Chapter 15 Cavernous Malformations Management Strategies
• Cerebral cavernous malformations (CCMs) are vascular lesions that contain a compact bundle of pathological capillary vessels without brain parenchyma between the vessels. CCMs are relatively common, affecting approximately 1 in every 200 individuals and accounting for 8% to 15% of all vascular malformations of the central nervous system (CNS). The majority of lesions occurs in the brain, with most being located in the supratentorial compartment and the minority infratentorially, to include the brainstem. Approximately 3% to 5% are discovered as intramedullary spinal CCM.
• Despite the high prevalence of CCM, most lesions are asymptomatic, being discovered incidentally, if ever. Only 20% to 30% of CCM patients will be symptomatic in their lifetimes, presenting to medical attention most commonly during their third to fifth decades of life with symptoms such as headaches, seizures, and focal neurological deficits due to lesion expansion following such events as thromboses and hemorrhages.
• Cavernous malformations can be either familial or sporadic. The familial form of the disease often manifests as multiple lesions in the setting of a family history of neurological disease. In the sporadic form, patients rarely have more than two lesions, and family history is typically absent. Mutations in three genes, CCM1, CCM2, and CCM3, have been discovered as being responsible for the familial disease, accounting for 96% of all mutations. De novo occurrence of lesions can occur and may impact long-term outcome.
• Complete surgical resection of CCM is the only management strategy that is curative. Medical management is limited to seizure control and symptomatic relief of headaches. The indication for radiosurgery treatment is controversial. In contrast, successful lesion resection immediately eliminates a patient’s hemorrhage risk, but up to 80% of patients achieve seizure control postoperatively. The main goal in managing CCM is to balance the risks of surgery with those of the natural history of the disease. Because both of these factors vary significantly, each CCM patient must be considered on an individual basis.
• The most serious complications of CCM natural history is intracerebral hemorrhage. Patients with posterior fossa cavernomas are reported to be 6.75 times more likely to present with a bleed and the rehemorrhage rate is higher than that for lesions in other locations. Not only were these repeat hemorrhages common but brainstem cavernomas can result in debilitating deficits due to the high density of critical tracts and nuclei in this region.
• As a result of devastating outcomes with untreated cavernomas in high-risk regions, surgical treatment must always be considered for these select patients. Four major criteria assist in determining which patients with infratentorial lesions are appropriate surgical candidates: (1) lesions that rise to the pial surface based on T1-weighted MRI, (2) lesions with repeated hemorrhages causing progressive neurological deficits, (3) lesions with acute hemorrhage extending outside the lesion capsule, and (4) significant mass effect produced with a large intralesional hemorrhage. Surgery is considered only when total resection can be achieved, because lesion remnants can grow and hemorrhage as well.
Description
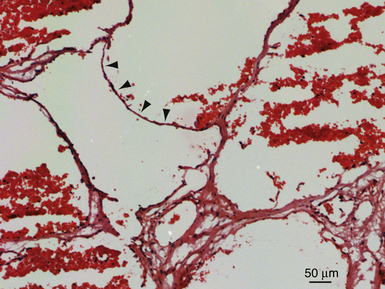
Cerebral cavernous malformations (CCMs), or cavernomas, are vascular lesions consisting of a compact bundle of dilated capillary-like channels lacking intervening neural parenchyma. They typically range in size from 1 mm to several centimeters and can be found anywhere in the central nervous system (CNS) as well as in other organs such as the skin and eye. Histologically, the lesions are composed of a single layer of endothelial cells and lack structural elements found in mature vessels, including smooth muscle and elastin. Other elements usually found in the blood-brain barrier, including astrocytic foot processes and pericytes, are also diminished or completely absent.1–3 Macroscopically, the lesions appear reddish purple. They are often multilobulated and can be encapsulated by a variable layer of fibrous adventitia, giving them their characteristic mulberry-like appearance.
CCMs are relatively common, affecting approximately 1 in every 200 individuals and accounting for 8% to 15% of all vascular malformations of the CNS.4,5 The majority of lesions occurs in the brain, with 63% to 90% of these being located in the supratentorial compartment and 7.8% to 35.8% infratentorially.4,6 Between 9% and 35% of infratentorial lesions are located in the brainstem.6
Despite the high prevalence of CCM, most lesions are asymptomatic, being discovered incidentally, if ever. Only 20% to 30% of CCM patients will be symptomatic in their lifetimes, presenting to medical attention most commonly during their third to fifth decades of life with symptoms such as headaches, seizures, and focal neurological deficits due to lesion expansion following such events as thromboses and hemorrhages.7 Asymptomatic patients will also occasionally present to health care providers after having one or multiple CCMs detected incidentally on imaging studies obtained for other purposes. Given this highly variable presentation and disease progression in patients with CCM, choosing the appropriate management strategy can be challenging. This chapter will explore the different treatment options available for CCM and provide suggestions as to the appropriate management in different circumstances. Before doing so, however, the natural history of these lesions will be discussed, as sound understanding of the natural history of the disease is paramount in any decision-making process with regard to treatment.
Natural History
The natural history of CCM can vary widely among patients. Although once believed to be congenital, it is now recognized that CCM can also occur de novo.8 Once present, these lesions are dynamic, expanding as lesions thrombose and hemorrhage, and regressing as they recanalize and as blood products from hemorrhages are resorbed.8,9 The most common symptom reported in these patients is seizures, which are especially common with frontal or temporal lobe lesions.10 Cavernoma lesions are typically surrounded by reactive gliosis which, at least in certain cases, is thought to serve as an epileptogenic focus. The estimated annual seizure risk is approximately 1% to 2%, and in patients with supratentorial lesions several seizure types including simple, complex partial, and generalized have been reported.10,11
Gross apoplectic hemorrhages are the most severe presentation of CCM. Because cavernomas are low-flow, low-pressure lesions, most bleeds are relatively small and result from blood extravasation from the leaky vascular channels of the lesion. Larger bleeds can and do occur, though, with an annual hemorrhage risk ranging between 0.25% and 6% depending on a variety of factors.11 Larger and deeper lesions have been reported to have an increased bleeding risk, as do lesions in older patients, pregnant patients, and patients who have suffered a previous bleed.7,12 Asymptomatic patients or those presenting with seizures typically have the lowest risk, which usually ranges from 0.4% to 2% annually.12 Symptoms from a hemorrhage are typically maximal at the time of the bleed and gradually improve as the hemorrhage undergoes organization and resorption. With repeat hemorrhages, however, neurological deficits often worsen, and the risk that such deficits will become permanent increases.13 Progressive neurological deficits are usually the most common presenting symptom of infratentorial CCM. Because this region has a higher density of eloquent neural structures compared to supratentorial regions, lesions here usually become symptomatic at smaller sizes.14
Management Options
Expectant Management
Expectant management consists of regular radiographic follow-up of lesions, usually every 1 to 2 years. Because MRI is the best modality for visualizing CCM, it is the imaging technique of choice when following lesions expectantly. Each new MRI is compared to prior ones in order to detect evidence of lesion changes over time. Of particular interest are signs of lesion expansion or hemorrhage. If present, there may be a need for intervention, especially for those lesions located in neurologically high-risk areas. For patients who are not operative candidates owing to their age or because of significant medical comorbid conditions, expectant management may be the only option. Asymptomatic patients, especially those with lesions in eloquent brain regions, are also best managed nonoperatively.
Medical Management
Recent in vitro and in vivo studies on animal models have shown activation of Rho GTPases in CCM lesions, suggesting that statin therapy, which is known to inhibit signaling through these molecules, might have a role in medical treatment.15 However, clinical trials are needed to test the validity of statin treatment in CCM patients.
Surgical Resection
Complete surgical resection of CCM is the only management strategy that can be fully curative. Not only does a successful lesionectomy immediately eliminate a patient’s hemorrhage risk, but up to 80% of patients achieve seizure control postoperatively.16 As the most invasive treatment option, however, surgical resection is also the riskiest, with potential complications including permanent neurological deficits and even death. When offered to appropriate patients, however, surgical interventions usually have good outcomes. A brief explanation of the techniques employed in surgical resection of CCM is provided here.
Stereotactic Radiosurgery
The use of stereotactic radiosurgery in the treatment of CCM remains controversial.14 As the only intervention available for cavernoma treatment aside from open surgery, radiosurgery seems like an attractive alternative for patients who are either not surgical candidates or who have lesions in surgically inaccessible areas. Current imagining techniques are unable to detect complete lesion occlusion, though, making it impossible to determine whether or not this treatment modality is curative.17 Efficacy therefore must be based on clinical data such as postprocedural hemorrhage rates or on histological results such as specimens that show complete occlusion following radiosurgical intervention. Several studies have looked at these parameters following radiosurgical intervention, but none of them has found evidence supporting the cure of CCM with this procedure. Although some studies report decreased hemorrhage rates following radiosurgery,18,19 others, including some of the same studies, report an increased complication rate following this intervention, such as permanent neurological deficits.20–22 Furthermore, patients in one study had to undergo open surgical resection of their lesions after radiosurgery failed to eliminate their cavernoma.21
In terms of histological studies, evaluation of resected cavernoma specimens from patients who had undergone radiosurgery anywhere from 1 to 10 years prior failed to show complete obliteration of their lesions. Instead, the main finding in these specimens was changes consistent with fibrinoid necrosis.23
Management Decisions
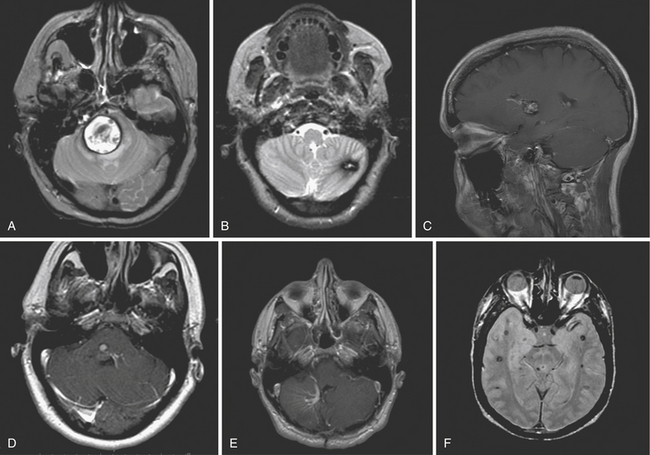
One of the most serious complications of CCM is intracerebral hemorrhage. Patients with posterior fossa cavernomas are reported to be 6.75 times more likely to present with a bleed than those with lesions in other locations.24 In addition to this increased initial bleeding risk, several studies have found an increased rehemorrhage rate among these patients. Not only were these repeat hemorrhages common, but 21% to 50% of rehemorrhages from brainstem cavernomas resulted in debilitating deficits25,26 owing to the high density of critical tracts and nuclei in this region (Table 15.1).
TABLE 15.1 Annual Hemorrhage Rates and Events Due to Cerebral Cavernous Malformations by Location∗
| Location | Hemorrhage Rate (%/yr) | Event Rate (%/yr) |
|---|---|---|
| Infratentorial | 3.8 | 10.6 |
| Supratentorial | 0.4 | 0.4 |
| Deep | 4.1 | 10.6 |
| Superficial | 0 | 0 |
∗ An event refers to neurological deterioration, defined as subjective worsening (new or increased neurological symptoms) accompanied by objective worsening of neurological findings, with or without radiologically proven hemorrhage.
Modified from Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190-197.
Given this potential for devastating outcomes with untreated cavernomas in these high-risk regions, surgical treatment must always be considered for these patients. However, the locations of these lesions also make the surgery higher risk, and this risk needs to be weighed heavily against the natural course of the disease when deciding whether to operate. In the acute setting, if a patient’s neurological status is rapidly deteriorating from mass effect related to a hemorrhage, immediate surgery might be warranted. In all other settings, four major criteria have been developed to assist in determining which patients with infratentorial lesions are appropriate surgical candidates: (1) lesions that abut the pial surface based on T1-weighted MRI, (2) lesions producing repeated hemorrhages causing progressive neurological deficits, (3) lesions with acute hemorrhage extending outside the lesion capsule, and (4) significant mass effect produced with a large intralesional hemorrhage. In addition, surgery is considered only when total resection can be achieved because lesion remnants can grow and hemorrhage as well. A study by Porter and associates looked at the results of 86 patients with brainstem cavernomas who satisfied these surgical criteria and underwent resection of their lesions. Thirty-five percent of these patients suffered temporary or permanent morbidity or death, with permanent or severe deficits occurring in 12%. The overall mortality rate was 8%, with 3.5% of deaths being surgically related. Nonetheless, patients undergoing surgery seemed to do better than those receiving conservative management. At late follow-up, 87% of surgically treated patients reported doing the same or better than before surgery, compared to 58% of patients in the nonsurgical group having this outcome. In addition, only 10% of surgical patients were worse at this late follow-up, as opposed to 42% of those not receiving surgery. Although risky, surgery is therefore justified in these cases.6 Gross and colleagues performed a systematic review of 78 studies that looked at CCM of the brainstem. Of the 745 cases examined, 683 (92%) of the cavernomas were completely resected. Although early postoperative morbidity rates ranged from 29% to 67%, it was often temporary. Of the 683 patients who received surgery, 85% had clinical improvement, 14% had deterioration of their symptoms, and 1.9% died as a result of complications related to surgery.27 These results suggest that, although risky, surgery benefits a majority of patients and might therefore be justified in carefully selected symptomatic patients.
Supratentorial lesions are reported to have a lower hemorrhage rate28 (Fig. 15.1, see Table 15.1). Although infrequent, however, overt hemorrhages might occur. In these situations, patients are usually taken to surgery based on their rate of neurological decline as well as the foreseeable consequences of mass effect from the bleed. Blood may interfere with the ability to visualize the cavernoma on imaging, in which case surgery may be deferred if the patient does not require immediate surgical decompression. In all other cases, two options can be considered. In the first, the patient can undergo elective exploratory surgery soon after the event, especially when the clot is subacute and thus soft. Otherwise, the patient can be managed expectantly until the diagnosis is confirmed.
More commonly, though, lesions in this location are associated with seizures. Antiepileptic drugs have traditionally been the initial management strategy for such patients, with surgery being considered for patients with seizures that are refractory to medication or that intensify during pharmacological treatment. Using this treatment approach, 65% of the 168 patients with supratentorial lesions in one study were free from their disabling seizures 3 years after surgery, with half of them remaining seizure free for this entire period.29 A few other studies suggest that this outcome can be improved with earlier surgical intervention. In one study, 100% of patients who had developed epilepsy within 2 months of surgery were completely free of seizures postoperatively. Patients who waited between 2 and 12 months and greater than 12 months after the onset of epilepsy to undergo surgery had seizure control rates of 76% and 52%, respectively.30 Regardless of when surgery is done, outcomes are usually good. For patients with solitary, superficial supratentorial CCM, the risks of surgical resection of cavernomas outside highly eloquent areas are only slightly higher than those of general anesthesia in trained hands. Furthermore, in the study of 168 patients discussed previously, which is one of the largest studies of supratentorial cavernomas, no deaths occurred, and mild postoperative neurological deficits were seen in only 12 patients.29
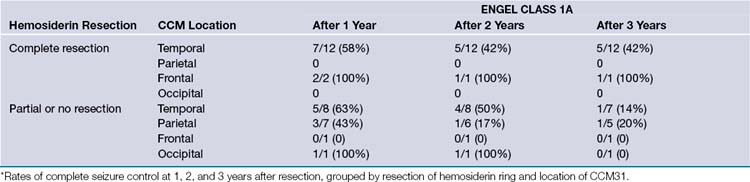
Another factor to consider when operating on patients with seizures is whether or not to resect any gliotic or hemosiderin-stained tissue surrounding the lesion. Although some studies report complete resolution of seizure activity with pure lesionectomies, others report a higher rate of seizure control with removal of additional elements surrounding the lesion. In a study of 31 patients, 64% of the 14 who underwent excision of the hemosiderin ring in addition to a lesionectomy were categorized as Engel class I (free of disabling seizures) 1 year later. Only 53% of patients who had undergone either a lesionectomy or a lesionectomy plus partial removal of the hemosiderin ring fell into this category. Fifty-nine percent and 46%, respectively, were still in this group 3 years postoperatively31 (Fig. 15.2). Another study had similar results, with 77.8% of patients being classified as Engel type Ia (free of all seizures) after a complete lesionectomy plus hemosiderin ring resection compared to 65.7% of those in the lesionectomy-only group, but the additional benefit of the hemosiderin ring resection was only seen in a subset of patients who received surgery within 2 years of their seizure onset32 (Table 15.2).
TABLE 15.2 Complete Seizure Control (Engel Class 1a—Free from All Seizures) in Relation to Cerebral Cavernous Malformation (CCM) Location and Hemosiderin Ring Resection∗
Although most CCMs are located in the brain, intramedullary spinal cavernomas account for 3% to 5% of lesions.33–35 These cavernomas can occur alone or in combination with cerebral lesions. Lesions are typically located in the thoracic cord, with cervical cavernomas being second most common, and lumbar or conus rare. Spinal CCMs usually present with a slowly progressive myelopathy causing deficits in sensation, motor skills, or both.36 Acute presentations of focal neurological deficits can also occur as a result of hematomyelia, intralesional hemorrhage, and cord compression.37 Although symptoms resulting from acute presentations often resolve spontaneously, those resulting from chronic myelopathy frequently do not improve even after surgery. Surgery will stop the progression of myelopathy, though, and therefore is warranted in symptomatic patients. In a chart review of 26 patients receiving surgery for spinal CCM, 12 (46%) were improved at long-term follow-up, 12 (46%) were unchanged, and only 2 (8%) had worsened neurological conditions.36 Surgery therefore might be the best option to prevent disease progression in select cases in order to potentially improve the patient’s condition.
Special Considerations
Another group requiring special consideration is patients with multiple lesions. Almost all cases of familial CCM and a small percentage of patients with sporadic cavernomas might have more than one lesion. Treatment of these patients should be handled expectantly, with intervention being reserved for symptoms that can be attributed to a specific lesion that is clinically active. For patients experiencing progressive neurological deficits, imaging can be helpful in determining which lesions may be active based on size changes over time. Seizure types and auras, as well as monitoring devices, can be helpful in identifying particular lesions that are symptomatic in patients presenting with seizures. If the clinically active seizures can be determined using these tools, surgical intervention may be warranted. In a recent study of 63 patients with medically refractory CCM, 11 patients had multiple lesions, with a mean of 3.7 lesions per patient. All of these patients had only one epileptogenic zone, and removal of the cavernoma at this site resulted in nine patients being classified as Engel class Ia and two as Engel Ib (free of disabling seizures) or IVc (seizure worsening postoperatively) 2 years later.38 Unfortunately de novo occurrence of lesions is common in these patients and can have a negative impact on the patient’s long-term outcome.
Genetic screening of patients with a family history of CCM is not recommended.39 The three genes currently recognized as being responsible for cavernous malformation account for 96% of familial mutations, making it possible that a fourth gene has yet to be discovered and consequently would not be detected during genetic screens.40 A study of 20 families affected by cavernomas failed to detect mutations in any of the three cavernous malformation genes in 12 of the families.41 Furthermore, the origin of sporadic CCM is unknown, and could be related to environmental factors either alone or in combination with genetic factors. Patients with a positive cavernoma family history should obtain an MRI if they experience any symptoms commonly associated with cavernomas, including headaches, seizures, and focal neurological deficits, or if they are diagnosed with a cerebral hemorrhage. In addition, genetic counseling should be offered to these patients.
Conclusion
The natural history of CCM can differ greatly among patients depending on various factors, including the location, biological state, and genetic background of the patient. Choosing an appropriate management strategy can therefore be difficult because different patients can be subject to vastly different clinical courses. In general the best treatment strategy is the one that minimizes the risks associated with the disease’s natural history while also having a low procedural risk. Therefore, infratentorial lesions with high rates of devastating hemorrhage are often treated surgically, and supratentorial lesions commonly causing seizures can either be treated medically or surgically depending on the patient’s surgical risks and seizure control. In experienced hands, outcomes from these procedures generally have low morbidity and mortality rates, and these statistics will only further improve as research provides new insight to the disease’s natural history and the long-term outcomes of different treatment options.
Baumann C.R., Acciarri N., Bertalanffy H., et al. Seizure outcome after resection of supratentorial cavernous malformations: a study of 168 patients. Epilepsia. 2007;48:559-563.
Brown R.D.Jr., Flemming K.D., Meyer F.B., et al. Natural history, evaluation, and management of intracranial vascular malformations. Mayo Clin Proc. 2005;80:269-281.
Kondziolka D., Lunsford L.D., Flickinger J.C., Kestle J.R. Reduction of hemorrhage risk after stereotactic radiosurgery for cavernous malformations. J Neurosurg. 1995;83:825-831.
Porter R.W., Detwiler P.W., Spetzler R.F., et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
Tu J., Stoodley M.A., Morgan M.K., Storer K.P. Ultrastructural characteristics of hemorrhagic, nonhemorrhagic, and recurrent cavernous malformations. J Neurosurg. 2005;103:903-909.
Please go to expertconsult.com to view the complete list of references.
1. Clatterbuck R.E., Eberhart C.G., Crain B.J., Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry. 2001;71:188-192.
2. Tu J., Stoodley M.A., Morgan M.K., Storer K.P. Ultrastructural characteristics of hemorrhagic, nonhemorrhagic, and recurrent cavernous malformations. J Neurosurg. 2005;103:903-909.
3. Wong J.H., Awad I.A., Kim J.H. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery. 2000;46:1454-1459.
4. Giombini S., Morello G. Cavernous angiomas of the brain. Account of fourteen personal cases and review of the literature. Acta Neurochir (Wien). 1978;40:61-82.
5. Lonjon M., Roche J.L., George B., et al. [Intracranial cavernoma. 30 cases. Presse Med. 1993;22:990-994.
6. Porter R.W., Detwiler P.W., Spetzler R.F., et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
7. Robinson J.R., Awad I.A., Little J.R. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709-714.
8. Clatterbuck R.E., Moriarity J.L., Elmaci I., et al. Dynamic nature of cavernous malformations: a prospective magnetic resonance imaging study with volumetric analysis. J Neurosurg. 2000;93:981-986.
9. Scott R.M., Barnes P., Kupsky W., Adelman L.S. Cavernous angiomas of the central nervous system in children. J Neurosurg. 1992;76:38-46.
10. Simard J.M., Garcia-Bengochea F., Ballinger W.E.Jr., et al. Cavernous angioma: a review of 126 collected and 12 new clinical cases. Neurosurgery. 1986;18:162-172.
11. Del Curling O.Jr., Kelly D.L.Jr., Elster A.D., Craven T.E. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702-708.
12. Brown R.D.Jr., Flemming K.D., Meyer F.B., et al. Natural history, evaluation, and management of intracranial vascular malformations. Mayo Clin Proc. 2005;80:269-281.
13. Samii M., Eghbal R., Carvalho G.A., Matthies C. Surgical management of brainstem cavernomas. J Neurosurg. 2001;95:825-832.
14. Bertalanffy H., Benes L., Miyazawa T., et al. Cerebral cavernomas in the adult. Review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev. 2002;25:1-53. discussion 4-5
15. Whitehead K.J., Chan A.C., Navankasattusas S., et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009;15:177-184.
16. Ferroli P., Casazza M., Marras C., et al. Cerebral cavernomas and seizures: a retrospective study on 163 patients who underwent pure lesionectomy. Neurol Sci. 2006;26:390-394.
17. Kim D.G., Choe W.J., Paek S.H., et al. Radiosurgery of intracranial cavernous malformations. Acta Neurochir (Wien). 2002;144:869-878. discussion 878
18. Hasegawa T., McInerney J., Kondziolka D., et al. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190-1197. discussion 1197-1198
19. Kondziolka D., Lunsford L.D., Flickinger J.C., Kestle J.R. Reduction of hemorrhage risk after stereotactic radiosurgery for cavernous malformations. J Neurosurg. 1995;83:825-831.
20. Amin-Hanjani S., Ogilvy C.S., Candia G.J., et al. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at the Harvard cyclotron. Neurosurgery. 1998;42:1229-1236. discussion 1236-1238
21. Karlsson B., Kihlstrom L., Lindquist C., et al. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88:293-297.
22. Pollock B.E., Garces Y.I., Stafford S.L., et al. Stereotactic radiosurgery for cavernous malformations. J Neurosurg. 2000;93:987-991.
23. Gewirtz R.J., Steinberg G.K., Crowley R., Levy R.P. Pathological changes in surgically resected angiographically occult vascular malformations after radiation. Neurosurgery. 1998;42:738-742. discussion 742-743
24. de Oliveira J.G., Rassi-Neto A., Ferraz F.A., Braga F.M. Neurosurgical management of cerebellar cavernous malformations. Neurosurg Focus. 2006;21:e11.
25. Abe M., Kjellberg R.N., Adams R.D. Clinical presentations of vascular malformations of the brainstem: comparison of angiographically positive and negative types. J Neurol Neurosurg Psychiatry. 1989;52:167-175.
26. Tung H., Giannotta S.L., Chandrasoma P.T., Zee C.S. Recurrent intraparenchymal hemorrhages from angiographically occult vascular malformations. J Neurosurg. 1990;73:174-180.
27. Gross B.A., Batjer H.H., Awad I.A., Bendok B.R. Brainstem cavernous malformations. Neurosurgery. 2009;64:E805-E818. discussion E818
28. Porter P.J., Willinsky R.A., Harper W., Wallace M.C. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190-197.
29. Baumann C.R., Acciarri N., Bertalanffy H., et al. Seizure outcome after resection of supratentorial cavernous malformations: a study of 168 patients. Epilepsia. 2007;48:559-563.
30. Cohen D.S., Zubay G.P., Goodman R.R. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg. 1995;83:237-242.
31. Baumann C.R., Schuknecht B., Lo Russo G., et al. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia. 2006;47:563-566.
32. Stavrou I., Baumgartner C., Frischer J.M., et al. Long-term seizure control after resection of supratentorial cavernomas: a retrospective single-center study in 53 patients. Neurosurgery. 2008;63:888-896. discussion 897
33. Furuya K., Sasaki T., Suzuki I., et al. Intramedullary angiographically occult vascular malformations of the spinal cord. Neurosurgery. 1996;39:1123-1130. discussion 1131-1132
34. Ghogawala Z., Ogilvy C.S. Intramedullary cavernous malformations of the spinal cord. Neurosurg Clin North Am. 1999;10:101-111.
35. Harrison M.J., Eisenberg M.B., Ullman J.S., et al. Symptomatic cavernous malformations affecting the spine and spinal cord. Neurosurgery. 1995;37:195-204. discussion 205
36. Jallo G.I., Freed D., Zareck M., et al. Clinical presentation and optimal management for intramedullary cavernous malformations. Neurosurg Focus. 2006;21:e10.
37. Labauge P., Bouly S., Parker F., et al. Outcome in 53 patients with spinal cord cavernomas. Surg Neurol. 2008;70:176-181. discussion 181
38. Rocamora R., Mader I., Zentner J., Schulze-Bonhage A. Epilepsy surgery in patients with multiple cerebral cavernous malformations. Seizure. 2009;18:241-245.
39. Revencu N., Vikkula M. Cerebral cavernous malformation: new molecular and clinical insights. J Med Genet. 2006;43:716-721.
40. Labauge P., Laberge S., Brunereau L., et al. Hereditary cerebral cavernous angiomas: clinical and genetic features in 57 French families. Sociètè Française de Neuroçhirurgie. Lancet. 1998;352:1892-1897.
41. Bergametti F., Denier C., Labauge P., et al. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet. 2005;76:42-51.