CHAPTER 17 Cervical Spine
Surgical Approaches
Surgical Anatomy
Surface Anatomy and Skin
An understanding of the relationship of surface landmarks to anatomic structures in the neck is useful for localizing vertebral levels. The hyoid bone lies at the level of C3, the thyroid cartilage lies at the level of C4, and the cricoid cartilage lies opposite C6.1 Gentle but firm palpation laterally allows inspection of the transverse processes. Superiorly, the transverse process of the atlas is most prominent and is found just anterior and inferior to the mastoid process. To help differentiate it from the skull, rotation of the head shows the atlas moving independently from the skull. The anterior tubercle of the transverse process of the sixth cervical vertebra, Chassaignac tubercle, is an important palpable landmark. Posteriorly, in the midline, the first bony prominence palpated inferior to the occiput is the spinous process of the second vertebra. The next palpable spinous processes are typically from the sixth and the seventh vertebrae, with the seventh being the most prominent.
Osseous Anatomy and Bony Articulation
The axis, or C2, is characterized by an odontoid process or dens that projects upward anteriorly, articulating with the posterior aspect of the anterior arch of the atlas as a synovial joint. At its narrowest portion, at the base of the dens, the coronal and sagittal plane diameters are 8 to 10 mm and 10 to 11 mm.2,3 Posteriorly, the axis has a large lamina and a bifid spinous process, which serve as attachments for the rectus major and inferior oblique muscles. The zone between the lamina and the lateral mass of the axis is indistinct, and posteriorly the neural arch connects to the body by large pedicles that are 8 mm wide and 10 mm long.4 Lying directly anterolateral to the pedicle is the vertebral artery, which runs through the foramen transversarium. The pedicle of the axis projects 30 degrees medially and 20 degrees superiorly from a posterior-to-anterior direction.3
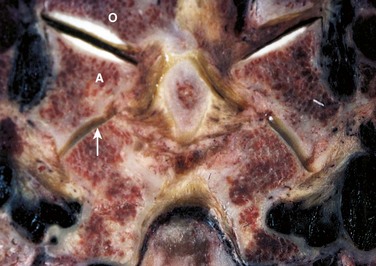
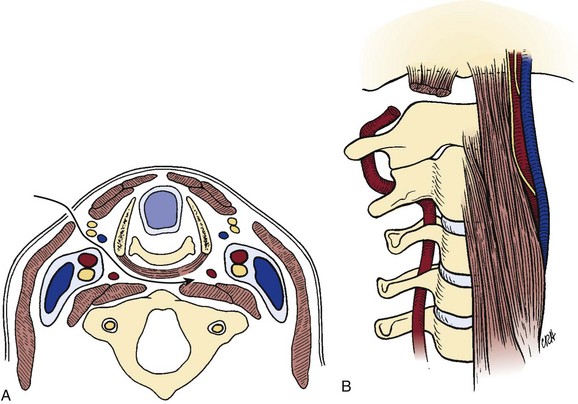
The bony articulations of the upper cervical spine (occiput-C1-C2) are unique and warrant special attention (Fig. 17–1). The atlanto-occipital articulation is a shallow ball-and-socket joint allowing for considerable motion mostly in flexion, extension, and lateral bending. The greatest degree of flexion and extension of any cervical articulation occurs at this level (25 degrees).5 Lateral displacement is minimized because the lateral wall of the cup-shaped articulation of the atlas is higher than the medial wall. The superior articular surface of the atlas projects cephalad and medially, articulating with the occipital condyle, which projects caudad and laterally. Conversely, the inferior articular surface of the atlas projects caudad and medially and articulates with the laterally projecting superior facet of the axis. As a result of this bony configuration, axial loads on the atlas tend to result in horizontal displacement of the lateral masses.6
The atlantoaxial articulation provides about 50% of rotatory motion of the cervical spine.5,7 The transverse ligament, which spans across the arch of the atlas, holds the odontoid process against the anterior arch of the atlas, creating a pivot joint with a synovial membrane and capsular ligaments anteriorly and posteriorly to the dens. This transverse ligament is the principal stabilizing structure for the atlantoaxial articulation and averages 21.9 mm in length.8 The transverse ligament has superior and inferior extensions, which form the cruciform ligament of the atlas, connecting it to the anterior edge of the foramen magnum and posterior aspect of the C2 body. To allow more rotatory motion, the inferior facets of the atlas are flatter and more circular than the superior facets and face inferiorly to articulate with the axis.
The inferior surface of the vertebral body is convex in the coronal plane and concave in the sagittal plane, with the anterior lip occasionally overlapping the inferior vertebra.8 Conversely, the superior surface of the vertebral body is convex or straight in the sagittal plane and concave in the coronal plane, creating projections on either side of the lateral superior surface, called the uncus, or hook. These processes project upward and conform to small grooves in the inferolateral border of the cephalad vertebra, forming the uncovertebral joints, or joints of Luschka. The width and depth of the vertebral surfaces average 17 mm and 15 mm from C2 to C6 and increase to about 20 mm and 17 mm at C7. Vertebral heights on the posterior wall in the mid-sagittal plane range from 11 to 13 mm.9
The pedicles project posterolaterally from the vertebral body and join the lamina to form the vertebral arch. From C3 to C7, the angulation of the pedicles varies from 8 degrees below to 11 degrees above the transverse plane and decreases from 45 degrees to 30 degrees in relation to the sagittal plane.9 The width and height of the pedicles increase slightly in size from C3 to C7, and average diameters are 5 to 6 mm and 7 mm. The lateral wall of the pedicle is thinner than the medial wall and should be taken into consideration if attempts at pedicle fixation are considered in this region.10–12
In the lower cervical spine, the neural foramina are bounded anteriorly by the uncinate process, the posterolateral aspect of the intervertebral disc, and the inferior portion of the vertebral body; posteriorly by the facet joint and superior articular process of the vertebral body below; and superiorly and inferiorly by adjacent pedicles. Vertebral notches located on the superior and inferior aspect of each pedicle contribute to the size of the neural foramina, which are 9 to 12 mm in height, 4 to 6 mm in width, and 4 to 6 mm in length and are aligned 45 degrees to the sagittal plane.13,14 They can be visualized radiographically with oblique views, with the right neural foramina outlined on the left posterior oblique view and the left neural foramina outlined on the right posterior oblique view.
The spinal canal is triangular and at all levels in the cervical spine is significantly greater in the medial-to-lateral dimension than in the anterior-to-posterior dimension. The cross-sectional area of the spinal canal is largest at C2 and smallest at C7, with a sagittal diameter of about 23 mm at C1 and 20 mm at C2, decreasing to 17 to 18 mm at C3-6 and to 15 mm at C7.7 This is one reason that the passage of sublaminar wires is safer in the upper cervical spine than in the lower cervical spine.
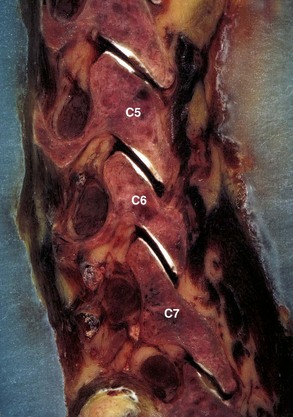
The lateral mass, an important structure for posterior cervical plate-screw systems, forms at the junction of the lamina and the pedicle and gives rise to the superior and inferior articular processes. These processes project upward and downward and are angled approximately 45 degrees cephalad from the transverse plane and gradually assume a more vertical position as they descend into the thoracic region (Fig. 17–2). The articular process of the superior facet faces posteriorly, whereas the inferior facet of the upper vertebra faces anteriorly, and the facets oppose one another to form a zygapophyseal joint. The facet joints are true diarthrodial joints with articular cartilage and menisci surrounded by a fibrous capsule lined by a synovial membrane. The interfacet distances are relatively constant between levels, with individual variations ranging from 9 to 16 mm (average 13 mm).4,15
The cervicothoracic junction is a transition region, with C7 having similar anatomic characteristics at T1 and T2. The dimensions of the vertebral body and the sizes of the transverse processes and spinous processes are larger at C6 and C7. Additionally, dimensions of the spinal canal decrease at C6 and C7, representing a distinct transition to the thoracic region. The articulating facet joint between C7 and T1 resembles the thoracic facet joint, and the lateral mass of C7 is thinner than that of upper levels. Morphologic characteristics of pedicles of C7, T1, and T2 were obtained with respect to diameters, depths, and medial angulations. Inner diameters of the pedicles at C7, T1, and T2 from medial to lateral plane averaged 5.2 mm, 6.3 mm, and 5.5 mm. Medial angulations were 34 degrees, 30 degrees, and 26 degrees at C7, T1, and T2.9,16 These morphologic characteristics should be remembered when performing transpedicular procedures in the cervicothoracic region.
Ligaments
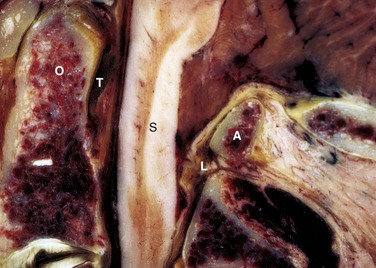
The transverse ligament is the major stabilizer of the atlantoaxial complex (Fig. 17–3). It attaches laterally to tubercles located on the posterior aspect of the anterior arch of C1, where it blends with the lateral mass. Secondary stabilizers include the thick alar ligament, which arises from the sides of the dens to the medial aspects of the condyles of the occipital bone, and the apical ligament, which arises from the apex of the dens to the anterior edge of the foramen magnum. In some individuals, an anterior atlantodental ligament exists connecting the base of the dens to the anterior arch of the atlas.17 The tectorial membrane, the superior continuation of the posterior longitudinal ligament, covers the dens and all the occipitoaxial ligaments and extends from the posterior body of C2 to the basilar portion of the occipital bone and the anterior aspect of the foramen magnum.
The bodies of the lower cervical vertebrae (C3-7) are connected by two longitudinal ligaments and the intervertebral discs. The anterior longitudinal ligament is a strong band that attaches from the skull, as the anterior atlanto-occipital membrane, and continues caudad over the entire length of the spine down to the sacrum. The anterior longitudinal ligament is thinner and more closely attached at the intervertebral disc margins than at the anterior vertebral surfaces.18 The anterior longitudinal ligament also sweeps around and envelops the lateral aspect of the vertebral bodies under the longus collis muscle, and the lateral extension is continuous with the deep layer of the posterior longitudinal ligament in the region of the intervertebral foramina.
The posterior longitudinal ligament, lying within the vertebral canal on the posterior aspect of the vertebral body and intervertebral disc, is wider in the upper cervical spine than the lower cervical spine.18 Superiorly, it is continuous with the tectorial membrane, and as it descends it widens over the intervertebral discs and narrows behind each vertebral body. The posterior longitudinal ligament supplies additional strength and stability to the posteromedial fibers of the anulus. There is an area of relative weakness in the posterolateral corners of the disc, however, at the junction of the posterior longitudinal ligament and uncinate process; as a result, it is the site of most cervical disc herniations.19 According to Hayashi and colleagues,20 the posterior longitudinal ligament is double-layered, and the deep layer sends fibers to the anulus fibrosus and continues laterally to the region of the intervertebral foramina. The superficial or more dorsal layer of the posterior longitudinal ligament is adjacent to the dura mater and continues as a connective tissue membrane, which envelops the dura mater, nerve roots, and vertebral artery, suggesting that this membrane may serve as a protective barrier.
Intervertebral Discs
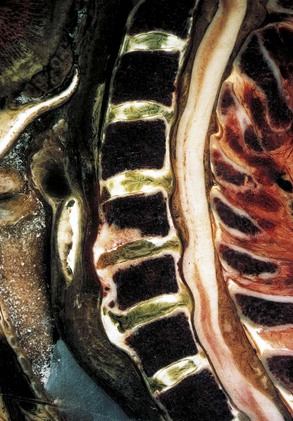
Intervertebral discs are present between vertebrae except at the atlantoaxial level. Each intervertebral disc is an avascular structure that consists of the nucleus pulposus at the interior of the disc, the outer anulus fibrosus, and the cartilaginous endplates adjacent to the vertebral surfaces. The nucleus pulposus functions as a shock absorber, and the anulus fibrosus maintains the stability of the motion segment. With increasing age, the margin between the nucleus pulposus and anulus fibrosus becomes less distinct, and often by age 50 the nucleus pulposus has become a fibrocartilaginous mass similar to the inner zone of the anulus fibrosus.21
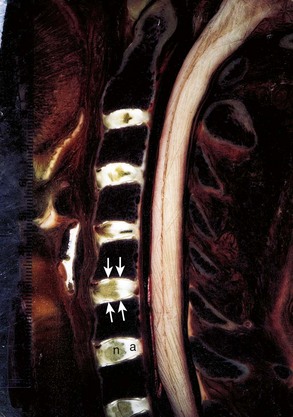
The anulus has an outer collagenous layer, in which the fibers are arranged in oblique layers of lamellae. The outermost fibers of the anulus fibrosus are contiguous with the anterior and posterior longitudinal ligaments and are firmly attached to the adjacent vertebral endplates. The fibers of the lamella run perpendicular to the fibers of the adjacent lamella. The collagen fibers in the posterior portion of the disc run more vertical than oblique, and this may account for the relative frequency of radial tears seen clinically. The discs are shaped to conform to the surface of the bodies; the superior surface of the disc is concave, and the inferior surface of the disc is correspondingly convex in the coronal plane. The discs are also slightly thicker anteriorly than posteriorly, which contributes to the lordotic posture of the cervical spine. The cervical intervertebral discs allow some translational movement in the sagittal plane, but the uncinate processes resist lateral movement. The uncinate process, located in the posterolateral aspect of the disc, also helps prevent disc herniations in this area. Degeneration of the anulus fibrosus (Fig. 17–4) in the cervical region is similar to the lumbar region in that concentric, transverse, and radial tears of the anulus occur, and the radial tear in the posterior aspect of the disc may be more clinically significant.
The cartilaginous endplate is a layer of hyaline cartilage resting on the subchondral bone and serves as a barrier between the pressure of the nucleus pulposus and the adjacent vertebral bodies. This cartilage is a growth plate and responsible for endochondral ossification during growth (Fig. 17–5). The cartilaginous endplates also allow the insertion of the inner fibers of the anulus fibrosus and the diffusion of nutrients from the subchondral bone to the disc.
Neural Elements
The cervical cord emerges from the foramen magnum as a continuation of the medulla oblongata. There is considerable variation in size of the spinal cord; however, in general, owing to the increased nerve supply to the upper limbs, the cervical cord enlarges from C3 and becomes maximal at C6. Maximal transverse diameters of 13 to 14 mm have been reported,22 with transverse areas ranging from 58.3 ± 6.7 mm2 at C623 to 85.8 ± 7.2 mm2 at C4-5.24
The dorsal sensory rootlets enter the cord through the lateral longitudinal sulcus, and the ventral motor rootlets exit the cord through the ventral lateral sulcus. The six or eight rootlets at each level leave the spinal cord laterally to lie in the lateral subarachnoid space bathed in the cerebrospinal fluid. The rootlets join to form the dorsal and ventral root, which together enter a narrow sleeve of arachnoid and pass through the dura to become a nerve root at each level. The cervical nerve roots that form from the ventral and dorsal nerve rootlet extend anterolaterally at a 45-degree angle to the coronal plane and inferiorly at about 10 degrees to the axial plane.14 The nerve roots enter the intervertebral foramina by passing directly laterally from the spinal canal adjacent to the corresponding disc and over the top of the corresponding pedicle. The anterior root lies anteroinferiorly adjacent to the uncovertebral joint, and the posterior root is close to the superior articular process. The nerve root is positioned at the tip of the superior articular process in the medial aspect of the neural foramen, and it courses more inferiorly to position over the pedicle in the lateral aspect of the neural foramen (Fig. 17–6).
The roots occupy about one third of the foraminal space in the normal spine but much more in the degenerative spine. The roots are located in the inferior half of the neural foramen normally, but the nerve roots occupy a more cranial part of the foramina, and the size of the foramen is diminished if the neck is fully extended.25 The upper half of the neural foramen contains fat and small veins.26 The nerve root is enlarged in the distal aspect of the intervertebral foramen, and the dorsal root ganglion is located just distal to the foramen.27 The dorsal root ganglion is located between the vertebral artery and a small concavity in the superior articular process. Just distal to the ganglion and outside the intervertebral foramen, the anterior and posterior roots join to form the spinal nerve. The spinal nerve divides into dorsal and ventral primary rami branches.
The gray rami from the sympathetic cervical ganglion join the ventral primary rami. There are interconnections between gray rami, the perivascular plexus around the vertebral artery, and the sympathetic trunk, all of which give contributions to the ventral nerve plexus to innervate the anterior longitudinal ligament, outer anulus fibrosus, and anterior vertebral body.28,29 The dorsal nerve plexus receives contributions from the sinuvertebral nerves, which originate from the gray rami and perivascular plexus of the vertebral artery. The dorsal nerve plexus innervates the posterior longitudinal ligament, and the sinuvertebral nerves give branches to the posterior part of the anulus and the ventral part of the dura. The sinuvertebral nerves innervate two or more discs or motion segments.
Vascular Structures
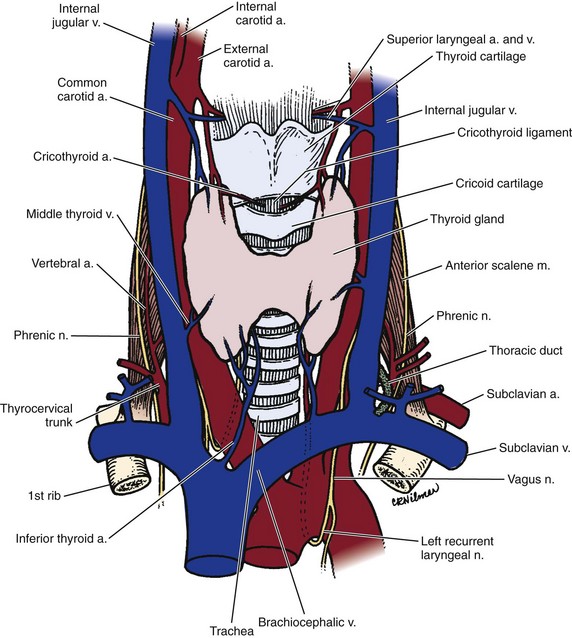
The major blood supply of the cervical cord and the cervical spine is the vertebral artery. Variations of the course of the vertebral artery have been reported.30 In most cases, the vertebral artery originates from the first part of the subclavian artery and begins its ascent behind the common carotid artery between the longus colli and the anterior scalene. In the lower cervical spine, the vertebral arteries are crossed by the inferior thyroid artery and on the left by the thoracic duct. The vertebral arteries course anterior to the ventral rami of the seventh and eighth cervical nerves and the C7 transverse process before entering the C6 transverse foramen, where they ascend within the transverse foramen of C6-C2.
In the foramen magnum region, the vertebral artery gives branches anteriorly that join together to form the single anterior spinal artery, whereas the paired posterior spinal arteries are branches from the posterior inferior cerebellar arteries. The anterior and posterior spinal arteries are the major blood supplies of the spinal cord. The posterior spinal arteries give rise to plexiform channels that are arranged transversely on the dorsum of the cord. The anterior spinal artery supplies most of the spinal cord except the posterior columns.31 The spinal cord also receives blood supplies from radicular arteries or medullary feeders from the vertebral arteries and ascending cervical arteries.31 The segmental arteries that are branches of the vertebral artery are present at each level to supply the vertebrae and surrounding tissues, but only a few segmental vessels give rise to radicular arteries or medullary feeders to the spinal cord. These vessels have a variable distribution, but medullary feeders are more commonly present at C6 and C3 from the left and C5 and T1 from the right.18
Musculature
The musculature of the cervical spine can be grouped into the anterolateral and posterior muscle groups. The anterolateral muscles of the neck include platysma muscle, sternocleidomastoid muscle, hyoid muscles, strap muscles of the larynx, scalene muscles, longus colli muscle, and longus capitis muscle. The posterior musculature is subdivided into superficial, intermediate, and deep muscle groups.32
The posterior muscles of the neck are divided into superficial, intermediate, and deep groups.32 The most superficial muscle is the trapezius, which originates from the external occipital protuberance and the medial nuchal line of C7-T12 spinous processes and inserts on the spine of the scapula, the acromion, and the lateral aspect of the clavicle. The trapezius is innervated by the 11th cranial nerve and functions to extend the head. The intermediate muscles beneath the trapezius muscle are the splenius capitis and splenius cervicis, which originate from the spinous processes of the lower cervical and upper thoracic spines and insert on the transverse processes of the upper cervical spine and the mastoid process. In the deep layer, the erector spinal muscles continue into the cervical region, which includes the iliocostalis laterally; the longissimus cervicis and longissimus capitis centrally; and the spinalis cervicis, semispinalis capitis, and semispinalis cervicis medially. Beneath the semispinalis muscles lie the multifidus from C4-7 and rotatores muscles, which cross only one segment from the transverse processes to the spinous processes.
Fascial Layers
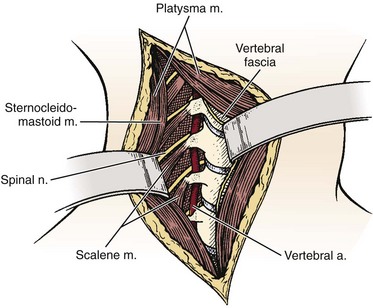
The key to understanding the anterior approach to the cervical spine lies in recognizing the fascial layers of the neck, which invest the muscles and viscera and separate them into different compartments.19 Anteriorly, the cervical fascia is divided into one superficial and four deep layers. The superficial fascia contains fat and areolar tissue, including the platysma muscle, external jugular vein, and cutaneous sensory nerves. The deep cervical fascia, including the outer investing layer of deep fascia, middle cervical fascia, and prevertebral fascia, compartmentalizes the structures deep to the superficial fascia. The superficial layer of the deep fascia extends from the trapezius muscle over the posterior triangle and splits to enclose the sternocleidomastoid muscle. The middle layers of the deep cervical fascia enclose the strap muscles and omohyoid and extend as far laterally as the scapula. The deeper middle layer is the visceral fascia that surrounds the thyroid gland, larynx, trachea, pharynx, and esophagus. The alar fascia spreads behind the esophagus and surrounds the carotid sheath structures laterally. The carotid sheath encloses the carotid artery, internal jugular vein, and vagus nerve. The deepest layer of the deep fascia is the prevertebral fascia, which covers the scaleni muscles, longus colli muscles, and anterior longitudinal ligament.
Triangles of the Neck
The cervical region is divided into two anatomic compartments, the anterior and posterior triangles, by the sternocleidomastoid. The anterior triangle is formed by the midline anteriorly, the anterior border of the sternocleidomastoid posteriorly, and the inferior border of the mandible superiorly. The posterior triangle is bound anteriorly by the posterior border of the sternocleidomastoid, posteriorly by the anterior border of the trapezius, and inferiorly by the middle third of the clavicle. Understanding the structures within the triangles and their complex relationship helps the surgeon to learn these important landmarks during surgical approaches to the neck.5
The posterior triangle is subdivided into the occipital and supraclavicular triangles by the inferior belly of the omohyoid muscle. The posterior triangle contains the accessory nerve, the brachial plexus, the third part of the subclavian artery, the dorsal scapular nerve, the long thoracic nerve, the nerve to the subclavius, the suprascapular nerve, and the transverse cervical artery.18
Surgical Approaches
Anterior Approaches to Upper Cervical Spine
The transoral approach provides anterior exposure to the atlantoaxial complex. Inferior exposure down to C3-4 can be obtained with the addition of a lip-splitting approach with mandibulotomy, whereas superior exposure up to the clivus of the occiput can be obtained by splitting the uvula, soft palate, and posterior pharyngeal wall.33,34 If necessary, a portion of the hard palate can also be cut with a rongeur. Thoughtful placement of a self-retaining retractor system also facilitates exposure by retraction of the hard and soft palate and tongue.
Several variations to the retropharyngeal exposure have been described and can be divided into anteromedial and anterolateral approaches depending on the relationship of the dissection to the carotid sheath.35–37 The anteromedial retropharyngeal approach uses the interval medial (anterior) to the carotid sheath, whereas the anterolateral approach uses the plane lateral (posterior) to the carotid sheath. In both cases, a thorough understanding of the local anatomy is imperative. For right-handed surgeons, the approach is typically from the patient’s right side. At this level, above C5 the recurrent laryngeal nerve has already crossed the surgical field from lateral to medial and runs safely within the tracheoesophageal groove.
Transoral Technique
For the transoral technique, prophylactic antibiotics are given immediately preoperatively and 72 hours postoperatively based on preoperative nasopharyngeal culture and sensitivity studies. Care must be exercised during this approach to stay in the midline and develop full-thickness pharyngomucosal flaps. A vertical incision is made through the posterior pharyngeal mucosa, the constrictors, and the longus colli muscle with the anterior arch of the atlas as the landmark. After adequate superior and inferior exposure is obtained, subperiosteal lateral dissection is done to expose the medial edge of the C1-2 facet joint. Dissection beyond the lateral edge of the C1-2 facet risks injury to the vertebral artery, which usually lies at a minimum of 20 mm from the midline.38
In a pure transoral approach, exposure is limited by the amount that the retractors can open the oral cavity. The addition of a mandibulotomy can increase exposure significantly. The mandibulotomy is achieved with a midline incision of the lower lip around the chin in a C-shaped fashion and then straight down to the hyoid bone to expose the mandible subperiosteally.39 When this is done, a reconstruction plate is bent, and the screw holes in the mandible are drilled before the mandibular osteotomy is done; this decreases the risk of postoperative malocclusion. The plate should be placed low on the mandible to decrease the risk of injuring the dental roots by the drill. The mandibulotomy is performed between the central incisors. If the decision is made also to split the tongue, this should be done in the midline, with care taken not to injure the epiglottis.39
Complications
Reported results with this exposure are variable. Although access to the upper cervical vertebra through this approach is relatively direct, the potential for significant morbidity and mortality exists owing to the risk of infection by pharyngeal flora, the confined working area, and the lack of extensile exposure.40 Complications can be minimized with careful patient selection and proper surgical technique.
Infection is a frequently reported complication with the transoral approach,41 particularly with extensive resections and use of bone graft. Direct contamination and septic encephalomeningitis can occur through direct exposure or opening of the dura. In a series reported by Fang and Ong41 in six patients who underwent extensive vertebral body resection and bone grafting, four developed wound infections, and one developed encephalomeningitis. Using perioperative antibiotics, limiting the use of bone graft when possible, and minimizing the exposure and resection can help decrease these risks. The use of a nasogastric tube postoperatively for 5 days until evidence of mucosal healing may decrease wound contamination.
Venous hemorrhage from epidural veins can typically be controlled with the use of cellulose and cottonoid patties. Arterial hemorrhage owing to injury to the vertebral artery or its branches can be more problematic. Excessive or uncontrolled bleeding can occur if the dissection is not performed subperiosteally or strays too lateral into the vertebral arteries. Life-threatening hemorrhage or basilar artery ischemia, especially in elderly patients, can result. Tamponade of the bleeding with hemostatic agents and bone wax may result in a false aneurysm or late bleeding requiring urgent surgery or balloon embolization.38 Uncontrolled bleeding often requires emergent balloon embolization or immediate surgical exposure of the vertebral artery in the foramen transversarium for ligation.
Anteromedial Retropharyngeal Technique
Described by deAndrade and McNab in 196936 and later by McAfee and colleagues in 1987,35 the anteromedial retropharyngeal approach is the superior extension of the anteromedial approach to the lower cervical spine as described by Southwick and Robinson.42 Similar to the anteromedial approach to the lower cervical spine, familiarity with the fascial planes is vital to understanding the approach. These planes include (1) the superficial fascia containing the platysma; (2) the superficial layer of the deep fascia extending from the sternocleidomastoid anteriorly and enclosing the trapezius posteriorly; (3) the middle layer of the deep fascia covering the strap muscles and omohyoid and visceral fascia surrounding the thyroid gland, larynx, trachea, pharynx, and esophagus; and (4) the deep layer of the cervical fascia, which includes the alar fascia connecting the two carotid sheaths laterally and fusing in the midline to the visceral fascia and the prevertebral fascia covering the scaleni and longus colli muscles and the anterior longitudinal ligament (Fig. 17–7).
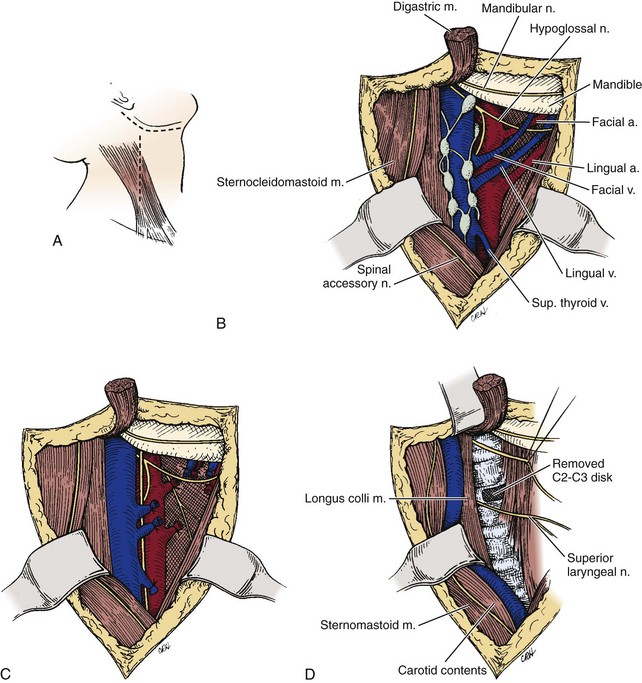
A transverse submandibular incision is used in this approach, or, alternatively, an incision is made along the anterior aspect of the sternocleidomastoid muscle and curved toward the mastoid process (Fig. 17–8A). The platysma and the superficial layer of the deep cervical fascia are divided in line with the incision to expose the anterior border of the sternocleidomastoid (Fig. 17–8B). With the help of a nerve stimulator, the marginal mandibular branch of the facial nerve (cranial nerve VII) is isolated and protected. Because the branches of the mandibular nerve are superficial to the lateral crossing veins, ligating the retromandibular vein as it joins the internal jugular vein and keeping the dissection deep and inferior to the vein during the exposure help protect the superficial branch of the facial nerve.
The retropharyngeal space is entered by using blunt dissection to develop the plane between the carotid sheath laterally and the visceral fascia containing the larynx and pharynx medially. Exposure can be improved by sequentially ligating tethering branches of the carotid artery and jugular vein, which may include the superior thyroid artery and vein, lingual artery and vein, ascending pharyngeal artery and vein, and facial artery and vein (Fig. 17–8C). The superior laryngeal nerve is identified, protected, and mobilized as it travels from its origin near the nodose ganglion into the larynx.
The prevertebral fascia overlying the vertebral body, intervertebral disc, and longus colli are now visible (Fig. 17–8D). The two longus colli converge in the midline on the anterior tubercle of the atlas. Because the hypoglossal, glossopharyngeal, vagus, and accessory nerves and the internal carotid artery and jugular vein are tethered to the occiput as they exit their respective foramina, they can be injured with vigorous retraction or greater than 2 cm lateral dissection from the midline. Additionally, excess anterior retraction of the pharynx can result in injury to the pharyngeal and laryngeal branches of the vagus nerve. At this point, a midline incision over the basiocciput, atlas, and axis can be performed, and the anterior longitudinal ligament and longus colli muscle can be dissected subperiosteally to obtain lateral exposure to the cervical spine.
Anterolateral Retropharyngeal Technique
Described by Whitesides and Kelly,43 the anterolateral retropharyngeal approach provides exposure of the upper cervical spine by partially transecting the sternocleidomastoid and proceeding laterally and posterior to the carotid sheath (Fig. 17–9). As a result, the major branches of the external carotid and laryngeal nerves are not disturbed. Although this exposure allows for distal extension to include T1, its superior extension is limited to the ring of the atlas. Because the internal carotid artery; jugular vein; and vagus, accessory, and hypoglossal nerves are tethered to the skull, adequate retraction necessary to expose the basiocciput would result in injury to these structures.
A longitudinal skin incision is made from the mastoid extending distally and anteriorly along the anterior aspect of the sternocleidomastoid muscle. The external jugular vein is identified and ligated, and the greater auricular nerve running parallel to the external jugular vein is spared if possible. The sternocleidomastoid now is prominent; if only a limited exposure (C1-2) is required, consideration can be given to preserving the sternocleidomastoid. In most cases, the sternocleidomastoid and splenius capitis muscles are detached from the mastoid, leaving a fascial edge for later repair. The spinal accessory nerve enters the sternocleidomastoid approximately 3 cm distal to the mastoid tip and should be identified and protected.43
Complications
Laryngeal and pharyngeal dysfunction can result from retraction of the laryngeal nerves. Patients should be advised preoperatively to expect difficulty with phonation and swallowing, especially in the early postoperative course. Problems can persist if the external branch of the superior laryngeal nerve is sacrificed or is transected. In three of five cases reported by deAndrade and McNab,36 persistent postoperative hoarseness, laryngeal fatigue, and inability to produce high tones persisted. Nerve injury to the spinal accessory nerve can occur particularly with the anterolateral retropharyngeal approach. Care should be taken to identify and protect this nerve intraoperatively because weakness to the sternocleidomastoid and trapezius muscle can result.
Anterior Exposure of Lower Cervical Spine
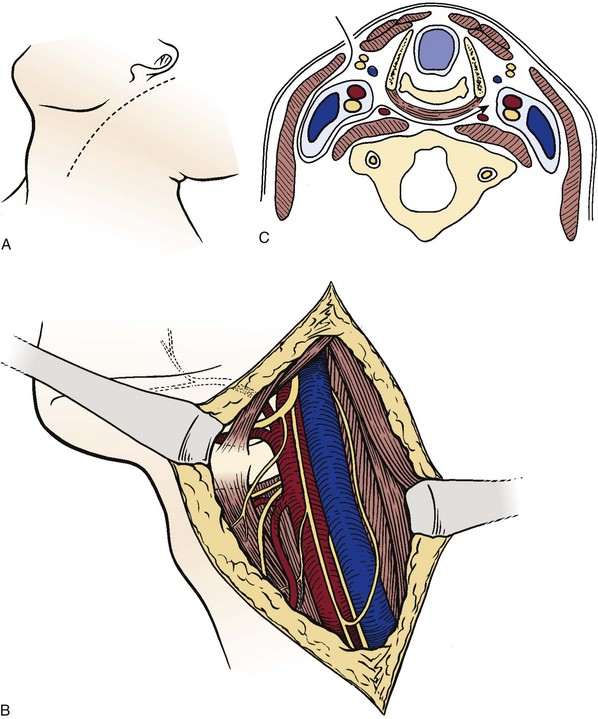
Similar to approaches to the upper cervical spine, anterior exposures to the lower cervical spine can be divided into anterolateral and anteromedial approaches based on their relationship to the carotid sheath. First described by Southwick and Robinson,42 the anteromedial approach employs the interval between the sternocleidomastoid laterally and the strap muscles and tracheoesophageal complex medially and is used in most cases. In special circumstances, the anterolateral approach described by Henry44 and Hogson45 may be used. Hogson45 described an approach to the lower cervical spine in which dissection was done posterior to the carotid sheath to expose the anterior and lateral aspects. Verbiest46 described a modification of the original approach for the exposure of the vertebral artery. Dissection anterior to the carotid sheath, as in the anteromedial Smith-Robinson technique, provides more lateral exposure to the cervical spine and may be better in cases in which the lesion is localized more laterally or if the vertebral artery must be exposed. The spinal nerve can also be identified posterior to the vertebral artery (Fig. 17–10).
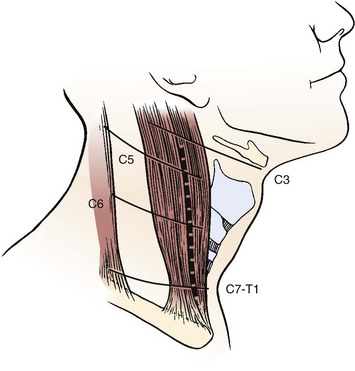
To minimize injury to the recurrent laryngeal nerve, the cervical spine is often approached from the left, particularly at the C6-T1 region. Although a right-handed surgeon may prefer the right-sided approach, the recurrent laryngeal nerve is at greater risk of injury because it may leave the carotid sheath at a higher level on the right side. The hyoid bone overlies the third vertebra, the thyroid cartilage overlies the C4-5 intervertebral disc space, and the cricoid ring is at the C6 vertebra (Fig. 17–11).5 In many cases, when the neck is in a significantly extended position, these landmarks may be displaced inferior in relationship to the vertebral bodies, and moving the incision slightly higher can help accommodate for the shift. A horizontal incision is used in most cases, but a vertical incision anterior to the sternocleidomastoid may be necessary in cases in which multiple levels need to be exposed.
Anteromedial Approach
An 18-gauge needle with two 90-degree bends to prevent spinal canal penetration is placed in the disc space, and a lateral radiograph is taken. When the correct level is confirmed, the exposure is completed by dividing the pretracheal fascia and anterior longitudinal ligament in the midline to minimize bleeding and prevent injury to the sympathetic chain and subperiosteal mobilization of the longus colli laterally. Too vigorous lateral dissection may damage the vertebral artery or nerve roots, especially at the level of the intervertebral disc space.19 At the level of the vertebral body, the anterior aspect of the foramen transversarium offers some protection to the vertebral artery.
Anterolateral Approach
By performing the dissection posterior to the carotid sheath, the anterolateral approach avoids the thyroid vessel, vagus nerve, and superior laryngeal nerve and provides access to the anterior and lateral aspect of the cervical spine. Superior extension of this approach allows access to the upper cervical spine as described by Whitesides and Kelly43 (see anterior retropharyngeal approach to upper cervical spine). A transverse or oblique skin incision is made from the right side. The subcutaneous tissue and the platysma muscle are divided, and the branches of the external jugular vein are ligated, but the cutaneous nerves should be protected if possible. The posterior border of the sternocleidomastoid muscle is identified, and blunt dissection should follow the fat pad through the posterior triangle of the cervical spine. The dissection should stay anterior to the anterior scalene muscle and anterior to the anterior tubercle of the transverse process to avoid injuries to the vertebral artery or nerve root. If retraction of the sternocleidomastoid muscle is difficult, the posterior third and the omohyoid muscle can be divided to enhance exposure. The cervical sympathetic plexus on the lateral aspect of the prevertebral musculature should be identified and protected. The prevertebral fascia and longus colli muscle are incised in the midline for subperiosteal exposure of the cervical spine. After palpation of the anterior tubercle of the transverse process, the anterior tubercle can be removed to gain access to the vertebral artery and venous plexus.
Complications
Esophageal perforation is a rare but serious complication of anterior cervical spine fusion, occurring in about 1 of 500 procedures. Sharp retractors must be avoided, and gentle handling of the medial soft structures is mandatory. In revision cases, the use of a nasogastric tube may help identify the esophagus intraoperatively. If perforation is suspected during surgery, methylene blue can be injected for better visualization. The perforation is frequently not recognized until the patient develops an abscess, tracheoesophageal fistula, or mediastinitis in the postoperative period.47 The usual treatment consists of intravenous antibiotics, nasogastric feeding, drainage, débridement, and repair. Early consultation with head and neck surgeons is recommended.
Anterior Approach to Cervicothoracic Junction
Anterior approaches to the cervicothoracic junction are challenging because of the proximity of the great vessels and overlying sternum and clavicle (Fig. 17–12). Three main approaches have been described to address access in this region: the modified anterior approach, the sternal-splitting approach, and the transthoracic approach.48–50 Each approach has its own advantages and disadvantages and should be chosen accordingly. Theoretically, the modified anterior approach can provide visualization and access to the anterior spinal structures from C4 to T4 but requires resection of the medial clavicle and sternoclavicular joint. Similarly, the sternal-splitting approach when combined with the anteromedial approach to the neck offers access from C4 to T4 through retraction of the great vessels. Although the transthoracic approach provides adequate exposure to the upper thoracic spine, access to the cervical spine is limited to C7 at best. This approach generally provides limited access to the cervical spine.
Posterior Approaches
Posterior exposures to the cervical spine are among the safest and most used exposures for management of cervical spine disorders, allowing direct access to the posterior elements from the occiput to the thoracic spine.5,51 The particular anatomy of the upper cervical spine and the transitional anatomy of the cervicothoracic junction should also be understood when approaching these regions posteriorly.
Posterior Approach to Upper Cervical Spine
The greater occipital nerve (C2) and the third occipital nerve cross the field and course laterally in the paracervical muscles. Subperiosteal dissection and avoidance of vigorous lateral dissection should prevent injury to these nerves. If occipital fixation is required, the inion is thickest at its prominence near the ridge, and the passage of wires is possible without violating both tables of the occiput. If screw fixation is being used, bicortical purchase is recommended for the occiput, and screw lengths of typically 10 to 12 mm can be accepted in this region.52
The passage of sublaminar wires at the C1-2 level is common because the spinal canal at this level is capacious, but passage at lower cervical levels is associated with increased risk of neurologic injury. The removal of the atlantoaxial ligament or atlanto-occipital membrane is not required except for laminectomy cases. Careful separation of the membrane or ligament from the bone is all that is usually needed to pass sublaminar wires. This separation can be performed with a small-angled curet or a small Freer elevator. Slight head flexion can also help by opening the space between the ring of C1 and the occiput. The mean thickness of the posterior ring is 8 mm, and the cortical bone is thin53; great care must be taken not to fracture the posterior ring of C1 while dissecting the ligamentum flavum.
An additional technique to expose the lateral aspect of C1 or C2 is to elevate the periosteum with a small Freer elevator. This allows the vertebral artery to be protected at the lateral aspect of the C1 arch. To avoid injury to the vertebral artery, lateral dissection should not exceed greater than 1.5 cm from the midline in an adult and 1 cm in a child. The vertebral artery is at risk during posterior exposures and lateral decompressions of C1 if the dissection is performed more than 15 mm from the midline of the posterior tubercle in adults or 10 mm in children.54,55 The vertebral artery courses over the arch of the atlas and pierces the lateral angle of the posterior atlanto-occipital membrane.
Brief consideration is given here to the regional anatomy for the C1-2 transarticular screw fixation (Magerl) technique,56–58 C1 lateral mass and C2 pedicle screw (Harms) technique,58,59 and C2 translaminar screw.58,60 A preoperative thin-cut CT scan with sagittal reconstructions should be obtained to evaluate the course of the vertebral artery. This imaging is especially important to obtain in rheumatoid patients in whom an anomalous or enlarged foramen transversarium is common, which may place the vertebral arteries at increased risk with this technique. Attention should be paid to the presence of a ponticulus posticus, an anomalous ossification overlying the vertebral artery as it runs in the superior sulcus of C1, which can occur in 15% of the population. Regardless of the technique used, the intraoperative use of anteroposterior and lateral fluoroscopy can provide screw inclination in the coronal and parasagittal plane.
Because of the amount of cephalad angulation required to place the C1-2 transarticular screw, subperiosteal exposure should extend down to C4.54 The main landmark is the medial part of the isthmus of the axis, which can be visualized directly by subperiosteal dissection of the C2 lamina proceeding along the bony contour around the spinal canal until the maximum width in the coronal plane is reached. A Kirschner wire can be used to retract the soft tissues containing the greater occipital nerve and accompanying the venous plexus. The drilling for the screw is strictly sagittal, 2 to 3 mm lateral to the inner border of the isthmus. The screw should perforate the atlantoaxial joint approximately in the posteromedial part entering the lateral mass of the atlas.61
In the case of placement of a C1 lateral mass screw, the C1-2 joint is the key anatomic landmark to be identified.59 This identification can be facilitated by caudal retraction of the C2 nerve, which exposes the posterior aspect of the lateral mass of C1.58 The starting point of the C1 lateral mass screw lies directly in the mid-portion in the lateral mass. The C2 pedicle screw is identified by delineating the medial border of the isthmus and pars of the axis as in the C1-2 transarticular screw; however, the trajectory of the C2 pedicle screw is more medial and follows the path of the pedicle as would be expected.58,59
Technical challenges associated with the C1-2 transarticular screw and C2 pedicle screw placement led to the development of the C2 translaminar screw. Use of this screw is possible because of the predictably large size of the C2 lamina combined with the fact that the use of this screw eliminates the possibility for vertebral artery injury.58,60 The starting point is identified as the junction of the C2 spinous process and the lamina, and the trajectory of the screw parallels the down slope of the dorsal aspect of the contralateral lamina. Care should be taken not to breach the ventral aspect of the lamina resulting in placement of the screw within the spinal canal and to ensure that the C2-3 facet joint is not violated by placement of a screw that is too long.60
Posterior Approach to Lower Cervical Spine
A reverse Trendelenburg position minimizes venous bleeding and reduces cerebrospinal fluid pressure (Fig. 17–13). The posterior approach uses a longitudinal midline incision that extends above and below the segments required for the procedure. This extension of the skin and subcutaneous tissues is necessary because the skin of the posterior neck is less mobile and thicker for retraction. The skin is incised sharply, and electrocautery is used to incise the ligamentum nuchae in the midline. With a wide flat periosteal elevator such as a Cobb, the dissection is carried subperiosteally down the spinous processes. Inadvertent penetration of instruments into the spinal canal can be minimized by examining preoperative films for evidence of spina bifida and other bony defects and by realizing that, in the cervical spine, the laminae do not override each other as much as in the thoracic spine, resulting in wider interlaminar spaces. Care should be taken to stay subperiosteal because the bifid nature of the spinous processes may result in a bulbous expanse, and the dissection may err into the paraspinal musculature. A superficial plexus of veins may be encountered and should be cauterized as needed.
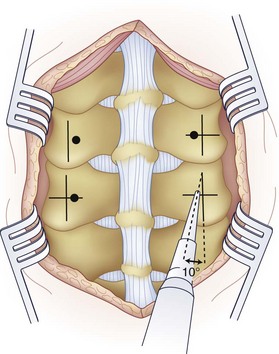
First popularized by Roy-Camille and colleagues,62 placement of posterior cervical screws requires a thorough understanding of the lateral mass anatomy to minimize injury to associated neurovascular structures. Different entry points and screw orientations have been recommended. In the original description by Roy-Camille and colleagues,62 the entry point was the center of the lateral mass with the screw angled 10 degrees laterally (Fig. 17–14), whereas Magerl recommended the drilling angle to be 25 degrees laterally and 45 degrees superiorly. An and colleagues4 found that by orienting the screw 15 degrees cephalad and 30 degrees laterally with an entry point 1 mm medial to the anatomic center of the lateral mass, the facet joint and nerve root are avoided.
Posterior Approach to Cervicothoracic Junction
Complications
Pearls
Pitfalls
Key Points
1 An HS, Cotler JM, editors. Spinal Instrumentation, 2nd ed, Philadelphia: Lippincott Williams & Wilkins, 1990.
2 Graham JJ. Complications of cervical spine surgery: A five-year report on a survey of the membership of the Cervical Spine Research Society by the Morbidity and Mortality Committee. Spine. 1989;14:1046.
3 Heller JG, Pedlow FX. Anatomy of the cervical spine. In: Clark CR, editor. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:3-36.
4 Miller MD, Chhabra AB, Hurwitz SR, et al, editors. Orthopaedic Surgical Approaches. Philadelphia: WB Saunders. 2008:211-329.
5 Southwick WO, Robinson RA. Surgical approaches to the vertebral bodies in the cervical and lumbar regions. J Bone Joint Surg Am. 1957;39:631-644.
1 Albert TJ. Anterior, middle, and lower cervical exposures. In: Albert TJ, Balderston RA, Northrup BE, editors. Surgical Approaches to the Spine. Philadelphia: WB Saunders; 1997:9-24.
2 Schaffler MB, Alson MD, Heller JG, et al. Morphology of the dens. Spine. 1992;17:738-743.
3 Xu R, Naduad MC, Ebraheim NA, et al. Morphology of the second cervical vertebra and the posterior projection of the cervical pedicle axis. Spine. 1995;20:259-263.
4 An H, Gordin R, Renner K. Anatomic considerations for plate-screw fixation of the cervical spine. Spine. 1991;16(Suppl):S548-S551.
5 Johnson RM, Murphy MJ, Southwick WO. Surgical approaches to the spine: Function and surgical anatomy of the neck. In: Herkowitz HN, Garfin SR, Balderston RA, et al, editors. Rothman-Simeone the Spine. 4th ed. Philadelphia: WB Saunders; 1999:1463-1571.
6 Jefferson G. Fracture of atlas vertebra: Report of four cases and review of those previously reported. Br J Surg. 1920;7:407.
7 An HS. Anatomy of the spine. In: An HS, editor. Principles and Techniques of Spine Surgery. Philadelphia: Lippincott Williams & Wilkins; 1998:1-30.
8 Heller JG, Pedlow FX. Anatomy of the cervical spine. In: Clark CR, editor. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:3-36.
9 Panjabi MM, Duranceau J, Goel V, et al. Cervical human vertebrae: Quantitative three-dimensional anatomy of the middle and lower regions. Spine. 1993;16:861-874.
10 Karaikovic EE, Kunakornsawat S, Daubs MD, et al. Surgical anatomy of the cervical pedicles: Landmarks for posterior cervical pedicle entrance localization. J Spinal Disord. 2000;13:63-72.
11 Karaikovic EE, Yingsakmongkol W, Gaines RWJr. Accuracy of cervical pedicle screw placement using the funnel technique. Spine. 2001;26:2456-2462.
12 Karaikovic EE, Yingsakmongko LW, Griffiths HJ, et al. Possible complications of anterior perforation of the vertebral body using cervical pedicle screws. J Spinal Disord Tech. 2002;15:75-78.
13 Czervionke LF, Daniels DL, Ho PSP, et al. Cervical neural foramina: Correlative anatomic and MR imaging study. AJNR Am J Neuroradiol. 1988;169:753-759.
14 Daniels DL, Hyde JS, Kneeland JB, et al. The cervical nerves and foramina: Local-coil MR imaging. AJNR Am J Neuroradiol. 1986;7:129-133.
15 Aebi M, Thalgott JS, Webb JK. Stabilization techniques: Lower cervical spine. In: Aebi M, Thalgott JS, Webb JK, editors. AO/ASIF Principles in Spine Surgery. New York: Springer; 1998:54-79.
16 Ebraheim NA, Xu R, Knight T, et al. Morphometric evaluation of the lower cervical pedicle and its projection. Spine. 1997;22:1-6.
17 Dvorak JPM. Functional anatomy of the alar ligaments. Spine. 1987;12:183-189.
18 Parke WW, Sherk HH. Normal adult anatomy. In: Sherk HH, Dunn EJ, Eismont FJ, editors. The Cervical Spine. Philadelphia: JB Lippincott; 1988:11-32.
19 An HS. Anatomy of the cervical spine. In: An HS, Simpson MJ, editors. Surgery of the Cervical Spine. London: Martin Dunitz; 1994:1-40.
20 Hayashi K, Yabuki T, Kurokawa T, et al. The anterior and posterior longitudinal ligaments of the lower cervical spine. J Anat. 1977;124:633-636.
21 Bland JH, Boushey DR. Anatomy and physiology of the cervical spine. Semin Arthritis Rheumatol. 1990;20:1-20.
22 Lang J. Clinical Anatomy of the Cervical Spine. New York: Thieme; 1993.
23 Kameyama T, Hashizume Y, Ando T, et al. Morphometry of the normal cadaveric cervical spinal cord. Spine. 1994;19:2077-2081.
24 Okada Y, Ikata T, Katoh S, et al. Morphologic analysis of the cervical spinal cord, dural tube and spinal canal by magnetic resonance imaging in normal adults and patients with cervical spondylotic myelopathy. Spine. 1994;19:2231-2235.
25 Rauschning W. Anatomy and pathology of the cervical spine. In: Frymoyer JW, editor. The Adult Spine. New York: Raven Press; 1991:907-929.
26 Flannigan BD, Lufkin RB, McGlade C, et al. MR imaging of the cervical spine: Neurovascular anatomy. AJR Am J Roentgenol. 1987;148:785-790.
27 Pech P, Daniels DL, Williams AL, et al. The cervical neural foramina: Correlation of microtomy and CT anatomy. Radiology. 1985;155:143-146.
28 Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine. 1982;7:319-320.
29 Gerbrand JG, Baljet B, Drukker J. Nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188:282-296.
30 Rickenbacher J, Landolt AM, Theiler K. Applied Anatomy of the Back. Berlin: Springer-Verlag; 1982.
31 Dommisse GF. The blood supply of the spinal cord. J Bone Joint Surg Br. 1974;56:225.
32 Hoppenfeld S, deBoer P. The spine. In: Hoppenfeld S, deBoer P, editors. Surgical Exposures in Orthopaedics: The Anatomic Approach. 2nd ed. Philadelphia: JB Lippincott; 1994:215-301.
33 Arbit E, Patterson RHJr. Combined transoral and median labiomandibular glossotomy approach to the upper cervical spine. Neurosurgery. 1981;8:672-674.
34 Ashraf J, Crockard HA. Transoral fusion for high cervical fractures. J Bone Joint Surg Br. 1990;72:76.
35 McAfee PC, Bohlman HH, Riley LHIII, et al. The anterior retropharyngeal approach to the upper part of the cervical spine. J Bone Joint Surg Am. 1987;69:1371.
36 deAndrade J, McNab I. Anterior occipitocervical fusion using extrapharyngeal approach. J Bone Joint Surg Am. 1969;51:1621.
37 Whitesides TE, McDonald P. Lateral retropharyngeal approach to the upper cervical spine. Orthop Clin North Am. 1978;9:115.
38 Mendoza N, Crockard HA. Anterior transoral procedures. In: An HS, Riley LHIII, editors. An Atlas of Surgery of the Spine. Philadelphia: Lippincott-Raven; 1998:55-69.
39 Rosen MR, Keane WM, Rosen D. Anterior upper cervical exposures. In: Albert TJ, Balderston RA, Northrup BE, editors. Surgical Approaches to the Spine. Philadelphia: WB Saunders; 1997:25-52.
40 Menezes AH. Complications of surgery at the craniovertebral junction: Avoidance and management. Pediatr Neurosurg. 1992;17:254.
41 Fang H, Ong G. Direct anterior approach to the upper cervical spine. J Bone Joint Surg Am. 1962;44:1588-1604.
42 Southwick WO, Robinson RA. Surgical approaches to the vertebral bodies in the cervical and lumbar regions. J Bone Joint Surg Am. 1957;39:631-644.
43 Whitesides TE, Kelly RP. Lateral approach to the upper cervical spine for anterior fusion. South Med J. 1966;59:879.
44 Henry AK. Extensile Exposure. Baltimore: Williams & Wilkins; 1959. p 53
45 Hogson AR. An approach to the cervical spine (C3-C7). Clin Orthop. 1965;39:129.
46 Verbiest H. Anterolateral operations for fractures and dislocations in the middle and lower parts of the cervical spine. J Bone Joint Surg. 1969;51:1489-1530.
47 Whitehill R. Late esophageal perforation from an autogenous bone graft: Report of a case. J Bone Joint Surg Am. 1985;67:644-645.
48 Kurz LT, Herkowitz HN. Anterior exposures of the cervicothoracic junction and upper thoracic spine. In: Albert TJ, Balderston RA, Northrup BE, editors. Surgical Approaches to the Spine. Philadelphia: WB Saunders; 1997:61-80.
49 Sundaresan N, Shah J, Foley KM, et al. An anterior surgical approach to the upper thoracic vertebrae. J Neurosurg. 1984;61:686-690.
50 Vaccaro AR, An HS. Anterior exposures of the cervicothoracic junction. In: An HS, Riley LHIII, editors. An Atlas of Surgery of the Spine. Philadelphia: Martin Dunitz; 1998:113-130.
51 Andreshak TG, An HS. Posterior cervical exposures. In: Albert TJ, Balderston RA, Northrup BE, editors. Surgical Approaches to the Spine. Philadelphia: WB Saunders; 1997:81-114.
52 Winter RB, Lonstein JW, Denis F, et al. Posterior upper cervical procedures. In: Winter RB, Lonstein JW, Denis F, et al, editors. Atlas of Spine Surgery. Philadelphia: WB Saunders; 1995:19-33.
53 Doherty B, Heggeness MH. The quantitative anatomy of the atlas. Spine. 1994;19:2497-2500.
54 An H, Xu R. Posterior cervical spine procedures. In: An H, Riley LIII, editors. An Atlas of Surgery of the Spine. Philadelphia: Lippincott-Raven; 1998:13-14.
55 Ebraheim N, Xu R, Ahmad M, et al. The quantitative anatomy of the vertebral artery groove of the atlas and its relation to the posterior atlantoaxial approach. Spine. 1998;23:320-323.
56 Magerl F, Seemann P. Stable posterior fusion of the atlas and axis by transarticular screw fixation. In: Kehr P, Weidner A, editors. Cervical Spine. New York: Springer-Verlag; 1987:322.
57 Grob D, Crisco J, Panjabi MM, et al. Biomechanical evaluation of four different posterior atlantoaxial fixation techniques. Spine. 1991;17:480-490.
58 Shen FH. Spine. In: Miller MD, Chhabra AB, Hurwitz SR, et al, editors. Orthopaedic Surgical Approaches. Philadelphia: WB Saunders; 2008:211-329.
59 Harms J, Melcher RP. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine. 2001;26:2467-2471.
60 Wright NM. Posterior C2 fixation using bilateral, crossing C2 laminar screws. J Spinal Disord Tech. 2004;17:158-162.
61 Grob D, An HS. Posterior occipital and C1/C2 instrumentation. In: An HS, Cotler JS, editors. Spinal Instrumentation. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1999:191-201.
62 Roy-Camille RR, Sailant G, Mazel C. Internal fixation of the unstable cervical spine by posterior osteosynthesis with plate and screws Cervical Spine Research Society (ed). The Cervical Spine, 2nd ed. Philadelphia: JB Lippincott. 1989:390-404.