Chapter 12 Cerebrovascular Occlusive Disease and Carotid Surgery
• Atherosclerotic occlusive disease is the most commonly seen cervical common carotid bifurcation and involves the common, internal carotid arteries (ICAs). Other causes of ischemia are intracranial atherosclerotic narrowing or occlusion; extracranial or intracranial dissection of the internal carotid, vertebral, and other arteries; and moyamoya disease.
• Ischemic disease becomes symptomatic owing to distal thromboembolism or diminished flow. Symptoms may include stroke and transient ischemic attacks.
• Workup of a patient presenting with ischemic stroke may include magnetic resonance imaging (MRI; diffusion-weighted images and MR perfusion), magnetic resonance angiography (MRA), computed tomography angiography (CTA), carotid duplex ultrasonography, transcranial Doppler angiography, and digital subtraction angiography (DSA).
• Patients with symptomatic cervical ICA stenosis greater than 50% benefit from carotid endarterectomy at high-volume centers with low complication rates.
• Endovascular carotid artery stenting (CAS) has been evaluated as an alternative to carotid endarterectomy in two randomized international trials. The short-term results indicate higher complication rates with CAS. It may be performed in selected patients, especially with very high carotid lesions.
• Moyamoya disease is caused by progressive occlusion of the intracranial ICA, and adjacent major branches, with accompanying dilation of small collateral arteries. It may manifest in children with transient ischemic attack or strokes and in adults with ischemic symptoms or hemorrhage. Prevention of further ischemic episodes may be accomplished by indirect revascularization (encephalodural/glial/myosynangiosis) or direct revascularization (extracranial bypass, most commonly superficial temporal artery to middle cerebral artery anastomosis).
• At present, randomized trials do not support the use of external carotid/internal carotid (EC-IC) arterial bypass for chronic atherosclerotic ischemic disease.
The central nervous system is metabolically demanding, receiving approximately 20% of cardiac output despite comprising only 2% of body weight. Cerebral blood flow (CBF) is directly proportional to the difference between mean arterial pressure (MAP) and intracranial pressure (ICP) and inversely related to cerebrovascular resistance (CVR) as per Ohm’s law. Alteration of cerebrovascular tone allows for maintenance of cerebral perfusion pressure over a wide range of mean arterial pressures. However, if cerebral perfusion pressure drops below 20 mm Hg, in the setting of arterial occlusion, for example, inadequate delivery of oxygen to brain tissue results in ischemia and subsequent infarction if CBF is not quickly returned to normal.1
The differential diagnosis for arterial occlusion includes both acute and chronic disease processes that may affect either intracranial or extracranial vessels. This chapter focuses on cerebrovascular occlusive disease processes commonly encountered and treated by the neurosurgeon—atherosclerotic cerebrovascular occlusive disease (with a specific emphasis on carotid artery stenosis), moyamoya disease, and cerebral arterial dissection. Following a brief review of clinical anatomy, the pathophysiology, clinical presentation, diagnosis, and management of each of the aforementioned conditions will be discussed. The final section of this chapter will detail the neurosurgical technique for carotid endarterectomy and superficial temporal artery to middle cerebral artery bypass, important surgical treatments for cerebrovascular occlusive disease in the armamentarium of the neurovascular surgeon.
Review of Clinical Anatomy
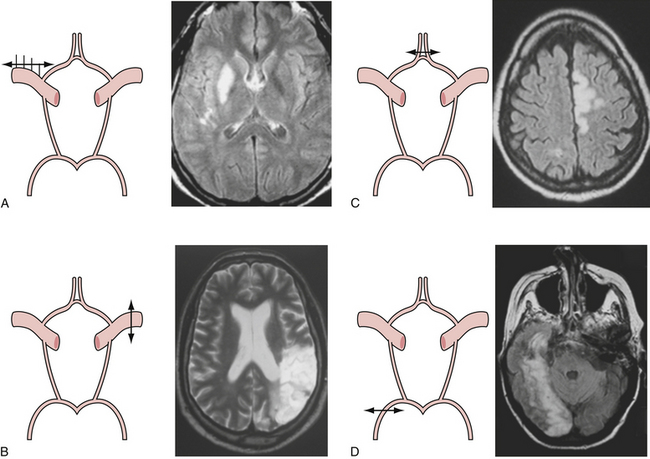
Presenting symptoms of acute occlusion reflect the respective vascular territories (Fig. 12.1). Anterior circulation involvement may manifest as monocular blindness and an absent pupillary light response; hemispheric signs such as contralateral homonymous hemianopia, hemiparesis, and hemisensory loss; specific signs of dominant hemispheric ischemia including aphasia, alexia, agraphia, acalculia, and dysarthria; and nondominant hemispheric symptoms including visuospatial neglect, constructional apraxia, loss of prosody of speech, and anosognosia. Posterior circulation symptoms, aside from alteration in level of consciousness, include motor deficits such as hemiparesis, tetraparesis, and facial paresis from brainstem lesions, vertigo, vomiting, pupillary abnormalities, ataxia, oculomotor signs, and pseudobulbar manifestations.
Atherosclerotic Cerebrovascular Occlusive Disease
Clinical Features
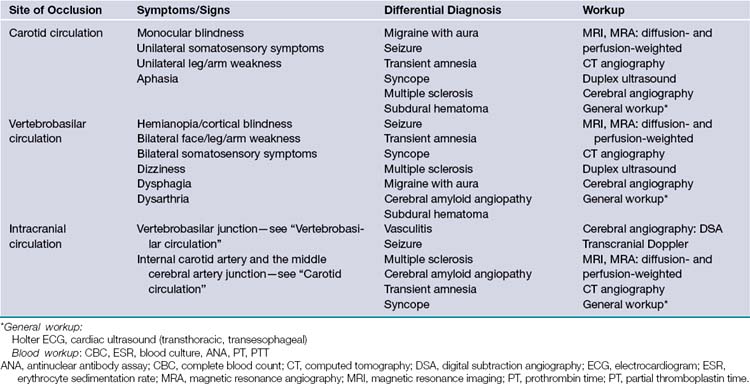
Given the chronic nature of cerebrovascular atherosclerosis, the cerebrovascular system can show a remarkable level of resilience prior to symptomatic presentation and often remains undiagnosed for many years. The remainder of the discussion will be focused on carotid disease given its responsiveness to neurosurgical intervention. Vertebrobasilar and intracranial atherosclerosis share a similar clinical presentation with carotid artery stenosis but specific neurological findings are localized to the vascular territories involved (Table 12.1).
Diagnosis
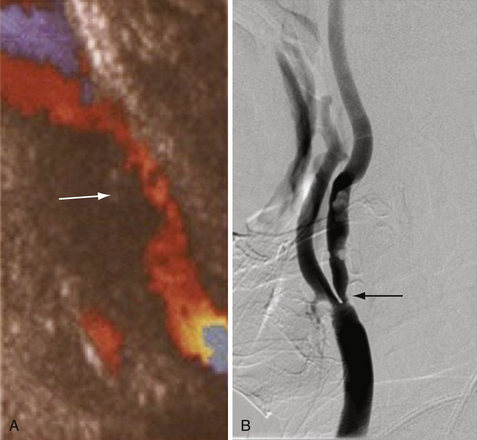
The radiological evaluation of cerebrovascular atherosclerotic disease, specifically disease of the carotid arteries, consists of identifying the level and location of stenosis/occlusion, defining the etiology of these lesions, surgical planning, and patient follow-up. The four major modalities (most invasive to the least invasive techniques) are digital subtraction cerebral angiography (CA), computed tomographic angiography (CTA), magnetic resonance angiography (MRA), and duplex ultrasound (DUS).2–4
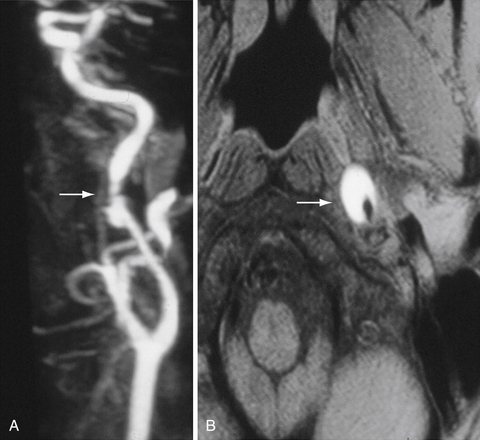
Cerebral angiography is the gold standard for imaging the carotid arteries (Fig. 12.2). Cerebral angiography has superior accuracy compared to noninvasive techniques, which may overestimate or underestimate the degree of stenosis, an important characteristic for accurately determining the extent of disease and for surgical planning. Moreover, more than one noninvasive modality is usually required to perform an accurate and comprehensive assessment of atherosclerotic disease. The advent of digital subtraction angiography (DSA) has reduced the size of catheter needed, the amount of contrast required, and the duration of this procedure. Although there is lower spatial resolution, DSA allows for dynamic visualization of blood flow at the site of stenosis as well as collateralization and flow around the vascular lesion; this information provides an indication of the clinical impact of the stenosis. Patients should be screened for history of adverse reaction to contrast agent and renal disease, as contrast nephropathy and allergy are potential complications of cerebral angiography.
CTA combines CT technique with venous injection of contrast dye to visualize the supra-aortic vessels (both intracranially and extracranially). Unlike CA and DSA, CTA provides an anatomical description of the surrounding structures in addition to the vasculature, which is extremely useful in identifying nonatherosclerotic causes of stenosis. This technique is less invasive than DSA but requires contrast bolus comparable to angiography, and so contrast allergy and nephropathy are possible complications. Furthermore, as the quality and accuracy of the obtained image depends on both the timing of the injection and the scan itself, CTA often suffers from overestimation or underestimation of the degree of disease.
Measurement of Carotid Artery Stenosis
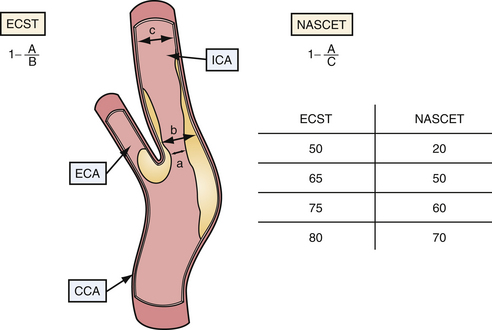
Current indications for surgical intervention of carotid atherosclerotic disease require objective and reproducible methods to evaluate the degree of stenosis. Two major methods of measuring carotid stenosis were developed for use in the major clinical trials evaluating the efficacy of carotid endarterectomy: the North American Symptomatic Carotid Endarterectomy Trial (NASCET)5 method and the European Carotid Surgery Trial (ESCT)6 method. The primary difference in these methods lies in how the observer estimates the diameter of the reference vessel. The NASCET utilizes the normal carotid wall just distal to the stenotic lesion as the reference vessel, and ESCT defines the reference as the estimated diameter of the carotid bulb. Figure 12.3 diagrams each method of measurement and the mathematical relationship between these two methods. Note that the ECST and NASCET approximations are comparable with severe disease, but that their values diverge when the stenosis is not as pronounced. In contemporary practice, most patients are determined to have high-grade (>60-70%) stenosis on the basis of noninvasive color flow Doppler, CTA, or MRA. When two of these three modalities agree on the degree of stenosis and no other modality questions the result, the correlation with catheter angiography is excellent. Given the risk of catheter angiography, this is generally reserved for patients in whom the studies are not concordant.
Management
Medical Management
Antiplatelet therapy has been shown to decrease the risk of ischemic stroke, although it does not eliminate this risk, presumably because of its multifactorial etiology. Antiplatelet therapy directly is thought to prevent the formation of mural-platelet aggregates that either lead to occlusion of large arteries or embolize to distal vessels. Aspirin (acetylsalicylic acid, ASA) is the typical agent used. The optimal dose prescribed remains controversial, although a daily dose of 325 mg has been shown to decrease the risk of stroke by 30% following a TIA. However, it should be noted that patients following carotid endarterectomy (CEA) have lower rates of morbidity and mortality with doses within the range of 81 mg to 325 mg when compared with patients using higher doses.7 For patients who cannot tolerate ASA, thienopyridines may be used. Clopidogrel is preferred over ticlodipine owing to the greater risk of severe neutropenia in the latter.
Surgical Therapy
The efficacy of carotid endarterectomy at reducing the risk of stroke for both symptomatic and asymptomatic patients has been demonstrated in several large, randomized clinical trials, most notably the NASCET5 and the Asymptomatic Carotid Artery Stenosis Trial (ACAST).8
The NASCET, which enrolled patients with transient ischemic attacks (TIAs) or mild stroke within 120 days of surgery, had an absolute risk reduction of ipsilateral stroke at 2 years of 17% when compared with best medical management (9% vs. 28%, respectively) for patients with severe stenosis defined as greater than 70%. A follow-up report found that patients with moderate stenosis (between 50% and 69%) demonstrated a modest risk reduction of 6.7% of any fatal or nonfatal stroke within the 5-year follow-up period for patients treated with surgery versus best medical management (15.7% vs. 22.2, respectively). The ACAST examined patients without symptomatic history and found that patients with greater than 60% stenosis had a 6.1% absolute reduction in risk of any ipsilateral stroke, perioperative stroke, or death at 5 years (5.1% vs. 11.0%, respectively).9
Contralateral stenosis is not uncommon, with one follow-up study of NASCET reporting approximately 8.6% with severe stenosis and 6.5% with complete occlusion of the contralateral carotid artery.10 Although randomized controlled trials are needed to determine the efficacy of operating on patients with bilateral carotid stenosis/occlusion, subgroup analyses of NASCET suggest that patients with occluded contralateral carotid arteries have improved outcomes with surgery when compared with best medical management despite a greater risk of perioperative stroke or death.
Patients with concomitant intracranial atherosclerosis may especially benefit from carotid surgery as this subset of patients with carotid disease; a NASCET subgroup analysis of patients with intracranial atherosclerosis showed that patients who received medical management had a greater risk of stroke, but the surgically treated patients with and without intracranial atherosclerosis did not differ in rates of stroke.11
Alternatives to Carotid Endarterectomy
Carotid Stenting
The advent of endovascular surgery offers an alternative treatment for carotid atherosclerosis in patients who are at high risk for open surgery. Several large randomized trials have attempted to compare stenting versus CEA, but the results have largely been equivocal. Two recent trials are worth noting, the International Carotid Stenting Study (ICSS)12 and the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).13
The ICSS examined outcome after carotid endarterectomy (CEA) and stenting in patients with recently symptomatic carotid artery stenosis. Short-term results (120 days) showed no significant difference in disabling stroke or death after CEA (3.2%) and stenting (4.0%). However, there was a significantly greater risk of stroke, death, or procedural myocardial infarction after stenting (8.5%) than with CEA (5.2%). Thirty-day results demonstrated that the incidence of any stroke, death, and fatal myocardial infarction in stenting patients exceeded twice the rate seen with CEA patients. CREST also studied outcomes after CEA and stenting in patients with both symptomatic and asymptomatic carotid artery stenosis. Preliminary results demonstrated no significant difference in the 30-day incidence of stroke, death, and myocardial infarction between the two treatment groups. However, the 4-year rate of stroke or death was significantly greater in the stenting versus surgery group with a hazard ratio of 1.5. Final, long-term results of both these trials are still pending, although it appears that carotid surgery remains the primary surgical intervention for both symptomatic and asymptomatic carotid stenosis but that carotid stenting may be used in select patients.14
External Carotid Artery/Internal Carotid Artery Bypass
External carotid/internal carotid (EC-IC) bypass surgery involves anastomosing a segment of the external carotid artery (most commonly, the superficial temporal artery, STA) to a segment of the internal carotid artery (usually the middle cerebral artery, MCA) using a venous graft and is an alternative surgical intervention for patients with atherosclerotic disease of the ICA (or MCA, for that matter). To date, the EC-IC Bypass Trial remains the only prospective randomized controlled trial evaluating the efficacy of bypass surgery in patients with atherosclerotic cerebrovascular disease compared with best medical management.15 In this study, patients who experienced at least one TIA or stroke ipsilateral to the diseased vessel within 3 months were randomized to either STA-MCA bypass or best medical management. The trial demonstrated that surgery did not reduce the risks of major or fatal strokes, any ipsilateral stroke, major ipsilateral stroke, or all strokes and death. Moreover, operative patients had greater rates of perioperative stroke and death when compared to medically treated patients. As such, the authors concluded that EC-IC bypass was not effective in preventing ischemia or infarction in patients with atherosclerotic cerebrovascular disease. However, many critics of the study argue that the patient inclusion criteria were too broad and that additional prospective trials with better patient stratification, particularly with respect to cerebrovascular hemodynamic status, may demonstrate the utility of EC-IC bypass for a subset of patients.16 The Carotid Occlusion Surgery Study (COSS) was a randomized trial that compared EC/IC bypass and best medical management in patients with hemispheric ischemia within the previous 120 days, and ipsilateral oxygen fraction (OEC) by positron emission tomography (PET). The trial was stopped early by the U.S. National Institute of Health owing to a much better outcome than expected in the medically treated group.17
Nonatherosclerotic Occlusive Disease
Moyamoya Disease
Moyamoya disease is a relatively rare cause of cerebrovascular occlusive disease, with a typical reported incidence of less than 1 per 100,000 per year.18 More common in females, moyamoya disease has a bimodal age distribution, with peak incidence in the first and fourth decades of life. Moyamoya disease manifests as chronic, progressive occlusion of the internal carotid artery at and distal to the carotid siphon that may also involve the proximal segments of the middle and anterior cerebral arteries. Over time, patients with moyamoya disease 3 develop networks of fragile, collateral vessels that resemble a “puff of smoke” on angiogram, hence its name.
Diagnosis
Cerebral angiography is the gold standard for diagnosing moyamoya disease. Hallmark features include narrowing of the C1 and C2 segments of the internal carotid artery and proximal involvement of the middle cerebral artery (MCA) and anterior cerebral artery (ACA) bilaterally. The pathognomonic, delicate collateral vessels typically are visualized near deep brain structures supplied by the thalamoperforating, lenticulostriate, and anterior choroidal vessels. The ethmoidal arteries may also be recruited to supply the ACA (ethmoidal moyamoya) and anastomoses between dural and pial vessels (vault moyamoya). As collateral vessels disappear following revascularization, cerebral angiography may be used to assess success of bypass during follow-up. MRA is a noninvasive alternative, and angiography need not be performed if MRA/MRI shows the following bilaterally: (1) stenosis/occlusion of the terminal ICA and proximal ACA and MCA; (2) abnormal vascular network within the basal ganglia.19
Management
Prevention of further ischemic or hemorrhagic events in moyamoya disease involves revascularization procedures.20 Direct revascularization involves creating anastomoses between a branch of the external carotid artery and a branch of the internal carotid artery (typically the superficial temporal artery and M3 or M4 segment of the middle cerebral artery, respectively). This results in an immediate restoration of flow to previously poorly perfused areas of the brain. See later discussion for in-depth description of the operative technique for EC-IC bypass.
Dissection
Cerebral arterial dissection results from a tear in the arterial wall and extravasation of blood between the intima and media layers, with subsequent luminal narrowing or occlusion. The etiology of arterial dissection is multifactorial but can be grossly categorized as traumatic or spontaneous, although spontaneous dissections may be due to minor trauma to an already predisposed vessel. Conditions commonly associated with cerebral arterial dissections include fibromuscular dysplasia, Marfan syndrome, polycystic kidney disease, and vasculitides. The annual incidence of dissection is approximately 3.6 per 100,000, and vertebrobasilar dissections occur more frequently than carotid dissections.21
The clinical manifestations of cranial arterial dissection vary, but patients often present with either ischemic stroke (more common with carotid) or subarachnoid hemorrhage (more common with vertebrobasilar); however, patients may only have mild complaints, including headache, neck pain, incomplete Horner syndrome, transient deficits, or neck swelling. Ischemic symptoms represent occlusion of the cerebral arteries and can occur secondary to embolization of thrombus formed at the site of dissection, from intraluminal thrombosis, or from expansion of the false lumen with occlusion of the true lumen. As with atherosclerotic cerebrovascular disease, the clinical symptoms reflect the vascular territory involved.
Diagnosis
Cerebral angiography is the gold standard for diagnosis of cerebral arterial dissection.21 Notable angiographic findings include a smooth, but irregular narrowing of the vessel, presence of a double lumen, or intimal flap, and arterial occlusion. The diagnosis may sometimes be confused with atherosclerosis or vasospasm in the setting of subarachnoid hemorrhage (SAH); the former can be distinguished by unusual location and relatively young age of the patient in dissection, and the latter occurs several days following the SAH. Angiography also has the advantage of allowing for immediate, endovascular intervention in select patients. However, there is a small, but real risk of ischemic stroke (<0.5%) as well as contrast nephropathy in patients with renal disease.21 CTA has high sensitivity for both extracranial carotid and vertebral dissections. Additionally, this quick diagnostic allows for rapid diagnosis of dissection in patients, which is ideal for patients presenting acutely. However, as with angiography, patients are at risk of contrast-induced nephropathy and are exposed to significant levels of radiation. MRI/MRA is gaining favor and has particularly high sensitivity for detecting extracranial carotid dissection. Moreover, MRI allows for visualization of ischemic brain lesions. However, MRI/MRA has less utility in detecting vertebrobasilar dissections and may be associated with significant motion artifact in the restless patient. Finally, Doppler ultrasound can be used at the bedside to rapidly to diagnose extracranial carotid dissection the good sensitivity, but is not useful at identifying other dissecting vessels.
Management
Management of cerebral arterial dissection remains controversial given the absence of large, randomized clinical trials.21 However, patients without evidence of hemorrhage and with extracranial disease (especially those with evidence of thromboembolic phenomena) may be treated with anticoagulation (intravenous heparin during the first week or so followed by several months of oral anticoagulation). Patients who do not respond to anticoagulation may benefit from endovascular or surgical intervention. Angioplasty, stenting, and intra-arterial thrombolysis are potential endovascular treatments. EC-IC bypass using high-flow conduit (either saphenous vein or radial artery grafts) with subsequent ligation of the ICA may be used in rare cases. Dissections that present with subarachnoid hemorrhage (usually intracranial cases involving the vertebral artery) usually require surgery. Options include sacrifice of the vertebral artery with or without vascular bypass, surgical clipping of dissection aneurysm, and endovascular coiling.
Surgical Management of Cerebrovascular Occlusive Disease
Carotid Endarterectomy
Choice of Anesthesia
Patients may undergo either local or general anesthesia per surgeon and patient preference, as previous studies have demonstrated that rates of poor short-term outcome following surgery are similar.22 Choice of anesthesia determines intraoperative monitoring. Patients undergoing local anesthesia may be followed clinically throughout the procedure by frequently assessing neurological function. For patients receiving general anesthesia, EEG monitoring may be used to monitor for warning signs of intraoperative ischemia.
Operative Technique
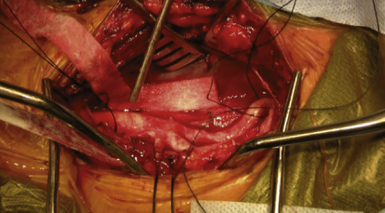
Patients are placed in the supine position, with the neck extended and rotated gently contralateral from the side of the lesion of interest to maximize exposure of the internal carotid artery (the degree of neck rotation needed for optimal exposure may be ascertained by preoperative imaging) (Fig. 12.4). Care must be taken not to cause trauma to the lesion and subsequent thromboembolism to distal cerebral vessels when the operative region is prepped and draped.
Once the carotid sheath is identified (Fig. 12.5), the jugular vein, which lies parallel and anteriorlateral to the carotid artery, is gently dissected from the carotid artery along its medial aspect. Division of the common facial vein enhances exposure of the carotid artery at the level of the bulb and the proximal common carotid, respectively. Following incision through the carotid sheath, the common, external, and internal segments of the carotid artery are identified and controlled using umbilical tape. Any hemodynamic instability noted during this time, principally bradycardia, can be treated with application of lidocaine to the carotid bulb. The patient is then heparinized and the internal, common, and external segments of the carotid artery are sequentially clamped. Cerebral shunting may be performed at this time, depending on surgeon preference; however, intraoperative neurological monitoring must be performed throughout the surgery and a shunt should be prepared and ready for insertion in the event that brain ischemia is suspected.
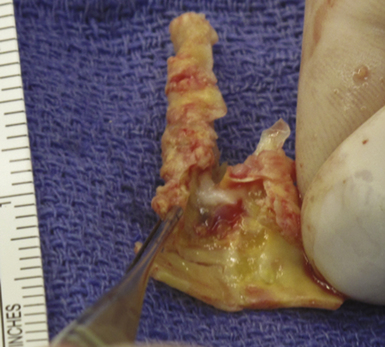
Longitudinal arterotomy of the common carotid near the bifurcation is made and extended proximally and distally through the internal segment. The atherosclerotic plaque (Fig. 12.6) must be carefully dissected away from the arterial wall; a smooth transition between normal and endarterectomized vessel should be made to avoid the creation of a false lumen of the artery.
Although primary closure of the arterotomy is preferred, a prosthetic or venous patch graft (usually harvested from the saphenous vein) may be indicated in patients with repeat endarterectomy or with unusually small carotid arteries. In the latter case, a patch graft may be avoided by visualizing the artery under the microscope during closure. Unclamping for flushing before complete closure is routine. Carotid segments are unclamped following closure of the arterotomy beginning with the external carotid artery, and then the common carotid artery and internal carotid artery, in that order. Prior to final wound closure, meticulous hemostasis is performed and sufficient time is allowed to pass to guarantee its success. A Jackson-Pratt drain may be placed to avoid formation of neck hematomas, and the platysma, fascial layer, and skin are sutured.
Postoperative Care
Patients should be monitored in an intensive care unit with an arterial line in place. Blood pressure lability is common within the first 24 hours postoperatively and pressures should be kept within 110 mm Hg to 150 mm Hg, ideally with short-acting agents to avoid prolonged rebound hypotension or hypertension. Antiplatelet agents should be held for 24 to 48 hours after surgery but may be restarted as soon as 24 hours after surgery. Frequent postoperative evaluations should focus on the identification of new neurological deficits, which may be due to iatrogenic nerve injury or postoperative stroke, evidence of hematoma development at surgical site, and cardiac abnormalities.
Postoperative Complications
Hyperperfusion, which occurs in approximately 9% to 14% of patients postoperatively, is defined as more than 100% increase in cerebral blood flow over preoperative baseline. Cerebral hyperperfusion syndrome is a less common but serious complication of CEA characterized by ipsilateral headache, seizure, and focal neurological deficits in the absence of evidence of ischemia; symptoms usually occur secondary to ipsilateral hemorrhage or edema.23 It occurs in approximately 0.75% to 3.0% of patients; it can occur at any time from hours to a month after surgery, but most commonly begins several days after surgery. Although the exact pathophysiology is unknown, a combination of ischemia-reperfusion injury, impaired cerebral autoregulation, and hypertension is thought to play a role. Hence, it is critical to maintain the systolic blood pressure less than 150 mm Hg following surgery to decrease the risk of developing this syndrome. Trans-cranial Doppler may be used to screen for patients at risk of developing hyperperfusion syndrome; urgent CT is warranted in suspected cases to rule out intracerebral hemorrhage.
External Carotid Artery/Internal Carotid Artery Bypass
The arachnoid should be incised to free the recipient vessel and small branches around the expected arterotomy site must be cauterized. A rubber dam is placed underneath the recipient vessel to isolate the vessel, define the operative field, and protect the underlying parenchyma. Continuous suction is applied to clear the operative field from excessive buildup of CSF.
Please go to expertconsult.com to view the complete list of references.
1. Powers W.J., Grubb R.L., Darriet D., Raichle M.E. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab. 1985;5(4):600-608.
2. Toole J.F., Castaldo J.E. Accurate measurement of carotid stenosis. Chaos in methodology. J Neuroimaging. 1994;4:222-230.
3. El-Saden S.M., Grant E.G., Hathout G.M., et al. Imaging of the internal carotid artery: the dilemma of total versus near total occlusion. Radiology. 2001;221:301-308.
4. Nederkoorn P.J., Mali W.P.T.M., Eikelboom B.C., et al. Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing. Stroke. 2002;33:2003-2008.
5. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445-453.
6. The European Carotid Surgery Trialists’ Collaborative Group. Randomized trial of endartectomy for recently symptomatic carotid stenosis. Lancet. 1998;351:1379-1387.
7. Taylor D.W., Barnett H.J., Haynes R.B., et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet. 1999;353(9171):2179-2184.
8. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273(18):1421-1428.
9. Barnett H.J., Taylor D.W., Eliasziw M., et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339(20):1415-1425.
10. Gasecki A.P., Eliasziw M., Ferguson G.G., et al. Long-term prognosis and effect of endarterectomy in patients with symptomatic severe carotid stenosis and contralateral carotid stenosis or occlusion: results from NASCET. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. J Neurosurg. 1995;83(5):778-782.
11. Kappelle L.J., Eliasziw M., Fox A.J., et al. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. The North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1999;30(2):282-286.
12. Ederle J., Dobson J., Featherstone R.L., et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375(9719):985-997.
13. Brott T.G., Hobson R.W., Howard G., et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11-23.
14. Komotar R.J., Starke R.M., Connolly E.S. Carotid endarterectomy vs endovascular stenting. Neurosurgery. 2010;66(6):N12-N13.
15. The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985;313(19):1191-1200.
16. Reichman O.H., Origitano T.C., Anderson D.E., Duckworth E.A. Lies, damned lies, and statistics: a neurosurgical perspective on the international randomized trial of extracranial to intracranial arterial bypass surgery. J Stroke Cerebrovasc Dis. 2009;18(5):389-397.
17. Powers WJ, Clarke WR, Grubb RC Jr, et al. Carotid Occlusion Study Investigators. Results of the Carotid Occlusion Study (COSS). Presented at the International Stroke Conference, Los Angeles, CA, Feb. 9-11, 2011.
18 Kuroda S., Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7(11):1056-1066.
19. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis. Clin Neurol Neurosurg. 1997;99:S238-S240.
20. Veeravagu A., Guzman R., Patil C.G., et al. Moyamoya disease in pediatric patients: outcomes of neurosurgical interventions. Neurosurg Focus. 2008;24(2):E16.
21. Goyal M.S., Derdeyn C.P. The diagnosis and management of supraaortic arterial dissections. Curr Opin Neurol. 2009;22(1):80-89.
22. Group G.T.C., Lewis S.C., Warlow C.P., et al. General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial. Lancet. 2008;372(9656):2132-2142.
23. Adhiyaman V., Alexander S. Cerebral hyperperfusion syndrome following carotid endarterectomy. Q J Med. 2007;100(4):239-244.