina and ST-segment and non–ST-segment elevation MI. The “classic” symptoms of ACS include chest pressure radiating to the left arm, nausea, and diaphoresis, but the history has several limitations with regard to the diagnosis of ACS. Although certain features (pain radiating down the right arm or both arms) are associated with a higher likelihood of ACS, and other characteristics (pleuritic, positional, or sharp pain) with a lesser likelihood, none of these can reliably confirm or exclude the diagnosis.6,7 Further complicating matters, diabetes, smoking, dyslipidemia, hypertension, and a family history predict the development of heart disease over years in asymptomatic patients but may be less useful in predicting ACS in patients with acute chest pain.8 Reduction in pain after the administration of nitroglycerin is also not a reliable indicator of cardiac chest pain.9 Thus, ACS should almost never be excluded as a cause of chest pain based on the history alone.
Pulmonary Embolism
Approximately 1% to 2% of ICU patients develop deep vein thrombosis (DVT) or PE, but the true incidence is probably higher.10 Unrecognized PE carries a high mortality, but survival improves dramatically with prompt diagnosis and treatment. Chest pain due to PE is usually pleuritic and often associated with dyspnea, hemoptysis, cough, or syncope.11 ICU patients usually have risk factors for PE including immobility, advanced age, recent surgery or trauma, malignancy, and central venous catheterization. Do not be deterred from working up PE in patients receiving subcutaneous heparin, as two-thirds of those with DVT and PE are receiving prophylaxis at the time of diagnosis.10
An elevated arterial-alveolar gradient may be noted on blood gas analysis, but this is a nonspecific finding in the critically ill. The ECG is often normal, but it may show sinus tachycardia, nonspecific ST-segment and T-wave changes, or a right bundle branch block.12 The CXR can be normal but more commonly reveals nonspecific findings such as pleural effusion, infiltrates, or atelectasis.13 Although D-dimer testing has been used to rule out venothromboembolic disease in outpatients with a low likelihood of this diagnosis, the D-dimer assay does not appear to be a particularly useful diagnostic tool in the ICU setting.14 The sensitivity of transthoracic echocardiography (TTE) for PE varies considerably, but the test can be useful in patients who have larger clots that are of hemodynamic significance.15 In such cases, TTE can be performed rapidly at the bedside when patients are unsafe for transport out of the ICU. TTE has the added benefit of assessing the response to thrombolytics by evaluating right heart function and changes in pulmonary artery pressure.15 A ventilation/perfusion scan can be time consuming and difficult to perform in mechanically ventilated patients; its interpretation may be obscured by other lung pathology.16 An IV contrast-enhanced CT of the chest can be performed rapidly, and newer scanners have high sensitivity and specificity, making this the diagnostic study of choice in most ICU patients.
Initial treatment of patients with confirmed PE involves anticoagulation with subcutaneous low-molecular-weight heparin or IV unfractionated heparin. Patients with hemodynamic instability due to PE may require thrombolysis or surgical embolectomy.17
Thoracic Aortic Dissection
Aortic dissection results from a tear in the aortic intima, allowing blood to dissect between the intima and adventitia. The Stanford system classifies dissections as type A (involving the ascending aorta) or type B (not involving the ascending aorta). Risk factors include hypertension, advanced age, atherosclerosis, cocaine use, intraaortic catheterization, Ehlers-Danlos syndrome, Turner syndrome, and giant cell arteritis.18 Patients younger than 40 years are more likely to have Marfan syndrome, bicuspid aortic valve, prior aortic surgery, or aortic aneurysm.19 The mortality rate is as high as 1% to 2% per hour from symptom onset, and the history remains critical to early diagnosis.19 Clinicians correctly suspect aortic dissection in more than 90% of cases when questions about quality, radiation, and intensity of the pain are asked. If one or more of these questions is omitted, the correct diagnosis is missed in over half of cases.2 Many patients complain of sudden onset of chest pain that radiates to the back or abdomen. Contrary to popular belief, patients more commonly describe their pain as sharp, rather than “tearing.”19 Dissection can extend into any of the major aortic branches, causing a multitude of clinical presentations owing to ischemia of the brain, heart, kidney, spinal cord, or gut.
Certain physical examination findings should raise the suspicion of aortic dissection. About one third of patients have pulse deficits in the carotid, radial, or femoral arteries, and some have focal neurologic deficits related to cerebral or spinal cord ischemia.18 Hypotension often occurs with type A dissection, whereas hypertension is more commonly seen in type B dissection.20 A significant difference in systolic blood pressure (>20 mm Hg) between the upper extremities may be seen with dissection, but this is not a pathognomonic finding. A diastolic murmur of aortic insufficiency can result from retrograde dissection into the aortic valve.
The ECG may be normal or show nonspecific ST-segment or T-wave changes or left ventricular hypertrophy (LVH) from hypertension. Rarely, the ECG reveals evidence of an MI from dissection into a coronary artery. Over 90% of patients will have some abnormality on CXR, such as widening of the mediastinum, an abnormal aortic contour, pleural effusion, or displacement of intimal aortic calcification from the outer border of the aortic knob.21 Therefore, it behooves the clinician to scour the CXR for these findings when considering aortic dissection as a cause of chest pain. The diagnosis can be confirmed with CT, magnetic resonance imaging (MRI), or transesophageal echocardiography, all of which have high sensitivity and specificity. The choice of diagnostic study will depend on physician preference and the risks involved. Initial management should focus on blood pressure control, usually with beta-blockers and a potent vasodilator such as nitroprusside.20
Pneumothorax
Pneumothorax is caused by air entry from the alveolar space or the atmosphere into the potential space between the parietal and visceral pleura. Pneumothorax in the ICU is often iatrogenic and results from mechanical ventilation (particularly with acute respiratory distress syndrome), attempts at central venous catheterization, thoracentesis, tracheostomy, or bronchoscopy.22 Virtually any lung pathology can contribute to a pneumothorax, but a ruptured bleb from chronic obstructive pulmonary disease is the most common culprit. Patients with pneumothorax typically complain of sudden onset of ipsilateral pleuritic chest pain with associated dyspnea.
CXRs are often performed in the semiupright or supine position in the ICU, and the classic finding of a visceral pleural line is often seen only on upright CXR. In supine patients, a deep sulcus sign may be seen where the costophrenic angle extends more inferiorly than normal as air collects in this space. Alternatively, a sharp delineation of the cardiac silhouette from the lucency of an anteromedial pneumothorax may be seen. In an experienced operator’s hands, ultrasound can effectively rule out a pneumothorax in seconds.23
Esophageal Rupture
A full-thickness tear of the esophagus carries high mortality, owing to the intense inflammatory response to gastric contents in the mediastinum, secondary bacterial infection, and subsequent sepsis and multisystem organ failure. Most cases of esophageal perforation are caused by upper gastrointestinal tract endoscopy.24 The risk of esophageal injury from a diagnostic endoscopy is low but increases dramatically when interventions such as dilation or stent placement are performed. Esophageal rupture may be caused by other procedures commonly performed in the ICU, including nasogastric or tracheal intubation. Spontaneous rupture of the esophagus (Boerhaave syndrome) occurs from a sudden increase in intraluminal pressure, usually from vomiting or retching. Patients with esophageal disease such as cancer, Barrett’s esophagus, strictures, prior radiation, and varices are particularly vulnerable to rupture. With thoracic perforations, the pain localizes to the substernal or epigastric area, but it may occur in the neck with cervical perforations. Other associated symptoms include dysphagia, odynophagia, and dyspnea.
The patient is often febrile. Crepitus can be felt in the neck with perforation of the cervical esophagus. Mediastinal emphysema can sometimes be detected by a crunching sound on cardiac auscultation, termed Hamman’s sign. A CXR often reveals subcutaneous emphysema, pneumomediastinum, pneumothorax, or pleural effusion. The CXR is abnormal in almost 90% of cases but may be normal early after the perforation occurs.24 A water-soluble contrast study of the esophagus or a CT scan of the chest can be performed in cases where there is a high clinical suspicion and the CXR is nondiagnostic.
Non–Life Threatening Causes of Chest Pain
Esophageal Disorders
In patients with noncardiac chest pain, gastroesophageal reflux disorder and esophageal motility disorders (e.g., esophageal spasm) are common. Esophageal disease is associated with pain precipitated by lying flat, postprandial pain, heartburn, or dysphagia. Owing to the shared innervation of the heart and esophagus, visceral pain originating from these two organs can be similar in character. Relief of symptoms after a “GI cocktail” cannot be relied upon to identify chest pain as noncardiac in origin.25 Confirmatory tests including esophageal manometry and esophageal pH monitoring can be performed, but a trial of a proton pump inhibitor may be a more practical diagnostic approach.26 Lastly, a nasogastric tube with the distal tip in the esophagus can produce chest pain; this is easily remedied by advancing the tube distally into the stomach.
Musculoskeletal Disorders
The chest wall is a common source of pain in patients without a cardiorespiratory etiology of their symptoms. Pain from costochondritis is often reproduced with palpation or with arm movement. Up to 15% of patients with MI also have chest wall tenderness, so this finding does not exclude ACS.27 Most cases of costochondritis are self-limiting and treated with nonsteroidal antiinflammatory drugs (NSAIDs). ICU patients may have other causes of chest wall pain, including rib fractures, chest tubes, postoperative pain after cardiothoracic surgery, or an intercostal muscle strain from coughing.
Pericarditis
Pericarditis is a relatively rare cause of chest pain in the inpatient setting.28 The condition most commonly results from viral or idiopathic causes, but other etiologies include bacterial infections, malignancy, tuberculosis, uremia, autoimmune diseases, transmural MI, and cardiac surgery. Chest pain from pericarditis is typically pleuritic, sharp, retrosternal, and radiates to the back, neck, or arms. The pain is often relieved by sitting forward and exacerbated by lying flat. Although uncomplicated pericarditis is not generally life threatening, pericardial inflammation can lead to pericardial effusion and cardiac tamponade if the effusion is large or acute.
ECG findings can clinch the diagnosis of pericarditis. Both MI and pericarditis may result in ST-segment elevation, but with pericarditis, ST-segment depression is typically absent in the reciprocal leads. Absence of Q waves, concave ST-segment elevation, and PR depression strongly favor pericarditis.28 Careful ECG review, auscultation, and history are key to distinguishing ACS from pericarditis and avoiding the potentially fatal complication of administering thrombolytics to a patient with pericarditis and precipitating hemotamponade. Electrical alternans and low voltage on the ECG, coupled with cardiomegaly on CXR, strongly favor pericardial effusion. Although the ECG and CXR findings of pericardial effusion can be useful, echocardiography should be performed to confirm the diagnosis.
Psychiatric Disorders
A significant number of patients with noncardiac chest pain suffer from panic disorder.29 In addition to chest pain, panic attacks can cause other symptoms that mimic MI, including diaphoresis, dyspnea, palpitations, and a sense of impending doom. A self-report of anxiety helps clue into the diagnosis of underlying panic disorder. Severe illness and its treatment with invasive procedures in the ICU can provoke profound psychological distress. The development of posttraumatic stress disorder is well described in ICU survivors, particularly in patients who experience episodes of extreme fear.30 Thus, the diagnosis of chest pain due to panic attack may not be acutely life threatening, but this condition should not be considered benign and must be treated. Benzodiazepines are helpful in this regard. Psychiatric patients with cardiac or pulmonary disease can be especially challenging to diagnose, and a thorough, empathetic history is essential.
Gajic O, Urrutia LE, Sewani H, et al. Acute abdomen in the medical intensive care unit. Crit Care Med. 2002;30(6):1187-1190.
Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499.
Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAAD): new insights into an old disease. JAMA. 2000;283(7):897-903.
Han JH, Lindsell CJ, Storrow AB, et al. The role of cardiac risk factor burden in diagnosing acute coronary syndromes in the emergency department setting. Ann Emerg Med. 2007;49(2):145-152. 52 e1
Marvel MK, Epstein RM, Flowers K, Beckman HB. Soliciting the patient’s agenda: have we improved? JAMA. 1999;281(3):283-287.
1 Marvel MK, Epstein RM, Flowers K, Beckman HB. Soliciting the patient’s agenda: have we improved? JAMA. 1999;281(3):283-287.
2 Rosman HS, Patel S, Borzak S, Paone G, Retter K. Quality of history taking in patients with aortic dissection. Chest. 1998;114(3):793-795.
3 Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499.
4 Rapezzi C, Longhi S, Graziosi M, et al. Risk factors for diagnostic delay in acute aortic dissection. Am J Cardiol. 2008;102(10):1399-1406.
5 Gajic O, Urrutia LE, Sewani H, Schroeder DR, Cullinane DC, Peters SG. Acute abdomen in the medical intensive care unit. Crit Care Med. 2002;30(6):1187-1190.
6 Goodacre S, Pett P, Arnold J, et al. Clinical diagnosis of acute coronary syndrome in patients with chest pain and a normal or non-diagnostic electrocardiogram. Emerg Med J. 2009;26(12):866-870.
7 Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294(20):2623-2629.
8 Han JH, Lindsell CJ, Storrow AB, et al. The role of cardiac risk factor burden in diagnosing acute coronary syndromes in the emergency department setting. Ann Emerg Med. 2007;49(2):145-152. 52 e1
9 Diercks DB, Boghos E, Guzman H, Amsterdam EA, Kirk JD. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005;45(6):581-585.
10 Patel R, Cook DJ, Meade MO, et al. Burden of illness in venous thromboembolism in critical care: a multicenter observational study. J Crit Care. 2005;20(4):341-347.
11 Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879. PMCID: 2071924
12 Brown G, Hogg K. Best evidence topic report. Diagnostic utility of electrocardiogram for diagnosing pulmonary embolism. Emerg Med J. 2005;22(10):729-730. PMCID: 1726554
13 Elliott CG, Goldhaber SZ, Visani L, DeRosa M. Chest radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry. Chest. 2000;118(1):33-38.
14 Crowther MA, Cook DJ, Griffith LE, Meade M, Hanna S, Rabbat C, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med. 2005;31(1):48-55.
15 Stawicki SP, Seamon MJ, Kim PK, et al. Transthoracic echocardiography for pulmonary embolism in the ICU: finding the “right” findings. J Am Coll Surg. 2008;206(1):42-47.
16 Cook D, Douketis J, Crowther MA, Anderson DR. The diagnosis of deep venous thrombosis and pulmonary embolism in medical-surgical intensive care unit patients. J Crit Care. 2005;20(4):314-319.
17 Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
18 Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg. 2009;37(2):149-159.
19 Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, et al. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol. 2004;43(4):665-669.
20 Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372(9632):55-66.
21 Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283(7):897-903.
22 Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290.
23 Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007;35(5 Suppl):S250-S261.
24 Wu JT, Mattox KL, Wall MJJr. Esophageal perforations: new perspectives and treatment paradigms. J Trauma. 2007;63(5):1173-1184.
25 Wrenn K, Slovis CM, Gongaware J. Using the “GI cocktail”: a descriptive study. Ann Emerg Med. 1995;26(6):687-690.
26 Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain?: a meta-analysis. Arch Intern Med. 2005;165(11):1222-1228.
27 Lee TH, Cook EF, Weisberg M, Sargent RK, Wilson C, Goldman L. Acute chest pain in the emergency room. Identification and examination of low-risk patients. Arch Intern Med. 1985;145(1):65-69.
28 Ariyarajah V, Spodick DH. Acute pericarditis: diagnostic cues and common electrocardiographic manifestations. Cardiol Rev. 2007;15(1):24-30.
29 Katerndahl DA. Chest pain and its importance in patients with panic disorder: an updated literature review. Prim Care Companion J Clin Psychiatry. 2008;10(5):376-383. PMCID: 2629063
30 Samuelson KA, Lundberg D, Fridlund B. Stressful memories and psychological distress in adult mechanically ventilated intensive care patients—a 2-month follow-up study. Acta Anaesthesiol Scand. 2007;51(6):671-678.