CHAPTER 12 Cell populations at the start of organogenesis
SPECIFICATION OF THE BODY AXES AND THE BODY PLAN
Axes may be conferred on the whole embryonic disc, which is initially flat and mainly two-dimensional. However, their subsequent orientation in the folded three-dimensional embryo will be completely different. The dorsal structures of the folded embryo form from a circumscribed central ellipse of the early flat embryonic disc (see Fig. 10.5). Lateral and ventral structures form from the remainder of the disc, and the peripheral edge of the disc eventually becomes constricted at the umbilicus (see Figs 10.9 and 10.10). Although the appearance of part of the epiblast is taken to specify the dorsal surface of the embryo, the inner layer, i.e. the hypoblast, is not by default a ventral embryonic structure.
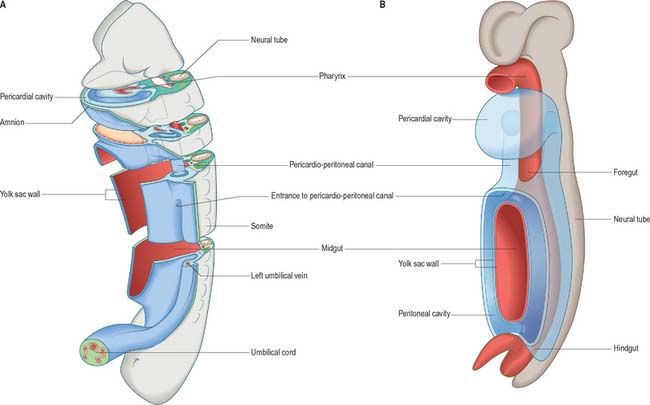
The development of all the body organs and systems, organogenesis, begins after the dramatic events of gastrulation, when the embryo has attained a recognizable body plan. In human embryos this corresponds to the end of stage 10 (Fig. 12.1). The head and tail folds are well formed, with enclosure of the foregut and hindgut (proenteron and metenteron), although the midgut (mesenteron) is only partly constricted from the yolk sac. The forebrain projection dominates the cranial end of the embryo, and the buccopharyngeal membrane and cardiac prominence are caudal and ventral to it. The cardiac prominence contains the transmedian pericardial cavity, which communicates dorsocaudally with right and left pericardioperitoneal canals. These pass dorsally to the transverse septum mesenchyme and open caudally into the extraembryonic coelom on each side of the midgut. The intraembryonic mesenchyme has begun to differentiate and the paraxial mesenchyme is being segmented into somites. Neural groove closure is progressing caudally, so that a neural tube is forming between the newly segmenting somites. Rostrally, the early brain regions, which have not yet fused, can be discerned. The neuroepithelium is separated from the dorsal aspect of the gut by the notochord. The earliest blood vessels have appeared, and a primitive tubular heart occupies the pericardium. The chorionic circulation is soon to be established, after which the embryo rapidly becomes completely dependent upon the maternal bloodstream for its requirements. The embryo is connected to the developing placenta by a mesenchymal connecting stalk in which the umbilical vessels develop, and which also contains the allantois, a hindgut diverticulum. The lateral body walls are still widely separated. The embryo has contact with three different vesicles: the amnion, which is in contact with the surface ectoderm; the yolk sac, which is in contact with the endoderm; and the chorionic cavity, containing the extraembryonic coelom, which is in contact with the intraembryonic coelomic lining (see Fig. 10.10).
The degree to which vertebrate embryos are developmentally constrained at this period of development is controversial. Comparative studies on the timing at which specific embryonic structures appear, heterochrony, have shown that other embryonic species do not follow the same developmental sequence as humans (Richardson & Keuck 2002). Although some developmental mechanisms are highly conserved, e.g. the homeobox gene codes, others may have been dissociated and modified in different vertebrate species during evolution.
EMBRYONIC CELL POPULATIONS AT THE START OF ORGANOGENESIS
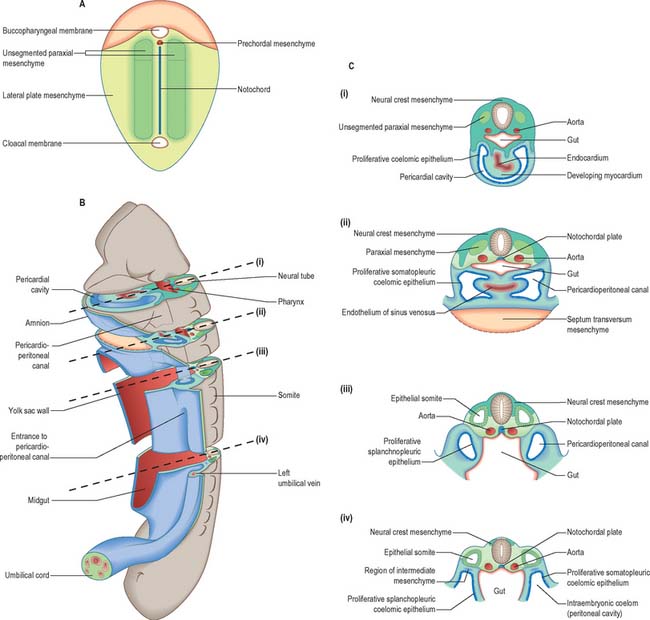
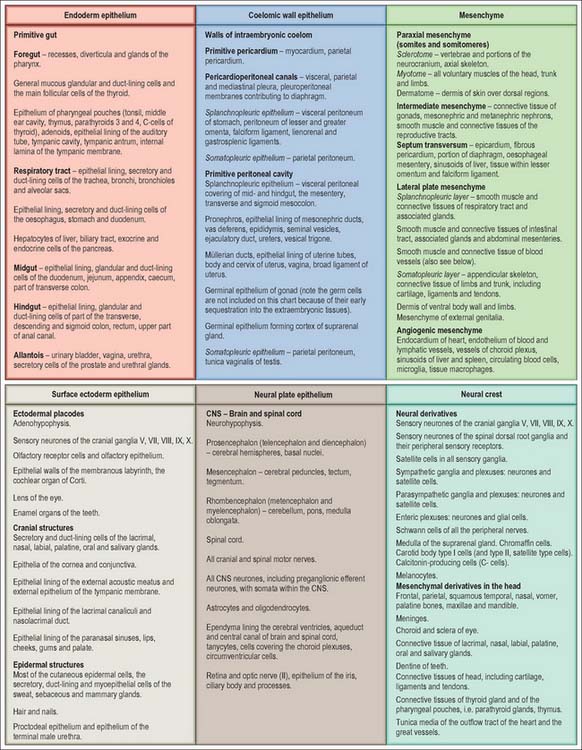
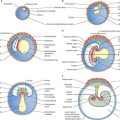
The developmental processes operating in the embryo between stages 5 and 9 enabled the construction of the bi- and tri-laminar embryonic disc, the intraembryonic coelom and new proliferative epithelia. From the end of stage 10, a range of local epithelial and mesenchymal populations now interact to produce viscera and appendages. The inductive influences on these tissues and their repertoire of responses are very different from those seen at the onset of gastrulation. The range of tissues present at the start of organogenesis, when the body plan is clear, is given below and shown in Figs 12.1 and 12.2. For a summary of the fates of the embryonic cell populations, see Fig. 12.3.
Epithelial populations in the embryo
Ectodermal ring and ectodermal placodes
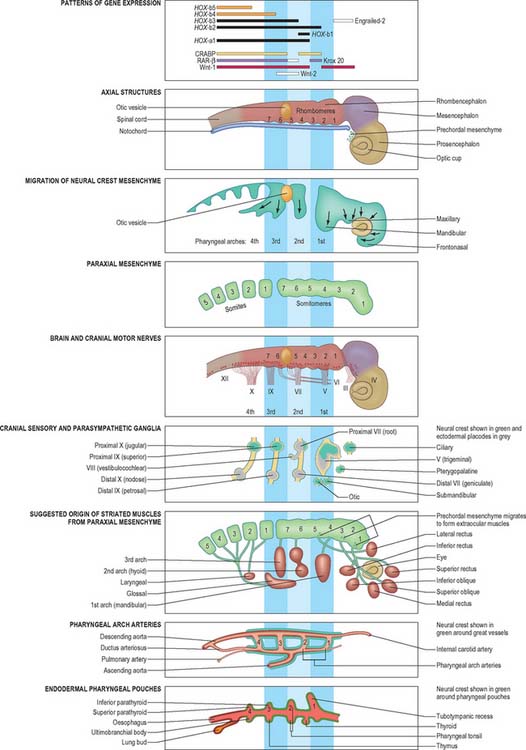
The ectoderm on the head and lateral borders of the embryo shows a zone of epithelial thickening, the ectodermal ring, which can be discerned from stage 10 and is completed by stage 12. Rostrally it contains populations of neuroectoderm that remain in the ectoderm after primary neurulation that are termed ectodermal placodes: these placodes may be considered to be neuroepithelial cells that remain within the surface ectoderm until central nervous system development has progressed sufficiently for their inclusion into sensory epithelia and cranial nerve ganglia. The neuronal placodes may invaginate in toto to form a vesicle, or remain as a neuronal layer, or contribute individually to neuronal structures with cells of other origins. The midline ectodermal thickening, the adenohypophysial placode, invaginates as Rathke’s pouch and forms a vesicle immediately rostral to the buccopharyngeal membrane. The ectodermal ring then passes bilaterally to encompass the olfactory and optic placodes, which give rise to the olfactory sensory epithelium and the lens of the eye respectively. It then overlays the pharyngeal arches where it gives rise to epibranchial placodes which remove themselves individually from the ectoderm at stage 10–11 and become associated with the neural crest cells within the cranial sensory ganglia supplying the arches. It also forms specializations of the ectoderm on the frontonasal, maxillary and mandibular processes which give rise to the tooth buds and the outer coating of the teeth. The paired otic placodes overlying the rhombencephalon at the lateral portion of the second pharyngeal arch invaginate to form the otic vesicles, which give rise to the membranous labyrinth of the ear. The ectodermal ring then passes over the occipital and cervicothoracic parts of the embryo, superficial to the four occipital somites and later to the occipito-cervical junction. Further caudally it is associated with the upper limb field, where it will give rise to the apical ectodermal ridge. O’Rahilly & Müller (1985) have called the portion of the ring between the upper and lower limbs the intermembral part. It overlies the underlying intraembryonic coelom, and later (between stages 12 and 13), the mesonephric duct and ridge. In stages 14 and 15, this portion of the ectodermal ring gives rise to the mammary line. Caudal to the lower limb field, in the unfolded embryo, the ring passes distal to the cloacal membrane. In the folded embryo, this region becomes superior to the cloacal membrane and corresponds to the ectoderm associated with the external genitalia, particularly the genital tubercle and urogenital swellings.
Notochord
During stage 10, the notochordal plate undergoes a process which is similar to, but a mirror image of, neurulation, and forms an epithelial tube from caudal to rostral, ending with the pharynx. The notochordal plate forms a deep groove, the vertical edges of the groove move medially and touch, and then the endodermal epithelium from each side fuses ventral to the notochord. The cells swell and develop an internal pressure (turgor) that confers rigidity on the notochord. The notochord is surrounded by a basal lamina, which is initially referred to as a perinotochordal sheath, but this term is subsequently applied to mesenchymal populations that surround the notochord. After stage 11, the tubular notochord is in contact with the neural tube dorsally and the endoderm ventrally. It is not a proliferative epithelium, but it has inductive effects on the overlying neural tube and the adjacent somites, and later provides a focus for sclerotomal migration.
Endoderm
The craniocaudal progression of development means that the endoderm of the early stomodeum develops ahead of other portions of the endodermal epithelium. The development of the pharyngeal arches and pouches (see Ch. 35) is closely associated with the development of the neural ectoderm and proliferation of the neural crest. The respiratory diverticulum arises slightly later when the postpharyngeal gut may also be distinguished (see pages 1033 and 1203). The endoderm gives rise to the epithelial lining of the respiratory and gastrointestinal tracts, the biliary system (see p. 1207), and the bladder and urethra (see pages 1307, 1310).
Coelomic epithelium
The coelomic epithelium lines the intraembryonic coelom, which is subdivided into a midline pericardial cavity, two bilateral pericardioperitoneal canals, and the initially bilateral peritoneal cavities; the latter are continuous with the extraembryonic coelom. The coelomic epithelium is a germinal epithelium. It produces the myocardium (see p. 189) and connective tissue populations for the viscera (see p. 1033), and also gives rise to the supporting cells for the germ cells (see p. 1314), the epithelial lining of the urogenital tracts (see p. 1313) and the mesothelial lining of the pericardial, pleural (see p. 1013) and peritoneal cavities (see p. 1314).
The relative dispositions of the neural epithelium, endodermal and coelomic epithelia are shown in Fig. 12.1.
Mesenchymal populations in the embryo
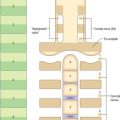
In the stage 10 embryo, the major mesenchymal populations are in place. Mesoblast is still being generated at the primitive streak and moving into the presomitic mesenchymal population adjacent to the notochord. Some mesoblast is also contributing to the lateral regions of the embryo. The different mesenchymal populations within the embryo from stage 10 onwards are described below. The relative dispositions of the early mesenchymal populations are shown in Fig. 12.2.
Axial mesenchyme
The first epiblast cells to ingress through the primitive streak form the endoderm and notochord and initially occupy a midline position. The earliest population of endodermal cells rostral to the notochordal plate is termed the prechordal plate. The notochordal cells remain medially and the endodermal cells subsequently flatten and spread laterally. The population of cells that remain mesenchymal in contact with the floor of the neural groove, just rostral to the notochordal plate, is termed prechordal mesenchyme (Fig. 12.4). These axial mesenchyme cells are tightly packed, unlike the more lateral paraxial cells, but unlike the notochord, they are not contained in an extracellular sheath. They are displaced laterally at the time of head flexion and form bilateral premandibular mesenchymal condensations. They become associated with the local paraxial mesenchyme. Orthotopic grafting has demonstrated that these cells leave the edges of the prechordal mesenchyme and migrate laterally into the periocular mesenchyme, where they give rise to all of the extrinsic ocular muscles (see p. 702).
Paraxial mesenchyme
Epiblast cells that migrate through the primitive node and rostral primitive streak during gastrulation form mesoblast cells which migrate to a position lateral to the notochord and beneath the developing neural plate. Cells that ingress through the primitive node form the medial part of this paraxial mesenchyme, and cells that ingress through the rostral streak form the lateral part (see Fig. 10.3). The paraxial mesenchyme extends cranially from the primitive streak to the prechordal plate immediately rostral to the notochord. Before somite formation, this mesenchyme is also termed presomitic or unsegmented mesenchyme in mammals (analogous to the segmental plate in birds). Paraxial mesenchyme rostral to the otic vesicle was previously believed not to segment. However, the mesenchyme in this region shows concentric rings of cell bodies and processes that form paired, bilateral cylinders, termed somitomeres.
Caudal to the otic vesicle, the paraxial mesenchyme on each side of the rhombencephalon segments into somites as the neural folds elevate and neurulation begins: somites are therefore post-otic. During somitogenesis the mesenchyme cells show changes in shape, and in cell–cell adhesion, and become organized into epithelial somites. This process begins at the eighth somitomere, which is just caudal to the midpoint of the notochordal plate. Somite one is also termed the first occipital somite. Somites can be seen on each side of the fusing neural tube in the human embryo from stage 9. Development proceeds in a craniocaudal direction, thus unsegmented paraxial mesenchyme is a transient structure. It forms somites from its cranial end, whilst mesenchyme is added to its caudal end by the regressing primitive streak. Somites give rise to the base of the skull (see p. 610); the vertebral column and ribs (see p. 764); and the skeletal muscle of the body, including that in the limbs (see p. 899).
Septum transversum
Early mesenchyme that invaginates through the middle part of the primitive streak comes to lie rostral to the buccopharyngeal membrane, where the cells form the epithelial wall of the pericardial coelom. As this epithelium proliferates, the visceral pericardial wall gives rise to the myocardium. The parietal pericardial wall forms mesenchyme, initially termed precardiac, or cardiac, mesenchyme, which is able to induce proliferation of hepatic endodermal epithelium. With further proliferation, the precardiac mesenchyme forms a ventral mass caudal to the heart, the septum transversum, which separates the foregut endoderm from the pericardial coelom (Fig. 12.2). By stage 11 the septum transversum extends dorsally on each side of the developing gut, and becomes continuous with the mesenchymal populations that proliferate from the walls of the pericardioperitoneal canals. Cells of the septum transversum give rise to the sinusoids of the liver (see p. 1207), the central portions of the diaphragm (see p. 1014) and the epicardium (see p. 1026).
Neural crest
Trunk neural crest
Trunk neural crest is formed as the neural tube closes cranio-caudally, which means that various stages of crest development can be found in the more caudal regions of an embryo. As the neural tube begins to fuse dorsally in the midline, the neural crest cells lose their epithelial characteristics and junctional connections and form a band of loosely arranged mesenchyme cells immediately dorsal to the neural tube and beneath the ectoderm. Initially, most of the crest cells lie with their long axes perpendicular to the long axis of the neural tube. Later, the cell population expands laterally and around the neural tube as a sheet. Trunk neural crest cells move from their position dorsal to the neural tube via three routes (see Fig. 24.11) dorsolaterally, to form dorsal root ganglia throughout the trunk; ventrally, to form sympathetic ganglia, enteric nerves and the suprarenal medulla; and rostrocaudally along the aorta, to form the preaortic ganglia. In a second migration route, crest cells pass dorsolaterally between the ectoderm and the epithelial plate of the somite into the somatopleure, where they eventually form melanocytes in the skin.
Head neural crest
Unlike its counterpart in the trunk, head neural crest migrates before the neural tube closes. Two populations of crest cells develop. Some cells retain a neuronal lineage and contribute to the somatic sensory and parasympathetic ganglia in the head and neck (see p. 364). Others produce extensive mesenchymal populations: the crest cell population arising from the head is larger than that found at any trunk level. Each brain region has its own crest population that migrates dorsolaterally around the sides of the neural tube to reach the ventral side of the head. Crest cells surround the prosencephalic and optic vesicles and occupy each of the pharyngeal arches (see Ch. 35). They provide mesenchyme cells which will produce the connective tissue in parts of the neuro- and viscero-crania. All cartilage, bone, ligament, tendon, dermal components and glandular stroma in the head are derived from the head neural crest. Head neural crest also contributes to the tunica media of the aortic arch arteries.
Lateral plate
Lateral plate is the term for the early unsegmented mesoblast population lateral to the paraxial mesenchyme. Mesoblastic cells, which arise from the middle of the primitive streak (primary mesenchyme), migrate cranially, laterally and caudally to reach their destinations, where they revert to epithelium and form a continuous layer that adheres to the ectoderm dorsally and the endoderm ventrally. The epithelium faces a new intraembryonic cavity, the intraembryonic coelom, which becomes confluent with the extraembryonic coelom and provides a route for the circulation of coelomic fluid through the embryo. Once formed, the intraembryonic coelomic wall becomes a proliferative epithelium which produces new populations of mesenchymal cells. The mesenchymal population subjacent to the ectoderm is termed somatopleuric mesenchyme, and is produced by the somatopleuric coelomic epithelium. The mesenchymal population surrounding the endoderm is termed splanchnopleuric mesenchyme, and is produced by the splanchnopleuric coelomic epithelium (Fig. 12.2).
Somatopleuric mesenchyme
Somatopleuric mesenchyme produces a mixed population of connective tissues and has a significant organizing effect at the level of the developing limbs. The pattern of limb development is controlled by information contained in the somatopleuric mesenchyme. Regions of the limb are specified by interaction between the surface ectoderm (apical ectodermal ridge) and underlying somatopleuric mesenchyme; together these tissues form the progress zone of the limb (see p. 899). The somatopleuric mesenchyme in the limb bud also specifies the postaxial border of the developing limb.
Muscles of the limbs are derived from paraxially derived somitic precursor muscle cells.
Splanchnopleuric mesenchyme
Splanchnopleuric mesenchyme surrounds the developing gut and respiratory tubes, contributing connective tissue cells to the lamina propria and submucosa, and smooth muscle cells to the muscularis mucosae and muscularis externa. It plays a patterning role in endodermal development, specifying the region and villus type in the gut (see p. 1203), and the branching pattern in the respiratory tract (see p. 1032).
Intermediate mesenchyme
Intermediate mesenchyme is a loose collection of cells found between the somites and the lateral plate (Fig. 12.2). Its development is closely related to the progress of differentiation of the somites and the proliferating coelomic epithelium from which it is derived. Intermediate mesenchyme is not present before somitogenesis or the formation of the eighth somite. In embryos with eight to ten somites, it is present lateral to the sixth somite, but does not extend cranially. The mesenchyme cells are arranged as layers, one continuous with the dorsal side of the paraxial mesenchyme and the somatopleure, the other with the ventral side of the paraxial mesenchyme and the splanchnopleure.
As development proceeds, the intermediate mesenchyme forms a loosely packed dorsolateral cord of cells, which lengthens at the caudal end and ultimately joins the cloaca. It gives rise to the nephric system, gonads and reproductive ducts (see pp. 1305, 1311).