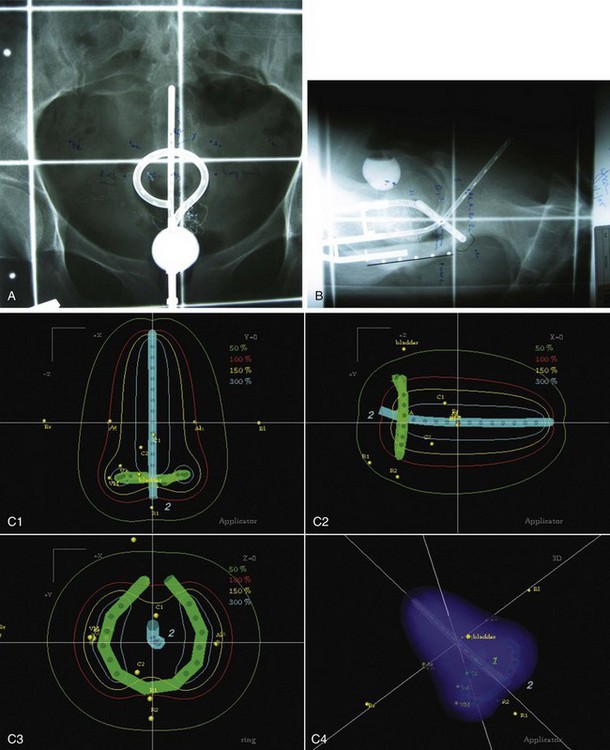
Chapter 56 Cervical Cancer
The incidence of and mortality rates from invasive cervical cancer have steadily declined in the United States over the past five decades, in part because of the successful implementation of screening programs that detect many cancers at a preinvasive stage. Unfortunately, cytologic screening programs have failed to comprehensively penetrate the population, with many medically underserved or uneducated communities at risk. Invasive cervical cancer continues to be a major public health problem internationally. In the United States, 60% of women with newly diagnosed invasive cervical cancers have not had a Papanicolaou (Pap) smear for 5 years or longer.1
Epidemiology and Etiology
In the United States, cervical cancer is responsible for about 2% of the cancer deaths in women, with about 12,200 new cases of invasive disease and 4210 deaths in 2010.2 Worldwide, invasive cervical cancer is the third most common malignant tumor in women (after breast and colorectal cancers) and accounts for nearly 371,000 cases and 190,000 deaths per year.3 Incidence rates range from as low as 3 to 4 per 100,000 in Israel to more than 80 per 100,000 in Recife, Brazil.4 Presumably, this wide range reflects a variation in epidemiologic risk factors that is compounded by the lack of adequate screening programs in poor communities. The relatively high international mortality rates may also reflect the greater number of women who present with advanced disease when screening programs and patient education are inadequate and access to medical resources is limited or nonexistent. Because of the lack of cancer registries, it is likely that reported incidence and mortality rates grossly underestimate the magnitude of the problem.
Risk factors for the development of carcinoma of the cervix and its intraepithelial precursors follow a pattern typical of sexually transmitted diseases. These include first coitus at a young age, multiple sexual partners, a history of other sexually transmitted diseases, and high parity.5 Weaker associations have been suggested with cigarette smoking and the use of oral contraceptives. In contrast, the risk of developing cervical cancer is low in women who are nulliparous or virginal. Among women with only one lifetime sexual partner, past and current high-risk sexual behavior by the male partner has a substantial role in the development of cervical carcinogenesis.6 Conversely, male circumcision is associated with a reduced prevalence of penile HPV infection and a reduced risk of cervical cancer in the current sexual partners.7
Until recently, the reasons for these associations were only the subject of speculation. Today, however, the epidemiologic findings, combined with the results of molecular studies (discussed in detail later), are sufficient to identify sexually acquired human HPV infection as the primary etiologic agent in the development of most cervical cancers.8,9
Although the epidemiology of cervical adenocarcinoma is somewhat less well understood, studies also reveal the presence of HPV DNA in most cases.10 It is interesting that in the United States, the absolute and relative incidence of adenocarcinoma appears to have increased in contrast to the steady decrease in the rate of invasive squamous carcinoma. This may reflect, in part, differences in the effectiveness of cytologic screening in detecting adenocarcinoma at a preinvasive stage.11 Some investigators have implicated oral contraceptives as a possible risk factor for adenocarcinoma, but this remains controversial.12
Clear cell carcinoma, a rare form of cervical and vaginal adenocarcinomas, has been clearly linked with prenatal exposure to diethylstilbestrol (DES), a drug that was used to prevent miscarriages in the 1940s and 1950s.13 Prenatal DES exposure arrests development of the transformation zone in the upper third of the vagina, which accounts for the location of these lesions. The average age at which cancer in DES-exposed patients was diagnosed was 19 years, much younger than the average age of patients with newly diagnosed squamous carcinoma or non–DES-related adenocarcinoma. New diagnoses of DES-related clear cell carcinoma have declined now that the youngest cohort of DES-exposed patients has passed the age of peak incidence.
Prevention and Early Detection
Although there have been significant changes in the fields of radiation and gynecologic oncology, the dramatic decrease in cervical cancer mortality from the 1940s to the 1980s is primarily due to the success of mass screening programs.14,15 The cervix is an ideal target for cancer screening because of its accessibility, the long average time interval from the initial DNA insult to the development of invasive cancer, and the high cure rate with appropriate treatment of preinvasive and early invasive lesions.
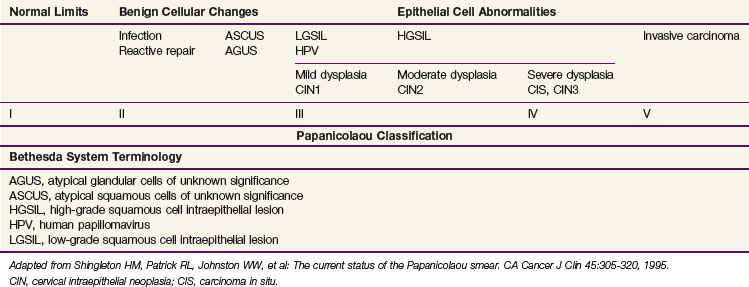
Squamous cell carcinomas originate at the squamocolumnar junction (transformation zone) of the cervix. Invasive lesions are frequently associated with adjacent preinvasive or in situ disease (low-grade squamous cell intraepithelial lesion [LGSIL] or high-grade squamous cell intraepithelial lesion [HGSIL]) (Table 56-1).
Longitudinal studies suggest a long average time to progression from HGSIL to invasive disease. Peterson16 described 127 patients with carcinoma in situ (CIS) (HGSIL) who were followed for at least 3 years. At the end of 10 years, invasive carcinoma had developed in about 30%. Koss and colleagues17 observed spontaneous regression of 25% in 67 patients with untreated CIS (HGSIL) who were observed for 3 years. In a later analysis of this series, the authors reported that invasive lesions had developed in 40% of the original 67 patients.18 In another longitudinal study of patients with untreated CIS (HGSIL), Kottmeier19 observed progression to invasive carcinoma in 71% and 80% of patients followed for 12 and 30 years, respectively.
Longitudinal studies have also demonstrated a relatively long time between the diagnosis of dysplasia and the development of HGSIL. One large prospective study reported mean times for progression to HGSIL development of 58, 38, and 12 months for patients with mild, intermediate, or severe dysplasia, respectively.20 These results are consistent with the finding that the mean age of women diagnosed with cervical intraepithelial neoplasia (HGSIL) is about 16 years younger than that of women diagnosed with invasive carcinoma.21
Although a number of classifications have been used, the Bethesda system is currently the most widely accepted method of categorizing cervical cytologic specimens in the United States.22 The relationship between this system and earlier classification systems is outlined in Table 56-2.
TABLE 56-2 International Federation of Gynecology and Obstetrics Staging of Carcinoma of the Cervix
| Stage I | The carcinoma is strictly confined to the cervix (extension to the corpus should be disregarded). |
| Stage IA | Invasive cancer identified only microscopically. All gross lesions, even with superficial invasion, are stage IB cancers. |
| Invasion is limited to measured stromal invasion with a maximum depth of 5 mm and no wider than 7 mm. (The depth of invasion should not be more than 5 mm taken from the base of the epithelium, either surface or glandular, from which it originates. Vascular space involvement, either venous or lymphatic, should not alter the staging.) | |
| Stage IA1 | Measured invasion of stroma no greater than 3 mm in depth and no wider than 7 mm. |
| Stage IA2 | Measured invasion of stroma greater than 3 mm and no greater than 5 mm in depth and no wider than 7 mm. |
| Stage IB | Clinical lesions confined to the cervix or preclinical lesions greater than IA. |
| Stage IB1 | Clinical lesions no greater than 4 cm in size. |
| Stage IB2 | Clinical lesions greater than 4 cm in size. |
| Stage II | The carcinoma extends beyond the cervix but has not extended onto the pelvic wall; the carcinoma involves the vagina but not as far as the lower third. |
| Stage IIA | No obvious parametrial involvement. |
| Stage IIA1 | Clinical lesions no greater than 4 cm in size. |
| Stage IIA2 | Clinical lesions greater than 4 cm in size. |
| Stage IIB | Obvious parametrial involvement. |
| Stage III | The carcinoma has extended onto the pelvic wall; on rectal examination there is no cancer-free space between the tumor and the pelvic wall; the tumor involves the lower third of the vagina; all cases with a hydronephrosis or nonfunctioning kidney should be included, unless they are known to be due to another cause. |
| No extension onto the pelvic wall but involvement of the lower third of the vagina. | |
| Stage IIIA | Extension onto the lower third of the vagina. |
| Stage IIIB | Extension onto the pelvic wall or hydronephrosis or nonfunctioning kidney. |
| Stage IV | The carcinoma has extended beyond the true pelvis or has clinically involved the mucosa of the bladder or rectum. |
| Stage IVA | Spread of the growth to adjacent organs. |
| Stage IVB | Spread to distant organs. |
From Pecorelli S, Zigliani L, Odicino F: Special communication. Revised FIGO staging for carcinoma of the cervix, Int J Gynecol Obstet 105:107-108, 2009.
The American College of Obstetricians and Gynecologists (ACOG) recommends that cervical cancer screening should begin at age 21 years regardless of age of onset of sexual intercourse. Cervical cytologic screening is recommended every 2 years for women aged 21 to 29 years, with either conventional or liquid-based methods. Women aged 30 years and older with three consecutive cervical cytologic test results may be screened every 3 years. Women with human immunodeficiency virus (HIV) infection, those who are immunosuppressed, those who have been exposed in utero to DES, or those who have been previously treated for cervical intraepithelial neoplasia (CIN) stage II or III or cancer may require more frequent screening. HPV DNA testing may be used as an adjunct or even a replacement for cytologic screening in women older than 30 years; women in this age-group with both negative cytologic and negative HPV DNA test results may be screened every 3 years. Cytologic screening may be discontinued in women after hysterectomy for benign indications (with no prior history of high-grade CIN) or at age 70 years if three consecutive cytologic screening test results have been negative and no abnormal test results have been seen in the previous 10 years.23 As compared with annual screening, mathematical modeling predicts the excess risk of cervical cancer with less frequent screening intervals to be approximately 3 in 100,000.24
Despite the proven efficacy of cytologic screening, many women remain unscreened. In the United States, 50% of women with newly diagnosed invasive cervical cancer have never had a Pap smear and another 10% have not had a Pap smear in 5 years.9 Women who tend to be underscreened in the United States fall into groups of postmenopausal, uninsured, ethnic minority, and, especially, elderly black and poor women in rural areas. In the United States, about 25% of the cervical cancer cases and 41% of the deaths occur in women who are aged 65 years or older. In addition, nearly 50% of women older than 60 years have not had a Pap smear in 3 years, even though many have seen a physician for other medical reasons.
Pathology and Pathways of Spread
Eighty percent to 90% of cervical cancers are squamous cell lesions. Squamous cell neoplasms are frequently subcategorized as either large cell keratinizing, large cell nonkeratinizing, or small cell carcinomas.25,26 The latter should not be confused with anaplastic small cell carcinomas, which have histologic features that resemble small cell neuroendocrine neoplasms of the lung and tend to have a particularly aggressive clinical course.27,28
The frequency of primary cervical adenocarcinoma is about 10% to 20%, but it appears to have been increasing recently, particularly in younger women.29,30 Cervical adenocarcinomas can originate high in the endocervical canal and can be missed with standard cytologic screening collection methods. The perceived increased frequency of cervical adenocarcinomas may be attributed to a decreased incidence of squamous cell carcinomas in the screened population without a concomitant decreased incidence of cervical adenocarcinomas. Whether conventional screening methods are insensitive to the detection of adenocarcinoma precursor lesions or whether such precursor lesions progress more quickly to invasive disease has not been determined. Nonetheless, women who participate in a cytologic screening program and develop cervical adenocarcinoma are more likely to have early-stage disease than an unscreened cohort. Most cervical adenocarcinomas are mucinous with features suggestive of endocervical glandular epithelium; about 20% demonstrate other müllerian neoplastic patterns. Others may be adenosquamous in type.
The cervix is richly supplied with lymphatics organized into three anastomosing plexuses that drain the mucosal, muscularis, and serosal layers.31 The most important lymphatic collecting trunks exit laterally from the uterine isthmus in three groups31,32: the upper branches originate in the anterior and lateral cervix and follow the uterine artery, the middle branches drain to the deeper hypogastric (obturator) nodes, and the lowest branches drain posteriorly to the inferior and superior gluteal, common iliac, presacral, and subaortic lymph nodes.
The incidences of pelvic lymph node involvement for patients with FIGO stages IB, IIB, and IIIB cervical cancer are approximately 15%, 30%, and 50%, respectively.33–36 The incidence of para-aortic lymph node metastasis also increases with tumor stage; about 5%, 20%, and 30% of patients with FIGO stage IB, IIB, and IIIB disease, respectively, have para-aortic lymph node metastasis at diagnosis.33 For each stage, the risk of lymph node involvement is further correlated with tumor size.37,38
Hematogenous metastases are rarely detectable at diagnosis, and recurrent pelvic disease is seen in about two thirds of patients who relapse after treatment. Distant metastases are also a common feature of disease relapse, however. In a study of disease relapse patterns from the Mallinckrodt Institute of Radiology, Fagundes and associates39 reported 10-year actuarial rates for distant metastasis of 16%, 31%, 26%, and 39% for patients treated with radiation for FIGO stages IB, IIA, IIB, and III disease, respectively. These may well be underestimated if pelvic disease is detected as the first site of relapse because systematic radiologic evaluation may not be performed. The most common site of extrapelvic metastasis was the lungs, followed by the para-aortic lymph nodes. Although the lumbar spine has been reported to be a relatively frequent site of skeletal metastasis, studies that include CT scanning suggest that patients who appear to have had metastases isolated to the lumbar spine may actually have had direct tumor extension from para-aortic disease.40
Prognostic Factors
With the exception of distant hematogenous metastases, the presence of retroperitoneal nodal involvement represents the most significant negative prognostic factor in patients with cervical cancer.34,41 The evaluation of other prognostic factors, however, is to a great degree predicated on whether patients have early-stage tumors that are often managed surgically or more locally advanced cancers that have received definitive chemoradiation. The availability of a hysterectomy specimen for full pathologic review allows finer assessment of primary associated and extrauterine risk factors, although these factors may be superseded in larger tumors with obvious extracervical extension.
In a prospective surgicopathologic evaluation of 645 patients with clinical stage IB disease who underwent radical hysterectomy and pelvic lymphadenectomy, independent prognostic factors for disease-free survival included pelvic nodal metastases, clinical tumor size (within stage IB classification), capillary-lymphatic space invasion, and relative and absolute depth of tumor infiltration into the cervical stroma.34
In more advanced lesions, a multivariate analysis of 642 patients entered into three Gynecologic Oncology Group (GOG) prospective clinical trials of definitive radiotherapy demonstrated that the presence of positive para-aortic nodes was the single most important independent predictor for relapse and survival, overwhelming all other risk factors. The next two most important prognostic factors (pelvic nodal involvement and tumor size) retained significance only in the absence of para-aortic metastases. Other weaker prognostic variables included clinical stage, patient age, and performance status. In this analysis of more advanced tumors, cell type, histologic grade, and pretreatment hematocrit and peritoneal cytologic findings were not significant prognostic factors when the preceding variables were taken into account.41
Molecular Biology
HPV is a small, double-stranded DNA virus that belongs to the papovavirus group. To date, more than 80 different strains of HPV have been isolated using polymerase chain reaction analysis.42 HPV infection occurs in the basal cell layer of the epithelium, which becomes a continuous reservoir of HPV DNA as the viral genome replicates itself in the dividing cells. As the epithelial cells mature, viral DNA replication continues in the absence of basal cell division, increasing the HPV DNA copy number per cell.43 Microscopically, this process is associated with koilocytosis, a finding considered pathognomonic of HPV infection.44
Of the many strains that have been characterized, HPV-16 and HPV-18 have been most commonly associated with squamous cell carcinoma and adenocarcinoma, respectively.45,46 HPV-31, HPV-33, and HPV-35 have also been associated with malignancy, whereas HPV-6 and HPV-11 are usually associated with benign viral condyloma or mild dysplastic epithelial changes such as CIN1.45,47–52
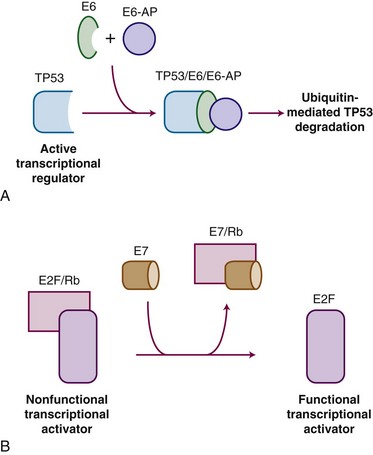
HPV carcinogenesis is mediated through oncogenes E6 and E7. Transgenic mouse experiments provide some of the strongest in vivo evidence linking E6 and E7 expression to the development of cervical cancer.53,54 Figure 56-1 outlines the mechanisms by which HPV influences oncogenic activity. The E6 protein of HPV oncogenes binds with the E6-associated protein (E6-AP) to the TP53 protein, which results in the degradation of TP53, an important negative regulator of cell growth. Thus, by binding and degrading the TP53 protein, E6 proteins contribute to the pathogenesis of cancer by removing the critical and protective function of TP53.55 The E6 proteins of oncogenes from a carcinogenic HPV strain, such as HPV-16 and HPV-18, bind TP53 more efficiently than oncogenes from HPV strains not involved in carcinogenesis, which possibly explains the differences in oncogenicity of these strains.56 By itself, E6 is not capable of inducing transformation, but it induces immortalization of primary human keratinocytes in conjunction with E7, the protein of which binds to and functionally inactivates the gene product of the retinoblastoma (RB) tumor suppressor gene. This binding results in the uncontrolled release of active transcription factors (E2F) and unregulated progression through the cell cycle. Biologic differences between low-risk and high-risk HPV viruses include observations that the latter are capable of inducing chromosome abnormalities in normal keratinocytes and are more likely to interfere with cell cycle regulatory proteins and checkpoints.57
The evidence implicating HPV in the pathogenesis of cervical cancer is conclusive. It includes (1) multiple epidemiologic studies demonstrating HPV infection as the most important risk factor for the development of squamous cell intraepithelial lesions and cervical carcinomas, (2) detection of HPV DNA in more than 90% of cervical cancers and their precursor lesions, (3) evidence of HPV transcriptional activity in neoplastic tissues, and (4) evidence that HPV oncogenes can mediate malignant transformation in transgenic mice.53,54 The precise rate of development for both low- and high-grade squamous intraepithelial lesions in women infected with HPV is unknown. Longitudinal studies have demonstrated that the incidence of HPV infections among cytologically normal sexually active young women is high.58 Most women infected with oncogenic HPV eliminate the infection and are at low or no risk for developing cervical neoplasia. The median duration of most new HPV infections is less than 1 year.58 More than one-third of women remain consistently or intermittently positive for HPV DNA, and many of these women will subsequently test positive for an HPV type that is different from the original HPV type.59 The transient nature of HPV infections in younger women substantiates the common observation that low-grade cervical neoplasia frequently regresses to normal.60
Theoretically, other cofactors may interact with HPV to attenuate the body’s immune response, promote persistent HPV infection, up-regulate E6/E7 expression, or enhance the genetic damage caused by HPV oncogene expression. Possible factors include smoking, oral contraceptive use, and infection with HIV.61 Several case-control studies have linked smoking behavior to an increased risk of cervical neoplasia.62,63 Carcinogens found in cigarette smoke are concentrated in cervical mucus,64 and women who smoke have decreased numbers of antigen-presenting cells in cervical epithelium.65 Thus smoking may increase the risk for cervical cancer via changes in local immunity. Immunosuppression has long been recognized as a risk factor for the development of cervical carcinoma.66 A positive link between oral contraceptive use and cervical cancer, however, is still uncertain.67 Although other cofactors may be involved, the acquisition and persistence of HPV infection are critical to the development of cervical neoplasia.
With this knowledge, specific vaccines could be developed. Koutsky and colleagues68 reported results from the first prospective, double-blind, placebo-controlled study of a monovalent HPV-16 virus-like vaccine. With a median duration of follow-up of 17 months, the incidence of persistent HPV-16 infection was 3.8 per 100 woman-years at risk in the placebo group versus 0 per 100 woman-years in the vaccine group.68
Subsequent, longer-term studies have confirmed the efficacy in preventing infection of HPV vaccines and their safety. A randomized, double-blind phase II study using a quadrivalent HPV (types 6, 11, 16, and 18) vaccine showed that the combined incidence of HPV infection or genital tract disease fell by 90% in those receiving prophylactic vaccine.69 Another long-term follow-up study of patients participating in a double-blind, randomized, placebo-controlled trial using a bivalent HPV (types 16 and 18) vaccine showed that, up to 4.5 years, the vaccine was highly immunogenic and safe, and induced a high degree of protection (100%) against HPV-16 or HPV-18 infection and associated cervical lesions.70 The quadrivalent and bivalent vaccines are both approved by the Food and Drug Administration (FDA) for women 9 to 26 years of age and are commercially available for intramuscular injection (three-dose sequence at 0, 2, and 6 months).
Using mathematical models, Kim and Goldie71 predicted that the cost-effectiveness of HPV vaccination programs in the United States will ultimately depend upon the duration of vaccine immunity and will be optimized by achieving high coverage in adolescent girls, targeting catch-up efforts for women up to 18 or 21 years of age, and revising screening practices. Unfortunately, research has shown that targeted approaches to HPV vaccination based on specific risk factors is a poor strategy for implementation, and, therefore, comprehensive strategies are recommended.72
Clinical Manifestations, Patient Evaluation, and Staging
Staging and Patient Evaluation
The current clinical staging system for cervical cancer by the International Federation of Gynecology and Obstetrics (FIGO) was updated in 200973,74 and is detailed in Table 56-2. The only changes made from the previous 1994 classification were the elimination of stage 0 (preinvasive disease) and the subdivision of stage IIA into IIA1 (tumor ≤4 cm in size) and IIA2 (tumor >4 cm). The subdivision of stage IIA parallels that of stage IB disease and reflects a partial appreciation of the prognostic impact of tumor size.
According to FIGO, only the results of palpation, inspection, colposcopy, endocervical curettage, hysteroscopy, cytoscopy, proctoscopy, intravenous urography, and plain films of the lungs and skeleton can be used to influence stage assignment. Suspected bladder or rectal involvement should be confirmed by biopsy. Because sophisticated imaging is unavailable for many in developing countries with a high incidence of locally advanced disease, FIGO staging clearly precludes the use of other imaging studies, such as lymphangiography; CT, MRI, or PET scanning; or the findings of operative procedures, including laparoscopy or open lymphadenectomy. These cannot be used to change the stage assignation. However, if CT is obtained, the urographic findings may be used to rule out hydronephrosis. The results of these other tests are of prognostic value and often influence treatment decisions, but institutions that publish reports on cervical cancer must adhere strictly to FIGO’s rules for staging to enable comparisons using consistent data among studies. Although FIGO continues to depend on a predominantly clinical staging approach, it recognizes other clinicopathologic risk factors, such as lymphovascular space invasion and lymph node status, and advises that these features, although not affecting staging per se, be recorded when determined. FIGO also acknowledges the potential benefits of contemporary imaging studies and surgical staging, where available, in helping guide treatment.74
Surgical staging of the pelvic and para-aortic lymph nodes has been used to obtain prognostic information, to identify patients who would benefit from extended-field irradiation, and to debulk grossly enlarged lymph nodes. Early reports of high complication rates in patients treated with radiation following transperitoneal lymphadenectomy discouraged the use of this procedure,75,76 but more recent studies have documented a significantly lower incidence of major complications if the operation is performed using a retroperitoneal approach.77 Laparoscopic biopsy of pelvic and aortic lymph nodes is less invasive, with shorter times to recuperation and less potential for adhesions, thereby decreasing the potential for treatment-related bowel toxicity.78,79 Using a variety of surgical approaches, Goff and colleagues80 reported that pretreatment surgical staging resulted in modifications in planned radiation therapy in 43% of patients. The value of pretreatment surgical debulking of tumor-involved lymph nodes is unproven, and although the information gained from surgical staging may be valuable in selected patients, its routine use should probably be limited to investigational settings.81
Other studies used to assess the extent of lymph node involvement include CT, MRI, lymphangiography, and PET. Lymphangiography has previously been used to assist the design of radiation fields; although it was determined to be a good imaging technique for assessing the status of the para-aortic lymph nodes,9 it is no longer available in most medical communities.
MRI of the pelvis is a relatively new modality that has been increasingly used to evaluate local tumor extent.82 MRI provides better definition of the extent of disease within the cervix than CT and may be valuable in detecting extension through the periphery of the cervix; it therefore may be particularly useful in assessing patients with disease confined to the cervix for whom surgery is being considered as primary treatment.83 MRI may also be useful for detecting extension into the uterine body. Pelvic MRI and CT are both useful for designing radiation portals, particularly in defining the position of the draining lymph nodes and, therefore, the borders of and shielding on conformal radiation fields.83,84 There appears to be no advantage with MRI over CT for detecting involved nodes.
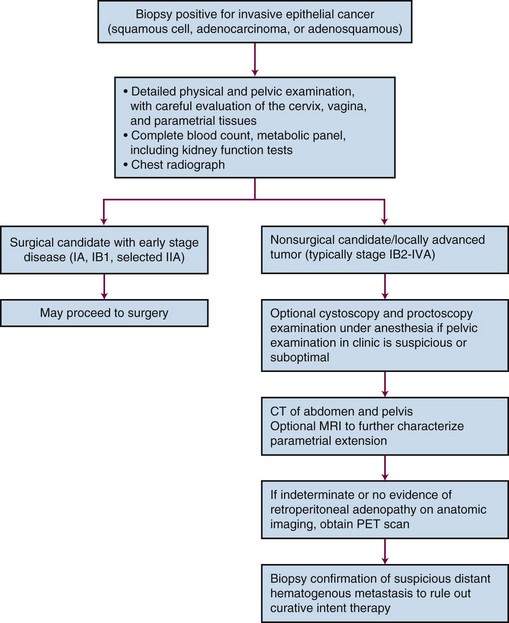
PET scanning has been compared with CT scanning for assessing lymph node status prior to planned radiation therapy. Grigsby and associates85 compared PET with CT in a retrospective study of 101 consecutive patients with newly diagnosed cervical carcinoma. CT demonstrated enlarged pelvic lymph nodes in 20% and aortic lymph nodes in 7% of patients. PET demonstrated abnormal uptake in pelvic lymph nodes in 67% and in aortic lymph nodes in 21% of patients. Surgical staging was not routinely performed to verify the accuracy of imaging findings. A multivariate analysis showed that the detection of positive aortic lymph nodes by PET imaging was the most important prognostic factor for progression-free survival. Yen and colleagues86 conducted a prospective evaluation of PET versus MRI and/or CT staging of patients with newly diagnosed (35%) or recurrent (65%) cervical cancer. Verification of lesions identified on imaging was obtained via surgical biopsy or clinical follow-up. Although its diagnostic accuracy was similar for local lesions, PET was superior to both MRI and CT in identifying metastatic lesions. As a follow-up to their previous retrospective study,87 investigators from the Mallinckrodt Institute of Radiology at Washington University have recently published a large prospective series of 560 cervical cancer patients who underwent pretreatment imaging with fluorodeoxyglucose (FDG) combined with PET. Overall, 47% of these unselected patients had lymph node involvement on FDG-PET, and the prognosis following therapy was highly correlated with positive lymph nodes as shown on PET, as well as the level of involved nodes.87 As of January 2005, the Centers for Medicare and Medicaid Services (CMS) has approved the use of PET scanning in the evaluation of newly diagnosed, locally advanced cervical cancer as an adjunct to conventional anatomic imaging. Its utility in staging early disease is limited, particularly due to the relatively low incidence of extrapelvic disease in this setting. A suggested approach in the workup of newly diagnosed cervical cancer is shown in Figure 56-2.
Of significant interest, but still requiring wider validation, is the potential use of FDG-PET imaging early (at 3 months) after completion of radiotherapy for early assessment of treatment efficacy, as well as possible identification of isolated failures amenable to aggressive salvage therapy.88
Primary Therapy
Because patients with cervical cancer usually present with disease that is clinically confined to the pelvis, locoregional disease control is the primary treatment challenge. Treatment with carefully tailored surgery or radiation therapy (RT) has produced impressive cure rates in patients with early-stage disease (Table 56-3).
| Stage | Primary Therapy | 5-yr Overall Survival |
|---|---|---|
| IA1 | Cone biopsy; simple hysterectomy; brachytherapy | >98% |
| IA2 | Radical hysterectomy plus pelvic node dissection (PND); irradiation (RT) | ≥95% |
| IB1/limited IIA1 | Radical hysterectomy plus PND; RT | ~90% |
| IB2/larger IIA1/IIA2 | ChemoRT | 80% to 85% |
| IIB | ChemoRT | 70% to 75% |
| III | ChemoRT | ~50% |
| IVA | ChemoRT; selective exenteration | 15% to 25% |
| IVB | Chemotherapy; palliative RT | ~0% |
ChemoRT, Chemoradiation; RT, radiotherapy.
* The choice of primary therapy and the overall survival (OS) rates are related to disease extent at the time of initial diagnosis and staging evaluation.
Radical RT or chemoradiation is effective for patients with locoregional confined cervical cancer of any stage. Treatment must be carefully tailored to the patient and to the extent of disease but usually consists of a combination of external beam irradiation (EBRT) with concurrent chemotherapy and brachytherapy. Overall, historically reported 5-year OS of patients treated with RT alone are 75% to 85% for FIGO stage IB, 65% to 70% for FIGO stage II, 30% to 40% for FIGO stage III, and 10% to 15% for FIGO stage IVA disease.89–93 Within stage subsets, cure rates are strongly correlated with the size of the primary tumor and the extent of regional involvement.38,94
Radiation Treatment Variables
Chemoradiation
Initial efforts to identify a drug providing radiosensitization focused on the potential role of hydroxyurea with RT. Although several studies reported possible benefits, a recent meta-analysis has concluded that there was no evidence to support the use of hydroxyurea combined with RT in the treatment of cervical cancer.95
With data postulating that tumor hypoxia represented a significant cause of treatment failure following RT in patients with bulky cervical cancer, multi-institutional phase III trials consistently showed no improvement in outcome with the use of the first-generation nitroimadazole hypoxic-cell radiosensitizers misonidazole or pimonidazole combined with RT.96–100 On this basis, in 1996 the National Institutes of Health Consensus Statement on Cervical Cancer stated that there was “no proven benefit to combining chemotherapy with radiation” in locally advanced cervical cancer.101
Subsequently, over a relatively short time span, there was a profound shift in the paradigm of RT in the management of cervical cancer. In February 1999, the National Cancer Institute (NCI) issued a clinical announcement stating that “strong consideration should be given to the incorporation of concurrent cisplatin-based chemotherapy with radiation therapy in women who require radiation therapy for treatment of cervical cancer.”102 This recommendation was based on the results of five phase III randomized clinical trials, conducted in North America under the aegis of the NCI-sponsored Clinical Trials Cooperative Groups. The five studies had different eligibility criteria, but in total included a broad spectrum of clinical presentations: (1) patients with locally advanced tumors for whom chemoradiation represented primary therapy, (2) bulky early-stage cancers in which chemoradiation was delivered prior to adjuvant hysterectomy, and (3) postradical hysterectomy cases with high-risk pathologic factors (positive lymph nodes, positive parametria, positive margins) for whom adjuvant chemoradiation was given. There was a consistent statistically significant survival advantage favoring the RT arm that included a concurrent cisplatin-based regimen, as compared with RT alone or RT combined with hydroxyurea, with a dramatic 30% to 50% decrease in the risk of death from cervical cancer.103–111 Although now a decade old, the outcome benefit represents the single largest contemporary therapeutic gain in the management of patients with cervical cancer, especially in those with locoregionally advanced disease. Several of these studies, which initially had relatively short follow-up intervals of approximately 3 years, have been updated with significantly longer follow-up periods.108–110 These confirm that the statistically significant survival advantage of cisplatin-based chemoradiation is maintained over the long term, and they validate the 1999 NCI clinical alert. They also suggest that for future trials in locally advanced cervical cancer, estimates of PFS and OS with median follow-up intervals of 3 years represent fairly mature results.
Cisplatin was a drug of interest because it is the single most active systemic cytotoxic agent in cervical cancer, demonstrated radiosensitizing properties in vitro, had limited adverse effects on hematopoiesis, and showed promise when combined with RT in pilot studies.112–114 The relatively nonmyelosuppressive properties of cisplatin are important when considering the impact of whole-pelvis irradiation on bone marrow function.
GOG Protocol 85/SWOG 8695
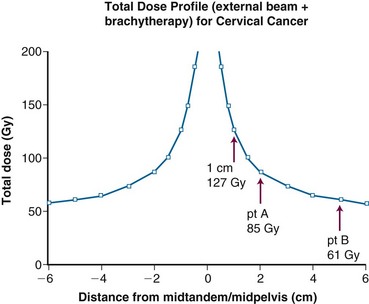
From 1986 to 1990, a total of 368 evaluable patients were entered into the two-arm joint phase III randomized trial of the Gynecologic Oncology Group (GOG) and the Southwest Oncology Group (SWOG).103 Eligible cases included locally advanced squamous cell cancer, adenocarcinoma, or adenosquamous carcinoma of the uterine cervix (FIGO stage IIB to IVA), with negative para-aortic nodes and negative intra-abdominal metastases based on surgical staging. Radiotherapy guidelines were identical in both arms. Patients with stage IIB tumors were prescribed 40.8 Gy of EBRT directed to the whole pelvis in 24 fractions (1.7 Gy per fraction), followed by low-dose-rate (LDR) brachytherapy in one or two applications, for an additional dose of 40 Gy to point A, or a cumulative point A dose of 80.8 Gy. Further limited supplemental parametrial EBRT was permitted to bring the point B dose to 55 Gy (combining both EBRT and brachytherapy contributions). Patients with stage III or IVA tumors received 51 Gy of EBRT in 30 fractions to the whole pelvis, followed by intracavitary brachytherapy delivering an additional dose of 30 Gy to point A (cumulative point A dose of 81 Gy), plus an optional parametrial boost to bring point B to a total dose of 60 Gy. Patients randomized to the control arm received hydroxyurea orally at a dose of 80 mg/kg twice weekly during EBRT. Patients in the experimental arm received cisplatin and 5-fluorouracil (5-FU) during weeks 1 and 5 of EBRT. Cisplatin was given as a slow intravenous bolus at 50 mg/m2 on days 1 and 29, and 5-FU was delivered as a 4-day infusion of 1000 mg/m2 per 24 hours on days 2 to 5 and 30 to 33 of EBRT.
With a median at-risk follow-up interval of 8.7 years, there were statistically significant improvements in disease-free survival (DFS) and OS favoring the patients treated with cisplatin and 5-FU (5-year OS of 62% in the cisplatin/5-FU arm vs. 50% in the hydroxyurea arm; p = .018) (Fig. 56-3). Analysis of patterns of first sites of relapse did not identify significant differences between the two treatment groups. The cisplatin/5-FU arm was associated with a lower incidence of severe or life-threatening leukopenia. Although there was a slight increase (not statistically significant) in acute gastrointestinal toxicity in the cisplatin/5-FU arm, the overall late complication rates at 3 years were identical for both patient cohorts.103
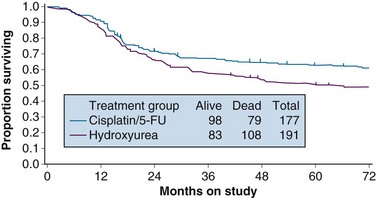
Figure 56-3 Overall survival by treatment arm in GOG 85 (p = .018).
From Whitney CS, Sause W, Bundy BN, et al: Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stages IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes. A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 17:1344, 1999, Figure 2. Reprinted with permission from the American Society of Clinical Oncology.
GOG Protocol 120
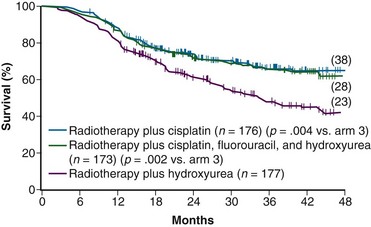
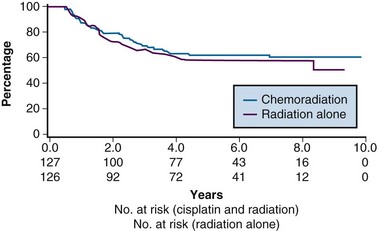
While awaiting the maturation of data from GOG 85/SWOG 8695, the GOG conducted another phase III randomized trial of concurrent chemoradiation in patients with locally advanced cervical cancer.104 Eligibility criteria for GOG 120 were similar to those in the previously described GOG 85, and included patients with locally advanced (FIGO stage IIB to IVA) cervical cancer with negative para-aortic nodal and intraperitoneal metastases following surgical staging. Radiation guidelines in this study were identical to those in GOG 85/SWOG 8695, and were held constant across all three study arms. Given that the results from GOG 85/SWOG 8695 were not yet known, the control arm was also set up as hydroxyurea given concurrently with RT, with the drug given at 3 g/m2 twice weekly during EBRT. The two experimental arms each contained cisplatin but with different schedules. In one arm, patients received cisplatin at 50 mg/m2, followed by infusional 5-FU at 1000 mg/m2/day for 4 days, starting on days 1 and 29 of EBRT (similar to GOG 85/SWOG 8695), and hydroxyurea at 2 g/m2 twice weekly throughout EBRT. In the other cisplatin treatment group, patients received cisplatin as the sole chemotherapy agent at 40 mg/m2 weekly during EBRT.
A total of 526 analyzable patients were entered into this trial from 1992 to 1997. With a median at-risk follow-up interval of 35 months, there was a significant improvement in PFS and OS favoring both cisplatin-containing arms over the arm with RT and hydroxyurea alone. Actuarial 3-year OS was 65% for both cisplatin-containing arms versus 47% for the hydroxyurea alone group (p <.005 for both cisplatin-containing arms compared with the hydroxyurea cohort) (Fig. 56-4). Patients in both cisplatin-treated groups had a significantly lower frequency of pelvic relapse (19% to 20%) compared with the patients receiving hydroxyurea alone (30%). There was also a slight reduction in distant failures favoring the cisplatin-treated patients, but this did not reach statistical significance. Although both cisplatin-containing arms had essentially identical PFS and OS, treatment with cisplatin alone resulted in significantly less acute toxicity than with the three-drug regimen, and hence it was selected as the preferred regimen from this study.104
A published update of this study, with a median at-risk follow-up interval of 106 months, confirmed prolonged improved PFS and OS favoring the cisplatin-containing chemoradiation arms, with the survival benefit seen for both stage IIB and IIIB presentations.109 Within the limitations of the available follow-up data, there was no observed increase in late toxicity with cisplatin-based chemoradiation.
RTOG Protocol 90-01
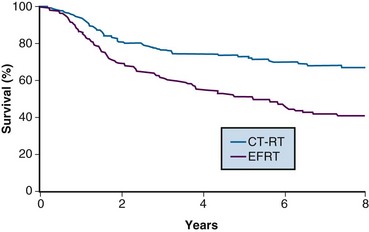
One of the many studies performed is of particular importance because it influences understanding of potential control of clinically occult para-aortic nodal disease. From 1990 to 1997, the Radiation Therapy Oncology Group (RTOG) conducted a phase III randomized trial (RTOG 90-01) comparing RT alone with concurrent chemoradiation105,108 (Fig. 56-5). Eligible patients included women with FIGO stage IIB to IVA locally advanced epithelial cervical cancers, as well as those with earlier-stage IB to IIA tumors if the primary lesion size was 5 cm or larger or if there were biopsy-proved pelvic nodal metastases. Patients with known extrapelvic disease, as evaluated by bipedal lymphangiography or retroperitoneal surgical staging, were ineligible. Based on an earlier RTOG study that indicated a benefit of prophylactic para-aortic nodal RT in patients with stage IB2 to IIB cancers,115 the control arm of RT alone included contiguous extended-field coverage to the L1 to L2 vertebral interspace. The pelvis and para-aortic nodes were prescribed a dose of 45 Gy in 25 fractions, supplemented by LDR intracavitary brachytherapy to boost the point A cumulative dose to at least 85 Gy. Institutions were allowed to initiate brachytherapy earlier, at an EBRT dose of 20 to 30 Gy, provided that the final point A dose specification was adhered to and that further pelvic EBRT be delivered with a midline block. In the experimental arm, patients were selected to receive pelvic RT only, with the superior field edge defined at the L4 to L5 vertebral interspace. With the exception of eliminating prophylactic para-aortic coverage, EBRT parameters for the experimental arm were similar to those of the control group. Concurrent with RT, patients randomized to the experimental group received three cycles of cisplatin/5-FU chemotherapy (cisplatin 75 mg/m2, 5-FU 1000 mg/m2/day for 4 days) administered at 3-week intervals, with the third cycle often given at the time of brachytherapy insertion.105,108
A total of 388 analyzable patients were included. At a median at-risk follow-up interval of 43 months, preceding the NCI clinical announcement, there was a statistically significant advantage for DFS and OS favoring the chemoradiation arm.105 Actuarial 5-year OS was 73% in patients treated with chemoradiation compared with 58% in patients treated with extended-field RT alone (p = .004).
The initial results of RTOG 90-01 were updated in the 388 analyzable patients with a median at-risk follow-up duration of 6.6 years.108 The significant outcome benefit of concurrent chemoradiation over RT alone has been maintained, with an 8-year OS of 67% versus 41%, respectively (p <.0001) (see Fig. 56-5). In a post hoc analysis, the advantage of chemoradiation was noted regardless of FIGO stage (IB and II vs. III and IVA), pelvic nodal involvement, or the method of pretherapy nodal evaluation (lymphangiography vs. retroperitoneal nodal staging). The rate of cumulative overall grade 3 or higher late complications was similar in both treatment groups (14% at 5 years). Analysis of patterns of failure noted a significant reduction in locoregional and distant metastasis (excluding the para-aortic nodes) relapse rates favoring the patients treated with RT and concurrent cisplatin/5-FU. Although there was a slightly higher para-aortic failure rate in patients receiving chemoradiation compared with extended-field RT alone, this did not reach statistical significance.
The patterns of failure recorded in RTOG 90-01 (Table 56-3) may allow the hypothesis that concurrent chemotherapy not only provides locoregional radiosensitization but also addresses preexisting micrometastases, although it should be recognized that the risk of distant metastases is closely linked to the incidence of pelvic failure, which was higher in the control arm.39 At the very least, this trial indicates that for patients at some but variable risk of para-aortic nodal metastases, albeit without grossly identifiable disease, chemoradiation with irradiation limited only to the pelvis achieves better outcomes than extended-field irradiation alone without chemotherapy.
GOG Protocol 123
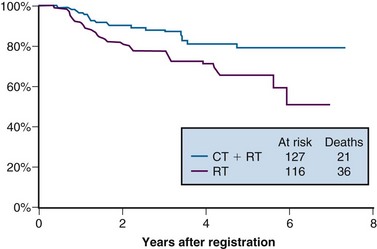
From 1992 to 1997, the GOG conducted a phase III randomized trial comparing preoperative treatment with RT versus RT and concurrent cisplatin in patients presenting with an earlier tumor stage than those included in the preceding three described studies.106 Eligible patients were those with stage IB2 cervical cancers (≥4 cm tumor diameter), with no evidence of retroperitoneal adenopathy on radiologic (CT or lymphangiography) or surgical assessment. Radiotherapy specifications were identical in both treatment arms. The pelvis was prescribed a dose of 45 Gy in 25 fractions with EBRT, followed by LDR intracavitary brachytherapy to boost the cumulative point A dose to 75 Gy. In the experimental arm, patients received weekly cisplatin at a dose of 40 mg/m2 (with a maximal dose of 70 mg/week) for up to six doses. The final dose of cisplatin could be given during brachytherapy insertion. Based on the interim results of a previous GOG trial that evaluated the role of adjuvant hysterectomy in centrally bulky tumors (GOG 71), all patients in this study underwent extrafascial hysterectomy following either RT alone or RT plus concurrent cisplatin.
A total of 369 patients were evaluated, with a median at-risk follow-up interval of 36 months. Patients who received concurrent cisplatin had a significantly higher incidence of pathologic complete response in the hysterectomy specimen, as well as improved rates of pelvic control, PFS, and OS compared with those treated without chemotherapy. Three-year OS was 83% and 74% in the chemoradiation versus RT arms, respectively (p = .008)106 (Fig. 56-6). Although there was an increase in acute toxicities in the patients treated with cisplatin and radiation, these reactions were predominantly of transient hematologic perturbations, and severe late toxicities were infrequent and equally divided between the two groups. An updated analysis of this study, with an extended at-risk follow-up duration of 101 months, confirmed that concurrent weekly cisplatin with RT maintained significant long-term PFS and OS benefit compared with RT alone, without a reported increase in serious late effects.110
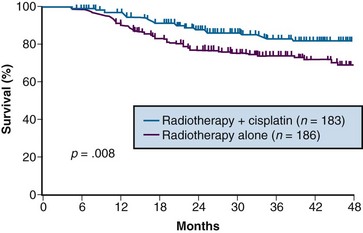
Figure 56-6 Overall survival by treatment arm in GOG 123 (p = .008).
From Keys HW, Bundy BN, Stehman FB, et al: A comparison of weekly cisplatin during radiation therapy versus irradiation alone, each followed by adjuvant hysterectomy in bulky stage IB cervical carcinoma. A randomized trial of the Gynecologic Oncology Group. N Engl J Med 340:1154-1161, 1999. Copyright © Massachusetts Medical Society. All rights reserved.
Intergroup 0107 (SWOG 8797/GOG 109/RTOG 91-12)
The last of the five phase III trials that formed the basis for the 1999 National Institutes of Health (NIH) clinical announcement investigated the role of chemoradiation as an adjunct to primary surgery for patients with clinical early-stage disease in whom postoperative high-risk pathologic features had been discovered.107 Patients eligible for this intergroup study were those with stage IA2, IB, or IIA carcinoma of the cervix who were initially treated with radical hysterectomy and pelvic lymphadenectomy and who were found to have positive pelvic lymph nodes, positive margins, and/or positive parametrial infiltration on microscopic evaluation. Taken together, these high-risk factors have the unifying theme of pathologically identified extracervical/extrauterine disease extension. In this two-arm trial, patients were randomized to pelvic RT alone (EBRT to 49.3 Gy in 29 fractions, without brachytherapy) or to the same irradiation combined with chemotherapy. Chemotherapy consisted of cisplatin (70 mg/m2) and 5-FU (1000 mg/m2/day for 4 days) given for four cycles beginning on days 1, 22, 43, and 64 of therapy. Two of the chemotherapy cycles were delivered concurrently with pelvic RT, followed by two additional cycles after completion of EBRT.
A total of 243 eligible patients were entered into the trial from 1991 to 1996. With a median at-risk follow-up time of 42 months, patients receiving combined adjuvant chemoradiation had statistically significant improvement in PFS and OS compared with those receiving RT alone. Estimated 4-year OS was 81% versus 71% for the chemoradiation and RT only arms, respectively (p = .007) (Fig. 56-7). The outcome benefit for adjuvant chemoradiation appeared to arise from reduction in both pelvic and distant failures, although comparisons of relapse patterns between treatment arms did not reach statistical significance. There was an increase in acute toxicities in the combined-modality arm, but these were mostly limited to transient and manageable hematologic and gastrointestinal effects.111
National Cancer Institute of Canada Trial
In contrast to the consistent therapeutic benefits seen for combining cisplatin-based chemoradiation in the preceding five phase III trials, the National Cancer Institute of Canada (NCIC) published the results of another randomized study that questioned the role of concurrent chemoradiation in locally advanced cervical cancers.111 Eligible patients included those with stage IIB to IVA squamous cell cancers or earlier-stage disease (IB to IIA) if the primary tumor was at least 5 cm in diameter or if there was histologically positive pelvic nodal involvement. Radiation specifications, which were identical in both treatment arms, prescribed a dose of 45 Gy in 25 fractions to the pelvis with EBRT. This was followed by intracavitary brachytherapy using various allowable dose rates, but each calibrated to provide an LDR biologically equivalent dose of 35 Gy to point A (total cumulative point A dose of 80 Gy in conventional equivalents). Careful quality control was maintained to complete all RT within 7 weeks. Patients randomized to receive chemotherapy were given weekly cisplatin at a dose of 40 mg/m2 for a total of five administrations concurrently with EBRT.
Between 1991 and 1996, 253 eligible patients were entered into the trial. With a median at-risk follow-up of 82 months, there were no observable differences in PFS or OS in the two treatment arms (Fig. 56-8).
Various reasons for the lack of a demonstrable effect of cisplatin concurrent with RT in the NCIC study have been promulgated by the authors and others. These include the lack of surgical staging, a relatively small sample size, the confounding factor of treatment-related anemia in the chemotherapy arm, and the omission of 5-FU (which may be synergistic with cisplatin) from the chemotherapy regimen. Perhaps most provocative is the suggestion that concurrent cisplatin-containing chemoradiation exhibits its greatest benefit primarily in patients with suboptimal and prolonged overall RT durations. In contrast to the studies reported by Whitney103 and Rose,104,109 where the median duration of the treatment course was 64 and 62 days, respectively, the timing for completion of RT in the NCIC study was carefully controlled, with a median duration of 51 days.111 The potential reduced impact of concurrent cisplatin with optimum RT schedules has been refuted by others, however.116 Nonetheless, the NCIC authors conclude that despite their negative trial, “the balance of evidence favors the use of combined-modality treatment for the types of patients studied in this trial. The best results are certainly achieved by careful attention to RT details, including dose and overall delivery time, the use of brachytherapy whenever possible, and probably the addition of concurrent cisplatin chemotherapy to RT.”111
Chemotherapy Concurrent with Irradiation: A Synopsis
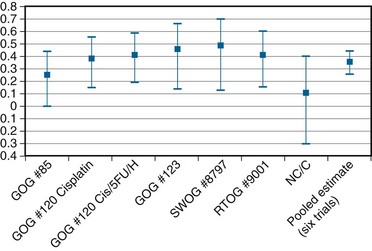
In an overview, Rose and Bundy116 summated the collective results of the six North American randomized trials (including the NCIC study) and showed a cumulative and statistically significant 36% reduction in the risk of death favoring combined cisplatin-based chemoradiation over RT alone or combined with hydroxyurea116 (Fig. 56-9). The generalizability of concurrent chemoradiation for cervical cancer to an unselected, non–study-limited patient population has also been raised. In part, this has been addressed by a provocative population-based cohort study of cervical cancer outcome in Ontario, Canada. In this analysis, Pearcey and colleagues117 showed a significant improvement in cervical cancer OS at the population level between 1992 and 2001, without a coincident change in stage presentation or an imbalance in cases selected for primary surgery. The investigators attribute the survival gains to the generalized and appropriate use of concurrent, predominantly cisplatin-based chemoradiation in the majority of patients receiving RT for cervical cancer.117
Although cisplatin-based chemoradiation has formed the basis for most discussions and conclusions regarding the superiority of combined-modality therapy, more recent data suggest that other nonplatinum agents, most notably 5-FU and/or mitomycin C, may also provide benefit when used concurrently with definitive radiotherapy. A recent updated and comprehensive meta-analysis of chemoradiation for locally advanced cervical cancer confirmed the general applicability of this treatment algorithm for all women and endorsed the NCI alert regarding the use of concomitant cisplatin-based chemoradiotherapy but also indicated that similar gains could be derived from non–platinum-based chemotherapy.118
It should be emphasized, however, that RT remains the single most effective modality in the management of patients with locally advanced cervical cancer, where surgical extirpation of tumor is infeasible or incomplete. Even in the absence of chemotherapy, RT alone is able to cure many patients with bulky cervical tumors. Some patients present with hydronephrosis and renal dysfunction or other medical comorbidities that preclude the use of cisplatin. The use of concurrent carboplatin, which avoids nephrotoxicity, has been suggested by some as an alternative to cisplatin, but its comparative efficacy remains unproven.119 Ultimately, the selection of concurrent cisplatin-based chemotherapy requires sound medical judgment, balancing the potential improvement in tumor control with patient physiology, disease presentation, and the increased acute toxicities (notably, hematologic, metabolic, and gastrointestinal) associated with the addition of chemotherapy.
Noncisplatin Chemoradiation Regimens
Although there is general acknowledgment of the efficacy of cisplatin in combination with RT, various investigators have formally evaluated other noncisplatin chemoradiation regimens, primarily infusion 5-FU. The GOG conducted a phase III trial (GOG 165) in which patients with locally advanced stage IIB to IVA tumors were randomized to weekly cisplatin at 40 mg/m2/week versus protracted venous infusion of 5-FU at 225 mg/m2/day for 5 days per week throughout the duration of EBRT. GOG 165 was closed before planned completion when a scheduled interim analysis indicated that there was no likelihood that the 5-FU arm would ever show improved outcomes compared with the control arm of weekly cisplatin.120 Other concurrent chemoradiation regimens tested that appear to have potential benefit include epirubicin121 and 5-FU plus mitomycin C.122
Balanced against the indeterminate individual trial results described above, a recent comprehensive meta-analysis has suggested that nonplatinum agents may also improve outcome when combined with RT and deserve further evaluation.118
Neoadjuvant Chemotherapy
Although concurrent cisplatin-based chemoradiation has become the widely accepted standard of care, caution is raised regarding the use of strategies employing neoadjuvant chemotherapy before RT. Multiple randomized trials, most with small patient numbers, have failed to identify an effective strategy of neoadjuvant chemotherapy followed by definitive RT. A systematic review and individual patient data meta-analysis showed no benefit and, indeed, an overall trend to detriment, for patients receiving neoadjuvant chemotherapy (predominantly cisplatin-based) before radical RT.123 In two of the randomized trials, it was reported that the neoadjuvant chemotherapy approach, although associated with a reasonable initial response, ultimately resulted in statistically significant inferior pelvic control and OS compared with definitive RT alone.124,125
A plausible explanation for the lack of benefit seen with chemotherapy preceding RT is that although responses can be seen with systemic cytotoxics alone, the remaining clonogens undergo accelerated repopulation, thereby offsetting the efficacy of RT.126 Questions remain regarding the potential benefit of initial chemotherapy in surgical candidates or where the dose and intercycle interval of systemic agents are optimized, but the use of neoadjuvant chemotherapy strategies should be considered only in the context of carefully designed clinical trials. Unless more effective cytotoxic agents are identified, there is no current reason to consider neoadjuvant chemotherapy followed by definitive RT or chemoradiation in patients with localized, curable cervical cancer.
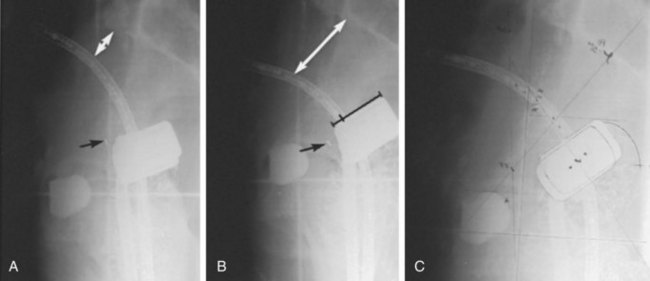
Brachytherapy Plus External Beam Irradiation
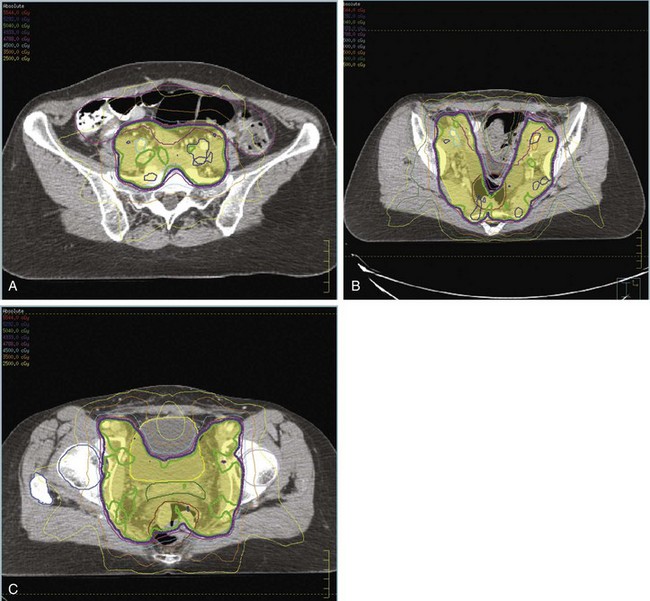
The importance of brachytherapy in the curative treatment of intact cervical cancer is indisputable. Clinicians have sometimes been inclined to deemphasize the use of brachytherapy in patients with FIGO stage III disease, arguing that disease at the pelvic wall is beyond the reach of traditional intracavitary therapy. Reports from the Patterns of Care Study127 and from the M.D. Anderson Cancer Center (MDACC),94 however, have demonstrated significantly better survival rates for patients treated with a combination of EBRT and intracavitary irradiation. In the MDACC report of 1007 patients treated with irradiation for FIGO stage IIIB disease, the disease-specific survival (DSS) was 43% for patients who had intracavitary irradiation versus 21% for those treated with EBRT alone. Patients treated during periods when policies emphasized the use of brachytherapy had significantly better survival rates. Patients who received higher doses of EBRT (and concomitantly lower doses of intracavitary irradiation) to the central pelvis had poorer survival rates. The rate of major complications was also correlated with the proportion of central pelvic treatment given with EBRT, and this correlation suggested that the therapeutic ratio narrows when the dose of EBRT given to the whole pelvis exceeds 45 Gy.94 Nonetheless, a careful balance between EBRT and brachytherapy components of therapy, accounting for tumor volume, anatomic presentation, and disease regression rate, represents the hallmark of appropriate cervical cancer radiotherapy planning.
Radiation Dose and Duration
The adverse impact of treatment duration protraction in cervical cancer has been evaluated by several investigators. There is general consensus that prolonging a course of radical RT beyond approximately 7 weeks is associated with a 0.5% to 1% decrease in pelvic control per extra day.128–133 Although debate remains about whether an increase in treatment duration adversely affects outcome or is simply a reflection of unfavorable tumor or patient characteristics, there is compelling radiobiologic demonstration of accelerated tumor repopulation that mitigates the efficacy of RT.126 In contrast, there is no evidence that prolongation of treatment duration results in a reduction of late normal tissue sequalae.133 Therefore it is now considered prudent to complete a course of definitive RT for cervical cancer within 7 to 8 weeks by eliminating all elective treatment breaks, delivering EBRT parametrial boosts between, or interdigitated with, brachytherapy insertions, and actively supporting patients through acute toxicities so as to avoid RT interruptions.
Role of Extended-Field Irradiation
The prognosis of patients with cervical carcinoma is inversely related to the extent of regional and nodal involvement. Because hematogenous metastasis is usually a late event in the course of this disease, however, patients with lymph node metastases often can be cured with regional EBRT if pelvic disease can be controlled. Even patients with documented para-aortic lymph node metastases have 5-year OS of 20% to 50%, depending on the extent of pelvic disease.134–139
Two groups have explored the use of prophylactic extended-field EBRT in patients who do not have overt para-aortic metastases. A trial from the European Oncology and Radiation Therapy Consortium (EORTC)140 was conducted prior to the use of concurrent chemotherapy. Randomized patients had more locally advanced pelvic disease (bulky FIGO stage IIB, FIGO stage III, or biopsy-proved pelvic lymph node metastases). DFS of patients in the two treatment arms was not significantly different at 4 years, although the rate of relapse in the para-aortic lymph nodes was higher in patients who had not received RT to this site. OS was not reported. Results from RTOG 90-01 were previously discussed.
Although the role of prophylactic para-aortic nodal RT remains to be fully defined, the results of RTOG 90-01 clearly indicate that concurrent chemotherapy with pelvic RT leads to significant outcome benefits over extended-field EBRT without chemotherapy.108 Underlying the question of benefits of prophylactic para-aortic RT is the assumption that the para-aortic nodes may represent isolated occult extrapelvic disease in some patients for whom sterilization by RT would translate to cure (assuming achievable pelvic control and no distant metastases). The results of RTOG 90-01, which showed significant reductions in both pelvic and distant relapse with chemoradiation, suggest that the use of concurrent chemotherapy to some degree addresses subclinical metastases beyond the volume of RT. Unanswered is the question of whether prophylactic extended-field RT in addition to systemic chemotherapy would provide additional therapeutic gain.
Practice patterns vary for patients with negative para-aortic nodes but who are deemed to be at elevated risk for occult disease in this nodal chain. At the University of Washington, prophylactic para-aortic nodal EBRT to 45 Gy, concurrent with chemotherapy (to the level of the renal vessels, or at the L1 to L2 vertebral interspace), is often considered for patients with the highest potential risk of subclinical para-aortic disease (i.e., those with common iliac adenopathy on surgical staging or those who have negative para-aortic nodes but extensive pelvic lymphadenopathy by radiologic imaging [recognizing the limitations of even the best imaging modalities to detect microscopic tumor]). Whereas the efficacy of such an extended-field prophylaxis approach has not been proven, especially in the era of chemoradiation, the use of concurrent cisplatin-based chemotherapy with extended-field RT has been shown to be tolerable in several phase II trials.138,139
Impact of Anemia and Tumor Hypoxia on Radiation Therapy Outcome: Potential as Therapeutic Targets
The adverse association of anemia on outcome following primary RT for cervical cancer has been well documented in many previous clinical reviews.140–142 Whether this association reflects cause and effect or whether anemia is simply a surrogate for worse prognostic and predictive variables, such as larger and more radiation-refractory tumors, is still being debated. Potential mechanisms for a negative impact of anemia on cervical cancer RT outcomes may be linked to tumor hypoxia and consequent radioresistance, as well as induction of angiogenesis, increased tumor aggressiveness, and enhanced metastatic potential.
Some retrospective reports have suggested that anemia is an independent negative prognostic factor, with the average hemoglobin level during RT being more predictive than the level before therapy.143,144 Only one small prospective randomized trial directly addressing the value of transfusion has been completed, in the 1970s. This study indicated improved pelvic control for irradiated patients whose hemoglobin level was corrected by transfusion, but the analysis was hampered by limited patient numbers and lack of stratification.145
In an attempt to provide definitive answers, the GOG conducted a prospective phase III trial (GOG protocol 191) in which patients with locally advanced cervical cancer undergoing primary chemoradiation (concurrent weekly cisplatin at a dose of 40 mg/m2) were randomized to hemoglobin maintenance at a level of 10 g/dL versus aggressive intervention to raise hemoglobin levels to 12 to 13 g/dL (by transfusion and erythropoietin). However, concerns about an increased incidence of thromboembolic events and a possible negative impact on survival associated with erythropoietin in several other disease sites, while not statistically significant in this trial, led to its early closure, leaving the impact of maintaining optimal hemoglobin levels on outcome following chemoradiation still undetermined.146 Although erythropoiesis-stimulating agents have been previously used to correct anemia, and may be considered in highly selected cases, their generalized use is currently discouraged, due to concerns of thromboembolism and possible tumor progression.147
Tumor hypoxia, as measured by oxygen electrodes, has been identified in many cervical cancers and is a predictor of poor outcome.148 Although there may be a link, the relationship between host status (anemia and hemoglobin level) and tumor milieu (hypoxia) remains ambiguous. To exploit hypoxia as a potential therapeutic target, the GOG opened a randomized phase III trial (GOG 219) that investigated the efficacy of a new-generation bioreductive drug, tirapazamine, added to the present standard of concurrent cisplatin and RT for locally advanced cervical cancer. In addition to its radiosensitizing effects, tirapazamine had generated significant interest because it is itself a potent and selective hypoxic cell cytotoxin and is also potentially synergistic with cisplatin.149,150 However, the lack of expected benefit for the combination of tirapazamine, cisplatin, and radiotherapy in a large phase III trial of head and neck cancer,151 and subsequent loss of sponsorship support, led to premature closure of GOG 219.
Recent studies have identified significant modulations of gene expression in hypoxic tumors that may be associated with tumor aggressiveness and progression, including hypoxia-inducible factor 1, TP53, vascular endothelial growth factor (VEGF), platelet-derived endothelial cell growth factor, nitric oxide synthase, and matrix metalloproteinases.152 These biomolecular markers provide potential new targets for future therapeutic investigations.
Smoking and Cigarette Cessation during Radiotherapy
Smoking is a known risk factor for the development of cervical cancer. Several retrospective series have shown conflicting correlations between smoking and cervical cancer prognosis. A recent prospective, multicenter analysis, however, strongly suggested that smoking during chemoradiation was associated with poorer outcomes compared with those in patients who had never smoked or who had quit smoking before treatment. An added strength of this study was the use of urine cotinine levels to confirm patient-reported smoking status.153 In another large retrospective review, smoking has also been associated with an increased risk of late complications following pelvic RT for cervical cancer.154 Although the mechanisms underlying smoking and treatment outcome and toxicity remain to be elucidated, and even though it is not a specific cancer treatment variable, it seems intuitive that concerted efforts to assist patients in smoking cessation during cervical cancer therapy would be reasonable and may contribute to better outcomes.
Systemic Therapy Intensification
Current delivery techniques, employing both EBRT and brachytherapy, are likely approaching the limits of RT doses to pelvic structures. It is also recognized that in patients with bulky locoregionally advanced cervical cancer, there remains an appreciable incidence of pelvic relapse, and the risk of distant relapse is as high, if not higher, than pelvic failures following chemoradiation.108,118 These findings have led some researchers to propose that adding further chemotherapy to a “backbone” of cisplatin and RT may provide further therapeutic benefit, in terms of both distant and locoregional tumor control.118
In a hypothesis-generating, post-hoc subset analysis performed in Intergroup 0107 (discussed above), Peters and colleagues107 suggested that the number of cycles of chemotherapy given with pelvic RT (including up to two cycles following completion of radiation therapy) correlated with an improved outcome. Duenas-Gonzalez and colleagues155 recently presented in abstract form the intriguing results of an international trial comparing standard cisplatin and RT versus cisplatin and gemcitabine both during and after RT, with an improvement in 3-year PFS and OS favoring the doublet chemotherapy arm, at the expense of increased but acceptable acute toxicity. The full publication of results of this trial is eagerly awaited. Still unanswered is whether the improved results are attributable to the addition of more chemotherapy during RT or as added systemic adjuvant therapy following RT, or both.155 Of added concern are reports from other investigators that the combination of cisplatin, gemcitabine, and pelvic irradiation results in unacceptable toxicity.156,157
Based on the observations of Intergroup 0107107 and a meta-analysis118 that suggested possible therapeutic benefit of extra cycles of chemotherapy given after RT, two international phase III trials have been activated to evaluate the role of additional chemotherapy (carboplatin and paclitaxel in four cycles) following cisplatin and RT (to avoid potential excessive acute toxicity concerns, chemotherapy is not escalated during the RT course). The first trial addresses high-risk posthysterectomy patients who have positive lymph nodes or parametrial extension (RTOG 0724), while the second evaluates outcome in patients with locally advanced intact cervical cancer (stage IB2 to IVA) treated with definitive chemoradiation (OUTBACK ANZGOG, Australian-led trial).
Primary Therapy by Disease Stage
Microinvasive Disease (FIGO Stage IA Disease)
Although early microinvasive carcinomas of the cervix (FIGO stage IA1 lesions) are usually treated with hysterectomy, patients with medical contraindications to surgery can be treated very effectively with brachytherapy alone, for which 10-year PFS of 98% to 100% have been reported. Treatment usually consists of one or more intracavitary insertions that deliver a total of 65 to 75 Gy to point A in LDR equivalents, with a maximal vaginal surface dose of 100 to 120 Gy, depending on the extent of microinvasive disease, size of the cervix, and normal tissue anatomy.158–160
Although the risk of regional lymph node spread from FIGO stage IA2 carcinomas is less than 5%, early reports of conservative surgery for IA2 cervical cancer yielded suboptimal cancer control results.161 Most patients with stage IA2 cervical cancer have, therefore, traditionally undergone radical hysterectomy with node dissection.
More recent analyses, with contemporary emphasis on quality of life, have led to a reappraisal of the surgical extent required for these early-stage tumors. Gadducci and colleagues162 reviewed the hospital records of 166 patients with microinvasive squamous cell carcinoma of the cervix. Among the 143 cases of stage IA1 and 23 cases of stage IA2 disease, there were 67 patients (stage IA1, 44; stage IA2, 23) who underwent pelvic lymph node dissection, and none had lymph node metastasis. For patients with stage IA2 cervical cancer, the authors concluded that nonradical hysterectomy might be adequate therapy and the need for lymph node dissection was questioned.162 In another clinicopathologic review of specimens from 394 patients with stage I squamous cell cervical carcinoma, none of the patients with depth of stromal invasion of less than 5 mm had pelvic lymph node metastasis and none of these patients died from recurrent cancer. The authors recommended simple hysterectomy for these early, stage IA tumors (or modified radical hysterectomy with pelvic lymph node dissection for cases of stage IA2 cervical squamous cell carcinoma where lymphovascular space involvement is present).163
Most reports of “microinvasive” cervical cancer have focused on squamous cell tumors. Ceballos and colleagues164 reported on a series of 32 patients with stage IA1 or IA2 cervical adenocarcinomas treated with hysterectomy (29 patients), radical trachelectomy (2 patients), or cervical conization (1 patient). Postoperative RT was administered to one patient. There were 27 patients who also underwent pelvic lymph node dissections, and none had lymph node metastasis. With a mean follow-up of 54 months, there were no relapses. The researchers recommended less radical surgery for these patients.164 In another series, none of the 48 women with stage IA1 or IA2 cervical adenocarcinomas had parametrial disease or involved lymph nodes on pathologic assessment of the operative specimens.164 Smith and co-workers165 identified 560 cases of early cervical adenocarcinoma (200 with stage IA1, 286 with stage IA2, and 74 with “localized” disease) in the SEER (Surveillance, Epidemiology, and End Results) registry. Simple hysterectomy was performed in 272 (49%) and radical hysterectomy in 210 (38%). Combining their data with all other published series of early cervical adenocarcinomas, they identified 1170 cases, including 585 stage IA1, 358 stage IA2, and 227 “others” with less defined early disease. Among 531 patients who underwent lymph node biopsy, 15 (1.3%) had one or more positive nodes; of these 15 patients, 11 (73%) later relapsed or died. For IA1 versus IA2 disease, there were no significant differences in the frequency of positive lymph nodes, relapse, or death. However, the cases that were considered “others” based on less well defined tumor dimensions, as well as those with lesions larger than IA2, were at increased risk of relapse.165 The authors concluded that well-defined early invasive adenocarcinoma (stages IA1 and IA2) has an excellent prognosis, and conservative surgery may be appropriate.166 These retrospective data suggest that the management of patients with stage IA1 and stage IA2 cervical adenocarcinomas need not differ from their counterparts with squamous cell lesions.
FIGO Stages IB and IIA Disease
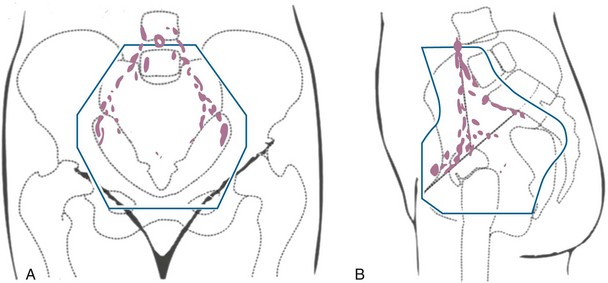
Primary surgical management is probably best suited for physiologically fit patients with small FIGO stage IB or IIA cervical cancers without clinical evidence of regional metastasis. Depending on the size of the cervical tumor, surgeons may vary somewhat in the extent of resection of the paracervical tissues and uterosacral ligaments (Fig. 56-10). The risk of major complications, particularly bladder and rectal dysfunction or ureteral injury, is proportional to the extent of surgical resection and may therefore be greater when radical hysterectomy is used to treat larger tumors. The ovaries of premenopausal women are usually not removed, which provides an advantage for radical surgery over irradiation in young women with relatively small tumors. Massive obesity and other medical problems that increase the risk of a major pelvic surgical procedure are usually considered relative contraindications to a primary surgical approach.
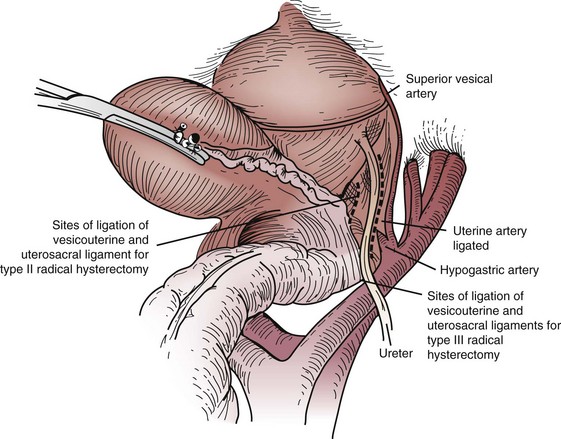
Figure 56-10 Sites of ligation of the vesicouterine and uterosacral ligaments for a type II or type III radical hysterectomy.
From Berek JS, Hacker NF: Practical Gynecologic Oncology. Baltimore, Williams & Wilkins, 1994, p 256.
For patients with FIGO stages IB and IIA disease, treatment results are strongly correlated with tumor size, and this factor thus influences the appropriate selection of primary therapy. Most FIGO stage IB1 tumors can be treated effectively with radical hysterectomy or combined EBRT and intracavitary irradiation; both have cure rates of 85% to 90%.167
In a report of the only randomized trial comparing radical surgery (with or without postoperative RT) with RT alone, Landoni and colleagues168 reported 5-year OS of 87% for patients with FIGO stage IB to IIA tumors of 4 cm or less in diameter treated with primary surgery compared with 90% for patients treated with definitive RT. Morbidity was somewhat greater in patients initially treated with surgery because many also received postoperative pelvic RT per the protocol for parametrial or lymph node involvement, positive tumor margins, or deep invasion into the stroma. In the same study, 5-year OS of patients with bulky FIGO stages IB2 and IIA tumors larger than 4 cm was similar for both treatments (70% for patients treated with surgery and 72% for patients treated with RT). However, 84% of patients with the larger lesions had the aforementioned high-risk features and received postoperative pelvic RT. As a result, morbidity was again greater in patients who had undergone surgery up front, as compared with those who had received definitive RT alone.168 The equivalent survival results were obtained even though the combined dose of EBRT and LDR intracavitary irradiation used to treat patients in this study (median cumulative dose to point A was 76 Gy) was lower than that recommended by other clinicians.
Although some authors recommend radical surgical treatment for patients with FIGO stage IB2 carcinomas regardless of size, the risk of pelvic recurrence is high for most of these patients unless they also receive adjuvant postoperative RT or CT-based RT. To avoid exposing these patients to the combined risks of a radical hysterectomy and RT, concurrent chemoradiation is often preferred as the primary treatment. For the same reason, other findings that have been associated with an increased risk of pelvic lymph node metastasis and pelvic recurrence (extensive lymphovascular space invasion, deep stromal penetration) are considered by some clinicians to be contraindications to surgery as primary disease management. Unfortunately, the presence or absence of these factors cannot be reliably determined with conventional clinical staging. A recent cohort analysis suggested that elevated preoperative squamous cell carcinoma antigen levels predicted an increased likelihood of postoperative RT and cancer relapse.169 Although intriguing, these observations should be validated with further studies.
Minimally Invasive Surgery
Several surgical innovators have applied their laparoscopic and/or vaginal surgical skills to the treatment of cervical cancer as an alternative to the abdominal operation. In a prospective study of various approaches to pelvic lymphadenectomy for surgical staging, consecutive patients scheduled for radical surgery were randomly assigned to transperitoneal, extraperitoneal, or laparoscopic pelvic lymphadenectomy. Extraperitoneal and laparoscopic approaches were feasible and as effective as transperitoneal lymphadenectomy. Although laparoscopic pelvic lymphadenectomy showed a significantly longer operative time, both extraperitoneal and laparoscopic approaches minimized some postoperative complications and reduced the length of stay compared with transperitoneal surgery.170
Sharma and colleagues171 analyzed their first 35 consecutive patients undergoing laparoscopic-assisted radical vaginal (Schauta) hysterectomy for early cervical cancer and 32 consecutive patients who underwent abdominal (Wertheim/Meigs) radical hysterectomy. Cohorts were similar in age, weight, previous abdominal surgery, histologic tumor subtype, FIGO stage, resection margins, lymph node count and status, length of follow-up, and recurrence. There were significant differences between laparoscopic-assisted vaginal and abdominal approaches for mean duration of surgery (160 minutes vs. 132 minutes), blood loss (479 mL vs. 715 mL), hospital stay (mean, 5 days vs. 9 days), postoperative complications (6 patients vs. 20 patients), and duration of bladder catheterization (mean, 4 days vs. 9 days).171 Steed and colleagues172 retrospectively compared 71 patients who underwent laparoscopic-assisted radical vaginal hysterectomy with 205 patients who underwent radical abdominal hysterectomy for stage IA to IB cervical carcinoma. Both groups were similar with respect to age and Quetelet (obesity) index. There were no differences in tumor size, grade, histologic type, depth of invasion, lymph node metastasis, or surgical margins. All laparoscopic procedures were completed successfully, with no conversions to laparotomy. Differences in laparoscopic versus abdominal factors included blood loss (300 mL vs. 500 mL; p <.001), operative time (3.5 hours vs. 2.5 hours; p <.001), and intraoperative complications (13% vs. 4%; p <.03). Intraoperative complications in the laparoscopic-assisted radical vaginal surgery group included cystotomy (7), ureter injury (1), and bowel injury (1). The median time to normal urine residual was 10 days versus 5 days (p <.001), and the median length of hospital stay was 1 day versus 5 days (p <.001). Two-year relapse-free survival (RFS) was 94% in both groups. These data showed that laparoscopic-assisted radical vaginal hysterectomy yielded similar efficacy and relapse rates to radical abdominal hysterectomy. The major benefits are less blood loss and a shorter hospital stay. Laparoscopic-assisted radical vaginal surgery is associated with an increase in intraoperative complications and an increased time to return to normal bladder function, however.172
A robot-assisted radical hysterectomy was compared with open radical hysterectomy by Boggess and associates173; it resulted in less blood loss, a shorter operative time, a higher lymph node yield, and a shorter length of hospital stay.173 Postoperative complications were lower in the robotic surgery group, but the difference was not statistically significant. Thirty-two consecutive patients undergoing robotic radical hysterectomy were compared with 17 patients undergoing laparoscopic radical hysterectomy and 14 patients undergoing abdominal radical hysterectomy by Estape and co-workers.174 Operative times were not significantly different between the three groups. Estimated blood loss for the robotic (130 mL) and laparoscopic (209 mL) groups was similar, and significantly less than that in the laparotomy group (621 mL). The mean length of hospital stay for the robotic, laparoscopic, and abdominal surgery groups was 2.6, 2.3, and 4 days, respectively. The incidence of postoperative complications was lowest and the lymph node yield highest among patients undergoing robotic surgery.174 In a review of 27 published studies, Cho and Nezhat175 concluded that robotic and laparoscopic surgery yielded comparable results. Compared with laparotomy, both minimally invasive approaches resulted in fewer wound complications, less blood loss, and a shorter length of hospital stay. They found good evidence that robotic surgery facilitates laparoscopic surgery.175
Obermair and colleagues176 have initiated a phase III randomized trial comparing laparoscopic or robotic radical hysterectomy with abdominal radical hysterectomy for the treatment of stage IA1 (with lymphovascular invasion), IA2, and IB1 cervical squamous cell carcinomas and adenocarcinomas. Researchers hope that this study will eliminate the selection bias of retrospective analyses and elucidate the optimal surgical approach for early-stage cervical carcinoma.176
Fertility-Preserving Surgery
Approximately 10% to 15% of cervical cancers will occur in women during the reproductive years, and some of these patients are hesitant to undergo radical pelvic surgery or RT with resulting permanent loss of fertility. Dargent and colleagues177 reported their pioneering experience with laparoscopic pelvic lymph node dissection and radical vaginal trachelectomy (for highly selected, nonbulky stage I and IIA patients), placing an isthmic cerclage around the lower uterine segment that is sutured to the vaginal cuff.
A number of investigators have subsequently reported their experiences with fertility-preserving surgery for early-stage (predominantly IA2 and IB tumors no larger than 2 cm) cervical cancer using both vaginal and abdominal approaches.178–180 In 2003, Bernardini and colleagues181 reported that 80 women had undergone radical trachelectomy at their institution. There were 39 women who subsequently attempted pregnancy, resulting in a total of 22 pregnancies among 18 patients. The rate of cancer relapse following these fertility-sparing procedures appeared to be comparable to that of women undergoing radical hysterectomy.181 Beiner and associates182 confirmed these findings in a larger cohort of 90 patients who underwent radical vaginal trachelectomy for cervical cancer of 2 cm or less with fertility-sparing intent, who were clinicopathologically matched to a similar group of patients treated with radical hysterectomy. Their analysis indicated that histologically typed adenocarcinoma can be treated safely with radical trachelectomy when patients are appropriately selected (this type was found in 50% of the patients, and its frequency is increasing in younger women). Although some have reported an increased relapse rate in patients with lymphovascular space invasion, these authors postulate that lymphovascular space invasion alone (found in 68% of their cases), without other adverse pathologic prognostic factors, did not jeopardize the outcome in patients undergoing fertility-sparing surgery.182
The rate of relapse following vaginal trachelectomy has historically been higher for larger tumors (>2 cm). Recent advances, however, including the adoption of abdominal trachelectomy techniques, as well as possible neoadjuvant chemotherapy in larger-sized lesions, may broaden the feasibility of fertility-sparing options for more patients.183 Nonetheless, women who desire fertility-preserving surgery for early cervical cancer need to be carefully selected and adequately counseled regarding risk of recurrence and potential problems with subsequent pregnancy.
Adjuvant Postoperative Irradiation
Most clinicians recommend postoperative pelvic RT when histologic examination of a specimen resected during radical hysterectomy reveals involved positive lymph nodes, parametrial involvement, or positive tumor margins. Historically, the use of adjuvant RT in these patients led to an improvement in pelvic control but without clear evidence of survival benefit.184,185 However, present evidence, based on a randomized controlled trial, clearly shows an outcome advantage, including an OS benefit, for combined chemoradiation in such patients, who may be collectively defined as having pathologic evidence of extracervical disease following radical hysterectomy for clinical early-stage disease.107 In the absence of any gross tumor residual, a dose of 50 Gy to the whole pelvis is typically prescribed as adjuvant treatment. Although some clinicians also add a vaginal brachytherapy boost, the added therapeutic benefit of this is unclear, and it should be remembered that the results from the aforementioned trial were achieved without the use of intravaginal brachytherapy. Tumor features associated with a high risk of local recurrence, especially nodal metastases, also tend to be predictors of distant metastasis, decreasing the benefit of improved local control on survival rates. In addition, a number of factors may reduce the efficacy of postoperative RT.186 Higher doses of irradiation may be required to sterilize microscopic disease in a disturbed operative site. Furthermore, clinicians’ fears of causing major complications in patients who have had an extensive transperitoneal pelvic surgical procedure often limit the irradiation dose that can be administered safely.187–190
More controversial indications for adjuvant postoperative RT may include findings of a single microscopically positive node, bulky primary disease, deep stromal penetration, invasion into the lymphovascular space, or high-grade adenocarcinoma. The GOG conducted a trial (GOG 92) of postoperative RT versus observation alone following radical hysterectomy in stage IB cervical cancer with risk factors limited to the primary tumor (without extracervical spread or nodal metastases).191 Eligible patients were those modeled to have an approximately 3-year risk of failure of 30% following surgery alone, based on varying combinations of tumor size, lymphovascular space involvement, and depth of cervical stromal invasion. They are sometimes referred to as “intermediate-risk” patients. A total of 277 eligible patients were entered into the study between 1988 and 1995. Patients randomized to postoperative RT (no concurrent chemotherapy) received 50.4 Gy of whole-pelvis RT, without a vaginal brachytherapy boost. There was a statistically significant improvement in RFS at 2 years (88% vs. 79%) favoring the RT arm over the observation-only controls. There was also a higher incidence of severe toxicity in the radiation therapy cohort (6%) compared with the surgery only cohort (2.1%). An updated analysis with approximately 10 years of at-risk follow-up confirmed that adjuvant pelvic RT significantly reduced the risk of relapse and prolonged the PFS time in intermediate-risk early-stage cervical cancer patients. Although there was a trend toward improved OS for adjuvant RT, this did not reach conventional statistical significance (p = .074), which may have reflected an underpowered trial.192 An interesting observation from GOG 92 was that postoperative RT seemed particularly beneficial for patients with non–squamous cell histologic findings (adenocarcinoma, adenosquamous cancer). Although not a primary analysis endpoint of the trial, and not adjusted for by multivariate analysis, the magnitude of the reduction in relapse for non–squamous cell versus squamous cell histologic types was statistically significant (p = .019).192 At present, the use of adjuvant RT following radical hysterectomy for node-negative patients with multiple risk factors confined to the cervix is generally recommended, but its use should be based on careful consideration of patient physiology, surgicopathologic findings, and multidisciplinary consultation between the patient, surgeon, and radiation oncologist.
An NCI consensus conference concluded that “primary therapy should avoid the routine use of both radical surgery and RT.”9 If clinical evaluation reveals risk factors that would require pelvic RT after radical hysterectomy, RT should be considered the primary treatment of choice. Although improvements in imaging technology allow better determination of nodal disease and may eventually permit evaluation of more subtle intratumoral risk features, such as depth of stromal invasion and lymphovascular space involvement, the only tumor parameter that can be routinely evaluated before hysterectomy is tumor size. It is clear that the larger the cervical lesion, the more likely the indications for postoperative RT following radical hysterectomy. For this reason, many clinicians avoid primary surgery in patients with tumor diameters greater than 4 cm (stage IB2) because 50% to 80% of such cases undergo recommended postoperative RT.168,193,194
Although it is standard practice to screen women for cervical cancer before a hysterectomy is performed for any reason, unsuspected invasive cancer is occasionally discovered in the resected specimen from a simple hysterectomy performed for benign disease or CIN. If cancer invasion is more than 3 mm or if there is extensive invasion into the lymphovascular space, then there is a significant risk of paracervical or pelvic lymph node involvement, and postoperative pelvic RT or chemoRT for risk factors is indicated. With this treatment, the prognosis is still very good, with 5-year OS of approximately 80% if the tumor appears to have been confined to the cervix and if surgical margins are negative.195
Adjunctive Surgery
In a retrospective study of cases from the University of Texas MDACC, Durrance and associates196 suggested that the rate of central disease recurrence after radiation therapy of bulky (≥6 cm) endocervical cancers may be reduced when irradiation is combined with an extrafascial hysterectomy. Authors of a subsequent report emphasized that acceptable complication rates could be achieved only if the dose of irradiation was reduced and if surgery was limited to a simple (type I) extrafascial hysterectomy performed with careful, sharp dissection.197 After these reports, the use of combined treatment increased, and some clinicians began arbitrarily extending the use of adjunctive hysterectomy to treat patients with smaller endocervical tumors (<6 cm in diameter) and tumors with an exophytic morphology.
In 1992, Thoms and colleagues198 reexamined the experience of the MDACC and concluded that biases that led to the selection of patients with more favorable disease for combined treatment made it impossible to compare meaningfully the relative efficacy of the two approaches. Mendenhall and colleagues199 compared the outcome of patients treated before or after treatment policy changed at the University of Florida to include planned adjuvant hysterectomy. No difference in outcome was seen between patients treated before or after the change in policy.
The GOG conducted a phase III trial (GOG 71) in patients with stage IB2 cervical cancer randomized to receive RT alone versus RT followed by extrafascial hysterectomy (this trial did not use concurrent chemotherapy). Although adjuvant hysterectomy following primary radiotherapy appeared to modestly reduce the rate of local recurrence, there was no improvement in OS.200
There are no clear data to support the routine role of adjuvant hysterectomy after primary RT for bulky tumors confined to the cervix, particularly after optimal doses of irradiation have been delivered. In a large retrospective analysis of almost 3000 patients with stage I to II squamous cell cervical cancer treated with definitive radiotherapy, Eifel and colleagues201 reported very low central disease (cervix, upper vaginal, proximal paracervical tissues) recurrence rates of 6.8%, 7.8%, and 9.6% at 5, 10, and 20 years, respectively. This indicated that very few patients might benefit from adjuvant hysterectomy following adequate radiotherapy.201 However, surgery may be selectively applied in patients with a poor tumor response to RT, in those whose tumor and pelvic anatomy precluded good brachytherapy geometry, or as salvage treatment for isolated cervical cancer recurrence.
Locally Advanced Disease and Palliation
The treatment of choice for most patients with locoregionally advanced disease (IIB to IVA) is definitive RT with concurrent, usually cisplatin-based, chemotherapy. Radiation therapy consists of a combination of EBRT and brachytherapy. Most authorities today consider optimal RT as a cumulative point A dose of 80 to 90 Gy (in conventional equivalents), with all treatment completed within 8 weeks. Five-year OS of patients treated with chemoradiation now approach 70% to 80% for stage IIB disease, 50% to 60% for stage IIIB disease, and perhaps 15% to 25% for stage IVA disease103–109 (see Table 56-4). With aggressive treatment, even patients with massive, locoregionally advanced tumors have a realistic chance of being cured.
TABLE 56-4 Patterns of Failure in RTOG 90-01, Comparing Pelvic Irradiation with Concurrent Chemotherapy (CT/RT) versus Extended-Field Radiotherapy without Chemotherapy (EFRT)
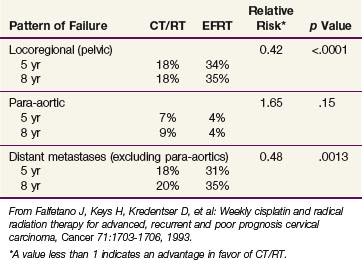
Salvage Therapy for Recurrent Disease
Following primary management for cervical cancer, a small percentage of patients will present with isolated locoregional failure for whom radical salvage therapy may be contemplated. Patient selection, careful “restaging,” and individualization of therapy are essential.202 The most important determinant of therapy options is the type of prior treatment the patient has received.
Patients who experience local recurrence after initial surgery alone may be salvaged by radical RT plus concurrent chemotherapy (as extrapolated from the primary management of locally advanced disease). Customized combinations of EBRT, often using highly conformal techniques including intensity-modulated radiation therapy (IMRT) and intracavitary or interstitial brachytherapy are used. Salvage RT is particularly effective for small central failures limited to the vagina or paravaginal tissues, with 5-year OS of 69% reported in one series.203,204
There are limited reports of radical reirradiation for patients who relapse following prior pelvic RT.205,206 However, radical surgery forms the primary cornerstone of salvage treatment for isolated pelvic recurrences without pelvic side wall disease in previously irradiated patients. In most cases, the only potentially curative option is pelvic exenteration. For highly selected patients with central recurrence, in whom pelvic exenteration is successfully completed, 5-year OS of approximately 50% may be achieved.207–209
Proximity or fixation of locally recurrent tumor to the pelvic side wall is an adverse prognostic finding, leading most investigators to suggest that attempted surgical salvage be abandoned with intraoperatively detected side wall involvement. However, some patients previously considered incurable with exenteration have survived with the incorporation of preoperative chemoradiation (EBRT is dose dependent on the amount of prior RT) followed by maximal resection plus intraoperative irradiation (IORT) directed to the initially involved pelvic side wall (i.e., to narrow or involved margins of resection).210,211 (See Chapter 16, Intraoperative Irradiation, for information on techniques for multiple disease sites, including the cervix, and their results.)
Advanced Disease and Palliation
Chemotherapy
Cisplatin has long been considered the most active single chemotherapy agent for the treatment of cervical carcinoma.212 Phase III studies comparing cisplatin 50 mg/m2 administered as a short infusion with other regimens have not confirmed an advantage with either higher doses or alternative infusion schedules.213,214 Furthermore, a phase II study conducted by the GOG suggested inferior response rates with the platinum analogs carboplatin and iproplatin.215 Subsequent phase II studies have identified a number of drugs with limited single-agent activity against cervical carcinoma, including mitolactol, ifosfamide, paclitaxel, topotecan, gemcitabine, vinorelbine, and bevacizumab216–222 (see web-only Table 56-1).
WEB-ONLY TABLE 56-1 Phase II Chemotherapy Trials in Metastatic or Recurrent Squamous Cell Carcinoma of the Cervix
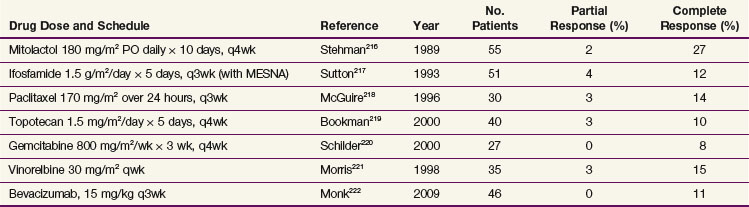
In an attempt to improve therapeutic efficacy, a number of phase III trials have investigated the use of drug combinations with cisplatin.223–227
Compared with cisplatin alone, Omura and colleagues223 showed a higher objective response rate and PFS with cisplatin plus ifosfamide; however, there was no improvement in OS, and toxicity rates with combination chemotherapy were significantly increased. Another GOG phase III trial demonstrated no advantage with the addition of bleomycin to the cisplatin plus ifosfamide combination.224 The GOG has compared single-agent cisplatin with a combination of cisplatin plus paclitaxel with quality-of-life assessments included among outcome measures. The combination of cisplatin plus paclitaxel resulted in a higher objective response rate, longer progression-free survival (PFS), and higher toxicity rate, with no apparent decrement in patient-reported quality of life.225 A prospective randomized trial comparing cisplatin with cisplatin plus topotecan demonstrated an improvement in OS with the combination regimen.226 Although statistically significant, many consider the gains clinically unimportant because they are of such short duration.
GOG 204 was a four-arm study comparing four cisplatin doublets with paclitaxel, topotecan, gemcitabine, and vinorelbine. After 513 patients were enrolled, a planned interim analysis recommended early closure of the trial based on the fact that none of the other arms would ever statistically show benefit over the control arm of cisplatin plus paclitaxel, demonstrating the challenge in achieving major progress for this patient population.227 PFS was as short as 4 to 6 months. Although multiagent systemic therapy remains an active area of investigation, the benefits of salvage chemotherapy remain modest at best (web-only Table 56-2).
WEB-ONLY TABLE 56-2 Phase III Trials of Cisplatin and Cisplatin-Containing Regimens in Advanced, Recurrent, or Metastatic Cervical Carcinoma
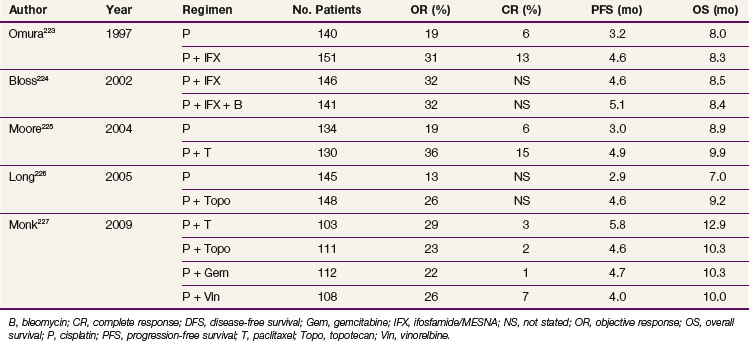
It is now recognized that a majority of patients who recur after initial therapy are not platinum-naive, having received platinum-based chemoradiation as definitive or adjuvant therapy. Previous exposure to platinum clearly influences the potential response when platinum is used in a palliative setting. In an analysis of prognostic factors for response to cisplatin-based systemic chemotherapy for recurrent or metastatic disease, Moore and colleagues228 identified five factors as independent predictors of a poor response: African-American patient, Eastern Cooperative Oncology Group (ECOG) performance status score greater than 0, pelvic disease, prior radiosensitizer, and time interval from diagnosis to first recurrence of less than 1 year. They suggest that with a simple prognostic index, which combines the risk factors, a subgroup of patients can be identified who are very unlikely to benefit from cisplatin-containing regimens and who should be evaluated for other novel investigational agents or best supportive care.228
Irradiation
Radiation therapy can also play an important role in the palliation of patients with incurable advanced disease. Hypofractionated pelvic irradiation usually controls bleeding and frequently provides some relief of pelvic pain. There is a paucity of data and no randomized trials on optimal palliative irradiation regimens. A variety of fractionation schemes have been used. No standardized instruments for measuring symptom relief have been used.229 The choice of dose and fractionation schedule will be influenced by expected survival times, patient performance status, symptoms, site(s) and bulk of tumor involvement, adjacent normal tissue considerations, medical comorbidities, and logistic considerations.
The RTOG reported the results of a phase I/II study in which patients with advanced pelvic malignant disease were given up to three fractions of 10 Gy at 1-month intervals with misonidazole.230 The overall objective response rate was 57%, but the rate of grade 3 and 4 toxicities was high (45%), although it is unclear if some of the complications may have been due to tumor progression. Studies of the MDACC experience with this method have demonstrated a symptomatic response rate of approximately 75% with two fractions of 10 Gy but have also revealed an unacceptable rate of major complications when a third fraction of 10 Gy was given.231,232
In a second RTOG study, accelerated split-course EBRT was used as palliative treatment for patients with advanced pelvic malignant disease. Patients received up to three courses of 14.8 Gy given in 3.7-Gy fractions twice daily over 2 days. A 3- to 6-week rest interval was given between courses. Patients who completed three courses of EBRT had an overall response rate of around 60%, with complete relief of pain achieved in 50% of patients.233
Irradiation Techniques/tolerance
External Beam Irradiation (EBRT)
Some radiation oncologists prefer to begin intracavitary irradiation as early as possible, even in patients with initial bulky disease, shielding central normal structures with a midline block on anteroposterior/posteroanterior (AP/PA) fields after 20 to 30 Gy. In the past, use of a midline block of 3 to 4 cm in diameter was considered standard.234 A review of urinary tract complications in patients treated with RT for FIGO stage I cervical cancers at the MDACC demonstrated a significantly higher rate of ureteral stenosis when parametrial EBRT was combined with relatively high doses of intracavitary therapy, presumably because the relatively narrow standard central blocks failed to protect the ureters from irradiation.235 For this reason, customized blocks are preferred when higher doses of parametrial irradiation are given.
To optimize the match between EBRT and intracavitary irradiation, Perez and colleagues236 developed an elaborate central gradient step-wedge block designed to compensate for the lateral dose gradient from intracavitary treatment. For this method, one of several standard gradient blocks is matched to the patient’s intracavitary distribution and used to shield central structures on AP/PA fields. They were able to deliver very high paracentral RT doses by using intracavitary therapy to give most of the central treatment. However, the gradient blocks can only be matched to the dose distribution calculated in a single plane (usually, in the center midcoronal plane of the implant). Theoretically, anterior and posterior structures (such as the uterosacral ligaments) may be underdosed with this method, although the survival rates reported by Perez and associates236 have generally been excellent.
In all cases, care must be taken to include all areas at risk for disease in the pelvis. Conventional field arrangements for treating the pelvis typically include the use of four fields (anterior, posterior, and two lateral fields). Occasionally, especially in thinner patients, anterior and posterior opposed fields are used, and this is generally tolerable as long as EBRT doses do not exceed 40 to 45 Gy. Historically, field borders that define the volume of the EBRT have been based on empirically derived bony anatomic landmarks. These bony landmarks, which are still widely promulgated in textbooks and radiotherapy protocol guidelines, represent the careful attempts of past clinical investigators to match skeletal anatomy, which can be readily appreciated radiographically, with potential soft tissue tumor extent, nodal drainage, and pathways of regional spread. These “standardized fields” for “field-based planning” are illustrated in Figure 56-11 for a four-field “box” technique. The anterior and posterior fields usually incorporated an inferior border placed at the mid or lower obturator foramina or at least 3 to 4 cm below the lowest extent of clinically appreciated cervical or vaginal disease, and lateral borders were placed approximately 1.5 to 2 cm beyond the bony margins of the true pelvis. Lateral fields generally were placed with an anterior border anterior to the symphysis pubis; the posterior border previously was historically described as passing through the S2 to S3 junction, but clinical examination and contemporary pelvic imaging have suggested that such a conventional posterior border would frequently shield gross tumor in patients with disease extending posteriorly, particularly in the uterosacral ligaments. Without the benefit of modern imaging techniques to approximate tumor extent, shielding of the anterior-superior border of the lateral fields must be done with care or possibly omitted because of the possibility of shielding disease in the upper external iliac nodes. Although broadly useful, the routine use of “standardized fields” based on bony anatomy has been questioned as lacking in precise individual case coverage based on intraoperative measurements and imaging studies.84,237–239
Many centers now use image-guided CT or MRI planning to design radiation volumes84 (Fig. 56-12). Although even these imaging techniques are inaccurate and may underestimate the extent of microscopic spread within the pelvis, they do at least allow a somewhat better approximation of the location of the nodes and gross disease. These imaging technologies clearly improve the ability to define gross tumor extent, but they also underscore several critical issues that need to be addressed. To take full advantage of these imaging modalities, radiation oncologists must have adequate training and formalized experience in interpreting radiologic studies. Findings from a detailed pelvic examination must be carefully incorporated into the treatment plan because there are areas of potential tumor extension, such as in the vagina and parametria, which are imprecisely defined by imaging alone. Planners must have a clear awareness of the potential pathways of regional and nodal spread from cervical cancer because it is not possible to obtain images of microscopic disease, and lack of such recognition may lead to inadequate coverage of the volume at risk. There are no data examining the necessity for inclusion of the whole uterine body, but most recommend its inclusion in the treated volume given the potential for myometrial extension of disease from the cervix.
Examples of EBRT ports for patients with cervical cancer based on contemporary CT simulation are shown in Figures 56-13 and 56-14. An example of a composite EBRT dosimetry plan is illustrated in Figure 56-15. Although CT-based treatment planning and field design allow customization based on individual patient findings, it should be noted that the general similarity of these fields to the traditional “standardized” bony anatomy–based fields serve as a testament to the clinical acumen and observational skills of previous clinicians who did not have access to the imaging modalities of today.
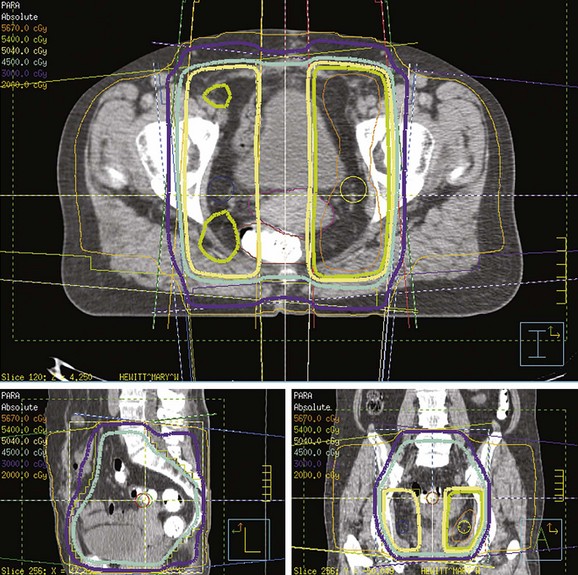
Figure 56-15 External beam dosimetry planning (for patient shown in Fig. 56-13). The prescribed dose was 45 Gy to the whole pelvis, with parametrial boosts to 50.4 Gy to the right and 54 Gy to the left (patient had bulkier disease on the left, with extension to the pelvic side wall). The central dose in this patient was supplemented by intracavitary brachytherapy.
Intensity-Modulated Irradiation (IMRT)
Exciting modern technologies in radiotherapy delivery such as intensity-modulated irradiation (IMRT) allow the delivery of highly conformal dose distributions unachievable using conventional approaches. They combine high-resolution imaging, advances in computer treatment software and linear accelerator collimation capabilities, inverse planning, and radiation beam flux modulation. IMRT has been most widely used in head and neck and prostate cancers, simultaneously allowing sparing of surrounding normal structures and dose intensification to the tumor target volume. The success of highly conformal radiotherapy such as IMRT in various tumor sites is heavily dependent on the accuracy of tumor delineation through imaging. Dosimetric evaluation of its use in gynecologic cancers suggests IMRT may significantly reduce unwanted radiation exposure to adjacent bowel and bladder while preserving tumor coverage.240 Early clinical experience at the University of Chicago has demonstrated a reduction in acute gastrointestinal toxicity for gynecologic cancer patients undergoing pelvic IMRT compared with contemporaneous historical controls treated with traditional standard techniques.241 Recent analysis has also indicated a decrease in acute hematologic suppression, favoring patients treated with IMRT, especially in those who also received chemotherapy.242 Although there is excitement about the potential eventual application of IMRT to definitive cervical cancer radiotherapy, questions remain about accurate target definitions, treatment standardization, intrapatient and interpatient reproducibility, and time-intensive requirements for treatment planning and delivery.
Currently, there is an emerging consensus that IMRT may be a useful technique in posthysterectomy irradiation where organ motion, particularly of the uterus and cervix, is absent. It may allow greater sparing of normal tissues displaced into the pelvis, provided that meticulous attention is paid to anatomic detail and contouring of targets. However, issues regarding dose heterogeneity within the target volume and broad “low-dose scatter” remain to be resolved.243,244 The RTOG atlas “Consensus Guidelines for the Delineation of the CTV in the Postoperative Pelvic Radiotherapy of Endometrial and Cervical Cancer” for posthysterectomy contouring is available at www.rtog.org/pdf_document/GYN-Atlas.pdf. Accrual has been completed for RTOG-0418, a phase II trial evaluating the use of pelvic IMRT in the posthysterectomy setting for patients with endometrial or cervical carcinoma. Preliminary analysis showed general feasibility for the use of posthysterectomy IMRT in multi-institutional trials when stringent guidelines are specified and quality assurance measures are employed.245 Current phase III trials in postoperative irradiation following radical hysterectomy for cervical cancer (RTOG 0724, GOG 263) permit the use of IMRT but require detailed institutional and individual investigator credentialing, as well as “real-time” central review of the first IMRT protocol case treated. An example of IMRT dosimetry in a patient undergoing adjuvant chemoradiation following radical hysterectomy for node-positive stage IB cervical cancer is shown in Figure 56-16; it shows highly conformal shaping of the higher isodoses, with relative sparing of small bowel, bladder, and some rectum compared with a more traditional four-field pelvic technique, despite the use of generous target volumes for the IMRT plan.
The role of IMRT for intact cervical cancer remains more nascent and must be considered investigational at present despite considerable current implementation. Target mobility, as well as tumor deformation/regression during a course of radiotherapy, is of greater concern in intact cancer cases than in the posthysterectomy setting.246–248 A preliminary attempt at target volume consensus in intact cervical cancer (based on a single case study) was recently published and showed appreciable variability in target volumes even when assessed by experienced gynecologic radiation oncologists.249 Prospective multicenter trials of IMRT in intact cervical cancer have been proposed but have not yet been activated.
Brachytherapy
Over the past two decades, manufacturers have withdrawn support for LDR systems. Comparison data from several randomized trials,250–253 reviews,254 and large single-institutional retrospective experiences255,256 have led to an increasing acceptance of the efficacy and tolerability of HDR brachytherapy for cervical cancer. Large clinical cooperative trial groups such as the GOG and RTOG accept that when performed appropriately and within clearly specified guidelines of dosing and fractionation, HDR and LDR intracavitary cervical brachytherapy regimens are therapeutically similar. Faced with issues related to LDR-associated personnel exposure, source regulation and storage, source replacement, and withdrawal of technical support from suppliers, most centers now use outpatient-based HDR brachytherapy for cervical cancer. In a patterns of care survey of cervical cancer cases treated between 1996 and 1999, 16.4% of patients who underwent brachytherapy had at least part of their therapy delivered using an HDR regimen.257 By 2005 to 2007, a new survey indicated that 55% of patients undergoing intracavitary brachytherapy for cervical cancer were treated with HDR techniques,258 and this percentage is undoubtedly higher now. Several major centers (e.g., the MDACC) have chosen to use pulsed-dose-rate (PDR) brachytherapy, arguing that it is more radiobiologically similar to LDR therapy and may cause fewer late complications.
Low-Dose-Rate Intracavitary Therapy
Many intracavitary applicator systems have been used to position sources in the uterus and vagina. All include a central uterine tandem; the major differences are in the design of the vaginal source holders. In the United States, most radiation oncologists use some variation of the Fletcher-Suit-Delclos applicator system that was first developed at the MDACC. The most characteristic feature of this system is the pair of cylindrical vaginal colpostats that are placed against the cervix on either side of the tandem. Ideally, these holders position the axis of the vaginal sources approximately perpendicular to the uterine tandem (Fig. 56-17). This positioning is designed to take advantage of the anisotropy of the sources, and, combined with the anterior and posterior tungsten shields incorporated in the design, to reduce the volume of bladder and rectum exposed to high doses of radiation while spreading the dose laterally to the parametria. For patients with a narrow vagina, unshielded miniovoids and Lucite cylinders of various sizes can be used to position the intravaginal sources. Another applicator system used successfully by a number of groups, including the Memorial Sloan-Kettering Cancer Center, is the Henschke applicator. With this system, the vaginal sources are positioned parallel to the axis of the intrauterine tandem.
Successful intracavitary therapy requires experience, skill, some pragmatism, and a willingness to persist until an “optimal placement” has been achieved.259 Radiation sources must be positioned in the uterus and vagina in a way that avoids underdosing areas in the cervix and paracervical tissues, with a sensitivity to the limits of mucosal tolerance. Every tumor presents a somewhat different challenge because of the wide variations in tumors and patient anatomy encountered clinically. A thorough discussion of the methods that can be used to address these variations is beyond the scope of this chapter, but some basic principles apply to most common clinical situations.
Although there has been considerable debate regarding the “optimal” intracavitary brachytherapy system, and there is no doubt that brachytherapy requires technical skill and attention to detail, it is also apparent that whether intracavitary brachytherapy is used is more important than the details of how it is placed. In the United States, most radiation oncologists are familiar with some variation of the Fletcher-Suit-Delclos applicator system, but other systems are also used. A recent dosimetric evaluation of the Fletcher-Suit-Delclos applicator system compared with the Henschke shielded applicator suggested that the latter system provided a dose-distribution advantage.260 Other large institutions in North America reported a similar therapeutic ratio using linear intrauterine sources alone, without colpostats, provided that the tandem sources extended to 1 cm below the cervix or the inferiormost extent of vaginal disease.261 What is clear is that the introduction of radioactive sources into the endocervical and endometrial canal permits a central tumor dose intensification that is unachievable by any EBRT technique, regardless of the nuances of source placement (Fig. 56-18). It is this very high radiation dose, placed in direct juxtaposition to the tumor, within a radiotolerant organ, that allows for control of even large cervical cancers.
The choice of vaginal applicator is determined by the vaginal capacity and by the distribution of any vaginal disease. Vaginal cylinders are usually reserved for patients who have a very narrow vagina or significant vaginal mucosal disease where the dose needs to be delivered inferiorly in the vagina. In general, when colpostats are used, their medial surfaces should be separated by 1 cm or less to avoid a central cold spot. Instead, to maximize the dose deliverable from intravaginal sources and optimize the relative depth dose, the vagina should be fitted with the largest ovoids that can be placed snugly against the cervix. The anterior and posterior lips of the cervix should always be marked with radiopaque seeds (see Fig. 56-17) so the relationship between the cervix and vaginal applicators can be verified on intraoperative films. Significant caudad displacement of the colpostats will dramatically reduce the dose to the cervical tumor and lead to increased vaginal stenosis and probable fistula risk. The colpostats should be centered over the cervical portion; unless the anterior or posterior lip is disproportionately involved, the colpostats should be bisected by the tandem on the lateral view. Very posterior displacement of the colpostats usually results in an underdosage to anterior disease and an unacceptable overdosage to the rectum, particularly to the portion of the rectum that is superior to the colpostat. The standard loadings for Fletcher-Suit-Delclos ovoids are 10 or 15 mgRaEq for 2 cm (small); 15 or 20 mgRaEq for 2.5 cm (medium); 20 or, rarely, 25 mgRaEq for 3 cm (large); and 5 to 10 mgRaEq for miniovoids. These loadings will result in a dose rate at the lateral vaginal surface of 75 to 110 cGy/hr. The higher-activity sources are used when the placement is excellent or when the patient has bulky central disease. The lower activities tend to be used for patients with small tumors, for suboptimal (but acceptable) positioning, and to keep the total vaginal surface dose beneath the cumulative maximum of 120 to 140 Gy.
Placement of the system can be performed under intravenous sedation with monitored anesthesia care; general or regional anesthesia may be preferred, unless the patient is at increased risk for anesthetic complications, because it permits a good examination of the pelvis and provides maximal relaxation of the perineal musculature during placement and packing of the intracavitary applicator. The system should be packed in place with lubricated gauze, preferably a type that contains a radiopaque thread, while the vagina is retracted by an assistant. Packing should be carefully placed anteriorly and posteriorly, displacing the rectum and bladder away from the system. Care should be taken not to force the cervix away from the colpostats during the packing. Gradual withdrawal of the retractors during the procedure permits placement of additional packing in the space occupied by the retractors. With firm packing, the risk of displacement during a 48- to 72-hour placement is minimal. However, when vaginal cylinders are used instead of packing or when a remote afterloading device is used, a stitch should be placed in the labia and secured to the system to prevent displacement. The accuracy of the applicator position should be confirmed while the patient is still under sedation by anteroposterior and lateral radiographs. If the placement is unsatisfactory, the system should be repositioned and repacked (see Fig. 56-17). For patients undergoing LDR brachytherapy with prolonged immobilization, thromboprophylaxis with subcutaneous heparin and sequential compression devices should be initiated as early as during anesthesia for insertion, given the elevated risk for deep venous thrombosis in this setting.
Dose Specification
Several conventional standardized methods have been used to specify the radiation dose delivered by an intracavitary system. Today, most clinicians describe treatment using one of several variations of the Manchester system. With this approach, the dose and dose rate are specified to a paracentral point (point A) and to a point near the pelvic wall (point B) (Fig. 56-19). Point A is placed 2 cm lateral and superior to the external cervical os along the axis of the intrauterine tandem, although a number of methods have been used to identify the position of this reference point. Because point A lies in a region of steep dose gradient, relatively small variations in its position can result in substantial differences in the calculated dose rate. For this reason, the use of point A or similar reference points was criticized by the International Commission on Radiation Units and Measurements (ICRU) in its 1985 report on intracavitary dose and volume specification.262 The commission recommended a system, used in some parts of Europe, in which the volume of tissue treated to a certain dose (usually, 60 Gy) and the reference air kerma (in micrograys [µGy] at 1 m) are specified. At the MDACC, a somewhat similar system is used, with additional emphasis on the vaginal surface dose, substituting mgRaEq hours for reference air kerma as a means of limiting pelvic integral dose and relying on a set of empirically derived rules concerning the position and activity of the radiation sources. When point A is used as a reference point for dosing and reporting, attention must also be given to the three-dimensional isodose distributions surrounding the entire implant.
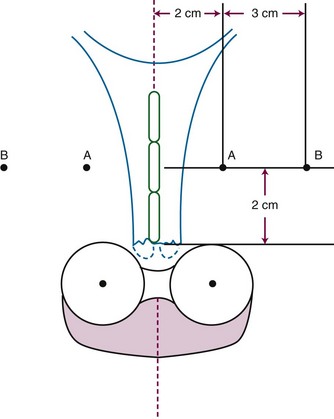
Figure 56-19 Location of point A and point B using the method recommended in ICRU 38.
From International Commission on Radiation Units and Measurements: Dose and Volume Specifications for Reporting Intracavitary Therapy in Gynecology. Vol 38. Bethesda, Maryland, ICRU, 1985, pp 1-23.
A well-placed LDR system loaded according to the preceding recommendations will produce a dose rate at point A of approximately 45 to 55 cGy/hr. Somewhat higher activity loadings have been recommended and used successfully. However, higher activity sources will result in higher dose rates and may have a somewhat greater biologic effect with the number of brachytherapy insertions limited to one or two; dose rates of more than 65 cGy/hr to point A should be used with caution. In a prospective randomized study, Haie-Meder and colleagues263 reported an increased rate of major complications when the dose rate to point A was increased from 40 to 80 cGy/hr.
LDR brachytherapy has been used successfully in combination with EBRT since the 1950s to treat thousands of patients and to achieve high local control and cure rates with a low risk of complications. The radiobiologic advantages of delivering brachytherapy at a low dose rate have been emphasized by many, and a number of clinicians continue to express skepticism about the comparability of HDR therapy, particularly in the treatment of patients with unfavorable anatomy, for whom the dose to critical normal tissues may be similar to the paracentral dose.264 On the other hand, increased acceptance of, experience with, and training in HDR brachytherapy techniques have led many to adopt outpatient-based HDR brachytherapy. Whether using LDR or HDR therapy, the importance of attention to anatomic detail, an understanding of normal tissue tolerance, an appreciation of radiobiologic principles, and an adherence to guidelines for loading and fractionation cannot be overemphasized.
High-Dose-Rate Brachytherapy
Investigators have been reporting their experiences with HDR brachytherapy for more than 20 years, describing thousands of patients with cervical cancer who were treated with this approach. The authors of numerous retrospective studies have demonstrated that HDR treatment is feasible, effective, and safe.254 In addition, there have been four randomized trials of HDR versus LDR brachytherapy250–253 (Table 56-5). Although each study had flaws, the data would suggest, in total, that HDR brachytherapy, within carefully applied dosing and fractionation guidelines, provides therapeutic equivalency to traditional LDR approaches.
TABLE 56-5 Randomized Trials of High-Dose-Rate (HDR) versus Low-Dose-Rate (LDR) Brachytherapy in Cervical Cancer
The LQ model has been used to describe a biologically effective dose (BED), given as
There is emerging evidence that small volumes of the rectum and bladder can tolerate a total of 130 to 140 Gy3. Ogino and associates265 did not observe any severe grade 4 rectal complications with a dose equivalent of less than 147 Gy3 to the rectum. Lower grade 2 to 3 rectal toxicity was reported in less than 5% of patients with a BED of less than 119 Gy3 and in less than 10% with a BED of less than 146 Gy3. Their current fractionation schedules are designed to keep the BED for late-responding tissues to less than 119 Gy3 for early-stage disease and to less than 147 Gy3 for locally advanced cancers, allowing a tailoring of dose to disease extent.265
HDR fractionation schedules reported in the literature vary markedly, with insertion numbers and point A dose fraction sizes ranging from 2 to 7 Gy and 3 to 14 Gy, respectively.254 Assuming an α/β ratio of 3 for late damage to normal tissues, Stitt and colleagues266 used the LQ model to predict how a dose of HDR brachytherapy would have to be divided so its effect on normal tissue was comparable to that of 70 Gy of LDR brachytherapy. According to their model, five or six fractions are sufficient if the dose to critical normal tissues is 80% or less of the dose to the paracentral reference point. If the anatomy is somewhat less favorable and the normal tissue dose is 90% of the tumor dose, however, more fractions may be considered.
A detailed analysis of 24 reported series studying HDR brachytherapy in locally advanced cervical cancer failed to identify a dose-response relationship for either tumor control or late tissue complications.267 Although this does not refute the importance of radiobiologic considerations and calculations in the implementation of HDR brachytherapy, it does underscore the complexity of patient- and treatment-related variables involved in the radiotherapeutic management of cervical cancer and the difficulty in reducing HDR dosing issues to a simple mathematical formula.
Viswanathan and colleagues268 reported on a recent survey of international HDR brachytherapy practice among members of the Gynecologic Cancer Intergroup (GCIG). The average EBRT dose to the pelvis approximated 45 Gy. For stage IB to IIA tumors, the three most common regimens were 6 Gy for five fractions, 6 Gy for four fractions, and 7 Gy for three fractions, with brachytherapy doses predominantly prescribed to point A. For stage IIB to IVA tumors, the three most common regimens were 6 Gy for five fractions, 7 Gy for four fractions, and 7 Gy for three fractions.231a For its most recent protocols in locally advanced cervical cancer (where HDR therapy was permitted), the GOG had prescribed a whole-pelvic dose of 45 Gy, supplemented by an HDR boost of 6 Gy to point A for five fractions, as well as a 5.4- to 9-Gy parametrial boost to the involved side(s). This regimen, when converted using the LQ model, corresponded to a calculated LDR/conventional equivalent dose of 85 Gy to point A. However, it has recently been recognized that absolute adherence to inflexible guidelines does not take into consideration the uncertainties in assumptions and applications of the LQ model, nor the specific tumor geometry and patient factors associated with each case, leading to potential increased toxicity. Although the LQ model is a useful tool for comparing fractionation schemes, no mathematical formula can take the place of clinical judgment and observation. Going forward, the GOG has proposed modifications of its dosing parameters in locally advanced cervical cancer as follows: a whole-pelvis dose of 41.4 to 45 Gy, followed by HDR brachytherapy of 5.4 to 6 Gy for five fractions, with optional parametrial boosting based on tumor regression during EBRT. This new “scheme,” while seeking to maintain a minimal LDR equivalent dose of 80 Gy to point A, provides for flexibility in HDR application and allows for dose escalation to 85 Gy as clinically appropriate. To preserve biologic equivalency dosing to late-reacting normal tissues, the GOG has recommended that the ICRU rectal dose point receive no more than 4.1 Gy (68% of the point A dose) and the bladder dose point no more than 4.6 Gy (77% of the point A dose) per insertion. The increased flexibility in dose shaping with HDR brachytherapy may be useful in optimizing tumor-to-normal-tissue dose ratios.
The literature describes a wide range of sedation methods (none involving spinal or general anesthesia) used during the administration of HDR brachytherapy. Most commonly, conscious sedation is used. This involves administration of intravenous midazolam and fentanyl by a trained nurse. To accommodate conscious sedation in the radiation oncology clinic, the patient must at least be continuously monitored by pulse oximetry and blood pressure measurements. The physician and nurse must be certified in conscious sedation procedures, be up to date on cardiopulmonary resuscitation measures, and have ready access to a resuscitation cart. In a review of 124 patients with cervical cancer treated at the University of Wisconsin with HDR brachytherapy, the median doses of midazolam and fentanyl given during each procedure were 8 mg (2 to 40 mg) and 200 µg (50 to 600 µg), respectively.269 After review of an MRI if available, the first HDR insertion in each patient may be facilitated with transabdominal ultrasound guidance for placement of the intrauterine tandem. If the endocervical card is difficult to negotiate for insertion of the tandem, an intrauterine Smit sleeve may be placed at the first implant to facilitate subsequent applications.
At present, most standard HDR loading schemes attempt to recapitulate the familiar and symmetric “pear-shaped” dose distribution typically used in LDR brachytherapy. This isodose distribution can be obtained by designating a series of dose points surrounding the tandem and colpostat and using the dosimetry program to calculate source dwell times in each source location to create the desired dose distribution (inverse planning). Alternatively, the physician may specify the relative weight of each selected dwell position in the brachytherapy apparatus, somewhat analogous to the loading of individual sources in an LDR system. Most HDR practitioners use tandem and ovoids that are based on the LDR Fletcher-Suit-Delclos applicator. Others, however, favor a tandem and ring system that has the advantage of having fixed geometry and potentially simplified dosimetric planning (Fig. 56-20).
A potential advantage of HDR brachytherapy that has yet to be fully exploited is the greater degree of dose optimization that may be achieved compared with LDR approaches. Extensive international efforts in three-dimensional image-guided and adaptive brachytherapy are leading, perhaps prematurely, to a shift from the present central symmetry of a classical “pear-shaped” distribution inherent in the point A system toward a more conformal and often asymmetric dose distribution biased toward residual tumor volume. Although CT imaging can help in delineating adjacent organs at risk, MRI-based brachytherapy is preferable for defining tumor target volumes.270,271 It has been suggested that MRI image-guided brachytherapy allows customization that decreases the dose to adjacent organs at risk when treating small tumors, while permitting dose escalation in larger tumors to improve local control.272–274 To further tailor the brachytherapy dose, investigators at the University of Vienna have developed an applicator that combines a tandem and ring with ring-templated parametrial interstitial needles, and have reported enhanced and effective brachytherapy coverage of asymmetric, bulky tumors.276 Although it is intriguing, the use of image-guided brachytherapy remains to be standardized and validated on a wide scale; this forms the basis for the ongoing IntErnational study on MRI-guided BRachytherapy in locally Advanced CErvical cancer (EMBRACE) trial.273 Interobserver reproducibility of target definitions to published principles (such as the GEC-ESTRO guidelines)271 and multi-institutional assessment of tumor control and complication rates will be evaluated. A clear danger would be underestimation of residual tumor volume for brachytherapy planning. Also of concern is that strict adherence to proposed dose volume constraints for organs at risk may result in tumor underdosage compared with that achieved with conventional dosing. Furthermore, there remain unanswered the issues of resource utilization and whether multiple MRI scans and complex planning are feasible for the majority of patients with cervical cancer. A recent survey of practice patterns by members of the American Brachytherapy Society showed that very few centers in the United States used MRI-based brachytherapy. Although the majority of physicians used CT postimplantation imaging rather than plain films for visualizing the gynecologic brachytherapy apparatus, most (76%) still prescribe to point A alone, indicating the need for further development and experience with three-dimensional image-guided brachytherapy dosimetry.277
Interstitial Brachytherapy
Interstitial brachytherapy has long been used to treat patients with vaginal disease recurrence after hysterectomy and to increase the dose of irradiation delivered to the distal vagina in the rare patient who presents with extensive vaginal disease. However, several groups have advocated a broader role for interstitial irradiation by reporting on the use of large interstitial implants, usually guided by a perineal template, for patients with a variety of clinical presentations, including poor anatomy, bulky disease, or extensive involvement in the parametrium or pelvic wall. Initial reports of this technique were enthusiastic, and high local control rates were reported278–280 (see Chapter 14, Brachytherapy). Unfortunately, despite having more than 20 years of clinical experience, researchers have published few reports of long-term outcomes in patients with primary cervical carcinoma treated with this technique. A 1995 report281 of the combined experiences of Stanford University and the Joint Center for Radiation Therapy at Harvard University was disappointing, with 3-year DFS of only 36% and 18% for patients with FIGO stages IIB and IIIB disease, respectively, and high rates of major complications. A review of 1992 to 1994 practice patterns from the Patterns of Care Study indicates that less than 6% of patients in the United States are treated with interstitial brachytherapy. Comparisons across series are rendered difficult by patient selection and the inherent case individualization in technique and dosimetry.
For selected patients with very difficult clinical presentations, this may, however, be a useful technique. Full understanding of its efficacy awaits further reports of mature clinical experiences, but its use should probably be limited to selected institutions with sufficient experience in using an interstitial approach. Because of the variability of interstitial approaches, the use of interstitial brachytherapy is currently not permitted in phase III randomized trials of irradiation or chemoradiation in cervical cancer. As noted earlier, a novel approach, using the Vienna applicator that combines both intracavitary and interstitial techniques, has been found effective in brachytherapy of selected patients with bulky, asymmetric tumors.276
Normal Tissue Effects of Irradiation
An appreciation of the tolerance of normal tissues to irradiation is needed to determine optimal treatment doses. Experience suggests that the upper third of the vaginal mucosa can tolerate radiation doses as high as 120 to 140 Gy, whereas the lower two-thirds usually should not receive more than 80 to 85 Gy. Most institutions do not exceed 75 to 80 Gy (combined EBRT and LDR intracavitary dose) to the ICRU bladder reference point and 70 to 75 Gy to the ICRU rectal point, based on orthogonal plain film dosimetry.282 These reference doses should be used only as general guidelines, however. It has long been recognized, using CT-based dosimetry, that small volumes of rectum and bladder routinely receive much higher doses than those calculated from orthogonal films.283 Also, concern about reference dose limits should always be balanced against the need to deliver a tumoricidal dose to the cancer.
Historical reports of severe late normal complications, usually defined as injury to the bladder, rectum, or small bowel requiring hospitalization or surgical intervention, typically range from 5% to 10% using crude estimates, but they may be higher using actuarial calculations.284,285 Complications are related to the presenting stage and volume of disease, as well as to the dose delivered to the individual normal tissues. The severe complications occur in the first 3 to 5 years after completion of RT, but there remains a small but continuous added risk thereafter.285 Smoking during RT has been associated with a significantly higher risk of late complications.154 Most reports of late morbidity have focused on severe gastrointestinal and genitourinary toxicities; only recently have rigorous efforts been made to monitor for less severe sequalae that, while not requiring surgery or extensive medical care, still have an impact on patients’ quality of life. Other late effects, such as pelvic insufficiency fracture, vaginal stenosis, lymphedema, and neuropathy, are now recognized as important consequences. The recent use of concurrent chemoradiation has been clearly shown to increase acute toxicities, especially with respect to hematologic and gastrointestinal function, compared with RT alone, but while there does not appear to be a coincident increase in late morbidity, accurate long-term follow up data are scant.286 Unfortunately, most prospective clinical trials have based much of their toxicity assessment on the NCI common toxicity criteria scales, which favor acute effects reporting. Newer trials that emphasize late effects, and employ validated quality-of-life assessment tools, will give us much needed and useful information.
Radiobiology principles may predict an unacceptable complication rate for patients treated with curative HDR brachytherapy if the rectum and bladder receive the same dose as point A. With careful retraction and packing, however, the doses to these normal tissues can usually be substantially reduced, and studies generally have not demonstrated an increased complication rate when the dose of HDR therapy is adequately fractioned.250,254 As with LDR brachytherapy, careful technique and the expertise of the brachytherapist may be as important as any other factor. These parameters are very difficult to evaluate. Studies have suggested that the complication rates from HDR treatment of cervical cancer tend to decrease with increasing institutional experience.287–289
Treatment Algorithm, Conclusions, and Future Possibilities
Aggressive radiation therapy, including a combination of EBRT and brachytherapy, given concurrently with cisplatin-based chemotherapy, is now the primary treatment of choice in the management of bulky and locoregionally advanced disease (Fig. 56-21). Irradiation remains the most active single modality for the treatment of cervical cancer not amenable to surgical resection or sterilization. Improvements in RT targeting and delivery are being realized by advances in imaging modalities, including PET scanning, careful conformal radiation treatment planning, and appropriate incorporation of brachytherapy. Current efforts in image-guided RT, including brachytherapy, may eventually provide confirmation that such techniques reduce toxicity and/or allow for appropriate tumor-directed dose escalation that can improve tumor control and cure.
1 Makuc DM, Freid VM, Kleinman JC. National trends in the use of preventive health care by women. Am J Public Health. 1989;79:21-26.
2 Jemal A, Siegal R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
3 Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33-64.
4 Whelan SL, Parkin DM, Masuyer E. Patterns of cancer in five continents. Lyon, France: International Agency for Research on Cancer; 1990.
5 Cannistra SA, Niloff JM. Cancer of the uterine cervix. N Engl J Med. 1996;334:1030-1038.
6 Agarwal SS, Sehgal A, Sardana S, et al. Role of male behavior in cervical carcinogenesis among women with one lifetime sexual partner. Cancer. 1993;72:1666-1669.
7 Castellsague X, Bosch FX, Munoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105-1112.
8 Munoz M, Bosch FX, deSanjose S, et al. The role of HPV in the etiology of cervical cancer. Mutat Res. 1994;305:293-301.
9 National Institutes of Health Consensus Development Conference Statement on Cervical Cancer. Gynecol Oncol. 1997;66:351-361.
10 Tenti P, Romagnoli S, Silini E, et al. Human papillomavirus types 16 and 18 infection in infiltrating adenocarcinoma of the cervix. PCR analysis of 138 cases and correlation with histologic type and grade. Am J Clin Pathol. 1996;106:52-56.
11 Sigurdsson K. Effect of organized screening on the risk of cervical cancer. Evaluation of screening activity in Iceland, 1964-1991. Int J Cancer. 1993;54:563-570.
12 Stubblefield PG. Oral contraceptives and neoplasia. J Reprod Med. 1984;29:524.
13 Herbst AL, Cole P, Norusis MJ. Epidemiologic aspects and factors related to survival in 384 registry cases of clear cell adenocarcinoma of the vagina and cervix. Am J Obstet Gynecol. 1979;135:876-886.
14 Devessa SS, Silverman DT, Young JLJ, et al. Cancer and mortality trends among whites in the United States. J Natl Cancer Inst. 1987;79:701-770.
15 Koss LG. The Papanicolaou test for cervical cancer detection. A triumph and a tragedy. JAMA. 1989;261:737-743.
16 Peterson O. Spontaneous course of cervical precancerous conditions. Am J Obstet Gynecol. 1956;72:1063.
17 Koss LG, Stewart FW, Foote FW, et al. Some histological aspects of behavior of epidermoid carcinoma in situ and related lesions of the uterine cervix. Cancer. 1963;16:1160.
18 Clemmesen J, Poulsen H: Report of the Ministry of the Interior, Document 3. Copenhagen, 1971.
19 Kottmeier HL. Evolution et traitment des epitheliomas. Rev Fr Gynecol Obstet. 1961;56:821.
20 Richart RM, Barron BA. A follow-up study of patients with cervical dysplasia. Am J Obstet Gynecol. 1969;105:386.
21 Walton RJ. Cervical cancer screening programs. Can Med Assoc J. 1982;127:953.
22 Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System. Terminology for reporting results of cervical cytology. JAMA. 2001;287:2114-2119.
23 ACOG Practice Bulletin no.109. Cervical cytology screening. Obstet Gynecol. 2009;114:1409-1420.
24 Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003;349:1501-1509.
25 Robert ME, Fu YS. Squamous cell carcinoma of the uterine cervix. A review with emphasis on prognostic factors and unusual variants. Semin Diagn Pathol. 1990;7:173.
26 Wentz WB, Reagan JW. Survival in cervical cancer with respect to cell type. Cancer. 1959;12:384-388.
27 Sheets EE, Berman ML, Hrountas CK, et al. Surgically treated, early-stage neuroendocrine small-cell cervical carcinoma. Obstet Gynecol. 1988;71:10-14.
28 Silva EG, Kott M, Ordonez NG. Endocrine carcinoma intermediate cell type of the uterine cervix. Cancer. 1984;54:1705.
29 Parazzini F, LaVecchia C. Epidemiology of adenocarcinoma of the cervix. Gynecol Oncol. 1990;39:40.
30 Peters RK, Chao A, Mack TM, et al. Increased frequency of adenocarcinoma of the uterine cervix in young women in Los Angeles County. J Natl Cancer Inst. 1986;1986:423.
31 Plentl AA, Friedman EA. Lymphatics of the cervix uteri. In: Lymphatic System of Female Genitalia. Philadelphia: WB Saunders; 1971:75-115.
32 Netter FH. The CIBA Collection of Medical Illustrations. Vol 2: Reproductive System, Summit. New Jersey: CIBA Pharmaceutical Products; 1988.
33 Berman ML, Keys H, Creasman W, et al. Survival and patterns of recurrence in cervical cancer metastatic to periaortic lymph nodes (a Gynecologic Oncology Group study). Gynecol Oncol. 1984;19:8-16.
34 Delgado G, Bundy BN, Fowler WC, et al. A prospective surgical pathological study of Stage I squamous carcinoma of the cervix. A Gynecologic Oncology Group study. Gynecol Oncol. 1989;36:314-320.
35 Lagasse LD, Creasman WT, Singleton HM, et al. Results and complications of operative staging in cervical cancer. Experiences of the Gynecologic Oncology Group. Gynecol Oncol. 1980;9:90-98.
36 Lee Y-N, Wang KL, Lin M-H, et al. Radical hysterectomy with pelvic lymph node dissection for treatment of cervical cancer. A clinical review of 954 cases. Gynecol Oncol. 1989;32:135-142.
37 Eifel PJ, Morris M, Wharton JT, et al. The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1994;29:9-16.
38 Hoskins WJ. Prognostic factors for risk of recurrence in Stages Ib and IIa cervical cancer. Baillière’s Clin Obstet Gynecol. 1988;2:817-828.
39 Fagundes H, Perez CA, Grigsby PW, et al. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992;24:197-204.
40 Kim RY, Weppelmann B, Salter MM, et al. Skeletal metastases from cancer of the uterine cervix. Frequency, patterns, and radio-therapeutic significance. Int J Radiat Oncol Biol Phys. 1987;13:705-708.
41 Stehman FB, Bundy BN, Disaia PJ, et al. Carcinoma of the cervix treated with radiation therapy. I. A multivariate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776-2785.
42 Los Alamos National Laboratory HPV sequence database. Available at www.stdgen.lanl.gov/stdgen-virus/hpv/
43 Durst M, Glitz D, Schneider A, et al. Human papillomavirus type 16 gene expression and DNA replication in cervical neoplasia analysis by in situ hybridization. Virology. 1992;189:132-140.
44 Ferenczy A, Winkler B. Cervical intraepithelial neoplasia and condyloma. In: Kurman R, editor. Blaustein’s Pathology of the Female Genital Tract. New York: Springer-Verlag; 1987:117-217.
45 Boshart M, Gissmann L, Ikenberg H, et al. A new type of papillomavirus DNA. Its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984;3:1151-1157.
46 Durst M, Gissman L, Ikenberg H, et al. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy specimens from different geographic regions. Proc Natl Acad Sci U S A. 1983;80:3812-3815.
47 Crum CP, Levine RU. Human papillomavirus infection and cervical neoplasia. New perspectives. Int J Gynecol Pathol. 1984;3:376-388.
48 Crum CP, Ikenberg H, Richart RM, et al. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984;310:880-883.
49 Crum CP, Mitao M, Levine RU, et al. Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol. 1985;54:675-681.
50 Koutsky LA, Holmes KK, Critchow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992;327:1272-1278.
51 Reid R, Crum CP, Herschmann BR, et al. Genital warts and cervical cancer. III. Subclinical papillomaviral infection and cervical neoplasia are linked by a spectrum of continuous morphologic and biologic change. Cancer. 1984;53:943-953.
52 Willett GD, Kurman RJ, Reid R, et al. Correlation of the histologic appearance of intraepithelial neoplasia of the cervix with human papillomavirus types. Emphasis on low grade lesions including so-called flat condyloma. Int J Gynecol Pathol. 1989;8:18-25.
53 Arbeit JM, Munger K, Howley PM, et al. Neuroepithelial carcinomas in mice transgenic with human papillomavirus type 16 E6/E7 ORFs. Am J Pathol. 1993;142:1187-1197.
54 Lambert PF, Pan H, Pitot HC, et al. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 cervical cancer oncogenes in the skin of transgenic mice. Proc Natl Acad Sci U S A. 1993;90:356-364.
55 Griep AE, Lanbert PF. Role of papillomavirus oncogenes in human cervical cancer. Transgenic animal studies. Proc Soc Exp Biol Med. 1994;206:24-34.
56 Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
57 Southern SA, Herrington CS. Disruption of cell cycle control by human papillomaviruses with special reference to cervical carcinoma. Int J Gynecol Oncol. 2000;10:263-274.
58 Ho GYF, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423-438.
59 Moscicki AB, Palefsky J, Smith G, et al. Variability of human papillomavirus DNA testing in a longitudinal cohort of young women. Obstet Gynecol. 1993;82:578-585.
60 Nasiell K, Roger V, Nasiell M. Behavior of mild cervical dysplasia during long-term follow-up. Obstet Gynecol. 1986;67:665-669.
61 Vernon SD, Hart CE, Reeves WC, et al. The HIV-1 tat protein enhances E2-dependent human papillomavirus 16 transcription. Virus Res. 1993;27:133-145.
62 Slattery ML, Robison LM, Schuman KL, et al. Cigarette smoking and exposure to passive smoke are risk factors for cervical cancer. JAMA. 1989;261:1593-1598.
63 Sierra-Torres CH, Tyring SK, Au WW. Risk contribution of sexual behavior and cigarette smoking to cervical neoplasia. Int J Gynecol Cancer. 2003;13:617-625.
64 Hellberg D, Nilsson S, Haley NJ, et al. Smoking and cervical intraepithelial neoplasia. Nicotine and cotinine in serum and cervical mucus in smokers and nonsmokers. Am J Obstet Gynecol. 1988;158:910-913.
65 Barton SE, Maddox PH, Jenkins D, et al. Effect of cigarette smoking on cervical epithelial immunity. A mechanism for neoplastic change? Lancet. 1988;2:652-654.
66 Halpert R, Fruchter RG, Sedlis A, et al. Human papillomavirus and lower genital neoplasia in renal transplant patients. Obstet Gynecol. 1986;68:251-258.
67 Piper JM. Review. Oral contraceptives and cervical cancer. Gynecol Oncol. 1985;22:1-14.
68 Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645-1651.
69 Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women. A randomized double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271-278.
70 Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18. Follow-up from a randomised control trial. Lancet. 2006;367:1247-1255.
71 Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359:821-832.
72 Dempsey AF, Gebremariam A, Koutsky LA, Manhart L. Using risk factors to predict human papillomavirus infection. Implications for targeted vaccination strategies in young adult women. Vaccine. 2008;26:1111-1117.
73 FIGO Committee on Gynecologic Oncology. Revised FIGO staging for carcinoma of the vulva, cervix and endometrium. Int J Gynecol Obstet. 2009;105:103-104.
74 Pecorelli S, Zigliani L, Odicino F. Special communication. Revised FIGO staging for carcinoma of the cervix. Int J Gynecol Obstet. 2009;105:107-108.
75 Piver MS, Barlow JJ. High dose irradiation to biopsy confirmed aortic node metastases from carcinoma of the uterine cervix. Cancer. 1977;39:1243-1246.
76 Wharton JT, Jones HWI, Day T, et al. Preirradiation celiotomy and extended field irradiation for invasive carcinoma of the cervix. Obstet Gynecol. 1977;49:333-338.
77 Weiser EB, Bundy BN, Hoskins WJ, et al. Extraperitoneal versus transperitoneal selective para-aortic lymphadenectomy in the pretreatment surgical staging of advanced cervical carcinoma (a Gynecologic Oncology Group study). Gynecol Oncol. 1989;33:283-289.
78 Querleau D, LeBlanc E, Castelain B. Laparoscopic pelvic lymphadenectomy in the staging of early carcinoma of the cervix. Am J Obstet Gynecol. 1991;164:579-581.
79 Benedetti-Panici P, Maneschi F, Cutillo G, et al. Laparoscopic abdominal staging in locally advanced cervical cancer. Int J Gynecol Cancer. 1999;9:194-197.
80 Goff BA, Muntz HG, Paley PJ, et al. Impact of surgical staging in women with locally advanced cervical cancer. Gynecol Oncol. 1999;74:436-442.
81 Moore DH, Stehman FB. What is the appropriate management of early stage cervical cancer (International Federation of Gynecology and Obstetrics Stages I and IIA), surgical assessment of lymph nodes, and role of therapeutic resection of lymph nodes involved with cancer? Monogr Natl Cancer Inst. 1996;21:43-46.
82 Russell AH, Shingleton HM, Jones WB, et al. Diagnostic assessments in patients with invasive cancer of the cervix. A national pattern of care study of the American College of Surgeons. Gynecol Oncol. 1996;63:159-165.
83 Hricak H, Powell CB, Yu KK, et al. Invasive cervical carcinoma. Role of MR imaging in pretreatment work-up. Cost minimization and diagnostic efficacy analysis. Radiology. 1996;198:403-409.
84 Russell AH, Walter JP, Anderson MW, et al. Sagittal magnetic resonance imaging in the design of lateral radiation treatment portals for patients with locally advanced squamous cancer of the cervix. Int J Radiat Oncol Biol Phys. 1992;23:449-455.
85 Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745-3749.
86 Yen TC, Ng KK, Ma SY, et al. Value of dual-phase 2-fluoro-2-deoxy-D-glucose positron emission tomography in cervical cancer. J Clin Oncol. 2003;21:3651-3658.
87 Kidd EA, Siegel BA, Dehdashti F, et al. Lymph node staging by positron emission tomography in cervical cancer. Relationship to prognosis. J Clin Oncol. 2010;28:2108-2113.
88 Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. J Am Med Assoc. 2007;209:2289-2295.
89 Barillot I, Horiot JC, Pigneux J, et al. Carcinoma of the intact uterine cervix treated with radiotherapy alone. A French Cooperative Study. Update and multivariate analysis of prognostic factors. Int J Radiat Oncol Biol Phys. 1997;38:969-978.
90 Lanciano RM, Won M, Coia L, et al. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix. A final report of the 1973 and 1978 Patterns of Care Studies. Int J Radiat Oncol Biol Phys. 1991;20:667-676.
91 Perez CA, Camel HM, Kuske RR, et al. Radiation therapy alone in the treatment of carcinoma of the uterine cervix. A 20-year experience. Gynecol Oncol. 1986;23:127-140.
92 Horiot JC, Pigneux J, Pourquier H, et al. Radiotherapy alone in carcinoma of the intact uterine cervix according to G. H. Fletcher guidelines. A French cooperative study of 1383 cases. Int J Radiat Oncol Biol Phys. 1988;14:605-611.
93 Perez CA, Grigsby PW, Nene SM, et al. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer. 1992;69:2796-2806.
94 Logsdon MD, Eifel PJ. FIGO IIIB squamous cell carcinoma of the cervix. An analysis of prognostic factors emphasizing the balance between external-beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Phys. 1999;43:763-775.
95 Symonds P, Kirwan J, Williams C, et al. Concomitant hydroxyurea plus radiotherapy versus radiotherapy for carcinoma of the uterine cervix (review). Cochrane Database Syst Rev. 2004;1:CD003918.
96 Overgaard J, Bentzen SM, Kolstad P, et al. Misonidazole combined with radiotherapy in the treatment of carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1989;16:1069-1072.
97 Stehman FB, Bundy BN, Thomas G, et al. Hydroxyurea versus misonidazole with radiation in cervical carcinoma. Long-term follow-up of a Gynecologic Oncology Group trial. J Clin Oncol. 1993;11:1523-1528.
98 Dische S, Chassagne D, Hope-Stone HF, et al. A trial of Ro 03-8799 (pimonidazole) in carcinoma of the uterine cervix. An interim report from the Medical Research Council Working Party on advanced carcinoma of the cervix. Radiother Oncol. 1993;26:93-103.
99 Grigsby PW, Winter K, Wasserman TH, et al. Irradiation with or without misonidazole for patients with stages IIIB and IVA carcinoma of the cervix. Final results of RTOG 80-05. Int J Radiat Oncol Biol Phys. 1999;44:513-517.
100 Chan P, Milosevic M, Fyles A, et al. A phase III randomized study of misonidazole plus radiation versus radiation alone for cervix cancer. Radiother Oncol. 2004;70:295-299.
101 National Institutes of Health. Cervical Cancer. NIH Consens Statement. 1996;14:1-38.
102 National Cancer Institute. Clinical Announcement: Concurrent Chemoradiation for Cervical Cancer. Bethesda, Maryland: National Institutes of Health; February 1999.
103 Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stages IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes. A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339-1348.
104 Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based chemoradiation improves progression-free and overall survival in advanced cervical cancer. Results of a randomized Gynecologic Oncology Group study. N Engl J Med. 1999;340:1144-1153.
105 Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy versus pelvic and para-aortic radiation for high-risk cervical cancer. A randomized Radiation Therapy Oncology Group clinical trial. N Engl J Med. 1999;340:1137-1143.
106 Keys HM, Bundy BN, Stehman FB, et al. A comparison of weekly cisplatin during radiation therapy versus irradiation alone, each followed by adjuvant hysterectomy in bulky stage IB cervical carcinoma. A randomized trial of the Gynecologic Oncology Group. N Engl J Med. 1999;340:1154-1161.
107 Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjunctive therapy after radical surgery in high-risk, early-stage carcinoma of the cervix. J Clin Oncol. 2000;18:1606-1713.
108 Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer. An update of Radiation Therapy Oncology Group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872-880.
109 Rose PG, Ali S, Watkins E, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer. A Gynecologic Oncology Group study. J Clin Oncol. 2007;25:2804-2810.
110 Stehman FB, Ali S, Keys HM, et al. Radiation therapy with or without weekly cisplatin for bulky stage IB cervical carcinoma. Follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol. 2007;197:503.e1-503.e6.
111 Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20:966-972.
112 Malfetano J, Keys H, Kredentser D, et al. Weekly cisplatin and radical radiation therapy for advanced, recurrent and poor prognosis cervical carcinoma. Cancer. 1993;71:3703-3706.
113 Potish RA, Twiggs LB, Adcock LL, et al. Effect of cis-platinum on tolerance to radiation therapy in advanced cervical cancer. Am J Clin Oncol. 1986;9:387-391.
114 Runowicz CD, Wadler S, Rodriguez-Rodriguez L, et al. Concomitant cisplatin and radiotherapy in locally advanced cervical carcinoma. Gynecol Oncol. 1989;34:387-391.
115 Rotman M, Pajak M, Choi K, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten-year treatment results of RTOG 79-20. JAMA. 1995;274:387-393.
116 Rose PG, Bundy BN. Chemoradiation for locally advanced cervical cancer. Does it help? J Clin Oncol. 2002;20:891-893.
117 Pearcey R, Miao Q, Kong W, et al. Impact of adoption of chemoradiotherapy on the outcome of cervical cancer in Ontario. Results of a population-based cohort study. J Clin Oncol. 2007;25:2383-2388.
118 Vale C, Tierney JF, Stewart LA, et al. for the Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration Group: Reducing uncertainties about the effect of chemoradiotherapy for cervical cancer. A systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802-5812.
119 Higgins RV, Naumann WR, Hall JB, Ha-ake M. Concurrent carboplatin with pelvic radiation therapy in the primary treatment of cervical cancer. Gynecol Oncol. 2003;89:499-503.
120 Lanciano R, Calkins A, Bundy BN, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer. A Gynecologic Oncology Group study. J Clin Oncol. 2005;23:8289-8295.
121 Wong LC, Ngan HYS, Cheung ANY, et al. Chemoradiation and adjuvant chemotherapy in cervical cancer. J Clin Oncol. 1999;17:2055-2060.
122 Lorvidhaya V, Chitapanarux I, Sangruchi S, et al. Concurrent mitomycin-C, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of the cervix. A randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:1226-1232.
123 Neoadjuvant Chemotherapy for Cervical Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer. A systematic review and meta-analysis of individual patient data from 21 randomized trials. Eur J Cancer. 2003;39:2470-2486.
124 Souhami L, Gil R, Allan S, et al. A randomized trial of chemotherapy followed by pelvic radiation therapy in Stage IIIB carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 1991;9:970-997.
125 Tattersall MHN, Lorvidhaya V, Vootiprux V, et al. Randomized trial of epirubicin and cisplatin chemotherapy followed by pelvic radiation in locally advanced cervical cancer. J Clin Oncol. 1995;13:444-451.
126 Withers HR, Taylor JMG, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131-146.
127 Lanciano RM, Martz K, Coia LR, et al. Tumor and treatment factors improving outcome in stage III-B cervix cancer. Int J Radiat Oncol Biol Phys. 1991;20:95-100.
128 Fyles A, Keane TJ, Barton M, Simm J. The effect of treatment duration in the local control of cervix cancer. Radiother Oncol. 1992;25:273-279.
129 Lanciano RM, Pajak TF, Martz K, Hanks GE. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation. A Patterns-of-Care study. Int J Radiat Oncol Biol Phys. 1993;25:391-397.
130 Girinsky T, Rey A, Roche B, et al. Overall treatment time in advanced cervical carcinomas. A critical parameter in treatment outcome. Int J Radiat Oncol Biol Phys. 1993;27:1051-1056.
131 Perez CA, Grigsby PW, Castro-Vita H, Lockett MA. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1995;32:1275-1288.
132 Petereit DG, Sarkaria JN, Chappell R, et al. The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys. 1995;32:1301-1307.
133 Perez CA, Grigsby PW, Castro-Vita H, Lockett MA. Carcinoma of the uterine cervix. II. Lack of impact of prolongation of overall treatment time on morbidity of radiation therapy. Int J Radiat Oncol Biol Phys. 1996;34:3-11.
134 Cunningham M, Dunton C, Corn B, et al. Extended-field radiation therapy in early-stage cervical carcinoma. Survival and complications. Gynecol Oncol. 1991;43:51-54.
135 Rubin SC, Brookland R, Mikuta JJ, et al. Para-aortic nodal metastases in early cervical carcinoma. Long-term survival following extended-field radiotherapy. Gynecol Oncol. 1984;18:213-217.
136 Komaki R, Mattingly RF, Hoffman RG, et al. Irradiation of para-aortic lymph node metastases from carcinoma of the cervix or endometrium. Preliminary results. Radiology. 1983;147:245-248.
137 Rotman M, Aziz H, Eifel PJ. Irradiation of pelvic and para-aortic nodes in carcinoma of the cervix. Semin Radiat Oncol. 1994;4:23-29.
138 Varia MA, Bundy BN, Deppe G, et al. Cervical carcinoma metastatic to para-aortic nodes. Extended field radiation therapy with concomitant 5-fluorouracil and cisplatin chemotherapy. A Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1998;42:1015-1023.
139 Malfetano JH, Keys H, Cunningham MJ, et al. Extended field radiation and cisplatin for stage IIB and IIIB cervical cancer. Gynecol Oncol. 1997;67:203-207.
140 Haie C, Pejovic MH, Gerbaulet A, et al. Is prophylactic para-aortic irradiation worthwhile in the treatment of advanced cervical carcinoma? Results of a controlled clinical trial of the EORTC radiotherapy group. Radiother Oncol. 1988;11:101-112.
141 Bush R. The significance of anemia in clinical radiation therapy. Int J Radiat Oncol Biol Phys. 1986;12:2047-2050.
142 Girinski T, Pejovic-Lenfant M, Bourhis J, et al. Prognostic value of hemoglobin concentrations and blood transfusions in advanced carcinoma of the cervix treated by radiation therapy. Results of a retrospective study of 386 patients. Int J Radiat Oncol Biol Phys. 1989;16:37-42.
143 Grogan M, Thomas GM, Melamed I, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86:1528-1536.
144 Kapp KS, Poschauko J, Geyer E, et al. Evaluation of the effect of routine packed red blood cell transfusion in anemic cervix cancer patients treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:58-66.
145 Bush RS, Jenkin RDT, Allt WEC, et al. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer. 1978;37(Suppl 3):302-306.
146 Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317-325.
147 Hockel M, Schlenger K, Aral B, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509-4515.
148 Fyles AW, Milosevic M, Wong R, et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149-156.
149 Dorie MJ, Brown JM. Tumor-specific, schedule-dependent interaction between tirapazamine (SR 4233) and cisplatin. Cancer Res. 1993;53:4633-4636.
150 Gatzemeier U, Rodriguez G, Treat J, et al. Tirapazamine-cisplatin. The synergy. Br J Cancer. 1998;77(Suppl 4):15-17.
151 Rischin D, Peters LJ, O’Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART). A phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28:2989-2995.
152 Dachs GU, Tozer GM. Hypoxia modulated gene expression. Angiogenesis, metastasis, and therapeutic exploitation. Eur J Cancer. 2000;36:1649-1660.
153 Waggoner SE, Darcy KM, Fuhrman B, et al. Association between cigarette smoking and prognosis in locally advanced cervical cancer treated with chemoradiation. A Gynecologic Oncology Group study. Gynecol Oncol. 2006;103:853-858.
154 Eifel PJ, Jhingran A, Bodurka DC, et al. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20:3651-3657.
155 Duenas-Gonzalez A, Zarba JJ, Alcedo JC, et al. A phase III study comparing concurrent gemcitabine (Gem) plus cisplatin (Cis) and radiation followed by adjuvant Gem plus Cis versus concurrent Cis and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2009;27(Suppl; Abstract CRA 5507):18s.
156 Swisher EM, Swensen RE, Greer B, et al. Weekly gemcitabine and cisplatin in combination with pelvic radiation in the primary therapy of cervical cancer. A phase I trial of the Puget Sound Oncology Consortium. Gynecol Oncol. 2006;101:429-435.
157 Rose PG, Degeest K, McMeekin S, Fusco N. A phase I study of gemcitabine followed by cisplatin concurrent with whole pelvic radiation therapy in locally advanced cervical cancer. A Gynecologic Oncology Group study. Gynecol Oncol. 2007;107:274-279.
158 Grigsby PW, Perez CA. Radiotherapy alone for medically inoperable carcinoma of the cervix. Stage IA and carcinoma in situ. Int J Radiat Oncol Biol Phys. 1991;21:375-378.
159 Kolstad P. Follow-up study of 232 patients with stage Ia1 and 411 patients with stage Ia2 squamous cell carcinoma of the cervix (microinvasive carcinoma). Gynecol Oncol. 1989;33:265-272.
160 Hamberger AD, Fletcher GH, Wharton JT. Results of treatment of early Stage I carcinoma of the uterine cervix with intracavitary radium alone. Cancer. 1978;41:980-985.
161 Burghardt E, Pickel H. Local spread and lymph node involvement in cervical cancer. Obstet Gynecol. 1978;52:138-145.
162 Gadducci A, Sartori E, Maggino T, et al. The clinical outcome of patients with stage Ia1 and Ia2 squamous cell carcinoma of the uterine cervix. A Cooperation Task Force (CTF) study. Eur J Gynaecol Oncol. 2003;24:513-516.
163 Kodama J, Mizutani Y, Hongo A, et al. Optimal surgery and diagnostic approach of stage IA2 squamous cell carcinoma of the cervix. Eur J Obstet Gynecol Reprod Biol. 2002;101:192-195.
164 Ceballos KM, Shaw D, Daya D. Microinvasive cervical adenocarcinoma (FIGO stage IA tumors). Results of surgical staging and outcome analysis. Am J Surg Pathol. 2006;30:370-374.
165 Smith HO, Qualls CR, Romero AA, et al. Is there a difference in survival for IA1 and IA2 adenocarcinoma of the uterine cervix? Gynecol Oncol. 2002;85:229-241.
166 Balega J, Michael H, Hurteau J, et al. The risk of nodal metastasis in early adenocarcinoma of the uterine cervix. Int J Gynecol Cancer. 2004;14:104-109.
167 Alvarez RD, Potter ME, Soong SJ, et al. Rationale for using pathologic tumor dimensions and nodal status to subclassify surgically treated stage IB cervical cancer patients. Gynecol Oncol. 1991;43:108-112.
168 Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535-540.
169 Reesink-Peters N, van der Velden J, ten Hoor KA, et al. Preoperative serum squamous cell carcinoma antigen levels in clinical decision making for patients with early-stage cervical cancer. J Clin Oncol. 2005;23:1455-1462.
170 Panici PB, Plotti F, Zullo MA, et al. Pelvic lymphadenectomy for cervical carcinoma. Laparotomy extraperitoneal, transperitoneal or laparoscopic approach? A randomized study. Gynecol Oncol. 2006;103:859-864.
171 Sharma R, Bailey J, Anderson R, Murdoch J. Laparoscopically assisted radical vaginal hysterectomy (Coelio-Schauta). A comparison with open Wertheim/Meigs hysterectomy. Int J Gynecol Cancer. 2006;16:1927-1932.
172 Steed H, Rosen B, Murphy J, et al. A comparison of laparoscopic-assisted radical vaginal hysterectomy and radical abdominal hysterectomy in the treatment of cervical cancer. Gynecol Oncol. 2004;93:588-593.
173 Boggess JF, Gehrig PA, Cantrell L, et al. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol. 2008;199:357e1-357e7.
174 Estape R, Lambrou N, Diaz R, et al. A case matched analysis of robotic radical hysterectomy with lymphadenectomy compared with laparoscopy and laparotomy. Gynecol Oncol. 2009;113:357-361.
175 Cho JE, Nezhat FR. Robotics and gynecologic oncology. Review of the literature. J Minim Invasive Gynecol. 2009;16:669-681.
176 Obermair A, Gebski V, Frumovitz M, et al. A phase III randomized clinical trial comparing laparoscopic or robotic radical hysterectomy with abdominal radical hysterectomy in patients with early stage cervical cancer. J Minim Invasive Gynecol. 2008;15:584-588.
177 Dargent D, Methevet P. Shauta’s vaginal hysterectomy combined with laparoscopic lymphadenectomy. Baillieres Clin Obstet Gynaecol. 1995;9:691-705.
178 Roy M, Plante M. Pregnancies after radical vaginal trachelectomy for early-stage cervical cancer. Am J Obstet Gynecol. 1998;179:1491-1496.
179 Covens A, Shaw P, Murphy J, et al. Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage IA-B carcinoma of the cervix? Cancer. 1999;86:2273-2279.
180 Rodriguez M, Guimares O, Rose PG. Radical abdominal trachelectomy and pelvic lymphadenectomy with uterine conservation and subsequent pregnancy in the treatment of early invasive cervical cancer. Am J Obstet Gynecol. 2001;185:370-374.
181 Bernardini M, Barrett J, Seaward G, Covens A. Pregnancy outcomes in patients with radical trachelectomy. Am J Obstet Gynecol. 2003;189:1378-1382.
182 Beiner ME, Hauspy J, Rosen B, et al. Radical vaginal trachelectomy vs. radical hysterectomy for small early stage cervical cancer. A matched case-control study. Gynecol Oncol. 2008;110:168-171.
183 Gien LT, Covens A. Fertility-sparing options for early stage cervical cancer. Gynecol Oncol. 2010;117:350-357.
184 Thomas GM, Dembo AJ. Is there a role for adjuvant pelvic radiotherapy after radical hysterectomy in early stage cervical cancer? Int J Gynecol Cancer. 1991;1:1-8.
185 Koh WJ, Panwala K, Greer B. Adjuvant therapy for high-risk, early stage cervical cancer. Semin Radiat Oncol. 2000;10:51-60.
186 Stock RG, Chen ASJ, Karasek K. Patterns of spread in node-positive cervical cancer. The relationship between local control and distant metastases. Cancer J Sci Am. 1996;2:256-262.
187 Bandy LC, Clarke-Pearson DL, Soper JT, et al. Long-term effects on bladder function following radical hysterectomy with and without postoperative radiation. Gynecol Oncol. 1987;26:160-168.
188 Barter JF, Soong SJ, Shingleton HM, et al. Complications of combined radical hysterectomy-postoperative radiation therapy in women with early stage cervical cancer. Gynecol Oncol. 1989;32:292-296.
189 Montz FJ, Holschneider CH, Solh S, et al. Small bowel obstruction following radical hysterectomy. Risk factors, incidence and operative findings. Gynecol Oncol. 1994;53:114-120.
190 Remy JC, Fruchter RG, Choi K, et al. Complications of combined radical hysterectomy and pelvic radiation. Gynecol Oncol. 1986;24:317-326.
191 Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy. A Gynecologic Oncology Group study. Gynecol Oncol. 1999;73:177-183.
192 Rotman M, Sedlis A, Peidmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features. Follow-up of a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 2006;65:169-176.
193 Finan MA, DeCesare S, Fiorica JV, et al. Radical hysterectomy for stage IB1 versus IB2 carcinoma of the cervix. Does the new staging system predict morbidity and survival? Gynecol Oncol. 1996;62:139-147.
194 Rutledge TL, Kamelle SA, Tillmanns TD, et al. A comparison of stages IB1 and IB2 cervical cancers treated with radical hysterectomy. Is size the real difference? Gynecol Oncol. 2004;95:70-76.
195 Roman LD, Morris M, Mitchell MF, et al. Prognostic factors for patients undergoing simple hysterectomy in the presence of invasive cancer of the cervix. Gynecol Oncol. 1993;50:179-184.
196 Durrance FY, Fletcher GH, Rutledge FN. Analysis of central recurrent disease in stages I and II squamous cell carcinomas of the cervix on intact uterus. Am J Roentgenol. 1969;106:831-838.
197 O’Quinn AG, Fletcher GH, Wharton JT. Guidelines for conservative hysterectomy after irradiation. Gynecol Oncol. 1980;9:68-79.
198 Thoms WW, Eifel PJ, Smith TL, et al. Bulky endocervical carcinomas. A 23-year experience. Int J Radiat Oncol Biol Phys. 1992;23:491-499.
199 Mendenhall WM, McCarty PJ, Morgan LS, et al. Stage IB-IIA-B carcinoma of the intact uterine cervix greater than or equal to 6 cm in diameter. Is adjuvant extrafascial hysterectomy beneficial? Int J Radiat Oncol Biol Phys. 1991;21:899-904.
200 Keys HM, Bundy BN, Stehman FB, et al. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma. A randomized trial of the Gynecologic Oncology Group. Gynecol Oncol. 2003;89:343-353.
201 Eifel PJ, Jhingran A, Brown J, et al. Time course and outcome of central recurrence after radiation therapy for carcinoma of the cervix. Int J Gynecol Cancer. 2006;16:1106-1111.
202 Koh WJ, Paley PJ, Comsia ND, Greer B. Radical management of recurrent cervical cancer. In: Eifel PJ, Levenback C, editors. Cancer of the Female Lower Genital Tract—American Cancer Society Atlas of Clinical Oncology. London: BC Decker; 2001:169-182.
203 Ijaz T, Eifel PJ, Burke T, Oswold MJ. Radiation therapy of pelvic recurrence after radical hysterectomy for cervical carcinoma. Gynecol Oncol. 1998;70:241-246.
204 Thomas GM, Dembo AJ, Myhr T, et al. Long-term results of concurrent radiation and chemotherapy for carcinoma of the cervix recurrent after surgery. Int J Gynecol Cancer. 1993;3:193-198.
205 Russell AH, Koh WJ, Markette K, et al. Radical reirradiation for recurrent or second primary carcinoma of the female reproductive tract. Gynecol Oncol. 1987;27:226-232.
206 Randall ME, Evans L, Greven KM, et al. Interstitial reirradiation for recurrent gynecologic malignancies. Results and analysis of prognostic factors. Gynecol Oncol. 1993;48:23-31.
207 Morley GW, Hopkins MP, Lindenauer SM, Roberts JA. Pelvic exenteration, University of Michigan. 100 patients at 5 years. Obstet Gynecol. 1989;74:934-943.
208 Shingleton HM, Soong SJ, Gelder MS, et al. Clinical and histopathologic factors predicting recurrence and survival after pelvic exenteration for cancer of the cervix. Obstet Gynecol. 1989;73:1027-1034.
209 Stanhope CR, Webb MJ, Prodratz KC. Pelvic exenteration for recurrent cervical cancer. Clin Obstet Gynecol. 1990;33:897-909.
210 Stelzer KJ, Koh WK, Greer BE, et al. The use of intraoperative radiation therapy in radical salvage for recurrent cervical cancer. Outcome and toxicity. Am J Obstet Gynecol. 1995;172:1881-1888.
211 Garton GR, Gunderson LL, Webb MJ, et al. Intraoperative radiation therapy in gynecologic cancer. Update of the experience at a single institution. Int J Radiat Oncol Biol Phys. 1997;37:839-843.
212 Thigpen JT, Singleton H, Homesley H, et al. cis-Platinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix. A phase II trial of the Gynecologic Oncology Group. Cancer. 1981;48:899-903.
213 Bonomi P, Blessing JA, Stehman FB, et al. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix. A Gynecologic Oncology Group study. J Clin Oncol. 1985;3:1079-1085.
214 Thigpen JT, Blessing JA, DiSaia PJ, et al. A randomized comparison of rapid versus prolonged (24 hr) infusion of cisplatin in therapy of squamous cell carcinoma of the uterine cervix. A Gynecologic Oncology Group study. Gynecol Oncol. 1989;32:198-202.
215 McGuire WP, Arseneau JC, Blessing JA, et al. A randomized comparative trial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix. A Gynecologic Oncology Group study. J Clin Oncol. 1989;7:1462-1468.
216 Stehman FB, Blessing JA, McGehee R, Barrett RJ. A phase II evaluation of mitolactol in patients with advanced squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. J Clin Oncol. 1989;7:1892-1895.
217 Sutton GP, Blessing JA, McGuire WP, et al. Phase II trial of ifosfamide and mesna in patients with advanced or recurrent squamous carcinoma of the cervix who had never received chemotherapy. A Gynecologic Oncology Group study. Am J Obstet Gynecol. 1993;168:805-807.
218 McGuire WP, Blessing JA, Moore DH, et al. Paclitaxel has moderate activity in squamous cervix cancer. A Gynecologic Oncology Group study. J Clin Oncol. 1996;14:792-795.
219 Bookman MA, Blessing JA, Hanjani P, et al. Topotecan in squamous cell carcinoma of the cervix. A phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2000;77:446-449.
220 Schilder RJ, Blessing JA, Morgan M, et al. Evaluation of gemcitabine in patients with squamous cell carcinoma of the cervix. A phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2000;76:204-207.
221 Morris M, Brader KR, Levenback C, et al. Phase II study of vinorelbine in advanced and recurrent squamous cell carcinoma of the cervix. J Clin Oncol. 1998;16:1094-1098.
222 Monk BJ, Sill MW, Burger RA, et al. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:1069-1074.
223 Omura GA, Blessing JA, Vaccarello L, et al. A randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix. A Gynecologic Oncology Group study. J Clin Oncol. 1997;15:165-171.
224 Bloss JD, Blessing JA, Behrens BC, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix. A Gynecologic Oncology Group study. J Clin Oncol. 2002;20:1832-1837.
225 Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix. A Gynecologic Oncology Group trial. J Clin Oncol. 2004;22:3113-3119.
226 Long HJ, Bundy BN, Grendys EC, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix. A Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4626-4633.
227 Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent or persistent cervical carcinoma. A Gynecologic Oncology Group study. J Clin Oncol. 2009;28:4649-4655.
228 Moore DH, Tian C, Monk BJ, et al. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma. A Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:44-49.
229 van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma: A systematic review. Radiother Oncol. 2011;98(3):287-291.
230 Spanos WJ, Wasserman T, Meoz R, et al. Palliation of advanced pelvic malignant disease with large fraction pelvic radiation and misonidazole. Final report of RTOG phase I/II study. Int J Radiat Oncol Biol Phys. 1987;13:1479-1482.
231 Adelson MD, Wharton JT, Delclos L, et al. Palliative radiotherapy for ovarian cancer. Int J Radiat Oncol Biol Phys. 1987;13:17-21.
232 Boulware RJ, Caderao JB, Delclos L, et al. Whole pelvis mega-voltage irradiation with single doses of 1000 rad to palliate advanced gynecologic cancers. Int J Radiat Oncol Biol Phys. 1979;5:333-338.
233 Spanos WJ, Perez CA, Marcus S, et al. Effect of rest interval on tumor and normal tissue response. A report of phase III study of accelerated split course palliative radiation for advanced pelvic malignancies (RTOG-8502). Int J Radiat Oncol Biol Phys. 1993;25:399-403.
234 Wolfson AH, Abdel-Wahab M, Markoe AM, et al. A quantitative assessment of standard vs. customized midline shield construction for invasive cervical carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:237-242.
235 McIntyre JF, Eifel PJ, Levenback C, et al. Ureteral stricture as a late complication of radiotherapy for stage IB carcinoma of the uterine cervix. Cancer. 1995;75:836-843.
236 Perez CA. Uterine cervix. In Perez CA, Brady LW, editors: Principles and Practice of Radiation Oncology, ed 2, Philadelphia: JB Lippincott, 1992.
237 Greer BE, Koh WJ, Figge DC, et al. Gynecologic radiotherapy fields defined by intraoperative measurements. Gynecol Oncol. 1990;38:421-424.
238 Kim RY, McGinnis S, Spencer SA, et al. Conventional four-field pelvic radiotherapy technique without computed tomography-reatment planning in cancer of the cervix. Potential geographic miss and its impact on pelvic control. Int J Radiat Oncol Biol Phys. 1994;31:109-112.
239 Bonin SR, Lanciano RM, Corn BW, et al. Bony landmarks are not an adequate substitute for lymphangiography in defining pelvic node location for the treatment of cervical cancer with radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:167-172.
240 Portalance L, Chao KSC, Grigsby PW, et al. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic radiation. Int J Radiat Oncol Biol Phys. 2001;51:261-266.
241 Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52:1330-1337.
242 Brixey CJ, Roeske JC, Lujan AE, et al. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;54:1388-1396.
243 Small W, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428-434.
244 Loiselle C, Koh WJ. The emerging use of IMRT for treatment of cervical cancer. J Natl Comp Cancer. Network. 2010;8:1425-1433.
245 Jhingran A, Winter K, Portelance L, et al. A phase II study of intensity modulated radiation therapy (IMRT) to the pelvis for post-operative patients with endometrial carcinoma (RTOG 0418). Int J Radiat Oncol Biol Phys. 2008;72(Abstract):S16.
246 van de Bunt L, van der Heide UA, Ketelaars M, et al. Conventional, conformal, and intensity-modulated radiation therapy treatment planning of external beam radiotherapy for cervical cancer. The impact of tumor regression. Int J Radiat Oncol Biol Phys. 2006;64:189-196.
247 van de Bunt L, Jurgenliemk-Schulz IM, de Kort GA, et al. Motion and deformation of the target volumes during IMRT for cervical cancer. What margins do we need? Radiother Oncol. 2008;88:233-240.
248 Beadle BM, Jhingran A, Salehpour M, et al. Cervix regression and motion during the course of external beam chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys. 2009;73:235-341.
249 Lim K, Small WJr, Portelance L, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79:348-355.
250 Patel FD, Sharma SC, Negi PS, et al. Low dose rate versus high dose rate brachytherapy in the treatment of carcinoma of the uterine cervix. A clinical trial. Int J Radiat Oncol Biol Phys. 1994;28:335-341.
251 Teshima T, Inoue T, Ikeda T, et al. High-dose rate and low-dose rate intracavitary therapy for carcinoma of the uterine cervix. Final results of Osaka University Hospital. Cancer. 1993;72:2409-2414.
252 Hareyama M, Sakata K, Oouchi A, et al. High-dose-rate versus low-dose-rate intracavitary therapy for carcinoma of the uterine cervix. A randomized trial. Cancer. 2002;94:117-124.
253 Lertsanguansinchai P, Lertbutsayanukul C, Shotelersuk K, et al. Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2004;59:1424-1431.
254 Orton CG, Seyedsadr M, Somnay A. Comparison of high and low dose rate remote afterloading for cervix cancer and the importance of fractionation. Int J Radiat Oncol Biol Phys. 1991;21:1425-1426.
255 Petereit DG, Sarkaria JN, Petter DM, Schink JC. High-dose-rate versus low-dose-rate brachytherapy in the treatment of cervical cancer. Analysis of tumor recurrence—the University of Wisconsin experience. Int J Radiat Oncol Biol Phys. 1999;45:1267-1274.
256 Arai T, Nakano T, Morita S, et al. High dose rate remote after-loading intracavitary radiation therapy for cancer of the uterine cervix. Cancer. 1992;69:175-180.
257 Eifel PJ, Moughan J, Erickson B, et al. Patterns of radiotherapy practice for patients with carcinoma of the uterine cervix. A patterns of care study. Int J Radiat Oncol Biol Phys. 2004;60:1144-1153.
258 Eifel PJ, Khalid N, Erickson B, et al. Patterns of radiotherapy practice for patients treated for intact cervical cancer 2005-2007. A QRRO study. Int J Radiat Oncol Biol Phys. 2010;78(3):S119.
259 Katz A, Eifel PJ. Quantification of intracavitary brachytherapy parameters and correlation with outcome in patients with carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48:1417-1425.
260 Thirion P, Kelly C, Salib O, et al. A randomized comparison of two brachytherapy devices for the treatment of uterine cervical carcinoma. Radiother Oncol. 2005;74:247-250.
261 Thomas G, Dembo A, Ackerman I, et al. A randomized trial of standard versus partially hyperfractionated radiation with or without concurrent 5-fluorouracil in locally advanced cervical cancer. Gynecol Oncol. 1998;69:137-145.
262 International Commission on Radiation Units and Measurements. Dose and Volume Specification for Reporting Intracavitary Therapy in Gynecology. Vol 38. Bethesda, Maryland: International Commission on Radiation Units and Measurements; 1985.
263 Haie-Meder C, Kramar A, Lambin P, et al. Analysis of complications in a prospective randomized trial comparing two brachytherapy low dose rates in cervical carcinoma. Int J Radiat Oncol Biol Phys. 1994;29:1195-1197.
264 Scalliet P, Gerbaulet A, Dubray B. HDR versus LDR gynecological brachytherapy revisited. Radiother Oncol. 1993;28:118-126.
265 Ogino I, Kitamura T, Okamoto N, et al. Late rectal complication following high dose rate intracavitary brachytherapy in cancer of the cervix. Int J Radiat Oncol Biol Phys. 1995;31:725-734.
266 Stitt JA, Fowler JF, Thomadsen BR, et al. High dose rate intracavitary brachytherapy for carcinoma of the cervix. The Madison system. I. Clinical and radiobiological considerations. Int J Radiat Oncol Biol Phys. 1992;24:383-386.
267 Petereit DG, Pearcey R. Literature analysis of high dose rate brachytherapy fractionation schedules in the treatment of cervical cancer. Is there an optimal fractionation schedule? Int J Radiat Oncol Biol Phys. 1999;43:359-366.
268 Viswanathan AN, Creutzberg CL, Craighead P, et al. International brachytherapy practice patterns. A survey of the Gynecologic Cancer Intergroup (GCIG). Int J Radiat Oncol Biol Phys. December 22, 2010. [Epub ahead of print.]
269 Brandt K, Petereit DG, Sarkaria J, et al. Conscious sedation in outpatient GYN HDR brachytherapy. Philadelphia: Proceedings of the First Annual Multidisciplinary Radiation Oncology Conference; 1996.
270 Viswanathan AN, Dimopoulos J, Kirisits C, et al. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy. Results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491-498.
271 Haie-Meder C, Potter R, Van Limbergen EV, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO working group (I). Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235-245.
272 Dimopoulos JC, Potter R, Lang S, et al. Dose-effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapy. Radiother Oncol. 2009;93:311-315.
273 Potter R, Kirisits C, Fidarova EF, et al. Present status and future of high-precision image guided adaptive brachytherapy for cervix carcinoma. Acta Oncol. 2008;47:1325-1336.
274 Georg P, Pötter R, Georg D, et al. Dose effect relationship for late side effects of the rectum and urinary bladder in magnetic resonance image-guided adaptive cervix cancer brachytherapy. Int J Radiat Oncol Biol Phys. February 22, 2011. [Epub ahead of print.]
275 Potter R, Kirisits C, Fidarova E, et al. Pesent status and future of high-precision image guided adaptive brachytherapy for cervix carcinoma. Acta Oncol. 2008;47:1325-1336.
276 Kirisits C, Lang S, Dimopoulos J, et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer. Design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;65(2):624-630.
277 Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy. A survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2010;76:104-109.
278 Aristizabal SA, Woolfitt B, Valencia A, et al. Interstitial parametrial implants in carcinoma of the cervix stage II-B. Int J Radiat Oncol Biol Phys. 1987;13:445-450.
279 Martinez A, Edmundson GK, Cox RS, et al. Combination of external-beam irradiation and multiple-site perineal applicator (MUPIT) for treatment of locally advanced or recurrent prostatic, anorectal, and gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1985;11:391-398.
280 Syed AMN, Puthwala AA, Neblett D, et al. Transperineal interstitial-intracavitary “Syed-Neblett” applicator in the treatment of carcinoma of the uterine cervix. Endocuriether Hyperther Oncol. 1986;2:1-13.
281 Hughes-Davies L, Silver B, Kapp D. Parametrial interstitial brachytherapy for advanced or recurrent pelvic malignancy. The Harvard/Stanford experience. Gynecol Oncol. 1995;58:24-27.
282 Eifel PJ, Morris M, Delclos L, et al. Radiation therapy for cervical carcinoma. In: Dilts PVJr, Sciarra JJ, editors. Gynecology and Obstetrics. Philadelphia: JB Lippincott; 1993:1-25.
283 Schoeppel SJ, LaVigne M, Martel MK, et al. Three-dimensional treatment planning of intracavitary gynecologic implants. Analysis of ten cases and implications for dose specification. Int J Radiat Oncol Biol Phys. 1993;28:277-283.
284 Perez CA, Grigsby PW, Lockett MA, et al. Radiation therapy morbidity in carcinoma of the uterine cervix. Dosimetric and clinical correlation. Int J Radiat Oncol Biol Phys. 1999;44:855-866.
285 Eifel PJ, Levenback C, Wharton JT, Oswald MJ. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1995;32:1289-1300.
286 Kirwan JM, Symonds P, Green JA, et al. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol. 2003;68:217-226.
287 Rotte K. Comparison of HDR afterloading and classical techniques for radiation therapy of cancer of the cervix uteri. Acta Select Brachyther (Suppl). 1991;2:42-46.
288 Kataoka M, Kawamura M, Nishiyama Y, et al. Results of the combination of external-beam and high dose rate intracavitary irradiation for patients with cervical carcinoma. Gynecol Oncol. 1992;44:48-52.
289 Utley J, von Essen C, Horn R, et al. High-dose-rate afterloading brachytherapy in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1984;10:2259-2263.