Chapter 41 Chemical Treatment of the Labyrinth
Despite some successes, the medical treatment of inner ear conditions, such as Meniere’s disease and sudden sensorineural hearing loss, is often frustrating to patients and physicians. Molecular studies of inner ear function have revealed some promising approaches to therapy. Chemoprotection strategies for exposure to noise,1 cisplatin,2 and aminoglycosides3 have generated considerable interest. Other than aminoglycoside treatment for vertigo in Meniere’s disease, no intratympanic treatment strategy has gained widespread acceptance as standard therapy. We review the status of this treatment and of intratympanic treatment with corticosteroids.
Most patients with Meniere’s disease can be managed satisfactorily with dietary salt restriction, diuretics, and vestibular suppressants. Only about 10% of patients become disabled in regard to work, domestic activities, travel, and the general enjoyment of life. The goals of treatment of Meniere’s disease are to control the definitive spells of vertigo and to preserve hearing.4
In 1944, streptomycin was isolated from cultures of a soil organism, Streptomyces griseolus.5 This drug displayed broad-spectrum antibacterial activity and was the first found to be effective against tuberculosis. Because effective treatment of tuberculosis with streptomycin required prolonged therapy, ototoxicity became evident soon after introduction of the drug. In 1948, streptomycin was used to treat patients with unilateral Meniere’s disease specifically on the basis of its vestibulotoxic effects.6
Mechanisms of aminoglycoside ototoxicity have been reviewed more recently.7 Aminoglycosides exert toxic effects on the hair cells of the inner ear by several mechanisms. First, aminoglycosides bind to the plasma membrane and displace calcium and magnesium. This event results in acute but reversible interference with calcium-dependent mechanicoelectric transduction channels.8 Second, dihydrostreptomycin enters the mouse outer hair cell through the mechanoelectric transduction channel.9,10 This channel can act as a one-way valve for aminoglycoside entry, promoting the accumulation of aminoglycoside within the cell. There seems to be a competition between the aminoglycoside and calcium for entry into the outer hair cell. The normal low endolymph concentration of calcium promotes intracellular accumulation of aminoglycosides.10 Third, aminoglycosides are transported into the cell by an energy-dependent process. Within the cell, the drug binds to phosphatidylinositol. This event is associated with progressive disruption of the plasma membrane and inhibition of the second messenger inositol triphosphate. With progressive disruption of the second messenger system and the plasma membrane, cell death occurs.11–13
The disruption of cell membranes and other intracellular components may be mediated by free radicals. More recent studies have shown that aminoglycosides form a complex with iron, and that this complex catalyzes the production of free radicals. Reactive oxygen species formation in vitro requires iron and polyunsaturated lipids, such as those found in cell membranes, as electron donors. Gentamicin and iron form ternary complexes with phospholipids. Oxidative damage to phospholipids can cause the release of arachidonic acid, which can also form complexes with gentamicin and iron. These complexes can lead to further lipid peroxidation, damaging membranes, proteins, and DNA to disrupt the function of the outer hair cell, leading to programmed cell death (apoptosis).14 Reactive oxygen species may promote the opening of the mitochondrial permeability pore.15 The Jun N-terminal kinase (JNK) pathway seems to play a role in the death from gentamicin of auditory and vestibular hair cells.16
The combination of iron chelators and free radical scavengers in animal experiments provides complete protection from gentamicin ototoxicity.17 Pretreatment with aspirin protected against amikacin ototoxicity in animals.18 More recent clinical trials in China showed protection against gentamicin-induced hearing loss in patients pretreated with salicylates.19
Aminoglycosides do not become concentrated in cochlear fluids, although the elimination half-life increases with long-term administration. These observations support the notion that intracellular sequestration of the drug occurs.10,20 Aran and associates21 confirmed that aminoglycosides undergo rapid uptake by cochlear and vestibular hair cells, and slow clearance from these cells.
Amikacin, dihydrostreptomycin, and kanamycin are primarily cochleotoxic, whereas gentamicin and streptomycin are primarily vestibulotoxic. At high doses, streptomycin is also cochleotoxic. Streptomycin, 25 mg/kg per day, administered systemically to cats resulted in loss of vestibular hair cells only, but at 100 mg/kg/day, vestibular and cochlear hair cells were lost.22
More recent animal experiments have tried to model the pharmacokinetics of intratympanic administration of gentamicin applied in a sustained-release vehicle of liquid fibrin glue. High levels of gentamicin were measured in perilymph within 8 hours of administration. These high levels persisted for at least 24 hours, then declined rapidly by 72 hours. The elimination rate for gentamicin was 1.04 mg/mL/hr.23
The hair cells of the cristae, the ampullae, and the cochlea degenerate to different degrees after the administration of aminoglycosides. The primary vestibular neurons, the cochlear nuclei, and the vestibular nuclei are not directly affected, even at high doses.22,24 The basal turn of the cochlea is the region most susceptible to permanent loss of hair cells, resulting in an initial loss of high-frequency hearing sensitivity. Although the mechanisms of this differential toxicity are incompletely understood, several contributing factors have been identified, including the route of administration, dose variables, and the specific aminoglycoside used. Mutations in the 12S ribosomal RNA gene have been identified in association with maternally inherited susceptibility to deafness from treatment with aminoglycosides.25 Affected individuals can sustain profound deafness from even a single dose administered systemically.
Damage to vestibular dark cells, which are thought to play a role in the production of endolymph, has been reported after administration of doses of aminoglycoside below the threshold for damage to hair cells. It is possible, but unproven, that impaired function of dark cells is beneficial in Meniere’s disease.26,27
INTRAMUSCULAR APPLICATION OF STREPTOMYCIN
Clinical Studies
Between 1948 and 1980, eight investigators reported a total of 49 patients treated with intramuscular streptomycin for unilateral or bilateral Meniere’s disease. In the first extensive studies of intramuscular streptomycin, Schuknecht28,29 administered 0.75 to 1.75 g intramuscularly every 12 hours. Treatment continued until there were no ice water caloric responses in the diseased ears. This treatment end point was assumed to represent total or near-total vestibular ablation. The total doses ranged from 13.5 to 89 g (mean 39 g). All patients became severely ataxic, and most had oscillopsia early in the course of treatment, but none experienced hearing loss; 35% had persistent ataxia, and 15% had persistent oscillopsia. Ninety-five percent of patients had no post-treatment vertigo. Hearing levels did not change or fluctuate in 90% of patients. When a totally ablative dose of intramuscular streptomycin was administered, vertigo was controlled, and hearing was preserved.30 Patients who undergo total vestibular ablation may still be disabled by chronic dysequilibrium, ataxia, and oscillopsia.31
To attempt to limit the chronic oscillopsia and ataxia that follow total bilateral vestibular ablation, subtotal or “titration” treatment protocols with streptomycin were developed.32,33 Rather than giving an ablative dose, streptomycin was administered until episodic vertigo was controlled.
Treatment Method for Subtotal Vestibulectomy with Intramuscular Streptomycin
Our suggestions have been adapted from established protocols and modified by our own experience.32,33
Indications
Intramuscular streptomycin may be considered for patients with disabling episodic vertigo caused by Meniere’s disease in the following situations: (1) simultaneously active Meniere’s disease in both ears, or when it is unclear from which ear the attacks of vertigo are arising; (2) Meniere’s disease in an only hearing ear; or (3) in the second ear after an ablative procedure on the opposite side, such as selective vestibular nerve section. It is essential to determine to what extent a patient may be disabled by the definitive episodic vertigo of Meniere’s disease as defined by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS).4 Patients disabled primarily by continuous dysequilibrium, ataxia, oscillopsia, or motion intolerance are generally not good candidates for vestibular destructive procedures of any type because their symptoms are due to a failure of central compensation, to the perception of dysequilibrium in the presence of normal balance performance, or both.34,35
It is important to identify patients with Cogan’s syndrome, luetic hydrops, and autoimmune disease of the ear because these patients may respond to nondestructive medical treatment, such as corticosteroids.36 Although reliable data are lacking, it is likely that the recognition of autoimmune inner ear disease and immunosuppressive treatment have resulted in reduction of the number of cases of bilateral Meniere’s disease treated with intramuscular streptomycin. Patients with markedly reduced caloric function before treatment with streptomycin should be managed with additional caution because they may develop permanent dysequilibrium or oscillopsia with the loss of additional vestibular function. Treating such patients with lower doses of streptomycin should be considered.
Pretreatment Evaluation and Patient Counseling
It is essential to distinguish clearly between two phenomena in patients with Meniere’s disease who are undergoing intramuscular treatment with aminoglycosides: (1) vertigo owing to the disease, and (2) the syndrome of acute bilateral vestibular loss caused by vestibulotoxicity. Vertigo is the hallucination of motion—the perception of motion when none is occurring. The vertigo of Meniere’s disease occurs without provocation, consists of a spinning or rotating sensation always with spontaneous nystagmus, lasts 15 minutes to several hours, and is accompanied by dysequilibrium and nausea that may last for hours.4
The syndrome of acute bilateral vestibular loss may include rotational vertigo, but the vertigo is related to treatment rather than being spontaneous. Commonly, patients experience discomfort associated with rapid head movements, a sense of disorientation in space, and ataxia. Patients may also manifest oscillopsia, a disturbing sensation of the visual field bobbling as the patient walks about or rides in a car. This phenomenon is due to impairment or loss of the vestibulo-ocular reflex, which helps to maintain a stable image on the retina during head movement. Oscillopsia may be temporary or permanent after bilateral loss of vestibular function.30,31,37
Results
The long-term results of intramuscular subtotal streptomycin therapy for bilateral Meniere’s disease are known from only one report.37 Nineteen patients were reviewed with follow-up from 2 to 9 years. Recurrent vertigo was controlled in 95% of patients within the first 6 to 18 months after treatment. At last follow-up, 63% of patients reported having had no recurrence of vertigo. Persistent mild dysequilibrium occurred in 60% of patients without recurrent vertigo, and oscillopsia persisted in 16% of patients.37
APPLICATION OF STREPTOMYCIN TO LATERAL SEMICIRCULAR CANAL
Experimental Studies
Kimura and colleagues38 reported that the application of gentamicin to the lateral semicircular canal of normal guinea pigs produced a selective vestibular lesion. There was sensory cell degeneration in the utricular maculae and all but one of the cristae of the superior, lateral, and posterior canals of 27 ears. The saccular maculae were less affected. All cochleas were normal except one that had a small lesion of outer hair cells at the basal turn. Fenestrated control inner ears were normal throughout. Kimura and colleagues39 repeated some of their experiments with streptomycin after producing experimental endolymphatic hydrops by occlusion of the endolymphatic duct. Hydropic ears sustained substantial cochlear and vestibular lesions when streptomycin was applied to the lateral semicircular canal. Fenestration of the lateral canal without drug application produced a significant cochlear lesion, but not a vestibular lesion.
Clinical Studies
The application of streptomycin to the labyrinth through the lateral semicircular canal (labyrinthotomy with streptomycin infusion [LSI]) was introduced by Shea.40,41 The rationale of this procedure was that application to the vestibular labyrinth might cause more drug to reach the vestibular hair cells than cochlear hair cells, and produce a more selective lesion with a single treatment. Use of this route of administration requires the performance of a mastoidectomy and opening the bony otic capsule.
Surgical Method
Shea and Norris40 described the technique of LSI. A simple mastoidectomy is performed, and the lateral semicircular canal is identified. The bone of the dome of the semicircular canal is gradually thinned with a diamond burr. A “double blue-line” technique is used to create a fenestration of the lateral semicircular canal.
A small amount of fluid containing streptomycin is slowly infused into the perilymphatic space of the lateral semicircular canal. Opening the lateral semicircular canal in Meniere’s disease results in enhancement of the ratio of the summating potential to the action potential of the electrocochleographic recording, primarily by reduction in the amplitude of the action potential. The ratio does not change during infusion of streptomycin, but sometimes declines slightly after the fenestration is closed.42
The volume of fluid, the composition of the diluent (lactated Ringer solution or other physiologic solution), the amount of streptomycin in the solution delivered, the amount of time during which the fluid is introduced, and whether the streptomycin solution is followed by a “rinse” of a physiologic solution without streptomycin are important technical variables that could affect the amount of streptomycin administered and the amount of trauma to the inner ear. After the drug is infused, the fenestration is closed with a thick piece of temporalis muscle and fascia. The postauricular wound is closed in the usual manner. On the basis of animal experiments, Shea40,41 initially recommended puncturing the lateral membranous canal in hydropic ears to release endolymph, possibly decompressing endolymphatic hydrops acutely, but later withdrew that recommendation.
Results
In 1989, a multicenter study was initiated to produce an independent study of LSI results for hearing preservation and control of vertigo. Preliminary data from this and other studies were reported.42,43
Preoperative pure tone averages ranged from 14 to 76 dB HL with a mean of 54 dB (SD 14). Postoperative pure tone averages ranged from 25 to 110 dB HL with a mean of 76 dB. Four patients (9%) had an early postoperative hearing result that was improved over the preoperative hearing level.44 In 11 patients (23%), the hearing was unchanged; in 32 patients (68%), hearing worsened. In 27 patients (57%), the postoperative pure tone level was 71 dB or worse (severe to profound hearing loss). Hearing outcome (change in pure tone average or word recognition) did not seem to be a function of patient age, sex, side of surgery, duration of hearing loss before surgery, or whether the patient had had a surgical procedure on the ear before the LSI, or which surgeon performed the procedure. A trend was identified suggesting that patients with milder preoperative losses may sustain less hearing damage from LSI. Opening the endolymphatic space of the lateral membranous canal resulted in more loss of hearing than when the membranous canal was not opened (P = .05).
Discussion
Silverstein33 reported that patients with good hearing seemed to be more resistant to hearing loss from streptomycin applied intratympanically. There seemed to be less postoperative hearing loss from LSI when the preoperative pure tone average was 40 dB or better. Findings in patients with vestibular disorders seem to corroborate findings in the guinea pig model; that is, the hydropic ear shows more sensitivity to aminoglycoside ototoxicity than the normal ear, and there is less selectivity of lesions between vestibular and cochlear hair cells in hydropic ears.39 The long-term results (≥2 years) for hearing preservation and control of vertigo by LSI have not been reported. Because of the high risk of associated hearing loss and treatment failures, and because of generally better results with intratympanic gentamicin therapy, LSI has mostly fallen out of use.
INTRATYMPANIC GENTAMICIN THERAPY
Experimental Studies
Intratympanic injection of aminoglycosides allows treatment of unilateral Meniere’s disease without producing systemic toxicity or effects on the contralateral ear. Tracer studies have shown that the primary route of entry into the inner ear is through the round window membrane.45–49 A secondary route of entry into the inner ear may be through the annular ligament of the stapes.38,50
After the intratympanic application of streptomycin to guinea pigs, the cristae of the semicircular canals showed the most degeneration, followed by the utricle, then the saccule and the cochlea.51 The selectivity of the lesion for vestibular versus cochlear hair cells after intratympanic injection was reduced at higher dosages. The application of 8 mg of streptomycin to the round window membrane of the cat produced only vestibular toxicity, whereas 20 to 40 mg produced cochlear and vestibular toxicity.52 These experimental results emphasize the potential for cochlear toxicity when the primary route of entry into the inner ear is through the round window membrane.
Clinical Studies
Schuknecht28,29 introduced the use of intratympanic aminoglycosides to induce a chemical labyrinthectomy for the treatment of unilateral Meniere’s disease. He reported the results of eight patients given large daily doses of streptomycin (150 to 600 mg/day) for 1 to 7 days. The treatment end point was the onset of signs and symptoms of vestibular ablation. The treatment was successful in controlling vertigo in five of eight patients, all of whom lost substantial hearing in the treated ear. Although hearing was preserved in the remaining three of eight patients, persistent vertigo necessitated surgical labyrinthectomy. Schuknecht28,29 concluded that complete control of vertigo with intratympanic streptomycin required abolition of ice water caloric responses, and that when this was accomplished, hearing was also lost.
Lange53,54 reported an extensive experience using various aminoglycosides to treat Meniere’s disease. In his group of 92 patients, Lange reported that 90% had no further episodes of severe vertigo, and hearing remained unchanged in 76% of patients. Many of the studies reported from 1956 to 1990 did not use standardized reporting methods (Table 41-1).
In a study to characterize the delayed effects of intratympanic gentamicin, Magnusson and Padoan55 treated five patients with two doses of gentamicin (30 mg/mL, pH 6.4), given 12 hours apart. The first symptom of an ototoxic reaction noted by the patients was a sensation of unsteadiness occurring 2 to 5 days (mean 3.2 days) after the injections. Vertigo and nystagmus were noted 3 to 8 days (mean 5.1 days) after the injections. With 1 year of follow-up, vertigo was controlled (with a loss of caloric responsiveness in the treated ear), and hearing was preserved in all five patients.55 This preliminary study raised the question of whether very low intratympanic doses of gentamicin may be effective in controlling vertigo and preserving hearing. Because this technique did not totally ablate vestibular function, vertigo may recur, but hearing was usually preserved. Additional gentamicin could be used if vertigo recurred.
Beck and Schmidt56 administered gentamicin, 30 mg/day, and stopped after 6 days or at the slightest indication of ototoxicity. In 40 patients treated with their regimen, control of vertigo occurred in 92.5% of patients; 85% of patients maintained hearing. Following the work of Beck and Schmidt, several investigators developed the hypothesis that if a “titration” dosage schedule were adopted, it might be possible to achieve satisfactory rates of control of vertigo while preserving hearing in more patients. Much of the ongoing controversy about intratympanic gentamicin treatment has been over the issue of whether such approaches achieve this goal, and how various protocols compare with each other.
Studies by several authors showed remarkably similar results for control of vertigo, ranging from 86% to 93% of patients treated (Table 41-2; see Table 41-1). Some patients required additional gentamicin injections or surgery. Authors have noted an association between loss of the ice water caloric response and complete control of vertigo.57,58
Reports showed considerable variation in rates of hearing preservation, ranging from 55% to 85%. The results suggest that the administration schedule and total dosage may be significant factors in hearing preservation, and that low-dose methods may be associated with a lower incidence of hearing loss.59 The variability in reports may also be due to differences in case selection because some series have more elderly patients or patients with worse pretreatment hearing levels than other series. It is suspected, but has not been well established, that there may be a tradeoff between control of vertigo and preservation of hearing.
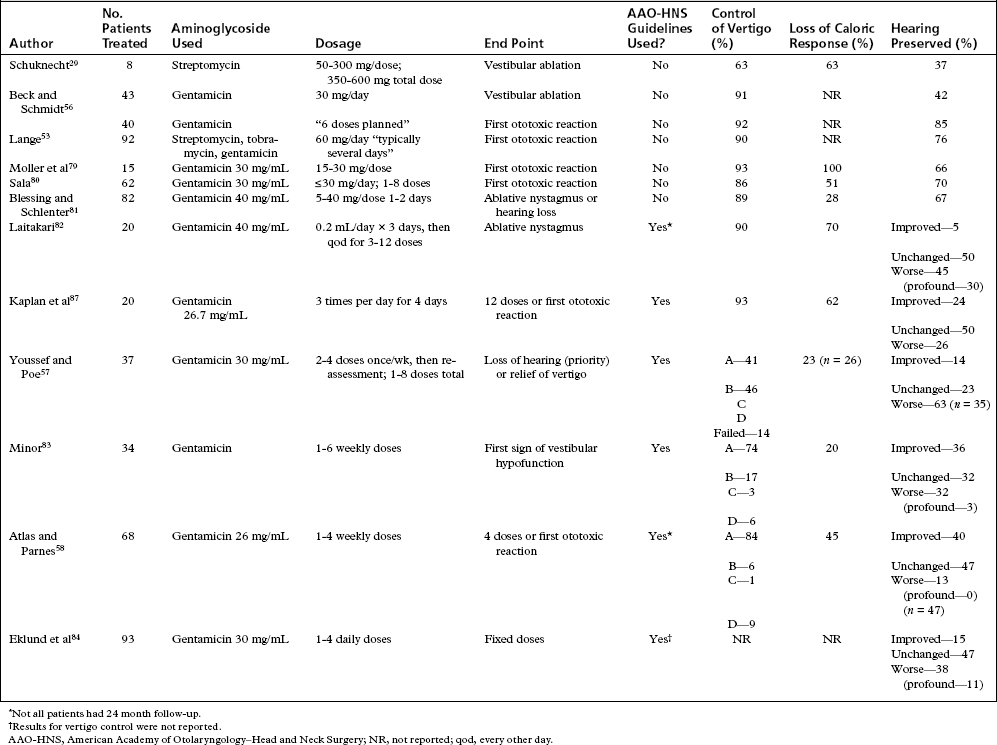
One of the authors (J.M.N.) has developed a fixed-dose protocol, which has been applied with consistent technique since 1988.60–62 A catheter assembly is constructed (Figs. 41-1 and 41-2). Gentamicin is buffered to a pH of 6.4 with sodium bicarbonate such that the final concentration is 26.7 mg/mL. The T-tube assembly is inserted into the middle ear so that the solution can easily access the round window niche. This procedure is done in the outpatient clinic under local anesthesia. If possible, the T-tube is inserted into the posteroinferior quadrant of the tympanic membrane. Three doses of approximately 0.7 mL of gentamicin are instilled daily for a total of 12 doses over 4 days. A family member or friend is instructed how to evacuate the catheter tubing before instilling the solution each morning and evening. Patients are seen in the office daily during treatment. The mid-day dose is administered during the office encounter, but only if there has been no been no history of vertigo, and no nystagmus is observed. The catheter and gentamicin solution produce a mild conductive hearing loss during the period of the instillations.
Early in the series, a daily bone conduction audiogram was performed; however, this is now done at completion of the treatment. The patient is seen on the morning of the 5th day, the catheter assembly is removed, and an audiogram is performed. The treatment is generally carried out over a Monday-to-Friday interval. The patient is advised that vertigo rarely occurs sooner than 2 to 3 days after completion of treatment. The patient is also seen by a physiotherapist who has a special interest in vestibular rehabilitation, and a suitable exercise program is recommended before discharge on day 5. All patients have been studied prospectively using the AAO-HNS guidelines for reporting treatment results in Meniere’s disease.4,44 All patients have electronystagmography before treatment. An audiogram and an electronystagmogram are performed 1 month after treatment. Comparison of the caloric response before and after treatment is viewed as a “barometer” of peripheral vestibular loss.
This protocol has produced consistent results. The first publication in 199262 has been followed by several others. In the initial cohort of 30 patients, complete control (vertigo class A) of vertigo was obtained in 83%, and substantial control (vertigo class B) was obtained in 17%. Hearing worsened in 27%, and 13% of patients sustained a profound hearing loss. An ice water caloric response was abolished in 53% of individuals. A subsequent study60 consisting of 90 patients reported complete vertigo control in 85% and substantial control in 9%.63 Hearing loss occurred in 23%; 16% patients became deaf. The most recent study has shown the effectiveness of long-term vertigo control using this protocol. Patients followed for 5 years or longer (15 year mean interval) have continued to be consistently free of vertigo.
One of the authors (S.P.C.) uses a low-dose titration method, which is described here.64 Before performing an ablative procedure, a full diagnostic assessment is recommended, including a complete history, physical examination, neurotologic examination, vestibular function testing (electronystagmography at a minimum, posturography and rotational chair testing if available), and audiometric evaluation. The neurotologic examination should include a search for spontaneous nystagmus (eyes open in light and dark), nystagmus after head shaking, and the head thrust test. Retrocochlear and metabolic disorders should be excluded. Because of the low total dose of gentamicin used, the need to withhold treatment based on abnormal renal function is rare.
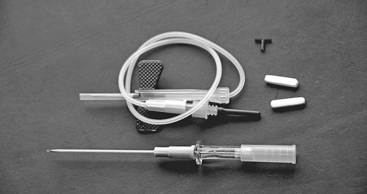
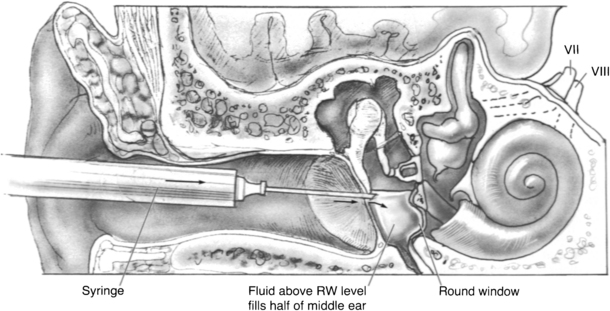
Low-dose titration chemical labyrinthectomy is performed by placing the patient in the supine position with the head turned 45 degrees away from the affected ear (Fig. 41-3). The eardrum is anesthetized with a small dot of topical phenol. Gentamicin (40 mg/mL) is injected using a tuberculin syringe with a 25 gauge 1½ inch needle; approximately 0.2 to 0.5 mL is injected into the middle ear space (Fig. 41-4). The patient is asked to remain for 30 minutes in the supine position and to refrain from excessive swallowing, which may contribute to early evacuation of the drug through the eustachian tube.
RESULTS
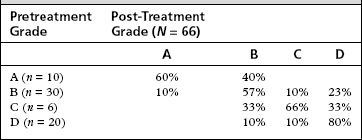
A total of 66 patients have been treated with 2 years or greater follow-up. The average age of treated patients is 60 years (range 34 to 89 years). The most common number of injections given is two (Table 41-3). The wide span in the number of injections reflects the individualized nature of the titration therapy. Eighty percent of patients have had complete control of vertigo; 94% have complete or substantial vertigo control (Table 41-4).
| No. Injections | No. Patients |
|---|---|
| 1 | 10 |
| 2 | 24 |
| 3 | 12 |
| 4 | 11 |
| ≥5 | 9 |
| Vertigo Control Grade (Numeric Value) | No. Patients (%) |
|---|---|
| A (complete) | 53 (80%) |
| B (1-40) | 9 (14%)∗ |
| C (41-80) | 1 (1.5%) |
| D (81-120) | 0 |
| E (≥121) | 0 |
| F (surgical treatment initiated) | 3 (4.5%) |
∗ 5 patients with class B results were retreated.
Pretreatment and post-treatment caloric testing was available for analysis in 48 patients. An absent ice water caloric response was found in 88% of patients. Bithermal caloric responses or a positive ice water response was found in 12% of patients. Three of five retreated patients and all three patients who required surgery for salvage had positive ice water response on caloric testing. Hearing results are presented according to the pretreatment Gardner-Robinson Hearing Scale (Table 41-5).65 In the Gardner-Robinson scale, patients with grade A hearing have a speech reception threshold (SRT) less than 30 dB and speech recognition greater than 70%. Patients with grade B have SRT less than 50 dB and speech recognition greater than 50%. Patients with grade C have SRT greater than 50 dB but less than 70 dB and speech recognition greater than 30% but less than 50%. Patients with grade D hearing have SRT greater than 70 dB and speech recognition less than 30%. Overall, using 10 dB SRT and 15% discrimination criteria, hearing was worse in 45% of patients; change in Gardner-Robinson grade occurred in 35%; loss of serviceable hearing (50 dB SRT and 50% speech recognition) occurred in 25%; and profound loss of hearing occurred in 3%.
Table 41-2 shows a comparison of the results from the fixed-dose protocol versus five studies following the clinical research reporting guidelines of the AAO-HNS for Meniere’s disease4 and with at least 25 subjects. The results are generally the same for control of vertigo among these studies, and are about the same as results for control of vertigo by selective vestibular nerve section. The results for hearing loss are also generally the same, although more patients may be deafened by the fixed dosage protocol. Most of the patients deafened had poor hearing before treatment. The advantage of the fixed dosage protocol is that it can be completed in less time and with fewer clinical visits for testing and evaluation than the titration protocols.
TREATMENT OF LABYRINTHINE DISORDERS WITH INTRATYMPANIC CORTICOSTEROIDS
Experimental Studies
The properties of glucocorticoids vary. Cortisone and prednisone have an 11-keto group, which must be enzymatically reduced in the liver before they are biologically active.53 Steroids such as prednisone and methylprednisolone have a shorter half-life than dexamethasone or betamethasone. In addition, the latter steroids are about seven times as potent as prednisone and prednisolone, and about five times as potent as methylprednisolone.53
Animal studies have shown that conditioning by sound to the harmful effects of noise exposure depends on the pituitary-hypothalamic-adrenal axis.65 Glucocorticoid and mineralocorticoid receptors have been identified in inner ear tissue.66 Endogenous steroids may stabilize junctions in the stria vascularis,67 and up-regulate the synthesis of glutathione, an endogenous antioxidant, in the spiral ganglion.68 Although clinical evidence is lacking, results have suggested the possibility that systemic steroids may protect against inner ear trauma during middle ear surgery and cochlear implantation.67,69
Clinical Studies
There is substantial clinical evidence that some otologic conditions are autoimmune in etiology and responsive to systemic immunosuppressive medications.36 Systemic corticosteroids have been shown to improve the outcome for hearing in bacterial meningitis70 and autoimmune inner ear disease,71 and have become accepted treatment for these conditions. In contrast, a more recent review cast doubt on the efficacy of systemic corticosteroids in sudden hearing loss.72 Immunosuppressants have undesirable systemic side effects, which limit their use. Consequently, there is considerable interest in applying immunosuppressive drugs, principally corticosteroids, intratympanically.
Much less is known about the pharmacokinetics and clinical effects of intratympanic corticosteroid administration than is known about aminoglycosides. It may be possible to achieve high concentrations in some otic tissues, but safety and efficacy in otologic disorders remain unproven. Intratympanic treatment of sudden hearing loss and Meniere’s disease with corticosteroids has been the subject of retrospective, uncontrolled studies and a few controlled studies with small numbers of subjects. Complications of infection, tympanic membrane perforation, and sensorineural hearing loss have been reported.73–75 One prospective, randomized, double-blind, crossover trial showed no benefit compared with placebo in Meniere’s disease.76
Larger, prospective, controlled studies with more rigorous attention to study methods are needed to evaluate the potential benefit and harms of intratympanic corticosteroid treatment.77 Rescue trials of intratympanic treatment after failure of oral steroid therapy also must be controlled because recovery may occur several months after onset of sudden hearing loss following oral corticosteroid therapy.78 A large-scale clinical trial funded by the National Institutes of Health is currently under way to assess the effectiveness of intratympanic steroid treatment in sudden sensorineural hearing loss (www.nidcd.nih.gov). Future studies may define safe and effective protocols for intratympanic treatment with corticosteroids and perhaps other medications in certain subsets of patients.
SUMMARY
Intratympanic application of gentamicin frequently results in treatment-related hearing loss, although episodic vertigo is usually well controlled. Control rates are comparable to control rates for selective vestibular nerve section. Patients with recurrent post-treatment vertigo can be treated with intratympanic gentamicin again. A current trend in intratympanic application is to administer one or two doses, rather than treating until ototoxicity is clinically evident. Just enough additional doses are applied to achieve control of attacks. The advantages of such titration protocols over fixed-dosage protocols have not been proven in long-term studies. Intratympanic gentamicin should be reserved for patients with classic Meniere’s disease who meet appropriate treatment criteria. Prolonged disabling dysequilibrium occurs about as frequently as after surgical labyrinthectomy or vestibular nerve section. Complete ablation and long-term control of vertigo are not achieved as reliably as with surgical labyrinthectomy.57 The safety and efficacy of corticosteroid intratympanic treatment of Meniere’s disease and other inner ear disorders are undefined at this time.
1. Yamashita D., Jiang H.Y., Le Prell C.G., et al. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005;134:633-642.
2. Dickey D.T., Muldoon L.L., Doolittle N.D., et al. Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin-induced toxicity in rat models. Cancer Chemother Pharmacol. 2007;62:235-241.
3. Chen Y., Huang W.G., Zha D.J., et al. Aspirin attenuates gentamicin ototoxicity: From the laboratory to the clinic. Hear Res. 2007;226:178-182.
4. Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Ménière’s disease. Otolaryngol Head Neck Surg. 1995;113:181-185.
5. Sande M., Mandell G. Antimicrobial agents. In: Gilman A., Rall T., Nies A., Taylor P., editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 8th ed. New York: Pergamon Press; 1999:1098-1116.
6. Fowler E. Streptomycin treatment of vertigo. Trans Am Acad Ophth Otolaryngol. 1948;52:239-301.
7. Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13:119-126.
8. Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985;359:189-217.
9. Marcotti W., van Netten S., Kros C. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567:505-521.
10. Waguespack J., Ricci A. Aminoglycoside ototoxicity: Permeant drugs cause permanent hair cell loss. J Physiol. 2005;567:359-360.
11. Rybak L. Ototoxic mechanisms. In: Altschuler R.A., Hoffman D.W., Bobbin R.P., editors. Neurobiology of Hearing. New York: Raven Press; 1986:441-454.
12. Williams S., Smith D., Schacht J. Characteristics of gentamicin uptake in the isolated crista ampullaris of the inner ear of the guinea pig. Biochem Pharmacol. 1987;36:89-95.
13. Williams S., Zenner H., Schacht J. Three molecular steps of aminoglycoside ototoxicity demonstrated in outer hair cells. Hear Res. 1987;30:11-18.
14. Lesniak W., Pecoraro V., Schacht J. Ternary complexes of gentamicin with iron and lipid catalyze formation of reactive oxygen species. Chem Res Toxicol. 2005;18:357-364.
15. Jacotot E., Costantini P., Laboureau E., et al. Mitochondrial membrane permeabilization during the apoptotic process. Ann N Y Acad Sci. 1999;887:18-30.
16. Ylikoski J., Xing-Qun L., Virkkala J., Pirvola U. Blockade of c-JUN N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;166:33-43.
17. Song B.-B., Anderson D., Schacht J. Protection from gentamicin toxicity by iron chelaters in guinea pig in vivo. J Pharmacol Exp Ther. 1999;282:369-377.
18. Lecain E., Omri B., Behar-Cohen F., et al. The role of PKCzeta in amikacin-induced apoptosis in the cochlea: Prevention by aspirin. Apoptosis. 2007;12:333-342.
19. Sha S.-H., Qiu J.-H., Schacht J. Aspirin attenuates gentamicin-induced hearing loss. N Engl J Med. 2006;354:1856-1857.
20. Henley C.I., Schacht J. Pharmacokinetics of aminoglycoside antibiotics in inner ear fluids and their relationship to ototoxicity. Audiology. 1988;27:137-147.
21. Aran J., Chappert C., Dulon D., et al. Uptake of amikacin by hair cells of the guinea pig cochlea and vestibule and ototoxicity in comparison with gentamicin. Hear Res. 1999;82:179-183.
22. McGee T., Olszewski J. Streptomycin sulfate and dihydrostreptomycin toxicity. Arch Otolaryngol. 1962;75:295-311.
23. Balough B., Hoffer M., Wester D., et al. Kinetics of gentamicin uptake in the inner ear of chinchilla langier after middle-ear administration in a sustained-release vehicle. Otolaryngol Head Neck Surg. 1998;119:427-431.
24. Berg K. The toxic effect of streptomycin on the vestibular and cochlear apparatus. Acta Otolaryngol. 1951;157:1-77.
25. Rodriguez-Ballesteros M., Olarte M., Aguirre L.S., et al. Molecular and clinical characterisation of three Spanish families with maternally inherited non-syndromic hearing loss caused by the 1494C→T mutation in the mitochondrial 12S rRNA gene. J Med Genet. 2006;43:e54.
26. Park J., Cohen G. Vestibular ototoxicity in the chick: Effects of streptomycin on equilibrium and on ampullary dark cells. Am J Otolaryngol. 1982;6:117-127.
27. Pender D. Gentamicin tympanoclysis: Effects on the vestibular secretory cells. Am J Otolaryngol. 1985;6:358-367.
28. Schuknecht H. Ablation therapy for the relief of Ménière’s disease. Laryngoscope. 1956;66:859-870.
29. Schuknecht H. Ablation therapy in the management of Ménière’s disease. Acta Otolaryngol (Stockh) Suppl. 1957;132:1-42.
30. Wilson W., Schuknecht S. Update on the use of streptomycin therapy for Ménière’s disease. Am J Otolaryngol. 1980;2:108-111.
31. “J.C.”: Living without a balance mechanism. N Engl J Med. 1952;246:458-460.
32. Graham M., Kemink J. Titration streptomycin therapy for bilateral Ménière’s disease: A progress report. Otolaryngol Head Neck Surg. 1984;92:440-447.
33. Silverstein H. Streptomycin treatment for Ménière’s disease. Ann Otol Rhinol Laryngol Suppl. 1984;112:44-88.
34. Konrad H. Intractable vertigo—when not to operate. Trans Am Acad Ophthalmol Otolaryngol. 1986;95:482-484.
35. Monsell E., Brackmann D., Linthicum F. Why do vestibular destructive procedures sometimes fail? Otolaryngol Head Neck Surg. 1988;99:472-479.
36. Harris J., Tomiyama S. Immunology/virology of Ménière’s disease. In: Harris J., editor. Ménière’s Disease. The Hague: Kugler Publications; 1999:123-138.
37. Langman A., Kemink J., Graham M. Titration therapy for bilateral Ménière’s disease, follow-up report. Ann Otol Rhinol Laryngol. 1990;99:923-926.
38. Kimura R., Iverson N., Southard R.E. Selective lesions of the vestibular labyrinth. Ann Otol Rhinol Laryngol. 1988 Nov-Dec;97(6 Pt 1):577-584. PMID: 3264488 [PubMed-indexed for MEDLINE]
39. Kimura R., Lee K.-S., Nye C., Trehey J. Effects of systemic and lateral semicircular canal administration of aminoglycosides on normal and hydropic inner ears. Acta Otolaryngol (Stockh). 1991;111:1021-1030.
40. Shea J., Norris C. Streptomycin perfusion of the labyrinth. In: Nadol J.B., editor. Second International Symposium on Ménière’s Disease. Cambridge, MA: Amsterdam, Kugler & Ghendini Publications, June 20-22, 1988. 1989
41. Shea J. Perfusion of the inner ear with streptomycin. Am J Otol. 1989;10:150-155.
42. Monsell E. Electrocochleographic recording in patients undergoing labyrinthotomy with streptomycin infusion. In: Arenberg I.K., editor. Surgery of the Inner Ear, Proceedings of the Third International Symposium and Workshops on Surgery of the Inner Ear. Snowmass, CO: Amsterdam, Kulger & Ghedini, July 29-August 4, 1990. 1991
43. Monsell E., Shelton C. Labyrinthotomy with streptomycin infusion: Early results of a multicenter study. Am J Otol. 1992;13:416-422.
44. Subcommittee on Equilibrium. Ménière’s disease: Criteria for diagnosis and evaluation of therapy for reporting. AAO-HNS Bull. 1985;7:6-7.
45. Smith B., Myers M. The penetration of gentamicin and neomycin into the perilymph across the round window membrane. Otolaryngol Head Neck Surg. 1978;87:888-891.
46. Saijo S., Kimura R. Distribution of HRP in the inner ear after injection into the middle ear cavity. Acta Otolaryngol (Stockh). 1999;97:593-610.
47. Goycoolea M., Carpenter A., Muchow D. Ultrastructural studies of the round-window membrane of the cat. Arch Otolaryngol. 1987;113:617-624.
48. Kawauchi H., DeMaria T., Lim D. Endotoxin permeability through the round window. Acta Otolaryngol (Stockh) Suppl. 1988;457:100-115.
49. Lundman L., Bagger-Sjöbäck D., Holmquist L., Juhn S. Round window membrane permeability. Acta Otolaryngol (Stockh) Suppl. 1988;457:73-77.
50. Jahnke K. Transtympanic application of gentamicin with cochlea protection. In: Nadol J.B., editor. Second International Symposium on Ménière’s Disease. Cambridge, MA: Amsterdam, Kugler & Ghendini Publications, June 20-22, 1988. 1989
51. Lindeman H. Regional differences in sensitivity of the vestibular sensory epithelia to ototoxic antibiotics. Acta Otolaryngol (Stockh). 1969;67:177-189.
52. Cass S., Bouchard K., Graham M. Controlled application of streptomycin to the round window membrane of the cat. Otolaryngol Head Neck Surg. 1990;103:223.
53. Lange G. Gentamicin and other ototoxic antibiotics for the transtympanic treatment of Ménière’s disease. Arch Otorhinolaryngol. 1989;246:269-270.
54. Lange G. Isolierte Medikamentose Ausschaltungeines Gleichgewichtsorganes beim Morbus Ménière mit Streptomycin-Ozothin. Arch Klin Exp Ohren-Nasen-Kehlkopfheilkd. 1999;191:545-549.
55. Magnusson M., Padoan S. Delayed onset of ototoxic effects of gentamicin in treatment of Ménière’s disease. Acta Otolaryngol. 1991;111:671-676.
56. Beck C., Schmidt C. Ten years of experience with intratympanically applied streptomycin (gentamicin) in the therapy of morbus Ménière. Arch Otorhinolaryngol. 1978;221:149-152.
57. Youssef T., Poe D. Intratympanic gentamicin injection for the treatment of Ménière’s disease. Am J Otol. 1998;19:435-442.
58. Atlas J., Parnes L. Intratympanic gentamicin titration therapy for intractable Ménière’s disease. Am J Otol. 1999;20:357-363.
59. Chia S.H., Gamst A.C., Anderson J.P., Harris J.P. Intratympanic gentamicin therapy for Ménière’s disease: A meta-analysis. Otol Neurotol. 2004;25:544-552.
60. Commins D., Nedzelski J. Topical drugs in the treatment of Ménière’s disease. Curr Opin Otolaryngol Head Neck Surg. 1996;4:319-323.
61. Hone S., Nedzelski J. Selective chemical ablation as treatment for Ménière’s disease. In: Harris J., editor. Ménière’s Disease. The Hague: Kugler Publications; 1999:381-389.
62. Nedzelski J., Schessel D., Bryce G., Pfleiderer A. Chemical labyrinthectomy: Local application for the treatment of unilateral Ménière’s disease. Am J Otol. 1992;13:18-22.
63. Bodmer D., Morong S., Stewart C., Alexander A., Chen J.M., Nedzelski J.M. Long-term vertigo control in patients after intratympanic gentamicin instillation for Ménière’s disease. Otol Neurotol. Dec 2007;28(8):1140-1144. PMID: 18084826 [PubMed-indexed for MEDLINE]
64. Cass S. Chemical labyrinthectomy using intratympanic gentamicin for treatment of disabling vertigo associated with Ménière’s disease. In: Lim D., editor. Ménière’s Disease and Inner Ear Homeostasis Disorders. Los Angeles: House Ear Institute, 2005.
65. Gardner G., Robertson J.H. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97:55-66.
66. Tahera Y., Meltser I., Johansson P., et al. Sound conditioning protects hearing by activating the hypothalamic-pituitary-adrenal axis. Neurobiol Dis. 2007;25:189-197.
67. Trune D.R., Kempton J.B., Gross N.D. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hear Res. 2006;212:22-32.
68. Hashimoto K., Seki M., Miyasaka H., Watanabe K. Effect of steroids on increased permeability of blood vessels of the stria vascularis after auditory ossicle vibration by a drill in otologic surgery. Ann Otol Rhinol Laryngol. 2006;115:769-774.
69. Nagashima R., Ogita K. Enhanced biosynthesis of glutathione in the spiral ganglion of the cochlea after in vivo treatment with dexamethasone in mice. Brain Res. 2006;117:101-108.
70. Ye Q., Tillein J., Hartmann R., et al. Application of a corticosteroid (Triamcinolon) protects inner ear function after surgical intervention. Ear Hear. 2007;28:361-369.
71. van de Beek D., de Gans J., McIntyre P., Prasad K. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 1, 2007. CD004405
72. Harris J.P., Weisman M.H., Derebery J.M., et al. Treatment of corticosteroid-responsive autoimmune inner ear disease with methotrexate: A randomized controlled trial. JAMA. 2003;290:1875-1883.
73. Conlin A.E., Parnes L.S. Treatment of sudden sensorineural hearing loss, II: A meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582-586.
74. Shulman A., Goldstein B. Intratympanic drug therapy with steroids for tinnitus control. A preliminary report. Int Tinnitus J. 2000;6:10-20.
75. Herr B., Marzo S.J. Intratympanic steroid perfusion for refractory sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2005;132:527-531.
76. Spandow O., Hellstrom S., Anniko M. Impaired hearing following instillation of hydrocortisone into the middle ear. Acta Otolaryngol (Stockh) Suppl. 1988;455:90-93.
77. Silverstein H., Isaacson J.E., Olds M.J., et al. Dexamethasone inner ear perfusion for the treatment of Ménière’s disease: A prospective, randomized, double-blind, cross-over trial. Am J Otol. 1998;19:196-201.
78. Doyle K.J., Bauch C., Battista R., et al. Intratympanic steroid treatment: A review. Otol Neurotol. 2004;25:1034-1039.
79. Slattery W.H., Fisher L.M., Iqbal Z., Liu N. Oral steroid regimens for idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2005;132:5-10.
80. Moller C., Odkvist L., Thell J., et al. Vestibular and audiologic functions in gentamicin-treated Ménière’s disease. Am J Otolaryngol. 1988;9:383-391.
81. Sala T. Transtympanic administration of aminoglycosides in patients with Ménière’s disease. Arch Otorhinolaryngol. 1988;245:293-296.
82. Blessing R., Schlenter W. Langzeitergebnisse der Gentamicin-Therapie Des Morbus Ménière. Laryngorhino-otology. 1989;68:657-660.
83. Laitakari K. Intratympanic gentamicin in severe Ménière’s disease. Clin Otolaryngol. 1990;15:545-548.
84. Minor L. Intratympanic gentamicin for control of vertigo in Ménière’s disease: Vestibular signs that specify completion of therapy. Am J Otol. 1999;20:209-219.
85. Eklund S., Pyykko I., Aalto H., et al. Effect of intratympanic gentamicin on hearing and tinnitus in Ménière’s disease. Am J Otol. 1999;20:356.
86. Hirsch B., Kammerer D. Intratympanic gentamicin therapy for Ménière’s disease. Am J Otol. 1997;18:44-51.
87. Kaasinen S., Pyykko I., Ishizaki H., Aalto H. Intratympanic gentamicin in Ménière’s disease. Acta Otolaryngol. 1998;118:294-298.
88. Kaplan D., Nedzelski J., Chen J., Shipp D. Intratympanic gentamicin for treatment of unilateral Ménière’s disease. Laryngoscope. 2000;110:1298-1305.