22 Cerebral Cortex
Most Cerebral Cortex Is Neocortex
Most areas of cerebral cortex are neocortex, meaning that they have six more or less distinct layers (numbered I through VI from the surface down). About 80% of all cortical neurons are pyramidal cells, shaped as their name implies. They have a long apical dendrite ascending toward the cortical surface, a series of basal dendrites, and an axon emerging from the base of the cell body. Nearly all of the axons that leave the cerebral cortex are axons of pyramidal cells. The remaining 20% of cortical neurons is an assortment of nonpyramidal cells, most of them small and most of them inhibitory interneurons with axons that do not leave the cortex.
Layer I contains few cells and many synapses (just as the superficial layer of cerebellar cortex (see Chapter 20) is a place where mossy and climbing fibers synapse on the dendrites of Purkinje cells). Layer VI contains spindle-shaped modified pyramidal cells. The four middle layers of neocortex are alternating layers of mostly small cells and mostly large pyramidal cells (THB6 Figure 22-5, p. 545). Cortical areas that do not emit many long axons, such as primary sensory areas, are full of small pyramidal and nonpyramidal cells and are called granular areas. Cortical areas that emit many long axons, such as motor cortex, have many large pyramidal cells and are called agranular areas.
Different Neocortical Layers Have Distinctive Connections
Neocortical Areas Are Specialized for Different Functions
Although neocortex has the same basic structure everywhere in terms of percentages of pyramidal and nonpyramidal cells, layering, and columns, areas still differ from one another in terms of things like the sizes of cells and the thickness of layers. These differences turn out to be correlated with differences in function and connections, and so they have led to systematic maps of cortical areas. Several mapping systems have been devised, and some of the numbers in the map devised by Brodmann are in common use (THB6 Figure 22-10, p. 550). Important Brodmann’s numbers are indicated in parentheses in the figures in this chapter.
There Are Sensory, Motor, Association, and Limbic Areas
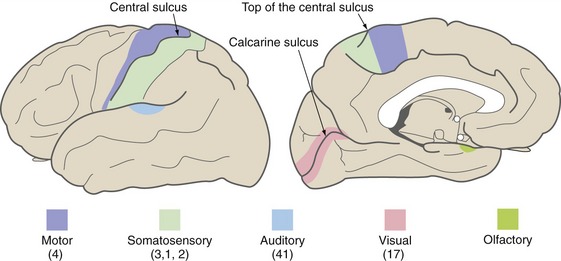
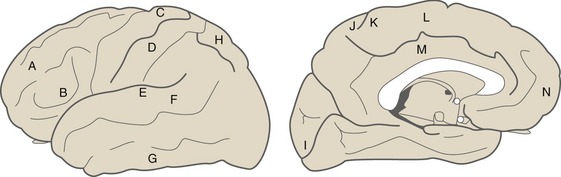
Primary areas (Fig. 22-1) of cortex are those most directly linked to the rest of the world, either through inputs from thalamic sensory relay nuclei or through outputs to the brainstem and spinal cord. Primary motor cortex occupies part of the precentral gyrus, primary somatosensory cortex the postcentral gyrus, primary auditory cortex the transverse temporal gyri, and primary visual cortex the banks of the calcarine sulcus. These primary areas contain precise but distorted somatotopic, tonotopic, or retinotopic maps with large representations of functionally important areas like the fingers, speech frequencies, and the fovea. There is also a primary gustatory area in the anterior insula and a primary vestibular area in the posterior insula, but relatively less is known about them. Primary olfactory cortex (see Chapter 13), in and near the anterior temporal lobe, is different in not being neocortical and not receiving its input from the thalamus.
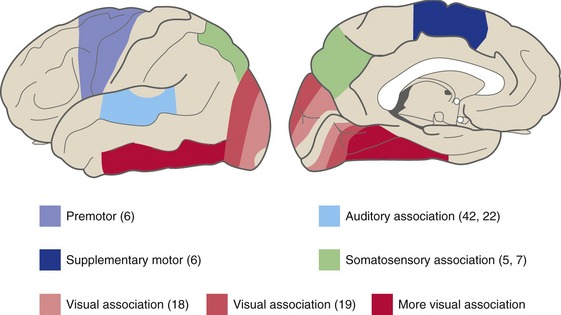
Adjacent to each of the primary cortical areas are areas that are involved in the same function and that receive projections from the primary area (and usually from the appropriate thalamic relay nucleus as well). They have less precise somatotopic, tonotopic, and retinotopic maps than the primary areas, but their cells have more complex response properties. These are referred to as unimodal (i.e., single-function) association areas (Fig. 22-2). Corresponding to the great importance of vision for primates, there is a particularly large expanse of visual association cortex. Damage to unimodal areas can cause different kinds of sensory-specific agnosia (from a Greek word meaning “lack of knowledge”), in which someone is unable to recognize objects or some of their properties using a particular sensory modality even though basic sensation using that modality is normal; loss of the ability to recognize faces or colors (THB6 Box 17-2, p. 451) are two examples. Premotor cortex and the supplementary motor area are comparable unimodal areas adjacent to primary motor cortex.
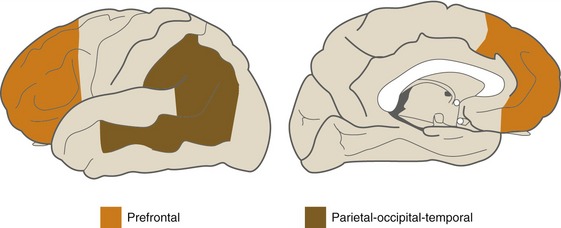
The single-function association areas send converging outputs to two large expanses of more complex association cortex (Fig. 22-3). The first is a parietal-occipital-temporal region surrounded by sensory areas; it receives thalamic inputs from the pulvinar (which also projects to unimodal sensory association areas). The second is anterior to premotor cortex and is called prefrontal cortex; it receives thalamic inputs from the dorsomedial nucleus. Neurons in these areas have still more complex properties; they may respond to multiple kinds of stimuli and may only respond under particular behavioral conditions. Lesions in these multimodal association areas can cause deficits more complex than simple weakness or diminished sensation. Apraxia refers to a condition in which someone is unable to perform a skilled movement in spite of wanting to move and not being weak or uncoordinated, and most often follows left parietal damage. Neglect syndromes in a way are sensory analogs of apraxia. These are conditions in which someone is unable to direct attention to one side and may be totally unaware of one entire side of his or her body. Contralateral neglect most often follows damage to the right parietal or temporal lobe.
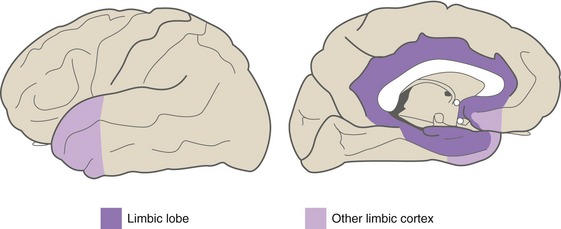
Limbic areas of cerebral cortex (Fig. 22-4), as described further in Chapter 23, operate in emotional and drive-related behavior by interconnecting the hypothalamus with other areas of cortex. The major limbic areas are the cingulate and parahippocampal gyri, with extensions onto the insula, the temporal pole, and the orbital surface of the frontal lobe.
Language Areas Border the Lateral Sulcus, Usually on the Left
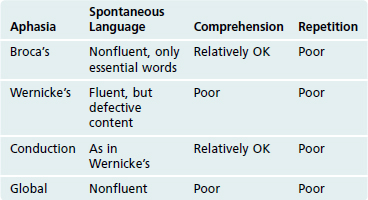
Nearly all right-handed people, as well as most left-handed people, have left hemispheres that are dominant for the production and comprehension of language. Two areas in the left hemisphere are particularly important (Table 22-1). The posterior part of the left inferior frontal gyrus, called Broca’s area, is involved in the production of both written and spoken language. Damage affecting Broca’s area and structures deep to it causes nonfluent (or motor, or expressive, or Broca’s) aphasia, in which comprehension is relatively intact but language is produced only with difficulty. The posterior part of the left superior temporal gyrus, called Wernicke’s area, is involved in the comprehension of both written and spoken language. Damage affecting Wernicke’s area and adjoining cortex causes fluent (or sensory, or receptive, or Wernicke’s) aphasia, in which language can be produced but its comprehension is relatively impaired. Affected individuals have difficulty comprehending even their own language, so it is produced fluidly but inaccurately; the result is incorrect words and meaningless phrases. Damage affecting the arcuate fasciculus (which interconnects Broca’s area and Wernicke’s area) and the overlying supramarginal gyrus causes conduction aphasia, in which an individual speaks and writes like a Wernicke’s aphasic but comprehends language relatively well. Damage that encompasses both Broca’s and Wernicke’s areas causes global aphasia, in which both production and comprehension of language are impaired. Damage outside this perisylvian language zone (so called because it borders the Sylvian fissure, another name for the lateral sulcus) can cause other forms of nonfluent or fluent aphasia by removing important inputs to Broca’s or Wernicke’s area; because connections between auditory cortex and these language areas are still intact, however, such patients are still able to repeat spoken words.
The Corpus Callosum Unites the Two Cerebral Hemispheres
Consciousness and Sleep Are Active Processes
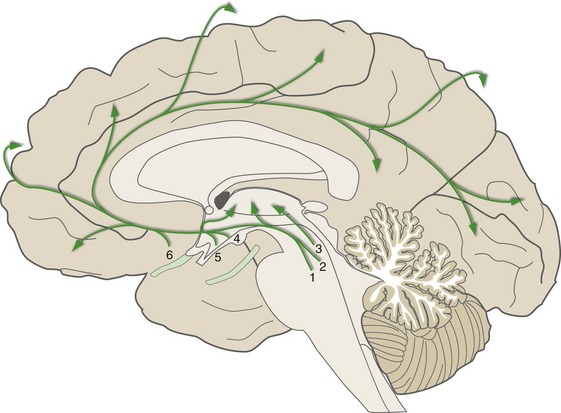
Consciousness—the waking state of self-awareness—is a result of interactions between the cerebral cortex and other parts of the nervous system. The content of consciousness is a reflection of moment-to-moment interactions between different cortical areas; the level of consciousness is regulated by diffuse modulatory projections to the thalamus and cortex (Fig. 22-5). Some of these, as mentioned in Chapter 11, are projections from the ascending reticular activating system (ARAS) in the brainstem; others arise in the hypothalamus and basal forebrain. Major components include chemically coded neurons in the rostral pons and midbrain (e.g., noradrenergic, serotonergic), hypothalamic neurons that use histamine or orexin (a neuropeptide) as a neurotransmitter, and cholinergic neurons in the basal nucleus and the midbrain reticular formation. Collectively, this network of modulatory neurons regulates the level of excitability of the cortex and helps switch thalamic neurons back and forth between tonic and bursting modes.
There Are Two Forms of Sleep
During wakefulness the electroencephalogram (EEG) typically is either a small, desynchronized signal (during attentiveness) or contains small, synchronized waves in an 8 to 13 Hz alpha rhythm (during relaxation). As we fall asleep, the waking desynchronized EEG gives way in stages to a more and more synchronized, slow-wave signal (delta waves, <4 Hz). These stages of non-REM sleep, culminating in slow-wave sleep, are interrupted periodically by intervals of REM sleep, in which there is a desynchronized EEG like that of wakefulness, accompanied by detailed dreams and bursts of rapid eye movements (Table 22-2).
| Non-REM Sleep | REM Sleep | |
|---|---|---|
| EEG | Progressively bigger, slower, more synchronized | Small, fast, desynchronized |
| Muscle tone | Somewhat reduced | Almost abolished |
| Arousal threshold | High | Higher |
| Mental activity | Vague dreams | Detailed, complex dreams |
| ANS activity | Increased parasympathetic; slow, regular pulse and respiration |
Increased sympathetic; irregular pulse and respiration |
Both Brainstem and Forebrain Mechanisms Regulate Sleep-Wake Transitions
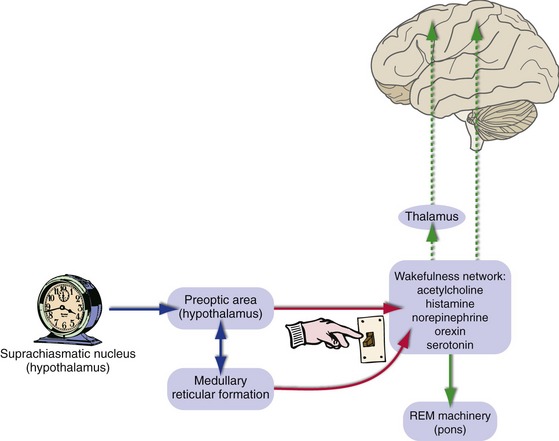
Coordinated activity of the most anterior part of the hypothalamus (the preoptic area; see Chapter 23) and the reticular formation of the caudal brainstem periodically inhibits the wakefulness-promoting network shown in Fig. 22-5, and we fall asleep (Fig. 22-6). Sleep is something we do regularly, with a period of about 24 hours. The clock that controls the sleep-wakefulness cycle is in the suprachiasmatic nucleus of the hypothalamus (see Chapter 23); its output gets in step with the day-night cycle through retinal inputs to the suprachiasmatic nucleus and is then passed along to the preoptic area. Reduced activity in the wakefulness-promoting network in turn reduces cortical activity, and slow-wave sleep is the result. REM sleep, in contrast, depends on neural machinery located in the pons that turns on and off about every 90 minutes during periods of slow-wave sleep (THB6 Figure 22-35, p. 576).