CHAPTER 69 Cervicogenic Headache
INTRODUCTION
It is a common experience in clinical practice to encounter syndromes that are diagnosed and treated using a variety of methods despite limited research and/or a lack of evidence-based medical consensus. Cervicogenic headache (CH) is one of these entities. Although there is long-standing notion that headaches can originate from structures in the neck and can be treated by interventions directed at the cervical spine, it is only during the past two decades that the topic has gained attention in mainstream medical literature. CH is a syndrome and not a distinct disease process. Its symptoms constitute a ‘final common pathway’ for pain emanating from a variety of cervical biomechanical and/or inflammatory disorders. It is a diagnosis of exclusion and more serious pathology should be ruled out. Specific spinal structures such as nerves, nerve root ganglia, uncovertebral joints, intervertebral discs, facet joints, ligaments, and even muscle may give rise to similar symptoms ultimately diagnosed as CH.1,2 The absence of pathognomonic symptoms, physical findings, or imaging studies makes the diagnosis and treatment of CH challenging to the clinician. This chapter will review the history, epidemiology, pathophysiological basis, diagnostic criteria, and treatment of CH.
HISTORICAL PERSPECTIVE
One of the earliest references describing a relationship between headache and the cervical spine was a series of lectures by Hilton occurring between 1860 and 1862, which were reported by Pearce.3 Almost 90 years later, a case series published by Hunter and Mayfield4 in 1949 formed the intellectual rationale for interventional techniques to treat cervicogenic headaches. Hunter and Mayfield reported that occipital neuralgia could be an important cause of headaches, with pain radiating from the occiput to the periorbital and jaw areas. Their theory was used to justify the injection of analgesics around the occipital nerves in an attempt to relieve these headaches. That same year, Bartschi-Rochaix5 coined the term ‘cervical migraine’ to label headaches presumed to derive from the neck. In 1955, Kovacs6 postulated that motion restriction of the cervical spine could lead to muscle spasm, thereby compromising the vertebral artery and nerves, and manifesting as a headache. This premise helped popularize osteopathic, chiropractic, and manual treatment of the cervical spine to relieve CH. Bogduk and Marsland7 in 1986 put forth their theory of ‘third occipital headache’ and advocated surgical intervention to treat it. This paper provided the first compelling scientific evidence that headaches could indeed develop as a consequence of a cervical spine biomechanical disorder.
The term ‘cervicogenic headache’ was initially introduced into the medical lexicon in 1983 by Sjaastad et al.,8 to describe patients with a headache associated with disorders of the neck. In 1988, the International Headache Society (IHS)9 amended its diagnostic classification system to include a category for CH. In 1990, Sjaastad et al.1 published specific diagnostic criteria for CH. Less stringent diagnostic criteria for CH was subsequently published by the International Association for the Study of Pain (IASP) in 1994,10 and by the Quebec Headache Study Group in 1995.11 In 1998, Sjaastad et al.12 revised their diagnostic criteria for CH based on more extensive clinical research.
EPIDEMIOLOGY
Few epidemiologic studies exist and these have only been done in the last decade.13–20 These studies support that CH is common. However, there is a great deal of variation in the perceived prevalence of CH. In the general population, for example, prevalence rates ranged from 0.4% to 2.5%,13,14 whereas studies looking at all patients with a complaint of headache reported estimates of 15–20%.9,14–16 The highest variation was among headache center patients, with prevalence estimates of 0.4–80%.17,18 The wide variation in reported prevalence can be attributed to the different diagnostic criteria used to define CH. The population pool each of the publications drew from was not comparable. Another factor influencing prevalence rates in headache centers is the overlap between the diagnosis of CH, tension-type headache (TTH), and common migraine headache (MH). Bono et al.19 report that 75% of patients fulfilling IHS criteria for MH also met most of the criteria for CH. One study of headache center patients reported that whereas only 16.1% were diagnosed with CH, an additional 20.1% were diagnosed with both MH and CH, for a total prevalence of 36.2%.16 Another study reported that 56.4% of CH diagnoses occur in combination with other headaches, including MH, TTH, and drug-induced headache.20
Analysis of patient descriptive data from studies where such information was given reveals that there is a preponderance of female patients with CH,21–23 with an average gender distribution of 79.1% female and 20.9% male. The mean age was noted to be 42.9 years, and the mean duration of symptoms was 6.8 years.24
NEUROANATOMIC BASIS AND PATHOPHYSIOLOGIC MECHANISMS
Convergence theory
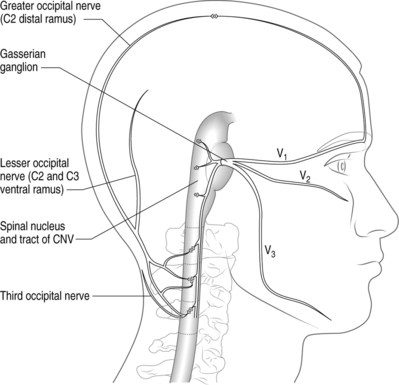
There is compelling circumstantial evidence that substantiates the theory of convergence to explain how cervical spine pathology can manifest as CH. The basic premise of convergence is that when primary afferents from two separate regions in the body converge on the same second-order neurons in the spinal cord, nociceptive activity along one of the afferent nerves can be perceived as pain in the other afferent nerve.25 Anatomically, both cervical and cranial afferent nerves innervate the head and face. The greater occipital, lesser occipital, and greater auricular nerves may innervate as far as the coronal sutures. Pain perceived in the forehead could be due to convergence between the trigeminal and upper cervical afferents. The cervicotrigeminal interneuron relay conveys nociceptive information to the upper cervical cord neurons. The trigeminal nucleus begins in the pyramidal decussations and descends as far caudad as C4 as the nucleus caudalis. The trigeminal nucleus is morphologically and functionally associated with the upper cervical cord and the cells form a column which is continuous with the posterior horn of the cervical cord. These anatomical relationships clearly demonstrate convergence between the trigeminal and cervical cord (Fig. 69.1). This convergence may also help to explain the systemic and sympathetic nervous system features accompanying CH.
Kerr26 discussed the relationship of the descending tract of the trigeminal nerve to the upper cervical roots following his dissection and analyses of adult cats. He observed that trigeminal afferents formed a bundle (trigeminosolitary bundle) with the solitary nucleus leading him to conclude that the trigeminal nerve provides a visceral component to the head and neck. The descending afferent trigeminal tract was identified as far caudal as the third cervical root. Trigeminal fibers were found at the low medullary, first, and third cervical cord levels. The second cervical root of the trigeminal fibers descends to the upper half of the cervical segment, but rapidly disappeared.26 Sectioned dorsal rhizotomy specimens demonstrated that the first cervical root traversed the trigeminal tract. There was no evidence of afferent fibers descending to the fourth through sixth cervical roots.26 The spinal tract of the trigeminal nerve entered the cervical cord in Lissauer’s tract as far down as the uppermost area of the third cervical cord. These cadaveric findings provide the anatomic basis for convergence theory (Kerr principle) and may explain why cervicogenic pain can occur ipsilaterally or bilaterally.
Almost every structure in the cervical spine has been implicated as a cause of headaches. Similarly, the mechanism of action may be due to degenerative changes, a direct result of trauma or without any underlying biomechanical basis. Current theories of anatomic causes of CH are based on retrospective observation, of either a reproducible finding on clinical examination, a response to stimulation of the structure, or relief of symptoms after treatment directed at the structure. Examples include the response of patients to surgery for disc disease,27 injections of posterior facets with local anesthetics,28 and injections of cervical muscles with botulinum toxin.29
Cervical facet joint
The zygapophyseal joints are implicated as a major source for cervicogenic headaches. The medial branch of the dorsal ramus above and below its location innervates the facet joints, except C2–3. The C2–3 is innervated by the superficial medial branch of the C3 dorsal ramus, also known as the third occipital nerve (TON).25,30 Pain patterns from stimulation of the cervical zygapophyseal joints have been studied in normal volunteers31 and from clinical evaluation.32,33 These studies suggest that the cervical zygapophyseal joints produce characteristic pain patterns according to the segmental distribution.31,32 The prevalence of zygapophyseal joint pain after whiplash has been reported as high as 50% in the C2–3 joint and 49% in the lower cervical joints.34,35
Cervical discs
Cervical discs have been implicated as a possible source of cervicogenic headache.25,36 Provocative discography is required to diagnose a painful cervical disc. In provocative discography, approximately 1 cc of radiopaque contrast is injected into the nucleus pulposus and the patient’s pain response is documented.37 Grubb and Kelly38 reported the prevalence of cervical pathology and referral patterns over a 12-year period. There were characteristic pain patterns depending on the level of the intervertebral disc. The C2–3 disc level referred pain into the upper cervical area, often extending into the occipital region and head, possibly accompanied by headaches in the occiput or frontal region. The pain pattern at the C3–4 level was similar to the C2–3 pain, but extended less into the occiput; and fewer patients experienced headache. More than 50% of the patients had three levels that had concordant responses, which may change clinical decisions to operate. Schellhas et al.,39 while correlating MRI findings with discography results, found a significant number of annular tears on discography that magnetic resonance imaging (MRI) was unable to detect. They concluded that magnetic resonance imaging is not reliable to delineate annular tears and should be used only as screening tool. Slipman et al.40 performed symptom mapping on 41 patients who underwent provocative discography at 101 levels to outline the pain referral patterns. The pain pattern on provocation of the C2–3, C3–4, C4–5, and C5–6 disc levels involved the occipital and/or facial areas, implicating the cervical discs as a possible source of CH.
Cervical segmental nerves
Diagnostic blocks of segmental nerves C2 and C3 have been routinely performed to confirm the diagnosis of cervicogenic headache.41 Bovin et al.28 performed an anesthetic blockade of the C2–5 spinal nerves to determine their involvement in the pathogenesis of cervicogenic headache. They reported that the most convincing relief occurred with a blockade of the C2 nerve. No patients responded completely to isolated blockades of nerves C3, C4, or C5. The C2 and C3 pain dermatomes were well described by Poletti in 1991.42
Occipital nerves
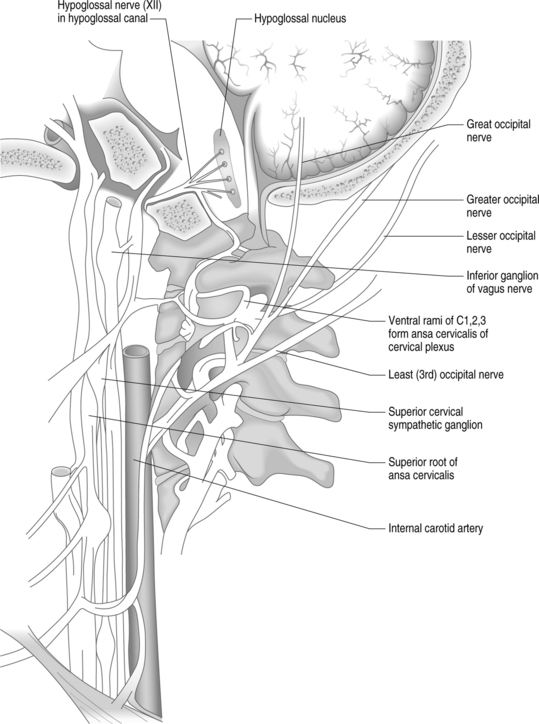
Another commonly implicated structure is the occipital nerve. The greater occipital nerve originates from the dorsal ramus of the C2 spinal nerve, the lesser occipital nerve from the ventral ramus of C2 and C3 spinal nerves via the cervical plexus, and the third (least) occipital nerve is the superficial medial branch of the C3 dorsal ramus (Fig. 69.2).43 Attributing occipital pain to irritation of the greater and lesser occipital nerve was common in the past. There is no compelling evidence that occipital pain is the result of irritation of the greater or lesser occipital nerve. Lancinating occipital neuralgia is recorded as a feature of temporal arteritis,44 in which case inflammation of the occipital artery could affect the companion nerve. In the majority of cases of so-called ‘occipital neuralgia,’ however, no such pathology is evident. The commonly held but inaccurate view is that occipital neuralgia is caused by entrapment of the greater occipital nerve where it pierces the trapezius. Surgical studies do not provide any evidence of this.45–47 The medial branch of C2 dorsal ramus, known conventionally as the greater occipital nerve, at first runs transversely across obliquus inferior. Near the origin of the obliquus inferior, the greater occipital nerve turns upwards across the dorsal surface of rectus capitis posterior major (see Fig. 69.2). Here it receives a communicating branch from the third occipital nerve. It emerges onto the scalp, not by piercing the trapezius, but by passing above an aponeurotic sling. This sling blends medially and laterally, with the aponeurotic insertions of trapezius and sternocleidomastoid, respectively, and thereby attaches to the superior nuchal line. Along its middle portion, the sling lies suspended from the superior nuchal line, leaving an aperture between it and the bone, through which the greater occipital nerve and the occipital artery emerge, leaving the plane deep to trapezius and sternocleidomastoid to enter the scalp.47 When the trapezius and sternocleidomastoid muscles contract, there is a ‘sling effect’ that actually relieves pressure on the greater occipital nerve.
The cardinal diagnostic criterion for greater occipital neuralgia seems to be response to blocks of the greater occipital nerve; but these blocks are not target specific when they involve volumes such as 5 mL45 or 10 mL.48,49 In such volumes, they do not selectively implicate the greater occipital nerve.
Bogduk and Marsland first explained the concept of the third occipital nerve (TON) headache.7 They provided a detailed description of the anatomy of the C3 dorsal ramus. The superficial medial branch of the C3 ramus (also known as the third occipital nerve) crosses the lateral and dorsal aspects of the lower half of the C2–3 zygapophyseal joint (see Fig. 69.2). It then passes across the lamina of C3 before turning backwards and upwards to pierce semispinalis capitis and splenius capitis to become cutaneous over the suboccipital area.7 In a later study, 100 consecutive patients with neck pain for more than 3 months were examined to determine the prevalence of TON headache.50 Seventy-one patients complained of headache associated with neck pain; in 40 of these patients, headache was the dominant complaint. In 31 patients, headache was the ‘secondary’ complaint. The prevalence of TON headache was 27%. Of those patients with headache as the dominant complaint, the prevalence was 53%.50
Dural attachments
Another theory of CH etiology comes from anatomical studies showing an attachment of the suboccipital tissues to the dura mater at the cervical–cranial junction, and the observation that mechanical traction on these tissues can cause movement of the dura.51–53 The rectus capitus posterior minor muscle53 and ligamentum nuchae52 have been shown to have direct connections to the suboccipital dura on very delicate dissection in a small number of cadavers. This suggests the possibility of the dura as a nociceptive structure in CH.
Inflammation
Recent studies have implicated inflammation as the cause of various spine-related conditions, including CH. When intervertebral discs are injured they have been found to release inflammatory mediators.54–56 Interleukin (IL)-1β and TNF-α increase the molecular events of inflammation.57 As well, a marked increase in the nitric oxide (NO) pathway has been demonstrated in patients with migraines or cluster headaches.58 Martelletti et al. observed increased levels of IL-1β and TNF-α in patients experiencing cervicogenic headaches during periods of spontaneous fluctuating basal pain and during mechanically induced attacks. There were statistically significant differences in cytokine levels as compared to controls.59,60 An increase in NO formation in the presence of reactive oxygen species may interact with IL-1β and TNF-α. This signals a cascade of proinflammatory/pain mediators such as prostaglandin and bradykinin, which play a role in neuronal sensitization. These interactions and responses implicate CH as an inflammatory consequence of cervical trauma. More importantly, they suggest that a myriad of pathological processes in different structures can manifest with similar or identical symptomatology (CH).
The inability to find a singular involved anatomic structure or pathology as the cause of CH has led some to believe that CH does not represent a single pathological entity, but rather a pain syndrome resulting from the nociceptive stimulation of almost any structure in the cervical spine.61
Differential diagnosis
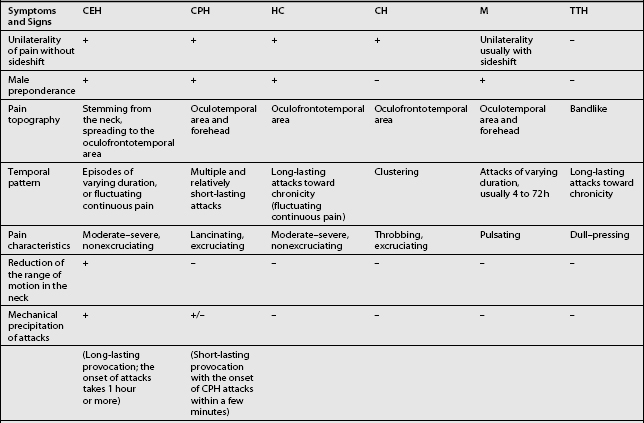
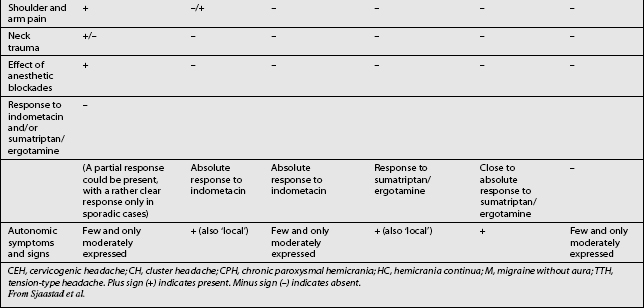
Other headaches, such as cluster headache, migraine (MH), chronic paroxysmal hemicrania (CPH), hemicrania continua (HC), and tension-type headache (TTH) must be included in the differential diagnosis (Table 69.1). Intracranial pathology, infection, neoplasm must be ruled out prior to assigning the patient the diagnosis of CH. Headaches associated with sinusitis, temporomandibular joint syndrome, and visual or auditory disturbances are rarely confused with CH because each possesses unique distinguishing characteristics.
CLINICAL PRESENTATION AND DIAGNOSTIC CRITERIA
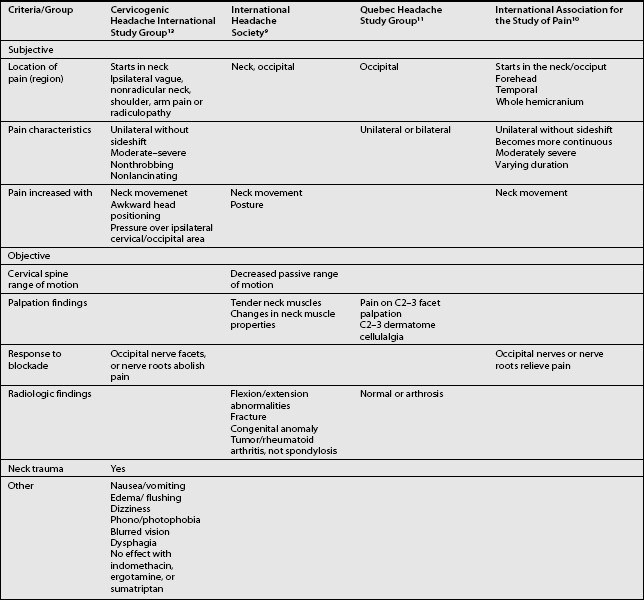
The term CH, although adopted by a number of organizations, is not universally accepted. Given this lack of consensus it is not surprising that a variety of labels are used to discuss headaches associated with disorders of the cervical spine. Perusal of literature published prior to 1983 emphasizes this point. Before that date, a number of terms such as vertebragenous headache,62 vertebrogenic headache,63 spondylotic headache,64 cervical spine syndrome,65 cervical migraine,66 cervical headache,67 cervicogenic syndrome,68 greater occipital neuralgia,69 and third occipital headache8 appear to have referred to the same clinical entity. Providing a consistent label for CH is not the only aspect of this entity that has been afflicted by a lack of unanimity. A similar problem arises when one considers the diagnostic criteria for CH. The most widely used diagnostic criteria are those proposed by Sjaastad in 1990, which were subsequently updated in 1998.12 These criteria have been adapted by the Cervicogenic Headache International Study Group (Table 69.2). Three other expert groups, the International Headache Society (Table 69.3),9 the Quebec Headache Group,11 and the International Association for the Study of Pain10 have published their own criteria. Table 69.4 summarizes the prominent features of the diagnostic criteria published by various expert groups.
Table 69.2 The Cervicogenic Headache International Study Group
| I. MAJOR CRITERIA OF CERVICOGENIC HEADACHE | |
From Sjaastad et al.13
Table 69.3 International Headache Society Criteria for Headache Associated with Disorder of the Neck
| Resistance to or limitation of passive neck movements |
| Changes in neck muscle contour, texture, tone, or response to active and passive stretching and contraction |
| Abnormal tenderness of neck muscles |
| Movement abnormalities in flexion/extension |
| Abnormal posture |
| Fractures, congenital abnormalities, bone tumors, rheumatoid arthritis, or other distinct pathology (not spondylosis or osteochondrosis) |
Comment: Cervical headaches are associated with movement abnormalities in cervical intervertebral segments. The disorder may be located in the joints or ligaments. The abnormal movement may occur in any component of intervertebral movement, and is manifest during either active or passive examination of the movement.
Adapted from IHS, Headache Classification Committee of the International Headache Society. Cephalalgia 1988.9
Obtaining an accurate history is the initial step in formulating a differential diagnosis. A history of neck/head trauma should be considered to be of importance, especially if there is a possible whiplash mechanism.2,12 It has been reported that whiplash injuries usually affect the cervical facet joint, intervertebral disc, cervical nerve root, or a combination of these structures.34,70–72 Prior to the modification of the diagnostic criteria by Sjaastad et al. in 1998,12 CH had been defined as a unilateral headache spreading to the neck and the ipsilateral shoulder/arm, triggered by head/neck movements and posture. A strict unilaterality requirement has been softened in the updated CH diagnostic criteria.12 Since CH is a syndrome, the pathologic process can involve the contralateral side, potentially presenting as a bilateral headache. Even in the typical unilateral case, pain may eventually spread to the opposite side particular when the headache becomes severe. Nevertheless, the symptom intensity will remain greater on the original side.12
Other diagnostic features of CH include signs and symptoms of neck involvement. Such signs are biomechanical precipitation of attacks, whether iatrogenically and subjectively induced, reduced active range of motion (ROM) in the neck in one or more directions, diffuse ipsilateral neck/shoulder/arm pain of nonradicular nature, and occasionally, seemingly radicular arm pain (see Table 69.2). Pain may be reproduced iatrogenically by external pressure (1) over the tendinous insertions in the occipital area, (2) along the course of the major occipital nerve, (3) over the groove immediately behind the mastoid process, (4) over the upper part of the sternocleidomastoid muscle on the symptomatic side, and (5) over the lateral aspect of a cervical zygapophyseal joint. Pain may be precipitated intrinsically by neck movements and/or sustained, awkward head positioning, especially during sleep.
The duration of pain attacks, a few hours to a few weeks, and pain intensity, vary widely in CH with a strong tendency toward chronicity. CH can present as episodic in the initial phase becoming chronic in later stages. The pain usually starts in the neck, eventually spreading to the oculofrontotemporal area. Symptoms may actually be more intense in this latter location than in the occipital or cervical region.1,12 The duration of pain episodes for CH is frequently longer than in common migraine; the pain intensity is moderate and nonexcruciating, unlike cluster headache, and it is usually of a nonthrobbing nature. Autonomic symptoms and signs, such as photophobia, phonophobia, nausea, vomiting, and ipsilateral periocular edema, are generally less frequent and less marked than in common migraine, but they can occur.1,2,12,21,22,73 Swallowing difficulty is reported, albeit rarely occurring associated phenomenon.74 There have also been cases with features consistent with a CH picture, but with additional dizziness/vertigo and vertebral drop-attacks.8,73
In the revised criteria, among the ‘Other Important Characteristics’ (see Table 69.2), the lack of complete response to indomethacin, sumatriptan, and ergotamine has also been introduced.
Authors’ recommendation
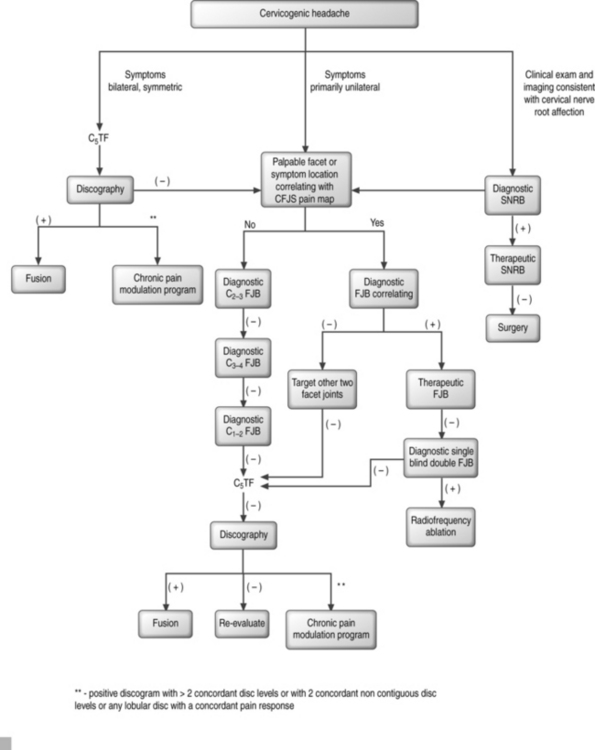
Formulating a probability analysis of the structures involved is the most important step in the diagnostic and therapeutic algorithm (Fig. 69.3). Unilateral symptoms of occipital headaches greater than neck pain following a traumatic event is more suggestive of cervical facet joint syndrome (CFJS) than internal disc disruption.34,70 Similarly, one may suspect CFJS more than internal disc disruption if there is focal tenderness following palpation of an isolated cervical joint or if the patient is able to point to the painful area corresponding to the distribution of pain reported for a particular facet joint.75 Examination finding of increased focal suboccipital pain with terminal cervical flexion and sequential lateral rotation suggests pain emanating from a C1–2 joint. Bilateral symptoms of neck pain with headaches would be more suggestive of cervical internal disc disruption (CIDD) syndrome. Reproduction of symptoms by provocative maneuvers, which facilitate closure or narrowing of the neuroforamina, are positive, then nerve root involvement rather than facet joint syndrome or internal disc disruption syndrome is of higher probability. In whiplash injuries, CFJS may be more common than upper cervical nerve root injury;34,76 however, this may be a consequence of the paucity of epidemiological data for whiplash-induced cervical nerve root injury.
Diagnostic testing
Cervical spine radiographs are not a sensitive method for diagnosing CH, because no specific radiologic abnormalities are usually found.77,78 In patients with a history of whiplash injury or traumatic insult, cervical flexion and extension radiographs should be obtained. CH is a diagnosis of exclusion, and more serious pathologies must be ruled out. Magnetic resonance imaging is indicated to search for causes of pain that may require surgery or other more aggressive forms of treatment (i.e. Arnold–Chiari malformation, herniated intervertebral disc, spinal or neural foraminal stenosis, vertebral or facet fracture, and intramedullary or extramedullary spinal tumors).79,80 Although MRI has a high sensitivity to detect focal disc protrusions, foraminal stenosis, facet joint arthropathy or other pathology, there are concerns about its clinical specificity. Several studies have demonstrated that a significant percentage of individuals with focal protrusions or foraminal stenosis are asymptomatic.39 This issue of clinical specificity underscores the importance of correlating each patient’s symptoms and examination findings with the imaging study to obtain an accurate clinical diagnosis. If the patient is claustrophobic, then a high-quality open MRI is obtained. If this is unavailable, then a multi-planar computerized tomography (CT) scan is requested.
Diagnostic anesthetic blockade is required to confidently render a diagnosis of cervicogenic headache. Diagnostic blockade can be directed to several anatomic structures such as the greater occipital nerve (dorsal ramus C2), lesser occipital nerve, atlanto-occipital joint, atlantoaxial joint, cervical segmental nerve roots, third occipital nerve (dorsal ramus C3), zygapophyseal joint(s), or intervertebral discs based on the clinical characteristics of the pain and the physical examination.41 Fluoroscopic guidance with and without contrast is necessary to assure accurate and specific localization of the pain source.28,81,82 Diagnostic blocks use small amounts of local anesthetic that are infused into or around the suspected nerve or structure with the goal of temporarily interrupting head pain. Because diffusion of the anesthetic to adjacent structures would muddle the results, it is essential to use the least amount of local anesthetic feasible to limit the anesthetic affect to the target site. The most recent diagnostic criteria for cervicogenic headache require the use of confirmatory nerve blocks.12 Although this approach is quite appealing it does not offer 100% accuracy.41,83 False-negative and false-positive responses to diagnostic blocks do occur and can result due to technical failure, placebo response (see Ch. 19), administration of sedative agents before or during the diagnostic block, concurrent pharmacologic treatment, and secondary psychosocial factors.
TREATMENT
The successful treatment of cervicogenic headache requires a multifaceted approach using pharmacologic, nonpharmacologic, manipulative, anesthetic, and occasionally surgical interventions (Table 69.5). Some have suggested that the multidisciplinary treatment team consist of a primary treating physician, interventional spine physician, a physical therapist or physician appropriately trained to provide manipulative treatments, and a psychologist.63,84–87 Medications alone are often ineffective or provide only modest benefit. Positive diagnostic blocks, as outlined in diagnostic testing, may direct treatment toward more invasive interventional or neuroablative therapy. The diagnostic and treatment algorithm as recommended by the authors is outlined in Figure 69.3.
Table 69.5 Potential Treatment Interventions for Cervicogenic Headache
| PHARMACOLOGIC | |
| Tricyclic antidepressants: amitriptyline, nortriptyline, doxepin, and others | |
| Antiepileptic drugs: gabapentin, topiramate, carbamazepine, divalproex sodium | |
| Muscle relaxants: tizanidine, baclofen, cyclobenzaprine, metaxalone, and others | |
| Nonsteroidal anti-inflammatory drugs | |
| Nonselective: ibuprofen, naproxen, indometacin, and others | |
| COX-2 selective: valdecoxib, celecoxib | |
| NONPHARMACOLOGIC | |
| Manipulative therapies | |
| Physical therapy | |
| Transcutaneous electrical nerve stimulation | |
| Biofeedback/relaxation therapies | |
| Individual psychotherapy | |
| INTERVENTIONAL | |
| Anesthetic blockades | |
| Spinal roots, nerves, rami, or branches | |
| Zygapophyseal joints | |
| Trigger points | |
| Neurolytic procedures | |
| Radiofrequency thermal neurolysis | |
| Cryoneurolysis | |
| Botulinum toxin injections | |
| SURGICAL | |
| Neurectomy | |
| Dorsal rhizotomy | |
| Microvascular decompression | |
| Nerve exploration and ‘release’ | |
| Zygapophyseal joint fusion | |
Interventional, anesthetic, and ablative treatment
Cervical zygapophyseal joints
Of the potential sources of CH, the zygapophyseal joints have been considered a common source of referral to the head and face. Prior studies have noted significant variations in the referral patterns of the zygapophyseal joints. Dwyer et al. distended the C2–3 to C6–7 zygapophyseal joints in asymptomatic patients to report that only the C2–3 zygapophyseal joint referred symptoms to the head.31 Aprill et al. investigated the C2–3 to C7–T1 zygapophyseal joints and reported that the C2–3, C3–4, and C4–5 zygapophyseal joints could refer to the head.32 Dreyfuss et al. stimulated the atlanto-occipital and atlantoaxial joints by injection of radiopaque contrast with subsequent distention of the joint capsule. Both joints tended to refer pain into the ipsilateral occipital or suboccipital area, with possible referral into the face.88 Bogduk et al. performed medial branch and zygapophyseal joints blocks in 24 symptomatic patients from C1–2 to C5–6 levels and reported that the C2–3 zygapophyseal joints refers to the head and face while the C3–4 zygapophyseal joints only refers to the head.89 Slipman et al., reviewing data from 100 patients demonstrated that C4–5 and C5–6 zygapophyseal joints can refer pain to the head and the C1–2, C2–3, and C3–4 zygapophyseal joints can also refer pain to the face.33
The cervical facet joints, except for the C2–3 level, are innervated by the medial branch of the dorsal ramus above and below its location. At the C2–3 level, two medial branches innervate the joint: the C3 medial branch (deep) and the C3 medial branch (superficial), also known as the TON.25,61,90 Confirmation of joint involvement in CH is through unequivocal relief of head pain after the local anesthetic block of the joint. When upper CFJS is suspected, diagnostic blocks are performed sequentially at C2–3, C3–4, and C1–2 levels, until the offending site is identified. This sequence is based on clinical experience and is supported by epidemiological studies.70
If a diagnostic facet joint block is positive, fluoroscopically guided therapeutic intra-articular steroid injections are offered. Barnsley et al. investigated the effectiveness of intra-articular corticosteroid injections for chronic pain in the cervical zygapophyseal joints.91 Less than half the patients reported relief of more than 1 week and less than one in five patients reported relief for more than a month. They concluded that intra-articular injections with betamethasone was an ineffective treatment for pain in the cervical zygapophyseal joints. However, this study used one outcome measure, i.e. verbal pain score, and only evaluated the efficacy of one intra-articular steroid injection per joint without restricting physical activities or physical therapy. In our experience, fluoroscopically guided therapeutic intra-articular steroid injections have been efficacious in the treatment of CFJS. Slipman et al.92 demonstrated good to excellent results in 61% of patients treated with intra-articular steroids who experienced daily unremitting headaches emanating from the C2–3 facet joint subsequent to a whiplash injury. In that study, the average duration of symptoms was 3 years and no patient obtained relief with any analgesics prior to the injections. Most patients received 1–3 injections per joint. Although the change in average pain score (5.5 at follow-up compared with 8.2 at the time of initial presentation) does not seem to be a significant clinical difference, the frequency of patient’s headaches and their responsiveness to analgesic use were clearly improved. Patients with previous employment restrictions were observed to return to full-time work status. During treatment, patients in this study92 were advised to avoid forceful, rapid, or sustained cervical extension or rotation whenever possible. The basis for such a strict protocol is the observation that CFJS, especially when associated with whiplash injury, may be associated with subchondral fractures,93,94 joint capsule ruptures,95,96 and intra-articular hemorrhages.71,95 These structural insults may be responsible in triggering zygapophyseal joint headaches when stressed by overactivity or exercise. When the symptoms are reduced, the patient gradually returned to engaging in normal physical tasks rather than letting the patient participate in unregulated physical activities. If a patient experiences greater than 90% relief of symptoms after a therapeutic intra-articular facet injection that lasts until the date of a planned second, third, or even fourth subsequent injection, then the intervention is cancelled. Such relief typically heralds the onset of continued symptom relief provided the patient adheres to specific activity prohibitions and patiently returns to a normal activity level. As previously alluded, this regimen is conducted under direct physician supervision and must be individualized. Overall, it can take 6–12 months after the final injection before premorbid activities and habits can be resumed.
Patients who have responded to diagnostic/therapeutic blocks of the zygapophyseal joints with unequivocal but unsustained relief of head and neck pain may be good candidates for radiofrequency (RF) neurotomy. Because the authors perform intra-articular injections following a single positive diagnostic injection, the major question that must be addressed when a patient fails to improve following therapeutic injections is whether the patient failed because of an incorrect diagnosis or if he or she is a true nonresponder with a false-positive placebo response. The former issue is raised because there is a false-positive rate of 27% with single diagnostic facet joint blocks.97 Therefore, it is conceivable the initial diagnostic block was a false-positive response. Accordingly, the authors advocate a double-blind, double-block diagnostic injection utilizing 2% lidocaine intra-articularly and normal saline extra-articularly for definitive confirmation of the diagnosis, before undertaking ablative procedure. This double block is considered positive if the lidocaine injection relieves the pain and the saline injection does not. In summary, all the patients progressing to double block have had a positive single block, have been treated with intra-articular steroids and failed to progress, and then participate in this double block procedure. So the authors are essentially doing a triple-block paradigm. If the double block is positive, the patient is a candidate for RF ablation of the medial branches of the dorsal rami supplying the involved facet joint. If the double block is negative, the next suspected structure in the diagnostic algorithm should be assessed.
The use of RF techniques in treating cervicogenic headache resulting from cervical zygapophyseal joint is described by Hapeslagh and van Kleef in Chapter 65. An RF cannula is inserted to the target nerve under fluoroscopic guidance. An RF generator then heats the surrounding tissue under controlled conditions that allows the formation of a discrete lesion to interrupt all sensory pathways to the joint. Two to three lesions at 75–80°C for 60–90 seconds are typically made because the exact location of the nerve may vary.98 In a randomized, double-blind trial, Lord et al.99 demonstrated that the pain relief realized following percutaneous RF neurotomy in patients with chronic cervical zygapophyseal joint pain confirmed with double-blind, placebo-controlled local anesthesia, is real and not a placebo effect. The median time that elapsed before the pain returned to at least 50% of the preneurotomy level in the active-treatment group was 9 months as compared to 1 week in the sham-control group. Using a prospective cohort methodology McDonald et al.100 subsequently reported complete relief of pain in 71% of properly selected patients. The median duration of relief after a first procedure was 219 days when failures were included, but 422 days when successful cases were considered. Repeat procedures were effective in each instance. They concluded that RF neurotomy provides a clinically significant period of freedom from pain, and its effects can be reinstated if pain recurs. Patients whose pain was not relieved by the first procedure did not respond well to a second procedure. It is interesting to note that the outcome was not affected by litigation, status, or type of diagnostic block used. The contention that the potential for secondary gain does not influence response to treatment was further demonstrated by Sapir and Gorup101 in their prospective study of 46 patients with cervical zygapophyseal joint pain from whiplash treated with RF medial branch neurotomy. Radiofrequency cervical medial branch neurotomy markedly reduced the pain from whiplash in the study patients (both litigation and nonlitigation group) with an overall reduction of VAS 5.7±1.8. At 1 year the combined reduction from baseline in VAS score of 4.6±1.8 was still significant. When evaluating the reduction in pain from baseline at 1 year there was a statistical difference between groups, with the litigant group having a tendency for greater return of pain. This difference, however, is only a change in a VAS score of 1.4 between groups, which would be clinically difficult to differentiate. The overall change at 1 year from the pre-RF (baseline) score was still greater than 4 VAS points for the litigation group compared with 5.5 VAS points for the nonlitigation group. Clinically both represent significant reductions in pain.
In a retrospective study evaluating the effectiveness of percutaneous radiofrequency neurotomy in the treatment of cervical zygapophyseal joint pain, Lord et al.98 noted a high failure rate of third occipital neurotomy for the treatment of C2–3 zygapophyseal joint pain. Of the 10 patients who underwent third occipital neurotomy, only four obtained relief lasting for more than 6 months. Ataxia was a regular side effect of third occipital neurotomy. It is unclear from the study whether ataxia was associated with long-lasting pain relief or occurred even in absence of sustained pain relief. All patients with ataxia were able to accommodate it by relying on visual cues, some within a few hours but others after 2–3 days. One patient continued to experience disorientation whenever challenged by visually complex situations, such as walking down stairs or turning quickly. The cause of ataxia is unclear. The rate of technical failure was considerably higher for third occipital neurotomies than for lower cervical medial branch neurotomies in the study by Lord et al.98 In this regard, Lord et al.98 recommend that third occipital neurotomy be abandoned until the technical problems can be overcome. Possible reasons for this high rate of failures include the relatively larger diameter of the nerve (1.5–2.0 mm) and its variable course. In contrast to the lower cervical medial branches that course around the concave waists of the cervical articular pillars, the TON curves around the convex capsule of the C2–3 joint. Thus, its course may vary, running higher or lower than the equator of the joint. In addition, because the TON lies superficial to the joint capsule, an electrode placed immediately adjacent to the bony margin of the joint may fail to incorporate the nerve by passing deep to the capsule, thereby displacing the nerve from the electrode. The authors routinely perform radiofrequency ablation of the TON for C2–3 joint pain, following positive diagnostic blocks and failure of steroid response as outlined above. In the authors’ experience, the results of third occipital neurotomies are comparable to lower cervical medial branch neurotomies and ataxia is not a major reported side effect. There is sufficient merit in the third occipital neurotomy procedure to justify a randomized, double-blind, controlled trial.
Atlanto-occipital and atlantoaxial joints
The atlanto-occipital and atlantoaxial joints are involved, respectively, in the flexion–extension and horizontal rotation of the head. These two joints are innervated by the C1, C2, and C3 spinal nerve roots and can be a source of CH.25,32,88 Racz et al.102 reported that the atlanto-occipital and atlantoaxial joint headaches are rarely seen and frequently misdiagnosed. Aprill et al.103 tested the hypothesis that C1–2 headaches are a rare entity. Thirty-four patients with suspected C1–2 pain underwent diagnostic blocks of the joint with a local anesthetic and steroid. Twenty-one patients obtained complete relief of headache. Pain relief lasted for the duration of the injected local anesthetic. The overall incidence of carefully selected patients who had pain in the occipital or suboccipital region resulting from atlantoaxial joint pain was reported as 16%.
The technique for injecting these joints has been described by Racz et al.102 and Dreyfuss et al.104 Potential complications include: (1) injury to the brain stem, vertebral artery, or spinal cord; (2) intravascular injection of anesthetic or steroids that results in central nervous system toxicity or stroke; and (3) inadvertent epidural and intrathecal injections. Because of the close proximity of these joints to major neural and vascular structures, these procedures should only be performed by physicians who have great experience in the use of fluoroscopic-guided injection techniques.
Apart from corticosteroid injections, there do not seem to be any other reliable therapeutic options. A specific RF procedure or other neuroablative techniques for the atlantoaxial and atlanto-occipital joints have not been developed.105
Cervical discs
Cervical discs have been implicated as a possible source of cervicogenic headache,25,36 and cervical epidural steroid injections (CESIs) are preferred treatment of discogenic pain by many clinicians. CESIs have been used with success rates ranging from 40% to 75% for treatment of axial and radicular neck symptoms.106,107 However, the use of CESIs in the diagnosis and treatment of CH remains controversial. Cronen and Waldman reported the efficacy of CESIs in the treatment of pain secondary to tension-type headaches108 and as a diagnostic tool in the evaluation of head, neck, and face pain.109 Martelleti et al., who advocated the theory of the role of inflammation in CH, presented a small series on CESI and reported some relief.110 Reale et al.111 further suggested that epidural steroids are an effective treatment for CH because of their antiinflammatory effects combined with their direct effect on C fibers to block their nociceptive transmission. They report short-term (2 months) pain relief with few risks or side effects. However, van Suijlekom41 pointed out that a cervical epidural nerve block is not selective, can result in life-threatening situations, and should not be used in the standard diagnostic work-up for cervicogenic headache. The authors concur with that view for two reasons. First, an epidural injection anesthetizes in a widespread and indiscriminate fashion, precluding any determination as to which anatomic structure is the offending site. Second, a large deposition of glucocorticoid will affect these numerous structures and may even have a systemic effect. Any alleviation of symptoms could very well be a consequence of this systemic response. Despite the absence of controlled, prospective, double-blind studies to assess the therapeutic benefit of CESIs in the treatment of head and neck pain, they are the preferred treatment of many clinicians. As elucidated in the chapter by Kenneth Botwin, extreme caution needs to be used when planning to do a cervical epidural injection. All CESIs should be performed under real-time fluoroscopic guidance using contrast to avoid intravascular injection which can result in potentially devastating neurological consequences. Potential complications of CESIs include: (1) inadvertent dural puncture with postdural puncture headache or intrathecal injection of local anesthetic and steroids; (2) intravascular injection, epidural hematoma, or abscess resulting in spinal cord compression; and (3) direct trauma to the spinal cord or nerve root. Similar to other nerve blocks in the cervical region, routine sedation should be avoided or kept to a minimum.
The authors prefer using the transforaminal approach to the epidural space. For those patients suspected of experiencing CH symptoms secondary to discogenic etiology, a fluoroscopically guided transforaminal steroid injection at the C5 level on the symptomatic ipsilateral side is recommended to bathe the posterior surface of the upper intervertebral discs, posterior longitudinal ligament, and other nearby innervated structures. The insertion point for this injection is predicated upon the locations of symptoms and the likely disc levels that are involved.112 The C5 level is chosen when the headaches extended beyond the posterior occiput. When the headache symptoms involve the prefrontal/supraorbital distribution or the face, a C6 approach is use. In the former circumstance the therapeutic agents need to cover from C5 and cephalad. In the latter circumstance, the spread should be viewed cephalad and at least one level caudal such that it reaches the C7 vertebral body. Another critical aspect is insuring that flow is ventral and not dorsal to the spinal cord. When the aforementioned steps have been undertaken and a steroid effect is not realized after two injections, the patient will require provocative cervical discography to determine if there is internal disc disruption, answering the questions of whether the diagnosis was accurate, which levels are involved and whether surgery is a viable alternative. If the discogram reveals one or two successive disc levels with concordant pain responses, the patient may be a candidate for surgical fusion. If the discogram demonstrates discs without concordant pain responses, the next suspected structure in the diagnostic algorithm should be tested. When a discogram reveals three or more concordant disc levels, two disc levels with an intervening normal disc, or if any concordantly painful discs are lobular, the patient is not considered as a surgical candidate.
Blume113 has reported the use of multiple RF lesions to the cervical disc at the C2–3 and C3–4 level. Six to eight lesions at 80°C for 3 minutes were performed. He described complete to partial relief of CH for 2–6 months. Side effects of cervical disc RF lesions include disc infection, nerve root injury, and temporary postoperative neuritis. Potential side effects and complications associated with this procedure, when weighed against the outcome, justify the lack of popularity of RF lesions of upper cervical discs for CH.
Cervical segmental nerve
Klein105 reported, in a nonpeer-reviewed publication, that percutaneous radiofrequency lesions of the cervical dorsal root ganglion at the C2 and C3 levels may be helpful in the treatment of C2- and C3-pattern headache. The goal of RF of the dorsal root ganglion is to interrupt the pain pathways carried by small fiber systems while maintaining sensory and motor function. He reported no significant side effects. However, this technique has not gained in popularity. The authors suspect this is because there is not a single peer-reviewed publication remotely substantiating this procedure.
Greater occipital nerve and lesser occipital nerve
Management of the greater and lesser occipital nerves has varied greatly. Diverse treatments have included infiltration of the affected nerves with local anesthetic and steroids,16,46,114 occipital neurectomy,115 occipital neurolysis,45,116 RF denaturation of the greater, lesser, and least occipital nerves,113 microsurgical C2 ganglionectomy,117 partial posterior rhizotomy of C1–3 nerve,118 and peripheral nerve stimulation (PNS).119,120 Many patients with occipital neuralgia will respond to infiltration of local anesthetic and steroids in the distribution of the GON and LON. A number of small case series have reported on the injection of the occipital nerves where short-term improvement was noted in 50–90% of patients.16,28 A more permanent neuroablative technique can be considered for intractable occipital neuralgia that consistently responds to diagnostic nerve block. Performing neurolytic blocks using alcohol, or phenol, and surgical occipital neurectomy have lost favor because of recurrence, deafferentation syndrome, and neuroma formation. Liberation of the greater occipital nerve initially relieves headache in approximately 80% of cases, but the relief has a median duration of only 3–6 months.45 Excision of the greater occipital nerve provides relief in approximately 70% of patients, but this has a median duration of only 244 days.46 Reports of the treatment of intractable, idiopathic occipital neuralgia are mixed. Dorsal rhizotomy at C1–3 or C1–4 has provided some patients with complete relief for 1–4 years; nevertheless some patients suffer recurrences.121 Partial posterior rhizotomy at C1–3 seems to achieve good relief while preserving touch sensation, but not all patients respond adequately.118 Unfortunately, these procedures are so radical that they provide little insight into the mechanisms of occipital neuralgia, except to warn that even complete deafferentation of the affected region does not guarantee relief of pain. Hence, the authors do not routinely include diagnostic work-up or treatment of the greater and lesser occipital nerves in the authors’ algorithm.
CONCLUSIONS
Despite a growing body of literature on CH and an increasing acceptance that headaches can originate from the cervical spine, there remains considerable controversy and confusion concerning most aspects of this topic. Anesthetic blockade plays an essential role in the diagnosis and treatment of cervicogenic headache. A positive or negative response to a diagnostic block must be considered in conjunction with the complexity of the patient with chronic headache, the placebo effect, and concurrent medical therapy before proceeding with more invasive interventional or neuroablative treatment. It is essential to have a profound knowledge of the anatomy of the cervical spine, an understanding of the pathophysiologic mechanisms of cervicogenic headache and a high degree of technical skill before performing these procedures. Serious life-threatening and neurologic injuries have been reported and this must be weighed against the benefits of treatment. Conservative therapeutic options should be attempted first. Acknowledging that conducting randomized, double-blind, controlled surgical trials are difficult, it is important that further studies are done if interventional techniques are to become standard and accepted therapies for cervicogenic headache. As the literature on this topic grows in volume and quality, the debate will intensify and, hopefully, will result in the clarification of the cause, diagnosis, and treatment of CH.
1 Sjaastad O. Cervicogenic headache: diagnostic criteria. Headache. 1990;30:725-726.
2 Sjaastad O. Cervicogenic headache a critical view on pathogenesis. Funct Neurology. 1998;13:71-74.
3 Pearce JM. Cervicogenic headache: an early description. J Neurol, Neurosurg Psychiatr. 1995;58(6):698.
4 Hunter C, Mayfield F. Role of the upper cervical roots in the production of pain in the head. Am J Surg. 1949;48:743-751.
5 Bärtschi-Rochaix W. Migraine cervicale, das encéphale Syndrome nach Halswirbeltrauma. Huber: Bern, 1949.
6 Kovacs A. Subluxation and deformation of the cervical apophyseal joints: a contribution to the etiology of headache. Acta Radiologia. 1955;43:1-16.
7 Bogduk N, Marsland A. On the concept of third occipital headache. J Neurol, Neurosurg Psychiatr. 1986;49(7):775-780.
8 Sjaastad O, Saunte C, Hovdahl H, et al. ‘Cervicogenic’ headache. A hypothesis. Cephalgia. 1983;3:249-256.
9 International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias, and facial pain. Cephalgia. 1988;8(Suppl 7):1-96.
10 Merskey H, Bogduk N. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. In Merskey H, Bogduk N, editors: Cervicogenic headache, 2nd edn., Seattle: IASP, 1994.
11 Meloche J, Bergeron Y, Bellavance A, et al. Painful intervertebral dysfunction: Robert Maigne’s original contribution to headache of cervical origin. The Quebec Headache Study Group. Headache. 1993;33(6):328-334.
12 Sjaastad O, Fredriksen T, Pfaffenrath V. Cervicogenic headache: diagnostic criteria. The Cervicogenic Headache International Study Group. Headache. 1998;38(6):442-445.
13 Sjaastad O, Fredriksen T. Cervicogenic headache: criteria, classification and epidemiology. Clin Exp Rheumatol. 2000;18(2Suppl 19):S3-S6.
14 Nilsson N. The prevalence of cervicogenic headache in a random population sample of 20–59 year olds. Spine. 1995;20:1884-1888.
15 Kränzlin P, Wälchli B. The concept of cervicogenic headache. Annual postgraduate course of the association of Swiss chiropractors. Interlaken, Switzerland. 1993:13.
16 Anthony M. Cervicogenic headache: prevalence and response to local steroid therapy. Clin Exp Rheumatol. 2000;18(2Suppl 19):S59-S64.
17 Leone M, D’Amico D, Grazzi L, et al. Cervicogenic headache: a critical review of the current diagnostic criteria. Pain. 1998;78(1):1-5.
18 Rothbart P. Cervicogenic headache: a pain in the neck. Can J Diagnostics. 1996;13(2):64-66. 71–76
19 Bono G, Antonaci F, Ghirmai S, et al. The clinical profile of cervicogenic headache as it emerges from a study based on the early diagnostic criteria (Sjaastad et al. 1990). Funct Neurology. 1998;13(1):75-77.
20 Pfaffenrath V, Kaube H. Diagnostics of cervicogenic headache. Funct Neurology. 1990;5(2):159-164.
21 Fredriksen T. ‘Cervicogenic headache’: clinical manifestation. Cephalgia. 1987;7:147-160.
22 Pfaffenrath V. Cervicogenic headache – the clinical picture, radiological findings and hypotheses on its pathophysiology. Headache. 1987;27:495-499.
23 Vincent M. Cervicogenic headache: a comparison with migraine and tension-type headache. Cephalgia. 1999;19(Suppl 25):11-16.
24 Haldeman S, Dagenais D. Cervicogenic headaches: a critical review. Spine J. 2001;1:31-46.
25 Bogduk N. Cervicogenic headache: anatomic basis and pathophysiologic mechanisms. Curr Pain Headache Rep. 2001;5:382-386.
26 Kerr FWL. Relation of the trigeminal spinal tract to upper cervical roots and the solitary nucleus in the cat. Exp Neurol. 1961;4:134-148.
27 Fredriksen T, Salvesen R, Stolt-Nielsen A, et al. Cervicogenic headache: long-term postoperative follow-up. Cephalgia. 1999;19(10):897-900.
28 Bovim G, Berg R, Dale L. Cervicogenic headache: anesthetic blockades of cervical nerves (C2–C5) and facet joint (C2/C3). Pain. 1992;49(3):315-320.
29 Freund B, Schwartz M. Treatment of chronic cervical-associated headache with botulinum toxin A: a pilot study. Headache. 2000;40(3):231-236.
30 Silverman S. Cervicogenic headache: Interventional, anesthetic, and ablative treatment. Curr Pain Headache Rep. 2002;6:308-314.
31 Dwyer S, April C, Bogduk N. Cervical zygapophyseal joints pain patterns I: A study in normal volunteers. Spine. 1990;15:453-457.
32 April C, Dweyer A, Bogduk N. Cervical zygapophyseal joint pain patterns II: A clinical evaluation. Spine. 1990;15:458-461.
33 Slipman C, Isaac Z, Thomas J, et al. Abstract: Cervical zygapophyseal joint syndrome and referral to the head and face. Preliminary data from 100 patients. Arch Phys Med Rehabil. 2002;83:1665.
34 Lord S, Barnsely L, Wallis B, et al. Chronic cervical zygapophyseal joint pain after whiplash. A placebo-controlled prevalence study. Spine. 1996;21(15):1737-1745.
35 Lord S, Barnsely L, Wallis B, et al. The prevalence of chronic cervical zygapophyseal joint pain after whiplash. Spine. 1995;20(1):20-23.
36 Biondi D. Cervicogenic headache: diagnostic evaluation and treatment strategies. Curr Pain Headache Rep. 2001;5:361-362.
37 April C. Diagnostic disk injection. In: Frye A, Moyer J, editors. The adult spine: principles and practice. New York: Raven Press; 1991:403-440.
38 Grubb S, Kelly C. Cervical discography: Clinical implications from 12 years of experience. Spine. 2000;25:1382-1389.
39 Schellhas K, Smith S, Gundry C, et al. Cervical discogenic pain. Prospective correlation of magnetic resonance imaging and discography in asymptomatic subjects and pain sufferers. Spine. 1996;21:300-312.
40 Slipman C, Bhagia S, Plastaras C. Provocative cervical discographic symptom mapping. Chicago: Presented at the Annual meeting of the Am Acad Phys Med Rehab; October 2003.
41 van Suijlekom J. Cervicogenic headache: techniques of diagnostic nerve blocks. Clin Expl Rheumatol. 2000;18(Suppl 19):S39-S44.
42 Poletti C. C2 and C3 pain dermatomes in man. Cephalgia. 1991;11:155-159.
43 Netter F. Suboccipital triangle. In: Netter F, ed. Atlas of human anatomy. 2nd edn. 2000: Plate 164.
44 Jundt J, Mock D. Temporal arteritis with normal erythrocyte sediment rates presenting as occipital neuralgia. Arthritis Rheum. 1991;34:217-219.
45 Bovim G. Neurolysis of the greater occipital nerve in cervicogenic headache:a follow-up study. Headache. 1992;32:175-179.
46 Anthony M. Headache and the greater occipital nerve. Clinical Neurol Neurosurg. 1992;94:297-301.
47 Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine. 1982;7(4):319-330.
48 Saadah H, Taylor F. Sustained headache syndrome associated with tender occipital nerve zones. Headache. 1987;27:201-205.
49 Gawel M, Rothbart P. Occipital nerve block in the management of headache and cervical pain. Cephalgia. 1992;12:9-13.
50 Lord S, Barsley L, Wallis B, et al. Third occipital nerve headache: a prevalence study. J Neurol Neurosurg Psychiatr. 1994;57:1187-1190.
51 Hack G. Cervicogenic headache: new anatomical discovery provides the missing link. Chiroprac Rep. 1998;12(3):1-3.
52 Mitchell B, Humphreys B, O’Sullivan E. Attachments of the ligamentum nuchae to cervical posterior spinal dura and the lateral part of the occipital bone. J Manipul Physiol Ther. 1998;21(3):145-148.
53 Alix M, Bates D. A proposed etiology of cervicogenic headache: the neurophysiologic basis and anatomic relationship between the dura mater and the rectus posterior capitis minor muscle. J Manipul Physiol Ther. 1999;22(8):534-539.
54 Saal J, Franson R, Dobrow R, et al. High levels of inflammatory phospholipase A2 activity in the lumbar disc herniation. Spine. 1990;15:674-678.
55 Franson R, Saal J, Saal J. Human disc phospholipase A2 is an inflammatory. Spine. 1992;17(Suppl):129-132.
56 Kang J, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271-275.
57 Martelletti P, La Tour D, Giacovazzo M. Spectrum of pathophysiological disease in cervicogenic headache and its therapeutic indications. Neuromusculoskeletal Symp. 1995;3:182-187.
58 Martelletti P, Stirparo G, Favilla M. Expression of NOS-2, COX-2, and Th1/Th2 cytokines in migraine. J Headache Pain. 2001;2:S51-S56.
59 Martelletti P. Proinflammatory pathways in cervicogenic headache. Clin Exp Rheumatol. 2000;18(2):S33-S38.
60 Martelletti P, Stirparo G, Giacovazzo M, et al. Proinflammatory cytokines in cervicogenic headache. Funct Neurol. 1999;14(3):159-162.
61 Bogduk N. The anatomical basis for cervicogenic headache. J Manipul Physiol Ther. 1992;15(1):67-70.
62 Grillo F. The differential diagnosis and therapy of headache. Swiss Ann Chiroprac. 1961;11:121-166.
63 Vernon H. Spinal manipulation and headaches of cervical origin. J Manipul Physiol Ther. 1989;12(6):455-468.
64 Bitterli J, Graf R, Robert F, et al. Objektivierung der manualtherapeutischen Beeinflussbarkeit des spondylogenen Kopfschmerzes. Nervenarzt. 1977;48(5):159-162.
65 Hogan L, Beland I. Cervical spine syndrome. Am J Nurs. 1976;76(7):1104-1107.
66 Dutton C, Riley L. Cervical migraine. Not merely a pain in the neck. Am J Med. 1969;47(1):141-148.
67 Bogduk N, Corrigan B, Kelly P, et al. Cervical headache. Med J Aust. 1985;143(5206–202):207.
68 Stevans J. The effects of remote locomotor rehabilitation in a chronic cervicogenic syndrome: a case report. Chiroprac Tech. 1996;8(3):121-124.
69 Chouret E. The great occipital neuralgia headache. Headache. 1967;7(1):33-34.
70 Barnsley LS, Wallis BJ, et al. Chronic zygapophyseal joint pain after whiplash: a prospective prevalence study. Spine. 1995;20:20-26.
71 Taylor JR. Acute injuries to cervical joints. An autopsy study of neck sprains. Spine. 1993;18:1115-1122.
72 Spitzer WO, Salmi LR, Cassidy JD, et al. Scientific monograph of the Quebec Taskforce on Whiplash associated discorders: Redifining whiplash and its management. Spine. 1995;20:10S-68S.
73 Fredriksen T. Cervicogenic headache (CEH): notes on some burning issues. Funct Neurol. 2000;15:199-203.
74 Michler R. Disorder in the lower cervical spine. A cause of unilateral headache? Headache. 1991;31:550-551.
75 Jull GB. The accuracy of manual diagnosis for cervical zygapophyseal joint pain syndromes. Med J Aust. 1988;148:233-236.
76 Slipman C, Plastaras CT, Huston CW, et al. Outcomes of nerve root blocks for whiplash induced cervical radiculitis. Presented at the 11th annual meeting of the North American Spine Society. 1996.
77 Pfaffenrath V, et al. Cervicogenic headache: results of a computer-based measurements of cervical spine mobility in 15 patients. Cephalgia. 1988;8:45-48.
78 Fredriksen T. Cervicogenic headache. Radiological investigation concerning head/neck. Cephalgia. 1989;9:139-146.
79 Delfini R, Salvati M, Passacantilli E, et al. Symptomatic cervicogenic headache. Clin Exp Rheumatol. 2000;18(Suppl 19):S29-S32.
80 Stovner L. Headache associated with the Chiari type I malformation. Headache. 1993;33:175-181.
81 Stolker R. The management of chronic spinal pain by blockades: a review. Pain. 1994;58:1-20.
82 Schellhas K. Facet nerve blockade and radiofrequency neurotomy. Neuroimag Clin N Am. 2000;10:493-501.
83 Hogan Q, Abram S. Neuroblockade for diagnosis and prognosis: a review. Anesthesiology. 1997;86:216-241.
84 Roberts A. Behavioral management of chronic pain and excess disability: long-term follow-up of an outpatient program. Clin J Pain. 1993;9:41-48.
85 Nilsson N. A randomized controlled trial of the effect of spinal manipulation in the treatment of cervicogenic headache. J Manipul Physiol Ther. 1995;18(7):435-440.
86 Nilsson N. The effect of spinal manipulation in the treatment of cervicogenic headache. J Manipul Physiol Ther. 1997;20:326-330.
87 Howe D, Newcombe R, Wade M. Manipulation of the cervical spine – a pilot study. J Roy Coll Gen Pract. 1983;33:574-579.
88 Dreyfuss P, Michaelsen M, Fletcher D. Atlanto-occipital and lateral axial joint pain patterns. Spine. 1994;19:1125-1131.
89 Bogduk N, Marsland A, Ffaracs D. The cervical zygapophyseal joints as a source of neck pain. Spine. 1988;13:610-617.
90 Barnsley L. Comparative local anesthetic blocks in the diagnosis of cervical zygapophyseal joint pain. Pain. 1993;55:99-106.
91 Barnsley L, Lord S, Wallis B, et al. Lack of effect of intraarticular corticosteroids for chronic pain in the cervical zygapophyseal joints. N Engl J Med. 1994;330:1047-1050.
92 Slipman C, Lipetz J, Plastaras C, et al. Therapeutic zygapophyseal joint injection for headaches emanating from the C2–3 joint. Am J Phys Med Rehabil. 2001;80(3):182-188.
93 Abel M. Moderately severe whiplash injuries of the cervical spine and their roentgenologic diagnosis. Clin Ortho. 1958;12:189-208.
94 Woodring JH. Fractures of articular processes of the cervical spine. Am J Roentgenol. 1982;139:341-344.
95 Jonsson HJr, Rauschning W, et al. Hidden cervical spine injuries in traffic accident victims with skull fractures. J Spinal Disord. 1991;4:251-263.
96 Buonocore E, Nelson CL. Cineradiograms of cervical spine in diagnosis of soft tissue injuries. JAMA. 1966;198:143-147.
97 Barnsley L, Lord SM, Wallis BJ, et al. False positive rates of cervical zygapophyseal joint blocks. Clin J Pain. 1993;9:124-130.
98 Lord S. Percutaneous radiofrequency neurotomy in the treatment of cervical zygapophyseal joint pain: a caution. Neurosurgery. 1995;36:732-739.
99 Lord S, Barnsley L, Wallis B, et al. Percutaneous radiofrequency neurotomy for chronic cervical zygapophyseal joint pain. N Engl J Med. 1996;335:1721-1726.
100 McDonald G. Long-term follow-up of patients treated with cervical radiofrequency neurotomy for chronic neck pain. Neurosurgery. 1999;45:61-67.
101 Sapir A, Gorup J. Radiofrequency medial branch neurotomy in litigant and non-litigant patients with cervical whiplash. Spine. 2001;26(12):E268-E273.
102 Racz G. Atlanto-occipital and atlantoaxial injections in the treatment of headache and neck pain. In: Waltman S, Winnie A, editors. Interventional pain management. Philadelphia: WB Saunders; 1996:219-222.
103 April C. Occipital headaches stemming from the lateral atlantoaxial (C1–C2) joint. Paper presented at North American Spine Society Proceedings 18th Annual Meeting, 2000; New Orleans.
104 Dreyfuss P. Atlanto-occipital joint pain. A report of 3 cases and description of an intra-articular joint block technique. Reg Anesth. 1994;19:344-351.
105 Klein M. Radiofrequency techniques in clinical practice. Philadelphia: WB Saunders, 1996.
106 Gordin V. Diagnostic and therapeutic injections for the nonoperative treatment of axial neck pain and cervical radiculopathy. Curr Opin Orthop. 2001;12:238-244.
107 Ferrante F, et al. Clinical classification as a predictor of therapeutic outcome after cervical epidural steroid injection. Spine. 1993;18:730-736.
108 Cronen M. Cervical steroid epidural nerve blocks in the palliation of pain secondary to tension-type headaches. J Pain Symptom Manage. 1990;5:379-381.
109 Waldman S. Cervical epidural nerve block. In: Waltman S, Winnie A, editors. Interventional pain management. Philadelphia: WB Saunders; 1996:275-282.
110 Martelletti P, Di Sabato F, Granata M, et al. Epidural corticosteroid blockade in cervicogenic headache. Eur Rev Med Pharmacol Sci. 1998;1:31-36.
111 Reale C, et al. Epidural steroids as a pharmacologic approach. Clin Exp Rheumatol. 2000;18(Suppl 19):S65-S66.
112 Slipman C, Plastaras C, Patel R, et al. Provocative cervical discographic symptom mapping. Spine J. in press.
113 Blume H. Radiofrequency denaturation and occipital pain: results in 450 cases. A Neurophysiol. 1982;45:543-548.
114 Kuhn W. Occipital neuralgias: clinical recognition of a complicated headache: a case series and literature review. J Orofacial Pain. 1997;11(Suppl 2):158-165.
115 Murphy J. Occipital neurectomy in the treatment of headache results in 30 cases. Maryland State Med J. 1969;8(6):62-66.
116 Magnusson T. Occipital nerve release in patients with whiplash trauma and occipital neuralgia. Headache. 1996;36(1):32-36.
117 Lozano A. Microsurgical C-2 ganglionectomy for chronic intractable occipital pain. J Neurosurg. 1998;89:359-365.
118 Dubuisson D. Treatment of occipital neuralgia by partial posterior rhizotomy at C1–C3. J Neurosurg. 1995;82:581-586.
119 Weiner R. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217-221.
120 Stojanovic M. Stimulation methods for neuropathic pain control. Curr Pain Headache Rep. 2001;5(2):130-137.
121 Horowitz M, Yonas H. Occipital neuralgia treated by intradural dorsal nerve root sectioning. Cephalgia. 1993;13:354-360.