Chapter 53 Cerebral Palsy
The term cerebral palsy (CP) (originally “cerebral paresis”) was first used in 1843 by English orthopedic surgeon William Little in a series of lectures entitled “Deformities of the Human Frame.”110,207 As a result, CP was known for many years as “Little’s disease.” Other notable contributions include Sir William Osler’s The Cerebral Palsies of Children (1889), and publications by neurologists Sachs and Peterson (1890), and Sigmund Freud (1893). Although the term CP is well recognized in both the lay community and medical literature, a precise definition of what comprises CP is still debated to this day. In 2004 an international multidisciplinary group met to revise previous definitions of CP, and their contribution is as follows207:
Implicit in the above definition are several points of clarification that warrant further analysis. The notion of a permanent disorder excludes transient disorders of child development. Permanent does not equate to static, however, and the intent was to recognize that children and adults with CP can have changing patterns of clinical manifestations.207 The term disorder of development distinguishes CP from acquired brain injury that occurs after basic motor development has occurred. Central to the definition of CP is the presence of abnormal movement and posture. This concept has been universal in previous definitions. Because CP comprises a heterogeneous group of disorders, multiple etiologic mechanisms can result in the final common pathway of disturbance of development in the developing fetal or infant brain. There is no explicit upper age limit specified for the onset of the disturbance of brain development. The first 2 or 3 years of life, however, are identified as the crucial period for insults resulting in CP.207 Another key point in the definition of CP has been the identification of the brain insult to be nonprogressive. The pathophysiologic mechanisms leading to CP are presumed to arise from a single inciting event or a discrete series of events that are no longer active at the time of diagnosis.207 The disruption of the orderly process of early brain development in CP initiates a cascade of physiologic consequences potentially affecting every organ system in the developing child. The issues that arise are lifelong, dynamic in nature and require an interdisciplinary team and a “medical home” to effectively manage.13,25
Epidemiology
The most frequently reported incidence of CP is between 1 and 2.3 per 1000 live births.146 The above incidence often excludes acquired CP, which represents between 5% and 60% of all cases, the proportion correlating inversely with the degree of development of the country.91 Although early diagnosis is helpful clinically to initiate services, it can falsely elevate the incidence of CP, because up to 50% of children with CP diagnosed before 2 years of age can have spontaneous resolution of symptoms.56,144 Surveying population-based registers is also complicated by the use of alternate terms such as neonatal encephalopathy, birth asphyxia, periventricular leukomalacia (PVL), hypoxic brain injury, stroke, traumatic brain injury, and shaken infant syndrome.25
The ability to accurately identify trends in CP is dependent on consistent application of diagnostic criteria, and the availability of sufficient longitudinal data. In developed countries where sufficient data exist, the general trend has indicated a relatively stable incidence over a 30-year period from the 1960s to the 1990s. The trend has been a slow decline in CP from the 1960s to the lowest incidence in the 1970s, just before the widespread use of assisted ventilation. The ability to keep premature low birth weight (<2500 g) to extremely low birth weight (<1000 g) infants viable who might otherwise not have survived without mechanical ventilation, resulted in an incidence spike in the 1970s that had not returned to its previous low by the late 1990s.22 In addition, of children with CP diagnosed in the 1980s and 1990s, a greater proportion were more severely impaired.
Etiology
Injury to the immature brain resulting in CP can occur in the prenatal, perinatal, or postnatal period in both term and preterm infants. It is now thought that most cases of CP stem from injury incurred in the prenatal period.146 Common prenatal causes include TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex, other) or other infections, intrauterine stroke (ischemic and hemorrhagic), toxemia, and genetic malformation. The perinatal etiologies of CP comprise injuries occurring at or near the time of birth and can include placental abruption, cord prolapse, uterine rupture or similar processes that result in birth asphyxia. Fortunately, these etiologies are relatively rare, and multiple, controlled population-based studies have shown that an interruption of oxygen supply to the fetus does not account for most cases of CP.51,143,145,146,218 There are numerous postnatal etiologies of CP, including central nervous system (CNS) infection, cerebrovascular insults (ischemic and hemorrhagic), acquired head injury, anoxia, kernicterus, and progressive hydrocephalus. The etiology or pathophysiology of the brain lesion differs with gestational age and will determine the resultant CP subtype and associated movement disorder.
Premature birth is a well-recognized risk factor for CP, which results in an increased risk per infant up to 100-fold. The overall incidence of preterm and very preterm birth is quite low, however, and prematurity is implicated in half or less of CP cases.15,143 The most common CP subtype in premature infants is spastic diplegia. Although prematurity is a risk factor, the etiology of the CP is often multifactorial and a direct result of one or multiple environmental, genetic, or maternal factors that caused the preterm delivery (Box 53-1).
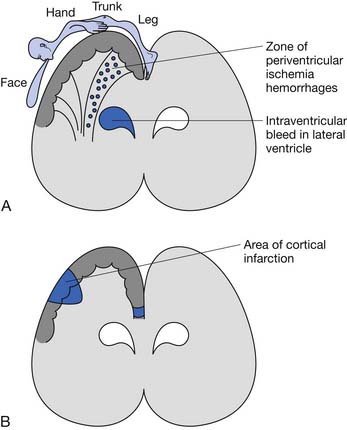
The underlying brain lesion in premature infants classically manifests as a white matter abnormality on neuroimaging. These white matter changes are a consequence of both arterial hemorrhage from fragile capillaries in the watershed zone next to the lateral ventricles known as the germinal matrix, and periventricular hemorrhagic infarction of venous origin. The highest risk for periventricular hemorrhage is between 23 and 32 weeks’ gestation.167 The extent of the bleeding into the germinal matrix is described by a grading system (Table 53-1). Intraventricular hemorrhage can be detected early in the clinical course via transfontanelle cerebral high-resolution ultrasound (US).44 Hemorrhagic infarction of venous origin in the periventricular region is usually asymmetric and located lateral to the external angle of the lateral ventricle.69,155 With healing of the periventricular hemorrhage, symmetric necrosis of the white matter adjacent to the lateral ventricles ensues, resulting in PVL (Figure 53-1). PVL is most commonly associated with a history of prematurity.16,102
Table 53-1 Grades of Intraventricular Hemorrhage in the Premature Brain
| Grade | Hemorrhage |
|---|---|
| 1 | Isolated to germinal matrix |
| 2 | With normal ventricular size |
| 3 | With ventricular dilatation |
| 4 | With parenchymal hemorrhage |
In term and near-term infants, hemiparetic and quadriparetic CP are the most common clinical subtypes of CP.143 Hemiplegic CP is frequently the result of a focal, or sometimes multifocal, lesion, most frequently caused by perinatal stroke or congenital malformation.16,219 The arm is usually more affected than the leg in hemiplegic CP, and seizures and cognitive issues caused by cortical injury can also be present. This is in contrast to PVL and diplegia, which is caused by a subcortical injury, and consequently is less likely to have associated seizures and significant cognitive sequelae.
Quadriplegic CP can be caused by any etiology that induces a diffuse bilateral insult to the brain, such as severe anoxic or ischemic brain injury. Intrapartum or birth asphyxia must be severe and prolonged to cause CP and accounts for less than 10% of quadriplegic CP cases.70,143 Because of the diffuse nature of injury to cortical and subcortical areas of the brain in quadriplegic CP, cognitive deficits, seizures, and the degree of disability are most severe in this group.
In addition to the brain lesions listed above, injury to particular areas of the brain can result in characteristic movement disorders such as those seen with basal ganglia lesions and athetoid CP. Athetoid CP is most often caused by hyperbilirubinemia and has seen a sharp decline in incidence with the institution of Rh incompatibility testing and treatment, and is now relatively rare.65 Basal ganglia and thalamic damage can also be associated with dystonia and associated dystonic subtypes of CP.16
Classification
The most appropriate way to classify children with CP continues to be debated. As early as 1862, Little111 described hemiplegic rigid, paraplegic, and generalized rigid types. In the 1950s, Perlstein156 and Minear134 discussed the importance of classifying children in a more comprehensive manner to include clinical symptoms, motor impairment, function, etiology, topography, degree of muscle tone, neuroanatomy, and therapeutic requirements. In 2000 the group for the Surveillance of Cerebral Palsy in Europe (SCPE; a network of CP surveys and registers formed in 14 centers in 8 countries across Europe) published its process of standardizing classification and descriptions of children with CP.204 Detailed neurologic and topographic categories include unilateral spastic CP, bilateral spastic CP, dystonic CP, choreoathetoid CP, ataxic CP, and nonclassifiable. This classification system is often used in the European literature, but further validity and reliability testing is needed.58
In the most commonly used classification system, tone patterns are described as spastic, dyskinetic, hypotonic, or mixed (Box 53-2), and distribution of limb involvement is characterized as diplegia (lower limbs affected more than upper limbs), hemiplegia (upper limb frequently more affected than lower limb), and quadriplegia (Figures 53-2 through 53-4). Triplegia has been used to describe a combination of diplegia and quadriplegia but with asymmetric upper limb involvement. As the upper limb involvement becomes more severe, inconsistencies in classifying the child as diplegic or quadriplegic arise. In addition, this classification system does not provide an adequate picture of the child’s functional or cognitive status.
The most widely used functional classification system is the Gross Motor Function Classification System (GMFCS), a five-level scale with four age bands that stratifies children based on gross motor ability (Box 53-3).150 This system has been shown to have excellent interrater reliability for both children younger than 2 years and children 2 to 12 years of age. The GMFCS does not describe the limbs affected or the type of movement disorder; consequently it is often used in combination with other descriptive classification systems. The GMFSC has been internationally recognized and is used frequently in research and clinical practice. Palisano et al.152 recently developed the expanded and revised GMFCS, which includes a 12- to 18-year age band and revision of the 6- to 12-year age band. Further validation and reliability testing is needed. An analogous scale to classify functional upper extremity use is the Manual Ability Classification System (MACS), a five-level scale that classifies how children with CP, ages 4 to 18 years, use their hands when handling objects in daily activities.49 It reflects the child’s typical manual performance and has been shown to have good validity and reliability. Its reliability in children younger than 5 years has recently been examined.160
BOX 53-3 Gross Motor Functional Classification System
Modified from Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39:214-223, 1997, with permission.
In 2004 an international multidisciplinary group met to critically review and revise the current classification system. A report on the “Definition and Classification of Cerebral Palsy April 2006” was subsequently published.169 It was recommended that four major dimensions of classification be used:
Diagnosis
The diagnosis of CP is based on the history and physical examination. Further diagnostic testing can help identify the most likely etiology, as well as potential comorbidities such as seizures. The diagnosis of CP is often made by 2 years of age12; however, children with the dystonic type or even mild hemiplegia might have more subtle findings that are overlooked until difficulty with more advanced skills becomes obvious.
History
Eliciting a thorough birth history (prenatal, perinatal, and postnatal) and developmental history is important in guiding one’s understanding of the etiology, determining underlying medical problems, determining the level of function, and developing a medical treatment plan. Delayed acquisition of motor milestones is a universal finding in children with CP. A delay in sitting is often a paramount concern of caregivers, and motor delay tends to increase with successive milestones.136 Early achievement of milestones such as rolling can be interpreted as precocious by caregivers but can be pathologic. A history of asymmetry in movement needs to be investigated. A history of regression in developmental milestones raises concern for progressive neurologic disease rather than CP, which is a static disorder. Other important aspects of the history include nutritional status, associated medical problems, visual and hearing status, therapies, equipment, education, and recreation. Obtaining a family history is essential. For example, children with slowly progressive familial spastic paraparesis can be clinically mistaken for having spastic diplegia, but an accurate family history can help differentiate the two.
Clinical Findings
The diagnosis of CP requires that a motor abnormality be present. Abnormalities in tone, reflexes, posture, and motor performance are seen in infancy.50 Other early predictive signs include weak cry and suck, irritability, and lethargy, although these are nonspecific. In infants born prematurely or of low birth weight, early neurologic abnormalities might not persist, such as transient dystonia.43,209 Spontaneous resolution of symptoms can occur in up to 50% of infants with CP diagnosed before 2 years of age.56,144 Because these early examinations might not predict future abnormality, repeated examinations and follow-up are necessary to ensure a correct diagnosis.
Persistent or obligatory primitive reflexes are an early sign of CP. Obligatory primitive reflexes are those that children cannot emerge from. These can often impede volitional motor control. Examples are the tonic neck reflexes including the asymmetric tonic neck reflex (Figure 53-5), symmetrical tonic neck reflex, and tonic labyrinthine reflex. The Moro (Figure 53-6), palmar, and plantar reflexes often persist in children with CP.138 An absent or inadequate Moro response on one side is found in infants with hemiplegia, brachial plexus palsy, or a fractured clavicle.
Abnormal tone can be manifested as decreased, increased, or fluctuating resistance to passive motion. Hypotonia is often a precursor to spasticity or athetosis.136 However, a prolonged period of hypotonia warrants further evaluation for a neuromuscular disorder such as congenital myopathy or spinal muscular atrophy. Only a small percentage of children are considered to have hypotonic CP.
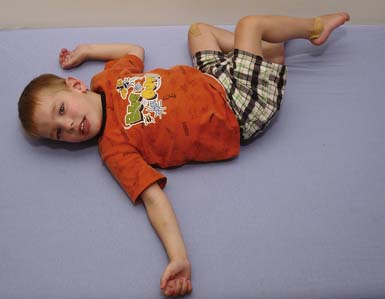
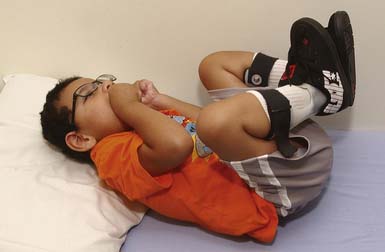
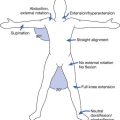
The spastic subtype of CP is the most common, affecting approximately 75% of children with CP. Spasticity is an increased resistance to passive motion that is velocity dependent.178 Upper motor neuron signs in these children include increased reflexes, clonus, and Babinski reflexes. Other findings include decreased strength and selective motor control with resultant impairments of coordination and balance. Common postural abnormalities are influenced by spasticity as well as primitive reflexes, and include lower limb extension and upper limb flexion patterns. The extension pattern of hip adduction, knee extension, and ankle plantar flexion can make diapering and dressing difficult. This pattern, also known as scissoring, is more prominent in weight-bearing or vertical suspension (Figure 53-7). Children with severe extensor tone can exhibit opisthotonic posturing with hyperextension of the neck and trunk (Figure 53-8). The upper limb flexor pattern consists of shoulder abduction, elbow flexion, wrist flexion, finger flexion, wrist pronation, and thumb adduction (Figure 53-9). This pattern becomes more pronounced when the child is stimulated or engaged in an activity resulting in a “high-guard” position. Abnormal fisting of the hand can be the only manifestation of increased tone in mildly affected children.
Fluctuating tone is more typical of dystonia, a movement disorder in which involuntary sustained or intermittent muscle contractions cause twisting and repetitive movements, abnormal postures, or both.178 It is often seen during rest. Spasticity and dystonia frequently coexist in CP, and differentiating the two movement patterns using clinical assessment is important to guide appropriate treatment. Children with dystonia have a greater degree of co-contraction, an increased resistance to external motion at slow velocities, and a greater impairment in muscle strength.108 When dystonia is present in infancy, one must have a high index of suspicion for treatable conditions such as glutaric aciduria type 1. Other dyskinesias include athetosis, an involuntary constant rotary or writhing movement pattern of the distal extremities (see Figure 53-9). Ataxia is rare, and if seen, should warrant further investigation into a degenerative process of the cerebellum. Progressive spinocerebellar diseases often present with a combination of ataxia and spasticity, and include Friedreich ataxia and ataxia telangiectasia
Patterns of movement can differ in children with CP. A combat crawling pattern is often used in children with CP because lower limb extensor tone makes reciprocal crawling difficult. However, this pattern is often used in “normally” developing children. Bunny hopping or knee walking is a pattern adopted for similar reasons. Toe walking is common in children with spasticity affecting bilateral lower limbs. A history of hand preference before 1 year of age, as opposed to normal handedness by the age of 2 years, or any asymmetry in movement should raise suspicion for a hemiplegia.136 Some children with hemiplegia scoot on their buttocks and propel themselves with their unaffected upper limb. Oral-motor abnormalities including drooling and tongue thrusting can be present.
Diagnostic Studies
Neuroimaging
Neuroimaging is part of the diagnostic evaluation of CP and can provide insight into the etiology, medical management, and neurodevelopmental outcome. For example, brain malformations found on magnetic resonance imaging (MRI) in children with spastic CP have been associated with epilepsy and mental retardation,103 and can warrant further investigation into a genetic etiology. Children with a congenital hemiparesis and a finding of a cerebrovascular infarct on neuroimaging might prompt further investigation and identification of a treatable coagulopathy.
The most commonly used modality in premature infants is cranial US for the evaluation of PVL. Guidelines for cranial US have been published by the American Academy of Neurology (AAN), and screening is recommended in premature infants less than 30 weeks’ gestation at 7 to 14 days of age and again at 36 to 40 weeks. US is used to counsel families about prognosis and has been correlated with neurodevelopmental impairment.8,215 However, the positive predictive value for CP with US in one study was only 48%.44 Diffuse, noncystic white matter abnormalities often seen in preterm infants are poorly visualized on US and are better seen on MRI.86
The AAN has recommended that routine neuroimaging, preferably with MRI over computed tomography, be performed in children with suspected CP if an etiology has not been determined.12 MRI has been shown to have a high yield of greater than 80% in identifying abnormalities in children with CP,12,167 and can aid in determining whether the injury was prenatal, perinatal, or postnatal in onset.222 Significant associations have been found between cerebral white matter and gray matter abnormalities on MRI and neurodevelopmental impairment at 2 years of age in preterm infants less than 30 weeks’ gestation.216 Significant correlations between MRI findings and GMFCS in children with diplegic and quadriplegic CP have been described.104 Wu et al.219 found that children with congenital hemiparesis and normal cranial imaging studies were more likely to outgrow clinical signs of hemiparesis by 3 years of age.
Coagulation Studies
Children with hemiplegic CP have a high incidence of prenatal and perinatal cerebral infarction. Studies show that cerebrovascular occlusion, often in the middle cerebral artery, occurs in up to 37% of children.139 Coagulation abnormalities are found frequently in these children and include factor V Leiden mutation, protein C or S deficiency, elevated lipoprotein-a levels, the presence of anticardiolipin or antiphospholipid antibodies, and G20210A mutation of prothrombin.68,185 The AAN recommends that diagnostic testing for a coagulation disorder be considered, and this has been supported in other studies.12
Functional Prognosis
Although CP is a nonprogressive disease, the differences in clinical course are considerable and characterized by a wide change in function over the years of growth.41 Motor milestones are not always met at a predictable rate, and caregivers frequently have questions regarding ultimate functional prognosis. Attempting to predict function becomes important when identifying future care, and therapeutic and equipment needs.
The attainment of independent sitting by 2 years of age is a good predictive sign for eventual ambulation, whereas children who are unable to sit by 4 years of age rarely ambulate.135 In addition, the presence of fewer than three primitive reflexes at 18 months of age is a good prognostic indicator for ambulation.138 Gross motor development has been shown to typically reach a plateau at 7 years of age.17,170 Molnar and Gordon138 reported that walking is achieved in 80% to 90% of children with diplegia, 50% of those with quadriplegia, and 75% of those with dyskinesia. In 2008, walking ability and predictive factors were described in a group of 9012 children with CP from the SCPE common database.17 By 5 years of age, walking with or without aides was seen in 96% of children with unilateral spasticity, 57% of the bilateral spastic group, 90% of the ataxic group, and 61% of the dyskinetic group. It is difficult to compare the statistics in the latter study to those reported by Molnar and Gordon because of the differences in CP classification and nomenclature; however, walking ability was significantly related to cognitive status in both studies. Independence in activities of daily living is often achieved in children with diplegia and those with quadriplegia who are ambulatory.136
Several studies have examined the stability of the GMFCS over time. It has been shown that 73% of children aged 16 months to 13 years remain in the same GMFSC level.151 Children in levels I and V are least likely to be reclassified, and children younger than 6 years tend to be reclassified to a lower level. The predictive value of the GMFCS enables health professionals to give families a more reliable functional prognosis. The GMFCS classification of infants is more challenging, however, because of their variability in development, making the prognostic value less in this group. It has been found that 42% of children younger than 2 years had a change of one or two GMFCS levels by 2 to 4 years of age.
As children reach adulthood, walking can become less efficient because of physiologic changes such as development of contractures, joint degeneration, or change in body weight. The energy cost of walking has been found to correlate with the GMFCS level.92 Fifty-two percent of adults, aged 24 to 76 years, with hemiplegic and diplegic CP report deterioration in walking function.149 Deterioration was associated with increased pain frequency and intensity, reduced balance, and fatigue. Stability of the GMFCS in adults with CP, aged 17 to 38 years, has also been examined and shows that the GMFCS level at 12 years of age is highly predictive of adult motor function.123
Medical Management
Feeding, Growth, and Nutrition
Growth and nutrition disorders are very common in children with CP and are the result of a combination of factors. It has been reported that 27% of children with moderate to severe CP are malnourished,174 which leads to slower growth than expected compared with age- and gender-based standards.175,176,198 Although the incidence of malnutrition is higher in persons with spastic quadriplegia and more severe disability, one study found that up to 30% of children with spastic hemiplegia and diplegia were undernourished.192 Malnutrition in CP is known to have a negative impact on the physiology of nearly every organ system and has been demonstrated to increase health care utilization, decrease societal participation, impair motor function, decrease life expectancy, negatively affect bone health, impair wound healing, and increase the risk for postoperative complications.105 The importance of optimizing nutritional health cannot be emphasized enough.
Poor oral-motor function results in dysphagia, malnutrition, and dehydration, and increases the risk for aspiration pneumonia. At least 75% of children with CP have been reported to have some sign or symptom of dysphagia.164,165,193 Gastrostomy tube (G-tube) placement is one means to ensure adequate nutrition intake and decrease the risk for aspiration, and is a routine practice in the medical management of CP. Despite proven efficacy, G-tubes are generally used only after other treatments have failed.26,39 Clinicians must be sensitive to some caretaker’s perception of G-tube feeds as unnatural.157 Despite initial reluctance, most caretakers are usually pleased with the outcome after surgery given the improvement in the child’s health.203
Although nutritional concerns have been demonstrated to be a significant contributor to poor growth in CP, they are certainly not the only factor. Hormone assays in children with CP have identified growth hormone deficiency.38 In addition, children with moderate to severe CP have been found to lack the dramatic pubertal growth spurt that accounts for a significant amount of adult height. Children with CP often experience early adrenarche that lasts longer than their peers.217 Additional work has suggested that pubertal girls with CP, even when adjusting for nutritional status, have decreased insulin-like growth factor-1 and growth hormone levels.105 The etiology and clinical significance of endocrine abnormalities in CP warrants further research.
Pulmonary
The leading cause of death in children with a severe disability, including CP, is chronic respiratory infection, and more than 90% of deaths in children with severe CP are caused by pneumonia.79,161 The etiology of pulmonary compromise in CP is multifactorial, including pulmonary aspiration, decreased mucociliary clearance, suppuration, kyphoscoliosis, and airway obstruction.54 Various tests are available to evaluate for aspiration, including the salivagram, barium videofluoroscopy, fiberoptic endoscopic evaluation of swallowing, and a radionuclide scan. If aspiration of saliva and drooling is deemed problematic, management can include oral-motor therapy, behavior modification via biofeedback, anticholinergic medications such as glycopyrrolate, benztropine, and scopolamine transdermal patches, botulinum toxin injections to the salivary glands, surgical resection of the salivary glands, or rerouting of the salivary ducts.23,130 Treatments to improve clearance of secretions can include inhalation therapy with normal or hypertonic saline, bronchodilators, and chest physiotherapy.
Pulmonary infections are common in CP, and a low threshold should be had for antibiotic use when there is evidence of suppurative lung disease. The antibiotic selection should be in response to sputum culture results and will often require a prolonged course of treatment (3 to 4 weeks).54 Immunization against influenza and pneumococcus is also recommended if not otherwise contraindicated. Kyphoscoliosis in CP can also contribute to pulmonary dysfunction usually as a result of structural restrictive lung disease. Airway obstruction is also a frequent contributor to respiratory dysfunction and can involve either the upper or lower airways. Upper airway obstruction can occur at the supraglottic level, with redundant aryepiglottic folds causing a clinical picture of laryngomalacia. Lower airway obstruction might be the result of reactive airway disease or asthma, or the consequence of intrinsic lung abnormalities. A trial of bronchodilators and asthma therapy is warranted if reactive airway dysfunction is thought to contribute, but should be monitored for efficacy and clinical response.
Respiratory dysfunction in CP might also affect sleep, and community-based surveys have identified a higher prevalence of sleep breathing problems in children with CP compared with healthy children.99 Treatment of the sleep disturbance involves directed therapy to address the identified contributing factors. The disruption of normal sleep patterns has an enormous effect not only on the cognition and quality of life of the child, but also on the caretaker, parents, and siblings who are involved in the provision of care.54
Neurologic Issues
The disruption of the normal orderly sequence of early brain development in CP is associated with a multitude of neurologic comorbidities. A practice parameter guideline by the AAN in 2004 recommended screening children with CP for associated mental retardation, ophthalmologic and hearing impairments, speech and language disorders, and oral-motor dysfunction.55 Children with CP also have an increased frequency of hydrocephalus, sleep disturbance, and epilepsy.
On average, 43% of children with CP develop epilepsy, and the risk is increased in those with neuroimaging abnormalities. The reported incidence of epilepsy is variable, with an incidence of 50% to 94% in spastic quadriplegia, 30% in hemiplegia, and 16% to 27% in diplegia or ataxic CP.12 Despite this, there is no evidence to support routine electroencephalogram (EEG) screening in children without a history of seizures, and EEG was not found to be helpful in identifying the etiology of CP.12
Mental retardation or the impairment of cognitive and neuropsychological function is commonly present in CP.53 In general terms the degree of motor impairment and the extent of cortical injury on imaging studies directly correlate with the severity of cognitive deficits, with the exception of dyskinetic CP where this association is lacking.53 There is also a strong correlation of greater intellectual impairment in children who have abnormal EEG findings or epilepsy.83,213 A comprehensive neurodevelopmental evaluation including speech-language assessment and neuropsychologic evaluation might help in guiding therapeutic intervention and tailoring the school program.
Visual impairment in CP is common (28%), and the relative risk is increased with the degree of imaging abnormality.12 The etiology is often multifactorial, including pathologic disorder. of the anterior afferent sensory visual pathways, ocular structures, cortical vision impairment (CVI), and disorders of ocular motility. CVI is commonly defined as a loss in visual function in the absence of damage to the anterior afferent visual pathways or ocular structures.95 The term CVI is not accurate because the visual impairment is not limited to the visual cortex but also usually involves white matter pathways such as the optic radiations in children with PVL.82 Neuroimaging findings have been found to correlate with the prognosis, as children with isolated striate cortex damage had a better prognosis than did those with isolated PVL.95 The most common etiologies for CVI are perinatal hypoxia (35%), prematurity (29%), hydrocephalus (19%), structural CNS abnormalities (11%), and seizures (10%).95 With the increased survival of premature and very sick term infants as a result of advances in modern perinatal care, the prevalence of CVI in childhood has been steadily rising over the past few decades. CVI is now the most common cause of acquired bilateral visual impairment in childhood in developed countries.55,195 The overall prognosis for significant improvement in visual function with CVI is poor, and children with structural abnormalities of the brain and afferent visual pathways are the least likely to improve.95
Hearing impairment is reported to occur in approximately 12% of children with CP.95 Risk factors include very low birth weight, kernicterus, neonatal meningitis, severe hypoxic-ischemic insults, and administration of ototoxic medications. The 2008 U.S. Preventive Services Task Force recommends universal screening for hearing loss in all newborn infants.211
Speech and language disorders are common in CP, with a reported incidence of articulation disorders and impaired speech intelligibility of approximately 38%.36,113 Anarthric or dysarthric speech is caused by oral-motor dysfunction as a result of bilateral corticobulbar dysfunction or lesions to cortical speech-language centers. Many children with CP have preserved receptive language despite being nonverbal or markedly dysarthric. Marked motor and speech impairment with relative preservation of intellect is the hallmark of athetoid CP secondary to subcortical basal ganglia lesions. This is important to remember because one should assume that children understand what is said to them or in their presence. In children with significant expressive language dysfunction, alternative means of communication including yes-no sign boards, picture boards, adaptive switches, or augmentative communication devices should be explored.
Genitourinary
Voiding dysfunction has been estimated to affect 36% of children with CP.129 Symptoms can include urinary frequency, incontinence, or difficulty urinating. In studies where urodynamic evaluation was performed, neurogenic detrusor overactivity with a low bladder capacity was seen in 47% of children with CP, while the finding of detrusor-sphincter dyssynergia was present in 11%.93 In symptomatic children, early referral for urodynamic evaluation and treatment might significantly improve urinary symptoms.
Gastrointestinal
Gastrointestinal symptoms are frequent in CP and are often attributable to impairments in colonic motility.153 Gastroesophageal reflux can be seen because of weakness of the lower esophageal sphincter and can result in emesis and aspiration. Malnutrition as a consequence of oral-motor dysfunction can further complicate the impaired colonic motility that results from inadequate fluid and dietary fiber intake. The consequence of impaired colonic motility is chronic constipation with possible long-term large bowel megacolon and volvulus, which are preventable with regular bowel evacuation. Abnormal segmental colonic transit times have been correlated with ambulatory function in CP.153 Fecal incontinence or defecation distress can occur because of anal sphincter or pelvic muscle incoordination. This dysfunction in the pelvic floor musculature is thought to be the result of abnormal neuromotor control and can be assessed with anorectal manometry.3
Osteoporosis
Peak bone mineral density (BMD) achieved by the end of adolescence determines the risk for later pathologic fractures and osteoporosis.172 In children with CP, bone mineralization can be adversely affected by a combination of factors, including malnutrition with vitamin D and calcium deficiencies, antiepileptic medications, immobility, and lack of dynamic loading via muscle forces and standing. Previous work by Henderson et al.75 demonstrated that in a cohort of 117 children with moderate to severe CP who were unable to stand by 9 years of age, 97% had osteopenia (BMD Z score <−2.0) as tested in the distal femur. Positive effects on BMD have been demonstrated with vitamin D and calcium supplementation as well as bisphosphonate therapy.89,162 Specific recommendations regarding indications for starting bisphosphonate therapy, age, optimal dosage, duration of therapy, and long-term effects are not known. Studies on increased weight-bearing via passive standing have failed to demonstrate a positive effect on BMD and the risk for pathologic fracture.33
Outcome Measures
Choosing the right tool is the key to comparing outcomes in rehabilitation research.64 Good validity and reliability as well as responsiveness to change are desirable. Table 53-2 depicts various outcome measures used in pediatric functional assessment.
Table 53-2 Outcome Measures for Cerebral Palsy Rehabilitation
| Outcome | Measure |
|---|---|
| Spasticity |
Therapeutic Management
The therapeutic management of a child with CP requires a thorough understanding of the multisystem involvement in addition to the obvious motor impairments. Therapeutic interventions can be undertaken in a facility, home, or school, but education of caregivers in a home program of therapeutic exercises and age-appropriate activities is an important component. Integration of the child into community activities such as karate, gymnastics, or dance is another important way to meet the child’s therapeutic goals.131 It is imperative that the family and child be active participants in the process of setting priorities and goals within the context of the impairment. The ultimate goal of any therapy program is to encourage the child’s maximum potential in motor, cognitive, and social realms.
Early Intervention
Early intervention is the process of providing services, education, and support to children from birth to 36 months of age who have delays in one or more areas of physical, cognitive, social, emotional, or adaptive development.131 An early intervention program includes not only therapy services and family training, but also health, psychologic, vision, assistive technology, social work, and care coordination services.131 Some studies have shown that early intervention programs for preterm infants have a positive influence on cognitive outcomes, but there is little evidence of an effect on motor outcomes.124,191 There is evidence that early intervention minimizes secondary complications and provides family support.72
Being well versed in special education law and federal legislation enables health care professional to advocate for children with disabilities.85 The Vocational Rehabilitation Act of 1973, or Public Law (PL) 93-112, provides services for the physically and mentally handicapped. Section 504 of this act protects against discrimination of service. It ensures that children who might not meet “special education” definitions have appropriate classroom accommodations, such as extra time for test taking or frequent breaks. PL 94-142, or the Education of All Handicapped Children Act of 1975, requires that all states provide a free and appropriate public education for children aged 3 to 21 years (except ages 3 to 5 years and 18 to 21 years were not mandatory) regardless of handicap, and requires that these students be placed in the least restrictive environment possible.48,85 It also mandates that every child with a disability have an individualized educational plan that, like a 504 plan, ensures appropriate accommodations, but also includes statements of present level of functioning, short- and long-term goals, specific educational services needed, participation in regular education programs, and assurance that the necessary services are available. The Education of the Handicapped Act Amendments of 1986, PL 99-457, expanded the provisions of PL 94-142 to include handicapped infants and preschool children from birth to 3 years of age, and extended mandatory free and appropriate public education to children aged 3 to 5 years. Included in this amendment was the requirement that all children receiving early intervention services have an individualized family service plan (IFSP). In addition to containing goals for the child, the IFSP includes information about the family’s strengths and needs related to the child’s development. The Individuals with Disabilities Education Act (IDEA) of 1990 or PL101-476, also known as the Education of the Handicapped Act Amendments of 1990, expanded on PL 94-142, provided funding for infant and toddler early intervention programs, and replaced the word “handicapped” with “disabled.” Infants and toddlers with disabilities from birth to 2 years of age who demonstrate developmental delay receive early intervention services under IDEA Part C, and children aged 3 to 21 years receive special education and services under Part B. For a state to participate in the program, it must ensure that early intervention will be available to all eligible children and their families. In 2004 the IDEA law was reauthorized by Congress and updated to include the requirement of preparing children with disabilities for further education, employment, and independent living.31,85
Therapy Approaches
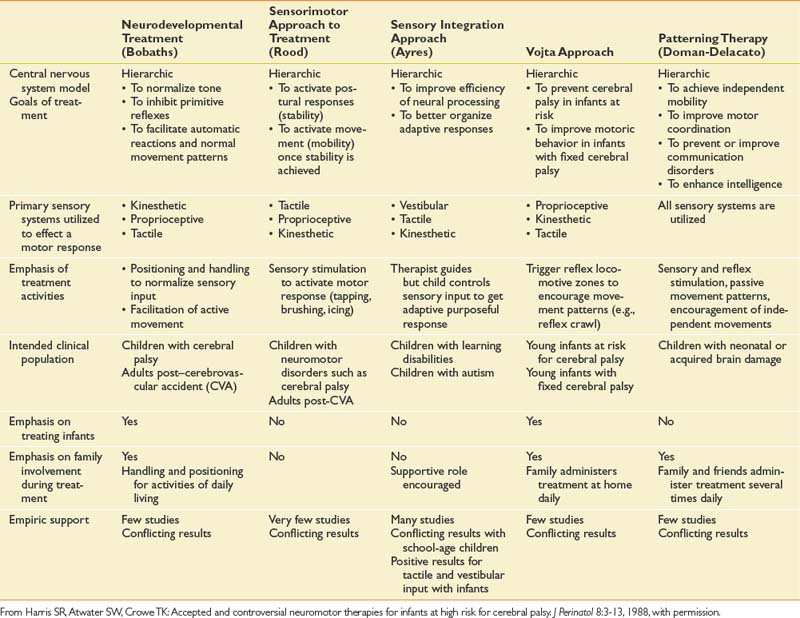
The goal of therapy intervention is to facilitate the development of fine motor, gross motor, and speech-language skills. Functional goal setting has become prominent since the 1980s, and progress is frequently monitored as a requisite to justifying continued services.88 A number of different therapy approaches have been advocated to facilitate the development of skills, although no one system has been proven to be superior to others (Table 53-3).73 Most therapy approaches were developed empirically from clinical observation, and a theoretic framework was devised later to explain the possible neurophysiologic principles.121,137 Most therapists do not adhere to a single therapy approach, and often an eclectic approach is used with tailoring of therapy interventions based on the needs and clinical response of the child.
Most studies that have attempted to quantify the effectiveness and benefit of therapy interventions have been inconclusive.11,77 The development of appropriate and sensitive outcome measures, adherence to randomized controlled studies, and the use of metaanalysis will be necessary to definitively demonstrate the benefit of therapy interventions over the natural history of CP without therapy. This process is methodologically difficult, however, because of the inherent problems with measuring treatment-related change within the background of expected developmental changes, compounded by the heterogeneity of the child with CP. Systematic reviews of physical, occupational, and speech and language therapy similarly show positive trends toward a beneficial treatment effect, but fail to definitively show benefit.10,154,197
Therapy interventions that are being examined currently for potential benefit in CP include partial body weight–supported treadmill training (PBWSTT), constraint-induced movement therapy (CIMT), hippotherapy, and functional electrical stimulation.78,142,187 However, efficacy studies are similarly plagued with the same methodologic barriers as conventional therapy interventions.
Specific Therapy Interventions
CIMT is a treatment for hemiparesis to improve motor function in the affected upper limb. CIMT has been shown to be efficacious in adults with stroke214 (see Chapter 50). In children with hemiplegic CP, the unaffected limb is restrained with a removable cast, typically for 3 weeks, and the child undergoes intensive, structured therapy in addition to daily activities and play. Studies show positive results, with new motor skills gained and increased arm use up to 6 months after CIMT.35,206 CIMT in stroke has been associated with significant neuroplasticity or cortical reorganization on neuroimaging.59,109,179 Evidence of cortical changes in children with CP is emerging.205
Electrical stimulation is a potential adjunctive therapy, and proponents advocate improvement in strength and function. Neuromuscular electrical stimulation is the application of an electrical current of sufficient intensity to elicit muscle contraction. When applied during a functional activity, it is referred to as functional electrical stimulation.94 In contrast, threshold electrical stimulation is a low-intensity, subthreshold electrical stimulus that has been theorized to increase blood flow and stimulate muscle growth (see Chapter 21). Evidence to support use of these modalities in CP is limited and with varied outcomes.40,94,188,212
PBWSTT is a therapy in which an overhead harness system is used to support the child’s body weight on a treadmill, while the therapist provides verbal and tactile cues to facilitate the kinematic, kinetic, and temporal features of walking.142 Although evidence of potential benefits from PBWSTT is emerging in adults with stroke and spinal cord injury, several recent systematic reviews showed limited evidence to support the use of PBWSTT in children with CP.120,142 Despite reported improvements in gross motor function, functional status, walking performance, and gait parameters, statistical significance was not reached in most of the studies.
Therapeutic horse riding (hippotherapy) has been gaining popularity and is considered by many families to be complementary to their traditional therapies. Improvements in gross motor function, postural control, pelvic movement, co-contraction, and joint stabilization have been shown; however, the strength of the evidence is variable.187,196
Durable Medical Equipment
Truncal hypotonia, impaired balance, proprioception deficits, and spasticity often delay if not preclude independent sitting and standing. Supportive or adaptive seating and standing can facilitate upper limb function, communication, and feeding by freeing the child’s hands to perform bimanual tasks, improving breath support, and optimizing the head and trunk position to facilitate a safe swallow. The optimal seating angle should be determined on an individual basis; however, available evidence supports a neutral to anterior seat inclination for functional tasks.128 Specialized seating devices are available for sitting on the floor, sitting on the toilet, feeding, and bathing, as well as for incorporation in a mobility device. Passive standers are also available as positioning devices, and although they might have the ability to be mobile, the high center of gravity and propensity to tip does not make them a practical or safe means of community mobility.
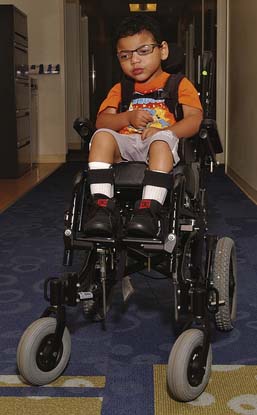
Use of a wheelchair becomes applicable when a child either outgrows commercially available strollers or additional support is necessary. The goal of supportive seating is to provide an upright seated posture to facilitate interaction with the environment and minimize deforming forces secondary to postural abnormalities (Figure 53-10). The clinician must consider both the seating goals and the family’s needs. One consideration unique to pediatrics is the decision to use either a conventional wheelchair base or a stroller base for the mobility device. The decision to use power mobility is dependent on motor control, vision, and cognition; however, it has been successfully used in children as young as 18 months.30 (See Chapter 17 for additional considerations in wheelchair seating prescriptions.)
For children with adequate head and trunk support, a gait trainer, walker, or crutches might help facilitate gait training. The gait trainer is a wheeled walker with a sling seat and various support options, which allows the patient to propel the device without necessarily having a coordinated, reciprocal gait pattern. Walkers provide external support for strength and balance, and can improve upright position.67 For the dependent child and adult, families might benefit from equipment to facilitate transfers and floor recovery, such as a mobile mechanical lift or overhead lift device.
Splinting and orthoses are commonly used in CP to manage spastic but flexible dynamic deformities of the extremities. The decision to brace and the type of orthosis to be used are dictated by the age of the child, functional level, motor control, type of deformity, and commitment to use.140 With lower limb orthoses the clinician should clearly identify the gait deviation and goals to be addressed, with special consideration of ankle-foot alignment, range of motion, and tone. Ankle-foot orthoses have been demonstrated to decrease the energy cost of walking in children with CP, compared with barefoot walking, and to improve gait parameters of stride length and velocity.1,27 (See Chapters 14 and 15 for further information on orthoses.)
Management of Hypertonia
Management of hypertonia in children with CP is challenging, and no standard approach exists. The most common form of hypertonicity in children with CP is spasticity, and it can occur globally affecting both the trunk and limbs or can occur more focally. Spasticity often coexists with dystonia, a more dynamic movement disorder, and using quantitative measures to differentiate between the two will ensure more appropriate treatment.63 The importance of physical therapy, orthoses and casting has been stressed by a consensus group,66 but these interventions can be augmented with oral medications as well as chemodenervation with botulinum toxin (BTX), phenol, or both. Neurosurgical procedures including intrathecal baclofen (ITB) and selective dorsal rhizotomy (SDR) can also be considered in carefully selected populations. (See Chapter 30 for a more comprehensive discussion of spasticity management.)
The impact of hypertonicity on a growing child needs to be continually reevaluated and the goals adjusted to help facilitate developmentally appropriate milestones. In some cases spasticity can be “useful”—for example, when it aids the ability to transfer in the face of underlying muscle weakness.208 Treatment goals for dystonia differ somewhat compared with those for spasticity.4 In children with dystonia, contractures occur less often, and dystonic arching of the trunk can affect sitting, caregiving, and comfort.
Oral Medications
Oral medications are often used as an early treatment for generalized spasticity. Commonly used medications include baclofen, diazepam, dantrolene sodium, tizanidine, clonazepam and clonidine. All of these medications, except dantrolene, exert their effect through the CNS and can cause sedation. Both baclofen and diazepam have been shown to be significantly better than placebo in reducing spasticity and improving selective motor control in children with CP.119,132,180 Pediatric dosing is quite variable and needs to be tailored to the response of the child. The choice of medication is often based on personal experience and the impact of benefit versus potential side effects.122
Oral medications used for treatment of dystonia in children with CP include trihexyphenidyl hydrochloride and levodopa-carbidopa. Severe, generalized secondary dystonia often responds poorly to oral medications, and ITB has been found to be more effective.4 The scientific evidence for the clinical use of trihexyphenidyl in children with CP remains equivocal.80,166,177 Adverse events such as sedation, dizziness, and dry mouth are common and can limit its tolerance.
Chemical Denervation
Focal hypertonicity can be managed with intramuscular BTX injections and phenol neurolysis. Injections are most effective in children with dynamic muscle shortening localized to specific muscles and those without significant contracture.66 Chemical denervation can be used in combination with systemic medications.
Botulinum Toxin
BTX is widely used in the management of CP. BTX-A is marketed as Botox and BTX-B as Myobloc. Dosing and dilution guidelines have not been established, but consensus statements and systematic reviews provide recommendations.66 Considerations used in dosing include the child’s weight, muscle size, location of muscle, and degree of spasticity.96 The period of clinical useful relaxation is usually 12 to 16 weeks, and it is recommended that injections be spaced a minimum of 3 months apart because of concern for neutralizing antibody formation.66 Adverse events related to BTX are rare and include injection site pain and excessive weakness of the injected muscle.66 Caution is recommended when injecting children with pseudobulbar palsy.
Muscles commonly injected include the gastrocnemius, hamstrings, hip adductors, and flexor synergy muscles of the upper limb. Improvement in gait and gross motor function has been demonstrated with isolated BTX-A injections into the gastrocnemius muscle for equinus foot deformity,20,98 as well as with multilevel lower limb injections.181 Studies evaluating the effectiveness of BTX-A injections combined with serial casting for dynamic equinus show mixed results.2,45 BTX-A injections into the upper limbs of children with hemiplegia have been shown to improve movement and function,114 and the use of three-dimensional kinematics is showing promise in detecting functional changes.115
Phenol
Phenol has been used for many years to reduce focal spasticity. Its low cost is a significant advantage over BTX, but the technical difficulty in isolating a nerve and a need for general anesthesia can incur more cost. Duration of improvement varies between 3 and 18 months.106,220 Phenol perineural injections or motor point blocks are typically used for large proximal muscles such as the biceps brachii, hip adductors, and hamstrings. Phenol motor point blocks can also be performed in the posterior tibialis and gastrocnemius muscles to minimize the risk for sensory dysesthesia. Injection into the anterior branch of the obturator nerve in children with CP has been shown to be safe and effective.106 Rare adverse events include sensory dysesthesias and cardiac dysrhythmias.61,97 BTX-A and BTX-B have been combined safely with phenol injections thus allowing simultaneous injection during a single injection episode. By using phenol and BTX in conjunction, one can maximize the dose and number of muscles treated by a finite amount of BTX.61,97
Intrathecal Baclofen
ITB is delivered to the intrathecal space via a catheter connected to an implanted pump in the abdomen. This method of delivery infers an advantage over oral baclofen in that sedative effects are minimized. ITB is most often used to treat children with generalized spasticity or generalized moderate to severe dystonia. Cost and maintenance can be prohibitive for some families. A screening lumbar bolus dose of baclofen can be used to evaluate responsiveness, but it is not always helpful, especially in cases of dystonia.4 Catheter tips are typically positioned at C5–T2, but can be placed intraventricularly for severe dystonia.5
Several studies demonstrate the effectiveness of ITB in children with CP, and one study showed a significant reduction in upper and lower extremity spasticity for up to 10 years.6,81 Significant improvements in gross motor function 1 year after ITB pump implant have been demonstrated.100 Children and their caregivers are generally satisfied with the results of ITB and report improved positioning, transfers, dressing, toileting and hygiene, and comfort.101 Speech, communication, and saliva control can improve after ITB.21
Potential complications of ITB include infections, cerebrospinal fluid leaks, and catheter problems such as disconnection and migration. Abrupt withdrawal is a medical emergency and needs to be managed aggressively.223 Urinary retention can be seen acutely, whereas constipation and weight gain tend to be more chronic complications.4,125 ITB has not been found to significantly affect the rate of progression of hip subluxation96 or scoliosis in children with CP.184,186
Selective Dorsal Rhizotomy
Selective dorsal rhizotomy (SDR) is a surgical procedure that has been widely used as a treatment for spasticity. The surgical technique involves single or multilevel osteoplastic laminectomies exposing the L2–S2 nerve roots. Typically, 25% to 70% of dorsal rootlets are selectively cut with the aid of electrophysiologic monitoring.74,194
Children 3 to 8 years of age with spastic diplegia, little upper limb involvement, good selective motor skills, and minimal contractures are the best candidates for SDR.127,194 Combined dorsal and ventral rhizotomies have been performed in a few children with severe spasticity and dystonia who were not candidates for ITB.4 Appropriate candidates for SDR must have sufficient underlying strength. The preoperative ability to rise from a squatted position with minimal support, and a younger child’s ability to crawl on hands and knees are positive predictors for a good outcome with SDR.194 Some degree of lower limb weakness can be unmasked postoperatively by reducing the spasticity, making intensive physical therapy a necessity. Children must have the cognitive and social capacity for such an intensive intervention.127
A metaanalysis of three randomized controlled trials evaluating the outcome of SDR in children with spastic diplegia showed that SDR plus physical therapy is more efficacious than physical therapy alone in reducing spasticity and has a small positive effect on gross motor function.127 Studies using three-dimensional gait analysis show improvement in gait parameters after SDR.37 Long-term outcomes 5 and 20 years after SDR in children show an improvement in the Gross Motor Function Measure and gait pattern.107,147 Quantifiable improvements in upper limb function have also been described after SDR.112
SDR can reduce the need for orthopedic surgeries, with 35% of children avoiding surgery, and this might be more likely if SDR is performed before the age of 5 years.148,194 Long-term complications such as sensory dysfunction, bladder or bowel dysfunction, or back pain are infrequent.194 A concern with multilevel laminectomies is the potential increased risk for spinal deformities, but there is no clear evidence to support this.
Orthopedic Management
Children with CP have abnormalities in muscle tone, strength, balance, and selective motor control. These abnormalities in muscle tone restrict the range of motion of affected joints, and when coupled with musculoskeletal growth, result in muscle contractures and associated bony deformities.18 Orthopedic surgical management of persons with CP can differ depending on the functional abilities of the individual, but share the common goal of maximizing quality of life and function by addressing the secondary musculoskeletal complications that result. Orthopedic surgical procedures in CP consist of a combination of muscle releases and lengthenings, split tendon transfers, osteotomies, and arthrodesis.18 The timing of surgical intervention is determined by CNS maturation, ambulation potential, and the rate at which the deformity is developing.
Hips
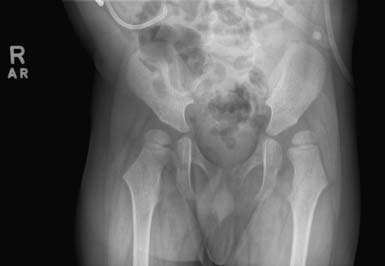
The goal of orthopedic surgical intervention for the hips is to prevent dislocation and achieve a stable and level pelvis to facilitate sitting, improve hygiene, prevent pain, and inhibit postural abnormalities that promote excessive pressure on bony prominences and scoliosis.18 It has been reported that approximately 60% of children who are not walking by the age of 5 years are likely to develop subluxation of the hip, with the greatest risk in those with severe neurologic involment.62 In contrast, Scrutton et al.183 found that no children with CP who walked 10 steps alone by 30 months of age required treatment of their hips by 5 years of age. Because children with spastic quadriplegic CP are at highest risk for hip pathology, guidelines have recommended radiographs of the hip by 18 months of age in this population.183,189 Surveillance radiographic imaging monitors the migration percentage of the femoral head as it subluxates, and the acetabular index, at an interval of 6 to 12 months, depending on the rate of progression and associated risk factors.46 Children with a migration percentage greater than 33% or an acetabular index greater than 30 degrees are likely to require further treatment, and hips with a migration percentage greater than 60% are unstable and require immediate attention.62
The deforming force of spasticity exerts its effect through the muscle tendon unit. “Soft tissue” surgeries are procedures involving muscles and tendons that serve to lengthen and weaken spastic and shortened muscles, and decrease their unopposed pull on the bony skeleton with the goal of balancing forces. Soft tissue surgeries used to counteract hip subluxation often include some combination of hip adductor tenotomy, psoas recession, iliopsoas tenotomy, or medial hamstring lengthening and are performed after the hip has demonstrated subluxation.163,189 Alternatively, BTX is also being used to decrease the deforming forces of the spastic hip adductor muscle and slow the progression, if not successfully prevent dislocation of the hip joint.159,221
The lack of weight-bearing through the hip joint in nonambulatory children also compounds the forces leading to instability, because axial weight-bearing serves to deepen the acetabulum and induces a progressive varus angle of the femoral neck with respect to the shaft. The osseous deformities of the hip that result include femoral anteversion caused by spastic overpull of the medial hamstring, as well as acetabular dysplasia and coxa valga resulting from a lack of axial weight-bearing.189 Treatment of these conditions includes periacetabular osteotomies to improve coverage of the femoral head, as well as proximal varus and external rotation osteotomies of the femur to address the angular and rotational deformities, respectively190 (Figures 53-11 and 53-12).
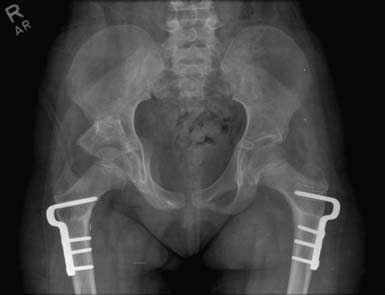
FIGURE 53-12 Proximal varus derotational osteotomies of the femurs and right periacetabular osteotomy.
The natural history of the severely subluxated hip has also been studied, with variable conclusions.190 The relationship between hip pain and subluxation or dislocation of one or both hips is highly variable. Although it is clear that a subset of children with neuromuscular dysplasia has hip pain, the relationship between radiographic and clinical findings remains elusive.190 For those with established pain attributed to hip dislocation, deformity of the femoral head and breakdown of articular cartilage, salvage procedures to include proximal femoral resection, osteotomies to redirect the femoral head, and hip arthroplasty have all been described.190 Total hip arthroplasty has been shown to relieve pain and improve function in 94% of adults with various severities of CP, but its lifelong efficacy is not known.29
Lower Limb
In the ambulatory child with CP, the complexity of forces involved in pathologic gait can be assessed with the help of three-dimensional gait and motion analysis, particularly to assist with surgical planning.57 Various soft tissue surgeries are used to maintain muscle length and prevent contracture.190 The practice of limiting surgeries to a single event addressing multiple areas of deformity and contracture (single-event multilevel surgery) has been shown to be effective in preserving functional ambulation, while minimizing interruptions in school and leisure activities.18
Children with CP have a variety of pathologic gait patterns that are compensatory responses to abnormal muscle tone, altered range of motion, motor imbalance, and bony deformity. Scissoring is a common frontal plane pattern of excessive lower extremity adduction caused by persistent femoral anteversion in the setting of hip adductor and medial hamstring spasticity. Management of scissoring can include focal spasticity management with BTX, and adductor tenotomy or myotomy, and is often combined with hamstring lengthenings. Scissoring might accompany sagittal plane deformities of “crouch gait” and “jump gait.” Jump gait is characterized by excessive hip flexion, knee flexion, and equinus at the ankle in stance, and is the most common sagittal plane pathologic finding in young diplegic children.18 The jump gait pattern is in contrast to “apparent” equinus in which the child adopts a similar toe walking pattern, but is caused by dynamic or fixed knee flexion rather than excessive ankle plantar flexion.34 Crouch gait is a pattern seen in older diplegic children and is characterized by excessive ankle dorsiflexion, excessive knee flexion, increased hip flexion, and variable pelvic position.168 The etiology of crouch gait and jump gait is often multifactorial but can include hamstring, psoas, and gastrocnemius tightness resulting from spasticity or contracture, weakness, and impaired balance.199
In contrast to crouch gait, “stiff knee gait” describes decreased dynamic range of motion of the knee throughout the gait cycle with the knee in an erect or extended position.34 The etiology of stiff knee gait is thought to be spasticity of the rectus femoris muscle firing inappropriately during swing phase, which is demonstrable with surface electromyography in the gait laboratory.34,158 Surgical options for stiff knee gait include transfer of the rectus femoris to improve dynamic knee flexion. Additional sagittal plane deformities are attributable to equinus at the ankle resulting from spasticity of the gastrocnemius and soleus muscles (exerting a plantar flexion moment at the ankle). Secondary to the plantar flexion–knee extension couple,57 plantar flexion overactivity can contribute to compensatory knee hyperextension (“recurvatum”), which can become painful with time.
The orthopedic interventions for the gait patterns described above are tailored to address the primary forces that are thought to contribute, and are therefore unique for each child and pattern. Management options include muscle strengthening of the antigravity muscles, external support with orthoses, and orthopedic operations to correct fixed soft tissue and bony musculoskeletal deformities at single or multiple levels.168 Dynamic deformities without fixed contracture are generally managed by focal spasticity management. A host of soft tissue and bony procedures are described to address the musculoskeletal deformities of the foot and ankle in CP with the goal of obtaining a plantigrade, braceable foot and stable base of support for standing and gait.
Upper Limb
Spasticity management and orthopedic intervention for the upper limb in CP follows a similar treatment algorithm to that of the lower limb. Dynamic posturing and tone patterns are addressed with stretching, splinting, and focal and systemic spasticity management. Fixed deformities must be addressed through surgical correction of soft tissue and bony abnormalities. Although extension spasticity patterns predominate in the lower limbs, flexion posturing of the elbow, wrist, and fingers is frequently seen in hemiplegic and quadriplegic CP. Wrist-hand orthoses are frequently used to reduce thumb-in-palm and wrist flexion deformities for hygiene and to achieve a more functional position for grip. They also, however, can serve to lessen sensory input to an already compromised hand. The most common soft tissue surgery to address wrist flexion deformities is the flexor carpi ulnaris transfer to either the extensor carpi radialis brevis, in which the problematic wrist flexor is reinserted into the wrist extensor tendon. Associated surgeries often performed at the same time to address the flexion synergy pattern of the upper limb include biceps release or lengthening, finger flexor lengthenings, thumb abductor augmentation, thumb adductor release, and pronator teres release or rerouting. Upper limb surgery for function should be delayed until the child is mature enough to cooperate with rehabilitation.18 Motion analysis and computer modeling of the upper extremity is also being increasingly used to aid in surgical planning, much like three-dimensional gait analysis for the lower extremities.
Spine
The reported incidence of scoliosis in CP has varied from 6.5% to 76%, and the severity has been found to directly correlate with the degree of involvement and the level of mental retardation, and inversely correlate with functional status and ambulation.116 Progression of scoliosis in CP has not been found to be affected by nonoperative treatments such as bracing.133 However, spinal orthoses can help provide postural control in children with truncal hypotonia to facilitate an upright posture for daily activities.
The presence of scoliosis or a spine deformity at an early age (<5 years) might suggest a congenital syndrome or spinous congenital anomaly. A physical examination should be performed every 6 to 12 months and radiographs obtained if a curve is detected. Standardizing the position of the child when follow-up radiographs are obtained will help minimize errors in measurement of progression. The treatment of scoliosis in CP is spinal instrumentation and fusion. Ideally this can be delayed in children with flexible deformities until they approach skeletal maturity. In contrast to idiopathic scoliosis, there is a high risk for progression in CP with curves of 40 degrees at skeletal maturity.118,171
Complementary and Alternative Medicine
Complementary and alternative medicine (CAM) is defined by the National Center for Complementary and Alternative Medicine on its website (www.nccam.nih.gov) as “a group of diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine.” CAM is an area that families of children with CP often explore, and a prevalence rate of 56% has been reported.84 Although CP is a nonprogressive condition, it is noncurable, and families might seek alternative treatments to ensure that they are doing everything possible to help their child. A greater severity of CP and a higher level of education completed by caregivers have been associated with a higher use of CAM.84,173
Information on these treatments is often sought on the Internet or through family networking, but families also seek advice from their physician. Organizations such as the National Center for Complementary and Alternative Medicine, United Cerebral Palsy, and the American Academy for Cerebral Palsy and Developmental Medicine provide web-based evidence reviews on current treatments. The American Academy of Pediatrics has developed policy statements on CAM that encourage physicians to become knowledgeable about the various complementary treatments, and to remain nonjudgmental as the family is guided through the risks, theoretic benefits, and any available credible research.7
There is often a gray zone between conventional and alternative treatments. For example, hyperbaric oxygen therapy (HBOT) can be considered as part of the conventional treatment for wound care, but is considered alternative when used in CP. Examples of CAM used in CP include massage therapy, aqua therapy, hippotherapy, spinal manipulation, conductive education, craniosacral therapy, Euromed Adeli Suit program, HBOT, acupuncture, Feldenkrais, and homeotherapy.84 A systemic review of evidence on the benefits of HBOT for CP was performed in 2007.126 HBOT and pressurized room air resulted in improvements in motor function compared with baseline. Adverse events included seizures and the need for ear pressure equalization tube placement.
Transition to Adulthood and Aging With Cerebral Palsy
The transition period is often difficult for young adults with CP, and follow-up with health and rehabilitation services is often dramatically reduced once children with CP reach adulthood.24 Adult health care providers may lack expertise and interest in childhood-onset disabling conditions, and services can be fragmented.19 Preserving wellness, function, independence, activity, participation in society, and quality of life needs to be the ultimate focus, not necessarily episodic or acute care management.
Adults with CP exhibit musculoskeletal or performance changes typical of advanced aging earlier than their nondisabled peers.141 Disabilities that have a long duration can produce excessive wear and tear on the musculoskeletal system. In a survey of adults with CP, 85% reported contractures, with 33% of these occurring in two to three joints.9 Chronic pain has been reported in 30% to 67% of adults, with the back and lower limbs the most commonly affected sites.87,182 Adults with dyskinesia tend to report more cervical pain.141 Improper biomechanical forces caused by spasticity, contractures, and physical stress can lead to overuse injuries.141 Many adults with CP and chronic pain experience limitations in their activities, social withdrawal, decreased self-esteem, depression, relationship strain, and emotional distress that lead to a cycle of despair and hopelessness.32,90 Most adults with CP do not seek help from health care providers for pain management.60
Subluxed or dislocated hips and severe scoliosis not managed in childhood can result in pelvic obliquity, pressure sores, and functional decline in adulthood. 118 The average progression rate of scoliosis in adults with CP ranges from 3 to 4.4 degrees per year, and this contributes to functional decline.118 (See the discussion of orthopedic management for further surgical information.)
Cervical myelopathy is a potentially devastating condition in adults with athetoid CP. Cervical disk degeneration tends to occur at a younger age and can be attributable to abnormal excessive neck motion.71 Short-term surgical outcome is favorable, but neurologic deterioration or development of kyphosis can occur greater than 5 years and occasionally greater than 10 years postoperatively.14
An increased incidence of gastrointestinal complaints has been reported in adults with CP.141,210 Adults with recurrent urinary tract infections or incontinence tend to have abnormal urodynamics, and these problems increase with a higher GMFCS.28
Aging can affect motor function and life skills. By 25 years of age, improvement in ambulation is unlikely and decline more likely, especially for those with a higher GMFCS.42 For those adults who are ambulatory, a significant decline occurs in late adulthood, and few of the 60-year-old persons who ambulate well maintain this skill over the following 15 years.200 Adults with CP and normal intelligence are less likely to live independently, have intimate relationships, and be employed than their able-bodied peers.47 Female gender, lower IQ, and transportation dependence are associated with unemployment.117 Despite these challenges, subjective well-being and general satisfaction with life are not reduced in adults with CP.76
Life expectancy in CP is influenced by the presence of severe mental retardation and reduced mobility,52,201,202 and the key predictors are lack of basic functional skills including mobility and feeding. Adults lacking these skills have much reduced life expectancies, as much as 11 years less than age-matched peers. for the worst functioning groups. By contrast, survival of high-functioning adults is close to that of the general population. Life expectancies of adults of a given age can differ by 40 years or more, according to their functional level.201
1. Abel M.F., Juhl G.A., Vaughan C.L., et al. Gait assessment of fixed ankle-foot orthoses in children with spastic diplegia. Arch Phys Med Rehabil. 1998;79:126-133.
2. Ackman J.D., Russman B.S., Thomas S.S., et al. Comparing botulinum toxin A with casting for treatment of dynamic equinus in children with cerebral palsy. Dev Med Child Neurol. 2005;47:620-627.
3. Agnarsson U., Warde C., McCarthy G., et al. Anorectal function of children with neurological problems. II. Cerebral palsy. Dev Med Child Neurol. 1993;35:903-908.
4. Albright A.L. Intrathecal baclofen for childhood hypertonia. Childs Nerv Syst. 2007;23:971-979.
5. Albright A.L., Ferson S.S. Intraventricular baclofen for dystonia: techniques and outcomes—clinical article. J Neurosurg Pediatr. 2009;3:11-14.
6. Albright A.L., Gilmartin R., Swift D., et al. Long-term intrathecal baclofen therapy for severe spasticity of cerebral origin. J Neurosurg. 2003;98:291-295.
7. American Academy of Pediatrics Committee on Children With Disabilities. Counseling families who choose complementary and alternative medicine for their child with chronic illness or disability. Pediatrics. 2001;107:598-601.
8. Ancel P.Y., Livinec F., Larroque B., et al. Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics. 2006;117:828-835.
9. Andersson C., Mattsson E. Adults with cerebral palsy: a survey describing problems, needs, and resources, with special emphasis on locomotion. Dev Med Child Neurol. 2001;43:76-82.
10. Anttila H., Autti-Ramo I., Suoranta J., et al. Effectiveness of physical therapy interventions for children with cerebral palsy: a systematic review. BMC Pediatr. 2008;8:14.
11. Anttila H., Suoranta J., Malmivaara A., et al. Effectiveness of physiotherapy and conductive education interventions in children with cerebral palsy: a focused review. Am J Phys Med Rehabil. 2008;87:478-501.
12. Ashwal S., Russman B.S., Blasco P.A., et al. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2004;62:851-863.
13. Ayyangar R. Health maintenance and management in childhood disability. Phys Med Rehabil Clin N Am. 2002;13:793-821.
14. Azuma S., Seichi A., Ohnishi I., et al. Long-term results of operative treatment for cervical spondylotic myelopathy in patients with athetoid cerebral palsy: an over 10-year follow-up study. Spine. 2002;27:943-948. discussion 948
15. Bax M. Diagnostic assessment of children with cerebral palsy. Lancet Neurol. 2004;3:395.
16. Bax M., Tydeman C., Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA. 2006;296:1602-1608.
17. Beckung E., Hagberg G., Uldall P., et al. Probability of walking in children with cerebral palsy in Europe. Pediatrics. 2008;121:e187-e192.
18. Berker A.N., Yalcin M.S. Cerebral palsy: orthopedic aspects and rehabilitation. Pediatr Clin North Am. 2008;55:1209-1225.
19. Binks J.A., Barden W.S., Burke T.A., et al. What do we really know about the transition to adult-centered health care? A focus on cerebral palsy and spina bifida. Arch Phys Med Rehabil. 2007;88:1064-1073.
20. Bjornson K., Hays R., Graubert C., et al. Botulinum toxin for spasticity in children with cerebral palsy: a comprehensive evaluation. Pediatrics. 2007;120:49-58.
21. Bjornson K.F., McLaughlin J.F., Loeser J.D., et al. Oral motor, communication, and nutritional status of children during intrathecal baclofen therapy: a descriptive pilot study. Arch Phys Med Rehabil. 2003;84:500-506.
22. Blair E., Watson L. Epidemiology of cerebral palsy. Semin Fetal Neonatal Med. 2006;11:117-125.
23. Blasco P.A. Management of drooling: 10 years after the Consortium on Drooling. Dev Med Child Neurol. 1990;2002(44):778-781.
24. Bottos M., Feliciangeli A., Sciuto L., et al. Functional status of adults with cerebral palsy and implications for treatment of children. Dev Med Child Neurol. 2001;43:516-528.
25. Braddom R.L., editor. Physical medicine & rehabilitation, ed 3, Philadelphia: Saunders, 2007.
26. Brant C.Q., Stanich P., Ferrari A.P.Jr. Improvement of children’s nutritional status after enteral feeding by PEG: an interim report. Gastrointest Endosc. 1999;50:183-188.
27. Brehm M.A., Harlaar J., Schwartz M. Effect of ankle-foot orthoses on walking efficiency and gait in children with cerebral palsy. J Rehabil Med. 2008;40:529-534.
28. Bross S., Honeck P., Kwon S.T., et al. Correlation between motor function and lower urinary tract dysfunction in patients with infantile cerebral palsy. Neurourol Urodyn. 2007;26:222-227.
29. Buly R.L., Huo M., Root L., et al. Total hip arthroplasty in cerebral palsy. long-term follow-up results, clin Orthop Relat Res. 1993:148-153.
30. Butler C. Effects of powered mobility on self-initiated behaviors of very young children with locomotor disability. Dev Med Child Neurol. 1986;28:325-332.
31. Cartwright J.D. Provision of educationally related services for children and adolescents with chronic diseases and disabling conditions. Pediatrics. 2007;119:1218-1223.
32. Castle K., Imms C., Howie L. Being in pain: a phenomenological study of young people with cerebral palsy. Dev Med Child Neurol. 2007;49:445-449.
33. Caulton J.M., Ward K.A., Alsop C.W., et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135.
34. Chambers H.G. Treatment of functional limitations at the knee in ambulatory children with cerebral palsy. Eur J Neurol. 2001;8(suppl 5):59-74.
35. Charles J.R., Wolf S.L., Schneider J.A., et al. Efficacy of a child-friendly form of constraint-induced movement therapy in hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2006;48:635-642.
36. Clarke W.M., Hoops H.R. Predictive measures of speech proficiency in cerebral palsied speakers. J Commun Disord. 1980;13:385-394.
37. Cole G.F., Farmer S.E., Roberts A., et al. Selective dorsal rhizotomy for children with cerebral palsy: the Oswestry experience. Arch Dis Child. 2007;92:781-785.
38. Coniglio S.J., Stevenson R.D., Rogol A.D. Apparent growth hormone deficiency in children with cerebral palsy. Dev Med Child Neurol. 1996;38:797-804.
39. Corwin D.S., Isaacs J.S., Georgeson K.E., et al. Weight and length increases in children after gastrostomy placement. J Am Diet Assoc. 1996;96:874-879.
40. Dali C., Hansen F.J., Pedersen S.A., et al. Threshold electrical stimulation (TES) in ambulant children with CP: a randomized double-blind placebo-controlled clinical trial. Dev Med Child Neurol. 2002;44:364-369.
41. Dan B. Progressive course in cerebral palsy? Dev Med Child Neurol. 2007;49:644.
42. Day S.M., Wu Y.W., Strauss D.J., et al. Change in ambulatory ability of adolescents and young adults with cerebral palsy. Dev Med Child Neurol. 2007;49:647-653.
43. De Vries A.M., de Groot L. Transient dystonias revisited: a comparative study of preterm and term children at 2 1⁄2 years of age. Dev Med Child Neurol. 2002;44:415-421.
44. De Vries L.S., Van Haastert I.L., Rademaker K.J., et al. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr. 2004;144:815-820.
45. Desloovere K., Molenaers G., Jonkers I., et al. A randomized study of combined botulinum toxin type A and casting in the ambulant child with cerebral palsy using objective outcome measures. Eur J Neurol. 2001;8(suppl 5):75-87.
46. Dobson F., Boyd R.N., Parrott J., et al. Hip surveillance in children with cerebral palsy. Impact on the surgical management of spastic hip disease. J Bone Joint Surg Br. 2002;84:720-726.
47. Donkervoort M., Wiegerink D.J., van Meeteren J., et al. Transition to adulthood: validation of the Rotterdam Transition Profile for young adults with cerebral palsy and normal intelligence. Dev Med Child Neurol. 2009;51:53-62.
48. Duby J.C. Role of the medical home in family-centered early intervention services. Pediatrics. 2007;120:1153-1158.
49. Eliasson A.C., Krumlinde-Sundholm L., Rosblad B., et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549-554.
50. Ellenberg J.H., Nelson K.B. Early recognition of infants at high risk for cerebral palsy: examination at age four months. Dev Med Child Neurol. 1981;23:705-716.
51. Evans K., Rigby A.S., Hamilton P., et al. The relationships between neonatal encephalopathy and cerebral palsy: a cohort study. J Obstet Gynaecol. 2001;21:114-120.
52. Eyman R.K., Grossman H.J., Call T.L., et al. Life expectancy in children with cerebral palsy. BMJ. 1995;310:665.
53. Fennell E.B., Dikel T.N. Cognitive and neuropsychological functioning in children with cerebral palsy. J Child Neurol. 2001;16:58-63.
54. Fitzgerald D.A., Follett J., Van Asperen P.P. Assessing and managing lung disease and sleep disordered breathing in children with cerebral palsy. Paediatr Respir Rev. 2009;10:18-24.
55. Flanagan N.M., Jackson A.J., Hill A.E. Visual impairment in childhood: insights from a community-based survey. Child Care Health Dev. 2003;29:493-499.
56. Ford G.W., Kitchen W.H., Doyle L.W., et al. Changing diagnosis of cerebral palsy in very low birthweight children. Am J Perinatol. 1990;7:178-181.
57. Gage J.R. The treatment of gait problems in cerebral palsy. London: Mac Keith Press; 2004.
58. Gainsborough M., Surman G., Maestri G., et al. Validity and reliability of the guidelines of the surveillance of cerebral palsy in Europe for the classification of cerebral palsy. Dev Med Child Neurol. 2008;50:828-831.
59. Gauthier L.V., Taub E., Perkins C., et al. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520-1525.
60. Gollust S.E., Thompson R.E., Gooding H.C., et al. Living with achondroplasia: attitudes toward population screening and correlation with quality of life. Prenat Diagn. 2003;23:1003-1008.
61. Gooch J.L., Patton C.P. Combining botulinum toxin and phenol to manage spasticity in children. Arch Phys Med Rehabil. 2004;85:1121-1124.
62. Gordon G.S., Simkiss D.E. A systematic review of the evidence for hip surveillance in children with cerebral palsy. J Bone Joint Surg Br. 2006;88:1492-1496.
63. Gordon L.M., Keller J.L., Stashinko E.E., et al. Can spasticity and dystonia be independently measured in cerebral palsy? Pediatr Neurol. 2006;35:375-381.
64. Gormley M.E.Jr., Krach L.E. Pediatric functional assessment. Choosing the right tools is the key to comparing outcomes. Rehab Manag. 1997;10:32-35. 103
65. Govaert P., Lequin M., Swarte R., et al. Changes in globus pallidus with (pre)term kernicterus. Pediatrics. 2003;112:1256-1263.
66. Graham H.K., Aoki K.R., Autti-Ramo I., et al. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture. 2000;11:67-79.
67. Greiner B.M., Czerniecki J.M., Deitz J.C. Gait parameters of children with spastic diplegia: a comparison of effects of posterior and anterior walkers. Arch Phys Med Rehabil. 1993;74:381-385.
68. Gunther G., Junker R., Strater R., et al. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
69. Guzzetta F., Shackelford G.D., Volpe S., et al. Periventricular intraparenchymal echodensities in the premature newborn: critical determinant of neurologic outcome. Pediatrics. 1986;78:995-1006.
70. Hankins G.D., Speer M. Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet Gynecol. 2003;102:628-636.
71. Harada T., Ebara S., Anwar M.M., et al. The cervical spine in athetoid cerebral palsy: a radiological study of 180 patients. J Bone Joint Surg Br. 1996;78:613-619.
72. Harris S.R. Evaluating the effects of early intervention: a mismatch between process and product? Am J Dis Child. 1993;147:12-13.
73. Harris S.R., Atwater S.W., Crowe T.K. Accepted and controversial neuromotor therapies for infants at high risk for cerebral palsy. J Perinatol. 1988;8:3-13.
74. Hays R.M., McLaughlin J.F., Bjornson K.F., et al. Electrophysiological monitoring during selective dorsal rhizotomy, and spasticity and GMFM performance. Dev Med Child Neurol. 1998;40:233-238.
75. Henderson R.C., Lark R.K., Gurka M.J., et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5.
76. Hergenroder H., Blank R. Subjective well-being and satisfaction with life in adults with spastic cerebral palsy: a pilot study of a randomized sample. Dev Med Child Neurol. 2009;51:389-396.
77. Herndon W.A., Troup P., Yngve D.A., et al. Effects of neurodevelopmental treatment on movement patterns of children with cerebral palsy. J Pediatr Orthop. 1987;7:395-400.
78. Hoare B.J., Wasiak J., Imms C., et al. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database Syst Rev. 2007;(2):CD004149.
79. Hollins S., Attard M.T., von Fraunhofer N., et al. Mortality in people with learning disability: risks, causes, and death certification findings in London. Dev Med Child Neurol. 1998;40:50-56.
80. Hoon A.H.Jr., Freese P.O., Reinhardt E.M., et al. Age-dependent effects of trihexyphenidyl in extrapyramidal cerebral palsy. Pediatr Neurol. 2001;25:55-58.
81. Hoving M.A., van Raak E.P., Spincemaille G.H., et al. Efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy: a randomised controlled trial. Eur J Paediatr Neurol. 2009;13:240-246.
82. Hoyt C.S. Visual function in the brain-damaged child. Eye. 2003;17:369-384.
83. Humphreys P., Deonandan R., Whiting S., et al. Factors associated with epilepsy in children with periventricular leukomalacia. J Child Neurol. 2007;22:598-605.
84. Hurvitz E.A., Leonard C., Ayyangar R., Nelson V.S. Complementary and alternative medicine use in families of children with cerebral palsy. Dev Med Child Neurol. 2003;45:364-370.
85. Hurwitz K.A. A review of special education law. Pediatr Neurol. 2008;39:147-154.
86. Inder T.E., Anderson N.J., Spencer C., et al. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24:805-809.
87. Jahnsen R., Villien L., Aamodt G., et al. Musculoskeletal pain in adults with cerebral palsy compared with the general population. J Rehabil Med. 2004;36:78-84.
88. Jansen L.M., Ketelaar M., Vermeer A. Parental experience of participation in physical therapy for children with physical disabilities. Dev Med Child Neurol. 2003;45:58-69.
89. Jekovec-Vrhovsek M., Kocijancic A., Prezelj J. Effect of vitamin D and calcium on bone mineral density in children with CP and epilepsy in full-time care. Dev Med Child Neurol. 2000;42:403-405.
90. Jensen M., Engle J., Schwarts L. Coping with cerebral palsy pain: a preliminary longitudinal study. Pain Med. 2006;7:30-37.
91. Johnson A. Cerebral palsies: epidemiology and causal pathways. Arch Dis Child. 2000;83:279A.
92. Johnston T.E. Energy cost of walking in children with cerebral palsy: relation to the gross motor function classification system. Dev Med Child Neurol. 2004;46:575.
93. Karaman M.I., Kaya C., Caskurlu T., et al. Urodynamic findings in children with cerebral palsy. Int J Urol. 2005;12:717-720.
94. Kerr C., McDowell B., McDonough S. Electrical stimulation in cerebral palsy: a review of effects on strength and motor function. Dev Med Child Neurol. 2004;46:205-213.
95. Khetpal V., Donahue S.P. Cortical visual impairment: etiology, associated findings, and prognosis in a tertiary care setting. J AAPOS. 2007;11:235-239.
96. Kinnett D. Botulinum toxin A injections in children: technique and dosing issues. Am J Phys Med Rehabil. 2004;83:S59-S64.
97. Kolaski K., Ajizian S.J., Passmore L., et al. Safety profile of multilevel chemical denervation procedures using phenol or botulinum toxin or both in a pediatric population. Am J Phys Med Rehabil. 2008;87:556-566.
98. Koman L.A., Brashear A., Rosenfeld S., et al. Botulinum toxin type A neuromuscular blockade in the treatment of equinus foot deformity in cerebral palsy: a multicenter, open-label clinical trial. Pediatrics. 2001;108:1062-1071.
99. Kotagal S., Gibbons V.P., Stith J.A. Sleep abnormalities in patients with severe cerebral palsy. Dev Med Child Neurol. 1994;36:304-311.
100. Krach L.E., Kriel R.L., Gilmartin R.C., et al. GMFM 1 year after continuous intrathecal baclofen infusion. Pediatr Rehabil. 2005;8:207-213.
101. Krach L.E., Nettleton A., Klempka B. Satisfaction of individuals treated long-term with continuous infusion of intrathecal baclofen by implanted programmable pump. Pediatr Rehabil. 2006;9:210-218.
102. Krageloh-Mann I., Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: a systematic review. Dev Med Child Neurol. 2007;49:144-151.
103. Kulak W., Sobaniec W., Goscik M., et al. Clinical and neuroimaging profile of congenital brain malformations in children with spastic cerebral palsy. Adv Med Sci. 2008;53:42-48.
104. Kulak W., Sobaniec W., Kubas B., et al. Spastic cerebral palsy: clinical magnetic resonance imaging correlation of 129 children. J Child Neurol. 2007;22:8-14.
105. Kuperminc M.N., Stevenson R.D. Growth and nutrition disorders in children with cerebral palsy. Dev Disabil Res Rev. 2008;14:137-146.
106. Kwon J.Y., Kim J.S. Selective blocking of the anterior branch of the obturator nerve in children with cerebral palsy. Am J Phys Med Rehabil. 2009;88:7-13.
107. Langerak N.G., Lamberts R.P., Fieggen A.G., et al. A prospective gait analysis study in patients with diplegic cerebral palsy 20 years after selective dorsal rhizotomy. J Neurosurg Pediatr. 2008;1:180-186.
108. Lebiedowska M.K., Gaebler-Spira D., Burns R.S., et al. Biomechanic characteristics of patients with spastic and dystonic hypertonia in cerebral palsy. Arch Phys Med Rehabil. 2004;85:875-880.
109. Liepert J., Bauder H., Wolfgang H.R., et al. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210-1216.
110. Little W. Lectures on the deformity of the human frame. Lancet. 1843;1:318-320.
111. Little W.J. On the influence of abnormal parturition, difficult labours, premature birth, and asphyxia neonatorum, on the mental and physical condition of the child, especially in relation to deformities. Clin Orthop Relat Res. 1966;46:7-22.
112. Loewen P., Steinbok P., Holsti L., et al. Upper extremity performance and self-care skill changes in children with spastic cerebral palsy following selective posterior rhizotomy. Pediatr Neurosurg. 1998;29:191-198.
113. Love R.J., Hagerman E.L., Taimi E.G. Speech performance, dysphagia and oral reflexes in cerebral palsy. J Speech Hear Disord. 1980;45:59-75.
114. Lowe K., Novak I., Cusick A. Repeat injection of botulinum toxin A is safe and effective for upper limb movement and function in children with cerebral palsy. Dev Med Child Neurol. 2007;49:823-829.
115. Mackey A.H., Miller F., Walt S.E., et al. Use of three-dimensional kinematic analysis following upper limb botulinum toxin A for children with hemiplegia. Eur J Neurol. 2008;15:1191-1198.
116. Madigan R.R., Wallace S.L. Scoliosis in the institutionalized cerebral palsy population. Spine. 1981;6:583-590.
117. Magill-Evans J., Galambos N., Darrah J., et al. Predictors of employment for young adults with developmental motor disabilities. Work. 2008;31:433-442.
118. Majd M.E., Muldowny D.S., Holt R.T. Natural history of scoliosis in the institutionalized adult cerebral palsy population. Spine. 1997;22:1461-1466.
119. Mathew A., Mathew M.C., Thomas M., et al. The efficacy of diazepam in enhancing motor function in children with spastic cerebral palsy. J Trop Pediatr. 2005;51:109-113.
120. Mattern-Baxter K. Effects of partial body weight supported treadmill training on children with cerebral palsy. Pediatr Phys Ther. 2009;21:12-22.
121. Matthews D.J. Controversial therapies in the management of cerebral palsy. Pediatr Ann. 1988;17:762-764.
122. Matthews D.J., Balaban B. [Management of spasticity in children with cerebral palsy]. Acta Orthop Traumatol Turc. 2009;43:81-86.
123. McCormick A., Brien M., Plourde J., et al. Stability of the Gross Motor Function Classification System in adults with cerebral palsy. Dev Med Child Neurol. 2007;49:265-269.
124. McCormick M.C., McCarton C., Tonascia J., et al. Early educational intervention for very low birth weight infants: results from the Infant Health and Development Program. J Pediatr. 1993;123:527-533.
125. McCoy A.A., Fox M.A., Schaubel D.E., et al. Weight gain in children with hypertonia of cerebral origin receiving intrathecal baclofen therapy. Arch Phys Med Rehabil. 2006;87:1503-1508.
126. McDonagh M.S., Morgan D., Carson S., et al. Systematic review of hyperbaric oxygen therapy for cerebral palsy: the state of the evidence. Dev Med Child Neurol. 2007;49:942-947.
127. McLaughlin J., Bjornson K., Temkin N., et al. Selective dorsal rhizotomy: meta-analysis of three randomized controlled trials. Dev Med Child Neurol. 2002;44:17-25.
128. McNamara L., Casey J. Seat inclinations affect the function of children with cerebral palsy: a review of the effect of different seat inclines. Disabil Rehabil Assist Technol. 2007;2:309-318.
129. McNeal D.M., Hawtrey C.E., Wolraich M.L., et al. Symptomatic neurogenic bladder in a cerebral-palsied population. Dev Med Child Neurol. 1983;25:612-616.
130. Meningaud J.P., Pitak-Arnnop P., Chikhani L., et al. Drooling of saliva: a review of the etiology and management options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:48-57.
131. Michaud L.J. Prescribing therapy services for children with motor disabilities. Pediatrics. 2004;113:1836-1838.
132. Milla P.J., Jackson A.D. A controlled trial of baclofen in children with cerebral palsy. J Int Med Res. 1977;5:398-404.
133. Miller A., Temple T., Miller F. Impact of orthoses on the rate of scoliosis progression in children with cerebral palsy. J Pediatr Orthop. 1996;16:332-335.
134. Minear W.L. A classification of cerebral palsy. Pediatrics. 1956;18:841-852.
135. Molnar G.E. Cerebral palsy: prognosis and how to judge it. Pediatr Ann. 1979;8:596-605.
136. Molnar G.E. Rehabilitation in cerebral palsy. West J Med. 1991;154:569-572.
137. Molnar G.E., Alexander M.A. Pediatric rehabilitation, ed 3. Philadelphia: Hanley & Belfus; 1999.
138. Molnar G.E., Gordon S.U. Cerebral palsy: predictive value of selected clinical signs for early prognostication of motor function. Arch Phys Med Rehabil. 1976;57:153-158.
139. Molteni B., Oleari G., Fedrizzi E., et al. Relation between CT patterns, clinical findings and etiological factors in children born at term, affected by congenital hemiparesis. Neuropediatrics. 1987;18:75-80.
140. Morris C., Newdick H., Johnson A. Variations in the orthotic management of cerebral palsy. Child Care Health Dev. 2002;28:139-147.
141. Murphy K.P., Molnar G.E., Lankasky K. Medical and functional status of adults with cerebral palsy. Dev Med Child Neurol. 1995;37:1075-1084.
142. Mutlu A., Krosschell K., Spira D.G. Treadmill training with partial body-weight support in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2009;51:268-275.
143. Nelson K.B. Causative factors in cerebral palsy. Clin Obstet Gynecol. 2008;51:749-762.
144. Nelson K.B., Ellenberg J.H. Children who “outgrew” cerebral palsy. Pediatrics. 1982;69:529-536.
145. Nelson K.B., Ellenberg J.H. Obstetric complications as risk factors for cerebral palsy or seizure disorders. JAMA. 1984;251:1843-1848.
146. Nelson K.B., Grether J.K. Causes of cerebral palsy. Curr Opin Pediatr. 1999;11:487-491.
147. Nordmark E., Josenby A.L., Lagergren J., et al. Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatr. 2008;8:54.
148. O’Brien D.F., Park T.S., Puglisi J.A., et al. Orthopedic surgery after selective dorsal rhizotomy for spastic diplegia in relation to ambulatory status and age. J Neurosurg. 2005;103:5-9.
149. Opheim A., Jahnsen R., Olsson E., et al. Walking function, pain, and fatigue in adults with cerebral palsy: a 7-year follow-up study. Dev Med Child Neurol. 2009;51:381-388.
150. Palisano R., Rosenbaum P., Walter S., et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214-223.
151. Palisano R.J., Cameron D., Rosenbaum P.L., et al. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48:424-428.
152. Palisano R.J., Rosenbaum P., Bartlett D., et al. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744-750.
153. Park E.S., Park C.I., Cho S.R., et al. Colonic transit time and constipation in children with spastic cerebral palsy. Arch Phys Med Rehabil. 2004;85:453-456.
154. Pennington L., Goldbart J., Marshall J. Speech and language therapy to improve the communication skills of children with cerebral palsy. Cochrane Database Syst Rev. 2004;(2):CD003466.
155. Perlman J.M., Rollins N., Burns D., et al. Relationship between periventricular intraparenchymal echodensities and germinal matrix-intraventricular hemorrhage in the very low birth weight neonate. Pediatrics. 1993;91:474-480.
156. Perlstein M.A. Infantile cerebral palsy; classification and clinical correlations. JAMA. 1952;149:30-34.
157. Petersen M.C., Kedia S., Davis P., et al. Eating and feeding are not the same: caregivers’ perceptions of gastrostomy feeding for children with cerebral palsy. Dev Med Child Neurol. 2006;48:713-717.
158. Piazza S.J., Delp S.L. The influence of muscles on knee flexion during the swing phase of gait. J Biomech. 1996;29:723-733.
159. Pidcock F.S., Fish D.E., Johnson-Greene D., et al. Hip migration percentage in children with cerebral palsy treated with botulinum toxin type A. Arch Phys Med Rehabil. 2005;86:431-435.
160. Plasschaert V.F., Ketelaar M., Nijnuis M.G., et al. Classification of manual abilities in children with cerebral palsy under 5 years of age: how reliable is the Manual Ability Classification System? Clin Rehabil. 2009;23:164-170.
161. Plioplys A.V., Kasnicka I., Lewis S., et al. Survival rates among children with severe neurologic disabilities. South Med J. 1998;91:161-172.
162. Plotkin H., Coughlin S., Kreikemeier R., et al. Low doses of pamidronate to treat osteopenia in children with severe cerebral palsy: a pilot study. Dev Med Child Neurol. 2006;48:709-712.
163. Presedo A., Oh C.W., Dabney K.W., et al. Soft-tissue releases to treat spastic hip subluxation in children with cerebral palsy. J Bone Joint Surg Am. 2005;87:832-841.
164. Ramage I.J., Simpson R.M., Thomson R.B., et al. Feeding difficulties in children with cerebral palsy. Acta Paediatr. 1997;86:336.
165. Reilly S., Skuse D., Poblete X. Prevalence of feeding problems and oral motor dysfunction in children with cerebral palsy: a community survey. J Pediatr. 1996;129:877-882.
166. Rice J., Waugh M.C. Pilot study on trihexyphenidyl in the treatment of dystonia in children with cerebral palsy. J Child Neurol. 2009;24:176-182.
167. Robinson M.N., Peake L.J., Ditchfield M.R., et al. Magnetic resonance imaging findings in a population-based cohort of children with cerebral palsy. Dev Med Child Neurol. 2009;51:39-45.
168. Rodda J.M., Graham H.K., Nattrass G.R., et al. Correction of severe crouch gait in patients with spastic diplegia with use of multilevel orthopaedic surgery. J Bone Joint Surg Am. 2006;88:2653-2664.
169. Rosenbaum P., Paneth N., Leviton A., et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8-14.
170. Rosenbaum P.L., Walter S.D., Hanna S.E., et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA. 2002;288:1357-1363.
171. Saito N., Ebara S., Ohotsuka K., et al. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351:1687-1692.
172. Samaniego E.A., Sheth R.D. Bone consequences of epilepsy and antiepileptic medications. Semin Pediatr Neurol. 2007;14:196-200.
173. Samdup D.Z., Smith R.G., Il Song S. The use of complementary and alternative medicine in children with chronic medical conditions. Am J Phys Med Rehabil. 2006;85:842-846.
174. Samson-Fang L., Butler C., O’Donnell M. Effects of gastrostomy feeding in children with cerebral palsy: an AACPDM evidence report. Dev Med Child Neurol. 2003;45:415-426.
175. Samson-Fang L., Fung E., Stallings V.A., et al. Relationship of nutritional status to health and societal participation in children with cerebral palsy. J Pediatr. 2002;141:637-643.
176. Samson-Fang L., Stevenson R.D. Linear growth velocity in children with cerebral palsy. Dev Med Child Neurol. 1998;40:689-692.
177. Sanger T.D., Bastian A., Brunstrom J., et al. Prospective open-label clinical trial of trihexyphenidyl in children with secondary dystonia due to cerebral palsy. J Child Neurol. 2007;22:530-537.
178. Sanger T.D., Delgado M.R., Gaebler-Spira D., et al. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:e89-e97.
179. Schaechter J.D., Kraft E., Hilliard T.S., et al. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326-338.
180. Scheinberg A., Hall K., Lam L.T., et al. Oral baclofen in children with cerebral palsy: a double-blind cross-over pilot study. J Paediatr Child Health. 2006;42:715-720.
181. Scholtes V.A., Dallmeijer A.J., Knol D.L., et al. The combined effect of lower-limb multilevel botulinum toxin type A and comprehensive rehabilitation on mobility in children with cerebral palsy: a randomized clinical trial. Arch Phys Med Rehabil. 2006;87:1551-1558.
182. Schwartz L., Engel J.M., Jensen M.P. Pain in persons with cerebral palsy. Arch Phys Med Rehabil. 1999;80:1243-1246.
183. Scrutton D., Baird G., Smeeton N. Hip dysplasia in bilateral cerebral palsy: incidence and natural history in children aged 18 months to 5 years. Dev Med Child Neurol. 2001;43:586-600.
184. Senaran H., Shah S.A., Presedo A., et al. The risk of progression of scoliosis in cerebral palsy patients after intrathecal baclofen therapy. Spine. 2007;32:2348-2354.
185. Senbil N., Yuksel D., Yilmaz D., et al. Prothrombotic risk factors in children with hemiplegic cerebral palsy. Pediatr Int. 2007;49:600-602.
186. Shilt J.S., Lai L.P., Cabrera M.N., et al. The impact of intrathecal baclofen on the natural history of scoliosis in cerebral palsy. J Pediatr Orthop. 2008;28:684-687.
187. Snider L., Korner-Bitensky N., Kammann C., et al. Horseback riding as therapy for children with cerebral palsy: is there evidence of its effectiveness? Phys Occup Ther Pediatr. 2007;27:5-23.
188. Sommerfelt K., Markestad T., Berg K., et al. Therapeutic electrical stimulation in cerebral palsy: a randomized, controlled, crossover trial. Dev Med Child Neurol. 2001;43:609-613.
189. Soo B., Howard J.J., Boyd R.N., et al. Hip displacement in cerebral palsy. J Bone Joint Surg Am. 2006;88:121-129.
190. Spiegel D.A., Flynn J.M. Evaluation and treatment of hip dysplasia in cerebral palsy. Orthop Clin North Am. 2006;37:185-196.
191. Spittle A.J., Orton J., Doyle L.W., et al. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst Rev. 2007;(2):CD005495.
192. Stallings V.A., Charney E.B., Davies J.C., et al. Nutritional status and growth of children with diplegic or hemiplegic cerebral palsy. Dev Med Child Neurol. 1993;35:997-1006.
193. Stathopolou E., Thomas A.G. Nutrition in disabled children. Acta Paediatr. 1997;86:670-671.
194. Steinbok P. Selective dorsal rhizotomy for spastic cerebral palsy: a review. Childs Nerv Syst. 2007;23:981-990.
195. Steinkuller P.G., Du L., Gilbert C., et al. Childhood blindness. J AAPOS. 1999;3:26-32.
196. Sterba J.A. Does horseback riding therapy or therapist-directed hippotherapy rehabilitate children with cerebral palsy? Dev Med Child Neurol. 2007;49:68-73.
197. Steultjens E.M., Dekker J., Bouter L.M., et al. Occupational therapy for children with cerebral palsy: a systematic review. Clin Rehabil. 2004;18:1-14.
198. Stevenson R.D., Conaway M., Chumlea W.C., et al. Growth and health in children with moderate-to-severe cerebral palsy. Pediatrics. 2006;118:1010-1018.
199. Stout J.L., Gage J.R., Schwartz M.H., et al. Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy. J Bone Joint Surg Am. 2008;90:2470-2484.
200. Strauss D., Ojdana K., Shavelle R., et al. Decline in function and life expectancy of older persons with cerebral palsy. NeuroRehabilitation. 2004;19:69-78.
201. Strauss D., Shavelle R. Life expectancy of adults with cerebral palsy. Dev Med Child Neurol. 1998;40:369-375.
202. Strauss D., Shavelle R., Reynolds R., et al. Survival in cerebral palsy in the last 20 years: signs of improvement? Dev Med Child Neurol. 2007;49:86-92.
203. Sullivan P.B., Juszczak E., Bachlet A.M., et al. Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy. Dev Med Child Neurol. 2004;46:796-800.
204. Surveillance of Cerebral Palsy in Europe (SCPE). Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816-824.
205. Sutcliffe T.L., Gaetz W.C., Logan W.J., et al. Cortical reorganization after modified constraint-induced movement therapy in pediatric hemiplegic cerebral palsy. J Child Neurol. 2007;22:1281-1287.
206. Taub E., Ramey S.L., DeLuca S., et al. Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics. 2004;113:305-312.
207. The definition and classification of cerebral palsy. Dev Med Child Neurol. 2007;49(suppl 109):1-44.
208. Tilton A. Management of spasticity in children with cerebral palsy. Semin Pediatr Neurol. 2009;16:82-89.
209. Touwen B.C., Hadders-Algra M. Hyperextension of neck and trunk and shoulder retraction in infancy: a prognostic study. Neuropediatrics. 1983;14:202-205.
210. Turk M.A., Geremski C.A., Rosenbaum P.F., et al. The health status of women with cerebral palsy. Arch Phys Med Rehabil. 1997;78:S10-S17.
211. US Preventive Services Task Force. Universal screening for hearing loss in newborns: US Preventive Services Task Force recommendation statement. Pediatrics. 2008;122:143-148.
212. Van der Linden M.L., Hazlewood M.E., Aitchison A.M., et al. Electrical stimulation of gluteus maximus in children with cerebral palsy: effects on gait characteristics and muscle strength. Dev Med Child Neurol. 2003;45:385-390.
213. Wallace S.J. Epilepsy in cerebral palsy. Dev Med Child Neurol. 2001;43:713-717.
214. Wolf S.L., Winstein C.J., Miller J.P., et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095-2104.
215. Wood N.S., Costeloe K., Gibson A.T., et al. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F134-F140.
216. Woodward L.J., Anderson P.J., Austin N.C., et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685-694.
217. Worley G., Houlihan C.M., Herman-Giddens M.E., et al. Secondary sexual characteristics in children with cerebral palsy and moderate to severe motor impairment: a cross-sectional survey. Pediatrics. 2002;110:897-902.
218. Wu Y.W., Backstrand K.H., Zhao S., et al. Declining diagnosis of birth asphyxia in California: 1991-2000. Pediatrics. 2004;114:1584-1590.
219. Wu Y.W., Lindan C.E., Henning L.H., et al. Neuroimaging abnormalities in infants with congenital hemiparesis. Pediatr Neurol. 2006;35:191-196.
220. Yadav S.L., Singh U., Dureja G.P., et al. Phenol block in the management of spastic cerebral palsy. Indian J Pediatr. 1994;61:249-255.
221. Yang E.J., Rha D.W., Kim H.W., et al. Comparison of botulinum toxin type A injection and soft-tissue surgery to treat hip subluxation in children with cerebral palsy. Arch Phys Med Rehabil. 2008;89:2108-2113.
222. Yin R., Reddihough D., Ditchfield M., et al. Magnetic resonance imaging findings in cerebral palsy. J Paediatr Child Health. 2000;36:139-144.
223. Zuckerbraun N.S., Ferson S.S., Albright A.L., et al. Intrathecal baclofen withdrawal: emergent recognition and management. Pediatr Emerg Care. 2004;20:759-764.