Chapter 26 Cautery-assisted palatal stiffening operation
1 INTRODUCTION
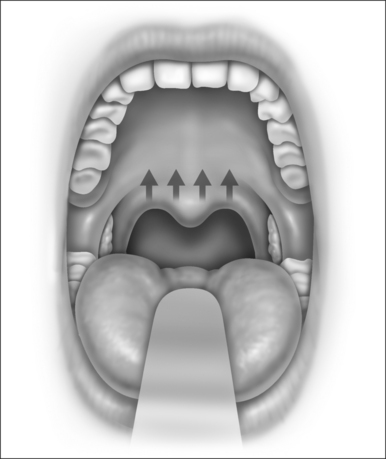
Snoring is caused by the vibration of the structures in the oral cavity and oropharynx – namely the soft palate, uvula, tonsils, base of tongue, epiglottis and pharyngeal walls. Most authorities would concur that over 80% of snoring is due to palatal flutter, caused by vibration of the uvula and the soft palate. Hence, it would be conceivable that techniques to stiffen the palate would be beneficial in reducing snoring. Different techniques using various instruments (e.g. the laser, cautery and coblator) have been used to achieve the same outcome. The palatal stiffening operation was first introduced by Ellis in 19941 and improvised by Mair in 2000.2 Both authors utilized cautery to stiffen the palate. The original cautery-assisted palatal stiffening operation (CAPSO) procedure was based on stripping a ‘diamond’ shaped area of mucosa off the soft palate and uvula, with the aid of cautery under local anesthesia (Figs 26.1 to 26.4). Although good results were reported, the procedure produced a stellate puckered scar on the soft palate that resulted in tenting of the lateral pharyngeal walls and therefore narrowing of the late-ral distance between the tonsillar pillars (Fig. 26.5). These anatomic manifestations may explain why some patients did not have any clear benefit from the procedure.
2 PATIENT SELECTION
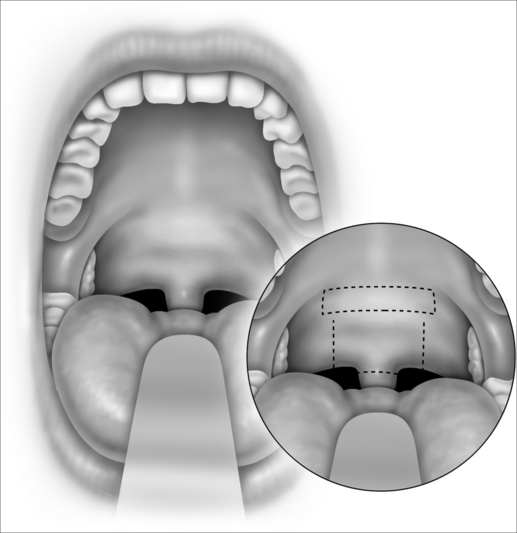
This modified CAPSO (Fig. 26.6) procedure is performed for patients with mild obstructive sleep apnea (OSA) (Apnea/Hypopnea Index (AHI) < 15) or patients who are primary snorers (AHI <5). The inclusion criteria include patients above 18 years of age, Body Mass Index (BMI) < 33, tonsil size grade 1 and 2, elongated uvula, all Mallampati grades, minimal base of tongue collapse (<25%) as seen on Mueller’s maneuver.
All patients undergo a thorough physical examination, nasoendoscopy, and a level I overnight attended poly-somnography (PSG). Patients also complete the Epworth Sleepiness Scale (ESS) and a visual analogue scale (VAS) for snoring before surgery and 7, 14, 30, 60 and 90 days after surgery. The sleep partner also completes a similar scale for snoring. Patients also complete a VAS for pain on postoperative days 1, 3, 7 and 14. Examination includes height, weight, neck circumference, BMI, and assessment of the nasal cavity, posterior nasal space, oropharyngeal area, soft palatal redundancy, uvula size and thickness, tonsillar size and Mallampati grade. Flexible nasoendoscopy is performed for all patients, and collapse during a Mueller’s maneuver is graded for the soft palate, lateral pharyngeal walls and base of tongue on a five-point scale.3
3 SURGICAL TECHNIQUE
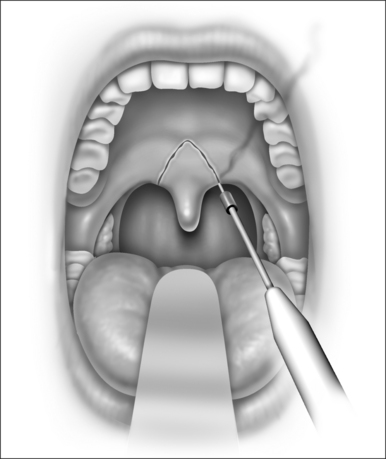
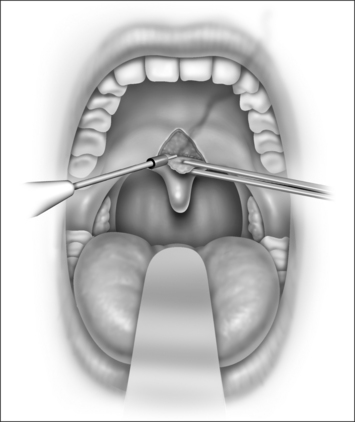
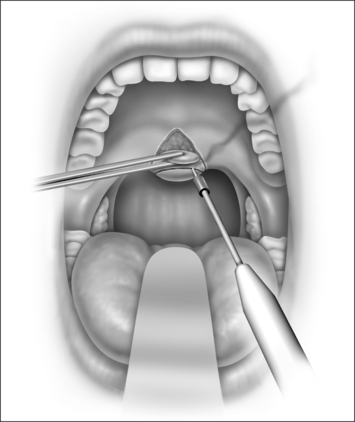
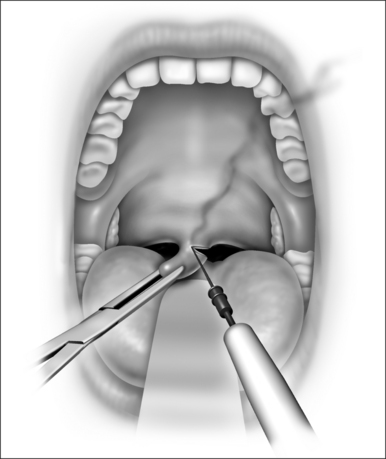
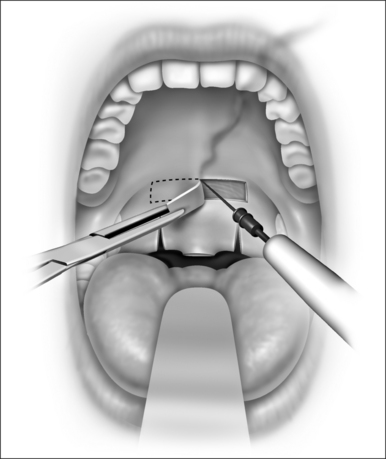
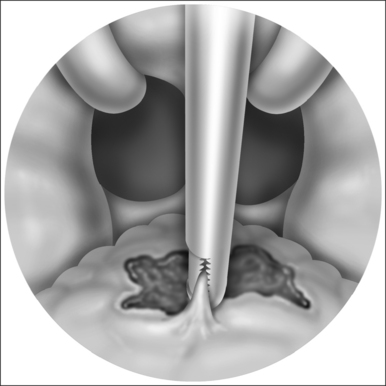
The procedure is done under local anesthesia in the office as an outpatient. The patient is seated in an examination chair with the mouth open. Topical benzocaine (14%) is used to anesthetize the palatal region. A total of 3 ml of 1:100,000 adrenaline and 2% xylocaine is injected into three sites of the soft palate. An uvulectomy is performed (Fig. 26.7), followed by vertical cuts on either side of the uvula (Fig. 26.8), through both soft palatal arches. A horizontal rectangular strip of mucosa is removed from the soft palate (50 mm in length by 7 mm in width), down to the muscle layer (Fig. 26.9). Hemostasis is achieved with electrocautery. All patients are prescribed with anesthetic gargles (Difflam) and lozenges (Difflam), non-steroidal anti-inflammatory agents (Naproxen sodium), narcotics (like codeine), and cyclo-oxygenase-2 inhibitors. The co-blator technique may also be used as an instrument for this technique.
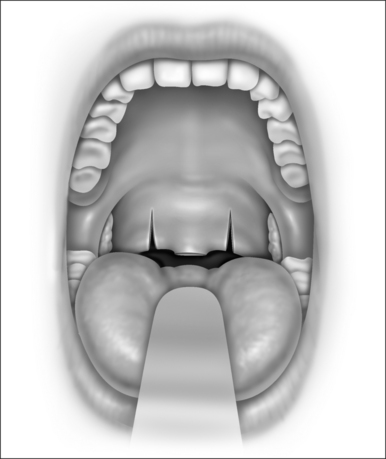
Fig. 26.8 (3 Oral cavity). Bilateral vertical trenches are created through-and-through mucosa and muscle.
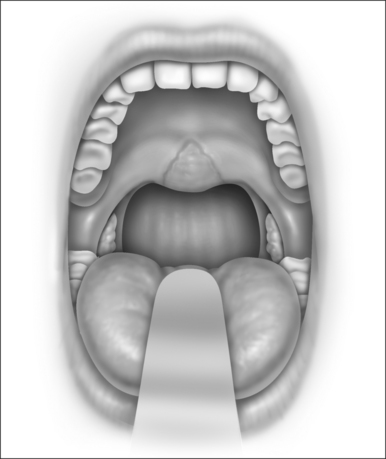
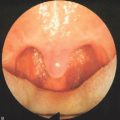
With the healing process, as the palatal tissue heals, a fiberoptic palate results and retracts superiorly, with the oropharyngeal airway widened superiorly (Fig. 26.10).
4 POSTOPERATIVE MANAGEMENT AND COMPLICATIONS
All patients are monitored in the recovery room for a further 30 minutes, with monitoring of their blood pressure, pulse rate and oxygen saturation. They are given adequate analgesia in the form of anesthetic gargles (Difflam)and lozenges (Difflam), non-steroidal anti-inflammatory agents (Naproxen sodium), narcotics (like codeine), and cyclo-oxygenase-2 inhibitors. All patients are advised to take adequate oral fluid hydration and soft blended diet in the first postoperative week.
4.1 SUCCESS RATE OF PROCEDURE
In this series, there were 13 patients who underwent this procedure for management of their snoring or mild OSA. All 13 patients were males, with a mean age of 35.7 years old (range of 24 to 47 years old). The mean BMI was 28.4 (range of 21.6 to 31.2). All patients were classified as Fried-man stage II and III,4 with tonsil size 0, 1 or 2. The mean preoperative AHI was 11.6 (range of 3.5 to 14.8), with a mean preoperative Apnea Index (AI) of 5.6 (range 0.5 to 9.1). The mean preoperative lowest oxygen saturation (LSAT) was 91.4% (range of 88% to 94%). All patients had a 3-month postoperative polysomnogram done. There were five patients who were simple snorers (mean AHI of 3.9) and eight patients who had mild OSA (mean AHI of 12.3).
Mair and Day2 reported a 77% success rate for reduction of snoring in 206 patients, at 1 year, who underwent the cautery-assisted palatal stiffening operation (CAPSO). Ellis1 similarly revealed excellent results with this procedure. Kamami first described the laser-assisted uvulopalatoplasty (LAUP)5 for patients with snoring. Kamami studied 417 snorers who underwent LAUP and found a reduction of snoring in 95% of the patients, after 1 year. Most authors report modest improvement after LAUP for patients with mild OSA, although a success rate as high as 75% was described by Walker et al.6
1. Ellis PD. Laser palatoplasty for snoring due to palatal flutter: a further report. Clin Otolaryngol Allied Sci. 1994;19(4):350-351.
2. Mair EA, Day RH. Cautery-assisted palatal stiffening operation. Otolaryngol Head Neck Surg. 2000;122(4):547-556.
3. Terris DJ, Hanasono MM, Liu YC. Reliability of the Muller maneuver and its association with sleep-disordered breathing. Laryngoscope. 2000;110:1819-1823.
4. Friedman M. Clinical staging. Uvulopalatopharyngoplasty. In: Snoring and Obstructive Sleep Apnea, 3rd edn, Chapter 9, Section 9.2. Fairbanks DNF et al., eds. Lippincott Williams & Wilkins; 2003:120–27.
5. Kamami YV. Outpatient treatment of sleep apnea syndrome with CO2 laser, LAUP: laser-assisted UPPP results on 46 patients. J Clin Laser Med Surg. 1994;12(4):215-219.
6. Walker RP, Grigg-Damberger MM, Gopalsami C. Laser-assisted uvulopalatoplasty for the treatment of mild, moderate, and severe obstructive sleep apnea. Laryngoscope. 1999;109(1):79-85.