Cells and Cellular Activities of the Immune System
Lymphocytes and Plasma Cells
At the conclusion of this chapter, the reader should be able to:
• Differentiate and compare the function of primary and secondary lymphoid tissues.
• Describe the structure and function of a lymph node.
• Explain the role of the thymus in T lymphocyte maturation.
• Describe the maturation of a B lymphocyte from origination to plasma cell development.
• Compare the function of T lymphocytes and B lymphocytes in immunity.
• Explain the function of natural killer (NK) cells.
• Define the term cluster of differentiation (CD) and explain the purpose of detecting this marker.
• Differentiate the characteristics of T lymphocyte subsets on the basis of antigen structures and function.
• Describe the evaluation of suspected lymphocytic or plasma cell defects.
• Name and compare disorders of immunologic (lymphocytic or plasma cell) origin.
• Compare various categories of immunodeficiency disorders.
• Analyze and apply knowledge from this chapter to a representative case study.
• Correctly answer case study–related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
Lymphoid and Nonlymphoid Surface Membrane Markers
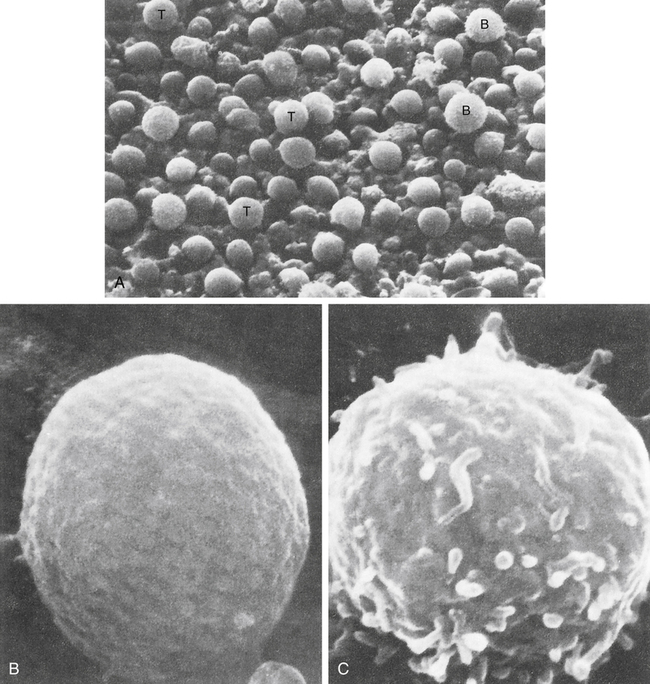
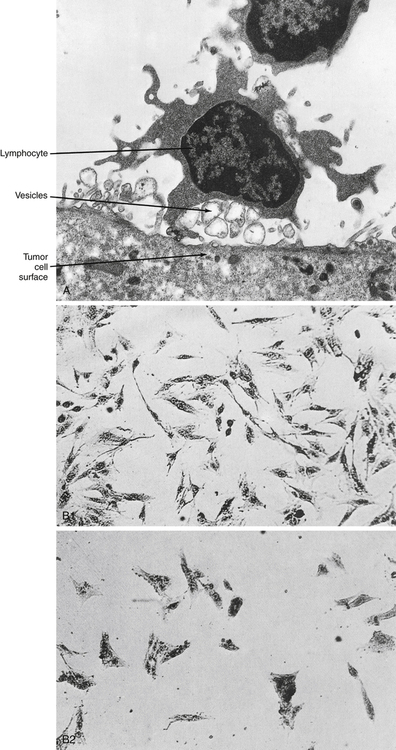
Before 1979, human lymphocytes could be classified as T or B cells based on observation of these cells with electron microscopy (Fig. 4-1). T lymphocytes have a relatively smooth surface compared with the rough pattern of the B lymphocytes.
The introduction of monoclonal antibody (MAb) testing (see Chapter 2) led to the present identification of surface membrane markers on lymphocytes and other cells. In practical terms, surface markers are used to identify and enumerate various lymphocyte subsets, establish lymphocyte maturity, classify leukemias, and monitor patients on immunosuppressive therapy.
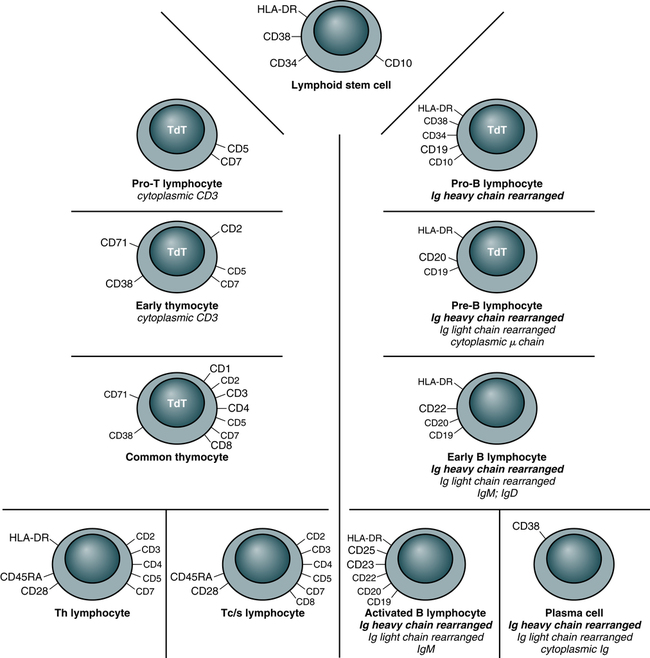
In this system, a surface marker that identifies a particular lineage or differentiation stage with a defined structure, and that can be identified with a group or cluster of MAbs, is called a member of a cluster of differentiation (CD; Fig. 4-2). Markers can be categorized as follows:
• Some markers are specific for cells of a particular lineage or maturational pathway.
• Some markers vary in expression, depending on the state of activation or differentiation of the same cells—for example, when CD antigen identification is used to classify lymphocyte subsets (e.g., CD4 and CD8).
• Promotion of cell to cell interactions and adhesion
• Transduction of signals that lead to lymphocyte activation
Sites of Lymphocyte Development
In mammalian immunologic development, the precursors of lymphocytes arise from progenitor cells of the yolk sac and liver (Fig. 4-3). Later in fetal development, and throughout the life cycle, the bone marrow becomes the sole provider of undifferentiated progenitor cells, which can further develop into lymphoblasts. Continued cellular development and proliferation of lymphoid precursors occur as the cells travel to the primary and secondary lymphoid tissues.
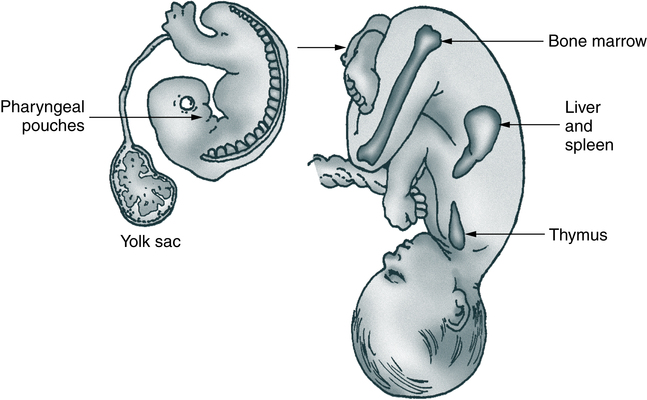
The anatomy of the human fetus illustrates the development of the mammalian immune system. Cells of the pharyngeal pouches migrate into the chest and form the thymus. Precursors of lymphocytes originate early in embryonic life in the yolk sac and eventually migrate to the bone marrow via the spleen and liver.
Primary Lymphoid Tissue
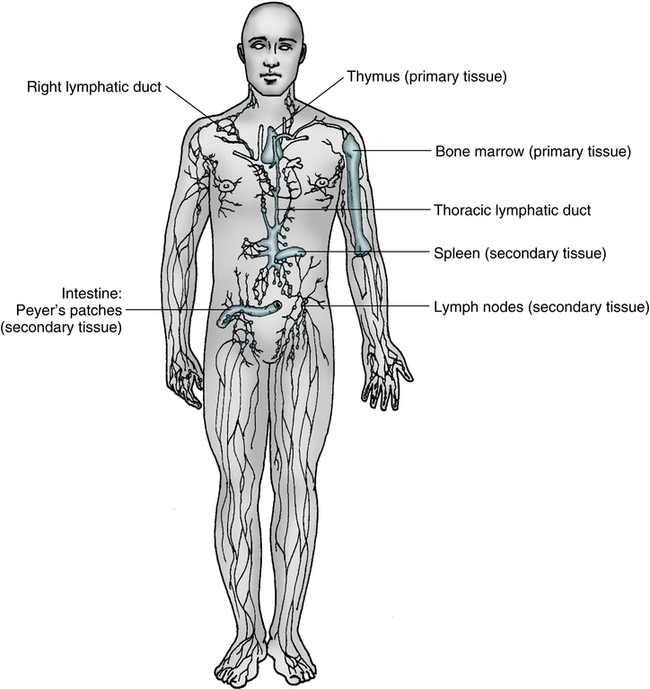
In mammals, both the bone marrow (and/or fetal liver) and thymus are classified as primary or central lymphoid organs (Fig. 4-4).
Thymus
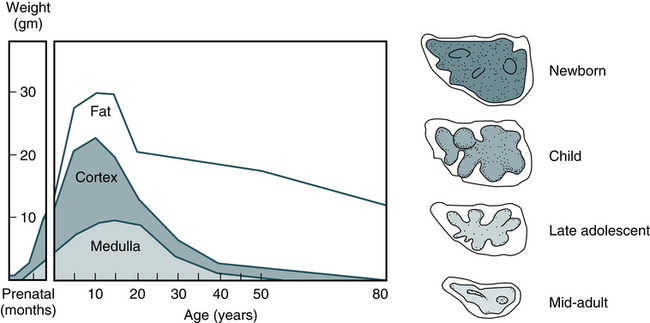
Involution of the thymus is the first age-related change occurring in the human immune system. In postnatal life, the thymus is the primary organ that produces naïve T cells for the peripheral T cell pool but production of cells declines as early as 3 months of age. The thymus gradually loses up to 95% of its mass during the first 50 years of life (Fig. 4-5). The accompanying functional changes of decreased synthesis of thymic hormones and the loss of ability to differentiate immature lymphocytes are reflected in an increased number of immature lymphocytes within the thymus and as circulating peripheral blood T cells. Most changes in immune function, such as dysfunction of T and B lymphocytes, elevated levels of circulating immune complexes, increases in autoantibodies, and monoclonal gammopathies are correlated with involution of the thymus (see Chapter 27). Immune senescence may account for the increased susceptibility of older adults to infections, autoimmune disease, and neoplasms.
Secondary Lymphoid Organs
Secondary lymphoid organs provide a unique microenvironment for the initiation and development of immune responses. The secondary lymphoid tissues include lymph nodes, spleen, GALT, thoracic duct, bronchus-associated lymphoid tissue (BALT), skin-associated lymphoid tissue, and blood. Mature lymphocytes and accessory cells (e.g., antigen-presenting cells) are found throughout the body, although the relative percentages of T and B cells vary in different locations (Table 4-1).
Table 4-1
Approximate Percentage of Lymphocytes in Lymphoid Organs
| Lymphoid Organ | T Lymphocytes (%) | B Lymphocytes (%) |
| Thymus | 100 | 0 |
| Blood | 80 | 20 |
| Lymph nodes | 60 | 40 |
| Spleen | 45 | 55 |
| Bone marrow | 10 | 90 |
Adapted from Claman HN: The biology of the immune response, JAMA 268:2790–2796, 1992.
Tumor necrosis factor (TNF) and lymphotoxin are essential to the formation and maintenance of secondary organs. These cytokines are produced by B and T lymphocytes. Proliferation of the T and B lymphocytes in the secondary or peripheral lymphoid tissues (Fig. 4-6) is primarily dependent on antigenic stimulation.
The T lymphocytes or T cells populate the following:
1. Perifollicular and paracortical regions of the lymph nodes
2. Medullary cords of the lymph nodes
The B lymphocytes or B cells multiply and populate the following:
1. Follicular and medullary (germinal centers) of the lymph nodes
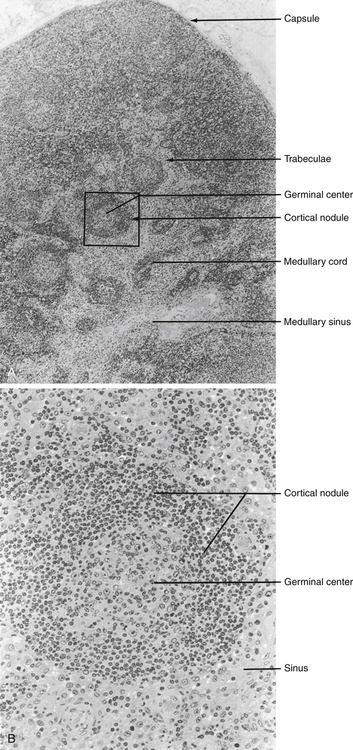
Lymph Nodes
Lymph nodes act as lymphoid filters in the lymphatic system. Lymph nodes respond to antigens introduced distally and routed to them by afferent lymphatics (Fig. 4-7). Generalized lymph node reactivity can occur after systemic antigen challenge (e.g., serum sickness).
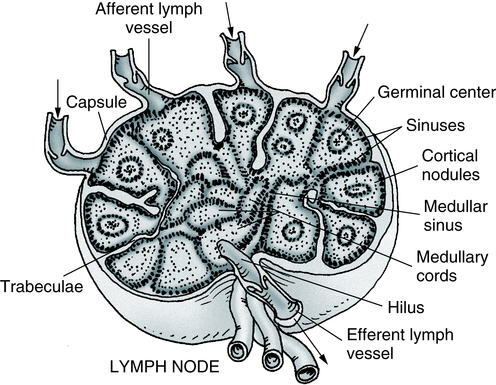
Several valved afferent lymphatics bring lymph to the node. An efferent lymphatic leaves the node at the hilus. Note that the artery and vein enter and leave at the hilus. (Adapted from Anthony CP, Thibodeau GA: Textbook of anatomy and physiology, ed 12, St Louis, 1987, Mosby.)
Circulation of Lymphocytes
Recirculation of lymphocytes back to the blood is through the major lymphatic ducts. Lymphocytes enter the lymph node from the blood circulation via arterioles and capillaries to reach the specialized postcapillary venules. From the venule, the lymphocytes enter the node and remain in the node or pass through the node and return to the circulating blood. Lymphatic fluid, lymphocytes, and antigens from certain body sites enter the lymph node through the afferent lymphatic duct and exit the lymph node through the efferent lymphatic duct (see Fig. 4-7).
Virgin or Naïve Lymphocytes
Memory cells are populations of long-lived T or B cells that have been stimulated by antigen. They can make a quick response to a previously encountered antigen. Memory B cells carry surface IgG as their antigen receptor; memory T cells express the CD45RO variant of the leukocyte common antigen and increased levels of cell-adhesion molecules (CAMs), chemical mediators involved in inflammatory processes throughout the body (Fig. 4-8).
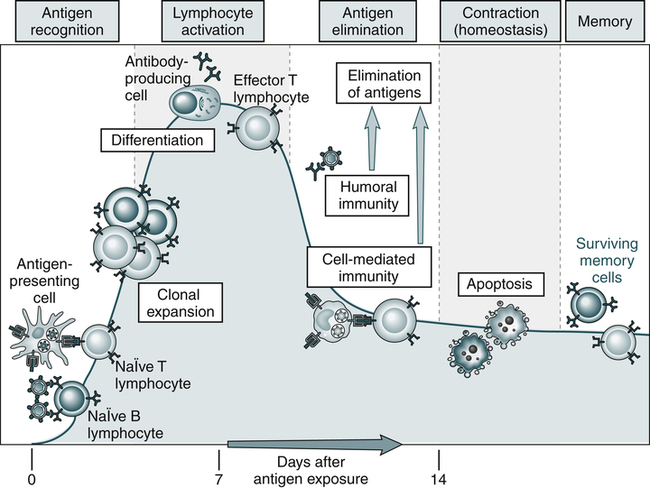
An adaptive immune response consists of distinct phases; the first three are the recognition of antigen, activation of lymphocytes, and elimination of antigen (effector phase). The response declines as antigen-stimulated lymphocytes die by apoptosis, restoring homeostasis, and the antigen-specific cells that survive are responsible for memory. The duration of each phase may vary in different immune responses. The y-axis represents an arbitrary measure of the magnitude of the response. These principles apply to humoral immunity (measured by B lymphocytes) and cell-mediated immunity (mediated by T lymphocytes). (From Abbas AK, Lichtman AH: Basic immunology: functions and disorders of the immune system, updated edition, ed 3, Philadelphia, 2011, Saunders.)
Development of T Lymphocytes
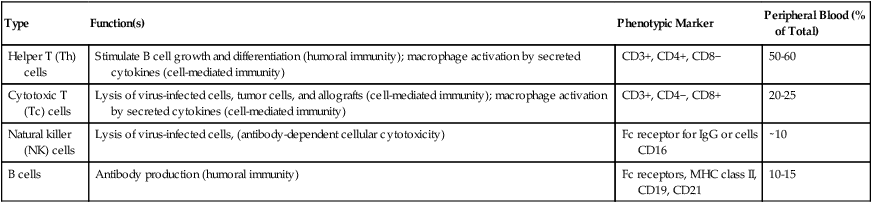
Most lymphocytes (see Color Plate 8) found in the circulating blood are T cells derived from bone marrow progenitor cells that mature in the thymus gland (Table 4-2). These cells are responsible for cellular immune responses and are involved in the regulation of antibody reactions in conjunction with B lymphocytes.
Table 4-2
| Type | Function(s) | Phenotypic Marker | Peripheral Blood (% of Total) |
| Helper T (Th) cells | Stimulate B cell growth and differentiation (humoral immunity); macrophage activation by secreted cytokines (cell-mediated immunity) | CD3+, CD4+, CD8− | 50-60 |
| Cytotoxic T (Tc) cells | Lysis of virus-infected cells, tumor cells, and allografts (cell-mediated immunity); macrophage activation by secreted cytokines (cell-mediated immunity) | CD3+, CD4−, CD8+ | 20-25 |
| Natural killer (NK) cells | Lysis of virus-infected cells, (antibody-dependent cellular cytotoxicity) | Fc receptor for IgG or cells CD16 | ∼10 |
| B cells | Antibody production (humoral immunity) | Fc receptors, MHC class II, CD19, CD21 | 10-15 |
Early Cellular Differentiation and Development
Helper T Lymphocytes
• Helper T type 1 (Th1) cells are responsible for cell-mediated effector mechanisms.
• Helper T type 2 (Th2) cells play a greater role in the regulation of antibody production.
• Regulatory T (Treg) cells are an immunoregulatory type of Th cells.
These divisions are not absolute, with considerable overlap or redundancy in function among the different subsets. This classification is based on the in vitro blends of cytokines that they produce. Th1 and Th2 cells can promote the development of cytotoxic cells and are believed to develop from Th0 cells. Th1 cells interact most effectively with mononuclear phagocytes; Th2 cells release cytokines that are required for B cell differentiation (Fig. 4-9).
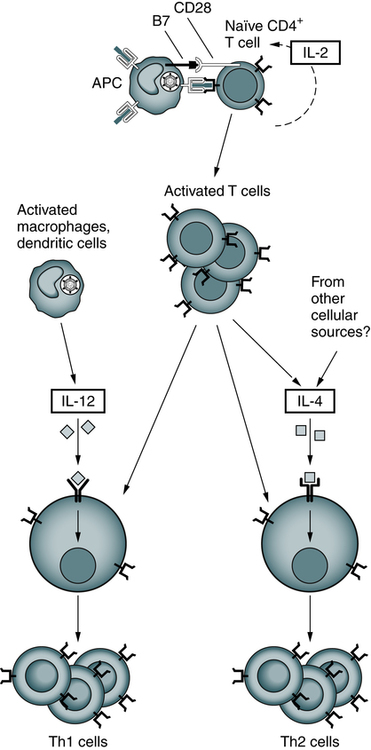
After their activation by antigen and costimulators, naïve helper T cells may differentiate into Th1 and Th2 cells under the influence of cytokines. IL-12 produced by microbe-activated macrophages and dendritic cells stimulates differentiation of CD4+ T cells into Th1 effectors. In the absence of IL-12, the T cells themselves (and perhaps other cells) produce IL-4, which stimulates their differentiation into Th2 effectors. (Adapted from Abbas AK, Lichtman AH: Basic immunology: functions and disorders of the immune system, ed 3, Philadelphia, 2008, Saunders.)
Characterized by high interferon-gamma (IFN-γ) production, Th1 responses promote the elimination of intracellular pathogens (Fig. 4-10, A). Characterized by IL-4 and IL-5, Th2 responses promote a different type of effector response that involves immunoglobulin E (IgE) production and eosinophils capable of eliminating larger extracellular pathogens, such as helminths (see Fig. 4-10, B). In situations of repeated pathogen exposure or persistent infection, the polarization of T cell responses serves to focus the antigen-specific response on a specific effector pathway.
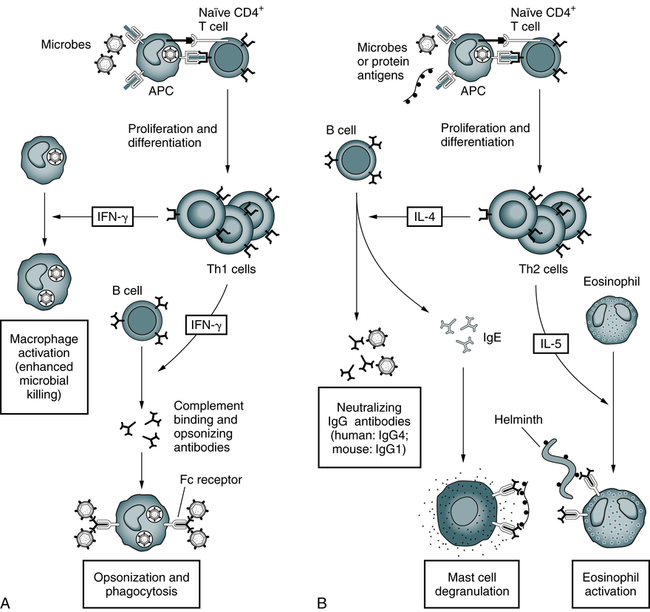
A, Th1 cells produce the cytokine IFN-γ, which activates phagocytes to kill ingested microbes and stimulates the production of antibodies that promote the ingestion of microbes by the phagocytes. B, Th2 cells specific for microbial or nonmicrobial protein antigens produce the cytokines IL-4, which stimulates the production of IgE antibody, and IL-5, which activates eosinophils. IgE participates in the activation of mast cells by protein antigens and coats helminths for destruction by eosinophils. Th2 cells also stimulate the production of other antibodies (IgG4 in humans) that neutralize microbes and toxins but do not bind to Fc receptors or activate complement efficiently. (Adapted from Abbas AK, Lichtman AH: Basic immunology: functions and disorders of the immune system, ed 3, Philadelphia, 2008, Saunders.)
The following factors can influence the terminal differentiation of lymphocytes:
Cytotoxic T Lymphocytes
Cytotoxic T lymphocytes, or T cytotoxic (Tc) cells, are effector cells found in the peripheral blood that are capable of directly destroying virally infected target cells. Most Tc cells are CD8+ and recognize antigen on the target cell surface associated with MHC class I molecules (e.g., human leukocyte antigen [HLA] types A, B, and C) or MHC class I alone. This process is demonstrated by the immune response to virus-infected cells or tumor cells (Fig. 4-11).
In addition to destruction of virally infected, MHC class I–bearing targets, Tc cells are major effectors in allograft organ rejection. Tc cells express CD4 or CD8, depending on the MHC antigen restriction that governs their antigen recognition (i.e., class I or II antigens; Fig. 4-12).
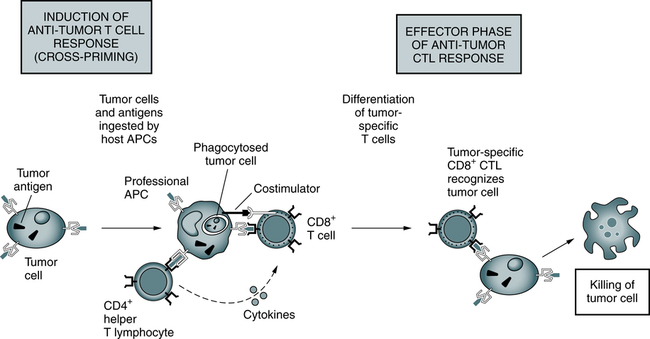
Responses to tumors may be induced by cross-priming (or cross-presentation), in which the tumor cells and tumor antigens are taken up by specialized (so-called professional) APCs, processed, and presented to T cells. In some cases, B7 costimulators expressed by the APCs provide the second signals for the differentiation of the CD8+ T cells. APCs may also stimulate CD4+ helper T cells, which provide the second signals for cytotoxic T lymphocyte (CTL) development. Differentiated CTLs kill tumor cells without a requirement for costimulation or T cell assistance. (From Abbas AK, Lichtman AH: Basic immunology: functions and disorders of the immune system, ed 3, Philadelphia, 2008, Saunders.)
Antigen Recognition by T Cells
The TCR of most T lymphocytes is composed of an alpha and beta polypeptide chain, with constant regions located close to the cell surface and the part that binds to the antigenic peptide of appropriate fit located away from the cell surface. The difference in structure of the distal regions of the alpha and beta chains allows the development of different clones of T cells. The TCR reacts with antigen in the context of MHC class I or II molecules on an APC (Fig. 4-13).
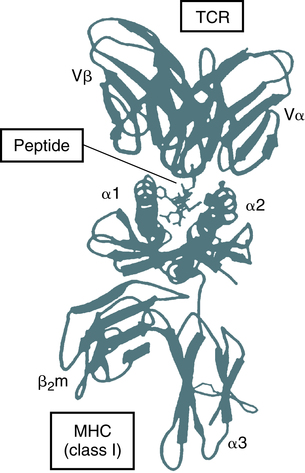
This ribbon diagram is drawn from the crystal structure of the extracellular portion of a peptide-MHC bound to a T cell antigen receptor (TCR) that is specific for the peptide displayed by the MHC molecule. The peptide can be seen attached to the cleft at the top of the MHC molecule, and one residue of the peptide contacts the variable (V) region of a TCR. (Adapted from Bjorkman PJ: MHC restriction in three dimensions: a view of T cell receptor/ligand interactions, Cell 89:167-170, 1997.)
T Cell Activation
T cell activation requires a minimum of two signals:
• Signal 1 is delivered by the TCR-CD3 complex through interaction of the TCR α and β chains as they recognize peptide presented by a class I CD8+ T cell or a class II CD4+ T cell MHC molecule.
• Signal 2 is usually provided by the engagement of CD28 on the T cell with the costimulatory molecule CD80 or CD86 on the APC. The surface markers CD 137 and CD134 also provide costimulation to T cells.
Activation of T cells can lead to the following:
Activated T cells frequently express activation antigens (Box 4-1). Expression of CD69 occurs within 12 hours of activation, followed by CD25 (IL-2 receptor) and CD71 (transferrin receptor) in 1 to 3 days. Alternatively, in the case of Tc cells, interaction with antigen through the specific TCR leads to destruction of target cells.
Natural Killer and K-Type Lymphocytes
Although these cells were previously classified as null cells, MAbs demonstrate that NK and K-type cells express a variety of surface membrane markers (Table 4-3). Most of these cells lack CD3 but express CD2, CD16, CD56, CD57 and, occasionally, CD8.
Table 4-3
| Components | Reference Interval (% Positive) |
| CD2 | 75-92 |
| CD3 | 63-84 |
| CD5 | 61-88 |
| CD7 | 73-94 |
| CD8 | 14-39 |
| CD16 | 1-12 |
| CD56 | 7-27 |
| CD57 | 1-26 |
Data from Associated Regional and University Pathologists (ARUP): Interpretive data guide, ed 2, Salt Lake City, Utah, 1999, Associated Regional and University Pathologists, p 473.
B Lymphocyte Subsets
Cell Surface Markers
In humans, there is evidence of four types of B cell surface markers:
1. Ig receptor is the best studied B cell surface marker. This receptor is actually an antibody molecule with antigenic specificity. According to the clonal selection theory, B cells exist in the body with Ig receptors specific for antigen before exposure to the antigenic substances. When specific antigen exposure does occur, the antigen will select the B cell having an Ig receptor with the best fit.
2. An Fc receptor that specifically binds the Fc portion of IgG antibody may function to aid B cells in binding to antigen already bound to antibody.
3. Receptors that bind fragments of the cleaved complement component C3 have been reported on the surface of approximately 75% of B cells. This receptor binds C3b, iC3b (inactivated C3b), and C3d, but the function of these receptors is not completely understood.
4. B cell surface antigens coded by the MHC class II genes are a fourth type of human B cell marker.
Plasma Cell Biology
The function of plasma cells (see Color Plate 9) is the synthesis and excretion of immunoglobulins. Plasma cells are not normally found in the circulating blood but are found in the bone marrow in concentrations that do not normally exceed 2%. Plasma cells arise as the end stage of B cell differentiation into a large, activated plasma cell.
Evaluation of Immunodeficiency Syndromes
Laboratory testing can then proceed with a complete blood cell (CBC) count, including a platelet count and erythrocyte sedimentation rate (ESR). These are among the most cost-effective screening tests. If the ESR is normal, chronic bacterial infection is unlikely. If the absolute neutrophil count is normal, congenital and acquired neutropenias and severe chemotactic defects are eliminated. If the absolute lymphocyte count is normal, the patient is not likely to have a severe T cell defect. The absolute lymphocyte count is the number of lymphocytes in the total white blood cell (WBC) population (Box 4-2).
Laboratory tests to screen for more common immunodeficiencies include immunoglobulin testing, complement testing, cell-mediated immunity testing, and the neutrophil function test. Additional laboratory testing should include a general metabolic panel to assess overall general health, HIV types 1 and 2, protein electrophoresis, sweat chloride, and pneumococcal antibody IgG titers pre- and postvaccine in patients with only recurrent sinopulmonary infections. Follow-up testing based on any initially abnormal results is presented in Tables 4-4 and 4-5. If all initial test results are normal, IL-1 receptor-associated kinase-4 (IRAK-4) deficiency screening or a Toll-like receptor function assay should be performed. If abnormal results are subsequently found, the diagnosis is an innate immune deficiency.
Table 4-4
Next Steps in Laboratory Evaluation of Suspected Immunodeficiency
| Abnormal Initial Laboratory Result | Follow-Up Testing |
| Protein electrophoresis | Immunofixation electrophoresis monoclonal protein detection; quantitation and characterization of IgA, IgG and IgM, and Bence Jones protein; depending on individual results, additional testing may be needed |
| Sweat chloride | Cystic fibrosis (CFTR)—32 mutations with reflex to sequencing |
| Positive for HIV-1, HIV-2 | HIV-1 antibody confirmation by Western blot |
| Pneumococcal antibodies absent after vaccination | Specific antibody deficiency |
Adapted from Associated Regional and University Pathologists (ARUP) Consult: Immunodeficiency evaluation for chronic infections in adults and older children testing algorithm, 2012 (http://www.arupconsult.com/Algorithms/ChronicInfections Adults.pdf).
Table 4-5
Next Steps in Evaluation of Suspected Immunodeficiency Based on Physical Findings
| Physical Manifestations | Follow-Up Laboratory Assays | Differential Diagnosis |
| Recurrent severe viral or fungal infections (e.g., candidiasis, herpes) | Lymphocyte subset for congenital immunodeficiencies, lymphocyte antigen and mitogen proliferation panel, proliferation panel with cytokine responses to mitogens; testing for 12 cytokines | T cell deficiency, HIV, CD4 deficiency, adenosine deaminase deficiency, nucleoside phosphorylase deficiency, chronic mucocutaneous candidiasis |
| Recurrent severe sepsis, Neisseria spp., Streptococcus pneumoniae infections | Rule out previous splenectomy and immunoglobulin abnormality; then order a total CAEI. If abnormal (low) activity, analyze C2-C5 components. | Complement deficiency |
| Abscesses, pneumonia, recurrent respiratory infections with or without diarrhea | Order quantitative IgM, IgG and IgA, IgE, CAEI, neutrophil oxidative burst assay (DHR), leukocyte adhesion deficiency panel, and myeloperoxidase stain. | Low IgM– or IgG–hypogammaglobulinemia Low complement—complement defect Abnormal DHR—chronic granulomatous disease Decreased CD11b/CD18—LAD-1, Decreased CD15—LAD-2 Increased IgE—Possible hyper-IgE syndrome (Job syndrome) but must be followed by Candida-specific IgE neutrophil chemotaxis and subsequent genetic testing Positive neutrophil antibody—autoimmune neutropenia, Absence of myeloperoxidase—myeloperoxidase deficiency |
Adapted from Associated Regional and University Pathologists (ARUP) Consult: Immunodeficiency evaluation for chronic infections in adults and older children testing algorithm, 2012 (http://www.arupconsult.com/Algorithms/ChronicInfections Adults.pdf).
Immunologic Disorders
A breakdown in any part of the immune mechanism can lead to disease. Disorders with an immunologic origin can involve progenitor cells, phagocytosis (see Chapter 3), T cells, B cells, or complement (see Chapter 5).
Immunologic disorders can be divided into primary processes (dysfunction in the immune organ itself) and acquired, or secondary, processes (disease or therapy causing an immune defect). A third category, diseases mediated through immune mechanisms, can also be included. Because of its complexity and contemporary importance, AIDS is discussed separately in Chapter 25. Other immunoproliferative and autoimmune disorders are discussed in Chapters 27 to 30.
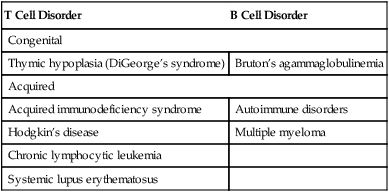
Immunodeficiency disorders may be caused by defects in the quality (defects) or quantity (deficiencies) of lymphocytes and may be congenital or acquired. These conditions may be combined disorders or may involve T cells or B cells (Table 4-6).
Table 4-6
| T Cell Disorder | B Cell Disorder |
| Congenital | |
| Thymic hypoplasia (DiGeorge’s syndrome) | Bruton’s agammaglobulinemia |
| Acquired | |
| Acquired immunodeficiency syndrome | Autoimmune disorders |
| Hodgkin’s disease | Multiple myeloma |
| Chronic lymphocytic leukemia | |
| Systemic lupus erythematosus | |
Primary Immunodeficiency Disorders
Primary immunodeficiencies (PID) are rare genetic disorders of the innate and adaptive immune system. Classic primary immunodeficiency disorders (PIDs) are usually monogenic (mendelian) disorders affecting host defenses (Box 4-3). More than 200 clinical phenotypes of PID have been described. Over 120 different gene mutations have been identified which cause impairment in the differentiation and/or function of immune cells with different degrees of severity. Diseases associated with a primary defect in the immune response are comprised of about 40% T cell disorders, 50% B cell disorders, 6% phagocytic abnormalities, and 4% complement alterations (Fig. 4-14). The most common T cell deficiency states are those associated with a concurrent B cell abnormality. Primary immunodeficiency disorders are predominantly seen (75%) in children younger than 5 years.
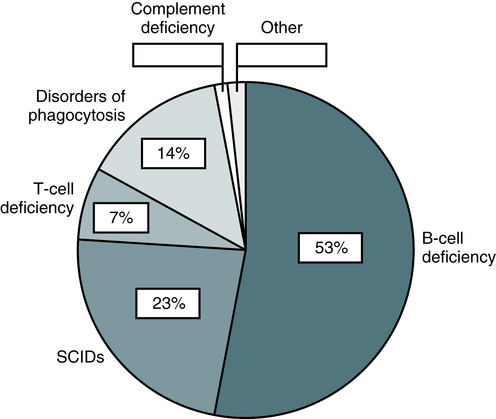
T Cell and Combined Immunodeficiency Disorders
Immunoglobulin Subclass Deficiencies
X-Linked Lymphoproliferative Disease (Duncan’s Disease)
Signs and Symptoms
The disease is characterized by an inadequate immune reaction to infection with Epstein-Barr virus (EBV). Infected patients are apparently healthy until they experience infectious mononucleosis (see Chapter 22). Two thirds of more than 100 patients studied died of overwhelming EBV-induced B cell proliferation during mononucleosis. Most patients surviving the primary infection developed hypogammaglobulinemia and/or B cell lymphomas.
Partial Combined Immunodeficiency Disorders
T Cell Activation Defects
Some patients with defective activation of T cells have experienced the following:
Other Primary Immunodeficiencies
Complement Deficiency
Deficiencies in all of the components of the complement system have been described (see Chapter 5). These deficiencies are genetic in origin. Unusual susceptibility to infection is characteristic of some of these components, particularly deficiencies involving C3, C5, C6, and C7 (Table 4-7).
Table 4-7
| Deficient Component | Common Types of Infections |
| C1 (r/q) | Gram-positive, mainly respiratory |
| C2 | Gram-positive, recurrent respiratory; meningitis, sepsis, tuberculosis |
| C3 | Gram-positive, recurrent |
| C4 | Gram-positive; sepsis, meningitis |
| C5 | Meningitis (Neisseria meningitidis), disseminated gonococcal infection |
| C6 | Meningitis (N. meningitidis), disseminated gonococcal infection |
| C7 | Meningitis (N. meningitidis) |
| C8 | Meningitis (N. meningitidis), disseminated gonococcal infection |
| C9 | Meningitis (N. meningitidis) |
A functional deficiency of polymorphonuclear neutrophil (PMN) leukocytes is chronic granulomatous disease (CGD; see Chapter 3). This fatal syndrome usually begins with the onset of symptoms during the first year of life.
Secondary Immunodeficiency Disorders
A secondary immunodeficiency can result from a disease process that causes a defect in normal immune function, which leads to a temporary or permanent impairment of one or multiple components of immunity in the host (Box 4-4). Patients with secondary immunodeficiencies, which are much more common than primary deficiencies, have an increased susceptibility to infections, as seen in the PIDs.
Immune-Mediated Disease
The immune system is normally efficient in eliminating foreign antigens. The nature of the antigen or the genetic makeup of the host, however, can cause alterations of the immune response that can be injurious and lead to immune-mediated disease (Table 4-8). In these disorders, the immune response is normal but the reactivity is heightened, prolonged, or inappropriate.
Table 4-8
| Type | Cause, Disease |
| Allergic hypersensitivity | Foods, drugs, aeroallergens (dust, pollens, molds) |
| Contact hypersensitivity | Poison ivy, nickel, cosmetics |
| Transfusion Reactions | |
| Autoimmune disease | Systemic lupus erythematosus, rheumatoid arthritis, vasculitis syndromes, hemolytic anemia, idiopathic thrombocytopenia, pernicious anemia, Goodpasture’s syndrome, myasthenia gravis, Graves’ disease |
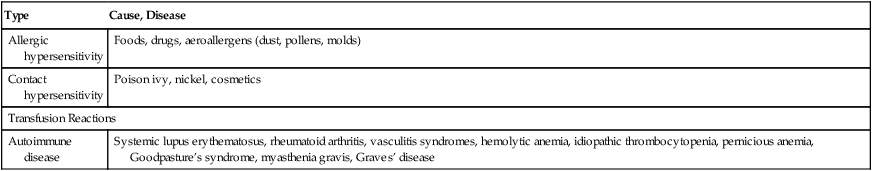
A major concern is allergic reactions, characterized by an immediate response on exposure to an offending antigen and the release of mediators (e.g., histamine, leukotrienes, prostaglandins) capable of initiating signs and symptoms (see Chapter 26). Although allergic reactions are associated with IgE, not all allergic reactions are IgE-mediated. Complement activation by immune complexes or through the alternative complement pathway has been shown to release complement C3a and C5a anaphylatoxins, which are capable of producing similar reactions.
Autoimmune disease is thought to be caused by antibody or T cell sensitization with autologous self-antigens (see Chapter 28). Postulated mechanisms of this process include the following:
• Altered antigen or neoantigen. These antigens may be created by chemical, physical, or biologic processes. Hemolytic anemia caused by a drug interaction is an example of this process occurring in RBCs.
• Shared or cross-reactive antigens. Evidence has suggested that poststreptococcal disease occurs through this mechanism.
Chapter Highlights
• Lymphocytes represent the cellular components of the specific system of body defense. These cells function cooperatively in cell-mediated or humoral immunity.
• The primary lymphoid organs in mammals are the bone marrow (and/or fetal liver) and thymus.
• The secondary lymphoid tissues include the lymph nodes, spleen, and Peyer’s patches in the intestine. Proliferation of the T and B lymphocytes in the secondary or peripheral lymphoid tissues is primarily dependent on antigenic stimulation.
• Several major categories of lymphocytes are recognized by the presence of cell surface membrane markers. These categories are T and B cells and natural killer (NK) and K-type lymphocytes.
• The function of plasma cells is the synthesis and excretion of immunoglobulins.
• Monoclonal antibody (MAb) testing led to the present identification of surface membrane markers. Relating MAbs to cell surface antigens now provides a method for classifying and identifying specific cellular membrane characteristics. The current method of testing uses flow cytometry with immunofluorescence.
• Immunologic disorders can be divided into primary, secondary (acquired), and those mediated through immune mechanisms. Diseases associated with a primary immunodeficiency are comprised of 40% T cell disorders, 50% B cell disorders, 6% phagocytic abnormalities, and 4% complement alterations. The most common T cell deficiency states are those associated with a concurrent B cell abnormality.