CHAPTER 36 Cervical Spondylosis
Pathophysiology, Natural History, and Clinical Syndromes of Neck Pain, Radiculopathy, and Myelopathy
Degenerative changes at the cervical discs and facet joints are ubiquitous in the adult population; these changes are a natural consequence of aging and are asymptomatic in most of the population. Spondylosis refers to these age-related degenerative changes within the spinal column.1 Most patients who present with cervical spondylosis are older than 40 years.2 Although most of these age-related degenerative changes remain asymptomatic, they can manifest as three main symptom complexes—axial neck pain, upper extremity radiculopathy, or myelopathy—or some combination thereof.1
Pathoanatomy
Degenerative changes within the cervical disc lead to loss of disc height, arthrosis in the uncovertebral and facet joints, and motion aberrations between two vertebral bodies.3 In most patients, desiccation of the disc initiates a cascade of degenerative changes.4 An alteration in proteoglycan content beginning in the 3rd decade diminishes the ability of the disc to maintain its hydration.1 The amount of keratin sulfate increases, and the amount of chondroitin sulfate decreases.4 With these changes in viscoelasticity, the periphery of the disc begins to bear an increasingly greater proportion of the load borne by the disc, with resultant loss of disc height and bulging of the anulus into the spinal canal.
As the disc loses height, the vertebral bodies approach each other, causing infolding of the ligamentum flavum and facet joint capsule and reducing the dimensions of the canal and the foramen.5 The anterior height of the disc is greater than the posterior height of the disc in a normally configured disc; with degeneration, the ventral portion of the disc loses height to a greater degree than the dorsal portion, and loss of cervical lordosis can occur.6 A positive feedback cycle ensues with greater force placed on the ventral aspect of the vertebral bodies leading to kyphosis.4 The uncovertebral and facet joints bear greater loads, accelerating the formation of osteophytes at these joints and at the peripheral vertebral endplate margins. Osteophytes, the posteriorly protruded disc material, and the infolded soft tissue within the canal and neuroforamina all diminish the space available for the spinal cord or nerve root. Radiographically, the C5-6 interspace is the most frequently affected level, followed closely by C6-7.7
Pathophysiology
Pathophysiology of Axial Neck Pain
Axial neck pain results from a multitude of potential causes and can be divided geographically into anterior neck pain, which usually stems from sprains and strains of the sternocleidomastoid and other strap muscles and their attachments, and posterior neck pain, which can be subdivided further into subaxial and suboccipital locations.8 In many patients, subaxial neck pain results from muscular or ligamentous imbalances related to poor posture, faulty ergonomics, or muscle fatigue or stress or both. Muscular pain often occurs as a result of postural adaptations to a primary pain source located in the shoulder, the craniovertebral junction, or the temporomandibular joint.5
The physiology of this pain process is not yet fully delineated. Patients with chronic myofascial pain have significantly lower levels of high-energy phosphates in the involved muscle tissue.9 It is unknown whether the diminished level of high-energy phosphates causes the pain or if it is a result of the pain. Unencapsulated free nerve endings in the neck musculature function as chemonociceptive units. Fatigued muscle generates anaerobic metabolites, which accumulate and can stimulate these chemonociceptive nerve endings. These free nerve endings also respond to non-neurogenic pain mediators released as a result of ischemia or injury, such as bradykinin, histamine, serotonin, and potassium ions. Primary muscle pain may result from sensitization of these nerve endings.
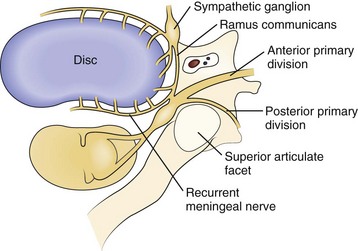
Axial neck pain should be attributed to degenerative changes in the cervical discs or facet joints only after careful consideration, owing to the ubiquitous nature of these changes in the spine. Nevertheless, multiple studies suggest that cervical discs and facet joints can generate pain.10–14 Nerve fibers and nerve endings, containing somatic afferent fibers, innervate the peripheral portion of the intervertebral disc (the outer third of the anulus) and offer a potential mechanism by which degenerative cervical discs generate pain directly. The sinuvertebral nerve, formed by branches of the ventral nerve root and by the sympathetic plexus, innervates the intervertebral disc (Fig. 36–1). When formed, the sinuvertebral nerve turns back into the intervertebral foramen along the posterior aspect of the disc, supplying portions of the anulus, posterior longitudinal ligament, periosteum of the vertebral body and pedicle, adjacent epidural veins, and dura mater.10 A review of a 12-year cervical discography experience suggests that stimulation of each disc results in consistent and predictable patterns of neck pain (Fig. 36–2).14
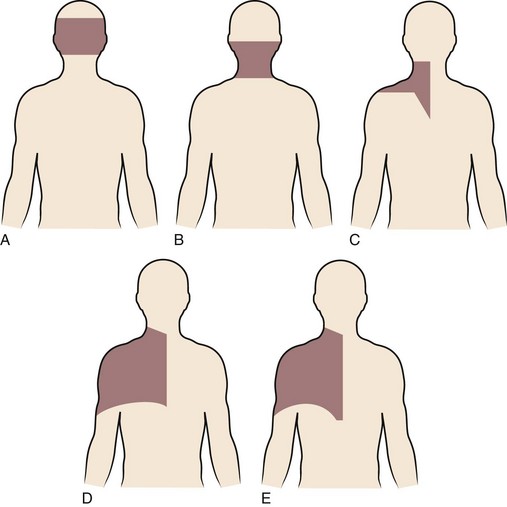
Degenerative changes at a cervical facet joint can be a source of axial neck pain. Provocative injections into the facet joints of asymptomatic volunteers result in a reproducible pattern of axial neck pain and shoulder girdle pain (Fig. 36–3).12 Controlled injection of anesthetic into the symptomatic facet joint or into the dorsal primary rami blocks these patterns of facet pain, suggesting that the facet joint plays a role in the development of axial neck pain. C3-4 to C8-T1 facet joints receive their innervation from the medial branches of the cervical dorsal rami above and below each joint, whereas the third occipital nerve innervates the C2-3 facet joint.11 The presence of mechanoreceptors and nociceptive nerve endings in cervical facet joint capsules further supports a possible role for these structures in the pathogenesis of cervical spine pain.15 Immunohistochemical studies show the presence of free nerve endings reactive for pain-related peptides located in the synovial folds of the human cervical facet joint.
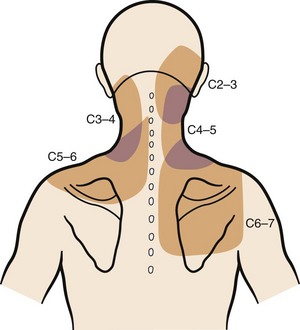
FIGURE 36–3 Composite map of axial pain patterns from facet joints at C2-3 to C6-7.
(From Dwyer A, Aprill C, Bogduk N: Cervical zygapophyseal joint pain patterns. I. A study in normal volunteers. Spine [Phila Pa 1976] 15:453-457, 1990.)
Suboccipital pain radiating down into the neck or to the back of the ear may be a manifestation of degenerative arthritis in the upper cervical spine. Injection of the atlanto-occipital and atlantoaxial joints results in a reproducible pain pattern in this region, with the atlanto-occipital joints showing the capacity to generate intense and diffuse pain.16 Wächli and colleagues17 reported unilateral headaches and atypical facial pain as a result of degenerative changes at the C2-3 level. Some patients with suboccipital headaches presumably have irritation of the greater occipital nerve, which originates from the posterior rami of C2, C3, and C4.18 The sinuvertebral nerves from C2 and C3 exist as another potential source of suboccipital pain, ascending proximally to innervate the atlantoaxial ligaments, tectorial membrane, and dura mater of the upper cervical cord and posterior cranial fossa.10
Pathophysiology of Radiculopathy
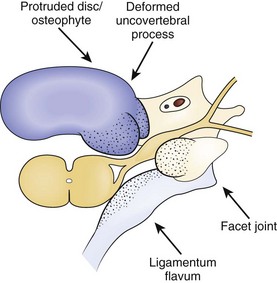
Radicular findings in the arm originate from the cervical nerve roots at some point between their origins as nerve rootlets from the spinal cord and their transition into peripheral nerves as they emerge from the neural foramen. Degenerative changes at the cervical motion segment, soft disc herniations, stenosis, intrinsic nerve root pathology, and trauma all can result in these symptoms. Loss of disc height leads to impingement on the nerve root origins from disc bulging, infolding of the facet joint capsule and ligamentum flavum, and osteophyte formation at the disc margins (hard disc formation) and at the uncovertebral and facet joints, all of which result in foraminal stenosis and radiculopathy (Fig. 36–4). Osteophytic spur formation may also compromise the blood supply to the nerve roots. Osteophytes may compress radicular arteries within the dural root sleeves leading to spasm and reduced vascular perfusion. Additionally, blockage of venous outflow may occur, resulting in edema and further compromise of the blood supply of the nerve roots.19
Mechanical deformation of the nerve root may lead to motor weakness or sensory deficits. The exact pathogenesis of radicular pain is unclear, but the general belief exists that in addition to the compression, an inflammatory response must occur for pain to develop. Within the compressed nerve root, intrinsic vessels show increased permeability, secondarily resulting in edema of the nerve root. Chronic edema and fibrosis (scar) within the nerve root play a role in altering the response threshold and heighten the sensitivity of the nerve root to pain.20 Neurogenic chemical pain mediators released from the sensory neuron cell bodies and non-neurogenic mediators released from disc tissue may initiate and perpetuate this inflammatory response (Table 36–1).21,22
| Neurogenic | Non-neurogenic |
|---|---|
| Substance P | Bradykinin |
| Somatostatin | Serotonin |
| Cholecystokininlike substance | Histamine |
| Vasoactive intestinal peptide | Acetylcholine |
| Calcitonin gene-related peptide | Prostaglandin E1 |
| Gastrin-releasing peptide | Prostaglandin E2 |
| Dynorphin | Leukotrienes |
| Enkephalin | diHETE |
| Gelanin | |
| Neurotensin | |
| Angiotensin II |
diHETE, dihydroxyeicosatetraenoic acid
From Chabot MC, Montgomery DM: The pathophysiology of axial and radicular neck pain. Semin Spine Surg 7:2-8, 1995.
Dynamic factors in the cervical spinal column affect the amount of nerve root compression. Flexion of the cervical spine lengthens the cervical neural foramina 18% to 31%, whereas extension shortens the foramina 16% to 22%.23 Rotation to the ipsilateral side narrows the foramen, whereas rotation to the contralateral side widens the foramen. The facet joint capsule and ligamentum flavum buckle with extension, narrowing the foraminal dimensions further. Translation or angulation between vertebral bodies in flexion or extension may result in increased stretch on the nerve root and predispose the individual to radicular symptoms. Patients who do not have nerve root compression with their necks in a static, neutral position may dynamically compress the nerve root during normal activities, resulting in radicular symptoms.
Changes in intrinsic tension within the nerve root have the ability to alter radicular pain. Davidson and colleagues24 postulated that the decrease in tension within the nerve root caused by a patient resting the hand atop the head—the shoulder abduction sign—relieves radicular pain. These investigators also postulated that this change in arm position lifts the sensory root, or dorsal root ganglion, directly cephalad or lateral to the source of compression and that this position decompresses the epidural veins, augmenting pain relief. Another study suggested that the abducted arm position allows relative laxity in the dural ligaments (of Hoffman), resulting in decreased tension on the nerve root.25
Often, patients present with radicular pain in an atypical distribution.26 An anatomic human cadaveric study confirmed the high incidence of intradural connections among C5, C6, and C7 dorsal rootlets (noted to be anatomic variants because of their high incidence rather than anatomic anomalies) and postulated that these variant intradural connections potentially explain the clinical variation and overlapping sensory symptoms frequently observed with cervical spine nerve root compression.27
Pathophysiology of Myelopathy
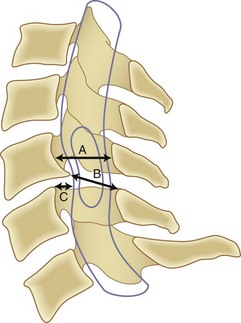
Although it is generally agreed that mechanical compression of the spinal cord is the primary pathophysiologic mechanism resulting in myelopathy, in many patients a combination of this static compression with dynamic factors secondary to motion between the vertebral bodies, a congenitally stenotic canal, changes in the intrinsic morphology of the spinal cord, and vascular factors contributes to the development of myelopathy. A developmentally narrow spinal canal in the anteroposterior plane can contribute to the development of cervical myelopathy. The normal anteroposterior diameter of the cervical spine measures 17 to 18 mm in adults, and the anteroposterior diameter of the spinal cord in the cervical region measures approximately 10 mm. An anteroposterior diameter of the spinal canal less than 13 mm defines congenital cervical stenosis, whereas a diameter greater than 16 mm suggests a relatively low risk of myelopathy (Fig. 36–5A).28,29 A congenitally narrow spinal canal lowers the threshold at which the cumulative effects of various degenerative structures encroaching on the spinal cord cause signs and symptoms of myelopathy.30
A strong association exists between flattening of the cord within the narrowed spinal canal and the development of cervical myelopathy. Penning and colleagues31 believed that symptoms of cord compression occurred when cross-sectional area of the cord had been reduced by a critical amount (30%) and the remaining transverse area of the cord was less than 60 mm2. Houser and colleagues32 contended that the extent and shape of flattening of the spinal cord serve as an indicator of neurologic deficit: 98% of their patients with severe stenosis manifested by a banana-shaped spinal cord had clinical evidence of myelopathy. Ono and colleagues33 described an anteroposterior cord compression ratio calculated by dividing the anteroposterior diameter of the cord by the transverse diameter of the cord. A lower anteroposterior compression ratio (<0.40) correlated well with the areas of most severe injury of the cord histologically. The Pavlov ratio, which is the anteroposterior diameter of the spinal canal divided by the anteroposterior diameter of the vertebral body at the same level, as measured on a lateral radiograph, also indicates static compression; a value of 0.8 or less indicates a developmentally narrow cervical canal and stenosis of the canal.30,34
Segmental motion of the cervical spinal column affects the development of cervical myelopathy. Hyperextension of the neck narrows the spinal canal by shingling the laminae and buckling the ligamentum flavum ventrally into the canal. Extension and flexion of the neck may alter the diameter of the canal by 2 mm.35 Angulation or translation between vertebral bodies in flexion or extension may result in narrowing of the space available for the cord (Fig. 36–5B). Particularly during extension, retrolisthesis of a vertebral body can pinch the spinal cord between the inferoposterior margin of a vertebral body and the superior edge of the lamina caudad to it. Forward slippage of a vertebral body may compress the spinal cord between the superoposterior margin of the vertebral body below and the lamina above.30 Flexion of the spinal column aggravates this forward slippage. Retrolisthesis and anterolisthesis often cause myelopathy in elderly (≥70 years old) patients (Fig. 36–5C).28,36 Additionally, hypermobility at the third and fourth cervical levels cephalad to a degenerated and stiffened C4-5 segment commonly exists in elderly individuals, potentially resulting in myelopathy at the hypermobile C3-4 level.37 Research using a spinal cord model showed that the cord is more vulnerable to dynamic, repeated minor loading compared with severe static loading.38
Cervical spine flexion and extension cause morphologic changes within the spinal cord itself. Breig and colleagues39 showed that the spinal cord thickens and shortens with extension, which renders it more susceptible to pressure from the infolded ligamentum flavum or lamina. The spinal cord stretches with flexion, which may subject the cord to higher intrinsic pressure if it presses against a disc or a vertebral body anteriorly. Flexion of the cervical spine may cause stretch (strain) injury to the axons through tensile loading, resulting in increased permeability and myelin injury, rendering these already injured axons more susceptible to secondary injury from other processes, including ischemia.40
Barre41 first proposed in 1924 the possibility that vascular factors play a significant role in the development of cervical myelopathy. The acute development or progression of findings suggests vascular involvement. In two separate canine experiments, cervical cord ischemia superimposed on compression of the cord resulted in a dramatic increase in neurologic findings.42,43 The effects of compression and ischemia were additive and responsible for the clinical manifestations of myelopathy. These investigations also led to the proposal that ischemia may play an important role in the irreversibility of spinal compression.42 In a separate dog study, obstruction of the peripheral arterial plexus caused structural changes within the spinal cord.44
The classic study by Breig and colleagues39 established that blood flow through the anterior spinal artery and anterior radicular arteries diminishes when those vessels are tented over a disc or a vertebral body, but that this position does not have a substantial impact on flow through the tortuous posterior spinal arteries. Vessels considered most vulnerable to reduced blood flow include the transverse intramedullary arterioles, arising from the anterior sulcal arteries. These vessels perfuse the gray matter and adjacent lateral columns.45 Ischemia may also occur from venous congestion.46 One cell type known to be particularly sensitive to ischemic injury, the oligodendrocyte, plays a principal role in insulating axons with a myelin sheath. Oligodendrocyte death caused by ischemic insult, likely through the mechanism of oligodendrocyte apoptosis, may explain the demyelination and subsequent irreversible neurologic deficit associated with chronic cervical myelopathy.40,47
Severe compression results in pathologic changes within the spinal cord. The central gray matter and the lateral columns show the most changes, with cystic cavitation, gliosis, and demyelination most pronounced caudad to the compression site. The posterior columns and posterolateral tracts show wallerian degeneration cephalad to the site of compression. The irreversibility of these changes may explain why some patients fail to recover after decompressive surgery. The anterior white columns are relatively resistant to infarction, even in cases of severe compression.48
Natural History and Epidemiology of Neck Pain, Radiculopathy, and Myelopathy
Axial Neck Pain
Neck pain commonly affects adults of all ages and both sexes almost equally. Few population-based studies exist on the prevalence of neck pain. A study of the Saskatchewan adult population showed that neck pain is more prevalent than commonly thought, with 66% of adults experiencing neck pain during their lifetime and 5% highly disabled by it.49 Another study showed a 9% point prevalence of neck and shoulder pain.50 The prevalence of neck pain increases in highly educated individuals and in individuals with a history of injury, headaches, low back pain, or medical comorbidities such as cardiovascular and digestive disorders.51
No true natural history studies of axial neck pain exist; all published studies involve some form of treatment in most patients. DePalma and Subin52 found that most patients with axial symptoms from cervical spondylosis respond favorably to nonoperative treatment. After 3 months of nonoperative treatment, 29% of their patients had total relief of symptoms, 49% improved, and 22% did not improve. Rothman and Rashbaum53 reported on the 5-year outcome of patients with “predominantly” axial neck pain treated conservatively. They found that 23% of these patients remained partially or totally disabled from their symptoms. At the 5-year follow-up, Rothman and Rashbaum53 found no significant difference between this group and another similar group of patients who underwent surgery and recommended nonoperative management for “non-neurogenic” axial neck pain symptoms.
Cervical Radiculopathy
In a study of a Midwestern U.S. population, the annual incidence of cervical radiculopathy was found to be 83 per 100,000 population, with a peak incidence in the 6th decade of life.54 The point prevalence of cervical radiculopathy was found to be 3.5 per 1000 population.55 As with the natural history of neck pain, no studies on the true natural history of radiculopathy exist, with some form of treatment being provided in most patients. With varying degrees of nonoperative management, 45% of patients with cervical radiculopathy have good resolution of symptoms within 6 weeks of onset; the remaining 55% continue to have a minor to moderate degree of long-term morbidity.
The reported outcomes of cervical radiculopathy vary depending on the study population. Population-based and primary care–based studies suggest a benign natural or nonoperative history of cervical radiculopathy. Good outcomes occur in 71% to 92% of patients after nonoperative measures such as medications, exercise or physical therapy, and traction.56–59 A population-based study in Rochester, Minnesota, reported that 90% of patients initially diagnosed with cervical radiculopathy were asymptomatic or minimally affected by their condition at 5.9 years of follow-up. Surgery was performed in 26% of this group, but the effects of intervention were not independently analyzed.54 Studies originating from tertiary referral centers or surgical practices show persistent symptoms and disability in a significant percentage of patients with cervical radiculopathy.
The 1963 study by Lees and Turner60 on the “natural history” of cervical spondylosis showed the natural history and prognosis to be generally favorable. At long-term follow-up (2 to 19 years) of 51 patients with radiculopathy, 45% had only a single episode of pain without recurrence, 30% had mild continuing symptoms, and 25% had persistent or worsening symptoms. Lees and Turner60 found that nonoperative treatment helped improve initial symptoms but did not affect end results. Of the patients followed for cervical radiculopathy, none had evidence of myelopathy on follow-up.
Cervical Myelopathy
The true incidence of cervical myelopathy is difficult to ascertain because of the subtle findings in its early stages and the fact that many of the clinical findings of myelopathy are attributed to old age. Similarly, no modern studies on the true natural history of spondylotic myelopathy exist because surgical intervention is so commonly part of the treatment.61 In 1963, Lees and Turner60 reported on 44 patients with radiologic and myelographic evidence of myelopathy followed for 3 to 40 years (22 patients for >10 years and 22 additional patients for <10 years). The authors concluded that cervical spondylotic myelopathy follows a protracted clinical course consisting of long periods of relatively stable symptoms punctuated by exacerbations of variable duration. Clark and Robinson62 showed in a study of 120 patients that the natural history of cervical spondylotic myelopathy consists of progressive deterioration of motor symptoms, with 75% of patients deteriorating stepwise with intervening variable periods of stable disease, 20% deteriorating gradually and steadily, and 5% experiencing a rapid onset of symptoms followed by a lengthy period of quiescence. None of the 120 patients had a return to a normal neurologic state or reversal of symptoms. Nurick63 retrospectively assessed 37 patients, noting that the neurologic disability manifests early in the course of the disease and subsequently consists of static periods lasting many years. He noted that the prognosis improved for patients presenting with mild disease and that the disability tended to progress in patients older than 60 years. Nurick63 concluded that spondylotic myelopathy is a benign condition with minimal risk of future neurologic deterioration. Finally, Symon and Lavender64 argued against the idea that spondylotic myelopathy constitutes a benign disease process and showed in their review that 67% of patients underwent relentless neurologic deterioration without periods of clinical stability. Their analysis of Lees and Turner’s data showed that when disability was used as a criterion, only 18% of patients showed improvement.
Clinical Syndromes of Axial Neck Pain, Cervical Radiculopathy, and Myelopathy
Axial Neck Pain
Pain along the posterior neck and trapezius muscles without radiation into the upper extremity is an extremely common, but nonspecific presenting symptom. Patients usually localize the pain to the posterior paraspinal musculature of the neck, with radiation toward the occiput or into the shoulder and periscapular regions. Patients may report stiffness in one or more directions and commonly complain of headaches.65 Radiating “referred” pain without a dermatomal distribution in the shoulder or arm may accompany the neck pain. Referred pain may be associated with a sensation of warmth or tingling and autonomic phenomena such as piloerection and sweating. Areas of localized pain and tenderness in the posterior muscles of the neck suggest a muscle sprain or a soft tissue injury. Deep palpation of these trigger points produces referred patterns of pain along the course of the myofascial structures.
Cervical Radiculopathy
Patients typically have severe neck and arm pain (frequently unilateral) that precludes them from finding a comfortable position. They may present with the head cocked to the side opposite to their arm pain and sometimes hold the arm over the head, typically resting the wrist or forearm on top of the head—the shoulder abduction sign.24 The Valsalva maneuver typically exacerbates patients’ pain complaints.66 Extension and lateral rotation of the head to the side of the pain usually aggravate the symptoms—the Spurling maneuver. Aggravation of the symptoms by neck extension often helps differentiate a radicular etiology from muscular neck pain or a shoulder pathologic process with secondary muscle pain in the neck. The Spurling maneuver is particularly useful in differentiating cervical radiculopathy from other upper extremity neck pain etiologies, such as peripheral nerve entrapment, because it stresses only structures located within the cervical spine by decreasing the size of the intervertebral foramen and impinging further on the involved nerve root.66,67
Multiple sources of pain in the neck and upper extremity commonly coexist, and the structures may be compressed at more than one site.68 Patients with metabolic disorders such as diabetes who have accompanying neuropathy may be more susceptible to radiculopathy and compressive neuropathy. Adaptations to an initial presentation of radiculopathy may result in secondary shoulder pathology, carpal tunnel syndrome, or ulnar nerve irritation persisting long after the initial radicular pain resolves (Table 36–2).
From Rao R: Neck pain, cervical radiculopathy, and cervical myelopathy: Pathophysiology, natural history and clinical evaluation. J Bone Joint Surg Am 84:1872-1881, 2002.
Henderson and colleagues29 reviewed clinical presentations in 736 patients with cervical radiculopathy: 99.4% had arm pain, 85.2% had sensory deficits, 79.7% had neck pain, 71.2% had reflex deficits, 68% had motor deficits, 52.5% had scapular pain, 17.8% had anterior chest pain, 9.7% had headaches, 5.9% had anterior chest and arm pain, and 1.3% had left-sided chest and arm pain, known as cervical angina. Neurologic deficits corresponded with the offending disc level in approximately 80% of patients with radiculopathy. Another study of 275 patients with cervical radiculopathy noted that 59% of these patients reported headache, frequently occurring ipsilateral to the radicular symptoms.18
Occasionally, patients with nerve root compression present with “referred” upper trapezial or interscapular pain without radiating arm pain. The absence of radiating symptoms in a dermatomal fashion does not rule out symptomatic nerve root compression. The clinician should perform a careful physical examination to identify any involved nerve roots, keeping in mind that crossover between myotomes and dermatomes may be present.67
C4 radiculopathy may also be a source of unexplained neck and shoulder pain. Patients occasionally have paresthesias or numbness in the lower neck extending laterally to the superior aspect of the shoulder. Diaphragmatic involvement may result from involvement of C3-5 nerve roots.69,70 Motor deficits in the diaphragm manifest as paradoxical respiration and can be confirmed by fluoroscopic evaluation of the diaphragm during respiration. With paradoxical respiration, the unaffected hemidiaphragm contracts and descends during inspiration; this downward excursion transmits pressure to the abdominal cavity, resulting in upward passive movement of the paralyzed side. A “sniff test,” done under fluoroscopy, detects paradoxical movement: Rapidly repeated inspirations through the nostrils normally result in the descent of both hemidiaphragms, but with unilateral diaphragmatic paralysis, there is paradoxical upward motion of the paralyzed side.71
C6 radiculopathy manifests as pain radiating from the base of the neck to the lateral aspect of the elbow, into the radial forearm and radial digits, more commonly involving the thumb. Numbness or paresthesias may exist in the same distribution. Motor deficits may be elicited in the wrist extensors, elbow flexion, and forearm supination. C6 compression most directly affects the brachioradialis reflex; however, subtle changes in the biceps reflex may exist as well.66 The sensory symptoms may mimic symptoms of carpal tunnel syndrome, which typically involves the radial three and a half digits and causes weakness in the thenar musculature.
C7 is the most frequently involved nerve root in cervical radiculopathy and results from C6-7 disc space pathology. Patients report pain radiating from the neck to the shoulder, down along the triceps, then along the dorsum of the forearm onto the dorsum of the middle finger. Patients usually pronate the forearm while trying to describe the radiation of their symptoms into the dorsum of the hand or long finger, a useful observation when attempting to differentiate the hand symptoms from carpal tunnel syndrome or C6 radiculopathy.5 Chronic breast pain also has been associated with C7 radiculopathy.72 Motor weakness exists in the triceps, wrist flexors, and finger extensors with C7 radiculopathy. The triceps reflex may be absent or diminished.
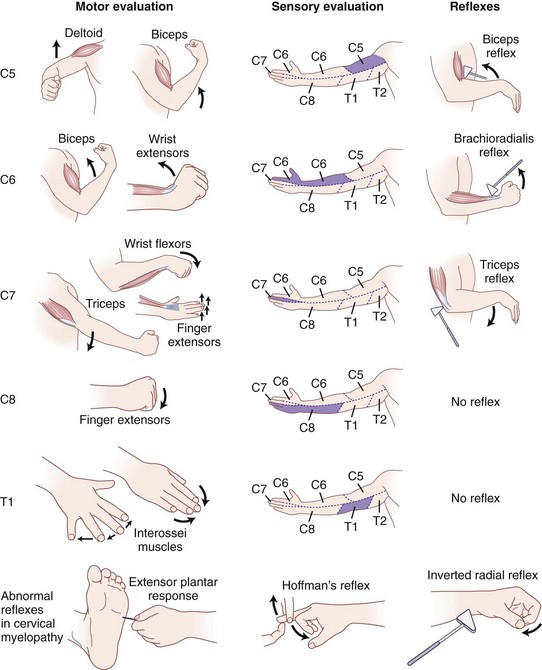
C8 radiculopathy occasionally results from disc herniation or spondylosis at the C7-T1 level. Patients present with paresthesias or pain in a dermatomal distribution along the ulnar border of the arm and forearm, radiating into the ulnar aspect of the hand to the small finger and the ring finger. Numbness usually involves the dorsal and volar aspects of the ulnar two digits and hand. The small muscles of the hand exhibit profound weakness, and patients report difficulty using their hands for routine daily activities. The clinician must differentiate between C8 radiculopathy and ulnar nerve entrapment. C8 radiculopathy may affect function of the flexor digitorum profundus in the index and long fingers and function of the flexor pollicis longus in the thumb, but ulnar nerve entrapment has no effect on these muscles. Ulnar nerve involvement spares all of the short thenar muscles except the adductor pollicis, whereas C8 radiculopathy affects these muscles (Fig. 36–6).
Cervical Myelopathy
The subtle nature of the clinical findings of early cervical spondylotic myelopathy makes diagnosis a challenge. The physical findings in cervical spondylotic myelopathy can vary significantly depending on the anatomic portion of the cord primarily involved. Sensory symptoms arise from compression at three discrete anatomic locations: (1) the spinothalamic tract, affecting contralateral pain and temperature sensation with light touch often preserved; (2) posterior columns, affecting ipsilateral position and vibration sense, possibly leading to gait disturbances; and (3) dorsal root compression, leading to decreased dermatomal sensation. The motor and reflex examination typically reveals lower motor neuron signs at the levels of the cervical lesions (hyporeflexia and weakness in the upper extremities) and upper motor neuron signs below the lesions (hyperreflexia and spasticity in the lower extremities).30
Crandall and Batzdorf73 described five general categories of cervical spondylotic myelopathy: (1) In transverse lesion syndrome, the corticospinal, spinothalamic, and posterior cord tracts are essentially equally involved. This myelopathy is associated with the longest duration of symptoms, suggesting this category may represent an end stage of the disease. (2) In motor system syndrome, corticospinal tracts and anterior horn cells are involved, resulting in spasticity. (3) In central cord syndrome, motor and sensory deficits affect the upper extremities more severely than the lower extremities. (4) Brown-Séquard syndrome consists of ipsilateral motor deficits with contralateral sensory deficits and seems to be the least advanced form of the disease. (5) Brachialgia and cord syndrome consists of radicular pain in the upper extremity along with motor or sensory long tract signs.
Ferguson and Caplan74 divided cervical spondylotic myelopathy into four syndromes: (1) medial syndrome, consisting primarily of long tract signs; (2) lateral syndrome, consisting primarily of radicular symptoms; (3) combined medial and lateral syndrome, which constitutes the most common presentation and includes aspects of cord and root involvement; and (4) vascular syndrome, which manifests with rapidly progressive myelopathy and likely represents vascular insufficiency of the cervical spinal cord. A clear-cut sensory or motor pattern may not be present with this syndrome because of the variable injury to the cord resulting from vascular ischemia. A fifth clinical presentation, anterior syndrome, has also been described, consisting of painless weakness in the upper extremities without accompanying symptoms in the lower extremities and without radicular or long tract signs.30
The findings in cervical spondylotic myelopathy vary with each patient. Patients may report an insidious onset of clumsiness in the hands or diffuse numbness in the hands resulting in worsening of handwriting or other fine motor skills over the past few months or weeks and difficulty with grasping or holding objects (i.e., trouble with manipulating buttons or zippers).75 Patients frequently experience increasing difficulty with balance that they attribute to age or arthritic hips; relatives may note that the patient’s gait has become increasingly clumsy, that the patient holds onto objects to help with balance, and that he or she sustains occasional falls. Nurick76 developed a system for grading the disability in cervical spondylotic myelopathy on the basis of gait abnormality. Spasticity, muscle weakness, and wasting in the lower extremities with superimposed loss of proprioception result in an unsteady, broad-based gait. Severely affected individuals can be quadriparetic or quadriplegic when first seen.
Physical examination shows exaggerated deep tendon reflexes, sustained clonus, absent or diminished superficial reflexes, and the presence of pathologic reflexes confirming an upper motor neuron lesion. Myelopathy caused by pathology in the region of the cord cephalad to C3 may result in a hyperactive scapulohumeral reflex (tapping of the spine of the scapula or acromion with a caudally directed force with the seated patient’s arm resting at the side results in brisk scapular elevation or humerus abduction or both). This response represents a stretch reflex of the trapezius muscle.77 Superficial reflexes, such as the abdominal or cremasteric reflex, are often diminished or absent in the presence of upper motor neuron lesions. The pathologic reflexes represent abnormal long tract signs and indicate cord compression.
Patients with moderate to severe spondylotic myelopathy typically exhibit the following pathologic reflexes to varying degrees: (1) the inverted radial reflex—indicative of cord compression at C6 and present when, during elicitation of a brachioradialis reflex, the brachioradialis is hyporesponsive and the ipsilateral fingers flex briskly at each hammer tap; (2) the Hoffman reflex—present if the ipsilateral interphalangeal joints of the thumb and index finger flex when the volar surface of the distal phalanx of the long finger is flicked into extension and strongly indicative of cord impingement when asymmetric; and (3) the extensor plantar reflex (also called the Babinski sign)—present when rubbing of the lateral sole of the foot from the heel along a curve to the metatarsal pads with a blunt object causes the hallux to dorsiflex and the lesser toes to fan out (see Fig. 36–6).30,66 Combined cervical and lumbar involvement is present in 13% of patients with spondylosis, resulting in a potentially confusing clinical picture of lower extremity lower motor neuron findings.78
Sensory findings in cervical spondylotic myelopathy also vary. Depending on the exact area of compromise of the cord or nerve root, pain, temperature, proprioception, vibratory, and dermatomal sensations all may be diminished. Presenting findings usually do not include sphincter disturbances. Patients may present with urinary complaints: hesitation, frequency, and, rarely, incontinence or retention. In the study by Crandall and Batzdorf73 of 62 patients with cervical spondylotic myelopathy, neck pain was present in less than 50% of the patients, and associated radicular pain was present in 38%. Generalized shocklike sensations in the trunk and upper and lower extremities resulting from quick flexion or extension of the neck—the Lhermitte sign—was present in 27% of patients, and sphincter disturbances were present in 44%.
In the past, hand disturbances were primarily attributed to radicular pathology. Several reports have shown findings specific to “myelopathy hand,” indicating a high cervical myelopathy above the C5 level.79,80 Diffuse numbness in the hands is extremely common and is often misdiagnosed as carpal tunnel syndrome or peripheral neuropathy. Clumsiness of the hands results in an inability to perform fine motor tasks. Marked wasting of the intrinsic hand muscles is usually present and progresses insidiously with weakness of finger extension and adduction.81 Ono and colleagues80 described two specific signs of myelopathy hand signifying pyramidal tract involvement: (1) the finger-escape sign—when the patient attempts to extend the digits fully with the palm facing down, the ulnar two or three digits tend to drift into abduction and flexion after 30 seconds’ duration; and (2) the grip-and-release test—a decreased ability to open and close the fist rapidly because of weakness and spasticity. Normal is greater than 20 grip-and-release movements in 10 seconds. To distinguish between upper motor neuron signs arising from brain pathology versus signs arising from cervical cord pathology, a jaw jerk test may be performed. Closing of the mouth (upward jerking of the mandible) caused by tapping of the lower jaw at a downward angle with the mouth held slightly open constitutes a positive jaw jerk test. This response signifies that the origin of the upper motor neuron findings may be higher up in the brain rather than in the spinal canal and specifically tests cranial nerve V.82
Many neurologic conditions resemble cervical spondylotic myelopathy. Multiple sclerosis has distinctive plaques that can be seen on magnetic resonance imaging (MRI) of the brain and spinal cord. The disease is a demyelinating disorder of the central nervous system and causes motor and sensory symptoms but typically has remissions and exacerbations and involvement of the cranial nerves. Amyotrophic lateral sclerosis results in upper and lower motor neuron symptoms, without alteration in sensation. Subacute combined degeneration seen with vitamin B12 deficiency causes corticospinal tract and posterior tract symptoms, with greater sensory involvement in the lower extremities. Patients with metabolic or idiopathic peripheral neuropathy have sensory symptoms that may mirror symptoms of myelopathy (Table 36–3).
TABLE 36–3 Differential Diagnoses of Cervical Spondylotic Myelopathy
From Rao R: Neck pain, cervical radiculopathy, and cervical myelopathy: Pathophysiology, natural history and clinical evaluation. J Bone Joint Surg Am 84:1872-1881, 2002.
Summary
Pearls
Key Points
1 Bogduk N, Windsor M, Inglis A. The innervation of the cervical intervertebral discs. Spine (Phila Pa 1976). 1988;13:2-8.
2 Cornefjord M, Olmarker K, Farley DB, et al. Neuropeptide changes in compressed spinal nerve roots. Spine (Phila Pa 1976). 1995;20:670-673.
3 Gore DR, Sepic SB, Gardner GM, et al. Neck pain: A long-term follow-up of 205 patients. Spine (Phila Pa 1976). 1987;12:1-5.
4 Lees F, Turner JW. Natural history and prognosis of cervical spondylosis. BMJ. 1963;2:1607-1610.
5 Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy: Pathophysiology, natural history, and clinical evaluation. J Bone Joint Surg Am. 2002;84:1872-1881.
1 Roh JS, Teng AL, Yoo JU, et al. Degenerative disorders of the lumbar and cervical spine. Orthop Clin North Am. 2005;36:255-262.
2 Truumees E, Herkowitz HN. Cervical spondylotic myelopathy and radiculopathy. Instr Course Lect. 2000;49:339-360.
3 Geck MJ, Eismont FJ. Surgical options for the treatment of cervical spondylotic myelopathy. Orthop Clin North Am. 2002;33:329-348.
4 Shedid D, Benzel EC. Cervical spondylosis anatomy: Pathophysiology and biomechanics. Neurosurgery. 2007;60:S7-S13.
5 Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy: Pathophysiology, natural history, and clinical evaluation. J Bone Joint Surg Am. 2002;84:1872-1881.
6 Lestini WF, Wiesel SW. The pathogenesis of cervical spondylosis. Clin Orthop Relat Res. 1989:69-93.
7 Gore DR, Sepic SB, Gardner GM, et al. Neck pain: A long-term follow-up of 205 patients. Spine (Phila Pa 1976). 1987;12:1-5.
8 Wieser ES, Wang JC. Surgery for neck pain. Neurosurgery. 2007;60:S51-S56.
9 Bengtsson A, Henriksson KG, Larsson J. Reduced high-energy phosphate levels in the painful muscles of patients with primary fibromyalgia. Arthritis Rheum. 1986;29:817-821.
10 Bogduk N, Windsor M, Inglis A. The innervation of the cervical intervertebral discs. Spine (Phila Pa 1976). 1988;13:2-8.
11 Bogduk N, Marsland A. The cervical zygapophysial joints as a source of neck pain. Spine (Phila Pa 1976). 1988;13:610-617.
12 Dwyer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns. I: A study in normal volunteers. Spine (Phila Pa 1976). 1990;15:453-457.
13 Aprill C, Dwyer A, Bogduk N. Cervical zygapophyseal joint pain patterns. II: A clinical evaluation. Spine (Phila Pa 1976). 1990;15:458-461.
14 Grubb SA, Kelly CK. Cervical discography: Clinical implications from 12 years of experience. Spine (Phila Pa 1976). 2000;25:1382-1389.
15 McLain RF. Mechanoreceptor endings in human cervical facet joints. Spine (Phila Pa 1976). 1994;19:495-501.
16 Dreyfuss P, Michaelsen M, Fletcher D. Atlanto-occipital and lateral atlanto-axial joint pain patterns. Spine (Phila Pa 1976). 1994;19:1125-1131.
17 Wächli B, Dvorak J, Grob D: Cervical spine disorders and headaches. Twenty-First Annual Meeting of the Cervical Spine Research Society, New York, December 1, 1993.
18 Persson LC, Carlsson JY, Anderberg L. Headache in patients with cervical radiculopathy: A prospective study with selective nerve root blocks in 275 patients. Eur Spine J. 2007;16:953-959.
19 Manifold SG, McCann PD. Cervical radiculitis and shoulder disorders. Clin Orthop Relat Res. 1999;368:105-113.
20 Cooper RG, Freemont AJ, Hoyland JA, et al. Herniated intervertebral disc-associated periradicular fibrosis and vascular abnormalities occur without inflammatory cell infiltration. Spine (Phila Pa 1976). 1995;20:591-598.
21 Chabot RG, Montgomery DM. The pathophysiology of axial and radicular neck pain. Semin Spine Surg. 1995:2-8.
22 Cornefjord M, Olmarker K, Farley DB, et al. Neuropeptide changes in compressed spinal nerve roots. Spine (Phila Pa 1976). 1995;20:670-673.
23 Muhle C, Resnick D, Ahn JM, et al. In vivo changes in the neuroforaminal size at flexion-extension and axial rotation of the cervical spine in healthy persons examined using kinematic magnetic resonance imaging. Spine (Phila Pa 1976). 2001;26:E287-E293.
24 Davidson RI, Dunn EJ, Metzmaker JN. The shoulder abduction test in the diagnosis of radicular pain in cervical extradural compressive monoradiculopathies. Spine (Phila Pa 1976). 1981;6:441-446.
25 Farmer JC, Wisneski RJ. Cervical spine nerve root compression: An analysis of neuroforaminal pressures with varying head and arm positions. Spine (Phila Pa 1976). 1994;19:1850-1855.
26 Anderberg L, Annertz M, Rydholm U, et al. Selective diagnostic nerve root block for the evaluation of radicular pain in the multilevel degenerated cervical spine. Eur Spine J. 2006;15:794-801.
27 Tanaka N, Fujimoto Y, An HS, et al. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine (Phila Pa 1976). 2000;25:286-291.
28 Rao RD, Gourab K, David KS. Operative treatment of cervical spondylotic myelopathy. J Bone Joint Surg Am. 2006;88:1619-1640.
29 Henderson CM, Hennessy RG, Shuey HMJr, et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: A review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
30 Bernhardt M, Hynes RA, Blume HW, et al. Cervical spondylotic myelopathy. J Bone Joint Surg Am. 1993;75:119-128.
31 Penning L, Wilmink JT, van Woerden HH, et al. CT myelographic findings in degenerative disorders of the cervical spine: Clinical significance. AJR Am J Roentgenol. 1986;146:793-801.
32 Houser OW, Onofrio BM, Miller GM, et al. Cervical spondylotic stenosis and myelopathy: Evaluation with computed tomographic myelography. Mayo Clin Proc. 1994;69:557-563.
33 Ono K, Ota H, Tada K, et al. Cervical myelopathy secondary to multiple spondylotic protrusions: A clinicopathologic study. Spine (Phila Pa 1976). 1977;2:109-125.
34 Pavlov H, Torg JS, Robie B, et al. Cervical spinal stenosis: Determination with vertebral body ratio method. Radiology. 1987;164:771-775.
35 Murone I. The importance of the sagittal diameters of the cervical spinal canal in relation to spondylosis and myelopathy. J Bone Joint Surg Br. 1974;56:30-36.
36 Kawaguchi Y, Kanamori M, Ishihara H, et al. Pathomechanism of myelopathy and surgical results of laminoplasty in elderly patients with cervical spondylosis. Spine (Phila Pa 1976). 2003;28:2209-2214.
37 Mihara H, Ohnari K, Hachiya M, et al. Cervical myelopathy caused by C3-C4 spondylosis in elderly patients: A radiographic analysis of pathogenesis. Spine (Phila Pa 1976). 2000;25:796-800.
38 Kadanka Z, Kerkovsky M, Bednarik J, et al. Cross-sectional transverse area and hyperintensities on magnetic resonance imaging in relation to the clinical picture in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2007;32:2573-2577.
39 Breig A, Turnbull I, Hassler O. Effects of mechanical stresses on the spinal cord in cervical spondylosis: A study on fresh cadaver material. J Neurosurg. 1966;25:45-56.
40 Henderson FC, Geddes JF, Vaccaro AR, et al. Stretch-associated injury in cervical spondylotic myelopathy: New concept and review. Neurosurgery. 2005;56:1101-1113.
41 Barre JA. Troubles pyramidaux et arthrite vertebrale chronique. Medecine. 1924;3:58-60.
42 Hukuda S, Wilson CB. Experimental cervical myelopathy: Effects of compression and ischemia on the canine cervical cord. J Neurosurg. 1972;37:631-652.
43 Gooding MR, Wilson CB, Hoff JT. Experimental cervical myelopathy: Effects of ischemia and compression of the canine cervical spinal cord. J Neurosurg. 1975;43:9-17.
44 Shimomura Y, Hukuda S, Mizuno S. Experimental study of ischemic damage to the cervical spinal cord. J Neurosurg. 1968;28:565-581.
45 Doppman JL. The mechanism of ischemia in anteroposterior compression of the spinal cord. Invest Radiol. 1975;10:543-551.
46 Baron EM, Young WF. Cervical spondylotic myelopathy: A brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60:S35-S41.
47 Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6:190S-197S.
48 Ogino H, Tada K, Okada K, et al. Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine (Phila Pa 1976). 1983;8:1-15.
49 Cote P, Cassidy JD, Carroll L. The Saskatchewan health and back pain survey: The prevalence of neck pain and related disability in Saskatchewan adults. Spine (Phila Pa 1976). 1998;23:1689-1698.
50 Lawrence JS. Disc degeneration: Its frequency and relationship to symptoms. Ann Rheum Dis. 1969;28:121-138.
51 Cote P, Cassidy JD, Carroll L. The factors associated with neck pain and its related disability in the Saskatchewan population. Spine (Phila Pa 1976). 2000;25:1109-1117.
52 DePalma AF, Subin DK. Study of the cervical syndrome. Clin Orthop Relat Res. 1965;38:135-142.
53 Rothman RH, Rashbaum RF. Pathogenesis of signs and symptoms of cervical disc degeneration. Instr Course Lect. 1978;27:203-215.
54 Radhakrishnan K, Litchy WJ, O’Fallon WM, et al. Epidemiology of cervical radiculopathy: A population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994;117:325-335.
55 Salemi G, Savettieri G, Meneghini F, et al. Prevalence of cervical spondylotic radiculopathy: A door-to-door survey in a Sicilian municipality. Acta Neurol Scand. 1996;93:184-188.
56 Honet JC, Puri K. Cervical radiculitis: Treatment and results in 82 patients. Arch Phys Med Rehabil. 1976;57:12-16.
57 Pain in the neck and arm: A multicentre trial of the effects of physiotherapy, arranged by the British Association of Physical Medicine. BMJ. 1966;1:253-258.
58 Martin GM, Corbin KB. An evaluation of conservative treatment for patients with cervical disk syndrome. Arch Phys Med Rehabil. 1954:87-92.
59 Rubin D. Cervical radiculitis: Diagnosis and treatment. Arch Phys Med Rehabil. 1960;41:580-586.
60 Lees F, Turner JW. Natural history and prognosis of cervical spondylosis. BMJ. 1963;2:1607-1610.
61 Rowland LP. Surgical treatment of cervical spondylotic myelopathy: Time for a controlled trial. Neurology. 1992;42:5-13.
62 Clarke E, Robinson PK. Cervical myelopathy: A complication of cervical spondylosis. Brain. 1956;79:483-510.
63 Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101-108.
64 Symon L, Lavender P. The surgical treatment of cervical spondylotic myelopathy. Neurology. 1967;17:117-127.
65 Travell JG, Simons DG. Myofascial Pain and Dysfunction: The Trigger Point Manual, vol 2. Baltimore: Williams & Wilkins. 1983.
66 An HS. Cervical root entrapment. Hand Clin. 1996;12:719-730.
67 Rhee JM, Yoon T, Riew KD. Cervical radiculopathy. J Am Acad Orthop Surg. 2007;15:486-494.
68 Massey EW, Riley TL, Pleet AB. Coexistent carpal tunnel syndrome and cervical radiculopathy (double crush syndrome). South Med J. 1981;74:957-959.
69 Cloward RB. Diaphragm paralysis from cervical disc lesions. Br J Neurosurg. 1988;2:395-399.
70 Buszek MC, Szymke TE, Honet JC, et al. Hemidiaphragmatic paralysis: An unusual complication of cervical spondylosis. Arch Phys Med Rehabil. 1983;64:601-603.
71 Malagori K, Fraser RG. The Thorax: Disease, 2nd ed. New York: Marcel Dekker; 1995.
72 LaBan MM, Meerschaert JR, Taylor RS. Breast pain: A symptom of cervical radiculopathy. Arch Phys Med Rehabil. 1979;60:315-317.
73 Crandall PH, Batzdorf U. Cervical spondylotic myelopathy. J Neurosurg. 1966;25:57-66.
74 Ferguson RJ, Caplan LR. Cervical spondylotic myelopathy. Neurol Clin. 1985;3:373-382.
75 Emery SE. Cervical spondylotic myelopathy: Diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:376-388.
76 Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87-100.
77 Shimizu T, Shimada H, Shirakura K. Scapulohumeral reflex (Shimizu): Its clinical significance and testing maneuver. Spine (Phila Pa 1976). 1993;18:2182-2190.
78 Edwards WC, LaRocca SH. The developmental segmental sagittal diameter in combined cervical and lumbar spondylosis. Spine (Phila Pa 1976). 1985;10:42-49.
79 Good DC, Couch JR, Wacaser L. “Numb, clumsy hands” and high cervical spondylosis. Surg Neurol. 1984;22:285-291.
80 Ono K, Ebara S, Fuji T, et al. Myelopathy hand: New clinical signs of cervical cord damage. J Bone Joint Surg Br. 1987;69:215-219.
81 Ebara S, Yonenobu K, Fujiwara K, et al. Myelopathy hand characterized by muscle wasting: A different type of myelopathy hand in patients with cervical spondylosis. Spine (Phila Pa 1976). 1988;13:785-791.
82 Rhee JM, Riew KD. Cervical spondylotic myelopathy: Including ossification of the posterior longitudinal ligament. In: Spivak J, editor. Orthopaedic Knowledge Update Spine. 3rd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006:235-249.