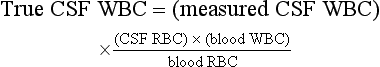Central Nervous System Infections
Perspective
Central nervous system (CNS) infections have always been among the most perplexing and devastating illnesses. “Epidemic cerebrospinal fever,” classically described by Vieusseux in 1805, was associated with almost universal mortality.1 The first American epidemic of meningococcal meningitis was recorded in 1806.2 Since that time, epidemiologic changes have occurred in concert with advances in understanding of disease processes and evolution of effective treatment strategies.
Likewise, diagnostic tools have been developed that allow precise pathogen identification, most recently by molecular technologies such as polymerase chain reaction (PCR) tests for viral nucleic acids in cerebrospinal fluid (CSF). The initial treatment methods began by demonstrating the efficacy of antiserum treatment by Flexner in 1913 and of antibiotics by Colebrook and Kenny in 1936.3,4 Mortality rates in CNS infections were decreased further with the use of high-dose penicillin by Dowling and colleagues in the 1940s.5 Unfortunately, despite historical advances, the morbidity and mortality of these disorders remain considerable.6 The use of pneumococcal, Haemophilus influenzae type b, and meningococcal vaccines has led to dramatic reductions in the incidence of meningitis caused by these bacteria.7–10
Definitions
This chapter focuses on the more common acute and subacute CNS infections. Infections of the nervous system with HIV or human T-lymphotropic virus, rabies virus, poliovirus, hepatitis viruses, Borrelia burgdorferi (Lyme disease), Treponema organisms (syphilis), parasites, or Rickettsia and the chronic and slow infections of the CNS (subacute sclerosing panencephalitis; progressive multifocal leukoencephalopathy; and the prion-mediated spongiform encephalopathies, such as Creutzfeldt-Jakob disease, bovine spongiform encephalopathy, and kuru) are not addressed in detail. Of note, the incidence of neurocysticercosis is on the rise in the United States.11
Epidemiology
Bacterial meningitis is a common disease worldwide. Meningococcal meningitis is endemic in parts of Africa, and epidemics commonly occur in other countries, including the United States. A variety of other pathogens are also causative.12–16 The overall incidence of bacterial meningitis in the United States is 5 to 10 cases per 100,000 people per year.17 Men are affected more often than women are.17 Approximately 80% of cases in the United States are caused by either Streptococcus pneumoniae or Neisseria meningitidis.18 In regions where vaccination is common, the epidemiology of bacterial meningitis has substantially changed.7–10,19 The incidence of bacterial meningitis increases in late winter and early spring, but the disease may occur at any time of the year.
Because most cases go unreported, the actual incidence of viral meningitis is unknown. It is estimated to affect between 11 and 27 individuals per 100,000 people.20 A prominent increase of cases is seen in summer months, which is concurrent with seasonal predominance of the enterovirus group of the picornaviruses.
The same organisms responsible for viral meningitis may also be associated with encephalitis. Encephalitis is far less common, however, and the ratio of cases of meningitis to encephalitis varies according to the specific pathogen. Arbovirus infection is transmitted by an insect vector, although clinical disease develops in only a small percentage of the people bitten. Before 1999, there were approximately 19,000 hospitalizations in the United States annually for cases of encephalitis. Since then, there has been a rapid increase because of emergence of West Nile virus (WNV). In 2003, more than 8000 additional hospitalizations were required because of WNV alone.21,22
Approximately 2000 cases of brain abscess occur in the United States annually.23 Although CNS abscesses may occur at any age and any time of year, they are seen more commonly in men than in women.24 CNS abscesses are associated with local contiguous and remote systemic infections, injection drug use, neurologic surgery, and cranial trauma. Brain abscess secondary to otitis media most often occurs in pediatric or older adult populations. When brain abscess is associated with sinusitis, it most often arises among young adults. Increasingly, CNS abscesses are seen in immunocompromised patients, particularly those with HIV infection, and among bone marrow and solid organ transplant recipients. However, antimicrobial prophylaxis of immunosuppressed patients and more aggressive treatment of otitis and sinusitis have decreased the overall incidence to 0.9 per 100,000 person-years.23
Principles of Disease
Meningitis
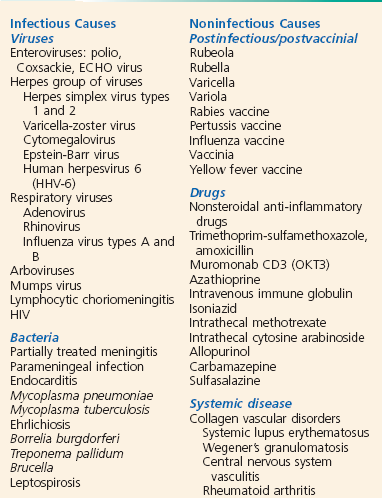
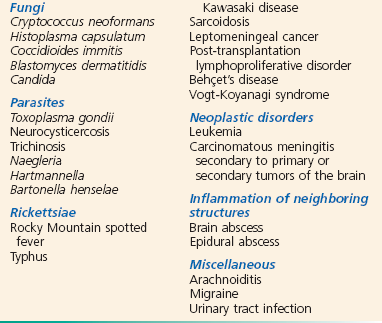
Meningeal inflammation may be caused by a variety of disease processes, but the infectious causes predominate. Among the bacterial agents, Streptococcus pneumoniae remains the predominant pathogen in adult patients, followed by Neisseria meningitidis and Listeria monocytogenes.25 N. meningitidis is the predominant organism in adults younger than 45 years. Five major serogroups cause most meningococcal disease worldwide (A, B, C, Y, and W-135). Serogroup A accounts for the majority of cases of meningococcal meningitis in developing nations.26 A new vaccine for serogroup A may potentially reduce the impact of this disease in nearly half a billion individuals at risk.27 Serogroup distribution for invasive disease has changed markedly in the United States, with B, C, and Y now most commonly responsible.28 These pathogens account for the bulk of cases in nontraumatic meningitis, although virtually any organism can be encountered, particularly among patients who are elderly, alcoholic, or immunosuppressed and those who have cancer. Interestingly, higher case fatality has been observed in N. meningitidis outbreaks versus sporadic cases, probably because of increased virulence of outbreak-related strains.29 Causes of aseptic meningitis, simply defined as all cases with CSF cultures negative for bacteria, are listed in Box 109-1.30
Viral meningitis may likewise be caused by a variety of etiologic agents.31 Enteroviruses are statistically encountered most commonly.32 Unfortunately, precise identification of the etiologic agent is often impossible. Fungal and parasitic meningitides are additional concerns, particularly among immunocompromised patients.14,15 Viral meningitis secondary to herpes simplex virus (HSV) infection is actually relatively benign, especially in comparison with HSV encephalitis.33
Encephalitis
Arboviruses and HSV, a human herpesvirus (HHV), are the most common causes of epidemic and sporadic cases of encephalitis, respectively. Children are the most vulnerable to infection with these viruses, although adults are also commonly affected. Epidemics of viral encephalitis have been attributed to a wide variety of viral agents. WNV, a flavivirus, first infected humans in the New York City area and rapidly spread to 47 states by 2003.15,34 Varicella, herpes zoster, HHV types 6 and 7, and Epstein-Barr virus have been increasingly reported to be the cause of encephalitis in immunocompetent hosts.35 Vaccinia encephalitis has been recognized in those receiving vaccination for smallpox.36 Postinfectious encephalomyelitis is also induced by a variety of viral pathogens, most commonly the measles virus.37 However, Mycoplasma pneumoniae and idiopathic causes are becoming more common in developed countries.
Central Nervous System Abscess
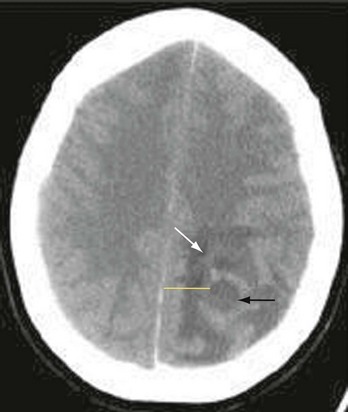
The causes of CNS abscess are multiple and reflect the primary infective process and the immune state of the human host. A variety of mixed pathogens may be responsible for intracranial abscesses. Streptococci, particularly the Streptococcus milleri group, have been identified in nearly 50% of brain abscesses.38 Anaerobic bacteria, predominantly Bacteroides species, are commonly seen when the primary infectious process is chronic otitis media or pulmonary disease. S. aureus and Propionibacterium are often identified, particularly after cranial penetration from surgery or trauma.39 The Enterobacteriaceae are an additional common isolate. Opportunistic fungal and parasitic agents, including Nocardia species, are often seen in the immunosuppressed.40 Culture of epidural and subdural abscesses more often yields a single organism; streptococci are most commonly seen in association with contiguous spread, and S. aureus and gram-negative rods are most commonly encountered after neurologic trauma (Fig. 109-1).15 Etiologic agents in spinal abscess are similarly varied, with S. aureus being the most commonly encountered.
Pathophysiology
The pathogenetic sequence in bacterial meningitis has been well characterized.14,15 The first step is nasopharyngeal colonization and mucosal invasion. Although colonization rates vary, virulent microbes use secretion of immunoglobulin A proteases and induce ciliostasis of mucosal cells. After penetration occurs by a variety of mechanisms, bacterial intravascular survival occurs because of evasion of the complement pathway. The varying capsular properties of each organism protect the bacteria. The third step occurs when the bacteria cross the blood-brain barrier to enter the CSF. The dural venous sinuses, cribriform plate area, and choroid plexus have all been implicated as potential sites of invasion. Although the mechanism of invasion is not completely understood, host defense mechanisms within the CSF are often ineffective; there are low levels of complement, immunoglobulin, and opsonic activity. Bacterial proliferation then occurs, which stimulates a convergence of leukocytes into the CSF.
Meningeal and subarachnoid space inflammation is also associated with the release of cytokines into the CSF, most notably tumor necrosis factor and interleukins 1 and 6.41 This results in increased permeability of the blood-brain barrier, cerebral vasculitis, edema, and increased intracranial pressure. A subsequent decrease in cerebral blood flow leads to cerebral hypoxia. Glucose transport into the CSF is decreased concomitantly with an increased use of glucose by the brain, bacteria, and leukocytes, which depresses CSF glucose concentrations. The increased permeability leads to increased CSF proteins.
Viral Meningitis and Encephalitis
Viruses enter the human host through the skin (i.e., insect vectors); through the respiratory, gastrointestinal, or urogenital tract; or by receipt of infected blood products or donor organs.42 Viral replication subsequently occurs outside the CNS, most often followed by hematogenous spread to the CNS. Additional routes into the CNS include retrograde transmission along neuronal axons and direct invasion of the subarachnoid space after infection of the olfactory submucosa.43,44
Fortunately, most systemic viral infections do not result in meningitis or encephalitis. The development and subsequent magnitude of viral infection depend on the virulence of the specific virus, the viral inoculum level, and the state of immunity of the human host. The tropism of the virus for specific CNS cell types also influences the focality of disease and its manifestations.43 Particular viruses may preferentially attack cortical, limbic, or spinal neurons, oligodendroglia, or ependymal cells. An example is the tropism of HSV for the temporal lobes and the development of temporal lobe seizures and behavioral changes in afflicted patients.
Central Nervous System Abscess
Intraparenchymal brain abscesses, subdural empyema, and intracranial or spinal epidural abscesses form by inoculation of the CNS from contiguous spread of organisms from a sinus, middle ear, or dental infection or metastatic seeding from a distant site, usually from pulmonary infection, endocarditis, or osteomyelitis.45 The primary infection can be identified in 75 to 85% of cases. These conditions may also follow surgery or penetrating cranial trauma, particularly when bone fragments are retained in brain tissue. Otogenic abscesses occur most commonly in the temporal lobe in adults and the cerebellum in children, whereas sinogenic abscesses typically occur in frontal areas.38 Multiple brain abscesses suggest hematogenous spread of organisms, although solitary lesions may also occur. The pulmonary system is the most common source of hematogenous spread.15
Clinical Features
Numerous host factors have been implicated in the acquisition of meningitis (Box 109-2).46 Although these factors alone and in combination increase the risk of meningitis, the disease often occurs in patients with none of these factors.
Many patients with meningitis present with advanced disease; in these patients, the diagnosis of acute meningitis is strongly suspected. The constellation of symptoms that may classically occur in an acute CNS infection consists of fever, headache, photophobia, nuchal rigidity, lethargy, malaise, altered sensorium, seizures, vomiting, and chills.13,46
Unfortunately, more subtle presentations are also common. Immunosuppressed and geriatric patients present a diagnostic challenge because the classic signs and symptoms of meningitis may not be present. Although some degree of fever is present in most patients, as are a headache and neck stiffness, meningitis should be carefully considered in any immunosuppressed patient with symptoms or signs of infectious disease. Often, the only presenting sign of meningitis in elders is an alteration of mental status. However, a meta-analysis suggested that the absence of fever, stiff neck, and mental status change excludes meningitis in immunocompetent adults.47 A systematic review of prospective data in children found several clinical factors that were useful in influencing the likelihood of bacterial meningitis within suspected cases.48 There was no combination of factors that could rule in or rule out the disease, which is not surprising given the diversity of presentations in children.
The presentation of fungal meningitis can be obscure even in the healthy adult population. Headache, low-grade fever, lassitude, and weight loss may be present but often to such a mild degree that the correct diagnosis is not initially considered.13 This is also true of tuberculous meningitis, which often has a protracted course and a vague nonspecific presentation consisting of fever, weight loss, night sweats, and malaise, with or without headache and meningismus.12
The physical findings in meningitis vary by the host, causative organism, and severity of the illness. Nuchal rigidity or discomfort on flexion of the neck is common. Kernig’s sign (the inability to straighten the leg to a position of full knee extension when the patient is lying supine with the hip flexed to a right angle) and Brudzinski’s sign (attempts to flex the neck passively are accompanied by flexion of the hips) are present in approximately 50% of adults.15 In the evaluation of patients with suspected meningitis, the sensitivity of Kernig’s sign, Brudzinski’s sign, and nuchal rigidity are 5%, 5%, and 30%, respectively, suggesting that these physical examination findings have little diagnostic value.49 Deep tendon reflexes may be increased, and ophthalmoplegia may be present, especially of the lateral rectus muscles.
The systemic findings may include an obvious source of infection, such as sinusitis, otitis media, mastoiditis, pneumonia, or urinary tract infection. Various manifestations of endocarditis may be present. Arthritis may be seen with N. meningitidis and occasionally with other bacteria.46 Petechiae and cutaneous hemorrhages are widely reported with meningococcemia but also occur with H. influenzae type b, pneumococcal organisms, L. monocytogenes, and ECHO virus infections in addition to staphylococcal endocarditis.46 Endotoxic shock with vascular collapse often develops in severe meningococcal disease, but shock may be present in the advanced stages of any bacterial meningitis. Any determination of a serious systemic infection should encourage rather than dissuade the clinician from considering the possibility of a concomitant CNS infection.
Patients with encephalitis may also have symptoms of meningeal irritation. An alteration of consciousness occurs in virtually all patients. Fever, headache, and a change of personality are also usually present.44 Hallucinations and bizarre behavior may precede motor, reflex, and other neurologic manifestations by several days, occasionally prompting an initial diagnosis of a psychiatric disorder. Because focal neurologic deficits and seizures occur much more commonly with encephalitis than with meningitis, there may also be diagnostic confusion with a brain abscess. It is difficult to distinguish the etiologic agent in encephalitis clinically, although HSV encephalitis results in a higher incidence of dysphasia and seizures.50 In some patients, WNV produces a myelitis that affects the anterior horn cells of the spinal column, resulting in a flaccid paralysis with a clear sensorium, similar to findings in poliomyelitis or Guillain-Barré syndrome.34
Patients with a subdural or epidural abscess most often have headache, fever, and focal signs, although more subtle presentations are common. Most of the patients with spinal abscess typically present with spinal pain and other symptoms and signs of cord compression but not necessarily with fever.51
Complications
The immediate complications of bacterial meningitis include coma (with loss of protective airway reflexes), seizures, cerebral edema, vasomotor collapse, disseminated intravascular coagulation, respiratory arrest, dehydration, syndrome of inappropriate secretion of antidiuretic hormone, pericardial effusion, and death (Box 109-3).16 Various delayed complications include multiple seizures, focal paralysis, subdural effusions, hydrocephalus, intellectual deficits, sensorineural hearing loss, ataxia, blindness, bilateral adrenal hemorrhage (Waterhouse-Friderichsen syndrome), peripheral gangrene, and death.46
The case fatality rate for pneumococcal meningitis averages 20 to 25%; higher fatality rates occur in patients with serious underlying or concomitant disease or advanced age.52 The prognosis is related to the degree of neurologic impairment on presentation. Overall, 20 to 30% of the survivors of pneumococcal meningitis have some residual neurologic deficit.46 The case fatality rate for Listeria meningitis may be as high as 40%.25
With the advent of antibiotic therapy, the mortality from meningococcal meningitis has markedly decreased to less than 20%, but it remains substantially higher in elders and those who have meningococcemia.52 Although most of the complications and sequelae are less common than with pneumococcal disease, the incidence of Waterhouse-Friderichsen syndrome is dramatically higher when meningococcemia is present.46 The overall mortality rate in community-acquired gram-negative meningitis has been less than 20% since the introduction of the third-generation cephalosporins.14 Hydrocephalus may develop in as many as 5% of patients with community-acquired meningitis; when this is present on admission, the proportion dead or with an unfavorable outcome approaches 50 to 70%.53 A delayed cerebral venous thrombosis was observed in several adults with an initially excellent recovery from pneumococcal meningitis, suggesting an immunologic vasculopathy.54
Viral Meningitis
With rare exceptions, the overall prognosis for complete recovery from viral meningitis is excellent. Various complications related to the systemic effects of the particular virus include orchitis, parotitis, pancreatitis, and various dermatoses. Usually, all of these complications resolve without sequelae.31 Interestingly, HSV meningitis is often associated with the initial outbreak of genital herpes.33
Viral Encephalitis
The outcomes in viral encephalitis are dependent on the infecting agent. Encephalitis caused by Japanese encephalitis virus, Eastern equine virus, and St. Louis encephalitis virus is severe, with high mortality rates and virtually universal neurologic sequelae among survivors.55 WNV produces encephalitis in only 0.5% of those infected, yet it resulted in 120 deaths in 2003.22 Western equine virus and California encephalitis virus cause milder infections, and death is rare. The incidence of neurologic sequelae is highly variable and appears to depend on both the host and the infecting agent.56 Reports have emerged of influenza A H1N1 encephalitis that bears striking resemblance to “encephalitis lethargica,” reported as a complication of influenza-like illnesses in the 1920s.57
The mortality from HSV encephalitis before the use of acyclovir was 60 to 70%. Acyclovir treatment has reduced the mortality to approximately 30%.37 Common complications observed among survivors include seizure disorders, motor deficits, and changes in mentation.
Tuberculous Meningitis
Death from tuberculous meningitis in the adult age group ranges from 10 to 50% of cases, with the incidence directly proportional to the patient’s age and the duration of symptoms before presentation. Focal ischemic stroke may result from the associated cerebral vasculitis. In advanced disease, up to 25% of patients may require some neurosurgical procedure for obstruction (ventriculoperitoneal shunt or drainage).58 Some neurologic deficit develops in most patients, but severe long-term sequelae among survivors are unusual.12
Fungal Meningitis
Common CNS complications with fungal meningitis include abscesses, papilledema, neurologic deficits, seizures, bone invasion, and fluid collections. Direct invasion of the optic nerve results in ocular abnormalities in up to 40% of patients with cryptococcal meningitis.13 The mortality rate is high but variable and is related to the timeliness of diagnosis, underlying illness, and therapeutic regimens.
Central Nervous System Abscess
With the early diagnosis afforded by the use of cranial computed tomography (CT), appropriate antimicrobial therapy, and combined management approaches with surgery, aspiration, and medical therapy, the mortality rate from brain abscess has declined dramatically from approximately 50% to less than 20%.45,59 A seizure disorder is the most common complication of an intracranial abscess, occurring in 80% of patients.14 Other neurologic findings of intracranial abscesses, including focal motor or sensory deficits and changes in mentation, are common. Complications of spinal abscess primarily result from cord compression, including paralysis, motor and sensory deficits, and bowel and bladder dysfunction. Generalized spread of CNS infection and death may also occur.51
Diagnostic Strategies
General Considerations
The possibility of the diagnosis of meningitis mandates lumbar puncture (LP) unless the procedure is contraindicated by the presence of infection in the skin or soft tissues at the puncture site or the likelihood of brain herniation.37 Adherence to this principle prevents a delay in diagnosis, which substantially increases the morbidity and mortality of the disease. Some patients have clinically obvious bacterial meningitis, and CSF examination serves primarily to help identify the organism, thereby facilitating the appropriate treatment. Most patients, however, present more of a diagnostic problem, and analysis of the CSF constitutes the critical step in confirming the presence of CNS infection.
Increased Intracranial Pressure
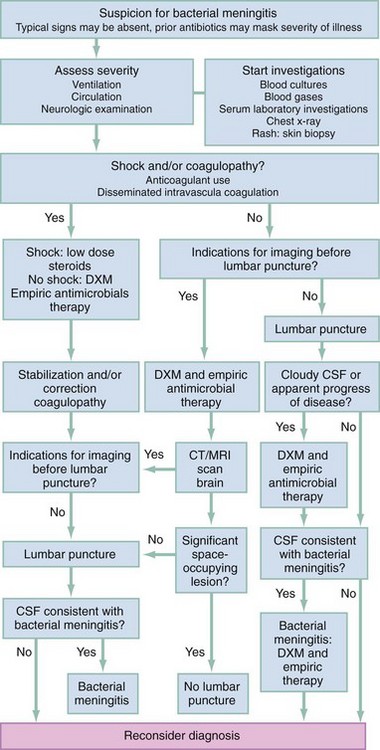
In most patients with bacterial meningitis, LP may be safely performed without antecedent neuroimaging studies. As this may not be the case in other brain diseases, in many circumstances it is advisable to obtain a CT scan of the head before LP is performed.60 An algorithm for diagnostic and therapeutic decision-making is presented in Figure 109-2.61
It has been conventionally asserted that LP in the presence of increased intracranial pressure may be harmful or fatal to the patient. Although data to address this concern are limited, the presence of focal neurologic signs does appear to be associated with a dramatic increase in complications associated with LP. These patients may deteriorate precipitously during or after the procedure.62–65
Patients with a markedly depressed sensorium that precludes careful neurologic examination or those with a focal neurologic deficit, papilledema, seizures, or evidence of head trauma must be considered to be at risk for a herniation syndrome that may be exacerbated by LP. If the presentation is an acute, fulminating, febrile illness and bacterial meningitis is the concerning diagnosis, early initiation of antimicrobial therapy is mandatory because of the association of prognosis and time to treatment.66 The algorithmic alternatives are therefore (1) immediate LP followed by initiation of antibiotic treatment before the results are obtained and (2) initiation of antibiotic treatment followed by a cranial CT scan and then LP. The latter choice of empirical treatment with antibiotics is now the routine in many institutions, although in some cases a third option could be considered: antibiotics and no LP despite an unremarkable CT scan.65 This reflects the efficacy of current methods of identification of causative organisms in CSF by means other than bacteriologic cultures that are less likely to be affected by antibiotic administration. The controversy emerging about not performing LP despite a lack of CT findings is based on some reviews and case reports. These describe a fulminant herniation syndrome temporally related to LP in patients with normal CT scans.63 Raised intracranial pressure may not be reliably detected by CT. Clinical signs of increased intracranial pressure, rapid change in consciousness, and recent seizures were identified as risk factors predicting deterioration despite a normal CT scan.65 The risks of ongoing empirical treatment with antibiotics without additional information from CSF analysis appears to be low as the yield from blood cultures and other diagnostic techniques such as PCR is relatively high. Therefore, this risk may be less than the risks of performing LP in certain very high-risk patients. In patients who have rapid neurologic deterioration, seizures, or signs of herniation, antibiotics should be given, a CT scan ordered, and the patient moved to intensive care. In this situation, LP may be deferred.
Cerebrospinal Fluid Analysis
The normal CSF pressure in an adult varies from 5 to 20 cm H2O. This value applies only to patients in the lateral recumbent position and may increase substantially when the patient is in the sitting position. The pressure is often elevated in bacterial, tuberculous, and fungal meningitides and a variety of noninfectious processes.44 Pressure may be falsely elevated when the patient is tense or obese or has marked muscle contraction.
Collection of Fluid
At least three sterile tubes each containing at least 1 to 1.5 mL of CSF are obtained and numbered in sequence. A fourth tube may be desirable should later studies, such as viral cultures or a Venereal Disease Research Laboratory (VDRL) test for syphilis, become necessary. The fluid is sent to the laboratory for immediate analysis of turbidity, xanthochromia, glucose, protein, cell count and differential, Gram’s stain, bacterial culture, and antigen testing (Table 109-1). In certain cases an India ink stain, a bacteriologic stain for acid-fast bacilli, or a VDRL test should be obtained. When only a small amount of fluid can be obtained, the most important studies are the cell count with differential, Gram’s stain, and bacterial cultures. Ideally, the cell count should be performed on both the first and third or fourth tubes to help differentiate true CSF pleocytosis from contamination of the specimen by a traumatic LP.
Table 109-1
Analysis of Cerebrospinal Fluid
| TEST | NORMAL VALUE | SIGNIFICANCE OF ABNORMALITY |
| Cell count | ≤5 WBC/mm3 ≤1 PMN/mm3 ≤1 eosinophil/mm3 |
Increased WBC counts are seen in all types of meningitis and encephalitis; increased PMN count suggests bacterial pathogen |
| Gram’s stain | No organism | Offending organism identified 80% of the time in bacterial meningitis, 60% if patient has been pretreated |
| Turbidity | Clear | Increased turbidity with leukocytosis, blood, or high concentration of microorganisms |
| Xanthochromia | None | Presence of RBCs in spinal fluid for 4 hr before lumbar puncture; occasionally caused by traumatic tap (if protein ≥150 mg/dL) or hypercarotenemia |
| CSF-to-serum glucose ratio | 0.6 : 1 | Depressed in pyogenic meningitis or hyperglycemia; lag time if glucose given intravenously |
| Protein | 15-45 mg/dL | Elevated with acute bacterial or fungal meningitis; also elevated with vasculitis, syphilis, encephalitis, neoplasms, and demyelination syndromes |
| India ink stain | Negative | Positive in one third of cases of cryptococcal meningitis |
| Cryptococcal antigen | Negative | 90% accuracy for cryptococcal disease |
| Lactic acid | ≤35 mg/dL | Elevated in bacterial and tubercular meningitis |
| Bacterial antigen tests | Negative | ≥95% specific for organism tested; up to 50% false-negative rate |
| Acid-fast stain | Negative | Positive in 80% of cases of tuberculous meningitis if ≥10 mL of fluid |
Turbidity
The CSF is assessed immediately for turbidity or cloudiness by the person performing the LP. Because normal CSF is completely clear and colorless and is indistinguishable from water, any degree of turbidity is pathologic. Leukocytosis is the most common cause of CSF turbidity; counts greater than 200 cells/mm3 usually cause clinically detectable changes in CSF clarity.67
Cell Count and Differential
Normal adult CSF contains no more than 5 leukocytes/mm3 with at most one granulocyte (polymorphonuclear [PMN] leukocyte)46,67,68; therefore, the presence of more than one PMN or a total cell count of more than 5 cells/mm3 should be considered evidence of CNS infection. In addition, the presence of any eosinophil in the CSF is abnormal, although basophils may occasionally be seen in the absence of disease.67 Pretreatment with a few doses of antibiotics, although possibly diminishing the yield of Gram’s staining and cultures, should not affect the CSF cell counts in meningitis.14,69,70
The cell counts in bacterial meningitis are usually markedly elevated, sometimes exceeding 10,000 cells/mm3, and demonstrate a dramatic granulocytic shift.46 In general, counts exceed 500 cells/mm3, with a preponderance of PMN leukocytes. However, the initial CSF analysis exhibits lymphocytosis (lymphocyte count greater than 50%) in 6 to 13% of all cases of bacterial meningitis. When only the patients with bacterial meningitis with fewer than 1000 cells/mm3 are considered, 24 to 32% have a predominance of lymphocytes.71,72 In addition, the same population of patients often has only a mild disturbance of CSF glucose and protein levels. In well-established viral meningitis and encephalitis, counts are usually less than 500 cells/mm3, with nearly 100% of the cells being mononuclear.32 Early (48 hours) presentations may reveal significant PMN pleocytosis and hence be indistinguishable from presentations in early bacterial meningitis.73
Similarly, normal cell counts and differentials, although reassuring, do not absolutely exclude bacterial meningitis.68 Any patient thought to have a clinical syndrome compatible with meningitis requires hospital admission with frequent reevaluation, repeated LP, and antimicrobial therapy. In some patients who have symptoms or signs of meningitis and have a normal initial CSF analysis, CSF pleocytosis may develop within 24 hours; the causative organism may be cultured from the original “normal” CSF.
A traumatic LP is suggested by the presence of a clot in one of the tubes or the clearing of the CSF and a decreasing red blood cell (RBC) count from tubes one to three. In the presence of a traumatic LP, one may estimate the true degree of CSF white blood cell (WBC) pleocytosis with the following formula67:
Gram’s Stain
A properly performed Gram’s stain of a centrifuged specimen of CSF identifies the causative organism approximately 80% of the time in cases of bacterial meningitis.70 Gram’s stain characteristics of the most commonly encountered organisms are described in Table 109-2. The yield from this procedure is diminished by 20 to 30% when there has been prior treatment with antibiotics.14 Misidentification of gram-positive organisms as gram negative is also known to occur more commonly among pretreated patients because organisms with damaged walls stain unpredictably.
Table 109-2
Gram’s Stain Characteristics of Selected Meningeal Pathogens
| PATHOGEN | TYPICAL CHARACTERISTICS |
| Staphylococci | Gram-positive cocci: singles, doubles, tetrads, clusters |
| Streptococcus pneumoniae | Gram-positive cocci: paired diplococci |
| Other streptococci | Gram-positive cocci: pairs and chains |
| Listeria monocytogenes | Gram-positive rods: single or chains |
| Neisseria meningitidis | Gram-negative cocci: negative paired diplococci; kidney or coffee bean appearance |
| Haemophilus influenzae | Gram-negative coccobacilli: “pleomorphic” bacilli |
| Enterobacteriaceae (including Escherichia coli) | Gram-negative rods |
| Pseudomonas aeruginosa | Gram-negative rods |
Xanthochromia
Xanthochromia refers to the yellowish discoloration of the supernatant of a centrifuged CSF specimen. Xanthochromia is abnormal and results from the lysis of RBCs and release of the breakdown pigments oxyhemoglobin, bilirubin, and methemoglobin into the CSF. This process normally begins within 2 hours, and pigments may persist up to 30 days; therefore, early analysis of the LP specimen is essential. If a traumatic tap has introduced enough plasma to raise the CSF protein level to 150 mg/dL or more, blood pigments may cause xanthochromia. If the CSF protein level is less than 150 mg/dL, however, and systemic hypercarotenemia does not exist, xanthochromia of a centrifuged CSF specimen should suggest that subarachnoid hemorrhage has occurred.67
Glucose
When the serum glucose concentration is normal, the CSF glucose concentration is usually between 50 and 80 mg/dL. The CSF glucose concentration is normally in a ratio of 0.6 : 1 to the serum glucose concentration, except with marked systemic hyperglycemia, when the ratio is closer to 0.4 : 1. Therefore, a CSF-to-serum glucose ratio of less than 0.5 in normoglycemic subjects or 0.3 in hyperglycemic subjects is abnormal and may represent the impaired glucose transport mechanisms and increased CNS glucose use associated with pyogenic meningitis.46,67 Mild decreases in the CSF glucose level may occur with certain viral and parameningeal processes. However, bacterial or fungal meningitis should be presumed to be the cause of low CSF glucose concentration, termed hypoglycorrhachia, until each is clearly excluded.74 If the serum glucose level has increased rapidly (e.g., after intravenous administration of 50% dextrose in water), equilibration in the CSF may take up to 4 hours, and therefore the interpretation of CSF-to-serum glucose ratios may be unreliable.
Protein
The normal CSF protein level in adults ranges from 15 to 45 mg/dL. An elevated CSF protein level, usually higher than 150 mg/dL, commonly occurs with acute bacterial meningitis.46 When a traumatic LP has occurred, the CSF protein level can be corrected for the presence of blood by subtracting 1 mg/dL of protein for each 1000 RBCs.67 Elevated CSF protein concentrations can result from any cause of meningitis, subarachnoid hemorrhage, CNS vasculitis, syphilis, viral encephalitis, neoplasms, and demyelination syndromes.67 A greatly elevated CSF protein level (>1000 mg/dL) in the presence of a relatively benign clinical presentation should suggest fungal disease.13
India Ink Preparation
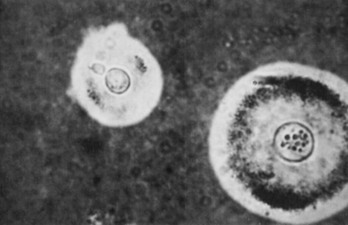
India ink staining of the CSF should be performed when a diagnosis of cryptococcal meningitis is being considered. The demonstration of budding organisms (Fig. 109-3) is virtually diagnostic for cryptococcal disease but occurs in only one third of the cases.13 A more definitive diagnostic test is the cryptococcal antigen, which appears to have a similar sensitivity when it is measured in serum, CSF, or urine.75
Lactic Acid
Although nonspecific, elevations in CSF lactic acid concentrations (>35 mg/dL) are potentially indicative of bacterial meningitis, and lactate may rise before the decline in glucose.76,77 Normal lactate levels (<35 mg/dL) are usually seen in patients with viral meningitides. A systematic review suggested that with respect to efficacy, CSF lactate outperforms other commonly used laboratory findings (glucose, protein, leukocyte count).78 The authors did observe that the CSF lactate level is less helpful if it is low but that higher values are highly associated with bacterial meningitis.
Antigen Detection
Antigen and antibody testing is also being used to identify viral and atypical pathogens. These tests have particular utility in HSV encephalitis. Enzyme-linked immunosorbent assays can detect HSV antibody production.79 Unfortunately, the appearance of antibody in CSF occurs too late to aid in any therapeutic decision analysis. PCR amplification and the identification of HSV DNA have demonstrated a sensitivity of 95 to 100% and a specificity of 100% early in the disease and have markedly decreased the need for diagnostic brain biopsy in this disorder.80 PCR has improved the diagnosis of tuberculous meningitis, with a sensitivity of 80 to 85% and a specificity of 97 to 100%, and is superior to standard techniques.81,82 PCR has additionally been shown to be superior in identifying bacteria, enteroviruses, and other viral etiologic agents in both immunocompromised and immunocompetent patients.83,84 Reported sensitivities of detection in CSF by PCR for N. meningitidis, H. influenzae, and S. pneumoniae are 88%, 100%, and 92%, with nearly 100% specificity.85,86 The sensitivities of bacteriologic culture are much lower, especially for N. meningitidis at 37 to 55% and H. influenzae at 50%.87,88
In addition, PCR assays have nearly tripled the yield of viral culture in identifying the etiologic agent.89 In studies of enteroviral meningitis, sensitivities and specificities for PCR ranged from 86 to 100% and 92 to 100%, respectively.90 PCR has been shown to be at least as sensitive as culture technique in detecting cryptococcal meningitis. Quantitative PCR may be of benefit in monitoring response to therapy in some forms of severe disease.35
The growing availability of these molecular techniques does not, however, suggest that they should be routinely used as initial diagnostic tests. Most cases of acute bacterial meningitis are readily diagnosed and treated on the basis of the standard Gram’s stain and culture. PCR should be reserved for less clear presentations, patients pretreated with antibiotics, and cases in which concern exists for tuberculous, cryptococcal, and treatable viral CNS infections.91 A reasonable approach is to save the CSF and to consider ordering PCR when acute infection is strongly suspected on the basis of the initial results from the cell count and Gram’s stain.
Neuroimaging Techniques
Cranial CT or magnetic resonance imaging (MRI) is indicated in the evaluation of any patient with presumed CNS infection in whom there is the possibility of an intracranial abscess, intracranial hemorrhage, or mass lesion. In the diagnostic evaluation of acute meningitis, however, a CT scan should not unnecessarily delay LP or antimicrobial therapy. The CT scan may also show hypodense lesions in the temporal lobes in patients with HSV encephalitis, although MRI reveals this abnormality much earlier in the disease process. A contrast-enhanced cranial CT scan or MRI scan is invaluable in the diagnosis of a CNS abscess.45 MRI is also helpful in the evaluation of other infectious and noninfectious encephalitides.
Additional Investigations
As with other infectious diseases, the complete blood count with differential is a nonspecific adjunct in the diagnostic evaluation of a patient thought to have a CNS infection. The peripheral cell counts are often normal in the presence of significant disease and may even be depressed, particularly in elders or immunosuppressed persons. A “normal” leukocyte count and differential should not dissuade one from performing a diagnostic LP, obtaining a CT scan, or otherwise pursuing the diagnosis of a CNS infection. Serum C-reactive protein is nonspecific, but a negative test result is potentially helpful.92 Procalcitonin is emerging as a promising serum marker in infectious disease; however, there is not convincing evidence at this point to advocate its use to attempt to rule out bacterial meningitis.93
As many as 50% of patients with pneumococcal meningitis also have evidence of pneumonia on an initial chest radiographic study. This association occurs in less than 10% of the cases of meningitis caused by H. influenzae type b and N. meningitidis and in approximately 20% of cases of meningitis caused by other organisms. The identification of a pulmonary infection on chest radiography may assist in identification of causative organisms and appropriate antimicrobial therapy in approximately 10% of cases of brain abscess.45
A number of characteristic but not pathognomonic electroencephalographic abnormalities have been associated with HSV type 1 encephalitis. Focal or lateralized electroencephalographic abnormalities in the presence of an encephalitis syndrome should be considered strong evidence supporting a diagnosis of HSV encephalitis.94
Differential Considerations
Acute meningitis encompasses patients with obvious signs and symptoms of meningitis who are evaluated in less than 24 hours after the onset of their symptoms and who rapidly deteriorate. In many of these patients the diagnosis of meningitis is not in doubt, and the crucial step is to initiate antimicrobial therapy immediately. The most likely pathogens in this syndrome are S. pneumoniae and N. meningitidis. Although H. influenzae has been reported in this context, it is not commonly implicated in the adult population.16,69
In the syndrome of subacute meningitis, the symptoms and signs causing the patient to seek care have developed during a period of 1 to 7 days. This syndrome includes virtually all cases of viral meningitis along with most of the bacterial and some of the fungal causes.14,15 The differential diagnosis depends on the symptoms and signs at presentation. Among elders and immunosuppressed individuals, a change in the patient’s mental status may be the only presenting sign in meningitis. Even when a fever is present, the patient’s change in mental status may be misattributed to another disease outside the CNS, such as pneumonia or urinary tract infection; neck stiffness may be misattributed to degenerative joint disease. Elders are at high risk for meningitis, and rather than constituting a diagnostic endpoint, the identification of an infection outside the CNS in elders is a clear indication for LP because of the risk of bacteremic seeding by the involved organisms.
The spectrum of chronic meningitis includes some of the viral meningitides as well as meningitis caused by tubercle bacilli, syphilis, and fungi. Many of the patients in this group have had symptoms for at least 1 week before presentation and generally have a prolonged indolent course marked by difficult and changing diagnoses and multiple therapies.12,13 Prediction rules have been both derived and validated and have not yet diffused into widespread practice, probably because of limitations in the models, shifts in the epidemiology of the causative organisms, and relatively small sample sizes.95
Management
Hypotension or shock is treated with isotonic crystalloid infusion, high-flow oxygen, and pressors. Intravenous administration of dextrose may be required for hypoglycemia secondary to depletion of glycogen stores. Alcoholic or nutritionally compromised patients should also receive 50 to 100 mg of thiamine intravenously (IV). In cases of moderate to severe hypotension, central venous pressure monitoring should be initiated on the basis of sepsis guidelines and used to determine additional intravenous fluids or vasopressors. Concern has been raised about brain edema with large amounts of crystalloid fluid in patients with meningitis. However, in children, once fluid volume and vital signs are normalized through resuscitation, there does not appear to be evidence to restrict fluids, and appropriate maintenance fluids should be instituted.96
Definitive Therapy
Until the pathogenetic organism is identified, broad-spectrum coverage of the most common pathogens is necessary (Table 109-3). Many authorities recommend cefotaxime or ceftriaxone, plus vancomycin to cover potentially resistant organisms.97 High-dose ampicillin is also added if concern exists about Listeria.97 In patients allergic to penicillin and cephalosporins, meropenem or chloramphenicol plus vancomycin may be effective while the outcome of desensitization techniques is awaited.97
Table 109-3
| PREDISPOSING FACTOR | COMMON BACTERIAL PATHOGENS | ANTIMICROBIAL THERAPY |
| Age | ||
| <1 month | Streptococcus agalactiae, Escherichia coli, Listeria monocytogenes, Klebsiella species | Ampicillin plus cefotaxime or ampicillin plus an aminoglycoside |
| 1-23 months | Streptococcus pneumoniae, Neisseria meningitidis, S. agalactiae, Haemophilus influenzae, E. coli | Vancomycin plus a third-generation cephalosporin*,† |
| 2-50 years | N. meningitidis, S. pneumoniae | Vancomycin plus a third-generation cephalosporin*,† |
| >50 years | S. pneumoniae, N. meningitidis, L. monocytogenes, aerobic gram-negative bacilli | Vancomycin plus ampicillin plus a third-generation cephalosporin*,† |
| Head trauma | ||
| Basilar skull fracture | S. pneumoniae, H. influenzae, group A β-hemolytic streptococci | Vancomycin plus a third-generation cephalosporin*,† |
| Penetrating trauma | Staphylococcus aureus, coagulase-negative staphylococci (especially Staphylococcus epidermidis), aerobic gram-negative bacilli (including Pseudomonas aeruginosa) | Vancomycin plus cefepime, vancomycin plus ceftazidime, or vancomycin plus meropenem |
| Postneurosurgery | Aerobic gram-negative bacilli (including P. aeruginosa), S. aureus, coagulase-negative staphylococci (especially S. epidermidis) | Vancomycin plus cefepime, vancomycin plus ceftazidime, or vancomycin plus meropenem |
| CSF shunt | Coagulase-negative staphylococci (especially S. epidermidis), S. aureus, aerobic gram-negative bacilli (including P. aeruginosa), Propionibacterium acnes | Vancomycin plus cefepime,‡ vancomycin plus ceftazidime,‡ or vancomycin plus meropenem‡ |
†Some experts would add rifampin if dexamethasone is also given.
‡In infants and children, vancomycin alone is reasonable unless Gram stains reveal the presence of gram-negative bacilli.
From Tunkel AR, et al: Practice guidelines for bacterial meningitis. Clin Infect Dis 39:1267-1284, 2004.
After the pathogen is identified, more targeted therapy can be instituted. It is prudent to refer to a current antimicrobial reference to guide therapy in all instances, given rapid changes in etiologic spectrum, drug resistance, and available agents. Duration of treatment varies, and in certain situations (namely, epidemics in sub-Saharan African), long-acting chloramphenicol or ceftriaxone is effective.98
Corticosteroid treatment is additionally recommended in adult acute bacterial meningitis, although the evidence of efficacy is becoming less compelling over time. Animal studies demonstrate the salutary effects of the administration of corticosteroids in experimental pneumococcal meningitis, including reduced brain edema, CSF pressure, and CSF lactate levels.99 Earlier resolution of the clinical and CSF stigmata of meningitis and a decrease in long-term hearing loss are observed in infants and children given dexamethasone with cefuroxime or ceftriaxone compared with those receiving the antibiotic alone, particularly when H. influenzae is the offending agent.100 In adult bacterial meningitis, an absolute risk reduction of 10% for unfavorable outcome is seen when dexamethasone is given either 15 minutes before or concomitantly with antibiotics and continued for 4 days at 6-hour intervals.101 This benefit is greatest in those with S. pneumoniae. Subgroup analyses for different causative organisms did not establish a benefit; however, the study was not designed with adequate power to detect improved outcomes. In addition, amoxicillin and penicillin were the most commonly used initial therapy at the time of that study because of the process of health care delivery in Europe, in which nearly all patients were seen initially in an office-based practice. A randomized controlled trial did not demonstrate a benefit of adjuvant dexamethasone in adult patients, even when only those with S. pneumoniae were included in a secondary analysis.102 In the current era of initial empirical parenteral therapy, rising β-lactam resistance, the possibility of the decreased CSF penetration of vancomycin after dexamethasone treatment, and shifts in the likely causative organisms secondary to vaccination campaigns, the true effectiveness of dexamethasone is unclear. The most recent meta-analysis suggests a benefit for patients in the developed (but not developing) world, but the included studies suffer from one or more of the aforementioned limitations.103 A pooled analysis of patient-level data was less conclusive, suggesting that the benefit of adjunctive dexamethasone for any “subgroup of patients with bacterial meningitis remains unproven.”104 Despite uncertainty from conflicting trials, an initial dose of dexamethasone 10 mg IV is recommended before or concurrent with empirical antibiotics in patients with suspected community-acquired meningitis and without signs of septic shock. Given the potential adverse effects of high-dose corticosteroids in patients with septic shock, the use of low-dose hydrocortisone at 50 mg IV instead of high-dose dexamethasone is a reasonable approach, although it has not been proved in randomized controlled trials of patients with both septic shock and meningitis.105,106
In pediatric meningitis, the evidence that adjunctive dexamethasone is helpful is less compelling. Invasive H. influenzae type b and pneumococcal infections have drastically been reduced by vaccination.19 A randomized trial of dexamethasone in childhood meningitis in sub-Saharan Africa did not demonstrate a benefit.107 A retrospective analysis from U.S. administrative data did not demonstrate any mortality benefit to adjunctive dexamethasone.108 Current recommendations are organism specific, which presents a major limitation as recommendations are to begin empirical therapy before laboratory results in suspicious cases. For H. influenzae type b (Hib): “dexamethasone may be beneficial for treatment of infants and children with Hib meningitis to diminish the risk of neurological sequelae, including hearing loss, if given before or concurrently with the first dose of antimicrobial agent(s). There probably is no benefit if dexamethasone is given more than 1 hour after antimicrobial agent(s).”109 For S. pneumoniae: “for infants and children 6 weeks of age and older, adjunctive therapy with dexamethasone may be considered after weighing the potential benefits and possible risks. Experts do not agree on a recommendation to use corticosteroids in pneumococcal meningitis; data are not sufficient to demonstrate a clear benefit in children. If used, dexamethasone should be given before or concurrently with the first dose of the antimicrobial agent.”110 The implication for frontline physicians caring for children is that unless the causative organism is known before antibiotic treatment, there is probably little role for adjunctive dexamethasone in children.
Viral Meningitis
No specific agents are available to treat most types of viral meningitis. Investigational agents in development may reduce symptoms in enterovirus meningitis111; however, with the exception of HSV meningitis, the viral meningitides contracted in the United States are generally characterized by a short, benign, self-limited course followed by a complete recovery. The primary therapeutic consideration in cases of viral meningitis is therefore the validity of the diagnosis. Early cases of viral meningitis may be indistinguishable from bacterial meningitis, and this confusion may not be resolved by CSF analysis; therefore, when any doubt exists about the veracity of the diagnosis, appropriate culture specimens should be obtained and the patient admitted to the hospital. Antimicrobial therapy for presumed bacterial meningitis may be initiated on the basis of the clinical presentation or may be withheld pending the outcome of close clinical observation and repeated LP in 8 to 12 hours.
Viral Encephalitis
Specific therapy for meningoencephalitis from HHV is available. Acyclovir remains the current choice and is capable of substantially improving the patient’s outcome. When the diagnosis of herpes meningoencephalitis is suspected or established, acyclovir should be administered in a dose of 10 mg/kg IV every 8 hours.81 Ganciclovir, foscarnet, and cidofovir are also effective in HHV infections, and pleconaril has been effective in enteroviral disease. Additional antiviral treatments are in development.34,35,111
Tuberculous Meningitis
Early chemotherapeutic intervention in acute tuberculous meningitis improves the patient’s prognosis. A strong clinical suggestion of this disease is an adequate indication to begin antituberculous therapy. A standard treatment regimen consists of isoniazid, rifampin, pyrazinamide, and ethambutol or streptomycin.97 Corticosteroids have also been shown to decrease secondary complications.97
Fungal Meningitis
The treatment of fungal meningitis is complex.13 Four agents are commonly used: amphotericin B, flucytosine, miconazole, and fluconazole. Of these, amphotericin B, either alone or in combination with flucytosine, is the most commonly recommended initial therapeutic regimen.97 These diseases are rarely acutely life-threatening but rather are slowly progressive. Prolonged therapy, often with multiple agents, is necessary. The initiation of antifungal therapy is rarely indicated in the emergency department.
Central Nervous System Abscess
The treatment of cerebral abscess is complex, and neurosurgical consultation is indicated. The location, size, and number of abscesses influence the choice of medical management, surgical excision, aspiration, or a combination of these modalities.38 In general, small multiple abscesses are more appropriately treated medically, whereas large, surgically accessible lesions should be excised. Empirical antimicrobial therapy before identification of specific organisms by aspiration or surgical excision should be guided by the principles of CSF penetration and the coverage of likely pathogens.
Otogenic and sinogenic abscesses are often treated with cefotaxime or ceftriaxone plus metronidazole.97 Abscesses with traumatic or neurosurgical causes should have antimicrobial coverage for S. aureus or methicillin-resistant S. aureus. Patients at high risk for tuberculous, fungal, or parasitic abscess should also receive coverage for the suspected etiologic agent. Corticosteroids should be reserved specifically for management of any attendant cerebral edema; in other circumstances, steroid use is associated with increased mortality. In cases in which the etiology is bacterial endocarditis, valve replacement is often required.112
Chemoprophylaxis
Among household contacts, the incidence of transmission of meningococcus is approximately 5%; therefore, it is recommended that household contacts of bacteriologically confirmed cases receive rifampin (adults, 600 mg; children older than 1 month, 10 mg/kg; children younger than 1 month, 5 mg/kg) orally every 12 hours for a total of four doses.97 In addition, these contacts should be advised to watch for fever, sore throat, rash, or any symptoms of meningitis. They should be hospitalized with appropriate intravenous antimicrobial therapy if there are signs that active meningococcal disease is developing because rifampin is ineffective against invasive meningococcal disease. Intimate, nonhousehold contacts who have had mucosal exposure to the patient’s oral secretions should also receive rifampin prophylaxis. Health care workers are not at increased risk for the disease and do not require prophylaxis unless they have had direct mucosal contact with the patient’s secretions, as might occur during mouth-to-mouth resuscitation, endotracheal intubation, or nasotracheal suctioning. Ciprofloxacin 500 mg by mouth (adults only) and ceftriaxone 250 mg intramuscularly (125 mg intramuscularly for children younger than 15 years) provide single-dose alternatives.97
There is no indication for chemoprophylaxis in pneumococcal meningitis. Rifampin prophylaxis for the contacts of patients with H. influenzae type b meningitis is recommended for nonpregnant household contacts when there are children younger than 4 years in the household97 (adults, 600 mg by mouth; children, 20 mg/kg by mouth daily for 4 days).
Immunoprophylaxis
A quadrivalent vaccine based on the polysaccharide capsule and conferring protection against group A, C, Y, and W-135 meningococci has been in routine use by the U.S. military since the 1980s.113 However, the capsular polysaccharide vaccines used to immunize adults are neither immunogenic nor protective in children younger than 2 years because of poor antibody response. In addition, no licensed vaccine is currently available against the serogroup B meningococcus.47 The serogroup B capsular polysaccharide has proved to be poorly immunogenic in both adults and children.114 The sequence variation of the surface proteins and cross-reactivity of the group B polysaccharide with human tissues have further impeded efforts to develop a successful vaccine. Efforts to enhance the immunogenicity and protective efficacy of meningococcal vaccines have focused on use of conjugate methods that link polysaccharides and carrier proteins. Serogroup C and serogroup C + Y conjugate vaccines have been developed and used effectively.115 Current recommendations for the quadrivalent vaccine are evolving. The vaccine is recommended in established meningococcal epidemics and for travelers to countries where meningococcal disease is currently epidemic. Elective vaccination of college freshmen has been recommended by the Advisory Committee on Immunization Practices (ACIP) in the United States and public health authorities in the United Kingdom.116,117 The United Kingdom has also implemented universal childhood immunization with a group C conjugate vaccine.115
The development of effective pneumococcal vaccines has been hampered by the large number of serotypes of the organism. A small number of serotypes, however, are responsible for most clinical pneumococcal disease, and a 23-valent vaccine effective against many of these principal serotypes has been developed.118 The recommendations for this polyvalent pneumococcal vaccine are targeted primarily at prevention of pneumonia, despite a potential beneficial effect for meningitis. A single dose of the vaccine should be considered for elderly or debilitated patients, especially those with pulmonary disease, and for patients with impaired splenic function, splenectomy, or sickle cell anemia.18 A heptavalent conjugated pneumococcal vaccine has also been developed and is recommended for universal childhood immunization by the ACIP.119
A conjugate vaccine effective against H. influenzae type b has been developed for use in the pediatric but not the adult population. It appears to be approximately 90% protective and has a very low incidence of adverse reactions.120 Modern childhood immunization against H. influenzae type b has raised the average age of patients afflicted with Haemophilus meningitis to 25 years and decreased the incidence of meningitis of any etiology by 55%.91
Vaccination is also available to confer immune protection against Japanese encephalitis virus, and it is recommended for people performing extensive outdoor activities or spending more than 30 days in endemic areas during transmission seasons.121 The reported protective efficacy of the vaccine is approximately 90%. Although there is no current human vaccine for WNV, vaccines for nonhuman mammals have been developed.34
References
1. Vieusseux, M. Mémoire sur le maladie qui a régné à Genève au printemps de 1805. J Med Chir Pharmacol. 1805;11:163.
2. Roos, KL. Acute bacterial meningitis. Semin Neurol. 2000;20:293–306.
3. Flexner, S. The results of serum treatment in 1300 cases of epidemic meningitis. J Exp Med. 1913;17:553.
4. Colebrook, L, Kenny, M. Treatment of human puerperal infections and experimental infections in mice with prontosil. Lancet. 1936;1:1279.
5. Dowling, H, et al. The treatment of pneumococcal meningitis with massive doses of systemic penicillin. Am J Med Sci. 1949;217:149.
6. Swartz, MN. Bacterial meningitis—a view of the past 90 years [see comment]. N Engl J Med. 2004;351:1826–1828.
7. Gjini, AB, et al. Changing epidemiology of bacterial meningitis among adults in England and Wales 1991-2002. Epidemiol Infect. 2006;134:567–569.
8. Albrich, WC, et al. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1569–1576.
9. Pollard, AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23(12 Suppl):S274–S279.
10. Dubos, F, et al. Decline in pneumococcal meningitis after the introduction of the heptavalent-pneumococcal conjugate vaccine in northern France. Arch Dis Child. 2007;92:1009–1012.
11. Wallin, MT, Kurtzke, JF. Neurocysticercosis in the United States: Review of an important emerging infection. Neurology. 2004;63:1559–1564.
12. Alvarez, S, McCabe, WR. Extrapulmonary tuberculosis revisited: A review of experience at Boston City and other hospitals. Medicine (Baltimore). 1984;63:25–55.
13. Salaki, JS, Louria, DB, Chmel, H. Fungal and yeast infections of the central nervous system. A clinical review. Medicine (Baltimore). 1984;63:108–132.
14. Lambert HP, ed. Infections of the Central Nervous System. Philadelphia: BC Decker, 1991.
15. Tyler KL, Martin JB, eds. Infectious Diseases of the Central Nervous System. Philadelphia: FA Davis, 1993.
16. Durand, ML, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21–28.
17. Fraser, DW, Geil, CC, Feldman, RA. Bacterial meningitis in Bernalillo County, New Mexico: A comparison with three other American populations. Am J Epidemiol. 1974;100:29–34.
18. Schuchat, A, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970–976.
19. Scheifele, D, et al. Invasive Haemophilus influenzae type b infections in vaccinated and unvaccinated children in Canada, 2001-2003. CMAJ. 2005;172:53–56.
20. Beghi, E, et al. Encephalitis and aseptic meningitis, Olmsted County, Minnesota, 1950-1981: I. Epidemiology. Ann Neurol. 1984;16:283–294.
21. Khetsuriani, N, Holman, RC, Anderson, LJ. Burden of encephalitis-associated hospitalizations in the United States, 1988-1997. Clin Infect Dis. 2002;35:175–182.
22. West Nile virus activity—United States, October 30–November 5, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1080.
23. Calfee, DP, Wispelwey, B. Brain abscess. Semin Neurol. 2000;20:353–360.
24. Mathisen, GE, Johnson, JP. Brain abscess. Clin Infect Dis. 1997;25:763–779.
25. Hussein, AS, Shafran, SD. Acute bacterial meningitis in adults. A 12-year review. Medicine (Baltimore). 2000;79:360–368.
26. Ahlawat, S, et al. Meningococcal meningitis outbreak control strategies. J Commun Dis. 2000;32:264–274.
27. Moszynski, P. New meningitis A vaccine is a “breakthrough” for 430 million people at risk. BMJ. 2010;341:c3552.
28. Summary of notifiable diseases, United States, 1997. MMWR Morb Mortal Wkly Rep. 1998;46:ii–vii.
29. Brooks, R, et al. Increased case-fatality rate associated with outbreaks of Neisseria meningitidis infection, compared with sporadic meningococcal disease, in the United States, 1994-2002. Clin Infect Dis. 2006;43:49–54.
30. Kumar, R. Aseptic meningitis: Diagnosis and management. Indian J Pediatr. 2005;72:57–63.
31. Specter, S, Bendinelli, M, Friedman, H. Neuropathogenic Viruses and Immunity. New York: Plenum; 1992.
32. Nowak, DA, Boehmer, R, Fuchs, HH. A retrospective clinical, laboratory and outcome analysis in 43 cases of acute aseptic meningitis. Eur J Neurol. 2003;10:271–280.
33. Logan, SA, MacMahon, E. Viral meningitis. BMJ. 2008;336:36–40.
34. Solomon, T, et al. West Nile encephalitis. BMJ. 2003;326:865–869.
35. Redington, JJ, Tyler, KL. Viral infections of the nervous system, 2002: Update on diagnosis and treatment. Arch Neurol. 2002;59:712–718.
36. Thorne, CD, et al. Emergency medicine tools to manage smallpox (vaccinia) vaccination complications: Clinical practice guideline and policies and procedures. Ann Emerg Med. 2003;42:665–680.
37. Rowland, LP, Kneass, W. Merritt’s Textbook of Neurology, 9th ed. Baltimore: Williams & Wilkins; 1995.
38. Wispelwey, B, Scheld, WM. Brain abscess. Semin Neurol. 1992;12:273–278.
39. McClelland, S, 3rd., Hall, WA. Postoperative central nervous system infection: Incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45:55–59.
40. Carpenter, J, Stapleton, S, Holliman, R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1–11.
41. Quagliarello, V, Scheld, WM. Bacterial meningitis: Pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–872.
42. Charatan, F. Organ transplants and blood transfusions may transmit West Nile virus. BMJ. 2002;325:566.
43. Johnson, RT. The pathogenesis of acute viral encephalitis and postinfectious encephalomyelitis. J Infect Dis. 1987;155:359–364.
44. Whitley, RJ, et al. Herpes simplex encephalitis. Clinical assessment. JAMA. 1982;247:317–320.
45. Yang, SY. Brain abscess: A review of 400 cases. J Neurosurg. 1981;55:794–799.
46. Geiseler, PJ, et al. Community-acquired purulent meningitis: A review of 1,316 cases during the antibiotic era, 1954-1976. Rev Infect Dis. 1980;2:725–745.
47. Attia, J, et al. The rational clinical examination. Does this adult patient have acute meningitis? JAMA. 1999;282:175–181.
48. Curtis, S, et al. Clinical features suggestive of meningitis in children: A systematic review of prospective data. Pediatrics. 2010;126:952–960.
49. Thomas, KE, et al. The diagnostic accuracy of Kernig’s sign, Brudzinski’s sign, and nuchal rigidity in adults with suspected meningitis. Clin Infect Dis. 2002;35:46–52.
50. Studahl, M, Bergstrom, T, Hagberg, L. Acute viral encephalitis in adults—a prospective study. Scand J Infect Dis. 1998;30:215–220.
51. Maslen, DR, et al. Spinal epidural abscess. Optimizing patient care. Arch Intern Med. 1993;153:1713–1721.
52. Wenger, JD, et al. Bacterial meningitis in the United States, 1986: Report of a multistate surveillance study. The Bacterial Meningitis Study Group. J Infect Dis. 1990;162:1316–1323.
53. Kasanmoentalib, ES, et al. Hydrocephalus in adults with community-acquired bacterial meningitis. Neurology. 2010;75:918–923.
54. Schut, ES, et al. Delayed cerebral thrombosis after initial good recovery from pneumococcal meningitis. Neurology. 2009;73:1988–1995.
55. Anderson, JR. Viral encephalitis and its pathology. Curr Top Pathol. 1988;76:23–60.
56. Sejvar, JJ, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–515.
57. Gadoth, A, Aizenstein, O, Mosek, A. Influenza A/H1N1 encephalitis. Neurology. 2010;75:666–667.
58. Kennedy, DH, Fallon, RJ. Tuberculous meningitis. JAMA. 1979;241:264–268.
59. Sennaroglu, L, Sozeri, B. Otogenic brain abscess: Review of 41 cases. Otolaryngol Head Neck Surg. 2000;123:751–755.
60. Hasbun, R, Abrahams, J, Jekel, J, Quagliarello, VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345:1727–1733.
61. van de Beek, D, et al. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44–53.
62. van Crevel, H, Hijdra, A, de Gans, J. Lumbar puncture and the risk of herniation: When should we first perform CT? J Neurol. 2002;249:129–137.
63. Shetty, AK, et al. Fatal cerebral herniation after lumbar puncture in a patient with a normal computed tomography scan. Pediatrics. 1999;103(6 Pt 1):1284–1287.
64. Greig, PR, Goroszeniuk, D. Role of computed tomography before lumbar puncture: A survey of clinical practice. Postgrad Med J. 2006;82:162–165.
65. Joffe, AR. Lumbar puncture and brain herniation in acute bacterial meningitis: A review. J Intensive Care Med. 2007;22:194–207.
66. Radetsky, M. Duration of symptoms and outcome in bacterial meningitis: An analysis of causation and the implications of a delay in diagnosis. Pediatr Infect Dis J. 1992;11:694–698.
67. Conly, JM, Ronald, AR. Cerebrospinal fluid as a diagnostic body fluid. Am J Med. 1983;75:102–108.
68. Onorato, IM, Wormser, GP, Nicholas, P. “Normal” CSF in bacterial meningitis. JAMA. 1980;244:1469–1471.
69. Luby, JP. Infections of the central nervous system. Am J Med Sci. 1992;304:379–391.
70. Pickens, S, et al. The effects of pre-admission antibiotics on the bacteriological diagnosis of pyogenic meningitis. Scand J Infect Dis. 1978;10:183–185.
71. Powers, WJ. Cerebrospinal fluid lymphocytosis in acute bacterial meningitis. Am J Med. 1985;79:216–220.
72. Arevalo, CE, et al. Cerebrospinal fluid cell counts and chemistries in bacterial meningitis. South Med J. 1989;82:1122–1127.
73. Huang, QS, et al. An echovirus type 33 winter outbreak in New Zealand. Clin Infect Dis. 2003;37:650–657.
74. Leonard, JM. Cerebrospinal fluid formula in patients with central nervous system infection. Neurol Clin. 1986;4:3–12.
75. Saha, DC, et al. Detection of Cryptococcus by conventional, serological and molecular methods. J Med Microbiol. 2009;58:1098–1105.
76. Watson, MA, Scott, MG. Clinical utility of biochemical analysis of cerebrospinal fluid. Clin Chem. 1995;41:343–360.
77. Jordan, GW, Statland, B, Halsted, C. CSF lactate in diseases of the CNS. Arch Intern Med. 1983;143:85–87.
78. Huy, N, et al. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: A systemic review and meta-analysis. Crit Care. 2010;14:R240.
79. Aurelius, E. Herpes simplex encephalitis. Early diagnosis and immune activation in the acute stage and during long-term follow-up. Scand J Infect Dis Suppl. 1993;89:3–62.
80. Aslanzadeh, J, Skiest, DJ. Polymerase chain reaction for detection of herpes simplex virus encephalitis. J Clin Pathol. 1994;47:554–555.
81. Kearns, AM, Freeman, R, Steward, M, Magee, JG. A rapid polymerase chain reaction technique for detecting M. tuberculosis in a variety of clinical specimens. J Clin Pathol. 1998;51:922.
82. Rafi, A, Naghily, B. Efficiency of polymerase chain reaction for the diagnosis of tuberculous meningitis. Southeast Asian J Trop Med Public Health. 2003;34:357–360.
83. Read, SJ, Kurtz, JB. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J Clin Microbiol. 1999;37:1352–1355.
84. Casas, I, et al. Viral diagnosis of neurological infection by RT multiplex PCR: A search for entero- and herpesviruses in a prospective study. J Med Virol. 1999;57:145–151.
85. Corless, CE, et al. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Microbiol. 2001;39:1553–1558.
86. Porritt, RJ, Mercer, JL, Munro, R. Detection and serogroup determination of Neisseria meningitidis in CSF by polymerase chain reaction (PCR). Pathology. 2000;32:42–45.
87. Richardson, DC, et al. Evaluation of a rapid PCR assay for diagnosis of meningococcal meningitis. J Clin Microbiol. 2003;41:3851–3853.
88. Singhi, SC, et al. Evaluation of polymerase chain reaction (PCR) for diagnosing Haemophilus influenzae b meningitis. Ann Trop Paediatr. 2002;22:347–353.
89. Hukkanen, V, Vuorinen, T. Herpesviruses and enteroviruses in infections of the central nervous system: A study using time-resolved fluorometry PCR. J Clin Virol. 2002;25(Suppl 1):S87–S94.
90. Romero, JR. Reverse-transcription polymerase chain reaction detection of the enteroviruses. Arch Pathol Lab Med. 1999;123:1161–1169.
91. Thomson, RB, Jr., Bertram, H. Laboratory diagnosis of central nervous system infections. Infect Dis Clin North Am. 2001;15:1047–1071.
92. Gerdes, LU, et al. C-reactive protein and bacterial meningitis: A meta-analysis. Scand J Clin Lab Invest. 1998;58:383–393.
93. Dubos, F, et al. Serum procalcitonin and other biologic markers to distinguish between bacterial and aseptic meningitis. J Pediatr. 2006;149:72–76.
94. Lai, CW, Gragasin, ME. Electroencephalography in herpes simplex encephalitis. J Clin Neurophysiol. 1988;5:87–103.
95. Nigrovic, LE, et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis [see comment]. JAMA. 2007;297:52–60.
96. Oates-Whitehead, RM, et al. Fluid therapy for acute bacterial meningitis. Cochrane Database Syst Rev. (3):2005.
97. Gilbert, DN, Moellering, RC, Sande, MA. Guide to Antimicrobial Therapy 2003. Hyde Park, Vt: Antimicrobial Therapy; 2003.
98. Nathan, N, et al. Ceftriaxone as effective as long-acting chloramphenicol in short-course treatment of meningococcal meningitis during epidemics: A randomised non-inferiority study. Lancet. 2005;366:308–313.
99. Tauber, MG, Khayam-Bashi, H, Sande, MA. Effects of ampicillin and corticosteroids on brain water content, cerebrospinal fluid pressure, and cerebrospinal fluid lactate levels in experimental pneumococcal meningitis. J Infect Dis. 1985;151:528.
100. McIntyre, PB, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. JAMA. 1997;278:925.
101. de Gans, J, van de Beek, D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549–1556.
102. Scarborough, M, et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med. 2007;357:2441–2450.
103. Brouwer, M, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. (9):2010.
104. van de Beek, D, et al. Adjunctive dexamethasone in bacterial meningitis: A meta-analysis of individual patient data. Lancet Neurol. 2010;9:254–263.
105. Annane, D, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871.
106. Sprung, CL, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124.
107. Molyneux, EM, et al. Dexamethasone treatment in childhood bacterial meningitis in Malawi: A randomised controlled trial. Lancet. 2002;360:211–218.
108. Mongelluzzo, J, et al. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299:2048–2055.
109. Haemophilus influenzae infections. In: Pickering LK, et al, eds. Red Book: 2006 Report of the Committee on Infectious Diseases. Elk Grove Village, Ill: American Academy of Pediatrics; 2006:310–318.
110. Pneumococcal infections. In: Pickering LK, et al, eds. Red Book: 2006 Report of the Committee on Infectious Diseases. Elk Grove Village, Ill: American Academy of Pediatrics; 2006:525–537.
111. Rotbart, HA, O’Connell, JF, McKinlay, MA. Treatment of human enterovirus infections. Antiviral Res. 1998;38:1–14.
112. Gillinov, AM, et al. Valve replacement in patients with endocarditis and acute neurologic deficit. Ann Thorac Surg. 1996;61:1125–1129.
113. Zangwill, KM, et al. Duration of antibody response after meningococcal polysaccharide vaccination in US Air Force personnel. J Infect Dis. 1994;169:847–852.
114. Morley, SL, et al. Immunogenicity of a serogroup B meningococcal vaccine against multiple Neisseria meningitidis strains in infants. Pediatr Infect Dis J. 2001;20:1054–1061.
115. Soriano-Gabarro, M, Stuart, JM, Rosenstein, NE. Vaccines for the prevention of meningococcal disease in children. Semin Pediatr Infect Dis. 2002;13:182–189.
116. The Changing Epidemiology of Meningococcal Disease in the United States with an Emphasis on College Health Issues. Englewood, Colo: Postgraduate Institute for Medicine, 1999.
117. Meningococcal disease and college students. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49:13–20.
118. Butler, JC, et al. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993;270:1826–1831.
119. Jacobson, RM, Poland, GA. The pneumococcal conjugate vaccine. Minerva Pediatr. 2002;54:295–303.
120. Vadheim, CM, et al. Eradication of Haemophilus influenzae type b disease in southern California. Kaiser-UCLA Vaccine Study Group. Arch Pediatr Adolesc Med. 1994;148:51–56.
121. Zhou, B, Jia, L, Xu, X. A large-scale study on the safety and epidemiological efficacy of Japanese encephalitis (JE) live vaccine (SA14-14-2) in the JE endemic areas [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 1999;20:38–41.