CHAPTER 10 Cell populations at gastrulation
CONCEPTUS WITH A BILAMINAR EMBRYONIC DISC
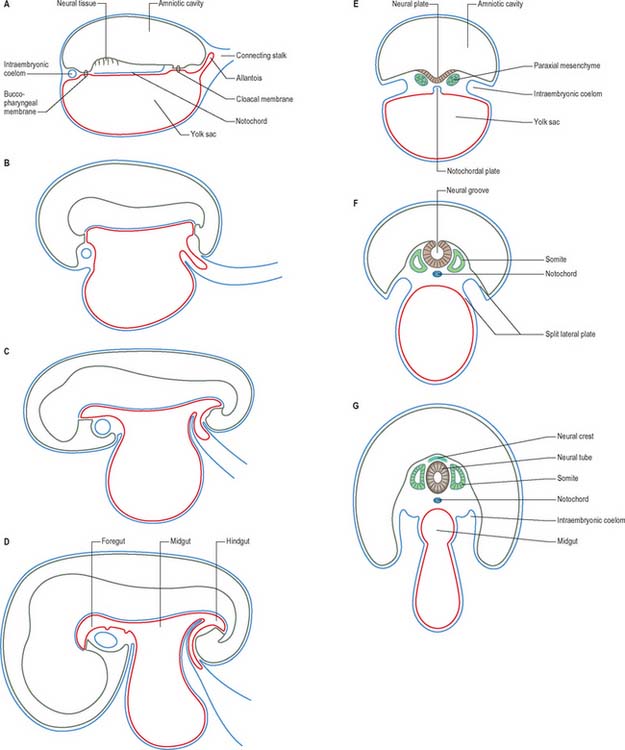
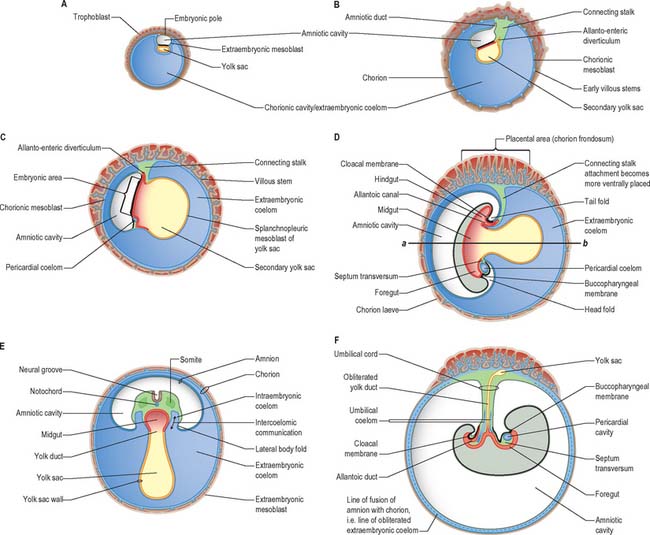
At stage 6 the conceptus is composed of the walls of three cavities: the large chorionic cavity is surrounded by a meshwork of trophoblast and developing villi and lined with extraembryonic mesoblast. The chorion, trophoblast and extraembryonic mesoblast enclose the extraembryonic coelom and contain the much smaller amniotic cavity and yolk sac (see Fig. 9.1). These latter cavities abut at the embryonic bilaminar disc where the epithelial epiblast and visceral hypoblast are approximated. A fourth cavity, the allantois, will form as a hypoblastic diverticulum in stage 7. The ‘bilaminar disc’ commonly referred to in embryology texts does not yet possess the definitive layers of embryonic ectoderm and endoderm that will give rise to embryonic structures. Only the epiblast will give rise to the embryo; all other layers produced so far are extraembryonic. The amnion and chorion (and surrounding mesoblast) are part of the extraembryonic somatopleure, whereas the yolk sac, allantois and surrounding extraembryonic mesoblast constitute extraembryonic splanchnopleure. At the junctional zone surrounding the margins of the embryonic area, where the walls of the amnion and yolk sac converge, the somatopleuric and splanchnopleuric layers of extraembryonic mesoblast are continuous.
The terms epiblast and hypoblast are used to make the distinction between the earliest bilaminar disc layers and the later embryonic layers. Epiblast and hypoblast contain mixed populations of cells with little restriction (see p. 193), which establish the placental structures and extraembryonic tissues before the production of embryonic cell lines at gastrulation. The older terminology depicting three germ layers that give rise to the skin, gut lining and intervening tissues is thus incorrect for the bilaminar and trilaminar embryonic disc. The application and the retention of this aged terminology for the early stages of embryology continues to cause confusion and inhibits the development of more pertinent descriptive language to describe these early events.
Primitive streak and node
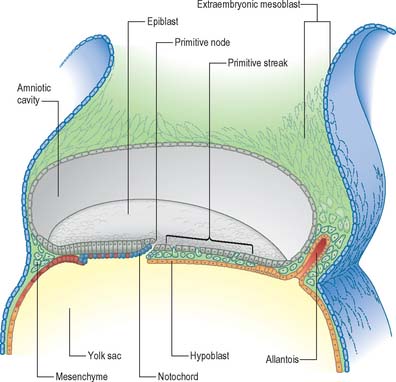
Seen from the dorsal (epiblastic) aspect, at stage 6, the embryonic disc appears elongated. The primitive streak is first seen in the caudal region of the embryonic disc at this stage as a collection of pluripotent cells, orientated along its long axis in the median position, conferring the future craniocaudal axis of the embryo (Figs 10.1 and 10.2). Although the future cranial and caudal regions of the embryo are well within the boundaries of the embryonic disc, it has become the practice to term the region of the disc closest to the streak ‘caudal’, and the region of the disc furthest from the streak ‘cranial’ or ‘rostral’. With the development of the streak, the terms medial and lateral can be used. The relative dimensions of the primitive streak and the fates of the cells that pass through it change with the developmental stage. Thus the streak extends half way along the disc in the stage 6 embryo, reaches its greatest relative length in stage 7 and its maximum length in stage 8.
Formation of the primitive streak is induced by the underlying visceral hypoblast which remains beneath the streak even at later stages. The primitive streak may be considered to be generally homologous with the blastopore of lower vertebrates (e.g. amphibia), with the nodal region corresponding to the dorsal lip. Experiments clearly show the lip of the blastopore to be a dynamic wave front on which cells are carried into the interior to form the roof of the archenteron, a situation analogous to ingression through the node of the prechordal plate and endoderm. The primitive streak similarly may be considered analogous to the coapted, or fused, lateral lips of the blastopore, and the cloacal membrane and its immediate environs are considered analogous to the ventral lip of the blastopore.
Position and time of ingression through the primitive streak
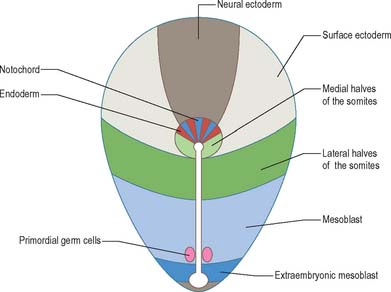
The position and time of ingression through either streak or node directly affect the developmental fate of cells. Passage through the streak is specified according to position, e.g. via the node, or rostral, middle or caudal regions of the streak. Cells that ingress through the primitive node give rise to the axial cell lines, the prechordal mesenchyme and notochord, and to the endoderm and the medial halves of the somites. The rostral portion of the primitive streak produces cells for the lateral halves of the somites, whereas the middle streak produces the lateral plate mesoblast. The adjacent caudal portion of the streak gives rise to the primordial germ cells, which can be distinguished histologically and histochemically, and the most caudal portion of the streak contributes cells to the extraembryonic mesoblast until the somites are visible. A composite of the information on the position of ingression through the streak and node is shown in Fig. 10.3. The epiblast cells that do not pass through the streak but remain instead within the epiblast population give rise to the neural and surface ectoderm of the embryo.
Prechordal plate
The earliest cells migrating through the primitive node and streak give rise to both the embryonic endoderm and the notochord. The prechordal plate is first seen at stage 7. It has been defined as a localized thickening of the endoderm rostral to the notochordal process, although it is seen as a highly developed mesenchymal mass in contact with the floor of the neural groove, rostral to the notochordal process, rather than as an epithelial layer. The prechordal plate is a temporary collection of cells which underlies the neural plate during stage 9. It is composed of cells that are similar to, or larger and more spherical than, the ingressing endodermal cells (Müller & O’Rahilly 2003). In stage 8 embryos the prechordal plate is up to eight cells deep and extends along the long axis of the embryonic disc. By stages 9 and 10 the cells at the lateral edge of the plate have begun to migrate laterally as free mesenchymal cells and the plate reduces in height to two cells deep. At stage 11 the migrating prechordal mesenchyme forms bilateral premandibular mesenchymal condensations and is no longer a median structure. The extent of prechordal cells remaining within the endoderm is not clear.
Notochord
The notochord, also called chordamesoderm, the head process or chorda, arises from epiblast cells of the medial part of the primitive node. It passes through several stages during development. The cells of the early notochordal process express myogenic markers transitorily as they migrate beneath the epiblast, but later they become epithelial, forming junctions and a basal lamina. The notochordal cells are intimately mixed with endodermal cells, as both cells lines ingress at the same time (Figs 10.1, 10.2 and 10.4). In the stage 8 embryo, the ingressing notochordal cells remain in the midline along the cephalocaudal axis. They form a rostral part, which is composed of a cell mass continuous with the prechordal mesenchyme, a mid portion in which cells are arranged in a tube with a central notochordal canal, and a caudal epithelial layer of cells, the notochordal plate, which is contiguous with the embryonic endoderm and forms a roof to the secondary yolk sac. There is a transitory opening between the primitive node (and amniotic cavity) and the secondary yolk sac called the neurenteric canal (so named because its upper opening is in the future caudal floor of the neural groove, and its lower opening is into the archenteron, which is the primitive gut); it may still be found at stage 9, and the site of the neurenteric canal can be recognized in stage 10 embryos. The ingression of notochordal cells at the primitive node is matched by specification of the overlying neural ectodermal cells, and the notochordal plate is thus matched in length by the future neural floor plate. Both the notochord and the region of the floor plate of the neural tube may arise from a common progenitor cell. The early notochord is important for the maintenance and subsequent development of the neural floor plate and the induction of motor neurones. Removal of the notochord results in elimination of the neural floor plate and motor neurones, and expression of sensory cells types.
Caudal eminence
From stages 9 and 10 the region between the neurenteric canal and the cloacal membrane (see below), including the primitive streak, is termed the caudal eminence. It consists of the caudal region of the trunk composed of mesenchyme derived from the primitive streak and epiblast, covered with surface ectoderm. Whereas ingression of cells through the primitive streak gives rise to the prechordal and notochordal plates, and cells rostral to the neurenteric canal (see below), the cells of the caudal eminence arise from local division of a mesenchymal population positioned caudal to the neurenteric canal. The caudal portions of the notochord, which form later in development when secondary neurulation processes begin, arise from these cell populations, sometimes termed the caudoneural hinge or junction (see p. 361). This tissue is thicker and more advanced in differentiation than the tissues derived from the early primitive streak.
Embryonic endoderm
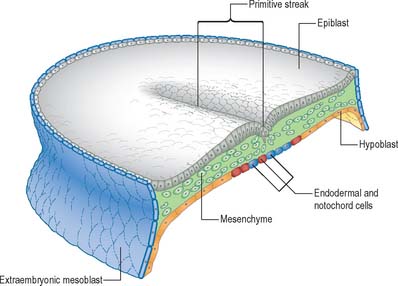
Before ingression, definitive embryonic endoderm cells are found in the epiblast located at the primitive node and rostral primitive streak. In the mouse, the endodermal cells lie beneaththe epiblast mainly in the midline, interspersed with presumptive notochordal cells, forming the roof of the secondary yolk sac. The ingressing endoderm displaces the visceral hypoblast into the secondary yolk sac wall by a dramatic territorial expansion that is brought about by a change in the morphology of the cells (Figs 10.1, 10.2 and 10.4). The putative endoderm cells are cuboidal epithelial cells within the node, but they become squamous in the endoderm layer; this could result in a four-fold increase in the surface area covered by the cells. A complete replacement of the visceral hypoblast has not yet been confirmed, and there may be a mixed population of cells in the endodermal layer in the early stages. Ingression of cells through the streak and node in the human is apparent at stage 6, and by stage 7 a population of endoderm and notochord cells is present beneath the epiblast (Figs 10.1, 10.2 and 10.4). During stages 6–11, the midline roof of the secondary yolk sac becomes populated mainly by the notochordal plate, which remains in direct lateral continuity with the endodermal cells. It is not until stage 11, after the definitive notochord is formed, that the endoderm cells can join across the midline. For the developmental fate of the embryonic endoderm, see Fig. 12.3.
Intraembryonic mesoblast (mesenchyme)
Epiblast cells ingress through the cranial and middle parts of the streak individually, maintaining their apical epithelial contacts while elongating ventrally. The cells become flask-shaped, with thin attenuated apical necks and broad basal regions. The basal and lateral surfaces form lamellipodia and filopodia and the apical contact is released. The cells are now free mesoblast cells, their fibroblastic, stellate morphology reflecting the release from the epithelial layer. Once through the streak, the cells migrate away from it, using the basal lamina of the overlying epiblast and extracellular matrix as a substratum. The cells contact one another by filopodia and lamellipodia, with which they also contact the basal lamina. Gap junctions have been observed between filopodia and cell bodies. With the appearance of the mesoblast, spaces form between the epiblast and visceral hypoblast that are filled with extracellular matrix rich in glycosaminoglycans. The migrating mesoblast has a leading edge of cells that open up the migration routes, and the following cells seem to be pulled along behind in a coordinated mass movement. Mesoblast formed by cells migrating through the primitive node and rostral primitive streak will form the paraxial mesenchyme, whereas cells migrating through the middle to caudal streak will form the lateral plate mesenchyme (Figs 10.1, 10.2; see Fig. 10.6).
Embryonic ectoderm
When the ingression of cells through the primitive streak is completed, the epithelial cells remaining in the epiblast layer are termed embryonic ectoderm cells. This layer still contains a mixed population, because both surface ectoderm cells and neural ectoderm cells are present. It is believed that these cells were originally in the cranial half of the disc when the primitive streak first appeared, at which time the neural-fated cells were closest to the streak, and the surface ectoderm cells were most cranial (Fig. 10.3). The process of primary neurulation relocates most of the neuroepithelial cells (see below).
Primordial germ cells
Although early studies on human embryos have reported primordial germ cells, and described their development from the early endoderm of the yolk sac and allantois, it is now clear from animal experimentation that the primordial germ cells arise from epiblast ingressing at the caudal end of the primitive streak (Fig. 10.3). It is not known whether these cells originate from rostral regions that migrate to the streak, or from local caudal regions. Extremely early segregation of the germ cells, when the epiblast layer consists of only 10–13 cells, has been demonstrated. It has been suggested that the primordial germ cells remain sequestered in the extraembryonic mesenchyme at the caudal end of the embryo until the embryonic endoderm has been produced and gastrulation completed, and that they start to migrate along the allantoic and hindgut endoderm as the folding of the embryo begins. The formation of the tail fold brings the proximal portion of the allantois within the body, so reducing the final distance over which the cells migrate to the genital ridges. Further development of the germ cells is described on page 1318.
TRILAMINAR DISC
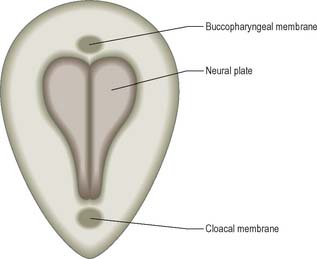
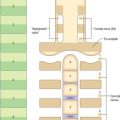
Although the stage 8 embryo is termed a trilaminar disc, the concept of three epithelial layers forming a trilaminar disc is incorrect: the middle, mesoblast, layer is several cells thick with intervening extracellular matrix. The embryo at this stage, approximately 23 days after ovulation, is pear-shaped, and broader cranially than caudally (Fig. 10.5). The upper epiblast cells are tall, and form a pseudostratified columnar epithelial layer with a basal lamina, except at the primitive streak, where the cells are ingressing to form the other layers. The more centrally placed epiblast will give rise to neural ectoderm (neurectoderm) and the more laterally placed epiblast will give rise to surface ectoderm. The future neural ectoderm is seen as a neural plate which matches the length of the notochordal plate directly beneath, being slightly wider near the prechordal plate. The lower embryonic endoderm, a simple squamous layer with a developing basal lamina, is not always complete at this stage, particularly in the midline caudal to the prechordal plate, which is still occupied by the notochordal process or plate.
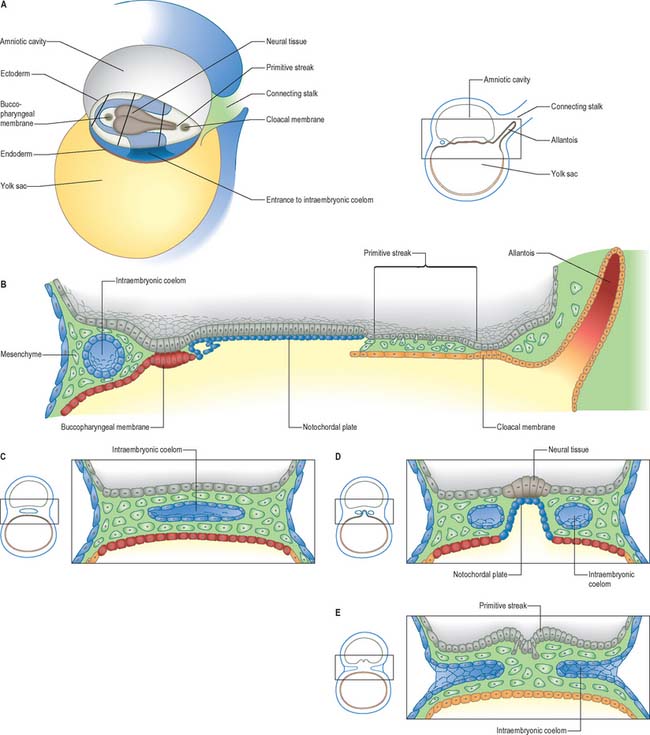
The middle, mesoblast, layer is composed of free cells migrating cranially, laterally and caudally from the primitive streak (Fig. 10.4). They produce extracellular matrix, which separates the epiblast and endoderm of the embryonic area and permits their passage. The streams of mesoblast extend between the epiblast and endoderm over all of the disc area except cranially at the buccopharyngeal membrane (where the endoderm and ectoderm become apposed once the prechordal mesenchyme has migrated laterally), and caudally at the cloacal membrane (a patch of thickened endoderm, similar to the buccopharyngeal membrane, caudal to the primitive streak). The mesoblast on each side of the notochord is termed paraxial mesenchyme.
Still further caudally, the embryonic disc develops a midline diverticulum adjacent to the cloacal membrane. This diverticulum, the allantois, projects into the extraembryonic connecting stalk (Fig. 10.4, see also Fig. 10.6 and p. 180). There is little information about which cells form the allantois, i.e. whether it is composed of visceral hypoblast, parietal hypoblast or embryonic endoderm. The allantois later develops a rich anastomotic blood supply around it, in the manner of the yolk sac.
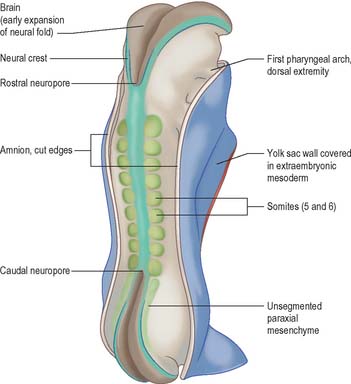
The generation of cells at the primitive node produces midline endoderm, notochord and the floor plate of the future neural tube. As the notochord grows and elongates, there is a matched growth of neural floor plate cells until both cell lines extend to the buccopharyngeal membrane. The epiblast lateral to the midline contains both future surface and neural ectoderm. The latter becomes arranged between the primitive node and buccopharyngeal membrane: cells destined to be in the neural plate lie medially, and those destined for the neural crest lie laterally (Fig. 10.5). A smaller subpopulation of neuronal cells, the ectodermal placodes, are arranged either close to the neural crest or within the rostral limit of the neural plate itself.
FOLDING OF THE EMBRYO
In a diagrammatic representation of the trilaminar disc prior to folding and viewed from the ectodermal aspect, all of the future external surface of the body is delimited (see Fig. 10.5). The ends of the gut tube are specified on the ectodermal surface at the buccopharyngeal and cloacal membranes, which are regions where the ectoderm and underlying endoderm are apposed without intervening mesoblast. In the midline between these membranes, proliferation of the neural ectoderm matches the underlying migration of mesoblast from the primitive streak, so that the neural plate covers the paraxial mesenchyme on each side of the notochord (Fig. 10.6E). As the paraxial mesenchyme segments (see p. 763), the formation of the epithelial somites elevates the edges of the neural plate and initiates primary neurulation (Figs 10.6F and 10.8). The neural plate itself undergoes concurrent morphological changes. The most medial cells become wedge-shaped, forming the neural groove. Further elevation of the edges of the neural groove permits fusion of the neuronal populations in the dorsal midline to form the neural tube. The surface ectoderm forms the putative dorsal epidermis (Figs 10.6G and 10.8). Cells at the lateral edge of the neural plate termed neural crest cells remain as a linearly arranged mesenchymal population between these two epithelia. Fusion of the neural tube begins in the future rhombencephalic region of the embryo and proceeds rostrally and caudally to about the level of somite 29. Neurulation is described further in Chapter 24. A population of neural epithelial cells remain within the surface ectoderm; at this stage they are termed ectodermal placodes.
The representation of a person on the trilaminar disc (Fig. 10.9) shows, to some extent, the way in which the positions of the main body structures are already specified in the unfolded embryo. The portion of ectoderm lateral to the neural plate and the paraxial mesenchyme will form structures within the back. The portion of the disc between the buccopharyngeal membrane and the edge of the disc will become the ventral thoracic wall and the ventral abdominal wall cranial to the umbilicus. Further caudally, midway along the neural axis, the lateral portions of the disc will become the lateral and ventral abdominal walls of the trunk. The portion of the disc beyond the cloacal membrane will form the ventral abdominal wall caudal to the umbilicus. The circumference of the disc, where the embryonic tissue meets the extraembryonic membranes, will become restricted to the connection between the ventral abdominal wall and the umbilical cord, i.e. the umbilicus.
Head folding begins at stage 9, when the fusing cranial neural plate rises above the surface ectoderm and the portion of the disc rostral to the buccopharyngeal membrane (which contains the cardiogenic mesenchyme) moves to lie ventral to the developing brain (Fig. 10.6). The prosencephalon and buccopharyngeal membrane are now the most rostral structures of the embryo. The previously flat region of endoderm, which may contain cells from the prechordal plate, is now modified into a deep tube, the primitive foregut. Tail folding can be seen in stage 10 embryos, when the entire embryo comes to rise above the level of the yolk sac. Similar movement of the part of the disc caudal to the cloacal membrane results in its repositioning ventral to the neural plate. Generally, as the embryo rises above the edges of the disc, the lateral regions of the disc are drawn ventrally and medially, contributing to the lateral folding of the embryo.
FORMATION OF THE INTRAEMBRYONIC COELOM
At and just before stage 9 (before formation of the head fold), vesicles appear between the mesenchymal cells cranial to the buccopharyngeal membrane and within the cranial lateral plate mesenchyme. At the periphery of the vesicles, the mesenchymal cells develop junctional complexes and apical polarity, and form an epithelium. The vesicles become confluent to form a horse-shoe shaped tube, the intraembryonic coelom, which extends caudally to the level of the first somite and laterally into the lateral plate mesenchyme towards the extraembryonic mesenchyme. The intra- and extra-embryonic coeloms do not communicate at this stage. The lateral plate mesenchyme thus develops somatopleuric coelomic epithelium subjacent to the ectoderm, and a splanchnopleuric coelomic epithelium next to the embryonic endoderm (Fig. 10.4; see also Fig. 12.2C(iv)).
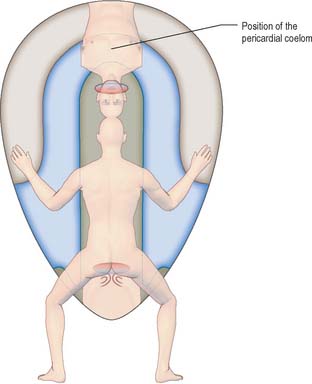
Compartments of the coelom that will later in development give rise to the body cavities can already be seen. The midline ventral portion, caudal to the buccopharyngeal membrane, becomes the pericardial cavity. The canals lateral to the foregut (pericardioperitoneal canals) become the pleural cavities and the uppermost part of the peritoneal cavity. The remaining portion of the coelom becomes the peritoneal cavity. By stage 11, the intraembryonic coelom within the lateral plate mesenchyme extends caudally to the level of the caudal wall of the yolk sac. The intra- and extra-embryonic coeloms communicate widely on each side of the midgut along the length of the embryo from the level of the 4th somite (Fig. 10.10; see also Fig. 12.1).
In spite of the importance of the coelom in defining the body cavities, and of the coelomic epithelium in the production of the major mesenchymal populations of the trunk (see Fig. 12.2), only a few workers have considered the overall contribution of the coelom and its epithelium to the embryo (Streeter 1942, Langemeijer 1976). The coelom can be described as a single, tubular organ which is comparable to the neural tube, in that it possesses a specialized wall that encloses a cavity. Certainly, the proliferating coelomic epithelium has many similarities to the neural ectoderm. It is pseudostratified columnar epithelium with an inner germinal layer from which cellular progeny migrate. After the germinal phase, both epithelia ultimately form the lining of a cavity, i.e. ependyma for the neural epithelium, and mesothelium for the coelomic epithelium. The coelomic epithelium, like the neural epithelium, produces cells destined for different fates from different sites and at different developmental times. Coelomic cells are like the neural epithelium, in that they have apical epithelial specializations and tapering basal processes that are in direct contact with the underlying mesenchyme, without an intervening basal lamina. The possibility of the tapering processes forming directional signals for migrating progeny, similar to radial glia of the neural tube, has not been examined.
EMBRYONIC CELL POPULATIONS AT GASTRULATION
Mesenchyme
The terms mesoblast and mesenchyme are used in this text in a specific manner and not interchangeably. Previously, cells forming a population between the epiblast and hypoblast were termed mesoderm and, more recently, mesenchyme. The terms primary and secondary mesenchyme have been used to distinguish between those cells which arise from ingression through the primitive streak and those which arise from neural crest ingression respectively. Primary mesenchymal cells revert to epithelia at their destinations. However, whereas some primary mesenchymal cells may become epithelial within a short time frame, e.g. somites and lateral plate, other cells may transform later, e.g. the epithelium lining blood vessels. To cope with these conflicts in terminology, the mixed population of epiblast cells that ingress through the primitive streak and come to lie between the epiblast and embryonic endoderm is termed mesoblast until the cells have migrated to their final position, at which time the populations of mesenchyme can be identified and their fates inferred.
Extracellular matrix
Fibronectin deposited extracellularly along a migration pathway will affect cells that touch it later, causing realignment of their intracellular actin filaments and thus of their orientation; it also induces cell migration. The receptors for extracellular matrix molecules such as fibronectin and laminin were originally termed integrins because they integrate extracellular proteins and intracellular cytoskeletal elements (via α and β subunits that span the cell membrane), allowing them to act together: the binding preference of integrins depends upon their combination of subunits and environmental conditions.
Transition between epithelial and mesenchyme states
Transformations of cell morphology from epithelium to mesenchyme, and vice versa, occur in specific places and times during development, and can be seen as ways of dispersing germinal centres with increasing restriction. The first epithelial-to-mesenchyme transition occurs at the primitive streak, a germinal epithelium which confers embryonic specification on the resultant mesoblast population. The mesoblast so formed migrates and the cells undergo mesenchyme-to-epithelial transitions when they reach their final destinations. Series of small epithelial germinal centres, the somites, are formed, as are larger, more extensive, germinal epithelial sheets which line the walls of the intraembryonic coelom. The coelomic walls, especially those derived from somatopleure and splanchnopleure, form germinal epithelia which give rise to the major mesenchymal populations that form the viscera. The early epithelial somites undergo further local epithelial-to-mesenchyme transitions to form the sclerotomes, and subsequently form several germinal epithelia in the epithelial plate of each somite. Later mesenchyme-to-epithelium transitions are not associated with the formation of germinal epithelia: the most common involve the transition of mesenchyme into the endothelium of the vascular system (see p. 1017). The nephrons of the mesonephric and metanephric systems also form from mesenchyme-to-epithelial transition (see p. 1308).
Müller F, O’Rahilly R. The prechordal plate, the rostral end of the notochord and nearby median features in staged human embryos. Cells Tissues Organs. 2003;173:1-20.
Langemeijer RATM. Le coelome et son revètement comme organoblasteme. Bull Ass Anat. 1976;60:547-558.
Streeter GL. Developmental horizons in human embryos. Descriptions of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites. Contrib Embryol Carnegie Inst Washington. 1942;30:211-245.