CHAPTER 41 Cervical Spondylotic Myelopathy
Surgical Management
Aging is associated with the development and progression of degenerative changes within the cervical spine. Degenerative changes are manifested in the cervical spine as disc height loss, facet and uncovertebral joint osteophytes, spondylotic bars, and hypertrophic ligamentum flavum.1,2 Neural compression from these structures may lead to the development of clinical symptoms consistent with the diagnosis of myelopathy. Although older patients may present with some degree of developmental stenosis from superimposed degenerative changes, younger patients may present with cord compression secondary to a large disc herniation. Although there is still debate regarding the etiology and pathophysiology of neuronal damage that occurs in the presence of spinal stenosis, direct mechanical compression and indirect vascular ischemia have been suggested as potential causes.3 In a cadaveric study, Ono and colleagues3 observed damage to gray and white matter, with cord atrophy, demyelination, and vascular infarction found in several segments removed from the site of maximal cord compression.
The clinical findings early in the natural course of cervical spondylotic myelopathy (CSM) are often very subtle, and delays in diagnosis are common owing to the insidious onset of these symptoms.4–8 Sadasivan and colleagues9 reported an average delay in diagnosis of approximately 6 years with gait disturbances manifesting as the earliest symptom in patients in his series. Often patients subjectively complain of a useless hand, and they notice a decline in fine motor skills. They also observe deterioration in penmanship and dexterity. Patients exhibit a loss of dexterity from atrophy of the thenar and hypothenar eminences, weakness of the extensors of the wrist, and loss of apposition of the thumb. Gait and balance disturbances from proximal lower extremity weakness are noticed and are often attributed to old age.1,10,11 Other signs of myelopathy include hyperactive deep tendon reflexes, clonus, pathologic reflexes such as bilateral Babinski responses, loss of proprioception and stereoanesthesia, and decreased pinprick and vibratory appreciation. Rarely, severely affected individuals may present with frank paralysis affecting bowel and bladder control.
Natural History
The natural history of CSM has been studied extensively in the literature. Clark and Robinson12 followed 120 patients with cervical spondylosis and myelopathy in an effort to describe the natural progression of the disease. These authors noted that of 120 patients, 5% showed a rapid onset of symptoms followed by long periods of remission, 20% showed a slow gradual decline in function without any periods of remission, and 75% showed stepwise deterioration in function followed by episodic periods of remission. In 1963, Lees and Turner13 documented their experience in the treatment of cervical myelopathy. Most patients in their series were noted to have periods of progression of disease mixed with static periods of unchanged symptoms. They also observed that 14 of the 15 patients in their series who presented with severe myelopathy continued to be disabled at 10 to 20 years of follow-up.
Nurick14,15 published his series on the natural history of cervical myelopathy following 36 patients who were treated nonoperatively. In patients who presented with mild clinical symptoms, he observed no significant clinical worsening of their condition at final assessment decades later. In addition, he noted that patients who presented with clinical symptoms at an older age tended to have a worse decline in functional status. The patients with the worst prognosis were patients who presented with severe disability, especially if they were of advanced age. Nurick15a also established a grading system for myelopathy that revolved around the patient’s ambulatory status (Table 41–1). Most series that document the long-term follow-up of patients with CSM report a progression of disease with a gradual deterioration in functional status over time.16–18
TABLE 41–1 Disability Classification of Cervical Spondylotic Myelopathy
| Grade | Description |
|---|---|
| 0 | Root signs and symptoms, no cord involvement |
| I | Signs of cord involvement, normal gait |
| II | Mild gait involvement, able to be employed |
| III | Gait abnormality prevents employment |
| IV | Able to ambulate only with assistance |
| V | Chair-bound or bedridden |
Indications for Surgical Intervention
As documented in natural history studies, patients presenting with moderate to severe symptoms are unlikely to experience regression of myelopathy. To alter the natural progression of the disease and prevent further neurologic deterioration, surgical intervention should be considered. In a review of surgical indications for cervical myelopathy, Law and colleagues19 identified several poor prognostic factors for conservative treatment, including progression of symptoms, presence of myelopathy for more than 6 months, compression ratio approaching 0.4 indicating flattening of the cord, and transverse area of the cord less than 40 mm2. The presence of any of these factors is an indicator for surgical intervention.
The goals of operative intervention include decompression of the spinal cord, stabilization of the spinal column, and reestablishment of the normal sagittal alignment. Preoperative findings that favor a successful surgical outcome include young age at presentation, duration of symptoms less than 1 year, presence of a Lhermitte sign, involvement of pathology limited to fewer vertebral segments, and presence of unilateral symptoms.20 A constellation of findings contributes to the surgeon’s decision to proceed with surgery. Factors that play a role in the decision-making process include duration of symptoms, degree of spinal cord dysfunction, general health of the patient, degree of functional deterioration, and radiographic findings.
The degree of spinal cord dysfunction is evaluated by looking for balance deficits, gait abnormalities, motor weakness, long tract signs, and changes in function. Studies by Okada and colleagues21 and Bohlman22 used clinical symptoms such as gait disturbance as the primary indication for operative treatment. Wada and colleagues23 recommended using a combination of the patient’s clinical findings, preoperative functional status as measured by the Japanese Orthopaedic Association (JOA) scale, and radiographic findings to determine the need for operative intervention. They recommended surgery when symptoms such as gait disturbances and loss of fine motor control were present in combination with a JOA score of less than 13 and radiographic evidence of spinal cord compression. In most reported studies, the neurologic results of laminoplasty are graded according to the JOA myelopathy score. A maximum score of 17 reflects normal function, and the recovery rate describes the extent to which the score returns to normal postoperatively (Table 41–2).
TABLE 41–2 Criteria for Evaluation of Operative Results of Patients with Cervical Myelopathy by Japanese Orthopaedic Association
| Function | Score (Maximum = 17) | Remarks |
|---|---|---|
| Motor | ||
| Upper extremity | 4 | Normal |
| 3 | Able to feed self with chopsticks regularly, but slightly awkwardly | |
| 2 | Able to feed self with chopsticks regularly, although awkwardly | |
| 1 | Able to feed self with a spoon, but not with chopsticks | |
| 0 | Unable to feed self with either spoon or chopsticks | |
| Lower extremity | 4 | Normal |
| 3 | Able to walk on level surface or climb stairs without cane or support but awkwardly | |
| 2 | Able to walk on level surface without cane or support but unable to climb stairs without either of them | |
| 1 | Needs cane or support even when walking on level surface | |
| 0 | Nonambulatory | |
| Sensory | ||
| Upper extremities | 2 | Normal |
| 1 | Slight sensory loss or numbness | |
| 0 | Definite sensory loss | |
| Lower extremities | 2 | Normal |
| 1 | Slight sensory loss or numbness | |
| 0 | Definite sensory loss | |
| Trunk | 2 | Normal |
| 1 | Slight sensory loss or numbness | |
| 0 | Definite sensory loss | |
| Bladder | 3 | Normal |
| 2 | Mild dysuria | |
| 1 | Severe dysuria | |
| 0 | Complete retention of urine | |
Multiple studies have shown that the degree of neurologic recovery depends highly on the preoperative duration of symptoms and the severity of the myelopathy at the time of intervention.24–27 Irreversible histologic and physiologic changes such as intraneural fibrosis and demyelination can occur within the spinal cord with prolonged compression.28 Tanaka and colleagues24 observed that the preoperative duration of symptoms strongly influenced the degree of functional recovery after surgical decompression. They recommended early surgical intervention in the treatment of CSM. Similarly, Suri and colleagues25 noted that patients with symptoms of less than 1 year’s duration showed a greater degree of postoperative motor recovery compared with patients with symptoms of longer duration. Patients with severe forms of myelopathy tend to experience less relative recovery compared with patients with mild symptoms. Bernard and Whitecloud29 also observed that patients with severe preoperative disability and a longer duration of symptoms had poorer outcomes after decompression.
The influence of age on neurologic recovery has also been investigated. Although most patients experience significant neurologic improvement after decompression, the degree of recovery tends to be mildly improved for younger age groups.21,24,26,27 Hasegawa and colleagues30 believed that this discrepancy occurred because elderly patients had a higher incidence of new neurologic dysfunction arising from different sources.
Radiographic studies also play an important role in the operative management of CSM. The normal mid-sagittal spinal canal diameter measures 17 mm in depth, and the normal spinal cord measures approximately 8 to 13 mm in its anteroposterior dimension.31,32 The addition of soft tissue structures, including the posterior longitudinal ligament and the ligamentum flavum, can occupy an additional 2 to 3 mm of the canal diameter.33 Patients with narrowing of the canal to 13 mm are considered to have relative stenosis, whereas patients with narrowing of 10 mm are considered to have absolute stenosis.34 Sites of maximal cord compression may manifest as high signal intensity on T2-weighted magnetic resonance imaging (MRI). These hyperintense signals in the cord may represent intraspinal edema or neuronal death and are generally referred to as myelomalacia. Evidence of myelomalacia on MRI has been associated with a greater degree of clinical disability and a poor prognostic finding for neurologic recovery after surgery.35,36 Suda and colleagues37 found that high cord signal intensity on MRI was associated with inferior surgical outcomes.
Radiographic evidence of dynamic stenosis as measured by translation between the vertebral bodies or by shingling of the lamina in hyperextension can transiently narrow the spinal canal.38 These structural changes in conjunction with the associated buckling of the ligamentum flavum during hyperextension of the cervical spine may decrease further the space available for the cord and may precipitate the development of myelopathy.39 Penning40 showed that cervical myelopathy should be strongly suspected if the canal space measured on dynamic lateral radiographs is reduced to less than 11 mm.
When the decision for operative intervention is made, the surgeon is faced with different approaches and operative techniques for the surgical treatment of cervical myelopathy. Regardless of whether a posterior or an anterior approach is used, the primary goal of surgical intervention in the treatment of CSM is to decompress the spinal canal. Appropriate expansion of the spinal canal has been shown to improve cord morphology and likely to maximize blood flow to the cord.41,42 Important factors that may influence the choice of approach include the sagittal alignment of the spinal column, the location of the compressive pathology, the presence of axial neck pain, the number of segments involved, and the presence of previous surgeries. The following sections discuss the different approaches and various operative techniques described in the treatment of CSM.
Anterior Approach
Indications for an Anterior Approach
The spinal cord can be compressed by herniated discs, spondylotic bars, and uncovertebral osteophytes. Direct decompression of the cord and nerve roots from these degenerative changes can be accomplished with an anterior approach. An anterior approach also allows the surgeon to relieve directly any compression on the anterior spinal artery that has been shown to supply the ventral 75% to 80% of the spinal cord. For most patients with stenosis confined to only one or two levels, an anterior approach provides adequate decompression and is the procedure of choice for most surgeons.43,44 Yonenobu and colleagues44 recommended that an anterior procedure should be confined to the treatment of spondylosis involving no more than three levels, whereas a posterior procedure should be reserved for the treatment of spondylosis involving four levels or more.
The sagittal alignment of the spinal column is an important factor when deciding on an anterior versus a posterior approach. Cervical kyphosis and degenerative instability are clear indications for an anterior approach.37,45 In patients who have lost the normal cervical lordotic curvature or who have developed a kyphotic deformity, a posterior decompression alone may destabilize the cervical spine and may lead to a progression of the deformity. Anterior surgery allows for direct decompression of the neural elements, fusion of the involved segments, and possibly reconstitution of the normal sagittal contours. An anterior approach may also be favored in patients who complain of preoperative neck pain. Two techniques described in anterior decompression and surgical treatment of cervical myelopathy include anterior cervical discectomy and fusion (ACDF) and anterior cervical corpectomy and fusion (ACCF).
Surgical Techniques
A transverse incision may be used for procedures involving one or two levels; an oblique, longitudinal incision along the course of the medial border of the sternocleidomastoid muscle may be needed for procedures involving three or more vertebral segments. The location of the transverse incision depends on what levels are involved in the surgical procedure (Fig. 41–1). Palpable landmarks of the anterior neck provide guidance as to the appropriate location for this incision. The C1-2 interspace is located at the angle of the mandible, whereas the hyoid bone usually lies anterior to the C3 level. The superior portion of the thyroid cartilage marks the position of the C4-5 interspace. The C6 level can be identified by palpation of the cricoid cartilage or by palpation of the carotid tubercle, which projects anteriorly from the transverse process of the C6 vertebral segment. Care should be taken to orient the transverse incision along the skin creases of the anterior neck to leave a cosmetic-appearing scar.
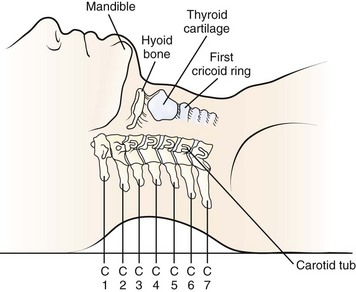
FIGURE 41–1 Palpable bony landmarks and their relationship to cervical spine.
(From Hoppenfeld S: Physical Examination of the Spine and Extremities. Norwalk, CT, Appleton & Lange, 1976, p 110.)
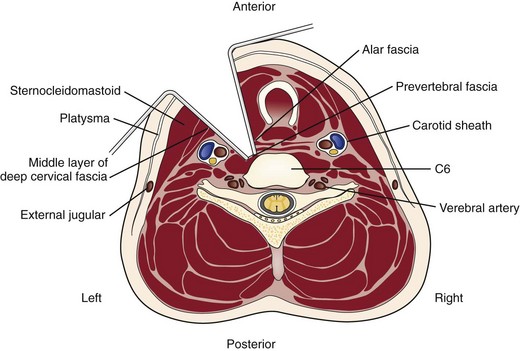
The skin and subcutaneous tissues are incised sharply with a scalpel. The platysma is divided in line with the skin incision with the transverse and longitudinal incisions. Superficial veins, especially the external jugular vein and its branches, must be protected or ligated if they cross the planes of dissection. Next, the medial border of the sternocleidomastoid is identified. Dissection is carried out through the superficial layers of the investing deep cervical fascia between the sternocleidomastoid and the medial visceral muscle column (Fig. 41–2).
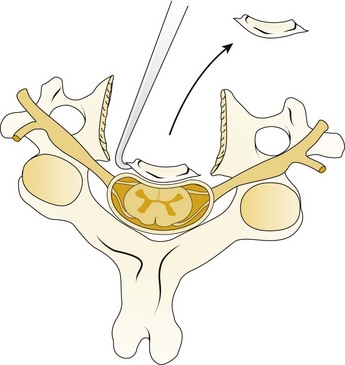
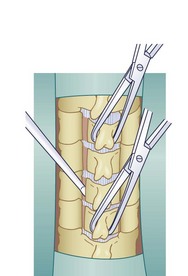
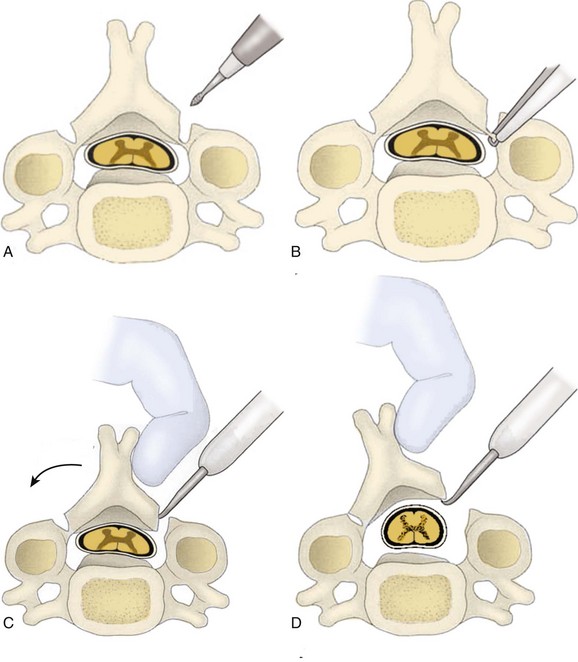
A Leksell rongeur is used to create the most ventral portion of the corpectomy trough (Fig. 41–3). The use of a motorized bur without the creation of this initial trough is dangerous and could result in injury to the carotid vessels or the esophagus if the bur “jumps” off of the anterior vertebral body cortex. After the anterior cortex of the vertebral bodies has been removed, a 5-mm power bur is used to widen and deepen the through. Starting and stopping the bur within the bony trough avoids potential injury to the surrounding soft tissue structures. Resection is continued posteriorly until the posterior cortex of the vertebral bodies is encountered. Bleeding from the cancellous bone can be controlled with bone wax. A smaller bur may be used to thin the posterior cortex and to remove the osteophytes at the uncovertebral joints. The remaining thin shell of the posterior cortex can be removed with small angled curets by pulling the bone away from the posterior longitudinal ligament and the dura (Fig. 41–4).
The posterior longitudinal ligament can be carefully removed with a nerve hook and a small Kerrison rongeur. One should use caution in the presence of ossification of the posterior longitudinal ligament (OPLL) because the ligament may be incorporated into the dura.46 Removal of the ligament should not be attempted in this situation because it may create a dural tear. The cartilage endplates at the superior and inferior extent of the corpectomy are removed with the use of a curet or a bur. A safe and adequate decompression of the spinal cord requires approximately a 15- to 19-mm wide trough.47,48 This trough can be measured directly with a caliper or estimated by comparing the width of the trough with the width of the surgeon’s finger. Foraminotomies may be performed at each of the decompressed levels if the patient’s pathology and symptoms warrant foraminal decompression.
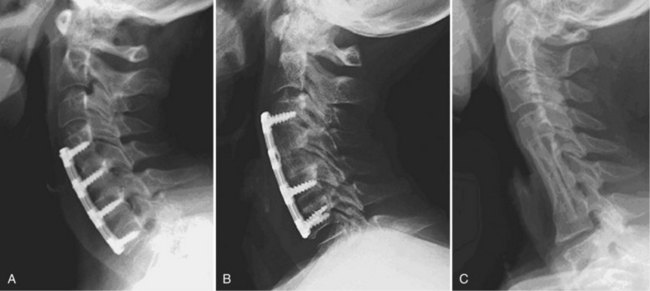
After complete decompression of the neural elements has been accomplished, the endplates are prepared for insertion of the graft. A bone tamp and mallet are used to tap the graft gently in the appropriate position while traction is being applied to the head (Fig. 41–5). Heller1 recommended a maximum of 25 lb of traction. Care must be taken to countersink the graft 1 to 2 mm behind the anterior cortex without forcing it into the canal posteriorly. A small nerve hook can be passed lateral to the graft in the trough to assess the space between the posterior longitudinal ligament and the posterior surface of the graft. The traction is released, and graft stability is assessed by manual flexion and rotation of the head by the anesthesiologist. If an anterior cervical plate is being used, it is placed at this time. The plate helps to prevent anterior migration of the graft and may provide graft compression to improve healing potential.
The wound is closed in layers over a suction drain, which is left in place for 1 to 2 days postoperatively to minimize the risk of postoperative airway compromise secondary to hematoma formation. The patient is placed into a cervical collar for additional immobilization. The choice of collar often depends on surgeon preference, number of levels fused, and presence or absence of instrumentation. After multilevel corpectomies with prolonged retractor times, many surgeons elect to keep the patient intubated overnight to minimize the possibility of respiratory distress from postoperative edema and swelling.49
Anterior Cervical Discectomy and Fusion
The use of anterior cervical decompression and fusion for the treatment of ventral pathology has been consistently reported to be a safe and effective procedure.50,51 Indications for ACDF in the treatment of cervical myelopathy include compression from any disk herniation or spondylotic degeneration that is confined to the disk level.
Although the removal of osteophytes has been reported to improve recovery in patients with CSM, controversy exists regarding the need for an osteophytectomy.52 Robinson and colleagues53 and Connolly and colleagues54 reported complete remodeling and resorption of osteophytes in the presence of a solid fusion. Bohlman22 reported excellent results in 16 of 17 patients who underwent ACDF and who were treated without any osteophyte removal. No patients experienced a loss of function, and all but one of the patients had improvement in functional status. Conversely, Stevens and colleagues55 reviewed CT myelograms of 53 patients who underwent ACDF and reported that at 12 years of follow-up no patients showed any evidence of osteophyte resorption. These authors recommended the systematic removal of all osteophytes to decrease the incidence of persistent postoperative symptoms.
The removal of a thickened ligament or an OPLL may also allow for a more thorough decompression of the spinal cord and may be necessary for the safe exploration and removal of disc fragments that may have become sequestered behind the posterior longitudinal ligament. The surgeon must always be mindful that the removal of the posterior longitudinal ligament increases the risk of developing a cord contusion or a postoperative hematoma.56 In the case of an OPLL, care must be exercised during the removal of the ligament because the dura of the spinal cord may also be ossified.46
Using ACDF in the treatment of 121 patients with CSM, Zhang and colleagues57 observed a 90% improvement in overall neurologic outcome. These authors noted an 85% fusion rate in association with the use of autogenous bone graft, whereas they noted a 50% fusion rate with the use of allograft. They also observed poorer clinical outcomes in patients who developed a pseudarthrosis. With an average number of 3.1 levels fused, Yang and colleagues58 reported 214 patients who underwent ACDF for treatment of CSM. At last follow-up, improved functional status was noted in 90% of patients, despite a pseudarthrosis rate of 37%. With a mean follow-up of 10 years, Irvine and Strachan59 retrospectively evaluated the long-term results of 46 patients who underwent ACDF for treatment of CSM. At last follow-up, 78% of the 46 patients had improved ability to ambulate, whereas 9% of patients experienced progression of their symptoms.
Anterior Cervical Corpectomy and Fusion
Bernard and Whitecloud29 evaluated multilevel cervical corpectomy. All 21 patients in this series had decompression of three or more vertebral levels with autograft. At an average of 32 months’ follow-up, these authors noted that 76% of patients had an improvement in functional outcome, and no patients developed pseudarthrosis. One patient in this series experienced graft dislodgment. In a study of 27 patients with CSM treated with multilevel corpectomy and fusion, Jamjoom and colleagues60 noted a 96% fusion rate with clinical improvement in 80% to 88% of patients. Although no patients experienced postoperative neurologic deterioration, three patients developed dislodgment of the strut graft.
Emery and colleagues50 reviewed their series of patients with CSM treated with different forms of anterior decompression and fusion procedures without the use of instrumentation. Of 108 patients, 58 were treated with ACCF using either fibular or iliac crest autograft without plate fixation. Six graft-related complications occurred in the corpectomy group, four of which occurred in patients who had been previously treated with cervical laminectomy. Of the patients who underwent corpectomy, a nonunion rate of 5% was observed, and the average improvement in Nurick grade ranged from 2.4 preoperatively to 1.2 at final follow-up.
Zdeblick and Bohlman61 reviewed their results of 14 patients with myelopathy and associated cervical kyphotic malalignment who were treated with anterior corpectomy and fusion using autograft without any plate fixation. The patients were stabilized postoperatively in a halo vest. At follow-up, 12 of the 14 patients had a solid arthrodesis. Nine patients had complete neurologic recovery, and only one patient failed to show any neurologic improvement. Correction of the kyphosis averaged 32 degrees. Zdeblick and Bohlman61 concluded that myelopathy in the setting of cervical kyphosis can be effectively and safely treated with multilevel corpectomy and strut graft reconstruction without instrumentation.
In 1998, Fessler and colleagues62 evaluated the outcomes of 93 patients with CSM who were treated with anterior corpectomy and fusion. Multisegmental involvement was noted in 31 of these patients. Fessler and colleagues62 reported that 86% of patients showed improvement in neurologic scores as measured by Nurick grade. In a more recent study published in 2005, Chibbaro and colleagues63 documented their experience in management of CSM with anterior cervical corpectomy. Using autograft and plate fixation, 54 patients received a one-level corpectomy, 11 patients received a two-level corpectomy, and 5 patients received a three-level corpectomy. At 16 weeks’ follow-up, the authors observed no evidence of pseudarthrosis, and they documented a 94% improvement in functional status. Using the modified JOA scale, no patients experienced any decline in neurologic function compared with their preoperative status.
The combination of corpectomy and adjacent discectomy has also been performed as a technique that can be used in treatment of CSM (Fig. 41–6). This method has been described as a hybrid decompression. It is indicated when spinal cord compression is present at a disc space and a vertebral body at different levels. Ashkenazi and colleagues64 published their series of 25 patients who underwent a hybrid decompression with anterior plate instrumentation for the treatment of multilevel CSM; 12 patients underwent a one-level corpectomy and adjacent one-level discectomy, and 13 patients underwent a two-level corpectomy with preservation of an intervening vertebral body. The investigators observed a 96% fusion rate, and 24 of the 25 patients reported either a neurologic improvement or an unchanged status.
Complications
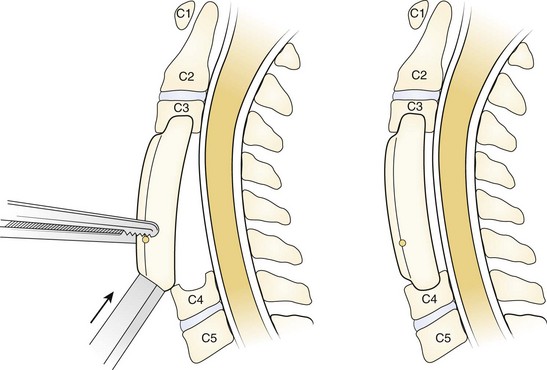
Although an anterior cervical approach has been shown to be a safe and effective procedure in treating patients with myelopathy, suboptimal outcomes have been reported when this approach is used to treat three or more levels.65–67 As more levels are involved in the attempted fusion, increased rates of nonunion have been noted in ACDF and ACCF procedures. Numerous studies have shown an inverse relationship between the fusion rate and the number of vertebral segments involved.68–71 Nonunion is determined by the absence of bridging bone across the graft-host interface on static radiographs and by the presence of motion on dynamic films.
The arthrodesis rate after ACDF without instrumentation was compared in a series by Bohlman and colleagues.72 They reported a fusion rate of 89% of 62 patients after a one-level fusion, 73% of 42 patients after a two-level fusion, and 73% of 11 patients after a three-level fusion. An unsuccessful four-level ACDF was performed in one patient in their series. Bohlman and colleagues72 attributed the lower fusion rate to increased number of fusion interfaces and increased motion as more levels are involved. Emery and colleagues50 reviewed their series of patients with CSM treated with different forms of anterior decompression and fusion procedures without the use of instrumentation. Of 108 patients, 45 were treated with anterior discectomy and fusion; the investigators reported 13 patients in the discectomy and fusion cohort who developed a nonunion. Similar to the observations made by Bohlman and colleagues,72 Emery and colleagues50 observed increased rates of nonunion as the numbers of fused levels increased. Better clinical outcomes were noted in patients who went on to a solid fusion.
Vaccaro and colleagues66 reported a series of patients who underwent an instrumented anterior corpectomy and fusion using a strut graft. They observed a 9% nonunion rate in two-level corpectomies, which increased to a 50% nonunion rate in three-level corpectomies. Sasso and colleagues73 noted a similar trend in increasing nonunion rates in their series of patients who underwent ACCF. They reported a 6% failure rate in two-level corpectomies that increased to a 71% failure rate when three levels were involved. When compared with multiple discectomy and fusion, Hilibrand and colleagues74 observed better fusion rates in patients treated with corpectomy and strut grafting. In their series, all reconstructions were performed with autograft and without anterior cervical plates. A 93% fusion rate was observed in the corpectomy cohort, whereas only a 66% fusion rate was noted in the ACDF cohort.
Although it is a more technically demanding surgery, corpectomy with fusion relies on an arthrodesis to occur at only two interfaces. Because a fusion is required between only two levels, the chance of developing a pseudarthrosis is decreased.61 Swank and colleagues75 reported 38 patients who were treated with multilevel ACDF and 26 patients who were treated with subtotal corpectomy. Allograft and anterior plate fixation were used in all patients. Among patients with two-level disease, the patients treated with two-level discectomy had a 64% fusion rate, whereas the patients treated with one-level corpectomy had a 90% fusion rate. Similarly, among patients with three-level disease, the patients treated with three-level ACDF had a fusion rate of 46%, whereas the patients treated with two-level corpectomy had a fusion rate of 56%.
In noninstrumented ACCF, immediate stability of the graft depends on the graft-host bone interface. The sculpting of the graft and the vertebral endplates and the impaction of the graft into place while the head is in traction contribute to the initial stability of the graft.29,76–78 Graft migration most commonly occurs at the inferior end of the construct. Wang and colleagues79 reported their findings in a retrospective review of 249 patients who underwent corpectomy and fusion with the use of autograft and no plate fixation over a 25-year period. Graft migration was observed in 16 of 249 patients. None of the 16 patients experienced any respiratory or neurologic complications as a result of the graft displacement. Wang and colleagues79 noted an increased rate of this complication occurring in patients undergoing longer fusions and in patients whose fusions ended at the C7 vertebral body.
To increase immediate postoperative stability, anterior plate instrumentation may be added. The addition of anterior plate fixation has been shown to improve the rate of fusion, reduce postoperative immobilization, reduce the incidence of segmental kyphosis, and reduce the prevalence of graft-related complications.54,80,81 The plate may act as a buttress to block graft migration physically.82–84 If plate fixation is performed, it is recommended that a minimum distance of 5 mm be maintained between the plate and the unaffected disc segment to minimize the potential of developing adjacent level disc ossification.85
The use of anterior instrumentation does not absolutely prevent graft complications. Sasso and colleagues73 noted that catastrophic construct failure occurred in five of seven patients who underwent a three-level corpectomy with autogenous iliac crest bone graft and anterior cervical locked plating. Most failures occurred with the graft cavitating into the vertebral body at the inferior end of the construct, which caused the graft to displace anteriorly. The biomechanical effects of long anterior cervical plates after three-level corpectomy and strut graft reconstruction were evaluated in two studies.86,87 The authors of the studies believed that the failures typically seen with instrumented multilevel corpectomies treated with long anterior cervical plates were the result of pistoning of the inferior aspect of the graft. Vaccaro and colleagues66 noted a 50% failure rate of the patients in their series who underwent three-level ACCF with anterior plate fixation. Based on their findings, they recommended the addition of a posterior stabilizing procedure to supplement multilevel ACCF.
Delayed fractures have been reported through fibular strut grafts after multilevel ACCF.88,89 Some authors support the use of a titanium cage to achieve immediate construct rigidity.90,91 The use of this construct may avoid the potential complication of late strut graft fracture. These cages are packed with bone and can be used in combination with anterior instrumentation after ACCF. Excellent fusion rates have been reported using this technique; however, caution must be exercised with the use of this construct in osteoporotic bone.90,91
Although graft complications have been reported extensively in the literature, they are not the only problem. Injury to the vertebral arteries has also been reported during ACCF procedures.61,74 In patients who underwent corpectomy, Eleraky and colleagues92 reported a prevalence of vertebral artery injury of 2% in 185 patients. It is imperative that a surgeon maintain a midline orientation during an anterior decompression to avoid any violation of the lateral wall and subsequent injury to the vertebral artery. This orientation is particularly important in patients who have tortuosity of the vertebral artery.93 Anatomic landmarks that have been discussed include the medial margin of the uncovertebral joint, medial margin of the longus colli muscle, and natural curve of the vertebral endplate. Studies have shown that by leaving a margin of approximately 5 mm to the medial border of the foramen transversarium, a total central decompression of approximately 15 mm at C3 and 19 mm at C6 can be performed safely.47,48
Individual patient characteristics or comorbidities may influence the surgeon’s decision to supplement an anterior decompression with allograft or autograft. Traditionally, the use of iliac crest autograft has been the “gold standard” in one-level and two-level anterior decompression and fusion procedures. For longer fusions, most surgeons prefer to use a structural fibula strut graft. The morbidity associated with the graft harvest is associated with an increased complication rate. Specific iliac crest donor site complications include neuroma formation, iliac crest fracture, cosmetic deformity, persistent pain, and infection.94–96 In addition to infection, harvesting autogenous fibula has been associated with injury to the peroneal nerve, contracture of the flexor hallucis longus and flexor digitorum longus tendons, development of lower extremity deep venous thrombosis, stress fractures of the tibia, and chronic ankle pain.97–100
To avoid the complications associated with the harvest of autograft, some authors advocate the use of allograft. The use of allograft in multilevel fusion has historically been associated with higher rates of nonunion.101 In a retrospective study of 126 multilevel discectomy and corpectomy cases, Fernyhough and colleagues102 compared the fusion rates between fibula strut autograft and fibula strut allograft. They noted a 27% nonunion rate in the autograft group versus a 41% nonunion rate in the allograft group. Conversely, MacDonald and colleagues103 reported a 97% fusion rate in their series of patients who underwent a decompressive corpectomy with the use of fibula strut allograft. More recently, Samartzis and colleagues104 published a series that showed equivalent rates of fusion between allograft and autograft in patients who underwent multilevel ACDF using cervical plates and current surgical techniques.
Adjacent segment degeneration in previously fused segments in the cervical spine has been reported in the literature. Based on a long-term follow-up study of 374 patients who underwent anterior cervical fusion, Hilibrand and colleagues105 reported a 25% risk of development of adjacent segment disease within 10 years. In their published series, these authors noted that the C5-6 and C6-7 levels were the most frequently involved. They reported that the risk of developing adjacent segment disease was more likely a manifestation of the natural aging process rather than a consequence of the fusion. More recently, Rao and colleagues106 reported that performing ACDF with plate fixation does not lead to the development of adjacent segment disease.
Postoperative radiculopathy is a well-recognized phenomenon that occurs after posterior decompression of the spinal cord.23,107,108 This complication has also been reported in the treatment of CSM in anterior decompressive procedures. Saunders and colleagues65 reported an incidence of C5 palsy of 20% in their series of 96 patients who underwent corpectomy to treat CSM. They recommended limiting the ventral trough to 14 or 15 mm. Yonenobu and colleagues108 reported a prevalence of postoperative radiculopathy of 3.9% of 204 patients after anterior procedures. A proposed etiology for the development of the palsy is secondary to an impingement of the ventral aspect of the spinal cord against the edges of the corpectomy trough. The removal of osteophytes can increase the risk of inadvertent injury to the spinal cord itself. Yonenobu and colleagues109 reported a single patient in their series of 75 patients who sustained an injury to the cord after the resection of posterior osteophytes during ACDF. Subsequently, these authors recommended that corpectomy be considered in patients with posterior osteophytes that are substantial enough to require removal.
Injuries to the soft tissue structures can occur during an anterior approach to the cervical spine. Swallowing difficulties are the most common postoperative problems encountered. Although a frequent complaint, the dysphagia most commonly follows a transient course.110 This problem seems to be related to esophageal dysmotility that can result from excessive retraction. Bazaz and colleagues110 reported a 50% prevalence of dysphagia at 1-month follow-up that was more frequently noted when multiple levels were involved in the fusion. At 1-year follow-up, of the 197 patients involved in this study, only 12.5% continued to complain of symptoms. Instrumentation has not been shown to increase the risk of dysphagia.110,111 Esophageal injury can occur secondary to excessive retraction, electrocautery, or perforation from sharp instruments. The incidence of esophageal perforation has been reported by Newhouse and colleagues112 to be approximately 0.25%.
Injuries to the recurrent laryngeal nerve have been reported among complications related to the dissection and mobilization of soft tissues in the neck. In a series of 650 patients who underwent an anterior cervical procedure, Frempong-Boadu and colleagues113 reported a 2% prevalence of injury to the recurrent laryngeal nerve. In contrast, Yue and colleagues114 reported a prevalence of recurrent laryngeal nerve injury of 11% in 85 patients. Injury to the laryngeal nerve is most commonly attributable to compression within the endolarynx.115 Apfelbaum and colleagues115 observed a significant decrease in the occurrence rate of this complication from 6.8% to 1.7% by deflating and reinflating the endotracheal cuff after the retractors were placed. After a review of the literature, Baron and colleagues116 reported an overall incidence of 4.9% for hoarseness and 1.4% for unilateral vocal cord paralysis. Other causes of nerve injury include direct trauma or an indirect pressure or stretch injury induced by a hematoma or by prolonged retractor placement.
Beutler and colleagues117 compared the rate of injury to the recurrent laryngeal nerve during right-sided and left-sided approaches. Although they found no difference in the rate of postoperative dysphonia between the two sides, they did observe a generally higher complication rate in the setting of revision surgery. For this reason, some surgeons advocate approaching the spine from the opposite side in a revision setting to avoid the frustration and dangers of operating through scar tissue. If a revision surgery is contemplated, a formal evaluation of vocal cord function must be done if the surgeon intends to approach the spine from the opposite side. In a situation in which the original surgery resulted in permanent unilateral vocal cord paralysis, an opposite-sided approach would be inadvisable because the potential for bilateral vocal cord paralysis would be catastrophic.
Prolonged retraction can also cause injury to the cervical sympathetic ganglion resulting in Horner syndrome, characterized by ipsilateral ptosis, miosis, and anhidrosis. This complication can also be seen in revision surgeries or in operations involving the cervicothoracic junction. Bertalanffy and Eggert56 reported an incidence of 1.1% in their series of 450 patients who underwent an anterior cervical fusion. To prevent this complication, the longus colli muscles should be dissected of the anterior vertebral body in a subperiosteal fashion, and retractors should be placed deep to the longus colli muscles.
Posterior Approach
Indications for a Posterior Approach
Before the 1950s, the operative treatment of degenerative cervical disorders primarily occurred through a posterior approach. The posterior approach to the cervical spine has continued to be a safe and effective treatment option in the surgical management of CSM. Indications for a posterior approach to the cervical spine include congenital cervical stenosis, multilevel cervical spondylosis, OPLL, ossification of the yellow ligament, and posterior compression caused by infolding of the ligamentum flavum.37,45,118,119 A posterior approach to the cervical spine relieves spinal cord compression through two distinct mechanisms: direct and indirect decompression. When the pathology causing the myelopathy is primarily due to ventral structures, posterior procedures are indirect methods of decompression. With indirect decompression, expansion of the canal allows the spinal cord to shift posteriorly away from the anterior impinging structures. In situations in which the myelopathy is secondary to a congenital stenosis or a redundant ligamentum flavum, the decompressive effect of a posterior procedure is more direct. The removal or relocation of the posterior impinging structures acts to decompress the spinal cord and is a form of direct decompression. Laminectomy and laminoplasty are two techniques of decompressing the cervical spine from a posterior approach.
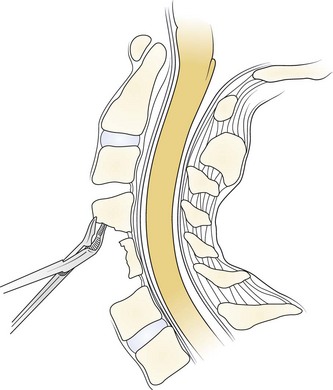
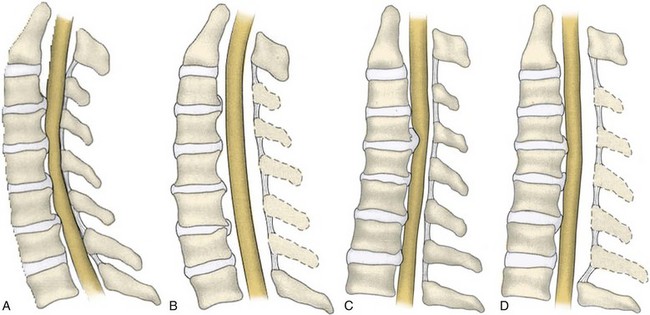
For an effective indirect spinal cord decompression to occur, certain prerequisites must exist. Posterior procedures are primarily indicated in the presence of a neutral or lordotic sagittal alignment to allow for the posterior translation of the spinal cord.120 As spondylosis progresses, there is a general trend toward loss of lordosis.121 In a patient with a kyphotic cervical spine, the sagittal alignment of the bony elements does not allow for the posterior translation of the spinal cord (Fig. 41–7). Even after a posterior decompression, the cord remains draped over the anterior compressive pathology. A minimal lordotic curvature of 10 degrees should be present if posterior decompression is considered.122 Studies have shown that measured from C2 to C7, the average lordotic curvature in a normal cervical spine is 14.4 degrees.123
Yamazaki and colleagues124 observed continued ventral compression after posterior decompression in patients with anterior canal encroachment greater than 7 mm and with lordotic sagittal alignments of less than 10 degrees. When a posterior decompression is performed in patients with a kyphotic cervical alignment, modest improvements in neurologic outcome can be expected. The recovery achieved from a decompression in the presence of a cervical kyphosis tends to be inferior, however, when compared with a posterior decompression that is performed in the presence of a cervical lordosis.37,125 These findings were echoed by Satomi and colleagues,125 who found that patients with maintenance of preoperative lordosis exhibited a superior improvement in JOA score compared with patients in whom the lordosis decreased by 10 degrees or more.
In patients who underwent decompressive laminoplasty, Sodeyama and colleagues45 showed that patients with lordotic spines had the greatest postoperative posterior translation of the spinal cord with an average shift of 3.1 mm. Patients who had a neutral alignment showed a peak posterior shift of less than 3 mm, and patients who had a kyphotic cervical alignment showed a peak posterior shift of less than 2 mm. In a study evaluating 114 patients with CSM after laminoplasty, Suda and colleagues37 observed that a preoperative local kyphosis exceeding 13 degrees provided patients with the worst prognosis for neurologic recovery. They concluded that cervical kyphosis should be regarded as a relative contraindication for a posterior decompression.
As a second prerequisite, indirect spinal cord decompression also requires a sufficient length of canal expansion. Decompression of the posterior structures at levels beyond areas of focal stenosis may allow for greater translation of the cord. The degree of canal expansion that is achieved via a posterior approach has been shown to correlate with the success of postoperative recovery.126–128 A statistically significant difference in recovery rate was reported by Hirabayashi and colleagues126 when the anteroposterior diameter of the spinal canal increased greater than 5 mm versus an increase of less than 2 mm. Ishida and colleagues127 observed better recovery rates in patients in whom posterior decompression increased the sagittal diameter of the canal to 15 mm or greater. Kohno and colleagues128 showed that expansion of the anteroposterior canal diameter to 12.8 mm correlated with a good postoperative recovery after laminoplasty. Shaffrey and colleagues129 reported an overall average increase in the spinal canal cross-sectional area of 55% after posterior decompression with an 88% increase at the most compressed level.
When adequately decompressed, the spinal cord has been reported to change in morphology from a flattened to a more natural oval shape. In a CT myelography study, Aita and colleagues120 described changes in spinal cord morphology in 38 patients after open-door laminoplasty. Postoperatively, the largest increase in mean spinal cord cross-sectional area was improved by 12%. In addition, the mean sagittal cord diameter was enlarged by 1 mm, and the mean coronal diameter was decreased by 1 mm.
The longitudinal height of the cervical spine as a prognostic indicator for neurologic recovery has also been evaluated. Chiba and colleagues130 noted that after extensive open-door laminoplasty, patients with CSM were more likely to have improved neurologic results compared with patients with OPLL. They observed a greater degree of preoperative cervical spine shortening owing to disc degeneration in patients with CSM. These authors attributed the improved recovery rate in these patients to the redundancy within the spinal cord after decompression.
Laminectomy and Laminectomy and Fusion
Laminectomy is indicated for multilevel decompression in elderly myelopathic patients with multiple medical comorbidities and preserved cervical lordosis. Decompressive laminectomy is typically performed at the junctions of the lateral masses and the laminae. If a patient also has concurrent radicular symptoms, a concomitant foraminotomy may be performed to decompress the associated foraminal stenosis. If a foraminotomy is performed, care must be taken not to remove much of the facet. Progressive removal of greater increments of the facet joint is correlated with greater degrees of instability.123 To preserve stability, it is crucial to resect no more than 25% of the facet joint.131 In addition, any level with radiographic evidence of stenosis should be included in the decompression. Kato and colleagues132 observed increased rates of recurrent symptoms resulting from progression of disease at adjacent segments not involved in the decompression. Kaptain and colleagues133 reported no added benefit in limiting the number of segments involved in the decompression because it did not influence the development of postlaminectomy kyphosis or instability.
Circumstances such as iatrogenic instability, flexible kyphosis, or focal instability require supplemental posterior cervical instrumentation. The goal of any fixation system is to provide structural support for a bony fusion mass to mature. The use of posterior instrumentation has been shown to maintain lordosis effectively after a posterior cervical laminectomy and fusion.134,135 Before the introduction of lateral mass screws, wiring techniques were the mainstay of treatment when a fusion was performed. The wire would be passed between neighboring facets or tied to bone grafts or metallic rods. With these techniques, acceptable fusion rates were obtained.136,137 With the introduction of lateral mass screws, wiring techniques have largely fallen out of favor.
Biomechanically, lateral mass screws have been proven to be a superior technique compared with wiring procedures. They produce a more rigid construct and show a better fusion potential.138 The early screw-plate systems were difficult to use and very inflexible because they could not adapt well to variations in patient anatomy. The lack of versatility these early designs offered has been overcome with the evolution of multiaxial screw and rod constructs.139 The newer systems are constrained and allow for more rigid fixation. Similarly, these new systems accommodate variations in the cervical anatomy without having to perform excessive rod contouring or having to compromise screw positioning.
A review of the literature reveals various lateral mass screw insertion techniques.140–143 The biomechanical characteristics and anatomic relationships of each of these techniques have been studied.144,145 Despite the various techniques described, clinically none have shown any difference in complication rate.146 In a cadaveric study, Seybold and colleagues147 showed no clear biomechanical advantage with bicortical placement of lateral mass screws versus unicortical placement of lateral mass screws. They showed an increased incidence of nerve root impingement and potential vertebral artery injury with the use of bicortical screws. These investigators reported no injuries using unicortical screws. Ultimately, an appropriate understanding and knowledge of anatomic relationships is needed when placing these screws.
Techniques for pedicle screw placement have also been described in the literature.148 Pedicle screws have been shown to have a higher pullout strength compared with lateral mass screws.149 Because of the small size and morphologic variation in the cervical pedicle, however, there is greater potential for injury with pedicle screw placement.150 Pedicle screw placement is usually reserved for levels with larger pedicles, such as the C7 vertebra, or when crossing the cervicothoracic junction. The technique for pedicle screw placement is not discussed in this chapter.
Technique
As with any patient with myelopathy, care should be taken to avoid any extremes of neck motion during intubation. Mean arterial pressure should be monitored during positioning of the patient, and hypotension should be avoided throughout the entire operative procedure. A Mayfield tong or three-pin head holder is affixed to the patient’s head, and the patient is positioned prone on the operating table. The head should be fixed in neutral or in slight extension. The trunk should be supported by a four-poster frame or by padded chest rolls. If a padded headrest with a laminectomy frame is used, care must be taken to avoid any force on the eyes because this may increase intraocular pressure and create an ischemic optic neuropathy resulting in visual loss.151 All bony prominences should be padded. The arms should be tucked in and secured at the patient’s side using arm sleds or sheets under the patient’s torso. Legs should be flexed at the knees, and the patient should be secured to the table using the appropriate straps. The table should be positioned in a reverse Trendelenburg position to reduce any venous congestion. After the patient is positioned, the suboccipital region is shaved, and the surgical field is sterilely prepared and draped.
Dissection is carried subperiosteally exposing the spinous processes and laminae of the appropriately selected cervical levels. Care should be exercised not to dissect the muscular attachments of C2 because its removal has been shown to create instability in the upper cervical spine.152 The extent of lateral dissection is minimized to just beyond the medial aspect of the facet joint, with care taken not to violate the facet capsules. A self-retainer is placed to mobilize the paraspinal musculature free from the surgical field. To avoid excessive damage to the paraspinal muscles, retractors should be intermittently released and repositioned. If an instrumentation and fusion is indicated, the exposure for lateral mass fixation requires the subperiosteal soft tissue dissection to extend to the far lateral margin of the facet. Care must be taken to avoid any compromise to the facet capsule of the most cranial and caudal aspects of the intended fusion.
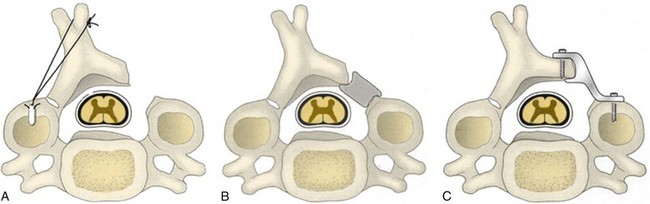
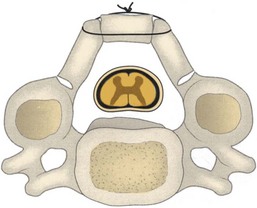
It is essential that the landmarks of the lateral mass be clearly identified to ensure accurate placement of the screws. The cranial and caudal boundaries of the lateral mass are defined by the adjacent facet joints, and the medial boundary is defined by the lamina-facet junction. After adequate exposure of the lateral masses is achieved, entry sites for the lateral mass screws are marked using a high-speed bur. Screws are placed depending on the surgeon’s choice of technique. After the screws are adequately placed, the lamina can be resected. Laminectomies are performed after the insertion of the lateral mass screws to protect the cord with its neural elements. Care must be taken to preserve the bone for use as local autograft. Removal of the spinous processes with preservation of a small remnant facilitates visualization and removal of the laminae. Bilateral trough laminotomies are created with the aid of a bur at the lamina-facet junction just medial to the facet complex (Fig. 41–8). During the laminotomies, 1 to 2 mm of thinned ventral bony cortex or hypertrophied yellow ligament dorsal to the underlying thecal sac is preserved.
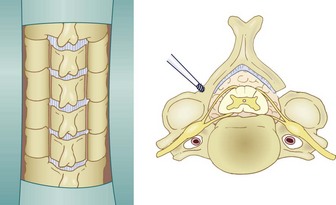
FIGURE 41–8 Bilateral trough laminotomies are created at lamina-facet junction medial to facet complex.
(From Komotar RJ, Mocco J, Kaiser MG: Surgical management of cervical myelopathy: Indications and techniques for laminectomy and fusion. Spine J 6:258S, 2006.)
Removal of the ventral cortex is accomplished with a low-speed 4-mm bur. The intraspinous ligament at the most cranial and caudal level is divided and resected. Following this, a towel clamp is placed on the most cranial and caudal spinous process, and the lamina and associated ligamentum flavum are gently lifted off the underlying dura (Fig. 41–9). The ligamentum flavum is divided, and the laminae are removed en bloc. Any residual osseous remnants are removed using a Kerrison punch, completing the laminectomy to the lateral edge of the thecal sac. In an extremely stenotic canal with no epidural fat and nearly no yellow ligament, there may be dense adhesions to the underlying dura. No instrument should be introduced in or near the midline to avoid cord contusion. In a patient with severe stenosis, the exclusive use of a high-speed bur may be advisable to minimize any further compression that may be caused by the footplate of a Kerrison punch.
Neurologic Outcomes
Laminectomy has been proven to be a successful procedure in the treatment of elderly myelopathic patients with multilevel cervical spondylosis and lordotic sagittal alignment. Of 90 patients who underwent cervical laminectomy in the series by Snow and Weiner,118 77% of patients showed neurologic improvement, 13% of patients remained unchanged, and 10% of patients experienced neurologic deterioration. In a review of 32 patients treated with multilevel laminectomy and posterior fusion using a lateral mass screw and plate construct, Huang and colleagues153 noted a postoperative improvement in Nurick grade of at least 1 point in 22 patients. The remaining nine patients showed no change in neurologic function, but more importantly no patients involved in the study showed any neurologic deterioration. More recently, Sekhon154 reviewed 50 patients with CSM who underwent posterior cervical decompression and fusion with an average follow-up of 30.1 months. All patients underwent fusion with a lateral mass screw plate and rod construct with local autograft. An average of 2.88 levels were fused. At last follow-up, no patients developed any symptomatic or radiographic evidence of pseudarthrosis. Postoperative MRI showed an absence of persistent compression at the operative levels in 100% of cases. Most patients improved at least one Nurick grade, and no myelopathic deterioration was noted postoperatively.
Complications
Although good to excellent results have been reported in patients after laminectomy, subsequent deterioration in outcome may occur.118,155,156 The development of a new-onset neurologic deficit from an injury to the nerve root or to the spinal cord itself is among the most feared complications involving the surgical management of CSM. Yonenobu and colleagues108 observed a 3.5% prevalence of spinal cord injury after laminectomy in their series of 85 patients. They attributed spinal cord dysfunction to postoperative swelling and to epidural hematoma formation that caused compression on the uncovered cord. In 287 patients undergoing multilevel cervical laminectomy, Dai and colleagues157 observed a 13% incidence of postoperative radiculopathy. Most patients in their series were found to have regained function within 6 months of surgery. The postoperative development of a sagittal malalignment or a segmental instability has been linked to the development of neurologic deterioration after laminectomy.152 Adequate decompression often requires the removal of important static and dynamic stabilizing structures, which can lead to an imbalance in sagittal alignment.133,158–160 Over time, the spinal cord may become tethered against anterior impinging structures, resulting in progressive spinal cord dysfunction.78,118,155,156
In adults, laminectomy alone significantly increases total cervical spine flexibility.159 This instability is increased further with the removal of the spinous process or lamina of C2 or C7. Removal of the posterior arch with the spinous process of C2 disrupts the semispinalis cervicis and the suboccipital muscles significantly weakening the posterior tension band of the cervical spine. Iizuka and colleagues152 showed that postoperative loss of sagittal alignment was strongly associated with dissection and subsequent nonhealing of the muscle insertion on C2. Younger individuals are particularly vulnerable to the development of a kyphotic sagittal malalignment after laminectomy. Bell and colleagues161 showed that 53% of patients younger than 18 years who underwent multilevel laminectomy developed a progressive kyphosis. The tendency for children to develop a symptomatic kyphotic deformity after extensive laminectomy may be due to the increased viscoelasticity of the posterior elements and the immaturity of the vertebral body.162 The horizontal orientation of the facet joints in the immature spine can lead to a less resistive force to flexion.163
The width of the facetectomy has also been shown to be a crucial determinant in the development of postoperative instability. Zdeblick and colleagues123 showed that facetectomy of more than 50% caused a statistically significant loss of stability of the cervical spine in flexion and torsion. Voo and colleagues164 observed a pronounced increase in angular rotation and intervertebral disc stress with resection of greater than 50% of the facet complexes bilaterally. Herkowitz82 noted a 25% increase in the incidence of postoperative kyphotic deformities within 2 years after cervical laminectomy with partial bilateral facetectomies. He concluded that if 50% or more of the facet has been violated, a concurrent stabilization procedure should be recommended. Nowinski and colleagues165 reinforced further the idea that in multilevel laminectomy a concurrent fusion should be performed if more than 25% of the facet is removed bilaterally.
In an effort to avoid any postoperative instability associated with laminectomy, some authors advocate performing a concurrent instrumentation and fusion, with the use of lateral mass screws.153,154 Complications using this technique have been reported. Complications with lateral mass fixation include neuronal injury, vertebral artery injury, and hardware failure secondary to pseudarthrosis. In an analysis of complications after lateral mass fixation with screws and plates, Heller and colleagues166 found a 0.6% incidence of nerve root injury per screw placed and a 1.1% incidence of screw loosening after instrumentation of 654 screws. They observed that 3.8% of patients had development of adjacent segment disease within 2.5 years after laminectomy and fusion. Of the 654 screws placed, they reported no vertebral artery injuries. In a review of 43 patients who had 281 screws placed, Wellman and colleagues167 showed no screw-related complications after a 25-month follow-up. They concluded that lateral mass fixation is a safe and effective means of posterior cervical stabilization.
Laminoplasty
The preservation of the posterior elements of the cervical spine provides a biologic covering to the spinal canal and a reattachment site for paraspinal muscles. Pal and Cooper168 tested load transmission in the anterior and posterior cervical column in cadavers. They showed that 36% of load transmission was through the anterior column, whereas 64% was through the posterior column. These findings highlight the importance of preserving the integrity of the posterior arch and facet complex. In addition, the posterior arch complex serves as an important static factor in the maintenance of cervical lordosis.169–171 With preservation of the natural static and dynamic restraints of the posterior cervical spine, iatrogenic instability is rarely encountered after laminoplasty.129,172 Of 171 patients treated with French-door laminoplasty, Shimamura and colleagues172 reported no cases of segmental instability at 5-year follow-up. Similarly, Kawakami and colleagues173 observed no evidence of segmental instability on dynamic radiographs at 3.5-year follow-up in their series of 67 patients who underwent laminoplasty for the treatment of CSM.
The procedure was first described by Oyama and colleagues in 1973.174 The technique involved a Z-plasty of each thinned lamina. Through the expansion of the modified lamina, a decompression of the spinal cord was performed, and the posterior arch was reconstructed. The original technique used a supplemental arthrodesis through the placement of bone graft over the expanded arch and facets. This technique never gained popularity owing to the prolonged time and painstaking detail required.
Two examples of laminoplasty techniques that are discussed in this chapter include the open-door laminoplasty technique described by Hirabayashi and colleagues175 and the mid-sagittal laminoplasty or French-door laminoplasty technique described by Kurokawa and colleagues.176 Comparative studies of these two procedures have failed to show a significant distinction with regard to neurologic outcomes.177,178 Okada and colleagues179 published a prospective randomized study comparing the clinical outcomes of open-door laminoplasty versus French-door laminoplasty. Although Okada and colleagues179 observed higher rates of perioperative complications and added complaints of postoperative axial pain in the open-door laminoplasty group, no significant differences in postoperative JOA scores and recovery rates were noted between the two groups.
Open-Door Laminoplasty
In 1978, Hirabayashi180 described the open-door laminoplasty technique. With this technique, the canal is expanded at consecutive levels by creating a bone hinge on one side of the canal while detaching the lamina at the opposite side. This technique enables the spinal cord to be adequately decompressed while maintaining stability of the spinal column. Advantages of the open-door procedure include a straightforward operative technique and shorter operative time. Advocates of this technique cite that entering the canal at its lateralmost extent, where spinal cord compression tends to be the least, protects the cord and provides for a greater margin of safety.
Disadvantages associated with the use of the open-door technique have been described. A greater degree of bleeding is encountered with this technique because of the vast plexus of epidural veins in the lateral portion of the cervical spinal canal. Cases have been documented in which the hinge prematurely closed resulting in radiologic and catastrophic neurologic deterioration. This complication was first described by Hirabayashi and colleagues175 and termed the spring-back phenomenon. To safeguard against this complication, Hirabayashi advocated the use of stay sutures to secure the spinous process to the facet capsule on the hinge side.175,181
The description of the spring-back phenomenon acted as a catalyst in the design of a newer generation of techniques to maintain a patent canal (Fig. 41–10). Itoh and Tsuji182 described the use of autograft spinous process bone blocks and stainless steel wiring to maintain canal patency. In 30 patients in their series, these authors reported no significant deterioration in 18 months of follow-up, and they observed an average of 4.1 mm of anteroposterior canal expansion. Various other strut materials have been employed, including contoured ceramic blocks, hydroxyapatite blocks, and allografts.129,183,184
The use of rigid internal fixation between the expanded laminae and the lateral masses has also been described as an alternative method of providing immediate stability. Specialized plates have been designed to maintain canal patency and to reduce the probability of hinge closure and restenosis. O’Brien and colleagues185 showed a significant improvement in sagittal canal diameter with no hardware failure with the use of titanium plates to maintain patency in their series of patients who underwent an open-door laminoplasty. Similarly, through the use of plate fixation and allograft, Shaffrey and colleagues129 reported an increase in sagittal canal diameter with an improvement in overall neurologic function in patients in their series. The long-term stability of these constructs is achieved with the eventual healing of the bone on the hinge side or the eventual healing of the host bone-strut graft interface on the open-door side, or both.
French-Door Laminoplasty
In 1978, Kurokawa and colleagues176 described a spinous process splitting or French-door laminoplasty. This novel technique provided for symmetrical expansion of the stenotic canal by dividing the spinous processes in the midline before hinging open each hemilamina at the lamina–lateral mass junction. Bone or synthetic blocks were placed as a strut to maintain the patency of the hinged hemilaminae (Fig. 41–11). Advocates of the French-door technique reported decreased bleeding because of the paucity of veins encountered in the midline of the canal. They also reported a more symmetrical expansion of the canal compared with the open-door technique.159,178 The French-door technique can be readily combined with lateral mass or pedicle screw fixation methods if a concurrent posterior fusion is necessary. Typically, the midline sagittal split in the spinous process is created with the use of a bur or a threadwire saw. Using the threadwire saw to perform the sagittal split in the spinous process, Tomita and colleagues186 reported a mean decrease in operating room time of 63 minutes and a mean blood loss of 70 mL less than if a bur was used. No matter which instrument is used, the surgeon must always be mindful that the introduction of instruments into the central portion of a stenotic canal can increase the danger of creating a catastrophic neurologic injury.176,186
Technique
Laminoplasty has proven to be a safe and reliable technique of decompressing the spinal cord. Although the described methods each have their own technical points, certain operative principles are applicable to all techniques. Patients are positioned prone, on the operative table. The use of a frame or rolls to support the trunk provides for decreased abdominal and chest pressure. Cranial tongs are used to secure the head, and the cervical spine is placed in a neutral or mildly flexed position. To minimize the bleeding from venous congestion and minimize facial or airway edema during surgery, the operative table is placed in 30 degrees of reverse Trendelenburg position.175 Care must be taken to avoid any pressure on the eyes.
A posterior midline approach is made along the nuchal ligament to the spinous processes. The paraspinal muscles are dissected from the posterior elements. Care is taken to preserve the insertion of the paraspinal muscles to the spinous process of C2. If significant tension of the supraspinous and interspinous ligaments is noted during the expansion of an open-door laminoplasty, a partial osteotomy at the base of the spinous process just below the caudal end of the expanded construct may be performed.187 The osteotomized spinous process is bent toward the hinge side to decrease the ligamentous tension. With an open-door laminoplasty from C3 to C7, significant ligamentous tension would be alleviated with an incomplete osteotomy through the base of the T1 spinous process. Other authors simply excise the spinous processes and their associated ligaments at the cranial and caudal ends to facilitate the opening of the spinal canal. The trough laminectomies are created at the lamina–lateral mass junction without disruption of the facet joints (Fig. 41–12).
Outcomes
Laminoplasty has been shown to be an effective treatment option in the decompression of patients with myelopathy secondary to OPLL. Numerous authors have reported, however, that an OPLL continues to increase in thickness even after a decompressive procedure is performed. In a series of 47 patients, Satomi and colleagues125 reported an average increase in thickness of the OPLL from 6.3 mm preoperatively to 7.5 mm after laminoplasty at 5-year follow-up. To accommodate any future extension of the OPLL, they recommended that decompression of the level above and the level below the compressive pathology be strongly considered.125,188 Often a dome laminectomy of C2 may be necessary. This technique involves the removal of bone from the lamina of C2, while maintaining the structural integrity of the arch and its important muscular insertions. Iwasaki and colleagues189 reported the results of 64 patients with OPLL over a 10-year minimal follow-up. They observed progression of cervical OPLL in 70% of patients, with the development of neurologic deterioration in only two patients. Similarly, in long-term follow-up greater than 10 years for patients with cervical myelopathy caused by OPLL, Kawaguchi and colleagues188 noted progression of OPLL in 73% of patients. Young age at the time of surgery has been identified as a risk factor for progression of OPLL, whereas an older age has been shown to be protective.188,189
Many short-term studies have been reported evaluating the clinical outcomes after cervical laminoplasty. The first of such reports was by Hirabayashi and colleagues.190 Using the JOA score, they noted a 54% improvement rate over an average follow-up of 3 years on patients who underwent an open-door laminoplasty for CSM. Similarly, Satomi and colleagues125 reported an average recovery rate of 61% in 18 patients with CSM over an 8-year follow-up. Using a French-door laminoplasty technique, Tomita and colleagues186 observed a 72% recovery rate over a 34-month follow-up.
Long-term studies have also been reported in the literature regarding clinical outcomes after laminoplasty. Over a period of greater than 10 years, Kawaguchi and colleagues191 studied the long-term outcome of 126 patients who underwent laminoplasty for the treatment of cervical myelopathy. They reported a preoperative JOA score of 9.1, which improved to 13.7 after 1 postoperative year. At last follow-up, Kawaguchi and colleagues191 found that the score was maintained at 13.4. Seichi and colleagues192 reported their results of 25 patients over a 12-year follow-up who underwent a laminoplasty. They noted mean JOA preoperative scores of 8.3, which at last follow up were observed to increase to 11.7. They also reported that late neurologic deterioration occurred in four patients, three of whom had athetoid cerebral palsy.
Complications
The most important role of any laminoplasty technique is to permit extensive decompression of the spinal canal through the expansion of the posterior arch. Early in the laminoplasty experience, cases of posterior arch closure were reported (Fig. 41–13).175 Satomi and colleagues125 observed postoperative lamina closure resulting in neurologic deterioration in 4% of 51 patients after open-door laminoplasty. Matsumoto and colleagues193 identified the presence of preoperative cervical kyphosis as a significant risk factor for the development of lamina closure. In their series, recovery rates were not influenced by lamina closure; however, patients with lamina closure expressed a greater degree of dissatisfaction with the surgery.
Postoperative motor root palsy is a clinically significant complication of cervical laminoplasty and has received significant attention in the literature.23,107,108 In most cases, the symptoms are not permanent, and complete to partial recovery can be expected. Obvious causes of neurologic deterioration include displacement of the posterior arch into the canal through strut graft or fixation failure, inadequate decompression, postoperative hematoma, and iatrogenic trauma to the cord. In cases in which no obvious abnormality is identified, the etiology of postoperative motor root palsy remains undetermined. Some authors believe that the mechanism of injury is associated with the tethering of the nerve roots secondary to the posterior migration of the spinal cord.108 A posterior translation of the spinal cord may stress the ventrally exiting nerve roots, causing mechanical injury and ischemic insult to the nerve root and the adjoining spinal cord parenchyma. A posterior shift of the spinal cord could also produce focal pressure on the nerve root at its entry into the foramen.194,195
Sasai and colleagues196,197 suggested that preexisting subclinical C5 root compression is a cause of C5 palsy. They found that preexisting subclinical radiculopathy as determined by abnormal preoperative electrophysiologic studies was predictive for the development of postoperative motor deficits. Sasai and colleagues196,197 also evaluated the role of prophylactic foraminotomies in decreasing the incidence of motor palsy. Based on their findings, they suggested that postoperative nerve palsy could be avoided if a selective foraminotomy was performed concurrently with the laminoplasty. The clinical presentation of most patients presenting with postoperative motor root palsy is often delayed hours to days after surgery and most commonly involves the C5 nerve root.107,198 Uematsu and colleagues107 reported on 20 of 365 patients who underwent laminoplasty and who developed postoperative motor palsy. They noted an 8% incidence of C5 root palsy, and most of their patients presented with symptoms within 1 week of surgery. Most of their patients presented with symptoms within 1 week of surgery. They recommended that expansion of the posterior arch be limited to prevent the development of anterior root tension.
Chiba and colleagues198 reported 15 patients in their series who developed postoperative segmental motor paralysis after open-door laminoplasty. The symptoms developed an average of 4.6 days postoperatively. All 15 patients showed a high T2 signal intensity in the spinal cord on MRI. Of the 15 patients, 10 were observed to display signal changes that corresponded to the level at the paralyzed segment. At last follow-up, paralysis resolved completely in 11 patients. The remaining patients regained their strength to a motor grade of 4 out of 5. Based on their findings, Chiba and colleagues198 concluded that impairments in the gray matter of the spinal cord might play an important role in the development of postoperative motor paralysis.
Laminoplasty has not been shown to be an effective procedure in the relief of axial neck pain in patients with myelopathy. In addition, patients who have undergone laminoplasty often complain of discomfort in the neck, the shoulders, and the intrascapular region in the postoperative period. The etiology of neck pain is undetermined, but it has been postulated to result secondary to posterior soft tissue dissection or to the inadvertent disruption of the facet joints.199,200 Hosono and colleagues201 noted that the prevalence of postoperative axial symptoms was significantly higher after laminoplasty than after anterior fusion. Axial symptoms were reported in 60% of patients after laminoplasty and 19% of patients after an anterior fusion. Similarly, in a study by Wada and colleagues23 comparing subtotal corpectomy and laminoplasty, 15% of patients in the corpectomy group versus 40% of patients in the laminoplasty group complained of axial pain postoperatively. More recently, using a visual analog scale pain questionnaire, Takeuchi and Yasuhiro202 reported the importance of preserving the C7 spinous process and the attached nuchal ligament to reduce postoperative axial symptoms after French-door laminoplasty.
Early in the development of the laminoplasty technique, most authors recommended a concomitant fusion to prevent the development of subsequent instability and recurrent stenosis.182,203 The practice of applying bone graft to the posterior elements led to a reduced range of motion and a higher rate of postoperative axial discomfort than necessary. Studies over the years have shown that cervical lordosis and segmental stability are maintained after laminoplasty without the need of a simultaneous fusion. Based on these findings, most authors currently recommend that laminoplasty be performed without fusion except in cases of known instability or correction of deformity.129,186,204
In the evaluation of long-term outcomes of laminoplasty, most authors have reported a significant loss of motion at final follow-up. Edwards and colleagues67 reported a 38% loss of C2-7 sagittal motion after laminoplasty. Similarly, Kimura and colleagues203 noted a postoperative loss of motion of 62% in their series of patients who underwent an open-door laminoplasty. Kawaguchi and colleagues191 observed a similar reduction in range of motion in their long-term series of patients; 61% of patients in their series developed some decrease in range of motion. More recently, Hyun and colleagues205 observed a decrease in range of motion that was time dependent in patients who underwent an open-door laminoplasty. In their series of 23 patients, they noted a gradual decrease in cervical range of motion that continued for 18 months before stabilizing. To diminish the potential for spontaneous facet fusion and to preserve motion, some authors recommend the application of bone wax to the exposed cancellous hinge surfaces.67 Many contemporary rehabilitation programs encourage early active range of motion with the goal of reducing postoperative neck stiffness and pain.206
The development of a kyphotic sagittal alignment after laminoplasty has been well reported.189,191 Fewer reports exist documenting the potential neurologic decline that can accompany a loss of normal cervical lordosis. Over a 10-year follow-up, Iwasaki and colleagues189 observed that 8% of 59 patients in their series developed a kyphotic sagittal alignment. No patients were reported to have developed any neurologic sequelae. Over the course of 10 years, Kawaguchi and colleagues191 observed 8 patients who developed a kyphotic deformity in a series of 126 patients who underwent laminoplasty for the treatment of CSM. They noted that the recovery rate in these eight patients was poor. Wada and colleagues23 observed a loss of lordosis that occurred in three patients in their series after laminoplasty. They found no relationship, however, between loss of lordosis and final JOA score. Sasai and colleagues207 observed that diminished lordosis occurred most prominently at C2-3 and C6-7 after C3-7 French-door laminoplasty. In a retrospective analysis of 64 patients 6 years after laminoplasty, Matsunaga and colleagues208 noted that the expansion of C2 led to the development of segmental kyphosis in 20% of patients. In contrast, they noted that the preservation of C2 was protective because only 2% of patients developed a segmental kyphosis.
Postoperative dysfunction of the paraspinal musculature is reported by numerous authors to be a causative agent in the decrease in lordosis noted after laminoplasty.209,210 Using MRI, Hayashi and colleagues209 found a significant postoperative reduction in the cross-sectional area of paraspinal musculature after laminoplasty. Matsuzaki and colleagues211 associated the paraspinal muscle dysfunction with the denervation of its nerve supply. They cautioned against dissection farther lateral than the medial third of the facet to prevent injury to the dorsal primary rami of the spinal nerves.
Comparison of Outcomes
Whether anterior or posterior, the appropriate approach for the treatment of CSM depends on a host of factors, including site of cord compression, sagittal alignment, and number of vertebral segments involved. In a comparison of clinical and radiographic outcomes, Heller and colleagues212 matched 13 patients who underwent laminectomy and fusion with 13 patients who underwent laminoplasty. Although not statistically significant, they reported a lower complication rate and a higher degree of functional improvement in patients managed with laminoplasty. Although nine patients in the laminectomy group developed complications, no complications were reported in the laminoplasty group. The complications observed included progression of myelopathy, nonunion, instrumentation failure, development of kyphosis, adjacent segment degeneration, and issues related to donor site morbidity. Although the sample size of this study was small, Heller and colleagues212 concluded that laminoplasty was a safer and more reliable procedure than laminectomy and fusion in the treatment of CSM.
When long-term outcomes after subtotal corpectomy and strut grafting were compared with outcomes after laminoplasty, Wada and colleagues23 showed no difference in neurologic recovery between the two groups. In the subtotal corpectomy group, they noted longer surgical times, more blood loss, and 26% pseudarthrosis rate. Of the 23 patients in the corpectomy cohort, 15% complained of postoperative axial pain. In contrast, of the 24 patients in the laminoplasty cohort 40% of patients complained of similar symptoms. Yonenobu and colleagues213 reported their results after a similar study that evaluated 41 patients after subtotal corpectomy and strut grafting and 42 patients after laminoplasty. They noted no difference in recovery rate and final JOA scores between the two groups; however, complications were seen more frequently in the corpectomy group with 29% of patients reporting a complication. In contrast, complications were reported in only 7% of patients in the laminoplasty group. In an earlier study, Yonenobu and colleagues44 compared the clinical outcomes of 50 patients treated with ACDF, 21 patients treated with corpectomy and fusion, and 24 patients treated with laminectomy in the treatment of CSM. These investigators observed that patients treated with corpectomy showed the best results clinically; however, they recommended a posterior approach for patients with disease at four segments or more.
In the treatment of multilevel cervical spondylotic radiculopathy Herkowitz82 compared the outcomes and complication rates between anterior cervical fusion, laminectomy, and laminoplasty. He noted a successful outcome in 92% of 33 patients in the anterior fusion group, 86% of 15 patients in the laminoplasty group, and 66% of 12 patients in the laminectomy group. Complication rate in the anterior procedure group was reported to be 71%, whereas the complication rate of the laminoplasty group was only 13%. For the treatment of multilevel CSM, Herkowitz82 recommended anterior decompression for most patients. For patients with myelopathy secondary to a congenitally narrow cervical spine, however, he recommended the use of laminoplasty as the decompressive procedure of choice. Kawano and colleagues214 noted a 78% improvement rate in 14 patients after anterior surgery versus a 46% improvement rate in 61 patients after laminoplasty or laminectomy. They reported that better long-term outcomes were observed after anterior decompressive procedures rather than posterior decompressive procedures.
In a comparison of laminectomy versus anterior decompression and fusion for the treatment of patients with spondylotic myelopathy, Ebersold and colleagues215 reported a greater risk of long-term worsening after laminectomy. In the immediate postoperative period, they observed 72% of 49 patients in the anterior decompression group noting an improvement and 68% of 51 patients in the laminectomy group noting an improvement. Although they observed comparable improvements in neurologic rates between the two groups in the immediate postoperative period, the frequency of delayed deterioration observed in the laminectomy group was far greater. Ebersold and colleagues215 observed delayed deterioration in 10% of patients in the laminectomy cohort versus 2% of patients in the ACDF cohort.
Combined Anterior-Posterior Procedures
Although many authors report satisfactory results using a solitary anterior or posterior approach in the treatment of multilevel CSM, pseudarthrosis, graft failure, progressive kyphotic deformity, and instrumentation complications remain significant concerns.77,167,216,217 Circumferential fusion provides immediate rigid stabilization and restoration of the sagittal balance of the cervical spine. It should be considered in patients requiring decompression involving multiple levels owing to spondylosis, OPLL, congenital stenosis, or kyphotic deformity. It should also be considered in patients with medical comorbidities or predisposing risk factors that are associated with poor bone quality or inability to heal.218 A posterior stabilization should be considered in cases of multilevel postlaminectomy kyphosis in which anterior stabilization has been previously performed.
Riew and colleagues216 reported their experience in treating postlaminectomy patients with ACCF and postoperative halo immobilization. They showed early graft-related complications in 56% of 16 postlaminectomy patients, including graft extrusion and nonunion. Because of the high complication rate, these authors recommended augmenting anterior corpectomy with posterior fusion and instrumentation. They proved that halo vest immobilization was inadequate to prevent graft extrusion after complex anterior reconstructions for the treatment of postlaminectomy kyphosis. When faced with a situation in which a three-level corpectomy must be performed, many surgeons choose to supplement an anterior procedure with posterior fixation to decrease the likelihood of graft migration and pseudarthrosis.66,73
Kim and Alexander219 described 35 combined anterior-posterior stabilization procedures performed at their institution over 5 years. They observed no graft-related or instrumentation-related complications, and they did not observe any development of nonunion. Using fibular strut allograft with anterior cervical plates supplemented with posterior instrumentation and lateral mass plates, Shultz and colleagues218 reported a 100% fusion rate in 72 patients who underwent single-stage multilevel anterior-posterior decompression and fusion. None of their patients required a reoperation, and there was no evidence of graft extrusion or clinically significant hardware complications. In a similar study, all 17 patients who underwent multilevel subtotal corpectomy with allograft strut fusion and concurrent posterior fusion with lateral mass plates achieved a solid arthrodesis.220 There was no visible radiographic change in postoperative sagittal alignment.
Spivak and colleagues221 observed that performing a combined anterior-posterior spinal fusion in a single setting resulted in decreased anesthesia, decreased operative times, decreased total blood loss, and an overall shorter hospital stay compared with a two-stage anterior-posterior fusion procedure. Epstein222 published a series of 22 patients who underwent a combined anterior-posterior decompression and fusion. Marked neurologic recovery was noted in all patients in his series. Two patients had subacute plate extrusion. In a more recent study, Konya and colleagues223 described the outcomes in their patients with multilevel CSM who underwent a combined anterior-posterior surgical approach. Of patients, 31 had four-level disease, and 9 patients had three-level disease. At 1-year follow-up neurologic function was improved in all 40 patients as assessed by the Nurick classification. A 97.5% fusion rate was obtained, and no complications related to instrumentation were noted.
Intraoperative Neurologic Monitoring
Historically, the most common method for spinal cord monitoring was the Stagnara wakeup test, which provided for an assessment of the patient’s overall motor function.224 This test could not be used in a continuous fashion, and it did not provide any information regarding individual nerve root function. New techniques in spinal cord assessment have revolutionized the practice of neurophysiologic monitoring. The ultimate goal of any monitoring technique is to minimize the potential for intraoperative neurologic deterioration. May and colleagues225 identified potential risk factors for neurologic deterioration as preoperative myelopathy, multilevel disease, upper cervical surgery, use of instrumentation, and use of manipulation.
Somatosensory evoked potentials (SSEPs) assess the function of the sensory pathways of the dorsal column of the spinal cord.226 The primary blood supply to the dorsal column of the spinal cord is derived from the posterior spinal artery. If a change in SSEPs is observed, resuscitative measures to promote spinal cord perfusion are encouraged. Resuscitative efforts should include increase in oxygenation, decrease in inhalation or intravenous anesthetics usage, use of warm irrigating solutions, and induction of hypertension. In addition, any manipulation causing undue tension on the cord should be stopped, which may include the release of distraction or the removal of an oversized graft. Parameters such as amplitude and latency are monitored continuously throughout the procedure, and any loss of amplitude of 50% to 60% is indicative of neuronal injury.227
Poorly defined or unrecordable SSEP tracings may occur in the presence of inhalation anesthetic agents. In an effort to overcome this complication, most spine centers avoid the use of any inhalation agents (e.g., isoflurane, desflurane, sevoflurane, nitrous oxide) and promote the use of an intravenous anesthetic regimen.228 For surgery involving the cervical spine, the ulnar nerve is the preferred stimulation site for SSEP monitoring because it assesses the entire cervical neural axis. In patients with severe myelopathy, Kombos and colleagues229 prospectively evaluated SSEP monitoring in 100 patients. They noted that SSEP changes occurred most commonly during positioning of the patient. These changes were most frequently seen in patients with myelopathy. The patient morbidity of 100 SSEP-monitored cervical procedures was compared by Epstein and colleagues230 with the morbidity of a historical control group of 218 patients who underwent cervical procedures without any neurologic monitoring. They observed that 3.7% of patients in the unmonitored group became quadriplegic, whereas no instances of quadriplegia were noted in the monitored group. They attributed this reduction in neurologic deficit to the early detection of cord compromise as a result of the neurologic monitoring.
The use of inhalation anesthetic agents and neuromuscular relaxation agents must be avoided when monitoring transcranial electrical motor evoked potentials. The intraoperative monitoring of transcranial electrical motor evoked potentials was described by Kitagawa and colleagues231 in a series of 20 patients who underwent surgery of the cervical spine. Five patients in their series were noted to have a 50% attenuation of transcranial electrical motor evoked potential parameters intraoperatively, which was immediately addressed with resuscitative measures. None of these patients developed any neurologic deficits postoperatively. One patient in this series developed complete quadriplegia and was noted to have complete loss of transcranial electrical motor evoked potentials intraoperatively.
Identification of an injury to a specific nerve root is best accomplished using electromyography (EMG). Spontaneous EMG is useful for assessing nerve root function during implant placement or nerve root manipulation, whereas stimulus-evoked EMG is useful for assessing cortical breach secondary to instrumentation. The nerve root is particularly susceptible to mechanical injury because it lacks a protective epineurium and perineurium. It is also susceptible to ischemic injury because there is a relatively hypovascular area between the proximal and middle third of the dorsal and ventral roots.232 In an effort to prevent postoperative C5 palsies during cervical surgery, Jimenez and colleagues233 evaluated the importance of monitoring continuous C5 EMG. These investigators compared 161 patients who were monitored with spontaneous EMG and stimulus-evoked EMG techniques with a historical control group of 55 patients who were monitored without the use of EMG techniques. They noted an incidence of postoperative C5 palsy in the monitored group of 0.9%, which was decreased from an incidence of 7.3% in the unmonitored control group.
Monitoring a single evoked potential parameter may not reflect the global status of the spinal cord. Motor and sensory pathways are separated in the spinal cord and have a varied blood supply. It is possible to have loss of SSEP secondary to an injury of the posterior spinal artery with complete sparing of motor function. Conversely, with injury to the anterior spinal artery, it is possible to have loss of motor function without any change in SSEP. For this reason, it is important to have multiple modalities assessing cord function during a decompressive cervical spine procedure. In an effort to reduce postoperative nerve root injuries, the complementary use of intraoperative transcranial electric motor evoked potentials and spontaneous EMG has been studied.234
With the use of multimodal intraoperative neuromonitoring, Hilibrand and colleagues235 reported their experience after a series of 427 cervical spine cases. Greater than 50% of the patients underwent surgery for CSM, and all were monitored with transcranial electric motor evoked potentials and SSEPs. In this study, 12 patients developed a substantial decrease in transcranial electric motor evoked potential amplitude that warranted prompt resuscitative efforts. Of the 12 patients, 10 had complete reversal of amplitude changes, whereas 2 patients awoke with a new motor deficit after surgery. Based on their findings, they strongly recommended the use of transcranial electric motor evoked potentials and SSEPs when operating on patients with CSM.
Summary
Pearls
Key Points
1 Wada E, Suzuki S, Kanazawa A, et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: A long-term follow-up study of over 10 years. Spine (Phila Pa 1976). 2001;26:1443-1447.
2 Emery SE, Bohlman HH, Bolesta MJ, et al. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy: Two to seventeen-year follow-up. J Bone Joint Surg Am. 1998;80:941-951.
This article describes the long-term follow-up of results and complications after ACDF.
3 Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine (Phila Pa 1976). 1988;13:774-780.
4 Yonenobu K, Hosono N, Iwasaki M, et al. Laminoplasty versus subtotal corpectomy: A comparative study of results in multisegmental cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1992;17:1281-1284.
5 Hirabayashi K, Watanabe K, Wakano K, et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976). 1988;8:693-699.
The results and complications after open-door laminoplasty in the treatment of CSM are reported.
1 Heller JG. The syndromes of degenerative cervical disease. Orthop Clin North Am. 1992;23:381-394.
2 Veidlinger OF, Colwell JC, Smyth HS, et al. Cervical myelopathy and its relationship to cervical stenosis. Spine (Phila Pa 1976). 1981;6:550-552.
3 Ono K, Ikata T, Yamada H, et al. Cervical myelopathy secondary to multiple spondylotic protrusions. Spine (Phila Pa 1976). 1977;2:218-221.
4 Clark CR. Cervical spondylotic myelopathy: History and physical findings. Spine (Phila Pa 1976). 1988;13:847-849.
5 Panjabi MM, Goel VK, Clark CR, et al. Biomechanical study of cervical spine stabilization with methylmethacrylate. Spine (Phila Pa 1976). 1985;10:198-203.
6 Lee S, Harris KG, Goel VK, et al. Spinal motion after cervical fusion: In vivo assessment with roentgen stereophotogrammetry. Spine (Phila Pa 1976). 1994;19:2336-2342.
7 Crandall P, Batzdorf U. Cervical spondylotic myelopathy. J Neurosurg. 1966;25:57-66.
8 Ferguson R, Caplan LR. Cervical spondylotic myelopathy. Neurol Clin. 1985;3:373-382.
9 Sadasivan KK, Reddy RP, Albright JA. The natural history of cervical spondylotic myelopathy. Yale J Biol Med. 1993;66:235-242.
10 Veidlinger OF, Colwell JC, Smyth HS, et al. Cervical myelopathy and its relationship to cervical stenosis. Spine (Phila Pa 1976). 1981;6:550-552.
11 Ebara S, Yonenobu K, Fujiwara K, et al. Myelopathy hand characterized by muscle wasting: A different type of myelopathy hand in patients with cervical spondylosis. Spine (Phila Pa 1976). 1988;13:785-791.
12 Clark E, Robinson P. Cervical myelopathy: A complication of cervical spondylosis. Brain. 1956;79:483.
13 Lees FT, Turner JW. Natural history and prognosis of cervical spondylosis. BMJ. 1963;2:1607-1610.
14 Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101-108.
15 Nurick S. Pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87-100.
15a Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87-100.
16 Epstein J, Epstein N. The surgical management of cervical spinal stenosis, spondylosis, and myeloradiculopathy by means of the posterior approach. In: Sherk H, Dunn EJ, Eismont FJ, et al, editors. The Cervical Spine: An Atlas of Surgical Procedures. Philadelphia: JB Lippincott; 1989:625-643.
17 Roberts A. Myelopathy due to cervical spondylosis treated by collar immobilization. Neurology. 1966;16:951-954.
18 Symon L, Lavender P. The surgical treatment of cervical spondylotic myelopathy. Neurology. 1967;17:117-126.
19 Law MDJr, Bernhardt M, White AA3rd. Cervical spondylotic myelopathy: A review of surgical indications and decision making. Yale J Biol Med. 1993;66:165-177.
20 Montgomery DM, Brower RS. Cervical spondylotic myelopathy: Clinical syndrome and natural history. Orthop Clin North Am. 1992;23:487-493.
21 Okada K, Shirasaki N, Hayashi H, et al. Treatment of cervical spondylotic myelopathy by enlargement of the spinal canal anteriorly, followed by arthrodesis. J Bone Joint Surg Am. 1991;73:352-364.
22 Bohlman HH. Cervical spondylosis with moderate to severe myelopathy: A report of 17 cases treated by Robinson anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 1977;2:151.
23 Wada E, Suzuki S, Kanazawa A, et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: A long-term follow-up study of over 10 years. Spine (Phila Pa 1976). 2001;26:1443-1447.
24 Tanaka J, Seki N, Doi K, et al. Operative results of canal-expansive laminoplasty for cervical spondylotic myelopathy in elderly patients. Spine (Phila Pa 1976). 1999;24:2308-2312.
25 Suri A, Chabbra RP, Mehta VS, et al. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3:33-45.
26 Satomi K, Nishu Y, Kohno T, et al. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 1994;19:507-510.
27 Handa Y, Kubota T, Ishii H, et al. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis: A retrospective comparison with younger patients. J Neurosurg Spine. 2002;96:173-179.
28 Al-Mefty O, Harkey HL, Marawi I, et al. Experimental chronic compressive cervical myelopathy. J Neurosurg. 1993;79:550-561.
29 Bernard TNJr, Whitecloud TSIII. Cervical spondylotic myelopathy and myeloradiculopathy: Anterior decompression and stabilization with autogenous fibula strut graft. Clin Orthop Relat Res. 1987;221:149-160.
30 Hasegawa K, Chiba Y, Hirano T, et al. Effects of surgical treatment for cervical spondylotic myelopathy in patients 70 years of age: A retrospective comparative study. J Spinal Disord. 2002;15:458-460.
31 Edwards WC, LaRocca H. The developmental segmental sagittal diameter of the cervical spinal canal in patients with cervical spondylosis. Spine (Phila Pa 1976). 1983;8:20-27.
32 Nordquist L. The sagittal diameter of the spinal cord and subarachnoid space in different age groups: A roentgenographic and post-mortem study. Acta Radiol. 1964;227:1-96.
33 Brieg A, Turnbull I, Hassler O. Effects of mechanical stresses on the spinal cord in cervical spondylosis: A study of fresh cadaver material. J Neurosurg. 1966;25:45-48.
34 Epstein NE. Circumferential surgery for the management of cervical ossification of the posterior longitudinal ligament. J Spinal Disord. 1998;11:200-207.
35 Morio Y, Teshima R, Nagashima H, et al. Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine (Phila Pa 1976). 2001;26:1238-1245.
36 Matsuda Y, Miyazaki K, Tada K, et al. Increased MR signal intensity due to cervical compression myelopathy: Analysis of 29 surgical cases. J Neurosurg. 1991;74:887-892.
37 Suda K, Abumi K, Ito M, et al. Local kyphosis reduces surgical outcomes of expansive open-door laminoplasty for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2003;28:1258-1261.
38 Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy: Pathophysiology, natural history, clinical evaluation. J Bone Joint Surg Am. 2002;84:1872-1881.
39 Chen IH, Vasavada A, Panjabi MM. Kinematics of the cervical spine canal: Changes with sagittal plane loads. J Spinal Disord. 1994;7:93-101.
40 Penning L. Some aspects of plain radiography of the cervical spine in chronic myelopathy. Neurology. 1962;12:513-519.
41 Bucciero A, Vizioli L, Carangelo B, et al. MR signal enhancement in cervical spondylotic myelopathy: Correlation with surgical results in 35 cases. J Neurosurg Sci. 1993;37:217-222.
42 Fujiwara K, Yonenobu K, Ebara S, et al. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71:393-398.
43 Kawakami M, Tamaki T, Iwasaki H, et al. A comparative study of surgical approaches for cervical compressive myelopathy. Clin Orthop Relat Res. 2000;381:129-136.
44 Yonenobu K, Fuji T, Ono K, et al. Choice of surgical treatment for multisegmental cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1985;10:710-716.
45 Sodeyama T, Goto S, Mochizuki M, et al. Effect of decompression enlargement laminoplasty for posterior shifting of the spinal cord. Spine (Phila Pa 1976). 1999;24:1527-1532.
46 Smith MD, Bolesta MJ, Leventhal M, et al. Postoperative cerebrospinal-fluid fistula associated with erosion of the dura: findings after anterior resection of ossification of the posterior longitudinal ligament in the cervical spine. J Bone Joint Surg Am. 1992;74:270-277.
47 Vaccaro AR, Ring D, Scuderi G, et al. Vertebral artery location in relation to the vertebral body as determined by two dimensional computed tomography evaluation. Spine (Phila Pa 1976). 1994;19:2637-2641.
48 Smith MD, Emery SE, Dudley A, et al. Vertebral artery injury during anterior decompression of the cervical spine: A retrospective review of ten patients. J Bone Joint Surg Br. 1993;75:410-415.
49 Emery SE, Smith MD, Bohlman HH. Upper-airway obstruction after multilevel cervical corpectomy for myelopathy. J Bone Joint Surg Am. 1991;73:544-551.
50 Emery SE, Bohlman HH, Bolesta MJ, et al. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy: Two to seventeen-year follow-up. J Bone Joint Surg Am. 1998;80:941-951.
51 Smith GW, Robinson RA. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40:607-623.
52 Kadoya S, Nakamura T, Kwak R. A microsurgical anterior osteophytectomy for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1984;9:437-441.
53 Robinson R, Walker AE, Ferlic DC, et al. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg Am. 1962;44:1569-1587.
54 Connolly PJ, Esses SI, Kostuik JP. Anterior cervical fusion: Outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202-206.
55 Stevens JM, Clifton AG, Whitear P. Appearances of posterior osteophytes after sound anterior interbody fusion in the cervical spine: A high-definition computed myelographic study. Neuroradiology. 1993;35:227-228.
56 Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien). 1989;99:41-50.
57 Zhang ZH, Yin H, Yang K, et al. Anterior intervertebral disc excision and bone grafting in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1983;8:16-19.
58 Yang KC, Lu XS, Cai QL, et al. Cervical spondylotic myelopathy treated by anterior multilevel decompression and fusion: Follow-up report of 214 cases. Clin Orthop Relat Res. 1987;221:161-164.
59 Irvine GB, Strachan WE. The long-term results of localised anterior cervical decompression and fusion in spondylotic myelopathy. Paraplegia. 1987;25:18-22.
60 Jamjoom A, Williams C, Cummins B. The treatment of spondylotic cervical myelopathy by multiple subtotal vertebrectomy and fusion. Br J Neurosurg. 1991;5:249-255.
61 Zdeblick TA, Bohlman HH. Cervical kyphosis and myelopathy: Treatment by anterior corpectomy and strut-grafting. J Bone Joint Surg Am. 1989;71:170-182.
62 Fessler RG, Steck JC, Giovanini MA. Anterior cervical corpectomy for cervical spondylotic myelopathy: A consecutive series with long term follow up evaluation. Neurosurgery. 1998;43:257-265.
63 Chibbaro S, Benvenuti L, Carnesecchi S, et al. Anterior cervical corpectomy for cervical spondylotic myelopathy: Experience and surgical results in a series of 70 consecutive patients. J Clin Neurosci. 2006;13:233-238.
64 Ashkenazi E, Smorgick Y, Rand N, et al. Anterior decompression combined with corpectomies and discectomies in the management of multilevel cervical myelopathy: A hybrid decompression and fixation technique. J Neurosurg Spine. 2005;3:205-209.
65 Saunders RL, Pikus HJ, Ball P. Four-level cervical corpectomy. Spine (Phila Pa 1976). 1998;23:2455-2461.
66 Vaccaro AR, Falatyn SP, Scuderi GJ, et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11:410-415.
67 Edwards C, Heller J, Morikami H, et al. Corpectomy versus laminoplasty for multi-level cervical myelopathy: An independent matched cohort study. Spine (Phila Pa 1976). 2002;27:1168-1175.
68 Lunsford LD, Bissonette DJ, Zorub DS. Anterior surgery for cervical disc disease: I. Treatment of lateral cervical disc herniation in 253 cases. J Neurosurg. 1980;53:1-11.
69 Robinson R, Walker AE, Ferlic DC, et al. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg Am. 1962;44:1569-1587.
70 Boni M, Cherubino P, Denaro V, et al. Multiple subtotal somatectomy: Technique and evaluation of a series of 39 cases. Spine (Phila Pa 1976). 1984;9:358-362.
71 Brigham CD, Tsahakis PJ. Anterior cervical foraminotomy and fusion: Surgical technique and results. Spine (Phila Pa 1976). 1995;20:766-770.
72 Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298-1307.
73 Sasso RC, Ruggiero RAJr, Reilly TM, et al. Early reconstruction failures after multilevel cervical corpectomy. Spine (Phila Pa 1976). 2003;28:140-142.
74 Hilibrand AS, Fye MA, Emery SE, et al. Increased rate of arthrodesis with strut grafting after multilevel anterior cervical decompression. Spine (Phila Pa 1976). 2002;27:146-151.
75 Swank ML, Lowery GL, Bhat AL, et al. Anterior cervical allograft arthrodesis and instrumentation: Multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6:138-143.
76 Hanai K, Fujiyoshi F, Kamei K. Subtotal vertebrectomy and spinal fusion for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1986;11:310-315.
77 Zdeblick TA, Hughes SS, Riew KD, et al. Failed anterior cervical discectomy and arthrodesis: Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79:523-532.
78 Dai L, Ni B, Yuan W, et al. Radiculopathy after laminectomy for cervical compression myelopathy. J Bone Joint Surg Br. 1998;80:846-849.
79 Wang JC, Hart RA, Emery SE, et al. Graft migration or displacement after multilevel cervical corpectomy and strut grafting. Spine (Phila Pa 1976). 2003;28:1016-1021.
80 Wang JC, McDononugh PW, Endow K, et al. The effect of cervical plating on single level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12:467-471.
81 Kaiser MG, Haid RWJr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229-238.
82 Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine (Phila Pa 1976). 1988;13:774-780.
83 Villas C, Martinez-Peric R, Preite R, et al. Union after multiple anterior cervical fusion: 21 cases followed for 1-6 years. Acta Orthop Scand. 1994;65:620-622.
84 Wetzel FT, Hoffman MA, Arcieri RR. Freeze-dried fibular allograft in anterior spinal surgery: Cervical and lumbar applications. Yale J Biol Med. 1993;66:263-275.
85 Park JB, Cho YS, Riew KD. Development of adjacent-level ossification in patients with an anterior cervical plate. J Bone Joint Surg Am. 2005;87:558-563.
86 DiAngelo DJ, Foley KT, Vossel KA, et al. Anterior cervical plating reverses load transfer through multilevel strut-grafts. Spine (Phila Pa 1976). 2000;25:783-795.
87 Foley KT, DiAngelo DJ, Rampersaud YR. The in vitro effects of instrumentation on multilevel cervical strut-graft mechanics. Spine (Phila Pa 1976). 1999;24:2366-2376.
88 Hanks SE, Kang JD. Late stress fracture of a well incorporated autologous fibula strut graft in the cervical spine: A case report. J Spinal Disord Tech. 2004;17:526-530.
89 Jones J, Yoo J, Hart R. Delayed fracture of fibular strut allograft following multilevel anterior cervical spine corpectomy and fusion. Spine (Phila Pa 1976). 2006;31:E595-E599.
90 Hee HT, Majd ME, Holt RT, et al. Complications of multilevel cervical corpectomies and reconstruction with titanium cages and anterior plating. J Spinal Disord Tech. 2003;16:1-8.
91 Majd ME, Vadhva M, Holt RT. Anterior cervical reconstruction using titanium cages with anterior plating. Spine (Phila Pa 1976). 1999;24:1604-1610.
92 Eleraky MA, Llanos C, Sonntag VK. Cervical corpectomy: Report of 185 cases and review of the literature. J Neurosurg. 1999;90(1 Suppl):35-41.
93 Curylo LJ, Mason HC, Bohlman HH, et al. Tortuous course of the vertebral artery and anterior cervical compression: A cadaveric and clinical case study. Spine (Phila Pa 1976). 2000;25:2860-2864.
94 Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003;28:134-139.
95 Schnee CL, Freese A, Weil RJ, et al. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine (Phila Pa 1976). 1997;22:2222-2227.
96 Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: A statistical evaluation. Spine (Phila Pa 1976). 1995;20:1055-1060.
97 Bohay DR, Manoli A2nd. Clawtoe deformity following vascularized fibula graft. Foot Ankle Int. 1995;16:607-609.
98 Bodde EW, de Visser E, Duysens JE, et al. Donor-site morbidity after free vascularized autogenous fibular transfer: Subjective and quantitative analyses. Plast Reconstr Surg. 2003;111:2237-2242.
99 Emery SE, Heller JG, Petersilge CA. Tibial stress fracture after a graft has been obtained from the fibula: A report of five cases. J Bone Joint Surg Am. 1996;78:1248-1251.
100 Vail TP, Urbaniak JR. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg Am. 1996;78:204-211.
101 Zdeblick TA, Ducker TB. The use of freeze-dried allograft bone for anterior cervical fusions. Spine (Phila Pa 1976). 1991;16:726-729.
102 Fernyhough JC, White JI, LaRocca H. Fusion rates in multi-level cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine (Phila Pa 1976). 1991;16(10 Suppl):S561-S564.
103 MacDonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86:990-997.
104 Samartzis D, Shen FH, Matthews DK, et al. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J. 2003;3:451-459.
105 Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519-528.
106 Rao RD, Wang M, McGrady LM, et al. Does anterior plating of the cervical spine predispose to adjacent segmental changes? Spine (Phila Pa 1976). 2005;30:2788-2793.
107 Uematsu Y, Tasuaki T, Matsuzaki H, et al. Radiculopathy after laminoplasty of the cervical spine. Spine (Phila Pa 1976). 1998;23:2057-2062.
108 Yonenobu K, Hosono N, Iwasaki M, et al. Neurologic complications of surgery for cervical compression myelopathy. Spine (Phila Pa 1976). 1991;13:1277-1282.
109 Yonenobu K, Okada K, Fuji T, et al. Causes of neurologic deterioration following surgical treatment of cervical myelopathy. Spine (Phila Pa 1976). 1986;11:818-823.
110 Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: A prospective study. Spine (Phila Pa 1976). 2002;27:2453-2458.
111 Smith-Hammond CA, New KC, Pietrobon R, et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: Comparison of anterior cervical, posterior cervical and lumbar procedures. Spine (Phila Pa 1976). 2004;29:1441-1446.
112 Newhouse KE, Lindsey RW, Clark CR, et al. Esophageal perforation following anterior cervical spine surgery. Spine (Phila Pa 1976). 1989;14:1051-1053.
113 Frempong-Boadu A, Houten JK, Osbom B, et al. Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: A prospective, objective preoperative and postoperative assessment. J Spinal Disord Tech. 2002;15:362-368.
114 Yue WM, Brodner W, Highland TR. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: A 5 to 11 year follow-up study. Eur Spine J. 2005;14:677-682.
115 Apfelbaum TI, Kriskovich MD, Haller JR. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine (Phila Pa 1976). 2000;25:2906-2912.
116 Baron EM, Soliman AM, Gaughan JP, et al. Dysphagia, hoarseness, and unilateral true vocal fold motion impairment following anterior cervical discectomy and fusion. Ann Otol Rhinol Laryngol. 2003;112:921-926.
117 Beutler WJ, Sweeney CA, Connolly PJ. Recurrent laryngeal nerve injury with anterior cervical spine surgery risk with laterality of surgical approach. Spine (Phila Pa 1976). 2001;26:1337-1342.
118 Snow RB, Weiner H. Cervical laminectomy and foraminotomy as surgical treatment of cervical spondylosis: A follow-up study with analysis of failures. J Spinal Disord. 1993;6:245-250.
119 Hukuda S, Mochizuki T, Ogata M, et al. Operations for cervical spondylotic myelopathy: A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67:609-615.
120 Aita I, Hayashi K, Wadano Y, et al. Posterior movement and enlargement of the spinal cord after cervical laminoplasty. J Bone Joint Surg Br. 1998;80:33-37.
121 Baba H, Maezawa Y, Furusawa N, et al. Flexibility and alignment of the cervical spine after laminoplasty for spondylotic myelopathy: A radiographic study. Int Orthop. 1995;19:116-121.
122 Hamanishi C, Tanaka S. Bilateral multilevel laminectomy with or without posterolateral fusion for cervical spondylotic myelopathy: Relationship to type of onset and time until operation. J Neurosurg. 1996;85:447-451.
123 Zdeblick TA, Zou D, Warden KE, et al. Cervical stability after foraminotomy: A biomechanical in vitro analysis. J Bone Joint Surg. 1992;74:22-27.
124 Yamazaki A, Homma T, Uchiyama S, et al. Morphologic limitation of posterior decompression by midsagittal splitting method for myelopathy caused by ossification of the posterior longitudinal ligament in the cervical spine. Spine (Phila Pa 1976). 1999;24:32-34.
125 Satomi K, Nishu Y, Kohno T, et al. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 1994;19:507-510.
126 Hirabayashi K, Toyama Y, Chiba K, et al. Expansive laminoplasty for myelopathy in ossification of the longitudinal ligament. Clin Orthop Relat Res. 1999;359:35-48.
127 Ishida Y, Suzuki K, Ohmori K, et al. Critical analysis of extensive cervical laminectomy. Neurosurgery. 1989;24:215-222.
128 Kohno K, Kumon Y, Oka Y, et al. Evaluation of prognostic factors following expansive laminoplasty for cervical spinal stenotic myelopathy. Surg Neurol. 1997;48:237-245.
129 Shaffrey C, Wiggins G, Piccirilli C, et al. Modified open-door laminoplasty for treatment of neurological deficits in younger patients with congenital spinal stenosis: Analysis of clinical and radiographic data. J Neurosurg. 1999;90:170-177.
130 Chiba K, Toyama Y, Watanabe M, et al. Impact of longitudinal distance of the cervical spine on the results of expansive open-door laminoplasty. Spine (Phila Pa 1976). 2000;25:2893-2898.
131 Raynor RB, Pugh J, Shapiro I. Cervical facetectomy and its effect on spine strength. J Neurosurg. 1985;63:278-282.
132 Kato Y, Iwasaki M, Fuji T, et al. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89:217-223.
133 Kaptain GJ, Simmons NE, Replogle RE, et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg. 2000;93:1999-2004.
134 Kumar VG, Rea GL, Mervis LJ, et al. Cervical spondylotic myelopathy: Functional and radiographic long-term outcome after laminectomy and posterior fusion. Neurosurgery. 1999;44:771-777.
135 Houten JK, Cooper PR. Laminectomy and posterior cervical plating for multi-level cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: Effects on cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery. 2003;52:1081-1088.
136 Weiland DJ, McAfee PC. Posterior cervical fusion with triple-wire strut graft technique: One hundred consecutive patients. J Spinal Disord. 1991;4:15-21.
137 Lovely TJ, Carl A. Posterior cervical spine fusion with tension-band wiring. J Neurosurg. 1995;83:631-635.
138 Gill K, Paschal S, Corin J, et al. Posterior plating of the cervical spine: A biomechanical comparison of different posterior fusion techniques. Spine (Phila Pa 1976). 1988;13:813-816.
139 Horgan MA, Kellogg JX, Chestnut RM. Posterior cervical arthrodesis and stabilization: An early report using a novel lateral mass screw and rod technique. Neurosurgery. 1999;44:1267-1271.
140 . Internal fixation of the unstable cervical spine by a posterior osteosynthesis with plates and screws The Cervical Spine. R Roy-Camille, G Saillant, C Mazel. New York: Lippincott. 1989:390-403.
141 Jeanneret B, Magerl F, Ward EH, et al. Posterior stabilization of the cervical spine with hook plates. Spine (Phila Pa 1976). 1991;16(3 Suppl):S56-S63.
142 An HS, Gordin R, Renner K. Anatomic considerations for plate-screw fixation of the cervical spine. Spine (Phila Pa 1976). 1991;16(10 Suppl):S548-S551.
143 Anderson PA, Henley MB, Grady MS, et al. Posterior cervical arthrodesis with AO reconstruction plates and bone graft. Spine (Phila Pa 1976). 1991;16(3 Suppl):S72-S79.
144 Montesano PX, Jauch E, Jonsson HJr. Anatomic and biomechanical study of posterior cervical spine plate arthrodesis: An evaluation of two different techniques of screw placement. J Spinal Disord. 1992;5:301-305.
145 Barrey C, Mertens P, Jund J, et al. Quantitative anatomic evaluation of cervical lateral mass fixation with a comparison of the Roy-Camille and the Magerl screw techniques. Spine (Phila Pa 1976). 2005;30:E140-E147.
146 Heller JG, Carlson GD, Abitbol JJ, et al. Anatomic comparison of the Roy-Camille and Magerl techniques for screw placement in the lower cervical spine. Spine (Phila Pa 1976). 1991;16(10 Suppl):S552-S557.
147 Seybold EA, Baker JA, Criscitiello AA, et al. Characteristics of unicortical and bicortical lateral mass screws in the cervical spine. Spine (Phila Pa 1976). 1999;24:2397-2403.
148 Abumi K, Kaneda K, Shono Y, et al. One-stage posterior decompression and reconstruction of the cervical spine by using pedicle screw fixation systems. J Neurosurg. 1999;90(1 Suppl):19-26.
149 Jones EL, Heller JG, Sicox DH, et al. Cervical pedicle screws versus lateral mass screws: Anatomic feasibility and biomechanical comparison. Spine (Phila Pa 1976). 2007;22:977-982.
150 Abumi K, Shono Y, Ito M, et al. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine (Phila Pa 1976). 2000;25:962-969.
151 Meyers MA, Hamilton SR, Bogosian AJ, et al. Visual loss as a complication of spine surgery: A review of 37 cases. Spine (Phila Pa 1976). 1997;22:1325-1329.
152 Iizuka H, Shimizu T, Tateno K, et al. Extensor musculature of the cervical spine after laminoplasty. Spine (Phila Pa 1976). 2001;26:2220-2226.
153 Huang RC, Girardi FP, Poynton AR, et al. Treatment of multilevel cervical spondylotic myeloradiculopathy with posterior decompression and fusion with lateral mass plate fixation and local bone graft. J Spinal Disord Tech. 2003;16:123-129.
154 Sekhon LH. Posterior cervical decompression and fusion for circumferential spondylotic cervical stenosis: Review of 50 consecutive cases. J Clin Neurosci. 2006;13:23-30.
155 Mikawa Y, Shikata J, Yamamuro T, et al. Spinal deformity and instability after multilevel cervical laminectomy. Spine (Phila Pa 1976). 1987;12:6-11.
156 Morimoto T, Okuno S, Nakase H, et al. Cervical myelopathy due to dynamic compression by the laminectomy membrane: Dynamic MR imaging study. J Spinal Disord. 1999;12:172-173.
157 Dai L, Ni B, Yuan W, et al. Radiculopathy after laminectomy for cervical compression myelopathy. J Bone Joint Surg Br. 1998;80:846-849.
158 Sim FH, Suien HJ, Bickel WH, et al. Swan neck deformity following extensive cervical laminectomy. J Bone Joint Surg Am. 1974;56:564-580.
159 Cusick J, Pintar FA, Yoganandan N, et al. Biomechanical alterations induced by multilevel cervical laminectomy. Spine (Phila Pa 1976). 1995;20:2392-2399.
160 Albert T, Vacarro A. Postlaminectomy kyphosis. Spine (Phila Pa 1976). 1998;23:2738-2745.
161 Bell DF, Walker JL, O’Connor G, et al. Spinal deformity after multiple-level cervical laminectomy in children. Spine (Phila Pa 1976). 1994;19:406-411.
162 Yasuoka S, Peterson HA, Laws ERJr, et al. Pathogenesis and prophylaxis of postlaminectomy deformity of the spine after multiple level laminectomy: Difference between children and adults. Neurosurgery. 1981;9:145-152.
163 Yasuoka S, Peterson HA, MacCarty CS, et al. Incidence of spinal column deformity after multilaminectomy in children and adults. J Neurosurg. 1982;57:441-445.
164 Voo LM, Kumaresan S, Yoganandan N, et al. Finite element analysis of cervical facetectomy. Spine (Phila Pa 1976). 1997;22:964-969.
165 Nowinski GP, Visarius H, Nolte LP, et al. A biomechanical comparison of cervical laminoplasty and cervical laminectomy with progressive facetectomy. Spine (Phila Pa 1976). 1993;18:1995-2004.
166 Heller JG, Silcox DH3rd, Sutterline CE3rd. Complications of posterior cervical plating. Spine (Phila Pa 1976). 1995;20:2442-2448.
167 Wellman BJ, Follett KA, Traynelis VC. Complications of posterior articular mass plate fixation of the subaxial cervical spine in 43 consecutive patients. Spine (Phila Pa 1976). 1998;23:193-200.
168 Pal PP, Cooper HH. The vertical stability of the cervical spine. Spine (Phila Pa 1976). 1988;13:447-449.
169 Tsuzuki N, Abe R, Saiki K, et al. Tension band laminoplasty of the cervical spine. Int Orthop. 1996;20:275-284.
170 Baba H, Uchida K, Maezawa Y, et al. Lordotic alignment and posterior migration of the spinal cord following en bloc open door laminoplasty for cervical myelopathy: A magnetic resonance imaging study. J Neurol. 1996;243:626-632.
171 Baba H, Uchida K, Maezawa Y, et al. Three-dimensional computed tomography for evaluation of cervical spinal canal enlargement after en bloc open-door laminoplasty. Spinal Cord. 1997;35:674-679.
172 Shimamura T, Kato S, Toba T, et al. Sagittal splitting laminoplasty for spinal canal enlargement for ossification of the spinal ligaments (OPLL and OLF). Semin Musculoskel Radiol. 2001;5:203-206.
173 Kawakami M, Tamaki T, Yamada H, et al. Relationships between sagittal alignment of the cervical spine and morphology of the spinal cord and clinical outcomes in patients with cervical spondylotic myelopathy treated with expansive laminoplasty. J Spinal Disord. 2002;15:391-397.
174 Oyama M, Hattori S, Moriwaki N, et al. A new method of posterior decompression [In Japanese]. Chubuseisaisi. 1973;16:792.
175 Hirabayashi K, Watanabe K, Wakano K, et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976). 1988;8:693-699.
176 Kurokawa T, Tsuyama N, Tanaka H, et al. Enlargement of spinal canal by the sagittal splitting of the spinous process. Bessatsu Seikei Geka. 1982;2:234-240.
177 Yue WM, Tan CT, Tan SB, et al. Results of cervical laminoplasty and a comparison between single and double trap-door techniques. J Spinal Disord. 2000;13:329-335.
178 Naito M, Ogata K, Kurose S, et al. Canal expansive laminoplasty in 83 patients with cervical myelopathy: A comparative study of three different procedures. Int Orthop. 1994;18:347-351.
179 Okada M, Minamide A, Endo T, et al. A prospective randomized study of clinical outcomes in patients with cervical compressive myelopathy treated with open-door or French-door laminoplasty. Spine (Phila Pa 1976). 2009;34:1119-1126.
180 Hirabayashi K. Expansive open-door laminoplasty for cervical spondylotic myelopathy [In Japanese]. Jpn J Surg. 1978;32:1159-1163.
181 Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976). 1981;6:354-364.
182 Itoh T, Tsuji H. Technical improvement and results of laminoplasty for compressive myelopathy in the cervical spine. Spine (Phila Pa 1976). 1985;10:729-736.
183 Kamo Y, Takemitsu Y, Hamada O, et al. Cervical laminoplasty by splitting the spinous process using a AW glass-ceramic lamina spacer. Rinsho Seikeigeka. 1992;10:1115-1122.
184 Nakano K, Harata S, Suetsuna F, et al. Spinous process splitting laminoplasty using hydroxyapatite spinous process spacer. Spine (Phila Pa 1976). 1992;17:41-43.
185 O’Brien MF, Petersen D, Casey AH, et al. A novel technique for laminoplasty augmentation of spinal canal area using titanium miniplate stabilization: A computerized morphometric analysis. Spine (Phila Pa 1976). 1996;21:474-484.
186 Tomita K, Kawahara N, Toribatake Y, et al. Expansive midline T-saw laminoplasty (modified spinous process-splitting) for the management of cervical myelopathy. Spine (Phila Pa 1976). 1998;23:32-37.
187 Hirabayashi K, Toyama Y, Chiba K, et al. Expansive laminoplasty for myelopathy in ossification of the longitudinal ligament. Clin Orthop Relat Res. 1999;359:35-48.
188 Kawaguchi Y, Kanamori M, Ishihara H, et al. Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am. 2001;83:1798-1802.
189 Iwasaki M, Kawaguchi Y, Kimura T, et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: More than 10 years follow up. J Neurosurg. 2002;96(Spine 2):180-189.
190 Hirabayashi K, Satomi K. Operative procedure and results of expansive open door laminoplasty. Spine (Phila Pa 1976). 1988;13:870-876.
191 Kawaguchi Y, Kanamori M, Ishihara H, et al. Minimum 10 year follow-up after en bloc cervical laminoplasty. Clin Orthop Relat Res. 2003;411:129-139.
192 Seichi A, Takeshita K, Ohishi I, et al. Long-term results of double-door laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 2001;26:479-487.
193 Matsumoto M, Watanabe K, Tsuji T, et al. Risk factors for closure of lamina after open-door laminoplasty. J Neurosurg Spine. 2008;9:530-537.
194 Itoh K, Miura Y, Imakure A, et al. Cervical radiculopathy occurring after expansive laminoplasty. Higashi Nippon Rinsho Seikeigeka Gakkai Zasshi. 1993;5:306-310.
195 Herkowitz HN. Cervical laminoplasty: Its role in the treatment of cervical radiculopathy. J Spinal Disord. 1988;1:179-188.
196 Sasai K, Saito T, Akagi S, et al. Clinical study of cervical radiculopathy after laminoplasty for cervical myelopathy. Nippon Seikeigeka Gakkai Zashi. 1995;69:1237-1247.
197 Sasai K, Saito T, Akagi S, et al. Preventing C5 palsy after laminoplasty. Spine (Phila Pa 1976). 2003;28:1972-1977.
198 Chiba K, Toyama Y, Matsumoto M, et al. Segmental motor paralysis after expansive open-door laminoplasty. Spine (Phila Pa 1976). 2002;27:2108-2115.
199 Moskovich R. Neck pain in the elderly: Common causes and management. Geriatrics. 1988;43:65-92.
200 Kawaguchi Y, Matsui H, Ishihara H, et al. Axial symptoms after en bloc cervical laminoplasty. J Spinal Disord. 1999;12:392-395.
201 Hosono N, Yonenobu K, Ono K, et al. Neck and shoulder pain after laminoplasty: A noticeable complication. Spine (Phila Pa 1976). 1996;21:1969-1973.
202 Takeuchi T, Yasuhiro S. Importance of preserving the C7 spinous process and attached nuchal ligament in French-door laminoplasty to reduce postoperative axial symptoms. Eur Spine J. 2007;16:1417-1422.
203 Kimura I, Shingu H, Nasu Y. Long-term follow-up of cervical spondylotic myelopathy treated by canal-expansive laminoplasty. J Bone Joint Surg Br. 1995;77:956-961.
204 Saruhashi Y, Hakuda S, Hatsuura A, et al. A long-term follow-up study of cervical spondylotic myelopathy treated by “French window” laminoplasty. J Spinal Disord. 1999;12:99-101.
205 Hyun SJ, Rhim SC, Roh SW, et al. The time course of range of motion loss after cervical laminoplasty. Spine (Phila Pa 1976). 2009;34:1134-1139.
206 Kawaguchi Y, Kanamori M, Ishiara H, et al. Preventive measures for axial symptoms following cervical laminoplasty. J Spinal Disord Tech. 2003;16:497-501.
207 Sasai K, Saito T, Akagi S, et al. Cervical curvature after laminoplasty for spondylotic myelopathy—involvement of yellow ligament, semispinalis cervicis muscle, and nuchal ligament. J Spinal Disord. 2000;13:26-30.
208 Matsunaga S, Sakou T, Nakanisi K, et al. Analysis of the cervical spine alignment following laminoplasty and laminectomy. Spinal Cord. 1999;37:20-24.
209 Hayashi N, Yoshida M, Iwahashi T, et al. The effect of cervical posterior decompression to cervical paravertebral muscles. Chuba Nippon Seikeigeka Saigaigeka Gakkai Zasshi. 1989;32:2467-2469.
210 Fujimura Y, Nishi Y. Atrophy of the nuchal muscle and change in cervical curvature after expansive open-door laminoplasty. Arch Orthop Trauma Surg. 1996;115:203-205.
211 Matsuzaki H, Hoshino M, Kiuchi T, et al. Dome like expansive laminoplasty for the second cervical vertebrae. Spine (Phila Pa 1976). 1989;14:1198-1203.
212 Heller JG, Edwards CC, Murakami H, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: An independent matched cohort analysis. Spine (Phila Pa 1976). 2001;26:1330-1336.
213 Yonenobu K, Hosono N, Iwasaki M, et al. Laminoplasty versus subtotal corpectomy: A comparative study of results in multisegmental cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1992;17:1281-1284.
214 Kawano H, Hand Y, Ishii H, et al. Surgical treatment for ossification of the posterior longitudinal ligament of the cervical spine. J Spinal Disord. 1995;8:145-150.
215 Ebersold MJ, Pare MC, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82:745-751.
216 Riew KD, Hiligrand AS, Palumbo MA, et al. Anterior cervical corpectomy in patients previously managed with a laminectomy: Short-term complications. J Bone Joint Surg Am. 1999;81:950-957.
217 Herman JM, Sonntag VKH. Cervical corpectomy and plate fixation for postlaminectomy kyphosis. J Neurosurg. 1994;80:963-970.
218 Schultz KDJr, McLaughlin MR, Haid RWJr, et al. Single stage anterior posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(Suppl 2):214-221.
219 Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(Suppl 1):299-307.
220 Swank ML, Sutterlin CEIII, Bossons CR, et al. Rigid internal fixation with lateral mass plates in multilevel anterior and posterior reconstruction of the cervical spine. Spine (Phila Pa 1976). 1997;22:274-282.
221 Spivak JM, Neuwirth MG, Giordano CP, et al. The perioperative course of combined anterior and posterior spinal fusion. Spine (Phila Pa 1976). 1994;19:520-525.
222 Epstein NE. The value of anterior cervical plating in preventing vertebral fracture and graft extrusion following multilevel anterior cervical corpectomy with posterior wiring/fusion: Indications, results, and complications. J Spinal Disord. 2000;13:9-15.
223 Konya D, Ozgen S, Gercek A, et al. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16:404-409.
224 Vauzelle C, Stagnara P, Jouvinrous P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop Relat Res. 1973;93:173-178.
225 May DM, Jones SJ, Crockard HA. Somatosensory evoked potential monitoring in cervical surgery: Identification of pre- and intraoperative risk factors associated with neurological deterioration. J Neurosurg. 1996;85:566-573.
226 Powers SK, Bolger CA, Edwards MB. Spinal cord pathways mediating somatosensory evoked potentials. J Neurosurg. 1982;57:472-478.
227 Devlin VJ, Anderson PA, Schwartz DM, et al. Intraoperative neurophysiologic monitoring: Focus on cervical myelopathy and related issues. Spine J. 2006;6(1 Suppl):212-224.
228 Scheufler KM, Zentner J. Total intravenous anesthesia for intraoperative monitoring of the motor pathways: An integral view combining clinical and experimental data. J Neurosurg. 2002;96:571-579.
229 Kombos T, Suess O, DaSilva C, et al. Impact of somatosensory evoked potential monitoring on cervical surgery. J Clin Neurophysiol. 2003;20:122-128.
230 Epstein NE, Danto JD, Nardi D. Evaluation of intraoperative somatosensory evoked potential monitoring on cervical surgery. J Clin Neurophysiol. 2003;20:122-128.
231 Kitagawa H, Itoh T, Takano H, et al. Motor evoked potential monitoring during upper cervical spine surgery. Spine (Phila Pa 1976). 1989;14:1078-1083.
232 Berthold CH, Carlstedt T, Corneliuson O. Anatomy of the nerve root at the central-peripheral transitional region. In: Dyck PJ, Thomas PK, Lambert EH, et al, editors. Peripheral Neuropathy. Philadelphia: WB Saunders; 1984:156-217.
233 Jimenez JC, Sani S, Braverman B, et al. Palsies of the fifth cervical nerve root after cervical decompression: Prevention using continuous intraoperative electromyography monitoring. J Neurosurg Spine. 2005;3:92-97.
234 Fan D, Schwartz DM, Vaccaro AR, et al. Intraoperative neurophysiologic detection of iatrogenic C5 nerve root injury during laminectomy for cervical compression myelopathy. Spine (Phila Pa 1976). 2002;27:2499-2502.
235 Hilibrand AS, Schwartz DM, Sethuraman V, et al. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical surgery. J Bone Joint Surg Am. 2004;86:1248-1253.