CHAPTER 166 Central Vestibular Disorders
The first task of an otolaryngologist in examining a patient with dizziness is to determine if the symptom is of central or peripheral origin1 (Table 166-1). Some central causes of acute vertigo can be life-threatening and may necessitate immediate intervention.2 The differentiation can almost always be made by physical examination based on the type of nystagmus, result of head thrust test, severity of imbalance, and presence or absence of other neurologic signs.
Table 166-1 Differentiation of Central and Peripheral Vertigo
| Feature | Central Origin | Peripheral Origin |
|---|---|---|
| Imbalance | Severe | Mild to moderate |
| Neurologic symptoms | Frequent | Rare |
| Nystagmus | Changes direction No change with fixation |
Unidirectional Decreases with fixation |
| Hearing loss | Rare | Frequent |
| Nausea | Variable | Severe |
| Recovery | Slow | Rapid |
Spontaneous nystagmus of peripheral origin typically is horizontal, or horizontal with a torsional component, and does not change direction with direction of gaze. Spontaneous nystagmus of central origin also can be purely horizontal but often is purely vertical or torsional and usually changes direction with changes in gaze.1
The head thrust test is performed as follows: The examiner grasps the patient’s head and applies a brief, high-acceleration head rotation, first in one direction and then the other.3 During these maneuvers, the patient fixates on the examiner’s nose and the examiner watches for corrective rapid eye movements (saccades), which are a sign of decreased vestibular response (i.e., the eyes move with the head, rather than remaining fixed in space). If a “catch-up” saccade occurs after head thrusts in one direction but not in the other, a peripheral (including the labyrinth and the eighth cranial nerve on its course from the brainstem) lesion on that side is likely.
Migraine
Migraine is characterized by recurrent headaches associated with nausea, vomiting, hypersensitivity to light, sound, and smell.4 This clinical entity is an important consideration in the diagnosis because patients with migraine often present with dizziness. Neurologic aura symptoms are present in one third of migraine patients. Migraine affects nearly 25% of women, 15% of men,5,6 and 5% of children.7 In the prepubertal period, boys with migraine slightly outnumber girls, but at puberty, migraine decreases in boys and increases in girls, so that a 2 : 1 female preponderance is established by adulthood.8 Migraine typically begins in young adulthood, with onset of symptoms after the age of 40 in only 10% of sufferers.
The diagnosis and classification of migraine and other headache disorders have been a controversial issue. The most recent effort of the Headache Classification Committee of the International Headache Society (IHS)4 sets up criteria that allow meaningful comparisons of groups of patients between centers. The IHS classifies the most common type of migraine as migraine with and migraine without aura (Box 166-1); other classified types of migraine headache have been designated but are not considered in this chapter because they will only rarely be encountered by the otolaryngologist.
Box 166-1 Diagnostic Criteria for Migraine
Migraine without Aura
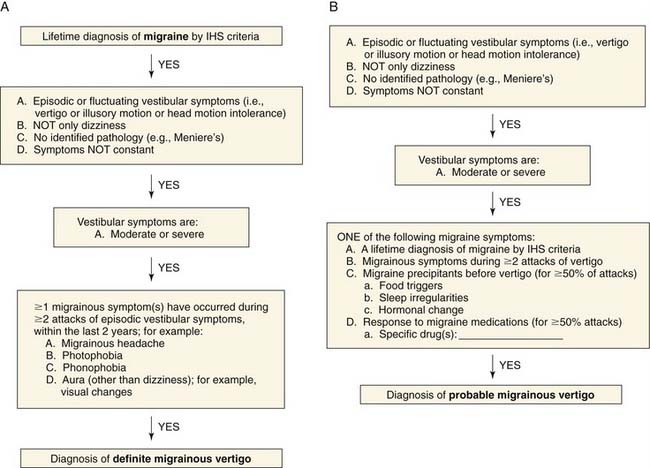
The definition of migrainous vertigo also has been controversial. Neuhauser and colleagues9 proposed criteria for definite migrainous vertigo as follows: (1) episodic vestibular symptoms (rotational vertigo, other illusory self or object motion, positional vertigo, head motion intolerance) of at least moderate severity; (2) migraine according to IHS criteria; (3) at least one of the following migrainous symptoms during at least two vertiginous attacks: migrainous headache, photophobia, phonophobia, visual or other auras; and (4) other causes ruled out by appropriate investigations. Probable migrainous vertigo was the designation chosen for patients who did not meet definite criteria but were still believed to have migrainous vertigo as the most likely diagnosis. This definition requires criteria 1 and 4 from the foregoing list, plus at least one of the following: migraine according to IHS criteria; migrainous symptoms during vertigo; migraine-specific precipitants of vertigo (e.g., specific foods, sleep irregularities, hormonal changes); and response to antimigraine drugs. A diagnostic algorithm10 for definite and probable migrainous vertigo (Fig. 166-1) has been proposed.
Vestibular Symptoms
Vertigo
Vertigo is extremely common in patients with migraine and occurred in 26.5% of Kayan and Hood’s group of patients with migraine, compared with 7.8% of those with tension headache (P < .001).11 Some studies have found the incidence of vertigo in classic migraine sufferers to be as high as 42%.12 The severity of vertigo in patients with migraine tends to be greater than in those suffering from tension headaches. Vertigo severe enough for the patient to seek medical attention for relief occurred in 5% of those with migraine but in less than 1% of those with tension headache.11
The temporal relationship of vertiginous spells to migraine headaches is important to ascertain. Vertigo does not usually manifest as an aura immediately preceding the headache as a prodrome. Of the 53 patients with vertigo and migraine interviewed by Kayan and Hood,11 the vertigo immediately preceded the headache in only 15%, whereas it occurred during the headache in 47% and during the headache-free interval in 36% of patients. In a series of 50 patients with basilar migraine, Olsson13 and Cuttler and Baloh14 also found vertigo to occur more often during the headache-free interval than as a prodrome before the headache.
Motion Sickness
The relationship between migraine and motion sickness is well established. In his seminal study of 9000 Swedish schoolchildren, Bille15 matched children with more pronounced migraine to children in a similar group without migraine. Severe motion sickness was present in 49% of the children with migraine and in only 10% of the control group. Barabas and colleagues found that 45% of 60 children with migraine experienced at least three episodes of motion sickness culminating in vomiting, compared with 5% to 7% of three similar-sized control groups of subjects with non-migraine headaches, seizure disorders, and learning disabilities or neurologic perceptual impairments.16 In adults, a similarly significantly greater incidence of motion sickness has been observed in patients with classic migraine than in patients with tension and cluster headaches.12 In the Kayan and Hood11 series of 200 unselected patients with migraine, 51% reported motion sickness, compared with 20% of the patients with tension headache.
Auditory Symptoms
Of the patients studied by Kayan and Hood,11 20% complained of hearing loss, tinnitus, or pitch distortion, and one fourth of the 20% had multiple auditory symptoms. In descending order of frequency, the temporal patterns for occurrence of these symptoms were during the headache, during the headache-free interval, and immediately before the headache. The temporal relationship of these symptoms mirror the relationship of vertigo to headache in this same series.
Hearing Loss
The association of hearing loss to migraine is reported to be approximately 7%.11 Hearing loss is a more prominent symptom in patients with basilar migraine. In Olsson’s study,13 52% of the patients noticed a change in hearing as part of the aura immediately preceding the migraine headache and 50% had a fluctuating low-frequency sensorineural hearing loss. A group of migraine patients in whom hearing loss thought to be due to vasospasm has been reported.17
Tinnitus
Tinnitus was experienced by 15% of migraine patients, but none with tension headache,11 and in more than 60% of basilar migraine patients.13
Distortion
Although distortion was noted by only 4% of patients in the Kayan and Hood series,11 its actual prevalence may be higher. More attention may be given to more bothersome symptoms, and auditory distortion may be ignored by physicians and patients alike as unimportant.
Phonophobia
Loud noise aversion distinguishes patients with migraine from those suffering other types of headaches. Only 12% of tension headache sufferers experienced phonophobia, but 81% of those with migraine had the symptom.11 Olsson13 detected phonophobia in patients with basilar migraine at a rate of 70% during headache and 76% during headache-free intervals. Furthermore, he documented an abnormal loudness discomfort level in 78% of these patients, whereas only 14% had abnormal speech reception thresholds.
Migraine Associated with Neuro-otologic Symptoms
Basilar Migraine
Bickerstaff18 described a form of migraine ascribed to ischemia in the distribution of the basilar artery. This syndrome currently is known as basilar migraine, replacing “basilar artery migraine” and “posterior fossa migraine,” which occasionally appear in the older literature. This subtype of migraine was similar to classic migraine in that it consisted of an aura followed by a severe headache. However, a majority of affected patients were adolescent girls, in whom the migraine attacks often occurred premenstrually. Later observers emphasized other aspects of the clinical picture such as stupor,19 loss of consciousness,20 neurologic symptoms,21 hearing loss, and vertigo.22 These symptoms are thought to be due to involvement of the brainstem, cerebellum, cranial nerve nuclei, and occipital lobe cortex.
Migraine without Headache
Migraine should not be eliminated as a cause for vertigo just because headaches are not present. Whitty23 reported on a series of patients with symptoms typically experienced during a migraine aura but no headaches. In these patients, typical headaches sometimes developed later in life, or auras that were only intermittently related to headaches were noted. Other observers have described similar patients using the term migraine equivalent24–26 or migraine accompaniments.27 A history of motion sickness, premenstrual clustering of attacks, or headaches associated with some of the attacks is frequent. Personal or family history of migraine also is helpful in diagnosing these patients.
Etiology
Historically migraine headache was thought to result from dilation of intracranial and dural arteries causing stretching of pain fibers in the walls of the arteries. The complex phenomena associated with migraine cannot be fully explained by a vascular theory. More recently, neurally based spreading of depression activity has been implicated,28,29 although the neurophysiology is far from completely understood.
A family history of migraine is present in approximately 40% to 90% of affected persons, compared with 5% to 20% of unaffected subjects. When migraine sufferers with and without aura are examined separately, patients who have migraine with aura are more likely to have affected family members. When twins are studied, conconcordance rates for migraine are only 10% to 34%, depending on the country, suggesting that both genetic and environmental factors play a role.30
Familial hemiplegic migraine (FHM) is a rare type of migraine that affects only 0.01% of the general population.31 However, the underlying cellular cause is relatively well understood. Unlike most types of migraine, FHM has a clear autosomal dominant pattern of inheritance. Thus far, mutations have been found in three different genes that determine neuronal excitability.
Management
Treatment of migraine has historically been focused on headache symptoms. Management of migraine headache can be divided into two categories: symptomatic and prophylactic. Some interventions are useful in ameliorating the symptoms of the acute attack, whereas others are designed to reduce the frequency or severity of attacks. Symptomatic management includes analgesics, antiemetics, antivertiginous drugs, sedatives, and vasoconstrictors. In many patients, over-the-counter analgesics such as ibuprofen, aspirin, or acetaminophen and rest are all that is needed to relieve headache symptoms. Decreased gastric motility occurs during and between migraine attacks, which may decrease absorption of oral drugs, contributing to nausea and vomiting.32 Metocopramide promotes normal gastric motility and may improve absorption of oral medication.
Nutrition may play a significant role in the frequency and severity of migraine headaches and migraine-associated dizziness. Riboflavin (vitamin B2) has been shown to be more effective than placebo in reducing the frequency of migraine headache when given in a dose of 400 mg daily.33 High-dose magnesium also has been found to have a therapeutic effect on migraine; suggested mechanisms have included inhibiting platelet aggregation, counteracting vasospasm, stabilizing cell membranes, and anti-inflammatory effects.34 Patients suffering from migraine symptoms have been found to have lower levels of magnesium, and infusion of magnesium can relieve symptoms.35 Magnesium given orally in a dose of 600 mg daily also has been shown to decrease headache symptoms36; however, diarrhea is a potential side effect at these doses.
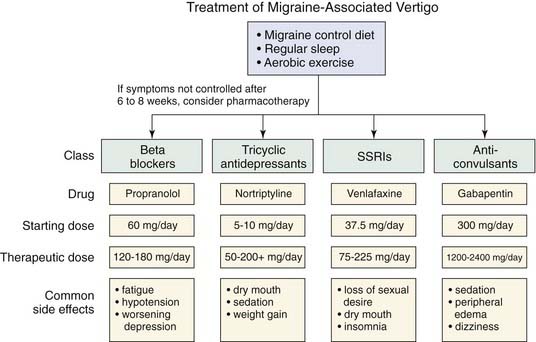
Food sensitivity may be a migraine trigger in a significant number of patients with migraine symptoms.34 Although some patients have observed that consuming a certain food will reliably provoke a headache, the effect of diet is more subtle. Foods that should be avoided include red wine, aged cheeses, beer, chocolate, caffeine, and yogurt.37 Alcohol itself does not seem to be a trigger, because vodka consumption does not provoke attacks.38 Dietary modification may be considered as first-line therapy for migraine prophylaxis (Fig. 166-2).
Vestibular migraine symptoms often are not directly related to the headache symptoms, and in some cases, dizziness as a manifestation of migraine can be present nearly continuously. Treatment of vestibular migraine has tended to mirror that of traditional migraine symptoms, and medications that alleviate headache symptoms also tend to be effective for vertigo symptoms.39 However, because the migraine-associated dizziness often is much more frequent than the headaches, prophylaxis usually is necessary.
Sumatriptan (Imitrex), a 5HT1D receptor agonist, has become the principal drug to abort severe migraine headache. Initial trials indicate that in 90% of cases the drug effectively aborts migraine headache symptoms within an hour of their onset when it is given subcutaneously; in 70% to 85%, symptoms may be aborted within 2 hours.40 Common side effects include sensations of heaviness and pressure in the chest, with coronary artery vasospasm more rarely reported. Although sumatriptan is remarkably effective in relief of early migraine headaches, breakthrough is common, and the drug has limited efficacy for concurrent vestibular symptoms.
Propranolol (Inderal) is the drug most commonly used for preventing migraine episodes. Approximately 50% to 70% of patients with headache symptoms derive benefit from prophylactic propranolol therapy, which also may be beneficial for vertigo symptoms.21 The medication is contraindicated in patients with asthma, congestive heart failure, peripheral vascular disease, diabetes, or hypothyroidism. Principal side effects include fatigue, lethargy, and dizziness, which usually occurs on rising from a seated or supine position. Side effects are minimized by slowly increasing the dose from a low starting level. Doses of 80 to 180 mg per day usually are effective. The medication should be continued for at least 2 to 3 months at the highest level of tolerance before it is considered a failure. On discontinuation, the drug should be gradually tapered over several days.
Calcium channel blockers (nifedipine, verapamil) also have been reported to reduce the frequency of migraine attacks, but the effect is likely to be only modest.41 Diltiazem (Cardizem) does not seem to have any effect on migraine prevention.
Tricyclic antidepressants also have been shown to decrease the frequency and severity of migraine attacks. Amitriptyline is perhaps the most-studied drug in this category, with several studies showing its efficacy.42 Starting doses usually are 10 to 25 mg, which usually is given at bedtime, to avoid the side effects of sedation and anticholinergic activity. Weight gain is a side effect that limits patient compliance with therapy in some populations. Therapeutic doses usually are 50 to 100 mg, with some patients who also suffer from depressive symptoms requiring doses of up to 400 mg. Nortriptyline is less sedating than amitriptyline and has a dosage profile similar to that of amitriptyline.
The role for selective serotonin reuptake inhibitors (SSRIs) for treatment of migraine remains controversial. Some evidence shows that they seem to be less effective than tricyclic antidepressants43 for headache symptoms. However, in patients with chronic subjective dizziness as their primary symptom, SSRIs seem to be effective.44
Clinical trials have shown gabapentin (Neurontin) to be effective at dosages of 1200 to 2400 mg per day,45 which usually is divided into three doses daily. Dizziness, peripheral edema, and somnolence are common side effects.
Acetazolamide (Diamox) has been shown to be effective in preventing migraine headaches and episodic vertigo in families who also exhibit essential tremor.46 However, it has not been shown to be of benefit in larger migraine headache populations.47 It may be effective in ameliorating motion-induced vestibular symptoms in patients with migraine.
Vestibular Disorders Associated with Migraine
Benign Paroxysmal Positional Vertigo
BPPV is the most common cause of peripheral vertigo. The disorder probably is due to free-floating particles in the semicircular canal (canalithiasis), which usually collect in the posterior semicircular canal owing its low point with respect to gravity. Classic symptoms include brief episodes (lasting less than 1 minute) of vertigo when the head is tilted backwards or during rolling over in bed. An extensive discussion of diagnosis and treatment of this common disorder is outside the scope of this chapter. Of note, however, migraine and motion sickness are three times more common in patients with BPPV than in the general population, with 59% of patients having a family history of migraine.48
Meniere’s Disease
Meniere’s disease classically manifests with unilateral low-frequency fluctuating hearing loss, a sense of aural fullness, and dizziness lasting several minutes to hours.49 However, the presence and severity of these symptoms are variable, which can overlap with those of migraine.50 Unselected patients with migraine have a 7% incidence of hearing loss and 26% incidence of vertigo.11 In patients with Meniere’s disease, the lifetime incidence of migraine is 56%, versus only 25% in controls.51 The frequent co-occurrence of Meniere’s disease and migraine suggests a pathophysiologic link between the two syndromes.52 Alternatively, the diseases may be separate processes that cause similar symptoms. It is likely that both Meniere’s disease and migraine are not homogeneous disorders but may be caused by a spectrum of genetic and environmental influences—so both explanations may be correct: Migraine may be one of many risk factors for Meniere’s disease, and vice versa.
Vestibular Disorders Related to Migraine
Benign Paroxysmal Vertigo of Childhood
Basser53 described a clinical entity in children younger than 4 years of age that he called benign paroxysmal vertigo. This should not be confused with the much more common benign paroxysmal positional vertigo (BPPV), which also is referred to as benign positional vertigo. Children with this disorder usually become suddenly frightened, cry out, cling to the parent, stagger as though drunk, and exhibit pallor, diaphoresis, and frequent vomiting. Symptoms are worsened by head motion. The episodes last less than 5 minutes, after which the child is normal again. The symptoms usually manifest at the age of 2 to 3 years and gradually decrease until they resolve before age 8. When the clinical picture is clear, treatment is not needed. On long-term follow-up, classic migraine eventually developed in six of seven patients with this disorder.54
Paroxysmal Torticollis
Snyder55 described a series of 12 infants who experienced recurrent attacks of head tilt before the age of 8 months. The spells lasted from a few hours to 3 days and occasionally were accompanied by pallor, vomiting, and agitation. Subsequent observers have suggested a familial occurrence56 and that symptoms also may involve the trunk and extremities.57 The spells usually stop spontaneously by age 5, and no treatment is needed.
Vascular Disorders
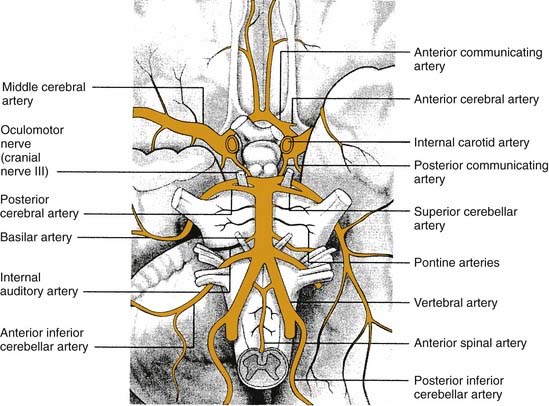
The blood supply to the inner ear, brainstem, and cerebellum arises from the vertebrobasilar system. Vertigo can occur from occlusion of any of the three major circumferential branches of the vertebral or basilar arteries: the posterior inferior cerebellar artery, the anterior inferior cerebellar artery, and the superior cerebellar artery (Fig. 166-3).
Specific Disorders
Vertebrobasilar Insufficiency
Vertebrobasilar insufficiency is a significant cause of vertigo in the elderly population.58,59 Vertigo due to vertebrobasilar insufficiency is of abrupt onset, usually lasts several minutes, and frequently is associated with nausea and vomiting. Presentation with vertigo symptoms alone is extremely uncommon and occurs in less than 1% of patients.60 Usually multiple symptoms are present, which often include headache, diplopia, ataxia, numbness, weakness, or oropharyngeal dysfunction. Repeated episodes of vertigo without other symptoms suggest another diagnosis.
Atherosclerosis of the subclavian, vertebral, or basilar arteries usually is the underlying cause of vertebrobasilar insufficiency. The distribution of large-artery atherosclerosis differs according to race and gender.61 European men tend to have atherosclerosis at the origin of the vertebral arteries near the subclavian. Intercranial large-artery atherosclerosis is more common among African Americans, Asians, and women. Hypertension increases the risk of lipohyalinotic thickening of these vessels, which increases the risk of infarction.
In most patients with nonacute presentations, treatment consists of controlling risk factors such as diabetes, hypertension, and hyperlipidemia. In patients with symptomatic intracranial stenosis with 50% to 99% occlusion, aspirin and warfarin are equally effective, but warfarin carries a higher risk of hemorrhagic events,62 so aspirin is the agent of choice. Anticoagulation with warfarin should be considered if the patient has previously had an embolic stroke of cardiac origin.58 Stenting of vertebral artery stenosis has been tried, with mixed results. Endarterectomy for extracranial vertebral artery disease also has been successfully performed, but indications for such procedures are still evolving.
Lateral Medullary Syndrome
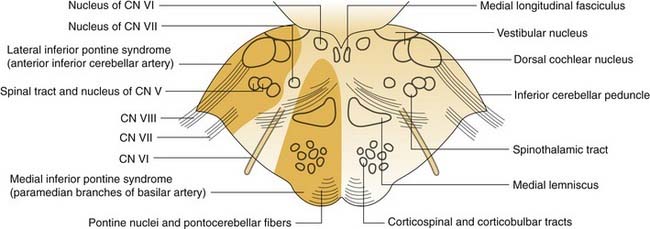
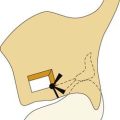
The zone of infarction producing the lateral medullary syndrome (Wallenberg’s syndrome) consists of a wedge of the dorsolateral medulla just posterior to the olive (Fig. 166-4). The syndrome usually results from occlusion of the ipsilateral vertebral artery and rarely from occlusion of the posterior inferior cerebellar artery.63 The classic presentation includes sensory deficits affecting the trunk and extremities on the opposite side of the infarct and sensory and motor deficits affecting the face and cranial nerves ipsilateral to the infarct. Characteristic symptoms include vertigo, ipsilateral facial pain, diplopia, dysphagia, and dysphonia. Neurologic examination may reveal a ipsilateral Horner’s syndrome, ipsilateral dysmetria, dysrhythmia, spontaneous nystagmus, and contralateral loss of pain and temperature sensation.
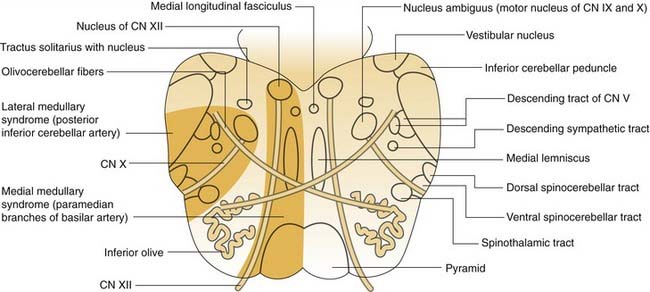
Figure 166-4. Medulla cross section with anatomic structures labeled on the right and vascular lesions on the left. CN, cranial nerve.
(Adapted from Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York: McGraw-Hill; 2000:1303.)
Some patients exhibit a prominent motor disturbance that causes the body and extremities to deviate toward the lesion site as if being pulled by an invisible force.64 This so-called lateral pulsion also affects the oculomotor system, producing excessively large saccades directed toward the side of the lesion and abnormally small saccades away from the lesion. Most patients with Wallenberg’s syndrome have major residual neurologic deficits months or even years after the acute infarction.
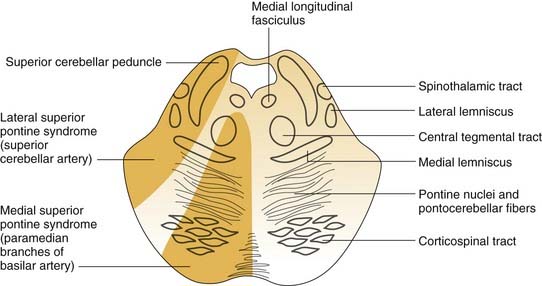
Lateral Pontomedullary Syndrome
Ischemia in the distribution of the anterior inferior cerebellar artery results in infarction of the dorsolateral pontomedullary region and the inferolateral cerebellum.65 The middle cerebellar peduncle typically is the core of the affected territory (Fig. 166-5). Because the labyrinthine artery originates from the anterior inferior cerebellar artery in 80% of patients, infarction of the membranous labyrinth is a common accompaniment. Severe vertigo, nausea, and vomiting are common initial symptoms. Other signs and symptoms include ipsilateral hearing loss, tinnitus, facial paralysis, and cerebellar asynergy. Spontaneous nystagmus is common. Contralateral loss of pain and temperature sensation may be caused by crossed spinothalamic fibers. Onset of symptoms is acute, followed by gradual improvement. Vertigo may persist for several weeks owing to damage of the central compensation mechanisms.
Cerebellar Infarction
Occlusion of the vertebral artery, the posterior inferior cerebellar artery, the anterior inferior cerebellar artery, or the superior cerebellar artery may result in infarction confined to the cerebellum without brainstem involvement66 (Fig. 166-6). Initial signs and symptoms are severe vertigo, vomiting, and ataxia. Lack of typical brainstem signs may cause an incorrect diagnosis of an acute peripheral labyrinthine disorder. The key to the diagnosis is the presence of prominent cerebellar signs such as gait ataxia and paretic gaze nystagmus. The diagnostic modality of choice is magnetic resonance imaging (MRI). After a latent interval of 24 to 96 hours, some patients exhibit signs of progressive brainstem dysfunction caused by compression by a swelling cerebellum. Progression to quadriplegia, coma, and death may follow unless surgical decompression is performed.
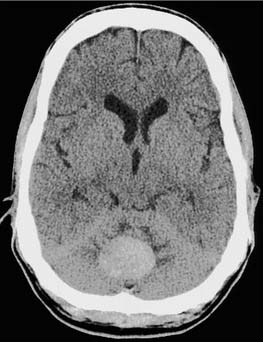
Cerebellar Hemorrhage
Spontaneous intraparenchymal hemorrhage into the cerebellum causes neurologic symptoms that often rapidly progress to coma and death.67–69 The initial manifestations are severe vertigo, vomiting, and ataxia. As with cerebellar infarction, brainstem signs may not initially be present, which may cause the condition to be confused with a peripheral vestibular problem. Modern imaging studies such as computed tomography (CT) and MRI have revolutionized the diagnosis of this condition (Fig. 166-7). Mortality rates range from as low as 20%70 up to 74%, depending on the series. The value of and indications for surgical intervention remain controversial and probably depend on the size and location of the hematoma as well as the patient’s symptoms.67
Evaluation and Management
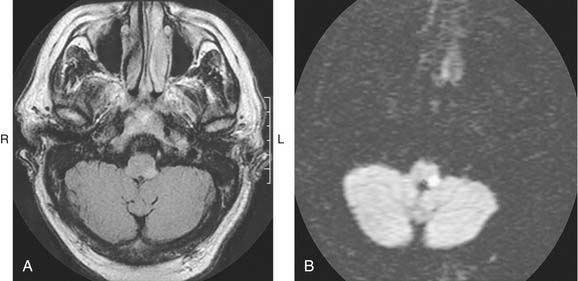
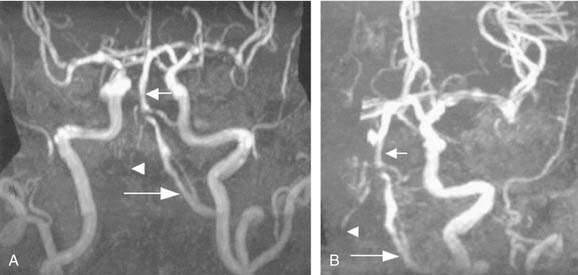
Cerebrovascular disease is a primary consideration in the differential diagnosis for acute spontaneous vertigo, particularly if the patient has vascular risk factors. If the history and examination findings suggest a vascular disorder, immediate neuroimaging is necessary. CT of the brain without contrast often is the first imaging test because it is widely available and shows intraparenchymal or subarachnoid blood. Hemorrhage can mimic any of the ischemic stroke syndromes and must be excluded with thin-section CT through the cerebellum, brainstem, and fourth ventricle. MRI and magnetic resonance angiography (MRA) are superior to CT for viewing the vertebrobasilar vessels and their supplied structures, because CT scans often are normal in appearance with cerebellar or brainstem infarction. MRI, on the other hand, detects ischemic strokes in the brainstem and cerebellum early on, as well as edema. Diffusion-weighted imaging with MRI can now detect infarcts within the first hour of ischemia (Fig. 166-8). MRA has begun to replace conventional angiography in certain circumstances. Vertebral artery dissection and vertebral or basilar artery stenosis or occlusion generally can be identified with MRA of the cervical vessels performed with contrast (Fig. 166-9).
Neoplasms
Vestibular Schwannomas
Vestibular schwannomas can manifest with vertigo symptoms, which can be disabling in some patients,71 although such symptoms are present in less than 20% of patients with these tumors.72 Hyperventilation-induced vertigo also may occur in the absence of hearing loss.73 Discussion of the diagnosis and treatment of vestibular neuromas can be found in Chapters 149 and 153.
Brainstem Neoplasms
Tumors originating in the fourth ventricle and compressing the vestibular nuclei in its floor also can produce vestibular symptoms. Medulloblastomas, occurring primarily in children and adolescents, are rapidly growing, highly cellular tumors that originate in the posterior midline or vermis of the cerebellum and invade the fourth ventricle and adjacent cerebellar hemispheres. Headaches and vomiting occur early from obstructive hydrocephalus and associated increased intracranial pressure. An attack of headache, vertigo, vomiting, and visual loss may result from a change in head position, producing transient cerebrospinal fluid (CSF) obstruction (Bruns’ syndrome). Positional vertigo and nystagmus may be the presenting manifestations.74,75 Other fourth ventricle tumors that produce a similar clinical picture include ependymomas, papillomas, teratomas, epidermoid cysts, and cysticercosis. The diagnosis of a fourth ventricular mass is made readily with MRI and CT scanning, but frequently the exact nature of the tumor cannot be determined before surgical exploration and biopsy. Complete removal of tumor should be achieved when possible. Medulloblastomas are particularly sensitive to radiotherapy.76
Cerebellar Neoplasm
Clinical manifestations of posterior fossa tumors include dizziness in 81% and vertigo in 44% of patients.77 One half of patients with posterior fossa tumors exhibit spontaneous nystagmus, and a third lack caloric responses. Other common symptoms and signs of cerebellar tumors include tinnitus, hearing loss, headache, nausea or vomiting, and ataxia. Gliomas of the cerebellum may be relatively silent until they become large enough to obstruct CSF circulation or compress the brainstem.78 Positional vertigo occasionally is the initial symptom of a cerebellar tumor.79,80 Paroxysmal positional nystagmus, when present, is atypical, because it can be induced in several different positions and is nonfatigable. Tumors that can produce these signs and symptoms include gliomas, teratomas, hemangiomas, and hemangioblastomas. Each of these tumors has a characteristic appearance on MRI and CT scans, although occasionally the exact tumor type cannot be determined before surgical biopsy.
Cervical Vertigo
Dizziness caused by disorders of the neck and cervical spine is poorly understood and relatively uncommon. Neck afferents are recognized to have a role in the coordination of eye, head, and body spatial orientation. Perception of head rotation can be driven by vestibular, proprioceptive, or visual inputs. Cervical vertigo must be by definition proprioceptive in nature, because the other inputs that cause vertigo do not exist in the neck. Laboratory experiments in humans that eliminate visual input with darkness and vestibular input by holding the head still have shown that trunk motion can produce the sensation of head turning81; however, this has not been shown to occur in more ordinary situations. Several studies have provided limited evidence for the existence of cervical vertigo. Unilateral local anesthesia of the cervical roots can cause ataxia and nystagmus in animals and ataxia without nystagmus in humans.82 Patients with chronic cervicobrachial neck pain also have worse results on posturography tests.83 These results are difficult to interpret because trauma that causes neck pain also can cause brain injury and damage to peripheral otolith organs. In clinical practice, it is necessary to exclude neurologic, vestibular, and psychosomatic disorders before a disorder of the craniovertebral junction can be given serious consideration. The diagnosis of cervical vertigo remains largely theoretical.84,85
Disorders of the Craniovertebral Junction
Pathophysiology
The physiologic problem common to these disorders is physical compression of the CNS at the level of the upper spinal cord and medulla (cervicomedullary compression).86–88 The rostrocaudal area of compression is variable, and the impingement can be ventral or dorsal, or (rarely) both. A second but uncommon potential mechanism is vascular insufficiency of the anterior spinal or vertebral arteries from angulation, stretching, or extrinsic obstruction.89,90
Classification
Basilar Impression
Basilar impression is an upward indentation or invagination of the clivus or posterior fossa floor.86,91 This defect causes the upper cervical spine to migrate cephalad toward the brainstem, as the floor of the skull collapses under the weight of the head. The normal anatomy of the foramen magnum, first cervical vertebra (atlas), second cervical vertebra (axis), and its odontoid process (dens) can become altered by disease, trauma, or congenital deformities. When a softening of the skull base results, the skull literally descends onto the spinal column and odontoid. As the odontoid projects intracranially, it compresses the ventral aspect of the medulla.
Disorders known to cause basilar impression include Paget’s disease, rheumatoid arthritis, osteomalacia, osteogenesis imperfecta, cretinism, and rickets.86 The diagnosis is confirmed when lateral radiographic views of the skull demonstrate that the tip of the odontoid either extends above Chamberlain’s line (a line extending from the posterior edge of the hard palate to the posterior lip of the foramen magnum)92 or projects posterior to Wackenheim’s clivus-canal line. The term platybasia has been used synonymously with basilar artery impression by some authors. Although the two conditions coexist, platybasia itself causes no symptoms.
Assimilation of the Atlas
Assimilation of the atlas (also called occipitalization of the atlas) is a bony union between the first cervical vertebra and the skull.93 The amount of union varies, but motion between the two structures does not occur. As a result, the odontoid often impinges on the effective anteroposterior diameter of the foramen. A frequent associated finding is fusion of the axis to the third cervical vertebra. The condition is frequently developmental in origin and is found in association with abnormalities of the jaw, cleft palate, auricular deformities, cervical ribs, and urinary tract anomalies.94 Klippel-Feil syndrome is one of the most common causes of cervical vertebral fusion.
Signs and symptoms of this syndrome are variable and typically appear fairly late and progress slowly. Symptoms may involve the brainstem, cerebellum, spinal cord, or lower cervical nerve roots or the vascular supply to these structures. These symptoms frequently precipitate a visit to a neurologist, but vertigo, facial paresis, dysphagia, hearing loss, and tongue atrophy may bring some patients to an otolaryngologist for initial evaluation. Although the disorder can be diagnosed with CT reconstructions, the key to diagnosis often is MRI, which allows direct coronal and sagittal imaging.95
Atlantoaxial Dislocation
Atlantoaxial instability is associated with a number of congenital and acquired disease processes. It occurs in 10% to 30% of patients with Down syndrome when diagnosed radiographically, with an atlantodens interval of 4 to 5 mm.96,97 The condition also is associated with spondyloepiphyseal dysplasia, Hurler’s syndrome, and Morquio syndrome, and in those with achondroplastic dwarfism. A high incidence of atlantoaxial instability, resulting from destruction of the normal stabilizing mechanisms by inflammatory tissue, has been reported in patients with rheumatoid arthritis.98 Similarly, ligamentous laxity can result from inflammatory conditions affecting the retropharyngeal soft tissues or cervical bony structures, such as osteitis, retropharyngeal abscess, or lymphadenitis. Atlantoaxial instability in association with pharyngeal infection has come to be known as Grisel’s syndrome.99 It is rare in adults and associated with pain and dysphagia.
Most patients with atlantoaxial instability of developmental origin are asymptomatic, and the instability does not usually worsen with time.97 Symptoms and signs develop in only 1% to 2% of patients with Down syndrome and usually appear between the ages of 5 and 15 years. Neck pain, sensory deficits, and difficulty with bladder control have been described; more subtle manifestations include decreased activity tolerance, changes in gait, and hyperreflexia.
Chiari Malformation
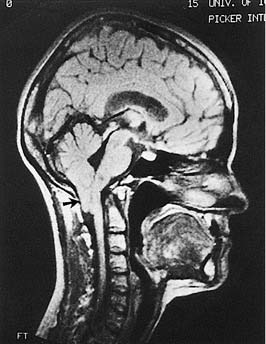
Chiari malformations are a group of developmental hindbrain and spinal cord abnormalities characterized by hernation of the contents of the posterior cranial fossa caudally through the foramen magnum into the upper cervical spine. These patients may present with symptoms related to the vestibular system such as ataxia, nystagmus, or vertigo.100 Chiari malformation can be divided into two forms, type I and type II. The Chiari type I malformation is more common and more mild. Symptoms of Chiari type I usually are delayed until the age of 25 to 35 years. Affected persons often have subtle neurologic symptoms that are not associated with other developmental defects. Diagnosis is made by CT or MR images reconstructed in the sagittal plane, which may demonstrate deformation of the cerebellar tonsils more than 5 mm caudally through the foramen magnum (Fig. 166-10). The Chiari type II malformation manifests in the first few months of life with associated hydrocephalus and other CNS malformations such as meningomyelocele.
Classic presentation symptoms of Chiari type I malformation include upper extremity weakness, sensory loss, and pain. Occipital headaches that are exacerbated by coughing sneezing, bending over, or lifting also are a common presenting feature. Ataxia and nystagmus are reported in 24% of patients, with vertigo being a complaint in only 8%.101 Patients also may have a vestibular test profile consistent with peripheral dysfunction but remain asymptomatic.100 These patients also may present to the otolaryngologist with difficulty swallowing, aspiration, or hoarseness due to involvement of the lower cranial nerves (cranial nerves IX through XII).
Multiple Sclerosis
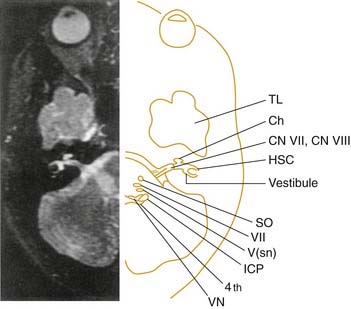
Multiple sclerosis (MS) is an inflammatory demyelinating CNS disorder of idiopathic origin with the onset in early adulthood. The disease affects women twice as often as men. The key to diagnosis is the presence of disseminated signs of CNS dysfunction manifested in an alternating and remitting and exacerbating course. Many symptoms can occur, depending on the location of the lesions. Vertigo is the initial symptom of MS in only approximately 5% of patients, although the symptom may be present in as many as one half of these patients at some point in the course of the disease.102 Lesions within the vestibular nuclei and in the entry zone of cranial nerve VIII represent the most common locations for demyelinating activity to cause vertigo in patients with MS103 (Fig. 166-11). However, benign paroxysmal positional vertigo remains the most common cause of vertigo in these patients,104 as in the general population.
The diagnosis of MS remains a clinical one, with no definitive imaging study or laboratory tests.105 Abnormalities in CSF can be identified in as many as 90% of patients with MS at some point in the course of the disease. Findings included increased gamma globulin level, oligoclonal banding of gamma globulin, and increased myelin basic protein. MRI demonstrates white matter lesions in more than 90% of patients, although similar lesions occasionally are seen in patients without MS.106
Cerebellar Ataxia Syndromes
Spinocerebellar atrophy encompasses a group of disorders in which cerebellar ataxia is a prominent feature. Many different genetically inherited subtypes have been identified by genetic linkage analysis.107
Cerebellar atrophy is characterized by gradual onset of ataxia, without other associated signs, beginning later in life. The disorder can be genetic or sporadic. Vestibular testing often reveals rebound nystagmus and spontaneous vertical nystagmus.108
Familiar episodic ataxia is an uncommon ataxia with a dominant inheritance pattern characterized by recurrent vertigo and ataxia. Episodes of ataxia often are triggered by stress, last minutes to hours, and begin between early childhood and early adulthood. Symptoms are markedly reduced or eliminated by acetazolamide.109
Paraneoplastic cerebellar degeneration (PCD) typically manifests as a subacute, rapidly progressive cerebellar syndrome. The development of anti-Purkinje cell antibodies (PCAs) in patients with undiagnosed and otherwise asymptomatic malignancies may result in the syndrome of PCD. PCD typically begins fairly abruptly and progresses rapidly through several months, with cerebellar-type ocular motor and vestibular abnormalities, dysarthria, and severe appendicular and gait ataxia. The cerebellum is not directly invaded by tumor, but the tumor produces remote effects on the cerebellum that probably are immune-mediated. It most commonly occurs in patients with carcinoma of the lung (small cell), breast, ovary, and Hodgkin’s lymphoma. However, the neurologic symptoms usually appear before the cancer is identified.110
Focal Seizure Disorders
Vertigo can be part of an aura of a focal seizure. Smith111 studied a group of patients with vestibular symptoms as part of their aura. The most common vestibular symptom was a sense of spinning, which occurred in 55% of these patients, followed by a sense of linear translation in 30%. However, episodes of vertigo as isolated manifestations of a seizure disorder occur rarely, if ever.
Normal Pressure Hydrocephalus
Idiopathic normal pressure hydrocephalus classically manifests with the triad of dementia, urinary incontinence, and gait or balance disturbance. However, these symptoms represent the more severe end of the clinical spectrum, and more subtle cases can manifest with only mild gait or balance symptoms.112 Cognitive symptoms also are common but may be mild. Patients usually become symptomatic after the age of 60 years. Diagnosis requires an imaging study that demonstrates nonobstructive ventricular enlargement disproportionate to the degree of cerebral atrophy.
Physiologic Dizziness
Mal de Débarquement Syndrome
Mal de débarquement (MDD), “sickness of disembarkment,” refers to inappropriate sensations of movement after exposure to motion. Although this unusual syndrome typically follows a sea voyage, MDD may occur after extended train, air, or car travel.113 The characteristic symptom is a sensation of persistent rocking or swaying of the body without rotational vertigo. Vague dysequilibrium or unsteadiness also may be a feature. MDD is distinguished from motion sickness in that patients with MDD are mostly symptom-free during the period of motion. Although many normal persons experience similar symptoms of “land sickness” for up to 48 hours after exposure to prolonged motion, most experts define MDD as a syndrome persisting for at least a month. During symptomatic periods, patients typically report improvement while in a moving vehicle or while walking that is lost when they stop moving, when symptoms worsen. MDD affects predominantly women, with a mean age at onset in the 40s. Symptoms may last for months to years. Some investigators have hypothesized that MDD is caused by a multisensorimotor adaptation and habituation to a new or abnormal motion environment. Benzodiazepines such as clonazepam or diazepam or amitriptyline may have a beneficial effect, but meclizine and scopolamine were ineffective.113
Baloh RW. Clinical practice. Vestibular neuritis. N Engl J Med. 2003;348:1027-1032.
Baloh RW, Foster CA, Yue Q, et al. Familial migraine with vertigo and essential tremor. Neurology. 1996;46:458-460.
Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol. 1997;18:350-354.
Buchholz D, Reich SG. Heal your headache. New York: Workman Publishing; 2002.
Chen CC, et al. Posterior cranial fossa tumors in young adults. Laryngoscope. 2006;116:1678-1681.
Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543-1554.
Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737-739.
Harker LA, Rassekh CH. Episodic vertigo in basilar artery migraine. Otolaryngol Had Neck Surg. 1987;96:239.
Harker LA, Rassekh CH. Migraine equivalent as a cause of vertigo. Laryngoscope. 1988;98:160.
Hotson JR, Baloh RW. Acute vestibular syndrome. N Engl J Med. 1998;339:680-685.
Kentala E, Pyykko I. Vestibular schwannoma mimicking Meniere’s disease. Acta Otolaryngol Suppl. 2000;543:17-19.
Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56:436-441.
Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology. 2002;59:1700-1704.
Thomsen LL, Olesen J. Sporadic hemiplegic migraine. Cephalalgia. 2004;24:1016-1023.
Viirre ES, Baloh RW. Migraine as a cause of sudden hearing loss. Headache. 1996;36:24.
1. Baloh RW. Clinical practice. Vestibular neuritis. N Engl J Med. 2003;348:1027-1032.
2. Hotson JR, Baloh RW. Acute vestibular syndrome. N Engl J Med. 1998;339:680-685.
3. Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737-739.
4. Silberstein SD, Olesen J, Bousser MG, et al. The International Classification of Headache Disorders, 2nd Edition (ICHD-II)—revision of criteria for 8.2 Medication-overuse headache. Cephalalgia. 2005;25:460-465.
5. Stewart WF, Simon D, Shechter A, et al. Population variation in migraine prevalence: a meta-analysis. J Clin Epidemiol. 1995;48:269-280.
6. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38:613-616.
7. Abu-Arafeh IA, Russell G. Epidemiology of headache and migraine in children. Dev Med Child Neurol. 1993;35:370-371.
8. Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J. Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol. 1995;24:612-618.
9. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56:436-441.
10. Furman JM, Marcus DA, Balaban CD. Migrainous vertigo: development of a pathogenetic model and structured diagnostic interview. Curr Opin Neurol. 2003;16:5-13.
11. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain. 1984;107(Pt 4):1123-1142.
12. Kuritzky A, Ziegler DK, Hassanein R. Vertigo, motion sickness and migraine. Headache. 1981;21:227-231.
13. Olsson JE. Neurotologic findings in basilar migraine. Laryngoscope. 1991;101:1-41.
14. Cutrer FM, Baloh RW. Migraine-associated dizziness. Headache. 1992;32:300-304.
15. Bille BS. Migraine in school children. Acta Paediatr Scand (Upps). 1962;136:1-151.
16. Barabas G, Matthews WS, Ferrari M. Childhood migraine and motion sickness. Pediatrics. 1983;72:188-190.
17. Viirre ES, Baloh RW. Migraine as a cause of sudden hearing loss. Headache. 1996;36:24-28.
18. Bickerstaff ER. Basilar artery migraine. Lancet. 1961;1:15.
19. Lee CH, Lance JW. Migraine stupor. Headache. 1977;17:32-38.
20. Lees F, Watkins SM. Loss of consciousness in migraine. Lancet. 1963;2:647-649.
21. Harker LA, Rassekh CH. Episodic vertigo in basilar artery migraine. Otolaryngol Head Neck Surg. 1987;96:239-250.
22. Love JT. Basilar artery migraine presenting as fluctuating hearing loss and vertigo. Otolaryngol Head Neck Surg. 1978;86:450-458.
23. Whitty CWM. Migraine without headache. Lancet. 1967;2:283-285.
24. Slater R. Benign recurrent vertigo. J Neurol Neurosurg Psychiatry. 1979;42:363-367.
25. Harker LA, Rassekh CH. Migraine equivalent as a cause of vertigo. Laryngoscope. 1988;98:160-164.
26. Brown JK. Migraine and migraine equivalents in children. Dev Med Child Neurol. 1977;19:683-692.
27. Fisher CM. Late-life migraine accompaniments—further experience. Stroke. 1986;17:1033-1042.
28. Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117:199-210.
29. Schoenen J. Neurophysiological features of the migrainous brain. Neurol Sci. 2006;27(suppl 2):S77-S781.
30. Mulder EJ, Van Baal C, Gaist D, et al. Genetic and environmental influences on migraine: a twin study across six countries. Twin Res. 2003;6:422-431.
31. Thomsen LL, Olesen J. Sporadic hemiplegic migraine. Cephalalgia. 2004;24:1016-1023.
32. Aurora SK, Kori SH, Barrodale P, et al. Gastric stasis in migraine: more than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46:57-63.
33. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470.
34. Sinclair S. Migraine headaches: nutritional, botanical and other alternative approaches. Altern Med Rev. 1999;4:86-95.
35. Mauskop A, Altura BM. Role of magnesium in the pathogenesis and treatment of migraines. Clin Neurosci. 1998;5:24-27.
36. Peikert A, Wilimzig C, Kohne-Volland R. Prophylaxis of migraine with oral magnesium: results from a prospective, multi-center, placebo-controlled and double-blind randomized study. Cephalalgia. 1996;16:257-263.
37. Buchholz D, Reich SG. Heal Your Headache. New York: Workman Publishing; 2002.
38. Littlewood JT, Gibb C, Glover V, et al. Red wine as a cause of migraine. Lancet. 1988;1:558-559.
39. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol. 1997;18:350-354.
40. Perrin VL, Färkkilä M, Goasguen J, et al. Overview of initial clinical studies with intravenous and oral GR43175 in acute migraine. Cephalalgia. 1989;9(suppl 9):63-72.
41. Albers GW, Simon LT, Hamik A, et al. Nifedipine versus propranolol for the initial prophylaxis of migraine. Headache. 1989;29:215-218.
42. Colombo B, Annovazzi PO, Comi G. Therapy of primary headaches: the role of antidepressants. Neurol Sci. 2004;25(suppl 3):S171-S175.
43. Moja PL, Cusi C, Sterzi RR, et al. Selective serotonin re-uptake inhibitors (SSRIs) for preventing migraine and tension-type headaches. Cochrane Database Syst Rev. 2005;3:CD002919.
44. Staab JP, Ruckenstein MJ. Chronic dizziness and anxiety: effect of course of illness on treatment outcome. Arch Otolaryngol Head Neck Surg. 2005;131:675-679.
45. Mathew NT, Rapoport A, Saper J, et al. Efficacy of gabapentin in migraine prophylaxis. Headache. 2001;41:119-128.
46. Baloh RW, Foster CA, Yue Q, et al. Familial migraine with vertigo and essential tremor. Neurology. 1996;46:458-460.
47. Vahedi K, Taupin P, Djomby R, et al. Efficacy and tolerability of acetazolamide in migraine prophylaxis: a randomised placebo-controlled trial. J Neurol. 2002;249:206-211.
48. Uneri A. Migraine and benign paroxysmal positional vertigo: an outcome study of 476 patients. Ear Nose Throat J. 2004;83:814-815.
49. Paparella MM, Mancini F. Vestibular Meniere’s disease. Otolaryngol Head Neck Surg. 1985;93:148-151.
50. Minor LB, Schessel DA, Carey JP. Meniere’s disease. Curr Opin Neurol. 2004;17:9-16.
51. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology. 2002;59:1700-1704.
52. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol. 2007;127:1241-1245.
53. Basser LS. Benign paroxysmal vertigo of childhood (a variety of vestibular neuronitis). Brain. 1964;87:141-152.
54. Lanzi G, Balottin U, Fazzi E, et al. Benign paroxysmal vertigo of childhood: a long-term follow-up. Cephalalgia. 1994;14:458-460.
55. Snyder CH. Paroxysmal torticollis in infancy. A possible form of labyrinthitis. Am J Dis Child. 1969;117:458-460.
56. Lipson EH, Robertson WCJr. Paroxysmal torticollis of infancy: familial occurrence. Am J Dis Child. 1978;132:422-423.
57. Sanner G, Bergstrom B. Benign paroxysmal torticollis in infancy. Acta Paediatr Scand. 1979;68(2):219-223.
58. Savitz SI, Caplan LR. Vertebrobasilar disease. N Engl J Med. 2005;352:2618-2626.
59. Williams D, Wilson TG. The diagnosis of the major and minor syndromes of basilar insufficiency. Brain. 1962;85:741-774.
60. Caplan LR, Wityk RJ, Glass TA, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389-398.
61. Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986;17:648-655.
62. Chimowitz MI, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488-1493.
63. Fisher CM, Karnes WE, Kubik CS. Lateral medullary infarction—the pattern of vascular occlusion. J Neuropathol Exp Neurol. 1961;20:323-379.
64. Bjerver K, Silfverskiold BP. Lateropulsion and imbalance in Wallenberg’s syndrome. Acta Neurol Scand. 1968;44:91-100.
65. Oas JG, Baloh RW. Vertigo and the anterior inferior cerebellar artery syndrome. Neurology. 1992;42:2274-2279.
66. Duncan GW, Parker SW, Fisher CM. Acute cerebellar infarction in the PICA territory. Arch Neurol. 1975;32:364-368.
67. Salvati M, Cervoni L, Raco A, et al. Spontaneous cerebellar hemorrhage: clinical remarks on 50 cases. Surg Neurol. 2001;55:156-161.
68. Ott KH, Kase CS, Ojemann RG, et al. Cerebellar hemorrhage: diagnosis and treatment. A review of 56 cases. Arch Neurol. 1974;31:160-167.
69. Freeman RE, Onofrio BM, Okazaki H, et al. Spontaneous intracerebellar hemorrhage. Diagnosis and surgical treatment. Neurology. 1973;23:84-90.
70. Luparello V, Canavero S. Treatment of hypertensive cerebellar hemorrhage—surgical or conservative management? Neurosurgery. 1995;37:552-553.
71. Godefroy WP, Hastan D, van der Mey AG. Translabyrinthine surgery for disabling vertigo in vestibular schwannoma patients. Clin Otolaryngol. 2007;32:167-172.
72. Kentala E, Pyykko I. Vestibular schwannoma mimicking Meniere’s disease. Acta Otolaryngol Suppl. 2000;543:17-19.
73. Chee NW, Tong HM. Acoustic neuroma presenting as exercise-induced vertigo. J Laryngol Otol. 2002;116:630-632.
74. Grand W. Positional nystagmus: an early sign in medulloblastoma. Neurology. 1971;21:1157-1159.
75. Watson P, Barber HO, Deck J, et al. Positional vertigo and nystagmus of central origin. Can J Neurol Sci. 1981;8:133-137.
76. Abacioglu U, Uzel O, Sengoz M, et al. Medulloblastoma in adults: treatment results and prognostic factors. Int J Radiat Oncol Biol Phys. 2002;54:855-860.
77. Chen CC, Cheng PW, Tseng HM, et al. Posterior cranial fossa tumors in young adults. Laryngoscope. 2006;116:1678-1681.
78. Morreale VM, Ebersold MJ, Quast LM, et al. Cerebellar astrocytoma: experience with 54 cases surgically treated at the Mayo Clinic, Rochester, Minnesota, from 1978 to 1990. J Neurosurg. 1997;87:257-261.
79. Shoman N, Longridge N. Cerebellar vermis lesions and tumours of the fourth ventricle in patients with positional and positioning vertigo and nystagmus. J Laryngol Otol. 2007;121:166-169.
80. Gregorius FK, Crandall PH, Baloh RW. Positional vertigo with cerebellar astrocytoma. Surg Neurol. 1976;6:283-286.
81. Mergner T, Siebold C, Schweigart G, et al. Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res. 1991;85:389-404.
82. de Jong PT, de Jong JM, Cohen B, et al. Ataxia and nystagmus induced by injection of local anesthetics in the Neck. Ann Neurol. 1977;1:240-246.
83. Karlberg M, Persson L, Magnusson M. Impaired postural control in patients with cervico-brachial pain. Acta Otolaryngol Suppl. 1995;520(Pt 2):440-442.
84. Brandt T. Cervical vertigo—reality or fiction? Audiol Neurootol. 1996;1:187-196.
85. Brandt T, Bronstein AM. Cervical vertigo. J Neurol Neurosurg Psychiatry. 2001;71:8-12.
86. Menezes AH, VanGilder JC, Graf CJ, et al. Craniocervical abnormalities. A comprehensive surgical approach. J Neurosurg. 1980;53:444-455.
87. Biesinger E. Vertigo caused by disorders of the cervical vertebral column. Diagnosis and treatment. Adv Otorhinolaryngol. 1988;39:44-51.
88. Galm R, Rittmeister M, Schmitt E. Vertigo in patients with cervical spine dysfunction. Eur Spine J. 1998;7:55-58.
89. Agrawal D, Gowda NK, Bal CS, et al. Have cranio-vertebral junction anomalies been overlooked as a cause of vertebro-basilar insufficiency? Spine. 2006;31:846-850.
90. Schneider RC, Schemm GW. Vertebral artery insufficiency in acute and chronic spinal trauma, with special reference to the syndrome of acute central cervical spinal cord injury. J Neurosurg. 1961;18:348-360.
91. Noske DP, van Royen BJ, Bron JL, et al. Basilar impression in osteogenesis imperfecta: can it be treated with halo traction and posterior fusion? Acta Neurochir (Wien). 2006;148:1301-1305.
92. Chamberlain WE. Basilar impression (platybasia). Yale J Biol Med. 1939;11:847.
93. Klimo PJr, Rao G, Brockmeyer D. Congenital anomalies of the cervical spine. Neurosurg Clin N Am. 2007;18:463-478.
94. VanGilder JC, Menezes AH, Dolan KD. The Craniovertebral Junction and Its Abnormalities. New York: Futura Publishing; 1987.
95. Smoker WR. Craniovertebral junction: normal anatomy, craniometry, and congenital anomalies. Radiographics. 1994;14:255-277.
96. Taggard DA, Menezes AH, Ryken TC. Instability of the craniovertebral junction and treatment outcomes in patients with Down’s syndrome. Neurosurg Focus. 1999;6:e3.
97. Ferguson RL, Putney ME, Allen BLJr. Comparison of neurologic deficits with atlanto-dens intervals in patients with Down syndrome. J Spinal Disord. 1997;10:246-252.
98. Clarke MJ, Cohen-Gadol AA, Ebersold MJ, et al. Long-term incidence of subaxial cervical spine instability following cervical arthrodesis surgery in patients with rheumatoid arthritis. Surg Neurol. 2006;66:136-140.
99. Welinder NR, Hoffmann P, Hakansson S. Pathogenesis of non-traumatic atlanto-axial subluxation (Grisel’s syndrome). Eur Arch Otorhinolaryngol. 1997;254:251-254.
100. Weber PC, Cass SP. Neurotologic manifestations of Chiari 1 malformation. Otolaryngol Head Neck Surg. 1993;109:853-860.
101. Dyste GN, Menezes AH, VanGilder JC. Symptomatic Chiari malformations. An analysis of presentation, management, and long-term outcome. J Neurosurg. 1989;71:159-168.
102. Noffsinger D, Olsen WO, Carhart R, et al. Auditory and vestibular aberrations in multiple sclerosis. Acta Otolaryngol Suppl. 1972;303:1-63.
103. Furman JM, Durrant JD, Hirsch WL. Eighth nerve signs in a case of multiple sclerosis. Am J Otolaryngol. 1989;10:376-381.
104. Frohman EM, Kramer PD, Dewey RB, et al. Benign paroxysmal positioning vertigo in multiple sclerosis: diagnosis, pathophysiology and therapeutic techniques. Mult Scler. 2003;9:250-255.
105. Poser CM, Brinar VV. Diagnostic criteria for multiple sclerosis: an historical review. Clin Neurol Neurosurg. 2004;106:147-158.
106. Traboulsee AL, Li DK. The role of MRI in the diagnosis of multiple sclerosis. Adv Neurol. 2006;98:125-146.
107. Margolis RL. The spinocerebellar ataxias: order emerges from chaos. Curr Neurol Neurosci Rep. 2002;2:447-456.
108. Moschner C, Perlman S, Baloh RW. Comparison of oculomotor findings in the progressive ataxia syndromes. Brain. 1994;117(Pt 1):15-25.
109. Griggs RC, Moxley RT, LaFrance RA, et al. Hereditary paroxysmal ataxia: response to acetazolamide. Neurology. 1978;28:1259-1264.
110. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543-1554.
111. Smith BH. Vestibular disturbances in epilepsy. Neurology. 1960;10:465-469.
112. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(suppl 3):S4-S16.
113. Hain TC, Hanna PA, Rheinberger MA. Mal de débarquement. Arch Otolaryngol Head Neck Surg. 1999;125:615-620.