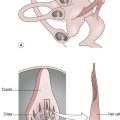
Cerebellum
Basic anatomy
The functional loops
Based on its function, the cerebellum can be broadly divided into four loops:
Afferent connections
The cerebellum receives information from many sources. Figure 12.1 shows the main afferent connections. Note that the vestibular and spinal inputs to the cerebellum remain ipsilateral as the information conveyed from these sources is also ipsilateral. However, the inputs from the cerebral cortex and the inferior olive (which contralateral) need to decussate (cross-over) before entering the cerebellum.
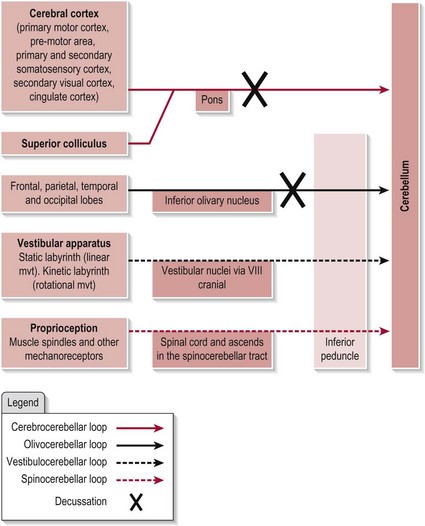
Figure 12.1 The afferent connections of the cerebellum.
Efferent connections
The efferent connections from the cerebellum are shown in Figure 12.2. Note that the pathway to the vestibular nuclei is a direct projection, however all other efferent pathways exit the cerebellum by one of the DCN.
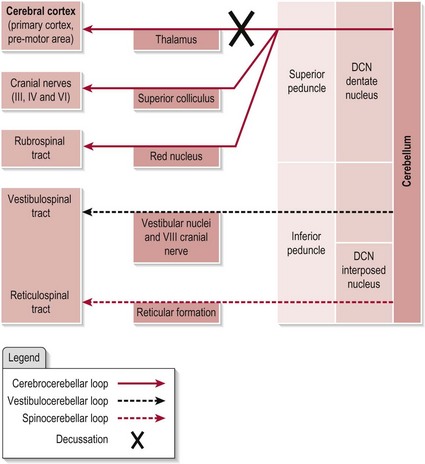
Figure 12.2 The efferent connections of the cerebellum.
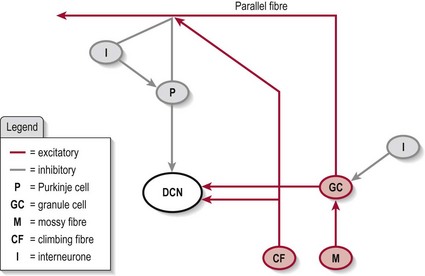
The intrinsic circuitry within the cerebellum
Mossy fibres, which transmit information into the cerebellum.
Granule cells, which form parallel fibres and make thousands of contacts with a Purkinje cell. This system also has a collateral which is excitatory to the DCN.
Purkinje cells, which transmit information out of the cerebellum. These cells are inhibitory at the DCN.
Climbing fibres, which form the inferior olivary nucleus. One climbing fibre has many excitatory contacts with one Purkinje cell and is excitatory to the DCN.
Basket cells, stellate cells, Golgi cells, which are found only within the cerebellar cortex and are inhibitory in output.
In brief, the cerebellar circuitry forms a loop between the input neurons (mossy fibres and granule cells/parallel fibres and climbing fibres) and the output neurons (Purkinje cells) (Fig. 12.3) – the final output being via the DCN. The Golgi, stellate and basket cells work as interneurons to control/modulate the flow of information through this loop with their inhibitory contacts on the Purkinje cell. Also, the whole circuit has various other connections which allow it to be self-modulating. The final output is decided by the balance of excitation and inhibition at the DCN (granule cell and climbing fibres excitatory and Purkinje cell inhibitory).
Function of the cerebellum
The three regions of the cerebellum have similar intrinsic circuitry (Fig. 12.3) but different functions which are a consequence of the origins and destinations of the input and output neurons, respectively. In brief, the cerebrocerebellum loop provides the cerebellum with knowledge about the intended plan for movement and the vestibulocerebellum and spinocerebellum loops monitor the actual position and motion of the body during the movement. Any mismatch/error is corrected and the movement is refined in terms of both timing and magnitude to meet the original goal. The correction is managed directly or indirectly via various descending tracts.
Cerebrocerebellum
This loop is specifically involved in the regulating, planning and timing of movement. Its connections with the superior colliculus also give it a role in visually guided coordination of voluntary movement. In addition, it has a further role in regulating distal limb movment and speech, with damage to the region presenting as limb ataxia (S3.18, 26) and dysarthria (S3.16), respectively.
Vestibulocerebellum
This loop is important in the regulation of posture and balance (S3.18, 32) via axial muscle control and vestibular reflexes. It also governs the eye movements associated with equilibrium (vestibulo-ocular reflex) (S2.10).
Spinocerebellum
This loop is primarily involved in the execution and control of axial and proximal muscle activity via the medial descending tracts. Therefore a role in postural control and balance is likely. Not surprisingly, damage to the area results in trunk ataxia (S3.26) but damage to the more lateral areas of the intermediate zone may also present with limb ataxia. The spinocerebellum also influences the level of muscle tone via the reticular system (S3.21). Recent studies are also challenging the view that this region participates only in the execution of movement and suggests that the intermediate zone also has a role in predictive control and adaptation.
References and Further Reading
Ausim, AS. And the olive said to the cerebellum: organization and functional significance of the olivo-cerebellar system. The Neuroscientist. 2007; 13:616–626.
Bakker, M, Allum, JH, Visser, JE, et al. Postural responses to multidirectional stance perturbations in cerebellar ataxia. Experimental Neurology. 2006; 202:21–35.
Bastian, AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Current Opinion in Neurobiology. 2006; 16:645–649.
Ilg, W, Giese, MA, Gizewski, ER, et al. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain. 2008; 131:2913–2927.
Kandel, ER, Schwartz, JH, Jessell, TM. Principles of neural science, ed 4. New York: McGraw-Hill Health Professions Division; 2000.
Lo, YL, Fook-Chong, S, Chan, LL, et al. Cerebellar control of motor activation and cancellation in humans: an electrophysiological study. The Cerebellum. 2009; 8:302–311.
Ohki, M, Kitazawa, H, Hiramatsu, T, et al. The role of primate cerebellar hemisphere in voluntary eye movement control revealed by lesion effects. Journal of Neurophysiology. 2009; 101:934–947.
Purves, D, Augustine, GJ, Fitzpatrick, D, et al. Neuroscience. Sunderland: Sinauer Associates; 2008.
Schoch, B, Dimitrova, A, Gizewski, AB, et al. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. Neuroimage. 2006; 30:36–51.
Shadmehr, R, Krakauer, JW. A computational neuroanatomy for motor control. Experimental Brain Research. 2008; 185:359–381.
Timmann, D, Konczak, J, Ilg, W, et al. Current advances in lesion-symptom mapping of the human cerebellum. Neuroscience. 2009; 1:1–16.
Voogd, J, Barmack, NH. Oculomotor cerebellum. Progress in Brain Research. 2005; 151:231–268.