CHAPTER 40 Cervical Radiculopathy
Anterior Surgical Approach
Radiographic Evaluation
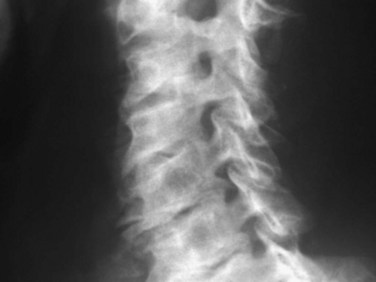
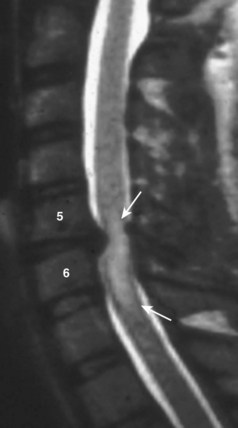
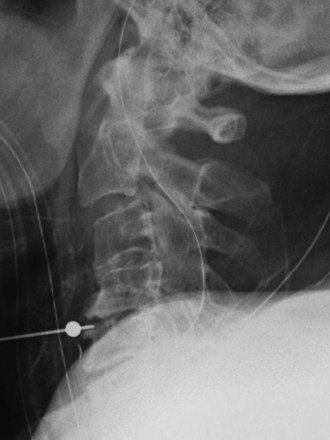
Radiographic evaluation usually begins with plain radiographs; lateral, anteroposterior, and oblique views are obtained. Global and segmental alignment can be easily assessed. Findings of degenerative disease include disc space narrowing, osteophytosis of the apophyseal joints, and spurring at the level the disc space. Degenerative spondylolisthesis of the cervical spine is common, and this usually involves facet arthropathy as well.1 Dynamic lateral radiographs can show segmental instability by assessing anteroposterior translation and angular motion. Foraminal stenosis may occur from uncovertebral arthrosis or degenerative subluxation. Typically, bony elements are visualized, but radiographs inherently are unable to assess neural elements. An indirect assessment of the neural foramina can be obtained with oblique views (Fig. 40–1). Although the soft tissue component of spinal cord compression cannot be visualized on lateral radiographs, the space available is easily assessed. Brain and Wilkinson2 reported an average space of 17 to 18 mm (range 14 to 23 mm).
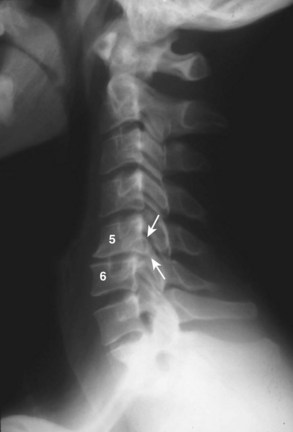
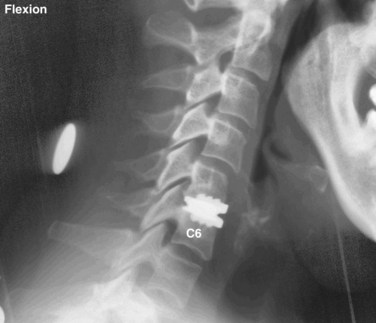
Several authors have reported on the use of lateral radiographs to assess cervical stenosis. A classic study by Edwards and LaRocca3 found the average sagittal diameter of asymptomatic individuals to be 17 mm. These authors reported that myelopathy was present in subjects with a diameter of less than 10 mm. A diameter of 10 to 13 mm was noted in patients without symptoms but who were likely to become symptomatic. Using radiographs, stenosis is present in about 5% of the population, and the rate increases with age to approximately 9% in patients older than 70 years.4 Torg ratio identifies stenosis and eliminates the variability and magnification associated with direct measurement of radiographs.5 A sagittal spinal canal diameter–to–vertebral body ratio of less than 0.8 is indicative of cervical spinal stenosis (Fig. 40–2). Several authors have questioned the reliability of Torg ratio.6–8
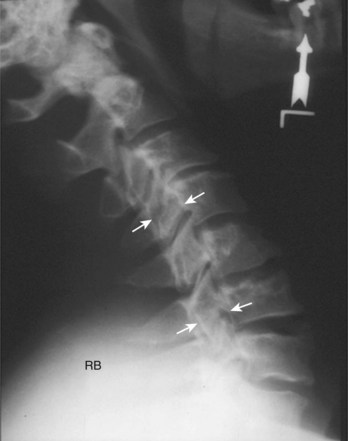
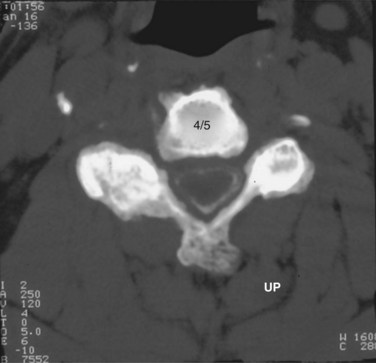
When more direct visualization of the neural elements is necessary, advanced imaging can be employed. Initially, spinal myelography was the test of choice. Water-soluble dye injected into the epidural space allows visualization of the neural elements by assessing flow patterns. Despite relatively high sensitivity, myelograms are nonspecific. Flow can be altered by soft disc herniations, hard disc (osteophyte formation), facet arthropathy, or various other pathologies. Computed tomography (CT) combined with myelography can significantly improve accuracy.9 Axial images and sagittal reconstructions improve the practitioner’s ability to determine the causes of compression. Altered filling of the nerve roots from bony compression can easily be assessed because of the bony detail produced with CT imaging (Fig. 40–3). CT myelography can be performed in patients who are not candidates for other procedures such as magnetic resonance imaging (MRI). Myelography has some disadvantages. A major concern is the invasiveness and morbidity associated with placement of the dye into the epidural space. Although CT myelography is more specific than radiographs, it is still very nonspecific for soft tissue causes of stenosis.
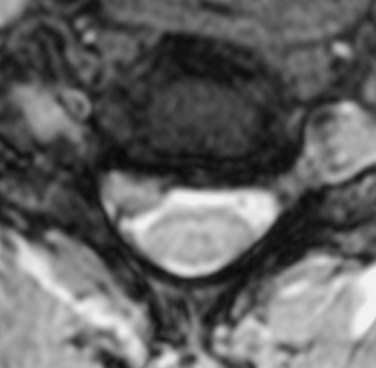
MRI offers several advantages. The neural elements and soft tissues are directly visualized, no radiation is required, and it is noninvasive. Meticulous inspection of the images can reveal soft disc herniations (Fig. 40–4) and sequestered fragments when they are present. Disruption of the posterior longitudinal ligament on sagittal MRI is indicative of a possibly sequestered fragment. Although MRI is best at visualizing soft tissue elements of the cervical spine, bony anatomy can be assessed. Additionally, MRI recognizes infiltrative soft tissue and vertebral body processes such as sarcoma or metastatic disease.
MRI scans offer excellent visualization of the lower brainstem and entire cervical spinal cord. The foramen magnum can be examined for Arnold-Chiari malformations. Basilar invagination or pannus formation from inflammatory arthritis can also be seen. Acute or chronic spinal cord compression may lead to signal change within the spinal cord, termed myelomalacia (Fig. 40–5). These changes may be important in predicting clinical outcomes after decompression.10,11
Although imaging has advanced greatly in recent years, a thorough history and physical examination still constitute the cornerstone of diagnosis and treatment. MRI and CT myelography must be correlated with the clinical evaluation of the patient. Boden and colleagues12 reported degenerative disc space changes in 25% of asymptomatic subjects younger than 40 years and almost 60% of subjects older than 60. A 10-year longitudinal study of healthy volunteers revealed that degenerative changes progressed, but symptoms developed in only 34% of subjects. Age was the only variable associated with the progression of spondylosis.13
Electrodiagnostic Studies
Electrodiagnostic studies are another diagnostic modality used to evaluate patients with radiculopathy. Peripheral nerve root entrapment can mimic cervical radiculopathy. Currently, electromyography (EMG) is the most useful modality. In some studies, 75% sensitivity has been reported.14–16 Because of the variable sensitivity of EMG, it is not an ideal screening tool. The diagnosis and location of pathology can be assessed with EMG, however. Compression causes pathophysiologic changes within the nerve root. These changes are shown on EMG as decreased motor unit potentials and fibrillation potentials. When attempting to distinguish between radiculopathy, peripheral neuropathy, and nerve root entrapment, neurophysiologic testing can be useful. When the diagnosis is clear from imaging and clinical examination, EMG is probably unnecessary. EMG and nerve conduction studies may be beneficial to distinguish specific nerve root compression when multilevel disease is present.
Surgical Indications
The indications for operative treatment of radiculopathy have not changed much over the years despite advances in operative techniques and devices. The most commonly accepted indications are (1) persistent or recurrent arm pain that is unresponsive to a trial of conservative treatment (3 months), (2) progressive neurologic deficit, (3) static neurologic deficit associated with significant radicular pain, and (4) confirmatory imaging studies consistent with the patient’s clinical findings. This chapter focuses on anterior approaches to surgical treatment. Smith and Robinson17 were the first to popularize the anterior approach.
Anterior Cervical Discectomy and Fusion: Surgical Technique
General endotracheal anesthesia provides the safest environment for anterior cervical discectomy and fusion (ACDF). The endotracheal tube should be taped on the side of the mouth opposite to the side on which the procedure is performed. Proper positioning is an essential part of this procedure. The authors typically place a small rolled towel longitudinally between the scapulae to allow the shoulders to fall back out of the way; this also positions the neck in mild hyperextension. Care must be taken in patients with myelopathy because iatrogenic worsening of myelopathy may occur with hyperextension. If symptoms are significantly worsened with hyperextension during the preoperative physical examination, this position should also be avoided. The arms are placed along the patient’s side and tucked. The shoulders can decrease visualization during exposure and during imaging. The authors use surgical tape to place longitudinal downward traction on the shoulders (Fig. 40–6).
More recently, it has been suggested that retractor displacement of the larynx against the endotracheal tube causes crush injury to the intralaryngeal segment of the recurrent laryngeal nerve, causing dysphonia. Apfelbaum and colleagues18 recommended deflating the endotracheal tube during the procedure to decrease pressure on the nerve and decrease the incidence of postoperative voice changes. Audu and colleagues19 performed a randomized, prospective, double-blind investigation of endotracheal tube manipulation. Postoperative examination with indirect laryngoscopy showed a 3.2% incidence of vocal cord paralysis. Endotracheal tube deflation did not reduce the incidence of vocal cord immobility. These authors concluded that tube manipulation has no effect on vocal cord injury.
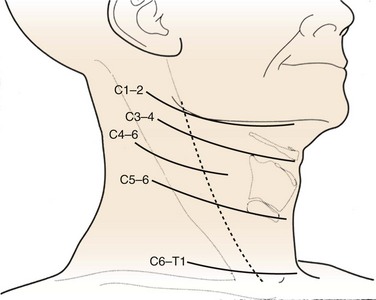
When a one-level or two-level discectomy is planned, a transverse incision is usually sufficient. These incisions tend to be more aesthetically pleasing. When an extensile approach is necessary, a longitudinal incision may be necessary. Patients with short, thick necks or who are undergoing revision surgery may benefit from longitudinal incisions. Fluoroscopy or superficial landmarks can be used to mark the appropriate skin level (Fig. 40–7). Typically, C3 is found at the level of the hyoid, C4-5 is found at the thyroid cartilage, and C6 is found at the cricoid cartilage. When exposing near the cervicothoracic junction (C7-T1), the inferior thyroid vessels and thoracic duct may have to be managed. A longitudinal incision may facilitate exposure at this level.
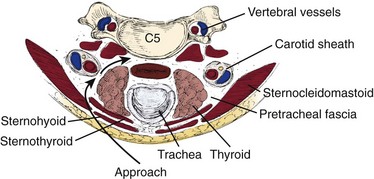
After the skin incision is complete, hemostasis can be obtained along the skin edges with electrocautery. The two edges of the skin can be elevated with either hand-held retractors or a small self-retaining retractor. The first muscle encountered is the platysma, which underlies the fascia. A curved hemostat is used to dissect bluntly underneath the platysma. Using electrocautery, the muscle is incised in line with the incision. Visualization is improved by opening the fascial planes underneath this layer; this also facilitates retractor placement to improve mobilization of the underlying structures. This is a crucial step in performing a safe dissection. The sternocleidomastoid should become visible. The dissection proceeds along the anteromedial aspect of the sternocleidomastoid. A blunt dissection should proceed between the carotid sheath and the trachea and esophagus. A finger should bluntly separate the medial border of the carotid sheath from the lateral border of the trachea and esophagus. Typically, the surgeon can easily discern the medial border of the tubular carotid. The pulsation of the carotid may not always be easily felt (Fig. 40–8).
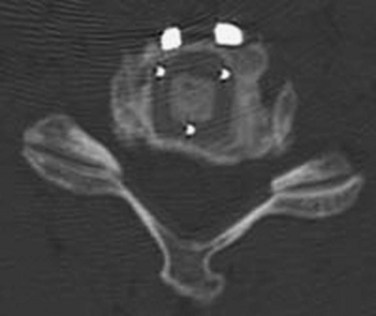
Correct identification of the proper level is imperative. Careful examination of radiographs may reveal anterior osteophytes. Palpation of these osteophytes on the anterior vertebral bodies can often lead to correct level identification. Lateral to C6 lies Chassaignac tubercle, which can be palpated and helps identify C5-6. Current patient safety recommendations by the North American Spine Society (NASS) recommend an intraoperative radiograph to verify the proper level. A standard spinal needle or specialized cervical marker should be placed in the intended operative level (Fig. 40–9). Identifying the level on a lateral radiograph may be difficult in the lower cervical spine or when the patient has either a short, thick neck or broad shoulders. The surgeon may elect to place a spinal needle at two levels, such that the superiormost needle can be visualized on the radiograph. Caution must be exercised if placement of two needles is required. A retrospective radiographic analysis of 87 consecutive patients by Nassr and colleagues20 showed a threefold increased risk of developing degenerative changes in disc levels that were previously marked with a needle for intraoperative level verification.
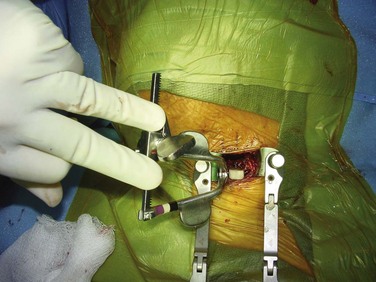
After the correct disc space is identified, the prevertebral fascia over the anterior vertebral body is divided; the longus colli muscles are then easily visualized. Dissection should begin in the midline and proceed laterally. The longus muscles are elevated bilaterally with a combination of electrocautery and mild blunt dissection. The exposure is held open with self-retaining retractors that are placed under the longus colli muscles (Fig. 40–10). The retractor can be secured by an assistant or with an arm secured to the operating table.
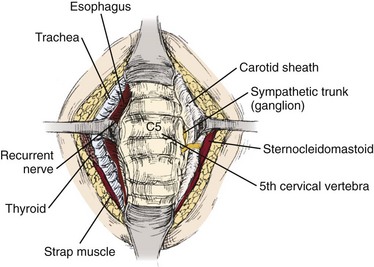
FIGURE 40–10 After elevation of longus colli muscles bilaterally, blunt retractors can be placed for remainder of procedure.
Visualization of the posterior half of the disc can be impaired by overhanging anterior osteophytes. These can be removed with a bur or rongeur to improve visualization (Fig. 40–11). It is imperative that the surgeon note the angle of the disc space on the intraoperative localization film, and care should be taken to perform the disc removal along the angle of the endplates to avoid inadvertent penetration of the vertebral bodies. The uncinate process, posterior longitudinal ligament, and bony endplates should be visible at the end of the discectomy. Inspection of the posterior longitudinal ligament is necessary to determine if a sequestered fragment is present.
The significance of posterior osteophyte removal is unknown. When they are causing obvious compression on the spinal cord, osteophytes should be removed (Fig. 40–12). After the neural elements have been decompressed and the endplates have been prepared, the anterior to posterior depth of the disc should be measured with a calibrated nerve hook or a small wire. Generally, the disc space is 10 to 15 mm wide, 12 to 17 mm deep, and 5 to 10 mm long. Regardless of the type used, the intervertebral graft should be several millimeters less than the actual depth to allow recession and avoid spinal cord compression.
The endplates should be punctured down to bleeding bone to facilitate bony union; various ways exist to accomplish this. Some surgeons prefer to use a bur to remove the endplates partially, whereas others use a small, angled curet to create holes in the adjacent sides. Regardless of the method, excessive endplate removal must be avoided. Endplate collapse and graft subsidence can occur when the structural integrity is weakened too much. When using tricortical iliac crest or patella, the graft should be shaped in a lordotic fashion with the anterior height being 1 to 2 mm taller than the posterior height (Fig. 40–13). This shape helps to avoid posterior retropulsion of the graft and subsequent cord compression and restores lordosis. The graft should be placed with the disc space under distraction to facilitate insertion. Longitudinal traction by the anesthesiologist is as effective as various intervertebral distractors. A tamp and mallet are used to position the graft (Fig. 40–14).
If an anterior cervical plate is not a planned part of the procedure, the bone graft is usually countersunk 1 to 2 mm in relation to the anterior margin of the adjacent vertebral bodies to decrease the chance of graft extrusion and esophageal compression (Fig. 40–15). When adding a plate, only minimal graft countersinking is necessary. A final lateral intraoperative radiograph is strongly suggested. Correct graft position can be verified along with proper plate position. The authors typically place a drain just anterior to the vertebral body. The fascia overlying the platysma is closed with simple absorbable sutures, and the skin is closed with a running absorbable subcuticular suture.
Consensus on postoperative management of patients after ACDF is lacking. Initially, most surgeons required patients to wear a rigid cervical collar for 6 to 8 weeks. Anterior instrumentation has eliminated many graft-related complications. Some surgeons have abandoned or significantly limited their use of postoperative immobilization because of the safety and effectiveness of current instrumentation. More recent studies have shown that a postoperative cervical brace does not improve the fusion rate or the clinical outcomes of patients undergoing single-level anterior cervical fusion with or without plating.21,22
When adequate internal fixation is obtained, a soft collar can be used for a short time in the initial postoperative period. Modern perioperative care and techniques allow patient mobilization on the day of surgery and discharge on postoperative day 1. Some surgeons have shown similar clinical outcomes and safety when performing ACDF in the outpatient setting.23–25 At 1 to 2 weeks, follow-up anteroposterior and lateral radiographs are taken. Lateral radiographs show a sufficient incorporation of the bone graft such that patients are allowed to begin range of motion exercises and strengthening exercises by 6 weeks. Within 3 months of the surgical procedure, most patients can expect return to full activity.
Anterior Cervical Plating
Anterior cervical plating was first introduced in the 1960s, when Bohler25a modified lower extremity hardware. Initially, he used the plates in trauma cases to restore stability. Exponential advancements have been made in the manufacture of anterior cervical instrumentation, biocompatibility, and ease of implantation. Currently, the use of an anterior cervical plate has been advocated to increase postoperative stability, decrease the incidence of graft extrusion, decrease the incidence of graft collapse, improve fusion rate, and obviate the need for postoperative halo brace placement after extensive multilevel corpectomies.
Biomechanical testing of bicortical anterior cervical instrumentation after single-level discectomy has shown a marked reduction in motion compared with the intact specimen.26 Additional testing by Grubb and colleagues27 showed that the Cervical Spine Locking Plate System (Synthes, Paoli, PA) using unicortical screws is biomechanically equivalent and in some cases more stable than the bicortical screw Caspar system. These results were noted after fixation of an experimental severe compression flexion injury in the cervical spine. The study by Spivak and colleagues28 showed that locking screws significantly increase rigidity of the hardware systems initially and after cyclic loading. Based on a combination of biomechanical and clinical studies at this time, most surgeons recommend the use of unicortical plating systems.
The clinical effectiveness of anterior cervical plate fixation must be judged not only by the successful fusion rate, but also by an improved clinical outcome. Over the past decade, several investigators have reported prospective and retrospective studies, looking at the two groups of patients fused with and without anterior cervical plates.29 Connolly and colleagues29 reported on 43 patients treated for cervical spondylosis with ACDF using autogenous iliac crest bone graft. The authors noted that there was no difference in the overall fusion rate or clinical results between these two groups of patients (plate vs. no plate).
Samartzis and colleagues30 reviewed 69 consecutive patients who had undergone single-level ACDF with and without rigid instrumentation. The nonplated group consisted of 38 patients, and the plated group consisted of 31 patients. All patients had tricortical iliac crest autograft as the interbody. The plated group wore hard collars for 3 to 4 weeks, and the nonplated group wore hard collars for 6 to 8 weeks. Fusion rates of 100% and 90% were achieved in the nonplated and plated groups. These rates were not significantly different. Mean blood loss was significantly more in the plated group. Clinical success was assessed using Odom criteria at an average of 21 months. Excellent and good outcomes was reported in 91.3% and 92.1% in the nonplated and plated groups. Three nonfused patients reported good clinical outcome. Of patients, 24 were smokers, and 23 had work-related injuries. Smokers achieved a fusion rate of 95.8%. Clinical outcome and fusion rate were similar between the groups in regard to smoking status and work-related injuries.
Current systems have incorporated the use of dynamic locking. Theoretically, these systems decrease stress shielding and allow load sharing to occur between the graft and the anterior column. The decrease in graft loading reduces the risk of plate or screw failure by recreating the natural load sharing of the anterior column.31–33 Some designs involve “dynamic screws,” and others involve “dynamic plates.”
Several more recent studies have shown that no significant clinical differences exist between static and dynamic plates.34–36 Pitzen and colleagues34 performed a prospective, controlled, randomized, multicenter study of 132 patients undergoing an anterior cervical discectomy and autograft fusion with either a dynamic plate or a rigid plate. They monitored patients for implant complications, segmental mobility, fusion, loss of lordosis, and clinical outcomes (visual analog scale [VAS] and Oswestry Disability Index [ODI]). No implant complications were seen in the dynamic group, whereas four implant complications occurred in the static group. There was no difference in fusion rate or clinical outcomes at 2 years. The loss of segmental lordosis was significantly greater in the dynamic group. By the criteria of Pitzen and colleagues,34 the dynamic group achieved fusion at a faster rate. These authors recommended using dynamic plates because of fewer implant-related revisions and a faster time to fusion.
Nunley and colleagues35 conducted a prospective, randomized study of static versus dynamic plates in single-level and multilevel ACDF. All patients were treated for radiculopathy. Multilevel procedures were performed in 38 of the 66 patients. When considering all study subjects, clinical outcome was equivalent between the dynamic and static groups. A clinically significant reduction in VAS and neck disability index (NDI) occurred in 73% of patients. A solid fusion was observed in 85% of patients. Clinical success predicted radiographic fusion, but fusion did not predict VAS and NDI reduction. Dynamic plates showed significantly better clinical outcomes in multilevel fusions than static plates. Nunley and colleagues35 concluded that plate design has no effect on clinical outcome in single-level constructs. Patients undergoing multilevel fusions experience better clinical outcomes with dynamic plating, however. The loss of lordosis may be related more to graft settling than to plate design, but this has not compromised outcomes.37
McLaughlin and colleagues38 detailed a cost analysis of 64 patients who underwent two-level ACDF for cervical radiculopathy. This study compared the cost of rigid internal fixation for patients with two-level ACDF with the cost in a similar population of patients who had an identical procedure without instrumentation. No difference was noted in the clinical outcome between the two groups, with both groups obtaining a 92% excellent or good result using Odom criteria. The investigators noted that patients who underwent rigid plating returned to light activities, driving, and unrestricted work sooner than noninstrumented patients. Although the overall cost was increased by approximately $3500 for the patients in the instrumentation group, the investigators believed that the increased cost was justified, owing to the significant clinical advantage to the patients and insurance disability providers, secondary to earlier mobilization and return to work.
Caspar and colleagues39 and Geisler and colleagues40 described one of the largest studies examining the results of plate fixation after ACDF. These two articles, describing the same group of patients, included 365 patients who underwent a one-level or two-level cervical arthrodesis performed with and without anterior cervical plate stabilization. The intended goal of this study was to determine the incidence of a second surgical intervention and whether the use of an anterior cervical plate decreased the need for subsequent surgical procedures. Although patients in these studies were not randomly assigned, and there was a mix of autograft and allograft procedures and multilevel fusions, the investigators reported that in the entire group 22 patients required repeat surgical intervention, with 20 of these patients initially in the noninstrumented group.
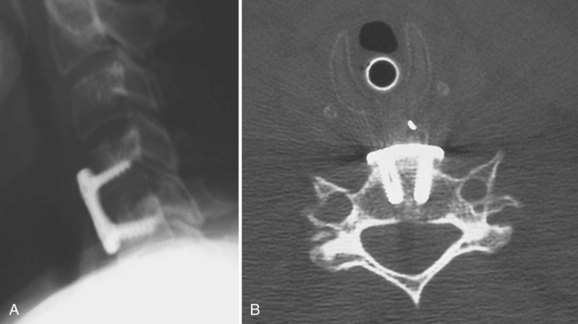
The addition of an anterior cervical plate using unicortical screw fixation after ACDF rarely leads to additional intraoperative complications. Plate fixation usually follows graft placement with care being taken to countersink the graft only minimally below the adjacent endplates. Typically, the exposure required for an ACDF is sufficient for placement of the cervical plate. Anterior osteophytes at the superior and inferior endplates need to be removed to provide a flat surface for plate placement. Placement of the cervical plate on top of anterior osteophytes may lead to prominence of the hardware with subsequent difficulty with postoperative swallowing. Unicortical screw placement can usually proceed without the use of fluoroscopy, with screw placement directed medially and away from the involved disc space (Fig. 40–16). Care should be taken to select a plate of the appropriate size so that the adjacent disc spaces are not violated by the screw (Fig. 40–17).
The authors recommend placing the plate at least 5 mm away from the adjacent disc space to decrease the risk of ossification (Fig. 40–18).41 Proper unicortical screw placement minimizes the risk of spinal cord injury. Intraoperative vertebral artery injury is extremely rare and occurs only with extreme screw misplacement. If sufficient surgical exposure has been obtained for the discectomy portion of the procedure, placement of the anterior cervical plate usually adds only 15 to 20 minutes to the entire operative time.
The use of an anterior cervical plate after a one-level ACDF is now common. Although the successful fusion rate may be higher with the use of instrumentation, clinical outcome has not yet been shown to be significantly improved with the addition of this hardware. As the complexity of cervical reconstructive surgical procedures increases, the use of cervical plates seems to be justified, owing to the higher fusion rate with instrumentation at two-level and three-level disease.42–44 Wang and colleagues44 compared the clinical and radiographic outcomes of 60 patients after two level ACDF with and without plating. The pseudarthrosis rate was significantly different at 25% for the nonplated group and 0% in the plated group. Age, gender, levels of surgery, tobacco use, and prior surgery had no correlation with fusion. A significant amount of segmental kyphosis and graft collapse was noted in the nonplated group. The complication rates were similar, however. Regardless of instrumentation, 87% of fused patients showed good to excellent results. Of patients with a pseudarthrosis, 58% reported fair results, and only 28% reported good to excellent results.
Results
In 1962, Robinson and colleagues45 were the first to publish the results of a large series of patients treated with ACDF. The series consisted of 55 patients who underwent anterior discectomy and fusion at 107 disc spaces. These authors hypothesized that preservation of the subchondral bone of the endplates was necessary to provide a bearing surface for the graft. (Emery and colleagues46 challenged this hypothesis and noted a pseudarthrosis rate of only 4.4% when partially removing the subchondral bone of the endplate with a bur.) Robinson and colleagues45 reported 46% excellent, 27% good, 22% fair, and 6% poor results. Patients with single-level fusions showed better outcomes than patients with multilevel fusions. More than 94% of patients with single-level fusions had an excellent or good result compared with 50% of patients undergoing three or more levels of fusion. Four patients continued to report symptoms that were believed to be associated with nonunion, and there were nine total pseudarthroses. Most importantly, only one additional surgery was necessary in a patient with a solid fusion.
Yue and colleagues47 performed a long-term retrospective review of 71 patients after ACDF using allograft and plating. The average follow-up was 7 years (range 5 to 11 years). Single-level and multilevel patients were included. More than 90% were primary procedures. Symptoms included neck pain (94%), radiculopathy (91%), and weakness or gait problems (79%). Greater than 95% of patients reported continued significant improvement in neck pain and radiculopathy at final follow-up. Clinical improvement was not related to fusion, smoking, number of levels, collapse, or subsidence. The pseudarthrosis rate was 9%. Smoking had no effect on fusion rate. Previous surgery increased the risk of pseudarthrosis, however. No patient underwent revision for pseudarthrosis. Adjacent segment degeneration occurred in 73% of patients. Only 16% required additional surgery, with half exhibiting degenerative changes before the index procedure. Global lordosis was increased with surgery and maintained at final follow-up.
Despite a large volume of literature reporting high fusion rates with single-level ACDF, multilevel procedures present a challenge for the spine surgeon. Fusion and clinical outcomes decrease with increasing levels of surgery. Emery and colleagues48 retrospectively reviewed 16 patients who underwent a modified Robinson ACDF at three operative levels. All patients had tricortical autogenous iliac graft placed at three levels with no internal fixation. Only 9 (56%) of the 16 patients went on to achieve solid arthrodesis at all levels. Of the seven patients with pseudarthrosis, two had severe pain requiring revision, two had moderate pain, and three had no pain. Of the nine patients with a solid fusion, three continued to have mild pain, and six had no pain. Emery and colleagues48 concluded that a three-level modified Robinson ACDF results in an unacceptably high rate of pseudarthrosis and recommended that these patients undergo additional or alternative measures to achieve a successful arthrodesis consistently.
Wright and Eisenstein 49 compared fusion rates in single-level and two-level anterior discectomy and fusion without plating. In the 54 single-level procedures, pseudarthrosis occurred in 11% compared with 28% in the two-level group. Postoperative neck pain was less likely in patients with a solid fusion.
In an effort to improve the successful fusion and patient outcome after multilevel ACDF, Bolesta and colleagues50 reported a prospective study of 15 patients who underwent a modified Smith-Robinson ACDF at three and four operative levels, stabilized with a unicortical anterior plate. All patients had autogenous iliac crest graft placed and had a minimum of 24 months of follow-up. Despite the addition of the anterior cervical plate, solid arthrodesis was achieved at all levels in only 7 (47%) of the 15 patients after the initial surgical procedure. Of the eight patients with pseudarthrosis, three had sufficient pain to necessitate revision surgery, one had pain without further surgery, and four had no pain. Of the seven patients with solid fusion, three had persistent pain, and four had none. Bolesta and colleagues50 also concluded that a three-level and four-level modified Robinson ACDF results in an unacceptably high rate of pseudarthrosis. These authors believed that the addition of an anterior cervical plate alone does not improve the arthrodesis rate.
Wang and colleagues44,51 also studied multilevel procedures. They reviewed 60 patients after two-level ACDF.44 Half of the patients received plate fixation, and half did not. The difference in fusion was significant. No pseudarthrosis occurred in the plated group, whereas seven pseudarthroses occurred in the nonplated group. The complication rate was similar between the groups. Pseudarthrosis was not related to demographics or smoking status. During this same period, Wang and colleagues51 reviewed three-level procedures as well. Of 59 patients followed for 3.2 years, pseudarthrosis occurred in 18% of plated patients versus 37% of nonplated patients. This difference was not significant, however, owing to the larger number of plated patients. Fused patients showed better clinical outcomes regardless of plating. Cauthen and colleagues52 reviewed 146 multilevel noninstrumented cases at an average of 5 years and found a 75% fusion rate. Fusion rates are lower but still acceptable for multilevel procedures when plating is employed.
The clinical effect of pseudarthrosis on patient outcome is controversial. Patients who have a successful fusion theoretically have improved cervical alignment, continued foraminal distraction, and prevention of collapse into kyphosis. The pseudarthrosis rate after single-level surgery ranges from 0% to 20%53,54 with the rates approaching 50% for patients undergoing multilevel surgery.55,56
Phillips and colleagues57 attempted to determine the natural history, risk factors, and treatment outcomes in a large population with documented pseudarthrosis after ACDF. They reported 48 patients with radiographically proven pseudarthrosis at an average of 5 years after the initial surgical procedure. Of the patients, 67% were symptomatic at latest follow-up or at the time of a required second surgical intervention. Several patients had a symptom-free period of up to 2 years after ACDF before redeveloping cervical symptoms after a minor traumatic episode. Analysis of the results revealed that patients who had surgery at a younger age had an increased likelihood of pseudarthrosis becoming symptomatic. Of 16 patients, 14 had successful repair of pseudarthrosis via an anterior approach, whereas 6 patients underwent posterior cervical fusion, with all going on to successful fusion. In patients in whom fusion was achieved with a second cervical operation, the results were excellent in 19 and good in 1. Phillips and colleagues57 concluded that the surgical repair of pseudarthrosis with either an anterior or a posterior approach results in a higher likelihood of a successful clinical outcome.
The etiology of pseudarthrosis after anterior cervical surgery is multifactorial. Various authors have cited multilevel fusions, tobacco use, and revision cervical surgery as relative risk factors for the development of pseudarthrosis. Multiple studies of lumbar spine fusions have shown that cigarette smoking creates a biologically challenging environment for a successful fusion. Hilibrand and colleagues58 compared the long-term radiographic and clinical results of smokers and nonsmokers who had undergone arthrodesis with autogenous bone graft after multilevel anterior cervical decompression for the treatment of cervical radiculopathy or myelopathy or both. After either corpectomy or multiple discectomies and interbody grafting, 190 patients were followed clinically and radiographically for at least 2 years. A subset of 40 of these patients were smokers who had undergone a cervical fusion, and only 20 had a solid fusion at all levels, whereas 69 of the 91 nonsmokers had a solid fusion at all levels (P < .02). This difference was more significant among patients who had a two-level interbody grafting procedure. Hilibrand and colleagues58 concluded that smoking had a significant negative impact on healing and clinical recovery after multilevel ACDF with autogenous interbody graft for radiculopathy or myelopathy.
A prospective study by Luszczyk and colleagues59 analyzed the fusion rates of smoking and nonsmoking patients who underwent single-level ACDF with allograft and a rigid locked anterior cervical plate. The study consisted of patients from the control groups of four separate studies evaluating cervical disc arthroplasty; 156 smokers and 417 nonsmokers were reviewed. Follow-up was at least 24 months in all patients. When analyzing all the patients, the fusion rate was 91.4%. The fusion rates were similar for the two subgroups at 91% and 91.6% for smokers and nonsmokers. These authors concluded that smoking status had no effect on the fusion status in single-level ACDF when using allograft and a locked plate.
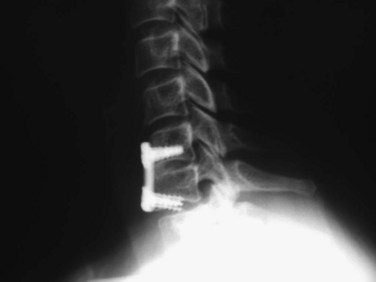
Long-term follow-up studies of patients treated with ACDF have shown that approximately 10% of patients undergo reoperation at an adjacent level, owing to progressive spondylolysis or a new disc herniation (Fig. 40–19).60–63 This adjacent segment disease has been theorized to be secondary to the increased biomechanical stresses on an unfused level adjacent to a solid fusion. Whether the biomechanical forces alone are sufficient to cause new disease or this is just a progression of the natural history of cervical spondylosis in a patient with significant degenerative changes remains to be determined. Treatment of this adjacent level disease is theoretically more difficult, however, because achievement of a solid fusion in these patients is hindered by the long lever arm of the prior fusion acting across the new operative level.
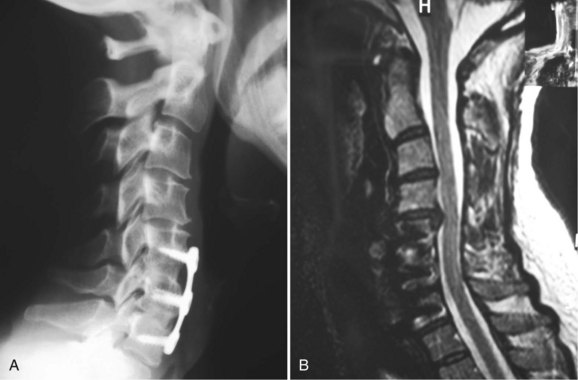
FIGURE 40–19 A and B, Lateral radiographs and sagittal MRI showing disc herniation adjacent to two-level fusion.
A retrospective review of all patients surgically treated for adjacent segment disease of the cervical spine over a 20-year period was performed by Hilibrand and colleagues63 to determine the clinical and radiographic outcome of discectomy with interbody grafting compared with corpectomy with strut grafting in the treatment of adjacent segment disease of the cervical spine. The investigators identified 38 patients who underwent surgical treatment for multilevel adjacent segment disease by either of these two procedures. The rate of arthrodesis in 24 patients treated with discectomy was only 63%, whereas patients treated with corpectomy and strut grafting had a fusion rate of 100%. This fusion rate for discectomy and interbody fusion is much lower than that reported in the literature for patients undergoing a primary cervical procedure. Hilibrand and colleagues63 concluded that the use of subtotal corpectomy (in patients who have had previous cervical surgery) has a higher fusion rate with strut grafting techniques. They believed that the strut of bone passing through a trough in the intermediate vertebrae across multiple motion segments may provide greater stability than multiple smaller pieces of tricortical iliac crest at each disc space. Secondarily, strut grafting requires bony union across only two surfaces compared with four or six surfaces for either two-level or three-level discectomy and fusion procedures.
Goldberg and colleagues64 assessed the effect of workers’ compensation claims on functional outcomes after ACDF. They retrospectively reviewed 80 patients; 30 had claims, and 50 did not have claims. Follow-up averaged 4 years. The functional outcomes were similar with 83% and 90% good or excellent results in patients with and without claims. Patients without claims returned to work on average 8 weeks earlier. Of patients, 97% with claims and 98% without claims returned to work. These authors concluded that workers’ compensation claims have no effect on long-term outcomes after ACDF.
Gore and Sepic65 performed one of the longest follow-up studies of ACDF to date. Radiographs and functional recovery were assessed at an average of 21 years after the index procedure. Initial improvement was reported by 96% of patients. At final follow-up, 64% of these patients reported no pain. Pain recurred in the remaining patients at an average of 7 years. Only a small percentage of these patients required additional surgery.
Klein and colleagues66 reviewed the outcomes of 28 patients with cervical radiculopathy treated with one-level or two-level ACDF. This prospective assessment used a health status questionnaire that is almost identical to the Medical Outcome Studies SF-36. The survey was self-administered and evaluated at an average follow-up interval of 21.8 months. The authors noted statistically significant improvement in postoperative scores for bodily pain, physical function, role function, and social function. Based on the results of the health status questionnaire, it was believed that ACDF performed on appropriate selected patients is a highly reliable surgical procedure for the management of cervical radiculopathy.
Complications
The complication rate of single-level ACDF with or without instrumentation is predictably low. Complications can occur at either the graft site during autograft harvest or in the neck. The initial report of Robinson and colleagues45 described complications in only 8 of the 56 patients. Four patients had temporary unilateral paralysis of the vocal cords, two patients had marked temporary dysphagia, and two patients had a transient Horner syndrome. A large series reported by DePalma and colleagues67 noted that the most common complication occurred at the iliac crest graft donor site. These researchers found 9% of patients developed a hematoma and 36% of patients continued to have persistent donor site pain 1 year after the surgical procedure. Silber and colleagues68 reviewed the long-term bone graft donor site morbidity. Average follow-up was 4 years. Although 92.5% were satisfied with the look of their donor site, 26% reported chronic pain at the site with half requiring pain medication. Donor site pain can be minimized by limiting the surgical exposure, with careful dissection of inner and outer tables of the iliac crest.69 The incision should be at least 2 fingerbreadths lateral to the anterior superior iliac spine to avoid injury to the lateral femoral cutaneous nerve, which can lead to postoperative numbness. The incidence of iliac crest donor site pain can be eliminated through the use of allograft.
Fountas and colleagues70 performed a large retrospective study of 1015 patients who had undergone primary ACDF. Final follow-up was at 1 year. About 10% of patients reported noticeable pain or difficulty swallowing initially; 95% reported resolution of symptoms within 7 days of surgery. The remaining patients reported significant improvement and resolutions by 4 weeks. Three-level procedures were significantly more likely to cause dysphagia. Dysphagia was similar between patients with and without plates. Surgical evacuation of a soft tissue hematoma was required in 24 patients (2.4%). The number of levels operated on was not related to the development of a hematoma. The addition of a plate also had no significant effect on the development of a hematoma.
Flynn71 compiled the results of 704 neurosurgeons performing 36,657 anterior cervical interbody fusions. The most common neurologic complication was recurrent laryngeal nerve palsy, which occurred in 52 cases. This condition compromised nearly 20% of all neurologic injuries. There has been some debate as to whether the side of surgical approach affects the development of recurrent laryngeal nerve injury. A large retrospective study by Kilburg and colleagues72 reviewed 415 patients undergoing one-level or two-level ACDF. Injury was diagnosed by observation of the vocal cords. The time from surgery to diagnosis of injury was similar between left-sided and right-sided approaches. Statistical analysis revealed no significant difference between left-sided and right sided approaches. Kilburg and colleagues72 concluded that the side of approach has no effect on the incidence of injury.
The most devastating complication that follows any type of spine surgery is spinal cord injury. Flynn71 reported 100 cases of significant permanent myelopathy or myeloradiculopathy in his large series. The deficit occurred immediately postoperatively in 75% of the 100 patients, whereas 25% of the patients developed a neurologic deficit in the postoperative recovery period. Analysis of his data led Flynn71 to conclude that regardless of the etiology of the neurologic deficit, reoperation had little effect on the ultimate status of the patient’s recovery. In addition, most surgeons were unable to determine the etiology of the neurologic deterioration.
Postoperative transient sore throat or dysphagia commonly occurs after anterior cervical surgery.73,74 Reported rates range from 28% to 57%.75–77 The exact etiology is controversial with the multiple possible mechanisms including postoperative soft tissue swelling, traction or pressure injury to the laryngeal nerves, hematoma formation, or prominent hardware. Sore throat or dysphagia is usually a transient problem that improves with time. Most surgeons report significant dysphagia persisting in less than 2% of patients at 2 years. Many practitioners believe this complication is minor, but many patients complain of significant difficulty swallowing for several weeks, with frequent need to chew their food fully before swallowing. Dull retractors and avoiding excessive retraction decrease soft tissue damage and pressure on the nerve. Yue and colleagues78 published a mid-term report of dysphonia and dysphagia. They reviewed 74 patients at an average of 7 years after index ACDF. At final follow-up, 35% of the patients reported persistent dysphagia. These authors concluded that dysphonia and dysphagia may exist in many patients at mid-term to long-term follow-up.
Esophageal injuries are extremely rare, and morbidity and mortality are significantly increased when they occur. Newhouse and colleagues79 reviewed 10,000 anterior cervical cases and reported an incidence of 0.25%. Sharp and motorized instruments were the cause of 30% of the injuries. These injuries were usually noted during the index procedure. If the esophageal perforation is noted at the time of the initial surgery, all attempts should be made for an acute repair. Graft extrusion, screw pullout or loosening, and plate migration were the most common causes of late perforations. The underlying reason for the perforation must be corrected first. These perforations are technically much more difficult to repair and often require prolonged use of nasogastric suction and parenteral hyperalimentation.
Autograft Versus Allograft
Brown and colleagues80 reported on one of the earliest studies comparing allograft with autograft bone for anterior cervical fusions. They noted there was no difference in fusion rates between the two types of graft, but there was a significant difference in graft collapse rate, with 28% of the patients receiving allograft showing radiographic collapse, whereas only 16% of patients receiving autograft had evidence of collapse on radiographs. Brown and colleagues80 found this trend to be true only for multilevel fusions, with single-level fusions comparable in their radiologic outcome.
Fernyhough and colleagues81 performed a retrospective review of 26 patients who had either autogenous or allograft fibular strut graft placed after decompression for multilevel cervical spondylosis. They reported that the combined nonunion rate for both groups ranged from 21% for two-level fusion to 50% for four-level fusion. Overall, the allograft group had a statistically significant higher rate of nonunion (41%) than the autograft group (27%). There was noted to be no correlation, however, between the clinical results and the rate of union in this study.
A retrospective study by Samartzis and colleagues82 compared 66 consecutive patients undergoing single-level ACDF with rigid plate fixation and allograft or autograft. Of the patients, 45% had work-related injuries, and 30% were smokers. The overall fusion rate was 95%. The fusion rates were similar between the two groups: 100% and 90% fusion were noted in the allograft and autograft groups. A good or excellent clinical outcome was reported by 90% of patients. The outcomes were similar regardless of the presence of a fusion and smoking status. These authors concluded that the use of allograft and a rigid plate yields a high fusion rate and good clinical outcomes.
Zdeblick and Ducker83 reviewed 88 consecutive patients who underwent a Smith-Robinson anterior cervical fusion. In 60 of these patients, an autogenous iliac crest graft was placed, and freeze-dried iliac crest grafts were inserted in the other 27 patients. The delayed union rate and nonunion rate were noted to be significantly higher in the allograft group compared with the autograft group. If only one-level fusions were examined, the nonunion rates were similar at 1 year. The union rate was noted to be dramatically lower with allograft (38% vs. 83%) only when examining two-level fusions. Additionally, freeze-dried allograft was noted to collapse en route to healing more frequently. The clinical results between the two groups were found to be similar. These authors also concluded that the use of freeze-dried iliac crest allograft in Smith-Robinson fusions is not recommended for multilevel procedures. When freeze-dried iliac crest graft is used in one-level fusions, radiographic collapse or lucency may persist despite clinical success.
Suchomel and colleagues84 performed a prospective study comparing allograft and autograft in single-level and two-level procedures. They followed 79 patients for at least 2 years. Similar fusion and graft collapse rates were noted regardless of graft type or number of levels fused. Smoking had no effect on fusion rate or collapse. No difference was noted between the subgroup of single-level allograft versus autograft. Likewise, no difference was noted for two-level procedures. The time to union was significantly longer in patients treated with allograft in single-level and two-level cases. Samartzis and colleagues85 compared allograft and autograft in two-level and three-level anterior cervical decompressions and fusions with plating. They followed 80 patients for an average of 16 months. Of patients, 45 were treated with autograft, and 35 were treated with allograft. These investigators reported a combined fusion rate of 97.5%. Nonunions occurred in the allograft group. Nearly 90% of patients reported a good or excellent outcome. Clinical outcome and fusion rate were similar regardless of graft type.
Bishop and colleagues86 evaluated the results of 132 patients after nonplated anterior cervical fusions who had at least a 1-year follow-up. Of the 83 patients who received autograft, 94% achieved a solid fusion. In the multilevel fusion group, the patients receiving autograft had an 87% successful fusion rate. Patients who received allograft had a lower fusion rate of 73% for single-level procedures and 53% for multilevel procedures.
Until more recently, the most common type of allograft used was tricortical iliac crest harvested from cadaveric iliac crest, similar in fashion to autogenous bone. In an effort to decrease the incidence of collapse rate, several surgeons have advocated the use of graft with superior structural properties, such as fibula shaft and patella.87
MacDonald and colleagues88 reported on 36 patients who had undergone an anterior cervical fusion using fibular shaft allograft. Although 15 of these patients also had placement of an anterior cervical plate, 96% of the patients at 2-year follow-up had a solid fusion. An even larger study by Martin and colleagues89 evaluated the use of freeze-dried fibular allograft. They reported on 269 patients who underwent either a single-level or a multilevel ACDF. A solid radiographic fusion was eventually achieved in 242 patients for a union rate of 90% at 2 years’ follow-up. A subset of these patients, who underwent two-level fusion, achieved a fusion rate of only 72%.
Cauthen and colleagues52 performed a large study comparing interbody graft types in noninstrumented fusions. They reviewed 348 patients with an average length of follow-up of more than 5 years. A literature review yielded historical data for comparison. Of the patients, 70% received allograft. Results revealed an overall fusion rate of 83%; this includes 75% fusion in multilevel cases and 88% in single-level cases. At an average of 5 years, 78% of patients were satisfied, and 83% returned to work. Cauthen and colleagues52 reported no correlation between graft type and fusion status. Outcome improved, however, with a solid fusion, fewer levels involved, higher education, and no secondary financial motives. These results show similar outcomes between allograft and autograft even at extended follow-up.
Polyetheretherketone
Polyetheretherketone (PEEK) cages have been advocated more recently as another alternative to autograft. Their radiolucency allows better assessment of the fusion than do metal devices (Fig. 40–20). Studies have shown PEEK to be more rigid than autograft90; this improves overall stiffness in compression and rotation.91 PEEK eliminates the concerns of disease transmission and poor donor quality graft. The morbidity of allograft and autograft may be avoided.
Several studies have shown PEEK to have fusion rates near 100% at 1 year with clinical outcomes of good to excellent in 95% or more of subjects.92–94 There were few complications. Kulkarni and colleagues95 retrospectively reviewed their experience with stand-alone PEEK cages in a single-level anterior discectomy and fusion. They reported a fusion rate of 93% at 6 months and 100% at 2 years. The immediate and final follow-up disc height were significantly greater than preoperative values. Significant subsidence occurred, however, between the immediate and final follow-up. The immediate postoperative segmental lordosis was greater than preoperatively but was not maintained at final follow-up. No cage-related complications were noted. Kulkarni and colleagues95 conceded that the use of anterior plating may have helped to maintain lordosis better and decrease the need for postoperative bracing.
Celik and colleagues96 performed a prospectively controlled randomized study of PEEK and iliac crest bone graft to assess changes in foraminal height and fusion rate. No anterior plating was employed. Postoperatively, both groups showed significant improvement in VAS and Japanese Orthopedic Association (JOA) scores. The groups had similar clinical outcomes, lordosis, and fusion rates at final follow-up of 18 months. The iliac crest group showed a significant increase in interspace and foraminal height initially. Interspace height was significantly decreased, however, and the foraminal height increase was not maintained at final follow-up. Celik and colleagues96 attributed these findings to graft subsidence, resorption, and compression. The PEEK group showed significant increases in interspace and foraminal height that were maintained at 18 months. These authors concluded that PEEK cages maintain foraminal decompression and sagittal alignment better than nonplated autograft.
PEEK may offer even more advantages when considering multilevel procedures. When using iliac crest, kyphosis has been noted even when a solid fusion develops.97,98 High rates of graft collapses have also been noted in multilevel fusions.98–100
A small prospective, nonrandomized study by Demircan and colleagues101 studied the safety and efficacy of PEEK used in multilevel fusions without screws, plates, or autogenous iliac crest. They packed PEEK cages with demineralized bone matrix. At final follow-up, all the patients showed a significant improvement in JOA score. Preoperative lordosis was maintained at 18-month follow-up. Fusion rate was 90%. The nonfusions occurred in three-level and four-level fusions. No one required reoperation at final follow-up.
Cho and colleagues102 performed a much larger prospective, randomized study of 180 patients. Subjects were randomly assigned to ACDF with PEEK cage, iliac crest autograft with plating, or iliac crest autograft without plating. All three groups were similar in demographics. The PEEK cages were packed with iliac crest bone marrow that was obtained with a hollow driver through a small 1-cm incision. The average follow-up was 2.5 years. There were no graft complications in the PEEK group; the plated group had a complication rate of 4%. Graft collapse, nonunion, and graft dislodgment resulted in a complication rate of 50% in the nonplated group. Fusion rates at 1 year of 100% and 98% for the PEEK and plated groups were significantly better than the rate of 87% for the nonplated group. The time to fusion was significantly faster in the PEEK and plated groups as well. Clinical outcome was similar between the PEEK and plated groups. The PEEK group was statistically better than the nonplated group. Cervical lordosis was increased in the PEEK and plated groups compared with a relative increase in kyphosis in the nonplated group. Although PEEK and plating provide good clinical and radiographic outcomes with few complications, PEEK may be advantageous because it has the fewest complications.
Bone Morphogenetic Proteins
Bone morphogenetic protein (BMP) has been available for use in the cervical spine for a few years. This is an off-label use, but nonetheless BMP has been used extensively. Buttermann103 directly compared allograft and BMP versus iliac crest bone graft in a nonrandomized study of 66 consecutive patients who had primary one-level, two-level, or three-level ACDF. He followed them prospectively over 2 to 3 years. No difference was seen in VAS, ODI index, pain medication use, opinion of treatment success, and neurologic recovery. Half of the BMP group had neck swelling and dysphagia versus 14% in the autograft group. BMP was more costly, and operative time was only modestly reduced. Buttermann103 concluded that BMP yields clinical and radiographic outcomes that are equivalent to autograft but at an increased cost with significant safety concerns.
Several other reports of complications have arisen in the literature.104–106 More than 20% of patients in these studies had significant neck swelling. This was significantly more than in patients who were not treated with BMP. One patient had to be reintubated more than 5 days after his index procedure.104 The swelling has been attributed to pharyngeal edema owing to inflammation and not hematoma formation. Vaidya and colleagues107 reviewed their complications with BMP in the anterior cervical spine. They compared 22 patients treated with BMP and PEEK cages with patients treated with allograft and demineralized bone matrix (DBM). Every patient treated with BMP showed endplate resorption during the first 6 weeks. Dysphagia was more severe and frequent in the BMP group. Anterior neck swelling was significantly greater in the BMP group. Clinical outcome was similar in both groups at 24 months. Cost analysis showed BMP and PEEK to be three times more expensive than allograft and DBM.
Anterior Cervical Foraminotomy
In an effort to eliminate the need for a fusion at the time of decompression, multiple authors108–110 have recommended anterior cervical microforaminotomy for nerve root decompression. Although there are multiple variations of this procedure, the exposure involves an anterolateral approach to the cervical spine with incisional splitting of the longus colli muscle medial to the anterior tubercle of the transverse process. This is followed by removal of the uncovertebral joint and subsequent removal of the pathologic elements compressing the nerve root. Several small studies have reported resolution of radicular symptoms with this procedure.111–113 Most of these studies have only short-term follow-up. Although one author109 reported excellent or good outcomes in 98% of 104 patients with cervical discogenic radiculopathy, there has not yet been widespread acceptance of this procedure. Potential complications more common to this procedure include injury to the sympathetic plexus and vertebral artery injury. This procedure typically can be performed unilaterally only. Further long-term studies by additional authors are needed before this procedure can be widely recommended. If this procedure is performed successfully, it obviates the need for fusion at the time of decompression with preservation of motion at the surgical level.
Anterior Discectomy Without Fusion
Anterior cervical discectomy without fusion developed as an alternative technique to cervical fusion, based on the premise that if successful results of anterior fusions occur with pseudarthrosis, discectomy can be performed without fusion. Several articles have advocated anterior cervical discectomy without fusion, reporting success rates similar to fusion.114–116 Murphy and Gadd114 reported 26 patients who underwent a one-level or two-level anterior cervical discectomy for radiculopathy secondary to a herniated cervical disc. Good results (neurologic deficit improved, pain alleviated, and patient able to return to normal activities) were reported in 24 of 26 patients. The two patients with poor results required reoperation within 2 weeks of the initial procedure. Murphy and Gadd114 noted that 72% of the patients developed a spontaneous fusion at the discectomy level, and all 20 patients developed some degree of postoperative kyphosis.
Murphy and Gadd114 and others115 neglected to mention specifically the postoperative incidence of neck pain. Several authors117–119 noted transient postoperative severe neck pain after cervical discectomy without fusion. This procedure defeats the principle of successful anterior cervical surgery, which is based on the premise of neuroforaminal distraction and reduction of buckling of the ligamentum flavum. In addition, by its very nature, this surgery is designed to create a pseudarthrosis that may lead to a less satisfactory result. Theoretically, the results of this surgery may be more successful for patients with soft disc herniation than for patients with spondylolysis, but there is an unacceptably high rate of persistent postoperative neck pain. Most series to date have a limited clinical follow-up and poor radiographic correlation to determine the development and significance of postoperative instability or kyphotic angulation.
Studies of anterior cervical discectomy with and without fusion120,121 have shown that the procedure without fusion decreases hospital cost and hospital stay and surgical morbidity. These early economic benefits must be weighed against long-term outcome, however.
Cervical Disc Arthroplasty
Most recently, the FDA has approved the use of cervical disc arthroplasty for single-level cervical radiculopathy. These devices have been studied in multiple prospective, randomized, multicenter clinical trials for noninferiority.122–124 Most of these studies have involved hundreds of patients randomly assigned to either disc arthroplasty or single-level ACDF (Fig. 40–21).
Generally, these devices have been studied for use in C3-7 in patients 18 to 60 years old without significant kyphosis, instability, inflammatory disease, significant disc height loss, or significant spondylosis. In a study of 463 patients, 242 received a cervical disc arthroplasty, and 221 underwent ACDF.122 Both groups showed improvement in clinical outcome that was maintained at 12 months and 24 months. Statistically significant reduction in NDI occurred in both groups. The arthroplasty group exhibited significantly greater reductions than the fusion group. Neck and arm pain were also significantly improved for each group at all time intervals. The study authors reported a greater improvement in NDI and overall success in the arthroplasty group. Adverse events including reoperation rates were similar between the groups. Arthroplasty patients on average returned to work 13 days earlier than fusion patients. When used in a workers’ compensation population, clinical outcomes were equivalent for patients treated with ACDF and patients undergoing disc arthroplasty.123 A trend was seen toward an earlier return to work for arthroplasty patients.
Cervical disc arthroplasty attempts to allow maintenance of cervical motion; it is thought that this decreases the abnormal adjacent level forces created by a fusion. This may lead to a decreased adjacent segment disease and lead to better long-term outcomes than fusion. The in vivo kinematics of two different cervical disc arthroplasty implants have been compared with controls.125 After analyzing the range of motion of the 51 implants to 200 healthy individuals’ discs, both implants reduced range of motion significantly. The range of motion between the implants was not significantly different. The center of rotation was in the normal range for both devices despite being influenced by the type of device. The study authors concluded that the two devices did not restore a normal range of motion.
These devices have been shown to maintain segmental mobility compared with fusion.122,124,126 Compared with intact specimens, disc arthroplasty can maintain motion coupling as well.127 The results of the Bryan disc (Medtronic, Memphis, TN) clinical trial, which was a prospective, randomized, and controlled study, were reviewed at 2 years.126 Motion analysis of the 242 Bryan artificial discs revealed no evidence of bridging bone across any implant, no implant migration, and no evidence of implant subsidence. The immediate postoperative range of motion of the disc replacement levels was maintained at final follow-up.
Sasso and Best128 performed a kinematic analysis of the adjacent segments in a subgroup of patients from this study. No difference in range of motion was shown at the level above and below either a fusion or arthroplasty. During flexion-extension, increased anterior and posterior translation at the level proximal to fusion levels was observed to be significant. Sasso and Best128 postulated that to achieve similar range of motion after fusion, increased translation is necessary at the adjacent segments. This increased translation may place excessive loads and forces across the adjacent disc.
Several other groups have studied segmental motion and intradiscal pressure at levels adjacent to a fusion or an arthroplasty.129–132 Cadaveric models showed that segmental mobility is increased at the levels adjacent to a fused level.130–132 Chang and colleagues129 evaluated the disc pressures and facet joint forces adjacent to a cervical arthroplasty and discectomy with fusion. They controlled their study by measuring intact specimens. After arthroplasty, the adjacent segment proximal and distal segments showed no significant change in disc pressure. The segment proximal to the ACDF group exhibited increased disc pressure of approximately 40%. Changes in facet joint force were minimal in all groups. Forces increased at the treated level and decreased at adjacent segments in the arthroplasty group, whereas the fusion group showed decreased forces at the treated level and increased forces at the adjacent segments. These authors believed that increased disc pressures may impair disc metabolism and contribute to disc degeneration. They concluded that although arthroplasty increases loading of the treated level facets, it maintains normal intradiscal pressures at adjacent segments. In a two-level model comparing disc arthroplasty with fusion, arthroplasty showed much lower disc pressures in adjacent segments than fusion.133 Fusion and arthroplasty alter cervical biomechanics. These alterations seem to be less pronounced with arthroplasty; however, more clinical studies and long-term study are warranted.
Complications have been reported with arthroplasty. Spontaneous fusion and heterotopic ossification causing restricted motion has been shown to occur in 10% of arthroplasty-treated levels.134 Anderson and colleagues135 compared the adverse events of a clinical trial of arthroplasty versus ACDF. Adverse medical events not related to surgery were slightly higher in the arthroplasty-treated patients: 35% versus 31% in the fusion group. Most of these adverse medical events were genitourinary and gastrointestinal. Dysphagia and superficial wound infections occurred in the arthroplasty group. Dysphagia was classified as a less severe complication. Anderson and colleagues135 postulated that the dysphagia may have been related to longer operative time in the arthroplasty group. When considering more severe adverse events, such as severe arm or neck symptoms, prolonged anesthesia, permanent neurologic injury, or death, significantly more occurred in the fusion group compared with the arthroplasty group. Reoperation was required in 5% of the arthroplasty group compared with 7% of the fusion group. Overall, the arthroplasty group required fewer reoperations, and complications were less severe compared with the fusion group.
Additional complications reported with arthroplasty include increased segmental kyphosis.136 Kim and colleagues137 reviewed 55 cervical disc replacements at 2 years. Global cervical lordosis was maintained in 86% of patients. Segmental lordosis was maintained in just 36% of the operated levels, however. The clinical importance of increased segmental kyphosis after arthroplasty is not well understood.
Artificial disc replacement combined with fusion may offer significantly better results than multilevel fusion. Phillips and colleagues138 reported on the use of disc replacement in patients with and without adjacent level fusion. Surgical time, blood loss, and clinical outcomes were similar between the groups. Both groups showed significant improvement in VAS and NDI. Two revisions were performed in each group. Shin and colleagues139 performed a prospective analysis of 40 patients. Half the patients underwent two-level ACDF, and the other half underwent hybrid construct of an ACDF and a disc arthroplasty. The hybrid group had significantly better NDI and decrease in neck pain compared with the multilevel ACDF group. Most importantly, Phillips and colleagues138 noted significantly increased range of motion in the segment inferior to the multilevel fusions. These studies suggest that arthroplasty adjacent to fusion offers similar short-term results to multilevel fusion, if not slightly improved results.
Several authors140–142 have studied multilevel cervical disc arthroplasty. A prospective, randomized study by Cheng and colleagues140 compared two-level ACDF with two-level arthroplasty. NDI and VAS were improved greatly in both groups; NDI improved more in the arthroplasty group. These authors concluded that two-level arthroplasty is safe and effective in treating two-level cervical disease. A large prospective, consecutive series by Pimenta and colleagues142 compared the outcomes of single-level versus multilevel arthroplasty. Single-level disc arthroplasty was performed in 71 patients, and two-level, three-level, and four-level arthroplasty was performed in 53, 12, and 4 patients. Although VAS reduction was similar, the 52% reduction in NDI for the multilevel group was significantly more compared with the 37% reduction in the single-level group. Complication rates including reoperation were similar with an implant retention rate of 94% at 3 years. Clinical success rates were similar, however: 93.9% for multilevel arthroplasties and 90.5% for single-level arthroplasties. These studies suggest that multilevel arthroplasty is as safe and effective as fusion for multilevel disease. Pseudarthrosis and complication rates increase with the number of levels fused; multilevel arthroplasty may decrease morbidity.143,144 Long-term clinical outcomes and rates of adjacent segment degeneration are still unknown.
Summary
Pearls
Pitfalls
Key Points
1 Smith G, Robinson R. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40:607-624.
2 Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: Long-term follow-up of one-hundred-and-twenty-two patients. J Bone Joint Surg Am. 1993;75:1298-1301.
3 McLaughlin MR, Purighalla V, Pizzi FJ. Cost advantages of two-level anterior cervical fusion with rigid internal fixation for radiculopathy and degenerative disease. Surg Neurol. 1997;48:560-565.
4 Malloy KM, Hilibrand AS. Autograft versus allograft in degenerative cervical disease. Clin Orthop Relat Res. 2002;394:27-38.
5 Flynn T. Neurologic complications of anterior cervical interbody fusion. Spine (Phila Pa 1976). 1982;7:536-539.
1 Dean CL, Gabriel JP, Cassinelli EH, et al. Degenerative spondylolisthesis of the cervical spine: Analysis of 58 patients treated with anterior cervical decompression and fusion. Spine J. 2009;9:439-446.
2 Brain L, Wilkinson M, editors. Cervical Spondylolisthesis and Other Disorders of the Cervical Spine. Philadelphia: WB Saunders, 1967.
3 Edwards WC, LaRocca H. The developmental segmental sagittal diameter of the cervical spinal canal in patients with cervical spondylosis. Spine (Phila Pa 1976). 1983;8:20-27.
4 Lee MJ, Cassinelli EH, Riew KD. Prevalence of cervical spine stenosis: Anatomic study in cadavers. J Bone Joint Surg Am. 2007;89:376-380.
5 Pavlov H, Torg JS, Robie B, et al. Cervical spinal stenosis: Determination with vertebral body ratio method. Radiology. 1987;164:771-775.
6 Blackley HR, Plank LD, Robertson PA. Determining the sagittal dimensions of the canal of the cervical spine: The reliability of ratios of anatomical measurements. J Bone Joint Surg Br. 1999;81:110-112.
7 Lim JK, Wong HK. Variation of the cervical spinal Torg ratio with gender and ethnicity. Spine J. 2004;4:396-401.
8 Prasad SS, O’Malley M, Caplan M, et al. MRI measurements of the cervical spine and their correlation to Pavlov’s ratio. Spine (Phila Pa 1976). 2003;28:1263-1268.
9 Bell GR, Ross JS. Diagnosis of nerve root compression. Orthop Clin North Am. 1992;22:405-419.
10 Chen CJ, Lyu RK, Lee ST, et al. Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: Prediction of prognosis with type of intensity. Radiology. 2001;221:789-794.
11 Shen Y, Zhang YZ, Wang LF. [Relation of MR T2 image signal intensity ratio of cervical spondylotic myelopathy with clinical manifestations and prognosis]. Zhonghua Yi Xue Za Zhi. 2008;88:3072-3076.
12 Boden SD, McCowin PR, Davis DO, et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects: A prospective investigation. J Bone Joint Surg Am. 1990;72:1178-1184.
13 Okada E, Matsumoto M, Ichihara D, et al. Aging of the cervical spine in healthy volunteers: A 10-year longitudinal magnetic resonance imaging study. Spine (Phila Pa 1976). 2009;34:706-712.
14 Ashkan K, Johnston P, Moore AJ. A comparison of magnetic resonance imaging and neurophysiological studies in the assessment of cervical radiculopathy. Br J Neurosurg. 2002;16:146-148.
15 Berger AR, Busis NA, Logigian EL, et al. Cervical root stimulation in the diagnosis of radiculopathy. Neurology. 1987;37:329-332.
16 Tsai CP, Huang CI, Wang V, et al. Evaluation of cervical radiculopathy by cervical root stimulation. Electromyogr Clin Neurophysiol. 1994;34:363-366.
17 Smith G, Robinson R. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40:607-624.
18 Apfelbaum RI, Kriskovich MD, Haller JR. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine (Phila Pa 1976). 2000;25:2906-2912.
19 Audu P, Artz G, Scheid S, et al. Recurrent laryngeal nerve palsy after anterior cervical spine surgery: The impact of endotracheal tube cuff deflation, reinflation, and pressure adjustment. Anesthesiology. 2006;105:898-901.
20 Nassr A, Lee JY, Bashir RS, et al. Does incorrect level needle localization during anterior cervical discectomy and fusion lead to accelerated disc degeneration? Spine (Phila Pa 1976). 2009;34:189-192.
21 Jagannathan J, Shaffrey CI, Oskouian RJ, et al. Radiographic and clinical outcomes following single-level anterior cervical discectomy and allograft fusion without plate placement or cervical collar. J Neurosurg Spine. 2008;8:420-428.
22 Campbell MJ, Carreon LY, Traynelis V, et al. Use of cervical collar after single-level anterior cervical fusion with plate: Is it necessary? Spine (Phila Pa 1976). 2009;34:43-48.
23 Liu JT, Briner RP, Friedman JA. Comparison of inpatient vs. outpatient anterior cervical discectomy and fusion: A retrospective case series. BMC Surg. 2009;9:3.
24 Erickson M, Fites BS, Thieken MT, et al. Outpatient anterior cervical discectomy and fusion. Am J Orthop. 2007;36:429-432.
25 Villavicencio AT, Pushchak E, Burneikiene S, et al. The safety of instrumented outpatient anterior cervical discectomy and fusion. Spine J. 2007;7:148-153.
25a Bohler J. Sofort-und Fruhbehandlong traumatischer querschnitt lahmungen. Zeitschrift fur Orthopadie und Ihre Grenzgebiete. 1967;103:512-529.
26 Schulte K, Clark C, Goel V. Kinematics of the cervical spine following discectomy and stabilization. Spine (Phila Pa 1976). 1989;14:1116-1121.
27 Grubb M, Currier B, Shih J, et al. Biomechanical evaluation of anterior cervical spine stabilization. Spine (Phila Pa 1976). 1998;23:886-892.
28 Spivak J, Chen D, Kummer F. The effect of locking fixation screws on the stability of anterior cervical plating. Spine (Phila Pa 1976). 1999;24:334-338.
29 Connolly PJ, Esses SI, Kostuik JP. Anterior cervical fusion: Outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202-206.
30 Samartzis D, Shen FH, Lyon C, et al. Does rigid instrumentation increase the fusion rate in one-level anterior cervical discectomy and fusion? Spine J. 2004;4:636-643.
31 Dvorak MF, Pitzen T, Zhu Q, et al. Anterior cervical plate fixation: A biomechanical study to evaluate the effects of plate design, endplate preparation, and bone mineral density. Spine (Phila Pa 1976). 2005;30:294-301.
32 Reidy D, Finkelstein J, Nagpurkar A, et al. Cervical spine loading characteristics in a cadaveric C5 corpectomy model using a static and dynamic plate. J Spinal Disord Tech. 2004;17:117-122.
33 Truumees E, Demetropoulos CK, Yang KH, et al. Effects of a cervical compression plate on graft forces in an anterior cervical discectomy model. Spine (Phila Pa 1976). 2003;28:1097-1102.
34 Pitzen TR, Chrobok J, Stulik J, et al. Implant complications, fusion, loss of lordosis, and outcome after anterior cervical plating with dynamic or rigid plates: Two-year results of a multi-centric, randomized, controlled study. Spine (Phila Pa 1976). 2009;34:641-646.
35 Nunley PD, Jawahar A, Kerr EJ3rd, et al. Choice of plate may affect outcomes for single versus multilevel ACDF: Results of a prospective randomized single-blind trial. Spine J. 2009;9:121-127.
36 Brodke DS, Klimo PJr, Bachus KN, et al. Anterior cervical fixation: Analysis of load-sharing and stability with use of static and dynamic plates. J Bone Joint Surg Am. 2006;88:1566-1573.
37 Ghahreman A, Rao PJ, Ferch RD. Dynamic plates in anterior cervical fusion surgery: Graft settling and cervical alignment. Spine (Phila Pa 1976). 2009;34:1567-1571.
38 McLaughlin MR, Purighalla V, Pizzi FJ. Cost advantages of two-level anterior cervical fusion with rigid internal fixation for radiculopathy and degenerative disease. Surg Neurol. 1997;48:560-565.
39 Caspar W, Geisler FH, Pitzen T, et al. Anterior cervical plate stabilization in one- and two-level degenerative disease: Over-treatment or benefit? J Spinal Disord. 1998;11:1-11.
40 Geisler FH, Caspar W, Pitzen T, et al. Re-operation in patients after anterior cervical plate stabilization in degenerative disease. Spine (Phila Pa 1976). 1998;23:911-920.
41 Park JB, Cho YS, Riew KD. Development of adjacent-level ossification in patients with an anterior cervical plate. J Bone Joint Surg Am. 2005;87:558-563.
42 Bolesta MJ, Rechtine GR2nd, Chrin AM. One- and two-level anterior cervical discectomy and fusion: The effect of plate fixation. Spine J. 2002;2:197-203.
43 Connolly PJ, Esses SI, Kostuik JP. Anterior cervical fusion: Outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202-206.
44 Wang JC, McDonough PW, Endow KK, et al. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2000;25:41-45.
45 Robinson R, Walker A, Ferlic D. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg Am. 1962;44:1569-1587.
46 Emery SE, Bolesta MJ, Banks MA, et al. Robinson anterior cervical fusion: Comparison of standard and modified techniques. Spine (Phila Pa 1976). 1994;19:660-663.
47 Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and plating: A 5- to 11-year radiologic and clinical follow-up study. Spine (Phila Pa 1976). 2005;30:2138-2144.
48 Emery SE, Fisher RS, Bohlman HH. Three-level anterior cervical discectomy and fusion: Radiographic and clinical results. Spine (Phila Pa 1976). 1997;22:2622-2625.
49 Wright IP, Eisenstein SM. Anterior cervical discectomy and fusion without instrumentation. Spine (Phila Pa 1976). 2007;32:772-774. discussion 775
50 Bolesta MJ, Rechtine GR, Chrin AM. Three- and four-level anterior cervical discectomy and fusion with plate fixation: A prospective study. Spine (Phila Pa 1976). 2000;25:2040-2046.
51 Wang JC, McDonough PW, Kanim LE, et al. Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2001;26:643-646. discussion 646-647
52 Cauthen JC, Kinard RE, Vogler JB, et al. Outcome analysis of noninstrumented anterior cervical discectomy and interbody fusion in 348 patients. Spine (Phila Pa 1976). 1998;23:188-192.
53 Aronson N, Filtzer DL, Bagan M. Anterior cervical fusion by the Smith-Robinson approach. J Neurosurg. 1968;29:397-404.
54 Riley LH, Robinson RA, Johnson KA, et al. The results of anterior interbody fusion of the cervical spine. J Neurosurg. 1969;30:127-133.
55 Connolly ES, Seymour RJ, Adams JE. Clinical evaluation of anterior cervical fusion for degenerative cervical disc disease. J Neurosurg. 1965;23:431-437.
56 White AA, Southwick WO, DuPonte R, et al. Relief of pain by anterior cervical spine fusion for spondylosis. J Bone Joint Surg Am. 1968;55:525-534.
57 Phillips FM, Carlson G, Emery SE, et al. Anterior cervical pseudarthrosis: Natural history and treatment. Spine (Phila Pa 1976). 1997;22:1585-1589.
58 Hilibrand AS, Fye MA, Emery SE, et al. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg Am. 2001;83:668-673.
59 Luszczyk M, Fischgrund J, Sasso R, et al: Does smoking have an impact on fusion rate in single level ACDF with allograft and rigid plate fixation? Cervical Spine Research Society meeting, 2008.
60 Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: Long-term follow-up of one-hundred-and-twenty-two patients. J Bone Joint Surg Am. 1993;75:1298-1301.
61 Clements DH, O’Leary PF. Anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 1990;15:1023-1025.
62 Gore DR, Sepic SB. Anterior cervical fusion for degenerated or protruded discs. Spine (Phila Pa 1976). 1984;9:667-671.
63 Hilibrand AS, Yoo JU, Carlson GD, et al. The success of anterior cervical arthrodesis adjacent to a previous fusion. Spine (Phila Pa 1976). 1997;22:1574-1579.
64 Goldberg EJ, Singh K, Van U, et al. Comparing outcomes of anterior cervical discectomy and fusion in workman’s versus non-workman’s compensation population. Spine J. 2002;2:408-414.
65 Gore DR, Sepic SB. Anterior discectomy and fusion for painful cervical disc disease: A report of 50 patients with an average follow-up of 21 years. Spine (Phila Pa 1976). 1998;23:2047-2051.
66 Klein GR, Vaccaro AR, Albert TJ. Health outcome assessment before and after anterior cervical discectomy and fusion for radiculopathy: A prospective analysis. Spine (Phila Pa 1976). 2000;25:801-803.
67 DePalma A, Rothman R, Lewinnek G, et al. Anterior interbody fusion for severe cervical disc degeneration. Surg Gynecol Obstet. 1972;134:755-758.
68 Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003;28:134-139.
69 Kurz LT, Garfin SR, Booth RE. Harvesting autogenous iliac bone grafts. Spine (Phila Pa 1976). 1989;12:1324-1331.
70 Fountas KN, Kapsalaki EZ, Smith BE, et al. Interobservational variation in determining fusion rates in anterior cervical discectomy and fusion procedures. Eur Spine J. 2007;16:39-45.
71 Flynn T. Neurologic complications of anterior cervical interbody fusion. Spine (Phila Pa 1976). 1982;7:536-539.
72 Kilburg C, Sullivan HG, Mathiason MA. Effect of approach side during anterior cervical discectomy and fusion on the incidence of recurrent laryngeal nerve injury. J Neurosurg Spine. 2006;4:273-277.
73 Herkowitz H. The surgical management of cervical spondylotic radiculopathy and myelopathy. Clin Orthop Relat Res. 1989;239:94-108.
74 Whitecloud T. Complications of anterior cervical fusion. In: Instructional Course Lectures. St Louis: CV Mosby; 1978.
75 Lee MJ, Bazaz R, Furey CG, et al. Risk factors for dysphagia after anterior cervical spine surgery: A two-year prospective cohort study. Spine J. 2007;7:141-147.
76 Smith-Hammond CA, New KC, Pietrobon R, et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: Comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine (Phila Pa 1976). 2004;29:1441-1446.
77 Edwards CC2nd, Karpitskaya Y, Cha C, et al. Accurate identification of adverse outcomes after cervical spine surgery. J Bone Joint Surg Am. 2004;86:251-256.
78 Yue WM, Brodner W, Highland TR. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: A 5- to 11-year follow-up study. Eur Spine J. 2005;14:677-682.
79 Newhouse KE, Lindsey RW, Clark CR, et al. Esophageal perforation following anterior cervical spine surgery. Spine (Phila Pa 1976). 1989;14:1051-1053.
80 Brown M, Malinin T, Davis P. A roentgenographic evaluation of frozen allografts versus autografts in anterior cervical spine fusions. Clin Orthop Relat Res. 1976;119:231-236.
81 Fernyhough J, White J, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine (Phila Pa 1976). 1991;16:5561-5564.
82 Samartzis D, Shen FH, Goldberg EJ, et al. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine (Phila Pa 1976). 2005;30:1756-1761.
83 Zdeblick T, Ducker T. The use of freeze-dried allograft bone for anterior cervical fusions. Spine (Phila Pa 1976). 1991;16:726-729.
84 Suchomel P, Barsa P, Buchvald P, et al. Autologous versus allogenic bone grafts in instrumented anterior cervical discectomy and fusion: A prospective study with respect to bone union pattern. Eur Spine J. 2004;13:510-515.
85 Samartzis D, Shen FH, Matthews DK, et al. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J. 2003;3:451-459.
86 Bishop RC, Moore KA, Hadley MN. Anterior cervical interbody fusion using autogenic and allogenic bone graft substrate: A prospective comparative analysis. J Neurosurg. 1996;85:206-210.
87 Malloy KM, Hilibrand AS. Autograft versus allograft in degenerative cervical disease. Clin Orthop Rel Res. 2002;394:27-38.
88 MacDonald RL, Fehlings M, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86:990-997.
89 Martin GJ, Haid RW, MacMillian M, et al. Anterior cervical discectomy with freeze-dried fibula allograft. Spine (Phila Pa 1976). 1999;24:852-859.
90 Shono Y, McAfee PC, Cunningham BW, et al. A biomechanical analysis of decompression and reconstruction methods in the cervical spine: Emphasis on a carbon-fiber-composite cage. J Bone Joint Surg Am. 1993;75:1674-1684.
91 Panjabi MM, Cholewicki J, Nibu K, et al. Critical load of the human cervical spine: An in vitro experimental study. Clin Biomech (Bristol, Avon). 1998;13:11-17.
92 Boakye M, Mummaneni PV, Garrett M, et al. Anterior cervical discectomy and fusion involving a polyetheretherketone spacer and bone morphogenetic protein. J Neurosurg Spine. 2005;2:521-525.
93 Cho DY, Lee WY, Sheu PC, et al. Cage containing a biphasic calcium phosphate ceramic (Triosite) for the treatment of cervical spondylosis. Surg Neurol. 2005;63:497-503. discussion 504
94 Mastronardi L, Ducati A, Ferrante L. Anterior cervical fusion with polyetheretherketone (PEEK) cages in the treatment of degenerative disc disease: Preliminary observations in 36 consecutive cases with a minimum 12-month follow-up. Acta Neurochir (Wien). 2006;148:307-312. discussion 312
95 Kulkarni AG, Hee HT, Wong HK. Solis cage (PEEK) for anterior cervical fusion: Preliminary radiological results with emphasis on fusion and subsidence. Spine J. 2007;7:205-209.
96 Celik SE, Kara A, Celik S. A comparison of changes over time in cervical foraminal height after tricortical iliac graft or polyetheretherketone cage placement following anterior discectomy. J Neurosurg Spine. 2007;6:10-16.
97 Das K, Couldwell WT, Sava G, et al. Use of cylindrical titanium mesh and locking plates in anterior cervical fusion. Technical note. J Neurosurg. 2001;94:174-178.
98 Katsuura A, Hukuda S, Imanaka T, et al. Anterior cervical plate used in degenerative disease can maintain cervical lordosis. J Spinal Disord. 1996;9:470-476.
99 Shapiro S. Banked fibula and the locking anterior cervical plate in anterior cervical fusions following cervical discectomy. J Neurosurg. 1996;84:161-165.
100 Shapiro S, Connolly P, Donnaldson J, et al. Cadaveric fibula, locking plate, and allogeneic bone matrix for anterior cervical fusions after cervical discectomy for radiculopathy or myelopathy. J Neurosurg. 2001;95:43-50.
101 Demircan MN, Kutlay AM, Colak A, et al. Multilevel cervical fusion without plates, screws or autogenous iliac crest bone graft. J Clin Neurosci. 2007;14:723-728.
102 Cho DY, Lee WY, Sheu PC. Treatment of multilevel cervical fusion with cages. Surg Neurol. 2004;62:378-385. discussion 385-386
103 Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426-435.
104 Perri B, Cooper M, Lauryssen C, et al. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: A case study. Spine J. 2007;7:235-239.
105 Smucker JD, Rhee JM, Singh K, et al. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976). 2006;31:2813-2819.
106 Shields LB, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976). 2006;31:542-547.
107 Vaidya R, Carp J, Sethi A, et al. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257-1265.
108 Grundy PL, Germon TJ, Gill SS. Transpedicular approaches to cervical uncovertebral osteophytes causing radiculopathy. J Neurosurg. 2000;93:21-27.
109 Jho HD, Kim MH, Kim WK. Anterior cervical microforaminotomy for spondylotic cervical myelopathy: II. Neurosurgery. 2002;51:54-59.
110 Johnson JP, Filler AG, McBride DQ, et al. Anterior cervical foraminotomy for unilateral radicular disease. Spine (Phila Pa 1976). 2000;25:905-909.
111 Yi S, Lim JH, Choi KS, et al. Comparison of anterior cervical foraminotomy vs arthroplasty for unilateral cervical radiculopathy. Surg Neurol. 2009;71:677-680. discussion 680
112 Koc RK, Menku A, Tucer B, et al. Anterior cervical foraminotomy for unilateral spondylotic radiculopathy. Minim Invasive Neurosurg. 2004;47:186-189.
113 Johnson JP, Filler AG, McBride DQ, et al. Anterior cervical foraminotomy for unilateral radicular disease. Spine (Phila Pa 1976). 2000;25:905-909.
114 Murphy M, Gadd M. Anterior cervical discectomy without interbody bone graft. J Neurosurg. 1972;37:71-74.
115 O’Laoire S, Thomas D. Spinal cord compression due to prolapse of cervical intervertebral disc (herniation of nucleus pulposus). J Neurosurg. 1983;59:846-853.
116 Rosenorn J, Hansen E, Rosenorn M. Anterior cervical discectomy with and without fusion. J Neurosurg. 1983;59:252-255.
117 Benini A, Krayenbuhl H, Bruder R. Anterior cervical discectomy without fusion: Microsurgical technique. Acta Neurochir. 1982;61:105-110.
118 Kadoya S, Nakamura T, Kwak R. A microsurgical anterior osteophytectomy for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1984;9:437-441.
119 Wilson D, Campbell D. Anterior cervical discectomy without bone graft. J Neurosurg. 1977;47:551-555.
120 Watters WCIII, Levinthal R. Anterior cervical discectomy with and without fusion: Results, complications and long-term follow-up. Spine (Phila Pa 1976). 1994;19:2343-2347.
121 Wirth FP, Dowd GC, Sanders HF, et al. Cervical discectomy: A prospective analysis of three operative techniques. Surg Neurol. 2000;53:340-348.
122 Heller JG, Sasso RC, Papadopoulos SM, et al. Comparison of Bryan cervical disc arthroplasty with anterior cervical decompression and fusion: Clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976). 2009;34:101-107.
123 Steinmetz MP, Patel R, Traynelis V, et al. Cervical disc arthroplasty compared with fusion in a workers’ compensation population. Neurosurgery. 2008;63:741-747. discussion 747
124 Nabhan A, Ahlhelm F, Shariat K, et al. The ProDisc-C prosthesis: Clinical and radiological experience 1 year after surgery. Spine (Phila Pa 1976). 2007;32:1935-1941.
125 Rousseau MA, Cottin P, Levante S, et al. In vivo kinematics of two types of ball-and-socket cervical disc replacements in the sagittal plane: Cranial versus caudal geometric center. Spine (Phila Pa 1976). 2008;33:E6-E9.
126 Sasso RC, Best NM, Metcalf NH, et al. Motion analysis of Bryan cervical disc arthroplasty versus anterior discectomy and fusion: Results from a prospective, randomized, multicenter, clinical trial. J Spinal Disord Tech. 2008;21:393-399.
127 Puttlitz CM, Rousseau MA, Xu Z, et al. Intervertebral disc replacement maintains cervical spine kinetics. Spine (Phila Pa 1976). 2004;29:2809-2814.
128 Sasso RC, Best NM. Cervical kinematics after fusion and Bryan disc arthroplasty. J Spinal Disord Tech. 2008;21:19-22.
129 Chang UK, Kim DH, Lee MC, et al. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine. 2007;7:33-39.
130 Eck JC, Humphreys SC, Lim TH, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine (Phila Pa 1976). 2002;27:2431-2434.
131 Lopez-Espina CG, Amirouche F, Havalad V. Multilevel cervical fusion and its effect on disc degeneration and osteophyte formation. Spine (Phila Pa 1976). 2006;31:972-978.
132 Park DH, Ramakrishnan P, Cho TH, et al. Effect of lower two-level anterior cervical fusion on the superior adjacent level. J Neurosurg Spine. 2007;7:336-340.
133 Laxer EB, Darden BV, Murrey DB, et al. Adjacent segment disc pressures following two-level cervical disc replacement versus simulated anterior cervical fusion. Stud Health Technol Inform. 2006;123:488-492.
134 Mehren C, Suchomel P, Grochulla F, et al. Heterotopic ossification in total cervical artificial disc replacement. Spine (Phila Pa 1976). 2006;31:2802-2806.
135 Anderson PA, Sasso RC, Riew KD. Comparison of adverse events between the Bryan artificial cervical disc and anterior cervical arthrodesis. Spine (Phila Pa 1976). 2008;33:1305-1312.
136 Pickett GE, Sekhon LH, Sears WR, et al. Complications with cervical arthroplasty. J Neurosurg Spine. 2006;4:98-105.
137 Kim SW, Shin JH, Arbatin JJ, et al. Effects of a cervical disc prosthesis on maintaining sagittal alignment of the functional spinal unit and overall sagittal balance of the cervical spine. Eur Spine J. 2008;17:20-29.
138 Phillips FM, Allen TR, Regan JJ, et al. Cervical disc replacement in patients with and without previous adjacent level fusion surgery: A prospective study. Spine (Phila Pa 1976). 2009;34:556-565.
139 Shin DA, Yi S, Yoon do H, et al. Artificial disc replacement combined with fusion versus two-level fusion in cervical two-level disc disease. Spine (Phila Pa 1976). 2009;34:1153-1159. discussion 1160-1161
140 Cheng L, Nie L, Zhang L, et al. Fusion versus Bryan Cervical Disc in two-level cervical disc disease: A prospective, randomised study. Int Orthop. 2009;33:1347-1351.
141 Liu J, Zhang H, Li K, et al. [Clinical effect of cervical artificial disc replacement on two-segment cervical spondylosis]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:385-388.
142 Pimenta L, McAfee PC, Cappuccino A, et al. Superiority of multilevel cervical arthroplasty outcomes versus single-level outcomes: 229 consecutive PCM prostheses. Spine (Phila Pa 1976). 2007;32:1337-1344.
143 Lowery GL, McDonough RF. The significance of hardware failure in anterior cervical plate fixation: Patients with 2- to 7-year follow-up. Spine (Phila Pa 1976). 1998;23:181-186. discussion 186-187
144 Swank ML, Lowery GL, Bhat AL, et al. Anterior cervical allograft arthrodesis and instrumentation: Multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6:138-143.