134 Central Nervous System Infections
Infections of the meninges can be subclassified by the acuity of onset of symptoms. Bacterial infections almost exclusively cause acute meningitis syndrome, characterized by rapid (<48 hours) progression of fever, headache, and meningismus. In contrast, the subacute meningitis syndrome, frequently due to viruses, fungi, or mycobacteria, is more slowly evolving, with symptoms developing over several days to weeks (Table 134-1). The following sections outline approaches to acute meningitis and subacute CNS infection syndromes. These approaches prioritize the competing needs of obtaining a precise etiologic diagnosis versus instituting early antimicrobial therapy.
TABLE 134-1 Causes of Acute and Subacute Central Nervous System Infection Syndromes
| Acute Meningitis Syndrome |
| Rapid onset (<24-48 h) of fever, headache, or meningismus, with early cognitive impairment |
| Common |
| Pyogenic meningitis (pneumococcal, meningococcal, Listeria, other) |
| Uncommon |
| Viral encephalitis (especially herpes simplex), subarachnoid bleed, brain abscess (with rupture) |
| Rare |
| Viral meningitis, granulomatous meningitis (cryptococcal, mycobacterial), carcinomatous meningitis, brain tumor |
| Subacute Central Nervous System Infection Syndrome |
| Subacute onset (>24-48 h) of fever, headache, or meningismus, with no or gradual cognitive impairment |
| Common |
| Viral meningitis, viral encephalitis, rickettsial infection |
| Uncommon |
| Brain abscess, brain tumor, granulomatous meningitis |
| Rare |
| Cerebrovascular accident, carcinomatous meningitis |
 Bacterial Meningitis
Bacterial Meningitis
Anatomy
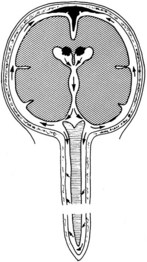
Bacterial meningitis is a pyogenic infection of the cerebral ventricles and the subarachnoid space, with bacteria usually confined to the nutrient-rich cerebrospinal fluid (CSF). CSF is formed in the choroid plexus of the ventricles, flows into the subarachnoid space at the cisterna magna and around the cerebral hemispheres, and is reabsorbed by the arachnoid villi (Figure 134-1). In adults, CSF is produced at a rate of approximately 500 mL/day, yet the CSF space averages only 140 mL in volume, consistent with rapid production and reabsorption. The cerebral and spinal subarachnoid spaces connect at the cisterna magna. Flow through the spinal subarachnoid space is of variable velocity and direction.
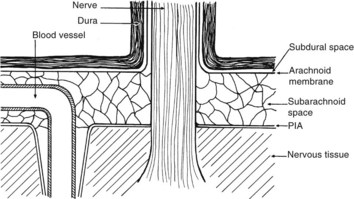
There are numerous potential and actual spaces among the layers of the meninges (Figure 134-2). Meningitis involves the actual space (i.e., the subarachnoid space), which consists of multiple interconnected compartments. The small size of the foramina of Luschka and Magendie allows unidirectional caudal flow toward the cisterna magna, where the CSF then moves either cephalad or into the spinal canal. This compartmentalization has implications for therapy, because the movement of medications and infectious agents depends on the rate and direction of CSF flow. A blockage at any of these levels may restrict entry of antibiotics into sites of ongoing infection.
Infectious agents can invade the CSF by at least three routes (Table 134-2). First, the vascular structures of the choroid plexus and pia and the vessels that traverse the subarachnoid space may serve as conduits during systemic bacteremia. A second less common route is direct invasion across the protective meninges. Physical disruption of the dura by trauma or surgery allows direct invasion of the subarachnoid space and should be considered in patients with a history of CSF leakage or rhinorrhea. Emissary veins provide another pathway for bacteria to spread from contiguous foci into the subarachnoid space. These veins traverse the skull and dura, directly connecting the soft tissues of the head and neck with the venous system of the brain and meninges, including the arachnoid villi. Although blood in the emissary veins usually flows away from the brain, the CNS veins and dural sinuses do not contain valves, and retrograde flow of bacteria is possible. Rarely, organisms may reach the ventricles or subarachnoid space from within the neural tissue; for example, rupture of a brain abscess into the ventricles may have disastrous effects.
TABLE 134-2 Routes by Which Bacteria May Enter the Subarachnoid Space
| Vascular (Blood-Brain Barrier) |
Pathophysiology
Neural damage occurs as a direct result of the host inflammatory response. The unique anatomy and composition of the CSF-filled compartments, combined with a paucity of host immunologic defenses, create a microenvironment that allows the persistence and proliferation of microorganisms.1 Polymorphonuclear leukocytes are not normal inhabitants of the CSF, and mobilization of these phagocytic cells is delayed during the early stages of infection. Similarly, the concentration of immunoglobulin in CSF is significantly less than that in serum, limiting the effectiveness of humoral immunity. Most important, complement, which plays a critical role in chemotaxis, phagocytosis, and intracellular killing, is virtually absent from normal CSF. Once in the CSF, bacteria induce leukocyte migration into the subarachnoid space, resulting in occlusion of cortical blood vessels, damage to nerve roots that traverse the subarachnoid space (see Figure 134-2), and impaired CSF flow (see Figure 134-1). Clinically, this manifests as cranial or spinal nerve dysfunction and hydrocephalus. Activation of leukocytes leads to an inflammatory cascade, with the release of cytokines, oxidants, and proteolytic enzymes. At the cellular level, this chain of events produces disruption of the blood-brain barrier and impaired cerebrovascular autoregulation.4 Increased intracranial pressure may result in transtentorial herniation or tissue hypoxia due to decreased tissue perfusion.
Clinical Course
Acute Meningitis Syndrome
Early recognition and therapy of acute meningitis syndrome are essential to minimize morbidity and mortality. The initial manifestation of the illness may be subtle, with a low-grade headache or fever. However, once meningeal symptoms (vomiting, severe headache, stiff neck) develop, the clinical course is dramatic. Patients appear “toxic,” and higher integrative functions may deteriorate rapidly. The classic clinical triad associated with bacterial meningitis, fever, neck stiffness, and altered mental status is present in only 44% of cases.6 A rash is highly suggestive of meningococcal infections; however, skin lesions are noted in only 64% of meningitis cases due to this organism.7 Other common signs and symptoms include headache and nausea. In the elderly, recognition of acute bacterial meningitis may be delayed by the absence of suggestive clinical findings. Nuchal rigidity, vomiting, and headache are significantly less frequently noted in the elderly; in contrast, seizures are present in 26% of patients ≥65 years of age.10 In addition, the elderly have a higher incidence of noninfectious conditions that may mimic acute meningitis syndrome (e.g., subarachnoid bleeding and malignancies involving the CNS) complicating the initial evaluation.
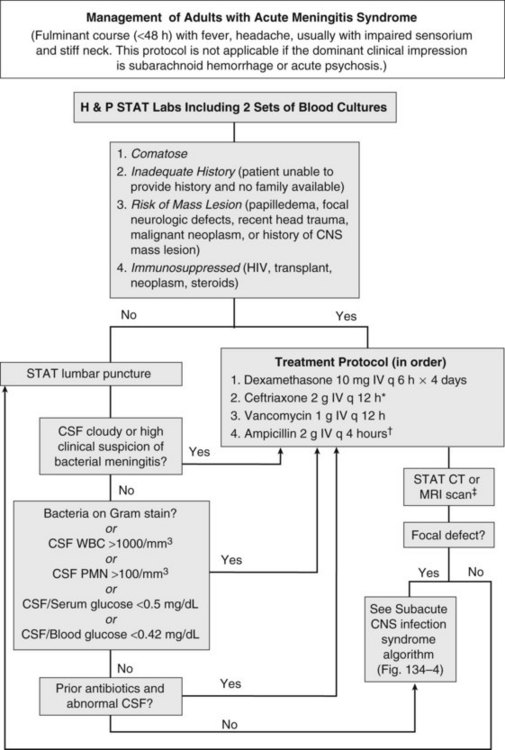
Acute meningitis syndrome represents an infectious disease emergency. Baseline predictors of adverse outcome in adults include advanced age, tachycardia (heart rate >120 beats/min), low Glasgow Coma Scale score, cranial nerve palsies, CSF WBC count less than 1000/mm3, and presence of gram-positive cocci on CSF Gram stain.8 This latter variable reflects the higher mortality associated with pneumococcal meningitis. Despite the availability of antibiotics active against all common causes of acute bacterial meningitis, in adults the overall mortality remains approximately 20%.6 A delay in antibiotic therapy increases the risk of an adverse outcome, particularly when progressive neurologic impairment occurs before receiving therapy.9 For this reason, recently published guidelines recommend the administration of empirical antibiotics to patients with a presumptive diagnosis of bacterial meningitis as soon as possible after presentation (Figure 134-3).5
Coupled with the need for urgent treatment is the need for urgent diagnosis. Identification of a pathogen allows the clinician to tailor the antibiotic regimen based on susceptibility patterns, and has prognostic and therapeutic implications. However, situations arise when lumbar puncture is unavoidably delayed. This may be due to anatomic factors that make lumbar puncture technically difficult or the need to perform neuroimaging studies to exclude a contraindication to lumbar puncture. If a significant delay in obtaining CSF is anticipated, antibiotics should be given immediately after peripheral blood cultures are obtained. The yield of CSF culture decreases within as little as 15 minutes following administration of antibiotics.11 Nevertheless, the risk of delaying treatment outweighs the need to make a microbiological diagnosis. Despite the inhibitory effect of prior antibiotics on bacterial culture and Gram stain, the absolute neutrophil count and neutrophilic pleocytosis remain suggestive of bacterial meningitis,12,13 and a full course of empirical therapy should be completed if CSF parameters are consistent with this diagnosis.
Historically, the perceived risk (and legal consequences) of uncal herniation following lumbar puncture in the presence of an intracranial mass lesion led to the ubiquitous use of neuroimaging. More recently, studies have challenged this practice, citing the potential deleterious effect of computed tomography (CT) scan–related delays in the initiation of therapy or the compromising effect of premature sterilization of CSF cultures.9,11,16 Even among patients with an abnormal CT scan, only a minority of cases have radiographic findings precluding lumbar puncture.14 For this reason, guidelines suggest that neuroimaging prior to lumbar puncture should be reserved for patients with compromised immune systems (e.g., human immunodeficiency virus [HIV] infection, use of immunosuppressive medications, or organ transplantation), history of an intracranial mass lesion, abnormal level of consciousness, papilledema, or focal neurologic deficit.5 In the absence of one of these features, the recommendation is to proceed directly to lumbar puncture, followed by immediate administration of empirical antibiotics.5
Subacute Central Nervous System Infection Syndrome
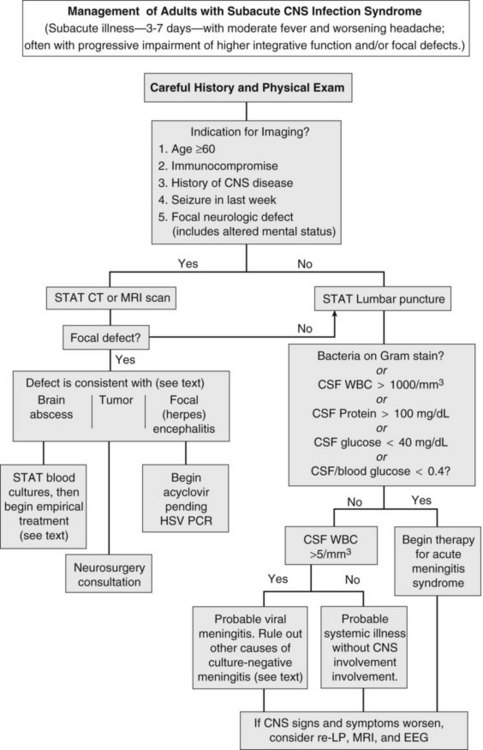
Herpes simplex encephalitis, brain abscess, and meningitis due to fungi, mycobacteria, fastidious bacteria (e.g., Rickettsia rickettsii, Treponema pallidum), or viruses all produce fever, worsening headache, and progressive impairment of higher integrative functions. On occasion, carcinomatous meningitis, brain tumor, and subarachnoid bleeding cause similar findings (see Table 134-1). To avoid inappropriate therapy and unnecessary hospitalization, the decision to institute antimicrobial therapy should be carefully weighed. However, if pyogenic meningitis is still a possibility, empirical antimicrobial therapy should be begun, as outlined in the previous section.
The first priority when managing subacute CNS syndrome is rapid diagnosis (as opposed to the rapid-therapy approach to acute meningitis syndrome). With this syndrome, the physician has time to carefully evaluate the patient and relevant laboratory data (Figure 134-4). Peripheral blood granulocytosis (>10,000/mm3), CSF cell counts over 1000/mm3, CSF protein concentration over 100 mg/dL, and CSF glucose concentrations below 40 mg/dL favor a bacterial cause, and these patients should be given empirical antibiotics for acute meningitis syndrome until a specific diagnosis is made or bacterial cultures return negative.
Additional diagnostic studies may be indicated for subacute infections. Serologic testing for HIV should be performed, because the spectrum of infectious agents is much broader among HIV-infected individuals. Testing for enteroviruses (CSF polymerase chain reaction [PCR] or viral culture), Cryptococcus (cryptococcal antigen), neurosyphilis (VDRL), mycobacterial infection (culture or PCR of CSF), herpes simplex virus (CSF PCR), tickborne infections (Ehrlichia, Rickettsia, Lyme disease), and arboviral encephalitides (West Nile virus) should be individualized based on patient characteristics, severity of illness, knowledge of local pathogens, and season. Despite intensive diagnostic testing, a pathogen is identified in only two-thirds of patients with subacute meningitis syndrome.17
Epidemiology
The epidemiology of bacterial meningitis has evolved in the last twenty years. The incidence of bacterial meningitis in the pediatric population has decreased markedly in the United States following the widespread use of conjugate vaccines active against Haemophilus influenzae type B, Neisseria meningitidis, and Streptococcus pneumoniae.18Conversely, the incidence of nosocomial bacterial meningitis caused by resistant strains of Enterobacteriaceae, Pseudomonas aeruginosa, and Staphylococcus aureus is increasing following surgery or instrumentation of the CNS.2
Therapy
Antibiotics
The choice of empirical antibiotics is based on knowledge of the likely causative agents, which vary based on host characteristics (e.g., age, immunocompromise), site of acquisition (nosocomial versus community acquired), and local resistance patterns. Recommendations for empirical therapy are listed in Table 134-3, with dosages commonly used for the treatment of CNS infections listed in Table 134-4.3,5 Pneumococci and meningococci remain the most common causes of community-acquired meningitis in immunocompetent adults younger than 50 years.3 In the last decade, pneumococci that are intermediately (minimum inhibitory concentration [MIC] >0.12 to 1 µg/mL) or highly (MIC >2 µg/mL) resistant to penicillin have emerged as important pathogens. Penicillin-resistant pneumococci are typically multidrug resistant; however, many isolates remain sensitive to third-generation cephalosporins, and all are susceptible to vancomycin.
TABLE 134-4 Antimicrobial Dosages for Central Nervous System Infections
| Drug | Dosage (by Total Body Weight) | Usual Dosage (for 70-kg Adult) |
|---|---|---|
| Acyclovir | 10 mg/kg IV q 8 h | 700 mg IV q 8 h |
| Ampicillin | 30 mg/kg IV q 4 h | 2 g IV q 4 h |
| Cefotaxime | 30 mg/kg IV q 6 h | 2 g IV q 6 h |
| Ceftazidime | 30 mg/kg IV q 8 h | 2 g IV q 8 h |
| Cefepime | 30 mg/kg IV q 8 h | 2 g IV q 8 h |
| Ceftriaxone | 30 mg/kg IV q 12 h | 2 g IV q 12 h |
| Meropenem | 40 mg/kg IV q 8 h* | 2 g IV q 8 h |
| Metronidazole | 7.5 mg/kg IV q 6 h | 500 mg IV q 6 h |
| Nafcillin | 30 mg/kg IV q 4 h | 2 g IV q 4 h |
| Penicillin G | 60,000-70,000 units/kg IV q 4 h | 4 million units IV q 4 h |
| Tobramycin or gentamicin† | 2 mg/kg IV load, then 1.7 mg/kg q 8 h‡ | 140 mg IV load, then 120 mg IV q 8 h‡ |
| Intrathecal | 0.1 mg/kg/d | 5-10 mg/d |
| Intraventricular | 0.1 mg/kg/d | 5-10 mg/d |
| Trimethoprim-sulfamethoxazole | 5 mg/kg IV q 6 h | 350 mg IV q 6 h§ |
| Vancomycin | 15 mg/kg IV q 6 h | 500 mg IV q 6 h‡ or 1 g IV q 12 h |
* Pediatric dosage. Adults should receive usual dosage.
† Regardless of which aminoglycoside is used, only preservative-free preparations should be used.
‡ Adjust dosage based on serum levels.
§ Dosage indicates trimethoprim component.
Antimicrobial therapy should be directed against the most common causes of bacterial meningitis. In the absence of a positive CSF Gram stain, initial therapy for adults with community-acquired meningitis should include a third-generation cephalosporin such as cefotaxime or ceftriaxone, as well as vancomycin. Vancomycin should never be used alone as initial therapy because of its marginal CNS penetration and lack of activity against gram-negative organisms. Empirical ampicillin therapy for Listeria monocytogenes should be added for adults aged 50 years or older or for patients with T-cell immunocompromise (e.g., on chronic steroid therapy), pregnant women, or patients with significant use of alcohol.3,5 If the CSF Gram stain shows gram-positive rods suggestive of Listeria, intravenous (IV) gentamicin should be added. For patients intolerant of penicillins, trimethoprim-sulfamethoxazole is an acceptable alternative for treatment of Listeria meningitis.
In contrast to community-acquired meningitis, organisms causing nosocomial meningitis reflect the highly resistant strains endemic to the hospital. Empirical therapy for patients suspected to have nosocomial meningitis must therefore be directed against staphylococcal species (both coagulase-positive and coagulase-negative strains) and multidrug-resistant strains of gram-negative bacilli, including P. aeruginosa and Acinetobacter baumannii (see Figure 134-3). Empirical treatment in this population should therefore include vancomycin as well as an antipseudomonal cephalosporin (ceftazidime or cefepime) or carbapenem. Imipenem is active against Pseudomonas and achieves therapeutic levels in the CSF; however, because this agent lowers the seizure threshold, it is relatively contraindicated for meningitis. Meropenem, a related carbapenem, is less epileptogenic and is therefore preferred for this indication.19
Initial antibiotic choices can be refined when sensitivity patterns become available, typically in 2 to 3 days. The duration of therapy in bacterial meningitis varies with the pathogen and the clinical response. Although there have been few randomized studies evaluating the optimal duration of therapy, 7 days of treatment for H. influenzae and N. meningitidis meningitis is typically sufficient,20 whereas S. pneumoniae requires 10 to 14 days of therapy.5,17 Adults with pneumococcal meningitis may have predisposing infections including pneumonia, sinusitis, otitis, or rarely endocarditis. Although therapy for meningitis usually treats the primary cause, endocarditis requires prolonged therapy with bactericidal antibiotics.
For all causes of bacterial meningitis, abnormalities of the CSF (high protein and cell counts) persist for days to weeks. Resolution of symptoms (e.g., fever, leukocytosis, meningismus) should serve as adequate evidence of successful therapy. In a patient who responds poorly to 48 hours of therapy, repeat lumbar puncture and head CT or MRI are indicated. Repeat lumbar puncture is particularly important for detecting clearance of bacteria from the CSF in patients with cephalosporin-resistant pneumococcal meningitis who demonstrate a slow clinical response.19 Patients with culture-negative pyogenic meningitis and suboptimal clinical response should also have repeat lumbar puncture to ensure response to empirical antibiotics. Ongoing or worsening CSF parameters suggest infection with either resistant bacteria or with a pathogen more typically associated with subacute meningitis syndrome (see Table 134-1).
Corticosteroids
Much of the morbidity of bacterial meningitis is caused by the host inflammatory response. Corticosteroids block inflammation, and animal studies have shown an improvement in outcome when corticosteroids are given as adjuvant therapy with antibiotics. A number of randomized controlled clinical trials have evaluated the use of steroids in patients with bacterial meningitis; however, the findings have varied based on the population studied. A large meta-analysis found use of adjuvant corticosteroids significantly decreased mortality as well as sequelae, including hearing loss and neurologic debility.21 Age stratification showed the survival benefits were restricted to adults, where the reduction in hearing loss was only seen among children. This may reflect the divergent microbiology among this age group; the decrease in mortality was restricted to patients with S. pneumoniae, a pathogen seen more frequently in adults, while the decrease in hearing loss was only found in patients with H. influenzae, who were almost exclusively pediatric. A more recent meta-analysis of individual patient data by the same authors failed to find a protective effect with adjunctive dexamethasone.22 Theoretical concerns regarding use of corticosteroids include decreased penetration of vancomycin across the meninges.
At present, treatment guidelines recommend adjuvant dexamethasone (0.15 mg/kg IV every 6 hours for 2-4 days) be given concomitantly with the first dose of antibiotics for adult patients with suspected or proven pneumococcal meningitis.5 Whether steroids are beneficial for other causes of bacterial meningitis remains unknown.
Complications
Systemic complications are frequent in acute bacterial meningitis. Forty percent of patients with pneumococcal meningitis have concomitant sepsis, typically from an extra-CNS infection such as pneumonia. Less commonly, sepsis represents seeding of the bloodstream from the infected meninges. The approach to sepsis associated with bacterial meningitis is similar to that in any patient in the intensive care unit (ICU): protection of the airway and hemodynamic support. Although many of these patients may meet the criteria for administration of activated protein C (drotrecogin alfa), limited data on its safety and efficacy in meningitis exist. Increased risk of intracranial hemorrhage among patients with severe sepsis and meningitis has been reported.23
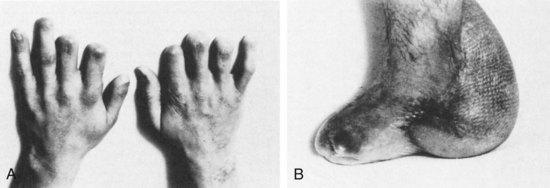
Meningococcal meningitis presents unique public health and infection control challenges. This diagnosis is suggested by the presence of a petechial or purpuric rash; however, this finding is neither sensitive nor specific.7 To prevent secondary cases of meningococcal meningitis among healthcare workers, all patients with presumed bacterial meningitis should initially be placed in respiratory isolation to prevent the spread of infection by droplet transmission.25 Complications specific to meningococcal meningitis include purpura fulminans and necrotizing vasculitis leading to skin necrosis and digital gangrene (Figure 134-5). Nonspecific complications associated with meningococcal as well as other forms of meningitis include adrenal insufficiency due to infarction (Waterhouse-Friderichsen syndrome), renal failure (due to acute tubular necrosis in the setting of hypotension), deafness, hydrocephalus, and cognitive impairment.
 Brain Abscess
Brain Abscess
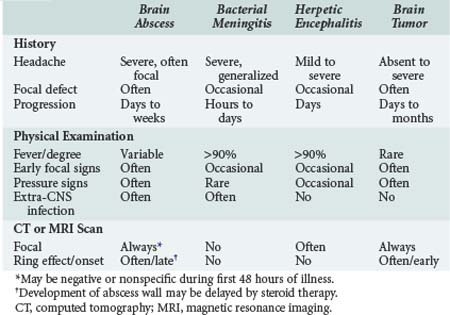
Pyogenic brain abscess is a localized suppurative infection of parenchymal CNS tissue and may involve any region of the CNS from the cerebral cortex to the conus medullaris. Differentiating brain abscess from other CNS infections or brain tumors may be challenging, as there is significant overlap in the clinical and radiologic presentation (Table 134-5). Even with a combined medical and surgical approach, mortality is significant. Rapid progression of symptoms and impaired mental status at presentation are predictors of an adverse outcome, with rupture of the abscess into the ventricle almost uniformly fatal.26
Pathophysiology
In the preantibiotic era, brain abscesses arose from extension of infection from contiguous foci (middle ear, mastoids, sinuses). With the availability of antibiotics, however, such complications have become less common. An increasing number of cases are due to distant foci of infection27 or are associated with local seeding following neurosurgery or trauma.15 Abscesses arising from hematogenous seeding tend to develop in the distribution of the middle cerebral artery. Brain abscesses associated with endocarditis are rare but, when present, are often multiple and small. Filtration of bacteria by the pulmonary vasculature protects the brain from hematogenous seeding. Therefore, when cardiac shunts or pulmonary arteriovenous fistulas are present, the risk of brain abscess is increased. In as many as a third of patients, there is no obvious source of infection.
The etiologic pathogen for brain abscesses differs according to the route of infection. Abscesses that arise from contiguous sites are frequently polymicrobial. In contrast, brain abscesses associated with hematogenous spread are usually due to a single pathogen. Infections following neurosurgery reflect nosocomial flora and often include multidrug-resistant organisms such as methicillin-resistant S. aureus (MRSA) or multidrug-resistant A. baumannii. The bacteria most often isolated from brain abscess include Enterobacteriaceae, streptococci, staphylococci, and pneumococci.27 Fastidious bacteria such as Nocardia, fungi such as Aspergillus, and even protozoa such as Toxoplasma can also be etiologic agents, particularly in immunosuppressed patients.
Therapy
In general, a combination approach of antimicrobials coupled with surgical drainage remains the standard approach for management of pyogenic brain abscesses. Choice of antimicrobials should be guided by culture results, given the diversity of potential pathogens and the need for prolonged therapy (e.g., 6-8 weeks). Because of the difficulty in getting therapeutic concentrations of antibiotics across the blood-brain barrier, additional pharmacologic considerations include CNS penetration and parenteral administration. Empirical therapy should be begun while awaiting culture results and should be guided by the likely microbiology based on the origin of the infection. In cases in which the source is unknown or metastatic spread from a distant focus is likely, empirical therapy with vancomycin, metronidazole, and a third-generation cephalosporin is suggested.15 An antipseudomonal cephalosporin should be substituted for the third-generation cephalosporin for postoperative infections or for an abscess arising from an otogenic site. Neurosurgical aspiration is invaluable in identifying specific pathogens, and sensitivity testing is crucial for narrowing therapy. Fungal and mycobacterial cultures should be obtained on all aspirates. Positive cultures from blood or extra-CNS suppurative foci occasionally establish a presumptive etiologic agent. Ancillary testing for a culture-negative brain abscess includes HIV serology, serum cryptococcal antigen, and toxoplasmosis titers.
In selected cases, brain abscesses can be treated with antimicrobials alone, particularly when the causative agent is known and the lesion measures less than 2.5 cm.28 Medical management without drainage may be necessary when the lesion is inaccessible or surgical intervention poses unacceptable risks. However, open or stereotactic drainage is indicated when (1) cultures of extra-CNS sites do not yield a pathogen, (2) deterioration from increased intracranial pressure occurs, and (3) there is no radiographic improvement on medical therapy. Patients treated without drainage may require a longer duration (e.g., 12 weeks) of parenteral antibiotics and should be followed closely for clinical and radiographic improvement. Steroids should be reserved for cases in which significant edema is present.
 Viral Infections of the Central Nervous System
Viral Infections of the Central Nervous System
Pathophysiology
Most viral infections of the CNS occur through hematogenous spread.29 The virus may initially traverse mucous membranes (e.g., enteroviruses) or be inoculated into subcutaneous tissue (e.g., arboviruses). After local replication within extraneural tissues, sustained viremia occurs. Alternatively, the virus may gain access to the CNS by direct neuronal invasion, as occurs when rabies virus spreads retrograde along peripheral nerves into the CNS. The olfactory tracts may provide a route of entry for herpes simplex virus type 1.
Acute Viral Meningitis
Although many viruses cause meningitis, in clinical practice, the specific pathogen is rarely identified. In most cases, extensive diagnostic evaluation is not indicated; viral meningitis is typically a self-limited syndrome and does not require treatment. Epidemiologic studies suggest that enteroviruses are the most common cause of viral meningitis17,30 and are particularly prevalent in children and young adults. Other viral causes of meningitis include arboviruses (see Viral Encephalitis), herpes simplex virus type 2, acute HIV infection, and lymphocytic choriomeningitis virus.
At the time of presentation, it may be difficult to differentiate viral meningitis from other forms of culture-negative meningitis that may be more aggressive or require directed therapy. The differential diagnosis for culture-negative or aseptic meningitis includes tickborne infections such as Ehrlichia or Rickettsia, secondary syphilis, mycobacterial or fungal infections, irritation from a parameningeal focus, and partially treated bacterial infections (see Figure 134-3). Signs and symptoms of viral and bacterial meningitis are indistinguishable. CSF findings suggestive of a viral cause include lymphocytic pleocytosis (typically with a total white blood cell count <1000), normal glucose, and normal to slightly elevated protein. Management is supportive, with fluid repletion for significant dehydration and pain control the mainstays of care. Meningeal symptoms usually resolve in the first 2 weeks, but malaise may be prolonged.
Viral Encephalitis
A host of viral agents infect the parenchyma of the brain or spinal cord to produce encephalitis or myelitis, respectively; however, despite intensive investigation, in the majority of cases, no organism is identified.24,31,32 Viral encephalitis is typically an acute febrile illness associated with headache, an altered level of consciousness disproportionate to systemic illness, behavioral or speech disturbances, and focal neurologic signs such as seizures or hemiparesis. In contrast, viral myelitis causes hemiparesis or hemiplegia but spares higher integrative functions. Overlap syndromes of encephalomyelitis can occur.
Viral encephalitis is caused by acute invasion of brain parenchyma. Clinically, viral encephalitis must be differentiated from acute disseminated encephalomyelitis (ADEM), an autoimmune phenomenon that typically occurs 5 to 21 days after a viral respiratory or gastrointestinal illness. MRI in ADEM reveals enhancing multifocal white matter lesions suggestive of demyelination.33 Neuroimaging to distinguish these entities is important because ADEM responds to high-dose steroids. With the exception of herpes simplex encephalitis (see later), management of viral encephalitides revolves around supportive care and control of seizures.24 Despite the lack of specific antiviral treatments, thorough evaluation is important to direct public health interventions (e.g., mosquito eradication for West Nile virus or other arboviruses) or provide prognostic information (e.g., rabies).
Herpes Simplex Encephalitis
Herpes simplex encephalitis (HSE) is the most common cause of sporadic encephalitis in the United States. The mortality of untreated HSE exceeds 70%; however, timely administration of acyclovir has been shown to significantly improve survival. While clinical, laboratory, or radiographic findings may be suggestive of HSE, no combination of presenting features is sufficiently sensitive, and empirical acyclovir should be given to all patients with encephalitis until definitive diagnostic studies are completed.24
Common signs and symptoms of HSE include fever, personality change, and dysphasia. Hemiparesis and seizures occur in approximately 40% of cases.34,35 Without treatment, progressive obtundation occurs. CSF typically exhibits a lymphocytic pleocytosis, but this is nonspecific. Suggestive findings include temporal lobe localization on neuroimaging studies, with MRI being superior to CT, and periodic lateralizing epileptiform discharges (PLEDS) on the electroencephalogram. Definitive diagnosis requires detection of herpes simplex in the brain or spinal fluid. PCR on CSF, a noninvasive test with a sensitivity greater than 95%, is now considered the gold standard for diagnosis.36 Empirical acyclovir can usually be discontinued if the PCR is negative, although there have been reports of false-negative results early in the course of the disease.37 For PCR-confirmed cases, acyclovir therapy should be continued for a minimum of 14 days, with treatment extended to 21 days if the CSF remains PCR-positive at the end of treatment.38
 Central Nervous System Infection and the AIDS Patient
Central Nervous System Infection and the AIDS Patient
CNS dysfunction is common in patients with HIV, found in as many as half of all infected patients during the course of the infection.45 Neuroimaging followed by lumbar puncture is indicated for any patient with acquired immunodeficiency syndrome (AIDS) who has significant headache or altered mental status, even when CNS symptoms do not dominate the picture. In addition to the standard diagnostic studies, CSF analysis in HIV/AIDS patients should include cytology, cryptococcal antigen, VDRL (for syphilis), and PCR for herpes viruses, JC virus, and Mycobacterium tuberculosis.45
This sequential approach to evaluation is recommended because mass lesions, including toxoplasmosis, lymphoma, and progressive multifocal leukoencephalopathy, are common. Ring-enhancing lesions on CT or MRI are suggestive of the first two entities, whereas focal white matter disease favors the last. Positive serologic testing for Toxoplasma gondii or the presence of multiple mass lesions increases the likelihood of CNS toxoplasmosis, and if either of these is present, empirical therapy for toxoplasmosis should be begun.39 Single-photon emission tomography (SPECT) imaging may be useful in differentiating lymphoma from infection for mass lesions ≥2cm46; however, definitive diagnosis requires tissue analysis. Surgical intervention is typically reserved for cases with signs of impending herniation or lack of radiographic response after 2 weeks of empirical therapy for toxoplasmosis.
Cryptococcal meningitis is also common in AIDS and typically presents as a slowly progressive syndrome marked by fever and headaches. Diagnosis of this infection can be made by either serum or CSF cryptococcal antigen testing, both of which have sensitivity greater than 90%. CSF testing provides additional important prognostic and therapeutic information, and an opening pressure should be obtained at the time of lumbar puncture. Treatment recommendations include induction therapy with amphotericin B and flucytosine for at least 2 weeks, followed by consolidation therapy with fluconazole.40
 Paradural Abscess
Paradural Abscess
The epidural space is between the dura and the bony structures of the skull and vertebral column; the subdural space is between the subarachnoid membrane and the dura (see Figure 134-2). Unlike the subarachnoid space, the paradural tissues are only potential spaces, with the arachnoid membrane and the dura limiting the spread of infection across their surfaces. Although subdural abscesses are more common within the cranium, and epidural abscesses are more common within the vertebral column, the causes, pathophysiology, and therapies are similar. These abscesses usually develop from a contiguous infection, surgery, or trauma.
Cranial Paradural Abscess
Cranial epidural abscesses most commonly occur adjacent to the frontal sinus, but untreated, infection can spread into the subdural or even parenchyma. Disease may be due to trauma, but most commonly is a complication of sinusitis, which is reflected in the microbiology of cranial epidural abscesses.47 Treatment requires emergent surgical drainage followed by antibiotics tailored against the bacteria cultured intraoperatively.
Cranial subdural empyema may be clinically indistinguishable from a brain abscess. Subdural abscess is usually associated with infection of the paranasal sinuses and, less commonly, the ears or mastoids.41 Trauma, surgical intervention, or hematogenous spread are responsible for the remaining cases. Organisms common to sinusitis, including streptococci, pneumococci, Haemophilus, anaerobes, and staphylococci, cause most infections. Gram-negative enteric bacilli may be associated with middle ear and mastoid infections. As with cranial epidural abscess, surgical drainage is crucial for treatment, followed by prolonged antibiotics.47
Spinal Paradural Abscess
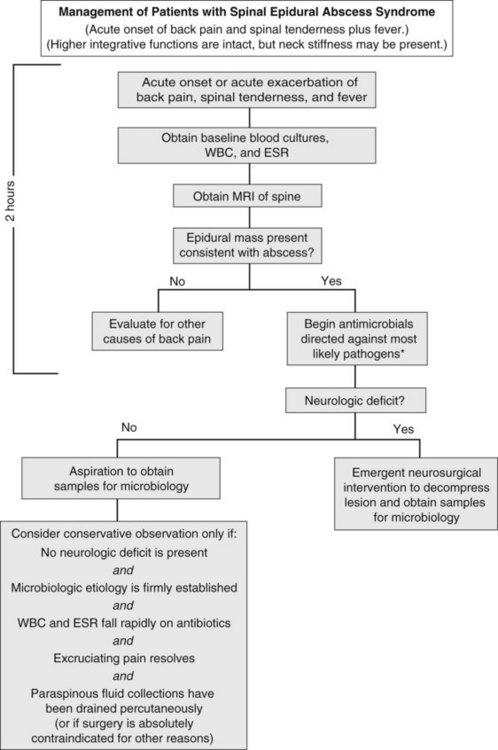
Spinal epidural abscess typically present with localized spinal pain, with fever present in less than 50% of cases.42–43 Cognition generally remains intact; systemic manifestations are rarely severe enough to cause cortical dysfunction. Symptoms usually progress through four clinical phases: spinal ache, nerve root pain, radicular weakness, and paralysis. The triad of back pain, fever, and progressive neurologic deficits strongly suggests this syndrome; however, the presence of any of these signs or symptoms should raise concern for the diagnosis.
Diagnosis hinges on visualization of a collection in the epidural space (Figure 134-6). The diagnostic study of choice is MRI, which defines cord compression and the presence and extent of abscess, identifies drainable paraspinal fluid collections, and detects concomitant vertebral osteomyelitis. Other procedures such as myelography and CT scanning may be used if MRI cannot be performed.
S. aureus accounts for more than two-thirds of cases of epidural abscess.42,44 Although most cases are community acquired, an increasing number are due to spinal instrumentation (surgery or nerve block), and nosocomial flora such as MRSA or Pseudomonas may be causative in this population. Other risk factors for spinal epidural abscess include IV drug use, diabetes mellitus, trauma, and comorbid conditions such as malignancy or alcohol use.42 Empirical therapy is directed against the most likely organisms and typically includes vancomycin and an antipseudomonal agent such as cefepime. Therapy should be refined once cultures confirm a causative pathogen.
Emergency neurosurgical intervention is considered mandatory for spinal paradural abscess if there is clinical evidence of cord compression. In a few selected cases, patients may be successfully treated with antibiotics alone. Nonsurgical management might be considered if a pathogen is identified by peripheral blood cultures or by needle biopsy; if the patient is neurologically intact and there is no progression of neurologic findings (e.g., weakness) on frequent examination; if pain improves with treatment; and if fever, peripheral white blood cell count, and sedimentation rate all decline on therapy. Unfortunately, some patients treated conservatively develop sudden neurologic impairment even weeks into conservative therapy, and retrospective data suggest a combined medical and surgical approach is associated with improved outcomes.42 Progressive weakness mandates the need for immediate MRI and neurosurgical consultation, because decompression within 24 hours offers the best chance of neurologic recovery.44
 Sepsis Syndrome with Central Nervous System Involvement
Sepsis Syndrome with Central Nervous System Involvement
In the sepsis syndrome, an acutely ill patient develops CNS dysfunction late in the course of the illness, typically in the setting of multiorgan system failure. Altered mental status, attributable to hypotension and hypoperfusion, ranges from confusion to obtundation. Seizures may occur due to metabolic abnormalities, ischemia, or hemorrhage. In treating such patients, general supportive measures take precedence over CNS concerns. After a brief assessment, general life-support measures should correct hypotension, hypoxia, and anuria. As soon as they are easily accessible, body fluid specimens are obtained for culture, and broad-spectrum antimicrobials should be administered. At this point, a careful history should be taken and physical examination performed. The patient is treated as outlined for subacute CNS infection syndrome (see Figure 134-4). Delays in directly assessing the CNS are justifiable only when the history is adequate to document that a clear-cut systemic illness preceded the onset of CNS symptoms and signs. Otherwise, a more aggressive use of lumbar puncture and CT or MRI is warranted (see Figures 134-3 and 134-4).
Key Points
Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
Van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;251:1849-1859.
Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303-327.
Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565-1577.
Curry WT, Hoh BL, Amin-Hanjani S, et al. Spinal epidural abscess: clinical presentation, management and outcome. Surg Neurol. 2005;63:364-371.
1 Koedel U, Klein M, Pfister HW. New understandings on the pathophysiology of bacterial meningitis. Curr Opin Infect Dis. 2010;23:217-223.
2 Van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362:146-154.
3 Van de Beek D, de Gans, Tunkel AR, et al. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44-53.
4 Scheld WM, Koedel U, Nathan B, et al. Pathophysiology of bacterial meningitis: Mechanism(s) of neuronal injury. J Infect Dis. 2002;186(Suppl 2):S225-S233.
5 Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
6 Van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;251:1849-1859.
7 Heckenberg SG, De Gans J, Brouwer MC, et al. Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: a prospective cohort study. Medicine (Baltimore). 2008;87:185-192.
8 Weisfelt M, van de Beek D, Spanjaard L, et al. A risk score for unfavorable outcome in adults with bacterial meningitis. Ann Neurol. 2008;63:90-97.
9 Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: Risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862.
10 Cabellos C, Verdaguer R, Olmo M, et al. Community-acquired bacterial meningitis in elderly patients: experience over 30 years. Medicine (Baltimore). 2009;88:115-119.
11 Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: Defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic treatment. Pediatrics. 2001;108:1169-1174.
12 Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122:726-730.
13 Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006;296:2012-2022.
14 Kastenbauer S, Winkler F, Pfister HW. Cranial CT before lumbar puncture in suspected meningitis. N Engl J Med. 2002;346:1248-1251.
15 Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1-11.
16 Hasbun R, Abrahams J, Jekel J, et al. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345:1727-1733.
17 Kupila L, Vuorinen T, Vainionpää R, et al. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology. 2006;66:75-80.
18 Nudelman Y, Tunkel AR. Bacterial meningitis: epidemiology, pathogenesis and management update. Drugs. 2009;69:2577-2596.
19 Bradley JS, Scheld WM. The challenge of penicillin-resistant Streptococcus pneumoniae meningitis: Current antibiotic therapy in the 1990s. Clin Infect Dis. 1997;24(Suppl 2):S213-S221.
20 Lin TY, Chrane DF, Nelson JD, et al. Seven days of ceftriaxone therapy is as effective as ten days’ treatment for bacterial meningitis. JAMA. 1985;253:3559-3563.
21 van de Beek D, de Gans J, McIntyre P, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2007;(1):CD004405.
22 van de Beek D, Farrar JJ, de Gans J, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol. 2010;9:254-263.
23 Vincent JL, Nadel S, Kutsogiannis DJ, et al. Drotrecogin alfa (activated) in patients with severe sepsis presenting with purpura fulminans, meningitis, or meningococcal disease: a retrospective analysis of patients enrolled in recent clinical studies. Crit Care. 2005;9:R331-R343. 24
24 Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303-327.
25 Siegel JD, Rhinehart E, Jackson M, Chiarello L, the Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Available at. http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf.
26 Seydoux C, Francioli P. Bacterial brain abscesses: Factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394-401.
27 Tsou TP, Lee PI, Lu CY, et al. Microbiology and epidemiology of brain abscess and subdural empyema in a medical center: a 10-year experience. J Microbiol Immunol Infect. 2009;42:405-412.
28 Mamelak AN, Mampalam TJ, Obana WG, et al. Improved management of multiple brain abscesses: A combined surgical and medical approach. Neurosurgery. 1995;36:76-85.
29 Bloch KC, Glaser CA, Tunkel A. Encephalitis and Myelitis. In: Cohen J, Opal SM, Powderley WG, editors. Infectious Diseases. 3rd ed. Elsevier Press. In Press.
30 Frantzidou F, Kamaria F, Dumaidi K, et al. Aseptic meningitis and encephalitis because of herpesviruses and enteroviruses in an immunocompetent adult population. Eur J Neurol. 2008;15:995-997.
31 Khetsuriani N, Holman RC, Anderson LJ. Burden of encephalitis-associated hospitalizations in the United States, 1988-1997. Clin Infect Dis. 2002;35:175-182.
32 Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: Clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565-1577.
33 Tenembaum S, Chitnis T, Ness J, et al. Acute disseminated encephalomyelitis. Neurology. 2007;68:S23-S36.
34 Whitley RJ, Soong SJ, Linneman CJr, et al. Herpes simplex encephalitis: Clinical assessment. JAMA. 1982;247:317-320.
35 Redington JJ, Tyler KL. Viral infections of the central nervous system, 2002: Update on diagnosis and treatment. Arch Neurol. 2002;59:712-718.
36 Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: Application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1995;171:857-863. (see comments)
37 Weil A, Glaser C, Amad Z, et al. Patients with suspected herpes simplex encephalitis: Rethinking an initial negative polymerase chain reaction result. Clin Infect Dis. 2002;34:1154-1157.
38 Tyler KL. Herpes simplex infection of the central nervous system: Encephalitis and meningitis including Mollaret’s. Herpes. 2004;11:S57a-64a.
39 Evaluation and management of intracranial mass lesions in AIDS. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1998;50:21-26.
40 Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
41 Greenlee JE. Subdural empyema. Curr Treat Options Neurol. 2003;5:13-22.
42 Curry WT, Hoh BL, Amin-Hanjani S, et al. Spinal epidural abscess: clinical presentation, management and outcome. Surg Neurol. 2005;63:364-371.
43 Kempthorne JT, Pratt C, Smale EL, et al. Ten-year review of extradural spinal abscesses in a New Zealand tertiary referral centre. J Clin Neurosci. 2009;16:1038-1042.
44 Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;232:175-204.
45 Berger JR. Pearls: Neurologic complications of HIV/AIDS. Semin Neurol. 2010;30:66-70.
46 Young RJ, Ghesani MV, Kagetsu NJ, et al. Lesion size determines accuracy of thallium-201 brain single photon emission tomography in differentiating between intracranial malignancy and infection in AIDS patients. AJNR Am J Neuroradiol. 2005;26:1975-1979.
47 Hall WA, Truwitt CL. The surgical management of infections involving the cerebrum. Neurosurgery. 2008;62:SHC519-SHC531.