CHAPTER 43 Cervical Disc Replacement
Background
Anterior cervical discectomy and fusion (ACDF) is a proven intervention for patients with radiculopathy and myelopathy.1 It has served as the standard by which other cervical and spinal disorders may be judged as the result of its high rate of success. The success of this technique is often judged based on its consistent ability to relieve symptoms related to neurologic dysfunction. In this sense, the clinical results with regard to the patient’s index complaint are outstanding. The radiographic results of this technique are also initially predictable with a high rate of fusion. Plating techniques have diminished the need for postoperative immobilization or eliminated them entirely.2 Because of limitations specific to this procedure, investigators have developed surgical alternatives to fusion that attempt to address the kinematic and biomechanical issues inherent in it.
A major concern related to the treatment of cervical degenerative disc disease and spondylosis with ACDF is the issue of adjacent segment degeneration. This event is manifest as the radiographic appearance of degenerative change at a level directly above or below a level treated with a surgical intervention—typically being associated with degeneration of a level adjacent to a fused level. The incidence of this phenomenon has been reported to be 92% by Goffin and colleagues,3 who wrote a long-term follow-up on patients after treatment with anterior interbody fusion.
Although some debate remains regarding the causation of adjacent segment degeneration—with a mix of postsurgical and naturally determined aging cited as root causes—there is little debate regarding the existence of this phenomenon. It is also relevant to note the clinical distinction between adjacent segment degeneration and adjacent segment disease. Adjacent segment disease is defined as adjacent segment degeneration that causes clinical symptoms (pain or neurologic disorders or both) severe enough to lead to patient complaint or require operative intervention.4 Although this distinction has not remained consistent in published literature, it is an important consideration with regard to the phenomenon that occurs in discs adjacent to discs that have undergone a surgical intervention. Numerous studies have made a consistent point of distinguishing between radiographic degeneration and symptomatic disease.3,5
There is clinical evidence to support the postsurgical nature of adjacent segment disease. In patients previously treated with fusion, adjacent segment disease has been documented at a rate of 2.9% of patients per annum by Hilibrand and colleagues,4 and 25% of patients undergoing cervical fusion have new onset of symptoms within 10 years of fusion. This study has received a great deal of attention and has led to further investigations into biomechanical causation. Other reports have focused on the recurrence of neurologic symptoms and degenerative changes adjacent to fused cervical levels.3,6 The concept that adjacent levels need to compensate for loss of motion in the fused segment may also be valid. Segments adjacent to a fusion have an increased range of motion and increased intradiscal pressures.7,8
Bone graft materials used in traditional ACDF procedures have also been a source of controversy. Current ACDF techniques make use of allograft bone, premanufactured allograft bone, and autologous iliac crest. Complications associated with autologous iliac crest harvest used as a fusion graft in ACDF are well documented. Sandhu and colleagues9 reported a complication rate of 1% to 25% with such procedures. Complications such as acute and chronic pain, infection, meralgia paresthetica, and pelvic fracture are known to occur at harvest donor sites.10,11
Although allograft removes the risks associated with the harvest of autograft, it has the detriment of having the theoretical risk of disease transmission. In practice, this risk is believed to be extremely minute, although the U.S. Food and Drug Administration (FDA) has taken this issue seriously. The issues of disease transmission and contaminated graft materials have been highlighted by allograft tissue recalls by the FDA in recent years.12 Although bone graft substitutes may play a role in the future practice of ACDF, this continues to be a minority stake in the overall graft selection of modern surgeons.
Pseudarthrosis is another complication encountered with anterior cervical fusion procedures. Pseudarthrosis is the failure of bony bridging or nonunion of a segment that has previously been treated with a bone graft or a bone graft substitute—an attempt at fusion has been made. In multilevel ACDF procedures, there is a relationship between the rate of pseudarthrosis and the number of levels fused. Brodke and Zdeblick13 reported a 97% fusion rate in single-level ACDF, which decreased to 83% with fusion at three levels. Bohlman and colleagues1 reported an 11% pseudarthrosis rate in single-level fusions that increased to 27% with multilevel fusions.
In recent years, the use of bone morphogenetic proteins has been proposed as an alternative or adjunct to traditional bone grafting techniques to combat the pseudarthrosis issue in patients deemed to be at higher risk for this complication.14,15 This off-label use has been associated with an increased incidence of swelling complications and concerns for graft resorption and migration of interbody implants.16–19 These issues serve to strengthen the argument for fusion alternatives in the treatment of discogenic pathology in the anterior cervical spine.
Total intervertebral disc replacement (TDR) is designed to preserve motion, avoid limitations of fusion, and allow patients to return quickly to routine activities. The primary goals of the procedure in the cervical spine are to restore disc height and segmental motion after removing local pathology that is deemed to be the source of a patient’s index complaint. A secondary intention is to preserve normal motion at adjacent cervical levels, which may be theorized to prevent later adjacent level degeneration. Cervical TDR avoids the morbidity of bone graft harvest.20,21 It also avoids complications such as pseudarthrosis, issues caused by anterior cervical plating, and cervical immobilization side effects.
History of Disc Arthroplasty and Device Design
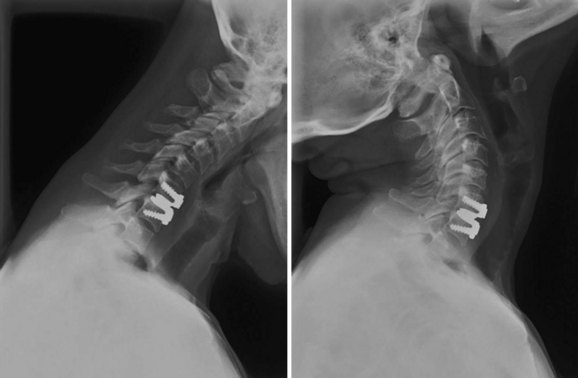
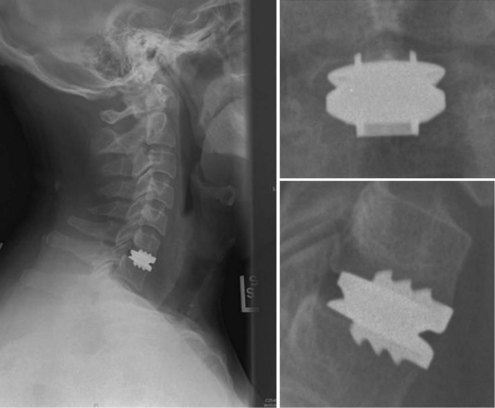
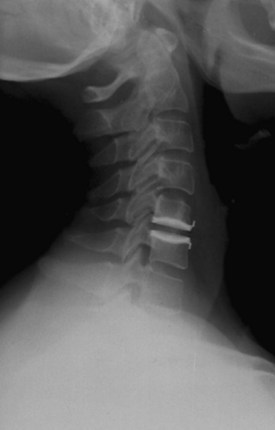
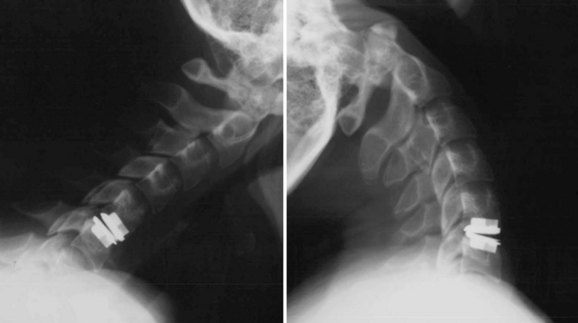
An understanding of the evolution of cervical TDR serves as an important lesson in the concepts of device design, TDR bearing and wear characteristics, and articular constraint. In the late 1980s, Cummins and colleagues22 developed a metal-on-metal ball-and-socket cervical disc replacement composed of 316L stainless steel. With the acquisition of this technology and the later development of new metal-on-metal devices came a rapid transition from this device to the most recent device, the PRESTIGE LP (Medtronic Sofamor Danek, Memphis, TN). A predecessor of this device, the PRESTIGE ST (Medtronic Sofamor Danek, Memphis, TN) is currently approved by the FDA for human use in the United States (Figs. 43-1 and 43-2). More recent additions to the metal-on-metal category of arthroplasty devices include the Kineflex-C disc (Spinal Motion, Mountain View, CA) and the CerviCore intervertebral disc (Stryker Spine, Allendale, NJ) (Figs. 43-3 and 43-4), which are in the process of U.S. FDA investigational device exemption (IDE) trials.
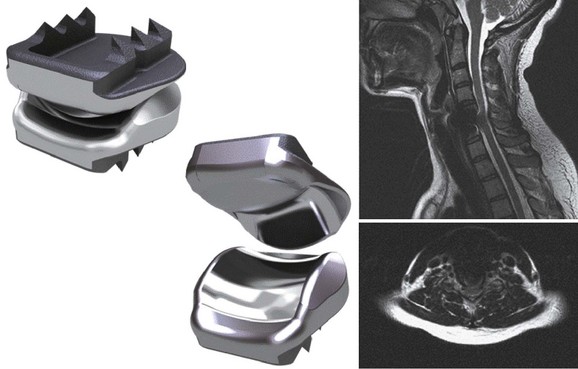
Numerous devices have evolved in parallel to the metal-on-metal implants, including the BRYAN cervical disc (Medtronic Sofamor Danek, Memphis, TN) (Figs. 43-5 through 43-7), the PCM (CerviTech, Rockaway, NJ), the DISCOVER (DePuy Spine, Raynham, MA), and the MOBI-C (LDR, Austin, TX). Each of these devices is in the process of limited human trials or U.S. FDA IDE submission and represents an alternative to metal-on-metal bearing surfaces, which have the potential for metal debris and systemic concentration of metal ions. To date, one such device, the Prodisc-C (Synthes Spine, Paoli, PA) (Figs. 43-8 and 43-9), has obtained approval for use in the United States.
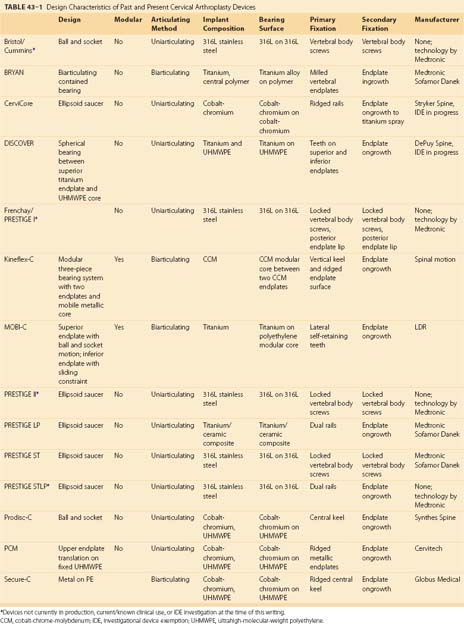
A summary of the design characteristics of each of these devices is presented in Table 43–1. Although the ideas of bearing surface, wear debris, and constraint are not new to discussions with regard to arthroplasty in general, they are relatively new in regard to the spine. A full understanding of the term constraint has not been agreed on because constraint may arise within the device or as a result of the local anatomy (e.g., facets, posterior longitudinal ligament). As the knowledge base in spine TDR increases, intelligent investigations and discussions are sure to include many of these concepts and may redefine understanding of them.
Indications for Use, Contraindications, and Complications
Cervical disc arthroplasty trials have included patients refractory to nonoperative treatment modalities with and without radiculopathy or myelopathy or both and with one-level and two-level degenerative disc disease or spondylosis.23–25 These indications have been retained throughout the FDA approval process. At the time of this writing, two devices, the PRESTIGE ST and the Prodisc-C, have achieved FDA approval for single-level use in the United States. Other devices are in various stages of the IDE and approval process (Table 43–2). ACDF may be discussed as part of the indication process for an arthroplasty procedure. The historical challenges associated with ACDF presented in this chapter may be weighed against the early nature of data with respect to cervical arthroplasty in a patient’s informed consent discussion.
TABLE 43–2 Current Status of Cervical Arthroplasty Devices in the United States*
| Device | Manufacturer | U.S. FDA Status |
|---|---|---|
| PRESTIGE-ST | Medtronic Sofamor Danek | Approved |
| Prodisc-C | Synthes Spine | Approved |
| BRYAN disc | Medtronic Sofamor Danek | IDE data submitted, approval pending |
| CerviCore disc | Stryker Spine | IDE in progress |
| DISCOVER disc | DePuy Spine | IDE in progress |
| Kineflex-C disc | Spinal Motion | IDE in progress |
| MOBI-C disc | LDR | IDE in progress |
| PCM disc | Cervitech | IDE in progress |
| PRESTIGE-LP | Medtronic Sofamor Danek | IDE in progress |
| SECURE-C | Globus Medical | IDE in progress |
IDE, investigational device exemption.
* Table current as of April 1, 2009.
The appropriate time to discuss complications related to cervical arthroplasty is at the time of informed consent. The approach-related risks of cervical arthroplasty are similar to ACDF and should be discussed as such. These risks have been adequately covered in other portions of this text. A unique risk of arthroplasty is the concern of heterotopic ossification in the region of the arthroplasty device.26–30 Heterotopic ossification may result in loss of motion or frank fusion of the index level. Heterotopic ossification may be associated with the physiologic response to the implantation process, the amount of bleeding or hemostasis necessary in a particular procedure, or the amount of bone debris created at the time of preparation for implantation. Postoperative use of nonsteroidal anti-inflammatory drugs has been suggested to moderate the prevalence of this risk.
Other complications may occur with cervical arthroplasty that are independent of the anterior cervical approach, including infection, implant migration, subsidence, continued or new neurologic findings, vascular injury, dural injury or cerebrospinal fluid leak, hematoma, and reoperation for adjacent level disease. Many complications associated with placement of arthroplasty devices have come to light as the result of reporting and analysis of the prospective randomized multicenter U.S. FDA IDEs and large studies performed outside the United States.23–25,31,32
Technique of Implantation
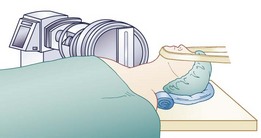
Intraoperatively, patient position is important. A “physiologic” or slightly lordotic cervical spine position is preferred.33 Assessment via fluoroscopy (C-arm) is crucial to patient positioning and implant insertion and fixation portions of these procedures. It is important to keep the head, neck, and shoulders in a stable and neutral position throughout this surgical procedure. A small towel roll may be placed under the neck to assist with appropriate positioning of the neck and shoulders and to keep a physiologic lordosis without creating a hyperlordosis (Fig. 43–10). This positioning technique differs from the typical placement of a roll under the shoulders or thoracic spine, which could place the cervical spine in hyperlordosis. The head is placed on a doughnut-type pillow or a folded towel to keep it from rolling during the procedure. The careful positioning of shoulders with a taping technique can also allow for less motion during this procedure and must be carefully weighed against the risk of traction to the shoulders. Taping of the shoulders differs from the commonly used wrist restraints in a typical ACDF procedure that are used to obtain additional longitudinal traction via a temporary “pull” on the arms.
After neurologic decompression, assessment for placement of an appropriately sized disc and planning for proper orientation of the implant are crucial to successful arthroplasty. To this end, it should be the surgeon’s goal to place as large a device (with respect to diameter) as possible in the prepared space.35 Device-specific tools may aid in this assessment.
Postoperative Imaging
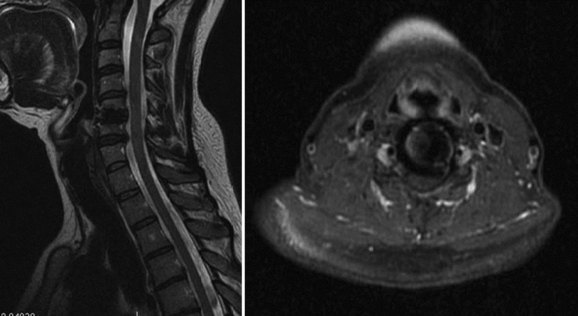
To moderate the risks of technique and radiation associated with CT myelography, MRI has been proposed as an alternative that allows for postoperative assessment of neurologic status adjacent to and at the level of a prior cervical arthroplasty procedure. Success with this technique has been described by Sekhon and colleagues34 in several devices, including the BRYAN and the PRESTIGE LP arthroplasty devices. Both of these devices use titanium alloy in their endplates, which was shown to produce less MRI artifact at the index and adjacent surgical levels than the metals associated with the manufacture of the Prodisc-C and PCM arthroplasty devices.34 The BRYAN prosthesis has a polyurethane core, and the PRESTIGE LP had a titanium carbide alloy (as tested in the study). Investigators had difficulty assessing the neural structures at the index and adjacent levels with devices manufactured with nontitanium metals (cobalt-chromium-molybdenum) used in the Prodisc-C and PCM devices.
Clinical Studies
PRESTIGE Disc
Wigfield and colleagues35 reported favorable results on a 2-year pilot study of the first-generation PRESTIGE disc designed to address the safety of the technique and to assess the stability of, and motion allowed by, the device. The investigators tried to target patients most at risk for adjacent segment disease. Inclusion in the study required radiculopathy or myelopathy secondary to herniated disc or uncovertebral osteophytes confirmed on CT or MRI adjacent to a surgically or congenitally fused segment. An additional inclusion category was patients with asymptomatic disc degeneration adjacent to the symptomatic level without presence of a fusion. The study enrolled 15 patients. Wigfield and colleagues35 concluded that the technique is safe because procedural complications were limited to two cases of transient hoarseness that resolved.
In a separate prospective nonrandomized study, Wigfield and colleagues36 compared the effects of the PRESTIGE disc and one-level anterior fusion on adjacent segment motion. No significant difference in adjacent segment motion was measured between the two groups preoperatively. Postoperatively, there was a significant increase in adjacent segment motion in the fusion group (mean increase of 9 degrees) compared with a slight reduction in adjacent level motion noted in the TDR group. In the fusion group, adjacent segment motion increased 5% at 6 months and 15% at 1 year. Subgroup analysis showed that increased motion occurs predominantly in normal rather than degenerative adjacent discs.
A prospective, randomized clinical trial was conducted by Porchet and Metcalf37 to compare the PRESTIGE II cervical disc with ACDF for the treatment of single-level degenerative disease. Standardized clinical outcome measures (NDI and SF-36) and radiographic examinations were used at prescribed postoperative intervals to compare the treatment groups. Of the 55 patients enrolled in the study, several had reached the final 24-month follow-up interval at the time of publication. Mean angular motion was 5.9 degrees at the disc level. Radiographic results showed that the PRESTIGE II disc maintained motion at the treated level without compromise of an adjacent segment. The authors concluded that use of the PRESTIGE II disc is as safe and as efficacious as standard ACDF at 24 months.
The most extensive report to date shows the results of the PRESTIGE ST device in a prospective randomized multicenter clinical trial.23 Data from this report have served as the basis for the current FDA approval of the PRESTIGE ST device in the United States. In this one-to-one randomization protocol, patients underwent either single-level arthroplasty or single-level ACDF. Data were reported up to and including 24 months and showed that the PRESTIGE ST device compared favorably with ACDF with regard to the study’s primary and secondary outcome measures. In addition, the device maintained an average of 7 degrees of angular motion and had no device failures or migration.
Mummaneni and colleagues31 published their clinical experience with the PRESTIGE LP device. This study focused on history, indications, patient positioning, surgical technique, complication avoidance, and revision strategies. As further evidence becomes available, the manufacturers of the PRESTIGE LP device hope to advance further on the early favorable results obtained by its predecessor, the PRESTIGE ST.23
Prodisc-C
Bertagnoli and colleagues38 published 1-year follow-up data on 27 patients who had single-level Prodisc-C implantation for treatment of one-level cervical degenerative disc disease. Standard preoperative and postoperative assessments of outcome were performed with NDI and VAS scores. Patients were also followed radiographically. Clinical outcome measures showed a sustained improvement at 1-year follow-up with a decrease in the NDI and VAS scores. Range of motion improved by 240% at 1 year compared with preoperative studies. Neck pain decreased by approximately 40%, arm pain frequency and intensity resolved to less than half of the original value, and no device-related or approach-related complications were noted.
The Prodisc-C has obtained approval by the U.S. FDA for use in single-level disc arthroplasty. Data from the multicenter human IDE trial have been published and to date represent the most significant compilation of outcomes with regard to this device.25 This prospective randomized multicenter study examined the results of the Prodisc-C cervical arthroplasty device compared with ACDF in patients treated for symptomatic single-level cervical degenerative disc disease. Demographic data were similar between the investigation and control treatment groups. A total of 24 months of outcome data were examined and showed that the arthroplasty device is safe and effective with regard to the outcome measures examined compared with the fusion cohort.
BRYAN Disc
Goffin and colleagues39 reported early results of a multicenter study of the BRYAN disc performed at single levels in 60 patients for the treatment of radiculopathy or myelopathy secondary to disc herniations or spondylosis failing at least 6 weeks of conservative treatment. Exclusion criteria included previous cervical spine surgery, axial neck pain as the sole symptom, significant anatomic deformity, and radiographic evidence of instability (translation >2 mm or >11 degrees of angulation compared with the adjacent level). Patient outcomes were determined by the Cervical Spine Research Society and SF-36 instruments. Clinical success rates at 6 months and 1 year were 86% and 90%, exceeding the study’s targeted success rate of 85%.
In a separate report, Goffin and colleagues40 published the intermediate-term results of this multicenter study. The study was expanded to include a second arm evaluating the treatment of two adjacent levels. The single-level arm had 103 patients enrolled, with 100 reaching the 1-year mark and 51 reaching 2-year follow-up. The bilevel study arm comprised 43 patients with 1-year data completed on 29 patients and 2-year data available on 1 patient. Success rates in the single-level study at 6 months, 12 months, and 24 months were 90%, 86%, and 90%. In the bilevel study, the success rate at 6 months was 82% and at 1 year was 96%. No device failures or subsidence was observed in any patient. At 1-year follow-up, flexion-extension range of motion per level averaged 7.9 degrees in the single-level arm and 7.4 degrees in the bilevel arm.
Sekhon41 reported early results of nine patients with cervical spondylotic myelopathy who were treated with anterior decompression and reconstruction with the BRYAN disc. Follow-up ranged from 1 to 17 months. On average, the Nurick grade improved by 0.72 points, and NDI scores improved by 51.4 points. Improvement in cervical lordosis was noted in 29% of the patients. No complications were reported.
In another small prospective study, Duggal and colleagues42 reported on 26 patients undergoing single-level or two-level implantation of the BRYAN artificial cervical disc for treatment of cervical degenerative disc disease resulting in radiculopathy or myelopathy or both. Patients were evaluated radiographically and via NDI and SF-36 at regular intervals. Segmental sagittal rotation from C2-3 to C6-7 was measured using quantitative motion analysis software. A total of 30 BRYAN discs were placed in 26 patients. Follow-up ranged from 1.5 to 27 months (mean 12.3 months). A statistically significant improvement in the mean NDI scores was seen between preoperative and late postoperative follow-up evaluations.
Anderson and colleagues33 described the follow-up results of 73 patients who had greater than 2-year follow-up status on a one-level BRYAN disc arthroplasty. Of these patients, 45 were rated as excellent, 7 as good, and 13 as fair. Only eight patients had a poor rating at the 2-year follow-up. SF-36 functional outcome data showed significant improvement from preoperative to 3-month postoperative time points. These outcomes remained stable 24 months after surgery. There was no radiographic evidence of subsidence of implants. 89% of all patients had at least 2 degrees of motion at 1 and 2 years. Average range of motion was 8 degrees. There was one early anterior device migration associated with a partially milled cavity.
This same report noted the results of 30 patients who had two-level disc arthroplasty and had reached the 1-year end point in follow-up. Of the patients, 21 were rated as excellent; 3, good; 5, fair; and 1, poor. A significant improvement in SF-36 functional outcome measures was noted postoperatively. There was no radiographic evidence of subsidence in the two-level patients. At 1 year, 84% of patients had at least 2 degrees of motion at both disc levels. The average amount of motion at each disc level was also 8 degrees. There was one posterior migration of a device, again associated with a partially milled cavity. Complications in the study as a whole included one cerebrospinal fluid leak, one esophageal injury, four hematoma evacuations, and three revision decompressions.33
Sasso and colleagues43,44 studied and reported on a group of patients regarding initial functional outcome results of the BRYAN artificial cervical disc replacement and compared them with fusion for patients with a cervical disc herniation or stenosis causing radiculopathy. Their prospective, three-center, randomized trial analyzed the data from three surgeons involved in the FDA IDE trial of the BRYAN cervical disc. Multiple outcome measures were used, including NDI, neck pain VAS, arm pain VAS, SF-36 physical component, SF-36 mental component, and range of motion flexion-extension.
The most comprehensive data to date stem from the full multicenter cohorts of the U.S. FDA IDE trial published by Heller and colleagues.24 This study presented the analysis of the multicenter groups using similar criteria and outcome measures as previously published in the studies by Sasso and colleagues.44,45 As shown in the prior three-cohort study, the multicenter study with 242 patients enrolled in the investigational group and 221 enrolled in the control group showed statistically greater improvement in many of the primary outcome variables. Although both groups improved compared with preoperative scores, the investigational group had statistically favorable results in several outcomes, including NDI, neck pain, and return to work. Arm pain, SF-36 physical and mental components, and rates of neurologic success, although significantly reduced in both cohorts compared with preoperative levels, did not show significant group-to-group differences.
Anderson and colleagues32 reported on a comparison of adverse events between BRYAN arthroplasty and ACDF. This report is a novel analysis of the 463-patient cohort enrolled in the BRYAN arthroplasty IDE.24 Adverse events were recorded at the intervals prescribed by the IDE study and categorized by severity and as medically or surgically related. Overall, no differences in adverse medical events occurred between groups. Surgically related adverse events were present at a higher rate in the BRYAN cohort than in the control cohort, largely owing to the increased incidence of dysphagia complaints and late medical events that occurred. An increased incidence of more severe events, World Health Organization grade 3 and 4, occurred in the ACDF group, however. This increased incidence was related to the revision treatment of pseudarthrosis and persistent symptoms after the index ACDF. Statistically more operations occurred in the control group. No deaths or deep infections were recorded in either cohort.
MOBI-C Disc
Early clinical results with the MOBI-C disc prosthesis have been reported in several studies.46,47 Kim and colleagues46 reported prospectively on a 23-patient cohort treated for cervical degenerative disc disease with 40 TDRs. Mean age was 43 years (range 31 to 62 years). Statistically significant improvement was observed in VAS arm and neck pain indices at the final follow-up interval of 6 months compared with the preoperative scores. Eight of the 23 patients were treated for an index complaint of cervical myelopathy and were assessed with the JOA myelopathy score. No statistically significant improvement was noted in myelopathy; however, the baseline presenting myelopathy for these 8 cases at the time of presentation was low. All patients were able to return to work within 1 month after surgery. No complications were observed in the entire cohort, and 95.6% of patients rated their outcome as either excellent or good by Odom criteria. Cervical mobility was preserved at the index and adjacent levels at the time of the final 6-month follow-up.
In a second study, Park and colleagues47 retrospectively evaluated 53 patients treated for cervical disc herniations. This study contained arthroplasty (21 patients) and control (ACDF; 32 patients) groups. Patient follow-up was 12 months. Mean operative time was similar in the two groups, as was patient satisfaction at the final follow-up. In both groups, NDI and VAS arm pain improved compared with the preoperative state at 12 months, but the groups showed no statistical difference compared with one another at this interval. Segmental range of motion at the index level was maintained overall at final follow-up. No complications were observed in the arthroplasty group, and no heterotopic ossification was noted.
PCM Disc
Current literature regarding the PCM arthroplasty device stems largely from two clinical publications48,49 and two publications that focus on cadaveric biomechanical50 and caprine biomechanical51 data. From a clinical standpoint, Pimenta and colleagues48 published the first 53 patient experiences in 2004—a single-cohort case series. In this investigation, 82 arthroplasty devices were implanted in 53 patients who were followed with the VAS pain scale, the NDI, the Treatment Intensity Gradient Test (TIGT), and radiographic studies. The average patient age was 45 years. Of patients, 28 received a single-level arthroplasty, 22 received a two-level arthroplasty (6 patients with noncontiguous placement), and 3 received a three-level arthroplasty. At 1 week, 80% of patients had good or excellent results by Odom criteria, improving to 90% at 3 months. One postoperative complication, a device migration with 4 mm of anterior displacement, was noted at the 3-month follow-up interval.
A second prospective, consecutive series of 229 PCM arthroplasty implantations in 140 patients was later reported by Pimenta and colleagues in 2007.49 This study reported the differences between two cohorts: patients with multilevel arthroplasty versus patients with single-level arthroplasty. In the index cohort, 71 patients received single-level arthroplasty; in the investigational cohort, 69 patients underwent 158 arthroplasties (multiple levels per patient). In the multilevel group, 53 patients received two-level arthroplasties, 12 patients received three-level arthroplasties, and 4 patients received four-level arthroplasties. Of the total surgeries, 21 were performed as arthroplasty revision procedures adjacent to a prior ACDF.
CerviCore, DISCOVER, Kineflex, and SECURE-C Devices
At the time of this writing, published clinical data are lacking on the outcomes of use of the CerviCore, DISCOVER, Kineflex, and SECURE-C cervical arthroplasty devices. Each of these devices remains in the process of human IDE trials in the United States. As data from these trials become available, it will be necessary and appropriate to compare these new data with the short-term and intermediate-term data that have been collected from devices farther along in the U.S. FDA approval process or devices with current approval. The design characteristics of these devices are summarized in Table 43–1, which serves as the basis for preliminary comparison of the devices with their peers.
Biomechanics
A primary goal of cervical disc replacement is to reproduce normal kinematics after implantation (see Figs. 43-2 and 43-9). Studies have been conducted to compare the biomechanics of cervical disc replacements on two kinematically different types of devices using identical protocols by the same laboratory.52,53 These studies may suggest which type of implant design would provide more kinematically accurate motion.
DiAngelo and colleagues52,53 investigated two different implant designs in human cadaveric spines to compare the motion of the harvested spine with the implanted spine. One of the designs was a semiconstrained device consisting of a ball-in-trough, which allows anterior-posterior translation (PRESTIGE cervical disc), whereas the other design was a constrained device consisting of a ball-in-socket with no anterior-posterior translation allowance (Prodisc-C). The results of the studies support the design rationale behind the PRESTIGE cervical disc. The semiconstrained device (PRESTIGE cervical disc) provided for normal kinematics in all ranges of motion tested, whereas the constrained device (Prodisc-C) failed to reproduce normal motion in extension.
Sasso and colleagues54 studied the kinematics of cervical motion from patients enrolled in a prospective, randomized, multicenter clinical trial. Radiographic data, including flexion, extension, and neutral lateral radiographs, were obtained preoperatively and at regular postoperative intervals up to 24 months. All patients had received either a single-level anterior cervical plate (Atlantis anterior cervical plate, n = 221) or a single-level artificial cervical disc (BRYAN cervical disc prosthesis, n = 242) at C3 to C7. Cervical vertebral bodies were tracked to calculate the functional spinal unit motion parameters. Parameters measured included flexion-extension range of motion and translation. These data were recorded at the index surgical level and, if visible, at the functional spinal units cranial and caudal to it.
The PCM disc has also undergone bench-top and nonhuman testing published in peer-reviewed literature. McAfee and colleagues50 established that the posterior longitudinal ligament provides a stabilizing influence to the cervical spinal segment. Biomechanical testing was performed using human cadaveric spines and a 6-degree-of-freedom spine simulator with additional optoelectronic motion measurement. The major finding was that biomechanical stability may be restored after complete anterior cervical discectomy with resection of the posterior longitudinal ligament via implantation of an arthroplasty device.
Wear Analysis
Failure of total joint arthroplasty has been largely attributed to wear debris from polymer-bearing surfaces. Wear debris induces an intense cellular inflammatory response, which results in the production of cytokines that cause resorption of bone.55,56 This process, known as aseptic loosening, leads to destabilization and mechanical failure of the joint. Factors that influence the loosening process include particle size, shape, number, material surface chemistry, and concentration and duration of exposure.57–61 The possibility of a similar process occurring after cervical disc replacement is concerning especially given the polymeric bearing surfaces of the several disc designs. In addition, the unknown effects of wear debris and inflammatory response in the spinal canal around neural structures are worrisome.
Anderson and colleagues33,57 published two pivotal studies that reported on the wear characteristics of the BRYAN disc in vitro in a cervical spine simulator and in vivo in goat and chimpanzee models. In the first report, the in vitro results of six discs were tested to 10 million or 40 million cycles in a cervical spine simulator by load and motion, and an additional three assemblies were tested only to load. Wear debris was reportedly produced at a rate of 1.2 mg/1 million cycles. Device heights decreased 0.02 mm/1 million cycles. Debris particles averaged 3.9 µ in diameter, larger than the particles typically observed in hip and knee arthroplasty.
In the second report, Anderson and colleagues33 examined the wear properties of BRYAN disc components in a custom cervical spine simulator and a goat model. The in vitro model involved simultaneous load and motion in six separate device assemblies with three control devices and was similar in design to the prior study.57 Nuclei were measured before and after the test for weight, height, and diameter. Wear debris was assessed with a standardized system. The caprine model was undertaken in a certified animal care facility with anterior discectomy and placement of the BRYAN cervical disc in 11 goats. Four goats underwent a similar discectomy with fusion using allograft and plating with a titanium cervical plate.
Hu and colleagues51 examined the in vivo results of the PCM device at 6 and 12 months in a caprine model. Each goat underwent single-level arthroplasty at C3-4 and was followed thereafter for the prescribed time. At both intervals, the device had no evidence of loosening, subluxation, or inflammatory reaction. CT did not significantly obscure visualization of the spinal canal. Preservation of segmental spinal motion was noted in all specimens in biomechanical testing under load. The bone-prosthesis interface was found to be osteointegrated. Histopathologic review showed no significant wear debris of a particulate nature in local and systemic tissues—no significant pathologic changes were noted in the analyzed tissues.
Conclusion
More recent clinical reports show promising early data suggesting that artificial disc replacement is comparable to fusion at least in the short-term.23–25,31,45 Wear studies suggest that there may be less potential for aseptic loosening than in large joint arthroplasty, although the reality of this will be borne out only with more follow-up time. Although early reports of success in the United States with the TDR have allowed several devices to gain FDA approval23,25 and suggest that the intended effects are being achieved, the final results of arthroplasty with these and other devices are pending the outcomes of long-term studies.
Pearls
Key Points
1 Mummaneni PV, Burkus JK, Haid RW, et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: A randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198-209.
2 Heller JG, Sasso RC, Papadopoulos SM, et al. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: Clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976). 2009;34:101-107.
3 Murrey D, Janssen M, Delamarter R, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275-286.
4 Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519-528.
5 Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298-1307.
1 Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298-1307.
2 Campbell MJ, Carreon LY, Traynelis V, et al. Use of cervical collar after single-level anterior cervical fusion with plate: Is it necessary? Spine (Phila Pa 1976). 2009;34:43-48.
3 Goffin J, Geusens E, Vantomme N, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79-85.
4 Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519-528.
5 Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: A prospective 2-year study. J Neurosurg Spine. 2005;3:417-423.
6 Goffin J, van LJ, Van CF, et al. Long-term results after anterior cervical fusion and osteosynthetic stabilization for fractures and/or dislocations of the cervical spine. J Spinal Disord. 1995;8:500-508. discussion 499
7 Eck JC, Humphreys SC, Lim TH, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine (Phila Pa 1976). 2002;27:2431-2434.
8 Fuller DA, Kirkpatrick JS, Emery SE, et al. A kinematic study of the cervical spine before and after segmental arthrodesis. Spine (Phila Pa 1976). 1998;23:1649-1656.
9 Sandhu HS, Grewal HS, Parvataneni H. Bone grafting for spinal fusion. Orthop Clin North Am. 1999;30:685-698.
10 Brown CA, Eismont FJ. Complications in spinal fusion. Orthop Clin North Am. 1998;29:679-699.
11 Summers BN, Eisenstein SM. Donor site pain from the ilium: A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677-680.
12 Mroz TE, Joyce MJ, Lieberman IH, et al. The use of allograft bone in spine surgery: Is it safe? Spine J. 2009;9:303-308.
13 Brodke DS, Zdeblick TA. Modified Smith-Robinson procedure for anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 1992;17:S427-S430.
14 Baskin DS, Ryan P, Sonntag V, et al. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine (Phila Pa 1976). 2003;28:1219-1225. discussion 1225
15 Lanman TH, Hopkins TJ. Early findings in a pilot study of anterior cervical interbody fusion in which recombinant human bone morphogenetic protein-2 was used with poly(L-lactide-co-D,L-lactide) bioabsorbable implants. Neurosurg Focus. 2004;16:E6.
16 Smucker JD, Rhee JM, Singh K, et al. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976). 2006;31:2813-2819.
17 Vaidya R, Sethi A, Bartol S, et al. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21:557-562.
18 Vaidya R, Carp J, Sethi A, et al. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257-1265.
19 Shields LB, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976). 2006;31:542-547.
20 Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003;28:134-139.
21 St John TA, Vaccaro AR, Sah AP, et al. Physical and monetary costs associated with autogenous bone graft harvesting. Am J Orthop. 2003;32:18-23.
22 Cummins BH, Robertson JT, Gill SS. Surgical experience with an implanted artificial cervical joint. J Neurosurg. 1998;88:943-948.
23 Mummaneni PV, Burkus JK, Haid RW, et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: A randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198-209.
24 Heller JG, Sasso RC, Papadopoulos SM, et al. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: Clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976). 2009;34:101-107.
25 Murrey D, Janssen M, Delamarter R, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275-286.
26 Bartels RH, Donk R. Fusion around cervical disc prosthesis: Case report. Neurosurgery. 2005;57:E194.
27 Leung C, Casey AT, Goffin J, et al. Clinical significance of heterotopic ossification in cervical disc replacement: A prospective multicenter clinical trial. Neurosurgery. 2005;57:759-763.
28 Parkinson JF, Sekhon LH. Cervical arthroplasty complicated by delayed spontaneous fusion: Case report. J Neurosurg Spine. 2005;2:377-380.
29 Heidecke V, Burkert W, Brucke M, et al. Intervertebral disc replacement for cervical degenerative disease—clinical results and functional outcome at two years in patients implanted with the Bryan cervical disc prosthesis. Acta Neurochir (Wien). 2008;150:453-459.
30 Mehren C, Suchomel P, Grochulla F, et al. Heterotopic ossification in total cervical artificial disc replacement. Spine (Phila Pa 1976). 2006;31:2802-2806.
31 Mummaneni PV, Robinson JC, Haid RWJr. Cervical arthroplasty with the PRESTIGE LP cervical disc. Neurosurgery. 2007;60:310-314.
32 Anderson PA, Sasso RC, Riew KD. Comparison of adverse events between the Bryan artificial cervical disc and anterior cervical arthrodesis. Spine (Phila Pa 1976). 2008;33:1305-1312.
33 Anderson PA, Sasso RC, Rouleau JP, et al. The Bryan Cervical Disc: Wear properties and early clinical results. Spine J. 2004;4(6 Suppl):303S-309S.
34 Sekhon LH, Duggal N, Lynch JJ, et al. Magnetic resonance imaging clarity of the Bryan, Prodisc-C, Prestige LP, and PCM cervical arthroplasty devices. Spine (Phila Pa 1976). 2007;32:673-680.
35 Wigfield CC, Gill SS, Nelson RJ, et al. The new Frenchay artificial cervical joint: Results from a two-year pilot study. Spine (Phila Pa 1976). 2002;27:2446-2452.
36 Wigfield C, Gill S, Nelson R, et al. Influence of an artificial cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosurg Spine. 2002;96:17-21.
37 Porchet F, Metcalf NH. Clinical outcomes with the Prestige II cervical disc: Preliminary results from a prospective randomized clinical trial. Neurosurg Focus. 2004;17:E6.
38 Bertagnoli R, Duggal N, Pickett GE, et al. Cervical total disc replacement, part two: Clinical results. Orthop Clin North Am. 2005;36:355-362.
39 Goffin J, Casey A, Kehr P, et al. Preliminary clinical experience with the Bryan Cervical Disc Prosthesis. Neurosurgery. 2002;51:840-845. discussion 845-847
40 Goffin J, Van CF, van LJ, et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan Cervical Disc Prosthesis: Single-level and bi-level. Spine (Phila Pa 1976). 2003;28:2673-2678.
41 Sekhon LH. Cervical arthroplasty in the management of spondylotic myelopathy. J Spinal Disord Tech. 2003;16:307-313.
42 Duggal N, Pickett GE, Mitsis DK, et al. Early clinical and biomechanical results following cervical arthroplasty. Neurosurg Focus. 2004;17:E9.
43 Sasso RC, Foulk DM, Hahn M. Prospective, randomized trial of metal-on-metal artificial lumbar disc replacement: Initial results for treatment of discogenic pain. Spine (Phila Pa 1976). 2008;33:123-131.
44 Sasso RC, Smucker JD, Hacker RJ, et al. Artificial disc versus fusion: A prospective, randomized study with 2-year follow-up on 99 patients. Spine (Phila Pa 1976). 2007;32:2933-2940.
45 Sasso RC, Smucker JD, Hacker RJ, et al. Clinical outcomes of BRYAN cervical disc arthroplasty: A prospective, randomized, controlled, multicenter trial with 24-month follow-up. J Spinal Disord Tech. 2007;20:481-491.
46 Kim SH, Shin HC, Shin DA, et al. Early clinical experience with the Mobi-C disc prosthesis. Yonsei Med J. 2007;48:457-464.
47 Park JH, Roh KH, Cho JY, et al. Comparative analysis of cervical arthroplasty using Mobi-C and anterior cervical discectomy and fusion using the solis cage. J Korean Neurosurg Soc. 2008;44:217-221.
48 Pimenta L, McAfee PC, Cappuccino A, et al. Clinical experience with the new artificial cervical PCM (Cervitech) disc. Spine J. 2004;4:315S-321S.
49 Pimenta L, McAfee PC, Cappuccino A, et al. Superiority of multilevel cervical arthroplasty outcomes versus single-level outcomes: 229 consecutive PCM prostheses. Spine (Phila Pa 1976). 2007;32:1337-1344.
50 McAfee PC, Cunningham B, Dmitriev A, et al. Cervical disc replacement-porous coated motion prosthesis: A comparative biomechanical analysis showing the key role of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2003;28:S176-S185.
51 Hu N, Cunningham BW, McAfee PC, et al. Porous coated motion cervical disc replacement: A biomechanical, histomorphometric, and biologic wear analysis in a caprine model. Spine (Phila Pa 1976). 2006;31:1666-1673.
52 DiAngelo DJ, Robertson JT, Metcalf NH, et al. Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech. 2003;16:314-323.
53 DiAngelo DJ, Foley KT, Morrow BR, et al. In vitro biomechanics of cervical disc arthroplasty with the ProDisc-C total disc implant. Neurosurg Focus. 2004;17:E7.
54 Sasso RC, Best NM, Metcalf NH, et al. Motion analysis of Bryan cervical disc arthroplasty versus anterior discectomy and fusion: Results from a prospective, randomized, multicenter, clinical trial. J Spinal Disord Tech. 2008;21:393-399.
55 Goodman SB, Lind M, Song Y, et al. In vitro, in vivo, and tissue retrieval studies on particulate debris. Clin Orthop Relat Res. 1998;352:25-34.
56 Goodman SB, Huie P, Song Y, et al. Cellular profile and cytokine production at prosthetic interfaces: Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br. 1998;80:531-539.
57 Anderson PA, Rouleau JP, Bryan VE, et al. Wear analysis of the Bryan Cervical Disc prosthesis. Spine (Phila Pa 1976). 2003;28:S186-S194.
58 Gelb H, Schumacher HR, Cuckler J, et al. In vivo inflammatory response to polymethylmethacrylate particulate debris: Effect of size, morphology, and surface area [erratum appears in J Orthop Res 12(4):598, 1994]. J Orthop Res. 1994;12:83-92.
59 Allen MJ, Myer BJ, Millett PJ, et al. The effects of particulate cobalt, chromium and cobalt-chromium alloy on human osteoblast-like cells in vitro. J Bone Joint Surg Br. 1997;79:475-482.
60 Gonzalez O, Smith RL, Goodman SB. Effect of size, concentration, surface area, and volume of polymethylmethacrylate particles on human macrophages in vitro. J Biomed Mater Res. 1996;30:463-473.
61 Goodman SB, Fornasier VL, Kei J. The effects of bulk versus particulate ultra-high-molecular-weight polyethylene on bone. J Arthroplasty. 1988;3(Suppl)):S41-S46.
62 Anderson PA, Sasso RC, Riew KD. Update on cervical artificial disk replacement. Instr Course Lect. 2007;56:237-245.
63 Goffin J. Complications of cervical disc arthroplasty. Semin Spine Surg. 2006;18:87-98.