Central and peripheral nervous systems
After reading this chapter, the reader will be able to:
1. Define key terms pertaining to the central and peripheral nervous systems
2. Classify the branches of the nervous system
3. Differentiate between the central, peripheral, and autonomic nervous systems
4. Discuss the use of neurotransmitters
5. Explain in detail the difference between the parasympathetic and sympathetic branches of the nervous system
6. Differentiate the effects of cholinergic and anticholinergic agents on the nervous system
7. Differentiate the effects of adrenergic and antiadrenergic agents on the nervous system
8. Discuss the various receptors in the airways
9. Differentiate between nonadrenergic, noncholinergic inhibitory, and excitatory nerves
Chemical produced by the body that is used in the transmission of nerve impulses. It is destroyed by the enzyme cholinesterase.
Refers to a drug stimulating a receptor for norepinephrine or epinephrine.
Signals that are transmitted to the brain and spinal cord.
Refers to a drug blocking a receptor for norepinephrine or epinephrine.
Refers to a drug blocking a receptor for acetylcholine.
System that includes the brain and spinal cord, controlling voluntary and involuntary acts.
Refers to a drug causing stimulation of a receptor for acetylcholine.
Signals that are transmitted from the brain and spinal cord.
Naturally occurring catecholamine, produced by the adrenal medulla, that has properties similar to epinephrine. It is used as a neurotransmitter in most sympathetic terminal nerve sites.
Agent blocking or inhibiting the effects of the parasympathetic nervous system.
Agent causing stimulation of the parasympathetic nervous system.
Peripheral nervous system (PNS)
Portion of the nervous system outside the CNS, including sensory, sympathetic, and parasympathetic nerves.
Agent blocking or inhibiting the effect of the sympathetic nervous system.
Agent causing stimulation of the sympathetic nervous system.
Nervous system
There are two major control systems in the body: the nervous system and the endocrine system. Both systems of control can be manipulated by drug therapy, which either mimics or blocks the usual action of the control system, to produce or inhibit physiologic effects. The endocrine system is considered separately in Chapter 11, which discusses the corticosteroid class of drugs. The nervous system is divided into the central nervous system (CNS) and the peripheral nervous system (PNS), both of which offer sites for drug action. The overall organization of the nervous system may be outlined as follows:
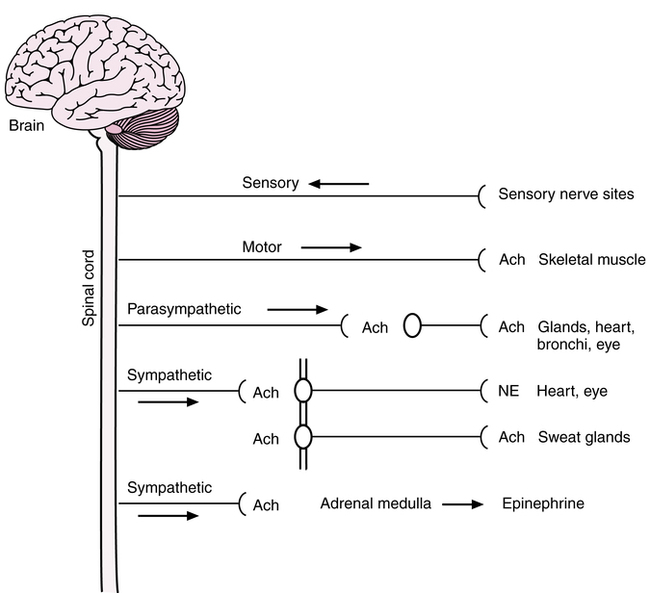
Figure 5-1 is a functional, but not anatomically accurate, diagram of the central and peripheral nervous systems. The sensory branch of the nervous system consists of afferent neurons from heat, light, pressure, and pain receptors in the periphery to the CNS. The somatic portion (or motor branch) of the nervous system is under voluntary, conscious control and innervates skeletal muscle for motor actions such as lifting, walking, or breathing. This portion of the nervous system is manipulated by neuromuscular blocking agents, to induce paralysis in surgical procedures or during mechanical ventilation. The autonomic nervous system is the involuntary, unconscious control mechanism of the body, sometimes said to control vegetative or visceral functions. For example, the autonomic nervous system regulates heart rate, pupillary dilation and contraction, glandular secretion such as salivation, and smooth muscle in blood vessels and the airway. The autonomic nervous system is divided into parasympathetic and sympathetic branches.
Autonomic branches
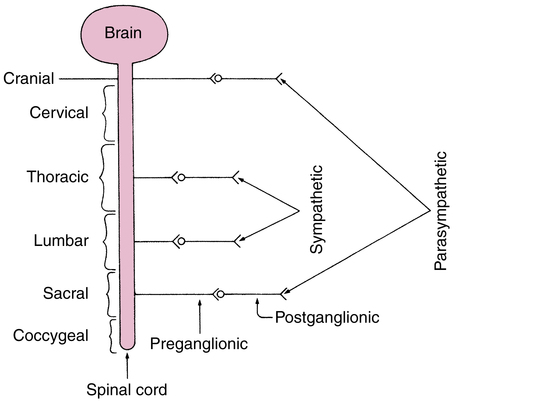
The parasympathetic branch arises from the craniosacral portions of the spinal cord and consists of two types of neurons—a preganglionic fiber leading from the vertebrae to the ganglionic synapse outside the cord and a postganglionic fiber from the ganglionic synapse to the gland or smooth muscle being innervated. The parasympathetic branch has good specificity, with the postganglionic fiber arising very near the effector site (e.g., a gland or smooth muscle). As a result, stimulation of a parasympathetic preganglionic neuron causes activity limited to individual effector sites, such as the heart or the eye. Figure 5-2 illustrates the portions of the spinal cord where the parasympathetic and sympathetic nerve fibers originate.
Neurotransmitters
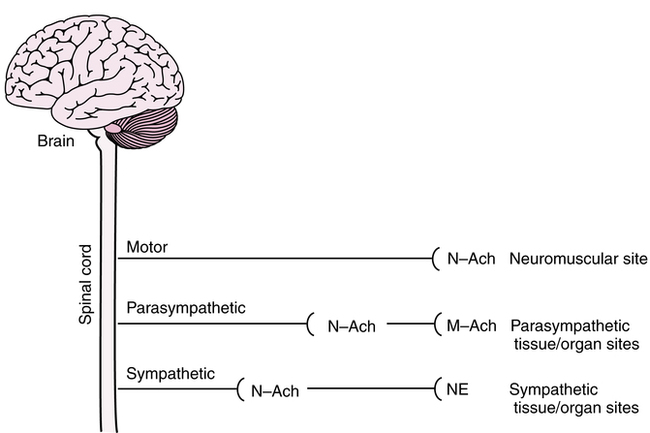
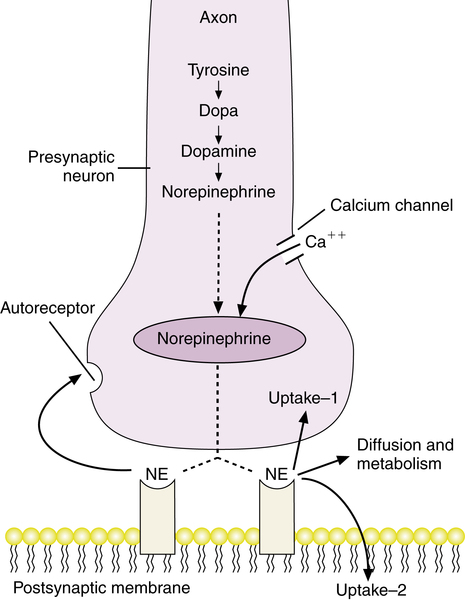
Another general feature of the autonomic nervous system, including sympathetic and parasympathetic branches, is the mechanism of neurotransmitter control of nerve impulses. Nerve impulse propagation is electrical and chemical (electrochemical). A nerve impulse signal is carried along a nerve fiber by electrical action potentials, caused by ion exchanges (sodium, potassium). At gaps in the nerve fiber between neurons (synapses), the electrical transmission is replaced by a chemical neurotransmitter. This is the chemical transmission of the electrical impulse, which occurs at the ganglionic synapses and at the end of the nerve fiber, termed the neuroeffector site. Identification of the chemical transmitters dates back to Loewi’s experiments in 1921 and is fundamental to understanding autonomic drugs and their classifications. The usual neurotransmitters in the PNS, including the ganglionic synapses and terminal sites in the autonomic branches, are shown in Figure 5-1 (Ach and norepinephrine).
Efferent and afferent nerve fibers
The autonomic system is generally considered an efferent system—that is, impulses in the sympathetic and parasympathetic branches travel from the brain and spinal cord out to the various neuroeffector sites, such as the heart, gastrointestinal tract, and lungs. Afferent nerves run alongside the sympathetic and parasympathetic efferent fibers and carry impulses from the periphery to the cord. The afferent fibers convey impulses resulting from visceral stimuli and can form a reflex arc of stimulus input–autonomic output analogous to the well-known somatic reflex arcs, such as the knee-jerk reflex. The mechanism of a vagal reflex arc mediating bronchoconstriction is discussed further in Chapter 7, in conjunction with drugs used to block the parasympathetic impulses.
Parasympathetic branch
Cholinergic neurotransmitter function
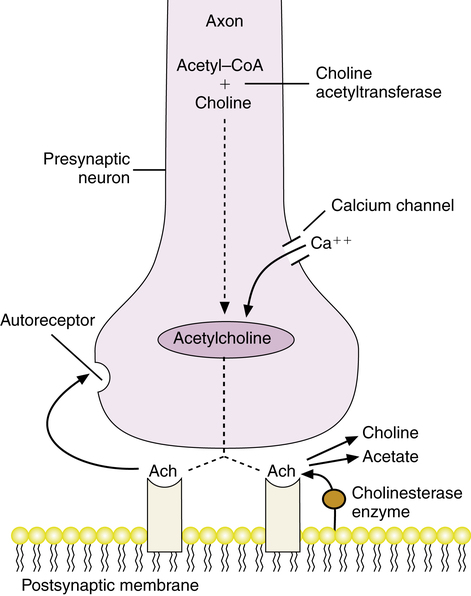
In the parasympathetic branch, the neurotransmitter Ach conducts nerve transmission at the ganglionic site and at the parasympathetic effector site at the end of the postganglionic fiber. This action is illustrated in Figure 5-3. The term neurohormone has also been used in place of neurotransmitter. Ach is concentrated in the presynaptic neuron (both at the ganglion and at the effector site). Ach is synthesized from acetyl-CoA and choline, catalyzed by the enzyme choline acetyltransferase. Ach is stored in vesicles as quanta of 1000 to 50,000 molecules per vesicle. When a nerve impulse (action potential) reaches the presynaptic neuron site, an influx of calcium is triggered into the neuron. Increased calcium in the neuron causes cellular secretion of the Ach-containing vesicles from the end of the nerve fiber. After release, the Ach attaches to receptors on the postsynaptic membrane and initiates an effect in the tissue or organ site.
Ach is inactivated through hydrolysis by cholinesterase enzymes, which split the Ach molecule into choline and acetate, terminating stimulation of the postsynaptic membrane. In effect, the nerve impulse is “shut off.” There are also receptors on the presynaptic neuron, termed autoreceptors, that can be stimulated by Ach to regulate and inhibit further neurotransmitter release from the neuron. The effects of the parasympathetic branch of the autonomic system on various organs are listed in Table 5-1. Drugs can mimic or block the action of the neurotransmitter Ach, to stimulate parasympathetic nerve ending sites (parasympathomimetics) or to block the transmission of such impulses (parasympatholytics). Both categories of drugs affecting the parasympathetic branch are commonly seen clinically. The effects of the parasympathetic system on the heart, bronchial smooth muscle, and exocrine glands should be mentally reviewed before considering parasympathetic agonists or antagonists (blockers):
TABLE 5-1
Effects of Parasympathetic Stimulation on Selected Organs or Sites
| ORGAN/SITE | PARASYMPATHETIC (CHOLINERGIC) RESPONSE |
| Heart | |
| SA node | Slowing of rate |
| Contractility | Decreased atrial force |
| Conduction velocity | Decreased AV node conduction |
| Bronchi | |
| Smooth muscle | Constriction |
| Mucous glands | Increased secretion |
| Vascular Smooth Muscle | |
| Skin and mucosa | No innervation* |
| Pulmonary | No innervation* |
| Skeletal muscle | No innervation† |
| Coronary | No innervation* |
| Salivary Glands | Increased secretion |
| Skeletal Muscle | None |
| Eye | |
| Iris radial muscle | None |
| Iris circular muscle | Contraction (miosis) |
| Ciliary muscle | Contraction for near vision |
| Gastrointestinal Tract | Increased motility |
| Gastrointestinal Sphincters | Relaxation |
| Urinary Bladder | |
| Detrusor | Contraction |
| Trigone sphincter | Relaxation |
| Glycogenolysis | |
| Skeletal muscle | None |
| Sweat Glands | None‡ |
| Lipolysis (Multiple Sites) | None |
| Renin Secretion (Kidney) | None |
| Insulin Secretion (Pancreas) | Increased |
AV, Atrioventricular; SA, sinoatrial.
*No direct parasympathetic nerve innervation; response to exogenous cholinergic agonists is dilation.
†Dilation occurs as a result of sympathetic cholinergic discharge or as a response to exogenous cholinergic agonists.
‡Sweat glands are under sympathetic control; receptors are cholinergic, however, and the response to exogenous cholinergic agonists is increased secretion.
Muscarinic and nicotinic receptors and effects
Two additional terms are used to refer to stimulation of receptor sites for Ach; they are derived from the action in the body of two substances, the alkaloids muscarine and nicotine. Receptor sites that are stimulated by these two chemicals are illustrated in Figure 5-4.
Subtypes of muscarinic receptors
Parasympathetic receptors and cholinergic receptors in general with or without corresponding nerve fibers are classified further into subtypes. These differences among cholinergic or muscarinic (M) receptors are based on different responses to different drugs, or recognition through use of DNA probes. Five muscarinic receptor subtypes have been identified: M1, M2, M3, M4, and M5. They are all G protein–linked (see Chapter 2). As G protein–linked receptors, these five subtypes of muscarinic receptors share a structural feature common to such receptors—a long, “serpentine” polypeptide chain that crosses the cell membrane seven times (illustrated for G protein receptors in Chapter 2). Table 5-2 summarizes the muscarinic receptor subtypes, with their predominant location and the type of G protein with which they are coupled. Additional details about muscarinic receptor location and function in the pulmonary system are presented in the final section of this chapter, which summarizes nervous control and receptors in the lung.
TABLE 5-2
Muscarinic Receptor Subtypes, Location, and G-Protein Linkage
| MUSCARINIC RECEPTOR TYPE | LOCATION | G-PROTEIN SUBTYPE |
| M1 | Parasympathetic ganglia, nasal submucosal glands | Gq |
| M2 | Heart, postganglionic parasympathetic nerves | Gi |
| M3 | Airway smooth muscle, submucosal glands | Gq |
| M4 | Postganglionic cholinergic nerves, possible effect on CNS | Gi |
| M5 | Possible effect on CNS | Gq |
Cholinergic agents
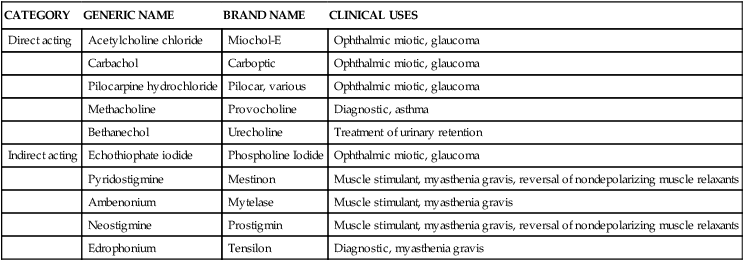
Cholinergic drugs mimic the action caused by Ach at receptor sites in the parasympathetic system and neuromuscular junction. Such agents can cause stimulation at the terminal nerve site (neuroeffector junction) by two distinct mechanisms, leading to their classification as direct acting or indirect acting. Table 5-3 lists cholinergic agents, categorized as direct acting or indirect acting, and their clinical uses. The terms cholinergic, cholinoceptor stimulant, and cholinomimetic are broader than parasympathomimetic and denote agents stimulating Ach receptors located in the parasympathetic system (muscarinic) or other sites, such as the neuromuscular junction (nicotinic). A cholinergic drug can activate muscarinic and nicotinic receptors.
TABLE 5-3
Examples of Direct-Acting and Indirect-Acting Cholinergic Agents
| CATEGORY | GENERIC NAME | BRAND NAME | CLINICAL USES |
| Direct acting | Acetylcholine chloride | Miochol-E | Ophthalmic miotic, glaucoma |
| Carbachol | Carboptic | Ophthalmic miotic, glaucoma | |
| Pilocarpine hydrochloride | Pilocar, various | Ophthalmic miotic, glaucoma | |
| Methacholine | Provocholine | Diagnostic, asthma | |
| Bethanechol | Urecholine | Treatment of urinary retention | |
| Indirect acting | Echothiophate iodide | Phospholine Iodide | Ophthalmic miotic, glaucoma |
| Pyridostigmine | Mestinon | Muscle stimulant, myasthenia gravis, reversal of nondepolarizing muscle relaxants | |
| Ambenonium | Mytelase | Muscle stimulant, myasthenia gravis | |
| Neostigmine | Prostigmin | Muscle stimulant, myasthenia gravis, reversal of nondepolarizing muscle relaxants | |
| Edrophonium | Tensilon | Diagnostic, myasthenia gravis |
Direct-acting cholinergic agents
Direct-acting cholinergic agents are structurally similar to Ach. As shown in Figure 5-3, direct-acting cholinergic agents mimic Ach, binding and activating muscarinic or nicotinic receptors directly. Examples of this group include methacholine, carbachol, bethanechol, and pilocarpine. Methacholine has been used in bronchial challenge tests by inhalation to assess the degree of airway reactivity in asthmatics and others. The parasympathetic effect is bronchoconstriction. Methacholine is a useful diagnostic agent to detect differences in degree of airway reactivity between asthmatics with hyperreactive airways and nonasthmatic individuals.
Indirect-acting cholinergic agents
Indirect-acting cholinergic agonists inhibit the cholinesterase enzyme, as seen in Figure 5-3. Because cholinesterase usually inactivates the Ach neurotransmitter, inhibiting this enzyme results in accumulation of endogenous Ach at the neuroeffector junction of parasympathetic nerve endings or the neuromuscular junction. More Ach is made available to attach to receptor sites and to stimulate cholinergic responses. If Ach receptors have been blocked, this increase in neurotransmitter can reverse the blockage by competing with the blocking drug for the receptors. Nerve transmission can then resume, either at the parasympathetic terminal site or at the neuromuscular junction.
The drug echothiophate (Phospholine), listed in Table 5-3, stimulates autonomic muscarinic receptors in the iris sphincter and ciliary muscle of the eye to produce pupillary constriction (miosis) and lens thickening. An increase in the neurotransmitter Ach at the neuromuscular junction makes drugs such as neostigmine useful in reversing neuromuscular blockade caused by paralyzing agents such as pancuronium or doxacurium (see Chapter 18). Neostigmine and edrophonium are also useful in increasing muscle strength in a neuromuscular disease such as myasthenia gravis, in which the cholinergic receptor is blocked by autoantibodies. The drug edrophonium (Tensilon) is used in the Tensilon test to determine whether muscle weakness is caused by overdosing with an indirect-acting cholinergic agent (causing ultimate receptor fatigue and blockade) or undertreatment with insufficient drug. Because edrophonium is short-acting (5 to 15 minutes, depending on the dose), it is useful as a diagnostic agent, rather than as a maintenance treatment in neuromuscular disease.
Anticholinergic agents
Atropine as a prototype parasympatholytic agent
Atropine is usually considered the prototype parasympatholytic, and there is renewed interest in the use of aerosolized analogues of atropine in respiratory care; this is discussed more fully in Chapter 7. Atropine occurs naturally as the levo isomer in Atropa belladonna, the nightshade plant, and in Datura stramonium, or jimsonweed. The drug is referred to as a belladonna alkaloid.
Parasympatholytic (antimuscarinic) effects
If the basic effects of the parasympathetic system are known, the effects of an antagonist such as atropine can be deduced. For example, if parasympathetic (vagal) stimulation slows the heart rate, parasympatholytic should increase the heart rate by blocking that innervation. Box 5-1 lists the effects and uses of parasympatholytic agents.
Sympathetic branch
Adrenergic neurotransmitter function
In the sympathetic branch of the autonomic nervous system, the usual neurotransmitter at the terminal nerve sites is norepinephrine, with the exceptions described previously (sweat glands and adrenal medulla). Figure 5-5 illustrates neurotransmitter function with norepinephrine. In the presynaptic neuron, tyrosine is converted to dopa and then to dopamine, which is converted by dopamine β-hydroxylase to norepinephrine, in the storage vesicles. An action potential in the nerve opens calcium channels, allowing an influx of calcium. Increased intracellular calcium leads to exocytosis of the vesicles containing norepinephrine, which attaches to receptors on the postsynaptic membrane. The exact physiologic effect depends on the site of innervation and the type of sympathetic receptor, which can also vary, as described subsequently.
The distinction between two types of uptake processes is due to research published by Iversen in 1965.1 The uptake-2 process is a mediated uptake of exogenous amines (chemicals such as norepinephrine) in nonneuronal tissues, such as cardiac muscle cells. Iversen and Salt2 distinguished details of the uptake-2 process:
• It is a mediated transport system.
• It is a low-affinity but high-capacity system.
• It is not as stereochemically specific as uptake-1.
• It is specific to catecholamines.
• The order of affinity for uptake of specific agents, in decreasing order, is as follows: isoproterenol > epinephrine > norepinephrine.
• Certain corticosteroids can inhibit the uptake-2 process, potentiating catecholamines.
The last effect of uptake-2 inhibition by corticosteroids is discussed more fully in Chapter 11. Table 5-4 lists the physiologic effects of sympathetic activation. The effects listed in Table 5-4 are given for the same organs as listed in Table 5-1 for the parasympathetic system for comparison.
TABLE 5-4
Effects of Sympathetic (Adrenergic) Stimulation on Selected Organs or Sites*
| ORGAN/SITE | SYMPATHETIC (ADRENERGIC) RESPONSE |
| Heart | |
| SA node | Increase in rate |
| Contractility | Increase in force |
| Conduction velocity | Increased AV node conduction |
| Bronchi | |
| Smooth muscle | Relaxation and dilation of airway diameter |
| Mucous glands | Increased secretion |
| Vascular Smooth Muscle | |
| Skin and mucosa | Vasoconstriction |
| Pulmonary | Dilation/constriction (two types of sympathetic receptors) |
| Skeletal muscle | Dilation (predominantly) |
| Coronary | Dilation/constriction (two types of sympathetic receptors) |
| Salivary Glands | Decreased secretion |
| Skeletal Muscle | Increased contractility |
| Eye | |
| Iris radial muscle | Contraction (mydriasis) |
| Iris circular muscle | None |
| Ciliary muscle | Relaxation for far vision† |
| Gastrointestinal Tract | Decreased motility |
| Gastrointestinal Sphincters | Contraction |
| Urinary Bladder | |
| Detrusor | Relaxation |
| Trigone sphincter | Contraction |
| Sweat Glands | Increased secretion‡ |
| Glycogenolysis | |
| Skeletal muscle | Increased |
| Lipolysis (Multiple Sites) | Increased |
| Renin Secretion (Kidney) | Increased |
| Insulin Secretion (Pancreas) | Decreased |
AV, Atrioventricular; SA, sinoatrial.
*Effects of sympathetic activation are mediated by direct innervation of nerve fibers and by circulating epinephrine released from the adrenal medulla.
†Relaxes as a result of circulating epinephrine, with sympathetic activation.
‡Innervated by sympathetic nerves with acetylcholine neurotransmitter (cholinergic receptors); response to exogenous cholinergic agent is increased sweating.
Enzyme inactivation
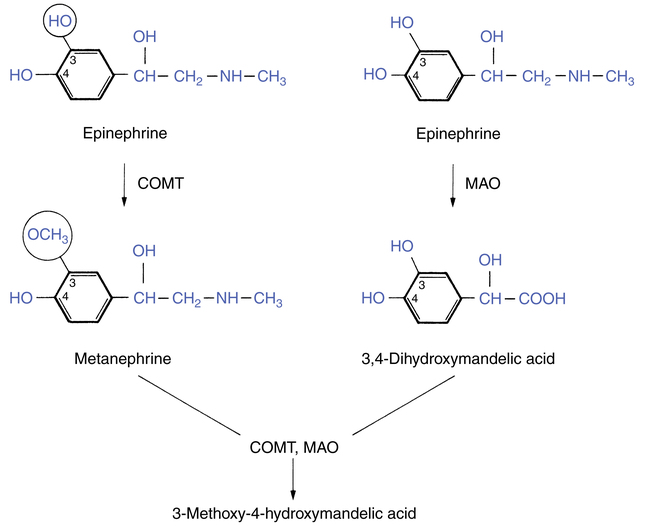
The enzymes that metabolize norepinephrine, epinephrine, and chemicals similar to these neurotransmitters are important for understanding differences in the action of the adrenergic bronchodilator group. Chemicals structurally related to epinephrine are termed catecholamines, and their general structure is outlined in Chapter 6 in the discussion of sympathomimetic (adrenergic) bronchodilators. Two enzymes are available that can inactivate catecholamines such as epinephrine: catechol O-methyltransferase (COMT) and monoamine oxidase (MAO). The action of both enzymes on epinephrine (Figure 5-6) is important because COMT is responsible for ending the action of catecholamine bronchodilators.
Sympathetic (adrenergic) receptor types
The effects of adrenergic receptors are mediated by coupling with G proteins, and they are identified as G protein–linked receptors. Adrenergic receptor subtypes, with examples of their location and the type of G protein with which they are coupled, are summarized in Table 5-5.
TABLE 5-5
Adrenergic Receptor Subtypes: Location and G-Protein Linkage
| RECEPTOR TYPE | LOCATION | G-PROTEIN SUBTYPE |
| α1 | Peripheral blood vessels | Gq |
| α2 | Presynaptic sympathetic neurons (autoreceptor), CNS | Gi |
| β1 | Heart | Gs |
| β2 | Smooth muscle (including bronchial), cardiac muscle | Gs |
| β3 | Lipocytes | Gs |
α and β receptors
In 1948, Ahlquist3 distinguished alpha (α) and beta (β) sympathetic receptors on the basis of differing responses to various adrenergic drugs, all of which were similar to norepinephrine with minor structural differences. These drugs included phenylephrine, norepinephrine, epinephrine, and isoproterenol. The two types of sympathetic receptors were distinguished as follows:
• α receptors: Generally excite, with the exception of the intestine and CNS receptors, where inhibition or relaxation occurs
• β receptors: Generally inhibit or relax, with the exception of the heart, where stimulation occurs
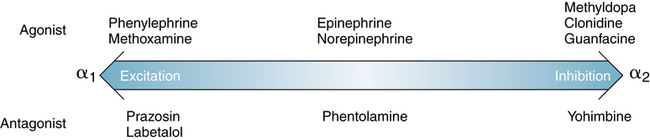
α-sympathetic receptors are found on peripheral blood vessels, and stimulation results in vasoconstriction. α-adrenergic agonists are frequently used for topical vasoconstriction of the nasal mucosa, to treat symptoms of nasal congestion caused by the common cold. β-adrenergic receptors are found on airway smooth muscle and in the heart. Drug activity of adrenergic stimulants (sympathomimetics) ranges along the spectrum seen in Figure 5-7.
As illustrated in Figure 5-7, phenylephrine is one of the purest α stimulants, and isoproterenol is an almost pure β stimulant. “Pure” reactions do not occur with any drug—that is, even phenylephrine may affect other sites. Epinephrine stimulates α and β sites equally, but norepinephrine has more of an α than β effect.
β1 and β2 receptors
In 1967, Lands and colleagues4 further differentiated β receptors into β1 and β2 subtypes. β1 receptors are found in cardiac muscle, and β2 receptors (which encompass all other β receptors) are found in bronchial, vascular, and skeletal muscle. The distinction among types of β receptors is as follows:
• β1 receptors: Increases the rate and force of cardiac contraction
• β2 receptors: Relaxes bronchial smooth muscle and vascular beds of skeletal muscle
β1 receptors constitute the exception to the general rule that β receptors cause relaxation. β2 receptors form the basis for the class of adrenergic bronchodilators, which act to relax bronchial smooth muscle by stimulation of these receptors. The β receptor, briefly characterized as an example of a G protein–linked receptor in Chapter 2 in the introduction to pharmacodynamics (drug-receptor interaction), is discussed in more detail in Chapter 6, which discusses β-adrenergic bronchodilators. A third type of β receptor, the β3 receptor, has also been distinguished as a β-receptor type found on lipocytes (fat cells) whose stimulation results in lipolysis.
α1 and α2 receptors
α receptors have also been differentiated into α1 and α2 receptors. This classification has been made on a morphologic basis (location of the receptors) and a pharmacologic basis (differences in response to various drugs). The pharmacologic differentiation of α1 and α2 receptors is similar to the distinction between α and β receptors (Figure 5-8). This differentiation is based on a response continuum ranging from excitation (α1) to inhibition (α2) as different drugs are administered. For example, phenylephrine causes vasoconstriction, as previously mentioned, whereas clonidine (Catapres) causes a lowering of blood pressure and sympathetic activity. Both agents are considered to be α-receptor agonists. Other agents such as prazosin (Minipress) or labetalol (Normodyne) cause a lowering of blood pressure, but yohimbine causes an increase in blood pressure. Yet all these agents are considered α-receptor antagonists. Blockade of α1-excitatory receptors by prazosin would prevent vasoconstriction and decrease blood pressure, whereas blockade of α2-inhibitory receptors by yohimbine would prevent vasodilation and increase blood pressure.
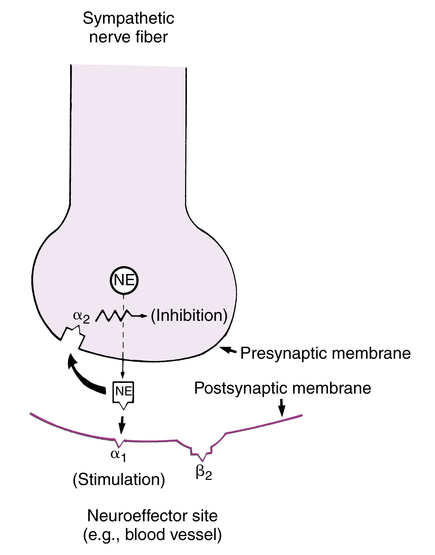
Because different α agonists can cause opposite effects, and different α blockers do the same, α receptors were subdivided into the two types described. The location-based, or morphologic, differentiation of α1 and α2 receptors is more complex. In peripheral nerves, α1 receptors are located on postsynaptic sites such as vascular smooth muscle, and α2 receptors are presynaptic (Figure 5-9). Stimulation of these peripheral α1 receptors causes excitation and vasoconstriction; activation of peripheral (presynaptic) α2 receptors causes inhibition of further neurotransmitter release. Peripheral α2 receptors perform a negative feedback control mechanism, referred to as autoregulation, which has been shown with sympathetic (adrenergic) neurons; they are referred to as autoreceptors (see Figure 5-5).5 Norepinephrine released from the nerve ending can activate α1 (postsynaptic) and α2 (presynaptic) receptors. Postsynaptic stimulation causes a cell response such as vasoconstriction, but presynaptic stimulation leads to inhibition of further neurotransmitter release. In the CNS, α2 receptors are generally considered to be on postsynaptic sites; this is the reverse of their location peripherally, where they are presynaptic. These central postsynaptic α2 receptors are the site of action for antihypertensive agents such as clonidine (Catapres) or methyldopa (Aldomet). These are discussed further and illustrated in Chapter 22.
Sympathomimetic (adrenergic) and sympatholytic (antiadrenergic) agents
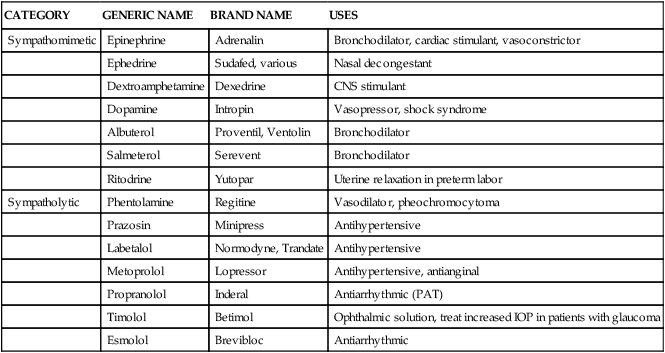
Drugs that stimulate the sympathetic system and produce adrenergic effects (sympathomimetics) and drugs that block adrenergic effects (sympatholytics) are discussed in greater detail in separate chapters. In this book, emphasis is placed on β-adrenergic agonists used for bronchodilation (see Chapter 6) and on adrenergic agonists used for cardiovascular effects such as cardiac stimulation (see Chapter 21) or vasoconstriction (see Chapter 22). Adrenergic blocking agents are considered for their antihypertensive and antianginal effects (see Chapter 20). To exemplify both sympathomimetic and sympatholytic agents, Table 5-6 provides selected examples of drugs categorized as agonists or antagonists of the sympathetic system, listing generic names, brand names, and common clinical uses.
TABLE 5-6
Examples of Adrenergic Agonists and Antagonists
| CATEGORY | GENERIC NAME | BRAND NAME | USES |
| Sympathomimetic | Epinephrine | Adrenalin | Bronchodilator, cardiac stimulant, vasoconstrictor |
| Ephedrine | Sudafed, various | Nasal decongestant | |
| Dextroamphetamine | Dexedrine | CNS stimulant | |
| Dopamine | Intropin | Vasopressor, shock syndrome | |
| Albuterol | Proventil, Ventolin | Bronchodilator | |
| Salmeterol | Serevent | Bronchodilator | |
| Ritodrine | Yutopar | Uterine relaxation in preterm labor | |
| Sympatholytic | Phentolamine | Regitine | Vasodilator, pheochromocytoma |
| Prazosin | Minipress | Antihypertensive | |
| Labetalol | Normodyne, Trandate | Antihypertensive | |
| Metoprolol | Lopressor | Antihypertensive, antianginal | |
| Propranolol | Inderal | Antiarrhythmic (PAT) | |
| Timolol | Betimol | Ophthalmic solution, treat increased IOP in patients with glaucoma | |
| Esmolol | Brevibloc | Antiarrhythmic |
CNS, Central nervous system; IOP, intraocular pressure; PAT, paroxysmal atrial tachycardia.
Neural control of lung function
In addition to autonomic nerve fibers and the receptors associated with them, sites in the lung (smooth muscle, glands, and vascular beds) may be affected by release of mediators from inflammatory cells, such as mast cells and platelets, or by release of epithelial factors, such as a relaxant factor, which can reduce airway contractility in response to spasmogens such as histamine, serotonin, or Ach.6 Receptors in the lung and airways for mediators released by inflammatory cells include the following:
• Histamine receptors: Especially the H1 type
• Prostaglandin receptors: Such as prostacyclin, prostaglandin D2 (PGD2), prostaglandin F2α (PGF2α), and thromboxane A2
• Leukotriene receptors: Such as LTB4 and the C4-D4-E4 series that comprise what was formerly termed slow-reacting substance of anaphylaxis (SRS-A)
• Platelet-activating factor (PAF) receptors
The mediators of inflammation and their receptors (e.g., histamine and prostaglandins) are discussed in the review of corticosteroids (Chapter 11) and other antiasthmatic drugs (Chapter 12) intended to inhibit or prevent an inflammatory response in the lung.
Sympathetic innervation and effects
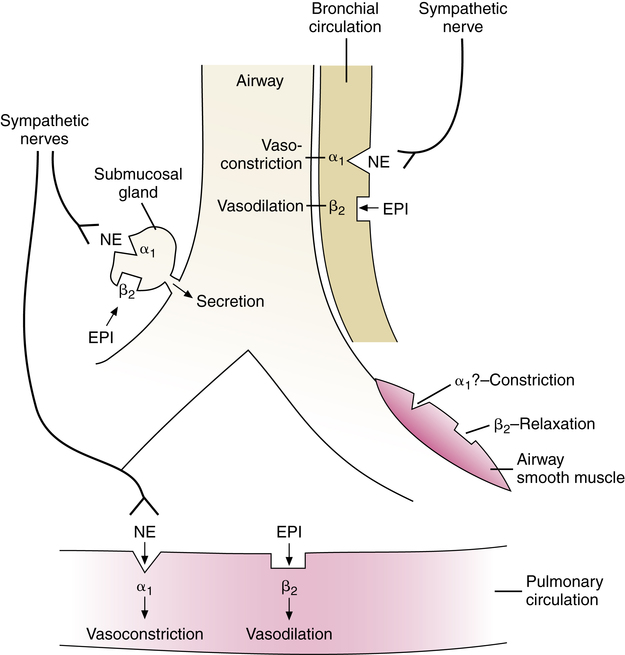
Sympathetic nerve fibers form ganglionic synapses outside the lung. Postganglionic sympathetic nerve fibers from the cervical and upper thoracic ganglia form plexuses at the hilar region of the lung and enter the lung mingled with parasympathetic nerves. Histochemical and ultrastructural studies show a relatively high density of sympathetic nerve fibers to submucosal glands and bronchial arteries but few or no nerve fibers to airway smooth muscle in human lung.7 Figure 5-10 illustrates sympathetic innervation and effects mediated directly by nerve action and indirectly by circulating epinephrine in the human lung.
Airway smooth muscle
There is little or no direct sympathetic innervation of airway smooth muscle in the human lung.8 The sympathetic nervous system controls bronchial smooth muscle tone by circulating epinephrine and norepinephrine, which act on α and β receptors on airway smooth muscle. Recall that epinephrine stimulates both α and β receptors, whereas norepinephrine acts primarily on α receptors.
β receptors.
β receptors mediate relaxation of airway smooth muscle. This action is mimicked by the class of β-adrenergic bronchodilators, introduced in Chapter 6. β receptors are distributed from the trachea to the terminal bronchioles, and the density of these receptors increases as airway diameter becomes smaller. β agonists can cause relaxation of small airways.
β2 receptors traditionally have been identified as the β-receptor subtype on the airway smooth muscle; this has been further verified for human lung by autoradiographic studies and molecular gene studies.9 There is species variation for the presence of β-receptor subtypes, however, with β1 and β2 receptors present in guinea pig and dog airways.
β1 and β2 receptors in the lung have also been distinguished as neuronal and hormonal receptors, respectively. This is based on the concept that β1 receptors are β receptors for sites where norepinephrine is released from sympathetic nerve terminal fibers (neuronal); β2 receptors are β receptors responsive to circulating epinephrine (hormonal). Both receptors cause airway smooth muscle relaxation when stimulated, either by sympathetic nerve release of norepinephrine or by circulating epinephrine, in species such as the dog or guinea pig, which have β1 receptors on airway smooth muscle.10 In the human lung, which has no sympathetic innervation of the airway smooth muscle, adrenergic receptors are all of the β2 type; β1 receptors have been identified by radioligand binding and autoradiographic studies of alveolar walls in the lung periphery.9 Using this terminology, relaxation of human airway smooth muscle would be accomplished by stimulation of hormonal β receptors, by circulating or exogenous catecholamines. β3 receptors, which have also been identified on lipocytes, have no known function in the human airway.11
α receptors.
α receptors exist in human lung in less quantity than β receptors and with no difference in distribution between large and small airways. Norepinephrine stimulates α receptors, but their effect in the airway seems to be minor. Evidence of sympathetic-induced bronchoconstriction has been provided by studies in which lung tissue was treated with a β blocker, or antagonist, such as propranolol, and then exposed to epinephrine, which stimulates α and β receptors.12 Because β receptors were blocked, the epinephrine attached to the free α receptors, and the result was contraction of the smooth muscle, providing evidence of the existence of α receptors and showing a contractile effect. The clinical use of α receptor–blocking agents such as dibenamine, thymoxamine, and phentolamine in cases of status asthmaticus has been reported for more than 40 years, lending support to the role of α receptors in bronchial contraction.13 The role of α receptors in controlling airway smooth muscle remains the subject of investigation.
Mucous glands
Human bronchial submucosal glands are innervated by sympathetic and parasympathetic nerves. α and β receptors are present on tracheal submucosal glands. Stimulation of these receptors causes an increase in secretion of fluid and mucus. Epithelial cells on the airway lining do not have direct sympathetic innervation but do possess β2 receptors whose stimulation can also increase secretion of fluid. Mucociliary clearance is enhanced, removing trapped particulate matter.14
Parasympathetic innervation and effects
Cholinergic nerve fibers in the lung are densest in the hilar region and decrease toward the airway periphery. Cholinergic muscarinic receptors also decrease in density in distal airways. Electrical stimulation of vagus nerves in dog studies caused more contraction in the intermediate bronchi than in the main bronchi or trachea.15
Muscarinic receptors in the airway
The genes for five subtypes of Ach, or muscarinic, receptors have been identified, designated M1 through M5. Only four of these subtypes, M1 to M4, have been identified by chemical (ligand)-binding studies pharmacologically. Three of these muscarinic receptor subtypes have been identified in human lung: M1, M2, and M3. Their location is illustrated in Figure 5-11, and the function of each is discussed.
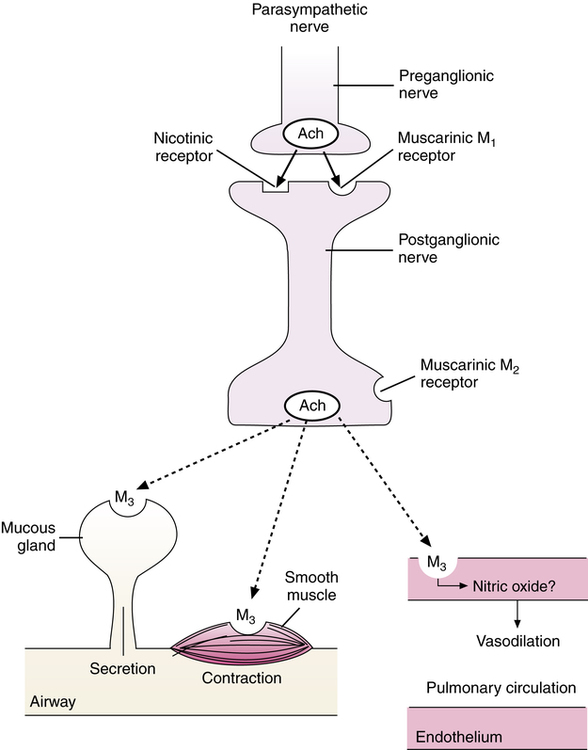
M2 receptors.
M2 receptors are localized to the presynaptic membrane of postganglionic parasympathetic nerve endings. These receptors are thought to be autoregulatory receptors whose stimulation by Ach inhibits further Ach release from the nerve ending, thereby limiting the cholinergic stimulation. This is analogous to the α2 receptor inhibiting further release of norepinephrine from sympathetic nerve endings, identifying it as an autoreceptor as discussed previously (see section on Cholinergic Neurotransmitter Function). Stimulation of prejunctional M2 receptors in human airways in vitro results in strong inhibition of cholinergic parasympathetic-induced bronchoconstriction. Pilocarpine, a direct-acting cholinergic agonist (parasympathomimetic), is a selective stimulant of M2 receptors.16 Inhalation of pilocarpine blocks cholinergic reflex bronchoconstriction caused by sulfur dioxide in nonasthmatic human subjects, verifying that M2 receptor stimulation can block cholinergic bronchoconstriction.17
M3 receptors.
M3 receptors are present on submucosal glands and airway smooth muscle and possibly on surface goblet cells. Stimulation of M3 receptors causes bronchoconstriction of smooth muscle and exocytosis and glandular secretion from submucosal mucous glands. M3 receptors may also be present on airway epithelial cells, to increase ciliary beat. Antagonism of M3 receptors is the basis for a class of bronchodilator agents, the anticholinergic bronchodilators (see Chapter 7).
Muscarinic receptors on blood vessels
Muscarinic M3 receptors are located on endothelial cells of both the bronchial and the pulmonary vasculature. Stimulation of M3 receptors causes release of an endothelium-derived relaxant factor.18 This relaxant factor, which produces vasodilation and is mediated by an increase in intracellular cyclic guanosine monophosphate (cGMP), has been identified as nitric oxide (NO) or a very similar nitrosocompound.19
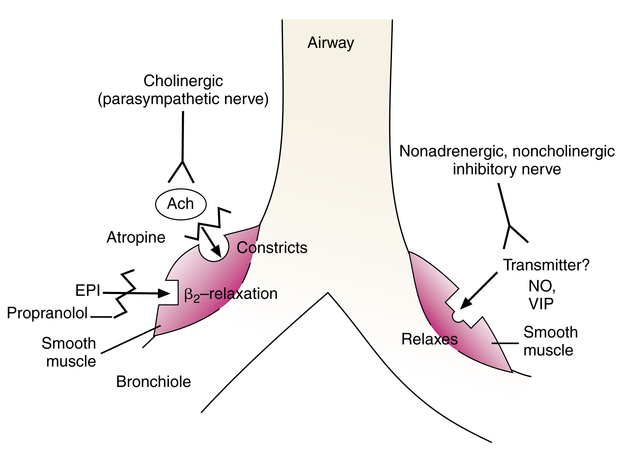
Nonadrenergic, noncholinergic inhibitory nerves
There is evidence of a branch of nerves that are neither parasympathetic (cholinergic) nor sympathetic (adrenergic), which can cause relaxation of airway smooth muscle. These nerves have been termed nonadrenergic, noncholinergic (NANC) inhibitory nerves.20 They are also referred to as simply nonadrenergic inhibitory nerves because adrenergic activity relaxes airway smooth muscle, and this is an additional but nonadrenergic neural method of relaxing such smooth muscle. Evidence of NANC inhibitory nerves is based on the following type of experimentation. When parasympathetic (cholinergic) receptors are blocked with an antagonist, such as atropine, and sympathetic (adrenergic) receptors are also blocked with a β blocker, such as propranolol, electrical field stimulation of the lung produces relaxation of bronchial smooth muscle. Katzung21 provides a more detailed description and evidence of this methodology. Figure 5-12 illustrates this inhibitory system that is neither adrenergic nor cholinergic and its possible neurotransmitter substances. A nonadrenergic inhibitory nervous system found in the gastrointestinal tract is primarily responsible for the relaxation of peristalsis and the internal anal sphincter. In the gastrointestinal tract, this system develops in conjunction with the parasympathetic branch. Embryologically, the gastrointestinal and respiratory tracts share a common origin, and the separation of the trachea and gut occurs around the 4th or 5th week of gestation. This common origin adds plausibility to the presence of a nonadrenergic inhibitory system in the lungs similar to that in the gastrointestinal tract.
The exact neurotransmitter responsible for relaxation responses mediated by NANC inhibitory nerves is under investigation; however, the neurotransmitter vasoactive intestinal peptide (VIP) is the current front-runner.22 VIP can relax mammalian airway smooth muscle. Another possible neurotransmitter causing airway smooth muscle relaxation is NO. The enzyme responsible for NO synthesis, nitric oxide synthase (NOS), has been found in nerve terminals around airway smooth muscle, and NO produces effects similar to those caused by NANC inhibitory nerve activation. Ricciardolo23 believed that NANC inhibition is mediated by NO, with the help of VIP. An NANC inhibitory neurotransmitter substance has not as yet been definitively identified.
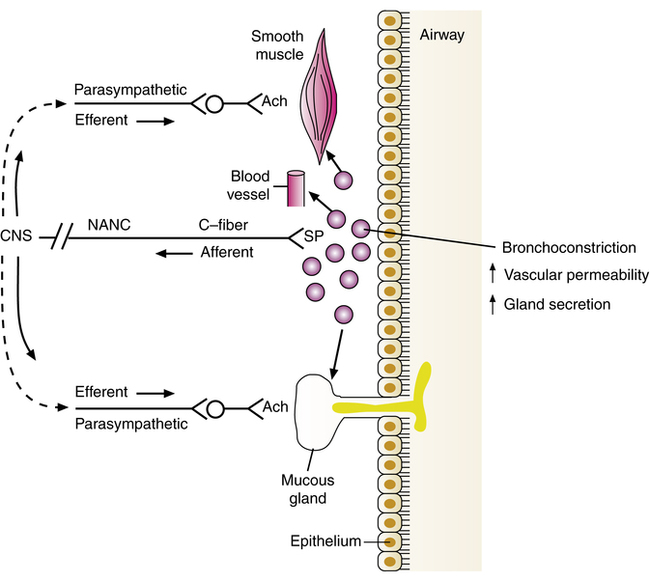
Nonadrenergic, noncholinergic excitatory nerves
The existence of nonadrenergic, noncholinergic excitatory nervous control of airway smooth muscle has also been shown using electrical field stimulation (EFS) techniques. This system is also referred to as simply noncholinergic excitatory nervous control because cholinergic activity contracts airway smooth muscle, and this is an additional but noncholinergic neural method of exciting and constricting such smooth muscle. Stimulation of NANC excitatory nerves causes bronchial contraction. Sensory afferent nerves termed C-fibers are present in the airways and around bronchial blood vessels and submucosal glands and within the airway epithelium. These afferent fibers follow vagal nerve tracts into the CNS, as shown in Figure 5-13.